Abstract
Reactive oxygen species (ROS) are mainly produced in mitochondria and are involved in various physiological activities of the ovary through signaling and are critical for regulating the ovarian cycle. Notably, the imbalance between ROS generation and the antioxidant defense system contributes to the development of ovarian diseases. These contradictory effects have critical implications for potential antioxidant strategies that aim to scavenge excessive ROS. However, much remains to be learned about how ROS causes various ovarian diseases to the application of antioxidant therapy for ovarian diseases. Here, we review the mechanisms of ROS generation and maintenance of homeostasis in the ovary and its associated physiological effects. Additionally, we have highlighted the pathological mechanisms of ROS in ovarian diseases and potential antioxidant strategies for treatment.
Keywords: Reactive oxygen species, Oxidative stress, Ovarian diseases, Antioxidant drugs, Hormones, Mesenchymal stem cells
Highlights
-
•
ROS play an indispensable role in various physiological activities of the ovary by mediating signaling.
-
•
The imbalance between ROS generation and the antioxidant defense system contributes to ovarian diseases.
-
•
Antioxidant drugs, hormones, and mesenchymal stem cells can alleviate ROS-mediated ovarian diseases.
1. Introduction
From a historical perspective, 1908 was the first milestone year in reactive oxygen species (ROS) biology, revealing the increased oxygen consumption of sea urchin eggs after fertilization [1]. The term oxidative stress (OS) was formally introduced in 1985 [2]. Numerous studies have broadened our understanding of ROS biology in the past century.
ROS are mainly produced by biochemical reactions during the cellular respiration process [3] and are a double-edged sword for ovarian health. Under normal conditions, the production of ROS and the antioxidant system are in balance and the body maintains physiologically required levels of ROS. ROS play a vital role in ovarian physiological activity as a secondary messenger for cellular signaling [4] and are involved in the regulation of the ovarian cycle, including in meiosis [5], ovulation [6], corpus luteum maintenance [7], and regression [8]. However, excessive ROS production might overwhelm the antioxidant defense system and induce OS [9], leading to the development of ovarian diseases including age-related ovarian dysfunction, ovarian cancer, polycystic ovary syndrome (PCOS), and ovarian endometriosis.
In recent years, there has been a growing interest in ROS and ovarian diseases and the application of antioxidant strategies. The combined efforts of biologists and chemists have uncovered the mechanisms by which ROS participate in diseases and the redox potential of chemical species, helping us to envisage viable antioxidant therapy. Currently, strategies with unique ROS regulatory properties, including antioxidant drugs, hormones, and mesenchymal stem cells (MSCs), have been extensively studied and are potential therapeutic options. However, we lack a comprehensive review of the mechanisms by which ROS levels influence ovarian diseases and the potential application of antioxidant therapy. Therefore, we comprehensively reviewed the role of ROS pathogenesis in various ovarian diseases and explored frontier antioxidant therapy for treating such diseases. The review begins with a description of ROS generation and the antioxidant defense system in the ovary, followed by a brief overview of the physiological role of ROS in the ovary and a comprehensive review of the available evidence for ROS involvement in ovarian diseases. Finally, the latest frontiers in treating ovarian diseases through antioxidant strategies are presented.
2. ROS generation and antioxidant defense system in the ovary
2.1. ROS generation
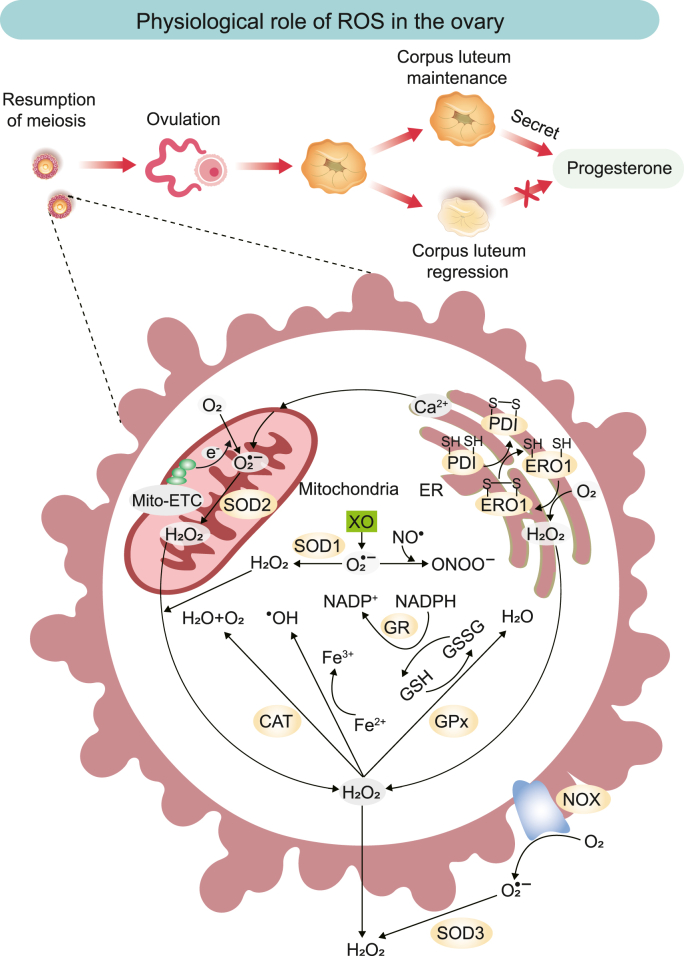
ROS are the chemical species formed when oxygen is incompletely reduced and include mainly superoxide anion (O2•−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radical (•OH) [10]. The ROS in the ovary are produced through multiple pathways. Mitochondria are the most abundant organelles in oocytes [11], and the respiratory electron transfer systems for aerobic metabolism are the primary sources of ROS production [12] (Fig. 1). Electrons leaking from the mitochondrial respiratory chain reduce O2 molecules to O2•− (primary ROS), which are further converted to molecules such as H2O2, •OH, and hypochlorite (secondary ROS) through various catalytic reactions [10]. In this process, chain complexes I and III produce mainly primary ROS (O2•−). The O2•− is then converted to H2O2 by mitochondrial Mn superoxide dismutase (SOD2) in the mitochondrial matrix or Cu–Zn superoxide dismutase (SOD1) in the intermembrane space [13]. In contrast, superoxide dismutase 3 (SOD3) catalyzes the production of H2O2 from O2•− outside the cell [14]. The endoplasmic reticulum (ER) is a site of protein synthesis and a source of ROS. The accumulation of misfolded proteins leads to ER stress, which activates the unfolded protein response (UPR) to restore protein homeostasis [15]. During the UPR, the ROS cascade reaction is triggered by the participation of protein disulfide isomerase, ER oxidoreductin 1, and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) complex (especially NOX4) [16]. ER stress also leads to a massive release of Ca2+ into the mitochondria, disrupting normal mitochondrial ROS and leading to an increase in O2•− [17]. Xanthine oxidase is also involved in the production of ROS; it reduces O2 to O2•−, which is not only converted to H2O2 by cytosolic SOD1, but also reacts with nitric oxide to form peroxynitrite [[18], [19], [20]].
Fig. 1.
Schematic illustration of ROS generation, its natural scavenging mechanisms, and physiological role in the ovary. ROS produced by the mitochondrial electron transport chain (Mito-ETC), the endoplasmic reticulum system, XO, and the NOX complex are eliminated by various antioxidant enzymes, thereby maintaining redox homeostasis in the ovary. Hydrogen peroxide (H2O2) can be converted to hydroxyl radicals (•OH) in the presence of transition metals, such as Fe2+. Nitric oxide (NO•) can react with superoxide (O2•−) to form peroxynitrite (ONOO−), a non-radical species that can modify the structure and function of proteins.
ROS, reactive oxygen species; Mito-ETC, mitochondrial electron transport chain; SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; GPx, glutathione peroxidase; NOX, nicotinamide adenine dinucleotide phosphate oxidase; NADPH, nicotinamide adenine dinucleotide phosphate; GSH, glutathione; GSSG, oxidized glutathione; XO, xanthine oxidase; PDI, protein disulfide isomerase; ERO1, endoplasmic reticulum oxidoreductin 1; ER, endoplasmic reticulum.
Ovarian aging [21], recurrent ovulation [22], and obesity [23] are among the physiological causes of ROS accumulation in the ovary, whereas environmental factors, including chemotherapeutic agents [24], smoking [25], drinking [26], and a high-sugar diet [8], can also contribute to the accumulation of ROS.
2.2. The antioxidant defense system
Excessive ROS generation can induce OS and lead to the development of ovarian diseases. The antioxidant defense system in the ovary maintains ovarian homeostasis through the timely removal of excessive ROS. The antioxidant defense system consists of enzymatic and non-enzymatic antioxidants.
In vivo enzymatic antioxidants, such as SOD, catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR), play critical roles in ROS homeostasis in the body [27]. The three SOD enzymes, SOD1, SOD2, and SOD3, are activated in the presence of a catalytic metal (Cu or Mn) and catalyze the conversion of O2•− to H2O2 [14]. GPx and GR are members of the glutathione (GSH) family of enzymes [28]. GPx utilizes GSH as a substrate providing H to degrade H2O2 to produce H2O, whereas GSH is converted to oxidized glutathione (GSSG). GSSG is subsequently reconverted to GSH using protons provided by NADPH, catalyzed by GR [4,29].
Non-enzymatic antioxidants in vivo mainly include vitamin C (VC), vitamin E (VE), β-carotene, carotenoids, selenium, zinc, taurine, and GSH [4], which play a vital role in ROS homeostasis in vivo.
3. Physiological role of ROS in the ovary
ROS regulates various physiological functions of the ovary through signaling, from oocyte growth to fertilization, including meiosis, ovulation, corpus luteum maintenance, and regression (Fig. 1). Among these regulating ROS, •OH and 1O2 have relatively short diffusion distances and can only exist in separated spaces. In addition, the instability of O2•− and its inability to diffuse across membranes limit its role as a signaling molecule. In contrast, H2O2 molecules, owing to their weak reactivity, high specificity, high diffusivity, and good membrane permeability, are crucial messengers in signaling [10].
The interactions between ROS and antioxidants in the ovary are complex. During meiosis, ROS and antioxidants play a crucial role in precisely regulating the arrest and resumption of oocyte meiosis [5]. Each month, only one dominant oocyte completes meiosis I, which is accompanied by an increase in ROS levels and inhibited antioxidant production [30,31]. Elevated ROS such as H2O2 might induce the resumption of meiosis by activating the adenosine monophosphate-activated protein kinase A or Ca2+-mediated pathway [31]. In contrast, meiosis II requires protection by enzymatic antioxidants such as CAT and SOD [30].
