Key Points
Question
What prolonged symptoms experienced by youth are most associated with SARS-CoV-2 infection?
Findings
Among 5367 participants in the RECOVER-Pediatrics cohort study, 14 symptoms in both school-age children (6-11 years) and adolescents (12-17 years) were more common in those with vs without SARS-CoV-2 infection history, with 4 additional symptoms in school-age children only and 3 in adolescents only. Empirically derived indices for PASC research and associated clustering patterns were developed.
Meaning
This study developed research indices for characterizing pediatric PASC. Symptom patterns were similar but distinguishable between school-age children and adolescents, highlighting the importance of characterizing PASC separately in different age groups.
Abstract
Importance
Most research to understand postacute sequelae of SARS-CoV-2 infection (PASC), or long COVID, has focused on adults, with less known about this complex condition in children. Research is needed to characterize pediatric PASC to enable studies of underlying mechanisms that will guide future treatment.
Objective
To identify the most common prolonged symptoms experienced by children (aged 6 to 17 years) after SARS-CoV-2 infection, how these symptoms differ by age (school-age [6-11 years] vs adolescents [12-17 years]), how they cluster into distinct phenotypes, and what symptoms in combination could be used as an empirically derived index to assist researchers to study the likely presence of PASC.
Design, Setting, and Participants
Multicenter longitudinal observational cohort study with participants recruited from more than 60 US health care and community settings between March 2022 and December 2023, including school-age children and adolescents with and without SARS-CoV-2 infection history.
Exposure
SARS-CoV-2 infection.
Main Outcomes and Measures
PASC and 89 prolonged symptoms across 9 symptom domains.
Results
A total of 898 school-age children (751 with previous SARS-CoV-2 infection [referred to as infected] and 147 without [referred to as uninfected]; mean age, 8.6 years; 49% female; 11% were Black or African American, 34% were Hispanic, Latino, or Spanish, and 60% were White) and 4469 adolescents (3109 infected and 1360 uninfected; mean age, 14.8 years; 48% female; 13% were Black or African American, 21% were Hispanic, Latino, or Spanish, and 73% were White) were included. Median time between first infection and symptom survey was 506 days for school-age children and 556 days for adolescents. In models adjusted for sex and race and ethnicity, 14 symptoms in both school-age children and adolescents were more common in those with SARS-CoV-2 infection history compared with those without infection history, with 4 additional symptoms in school-age children only and 3 in adolescents only. These symptoms affected almost every organ system. Combinations of symptoms most associated with infection history were identified to form a PASC research index for each age group; these indices correlated with poorer overall health and quality of life. The index emphasizes neurocognitive, pain, and gastrointestinal symptoms in school-age children but change or loss in smell or taste, pain, and fatigue/malaise–related symptoms in adolescents. Clustering analyses identified 4 PASC symptom phenotypes in school-age children and 3 in adolescents.
Conclusions and Relevance
This study developed research indices for characterizing PASC in children and adolescents. Symptom patterns were similar but distinguishable between the 2 groups, highlighting the importance of characterizing PASC separately for these age ranges.
This observational cohort study examines the symptoms experienced by children after SARS-CoV-2 infection and how these symptoms differ by age (6-11 years vs 12-17 years).
Introduction
Long COVID, or postacute sequelae of SARS-CoV-2 infection (PASC), has been broadly defined as symptoms, signs, and conditions that develop, persist, or relapse over time after SARS-CoV-2 infection.1,2 These symptoms can last weeks, months, or years after the acute infection resolves and can have debilitating effects. Some experts believe that worldwide, an estimated 65 million people are living with PASC,3 with impacts on population-level health anticipated to last for decades. Most research characterizing PASC has focused on adults,4 leading to misperception that pediatric PASC is rare or presents similarly to PASC in adults.5,6 This may lead clinicians to miss symptoms or misdiagnose children. Consistent with the life course framework in which developmental stage influences health outcomes,7 PASC may present in both similar and different ways compared with adults.
Studies of pediatric PASC have documented a wide range of symptoms involving every organ system.8,9,10,11 Most pediatric research has focused on individual symptoms and either pooled data from different ages or focused on adolescents. Little is known about differences in PASC symptoms between school-age children (6-11 years) and adolescents (12-17 years).12,13 The absence of a consistent analytic approach to objectively identify children with PASC hinders the research needed to identify underlying mechanisms of disease and treatment targets. The National Institutes of Health–funded Researching COVID to Enhance Recovery (RECOVER) Initiative aims to fill these gaps by bringing together researchers, clinicians, communities, and families in a comprehensive study of PASC in children.14 The aims of this analysis of the RECOVER-Pediatrics cohort were to identify (1) common prolonged symptoms experienced by children (6 to 17 years old) after SARS-CoV-2 infection, (2) how these symptoms differ by age (school-age vs adolescents), (3) how symptoms cluster into phenotypes, and (4) what symptoms in combination could be used as an empirically derived index to help researchers consistently assess the likely presence of PASC. These indices, like the one previously developed for the RECOVER-Adult cohort (18 years or older),15 were intended to be used to identify factors that distinguish children who likely have developed PASC from those who may not have and to help evaluate risk factors for developing PASC, elucidate its pathophysiology, and enable follow-up to analyze recovery and relapse.
Methods
Study Design
The RECOVER Pediatric Observational Cohort Study (RECOVER-Pediatrics)14 is a combined retrospective and prospective longitudinal study including 4 cohorts. Data presented are from 2 cohorts: the de novo RECOVER cohort, including participants from birth through 25 years with and without SARS-CoV-2 infection history newly recruited from health care and community settings, and the extant National Institutes of Health–funded Adolescent Brain Cognitive Development cohort,16,17,18 the largest long-term US study of brain development in adolescence. The protocol and statistical analysis plan for RECOVER-Pediatrics were previously described19 (see Supplements 1 and 2). Data were obtained from more than 60 sites (eTable 1 in Supplement 3). The study received institutional review board approval from NYU Grossman School of Medicine (de novo cohort) or UC San Diego Human Research Protections Program (Adolescent Brain Cognitive Development cohort), with other institutions relying on these single institutional review boards. Caregiver-child pairs provided informed consent and age-appropriate assent. Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed.
RECOVER-Pediatrics Participants
Inclusion Criteria
The analytic sample included individuals aged 6 to 17 years enrolled between March 16, 2022, and December 16, 2023, with and without known SARS-CoV-2 infection history (infected and uninfected, respectively). Child age was recorded at symptom survey completion.
For these analyses, the infected group included participants who completed their survey about prolonged symptoms at least 90 days after their first infection, reported by their caregivers (eMethods in Supplement 3). SARS-CoV-2 antibodies were not required. The uninfected group was defined by caregiver report and required confirmation of negative nucleocapsid antibodies at enrollment. Those thought to be uninfected but found to be antibody-positive (Ab+) within 30 days of survey completion were analyzed separately to understand asymptomatic infection.20 Throughout, uninfected refers strictly to uninfected participants who were confirmed to be nucleocapsid antibody–negative.
Exclusion Criteria
Infected participants with an unknown date for their first infection, participants with history of multisystem inflammatory syndrome in children (because this is a well-characterized entity),21,22,23,24,25 uninfected participants without antibody testing, and participants with missing symptom surveys (defined as <50% of questions completed) were excluded.
