Abstract
R-loops or DNA–RNA hybrids are prominent nucleic acid structures that commonly arise during transcription. These structures play important biological functions, such as regulating gene expression and DNA repair. However, when unresolved by nucleic acid processing factors, pathological R-loops can be harmful and lead to genome instability. N6-Methyladenosine (m6A), the most prevalent modification in messenger RNA, has been recently identified to be crucial for regulating R-loop balance and maintaining genome stability. Strikingly, m6A-modified R-loop formation can have opposing consequences, either stabilization or resolution, depending on the biological context. In this review, we discuss the current knowledge of the regulatory roles of m6A on R-loops across various processes, including gene transcription, DNA repair, and centromere and telomere stability. Additionally, we explore other m6A-mediated processes, such as nascent transcription and chromatin landscape, that potentially affect R-loop dynamics. Finally, we discuss the current limitations and future directions of studying the m6A–R-loop axis, as well as the opportunities to target this pathway as a potential therapeutic strategy.
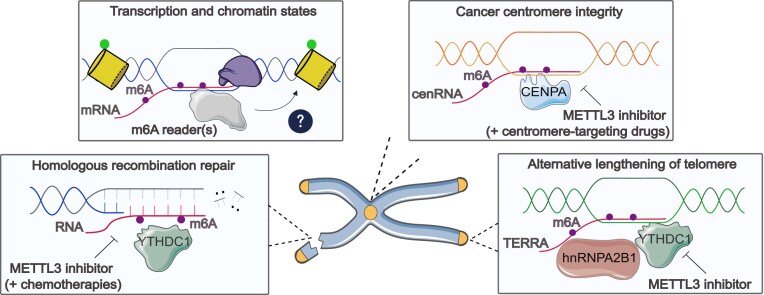
Graphical Abstract
Graphical Abstract.
Introduction
R-loops are three-stranded nucleic acid structures consisting of a nascent RNA strand that hybridizes to a template DNA strand with a displaced non-template DNA strand. They are prevalent in mammalian genomes covering up to 5% of the human genome [1]. R-loops have been proposed to serve regulatory roles in the cell, including the patterning of promoter chromatin, the facilitation of transcription termination, double-strand break (DSB) repair, and chromosome segregation [2–4, 5]. Despite their regulatory functions, unscheduled R-loops can be deleterious to transcription, interfere with DNA replication and repair, which can be an important source of genome instability, and contribute to diseases such as cancer or neurological disorders [6, 7]. It is thus important to understand the mechanisms and the factors regulating R-loops, as this may provide novel therapeutic targets for disease treatment.
Numerous studies have identified and characterized factors that contribute to the timely prevention of deleterious R-loops. These factors include co-transcriptional processing proteins, splicing factors, endo/exo-nucleases, DEAD-box helicases, topoisomerases, chromatin remodelers, and DNA repair factors (reviewed by [8]). In 2019, Abakir et al. and Yang et al. simultaneously discovered, for the first time, the presence of N6-methyladenosine (m6A) modification on the RNA moiety of R-loops in human induced pluripotent stem cells and HeLa cells, respectively, adding another layer to the growing list of R-loop regulators [9, 10]. Using the S9.6 antibody, which recognizes DNA–RNA hybrids, along with m6A-specific antibody to pull down genomic DNA for sequencing, the authors showed that most R-loop peaks overlap with m6A peaks, suggesting that most R-loops are methylated [9]. Hereafter, m6A is also detected in other subclasses of R-loops, such as DNA–RNA hybrids at DSBs [11], antisense R-loop at the floral repressor locus in Arabidopsis [12], and R-loop enriched telomeric-repeat-containing RNA (TERRA) at telomeres [13, 14].
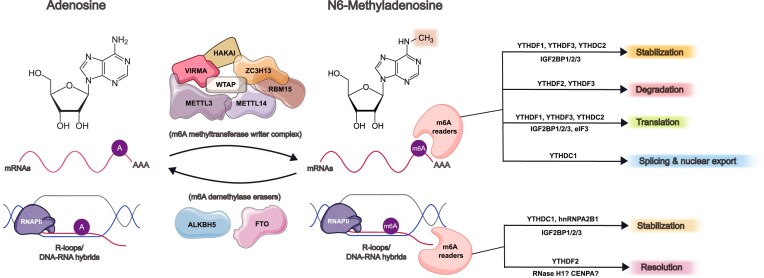
m6A is the most abundant modification on messenger RNAs (mRNAs), catalyzed by the methyltransferase (‘writer’) complex (MTC), which consists of the core catalytic subunits METTL3 and METTL14 together with its regulatory cofactors WTAP, VIRMA, RBM15, ZC3H13, and HAKAI [15–17] (Fig. 1). The fate of modified RNAs is then regulated through the recognition of m6A reader proteins, such as the YT521-B homology (YTH) domain-containing proteins, insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs), or heterogeneous nuclear ribonucleoproteins (hnRNP) families, to mediate downstream regulation of RNA stability, splicing, nuclear export, and translation [18]. Modified RNAs can also be dynamically demethylated by m6A erasers such as alkB homolog 5 (ALKBH5) or fat mass and obesity-associated protein (FTO). Depletion of FTO in mouse embryonic stem cells (mESCs) decreases DNA association of LINE1 RNAs and their R-loop formation [19], implying that this eraser may play a role in regulating R-loop dynamics. Several m6A readers, such as YTHDC1, hnRNPA2B1, and YTHDF2, have been found to bind R-loops through proteomics approaches in different cell models [20–22], suggesting that m6A modulates R-loop levels through such readers, in a similar manner as its diverse role in mRNA fate regulation (Fig. 1). Although the presence of m6A is widespread, its exact regulatory mechanism on R-loop homeostasis remains unclear.
Figure 1.
Overview of m6A regulators on mRNA and R-loops/DNA–RNA hybrids. The methyltransferase writer complex includes the core components (METTL3, METTL14, and WTAP) and the regulatory proteins (VIRMA, HAKAI, RBM15, and ZC3H13). The writer complex methylates adenosine at the N6 position of an mRNA transcript and a DNA–RNA hybrid transcribed by RNA polymerase II (RNAPII). The m6A erasers include ALKBH5 and FTO, which remove the methylation of adenosine from target mRNAs. Modified mRNAs are recognized by m6A readers, with each reader playing a different role in RNA metabolism. Modified DNA–RNA hybrids or R-loops are also stabilized or resolved by different m6A readers. The endonuclease RNase H1 and the histone H3 variant CENPA are also implicated as potential m6A readers (in question mark).
