Abstract
The Polygonati Rhizoma have generated significant market attention for their medicinal and culinary applications. However, morphological similarities and ambiguous species boundaries complicate the identification of genera and species, thereby impeding product development and utilization within Polygonatum sensu lato. Despite the widespread application of the chloroplast genome for taxonomic boundary revisions for Polygonatum s.l., a critical gap persist regarding their genomic applicability and the lack of standardized pipelines for developing species-specific molecular markers capable of rapid discrimination among species. This study aims to assess the effectiveness of chloroplast genomes in clarifying the current taxonomic status of the genera and species of Polygonatum s.l., and develop a reliable process for rapid identification of designated species from other species. A total of 21 chloroplast genomes were sequenced and assembled, and subsequent analyses included phylogenetic inference, multiple molecular species delimitation methods, and an automated screening framework were employed for subsequent analysis. Comparative analyses revealed relatively conserved chloroplast genomes, with notable variation limited primarily to the length of IR and LSC regions. By integrating multiple delimitation methods, the chloroplast genome validated 82.46% of the current classifications of Polygonatum s.l., demonstrating strong support (90.63%) for species represented by multiple sequences, yet only moderate support (70%) for those with single-sequence representation. Additionally, this study established and validated a scalable molecular marker development framework, spanning from identification of species-specific SNPs/InDels to the design of high-resolution molecular markers, illustrated through case studies involving Heteropolygonatum and three medicinally significant Polygonatum species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-12012-y.
Keywords: DNA barcoding, Polygonati rhizoma, Molecular marker, Heteropolygonatum
Introduction
Polygonatum sensu lato (s.l.), comprising Polygonatum Mill. and Heteropolygonatum M. N. Tamura & Ogisu, includes over 80 species distributed predominantly across temperate regions of Eurasia and North America [1–5]. Several species, notably P. sibiricum, P. cyrtonema, and P. kingianum, possess significant economic value as multipurpose resources in traditional medicine [6, 7]. The rhizomes (Polygonati Rhizoma) of these species are rich in bioactive compounds, including polysaccharides, saponins, and flavonoids [8, 9]. Furthermore, these rhizomes also exhibit a wide spectrum of pharmacological activities, such as modulation of intestinal microbiota, immune enhancement, antioxidant, anti-inflammatory, antidiabetic, antitumor, hypolipidemic, anti-fatigue, neuroprotective, and osteoporosis-preventing effects [8, 10, 11]. These therapeutic attributes, coupled with their historical utilization in Chinese herbal formulations for over two millennia, have driven modern applications ranging from nutraceutical development to innovative processing techniques like the traditional ‘nine-steam-nine-bask’ method [7, 12].
Accurate species identification is essential for ensuring the quality of economic crops, conserving germplasm resources, supporting industrial applications, and advancing scientific research [13–15]. Currently, species delimitation within Polygonatum s.l. predominantly relies on morphological characterization of diagnostic traits such as rhizome, phyllotaxis, and inflorescence [1]. However, this method of morphological identification remains challenging because many scenarios rely solely on Polygonatum rhizomes, complicating quality control in herbal product manufacturing [16, 17]. These persistent challenges have led to adjustments in species boundaries for more than seven species between Polygonatum and Heteropolygonatum in the past two decades, resulting in substantial taxonomic ambiguities [18–22]. Diagnostic features differentiating these two genera are challenging to observe directly. Polygonatum is characterized by a chromosome number of x = 9–15 and valvate tepals, while Heteropolygonatum exhibits chromosome numbers of x = 16, 32 with imbricate tepals [18, 20]. Previous phylogenetic studies employing multi-locus chloroplast markers have effectively resolved generic-level delimitation between Polygonatum and Heteropolygonatum, and have identified three major lineages within Polygonatum [23, 24]. However, these methods have demonstrated limited resolution at the species level. Recent advances in phylogenomics and phylogeographic analyses utilizing whole-chloroplast genomes have demonstrated greater potential in resolving species boundaries [2, 25, 26]. Crucially, whole-chloroplast genomes enable comprehensive detection of structural variants and provide an increased number of variable sites, thereby offering unprecedented resolution at the species level [17]. However, critical gaps persist in translating these evolutionary insights and their application potential into practical frameworks for authenticating medicinal species [27, 28]. This gap underscores the urgent need to bridge phylogenetic frameworks and practical identification tools, particularly for high-value species within Polygonatum, including the three traditional medicinal species [17, 29, 30].
The past decade has witnessed a surge in genetic methods for species identification, laying a critical foundation for systematically evaluating the utility of chloroplast genomes for clarifying current taxonomic classifications [31]. Current validation frameworks predominantly rely on single-locus datasets and comparative analyses of genetic distances, supplemented by multiple molecular species delimitation methods [32–34]. However, divergent taxonomic conclusions frequently occur due to data heterogeneity (e.g., cytonuclear discordance) and variable sampling densities across studies [28, 35]. These discrepancies highlight the imperative need for evaluating the taxonomic applicability of chloroplast genomes. Furthermore, empirical comparisons of these delimitation algorithms underscore the necessity of integrative validation frameworks to quantitatively assess support from molecular datasets for current species boundaries [31].
The integration of rigorously validated genomic datasets facilitates the development of species-specific molecular markers, significantly enhancing efficiency in species identification for morphologically cryptic lineages. These markers, including InDel regions and species-specific sites resulting from single nucleotide polymorphisms (SNPs), insertions or deletions at identical genomic loci across related species [36], are effective for species identification and combating herbal adulteration in commercial markets [37, 38]. For example, the development of InDel primers for Cynanchum wilfordii and C. auriculatum has effectively reduced adulteration of herbal materials [39], while species-specific primers for Arnebiae Forssk. species have enhanced identification accuracy [40]. Combining chloroplast genome analysis with these two types of species-specific markers offers a more versatile and reliable species identification approach, providing a foundation for quality control and sustainable cultivation of economically important species. This integrated strategy ensures taxonomic accuracy and enhances both scientific research and practical industrial applications. In this study, we sequenced 21 complete chloroplast genomes from Polygonatum s.l., prioritized to augment underrepresented groups, and integrated 102 publicly available datasets (comprising 11 from Heteropolygonatum, 90 from Polygonatum, and one outgroup) from public databases. Collectively, these sequences represent all three major clades within Polygonatum (sect. Verticillata, sect. Sibirica, and sect. Polygonatum), species phylogenetically closely related to the three medicinal Polygonatum species, and additional intraspecific variation. The objectives of this study were: (1) to identify patterns of chloroplast genome variation in Polygonatum s.l.; (2) to evaluate the effectiveness of chloroplast genome in delineating the currently accepted species of Polygonatum s.l. using various molecular species delimitation programs; and (3) to explore the distribution of species-specific sites for developing and validating specific primers to facilitate accurate genus and traditional medicinal species identification. Our study provides a comprehensive evaluation of chloroplast genomes, offering new insights and methodological frameworks for resolving taxonomic uncertainties at various levels in key medicinal plant species.
Materials and methods
Plant material and DNA sequencing
From 2020 to 2023, fieldwork was conducted in Anhui, Guizhou, Shandong, Shaanxi, Sichuan, Yunnan, and Zhejiang Provinces, China, where samples were collected from 21 populations of 13 species belonging to Polygonatum s.l. (Table 1; Fig. 1). These samples were identified by Yingfeng Hu and Prof. Jianwen Shao based on morphological characterization, and were deposited at the herbarium of Anhui Normal University (ANUB). One population of Heteropolygonatum, morphologically similar to H. alternicirrhosum (Hand.-Mazz.) Floden, but distinguished by coarse hairs on its stems and peduncles, was temporarily designated as Heteropolygonatum sp. Kangding in this study (Table 1). To evaluate chloroplast genome efficacy in species delimitation via genetic distance analysis, targeted sampling was conducted (Table 1) for taxa in Polygonatum s.l. with insufficient genomic data, ensuring at least two sequences per species, and prioritizing underrepresented groups. This optimized dataset enhanced phylogenetic resolution while expanding the chloroplast genomic repository, thus strengthening the validity of species boundary determination [17].
Table 1.
