Abstract
AIM
To investigate changes of choroidal thickness (ChT) in children with myopia and the effect of current myopia control interventions on ChT.
METHODS
Major literature databases were searched for studies relevant to myopia in children. All studies used swept-source optical coherence tomography (SS-OCT) or enhanced depth imaging optical coherence tomography (EDI-OCT) to measure the ChT value. The weighted mean difference (WMD) and 95% confidence interval (CI) were pooled to evaluate ChT in myopia children.
RESULTS
A total of 11 eligible articles, including 1693 myopic and 1132 non-myopic eyes, were included in the first Meta-analysis. The sub-foveal choroidal thickness (SFCT; WMD=-40.06, 95%CI, -59.36 to -20.75, P<0.001) and ChT at other sectors were significantly thinner in myopic eyes compared with the non-myopic eyes. The Meta-analysis revealed that the ChT decreased horizontally from the temporal sector toward the nasal sector in the pediatric myopia population. Another 11 studies reporting the effect of myopia control interventions were included in the second Meta-analysis for the relationship between myopia control treatments and ChT. SFCT significantly increased after orthokeratology (OK) treatment and OK combined with 0.01% atropine (OKA) treatment (WMD=19.47, 95%CI, 15.96 to 22.98, P<0.001; WMD=21.81, 95%CI, 12.92 to 29.70, P<0.001, respectively). The forest plots showed that SFCT changed little in myopic children receiving 0.01% atropine (P=0.30). Furthermore, the Meta-analysis showed that OK treatment had a stronger effect on the value of SFCT in myopic children as compared with 0.01% atropine (WMD=9.86; 95%CI, -0.21 to 19.93, P=0.05). There is no difference between the treatment with OK and OKA treatment in ChT in myopic children (P=0.37).
CONCLUSION
The ChT in myopic eyes is thinner than that in non-myopic eyes in pediatric population. Myopia control interventions including OK and OKA lead to ChT thickening, but other treatments such as 0.01% atropine did not show an increase in ChT.
Keywords: choroidal thickness, myopia, orthokeratology, atropine, children, Meta-analysis
INTRODUCTION
Myopia has become a significant public health problem in China, due to the growing number of myopic children and adolescents. It is estimated that approximately 50% of the global population will be affected by myopia by the mid 21-century[1]–[3]. At the same time, there are an increasing number of articles related to clinical science and methods to slow myopia progression. The choroid has been suggested to play a role in the development of refractive error and emmetropization in humans, and it is regarded a medium in the visual signaling pathways between the retina and sclera[4]. Many ocular diseases, such as central serous chorioretinopathy and age-related degeneration, are associated with structural and functional abnormalities of the choroid[5]–[6].
A number of myopia-promoting visual signal pathways traverse the retinal pigment epithelium (RPE) and vascular choroid and have an impact on sclera remodeling processes, increasing axial length and promoting myopia[7]–[8]. Dopamine, a neurotransmitter, plays an important role in development of the eye and in the growth of the eyeball. Dopamine receptors have been identified in retinal cells and divided into two subtypes: D1-like receptors (D1R/D5R) and D2-like receptors (D2R, D3R and D4R). Zhou et al[9] speculated that the balance of D1-like and D2-like receptor activation could modulate the refractive status of the eye, such that over activation of D2-like receptors results in myopia while over activation of D1-like receptors leads to hyperopia. There are some strong evidences that have suggested dopamine could affect choroidal thickness[10]. However, there is no evidence to indicate that dopamine receptors are expressed in the choroid. This indicates that the RPE may be able to translate dopamine signaling to changes in the choroid, which could influence the elongation of axial length and contribute to the development of myopia. Retinoic acid (RA) is found to play an important role in the development of myopia. All-trans retinoic acid (ATRA) could upregulate transforming growth factor beta 2 (TGF-β2) expression, which can affect collagen production, scleral fibroblast proliferation and finally control myopia development[11]. ATRA can be produced in the choroid and transported to the sclera may be able to influence scleral remodeling[12]. Scleral hypoxia is a common feature of myopia. Hypoxia-inducible factor 1-alpha (HIF-1α) has recently emerged as a new target of myopia research. Zhao et al[13] analyzed human gene databases and revealed association with the scleral HIF-1α signaling pathway in high myopia individuals. Scleral HIF-1 can promote myofibroblast transdifferentiation in human scleral fibroblasts and decrease type I collagen expression, leading to myopia[14]. Additionally, reduced choroidal blood perfusion was also found to induce myopia in animal[15]. Based on the previous studies, it is speculated that the choroid could be an fundamental point where various visual signaling pathways intersect and contribute to the development of myopia[7].
Nowadays, rapid development of swept-source coherence tomography (SS-OCT) and enhanced depth imaging optical coherence tomography (EDI-OCT) has allowed analysis of choroidal thickness (ChT) quantitatively precisely in vivo[16]. Several studies have reported that myopia is associated with a significantly thinner choroid in adults among different population[17]–[21]. Other studies have demonstrated that the value of ChT in children differs with age ranges[22]–[24]. A growing number of studies have examined the ChT of myopia in pediatric population and found that ChT in myopia children was thinner[25]–[26], however, no association between ChT and refractive error has also been reported[27]. Current myopia control interventions includes orthokeratology (OK), low concentration atropine, soft contact lenses and spectacles[28]. While the effectiveness of these interventions has been demonstrated, the effect on the thickness of choroidal remains controversial.
In this study, we performed Meta-analysis to detect the association between ChT change and myopia in children. First, we examine the existing evidence obtained by EDI-OCT or SS-OCT for the detection of choroidal changes in myopic children by Meta-analysis. Furthermore, we conducted a Meta-analysis to explore the effect of current myopia control interventions on ChT in the pediatric population.
MATERIALS AND METHODS
Literature Search
This Meta-analysis was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines[29].
Two investigators (Meng QY and Miao ZQ) independently searched PubMed, Embase, Web of Science, Cochrane Library databases, and ClinicalTrials.gov for publications up to June 2022. Headings and keywords for “myopia”, “myopia control”, “orthokeratology” or “OK”, “atropine”, “optical coherence tomography or OCT” and “choroidal thickness (ChT)” or “sub-foveal choroidal thickness (SFCT)” were used in the search strategy. The searches were modified to accommodate the unique terminology and syntax of each database. All articles in English were considered eligible.
Inclusion and Exclusion Criteria
Inclusion criteria for clinical trials included: 1) use of EDI-OCT or SS-OCT to evaluate ChT (sub-foveal or around the foveal area); 2) children with myopia were included, and all children underwent subjective refraction to obtain the value of spherical equivalent (SE=sphere+0.5×cylinder). Myopia was defined as SE≤-0.5 D. High myopia (SE≤-6.0 D), amblyopia or children with syndromic myopia were excluded. 3) comparison of ChT in children with and without myopia; or reporting ChT change after myopia control interventions in children. These interventions include atropine, OK, and atropine combined with orthokeratology (OKA). 4) the design of the case-control, cross-sectional, cohort and randomized controlled trial (RCT) was included; 5) the studies were published as original articles. Exclusion criteria included the following: 1) conference abstracts, letters, or reviews; 2) unretrievable data; 3) duplicate articles. In studies of the same population, only the most complete studies were included in the Meta-analysis.