The regulatory roles of m6A on R-loops have attracted significant research interest and several reviews on this topic have been published [23–26]. Here, we provide a timely and comprehensive overview of recent advances in understanding how m6A influences R-loop dynamics in various processes including transcription regulation, DNA repair, and centromere and telomere stability, among others. We also highlight the conflicting results regarding whether m6A promotes or resolves DNA–RNA hybrids and discuss recent findings on m6A function in chromatin and transcription regulation, which could indirectly contribute to R-loop alterations. Finally, we address the limitations of current studies and propose perspectives for methodological improvements and future investigations, particularly the potential of targeting the m6A–R-loop axis as a strategy for cancer treatment.
N 6-Methyladenosine regulates R-loop balance: stabilization or resolution?
Abakir et al. proposed that m6A plays a role in resolving R-loops via the reader YTHDF2, as its depletion resulted in R-loop-dependent DNA damage [9]. However, it remains unclear how this cytoplasmic reader mechanistically senses and removes R-loops as it does not possess any known resolving or unwinding functions. Conversely, other studies in HeLa cells identified an R-loop-stabilizing function of m6A, which promotes R-loop formation at transcription termination sites (TTS) to ensure proper RNAPII termination [10, 27]. Depletion of individual components of the m6A writer complex resulted in total R-loop reduction, accompanied by transcription readthrough [10]. Additionally, a study in prostate cancer cells identified IGF2BPs as readers that preferentially bind to m6A-modified R-loops and overexpression of IGF2BPs induced R-loop accumulation, indicating their stabilizing role [28]. In the case of DSB repair, the nuclear reader YTHDC1 recognizes and stabilizes methylated DNA–RNA hybrids at DSBs to facilitate RAD51 loading to perform homologous recombination (HR) [11]. At telomeres, YTHDC1 or hnRNPA2B1 stabilize R-loop-forming TERRA to maintain telomere length in alternative lengthening of telomere-positive (ALT+) cancer cells [13, 14].
These findings suggest that different reader proteins, such as YTHDC1 or YTHDF2, recognize m6A–R-loops to either stabilize or resolve them in a context-dependent manner. Therefore, m6A is implicated to have a dual role in R-loop regulation, potentially through context- or cell type-dependent mechanisms involving different readers and cofactors. It is also possible that m6A contributes to both roles in a stepwise manner, for instance, by promoting R-loop formation followed by resolution during different stages of transcription, similar to its role in DSB repair [11, 29] (see discussion below). In addition, m6A and the MTC are involved in the regulation of numerous processes independent of R-loops, such as transcription, splicing, chromatin dynamics, and DNA methylation, adding further complications in studying their direct roles in R-loop metabolism.
N 6-Methyladenosine regulates nascent transcription and co-transcriptional splicing
m6A has previously been shown to be deposited co-transcriptionally on nascent RNAs [30–32] (Fig. 2A). Xu et al. showed that, in MCF7 breast cancer cells, the MTC binds promoters and enhancers to install m6A on nascent transcripts, preventing premature termination by the Integrator complex, a large multimeric protein complex that interacts with RNAPII and plays a role in promoter-proximal termination of nascent transcripts [33]. m6A marks at 5′-end RNAs are protected by nuclear readers such as hnRNPG or YTHDC1 from Integrator-mediated cleavage, thus stimulating RNA transcription. In another study conducted in Drosophila melanogaster S2 cells, the MTC binds gene promoters to induce RNAPII pause release and this activity depends on the catalytic function of METTL3 [34]. This suggests that MTC and m6A have a positive role in promoting transcription. Although these studies did not specifically investigate R-loops, the m6A-mediated transcription changes are likely associated with R-loop alterations as R-loops are mainly by-products of transcription. Interestingly, the Integrator complex has been shown to play a role in resolving R-loop and RNAPII accumulation near the transcription start site (TSS), thereby preventing codirectional transcription–replication conflicts (TRCs) to ensure faithful DNA replication [35]. It is tempting to speculate that, in the absence of m6A, paused RNAPII and R-loop accumulation at TSS may recruit Integrator to prevent TRCs during replisome passage. Furthermore, m6A is deposited on promoter upstream antisense RNAs and enhancer RNAs (eRNAs), which are short-lived non-coding RNA species that promote enhancer–promoter interaction and transcriptional activation [36]. Modified eRNAs are highly active and bound by YTHDC1 to phase separate into condensates, facilitating bromodomain-containing protein 4 recruitment and condensation to activate transcription.
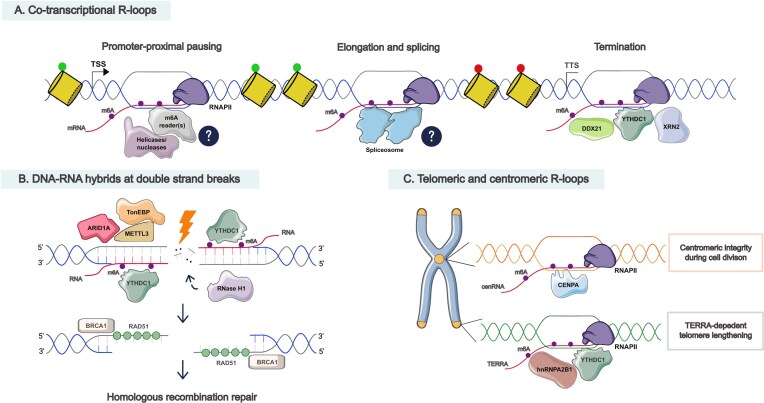
Figure 2.