Sampling information of the newly sequenced samples in this study
| Scientific name | GenBank number | Location | Longitude | Latitude | Herbarium accession numbers |
|---|---|---|---|---|---|
| H. alternicirrhosum | PQ591744 | Dagang Village, Kangding City, Sichuan Province | 102.1457E | 30.0705 N | ANUB100117 |
| H. binatifolium | PQ436974 | Baifotai, Fanjing Mountain, Tongren City, Guizhou Province | 108.6646E | 27.9089 N | ANUB100118 |
| H. binatifolium | PQ436975 | Jindaoxia, Fanjing Mountain, Tongren City, Guizhou Province | 108.6742E | 27.9163 N | ANUB100119 |
| H. binatifolium | PQ591745 | Diecuixi, Longcanggou Town, Yaan City, Sichuan Province | 102.8919E | 29.6163 N | ANUB100120 |
| H. binatifolium | PQ591746 | Renshenggou, Yaan City, Sichuan Province | 102.8945E | 29.6154 N | ANUB100121 |
| Heteropolygonatum. sp. Kangding | PQ436973 | Yulin Village, Kangding City, Sichuan Province | 101.9613E | 29.9677 N | ANUB100122 |
| P. curvistylum | PV055066 | Qingfengxia, Baoji City, Shanxi Province | 107.4463E | 34.0353 N | ANUB100123 |
| P. caulialatum | PV055067 | Baiyangwan, Kangding City, Sichuan Province | 101.9587E | 29.9665 N | ANUB100124 |
| P. cyrtonema var. gutianshanicuym | PV055063 | Suzhuang Town, Quzhou City, Zhejiang Province | 118.1248E | 29.1741 N | ANUB100125 |
| P. cyrtonema var. gutianshanicuym | PV055053 | Hongtan Town, Huangshan City, Anhui Province | 117.8611E | 30.0911 N | ANUB100126 |
| P. cyrtonema var. gutianshanicuym | PV055064 | Yanglin Village, Huangshan City, Anhui Province | 117.5097E | 29.9017 N | ANUB100127 |
| P. franchetii | PV055065 | Wulixia, Baoji City, Shanxi Province | 107.4367E | 33.9881 N | ANUB100128 |
| P. jinzhaiense | PV055061 | Yaoluoping, Anqing City, Anhui Province | 116.0859E | 30.9834 N | ANUB100129 |
| P. jinzhaiense | PV055062 | Tiantangzhai, Liuan City, Anhui Province | 115.7711E | 31.1237 N | ANUB100130 |
| P. langyanese | PV055060 | Langya Mountain, Chuzhou City, Anhui Province | 118.2866E | 32.2778 N | ANUB100131 |
| P. macropodum | PV055059 | Mount Tai, Taian City, Shandong Province | 117.1428E | 36.2995 N | ANUB100132 |
| P. nodosum | PV055057 | Yaochiba, Jinfo Mountain, Chongqing City | 107.1273E | 29.0492 N | ANUB100133 |
| P. nodosum | PV055058 | Dujuan Park, Jinfo Mountain, Chongqing City | 107.1839E | 29.0168 N | ANUB100134 |
| P. punctatum | PV055055 | Dawei Mountain, Honghe City, Yunnan Province | 103.8806E | 22.7381 N | ANUB100135 |
| P. punctatum | PV055056 | Dawei Mountain, Honghe City, Yunnan Province | 103.8806E | 22.7381 N | ANUB100136 |
| P. zanlanscianense | PV055054 | Wumei Village, Chizhou City, Anhui Province | 117.9712E | 30.5829 N | ANUB100137 |
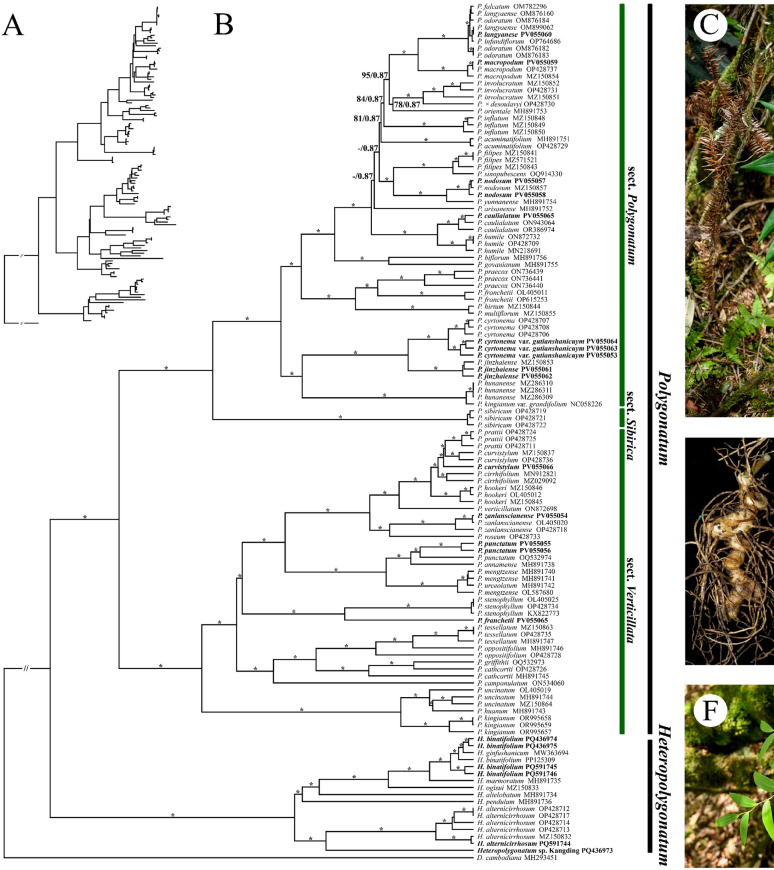
Fig. 1.
Phylogenetic relationships trees of Polygonatum and Heteropolygonatum based on 78 protein-coding genes. Phylogenetic tree with branch lengths based on Maximum Likelihood Method (A). A phylogenetic tree without branch lengths, generated using both Maximum Likelihood and Bayesian Inference (B); Asterisks represent BS = 100 / PP = 1; Branches with no support value; Samples highlighted in bold were newly generated in this study. Habitat (C) and rhizome (D) of P. cyrtonema. Rhizome (E) and habitat (F) of H. binatifolium
Fresh leaves from several individuals in each population were collected and dried in silica gel for subsequent molecular analysis. Total genomic DNA was extracted using the Tiangen DNA-secure Plant Kit (DP320) according to the manufacturer’s protocol, and DNA quality was evaluated using 2% agarose gel electrophoresis. For each sample, 350-bp DNA libraries were prepared using the MagicSeq DNA Library Prep Kit (M319), followed by sequencing with 150-bp paired-end reads on the BGI DNBSEQ-T7 platform at the Germplasm Bank of Wild Species in Southwest China (GBOWS, Kunming, China). After filtering low-quality reads with Fastp v0.23.2 [41] under default parameters, approximately 3 Gb of clean data was generated for each sample.
Complete Chloroplast genome assembly, annotation and comparison analysis
All 21 complete chloroplast genomes were de novo assembled using GetOrganelle v1.7.6.1 with K-mer values of 21, 85, and 127 [42] while other parameters were kept at default settings. These newly obtained chloroplast genomes, along with those downloaded from public databases, were annotated and re-annotated by PGA [43], with H. alternicirrhosum (MZ150832) as a reference. Start and stop codons for each protein-coding gene (PCG) were manually verified and corrected using Geneious v10.2.3 (Biomatters Ltd, Auckland, New Zealand). All newly generated chloroplast genomes were visualized using OGDRAW [44] and deposited in GenBank (https://www.ncbi.nlm.nih.gov/; Table 1).
Integrated with public databases, the chloroplast genomes of 57 species, including seven Heteropolygonatum and 50 Polygonatum, were analyzed. To identify potential structural variations and sequence lengths discrepancies within the chloroplast genomes, ProgressiveMAUVE v.2.4.0 [45] was utilized to compare variations among these 57 species (Table S1). The expansions and contractions of the inverted repeat (IR) regions were examined by analyzing the junctions between the large single-copy (LSC)/IR and small single-copy (SSC)/IR regions, as well as their adjacent genes, using CPJSdraw v0.0.1 [46]. Additionally, the lengths of the LSC, SSC, IR regions, and the total chloroplast genomes for all samples were statistically analyzed in Geneious v10.2.3 (Biomatters Ltd, Auckland, New Zealand). Differences in sequence lengths were assessed using an independent sample t-test to evaluate statistical significance for Polygonatum and Heteropolygonatum by the t-test plugin in Rstudio v.2021.09.1.