Outcomes
Caregivers completed a comprehensive symptom survey remotely (interviewer-administered if needed) assessing 89 prolonged symptoms across 9 domains, using health literacy–informed principles and plain-language descriptions (eTable 2 in Supplement 3).19,26 Some symptoms describing a similar phenotype were combined into composites, resulting in 75 symptoms (eMethods in Supplement 3): general (12 symptoms), eyes/ears/nose/throat (15 symptoms), heart/lungs (10 symptoms), gastrointestinal (6 symptoms), dermatologic (5 symptoms), musculoskeletal (3 symptoms), neurologic (6 symptoms), behavioral/psychological (14 symptoms), and menstrual (4 symptoms). The same symptoms were assessed in both age groups (except panic attacks, which were assessed in adolescents only). Menstrual symptoms were assessed in those assigned female or intersex at birth and who started menstruating (reported only among adolescents).
The primary outcome was a prolonged symptom lasting for more than 4 weeks that started or became worse since the beginning of the pandemic and was present at the time of survey completion (at least 90 days after infection). If a symptom lasted for more than 4 weeks but was absent at survey completion, it was not counted as a prolonged symptom.
Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health Scales were assessed, measuring caregiver perception of the child’s overall health, physical health, and quality of life.27
Covariates
The main exposure variable was SARS-CoV-2 infection. Other variables included sex, race and ethnicity, geographic origin, time since SARS-CoV-2 infection, calendar time of enrollment, and SARS-CoV-2 vaccination status (eMethods in Supplement 3). Like other variables, race and ethnicity were collected via caregiver report based on prespecified categories and measured to enhance understanding of racial and ethnic differences in PASC. Caregiver variables included relationship to child and educational attainment.
Statistical Analyses
Statistical analyses were modeled after those published for RECOVER-Adult and were age-stratified.15 The analysis calculated the proportion of participants who reported each prolonged symptom and who reported experiencing at least 1 prolonged symptom among infected and uninfected participants separately (eTable 3 in Supplement 3). For symptoms present in at least 5% of infected participants (candidate symptoms), the risk difference, odds ratio, and relative risk for infected vs uninfected participants were estimated using linear, logistic, and Poisson regression, respectively, adjusting for sex and race and ethnicity (eMethods in Supplement 3). Second, to identify combinations of symptoms that could be used for research, a penalized logistic regression approach (least absolute shrinkage and selection operator [LASSO])28 was used to identify what candidate symptoms (predictors) were best at differentiating participants with or without an infection history (outcome).15 Because all sexes were combined for this analysis, menstrual symptoms were excluded. Based on the model fit, each symptom was assigned a score corresponding to the estimated log odds ratio, where a higher symptom score indicated a stronger association with infection. A total index was calculated for each participant by summing the individual scores for each symptom reported. An optimal index threshold for identifying PASC was selected based on the proportion of uninfected participants who were likely misclassified as having PASC (eMethods in Supplement 3). Participants meeting the index threshold were categorized as PASC-probable and others were categorized as PASC-unspecified. PASC rates were reported among infected and uninfected participants separately. Among infected participants, these rates were also reported by whether they were infected by December 1, 2021 (when the Omicron variant became the dominant US strain).
Third, the analysis examined correlations between PASC indices and caregiver-reported overall child health, quality of life, and physical health and symptoms selected by LASSO. Further, the frequency of all symptoms was reported in infected PASC-probable, infected PASC-unspecified, and uninfected participants separately. Fourth, symptom patterns were investigated among infected participants categorized as PASC-probable. Correlations between symptoms contributing to the PASC index among infected PASC-probable participants were calculated. K-means consensus clustering was performed based on symptoms contributing to the PASC index to identify distinct PASC symptom profiles.29 The number of different systems affected among infected PASC-probable participants was then summarized by counting the systems in which at least 1 prolonged symptom was reported. Fifth, we summarized the characteristics and symptomatology of uninfected participants found to be Ab+.
Results
This study included 751 infected and 147 uninfected school-age children and 3109 infected and 1369 uninfected adolescents (see cohort identification details in eFigure 1 in Supplement 3). The Table and eTable 4 in Supplement 3 contain demographic and infection history characteristics, respectively. eTable 5 in Supplement 3 contains demographic characteristics for the adolescent cohort, stratified by recruiting cohort (Adolescent Brain Cognitive Development vs de novo).
Table. Demographic Characteristics of the Primary Analysis Cohort Participants Stratified by Age Group and SARS-CoV-2 Infection Status.
| Participant characteristic | No. (%) | |||
|---|---|---|---|---|
| School-age children (6-11 y) | Adolescents (12-17 y) | |||
| Infected (n = 751) | Uninfected (n = 147) | Infected (n = 3109) | Uninfected (n = 1369) | |
| Age, mean (SD), y | 9 (2) | 8 (2) | 15 (1) | 15 (1) |
| Sex assigned at birth | ||||
| Female | 362 (48) | 78 (53) | 1537 (49) | 592 (43) |
| Male | 389 (52) | 69 (47) | 1572 (51) | 776 (57) |
| Intersex | 0 | 0 | 0 | 1 (<0.1) |
| Race and ethnicitya | 747 | 142 | 3096 | 1360 |
| American Indian or Alaska Native | 21 (3) | 4 (3) | 81 (3) | 35 (3) |
| Asian | 58 (8) | 10 (7) | 202 (7) | 110 (8) |
| Black or African American | 92 (12) | 9 (6) | 405 (13) | 192 (14) |
| Hispanic, Latino, or Spanish | 255 (34) | 43 (30) | 689 (22) | 244 (18) |
| Native Hawaiian or Other Pacific Islander | 2 (<0.1) | 3 (2) | 16 (1) | 8 (1) |
| White | 434 (58) | 97 (68) | 2241 (72) | 1003 (74) |
| None of these fully describe me | 6 (1) | 1 (1) | 29 (1) | 5 (0.4) |
| English is primary language | 659/743 (89) | 133/146 (91) | 2974/3092 (96) | 1322/1355 (98) |
| Birth in the US | 723/746 (97) | 146 (99) | 2995/3095 (97) | 1331/1364 (98) |
| Population referral source | 751 | 147 | 3109 | 1369 |
| Existing non-COVID research or clinical cohortb | 60 (8) | 6 (4) | 2258 (73) | 1228 (90) |
| Community outreach | 209 (28) | 65 (44) | 225 (7) | 52 (4) |
| Participant tested/treated in the health system | 182 (24) | 11 (7) | 246 (8) | 25 (2) |
| Self-referral from RECOVER website or other unsolicited self-referral | 152 (20) | 44 (30) | 185 (6) | 34 (2) |
| Community health center | 66 (9) | 10 (7) | 58 (2) | 18 (1) |
| Public health department list | 16 (2) | 0 | 46 (1) | 2 (0.1) |
| Long COVID clinic | 15 (2) | 1 (1) | 41 (1) | 0 |
| Existing, prospectively followed up COVID cohort | 19 (3) | 2 (1) | 7 (0.2) | 1 (<0.1) |
| Other | 32 (4) | 8 (5) | 43 (1) | 9 (1) |
| From a medically underserved area | 295 (39) | 49 (33) | 789 (25) | 374 (27) |
| From a rural area | 39 (5) | 11 (7) | 136 (4) | 46 (3) |
| Prevalent SARS-CoV-2 strain at first infection is before Omicron (before Dec 1, 2021) | 391 (52) | 1636 (53) | ||
| Vaccination status at first infection (infected) or enrollment (uninfected)c | ||||
| Fully vaccinated | 194 (26) | 99 (67) | 1345 (43) | 1048 (77) |
| Partially vaccinated | 91 (12) | 2 (1) | 217 (7) | 46 (3) |
| Not eligible for vaccination | 337 (45) | 0 | 1048 (34) | 0 |
| Vaccinated but missing information | 23 (3) | 11 (7) | 90 (3) | 88 (6) |
| Not vaccinated | 106 (14) | 35 (24) | 409 (13) | 187 (14) |
| Time between first infection (infected) or Mar 1, 2020 (uninfected) and symptom survey, median (IQR), d | 504 (298-812) | 701 (580-857) | 519 (334-814) | 774 (691-842) |
| Primary caregiver characteristics | ||||
| Relationship to child: mother | 669/719 (93) | 131/139 (94) | 2785/3074 (91) | 1220/1361 (90) |
| Educational attainment: college or higher | 368/634 (58) | 84/127 (66) | 1891/2880 (66) | 891/1325 (67) |
See eMethods in Supplement 3 for additional details on how race and ethnicity were collected (self-report); note that participants could select all race and ethnicity groups that applied.
