Abstract
Tissue engineering (TE) is currently considered a cutting‐edge discipline that offers the potential for developing treatments for health conditions that negatively affect the quality of life. This interdisciplinary field typically involves the combination of cells, scaffolds, and appropriate induction factors for the regeneration and repair of damaged tissue. Cell fate decisions, such as survival, proliferation, or differentiation, critically depend on various biochemical and biophysical factors provided by the extracellular environment during developmental, physiological, and pathological processes. Therefore, understanding the mechanisms of action of these factors is critical to accurately mimic the complex architecture of the extracellular environment of living tissues and improve the efficiency of TE approaches. In this review, we recapitulate the effects that biochemical and biophysical induction factors have on various aspects of cell fate. While the role of biochemical factors, such as growth factors, small molecules, extracellular matrix (ECM) components, and cytokines, has been extensively studied in the context of TE applications, it is only recently that we have begun to understand the effects of biophysical signals such as surface topography, mechanical, and electrical signals. These biophysical cues could provide a more robust set of stimuli to manipulate cell signaling pathways during the formation of the engineered tissue. Furthermore, the simultaneous application of different types of signals appears to elicit synergistic responses that are likely to improve functional outcomes, which could help translate results into successful clinical therapies in the future.
Keywords: biochemical cues, biophysical cues, differentiation, induction factors, tissue engineering
Abbreviations
- ADSCs
adipose‐derived stem cells
- BDNF
brain‐derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BMPs
bone morphogenic proteins
- CS
chitosan
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GDNF
glial cell line‐derived neurotrophic factor
- GMP
good manufacturing practices
- HA
hyaluronic acid
- IL
interleukin
- miRNA
microRNA
- mRNA
messenger RNA
- MSCs
mesenchymal stem cells
- NGF
nerve growth factor
- PCL
poly caprolactone
- PDGF
platelet‐derived growth factor
- PEMFs
pulsed electromagnetic fields
- PLGA
poly lactic‐co‐glycolic acid
- PLLA
poly l‐lactic acid
- TE
tissue engineering
- TGF‐β
transforming growth factor‐beta
- TNF
tumor necrosis factor
- USWs
ultrasound standing waves
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Tissue engineering (TE) is a multifaceted and interdisciplinary field that is supported by the broad principles of engineering, natural sciences, and biology. The goal of TE is to develop functional substitutes for a broad wide of biomedical applications, including tissues for repairing damaged organs and in vitro models for pharmacological research. One of the key elements for the success of any TE approach is the creation of a native‐like microenvironment for the cells to recapitulate the processes occurring during development and under physiological and pathological conditions. 1 During these processes, for instance, angiogenesis, inflammation, and wound healing, the fate of the cells is tightly controlled by several biochemical and biophysical signals from the cell microenvironment. 2 Therefore, TE approaches have exploited inductive molecules, bioactive ligands, mechanical stimulation, and stiffness gradients to efficiently manipulate cell adhesion, migration, and lineage specification. 3 , 4 While significant progress has been obtained in recent years, a fascinating challenge in TE remains the accurate control of the microenvironmental cues that modulate cell fate. 5 , 6 Since the signaling networks that determine cell fate decisions are multiple and complex, the implementation of an appropriate natural‐like milieu requires careful consideration of the cross‐talk between the various pathways regulated by biochemical and biophysical cues. As studies have shown, the combination of biochemical and biophysical induction signals can be instrumental for efficient tissue regeneration and repair. 7 , 8 In this direction, there has been an increasing interest in the simultaneous application of biochemical and biophysical induction factors to synergistically promote the efficiency of TE approaches.
This review aims to summarize the main types of biochemical and biophysical stimulating factors, effective approaches, and related concepts to manipulate cell fate decisions in TE. Additionally, the review presents and discusses current advances regarding the synergistic application of these induction factors for engineering functional tissues.
2. BIOCHEMICAL FACTORS
Biochemical induction factors include growth factors, cytokines, 9 , 10 small molecules, 11 polynucleotides, 12 and other bioactive agents. Growth factors are a superfamily of peptides that regulate a large amount of cell signaling processes and mediate cell proliferation, migration, and differentiation. 13 Cytokines are a group of proteins creating a more stimulating microenvironment for better integration of implantable systems. 14 A wide range of small molecules regulates many intracellular processes, resulting in changing the transcription of specific genes, and subsequently, the expression of various proteins. 15 Unlike growth factors and cytokines, the extremely small size of small molecules leads to easy penetration through the cell membrane and affects intracellular signaling pathways. 15 , 16 , 17 Due to the participation of ECM proteins in cell fate determination mediated by cell–ECM interactions, the investigation of the ECM‐derived peptides and motifs as exogenous agents provides a great opportunity for mimicking the natural cell environment. 3 Other advanced techniques to control the level of soluble signaling molecules in TE approaches include introducing polynucleotides that alter the gene expression of selected signaling molecules on target cells. 12 , 18 , 19 , 20 The main types of biochemical factors are presented and discussed in the following subsections and summarized in Figure 1.
FIGURE 1.
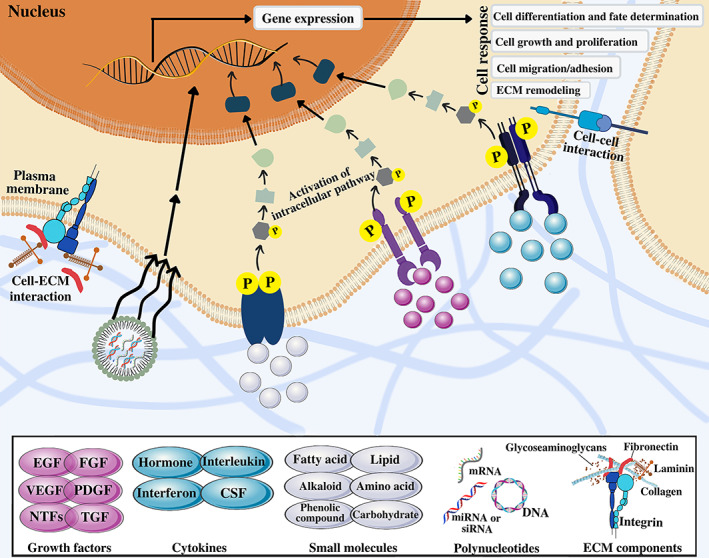
Biochemical stimulating factors involved in manipulating cell fate decisions in tissue engineering. Biochemical factors including growth factors, cytokines, small molecules, polynucleotides, and ECM components induce activation of various downstream regulatory molecules in intracellular signaling pathways which alter gene expression and subsequently lead to fate determination and different cell responses such as differentiation, growth, proliferation, migration, and ECM remodeling. CSFs, colony‐stimulating factors; EGF, epidermal growth factor; FGF, fibroblast growth factor; NTFs, neurotrophic factors; PDGF, platelet‐derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor
2.1. Growth factors
Growth factors are polypeptides secreted by a wide range of cell types that can stimulate or inhibit cellular functions such as proliferation, differentiation, gene expression, and migration. 21 Scientists have identified hundreds of growth factors that have been classified into 20 families and superfamilies depending on their structural similarities. 22 When the growth factor binds to its cell surface receptor, the signal will amplify and transfer through the cell membrane and eventually change cell function via modifying gene expression.
The action of growth factors is dependent on many factors like concentration, duration, cell location, and cell cycle state. 23 Although using growth factors at optimal concentrations is critical for efficient tissue regeneration, their short half‐life, poor stability, enzymatic inactivation under physiologic conditions, and toxicity in high doses are the major obstacles that raise serious concerns for their clinical applications. On the other hand, direct in vivo injection of biochemical factors or supplementation of soluble growth factors to the in vitro culture medium result in some side effects; therefore, researchers simultaneously use integrated methods or regulatory agents to release them under control in damaged tissue. Even though designing the appropriate delivery system for growth factors is a challenging process, there are some state‐of‐the‐art strategies that provide the opportunity for precise and spatiotemporal control of growth factors release. Some strategies that hold great promise for the controlled release of growth factors include incorporating growth factors in the hydrogel matrix, hydrophilic materials, compositions, microencapsulation, physical or chemical surface immobilization, and triggered delivery. 24 , 25 , 26
2.1.1. Epidermal growth factor
Epidermal growth factor (EGF) is a 53 amino acid mitogenic polypeptide that can affect the rate of wound healing. 27 The ERK/MAPK pathway controls gene expression, cell cycle, and proliferation, and the PI3K/Akt and JAK/STAT pathways, which trigger a range of anti‐apoptotic and pro‐survival signals and are the major intracellular signaling pathways activated by EGF signal transduction. 28 Specifically, binding the EGF receptor to its ligand activates the receptor's tyrosine kinase activity, which stimulates downstream signaling cascades such as Ras activation and mitogen‐activated protein kinase (MAPK) activation. 29 Subsequently, the receptor is internalized by clathrin‐coated endocytosis and EGF enters the cell to exert its effects (e.g., activation of stem cell proliferation and migration). 29 Activation of the EGF receptor or HER‐1 leads to increased production of specific proteins related to the endothelium, keratinocytes, and corneal epithelial differentiation in vivo and in vitro. 30 , 31 Another role of EGF on mesenchymal stem cells (MSCs) has been reported in partial wounds and surgical incisions by increasing proliferation and subsequently improving the tensile strength of the dermis. 32 In another study, a polylactic acid and glycolic acid (PLGA) nanofiber membrane containing EGF and Aloe vera extract promoted re‐epithelialization and wound healing. 33 Evaluation of EGF‐loaded PLGA nanofibers in another study showed that the controlled release of encapsulated EGF from this core–shell structure can effectively accelerate the proliferation of human fibroblasts. 34 Recent studies have shown that a combination of gelatin methacryloyl hydrogel (GelMA) with electrospun EGF‐loaded poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) (PHBV) promotes cell proliferation, migration, and angiogenesis. The researchers proposed this gelMA/PHBV/EGF patch as a promising tool for diabetic wound healing. 35 Besides, several studies have found that EGF serves as a critical growth factor during satellite cell myogenesis and the formation of sarcomeric structure. Wroblewski et al. exposed scaffold‐free skeletal muscle units with EGF, enhancing skeletal muscle cell differentiation and promoting contractile function. 36
2.1.2. Fibroblast growth factor
Fibroblast growth factor (FGF) family consists of more than 20 members involved in various biological functions such as morphogenesis, brain patterning, and muscle regeneration. Also, FGFs influence various cell types like fibroblasts, astrocytes, endothelial cells, chondrocytes, and smooth muscle cells. 37 The interaction between FGFs and their receptors activates downstream signaling pathways like RAS‐MAP and PI3K‐AKT. 38 Two important members of this family, FGF‐1 (16 kDa) as an acidic fibroblast growth factor and FGF‐2 (17 kDa) as a basic fibroblast growth factor (bFGF) have been well fully characterized. 39 Due to the rapid degradation of injected bFGF in the body, 40 it should bind to heparin‐Sepharose beads for a long time release. It is reported that high doses of FGF and heparin increased the DNA replication of the fibroblasts. 41 Also, bFGF can regulate blood vessel formation and has an important function in angioblasts induction. 42 Among this family, FGF‐2, FGF‐9, and FGF‐18 play major roles in bone formation. 43 Incorporation of FGF‐18 into chitin–PLGA/CaSO4 hydrogel is a promising technique for craniofacial bone defect regeneration since it has shown great improvements in bone formation. 44 In another study, researchers designed a scaffold‐based delivery system for FGF‐2 to promote bone regeneration. They fabricated FGF‐2 loaded poly 2‐hydroxyethyl methacrylate/trimethylolpropane trimethacrylate beads and then encapsulated them into a resin. Their results showed an increase in osteoblasts proliferation and bone regeneration in calvaria defects in animal models, suggesting this platform is an effective delivery system for growth factor delivery and TE applications. 45
2.1.3. Transforming growth factor‐β
Transforming growth factor‐β (TGF‐β) is an important protein that regulates cell differentiation, proliferation, and metabolism of ECM proteins. TGF‐β superfamily consists of three main groups including the TGF‐β, the bone morphogenic proteins (BMPs), and the activins. 46 The active form of TGF‐β (25 kDa) is a homodimer with disulfide bonds. 47 TGF‐β signaling pathway is regulated by its bioavailability and releasing process into ECM through a multi‐steps proteolytic procedure. Also, it has an important role in regulating inflammatory responses. 48 Until now, many functions of TGF‐β, such as stimulation of mesenchymal cells, inhibition of ectodermal cells, controlling fibrosis‐related chronic inflammatory diseases, improvement of tissue healing, and autoimmune diseases suppression, have been indicated. 49 The impressive enhancement in matrix synthesis via endothelial cells and vascular smooth muscle which were seeded on a hydrogel, has been detected under the TGF‐β treatment. 50
Other members of the TGF‐β superfamily are BMPs. Until now, more than 40 BMPs have been identified. These hydrophobic acid glycoproteins play different biological roles during morphogenesis and embryonic patterning. Also, they can orchestrate tissue formation throughout the body. MAPK, Smad, STAT, ERK 1/2, and PI3K‐PKB pathways are some of the cell signaling pathways regulated by these factors in the regeneration and repair of damaged tissues. Besides, in bone and cartilage TE, BMPs are extensively employed. 10 , 51 BMP‐2, BMP‐4, and BMP‐7 are the most widely used as osteogenic induction agents for stimulation of bone regeneration in fractures and ectopic site repair. 52 BMP‐2 is an osteoinductive factor that enhances the proliferation, migration, and differentiation of MSCs and the endochondral ossification process. 53 Besides, it can potentially stimulate angiogenesis. However, the natural short half‐life of this factor is an important limitation for its clinical application. To circumvent this issue, some studies suggest applying the scaffold‐based system for its delivery. In one study, an unmodified and modified nanofibrous polycaprolactone (PCL) scaffold with sulfated chitosan (CS) for the prolonged releasing time of BMP‐2 was investigated. The authors demonstrated a considerable increment in releasing time of BMP‐2. 54 In another study, Chiu et al. compared the function of surface‐modified PCL scaffold with heparan sulfate/perlecan as a platform for sustained release of BMP‐2. 55
Duruel et al. developed a CS/alginate/PLGA hydride scaffold loaded with insulin‐like growth factor (IGF) and BMP‐6 to examine the effect of the sequential release of these factors in periodontal regeneration. The results demonstrated a great improvement in proliferation and osteogenic differentiation of cementoblasts and suggested a new platform for efficient periodontal regeneration. 56
2.1.4. Platelet‐derived growth factor
Platelet‐derived growth factor (PDGF) is a growth‐promoting factor for fibroblasts that consists of two disulfide‐linked peptide chains that can be formed as homodimers (PDGF‐AA and PDGF‐BB) or as heterodimers (PDGF‐AB). 57 The interaction of PDGF with various ECM and plasma proteins regulates its biological functions. 58 The biological half‐life of intravenously injected PDGF is less than 2 min. 22 PDGF leads to initiating a biological response via binding to its receptor, activation of protein tyrosine kinase, and subsequent generation of phosphorylation‐mediated signals. 59 , 60 PDGF can promote tissue regeneration by increasing the production of autocrine factors, fibronectin, and hyaluronic acid. 61 It also plays an important role in bone regeneration and can effectively increase cartilage formation. 62 Lee et al. immobilized PDGF‐coated poly l‐lactic acid (PLLA) fibers in spheroids of adipose‐derived stem cells (ADSCs) to investigate the effect of this controlled‐release strategy on promoting osteogenic and endothelial differentiation of ADSCs. Subsequently, in vivo experiments demonstrated enhanced bone regeneration and new vessel formation in the mouse calvarial defect model. 63
2.1.5. Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF; 38.2 kDa) has an influence on the migration, proliferation, and growth of vascular endothelial cells. 23 VEGF as a master regulator of blood vessel formation can effectively initiate morphogenesis, promote the first vascular pattern, and increase endothelial precursor cell population in vivo. 64 Creating blood vessel networks is an essential and challenging step in TE as the diffusion of nutrients and oxygen is a major limit to the complexity and size of engineered constructs. 65 As an important factor for forming blood vessels and vascular permeability, VEGF has recently been introduced as a therapeutic factor for tissue repair. 66 , 67 Tsao et al. proposed a dual growth factor delivery system including porous PLGA/silica nanoparticles loaded with VEGF and PDGF and electrospun gelatin patch. Sequential delivery of these factors promoted localized neovascularization. 68 Park et al. reported a multi‐head 3D bioprinting method for heterogeneous TE applications. They represented a 3D‐printed prevascularized platform combined with human dental pulp stem cells for the spatiotemporal release of BMP‐2 and VEGF. After 28 days of grafting into rat cranial bone defects, the synergetic effect of BMP‐2 and VEGF considerably enhanced the regeneration of vascularized bone. 69 In another example, a gelatin/alginate/β‐TCP scaffold was fabricated with 3D‐printing technique with subsequent entrapment of VEGF‐loaded PLGA microspheres. Supporting cell viability and proliferation, this delivery system was suggested as a novel platform for craniofacial TE. 70
2.1.6. Neurotrophic factors
Neurotrophic factors (NTFs) are endogenous soluble proteins responsible for regulating cellular growth, survival, proliferation, re‐myelination, migration, and morphological plasticity. Nerve growth factor (NGF), glial cell line‐derived neurotrophic factor (GDNF), brain‐derived neurotrophic factor (BDNF), neurotrophin‐3 or neurotrophin‐4, and ciliary neurotrophic factor are some of the most well‐known neurotrophic factors that are extensively used in neuronal regenerative medicine. These factors modulate intracellular signaling pathways through specific neurotrophic receptors such as tyrosine receptor kinase and p75 receptor. 71 Due to in vivo short half‐life of neurotrophic factor, sustained scaffold‐based delivery strategies could enhance the regeneration capacity of nerve defects.
NGF plays an essential role in the growth and developmental plasticity in the vertebrate peripheral and central nervous system resulting in its vast applications in neural TE. 22 , 72 , 73 , 74 , 75 For example, encapsulation of NGF into chitosan nanoparticles (NGF‐nCS) effectively promoted the trans‐differentiation into neuron‐like cells. 76 In another study, immobilization of NGF and BDNF on the NH2 +/heparin bioactive surfaces induced neurite outgrowth in dorsal root ganglions in vitro. 77 Xia et al. loaded VEGF and NGF into the core–shell PLLA electrospun scaffold. Sequential release of NGF and VEGF enhanced the neural differentiation of stem cells in vitro. Furthermore, postoperation experiments showed neovascularization along with nerve regeneration in the rat sciatic nerve model. 78 Another in vitro investigation illustrated that immobilization of NGF via polydopamine‐coated PCL/CS scaffold improved adhesion, proliferation, and neurite outgrowth of PC12 cells. 79 Lackington et al. assessed the nerve regeneration capacity of biodegradable nerve conduits incorporating NGF and BDNF in PLGA microparticles. This dose‐dependent delivery system could effectively promote neurite and axonal outgrowth and Schwann cell migration in vitro. Besides, significant enhancement in functional nerve recovery in sciatic nerve defect of animal models was confirmed its therapeutic potential for peripheral nerve repair. 80
Hu et al. developed a thermoresponsive heparin‐poloxamer hydrogel for the controlled release of NGF and bFGF into the lesioned spinal cord. In vitro and in vivo studies showed excellent enhancement of axon regeneration and functional recovery significantly along with decreased neurons apoptosis and glial scar formation. This neuroregenerative effect was related to PI3K/Akt and MAPK/ERK signaling pathways. 81
Zhang et al. designed collagen/β‐tricalcium phosphate conduits combined with NGF to bridge a nerve gap in vivo. Electrophysiological and histomorphometric tests demonstrated that this biomedical system could improve functional recovery and axonal regeneration. 82 Taken together, these experiments confirmed the potential application of NGF and other neurotrophic factors, especially in peripheral TE.
