Abstract
Background
Mixed invasive ductal lobular carcinoma (mDLC) remains a poorly understood subtype of breast cancer composed of coexisting ductal and lobular components.
Methods
We sought to describe clinicopathologic characteristics and determine whether mDLC is clinically more similar to invasive ductal carcinoma (IDC) or invasive lobular carcinoma (ILC), using data from patients seen at the University of Pittsburgh Medical Center.
Results
We observed a higher concordance in clinicopathologic characteristics between mDLC and ILC, compared to IDC. There is a trend for higher rates of successful breast-conserving surgery after neoadjuvant chemotherapy in patients with mDLC compared to patients with ILC, in which it is known to be lower than in those with IDC. Metastatic patterns of mDLC demonstrate a propensity to develop in sites characteristic of both IDC and ILC. A meta-analysis evaluating mDLC showed shared features with both ILC and IDC with significantly more ER-positive and fewer high grades in mDLC compared to IDC, although mDLCs were significantly smaller and included fewer late-stage tumours compared to ILC.
Conclusions
These findings support clinicopathologic characteristics of mDLC driven by individual ductal vs lobular components and given the dominance of lobular pathology, mDLC features are often more similar to ILC than IDC. This study exemplifies the complexity of mixed disease.
Subject terms: Breast cancer, Breast cancer
Background
Breast cancer remains a common cancer in women in both the US [1] and worldwide [2], and has recently surpassed lung cancer as the most frequently diagnosed cancer [3]. With regard to histologic classification, the majority (>75%) of invasive breast cancers are of no special type (NST) frequently referred to as invasive ductal carcinoma (IDC). Invasive lobular carcinoma (ILC) is the most common special subtype, observed in approximately 15% of cases based upon recent estimates [4]. Various less frequent subtypes contribute to the remaining cases and are relatively understudied. Mixed invasive breast carcinomas are an elusive category of disease, which per the World Health Organization (WHO) classification in 2019 are defined as those exhibiting a specialised histologic pattern (such as ILC) occupying 10–90% of the tumour area and a non-specialised type (like NST or IDC) occupying at least 10% of the tumour [5]. It is important to note that this definition, as outlined in the recent 5th edition of the WHO classification of breast cancer, differs significantly from the definition provided in the 4th edition, published in 2012 [6]. The previous definition required that a specialised histologic subtype occupy >50% of the tumour area in order to be classified as a mixed tumour. This change in definition, while welcome, reflects the fluid interpretation of the mixed tumour concept and may make a comparison of ongoing and past studies of this entity more complex. Regardless of the change in criteria for mixed tumours, those composed of ILC and IDC patterns are the most common and have been referred to in the literature in various ways but are abbreviated here as mixed invasive ductal and lobular carcinoma (mDLC).
Distinguishing features between IDC and ILC are increasingly being appreciated beyond their well-defined differences in tissue architecture and immunohistochemical staining. Notably, ILC lacks E-cadherin expression and demonstrates cytoplasmic p120 rather than membrane staining seen in IDC [7]. Clinically, ILC more commonly presents as a larger tumour at diagnosis, occurs in older women, and is more likely to be hormone receptor positive, HER2 negative, and lower grade as compared to IDC [8–11]. ILC further demonstrates a predilection for metastatic spread to unique sites including the peritoneum, ovaries, and gastrointestinal tract, in addition to those shared with IDC [10–13].
The literature surrounding mDLC is limited, with the identification of cohorts largely reliant (with some exceptions) on ICD codes within cancer registries, which relies on abstraction using ever-changing algorithms that may not provide a sufficient definition for all nuances in a wide range of cases. mDLC has generally been found to display hormone receptor and HER2 receptor status between that of ILC and IDC, with higher likelihood to be hormone receptor positive and lower likelihood to be HER2 positive [14–20]. Previous studies have shown that mDLC, similar to ILC, are typically more often present as larger tumours than IDC and occur in patients at a median age younger than ILC [21, 22]. Differences in hormone receptor positivity and proliferative status are thought to contribute to the increased response of IDC to neoadjuvant chemotherapy as compared to ILC [23–25]. Given the mixed composition of mDLC, it is of interest—yet, unknown—whether the response to neoadjuvant therapy is associated with a dominant underlying histology.
In the retrospective analysis provided herein, we evaluated the clinicopathologic characteristics of mDLC including metastatic pattern of spread, as well as responses to neoadjuvant therapy as compared to IDC and ILC using clinical data from our institution. We sought to determine whether mDLC demonstrates clinical and pathologic characteristics more similar to either IDC or ILC, which may suggest that one histologic component is more dominant when both co-exist. Finally, we performed a meta-analysis of key defining parameters, comprehensively including all available mDLC literature with cases fitting inclusion criteria for the analysis.
Materials and methods
Objectives
The primary objective of this study is to perform a comprehensive clinicopathologic characterisation of mDLC in reference to the better-understood and more common invasive breast carcinoma subtypes of IDC (NST) and ILC. Key clinical and pathologic parameters are compared as the primary objective with a secondary goal of evaluating the metastatic pattern of spread and relative response to chemotherapy of mDLC. Secondary goals of this study included meta-analysis of available literature to determine the concordance of our findings with prior studies given the evolving definition of mDLC over time.
Study population
Institutional review board approval was obtained from the University of Pittsburgh prior to the initiation of the study. Patients diagnosed with and treated for invasive breast carcinoma between 1990 and 2017 at UPMC Magee-Womens Hospital were identified from the UPMC Network Cancer Registry using ICD-O-3 codes. Inclusion criteria included patients with Stage I–IV disease with either infiltrating ductal carcinoma (ICD-O-3 85003), infiltrating lobular carcinoma (ICD-O-3 85203), or infiltrating ductal and lobular carcinoma (ICD-O-3 85223). A total of 12,979 patients with IDC, 1569 patients with ILC, and 803 patients with mDLC were identified based on the aforementioned ICD-O-3 coding, which in turn was reliant on the interpretation of histology by the pathologist of record at the time of diagnosis. Histology classification was not changed or re-interpreted and any potential error due to changing guidelines was not accounted for in our study.
Clinical and tumour characteristics including patient race, age at diagnosis, menopausal status, tumour size, grade, stage, and oncotype Dx score were provided in the cancer registry. ER, PR, and HER2 statuses were also available in the database and entered as per standard guidelines at the time of diagnosis. Survival data was determined based on the date of diagnosis and date of disease recurrence or death for disease-free survival (DFS) and overall survival (OS), respectively.