Data Extraction and Clinical Outcome
Two authors (Meng QY and Miao ZQ) independently extracted the data from the studies into an electronic database according to a customized protocol. If there were any discrepancies in data inclusion, the studies would be addressed by the third author (Guo LL). The following data were extracted from each study: 1) first author; 2) year of publication; 3) country; 4) study design; 5) number of myopias and non-myopias; 6) SE; 7) mean age; 8) myopia control interventions; 9) ChT value at sub-foveal and other sectors; 10) axial length; 11) type of OCT; We used means and standard deviations (SD) to calculate the standard mean difference (SMD) and the weighted mean difference (WMD).
Quality Assessment
Two authors (Meng QY and Miao ZQ) assessed the quality score independently. If there were any discrepancies, the studies would be addressed by the third author (Guo LL). The Cochrane Collaboration's tool was applied to assess the risk of bias of RCT[30]. For case-cohort and case-control studies, the Newcastle-Ottawa Scale (NOS) was applied[31]. Studies awarded with 6 or more stars were regarded as high-quality studies. The Agency for Healthcare Research and Quality (AHRQ) methodology checklist was used to assess cross-sectional studies[32]. The maximum AHRQ score was 11, and the quality of the articles was assessed as follows quality: 1-3, moderate quality: 4-7, high quality: 8-11.
Statistical Analysis
For continuous outcomes, we quantified with WMD and their 95% confidence intervals (CI). P<0.05 was considered statistically significant. In the Meta-analysis of the effect of myopia control interventions on ChT, we also directly extracted the calculated value of ChT changes from baseline to final follow-up months in myopic intervention in some studies. And for other studies, we calculated the values of changes in ChT from baseline to final follow-up in those participants. ChT changes are calculated using the following formula: Mean difference (MD)=MDfinal–MDbaseline; Standard deviation2 (SD2)=SDfinal2+SDbaseline2–2×correlation coefficient×SDfinal×SDbaseline; We took the correlation coefficient =0.5 in this study.
Heterogeneity was assessed and quantified using the Chi-square based Q statistic test and the I2 test. For a significant heterogeneity (I2>50%, P<0.1), a random-effect model was selected to analyze the data. Alternatively, a fixed-effect model was used. Furthermore, funnel plots and Egger's test were used to evaluate the potential publication bias. The sensitivity analysis was performed by eliminating studies one by one to verify whether the results would change. Analyses were performed with STATA version 15.0 (Stata Corp, TX, USA).
RESULTS
Meta-analysis of Choroidal Thickness in Children with Myopia
Literature search and characteristics of studies
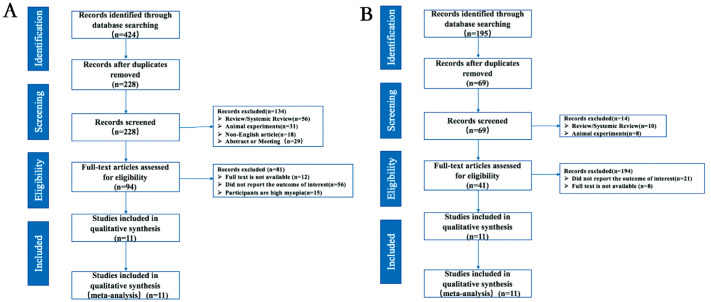
A flow chart showing the literature search and selection is presented in Figure 1A. Our initial search identified 424 potentially relevant reports, of which 194 were removed due to duplication. After applying the inclusion and exclusion criteria, 11 eligible articles including 1693 myopic and 1132 non-myopic eyes were included for the first Meta-analysis[26],[33]–[44]. These studies were published between 2016 and 2022, and 6 of them were conducted in China. The demographic and clinical characteristics of the included articles are summarized in Table 1. The age of the participants included in this Meta-analysis ranged from 6.4 to 15.4 years old. All studies were assessed as high-quality studies according to AHRQ and NOS.
Figure 1. PRISMA flow diagram of studies included in the Meta-analysis.
A: Screening process for Meta-analysis of choroidal changes in myopic children; B: Screening process for Meta-analysis of the effect of current myopia control interventions on choroidal thickness in pediatric population.
Table 1. Characteristics of the 11 studies included in the analysis.
| Study | Location | Year | Sample size (n) |
Spherical equivalent (D) |
Age (y) |
Type of OCT | Quality score | |||
| Myopia | Non-myopia | Myopia | Non-myopia | Myopia | Non-myopia | |||||
| Li[33] | China | 2016 | 65 | 71 | -1.50±1.0 | 0±0.5 | 11.4±1.5 | 11.5±1.7 | Cirrus-HD OCT | 8b |
| Bulut[26] | Turkey | 2016 | 53 | 64 | -2.1±1.0 | 0.0±0.3 | 10.9±3.4 | 11.7±2.7 | Cirrus HD | 8b |
| Jin[34] | China | 2016 | 86 | 91 | -2.00±1.45 | 0.18±0.26 | 10.1±1.1 | 10.1±1.1 | SS-OCT Topcon DRI OCT-1 | 9b |
| Lundberg[36] | Denmark | 2018 | 55 | 252 | -1.77±1.6 | 0.75±0.6 | 15.4±0.6 | 15.4±0.7 | Spectralis OCT | 9b |
| Lee[37] | Korea | 2017 | 28 | 39 | -2.83±1.17 | 0.08±0.50 | 8.4±1.7 | 8.1±1.8 | Spectralis OCT | 9b |
| Deng[38] | China | 2019 | 222 | 49 | -2.96±1.67 | 0.05±0.24 | 12.37±1.80 | 11.45±1.53 | SS-OCT Topcon DRI OCT-1 | 8b |
| Guo[39] | China | 2019 | 1020 | 410 | -2.88±2.63 | -0.25± 0.63 | 13.0±1.0 | 13.0±1.0 | Spectralis OCT | 9b |
| Read[41] | Australia | 2013 | 41 | 60 | -2.39±1.51 | +0.33±0.31 | 13±1.5 | 13.1±1.2 | Spectralis OCT | 9b |
| Jiang[42] | China | 2021 | 43 | 28 | - | - | 6.51±0.51 | 6.43±0.50 | Optovue SD-OCT | 7a |
| Matalia[43] | India | 2017 | 40 | 46 | - | - | - | - | Optovue SD-OCT | 8b |
| Chang[44] | China | 2022 | 40 | 19 | -2.21±1.26 | 0.35±0.46 | 8.55±1.63 | 8.18±2.44 | VG200S; SVision Imaging | 10b |
aThe Newcastle Ottawa Scale was used for quality assessment in the included studies; bThe Agency for Healthcare Research and Quality was used for quality assessment in the included studies. OCT: Optical coherence tomography.
Changes in sub-foveal choroidal thickness in myopic eyes
As shown in Figure 2 and Table 2, the SFCT of the myopic eyes was significantly thinner than that of the non-myopic eyes (WMD=-40.06, 95%CI of -59.36 to -20.75, P<0.001). According to location of the study, we divided the studies into two groups: China and non-China. The results did not change after subgroup analysis according to the location of the study.
Figure 2. Meta-analysis of sub-foveal choroidal thickness between the myopic and non-myopic eyes in pediatric population.