2.2. Cytokines
Cytokines are secreted factors that modulate cell growth, differentiation, and immunity. Usually, they are classified into two types: type I includes hormones, interleukins (ILs), neurotrophic factors, and colony‐stimulating factors, whereas interleukin‐10 and interferons are categorized in type II. 83 Cytokine‐secreting cells are usually related to the immune system and are often released by T cells and macrophages in the damaged area. 14 The most effective cytokines in TE include ILs and tumor necrosis factor (TNF). 10 , 14 In the first 24 h after tissue damage, cytokines, including ILs (1–6), are first activated by macrophages and begin the regeneration process by re‐calling the related angiogenesis factors. In the early stages, the TNF‐α as a cytokine facilitates the regeneration process by collecting and destroying damaged parts and attracting more stem cells to the site. These factors help regenerate damaged tissue in three stages: inflammation, angiogenesis, and finally transformation with new cells. 10 Mountziaris et al. designed a microfibrous PCL mesh containing TNF‐α to investigate the effect of TNF‐α on osteogenic differentiation of MSCs. The results demonstrated that temporal patterns of TNF‐α delivery supported the bone regeneration process. 84 In another study, an IL‐4‐loaded hybrid bilayer scaffold was fabricated to examine the anti‐inflammatory effect of IL‐4 on articular cartilage and subchondral bone repair. Both in vitro and in vivo experiments revealed decreased inflammatory effects of IL‐1β and macrophages on chondrocytes resulting in better osteochondral regeneration. 85
2.3. ECM components
The ECM provides a microenvironment that fully supports the structure and function of the tissue and meets its biological and physical needs. The ECM is produced and maintained by the cells in a dynamic equilibrium. 86 , 87 Investigations have shown that ECM proteins such as elastin, collagen, laminin, and fibronectin are fundamental for the survival and growth of many cell types. Collagen is the most abundant protein found in mammalian organisms, playing an important function in providing tensile strength to various tissues and supporting the function of multiple cell types. With its triple helix structure, collagen can significantly affect key cellular functions such as adhesion, proliferation, and migration. 88 Fibronectin can enhance cell adhesion through its various binding domains. 89 Some studies demonstrated that fibronectin‐conjugated scaffolds could effectively improve cell adhesion and infiltration depth. 90
Hyaluronic acid (HA), as an ECM glycosaminoglycan, participates in wound repair and cell migration. The biocompatibility, biodegradability, hydrophilicity, and limited immunogenicity of HA make it an ideal choice for cartilage, bone, vascular, and skin TE. 91 , 92 Park et al. produced an injectable HA hydrogel loaded with a BMP‐2 mimetic peptide for osteoinduction of human dental pulp stem cells. 93 Choi et al. revealed that encapsulated chondrocytes in HA/agarose hydrogels provided an enhanced microenvironment for chondrogenesis. 94 As an articular cartilage‐specific proteoglycan, Aggrecan is related to the load‐bearing properties of cartilage. Ingavle et al. designed an interpenetrating network hydrogels containing aggrecan and chondroitin sulfate as bioactive signals to enhance encapsulated chondrocytes performance. 95
Due to the appropriate biocompatibility and biodegradability of fibrin, it serves in cell delivery platforms. Montalbano et al. formulated a thermo‐responsive collagen/alginate/fibrin hydrogel to mimic the native ECM microenvironment for reconstitution of pseudo‐islets. Their results offered a tunable composite for pancreas TE and musculoskeletal regeneration. 96
Although purified ECM proteins can be utilized for TE applications, 97 the production of peptide sequences is simpler and more cost‐effective as compared to full‐length proteins. RGD, IKVAV, YIGSR, DGEA, PHRSN, and PRARI peptides, as modifier peptides, are usually derived from structural ECM protein domains particularly collagen, laminin, fibronectin, and elastin. 4 Considering good solubility and stability as compared to full‐length ECM proteins, peptide sequences have been widely employed to mimic the natural ECM. 98 , 99 These biomolecules can effectively promote various cell functions and stimulate specific signaling pathways due to their specific signature derived from the ECM proteins.
Among the mentioned peptides, the RGD motif (Arg‐Gly‐Asp) derived from collagen, fibronectin, laminin, gelatin, vitronectin, fibrinogen, osteopontin, and sialoprotein, specifically targets a number of integrin receptors. 100 , 101 Integrins are heterodimeric transmembrane complexes consisting of two subunits, the α‐chain and the β‐chain, which are present on cell membranes and associated with various components of the ECM. 102 , 103 The binding of cells to the ECM through the α‐chain of integrins at focal adhesion sites is mediated by calcium and is essential not only for cell‐ECM adhesion but also for cell–cell adhesion. 102 , 103 Integrin–ligand binding mediates the interaction between external forces and the actin cytoskeleton, leading to the clustering of cytoplasmic proteins (such as FAK, vinculin, or talin) and regulating intracellular signaling cascades such as the MAPK pathway, which influence important processes including proliferation, differentiation, and migration. 104 RGD‐modified materials have been used in cancer therapy, TE, and regenerative medicine studies. 100 , 102 For example, Wang et al. investigated the synergistic effect of topographic cues with peptide presentations (RGD/YIGSR) as a new approach to scaffold design for skeletal muscle TE. This study showed that surface‐mediated PLGA scaffolds can significantly affect myoblast proliferation and differentiation. 105 Another important integrin‐binding peptide motif is the IKVAV (Ile‐Lys‐Val‐Ala‐Val), which is derived from laminin and can act as a biofunctional epitope. 106 Cheng et al. reported that RADA16‐IKVAV self‐assembling peptide hydrogel could be used as a gap filler in nerve injury by positively affecting surviving neural stem cells and reducing glial astrocyte formation. 107 In another study, the incorporation of IKVAV and RGD peptides into a protein nanostructured scaffold provides the chemical and structural support essential for the myogenic differentiation of the C2C12 cells. 108 Besides, many studies focused on the effect of laminin‐derived IKVAV epitope on neuronal differentiation and neurite extension, which is a key point for neural TE. 109
2.4. Polynucleotides
Due to the high cost of exogenous factors and their potential cytotoxic effects, the introduction of DNA or RNA molecules into the cells is applied for gene expression manipulation. These methods can also be used to achieve sustained gene expression in the stem cells, with the aim of improving their properties for therapeutic applications. 110 , 111 In this context, the role of genetically modified cells in restoring the normal function of various types of tissues has been explored by several research groups. There are a number of proteins that can be overexpressed to induce a specific cell phenotype, such as FGF‐2, BMPs, TGF‐β, IGFs (insulin‐like growth factors), VEGF, and PDGF. 112 , 113
The improved survival, proliferation, and metabolism of these engineered cell lines have opened the perspective of translating preclinical studies into efficient and safe therapies for numerous diseases and deficiencies, including multiple sclerosis, Parkinson's disease, heart disease, kidney injury, bone and cartilage disorders, and amyotrophic lateral sclerosis. 114 , 115 In addition, there have been several studies on cancer therapy based on transformed stem cells, which have been shown to reduce tumor growth and increase patient survival in various malignancies including melanomas, brain, liver, and breast tumors. 112
Approaches for introducing genes into the desired cells are generally divided into nonviral methods (e.g., liposomes and polycations) and viral methods (e.g., adeno‐associated viruses, retroviruses, herpes viruses, and others). Each approach offers advantages and disadvantages that must be carefully considered. For example, viral delivery allows for sustained expression and highly efficient transfection, but may also trigger immune responses. On the other hand, nonviral gene transfer vectors are easy to produce, safer than the viral methods, and result in more transgenes. But, some nonviral polymeric vectors, such as polycations, may display toxicity and low efficiency. Nevertheless, nonviral vectors have lower immunogenicity and cytotoxicity, making them the method of choice in most studies. 7 , 111 , 112
Despite the clear potential of gene transfer techniques, the clinical application still encounters some serious limitations that need to be addressed. These include loss of paracrine activity, rapid in vivo degradation of RNAs, poor in vivo cell viability, risk of carcinogenesis, viral infections, and off‐targeting. 18 , 116 Remarkably, long‐term controlled release of polynucleotides in vivo does not match in vitro conditions. The integrity of the vectors in vivo, both postinjection and in the long‐term, should also be investigated to avoid the leakage of polynucleotides. To date, the optimal kinetics and duration of polynucleotide release from biomaterial scaffolds have not been fully elucidated. Moreover, the survival and proliferation of stem cells during their expansion ex vivo is reported to be affected by oxidative stress. 117 Other obstacles to this approach include electrostatic repulsion, which occurs when nucleic acids cross membranes with negative charge, and that delivery methods are short‐lived and do not provide sustained expression levels. Accordingly, numerous challenges must be considered, such as the optimal cell source, cultivation methods, vector type, promoter efficiency, dosage, and route of injection. 111 , 112 , 118 , 119 This section summarizes some well‐defined genetically cell mediation strategies employed in TE and regenerative medicine approaches.
2.4.1. DNA
The interplay between gene delivery and regenerative medicine suggests the versatile therapeutic method. In recent years, polymeric scaffolds have been used as vehicles for gene delivery into specific cells to stimulate sustained transgene expression. 120 Various cells are engineered to overexpress bioactive molecules that play crucial roles in tissue formation and repair. 121 , 122 Gonzalez‐Fernandez et al. have investigated the functionality of alginate hydrogels as supporters for encapsulating gene‐mediated MSCs with TGF‐β3 and BMP2 DNA plasmids; this complex caused rising secretion of sulfated glycosaminoglycan and collagen. 123 In an in vivo study, glycosaminoglycan, containing osteogenic and angiogenic inducer genes (BMP‐2 and VEGF, respectively), was integrated into chitosan‐based scaffolds, resulting in total bone repair defects. 124 In another investigation, Lackington et al. have focused on localized delivery of some neurotrophic factors via polyethyleneimine–pDNA nanoparticles incorporated into nerve guide conduit. Their results showed efficient delivery of NGF, GDNF, and c‐Jun transcription factor genes in both Schwann and neuronal cells. In vitro investigations presented enhancing regenerative cellular processes and neurite outgrowth. 125
2.4.2. RNA
Various types of RNA, including messenger RNA (mRNA), microRNAs (miRNAs), small interference RNAs (siRNAs), and small hairpin RNA (shRNA), can be used for orchestrating tissue regeneration signaling pathways through gene transcription modification, posttranscriptional gene silencing, and protein translation regulating in eukaryotic cells. 12 , 126 Due to better spatiotemporal control and safety concerns about direct gene delivery methods, RNA‐level gene expression modification provides an excellent opportunity for clinical applications. 127 , 128 , 129 , 130
Depending on the upregulation or downregulation of mRNA after tissue injury, various technologies such as miRNA‐based therapies and anti‐sense technologies can be used to manipulate and remodel the microenvironment of damaged tissue. 131 Synthetic oligonucleotides, known as anti‐microRNAs, can specifically inhibit miRNA–mRNA interaction (Table 1). 132 , 133
TABLE 1.
Some miRNA grafted scaffolds for tissue engineering purposes
| Engineered tissue | Scaffold | Animal model (in vivo analysis) | Cell type (in vitro analysis) | miRNA | Molecular consequences | Result | References |
|---|---|---|---|---|---|---|---|
| Bone | PLGA/PLA | Mouse | Mouse osteoblasts | miRNA‐26a | Downregulation of Gsk‐3β and activation of Wnt/β‐catenin pathway | Upregulation of the osteoblastic activity of endogenous stem cells and bone repair | 134 |
| PEG hydrogel | Rat | Human mesenchymal stem Cells | miRNA‐20a/siNoggin | Overexpression of BMP2 | Promoting osteogenic differentiation | 135 | |
| Collagen | Rat | Rat bone marrow stem cells | miR‐148b | BMP signaling cascade | Activating osteogenesis and bone repair | 136, 137 | |
| Poly sebacoyl | Rat | Rat adipose‐derived stem cells | miR‐135 | Repressing Hoxa2 expression/activating Runx2 pathway | Activating osteogenesis | 138 | |
| Neural | PCL fibers | – | Rat oligodendroglial precursor cells | miR‐219/miR‐338 | Inducing MBP expression | Promoting oligodendrocytes maturation | 139 |
| PLLA/PCL | – | Trabecular meshwork mesenchymal stem cells | miR‐7 | Overexpression of Nestin, GFAP, and MAP‐2 | Enhancing glial and neural differentiate | 140 | |
| Cardiovascular | PELCL/PCL/gelatin | Rabbit | Human umbilical vein endothelial cells | miR‐126 | Downregulation of SPRED‐1 and PIK3R2 | Acceleration of vascular endothelial cell proliferation | 141 |
| PLGA/cRGD | – | Human umbilical vein ECs | miR‐132 | Activation of Ras GAP‐1 | Improving vessel‐forming | 142 | |
| Atelocollagen | Rat | Mouse myoblast | miR‐1/miR‐206/anti miR‐133 | Overexpression of myogenic markers, downregulation of HDAC4 and MEF2C | Preventing fibrosis, promoting myotube differentiation | 143 |
Target miRNAs that contribute to cell differentiation are usually detected by various screening methods. 144 For example, blocking miR‐203 (sirtuin1 inhibitor) and miR‐449a (inhibitor of myeloid cell leukemia 1 factor) by their anti‐miR resulted in enhanced chondrogenesis in animal models. 145 , 146
In general, there are two ways to transform differentiated adult cells into other cell types: iPSC reprogramming and transdifferentiation, both of which require the expression of transcription factors. Synthetic mRNAs have shown great potential to facilitate cell engineering and reprogramming. They can be introduced into various somatic cells such as cardiomyocytes and translated directly into transcription factors by the cell's translation machinery. 127 , 147
Synthetic mRNAs can also be used for stem cell engineering because they can mediate safe, robust, and potent surface expression of molecules related to homing of stem cells. This is of great importance because when cultured in vitro, MSCs lack the potential for homing and cannot reach the target site, limiting their therapeutic efficiency. 127 , 147
With regard to spatial and temporal control of RNA delivery, injectable and in situ curing materials (e.g., hydrogels) are the most favorable candidates that allow minimally invasive approaches, reducing tissue damage, infection risks, and minimizing complications. 148 Steinle et al. synthesized an injectable chitosan/alginate hydrogel loaded with HEK293 cells and synthetic mRNA to achieve exogenous protein synthesis in the target tissue. 149 In another study, MSCs loaded with anti‐miR‐221 in fibrin and hyaluronan hydrogel were effectively converted into a chondrogenic line without using chondrogenic growth factors. 150 Another group proposed an injectable thermoresponsive hydrogel to enhance local and sustained delivery of siRNA for tumor treatment. 151 Balmayor et al. investigated the synergistic effects of fibrin gel/biphasic calcium phosphate granules and chemically modified BMP‐2 mRNA on stem cell osteogenesis. They showed that sustained release of BMP‐2 mRNA significantly promoted osteogenesis and mineral deposition. 148 Neural tissue is an example of tissue type with limited regeneration capacity that may be a good candidate for RNA delivery for therapeutic purposes. Ma et al. demonstrated the role of miR‐29a in regulating ECM protein synthesis, neurite growth, and neural stem cell recruitment in the hippocampus. 152 Upregulation of miR‐7 expression in human ocular MSCs seeded onto a nanofibrous PLLA/PCL scaffold resulted in differentiation of the cells into glial and neural progenitor cells. 140 Although there are promising results on nucleic acid‐mediated scaffolds at TE, this field is still in its early stages. Synthetic mRNAs still present several disadvantages that limit their applicability, including the lack of a standardized and efficient synthesis protocol, low stability, and potential immunogenicity. 127 , 147 These limitations need to be addressed in the future to develop robust and efficient methods that can be translated into the clinical context.
2.5. Small molecules
Small molecules with low molecular weights (<1000 Da) include carbohydrates, lipids, amino acids, fatty acids, phenolic compounds, and alkaloids. 153 , 154 Considering their well‐identified physicochemical properties, good permeability, reproducible, low‐cost synthesis, and low immunogenicity reactions, small molecules can be used as pharmaceutical substances and cell differentiation factors. 17 , 155 , 156 It is demonstrated that they can influence the endogenous stem cell lineage commitment, modulate specific intracellular processes and improve cell–cell and cell–scaffold interactions. Due to their function in cell manipulation, their application in TE is increasing. Some potential small molecules for use in TE are listed in Table 2. 10 , 17 , 155 , 157
TABLE 2.
