Abstract
Multicellular organisms arise from a single genome template in the zygote, necessitating the cells of the developing embryo to up- and downregulate specific genes to establish and maintain their identity. This template is maintained, propagated, and interpreted as chromatin, a polymer of nucleic acids and associated structural and regulatory proteins. Recent genome-wide surveys documented a wealth of disease-associated mutations in chromatin factors, indicating their fundamental significance and potential for therapeutic targeting. However, chromatin factors exist in a complex balance, with a single deficiency often leading to pleiotropic downstream effects. Here, we review the mechanisms of chromatin regulation and partitioning, highlighting examples of how these processes are altered in human diseases. We argue that loss of chromatin fidelity, both locally at specific genes and regulatory elements, and globally at the megabase-scale, contributes to many pathological states and may thus represent an intriguing target for corrective interventions.
Graphical Abstract
Graphical Abstract.
Introduction
How the zygote gives rise to multiple cell types during development, while preserving largely identical genome across lineages has been studied since the dawn of modern developmental biology [1]. Both biochemistry and molecular biology rapidly converged on gene promoters as the defining regulatory features of the genome and initiated decades of discovery in their respective fields, including the identification of “master regulator” transcription factors (TFs), lineage-defining regulators sufficient to drive gene expression programs toward specific cell fates. From the discovery of MyoD by the Weintraub lab in the late 1980s as a key factor in myogenesis [2, 3], to the subsequent identification of the four factors sufficient for pluripotency reprograming by Takahashi and Yamanaka [4], TFs are rightly regarded as key drivers in cell fate decisions. A recent survey of the human genome suggests that ∼1,600 out of ∼23,000 genes encode TFs [5], allowing for nearly infinite combinations, limited only by the availability of specific TF binding sites on the DNA and the presence of the TFs themselves. However, even the groundbreaking Yamanaka experiment offers two interpretations: on the one hand, ectopic TFs dramatically altered cell fate in the experiment, thus fulfilling the sufficiency criteria. On the other hand, only an estimated 0.02% of fibroblasts expressing the four factors successfully converted into stem cells [4]. This together with later observations that additional factors alter cell fate plasticity [6, 7] suggests that the promoter-centric paradigm does not sufficiently account for all observations, and additional mechanisms ensuring the stability of transcriptional programming must exist.
The genome is propagated, maintained, and interpreted within the context of the chromatin fiber—a polymer complex whose main components are DNA and histone proteins. The ∼146 base pairs of DNA wrapped around the octamer of histone proteins, H3/H4 tetramer and two H2A/H2B dimers, form the nucleosome core particle, the basic repeating unit of chromatin [8–10]. Other factors, including linker histones (H1) and high mobility group (HMG) proteins, as well as non-coding RNAs, further contribute to chromatin fiber compaction [11–13]. Crucially, while the DNA sequence (with a few notable exceptions [14–16]) is largely invariant and forms the basis of Mendelian inheritance, dynamic modifications of the chromatin fiber, as well as the degree of compaction and three-dimensional topology within the nucleus, are both variable across cell lineages and instructive to the gene expression [17, 18]. This layer of non-sequence-dependent regulation in the cell is often referred to as epigenetic; however, the strict definition of the term implies transgenerational inheritance of acquired traits, which is the subject of some controversy [19], and we favor the use of “chromatin biology” over “epigenetics” to denote the broader field of study.
For the information to be encoded in the genome in a sequence-independent manner, three core conditions must be met. First, a robust mechanism for establishing such modifications must be present. These factors have been extensively studied since the transcriptional activator GCN5 was shown to be a histone lysine acetyltransferase [20], and dozens of post-translational modifications in all components of the chromatin fiber have been characterized in detail [21]. Enzymes implicated in establishment and removal of specific modifications are commonly referred to as “writers” and “erasers,” respectively. Importantly, these are frequently located downstream of the sequence-specific transcriptional activators and repressors, highlighting the close connection between sequence-dependent and -independent regulation [22, 23]. Second, these modifications must be interpreted by cellular machinery. Indeed, a vast array of “reader” domains has been identified since the discovery of p300/CBP-associated factor (PCAF) bromodomain interaction with acetyl-lysine on H4 N-terminal tail [24]. Combinations of multiple reader domains within a single protein or protein complex enable logical connections (“AND” and “NOT”) for specificity, local separation of function, and redundancy [25, 26]. Third, maintenance mechanisms are required to ensure that specific information is retained throughout development. These are the more enigmatic of the three mechanisms: while the DNA structure itself provides clues to its semiconservative replication, the maintenance of specific chromatin modifications in situ relies on many distinct mechanisms that vary depending on the cell type (e.g. mitotically active versus non-dividing cells), substrate (e.g. modifications of DNA or histone proteins), and chemical nature of modification. Thus, while the role of the DNA repair mechanism became clear soon after the Avery experiment [27] and remains the focus of intense investigation [28], the mechanisms ensuring fidelity of chromatin modifications are still less well understood.
Recent methodological advances open exciting opportunities to understand the chromatin maintenance mechanisms in development and disease. Here, we review select examples that contribute to the stability of transcriptional programing, focusing on the molecular basis of broad domain establishment and maintenance, and their relationship to the topological compartmentalization of the nucleus in three-dimensional (3D) space. We then discuss how these processes relate to human disease, particularly aging, neurodegenerative, and metabolic disorders.
Chromatin modifications are established and interpreted as ensembles
Genomic regions with specific activity and function, including architectural chromosomal elements and cis-regulatory modules, as well as transient events such as DNA replication and repair, are demarcated by distinct chromatin modifications. The incorporation of histone variants into the nucleosome core particle, DNA modifications, and post-translational histone modifications have been extensively studied in disease context, and we will refer the reader to many excellent reviews published in the recent years [29–31]. Here, we will only briefly describe recent studies of how distinct states of chromatin are established and maintained in the context of their nuclear surroundings, focusing mainly on developmental regulation by Polycomb group (PcG) complex.
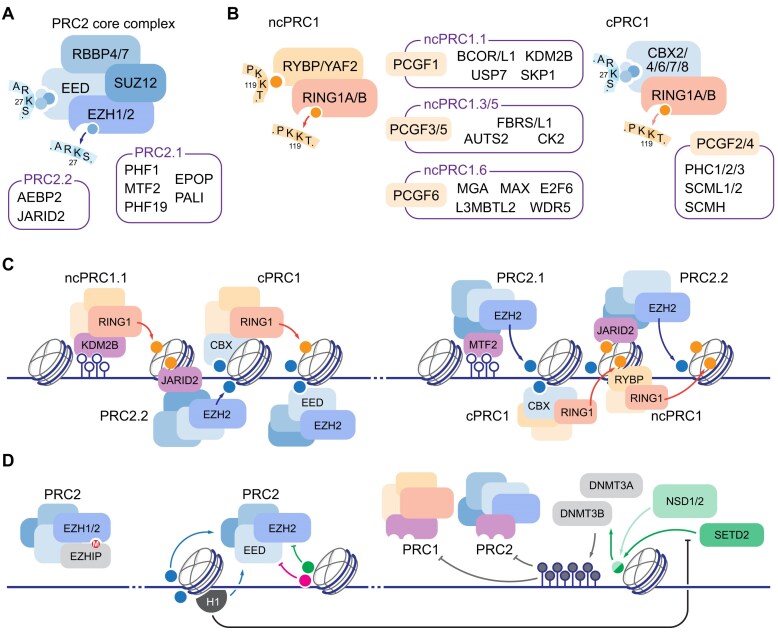
A convenient classification of factors operating in chromatin into functionally distinct “readers” and “writers” of post-translational modifications is complicated by the close relationship between the two: writers frequently incorporate reader subunits and domains, and/or are sensitive to the modification state of the neighboring amino acid residues. A paradigmatic example of such a relationship is the PcG complex, which is best known for its developmentally regulated repressor function [32]. In humans, core Polycomb Repressor Complex 2 (PRC2) contains the critical histone H3 lysine 27 (H3 K27) methyltransferase “writer” subunit Enhancer of Zeste Homolog 1 or 2 (EZH1/2), two structurally critical factors: Suppressor of Zeste 12 homolog (SUZ12) and Retinoblastoma-Associated Protein 46/48 (RBAP46/48, also known as RBBP4/7), and an essential “reader” component Embryonic Ectoderm Development (EED) that recognizes methylated H3 K27. This arrangement enables a feed-forward mechanism in which H3 K27 methylation, established by PRC2, in turn recruits PRC2 for processive enzymatic activity. This is additionally supported by allosteric activation mechanisms, including via PRC2 subunits JARID2 and AEBP2 or PALI1 [33, 34] (Fig. 1A). Remarkable structures of PRC2 in complex with the chromatin substrate reveal how the EED “reader” anchors the core complex while EZH2 methyltransferase extends to the adjacent nucleosome [35]. Together with the single-molecule studies of Polycomb processivity [36], these results suggest that PRC2-dependent spreading of H3 K27me3 may be sensitive to the distance between neighboring nucleosomes and is thus regulated by pre-existing state of the chromatin substrate.
Figure 1.
Mechanisms of genome partitioning by Polycomb Group complexes. (A) Core PRC2 complex consists of four subunits, EED and EZH1/2 representing “reader” and “writer” modules for H3 K27 methylation. Sequence context of H3 tail is shown. Subunits associated with PRC2.1 and PRC2.2 variants are listed. (B) Two major PRC1 complexes are characterized by distinct subunit composition: non-canonical PRC1 (ncPRC1, left) contains H2A K119 “reader” RYBP/YAF2, and canonical PRC1 (cPRC1, right) contains one of several Chromobox proteins (CBX2/4/6/7/8) associated with H3 K27me3. Both complexes include RING1 E3 ubiquityl ligase “writer” module, with ncPRC1 characterized by higher enzymatic activity, and distinct complement of additional factors, anchored by PCGF subunits, listed in corresponding boxes. Sequence context of H3 and H2A tails are shown. (C) Recruitment and spreading of PcG domains in the vertebrate genome: left, unmethylated CpG islands are recognized by ncPRC1.1 subunit KDM2B, leading to initial H2A K119 ubiquitylation (orange circles) recognized by PRC2.2 via JARID2 subunit; methylated H3 K27 is in turn recognized by cPRC1 for compaction and/or further H2A ubiquitylation, and processive spreading of H3 K27 methylation is established by coordinate EED “reader” and EZH2 “writer” activity of PRC2; right, unmethylated CpGs associate with PRC2.1 subunit MTF2; initial H3 K27 methylation in turn recruits cPRC1 via CBX subunits, and PRC2 recruitment is propagated via H2A K119 recognition via JARID2, or H3 K27me3 recognition via EED. (D) Positive feedback regulation of PcG is balanced by incorporation of negative regulator subunits (EZHIP, left), incorporation of histone modifications that prevent H3 K27 methylation in cis (green and magenta circles correspond to H3 K36 and H3 K4 methylations; other modifications, including H3 phosphorylations, may act in similar fashion [53], middle), and activity of DNA methyltransferases, recruited in part by H3 K36 di- and tri-methylation by NSD1/2 and SETD2 enzymes, respectively (right). Direct stimulation of PRC2 by H1 incorporation is thus reinforced by reducing NSD1/2 activity in chromatin [6, 52].
PRC1 is the second component of the PcG system and also possesses distinct “reader” and “writer” functions characterized by a number of self-reinforcing mechanisms. All PRC1 subcomplexes share the RING1 E3 ubiquityl ligase, which catalyzes H2A K119 monoubiquitylation. “Canonical” PRC1 contains one of five H3K27me3-binding Chromobox (CBX) 2/4/6/7/8 subunits, a self-assembling Polyhomeotic (PHC) 1/2/3 subunit, and PHC-binding PCGF subunit 2 or 4. “Non-canonical” PRC1, on the other hand, is characterized by the incorporation of the RYBP or YAF2 subunit, as well as PCGF 1/3/5 or 6, all of which stimulate H2A ubiquitylation by RING1. Additional PRC1 subcomplexes with distinct cell-specific accumulation profiles and developmental functions are known (reviewed in [37], Fig. 1B). Recent structural study of non-canonical PRC1 in complex with a dinucleosome substrate shows a mechanism remarkably similar to that of PRC2 processivity: while the paired EED “reader” and EZH2 “writer” mediate trans-nucleosomal engagement in PRC2, RYBP binds to H2A K119Ub, while RING1 deposits ubiquitylation on the adjacent nucleosome, thus similarly allowing for processive spread of Polycomb domain [38].
Several non-exclusive models of PcG recruitment in vertebrates have been proposed, employing both PRC1- and PRC2-dependent targeting mechanisms. PRC1-dependent targeting is initiated by the recruitment of variant PRC1 to unmethylated CpG islands, followed by demethylation of H3 K36 by KDM2B and RING1-dependent H2A K119 ubiquitylation. This modification is recognized by JARID2, a regulatory subunit of PRC2 that in turn stimulates its K27me3 activity (reviewed in [39]). PRC2-dependent targeting is established via MTF2, a subunit of Polycomb-like (PCL) family, which directly recruits PRC2 to primary target sites defined by high CpG density, low 5-methyl-cytosine (5-mC) content, and distinct DNA shape [40] (Fig. 1C). Methylation of histone H3 K27 is then recognized by canonical PRC1 complexes via Chromobox (CBX) “readers,” of which CBX2, 4, 6, and 8 are associated with high nucleosome compaction activity via a distinct Compaction and Phase-Separation (CaPS) domain, whereas CBX7 appears to completely lack CaPS [41]. Since expression of the CBX subunits varies depending on tissues and developmental stages, other mechanisms of Polycomb-dependent repression have been proposed, including PRC2-dependent compaction via EZH1-dependent dimerization [42]. Further, PRC1 and PRC2 have both overlapping and unique functions in developmental gene regulation: loss of PRC2 is dispensable for maintaining the pluripotency of embryonic stem cells in culture [43], while loss of PRC1 causes immediate loss of pluripotency due to derepression of the differentiation program [44]. Although both PRC1 and PRC2 appear together in the last eukaryotic common ancestor (LECA), the complexes evolved independently, suggesting that the convergence of regulatory function may be a secondary acquisition [45].