Phylogenetic analysis
To reconstruct the phylogenetic relationships of Polygonatum s.l., all chloroplast genome sequences involved in this study, including 17 Heteropolygonatum and 105 Polygonatum sequences, representing seven species of Heteropolygonatum and 50 species of Polygonatum (Table 1, S1). Dracaena cambodiana (MH293451) was chosen as the outgroup. These sequences covered all three major clades within Polygonatum (sect. Verticillata, sect. Sibirica, and sect. Polygonatum), and included phylogenetically proximate species to the three medicinal Polygonatum taxa. A total of 78 shared protein-coding genes (PCGs) and complete chloroplast genome sequences were extracted and aligned using MAFFT v7.480 in Geneious v10.2.3 with default settings [47]. The phylogenetic relationships of Polygonatum s.l. were inferred using both Maximum Likelihood (ML) and Bayesian Inference (BI) methods. The best substitution model was selected using ModelFinder in PhyloSuite v1.2.2 [48]. For the ML analysis, RAxML v2.0.0 was used with the GTRGAMMA model and 1000 bootstrap replicates [49]. BI analysis was performed in MrBayes v3.2 using the Markov Chain Monte Carlo (MCMC) algorithm, running for 20 million generations with trees sampled every 1000 generations [50]. Convergence was assessed by confirming that the average standard deviation of split frequencies was below 0.01. The first 25% of the sampled trees were discarded as burn-in, and the remaining trees were used to construct a consensus tree and estimate posterior probabilities (PP).
Evaluation of species identification based on chloroplast genome
Genetic distance analysis for over two sequences per species followed the methodology outlined by Wang et al. [17]. When the minimum interspecific genetic distance exceeded the maximum intraspecific genetic distance and a barcoding gap was detected, successful species identification was considered achieved [51, 52]. To assess genetic variation within and among the suspicious species, pairwise K2P (Kimura 2-parameter) distances were calculated using MEGA X [53]. Scatter plots were generated to visualize the minimum interspecific K2P distance versus the maximum intraspecific K2P distance for each species, providing a clear illustration of whether the species were accurately defined.
Three molecular species assessment methods, one distance-based and two coalescent-based, were also employed for species delimitation by all available sequences in this study. First, the tree generated using the Bayesian Inference (BI) was converted to NEXUS format and applied to the Bayesian Poisson Tree Processes (bPTP) and Generalized Mixed Yule Coalescent (GMYC) models for automatic species identification using default parameters [32, 34]. Next, the Assemble Species by Automatic Partitioning (ASAP) approach was applied directly to the aligned complete chloroplast genomes for species delimitation with default parameters [33]. Combined with genetic distance analysis, a total of four results for species delimitation were obtained, and the following principles were used to conduct a comprehensive evaluation: (i) if more than or equal to half of the methods supported a particular result, it was considered as the initial assessment; (ii) if the initial assessment aligned with the accepted species classification, it was deemed a trustworthy result; otherwise, the species was considered suspicious. Finally, based on the species delimitation results from each method, this study aimed to evaluate the validity of the currently accepted species delimitations for Polygonatum s.l., considering whether species were overly subdivided or misidentified.
Identification of species-specific makers for genera and species of Polygonatum s.l
To establish contamination-free authentication of three medicinal significant Polygonatum species (P. cyrtonema, P. sibiricum and P. kingianum) against Heteropolygonatum adulterants, we engineered an automated screening framework that identifies taxon-specific diagnostic markers through comparative alignment analysis. The pipeline was designed to detect species-specific sites from aligned sequence files, which were categorized as candidate single nucleotide polymorphisms (SNPs) and insertion-deletion (InDel) regions. InDel molecular markers, known for their rich genetic information and cost-effectiveness, are a pivotal tool in species identification [54–56]. For subsequent InDel-specific primer development and validation [57, 58], the following criteria were applied to filter InDel regions: (i) Avoidance of repetitive regions: InDels must not reside within genomic repetitive elements; (ii) Multi-allelic nature: InDels should exhibit multiple allelic states to enhance discriminatory power; (iii) Stability across samples: InDels must exhibit consistent presence/absence patterns across all samples within both Polygonatum and its sister genera; (iv) Size threshold: InDel lengths must exceed 50 bp to ensure reliable amplification and detection. For regions that failed to meet the aforementioned criteria, this study prioritized genomic regions harboring overlapping diagnostic loci capable of unambiguously authenticating three medicinal Polygonatum. These candidate regions were subsequently validated using the ASAP molecular species delimitation framework.
Development and validation of species-specific sites as molecular barcodes
Primers were designed using Primer Premier v6.0 (http://www.premierbiosoft.com/), with the universal primer trnH-psbA (5’-ACTGCCTTGATCCACTTGGC-3’ and 5’-CGAAGCTCCATCTACAAATGG-3’) for Polygonatum included as a control [23, 55]. Although previous studies had validated the effectiveness of chloroplast genome extraction and assembly from the rhizomes of Polygonatum s.l. [17], fresh rhizomes were also used in this study for DNA extraction to ensure the universality of this method in practical applications. PCR amplification was performed following a specific protocol described by Cao et al. [59], and 2% agarose gel electrophoresis was conducted for three medicinal Polygonatum (P. cyrtonema, P. sibiricum, and P. kingianum) and three Heteropolygonatum (representing three main evolutionary clades respectively: H. alternicirrhosum, H. binatifolium, and Heteropolygonatum sp. Kangding). Each species was tested in triplicate to ensure the stability and reproducibility of the results.
Validated regions containing species-specific sites were also subsequently utilized for primer design, serving as species-specific barcodes for robust authentication of medicinal Polygonatum materials. PCR amplification and subsequent sequencing protocols were rigorously executed in accordance with the methodology outlined by Dong et al. [60].
Results
General characteristics of newly generated complete Chloroplast genomes within Polygonatum s.l
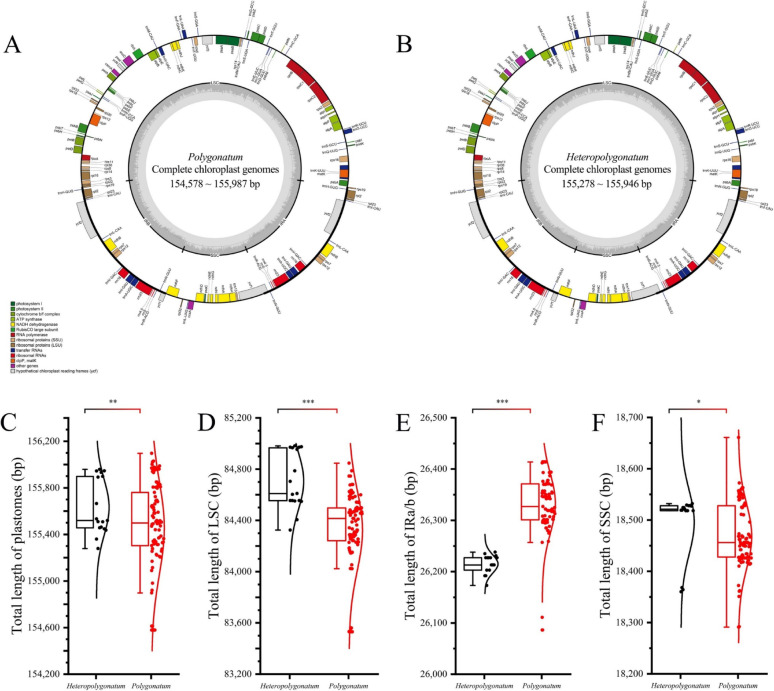
All 21 newly sequenced complete chloroplast genomes from 13 species of Polygonatum s.l. exhibited the typical quadripartite structure. Within the Polygonatum genus, genome lengths ranged from 154,578 bp (P. langyaense) to 155,987 bp (P. zanlanscianense), and within the Heteropolygonatum genus, they ranged from 155,278 bp (H. binatifolium) to 155,946 bp (H. alternicirrhosum; Table 2). The newly obtained lengths of the LSC region lengths in Polygonatum ranged from 83,530 to 84,676 bp, while those in Heteropolygonatum spanned from 84,324 to 84,974 bp. Similarly, the lengths of the IRa/b regions ranged from 26,295 to 26,414 bp in Polygonatum and 26,192 to 26,227 bp in Heteropolygonatum (Table 2). Significant intergeneric variation in the lengths of both the LSC and IR regions between the two genera. A t-test comparing all sequences in this study revealed significant differences (P < 0.01) in the lengths of the LSC and IR regions between Heteropolygonatum and Polygonatum. In contrast, no significant variation was observed in the SSC regions or other conserved regions of chloroplast genomes (P > 0.01; Fig. 2).