A list of small molecules for tissue engineering purposes
| Engineered tissue | Small molecule | Target gene/pathway | Result | References |
|---|---|---|---|---|
| Bone | Bisphosphonates | Upregulation of BMP‐2, collagen I, osteocalcin, and alkaline phosphatase genes | Improving of osteoblast proliferation and inhibition of bone resorption | 158, 159 |
| Doxycycline | Inhibiting of matrix metalloproteinase/neutralizing Dkk‐1/activation of Wnt signaling | Inhibiting osteoclast‐relating genes and bone resorption | 160 | |
| Simvastatin, Lovastatin, Rosuvastatin, Fluvastatin | Stimulating BMP/Smad pathway, activating BMP‐2 gene expression | Promoting neovascularization and osteogenesis | 161, 162, 163 | |
| Cartilage | Compound‐6 (sulfonamide based) | Overexpression of aggrecan, activating MEK/ERK pathway | Promoting chondrogenesis | 164 |
| Kartogenin | Overexpression of aggrecan and collagen II | Acceleration of MSCs chondrogenesis | 165, 166 | |
| Calcium silicate | Increasing glycosaminoglycan and collagen expression | Improving cell attachment and chondrogenesis | 167 | |
| Skin | Pyrvinium | Inhibiting Wnt/β‐catenin signaling and activating casein kinase | Reducing myofibroblasts, fibrosis formation, and wound size | 168 |
| XAV‐939 | Inhibiting Wnt/β‐catenin and tankyrase | Reducing the number of myofibroblast and fibrosis formation | 168 | |
| Nitric oxide | Regulating of collagen production in fibroblasts | Accelerating re‐epithelialization, wound closure and hair follicle regeneration | 169, 170 | |
| Neural | Isoxazole 9 | Triggering of calcium‐activated pathway, CaMK phosphorylation and myocyte‐enhancer factor‐2 | Enabling trans‐differentiation of astrocytes into neural cells/increasing neuron recruitment in the hippocampal dentate gyrus | 171, 172 |
| Metphormin | Activating of aPKC‐CBP pathway | Stimulating neural stem cells recruitment and neurogenesis/improving spatial memory | 173 | |
| Forskolin | Activating of cAMP/PKA pathway and increasing intracellular cAMP | Promoting neurite outgrowth and axon polarization | 174 | |
| Cardiovascular | Cardionogen | Inhibiting Wnt/β‐catenin signaling | Enhancing cardiogenesis | 175 |
| Cyclosporin A | Inactivation of Calcineurin and NF/AT transcription factors | Increasing cardiomyogenesis and ESCs differentiation to cardiac cells | 176 | |
| CW209E | Over expression of cardiomyogenesis genes | Increasing number of beating embryoid bodies from human stem cells | 177 |
Many small molecules specifically modulate intracellular signaling pathways for guided differentiation and determined stem cell fate. For example, Wnt/β‐catenin inhibitors including KY02111, IWR‐1, and XAV939, can robustly stimulate the differentiation of pluripotent stem cells to cardiomyocytes. 155 , 156 , 178 Purmorphamine (an activator of the Sonic Hedgehog pathway) and DMH1 (an inhibitor of the BMP pathway) enable stem cells to differentiate into neuron‐like cells. 179 , 180
Small molecules are also commonly used for bone regeneration. These compounds can stimulate osteogenesis while effectively inactivating osteoclastogenesis. 181 Simvastatin, lovastatin, and rosuvastatin are some members of the statin family that have been extensively studied for bone repair via affecting the BMP/Smad pathway. Tai et al. synthesized a PLGA/hydroxyapatite scaffold with simvastatin and found that it promoted neovascularization, cell growth, and osteogenesis. 161 , 162 In an in vivo experiment, lovastatin was encased in a polyurethane scaffold and injected into a bone defect model. 163 Another study showed that purmorphamine and CW008, activators of the PKA/CREB pathway, promoted osteogenic differentiation of human MSCs. 182 , 183 Another study showed that a PLGA scaffold coated with FTY720 (an osteoinductive small molecule) significantly promoted the healing of cranial bone defects. 184
2.6. Herbal extracts
In addition to synthetic agents, naturally derived small molecules, such as plant and bacterial secondary metabolites, could also effectively improve the biocompatibility of tissue‐engineered constructs. 185 , 186 Natural substances have shown great potential for bone TE because of their many advantages, such as their cost‐effectiveness and lack of side effects. 187 Herbal extracts containing different types of phytochemicals (e.g., phenolic compounds, terpenes, phytohormones, and alkaloids) are attractive natural compounds that have achieved many investigations for healing purposes. 186 , 188 Some herbal extracts have been used in studies of stem cell proliferation and differentiation, including the aqueous extract of Alpinia oxyphllae, 163 the extract of Morinda citrifolia, which induces osteogenesis differentiation, 189 , 190 and the extract of Bacopa monnieri, which promotes neurogenesis. 191 Some small bioactive molecules such as gingerols and ginger essential oils (phenolic secondary metabolites in Zingiber officinale) have anti‐inflammatory, antibacterial, anticancer, hypoglycemic, and cardiotonic effects. 192 In this context, Mohammadi et al. synthesized nanoliposomes containing ginger extract with pro‐angiogenic and antifungal effects and promoted wound healing process. 193 In another study, an environmentally friendly method for developing ginger‐based nanofiber hydrogels as wound dressing with antibacterial activity was presented. 194 Curcumin, the major secondary metabolite of Curcuma longa with antioxidant and anti‐inflammatory activity, enhances osteoblastic activity in addition to its antiosteoclastic effect. 195 Bose et al. proposed novel 3D‐printed calcium phosphate scaffolds loaded with curcumin to improve the osteogenic potential of bone grafts. The results showed that liposomal release of curcumin could effectively promote osteoblast cell viability and proliferation in vitro and mineralized bone regeneration in vivo. 196
Brahatheeswaran et al. prepared controlled‐release nanofibers loaded with curcumin, which resulted in enhanced adhesion and proliferation of fibroblasts. 197 , 198 Another example is garlic (Allium sativum), which contains various biologically functional secondary metabolites such as allicin (diallylthiosulfinate) that can be used for the treatment of wounds. 199 Acemannan, a bioactive component of Aloe vera, has demonstrated excellent antibacterial, antioxidant, and immunomodulatory effects. 200 , 201 When used in combination with ADSCs, Aloe vera extracts incorporated in a hydrogel have been shown to significantly promote the healing of burn wounds. 202
In recent decades, the use of herbal extracts in scaffolds has advanced modern TE technologies due to their bioactive, nontoxic, nonimmunogenic, and cost‐effective properties, as well as their mechanical strength. 186 , 187 , 188 Therefore, herbal extracts have been used in approaches to promote tissue growth, to optimize bone and skin TE. 187 Natural products in combination with polymer‐based scaffolds (e.g., chitosan and gelatin) can improve scaffold properties, including biodegradability and biocompatibility, enhance their physicochemical properties (e.g., wettability, thermal properties, morphology, porosity, and cell adhesion), modulate the response of cells, regulate immune responses, promote tissue formation at the site of injury or defect, and improve healing and regeneration. 186 , 187 , 188 Remarkably, herbal extracts add anti‐inflammatory (inhibition of cytokine secretion and nitric oxide pathway), antioxidant, and antimicrobial (blocking DNA replication and metabolic pathways and inhibiting the synthesis of important proteins in bacteria) properties to engineered tissues to facilitate differentiation into the desired cell phenotype and tissue regeneration. 186 , 188 These properties are of great importance because infection, inflammation, sepsis, or oxidative stress are likely to compromise cell survival and, consequently, the efficiency of TE approaches. 188 Alginate, starch, cellulose, and agar are plant polysaccharides that are also used for scaffold construction to make the biomedical use of scaffolds more efficient in terms of supporting cell growth, angiogenesis, and differentiation. 186
These naturally derived scaffolds also provide a robust system for drug delivery by combining the advantages of scaffolds and plant extracts, allowing controlled release of the drug, with less toxicity and side effects. 187 Some manufacturing technologies have been developed for drug delivery into biomaterials, such as freeze‐drying, solvent casting, 3D printing, electrospinning, and hydrogel formation, but there are still concerns about potential unsafe interactions at the implantation site and undesirable side effects. 187 , 188 Some potential herbal medicines for use in TE are listed in Table 3.
TABLE 3.
A list of herbal medicine for tissue engineering purposes
| Engineered tissue | Herbal medicine | Figure | Target gene/pathway | Result | References |
|---|---|---|---|---|---|
| Bone | Alpinia oxyphllae |

|
Suppression of RANKL/RANK pathway | Inhibition of osteoclast differentiation and bone destruction | 163 |
| Morinda citrifolia |

|
Activation of Wnt/βcatenin signaling and enhancement in ALP, Runx2, and OCN expression | Improvement of osteoblastic differentiation | 189, 190 | |
| Curcuma longa |

|
– | Osteoblastic activity enhancement | 195, 196 | |
| Equisetum arvense |

|
Enhancement in Runx2, Col I, and OPN expression | Increasing osteogenic differentiation, ALP activity, and mineralization content | 203, 204 | |
| Spinacia oleracea |

|
– | Prevention of loss of bone in osteoporosis | 205 | |
| Cissus quadrangularis |

|
The activation of the early bone marker (ALP) | Invoking biomineralization and osteogenesis | 206 | |
| Nerve | Bacopa monnieri |

|
Activation of Akt and ERK1/2 signaling | Promotion of neural progenitor cells proliferation | 191 |
| Skin | Zingiber officinale |

|
– | Enhancement in fibroblast cells proliferation | 192, 193 |
| Curcuma longa |

|
Inhibition of nuclear factor‐kappa B and effects on TGF‐β and MAPK pathways | Improvement of attachment and growth of fibroblast cells with free radical scavenging activity and reducing inflammation | 197, 207, 208 | |
| Allium sativum |

|
Affecting tight junctions and cytoskeleton distribution | High wound healing potential | 199 | |
| Aloe vera |

|
Increment in collagen synthesis and decrease in the collagen‐degrading MMP‐1 gene expression | Antibacterial, antioxidant, and immunomodulatory effects, improving wound healing | 200, 201 |
2.7. Microbial‐derived compounds
Bacterial cells produce functional compounds that are suitable for many biomedical applications. An important advantage of microorganisms is that they can be genetically engineered to synthesize the desired compound for specific purposes. For microbial compounds to be used in TE, cost‐effectiveness, nontoxicity, and ease of processing are of paramount importance. 209 Microbial polymers found in recent years include cellulose, alginic acid, agar, carrageenan, chitin, dextran, gellan gum, pullulan, and hyaluronic acid. 209 Microbial cellulose fibers are mainly derived from a gram‐negative bacterium called Acetobacter xylinum. These fibers offer many advantages in terms of elasticity, safety, and high wettability, making them strong candidates for TE. Scaffolds for TE based on bacterial cellulose have been shown to improve chondrogenesis, osteogenesis, and stem cell differentiation compared to other bacterial components such as alginate and cellulose‐free scaffolds. In addition, the pellicle of bacterial cellulose could mimic the native collagen fibers of blood vessels, providing a suitable platform for muscle cell growth. 210 Dextran, another component derived from bacteria, is used to develop scaffolds in combination with synthetic degradable polymers such as polyethylene glycol and polylactic acid. Scaffolds containing dextran can support proliferation and adhesion of cells and degrade in vivo. Moreover, these scaffolds showed an increase in Young's modulus by combining with nanohydroxyapatite. 210
Poly‐gamma‐glutamic acid and polyhydroxyalkanoates are other bacteria‐derived polymers of great interest in scaffold development and drug delivery because of their superior properties in homeostasis, cell adhesion, proliferation, and survival. 209 Microbial secondary metabolites, such as antibiotics, growth hormones, and antitumor agents, have great potential for medical applications. For example, in one study, incorporating the fluoroquinolone antibiotic levofloxacin into the electrospun hybrid scaffold promoted cellular activities and reduced potential postoperative inflammation and infection. 211 The sustained release of the polyketide antibiotic tetracycline from the core–mantle fibers could inhibit microbial infections. 212 In another study, Ramalingam et al. investigated the synergistic effects of the tetracycline antibiotic minocin and G. sylvestre extracts on re‐epithelialization and collagen organization during wound healing by a core–shell mat. 213 In addition, biopolymers derived from bacteria, polysaccharides (such as alginate, dextran, gellan gum), polyesters such as polyhydroxyalkanoates, polyamides (such as poly(γ‐glutamic acid), poly(ε‐l‐lysine)), and inorganic polyanhydrides (such as polyphosphates) are biocompatible and biodegradable candidates for potential TE applications. 214 , 215
In addition to microbial compounds, probiotics, living organisms that benefit host health, have also been found to hold promise for TE. 216 Probiotics are a group of living microorganisms that are effective in sufficient quantities for cancer, inflammatory diseases, and gastrointestinal infections. However, probiotics are sensitive to high temperatures, acidic conditions, and high oxygen levels, so encapsulation of probiotics serves to protect the bacteria and maintain their metabolic activity. The function of the encapsulation carrier is to provide a suitable microenvironment in which the bacteria can survive during processing and storage and be released at the desired site in the gastrointestinal tract. Probiotics can be used for wound healing, protection from harmful radiation, and enhancement of innate immunity. 217 In one study, the effective role of E. mundtii against infections was investigated. In vivo results showed a synergistic role of probiotic matrix and nanostructure to improve wound healing after burns. 218 Scientists studying the role of scaffolds functionalized with probiotics have shown that they have an anti‐inflammatory effect and thus increase the healing rate. 216
3. BIOPHYSICAL FACTORS
Although biochemical induction factors are widely used to create ECM‐like microenvironments, nowadays, growing studies report significant contributions of biophysical inducers in TE. There are numerous reports about the effects of heat, 219 , 220 , 221 ultrasound waves, 222 magnetic fields, 223 electrical, 224 , 225 , 226 mechanical, 227 , 228 , 229 and light stimulation on cellular phenotype and function. 230 , 231 Furthermore, different topographical aspects of the matrix, such as surface stiffness, elasticity, and porosity, could be considered as inductive factors to modify cell fate determination. Various methods are used to functionalize the structure of the scaffold to induce favorable cell behavior. 19 , 232
The selection of appropriate induction factors must take into account the structural and functional features of the target tissue. Due to the physiological nature of electrical stimulation in the normal function of some tissues such as nerves, bone, muscles, and heart, electrical stimulation and electroconductive polymers have been remarkable research topics in recent decades. 226 Based on the electromechanical properties of cardiac tissue, the combination of electro‐conductive materials with suitable mechanical properties and electrical stimulation could be considered a promising tool in cardiac TE. 11 , 233
Overlaps between biophysical and biochemical signals regulate various aspects of a cell life cycle. Biophysical factors regulate various intracellular signaling pathways by affecting sensitive receptors on the cell surface. However, the molecular mechanisms of physical cues are not fully understood. Due to the importance of the physical and mechanical properties of tissues to their natural function, the use of biophysical inducers has recently been emphasized for more effective TE. These biophysical stimuli may provide cost‐effective, safe, and nondestructive tools for regenerative medicine. In this section, the effects of various physical and mechanical stimuli on cell fate determination, cell function, and cell–cell integrity are presented and discussed in the following subsections and summarized in Figure 2.
FIGURE 2.
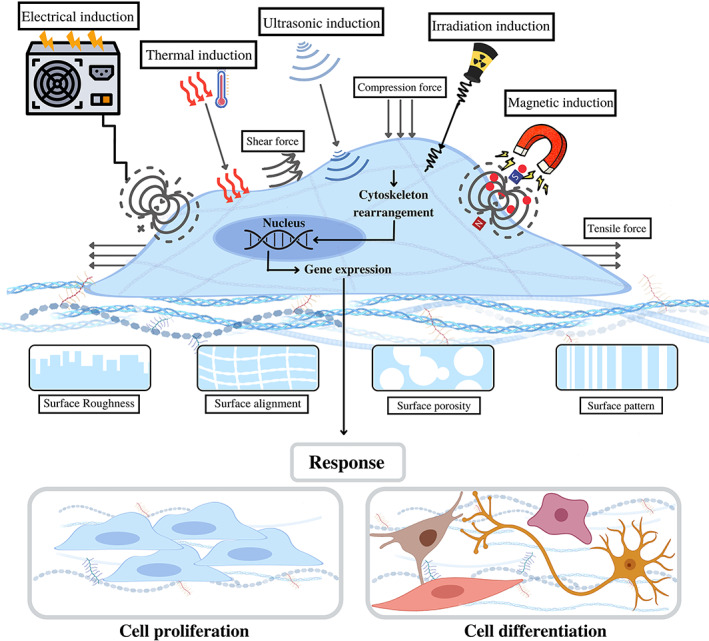
Biophysical stimulating factors involved in manipulating cell fate decisions in tissue engineering. Various biophysical stimuli can be applied to cells to obtain the most preferable cell behavior and characteristics; magnetic, thermal, ultrasound, irradiation, electric, and mechanical (shear stress, tensile, fluid flow, hydrostatic pressure, and compression forces) induction are among these stimuli. Moreover, various surface topographies give rise to different cells responses. Stimulatory factors upon affecting sensitive receptors of the cell surface, contribute to rearrangement and reorientation of cell cytoskeletons. Once in the nucleus, the signals generated by cytoskeleton rearrangement leads to alternation in the gene expression which determines the cell behavior consequently
3.1. Surface topography
The topographic properties of a surface encompass three general concepts: roughness, pattern, and porosity (Figure 3). 234 Scientists have taken advantage of these properties to efficiently engineer various tissue types by harnessing the effect of surfaces in controlling cell behavior, including cell proliferation, migration, adhesion, and differentiation. 234 , 235 , 236 , 237 Therefore, elucidating how surface features control cell fate is key to developing optimal implants for TE. Interestingly, these topographic features have been observed to act synergistically with soluble factor‐mediated signaling. 235 The methods used to engineer the surface topography generally fall into two groups: creating protrusions and/or depressions on a micro/nanoscale, or altering the mechanical properties of the material in a predefined pattern. These patterns can be arranged isotropically (e.g., columns, pits, or tubes) or anisotropically (e.g., grooves and ridges). Micro/nanoscale patterns have been shown to affect stem cell attachment by altering cytoskeletal orientation and intracellular focal adhesions that can modulate intracellular signaling and mechanotransduction pathways. 7 , 234 , 236 , 238 , 239 , 240 Surface patterning allows the regulation of biomaterial–host tissue interactions, enabling their application in the development of various tissues. 234 , 235 Lee et al. synthesized nanostructured ridge/groove pattern arrays to study the effects of controlling topological dimensions and orientations on neurogenesis of hESCs. 236 Interestingly, neural progenitor cells may respond differently to the physical presence of cues, as anisotropic topographies have been shown to significantly facilitate neural differentiation, whereas isotropic topographies promote glial differentiation. 236 Lee et al. synthesized the nanostructured ridge/groove pattern arrays to study the effect of controlling topological dimensions and alignments on hESCs neurogenesis. 241 Interestingly, neural progenitor cells may respond differently to the physical presence of cues, as anisotropic topographies have been shown to significantly facilitate neural differentiation, whereas isotropic topographies promote glial differentiation. 242 Surface patterns are also critical for bone tissue homeostasis, as they regulate osteoblasts in maintaining their characteristic function. The major signaling mechanism that appears to be involved in the transmission of microtopographic information to osteoblasts is the RhoA/ROCK signaling pathway. 243
FIGURE 3.
