Abstract
We report the identification and molecular characterization of Pex19p, an oleic acid-inducible, farnesylated protein of 39.7 kDa that is essential for peroxisome biogenesis in Saccharomyces cerevisiae. Cells lacking Pex19p are characterized by the absence of morphologically detectable peroxisomes and mislocalization of peroxisomal matrix proteins to the cytosol. The human HK33 gene product was identified as the putative human ortholog of Pex19p. Evidence is provided that farnesylation of Pex19p takes place at the cysteine of the C-terminal CKQQ amino acid sequence. Farnesylation of Pex19p was shown to be essential for the proper function of the protein in peroxisome biogenesis. Pex19p was shown to interact with Pex3p in vivo, and this interaction required farnesylation of Pex19p.
Eukaryotic cells have evolved an elaborate mechanism for the biogenesis of peroxisomes which includes targeting and import of proteins to the peroxisomal matrix, formation of the peroxisomal membrane, proliferation, and mitotic inheritance of the organelles (16, 47, 59, 70).
Peroxisomal matrix proteins are synthesized on free ribosomes and imported posttranslationally into preexisting peroxisomes (47). Different types of peroxisomal targeting signals (PTS) can direct proteins from the cytosol to the peroxisomal matrix. PTS1, which comprises the C-terminal 3 amino acids of the majority of peroxisomal matrix proteins, consists of species-specific and protein context-dependent variations of the tripeptide consensus Ser-Lys-Leu (34; for a review, see reference 54). PTS2 is used by a smaller subset of peroxisomal matrix proteins, and it consists of a conserved nonapeptide typically localized at the N terminus of a protein (57, 72; for a review, see reference 15). The different PTS are recognized by distinct import receptors (9, 52, 60, 80; for a review, see reference 59) which have been suggested to target proteins from the cytosol to putative docking sites at the peroxisomal membrane and then might shuttle back to the cytosol (17, 51). However, the functional role of the import receptors is still controversially discussed in the field (73, 79; for a review, see reference 59). In line with the idea that the import receptors shuttle between the cytosol and peroxisomes, putative binding proteins for the PTS receptors have been identified at the peroxisomal membrane (1, 19, 26, 35). Recent evidence suggests that both the PTS1- and PTS2-dependent import pathways for matrix proteins converge at the peroxisomal membrane (1). How translocation of matrix proteins proceeds from there on is not yet known, but, interestingly, since peroxisomes have been shown to import proteins in a folded state (32, 37, 53, 75; for a review, see reference 54), the mechanism might be different from that used to import proteins into the endoplasmic reticulum or mitochondria.
The formation of the peroxisomal membrane also requires an elaborate targeting and insertion of proteins. It is well documented that a subset of peroxisomal membrane proteins is synthesized on free polysomes and imported posttranslationally into peroxisomes (for a review, see reference 47), but several lines of evidence suggest that this pathway is different from the PTS1- and PTS2-dependent import routes (for a review, see reference 27). For instance, in cells lacking components of the common translocation complex for matrix proteins, posttranslational targeting and insertion of peroxisomal membrane proteins are still functional (1, 19, 26, 35). Moreover, a PTS for peroxisomal membrane proteins which is entirely different from PTS1 and PTS2 has recently been identified (18). The constituents of the posttranslational import pathway for peroxisomal membrane proteins are still unknown.
Yeast mutants have been valuable tools in the identification of proteins involved in the biogenesis of peroxisomes, the so-called peroxins (for reviews, see references 16, 20, 23, and 46). For most of the 15 peroxins identified to date, the role played in peroxisome biogenesis remains to be elucidated. Five of the peroxins have been shown to fulfill a function in PTS1- and PTS2-dependent protein import (for a review, see reference 27). So far, only one peroxin, Pex3p, has been suggested to be required for the biogenesis of the peroxisomal membrane (3, 77).
Here we report on the isolation and phenotypic characterization of a pex19 mutant. Mutant cells exhibit characteristic defects in peroxisome biogenesis, including the absence of normal peroxisomes and mislocalization of peroxisomal matrix enzymes to the cytosol. We describe the cloning and sequencing of the PEX19 gene and the identification and characterization of the PEX19 gene product. Pex19p is a newly identified protein essential for peroxisome biogenesis. We show that Pex19p is farnesylated in vivo, and we provide evidence that the protein physically interacts with Pex3p, a peroxin localized at the peroxisomal membrane (39).
MATERIALS AND METHODS
Strains, media, and general methods.
The yeast strains used in this study are shown in Table 1. The complete (YPD) and minimal (SD) yeast media used have been described earlier (22). Oleic acid medium (YNO) contained 0.1% oleic acid, 0.05% Tween 40, 0.1% yeast extract, and 0.67% yeast nitrogen base without amino acids, adjusted to pH 6.0. For oleic acid induction, cells were precultured in SD containing 0.3% dextrose to mid-log phase, shifted to YNO medium, and incubated for 13 to 15 h. When necessary, auxotrophic requirements were added as described previously (2).
TABLE 1.
Yeast strains used in this study
| Yeast strain | Genotype | Source or reference |
|---|---|---|
| UTL-7A | MATa ura3-52 trp1 leu2-3/112 | W. Duntze (Bochum, Germany) |
| JKR101 | MATα ura3-52 leu2-3/112 ade2 his4 | G. Schatz (Basel, Switzerland) |
| pex19-1 | MATa ura3-52 leu2-3/112 ade2-1 pex19 | This study |
| pex19Δ | MATa ura3-52 trp1 pex19::LEU2 | This study |
| ram1 | MATα ram1-1 cyrRleu2-3/112 try0trp1 his4aSUP4-3 | W. Duntze (Bochum, Germany) |
| PCY2 | MATα gal4Δ gal80Δ URA3::GAL1-lacZ lys2-80amberhis3-200Δ trp1-63Δ leu2 ade2-101ochre | P. M. Chevray (Baltimore, Md.) |
Recombinant DNA techniques were performed essentially as described previously (2, 49).
Isolation of the pex19 mutant.
The pex19 mutant was obtained after mutagenesis of UTL-7A cells with ethyl methanesulfonate (67). The screening protocol included replica plating on YNO agar plates, fractionation of yeast cells, and morphological characterization as described previously (22). Genetic analysis was performed by standard yeast techniques (2).
Cloning and characterization of the PEX19 gene.
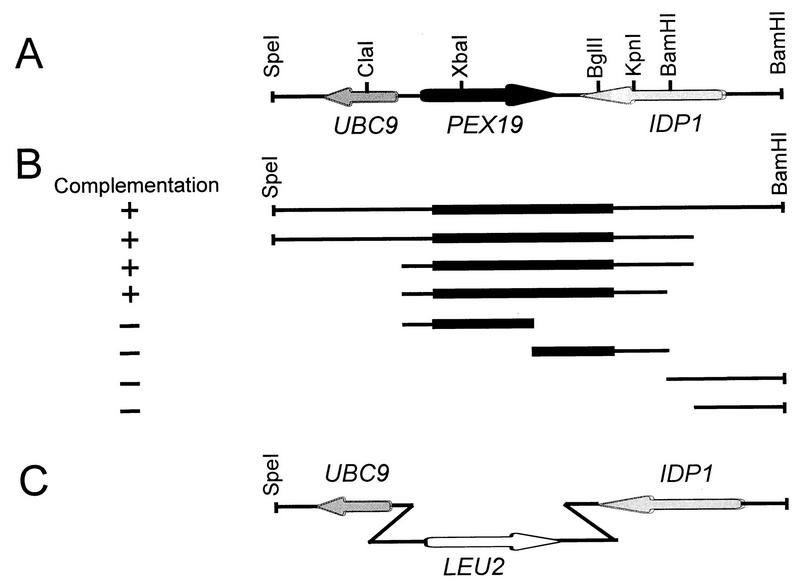
PEX19 was cloned by functional complementation of the pex19 mutant with a genomic library of Saccharomyces cerevisiae contained in the Escherichia coli-yeast shuttle vector YCp50 (61). Transformation was carried out by a modified lithium acetate method (30). Transformants were screened on YNO agar plates for their ability to utilize oleic acid as the sole carbon source. The complementing region of the plasmid isolated, YCpPEX19 (insert, 13.5 kb), was narrowed down to a 1.8-kb ClaI-KpnI fragment which contained the entire PEX19 gene. The 1.8-kb ClaI-KpnI fragment was subcloned into the CEN plasmid pRS316 (68), resulting in pRSPEX19. For overexpression of PEX19, the 1.8-kb ClaI-KpnI fragment was cloned into the episomal plasmid YEp352 (38), resulting in YEpPEX19.
DNA sequencing.
For DNA sequencing, the complementing 1.8-kb ClaI-KpnI fragment was subcloned into the pBluescript (KS+) vector (Stratagene), resulting in pKS-PEX19. Defined restriction fragments and deletion fragments generated with exonuclease III were subcloned into pBluescript (KS+), and nucleotide sequence analysis of both strands was performed by the dideoxy sequencing method (65). Computer analysis of DNA and amino acid sequences was performed with the GENPRO program (Riverside Scientific Enterprises, Seattle, Wash.).
Gene replacement.
The 0.182-kb ClaI-DraI fragment of the 5′ noncoding region was cloned into ClaI- and SmaI-digested pBluescript (SK+), resulting in pSKG20. The 2-kb XbaI-SacI fragment of pJJ283 (42) that contained the LEU2 gene was introduced into pSKG20, leading to pSKG21. In a third step, the 0.431-kb BglII-SacI fragment consisting of sequences flanking the 3′ end of PEX19 was inserted into BamHI- and SacI-digested pSKG21, resulting in pSKGD12, which was linearized with SacI and transformed into the wild-type strain UTL-7A. The resulting leucine-prototrophic transformant pex19Δ was crossed with wild-type strain JKR101. The resulting diploid was induced to sporulate, and the meiotic progenies were examined by standard tetrad analysis. The deletion was confirmed by Southern blot analysis.
Construction and expression of Pex19p-HK33 chimers.
Four chimeric genes expressing fusion proteins consisting of different parts of the yeast Pex19p protein and the human HK33 gene product were created by splice overlap extension PCR (40). The fusion primers which were used and the parts of the yeast and human proteins in the resulting chimeras are summarized in Table 2. The outside primers were KU82 (5′ PEX19; 5′CGCGGATCCTCCCGGGATGCCAAACATACAACAC3′), KU74 (3′ PEX19; 5′CCATCGATACTAGTACTTTATTGTTGTTTGCAACC3′), KU73 (5′ HK33; 5′CGCGGATCCTCCCGGGATGGCCGCCGCTGAGGAAGGC3′), and KU75 (3′ HK33; 5′CCATCGATACTAGTACTCATGATCAGACACTGTTC3′). Fragments were amplified with Pwo DNA polymerase (Boehringer, Mannheim, Germany) in a reaction mixture containing 10 ng of template plasmid and 100 pmol each of outside and corresponding fusion primers according to the manufacturer’s protocol. Templates were either pRSPEX19 or pYADE4-HK33, which was kindly provided by A. Roscher (Munich, Germany). Equimolar amounts of the resulting fragments were mixed and subjected to a second PCR with corresponding 5′ and 3′ outside primers. The resulting fusion genes were subcloned into EcoRV-digested pBluescript (SK+). The fusions were confirmed by DNA sequencing and subcloned into the yeast shuttle vector pYPGE15 (10), using the primer-derived BamHI and SalI restriction sites. The nonfused fragment encoding amino acids 1 to 231 of S. cerevisiae Pex19p was amplified by PCR with primers KU82 and KU79, subcloned into pBluescript (SK+), and subsequently cloned into pYPGE15, using the primer-derived BamHI site and the SalI site of the pBluescript (SK+) polylinker. Chimeras were tested for their ability to restore the mutant defects of pex19Δ cells.
TABLE 2.
Chimeras of human HK33p and yeast Pex19p
| Chimera name | Oligonucleotides used for PCR fusion | Amino acids from:
|
|
|---|---|---|---|
| S. cerevisiae Pex19p | HK33p | ||
| YH1 | KU70, 5′AAATGACGTCAAAAGAAGTGCTGTACCCATCACT3′ (sense); KU79, 5′AGTGATGGGTACAGCACTTCTTTTGACGTCATTT3′ (antisense) | 1–231 | 187–299 |
| YH2 | KU134, 5′TAGATTTGCAAAATGGATTCGAGAAGGCAATGAA3′ (sense); KU135, 5′TTCATTGCCTTCTCGAACTCATTTTGCAAATCTA3′ (antisense) | 1–86 | 87–299 |
| YH3 | KU136, 5′ACATTGTTTCCAATACGCTAAGTGGATTAGCCAA3′ (sense); KU137, 5′TTGGCTAATCCACTTAGTGTATTGGAAACAATGT3′ (antisense) | 1–154 | 133–299 |
| HY1 | KU71, 5′ACCTACTCTCCAAGGATGTATTATATGAGCCTAT3′ (sense); KU80, 5′ATAGGCTCATATAATACATCCTTGGAGAGTAGGT3′ (antisense) | 232–350 | 1–186 |
Fractionation of yeast homogenates.