The category “Existing non-COVID research or clinical cohort” includes the Adolescent Brain Cognitive Development cohort.
See eMethods in Supplement 3 for additional details on how vaccination categories were defined. Infected participants were ineligible for vaccination if their first infection date preceded when vaccines were available for children their age.
Overall, 45% of infected (338/751) and 33% of uninfected (48/147) school-age children and 39% of infected (1219/3109) and 27% of uninfected (372/1369) adolescents reported having at least 1 prolonged symptom. Twenty-six symptoms in infected school-age children and 18 symptoms in infected adolescents were prolonged in at least 5% of participants (Figure 1). The lower 95% confidence bound of the adjusted odds ratio exceeded 0 for 14 symptoms in both school-age children and adolescents, with 4 additional symptoms in school-age children only and 3 in adolescents only (Figure 1). The frequency of each symptom among infected participants did not differ after stratification into quintiles based on time between first infection and symptom survey date (eFigure 2 in Supplement 3).
Figure 1. Participants With Each Prolonged Symptom, Adjusted Odds Ratios, and Adjusted Risk Differences Comparing Infected vs Uninfected School-Age Children and Adolescents.
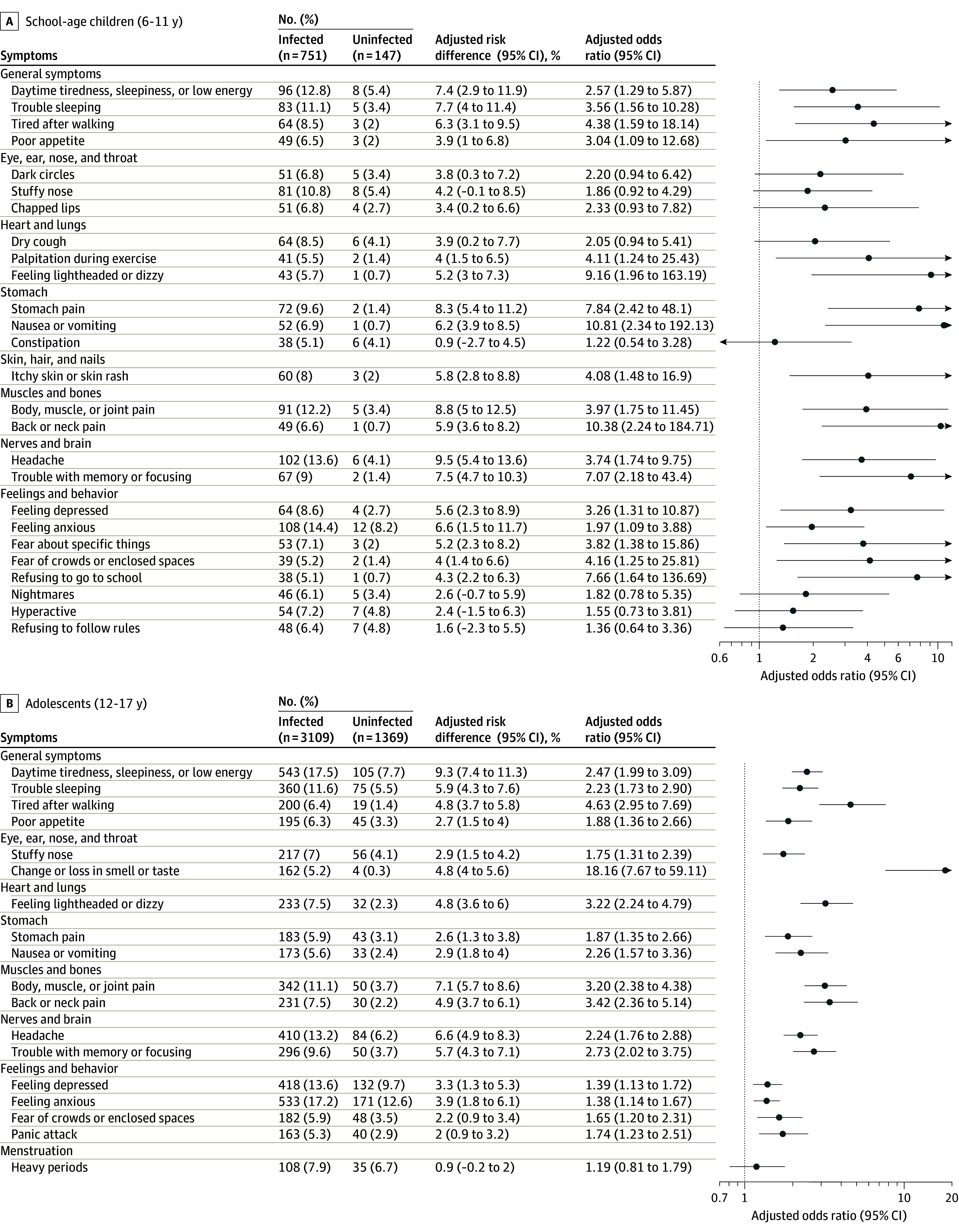
A symptom was included if at least 5% of infected or uninfected participants reported experiencing that symptom. Adjusted odds ratios and risk differences were estimated from models that included infection status as the exposure and the presence of each prolonged symptom as the outcome, with adjustment for sex assigned at birth and race and ethnicity (see eMethods in Supplement 3).
The LASSO analysis identified 10 symptoms in school-age children and 8 in adolescents that were most associated with infection history (Figures 2A and 3A). Optimal index thresholds of 5.5 in school-age children and 5.0 in adolescents were identified (Figures 2B and 3B). Overall, 152 infected (20%) and 6 uninfected (4%) school-age children and 445 infected (14%) and 44 uninfected (3%) adolescents met or exceeded this index threshold (eTable 6 in Supplement 3). This percentage was higher for participants infected before vs after the emergence of Omicron (21% vs 14% for school-age children; 17% vs 7% for adolescents). Correlations between symptoms that contributed to the index are shown in eFigure 3 in Supplement 3. Correlations between these symptoms and those that did not contribute to the index are shown in eTable 7 in Supplement 3. Some uninfected participants may have met the index threshold due to misclassification or due to having other symptoms.