Table 2.
Genome information of the newly sequenced samples in this study
| Scientific name | GenBank number | The length of chloroplast genome (bp) | The length of IRa/b (bp) | The length of SSC (bp) | The length of LSC (bp) | GC content (%) | Genes/kb |
|---|---|---|---|---|---|---|---|
| H. alternicirrhosum | PQ591744 | 155,946 | 26,227 | 18,518 | 84,974 | 37.6 | 0.846 |
| H. binatifolium | PQ436974 | 155,456 | 26,192 | 18,518 | 84,554 | 37.6 | 0.849 |
| H. binatifolium | PQ436975 | 155,463 | 26,192 | 18,528 | 84,551 | 37.6 | 0.849 |
| H. binatifolium | PQ591745 | 155,359 | 26,213 | 18,528 | 84,405 | 37.6 | 0.850 |
| H. binatifolium | PQ591746 | 155,278 | 26,213 | 18,528 | 84,324 | 37.6 | 0.850 |
| Heteropolygonatum. sp. Kangding | PQ436973 | 155,519 | 26,227 | 18,518 | 84,547 | 37.6 | 0.849 |
| P. curvistylum | PV055066 | 155,965 | 26,413 | 18,551 | 84,588 | 37.7 | 0.846 |
| P. caulialatum | PV055067 | 155,335 | 26,302 | 18,464 | 84,267 | 37.7 | 0.850 |
| P. cyrtonema var. gutianshanicuym | PV055063 | 155,630 | 26,370 | 18,430 | 84,460 | 37.7 | 0.848 |
| P. cyrtonema var. gutianshanicuym | PV055053 | 155,635 | 26,370 | 18,432 | 84,463 | 37.7 | 0.848 |
| P. cyrtonema var. gutianshanicuym | PV055064 | 155,630 | 26,370 | 18,430 | 84,460 | 37.7 | 0.848 |
| P. franchetii | PV055065 | 155,931 | 26,353 | 18,549 | 84,676 | 37.7 | 0.847 |
| P. jinzhaiense | PV055061 | 155,515 | 26,378 | 18,292 | 84,467 | 37.7 | 0.849 |
| P. jinzhaiense | PV055062 | 155,520 | 26,378 | 18,292 | 84,472 | 37.7 | 0.849 |
| P. langyanese | PV055060 | 154,578 | 26,296 | 18,456 | 83,530 | 37.7 | 0.854 |
| P. macropodum | PV055059 | 154,613 | 26,295 | 18,463 | 83,560 | 37.7 | 0.854 |
| P. nodosum | PV055057 | 155,211 | 26,319 | 18,428 | 84,145 | 37.7 | 0.850 |
| P. nodosum | PV055058 | 155,205 | 26,319 | 18,421 | 84,146 | 37.7 | 0.850 |
| P. punctatum | PV055055 | 155,274 | 26,302 | 18,361 | 84,309 | 37.7 | 0.850 |
| P. punctatum | PV055056 | 155,329 | 26,302 | 18,362 | 84,363 | 37.7 | 0.850 |
| P. zanlanscianense | PV055054 | 155,987 | 26,414 | 18,661 | 84,498 | 37.6 | 0.846 |
Fig. 2.
Complete chloroplast genome map of Polygonatum (A) and Heteropolygonatum (B) and distribution of lengths and t-test results for total chloroplast genomes (C), large single-copy (D), inverted repeat (E), and small single-copy (F) regions of Polygonatum and Heteropolygonatum. Three asterisks indicate P < 0.001; Two asterisks indicate 0.01 > P > 0.001; one asterisk indicates P > 0.01
Comparative analysis of complete Chloroplast genomes
Each chloroplast genome contained 112 unique genes, including four rRNA genes, 30 tRNA genes, and 78 PCGs. Of these, 15 genes contained a single intron, while rps12, clpP1, and ycf3 each included two introns. The IR regions of each chloroplast genome contain 20 genes, consisting of eight PCGs, eight tRNA genes, and four rRNA genes. The average gene density was consistent across all chloroplast genomes, with approximately 0.85 genes per kb and GC content ranging from 37.6 to 37.7% (Table 2). Our comparative analysis of 57 chloroplast genome sequences revealed no structural variations within both Polygonatum and Heteropolygonatum (Fig. S1). The JLB (LSC/IRb) junction, typically located between the rps19 and rpl22 genes was conserved across most plastids in this study, except in P. sibiricum and P. sinopubescens, which contained 60 bp and 7 bp of rps19 pseudogenes at the JLB border, respectively (Fig. S2). In contrast, the JSB (IRb/SSC) junction generated ndhF pseudogenes ranging from 22 to 35 bp in most samples, with the exception of H. ogisui. The JSA (SSC/IRa) junction between ycf1 and trnN-GUU, and the JLA (IRa/LSC) junction between rps19 and trnK-UUU were found to be highly consistent across all analyzed sequences (Fig. S2).
Phylogenetic analysis
This study reconstructed a phylogenetic framework based on 17 Heteropolygonatum and 105 Polygonatum sequences, consisting of 57 distinct taxa. The phylogenetic tree topologies, derived from both 78 concatenated PCGs and complete chloroplast genome sequences, were nearly identical. Both Polygonatum (BS = 100, PP = 1.00) and Heteropolygonatum (BS = 100, PP = 1.00) formed well-supported monophyletic clades, sister to each other (Fig. 1B, S3). Additionally, the inferred phylogeny recovered three sections within Polygonatum, including sect. Verticillata, sect. Sibirica, and sect. Polygonatum, with each forming a well-supported monophyletic group (Fig. 1B, S3; BS = 100, PP = 1.00). The newly reported sequences P. cyrtonema var. gutianshanicuym shared close phylogenetic affinity with the medicinal plant P. cyrtonema (BS = 100, PP = 1.00), clustering with P. jinzhaiensis in a robust clade (BS = 100, PP = 1.00). In contrast, P. sibiricum, another medicinal species, was placed in sect. Sibirica as the only member of an independent lineage (Fig. 1B). Despite taxonomic debates continue regarding whether P. uncinatum and P. huanum should be treated as synonyms of P. kingianum, our analysis unambiguously resolved them within the P. kingianum clade (BS = 100, PP = 1.00) (Fig. 1B, S3). Heteropolygonatum sp. Kangding was resolved into a distinct lineage (Fig. 1B, S3). Furthermore, the analysis revealed that P. franchetii (PV055065) differed from previous studies, with these sequences being classified into sect. Verticillata and sect. Polygonatum, respectively (Fig. 1B, S3).