Ovulation is one of the most crucial functions of the ovary, and this process is similar to inflammation. Increased secretion of the luteinizing hormone before ovulation leads to increased inflammatory precursors in the ovary, which results in excessive ROS production [8]. The increased ROS are involved in cumulus expansion, progesterone production, the expression of preovulatory genes, and the activation of signals contributing to ovulation [6]. Conversely, the use of antioxidant enzymes could significantly reduce ovulation efficiency and prolong ovulation time [32], which further demonstrates the role of ROS in ovulation. In the ovary, the corpus luteum which forms after ovulation can produce progesterone. If fertilization occurs after ovulation, progesterone maintains the pregnancy by improving the uteroplacental circulation, suppressing the immune response, and inhibiting uterine contractions [33]. Conversely, an absence of fertilization after ovulation requires corpus luteum regression and a decline in progesterone to create the conditions for follicular development in the next physiological cycle [34]. ROS and scavenger SOD are vital in regulating the corpus luteum function. Increased expression of the antioxidant enzyme SOD enhances the ability to scavenge ROS, thereby prolonging the function of the corpus luteum and progesterone secretion [7,35]. Conversely, under reduced SOD activity, ROS induce the corpus luteum regression by apoptosis of luteinized granulosa cells (GCs) through OS [8]. ROS and SOD are involved in ovarian cycle control through these steps, demonstrating their crucial roles in ovarian physiology.
4. Pathological role of ROS in ovarian diseases
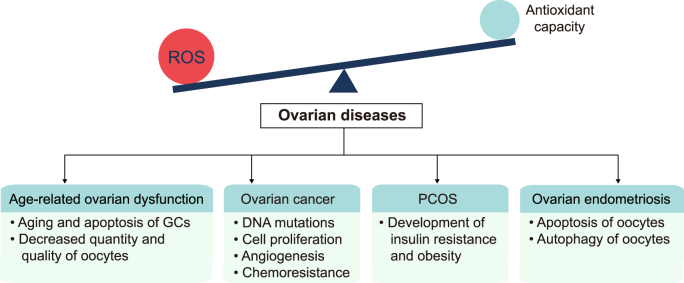
ROS are essential for ovarian physiological function; however, excessive ROS levels can induce the development of ovarian diseases (Fig. 2). Increased ROS production owing to factors such as aging and drinking, beyond the antioxidant capacity can induce OS and lead to the oxidative damage of lipids, proteins, and DNA [[36], [37], [38]]. Excessive ROS production can directly affect the target of signaling pathways and also induce abnormal results by interacting with intermediate reaction steps as second messengers [39]. This oxidative damage and the abnormal signaling pathways finally manifest in age-related ovarian dysfunction, ovarian cancer, PCOS, and ovarian endometriosis.
Fig. 2.
The imbalance between ROS generation and the antioxidant defense system contributes to ovarian diseases, including age-related ovarian dysfunction, ovarian cancer, PCOS, and ovarian endometriosis.
ROS, reactive oxygen species; GCs, granulosa cells; PCOS, polycystic ovary syndrome.
4.1. ROS and age-related ovarian dysfunction
The delayed childbearing age of modern women can lead to the development of age-related ovarian dysfunction and the simultaneous failure of pregnancy aspirations. OS is a critical factor in the decline of ovarian function with age, driving the ovarian aging process [40]. Ovarian aging is manifested by a progressive decrease in the quantity and quality of oocytes [[41], [42], [43]]. The occurrence of intracellular OS-induced damage is attributed to the downregulation of ovarian enzymatic antioxidant defenses and increased ROS accumulation. Antioxidant enzymes in follicular fluid, GCs, and cumulus cells play a key role in protecting oocytes from OS [40,44]. However, compared to younger women, the older women show reduced activity of enzymatic antioxidants such as CAT and glutathione transferase in follicular fluid [45], decreased mRNA and protein levels of antioxidants in GCs, and decreased SOD activity and SOD1 levels in the cumulus cells [40]. These overall decreases in age-related ROS scavenging capacity contribute to OS development. In addition, aging, smoking, high-sugar diet, stress, superovulation, chemotherapy, pollution, and inflammation contribute to the overproduction of ROS, resulting in an imbalance between ROS and the antioxidant system [8,46].
The increased ROS promote ovarian aging through various signaling pathways. Ovarian GCs play a crucial role in the maturation of oocytes and maintenance of the ovarian cycle through the secretion of steroid hormones, estradiol and progesterone [47]. ROS can reduce isocitrate dehydrogenase-1 expression through activation of the mitogen-activated protein kinase signaling pathway to inhibit GCs proliferation and accelerate their aging [48]. Furthermore, H2O2 can cause GCs oxidative damage and apoptosis via the hypoxia-inducible factor-1α (HIF-1α)-vascular endothelial growth factor (VEGF) signaling pathway [49]. The formation of these oxidative damage signaling pathways promotes ovarian aging, while the absence or impairment of protective pathways also accelerates ovarian aging. The nuclear factor erythroid 2-related factor 2 (Nrf2) and sirtuins (SIRT) signaling pathways are involved in the synthesis of antioxidant enzymes and the regulation of OS; their absence or impairment can lead to ovarian OS [8]. OS leads to oxidative damage to lipids, proteins, and DNA, as well as chromosomal abnormalities and telomere shortening, ultimately leading to a decrease in the quantity and quality of oocytes [21,50]. There are currently no clinical treatments for age-related ovarian dysfunction and the application of antioxidant therapy may be an effective strategy.
4.2. ROS and ovarian cancer
Ovarian cancer is a common gynecological tumor with the highest mortality rate among gynecological malignancies [51]. ROS are active contributors to the development, progression, and metastasis of ovarian cancer [52]. Excess ROS in the body induce DNA mutations and pro-oncogenic signaling pathways through complex biochemical reactions promoting tumor formation [53]. Tumor cells produce higher levels of ROS than normal cells mainly through mitochondria and NOX [54]. These increased ROS act as signaling molecules, thereby contributing to cancer progression and metastasis through several mechanisms.
ROS activate the extracellular signal-related kinase 1 and 2 signaling pathways in ovarian cancer cells to promote cell proliferation [55]. Angiogenesis, which transports nutrients and removes metabolic waste, is essential for the survival and development of tumor cells and plays a vital role in tumor metastasis [56]. H2O2 production in ovarian cancer cells promotes tumor angiogenesis by activating the protein kinase B (AKT) and p70S6K1 to regulate the expression of HIF-1α and VEGF [57,58]. Additionally, ROS are strongly associated with the occurrence of chemoresistance in ovarian cancer. Under normal conditions, Nrf2 binds to the Kelch Like ECH Associated Protein 1 (KEAP1)/Cullin 3/RING box protein 1 E3-ubiquitin ligase complex that targets Nrf2 for proteasomal degradation. However, under OS conditions, ROS binds to KEAP1, inducing its conformational change that leads to Nrf2 nuclear translocation and increased expression of antioxidant enzymes; this is one of the most critical mechanisms for developing chemoresistance, especially for platinum drugs [24,59]. Notably, ROS production also induces apoptotic signaling in cancer cells when the cytotoxicity threshold is exceeded [38]. The above study illustrated that ROS are also a double-edged sword for ovarian tumor cells, as low levels of ROS can promote tumor cell proliferation and development, whereas excessive ROS can induce apoptosis. This suggests that eliminating or enhancing ROS production may be a potentially effective therapeutic approach for ovarian cancer.
4.3. ROS and PCOS
PCOS is one of the most common complex endocrine disorders and a major cause of anovulatory infertility [60]. PCOS is often associated with insulin resistance (IR), hyperandrogenemia, and obesity, and its pathogenesis remains poorly elucidated, but it is known that ROS play an essential role [[61], [62], [63]]. Patients with PCOS exhibit more robust OS than that in healthy people [64,65], which can be attributed to several reasons. IR is closely associated with hyperandrogenemia, obesity, and ovulatory dysfunction and is a central factor in PCOS pathogenesis [66]. Hyperglycemia and IR are critical factors for increased OS in PCOS patients [67]. Hyperglycemia in IR patients leads to increased ROS production via the p47(phox) component of the NADPH oxidase [68,69]. In addition, hyperglycemia promotes the release of tumor necrosis factor-α, a known mediator of IR, from mononuclear cells and increases the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [70,71]. NF-κB further contributes to OS by activating NADPH oxidase, promoting ROS production and sustaining inflammatory response [63]. Hyperandrogenemia sensitizes leukocytes to hyperglycemia and contributes to OS [72]. NADPH oxidase also plays a vital role in ROS production in obese patients. In adipocytes, elevated fatty acid levels increase OS through NADPH oxidase activation [73]. Conversely, ROS production can be reduced by inhibiting NADPH oxidase, which can improve symptoms of diabetes, hyperlipidemia, and hepatic steatosis [73].
The increased ROS through these mechanisms can promote the development of PCOS. OS reduces the sensitivity of skeletal muscle to insulin, resulting in the development of IR [67]. Additionally, OS impairs glucose uptake in muscle and adipose tissue and induces pancreatic β-cell dysfunction [68,74]. Elevated OS exacerbates PCOS progression by increasing the size of mature adipocyte, inducing obesity-promoting preadipocyte proliferation and adipocyte differentiation [74]. These associations suggest that ROS play a crucial role in the pathogenesis of PCOS, thereby providing a novel perspective into the treatment of PCOS patients: the inhibition of ROS production through antioxidant therapy to alleviate PCOS symptoms.
4.4. ROS and ovarian endometriosis
Ovarian endometriosis is the most common lesion of endometriosis, which causes not only severe menstrual pain but also infertility [75]. The monthly conception rate in healthy women is 15–20% compared with 2–10% in women with endometriosis [76]. OS plays a vital role in endometriosis-related infertility [77]. In patients with ovarian endometriosis, endometriotic tissue causes increased expression of OS markers in the surrounding normal ovarian cortex, suggesting that OS was occurring [78]. This result is consistent with the following reports: most OS-related genes are upregulated in endometriotic oocytes compared with those from healthy donors [79]; a possible explanation for this is that the cysts stimulate an ovarian inflammatory response or that components of the cysts (e.g. iron) diffuse into the surrounding tissue, leading to ROS production [80]. The previous section described the critical role of ROS in various physiological stages of the oocytes; however, these supraphysiological ROS levels negatively impact oocytes and fertilization outcomes [81,82]. Excessive ROS induce apoptosis and autophagy in oocytes [83,84], which may at least partly explain the poor oocyte quality and infertility associated with ovarian endometriosis. Endometriosis-related infertility might result from a combination of factors [77], suggesting that a single model is insufficient to describe its pathogenesis. However, treatment targeting OS-induced infertility mechanisms might improve the associated symptoms.