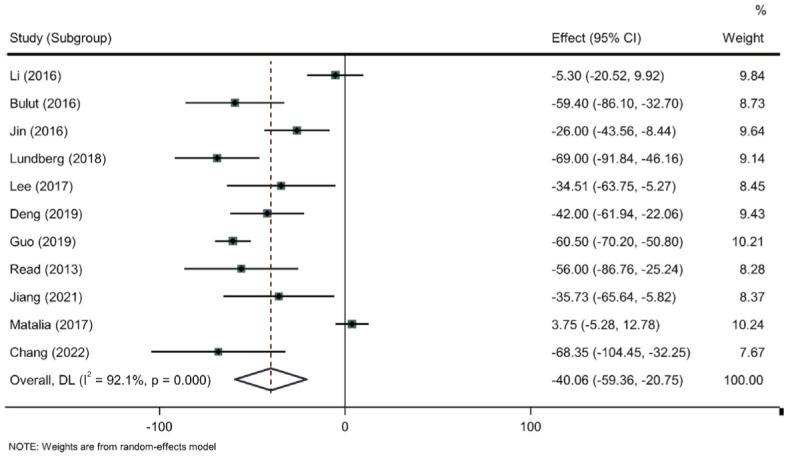
Table 2. The result of choroidal thickness at different sectors in the eligible articles.
| Study | Myopia |
Non-myopia |
||||||||
| Subfoveal | Temporal | Nasal | Superior | Inferior | Subfoveal | Temporal | Nasal | Superior | Inferior | |
| Li[33]a | 266.7±45.4 | 262.8±41.2 | 225.8±59.6 | 246.9±40.7 | 256.2±43.9 | 272.0±43.1 | 263.9±43.1 | 243.0±49.5 | 250.2±43.3 | 262.5±44.7 |
| Bulut[26]a | 306.1±80.8 | 348.0±79.3 | 271.7±76.9 | 356.5±63.2 | 382.9±69.5 | 375.5±69.8 | ||||
| Jin[34]b | 227±61 | 244±63 | 199±60 | 225±63 | 233±61 | 253±58 | 267±57 | 222±56 | 250±55 | 254±58 |
| Lundberg[36]a | 313±77 | 286±62 | 284±81 | 321±79 | 310±73 | 382±84 | 339±69 | 353±82 | 384±82 | 370±86 |
| Lee[37]a | 267.5±63.1 | 278.7±67.6 | 229.9±58.5 | 267.1±57.8 | 262.1±54.6 | 301.9±55.9 | 308.3±56.3 | 264.1±55.8 | 299.8±54.9 | 293.8±49.3 |
| Deng[38]a | 208±57 | 225±53 | 181±51 | 210±54 | 213±58 | 250±66 | 265±64 | 222±62 | 247± 60 | 259±68 |
| Guo[39]b | 250.0±82.0 | 148±62 | 266±73 | 310.5±84.5 | 179±77 | 309±73.5 | ||||
| Read[41]a | 303±79 | 312±79 | 269±71 | 314±74 | 299±79 | 359±77 | 354±69 | 327±82 | 371±78 | 353±76 |
| Jiang[42]a | 279.9±47.1 | 290.5±40.7 | 244.6±50.3 | 279.2±41.6 | 275.1±45.5 | 315.6±71.2 | 327.3±65.7 | 272.8±79.8 | 312.6±59.0 | 303.5±63.4 |
| Matalia[43]b | 314.9±21.7 | 298.6±28.8 | 285.7±22.1 | 321.1±29.6 | 323.3±34.8 | 311.2±20.9 | 303.6±27.6 | 279.7±21.2 | 315.0±28.4 | 323.5±21.8 |
| Chang[44]a | 247.0±41.6 | 257.2±42.9 | 222.2±6.4 | 236.6±38.9 | 244.3±39.1 | 315.4±74.9 | 320.1±69.6 | 2858±6.1 | 309.8±59.1 | 309.2±66.2 |
aChoroid thickness measured at sub-foveal and inner foveal region at a distance of 0.75-1 mm from the fovea in the temporal, superior, nasal, and inferior regions; bChoroid thickness measured at sub-foveal and parafoveal region at a distance of 2.5-3 mm from the fovea in the temporal, superior, nasal, and inferior regions.
mean±SD, µm
Choroidal thickness at other positions in myopic eyes
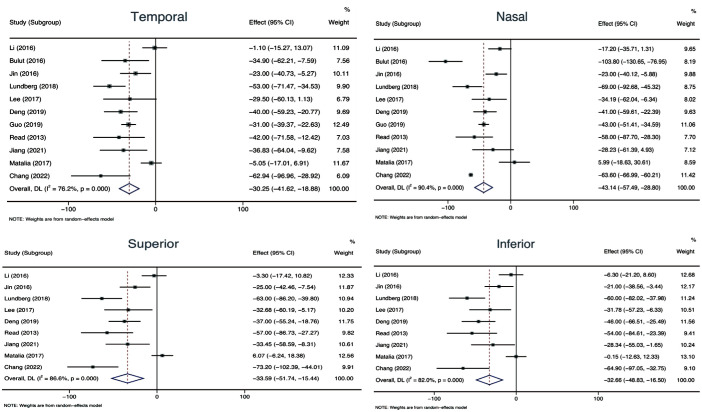
As shown in Table 2 and Figure 3, the temporal ChT of the myopic eyes was thinner than that of the non-myopic eyes (WMD=-30.25, 95%CI, -41.62 to -18.88, P<0.001); similarly, the myopia has thinner nasal ChT than non-myopia (WMD=-43.14, 95%CI, -57.49 to -28.80, P<0.001). The superior ChT of the myopic eyes was thinner than that of the non-myopic eyes (WMD=-33.59, 95%CI, -51.74 to -15.44, P<0.001). Similarly, myopic eyes had thinner inferior ChT compared to non-myopic eyes (WMD=-32.66, 95%CI, -48.83 to -16.50, P<0.001). The Meta-analysis revealed that the ChT decreased horizontally from the temporal sector toward the nasal sector in the pediatric myopia population; No significant difference was detected between the superior and inferior ChT.
Figure 3. Meta-analysis of choroidal thickness at different sectors between the myopic and non-myopic eyes in pediatric population.
Meta-regression analysis
A Meta-regression analysis was conducted to assess the impact of the study characteristics on the Meta-analysis, including age, SE, study location (China or non-China) and OCT type. According to the Meta regression analysis, the OCT type was the primary cause of heterogeneity (P=0.03 after 10 000 Monte Carlo simulation permutations).
Meta-analysis of Myopia Control Intervention on Choroidal Thickness in Pediatric Population
Literature search and characteristics of studies
A flow chart showing the literature search and selection is presented in Figure 1B. A total of 195 potentially relevant reports were initially identified in the literature search, of which 69 were removed due to duplication. After applying the inclusion and exclusion criteria, 11 eligible articles were included for the second Meta-analysis[45]–[55]. The demographic and clinical characteristics of the included articles are summarized in Table 3. The 11 studies were conducted in China and published between 2016 and 2022. Seven of these studies were RCTs and four were prospective cohort studies. The quality of the included cohort studies was generally high according to NOS.
