Abstract
Background:
Resistance to novel androgen signalling inhibition and metastatic castration resistant prostate cancer (mCRPC) progression is likely dependent on tumor microenvironment interactions. The Src pathway and neoangiogenesis have been implicated in prostate cancer progression. We studied the effect of adding the targeted agents Dasatinib and Sunitinib to Abiraterone acetate (AA) in men with mCRPC.
Patients and Methods:
In this open-label, randomized, phase II study, mCRPC patients received AA. Upon resistance to AA, they were randomized 1:1 to combination with (AA-D) Dasatinib or (AA-S) Sunitinib. Upon second progression, patients crossed over. Primary endpoint was time to treatment failure (TTF), defined as time to progression or death. Secondary endpoints included overall survival (OS) and safety.
Results:
From 03/2011 to 02/2015, 179 patients were enrolled and 132 subsequently randomized. Median TTF was 5.7 months in the Dasatinib and 5.5 months in the Sunitinib group. There was no difference between the two groups in terms of TTF (Hazard ratio (HR) 0.85, 95% Confidence Interval (CI) 0.59–1.22). Median OS from study entry was 26.3 months in the Dasatinib group and 27.7 months in the Sunitinib group (HR 1.02, 95% CI 0.71–1.47). Adverse events ≥Grade 3 related to study medication were more frequent with Sunitinib (n=44, 46%) compared to Dasatinib (n=26, 24%). At data cut off, 7 patients were demonstrating a continuous response to AA, with a median duration of treatment 5.7 years.
Conclusion:
There is no difference in OS and TTF between Dasatinib and Sunitinb combined with Abiraterone in the treatment of patients with bone mCRPC.
Keywords: metastatic castration-resistant prostate cancer, tumor microenvironment, targeted agents, androgen signaling inhibition, Abiraterone acetate, Dasatinib, Sunitinib malate
MicroAbstract
The Src pathway and neoangiogenesis have been implicated in prostate cancer progression. In an open-label, randomized, phase II study, no difference was reported in overall survival (OS) and time to treatment failure (TTF) between Dasatinib and Sunitinb combined with Abiraterone, after progression on Abiraterone monotherapy, in patients with bone metastatic castration resistant prostate cancer.
1. Introduction
Men with metastatic castration resistant prostate cancer (mCRPC) have a median survival of approximately 3 years based on phase III study data. (1, 2) As the disease progresses from an endocrine to a paracrine driven subtype, tumor microenvironment interactions maybe integral to treatment resistance. (3, 4)
Abiraterone acetate (AA) with prednisone has been established as a first-line treatment option in men with advanced prostate cancer. (2, 5–7) Through inhibition of 17α-hydroxylase and C17, 20-lyase, the drug achieves maximal androgen depletion, blocking not only endocrine (testicular and adrenal) but importantly “intracrine” (autocrine /paracrine) androgen production. Ensuing resistance to treatment may be accounted for by different mechanisms not fully elucidated to date, including both genomic and non-genomic alterations. Tumor microenvironment interactions, especially those within the bone microenvironment, may also contribute to such resistance. It is noteworthy that disease in lymph nodes is exquisitely and uniformly responsive to novel androgen signaling inhibitors, unlike the case for bone metastatic lesions that exhibit variable patterns of response. (2, 8)
Src-family kinases (SFK) are a group of non-receptor protein tyrosine kinases implicated in the pathogenesis and progression of prostate cancer to castration resistant disease. (9–11) Dasatinib is a potent SFK inhibitor, which also targets BCR-Abl, Fyn, c-Kit, platelet-derived growth factor receptor (PDGFR) alpha and –beta, and the ephrin receptor kinase. (12) Activity of Dasatinib against prostate cancer has been demonstrated pre-clinically both in vitro and in vivo. (13, 14) Additionally, several phase I and II clinical trials have documented an acceptable safety profile and activity of Dasatinib in patients with CRPC. A phase III trial evaluating the activity of Dasatinib in combination with docetaxel did not yield positive results. (15–19)
The role of neo-angiogenesis in prostate cancer progression is implied by the expression of vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) in the tumors and the association of increasing VEGF plasma levels with disease progression. (20–23) Sunitinib malate is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor, which targets VEGFRs, PDGFRs, c-KIT and other receptor tyrosine kinases. The efficacy and tolerability of the drug in CRPC has been investigated in several phase II and III clinical trials. A randomized phase III trial reported evidence of progression free survival (PFS) benefit but without confirmation of added survival benefit -which was the primary endpoint. (24–28)
Targeting pathways of interaction within tumor microenvironment may lead to an effective and prolonged suppression of tumor progression. This randomized, phase II study compared two different combinations of targeted agents (Dasatinib or Sunitinib) with Abiraterone acetate in patients with mCRPC.
2. Patients and Methods
2.1. Study design and Conduct
A prospective, open-label, randomized study was conducted at the University of Texas MD Anderson Cancer Center in Houston (UTMDACC) (TX), USA. The study (NCT01254864) was approved by the institutional review board of the UTMDACC and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent.
2.2. Patients and Interventions
Men with histologically confirmed prostate adenocarcinoma and documented bone-metastatic disease were eligible. Patients were required to have prostate cancer progression documented either by PSA according to Prostate Cancer Working Group (PCWG) 2 criteria or radiographically according to modified Response Evaluation Criteria in Solid Tumors (RECIST), while being surgically or medically castrated with testosterone levels ≤ 50 ng/dL (≤ 2.0 nM). Previous treatment with docetaxel was allowed. A list of inclusion and exclusion criteria is available in the Data Supplement.
All patients were treated with AA 1g orally daily in combination with prednisone 5mg orally twice daily. Upon resistance to AA, patients were randomized 1:1 to receive additional Sunitinib 37.5 mg orally daily for two weeks followed by a week of rest or Dasatinib 100 mg orally daily. Patients continued treatment with AA and prednisone.
Patients remained on treatment until progression per PCWG2 criteria on the combined treatment or excess toxicity. A washout period for a total of 4 weeks or until full recovery from treatment related adverse events (AEs) followed. Patients then crossed over to the alternate agent. Patients were taken off study upon further progression or excess toxicity.
2.3. Outcomes
Primary outcome measure was time to treatment failure (TTF), defined as time from randomization (AA-D or AA-S) to progression per PCWG2 criteria or death. Secondary endpoints included safety for combinations, overall survival from randomization, overall survival from treatment initiation for patients that received at least one of the subsequent treatments as well as for patients that received both targeted agents in sequence. Clinical endpoints and tissue based correlative studies with a focus on primary resistance to abiraterone are reported separately. ExploratoryPSA endpoints included assessment of long term response to abiraterone.
