SUMMARY
Adipose tissue exhibits remarkable plasticity with capacity to change in size and cellular composition under physiological and pathophysiological conditions. The emergence of single cell transcriptomics has rapidly transformed our understanding of the diverse array of cell types and cell states residing in adipose tissues and has provided insight into how transcriptional changes in individual cell types contribute to tissue plasticity. Here, we present a comprehensive overview of the cellular atlas of adipose tissues focusing on the biological insight gained from single cell and single nuclei transcriptomics of murine and human adipose tissues. We also offer our perspective on the exciting opportunities for mapping cellular transitions and crosstalk, which have been made possible by single cell technologies.
Keywords: Single-cell sequencing, single-nuclei sequencing, cellular heterogeneity, adipose tissue plasticity, adipocytes, macrophages, preadipocytes, fibro-adipogenic progenitors, adipogenesis, inflammation
INTRODUCTION
Adipose tissues are loose connective tissues with a high number of adipocytes. These specialized cells store metabolic energy in the form of large cytoplasmic lipid droplets and at the same time regulate several aspects of organismal physiology through their endocrine functions. Mammals harbor two main classes of adipose tissues, white and brown, that can be classified based on their primary function 1. White adipose tissue (WAT) serves as the principal site for long-term energy storage and is characterized by the presence of large unilocular white adipocytes, which store surplus metabolic energy as triacylglycerides (TAGs) under anabolic conditions and release that energy as free fatty acids (FFAs) to fuel peripheral tissues under catabolic conditions. WAT is found at many discrete anatomical locations throughout the body of mammals with the major depots broadly sub-characterized as subcutaneous (i.e., located under the skin) or intra-abdominal (within the peritoneum; associated with internal organs). In addition, multiple smaller depots are found at other locations, including the bone marrow, subdermal, perivascular, epicardial, peri- and inter-muscular regions 2–4. In addition, to its energy storing function, WAT serves numerous nonmetabolic functions, including thermal insulation, and support and cushioning of organs 4.
The second major class of adipose tissue in eutherian mammals is brown adipose tissue (BAT), which plays a major role in thermogenesis and are found in smaller depots at several distinct anatomical locations, including anterior cervical, supraclavicular, intrascapular, perirenal and perivascular in mice and humans 5,6. Brown adipocytes are characterized by multi-locular lipid droplets, abundance of mitochondria, and expression of uncoupling protein 1 (UCP1), which acts as a proton carrier in the inner mitochondrial membrane 1,7,8. The presence of UCP1 along with other programs of futile energy cycling allow brown adipocytes to burn excess energy to produce heat and act as a metabolic sink 9. In adult humans, BAT depots account for maximally 1–2% of total adipose tissue 10; however, the abundance of BAT in small hibernating mammals or human neonates can constitute up to 5% of total body mass 11.
What separates adipose tissue from most other tissues is its remarkable capacity to change in size and cellular composition under physiological and pathophysiological conditions 12. The adaptation to environmental and physiological challenges not only involves changes in adipocyte size, number, and function, but also long-lasting quantitative and qualitative changes in the cellular composition of the tissue. The “stromal-vascular” compartment is the term used to describe the non-adipocytes in the adipose tissue, and this consist of highly heterogeneous populations of immune cells, mesenchymal stromal cells, and endothelial cells as well as small populations of other cell types including neurons 12,13. The development of single cell transcriptomics has rapidly transformed our understanding of the diverse array of cell types and cell states residing in adipose tissue and have shown how they influence the ability of WAT and BAT to exert its multiple functions. In this review, we provide a brief introduction to adipose tissue plasticity and the importance for human health, followed by a comprehensive review of the rapidly growing body of literature describing the cellular heterogeneity of murine and human adipose tissue. We highlight the biological insights gained specifically from single cell transcriptomics and discuss the new opportunities offered by single cell approaches to acquire mechanistic insight into the regulation of adipose tissue plasticity.
SHORT-TERM METABOLIC PLASTICITY OF ADIPOSE TISSUES
Adipocytes can sense and quickly adapt to acute changes in the metabolic needs of the body. The classical example of a short-term change in adipocyte function is the adaptation that occurs during cycles of feeding and fasting. In the postabsorptive state, insulin acts as the major anabolic hormone stimulating both glucose and fatty acid uptake as well as de novo lipogenesis and triglyceride synthesis. Furthermore, insulin inhibits adipocyte lipolysis by stimulating phosphodiesterase 3B (PDE-3B), thereby decreasing cAMP levels 12,14. As such, insulin sensitivity is essential for adipocyte storage capacity and function. Mouse models with deletion of the insulin receptor specifically in mature adipocytes display WAT atrophy and ectopic lipid deposition 15. In the fasting state, when circulating insulin levels decline, inhibition of lipolysis is alleviated. Furthermore, release of noradrenaline from the sympathetic nervous system activates β-adrenergic receptors, thereby increasing PKA activity and stimulating lipolysis in adipocytes 16. This balance between lipogenic and lipolytic signals is critical for the ability of adipose tissue to buffer the daily flux of nutrients and for maintenance of whole body metabolic homeostasis 17.
Another classical example of short-term metabolic plasticity is the activation of brown adipose tissue in response to acute cold exposure. Here norepinephrine released from the sympathetic nervous systems innervating the tissue leads to activation of β-adrenergic receptors and stimulation of the cAMP/PKA axis and induction of lipolysis in brown adipocytes. The fatty acids released by lipolysis simultaneously fuels mitochondrial β-oxidation and activates UCP1-mediated thermogenesis. Furthermore, PKA drives transcriptional activation of gene programs that support a sustained catabolic activity and mitochondrial uncoupling in brown adipocytes 12,18. Interestingly, it was recently shown that the transcriptional effects are highly dependent on lipolysis which signals through peroxisome proliferator-activated receptor γ (PPARγ) as well as several other transcription factors 19. Thus, metabolism and transcription are closely linked during thermogenic activation of brown adipocytes.
LONG-TERM CELLULAR AND COMPOSITIONAL PLASTICITY OF ADIPOSE TISSUES
“Browning” and “Whitening” of Adipose Depots
Chronic exposure to environmental and physiological stressors can elicit lasting changes of the cellular composition of adipose tissue and/or modulate the programming of cells within the tissue. One of the most striking examples of the long-term plasticity of adipose tissue is the adaptation to changes in environmental temperatures. In response to cold temperatures, WAT in many mammalian species can adopt a BAT-like phenotype with the emergence of thermogenic brown-like adipocytes commonly referred to as “Beige” or “BRITE adipocytes” 20–22. This “browning” of WAT can occur upon reactivation of existing dormant (i.e., unilocular) beige adipocytes, by trans-differentiation of white adipocytes, as well as through de novo differentiation of beige adipocytes from progenitor cells 23–26. In rodents, cold exposure leads to marked browning primarily of subcutaneous inguinal WAT; however, prolonged cold exposure can also drive beige adipocyte accrual in other WAT depots 27. Moreover, WAT browning can be observed in rodents following calorie restriction, intermittent fasting, gastric bypass surgery, and cachexia 28–32. The human relevance of WAT browning is not clear; however, beige adipocyte accumulation has been observed in human WAT in response to conditions with increased sympathetic activity, such as severe burns and catecholamine-producing tumors 33–35 as well as in response to cachexia 30. Cold-induced browning of WAT is driven predominantly by norepinephrine released by sympathetic neurons; however, several other inducers have been reported 36,37. Catecholamines can act directly on adipocytes to activate a thermogenic gene program 27, or on adipocyte progenitors to drive beige adipogenesis 23. However, this response is heavily augmented by local stromal cells and cells of the adaptive and innate immune systems 6,38–44.
Importantly, brown and beige adipocytes depend on a continued β-adrenergic tone to maintain their function. Denervation of BAT leads to a loss of thermogenic and oxidative capacity, along with a morphological “whitening” of the depot characterized by the presence of white-like unilocular adipocytes 45. This “whitening” of BAT occurs naturally in association with aging, obesity, and shifts to warm environmental temperature, facilitated by the presence of inhibitory signals that interfere with β-adrenergic signaling 46–48. The browning of WAT can also be reversed upon cessation of cold exposure or other thermogenic stimuli. As mice transition from cold temperatures to a warm environment, beige adipocytes slowly revert to a unilocular white adipocyte state with the gross molecular features of white adipocytes. However, these “dormant” beige adipocytes retain an epigenetic memory of their prior cold exposure, allowing them to rapidly reactivate their beige phenotype upon subsequent cold exposure 49.
Adipose Tissue to Mammary Gland Transition during Pregnancy and Lactation
The response of mammary adipose tissue to pregnancy represents another striking example of adipose tissue plasticity 50,51. In rodents, the mammary gland is contained in the inguinal WAT, and during pregnancy this tissue undergoes a major transformation in preparation for lactation. Lipid-filled adipocytes essentially disappear coincident with a rapid expansion of the mammary epithelium. Adipocytes become delipidated during this time and lineage tracing indicates that cells undergo de-differentiation to a preadipocyte-like state 52,53. As mammary ducts involute at the end of lactation the small adipocytes undergo hypertrophy as adipocytes take up the milk lipids, and adipocyte-derived preadipocytes re-differentiate 50,53.
Obesity Associated Change in Adipose Tissue Mass
The capacity of WAT to expand and contract in size is arguably unparalleled in adulthood. A chronic state of positive energy balance leads to a significant enlargement of most adipose tissues. WAT expansion is driven by a combination of adipocyte hypertrophy and adipocyte hyperplasia. Mature adipocytes do not undergo cell division; therefore, adipocyte hyperplasia is mediated by de novo adipocyte differentiation, or “adipogenesis,” from resident adipocyte precursor cells (APCs). In humans with normal range of body mass index (BMI), WAT constitute roughly 20% and 30% of the body weight in men and women, respectively. In individuals with obesity, body fat percentage can rise to 50%, primarily due to expansion of the subcutaneous and intraabdominal visceral WAT 54–57. The expansion of WAT during development of obesity is associated with substantial changes in the cellular composition, referred to as “tissue remodeling”. This includes vascular expansion, extracellular matrix (ECM) remodeling, recruitment of monocytes and activation to pro-inflammatory macrophages, and changes in the mesenchymal stromal cell phenotypes 58.
Conversely, during a negative energy balance, some adipose tissues contract in mass due to adipocyte size reduction and elimination. The remodeling of adipose tissue during weight loss is less well understood; however, increased immune cell infiltration during the early stages of acute weight loss has been observed in both human and rodent WAT59–61. On the other hand, decreased adiposity by very low-calorie consumption in rodents and bariatric surgery in humans has been reported to be associated with reduced crown-like structures (CLSs) and improved systemic metabolic parameters 62,63. Interestingly however, persistent inflammation and metabolic defects of WAT has been reported during prolonged weight loss in some studies in mice and humans, indicating the existence of an obesity memory in the tissue despite improved systemic metabolism 64. The mechanisms and functional consequences of a obesity memory and how it might affect weight re-gain are currently unknown.
ADIPOSE TISSUE DYSFUNCTION IN HUMAN DISEASES
The importance of adipose tissue in human health is highlighted by the condition of lipodystrophy, a diverse group of metabolic disorders commonly characterized by insufficient capacity to form functional adipose tissue. Adipose deficiency can either be the result of heritable mutations in genes driving adipocyte differentiation and lipid storage or acquired secondary to other conditions or medication 65,66. The absence of functional adipose tissue leads to deleterious lipid accumulation in peripheral organs, including liver, skeletal muscle, heart, and the endocrine pancreas, resulting in insulin resistance and cardiovascular diseases.
On the other end of the spectrum, elevated BMI significantly confers increased risk of developing several life-threatening comorbidities including type 2 diabetes 67, cardiovascular diseases, fatty liver diseases 68,69, as well as increased risk of certain cancers 69,70. Importantly however, some individuals with obesity avoid many features of metabolic syndrome, at least for a period. The identification and study of this so-called “metabolically healthy” obesity underscores the notion that factors beyond elevated BMI per se lead to the metabolic syndrome 71. Comparisons to those individuals with obesity and insulin resistance (i.e., “pathologic obesity”) reveals that body fat distribution plays an important role in disease susceptibility. The incidence and severity of cardiometabolic comorbidities are primarily associated with expansion of visceral and abdominal subcutaneous WAT, whereas femoral and gluteal adipose tissues appear to be protective when corrected for fat mass 72. Genome-wide association studies (GWAS) discovered that genes associated with body fat distribution and healthy obesity are highly enriched for adipocyte genes 73, indicating that intrinsic properties of adipocytes, and their ability to remain functional, play an important role in determining body fat distribution and cardiometabolic diseases.
Furthermore, the way individual WAT depots expand and remodel in the setting of overnutrition impacts overall metabolic health 74. Pathologic obesity is often characterized by the presence of hypertrophic adipocytes, excess ECM accumulation (fibrosis), the accumulation of pro-inflammatory immune cells, and decreased expression of protective adipokines (e.g., Adiponectin). Macrophage infiltration and proinflammatory macrophage polarization contribute to adipose tissue dysfunction and may enhance cardiometabolic disorders 75–77. Healthy adipose tissue expansion is characterized by adipocyte hyperplasia, indicative of increased de novo adipogenesis, as well as a relatively lower degree of inflammation and fibrosis. In fact, fibrosis has emerged as a key factor that distinguishes metabolically healthy vs. metabolically unhealthy obesity of humans 78. As such, lipodystrophy and obesity manifest itself with many of the same cardiometabolic comorbidities, underscoring the notion that limited adipocyte function and storage capacity play a major role in the development of these diseases 79.
The realization that maintaining healthy and functional adipocytes with a high storage capacity is critical for human health emphasizes the need to understand the regulation of adipocyte function in different human depots. Although intrinsic properties of adipocytes have been shown to be major determinants of overall adipose tissue function 73, cells of the tissue microenvironment, including immune cells 80, endothelial cells 81, and neurons 82,83, all play a critical role in controlling adipose tissue plasticity and adipocyte function. Thus, the understanding of human diseases relating to adipose tissue storage depends on in-depth investigations of the molecular properties of adipocytes in their tissue context and understanding how they interact with the array of other cell types in the tissue microenvironment.
CLASSICAL METHODS FOR STUDYING ADIPOSE STRUCTURE AND FUNCTION
The study of adipose tissue has historically been facilitated by classical techniques in bioimaging, cell culture, flow cytometry, and mouse genetics (Figure 1). For nearly a century, critical insights into the morphology and cellularity of adipose tissues have been gained through microscopic examination of tissue sections 84,85. Some of the earliest observations include hypertrophy of adipocytes and the perivascular localization of pre-adipocytes 86, as well as cold-induced lipid droplet and mitochondrial remodeling in brown adipocytes 87. These pioneering histological findings have laid the foundation for our current understanding of adipocyte differentiation and adipose tissue plasticity 87–91. The development of the adipose tissue fractionation approach by Rodbell in the 1960’s facilitated the study of adipocyte metabolism in isolation as well as the ability to study cells of the “stromal vascular fraction” 92. The derivation of clonal adipogenic fibroblast cells lines (e.g., 3T3-L1) in the 1970’s 93,94 was the key to gaining rapid insight into the functional properties of adipocytes and the molecular basis of adipocyte differentiation. These and other immortalized cell lines have enabled the discovery of adipocyte metabolic pathways and lineage selective genes 95–99 as well as the transcriptional regulators of adipocyte differentiation and function including the master regulator peroxisome proliferator-activated receptor γ (PPARγ) 100 and CCATT-enhancer binding protein α(C/EBPα)101. Furthermore, with the advent of next generation sequencing, immortalized cells have been instrumental for mapping the transcriptional network and epigenomic mechanisms driving lineage determination and differentiation of 102–104 as well as browning of adipocytes 105. Importantly, despite the artificial conditions of cell culture adipogenesis, many of the basic mechanisms and transcription factors identified also play a role for in vivo adipogenesis 104.
Figure 1: Classical techniques for studying adipose tissue.
