Abstract
Cardiovascular disease (CVD) is a serious health challenge, causing more deaths worldwide than cancer. The vascular endothelium, which forms the inner lining of blood vessels, plays a central role in maintaining vascular integrity and homeostasis and is in direct contact with the blood flow. Research over the past century has shown that mechanical perturbations of the vascular wall contribute to the formation and progression of atherosclerosis. While the straight part of the artery is exposed to sustained laminar flow and physiological high shear stress, flow near branch points or in curved vessels can exhibit ‘disturbed’ flow. Clinical studies as well as carefully controlled in vitro analyses have confirmed that these regions of disturbed flow, which can include low shear stress, recirculation, oscillation, or lateral flow, are preferential sites of atherosclerotic lesion formation. Because of their critical role in blood flow homeostasis, vascular endothelial cells (ECs) have mechanosensory mechanisms that allow them to react rapidly to changes in mechanical forces, and to execute context-specific adaptive responses to modulate EC functions. This review summarizes the current understanding of endothelial mechanobiology, which can guide the identification of new therapeutic targets to slow or reverse the progression of atherosclerosis.
Keywords: Cardiovascular disease, Shear stress, Endothelial cells, Mechanotransduction, Antiatherogenic drugs
1. Introduction
1.1. Endothelial Mechanobiology
Endothelial cells (ECs) in the lining of blood vessels create a selective barrier for fluid and biomolecule transport. In normal development and adult physiology, ECs activate morphogenic programs to form vascular structures in the embryo, remodel existing vasculature, or create new blood vessels during tissue repair, while endothelial dysfunction leads to pathophysiological states that contribute to vascular disorders such as atherosclerosis and thrombosis.1
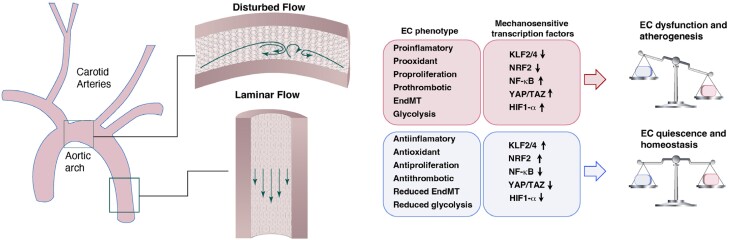
ECs are exquisitely sensitive to fluid shear stress (FSS), which is a critical determinant of homeostasis but can also be an instigator of disease2,3 (Figure 1). FSS is the tangential component of frictional forces generated at a surface (e.g. the vessel wall) by the flow of a viscous fluid (e.g. blood).4–6 The blood vascular wall has three mechanical force loadings, i.e. shear stress (SS), normal stress, and circumferential stress. SS used in this article denotes wall SS, which is the component of frictional forces arising from the blood flow and acts parallel to the vessel luminal surface.5–7 SS is an outcome of fluid viscosity and the velocity gradient between adjacent layers of the flowing blood. Imposed with the pulsatile characteristic of the flow, SS spans a range of spatiotemporal scales. The magnitude of SS is expressed using interchangeable units (1 Pa = 1 N/m2 = 10 dynes/cm2), and the value is affected by changes in blood flow velocity, the inner radius of vessel, and the viscosity of blood. Cells face diverse physical, biochemical, and biomechanical environments and possess the ability to respond to these cues to maintain appropriate biological functions. Mechanobiology refers to mechanisms by which cells sense, transduce, and respond to mechanical forces and modulate their functions. Cells achieve this through mechanotransduction—the transmission of mechanical forces through sensing structures, which results in the induction of biochemical signals.8
Figure 1.
Hemodynamic SS and its role in vessel pathophysiology. The straight part of the artery is exposed to sustained laminar flow and physiological high SS, while flow near branch points or in curved vessels can exhibit disturbed flow. ECs subjected to laminar flow show anti-inflammatory, antioxidant, and antiproliferative phenotypes accompanied by reduced EndMT and glycolysis, thereby maintaining EC quiescence and vascular homeostasis. Corresponding flow-responsive mechanisms include KLF2, KLF4, NRF2, and other protective pathways. By contrast, ECs in regions of disturbed flow show a proinflammatory, prooxidant, proproliferative response and an enhanced endothelial to EndMT phenotype. As a consequence, disturbed flow leads to EC dysfunction and atherogenesis. The mechanisms responsible for disturbed flow-mediated endothelial dysfunction involves activation of NF-κB, YAP/TAZ, and HIF-1 among other pathways. ↑: upregulation; ↓: downregulation.
Geometrical properties of arteries and pulsatile flow conditions determine the detailed blood flow patterns. Pulsatile blood flow results in a positive mean flow rate, but the velocity of the fluid oscillates with the frequency of the heartbeat, and the flow exerts pulsatile SS on the endothelium.9 In straight segments of arteries, blood flows in ordered laminar patterns in a pulsatile fashion dependent on the cardiac cycle. ECs are subjected to pulsatile laminar SS with fluctuations in magnitude that yields a mean positive SS.8,10,11 Regions of such laminar and physiological levels of SS are generally not prone to formation of atherosclerotic lesions. In the context of physiological laminar flow, ECs assume a quiescent state with characteristics of anti-inflammation, antiproliferation, antiapoptosis, antilipid infiltration, antileucocyte adhesion and migration, antithrombosis, and reduced endothelial to mesenchymal transition (EndMT) and glycolysis.2,9,12–14 Laminar SS maintains EC quiescence and vascular homeostasis by inducing endothelial production of a number of factors that promote vasodilation [e.g. endothelial nitric oxide synthase (eNOS) and nitric oxide (NO)] and downregulating proatherogenic genes encoding adhesion molecules and chemokines [e.g. vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule-1 (ICAM-1), and monocyte chemotactic protein-1 (MCP-1)], which inhibit the adherence of circulating blood elements.9,15 Corresponding flow-responsive mechanisms include Kruppel-like factor 2 (KLF2),16,17 Kruppel-like factor 4 (KLF4),18,19 nuclear factor (erythroid-derived 2)–like 2 (NRF2),20,21 and other protective pathways; these pathways synergistically contribute to the atheroprotective effects of laminar flow.22
In contrast, atherosclerosis-susceptible regions of arterial branches, bifurcations, and curvatures are characterized by disturbed flow and low time–averaged SS.8,11,23 Disturbed flow is a complex flow pattern that includes recirculation eddies and changes in direction with space (flow separation and reattachment) and time (reciprocating flow).24 ECs in these atheroprone sites experience oscillatory and low SS and exhibit low NO production, reduced barrier function, increased proadhesive, procoagulant and proproliferative properties, and an enhanced endothelial to EndMT phenotype.2,7,25–27 Upon exposure to low and oscillatory SS, activated ECs express atherogenic molecules, including MCP-1, which recruits monocytes into the arterial wall, and platelet-derived growth factors (PDGFs), which stimulate EC proliferation and migration of vascular smooth muscle cells (VSMCs) into the subintimal space.7,25 These changes in vessel biology are evidently related to inflammatory and tissue repair programs. The mechanisms responsible for disturbed flow-mediated endothelial dysfunction involve activation of nuclear factor κB (NF-κB),28–30 Yes-associated protein (YAP)/WW domain-containing transcription regulator 1 (TAZ),31–33 and hypoxia-inducible factor 1α (HIF-1α)34–36 among other pathways.
Importantly, exercise training is a well-established and potent physiological stimulus which reduces cardiovascular events.37 Endothelial dysfunction is associated with an impaired dilatory response to increased blood flow-associated SS [flow-mediated dilation (FMD)].38 Exercise (e.g. handgrip exercise and cycling exercise) can create a sustained, contraction intensity-dependent increase in SS, which potentiates the subsequent brachial artery FMD response to a reactive hyperaemia (RH)–induced increase in SS (RH-FMD; standard test, typically denoted simply as ‘FMD’).39–41 The mechanisms responsible for the benefits of exercise training in terms of endothelial function may be related to either direct hemodynamic effects, or secondary effects, mediated through risk factor modification.42,43 And perturbed flow dynamics are also induced in postsurgical neointimal hyperplasia and predispose to pathophysiologies such as in-stent restenosis, vein bypass graft failure, transplant vasculopathy, and vascular calcification.
1.2. Methodologies for studying the effects of FSS on ECs
To study the mechanisms of EC responses to SS, various models have been developed to mimic in vivo flow environments (Table 1). In vitro models can provide well-controlled conditions for studying endothelial biology over time. However, these models are limited in that they do not fully recapitulate vessel anatomy or mechanical properties, which is significantly more complicated. On the other hand, in vivo models are more relevant to disease processes, but fluid dynamics are difficult to accurately measure or control in animal models. Ultimately, a combination of in vivo and in vitro approaches has led to our current understanding of endothelial mechanobiology and atherosclerosis.
Table 1.
The pros and cons for in vitro and in vivo methods modelling SS
| In vitro models | Pros | Cons |
|---|---|---|
| Cone-and-plate system | Readily programmable and reproduce different SS waveforms under controlled conditions | Has a relatively low surface area, and hence, a low number of replicate studies can be simultaneously performed |
| Shear ring model | Effectively limits ‘mixed’ shear and provide unidirectional and periodic flow patterns within patho/physiological levels | The intrinsic inability to precisely control flow in the shear ring |
| Parallel plate chamber | The ability to generate steady unidirectional or oscillatory SS in a pulsatile manner in an apparatus | Relatively lengthy and complex setup time, low surface areas, requirements for pumps, and pressurization often requiring sealants and gaskets |
| Microfluidic bifurcated channel | Model gradient SS regions and changes in pressure or growth factor gradients | Linear rectangular channels that are optically transparent and permeable to gases |
| Microfluidic cylindrical channel | Incorporate ECM components and more physiologically relevant | Cylindrical, linear channels fail to accurately model the effects of disturbed flow in bifurcating vessels |
| In vivo models | Pros | Cons |
| Perivascular cuff model | Easy to induce vortex shedding, highly turbulent regions, and recirculation zones | Less physiologically relevant due to the acute flow alteration and the dissociation of oscillatory shear from low SS. Cuff placement may evoke intraplaque haemorrhage and plaque rupture with fibrin-positive luminal thrombus |
| Partial ligation model | More physiologically relevant due to associated chronic flow alteration and colocalization of both low and oscillatory SS. Allows for easy and rapid intimal RNA isolation and provide sufficient quantity of endothelial RNA for genome-wide microarray studies. Short study duration | The presence of thrombus, endothelial denudation, and decreased vessel diameter |
| AVF model | High clinical relevance of AVF to vascular access in haemodialysis | High cost and technical complexities |
1.2.1. In vitro approaches
Early efforts in this area used modified cone and plate viscometer systems to create a well-defined and constant SS44–47 (Figure 2A). In this approach, flow is generated using a cone, which rotates around a central axis that is perpendicular to a fixed plate on which the cells are seeded. Another approach to create SS is the ‘shear ring’ device, which can provide unidirectional and periodic flow patterns within physiological and pathophysiological ranges using a shaker plate48 (Figure 2B). By restricting the flow pathway within a circular culture dish to the periphery through the placement of an inner ring, the shear ring model can effectively alleviate ‘mixed’ cellular shear-induced phenotypes.
Figure 2.
Methods for investigating endothelial SS in vitro. (A) Modified cone and plate viscometer system for applying spatially uniform SS. (B) Shear ring device constructed from petri dishes, with flow driven by placement on a shaker plate. (C) Parallel plate flow chamber; flow is controlled by a syringe pump (not shown). (D) Step flow chamber. In this modification of the parallel plate flow chamber, a step expansion is introduced to produce localized flow separation/disturbance at the EC surface. (E) Bifurcating channel in a microfluidic device. (F) Cylindrical channel cast in a hydrogel (e.g. collagen I) and then coated with ECs.
To more accurately control fluid exchange over the ECs, parallel plate flow chambers were developed. This apparatus uses a syringe pump or peristaltic pump to pass fluid from a reservoir through the chamber, creating steady unidirectional, pulsatile, or oscillatory flow.26,49,50 Channels with rectangular cross-section and uniform height along the flow path can be used to study steady or pulsatile laminar flow (Figure 2C). Disturbed flow is achieved by introducing a vertical step expansion near the entrance, where the channel height suddenly increases (Figure 2D). As fluid passes the step, there is a region of flow separation with low SS where flow recirculation and reattachment occur. Downstream, the disturbed flow transits to laminar flow where the channel has uniform height. Thus, the step flow chamber can be used to examine EC responses to disturbed flow in the vicinity of the reattachment area and laminar flow downstream in the same device.7,26
Microfabrication techniques greatly advance the possible complexities of FSS models. Microfabricated channels have been used to mimic vessel branching (Figure 2E). Microfluidic devices with bifurcated channels produce regions with SS gradients, allowing detailed studies of the impact of chronic SS spatial gradients on ECs.51–53 More recently, microfluidic systems allow growth of ECs in 3D microchannels with dimensions similar to vessels in vivo using microfabrication techniques and enable integration of important cells and structural properties such as mural cells, extracellular matrix (ECM), or mechanical deformations (e.g. vessel hoop stress). Microfabricated polydimethylsiloxane (PDMS) hydrogel hybrids have been developed using PDMS scaffolds or channels that contain hydrogels (Figure 2F). Cells can then be seeded on the hydrogel, providing a more biologically relevant substrate for studies of EC functions. Such systems have been widely used to investigate capillary sprouting in response to changes in pressure or growth factor gradients.54–56 Although the flexibility of microfabrication technology helps to recapitulate more realistic vascular features in vitro, further modifications are needed to reproduce the correct microanatomy of the vessel wall.
1.2.2. In vivo methods
Over the past decades, several animal models have been utilized for studying the effects of atheroprone SS on ECs, including (i) exogenous constriction of a segment of a large vessel, (ii) partial ligation of the carotid artery, and (iii) creation of an arteriovenous fistula (AVF).
One of the most common strategies to induce disturbed flow and the associated low and reciprocating SS in vivo is to introduce a local constriction to recapitulate the effects of a stenosis in a segment of a large vessel such as the carotid artery or abdominal aorta (Figure 3A, left panel). Mimicking a stenosis (40–60% reduction in diameter) in this way leads to flow separation and low-velocity recirculation in the region immediately downstream from the constriction, and laminar with a relatively higher SS upstream of the constriction and in distal downstream segments.57,58 Computational fluid dynamics (CFD) studies for the perivascular cuff model further showed that the constrictive cuff results in three distinct regions of SS: relatively lower laminar shear upstream from the stenosis, relatively higher shear within the stenosis, and low oscillatory shear downstream from the stenosis59 (Figure 3A, right panel).
Figure 3.
In vivo methods for modulating endothelial SS. (A) In the SS–modifying cuff model, a restrictive cuff is placed around the right carotid artery (RCA) to modify blood flow. The resulting changes in SS can be estimated using CFD (at right).59 There is decreased laminar SS upstream and oscillatory SS downstream. (B) The partial ligation model. Three branches of the left carotid artery (LCA) are ligated, thus reducing SS in the LCA. (C) AVF model. A connection is made between the common carotid artery (CCA) and the external jugular vein (EJV) causing disturbed flow in multiple regions near the anastomosis. Right panel in part A is adapted from Mohri59; right panel in part (B) is adapted from Mitra61; right panel in part (C) is adapted from Bai.63.