While several elegant positive feedback mechanisms explain PRC1/2 propagation, what negative regulatory mechanisms limit PcG activity and how the boundaries to PcG-demarcated domains are established, are critical questions that yet remain to be fully deciphered. The accumulation of PRC variant complexes with reduced enzymatic activity, exemplified in the extreme case by incorporation of the autoinhibitory PRC2 subunit EZHIP/CXorf67, provides a global, cell-type specific mechanism [46–48]. Locally, endogenous lysine 36 (K36) methylation on histone H3, another broadly distributed core histone modification, appears to play an important role (Fig. 1D). Somatic loss of H3K36me in rare tumors expressing dominant negative K36M “oncohistone” leads to expansion of PRC2-dependent K27me and loss of PRC1-mediated repression by “reader” dilution [49]. A similar mechanism drives a subset of head and neck squamous cell carcinomas, associated with either loss of H3 K36-specific dimethylase NSD1 or, rarely, H3 K36M mutations [50]. Conversely, expansion of H3 K36me2 by the MMSET (NSD2) methyltransferase alters H3 K27me landscape in multiple myeloma, restricting PRC2 activity to narrow regions that undergo stronger repression [51]. Aspects of this regulation are reflected in a class of lymphomas driven by missense mutations in H1 linker histones: global loss of H1 in germinal center B cells broadly increases chromatin accessibility, with concomitant expansion of H3 K36me2 and contraction of H3 K27me-demarcated domains. This, in turn, drives derepression of early stem cell programs in terminally differentiated B cells, with resulting transcriptional profiles remarkably similar to both multiple myeloma and hematopoietic stem cells [6]. While H1 incorporation stimulates PRC2 activity and counters NSD2 function in vitro [6, 52], the hierarchy of events in chromatin upon H1 loss in vivo remains unknown. In addition to K36 methylation, other H3 modifications regulate PRC2 activity, including serine 28 phosphorylation, representing a well-characterized methyl-phos “switch” [53], lysine 4 methylation, and tail arginine methylations [54]. Interestingly, cancer-associated missense mutations in H3 R2 and R26 inhibit PRC2 activity in cis [55], thus potentially destabilizing the transcriptional repression established by broad Polycomb-dependent domains during development.
DNA methylation plays both direct and indirect roles in Polycomb regulation, restricting the PcG seeding to unmethylated CpG islands and limiting spread to unmethylated regions through its connection to H3 K36 methylation (Fig. 1D). The latter mechanism is particularly intriguing, as vertebrate de novo DNA methyltransferases DNMT3A and DNMT3B are distinctly recruited to di- and tri-methylated H3 K36, respectively [56, 57]. Further, an alternative isoform of DNMT3A was recently reported to contain a ubiquitin-dependent recruitment region (UDR) within its N-terminus; while targeting of DNMT3A to H3 K36-methylated regions takes precedence under normal conditions, both global loss of K36me2 or disease-associated missense mutations re-target DNMT3A to PRC1-dependent H2A K119-ubiquitylated regions [58]. While the physiological significance of this mechanism in normal development is yet unclear, this cryptic UDR domain is a conserved feature of a wild type protein, and ability to target de novo DNA methyltransferase activity to Polycomb domains may provide a much-needed mechanism to interrupt the feed-forward regulation.
Hemimethylated CpG dinucleotides are re-methylated upon DNA replication by maintenance methyltransferase DNMT1 and its cofactor Ubiquitin-like PHD and RING finger domain-containing protein 1 (UHRF1), while de novo methyltransferases DNMT3A and 3B, together with the catalytically inactive DNMT3L or DNMT3B3 cofactors, as well as UHRF1 [59], establish new methylation patterns, and ensure fidelity of DNMT1-dependent remethylation [60]. Importantly, histone modifications represent a key role in targeting DNMTs to distinct genome compartments, thus ensuring the fidelity of methylation [60]. A remarkable recent structural study of DNMT3A2/3B3 heterotetramer with the dinucleosome substrate demonstrate both the targeting and allosteric mechanisms of the complex, where the enzymatic activity is regulated by simultaneous presence of H3 K36 methylation and absence of K4 methylation, and is restricted to the linker DNA outside the nucleosome core particle [61]. As DNMT3 complex prefers short internucleosomal linker DNA, these studies echo the observations that actively transcribed and H3 K36 methylated regions are characterized by shorter internucleosomal distances, while longer nucleosome repeat length in H3 K27-methylated chromatin allows for linker histone H1 incorporation and more efficient higher-order compaction [62]. Loss of H1 indeed reduced the nucleosome repeat length and increased the global H3 K36 dimethylation [6, 63]; whether DNMT3A activity was altered remains to be tested.
Fundamentally, the regulatory circuit between Polycomb complexes, DNA methyltransferases, and their cognate substrates functions as a neural network, with multiple nodes represented by specific modifications, edges corresponding to positive or negative regulation, and directionality imposed by biochemical constraints. Positive feedback regulation, prevalent in enzymes operating to establish broad chromatin domains, necessitates such constraint. In fact, in silico modeling of epigenetic modifications established by “reader-writer” complexes demonstrate that negative regulation—by limiting the enzyme concentration, or via negative regulation of its activity—is essential for the stable maintenance of heritable patterns [64]. Account of the negative regulators is thus essential to better understand how chromatin landscape is established and maintained.
Genome organization is maintained in three dimensions
Distinct chromatin modifications co-segregate within the three-dimensional nuclear space, mirroring the organization of genomic DNA. Both histone modifications established by “reader-writer” machinery, and non-histone proteins associated with regulatory elements partition into distinct compartments, mediated by a combination of localized enzymatic activity anchored by “reader” modules [25] and charge-based interactions between intrinsically disordered regions [65]. Importantly, these two mechanisms are not mutually exclusive, as exemplified by presence of both H3 K27 methyl-“reader” chromodomain and phase-separating CaPS domain in several CBX subunits of PRC1 complex [41], and observations that association of yeast Heterochromatin protein 1 (HP1) homolog Swi6 with H3 K9-methylated nucleosome alters the nucleosome core conformation to facilitate partitioning of HP1-bound chromatin fiber into phase-separated condensates [66]. These interactions are typically confined to megabase-scale topologically associated domains (TADs) anchored by architectural proteins, in mammals represented by the zinc finger insulator protein CTCF and Cohesin complex, implicated in chromatin loop extrusion in interphase chromosomes [67]. More fine-grained interactions between cis-regulatory elements and target promoters are established by additional factors, including Mediator complex [68].
With average enhancer–promoter distance in vertebrates estimated at 20–50 kb, and occasionally exceeding hundreds of kilobases [69], how the regulatory elements find their targets is a fundamental question. Remarkably, not only the arrays of regulatory elements are brought to target promoters, but co-transcribed genes themselves are coupled in three-dimensional space, assembling into “topological operons” [70]. Curiously, while acute depletion experiments demonstrated near-complete disappearance of TADs upon CTCF or Cohesin loss [71] only modest effects on enhancer–promoter communication and resulting gene expression were observed under steady state [71, 72]. These observations suggest that, once established, enhancer–promoter communication is maintained independent of TAD anchors. Further, multiply-rearranged “balancer” chromosomes widely used in fruit fly genetic studies demonstrate distinct TAD organization yet remarkably few inappropriate enhancer–promoter activations [73]. Together, these observations suggest that TAD organization may be redundant with other mechanisms of gene regulation and is not the sole driver of transcriptional fidelity.
Lysine methylations of the histone H3 N-terminal tail have been extensively implicated in 3D genome organization. While both H3 K9me and K27me were extensively documented at lamina-associated domains (LADs) [74, 75], recent studies integrating bisulfite conversion into HiC workflow to map protein–DNA interactions (LIMe-HiC) demonstrated antagonistic role for H3 K27me3 in LAD organization [76]. Loss of PRC2 resulted in bipartite reorganization of facultative heterochromatin: one subset of genes sustained repression in absence of PRC2 by moving into LADs and gaining H3 K9me3, while another subset retained at the nuclear interior was derepressed. These studies highlight multiple compensatory mechanisms of nuclear organization, with simultaneous loss of several factors required for fully penetrant phenotype. Additional nuclear landmarks were integrated into the functional analyses of genome organization using optical microscopy coupled with analyses of histone modifications and replication timing [77]. In these studies, speckle attachment regions were identified as additional zones correlating with genome activity, and dynamics of chromatin domains were determined by both histone modifications and nuclear lamina composition.
Chromatin conformation is linked to cell specialization, with closely related cell types demonstrating distinct three-dimensional organization. Extensive reorganization of chromatin topology upon activation of specific programs in pyramidal neurons in the hippocampus and dopaminergic neurons in ventral tegmental area preferentially impacted genes implicated in synaptic plasticity and addiction, respectively [78]. Intact function of Topoisomerase 1 (TOP1) is required for such reorganization, and long genes are preferentially sensitive to TOP1 inhibition [79]. Remarkably, many of such genes are implicated in neurodevelopmental disorders. Cell-specific chromatin (mis)organization may thus be both the underlying cause of disease and potential target of therapeutic interventions.
Chromatin is maintained across the cell cycle
Every nucleated human cell contains millions of nucleosomes, each presenting a barrier for all DNA-templated processes, and requiring a reassembly mechanism to ensure preservation of epigenetic information through cell divisions. Whereas semiconservative nature of DNA replication provides a robust template to establish methylation patterns at palindromic CpGs, the problem presented by nucleosome transfer is orders of magnitude more complex, both due to the vast number of histone variants and modifications, and a lack of sequence specificity encoded in the nucleosome structure itself. Many chaperones and histone modifications involved in local recycling of nucleosomes are documented, in particular within genetically tractable yeast models, from initial observations that labeled nucleosomes are largely retained at their loci during replication [80], to recent studies taking advantage of AI structure predictions to identify new chaperones and surfaces involved in this process [81–83].
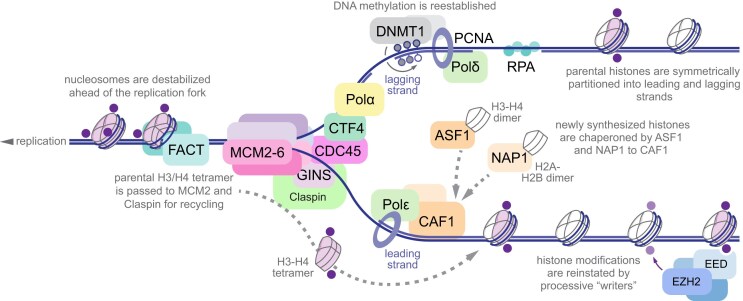
Eukaryotic replication machinery, referred to as Replisome Progression Complex (RPC), contains molecular mechanisms for both DNA synthesis and chromatin assembly, including CMG helicase complex containing Cdc45, Minichromosome maintenance (Mcm)2–7 hexamer, and go ichi ni san (GINS) complex of Psf 1, 2, 3 and Sld5 subunits, as well as Pol α-primase coupled to CMG by Ctf4 trimer, checkpoint effector Mrc1, Tof1-Csm3 pause complex, H2A/H2B histone chaperone Facilitates Chromatin Transcription (FACT), and Topoisomerase I [84–86]. Single-stranded DNA is protected by Replication protein A (RPA) assemblies [87], and DNA Polymerases δ and ϵ are clamped to lagging and leading strands via Proliferating Cell Nuclear Antigen (PCNA) trimer [88], which directly interacts with DNMT1 for remethylation of nascent DNA strands [89, 90]. Anti-silencing factor 1 (Asf1) is associated with Chromatin Assembly Factor 1 (CAF-1) complex, and together with Rtt106 orchestrates reassembly of H3/H4 tetramer following replication [91, 92]. Another H3/H4 chaperone NPM1 is recruited to RPC via Mcm2, and recruits PRC2 complex to re-establish the integrity of H3 K27me-demarcated domains upon nucleosome reassembly [93] (Fig. 2). Importantly, even perfectly efficient redistribution of old histones between the leading and lagging strands would result in a 2-fold dilution of all pre-existing modifications apart from ones established on unincorporated histones; the cell-cycle specific regulation of maintenance “writers” is thus of significant interest.
Figure 2.
Chromatin memory at the replication fork. Replication fork is shown progressing right to left, with lagging strand on the top and leading strand on the bottom. Parental histones are shown in light purple, with pre-existing post-translational modifications in dark purple. Nucleosomes are destabilized by FACT and parental H3/H4 tetramers are passed onto MCM2 and Claspin for symmetric recycling between the leading and lagging strands. Newly synthesized histones (white) are chaperoned by ASF1, NAP1 and CAF1 on both strands to maintain the nucleosome density. Histone modifications are reinstated by processive “reader”-“writer” activity of the cognate enzymes (light purple, only PRC2 is shown for clarity). DNA methylation is reestablished by maintenance methyltransferase DNMT1 via recognition of hemimethylated CpGs. Histone chaperones and DNMT1 operate on both strands but are omitted from lagging or leading strand for clarity.