Molecular species delimitation
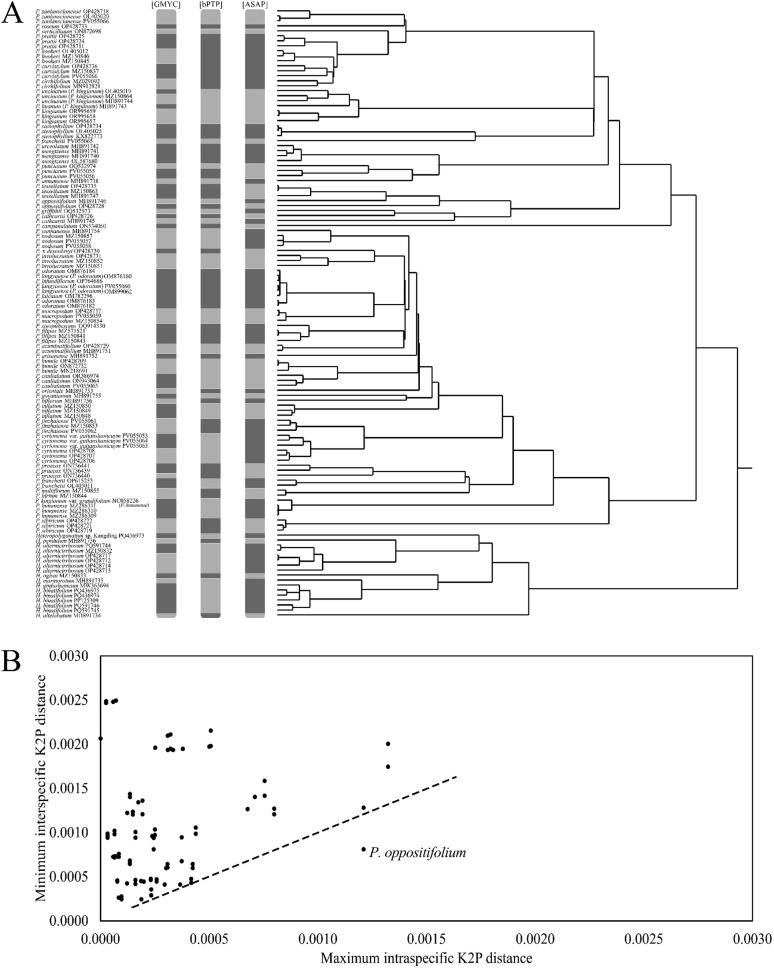
In this study, 57 accepted species of Polygonatum s.l. were analyzed, of which 30 species were represented by two or more sequences, with no issues in classifying the two genera (Polygonatum vs. Heteropolygonatum) and three groups (sect. Polygonatum, sect. Sibirica, and sect. Verticillata) across all methods. However, only 47 species were consistently supported by the species delimitation analyses, accounting for 82.46% of the total. Specifically, 64.91% of species were accepted by the ASAP method, 66.67% by PTP, 71.93% by GMYC, and 96.88% by genetic distance (Fig. 3; Table 3). All evaluation analyses failed to confirm independence in these eight Polygonatum species (P. falcatum, P. hirtum, P. infundiflorum, P. multiflorum, P. sinopubescens, P. urceolatum, P. oppositifolium, and H. ginfushanicum). Meanwhile, P. curvistylum, P. cathcartii, and H. marmoratum showed partial support across methods, but did not pass the final evaluation threshold for species recognition (Fig. 3; Table 3).
Fig. 3.
Results of molecular species assessment. The different blocks in these columns represent the results of the Bayesian Poisson Tree Processes (bPTP), Generalized Mixed Yule Coalescent (GMYC), and Assemble Species by Automatic Partitioning (ASAP), respectively; Genetic distance relationships obtained by the ASAP method are shown on the right (A). Scatter plots of maximum intraspecific Kimura 2-parameter (K2P) distance vs. minimum interspecific K2P distance for complete chloroplast genomes for over two sequences per species (B)
Table 3.
Results of multiple molecular species assessments
| Species | Sample size | GMYC | PTP | ASAP | Genetic distance | Assessment result |
|---|---|---|---|---|---|---|
| H. altelobatum | 1 | Yes | Yes | Yes | - | 100% |
| H. ginfushanicum | 1 | No | No | No | - | 0% |
| H. marmoratum | 1 | Yes | No | No | - | 33% |
| H. ogisui | 1 | Yes | Yes | Yes | - | 100% |
| H. pendulum | 1 | Yes | Yes | Yes | - | 100% |
| Heteropolygonatum sp. Kangding | 1 | Yes | Yes | Yes | - | 100% |
| P. annamense | 1 | Yes | Yes | Yes | - | 100% |
| P. arisanense | 1 | Yes | Yes | Yes | - | 100% |
| P. biflorum | 1 | Yes | Yes | Yes | - | 100% |
| P. campanulatum | 1 | Yes | Yes | Yes | - | 100% |
| P. falcatum | 1 | No | No | No | - | 0% |
| P. govanianum | 1 | Yes | Yes | Yes | - | 100% |
| P. griffithii | 1 | Yes | Yes | Yes | - | 100% |
| P. hirtum | 1 | No | No | No | - | 0% |
| P. huanum (P. kingianum) | 1 | No | Yes | Yes | - | 66% |
| P. infundiflorum | 1 | No | No | No | - | 0% |
| P. kingianum var. grandifolium (P. hunanense) | 1 | Yes | Yes | Yes | - | 100% |
| P. multiflorum | 1 | No | No | No | - | 0% |
| P. orientale | 1 | Yes | Yes | Yes | - | 100% |
| P. roseum | 1 | Yes | Yes | Yes | - | 100% |
| P. siNopubescens | 1 | No | No | No | - | 0% |
| P. urceolatum | 1 | No | No | No | - | 0% |
| P. verticillatum | 1 | Yes | Yes | Yes | - | 100% |
| P. x desoulavyi | 1 | Yes | Yes | No | - | 66% |
| P. yunnanense (P. Nodosum) | 1 | Yes | Yes | Yes | - | 100% |
| P. acuminatifolium | 2 | Yes | Yes | Yes | Yes | 100% |
| P. cathcartii | 2 | No | No | No | Yes | 25% |
| P. cirrhifolium | 2 | Yes | No | No | Yes | 50% |
| P. oppositifolium | 2 | No | No | No | No | 0% |
| P. caulialatum | 3 | Yes | No | No | Yes | 50% |
| P. curvistylum | 3 | Yes | No | No | Yes | 50% |
| P. cyrtonema | 3 | Yes | No | No | Yes | 50% |
| P. cyrtonema var. gutianshanicuym | 3 | Yes | No | No | Yes | 50% |
| P. filipes | 3 | Yes | Yes | Yes | Yes | 100% |
| P. franchetii | 3 | Yes | Yes | Yes | Yes | 100% |
| P. hookeri | 3 | Yes | No | No | Yes | 50% |
| P. humile | 3 | Yes | No | No | Yes | 50% |
| P. hunanense | 3 | Yes | Yes | Yes | Yes | 100% |
| P. inflatum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. involucratum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. jinzhaiense | 3 | Yes | Yes | No | Yes | 75% |
| P. kingianum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. langyanese (P. odoratum) | 3 | Yes | Yes | Yes | Yes | 100% |
| P. macropodum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. mengtzense (P. punctatum) | 3 | Yes | Yes | Yes | Yes | 100% |
| P. Nodosum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. odoratum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. praecox | 3 | No | Yes | Yes | Yes | 75% |
| P. prattii | 3 | Yes | No | No | Yes | 50% |
| P. punctatum | 3 | No | No | Yes | Yes | 50% |
| P. sibiricum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. steNophyllum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. tessellatum | 3 | Yes | Yes | Yes | Yes | 100% |
| P. uncinatum (P. kingianum) | 3 | No | Yes | Yes | Yes | 75% |
| P. zanlanscianense | 3 | Yes | Yes | Yes | Yes | 100% |
| H. binatifolium | 5 | Yes | Yes | Yes | Yes | 100% |
| H. alternicirrhosum | 6 | No | Yes | Yes | Yes | 75% |
| Total | 122 | 71.93% | 66.67% | 64.91% | 96.88% | 82.46% |
* The value of assessment result ≥ 50% were recognized as a confirmed species
Identification of species-specific makers for genera and species of Polygonatum s.l
This study identified a total of 563 species-specific sites from Heteropolygonatum, 288 from P. sibiricum, 259 from P. kingianum, and 18 from P. cyrtonema through a comparative genomic pipeline (Fig. 4A). Among these, three InDel regions meeting stringent criteria were exclusively identified in Heteropolygonatum. Two of these regions exhibited mirror-image symmetry, localized within the inverted repeat (IR) regions of the chloroplast genome, and the longest InDel (156 bp) from three regions was selected for subsequent primer design and validation (Fig. S4). To further distinguish these three traditional medicinal Polygonatum species from other species, four candidate regions containing species-specific sites for all three species were screened from the total 565 sites (Fig. 4B). Following the ASAP molecular species delimitation method, only region b (located in the rps11 gene) consistently demonstrated high discriminatory power across multiple replicates (Fig. 4C). This genomic region exhibits dual functionality: (i) It enables reliable differentiation between these three medicinal Polygonatum species and phylogenetically related taxa, and (ii) it provides species-specific diagnostic resolution for unambiguous identification of three individual medicinal Polygonatum species (P. sibiricum, P. kingianum, and P. cyrtonema).