The partial ligation model provides a powerful approach to uncover the pathogenesis of disturbed flow–induced atherosclerosis (Figure 3B, left panel). By ligating three out of four branches of the left carotid artery, the partial ligation model causes pathophysiologically relevant disturbed flow with characteristics of low and oscillatory wall SS in the common carotid artery.60 CFD models have incorporated the geometry of mouse carotid arteries as determined by ultrasound imaging to predict whether partial ligation changes SS levels and direction. Mitra et al.61 further revealed that the SS level is significantly reduced during diastole compared with that of the right common carotid artery (RCA) and becomes negative (because of flow reversal) during diastole in the ligated left common carotid artery (LCA) (Figure 3B, right panel). The disturbed flow induces rapid development of atherosclerosis in 2 weeks and advanced lesions by 4 weeks in apolipoprotein E-deficient (ApoE−/−) mice.60,62
AVF is another approach to obtain an experimental model of perturbed flow in vivo (Figure 3C, left panel). This is often created by forming a connection between the carotid artery and the jugular vein or between the femoral artery and the femoral vein of rodents, rabbit, or swine. CFD simulations showed that areas of low SS are found along the wall of the anastomotic floor, near the anastomosis heel on the inner wall of the vein and to a lesser extent on the inner wall after the curvature of the vein63 (Figure 3C, right panel). Despite the high flow rates present in AVF after maturation, low and oscillatory SS exists in zones where flow stagnation occurs on the outer wall of the artery and on the inner wall of the juxta-anastomotic site, thereby resulting in vessel stenosis in regions with flow perturbations.64–66
Of note, AVF-induced alteration of SS causes acute and chronic structural remodelling in the artery and vein, although the vein has received more attention in AVF research studies. AVF leads to neointima formation and stenosis in the venous region of disturbed flow induced by shunting of arterial blood flow directly into the vein.67,68 Like arterial endothelium responses to oscillating flow, ECs in such venous segments undergo proinflammatory activation associated with upregulation of MCP-1 and induction of the proinflammatory transcription NF-κB.67,69 In addition, the creation of AVFs induces high flow rates in the arterial segment proximal to the AVF, which leads to arterial remodelling. ECs exposed to high flow conditions show significantly increased cell density and upregulated expression of vascular endothelial growth factor (VEGF), a potent angiogenic factor that stimulates EC proliferation and migration.70
In summary, sophisticated methods for modelling shear forces in vitro and in vivo have greatly advanced our understanding of EC mechanics. The choice of a suitable model to study the effects of FSS on ECs is dependent on the specific endothelial process to be studied. By characterizing the FSS profiles generated by in vitro models and pairing them to the FSS profiles identified in atherosclerosis, we can characterize in more detail the contribution of mechanical forces on endothelial health and pathophysiology.
2. Response of ECs to FSS
2.1. EC morphology
In vitro and in vivo studies have clearly identified that ECs in areas of high laminar SS elongate and align in the direction of flow, with the well organized and parallel actin stress fibres in the central region.11,15,71 Whereas static cultures or cells exposed to disturbed flow are randomly oriented and tend to adopt a more polygonal shape; their actin filaments are short and localized mainly at the cell periphery.7,11,72,73 It is likely that EC alignment parallel to the flow direction is an adaption response that can reduce flow resistance and induce prosurvival signals in the endothelium.49,74 Disturbed flow or low SS results in less alignment of the ECs, thus exposing them to higher SS gradients and potentially priming these regions for atherosclerosis.75 Changes in EC morphology in response to SS are mediated by activation of the Rho family of small GTPases and upregulation of intercellular adhesion molecules, which are associated with structural reorganization of the cytoskeleton, cell-cell junctions, and focal adhesions (FAs).76
2.2. EC turnover
Laminar flow leads to reduced EC turnover rate and keeps ECs in a relatively nonproliferative state, which is associated with DNA synthesis inhibition and cell cycle progression suppression with the majority of cells being arrested in the G0 or G1 phase.77 Mechanically, laminar flow enhances the activity of histone deacetylase 1 (HDAC1) and its association with p53 resulting in deacetylation of p53 at Lys 320 and Lys 373. HDAC-deacetylated p53 then activates the growth arrest proteins GADD45 (growth arrest and DNA damage–inducible protein 45) and p21, which cause a decrease in cyclin-dependent kinase activity and then hypophosphorylation of retinoblastoma protein (Rb).77,78 Rb phosphorylation undergoes periodic oscillations during the cell cycle and predominates in G0/G1 phases. The hypophosphorylated Rb can bind several transcription factors essential for DNA synthesis to prevent their initiation of transcription, thus inhibiting cell proliferation. In contrast, oscillatory/low SS promotes EC turnover with characteristics of increased DNA synthesis and proliferation via G0/G1-S transition by suppressing p21.7,79 Such accelerated cell turnover would lead to an enhanced macromolecular permeability and contribute to the increases in lipid uptake at regions of disturbed flow.9,80 Although involved in p53/p21 signalling in EC turnover and senescence, SS––induced cell turnover can be reversed, which differs from cellular senescence, a state of irreversible growth arrest after successive cell division–induced telomere shortening that ultimately triggers DNA damage responses.81,82
2.3. EC permeability
Shear force is a major determinant of EC permeability and endothelial barrier function. In the context of physiological high laminar SS, the adjacent ECs are closely connected and restrict the passage of macromolecules such as lipoproteins.83 Conversely, disturbed flow causes barrier disruption and increases permeability to macromolecules. Perturbed flow results in a large intimal clearance of low-density lipoprotein (LDL) and thus a large mass influx of LDL into the intima.83 Disruption of the junctional complex proteins such as connexins and VE-cadherins is largely responsible for flow-mediated alterations in permeability.25,84 Interestingly, state-of-the-art morphological observations of human coronary atheroma reveal microscopic breaches in intimal endothelial continuity and the accumulation of erythrocyte components and polymorphonuclear leucocytes within the region of flow disturbances.85 The observations suggest that hemodynamically related microscopic lesions are an early contributor to increased permeability and atherogenesis. In addition, the enhanced cell turnover rate, as well as the morphological changes of ECs from elongated to more rounded shape caused by disturbed SS, might also affect cell–cell junctions and contribute to the greater permeability of ECs in atheroprone areas.26,49,73 Low/oscillatory SS increases the permeability of the internal elastic lamella and elastic lamellae by reducing fenestrae, thus increasing the accumulation of macromolecules in the arterial wall.86 More recently, a study by Lu et al. established a functional link between the activation of mechanosensitive Ca2+ channels, transient receptor potential vanilloid subtype 4 (TRPV4), and endothelial hyperpermeability through the phosphorylation and mitochondrial redistribution of eNOS mediated by PKC (protein kinase C).87
Moreover, the shear dependence of macromolecule uptake such as albumin and LDL in the vessel wall has been reported. Compared with the uptake by cells under static conditions, lower SS leads to an increased uptake of LDL into ECs, whereas there is decreased uptake at higher SS.88 Mechanistically, the endothelial glycocalyx is involved in regulation of shear-dependent uptake of macromolecules into ECs, since neutralization of the glycocalyx induces an increase in albumin uptake by cultured ECs.89,90 It is thought that the size and steric hindrance as well as electrostatic charges of the glycocalyx and the permeating substance contribute to glycocalyx-dependent permeability.91
2.4. EndMT
EndMT, a complex biological process with characteristics of EC transition to mesenchymal phenotype, has been identified to be regulated by mechanical forces. While laminar SS is protective against EndMT, ECs are exposed to disturbed flow undergo EndMT, contributing to atherogenesis.92 Extensive studies have shown that the multifunctional cytokine transforming growth factor-β (TGF-β) is the main inducer of EndMT,93 but the processes leading to activation of its signalling remain poorly defined. Analysis of human coronary arteries indicates a strong correlation among reduced fibroblast growth factor receptor 1 (FGFR1) expression, activated TGF-β signalling, the occurrence of EndMT, and the severity of atherosclerosis.94 Using the parallel plate system and partial carotid ligation model, a recent study has confirmed that ALK5 (TGFβR1) is a receptor responsible for mechano-EndMT and Shc acts as a critical downstream driver of EndMT and atherosclerosis in areas of disturbed SS.95 The activated TGF-β receptor complex transduces its signal by phosphorylating Smad transcription factors, which subsequently translocate into the nucleus and bind to promoters of EndMT inducing genes. These genes include the key transcription factors GATA4, TWIST1, and Snail.96,97 In addition to modulating transcription factors, induction of EndMT leads to cytoskeletal remodelling via activation of the Rho GTPase family (RhoA, RAC1, and CDC42) through Smad-dependent and Smad-independent mechanisms.98 This cytoskeletal reorganization alters the endothelial apicobasal polarity and the cells form spindle shapes with enhanced migration, thus predisposing to the development of atherosclerosis.99 Moreover, oscillatory SS in a three-dimensional microengineered human coronary-artery-on-a-chip induces transition of ECs into a proinflammatory EndMT phenotype and contributes to atherogenesis, which is mediated by the Notch1/p38 MAPK-NF-κB signalling pathways.92
2.5. Anticoagulant activity
Physiological laminar SS promotes anticoagulant characteristics consistent with an atheroprotected phenotype of ECs. Prostacyclin is the first inhibitor of platelet aggregation shown to be released from ECs in response to shear force.100 Laminar SS–induced release of the vasodilator NO is also responsible for the antiplatelet aggregation properties of ECs.101 More recently, studies have identified that thrombomodulin (TM), which is a key player in the regulation of coagulation and thrombosis, responds to shear force through interaction with protein C and protein S to inactivate certain clotting factors, thus exerting anticoagulant activity.102,103 Under laminar flow with high SS, KLF2-mediated upregulation of TM is an important protective antithrombotic and antiatherosclerotic mechanism in large arteries.102,104 TM is reversibly regulated by mechanical forces in an EC-specific, magnitude-dependent, and hysteresis-free manner.105 Besides, laminar SS has been shown to stimulate expression of tissue plasminogen activator (tPA) and reduce secretion of plasminogen activator inhibitor type-1.106–108 Importantly, ECs exposed to turbulent flow fail to show increases in TM and tPA.109,110
2.6. Leukocyte adhesion and migration
Adhesion of circulating leukocytes to and subsequent transmigration across the EC monolayer are key events in atherogenesis. The adhesive interactions between leukocytes and ECs are modulated by local blood flow.111 Circulating leukocytes tether and roll along the vessel wall by establishing transient selectin–mediated interactions with ECs.112 This initial contact facilitates recognition of chemoattractants (mostly chemokines), immobilized on the apical surface of ECs, by rolling leukocytes. Extensive studies have shown that laminar flow is associated with less adhesion of circulating leukocytes to ECs. This process involves inhibition of expression of adhesion molecules and chemotactic proteins (e.g. VCAM-1 and MCP-1) in ECs. In contrast, disturbed flow with altered SS promotes leukocyte binding and transmigration, thus causing leukocyte accumulation in the arteries and increased propensity for atherogenesis.73 Increased leukocyte–EC adhesion under disturbed flow may be attributed to (i) the altered expression of adhesive proteins such as ICAM-1, E-selectin, and MCP-1 on EC surfaces and (ii) the enhanced collisions and prolonged contacts between the circulating leukocytes and ECs.113 Furthermore, accumulating evidence demonstrates that shear forces participate in integrin-mediated leukocyte arrest.111,114 Ultimately, arrested leukocytes crawl along the endothelium and migrate across ECs (diapedesis or transendothelial migration), either at intercellular junctions (paracellular pathway), by engaging adhesion molecules including platelet–endothelial cell adhesion molecule-1 (PECAM-1), junctional adhesion molecules (JAMs), and molecules of the cluster of differentiation CD99 or through the EC body (transcellular route).115,116
Additionally, hemodynamic perturbations can activate neutrophils and induce release of neutrophil extracellular traps (NETs), which are comprised of web-liked DNA fibres containing histones and granular proteins.117 The process of NET formation is called NETosis. During NETosis, neutrophils decondensate and release their nuclear DNA in long chromatin filaments to form NETs.118 In addition to their known role in the elimination of pathogens, NETs have been newly described as potent inducers of leukocyte extravasation at sites of inflammation.119,120 NETs can trigger neutrophil rolling, firm adhesion, and emigration in the microvasculature in vivo, which can be abrogated by immunoneutralization of P-selectin and its major counter receptor PSGL-1 (P-selectin glycoprotein ligand-1).120
2.7. Lipid metabolism
Lipid accumulation in the artery wall is a common clinical manifestation of atherosclerosis and is largely impacted by local hemodynamics.121 Laminar flow inhibits lipid accumulation in ECs because it decreases LDL permeability as a result of the upregulation of growth arrest genes and the lower lipid uptake and synthesis as a result of the downregulation of sterol regulatory element binding protein 1 (SREBP1). On the contrary, disturbed flow induces sustained activation of SREBP1 and hence leads to an increase in SRE-mediated transcriptional activation of EC genes that promote lipid uptake and lipid synthesis.26,73 Activated SREBP1 is associated with enhanced expression of genes encoding for the LDL receptor, cholesterol synthase, and fatty acid synthase, thus increasing the intracellular sterol level.122 The shear-dependent activation of SREBP1 is abolished by blockade of β1 integrin with AIIB2 blocking-type mAb or disruption of actin cytoskeleton with cytochalasin D, suggesting the importance of integrin and actin cytoskeleton in the modulation of EC lipid metabolism in response to SS.123,124
2.8. SS and EC interactions with neighbouring cells
Since ECs do not exist alone in the vessel wall, there is crosstalk between ECs and other cells in the vessel wall, and interactions between these cell types are important for proper vascular function. Previous studies suggest that ECs can transmit SS–induced signals to VSMCs, and VSMCs exhibit feedback control to ECs as well.125,126 The net arterial remodelling under mechanical stimulation is controlled by a dynamic interplay between growth inhibitory signals from ECs and growth stimulatory signals from VSMCs.9,127 These results highlight the significance of considering cell–cell communication in studying endothelial mechanobiology and vascular pathologies. Because these interactions are challenging to isolate in vivo, they may be more easily studied using in vitro systems that have the potential to integrate multiple in vivo components including circulating blood cells, ECM, and other vascular cells of the arterial wall (e.g. VSMCs and fibroblasts). For example, artificial vessels surrounded by ECM can be created through the self-assembly of a mixture of ECs and VSMCs. These novel engineered blood vessels provide the correct configuration of lumen, an inner lining of ECs, and outer sheath of VSMCs and exhibit properties such as quiescence, perfusability, and vasoactivity expected for functional vessels.128 Advances in this field will greatly improve our understanding of the interplay between cell types in the atherogenic vascular wall.
In summary, ECs respond to laminar and disturbed flow by structural and functional adaption which involves perturbations of EC morphology and reprogramming of gene expression to modulate cellular functions (e.g. proliferation, permeability, EndMT, leukocyte extravasation, coagulation, and lipid accumulation) (Figure 4). These responses enable vessel stabilization as well as homeostatic remodelling and are critical determinants of vascular architecture during development.
Figure 4.
Endothelial response to SS. ECs exposed to laminar flow are spindle shaped, aligned, and elongated parallel to the flow direction, with characteristics of lower turnover rate, increased anticoagulant activity, hypopermeability and less lipid accumulation, reduced leukocyte adhesion and migration, and inhibited EndMT, hence maintaining endothelial barrier function, while disturbed flow leads to a more polygonal morphology of ECs with random orientation and endothelial barrier disruption, which is characterized by accelerated turnover, decreased anticoagulant activity, hyperpermeability and lipid accumulation, enhanced leukocyte adhesion to endothelium, and the occurrence of EndMT.
3. Endothelial mechanosensors
Numerous membrane-associated molecules and microdomains have been identified as mechanosensors in ECs and mediate conversion of mechanical stimuli to intracellular signals, including ion channels, FAs, receptor–tyrosine kinases [e.g. vascular endothelial growth factor receptor (VEGFR)], G-protein-coupled receptors (GPCRs), the glycocalyx, primary cilia, and caveolae (Figure 5). Typically, mechanosensors present on the EC membrane directly sense blood flow and transduce the mechanosignal into the cells.11,129 Altered expression/structure of these mechanosensors leads to altered mechanotransduction, and the occurrence of endothelial dysfunction, evidenced by endothelial injury, leukocyte adhesion to activated endothelium, EndMT, heightened oxidative stress/inflammatory response, senescence, and hyperpermeability.22,80,130 Dysfunctional status of ECs thus predispose to the development of atherosclerotic plaques and other vascular diseases.
Figure 5.
Mechanosensors in the endothelium that sense and convert biomechanical cues into biological signals. The glycocalyx, primary cilia, ion channels, cell–cell and cell–substrate adhesion complexes, G-protein–coupled sensors, caveolae, and NE proteins (e.g. LINC complexes) have been implicated in the transduction of fluid forces.