Molecular and potential functions of m6A on different classes of R-loops and DNA–RNA hybrids. (A) m6A deposition occurs co-transcriptionally at different stages of transcription. At TSSs, m6A-modified R-loops from paused RNAPII might be recognized by m6A readers (such as YTHDC1) that recruit helicases or nucleases (such as RNase H1) for R-loop resolution to promote elongation. m6A might regulate co-transcriptional splicing, which can influence transcription processing and R-loop levels. At TTSs, R-loops are unwound by DDX21 for m6A deposition, which is recognized by the reader YTHDC1, potentially recruiting XRN2 exonuclease for RNAPII termination. The m6A writers and readers also regulate active (green) or repressive (red) histone marks, contributing to R-loop metabolism. (B) Upon DSBs, ARID1A as well as TonEBP helps the recruitment of METTL3 to methylate RNA-associated DSBs. YTHDC1 stabilizes m6A-modified hybrids, which attracts RAD51 to coat single-strand DNA overhangs and BRCA1 to promote HR repair. RNase H1 is also recruited, either directly or indirectly by m6A, to resolve the hybrids for efficient repair. (C) In centromeres, m6A-modified centromeric RNAs (cenRNA), potentially forming R-loops, are recognized and bound by CENPA to ensure centromeric integrity during cell division. In telomeres, telomeric-repeat-containing RNA (TERRA) is m6A marked and stabilized by YTHDC1 or hnRNPA2B1 readers to form R-loops, promoting HR to maintain telomere length in alternative lengthening of telomere (ALT) pathway.
In addition to promoting transcription, m6A is also involved in controlling splicing efficiency co-transcriptionally and post-transcriptionally (Fig. 2A). By mapping m6A in bromouridine-labelled nascent RNA, Louloupi et al. reported that m6A is deposited at splice junctions and at introns, with the former contributing to fast constitutive splicing and the latter associating with slow alternative splicing [37]. In post-transcriptional splicing regulation, various studies have also demonstrated the role of different m6A readers. In 293T cells, YTHDC1 promotes exon inclusion in pre-mRNA splicing by facilitating the splicing factor SRSF3 while antagonizing SRSF10 on m6A-modified targets [38]. IGF2BPs have been shown to be associated with alternative splicing events, promoting oncogenic progression in leukaemia and hepatocellular carcinoma [39, 40]. The hnRNP family, such as hnRNPG and hnRNPA2B1, selectively binds to the m6A ‘RGAC’ motif to regulate alternative splicing in different cancer types [41–43]. Co-transcriptional R-loops can accumulate in response to splicing perturbations, such as treatment with SF3B1 inhibitor pladienolide B [44] or depletion of splicing factor SRSF1 [45]. Whether there is an interplay among these m6A readers in co-transcriptional splicing regulation, which potential subclass of RNAs is targeted by a particular reader, and the possible role of DNA–RNA hybrids during this process remain open questions for future research.
R-loop formation has been implicated in transcription termination and m6A also plays an important role in this process [23]. Specifically, the nucleolar helicase DDX21 acts as an upstream factor recruiting the MTC to R-loops and unwinds them to provide single-stranded RNA for m6A deposition. Modified R-loops then recruit the 5′–3′ exonuclease XRN2 to promote transcription termination, potentially through the interaction with the reader YTHDC1. Depletion of either DDX21 or METTL3 results in global transcription readthrough, causing DNA damage at certain readthrough sites where transcription collides with the replisome.
As most R-loops are co-transcriptional, any changes in R-loop levels are likely to be attributed to perturbed nascent transcription, a process in which m6A plays a prominent role. Many deleterious R-loop accumulation instances have been reported to be due to alterations in co-transcriptional processes such as splicing, nuclear export, and termination [46–48]. Given that m6A is already deposited on nascent RNA, it is not surprising that this mark is also present on DNA–RNA hybrids, which co-occur during transcription. Evidence of m6A regulatory role in nascent transcription, chromatin states, and splicing control has been supported by several studies in different cellular models, whereas its role in R-loop regulation remains less well understood. Indeed, a major challenge in the field of R-loops is to disentangle a factor’s role in regulating R-loop from its involvement in nascent transcription processing. Future studies that investigate the association of genome-wide R-loop distribution changes with altered nascent transcription and splicing, in the absence of m6A, should help clarify this issue. Another interesting question is whether m6A deposition occurs on nascent RNAs before or after R-loop formation, and how to experimentally distinguish m6A on nascent RNA prior to R-loop formation from m6A occurring after R-loops have already formed. Hao et al. demonstrated that overexpression of RNase H1 impairs METTL3 recruitment to chromatin and reduces m6A levels on chromatin-associated RNAs, suggesting that R-loops may be a prerequisite for m6A deposition and that methylation may occur post-formation [27]. However, RNase H1 overexpression might already prevent nascent RNAs from tethering to chromatin and indirectly hinders m6A deposition.
N 6-Methyladenosine and the MTC regulate chromatin landscape and DNA methylation
Beyond regulating RNA-dependent processes, the MTC and m6A can regulate chromatin states and influence histone mark modifications, although their connection to R-loop formation remains to be elucidated. In mESCs, METTL3 chromatin binding is strongly correlated with heterochromatin mark H3K9me3 and m6A modification is important for maintaining repressive chromatin [49]. Similarly, depletion of METTL3 or YTHDC1 in mESCs increases chromatin accessibility and active transcription by reducing the degradation of m6A-marked chromosome-associated regulatory RNAs [50], which may or may not occur in R-loops. These results contrast sharply with the observations made in cancer and other cell lines, where the MTC binds to the promoters of protein-coding genes, promoting transcription by preventing premature termination [33] or by inducing RNAPII elongation transition from paused states [34]. Another study showed that m6A co-transcriptionally recruits KDM3B demethylase to chromatin via YTHDC1 binding, leading to H3K9me2 demethylation and promoting gene transcription [51]. It is possible that the MTC exhibits temporal functions, transitioning from associating with heterochromatin regions during early embryonic development to interacting with euchromatin regions in later differentiated stages. Interestingly, m6A-independent functions of METTL3 and METTL14 in chromatin regulation have been demonstrated. For instance, METTL14 binds and regulates bivalent domains marked by H3K27me3 and H3K4me3, independently of METTL3 and m6A modification, to maintain mESCs pluripotency [52]. In IMR90 cells with oncogenic RAS-induced senescence, METTL14 binds to enhancers and METTL3 localizes to NF-kB sites within promoters of senescence-associated secretory phenotype genes to drive their expression, in an m6A-independent manner [53]. The functions of the MTC and m6A in chromatin organization are, therefore, likely to be cell-type specific and dependent on different developmental stages.