Preparation and fractionation of yeast homogenates by differential centrifugation were performed as described previously (22). For subfractionation by isopycnic sucrose density centrifugation, homogenates or resuspended 25,000 × g organellar pellets were loaded onto continuous 20 to 53% or 32 to 54% (wt/wt) sucrose density gradients (24-ml volume). Centrifugation, fractionation of the gradient, and preparation of samples for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were carried out as described previously (39).
Pex19p antibodies.
The protein fusion and purification system of New England BioLabs (Beverly, Mass.) was used for overexpression of a maltose binding protein-Pex19p fusion protein in E. coli TB1 [araΔ (lac proAB) rpsL(φ80d lacZΔM15) hsd]. A 0.330-kb SmaI-XbaI fragment of the exonuclease III-derived plasmid p9-65 (see below) encoding amino acids 4 to 112 of Pex19p was introduced into StuI- and XbaI-digested vector pMal-p. The expression was induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and cold osmotic shock of the periplasmatic fraction and affinity purification of the maltose binding protein-Pex19p fusion protein on amylose resin were performed according to the manufacturer’s protocol. Polyclonal antibodies were raised against the fusion protein (Eurogentec, Seraing, Belgium). Antisera were affinity purified against a 156-kDa Pex19p–β-galactosidase fusion protein which was expressed from pURPEX19, a pUR291 (64) derivative containing the 1.5-kb SmaI-HindIII fragment of plasmid p9-65 (see below) that encodes amino acids 4 to 350 of Pex19p. Affinity purification of antisera was performed as described earlier (39).
Immunoblot analysis.
Immunoblot analysis was performed according to standard protocols (36) with alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG) or horseradish peroxidase-conjugated anti-rabbit IgG as the secondary antibody. Protein-antibody complexes were visualized by treatment with color or chemiluminescence developing reagents (ECL System; Amersham, Braunschweig, Germany). Synthetic peptides were used for immunization of rabbits to generate polyclonal antibodies against peroxisomal Pex11p (25, 50) (Eurogentec, Seraing, Belgium). The polyclonal antibodies against Pex19p or Pex3p (39) were affinity purified prior to Western blot analysis.
In vitro isoprenylation assay.
Mutant pex19Δ strains overexpressing either Pex19p (pex19Δ[YEpPEX19]) or Pex19p-C347S (pex19Δ[YEpPEX19-C347S]), in which the cysteine at position 347 has been replaced by serine, were grown in YNO for 12 h. Cells were harvested, divided into 500-mg portions, and frozen at −20°C, thereby inactivating the endogenous protein prenyltransferase activity (data not shown). For the preparation of cell extracts containing active prenyltransferase, cells were grown in YPD to mid-log phase and were always used immediately to prevent the loss of enzyme activity. Cells were disrupted in 500 μl of a solution consisting of 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 0.5 mM EGTA, and 0.5 mM dithiothreitol plus proteinase inhibitors (1 mM phenylmethylsulfonyl fluoride; leupeptin, pepstatin, and chymostatin, each at 1 μg/ml) by vortexing with glass beads (0.4-mm diameter). Insoluble material was removed by centrifugation in a microcentrifuge for 10 min at 4°C.
Isoprenylation assay conditions were essentially as described previously (12). A 50-μl reaction volume contained 50 mM Tris-HCl (pH 8.0), 20 mM KCl, 5 mM MgCl2, 10 μM ZnCl2, 10 mM dithiothreitol, S. cerevisiae extract (100 μg) from thawed cells containing the target protein, 70 μg of S. cerevisiae extract from fresh cells that served as the source of protein prenyltransferase, and 2 μM [3H]farnesylpyrophosphate (triammonium salt; 16.5 Ci/mmol; Amersham). Reaction mixtures were incubated for 1 h at 37°C, and reactions were terminated by addition of SDS-PAGE sample buffer. Aliquots of the samples were analyzed by SDS-PAGE. Gels were fixed in 7% acetic acid–20% methanol for 30 min, washed twice with water, and immersed in Amplify fluorographic reagent (Amersham) for 30 min prior to fluorography.
Mutagenesis of Pex19p.
Mutagenesis was performed by PCR with pKS-PEX19 as the template. The sense primer was 5′AATACGACTCACTATAG3′; antisense primers were KG1 (5′TATATAAGCTTTCCCGGGAGATCTTCAACCGTCGGTTAATTC3′) for the deletion of the four carboxy-terminal amino acids, KG2-1 (5′TATATAAGCTTTCCCGGGAGATCTTTATTGTTGTTTGCGACCGTCGGTTAATTC3′) for the replacement of Cys347 with Arg347, and KG2-2 (5′TATATAAGCTTTCCCGGGAGATCTTTATTGTTGTTTGCTACCGTCGGTTAATTC3′) for the Cys347-to-Ser347 change. PCR fragments were subcloned into pBluescript (SK+), and regions to be subcloned further were confirmed by DNA sequence analysis. Taking advantage of the internal AccI site of PEX19 and the primer-derived HindIII site, the last 0.240 kb of the original PEX19 open reading frame was replaced with the corresponding regions of the PCR-derived fragments. This was done by ligation of AccI- and HindIII-restricted PCR fragments to the SacII-AccI fragment of pSK-PEX19 and subsequent subcloning into SacII- and HindIII-digested pBluescript (SK+). The CYC1 terminator (69) of pRSterm (21) was subcloned into YEp352 (38), resulting in YEpterm. The CYC1 terminator of YEpterm was subcloned behind the mutated PEX19 genes, taking advantage of the vector-derived SalI and KpnI sites. The PEX19 gene-CYC1 terminator constructs were subcloned into SacII- and KpnI-digested pRS316 (68). For overexpression, the constructs were subcloned into SacI- and KpnI-digested YEp352 (38). The resulting plasmids pRSPEX19-C347S, pRSPEX19-C347R, pRSPEX19-C347*, YEpPEX19-C347S, YEpPEX19-C347R, and YEpPEX19-C347* encoded Pex19p proteins with the indicated mutations of the CAAX box (with an asterisk indicating the deletion of the entire CAAX box), and expression was under the control of the PEX19 promoter.
Immunofluorescence, electron, and immunoelectron microscopy.
Immunofluorescence microscopy was performed essentially according to the procedure of Rout and Kilmartin (63) with modifications as previously described (21). Rabbit antisera against yeast thiolase (24) and yeast Pcs60p (7) were used at dilutions of 1:3,000; monoclonal 12CA5 antiserum against the hemagglutinin tag (BAbCO, Richmond, Calif.) was used at a dilution of 1:20. For detection, 6-μg/ml solutions of CY3-conjugated donkey anti-mouse IgG (cross-absorbed against rabbit IgG) and fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.) were used.
For electron microscopy, washed cells were fixed with 1.5% KMnO4 for 20 min at room temperature. After dehydration in a graded ethanol series, the samples were embedded in Epon 812, and ultrathin sections were cut with a diamond knife and examined. Immunogold labeling was performed as described previously (5).
Two-hybrid methodology.
The open reading frame of PEX19 was amplified by PCR with sense primer KU34 (5′CCATCGATAAGATCTCCAGTACTATGCCAAACATACAACAC3′), antisense primer KU35 (5′GGAATTCGAAGCTTATTGTTGTTTGCAACC3′), and pKS-PEX19 as the template. By taking advantage of the primer-derived ClaI site and the internal XbaI site of the resulting PCR amplification product, the fragment encoding the N-terminal region of Pex19p was subcloned into the pBluescript (SK+) vector, and its presence was confirmed by DNA sequence analysis. The fragment was excised with KpnI and XbaI and ligated to an XbaI-BamHI fragment containing the 3′ complement of PEX19. The ligation product was first subcloned into KpnI- and BamHI-digested pBluescript (SK+) vector, excised with BglII and SacII, and subcloned into pPC86. The resulting plasmid, pPCPEX19, contained an in-frame fusion of PEX19 and the activation domain encoding part of GAL4 (13). For introduction of the mutation of the CAAX box, the XbaI-SacII fragment of pPCPEX19 was replaced by the corresponding region of PEX19 encoding the C-terminal region of Pex19p with the Cys347-to-Ser347 change. The resulting plasmid was designated pPCPEX19-C347S. The constructs encoding fusions of peroxins with the DNA-binding domain in pPC97 have been described previously (1, 26, 43).
Cotransformation of two-hybrid vectors into strain PCY2 (13) was performed as described previously (60). Transformed yeast cells were plated on SD synthetic medium lacking tryptophan and leucine. The β-galactosidase filter assay has been described earlier (60).
Epitope tagging of Pex19p.
The clone p9-65 was derived by exonuclease III treatment of pKS-PEX19 and contained bp 10 to 1053 of the PEX19 open reading frame followed by 450 bp of the 3′ noncoding region of the gene. DNA sequencing revealed that the fourth codon of the PEX19 open reading frame was adjacent to the SmaI site of the multiple cloning site of the pBluescript vector (5′GGATCCCCCGGGATACAACACGAA3′). The vector-derived BamHI-KpnI sites were used to subclone the PEX19-containing fragment of clone p9-65 into BglII- and KpnI-digested SK/mycP7 (51), resulting in SK/myc19. The resulting plasmid contained the CUP1 promoter (11) in front of an in-frame fusion of the myc epitope-encoding sequence with codon 4 of the PEX19 open reading frame. For expression in S. cerevisiae, the expression cassette containing the promoter and the fusion gene was excised using vector-derived SacI and KpnI sites and subcloned into the yeast episomal plasmid YEp352 (38), resulting in YEp-mycPEX19. The fusion gene encoded N-terminally myc-tagged Pex19p under the control of the CUP1 promoter. Expression restored the peroxisome biogenesis and oleic acid growth defects of pex19Δ cells, suggesting that the tagged Pex19p is functional.
Coimmunoprecipitation.
Yeast cells expressing myc-tagged Pex19p were grown on 0.3% SD medium to late log phase and, subsequently, for 15 h in YNOG (0.1% glucose, 0.1% oleic acid, 0.05% Tween 40, 0.1% yeast extract, and 0.67% yeast nitrogen base). The CUP1 promoter was induced with 0.025-g/liter CuSO4 as described previously (51). Cells were stored at −70°C, and 1 g of them was used per immunoprecipitation experiment. A 3-ml aliquot of solution A (50 mM Tris-HCl [pH 7.5], 50 mM NaCl), protease inhibitors (0.5 mM NaF, 0.02% phenylmethylsulfonyl fluoride [Serva]), 15 μg of bestatin per ml, 1.5 μg of pepstatin per ml, 1 μg of leupeptin per ml, 0.1 μg of chymostatin per ml [all from Boehringer]), and 3 g of glass beads (0.5-mm diameter) were added, and the mixture was vortexed on ice eight times for 30 s each with at least 30 s between vortexings (45). Samples were filtered through cotton wool, and the filtrate was transferred to Corex tubes and centrifuged at 1,000 × g for 30 min. The resulting supernatant was centrifuged again at 100,000 × g for 30 min. The pellet was resuspended in 3 ml of solution B (solution A with 0.4% [wt/vol] Triton X-100), incubated on ice for 10 min, and centrifuged as described above. Supernatants were normalized for protein and volume and incubated with 50 μl of sheep anti-mouse IgG Dynabeads (Dynal, Hamburg, Germany) covered with monoclonal anti-myc IgG (serum 9E10) (28) for 2 h at 4°C. The Dynabeads were washed three times with 1 ml of solution B, and Dynabead-bound proteins were eluted with 60 μl of SDS-PAGE sample buffer. For the decoration of Dynabeads with anti-myc antibodies, 50 μl of Dynabeads was blocked with 5% bovine serum albumin in phosphate-buffered saline for 2 hours, washed five times with 10 volumes of phosphate-buffered saline, and saturated with the anti-myc antiserum at 4°C overnight. The supernatant was removed, and the beads were washed five times with 1 ml of solution B and then resuspended in 50 μl of solution B.
Analytical procedures.