How combinations of histone modifications regulate the engagement of replication machinery, and whether the decision to recycle paternal histones locally or replace the nucleosome with newly synthesized and thus minimally modified histones is driven by local chromatin landscape, are among the key questions in chromatin biology. Retention of both H3 K4 and K27 trimethylation post-replication was documented by a combination of chromatin immunoprecipitation (ChIP) with thymidine analog EdU pulse labeling to distinguish newly synthesized strands after replication (chromatin occupancy after replication, ChOR-Seq) [94]. In a complementary approach, local nucleosome labeling by biotin ligase fused to catalytically inactive Cas9 (dCas9) followed by extensive ChIP profiling documented local recycling of biotinylated histones at transcriptionally silent, but not actively transcribed regions [95]. Importantly, S-phase arrest blocked histone exchange at actively transcribed genes, indicating that DNA replication is the sole major contributor to nucleosome exchange, at least in the time span of a single cell cycle. Further, switching the gene activity rapidly altered the nucleosome retention profile, with induced genes losing parental nucleosomes within a single cell cycle—arguing for a local, and likely histone modification-dependent, mechanism of nucleosome eviction and reassembly [95]. A follow-up to these studies identified Nucleophosmin (NPM1) bridging the Mcm2 subunit of the replisome to PRC2, with acute NPM1 depletion resulting in loss of H3 K27me3, exacerbated by treatment with PRC2 inhibitors [93]. Mcm2 additionally interacts with Mrc1, which ensures symmetric distribution of parental H3/H4 tetramer between the leading and lagging strands [81, 82]. These components are remarkably conserved from yeast to vertebrates, and mutations in Mrc1 ortholog CLASPIN were recently linked to the defect in symmetric histone inheritance in mouse embryonic stem cells [83]. CryoEM structural studies of endogenous yeast replisomes isolated in early S phase demonstrated sequential disassembly of the histone octamer into H2A/H2B dimer, and hexamer containing H3/H4 tetramer core and residual dimer of H2A/H2B, which then gets chaperoned to the lagging strand by Mcm2 and Tof1, suggesting that at least some of the H2A/H2B modifications or variants may be more stably retained in situ than previously assumed [96]. Molecular dynamics simulations suggest that additional parameters of the leading and lagging strand, including DNA bending and amount of RPA, contribute to recycling of parental histones [97].
Transfer of parental H3 further extends to H3.3 variant, enabling variant-specific genome demarcation to persist through replication. This occurs independent of HIRA and is modestly reduced by DAXX deletion, thus indicating that H3.3 recycling is largely independent of variant-specific chaperones [98]. In contrast, inheritance of the more divergent centrosome-specific H3 variant CENP-A requires variant-specific chaperone HJURP at the replisome via association with Mcm2 subunit of the helicase [99], highlighting that distinct factors are recruited to the replisome to operate in specific genomic regions. Further, recycling of parental histones may be related to distinct replication timing between the transcriptionally active and repressed compartments [100, 101]. Temporal segregation of histone variants and modifications thus creates two pools of parental histones, further contributing to partitioning of distinct genome functions. As origin synchronization in vertebrates is established in early embryogenesis during zygotic genome activation (ZGA) [102], the first three cell divisions occurring prior to ZGA would lack this mechanism and thus may be uniquely suited to erase the nucleosome-dependent remnants of parental epigenetic memory, coincident with rapid erasure of DNA methylation post-fertilization [103]. Likewise, cellularization of a Drosophila embryo is achieved through fourteen synchronized cycles of mitotic divisions with S phase as short as 4 min [104]; as such, no distinction between “early” and “late” replicating chromatin yet exists, and histones are replaced near exclusively with maternally loaded copies carrying no position-specific information.
Asymmetric division with capacity for both self-renewal and differentiation is a fundamental property of tissue stem cell populations. Remarkably, mouse embryonic stem cells engineered to carry Mcm2 mutation asymmetrically redirecting parental histones to the leading strand (MCM-2A) demonstrate reduced differentiation competency, degradation of H3 K9me3-demarcated heterochromatin domains, and transcriptional signatures associated with two-cell-like state [105]. Yet while breaking the symmetry of leading and lagging strand distribution alters local retention of parental histones this mechanism does not account for asymmetric inheritance of the old or new histones globally, as equivalent amounts of DNA are synthesized from leading and lagging strands during each S phase.
Global asymmetry in histone inheritance was first documented in Drosophila male germline, where germline stem cells are anchored at the somatic niche and divide asymmetrically to produce one self-renewing stem cell and one differentiating gonialblast. Using genetically encoded labels, the authors documented preferential retention of parental (old) replication-dependent H3 in the stem cell pool, while differentiating gonialblast acquired newly synthesized histones [106]. Curiously, H3.3 variant, associated with active regulatory regions, was distributed symmetrically, which may be attributed to a distinct abundance of variant-specific modifications that contribute to this process [107]. Indeed, H3 threonine 3 (T3) phosphorylation by Haspin was identified in the same system as an asymmetrically distributed modification, with phosphorylated histones preferentially retained in the non-differentiating stem cell pool [108]. A similar phenomenon was recently reported in Drosophila intestinal stem cells [109] but not in cultured mouse embryonic stem cells [110] or Drosophila female germline [111], suggesting both organism- and tissue-specific mechanisms may be involved. Together, these studies demonstrate the emerging complexity of retention of epigenetic information during cell division, and diverse, organism- and cell-specific solutions to this problem.
While progressing through the S-phase offers a chance to reset minor deficiencies in chromatin fidelity, long-lived non-cycling cells face a different challenge, where replication-dependent histone synthesis is largely absent, and a distinct complement of histone isoforms and chaperones maintains the integrity of the chromatin fiber over the lifespan of the organism. As histones are among the slowest-exchanging proteins in both neurons and astrocytes [112], epigenetic information is continuously written, erased, and re-written again over the same stretch of chromatin palimpsest, both highlighting the need for efficient “erasers” in these cell types, and raising the question whether partial retention of previous states may eventually impair normal function. Remarkably, replication-independent H3.3 variant is accumulated in neurons and astrocytes, constituting from a third of the total H3 pool at birth to nearly 100% by 20 years of age in humans [113]. H3.3 pool is slowly exchanged during lifetime, and impairment of this process via depletion of H3.3 or loss of HIRA chaperone alters both transcriptional programs and functional output of mammalian brain, implicating histone turnover in fundamental neurologic functions. Further, these findings indicate that mature neurons and glia must have unique mechanisms of genome partitioning, as H3.1–H3.3 distinction is lost in these cells [113]. Whether mature neurons and glia employ additional factors to demarcate cis-regulatory elements typically delineated by higher H3.3 content or rely on stability of three-dimensional architecture established early in development, remains to be investigated.
Chromatin fidelity is lost in disease
The establishment and maintenance of chromatin modifications present a unique challenge, as many cell- and site-specific mechanisms must work in concert. While failsafe systems and layers of redundancy exist, these mechanisms are nonetheless sensitive to germline and somatic mutations, changes in metabolic state, and age-related degeneration. Emerging evidence implicates progressive disorganization of chromatin in human disease, and examples of chromatin alterations observed in specific disorders are discussed below.
Aging
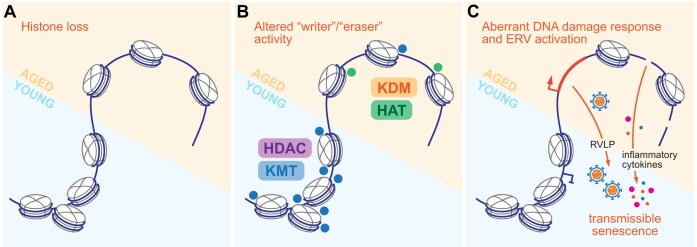
Progressive alterations in chromatin composition are among the hallmarks of aging observed in diverse organisms from invertebrate models to humans [114]. Early studies noted that loss of histone chaperone ASF1 resulted in near four-fold decrease of replicative life span in Saccharomyces cerevisiae, accompanied by aberrant transcription in telomeric regions, loss of silencing, and increased sensitivity to hydroxyurea pulse-chase indicative of a chromatin assembly defect [115]. This phenotype was subsequently linked to reduced expression of histone genes in aging, with ectopic overexpression of just H3 and H4 core histones extending the median lifespan of wild-type yeast by 30% [116]. Increased histone accumulation did not confer significant resistance to hydroxyurea or peroxide treatments, suggesting that lifespan extension in this model is likely not due to additional protection from DNA damage and chromosomal instability. Of note, in Metazoa each histone isoform is encoded by several genes, from two for each of the core histones in yeast, to dozens of near-identical paralogs in vertebrates, regulated by dosage compensation mechanisms [63, 117]. As loss of dosage compensation fidelity was reported during aging in both invertebrate and vertebrate models [118, 119], it is conceivable that changes in histone gene expression may both indirectly result from, and directly contribute to, the age-related chromatin phenotypes (Fig. 3A).
Figure 3.
Chromatin alterations in aging. (A) Histone loss and stochastic increase in chromatin accessibility are frequent in aging. (B) Young and aged cells are characterized by distinct activity of “writer” and “eraser” enzymes, with balance shift from lysine methyltransferases (KMT) and deacetylases (HDAC) in young cells and organisms to lysine demethylases (KDM) and acetyltransferases (HAT) in aged subjects. (C) Aberrant DDR and endogenous retroviruses are derepressed in aging cells, with shedded retrovirus-like particles (RVLPs) and secreted pro-inflammatory cytokines contributing to overall tissue senescence.
Numerous alterations in chromatin modifications are associated with aging. Loss of H3 K9me-demarcated constitutive heterochromatin was documented in both progeroid Werner syndrome fibroblasts, and mesenchymal stem cells derived from healthy older subjects [120]. While constitutive loss of H3K9me “writer” enzymes severely impairs early development and viability in mouse model [121], recent studies employed conditional alleles to generate inducible knock-out of the three methyltransferases responsible for H3 K9me3 deposition [122]. Loss of H3 K9me “writer” enzymes in vivo induced behavioral and morphological features of premature aging, including degeneration of organs and tissues, emergence of senescence-associated transcriptional and DNA methylation signatures, and severely reduced lifespan. While the organism-wide deletion provided the first causative model of heterochromatin depletion in aging, future studies will refine the primary effectors of the phenotype using tissue- and cell-type-specific Cre drivers.
Age-associated changes in developmentally regulated gene expression were linked to aberrant H3 K27me accumulation in Drosophila intestinal stem cells [123]. Curiously, significant H3 K27me2 expansion was documented by mass spectrometry, with minimal effect on K27me3—whether this indicates defects in PRC2 targeting, or increased activity of K27-specific demethylases, is unclear. Transcriptional effects were alleviated by depletion of Pc, a fly homolog of PRC1-associated K27me3 “reader” Cbx, further implicating PcG dysfunction in progressive tissue dysplasia during aging. Studies in mouse model of ulcerative colitis documented loss of CBX3/HP1γ, an H3 K9me3 “reader” protein implicated in constitutive heterochromatin organization [124]. CBX3 loss triggered inflammatory phenotypes in the gut epithelia, and activated cryptic splicing sites at multiple transcripts, including progerin, an aberrant prelamin A variant accumulated in Hutchinson-Gilford progeria. Interestingly, progressive splicing defects are documented in several models of aging and directly contribute to loss of proteostasis [125, 126]. Alteration of the chromatin landscape thus affects cellular homeostasis through transcriptional programing, and by altering fidelity of co-transcriptional pre-mRNA processing.
Loss and redistribution of repressive H3 K9 and K27 methylations in aging cells are balanced by gain of modifications associated with active genome. Nearly 1/5th of the genome in senescent IMR90 fibroblasts was occupied by H3 K4me3, a mark canonically limited to active transcription start sites [127]. Effects of this expansion on genome regulation are unclear, as K4 methylation directly recruits multiple “reader” factors via Chromo, Tudor, or Plant Homeodomain (PHD) [128]. Expansion of H3 K4 methylation is predicted to reduce the spread of PRC2-dependent K27 methylation by competition for an allosteric site on EED subunit [129]. Further, the state of H3 K4 is “read” by ATRX-DNMT3-DNMT3L (ADD) domain, with unmethylated K4 critical to the local activation of de novo methyltransferases and recruitment of chromatin remodeler and histone chaperone ATRX [130–132]. As such, effects of ectopic H3 K4 methylation likely extend beyond promoter activation by K4me3 “reader” recruitment, further exacerbating the degradation of repressive chromatin.
Aberrant histone acetylation has been linked to aging phenotypes, with excess acetylation at multiple H3 and H4 residues generally associated with age. Loss of acetyltransferase activity has been correlated with reduced senescence and extended healthy life span [133], while increased levels or activity of “eraser” enzymes have extended lifespan in invertebrate and select vertebrate models [134], although the latter notion has been challenged [135, 136]. Determining the molecular cascades implicated in ectopic and aberrant acetylation is further complicated by generally promiscuous activity of related enzymes [137], redundancies of many paralogous “writers” and “erasers,” and concerns about the specificity of antibodies essential for genome-wide analyses of specific modifications [138]. It seems apparent though that age-related alterations in histone modifications are not driven by a single principal factor but rather represent a coregulated ensemble. This is supported by recent findings that age predictor models trained on one set of histone modifications retain predictive power for other histone modifications, e.g. a model trained on H3 K27 acetylation is capable of predicting the sample age based on H3 K9ac or K4me3 as well [139] (Fig. 3B). While histone modification-based predictors of biological age are limited by the quality of genome-wide data, chromatin maintenance in aging can be estimated by DNA methylation- [140] or accessibility-based “epigenetic clocks” [141], further demonstrating profound connections between the chromatin maintenance mechanisms.
Functional outcomes of these diverse alterations include concurrent aberrant transcriptional activation and repression; while transcriptional noise has long been postulated to increase with age, recent analyses show it may not be a universal hallmark of aging [142]. More consistent are reports of DNA damage, activation of aberrant secretory phenotypes and endogenous non-long terminal repeat LINE-1 retrotransposons and retroviruses (ERVs) [143–145]. Further, ERV particle release by senescent cells induces similar phenotypes in young mesenchymal progenitors via innate cyclic GMP-AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway, suggesting cellular heterogeneity may dominantly exacerbate aging phenotypes [146] (Fig. 3C).