Fig. 4.
Identification and validation of species-specific sites for three medicinal Polygonatum species. Distribution (A) and categories (B) of species-specific sites; Validation of ASAP molecular species for region b (C)
Development and validation of indels and species-specific primers
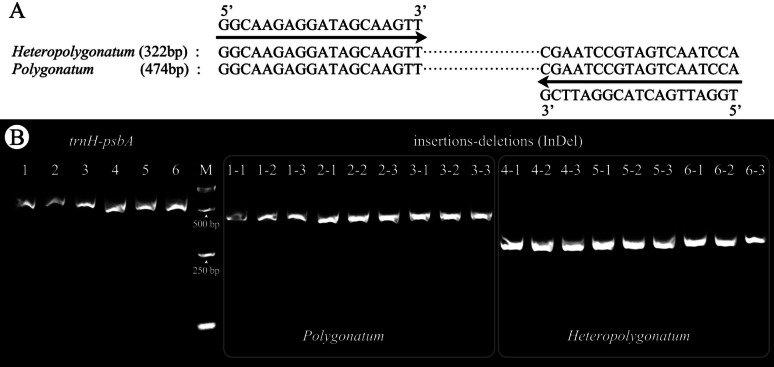
To obtain reliable molecular identification markers, InDel primer (5’-GGCAAGAGGATAGCAAGTT-3’ and 5’-TGGATTGACTACGGATTCG-3’) and SNPs primer (5’-TAATGCTGCGTCTCTTCC-3’ and 5’-TGTTACAGATGTACGAGGTC-3’) were designed separately for the longest InDel regions and region b. For InDel primer, PCR amplification consistently yielded strong bands, demonstrating the high applicability of the primers (Fig. 5, Fig. S4). Electrophoresis results revealed distinct amplification lengths for Polygonatum (ca. 474 bp) and Heteropolygonatum (ca. 322 bp), detecting potential contamination of Heteropolygonatum and simplifying the process with agarose gel electrophoresis. For the SNP-based primer targeting region b within the rps11 gene, the marker’s specificity and stability were validated across multiple experimental replicates (Fig. 6), further demonstrating its robustness for species-level identification.
Fig. 5.
InDel primer sequences and electrophoretograms for Polygonatum and Heteropolygonatum. InDel primer sequences, with left and right arrows indicating forward and reverse primers, respectively (A). Electrophoretogram of trnH-psbA and InDel sequence fragments in 6 species: P. cyrtonema (1), P. sibiricum (2), P. kingianum (3), H. alternicirrhosum (4), H. ginfushanicum (5), and H. binatifolium (6), each species was tested in triplicate (B)
Fig. 6.
Species-specific primers and validation results for three medicinal Polygonatum species. Species-specific primers sequence (A) and validation results (B)
Discussion
New insights into taxonomy assessments of Polygonatum s.l. Through Chloroplast genome
Chloroplast genomes have been extensively utilized for species delimitation and phylogenetic reconstruction due to their conserved structure and substantial sequence length [61–64]. In this study, 21 newly sequenced genomes exhibited genomic size, structural organization, and GC content comparable to those reported in previous monocotyledonous plant research, thereby reinforcing the conserved nature of chloroplast genomes across the Polygonatum s.l. at a broader taxonomic scale [65–69]. While earlier investigations primarily on infrageneric lineage classification within Polygonatum, few studies have systematically evaluated the efficacy of chloroplast genomes for species identification, especially among medicinally important taxa [2, 70, 71]. To address this gap, we compiled a dataset of 57 species by integrating public databases, representing the most comprehensive plastid-based phylogenetic analysis of Polygonatum s.l. to date (Fig. 1; Table S1). Notably, almost all species resolved into distinct, highly supported monophyletic clades (bootstrap values > 99%), validating the three morphological defined infrageneric lineages [2, 24].
Furthermore, multiple molecular species delimitation approaches (ASAP, bPTP, and GMYC) alongside genetic distance thresholds, which improved accuracy and reduced potential misclassification [17]. Three traditional medicinal Polygonatum species (P. sibiricum, P. cyrtonema, and P. kingianum) consistently formed distinct clades, even though preliminary analyses showed incongruence (Fig. 3). P. sibiricum maintained its distinct lineage, while P. cyrtonema var. gutianshanicuym, previously considered a variety of P. cyrtonema, was resolved as its closest relative, though both retained independent evolutionary lineages [17]. Interestingly, P. cyrtonema and its two phylogenetically proximate species (P. cyrtonema var. gutianshanicuym and P. jinzhaiense) have established three major geographical indication brands. Although no public reports exist of adverse medical events from species mixing, the potential variations in bioactive components—particularly polysaccharides and steroidal saponins—warrant further toxicopharmacological evaluation. Furthermore, integrative taxonomy approaches are recommended to resolve potential over-splitting in these complexes. Such as, the merger of P. kingianum, P. huanum and P. uncinatum was strongly supported in our assessments. These results underscore the power of using comprehensive assessments in resolving conflicts from single-method inferences, and provide a foundation for genetic resource development and conservation prioritization [1]. Furthermore, these findings also support the recent taxonomic revision of H. binatifolium as a new combination within Heteropolygonatum [21, 72]. The distinct phylogenetic position of, Heteropolygonatum sp. Kangding highlights a potentially novel lineage that merits further taxonomic investigation. Such discoveries underscore the value of molecular systematics in biodiversity studies and their application to medicinal resource management.
Novel markers for genera and species identification
Accurate species identification is critical for both conservation and clinical applications of medicinal plants [40]. In Polygonatum (including P. sibiricum, P. cyrtonema, and P. kingianum) — three widely used medicinal plants in traditional Chinese medicine — morphological characteristics serve as primary diagnostic markers in their natural state [1]. However, processing for herbal use often erases these traits, especially in tuberous materials. While chloroplast superbarcodes have have proven powerful for species delimitation, their practical implementation faces challenges such as experimental complexity, prolonged processing times, and high costs [17, 73, 74]. Historically, P. praecox —a recently described species phenotypically resembling P. cyrtonema—was indiscriminately harvested and utilized as the latter in herbal markets [27]. However, integrated evidence from plastid phylogenomics and morphological synapomorphies robustly supports the independent evolutionary status of P. praecox [27]. Given its small and restricted population, such misidentification could lead to pharmacovigilance issues and misguided conservation priorities, wasting limited resources.
Traditional barcoding focused on highly variable loci, which often fail in closely related, taxonomically complex genera such as Polygonatum [40, 71]. To address these limitations, we designed two new chloroplast-derived molecular markers: (i) InDel primers: genus-level markers for distinguishing Polygonatum from phylogenetically proximate genera (e.g., Heteropolygonatum), and (ii) species-specific primers: species-level markers targeting three medicinally significant Polygonatum species. Compared to prior approaches, our InDel primers allowed rapid genus-level identification (within 2 h) and detection of potential Heteropolygonatum contamination using simple gel electrophoresis. Additionally, species-specific primers yielded accurate identification of three medicinal Polygonatum species through a single round of Sanger sequencing [39, 70, 75].
Further molecular markers enable rapid screening of wild-collected material, combating illegal harvesting by verifying species identity in confiscated products—a key enforcement tool for protecting overexploited medicinal species. This strategy also offers a model for molecular identification in other medicinal genera, with clear advantages in clinical quality assurance and regulatory frameworks.