Acetyl-coenzyme A (acetyl-CoA) acyltransferase (3-oxoacyl-CoA thiolase; EC 2.3.1.16), catalase (EC 1.11.1.6), and fumarate hydratase (fumarase; EC 4.2.1.2) were assayed by established procedures (55). Protein concentrations were determined by using the bicinchoninic acid protein assay reagent (Pierce Chemical Co.) with bovine serum albumin as a standard.
Nucleotide sequence accession number.
The nucleotide sequence of the PEX19 gene has been submitted to the EMBL-GenBank-DDBJ database and has been assigned accession no. Z74113.
RESULTS
Isolation of the pex19 mutant and cloning of the PEX19 gene.
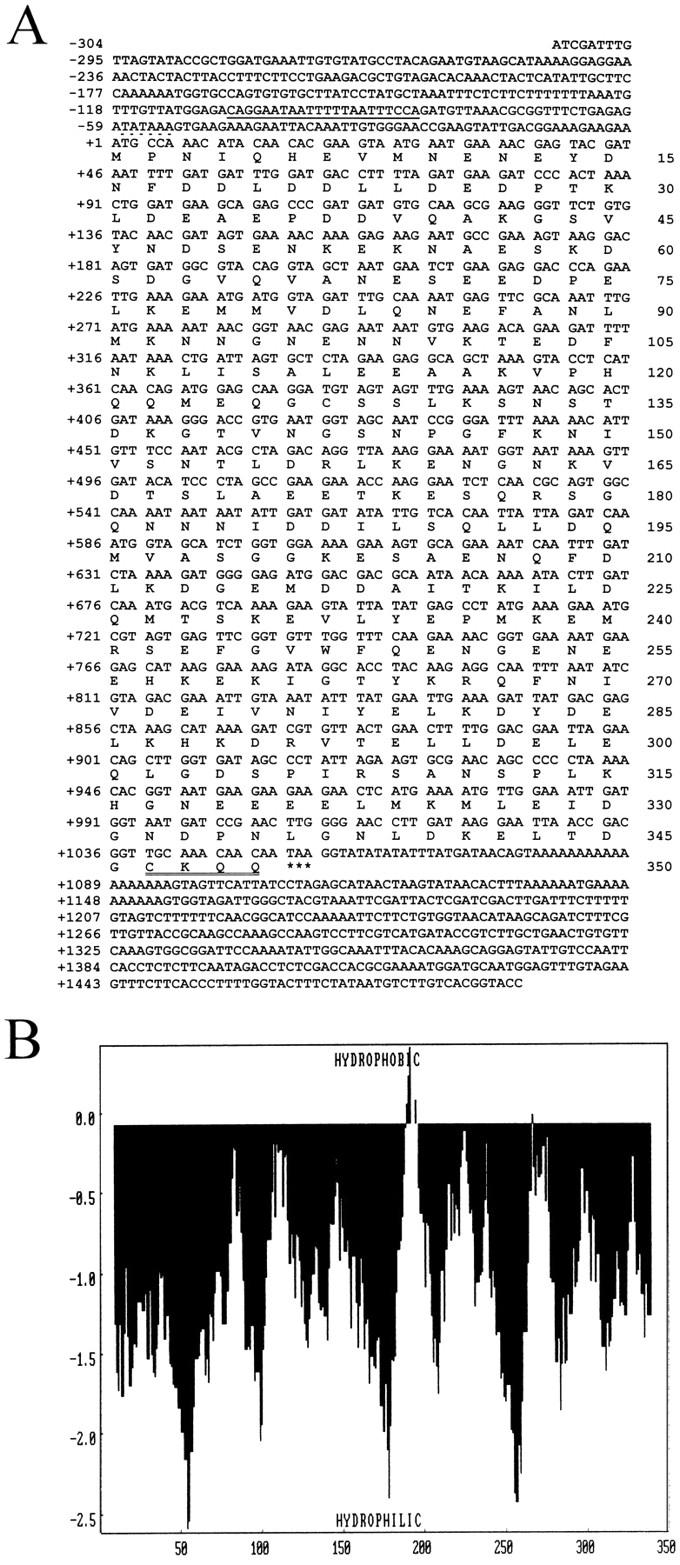
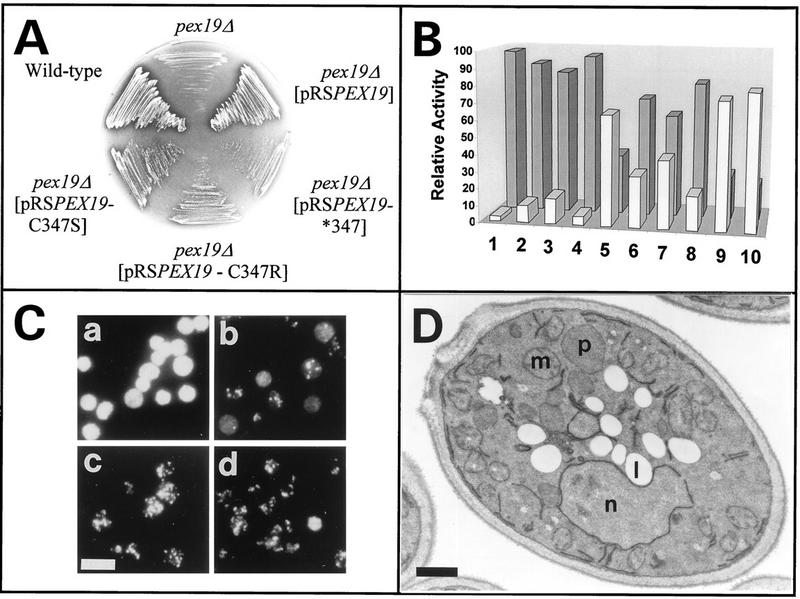
The pex19-1 mutant strain was identified by its inability to grow on oleic acid as the sole carbon source and by mislocalization of peroxisomal matrix enzymes to the cytosol, characteristic of a defect in peroxisome biogenesis (see below). The meiotic segregation behavior revealed that the defect was caused by a single gene. The diploids resulting from backcrossing the mutant strain to wild-type cells did not show the mutant phenotype, confirming the pex19-1 mutation to be recessive. The corresponding PEX19 wild-type gene was cloned by functional complementation of the pex19-1 mutant with a genomic library (Fig. 1). Nucleotide sequencing of the smallest complementing insert (a 1.793-kb ClaI-KpnI fragment) revealed an open reading frame of 1.050 kb encoding a protein with a calculated molecular mass of 39.7 kDa (Fig. 2A). Transformation of PEX19 resulted in functional complementation of the mutant phenotype of pex19-1, demonstrating that the authentic PEX19 gene had been cloned. More recently, this gene has also been sequenced as part of the S. cerevisiae genome sequencing project (7a). Hydropathy analysis of the deduced amino acid sequence of PEX19 (Fig. 2B) revealed the extremely hydrophilic nature of Pex19p, with no region fulfilling the requirements for a membrane spanning segment (44). Most interestingly, at the extreme C terminus is the tetrapeptide CKQQ, which resembles the consensus sequence for the so-called CAAX motif, the recognition sequence for protein farnesyltransferases (56).
FIG. 1.
(A) Location of the PEX19 gene within the 4.8-kb genomic SpeI-BamHI fragment of chromosome IV. The arrows denote the orientations of PEX19 and adjacent genes. (B) Complementation analysis of the PEX19 genomic region. Subclones of the 4.8-kb SpeI-BamHI genomic fragment are shown along with their ability to restore growth of the pex19 mutant on oleic acid. −, complementing activity was not shown; +, full complementing activity was shown. The location of the PEX19 open reading frame within each subclone is indicated by the black bars. The smallest complementing region identified was the 1.793-kb ClaI-KpnI fragment, which was subjected to nucleotide sequence analysis. (C) Targeted gene disruption strategy for replacement of PEX19 with LEU2.
FIG. 2.
(A) Nucleotide sequence of the PEX19 gene and deduced amino acid sequence of Pex19p. The potential oleic acid response element of the PEX19 promoter is underlined, and the putative TATA box is above the broken line. The CAAX box for farnesylation is double underlined. Asterisks indicate the stop codon. These sequence data are available from EMBL-GenBank-DDBJ under accession no. Z74113. (B) Hydropathy analysis of Pex19p. A hydropathy profile of the predicted amino acid sequence of Pex19p was calculated (44) with a window size of 17 amino acids. The analysis showed that the PEX19 gene product is extremely hydrophilic, with no apparent hydrophobic domains with the potential to span a membrane. X, axis, amino acids; y axis, hydrophobicity values.
A search of protein databases revealed a significant overall amino acid sequence similarity between Pex19p and a number of proteins from different organisms, including PxF, a prenylated peroxisomal protein from the Chinese hamster (41). The proteins aligned in Fig. 3 are characterized by a C-terminal CAAX box, suggesting that they all might be farnesylated. The overall sequence similarity and the presence of the putative farnesylation sites opened the possibility that these proteins might represent orthologs of Pex19p. For the human housekeeping gene HK33 (8), this assumption was further supported by functional studies in S. cerevisiae (see below).
FIG. 3.
Alignment of deduced amino acid sequences of the products of S. cerevisiae PEX19 (ScPex19p), Caenorhabditis elegans open reading frame F54F2.8 (CeF54F2.8), Chinese hamster PxF (CgPxF) (41), and the human HK33 gene (HsHK33) (8). Amino acids identical or similar in S. cerevisiae Pex19p and at least one of the three other proteins are indicated by a black background. Similarity rules were as follows: G = A = S, A = V, V = I = L = M, I = L = M = F = Y = W, K = R = H, D = E = Q = N, and S = T = Q = N. Dashes indicate gaps.
Cells lacking PEX19 are affected in peroxisome biogenesis.
A PEX19 deletion mutant (pex19Δ) was generated by replacing the entire PEX19 open reading frame with LEU2 as shown in Fig. 1C. Backcrosses of pex19Δ with the original pex19-1 mutant resulted in diploids exhibiting the pex phenotype, indicating that the cloned PEX19 gene is allelic to the original mutation and not a suppressor.
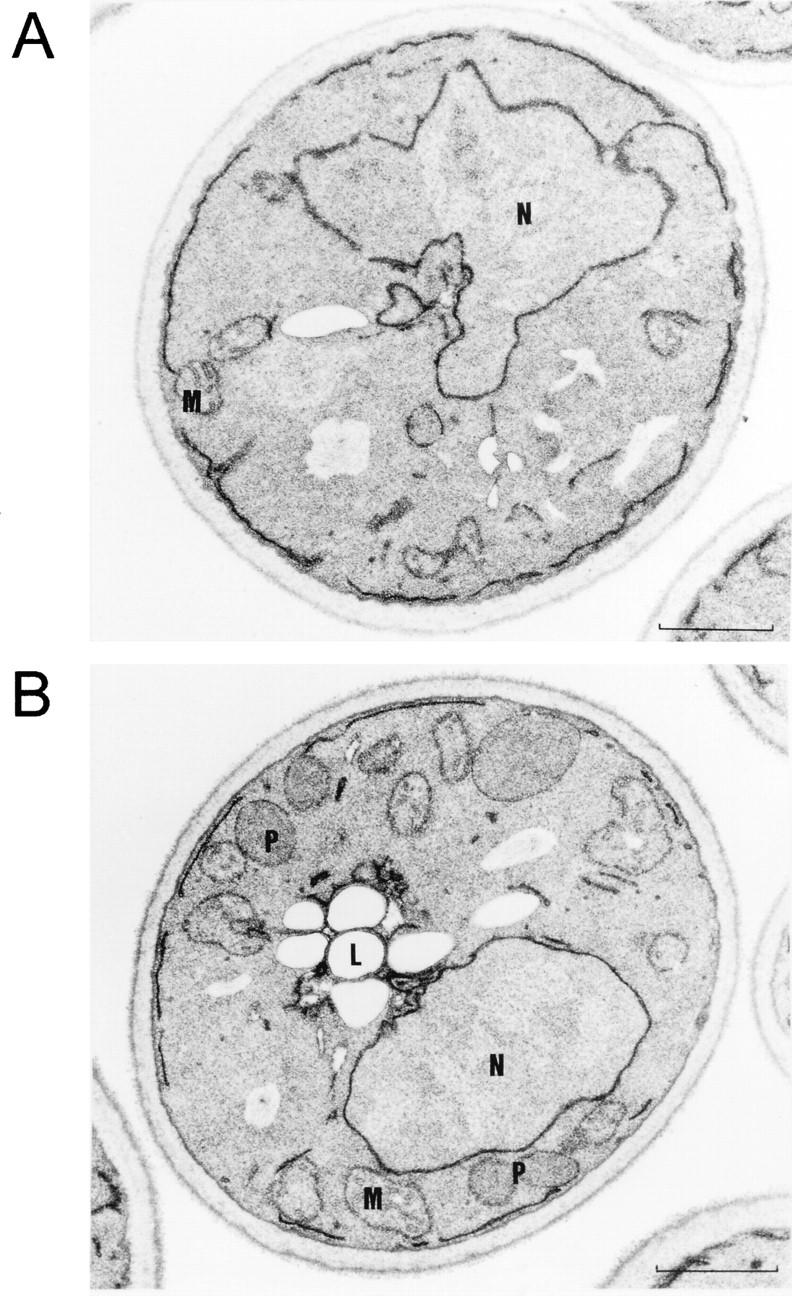
In S. cerevisiae, growth on oleic acid requires functional peroxisomes and is usually accompanied by a massive increase in the size and number of these organelles (74). Cells deficient in PEX19 were viable on YPD, SD, and ethanol media but were unable to grow on media with oleic acid as the sole carbon source (see below), typical for S. cerevisiae mutant strains that are defective in proteins essential for either peroxisome metabolism or biogenesis (oleic acid-nonutilizing [onu] phenotype [22, 23]). The assumption that Pex19p is more likely involved in peroxisome biogenesis than in peroxisome metabolism is supported by the ultrastructural appearance of the oleic acid-induced pex19Δ mutant strain, which is characterized by the absence of morphologically recognizable peroxisomes (Fig. 4A). Peroxisomes were restored upon transformation with the PEX19 gene (Fig. 4B). The involvement of Pex19p in peroxisome biogenesis is further supported by the evidence of mislocalization of peroxisomal matrix proteins to the cytosol which is observed by immunofluorescence microscopy (Fig. 5A). Wild-type cells exhibited a peroxisome-characteristic punctate pattern when stained for the PTS1 protein Pcs60p (7) or the PTS2 protein thiolase (21, 32). In contrast, a diffuse staining pattern for both peroxisomal matrix proteins was observed in pex19Δ cells, indicating their mislocalization to the cytosol. A punctate staining pattern for both proteins was restored in mutant cells expressing PEX19. These data suggest that pex19Δ cells exhibit a defect in import of peroxisomal matrix proteins of the PTS1 as well as the PTS2 variety.