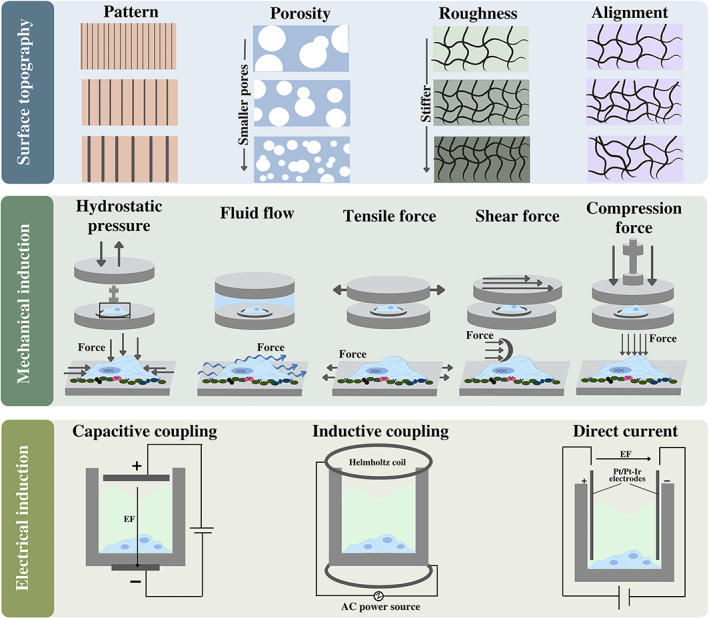
Various aspects of surface topography, Different types of mechanical stresses, and electrical stimulations in tissue engineering. (Top) Different surface topographic textures have different impacts on cell activities and responses. Four different aspects of topography demonstrated in the figure, are surface patterns, roughness, alignment, and pore size. (Middle) Mechanical forces in cell niches such as compression, shear, fluid flow, hydrostatic pressure, and tensile can be sensed by cells through diverse mechanisms. The direction of arrows illustrates the direction of mechanical force. (Below) Electrical stimulation along with parameters involved in electric current play crucial roles in the cell behavior. Three methods to induce electric current are direct coupling, capacitive coupling, and inductive coupling
The other aspect of surface topography, porosity, plays an essential role in cell functionality, nutrient transport, cell fate determination, cell migration, adherence, and growth. 234 In this sense, scaffolds for bone tissue with appropriate porosity led to osteogenesis, angiogenesis, and enhanced oxygen transfer to cells. 234 Pore size, shape, uniformity, and the degree of interconnectivity between pores, are some of the critical elements to consider. In addition, the influence of pore size seems to depend on the cell type and biomaterial of the matrix. 7 , 234 , 244 Zhang et al. indicated that pore size alters the proliferation rate of MSCs 245 and in another study, culture of MSCs in collagen/hyaluronic acid scaffolds with three different pore sizes showed the highest expression of cartilage marker genes and deposition of cartilage‐like matrix in the substrate with the largest pore size. 246 Accordingly, increasing porosity has a positive effect on nutrient diffusion and better removal of cellular debris, leading to better proliferation rate and osteogenesis of MSCs in vivo. 247 Brennan et al. fabricated a fibrous scaffold architecture to investigate the effect of different scaffold pore sizes (100, 200, and 300 μm) on osteogenesis of MSCs. Their results showed that cell infiltration into these porous structures was better than into high‐packed random fibrous scaffolds. In addition, a matrix with a pore size of 100 μm significantly promoted cell infiltration, spreading, and mineralization. 248
The other topographic parameter, roughness, is defined as the extent of protrusions and depressions on the surface of the biomaterial Chen et al. investigated the effects of fiber surface roughness by changing the moisture ratio during electrospinning. The results suggest that hMSCs seeded on the roughest surface produced more osteogenic markers including osteopontin, BMPs, and Runx2. Yang et al. prepared hyaluronic acid matrices with a different average roughness (ranging from 0.2 to 1.65 μm) and average spacing between tips (ranging from 89.7 to 18.6 μm) to study the osteogenic differentiation of MSCs. Their results showed that matrices with a roughness of 0.7–1.0 μm and peak spacing of 53.9–39.3 μm had better osteogenesis potential. 249 Interestingly, it appears that cell behavior is controlled by the combined contribution of the micro and nano‐roughness of the substrate. 240
3.2. Mechanical cues
Cells can sense the mechanical properties of their environment through diverse mechanisms including mechanoreceptors, focal adhesions, actin cytoskeleton, and cell–cell interactions. Matrix–cell adhesion mechanosensors connect the ECM and the actin cytoskeleton by transmembrane connections. Inside the cells, mechanical stimuli are converted to arrays of biochemical cascades transforming information to nuclei, leading to reorientation, and reorganization of the cell cytoskeleton. 229 These force‐induced modifications influence gene expression and change cell protein profiling. Rho/ROCK, Rac/MAPK, Src/FAK, PI3K/Akt, and Wnt/β‐catenin are some of the key mechanotransduction pathways that transform mechanical information into nuclei and reorganize cytoskeletal network. 5 , 250 So, the mechanical properties of cell niches significantly impact cell quiescence, division, and differentiation (Figure 3). During embryonic development, mechanical forces are involved in tissue formation, and organogenesis by affecting cell migration, fate determination, and matrix composition reorganizing. In response to mechanical stress, cells begin to produce more tissue‐specific ECM components. 251 In a study, short bursts of compression to human embryonic mesodermal progenitor cells led to increased mineral deposition and osteogenic differentiation. 252
Accordingly, forces such as fluid shear stresses, hydrostatic compression, mechanical tension, and bending take part to participate in cell fate determination. 253 , 254 The major influence of matrix stiffness on cell differentiation and migration is also reported. 255 , 256 The stiffness of the matrix regulates the type and the intensity of cell–substrate forces. On a stiff substrate, cells generate a large force at the focal adhesion, that affects the lineage specification and commitment. 257 A study showed that elastic environments favored differentiation of MSC into adipocytes, while osteogenesis was enhanced on stiffer substrates. 255 Sun et al. demonstrated that MSCs cultured on soft hydrogels, significantly increased the activation of b1‐integrin compared to stiffer matrix, playing an important role in modulating differentiation and self‐renewal of MSCs. 258
A variety of mechanical stimuli can be applied to cells using flexible culture substrates as well as scaffolds or bioreactors with suitable mechanical properties. 87 , 259 Axial, biaxial and cyclic static stresses have been used to induce cell proliferation. 251 Studies have shown that cyclic mechanical strain is determinant in the control of the assembly of cells grown on flexible substrates. 260 , 261 In an in vitro study, Pennisi et al. provided evidence on the role of uniaxial cyclic tensile strain (CTS) in the differentiation of skeletal myocytes. They found that applying CTS on cells resulted in a highly aligned array of cross‐striated fibers to the direction of strain and accelerated maturation of myoblasts. 262 Another study reported that stimulated MSCs with cyclic stretching represented increased proliferation and cardiomyogenic differentiation. 263 In continuation, cyclic stretching showed a negative effect on cell proliferation but a positive effect on osteogenesis. 264 Kong et al. developed a micro‐device for applying cyclic compressions to cardiac fibroblasts (CFs) encapsulated in hydrogels resulting in the strain–response correlation of mechanical stimulation in CFs phenotypic remodeling. 227 In addition, stress relaxation of the substrate can be an important mechanical parameter that influences on cell behavior. 265 Seeding of murine MSC in a tunable stress relaxation hydrogel revealed that the proliferation and spreading rate of MSCs on the fast stress relaxation substrate were extremely higher than the low relaxation hydrogel. As well, the rapid stress relaxation matrix increased the bone formation and osteogenesis of MSCs. 266 In another study, the simultaneous investigation of stiffness and topography of different polyesters introduced the nanopillar structures with lower stiffness with better conditions for chondrogenesis than the others. 267
3.3. Electrical cues
Electrical currents generated by the cells and ECM components during development, growth, or repair are known as bioelectric phenomena. Cells display a resting membrane potential which is mainly due to the differential concentration of ions across the cytoplasmic membrane. At rest, ion channels help maintain the electrochemical gradient of ions across the membrane. Activation of ion channels can lead to changes in the membrane potential, which is typically associated with a functional response in the cells. Many biological processes such as neuron synapses, muscle contraction, bone formation, and wound healing in human body are modulated by electrical fields. 233 , 268 Electrical stimulation has been used in combination with various inductive factors such as growth factors, 269 small molecules, 11 electro‐conductive scaffolds, 270 and even mechanical stimuli for efficient TE (Figure 3). 271
Usually, one of the three following electrical stimulation methods is applied: direct, capacitive, or inductive coupling. Direct coupling is limited by the fact that stimulation electrodes can produce cytotoxic by‐products and alter the pH. Capacitive coupling produces a uniform electric field by two opposite electrodes, without the need for electroconductive scaffolds. Inductive coupling can be produced by an electromagnetic field created by an external coil. In general, studies have shown that parameters such as frequency, intensity, and duration of the stimulation play an important role in cell behavior. 268 , 272
Due to the natural function of electrical pulses in cardiac and neural tissues as well as osteogenesis‐related processes, the use of conductive scaffolds and electrical stimulation in these areas has been very effective. 11 , 233 A study has shown that using a high‐intensity electrical field stimulation with conductive scaffolds provided an excellent stimulant to improve wound healing in tissue injuries due to its effect on cell membrane permeability. 224 Another study demonstrated that biphasic square pulses stimulated proliferation and enhanced differentiation of ADSCs to osteoblast. 268 Abedi et al. showed that the combined application of conductive scaffold and electrical stimulation significantly increased in the expression of cardiac marker genes in cardiac progenitor cells. 11 Another study in neural TE reported that synergic use of electrical stimulation and conductive scaffolds caused the upregulation of neural marker genes in stem cells. 233 Zhao et al. presented that the in vivo growth and regeneration of axons were effectively increased on conductive polypyrrole/silk fibroin scaffolds under electrical stimulation, while the signal transduction pathway of MAPKs was activated. 273 Bjorninen et al. presented a 3D, reusable and cost‐effective electrical stimulation device to differentiate ADSCs to SMCs on polytrimethylene carbonate scaffolds coated with polypyrrole. The use of biphasic electric current with a pulse width of 1 ms increased the number of living cells compared to unstimulated ADSCs and supported the expression of SMCs markers. 274
3.4. Low‐frequency magnetic fields
Most molecular materials are diamagnetic, a weak form of magnetism created by a magnetic field as a result of the change in the orbital motion of the electrons. The force that arises from the motion of electrons at the molecular level is very small, so the effect of diamagnetism can be increased by applying magnetic fields. 275 Studies on fibroblasts and osteoblasts seeded on magnetic polymer nanofibers have shown improvement in cell alignment and proliferation. Another study demonstrated increased chondrogenesis of MSCs by stimulating an external magnetic field on hydrogel scaffolds. 276 Pulsed electromagnetic fields (PEMFs) can accelerate fracture healing and osteogenesis in vivo, probably due to the activation of the biological cascade. Improvement of cytosolic Ca2+ and calmodulin activation is one of the impacts of PEMF exposure, which enhances the osteogenesis process. Efforts to investigate the differences between static magnetic fields and PEMFs have shown that using static magnetic fields with moderate intensity have more beneficial results in metabolic disorders treatment, while in musculoskeletal and neurological disorders, PEMFs have more favorable effects. It is noteworthy to mention that stimulation intensity, frequency, and duration of the magnetic field are important parameters to consider. 277 , 278 Özgün et al. investigated the role of N‐methyl‐d‐aspartate receptors in neural differentiation by exposing differentiated human neural progenitor cells under extremely low‐frequency magnetic field; an increase in the levels of neuronal markers expression and neurites outgrowth was observed. 279
3.5. Ultrasonic fields
The ultrasound wave is a high‐frequency mechanical wave that is widely used for medical applications due to its noninvasive, deep tissue penetration and remote controllability. This nondestructive stimulation approach can be passed through human tissues as a biophysical signal in a controlled and systematic manner. It can also stimulate complex biochemical events in cells. Small‐scale tissue models are used to improve our understanding of biological systems. In this regard, ultrasound has the capability to arrange cells in favorable patterns with micrometer‐sized features. 280 Ultrasound waves alter cellular metabolism by applying energy to the cellular cytoskeleton. 222 Recently, 2D and 3D acoustic cell patterns have been considered to stimulate biological processes including neuron conduction, neural differentiation, cardiomyocyte contractility, and angiogenesis. 281 Studies of low‐intensity pulsed ultrasound stimulation (LIPUS) to induce mechanical strain and, consequently, biochemical changes have shown that LIPUS stimulation can increase osteoblasts activity and bone differentiation of precursor stem cells. 282 Also, LIPUS could regulate the cell cycle by activating ERKs/MAPKs pathways and also affecting fibroblast proliferation. 283 Cheng et al. for the first time presented a simple, cost‐effective, reproducible ultrasound standing waves (USWs) system to repair the peripheral nerve. USWs radiation did not have any adverse effect on cell viability, differentiation, and proliferation. Axonal growth of differentiated PC12 cells by USWs had a higher directional uniformity than the control group. 284 USWs have been utilized in tissue explant cultures, drug delivery, tissue modeling, immunology, and cancer studies. USW particle manipulation provides aggregation of cells. Depending on actuation schemes, the pressure nodes defined by the USW are stable and can be both static and dynamic, while usually the cells are trapped in the mentioned nodes. 280 The components of the ultrasonic device include a signal generator and an ultrasonic transducer that can produce constant ultrasonic frequencies depending on the power and excitation time. 285
3.6. Thermal energy
Chemical reactions in cellular processes are temperature sensitive, both for protein activation or inactivation and for subsequent signaling pathways. Hyperthermia, in general, is used for cancer treatment due to its deleterious effects on cells, 286 , 287 whereas moderate temperature elevations could find application in TE. Ma et al. developed a new temperature‐sensitive hydroxyapatite/graphene oxide/CS platform to kill osteosarcoma cells at a temperature of 48°C by near‐infrared radiation. 288
Several studies described the beneficial effects of controlled hyperthermic therapy and mild heat stress on myogenic and osteogenic differentiation. For instance, incubation of cultured myoblasts at 39°C showed an increase in myotube diameter (skeletal muscle hypertrophy). 220 Another study examined the effect on myotubes of mild temperature increments (within the physiological range), produced by near‐infrared radiation using gold nanoshells, The result was a significant positive and protective effect of heating on C2C12 myotube contractility. 289 An in vivo study reported elevated temperature from 1.5°C to 3°C more than body temperature motivated bone growth. 290 Tong et al. investigated the effect of low intensity and periodic near‐infrared radiation on osteogenic differentiation of seeding cells on black phosphorus nanosheets and PLGA substrate. They found that moderate heat stimulation promoted heat shock protein expression following osteogenesis and bone healing acceleration in vitro and in vivo. 291 Sanchez‐Casanova et al. explored the influence of photo‐induced hyperthermia on the liberation of BMP‐2 from near‐infrared radiation‐responsive hydrogels for bone TE, which led to the controlled release of BMP‐2 for enhanced performance bone formation and mineralization in vivo. 292
3.7. Nonionizing, nonthermal light treatment
Stimulation of cells by nonionizing radiation leads to the absorption of energy by various cellular mechanisms. Recently, light radiation emanating from light‐emitting diodes (LEDs), has been considered as a physical agent with an extraordinary ability to modulate the proliferation and differentiation of various cell types. The term photobiomodulation, as a nonthermal method, refers to the biological effects that occur after electromagnetic waves in the visible and infrared range interact with molecules and cells. Photobiomodulation has the ability to turn on or off signaling mechanisms related to cell growth and metabolic activities by changing the parameters of wavelength and energy density. 230 , 293 It was reported that photobiomodulation effectively prevented or mitigated complications of radiotherapy by preconditioning the cells to reduce inflammation and promote tissue healing. 294 LED light, by stimulating the mitochondrial ATP synthesis, has been shown to significantly modify cellular metabolism. This method has been used to promote the proliferation of osteoblasts and accelerate bone repair. 295
Photobiomodulation also enhances the rate of tissue healing by increasing the differentiation of sensitive and precursor stem cells. 296 Ruan et al. showed that high‐intensity red LED irradiations positively affected the osteogenesis of hMSCs through activation of the Wnt/β‐catenin signaling pathway. However, it did not have a significant effect on cell proliferation after long‐term culture. 297 In another investigation, irradiation with 660 nm wavelength on astrocytes enhanced nestin and aldh1L1 expression and reduced Oct4 and GFAP co‐expression simultaneously. 298 Mokoena et al. used photobiomodulation with a wavelength of 660 nm to trans‐differentiate fibroblasts to myofibroblasts for diabetic wound healing. The results confirmed that wound closure occurred even without proper activation of the TGF‐β/Smad pathway, so that a different signaling pathway was activated during the transdifferentiation of fibroblasts to myofibroblasts. 299 In another study, Funch et al. examined the effect of 660 nm wavelength on the trans‐differentiation of ADSCs to fibroblasts and chondrocytes. Their findings suggested the potential use of using light stimulation at this wavelength for the treatment of temporomandibular disorder. 300
Noninvasive laser radiation can modulate cellular behavior and tissue repair by creating a photomodulator effect on cells and tissues. 230 , 231 One recent study suggested applying a noninvasive photobiomodulation therapy in the range of 820–840 nm wavelength to accelerate bone regeneration processes combined with the alloplastic ceramic and fibrin biopolymer scaffolds. After 42 days, progressive incensement in bone repair revealed the potential application of this designed platform for bone TE. 301
4. FUNCTIONAL SYNERGY BETWEEN DIFFERENT STIMULI
Since cell adhesion, proliferation, and differentiation are multifactorial mechanisms that are triggered or controlled by various biochemical and biophysical stimuli, recent studies have focused on the functional synergy between these different stimuli to establish efficient TE approaches for clinical application. For instance, a study reported the ability of an electro‐conductive scaffold integrated with BMP‐4 plasmid for efficient bone regeneration. 302 Zhang et al. investigated the synergistic effects of topographical cue of the scaffold, electrical stimulation, and neural growth factor on PC12 cells simultaneously. The results demonstrated that sustained release of NGF from core–shell nanofibrous scaffold under electrical stimulation considerably improved cell proliferation and neurite outgrowth. 303 In another research, the synergistic effects of electrical stimulation and small molecules (including IWP2, CHIR99021, purmorphamine, and SB431542) resulted in the overexpression of main cardiac marker genes that was suggested as a suitable cardiac repair platform. 11 Another investigation has focused on the synergistic effects of chemical and mechanical stimulations on cartilage regeneration. After preconditioning of MSCs in chondrogenic differentiation media, cells were loaded in a 3D hydrogel with subsequent dynamic compressive loading. The implantation of this construct in an osteochondral defect of a rat model showed great enhancement in cartilage repair. 304 Zhang et al. designed a multifunctional hollow‐pipe structure to control ions release for vascularized bone TE. Due to the remarkable synergistic effects of the structure and bioactive ionic composition of the Ca7MgSi4O16 scaffold, the angiogenesis and bone regeneration rate were significantly promoted. Their findings revealed this multifunctional platform not only improved the infiltration of host blood vessels into the hollow struts but also showed great opportunity for cells and growth factors delivery. 305 Synthesized thermally induced hydrogel/curcumin by Pham et al. showed promoted fibroblast adhesion and faster wound closure process. 306 Another study demonstrated that the combined application of TGF‐β1 and cyclic stretching could enhance tendon healing. 307 Taken together, these studies highlight the importance of synergism between various biochemical and biophysical inducers for more effective mimicking of the natural niche. Investigation of synergism between different stimulators and optimized exploitation of the interactions could lead to more efficient differentiation protocols for TE. 226
5. CLINICAL TRIALS AND CHALLENGES TO TRANSLATION
The first clinical attempts to use tissue engineering took place during 1980s to repair skin and articular, and cartilaginous surfaces in human and animal specimens. 308 Data analysis of tissue engineering and regenerative medicine patents by machine learning has shown that most patents focus on the integration of stem cells into scaffolds based on natural polymers (e.g., chitosan, collagen). 309 The development of a commercial tissue‐engineered construct generally requires the performance of multiple preclinical and clinical studies. In addition, regulatory agencies such as the Food and Drug Administration (FDA) have precise requirements for clinical efficacy and safety studies. 310 The use of tissue engineering platforms for clinical translation remains challenging in terms of producing sufficient tissue engineering tissues in compliance with good manufacturing practices (GMP). Despite significant efforts to advance tissue engineering and regenerative medicine, few products have been successfully translated into clinical practice; of those, only about 14% have been able to obtain FDA approval. This failure to implement tissue engineering and regenerative medicine has several causes, for example, lack of funding, financing, and clinical approval. 311
Wound healing scaffolds, nerve and small vessel grafts are tissue engineering based products currently on the market; however, they have not had significant regulatory success. 312 Among the therapies that have paved their way to the clinics, autologous cell‐based therapies (e.g., Carticel, autologous chondrocytes for cartilage repair) and decellularized scaffolds have demonstrated the most promising outcomes. 313 , 314 However, the need for one‐to‐one transfer, adequate cell density, requirements for sophisticated clinical bioreactors, and the sterilization process during scaffold manufacture pose a significant problem for clinical implementation. 315 Some clinical trials of cell‐based tissue‐engineered products are mentioned in Table 4.