Figure 2. Development of the Postacute Sequelae of SARS-CoV-2 Infection (PASC) Research Index and Threshold for School-Age Children (6-11 Years).
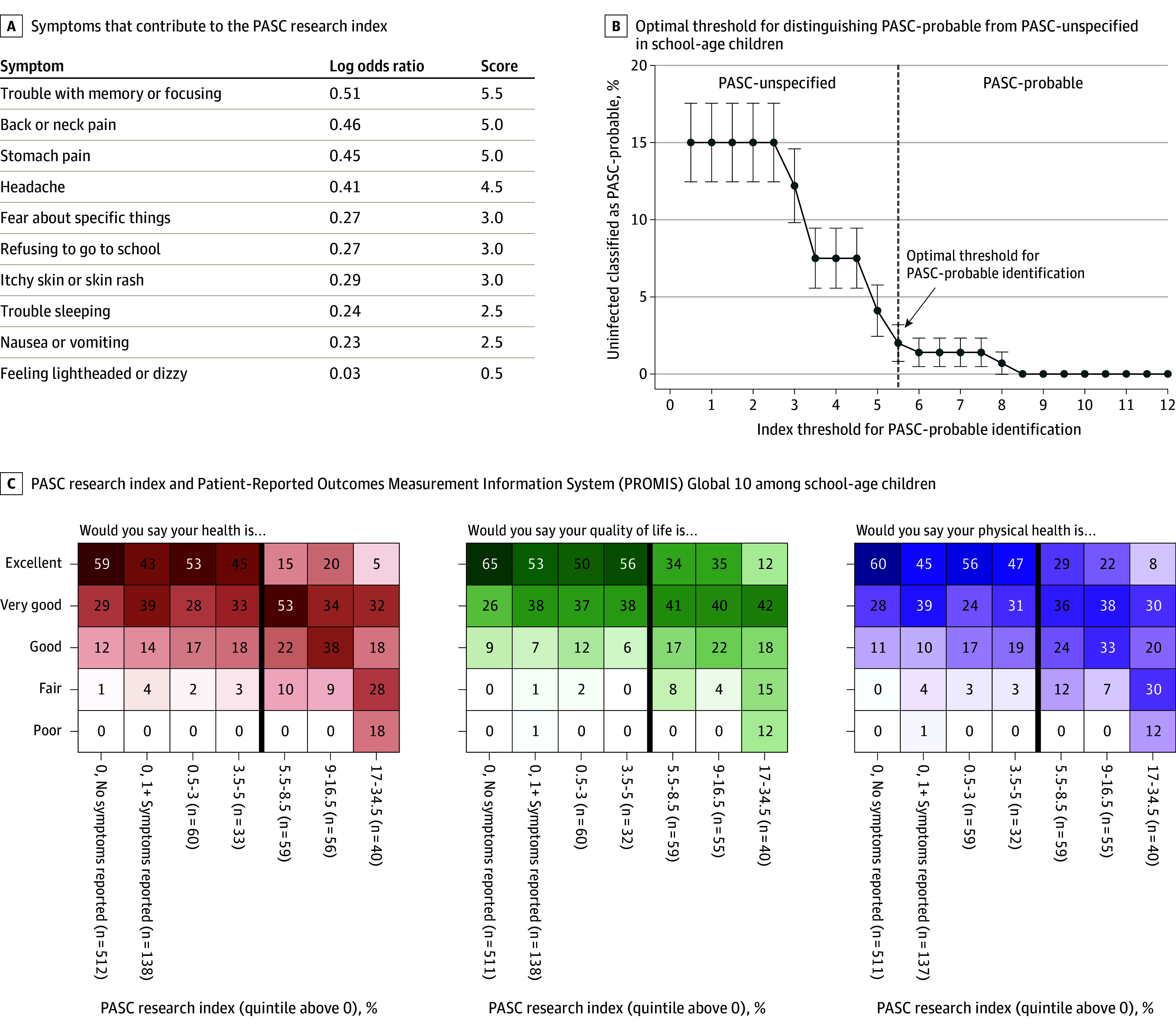
A, Least absolute shrinkage and selection operator (LASSO) was used to fit a logistic regression model to identify which symptoms could be used to identify individuals likely to have PASC. Estimated log odds ratios were divided by 0.10 and rounded up to the nearest 0.5 to calculate symptom scores. An individual’s PASC research index is calculated by summing the scores for each prolonged symptom a participant reported (ie, the participant experienced the symptom for 4 weeks since the beginning of the pandemic and is currently experiencing it at the time of the survey). B, The optimal index threshold for PASC was selected using bootstrapping to estimate standard error bars. An approximation of the “elbow” method was used to identify the cutoff where the number of uninfected participants misclassified as PASC-probable stabilized (eMethods in Supplement 3). The threshold (index of at least 5.5) can be used to identify school-age children with PASC for research purposes. Using this threshold, the percentage of infected PASC-probable school-age children with each symptom was as follows: headache, 55%; trouble with memory or focusing, 45%; trouble sleeping, 44%; stomach pain, 43%; nausea or vomiting, 34%; back or neck pain, 30%; itchy skin or skin rash, 29%; fear about specific things, 26%; feeling lightheaded or dizzy, 26%; and refusing to go to school, 23%. C, Participant responses to 3 questions from the Patient-Reported Outcomes Measurement Information System (PROMIS) Global 10 survey, stratified into 7 groups: participants with a zero PASC research index and no prolonged symptoms, zero PASC research index but at least 1 prolonged symptom, and participants with nonzero PASC index, divided into quintiles. The dark vertical line indicates the index threshold for PASC. Each cell is shaded according to the frequency of each response within each column, ranging from 0% to 100%.
Figure 3. Development of the Postacute Sequelae of SARS-CoV-2 Infection (PASC) Research Index and Threshold for Adolescents (Ages 12 to 17 Years).