FIG. 4.
Mutant pex19Δ cells lack morphologically detectable peroxisomes. Shown are electron micrographs of oleic acid-induced cells of null-mutant pex19Δ lacking Pex19p (A) and pex19Δ cells complemented with the isolated PEX19 gene on a single-copy plasmid (pRS-PEX19) (B). In the case of the complemented mutant, growth on oleic acid resulted in marked peroxisome proliferation. Peroxisomes were not detectable in sections of cells of the pex19Δ mutant. L, lipid droplet; M, mitochondrion; N, nucleus; P, peroxisome. Bar, 1 μm.
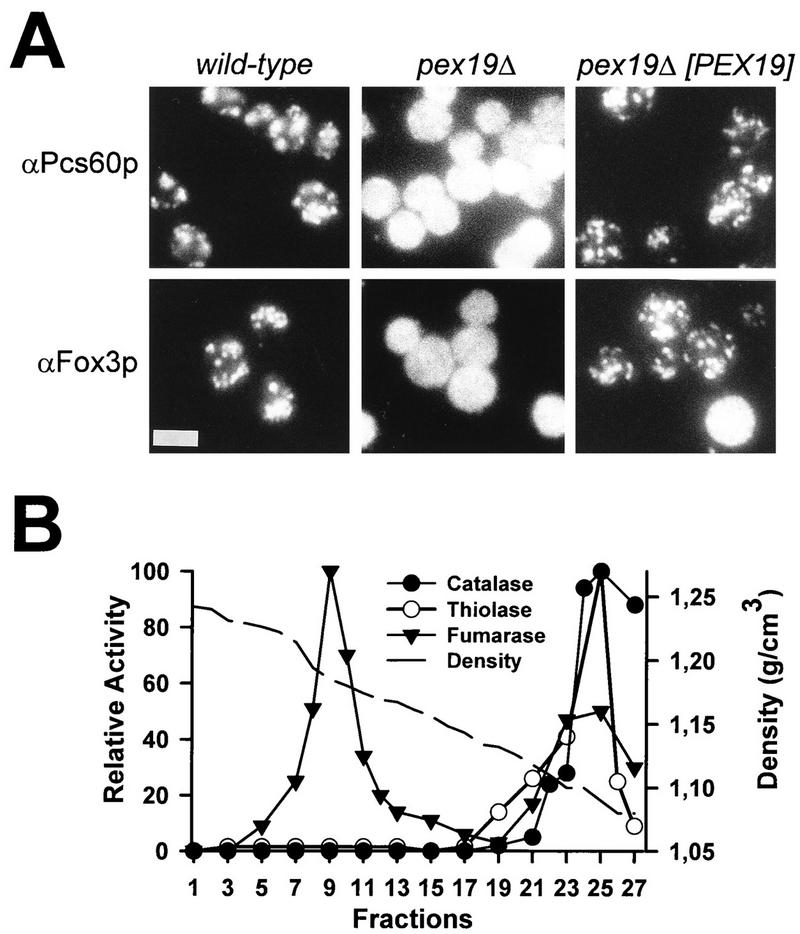
FIG. 5.
Mutant pex19Δ cells are defective in peroxisomal matrix protein import. (A) Immunofluorescence microscopy localization of PTS2-containing thiolase (Fox3p) and PTS1-containing Pcs60p in wild-type, pex19Δ mutant, and complemented pex19Δ mutant cells expressing pRS-PEX19. Bar, 5 μm. (B) Localization of peroxisomal matrix proteins in pex19Δ cells. A homogenate of oleic acid-induced pex19Δ cells was separated on a 20 to 54% (wt/wt) sucrose gradient by equilibrium density centrifugation. Peroxisomal marker enzymes catalase and 3-oxoacyl-CoA thiolase as well as the mitochondrial marker fumarase in gradient fractions were monitored by activity measurements. Mitochondria peaked in fraction 9 at a density of 1,185 g/cm3. Peroxisomal matrix enzymes were nearly exclusively found in the loading zone of the gradient, consistent with their mislocalization to the cytosol.
To quantify the import defect, the subcellular distributions of the peroxisomal matrix enzymes catalase and thiolase (Fox3p) as well as that of the mitochondrial fumarase were determined by cell fractionation analysis of both wild-type and pex19Δ cells. Organelles of oleic acid-induced cells were separated by differential centrifugation, and peroxisomal and mitochondrial marker enzyme activities of the sediment and supernatant fractions were determined (Table 3). The mitochondrial fumarase activity served as a control for the quantification of organelle breakage during homogenization. In wild-type cells, the majority of the peroxisomal and mitochondrial enzymes were detected in the organellar pellet. However, in pex19Δ cells, the peroxisomal matrix proteins were predominately found in the soluble fraction, consistent with the immunofluorescence microscopy data that suggested their mislocalization to the cytosol. This observation was substantiated by isopycnic sucrose density centrifugation of pex19Δ cell homogenates and subsequent detection of peroxisomal and mitochondrial marker enzymes in gradient fractions (Fig. 5B). Based on the localization of fumarase, mitochondria peaked at a density of 1.18 g/cm3. The peroxisomal marker enzymes catalase and thiolase were almost exclusively found in the loading zone of the gradient, indicating that they had been mislocalized to the cytosol. Neither catalase nor thiolase activity was detected in fractions with a density of 1.23 g/cm3, the typical density for peroxisomes. Taken together, the data on the subcellular localization of peroxisomal matrix proteins suggest that pex19Δ mutant cells exhibit a defect in import of PTS1- and PTS2-containing peroxisomal matrix proteins.
TABLE 3.
Distribution pattern of peroxisomal and mitochondrial marker enzymes in supernatant and pellet fractions from a 25,000 × g centrifugation of homogenates of oleic acid-induced wild-type, pex19Δ, and complemented pex19Δ cells
| Strain | Enzyme | Activity (nkat) in:
|
A1/A2 ratio | |
|---|---|---|---|---|
| Supernatant fraction (A1) | Pellet fraction (A2) | |||
| Wild type | Thiolase | 10.0 | 29.6 | 0.3 |
| Catalase | 15.4 × 103 | 65.5 × 103 | 0.2 | |
| Fumarase | 1.4 | 11.1 | 0.1 | |
| pex19Δ | Thiolase | 17.8 | 0.9 | 20.0 |
| Catalase | 97.1 × 103 | 2.7 × 103 | 39.0 | |
| Fumarase | 8.0 | 13.5 | 0.6 | |
| pex19Δ(pRSPEX19) | Thiolase | 25.6 | 34.1 | 0.7 |
| Catalase | 35.8 × 103 | 76 × 103 | 0.3 | |
| Fumarase | 2.9 | 8.9 | 0.3 | |
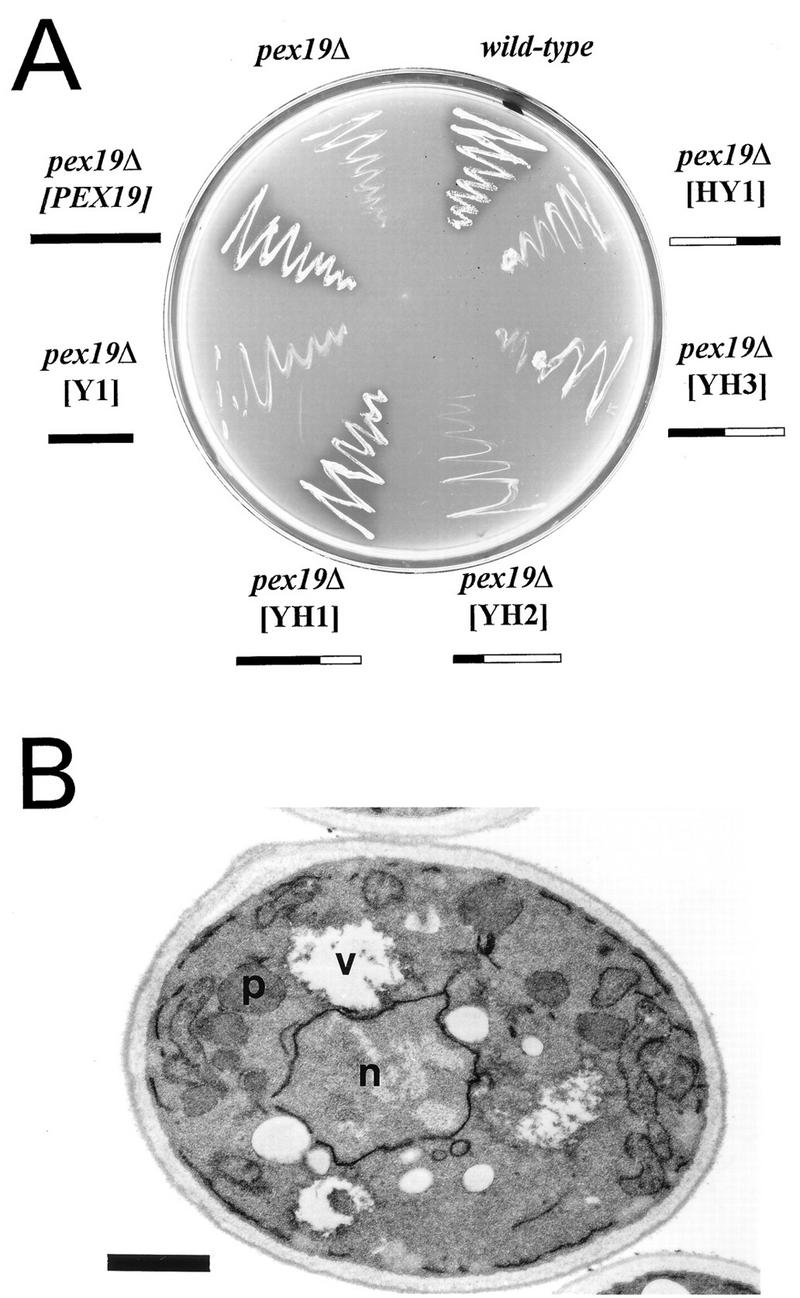
Functional analysis of chimeras of yeast Pex19p and the human HK33 gene product.