In addition to histone modifications, emerging evidence has pointed to the link between m6A RNA modification and DNA methylation. METTL3-deposited m6A on RNA recruits a reader FXR1, which attracts DNA 5-methylcytosine (5mC) dioxygenase TET1 to demethylate DNA [54]. In human embryonic stem cells, the m6A reader YTHDC2 binds to primate-specific transposable elements (TEs) to recruit TET1 to remove 5mC from m6A-marked TE RNAs [55]. A recent study further clarifies the interplay of epigenetic and epitranscriptomic marks in gene expression regulation in ESCs [56]. The METTL3–METT14 complex recruits the DNA methyltransferase DNMT1, independently of m6A, to chromatin to promote gene body methylation. Both m6A and 5mC play an important role in ESC differentiation, with increased co-transcriptional 5mC and decreased post-transcriptional m6A leading to upregulation of key differentiation genes.
N 6-Methyladenosine promotes R-loop-dependent homologous recombination repair
R-loops have been widely regarded as a source of genome instability, by stalling transcription and contributing to TRCs, which can result in DSBs, one of the most deleterious lesions. In contrast, R-loops can also participate in HR repair of DSBs in certain contexts. First discovered in yeast, transient DNA–RNA hybrids form as part of the HR repair intermediates and RNase H1 is important for their resolution and efficient repair [4]. The role of m6A modification in DSB repair was first demonstrated in A375 melanoma and HeLa cells, where m6A is rapidly deposited on RNAs by METTL3 in response to UV damage [57]. Subsequent studies further link m6A and DNA–RNA hybrids as an essential mediator of HR repair. In response to break-induced Zeocin treatment, METTL3 is phosphorylated by ataxia-telangiectasia mutated kinase (ATM) to localize and deposit m6A on DNA-damage-associated RNAs. This modification recruits YTHDC1 to stabilize DNA–RNA hybrids to subsequently facilitate the recruitment of RAD51 and BRCA1 to perform HR repair [11] (Fig. 2B). Interestingly, depletion of METTL3 impairs HR repair efficiency, while non-homologous end joining (NHEJ) repair remains unaffected [11]. It is unclear whether m6A acts as a signal favoring HR repair and whether the cells switch to NHEJ or other alternative repair pathways in the absence of m6A. Another study further explored the role of AT-rich interaction domain 1A (ARID1A), a SWI/SNF family member that regulates chromatin structure. ARID1A plays a central role in sensing R-loops associated with laser-generated DSBs and helps the recruitment of METTL3 to DSBs to deposit m6A, which attracts RNase H1 to promote R-loop clearance and efficient repair [29]. In vitro assays showed that RNase H1 preferentially binds to m6A-modified R-loops compared to unmodified ones, and this endonuclease’s recruitment to damage sites depends on the catalytic activity of METTL3 [29]. However, it remains unclear whether RNase H1 directly recognizes m6A-marked R-loops in vivo or whether this recruitment is mediated through an m6A reader. Consistent with these findings, the tonicity-responsive enhancer binding protein (TonEBP) is another factor that recognizes R-loops generated by DNA damaging agents and recruits METTL3 for m6A methylation and RNase H1 for R-loop resolution [58]. In general, m6A-marked R-loops need to form transiently after DNA end resection to recruit repair factors, which are subsequently removed by RNase H1 or other helicases, to allow RAD51 loading on single-stranded DNA for strand invasion and HR repair. The findings from these studies reinforce the concept that m6A plays a central role in either forming or resolving R-loops timely, the failure of which results in defective repair and genome instability.
N 6-Methyladenosine promotes R-loop-forming TERRA at telomeres and cenRNAs at centromeres
TERRA is a type of long non-coding RNA transcribed by RNAPII at subtelomeric regions that binds to telomeres and plays an essential role in telomerase-dependent and -independent lengthening of telomeres. A proportion of cancers are ALT+, in which TERRA forms R-loops to promote HR mechanism to maintain telomere length [59]. Depletion of RNase H1 induces telomeric R-loop gain and replication stress, while overexpressing RNase H1 reduces HR and telomere shortening in ALT+ cells [60]. This indicates maintaining a balance of R-loop levels is critical for telomere stability. It has been discovered that METTL3 methylates TERRA to form R-loops, which are stabilized by YTHDC1 or hnRNPA2B1, allowing HR-dependent telomere length maintenance in ALT+ cancer cells [13, 14] (Fig. 2C). In the absence of m6A or the readers, TERRA formation is compromised and telomeric DNA damage accumulates. ALT+ neuroblastoma cells exhibit higher levels of m6A-modified TERRA compared to ALT cells, implying that ALT+ cells may be more dependent on m6A for telomeric integrity.
Furthermore, in centromeres, DNA repeats are transcribed by RNAPII into non-coding cenRNAs, which play an important role in kinetochore assembly [61, 62]. These cenRNAs have been shown to form R-loops at centromeric DNA in both yeast and mammalian cells [5, 63]. Centromeric R-loops play a positive role in recruiting Aurora kinase B to orchestrate proper mitotic processes such as spindle assembly and the attachment of microtubules to kinetochores [5]. Conversely, persistent R-loops can trigger DSBs, anaphase bridges, and translocation events at centromeres during replication [64]. Interestingly, m6A has been recently discovered to be strongly enriched on cenRNAs in cancer cells compared to non-cancerous cells [65] (Fig. 2C). CENPA, a major H3 variant in maintaining centromere stability and function during mitosis, recognizes and binds to m6A-modified cenRNAs during S-phase to fulfill its function in the centromere. CENPA has been identified as a novel m6A reader of cenRNAs through its leucine 61 and arginine 63 residues [65]. Disruption of the CENPA–m6A–cenRNA axis leads to centromere instability, defective chromosome segregation, and tumour proliferation. The reason why cancer cells are more dependent on this specific pathway for centromere stability, while CENPA is considered a general m6A reader shown in this study, should be further investigated in future research. Based on these findings, m6A may play an important role in regulating R-loop-prone cenRNAs to safeguard centromeric integrity.