TABLE 4.
Clinical trials of cell‐based tissue‐engineered products
| NTC number | Phase | Indication | Intervention | Cell type | Outcome | References |
|---|---|---|---|---|---|---|
| NCT02698813 | I | Scar, senescence wrinkles | Intradermal injection of HA and UC‐MSCs | UC‐MSCs | Improvement of wrinkles, acne, and pitting scar | Web ref: 1 |
| NCT01981330 | I | Vocal fold scarring and hoarseness | Injection of BM‐MSCs with a carrier hyaluronan gel | BM‐MSCs | Healing scarred vocal folds | Web ref: 2 |
| NCT02123368 | I/II | Osteoarthritis | IA injection of BM‐MSCs and HA | BM‐MSCs | Improvement of the osteoarthritis | Web ref: 3 |
| NCT03384433 | I/II | Acute ischemic stroke | Intraparenchymal administration of allogenic MSC‐EVs with miR‐124 | Allogenic MSC‐EVs | Able to look after own affairs without assistance | Web ref: 4 |
| NCT04173650 | II | Dystrophic Epidermolysis Bullosa | Allogenic locally injection BMMSC‐EVs | BMMSC‐EVs | Not published | Web ref: 5 |
| NCT03857841 | I | Bronchopulmonary dysplasia | Intravenous infusion of BMMSC‐EVs | BMMSC‐EVs | Improvement of the disease | Web ref: 6 |
Note: Web References—1. https://clinicaltrials.gov/ct2/show/NCT02698813; 2. https://clinicaltrials.gov/ct2/show/NCT01981330; 3. https://clinicaltrials.gov/ct2/show/NCT02776943; 4. https://clinicaltrials.gov/ct2/show/NCT03384433; 5. https://clinicaltrials.gov/ct2/show/NCT04173650; 6. https://clinicaltrials.gov/ct2/show/NCT03857841.
Notably, numerous scaffolds have been developed clinically for the treatment of bone, skin, cartilage, and bladder injuries. 315 , 316 A 3D‐bioprinted cell‐free poly‐ε‐caprolactone implant has received FDA approval as a bioresorbable airway splint for use in lower airway damage. 317 Allogeneic scaffolds from the iliac vein, incorporating autologous smooth muscle cells and endothelial cells, are also used in clinics to treat extrahepatic portal vein obstruction. 6 In 2002, the incorporation of BMP‐2 growth factor into a collagen sponge to support bone regeneration in the INFUSE bone graft was approved by the FDA. 318 Another FDA‐approved collagen‐based scaffold named Osigraft® (Stryker Biotech) contains recombinant BMP‐7 used to treat tibia fractures. 319 The Integra® Template, made of collagen, glycosaminoglycans, and polysiloxane, is another FDA‐approved device for burn treatment and wound healing that uses ECM‐derived components for clinical purposes. 320 Dermagraft® is a cryopreserved dermal substitute developed by culturing neonatal fibroblasts onto a bioabsorbable polyglactin scaffold that has been approved for marketing in the US for diabetic foot ulcer treatment. 321
Despite the enormous potential of using biophysical stimuli in TE, little research has been done on the use of these triggers at the clinical level. Scientists' priority on product efficacy and lack of attention to regulations for a product's clinical and commercial applicability is a major reason why tissue engineering‐based products fail to meet clinical criteria. For example, bioprinted 3D tissues lack the necessary regulations for clinical trials. 309 These regulations are factors such as administration time, price, and ethics. Therefore, it is imperative that scientists and investigators follow clinical criteria regulations and apply a bedside to bench approach and back again to overcome the barriers to clinical acceptance of tissue engineering‐based therapy and increase its clinical impact. 311
Overall, barriers to the introduction of tissue engineering‐based therapies and products into clinics and the marketplace fall into three categories: technical challenges (e.g., optimal cell source, immune rejection, appropriate microenvironment, proper vascularization, and optimized delivery), manufacturing challenges (e.g., scale‐up, timelines, shelf life, and price), and regulatory challenges (e.g., low percentage of approvals and guidelines). 309 To address these challenges, the tissue engineering community has undertaken numerous efforts in three main areas, including the differentiation of iPSCs, the development of biomaterials, morphogens, and growth factors, and the use of decellularized ECM and bioprinting to create suitable tissue structures. 309
A group of researchers argues that many of the efforts in the field of tissue engineering will not have clinical applications because of the lack of FDA approval for biomaterials and their components. Requirements for a commercial biomaterials template include biodegradability of biomaterials with appropriate kinetics, ability to permanently adapt to the changing environment of the body, transmission of molecular signals to target cells by biomaterials, appropriate mechanical properties by adapting to the target tissue, ability to inject into target tissues on demand, optimization of the transfer of nutrients and biological molecules and gases to cells, ability to facilitate the development of blood vessels and neural networks, adaptation to modern manufacturing techniques such as 3D printing, nontoxicity and immunogenicity in the body. 322 On the other hand, some reports attribute the limited use of TE technology in the clinical field to the economic conditions in the industry and the high cost of TE research, which makes many investors reluctant to invest. 311 , 323
With the introduction of modern technologies in tissue engineering, such as microfluidics and advanced scaffold fabrication methods such as 3D printing, hopes for clinical application have increased. However, this process is still slow, and obstacles such as the lack of scalability of biomaterials for use in clinical cases, the lack of a unified monitoring protocol for scaffold fabrication processes and supply of materials and cell banks, and the lack of clear instructions for the production of standard biomaterials that do not elicit an immune response are the main issues to be overcome. Also, the inefficiency of sensitive and expensive laboratory solutions for physicians and surgeons, who are looking more for solutions with greater reliability, ease of use, and, at the same time, lower costs, are another obstacle to the nonentry of tissue engineering achievements into commercialization and have led to the fact that classical and old methods are more welcomed by surgeons. 323
Despite the above limitations, the value of tissue engineering techniques in medicine will not diminish, and with technological progress, tissue engineering has the opportunity to improve and develop for therapeutic purposes and organ replacement.
6. SUMMARY, CONCLUSIONS, AND PERSPECTIVES
Mimicking the natural cell environment, both as a physical supporter and regulator of cell behavior, should be considered for efficient tissue regeneration. An ideal TE construct should synchronously simulate the biological activity and mechanical properties of the target tissue and properly integrate with the adjacent tissue to create a natural ECM‐like environment. Here, we provide an overview of various biochemical and biophysical triggers that affect cell adhesion, proliferation, differentiation, and cell–cell communication, as well as the associated cellular mechanisms and outcomes (Table 5).
TABLE 5.
Different biochemical and biophysical cues in tissue engineering and their roles in various tissues
| Factors | Roles in various tissues | References | ||
|---|---|---|---|---|
| Biochemical factors | Growth factors | EGF |
|
27, 28, 29, 30, 31, 32, 33, 34, 35, 36 |
| FGF |
|
37, 38, 39, 40, 41, 42, 43, 44, 45 | ||
| TGF‐β |
|
10, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 | ||
| BMP |
|
52, 53, 54, 55, 56 | ||
| PDGF |
|
22, 57, 58, 59, 60, 61, 62, 63 | ||
| VEGF |
|
64, 65, 66, 67, 68, 69, 70 | ||
| NTF |
|
71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82 | ||
| Cytokines |
|
10, 14, 83, 84, 85 | ||
| ECM components |
|
86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 | ||
| Polynucleotides | DNA |
|
120, 121, 122, 123, 124, 125 | |
| RNA |
|
126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143 | ||
| Small molecules |
|
144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158 | ||
| Herbal extracts |
|
156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177 | ||
| Microbial derived compounds |
|
178, 179, 180, 181, 182, 183, 184, 185, 186, 187 | ||
| Biochemical factors | Surface topography |
|
203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218 | |
| Mechanical Cues |
|
5, 87, 198, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236 | ||
| Electrical cues |
|
193, 202, 237, 238, 239, 240, 241, 242, 243, 244 | ||
| Low‐frequency magnetic fields |
|
245, 246, 247, 248, 249 | ||
| Ultrasonic fields |
|
250, 251, 252, 253, 254, 255 | ||
| Thermal energy |
|
256, 257, 258, 259, 260, 261, 262 | ||
| Nonionizing, nonthermal light treatment |
|
263, 264, 265, 266, 267, 268, 269, 270, 271 | ||
Soluble biochemical inducers such as growth factors or cytokines are commonly used to regulate stem cell fate and tissue regeneration. Due to limitations in the use of biochemical factors, such as cost, off‐target activities, rapid degradation, and difficulty in determining optimal release kinetics, the focus has shifted to altering the biophysical properties of the cell microenvironment. Many studies have demonstrated the effects of biophysical factors such as electric and magnetic fields, mechanical forces, topographical features, and the use of electromagnetic, thermal, and ultrasonic energy on cell behavior. However, recent studies have shown that the simultaneous use of biochemical and biophysical signals can synergistically enhance the efficiency of TE approaches.
Remarkable progress has been made in the development of scaffold‐based platforms with well‐defined, spatially and temporally controlled physicomechanical signals. However, there are still some challenges and uncertainties that should be addressed for safer and more effective clinical implementation, such as controlling the dose of these factors to avoid adverse differentiation or cell transformation. Considering the dynamic extracellular environment that has viscoelastic properties, novel approaches focus on platforms with dynamic and tunable mechanical properties.
Many studies have shown that biochemical and biophysical cues can induce phenotypic changes in the cell. However, the subsequent changes in epigenetic state, including noncoding RNAs and DNA methylation, remain unclear. In this context, it is foreseeable that high‐throughput tools such as genomic, transcriptomic, epigenomic, and proteomic technologies could contribute significantly to determining the influence of the above factors on intracellular signaling pathways and gene expression at the single‐cell level.
With technological advances, the optimal conditions for the application of biophysical and biochemical factors could be simulated using advanced computational tools that simulate the relationship between cells and their extracellular matrices. Because any cellular change is likely to affect several intracellular pathways simultaneously, elucidation of the intracellular mechanisms underlying proliferation or differentiation is important not only to give us an overall picture of tissue biology but also to develop approaches to tissue regeneration and repair. In addition, understanding how the host immune system responds to the biochemical and biophysical cues is a new investigation in the field of TE to ensure the manipulation of cells in an immune‐modulated environment along with protection from inflammation. 236
Despite the numerous studies and experiments published in this field and the large amount of knowledge and progress achieved in the last decades, the clinical implementation of TE strategies still faces many challenges, including regulatory and economic barriers. 324 Indeed, there is ample room for further studies to address some critical questions about the wide range of inducing factors and their mutual effects. Clearly, cell behavior is very complex, so single factors have shown to be less efficient in cell stimulation than a combination of them. 325 By and large, a thorough understanding of the role of the various biophysical and biochemical factors and their synergistic effects will increase the pace of research and experimentation to eventually achieve optimal scaffold design for TE.
AUTHOR CONTRIBUTIONS
Behnaz Bakhshandeh: Conceptualization (lead); data curation (lead); project administration (lead); supervision (lead); writing – original draft (equal); writing – review and editing (lead). Nika Ranjbar: Conceptualization (equal); data curation (lead); writing – original draft (lead). Ardeshir Abbasi: Data curation (equal); writing – original draft (equal). Elahe Amiri: Data curation (equal); writing – original draft (equal). Ali Abedi: Data curation (equal); writing – original draft (equal). Mohammad‐Reza Mehrabi: Data curation (equal); writing – original draft (equal). Zahra Dehghani: Data curation (equal); writing – original draft (equal). Cristian Pablo Pennisi: Data curation, project administration, supervision, writing original draft, writing review editing.
FUNDING INFORMATION
The authors received no specific funding for this work.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10383.
Bakhshandeh B, Ranjbar N, Abbasi A, et al. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng Transl Med. 2023;8(2):e10383. doi: 10.1002/btm2.10383
Contributor Information
Behnaz Bakhshandeh, Email: b.bakhshandeh@ut.ac.ir.
Cristian Pablo Pennisi, Email: cpennisi@hst.aau.dk.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bakhshandeh B, Zarrintaj P, Oftadeh MO, et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev. 2017;33(2):144‐172. [DOI] [PubMed] [Google Scholar]
- 2. Di Nardo P, Minieri M, Ahluwalia A. Engineering the stem cell niche and the differentiative micro‐and macroenvironment: technologies and tools for applying biochemical, physical and structural stimuli and their effects on stem cells. Stem Cell Engineering. Springer; 2011:41‐59. [Google Scholar]
- 3. Quarto R, Giannoni P. Bone tissue engineering: past–present–future. Mesenchymal Stem Cells. Springer; 2016:21‐33. [DOI] [PubMed] [Google Scholar]
- 4. Huettner N, Dargaville TR, Forget A. Discovering cell‐adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 2018;36(4):372‐383. [DOI] [PubMed] [Google Scholar]
- 5. Yim EK, Sheetz MP. Force‐dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 2012;3(5):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boudou T, Andersen T, Balland M. On the spatiotemporal regulation of cell tensional state. Exp Cell Res. 2019;378(1):113‐117. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Liu Y, Zhang Y, et al. Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front Cell Dev Biol. 2021;9:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding S, Kingshott P, Thissen H, Pera M, Wang PY. Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: a review. Biotechnol Bioeng. 2017;114(2):260‐280. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Liu J, Guan M, Zhou T, Duan X, Xiang Z. Growth factor and its polymer scaffold‐based delivery system for cartilage tissue engineering. Int J Nanomedicine. 2020;15:6097‐6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kowalczewski CJ, Saul JM. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front Pharmacol. 2018;9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abedi A, Bakhshandeh B, Babaie A, et al. Concurrent application of conductive biopolymeric chitosan/polyvinyl alcohol/MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering. Mater Chem Phys. 2021;258:123842. [Google Scholar]
- 12. Sadeghi M, Bakhshandeh B, Dehghan MM, Mehrnia MR, Khojasteh A. Functional synergy of anti‐mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J Mater Sci Mater Med. 2016;27(8):132. [DOI] [PubMed] [Google Scholar]
- 13. Chen Y‐W, Wang K, Ho CC, Kao CT, Ng HY, Shie MY. Cyclic tensile stimulation enrichment of Schwann cell‐laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater Design. 2020;195:108982. [Google Scholar]
- 14. Zhang J‐M, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45(2):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo KW, Ashe KM, Kan HM, Laurencin CT. The role of small molecules in musculoskeletal regeneration. Regen Med. 2012;7(4):535‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goonoo N, Bhaw‐Luximon A. Mimicking growth factors: role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Adv. 2019;9(32):18124‐18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sidal H, Colakoglu Erkan P, Uslu M, Kocabas F. Development of small‐molecule‐induced fibroblast expansion technologies. J Tissue Eng Regen Med. 2020;14(10):1476‐1487. [DOI] [PubMed] [Google Scholar]
- 18. Sheyn D, Mizrahi O, Benjamin S, Gazit Z, Pelled G, Gazit D. Genetically modified cells in regenerative medicine and tissue engineering. Adv Drug Deliv Rev. 2010;62(7–8):683‐698. [DOI] [PubMed] [Google Scholar]
- 19. Amiri H, Mohammadi I, Afshar A. Electrophoretic deposition of nano‐zirconia coating on AZ91D magnesium alloy for bio‐corrosion control purposes. Surf Coat Technol. 2017;311:182‐190. [Google Scholar]
- 20. Han SB, Kim JK, Lee G, Kim DH. Mechanical properties of materials for stem cell differentiation. Adv Biosyst. 2020;4:2000247. [DOI] [PubMed] [Google Scholar]
- 21. McKay I. Growth Factors: A Practical Approach. Oxford University Press; 1993. [Google Scholar]
- 22. Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med J. 2000;41(6):704‐719. [DOI] [PubMed] [Google Scholar]
- 23. Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor β‐binding protein 1 interacts with fibrillin and is a microfibril‐associated protein. J Biol Chem. 2003;278(4):2750‐2757. [DOI] [PubMed] [Google Scholar]
- 24. Aguilar LMC, Silva SM, Moulton SE. Growth factor delivery: defining the next generation platforms for tissue engineering. J Control Release. 2019;306:40‐58. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials. 2017;9(10):e435. [Google Scholar]
- 27. Dibiase M, Rhodes C. The design of analytical methods for use in topical epidermal growth factor product development. J Pharm Pharmacol. 1991;43(8):553‐558. [DOI] [PubMed] [Google Scholar]
- 28. Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal. 2006;18(12):2089‐2097. [DOI] [PubMed] [Google Scholar]
- 29. Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol. 2010;2010:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tripathi RC, Raja SC, Tripathi BJ. Prospects for epidermal growth factor in the management of corneal disorders. Surv Ophthalmol. 1990;34(6):457‐462. [DOI] [PubMed] [Google Scholar]
- 31. Brown GL, Nanney LB, Griffen J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321(2):76‐79. [DOI] [PubMed] [Google Scholar]