From the cohorts analysed, 1568 patients with IDC, 113 patients with ILC, and 26 patients with mDLC were identified who had received neoadjuvant therapy based on treatment sequence data available in the database. A 249 case sub-cohort of the 1568 patients with IDC was selected, which was matched to the ILC cohort by age, ER, and HER2 status. Patients with prior history of in situ disease receiving surgical or radiation intervention were excluded. Cases in which definitive surgery was not performed, and cases where a concomitant invasive breast tumour of different histology was present were excluded. Individual chart review of the remaining 218 IDC, 82 ILC, and 25 mDLC cases was performed to determine type of neoadjuvant therapy, per cent tumour volume reduction, and rate of successful breast-conserving therapy.
From the original cohorts evaluated, follow-up data were available to identify 1131 patients with IDC, 145 patients with ILC, and 46 patients with mDLC who had developed metastatic spread throughout the course of their disease. Metastasis data were available in the cancer registry for bone, liver, lung, CNS, pleura and distant lymph nodes. Manual chart review was performed for cases in which additional metastatic sites were present but where the location was not specified in the Cancer Registry (6 mDLC, 23 ILC, 72 IDC).
Meta-analysis
For purposes of meta-analysis, clinicopathologic data abstracted as above were compared to data available in the literature pertaining to hormone receptor status, tumour size, grade, and stage at the time of diagnosis. To identify eligible publications, we accessed Pubmed.gov on 21 May 2020 and used the search term ‘mixed invasive ductal lobular breast cancer’ yielding 200 results. All entries were manually reviewed with publications containing data pertaining to any or all parameters of interest such as age, stage, grade, tumour size, ER/PR status, and HER2 status being included in the meta-analysis. Studies in which data were presented in a format that precluded statistical comparison with other studies were excluded. Publications included were limited to studies encompassing at least 10 patients per cohort, and directly comparing mDLC to either IDC, ILC, or both. Two additional studies meeting inclusion criteria that were identified in prior literature searches were included. Ultimately, 23 individual studies comprising data for almost 50,000 patients with mDLC were included in the meta-analysis (Supplementary Fig. S1 and Supplementary Table S1).
Immunohistochemistry
The slides are deparaffinised and rehydrated using a standard histology protocol. Antigen retrieval was performed using a citrate buffer (Agilent/Dako, Carpinteria, CA). The slides were stained using an Autostainer Plus (Dako) platform with TBST rinse buffer (Dako). The E-Cadherin ([EP913(2)Y], Abcam (Cambridge, MA), Cat# ab76319) was applied using a 1:100 dilution at room temperature. The detection reagent consisted of Mach 2 Rabbit HRP polymer, (Biocare Medical, Pacheco, CA). For p120 staining, p120 (Catenin δ-1 (D7S2M, Cell Signaling (Danvers, MA), Cat# ab59854) was applied using a 1:100 dilution at room temperature. The detection reagent consisted of SignalStain Boost Rabbit HRP polymer, (Cell Signaling, Danvers, MA). The substrate used was 3,3, Diaminobenzidine+ (Dako). Lastly, the slides were counterstained with Hematoxylin (Dako).
Statistical analysis
Descriptive and survival analysis: To compare the difference of demographic and clinicopathologic characteristics among the three cohorts, Wilcoxon and χ2 tests were used for the continuous variables (age, BMI and tumour size) and all the remaining categorical variables, respectively. Similarly, we applied χ2 test to compare locoregional treatment and systemic therapy, as well as the responses to neoadjuvant therapy, in the three cohorts. Kaplan–Meier was used for the estimation of OS and DFS. The p value was calculated by the log-rank test. DFS analysis was limited to patients with Stage I-III disease, while OS was inclusive of patients with all stages.
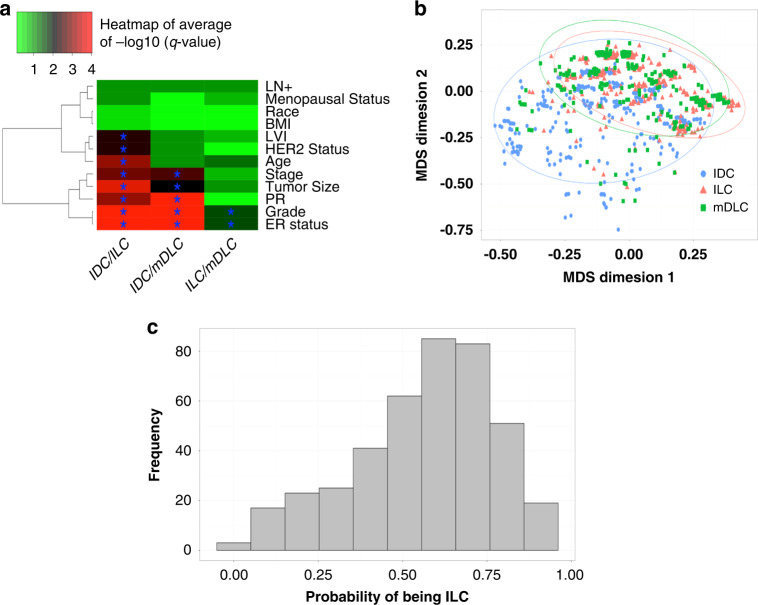
Dimension reduction and statistical learning: to systematically determine whether mDLC has more concordance with IDC or ILC, we used clinical variables (LN+, menopausal status, race, BMI, lymphovascular invasion (LVI), Her2 status, age, stage, tumour size, PR, grade and ER status) to build several unified models. First, 50 times subsampling was conducted and each time we subsampled 409 IDC and ILC in order to use the same size cohorts for the analysis. For each subsampled data, χ2 test and Wilcoxon test were conducted to calculate the p value between each pair of cohorts for categorical variables and continuous variables (age, BMI and tumour size), respectively. The raw p values are transformed into adjusted p values (q values) by Benjamini–Hochberg procedure and –log10 (q values) are utilised to draw the heatmap (Fig. 3a) to show the similarity among the three cohorts. Adjusted p value smaller than 0.05 are labelled with (*) symbol and for visualisation purpose, any q value smaller than 10–4 is cut at 10–4. Multidimensional scaling (MDS) plot was performed to visualise the data in two dimensions using the variables which distinguished the three subgroups (i.e., average p values among 50 subsampling smaller than 0.01 for any pair of cohorts). Finally, we built a classifier using an elastic-net regression model where we use the same variables in MDS with the exception of tumour size, due to too many missing values (N = 55 in mDLC). The best tuning parameter was determined by 10-fold cross-validation using R package ‘glmnet’ [26], the classifier was applied to mDLC data and the probability of the tumour being ILC is presented in a histogram.