N 6-Methyladenosine on R-loops as a potential target for cancer treatment
The m6A regulators have been implicated in the oncogenesis of several cancer types such as leukaemia [66], hepatocellular carcinoma [67], breast cancer [68], and glioblastoma [69]. Previous studies suggest that dysregulated m6A pathways can lead to abnormal expression of oncogenes or tumour suppressor genes, thereby contributing to cancer progression [70]. In addition to regulating gene expression, growing evidence has demonstrated the critical functions of m6A methylation in maintaining genome stability in cancer cells through several mechanisms, including R-loop-dependent HR repair of DSBs, TERRA-mediated ALT, and centromere integrity (Table 1). In ALT-positive neuroblastoma cells, treatment with a specific METTL3 inhibitor, STM2457, induces telomere shortening and telomeric DNA damage, suggesting m6A as a potential target for ALT-driven neuroblastoma [14]. Whether other ALT-positive cancers are dependent on the m6A–R-loop axis for telomere maintenance requires further investigation. Regarding HR repair, depletion of METTL3 sensitizes oral squamous carcinoma CAL27 cells and osteosarcoma U2OS cells as well as murine xenografts to cisplatin treatment and X-ray irradiation [11]. This suggests the potential of combination therapies of METTL3 inhibitors with chemotherapies, particularly those targeting DSB repair pathways. Combination of anti-METTL3–STM2457 compound with centromere-interfering drugs yields higher synergy in A375 melanoma cells compared to combinations with DNA damage-inducing drugs [65]. Strikingly, METTL3 depletion in normal (i.e. non-tumorigenic) cell lines, such as HEK293T (immortalized human embryonic kidney cells) and IMR90 (fibroblasts isolated from normal lung tissue), does not induce DNA damage, centromeric defects, altered CENPA expression, or cell proliferation [65]. This selectivity suggests that disrupting CENPA–m6A–cenRNA axis can potentially synergize with centromere-targeting agents and overcome drug resistance in cancers reliant on this pathway.
Table 1.
Targeting the m6A–R-loop axis and other m6A-mediated pathways as potential cancer therapies
| Target molecule(s) | Target pathway(s) | Cancer type(s) | Clinical trial | Reference(s) |
|---|---|---|---|---|
| METTL3 inhibitor (STM2457) + DNA damage chemotherapies | Homologous recombination repair (m6A on DNA–RNA hybrids) | Head and neck squamous carcinoma, ARID1A-mutant cancers | – | [11, 29] |
| METTL3 inhibitor (STM2457) | Alternative lengthening of telomeres (m6A on R-loop forming TERRA) | ALT-positive neuroblastoma | – | [14] |
| METTL3 inhibitor (STM2457) + centromere-targeting drugs (AZD1152, Tozasertib, etc.) | Centromere integrity (m6A on centromeric RNAs) | Melanoma and other cancers | – | [65] |
| METTL3 inhibitor (STC-15) | Interferon response | Solid tumours | Phase 1 (NCT05584111) | [71] |
| FTO inhibitor (bisantrene) | Cancer stem cell renewal, immune evasion | Acute myeloid leukaemia | Phase 2 (NCT04989335) | [72] |
Abnormal expression of m6A modifiers has been associated with tumour progression, poor prognosis, and drug resistance [70]. Cancer cells with aberrant R-loop levels have been shown to be more sensitive to DNA damage-inducing agents such as ATR inhibitors, BET inhibitors, and G-quadruplex stabilizers [8]. The potential of targeting m6A–R-loop axis to induce cancer-specific genome instability is promising and poised to improve precision oncology. However, the relationship between m6A-modified R-loop levels and patient malignancy remains unclear. As m6A plays a major role in regulating RNA metabolism, merely inhibiting m6A writers or erasers is likely to perturb normal cellular functions. It is thus paramount to identify certain cancer (sub)types that are more particularly dependent on the m6A–R-loop axis as well as the effectors involved in this process. A potential strategy could be drug combination screens of m6A-targeting drugs with other compounds targeting R-loop modulators or repair factors, in cancer and normal cell lines or organoids, which could identify synergistic combination pairs and ensure therapeutic efficacy while minimizing toxicity. Importantly, new drugs targeting specific reader proteins and their functional/interacting domains, exclusively involved in the processing of R-loops and separated from the m6A-mediated RNA regulation, will likely help achieve treatment specificity.
It should be noted that m6A has been targeted as a therapeutic strategy through other mechanisms, for instance, by triggering anti-tumour immune response (Table 1). Guirguis et al. developed a second generation of METTL3 inhibitor, STM3006, with increased potency compared to the previous compound STM2457 [71]. The authors show that METTL3 inhibition, in mouse AT3 triple-negative breast cancer cells and B16 melanoma cells, results in double-stranded RNA formation, triggers a cell-intrinsic interferon response stimulating T-cell activity, and enhances anti-tumour immunity when combined with anti-PD1 therapy [71]. Based on this finding, a more recent METTL3 inhibitor STC-15, with improved oral bioavailability, is undergoing phase 1 clinical trial for advanced solid tumours (NCT05584111). Similarly, two potent FTO inhibitors (bisantrene and brequinar) have been shown to suppress leukaemic stem cell renewal, reduce expression of immune checkpoint genes and increase sensitivity to T-cell cytotoxicity, particularly in highly expressed FTO tumours [72]. Bisantrene is currently under phase 2 clinical trial for resistant acute myeloid leukaemia (NCT04989335). Interestingly, cytosolic DNA fragments or micronuclei stemmed from replication stress and cytoplasmic R-loop accumulation can activate the cyclic GMP–AMP synthase/stimulator of interferon genes (cGAS/STING) pathway, promoting an immune response through interferon type I stimulation [73, 74]. This opens a potential link between m6A–R-loop and anti-tumour immunity, which warrants further mechanistic research and is likely to contribute to new targeted therapy development.
Concluding remarks and perspectives
The role of m6A modification in mediating R-loop homeostasis has attracted significant research interest and its importance in genome maintenance is increasingly appreciated. Despite these efforts, how m6A regulates R-loop balance in physiological versus pathological conditions, as well as the contribution of different factors, such as m6A readers and/or helicases, to this process remains elusive. It is also unclear how m6A correlates with R-loop levels in different cell types, which subsets of R-loops are selectively modified, and whether R-loops are preferentially methylated compared to nascent RNA. New techniques that can directly detect and quantify m6A on R-loops at single-nucleotide resolution should help address these questions. The diverse and complex functions of the MTC and m6A modification further pose a great challenge to R-loop studies, as chromatin states, histone marks, DNA methylation, and nascent transcription are all interconnected and each can impact R-loop balance. Additionally, a notable limitation of previous studies is the possible indirect effects from perturbing the m6A pathway. Depletion of any of the writers or readers can impact not only R-loop metabolism but also the transcriptome and nascent transcription processing. For example, altered expression of m6A-depleted transcripts, involved in transcription regulation or DNA damage response, could contribute to the observed effect on R-loop alterations and genome instability, thereby masking the direct effect of m6A on R-loops. The fact that these potential confounding factors have not been considered may explain the discrepancies observed among previous studies.