- 32. Brown GL, Curtsinger LJ, White M, et al. Acceleration of tensile strength of incisions treated with EGF and TGF‐beta. Ann Surg. 1988;208(6):788‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia‐Orue I, Gainza G, Gutierrez FB, et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm. 2017;523(2):556‐566. [DOI] [PubMed] [Google Scholar]
- 34. Norouzi M, Shabani I, Atyabi F, Soleimani M. EGF‐loaded nanofibrous scaffold for skin tissue engineering applications. Fibers Polym. 2015;16(4):782‐787. [Google Scholar]
- 35. Augustine R, Hasan A, Dalvi YB, et al. Growth factor loaded in situ photocrosslinkable poly (3‐hydroxybutyrate‐co‐3‐hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater Sci Eng C. 2021;118:111519. [DOI] [PubMed] [Google Scholar]
- 36. Wroblewski OM, Vega‐Soto EE, Nguyen MH, Cederna PS, Larkin LM. Impact of human epidermal growth factor on tissue‐engineered skeletal muscle structure and function. Tissue Eng Part A. 2021;27:1151‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baird A, Walicke PA. Fibroblast growth factors. Br Med Bull. 1989;45(2):438‐452. [DOI] [PubMed] [Google Scholar]
- 38. Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14(3):166‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J‐S. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive or conductive implants. Acta Orthop Scand. 1996;67(sup269):1‐33. [DOI] [PubMed] [Google Scholar]
- 40. Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619‐626. [DOI] [PubMed] [Google Scholar]
- 41. DeBlois C, Côté M‐F, Doillon CJ. Heparin‐fibroblast growth factorfibrin complex: in vitro and in vivo applications to collagen‐based materials. Biomaterials. 1994;15(9):665‐672. [DOI] [PubMed] [Google Scholar]
- 42. Pepper M, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189(2):824‐831. [DOI] [PubMed] [Google Scholar]
- 43. Charoenlarp P, Rajendran AK, Iseki S. Role of fibroblast growth factors in bone regeneration. Inflamm Regen. 2017;37(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sivashanmugam A, Charoenlarp P, Deepthi S, et al. Injectable shear‐thinning CaSO4/FGF‐18‐incorporated chitin–PLGA hydrogel enhances bone regeneration in mice cranial bone defect model. ACS Appl Mater Interfaces. 2017;9(49):42639‐42652. [DOI] [PubMed] [Google Scholar]
- 45. Tsuboi R, Sasaki JI, Kitagawa H, Yoshimoto I, Takeshige F, Imazato S. Development of a novel dental resin cement incorporating FGF‐2‐loaded polymer particles with the ability to promote tissue regeneration. Dent Mater. 2018;34(4):641‐648. [DOI] [PubMed] [Google Scholar]
- 46. Attisano L, Wrana JL, López‐Casillas F, Massagué J. TGF‐β receptors and actions. Biochim Biophys Acta Mol Cell Res. 1994;1222(1):71‐80. [DOI] [PubMed] [Google Scholar]
- 47. Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105(5):1323‐1332. [DOI] [PubMed] [Google Scholar]
- 48. Kwak E‐A, Lee NY. Synergetic roles of TGF‐β signaling in tissue engineering. Cytokine. 2019;115:60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor‐β (TGF‐β). Growth Factors. 1993;8(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 50. Browne S, Jha AK, Ameri K, Marcus SG, Yeghiazarians Y, Healy KE. TGF‐β1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels. PLoS One. 2018;13(3):e0194679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Witte T‐M, Fratila‐Apachitei LE, Zadpoor AA, Peppas NA. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater. 2018;5(4):197‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reddi AH, Reddi A. Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev. 2009;20(5–6):341‐342. [DOI] [PubMed] [Google Scholar]
- 53. Oryan A, Alidadi S, Moshiri A, Bigham‐Sadegh A. Bone morphogenetic proteins: a powerful osteoinductive compound with non‐negligible side effects and limitations. Biofactors. 2014;40(5):459‐481. [DOI] [PubMed] [Google Scholar]
- 54. Cao L, Yu Y, Wang J, Werkmeister JA, McLean KM, Liu C. 2‐N,6‐O‐sulfated chitosan‐assisted BMP‐2 immobilization of PCL scaffolds for enhanced osteoinduction. Mater Sci Eng C. 2017;74:298‐306. [DOI] [PubMed] [Google Scholar]
- 55. Chiu Y‐C, Fong EL, Grindel BJ, Kasper FK, Harrington DA, Farach‐Carson MC. Sustained delivery of recombinant human bone morphogenetic protein‐2 from perlecan domain I‐functionalized electrospun poly (ε‐caprolactone) scaffolds for bone regeneration. J Exp Orthop. 2016;3(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duruel T, Çakmak AS, Akman A, Nohutcu RM, Gümüşderelioğlu M. Sequential IGF‐1 and BMP‐6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int J Biol Macromol. 2017;104:232‐241. [DOI] [PubMed] [Google Scholar]
- 57. Deuel TF. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3(1):443‐492. [DOI] [PubMed] [Google Scholar]
- 58. Nimni M. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18(18):1201‐1225. [DOI] [PubMed] [Google Scholar]
- 59. Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57(1):443‐478. [DOI] [PubMed] [Google Scholar]
- 60. Williams LT. Signal transduction by the platelet‐derived growth factor receptor. Science. 1989;243(4898):1564‐1570. [DOI] [PubMed] [Google Scholar]
- 61. Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ. Controlled release of platelet‐derived growth factor‐BB from chondroitin sulfate–chitosan sponge for guided bone regeneration. J Control Release. 2000;67(2–3):385‐394. [DOI] [PubMed] [Google Scholar]
- 62. Lohmann C, Schwartz Z, Niederauer GG, Carnes DL Jr, Dean DD, Boyan BD. Pretreatment with platelet derived growth factor‐BB modulates the ability of costochondral resting zone chondrocytes incorporated into PLA/PGA scaffolds to form new cartilage in vivo. Biomaterials. 2000;21(1):49‐61. [DOI] [PubMed] [Google Scholar]
- 63. Lee J, Lee S, Ahmad T, et al. Human adipose‐derived stem cell spheroids incorporating platelet‐derived growth factor (PDGF) and bio‐minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192. [DOI] [PubMed] [Google Scholar]
- 64. Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198‐1202. [DOI] [PubMed] [Google Scholar]
- 65. Mastrullo V, Cathery W, Velliou E, Madeddu P, Campagnolo P. Angiogenesis in tissue engineering: as nature intended? Front Bioeng Biotechnol. 2020;8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89(2):607‐648. [DOI] [PubMed] [Google Scholar]
- 67. Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6(2):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsao CJ, Pandolfi L, Wang X, et al. Electrospun patch functionalized with nanoparticles allows for spatiotemporal release of VEGF and PDGF‐BB promoting in vivo neovascularization. ACS Appl Mater Interfaces. 2018;10(51):44344‐44353. [DOI] [PubMed] [Google Scholar]
- 69. Park JY, Shim JH, Choi SA, et al. 3D printing technology to control BMP‐2 and VEGF delivery spatially and temporally to promote large‐volume bone regeneration. J Mater Chem B. 2015;3(27):5415‐5425. [DOI] [PubMed] [Google Scholar]
- 70. Fahimipour F, Rasoulianboroujeni M, Dashtimoghadam E, et al. 3D printed TCP‐based scaffold incorporating VEGF‐loaded PLGA microspheres for craniofacial tissue engineering. Dent Mater. 2017;33(11):1205‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Madhusudanan P, Raju G, Shankarappa S. Hydrogel systems and their role in neural tissue engineering. J R Soc Interface. 2020;17(162):20190505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bax B, Blundell TL, Murray‐Rust J, McDonald NQ. Structure of mouse 7S NGF: a complex of nerve growth factor with four binding proteins. Structure. 1997;5(10):1275‐1285. [DOI] [PubMed] [Google Scholar]
- 73. Tian L, Prabhakaran MP, Hu J, Chen M, Besenbacher F, Ramakrishna S. Coaxial electrospun poly (lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cells. RSC Adv. 2015;5(62):49838‐49848. [Google Scholar]
- 74. Zhang P‐X, Han N, Kou YH, et al. Tissue engineering for the repair of peripheral nerve injury. Neural Regen Res. 2019;14(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu C, Wang C, Zhao Q, et al. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed Mater. 2018;13(4):044107. [DOI] [PubMed] [Google Scholar]
- 76. Mili B, das K, Kumar A, et al. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J Mater Sci Mater Med. 2018;29(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 77. Sandoval‐Castellanos AM, Claeyssens F, Haycock JW. Biomimetic surface delivery of NGF and BDNF to enhance neurite outgrowth. Biotechnol Bioeng. 2020;117(10):3124‐3135. [DOI] [PubMed] [Google Scholar]
- 78. Xia B, Lv Y. Dual‐delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater Sci Eng C. 2018;82:253‐264. [DOI] [PubMed] [Google Scholar]
- 79. Afrash H, Nazeri N, Davoudi P, Majidi R, Ghanbari H. Development of a bioactive scaffold based on NGF containing PCL/chitosan nanofibers for nerve regeneration. Biointerface Res Appl Chem. 2021;11(5):12606‐12617. [Google Scholar]
- 80. Lackington WA, Kočí Z, Alekseeva T, et al. Controlling the dose‐dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects. J Control Release. 2019;304:51‐64. [DOI] [PubMed] [Google Scholar]
- 81. Hu X, Li R, Wu Y, et al. Thermosensitive heparin‐poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury. J Cell Mol Med. 2020;24(14):8166‐8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang Z, Li X, Li Z, et al. Collagen/nano‐sized β‐tricalcium phosphate conduits combined with collagen filaments and nerve growth factor promote facial nerve regeneration in miniature swine: an in vivo study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128(5):472‐478. [DOI] [PubMed] [Google Scholar]
- 83. Calò V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197(2):157‐168. [DOI] [PubMed] [Google Scholar]
- 84. Mountziaris PM, Dennis Lehman E, Mountziaris I, Sing DC, Kurtis Kasper F, Mikos AG. Effect of temporally patterned TNF‐α delivery on in vitro osteogenic differentiation of mesenchymal stem cells cultured on biodegradable polymer scaffolds. J Biomater Sci Polym Ed. 2013;24(15):1794‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gong L, Li J, Zhang J, et al. An interleukin‐4‐loaded bi‐layer 3D printed scaffold promotes osteochondral regeneration. Acta Biomater. 2020;117:246‐260. [DOI] [PubMed] [Google Scholar]
- 86. O'brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88‐95. [Google Scholar]
- 87. Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Mater Today. 2010;13(1–2):14‐22. [Google Scholar]
- 88. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Klingberg F, Chau G, Walraven M, et al. The fibronectin ED‐A domain enhances recruitment of latent TGF‐β‐binding protein‐1 to the fibroblast matrix. J Cell Sci. 2018;131(5):jcs201293. doi: 10.1242/jcs.201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dubey G, Mequanint K. Conjugation of fibronectin onto three‐dimensional porous scaffolds for vascular tissue engineering applications. Acta Biomater. 2011;7(3):1114‐1125. [DOI] [PubMed] [Google Scholar]
- 91. Sionkowska A, Gadomska M, Musiał K, Piątek J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: a review. Molecules. 2020;25(18):4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhu Z, Wang YM, Yang J, Luo XS. Hyaluronic acid: a versatile biomaterial in tissue engineering. Plastic Aesthetic Res. 2017;4:219‐227. [Google Scholar]
- 93. Park SH, Park JY, Ji YB, Ju HJ, Min BH, Kim MS. An injectable click‐crosslinked hyaluronic acid hydrogel modified with a BMP‐2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020;117:108‐120. [DOI] [PubMed] [Google Scholar]
- 94. Choi JH, Kim JS, Kim WK, et al. Evaluation of hyaluronic acid/agarose hydrogel for cartilage tissue engineering biomaterial. Macromol Res. 2020;28(11):979‐985. [Google Scholar]
- 95. Ingavle GC, Frei AW, Gehrke SH, Detamore MS. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng Part A. 2013;19(11–12):1349‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Montalbano G, Toumpaniari S, Popov A, et al. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater Sci Eng C. 2018;91:236‐246. [DOI] [PubMed] [Google Scholar]
- 97. Xing H, Lee H, Luo L, Kyriakides TR. Extracellular matrix‐derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Klimek K, Ginalska G. Proteins and peptides as important modifiers of the polymer scaffolds for tissue engineering applications—a review. Polymers. 2020;12(4):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hosoyama K, Lazurko C, Muñoz M, McTiernan CD, Alarcon EI. Peptide‐based functional biomaterials for soft‐tissue repair. Front Bioeng Biotechnol. 2019;7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bellis SL. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials. 2011;32(18):4205‐4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Arnaout M, Mahalingam B, Xiong J‐P. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381‐410. [DOI] [PubMed] [Google Scholar]
- 102. Wang F, Li Y, Shen Y, Wang A, Wang S, Xie T. The functions and applications of RGD in tumor therapy and tissue engineering. Int J Mol Sci. 2013;14(7):13447‐13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bačáková L, Filová E, Rypácek F, Svorcík V, Starý V. Cell adhesion on artificial materials for tissue engineering. Physiol Res. 2004;53(Suppl 1):S35‐S45. [PubMed] [Google Scholar]
- 104. Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028‐1033. [DOI] [PubMed] [Google Scholar]
- 105. Wang P‐Y, Wu TH, Tsai WB, Kuo WH, Wang MJ. Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloids Surf B Biointerfaces. 2013;110:88‐95. [DOI] [PubMed] [Google Scholar]
- 106. Negah SS, Khooei A, Samini F, Gorji A. Laminin‐derived Ile‐Lys‐Val‐ala‐Val: a promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res. 2018;371(2):223‐236. [DOI] [PubMed] [Google Scholar]
- 107. Cheng T‐Y, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self‐assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34(8):2005‐2016. [DOI] [PubMed] [Google Scholar]
- 108. Yasa IC, Gunduz N, Kilinc M, Guler MO, Tekinay AB. Basal lamina mimetic nanofibrous peptide networks for skeletal myogenesis. Sci Rep. 2015;5(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Patel R, Santhosh M, Dash JK, et al. Ile‐Lys‐Val‐ala‐Val (IKVAV) peptide for neuronal tissue engineering. Polym Adv Technol. 2019;30(1):4‐12. [Google Scholar]
- 110. Hu K. All roads lead to induced pluripotent stem cells: the technologies of iPSC generation. Stem Cells Dev. 2014;23(12):1285‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mallanna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol. 2010;344(1):16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hassanzadeh A, Shamlou S, Yousefi N, Nikoo M, Verdi J. Genetically‐modified stem cell in regenerative medicine and cancer therapy; a new era. Curr Gene Ther. 2022;22(1):23‐39. [DOI] [PubMed] [Google Scholar]
- 113. Dang M, Saunders L, Niu X, Fan Y, Ma PX. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018;6(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Damasceno PKF, de Santana TA, Santos GC, et al. Genetic engineering as a strategy to improve the therapeutic efficacy of mesenchymal stem/stromal cells in regenerative medicine. Front Cell Dev Biol. 2020;8:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gugjoo M, Rasool E, Pal A. Mesenchymal stem cell genetic engineering and regenerative medicine. Mesenchymal Stem Cell in Veterinary Sciences. Springer; 2020:89‐98. [Google Scholar]
- 116. Kotterman MA, Schaffer DV. Engineering adeno‐associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Andrée L, Yang F, Brock R, Leeuwenburgh SCG. Designing biomaterials for the delivery of RNA therapeutics to stimulate bone healing. Mater Today Bio. 2021;10:100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bakhshandeh B, Soleimani M, Hafizi M, Ghaemi N. A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells. Cytotechnology. 2012;64(5):523‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fang Y, Chen X, WT G. Gene delivery in tissue engineering and regenerative medicine. J Biomed Mater Res B Appl Biomater. 2015;103(8):1679‐1699. [DOI] [PubMed] [Google Scholar]
- 121. O'Rorke S, Keeney M, Pandit A. Non‐viral polyplexes: scaffold mediated delivery for gene therapy. Prog Polym Sci. 2010;35(4):441‐458. [Google Scholar]
- 122. Suárez‐González D, Lee JS, Diggs A, et al. Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity. Tissue Eng Part A. 2014;20(15–16):2077‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gonzalez‐Fernandez T, Tierney EG, Cunniffe GM, O'Brien FJ, Kelly DJ. Gene delivery of TGF‐β3 and BMP2 in an MSC‐laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng Part A. 2016;22(9–10):776‐787. [DOI] [PubMed] [Google Scholar]
- 124. Raftery RM, Walsh DP, Blokpoel Ferreras L, et al. Highly versatile cell‐penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: from development to application in tissue engineering. Biomaterials. 2019;216:119277. [DOI] [PubMed] [Google Scholar]
- 125. Lackington WA, Raftery RM, O'Brien FJ. In vitro efficacy of a gene‐activated nerve guidance conduit incorporating non‐viral PEI‐pDNA nanoparticles carrying genes encoding for NGF, GDNF and c‐Jun. Acta Biomater. 2018;75:115‐128. [DOI] [PubMed] [Google Scholar]
- 126. Bakhshandeh B, Soleimani M, Hafizi M, Paylakhi SH, Ghaemi N. MicroRNA signature associated with osteogenic lineage commitment. Mol Biol Rep. 2012;39(7):7569‐7581. [DOI] [PubMed] [Google Scholar]
- 127. Kwon H, Kim M, Seo Y, et al. Emergence of synthetic mRNA: in vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials. 2018;156:172‐193. [DOI] [PubMed] [Google Scholar]
- 128. Patel S, Athirasala A, Menezes PP, et al. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng Part A. 2019;25(1–2):91‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hannon GJ. RNA interference. Nature. 2002;418(6894):244‐251. [DOI] [PubMed] [Google Scholar]
- 130. Tuschl T. RNA interference and small interfering RNAs. ChemBioChem. 2001;2(4):239‐245. [DOI] [PubMed] [Google Scholar]
- 131. Bai Z, Wei J, Yu C, et al. Non‐viral nanocarriers for intracellular delivery of microRNA therapeutics. J Mater Chem B. 2019;7(8):1209‐1225. [DOI] [PubMed] [Google Scholar]
- 132. Weiler J, Hunziker J, Hall J. Anti‐miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13(6):496‐502. [DOI] [PubMed] [Google Scholar]
- 133. Bakhshandeh B, Hafizi M, Ghaemi N, Soleimani M. Down‐regulation of miRNA‐221 triggers osteogenic differentiation in human stem cells. Biotechnol Lett. 2012;34(8):1579‐1587. [DOI] [PubMed] [Google Scholar]
- 134. Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell‐free 3D scaffold with two‐stage delivery of miRNA‐26a to regenerate critical‐sized bone defects. Nat Commun. 2016;7(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nguyen MK, Jeon O, Dang PN, et al. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018;75:105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Moncal KK, Aydin RST, Abu‐Laban M, et al. Collagen‐infilled 3D printed scaffolds loaded with miR‐148b‐transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater Sci Eng C. 2019;105:110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liao Y‐H, Chang YH, Sung LY, et al. Osteogenic differentiation of adipose‐derived stem cells and calvarial defect repair using baculovirus‐mediated co‐expression of BMP‐2 and miR‐148b. Biomaterials. 2014;35(18):4901‐4910. [DOI] [PubMed] [Google Scholar]