Fig. 3. Concordance of clinicopathologic parameters comparing mDLC, IDC, and ILC.
a Heatmap visualisation of concordance of clinicopathologic parameters comparing subsampled mDLC, IDC, ILC. The values represent average –log10 (q value) for all comparisons using subsampled data (50 times). χ2 test and wilcoxon test were conducted to calculate the p value between each pair of cohorts for categorical variables and continuous variables. Adjusted p values (q values) are calculated by Benjamini–Hochberg (BH) correction to control false discovery rate and adjusted p values (q value) less than 0.05 are highlighted (*). A larger value (red) indicates a smaller and more significant p value for the comparison between two groups reflecting more differences. A smaller value (green) indicates a larger and less significant p value between two groups reflecting more similarities between the two histological subtypes. b Multidimensional scaling (MDS) incorporating clinicopathologic characteristics from 1 out of 50 subsampling, using the variables which can distinguish the three subgroups (i.e., average p value among 50 subsampling is smaller than 0.01 for any pair of cohorts). c Elastic-net regression predicting probability of mDLC to align with ILC vs IDC. All IDC and ILC data are taken to build a classifier by elastic-net regression model where we use the same variables in MDS except tumour size, due to too many missing values. The best tuning parameter is determined by 10-fold cross-validation. The classifier is applied to mDLC data and the probability of being ILC is drawn in this figure.
For the meta-analysis, categorical variables (stage, tumour size [transformed to T1, T2 and T3], hormone receptor status and grade) were compared and the log odds ratio was calculated (e.g., grade 1 versus grade 2 + 3, size T1 versus size T2 + T3, etc.). The log odds ratio from each study was combined by random-effects model using the ‘rma’ function with the default estimation method ‘REML’ in the R package ‘metafor’ [27] and the forest plot was generated from the ‘ggforestplot’ package for visualisation.
Results
Patient and clinicopathologic characteristics
Manual chart review of 803 potential mDLC cases was performed and critical review of the pathology reports led to the exclusion of 393 cases that were not consistent with mDLC classification. Analysis was limited to the remaining 410 cases of mDLC which were described by the interpreting pathologist as either mixed invasive carcinoma (N = 248), IDC with lobular features (N = 138), or ILC with ductal features (N = 24) (Supplementary Table S2 and Supplementary Fig. S2). Representative histologic images of mDLC are shown in Fig. 1a. We further excluded cases with pathological ‘stage 0’ or grade 4 (137 IDC, 7 ILC and 1 mDLC). The total sample size for the remaining analysis is 12,842 IDC, 1562 ILC and 409 mDLC, unless specifically mentioned.
Fig. 1. Mixed invasive ductal lobular carcinoma contains co-existing ductal and lobular histologies. a.
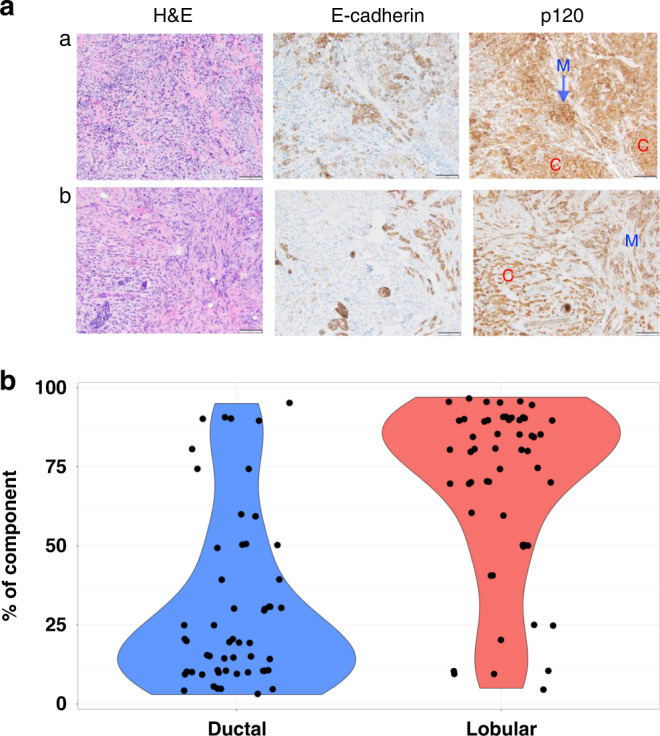
Histology examples of mixed invasive ductal lobular carcinoma. Left panel: H&E images of mDLC. Middle and right panel: IHC for E-Cadherin and b-catenin/p120. Loss of functional E-Cadherin results in the loss of lack of membranous (M) staining, and instead staining of p120 that is now accumulated in the cytoplasm (C). b Distribution of ductal and lobular components within individual mDLC tumours demonstrated by violin plot. Dots represent proportion of indicated histology within individual mDLC tumours and represent N = 54 cases. The p value from paired t-test of two components is smaller than 0.001.
Within the mDLC cohort, the pathology reports of 54/409 (13%) cases at diagnosis provided an estimate of the percentage of ductal vs lobular components by the interpreting pathologist. mDLC cases were on average composed of 31% ductal and 69% lobular components, and the distribution is shown in Fig. 1b.
From the total of 14,813 cases, we compared 409 cases of mDLC (2.7%) with N = 1562 cases of ILC (10.4%), and N = 12,842 cases of IDC (86.7%) (Table 1). Our main question was the comparison of clinicopathological features of mDLC with ILC and with IDC, and to allow comprehensive evaluation we also included a comparison of IDC vs ILC (Supplementary Table S3). Sizes of mDLC (19 mm) were on average larger than IDC (16 mm) (p < 0.001) yet smaller than ILC (20 mm) (p = 0.036). Similarly, patients with mDLC (59 years) were older in age than in patients with IDC (57 years) (p = 0.014) yet younger than those with ILC (61 years) (p = 0.006). The rate of ER positivity in mDLC (92%) fell between IDC (78%) (p < 0.001) and ILC (96%) (p < 0.001). Similar to ILC (6%), mDLC was less likely to be HER2 positive compared to IDC (8% vs 15%, p = 0.04).
Table 1.