Therefore, researchers should carefully consider the primary and secondary effects of m6A perturbation in future research. Acute proteasomal degradation approaches on m6A writers or readers, such as the auxin-degron system [75], the dTAG system [76], or proteolysis-targeting chimera (PROTAC) degraders [77], could help minimize such secondary effects and allow for rapid assessment of R-loop alterations. A more precise strategy involves m6A editing tools based on the clustered regularly interspaced short palindromic repeat. These techniques use programmable single guide RNAs (sgRNAs) for site-specific recruitment of Cas protein-fused with either an m6A writer or eraser to the target loci to install or remove m6A [78]. This would allow for localized m6A alteration at sgRNA-targeted R-loop regions without affecting the transcriptome. However, it remains unclear whether a pre-existing DNA–RNA hybrid may hinder sgRNA binding and Cas targeting. The fate of m6A-modified R-loops is potentially mediated by m6A readers and their downstream effectors. Proteomics mapping approaches such as proximity labelling or co-immunoprecipitation coupled with mass spectrometry, under R-loop accumulation condition, could help pinpoint the factors involved in the m6A–R-loop axis. Future studies utilizing such acute and targeted approaches will be pivotal in dissecting the exact mechanism by which m6A regulates R-loop balance and will contribute to the development of m6A- and R-loop-targeting cancer therapies.
In addition to m6A, other RNA modifications, such as N1-methyladenosine [79], m5C, and 3-methylcytosine, have also been recently shown to play an important role in R-loop function (reviewed by [26]). The potential interplay and crosstalk between m6A with other modifications in R-loop homeostasis open exciting avenues for future research and warrant further investigation.
Acknowledgements
The authors would like to acknowledge Yea-Lih Lin for critical reading of the manuscript.
Author contributions: Minh-Anh Vu (Funding acquisition [equal], Investigation [equal], Visualization [lead], Writing—original draft [lead], Writing—review & editing [equal]), Manuela Spagnuolo (Investigation [equal], Supervision [equal], Writing—review & editing [equal]), and Chun-Long Chen (Conceptualization [lead], Funding acquisition [lead], Investigation [equal], Project administration [lead], Supervision [lead], Visualization [equal], Writing—review & editing [equal]).
Contributor Information
Minh-Anh Vu, Institut Curie, PSL Research University, CNRS UMR3244, Dynamics of Genetic Information, Sorbonne Université, 75005 Paris, France.
Manuela Spagnuolo, Institut Curie, PSL Research University, CNRS UMR3244, Dynamics of Genetic Information, Sorbonne Université, 75005 Paris, France.
Chun-Long Chen, Institut Curie, PSL Research University, CNRS UMR3244, Dynamics of Genetic Information, Sorbonne Université, 75005 Paris, France.
Conflict of interest
M.A.V., M.S., and C.L.C. have filed a patent entitled ‘Method for the treatment of estrogen-dependent cancer’ (European Patent Application number EP24 305 746.0, 14 May 2024).
Funding
C.L.C.’s team was supported by the ATIP-Avenir programme from the Centre national de la recherche scientifique (CNRS) and Plan Cancer from INSERM [ATIP/AVENIR: No. 18CT014-00), the Agence Nationale de la Recherche (ANR) (ReDeFINe—19-CE12-0016-02, TELOCHROM—19-CE12-0020-02, SMART—21-CE12-0033-02, ReSPoND—23-CE12-0020-02), the Institut National du Cancer (INCa) (PLBIO19-076), and the Impulscience programme of the Bettencourt Schueller Foundation. M.A.V. was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement [847718 (Institut Curie’s EuReCa PhD Programme)] and by the Fondation ARC pour la recherche sur le cancer.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Sanz LA, Hartono SR, Lim YW et al. Prevalent, dynamic, and conserved R-loop structures associate with specific epigenomic signatures in mammals. Mol Cell. 2016; 63:167–78. 10.1016/j.molcel.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ginno PA, Lim YW, Lott PL et al. GC skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013; 23:1590–600. 10.1101/gr.158436.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skourti-Stathaki K, Kamieniarz-Gdula K, Proudfoot NJ R-loops induce repressive chromatin marks over mammalian gene terminators. Nature. 2014; 516:436–9. 10.1038/nature13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohle C, Tesorero R, Schermann G et al. Transient RNA–DNA hybrids are required for efficient double-strand break repair. Cell. 2016; 167:1001–13. 10.1016/j.cell.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 5. Kabeche L, Nguyen HD, Buisson R et al. A mitosis-specific and R loop-driven ATR pathway promotes faithful chromosome segregation. Science. 2018; 359:108–14. 10.1126/science.aan6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li D, Shao F, Li X et al. Advancements and challenges of R-loops in cancers: biological insights and future directions. Cancer Lett. 2025; 610:217359. 10.1016/j.canlet.2024.217359. [DOI] [PubMed] [Google Scholar]
- 7. Kannan A, Gangadharan Leela S, Branzei D et al. Role of senataxin in R-loop-mediated neurodegeneration. Brain Commun. 2024; 6:fcae239. 10.1093/braincomms/fcae239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petermann E, Lan L, Zou L Sources, resolution and physiological relevance of R-loops and RNA–DNA hybrids. Nat Rev Mol Cell Biol. 2022; 23:521–40. 10.1038/s41580-022-00474-x. [DOI] [PubMed] [Google Scholar]
- 9. Abakir A, Giles TC, Cristini A et al. N6-Methyladenosine regulates the stability of RNA:DNA hybrids in human cells. Nat Genet. 2020; 52:48–55. 10.1038/s41588-019-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X, Liu Q-L, Xu W et al. m6A promotes R-loop formation to facilitate transcription termination. Cell Res. 2019; 29:1035–8. 10.1038/s41422-019-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Chen L, Peng D et al. METTL3 and N6-methyladenosine promote homologous recombination-mediated repair of DSBs by modulating DNA–RNA hybrid accumulation. Mol Cell. 2020; 79:425–42. 10.1016/j.molcel.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 12. Xu C, Wu Z, Duan H-C et al. R-loop resolution promotes co-transcriptional chromatin silencing. Nat Commun. 2021; 12:1790. 10.1038/s41467-021-22083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Zhang C, Ma W et al. METTL3-mediated m6A modification stabilizes TERRA and maintains telomere stability. Nucleic Acids Res. 2022; 50:11619–34. 