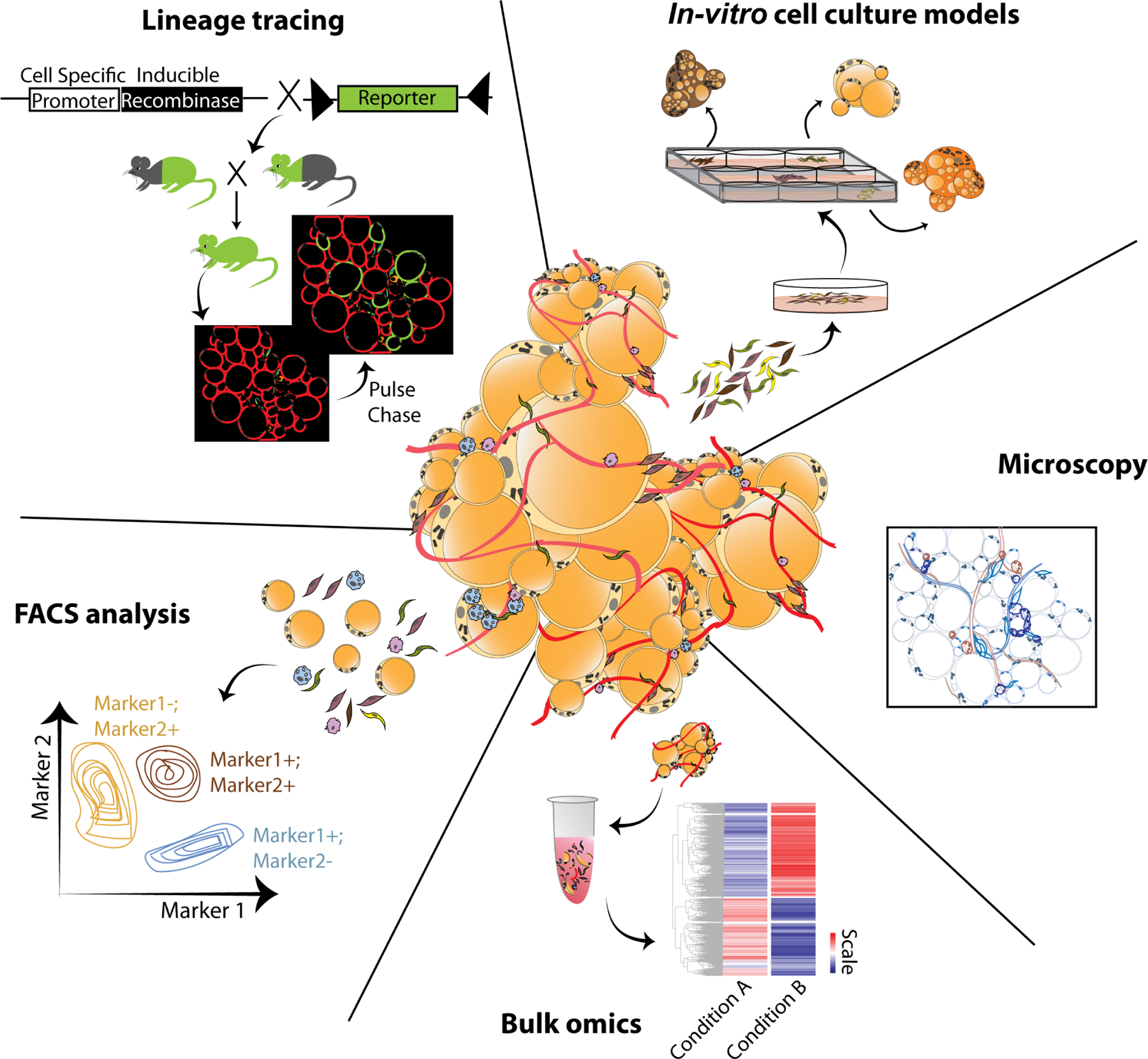
Conventionally applied microscopy, FACS analyses, genetic lineage tracing, in vitro cell culture models, and whole tissue (bulk) omics (genomics/proteomics/metabolomics/lipidomics) represent classical tools for adipose tissue biologists and have been instrumental for our current understanding of the morphological, compositional, functional, and physiological aspects of adipose tissue.
| Conventional technologies for studies of adipose tissue | ||
|---|---|---|
| Whole tissue technologies | Pros | Cons |
| Microscopy (immunostaining/RN A FISH) |
|
|
| Bulk tissue omics |
|
|
| Studies of cell types | ||
| FACS |
|
|
| Genetic lineage tracing |
|
|
| Cell culture models |
|
|
At the tissue level high throughput profiling of e.g., the transcriptome, proteome and metabolome have provided unbiased insight into the molecular composition of adipose tissue 106–109. Biochemical approaches have been used to localize (e.g., immunohistochemistry) and isolate (e.g., flow cytometry) different cell types 7,110,111, whereas genetic approaches (Cre-LoxP) have been used to manipulate or label selective cell types 25,112,113. Each approach has unique strengths and collectively they continue to be essential for studying aspects of adipose tissue biology. Nevertheless, as detailed below, the growing appreciation of cellular heterogeneity has also highlighted the limitations of these approaches (Figure 1). For instance, immunohistochemistry and flow cytometry rely on knowledge of antigens that discriminate cell populations and the availability of specific antibodies. Major cell types in the adipose tissue (e.g., hematopoietic cells, endothelial cells, mesenchymal stromal cells) can easily be distinguished from one another using a handful of markers; however, further subclassification of these major cell types relies on prior knowledge of these putative subpopulations and the identification of appropriate imaging/sorting strategies. Likewise, current gene targeting approaches in mice leverage the Cre-loxP recombination system, with Cre-recombinase expression ultimately directed by enhancers and promoters that are selectively activated in mature adipocytes (e.g., Adiponectin-enhancer and promoter) 114, thermogenic brown/beige adipocytes (Ucp1-enhancer and promoter) 115, or a broad pool of mesenchymal cells containing adipose progenitors (Pdgfra or Pdgfrb enhancer promoter) 113,116. Current Cre-drivers are limited by the fact that they target broad pools of adipocytes or stromal cells. As such, the classical toolbox for the study of adipose tissue and nearly all other tissues have significant limitations for studies of tissue/cellular heterogeneity and plasticity.
SINGLE-CELL TECHNOLOGIES: PROMISING PATH TO RESOLVING ADIPOSE TISSUE FUNCTION AND HETEROGENEITY
The advent of next-generation sequencing (NGS)-based single-cell technologies has allowed for genomic and transcriptomic profiling of tissues at single cell resolution 117, thus transforming the toolbox for adipose tissue biologists. The resolving power of these methods carry the potential to uncover novel biological insights that may otherwise be hidden when analyzing cells in bulk. In particular, single-cell RNA sequencing (scRNA-seq) methods have proven extremely powerful. Transcriptomics at single-cell resolution can reveal the existence of distinct cellular states in populations of cells that might otherwise appear homogenous. Elucidating these cellular states as distinct snapshots of a dynamic process allows for the reconstruction of biological function at the single cell level. This has led to discoveries of novel lineage hierarchies driving developmental processes, such as differentiation events 118,119 (Figure 2). Thus, single cell technologies have challenged the concept of cellular identity and plasticity.
Figure 2: Deconvoluting adipose tissue through single cell/nucleus technologies.
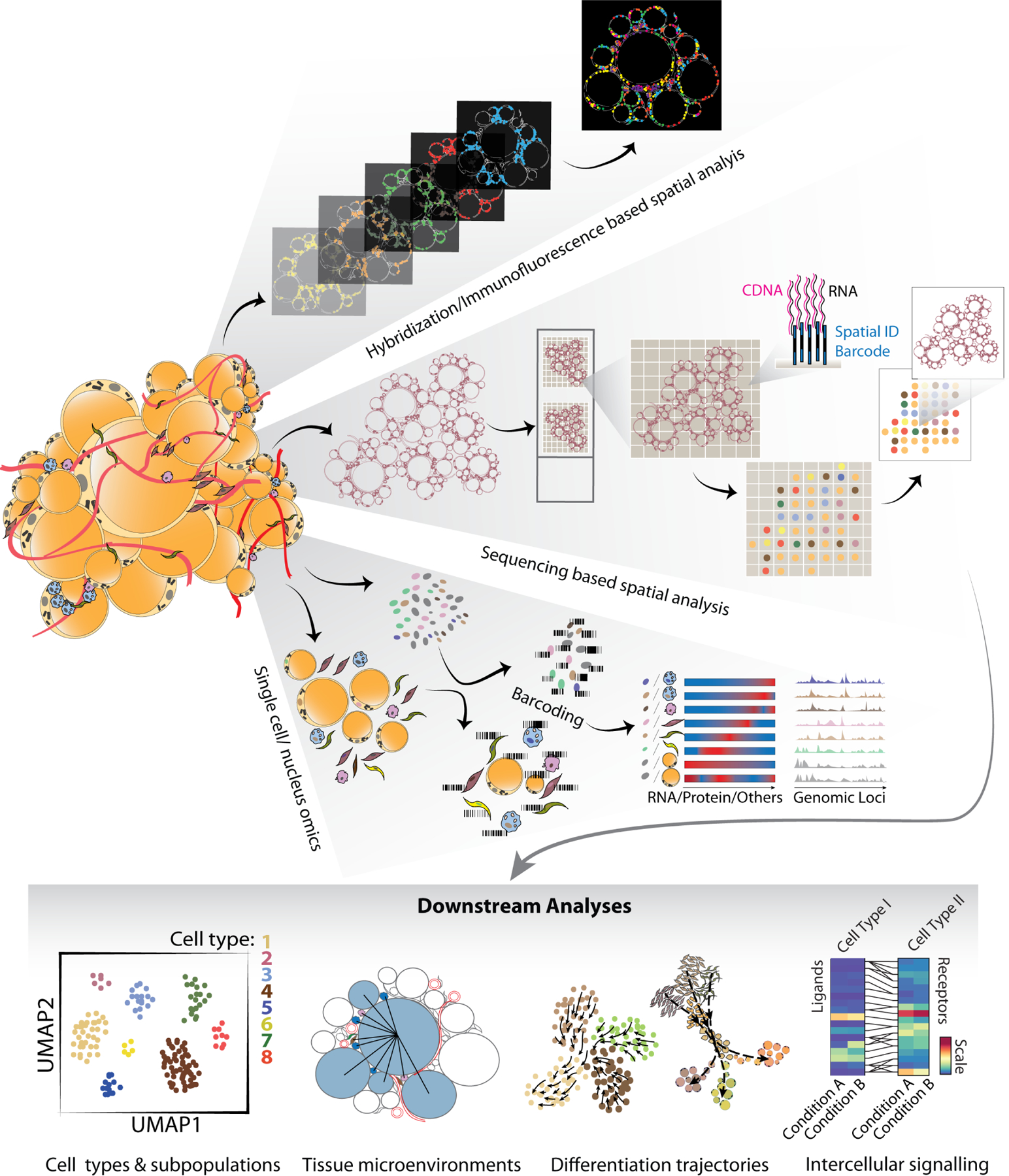
Summary of the current available single cell/nucleus technologies that are already in use, or can be implemented, to study adipose tissue functional and compositional heterogeneity. Single cell/nucleus omics based on various cell/nucleus barcoding have been widely applied for whole adipose tissue as well as the stromal vascular fraction of the tissue. Emerging spatial transcriptomics technologies include next generation sequencing-based relying on unique positional barcodes and bioimaging-based approaches rely either on in situ sequencing or hybridization of fluorescent probes to transcripts.
| Single cell/nuclei analyses of adipose tissue | ||
|---|---|---|
| Whole tissue technologies | Pros | Cons |
| Single cell/nuclei sequencing (e.g., 10x Genomics and other droplet-based methods) |
|
|
| Sequencing-based spatial transcriptomics (e.g., GeoMx® Digital Spatial Profiler, Illumina Visium) |
|
|
| Hybridization/antibody-based spatial analyses (e.g., MERFISH, CosMx™, 10x Genomics Xenium) |
|
|
With respect to adipose tissue, the use of single-cell technologies is complicated by the buoyant and fragile nature of isolated adipocytes. Many adipocytes are relatively large (up to 300 microns in humans 120) and therefore incompatible with conventional droplet-based single-cell platforms, where the cell size is restricted by the width of the microfluidic channels (e.g., 50–60 microns for the Chromium platform from 10x Genomics 121). Furthermore, due to the high lipid content, adipocytes float and cannot be pelleted with other cells in the tissue. Finally, even for strategies that do not involve droplet-based sequencing, the high lipid content interferes with enzymatic reactions. These challenges initially restricted adipose tissue single-cell analyses to non-parenchymal cell types. More recently, development of single-nucleus sequencing-based methods have allowed for inclusion of adipocytes in single-cell studies 122–124. Single-nucleus RNA-seq (snRNA-seq) is based on isolation of nuclei from tissues/cell populations, followed by sequencing of the RNA from individual nuclei 125–127 (Figure 2). The technique poses a fundamental challenge of low amounts of mature RNA transcripts and comes with an inherent bias for changes in transcript levels that are imposed by differential transcription over those imposed by differential RNA processing. However, recent work comparing snRNA-seq and scRNA-seq for the adipocyte lineage based on cell culture studies found an overall good alignment 128. A major advantage of snRNA-seq over scRNA-seq is that it does not require tissue dissociation to release individual cells from their native tissue environment, a process which can lead to cell type biases and to dissociation-induced artifacts at the transcriptomic level 129,130. Furthermore, high quality nuclei can be isolated from frozen tissue, thereby avoiding the need for processing of freshly isolated tissues 131. Nevertheless, a major challenge for snRNA-seq is that it is highly sensitive to transcript contamination (termed ambient RNA) from broken/damaged nuclei or the cytoplasm 129,132. Therefore, snRNA-seq studies require high quality nuclei preparations and careful computational analyses to ensure that only high-quality droplets are included and that high abundance ambient transcripts are computationally removed prior to clustering 124,132.
In addition to technologies for high throughput single-cell transcriptomics, a large number of technologies for epigenomic profiling of chromatin accessibility, histone marks, and DNA methylation at single cell resolution have been developed 133. The integration of single cell transcriptomics and epigenomics data provides a powerful strategy for inferring transcriptional mechanisms at single cell level 134,135 (Figure 2). Furthermore, integration with single cell proteomics allows for in-depth phenotypic characterization of cell states 136–138. Future results from such integrated analyses are expected to lead to many new mechanistic discoveries in the field of adipose tissue function.
An important development in the single-cell omics field is the introduction of spatial transcriptomics which provides gene expression matrices that can be integrated with information about tissue architecture, cellular features, and relative position of cells 139,140. These technologies can be categorized into, 1) next generation sequencing-based approaches that are extensions of the scRNA-seq combined with unique positional barcodes; and 2) bioimaging-based approaches that rely on in situ sequencing or hybridization of probes to transcripts. Given the structure of the adipose tissue with many different types of small stromal vascular cells interspersed between large lipid-filled adipocytes, where most of the volume is devoid of transcripts, techniques based on positional barcoding based on a fixed array slide could be challenging. Here techniques providing true single cell resolution, such as those based on bioimaging, and hybridization are likely to be required. Promising techniques include multiplexed error-robust fluorescence in situ hybridization (MERFISH), which uses massively multiplexed single-molecule FISH measurements and provides true single-cell resolution and the ability to quantify the spatial distribution of hundreds to tens of thousands of RNA species at single-cell resolution 139,141,142. Implementation of such high-resolution bioimaging-based technologies will be important for understanding adipose plasticity and the importance of the cellular microenvironment (Figure 2).
Single-cell omics technologies create a “still picture” of the state of all captured cells in the sample. However, due to the large number of cells, it is also possible to infer relationships between cells. For example, the developmental trajectory or state transitions of cells can be mapped from single cell RNA-seq using a variety of tools that order the cells according to a similarity-based trajectory 143,144 recently combined with directional information 145. Moreover, cellular crosstalk can be inferred using tools such as CellPhoneDB, NicheNet etc., which predicts crosstalk based on a public repository of ligands, receptors and their interactions 146,147. Such tools allow researchers to obtain pseudo-time and mechanistic insight into cellular differentiation and tissue plasticity. The combination with the emerging spatial technologies with single cell technologies will provide unique insight into the cellular crosstalk and tissue microenvironments. Finally, single cell transcriptome and/or epigenome data from tissues can be integrated with genetic data to map eQTLs (expression quantitative trait loci) across different cell types and cell states 148 and to infer disease mechanisms 149,150.
Single-cell and single-nuclei transcriptomics analyses have greatly expanded our knowledge of the cellular composition and heterogeneity of adipose tissues. In the sections that follow, we summarize the current knowledge of the cellular atlas of adipose tissue, focusing specifically on insight gained through the employment of these new technologies.
ADIPOCYTE HETEROGENEITY
White Adipocyte Heterogeneity
The importance of adipocytes for whole body physiology and the finding that adipose depots differ in their metabolic functions begs the question how adipocyte functions are regulated by the tissue microenvironment. Interestingly, recent studies mapping the single cell-resolved transcriptome of adipose tissue have revealed that there are multiple adipocyte subpopulations within individual WAT depots in humans as well as in mice 122,124,151 (Table 1). These subpopulations are characterized by expression of subpopulation-specific markers in addition to the canonical adipocyte-identity markers, such as Adiponectin and Perilipin1. First, snRNA-seq of epididymal WAT from mice, identified three distinct subpopulations of mature white adipocytes differing in their expression of metabolic and inflammatory genes 124. These subpopulations were denoted 1) lipogenic adipocytes (LGAs) expressing high levels of genes relating to de novo fatty acid synthesis, e.g., Acaca and Acly; 2) lipid-scavenging adipocytes (LSAs) expressing high levels of genes relating to lipid uptake and transport, e.g., Cd36 and Apoe; and 3) stressed LSAs (SLSAs) which in addition express genes relating to hypoxia and autophagy, e.g., Hif1a and Rab7. Interestingly, the relative proportions of LSA and SLSA subpopulations are increased in high-fat diet (HFD)-induced obesity at the expense of LGAs. Comparison of cell sizes showed that the SLSAs are the largest, whereas LGAs display the smallest average cell size, consistent with the notion that small adipocytes are more insulin sensitive 152–154. The cellular and molecular mechanisms underlying changes between the relative abundance of subpopulations are unclear; however, it is likely that adipocytes can switch between “cell states” in response to extrinsic and intrinsic signals. The reduction in LGA in response to HFD-induced obesity may explain the decrease in de novo lipogenesis and insulin sensitivity that has previously been reported in obese human and mouse WAT 155–157. More recently, snRNA-seq analyses of murine inguinal and perigonadal WAT depots, including both epididymal and periovarian WAT, identified six subpopulations of mature adipocytes with no notable enrichment across depots or sex 122. Some subpopulations could be associated with a lipogenic signature (mAd1, mAd4 and mAd5) similar to the LGAs reported previously; however, the abundance of these subpopulations increase rather than decrease in the obese condition. The discrepancy may relate to the different age and sex of the mice as well as the duration of the HFD-feeding in these studies. More careful time course studies are required to determine and resolve the temporal change in adipocyte plasticity in different depots in response to HFD.
Table 1.