- 138. Xie Q, Wang Z, Zhou H, et al. The role of miR‐135‐modified adipose‐derived mesenchymal stem cells in bone regeneration. Biomaterials. 2016;75:279‐294. [DOI] [PubMed] [Google Scholar]
- 139. Diao HJ, Low WC, Lu QR, Chew SY. Topographical effects on fiber‐mediated microRNA delivery to control oligodendroglial precursor cells development. Biomaterials. 2015;70:105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jedari B, Rahmani A, Naderi M, Nadri S. MicroRNA‐7 promotes neural differentiation of trabecular meshwork mesenchymal stem cell on nanofibrous scaffold. J Cell Biochem. 2020;121(4):2818‐2827. [DOI] [PubMed] [Google Scholar]
- 141. Zhou F, Jia X, Yang Y, et al. Nanofiber‐mediated microRNA‐126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016;43:303‐313. [DOI] [PubMed] [Google Scholar]
- 142. Devalliere J, Chang WG, Andrejecsk JW, et al. Sustained delivery of proangiogenic microRNA‐132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. 2014;28(2):908‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle‐specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010;14(10):2495‐2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bakhshandeh B, Soleimani M, Paylakhi SH, Ghaemi N. A microRNA signature associated with chondrogenic lineage commitment. J Genet. 2012;91(2):171‐182. [DOI] [PubMed] [Google Scholar]
- 145. Baek D, Lee KM, Park KW, et al. Inhibition of miR‐449a promotes cartilage regeneration and prevents progression of osteoarthritis in in vivo rat models. Mol Ther Nucl Acids. 2018;13:322‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhao C, Wang Y, Jin H, Yu T. Knockdown of microRNA‐203 alleviates LPS‐induced injury by targeting MCL‐1 in C28/I2 chondrocytes. Exp Cell Res. 2017;359(1):171‐178. [DOI] [PubMed] [Google Scholar]
- 147. Badieyan ZS, Evans T. Concise review: application of chemically modified mRNA in cell fate conversion and tissue engineering. Stem Cells Transl Med. 2019;8(8):833‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Balmayor ER. Synthetic mRNA‐emerging new class of drug for tissue regeneration. Curr Opin Biotechnol. 2022;74:8‐14. [DOI] [PubMed] [Google Scholar]
- 149. Steinle H, Ionescu TM, Schenk S, et al. Incorporation of synthetic mRNA in injectable chitosan‐alginate hybrid hydrogels for local and sustained expression of exogenous proteins in cells. Int J Mol Sci. 2018;19(5):1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lolli A, Sivasubramaniyan K, Vainieri ML, et al. Hydrogel‐based delivery of antimiR‐221 enhances cartilage regeneration by endogenous cells. J Control Release. 2019;309:220‐230. [DOI] [PubMed] [Google Scholar]
- 151. Fliervoet LA, Zhang H, van Groesen E, et al. Local release of siRNA using polyplex‐loaded thermosensitive hydrogels. Nanoscale. 2020;12(18):10347‐10360. [DOI] [PubMed] [Google Scholar]
- 152. Ma R, Wang M, Gao S, et al. miR‐29a promotes the neurite outgrowth of rat neural stem cells by targeting extracellular matrix to repair brain injury. Stem Cells Dev. 2020;29(9):599‐614. [DOI] [PubMed] [Google Scholar]
- 153. Mombini S, Mohammadnejad J, Bakhshandeh B, et al. Chitosan‐PVA‐CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int J Biol Macromol. 2019;140:278‐287. [DOI] [PubMed] [Google Scholar]
- 154. Vahdat S, Pahlavan S, Mahmoudi E, et al. Expansion of human pluripotent stem cell‐derived early cardiovascular progenitor cells by a cocktail of signaling factors. Sci Rep. 2019;9(1):16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Karakikes I, Senyei GD, Hansen J, et al. Small molecule‐mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med. 2014;3(1):18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Minami I, Yamada K, Otsuji TG, et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine‐and xeno‐free conditions. Cell Rep. 2012;2(5):1448‐1460. [DOI] [PubMed] [Google Scholar]
- 157. Vahdat S, Pahlavan S, Aghdami N, Bakhshandeh B, Baharvand H. Establishment of a protocol for in vitro culture of cardiogenic mesodermal cells derived from human embryonic stem cells. Cell J. 2019;20(4):496‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cui Y, Zhu T, Li D, et al. Bisphosphonate‐functionalized scaffolds for enhanced bone regeneration. Adv Healthc Mater. 2019;8(23):1901073. [DOI] [PubMed] [Google Scholar]
- 159. Yuan W, Li Z, Xie X, Zhang ZY, Bian L. Bisphosphonate‐based nanocomposite hydrogels for biomedical applications. Bioactive Mater. 2020;5(4):819‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gomes KDN, Alves APNN, Dutra PGP, Viana GSB. Doxycycline induces bone repair and changes in Wnt signalling. Int J Oral Sci. 2017;9(3):158‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tai I‐C, Fu YC, Wang CK, Chang JK, Ho ML. Local delivery of controlled‐release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomedicine. 2013;8:3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ito T, Takemasa M, Makino K, Otsuka M. Preparation of calcium phosphate nanocapsules including simvastatin/deoxycholic acid assembly, and their therapeutic effect in osteoporosis model mice. J Pharm Pharmacol. 2013;65(4):494‐502. [DOI] [PubMed] [Google Scholar]
- 163. Yoshii T, Hafeman AE, Esparza JM, Okawa A, Gutierrez G, Guelcher SA. Local injection of lovastatin in biodegradable polyurethane scaffolds enhances bone regeneration in a critical‐sized segmental defect in rat femora. J Tissue Eng Regen Med. 2014;8(8):589‐595. [DOI] [PubMed] [Google Scholar]
- 164. Choi E, Lee J, Lee S, et al. Potential therapeutic application of small molecule with sulfonamide for chondrogenic differentiation and articular cartilage repair. Bioorg Med Chem Lett. 2016;26(20):5098‐5102. [DOI] [PubMed] [Google Scholar]
- 165. Yang W, Zheng Y, Chen J, et al. Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded kartogenin for cartilage regeneration. Mater Sci Eng C. 2019;99:1362‐1373. [DOI] [PubMed] [Google Scholar]
- 166. Li X, Ding J, Zhang Z, et al. Kartogenin‐incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces. 2016;8(8):5148‐5159. [DOI] [PubMed] [Google Scholar]
- 167. Wu J, Sun J, Liu J. Evaluation of PHBV/calcium silicate composite scaffolds for cartilage tissue engineering. Appl Surf Sci. 2014;317:278‐283. [Google Scholar]
- 168. Bastakoty D, Saraswati S, Cates J, Lee E, Nanney LB, Young PP. Inhibition of Wnt/β‐catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015;29(12):4881‐4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhu H, Wei X, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. J Burn Care Res. 2008;29(5):804‐814. [DOI] [PubMed] [Google Scholar]
- 170. Frank S, Kämpfer H, Wetzler C, Pfeilschifter J. Nitric oxide drives skin repair: novel functions of an established mediator. Kidney Int. 2002;61(3):882‐888. [DOI] [PubMed] [Google Scholar]
- 171. Gao L, Guan W, Wang M, et al. Direct generation of human neuronal cells from adult astrocytes by small molecules. Stem Cell Rep. 2017;8(3):538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bettio LE, Patten AR, Gil‐Mohapel J, et al. ISX‐9 can potentiate cell proliferation and neuronal commitment in the rat dentate gyrus. Neuroscience. 2016;332:212‐222. [DOI] [PubMed] [Google Scholar]
- 173. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC‐CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23‐35. [DOI] [PubMed] [Google Scholar]
- 174. Xu H, Ferreira MM, Heilshorn SC. Small‐molecule axon‐polarization studies enabled by a shear‐free microfluidic gradient generator. Lab Chip. 2014;14(12):2047‐2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sachinidis A, Schwengberg S, Hippler‐Altenburg R, et al. Identification of small signalling molecules promoting cardiac‐specific differentiation of mouse embryonic stem cells. Cell Physiol Biochem. 2006;18(6):303‐314. [DOI] [PubMed] [Google Scholar]
- 176. Willems E, Cabral‐Teixeira J, Schade D, et al. Small molecule‐mediated TGF‐β type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell. 2012;11(2):242‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Oh S‐W, Lee JB, Kim B, et al. Peptidomimetic small‐molecule compounds promoting cardiogenesis of stem cells. Arch Pharm Res. 2012;35(11):1979‐1988. [DOI] [PubMed] [Google Scholar]
- 178. Wang H, Hao J, Hong CC. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β‐catenin signaling. ACS Chem Biol. 2011;6(2):192‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Hu B‐Y, Zhang S‐C. Directed differentiation of neural‐stem cells and subtype‐specific neurons from hESCs. Cellular Programming and Reprogramming. Springer; 2010:123‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Neely MD, Litt MJ, Tidball AM, et al. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction. ACS Chem Neurosci. 2012;3(6):482‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Egusa H, Saeki M, Doi M, et al. A small‐molecule approach to bone regenerative medicine in dentistry. J Oral Biosci. 2010;52(2):107‐118. [Google Scholar]
- 182. Gellynck K, Shah R, Parkar M, Young A, Buxton P, Brett P. Small molecule stimulation enhances bone regeneration but not titanium implant osseointegration. Bone. 2013;57(2):405‐412. [DOI] [PubMed] [Google Scholar]
- 183. Kim JM, Choi JS, Kim YH, et al. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol. 2013;228(3):617‐626. [DOI] [PubMed] [Google Scholar]
- 184. Aronin CEP, Shin SJ, Naden KB, et al. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1‐phosphate receptor targeted drug FTY720. Biomaterials. 2010;31(25):6417‐6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sefcik LS, Petrie Aronin CE, Botchwey EA. Engineering vascularized tissues using natural and synthetic small molecules. Organogenesis. 2008;4(4):215‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Indurkar A, Pandit A, Jain R, Dandekar P. Plant‐based biomaterials in tissue engineering. Bioprint. 2021;21:e00127. [DOI] [PubMed] [Google Scholar]
- 187. Bose S, Sarkar N. Natural medicinal compounds in bone tissue engineering. Trends Biotechnol. 2020;38(4):404‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Agarwal T, Tan SA, Onesto V, et al. Engineered herbal scaffolds for tissue repair and regeneration: recent trends and technologies. Biomed Eng Adv. 2021;2:100015. [Google Scholar]
- 189. Gu H, Boonanantanasarn K, Kang M, et al. Morinda citrifolia leaf extract enhances osteogenic differentiation through activation of wnt/β‐catenin signaling. J Med Food. 2018;21(1):57‐69. [DOI] [PubMed] [Google Scholar]
- 190. Hussain S, Ramasamy Tamizhselvi LG, Manickam V. Assessment of the role of noni (Morinda citrifolia) juice for inducing osteoblast differentiation in isolated rat bone marrow derived mesenchymal stem cells. Int J Stem Cells. 2016;9(2):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pham HTN, Tran HN, Nguyen PT, et al. Bacopa monnieri (L.) Wettst. Extract improves memory performance via promotion of neurogenesis in the hippocampal dentate gyrus of adolescent mice. Int J Mol Sci. 2020;21(9):3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Mashhadi NS, Ghiasvand R, Askari G, Hariri M, Darvishi L, Mofid MR. Anti‐oxidative and anti‐inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med. 2013;4(Suppl 1):S36‐S42. [PMC free article] [PubMed] [Google Scholar]
- 193. Mohammadi M, Haghirosadat BF, Larypoor M, et al. Synthesis, characterization and evaluation of Liponiosome containing ginger extract as a new strategy for potent antifungal formulation. J Cluster Sci. 2020;31(5):971‐981. [Google Scholar]
- 194. Squinca P, Berglund L, Hanna K, et al. Multifunctional ginger nanofiber hydrogels with tunable absorption: the potential for advanced wound dressing applications. Biomacromolecules. 2021;22(8):3202‐3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rohanizadeh R, Deng Y, Verron E. Therapeutic actions of curcumin in bone disorders. BoneKEy Rep. 2016;5:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bose S, Sarkar N, Banerjee D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Mater Today Chem. 2018;8:110‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Brahatheeswaran D, Mathew A, Aswathy RG, et al. Hybrid fluorescent curcumin loaded zein electrospun nanofibrous scaffold for biomedical applications. Biomed Mater. 2012;7(4):045001. [DOI] [PubMed] [Google Scholar]
- 198. Ahangari N, Kargozar S, Ghayour‐Mobarhan M, et al. Curcumin in tissue engineering: a traditional remedy for modern medicine. Biofactors. 2019;45(2):135‐151. [DOI] [PubMed] [Google Scholar]
- 199. Liu Y, Song R, Zhang X, Zhang D. Enhanced antimicrobial activity and pH‐responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int J Biol Macromol. 2020;161:1405‐1413. [DOI] [PubMed] [Google Scholar]
- 200. Rahman S, Carter P, Bhattarai N. Aloe vera for tissue engineering applications. J Funct Biomater. 2017;8(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Agnes Mary S, Giri Dev V. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J Textile Inst. 2015;106(8):886‐895. [Google Scholar]
- 202. Oryan A, Alemzadeh E, Mohammadi AA, Moshiri A. Healing potential of injectable Aloe vera hydrogel loaded by adipose‐derived stem cell in skin tissue‐engineering in a rat burn wound model. Cell Tissue Res. 2019;377(2):215‐227. [DOI] [PubMed] [Google Scholar]
- 203. Khakestani M, Jafari SH, Zahedi P, Bagheri R, Hajiaghaee R. Physical, morphological, and biological studies on PLA/n HA composite nanofibrous webs containing E quisetum arvense herbal extract for bone tissue engineering. J Appl Polym Sci. 2017;134(39):45343. [Google Scholar]
- 204. Marvi MS, Nourmohammadi J, Ataie M, Negahdari B, Naderi M. Surface modification of titanium implants via electrospinning of sericin and Equisetum arvense enhances the osteogenic differentiation of stem cells. Int J Polym Mater Polym Biomater. 2021;71:1‐12. [Google Scholar]
- 205. Sharmila G, Muthukumaran C, Kirthika S, Keerthana S, Kumar NM, Jeyanthi J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int J Biol Macromol. 2020;156:430‐437. [DOI] [PubMed] [Google Scholar]
- 206. Nair PR, Sreeja S, Sailaja G. In vitro biomineralization and osteogenesis of Cissus quadrangularis stem extracts: An osteogenic regulator for bone tissue engineering. J Biosci. 2021;46(4):1‐14. [PubMed] [Google Scholar]
- 207. Thangapazham RL, Sharad S, Maheshwari RK. Skin regenerative potentials of curcumin. Biofactors. 2013;39(1):141‐149. [DOI] [PubMed] [Google Scholar]
- 208. Liu X, You L, Tarafder S, et al. Curcumin‐releasing chitosan/aloe membrane for skin regeneration. Chem Eng J. 2019;359:1111‐1119. [Google Scholar]
- 209. Bhaskar B, Sreenivasa Rao P, Kasoju N, Nagarjuna V, Baadhe RR. Biomaterials in Tissue Engineering and Regenerative Medicine. Springer; 2021. [Google Scholar]
- 210. Ullah S, Chen X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl Mater Today. 2020;20:100656. [Google Scholar]
- 211. Ding Y, Li W, Correia A, et al. Electrospun polyhydroxybutyrate/poly (ε‐caprolactone)/sol–gel‐derived silica hybrid scaffolds with drug releasing function for bone tissue engineering applications. ACS Appl Mater Interfaces. 2018;10(17):14540‐14548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Chuysinuan P, Pengsuk C, Lirdprapamongkol K, Techasakul S, Svasti J, Nooeaid P. Enhanced structural stability and controlled drug release of hydrophilic antibiotic‐loaded alginate/soy protein isolate core‐sheath fibers for tissue engineering applications. Fibers Polym. 2019;20(1):1‐10. [Google Scholar]
- 213. Ramalingam R, Dhand C, Mayandi V, et al. Core–shell structured antimicrobial nanofiber dressings containing herbal extract and antibiotics combination for the prevention of biofilms and promotion of cutaneous wound healing. ACS Appl Mater Interfaces. 2021;13(21):24356‐24369. [DOI] [PubMed] [Google Scholar]
- 214. Aavani F, Biazar E, Heshmatipour Z, Arabameri N, Kamalvand M, Nazbar A. Applications of bacteria and their derived biomaterials for repair and tissue regeneration. Regen Med. 2021;16(6):581‐605. [DOI] [PubMed] [Google Scholar]
- 215. Mokhtarzadeh A, Alibakhshi A, Hejazi M, Omidi Y, Ezzati Nazhad Dolatabadi J. Bacterial‐derived biopolymers: advanced natural nanomaterials for drug delivery and tissue engineering. Trends Anal Chem. 2016;82:367‐384. [Google Scholar]
- 216. Zhang LDH. Recent advances in probiotics encapsulation by electrospinning. ES Food Agroforest. 2020;2:3‐12. [Google Scholar]
- 217. Khan MA, Hussain Z, Ali S, Qamar Z, Imran M, Hafeez FY. Fabrication of electrospun probiotic functionalized nanocomposite scaffolds for infection control and dermal burn healing in a mice model. ACS Biomater Sci Eng. 2019;5(11):6109‐6116. [DOI] [PubMed] [Google Scholar]
- 218. Deng L, Zhang H. Recent advances in probiotics encapsulation by electrospinning. ES Food Agroforest. 2020;2:3‐12. [Google Scholar]
- 219. Alqhtani N, Alenazi A, Nasyam FA, Mehaji S. Influencing effect of heat therapy on osteoblasts growth and differentiation following treatment with bone antiresorptive drugs—an in vitro study. J Young Pharm. 2019;11(4):395‐398. [Google Scholar]
- 220. Ikeda K, Ito A, Sato M, Kanno S, Kawabe Y, Kamihira M. Effects of heat stimulation and l‐ascorbic acid 2‐phosphate supplementation on myogenic differentiation of artificial skeletal muscle tissue constructs. J Tissue Eng Regen Med. 2017;11(5):1322‐1331. [DOI] [PubMed] [Google Scholar]
- 221. Orlacchio R, le Page Y, le Dréan Y, et al. Millimeter‐wave pulsed heating in vitro: cell mortality and heat shock response. Sci Rep. 2019;9(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Samandari M, Abrinia K, Mokhtari‐Dizaji M, Tamayol A. Ultrasound induced strain cytoskeleton rearrangement: An experimental and simulation study. J Biomech. 2017;60:39‐47. [DOI] [PubMed] [Google Scholar]
- 223. Filippi M, Dasen B, Guerrero J, et al. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose‐derived cells. Biomaterials. 2019;223:119468. [DOI] [PubMed] [Google Scholar]
- 224. Kolosnjaj‐Tabi J, Gibot L, Fourquaux I, Golzio M, Rols MP. Electric field‐responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv Drug Deliv Rev. 2019;138:56‐67. [DOI] [PubMed] [Google Scholar]
- 225. Zarrintaj P, Bakhshandeh B, Rezaeian I, Heshmatian B, Ganjali MR. A novel electroactive agarose‐aniline pentamer platform as a potential candidate for neural tissue engineering. Sci Rep. 2017;7(1):17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Oftadeh MO, Bakhshandeh B, Dehghan MM, Khojasteh A. Sequential application of mineralized electroconductive scaffold and electrical stimulation for efficient osteogenesis. J Biomed Mater Res A. 2018;106(5):1200‐1210. [DOI] [PubMed] [Google Scholar]