10.1093/nar/gkac1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaid R, Thombare K, Mendez A et al. METTL3 drives telomere targeting of TERRA lncRNA through m6A-dependent R-loop formation: a therapeutic target for ALT-positive neuroblastoma. Nucleic Acids Res. 2024; 52:2648–71. 10.1093/nar/gkad1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer KD, Saletore Y, Zumbo P et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012; 149:1635–46. 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dominissini D, Moshitch-Moshkovitz S, Schwartz S et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012; 485:201–6. 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Lu Z, Gomez A et al. N6-Methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014; 505:117–20. 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong J, Xu K, Lee JH Biological roles of the RNA m6A modification and its implications in cancer. Exp Mol Med. 2022; 54:1822–32. 10.1038/s12276-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei J, Yu X, Yang L et al. FTO mediates LINE1 m6A demethylation and chromatin regulation in mESCs and mouse development. Science. 2022; 376:968–73. 10.1126/science.abe9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cristini A, Groh M, Kristiansen MS et al. RNA/DNA hybrid interactome identifies DXH9 as a molecular player in transcriptional termination and R-loop-associated DNA damage. Cell Rep. 2018; 23:1891–905. 10.1016/j.celrep.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang IX, Grunseich C, Fox J et al. Human proteins that interact with RNA/DNA hybrids. Genome Res. 2018; 28:1405–14. 10.1101/gr.237362.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu T, Nance J, Chu F et al. Characterization of R-loop–interacting proteins in embryonic stem cells reveals roles in rRNA processing and gene expression. Mol Cell Proteomics. 2021; 20:100142. 10.1016/j.mcpro.2021.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhigalova NA, Oleynikova KY, Ruzov AS et al. The functions of N6-methyladenosine in nuclear RNAs. Biochemistry (Mosc). 2024; 89:159–72. 10.1134/S0006297924010103. [DOI] [PubMed] [Google Scholar]
- 24. Qiu Y, Man C, Zhu L et al. R-loops’ m6A modification and its roles in cancers. Mol Cancer. 2024; 23:232. 10.1186/s12943-024-02148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abakir A, Ruzov A A model for a dual function of N6-methyladenosine in R-loop regulation. Nat Genet. 2024; 56:1995–8. 10.1038/s41588-024-01905-5. [DOI] [PubMed] [Google Scholar]
- 26. Wu Y, Lin S, Chen H et al. Cross-regulation of RNA methylation modifications and R-loops: from molecular mechanisms to clinical implications. Cell Mol Life Sci. 2024; 82:1. 10.1007/s00018-024-05536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hao J-D, Liu Q-L, Liu M-X et al. DDX21 mediates co-transcriptional RNA m6A modification to promote transcription termination and genome stability. Mol Cell. 2024; 84:1711–26. 10.1016/j.molcel.2024.03.006. [DOI] [PubMed] [Google Scholar]
- 28. Ying Y, Wu Y, Zhang F et al. Co-transcriptional R-loops-mediated epigenetic regulation drives growth retardation and docetaxel chemosensitivity enhancement in advanced prostate cancer. Mol Cancer. 2024; 23:79. 10.1186/s12943-024-01994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Chen F, Tang M et al. The ARID1A–METTL3–m6A axis ensures effective RNase H1-mediated resolution of R-loops and genome stability. Cell Rep. 2024; 43:113779. 10.1016/j.celrep.2024.113779. [DOI] [PubMed] [Google Scholar]
- 30. Slobodin B, Han R, Calderone V et al. Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell. 2017; 169:326–37. 10.1016/j.cell.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knuckles P, Carl SH, Musheev M et al. RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat Struct Mol Biol. 2017; 24:561–9. 10.1038/nsmb.3419. [DOI] [PubMed] [Google Scholar]
- 32. Ke S, Pandya-Jones A, Saito Y et al. m6 A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017; 31:990–1006. 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu W, He C, Kaye EG et al. Dynamic control of chromatin-associated m6A methylation regulates nascent RNA synthesis. Mol Cell. 2022; 82:1156–68. 10.1016/j.molcel.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akhtar J, Renaud Y, Albrecht S et al. m6A RNA methylation regulates promoter-proximal pausing of RNA polymerase II. Mol Cell. 2021; 81:3356–67. 10.1016/j.molcel.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 35. Bhowmick R, Mehta KPM, Lerdrup M et al. Integrator facilitates RNAPII removal to prevent transcription–replication collisions and genome instability. Mol Cell. 2023; 83:2357–66. 10.1016/j.molcel.2023.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J-H, Wang R, Xiong F et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol Cell. 2021; 81:3368–85. 10.1016/j.molcel.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louloupi A, Ntini E, Conrad T et al. Transient N-6-methyladenosine transcriptome sequencing reveals a regulatory role of m6A in splicing efficiency. Cell Rep. 2018; 23:3429–37. 10.1016/j.celrep.2018.05.077. [DOI] [PubMed] [Google Scholar]
- 38. Xiao W, Adhikari S, Dahal U et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016; 61:507–19. 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Yang F, Shang J et al. Integrative analysis reveals the prognostic value and functions of splicing factors implicated in hepatocellular carcinoma. Sci Rep. 2021; 11:15175. 10.1038/s41598-021-94701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tran TM, Philipp J, Bassi JS et al. The RNA-binding protein IGF2BP3 is critical for MLL-AF4-mediated leukemogenesis. Leukemia. 2022; 36:68–79. 10.1038/s41375-021-01346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu N, Zhou KI, Parisien M et al. N6-Methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017; 45:6051–63. 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang J, Chen Z, Wang Q et al. hnRNPA2B1 promotes colon cancer progression via the MAPK pathway. Front Genet. 2021; 12:666451. 10.3389/fgene.2021.666451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alarcón CR, Goodarzi H, Lee H et al. HNRNPA2B1 Is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015; 162:1299–308. 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen HD, Yadav T, Giri S et al. Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol Cell. 2017; 65:832–47. 10.1016/j.molcel.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tresini M, Warmerdam DO, Kolovos P et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature. 2015; 523:53–8. 10.1038/nature14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aguilera A, García-Muse T R loops: from transcription byproducts to threats to genome stability. Mol Cell. 2012; 46:115–24. 