5. Antioxidant therapeutic strategies in ovarian diseases
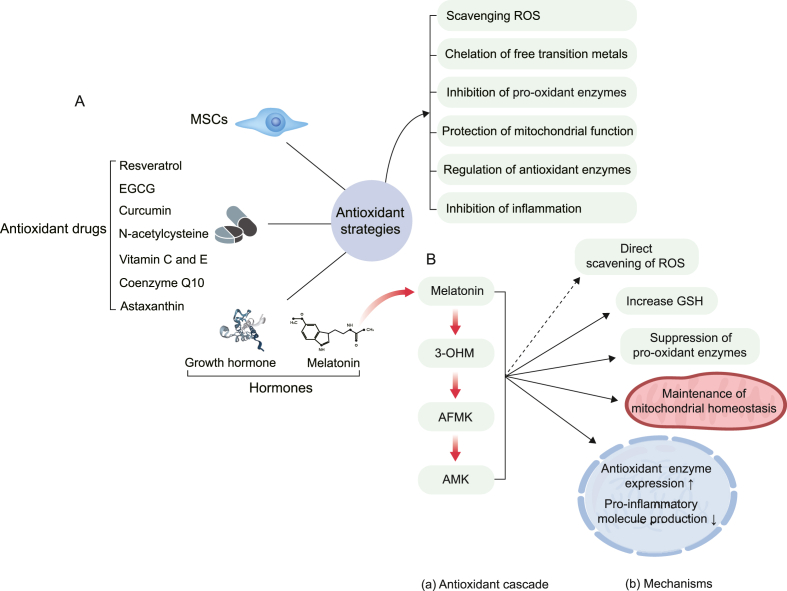
ROS levels are closely associated with the development of several ovarian diseases. Concordantly, we can treat ovarian diseases by modulating the levels of ovarian ROS. In contrast to conventional approaches, antioxidant therapy efficiently interferes with disease development directly at the signaling mechanism. The following sections describe the action mechanism of representative antioxidant strategies and recent studies on treating ovarian diseases using antioxidant therapy, including antioxidant drugs, hormones, and MSCs (Fig. 3A).
Fig. 3.
(A) Classification of antioxidant strategies and their antioxidant mechanisms. (B) Melatonin's antioxidant cascade and mechanisms of protection against oxidative damage. We have listed only well-studied melatonin metabolites that have significant antioxidant effects. The direct and indirect antioxidant effects of melatonin and its metabolites are presented with dashed and solid arrows, respectively.
EGCG, epigallocatechin-3-gallate; MSCs, mesenchymal stem cells; ROS, reactive oxygen species; 3-OHM, cyclic 3-hydroxymelatonin; AFMK, N(1)-acetyl-N(2)-formyl-5-methoxykynuramine; AMK, N(1)-acetyl-5-methoxykynuramine; GSH, glutathione.
5.1. Antioxidant drugs
Antioxidant drugs have been widely studied owing to their high safety profile in humans and low threshold for use. They are used to treat ovarian diseases by scavenging excess ROS through various pathways and have undergone animal and human trials (Table 1). In recent years, developments in materials science have further advanced the use and efficacy of antioxidant drugs; notably, combining some antioxidant drugs and materials has demonstrated promising results.
Table 1.
Testing of antioxidant drugs in animals and humans.
| Drugs | Diseases | Test subjects | Therapeutic effects | Mechanism | References |
|---|---|---|---|---|---|
| Resveratrol | Ovarian aging | Mice | Relieves oogonial stem cells loss | Reduces oxidative levels in ovaries; reduces H2O2-induced cytotoxicity and OS injury by activating Nrf2 and SOD2 | [156] |
| Resveratrol | Age-related infertility | Mice | Retains the capacity to reproduce; increases the quantity and quality of oocytes | Increases telomerase activity and telomere length | [89] |
| Resveratrol | POF | Mice | Increases survival of FGSCs and follicle number; improves the ovarian function | Relieves OS and inflammation; increases SOD, GPx, and CAT | [90] |
| Resveratrol | PCOS | Rats | Improves insulin resistance | Reduces MDA and lipid peroxidation; increases TAC | [91] |
| Resveratrol and metformin | PCOS | Rats | Improves weight gain, hormone profile, and ovarian follicular cell architecture | Induces antioxidant and antiinflammatory systems via SIRT1 and AMPK activation | [157] |
| Resveratrol | PCOS | Human | Decreases pro-inflammatory and endoplasmic reticulum stress markers | The suppression of NF-κB and NF-κB-regulated gene products; alters the expression of genes involved in UPR process | [92] |
| Resveratrol | PCOS | Human | Improves menstrual cyclicity and hair loss | Not available | [93] |
| Green tea extract | PCOS | Rat | Reduces insulin resistance index; improves the symptoms of PCOS | Not available | [99] |
| EGCG | Endometriosis | Mice | Inhibits the development of endometriosis and angiogenesis | Reduces mRNA for VEGF-A | [100] |
| EGCG | Endometriosis | Mice | Inhibits angiogenesis | Suppresses VEGF-C/VEGFR2 expression | [101] |
| Green tea | PCOS | Human | Reduces weight, fasting insulin, and the level of free testosterone | Not available | [158] |
| Curcumin | Ovarian aging | Mice | Delays ovarian aging | Reduces oxidative stress; increases AMH and estrogen; decreases FSH | [109] |
| Curcumin | PCOS | Human | Reduces OS-related complications in patients with PCOS | Increases gene expression of PGC1α and activity of the GPx enzyme | [110] |
| NAC | Ovarian aging | Mice | Improves the quality of oocytes and early embryo development | Increases expression of SIRT, telomerase activity, and telomere length | [117] |
| NAC | Ischemia-reperfusion injury in xenotransplanted human ovarian tissue | Mice | Reduces the loss of ovarian follicles; increases follicle density | Increases expression of SOD1, heme oxygenase-1, and CAT; anti-inflammatory, and antiapoptotic effects | [118] |
| NAC | Ovarian endometriosis | Human | Reduces cyst mean diameter | Not available | [119] |
| NAC | Infertility | Human | Increases the number of high-quality blastocysts | Increases GSH content in the follicular fluid | [120] |
| VC | PCOS | Mice | Reduces ovarian weight; improves cysts in the ovaries and congestion in the uterus | Increases TAC and expression of antioxidant enzyme genes; reduces MDA | [123] |
| VC | Ovarian aging | Mice | Restores ovarian follicular reservation | Not available | [159] |
| Selenium and VE | OPOI | Human | Increases AMH, antral follicle count, and mean ovarian volume | Not available | [125] |
| CoQ10 | PCOS | Human | Improves glucose and lipid metabolism | Not available | [127] |
| Astaxanthin | Ovarian damage | Rats | Reduces histopathological ovarian damage | Increases TAC and GSH; reduces MDA | [160] |
| Astaxanthin | PCOS | Human | Increases the MII oocyte and high-quality embryo rate | Increases levels of serum CAT, TAC, and expression of Nrf2 | [129] |
Abbreviations: OS, oxidative stress; Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; POF, premature ovarian failure; GPx, glutathione peroxidase; CAT, catalase; PCOS, polycystic ovary syndrome; MDA, malondialdehyde; TAC, total antioxidant capacity; FGSCs, female germline stem cells; SIRT, sirtuins; AMPK, AMP-dependent protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; UPR, unfolding protein response; EGCG, epigallocatechin-3-gallate; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; PGC1α, peroxisome proliferator activated receptor gamma coactivator 1α; NAC, N-acetylcysteine; GSH, glutathione; VC, vitamin C; VE, vitamin E; OPOI, occult premature ovarian insufficiency; CoQ10, coenzyme Q10.
5.1.1. Resveratrol
Resveratrol is a plant-derived polyphenol with antioxidant and anticancer effects [85]. The antioxidant activity of resveratrol is related to the promotion of antioxidant enzyme synthesis and the involvement of its hydroxyl group in ROS scavenging, as well as to the reduction of copper-mediated oxidation and the prevention of low-density lipoproteins and cell membrane lipid peroxidation [86]. In addition, resveratrol can also protect mitochondrial function by activating the SIRT1 and SIRT3 signaling pathways and reduce oxidative damage by regulating mitochondrial ROS homeostasis in vivo [87,88].
The antioxidant properties of resveratrol have been demonstrated in animals. Mice fed resveratrol for 12 months exhibited improved reproductive capacity, follicular pools, telomerase activity, and telomere length compared with that in control mice, and these results highlight the role of resveratrol in delaying ovarian aging [89]. In another study, researchers lavaged premature ovarian failure (POF) model mice with resveratrol. They observed elevated levels of GPx, SOD2, and CAT, and a significant increase in ovarian weight as well as follicle number in POF mice, suggesting that resveratrol is a potential anti-POF agent [90]. Resveratrol can also be administered by intraperitoneal injection. Intraperitoneal injection of resveratrol reduced serum malondialdehyde (MDA, a major biomarker for the assessment of lipid peroxidation) levels and IR, increased serum total antioxidant capacity (TAC), and alleviated complications in PCOS mice [91]. In human-controlled trials, the resveratrol-treated PCOS group had reduced pro-inflammatory and ER stress markers [92], and improved menstrual cyclicity [93], suggesting that resveratrol might reduce complications in PCOS patients. In addition to these antioxidant effects, resveratrol might also inhibit human ovarian cancer progression and angiogenesis by inhibiting HIF-1α and VEGF expression, providing a new potential therapy for the treatment of ovarian cancer [94].
Notably, resveratrol is a low water-soluble drug, which limits its efficacy [95]. The therapeutic efficiency of resveratrol in hamster ovaries may be improved using synthesizing phosphate monoester anionic surfactant-mesoporous silica nanoparticles as drug carriers [96], thereby expanding the application of the drug. These findings suggest that resveratrol is a promising antioxidant with potential clinical applications.
5.1.2. Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is the most physiologically active catechin in green tea, containing potent antioxidant properties [97]. EGCG acts as an antioxidant through several mechanisms: chelation of free transition metals to reduce ROS production, scavenging of ROS by groups on EGCG, inhibition of inducible nitric oxide synthase, inhibition of pro-oxidant enzyme production, activation of antioxidant enzymes, and inhibition of inflammation [98]. Green tea extract improved IR and increased follicle number in a rat model of PCOS [99], demonstrating the role of EGCG in the ovary. Angiogenesis is widely believed to play a crucial role in the development of endometriosis lesions. Levels of VEGF A and C mRNA decreased after EGCG therapy, which suggested that EGCG inhibited angiogenesis to reduce endometriosis damage [100,101]. However, EGCG is easily oxidized in neutral or alkaline environments, and its bioavailability in humans is low [102].