3.1. Ion channels
Activation of mechanosensitive ion channels is one of the most rapid reactions of ECs exposed to flow. Piezo1 and Piezo2 proteins are the most widely investigated flow-responsive cation channels. SS mechanically changes the conformation of Piezo1 and Piezo2, increasing ion transport, which then induces protease activation and cytoskeletal reorganization.131,132 Disturbed flow, but not laminar flow, triggers Piezo1- and Gq/G11-mediated integrin activation, resulting in focal adhesion kinase (FAK)–dependent NF-κB activation. Mice with endothelium-specific deficiency of Piezo1 or Gαq/Gα11 exhibit reduced integrin activation, inflammatory signalling, and progression of atherosclerosis in atheroprone areas.133 Beyond that, TRPV4, a Ca2+ entry channel, is also implicated in EC mechanotransduction by forming mechanosensitive heteromeric channels with other transient receptor potential channels such as TRPC1 and TRPP2.134 Mechanical stimulation induces dynamic microcompartmentation of caveolin-1/TRPV4/KCa in caveolae of ECs, a process that participates in regulation of flow-induced vasodilation.135
To date, the mechanism involved in shear-mediated alteration of ion channel activity remains controversial. Previous studies have mainly focused on direct deformation of the ion channels in response to SS. However, another potential mechanism involves indirect deformation of the channels due to their mechanical coupling to other structures that are strained by fluid forces, including cytoskeletal structures.136
3.2. FAs and integrins
FAs serve as linkages between the ECM and cell cytoskeleton and are essential regulators of EC responses to flow. Studies have proposed that SS activates FA signalling pathways mainly in two ways: (i) by triggering the rapid reorganization of FAs and the formation of aggregates of integrins and (ii) by directly activating FA signalling proteins.137–139 Nuclear mechanics also participate in FA mechanosensing, as disruption of nuclear–cytoskeletal connections in ECs interferes with adaption to SS.140 This is accompanied by altered cell–cell adhesion, barrier function, cell–matrix adhesion, and FA dynamics.140
Integrins, composed of α and β subunits, are major mediators of mechanosensing and mechanotransduction in ECs. By interacting dynamically with ECM proteins, the mechanosensitive integrins activate Rho small GTPase and many signalling events in FAs and the actin-based cytoskeleton to modulate vascular biology.141 Studies have demonstrated that shear-elicited activation of integrins and the associated RhoA small GTPase can induce ERK activation142 and stimulate tyrosine phosphorylation of FAK and paxillin,143,144 thus regulating flow-induced stress fiber formation and actin reorganization.
Additionally, binding of endothelial integrin α5β1 with fibronectin under low/oscillatory SS induced by a parallel plate flow system or partial ligation of ApoE−/− mice causes phosphorylation of the cytosolic nonreceptor protein kinase c-Abl, which then induces tyrosine phosphorylation (at Y357) and nuclear translocation of YAP, leading to proatherogenic gene expression and EC activation.145 Furthermore, Piezo1-dependent activation of endothelial annexin A2 uniquely binds to integrin α5 subunits and drives integrin movement into lipid rafts under partial ligation-induced oscillatory SS, thus mediating the translocation and activation of integrin α5β1.146 Once activated, integrin α5β1 stimulates FAK–dependent NF-κB signalling, which resulted in inflammation and formation of atherosclerotic plaques146. Integrin α5+/− mice are viable and display significant resistance to disturbed flow-induced EC dysfunction and atherosclerosis.147
Currently, most studies of integrin involvement in cardiovascular disease (CVD) have focused on the mechanistic relationship between abluminal integrins and endothelial inflammation under flow conditions. Interestingly, evidence suggested that integrins localized on the apical surface of ECs sense and respond to SS signals as well.148 Apical β1 integrin is activated by unidirectional SS but not oscillatory SS, potentially contributing to flow direction sensing.149 The activation of luminal β1 integrins by SS involves caveolae rather than cytoskeleton dynamics.150 However, the role of apically expressed integrins in mechanotransduction and whether there is coordination between luminal and abluminal integrins require further investigation.
3.3. Cell–cell junctions
High laminar SS promotes the formation of PECAM-1/VE-cadherin/VEGFR2–mechanosignaling complex at cell–cell junctions, which transduces mechanical force into the activation of PI3K (phosphoinositide 3-kinase) and AKT, stimulating NO production from eNOS.151 VE-cadherin is important for endothelial reorientation and gene expression modulation in response to flow, whereas PECAM-1 serves as a force transducer leading to activation of signalling by VEGFR2 and PI3K.152 The transmission of mechanical cues through the junctional complex is mediated by phosphorylation of a small pool of VE-cadherin on Y658. Y658 phosphorylation dissociates p120ctn, allowing binding of the polarity protein LGN, which is essential for multiple flow responses in vitro and in vivo.153 Additionally, using the in vitro flow chamber or cone-and-plate viscometer system and hypercholesterolaemic ApoE−/− mice model, a novel mechanocomplex of plexin D1/neuropilin-1/VEGFR2 has been identified in ECs, which regulates flow-mediated junction signalling, integrin function, and downstream cellular responses, ultimately regulating the site-specific distribution of atherosclerosis.154
3.4. GPCRs and G-proteins
GPCRs are sensitive to mechanical stimuli and are linked to a wide range of signalling molecules and effector systems by coupling to G-protein. The Gαq/11-coupled receptor GPR68 knockdown interferes with the response to mechanical forces, and the mechanosensitivity of GPCRs is attributed to its essential structural motif c-terminal helix 8 (H8).155,156 Unidirectional flow applied to ECs enhances the phosphorylation of both AKT-1 and its downstream effector GSK-3β (glycogen synthase kinase 3β), while silencing of Gαq/11 completely abrogates this effect.157 Moreover, Gαq/11 and PECAM-1 form a mechanosensitive complex at the cell–cell junction under flow condition. The Gαq/11/PECAM-1 complex experiences a rapid dissociation and reassociation in response to temporal gradients of SS.158 Another study revealed that PECAM-1 mediates GSK-3β phosphorylation during SS stimulation using an orbital shaker.159 Collectively, these studies indicated that Gαq/11 and PECAM-1 participated in regulating flow-induced activation of the AKT-GSK-3β signalling pathway. Additionally, steady SS exerted by a conventional parallel-plate flow chamber induces rapid activation of Gαq/11 without dephosphorylation of β-arrestin-1 or internalization of Gαq/11 receptor S1P3, suggesting that Gαq/11 can also respond to mechanical force, independent of GPCR activation.160
3.5. Glycocalyx and primary cilia
The EC surface is covered with a hydrated gel-like structure called the glycocalyx which contributes to the integrity of the endothelial barrier.161 Endothelial glycocalyx consists of sulfated proteoglycans—transmembrane syndecans and membrane-bound glypicans, with their covalently bound glycosaminoglycans (GAGs)—heparan sulfate (HS) and chondroitin sulfate (CS) and the nonsulfated GAG hyaluronic acid (HA), as well as glycoproteins bearing sialic acids (SA) and plasma proteins.162 Multiple studies have concluded that the glycocalyx is a key mechanosensor participating in endothelial mechanotransduction mechanisms and itself is physically modulated by the flow.163 The endothelial glycocalyx is relatively thick and substantially covers the endothelial surface in the common carotid region, where the endothelium is exposed to laminar flow. In contrast, flow perturbances at lesion-prone sites of arterial bifurcations are associated with scarce expression of glycocalyx.164,165 This renders the endothelium more vulnerable, resulting in disease-like cellular and molecular accumulation in the endothelium or within the blood vessel wall.166,167
Changes in blood flow influence conformation of glycocalyx, and the signal is transmitted to cytoskeleton through the intracellular domain of glycocalyx or transduced into biochemical signals through changes in local microenvironment. Disruption of glycocalyx affects flow-mediated actin cytoskeleton reorganization and FA localization.168 Researchers propose a ‘bumper-car’ model for the role of glycocalyx, in which the actin cortical web and dense peripheral actin bands (DPABs) are only loosely connected to basal attachment sites, allowing for two distinct cellular signalling pathways reacting to SS: one transmitted by glycocalyx core proteins as a torque that acts on the actin cortical web (ACW) and DPAB, and the other emanating from FAs and stress fibres at the basal and apical membranes of the cell.168
Additionally, endothelial glycocalyx is intimately involved in vascular homeostasis, exerting multiple antiatherogenic effects by inhibiting coagulation and leucocyte adhesion, by contributing to the vascular permeability barrier and by mediating SS–induced NO release.161 These qualities distinguish the glycocalyx as an essential element of a functional endothelium, and glycocalyx can be used to dynamically assess EC barrier function under flow.169 Studies to date have made great progress in imaging the glycocalyx under flow, its effect on barrier function, and the interplay between blood components (e.g. leucocytes) and the glycocalyx in both static and perfused in vitro models. However, ideal in vitro models that integrate all of these aspects have yet to be demonstrated.
Primary cilia are mechanical structures that extend from the apical surface of endothelial or epithelial cells. Endothelial primary cilia–mediated SS sensing is coupled to Ca2+ signalling and NO production, ciliary length, and cilia distribution.170 Under conditions of low shear flow, endothelial cilia act together with bone morphogenic protein 9 (BMP9) to keep immature vessels open before the onset of high SS–mediated remodeling.171 However, exposure of ECs to high laminar SS in a flow chamber leads to disassembly of primary cilia, suggesting that cilia cannot tolerate extreme levels of SS.172 Additionally, work conducted on zebrafish embryos indicates that endothelial cilia actively recruit vascular mural cells to control the development and maturation of the vertebrate vascular system through a mechanism that involves ciliary regulation of Notch activation and transcription factor foxc1b expression.173
3.6. Caveolae
Caveolae, cell membrane invaginations that are rich in cholesterol, sphingolipids, and a variety of signalling molecules, are mechanotransducers that reportedly respond to changes in blood flow.174–177 Signalling molecules that have been implicated in rapid EC response to flow include Src family tyrosine kinases, eNOS, Ras, and select heterotrimeric G proteins that are enriched in EC caveolae.178,179 The caveolae coat protein caveolin-1 knockout mice have impaired flow-dependent arterial remodelling, defects in flow-induced vasodilation, and blunted eNOS activation, which can be rescued with the reconstitution of endothelial specific caveolin-1.175 Compared to static conditions, exposure of ECs to atheroprotective shear leads to increased caveolae number, achieved by translocation of caveolin-1 from the Golgi to the luminal plasma membrane, while also increasing caveolae/caveolin-1 polarization to the portion of ECs that lies upstream of the flow direction.180,181 However, it remains obscure how caveolae mediate endothelial mechanotransduction, but these structures may be functionally linked to other mechanosensitive molecules. SS–induced integrinβ1 activation results in Src-family kinase–dependent phosphorylation of caveolin-1, suggesting that caveolae and integrins are functionally linked.150,182 Other evidence supports that both junctional complexes (VE-cadherin and PECAM-1) and FAK most likely collaborate with resident caveolar proteins (caveolin-1, VEGFR2, and eNOS) to integrate flow-dependent responses in vivo.183,184 Moreover, it is reported that the adaptation of caveolae/caveolin-1 to protective or atherogenic flow may involve endothelial glycocalyx and possibly other elements.164,185
3.7. The nucleus in mechanotransduction
The cell nucleus is the storage house of genetic information and also integrates epigenetic mechanisms that participate in regulation of transcription. This epigenetic regulation is central to mechanobiology186,187 and involves DNA methylation, histone modifications, and RNA-based mechanisms, which result in an altered chromatin structure and gene expression and play an important role in the pathogenesis of atherosclerosis.188–190 In the field of shear force transmission in ECs, a particularly exciting concept is the emerging role of nuclear envelope (NE) proteins in vascular mechanotransduction.191,192 LINC (linker of nucleoskeleton and cytoskeleton) complexes are the nuclear membrane proteins which cross the nuclear membrane and physically connect with both the cytoskeleton and the nuclear lamina which forms the scaffold beneath the inner nuclear membrane.193 Recent studies reported that the LINC complexes and lamina of NE may act as direct force-responsive structures and participate in multiple cell functions, including chromatin organization, DNA replication, and gene transcription.194,195 Specifically, nesprins, which are KASH (Klarsicht–ANC-1–SYNE homology) domain proteins located on the outer nuclear membrane, are LINC components connected to actin filaments as well as to microtubules and intermediate filaments. The nesprin proteins are also connected directly to LINC proteins at the inner nuclear membrane—the SUN (Sad1p–UNC-84) domain proteins—thereby forming a physical bridge between the outer and inner nuclear membranes. The SUN domain proteins are in turn connected to the nuclear lamina and to chromatin through a number of adaptor proteins, thereby providing a direct mode of physical signal transmission from the cell membrane into the nucleus.186,196–198 Low SS reduces the expressions of nesprin2 and lamin A, which affects the activation of transcription factors AP-2, TFIID, and Stat1, 3, 5, 6. In this way, SS regulates the mRNA levels of downstream target genes to modulate EC proliferation and apoptosis.194,199 Studies of NE mechanotransduction have revealed that different components sense the mechanical force directly from extracellular environment or via the cytoskeleton.194,196,199 However, this is an emerging area of study, and the details of the structure of the NE, its involvement in mechanotransduction, and the implications for vascular biology remain to be elucidated.
In short, mechanical cues perceived by ECs can be relayed from the cell membrane to nucleus via multiple flow-responsive molecular elements. The cytoskeleton links the luminal surface to cell–matrix adhesion sites, intercellular junctions, and the nuclear membrane, providing a structural framework for EC mechanotransduction.200 Importantly, different mechanosensing elements are interconnected in the initiation of the mechanotransduction process, e.g. the links between integrins, GPCRs, and nucleus,201–203 as well as synergistic effects between Piezo1 and TRPV4 in response to shearing.204 Hence, cellular responses to external forces are complex, and dynamic changes in cell phenotype are determined by integration of multiple mechanotransduction axes.
4. Mechanisms of mechanotransduction in ECs
Endothelial mechanosensors discussed above sense and respond to mechanical stresses either directly through conformational changes that alter protein activity or ion transport, or by initiating a complex network of signal transduction pathways that affect gene transcription, cell cycle, and other programs relevant to cardiovascular pathophysiology (Figure 6).
Figure 6.
EC mechanotransduction pathways involved in FSS–mediated endothelial function.
4.1. KLF2/4
Flow-responsive transcription factors KLF2/4 play an essential role in maintaining EC quiescence. Atheroprotective laminar flow, but not disturbed flow, upregulates expression of KLF2 and KLF4. KLF2/4 activate anti-inflammatory mediators (e.g. eNOS and TM) and repress NF-κB activity, which decreases expression of adhesion (e.g. ICAM-1 and VCAM-1) and prothrombotic [e.g. plasminogen activator inhibitor 1 (PAI-1)] molecules, thus conferring antiatherosclerotic effects.205 Mechanically, laminar flow increases KLF2 expression via the MEK5/ERK5/MEF2 signalling pathway, while oscillatory flow downregulates KLF2 expression through SMAD1/5 activation to mediate cell proliferation and cell cycle progression.206,207 Laminar SS modulates endothelial hyaluronan (HA) production through increased KLF2 and subsequent membrane localization of hyaluronan synthase 2 (HAS2) and UDP-sugar availability.165 Epigenetic modulation is also involved, as laminar flow stimulates HDAC5 phosphorylation and nuclear export in ECs, thus dissociating MEF2 to activate KLF2 and eNOS expression.208 Moreover, disturbed flow in atherosusceptible regions reduces Foxp (forkhead box P) transcription factor 1 (Foxp1) expression, which activates the NLRP3 inflammasome and promotes interleukin-1β (IL-1β) secretion, thereby impairing endothelial function and promoting monocyte adhesion and infiltration into the vessel wall to become proinflammatory foam cells and to facilitate atherosclerotic lesion formation.209
Studies to date suggest that there is significant overlap between the endothelial targets and functional effects of KLF2 and KLF4. Future studies should further evaluate whether KLF4 and KLF2 have competitive or cooperative effects on the promoters they regulate and how this regulation changes under conditions of SS.