While it is likely that not all changes listed above are directly causative to aging, substantial evidence points to the fundamental role of chromatin dysfunction in degeneration of nuclear function. In particular, Mendelian disorders recapitulate many, albeit not all, features of normal aging. Mesenchymal stem cells derived from patients with an adult-onset progeroid Werner syndrome (OMIM: 277700) are characterized by reduced replicative potential and premature senescence, accompanied by global disorganization of nuclear DNA density documented by confocal imaging analyses, and loss of constitutive and facultative heterochromatin in genic regions and near telomeres [120]. Likewise, progeroid Cockayne syndrome B (CSB, OMIM: 133540) resulting from ERCC6 protein implicated in transcription-coupled excision repair, is characterized by reduced H3 K9 methylation and decrease in cognate SUV39H1 and SETDB1 methyltransferase levels [147]. Curiously, reduced expression of histone H3 genes was also reported, mirroring the observation of reduced histone dosage in aging cells [116, 147]. On the contrary, progerias driven by nuclear lamina defects due to LMNA and BANF1 mutations show increased accumulation of H3 K9me and K27me, coupled with global loss of chromatin accessibility [148, 149]. These observations indicate that genomic mislocalization, but not the absolute abundance of aberrant histone modifications, is a likely driver of disease progression. Alternatively, these chromatin phenotypes may converge on the similar functional output by the “reader dilution” mechanism, wherein both loss and global gain of the modification results in similar functional outcomes [49, 51, 64].
Whether these alterations represent actionable therapeutic targets remains unclear, as multi-decade human trials are unlikely to proceed due to design complexity and cost, and studies in vertebrate models remain controversial [135, 150, 151]. Advances in functional genomics and organoid technology may alleviate some of the challenges presented by model organism research, and while we are cautiously optimistic about future advances in chromatin biology of aging, it is clear that no “silver bullet” will resolve every aspect of this complex process.
Neurodegeneration
Closely linked to pathological aging is neurodegeneration, the loss of neuronal function culminating in premature cell death and manifesting as progressive neurological impairment. This group of disorders with a complex and often unclear etiology and diverse pathogenesis can be triggered by primary autoimmune or cerebrovascular deficits or arise autonomously as a result of progressive dysfunction of lineage fidelity mechanisms in non-dividing brain cell types, including neurons, microglia and astrocytes.
Extreme longevity of neurons and astrocytes imposes distinct constraints on genome fidelity maintenance. Cytosine methylation, both in symmetrical CpG dinucleotides, and non-CpG (CpH) context, is remarkably high in adult brain, particularly in neurons [152]. Coincidentally both DNMT3A “writer” and Methyl-Cytosine binding Protein 2 (MeCP2) “reader” of CpH methylations emerged at the onset of vertebrate lineage, suggesting this may have contributed to anatomical innovations, in turn facilitating behavioral and cognitive complexity of the vertebrates [153]. Indeed, de novo DNA methylation is required for maturation of adult-born neurons, controlling dendrite branching and synaptogenesis formative to behavior and cognition [154]. Likewise, histone isoform and post-translational modification landscapes are distinct in the brain, with replication-independent histone variants progressively making up a greater proportion of total pool in aging neurons [113], and unique modifications acting in circuit-specific manner to facilitate distinct transcriptional programs [155, 156]. Neuronal function is thus particularly reliant on a unique complement of histone chaperones and “writer” enzymes [113, 157].
Single-cell analyses of transcriptional activity and chromatin accessibility landmarks altered in Alzheimer’s disease (AD) revealed erosion of epigenomic constraints and dysregulation of cell identity programs in multiple cell types in the prefrontal cortex [158]. These were accompanied by impaired DNA damage response (DDR) and altered compartmentalization of DDR foci, coupled with misexpression of cohesin subunits and chromatin chaperones and remodelers, including ATRX and subunits of the BAF complex [159]. While two core characteristics of AD are extracellular plaques of β-amyloid and neurofibrillary tangles of Tau protein in the neuron body, genome-wide analyses revealed a complex phenotype affecting multiple cell types and processes, including nuclear function [160]. Several studies suggest that aberrant DDR contributes to Tau neurotoxicity [161]. Interestingly, this effect was linked to the reentry of post-mitotic neurons into the cell cycle: reduction of ATM and Chk2 kinases and TF p53 exacerbated Tau pathology, whereas increased p53 accumulation had a surprising neuroprotective effect [162, 163]. The mechanism upstream of cell cycle reentry was determined in subsequent studies in Drosophila model, where global reduction of H3 K9me and the reader protein HP1 was induced by Tau overexpression [164]. Tau-induced toxicity was alleviated by restoration of repressive heterochromatin using genetic tools; remarkably, a similar rescue was achieved by manipulating the Ago3/PIWIL1 components of the piRNA biogenesis pathway, suggesting aberrant transposon reactivation in Tau pathology, observations subsequently validated in vertebrate models and patient samples [164, 165].
Oxidative stress
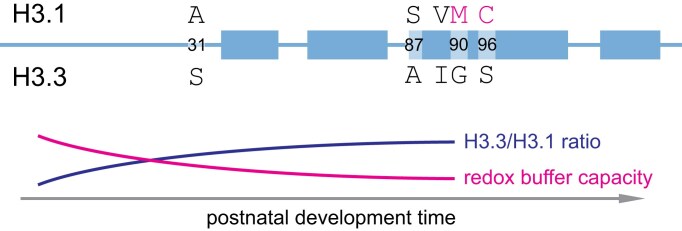
Mechanistic, etiological connection between neurodegeneration and advanced age has been postulated from the strong correlation between the two. Progressive accumulation of free radical-induced damage is thus likely to specifically disrupt the function of extremely long-lived proteins. Protein turnover rates are assessed by stable isotope labeling (SILAC)—a mass-spectrometry technique for quantification of distinct isotope incorporation into newly synthesized proteins. Experiments in cell culture and animal models, as well as remarkable studies of post-mortem human material matching carbon 13 (C13) levels in specific proteins to atmospheric levels that briefly rose during the widespread nuclear testing in the mid-twentieth century revealed remarkably slow exchange rate of histones and many histone-binding proteins, indicating these may be more susceptible to damage over long periods of time [112, 113, 166]. As chromatin in each human cell represents a polymer of an estimated 20 million nucleosome subunits with limited capacity for self-renewal in non-dividing cells, oxidative damage of even a small proportion of incorporated histones may lead to significant dysfunction over time. Further, exchange of replication-dependent histone H3.1/2 isoform for replication-independent H3.3 in postnatal neurons and glia may represent a curious vulnerability to free radical detoxification. Copper is a critical microelement for oxidative phosphorylation in mitochondria and reactive oxygen species (ROS) detoxification by superoxide dismutase 1 (SOD1) [167]. Mutations in copper homeostasis factors cause developmental disorders, including Menkes and Wilson’s diseases, with the central nervous system particularly susceptible to damage [168], and copper imbalance has been extensively studied in AD pathogenesis [169]. Remarkably, copper directly associates with the nucleosome, and several amino acids in the histone fold of H3, including C110, H113, and L126 are implicated in copper reductase activity of the H3/H4 tetramer, catalyzing the Cu2+ to Cu1+ reaction [170–172]. H3.3, the predominant H3 variant in adult post-mitotic neurons and astrocytes, replaces two adjacent oxidizable amino acids, M90 and C96 of H3.1/2, with non-oxidizable G90 and S96 (Fig. 4). As G90 is critical for H3.3/H4 replacement by DAXX/ATRX chaperone [173], these observations raise the question whether post-mitotic cells have a reduced capacity for copper buffering and thus may be specifically vulnerable to damage by ROS. We predict that future studies will identify mechanisms of disease progression beyond the canonical protein aggregates targeted by current therapeutic interventions.
Figure 4.
Histone H3 variant accumulation may alter redox capacity of the nucleosome. Replication-dependent H3.1 and replication independent H3.3 are shown schematically, with α-helices of histone fold represented as thicker bars. Five amino acids unique to each isoform are indicated, and H3.1-specific M90 and C96 are highlighted in magenta. Replacement of H3.1 with H3.3 in postnatal brain may reduce the redox buffering capacity in the aging post-mitotic cells.
Metabolism
Catalytic activities of enzymes operating in chromatin are critically dependent on small molecule cofactors, linking metabolic pathways to genome regulation. While the evolutionary advantage of this connection is intuitive, allowing for the rewiring of global transcriptional and replicative states in response to nutrient abundance, pathological states characterized by metabolic dysfunction directly impact chromatin landscape as well.
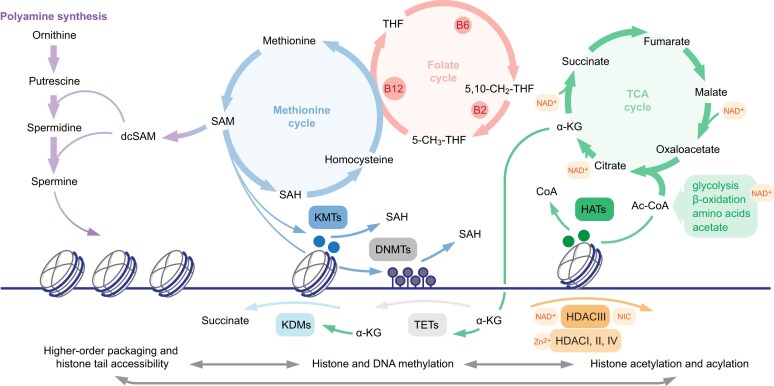
Acetylation of newly synthesized histones is established by type B acetyltransferases in the cytoplasm, followed by transfer into the nucleus where at least three distinct classes of type A acetyltransferases operate in context of chromatin [174]. Both enzyme types utilize acetyl-coenzyme A (ac-CoA) in the reaction, with ac-CoA availability directly related to cytoplasmic histone processing and specific lysine modifications in chromatin [175] (Fig. 5). Longer-chain acylations originate from short-chain fatty acids (SCFAs) incorporated into CoA by acyl-CoA synthetase ACSS2 [176]. While their abundance is orders of magnitude lower than that of acetyl-CoA in most contexts, microbiota-derived SCFAs in intestinal lumen provide a unique environment which shapes the chromatin landscape of intestinal epithelial cells [177]. Curiously, SCFAs additionally inhibit the expression of histone deacetylases (HDACs), thus indirectly increasing the levels of acetylated (and acylated) histones [178]. Acylation of lysine amino group causes two non-exclusive effects: first, it reversibly negates the positive charge of basic amino acid side chain thus altering electrostatic histone–DNA and histone–histone interactions, and, second, provides a platform for specific “reader” association [179]. While acyl-dependent chromatin decompaction is generally associated with active transcription, recent studies point to a more nuanced relationship, where specific acylations may recruit context-dependent effectors [179, 180].
Figure 5.
Metabolic pathways are essential for chromatin fidelity. Key metabolic pathways directly implicated in chromatin regulation are shown above, with enzymes and additional crosstalk omitted for clarity. Left, polyamines are implicated in nucleosome organization and histone tail accessibility; middle, methionine and coupled folate cycle are the source of S-adenosyl-methionine (SAM) donor for histone and DNA methylation; right, TCA cycle is the source of α-KG cofactor for histone and DNA demethylases, with the exception of FAD-dependent LSD1 histone demethylase. Reciprocal relationships between modifications are illustrated below. dcSAM, decarboxylated S-adenosyl-methionine; SAH, S-adenosyl-homocysteine; THF, tetrahydrofolate; B2, B6, and B12 are critical cofactors of the folate cycle, with B12 coupling methionine and THF biosynthesis; 5,10-CH2-THF, 5,10-methylene-tetrahydrofolate; 5-CH3-THF, 5-methyl-tetrahydrofolate; Ac-CoA, acetyl-Coenzyme A; KMTs, histone lysine methyltransferases; DNMTs, DNA methyltransferases; KDMs, histone lysine demethylases; TETs, ten–eleven translocation (DNA demethylases); HDACs, histone lysine deacetylases; NAD+, nicotinamide adenine dinucleotide (oxidized form); NIC, nicotinamide.
Ac-CoA is essential in carbon metabolism, including tricarboxylic acid (TCA) cycle and lipid biosynthesis, yet its distinct role in chromatin is highlighted by a nuclear pool of ac-CoA synthesis machinery, including pyruvate decarboxylase (PDC), ATP citrate lyase (ACLY), and ACSS2 [181–183]. In a recent study, non-uniform metabolic flux within the tissue was connected to distinct histone acetylation profiles of nuclei at distinct spatial locations. In a Drosophila wing disc model, enhanced availability of acetate in the layer at the tissue edge drives increased ac-CoA synthesis and histone acetylation [184]. Whether such positional signals are conserved in other developmental contexts or pathological conditions remains to be elucidated.
Erasure of acetylation and related acylations in histones is accomplished by several classes of deacetylase enzymes (HDACs), including class I, II, and IV “classical” HDACs and class III sirtuins, named after yeast homolog Silent mating-type information regulation 2 (Sir2). Sirtuins have been widely studied in context of lifespan and aging, and are colloquially referred to as “longevity proteins” [185]. Yet, the role of sirtuins in aging remains controversial, as overexpression of sirtuins in Caenorhabiditis elegans and Drosophila failed to increase the lifespan [186, 187], and effects of transgenic SIRT1 in mouse model were modest [188]. These findings have been explained by confounding effects of background mutations in laboratory animals, as well as the dependence of Sirtuins on NAD+ cofactor, such that overexpression alone may not be sufficient to substantially increase their enzymatic activity [189]. Increase in NAD+ levels via nicotinamide riboside supplementation indeed extended the replicative lifespan in the yeast model [190], although whether these effects are sirtuin-dependent remains a subject of debate [136].
Histone methylation represents another abundant modification with profound connections to metabolic pathways. While acylation is specific to lysines and negates the charge of the amino acid side chain, methyl groups can be installed on both lysines and arginines, and do not affect the charge. As such methylations exert their function primarily via recruitment of specific “reader” modules and are implicated in both activation and repression: histone H3 K9 and K27 methylations are foundational to formation of constitutive and facultative heterochromatin, respectively, while K4, K36, and K79 methylations are associated with active regulatory elements, highly interactive compartment A, and productive elongation [29]. Notably, relative quantification of methylation abundance by mass-spectrometry documented remarkable abundance of H3 K9, K27, and K36 methylations in cycling cells, reflecting broad distribution of these modifications in the genome. Further, as single lysine residue may carry up to three methyl groups, chromatin acts as a major rheostat in one-carbon metabolism pathways.