Research is currently focused on improving the bioavailability of EGCG. Combining poly(acrylic acid) and EGCG to form poly(acrylic acid)-EGCG conjugates, produces nanoparticles with stronger oxidative stability and ROS scavenging ability than natural EGCG [103]. EGCG also has potent anticancer potential [104], and its combination with materials for cancer treatment is gaining research interest. Nanogels consisting of hyaluronic acid-EGCG conjugate, the cytotoxic protein Granzyme B (GzmB), and linear polyethyleneimine could be used for intracellular delivery of GzmB in targeted cancer therapy [105]. In another study, researchers mixed hyaluronic acid-EGCG conjugate with cisplatin to form micellar nanocomplexes with a spherical core-shell structure, in which the antioxidant effect of the EGCG molecules protected non-target organs from cisplatin-induced OS [106]. These studies demonstrate the promising potential of EGCG for treating ovarian diseases.
5.1.3. Curcumin
Curcumin is a phenolic compound of plant origin and a natural antioxidant [107]. The antioxidant properties of curcumin are due to the direct involvement of its functional group in the scavenging of ROS and the upregulation of gene expression of the antioxidant enzymes SOD, CAT, GR, and GPx through the Nrf2 pathway [108]. Curcumin can reduce OS to postpone ovarian aging in mice [109] and improve complications in PCOS patients [110]. In addition, curcumin reportedly exerts anticancer properties through multiple biological pathways involved in cancer apoptosis, progression, and metastasis [111]. Curcumin is lipophilic and can freely cross cell membranes, meanwhile, it has a substantially low water solubility, resulting in considerably low bioavailability [112]. Therefore, improving the bioavailability of curcumin for cancer treatment has attracted research interest. Nanocarriers such as polymeric micelles, nanoparticles, liposomes, conjugates, and peptide carriers have been developed to improve the bioavailability of curcumin [112]. Encapsulation of curcumin in poly(lactic-co-glycolide) nanoparticles by nano-precipitation techniques can effectively enhance the bioavailability and pharmacokinetics of curcumin, thereby improving drug uptake and retention in A2780CP ovarian cancer cells (a cisplatin-resistant/metastatic ovarian cancer cell line) [113]. Super-paramagnetic iron oxide nanoparticles loaded with curcumin reduce degradation during drug transport, increase bioavailability, and reduce ovarian damage and apoptosis in PCOS [114]. These studies highlight the potential therapeutic value of curcumin in ovarian diseases.
5.1.4. N-acetylcysteine
N-acetylcysteine (NAC) is a potent antioxidant. It can act as a precursor to increasing levels of GSH in vivo, which act as a direct antioxidant and a substrate for several antioxidant enzymes [115,116]. NAC-fed mice have higher quality oocytes and telomerase activity as well as longer telomere length, demonstrating that it can delay the onset of ovarian aging [117]. NAC increased the expression of antioxidant enzymes such as SOD1 and CAT and decreased pro-inflammatory cytokines and apoptotic factors, suggesting that NAC can treat OS injury in mice transplanted with human ovarian tissue [118]. The therapeutic effect of NAC has also been demonstrated in controlled clinical trials. In patients with endometriosis, NAC reduced the mean diameter of ovarian cysts compared to the control group [119]. NAC treatment increased GSH levels in follicular fluid and improved blastocyst quality in older women [120]. In addition, a systematic evaluation and meta-analysis revealed that NAC significantly improved pregnancy and ovulation rates in patients with PCOS [121], suggesting its potential as an antioxidant in treating ovarian diseases.
5.1.5. Other antioxidant drugs
In addition to the antioxidant drugs mentioned in the earlier sections, VC, VE, coenzyme Q10 (CoQ10), and astaxanthin are used in ovarian disease treatment. VC is a water-soluble natural antioxidant [122]. In mice, VC increased TAC and alleviated OS damage in PCOS by upregulating the expression of antioxidant enzymes, suggesting a possible use in treatment [123]; VE is a fat-soluble antioxidant that protects tissues from lipid peroxidation [124]. Co-administration of VE and selenium alleviated ovarian OS, increased the anti-Müllerian hormone index and antral follicle count in women with occult premature ovarian insufficiency, and partially restored ovarian function [125]; CoQ10 is a potent lipophilic antioxidant [126]. In controlled clinical trials, glucose and lipid metabolism were improved in PCOS patients in the CoQ10 treatment group, demonstrating a beneficial effect [127]; Astaxanthin is a carotenoid with unusual antioxidant activity [128]. In a controlled human trial, astaxanthin supplementation increased serum CAT and TAC levels in PCOS patients, while increasing high-quality embryo rates [129].
5.2. Hormones
Over the last two decades, hormone replacement therapy (HRT) has gained much attention as a new strategy to reduce OS and treat ovarian diseases. The main hormones used as antioxidants include melatonin and growth hormone, with melatonin being the most interesting to researchers.
5.2.1. Melatonin
Melatonin is an endogenous radical scavenger, synthesized and secreted mainly by the pineal gland and distributed in various tissues [130]. Melatonin can reduce OS by scavenging ROS in several ways (Fig. 3B). Melatonin can be directly involved in ROS scavenging through electron donation, and its derivatives also have an antioxidant capacity [131]. In addition, melatonin metabolites such as N(1)-acetyl-N(2)-formyl-5-methoxykynuramine and N(1)-acetyl-5-methoxykynuramine also have a strong ROS scavenging capacity and form an antioxidant cascade with melatonin to maintain redox homeostasis [131,132]. In addition to its direct involvement in the scavenging of ROS, melatonin also enhances the cellular antioxidant defense system in several indirect ways (Table 2). These mechanisms of action provide a theoretical basis for using melatonin as an antioxidant.
Table 2.
Melatonin indirectly enhances the antioxidant defense system of cells.
| Test subjects | Antioxidant methods | Mechanism | References |
|---|---|---|---|
| ECV304 cell line | Increases antioxidant GSH | Increases the expression of gamma-glutamylcysteine synthetase | [161] |
| Brain homogenates from rats | Suppresses pro-oxidant enzymes | Reduces the activity of pro-oxidant enzymes such as XO and MPO | [162] |
| Cells from mice | Anti-inflammatory | Exerts anti-inflammatory effect via the ERK/Nrf2/HO-1 signaling | [163] |
| Oocytes from mice | Increases antioxidant enzyme | Increase the expression of GPx1/SOD1 | [164] |
| Mice | Increases antioxidant enzyme | Enhances the SIRT3 activity; increases the binding affinity of FoxO3a to the promoters of both SOD2 and CAT | [165] |
| Mice | Increases antioxidant enzyme | Increases in total antioxidative capacity and SOD level via MT1/AMPK pathway | [166] |
| Rats | Maintenance of mitochondrial homeostasis | Increases the activity of the respiratory chain complexes I and IV; reduces electron leakage and ROS generation | [167] |
Abbreviations: ECV304 cell line, human umbilical vascular endothelial cell line; GSH, glutathione; XO, xanthine oxidase; MPO, myeloperoxidase; ERK, extracellular regulated kinase; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; GPx1, glutathione peroxidase 1; SOD, superoxide dismutase; FoxO3a, forkhead box O 3a; CAT, catalase; MT1, melatonin membrane receptor 1; AMPK, AMP-dependent protein kinase; ROS, reactive oxygen species.
In addition to mechanistic studies, melatonin has also demonstrated excellent performance in trials of ovarian diseases (Table 3). Telomere length, fertilization, blastocyst rates, and follicle numbers were increased in mice, with melatonin supplementation, demonstrating that melatonin delays ovarian aging and fertility decline [133]. Clinical studies have indicated that melatonin supplementation in patients with infertility significantly increased the TAC of follicular fluid and rebalanced the oxidative status of the follicles, thereby improving oocyte quality and increasing the conception rate [134]. In an observational clinical study, PCOS patients supplemented with melatonin had improved menstrual cycles and hyperandrogenism [135] and increased conception rates after intrauterine insemination [136], demonstrating its potential clinical value. In addition, melatonin has a potential therapeutic effect on ovarian cancer [137]; melatonin can suppress ovarian cancer cell progression and metastasis through the norepinephrine/AKT/β-catenin/SLUG axis [138]. These molecular mechanisms and trials suggested that melatonin has considerable potential as an antioxidant for ovarian disease treatment.
Table 3.
Clinical and animal studies on the antioxidant role of melatonin in ovarian diseases.
| Diseases | Test subjects | Dose | Therapeutic effects | Mechanism | References |
|---|---|---|---|---|---|
| Ovarian aging | Mice | 100 μg/mL water | Increases fertilization rate, blastocyst rate, and telomere length; delays ovarian aging | Reduces autophagy; maintains telomeres; stimulates SIRT expression and ribosome function; antioxidant action | [133] |
| Ovarian aging | Mice | 10 mg/kg | Delays ovarian aging; improves age-induced fertility decline | Reduces mitochondrial ROS; increases SIRT3 activity; increases SOD2 and CAT level | [165] |
| Ovarian cancer | Rat | 200 μg/100 g/d | Reduces angiogenesis | Increases serum levels of melatonin; reduces TGFβ1, VEGF, and levels of VEGF receptor | [168] |
| PCOS | Human | 2 mg/d | Restores menstrual cyclicity | Reduces androgens and AMH levels; increases FSH levels | [135] |
| PCOS | Human | 3 mg/d | Improves the rate of chemical pregnancy | Not available | [136] |
| PCOS | Human | 6 mg/d | Improves hirsutism | Reduces serum TNF-α; increases TAC levels | [169] |
| Infertility | Human | 3 mg/d | Improves oocyte quality and fertilization rates | Protects oocytes from OS | [81] |
| Infertility | Human | 3 mg/d | Increases fertilization rate | Increases intra-follicular melatonin concentrations; reduces intra-follicular oxidative damage | [170] |
| Infertility | Human | 3 or 6 mg/day | Improves oocyte quality | Increases SOD activity and TAC as well as intrafollicular concentrations of melatonin; improves intrafollicular oxidative balance | [134] |
| Infertility | Human | 3 mg/d | Improves oocyte quality and ART outcomes | Down-regulated genes associated with cell death and OS; up-regulated genes associated with angiogenesis and steroidogenesis | [171] |
Abbreviations: SIRT, sirtuins; ROS, reactive oxygen species; SOD, superoxide dismutase; CAT, catalase; TGFβ1, transforming growth factor-beta1; VEGF, vascular endothelial growth factor; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; PCOS, polycystic ovary syndrome; TNF-α, tumor necrosis factor-α; TAC, total antioxidant capacity; OS, oxidative stress; ART, assisted reproductive technology.