Based on sequence similarity, the HK33 gene product (8) was a candidate human ortholog of the yeast Pex19p (Fig. 3). To substantiate this assumption, the HK33 gene product was tested for its ability to functionally replace S. cerevisiae Pex19p in peroxisome biogenesis. Expression of the human protein in mutant pex19Δ did not complement the growth defect on oleic acid medium, suggesting that the yeast and human proteins might not be interchangeable (data not shown). However, growth of the mutant on oleic acid was restored to normal upon expression of the chimeric protein YH1 (Fig. 6A), containing amino acids 1 to 231 of Pex19p and amino acids 187 to 299 of the HK33 gene product, which correspond to amino acids 232 to 350 of the yeast protein (Fig. 3). Morphological characterization of the pex19Δ mutant expressing the chimeric YH1 protein revealed the presence of normal-looking peroxisomes (Fig. 6B). These observations suggested that the chimeric YH1 protein was functionally active. Since the yeast portion of the fusion protein alone (Y1) did not possess complementing activity (Fig. 6A), these data indicate that the corresponding C-terminal regions of the yeast and human proteins might be interchangeable. Chimeric proteins containing larger portions of the HK33 gene product (YH2, YH3, and HY1) did not retain complementing activity. The outcome of the complementation studies supports the notion of the HK33 gene product being the human ortholog of S. cerevisiae Pex19p.
FIG. 6.
The HK33 gene product is the putative human ortholog of Pex19p. (A) Growth of pex19Δ transformants expressing S. cerevisiae PEX19-human HK33 chimeras on oleic acid medium. Expression of the YH1 construct complements the growth defect of pex19Δ cells, suggesting that the fusion protein is functionally active. The yeast and human protein amino acid (aa) regions fused are as follows: YH1 (yeast aa 1 to 231, human aa 187 to 299), YH2 (yeast aa 1 to 86, human aa 87 to 299), YH3 (yeast aa 1 to 154, human aa 133 to 299), HY1 (human aa 1 to 186, yeast aa 232 to 350), and Y1 (yeast aa 1 to 231). The solid boxes indicate the S. cerevisiae Pex19p portion; the open boxes indicate the human HK33 gene product portion. (B) Electron micrograph of oleic acid-induced pex19Δ cells expressing fusion construct YH1. Complementation of the mutant strain is indicated by the presence of peroxisomes. p, peroxisome; n, nucleus, v, vacuole. Bar, 1 μm.
Time course of oleic acid induction.
Polyclonal antibodies raised against bacterially expressed Pex19p were used to detect Pex19p in whole-cell lysates from wild-type S. cerevisiae and the corresponding pex19Δ null mutant as shown in Fig. 7A. Two polypeptides of 44 and 46 kDa were detected in wild-type extracts, but not in the extracts from the pex19Δ deletion strain, suggesting that the antiserum specifically recognized Pex19p. Since a putative farnesylation site has been predicted by the primary sequence of Pex19p, the simplest explanation for the appearance of the double band is the simultaneous intracellular presence of prenylated and nonprenylated Pex19p (see below).
FIG. 7.
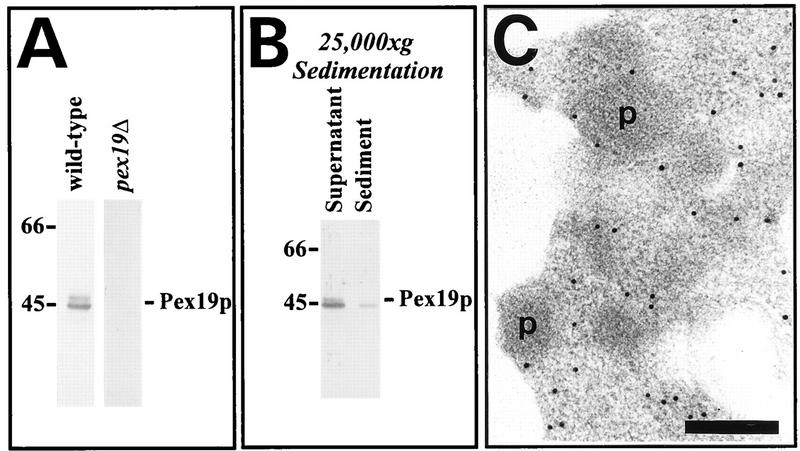
(A) Immunological detection of Pex19p. Equal amounts of oleic acid-induced wild-type and pex19Δ homogenates (50 μg of protein) were subjected to immunoblot analysis with rabbit antiserum against Pex19p. Pex19p was detected as a doublet of 44 and 46 kDa. (B) Immunoblot analysis of cell fractions obtained by centrifugation of cell homogenates from oleic acid-induced wild-type cells at 25,000 × g. Equal proportions of the supernatant and pellet fractions were loaded on the gel. Molecular mass standards (in kilodaltons) are indicated on the left. (C) Subcellular localization of myc-tagged Pex19p by immunogold labeling. Sections of pex19Δ cells expressing myc-PEX19 from the multicopy plasmid YEp-mycPEX19 were probed with polyclonal antiserum against Pex19p and goat anti-rabbit antibodies coupled to 10-nm-diameter gold particles. p, peroxisome. Bar, 0.2 μm.
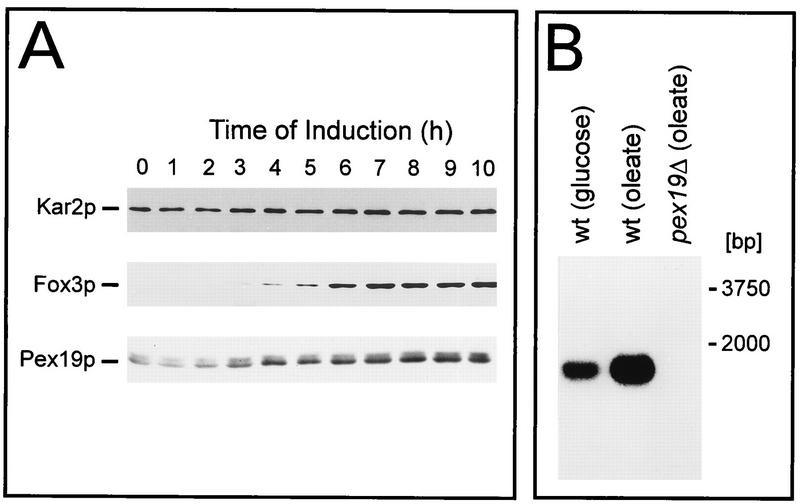
S. cerevisiae cells were shifted from growth in glucose-containing medium to growth in oleic acid-containing medium, which resulted in a massive proliferation of peroxisomes (74). At various time points, cell homogenates were prepared and probed for Kar2p (a matrix protein of the endoplasmic reticulum), Fox3p (a peroxisomal matrix protein), and Pex19p. Even before the shift to oleic acid medium, cells contained readily detectable levels of both forms of Pex19p, whereas Fox3p was not detectable (Fig. 8A, lane 1). Upon oleic acid induction, the amounts of both Pex19p forms increased approximately fivefold over the entire induction period (Fig. 8A), whereas Fox3p increased from nondetectable to clearly detectable levels. Interestingly, the profile of oleic acid induction of Pex19p is similar to the profile for Pex3p (25), which is a binding partner of Pex19p (see below). The results of the immunoblot analysis were also reflected by Northern blot analysis, as shown in Fig. 8B. A 1.8-kb transcript was detected in both glucose-repressed and oleic acid-induced cells, but not in the pex19Δ cells, with the transcript being more prominent upon oleic acid induction. Consistent with this observation, a putative oleic acid-responsive element is found at the 5′ noncoding region of the PEX19 gene (Fig. 2A).
FIG. 8.
Time course of Pex19p induction during growth on oleic acid. (A) Wild-type cells were precultured in 0.3% SD and subsequently shifted to YNO. At the indicated time points, whole-cell extracts were prepared for immunological detection of oleic acid-inducible peroxisomal thiolase (Fox3p) (24), constitutively expressed Kar2p (62), and Pex19p. The amount loaded per lane corresponds to 0.3 mg of cells. (B) Northern blot analysis of total RNA from wild-type (wt) and pex19Δ mutant cells grown on either glucose (i.e., SD) or oleate (i.e., YNO) as indicated. A radiolabeled internal fragment of the PEX19 open reading frame was used as a probe. Fifty micrograms of total RNA was loaded per lane.
Subcellular localization of Pex19p.
To determine the subcellular localization of Pex19p, a whole-cell homogenate from wild-type cells was separated into a supernatant, consisting predominantly of cytosol and microsomes, and a pellet, containing mitochondria and peroxisomes, by centrifugation at 25,000 × g (Fig. 7B). Only a minute amount of Pex19p was found in the organellar pellet; the majority of both farnesylated and nonfarnesylated Pex19p was found in the supernatant. A subsequent centrifugation of the supernatant at 100,000 × g did not result in additional sedimentation of Pex19p (42a). To examine the localization of the sedimentable portion of Pex19p, the 25,000 × g sediment was further fractionated by sucrose density gradient centrifugation. While peroxisomes and mitochondria were nicely separated, the Pex19p applied to the gradient was distributed throughout all fractions and, hence, could not be assigned to a specific organelle (data not shown). Since the majority (>95%) of Pex19p did not sediment at 25,000 × g or at 100,000 × g, it is presumed that the protein predominately resides in the cytosol in oleic acid-induced wild-type yeast cells. However, the association of at least a portion of Pex19p with organelles was also supported by immunofluorescence microscopy localization of the endogenous protein, which revealed a weak punctate pattern that could not be assigned to a specific organelle (data not shown). The intracellular localization of Pex19p was further analyzed by immunocytochemical detection of the protein with polyclonal anti-Pex19p antibodies. No labeling was observed in cells lacking Pex19p, indicating that the antibodies specifically recognized Pex19p (data not shown). Immunogold labeling of the endogenous Pex19p of oleic acid-induced wild-type cells was very weak, but the few gold particles found were localized in the cytosol (data not shown). An overexpressed myc-tagged version of Pex19p was also primarily detected in the cytosol (Fig. 7C). Gold particles, however, were also found at the peroxisomal membranes, indicating that a portion of Pex19p might be associated with that organelle (Fig. 7C). Since overexpression of the tagged Pex19p resulted in a functional complementation of the growth defect of the pex19Δ mutant on oleic acid medium (data not shown) accompanied by the presence of normal-looking peroxisomes (Fig. 7C), neither the tagging nor the overexpression apparently influenced the function of Pex19p. Therefore, the subcellular localization of the tagged Pex19p could be expected to closely mirror that of wild-type Pex19p. However, the experiment still has to be interpreted with caution, since it is well known that overexpression can lead to an abnormal intracellular localization of proteins.
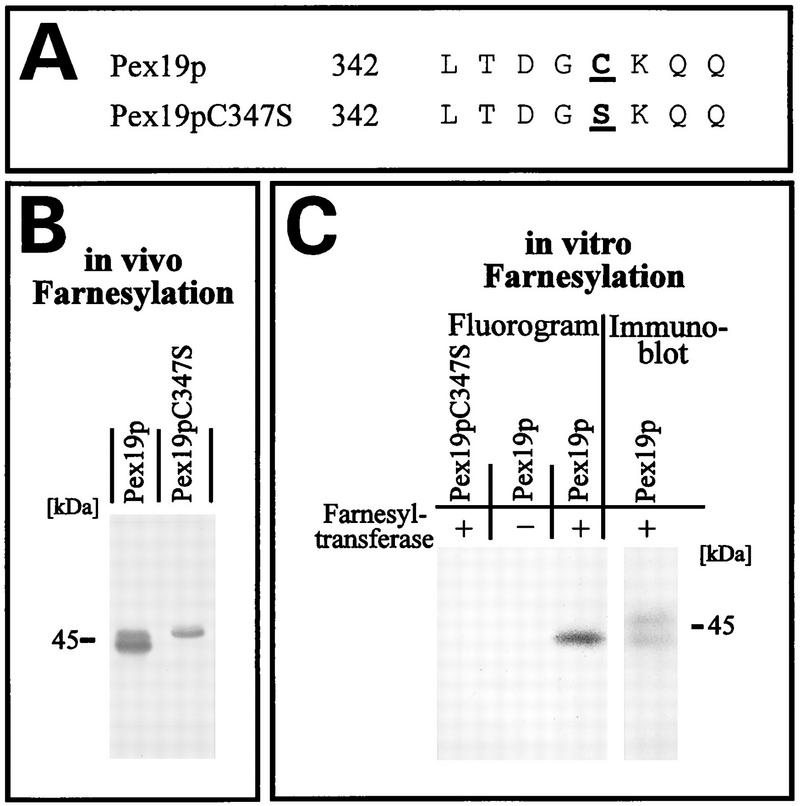
Pex19p is farnesylated at the C terminus.