Table 3. The eligible studies of myopia control interventions on choroidal thickness in pediatric population.
| Author | Study design and quality score | Location | Year | Intervention | Sample size | Age (y)a | Type of OCT | SE (D)a | SFCT(µm)a |
||
| Baseline | The end of treatment | Treatment duration | |||||||||
| Chen[45] | Case-cohort; 7c | China | 2016 | OK | 39 | 10.6±2.5 | RS-3000, NIDEK | -2.90±1.08 | 282±43 | 306±54 | 1mo |
| SVL | 38 | 10.0±2.7 | -2.75±1.08 | 275±60 | 276±64 | ||||||
| Li[46] | Case-cohort; 8c | China | 2019 | OK | 29 | 12.31±1.71 | Spectralis HRA+OCT | -3.16±0.85 | 228±56 | 21.03±12.74 | 12mo |
| SVL | 21 | 11.52±1.69 | -2.98±1.34 | 248±51 | -2.50±14.43 | ||||||
| Jin[47] | Case-cohort; 8c | China | 2018 | OK | 30 | 11.3±1.7 | Cirrus-HD OCT | -2.9±1.1 | 253.1±38.6 | 262.8±44.3 | 3mo |
| Li[48] | Case-cohort; 7c | China | 2020 | 0.01%A | 59 | 9.31±2.43 | SS-OCT, Topcon | -1.39±0.65 | 235.10±30.96 | 255.85±31.71 | 2mo |
| Ye[49] | RCT | China | 2020 | 1%A | 98 | 8.94±1.55 | Topcon Corp. | -2.12±1.09 | 214±45 | 27±23b | 6mo |
| 0.01%A | 87 | 8.84±1.65) | -2.12±1.09 | 218±39 | -5±17b | ||||||
| Zhao[50] | RCT | China | 2021 | 0.01%A | 42 | 9.96±1.03 | Spectralis HRA+OCT | -3.01±1.22 | 251.12±44.76 | 256.61±46.55 | 1mo |
| OK | 36 | 10.33±1.65 | -2.74±1.06 | 266.74±57.50 | 276.17±59.10 | ||||||
| OKA | 39 | 10.23±1.11 | -3.12±1.20 | 263.17±46.55 | 277.28±46.04 | ||||||
| SVL | 37 | 9.73±1.04 | -3.25±1.10 | 258.05±52.34 | 253.24±50.67 | ||||||
| Hao[51] | RCT | China | 2021 | 0.01%A | 22 | 9.77±1.27 | CIRRUS HD-OCT | -3.62±0.57 | 240.64±19.93 | 248.73±21.06 | 12mo |
| OK | 24 | 10.13±1.19 | -3.66±0.60 | 236.83±16.78 | 256.17±19.03 | ||||||
| OKA | 21 | 10.10±1.22 | -4.07±0.74 | 235.14±20.33 | 259.29±21.73 | ||||||
| Xiong[52] | RCT | China | 2021 | OK | 81 | 10.88±1.92 | Carl Zeiss | -3.42±1.28 | 284.36±72.58 | 299.33±73.65 | 6mo |
| LLLT+SVL | 74 | 10.22±2.38 | -3.42±1.28 | 288.61±59.59 | 323.91±65.63 | ||||||
| SVL | 74 | 10.33±2.03 | -3.32±1.36 | 286.81±63.67 | 269.97±64.11 | ||||||
| Kong[53] | RCT | China | 2021 | 0.01%A | 50 | 9.12±1.39 | RS-3000, NIDEK | -2.25±1.14 | 233.45±22.95 | -9.18±19.52b | 6mo |
| AAS+0.01%A | 50 | 8.96±1.38 | -2.14±1.27 | 233.45±22.95 | -7.92±20.65b | ||||||
| Wang[54] | RCT | China | 2022 | 0.01%A | 21 | 9.90±1.58 | Spectralis HRA+OCT | -2.38±1.46 | 249.98±38.26 | 261.10±43.25 | 3mo |
| SVL | 19 | 9.89±1.94 | -2.36±1.87 | 229.78±46.73 | 235.31±46.43 | ||||||
| Xu[55] | RCT | China | 2022 | PPALs | 59 | 9.2±1.1 | Cirrus HD-OCT | -2.38±0.61 | 231.60±51.84 | 202.17±56.78 | 24mo |
| FPALs | 48 | 9.4±1.1 | -2.38±0.61 | 230.46±57.36 | 210.87±68.29 | ||||||
| SVL | 61 | 9.2±1.1 | -2.38±0.61 | 231.65±56.77 | 209.51±64.98 | ||||||
aData was shown in means±SD; bData was shown in change values from final to baseline; cThe Newcastle Ottawa Scale was used for quality assessment in the included studies. RCT: Randomized controlled trial; OCT: Optical coherence tomography; SE: Spherical equivalent; SFCT: Sub-foveal choroidal thickness; A: Atropine; OK: Orthokeratology; OKA: Orthokeratology combined with 0.01% atropine; SVL: Single-vision lens; LLLT: Low-intensity, long-wavelength red light therapy; PPALs: Personalized progressive addition lens; FPALs: Fixed progressive addition lenses; ACS: Auricular acupoint stimulation.
Effect of atropine on the thickness of the choroidal in children with myopia
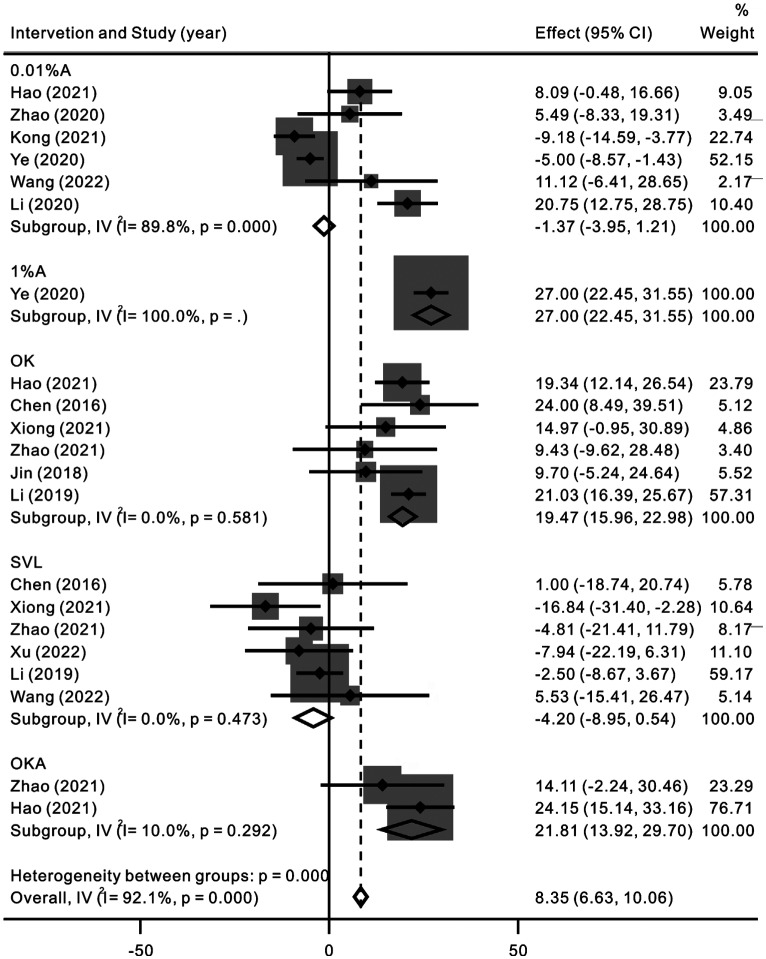
This Meta-analysis was performed for 6 of 11 eligible studies including 379 myopic children[48]–[51],[53]–[54]. There were 281 participants who received 0.01% atropine as myopia control intervention and 98 participants in 1 study received 1% atropine for myopia control. The forest plots in Figure 4 showed that the SFCT of the myopic children did not show significant difference after receiving 0.01% atropine (WMD=-1.37, 95%CI, -3.95 to 1.21, P=0.30). However, one study found that SFCT increased 27 µm after receiving 1% atropine participants.