4.2. NRF2
Antioxidant transcription factor NRF2 is a downstream target of KLF2 and functions synergistically with KLF2 to induce quiescence of ECs by the reduction of inflammatory responses.210 Laminar SS induces binding of NRF2 to the antioxidant response element (ARE) present in the promoters of many antioxidant and phase II detoxifying genes, such as glutathione-S-transferase, heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase (NQO1), peroxiredoxin 1, glutamate-cysteine ligase modifier subunit (GCLM), and glutamate-cysteine ligase catalytic subunit (GCLC).211 Using a parallel-plate flow chamber, a study found that flow-mediated NRF2 nucleus translocation and inflammation suppression involves inhibition of the p38 MAPK-VCAM-1 signalling axis and ERK5 activation.212 In contrast, disturbed flow significantly diminishes the binding of NRF2 to the NQO1 ARE, which involves deacetylation of NRF2 by class I HDACs.213 However, the role of NRF2 in atherogenesis seems to be genotype dependent. Myeloid deletion of NRF2 in LDL receptor–deficient mice (Ldlr−/−) leads to increased proinflammatory gene expression and atherosclerosis.214 While global NRF2 knockout in ApoE−/− mice unexpectedly decreased atherosclerotic lesion formation compared with wild-type ApoE−/− mice.215,216 Consistently, the absence of NRF2 in the bone marrow-derived cells decreases the lesion size, exerting a protective effect at the advanced stage of atherosclerosis.217 Additional work is needed to fully elucidate the complex role of NRF2 in these various models.
4.3. YAP/TAZ
Disturbed flow in a rat abdominal aorta crossclamping model induces YAP/TAZ activation to promote inflammation and atherogenesis by enhancing JNK (c-Jun N-terminal kinase) activity, whereas unidirectional SS inhibits YAP/TAZ activity by modulating the integrin–Gα13–RhoA pathway.203 Oscillatory SS by partial ligation of the carotid artery activates integrin α5β1 and its downstream kinase c-Abl in ECs to induce phosphorylation of YAP at Y357 and strong, continuous YAP nuclear translocation, thereby promoting the proatherogenic responses.145 Interestingly, a study using zebrafish embryos and an in vitro parallel plate–type apparatus found that short-term unidirectional laminar flow (15 dyne/cm2 for 10 min) increases the nuclear localization of YAP in a LATS1/2-independent but an angiomotin-regulated manner.32 Furthermore, mechanoregulated YAP/TAZ transcriptional activity is inhibited by interaction of the ARID1A-containing SWI/SNF complex and YAP/TAZ inside the nucleus, while nuclear F-actin competitively binds to SWI/SNF to interfere with the formation of the ARID1A–SWI/SNF–YAP complex and facilitates association of YAP/TAZ with TEAD (TEA domain transcription factor (TEAD).218 The identification of the transcription factors YAP and TAZ as EC mechanotransducers has helped to clarify how mechanical cues are sensed and transduced at the molecular level to regulate gene expression in atherosclerosis. However, the crosstalk of YAP/TAZ activation with endothelial apoptosis and enhanced lipid uptake in the vessel remains poorly understood.
4.4. BMPs
Laminar SS inhibits expression of BMP4 in ECs to exert antiatherogenic effects, while oscillatory SS activates BMP4 in atherosclerotic lesions, associating with NF-κB–mediated increase of ICAM-1 expression and monocyte adhesion.219,220 In the experimentally stenosed rat abdominal aorta and a parallel-plate flow chamber, atheroprone flow induces sustained activation of bone morphogenetic protein receptor (BMPR)–specific SMAD1/5 in ECs through activation of mammalian target of rapamycin and p70S6 kinase, leading to upregulation of cyclin A and downregulation of p21CIP1 and p27KIP1 and, hence, EC cycle progression.221 Low SS activates SMAD2/3 to regulate inward remodelling in atherosclerotic vessels through the type I TGF-β family receptor ALK5 and the transmembrane protein neuropilin-1, which together increase sensitivity to circulating BMP9.222 Interestingly, disturbed flow by rotation of a Teflon cone stimulates ECs to coexpress BMP antagonists and BMP4, the balance of which controls inflammation in atherosclerosis.223
4.5. Notch
Generally, laminar SS activates endothelial Notch1 and leads to polarization and Notch1 intracellular domain (NICD) translocation, thereby promoting stabilization of endothelial functions.224 Independent of canonical Notch signalling, high SS induces the cleavage of Notch1 and formation of a mechanosensory junctional complex consisting of Notch 1 transmembrane domain (TMD), the transmembrane protein tyrosine phosphatase LAR, and VE-cadherin, which further recruits the RAC1 guanidine exchange factor TRIO and RAC1 to control downstream cortical actin, thus resulting in barrier reinforcement.225 Recent work examining both postnatal retina neovascularization and cultured ECs in a parallel plate flow chambers describes a novel force-responsive pathway, in which arterial shear activates Notch signalling and causes upregulation of GJA4 (commonly known as connexin37) and the downstream cell cycle inhibitor CDKN1B (p27), promoting EC cycle arrest to enable arterial gene expression.226 However, evidence also showed that low SS by cast-induced stenosis of common carotid artery activates the Notch1 signalling pathway via caveolin-1 and that inhibition of Notch1 significantly alleviates low SS–induced plaque formation and inflammatory response through NF-κB.227 Together, these observations suggest that the effects of flow-induced Notch1 activation on EC physiology are highly context dependent.
4.6. WNT
Loss of WNT5a/WNT11 enhances endothelial shear sensitivity, resulting in axial polarization and migration against flow under low shear conditions.228 Canonical WNT signalling is implicated in oscillatory SS-induced angiopoietin-2 expression, which controls vascular development and repair.229 Consequently, inhibition of canonical WNT signalling suppresses systemic inflammation and atherosclerosis progression in angiotensin II–infused ApoE−/− mice.230 In addition, atheroprone flow in ApoE−/− mice and by a cone-and-plate system constitutively induces endothelial β-catenin nuclear localization, T-cell-specific transcription factor (TCF) activation and downstream expression of the proinflammatory molecule fibronectin to precede atherosclerosis lesion development, acting through the PECAM-1/GSK-3β pathway.231 As might be expected, a recent study involving multiple, single laboratory microarray analyses of shear force-responsive endothelial gene expression suggests that there is cross talk among WNT, TGF-β, and Notch signalling in ECs.232
4.7. NF-κB
The transcription factor NF-κB family, consisting of RelA (p65), RelB, c-Rel, and the precursor proteins NF-κB1 (p105) and NF-κB2 (p100), is central to the cell’s response to disturbed flow and its activity regulates plaque progression.233 In a flow-altering, constrictive cuff model, atheroprone flow induces NF-κB activation, which increases the expression of proinflammatory molecules (e.g. ICAM-1, VCAM-1 and MCP-1). This is in part controlled by JNK-ATF2 signalling, which positively regulates the expression of the RelA NF-κB subunit in response to low/oscillatory SS and initiates focal arterial inflammation at atherosusceptible sites.234 Moreover, oscillatory SS, but not atheroprotective physiological SS, upregulates miR-34a expression to promote endothelial inflammation, which depends on increased acetylation of RelA at residue Lys310.235 Additionally, in the partially ligated ApoE−/− mice model and in vitro orbital shaking or a parallel plate flow system, low SS–mediated NF-κB activation leads to HIF-1α accumulation, which promotes lesion initiation by enhancing inflammation and inducing excessive EC proliferation.35
4.8. MAPKs
MAPK pathways are involved in converting mechanical cues into a wide range of cellular responses. Using a parallel-plate flow chamber, short-term laminar SS exerted on ECs (30 min) triggers an eightfold increase of JNK activity compared to cells in static culture, resulting in actin remodelling and cell alignment.236 However, other studies showed that laminar flow inhibits the activation of JNK induced by inflammatory cytokines (e.g. TNF-α and IL-1) through the MEK5–BMK1/ERK5 pathway, which inhibits the MAP kinase kinase kinase (MAPKKK) and apoptosis signal–regulating kinase (ASK1) by inducing thioredoxin-interacting protein.237–239 In-depth research provided an explanation for this discrepancy in the response of JNK to laminar SS generated in a parallel plate flow chamber. Hahn et al. showed that JNK activation in response to laminar and long-term oscillatory flow is matrix specific, preferentially occurring at high-fibronectin deposition sites, which are regulated by the new binding of integrin with fibronectin as well as the downstream MAPK kinases MKK4 and p21-activated kinase.240
4.9. PI3K/AKT
Laminar flow in a cone–plate viscometer induces eNOS phosphorylation and AKT activation via a PI3K-dependent pathway and promotes NO production and an antiatherogenic phenotype in ECs.241 There is an initial rapid Src kinase-dependent and VEGFR2-dependent tyrosine phosphorylation of Grb2-associated binder 1 (Gab1) in response to laminar flow (12 dynes/cm2). Subsequently, Gab1 interacts with PI3K subunit p85 to activate PI3K/AKT/eNOS signalling.242 Under conditions of perturbed flow in a parallel-plate flow chamber, pretreating ECs with PI3K inhibitors LY294002 suppresses the association of HDAC-1/2/3 with NRF2 and HDAC-3/5/7 with MEF2 and its deacetylation, thereby upregulating NQO1 and KLF2 expression in ECs.213
In addition, PI3K has been implicated in SS–induced integrin activation in ECs. Using a monoclonal antibody Fab fragment (WOW-1) that binds selectively to high-affinity αv integrins, studies revealed that treatment with PI3K inhibitors LY294002 and wortmannin completely blocks integrin activation.152,243 Interestingly, the PI3K/AKT pathway can converge with the same integrins that the MAPKs interact with and can lead to activation of eNOS.123,141
Collectively, studies to date have revealed a complex signalling network by which different hemodynamic forces impact on EC functions and atherogenesis. Although informative, a long list of mechanosensors has accumulated, and the mechanisms for some of these remain to be elucidated. In addition, different mechanosensing elements across the cell are integrated and form molecular circuits that coordinate the cellular responses to mechanical cues. More work is needed to deconvolve the signal transduction networks to identify better targets for the treatment of vascular diseases.
5. Pharmacological targeting of mechanotransduction pathways in atherosclerosis
As described above, many mechanotransduction pathways are involved in endothelial responses to flow and SS–induced atherosclerosis. In this section, we overview the current antiatherosclerotic agents that can alter mechanotransduction in ECs (Table 2).
Table 2.
Pharmacological treatments based on mechanotransduction pathways
| Drug | Target | Pathway | Status | Ref |
|---|---|---|---|---|
| Statins | (+) KLF2 (+) NRF2 (−) YAP/TAZ (−) NF-κB |
(+) MEK5/ERK5/MEF2 (+) NRF2/HO-1 (−) RhoA/YAP/TAZ (−) PI3K/AKT |
Approved | 203,244–248 |
| Suberanilohydroxamic acid (SAHA, vorinostat) | (+) KLF2 | (+) MEF2 | Approved for T cell lymphoma Preclinical trials |
249 |
| Montelukast (singular) | (+) KLF2 | (+) ERK5 | Approved for asthma and allergies Clinical trials (NCT00351364) |
250,251 |
| Liraglutide (Victoza, Saxenda) |
(+) KLF2 (−) NF-κB |
(+) ERK5 (−) NF-κB |
Approved for diabetes and weight management Clinical trials (NCT04881110; NCT04146155) |
252–254 |
| Metformin | (+) KLF2 | (+) AMPK/HDAC5 (+) Autophagy |
Approved for diabetes Clinical trials (NCT03962686) |
255,256 |
| Laquinimod | (+) KLF2 | (+) ERK5 | Clinical trials (NCT01047319) | 257 |
| Dimethyl fumarate (Tecfidera, Fumaderm) | (+) NRF2 | (+) NRF2/ARE | Approved for psoriasis, multiple sclerosis Preclinical trials |
258 |
| Bosutinib | (−) YAP | (−) Integrin α5β1/c-Abl/YAP | Approved for chronic myeloid leukaemia Preclinical trials |
145 |
| Verteporfin | (−) YAP/TAZ | (−) YAP/TEAD | Approved for macular degeneration Preclinical trials |
259,260 |
| Methotrexate | (−) YAP/TAZ | (−) YAP/TAZ | Approved for psoriasis Clinical trials (NCT02312219; NCT02576067) |
33 |
| Niacin | (−) YAP/TAZ (−) NF-κB |
(−) YAP/TAZ (−) Notch1/NF-κB p65 |
Approved | 203,261,262 |
| Apelin agonist (BMS-986224) | (−) YAP/TAZ | (−) YAP/TAZ | Clinical trials (NCT03281122) | 263 |
| ApoA-I Milano (MDCO-216) | (−) YAP/TAZ | (−) YAP/TAZ | Clinical trials (NCT02678923) | 264,265 |
| Aspirin | (−) NF-κB | (−) NF-κB/CX3CL1 | Approved | 266–268 |
| Canakinumab | (−) IL-1β | (−) Nck1/IRAK-1/NF-κB | Approved for cryopyrin-associated periodic syndromes and Still’s disease Clinical trials (NCT00995930; NCT01327846) |
269,270 |
| Anakinra | (−) IL-1β | (−) p38 MAPK/NF-κB | Approved for rheumatoid arthritis Clinical trials (NCT02126280) |
269,271 |
| Investigational naturally occurring compounds | Target | Pathway | Status | Ref |
|---|---|---|---|---|
| Vinpocetine | (−) NF-κB | (−) IKK activity (+) AKT dephosphorylation |
Clinical trials (NCT02878772) | 272–274 |
| Resveratrol | (+) KLF2 (+) NRF2 (−) NF-κB |
(+) AMPK/ERK5/MEF2 (+) SIRT1/MEK5/MEF2 (+) NRF2/ARE (+) SIRT1/RelA/p65 deacetylation |
Approved | 275–280 |
| Tannic acid | (+) KLF2 | (+) MEK5/MEF2 | Preclinical trials | 281 |
| Sulforaphane | (+) NRF2 | (−) p38/VCAM-1 | Clinical trials (NCT01114399) | 212,282–284 |
| Colchicine | (−) NF-κB | (−) NF-κB/IκB | Clinical trials (NCT02162303; NCT05347316) |
285 |
| Diosgenin | (−) NF-κB | (−) IKKβ/NF-KB | Preclinical trials | 286 |
(+), activation; (−), inhibition; KLF2/4, Krüppel-like factor 2/4; NRF2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor-κB; YAP/TAZ, Yes-associated protein/transcriptional coactivator with PDZ-binding motif; TEAD: TEA-domain transcription factor; MEK5, MAP/ERK kinase 5; ERK5, extracellular signal-regulated kinases 5; MEF2, myocyte enhancer factor 2; HO-1, heme oxygenase-1; PI3K, phosphoinositide 3-kinases; AKT, serine/threonine protein kinase B; AMPK, AMP-activated protein kinase; HDAC5, histone deacetylase 5; IRAK-1, interleukin-1 receptor–associated kinase 1; ARE, antioxidant response element; c-Abl, cytosolic nonreceptor protein kinase; IKK, IκB kinase; SIRT1, sirtuin1; VCAM-1, vascular cell adhesion protein-1; IKKβ, nuclear factor kappa B kinase beta subunit.