Both histone and DNA methylation reactions require S-adenosylmethionine (SAM), the universal methyl donor generated at the convergence of folate and methionine biosynthesis cycles (Fig. 5). Dietary or redox state-driven deficiency of vitamin B coenzymes, in particular B12 and B6, thus has profound effects on SAM availability and downstream activity of methyltransferase enzymes in chromatin [191], and vitamin B12 supplementation may have neuroprotective effects in models of traumatic brain injury and ischemic stroke [192]. Erasure of methylation is accomplished via oxidation of the methyl group, either by flavin adenine dinucleotide (FAD)-dependent KDM1 enzyme, or a family of α-ketoglutarate (α-KG) dependent Jumonji-domain enzymes, including KDM2-6 histone demethylases and Ten-eleven translocation (Tet) family DNA demethylases. Availability of α-KG, ratio of α-KG to succinate, and presence of coenzyme cofactors, including iron and vitamin C, are critical for histone and DNA demethylation, and act as major regulator of cell fate in development and disease. Efficient demethylation is essential for pluripotency maintenance, as α-KG supplementation improves stem cell self-renewal in culture [193]. Simultaneously, both vitamin C and α-KG supplementation antagonize tumor progression in leukemia and pancreatic carcinoma models via activation of Tet-dependent DNA demethylation [194, 195]. Recently, maternal iron deficiency was causally linked to loss of H3 K9me “eraser” KDM3A activity in developing gonads: depletion of Fe2+ ion in utero results in accumulation of H3 K9me2 upstream of the Sex-determining region Y (Sry) gene in male embryonic gonads, inducing male-to-female sex reversal by suppressing Sry expression [196]. Together, these data highlight complex and context-dependent effects of metabolic interventions.
SAM availability has further implications for the biosynthesis of polyamines, ubiquitously abundant polycationic metabolites (Fig. 5). Aminopropyl group from decarboxylated SAM is transferred onto ornithine and spermidine by spermidine synthase (SRM) or spermine synthase (SMS), respectively. Polyamine recycling in turn is initiated by spermidine/spermine N-acetyltransferase utilizing ac-CoA as a cofactor [197]. Polyamine imbalance due to SMS mutation results in mendelian Snyder Robinson syndrome (SRS, OMIM: 309583) characterized by neurological deficits, mesenchymal malformations, and premature aging. While the primary cause remains unclear, SMS deficiency alters mitochondrial function, impairing glucose metabolism, and drives broad transcriptional misregulation in mesenchymal progenitor cells, limiting their proliferative capacity [198]. On the contrary, several malignancies including pancreatic ductal adenocarcinoma, midline gliomas, and acute leukemias upregulate polyamine biosynthesis pathway to support tumor growth, and inhibitors of synthesis and reuptake have shown promise in early trials [199–201]. In addition to multiple cytoplasmic activities (reviewed in [202]), polyamines are abundant in the nucleus, their polycationic nature suggesting extensive electrostatic interactions with negatively charged backbone of nucleic acids. Indeed, in vitro experiments demonstrated extensive association of polyamines with DNA [203], and polyamine supplementation enhances homology-directed repair, stabilizing synaptic complex formation by the RAD51 and single stranded DNA filament [204]. Recent studies investigated the role of polyamines in regulation of chromatin structure and histone modification landscape, revealing global disorganization of chromatin condensation upon induced polyamine deficiency [205]. Locally, polyamine depletion collapses the histone tails onto the nucleosomal DNA, reducing their accessibility to “writer” and “eraser” enzymes; globally altered modification landscape affected differentiation and reprogramming efficiency in cell culture [206]. These observations echo findings in linker histone deficient cells, wherein chromatin decondensation altered substrate preference of several “writer” complexes [6, 207], and further expand the connections between metabolic states, chromatin organization, and lineage fidelity.
Metabolic disorders thus have profound connections to chromatin-dependent regulation. While causal connections between chromatin alterations and metabolic disease are largely limited to Mendelian disorders, “metabolic imprinting” is increasingly seen as a potential cause of metabolic disorders in adults and seniors [208]. Interestingly, recent studies identified two populations of pancreatic endocrine β-cells characterized by starkly distinct H3 K27me3 levels and capacity for insulin secretion, suggesting epigenome modulation may have a role in metabolic syndrome [209]. Chromatin dysfunction as a consequence of metabolic abnormalities appears a more common occurrence, whereby imbalance of metabolites produced by intracellular pathways and microbiota alters activity of many “writer” and “eraser” enzymes, in turn exacerbating the pathologic state [210].
Malignancy
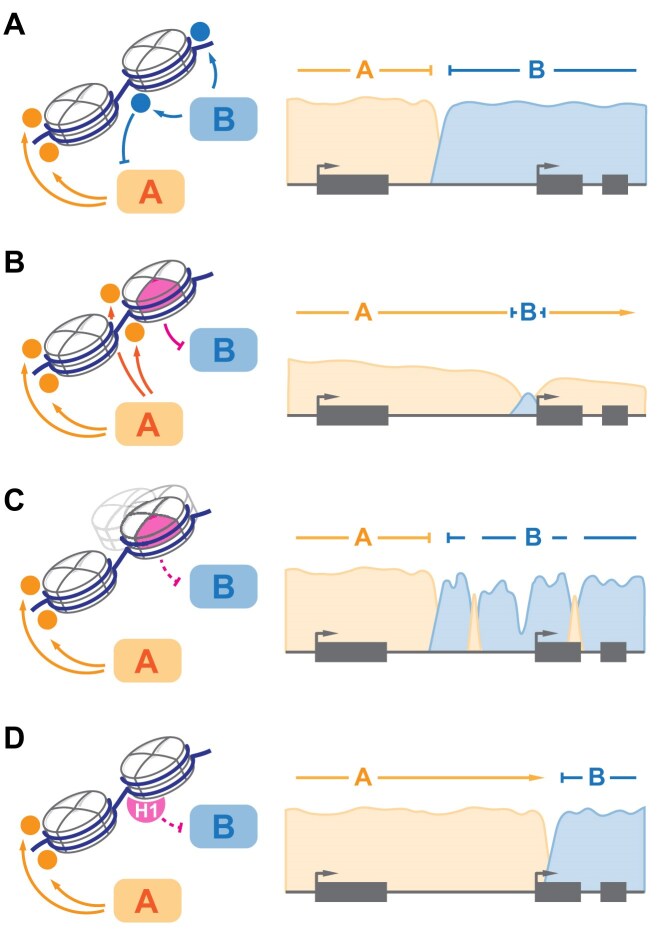
Somatic, i.e. tumor-specific, mutations in “reader” and “writer” factors are among the most prevalent oncogenic drivers [211, 212] and represent a promising class of therapeutic targets [213]. More recently, a number of mutations in core histone genes have been identified, initially in a few rare tumors [49, 214], and later broadly across multiple diverse malignancies [215–217]. These mutants, aptly termed “oncohistones,” appear to broadly fall into three classes, based on the mechanism of action and prevalence in specific disorders (Fig. 6). First, specific missense mutations affecting key residues in the N-terminus of H3 and either dominantly inhibiting cognate SET domain methyltransferases in trans (H3 K27M/I and H3 K36M) [49, 214] or inhibit H3 K36 methylation in cis resulting in altered DNMT3A recruitment (H3 G34 mutations) [218]. These mutations are found in specific malignancies, including pediatric gliomas (DIPG) and sarcomas, and drive unique transcriptional and cellular phenotypes associated with significant, albeit not complete, loss of specific “writer” activity. The latter observation prompted a hypothesis that residual activity of PRC2 represents a specific vulnerability in H3 K27M mutant gliomas [219]. Second, mutations in specific residues within the histone fold domain alter structural stability of the nucleosome core particle, enhance nucleosome sliding, and impair stable cell differentiation in vitro [215–217, 220]. These mutations show limited tumor specificity, reminiscent of BAF remodeler complex mutations prevalent across many malignancies [211]. Similarly, several cancer-associated missense variants in H3 N-terminal tail appear to act in cis, impairing local PRC2 activity and lineage fidelity [55]. Third, broadly distributed missense mutations in linker histone H1 appear to largely represent loss-of-function and are uniquely frequent in peripheral B-cell malignancies [6, 52, 207]. Chromatin decompaction upon H1 loss results in ectopic reactivation of stem-cell-like transcriptional programs associated with early hematopoiesis, constituting a distinct class of lymphomas. Remarkably, H1 heterogeneity was reported in solid tumors, and likewise correlates inversely with accumulation of cancer stem cell markers [221]. Together, these studies exemplify the role of chromatin in enforcing lineage fidelity during cell division and differentiation.
Figure 6.
Chromatin partitioning is altered in malignancy. (A) balanced activity of “writer” enzymes (A and B, left) establishes distinct chromatin domains (A and B, right). (B) inhibition of “writer” B by type 1 “oncohistones” in trans leads to expansion of domain A and may dilute the density of modifications established by A or associated “readers.” (C) reduced local activity of “writer” B due to effects of type 2 “oncohistones” in cis leads to stochastic disruption of domain B, with spurious accumulation of ectopic modifications. (D) altered compaction of chromatin fiber in H1 mutant malignancies drives redistribution of A and B-dependent modifications.
Aberrant differentiation phenotypes induced by histone mutations in malignancy are echoed by observations of altered 3D chromatin topology driving tumorigenesis. Hypermethylation of CTCF binding sites in CpG island methylator phenotype gliomas carrying Isocitrate Dehydrogenase (IDH) “oncometabolite” mutations alters insulation between TADs and promotes aberrant long-distance interaction between ectopic enhancer and PDFRA oncogene [222]. Likewise, reactivation of transposable elements due to failure of chromatin-dependent repression enables oncogene expression via cryptic regulatory elements in many malignancies [223], a process mimicked by hemizygous loss of cohesin cofactor NIPBL and thus driven by broad dysregulation of genome topology [224]. It stands to reason that such defects similarly contribute to other progressive disorders beyond malignancy.
Concluding remarks
The exponential adoption of genomic technologies, population-wide descriptive studies, and high-throughput functional analyses, together with emerging in vivo tools for chemical biology and single-molecule techniques, have allowed chromatin biology to look far beyond the textbook “on/off switch” of transcriptional regulation. While interdisciplinary collaborations enabling truly “greater than the sum of parts” insights are to be celebrated, the new challenge lies in making highly complex results accessible to a broader audience while retaining sufficient information about controls, data transformations, and biologically relevant background. Likewise, the growing number of studies coupled with the increasing technical complexity of experimental and analytical approaches inevitably results in occasionally contradictory observations. The role of sirtuins in aging and disease [134, 136, 186], the contribution of three-dimensional chromatin organization to development and cancer [67, 72, 73], the connections between metabolism and histone modifications [225, 226], and the very nature of transcriptional regulation by chromatin [227] are debated. We believe these conversations represent a critical part of healthy scientific process and are a positive sign that the field is not dominated by a single dogma. As new technologies permeate chromatin biology, we expect that new findings will continue to refute, improve, and extend existing foundations.
Although chromatin dysfunction had been extensively documented in virtually early stage of disease state—from developmental disorders to malignancy to acute viral infections [228]—translating these mechanistic insights into the clinic has been challenging. The introduction of genotype-guided approaches, for example, through the design of basket studies, and subsampling of patient groups with distinct molecular vulnerabilities have proven promising in specific cases [229, 230]. More comprehensive interventions, including restriction of polyamine availability by simultaneously restricting synthesis and uptake, as well as targeting histone acetylation by HDAC inhibition, have shown positive results in combination therapies for various solid tumors and lymphoid malignancies [231, 232]. The discovery of novel regulatory pathways may further expand the repertoire of available interventions. For example, understanding the mechanisms of dosage compensation by histone genes, where the loss of a gene drives compensatory upregulation of its paralogs, could enable targeted upregulation of histone accumulation in aging cells [233]. Likewise, chromatin dysfunctions in malignancy and neurodevelopmental disorders might prove amenable to manipulation of compaction states [207, 234] or specific enzymes associated with distinct vulnerabilities [49, 219]. However, with the exception of rare disorders driven by a singular deficit, we do not view chromatin targeting in therapy as a panacea, but rather as one facet of combinatorial interventions where a multipronged approach would offer synergistic benefits. Understanding the subtle mechanisms of chromatin fidelity will undoubtedly help address this challenge.
Acknowledgements
We thank Gauri Raje, Natalie Redding, Tiffany Bastos, and Ksenia Dydo for their helpful suggestions during the preparation of the manuscript. We acknowledge the contributions of many outstanding groups and individuals to the growing body of knowledge in the field of chromatin biology and apologize to the authors whose work was not cited due to space constraints or by unfortunate omission.
Author contributions: Dustin R. Fetch (Visualization [supporting], Writing—original draft [equal], Writing—review & editing [equal]), Amina Jumamyradova (Writing—original draft [supporting], Writing—review & editing [supporting]), Cameron M. Chapa (Writing—original draft [supporting], Writing—review & editing [supporting]), Yong Ge (Writing—review & editing [equal]), Mansour Mohamadzadeh (Writing—review & editing [equal]), and Alexey A. Soshnev (Conceptualization [lead], Funding acquisition [lead], Visualization [lead], Writing—original draft [lead], Writing—review & editing [supporting]).
Notes
Present address: The David Rockefeller Graduate Program in Bioscience, The Rockefeller University, 1230 York Ave, New York City, NY 10065, United States
Contributor Information
Dustin R Fetch, Graduate Program in Developmental and Regenerative Sciences, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, United States.