2.4. Assessments
Patients were assessed for response according to modified RECIST version 1.1 criteria with the use of CT scan of abdomen and pelvis, chest x-ray and bone scan at baseline, change of treatment and at other time points when clinically indicated (29), and according to the PCWG2 criteria with PSA measurements at baseline and every 4–8 weeks during treatment. (30)
Resistance to AA was defined by PCWG2 criteria for progression (PSA, imaging and/or symptom progression) or failure to achieve PSA response by 8 weeks or plateau of PSA demonstrated as failure to further suppress PSA measured at 4-week intervals during treatment.
Adverse events were assessed at 4-week intervals according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
2.5. Statistical Analysis
The sample size was calculated to be 180 patients, which would provide a power of 90% to detect a 75% increase in the mean time to final treatment failure, from 7.6 months to 13.3 months. In these calculations, we assumed a mean TTF of 4 months for patients in each treatment group and that the probability of progression and not death at final treatment failure is 0.9. All computations were carried out using East version 5 (Cytel Corporation).
Randomization after progression on AA was dynamically balanced on three binary patient covariates using the method of Pocock and Simon. The covariates were (i) quality of response to AA, defined as time to progression being either > 3 months or < 3 months, (ii) alkaline phosphatase either > 120 or < 120, and (iii) performance status either 0, 1 or > 1. All safety analyses were based on data from patients who received at least one dose of any study therapy.
Patient and clinical characteristics were summarized using descriptive statistics. The probabilities of TTF and OS were estimated using the Kaplan-Meier method. The difference in TTF and OS between treatment groups was assessed with the log-rank test. Cox proportional regression analyses were performed to assess the association between patient characteristics and TTF/OS. Statistical analyses were performed using SAS 9.4 [The SAS Institute, Cary, NC] and Splus 8.2 [TIBCO, Palo Alto, CA].
3. Results
Between March 2011 and February 2015, 179 patients were enrolled in the study and received treatment with AA. One hundred and thirty-two patients were randomized to AA-S(n=64) or AA-D(n=68) upon resistance to AA (as predefined), while continuing treatment with AA. Upon progression, only 71 patients crossed over to the alternate treatment arm. (CONSORT diagram, Supplementary Table 1). At data cut-off in July 2019, 7 patients remained on AA and prednisone. The most common reason for discontinuation was physician decision (58%), followed by toxicity (3% AA alone, 10% AA+ Sunitinib and 8% AA+ Dasatinib), withdrawal of consent by patients (13%) and other co-morbidities leading to treatment discontinuation (3%).
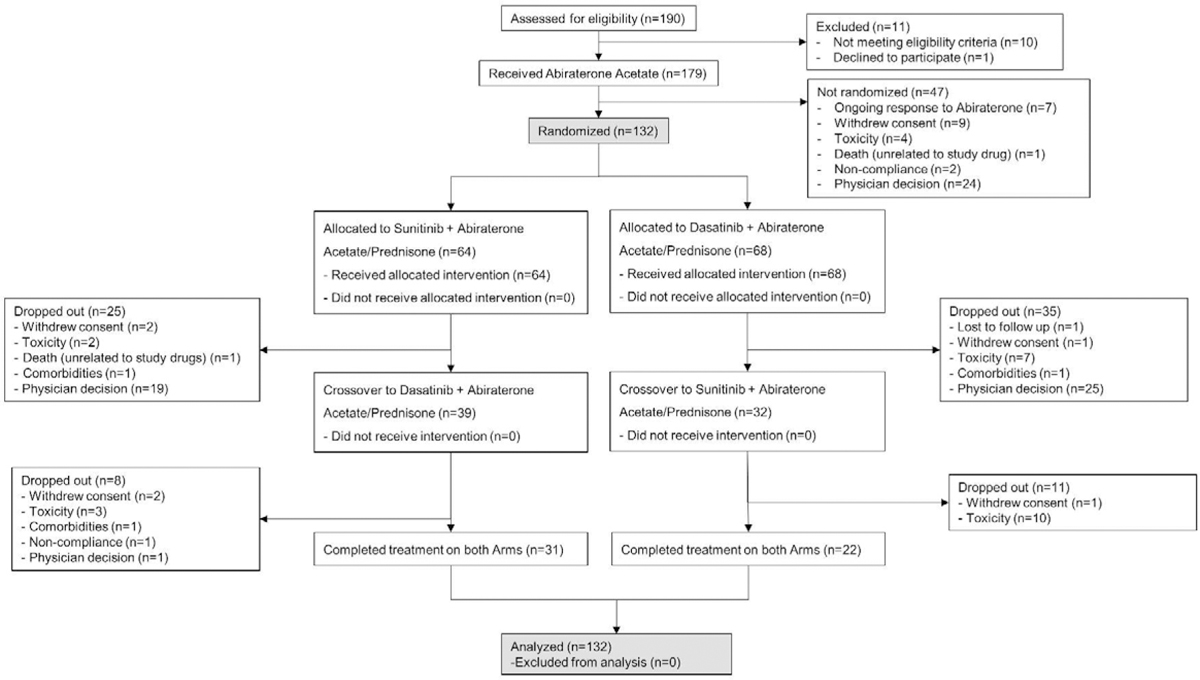
CONSORT Diagram
Baseline demographic, tumor and treatment characteristics were well balanced as depicted in Table 1. Patients median age was 66 years (range 48–87) and median ECOG PS 1 (range 0–1). The majority of tumors (62%) were histologically high-risk at initial diagnosis. At study entry, 26 patients (15%) had visceral metastases and 54 (30%) had 20 or more bone metastases. Approximately two thirds of the patients (N= 105, 59%) had undergone radical prostatectomy or External beam radiation therapy of primary. Forty-one (23%) had received prior chemotherapy. Most had received several lines of prior hormonal manipulation but no prior novel androgen signaling inhibition.