Conclusions
We generated 21 complete chloroplast genomes of Polygonatum s.l., expanding current genomic resources to cover over 50% of the taxonomically recognized species in the genus. Phylogenomic analysis resolved previously ambiguous taxa, confirming the distinct evolutionary status of P. cyrtonema var. gutianshanicum with strong support. The chloroplast genomes showed high discriminatory power, achieving 82.46% identification accuracy across Polygonatum s.l. using multiple molecular species delimitation methods. Based on these results, two molecular identification markers were developed: InDel primers targeting a 156-bp indel in the IR region that effectively differentiate Polygonatum from Heteropolygonatum through gel-based length polymorphism, and species-specific primers in the conserved rps11 gene, enabling simultaneous identification of three medicinal species (P. cyrtonema, P. kingianum, and P. sibiricum) via a single Sanger sequencing reaction. Furthermore, the findings support prior taxonomic revisions of P. kingianum var. grandifolium and H. binatifolium, and also suggest that Heteropolygonatum sp. Kangding represents a distinct lineage that warrants further investigation. Overall, this integrated approach combining chloroplast genome comparison and marker development provides a robust framework for species authentication and has significant implications for quality control in medicinal applications.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Y. Hu and J. Shao wrote the main manuscript text. S. Wang, Z. Xu, S. Yan, and M. Ali contributed to data validation, investigation, and formal analysis. Z. Li contributed to conceptualization, data curation, and formal analysis. M. Ali also participated in writing – review & editing. J. Shao was responsible for project administration, validation, supervision, and funding acquisition. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32070370 and 32470380).
Data availability
All newly annotated complete chloroplast genome sequences in this study are available from the Genbank with accession numbers: PV055053.to PV055067, PQ436973 to PQ436975 and PQ591744 to PQ591746.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhizhong Li, Email: lizhizhong@ahnu.edu.cn.
Jianwen Shao, Email: shaojw@ahnu.edu.cn.
References
- 1.Chen XQ, Tamura MN. Polygonatum. In: Wu ZY, Peter PH, editors. Flora of China. Beijing: Science; St. Louis: Missouri Botanical Garden Press;; 2000. pp. 223–32. [Google Scholar]
- 2.Floden AJ, Schilling EE. Using phylogenomics to reconstruct phylogenetic relationships within tribe polygonateae (Asparagaceae), with a special focus on Polygonatum. Mol Phylogenet Evol. 2018;129:202–13. [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey C. The genus Polygonatum (Liliaceae) in Eastern Asia. Kew Bull. 1980;34:435–71. [Google Scholar]
- 4.Jeffrey C. Further note on Eastern Asian Polygonatum (Liliaceae). Kew Bull. 1982;37:335–9. [Google Scholar]
- 5.Tamura MN, Ogisu M, Xu J. Heteropolygonatum, a new genus of the tribe polygonateae (Convallariaceae) from West China. Kew Bull. 1997;52:949–56. [Google Scholar]
- 6.Shan F, Huang LQ, Guo J, Chen M. History and development of one root of medicine and food. Chin Bull Life Sci. 2015;27:1061–9. [Google Scholar]
- 7.Shi Y, Liu JJ, Si D, Golding JB, Pristijono P, Li YX, He FL, Zhang XF, Han ZG, Wu LS, Chen DH, Si JP. Huangjing—From medicine to healthy food and diet. Food Front. 2023;4:1068–90. [Google Scholar]
- 8.Li XL, Ma RH, Ni ZJ, Thakur K, Cespedes-Acuna CL, Wang S, Zhang JG, Wei ZJ. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial–dependent signaling pathway. Food Chem Toxicol. 2021;148:111941. [DOI] [PubMed] [Google Scholar]
- 9.Shen WD, Li XY, Deng YY, Zha XQ, Pan LH, Li QM, Luo JP. Polygonatum Cyrtonema Hua polysaccharide exhibits anti–fatigue activity via regulating osteocalcin signaling. Int J Biol Macromol. 2021;175:235–41. [DOI] [PubMed] [Google Scholar]
- 10.He LL, Yan BX, Yao CY, Chen XY, Li LW, Wu YJ, Song ZJ, Song SS, Zhang ZF, Luo P. Oligosaccharides from Polygonatum Cyrtonema hua: structural characterization and treatment of LPS–induced peritonitis in mice. Carbohydr Polym. 2021;255:117392. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Yuan HY, Li WQ, Liu XY, Zhang XJ, Xiang DX, Luo SL. Polysaccharides protect against MPP–induced neurotoxicity via the akt/mtor and Nrf2 pathways. Oxid Med Cell Longev. 2021;8843899. [DOI] [PMC free article] [PubMed]
- 12.Si JP, Zhu YX. Polygonati rhizome–A new high–quality crop with great potential and not occupying farmland. Sci Sin Vitae. 2021;51:1477–84. [Google Scholar]
- 13.De Queiroz K. Species concepts and species delimitation. Syst Biol. 2007;56:879–86. [DOI] [PubMed] [Google Scholar]
- 14.Liu JQ. The integrative species concept and species on the speciation way. Biodiv Sci. 2016;24:1004–8. [Google Scholar]
- 15.Hong DY. Gen–morph species concept-A new and integrative species concept for outbreeding organisms. J Syst Evol. 2020;58:725–42. [Google Scholar]
- 16.Chen SL, Pang XH, Song JY, Shi LC, Yao H, Han JP, Leon C. A renaissance in herbal medicine identification: from morphology to DNA. Biotechnol Adv. 2014;32:1237–44. [DOI] [PubMed] [Google Scholar]
- 17.Wang SY, Zhou N, Shi NX, Zhang GF, Liu HY, Guo XR, Ji YH. Testing and using complete plastomes for authentication of medicinal Polygonatum species (Asparagaceae). Ind Crops Prod. 2023;197:116557. [Google Scholar]
- 18.Tamura MN, Chen X, Turland NJ. A new combination in heteropolygonatum (Convallariaceae, Polygonateae). Novon. 2000;2:156–7. [Google Scholar]
- 19.Floden AJ. A new combination in Polygonatum (Asparagaceae) and the reinstatement of P. mengtzense. Ann Botanici Fennici. 2014;51:106–16. [Google Scholar]
- 20.Floden AJ. New names in Heteropolygonatum. (Asparagaceae) Phytotaxa. 2014;188:218–26. [Google Scholar]
- 21.Zhu HX, Huang FY, Cheng HY, Zhang HJ, Dai QL, Yi SR. Polygonatum binatifolium, a new species of Polygonatum (Asparagaceae) from guizhou, China. Phytotaxa. 2022;549:247–52. [Google Scholar]
- 22.Zhou YL, Jiang H, Hu GW, Wang QF. Heteropolygonatum binatifolium, a new combination in Heteropolygonatum (Asparagaceae, Polygonateae). Phytotaxa 2025;682:274–280.
- 23.Zhao LH, Zhou SD, He XJ. A phylogenetic study of Chinese Polygonatum (Polygonateae, Asparagaceae). Nord J Bot. 2019;e02019.