Adipocyte subpopulations in white adipose tissue
| Species | Gender | Depot | Subpopulationa | Markers | Pathways | Reference |
|---|---|---|---|---|---|---|
| Mouse (C57BL/6J) | males | WAT (epididymal) | lipogenic adipocyte (LGA) | Acaca, Acly, Fasn, Dgat1/2 | triglyceride biosynthesis | Sárvári et al.123 |
| lipid-scavenging adipocyte (LSA) | Car3, Apoe, Cd36 | triglyceride handling | ||||
| stressed lipid-scavenging adipocyte (SLSA) | Apoe, Cd36, Hif1a, Ddr2 | leptin synthesis + triglyceride handling + stress responses | ||||
| male + female | WAT (inguinal + perigonadal) | mAd1 | Ebf2, Pck1 | insulin signaling + lipid handling + thermogenesis | Emont et al.121 | |
| mAd2 | Sorbs2, Crim1 | Ca2+ signaling + organization of cell-substrate junction | ||||
| mAd3 | Apoe, campk1d | OxPhos + lipid handling | ||||
| mAd4 | Cacna1a, Fgf1, Lep | PPAR and leptin signaling + ECM remodeling + stress response signaling | ||||
| mAd5 | Acss2, Prune2, Acly | lipogenesis + triglyceride biosynthesis | ||||
| mAd6 | Mt2, Casp4, Bcl3 | stress response + autophagy | ||||
| Human | men + women | WAT (subcutaneous + abdominal) | AdipoLEP | LEP, DDR2, TNS1 | leptin signaling + ECM remodeling | Bäckdahl et al.150 |
| AdipoPLIN | PLIN1/4, LIPE, DGAT1/2 | lipid biosynthesis and lipolysis | ||||
| AdipoSAA | SAA1/2, MGLL, AGPAT2 | retinol metabolism + FA handling | ||||
| men + women | WAT (subcutaneous + abdominal) | hAd1 | AFF3, WDPCP | organization of cell-substrate junction + MAPK/Rap1/EGFR signaling | Emont et al.121 | |
| hAd2 | NAV2, FABP4 | immune response to infection + autophagy | ||||
| hAd3 | SAA1, CAV2 | triglyceride biosynthesis + retinol metabolism | ||||
| hAd4 | GRIA4, ANKRD30A | leptin signaling + lipid handling + synaptic control | ||||
| hAd5 | ATP1B3, PGAP1 | insulin signaling + lipolysis regulation + HIF1a signaling | ||||
| hAd6 | EBF2, ZNF804A | lipolysis + thermogenic signaling + axon guidance | ||||
| hAd7 | THSD7B, AGMO | hormonal secretion + Ca2+ ion handling |
Distinguishing marker genes and pathways enriched in adipocyte subpopulations that have been identified in different depots of white adipose tissue using sc/snRNA-seq studies across mice and humans. LGA, lipogenic adipocyte; LSA, lipid-scavenging adipocyte; SLSA, stressed lipid-scavenging adipocyte.123 mAd1–6 are the mouse adipocyte subpopulations and hAd1–7 are the human adipocyte subpopulations defined in Emont et al.121 AdipoLEP, AdipoPLIN, and AdipoSAA subpopulations were defined by their expression of high levels of LEP, PLIN1/4, and SAA1/2, respectively.150
The subpopulations that seem to have similar markers and gene programs identified across different studies can be grouped as LGA, mAd1, AdipoPLIN, hAD4; LSA, mAd3/4, AdipoSAA, hAd3; SLSA, mAd5/6, hAd2/5; mAd2, hAd1/7.
snRNA-seq of WAT from human visceral and subcutaneous adipose tissue depots identified seven subpopulations of mature adipocytes 122, several of which are associated with a lipogenic gene signature, including expression of FASN, ACACA and DGAT1/2. Generally, the overlap between human and mouse subpopulations in this study is limited; however, similar to the decrease in LGA abundance in obese mouse epididymal WAT, the abundance of one of the human lipogenic subpopulations correlates negatively with increased BMI in the subcutaneous WAT depot. A smaller, yet significant, population of adipocytes seem to be associated with functions related to smooth muscle cells, such as calcium ion handling/signaling along with focal adhesion and cell substrate junctions.
Spatial transcriptomics of human subcutaneous abdominal WAT using Visium spatial arrays showed that despite the limited resolution of the arrays, three subpopulations of white mature adipocytes displaying distinct transcriptomic signatures (Table 1) and spatial organization could be detected 151. Interestingly, the AdipoPLIN subpopulation, (high expression of PLIN1/4), has a transcriptomic signature similar to the LGAs and is correlated with higher in vivo insulin sensitivity. Another subpopulation, AdipoSAA, are enriched for genes involved in lipid uptake and handling, thereby resembling LSAs in mice, and the AdipoLEP subpopulation and SLSAs seemed to share common enriched pathways related to leptin synthesis/signaling and ECM remodeling (Table 1). Similar to what was observed in mice, the relative abundance of the AdipoPLIN adipocytes were reduced in obese subjects. Taken together, all studies agree that mice as well as human adipose tissue contain several white adipocyte subpopulations with one or more being more lipogenic and others being more involved in lipid uptake (Figure 3). Going forward, it will be important to gain insight into the developmental and transitional trajectory of these subpopulations to determine for example whether some subpopulations of adipogenic progenitors are more likely to give rise to specific adipocyte subpopulations and to determine how the tissue microenvironment influence adipocyte cell states. Furthermore, it will be important to determine the role of different adipocyte subpopulations in defining short-term and long-term plasticity of WAT.
Figure 3: White adipocyte heterogeneity.
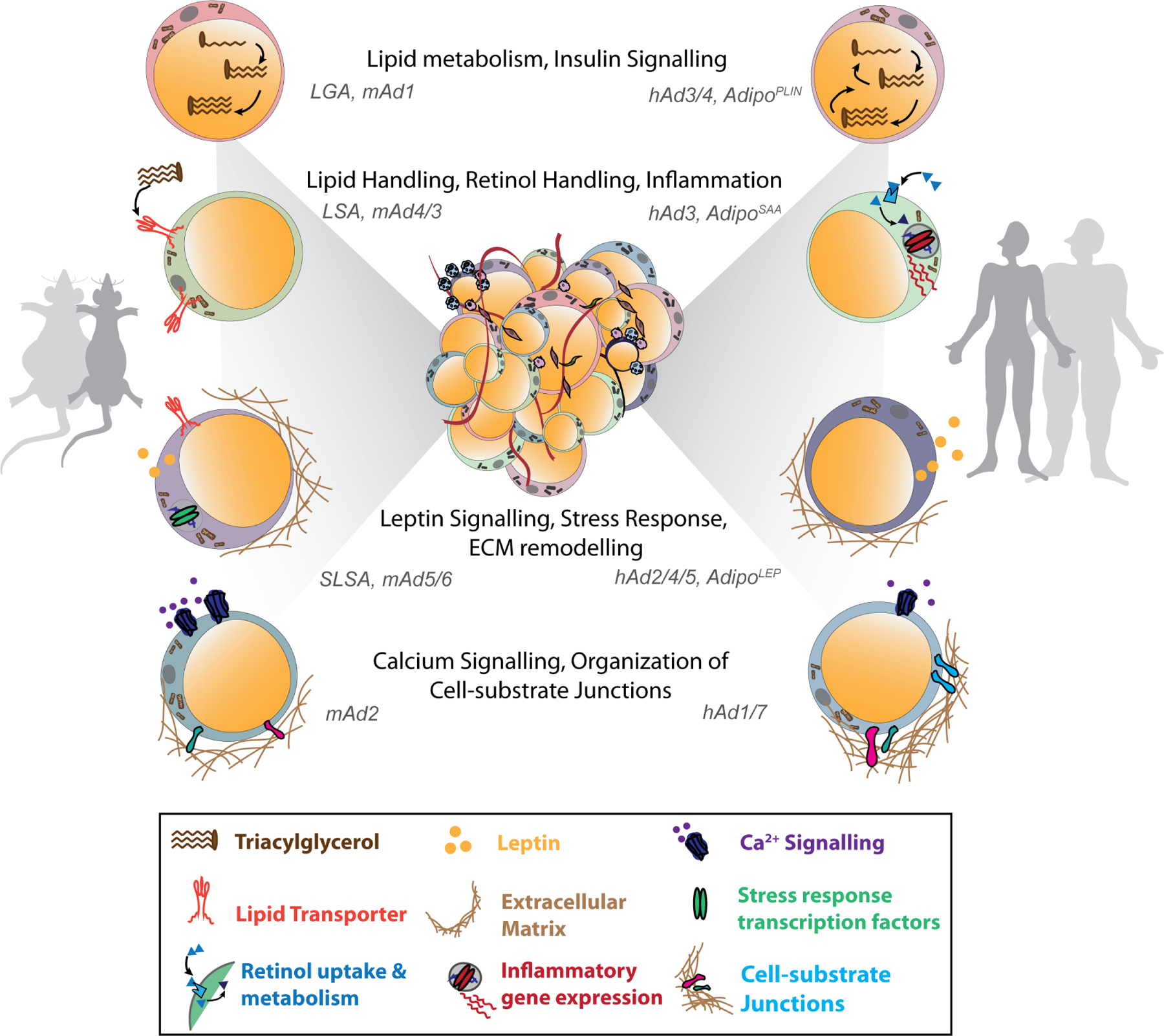
Subpopulations of white adipocytes identified across different studies in mice and humans, classified based on their gene expression and enriched pathways. The four different subpopulations identified across different studies in mouse and human WAT are shown. Abbreviations: LGA, lipogenic adipocyte; LSA, lipid-scavenging adipocyte; SLSA, stressed lipid-scavenging adipocyte 124. mAd1–6 are the mouse adipocyte subpopulations and hAd1–7 are the human adipocyte subpopulations defined in 122. AdipoLEP, AdipoPLIN and AdipoSAA subpopulations were defined by expressing high levels of LEP, PLIN1/4 and SAA1/2, respectively, in 151.
Thermogenic Adipocyte Heterogeneity
Investigation of mouse interscapular BAT have identified 10 subpopulations of brown adipocytes many of which are selectively enriched under specific temperature settings 123. One subpopulation (P4) marked by Cyp2e1 and Aldh1a1 increased in abundance in thermoneutrality and appears to be involved in inhibition of thermogenic capacity by a mechanism that involves a paracrine effect of acetate synthesized by aldehyde dehydrogenase 1 A1. Adipocytes with a similar transcriptomic signature were also found in mouse inguinal adipose tissue. In snRNA-seq of human BAT, several of the 8 adipocyte subpopulations display a P4-like transcriptomic signature but the functional relevance of this remains to be determined.
Previous histological and lineage tracing studies in mouse intrascapular BAT have indicated the existence of two distinct subpopulations of brown adipocytes with high (H) and low (L) expression of Ucp1 and adiponectin (termed “BA-H” and “BA-L”). These subpopulations display differential functional parameters like mitochondrial numbers and lipid droplet sizes 158,159. Multidimensional reconstruction of published snRNA-seq of mouse iWAT adipocytes 40, identified a unique thermogenic subpopulation (Ad1-Ucp1High) induced in response to cold or β-adrenergic activation 160. This population is similar to the mAd1 mouse iWAT adipocyte subpopulation and to the human hAd6 adipocyte subpopulation characterized by others as by the higher expression of browning markers such as EBF2, ESRRG and PPARGC1A 122. These subpopulations may represent beige adipocytes, which, as noted above, are remarkable in their plasticity to revert between a dormant and thermogenic state 49,158. Future studies employing combined transcriptomics with chromatin accessibility profiling (e.g., snATAC-seq) may aid in predicting and understanding the major transcriptional drivers for such change in cell state.
MESENCHYMAL STROMAL CELL HETEROGENEITY
Preadipocytes and Fibro-adipogenic Progenitors
The subcutaneous inguinal WAT (iWAT) and intra-abdominal perigonadal WAT (gWAT) depots of mice were the focus of several of the first published adipose scRNA-seq analyses designed to interrogate stromal cell heterogeneity. The consensus from these studies is that there are at least two molecularly and functionally distinct subclusters of adipocyte progenitors reside within the iWAT and gWAT depots of adult mice 122,124,161–163. One subcluster represents cells with a high degree of adipogenic potential in vitro and upon transplantation in vivo and with expression of adipocyte marker genes (e.g., Pparg, LpL, Cd36) along with the common mesenchymal markers used for lineage tracing such as both Pdgfra and Pdgfrb, and thus can be considered, “preadipocytes”. These cells have been referred to by many names across both depots (Table 2), including “APCs” (adipocyte precursor cells) 164, “(ASC 1)” (adipocyte stem cell 1) 165, “VmSC4/5” (VAT mesenchymal stromal cell 4/5) 166, “FAP1/2” (fibro-adipogenic progenitor 1/2)124, “ES2/3” 167, and “mASPC1/5” (mouse adipose stem and progenitor cell 1/5) 122. Furthermore, some studies observed a highly committed preadipocyte subpopulation that expresses Adipoq and Lep, two lineage-defining transcripts associated with terminal differentiation of adipocytes 124,167. Bioimaging studies indicated that the committed preadipocyte subpopulation appear in proximity to the adipose vasculature, consistent with the long-standing hypothesis that preadipocytes are a subset of adipose perivascular mesenchymal cells 161,163.
Table 2.
Adipose stromal subpopulations identified by single-cell transcriptomics
| Subtypes | Depot | Published nomenclature | Signature transcripts | Functional properties | Human correlate (blank if unknown) | Published isolation strategies (CD45− CD31− = Lin−) | Tissue localization |
|---|---|---|---|---|---|---|---|
| Preadipocytes (proposed nomenclature) | iWAT | • P2167 • Group2/committed preadipocytes/ICAM1+ cells160 • ASC1164 • DPP4− PDGFRβ+ cells23 • CD81High APCs168 • IS3166 |
Pparg
Icam1 Pref1 Col4a2 |
committed preadipocytes | yes | Lin− ICAM1+ CD142− (Merrick et al.160) Lin− PDGFRβ+ DPP4−(Shao et al.162) |
perivascular |
| eWAT | • APCs74 • ASC1161 • VmSC4/5165 • FAP1/2123 • ES2/3166 • mASPC1/5121 |
Pparg
Cd36 Fabp4 Cd34 Lo |
• committed preadipocytes | yes | Lin− PDGFRβ+ LY6C− CD9− (Hepler et al.163) | perivascular | |
| FAPs (proposed nomenclature) | iWAT | • P1167 • Group1/interstitial progenitor cells/DPP4+ cells160 • ASC2164 • DPP4+ PDGFRβ+ cells23 • CD81Low cell168 • IS1166 |
Dpp4
Wnt2 Anxa3 Pi16 Cd55 |
• multi-mesenchymal lineage potential in vitro • adipogenic in vivo; give rise to preadipocytes • cold responsive; secrete IL-33 |
yes | Lin− DPP4+ CD142− (Merrick et al.160) Lin− PDGFRβ+ DPP4+ (Shao et al.162) Lin− SCA1+ CD55+ (Schwalie et al.167) |
reticular interstitium proximity to blood vessels |
| eWAT | • FIPs74 • ASC2161 • VmSC1/2/3165 • FAP3/4123 • CD34High cells169 • ES1166 • mASPC2/3121 |
Dpp4
Ly6c Fn1 Pi16 Il33 CD55 Cd34 High |
• resistant to adipogenesis (adulthood) • anti-adipogenic (adulthood) • robust proinflammatory response; promote macrophage accrual • robust collagen deposition • secrete IL-33; promote Treg accrual |
yes | Lin− PDGFRβ+ LY6C+ (Hepler et al.163) | within tissue mesothelium proximity to blood vessels | |
| Additional stromal subpopulations | iWAT | • Aregs160 • Group3160 |
Cd142, Clec11a
Fmo2 |
• secrete RSPO2, inhibit preadipocyte commitment (adulthood) | yes | Aregs: Lin− SCA1+ CD142++ (Dong et al.170) | – |
| ARCs171 |
CD36, Lgals3
Ccl6 |
• secrete CCL6; inhibit AP differentiation and proliferation | yes | Lin− CD36+ Lgals3+ | – | ||
| Brown adipocyte precursors | Interscapular BAT | ASC1172 |
Col5a3
Cxcl14 Bmper |
• ASC1: upregulation of proliferation and adipogenesis gene profiles upon cold exposure | – | mixed location: tissue fascia; interstitial; surrounding vessels; contact F4/80+ immune cells | |
| ASC2172 |
DPP4
Pi16 |
||||||
| ASC3172 |
Clec11a
Gdf10 |
||||||
| Trpv1+ VSM-APCs173 |
Trpv1 Sca1− Cd81− |
• proliferate upon cold exposure • give rise to brown and white adipocytes |
yes | Trpv1Cre reporter mice | vascular smooth muscle cells | ||
| perivascular BAT (PVAT) | intermediate cells174 |
Vipr2
Gli1 |
• resistant to adipogenesis | – | PDGFRa+ CD200+ | in between the vascular SMCs and adventitial progenitors | |
| SMC-2 (adult)174 |
Pparg
Trpv1 Myh11 |
• adipogenesis | yes | MCAM+ CD200− | aortic adventitia/vascular smooth muscle cells | ||
| Skeletal bone marrow APCs | bone marrow adipose tissue | MALPs175 |
Adipoq
Pparg Vegfa |
• committed preadipocytes lacking Plin1 expression and lipid droplets • expressed RANKL and promotes osteoclastogenesis |
– | – | bone marrow pericytes |
| EMPs175 |
Ly6a
Cd34 Thy1 Mfap5 Clec3b |
• number reduces with age • prefer adipogenesis to osteogenesis with age |
– | – | – |
Single-cell/nuclei RNA sequencing identified adipose stromal cell populations in WAT and BAT depots. Proposed nomenclature, published nomenclature, defining transcripts, proposed presence in human adipose tissue, experimentally validated isolation strategy, and tissue localization are indicated.