Amina Jumamyradova, Department of Neuroscience, Developmental and Regenerative Biology, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, United States.
Cameron M Chapa, Department of Neuroscience, Developmental and Regenerative Biology, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, United States.
Yong Ge, Department of Microbiology, Immunology & Molecular Genetics, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MC 7758 San Antonio, TX 78229, United States.
Mansour Mohamadzadeh, Department of Microbiology, Immunology & Molecular Genetics, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MC 7758 San Antonio, TX 78229, United States.
Alexey A Soshnev, Graduate Program in Developmental and Regenerative Sciences, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, United States; Department of Neuroscience, Developmental and Regenerative Biology, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, United States.
Conflict of interest
None declared.
Funding
The Soshnev laboratory is supported by the National Institutes of Health [NCI R01CA234561], American Cancer Society [IRG-21-147-01-IRG], Cancer Prevention and Research Institute of Texas [IIRA RP240068, HIHR RP240446], UT Rising STARs, UTSA NDRB seed funding, and UTSA startup. C.M.C. is supported by the David Rockefeller Graduate Program at The Rockefeller University and a Rockefeller University Women & Science Graduate Fellowship. Mohamadzadeh laboratory is supported by NIH R01 DK109560C, NIH R01 AI154630-01, and VA BX006310-01A.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Liberali P, Schier AF The evolution of developmental biology through conceptual and technological revolutions. Cell. 2024; 187:3461–95. 10.1016/j.cell.2024.05.053. [DOI] [PubMed] [Google Scholar]
- 2. Lassar AB, Paterson BM, Weintraub H Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986; 47:649–56. 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 3. Tapscott SJ, Davis RL, Thayer MJ et al. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988; 242:405–11. 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi K, Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–76. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5. Lambert SA, Jolma A, Campitelli LF et al. The Human transcription factors. Cell. 2018; 172:650–65. 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 6. Yusufova N, Kloetgen A, Teater M et al. Histone H1 loss drives lymphoma by disrupting 3D chromatin architecture. Nature. 2021; 589:299–305. 10.1038/s41586-020-3017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Dwyer MR, Azagury M, Furlong K et al. Nucleosome fibre topology guides transcription factor binding to enhancers. Nature. 2024; 638:251–260. 10.1038/s41586-024-08333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olins AL, Olins DE Spheroid chromatin units (v bodies). Science. 1974; 183:330–2. 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- 9. Woodcock CL, Safer JP, Stanchfield JE Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp Cell Res. 1976; 97:101–10. 10.1016/0014-4827(76)90659-5. [DOI] [PubMed] [Google Scholar]
- 10. Luger K, Mader AW, Richmond RK et al. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997; 389:251–60. 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 11. Hock R, Furusawa T, Ueda T et al. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007; 17:72–9. 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fyodorov DV, Zhou BR, Skoultchi AI et al. Emerging roles of linker histones in regulating chromatin structure and function. Nat Rev Mol Cell Biol. 2018; 19:192–206. 10.1038/nrm.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall LL, Carone DM, Gomez AV et al. Stable C0T-1 repeat RNA is abundant and is associated with euchromatic interphase chromosomes. Cell. 2014; 156:907–19. 10.1016/j.cell.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Noia JM, Neuberger MS Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007; 76:1–22. 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 15. Bassing CH, Swat W, Alt FW The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002; 109:S45–55. 10.1016/S0092-8674(02)00675-X. [DOI] [PubMed] [Google Scholar]
- 16. Smith JJ, Timoshevskaya N, Ye C et al. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat Genet. 2018; 50:270–7. 10.1038/s41588-017-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millan-Zambrano G, Burton A, Bannister AJ et al. Histone post-translational modifications—cause and consequence of genome function. Nat Rev Genet. 2022; 23:563–80. 10.1038/s41576-022-00468-7. [DOI] [PubMed] [Google Scholar]
- 18. Sinha KK, Bilokapic S, Du Y et al. Histone modifications regulate pioneer transcription factor cooperativity. Nature. 2023; 619:378–84. 10.1038/s41586-023-06112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slatkin M Epigenetic inheritance and the missing heritability problem. Genetics. 2009; 182:845–50. 10.1534/genetics.109.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brownell JE, Zhou J, Ranalli T et al. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996; 84:843–51. 10.1016/S0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 21. Zhao Y, Garcia BA Comprehensive catalog of currently documented histone modifications. Cold Spring Harb Perspect Biol. 2015; 7:a025064. 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian TV, Di Stefano B, Stik G et al. Whsc1 links pluripotency exit with mesendoderm specification. Nat Cell Biol. 2019; 21:824–34. 10.1038/s41556-019-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu M, Mazor T, Huang H et al. Direct recruitment of polycomb repressive complex 1 to chromatin by core binding transcription factors. Mol Cell. 2012; 45:330–43. 10.1016/j.molcel.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhalluin C, Carlson JE, Zeng L et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999; 399:491–6. 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 25. Ruthenburg AJ, Li H, Milne TA et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell. 2011; 145:692–706. 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marunde MR, Popova IK, Hall NW et al. Nucleosome context regulates chromatin reader preference. bioRxiv29 April 2025, preprint: not peer reviewed 10.1101/2025.04.29.651129. [DOI]
- 27. Avery OT, Macleod CM, McCarty M Studies on the chemical nature of the substance inducing transformation of pneumococcal types : induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type iii. J Exp Med. 1944; 79:137–58. 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson SP, Bartek J The DNA-damage response in human biology and disease. Nature. 2009; 461:1071–8. 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao S, Allis CD, Wang GG The language of chromatin modification in human cancers. Nat Rev Cancer. 2021; 21:413–30. 10.1038/s41568-021-00357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allis CD, Jenuwein T The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016; 17:487–500. 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 31. Yu JR, Lee CH, Oksuz O et al. PRC2 is high maintenance. Genes Dev. 2019; 33:903–35. 10.1101/gad.325050.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piunti A, Shilatifard A Author correction: the roles of polycomb repressive complexes in mammalian development and cancer. Nat Rev Mol Cell Biol. 2022; 23:444. 10.1038/s41580-022-00495-6. [DOI] [PubMed] [Google Scholar]
- 33. Kasinath V, Beck C, Sauer P et al. JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science. 2021; 371:eabc3393. 10.1126/science.abc3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Q, Agius SC, Flanigan SF et al. PALI1 facilitates DNA and nucleosome binding by PRC2 and triggers an allosteric activation of catalysis. Nat Commun. 2021; 12:4592. 10.1038/s41467-021-24866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poepsel S, Kasinath V, Nogales E Cryo-EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes. Nat Struct Mol Biol. 2018; 25:154–62. 10.1038/s41594-018-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leicher R, Ge EJ, Lin X et al. Single-molecule and in silico dissection of the interaction between polycomb repressive complex 2 and chromatin. Proc Natl Acad Sci USA. 2020; 117:30465–75. 10.1073/pnas.2003395117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JJ, Kingston RE Context-specific polycomb mechanisms in development. Nat Rev Genet. 2022; 23:680–95. 10.1038/s41576-022-00499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez VG, Valencia-Sanchez MI, Abini-Agbomson S et al. Read-write mechanisms of H2A ubiquitination by polycomb repressive complex 1. Nature. 2024; 636:755–61. 10.1038/s41586-024-08183-5. [DOI] [PubMed] [Google Scholar]
- 39. Blackledge NP, Klose RJ The molecular principles of gene regulation by polycomb repressive complexes. Nat Rev Mol Cell Biol. 2021; 22:815–33. 10.1038/s41580-021-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perino M, van Mierlo G, Karemaker ID et al. MTF2 recruits polycomb repressive complex 2 by helical-shape-selective DNA binding. Nat Genet. 2018; 50:1002–10. 10.1038/s41588-018-0134-8. [DOI] [PubMed] [Google Scholar]
- 41. Jaensch ES, Zhu J, Cochrane JC et al. A polycomb domain found in committed cells impairs differentiation when introduced into PRC1 in pluripotent cells. Mol Cell. 2021; 81:4677–91. 10.1016/j.molcel.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grau D, Zhang Y, Lee CH et al. Structures of monomeric and dimeric PRC2:EZH1 reveal flexible modules involved in chromatin compaction. Nat Commun. 2021; 12:714. 10.1038/s41467-020-20775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chamberlain SJ, Yee D, Magnuson T Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008; 26:1496–505. 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Endoh M, Endo TA, Endoh T et al. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development. 2008; 135:1513–24. 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- 45. de Potter B, Raas MWD, Seidl MF et al. Uncoupled evolution of the polycomb system and deep origin of non-canonical PRC1. Commun Biol. 2023; 6:1144. 10.1038/s42003-023-05501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hubner JM, Muller T, Papageorgiou DN et al. EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro Oncol. 2019; 21:878–89. 10.1093/neuonc/noz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jain SU, Do TJ, Lund PJ et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019; 10:2146. 10.1038/s41467-019-09981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ragazzini R, Perez-Palacios R, Baymaz IH et al. EZHIP constrains polycomb repressive complex 2 activity in germ cells. Nat Commun. 2019; 10:3858. 10.1038/s41467-019-11800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu C, Jain SU, Hoelper D et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science. 2016; 352:844–9. 10.1126/science.aac7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papillon-Cavanagh S, Lu C, Gayden T et al. Impaired H3K36 methylation defines a subset of head and neck squamous cell carcinomas. Nat Genet. 2017; 49:180–5. 10.1038/ng.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Popovic R, Martinez-Garcia E, Giannopoulou EG et al. Histone methyltransferase MMSET/NSD2 alters EZH2 binding and reprograms the myeloma epigenome through global and focal changes in H3K36 and H3K27 methylation. PLoS Genet. 2014; 10:e1004566. 10.1371/journal.pgen.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Willcockson MA, Healton SE, Weiss CN et al. H1 histones control the epigenetic landscape by local chromatin compaction. Nature. 2021; 589:293–8. 10.1038/s41586-020-3032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Josefowicz SZ, Shimada M, Armache A et al. Chromatin kinases act on transcription factors and histone tails in regulation of inducible transcription. Mol Cell. 2016; 64:347–61. 10.1016/j.molcel.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmitges FW, Prusty AB, Faty M et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011; 42:330–41. 10.1016/j.molcel.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 55. Nacev BA, Dabas Y, Paul MR et al. Cancer-associated histone H3 N-terminal arginine mutations disrupt PRC2 activity and impair differentiation. Nat Commun. 2024; 15:5155. 10.1038/s41467-024-49486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baubec T, Colombo DF, Wirbelauer C et al. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature. 2015; 520:243–7. 10.1038/nature14176. [DOI] [PubMed] [Google Scholar]
- 57. Weinberg DN, Papillon-Cavanagh S, Chen H et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature. 2019; 573:281–6. 10.1038/s41586-019-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weinberg DN, Rosenbaum P, Chen X et al. Two competing mechanisms of DNMT3A recruitment regulate the dynamics of de novo DNA methylation at PRC1-targeted CpG islands. Nat Genet. 2021; 53:794–800. 10.1038/s41588-021-00856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamaguchi K, Chen X, Rodgers B et al. Non-canonical functions of UHRF1 maintain DNA methylation homeostasis in cancer cells. Nat Commun. 2024; 15:2960. 10.1038/s41467-024-47314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones PA, Liang G Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009; 10:805–11. 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie X, Liu M, Zhou XE et al. The structural basis for de novoDNA methylation in chromatin. bioRxiv21 December 2024, preprint: not peer reviewed 10.1101/2024.12.19.629503. [DOI]
- 62. Dombrowski M, Engeholm M, Dienemann C et al. Histone H1 binding to nucleosome arrays depends on linker DNA length and trajectory. Nat Struct Mol Biol. 2022; 29:493–501. 10.1038/s41594-022-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fan Y, Nikitina T, Zhao J et al. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005; 123:1199–212. 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 64. Owen JA, Osmanovic D, Mirny L Design principles of 3D epigenetic memory systems. Science. 2023; 382:eadg3053. 10.1126/science.adg3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lyons H, Veettil RT, Pradhan P et al. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell. 2023; 186:327–45. 10.1016/j.cell.2022.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanulli S, Trnka MJ, Dharmarajan V et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature. 2019; 575:390–4. 10.1038/s41586-019-1669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wutz G, Varnai C, Nagasaka K et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 2017; 36:3573–99. 10.15252/embj.201798004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramasamy S, Aljahani A, Karpinska MA et al. The mediator complex regulates enhancer–promoter interactions. Nat Struct Mol Biol. 2023; 30:991–1000. 10.1038/s41594-023-01027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Furlong EEM, Levine M Developmental enhancers and chromosome topology. Science. 2018; 361:1341–5. 10.1126/science.aau0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levo M, Raimundo J, Bing XY et al. Transcriptional coupling of distant regulatory genes in living embryos. Nature. 2022; 605:754–60. 10.1038/s41586-022-04680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schwarzer W, Abdennur N, Goloborodko A et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 2017; 551:51–6. 10.1038/nature24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hsieh TS, Cattoglio C, Slobodyanyuk E et al. Enhancer–promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat Genet. 2022; 54:1919–32. 10.1038/s41588-022-01223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ghavi-Helm Y, Jankowski A, Meiers S et al. Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat Genet. 2019; 51:1272–82. 10.1038/s41588-019-0462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Steensel B, Belmont AS Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017; 169:780–91. 