- 24.Meng Y, Nie ZL, Deng T, Wen J, Yang YP. Phylogenetics and evolution of phyllotaxy in the solomon’s seal genus Polygonatum (Asparagaceae: Polygonateae). Bot J Linn Soc. 2014;176:435–51. [Google Scholar]
- 25.Qin YQ, Zhang MH, Yang CY, Nie ZL, Wen J, Meng Y. Phylogenomics and divergence pattern of Polygonatum (Asparagaceae: Polygonateae) in the North temperate region. Mol Phylogenet Evol. 2024;190:107962. [DOI] [PubMed] [Google Scholar]
- 26.Xia M, Liu Y, Liu J, Chen D, Shi Y, Chen Z, Chen D, Jin R, Chen H, Zhu S. Out of the Himalaya–Hengduan mountains: phylogenomics, biogeography and diversification of Polygonatum mill. (Asparagaceae) in the Northern hemisphere. Mol Phylogenet Evol. 2022;169:107431. [DOI] [PubMed] [Google Scholar]
- 27.Hu YF, Liu YJ, Ali M, Wu W, Li XH, Cheng LS, Shao JW. Polygonatum praecox (Asparagaceae), a new species from mid–eastern China revealed by morphological and molecular evidence. PhytoKeys. 2022;211:125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Ren J, Jiang H, Wanga VO, Dong X, Hu G. Comparative and phylogenetic analysis of the complete Chloroplast genomes of six Polygonatum species (Asparagaceae). Sci Rep. 2023;13:7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo XR, Shi NX, Xie PX, Zhang GF, Liu HY, Ji YH. Plastome sequencing for accurate and effective authentication of Polygonatum Kingianum (Asparagaceae). Ind Crops Prod. 2022;184:115056. [Google Scholar]
- 30.Yan MX, Dong SJ, Gong QY, Xu Q, Ge YQ. Comparative Chloroplast genome analysis of four Polygonatum species insights into DNA barcoding, evolution, and phylogeny. Sci Rep. 2023;13:16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo AR, Ling C, Ho SYW, Zhu CD. Comparison of methods for molecular species delimitation across a range of speciation scenarios. Syst Biol. 2018;67:830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisawa T, Barraclough TG. Delimiting species using single–locus data and the generalized mixed Yule coalescent approach: a revised method and evaluation on simulated data sets. Syst Biol. 2013;62:707–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puillandre N, Brouillet S, Achaz G. ASAP: assemble species by automatic partitioning. Mol Ecol Resour. 2021;21:609–20. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong W, Liu Y, Li E, Xu C, Sun J, Li W, Zhou S, Zhang Z, Suo Z. Phylogenomics and biogeography of Catalpa (Bignoniaceae) reveal incomplete lineage sorting and three dispersal events. Mol Phylogenet Evol. 2022;166:107330. [DOI] [PubMed] [Google Scholar]
- 36.Bennett EP, Petersen BL, Johansen IE, Niu Y, Yang Z, Chamberlain CA, Met O, Wandall HH, Frodin M. INDEL detection, the ‘achilles heel’ of precise genome editing: a survey of methods for accurate profiling of gene editing induced indels. Nucleic Acids Res. 2020;48:11958–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo JH, Dhungana SK, Kang BK, Baek IY, Sung JS, Ko JY, Jung CS, Kim KS, Jun TH. Development and validation of SNP and indel markers for Pod–Shattering tolerance in soybean. Int J Mol Sci. 2022;23:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivaprasad KM, Aski M, Mishra GP, Sinha SK, Gupta S, Mishra DC, Singh AK, Singh A, Tripathi K, Kumar RR, Kumar A, Kumar S, Dikshit HK. Genome–wide discovery of indels and validation of PCR-Based indel markers for earliness in a RIL population and genotypes of lentil (Lens culinaris Medik). PLoS ONE. 2024;19:e0302870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Choi H, Shin J, Jo A, Lee KE, Cho SS, Hwang YP, Choi C. Molecular discrimination of Cynanchum wilfordii and Cynanchum auriculatum by indel markers of Chloroplast DNA. Molecules. 2018;23:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Wang YH, Sun JH, Cui XY, Li E, Wang RS, Li Q, Zhang PF, Dong WP, Guo LP, Huang LQ. Comparative Chloroplast genome analyses provide new insights into molecular markers for distinguishing arnebiae radix and its substitutes (tribe lithospermeae, Boraginaceae). Phytomedicine. 2025;136:156338. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de Novo assembly of organelle genomes. Genome Biol. 2020;21:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greiner S, Lehwark P, Bock R. Organellar genome DRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019;47:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darling AE, Mau B, Perna NT. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Guo Q, Xu L, Gao H, Liu L, Zhou X. CPJSdraw: analysis and visualization of junction sites of Chloroplast genomes. PeerJ. 2023;11:e15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2020;20:348–55. [DOI] [PubMed] [Google Scholar]
- 49.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post–analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBaYes 3.2: efficient baYesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins R, Cruickshank R. The seven deadly sins of DNA barcoding. Mol Ecol Resour. 2013;13:969–75. [DOI] [PubMed] [Google Scholar]
- 52.Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc Biol Sci. 2003;270:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv Y, Liu Y, Zhao H. mInDel: a high-throughput and efficient pipeline for genome–wide indel marker development. BMC Genomics. 2016;17:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton MB. Four primer pairs for the amplification of Chloroplast non-coding regions with intraspecific variation. Mol Ecol. 1999;8:521–3. [PubMed] [Google Scholar]
- 56.Zhao LH, Zhou SD, He XJ. A phylogenetic study of Chinese Polygonatum (Polygonateae, Asparagaceae). Nord J Bot. 2019;2019:e02019. [Google Scholar]
- 57.Jain A, Roorkiwal M, Kale S, Garg V, Yadala R, Varshney RK. InDel markers: an extended marker resource for molecular breeding in Chickpea. PLoS ONE. 2019;14:e0213999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu XY, Xu P, Wu XH, Wang BG, Lu ZF, Li GJ. Development of insertion and deletion markers for bottle gourd based on restriction Site-associated DNA sequencing data. Hortic Plant J. 2017;3:13–6. [Google Scholar]
- 59.Cao DL, Zhang XJ, Xie SQ, Fan SJ, Qu XJ. Application of Chloroplast genome in the identification of traditional Chinese medicine viola Philippica. BMC Genomics. 2022;23:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong W, Liu H, Xu C, Zuo Y, Chen Z, Zhou S. A Chloroplast genomic strategy for designing taxon specific DNA mini–barcodes: a case study on ginsengs. BMC Genet. 2014;15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed I, Bigg PJ, Matthews PJ, Collins LJ, Hendy MD, Lockhart PJ. Mutational dynamics of Aroid Chloroplast genomes. Genom Biol Evol. 2012;4:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henriquez CL, Abdullah, Ahmed I, Carlsen MM, McKain MR. Evolutionary dynamics of Chloroplast genomes in subfamily Aroideae (Araceae). Genomics. 2020;112:2349–60. [DOI] [PubMed] [Google Scholar]
- 63.Shahzadi I, Abdullah, Mehmood F, Ali Z, Ahmed I, Mirza B. Chloroplast genome sequences of Artemisia maritima and Artemisia absinthium: comparative analyses, mutational hotspots in genus Artemisia and phylogeny in family Asteraceae. Genomics. 2020;112:1454–63. [DOI] [PubMed] [Google Scholar]
- 64.Yu X, Zuo L, Lu D, Lu B, Yang M, Wang J. Comparative analysis of Chloroplast genomes of five Robinia species: genome comparative and evolution analysis. Gene. 2019;689:141–51. [DOI] [PubMed] [Google Scholar]
- 65.Cui G, Wang C, Wei X, Wang H, Wang X, Zhu X, Li J, Yang H, Duan H. Complete Chloroplast genome of Hordeum brevisubulatum: genome organization, synonymous codon usage, phylogenetic relationships, and comparative structure analysis. PLoS ONE. 2021;16:e0261196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freudenberg J, Wang M, Yang Y, Li W. Partial correlation analysis indicates causal relationships between GC–content, exon density and recombination rate in the human genome. BMC Bioinformatics. 2009;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang H, Zhang Y, Deng J, Gao G, Ding C, Zhang L, Yang R. The complete Chloroplast genome sequences of 14 Curcuma species: insights into genome evolution and phylogenetic relationships within zingiberales. Front Genet. 2020;11:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin RC, Ferreira BT, Yuan YW. The molecular basis of phenotypic evolution: beyond the usual suspects. Trends Genet. 2024;40:668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Kasif S, Cantor CR, Broude NE. GC/AT-content spikes as genomic Punctuation marks. Proc Natl Acad Sci U S A. 2004;101:16855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Mauleon R, Hu Z, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018;557:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Qian J, Jiang Y, Chen X, Zheng B, Chen S, Yang F, Xu Z, Duan B. Comparative analysis of Chloroplast genome and new insights into phylogenetic relationships of Polygonatum and tribe polygonateae. Front Plant Sci. 2022;13:882189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou YL, Jiang H, Hu GW, Wang QF. Heteropolygonatum binatifolium, a new combination in Heteropolygonatum (Asparagaceae, Polygonateae). Phytotaxa. 2025;682:274–80. [Google Scholar]
- 73.Hollingsworth PM, Li DZ, Michelle VDB, Twyford AD. Telling plant species apart with DNA: from barcodes to genomes. Philos Trans R Soc B Biol Sci. 2016;371:20150338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kane NS, Sveinsson S, Dempewolf H, Yang JY, Zhang D, Engels JMM, Cronk Q. Ultra-barcoding in Cacao (Theobroma spp.; Malvaceae) using whole Chloroplast genomes and nuclear ribosomal DNA. Am J Bot. 2012;99:320–9. [DOI] [PubMed] [Google Scholar]
- 75.Tian X, Guo J, Zhou X, Ma K, Ma Y, Shi T, Shi Y. Comparative and evolutionary analyses on the complete plastomes of five Kalanchoe horticultural plants. Front Plant Sci. 2021;12:705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All newly annotated complete chloroplast genome sequences in this study are available from the Genbank with accession numbers: PV055053.to PV055067, PQ436973 to PQ436975 and PQ591744 to PQ591746.