The second major cluster of mesenchymal stromal cells lacks Pparg expression and instead is enriched in gene signatures associated with extracellular matrix remodeling and inflammation. This cell cluster has also been referred to by many names across both the iWAT and gWAT depots (Table 2), including “fibro-inflammatory precursors” or “FIPs” 164, Group 1/"Interstitial progenitor cells” (IPCs), “adipocyte stem cell 2” (ASC 2)” 165, “VmSC1/2” 166, “FAP3/4” 124, “CD34High” cells 168, “ES1” 167, “collagen-rich (C09) progenitors” 151, and “mASPC2/3” 122. The proposed functions of these cells in iWAT and/or gWAT include the regulation of inflammation, collagen deposition, and adipogenesis. As such, this distinct subcluster of stromal cells exhibits many properties of interstitial skeletal muscle “fibro-adipogenic progenitors,” or “FAPs,” and could thus be considered the corresponding “FAPs” of adipose tissue 169. In iWAT and gWAT, FAPs can be identified based on DPP4 expression (DPP4+). FAPs have been observed in proximity of the adipose vasculature 163; however, they are readily apparent within the interstitial region on the outer edge of the depots 161. As such, adipose progenitor cells reside in multiple anatomical progenitor niches. Importantly, human subcutaneous adipose tissue harbors FAPs (DPP4+) and preadipocyes (DPP4-) populations that closely resemble the murine populations, suggesting that distinct progenitor subpopulations also exist in adult humans and can be selected based on DPP4 expression 161,170.
Depot, age, and sex-dependent Properties of Adipocyte Progenitor Cells.
Transcriptomic analyses based on scRNA-seq suggest that subcutaneous iWAT preadipocytes and FAPs are highly similar to their counterparts in intra-abdominal gWAT; however, functional analyses of these distinct subpopulations upon isolation reveal striking depot dependent properties. One notable depot difference lies in the adipogenic potential of isolated FAPs. Both preadipocytes and FAPs isolated from iWAT exhibit a strong capacity to differentiate into adipocytes in vitro and upon transplantation into mice. Here, iWAT FAPs give rise to committed preadipocytes in the process of forming adipocytes 161. This lineage relationship was predicted by cell trajectory analyses of single-cell transcriptomic profiles and supports the existence of a progenitor cell hierarchy 111,171. Cell trajectory analysis also suggests a hierarchical developmental relationship between gWAT preadipocytes and FAPs 124,167; however, multiple studies have demonstrated that adult gWAT FAPs lack significant adipogenic potential in vitro, even in the presence of strong pro-adipogenic stimuli such as PPARγ agonists. Moreover, gWAT FAPs, unlike iWAT FAPs, do not differentiate into adipocytes upon transplantation into lipodystrophic mice 164,166. Importantly, these in vitro assays and in vivo transplantation studies do not preclude the possibility that gWAT FAPs undergo adipocyte differentiation in vivo under some conditions. In fact, genetic lineage tracing indicates that FAPs expressing DPP4 in eWAT do undergo de novo adipocyte differentiation at a low frequency in association with HFD feeding 172. Additional studies will be needed to fully understand the developmental relationship between FAPs and preadipocytes in each depot and the relative contribution of each subpopulation to the maintenance and expansion of the adipocyte pool in vivo.
One of the surprising findings from scRNA-seq studies and the functional analyses of isolated subpopulations is the age-dependent changes in function of both preadipocytes and FAPs. In iWAT, a small subpopulation of stromal cells resembling preadipocytes emerges early in the postnatal period. After four weeks of age, this subpopulation, referred to as Aregs, lacks intrinsic adipogenic capacity and can exert an anti-adipogenic effect on adipose stromal cells in vitro and upon transplantation in vivo 173. Cells bearing the markers of Aregs can also be found in human adipose tissue; however, the functional properties of these are unknown. Another aging-associated stromal cell subpopulation, termed “aging-dependent regulatory cells (ARCs),” was also described 174. ARCs arise during aging in mice only in the iWAT depot. ARCs secrete CCL6 and other cytokines to inhibit the differentiation and proliferation of neighboring adipocyte progenitors. The emergence of these cells may contribute to the well-known reduction of subcutaneous adipose tissue mass and plasticity observed in human aging. Interestingly, isolated gWAT FAPs from adult mice have the capacity to inhibit preadipocyte differentiation through the production of secreted factors. This anti-adipogenic property of FAPs is not apparent at the perinatal stage but rather develops with age 175. Moreover, perinatal gWAT FAPs have a greater potential to undergo adipogenesis when Pparg is overexpressed or when exposed to PPARγ agonist compared to their adult counterparts 175.
Sex differences in adipose tissue remodeling and function are widely appreciated; however, less consideration has been given to the intrinsic sex differences in preadipocytes or FAP function. Bulk proteomics analyses of isolated stromal subclusters also highlight considerable sex differences in the expression of genes related to mitochondrial function and lipid metabolism 176. These findings should serve as a caution to investigators that the functional and molecular properties of adipose progenitors are depot, age, and sex dependent. Age and depot differences in the function of adipose stromal subpopulations may not be readily discerned by single-cell transcriptomics alone 176. As such, careful consideration should be made to these relevant biological variables in both the design, integration, and interpretation, of studies related to stromal cells.
Beige Adipocyte Progenitors
scRNA-seq has also been utilized to identify putative beige/BRITE adipocyte progenitors in murine iWAT. Fluidigm C1 system-based scRNA-seq identified a subset of PDGFRa+ Sca1+ cells marked by high levels of CD81 gene expression (CD81High) 177. CD81High stromal cells exhibit a vascular smooth muscle cell (VSMC) expression profile (enriched in Acta2 and Sm22 transcripts), congruent with prior lineage tracing studies, suggesting a contribution of the smooth muscle cell lineage to beige adipogenesis. In comparison to CD81Low cells, CD81High cells are more adipogenic in vitro and differentiate into beige adipocytes upon transplantation and cold stimulation in vivo. Genetic lineage tracing (CD81-CreERT2) of CD81+ cells provide clear evidence supporting the proliferation and differentiation of native CD81 expressing cells into iWAT beige adipocytes upon cold exposure. Notably, CD81 represents a functional marker of beige progenitor cells. CD81 forms a complex with integrins that mediate the integrin-FAK signaling cascade induced by irisin 177. Importantly, CRISPR-mediated inactivation of Cd81 attenuates cold-induced beige adipogenesis. Moreover, in line with the rodent data, reduced frequency of CD81+ cells in human subcutaneous WAT is an indicator of metabolic risk, indicating that beige adipocyte biogenesis influences systemic metabolic health. CD81 expression is present within a high proportion of iWAT PDGFRα+ cells, enriched within the aforementioned DPP4-preadipocyte pool. As such, whether CD81+ progenitors also give rise to white adipocytes under permissive conditions remains unclear.
Classical brown adipocyte progenitors
In adult rodents, the adaptation to cold environmental temperatures involves activation of preexisting brown adipocytes within BAT as well as de novo differentiation of brown fat cells from resident progenitor cells. Single-cell RNA-seq of BAT stromal vascular cells from mice exposed to cold temperatures (5°C) for either 2 or 7 days followed by cell lineage trajectory analysis pointed to vascular smooth muscle cells (VSMCs) expressing TRPV1 as possible progenitors to cold-induced brown adipocytes 178. Lineage tracing using the Trpv1-Cre line, supported this hypothesis and showed that genetically labeled Trpv1-lineage cells proliferate upon cold exposure and differentiate into labeled thermogenic adipocytes. Trpv1 expression in BAT appears quite specific to VMSCs and these data therefore support the notion of a mural cell origin of adipocytes 179.
Independent single-cell studies of thermogenic adipose depots raise the likelihood that multiple pools of distinct stromal subpopulations give rise to thermogenic adipocytes. scRNA-seq analysis of BAT stromal vascular cells following cold exposure identified multiple fibroblastic Pdgfra-expressing subpopulations with distinct tissue localization 180. One subpopulation, termed “ASC1,” proliferates upon cold exposure and appear poised for differentiation. Genetic pulse-chase lineage tracing confirms that Pdgfra-expressing cells undergo brown adipogenesis upon cold exposure. Importantly, Pdgfra-expressing fibroblasts are distinct from Trpv1+ VSMCs, indicating that multiple progenitor cells populations contribute to cold-induced brown adipogenesis in iBAT.
Aortic perivascular adipose tissue (PVAT) shares several characteristics with iBAT and harbors distinct progenitor cells committed to the brown adipocyte lineage 181. scRNA-seq analysis of perinatal mouse PVAT supported the notion of brown adipocyte progenitor heterogeneity and indicated that VSMCs as well as multiple Pdgfra-expressing fibroblastic subpopulations, including committed preadipocytes (Pparg+) and stem cell-like mesenchymal progenitors bearing resemblance to the multipotent cells identified in iWAT (Pi16+) 182. At this stage of development, the preadipocytes and FAPs, but not the VSMCs, are able to differentiate to brown adipocytes in vitro. The same progenitor subpopulations are present in adult mouse aortic PVAT; however, here Trpv1+ VSMCs can also undergo brown adipocyte differentiation. Lineage tracing using the Myh11-Cre confirmed a contribution of VSMCs to the maintenance of brown adipocyte number in adult PVAT 182. Collectively, these data support a model in which multiple progenitor populations are utilized within a tissue, with adipogenic PDGFRα+ stromal cells driving tissue genesis, and adipogenic VSMCs serving to help maintain tissue homeostasis in adults. Moreover, these data further highlight how the adipogenic potential of cell populations can change over time.
Non-classical adipose depots: Dermal and skeletal adipose stromal cells
Some of the most plastic WAT depots are relatively understudied and one such depot is the dermal white adipose depot (dWAT). dWAT plays an important role in a wide range of processes including local infection, hair cycling, and wound healing 183–186, and dWAT mass expands and contracts in association with hair cycling and wound healing 187. scRNA-seq studies have fueled the discovery of an unexpected plasticity of adipocytes within dWAT. Lineage tracing of mature adipocytes and scRNA-seq analysis revealed that mature adipocytes can “de-differentiate” under pathophysiological and physiological conditions into FAP-like cells that can subsequently re-differentiate into mature adipocytes 187,188. This phenomenon of mature adipocytes reverting to a more primitive lineage state was also unveiled through single cell transcriptomics of mammary gland remodeling during lactation and involution as well as during adipose tissue remodeling in response to a tumor microenvironment 52,189. As such, scRNA-seq has been instrumental in revealing previously unrecognized lineage relationships.
Bone marrow adipose tissue (BMAT) represents another depot with tremendous plasticity and heterogeneity 190–192. Here, scRNA-seq has been utilized to explore the heterogeneity of skeletal mesenchymal stromal cells 193. Cell trajectory analysis identified multiple progenitor subpopulations representing different stages of lineage commitment, and the study revealed the presence of a unique population of committed preadipocytes expressing Adipoq, Pparg, Cebpa, and Lpl, but devoid of Plin1 and lipid droplets. These cells, termed “marrow adipogenic lineage precursors” (MALPs) closely resemble pericytes. MALPs are readily found within bone marrow capillaries where they express Pdgfrb and share a basement membrane with endothelial cells. MALPs express notable angiogenic factors, including Vegfa, and genetic ablation of these cells resulted in a loss of overall blood vessel density. The identification of MALPs reinforces the notion that pericyte-like cells contribute to the adipogenic lineage and further highlights the multifaceted roles of adipose progenitors in the regulation of tissue homeostasis.
IMMUNE CELL HETEROGENEITY
Obesity-associated adipose tissue macrophages
Adipose tissue macrophages (ATMs) play an important role in adipose tissue homeostasis and adaptive remodeling 76,194. Obesity is associated with a major increase in the recruitment of bone marrow-derived monocytes into adipose tissues, contributing to a dramatic accumulation of ATMs promoting metabolic inflammation and insulin resistance 195. ATMs were initially thought to undergo polarization towards proinflammatory M1 macrophages under obese conditions 196,197; however, in-depth analyses of adipose immune cell populations from mice and humans challenged this view and indicated that obesity rather programs ATMs towards lipid uptake, storage, and catabolism 198–200. Recently, single cell technologies have made it possible to resolve the full heterogeneity and plasticity of the adipose immune cell compartment (Figure 4), and these studies all support the notion that ATMs are not strongly polarized towards the classical M1 and M2 macrophage states. Instead, scRNA-seq of human and mouse adipose immune cells or snRNA-seq of whole adipose tissues indicate that macrophage subpopulations constitute a continuum of cell states, where some are more proinflammatory, and others are more metabolically active 124,165,201–204. Importantly, careful computational analyses using the MacSpectrum tool to classify all macrophages according to their polarization and differentiation indexes support the absence of classical M1 and M2 states 205.
Figure 4: Immune cell (myeloid lineage) composition of adipose tissue.
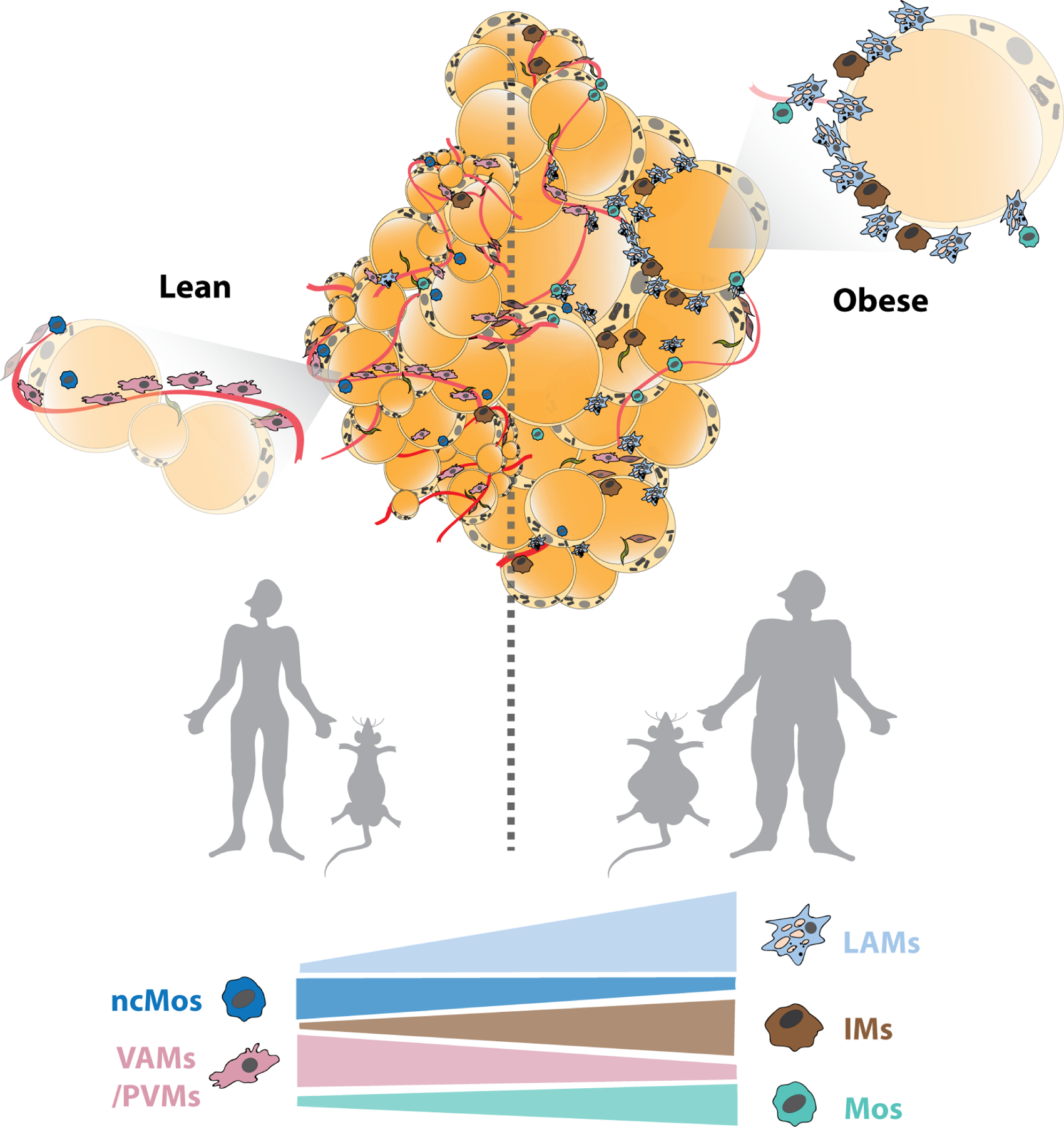
Obesity-associated remodeling of the myeloid-cell immune compartment of adipose tissue in mice and humans. VAMs/PVMs and ncMos are abundant in the lean adipose tissue, whereas the balance tips towards an enrichment of LAMs, IMs and Mos during obesity progression in mice as well as humans. Abbreviations: LAM, lipid associated macrophage; PVM, perivascular macrophage124; IM, inflammatory macrophage 206; Mos, Monocyte subpopulations Mo1 and Mo2; ncMos, non-canonical monocytes 203.