Figure 4. Forest plot depicting the changes in sub-foveal choroidal thickness values following the myopia control interventions in pediatric population.
OK: Orthokeratology; SVL: Single vision lens; A: Atropine; OKA: Orthokeratology combined with 0.01% atropine.
Effect of orthokeratology on the thickness of the choroidal in children with myopia
A Meta-analysis in relation to the effect of OK on SFCT was performed for 6 of the 11 studies[45]–[47],[50]–[52]. A total of 239 myopic children were included in this analysis. We used a fixed effect model based on the heterogeneity results (I2=0, P=0.58). The forest plots showed that SFCT increased significantly after OK treatment (WMD=19.43, 95%CI, 15.96 to 22.98, P<0.001), as shown in Figure 4.
Effect of atropine combined with orthokeratology on the thickness of the choroidal in children with myopia
In the 2 eligible studies, 60 myopic eyes treated with OKA were enrolled in this Meta-analysis[50]–[51]. We employed a fixed effect model based on the heterogeneity results (I2=10.0%, P=0.29). The results in Figure 4 showed that SFCT increased significantly with the OKA intervention (WMD=21.81, 95%CI, 13.92 to 29.70, P<0.001).
Comparison of the effect on choroidal thickness between orthokeratology and single vision lens in myopic eyes
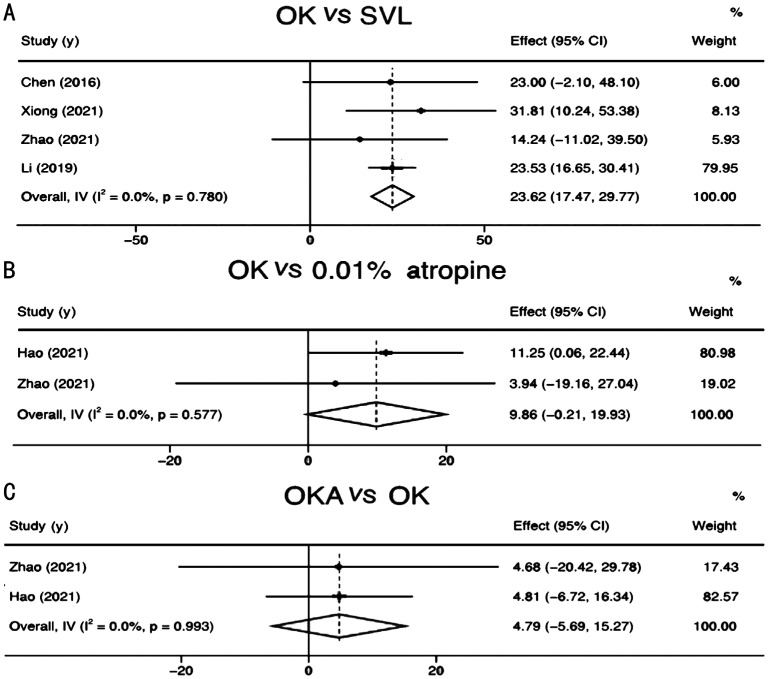
Of the 11 eligible studies, there were 4 studies that included 365 participants and compared the effect of OK with SVL on SFCT[45]–[46],[50],[52]. The fixed-effects Meta-analysis demonstrated that compared to SVL, OK treatment could significantly increase SFCT in myopic children (overall WMD=23.62, 95%CI, 17.47 to 29.77, P<0.001; Figure 5A).
Figure 5. Meta-analysis of SFCT value changes between OK and SVL, OK and 0.01% atropine, OK and OKA in myopic children.
A: Comparison the effect on SFCT between OK and SVL in myopic eyes; B: Comparison the effect on SFCT between OK and 0.01% atropine in myopic eyes; C: Comparison the effect on SFCT between OKA and OK in myopic eyes. SFCT: Sub-foveal choroidal thickness; OK: Orthokeratology; SVL: Single vision lens; OKA: Orthokeratology combined with 0.01% atropine.
Comparison of the effect on choroidal thickness between orthokeratology and 0.01% atropine in myopic eyes
Of the 11 eligible studies, there were 2 studies that compared the effect of OK with atropine on SFCT[50]–[51]. Fixed-effects Meta-analysis demonstrated a significant increase in SFCT in myopic children with the OK intervention as compared with 0.01% atropine intervention (overall WMD=9.86; 95%CI, -0.21 to 19.93, P=0.05; Figure 5B).
Comparison of the effect on choroidal thickness between orthokeratology combined with atropine and orthokeratology in myopic eyes
There were 2 studies that included 120 participants comparing the effect of OKA and OK on SFCT[50]–[51]. The Meta-analysis results showed that compared with OK alone, OKA did not have a stronger effect on SFCT (overall WMD=4.79, 95%CI, -5.69 to 15.27, P=0.37; Figure 5C).
Publication Bias
Begg's tests and Egger's tests showed that there was no obvious evidence of bias. It is worth mentioning that publication bias is only one of possible explanations for funnel plot asymmetry.
DISCUSSION
In recent years, the knowledge of the human choroid has expanded dramatically thanks to the developments in OCT technology that allow the structure of the choroid to be imaged and measured in vivo. By examining the relationship between age in childhood and ChT, studies have noted that ChT tends to increase from early childhood to adolescence in normal children without any refractive error, then reaching a peak in young adulthood; however, the ChT decreases with age in older population[22],[56]. In non-myopic children, the average thickness of the macular choroidal showed that the choroid is thickest in central regions and thinnest in nasal regions around the optic nerve[34],[57].
Our Meta-analysis showed that SFCT was thinner in myopic children, regardless of the foveal and parafoveal region. According to our Meta-analysis, topographical variation in ChT also existed in the myopia population. The ChT decreased horizontally from the temporal toward the nasal region, and there was no significant difference between the ChT of superior and inferior region. In the current study, the OCT type was the primary cause of heterogeneity. It is suggested that ChT measured on SS-OCT was 5.9 to 49.3 µm thinner than that on spectral domain OCT, and there was a significant inter-device difference of ChT measurements in normal children[58].
Fontaine et al[35] found that ChT tends to increase from early childhood to adolescence in normal children without refractive errors, peaking in young adulthood, but then decreases with age in older populations. Our results suggest that in myopic children, the choroidal layer was thinner than that of non-myopic children, suggesting that choroidal appears to play an important role during the development and progression of myopia. ChT appears to be a biomarker of eye growth, since more rapid eye growth is associated with choroidal thinning and slower eye growth with a thickening of the choroid over time. However, further work is required to determine the causal relationship between ChT and myopia. Some researchers proposed that the incremental retinal-defocus theory (IRDT) may contribute to myopia development[59]. The time-averaged decrease in retinal-image defocus area decreases the release of growth factors and molecules to the sclera, leading to decreased the choroidal blood flow, and contributes to choroidal thinning and sclera hypoxia which in turn promotes scleral extracellular matrix remodeling and myopia development[14].