Table 1:
Baseline patient and disease characteristics
| Characteristic | All Patients | Randomized Sunitinib | Randomized to Dasatinib | P valuea,b |
|---|---|---|---|---|
| Evaluable patients, n (%) | 179 (100) | 64 (48) | 68 (52) | |
|
| ||||
| Median age (range), years | 66 (48–87) | 67 (48–85) | 68 (52–80) | 0.044a |
|
| ||||
| Race, n (%) | ||||
| White | 146 (81) | 44 (69) | 58 (85) | 0.093b |
| Black/African American | 20 (11) | 13 (20) | 5 (7) | |
| Hispanic | 10 (6) | 4 (6) | 5 (7) | |
| Asian | 3 (2) | 3 (5) | 0 (0) | |
|
| ||||
| Median ECOG PS (range) | 1 (0–3) | 1 (0–1) | 1 (0–1) | 0.726a |
|
| ||||
| Median PSA (range), ng/mL | 20.6 (0.5 – 2427.5) | 28.45 (0.8 – 1195.6) | 19.35 (0.8 – 627.8) | 0.107a |
|
| ||||
| Gleason score at diagnosis, n (%) | ||||
| ≤ 7 | 41 (23) | 13 (20) | 18 (26) | 0.551b |
| ≥ 8 | 110 (61) | 40 (63) | 43 (63) | |
| Not Available | 28 (16) | 11 (17) | 7 (10) | |
|
| ||||
| >20 Bone Metastases, n (%) | ||||
| Yes | 54 (30) | 22 (34) | 17 (25) | 0.238b |
| No | 125 (70) | 42 (66) | 51 (75) | |
|
| ||||
| Visceral Metastases, n (%) | ||||
| Yes | 25 (14) | 8 (14) | 11 (16) | 0.655b |
| No | 154 (86) | 51 (86) | 56 (84) | |
|
| ||||
| Prior prostate cancer related treatments | ||||
| Median hormonal treatment lines (range) | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.562a |
| Anti-androgens, n (%) | 123 (69) | 45 (70) | 51 (75) | 0.546b |
| Chemotherapy, n (%) | 41 (23) | 20 (31) | 13 (19) | 0.108b |
| EBRT, n (%) | 58 (32) | 21 (33) | 23 (34) | 0.902b |
| Brachytherapy, n (%) | 7 (4) | 3 (5) | 3 (4) | 0.939b |
| Surgery, n (%) | 77 (43) | 30 (47) | 29 (43) | 0.625b |
| Cryoablation, n (%) | 4 (2) | 1 (2) | 2 (3) | 0.595b |
EBRT= External Beam Radiation Therapy, ECOG PS = Eastern Cooperative Oncology Group performance status, PSA= Prostate specific antigen
P value from Mann-Whitney U test comparing patients randomized to sunitinib versus dasatinib
P value from Chi square test comparing patients randomized to sunitinib versus dasatinib
Time to AA resistance (per protocol definition) was well balanced between the 2 treatment groups: median 5.7 (interquartile range (IQR) 3.7–10.7) months in the AA-D group and median 5.7 (IQR 3.6–9.6) months in the AA-S.
As of July 2019, 7 patients were still on ongoing treatment with AA and have maintained a PSA concentration of 0 ng/mL. Median duration of treatment in this subset was 5.7 years at data cut off. Five out of seven patients have history of prior local therapy of the prostate and only 1 has received prior chemotherapy. Median age at study entry was 62 years (range 50–74) and median ECOG was 1. All patients had < 20 bone metastatic sites. One had retroperitoneal lymph node metastases. Median PSA was 8.2 (range 0.6 –145.3). Baseline characteristics of this subset of patients are presented in supplementary table 2.
Median TTF was 5.7 months (95% confidence interval (CI) 3.8–7.4) in AA-D group and 5.5 (95% CI 4.6–6.8) in AA-S group. (HR 0.85, 95% CI 0.59–1.22) (Figure 1a). We also analyzed after excluding patient with primary resistance (confirmed disease progression within 4 months) to AA (n=29, 22 %) (HR 0.73, 95% CI 0.48–1.11) (Figure 1b). In the exploratory proportional hazards regression model performed, only prior chemotherapy was significantly associated with increased risk for progression or death (p=0.001). No association between treatment group and TTF was found. The model yielded similar results after exclusion of patients with primary resistance to AA. (Figure 2a, b)
Figure 1:
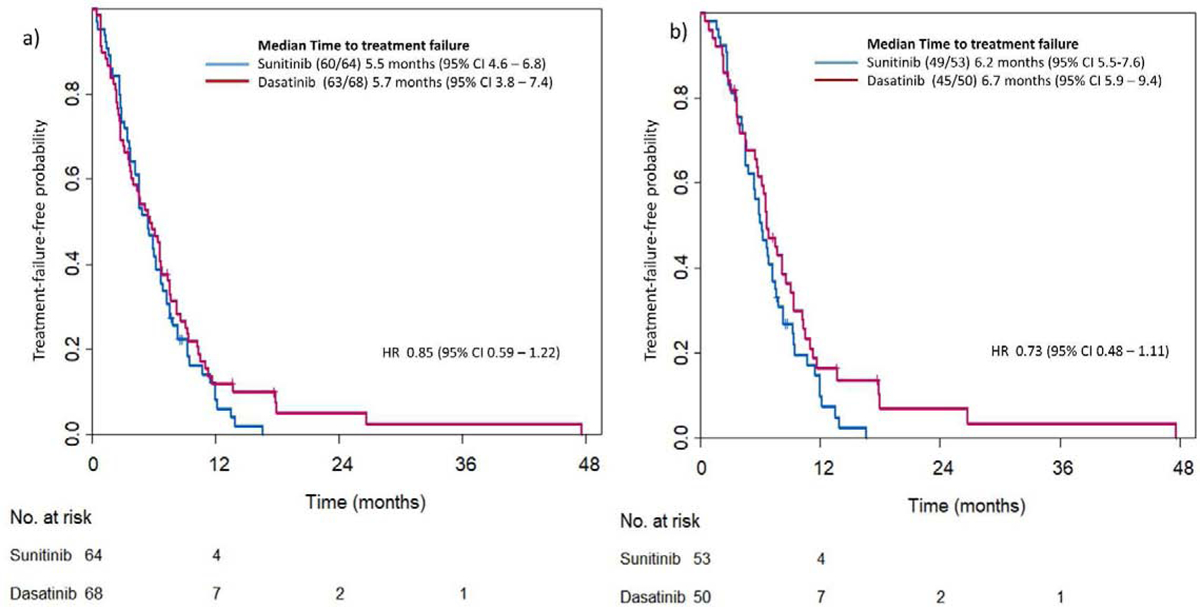
Kaplan-Meier estimates for time to treatment failure (TTF) by treatment group
A: In all patients randomized (n=132)
B: in patients without primary resistance to Abiraterone Acetate (n=103).