Interestingly, recent results from high-resolution scRNA-seq of Lin+ cells in murine and human obesogenic conditions 203,206, CITE-seq analyses of mouse eWAT 61, and whole tissue snRNA-seq of mouse eWAT 122,124 and human WAT 122 showed that obesity induces a unique ATM subpopulation marked by Cd9 and Trem2. This subpopulation expresses high levels of lipid handling genes including Cd36, Lpl and Lipa, and is now consensually termed lipid-associated macrophages (LAMs) (Table 3). LAMs are enriched at crown-like structures, where they appear to play an important and Trem2-dependent role in adipose tissue remodeling 203. Intriguingly, some studies report a smaller subpopulation of LAMs that expresses proliferative genes, hence termed “proliferative-LAMs” (P-LAMs) 124 or “cell-cycling macrophages” (CMs) 61, indicating that the pool of LAMs can expand by local proliferation and not just by increased recruitment and activation of monocytes. In addition to LAMs, “inflammatory macrophage” (IM) subpopulations with high expression of inflammatory gene programs have been shown to be positively associated with obesity 206,207.
Table 3.
Macrophage subpopulations in adipose tissue
| Species | Gender | Depot | Subpopulationa | Markers | Pathways/polarization | Obesity correlation (−negative/+positive) | Reference |
|---|---|---|---|---|---|---|---|
| Mouse (C57BL/6J) | males | WAT (epididymal) | CD11b+ Ly6c+ | Ly6c, Cd74, Dab2, Mgl2 | vascular development and organization | ++ | Xue et al.200 |
| Ly6c− CD9− CD206+ | CD206 | perivascular-like | − | ||||
| Ly6c− CD9+ | Cd9, Lpl, Plin2, Lamp2 | proinflammatory pathway + lysosomal pathways + lipid handling | ++ | ||||
| Mac1 | Cd163, Lyve1, Cd209f | perivascular | − | Jaitin et al.201 | |||
| Mac2 | Cd9, Nceh1 | not reported | + | ||||
| Mac3 | Cd9, Spp1, Trem2, Lipa, Lpl | lipid-associated | ++ | ||||
| LAM | Trem2, Cd9, Lpl | phagocytosis + lipid handling+ chemokine signaling | ++ | Sárvári et al.123 | |||
| PVM | Lyve1, Cd163 | statin pathway + EGFR signaling + oxidative stress and redox | − | ||||
| NPVM | Cd74, Fcrls, Ear5 | phagocytosis + chemokine signaling + PPAR signaling | − | ||||
| CEM | Col5a2, Tgfbr3, Col3a1 | focal adhesion + PI3K-Akt-mTOR signaling | + trend | ||||
| P-LAM | Pola1, Kif11, Kif15 | proliferative pathways + statin pathway | + | ||||
| RM | Prg4, Tgfb2, Ltbp1 | eicosanoid synthesis + chemokine signaling | no correlation | ||||
| TRM | Klf4, Cbr2, and Stab1, Selenop | perivascular-like | − | Cottam et al.61 | |||
| LAM | Trem2, Cd9, Lpl | lipid handling | + | ||||
| CM | Stmn1, Pclaf | cell cycling | + | ||||
| BAT (intrascapular) | matrix macrophages | Ecm1, MMP12, MMP19, Fn1 | tissue remodeling | not reported | Silva et al.208 | ||
| macrophages M2-like | Mrc1, Clec10a, C1qa, C1qb | M2-polarized | |||||
| macrophages Lplhi | CD36, Lpl, Lipa | lipid-associated | |||||
| macrophages Plin2hi | Fabp4, Trem2, Plin2 | lipid-associated | |||||
| IS2 | Cd9, Cd68, Lpl, Lipa, Cd36 | lipid metabolism | + | Li et al.207 | |||
| WAT (inguinal + perigonadal) | IS3 | Cxcl2, Cxcl3, CCL3 | proinflammatory macrophages | not reported | |||
| IS9 | FOLR2, Klf4 | M2-like | not reported | ||||
| males + females | mMac1 | Fgf13 | not reported | ++ in epididymal | Emont et al.121 | ||
| mMac2 | Plekhg5 | − in epididymal | |||||
| mMac3 | Trem2 | + in epididymal | |||||
| mMac4 | Prg4 | − in epididymal and periovarian | |||||
| Human | men + women | WAT (subcutaneous + abdominal) | Macrophage (M1-like) | CD68, TREM2 | not reported | not reported | Bäckdahl et al.150 |
| Macrophage (M2-like) | FOLR2, CD163 | ||||||
| hMac1 | PLEKHG5 | not reported | no correlation | Emont et al.121 | |||
| hMac2 | TREM2 | no correlation | |||||
| hMac3 | PROS1, CLEC10A | + | |||||
| women | WAT (subcutaneous) | LAM | TREM2, CD9, LPL | lipid-associated | + | Weinstock et al.204 | |
| PVM | LYVE1, SELENOP, C1Q | perivascular | − | ||||
| IM | CCL3L1, TNF, CXCL3 | inflammatory | ++ |
Adipose tissue macrophage (ATM) subpopulations in mouse and human WAT and BAT depots. Proposed nomenclature and expression of specific marker genes, enriched pathways, and/or polarization are indicated. Association with obesity, wherever reported, is depicted as + (positive correlation), ++ (strong positive correlation), – (negative correlation), or ‘‘no correlation.’’ Mac1–3, mMac1–4 (mouse), and hMac1–3 (human) are macrophage subpopulations identified in Emont et al.121 IS2/3/9 are immune subpopulations defined as macrophages in Vijay et al.207 LAM, lipid-associated macrophage; PVM, perivascular macrophage123; NPVM, non-perivascular macrophage; CM, collagen-expressing macrophage; P-LAM, proliferating LAM; RM, regulatory macrophage123; TRM, tissue resident macrophage; CM, cycling macrophage61; IM, inflammatory macrophage.206
The subpopulations that seem to have similar markers and gene programs identified across different studies can be grouped as CD11b+ Ly6c+, Ly6c-CD9-CD206+, Mac1, PVM, TRM, macrophages M2-like, macrophage(M2-like), hMac3; Ly6c− CD9+, mac3, LAM, P-LAM, macrophages Lplhi, macrophages Plin2hi, IS2, mMac3, macrophage(M1-like), hMac2; CEM, matrix macrophages; IS3, IM.
Single cell studies have also investigated macrophage subpopulations during obesity regression. Analysis of mouse eWAT by CITE-seq during weight loss demonstrated a decrease in LAMs with weight loss; however, despite the complete normalization of body and tissue weight, the abundance of LAMs in the tissue was higher than that of the lean controls 61. In addition, the weight regain group in the study showed a rapid increase in LAMs, over and above the obese cohort, suggesting that these cells may encode an obesogenic anticipation or memory.
Despite the clear depot and species differences between obesity-associated ATMs, the metabolic and inflammatory programming is relatively conserved across WAT depots and species (Table 3). A recent direct comparison of mouse and human WAT depots 122 reported differences in the relative abundance of myeloid and lymphoid cell populations across species and depots (Table 3); however, the transcriptomic profiles of the individual macrophage populations were notably similar. Surprisingly, however, this study did not seem to detect the lipid handling gene program in their Trem2+ LAMs.
Vascular associated/perivascular macrophages
Various studies across depots and species have detected subpopulations of macrophages that express genes relevant for vascular development and/or matrix remodeling and maintenance 124,201,208–210. The consensus across studies suggests that these “vascular-associated macrophages” or “perivascular macrophages” (VAMs or PVMs), marked by Lyve1, are likely the most abundant tissue resident macrophages in lean WAT. The relative abundance of VAMs and PVMs have been shown to decrease with obesity; however it is unclear whether this is due to an increased conversion of PVMs to IMs, as suggested by RNA velocity analyses 206, or primarily due to the large increase in LAM and IM populations. Interestingly, a reduction in the abundance of VAMs in eWAT was observed in response to acute fasting 210. Notably, the VAMs and PVMs co-express M2-like markers (such as Mrc1, Clec10a, and Cd163) and genes involved in the regulation of complement function and angiogenesis (Table 3). Finally, a minor subpopulation of ATMs with high levels of collagens, especially Col3a1 and Col5a2, have been identified as collagen-expressing macrophages (CEMs) in mouse eWAT124.
Monocyte heterogeneity and link to ATM subpopulations
Single cell studies have also identified two subpopulations of monocytes in mouse eWAT, termed “Mon 1” and “Mon 2” 203, or “classical” and “nonclassical” 61, whereas three subpopulations have been reported in human sWAT (“Mo-1”, “Mo-2” and “non-canonical”) 206 and mouse iBAT (Ly6clow, Ly6cint, Ly6chigh) 211 (Table 4). Interestingly, RNA velocity projections indicate that Mo-1 give rise to PVM and IMs in human sWAT, whereas Mo-2s represent an additional transition state between Mo-1s and IMs in obesity. During obesity, Mo-1 appears to preferentially transition to IMs, whereas PVMs transition to LAMs 206.
Table 4.
Other myeloid cell subpopulations in adipose tissue
| Myeloid cell type | Species | Gender | Depot | Subpopulation | Markers | Obesity correlation (−negative/+posotive) | Reference |
|---|---|---|---|---|---|---|---|
| Monocytes | mouse (C57BL/6J) | males | WAT (epididymal) | Mon1 | Fn1, Retnla | – | Jaitin et al.201 |
| Mon2 | Plac8, Clec4e | no correlation | |||||
| classical | Ccr2, Ly6c2 | no correlation | Cottam et al.61 | ||||
| non-classical | Ear2, Cx3cr1 | no correlation | |||||
| BAT (intrascapular) | monocytes Ly6clow | Treml4 | not reported | Silva et al.208 | |||
| monocytes Ly6cint | Plac8 | not reported | |||||
| monocytes Ly6chi | Ly6c2 | not reported | |||||
| human | women | WAT (subcutaneous) | ncMos | FCGR3A, HES4 | – | Weinstock et al.204 | |
| Mo-1 | FCER1A | + | |||||
| Mo-2 | CSF3R, FCAR, SELL | + | |||||
| Dendritic cells | mouse (C57BL/6J) | males | WAT (epididymal) | cDC1 | Clec9a, Xcr1 | + | Cottam et al.61 |
| activated cDC1 | Clec9a, Xcr2, Ccr7 | + | |||||
| cycling cDC1 | Clec9a, Xcr1, Pclaf, Stmn 1 | no correlation | |||||
| cDC2 | Sirpa, Cd209 | − | |||||
| activated cDC2 | Sirpa, Cd210, Ccr7 | + | |||||
| cycling cDC2 | Sirpa, Cd209, Pclaf, Stmn 1 | no correlation | |||||
| moDCs | Ear2 | − | |||||
| human | women | WAT (subcutaneous) | cDC1 | IRF8, DPP4, CADM1, XCR1 | + | Weinstock et al.204 | |
| cDC2A | CD1C, IRF4, IL7R, LAMP3 | + | |||||
| cDC2B | CD1C, IRF4, FCER1A, CLEC10A | + |
Subpopulations of adipose tissue monocytes and dendritic cells across white and brown adipose depots in mice and humans, defined by the expression of specific marker genes. Their association with obesity, wherever reported, is depicted as + (positive correlation), − (negative correlation), −− (strong negative correlation), or ‘‘no correlation.’’ Mon1/2 and Mo1/2, monocyte subpopulations; ncMos, non-canonical monocytes; cDCs, conventional dendritic cells.201
ATM subpopulations in BAT
Single cell sorting and transcriptome analyses have also enabled an extensive characterization of the monocyte and macrophage subpopulations of BAT 211, many of which seem to be concordant with those identified in WAT. For example, BAT also contains lipid-associated Lplhi and Plin2hi subpopulations, as well as M2-like and matrix macrophages analogous to the LAMs, M2-like, and CMs, of eWAT 211(Table 3). However, further analyses are required to determine the functional similarities between these subpopulations across depots. Trajectory analysis of monocyte subpopulations in BAT indicates that Ly6chigh monocytes give rise to LAMs, which further bifurcates into terminally differentiated M2-like or matrix macrophages 211.
Dendritic cells
Adipose tissue dendritic cells (ATDCs) have been previously associated with obesity 212, and single cell/nuclei analyses have now identified immuno-regulatory subpopulations in mouse and human WAT 61,206. Two major subpopulations of conventional DCs (cDC1 and cDC2) have been found across mouse eWAT and human sWAT. While in humans two further subclusters of cDC2 (cDC2A and cDC2B) were defined based on specific gene expression modules (Table 4), mouse ATDCs were further assigned to subclusters based on their activation state (Ccr7 expression) and proliferation markers. In addition, monocyte-derived DCs (moDCs) were found to be enriched in lean mouse WAT. Interestingly, activated cDCs were elevated in obesity and failed to regress upon weight loss in mice 61.
Lymphoid cells
The heterogeneity and plasticity of the lymphoid immune compartment of adipose tissue is much less understood. Recent studies have indicated that the adipose tissue lymphoid cells also form subpopulations with some being more responsive to thermogenic cues and others being more responsive to obesogenic cues. Multiple subpopulations of tissue resident T (Naïve, memory and regulatory) and B lymphocytes were reported in mouse iWAT 40, eWAT 61, and human sWAT 206. In addition, subpopulations of effector memory T cells in the human subcutaneous and omental adipose tissue (CD8+, CCL5 expressing cells), as well as in mouse eWAT (CD8+ TEM) were shown to increase with obesity 61,206,207. Furthermore, single-cell analyses of human sWAT-resident immune cells demonstrated subpopulations of adipose-resident Natural Killer (NK) cells and innate lymphoid cells (ILCs) 206. Investigation of the developmental trajectories and inflammatory interactome of the different ILCs identified the ILC3 subpopulation to be positively linked to obesity. Interestingly, iWAT-resident immune cell subpopulations have been shown to undergo cold-induced myeloid-to-lymphoid transition 41.
MESOTHELIAL AND ENDOTHEIAL CELL HETEROGENEITY
Intra-abdominal WAT depots in both humans and rodents contain a defined mesothelium composed of a cobblestone-like monolayer of cells with a mixed mesenchymal/epithelial expression profile. The primary function of this layer is to provide a protective, non-adhesive surface within the abdominal cavity 213; however, several additional functions of the adipose-associated mesothelium have been hypothesized 214. Studies of human WAT suggest that these cells adopt a pro-inflammatory phenotype in obesity 215.
Lineage tracing studies in mice have suggested a mesothelial cell origin of intra-abdominal adipocytes 216; however, recent studies utilizing a more specific Cre-driver targeting epithelial cells (Krt19-Cre) did not find evidence of a mesothelial origin of fat cells. Single cell/nuclei transcriptomics have offered an unprecedented view of these cells and have identified distinct mesothelial subpopulations which are largely conserved between mice and humans and appears to be differentially regulated in obesity 122,207.
Endothelial cells represent another important epithelial cell type within adipose tissues. The importance of endothelial cells in the regulation of nutrient transport, hormonal signals, and inflammation is clear; however, the potential heterogeneity of these cells within adipose tissue is still largely unexplored. Single-cell transcriptomic studies have highlighted the presence of multiple endothelial subpopulations in mouse and human WAT 122,207,217, likely representing arteriolar, venular, and lymphatic endothelial cells. One subpopulation expresses high levels of CD36 and other genes involved in lipid metabolism, possibly representing “lipid handling” endothelial cells 207. Endothelial cell diversity has also been studied through scRNA analyses of several other tissues 218,219. Integrating these data with single cell transcriptomics data from adipose tissues may help unveil WAT-specific properties of endothelial cells.
MECHANISTIC INSIGHTS FROM SINGLE CELL OMICS
Intercellular Communication
Intercellular communication between adipocytes and various cellular constituents of the adipose microenvironment is critical for adipose tissue homeostasis and the adaptation to environmental and physiological challenges. The ability to profile adipose tissue transcriptomes at single cell resolution, combined with emerging computational tools, allows for prediction of previously unrecognized intercellular signals. Such predictions can be made based on the expression of known ligand-receptor pairs across different cell types 146,147. This approach was used to predict potential pro-adipogenic ligands acting on preadipocyte receptors in eWAT 124, and to predict interactions between adipocytes and adipose progenitors and endothelial cells in both murine and human obesity 122. Similarly, ligand-receptor expression was used to infer cellular crosstalk within adipose tissue as well as potential muscle-adipose crosstalk during exercise training 220. These computational approaches have huge hypothesis-generating potential; however, experimental studies are required to further investigate and carefully validate predictions.
Recently, several more concrete examples of intercellular communication have emerged involving newly identified stromal cell subpopulations in adipose tissue (Figure 5). For instance, R-spondin2 (RSPO2), an enhancer of WNT signaling, was identified as a secretory protein enriched in the aforementioned Areg subpopulation. RSPO2 targets the Leucine Rich Repeat Containing G Protein-Coupled Receptor 4 (LGR4) in FAPs to block their transition to a more committed preadipocyte state 221. Likewise, scRNA-seq and follow-up multi-omics analyses of adipose progenitors identified a hypoxia-inducible factor (HIF)1α-dependent inhibitory signaling mechanism underlying sex and depot differences in preadipocyte activity in mouse iWAT and gWAT following the onset of HFD feeding 163. Activated HIF1α signaling drives the production and secretion of PDGFs, which act in an autocrine/paracrine manner to drive a PDGFR-ERK signaling cascade culminating in inhibitory phosphorylation of PPARγ at serine 112 163.
Figure 5: Critical insights from single-cell omics analyses.