5.1. Pharmacological targets for the KLF2 pathway
Because KLF2 is a master regulator of SS–induced EC homeostasis, it represents a promising pharmacological target for atherosclerosis treatment. Statins are the most widely used agents for atherosclerotic vascular diseases and act by decreasing circulating lipids and inflammatory factors. This involves KLF2 pathways, as genetic silencing of KLF2 reduces the beneficial effects of statins, including eNOS and TM production.244 Statins also affect ERK5 and MEF2-dependent signalling.245,246 Likewise, resveratrol and tannic acid promote endothelial vasoprotection in part by increasing KLF2 expression in a MEK5/MEF2-dependent, ERK5-independent pathway.279–281 Laquinimod, an experimental immunomodulator, has a clinical potential application as an antiatherosclerotic agent. The mechanism of action of laquinimod is to reduce the expression of adhesion molecules (VCAM-1 and E-selectin) and central inflammatory cytokines and chemokines (IL-6 and MCP-1) via upregulating KLF2 expression in an ERK5-dependent manner.257
Additionally, several other well-studied drugs exert cardioprotective effects by KLF2 induction. For example, suberanilohydroxamic acid (SAHA, also known as vorinostat or MK0683), an approved anticancer drug, has been shown to activate KLF2, reduce monocyte adhesion, and limit vascular inflammation and atherosclerosis in a MEF2–dependent manner.249 Montelukast—another drug commonly used for treatment of inflammatory diseases in the airway—can activate KLF2 in an ERK5–dependent manner, leading to reduced monocyte adhesion and suppression of adhesion molecules (VCAM-1 and E-selectin) in the early stages of atherosclerosis.250,251 Similarly, liraglutide, a clinically approved glucagon-like peptide 1 (GLP-1) receptor agonist, can reduce endothelial inflammation via ERK5-dependent KLF2 activation.252,253 Metformin pretreatment can alleviate endothelial inflammatory responses, partially ascribed to AMP–activated protein kinase (AMPK) activation–mediated HDAC5 phosphorylation and KLF2 upregulation.255 Other cellular programs may also be involved, as metformin-induced upregulation of KLF2 can inhibit foam cell formation and alleviate atherosclerosis in ApoE−/− mice by enhancing autophagy.256 However, their therapeutic effectiveness for atherosclerosis and other vascular diseases warrants further assessment of clinical efficacy.
5.2. Pharmacological targets for the NRF2 pathway
Despite the controversial role of NRF2 in atherogenesis in preclinical animal models, pharmacological activation of NRF2 appears to be generally related to atheroprotection. Statins activate the NRF2 pathway and induce a significant increase of cytoprotective genes such as HO-1.247 Resveratrol enhances NRF2 activity in a dose-dependent manner and significantly upregulates its target genes NQO1, GCLC, and HO-1, thereby conferring endothelial protective effects.275 Sulforaphane can repress atherosclerosis progression and improve endothelial dysfunction.212,282–284 The atheroprotective mechanism involves NRF2–mediated inhibition of p38–VCAM-1 signalling in ECs.212 In addition, dimethyl fumarate, a drug commonly used in multiple sclerosis treatment, can significantly reduce the plaque area through the NRF2/ARE pathway.258
5.3. Pharmacological targets for the YAP/TAZ/TEAD pathway
Pharmacological inhibition of YAP/TAZ signalling can prevent atherosclerosis, and statins are among the most potent YAP inhibitors of the 640 clinically applied drugs.287 Statins suppress YAP/TAZ transactivation through inhibition of RhoA, an upstream activator of YAP/TAZ, thus reducing EC proliferation and inflammation caused by disturbed flow.203 Moreover, constitutive activation of YAP/TAZ in ECs can partially antagonize the anti-inflammatory effect of simvastatin, further supporting that YAP/TAZ inhibition contributes to antiatherogenic effects of statins.31 Bosutinib, a tyrosine kinase inhibitor, profoundly reduces YAPY357 phosphorylation through blunting oscillatory SS– or fibronectin-mediated activation of integrin α5β1 and c-Abl, hence, completely inhibiting lesion formation in aortic arch but not thoracic aorta in ApoE−/− mice.145 Verteporfin, a second-generation photosensitizer, is the first identified pharmacological inhibitor of YAP that acts by disrupting the interaction between YAP and TEAD.259 Intra-arterial administration of verteporfin concurrent with photoactivation can lead to rapid and high accumulation of verteporfin in the plaque, which then inhibits atherosclerosis.260
In addition, methotrexate, a chemotherapeutic and anti-inflammatory drug used to treat rheumatoid arthritis, exhibits atheroprotective effects by interfering with endothelial YAP/TAZ activation under disturbed flow, reducing expression of YAP/TAZ–associated inflammatory genes, and decreasing monocyte adhesion.33 Apart from these drugs, other compounds such as niacin,203,261 apelin,263 and ApoA-I Milano (a mutational variant of ApoA1)264,265 have shown promise in preclinical and clinical studies, acting through YAP/TAZ to reduce inflammatory effects. However, the role of Apelin in atherosclerosis is controversial. Other studies have found that Apelin could induce adhesion of monocytes–ECs by inducing the expression of adhesion molecules and chemokines through activation of the PI3K and NF-κB/JNK signal pathway.288,289
5.4. Pharmacological targets for the NF-κB pathway
The NF-κB pathway is a representative mechanism that is enhanced by disturbed flow. Hence, endothelium-restricted inhibition of NF-κB is also a potential therapeutic strategy against atherosclerosis. Statins can prevent TNF-α–induced NF-κB activation in ECs through a mechanism independent of the classical IKK cascade; the observed suppression of NF-κB depends on impaired transport of the p65 subunit of NF-κB into the nucleus, induced by decreased endothelial AKT phosphorylation.248 Similarly, resveratrol effectively interferes with proinflammatory cytokine–induced NF-κB activation through the blockade of p65 subunit phosphorylation and consequent prevention of NF-κB nuclear translocation.276,277 Moreover, resveratrol regulates the transcriptional activity of NF-κB via activation of sirtuin1 (SIRT1), which is a nicotinamide adenosine dinucleotide-dependent HDAC.278 SIRT1 physically interacts with the RelA/p65 subunit of the NF-κB complex, leading to RelA/p65 deacetylation at lysine 310 and NF-κB transcriptional inhibition.290
Colchicine, an anti-inflammatory compound showing potential benefits for CVDs, can decrease the expression of NF-κB in ECs or NF-κB in nuclear fraction and thereby regulates the expression of inflammatory cytokines.285 Aspirin and diosgenin, antithrombotic agents that can reduce platelet activation, exert antiatherogenic effects through NF-κB inhibition.267,268,286 Low-dose aspirin administration inhibits NF-κB activation and subsequent expression of fractalkine (CX3CL1), another NF-κB target gene important in atherosclerosis. This reduces atherosclerotic plaque size in hyperlipidaemic mice.266 However, high-dose aspirin usage is associated with gastrointestinal bleeding. GLP-1 hormone is involved in immunomodulation of atherosclerosis.291 Liraglutide promotes eNOS expression and suppresses endothelin-1 (ET-1) expression by inhibiting NF-κB phosphorylation and its translocation from the cytoplasm to the nucleus.254 Canakinumab and anakinra, immunomodulators that act through IL-1β suppression, reduced cardiovascular events in a high-risk patient cohort. The protective effects were ascribed to suppressed activation of NF-κB and decreased production of proinflammatory cytokines triggered by the Toll/interleukin-1 receptor homology domain and the adaptor protein myeloid differentiation primary response 88 (MyD88).269–271
Additionally, niacin, a water-soluble vitamin that acts as a broad-spectrum lipid-regulating medication, has been shown to inhibit inflammation by suppressing NF-κB p65 and Notch1 expression in ECs.262 Vinpocetine can attenuate high-fat diet–induced atherosclerosis in ApoE−/− mice.272,273 Vinpocetine inhibits NF-κB–mediated inflammatory responses by directly affecting IKK activity, independent of its well-known action on cyclic nucleotide phosphodiesterase 1 (PDE-1).274 AKT dephosphorylation in part contributes to the inhibitory effects of vinpocetine on the NF-κB cascade.273
6. Conclusions
Interactions between flowing blood and ECs play important roles in the regulation of vascular integrity and homeostasis. Generally, ECs maintain quiescence in laminar, pulsatile flow but respond to disturbed flow by initiating programs for structural and functional adaption. Specifically, sustained SS alternation leads to EC dysfunction in a series of changes that involve the morphology, cell turnover rate (proliferation and apoptosis), macromolecular permeability, and inflammation.7
CVDs and their clinical sequelae remain the major cause of morbidity and mortality worldwide. The significance of mechanical stimuli in the physiopathology of CVDs such as atherosclerosis has catalyzed much research in the past decades. However, despite our increased understanding of the effects of blood flow on endothelial biology, numerous questions in this field remain unanswered. Dysregulation of mechanosensing mechanisms in ECs is implicated in a wide range of vascular diseases, especially in atherogenesis. Many recent studies discussed above indicate that drugs targeting key mechanosensitive transcription factors such as KLF2/4, NRF2, YAP/TAZ/TEAD, NF-κB, and others can produce antiatherosclerosis effects. Nevertheless, it remains challenging to translate findings from mechanobiological studies into interventions that actively target dysregulated mechanosensing mechanisms to treat diseased vasculature. This may be ascribed to the existence of multiple mechanotransduction mechanisms and complex interactions between distinct mechanisms. Thus, the interplay of redundant or complementary mechanotransduction pathways has to be viewed in a ‘systems biology’ context.
The vast majority of cellular response studies have been performed on cultured cells in vitro. In general, in vitro systems for studying endothelial biology are simplified, do not fully recapitulate the mechanics in biological systems, and require rigorous validation using in vivo methods. Novel experimental techniques such as microfabrication technology have greatly facilitated the study of cell mechanics and mechanobiology in vitro but still have limitations. For example, many of these studies use ECs adhered to artificial substrates such as PDMS, so vessel mechanics are not accurately reproduced. Other aspects of vessel mechanics are also generally lacking; ECs are exposed to not only FSS but also cyclic stretch, so techniques that incorporate appropriate tissue mechanics and fluid forces would improve physiological relevance. There are additional limitations related to the ability to integrate multidimensional in vivo components such as the ECM, mechanical stimulus, and chemical signals. Efforts to recapitulate the vessel wall structure in vitro, including the anatomy of the smooth muscle layers and pericytes, are promising, but still in the development stage.128 Finally, while the adhesion of circulating leukocytes, including monocytes, has been extensively studied using in vitro technologies, additional examination of the subsequent effects on the generation of foam cells and atherosclerosis is needed.
For in vivo models, there are exciting advances that allow control of flow in the microvasculature of live animals and the monitoring of specific biological responses including microvascular permeability and intracellular calcium.292,293 It is also possible to study the endothelial glycocalyx in vivo by using a variety of imaging methods.294 Such studies open the door for future investigations of flow-responsive events in vivo. However, access to the microvasculature is considerably easier than that to the medium and large arteries in which atherosclerosis develops; therefore, new tools need to be developed in order to provide the capability of monitoring flow-induced EC responses in larger arteries in vivo.
Multiple mechanosignalling pathways have been implicated in the regulation of EC function in physio/pathological conditions. Although informative, these hypothesis-based studies on the mechanisms that control focal lesion formation have only partially revealed the complexity of flow-induced pathways. Furthermore, there is mounting evidence that different mechanosensing mechanisms can interact with one another to orchestrate mechanotransduction signalling and cell function. For example, the interactions of KLF2 with NRF2,210,295,296 and NF-κB with HIF-1α,34,35 as well as KLF2/4 with NF-κB34 have been described recently. The current challenge is to determine how these systems work together, and to identify specific mechanosensitive pathways that can be targeted to alleviate or reverse vascular pathologies. A useful approach could be to determine how mechanotransduction occurs as an intracellular ‘systems’ response for each physiological or pathological context of interest, with appreciation of the potential redundancy of elements within the system. By discovering how ECs coordinate and integrate their response to mechanical signals, we can identify novel therapeutic targets to slow or reverse the progression of atherosclerosis.
The complexities of endothelial mechanobiology present both opportunities and challenges in the battle against CVDs. On one hand, new mechanosensitive pathways are being discovered and implicated in endothelial dysfunction during atherogenesis. These pathways may reveal new targets for preventing or reversing CVDs. On the other hand, the complexity of the system requires new experimental tools and conceptual advances to advance our understanding of how endothelial mechanobiology contributes to homeostasis in atheroprotective environments, but disease progression in atheroprone regions. This distinction is subtle, but key to the development of robust therapeutics with minimal toxicity.
Contributor Information
Xiaoli Wang, Institute of Biomedical Engineering, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, Sichuan 610041, China; Department of Cardiology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310020, China.
Yang Shen, Institute of Biomedical Engineering, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, Sichuan 610041, China.
Min Shang, Department of Cardiology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310020, China.
Xiaoheng Liu, Institute of Biomedical Engineering, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, Sichuan 610041, China.
Lance L Munn, Steele Laboratories, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Author contributions
X.W. (conceptualization: equal; writing—original draft: lead; writing—review & editing: equal), Y.S. (writing—review & editing: equal), M.S. (writing—review & editing: equal), X.L. (conceptualization: supporting; writing—review & editing: equal), L.L.M. (conceptualization: equal; writing—review & editing: lead).
Funding
The authors are funded by the National Natural Science Foundation of China (11932014, 31971239, 32071312 and 31870939; X.L. and Y.S.) and the National Institutes of Health (R21EB031982 and U01CA261842; L.L.M.)
Data availability
No new data were generated or analyzed in support of this manuscript.