Patient demographic and tumour clinicopathologic characteristics.
| mDLC (N = 409) | ILC (N = 1562) | p value | IDC (N = 12,842) | p value | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.73 (23.63, 31.13) | 26.99 (23.61, 31.82) | 0.54 | 26.91 (23.29, 31.56) | 0.98 |
| Unknown/missing | 35 | 138 | 1313 | ||
| Tumour size (mm) | 19 (12, 27.75) | 20 (12, 35) | 0.036 | 16 (10, 25) | <0.001 |
| Unknown/missing | 55 | 213 | 1388 | ||
| Age at diagnosis (years) | 59 (49, 68) | 61 (51, 70) | 0.006 | 57 (48, 67) | 0.014 |
| Menopausal status | |||||
| Pre/perimenopausal | 123 (32%) | 381 (26%) | 0.028 | 3914 (33%) | 0.69 |
| Postmenopausal | 266 (68%) | 1091 (74%) | 8050 (67%) | ||
| Unknown/missing | 20 | 90 | 878 | ||
| Race | |||||
| White | 386 (95%) | 1476 (95%) | 0.97 | 11,957 (93%) | 0.59 |
| Black | 18 (4%) | 69 (4%) | 713 (6%) | ||
| Other | 5 (1%) | 17 (1%) | 172 (1%) | ||
| ER | |||||
| Positive | 372 (92%) | 1455 (96%) | <0.001 | 9579 (78%) | <0.001 |
| Negative | 32 (8%) | 56 (4%) | 2779 (22%) | ||
| Unknown/missing | 5 | 51 | 484 | ||
| PR | |||||
| Positive | 339 (84%) | 1229 (82%) | 0.36 | 8530 (70%) | <0.001 |
| Negative | 63 (16%) | 266 (18%) | 3736 (30%) | ||
| Unknown/missing | 7 | 67 | 576 | ||
| HER2 | |||||
| Positive | 11 (8%) | 43 (6%) | 0.49 | 814 (15%) | 0.04 |
| Negative | 126 (89%) | 711 (92%) | 4316 (81%) | ||
| Equivocal | 4 (3%) | 16 (2%) | 190 (4%) | ||
| Unknown/missing | 268 | 792 | 7522 | ||
| Stage (pathologic) | |||||
| I | 157 (42%) | 601 (44%) | 0.053 | 6245 (57%) | <0.001 |
| II | 167 (45%) | 527 (38%) | 3727 (34%) | ||
| III | 42 (11%) | 215 (16%) | 812 (7%) | ||
| IV | 5 (2%) | 27 (2%) | 151 (2%) | ||
| Unknown/missing | 38 | 192 | 1907 | ||
| Grade | |||||
| 1 | 45 (12%) | 192 (16%) | <0.001 | 1634 (15%) | <0.001 |
| 2 | 263 (70%) | 937 (74%) | 5394 (47%) | ||
| 3 | 66 (18%) | 130 (10%) | 4380 (38%) | ||
| Unknown/missing | 35 | 303 | 1434 | ||
| Oncotype Dx RS | |||||
| Low risk (0–17) | 46 (65%) | 226 (67%) | 0.79 | 1105 (61%) | 0.13 |
| Intermediate risk (18–30) | 20 (29%) | 86 (26%) | 457 (25%) | ||
| High risk (>30) | 4 (6%) | 25 (7%) | 257 (14%) | ||
| Unknown/missing | 339 | 1225 | 11,023 | ||
| Lymphovascular invasion | |||||
| Yes | 55 (0.19) | 125 (0.14) | 0.056 | 2074 (0.25) | 0.02 |
| No | 239 (0.81) | 775 (0.86) | 6271 (0.75) | ||
| Unknown/missing | 116 | 669 | 4634 | ||
Median (interquartile) is shown for BMI, tumour size and age at diagnosis, and frequency (%) is shown for all remaining categorical characteristics.
Among the 70 mDLC, 337 ILC, and 1819 IDC cases in which information on Oncotype Dx was available, there were no significant differences between mDLC and other groups (Table 2), although IDC had proportionately more high-risk RS tumours compared to ILC (p = 0.003, Supplementary Table S3). Similar to the distribution of RS in ILC, mDLC demonstrated a higher proportion of tumours with low and intermediate-risk RS and less frequent high-risk RS tumours (ns).
Table 2.
Response rates in mDLC patients treated with neoadjuvant therapy compared to IDC and ILC.
| Neoadjuvant chemotherapy | |||||
| mDLC (N = 17) | ILC (N = 57) | p value | IDC (N = 180) | p value | |
| Volume reduction (%) | 78.5 (62.38, 92.5) | 76 (26.5, 90.5) | 0.22 | 75 (40.75, 95.62) | 0.29 |
| BCS attempted | |||||
| Yes | 9 (53%) | 19 (33%) | 0.16 | 70 (39%) | 0.34 |
| No | 8 (47%) | 38 (67%) | 110 (61%) | ||
| Rate of successful BCS | |||||
| Yes | 5 (56%) | 6 (32%) | 0.41 | 49 (70%) | 0.45 |
| No | 4 (44%) | 13 (68%) | 21 (30%) | ||
| pCR | |||||
| Yes | 3 (18%) | 4 (7%) | 0.34 | 24 (13%) | 0.71 |
| No | 14 (82%) | 52 (93%) | 156 (87%) | ||
| Neoadjuvant endocrine therapy | |||||
| Rate of successful BCS | mDLC (N = 7) | ILC (N = 21) | p value | IDC (N = 37) | p value |
| Yes | 2 (40%) | 6 (60%) | 0.61 | 14 (67%) | 0.34 |
| No | 3 (60%) | 4 (40%) | 7 (33%) | ||
| pCR | |||||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | ||
| No | 7 (100%) | 21 (100%) | 37 (100%) | ||
Volume reduction is presented as a percentage. Median (interquartile) is shown for volume reduction and frequency (%) is shown for other categorical variables.
BCS breast-conserving surgery, pCR pathologic complete response.
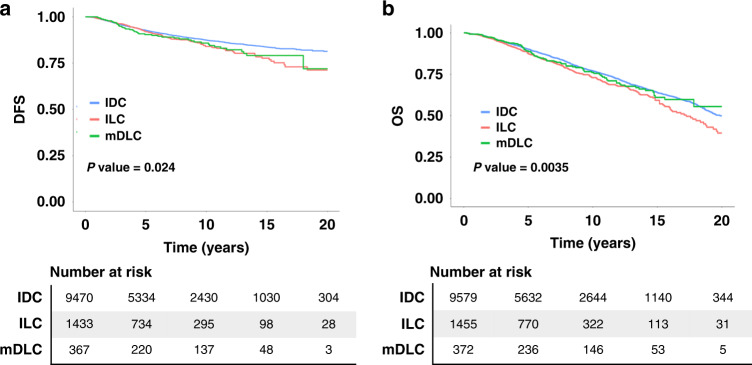
DFS and OS of patients with mDLC were not significantly different from ILC or IDC (data not shown), although patients with IDC demonstrated superior outcomes compared to the ILC cohort when the analysis was limited to ER+ cases (Fig. 2). DFS for patients with ER+ IDC vs ER+ ILC are 93% and 92% at 5 years, 87% and 84% at 10 years, 84% and 78% at 15 years, and 81% and 71% at 20 years (p = 0.006).