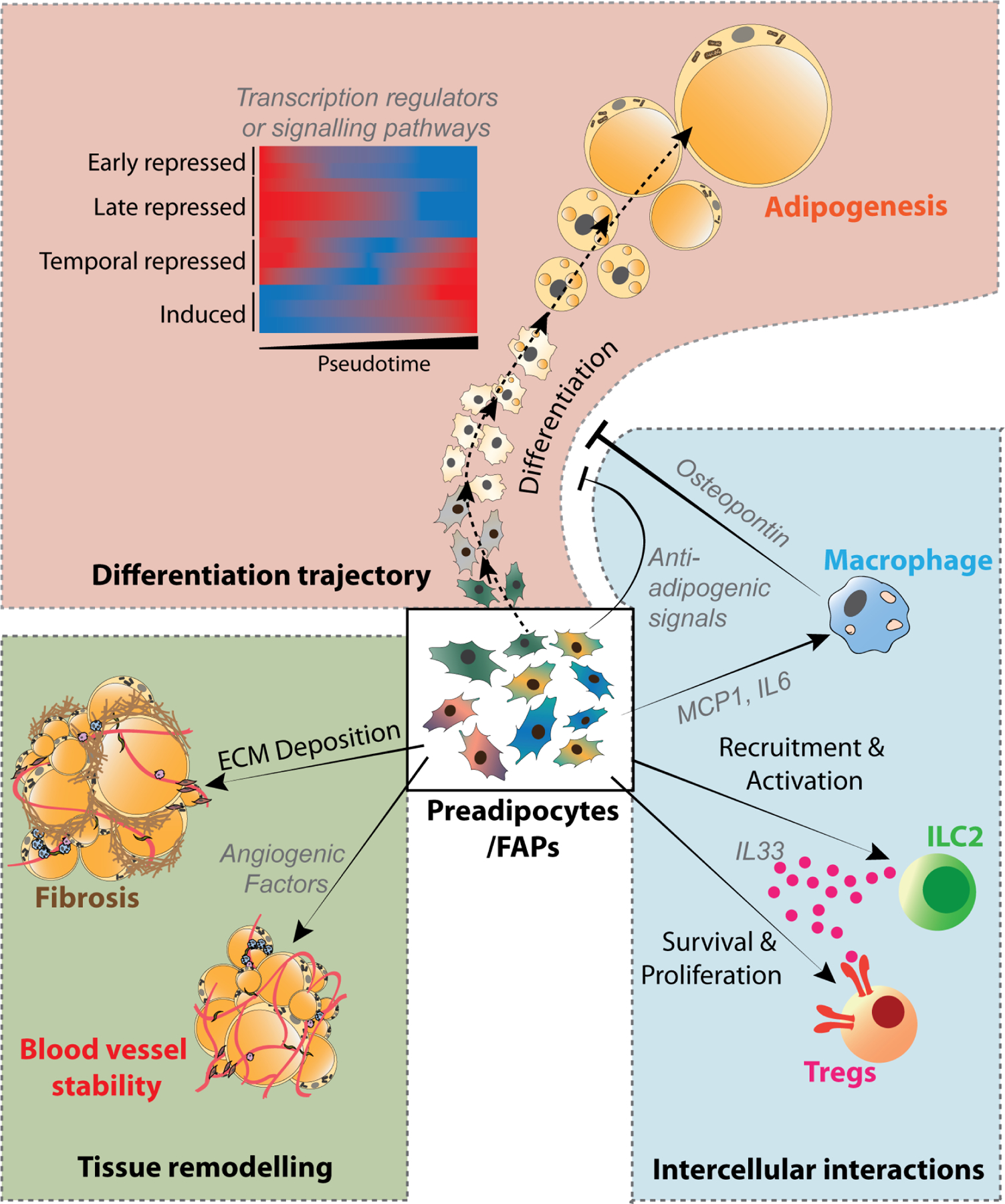
Single-cell omics has revealed novel insight into in vivo adipogenesis, stromal cell-immune cell crosstalk, and intrinsic regulators of tissue remodeling. Pseudo time analysis of gene programs revealed distinct gene modules that are early/late repressed, induced or transiently repressed during the progression of adipogenesis 124. Mesenchymal stromal cells, including preadipocytes and FAPs, secrete numerous collagens and modifiers of the extracellular matrix (ECM), thereby contributing to the pathologic tissue fibrosis observed in association with obesity 163,222. These cells can also secrete angiogenic factors that promote blood vessel growth/stability 193. Moreover, adipose stromal cells secrete proteins that act in an autocrine or paracrine manner to stimulate or suppress adipocyte differentiation 163,221, and they also modulate the local immune cell composition through the production of pro- (e.g. MCP1 and IL6) 223 and anti-inflammatory factors (e.g. IL-33) 166,224,225. Importantly, macrophages can influence the adipogenic and fibrogenic activity of adipose progenitors through the production of secreted factors (e.g., Osteopontin) 222.
Another emerging theme is the importance of intercellular communication between select stromal subpopulations and distinct immune cell types. By applying NicheNet to single-cell transcriptomic data it was proposed that senescent CD9+ macrophages accumulating in obesity block the adipogenic potential of stromal cells via secreted factors (e.g., Osteopontin) 222. The communication between immune cells and adipose progenitors appears bi-directional. Within the first few days of HFD feeding, eWAT FAPs activate the expression of notable pro-inflammatory cytokines (e.g., Il6, Ccl2) and extracellular matrix components to a much greater degree than preadipocytes 223. Genetic mouse models support a critical role for activated FAPs in the development of metabolic adipose tissue inflammation associated with diet-induced obesity 223. Other scRNA-seq studies of stromal cells from eWAT have revealed the selective expression of IL-33 in FAPs of lean adult mice 166,224. IL-33 promotes the activity of Tregs, immune cells that are critical to maintain an anti-inflammatory phenotype in adipose tissue. Inactivation of Il33 in Pdgfra-expressing cells (which include FAPs) attenuates Treg accumulation in gonadal adipose tissue 166,224. These results indicate that, eWAT FAPs play a role in regulating adipose immune cell composition under homeostatic conditions.
Within iWAT, FAPs represent the principal source of IL-33 225. Following acute cold exposure, Il33 is induced in these cells through activation of β-adrenergic receptor/CREB signaling 225. In the context of thermogenic remodeling, IL-33 drives the recruitment and activation of ILC2s to enhance beige adipocyte accrual 226. Contemporaneous studies support and extend these observations and show that IL-33 expression is enriched within the Bst2High subset of FAPs, which are suggested to reside in proximity to the lymph node within iWAT 167. This is a region known to be enriched for beige adipocytes following cold exposure. Surgical dissection of the iWAT lymph node attenuated beige adipocyte accumulation, suggesting that the lymph node might provide key signals that modulate thermogenic tissue remodeling 167. Taken together, scRNA-seq studies have highlighted the important role of crosstalk between adipose progenitors and immune cells in regulating tissue homeostasis and the adaptation to environmental challenges.
Differentiation Trajectories and Transcriptional Drivers
One of the exciting outcomes of snRNA-seq analyses of adipose tissue is the ability to apply pseudotime trajectory analysis tools to map the different stages of in vivo adipogenesis. Using this approach, the adipogenic trajectory from early preadipocytes, late preadipocytes and transitioning cells to mature adipocytes could be mapped in lean and obese eWAT 124. The finding that very few transitioning cells can be detected and that late preadipocytes appear to accumulate at the state just prior to the transition to mature adipocytes suggested the existence of a metastable state highly sensitive to differentiation cues. Furthermore, these findings indicate a switch-like mechanism for induction of differentiation. One of the interesting questions is which are the cues and the drivers that control this switch. Based on more than two decades of analyses of in vitro adipogenesis, it seems likely that a main driver of this switch is activation of the master regulator PPARγ above a certain threshold level. Projection of differential gene expression on the pseudo time scale revealed the sequential expression of gene programs during in vivo adipogenesis, including the temporal expression of transcriptional regulators that are likely to drive activation of adipocyte genes 124. This type of analyses combined with single cell epigenomic studies will be instrumental for gaining insight into the transcriptional mechanisms driving cellular transitions, including adipogenesis, in vivo.
PERSPECTIVES, CHALLENGES, AND OPPORTUNITIES
The rapid development of single-cell transcriptomics and associated analytical tools has completely reshaped our understanding of the distinct subpopulations of adipocytes, stromal cells, and immune cells, that reside in adipose tissues. The discovery of these new cellular subpopulations has provided new insight into the complex mechanisms by which adipose tissues adapt and remodel under various conditions depending on age, anatomical location, and sex. Moving forward it will be important to determine the role of different cellular subpopulations in defining sex, age, and depot-specific functions of WAT and in regulating short-term and long-term plasticity including metabolic functions of WAT.
The identification of subpopulation selective markers has enabled refinements of the classical strategies to study adipose tissue biology. In particular, the identification of new cell surface markers of stromal cells has fueled the development of new strategies for the isolation and functional analysis of distinct subpopulations by FACS. This now allows investigators to complement classical cell culture studies of primary cells and immortalized preadipocyte cell lines.
Nevertheless, the growing appreciation for intra-and inter-depot heterogeneity now presents numerous challenges to investigators in the field and raises important questions. For instance, do the distinct subpopulations of adipocytes and adipocyte progenitors cells identified within a given depot represent distinct cellular subtypes with distinct cellular origins and stable intrinsic properties (e.g., brown adipocytes vs. white adipocytes), or do these distinct cell subpopulations represent interconvertible “cell states”? Is there even more heterogeneity than current approaches can delineate? The depth of sequencing for individual genes within a single cell can limit the sensitivity of defining subtle differences within cell populations. Furthermore, what is the functional significance of the individual cellular subpopulations? Addressing the functional importance of cell populations and their signaling properties in vivo is an enormous challenge given the lack of subpopulation specific strategies for targeting adipocytes, adipose progenitors, or immune cells. The development of intersectional-CRE strategies to target cell subpopulations based on the expression of two defining genes may help gain specificity 227. Moreover, cell type specific targeting based on cell surface receptors may also constitute a valuable avenue 228.
A major challenge moving forward will be to understand to intercellular crosstalk and the role of this in shaping cellular subpopulations. The emergence of high-resolution spatial omics including transcriptomics approaches constitutes an important next step in understanding cellular subpopulations with their microenvironment. The integration of spatial omics data with single nucleus transcriptomics and epigenomics data constitutes a powerful future approach for obtaining detailed insight into the role of intercellular signaling mechanisms in adipose tissue plasticity.
Finally, as the field moves forward, the ability to integrate datasets gathered from independent studies will rely on unified nomenclature and accurate metadata that enables an accounting for these biological variables. The challenges associated with complexity of adipose tissue can undoubtedly be daunting; however, embracing these challenges may provide opportunities to unravel the natural cellular and molecular mechanisms that allow adipose tissue to maintain its plasticity and function, and identify those mechanisms which are disrupted in obesity. Such efforts may lead to the development of new therapeutic strategies to shape the health and function of adipose tissue as part of an effort to prevent and treat metabolic diseases.
ACKNOWLEDGEMENTS
B.M. and S.M. are supported by the grants from the Danish National Research Foundation (DNRF grant no. 141 to the Center for Functional Genomics and Tissue Plasticity [ATLAS]) and grants from the Novo Nordisk Foundation (NNF18OC0033444 Challenge Grant to the Center for Adipocyte Signaling). Furthermore, B.M. is supported by the EMBO post-doctoral fellowship (ALTF 146-2021). Q.Z. and R.K.G. are supported by National Institutes of Health NIDDK awards R01 DK104789, RC2 DK118620, and R01 DK119163 to R.K.G. B.M and S.M. would like to acknowledge Elvira Laila van Hauwaert as well as other member of Mandrup Group for the discussions and inputs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
All the authors declare that they have no competing financial interests.
REFERENCES
- 1.Rosen ED, and Spiegelman BM (2014). What we talk about when we talk about fat. Cell 156, 20–44. 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, and Kirkland JL (2013). Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17, 644–656. 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagchi DP, Forss I, Mandrup S, and MacDougald OA (2018). SnapShot: Niche Determines Adipocyte Character I. Cell Metab 27, 264–264.e261. 10.1016/j.cmet.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwick RK, Guerrero-Juarez CF, Horsley V, and Plikus MV (2018). Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metabolism 27, 68–83. 10.1016/j.cmet.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, Zhu Y, Kusminski CM, Hassan G, Gupta RK, et al. (2018). An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab 27, 252–262.e253. 10.1016/j.cmet.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P, and Kajimura S (2021). The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol 10.1038/s41580-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinti S (2005). The adipose organ. Prostaglandins Leukot Essent Fatty Acids 73, 9–15. 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard J, and Cannon B (2018). Brown adipose tissue as a heat-producing thermoeffector. Handb Clin Neurol 156, 137–152. 10.1016/B978-0-444-63912-7.00009-6. [DOI] [PubMed] [Google Scholar]
- 9.Chouchani ET, Kazak L, and Spiegelman BM (2019). New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab 29, 27–37. 10.1016/j.cmet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Chait A, and den Hartigh LJ (2020). Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 7, 22. 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger MA, and Andrews MT (2018). Nature's fat-burning machine: brown adipose tissue in a hibernating mammal. J Exp Biol 221. 10.1242/jeb.162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakers A, De Siqueira MK, Seale P, and Villanueva CJ (2022). Adipose-tissue plasticity in health and disease. Cell 185, 419–446. 10.1016/j.cell.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilherme A, Pedersen DJ, Henchey E, Henriques FS, Danai LV, Shen Y, Yenilmez B, Jung D, Kim JK, Lodhi IJ, et al. (2017). Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Molecular Metabolism 6, 781–796. 10.1016/j.molmet.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen MC, and Shulman GI (2018). Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev 98, 2133–2223. 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi M, Fujisaka S, Cai W, Winnay JN, Konishi M, O'Neill BT, Li M, García-Martín R, Takahashi H, Hu J, et al. (2017). Adipocyte Dynamics and Reversible Metabolic Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab 25, 448–462. 10.1016/j.cmet.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, and Sul HS (2007). Regulation of lipolysis in adipocytes. Annu Rev Nutr 27, 79–101. 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frayn KN (2002). Adipose tissue as a buffer for daily lipid flux. Diabetologia 45, 1201–1210. 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 18.Cannon B, and Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359. 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 19.Markussen LK, Rondini EA, Johansen OS, Madsen JGS, Sustarsic EG, Marcher A-B, Hansen JB, Gerhart-Hines Z, Granneman JG, and Mandrup S (2022). Lipolysis regulates major transcriptional programs in brown adipocytes. Nature Communications 13, 3956. 10.1038/s41467-022-31525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonet ML, Oliver P, and Palou A (2013). Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 1831, 969–985. 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Loncar D (1991). Convertible adipose tissue in mice. Cell and tissue research 266, 149–161. [DOI] [PubMed] [Google Scholar]
- 23.Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, and Gupta RK (2019). Cellular Origins of Beige Fat Cells Revisited. Diabetes 68, 1874–1885. 10.2337/db19-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YH, Petkova AP, Konkar AA, and Granneman JG (2015). Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29, 286–299. 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang QA, Tao C, Gupta RK, and Scherer PE (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19, 1338–1344. 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenwald M, Perdikari A, Rülicke T, and Wolfrum C (2013). Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15, 659–667. 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 27.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, and Cinti S (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298, E1244–1253. 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 28.Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, and Trajkovski M (2016). Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab 24, 434–446. 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, and Clegg DJ (2015). Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab 4, 427–436. 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. (2014). A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20, 433–447. 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, et al. (2017). Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab 26, 801. 10.1016/j.cmet.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KH, Kim YH, Son JE, Lee JH, Kim S, Choe MS, Moon JH, Zhong J, Fu K, Lenglin F, et al. (2017). Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res 27, 1309–1326. 10.1038/cr.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betz MJ, Slawik M, Lidell ME, Osswald A, Heglind M, Nilsson D, Lichtenauer UD, Mauracher B, Mussack T, Beuschlein F, and Enerback S (2013). Presence of brown adipocytes in retroperitoneal fat from patients with benign adrenal tumors: relationship with outdoor temperature. J Clin Endocrinol Metab 98, 4097–4104. 10.1210/jc.2012-3535. [DOI] [PubMed] [Google Scholar]