Pex19p contains at its C terminus the sequence CKQQ, resembling the consensus sequence for a CAAX motif, which has been shown to be the target for isoprenylation in a number of proteins (56). The amino acid in position X of the CAAX box determines the nature of the isoprenyl group transferred to the protein. Proteins with alanine, serine, methionine, cysteine, or glutamine at position X are usually farnesylated, whereas a leucine marks the protein for the transfer of a geranylgeranyl group (56). The C-terminal glutamine of the Pex19p CAAX box suggests that the protein is farnesylated, usually via a thioether linkage to the conserved cysteine of the CAAX box. Consistent with a putative farnesylation of Pex19p, two forms of the protein (44 and 46 kDa) were detected by immunoblot analysis of whole-cell extracts (Fig. 7A). Previous studies of protein prenylation had shown that this kind of modification typically results in slight increases in the electrophoretic mobilities of these proteins (12). According to this observation, the 44-kDa protein was expected to represent the farnesylated Pex19p. To test this assumption, we generated a mutated Pex19p in which serine was substituted for the conserved cysteine at position 347 (Pex19p-C347S) (Fig. 9A). When the mutated Pex19p was expressed in cells lacking endogenous Pex19p, only the 46-kDa form of Pex19p was detectable in yeast lysates, while expression of wild-type Pex19p resulted in the occurrence of both protein forms (Fig. 9B). The absence of the 44-kDa form upon expression of the mutated Pex19p strongly suggested that Pex19p is farnesylated at Cys347.
FIG. 9.
In vivo and in vitro farnesylation of Pex19p. (A) C-terminal amino acid sequence of wild-type Pex19p and mutated Pex19p-C347S. The letters in boldface type indicate amino acid substitutions within the CAAX box. (B) Immunoblot analysis of Pex19p in whole-cell lysates from oleic acid-induced pex19Δ mutant cells expressing wild-type or mutated Pex19p from pRSPEX19 or pRSPEX19-C347S, respectively. Note the disappearance of the faster-migrating form of Pex19p upon expression of the mutated Pex19p, suggesting that it represents the farnesylated Pex19p. (C) Fluorogram and immunoblot results of in vitro farnesylation assays. Yeast homogenates expressing wild-type or mutated Pex19p from YEpPEX19 or YEpPEX19-C347S, respectively, were subjected to an in vitro assay with [3H]farnesyldiphosphate in the absence (−) or presence (+) of farnesyltransferase as indicated. Comparison with the immunoblot shows that the faster-migrating Pex19p form had incorporated the farnesyl moiety. The absence of farnesylated Pex19p upon expression of the mutated Pex19p indicated that the CAAX box of Pex19p is essential for its farnesylation. Positions of molecular mass standards are indicated for both panels B and C.
To confirm the farnesylation of Pex19p, we performed an in vitro farnesylation assay. In the presence of [3H]farnesylpyrophosphate, cell extracts containing overexpressed wild-type or mutated Pex19p-C347S were incubated with extracts from either pex19Δ cells harboring the farnesyltransferase or ram1 cells lacking the farnesyltransferase (58, 78). Farnesylation of Pex19p was monitored by fluorography as shown in Fig. 9C. In the presence of the farnesyltransferase, the wild-type Pex19p, but not the mutant Pex19p, incorporated the [3H]farnesylpyrophosphate. Of the 44- and 46-kDa wild-type forms of Pex19p, only the 44-kDa band had incorporated the radioactivity, consistent with the idea that the increased electrophoretic mobility was due to farnesylation of the protein.
Farnesylation of Pex19p is essential for its proper biological activity.
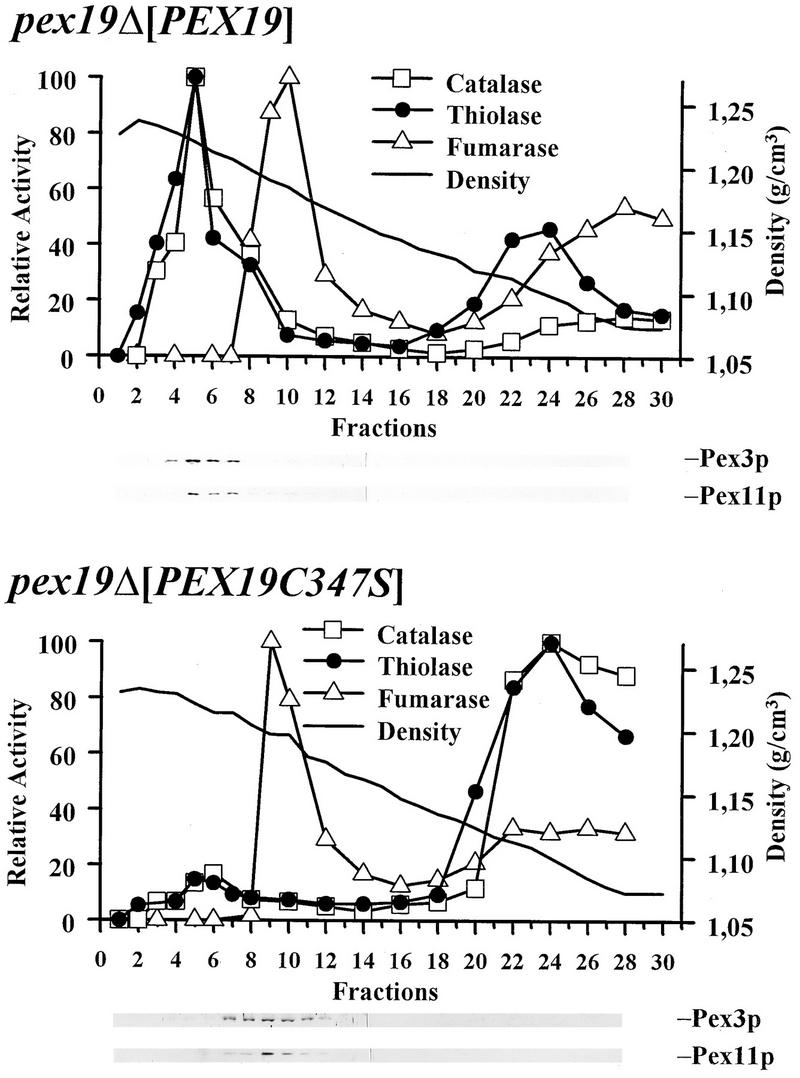
To analyze the significance of farnesylation for its biological activity, nonfarnesylated Pex19p species were tested for their ability to complement cells lacking endogenous Pex19p. In the constructs tested, cys347 of the CAAX box was replaced by either serine (Pex19p-C347S) or arginine (Pex19p-C347R) or the entire CAAX box was deleted (Pex19p-C347*). The pex19Δ cells which expressed the mutated Pex19p species from a low-copy-number CEN plasmid under the control of the PEX19 promoter grew on oleic acid as the sole carbon source (Fig. 10A). However, growth was weak in comparison to that of cells complemented with the wild-type gene (Fig. 10A). Organellar and cytosolic fractions were prepared from pex19Δ cells harboring either the wild-type or mutated Pex19p and were analyzed for the presence of peroxisomal matrix enzymes. Between 85 and 95% of the peroxisomal marker enzyme catalase was found in the supernatant fraction of transformants expressing nonfarnesylated Pex19p (Fig. 10B, lanes 2 and 3). This observation was substantiated by immunofluorescence microscopy localization of the peroxisomal marker Pcs60p in these cells, which revealed a punctate staining pattern above a cytosolic background labeling (Fig. 10C, panel b). These data suggested that the complementation of pex19Δ cells with nonfarnesylated Pex19p was only partial. Immunoblot analysis of whole-cell lysates revealed that the nonfarnesylated Pex19p species were expressed at a slightly higher level than the endogenous wild-type protein, indicating that a low expression level did not account for the low complementing activity of the constructs (data not shown). Nevertheless, the low complementing activity of nonfarnesylated Pex19p could be partially overcome by a massive overexpression of the protein. The ability of transformants expressing nonfarnesylated Pex19p species from multicopy plasmids to grow on oleic acid medium was nearly indistinguishable from that of wild-type cells (data not shown). In these cells, the immunofluorescence microscopy localization of the peroxisomal marker Pcs60p was also comparable to that of fully complemented cells (Fig. 10C, panels c and d). Morphological characterization of these transformants revealed the presence of normal-looking peroxisomes (Fig. 10D). The biochemical analysis depicted in Fig. 10B, however, showed that in pex19Δ cells complemented with overexpressed nonfarnesylated Pex19p, the majority of the peroxisomal matrix marker catalase was still found in the supernatant fraction. To monitor the complementation activity of overexpressed nonfarnesylated Pex19p in more detail, we examined the extent to which peroxisomal matrix proteins are localized in peroxisomes in these apparently complemented cells. Homogenates from transformants overexpressing either the wild-type or nonfarnesylated Pex19p were separated on sucrose density gradients, and fractions were probed for the peroxisomal matrix markers catalase and thiolase (Fox3p) and the membrane markers Pex3p and Pex11p (Fig. 11). Peroxisomes from fully complemented cells peaked at a density of 1.22 g/cm3 and contained the majority of the peroxisomal matrix and membrane enzymes. The peroxisomes of the cells expressing nonfarnesylated Pex19p also peaked at a density of 1.22 g/cm3 but contained only a minute amount of the peroxisomal matrix and membrane proteins. The majority of the peroxisomal matrix protein was found in the loading zone of the gradient; the peroxisomal membrane proteins were predominately found in fractions of 1.18 g/cm3, cosegregating with mitochondria (Fig. 11). This result clearly demonstrated that expression of the nonfarnesylated Pex19p resulted in only a partial complementation of pex19Δ cells. The small amount of correctly targeted peroxisomal proteins might be sufficient to allow transformed pex19Δ cells to grow on oleic acid medium. However, our results do not exclude the possibility that in vivo the majority of peroxisomal protein is correctly targeted to peroxisomes, as suggested by the immunofluorescence microscopy data (Fig. 10C, panel c). The observed presence of peroxisomal markers in soluble fractions in the subcellular localization fractionation studies could also be explained by the assumption that the peroxisomes of the partially complemented cells are more fragile than wild-type peroxisomes and thus would release their contents more easily during homogenization. The presence of the peroxisomal membrane proteins Pex3p and Pex11p in mitochondrial fractions might be due to mitochondrial mislocalization or aggregation; however, it could also indicate the presence of light peroxisomes or peroxisomal membrane ghosts. In any case, the inability of the nonfarnesylated Pex19p to fully replace the wild-type protein strengthens the importance of farnesylation for Pex19p function in peroxisome biogenesis.
FIG. 10.
Farnesylation is essential for proper function of Pex19p in peroxisome biogenesis. (A) Growth behavior on oleic acid medium of wild-type cells, pex19Δ mutant cells, and mutant cells expressing wild-type Pex19p or Pex19p containing the indicated mutations of the CAAX box. The pex19Δ null mutant was not able to grow on oleic acid medium. The mutant cells regained the wild-type growth behavior upon transformation with the wild-type PEX19 gene. Cells expressing Pex19p containing mutations in the CAAX box are characterized by a slow-growth phenotype on oleic acid medium. The plasmids used for the expression were pRSPEX19, pRSPEX19-C347S, pRSPEX19-C347R, and pRSPEX19-C347*. (B) Relative amount of catalase in supernatant (gray bars) and pellet (white bars) fractions derived by 25,000 × g centrifugation of cell homogenates from pex19Δ mutant (lane 1) and nontransformed wild-type (lane 10) cells and from pex19Δ mutant cells expressing wild-type or mutant PEX19 from plasmids pRSPEX19-C347S (lane 2), pRSPEX19-C347R (lane 3), pRSPEX19-C347* (lane 4), pRS-PEX19 (lane 5), YEpPEX19-C347S (lane 6), YEpPEX19-C347R (lane 7), YEpPEX19-C347* (lane 8), and YEp-PEX19 (lane 9). (C) Immunofluorescence microscopy localization of PTS1-containing Pcs60p in pex19Δ cells expressing pRS316 (a), pRSPEX19-C347S (b), YEpPEX19-C347S (c), or YEpPEX19 (d). Bar, 10 μm. (D) Electron micrograph of oleic acid-induced pex19Δ cells expressing YEpPEX19-C347S. Complementation of the mutant strain is suggested by the presence of peroxisomes (p). m, mitochondria; n, nucleus; l, lipid droplets. Bar, 0.5 μm.
FIG. 11.