Fig. 2. Disease free survival and overall survival of patients with mDLC falls between what is observed for patients with IDC and ILC.
Disease-free survival (a) and overall survival (b) of patients with ER-positive cases of mDLC, ILC, and IDC. The p values are calculated by log-rank test. The p values of pairwise comparison are adjusted by Bonferroni correction. Disease-free survival analysis is limited to patients with Stage I–III disease.
Concordance of clinicopathologic parameters between mDLC and ILC vs IDC
Next, we sought to assess concordance between the groups based on the overlap of key clinical and pathologic characteristics using machine-learning models. Heatmap visualisation of results from χ2 test of subsampled data is presented in Fig. 3a, visualised by a gradient from green, signifying no significant differences, to red, indicating highly significant differences (adjusted p value ≤10–4. These data demonstrate strong concordance between mDLC and ILC in most of the parameters, and lesser concordance between mDLC and IDC in most of the parameters (Fig. 3a).
Incorporating data from tumour size, age at diagnosis, ER, PR, HER2, stage, grade, and LVI parameters, dimension reduction of subsampled data by MDS also demonstrated significant overlap of the mDLC and ILC cohorts (Fig. 3b). The IDC cohort has a larger variance and a part of IDC cohort does not align with ILC and mDLC cohorts. An elastic-net regression model based on clinicopathologic parameters displaying moderate separation between IDC and ILC (cross-validation area-under-the-curve of IDC and ILC = 0.75) was devised. mDLC was predicted to align more closely with ILC than IDC, with 272 cases showing a probability larger than 0.5 and 198 cases with a probability larger than 0.6 (Fig. 3c).
Comparison of adjuvant systemic, surgical, and radiation therapies
There were no significant differences in adjuvant treatment for patients with mDLC vs ILC (Supplementary Table S4). Compared to patients with IDC, patients with mDLC were less likely to be treated with chemotherapy (45% vs 52%, p = 0.007), and more likely to receive hormone therapy (89% vs 73%, p < 0.001) (Supplementary Table S4). The difference in rates of chemotherapy received between mDLC and IDC was lost once analysis was limited to patients with ER+ disease; however, patients with ER+ mDLC received more hormone therapy (95%) than patients with ER+ IDC (90%) (p = 0.004) (Supplementary Table S5). Within ER+ cohorts, patients with ILC were less likely to receive chemotherapy, and more likely to receive hormone therapy compared to IDC (Supplementary Table S4). There were no significant differences between the different cohorts in receiving radiation therapies.
In contrast, surgical treatments for patients with mDLC were closer to those with IDC (Supplementary Tables S4 and S5). Specifically, patients with ER+ mDLC were more likely to undergo breast-conserving surgery (BCS) (64%) vs mastectomy (36%) which was the same as in patients with ER+ IDC. In contrast, patients with ER+ ILC were more likely to undergo mastectomy (51%) than BCS (49%). This was significantly different compared to patients with ER+ IDC (p < 0.001). Finally, as expected, patients with ILC were less likely than those with IDC to undergo initial BCS vs mastectomy (49% vs 64%, p < 0.001, Supplementary Table S6).
Response to neoadjuvant therapy
Patients with mDLC (N = 26), ILC (N = 113), and IDC (N = 1568) that received neoadjuvant therapy were identified from the original cohort of patients. Some patients were excluded from further analysis as described in the consort diagram shown in Supplementary Fig. S3. Within the IDC cohort, a smaller sub-cohort of patients matched for age, ER and HER2 status were selected for this analysis requiring chart review. 23/25 mDLC, 77/82 ILC and 209/218 IDC cases were ER-positive.
There were no differences in tumour volume reduction or pathological complete response rates (pCR) between the groups receiving neoadjuvant chemotherapy (Table 2). BCS was attempted at a similar rate in patients with mDLC compared to those with ILC or IDC. Successful BCS with no further need for subsequent surgical intervention was achieved in 56% mDLC cases, compared to 70% IDC and 32% ILC (mDLC vs ILC and IDC ns; IDC vs ILC p = 0.003; Table 2 and Supplementary Table S7).
A limited number of patients had received neoadjuvant endocrine therapy with no significant differences noted between cohorts with regard to successful BCS or pCR rates. pCR was not achieved with neoadjuvant endocrine therapy in either of the cohorts (Table 2). There were no significant differences in DFS or OS in patients receiving neoadjuvant endocrine therapy, although a trend for inferior outcomes in patients with IDC as compared to ILC was persistently noted (Supplementary Fig. S4). Ki-67 indices of mDLC tumours (20%) from patients receiving neoadjuvant therapy were the same as those ILC (20%), but lower than those compared to IDC (40%) (p = 0.008) (Supplementary Fig. S5A). There was no significant difference in average ER H-score or PR H-score between these sub-cohorts (Supplementary Fig. S5B, C).
Metastatic pattern of spread
The Cancer Registry contains information on recurrence including sites of metastases, although these data are incomplete due to the nature of cancer registries being designed to collect comprehensive information for primary and not metastatic disease. Acknowledging the limitations, we extracted data on recurrences when available. From the original cohort we identified 46 mDLC, 145 ILC, and 1,131 IDC in which distant metastasis had occurred.
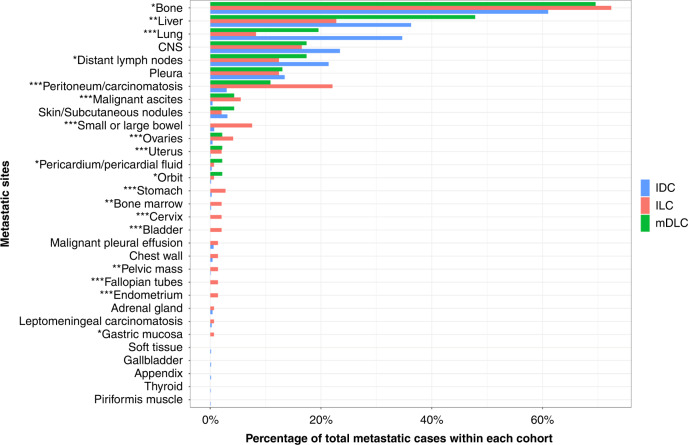
Patients with mDLC developed metastasis in common sites including bone, lung, liver, and CNS at an incidence similar to ILC and IDC, and included a pattern of spread to unique sites including the pericardium, ovaries, uterus, and orbit (Fig. 4 and Supplementary Table S8). mDLC demonstrated a pattern of metastatic spread with no statistically significant differences in incidence at any site compared to ILC. Compared to IDC, mDLC was proportionately more likely to metastasise to the peritoneum (p = 0.02), pericardium (p = 0.046), and gynaecologic sites (p = 0.034), while ILC was less likely to metastasise to the liver (p = 0.001) and lung (p < 0.001), and more likely to metastasise to the peritoneum (p < 0.001) and gastrointestinal sites (p = 0.019). Upon limiting the analysis to ER-positive cases, the aforementioned differences between mDLC and IDC or ILC did not reach statistical significance.