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 47. Stirling PC, Chan YA, Minaker SW et al. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012; 26:163–75. 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu ZS, Sinha S, Bannister M et al. R-loop accumulation in spliceosome mutant leukemias confers sensitivity to PARP1 inhibition by triggering transcription–replication conflicts. Cancer Res. 2024; 84:577–97. 10.1158/0008-5472.CAN-23-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu W, Li J, He C et al. METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature. 2021; 591:317–21. 10.1038/s41586-021-03210-1. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Dou X, Chen C et al. N6-Methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020; 367:580–6. 10.1126/science.aay6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Xia L, Tan K et al. N6-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat Genet. 2020; 52:870–7. 10.1038/s41588-020-0677-3. [DOI] [PubMed] [Google Scholar]
- 52. Mu M, Li X, Dong L et al. METTL14 regulates chromatin bivalent domains in mouse embryonic stem cells. Cell Rep. 2023; 42:112650. 10.1016/j.celrep.2023.112650. [DOI] [PubMed] [Google Scholar]
- 53. Liu P, Li F, Lin J et al. m6A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype. Nat Cell Biol. 2021; 23:355–65. 10.1038/s41556-021-00656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng S, Zhang J, Su J et al. RNA m6A regulates transcription via DNA demethylation and chromatin accessibility. Nat Genet. 2022; 54:1427–37. 10.1038/s41588-022-01173-1. [DOI] [PubMed] [Google Scholar]
- 55. Sun T, Xu Y, Xiang Y et al. Crosstalk between RNA m6A and DNA methylation regulates transposable element chromatin activation and cell fate in human pluripotent stem cells. Nat Genet. 2023; 55:1324–35. 10.1038/s41588-023-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quarto G, Li Greci A, Bizet M et al. Fine-tuning of gene expression through the Mettl3–Mettl14–Dnmt1 axis controls ESC differentiation. Cell. 2025; 188:998–1018. 10.1016/j.cell.2024.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xiang Y, Laurent B, Hsu C-H et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017; 543:573–6. 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kang HJ, Cheon NY, Park H et al. TonEBP recognizes R-loops and initiates m6A RNA methylation for R-loop resolution. Nucleic Acids Res. 2021; 49:269–84. 10.1093/nar/gkaa1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bettin N, Oss Pegorar C, Cusanelli E The emerging roles of TERRA in telomere maintenance and genome stability. Cells. 2019; 8:246. 10.3390/cells8030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arora R, Lee Y, Wischnewski H et al. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commun. 2014; 5:5220. 10.1038/ncomms6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferri F, Bouzinba-Segard H, Velasco G et al. Non-coding murine centromeric transcripts associate with and potentiate Aurora B kinase. Nucleic Acids Res. 2009; 37:5071–80. 10.1093/nar/gkp529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quénet D, Dalal Y A long non-coding RNA is required for targeting centromeric protein A to the human centromere. eLife. 2014; 3:e03254. 10.7554/elife.03254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mishra PK, Chakraborty A, Yeh E et al. R-loops at centromeric chromatin contribute to defects in kinetochore integrity and chromosomal instability in budding yeast. MBoC. 2021; 32:74–89. 10.1091/mbc.E20-06-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giunta S, Hervé S, White RR et al. CENP-A chromatin prevents replication stress at centromeres to avoid structural aneuploidy. Proc Natl Acad Sci USA. 2021; 118:e2015634118. 10.1073/pnas.2015634118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang Z, Li R, Liu C et al. m6A-modified cenRNA stabilizes CENPA to ensure centromere integrity in cancer cells. Cell. 2024; 187:6035–54. 10.1016/j.cell.2024.08.040. [DOI] [PubMed] [Google Scholar]
- 66. Barbieri I, Tzelepis K, Pandolfini L et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017; 552:126–31. 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen M, Wei L, Law C et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018; 67:2254–70. 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 68. Zhang C, Samanta D, Lu H et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016; 113:E2047–56. 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cui Q, Shi H, Ye P et al. A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017; 18:2622–34. 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deng X, Qing Y, Horne D et al. The roles and implications of RNA m6A modification in cancer. Nat Rev Clin Oncol. 2023; 20:507–26. 10.1038/s41571-023-00774-x. [DOI] [PubMed] [Google Scholar]
- 71. Guirguis AA, Ofir-Rosenfeld Y, Knezevic K et al. Inhibition of METTL3 results in a cell-intrinsic interferon response that enhances antitumor immunity. Cancer Discov. 2023; 13:2228–47. 10.1158/2159-8290.CD-23-0007. [DOI] [PubMed] [Google Scholar]
- 72. Su R, Dong L, Li Y et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020; 38:79–96. 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin Y-L, Pasero P Replication stress: from chromatin to immunity and beyond. Curr Opin Genet Dev. 2021; 71:136–42. 10.1016/j.gde.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 74. Crossley MP, Song C, Bocek MJ et al. R-loop-derived cytoplasmic RNA–DNA hybrids activate an immune response. Nature. 2023; 613:187–94. 10.1038/s41586-022-05545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yesbolatova A, Saito Y, Kitamoto N et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat Commun. 2020; 11:5701. 10.1038/s41467-020-19532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nabet B, Roberts JM, Buckley DL et al. The dTAG system for immediate and target-specific protein degradation. Nat Chem Biol. 2018; 14:431–41. 10.1038/s41589-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Békés M, Langley DR, Crews CM PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022; 21:181–200. 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu X-M, Zhou J, Mao Y et al. Programmable RNA N6-methyladenosine editing by CRISPR–Cas9 conjugates. Nat Chem Biol. 2019; 15:865–71. 10.1038/s41589-019-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsao N, Lombardi PM, Park A et al. YTHDC1 cooperates with the THO complex to prevent RNA-damage-induced DNA breaks. Mol Cell. 2025; 85:1085–100. 10.1016/j.molcel.2025.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.