Atropine is currently the most potent therapy for myopia control. Ha et al[60] revealed that the efficacy of atropine was not proportional to dose, whereas the adverse effects are dose dependent. Therefore, low-concentration atropine is a preferred choice for clinicians. Unfortunately, the exact mechanism of atropine in slowing progression is still a matter of speculation. Atropine is a non-specific muscarinic receptor antagonist and could decrease acetylcholine level. However, McBrien et al[61] reported that retinal acetylcholine levels remained unchanged in the experimental myopia animal model. The latest research on the mechanism of atropine's anti-myopia effects suggested that cholinergic agonists inhibited form-deprivation myopia, indicating that atropine prevents myopia progression through a non-cholinergic mechanism[62]. In our Meta-analysis, SFCT changed little after applying 0.01% atropine in myopic children and 1% atropine would increase SFCT. Our results indicated that the effect of atropine on the choroid may be dose-dependent. The exact mechanism of increasing ChT after atropine treatment needs further research. Some evidence indicated that atropine would stimulate the release of dopamine in the retina in animal, and then result in choroidal thickening[63]. But the latest research showed that atropine does not need to modulate dopamine release to be effective at inhibiting myopia[62]. Other animal studies showed that nitric oxide may participate in the anti-myopic role of atropine in chicks and by relaxing the choroidal vascular and increasing the blood flow of the choroid, leading to changes in ChT.
Our Meta-analysis also revealed that OK treatment could induce significant sub-foveal choroidal thickening in myopic children. Compared to atropine or SVL, the increase in SFCT in the OK group was also significant in the current study. There are several hypotheses for the mechanism by which OK increases ChT, and one is that OK could induce the relaxation of large choroidal vessels and lead to increase blood supply of the choroidal. Alshareef et al[64] found that the large choroidal vascular layer (LCVL) and medium choroidal vessel thickness (MCVT) were much thinner in myopic eyes. Li et al[65] found that LCVL thickness was significantly increased in the OK group after 6mo of treatment. They also found that the change in LCVL accounted for 80% of the thickening of SFCT. Therefore, it is speculated that during OK treatment, the blood supply to the choriocapillaris and medium choroidal vessel layer increased, leading to choroidal thickening. Another explanation for the effect of OK on ChT could be due to the myopic defocus on the peripheral retina. Several studies verified that OK intervention could induce peripheral myopic defocus in myopic children. Moderiano et al[66] showed that exposure to optical myopic defocus could cause a significant thickening of the sub-foveal choroid. The Atropine for the Treatment of Myopia 2 (ATOM2) study reported that 0.01% atropine could also cause pupil dilation by 1 mm[67], which would increase the magnitude of myopia defocus after OK treatment. However, OKA treatment did not show a stronger effect on SFCT thickening compared with OK alone in our Meta-analysis. The exact mechanism underlying the results remains to be explored further.
Repeated low-level red-light (RLRL) therapy has recently emerged as a treatment for myopia control. A multi-center RCT in China reported a 76.6% reduction in myopia progression and a 69.4% reduction in axial length progression in children using RLRL therapy, compared with wearing spectacles only[68]. Participants in the spectacles group were found to have a reduction in axial elongation and myopia progression after starting the RLRL therapy during the second year[69]. Up to now, several studies have proven the effectiveness of RLRL in myopia control in different districts in China[70]–[71]. The exact mechanism of RLRL in myopia control is still unclear. Xiong et al[52] found that RLRL could increase ChT in myopic children, and even has a stronger effect on choroid than OK treatment. It is speculated that RLRL could induces nitric oxide release in animal studies[72]. Further studies with larger sample and long period in other ethnicities are needed to determine the role and mechanism of RLRL in myopia control.
Our study has several limitations. First, we did not include unpublished articles or papers written in languages other than English, which may introduce some bias. Second, the changes in ChT is related to circadian rhythms, and previous research has shown that the choroid was thicker at night and thinner in daytime[73]. The time when ChT was measured differed from the studies, which may also contribute to bias. Third, the duration of myopic intervention varies in different studies, which might cause bias. Depending on the duration time, we divided the eligible studies into two groups: short (duration <6mo) and long (duration ≥6mo), and conducted a subgroup analysis. Despite this analysis, we found that the effect of myopia intervention on ChT did not change. Last but not the least, some studies were excluded due to lack of necessary information, and data from these studies could have influenced the results.
In conclusion, our study found in the pediatric population, SFCT in the myopic eyes was thinner than that in the non-myopic eyes examined by SS-OCT and EDI-COT. Furthermore, we observed that the ChT decreased horizontally from the temporal sector toward the nasal sector in myopia population. Myopia control interventions including OK and OKA were found to increase the thickness of the choroidal. But others, such as 0.01% atropine alone, did not show an increase in SFCT. Further research is necessary to confirm the clear causal relationship between choroid and myopia.
Acknowledgments
The authors thank Huixin Liu from the Peking University People's Hospital for his assistance with the statistic method.
Authors' contributions: Study design and concept (Guo LL, Meng QY); database search (Meng QY, Miao ZQ), data extracting (Meng QY, Miao ZQ, Guo LL), data analysis (Meng QY, Liang ST), supervision (Zhao MW, Guo LL), manuscript writing (Meng QY), manuscript revising (Wu X, Wang LJ, Zhao MW, Guo LL).
Foundations: Supported by the National Natural Science Foundation of China (No.31427801); National Key R&D Program of China (No.2020YFC2008200).
Conflicts of Interest: Meng QY, None; Miao ZQ, None; Liang ST, None; Wu X, None; Wang LJ, None; Zhao MW, None; Guo LL, None.
REFERENCES