Fig. 4. Distribution of metastatic spread compared between mDLC, ILC, and IDC.
x axis includes sites of metastatic disease. y axis presents percentage of total metastatic cases within mDLC, ILC, and IDC cohorts. * indicates the significance among three subgroups (*p value < 0.1, **p value < 0.01 and ***p value < 0.001).
Meta-analysis of mDLC clinicopathologic parameters
Finally, we performed a meta-analysis comparing mDLC with ILC and IDC, focusing on hormone receptor status, tumour size, grade, and stage at the time of diagnosis. 23 individual studies comprising data for almost 50,000 patients with mDLC were included in the meta-analysis after a thorough review of available literature (Supplementary Fig. S1 and Supplementary Table S1).
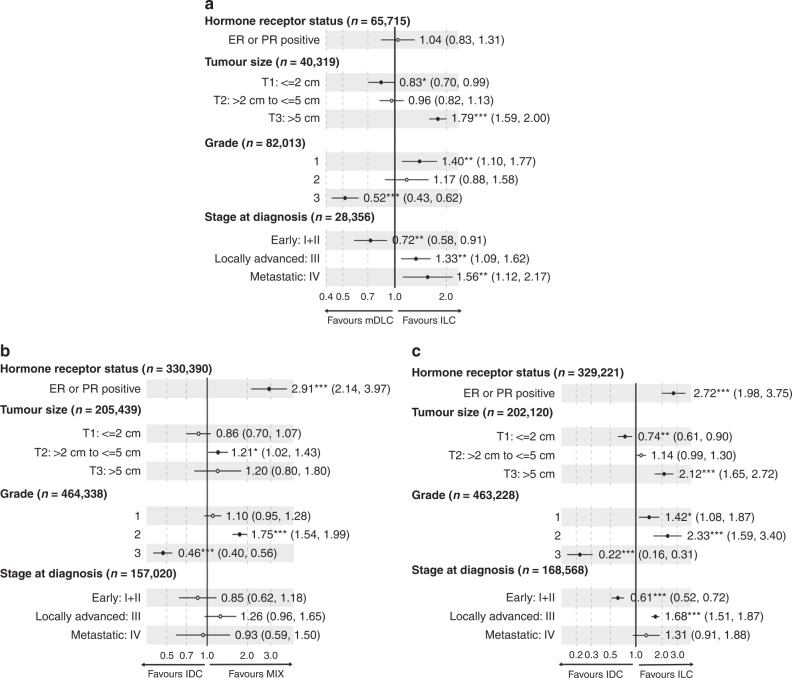
For the meta-analysis, clinical data were abstracted for one or more parameters as available in each of the 23 studies, which is reflected in total sample size of each parameter. The analysis showed that as compared to ILC, mDLC is less likely to be diagnosed at advanced stages of disease and present at T3 size, yet is more likely to be Grade 1 (Fig. 5a). There were no significant differences in hormone receptor status between mDLC and ILC. In contrast, when compared to IDC, mDLC were more likely to be hormone receptor positive (Fig. 5b). In addition, mDLCs were less likely than IDC to be Grade 3 tumours. There were no significant differences at the stage of diagnosis between mDLC and IDC. Using data from the same studies, a comparison of IDC and ILC revealed significantly higher hormone receptor positivity, more advanced tumours i.e. of larger size and later Stage while being of significantly lower grade (Fig. 5c).
Fig. 5. Meta-analysis of clinicopathologic parameters comparing mDLC, IDC, and ILC.
Forest plots of odds ratios (ORs) for individual subgroups comparing mDLC vs ILC (a), mDLC vs IDC (b). and IDC to ILC (c).
Discussion
Despite accumulating reports describing cellular, molecular and clinical heterogeneity and distinguishing differences between histological subtypes, treatment planning largely follows algorithms based upon molecular/immunohistochemical subtypes, i.e. hormone receptor-positive, HER2-positive, triple-negative breast cancer. ILC has distinct features from IDC, notable for higher ER positivity rates [8–10], associated difficulties with detection [28], large size and multifocal nature at diagnosis [8–10], and spread to atypical sites [10–13]. Furthermore, it is well documented that post-neoadjuvant chemotherapy pCR and successful BCS rates are significantly lower in ILC as compared to IDC [24, 29, 30]. Inferior outcomes reported in patients with ILC vs. IDC across multiple studies [11, 17, 31, 32] argue for the consideration of more tailored treatment planning based on underlying histology. The unique mixed composition of mDLC with generally poorly understood clinical behaviour further emphasises this need.
Owing to its rarity and in the absence of a widely accepted and changing definition, precise and consistent identification of mDLC has hindered its characterisation. As elaborated earlier, the current definition of mDLC is based on the 2019 classification by the WHO, which was updated from an initial description published in 2013 [6]. With regard to the definition of mixed tumours prior to 2013, few references are available on this topic. In the Breast Pathology 1st Edition 2012 textbook, mixed ductal tumours are described as consisting of 10–49% ductal NST pattern with over 50% containing a nonspecialised pattern [33]. Several other studies and textbooks describing the histology of mixed tumours [34–36] provide a descriptive characterisation of mixed tumours but often do not specify a more formal definition. Thus, we acknowledge the subjective nature associated with establishing the diagnosis of mDLC and believe this further necessitates studies such as those set forth by our group to better understand this elusive subtype of breast cancer.