5.2.2. Growth hormone
Growth hormone has been a familiar growth regulator for over a century. In recent years, growth hormone has gained interest as an antioxidant in treating patients with PCOS and poor ovarian response (POR). In PCOS patients, growth hormone can reduce OS damage by inhibiting ROS accumulation and apoptosis in GCs by activating the phosphatidylinositol 3-kinase/AKT signaling pathway [139]; it can also improve GCs mitochondrial function and reduce OS improving oocyte quality [140]. In POR patients, growth hormone improves TAC levels in follicular fluid and reduces intracellular ROS levels, improving oocyte quality and the in vitro fertilization outcomes [141]. Furthermore, in addition to treating ovarian diseases, growth hormone can reduce cisplatin-induced apoptosis in ovarian GCs by alleviating OS and enhancing mitochondrial function through the SIRT-SOD2 pathway [142]. Currently, there is a lack of sufficient mechanistic and clinical studies on using growth hormone for antioxidant therapy in the ovary; nonetheless, the findings from the existing studies exhibit promise.
5.3. MSCs: a novel antioxidant strategy
MSCs are multipotent stem cells with the capacity to self-renew [143,144]. Their primary source is the bone marrow, and they can differentiate into various cell types that make up mesenchymal tissues [144]. MSCs were initially used for tissue repair and regeneration, but there is now increasing interest in their antioxidant therapy. MSCs can exert antioxidant effects in several ways: MSCs can directly exhibit antioxidant properties by secreting antioxidant enzymes to scavenge free radicals [145,146]; they can also indirectly upregulate antioxidant defenses in other cells [147,148]. Notably, this indirect antioxidant capacity is non-dose dependent; low and high doses had similar effects on antioxidant and pro-oxidant enzyme mRNA expression, suggesting that both have similar antioxidant effects [147]. In addition, MSCs can improve the regulation of inflammatory responses, enhance cellular respiration and mitochondrial function, or donate their mitochondria to exert antioxidant action [149]. MSCs reduce OS through these mechanisms, partially explaining the therapeutic role of MSCs in various disease models.
In addition to mechanistic studies, the antioxidant properties of MSCs have been demonstrated in various ovarian disease models. Transplanted MSCs restored ovarian function in an ovariectomized rat model by reducing oxidative damage through the upregulated expression of antioxidant factors [148]. MSCs increased serum SOD activity in autologously transplanted ovarian tissue, thereby reducing ischemia-reperfusion-induced OS damage, which improved ovarian structure and function in mice [150]. Exosomal miRNA-17–5p from MSCs attenuated ROS accumulation by inhibiting SIRT7 expression, thereby improving ovarian function in premature ovarian insufficiency [151]. The mechanisms by which increased ROS production leads to the development of PCOS are described in the earlier sections. MSCs increase ovarian TAC levels and decrease MDA levels, which improves folliculogenesis in PCOS mice [152]. These findings provide novel insights into the potential of MSCs and offer new avenues for developing more effective therapies for ovarian diseases.
6. Practical challenges in the clinical application of antioxidant strategies
While the preclinical studies describe the potential value of antioxidant strategies in treating ovarian diseases, these novel strategies also imply new issues that need to be addressed. Practical challenges remain in the translation of these antioxidant strategies into clinical application.
First, although antioxidant strategies have demonstrated therapeutic promise in preclinical research, clinical trial outcomes have often been disappointing. One of the primary reasons for this is that the therapeutic potency of antioxidant strategies is restricted by the extent to which OS plays a role in pathology [20]. Therefore, it is necessary to determine whether OS plays a key role in ovarian disease development before applying antioxidant strategies. Second, there are limitations to the antioxidant strategies. Although significant progress has been made in implementing antioxidant drugs in recent years, issues remain, including the low bioavailability and weak redox-modulating ability of traditional drugs. How to overcome biocompatibility and evaluate the therapeutic effect of antioxidant drugs when combined with others materials is an issue that needs to be addressed before clinical application. The role of antioxidants in combination with others materials in improving ovarian function is mostly based on animal studies; however, animal models differ from humans in biology, physiology, and immunology, which may lead to an unsuccessful transition from animal to human therapy. Notably, using some natural or synthetic compounds could efficiently protect normal cells from OS-induced diseases, such as ovarian cancer. Still, their use in cancer patients may reduce the efficiency of chemotherapy because the primary target of these drugs is the activation of Nrf2/KEAP1 signaling, which is a critical factor involved in the occurrence of chemoresistance [24,59]. It is necessary to develop new strategies to address the conflicting effects of antioxidant drugs. HRT has been extensively studied as an essential part of the antioxidant strategy, but some controversy remains. Melatonin is associated with an increased risk of daytime sleepiness and headache [153]. Growth hormone is associated with the progression of several cancers [154]. After entering the body, these hormones act on non-target organs, causing side effects that pose a challenge to their clinical application. Furthermore, there are several outstanding issues regarding the application of MSCs, and the heterogeneity, immunocompatibility, stability, differentiation, and migratory capacity of MSCs may all be barriers to clinical translation [155].
Collectively, antioxidant therapy should be performed with safety as a priority. As the organ that exercises fertility, the ovary affects the health of women as well as the safety of future generations. Therefore, strategies for treating ovarian diseases require long-term scientific research before their clinical application. We believe that scientific and technological advancements will provide theoretical support for implementing such antioxidant strategies.
7. Conclusions and perspective
In conclusion, we have comprehensively reviewed the generation, homeostasis, and physiological role of ROS in the ovary, as well as highlighted its role in ovarian disease pathogenesis and the advances in antioxidant treatment. Biologists and chemists have long explored the generation, homeostasis, physiology, pathology, and scavenging strategies of ovarian ROS, leading to a clearer understanding of the antioxidant therapy for ovarian diseases such as age-related ovarian dysfunction, ovarian cancer, PCOS, and ovarian endometriosis. Some traditional antioxidant drugs have been combined with materials to break the boundaries of antioxidant therapy. This “new use of old drugs” has demonstrated promise in treating various ovarian diseases, particularly ovarian cancer. As the antioxidant mechanisms of hormones and MSCs become clearer, their clinical application is becoming more promising. In short, we believe that further substantial developments in these areas will significantly contribute to the treatment of ovarian diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
JL, DL, and ZN contributed to the manuscript writing. JL, YG, ZF, and BZ conducted the image production and manuscript editing. DL and ZN reviewed and supervised the manuscript. All authors have contributed to the manuscript and approved the submitted version.
Funding
National Natural Science Foundation of China (No. 82071607); LiaoNing Revitalization Talents Program (No. XLYC1907071); Fok Ying Tung Education Foundation (No. 151039); Key Research and Development Program of Liaoning Province (No. 2018225062); Outstanding Scientific Fund of Shengjing Hospital (No. 202003).
Contributor Information
Zhijing Na, Email: sharon_na@foxmail.com.
Da Li, Email: leeda@ymail.com.
Abbreviations
- AKT
protein kinase B
- AMH
anti-Müllerian hormone
- AMPK
AMP-dependent protein kinase
- ART
assisted reproductive technology
- CAT
catalase
- CoQ10
coenzyme Q10
- ECV304 cell line
human umbilical vascular endothelial cell line
- ER
endoplasmic reticulum
- ERK
extracellular regulated kinase
- ERO1
endoplasmic reticulum oxidoreductin 1
- FGSCs
female germline stem cells
- FoxO3a
forkhead box O 3a
- FSH
follicle-stimulating hormone
- GCs
granulosa cells
- GSH
glutathione
- GSSG
oxidized glutathione
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GzmB
granzyme B
- HIF-1α
hypoxia-inducible factor-1α
- H2O2
hydrogen peroxide
- HO-1
heme oxygenase-1
- HRT
hormone replacement therapy
- IR
insulin resistance
- KEAP1
kelch Like ECH Associated Protein 1
- MDA
malondialdehyde
- MPO
myeloperoxidase
- MSCs
mesenchymal stem cells
- MT1
melatonin membrane receptor 1
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- 1O2
singlet oxygen
- O2•−
superoxide anion
- •OH
hydroxyl radical
- OPOI
occult premature ovarian insufficiency
- OS
oxidative stress
- PCOS
polycystic ovary syndrome
- PDI
protein disulfide isomerase
- PGC1α
peroxisome proliferator activated receptor gamma coactivator 1α
- POF
premature ovarian failure
- POR
poor ovarian response
- ROS
reactive oxygen species
- SIRT
sirtuins
- SOD1
Cu–Zn superoxide dismutase
- SOD2
mitochondrial Mn superoxide dismutase
- SOD3
superoxide dismutase 3
- TAC
total antioxidant capacity
- TGFβ1
transforming growth factor-beta1
- TNF-α
tumor necrosis factor-α
- UPR
unfolded protein response
- VC
vitamin C
- VE
vitamin E
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- XO
xanthine oxidase
Data availability
No data was used for the research described in the article.