Figure 2:
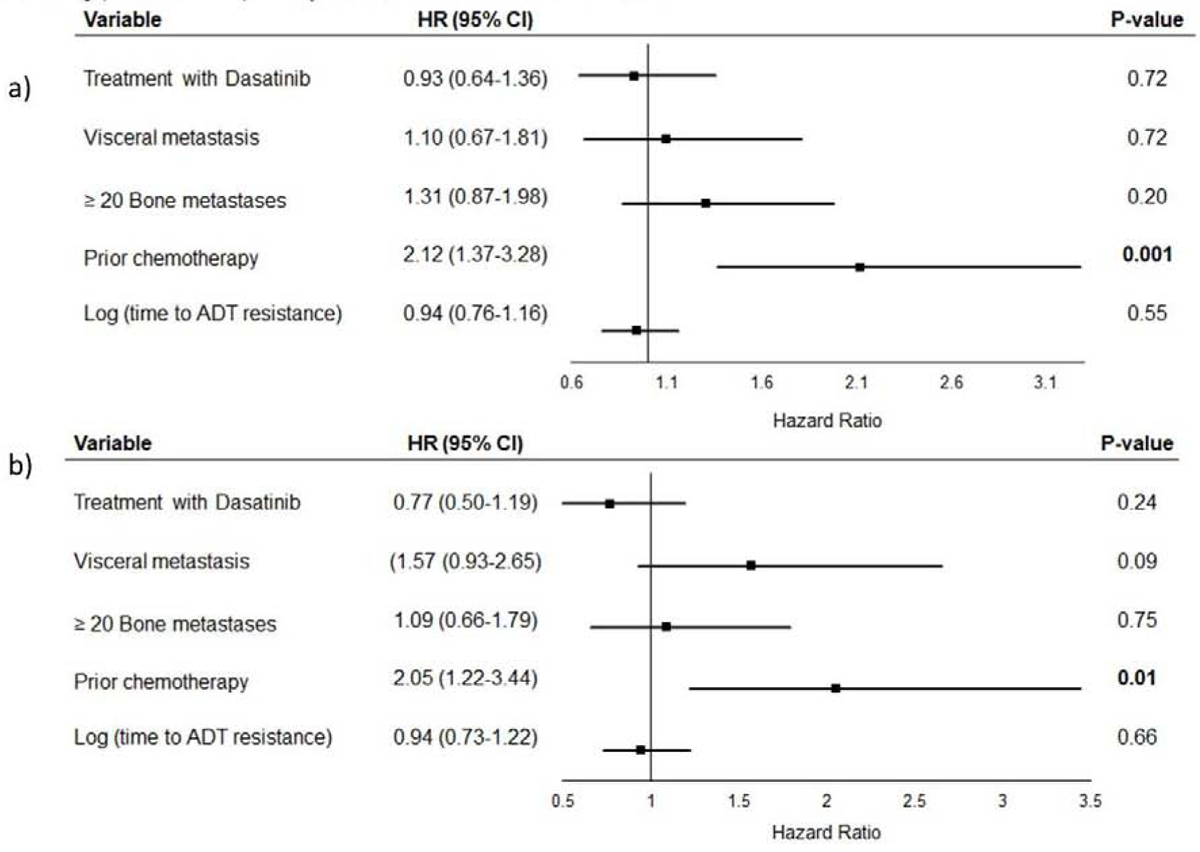
Multivariate cox proportional hazards regression model for time to treatment failure
A: In all patients randomized
B: After excluding patients with primary resistance to Abiraterone Acetate
Median OS from randomization was 20.8 months (95% CI 16.7–28.1) in the Dasatinib group and 22.9 months (95% CI 16.9–27.8) in the Sunitinib group. No significant difference in OS was evident between the two treatment groups (HR 1.04, 95% CI 0.72–1.49) (Figure 3a). This remained true also after exclusion of patients with primary resistance to AA (n=29, 22 %) (HR 0.92, 95% 0.61–1.41) (Figure 3b). At data cut off, 59 patients (87%) initially randomized to Dasatinib and 58 (91%) initially randomized to Sunitinib had died. Exploratory multivariable regression analysis accounting also for baseline disease characteristics demonstrated that ≥20 bone metastases and prior chemotherapy were significantly associated with the risk of death (p=0.002 and p<0.0001, respectively). No association between treatment group and risk of death was detected. Similar results yielded the same model after excluding patients with primary resistance to AA. (Figure 4 a, b)
Figure 3:
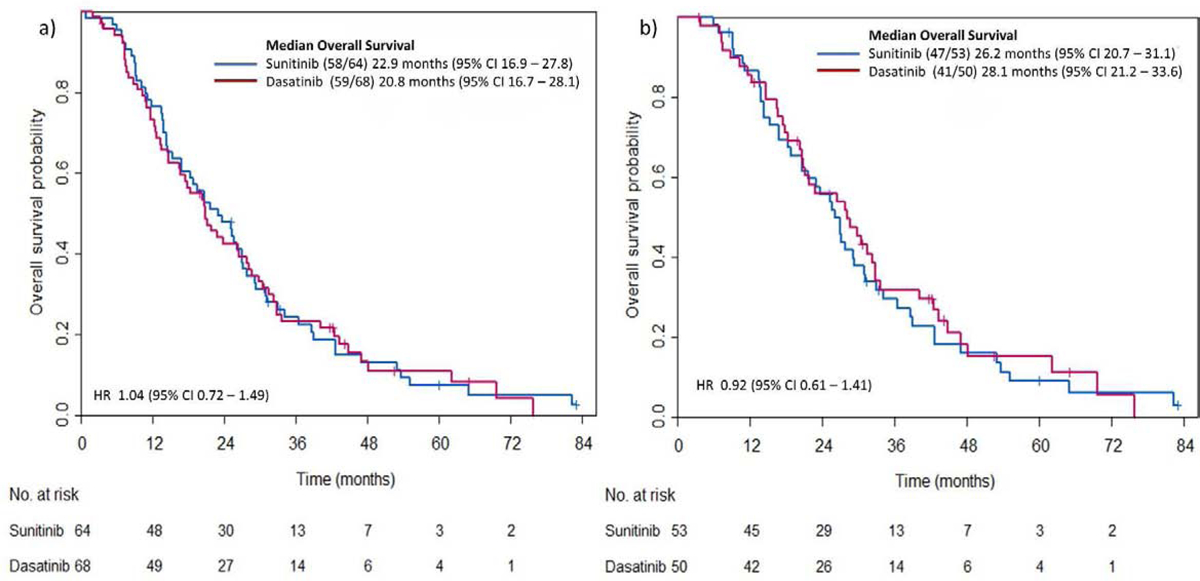
Kaplan-Meier estimates for overall survival by treatment group
A: In all patients randomized
B: After excluding patients with primary resistance to Abiraterone Acetate.