Despite ICD coding of cases as mDLC based on detailed algorithms, a careful review of data from our institution suggests that roughly half of these cases did not meet the WHO histopathologic criteria for categorisation as mDLC. Inaccurate classification of these cases based on similar ICD coding algorithms across institutions raises concerns regarding the validity of data analysis from prior studies in which pathologic interpretation was not independently assessed. While pathology reports were individually evaluated for included cases, histology was not independently reassessed, changed, or re-interpreted due to changing guidelines. Given the lack of central pathology review, the fact that the proportion of tumour that was ductal vs lobular is unknown for 87% of our mDLC cases, and the large time period covered of our study naturally associated with a number of pathologists diagnosing the cases, the true biology of these tumours remains poorly understood. We acknowledge this as a weakness of our study.
Our study exemplifies the challenging complexity of mixed disease. In general, our data suggest that mDLC behaves clinically more similar to ILC, consistent with a sampling of mDLC tumours showing the distribution of histologies skewed towards a higher volume of lobular histology. Arps et al. similarly reported a higher percentage of mDLC with predominantly lobular (83/148) vs ductal (65/148) histology [18]. In a smaller sample of mDLC cases evaluated by Suryadevara et al., however, the lobular component made up an average of 42% among 7 cases [22]. An underlying caveat complicating interpretation of individual studies lies in the evolving definition of mDLC. The absence of a formal definition for mDLC up until 2012 calls into question the variable criteria utilised by pathologists to identify and distinguish this subtype. The most recent definition of mDLC provided by the 5th edition of WHO classification of cancer [5] includes tumours in which the special subtype occupies at least 10%. This encompasses significantly more tumours than was previously defined by the 4th edition requiring 50% of the tumour to be of the special subtype [6]. It remains unclear, however, if the current definition sufficiently describes the spectrum of pathology seen in these tumours and how this change will affect conclusions made in prior studies including those made in this current manuscript. An even more important issue in the classification of ductal and lobular carcinomas is inconsistent use of E-cadherin (and other immunohistochemical stains such as p120 and beta-catenin) worldwide on tumours with ambiguous histology. Lack of e-cadherin expression with concomitant cytoplasmic accumulation of p120 is a characteristic finding seen in lobular cancers that molecularly correlate with biallelic inactivation of the CDH1 gene. At our institution, almost all invasive carcinomas with single-cell infiltrative or lobular-like nested growth patterns are confirmed with E-cadherin staining. We believe a more consistent immunohistochemical approach in routine practice is necessary for improving and standardising diagnosis of lobular disease.
Meta-analysis of the literature reported to date demonstrates similarities between mDLC and ILC with regard to hormone receptor positivity, although data herein show slightly higher positivity rates in ILC. Congruent to meta-analysis results, we report that, on average, mDLC tumours are larger in size upon diagnosis as compared to IDCs, and smaller than ILCs. Majority of mDLC have Grade 2 tumours similar to ILC.
Although mDLC behaves clinically more like ILC their survival outcomes are not exactly the same as ILC. Limited sample size is potentially a factor in our inability to detect a statistically significant difference between mDLC and IDC. We do however show inferior outcomes in patients with ILC as compared to patients with IDC, which is in line with several reports in the literature [8, 11, 31, 32, 37, 38].
Higher congruence between mDLC and ILC with regard to key clinicopathologic parameters suggests mDLC may respond to hormonal versus chemotherapy in a corresponding manner such that responses to chemotherapy are likely to be suboptimal. Within our patient population, we demonstrate lower rates of successful BCS in mDLC compared to IDC, yet higher than that observed in ILC. This trend did not reach statistical significance likely due to small sample sizes. These findings parallel the trend in ER positivity rates in mDLC, which were accordingly higher than IDC and lower than ILC. We acknowledge that very few patients with mDLC received neoadjuvant systemic therapy and thus these data are exploratory in nature and should be interpreted with caution.
Comparison of Oncotype DX RS between the cohorts identified a significantly different distribution of RS in ILC compared to IDC, similar to what has previously been shown [11, 39, 40]. High RS was found in 7% of ILC vs 14% in IDC (p < 0.003). High RS was seen in 6% mDLC, but this difference was not significant again likely a result of limited sample size. Data suggest that RS does have prognostic and predictive value in ILC. Furthermore, chemotherapy does confer OS benefit in patients with ILC and high RS [39]. With regard to mDLC, data are limited although several publications report a lesser frequency of high RS similar to what is seen in ILC [41, 42], which is consistent with the results in our study. A correlation to poorer breast cancer-specific survival with high RS has been demonstrated in one study for not only IDC and ILC, but was additionally noted in an mDLC cohort [42].
Conclusions
As our understanding of the clinical behaviour of mDLC deepens, fundamental questions remain regarding the origins of this elusive subtype. This of course requires a better understanding of the differences and origins of disease for ILC and IDC. Recognition of shared and unique pathways within individual ductal and lobular components of mDLC causing tumorigenesis and disease progression will be essential in determining the most effective approach to treatment. It will be essential to make frequent use of immunohistochemistry and promising new technologies including spatial molecular analyses and digital pathology to improve understanding of mDLC. In addition, working in national and international collaborations will help to obtain sufficiently large number of cases to generalise and solidly the findings and to standardise diagnoses which will ultimately help to improve outcomes in patients.
Supplementary information
Author contributions
Conceptualisation: AN, JMA, SO, JSL, PFM, GT, AVL, PCL. Manuscript preparation: AN, YL, YF. Data collection and analysis: AN, YL, YF. All authors read, edited, and approved the final manuscript.
Funding
AN acknowledges support from the Gianni Bonadonna Breast Cancer Research Fellowship provided by the Conquer Cancer Foundation of the American Society of Clinical Oncology, and SO acknowledges support from the Breast Cancer Research Foundation (BCRF). In addition, the study was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH; P30CA047904) and the Dynami Foundation.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Institutional review board approval was obtained from the University of Pittsburgh prior to the initiation of the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Azadeh Nasrazadani, Yujia Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02131-8.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed]
- 4.American Cancer Society. Breast cancer facts & figures. Atlanta, GA: The Society. p.v.
- 5.Board WCoTE. WHO Classification of Breast Tumours: WHO Classification of Tumours, 2: World Health Organization; 2019.