References
- 1.Warburg O. Beobachtungen über Die Oxydationsprozesse Im Seeigelei. 1908;57(1–2):1–16. doi: 10.1515/bchm2.1908.57.1-2.1. [DOI] [Google Scholar]
- 2.Milne G.L. Classifying oxidative stress by F2-isoprostane levels in human disease: the Re-imagining of a biomarker. Redox Biol. 2017;12:897–898. doi: 10.1016/j.redox.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juan C.A., Pérez de la Lastra J.M., Plou F.J., Pérez-Lebeña E. The chemistry of reactive oxygen species (Ros) Revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021;(9):22. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrman H.R., Kodaman P.H., Preston S.L., Gao S. Oxidative stress and the ovary. J. Soc. Gynecol. Invest. 2001;8(1 Suppl Proceedings):S40–S42. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 6.Shkolnik K., Tadmor A., Ben-Dor S., Nevo N., Galiani D., Dekel N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. U. S. A. 2011;108(4):1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takiguchi S., Sugino N., Kashida S., Yamagata Y., Nakamura Y., Kato H. Rescue of the corpus luteum and an increase in luteal superoxide dismutase expression induced by placental luteotropins in the rat: action of testosterone without conversion to estrogen. Biol. Reprod. 2000;62(2):398–403. doi: 10.1095/biolreprod62.2.398. [DOI] [PubMed] [Google Scholar]
- 8.Yan F., Zhao Q., Li Y., Zheng Z., Kong X., Shu C., et al. The role of oxidative stress in ovarian aging: a review. J. Ovarian Res. 2022;15(1):100. doi: 10.1186/s13048-022-01032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B., Chen Y., Shi J. Reactive oxygen species (Ros)-Based nanomedicine. Chem. Rev. 2019;119(8):4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 11.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128(3):269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 12.Rigoulet M., Yoboue E.D., Devin A. Mitochondrial Ros generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxidants Redox Signal. 2011;14(3):459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.E., Kim J. Ros-scavenging therapeutic hydrogels for modulation of the inflammatory response. ACS Appl. Mater. Interfaces. 2021 doi: 10.1021/acsami.1c18261. Epub 2021/12/29. [DOI] [PubMed] [Google Scholar]
- 14.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marciniak S.J., Chambers J.E., Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022;21(2):115–140. doi: 10.1038/s41573-021-00320-3. [DOI] [PubMed] [Google Scholar]
- 16.Zeeshan H.M.A., Lee G.H., Kim H.-R., Chae H.-J. Endoplasmic reticulum stress and associated Ros. Int. J. Mol. Sci. 2016;17(3):327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senft D., Ronai ZeA. Upr, autophagy, and mitochondria crosstalk underlies the Er stress response. Trends Biochem. Sci. 2015;40(3):141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson A.J., Gill E.K., Abudalo R.A., Edgar K.S., Watson C.J., Grieve D.J. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart. 2018;104(4):293–299. doi: 10.1136/heartjnl-2017-311448. [DOI] [PubMed] [Google Scholar]
- 19.Sárközy M., Kovács Z.Z.A., Kovács M.G., Gáspár R., Szűcs G., Dux L. Mechanisms and modulation of oxidative/nitrative stress in type 4 cardio-renal syndrome and renal Sarcopenia. Front. Physiol. 2018;9:1648. doi: 10.3389/fphys.2018.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forman H.J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20(9):689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki H., Hamatani T., Kamijo S., Iwai M., Kobanawa M., Ogawa S., et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 2019;10:811. doi: 10.3389/fendo.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto K., Sato E.F., Kasahara E., Jikumaru M., Hiramoto K., Tabata H., et al. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010;49(4):674–681. doi: 10.1016/j.freeradbiomed.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Bloomer R.J., Fisher-Wellman K.H. Systemic oxidative stress is increased to a greater degree in young, obese women following consumption of a high fat meal. Oxid. Med. Cell. Longev. 2009;2(1):19–25. doi: 10.4161/oxim.2.1.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tossetta G., Fantone S., Montanari E., Marzioni D., Goteri G. Role of Nrf2 in ovarian cancer. Antioxidants. 2022;11(4) doi: 10.3390/antiox11040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niemann B., Rohrbach S., Miller M.R., Newby D.E., Fuster V., Kovacic J.C. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017;70(2):230–251. doi: 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier T.W., Kable J.A., Burwell L., Coles C.D., Brown L.A.S. Maternal alcohol use during pregnancy causes systemic oxidation of the glutathione redox system. Alcohol Clin. Exp. Res. 2010;34(1):123–130. doi: 10.1111/j.1530-0277.2009.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Y., Zhang X., Xu T., Li X., Zhang H., Wu A., et al. Physiological and biochemical adaptations to high altitude in Tibetan frogs. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.942037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Lu Y., Woods K., Di Trapani G., Tonissen K.F. Investigating the thioredoxin and glutathione systems' response in lymphoma cells after treatment with [Au(D2pype)2]Cl. Antioxidants. 2021;10(1) doi: 10.3390/antiox10010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2011;15(7):1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J., Bao Y., Zhou X., Zheng L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019;17(1):67. doi: 10.1186/s12958-019-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey A.N., Tripathi A., Premkumar K.V., Shrivastav T.G., Chaube S.K. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J. Cell. Biochem. 2010;111(3):521–528. doi: 10.1002/jcb.22736. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T., Sueoka K., Dharmarajan A.M., Atlas S.J., Bulkley G.B., Wallach E.E. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in-vitro perfused rabbit ovary. J. Reprod. Fertil. 1991;91(1):207–212. doi: 10.1530/jrf.0.0910207. [DOI] [PubMed] [Google Scholar]
- 33.Di Renzo G.C., Giardina I., Clerici G., Brillo E., Gerli S. Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Invest. 2016;27(1):35–48. doi: 10.1515/hmbci-2016-0038. [DOI] [PubMed] [Google Scholar]
- 34.Sugino N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005;4(1):31–44. doi: 10.1007/BF03016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson J.C., Sawada M., Boone D.L., Stauffer J.M. Stimulation of progesterone secretion in dispersed cells of rat corpora lutea by antioxidants. Steroids. 1995;60(3):272–276. doi: 10.1016/0039-128x(94)00053-f. [DOI] [PubMed] [Google Scholar]
- 36.Barzilai A., Yamamoto K.-I. DNA damage responses to oxidative stress. DNA Repair. 2004;3(8–9):1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Berlett B.S., Stadtman E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 38.Schieber M., Chandel N.S. Ros function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal A., Gupta S., Sekhon L., Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxidants Redox Signal. 2008;10(8):1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 40.Yang L., Chen Y., Liu Y., Xing Y., Miao C., Zhao Y., et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.617843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M., Guo Y., Wei S., Xue L., Tang W., Chen D., et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnol. 2022;20(1):374. doi: 10.1186/s12951-022-01566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timóteo-Ferreira F., Abreu D., Mendes S., Matos L., Rodrigues A.R., Almeida H., et al. Redox imbalance in age-related ovarian dysfunction and perspectives for its prevention. Ageing Res. Rev. 2021;68 doi: 10.1016/j.arr.2021.101345. [DOI] [PubMed] [Google Scholar]
- 43.Timóteo-Ferreira F., Mendes S., Rocha N.A., Matos L., Rodrigues A.R., Almeida H., et al. Apocynin dietary supplementation delays mouse ovarian ageing. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/5316984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mihalas B.P., Redgrove K.A., McLaughlin E.A., Nixon B. Molecular mechanisms responsible for increased vulnerability of the ageing oocyte to oxidative damage. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbone M.C., Tatone C., Delle Monache S., Marci R., Caserta D., Colonna R., et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol. Hum. Reprod. 2003;9(11):639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Hu C., Ye H., Luo R., Fu X., Li X., et al. Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019;2019 doi: 10.1155/2019/8069898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury I., Thompson W.E., Welch C., Thomas K., Matthews R. Prohibitin (Phb) inhibits apoptosis in rat granulosa cells (Gcs) through the extracellular signal-regulated kinase 1/2 (Erk1/2) and the Bcl family of proteins. Apoptosis. 2013;18(12):1513–1525. doi: 10.1007/s10495-013-0901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Guo Y., Fan Y., Wang Q., Zhang Q., Lai D. Decreased expression of Idh1 by chronic unpredictable stress suppresses proliferation and accelerates senescence of granulosa cells through ros activated mapk signaling pathways. Free Radic. Biol. Med. 2021;169:122–136. doi: 10.1016/j.freeradbiomed.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z., Hong W., Zheng K., Feng J., Hu C., Tan J., et al. Chitosan oligosaccharides alleviate H2o2-stimulated granulosa cell damage via Hif-1α signaling pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/4247042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim J., Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011;84(4):775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellone S., Tassi R., Betti M., English D., Cocco E., Gasparrini S., et al. Mammaglobin B (Scgb2a1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: implications for ovarian cancer immunotherapy. Br. J. Cancer. 2013;109(2):462–471. doi: 10.1038/bjc.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen L., Zhan X. Mitochondrial dysfunction pathway alterations offer potential biomarkers and therapeutic targets for ovarian cancer. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/5634724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 54.Moloney J.N., Cotter T.G. Ros signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Chan D.W., Liu V.W.S., Tsao G.S.W., Yao K.-M., Furukawa T., Chan K.K.L., et al. Loss of Mkp3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis. 2008;29(9):1742–1750. doi: 10.1093/carcin/bgn167. [DOI] [PubMed] [Google Scholar]
- 56.Jiang X., Wang J., Deng X., Xiong F., Zhang S., Gong Z., et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020;39(1):204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L.-Z., Hu X.-W., Xia C., He J., Zhou Q., Shi X., et al. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of Akt and P70s6k1 in human ovarian cancer cells. Free Radic. Biol. Med. 2006;41(10):1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Xia C., Meng Q., Liu L.-Z., Rojanasakul Y., Wang X.-R., Jiang B.-H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67(22):10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 59.Tossetta G., Marzioni D. Natural and synthetic compounds in ovarian cancer: a focus on Nrf2/Keap1 pathway. Pharmacol. Res. 2022;183 doi: 10.1016/j.phrs.2022.106365. [DOI] [PubMed] [Google Scholar]
- 60.Moore A.M., Campbell R.E. Polycystic ovary syndrome: understanding the role of the brain. Front. Neuroendocrinol. 2017;46 doi: 10.1016/j.yfrne.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (Pcos) Hum. Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 62.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/8589318. Epub 2016/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papalou O., Victor V.M., Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr. Pharmaceut. Des. 2016;22(18):2709–2722. doi: 10.2174/1381612822666160216151852. [DOI] [PubMed] [Google Scholar]