- 34.Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, Guerrieri M, and Cinti S (2013). White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta 1831, 950–959. 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, and Jeschke Marc G. (2015). Burn Induces Browning of the Subcutaneous White Adipose Tissue in Mice and Humans. Cell Reports 13, 1538–1544. 10.1016/j.celrep.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartelt A, and Heeren J (2014). Adipose tissue browning and metabolic health. Nature reviews. Endocrinology 10, 24–36. 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, and Seale P (2016). Control of brown and beige fat development. Nat Rev Mol Cell Biol 17, 691–702. 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jun H, Yu H, Gong J, Jiang J, Qiao X, Perkey E, Kim DI, Emont MP, Zestos AG, Cho JS, et al. (2018). An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med 24, 814–822. 10.1038/s41591-018-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajbhandari P, Thomas BJ, Feng AC, Hong C, Wang J, Vergnes L, Sallam T, Wang B, Sandhu J, Seldin MM, et al. (2018). IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell 172, 218–233 e217. 10.1016/j.cell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajbhandari P, Arneson D, Hart SK, Ahn IS, Diamante G, Santos LC, Zaghari N, Feng AC, Thomas BJ, Vergnes L, et al. (2019). Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. Elife 8. 10.7554/eLife.49501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabhi N, Belkina AC, Desevin K, Cortez BN, and Farmer SR (2020). Shifts of Immune Cell Populations Differ in Response to Different Effectors of Beige Remodeling of Adipose Tissue. iScience 23, 101765. 10.1016/j.isci.2020.101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamsi F, Tseng YH, and Kahn CR (2021). Adipocyte Microenvironment: Everybody in the Neighborhood Talks about the Temperature. Cell Metab 33, 4–6. 10.1016/j.cmet.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D, and Spiegelman BM (2020). gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614. 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriques F, Bedard AH, Guilherme A, Kelly M, Chi J, Zhang P, Lifshitz LM, Bellve K, Rowland LA, Yenilmez B, et al. (2020). Single-Cell RNA Profiling Reveals Adipocyte to Macrophage Signaling Sufficient to Enhance Thermogenesis. Cell Rep 32, 107998. 10.1016/j.celrep.2020.107998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartness TJ, Vaughan CH, and Song CK (2010). Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 34 Suppl 1, S36–42. 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, Cecchini MP, Kershaw EE, Barbatelli G, Haemmerle G, et al. (2018). Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 59, 784–794. 10.1194/jlr.M079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu I, and Walsh K (2015). The Whitening of Brown Fat and Its Implications for Weight Management in Obesity. Curr Obes Rep 4, 224–229. 10.1007/s13679-015-0157-8. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, and Walsh K (2014). Vascular rarefaction mediates whitening of brown fat in obesity. The Journal of Clinical Investigation 124, 2099–2112. 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roh HC, Tsai LTY, Shao M, Tenen D, Shen Y, Kumari M, Lyubetskaya A, Jacobs C, Dawes B, Gupta RK, and Rosen ED (2018). Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab 27, 1121–1137.e1125. 10.1016/j.cmet.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cinti S (2018). Pink Adipocytes. Trends Endocrinol Metab 29, 651–666. 10.1016/j.tem.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Wang QA, and Scherer PE (2019). Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution. J Mammary Gland Biol Neoplasia 24, 207–212. 10.1007/s10911-019-09434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, Jiang L, Ye R, Shao M, Tao C, et al. (2018). Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell Metab 28, 282–288.e283. 10.1016/j.cmet.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zwick RK, Rudolph MC, Shook BA, Holtrup B, Roth E, Lei V, Van Keymeulen A, Seewaldt V, Kwei S, Wysolmerski J, et al. (2018). Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat Commun 9, 3592. 10.1038/s41467-018-05911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karastergiou K, Smith SR, Greenberg AS, and Fried SK (2012). Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 3, 13. 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huffman DM, and Barzilai N (2010). Contribution of adipose tissue to health span and longevity. Interdiscip Top Gerontol 37, 1–19. 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- 56.Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, and Bell JD (1998). Magnetic resonance imaging of total body fat. J Appl Physiol (1985) 85, 1778–1785. 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- 57.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787. 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 58.Sun K, Kusminski CM, and Scherer PE (2011). Adipose tissue remodeling and obesity. J Clin Invest 121, 2094–2101. 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, and Ferrante AW Jr. (2010). Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120, 3466–3479. 10.1172/jci42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alemán JO, Iyengar NM, Walker JM, Milne GL, Da Rosa JC, Liang Y, Giri DD, Zhou XK, Pollak MN, Hudis CA, et al. (2017). Effects of Rapid Weight Loss on Systemic and Adipose Tissue Inflammation and Metabolism in Obese Postmenopausal Women. J Endocr Soc 1, 625–637. 10.1210/js.2017-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cottam MA, Caslin HL, Winn NC, and Hasty AH (2022). Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat Commun 13, 2950. 10.1038/s41467-022-30646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. (2005). Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54, 2277–2286. 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 63.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, et al. (2004). Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. Faseb j 18, 1657–1669. 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 64.Schmitz J, Evers N, Awazawa M, Nicholls HT, Brönneke HS, Dietrich A, Mauer J, Blüher M, and Brüning JC (2016). Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab 5, 328–339. 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broekema MF, Savage DB, Monajemi H, and Kalkhoven E (2019). Gene-gene and gene-environment interactions in lipodystrophy: Lessons learned from natural PPARγ mutants. Biochim Biophys Acta Mol Cell Biol Lipids 1864, 715–732. 10.1016/j.bbalip.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Fiorenza CG, Chou SH, and Mantzoros CS (2011). Lipodystrophy: pathophysiology and advances in treatment. Nature Reviews Endocrinology 7, 137–150. 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Despres JP (2006). Is visceral obesity the cause of the metabolic syndrome? Ann Med 38, 52–63. 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 68.Khaodhiar L, McCowen KC, and Blackburn GL (1999). Obesity and its comorbid conditions. Clin Cornerstone 2, 17–31. 10.1016/s1098-3597(99)90002-9. [DOI] [PubMed] [Google Scholar]
- 69.Quail DF, and Dannenberg AJ (2019). The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 15, 139–154. 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, and Martinez JA (2017). Obesity. Nat Rev Dis Primers 3, 17034. 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 71.Smith GI, Mittendorfer B, and Klein S (2019). Metabolically healthy obesity: facts and fantasies. J Clin Invest 129, 3978–3989. 10.1172/jci129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karpe F, and Pinnick KE (2015). Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nature Reviews Endocrinology 11, 90–100. 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 73.Huang LO, Rauch A, Mazzaferro E, Preuss M, Carobbio S, Bayrak CS, Chami N, Wang Z, Schick UM, Yang N, et al. (2021). Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities. Nat Metab 3, 228–243. 10.1038/s42255-021-00346-2. [DOI] [PubMed] [Google Scholar]
- 74.Hepler C, and Gupta RK (2017). The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol Cell Endocrinol 445, 95–108. 10.1016/j.mce.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reilly SM, and Saltiel AR (2017). Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13, 633–643. 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 76.Chakarov S, Blériot C, and Ginhoux F (2022). Role of adipose tissue macrophages in obesity-related disorders. J Exp Med 219. 10.1084/jem.20211948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Baak MA, and Mariman ECM (2019). Mechanisms of weight regain after weight loss - the role of adipose tissue. Nat Rev Endocrinol 15, 274–287. 10.1038/s41574-018-0148-4. [DOI] [PubMed] [Google Scholar]
- 78.Cifarelli V, Beeman SC, Smith GI, Yoshino J, Morozov D, Beals JW, Kayser BD, Watrous JD, Jain M, Patterson BW, and Klein S (2020). Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J Clin Invest 130, 6688–6699. 10.1172/jci141828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim K, Haider A, Adams C, Sleigh A, and Savage DB (2021). Lipodistrophy: a paradigm for understanding the consequences of “overloading” adipose tissue. Physiological Reviews 101, 907–993. 10.1152/physrev.00032.2020. [DOI] [PubMed] [Google Scholar]
- 80.Kane H, and Lynch L (2019). Innate Immune Control of Adipose Tissue Homeostasis. Trends in Immunology 40, 857–872. 10.1016/j.it.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Graupera M, and Claret M (2018). Endothelial Cells: New Players in Obesity and Related Metabolic Disorders. Trends Endocrinol Metab 29, 781–794. 10.1016/j.tem.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Larabee CM, Neely OC, and Domingos AI (2020). Obesity: a neuroimmunometabolic perspective. Nature Reviews Endocrinology 16, 30–43. 10.1038/s41574-019-0283-6. [DOI] [PubMed] [Google Scholar]
- 83.Guilherme A, Pedersen DJ, Henchey E, Henriques FS, Danai LV, Shen Y, Yenilmez B, Jung D, Kim JK, Lodhi IJ, et al. (2017). Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol Metab 6, 781–796. 10.1016/j.molmet.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wasserman F (1926). The fat organs of man: development, structure and systematic place of the so-called adipose tissue. A. Zellforsch. Microskop. Anat. Abt. Histochem 3, 325–329. [Google Scholar]
- 85.Wasserman F (1960). Electron microscopic investigation of the surface structures of the fat cell and of their changes during depletion of cell. Z. Zellforsch. Microskop. Anat. Abt. Histochem 52, 778–787. [DOI] [PubMed] [Google Scholar]
- 86.Hausman GJ, and Richardson RL (1983). Cellular and vascular development in immature rat adipose tissue. J Lipid Res 24, 522–532. [PubMed] [Google Scholar]
- 87.Suter ER (1969). The fine structure of brown adipose tissue. I. Cold-induced changes in the rat. J Ultrastruct Res 26, 216–241. 10.1016/s0022-5320(69)80003-1. [DOI] [PubMed] [Google Scholar]
- 88.Napolitano L (1963). THE DIFFERENTIATION OF WHITE ADIPOSE CELLS. AN ELECTRON MICROSCOPE STUDY. J Cell Biol 18, 663–679. 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Napolitano LM (1965). Observations on the fine structure of adipose cells. Ann N Y Acad Sci 131, 34–42. 10.1111/j.1749-6632.1965.tb34777.x. [DOI] [PubMed] [Google Scholar]
- 90.Slavin BG, and Ballard KW (1978). Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec 191, 377–389. 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- 91.Cinti S (1999). Adipose tissues and obesity. Ital J Anat Embryol 104, 37–51. [PubMed] [Google Scholar]
- 92.Rodbell M (1964). Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J Biol Chem 239, 375–380. [PubMed] [Google Scholar]
- 93.Green H, and Meuth M (1974). An established pre-adipose cell line and its differentiation in culture. Cell 3, 127–133. [DOI] [PubMed] [Google Scholar]
- 94.Green Ha.K., O (1974). Sublines of mouse 3T3 cells that accumulate lipid. Cell 1, 113–116. [Google Scholar]
- 95.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos-MC J, and Londos C (1992). Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc.Natl.Acad.Sci.U.S.A. 89, 8537–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Student AK, Hsu RY, and Lane MD (1980). Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. Journal of Biological Chemistry 255, 4745–4750. [PubMed] [Google Scholar]
- 97.Hu E, Liang P, and Spiegelman BM (1996). AdipoQ is a novel adipose-specific gene dysregulated in obesity. J.Biol.Chem 271, 10697–10703. [DOI] [PubMed] [Google Scholar]
- 98.Bernlohr DA, Doering TL, Kelly TJ Jr, and Lane MD (1985). Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun 132, 850–855. [DOI] [PubMed] [Google Scholar]
- 99.Cook KS, Groves DL, Min HY, and Spiegelman BM (1985). A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc.Natl.Acad.Sci.U.S.A. 82, 6480–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tontonoz P, Hu E, Graves RA, Budavari AI, and Spiegelman BM (1994). mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8, 1224–1234. [DOI] [PubMed] [Google Scholar]
- 101.Lin FT, and Lane MD (1992). Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 6, 533–544. [DOI] [PubMed] [Google Scholar]
- 102.Lefterova MI, Haakonsson AK, Lazar MA, and Mandrup S (2014). PPARγ and the global map of adipogenesis and beyond. Trends in endocrinology and metabolism: TEM 25, 293–302. 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siersbaek R, and Mandrup S (2011). Transcriptional Networks Controlling Adipocyte Differentiation. Cold Spring Harbor Symposia on Quantitative Biology, sqb [DOI] [PubMed] [Google Scholar]
- 104.Rauch A, and Mandrup S (2021). Transcriptional networks controlling stromal cell differentiation. Nature Reviews Molecular Cell Biology 22, 465–482. 10.1038/s41580-021-00357-7. [DOI] [PubMed] [Google Scholar]
- 105.Loft A, Forss I, and Mandrup S (2017). Genome-Wide Insights into the Development and Function of Thermogenic Adipocytes. Trends Endocrinol Metab 28, 104–120. 10.1016/j.tem.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Marcher A-B, Loft A, Nielsen R, Vihervaara T, Madsen Jesper Grud S., Sysi-Aho M, Ekroos K, and Mandrup S (2015). RNA-Seq and Mass-Spectrometry-Based Lipidomics Reveal Extensive Changes of Glycerolipid Pathways in Brown Adipose Tissue in Response to Cold. Cell Reports 13, 2000–2013. 10.1016/j.celrep.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 107.Mardinoglu A, Kampf C, Asplund A, Fagerberg L, Hallström BM, Edlund K, Blüher M, Pontén F, Uhlen M, and Nielsen J (2014). Defining the human adipose tissue proteome to reveal metabolic alterations in obesity. J Proteome Res 13, 5106–5119. 10.1021/pr500586e. [DOI] [PubMed] [Google Scholar]
- 108.Honecker J, Ruschke S, Seeliger C, Laber S, Strobel S, Pröll P, Nellaker C, Lindgren CM, Kulozik U, Ecker J, et al. (2022). Transcriptome and fatty-acid signatures of adipocyte hypertrophy and its non-invasive MR-based characterization in human adipose tissue. EBioMedicine 79, 104020. 10.1016/j.ebiom.2022.104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, and Mann M (2009). Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab 10, 324–335. 10.1016/j.cmet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 110.Chi J, Wu Z, Choi CHJ, Nguyen L, Tegegne S, Ackerman SE, Crane A, Marchildon F, Tessier-Lavigne M, and Cohen P (2018). Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab 27, 226–236.e223. 10.1016/j.cmet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Rodeheffer MS, Birsoy K, and Friedman JM (2008). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249. 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 112.Wang F, Mullican SE, DiSpirito JR, Peed LC, and Lazar MA (2013). Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proceedings of the National Academy of Sciences 110, 18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK (2016). Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab 23, 350–359. 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, and Rosen ED (2011). Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13, 249–259. 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. (2014). IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell 158, 69–83. 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li R, Bernau K, Sandbo N, Gu J, Preissl S, and Sun X (2018). Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. Elife 7. 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hwang B, Lee JH, and Bang D (2018). Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 50, 1–14. 10.1038/s12276-018-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, and Quake SR (2014). Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509, 371–375. 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu WY, Hu DP, Xie L, Nonn L, Lu R, Abern M, Shioda T, and Prins GS (2021). Keratin Profiling by Single-Cell RNA-Sequencing Identifies Human Prostate Stem Cell Lineage Hierarchy and Cancer Stem-Like Cells. Int J Mol Sci 22. 10.3390/ijms22158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stenkula KG, and Erlanson-Albertsson C (2018). Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol 315, R284–R295. 10.1152/ajpregu.00257.2017. [DOI] [PubMed] [Google Scholar]
- 121.University of Utah Health. Single Cell Sequencing - 10X Genomics 3’ Gene Expression https://uofuhealth.utah.edu/huntsman/shared-resources/gba/htg/single-cell/genomics-10x.php.