Figure 4:
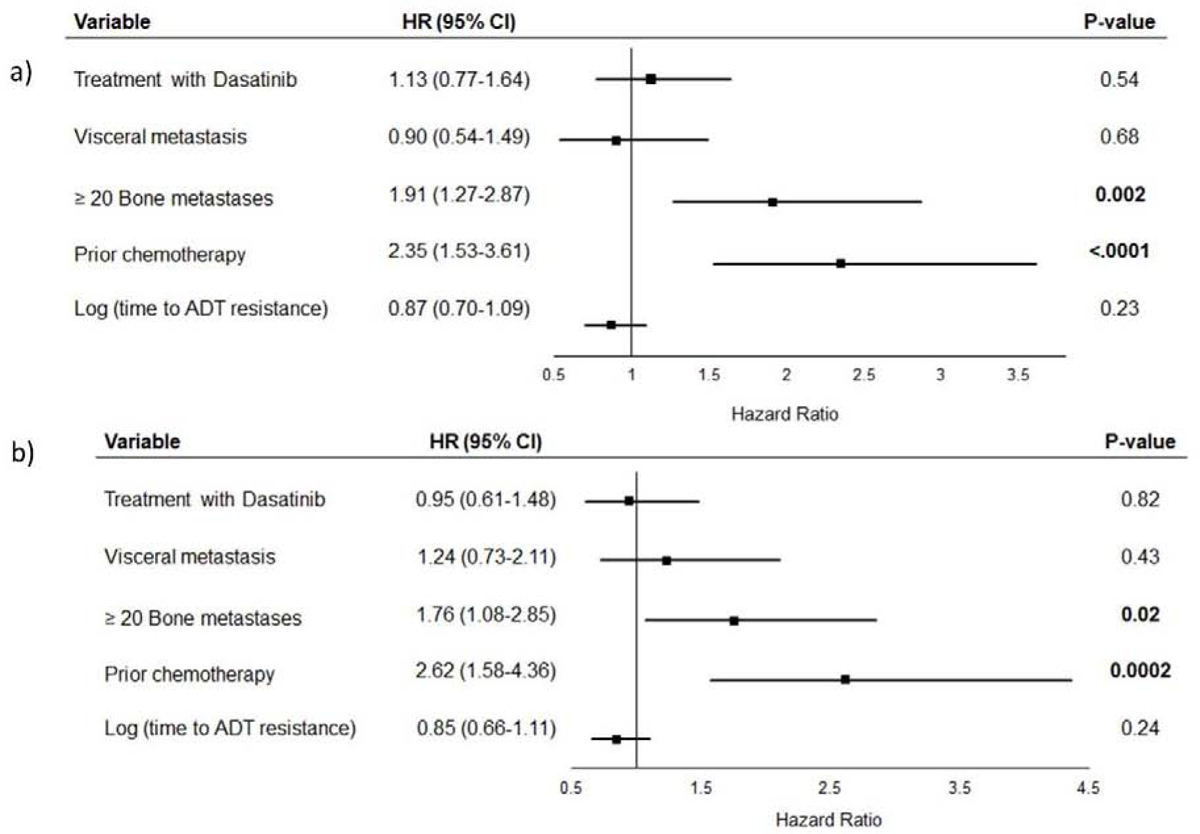
Multivariate cox proportional hazards regression model for overall survival
A: In all patients randomized
B: After excluding patients with primary resistance to Abiraterone Acetate.
Regarding OS from study entry (i.e. from start of AA), median OS was 26.3 months (95% CI 23.5–38.4) in the Dasatinib group and 27.7 months (95% CI 23.3–37.1) in the Sunitinib group. Exploratory analysis excluding patients who had received prior chemotherapy revealed longer OS in the chemotherapy-naïve subset (median OS 28.5 months (95% CI 23.5–41.8) with Dasatinib and 36.8 months (95% CI 26.4–46.3) with Sunitinib. (Supplementary Figure 1 a, b) Median OS from study entry for patients who received both targeted agents in sequence was 36.6 months (95% CI 27.2–45.5) in the subset randomized to Sunitinib followed by Dasatinib and 35.0 months (CI 95% 26.3–45.7) in the group of patients that received Dasatinib followed by Sunitinib. (Supplementary Figure 2 a, b)
Safety evaluation for Dasatinib and Sunitinib included all patients that received each drug at any time point (i.e. at randomization or after cross-over). Adverse events (AEs) of any grade were reported in 90 patients (84%) that received Dasatinib and 92 (96%) that received Sunitinib. The most frequent AEs in the Dasatinib and Sunitinib groups were fatigue (50% and 54%, respectively), abnormal liver function tests (40% and 50%, respectively) and nausea/vomiting (29% and 45%, respectively). (Table 2) Ten patients (9%) discontinued treatment due to adverse events while on Dasatinib and 12 (13%) while on Sunitinib. AEs related to AA presented a profile consistent with reported AA safety. AE ≥Grade 3 related to AA are summarized in Supplementary Table 3.
Table 2.