- 64.Murri M., Luque-Ramírez M., Insenser M., Ojeda-Ojeda M., Escobar-Morreale H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (Pcos): a systematic review and meta-analysis. Hum. Reprod. Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 65.Hilali N., Vural M., Camuzcuoglu H., Camuzcuoglu A., Aksoy N. Increased prolidase activity and oxidative stress in Pcos. Clin. Endocrinol. 2013;79(1):105–110. doi: 10.1111/cen.12110. [DOI] [PubMed] [Google Scholar]
- 66.Barber T.M. Why are women with polycystic ovary syndrome obese? Br. Med. Bull. 2022;143(1) doi: 10.1093/bmb/ldac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao Q., Zou X., Liu S., Wu H., Shen Q., Kang J. Oxidative stress as a contributor to insulin resistance in the skeletal muscles of mice with polycystic ovary syndrome. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malin S.K., Kirwan J.P., Sia C.L., González F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic Β-cell dysfunction in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014;99(1):322–329. doi: 10.1210/jc.2013-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan D., Oliveira-Emilio H.R., Keane D., Hirata A.E., Santos da Rocha M., Bordin S., et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like nadph oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50(2):359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 70.González F., Minium J., Rote N.S., Kirwan J.P. Hyperglycemia alters tumor necrosis factor-alpha release from mononuclear cells in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90(9):5336–5342. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 71.Lu J., Wang Z., Cao J., Chen Y., Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018;16(1):80. doi: 10.1186/s12958-018-0391-5. Epub 2018/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González F., Nair K.S., Daniels J.K., Basal E., Schimke J.M., Blair H.E. Hyperandrogenism sensitizes leukocytes to hyperglycemia to promote oxidative stress in lean reproductive-age women. J. Clin. Endocrinol. Metab. 2012;97(8):2836–2843. doi: 10.1210/jc.2012-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mancini A., Bruno C., Vergani E., d'Abate C., Giacchi E., Silvestrini A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: controversies and new insights. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayashi S., Nakamura T., Motooka Y., Ito F., Jiang L., Akatsuka S., et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Da Broi M.G., Navarro P.A. Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016;364(1):1–7. doi: 10.1007/s00441-015-2339-9. [DOI] [PubMed] [Google Scholar]
- 77.Corachán A., Pellicer N., Pellicer A., Ferrero H. Novel therapeutic targets to improve Ivf outcomes in endometriosis patients: a review and future prospects. Hum. Reprod. Update. 2021;27(5):923–972. doi: 10.1093/humupd/dmab014. [DOI] [PubMed] [Google Scholar]
- 78.Matsuzaki S., Schubert B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010;93(7):2431–2432. doi: 10.1016/j.fertnstert.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 79.Ferrero H., Corachán A., Aguilar A., Quiñonero A., Carbajo-García M.C., Alamá P., et al. Single-cell Rna sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 2019;34(7):1302–1312. doi: 10.1093/humrep/dez053. [DOI] [PubMed] [Google Scholar]
- 80.Sanchez A.M., Viganò P., Somigliana E., Panina-Bordignon P., Vercellini P., Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update. 2014;20(2):217–230. doi: 10.1093/humupd/dmt053. [DOI] [PubMed] [Google Scholar]
- 81.Tamura H., Takasaki A., Miwa I., Taniguchi K., Maekawa R., Asada H., et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008;44(3):280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 82.Artini P.G., Scarfò G., Marzi I., Fusi J., Obino M.E., Franzoni F., et al. Oxidative stress-related signaling pathways predict oocytes' fertilization in vitro and embryo quality. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaube S.K., Prasad P.V., Thakur S.C., Shrivastav T.G. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10(4):863–874. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]
- 84.Yadav A.K., Yadav P.K., Chaudhary G.R., Tiwari M., Gupta A., Sharma A., et al. Autophagy in hypoxic ovary. Cell. Mol. Life Sci. 2019;76(17):3311–3322. doi: 10.1007/s00018-019-03122-4. Epub 2019/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kisková T., Kassayová M. Resveratrol action on lipid metabolism in cancer. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu X.-L., Deng S.-L., Lian Z.-X., Yu K. Resveratrol targets a variety of oncogenic and oncosuppressive signaling for ovarian cancer prevention and treatment. Antioxidants. 2021;10(11) doi: 10.3390/antiox10111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V., Martin-Montalvo A., North B.J., et al. Sirt1 is required for Ampk activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabol. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou X., Chen M., Zeng X., Yang J., Deng H., Yi L., et al. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5(12) doi: 10.1038/cddis.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu M., Yin Y., Ye X., Zeng M., Zhao Q., Keefe D.L., et al. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013;28(3):707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- 90.Jiang Y., Zhang Z., Cha L., Li L., Zhu D., Fang Z., et al. Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int. J. Mol. Sci. 2019;20(14) doi: 10.3390/ijms20143605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghowsi M., Khazali H., Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: an experimental study. Int J Reprod Biomed. 2018;16(3):149–158. [PMC free article] [PubMed] [Google Scholar]
- 92.Brenjian S., Moini A., Yamini N., Kashani L., Faridmojtahedi M., Bahramrezaie M., et al. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am. J. Reprod. Immunol. 2020;83(1) doi: 10.1111/aji.13186. [DOI] [PubMed] [Google Scholar]
- 93.Mansour A., Samadi M., Sanginabadi M., Gerami H., Karimi S., Hosseini S., et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with Pcos. Clin. Nutr. 2021;40(6):4106–4112. doi: 10.1016/j.clnu.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Cao Z., Fang J., Xia C., Shi X., Jiang B.-H. Trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin. Cancer Res. 2004;10(15):5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 95.Wu M., Zhong C., Deng Y., Zhang Q., Zhang X., Zhao X. Resveratrol loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles for tail vein injection Ii: pharmacokinetics, tissue distribution and bioavailability. Drug Deliv. 2020;27(1):81–90. doi: 10.1080/10717544.2019.1704944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai C.-H., Vivero-Escoto J.L., Slowing, Fang I.J., Trewyn B.G., Lin V.S.Y. Surfactant-assisted controlled release of hydrophobic drugs using anionic surfactant templated mesoporous silica nanoparticles. Biomaterials. 2011;32(26):6234–6244. doi: 10.1016/j.biomaterials.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 97.Roychoudhury S., Agarwal A., Virk G., Cho C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online. 2017;34(5):487–498. doi: 10.1016/j.rbmo.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Mokra D., Adamcakova J., Mokry J. Green tea polyphenol (-)-Epigallocatechin-3-Gallate (egcg): a time for a new player in the treatment of respiratory diseases? Antioxidants. 2022;(8):11. doi: 10.3390/antiox11081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghafurniyan H., Azarnia M., Nabiuni M., Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran. J. Pharm. Res. (IJPR) 2015;14(4):1215–1233. [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H., Lui W.T., Chu C.Y., Ng P.S., Wang C.C., Rogers M.S. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009;24(3):608–618. doi: 10.1093/humrep/den417. [DOI] [PubMed] [Google Scholar]
- 101.Xu H., Becker C.M., Lui W.T., Chu C.Y., Davis T.N., Kung A.L., et al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011;96(4):1021–1028. doi: 10.1016/j.fertnstert.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Hung S.W., Li Y., Chen X., Chu K.O., Zhao Y., Liu Y., et al. Green tea epigallocatechin-3-gallate regulates autophagy in male and female reproductive cancer. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.906746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bae K.H., Chan K.H., Kurisawa M. Autoxidation-resistant, Ros-scavenging, and anti-inflammatory micellar nanoparticles self-assembled from poly(acrylic acid)-green tea catechin conjugates. ACS Macro Lett. 2022;11(7):835–840. doi: 10.1021/acsmacrolett.2c00239. [DOI] [PubMed] [Google Scholar]
- 104.Alam M., Ali S., Ashraf G.M., Bilgrami A.L., Yadav D.K., Hassan M.I. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- 105.Liang K., Ng S., Lee F., Lim J., Chung J.E., Lee S.S., et al. Targeted intracellular protein delivery based on hyaluronic acid-green tea catechin nanogels. Acta Biomater. 2016;33:142–152. doi: 10.1016/j.actbio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 106.Bae K.H., Tan S., Yamashita A., Ang W.X., Gao S.J., Wang S., et al. Hyaluronic acid-green tea catechin micellar nanocomplexes: fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials. 2017;148:41–53. doi: 10.1016/j.biomaterials.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 107.Treml J., Šalamúnová P., Hanuš J., Hošek J. The effect of curcumin encapsulation into yeast glucan particles on antioxidant enzyme expression. Food Funct. 2021;12(5):1954–1957. doi: 10.1039/d0fo03237a. [DOI] [PubMed] [Google Scholar]
- 108.Trujillo J., Granados-Castro L.F., Zazueta C., Andérica-Romero A.C., Yi Chirino, Pedraza-Chaverrí J. Mitochondria as a target in the therapeutic properties of curcumin. Arch. Pharm. (Weinheim) 2014;347(12):873–884. doi: 10.1002/ardp.201400266. [DOI] [PubMed] [Google Scholar]
- 109.Azami S.H., Nazarian H., Abdollahifar M.A., Eini F., Farsani M.A., Novin M.G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod. Fertil. Dev. 2020;32(3):292–303. doi: 10.1071/RD18472. [DOI] [PubMed] [Google Scholar]
- 110.Heshmati J., Golab F., Morvaridzadeh M., Potter E., Akbari-Fakhrabadi M., Farsi F., et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor Γ coactivator 1α gene expression in polycystic ovarian syndrome (Pcos) patients: a randomized placebo-controlled clinical trial. Diabetes Metabol. Syndr. 2020;14(2):77–82. doi: 10.1016/j.dsx.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 113.Yallapu M.M., Gupta B.K., Jaggi M., Chauhan S.C. Fabrication of curcumin encapsulated plga nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010;351(1):19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 114.Fatemi Abhari S.M., Khanbabaei R., Hayati Roodbari N., Parivar K., Yaghmaei P. Curcumin-loaded super-paramagnetic iron oxide nanoparticle affects on apoptotic factors expression and histological changes in a prepubertal mouse model of polycystic ovary syndrome-induced by dehydroepiandrosterone - a molecular and stereological study. Life Sci. 2020;249 doi: 10.1016/j.lfs.2020.117515. [DOI] [PubMed] [Google Scholar]
- 115.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., et al. N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 2018;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 117.Liu J., Liu M., Ye X., Liu K., Huang J., Wang L., et al. Delay in oocyte aging in mice by the antioxidant N-Acetyl-L-Cysteine (nac) Hum. Reprod. 2012;27(5):1411–1420. doi: 10.1093/humrep/des019. [DOI] [PubMed] [Google Scholar]
- 118.Olesen H.Ø., Pors S.E., Jensen L.B., Grønning A.P., Lemser C.E., Nguyen Heimbürger M.T.H., et al. N-acetylcysteine protects ovarian follicles from ischemia-reperfusion injury in Xenotransplanted human ovarian tissue. Hum. Reprod. 2021;36(2):429–443. doi: 10.1093/humrep/deaa291. [DOI] [PubMed] [Google Scholar]