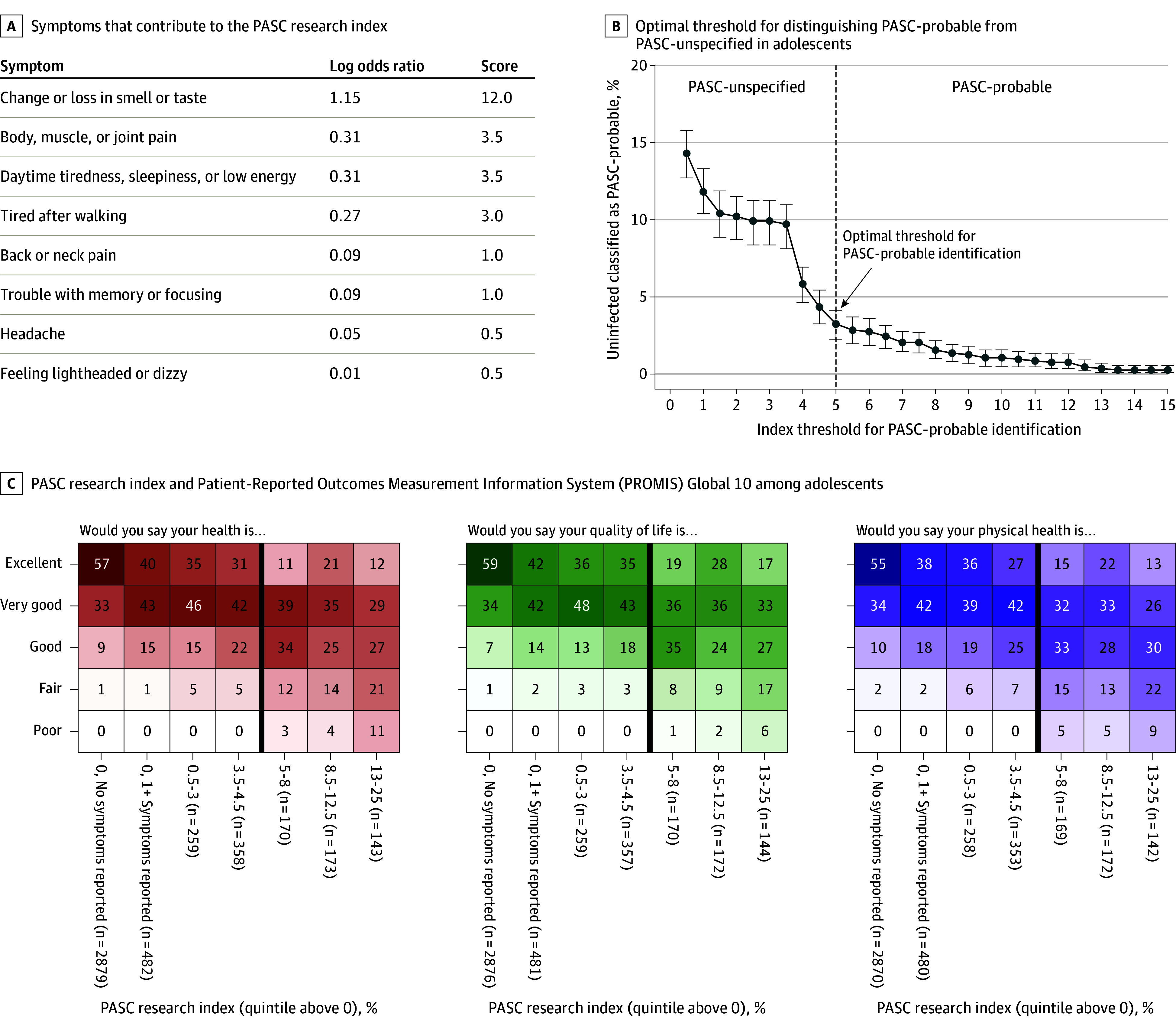
A, Least absolute shrinkage and selection operator (LASSO) was used to fit a logistic regression model to identify which symptoms could be used to identify individuals likely to have PASC. Estimated log odds ratios were divided by 0.10 and rounded up to the nearest 0.5 to calculate symptom scores. An individual’s PASC research index is calculated by summing the scores for each prolonged symptom a participant reported (ie, the participant experienced the symptom for 4 weeks since the beginning of the pandemic and is currently experiencing it at the time of the survey). B, The optimal index threshold for PASC was selected using 95% CIs to estimate error bars. An approximation of the “elbow” method was used to identify the cutoff where the number of uninfected participants misclassified as PASC-probable stabilized (eMethods in Supplement 3). The threshold (index of at least 5) can be used to identify adolescents with PASC for research purposes. Using this threshold, the percentage of infected PASC-probable adolescents with each symptom was as follows: daytime tiredness/sleepiness or low energy, 80%; body, muscle, or joint pain, 61%; headache, 56%; trouble with memory or focusing, 47%; tired after walking, 42%; back or neck pain, 40%; feeling lightheaded or dizzy, 39%; and change or loss in smell or taste, 34%. C, Participant responses to 3 questions from the Patient-Reported Outcomes Measurement Information System (PROMIS) Global 10 survey, stratified into 7 groups: participants with a zero PASC research index and no prolonged symptoms, zero PASC research index but at least 1 prolonged symptoms, and participants with nonzero PASC index, divided into quintiles. The dark vertical line indicates the index threshold for PASC (to the left is PASC-unspecified, to the right is PASC-probable). Each cell is shaded according to the frequency of each response within each column, ranging from 0% to 100%.
In both age groups, higher PASC research indices were correlated with worse PROMIS scores (Figures 2C and 3C). The number of systems affected among infected PASC-probable participants (eFigure 4 in Supplement 3) indicated substantial multisystem burden.
Figure 4 shows the percentage of participants in each age group experiencing each symptom after stratification into 3 subgroups: infected PASC-probable, infected PASC-unspecified, and uninfected. The most common prolonged symptom among PASC-probable school-age children that also contributed to the PASC research index (Figures 2B and 4) was headache (57%), followed by trouble with memory/focusing and trouble sleeping (44%) and stomach pain (43%). Among symptoms that did not contribute to the index, body/muscle/joint pain (51%), daytime tiredness/sleepiness or low energy (49%), and feeling anxious (47%) were the most common (Figure 4). The distribution of symptoms was similar between PASC-unspecified and uninfected school-age children.
Figure 4. Frequency of Prolonged Symptoms Among School-Age Children and Adolescents Stratified by Infection and PASC Status.
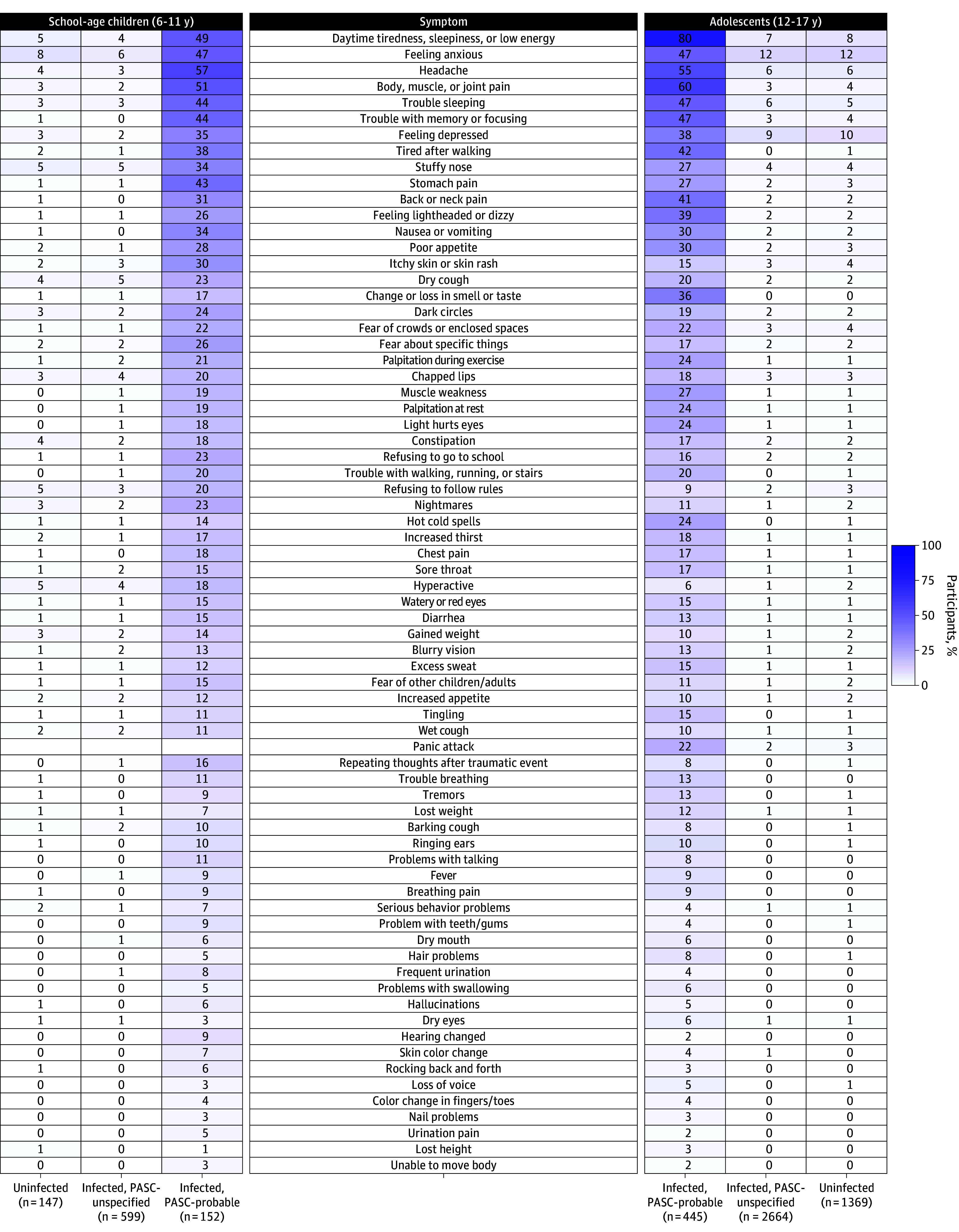
Symptoms, sorted from most to least common in the study population overall, are in the center column. Left columns correspond to school-age children in 3 groups: uninfected, infected and not meeting the PASC research index threshold (infected, PASC-unspecified), and infected and meeting the PASC research index threshold (infected, PASC-probable). The columns on the right correspond to adolescents with columns in the reverse order. Note that school-age children were not asked about panic attacks. Frequency of each prolonged symptom is indicated by shading, from 0% to 100%.