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kind J, Pagie L, Ortabozkoyun H et al. Single-cell dynamics of genome-nuclear lamina interactions. Cell. 2013; 153:178–92. 10.1016/j.cell.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 76. Siegenfeld AP, Roseman SA, Roh H et al. Polycomb-lamina antagonism partitions heterochromatin at the nuclear periphery. Nat Commun. 2022; 13:4199. 10.1038/s41467-022-31857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gholamalamdari O, van Schaik T, Wang Y et al. Major nuclear locales define nuclear genome organization and function beyond A and B compartments. eLife. 2025; 13:RP99116. 10.7554/eLife.99116.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Winick-Ng W, Kukalev A, Harabula I et al. Cell-type specialization is encoded by specific chromatin topologies. Nature. 2021; 599:684–91. 10.1038/s41586-021-04081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. King IF, Yandava CN, Mabb AM et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature. 2013; 501:58–62. 10.1038/nature12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schlissel G, Rine J The nucleosome core particle remembers its position through DNA replication and RNA transcription. Proc Natl Acad Sci USA. 2019; 116:20605–11. 10.1073/pnas.1911943116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Toda T, Fang Y, Shan CM et al. Mrc1 regulates parental histone segregation and heterochromatin inheritance. Mol Cell. 2024; 84:3223–36. 10.1016/j.molcel.2024.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu J, Zhang Y, Fang Y et al. A replisome-associated histone H3–H4 chaperone required for epigenetic inheritance. Cell. 2024; 187:5010–28. 10.1016/j.cell.2024.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Charlton SJ, Flury V, Kanoh Y et al. The fork protection complex promotes parental histone recycling and epigenetic memory. Cell. 2024; 187:5029–47. 10.1016/j.cell.2024.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li H, O’Donnell ME The eukaryotic CMG helicase at the replication fork: emerging architecture reveals an unexpected mechanism. Bioessays. 2018; 40:10.1002/bies.201700208. 10.1002/bies.201700208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gambus A, Jones RC, Sanchez-Diaz A et al. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol. 2006; 8:358–66. 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 86. Simon AC, Zhou JC, Perera RL et al. A Ctf4 trimer couples the CMG helicase to DNA polymerase alpha in the eukaryotic replisome. Nature. 2014; 510:293–7. 10.1038/nature13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pike AM, Friend CM, Bell SP Distinct RPA functions promote eukaryotic DNA replication initiation and elongation. Nucleic Acids Res. 2023; 51:10506–18. 10.1093/nar/gkad765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chilkova O, Stenlund P, Isoz I et al. The eukaryotic leading and lagging strand DNA polymerases are loaded onto primer-ends via separate mechanisms but have comparable processivity in the presence of PCNA. Nucleic Acids Res. 2007; 35:6588–97. 10.1093/nar/gkm741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Iida T, Suetake I, Tajima S et al. PCNA clamp facilitates action of DNA cytosine methyltransferase 1 on hemimethylated DNA. Genes Cells. 2002; 7:997–1007. 10.1046/j.1365-2443.2002.00584.x. [DOI] [PubMed] [Google Scholar]
- 90. Jimenji T, Matsumura R, Kori S et al. Structure of PCNA in complex with DNMT1 PIP box reveals the basis for the molecular mechanism of the interaction. Biochem Biophys Res Commun. 2019; 516:578–83. 10.1016/j.bbrc.2019.06.060. [DOI] [PubMed] [Google Scholar]
- 91. Li Q, Zhou H, Wurtele H et al. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell. 2008; 134:244–55. 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tyler JK, Collins KA, Prasad-Sinha J et al. Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol Cell Biol. 2001; 21:6574–84. 10.1128/MCB.21.19.6574-6584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Escobar TM, Yu JR, Liu S et al. Inheritance of repressed chromatin domains during S phase requires the histone chaperone NPM1. Sci Adv. 2022; 8:eabm3945. 10.1126/sciadv.abm3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reveron-Gomez N, Gonzalez-Aguilera C, Stewart-Morgan KR et al. Accurate recycling of parental histones reproduces the histone modification landscape during DNA replication. Mol Cell. 2018; 72:239–49. 10.1016/j.molcel.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Escobar TM, Oksuz O, Saldana-Meyer R et al. Active and repressed chromatin domains exhibit distinct nucleosome segregation during DNA replication. Cell. 2019; 179:953–63. 10.1016/j.cell.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li N, Gao Y, Zhang Y et al. Parental histone transfer caught at the replication fork. Nature. 2024; 627:890–7. 10.1038/s41586-024-07152-2. [DOI] [PubMed] [Google Scholar]
- 97. Nagae F, Murayama Y, Terakawa T Molecular mechanism of parental H3/H4 recycling at a replication fork. Nat Commun. 2024; 15:9485. 10.1038/s41467-024-53187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xu X, Duan S, Hua X et al. Stable inheritance of H3.3-containing nucleosomes during mitotic cell divisions. Nat Commun. 2022; 13:2514. 10.1038/s41467-022-30298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zasadzinska E, Huang J, Bailey AO et al. Inheritance of CENP-A nucleosomes during DNA replication requires HJURP. Dev Cell. 2018; 47:348–62. 10.1016/j.devcel.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Takahashi S, Miura H, Shibata T et al. Genome-wide stability of the DNA replication program in single mammalian cells. Nat Genet. 2019; 51:529–40. 10.1038/s41588-019-0347-5. [DOI] [PubMed] [Google Scholar]
- 101. Raghuraman MK, Winzeler EA, Collingwood D et al. Replication dynamics of the yeast genome. Science. 2001; 294:115–21. 10.1126/science.294.5540.115. [DOI] [PubMed] [Google Scholar]
- 102. Nakatani T, Schauer T, Altamirano-Pacheco L et al. Emergence of replication timing during early mammalian development. Nature. 2024; 625:401–9. 10.1038/s41586-023-06872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Greenberg MVC, Bourc’his D The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019; 20:590–607. 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- 104. Hua BL, Orr-Weaver TL DNA replication control during drosophila development: insights into the onset of S phase, replication initiation, and fork progression. Genetics. 2017; 207:29–47. 10.1534/genetics.115.186627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wenger A, Biran A, Alcaraz N et al. Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity. Nat Genet. 2023; 55:1567–78. 10.1038/s41588-023-01476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tran V, Lim C, Xie J et al. Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution. Science. 2012; 338:679–82. 10.1126/science.1226028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Garcia BA, Barber CM, Hake SB et al. Modifications of human histone H3 variants during mitosis. Biochemistry. 2005; 44:13202–13. 10.1021/bi050906n. [DOI] [PubMed] [Google Scholar]
- 108. Xie J, Wooten M, Tran V et al. Histone H3 threonine phosphorylation regulates asymmetric Histone inheritance in the drosophila male germline. Cell. 2015; 163:920–33. 10.1016/j.cell.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zion EH, Ringwalt D, Rinaldi K et al. Old and newly synthesized histones are asymmetrically distributed in Drosophila intestinal stem cell divisions. EMBO Rep. 2023; 24:e56404. 10.15252/embr.202256404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Evano B, Khalilian S, Le Carrou G et al. Dynamics of asymmetric and symmetric divisions of muscle stem cells In vivo and on artificial niches. Cell Rep. 2020; 30:3195–206. 10.1016/j.celrep.2020.01.097. [DOI] [PubMed] [Google Scholar]
- 111. Kahney EW, Zion EH, Sohn L et al. Characterization of histone inheritance patterns in the Drosophila female germline. EMBO Rep. 2021; 22:e51530. 10.15252/embr.202051530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Toyama BH, Savas JN, Park SK et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013; 154:971–82. 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Maze I, Wenderski W, Noh KM et al. Critical role of histone turnover in neuronal transcription and plasticity. Neuron. 2015; 87:77–94. 10.1016/j.neuron.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lopez-Otin C, Blasco MA, Partridge L et al. Hallmarks of aging: an expanding universe. Cell. 2023; 186:243–78. 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 115. Tyler JK, Adams CR, Chen SR et al. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature. 1999; 402:555–60. 10.1038/990147. [DOI] [PubMed] [Google Scholar]
- 116. Feser J, Truong D, Das C et al. Elevated histone expression promotes life span extension. Mol Cell. 2010; 39:724–35. 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. McKay DJ, Klusza S, Penke TJ et al. Interrogating the function of metazoan histones using engineered gene clusters. Dev Cell. 2015; 32:373–86. 10.1016/j.devcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hartman PS, Ishii N Chromosome dosage as a life span determinant in Caenorhabiditis elegans. Mech Ageing Dev. 2007; 128:437–43. 10.1016/j.mad.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 119. Bennett-Baker PE, Wilkowski J, Burke DT Age-associated activation of epigenetically repressed genes in the mouse. Genetics. 2003; 165:2055–62. 10.1093/genetics/165.4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang W, Li J, Suzuki K et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015; 348:1160–3. 10.1126/science.aaa1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Peters AH, O’Carroll D, Scherthan H et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001; 107:323–37. 10.1016/S0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 122. Mrabti C, Yang N, Desdin-Mico G et al. Loss of H3K9 trimethylation leads to premature aging. bioRxiv24 July 2024,preprint: not peer reviewed 10.1101/2024.07.24.604929. [DOI]
- 123. Tauc HM, Rodriguez-Fernandez IA, Hackney JA et al. Age-related changes in polycomb gene regulation disrupt lineage fidelity in intestinal stem cells. eLife. 2021; 10:e62250. 10.7554/eLife.62250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mata-Garrido J, Xiang Y, Chang-Marchand Y et al. The heterochromatin protein 1 is a regulator in RNA splicing precision deficient in ulcerative colitis. Nat Commun. 2022; 13:6834. 10.1038/s41467-022-34556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Heintz C, Doktor TK, Lanjuin A et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature. 2017; 541:102–6. 10.1038/nature20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma S, Avanesov AS, Porter E et al. Comparative transcriptomics across 14 Drosophila species reveals signatures of longevity. Aging Cell. 2018; 17:e12740. 10.1111/acel.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shah PP, Donahue G, Otte GL et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013; 27:1787–99. 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vermeulen M, Timmers HT Grasping trimethylation of histone H3 at lysine 4. Epigenomics. 2010; 2:395–406. 10.2217/epi.10.11. [DOI] [PubMed] [Google Scholar]
- 129. Cookis T, Lydecker A, Sauer P et al. Structural basis for the inhibition of PRC2 by active transcription histone posttranslational modifications. Nat Struct Mol Biol. 2025; 32:393–404. 10.1038/s41594-024-01452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Noh KM, Wang H, Kim HR et al. Engineering of a histone-recognition domain in Dnmt3a alters the epigenetic landscape and phenotypic features of mouse ESCs. Mol Cell. 2015; 59:89–103. 10.1016/j.molcel.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Noh KM, Maze I, Zhao D et al. ATRX tolerates activity-dependent histone H3 methyl/phos switching to maintain repetitive element silencing in neurons. Proc Natl Acad Sci USA. 2015; 112:6820–7. 10.1073/pnas.1411258112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ooi SK, Qiu C, Bernstein E et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007; 448:714–7. 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang W, Zheng Y, Sun S et al. A genome-wide CRISPR-based screen identifies KAT7 as a driver of cellular senescence. Sci Transl Med. 2021; 13:eabd2655. 10.1126/scitranslmed.abd2655. [DOI] [PubMed] [Google Scholar]
- 134. Kanfi Y, Naiman S, Amir G et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012; 483:218–21. 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 135. Timmons JA, Brenner C The information theory of aging has not been tested. Cell. 2024; 187:1101–2. 10.1016/j.cell.2024.01.013. [DOI] [PubMed] [Google Scholar]
- 136. Brenner C Sirtuins are not conserved longevity genes. Life Metab. 2022; 1:122–33. 10.1093/lifemeta/loac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Allis CD, Berger SL, Cote J et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007; 131:633–6. 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 138. Rothbart SB, Dickson BM, Raab JR et al. An Interactive Database for the assessment of histone antibody specificity. Mol Cell. 2015; 59:502–11. 10.1016/j.molcel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. de Lima Camillo LP, Asif MH, Horvath S et al. Histone mark age of human tissues and cell types. Sci Adv. 2025; 11:eadk9373. 10.1126/sciadv.adk9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Horvath S DNA methylation age of human tissues and cell types. Genome Biol. 2013; 14:R115. 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Morandini F, Rechsteiner C, Perez K et al. ATAC-clock: an aging clock based on chromatin accessibility. GeroScience. 2024; 46:1789–806. 10.1007/s11357-023-00986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ibanez-Sole O, Ascension AM, Arauzo-Bravo MJ et al. Lack of evidence for increased transcriptional noise in aged tissues. eLife. 2022; 11:e80380. 10.7554/eLife.80380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. De Cecco M, Ito T, Petrashen AP et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019; 566:73–8. 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Benayoun BA, Pollina EA, Singh PP et al. Remodeling of epigenome and transcriptome landscapes with aging in mice reveals widespread induction of inflammatory responses. Genome Res. 2019; 29:697–709. 10.1101/gr.240093.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jonsson ME, Garza R, Sharma Y et al. Activation of endogenous retroviruses during brain development causes an inflammatory response. EMBO J. 2021; 40:e106423. 10.15252/embj.2020106423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liu X, Liu Z, Wu Z et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell. 2023; 186:287–304. 10.1016/j.cell.2022.12.017. [DOI] [PubMed] [Google Scholar]
- 147. Lee JH, Demarest TG, Babbar M et al. Cockayne syndrome group B deficiency reduces H3K9me3 chromatin remodeler SETDB1 and exacerbates cellular aging. Nucleic Acids Res. 2019; 47:8548–62. 10.1093/nar/gkz568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kohler F, Bormann F, Raddatz G et al. Epigenetic deregulation of lamina-associated domains in Hutchinson–Gilford progeria syndrome. Genome Med. 2020; 12:46. 10.1186/s13073-020-00749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Marcelot A, Rodriguez-Tirado F, Cuniasse P et al. A de novo sequence variant in barrier-to-autointegration factor is associated with dominant motor neuronopathy. Cells. 2023; 12:847. 10.3390/cells12060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yang JH, Hayano M, Griffin PT et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023; 186:305–26. 10.1016/j.cell.2022.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yang JH, Hayano M, Rajman LA et al. Response to: the information theory of aging has not been tested. Cell. 2024; 187:1103–5. 10.1016/j.cell.2024.01.014. [DOI] [PubMed] [Google Scholar]