References
- 1. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, Harding IC, Ebong EE, Cameron SJ, Stewart AG, Weng J. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 2021;73:924–967. [DOI] [PubMed] [Google Scholar]
- 2. Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H, Evans PC. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol 2020;17:52–63. [DOI] [PubMed] [Google Scholar]
- 3. He L, Zhang C-L, Chen Q, Wang L, Huang Y. Endothelial shear stress signal transduction and atherogenesis: from mechanisms to therapeutics. Pharmacol Therapeut 2022;235:108152. [DOI] [PubMed] [Google Scholar]
- 4. Fung YC. Biomechanics: circulation. Shock 1998;9:155. [Google Scholar]
- 5. Fung YC. Stress, strain, growth, and remodeling of living organisms. Z Angew Math Phys 1995;46:S469–S482. [Google Scholar]
- 6. Fung Y, Cowin S. Biomechanics: motion, flow, stress, and growth. J Appl Mech 1993;60:567. [Google Scholar]
- 7. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humphrey JD, Schwartz MA. Vascular mechanobiology: homeostasis, adaptation, and disease. Annu Rev Biomed Eng 2021;23:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol 2017;219:382–408. [DOI] [PubMed] [Google Scholar]
- 10. Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 2016;13:210–220. [DOI] [PubMed] [Google Scholar]
- 11. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 2009;6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Souilhol C, Harmsen MC, Evans PC, Krenning G. Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc Res 2018;114:565–577. [DOI] [PubMed] [Google Scholar]
- 13. Maurya MR, Gupta S, Li JY-S, Ajami NE, Chen ZB, Shyy JYJ, Chien S, Subramaniam S. Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proc Natl Acad Sci U S A 2021;118:e2023236118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Fang Y, Wu D. Mechanical forces and metabolic changes cooperate to drive cellular memory and endothelial phenotypes. Curr Top Membr 2021;87:199–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 2016;126:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang J, Pu Y, Zhang H, Xie L, He L, Zhang CL, Cheng CK, Huo Y, Wan S, Chen S, Huang Y, Lau CW, Wang L, Xia Y, Huang Y, Luo JY. KLF2 mediates the suppressive effect of laminar flow on vascular calcification by inhibiting endothelial BMP/SMAD1/5 signaling. Circ Res 2021;129:e87–e100. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Chen J, Tao X, Fu M, Cheng B, Chen X. Activation of GPR30 with G1 inhibits oscillatory shear stress-induced adhesion of THP-1 monocytes to HAECs by increasing KLF2. Aging 2021;13:11942–11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moonen JR, Chappell J, Shi M, Shinohara T, Li D, Mumbach MR, Zhang F, Nair RV, Nasser J, Mai DH, Taylor S, Wang L, Metzger RJ, Chang HY, Engreitz JM, Snyder MP, Rabinovitch M. KLF4 recruits SWI/SNF to increase chromatin accessibility and reprogram the endothelial enhancer landscape under laminar shear stress. Nat Commun 2022;13:4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han Y, He M, Marin T, Shen H, Wang WT, Lee TY, Hong HC, Jiang ZL, Garland T Jr, Shyy JY, Gongol B, Chien S. Roles of KLF4 and AMPK in the inhibition of glycolysis by pulsatile shear stress in endothelial cells. Proc Natl Acad Sci U S A 2021;118:e2103982118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishii T, Warabi E, Mann GE. Mechanisms underlying unidirectional laminar shear stress-mediated Nrf2 activation in endothelial cells: amplification of low shear stress signaling by primary cilia. Redox Biol 2021;46:102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Psefteli PM, Kitscha P, Vizcay G, Fleck R, Chapple SJ, Mann GE, Fowler M, Siow RC. Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells. Redox Biol 2021;38:101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niu N, Xu S, Xu Y, Little PJ, Jin Z-G. Targeting mechanosensitive transcription factors in atherosclerosis. Trends Pharmacol Sci 2019;40:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwak BR, Back M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 2014;35:3013–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 2013;99:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peng Z, Shu B, Zhang Y, Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol 2019;39:e233–e243. [DOI] [PubMed] [Google Scholar]
- 26. Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 2008;36:554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng H, Zhong W, Wang L, Zhang Q, Ma X, Wang Y, Wang S, He C, Wei Q, Fu C. Effects of shear stress on vascular endothelial functions in atherosclerosis and potential therapeutic approaches. Biomed Pharmacother 2023;158:114198. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Ren P, Li Q, Umar SA, Yang T, Dong Y, Yu F, Nie Y. Low shear stress upregulates CX3CR1 expression by inducing VCAM-1 via the NF-κB pathway in vascular endothelial cells. Cell Biochem and Biophys 2020;78:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang F, Feng T, Li J, Zhang H, Wang Q, Chen Y, Wang G, Shen Y, Liu X. Cathepsin K contributed to disturbed flow-induced atherosclerosis is dependent on integrin-actin cytoskeleton–NF–κB pathway. Genes Dis 2022;10:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qu D, Wang L, Huo M, Song W, Lau CW, Xu J, Xu A, Yao X, Chiu JJ, Tian XY, Huang Y. Focal TLR4 activation mediates disturbed flow-induced endothelial inflammation. Cardiovasc Res 2020;116:226–236. [DOI] [PubMed] [Google Scholar]
- 31. Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 2016;113:11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, Ando K, Miyazaki T, Yokota Y, Schmelzer E, Belting HG, Affolter M, Lecaudey V, Mochizuki N. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev Cell 2017;40:523–536.e6. [DOI] [PubMed] [Google Scholar]
- 33. Liu D, Lv H, Liu Q, Sun Y, Hou S, Zhang L, Yang M, Han B, Wang G, Wang X, Du W, Nie H, Zhang R, Huang X, Hou J, Yu B. Atheroprotective effects of methotrexate via the inhibition of YAP/TAZ under disturbed flow. J Transl Med 2019;17:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, Mutlu GM. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 2017;6:e25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng S, Bowden N, Fragiadaki M, Souilhol C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, Thompson AAR, Jo H, Weber C, Ridger V, Schober A, Evans PC. Mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arterioscler Thromb Vasc Biol 2017;37:2087–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X, Weintraub DS, Fulton DJR. Beyond impressions:. how altered shear stress connects hypoxic signaling to endothelial inflammation. Arterioscler Thromb Vasc Biol 2017;37:1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez Matthew W, Kim Jonathan H, Shah Ankit B, Phelan D, Emery Michael S, Wasfy Meagan M, Fernandez Antonio B, Bunch TJ, Dean P, Danielian A, Krishnan S, Baggish Aaron L, Eijsvogels Thijs MH, Chung Eugene H, Levine Benjamin D. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease. J Am Coll Cardiol 2021;78:1453–1470. [DOI] [PubMed] [Google Scholar]
- 38. Tremblay JC, Pyke KE. Flow-mediated dilation stimulated by sustained increases in shear stress: a useful tool for assessing endothelial function in humans? Am J Physiol Heart Circ Physiol 2017;314:H508–H520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atkinson CL, Carter HH, Dawson EA, Naylor LH, Thijssen DHJ, Green DJ. Impact of handgrip exercise intensity on brachial artery flow-mediated dilation. Eur J Appl Physiol 2015;115:1705–1713. [DOI] [PubMed] [Google Scholar]
- 40. Grzelak P, Olszycki M, Majos A, Czupryniak L, Strzelczyk J, Stefańczyk L. Hand exercise test for the assessment of endothelium-dependent vasodilatation in subjects with type 1 diabetes. Diabetes Technol The 2010;12:605–611. [DOI] [PubMed] [Google Scholar]
- 41. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 2018;15:731–743. [DOI] [PubMed] [Google Scholar]
- 43. Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 2017;97:495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blackman BR, Barbee KA, Thibault LE. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Ann Biomed Eng 2000;28:363–372. [DOI] [PubMed] [Google Scholar]
- 45. Bussolari SR, Dewey CF Jr, Gimbrone MA Jr. Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum 1982;53:1851–1854. [DOI] [PubMed] [Google Scholar]
- 46. Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 1981;103:177–185. [DOI] [PubMed] [Google Scholar]
- 47. Schnittler HJ, Franke RP, Akbay U, Mrowietz C, Drenckhahn D. Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells. Am J Physiol 1993;265:C289–C298. [DOI] [PubMed] [Google Scholar]
- 48. White LA, Stevenson EV, Yun JW, Eshaq R, Harris NR, Mills DK, Minagar A, Couraud PO, Alexander JS. The assembly and application of ‘shear rings’: a novel endothelial model for orbital, unidirectional and periodic fluid flow and shear stress. J Vis Exp 2016;16:e54632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 2007;292:H1209–H1224. [DOI] [PubMed] [Google Scholar]
- 50. Nauman EA, Risic KJ, Keaveny TM, Satcher RL. Quantitative assessment of steady and pulsatile flow fields in a parallel plate flow chamber. Ann Biomed Eng 1999;27:194–199. [DOI] [PubMed] [Google Scholar]
- 51. Sobey IJ, Drazin PG. Bifurcations of two-dimensional channel flows. J Fluid Mech 1986;171:263–287. [Google Scholar]
- 52. Janakiraman V, Sastry S, Kadambi JR, Baskaran H. Experimental investigation and computational modeling of hydrodynamics in bifurcating microchannels. Biomed Microdevices 2008;10:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim TH, Lee JM, Ahrberg CD, Chung BG. Development of the microfluidic device to regulate shear stress gradients. Biochip J 2018;12:294–303. [Google Scholar]
- 54. Jeong GS, Han S, Shin Y, Kwon GH, Kamm RD, Lee SH, Chung S. Sprouting angiogenesis under a chemical gradient regulated by interactions with an endothelial monolayer in a microfluidic platform. Anal Chem 2011;83:8454–8459. [DOI] [PubMed] [Google Scholar]
- 55. Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 2011;108:15342–15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A 2013;110:6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levesque MJ, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis 1986;6:220–229. [DOI] [PubMed] [Google Scholar]
- 58. Hutchison KJ. Endothelial cell morphology around graded stenoses of the dog common carotid artery. J Vasc Res 1991;28:396–406. [DOI] [PubMed] [Google Scholar]
- 59. Mohri Z, Rowland EM, Clarke LA, De Luca A, Peiffer V, Krams R, Sherwin SJ, Weinberg PD. Elevated uptake of plasma macromolecules by regions of arterial wall predisposed to plaque instability in a mouse model. PLoS One 2014;9:e115728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 2009;297:H1535–H1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mitra R, Qiao J, Madhavan S, O'Neil GL, Ritchie B, Kulkarni P, Sridhar S, van de Ven AL, Kemmerling EMC, Ferris C, Hamilton JA, Ebong EE. The comparative effects of high fat diet or disturbed blood flow on glycocalyx integrity and vascular inflammation. Transl Med Commun 2018;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Merino H, Parthasarathy S, Singla DK. Partial ligation-induced carotid artery occlusion induces leukocyte recruitment and lipid accumulation–a shear stress model of atherosclerosis. Mol Cell Biochem 2013;372:267–273. [DOI] [PubMed] [Google Scholar]
- 63. Bai H, Sadaghianloo N, Gorecka J, Liu S, Ono S, Ramachandra AB, Bonnet S, Mazure NM, Declemy S, Humphrey JD, Dardik A. Artery to vein configuration of arteriovenous fistula improves hemodynamics to increase maturation and patency. Sci Transl Med 2020;12:eaax7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong CY, de Vries MR, Wang Y, van der Vorst JR, Vahrmeijer AL, van Zonneveld AJ, Roy-Chaudhury P, Rabelink TJ, Quax PH, Rotmans JI. Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J Vasc Surg 2014;59:192–201.e1. [DOI] [PubMed] [Google Scholar]
- 65. Ene-Iordache B, Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant 2012;27:358–368. [DOI] [PubMed] [Google Scholar]
- 66. Rajabi-Jagahrgh E, Krishnamoorthy MK, Wang Y, Choe A, Roy-Chaudhury P, Banerjee RK. Influence of temporal variation in wall shear stress on intima-media thickening in arteriovenous fistulae. Semin Dial 2013;26:511–519. [DOI] [PubMed] [Google Scholar]
- 67. Franzoni M, Cattaneo I, Longaretti L, Figliuzzi M, Ene-Iordache B, Remuzzi A. Endothelial cell activation by hemodynamic shear stress derived from arteriovenous fistula for hemodialysis access. Am J Physiol Heart Circ Physiol 2016;310:H49–H59. [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model–anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant 2008;23:525–533. [DOI] [PubMed] [Google Scholar]
- 69. Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol 2011;22:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sho E, Komatsu M, Sho M, Nanjo H, Singh TM, Xu C, Masuda H, Zarins CK. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol 2003;75:1–11. [DOI] [PubMed] [Google Scholar]
- 71. Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 2014;34:2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim DW, Gotlieb AI, Langille BL. In vivo modulation of endothelial F-actin microfilaments by experimental alterations in shear stress. Arteriosclerosis 1989;9:439–445. [DOI] [PubMed] [Google Scholar]
- 73. Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med 2009;41:19–28. [DOI] [PubMed] [Google Scholar]
- 74. Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol 2013;33:2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Heo KS, Fujiwara K, Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J 2011;75:2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Citi S. The mechanobiology of tight junctions. Biophys Rev 2019;11:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lin K, Hsu PP, Chen Benjamin P, Yuan S, Usami S, Shyy John YJ, Li Y-S, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A 2000;97:9385–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zeng L, Zhang Y, Chien S, Liu X, Shyy JY. The role of p53 deacetylation in p21Waf1 regulation by laminar flow. J Biol Chem 2003;278:24594–24599. [DOI] [PubMed] [Google Scholar]
- 79. Davies PF. Spatial hemodynamics, the endothelium, and focal atherogenesis: a cell cycle link? Circ Res 2000;86:114–116. [DOI] [PubMed] [Google Scholar]
- 80. Charbonier FW, Zamani M, Huang NF. Endothelial cell mechanotransduction in the dynamic vascular environment. Adv Biosyst 2019;3:1800252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Song J, Hu B, Qu H, Wang L, Huang X, Li M, Zhang M. Upregulation of angiotensin converting enzyme 2 by shear stress reduced inflammation and proliferation in vascular endothelial cells. Biochem Biophys Res Commun 2020;525:812–818. [DOI] [PubMed] [Google Scholar]
- 82. Regnault V, Challande P, Pinet F, Li Z, Lacolley P. Cell senescence: basic mechanisms and the need for computational networks in vascular ageing. Cardiovasc Res 2021;117:1841–1858. [DOI] [PubMed] [Google Scholar]
- 83. Michel JB, Lagrange J, Regnault V, Lacolley P. Conductance artery wall layers and their respective roles in the clearance functions. Arterioscler Thromb Vasc Biol 2022;42:e253–e272. [DOI] [PubMed] [Google Scholar]
- 84. Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res 2017;120:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Franck G, Even G, Gautier A, Salinas M, Loste A, Procopio E, Gaston A-T, Morvan M, Dupont S, Deschildre C, Berissi S, Laschet J, Nataf P, Nicoletti A, Michel J-B, Caligiuri G. Haemodynamic stress-induced breaches of the arterial intima trigger inflammation and drive atherogenesis. Eur Heart J 2019;40:928–937. [DOI] [PubMed] [Google Scholar]
- 86. Guo ZY, Yan ZQ, Bai L, Zhang ML, Jiang ZL. Flow shear stress affects macromolecular accumulation through modulation of internal elastic lamina fenestrae. Clin Biomech 2008;23:S104–S111. [DOI] [PubMed] [Google Scholar]
- 87. Lu Q, Zemskov EA, Sun X, Wang H, Yegambaram M, Wu X, Garcia-Flores A, Song S, Tang H, Kangath A, Cabanillas GZ, Yuan JX, Wang T, Fineman JR, Black SM. Activation of the mechanosensitive Ca(2+) channel TRPV4 induces endothelial barrier permeability via the disruption of mitochondrial bioenergetics. Redox Biol 2021;38:101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang K, Chen Y, Zhang T, Huang L, Wang Y, Yin T, Qiu J, Gregersen H, Wang G. A novel role of Id1 in regulating oscillatory shear stress-mediated lipid uptake in endothelial cells. Ann Biomed Eng 2018;46:849–863. [DOI] [PubMed] [Google Scholar]
- 89. Butler MJ, Down CJ, Foster RR, Satchell SC. The pathological relevance of increased endothelial glycocalyx permeability. Am J Pathol 2020;190:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pillinger NL, Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth Intens Care 2017;45:295–307. [DOI] [PubMed] [Google Scholar]
- 91. Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 2010;105:687–701. [DOI] [PubMed] [Google Scholar]
- 92. Zhao P, Yao QZ, Ao LH, Jarrett M, Zhang PJ, The E, Zhai YF, Fullerton D, Meng XZ. Shear stress-induced endothelial inflammatory response and EndMT promote adverse remodeling in a microengineered coronary-artery-on-a-chip. Circulation 2020;142:A14895. [Google Scholar]
- 93. Ma J, Sanchez-Duffhues G, Goumans M-J, Ten Dijke P. TGF-β-induced endothelial to mesenchymal transition in disease and tissue engineering. Front Cell Dev Biol 2020;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 2015;125:4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mehta V, Pang KL, Givens CS, Chen Z, Huang J, Sweet DT, Jo H, Reader JS, Tzima E. Mechanical forces regulate endothelial-to-mesenchymal transition and atherosclerosis via an Alk5-Shc mechanotransduction pathway. Sci Adv 2021;7:eabg5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mahmoud MM, Kim HR, Xing R, Hsiao S, Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire R, Ariaans M, Francis SE, Weinberg PD, van der Heiden K, Jones EA, Chico TJ, Ridger V, Evans PC. TWIST1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circ Res 2016;119:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev 2019;99:1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wesseling M, Sakkers TR, de Jager SCA, Pasterkamp G, Goumans MJ. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vasc Pharmacol 2018;106:1–8. [DOI] [PubMed] [Google Scholar]