Among PASC-probable adolescents, the most common prolonged symptoms contributing to the index (Figures 3B and 4) were daytime tiredness/sleepiness or low energy (80%), body/muscle/joint pain (60%), headaches (55%), and trouble with memory/focusing (47%). Among symptoms that did not contribute to the index, trouble sleeping (47%), feeling anxious (47%), and feeling sad/depressed (38%) were the most common (Figure 4). The distribution of symptoms was similar between PASC-unspecified and uninfected adolescent participants.
Among school-age children, 4 symptom clusters were identified (Figure 5). Cluster 1 had high rates of many symptoms and the highest symptom burden. Cluster 2 was characterized by high rates of headache (95%), body/muscle/joint pain (60%), and daytime tiredness/sleepiness or low energy (52%). Cluster 3 was characterized by higher rates of trouble sleeping (64%) and trouble with memory/focusing (62%). Cluster 4 was characterized predominantly by stomach pain (100%) and nausea/vomiting (61%). Among adolescents, 3 clusters were identified (Figure 5). Cluster 1 had high rates of many symptoms, similar to the first school-age cluster. Cluster 2 was characterized by high rates of daytime tiredness/sleepiness or low energy (89%) and body/muscle/joint pain (87%). Cluster 3 was characterized by having change/loss in smell or taste (100%), with relatively low rates of all other symptoms. The clusters with the most symptoms in both school-age children and adolescents (cluster 1) had the highest mean number of systems affected (eTable 8 in Supplement 3) and were correlated with poorer overall health and quality of life (eFigure 5 in Supplement 3).
Figure 5. Defining Subgroups of Postacute Sequelae of SARS-CoV-2 Infection (PASC)–Probable Participants.
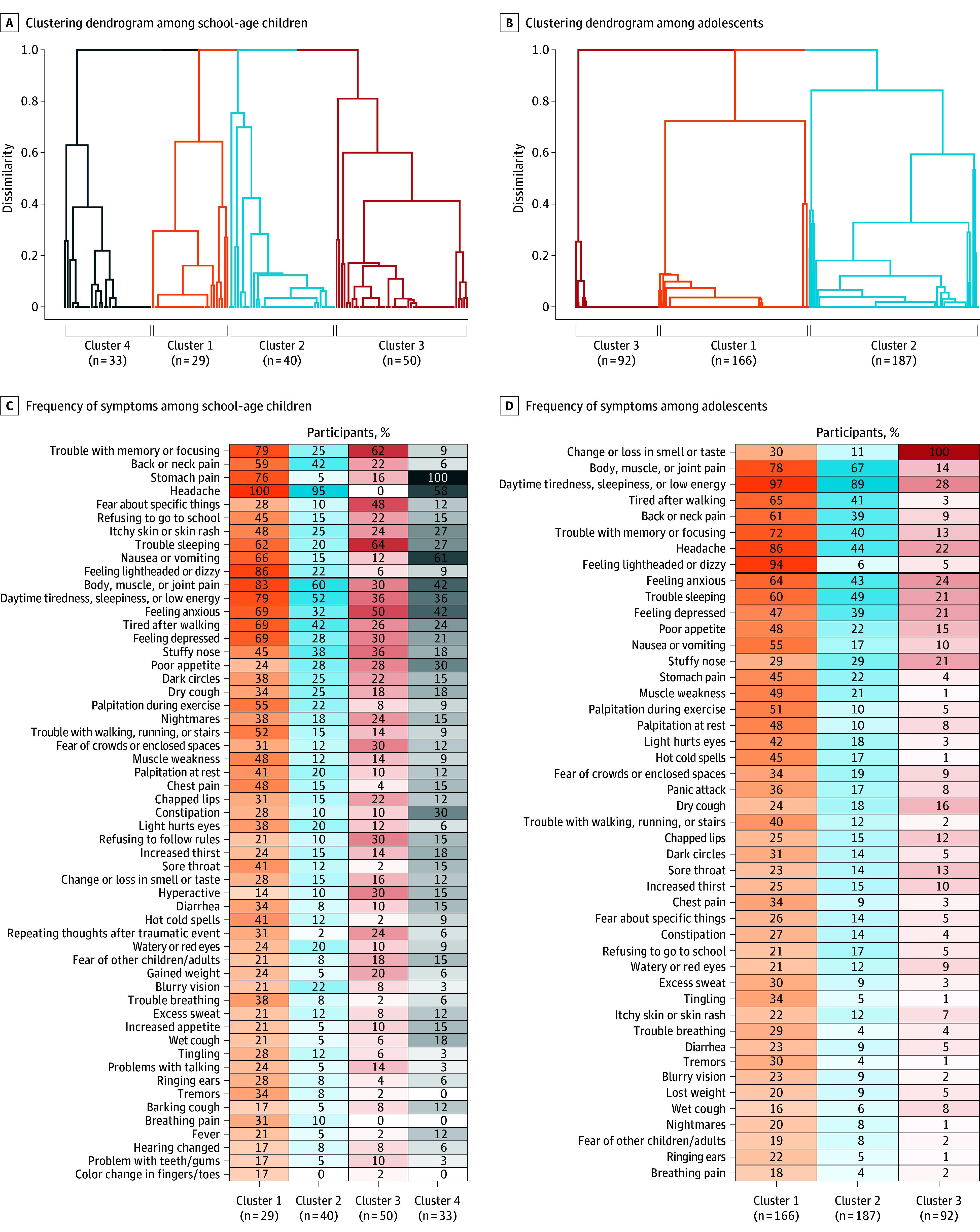
A and B, Subgroups formed using consensus clustering to group participants with similar symptom profiles (based on prolonged symptoms contributing to the PASC research index only). Four clusters were identified in PASC-probable school-age children and 3 clusters among adolescents. C and D, Frequencies of each prolonged symptom are shown for each cluster, where shading indicates frequency from 0%-100%. Symptoms that contribute to the PASC research index are above the dark horizontal line, and those below do not contribute to the PASC research index, sorted in decreasing frequency among all PASC-probable participants. Symptoms present in <5% of participants in every cluster were omitted. The full set of symptoms is in eFigure 6 in Supplement 3.