Activity of peroxisomal and mitochondrial marker enzymes in fractions derived by continuous 20 to 54% (wt/wt) sucrose density gradient centrifugation of cell homogenates from pex19Δ mutant cells expressing either wild-type or nonfarnesylated Pex19p. Expression was from YEpPEX19 or YEpPEX19-C347S, respectively. Expression of wild-type Pex19p resulted in the comigration of the majority of the peroxisomal enzymes and peroxisomal membrane proteins at a density of 1.23 g/cm3, typical for wild-type peroxisomes. Upon expression of mutated Pex19p, only a minor portion of the peroxisomal markers was found at 1.23 g/cm3, suggesting that nonfarnesylated Pex19p cannot fully complement the pex19Δ mutation. The majority of the peroxisomal membrane protein was found in fractions of 1.18 g/cm3, cosegregating with mitochondrial fumarase activity. Peroxisomal and mitochondrial proteins were monitored by enzyme activity measurements. Equal volumes of each fraction were analyzed for the presence of peroxisomal membrane proteins Pex3p (39) and Pex11p (25, 50) by immunoblotting.
Identification of Pex3p as a binding partner of Pex19p.
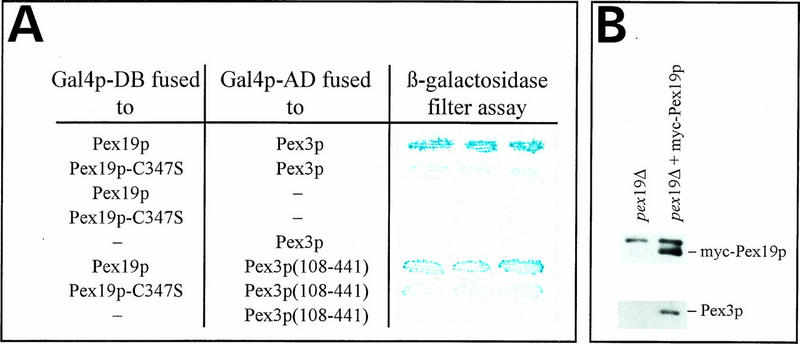
Pex19p is one of 15 peroxins which have so far been shown to be involved in peroxisome biogenesis in S. cerevisiae (16). There is striking evidence that some of these proteins require interaction with other peroxins for their function in peroxisome biogenesis (1, 27). Therefore, we used the two-hybrid system (14, 29) to detect putative binding partners of Pex19p. Fusion constructs were prepared by cloning PEX genes into plasmids encoding either the activation or the DNA-binding domains of Gal4p. Physical interactions of Pex19p with peroxins were expected to result in the activation of lacZ transcription in transformants. Yeast cells coexpressing Pex19p and Pex3p fused to the corresponding Gal4p domains expressed significant amounts of β-galactosidase, demonstrating that Pex19p is capable of binding Pex3p in vivo (Fig. 12A). Interaction of both proteins was strongly increased by farnesylation of Pex19p. However, although weak, the interaction of nonfarnesylated Pex19p with Pex3p was still significant, suggesting that a simple hydrophobic interaction of both proteins is rather unlikely. This interpretation is further supported by the observation that Pex19p still interacts with a truncated Pex3p lacking the N-terminal amino acids 1 to 107 that comprise both hydrophobic regions of the protein. The controls included in the assay shown in Fig. 12A indicate that coexpression of either of the fusion proteins, together with the respective Gal4p domains encoded by pPC86 and pPC97, did not support activation of transcription of the reporter genes. The yeast peroxins Pex1p, Pex4p, Pex5p, Pex6p, Pex7p, Pex8p, Pex13p, and Pex14p did not interact with Pex19p in the two-hybrid system, since transformants showed β-galactosidase activities in the range of the negative controls of Fig. 12A (data not shown).
FIG. 12.
Physical interaction of Pex19p with Pex3p. (A) PCY2 double transformants expressing the indicated combinations of fusion proteins were tested for β-galactosidase expression. The color intensities of these strains after the β-galactosidase filter assay are shown. (B) Coimmunoprecipitation of myc-Pex19p with Pex3p. Immunoprecipitations were performed with antibodies against the c-myc epitope and solubilized membranes prepared from pex19Δ cells and from pex19Δ cells expressing myc-Pex19p. The upper band that appears in both lanes corresponds to the heavy chain of IgG. Equal amounts of immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis with monoclonal antibodies against the myc epitope and polyclonal antibodies against Pex3p.
The interaction of Pex19p with Pex3p was independently confirmed by coimmunoprecipitation (Fig. 12B). Pex3p could be coimmunoprecipitated with myc-tagged Pex19p from solubilized membranes of transformants expressing the fusion protein but not from those of control strains. The immunoprecipitates contained neither the highly expressed peroxisomal membrane protein Pex11p (25, 50) nor the peroxisomal matrix protein Fox3p (24), indicating that micelle-associated preexisting or artifactual mixtures of proteins were not retained nonspecifically (data not shown).
DISCUSSION
Here we have reported the molecular characterization of Pex19p, an oleic acid-inducible farnesylated protein essential for peroxisome biogenesis. Consistent with this function, cells deficient in Pex19p do not grow on oleic acid as the sole carbon source, lack morphologically detectable peroxisomes, and are characterized by mislocalization of peroxisomal matrix enzymes of the PTS1 and PTS2 variety to the cytosol. Binding studies identified the interaction of Pex19p with the peroxin Pex3p, an integral protein of the peroxisomal membrane.
The gene product of the human housekeeping gene HK33 (8) and the Chinese hamster peroxisomal prenylated protein PxF (41) were identified as putative orthologs of the yeast peroxin (Fig. 3). Supporting the notion that the HK33 gene product is a true ortholog of Pex19p, the C-terminal region, which is essential for the function of yeast Pex19p, could be replaced by the corresponding region of the human HK33 gene product. The chimeric protein retained the ability to functionally complement the growth defect of the pex19Δ mutant on oleic acid medium (Fig. 6). The possibility that the HK33 gene product is a true ortholog of the peroxin Pex19p is of considerable clinical interest, since mutations in several peroxins have been demonstrated to cause peroxisome biogenesis disorders, which are a heterogeneous group of autosomal recessive diseases that are lethal in early infancy (48). Cells from patients with peroxisome biogenesis disorders are characterized by defects in peroxisomal protein import, thus phenotypically resembling most yeast pex mutants (16). Eleven complementation groups of these disorders have been defined, and for six of them the mutated gene has not yet been identified (71).
A striking feature of the Pex19p sequence is the C-terminal motif referred to as the CAAX box that is contained in numerous proteins in which the cysteine represents the site of prenylation. Proteins of S. cerevisiae in which the X residue is alanine, serine, cysteine, methionine, or glutamine are farnesylated by the farnesyl protein transferase. Several lines of evidence suggest that Pex19p is farnesylated at the cysteine of the C-terminal CKQQ sequence. Two forms of Pex19p, distinguishable by their different electrophoretic mobilities, were detected by Western blot analysis of whole-cell lysates (Fig. 7 to 9). Replacement of the invariant cysteine of the CKQQ sequence by either arginine or serine resulted in the disappearance of the faster-migrating form, suggesting that it represents the farnesylated Pex19p (Fig. 9B). In S. cerevisiae, farnesyl protein transferase activity requires the RAM1 gene product. The dependence of Pex19p farnesylation on the presence of Ram1p was confirmed by in vitro incorporation of the farnesyl moiety into wild-type Pex19p (Fig. 9C). In oleic acid-induced wild-type S. cerevisiae, both farnesylated and nonfarnesylated Pex19p are present, with the farnesylated form being the dominant species (Fig. 7A). One of the most frequently suggested functional roles for protein isoprenylation is facilitation of membrane binding. In S. cerevisiae, proteins like Ypt1p, Sec4p, Ste18p, and Cdc42p require posttranslational prenylation for membrane association (4, 56, 76). However, if both the farnesylated and nonfarnesylated forms of Pex19p predominately reside in the cytosol, as suggested by our biochemical data (Fig. 7), Pex19p farnesylation might not directly mediate an association of the protein with the membrane. In this respect, it is interesting that the physical association of prenylated proteins with the target membranes does not necessarily occur via the prenyl group. Prenylated Ras, for example, is cytosolic; additional palmitoylation is needed to target this protein to the plasma membrane (6). Based on our biochemical data, farnesylated Pex19p may also predominately reside in the cytosol. Furthermore, all of the above-mentioned prenylated proteins are targeted to different subcellular locations; consequently, the targeting information may reside within the protein rather than in the prenyl groups. It has been suggested that targeting to the appropriate location is realized by the specific adherence of defined prenylated proteins to organelle-specific membrane receptors (31, 33, 66). In agreement with this assumption, Pex19p was found to interact with the peroxisomal membrane protein Pex3p (Fig. 12). The interaction of Pex19p and Pex3p did strongly depend on the presence of the farnesyl group, consistent with the idea that the primary role of the farnesyl moiety may be to trigger the binding properties of Pex19p. A precedent for a protein-protein interaction enhanced by farnesylation is found in the yeast Ras-dependent signal transduction pathway. Evidence that the essential role of the farnesyl moiety of yeast Ras is to enhance the interaction with its downstream target, adenylate cyclase, rather than to localize Ras to the plasma membrane has been provided (6). Whether Pex3p targets Pex19p to the peroxisomal membrane remains to be shown, especially since biochemical data suggested that only a fraction of the endogenous Pex19p is associated with the peroxisomal membrane in S. cerevisiae (Fig. 7). However, on the basis of immunofluorescence microscopy data and immunogold labeling, PxF, the putative mammalian ortholog of Pex19p, has been determined to reside at the peroxisomal membrane (41). Interestingly, in agreement with our observations for Pex19p, the association of PxF with the peroxisomal membrane was no longer detectable by biochemical means, which was interpreted to indicate that the protein was attached loosely to the outer surface of peroxisomes. This is possible as well for the yeast Pex19p protein. The idea of a peroxisomal association of Pex19p is also supported by other observations. Since Pex19p and Pex3p were coimmunoprecipitated from sedimented membranes, at least a portion of Pex19p is apparently membrane associated. This would also explain the punctate pattern observed upon immunofluorescence localization of the endogenous protein (data not shown) and the immunocytochemically observed association of myc-tagged Pex19p with the peroxisomal membrane (Fig. 7C). However, even a different subcellular localization of the two binding partners, Pex3p and Pex19p, in S. cerevisiae appears less disturbing if we take into consideration the possibility that the interaction between Pex19p and Pex3p is transient rather than stable. As a working model, a transient interaction of the two proteins could result in the modulation of one of the binding partners, which might trigger its function in peroxisome biogenesis.
Peroxisome biogenesis includes of matrix protein import, formation of the peroxisomal membrane, and proliferation of the organelle (16). To date, we do not know at which point Pex19p fulfills its functional role. Pex3p has been reported to be required for the maintenance of the peroxisomal membrane (3) and has been suggested to be involved in the topogenesis of at least some peroxisomal membrane proteins (77). Based on the observed interaction of Pex19p with Pex3p, it is tempting to speculate that both proteins act in tandem at the same step in peroxisome biogenesis, supposedly the formation of the peroxisomal membrane.
ACKNOWLEDGMENTS
K.G., W.G., and M.L. contributed equally to this work.
We are grateful to all members of our labs for fruitful discussions. We thank Adalbert Roscher for kindly providing the HK33 gene. We thank Gabriele Dodt, Peter Rehling, and Michael Schwierskott for reading the manuscript.
This work was funded by grants from the Deutsche Forschungsgemeinschaft (Er178/2-1, Ku329/17-1, Ku329/17-2, and Ro 727/1-2) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Albertini M, Rehling P, Erdmann R, Girzalsky W, Kiel J A K W, Veenhuis M, Kunau W-H. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell. 1997;89:1–20. doi: 10.1016/s0092-8674(00)80185-3. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F J, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1989. [Google Scholar]
- 3.Baerends R J S, Rasmussen S W, Hilbrands R E, van der Heide M, Faber K N, Reuvekamp P T W, Kiel J A K W, Cregg J M, van der Klei I J, Veenhuis M. The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J Biol Chem. 1996;271:8887–8894. doi: 10.1074/jbc.271.15.8887. [DOI] [PubMed] [Google Scholar]
- 4.Balch W E. Low molecular weight GTP-binding proteins (LMGPs) involved in vesicular transport: binary switches or biological transducers? Trends Biochem Sci. 1990;15:469–472. [Google Scholar]
- 5.Baumgart E. Morphology of peroxisomes in light- and electron microscopy. In: Bugaut M, Latruffe N, editors. Peroxisomes: biochemistry, molecular biology and genetic diseases. Heidelberg, Germany: Springer-Verlag; 1994. pp. 37–57. [Google Scholar]
- 6.Bhattacharya S, Chen L, Broach J R, Powers S. Ras membrane targeting is essential for glucose signaling but not for viability in yeast. Proc Natl Acad Sci USA. 1995;92:2984–2988. doi: 10.1073/pnas.92.7.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blobel F, Erdmann R. Identification of a peroxisomal member of the AMP-binding protein family. Eur J Biochem. 1996;240:468–476. doi: 10.1111/j.1432-1033.1996.0468h.x. [DOI] [PubMed] [Google Scholar]
- 7a.Bloecker, H., and P. Brandt. Unpublished data.