- 1.Resnikoff S, Jonas JB, Friedman D, He M, Jong M, Nichols JJ, Ohno-Matsui K, Smith ELI, Wildsoet CF, Taylor HR, Wolffsohn JS, Wong TY. Myopia - A 21st century public health issue. Invest Ophthalmol Vis Sci. 2019;60(3):Mi–Mii. doi: 10.1167/iovs.18-25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong PTVM. Myopia: its historical contexts. Br J Ophthalmol. 2018;102(8):1021–1027. doi: 10.1136/bjophthalmol-2017-311625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan IG, French AN, Ashby RS, Guo XX, Ding XH, He MG, Rose KA. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan CS, Ngo WK, Cheong KX. Comparison of choroidal thicknesses using swept source and spectral domain optical coherence tomography in diseased and normal eyes. Br J Ophthalmol. 2015;99(3):354–358. doi: 10.1136/bjophthalmol-2014-305331. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Q, Yao Y, Tu S, Zhao M. Quantitative analysis of choroidal vasculature in central serous chorioretinopathy using ultra-widefield swept-source optical coherence tomography angiography. Sci Rep. 2022;12(1):18427. doi: 10.1038/s41598-022-23389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DM, Mazade R, Clarkson-Townsend D, Hogan K, Datta Roy PM, Pardue MT. Candidate pathways for retina to scleral signaling in refractive eye growth. Exp Eye Res. 2022;219:109071. doi: 10.1016/j.exer.2022.109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Ouyang X, Fu H, Hou X, Liu Y, Xie Y, Yu H, Wang G. Advances in biomedical study of the myopia-related signaling pathways and mechanisms. Biomed Pharmacother. 2022;145:112472. doi: 10.1016/j.biopha.2021.112472. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: What are the key challenges. Prog Retin Eye Res. 2017;61:60–71. doi: 10.1016/j.preteyeres.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathis U, Feldkaemper M, Liu H, Schaeffel F. Studies on the interactions of retinal dopamine with choroidal thickness in the chicken. Graefes Arch Clin Exp Ophthalmol. 2023;261(2):409–425. doi: 10.1007/s00417-022-05837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Deng Z, Tan J, Liu S, Hu S, Tao H, Tang R. All-trans retinoic acid stimulates the secretion of TGF-β2 via the phospholipase C but not the adenylyl cyclase signaling pathway in retinal pigment epithelium cells. BMC Ophthalmol. 2019;19(1):23. doi: 10.1186/s12886-018-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summers JA, Cano EM, Kaser-Eichberger A, Schroedl F. Retinoic acid synthesis by a population of choroidal stromal cells. Exp Eye Res. 2020;201:108252. doi: 10.1016/j.exer.2020.108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao F, Zhang D, Zhou Q, et al. Scleral HIF-1α is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine. 2020;57:102878. doi: 10.1016/j.ebiom.2020.102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115(30):E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Zhang S, Yang F, Yang YZ, Huang Q, Huang CJ, Qu J, Zhou XT. Decreased choroidal blood perfusion induces myopia in Guinea pigs. Invest Ophthalmol Vis Sci. 2021;62(15):30. doi: 10.1167/iovs.62.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhablani J, Barteselli G, Wang HY, El-Emam S, Kozak I, Doede AL, Bartsch DU, Cheng LY, Freeman WR. Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(4):2274–2280. doi: 10.1167/iovs.12-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta P, Saw SM, Cheung CY, Girard MJ, Mari JM, Bhargava M, Tan C, Tan M, Yang A, Tey F, Nah G, Zhao P, Wong TY, Cheng CY. Choroidal thickness and high myopia: a case-control study of young Chinese men in Singapore. Acta Ophthalmol. 2015;93(7):e585–e592. doi: 10.1111/aos.12631. [DOI] [PubMed] [Google Scholar]
- 18.Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120(1):175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Ho M, Liu DT, Chan VC, Lam DS. Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology. 2013;120(9):1909–1914. doi: 10.1016/j.ophtha.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg D, Moisseiev E, Goldstein M, Loewenstein A, Barak A. Enhanced depth imaging optical coherence tomography: choroidal thickness and correlations with age, refractive error, and axial length. Ophthalmic Surg Lasers Imaging. 2012;43(4):296–301. doi: 10.3928/15428877-20120426-02. [DOI] [PubMed] [Google Scholar]
- 21.Hoseini-Yazdi H, Vincent SJ, Collins MJ, Read SA, Alonso-Caneiro D. Wide-field choroidal thickness in myopes and emmetropes. Sci Rep. 2019;9(1):3474. doi: 10.1038/s41598-019-39653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong F, Tu J, Mao T, Yu L, Lin N, Liao H. Subfoveal choroidal thickness in myopia: an OCT-based study in young Chinese patients. J Ophthalmol. 2020;2020:5896016. doi: 10.1155/2020/5896016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biyik KZ, Tideman JWL, Polling JR, Buitendijk GHS, Jaddoe VVW, Larsen M, Klaver CCW. Subfoveal choroidal thickness at age 9 years in relation to clinical and perinatal characteristics in the population-based Generation R Study. Acta Ophthalmol. 2020;98(2):172–176. doi: 10.1111/aos.14178. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JM, Wu JF, Chen JH, Wang L, Lu TL, Sun W, Hu YY, Jiang WJ, Guo DD, Wang XR, Bi HS, Jonas JB. Macular choroidal thickness in children: the Shandong children eye study. Invest Ophthalmol Vis Sci. 2015;56(13):7646–7652. doi: 10.1167/iovs.15-17137. [DOI] [PubMed] [Google Scholar]
- 25.El-Shazly AA, Farweez YA, ElSebaay ME, El-Zawahry WMA. Correlation between choroidal thickness and degree of myopia assessed with enhanced depth imaging optical coherence tomography. Eur J Ophthalmol. 2017;27(5):577–584. doi: 10.5301/ejo.5000936. [DOI] [PubMed] [Google Scholar]
- 26.Bulut A, Öner V, Büyüktarakçı Ş, Kaim M. Associations between choroidal thickness, axial length and spherical equivalent in a paediatric population. Clin Exp Optom. 2016;99(4):356–359. doi: 10.1111/cxo.12353. [DOI] [PubMed] [Google Scholar]
- 27.Park KA, Oh SY. Choroidal thickness in healthy children. Retina. 2013;33(9):1971–1976. doi: 10.1097/IAE.0b013e3182923477. [DOI] [PubMed] [Google Scholar]
- 28.Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. doi: 10.1016/j.preteyeres.2020.100923. [DOI] [PubMed] [Google Scholar]
- 29.Parums DV. Editorial: review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Monit. 2021;27:e934475. doi: 10.12659/MSM.934475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Zhou X, Wang Z, Zhu J, Shen W, Jiang B. Assessment of retinal and choroidal measurements in Chinese school-age children with cirrus-HD optical coherence tomography. PLoS One. 2016;11(7):e0158948. doi: 10.1371/journal.pone.0158948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin P, Zou H, Zhu J, Xu X, Jin J, Chang TC, Lu L, Yuan H, Sun S, Yan B, He J, Wang M, He X. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol. 2016;168:164–176. doi: 10.1016/j.ajo.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Fontaine M, Gaucher D, Sauer A, Speeg-Schatz C. Choroidal thickness and ametropia in children: a longitudinal study. Eur J Ophthalmol. 2017;27(6):730–734. doi: 10.5301/ejo.5000965. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg K, Vestergaard AH, Jacobsen N, Suhr Thykjaer A, Søgaard Hansen R, Goldschmidt E, Peto T, Halekoh U, Wedderkopp N, Grauslund J. Choroidal thickness and myopia in relation to physical activity - the CHAMPS Eye Study. Acta Ophthalmol. 2018;96(4):371–378. doi: 10.1111/aos.13640. [DOI] [PubMed] [Google Scholar]
- 37.Lee GY, Yu S, Kang HG, Kim JS, Lee KW, Lee JH. Choroidal thickness variation according to refractive error measured by spectral domain-optical coherence tomography in Korean children. Korean J Ophthalmol. 2017;31(2):151–158. doi: 10.3341/kjo.2017.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J, Jin J, Lv M, Jiang W, Sun S, Yao C, Zhu J, Zou H, Wang L, He X, Xu X. Distribution of scleral thickness and associated factors in 810 Chinese children and adolescents: a swept-source optical coherence tomography study. Acta Ophthalmol. 2019;97(3):e410–e418. doi: 10.1111/aos.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Liu L, Zheng D, et al. Prevalence and associations of fundus tessellation among junior students from greater Beijing. Invest Ophthalmol Vis Sci. 2019;60(12):4033–4040. doi: 10.1167/iovs.19-27382. [DOI] [PubMed] [Google Scholar]
- 40.Liu WQ, Wang DD, Yang XX, Pan YY, Song X, Hou YS, Wang CX. Topographic distribution features of the choroidal and retinal nerve fiber layer thickness in Chinese school-aged children. Int J Ophthalmol. 2020;13(9):1459–1466. doi: 10.18240/ijo.2020.09.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(12):7578–7586. doi: 10.1167/iovs.13-12772. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Y, Zhang Z, Wu Z, Sun S, Fu Y, Ke B. Change and recovery of choroid thickness after short-term application of 1% atropine gel and its influencing factors in 6-7-year-old children. Curr Eye Res. 2021;46(8):1171–1177. doi: 10.1080/02713683.2020.1863431. [DOI] [PubMed] [Google Scholar]