Overall, 64 school-age children and 781 adolescents enrolled as uninfected but were Ab+ (ie, asymptomatically infected; eFigure 1 and eTable 9 in Supplement 3). Among school-age children, 6 (9%) met the index threshold whereas 18 (28%) reported experiencing at least 1 prolonged symptom. Among adolescents, 29 (4%) met the index threshold and 175 (22%) reported at least 1 prolonged symptom.
Symptom frequencies for all groups (infected, uninfected, and uninfected Ab+), including estimated risk ratios and odds ratios, are shown in eTable 10 in Supplement 3.
Discussion
In this large-scale study, children with probable PASC experienced prolonged symptoms in almost every organ system, with the majority having multisystem involvement. A clear pattern of symptom differences was identified between school-age children and adolescents, underscoring the importance of characterizing PASC separately in these 2 age groups.
This study developed an empirically derived index that can be used to help researchers identify children likely to have PASC, which was associated with overall health, physical health, and quality of life. This PASC research index, distinct for each age group, used combinations of 10 symptoms in school-age children and 8 symptoms in adolescents to indicate the likelihood of PASC. Although many other symptoms were more common in infected compared with uninfected participants, symptoms selected for the index were those that were most associated with infection history. Because these other symptoms were highly associated with the symptoms selected for the index (eTable 7 in Supplement 3), it was rare for participants not meeting the index threshold to have these other symptoms (Figure 4). In this cohort, 20% of infected school-age children exceeded the PASC symptom threshold, while 14% of adolescents exceeded the threshold. PASC symptoms clustered into 4 distinct clusters in school-age children and 3 in adolescents.
The PASC research index presents a framework for future studies and can be used as a continuous or binary outcome variable (based on derived thresholds) to determine risk factors for developing PASC and the trajectory of PASC and its resolution (or relapse). Although this provisional index may be used for research, it is not intended for clinical practice, and 1 symptom may be sufficient to indicate PASC in any given child.
This study makes a substantial contribution to the understanding of pediatric PASC. Most research to understand PASC symptoms has focused on adults, potentially due to the misperception that children were not severely affected by COVID-19, leaving childhood symptoms less understood. Most prior pediatric studies have relied on electronic health records.30,31 The current study had the advantage of comprehensively assessing caregiver-reported symptoms across every organ system, examining them in combination, and comparing them directly to an uninfected seronegative control group. The symptoms identified as being related to PASC were associated with infection, not only symptoms that became more common during the pandemic.
This study identified separate PASC research indices for school-age children and adolescents based on symptoms most likely to differentiate between those with and without an infection history. Higher indices were correlated with worse functional outcomes, and those with indices meeting the PASC threshold reported many prolonged symptoms, not just those selected by LASSO.28 The strongest differentiators of infection history in adults (RECOVER-Adult study)15 and adolescents overlapped considerably. There was less overlap between adults and school-age children. These findings underscore the need for separate assessments in different age groups. This may be one reason that younger children with PASC are being undercounted in studies and/or undiagnosed clinically, although undercounting may also be due to younger children being less able to recognize and report symptoms. The pathophysiology behind these age-related differences warrants future study, given substantial changes in growth, development, immunological factors, and pubertal hormones that occur across the life course.11
Among infected participants, there was a wide range of time elapsed between infection and survey completion (median [IQR] time was 501 [297-801] days for school-age children and 518 [333-810] days for adolescents). However, symptom frequency did not change meaningfully when comparing different times between infection and survey completion, underscoring the usefulness of the PASC index for any child in the postacute phase of SARS-CoV-2 infection.
Four symptom clusters in school-age children and 3 in adolescents were identified. In both age groups, there was a single cluster with high symptom burden (as in adults) and a cluster predominated by fatigue and pain symptoms. Other clusters differed by age. School-age children had a cluster with neuropsychological and sleep impacts and another with gastrointestinal predominance. Adolescents had a cluster that was primarily loss of taste and smell,32 similar to that found in adults, which was not noted in the school-age clusters. Clusters predominated by respiratory symptoms were not identified, possibly related to community recruitment or few participants with severe acute illness. Future research should evaluate whether these pediatric clusters are associated with different pathophysiology from adults,33,34,35 which will be critical for identifying the treatment targets needed for clinical trials.36,37,38,39,40
Limitations
This study has limitations. First, the research index is not intended for use in clinical practice to diagnose PASC. Rather it must be considered with clinical judgement because children may have PASC without meeting the index threshold. There are many prolonged symptoms that differ between those previously infected and uninfected with SARS-CoV-2 that are not part of this index. It remains unknown how many children with other diagnoses would have similar prolonged symptoms. This index may evolve over time with changing variants and population immunity. Although children with higher PASC indices report worse quality of life, the cross-sectional analyses preclude causal inference. If a symptom lasted more than 4 weeks but was absent at survey completion, it was not included as a prolonged symptom because this index was not meant to describe incidence. However, it can be used for longitudinal follow-up of recovery and relapse, which would not be possible if resolved symptoms were used in the calculations.
Second, the population prevalence of pediatric PASC cannot be determined with the current design because participants with more prolonged symptoms may have been more inclined to enroll. To mitigate differences that may have resulted from having an extant adolescent cohort, community outreach within the school-age group was encouraged.
Third, some participants in the infected and uninfected groups could have been misclassified. Infected participants were not required to have evidence of SARS-CoV-2 infection; this study relied on caregiver-reported COVID-19 infection history, given variable access to testing. Uninfected children were confirmed to not have SARS-CoV-2 antibodies, but it is possible that some may have been unknowingly infected without developing antibodies or their immunity waned.41 Uninfected participants may have another postviral syndrome or other conditions that may have symptoms and even pathophysiology that overlaps with PASC.42 Despite this uncertainty, important differences between infected and uninfected groups were detected.
Fourth, given that symptoms were caregiver-reported, recall bias is possible. In addition, caregiver perceptions of their adolescents’ symptoms may differ from those of the adolescents themselves. However, to enable valid comparisons across age groups, data collection methods were standardized. Future analyses will combine caregiver-reported surveys with objective measures collected during the in-person longitudinal study phase.19
Fifth, this empirically derived index is a framework that identified commonalities for research purposes. Iterative adaptation of how PASC is assessed may occur as more RECOVER data are collected and as children are followed up. Future analyses will examine PASC symptoms in early childhood (birth to 5 years) and the effects of SARS-CoV-2 on worsening underlying conditions and increasing new conditions,43,44,45 such as diabetes,46 autoimmune diseases,47 neurocognitive disorders, and postinfectious syndromes.11
Conclusions
In this large-scale study, symptoms that characterized pediatric PASC differed by age group, and several distinct phenotypic PASC presentations were described. The research indices developed here will help researchers identify children and adolescents with high likelihood of PASC. Although these indices will require further research and validation, this work provides an important step toward a clinically useful tool for diagnosis with the ultimate goal of supporting optimal care for youth with PASC.