- 119.Porpora M.G., Brunelli R., Costa G., Imperiale L., Krasnowska E.K., Lundeberg T., et al. A promise in the treatment of endometriosis: an observational cohort study on ovarian endometrioma reduction by N-acetylcysteine. Evid. Based Compl. Alternat. Med. 2013;2013 doi: 10.1155/2013/240702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X., Wang Z., Wang H., Xu H., Sheng Y., Lian F. Role of N-acetylcysteine treatment in women with advanced age undergoing Ivf/Icsi cycles: a prospective study. Front. Med. 2022;9 doi: 10.3389/fmed.2022.917146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thakker D., Raval A., Patel I., Walia R. N-acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Obstet. Gynecol. Int. 2015;2015 doi: 10.1155/2015/817849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salem N., Salem M.Y., Elmaghrabi M.M., Elawady M.A., Elawady M.A., Sabry D., et al. Does vitamin C have the ability to augment the therapeutic effect of bone marrow-derived mesenchymal stem cells on spinal cord injury? Neural Regen. Res. 2017;12(12):2050–2058. doi: 10.4103/1673-5374.221163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bafor E.E., Uchendu A.P., Osayande O.E., Omoruyi O., Omogiade U.G., Panama E.E., et al. Ascorbic acid and alpha-tocopherol contribute to the therapy of polycystic ovarian syndrome in mouse models. Reprod. Sci. 2021;28(1):102–120. doi: 10.1007/s43032-020-00273-9. [DOI] [PubMed] [Google Scholar]
- 124.Galli F., Bonomini M., Bartolini D., Zatini L., Reboldi G., Marcantonini G., et al. Vitamin E (Alpha-Tocopherol) metabolism and nutrition in chronic kidney disease. Antioxidants. 2022;11(5) doi: 10.3390/antiox11050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Safiyeh F.D., Mojgan M., Parviz S., Sakineh M.A., Behnaz S.O. The effect of selenium and vitamin E supplementation on anti-mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: a randomized controlled clinical trial. Compl. Ther. Med. 2021;56 doi: 10.1016/j.ctim.2020.102533. [DOI] [PubMed] [Google Scholar]
- 126.Masotta N.E., Martinefski M.R., Lucangioli S., Rojas A.M., Tripodi V.P. High-dose coenzyme Q10-loaded oleogels for oral therapeutic supplementation. Int. J. Pharm. 2019:556. doi: 10.1016/j.ijpharm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 127.Samimi M., Zarezade Mehrizi M., Foroozanfard F., Akbari H., Jamilian M., Ahmadi S., et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, Placebo-controlled trial. Clin. Endocrinol. 2017;86(4):560–566. doi: 10.1111/cen.13288. [DOI] [PubMed] [Google Scholar]
- 128.Nishida Y., Nawaz A., Kado T., Takikawa A., Igarashi Y., Onogi Y., et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of Ampk pathway. J. Cachexia Sarcopenia Muscle. 2020;11(1):241–258. doi: 10.1002/jcsm.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gharaei R., Alyasin A., Mahdavinezhad F., Samadian E., Ashrafnezhad Z., Amidi F. Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J. Assist. Reprod. Genet. 2022;39(4) doi: 10.1007/s10815-022-02432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yong W., Ma H., Na M., Gao T., Zhang Y., Hao L., et al. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112001. [DOI] [PubMed] [Google Scholar]
- 131.Reiter R.J., Rosales-Corral S., Tan D.X., Jou M.J., Galano A., Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life Sci. 2017;74(21):3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Galano A., Tan D.X., Reiter R.J. On the free radical scavenging activities of melatonin's metabolites, Afmk and Amk. J. Pineal Res. 2013;54(3):245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 133.Tamura H., Kawamoto M., Sato S., Tamura I., Maekawa R., Taketani T., et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017;62(2) doi: 10.1111/jpi.12381. [DOI] [PubMed] [Google Scholar]
- 134.Espino J., Macedo M., Lozano G., Ortiz Á., Rodríguez C., Rodríguez A.B., et al. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. 2019;8(9) doi: 10.3390/antiox8090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tagliaferri V., Romualdi D., Scarinci E., Cicco S.D., Florio C.D., Immediata V., et al. Melatonin treatment may Be able to restore menstrual cyclicity in women with pcos: a pilot study. Reprod. Sci. 2018;25(2):269–275. doi: 10.1177/1933719117711262. [DOI] [PubMed] [Google Scholar]
- 136.Mokhtari F., Akbari Asbagh F., Azmoodeh O., Bakhtiyari M., Almasi-Hashiani A. Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int. J. Fertil. Steril. 2019;13(3):225–229. doi: 10.22074/ijfs.2019.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chuffa LGdA., Reiter R.J., Lupi L.A. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38(10):945–952. doi: 10.1093/carcin/bgx054. [DOI] [PubMed] [Google Scholar]
- 138.Bu S., Wang Q., Sun J., Li X., Gu T., Lai D. Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via Ne/Akt/Β-Catenin/Slug Axis. Cell Death Dis. 2020;11(8):644. doi: 10.1038/s41419-020-02906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong Y., Luo S., Fan P., Zhu H., Li Y., Huang W. Growth hormone activates Pi3k/Akt signaling and inhibits Ros accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020;18(1):121. doi: 10.1186/s12958-020-00677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gong Y., Luo S., Fan P., Jin S., Zhu H., Deng T., et al. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-75107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gong Y., Zhang K., Xiong D., Wei J., Tan H., Qin S. Growth hormone alleviates oxidative stress and improves the Ivf outcomes of poor ovarian responders: a randomized controlled trial. Reprod. Biol. Endocrinol. 2020;18(1):91. doi: 10.1186/s12958-020-00648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J., Wu J., Zhang Y., Zhang J., Xu W., Wu C., et al. Growth hormone protects against ovarian granulosa cell apoptosis: alleviation oxidative stress and enhancement mitochondrial function. Reprod. Biol. 2021;21(2) doi: 10.1016/j.repbio.2021.100504. [DOI] [PubMed] [Google Scholar]
- 143.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 144.Timaner M., Tsai K.K., Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin. Cancer Biol. 2020;60:225–237. doi: 10.1016/j.semcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Kemp K., Gray E., Mallam E., Scolding N., Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. Rep. 2010;6(4):548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 146.Kim W.-S., Park B.-S., Kim H.-K., Park J.-S., Kim K.-J., Choi J.-S., et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J. Dermatol. Sci. 2008;49(2):133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 147.Park H., Seok J., You J.H., Lee D.H., Lim J.-Y., Kim G.J. Can a large number of transplanted mesenchymal stem cells have an optimal therapeutic effect on improving ovarian function? Int. J. Mol. Sci. 2022;23(24) doi: 10.3390/ijms232416009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seok J., Park H., Choi J.H., Lim J.-Y., Kim K.G., Kim G.J. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants. 2020;9(7) doi: 10.3390/antiox9070591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stavely R., Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020;9(9) doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shojafar E., Soleimani Mehranjani M., Shariatzadeh S.M.A. Adipose-derived mesenchymal stromal cell transplantation at the graft site improves the structure and function of autografted mice ovaries: a stereological and biochemical analysis. Cytotherapy. 2018;20(11):1324–1336. doi: 10.1016/j.jcyt.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 151.Ding C., Zhu L., Shen H., Lu J., Zou Q., Huang C., et al. Exosomal mirna-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating Sirt7. Stem Cell. 2020;38(9):1137–1148. doi: 10.1002/stem.3204. [DOI] [PubMed] [Google Scholar]
- 152.Kalhori Z., Azadbakht M., Soleimani Mehranjani M., Shariatzadeh M.A. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy. 2018;20(12):1445–1458. doi: 10.1016/j.jcyt.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 153.Besag F.M.C., Vasey M.J., Lao K.S.J., Wong I.C.K. Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs. 2019;33(12):1167–1186. doi: 10.1007/s40263-019-00680-w. [DOI] [PubMed] [Google Scholar]
- 154.Brittain A.L., Basu R., Qian Y., Kopchick J.J. Growth hormone and the epithelial-to-mesenchymal transition. J. Clin. Endocrinol. Metab. 2017;102(10):3662–3673. doi: 10.1210/jc.2017-01000. [DOI] [PubMed] [Google Scholar]
- 155.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14(1):24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu M., Ma L., Xue L., Ye W., Lu Z., Li X., et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany NY) 2019;11(3):1030–1044. doi: 10.18632/aging.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Furat Rencber S., Kurnaz Ozbek S., Eraldemır C., Sezer Z., Kum T., Ceylan S., et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in Pcos: an experimental study. J. Ovarian Res. 2018;11(1):55. doi: 10.1186/s13048-018-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tehrani H.G., Allahdadian M., Zarre F., Ranjbar H., Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: a clinical trial. J. Educ. Health Promot. 2017;6:36. doi: 10.4103/jehp.jehp_67_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abdollahifar M.-A., Azad N., Sajadi E., Shams Mofarahe Z., Zare F., Moradi A., et al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat. Cell Biol. 2019;52(2):196–203. doi: 10.5115/acb.2019.52.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kükürt A., Karapehlivan M. Protective effect of astaxanthin on experimental ovarian damage in rats. J. Biochem. Mol. Toxicol. 2022;36(3) doi: 10.1002/jbt.22966. [DOI] [PubMed] [Google Scholar]
- 161.Urata Y., Honma S., Goto S., Todoroki S., Iida T., Cho S., et al. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999;27(7–8):838–847. doi: 10.1016/s0891-5849(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 162.Teixeira A., Morfim M.P., de Cordova C.A.S., Charão C.C.T., de Lima V.R., Creczynski-Pasa T.B. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J. Pineal Res. 2003;35(4):262–268. doi: 10.1034/j.1600-079x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 163.Hu W., Liang J.-W., Liao S., Zhao Z.-D., Wang Y.-X., Mao X.-F., et al. Melatonin attenuates radiation-induced cortical bone-derived stem cells injury and enhances bone repair in postradiation femoral defect model. Mil. Med. Res. 2021;8(1):61. doi: 10.1186/s40779-021-00355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nikmard F., Hosseini E., Bakhtiyari M., Ashrafi M., Amidi F., Aflatoonian R. The boosting effects of melatonin on the expression of related genes to oocyte maturation and antioxidant pathways: a polycystic ovary syndrome- mouse model. J. Ovarian Res. 2022;15(1):11. doi: 10.1186/s13048-022-00946-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Song C., Peng W., Yin S., Zhao J., Fu B., Zhang J., et al. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016;6 doi: 10.1038/srep35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang L., Zhang Z., Wang J., Lv D., Zhu T., Wang F., et al. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via Mt1/Ampk pathway. J. Pineal Res. 2019;66(3) doi: 10.1111/jpi.12550. [DOI] [PubMed] [Google Scholar]
- 167.Martín M., Macías M., Escames G., Reiter R.J., Agapito M.T., Ortiz G.G., et al. Melatonin-induced increased activity of the respiratory chain complexes I and Iv can prevent mitochondrial damage induced by Ruthenium red in vivo. J. Pineal Res. 2000;28(4):242–248. doi: 10.1034/j.1600-079x.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- 168.Zonta Y.R., Martinez M., Camargo I.C.C., Domeniconi R.F., Lupi Júnior L.A., Pinheiro P.F.F., et al. Melatonin reduces angiogenesis in serous papillary ovarian carcinoma of ethanol-preferring rats. Int. J. Mol. Sci. 2017;18(4) doi: 10.3390/ijms18040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mousavi R., Alizadeh M., Asghari Jafarabadi M., Heidari L., Nikbakht R., Babaahmadi Rezaei H., et al. Effects of melatonin and/or magnesium supplementation on biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2022;200(3):1010–1019. doi: 10.1007/s12011-021-02725-y. [DOI] [PubMed] [Google Scholar]
- 170.Tamura H., Takasaki A., Taketani T., Tanabe M., Kizuka F., Lee L., et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012;5:5. doi: 10.1186/1757-2215-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tamura I., Tamura H., Kawamoto-Jozaki M., Shirafuta Y., Fujimura T., Doi-Tanaka Y., et al. Effects of melatonin on the transcriptome of human granulosa cells, fertilization and blastocyst formation. Int. J. Mol. Sci. 2022;23(12) doi: 10.3390/ijms23126731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.