Adverse events related to Sunitinib and Dasatinib
| Adverse events | Sunitiniba (N=96) |
Dasatiniba (N=107) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Any grade | Grade 1–2 | ≥Grade 3b | Any grade | Grade 1–2 | ≥Grade 3b | |
|
Patients, n (%) | ||||||
| Any | 92 (96) | 48 (50) | 44 (46) | 90 (84) | 64 (60) | 26 (24) |
|
| ||||||
| Most common (≥5% of patients) c | ||||||
|
| ||||||
| Fatigue | 52 (54) | 40 (42) | 12 (13) | 54 (50) | 48 (45) | 6 (6) |
|
| ||||||
| Abnormal liver function testsd | 48 (50) | 45 (47) | 3 (3) | 43 (40) | 43 (40) | - |
|
| ||||||
| Nausea/Vomiting | 43 (45) | 40 (42) | 3 (3) | 31 (29) | 29 (27) | 2 (2) |
|
| ||||||
| Diarrhea | 30 (31) | 29 (30) | 1 (1) | 26 (24) | 25 (23) | 1 (1) |
|
| ||||||
| Anemia | 17 (18) | 16 (17) | 1 (1) | 25 (23) | 19 (18) | 6 (6) |
|
| ||||||
| Dyspnea | 10 (10) | 8 (8) | 2 (2) | 23 (21) | 19 (18) | 4 (4) |
|
| ||||||
| Edema | 9 (9) | 9 (9) | - | 22 (21) | 21 (20) | 1 (1) |
|
| ||||||
| Electrolyte disorders | ||||||
| Hypokalemia | 20 (21) | 16 (17) | 4 (4) | 21 (20) | 21 (20) | - |
| Other | 29 (30) | 27 (28) | 2 (2) | 17 (16) | 14 (13) | 3 (3) |
|
| ||||||
| Anorexia | 8 (8) | 8 (8) | - | 14 (13) | 13 (12) | 1 (1) |
|
| ||||||
| Dyspepsia | 13 (14) | 13 (14) | - | 10 (9) | 9 (8) | 1 (1) |
|
| ||||||
| Hypertension | 33 (34) | 15 (16) | 18 (19) | 9 (8) | 7 (7) | 2 (2) |
|
| ||||||
| Dysgeusia | 33 (34) | 33 (34) | - | 9 (8) | 9 (8) | - |
|
| ||||||
| Pleural Effusion | - | - | - | 8 (7) | 8 (7) | - |
|
| ||||||
| Platelet count decreased | 19 (20) | 16 (17) | 3 (3) | 7 (7) | 7 (7) | - |
|
| ||||||
| Constipation | 6 (6) | 6 (6) | - | 7 (7) | 7 (7) | - |
|
| ||||||
| Rash maculo-papular | 11 (11) | 10 (10) | 1 (1) | 7 (7) | 7 (7) | - |
|
| ||||||
| Cardiac Disorderse | 5 (5) | 4 (4) | 1 (1) | 5 (5) | 3 (3) | 2 (2) |
|
| ||||||
| White blood cell count decreased | 29 (30) | 27 (28) | 2 (2) | 5 (5) | 5 (5) | - |
| Neutrophil count decreased | 19 (20) | 15 (16) | 4 (4) | 2 (2) | 2 (2) | - |
| Lymphocyte count increased | 6 (6) | 5 (5) | 1 (1) | - | - | - |
|
| ||||||
| Headache | 10 (10) | 9 (9) | 1 (1) | 5 (5) | 5 (5) | - |
|
| ||||||
| Dizziness | 8 (8) | 8 (8) | - | 5 (5) | 5 (5) | - |
|
| ||||||
| Dry skin | 4 (4) | 4 (4) | - | 5 (5) | 5 (5) | - |
|
| ||||||
| Generalized muscle weakness | 5 (5) | 5 (5) | - | 4 (4) | 3 (3) | 1 (1) |
|
| ||||||
| Weight loss | 5 (5) | 4 (4) | 1 (1) | 3 (3) | 3 (3) | - |
|
| ||||||
| Skin Hypopigmentation | 17 (18) | 17 (18) | - | 2 (2) | 2 (2) | - |
|
| ||||||
| Creatinine increased | 8 (8) | 8 (8) | - | 1 (1) | - | 1 (1) |
|
| ||||||
| Mucositis Oral | 25 (26) | 23 (24) | 2 (2) | 1 (1) | 1 (1) | - |
|
| ||||||
| Gastroesophagel reflux | 6 (6) | 6 (6) | - | 1 (1) | 1 (1) | - |
|
| ||||||
| Bruising | 5 (5) | 5 (5) | - | 1 (1) | 1 (1) | - |
|
| ||||||
| Palmar-plantar erythrodysesthesia syndrome | 14 (15) | 14 (15) | - | 1 (1) | 1 (1) | - |
|
| ||||||
| Dry mouth | 5 (5) | 5 (5) | - | - | - | - |
Table includes all patients who received Sunitinib/Dasatinib at any time point (i.e. after randomization or cross-over)
No Grade 5 adverse events related to Dasatinib were reported. 1 Grade 5 adverse event related to Sunitinib was reported (pneumonia).
Listed are the adverse events occurring in at least 5% in either group.
Abnormal liver function tests include alanine aminotransferase (ALT) increase, aspartate aminotransferase (AST) increase, alkaline phosphatase increase, and bilirubin increase.
Cardiac disorders include cardiac chest-pain, pericardial effusion, palpitations, atrial fibrillation, and other, bigeminal premature atrial contractions.
4. Discussion
This phase 2 study of AA followed by randomization to addition of Dasatinib or Sunitinib in men with mCRPC showed no difference in TTF and OS between the two treatment arms. AEs ≥Grade 3 related to the study medication were more frequent with Sunitinib compared to Dasatinib.
Several clinical trials have studied the effect of Dasatinib and Sunitinib in chemotherapy naïve as well as pre-treated mCRPC patients. Although the role of Src and neo-angiogenesis in prostate cancer progression have been supported by pre-clinical data, (13, 14, 20–23) clinical trials involving their inhibitors in unselected mCRPC patient populations have failed to yield overall survival benefit. Dasatinib has shown a reasonable safety profile and some evidence of activity in bone metastatic disease (17, 18) in patients with mCRPC. Randomized trials, however, have not demonstrated a benefit in OS or PFS. (15, 16, 19, 31, 32) A recently published phase II trial comparing the combination of AA with Dasatinib vs. placebo in patients with mCRPC did not report significant PFS or OS benefit to favor the combinatorial strategy. (31) Sunitinib has been studied in combination with docetaxel and as monotherapy in mCRPC, exhibiting moderate tolerability. Reported response rates were promising, with imaging responses of note not couple with PSA decline. (24, 28) A phase III trial of Sunitinib vs. placebo in progressive mCRPC demonstrated a significant PFS improvement in patients receiving Sunitinib, as well as higher objective response rates in this treatment group. OS, however, was not significantly prolonged.(25)
Resistance to novel androgen signaling inhibition and mCRPC progression are likely dependent on tumor microenvironment interactions, with the Src and neoangiogenesis pathways being implicated in prostate cancer progression. We hypothesized that the addition of Dasatinib or Sunitinib in patients who exhibit resistance to treatment with AA would prolong clinical benefit form androgen signalling inhibition by targeting these candidate pathways of resistance. The median OS in the chemotherapy-naïve subset of our patients (77% of the overall trial population) was 28.5 months (95% CI 23.5 – 41.8) with Dasatinib and 36.8 months (95% CI 26.4 – 46.3) with Sunitinib, markedly longer compared to the OS of the entire study population and comparable to the OS with AA reported in the phase III COU-AA-302 trial in chemotherapy-naïve mCRPC patients (median 34.7 months, 95% CI 32.7–36.8).(2) Although our trial was not powered for this analysis, there might be a positive signal towards improved outcome with AA + Sunitinib. The wide range of responses is dominantly indicative of disease heterogeneity. However, there is a potential subset of patients in whom specific microenvironment interactions likely play an active role in androgen signaling resistance and progression. This subset likely derived benefit from targeted therapy with Sunitinib in this trial, consistent with the activity signal observed in the phase III trial of Sunitinib monotherapy (25).