Trial protocol
Statistical analysis plan
eMethods
Nonauthor contributors
Data sharing statement
References
- 1.World Health Organization . Post COVID-19 condition (long COVID). December 7, 2022. Accessed February 27, 2024. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition
- 2.Centers for Disease Control and Prevention (CDC) . Long COVID . Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 3.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 Infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce CA, Herold KC, Herold BC, et al. COVID-19 and children. Science. 2022;377(6611):1144-1149. doi: 10.1126/science.ade1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long COVID and kids: more research is urgently needed. Nature. 2022;602(7896):183. doi: 10.1038/d41586-022-00334-w [DOI] [PubMed] [Google Scholar]
- 7.Elder GH. Perspectives on the life course. Life Course Dynamics: Trajectories and Transitions. Cornell University Press; 1995:23-49. [Google Scholar]
- 8.Funk AL, Kuppermann N, Florin TA, et al. ; Pediatric Emergency Research Network–COVID-19 Study Team . Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open. 2022;5(7):e2223253. doi: 10.1001/jamanetworkopen.2022.23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao S, Lee GM, Razzaghi H, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022;176(10):1000-1009. doi: 10.1001/jamapediatrics.2022.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12(1):9950. doi: 10.1038/s41598-022-13495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao S, Gross RS, Mohandas S, et al. Postacute sequelae of SARS-CoV-2 in children. Pediatrics. 2024;153(3):e2023062570. doi: 10.1542/peds.2023-062570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84(2):158-170. doi: 10.1016/j.jinf.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Romieu AC, Carton TW, Saydah S, et al. Prevalence of select new symptoms and conditions among persons aged younger than 20 years and 20 years or older at 31 to 150 days after testing positive or negative for SARS-CoV-2. JAMA Netw Open. 2022;5(2):e2147053. doi: 10.1001/jamanetworkopen.2021.47053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVER (Researching COVID to Enhance Recovery) . The RECOVER Initiative. Accessed February 26, 2024. https://recovercovid.org/
- 15.Thaweethai T, Jolley SE, Karlson EW, et al. ; RECOVER Consortium . Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi: 10.1001/jama.2023.8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. 2018;32:55-66. doi: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16-22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4-7. doi: 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross RS, Thaweethai T, Rosenzweig EB, et al. ; RECOVER-Pediatric Consortium . Researching COVID to enhance recovery (RECOVER) pediatric study protocol: rationale, objectives and design. PLoS One. 2024;19(5):e0285635. doi: 10.1371/journal.pone.0285635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22-e23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334-346. doi: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074-1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son MBF, Murray N, Friedman K, et al. ; Overcoming COVID-19 Investigators . Multisystem inflammatory syndrome in children: initial therapy and outcomes. N Engl J Med. 2021;385(1):23-34. doi: 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group . Incidence of multisystem inflammatory syndrome in children among us persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420. doi: 10.1001/jamanetworkopen.2021.16420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harahsheh AS, Portman MA, Khoury M, et al. Management of multisystem inflammatory syndrome in children: decision-making regarding a new condition in the absence of clinical trial data. Can J Cardiol. 2023;39(6):803-814. doi: 10.1016/j.cjca.2022.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis TC, Gazmararian J, Kennen EM. Approaches to improving health literacy: lessons from the field. J Health Commun. 2006;11(6):551-554. doi: 10.1080/10810730600835517 [DOI] [PubMed] [Google Scholar]
- 27.Forrest CB, Bevans KB, Pratiwadi R, et al. Development of the PROMIS ® pediatric global health (PGH-7) measure. Qual Life Res. 2014;23(4):1221-1231. doi: 10.1007/s11136-013-0581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; 2009. [Google Scholar]
- 29.Monti STP, Mesirov J, Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52(1):91-118. doi: 10.1023/A:1023949509487 [DOI] [Google Scholar]
- 30.Lorman V, Razzaghi H, Song X, et al. A machine learning-based phenotype for long COVID in children: an EHR-based study from the RECOVER program. PLoS One. 2023;18(8):e0289774. doi: 10.1371/journal.pone.0289774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorman V, Rao S, Jhaveri R, et al. Understanding pediatric long COVID using a tree-based scan statistic approach: an EHR-based cohort study from the RECOVER Program. JAMIA Open. 2023;6(1):ooad016. doi: 10.1093/jamiaopen/ooad016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellford SA, Moseman EA. Olfactory immune response to SARS-CoV-2. Cell Mol Immunol. 2024;21(2):134-143. doi: 10.1038/s41423-023-01119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson T, Pinto Pereira SM, Shafran R, et al. ; CLoCk Consortium . Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6(4):230-239. doi: 10.1016/S2352-4642(22)00022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zavala M, Ireland G, Amin-Chowdhury Z, Ramsay ME, Ladhani SN. Acute and persistent symptoms in children with polymerase chain reaction (PCR)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection compared with test-negative children in England: active, prospective, national surveillance. Clin Infect Dis. 2022;75(1):e191-e200. doi: 10.1093/cid/ciab991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahanic S, Tymoszuk P, Luger AK, et al. COVID-19 and its continuing burden after 12 months: a longitudinal observational prospective multicentre trial. ERJ Open Res. 2023;9(2):00317-2022. doi: 10.1183/23120541.00317-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machhi J, Herskovitz J, Senan AM, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15(3):359-386. doi: 10.1007/s11481-020-09944-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohandas S, Jagannathan P, Henrich TJ, et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12:e86014. doi: 10.7554/eLife.86014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12:e86002. doi: 10.7554/eLife.86002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malone LA, Morrow A, Chen Y, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of postacute sequelae of SARS-CoV-2 infection (PASC) in children and adolescents. PM R. 2022;14(10):1241-1269. doi: 10.1002/pmrj.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonilla H, Peluso MJ, Rodgers K, et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front Immunol. 2023;14:1129459. doi: 10.3389/fimmu.2023.1129459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi H, Liu B, Wang X, Zhang L. The humoral response and antibodies against SARS-CoV-2 infection. Nat Immunol. 2022;23(7):1008-1020. doi: 10.1038/s41590-022-01248-5 [DOI] [PubMed] [Google Scholar]
- 42.Minotti C, McKenzie C, Dewandel I, et al. How does post COVID differ from other post-viral conditions in childhood and adolescence (0-20 years old)? a systematic review. EClinicalMedicine. 2024;68:102436. doi: 10.1016/j.eclinm.2024.102436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph P, Singh I, Oliveira R, et al. Exercise pathophysiology in myalgic encephalomyelitis/chronic fatigue syndrome and postacute sequelae of SARS-CoV-2: more in common than not? Chest. 2023;164(3):717-726. doi: 10.1016/j.chest.2023.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe K. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in adolescents: practical guidance and management challenges. Adolesc Health Med Ther. 2023;14:13-26. doi: 10.2147/AHMT.S317314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchhorn R. Therapeutic approaches to dysautonomia in childhood, with a special focus on long COVID. Children (Basel). 2023;10(2):316. doi: 10.3390/children10020316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014. doi: 10.1001/jamanetworkopen.2022.33014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155-162. doi: 10.1097/BOR.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
Nonauthor contributors
Data sharing statement