- 227. Kong M, Lee J, Yazdi IK, et al. Cardiac fibrotic remodeling on a Chip with dynamic mechanical stimulation. Adv Healthc Mater. 2019;8(3):e1801146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Rinoldi C, Fallahi A, Yazdi IK, et al. Mechanical and biochemical stimulation of 3D multilayered scaffolds for tendon tissue engineering. ACS Biomater Sci Eng. 2019;5(6):2953‐2964. [DOI] [PubMed] [Google Scholar]
- 229. Andersen JI, Pennisi CP, Fink T, Zachar V. Focal adhesion kinase activation is necessary for stretch‐induced alignment and enhanced differentiation of myogenic precursor cells. Tissue Eng Part A. 2018;24(7–8):631‐640. [DOI] [PubMed] [Google Scholar]
- 230. Fekrazad R, Asefi S, Eslaminejad MB, Taghiar L, Bordbar S, Hamblin MR. Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: proliferation and differentiation to bone or cartilage. Lasers Med Sci. 2019;34(1):115‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Staffoli S, Romeo U, Amorim RNS, et al. The effects of low level laser irradiation on proliferation of human dental pulp: a narrative review. Clin Ter. 2017;168(5):e320‐e326. [DOI] [PubMed] [Google Scholar]
- 232. Bakhshandeh B, Soleimani M, Ghaemi N, Shabani I. Effective combination of aligned nanocomposite nanofibers and human unrestricted somatic stem cells for bone tissue engineering. Acta Pharmacol Sin. 2011;32(5):626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Babaie A, Bakhshandeh B, Abedi A, et al. Synergistic effects of conductive PVA/PEDOT electrospun scaffolds and electrical stimulation for more effective neural tissue engineering. Eur Polym J. 2020;140:110051. [Google Scholar]
- 234. Cun X, Hosta‐Rigau L. Topography: a biophysical approach to direct the fate of mesenchymal stem cells in tissue engineering applications. Nanomaterials. 2020;10(10):2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Gasiorowski JZ, Murphy CJ, Nealey PF. Biophysical cues and cell behavior: the big impact of little things. Annu Rev Biomed Eng. 2013;15:155‐176. [DOI] [PubMed] [Google Scholar]
- 236. Wan X, Liu Z, Li L. Manipulation of stem cells fates: the master and multifaceted roles of biophysical cues of biomaterials. Adv Funct Mater. 2021;31(23):2010626. [Google Scholar]
- 237. Wang S, Li J, Zhou Z, Zhou S, Hu Z. Micro‐/Nano‐scales direct cell behavior on biomaterial surfaces. Molecules. 2018;24(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Eftekhari BS, Eskandari M, Janmey PA, Samadikuchaksaraei A, Gholipourmalekabadi M. Surface topography and electrical signaling: single and synergistic effects on neural differentiation of stem cells. Adv Funct Mater. 2020;30(25):1907792. [Google Scholar]
- 239. Metavarayuth K, Sitasuwan P, Zhao X, Lin Y, Wang Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater Sci Eng. 2016;2(2):142‐151. [DOI] [PubMed] [Google Scholar]
- 240. Dolatshahi‐Pirouz A, Kolind K, Pennisi CP, et al. Synthesis of Nano‐and micro‐scale topographies by combining colloidal lithography and glancing angle deposition (GLAD). Adv Eng Mater. 2015;17(1):8‐13. [Google Scholar]
- 241. Lee MR, Kwon KW, Jung H, et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31(15):4360‐4366. [DOI] [PubMed] [Google Scholar]
- 242. Moe AAK, Suryana M, Marcy G, et al. Microarray with micro‐and nano‐topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small. 2012;8(19):3050‐3061. [DOI] [PubMed] [Google Scholar]
- 243. Moerke C, Mueller P, Nebe JB. Sensing of micropillars by osteoblasts involves complex intracellular signaling. J Mater Sci Mater Med. 2017;28(11):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shinitzky M, Dianoux AC, Gitler C, Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic Micelles. Biochemistry. 1971;10(11):2106‐2113. [DOI] [PubMed] [Google Scholar]
- 245. Clarke TM, Durrant JR. Charge photogeneration in organic solar cells. Chem Rev. 2010;110(11):6736‐6767. [DOI] [PubMed] [Google Scholar]
- 246. Matsiko A, Gleeson JP, O'Brien FJ. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A. 2015;21(3–4):486‐497. [DOI] [PubMed] [Google Scholar]
- 247. Zhao D, Zhu T, Li J, et al. Poly (lactic‐co‐glycolic acid)‐based composite bone‐substitute materials. Bioactive Mater. 2021;6(2):346‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Brennan C, Eichholz K, Hoey D. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed Mater. 2019;14(6):065016. [DOI] [PubMed] [Google Scholar]
- 249. Yang W, Han W, He W, et al. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C. 2016;60:45‐53. [DOI] [PubMed] [Google Scholar]
- 250. Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fahy N, Alini M, Stoddart MJ. Mechanical stimulation of mesenchymal stem cells: implications for cartilage tissue engineering. J Orthop Res. 2018;36(1):52‐63. [DOI] [PubMed] [Google Scholar]
- 252. Brunelli M, Perrault C, Lacroix D. Short bursts of cyclic mechanical compression modulate tissue formation in a 3D hybrid scaffold. J Mech Behav Biomed Mater. 2017;71:165‐174. [DOI] [PubMed] [Google Scholar]
- 253. Hao J, Zhang Y, Jing D, et al. Mechanobiology of mesenchymal stem cells: perspective into mechanical induction of MSC fate. Acta Biomater. 2015;20:1‐9. [DOI] [PubMed] [Google Scholar]
- 254. Bakhshandeh B, Nateghi SS, Gazani MM, Dehghani Z, Mohammadzadeh F. A review on advances in the applications of spider silk in biomedical issues. Int J Biol Macromol. 2021;192:258‐271. [DOI] [PubMed] [Google Scholar]
- 255. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677‐689. [DOI] [PubMed] [Google Scholar]
- 256. Nallanthighal S, Heiserman JP, Cheon DJ. The role of the extracellular matrix in cancer Stemness. Front Cell Dev Biol. 2019;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. d'Angelo M, Benedetti E, Tupone MG, et al. The role of stiffness in cell reprogramming: a potential role for biomaterials in inducing tissue regeneration. Cell. 2019;8(9):1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Sun M, Chi G, Xu J, et al. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem Cell Res Ther. 2018;9(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Padhi A, Nain AS. ECM in differentiation: a review of matrix structure, composition and mechanical properties. Ann Biomed Eng. 2020;48(3):1071‐1089. [DOI] [PubMed] [Google Scholar]
- 260. Lee W‐CC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose‐derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6(4):265‐273. [DOI] [PubMed] [Google Scholar]
- 261. Pfister BJ, Weihs TP, Betenbaugh M, Bao G. An in vitro uniaxial stretch model for axonal injury. Ann Biomed Eng. 2003;31(5):589‐598. [DOI] [PubMed] [Google Scholar]
- 262. Pennisi CP, Olesen CG, de Zee M, Rasmussen J, Zachar V. Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng Part A. 2011;17(19–20):2543‐2550. [DOI] [PubMed] [Google Scholar]
- 263. Yoon J‐K, Lee TI, Bhang SH, Shin JY, Myoung JM, Kim BS. Stretchable piezoelectric substrate providing pulsatile mechanoelectric cues for cardiomyogenic differentiation of mesenchymal stem cells. ACS Appl Mater Interfaces. 2017;9(27):22101‐22111. [DOI] [PubMed] [Google Scholar]
- 264. Tan J, Xu X, Tong Z, et al. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res. 2015;3(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Dey K, Agnelli S, Sartore L. Dynamic freedom: substrate stress relaxation stimulates cell responses. Biomater Sci. 2019;7(3):836‐842. [DOI] [PubMed] [Google Scholar]
- 266. Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Wu Y, Yang Z, Law JBK, et al. The combined effect of substrate stiffness and surface topography on Chondrogenic differentiation of mesenchymal stem cells. Tissue Eng Part A. 2017;23(1–2):43‐54. [DOI] [PubMed] [Google Scholar]
- 268. Leppik L, Oliveira KMC, Bhavsar MB, Barker JH. Electrical stimulation in bone tissue engineering treatments. Eur J Trauma Emerg Surg. 2020;46(2):231‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kwon HJ, Lee GS, Chun H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci Rep. 2016;6:39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zarrintaj P, Bakhshandeh B, Saeb MR, et al. Oligoaniline‐based conductive biomaterials for tissue engineering. Acta Biomater. 2018;72:16‐34. [DOI] [PubMed] [Google Scholar]
- 271. Kitsara M, Blanquer A, Murillo G, et al. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale. 2019;11(18):8906‐8917. [DOI] [PubMed] [Google Scholar]
- 272. Fonseca JHJ, Bagne L, Meneghetti DH, et al. Electrical stimulation: complementary therapy to improve the performance of grafts in bone defects? J Biomed Mater Res B Appl Biomater. 2019;107(4):924‐932. [DOI] [PubMed] [Google Scholar]
- 273. Zhao Y, Liang Y, Ding S, Zhang K, Mao HQ, Yang Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials. 2020;255:120164. [DOI] [PubMed] [Google Scholar]
- 274. Bjorninen M, Gilmore K, Pelto J, et al. Electrically stimulated adipose stem cells on polypyrrole‐coated scaffolds for smooth muscle tissue engineering. Ann Biomed Eng. 2017;45(4):1015‐1026. [DOI] [PubMed] [Google Scholar]
- 275. Radvar E, Shi Y, Grasso S, et al. Magnetic field‐induced alignment of Nanofibrous supramolecular membranes: a molecular design approach to create tissue‐like biomaterials. ACS Appl Mater Interfaces. 2020;12(20):22661‐22672. [DOI] [PubMed] [Google Scholar]
- 276. Vikingsson L, Vinals‐Guitart A, Valera‐Martínez A, et al. Local deformation in a hydrogel induced by an external magnetic field. J Mater Sci. 2016;51(22):9979‐9990. [Google Scholar]
- 277. Xia Y, Sun J, Zhao L, et al. Magnetic field and nano‐scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151‐170. [DOI] [PubMed] [Google Scholar]
- 278. Kuan‐Jung Li J, Cheng‐An Lin J, Liu HC, et al. Comparison of ultrasound and electromagnetic field effects on osteoblast growth. Ultrasound Med Biol. 2006;32(5):769‐775. [DOI] [PubMed] [Google Scholar]
- 279. Özgün A, Marote A, Behie LA, Salgado A, Garipcan B. Extremely low frequency magnetic field induces human neuronal differentiation through NMDA receptor activation. J Neural Transm. 2019;126(10):1281‐1290. [DOI] [PubMed] [Google Scholar]
- 280. Olofsson K, Hammarstrom B, Wiklund M. Ultrasonic based tissue modelling and engineering. Micromachines (Basel). 2018;9(11):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Armstrong JPK, Puetzer JL, Serio A, et al. Engineering anisotropic muscle tissue using acoustic cell patterning. Adv Mater. 2018;30(43):1802649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Maung WM, Nakata H, Miura M, et al. Low‐intensity pulsed ultrasound stimulates osteogenic differentiation of periosteal cells in vitro. Tissue Eng Part A. 2021;27(1‐2):63‐73. [DOI] [PubMed] [Google Scholar]
- 283. de Lucas B, Pérez LM, Bernal A, Gálvez BG. Ultrasound therapy: experiences and perspectives for regenerative medicine. Gene. 2020;11(9):1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Cheng KW, Alhasan L, Rezk AR, et al. Fast three‐dimensional micropatterning of PC12 cells in rapidly crosslinked hydrogel scaffolds using ultrasonic standing waves. Biofabrication. 2019;12(1):015013. [DOI] [PubMed] [Google Scholar]
- 285. Zhang L, Liu X, Gao L, et al. Activation of Piezo1 by ultrasonic stimulation and its effect on the permeability of human umbilical vein endothelial cells. Biomed Pharmacother. 2020;131:110796. [DOI] [PubMed] [Google Scholar]
- 286. Mohapatra A, Uthaman S, Park IK. External and internal stimuli‐responsive metallic Nanotherapeutics for enhanced anticancer therapy. Front Mol Biosci. 2020;7:597634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Rao W, Zhang W, Poventud‐Fuentes I, et al. Thermally responsive nanoparticle‐encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2014;10(2):831‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ma L, Feng X, Liang H, et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater Today. 2020;36:48‐62. [Google Scholar]
- 289. Marino A, Arai S, Hou Y, et al. Gold nanoshell‐mediated remote myotube activation. ACS Nano. 2017;11(3):2494‐2508. [DOI] [PubMed] [Google Scholar]
- 290. Chen J, Shi ZD, Ji X, et al. Enhanced Osteogenesis of human mesenchymal stem cells by periodic heat shock in self‐assembling peptide hydrogel. Tissue Eng Part A. 2013;19(5–6):716‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Tong L, Liao Q, Zhao Y, et al. Near‐infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1‐11. [DOI] [PubMed] [Google Scholar]
- 292. Sanchez‐Casanova S, Martin‐Saavedra FM, Escudero‐Duch C, et al. Local delivery of bone morphogenetic protein‐2 from near infrared‐responsive hydrogels for bone tissue regeneration. Biomaterials. 2020;241:119909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Borzabadi‐Farahani A. Effect of low‐level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B. 2016;162:577‐582. [DOI] [PubMed] [Google Scholar]
- 294. Zecha JA, Raber‐Durlacher JE, Nair RG, et al. Low‐level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer. 2016;24(6):2793‐2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Amaroli A, Colombo E, Zekiy A, Aicardi S, Benedicenti S, de Angelis N. Interaction between laser light and osteoblasts: photobiomodulation as a trend in the management of socket bone preservation—a review. Biology. 2020;9(11):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Dompe C, Moncrieff L, Matys J, et al. Photobiomodulation‐underlying mechanism and clinical applications. J Clin Med. 2020;9(6):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Ruan Y, Kato H, Taguchi Y, Yamauchi N, Umeda M. Irradiation by high‐intensity red light‐emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/beta‐catenin signaling pathway. Lasers Med Sci. 2020;36:55‐65. [DOI] [PubMed] [Google Scholar]
- 298. Yoon S‐R, Hong N, Lee MY, Ahn JC. Photobiomodulation with a 660‐nanometer light‐emitting diode promotes cell proliferation in astrocyte culture. Cell. 2021;10(7):1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Mokoena DR, Houreld NN, Dhilip Kumar SS, Abrahamse H. Photobiomodulation at 660 nm stimulates fibroblast differentiation. Lasers Surg Med. 2020;52(7):671‐681. [DOI] [PubMed] [Google Scholar]
- 300. Karic V, Chandran R, Abrahamse H. Laser‐induced differentiation of human adipose‐derived stem cells to temporomandibular joint disc cells. Lasers Surg Med. 2021;53(4):567‐577. [DOI] [PubMed] [Google Scholar]
- 301. Della Coletta BB, Jacob TB, Moreira LAC, et al. Photobiomodulation therapy on the guided bone regeneration process in defects filled by biphasic calcium phosphate associated with fibrin biopolymer. Molecules. 2021;26(4):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Cui L, Zhang J, Zou J, et al. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials. 2020;230:119617. [DOI] [PubMed] [Google Scholar]
- 303. Zhang J, Qiu K, Sun B, et al. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J Mater Chem B. 2014;2(45):7945‐7954. [DOI] [PubMed] [Google Scholar]
- 304. Lin S, Lee WYW, Feng Q, et al. Synergistic effects on mesenchymal stem cell‐based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res Ther. 2017;8(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Zhang W, Feng C, Yang G, et al. 3D‐printed scaffolds with synergistic effect of hollow‐pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials. 2017;135:85‐95. [DOI] [PubMed] [Google Scholar]
- 306. Pham L, Dang LH, Truong MD, et al. A dual synergistic of curcumin and gelatin on thermal‐responsive hydrogel based on chitosan‐P123 in wound healing application. Biomed Pharmacother. 2019;117:109183. [DOI] [PubMed] [Google Scholar]
- 307. Zhang B, Luo Q, Deng B, Morita Y, Ju Y, Song G. Construction of tendon replacement tissue based on collagen sponge and mesenchymal stem cells by coupled mechano‐chemical induction and evaluation of its tendon repair abilities. Acta Biomater. 2018;74:247‐259. [DOI] [PubMed] [Google Scholar]
- 308. Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3(10):589‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ghaemi RV, Siang LC, Yadav VG. Improving the rate of translation of tissue engineering products. Adv Healthc Mater. 2019;8(19):1900538. [DOI] [PubMed] [Google Scholar]
- 310. Goldstein SA. Tissue engineering: functional assessment and clinical outcome. Ann N Y Acad Sci. 2002;961(1):183‐192. [DOI] [PubMed] [Google Scholar]
- 311. O'Donnell BT, Ives CJ, Mohiuddin OA, Bunnell BA. Beyond the present constraints that prevent a wide spread of tissue engineering and regenerative medicine approaches. Front Bioeng Biotechnol. 2019;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Jensen G, Morrill C, Huang Y. 3D tissue engineering, an emerging technique for pharmaceutical research. Acta Pharm Sin B. 2018;8(5):756‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Otto I, Breugem CC, Malda J, Bredenoord AL. Ethical considerations in the translation of regenerative biofabrication technologies into clinic and society. Biofabrication. 2016;8(4):042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Harrison RH, St‐Pierre J‐P, Stevens MM. Tissue engineering and regenerative medicine: a year in review. Tissue Eng Part B Rev. 2014;20(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 316. Thesleff T, Lehtimäki K, Niskakangas T, et al. Cranioplasty with adipose‐derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68(6):1535‐1540. [DOI] [PubMed] [Google Scholar]
- 317. Bracci R, Maccaroni E, Cascinu S. Bioresorbable airway splint created with a three‐dimensional printer. N Engl J Med. 2013;368(21):2043‐2045. [DOI] [PubMed] [Google Scholar]
- 318. McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein‐2 (INFUSE® bone graft). Int Orthop. 2007;31(6):729‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Calori G, Tagliabue L, Gala L, d'Imporzano M, Peretti G, Albisetti W. Application of rhBMP‐7 and platelet‐rich plasma in the treatment of long bone non‐unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39(12):1391‐1402. [DOI] [PubMed] [Google Scholar]
- 320. Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra® dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 321. Hart CE, Loewen‐Rodriguez A, Lessem J. Dermagraft: use in the treatment of chronic wounds. Adv Wound Care. 2012;1(3):138‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Williams DF. Challenges with the development of biomaterials for sustainable tissue engineering. Front Bioeng Biotechnol. 2019;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403‐430. [DOI] [PubMed] [Google Scholar]
- 324. Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99(3):203‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Kim HS, Kumbar SG, Nukavarapu SP. Biomaterial‐directed cell behavior for tissue engineering. Curr Opin Biomed Eng. 2021;17:100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