Interestingly, the subset of patients who received both targeted agents in sequence after randomization demonstrated markedly longer OS compared to the overall study population, with the median OS being up to 10 months longer. No significant difference was detected between the two different sequences (Sunitinib followed by Dasatinib vs. Dasatinib followed by Sunitinib). Taking into consideration that this subset had comparable baseline characteristics and proportion of chemotherapy-naïve patients to the entire study population (79%), this observation might be indicative of a potentiation of therapeutic efficacy with the sequencing of mechanistically different, microenvironment targeting strategies. Although sequential application of targeted agents has become routine in other cancer entities such as the renal cell carcinoma, this needs to be further studied in prostate cancer. However, this observation may also be caused by a biased selection of patients who just have a more indolent disease course.
It is noteworthy that 7 patients have exhibited an exceptional ongoing response to AA ranging from 4.7 to 8.2 years. These patients have all achieved a sustained response to AA with undetectable PSA. More specifically, the median time to PSA progression reported in COU-AA-302 was 11.1 months with AA, while the median radiographic progression free survival (rPFS) was 16.5 months. Similarly, the median time to PSA progression with Enzalutamide in the PREVAIL trial was 11.2 months, with a median rPFS of 20 months. (33, 34) Compared to the overall patient population of our trial, these patients were younger (median age 62 years vs 66 years overall) and had a lower median PSA concentration (8.2 ng/mL vs 20.6 ng/mL) at study entry. The burden of bone metastatic disease in this subset was considerably lower, and 6 were chemotherapy-naïve. This is in line with literature indicating several factors to be associated with outcome with AA, including age, baseline PSA concentration, LDH and alkaline phosphatase, Gleason score, presence of numerous bone metastases, presence of visceral metastases and pain level (evaluated by the Brief Pain Inventory-Short Form). (35, 36) The specific characteristics of patients with an exceptional response to AA remain to be elucidated. This could guide the selection of the subset of patients that will derive maximum and prolonged benefit from novel androgen synthesis inhibitors and inform clinical decisions.
The safety profile of Dasatinib and Sunitinib in combination with AA is similar to that reported in previous phase III trials in patients with mCRPC (19, 25), as well as in other malignancies, such as chronic myeloid leukemia, renal cell carcinoma, gastrointestinal stromal tumor, and pancreatic neuroendocrine tumors. (37–40) In our study, we reported abnormal liver function tests (including alanine aminotransferase (ALT) increase, aspartate aminotransferase (AST) increase, and bilirubin increase) in 43% of patients receiving Dasatinib and 48% of patients receiving Sunitinib, which is more frequent than previously reported. This is likely attributable to the combination of the drugs with AA, as depicted by the occurrence of ≥ grade 3 ALT increase in patients receiving AA monotherapy before randomization (Supplementary table 3). The only clinical trial combining Dasatinib with AA to our knowledge reported an increase in ALT and AST in 2/14 patients (14%) receiving the combination. (31) This lower rate may be attributed to the small sample size. Between the two treatment arms, more AEs were reported with Sunitinib (96% vs. 84% with Dasatinib). Adverse events ≥ grade 3 were reported in a higher frequency in patients receiving Sunitinib (46% vs 24% with Dasatinib), underscoring the more toxic profile of the drug.
A limitation of our study was that 40 patients failed to be randomized, hence not providing the pre-planned power. Only patients with bone-metastatic disease were eligible for participation in this study and only a small proportion of the enrolled patients (14%) had additional visceral metastases. Even though our findings might thus not be generalizable to the entire mCRPC population, bone metastatic patients with or without visceral metastases is by far the largest patient group in the mCRPC setting.
5. Conclusion
Overall, there was no significant difference in TTF and OS between Dasatinib and Sunitinib combined with AA in the treatment of patients with bone metastatic CRPC who had exhibited resistance to AA. Amongst the chemotherapy-naïve patients, there is a subset that potentially derived benefit from targeting the neoangiogenesis pathway with Sunitinib upon resistance to AA. The safety profile of both drugs when co-administered with AA is similar to that reported in previous clinical trials, with Sunitinib demonstrating a higher frequency of adverse events. Characterization of patients with sustained response to AA is critical for the selection of the optimal strategy.
6. Clinical Practice Points
Treatment with Dasatinib and Sunitinib does not overcome resistance to AA in patients with mCRPC. There is a subset benefiting from the addition of Sunitinib to AA, implicating microenvironment interactions as a potential effective target. A subgroup of patients has shown an exceptionally durable response with AA monotherapy for a median of 5.7 years. The specific characteristics of patients that would derive maximum benefit from novel androgen signaling inhibitors and targeted agents remain to be deciphered, guiding towards a personalized optimal treatment selection for individual patients.
Supplementary Material
Highlights:
Resistance to novel androgen signalling inhibition and metastatic castration resistant prostate cancer (mCRPC) progression is likely dependent on tumor microenvironment interactions, including the Src pathway and neoangiogenesis.
In an open-label, randomized, phase II study, no difference was reported in overall survival (OS) and time to treatment failure (TTF) between Dasatinib and Sunitinb combined with Abiraterone in the treatment of patients with bone mCRPC.
A subgroup of patients has shown an exceptionally durable response with Abiraterone monotherapy.
The specific characteristics of patients that would derive maximum benefit from novel androgen signaling inhibitors remain to be deciphered, guiding towards a personalized optimal treatment selection for individual patients.
Acknowledgments
The authors acknowledge all the participating physicians, research support staff, and patients who participated in this study for their valuable contribution.