- 99. Man S, Sanchez Duffhues G, Ten Dijke P, Baker D. The therapeutic potential of targeting the endothelial-to-mesenchymal transition. Angiogenesis 2019;22:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science 1985;227:1477–1479. [DOI] [PubMed] [Google Scholar]
- 101. Yin W, Rouf F, Shanmugavelayudam SK, Rubenstein DA. Endothelial cells modulate platelet response to dynamic shear stress. Cardiovasc Eng Techn 2014;5:145–153. [Google Scholar]
- 102. Peghaire C, Dufton NP, Lang M, Salles-Crawley II, Ahnström J, Kalna V, Raimondi C, Pericleous C, Inuabasi L, Kiseleva R, Muzykantov VR, Mason JC, Birdsey GM, Randi AM. The transcription factor ERG regulates a low shear stress-induced anti-thrombotic pathway in the microvasculature. Nat Commun 2019;10:5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Giri H, Panicker SR, Cai X, Biswas I, Weiler H, Rezaie AR. Thrombomodulin is essential for maintaining quiescence in vascular endothelial cells. Proc Natl Acad Sci U S A 2021;118:e2022248118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nayak L, Shi H, Atkins GB, Lin Z, Schmaier AH, Jain MK. The thromboprotective effect of bortezomib is dependent on the transcription factor Kruppel-like factor 2 (KLF2). Blood 2014;123:3828–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Malek AM, Jackman R, Rosenberg RD, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res 1994;74:852–860. [DOI] [PubMed] [Google Scholar]
- 106. Bellien J, Iacob M, Richard V, Wils J, Le Cam-Duchez V, Joannidès R. Evidence for wall shear stress-dependent t-PA release in human conduit arteries: role of endothelial factors and impact of high blood pressure. Hypertension Res 2021;44:310–317. [DOI] [PubMed] [Google Scholar]
- 107. Hosokawa K, Ohnishi-Wada T, Sameshima-Kaneko H, Nagasato T, Miura N, Kikuchi K, Koide T, Maruyama I, Urano T. Plasminogen activator inhibitor type 1 in platelets induces thrombogenicity by increasing thrombolysis resistance under shear stress in an in-vitro flow chamber model. Thromb Res 2016;146:69–75. [DOI] [PubMed] [Google Scholar]
- 108. Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science 1989;243:1483–1485. [DOI] [PubMed] [Google Scholar]
- 109. Rajeeva Pandian NK, Walther BK, Suresh R, Cooke JP, Jain A. Microengineered human vein-chip recreates venous valve architecture and its contribution to thrombosis. Small 2020;16:2003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 1998;18:677–685. [DOI] [PubMed] [Google Scholar]
- 111. Hu S, Liu Y, You T, Heath J, Xu L, Zheng X, Wang A, Wang Y, Li F, Yang F, Cao Y, Zhang H, van Gils JM, van Zonneveld AJ, Jo H, Wu Q, Zhang Y, Tang C, Zhu L. Vascular semaphorin 7A upregulation by disturbed flow promotes atherosclerosis through endothelial β1 integrin. Arterioscler Thromb Vasc Biol 2018;38:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 2015;107:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hordijk PL. Recent insights into endothelial control of leukocyte extravasation. Cell Mol Life Sci 2016;73:1591–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wen L, Moser M, Ley K. Molecular mechanisms of leukocyte beta2 integrin activation. Blood 2022;139:3480–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol 2015;15:692–704. [DOI] [PubMed] [Google Scholar]
- 116. Schmitt MMN, Megens RTA, Zernecke A, Bidzhekov K, van den Akker NM, Rademakers T, van Zandvoort MA, Hackeng TM, Koenen RR, Weber C. Endothelial junctional adhesion molecule-A guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation 2014;129:66–76. [DOI] [PubMed] [Google Scholar]
- 117. Yu X, Tan J, Diamond SL. Hemodynamic force triggers rapid NETosis within sterile thrombotic occlusions. J Thromb Haemost 2018;16:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134–147. [DOI] [PubMed] [Google Scholar]
- 119. Grässle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaría C, Baldauf C, Gräter F, Schneppenheim R, Obser T, Schneider SW. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol 2014;34:1382–1389. [DOI] [PubMed] [Google Scholar]
- 120. Wang Y, Du F, Hawez A, Mörgelin M, Thorlacius H. Neutrophil extracellular trap-microparticle complexes trigger neutrophil recruitment via high-mobility group protein 1 (HMGB1)-Toll-like receptors(TLR2)/TLR4 signalling. Brit J Pharmacol 2019;176:3350–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Venturini G, Malagrino PA, Padilha K, Tanaka LY, Laurindo FR, Dariolli R, Carvalho VM, Cardozo KHM, Krieger JE, Pereira A. Integrated proteomics and metabolomics analysis reveals differential lipid metabolism in human umbilical vein endothelial cells under high and low shear stress. Am J Physiol Cell Physiol 2019;317:C326–C338. [DOI] [PubMed] [Google Scholar]
- 122. Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997;89:331–340. [DOI] [PubMed] [Google Scholar]
- 123. Liu Y, Chen BPC, Lu M, Zhu Y, Stemerman MB, Chien S, Shyy JYJ. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol 2002;22:76–81. [DOI] [PubMed] [Google Scholar]
- 124. Lin T, Zeng L, Liu Y, DeFea K, Schwartz MA, Chien S, Shyy JYJ. Rho-ROCK-LIMK-Cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins. Circ Res 2003;92:1296–1304. [DOI] [PubMed] [Google Scholar]
- 125. Zhou J, Li YS, Nguyen P, Wang K-C, Weiss A, Kuo YC, Chiu JJ, Shyy JY, Chien S. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res 2013;113:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Balcells M, Martorell J, Olive C, Santacana M, Chitalia V, Cardoso AA, Edelman ER. Smooth muscle cells orchestrate the endothelial cell response to flow and injury. Circulation 2010;121:2192–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-β signaling pathway. Circ Res 2008;103:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Andrique L, Recher G, Alessandri K, Pujol N, Feyeux M, Bon P, Cognet L, Nassoy P, Bikfalvi A. A model of guided cell self-organization for rapid and spontaneous formation of functional vessels. Sci Adv 2019;5:eaau6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fang Y, Wu D, Birukov KG. Mechanosensing and mechanoregulation of endothelial cell functions. Compr Physiol 2019;9:873–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yamashiro Y, Yanagisawa H. The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin Sci 2020;134:2399–2418. [DOI] [PubMed] [Google Scholar]
- 131. Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018;554:487–492. [DOI] [PubMed] [Google Scholar]
- 132. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010;330:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. Piezo1 and G(q)/G(11) promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med 2018;215:2655–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J 2014;28:4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Goedicke-Fritz S, Kaistha A, Kacik M, Markert S, Hofmeister A, Busch C, Bänfer S, Jacob R, Grgic I, Hoyer J. Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur J Cell Biol 2015;94:391–400. [DOI] [PubMed] [Google Scholar]
- 136. Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. BBA-Biomembranes 2014;1838:682–691. [DOI] [PubMed] [Google Scholar]
- 137. Shahzad KA, Qin ZJ, Li Y, Xia DL. The roles of focal adhesion and cytoskeleton systems in fluid shear stress-induced endothelial cell response. Biocell 2020;44:137–145. [Google Scholar]
- 138. Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol 2007;293:C1616–C1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rossier O, Octeau V, Sibarita JB, Leduc C, Tessier B, Nair D, Gatterdam V, Destaing O, Albiges-Rizo C, Tampe R, Cognet L, Choquet D, Lounis B, Giannone G. Integrins beta1 and beta3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat Cell Biol 2012;14:1057–1067. [DOI] [PubMed] [Google Scholar]
- 140. Denis KB, Cabe JI, Danielsson BE, Tieu KV, Mayer CR, Conway DE. The LINC complex is required for endothelial cell adhesion and adaptation to shear stress and cyclic stretch. Mol Biol Cell 2021;32:1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 2002;91:769–775. [DOI] [PubMed] [Google Scholar]
- 142. Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem 1996;271:21691–21694. [DOI] [PubMed] [Google Scholar]
- 143. Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest 1999;103:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Flinn HM, Ridley AJ. Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J Cell Sci 1996;109:1133–1141. [DOI] [PubMed] [Google Scholar]
- 145. Li B, He J, Lv H, Liu Y, Lv X, Zhang C. Zhu Y and Ai D. c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J Clin Invest 2019;129:1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang C, Zhou T, Chen Z, Yan M, Li B, Lv H, Wang C, Xiang S, Shi L, Zhu Y, Ai D. Coupling of integrin alpha5 to annexin A2 by flow drives endothelial activation. Circ Res 2020;127:1074–1090. [DOI] [PubMed] [Google Scholar]
- 147. Sun X, Fu Y, Gu M, Zhang L, Li D, Li H, Chien S, Shyy JY, Zhu Y. Activation of integrin alpha5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc Natl Acad Sci U S A 2016;113:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 2006;119:508–518. [DOI] [PubMed] [Google Scholar]
- 149. Xanthis I, Souilhol C, Serbanovic-Canic J, Roddie H, Kalli AC, Fragiadaki M, Wong R, Shah DR, Askari JA, Canham L, Akhtar N, Feng S, Ridger V, Waltho J, Pinteaux E, Humphries MJ, Bryan MT, Evans PC. Β1 integrin is a sensor of blood flow direction. J Cell Sci 2019;132:jcs229542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yang B, Rizzo V. Shear stress activates eNOS at the endothelial apical surface through β1 containing integrins and caveolae. Cell Mol Bioeng 2013;6:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 2005;118:4103–4111. [DOI] [PubMed] [Google Scholar]
- 152. Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005;437:426–431. [DOI] [PubMed] [Google Scholar]
- 153. Conway DE, Coon BG, Budatha M, Arsenovic PT, Orsenigo F, Wessel F, Zhang J, Zhuang Z, Dejana E, Vestweber D, Schwartz MA. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol 2017;27:2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mehta V, Pang K-L, Rozbesky D, Nather K, Keen A, Lachowski D, Kong Y, Karia D, Ameismeier M, Huang J, Fang Y, Del Rio Hernandez A, Reader JS, Jones EY, Tzima E. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 2020;578:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang F-L, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, Patapoutian A. GPR68 senses flow and is essential for vascular physiology. Cell 2018;173:762–775.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Erdogmus S, Storch U, Danner L, Becker J, Winter M, Ziegler N, Wirth A, Offermanns S, Hoffmann C, Gudermann T, Mederos YSM. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat Commun 2019;10:5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Melchior B, Frangos JA. Distinctive subcellular Akt-1 responses to shear stress in endothelial cells. J Cell Biochem 2014;115:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 2009;587:2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol 2006;169:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. dela Paz NG, Melchior B, Frangos JA. Shear stress induces Gαq/11 activation independently of G protein-coupled receptor activation in endothelial cells. Am J Physiol Cell Physiol 2017;312:C428–C437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Weinbaum S, Cancel LM, Fu BM, Tarbell JM. The glycocalyx and its role in vascular physiology and vascular related diseases. Cardiovasc Eng Techn 2021;12:37–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121–167. [DOI] [PubMed] [Google Scholar]
- 163. Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 2014;16:505–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Harding IC, Mitra R, Mensah SA, Herman IM, Ebong EE. Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation. J Transl Med 2018;16:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang G, Kostidis S, Tiemeier GL, Sol W, de Vries MR, Giera M, Carmeliet P, van den Berg BM, Rabelink TJ. Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler Thromb Vasc Biol 2020;40:350–364. [DOI] [PubMed] [Google Scholar]
- 166. Harding IC, Mitra R, Mensah SA, Nersesyan A, Bal NN, Ebong EE. Endothelial barrier reinforcement relies on flow-regulated glycocalyx, a potential therapeutic target. Biorheology 2019;56:131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bar A, Targosz-Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, Sternak M, Przybyło M, Kurpińska A, Walczak M, Kostogrys RB, Szymonski M, Chlopicki S. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/low-density lipoprotein receptor-deficient mice. J Am Heart Assoc 2019;8:e011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A 2004;101:16483–16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Haymet AB, Bartnikowski N, Wood ES, Vallely MP, McBride A, Yacoub S, Biering SB, Harris E, Suen JY, Fraser JF. Studying the endothelial glycocalyx in vitro: what is missing? Front Cardiovasc Med 2021;8:647086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Luu VZ, Chowdhury B, Al-Omran M, Hess DA, Verma S. Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis 2018;275:196–204. [DOI] [PubMed] [Google Scholar]
- 171. Vion AC, Alt S, Klaus-Bergmann A, Szymborska A, Zheng T, Perovic T, Hammoutene A, Oliveira MB, Bartels-Klein E, Hollfinger I, Rautou PE, Bernabeu MO, Gerhardt H. Primary cilia sensitize endothelial cells to BMP and prevent excessive vascular regression. J Cell Biol 2018;217:1651–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 2004;164:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chen X, Gays D, Milia C, Santoro MM. Cilia control vascular mural cell recruitment in vertebrates. Cell Rep 2017;18:1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Del Pozo MA, Lolo F-N, Echarri A. Caveolae: mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr Opin Cell Biol 2021;68:113–123. [DOI] [PubMed] [Google Scholar]
- 175. Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 2006;116:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rausch V, Bostrom JR, Park J, Bravo IR, Feng Y, Hay DC, Link BA, Hansen CG. The Hippo pathway regulates caveolae expression and mediates flow response via caveolae. Curr Biol 2019;29:242–255.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Torrino S, Shen W-W, Blouin CM, Mani SK, Viaris de Lesegno C, Bost P, Grassart A, Köster D, Valades-Cruz CA, Chambon V, Johannes L, Pierobon P, Soumelis V, Coirault C, Vassilopoulos S, Lamaze C. EHD2 Is a mechanotransducer connecting caveolae dynamics with gene transcription. J Cell Biol 2018;217:4092–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res 2004;94:1408–1417. [DOI] [PubMed] [Google Scholar]
- 179. Lamaze C, Tardif N, Dewulf M, Vassilopoulos S, Blouin CM. The caveolae dress code: structure and signaling. Curr Opin Cell Biol 2017;47:117–125. [DOI] [PubMed] [Google Scholar]
- 180. Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 2003;285:H1113–H1122. [DOI] [PubMed] [Google Scholar]
- 181. Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 2003;285:H1720–H1729. [DOI] [PubMed] [Google Scholar]
- 182. Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fässler R. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 2010;19:574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Noel J, Wang H, Hong N, Tao J-Q, Yu K, Sorokina EM, DeBolt K, Heayn M, Rizzo V, Delisser H, Fisher AB, Chatterjee S. PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium. Am J Physiol-Lung C 2013;305:L805–L818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Givens C, Tzima E. Endothelial mechanosignaling: does one sensor fit all? Antioxid Redox Sign 2016;25:373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 2016;252:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol 2018;20:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci 2019;26:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. He M, Huang TS, Li S, Hong H-C, Chen Z, Martin M, Zhou X, Huang HY, Su SH, Zhang J, Wang WT, Kang J, Huang H-D, Zhang J, Chien S, Shyy J. Atheroprotective flow upregulates ITPR3 (inositol 1,4,5-trisphosphate receptor 3) in vascular endothelium via KLF4 (Krüppel-like factor 4)-mediated histone modifications. Arterioscler Thromb Vasc Biol 2019;39:902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chien C-S, Li JY-S, Chien Y, Wang M-L, Yarmishyn AA, Tsai P-H, Juan C-C, Nguyen P, Cheng H-M, Huo T-I, Chiou S-H, Chien S. METTL3-dependent N6-methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium. Proc Natl Acad Sci U S A 2021;118:e2025070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Stanicek L, Lozano-Vidal N, Bink DI, Hooglugt A, Yao W, Wittig I, van Rijssel J, van Buul JD, van Bergen A, Klems A, Ramms AS, Le Noble F, Hofmann P, Szulcek R, Wang S, Offermanns S, Ercanoglu MS, Kwon H-B, Stainier D, Huveneers S, Kurian L, Dimmeler S, Boon RA. Long non-coding RNA LASSIE regulates shear stress sensing and endothelial barrier function. Commun Biol 2020;3:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Aureille J, Belaadi N, Guilluy C. Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr Opin Cell Biol 2017;44:59–67. [DOI] [PubMed] [Google Scholar]
- 192. Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Gene Dev 2015;29:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Janota CS, Calero-Cuenca FJ, Gomes ER. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol 2020;63:204–211. [DOI] [PubMed] [Google Scholar]
- 194. Qi YX, Han Y, Jiang ZL. Mechanobiology and vascular remodeling: from membrane to nucleus. Adv Exp Med Biol 2018;1097:69–82. [DOI] [PubMed] [Google Scholar]
- 195. Maurer M, Lammerding J. The driving force: nuclear mechanotransduction in cellular function, fate, and disease. Annu Rev Biomed Eng 2019;21:443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Uhler C, Shivashankar GV. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol 2017;18:717–727. [DOI] [PubMed] [Google Scholar]