- 6.Sinn HP, Kreipe H. A brief overview of the WHO Classification of Breast Tumors, 4th Edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013;8:149–54. doi: 10.1159/000350774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbs DJ, Bhargava R, Chivukula M. Lobular versus ductal breast neoplasms: the diagnostic utility of p120 catenin. Am J Surg Pathol. 2007;31:427–37. doi: 10.1097/01.pas.0000213386.63160.3f. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Yang J, Li S, Lv M, Shen Y, Wang B, et al. Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12:e0182397. doi: 10.1371/journal.pone.0182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biglia N, Maggiorotto F, Liberale V, Bounous VE, Sgro LG, Pecchio S, et al. Clinical-pathologic features, long term-outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) Eur J Surg Oncol. 2013;39:455–60. doi: 10.1016/j.ejso.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterreich S, Nasrazadani A, Zou J, Carleton N, Onger T, Wright MD, et al. Clinicopathological features and outcomes comparing patients with invasive ductal and lobular breast cancer. J Natl Cancer Inst. 2022;114:1511–22. [DOI] [PMC free article] [PubMed]
- 12.Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991;48:28–33. doi: 10.1002/jso.2930480106. [DOI] [PubMed] [Google Scholar]
- 13.Mathew A, Rajagopal PS, Villgran V, Sandhu GS, Jankowitz RC, Jacob M, et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017;77:660–6. doi: 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharat A, Gao F, Margenthaler JA. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg. 2009;198:516–9. doi: 10.1016/j.amjsurg.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77:113–20. doi: 10.1002/(SICI)1097-0142(19960101)77:1<113::AID-CNCR19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Rakha EA, Gill MS, El-Sayed ME, Khan MM, Hodi Z, Blamey RW, et al. The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res Treat. 2009;114:243–50. doi: 10.1007/s10549-008-0007-4. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Ma D, Ruan M, Zhao S, Liu XY, Jiang YZ, et al. Mixed invasive ductal and lobular carcinoma has distinct clinical features and predicts worse prognosis when stratified by estrogen receptor status. Sci Rep. 2017;7:10380. doi: 10.1038/s41598-017-10789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arps DP, Healy P, Zhao L, Kleer CG, Pang JC. Invasive ductal carcinoma with lobular features: a comparison study to invasive ductal and invasive lobular carcinomas of the breast. Breast Cancer Res Treat. 2013;138:719–26. doi: 10.1007/s10549-013-2493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duraker N, Hot S, Akan A, Nayir PO. A comparison of the clinicopathological features, metastasis sites and survival outcomes of invasive lobular, invasive ductal and mixed invasive ductal and lobular breast carcinoma. Eur J Breast Health. 2020;16:22–31. doi: 10.5152/ejbh.2019.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phipps AI, Li CI, Kerlikowske K, Barlow WE, Buist DS. Risk factors for ductal, lobular, and mixed ductal-lobular breast cancer in a screening population. Cancer Epidemiol Biomark Prev. 2010;19:1643–54. doi: 10.1158/1055-9965.EPI-10-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger-Filho O, Ferreira AR, Jeselsohn R, Barry WT, Dillon DA, Brock JE, et al. Mixed invasive ductal and lobular carcinoma of the breast: prognosis and the importance of histologic grade. Oncologist. 2019;24:e441–9. doi: 10.1634/theoncologist.2018-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suryadevara A, Paruchuri LP, Banisaeed N, Dunnington G, Rao KA. The clinical behavior of mixed ductal/lobular carcinoma of the breast: a clinicopathologic analysis. World J Surg Oncol. 2010;8:51. doi: 10.1186/1477-7819-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiere JM, Bouita L, Cohen-Solal C, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17:1228–33. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- 24.Truin W, Vugts G, Roumen RM, Maaskant-Braat AJ, Nieuwenhuijzen GA, van der Heiden-van der Loo M, et al. Differences in response and surgical management with neoadjuvant chemotherapy in invasive lobular versus ductal breast cancer. Ann Surg Oncol. 2016;23:51–7. doi: 10.1245/s10434-015-4603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat. 2013;142:227–35. doi: 10.1007/s10549-013-2751-3. [DOI] [PubMed] [Google Scholar]
- 26.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 28.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics. 2009;29:165–76. doi: 10.1148/rg.291085100. [DOI] [PubMed] [Google Scholar]
- 29.Wenzel C, Bartsch R, Hussian D, Pluschnig U, Altorjai G, Zielinski CC, et al. Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel + G-CSF. Breast Cancer Res Treat. 2007;104:109–14. doi: 10.1007/s10549-006-9397-3. [DOI] [PubMed] [Google Scholar]
- 30.Cocquyt VF, Blondeel PN, Depypere HT, Praet MM, Schelfhout VR, Silva OE, et al. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29:361–7. doi: 10.1053/ejso.2002.1404. [DOI] [PubMed] [Google Scholar]
- 31.Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006–14. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 32.Adachi Y, Ishiguro J, Kotani H, Hisada T, Ichikawa M, Gondo N, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. doi: 10.1186/s12885-016-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabbs DJ, editor. Breast pathology. 1st ed. Philadelphia, PA: Elsevier; 2012.
- 34.Rosen P, editor. Breast pathology. 3rd ed. United Kingdom: Wolters Kluwer Health; 2009.
- 35.Collins SJSaL, editor. Biopsy interpretation of the breast. 2nd ed. United States: Wolters Kluwer Health; 2009.
- 36.Lawton TJ, editor. Breast Cambridge illustrated surgical pathology. 1st ed. United Kingdom: Cambridge University Press; 2009.
- 37.Engstrom MJ, Opdahl S, Vatten LJ, Haugen OA, Bofin AM. Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology. 2015;66:409–19. doi: 10.1111/his.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang LY, Yang LP, Zhu B. Clinicopathological characteristics and survival outcomes of invasive lobular carcinoma in different races. Oncotarget. 2017;8:74287–98. doi: 10.18632/oncotarget.19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser R, Polychronopoulou E, Hatch SS, Haque W, Ghani HA, He J, et al. Adjuvant chemotherapy in patients with invasive lobular carcinoma and use of the 21-gene recurrence score: A National Cancer Database analysis. Cancer. 2022;128:1738–47. doi: 10.1002/cncr.34127. [DOI] [PubMed] [Google Scholar]
- 40.Felts JL, Zhu J, Han B, Smith SJ, Truica CI. An analysis of oncotype DX recurrence scores and clinicopathologic characteristics in invasive lobular breast cancer. Breast J. 2017;23:677–86. doi: 10.1111/tbj.12751. [DOI] [PubMed] [Google Scholar]
- 41.Hanna MG, Bleiweiss IJ, Nayak A, Jaffer S. Correlation of oncotype DX recurrence score with histomorphology and immunohistochemistry in over 500 patients. Int J Breast Cancer. 2017;2017:1257078. doi: 10.1155/2017/1257078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, He ZY, Dong Y, Sun JY, Zhang WW, Wu SG. The distribution and outcomes of the 21-gene recurrence score in T1-T2N0 estrogen receptor-positive breast cancer with different histologic subtypes. Front Genet. 2018;9:638. doi: 10.3389/fgene.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.