This study received financial support by the Prostate Cancer Foundation. Study drugs were provided by Janssen, Bristol-Myers Squibb Company (BMS), and Pfizer.
Funding
This study received financial support by the Prostate Cancer Foundation. Study drugs were provided by Janssen, Bristol-Myers Squibb Company (BMS), and Pfizer.
L.P. has received research funding from Pfizer, Roche/Genentech, Exelixis, and Merck and travel from Merck. J.K. is an employee and owns stocks at Merck. S.S. has consulting/advisory relationship with Valeant, Dendreon, Apricity Health, Janssen, Polaris, Amgen, Bayer, and Exelixis, has received research funding from Janssen, Bristol-Myers Squibb, and AstraZeneca, has received honoraria from Compugen, Apricity Health, Janssen, Dendreon, Polaris, Parker Institute of Cancer Immunotherapy, and Society for Immunotherapy of Cancer, and has ownership interest with Apricity Health. N.T. has consulting/advisory relationship with Bristol-Myers-Squibb and Pfizer, has received research funding from Bristol-Myers-Squibb and honoraria from Bristol-Myers-Squibb and Pfizer, and has participated in scientific advisory committees for Pfizer. C.J.L. has consulting/advisory relationship with Janssen, has received research funding from Bristol-Myers-Squibb, Janssen and Pfizer, has received honoraria from Janssen, and has participated in scientific advisory committees for Pfizer. E.E. has received research grant support, ad board, honoraria and travel from Sanofi, Janssen, Astellas, Tolmar, Bayer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
M.B., N.S., J.A.W., A.T., A.H., A.A., S.M.T., J.C.A., A.J.Z., P.C., J.W., P.T. and S.W. have no conflict of interest to declare.
Trial registration: ClinicalTrials.gov NCT01254864.
References
- 1.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 2017;71(2):151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16(2):152–60. [DOI] [PubMed] [Google Scholar]
- 3.Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res 2010;16(4):1100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logothetis CJ, Gallick GE, Maity SN, Kim J, Aparicio A, Efstathiou E, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov 2013;3(8):849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13(10):983–92. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20(5):686–700. [DOI] [PubMed] [Google Scholar]
- 7.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017;377(4):338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev 2017;57:16–27. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay I, Wang J, Qin M, Gao L, Holtz R, Vessella RL, et al. Src promotes castration-recurrent prostate cancer through androgen receptor-dependent canonical and non-canonical transcriptional signatures. Oncotarget 2017;8(6):10324–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K The role of Src in prostate cancer. Ann Oncol 2007;18(11):1765–73. [DOI] [PubMed] [Google Scholar]
- 11.Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res 2009;15(10):3540–9. [DOI] [PubMed] [Google Scholar]
- 12.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004;47(27):6658–61. [DOI] [PubMed] [Google Scholar]
- 13.Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101(2):263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res 2008;68(9):3323–33. [DOI] [PubMed] [Google Scholar]
- 15.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer. 2012;118(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twardowski PW, Beumer JH, Chen CS, Kraft AS, Chatta GS, Mitsuhashi M, et al. A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anticancer Drugs. 2013;24(7):743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu EY, Massard C, Gross ME, Carducci MA, Culine S, Hudes G, et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77(5):1166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res 2009;15(23):7421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo JC, Trudel GC, Saad F, Armstrong AJ, Yu EY, Bellmunt J, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013;14(13):1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duque JL, Loughlin KR, Adam RM, Kantoff P, Mazzucchi E, Freeman MR. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61(5):401–8. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer FA, Miller LJ, Lindquist R, Kowalczyk P, Laudone VP, Albertsen PC, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54(3):567–72. [DOI] [PubMed] [Google Scholar]
- 22.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J Clin Oncol 2004;22(9):1655–63. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y, Opeskin K, Goad J, Williams ED. Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer Res 2006;66(19):9566–75. [DOI] [PubMed] [Google Scholar]
- 24.Dror Michaelson M, Regan MM, Oh WK, Kaufman DS, Olivier K, Michaelson SZ, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol 2009;20(5):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaelson MD, Oudard S, Ou YC, Sengelov L, Saad F, Houede N, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol 2014;32(2):76–82. [DOI] [PubMed] [Google Scholar]
- 26.Parimi S, Eliasziw M, North S, Trudeau M, Winquist E, Chi KN, et al. Sunitinib maintenance therapy after response to docetaxel in metastatic castration resistant prostate cancer (mCRPC). Invest New Drugs. 2016;34(6):771–6. [DOI] [PubMed] [Google Scholar]
- 27.Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, Galsky MD, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol 2010;21(2):319–24. [DOI] [PubMed] [Google Scholar]
- 28.Zurita AJ, George DJ, Shore ND, Liu G, Wilding G, Hutson TE, et al. Sunitinib in combination with docetaxel and prednisone in chemotherapy-naive patients with metastatic, castration-resistant prostate cancer: a phase 1/2 clinical trial. Ann Oncol 2012;23(3):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 30.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26(7):1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorff TB, Quinn DI, Pinski JK, Goldkorn A, Sadeghi S, Tsao-Wei D, et al. Randomized Phase II Trial of Abiraterone Alone or With Dasatinib in Men With Metastatic Castration-resistant Prostate Cancer (mCRPC). Clin Genitourin Cancer. 2019;17(4):241–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spreafico A, Chi KN, Sridhar SS, Smith DC, Carducci MA, Kavsak P, et al. A randomized phase II study of cediranib alone versus cediranib in combination with dasatinib in docetaxel resistant, castration resistant prostate cancer patients. Invest New Drugs. 2014;32(5):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66(5):815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boegemann M, Khaksar S, Bera G, Birtle A, Dopchie C, Dourthe LM, et al. Abiraterone acetate plus prednisone for the Management of Metastatic Castration-Resistant Prostate Cancer (mCRPC) without prior use of chemotherapy: report from a large, international, real-world retrospective cohort study. BMC Cancer. 2019;19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller K, Carles J, Gschwend JE, Van Poppel H, Diels J, Brookman-May SD. The Phase 3 COU-AA-302 Study of Abiraterone Acetate Plus Prednisone in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Stratified Analysis Based on Pain, Prostate-specific Antigen, and Gleason Score. Eur Urol 2018;74(1):17–23. [DOI] [PubMed] [Google Scholar]
- 37.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362(24):2260–70. [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356(2):115–24. [DOI] [PubMed] [Google Scholar]
- 40.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6):501–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