- 197. Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 2010;26:421–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 2011;286:26743–26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Han Y, Huang K, Yao Q-P, Jiang Z-L. Mechanobiology in vascular remodeling. Natl Sci Rev 2018;5:933–946. [Google Scholar]
- 200. Harris AR, Jreij P, Fletcher DA. Mechanotransduction by the actin cytoskeleton: converting mechanical stimuli into biochemical signals. Ann Rev Biophys 2018;47:617–631. [Google Scholar]
- 201. Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside–outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol 2012;24:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Yu FX, Zhao B, Panupinthu N, Jewell Jenna L, Lian I, Wang Lloyd H, Zhao J, Yuan H, Tumaneng K, Li H, Fu X-D, Mills Gordon B, Guan K-L. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012;150:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016;540:579–582. [DOI] [PubMed] [Google Scholar]
- 204. Swain SM, Liddle RA. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J Biol Chem 2021;296:100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sweet DR, Fan L, Hsieh PN, Jain MK. Kruppel-like factors in vascular inflammation: mechanistic insights and therapeutic potential. Front Cardiovasc Med 2018;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 2006;116:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun 2006;341:1244–1251. [DOI] [PubMed] [Google Scholar]
- 208. Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 2010;115:2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhuang T, Liu J, Chen X, Zhang L, Pi J, Sun H, Li L, Bauer R, Wang H, Yu Z, Zhang Q, Tomlinson B, Chan P, Zheng X, Morrisey E, Liu Z, Reilly M, Zhang Y. Endothelial foxp1 suppresses atherosclerosis via modulation of nlrp3 inflammasome activation. Circ Res 2019;125:590–605. [DOI] [PubMed] [Google Scholar]
- 210. Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 2008;28:1339–1346. [DOI] [PubMed] [Google Scholar]
- 211. McSweeney SR, Warabi E, Siow RC. Nrf2 as an endothelial mechanosensitive transcription factor: going with the flow. Hypertension 2016;67:20–29. [DOI] [PubMed] [Google Scholar]
- 212. Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol 2009;29:1851–1857. [DOI] [PubMed] [Google Scholar]
- 213. Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, Chien S, Chiu JJ. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A 2012;109:1967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Collins AR, Gupte AA, Ji R, Ramirez MR, Minze LJ, Liu JZ, Arredondo M, Ren Y, Deng T, Wang J, Lyon CJ, Hsueh WA. Myeloid deletion of nuclear factor erythroid 2-related factor 2 increases atherosclerosis and liver injury. Arterioscler Thromb Vasc Biol 2012;32:2839–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol 2011;31:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One 2008;3:e3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Harada N, Ito K, Hosoya T, Mimura J, Maruyama A, Noguchi N, Yagami K, Morito N, Takahashi S, Maher JM, Yamamoto M, Itoh K. Nrf2 in bone marrow-derived cells positively contributes to the advanced stage of atherosclerotic plaque formation. Free Radic Biol Med 2012;53:2256–2262. [DOI] [PubMed] [Google Scholar]
- 218. Chang L, Azzolin L, Di Biagio D, Zanconato F, Battilana G, Lucon Xiccato R, Aragona M, Giulitti S, Panciera T, Gandin A, Sigismondo G, Krijgsveld J, Fassan M, Brusatin G, Cordenonsi M, Piccolo S. The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature 2018;563:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker EN, Jo H, Gardner J, Orosz Z, Ungvari Z. Downregulation of bone morphogenetic protein 4 expression in coronary arterial endothelial cells: role of shear stress and the cAMP/protein kinase A pathway. Arterioscler Thromb Vasc Biol 2007;27:776–782. [DOI] [PubMed] [Google Scholar]
- 220. Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 2003;278:31128–31135. [DOI] [PubMed] [Google Scholar]
- 221. Zhou J, Lee PL, Tsai CS, Lee CI, Yang TL, Chuang HS, Lin WW, Lin TE, Lim SH, Wei SY, Chen YL, Chien S, Chiu JJ. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc Natl Acad Sci USA 2012;109:7770–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Deng H, Min E, Baeyens N, Coon BG, Hu R, Zhuang ZW, Chen M, Huang B, Afolabi T, Zarkada G, Acheampong A, McEntee K, Eichmann A, Liu F, Su B, Simons M, Schwartz MA. Activation of Smad2/3 signaling by low fluid shear stress mediates artery inward remodeling. Proc Natl Acad Sci U S A 2021;118:e2105339118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 2007;116:1258–1266. [DOI] [PubMed] [Google Scholar]
- 224. Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragón RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, Iruela-Arispe ML. NOTCH1 Is a mechanosensor in adult arteries. Nat Commun 2017;8:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 2017;552:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 2017;8:2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Qin WD, Zhang F, Qin XJ, Wang J, Meng X, Wang H, Guo HP, Wu QZ, Wu DW, Zhang MX. Notch1 inhibition reduces low shear stress-induced plaque formation. Int J Biochem Cell B 2016;72:63–72. [DOI] [PubMed] [Google Scholar]
- 228. Franco CA, Jones ML, Bernabeu MO, Vion AC, Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP, Coveney PV, Lang RA, Gerhardt H. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. Elife 2016;5:e07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Li R, Beebe T, Jen N, Yu F, Takabe W, Harrison M, Cao H, Lee J, Yang H, Han P, Wang K, Shimizu H, Chen J, Lien CL, Chi NC, Hsiai TK. Shear stress-activated Wnt-angiopoietin-2 signaling recapitulates vascular repair in zebrafish embryos. Arterioscler Thromb Vasc Biol 2014;34:2268–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Krishna SM, Seto SW, Jose RJ, Li J, Morton SK, Biros E, Wang Y, Nsengiyumva V, Lindeman JH, Loots GG, Rush CM, Craig JM, Golledge J. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb Vasc Biol 2017;37:553–566. [DOI] [PubMed] [Google Scholar]
- 231. Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol 2011;31:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Abuammah A, Maimari N, Towhidi L, Frueh J, Chooi KY, Warboys C, Krams R. New developments in mechanotransduction: cross talk of the Wnt, TGF-β and Notch signalling pathways in reaction to shear stress. Curr Opin Biomed Eng 2018;5:96–104. [Google Scholar]
- 233. Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A 2000;97:9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Cuhlmann S, Van der Heiden K, Saliba D, Tremoleda JL, Khalil M, Zakkar M, Chaudhury H, Luong le A, Mason JC, Udalova I, Gsell W, Jones H, Haskard DO, Krams R, Evans PC. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-κB regulation that promotes arterial inflammation. Circ Res 2011;108:950–959. [DOI] [PubMed] [Google Scholar]
- 235. Fan W, Fang R, Wu X, Liu J, Feng M, Dai G, Chen G, Wu G. Shear-sensitive microRNA-34a modulates flow-dependent regulation of endothelial inflammation. J Cell Sci 2015;128:70–80. [DOI] [PubMed] [Google Scholar]
- 236. Mengistu M, Brotzman H, Ghadiali S, Lowe-Krentz L. Fluid shear stress-induced JNK activity leads to actin remodeling for cell alignment. J Cell Physiol 2011;226:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Li L, Tatake RJ, Natarajan K, Taba Y, Garin G, Tai C, Leung E, Surapisitchat J, Yoshizumi M, Yan C, Abe J, Berk BC. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun 2008;370:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Yamawaki H, Lehoux S, Berk BC. Chronic physiological shear stress inhibits tumor necrosis factor-induced proinflammatory responses in rabbit aorta perfused ex vivo. Circulation 2003;108:1619–1625. [DOI] [PubMed] [Google Scholar]
- 239. Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A 2001;98:6476–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res 2009;104:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- 242. Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem 2005;280:12305–12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Pampori N, Hato T, Stupack DG, Aidoudi S, Cheresh DA, Nemerow GR, Shattil SJ. Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J Biol Chem 1999;274:21609–21616. [DOI] [PubMed] [Google Scholar]
- 244. Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA Jr, García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem 2005;280:26714–26719. [DOI] [PubMed] [Google Scholar]
- 245. Sen-Banerjee S, Mir S, Lin ZY, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005;112:720–726. [DOI] [PubMed] [Google Scholar]
- 246. Le NT, Takei Y, Izawa-Ishizawa Y, Heo KS, Lee H, Smrcka AV, Miller BL, Ko KA, Ture S, Morrell C, Fujiwara K, Akaike M, Abe J. Identification of activators of ERK5 transcriptional activity by high-throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. J Immunol 2014;193:3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ali F, Zakkar M, Karu K, Lidington EA, Hamdulay SS, Boyle JJ, Zloh M, Bauer A, Haskard DO, Evans PC, Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem 2009;284:18882–18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Hölschermann H, Schuster D, Parviz B, Haberbosch W, Tillmanns H, Muth H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006;185:240–245. [DOI] [PubMed] [Google Scholar]
- 249. Xu Y, Xu S, Liu P, Koroleva M, Zhang S, Si S, Jin ZG. Suberanilohydroxamic acid as a pharmacological Kruppel-like factor 2 activator that represses vascular inflammation and atherosclerosis. J Am Heart Assoc 2017;6:e007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. El-Kashef DH, Abdelrahman RS. Montelukast ameliorates concanavalin A-induced autoimmune hepatitis in mice via inhibiting TNF-α/JNK signaling pathway. Toxicol Appl Pharm 2020;393:114931. [DOI] [PubMed] [Google Scholar]
- 251. Di X, Tang X, Di X. Montelukast inhibits oxidized low-density lipoproteins (ox-LDL) induced vascular endothelial attachment: an implication for the treatment of atherosclerosis. Biochem Biophys Res Commun 2017;486:58–62. [DOI] [PubMed] [Google Scholar]
- 252. Yue W, Li Y, Ou D, Yang Q. The GLP-1 receptor agonist liraglutide protects against oxidized LDL-induced endothelial inflammation and dysfunction via KLF2. IUBMB Life 2019;71:1347–1354. [DOI] [PubMed] [Google Scholar]
- 253. Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, Node K. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012;221:375–382. [DOI] [PubMed] [Google Scholar]
- 254. Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc Drugs Ther 2013;27:371–380. [DOI] [PubMed] [Google Scholar]
- 255. Tian R, Li R, Liu Y, Liu J, Pan T, Zhang R, Liu B, Chen E, Tang Y, Qu H. Metformin ameliorates endotoxemia-induced endothelial pro-inflammatory responses via AMPK-dependent mediation of HDAC5 and KLF2. BBA-Mol Basis 2019;1865:1701–1712. [DOI] [PubMed] [Google Scholar]
- 256. Wu H, Feng K, Zhang C, Zhang H, Zhang J, Hua Y, Dong Z, Zhu Y, Yang S, Ma C. Metformin attenuates atherosclerosis and plaque vulnerability by upregulating KLF2-mediated autophagy in ApoE(-/-) mice. Biochem Biophys Res Commun 2021;557:334–341. [DOI] [PubMed] [Google Scholar]
- 257. Jiang T, Zhang W, Wang Z. Laquinimod protects against TNF-α-induced attachment of monocytes to human aortic endothelial cells (HAECs) by increasing the expression of KLF2. Drug Des Dev Ther 2020;14:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Luo M, Sun Q, Zhao H, Tao J, Yan D. The effects of dimethyl fumarate on atherosclerosis in the apolipoprotein E-deficient mouse model with streptozotocin-induced hyperglycemia mediated by the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway. Med Sci Monit 2019;25:7966–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Gene Dev 2012;26:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Jain M, Zellweger M, Frobert A, Valentin J, van den Bergh H, Wagnières G, Cook S, Giraud MN. Intra-Arterial drug and light delivery for photodynamic therapy using Visudyne®: implication for atherosclerotic plaque treatment. Front Physiol 2016;7:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. D'Andrea E, Hey SP, Ramirez CL, Kesselheim AS. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Net Open 2019;2:e192224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Si Y, Zhang Y, Zhao J, Guo S, Zhai L, Yao S, Sang H, Yang N, Song G, Gu J, Qin S. Niacin inhibits vascular inflammation via downregulating nuclear transcription factor-κB signaling pathway. Mediat Inflamm 2014;2014:263786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA, Quertermous T. Apelin signaling antagonizes ang II effects in mouse models of atherosclerosis. J Clin Invest 2008;118:3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292–2300. [DOI] [PubMed] [Google Scholar]
- 265. Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc Diabetol 2007;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Liu H, Jiang D, Zhang S, Ou B. Aspirin inhibits fractalkine expression in atherosclerotic plaques and reduces atherosclerosis in ApoE gene knockout mice. Cardiovasc Drugs Ther 2010;24:17–24. [DOI] [PubMed] [Google Scholar]
- 267. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998;396:77–80. [DOI] [PubMed] [Google Scholar]
- 268. Cyrus T, Sung S, Zhao L, Funk CD, Tang S, Praticò D. Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation 2002;106:1282–1287. [DOI] [PubMed] [Google Scholar]
- 269. Malcova H, Strizova Z, Milota T, Striz I, Sediva A, Cebecauerova D, Horvath R. IL-1 Inhibitors in the treatment of monogenic periodic fever syndromes: from the past to the future perspectives. Front Immunol 2020;11:619257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Alfaidi M, Acosta CH, Wang D, Traylor JG, Orr AW. Selective role of Nck1 in atherogenic inflammation and plaque formation. J Clin Invest 2020;130:4331–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ku EJ, Kim B-R, Lee J-I, Lee YK, Oh TJ, Jang HC, Choi SH. The anti-atherosclerosis effect of anakinra, a recombinant human interleukin-1 receptor antagonist, in apolipoprotein E knockout mice. Int J Mol Sci 2022;23:4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Cai Y, Li JD, Yan C. Vinpocetine attenuates lipid accumulation and atherosclerosis formation. Biochem Biophys Res Commun 2013;434:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zhuang J, Peng W, Li H, Lu Y, Wang K, Fan F, Li S, Xu Y. Inhibitory effects of vinpocetine on the progression of atherosclerosis are mediated by Akt/NF-κB dependent mechanisms in apoE-/- mice. PLoS One 2013;8:e82509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, Yan C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A 2010;107:9795–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 2010;299:H18–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 2006;291:H1694–H1699. [DOI] [PubMed] [Google Scholar]
- 277. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 2000;164:6509–6519. [DOI] [PubMed] [Google Scholar]
- 278. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196. [DOI] [PubMed] [Google Scholar]
- 279. Gan W, Dang Y, Han X, Ling S, Duan J, Liu J, Xu JW. ERK5/HDAC5-mediated, resveratrol-, and pterostilbene-induced expression of MnSOD in human endothelial cells. Mol Nutr Food Res 2016;60:266–277. [DOI] [PubMed] [Google Scholar]
- 280. Gracia-Sancho J, Villarreal G Jr, Zhang Y, García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res 2010;85:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Xu Y, Liu P, Xu S, Koroleva M, Zhang S, Si S, Jin ZG. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci Rep 2017;7:6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002;62:5196–5203. [PubMed] [Google Scholar]
- 283. Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 2008;57:2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Shehatou GS, Suddek GM. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med 2016;241:426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Cimmino G, Conte S, Morello A, Pellegrino G, Marra L, Calì G, Golino P, Cirillo P. Colchicine inhibits the prothrombotic effects of oxLDL in human endothelial cells. Vasc Pharmacol 2021;137:106822. [DOI] [PubMed] [Google Scholar]
- 286. Binesh A, Devaraj SN, Halagowder D. Atherogenic diet induced lipid accumulation induced NFκB level in heart, liver and brain of Wistar rat and diosgenin as an anti-inflammatory agent. Life Sci 2018;196:28–37. [DOI] [PubMed] [Google Scholar]
- 287. Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014;16:357–366. [DOI] [PubMed] [Google Scholar]
- 288. Mao X, Su T, Zhang X, Li F, Qin X, Li X, Liao D. Apelin-13 promote monocytes adhesion to HUVECs via PI3K signaling. J Prog Biochem Biophys 2011;38:1162–1170. [Google Scholar]
- 289. Lu Y, Zhu X, Liang GX, Cui RR, Liu Y, Wu SS, Liang QH, Liu GY, Jiang Y, Liao XB, Xie H, Zhou HD, Wu XP, Yuan LQ, Liao EY. Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-κB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids 2012;43:2125–2136. [DOI] [PubMed] [Google Scholar]
- 290. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Alonso N, Julián MT, Puig-Domingo M, Vives-Pi M. Incretin hormones as immunomodulators of atherosclerosis. Front Endocrinol 2012;3:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+] i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 2005;288:H2869–H2877. [DOI] [PubMed] [Google Scholar]
- 293. Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res 2010;87:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res 2009;104:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Kim M, Kim S, Lim JH, Lee C, Choi HC, Woo CH. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem 2012;287:40722–40731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Kuosmanen SM, Kansanen E, Kaikkonen MU, Sihvola V, Pulkkinen K, Jyrkkanen HK, Tuoresmaki P, Hartikainen J, Hippelainen M, Kokki H, Tavi P, Heikkinen S, Levonen AL. NRF2 Regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res 2018;46:1124–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this manuscript.