- 43.Matalia J, Anegondi NS, Veeboy L, Roy AS. Age and myopia associated optical coherence tomography of retina and choroid in pediatric eyes. Indian J Ophthalmol. 2018;66(1):77–82. doi: 10.4103/ijo.IJO_652_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang X, Li M, Lv L, Yan X, Liu Y, Zhu M, Wang J, Wang P, Xiang Y. Assessment of choroidal vascularity and choriocapillaris blood perfusion after accommodation in myopia, emmetropia, and hyperopia groups among children. Front Physiol. 2022;13:854240. doi: 10.3389/fphys.2022.854240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Xue F, Zhou J, Qu X, Zhou X. Effects of orthokeratology on choroidal thickness and axial length. Optom Vis Sci. 2016;93(9):1064–1071. doi: 10.1097/OPX.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019;97(3):e454–e459. doi: 10.1111/aos.13866. [DOI] [PubMed] [Google Scholar]
- 47.Jin WQ, Huang SH, Jiang J, Mao XJ, Shen MX, Lian Y. Short term effect of choroid thickness in the horizontal meridian detected by spectral domain optical coherence tomography in myopic children after orthokeratology. Int J Ophthalmol. 2018;11(6):991–996. doi: 10.18240/ijo.2018.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Jiang R, Zhu Y, Zhou J, Cui C. Effect of 0.01% atropine eye drops on choroidal thickness in myopic children. J Fr Ophtalmol. 2020;43(9):862–868. doi: 10.1016/j.jfo.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Ye L, Shi Y, Yin Y, Li S, He J, Zhu J, Xu X. Effects of atropine treatment on choroidal thickness in myopic children. Invest Ophthalmol Vis Sci. 2020;61(14):15. doi: 10.1167/iovs.61.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao W, Li Z, Hu Y, Jiang J, Long W, Cui D, Chen W, Yang X. Short-term effects of atropine combined with orthokeratology (ACO) on choroidal thickness. Cont Lens Anterior Eye. 2021;44(3):101348. doi: 10.1016/j.clae.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Hao Q, Zhao Q. Changes in subfoveal choroidal thickness in myopic children with 0.01% atropine, orthokeratology, or their combination. Int Ophthalmol. 2021;41(9):2963–2971. doi: 10.1007/s10792-021-01855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong F, Mao T, Liao H, Hu X, Shang L, Yu L, Lin N, Huang L, Yi Y, Zhou R, Zhou X, Yi J. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int. 2021;2021:8915867. doi: 10.1155/2021/8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong XH, Zhao Y, Chen Z, Zeng L, Han R, Dong XQ, Guo XC, Shi Z, Yang G, Yang YT, Zhang D, Zhou XT, Ma XP. A randomized controlled trial of the effect of 0.01% atropine eye drops combined with auricular acupoint stimulation on myopia progression. J Ophthalmol. 2021;2021:5585441. doi: 10.1155/2021/5585441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Zhu X, Xuan Y, Wang M, Zhou X, Qu X. Short-term effects of atropine 0.01% on the structure and vasculature of the choroid and retina in myopic Chinese children. Ophthalmol Ther. 2022;11(2):833–856. doi: 10.1007/s40123-022-00476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu M, Yu X, Wan M, Feng K, Zhang J, Shen M, Drobe B, Chen H, Qu J, Bao J. Two-year longitudinal change in choroidal and retinal thickness in school-aged myopic children: exploratory analysis of clinical trials for myopia progression. Eye Vis (Lond) 2022;9(1):5. doi: 10.1186/s40662-022-00276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bidaut-Garnier M, Schwartz C, Puyraveau M, Montard M, Delbosc B, Saleh M. Choroidal thickness measurement in children using optical coherence tomography. Retina. 2014;34(4):768–774. doi: 10.1097/IAE.0b013e3182a487a4. [DOI] [PubMed] [Google Scholar]
- 57.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci. 2013;54(5):3586–3593. doi: 10.1167/iovs.13-11732. [DOI] [PubMed] [Google Scholar]
- 58.Lee CO, Zhang X, Yuan N, Tang S, Chen LJ, Cheung CY, Yam JC. Comparison of choroidal thickness measurements between spectral domain optical coherence tomography and swept source optical coherence tomography in children. Sci Rep. 2021;11(1):13749. doi: 10.1038/s41598-021-92980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung GK, Ciuffreda KJ. Incremental retinal-defocus theory of myopia development--schematic analysis and computer simulation. Comput Biol Med. 2007;37(7):930–946. doi: 10.1016/j.compbiomed.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129(3):322–333. doi: 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 61.McBrien NA, Cottriall CL, Annies R. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis Neurosci. 2001;18(4):571–580. doi: 10.1017/s0952523801184075. [DOI] [PubMed] [Google Scholar]
- 62.Thomson K, Kelly T, Karouta C, Morgan I, Ashby R. Insights into the mechanism by which atropine inhibits myopia: evidence against cholinergic hyperactivity and modulation of dopamine release. Br J Pharmacol. 2021;178(22):4501–4517. doi: 10.1111/bph.15629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathis U, Feldkaemper MP, Schaeffel F. Effects of single and repeated intravitreal applications of atropine on choroidal thickness in alert chickens. Ophthalmic Res. 2021;64(4):664–674. doi: 10.1159/000515755. [DOI] [PubMed] [Google Scholar]
- 64.Alshareef RA, Khuthaila MK, Januwada M, Goud A, Ferrara D, Chhablani J. Choroidal vascular analysis in myopic eyes: evidence of foveal medium vessel layer thinning. Int J Retina Vitreous. 2017;3:28. doi: 10.1186/s40942-017-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Z, Cui D, Hu Y, Ao S, Zeng J, Yang X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017;40(6):417–423. doi: 10.1016/j.clae.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Moderiano D, Do M, Hobbs S, Lam V, Sarin S, Alonso-Caneiro D, Chakraborty R. Influence of the time of day on axial length and choroidal thickness changes to hyperopic and myopic defocus in human eyes. Exp Eye Res. 2019;182:125–136. doi: 10.1016/j.exer.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Y, Zhu Z, Tan X, Kong X, Zhong H, Zhang J, Xiong R, Yuan Y, Zeng J, Morgan IG, He M. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129(5):509–519. doi: 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 69.Xiong R, Zhu Z, Jiang Y, Kong X, Zhang J, Wang W, Kiburg K, Yuan Y, Chen Y, Zhang S, Xuan M, Zeng J, Morgan IG, He M. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: a 2-year post-trial follow-up study. Clin Exp Ophthalmol. 2022;50(9):1013–1024. doi: 10.1111/ceo.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Xiong R, Chen X, Zhang J, Bulloch G, Lin X, Wu X, Li J. Efficacy comparison of repeated low-level red light and low-dose atropine for myopia control: a randomized controlled trial. Transl Vis Sci Technol. 2022;11(10):33. doi: 10.1167/tvst.11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou L, Xing C, Qiang W, Hua C, Tong L. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic Physiol Opt. 2022;42(2):335–344. doi: 10.1111/opo.12939. [DOI] [PubMed] [Google Scholar]
- 72.Ojaghi R, Sohanaki H, Ghasemi T, Keshavarz F, Yousefifard M, Sadeghipour HR. Role of low-intensity laser therapy on naloxone-precipitated morphine withdrawal signs in mice: is nitric oxide a possible candidate mediator? Lasers Med Sci. 2014;29(5):1655–1659. doi: 10.1007/s10103-014-1530-7. [DOI] [PubMed] [Google Scholar]
- 73.Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K, Iida T. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53(4):2300–2307. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]