- 152. Guo JU, Su Y, Shin JH et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci. 2014; 17:215–22. 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. de Mendoza A, Poppe D, Buckberry S et al. The emergence of the brain non-CpG methylation system in vertebrates. Nat Ecol Evol. 2021; 5:369–78. 10.1038/s41559-020-01371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zocher S, Overall RW, Berdugo-Vega G et al. De novo DNA methylation controls neuronal maturation during adult hippocampal neurogenesis. EMBO J. 2021; 40:e107100. 10.15252/embj.2020107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Al-Kachak A, Di Salvo G, Fulton SL et al. Histone serotonylation in dorsal raphe nucleus contributes to stress- and antidepressant-mediated gene expression and behavior. Nat Commun. 2024; 15:5042. 10.1038/s41467-024-49336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zheng Q, Weekley BH, Vinson DA et al. Bidirectional histone monoaminylation dynamics regulate neural rhythmicity. Nature. 2025; 637:974–82. 10.1038/s41586-024-08371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wenderski W, Maze I Histone turnover and chromatin accessibility: critical mediators of neurological development, plasticity, and disease. Bioessays. 2016; 38:410–9. 10.1002/bies.201500171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xiong X, James BT, Boix CA et al. Epigenomic dissection of Alzheimer’s disease pinpoints causal variants and reveals epigenome erosion. Cell. 2023; 186:4422–37. 10.1016/j.cell.2023.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mathys H, Peng Z, Boix CA et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer's disease pathology. Cell. 2023; 186:4365–85. 10.1016/j.cell.2023.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Luo W, Qu W, Gan L The AD odyssey 2023: tales of single cell. Cell. 2023; 186:4257–9. 10.1016/j.cell.2023.09.001. [DOI] [PubMed] [Google Scholar]
- 161. Delint-Ramirez I, Madabhushi R DNA damage and its links to neuronal aging and degeneration. Neuron. 2025; 113:7–28. 10.1016/j.neuron.2024.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wersto RP, Cardozo-Pelaez F, Smilenov L et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004; 41:549–61. [DOI] [PubMed] [Google Scholar]
- 163. Khurana V, Merlo P, DuBoff B et al. A neuroprotective role for the DNA damage checkpoint in tauopathy. Aging Cell. 2012; 11:360–2. 10.1111/j.1474-9726.2011.00778.x. [DOI] [PubMed] [Google Scholar]
- 164. Frost B, Hemberg M, Lewis J et al. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014; 17:357–66. 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sun W, Samimi H, Gamez M et al. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci. 2018; 21:1038–48. 10.1038/s41593-018-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dorrbaum AR, Kochen L, Langer JD et al. Local and global influences on protein turnover in neurons and glia. eLife. 2018; 7:e34202. 10.7554/eLife.34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Chen L, Min J, Wang F Copper homeostasis and cuproptosis in health and disease. Sig Transduct Target Ther. 2022; 7:378. 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Czlonkowska A, Litwin T, Dusek P et al. Wilson disease. Nat Rev Dis Primers. 2018; 4:21. 10.1038/s41572-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gaggelli E, Kozlowski H, Valensin D et al. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev. 2006; 106:1995–2044. 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 170. Palma FR, Coelho DR, Pulakanti K et al. Histone H3.1 is a chromatin-embedded redox sensor triggered by tumor cells developing adaptive phenotypic plasticity and multidrug resistance. Cell Rep. 2024; 43:113897. 10.1016/j.celrep.2024.113897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tod NP, Vogelauer M, Cheng C et al. The role of histone H3 leucine 126 in fine-tuning the copper reductase activity of nucleosomes. J Biol Chem. 2024; 300:107314. 10.1016/j.jbc.2024.107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Attar N, Campos OA, Vogelauer M et al. The histone H3–H4 tetramer is a copper reductase enzyme. Science. 2020; 369:59–64. 10.1126/science.aba8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Elsasser SJ, Huang H, Lewis PW et al. DAXX envelops a histone H3.3–H4 dimer for H3.3-specific recognition. Nature. 2012; 491:560–5. 10.1038/nature11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Parthun MR Histone acetyltransferase 1: more than just an enzyme?. Biochim Biophys Acta. 2012; 1819:256–63. 10.1016/j.bbagrm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Eckel-Mahan KL, Patel VR, de Mateo S et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013; 155:1464–78. 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sabari BR, Tang Z, Huang H et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell. 2015; 58:203–15. 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Gates LA, Reis BS, Lund PJ et al. Histone butyrylation in the mouse intestine is mediated by the microbiota and associated with regulation of gene expression. Nat Metab. 2024; 6:697–707. 10.1038/s42255-024-00992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Farhadipour M, Arnauts K, Clarysse M et al. SCFAs switch stem cell fate through HDAC inhibition to improve barrier integrity in 3D intestinal organoids from patients with obesity. iScience. 2023; 26:108517. 10.1016/j.isci.2023.108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li Y, Sabari BR, Panchenko T et al. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol Cell. 2016; 62:181–93. 10.1016/j.molcel.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu N, Konuma T, Sharma R et al. Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin. Mol Cell. 2023; 83:2206–21. 10.1016/j.molcel.2023.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sutendra G, Kinnaird A, Dromparis P et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014; 158:84–97. 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 182. Sivanand S, Rhoades S, Jiang Q et al. Nuclear acetyl-CoA production by ACLY promotes homologous recombination. Mol Cell. 2017; 67:252–65. 10.1016/j.molcel.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mews P, Donahue G, Drake AM et al. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. 2017; 546:381–6. 10.1038/nature22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Willnow P, Teleman AA Nuclear position and local acetyl-CoA production regulate chromatin state. Nature. 2024; 630:466–74. 10.1038/s41586-024-07471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Imai S, Armstrong CM, Kaeberlein M et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000; 403:795–800. 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 186. Burnett C, Valentini S, Cabreiro F et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011; 477:482–5. 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Viswanathan M, Guarente L Regulation of Caenorhabditis elegans lifespan by sir-.477:E1–2.1 transgenes. Nature. 2011; E1–2. 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- 188. Bordone L, Cohen D, Robinson A et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007; 6:759–67. 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 189. Lombard DB, Pletcher SD, Canto C et al. Ageing: longevity hits a roadblock. Nature. 2011; 477:410–1. 10.1038/477410a. [DOI] [PubMed] [Google Scholar]
- 190. Belenky P, Racette FG, Bogan KL et al. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007; 129:473–84. 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 191. Dai Z, Ramesh V, Locasale JW The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet. 2020; 21:737–53. 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ge Y, Yang C, Zadeh M et al. Functional regulation of microglia by vitamin B12 alleviates ischemic stroke-induced neuroinflammation in mice. iScience. 2024; 27:109480. 10.1016/j.isci.2024.109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Carey BW, Finley LW, Cross JR et al. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015; 518:413–6. 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Morris JPt, Yashinskie JJ, Koche R et al. Alphaketoglutaratee links p53 to cell fate during tumour suppression. Nature. 2019; 573:595–9. 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Cimmino L, Dolgalev I, Wang Y et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017; 170:1079–95. 10.1016/j.cell.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Okashita N, Maeda R, Kuroki S et al. Maternal iron deficiency causes male-to-female sex reversal in mouse embryos. Nature. 2025; 643:262–70. 10.1038/s41586-025-09063-2. [DOI] [PubMed] [Google Scholar]
- 197. Pegg AE Functions of polyamines in mammals. J Biol Chem. 2016; 291:14904–12. 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ramsay AL, Alonso-Garcia V, Chaboya C et al. Modeling Snyder–Robinson syndrome in multipotent stromal cells reveals impaired mitochondrial function as a potential cause for deficient osteogenesis. Sci Rep. 2019; 9:15395. 10.1038/s41598-019-51868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Khan A, Gamble LD, Upton DH et al. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat Commun. 2021; 12:971. 10.1038/s41467-021-20896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lee MS, Dennis C, Naqvi I et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer. Nature. 2023; 616:339–47. 10.1038/s41586-023-05891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Gao W, Karsa M, Xiao L et al. Polyamine depletion limits progression of acute leukaemia. Intl J Cancer. 2025; 156:2360–76. 10.1002/ijc.35362. [DOI] [PubMed] [Google Scholar]
- 202. Madeo F, Eisenberg T, Pietrocola F et al. Spermidine in health and disease. Science. 2018; 359:eaan2788. 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 203. van Dam L, Korolev N, Nordenskiold L Polyamine-nucleic acid interactions and the effects on structure in oriented DNA fibers. Nucleic Acids Res. 2002; 30:419–28. 10.1093/nar/30.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lee CY, Su GC, Huang WY et al. Promotion of homology-directed DNA repair by polyamines. Nat Commun. 2019; 10:65. 10.1038/s41467-018-08011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Park S, Merino-Urteaga R, Karwacki-Neisius V et al. Native nucleosomes intrinsically encode genome organization principles. Nature. 2025; 643:572–81. 10.1038/s41586-025-08971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Emmons-Bell M, Forsyth G, Sundquist A et al. Polyamines regulate cell fate by altering the activity of histone-modifying enzymes. bioRxiv3 July 2024, preprint: not peer reviewed 10.1101/2024.07.02.600738. [DOI]
- 207. Soshnev AA, Allis CD, Cesarman E et al. Histone H1 mutations in lymphoma: a link(er) between chromatin organization, developmental reprogramming, and cancer. Cancer Res. 2021; 81:6061–70. 10.1158/0008-5472.CAN-21-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hanley B, Dijane J, Fewtrell M et al. Metabolic imprinting, programming and epigenetics—a review of present priorities and future opportunities. Br J Nutr. 2010; 104:S1–S25. 10.1017/S0007114510003338. [DOI] [PubMed] [Google Scholar]
- 209. Dror E, Fagnocchi L, Wegert V et al. Epigenetic dosage identifies two major and functionally distinct beta cell subtypes. Cell Metab. 2023; 35:821–36. 10.1016/j.cmet.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Park YJ, Han SM, Huh JY et al. Emerging roles of epigenetic regulation in obesity and metabolic disease. J Biol Chem. 2021; 297:101296. 10.1016/j.jbc.2021.101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kandoth C, McLellan MD, Vandin F et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013; 502:333–9. 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Hanahan D Hallmarks of cancer: new dimensions. Cancer Discov. 2022; 12:31–46. 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 213. Topper MJ, Vaz M, Marrone KA et al. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol. 2020; 17:75–90. 10.1038/s41571-019-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lewis PW, Muller MM, Koletsky MS et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013; 340:857–61. 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Nacev BA, Feng L, Bagert JD et al. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature. 2019; 567:473–8. 10.1038/s41586-019-1038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bagert JD, Mitchener MM, Patriotis AL et al. Oncohistone mutations enhance chromatin remodeling and alter cell fates. Nat Chem Biol. 2021; 17:403–11. 10.1038/s41589-021-00738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Arimura Y, Ikura M, Fujita R et al. Cancer-associated mutations of histones H2B, H3.1 and H2A.Z.1 affect the structure and stability of the nucleosome. Nucleic Acids Res. 2018; 46:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Khazaei S, Chen CCL, Andrade AF et al. Single substitution in H3.3G34 alters DNMT3A recruitment to cause progressive neurodegeneration. Cell. 2023; 186:1162–78. 10.1016/j.cell.2023.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Mohammad F, Weissmann S, Leblanc B et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med. 2017; 23:483–92. 10.1038/nm.4293. [DOI] [PubMed] [Google Scholar]
- 220. Bennett RL, Bele A, Small EC et al. A mutation in histone H2B represents a new class of oncogenic driver. Cancer Discov. 2019; 9:1438–51. 10.1158/2159-8290.CD-19-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Torres CM, Biran A, Burney MJ et al. The linker histone H1.0 generates epigenetic and functional intratumor heterogeneity. Science. 2016; 353:aaf1644. 10.1126/science.aaf1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Flavahan WA, Drier Y, Liau BB et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016; 529:110–4. 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Jang HS, Shah NM, Du AY et al. Transposable elements drive widespread expression of oncogenes in human cancers. Nat Genet. 2019; 51:611–7. 10.1038/s41588-019-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wong EWP, Sahin M, Yang R et al. Disruption of TAD hierarchy promotes LTR co-option in cancer. Nat Genet. 2025; 57:1754–65. 10.1038/s41588-025-02239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gaffney DO, Jennings EQ, Anderson CC et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol. 2020; 27:206–13. 10.1016/j.chembiol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang D, Tang Z, Huang H et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019; 574:575–80. 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Henikoff S, Shilatifard A Histone modification: cause or cog?. Trends Genet. 2011; 27:389–96. 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 228. Kee J, Thudium S, Renner DM et al. SARS-CoV-2 disrupts host epigenetic regulation via histone mimicry. Nature. 2022; 610:381–8. 10.1038/s41586-022-05282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hobbs BP, Pestana RC, Zabor EC et al. Basket Trials: review of current practice and innovations for future trials. JCO. 2022; 40:3520–8. 10.1200/JCO.21.02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Soto-Feliciano YM, Sanchez-Rivera FJ, Perner F et al. A molecular switch between mammalian MLL complexes dictates response to menin-MLL inhibition. Cancer Discov. 2023; 13:146–69. 10.1158/2159-8290.CD-22-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Bondarev AD, Attwood MM, Jonsson J et al. Recent developments of HDAC inhibitors: emerging indications and novel molecules. Brit J Clin Pharma. 2021; 87:4577–97. 10.1111/bcp.14889. [DOI] [PubMed] [Google Scholar]
- 232. Casero RAJr, Murray Stewart T, Pegg AE Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018; 18:681–95. 10.1038/s41568-018-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Dubey SK, Dubey R, Prajapati SC et al. Histone deficiency and hypoacetylation in the aging retinal pigment epithelium. Aging Cell. 2024; 23:e14108. 10.1111/acel.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Guy J, Gan J, Selfridge J et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007; 315:1143–7. 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.