- 122.Emont MP, Jacobs C, Essene AL, Pant D, Tenen D, Colleluori G, Di Vincenzo A, Jørgensen AM, Dashti H, Stefek A, et al. (2022). A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933. 10.1038/s41586-022-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, Giordano A, Kovanicova Z, Stefanicka P, Balazova L, et al. (2020). snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102. 10.1038/s41586-020-2856-x. [DOI] [PubMed] [Google Scholar]
- 124.Sárvári AK, Van Hauwaert EL, Markussen LK, Gammelmark E, Marcher AB, Ebbesen MF, Nielsen R, Brewer JR, Madsen JGS, and Mandrup S (2021). Plasticity of Epididymal Adipose Tissue in Response to Diet-Induced Obesity at Single-Nucleus Resolution. Cell Metab 33, 437–453.e435. 10.1016/j.cmet.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 125.Ding J, Adiconis X, Simmons SK, Kowalczyk MS, Hession CC, Marjanovic ND, Hughes TK, Wadsworth MH, Burks T, Nguyen LT, et al. (2020). Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat Biotechnol 38, 737–746. 10.1038/s41587-020-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bakken TE, Hodge RD, Miller JA, Yao Z, Nguyen TN, Aevermann B, Barkan E, Bertagnolli D, Casper T, Dee N, et al. (2018). Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS One 13, e0209648. 10.1371/journal.pone.0209648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lake BB, Codeluppi S, Yung YC, Gao D, Chun J, Kharchenko PV, Linnarsson S, and Zhang K (2017). A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci Rep 7, 6031. 10.1038/s41598-017-04426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gupta A, Shamsi F, Altemose N, Dorlhiac GF, Cypess AM, White AP, Yosef N, Patti ME, Tseng Y-H, and Streets A (2022). Characterization of transcript enrichment and detection bias in single-nucleus RNA-seq for mapping of distinct human adipocyte lineages. Genome Research. 10.1101/gr.275509.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denisenko E, Guo BB, Jones M, Hou R, de Kock L, Lassmann T, Poppe D, Clement O, Simmons RK, Lister R, and Forrest ARR (2020). Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol 21, 130. 10.1186/s13059-020-02048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den Brink SC, Sage F, Vertesy A, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C, and van Oudenaarden A (2017). Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods 14, 935–936. 10.1038/nmeth.4437. [DOI] [PubMed] [Google Scholar]
- 131.Van Hauwaert EL, Gammelmark E, Sárvári AK, Larsen L, Nielsen R, Madsen JGS, and Mandrup S (2021). Isolation of nuclei from mouse white adipose tissues for single-nucleus genomics. STAR Protoc 2, 100612. 10.1016/j.xpro.2021.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alvarez M, Rahmani E, Jew B, Garske KM, Miao Z, Benhammou JN, Ye CJ, Pisegna JR, Pietilainen KH, Halperin E, and Pajukanta P (2020). Enhancing droplet-based single-nucleus RNA-seq resolution using the semi-supervised machine learning classifier DIEM. Sci Rep 10, 11019. 10.1038/s41598-020-67513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stuart T, and Satija R (2019). Integrative single-cell analysis. Nature Reviews Genetics 20, 257–272. 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- 134.Avsec Ž, Weilert M, Shrikumar A, Krueger S, Alexandari A, Dalal K, Fropf R, McAnany C, Gagneur J, Kundaje A, and Zeitlinger J (2021). Base-resolution models of transcription-factor binding reveal soft motif syntax. Nature Genetics 53, 354–366. 10.1038/s41588-021-00782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao J, Cusanovich DA, Ramani V, Aghamirzaie D, Pliner HA, Hill AJ, Daza RM, McFaline-Figueroa JL, Packer JS, Christiansen L, et al. (2018). Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385. doi: 10.1126/science.aau0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schoof EM, Furtwängler B, Üresin N, Rapin N, Savickas S, Gentil C, Lechman E, Keller Ua.d., Dick, J.E., and Porse, B.T. (2021). Quantitative single-cell proteomics as a tool to characterize cellular hierarchies. Nature Communications 12, 3341. 10.1038/s41467-021-23667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Labib M, and Kelley SO (2020). Single-cell analysis targeting the proteome. Nature Reviews Chemistry 4, 143–158. 10.1038/s41570-020-0162-7. [DOI] [PubMed] [Google Scholar]
- 138.Brunner A-D, Thielert M, Vasilopoulou C, Ammar C, Coscia F, Mund A, Hoerning OB, Bache N, Apalategui A, Lubeck M, et al. (2022). Ultra-high sensitivity mass spectrometry quantifies single-cell proteome changes upon perturbation. Molecular Systems Biology 18, e10798. 10.15252/msb.202110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marx V (2021). Method of the Year: spatially resolved transcriptomics. Nat Methods 18, 9–14. 10.1038/s41592-020-01033-y. [DOI] [PubMed] [Google Scholar]
- 140.Rao A, Barkley D, França GS, and Yanai I (2021). Exploring tissue architecture using spatial transcriptomics. Nature 596, 211–220. 10.1038/s41586-021-03634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia C, Babcock HP, Moffitt JR, and Zhuang X (2019). Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci Rep 9, 7721. 10.1038/s41598-019-43943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen KH, Boettiger AN, Moffitt JR, Wang S, and Zhuang X (2015). Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Z, and Zhang X (2021). Inference of high-resolution trajectories in single-cell RNA-seq data by using RNA velocity. Cell Rep Methods 1, 100095. 10.1016/j.crmeth.2021.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.DeTomaso D, Jones MG, Subramaniam M, Ashuach T, Ye CJ, and Yosef N (2019). Functional interpretation of single cell similarity maps. Nat Commun 10, 4376. 10.1038/s41467-019-12235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lange M, Bergen V, Klein M, Setty M, Reuter B, Bakhti M, Lickert H, Ansari M, Schniering J, Schiller HB, et al. (2022). CellRank for directed single-cell fate mapping. Nature Methods 19, 159–170. 10.1038/s41592-021-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Efremova M, Vento-Tormo M, Teichmann SA, and Vento-Tormo R (2020). CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nature Protocols 15, 1484–1506. 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 147.Browaeys R, Saelens W, and Saeys Y (2020). NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods 17, 159–162. 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 148.van der Wijst MGP, de Vries DH, Groot HE, Trynka G, Hon CC, Bonder MJ, Stegle O, Nawijn MC, Idaghdour Y, van der Harst P, et al. (2020). The single-cell eQTLGen consortium. eLife 9, e52155. 10.7554/eLife.52155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bryois J, Calini D, Macnair W, Foo L, Urich E, Ortmann W, Iglesias VA, Selvaraj S, Nutma E, Marzin M, et al. (2022). Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat Neurosci 25, 1104–1112. 10.1038/s41593-022-01128-z. [DOI] [PubMed] [Google Scholar]
- 150.Yazar S, Alquicira-Hernandez J, Wing K, Senabouth A, Gordon MG, Andersen S, Lu Q, Rowson A, Taylor TRP, Clarke L, et al. (2022). Single-cell eQTL mapping identifies cell type-specific genetic control of autoimmune disease. Science 376, eabf3041. 10.1126/science.abf3041. [DOI] [PubMed] [Google Scholar]
- 151.Backdahl J, Franzen L, Massier L, Li Q, Jalkanen J, Gao H, Andersson A, Bhalla N, Thorell A, Ryden M, et al. (2021). Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metab 33, 1869–1882 e1866. 10.1016/j.cmet.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 152.Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, and Blüher M (2010). Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299, E506–515. 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 153.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, and Smith U (2015). Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab 26, 193–200. 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 154.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, and Cushman SW (2007). Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50, 1707–1715. 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 155.Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, Li H, Edwards YJK, Wolfrum C, Metallo CM, et al. (2018). Brown Fat AKT2 Is a Cold-Induced Kinase that Stimulates ChREBP-Mediated De Novo Lipogenesis to Optimize Fuel Storage and Thermogenesis. Cell Metab 27, 195–209 e196. 10.1016/j.cmet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang Y, Wallace M, Sanchez-Gurmaches J, Hsiao WY, Li H, Lee PL, Vernia S, Metallo CM, and Guertin DA (2016). Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun 7, 11365. 10.1038/ncomms11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, Pinnschmidt HO, Rensen SS, Wolf AM, Bartelt A, et al. (2013). De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 4, 1528. 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cinti S, Cancello R, Zingaretti MC, Ceresi E, De Matteis R, Giordano A, Himms-Hagen J, and Ricquier D (2002). CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J Histochem Cytochem 50, 21–31. 10.1177/002215540205000103. [DOI] [PubMed] [Google Scholar]
- 159.Spaethling JM, Sanchez-Alavez M, Lee J, Xia FC, Dueck H, Wang W, Fisher SA, Sul JY, Seale P, Kim J, et al. (2016). Single-cell transcriptomics and functional target validation of brown adipocytes show their complex roles in metabolic homeostasis. Faseb j 30, 81–92. 10.1096/fj.15-273797. [DOI] [PubMed] [Google Scholar]
- 160.Biagi CAO Jr., Cury SS, Alves CP, Rabhi N, Silva WA Jr., Farmer SR, Carvalho RF, and Batista ML Jr. (2021). Multidimensional Single-Nuclei RNA-Seq Reconstruction of Adipose Tissue Reveals Adipocyte Plasticity Underlying Thermogenic Response. Cells 10. 10.3390/cells10113073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, and Seale P (2019). Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364. 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rondini EA, and Granneman JG (2020). Single cell approaches to address adipose tissue stromal cell heterogeneity. Biochem J 477, 583–600. 10.1042/bcj20190467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shao M, Hepler C, Zhang Q, Shan B, Vishvanath L, Henry GH, Zhao S, An YA, Wu Y, Strand DW, and Gupta RK (2021). Pathologic HIF1alpha signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell 28, 685–701 e687. 10.1016/j.stem.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, and Gupta RK (2018). Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife 7. 10.7554/eLife.39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, and Granneman JG (2018). Deconstructing Adipogenesis Induced by beta3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab 28, 300–309 e304. 10.1016/j.cmet.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, and Mathis D (2019). Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol 4. 10.1126/sciimmunol.aaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nahmgoong H, Jeon YG, Park ES, Choi YH, Han SM, Park J, Ji Y, Sohn JH, Han JS, Kim YY, et al. (2022). Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics. Cell Metab 34, 458–472 e456. 10.1016/j.cmet.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 168.Buffolo M, Pires KM, Ferhat M, Ilkun O, Makaju A, Achenbach A, Bowman F, Atkinson DL, Holland WL, Amri EZ, et al. (2019). Identification of a Paracrine Signaling Mechanism Linking CD34(high) Progenitors to the Regulation of Visceral Fat Expansion and Remodeling. Cell Rep 29, 270–282 e275. 10.1016/j.celrep.2019.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Giuliani G, Rosina M, and Reggio A (2022). Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. Febs j 289, 6484–6517. 10.1111/febs.16080. [DOI] [PubMed] [Google Scholar]
- 170.Hatzmann FM, Grossmann S, Waldegger P, Wiegers GJ, Mandl M, Rauchenwald T, Pierer G, and Zwerschke W (2022). Dipeptidyl peptidase-4 cell surface expression marks an abundant adipose stem/progenitor cell population with high stemness in human white adipose tissue. Adipocyte 11, 601–615. 10.1080/21623945.2022.2129060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berry R, and Rodeheffer MS (2013). Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15, 302–308. 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stefkovich M, Traynor S, Cheng L, Merrick D, and Seale P (2021). Dpp4+ interstitial progenitor cells contribute to basal and high fat diet-induced adipogenesis. Mol Metab 54, 101357. 10.1016/j.molmet.2021.101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, Soldati G, et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108. 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 174.Nguyen HP, Lin F, Yi D, Xie Y, Dinh J, Xue P, and Sul HS (2021). Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev Cell 56, 1437–1451 e1433. 10.1016/j.devcel.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Q, Shan B, Guo L, Shao M, Vishvanath L, Elmquist G, Xu L, and Gupta RK (2022). Distinct functional properties of murine perinatal and adult adipose progenitor subpopulations. Nat Metab 4, 1055–1070. 10.1038/s42255-022-00613-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shan B, Barker CS, Shao M, Zhang Q, Gupta RK, and Wu Y (2022). Multilayered omics reveal sex- and depot-dependent adipose progenitor cell heterogeneity. Cell Metab 34, 783–799 e787. 10.1016/j.cmet.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, Brown Z, Pradhan RN, Tajima K, Yoneshiro T, et al. (2020). CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 182, 563–577 e520. 10.1016/j.cell.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shamsi F, Piper M, Ho LL, Huang TL, Gupta A, Streets A, Lynes MD, and Tseng YH (2021). Vascular smooth muscle-derived Trpv1(+) progenitors are a source of cold-induced thermogenic adipocytes. Nat Metab 3, 485–495. 10.1038/s42255-021-00373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, and Graff JM (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586. 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Burl RB, Rondini EA, Wei H, Pique-Regi R, and Granneman JG (2022). Deconstructing cold-induced brown adipocyte neogenesis in mice. Elife 11. 10.7554/eLife.80167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tran KV, Fitzgibbons T, Min SY, DeSouza T, and Corvera S (2018). Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol Metab 9, 199–206. 10.1016/j.molmet.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Angueira AR, Sakers AP, Holman CD, Cheng L, Arbocco MN, Shamsi F, Lynes MD, Shrestha R, Okada C, Batmanov K, et al. (2021). Defining the lineage of thermogenic perivascular adipose tissue. Nat Metab 3, 469–484. 10.1038/s42255-021-00380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, and Horsley V (2011). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771. 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guerrero-Juarez CF, and Plikus MV (2018). Emerging nonmetabolic functions of skin fat. Nat Rev Endocrinol 14, 163–173. 10.1038/nrendo.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al. (2017). Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752. 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, and Gallo RL (2015). Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347, 67–71. 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben AL, Wang MY, Li N, et al. (2019). Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129, 5327–5342. 10.1172/JCI130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shook BA, Wasko RR, Mano O, Rutenberg-Schoenberg M, Rudolph MC, Zirak B, Rivera-Gonzalez GC, Lopez-Giraldez F, Zarini S, Rezza A, et al. (2020). Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 26, 880–895 e886. 10.1016/j.stem.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu Q, Zhu Y, Hepler C, Zhang Q, Park J, Gliniak C, Henry GH, Crewe C, Bu D, Zhang Z, et al. (2022). Adipocyte mesenchymal transition contributes to mammary tumor progression. Cell Rep 40, 111362. 10.1016/j.celrep.2022.111362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Z, Hardij J, Bagchi DP, Scheller EL, and MacDougald OA (2018). Development, regulation, metabolism and function of bone marrow adipose tissues. Bone 110, 134–140. 10.1016/j.bone.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, and MacDougald OA (2016). Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab 27, 392–403. 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, Sinton MC, Ramage LE, McDougald WA, Lovdel A, et al. (2020). Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun 11, 3097. 10.1038/s41467-020-16878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, Shrestha R, Wang L, Yu W, Holdreith N, et al. (2020). Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife 9. 10.7554/eLife.54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grosjean A, Venteclef N, and Dalmas E (2021). Understanding the heterogeneity and functions of metabolic tissue macrophages. Seminars in Cell & Developmental Biology 119, 130–139. 10.1016/j.semcdb.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 195.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808. 10.1172/jci19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li P, Lu M, Nguyen MTA, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. (2010). Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem 285, 15333–15345. 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lumeng CN, Bodzin JL, and Saltiel AR (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117, 175–184. 10.1172/jci29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, and Ferrante AW Jr. (2013). Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab 18, 816–830. 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, et al. (2014). Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20, 614–625. 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. (2014). Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, et al. (2018). Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A 115, E5096–e5105. 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sharma M, Schlegel M, Brown EJ, Sansbury BE, Weinstock A, Afonso MS, Corr EM, van Solingen C, Shanley LC, Peled D, et al. (2019). Netrin-1 Alters Adipose Tissue Macrophage Fate and Function in Obesity. Immunometabolism 1. 10.20900/immunometab20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. (2019). Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 178, 686–698.e614. 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Weinstock A, Brown EJ, Garabedian ML, Pena S, Sharma M, Lafaille J, Moore KJ, and Fisher EA (2019). Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells. Immunometabolism 1. 10.20900/immunometab20190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li C, Menoret A, Farragher C, Ouyang Z, Bonin C, Holvoet P, Vella AT, and Zhou B (2019). Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI Insight 5. 10.1172/jci.insight.126453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hildreth AD, Ma F, Wong YY, Sun R, Pellegrini M, and O'Sullivan TE (2021). Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol 22, 639–653. 10.1038/s41590-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ, Cheung WA, Belden B, Pramatarova A, Biertho L, Gibson M, et al. (2020). Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab 2, 97–109. 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Félix I, Jokela H, Karhula J, Kotaja N, Savontaus E, Salmi M, and Rantakari P (2021). Single-Cell Proteomics Reveals the Defined Heterogeneity of Resident Macrophages in White Adipose Tissue. Front Immunol 12, 719979. 10.3389/fimmu.2021.719979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, Zhang XM, Foo S, Nakamizo S, Duan K, et al. (2019). Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363. 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 210.Silva HM, Báfica A, Rodrigues-Luiz GF, Chi J, Santos PDA, Reis BS, Hoytema van Konijnenburg DP, Crane A, Arifa RDN, Martin P, et al. (2019). Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med 216, 786–806. 10.1084/jem.20181049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gallerand A, Stunault MI, Merlin J, Luehmann HP, Sultan DH, Firulyova MM, Magnone V, Khedher N, Jalil A, Dolfi B, et al. (2021). Brown adipose tissue monocytes support tissue expansion. Nat Commun 12, 5255. 10.1038/s41467-021-25616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O'Rourke RW, and Lumeng CN (2016). Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J Immunol 197, 3650–3661. 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ksiazek K (2013). Mesothelial cell: a multifaceted model of aging. Ageing Res Rev 12, 595–604. 10.1016/j.arr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 214.Gupta OT, and Gupta RK (2015). Visceral Adipose Tissue Mesothelial Cells: Living on the Edge or Just Taking Up Space? Trends Endocrinol Metab 26, 515–523. 10.1016/j.tem.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 215.Darimont C, Avanti O, Blancher F, Wagniere S, Mansourian R, Zbinden I, Leone-Vautravers P, Fuerholz A, Giusti V, and Mace K (2008). Contribution of mesothelial cells in the expression of inflammatory-related factors in omental adipose tissue of obese subjects. Int J Obes (Lond) 32, 112–120. 10.1038/sj.ijo.0803688. [DOI] [PubMed] [Google Scholar]
- 216.Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. (2014). Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16, 367–375. 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Whytock KL, Sun Y, Divoux A, Yu G, Smith SR, Walsh MJ, and Sparks LM (2022). Single cell full-length transcriptome of human subcutaneous adipose tissue reveals unique and heterogeneous cell populations. iScience 25, 104772. 10.1016/j.isci.2022.104772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, et al. (2020). Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 180, 764–779 e720. 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 219.Trimm E, and Red-Horse K (2022). Vascular endothelial cell development and diversity. Nat Rev Cardiol 10.1038/s41569-022-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang J, Vamvini M, Nigro P, Ho LL, Galani K, Alvarez M, Tanigawa Y, Renfro A, Carbone NP, Laakso M, et al. (2022). Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells. Cell Metab 34, 1578–1593 e1576. 10.1016/j.cmet.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dong H, Sun W, Shen Y, Baláz M, Balázová L, Ding L, Löffler M, Hamilton B, Klöting N, Blüher M, et al. (2022). Identification of a regulatory pathway inhibiting adipogenesis via RSPO2. Nat Metab 4, 90–105. 10.1038/s42255-021-00509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rabhi N, Desevin K, Belkina AC, Tilston-Lunel A, Varelas X, Layne MD, and Farmer SR (2022). Obesity-induced senescent macrophages activate a fibrotic transcriptional program in adipocyte progenitors. Life Sci Alliance 5. 10.26508/lsa.202101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shan B, Shao M, Zhang Q, Hepler C, Paschoal VA, Barnes SD, Vishvanath L, An YA, Jia L, Malladi VS, et al. (2020). Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat Metab 2, 1332–1349. 10.1038/s42255-020-00301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mahlakoiv T, Flamar AL, Johnston LK, Moriyama S, Putzel GG, Bryce PJ, and Artis D (2019). Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol 4. 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shan B, Shao M, Zhang Q, An YA, Vishvanath L, and Gupta RK (2021). Cold-responsive adipocyte progenitors couple adrenergic signaling to immune cell activation to promote beige adipocyte accrual. Genes Dev 35, 1333–1338. 10.1101/gad.348762.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, and Artis D (2015). Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246. 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Han X, Zhang Z, He L, Zhu H, Li Y, Pu W, Han M, Zhao H, Liu K, Li Y, et al. (2021). A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell 28, 1160–1176 e1167. 10.1016/j.stem.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 228.Zhao Z, Ukidve A, Kim J, and Mitragotri S (2020). Targeting Strategies for Tissue-Specific Drug Delivery. Cell 181, 151–167. 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]