- 8.Braun A, Kammerer S, Weissenhorn W, Weis E H, Cleve H. Sequence of a putative human housekeeping gene (HK33) localized on chromosome 1. Gene. 1994;146:291–295. doi: 10.1016/0378-1119(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 9.Brocard C, Kragler F, Simon M M, Schuster T, Hartig A. The tetratricopeptide repeat-domain of the Pas10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal SKL. Biochem Biophys Res Commun. 1994;204:1016–1022. doi: 10.1006/bbrc.1994.2564. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli J P, Pall M L. A series of yeast vectors for expression of cDNAs and other DNA sequences. Yeast. 1993;9:1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- 11.Butt T R, Sternberg E J, Gorman J A, Clark P, Hamer D, Rosenberg M, Crooke S T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci USA. 1984;81:3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan A J, Tsai J, Casey P J, Douglas M G. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J Biol Chem. 1992;267:18890–18895. [PubMed] [Google Scholar]
- 13.Chevray P M, Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien C T, Bartel P L, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Hoop H J, Ab G. Import of proteins into peroxisomes and other microbodies. Biochem J. 1992;286:657–669. doi: 10.1042/bj2860657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Distel B, Erdmann R, Gould S J, Blobel G, Crane D I, Cregg J M, Dodt G, Fujiki Y, Goodman J M, Just W W, Kiel J A K W, Kunau W-H, Lazarow P B, Mannaerts G P, Moser H W, Osumi T, Rachubinski R A, Roscher A, Subramani S, Tabak H F, Tsukamoto T, Valle D, van der Klei I, van Veldhoven P P, Veenhuis M. A unified nomenclature for peroxisome biogenesis. J Cell Biol. 1996;135:1–3. doi: 10.1083/jcb.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodt G, Gould S J. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol. 1996;135:1763–1774. doi: 10.1083/jcb.135.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer J M, McNew J A, Goodman M. The sorting sequence of the peroxisomal integral membrane protein PMP47 is contained within a short hydrophilic loop. J Cell Biol. 1996;133:269–280. doi: 10.1083/jcb.133.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elgersma Y, Kwast L, Klein A, Voorn-Brouwer T, van den Berg M, Metzig B, America T, Tabak H F, Distel B. The SH3 domain of the Saccharomyces cerevisiae peroxisomal membrane protein Pex13p functions as a docking site for Pex5p, a mobile receptor for the import of PTS1 containing proteins. J Cell Biol. 1996;135:97–109. doi: 10.1083/jcb.135.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elgersma Y, Tabak H F. Proteins involved in peroxisome biogenesis and functioning. Biochim Biophys Acta. 1996;1286:269–283. doi: 10.1016/s0304-4157(96)00012-3. [DOI] [PubMed] [Google Scholar]
- 21.Erdmann R. The peroxisomal targeting signal of 3-oxoacyl-CoA thiolase from Saccharomyces cerevisiae. Yeast. 1994;10:935–944. doi: 10.1002/yea.320100708. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann R, Veenhuis M, Mertens D, Kunau W-H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1989;86:5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdmann R, Kunau W-H. A genetic approach to the biogenesis of peroxisomes in the yeast Saccharomyces cerevisiae. Cell Biochem Funct. 1992;10:167–174. doi: 10.1002/cbf.290100306. [DOI] [PubMed] [Google Scholar]
- 24.Erdmann R, Kunau W-H. Purification and immunolocalization of the peroxisomal 3-oxoacyl-CoA thiolase from Saccharomyces cerevisiae. Yeast. 1994;10:1173–1182. doi: 10.1002/yea.320100905. [DOI] [PubMed] [Google Scholar]
- 25.Erdmann R, Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J Cell Biol. 1995;128:509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdmann R, Blobel G. Identification of a peroxisomal membrane receptor for the C-terminal tripeptide signal recognition factor. J Cell Biol. 1996;135:111–121. doi: 10.1083/jcb.135.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdmann R, Veenhuis M, Kunau W-H. Peroxisomes: organelles at the cross-roads. Trends Cell Biol. 1997;7:400–407. doi: 10.1016/S0962-8924(97)01126-4. [DOI] [PubMed] [Google Scholar]
- 28.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields S, Song O K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 30.Gietz R D, Sugino A. New yeast-E. coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 31.Glomset J A, Gelb M H, Farnsworth C C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990;15:139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- 32.Glover J R, Andrews D W, Rachubinski R A. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc Natl Acad Sci USA. 1994;91:10541–10545. doi: 10.1073/pnas.91.22.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein L J, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 34.Gould S J, Keller G A, Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987;105:2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould S J, Kalish J E, Morrell J E, Bjorkman J, Urquhart A J, Crane D I. An SH3 protein in the peroxisome membrane is a docking factor for the PTS1 receptor. J Cell Biol. 1996;135:85–95. doi: 10.1083/jcb.135.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harlow E, Lane D. Antibodies—a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 37.Häusler T, Stierhof Y D, Wirtz E, Clayton C. Import of a DHFR hybrid protein into glycosomes in vivo is not inhibited by the folate-analogue aminopterin. J Cell Biol. 1996;132:311–324. doi: 10.1083/jcb.132.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill J E, Myers A M, Koerner T J, Tzagaloff A. Yeast E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- 39.Höhfeld J, Veenhuis M, Kunau W-H. PAS3, a Saccharomyces cerevisiae gene encoding a peroxisomal integral membrane protein essential for peroxisome biogenesis. J Cell Biol. 1991;114:1167–1178. doi: 10.1083/jcb.114.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 41.James G L, Goldstein J L, Pathak R K, Anderson R G W, Brown M S. PxF, a prenylated protein of peroxisomes. J Biol Chem. 1994;269:14182–14190. [PubMed] [Google Scholar]
- 42.Jones J S, Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990;6:363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- 42a.Köhl, R., and W.-H. Kunau. Unpublished data.
- 43.Krause T. Investigation on membrane proteins of Saccharomyces cerevisiae with the focus on Pas3p. Ph.D. thesis. Bochum, Germany: Ruhr-Universität Bochum; 1995. [Google Scholar]
- 44.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 45.Lamb J R, Michaud W A, Sikorski R S, Hieter P A. Cdc16p, Cdc23p and Cdc27p form a complex for mitosis. EMBO J. 1994;13:4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarow P B. Genetic approaches to study peroxisome biogenesis. Trends Cell Biol. 1993;175:105–132. doi: 10.1016/0962-8924(93)90079-g. [DOI] [PubMed] [Google Scholar]
- 47.Lazarow P B, Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- 48.Lazarow P B, Moser H W. Disorders in peroxisome biogenesis. In: Scriver C R, Beaudet C R, Sly W S, Valle D, editors. The metabolic and molecular bases of inherited disease. 7th ed. Vol. 2. New York, N.Y: McGraw-Hill Book Co.; 1995. pp. 2287–2324. [Google Scholar]
- 49.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 50.Marshall P A, Krimkevich Y I, Lark R H, Deyer J M, Veenhuis M, Goodman J M. PMP27 promotes peroxisome proliferation. J Cell Biol. 1995;129:345–355. doi: 10.1083/jcb.129.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marzioch M, Erdmann R, Veenhuis M, Kunau W-H. PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J. 1994;13:4908–4918. doi: 10.1002/j.1460-2075.1994.tb06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCollum D, Monosov E, Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein deficiencies of Zellweger syndrome cells—the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J Cell Biol. 1993;121:761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNew J A, Goodman J M. An oligomeric protein is imported into peroxisomes in vivo. J Cell Biol. 1994;127:1245–1257. doi: 10.1083/jcb.127.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNew J A, Goodman J M. The targeting and assembly of peroxisomal proteins: some old rules do not apply. Trends Biochem Sci. 1996;21:54–58. [PubMed] [Google Scholar]
- 55.Moreno de la Garza M, Schultz-Borchardt U, Crabb J W, Kunau W-H. Peroxisomal β-oxidation system of Candida tropicalis. Eur J Biochem. 1985;148:285–291. doi: 10.1111/j.1432-1033.1985.tb08837.x. [DOI] [PubMed] [Google Scholar]
- 56.Omer C A, Gibbs J B. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol Microbiol. 1994;11:219–225. doi: 10.1111/j.1365-2958.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 57.Osumi T, Tsukamoto T, Hata S, Yokota S, Miura S, Fujiki Y, Hijikata M, Miyazawa S, Hashimoto T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem Biophys Res Commun. 1991;181:947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- 58.Powers S, Michaelis S, Broek D, Santa Anna S, Field J, Herskowitz I, Wigler M. RAM1, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell. 1986;47:413–422. doi: 10.1016/0092-8674(86)90598-2. [DOI] [PubMed] [Google Scholar]
- 59.Rachubinski R A, Subramani S. How proteins penetrate peroxisomes. Cell. 1995;83:525–528. doi: 10.1016/0092-8674(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 60.Rehling P, Marzioch M, Niesen F, Wittke E, Veenhuis M, Kunau W-H. The import receptor for the peroxisomal targeting signal 2 (PTS2) in Saccharomyces cerevisiae is encoded by the PAS7 gene. EMBO J. 1996;15:2901–2913. [PMC free article] [PubMed] [Google Scholar]
- 61.Rose M D, Novick P, Thomas J H, Bothstein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 62.Rose M D, Misra L M, Vogel J P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 63.Rout M P, Kilmartin J V. Components of the yeast spindle pole body. J Cell Biol. 1990;111:1913–1927. doi: 10.1083/jcb.111.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rüther U, Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2:1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafer W R, Rine J. Protein prenylation: genes, enzymes, targets and functions. Annu Rev Genet. 1992;30:209–237. doi: 10.1146/annurev.ge.26.120192.001233. [DOI] [PubMed] [Google Scholar]
- 67.Sherman F, Fink G R, Hicks J. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 68.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith M, Leung D W, Gillam S, Astell C R, Montgomery D L, Hall B D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979;16:753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- 70.Subramani S. Protein translocation into peroxisomes. J Biol Chem. 1996;271:32483–32486. doi: 10.1074/jbc.271.51.32483. [DOI] [PubMed] [Google Scholar]
- 71.Subramani S. PEX genes on the rise. Nat Genet. 1997;15:331–333. doi: 10.1038/ng0497-331. [DOI] [PubMed] [Google Scholar]
- 72.Swinkels B W, Gould S J, Bodnar A G, Rachubinski R A, Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991;10:3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szilard K R, Titorenko V I, Veenhuis M, Rachubinski R A. Pay32p of the yeast Yarrowia lipolytica is an intraperoxisomal component of the matrix protein translocation machinery. J Cell Biol. 1995;131:1453–1469. doi: 10.1083/jcb.131.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veenhuis M, Mateblowski M, Kunau W-H, Harder W. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast. 1987;3:77–84. doi: 10.1002/yea.320030204. [DOI] [PubMed] [Google Scholar]
- 75.Walton P A, Hill P E, Subramani S. Import of stably folded proteins into peroxisomes. Mol Biol Cell. 1995;6:675–683. doi: 10.1091/mbc.6.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walworth N C, Goud B, Kastan Kabcenell A, Novick P J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989;8:1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiemer E A C, Luers G H, Faber K N, Wenzel T, Veenhuis M, Subramani S. Isolation and characterization of Pas2p, a peroxisomal membrane protein essential for peroxisome biogenesis in the methylotrophic yeast Pichia pastoris. J Biol Chem. 1996;271:18973–18980. doi: 10.1074/jbc.271.31.18973. [DOI] [PubMed] [Google Scholar]
- 78.Wilson K L, Herskowitz I. STE16, a new gene required for pheromone production by cells of Saccharomyces cerevisiae. Genetics. 1987;115:441–449. doi: 10.1093/genetics/115.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J W, Lazarow P B. PEB1(PAS7) in Saccharomyces cerevisiae encodes a hydrophilic, intra-peroxisomal protein that is a member of the WD repeat family and is essential for the import of thiolase into peroxisomes. J Cell Biol. 1995;129:65–80. doi: 10.1083/jcb.129.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J W, Lazarow P B. PEB1(PAS7) is an intraperoxisomal receptor for the NH2-terminal, type 2, peroxisomal targeting sequence of thiolase: Peb1p itself is targeted to peroxisomes by an NH2-terminal peptide. J Cell Biol. 1996;132:325–334. doi: 10.1083/jcb.132.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]