Abstract
Psychological distress is prevalent in people with COPD and relates to a worse course of disease. It often remains unrecognised and untreated, intensifying the burden on patients, carers and healthcare systems. Nonpharmacological management strategies have been suggested as important elements to manage psychological distress in COPD. Therefore, this review presents instruments for detecting psychological distress in COPD and provides an overview of available nonpharmacological management strategies together with available scientific evidence for their presumed benefits in COPD. Several instruments are available for detecting psychological distress in COPD, including simple questions, questionnaires and clinical diagnostic interviews, but their implementation in clinical practice is limited and heterogeneous. Moreover, various nonpharmacological management options are available for COPD, ranging from specific cognitive behavioural therapy (CBT) to multi-component pulmonary rehabilitation (PR) programmes. These interventions vary substantially in their specific content, intensity and duration across studies. Similarly, available evidence regarding their efficacy varies significantly, with the strongest evidence currently for CBT or PR. Further randomised controlled trials are needed with larger, culturally diverse samples and long-term follow-ups. Moreover, effective nonpharmacological interventions should be implemented more in the clinical routine. Respective barriers for patients, caregivers, clinicians, healthcare systems and research need to be overcome.
Short abstract
Psychological distress is common in COPD but remains mostly undetected and untreated despite several available management options. Increased research and clinical efforts are needed to further improve diagnostic and clinical treatment routines. https://bit.ly/3XjTyos
Introduction
COPD is a prevalent, preventable and treatable disease. It is characterised by persistent respiratory symptoms and airflow limitation, which result from airway and/or alveolar abnormalities that are typically provoked by exposure to noxious particles and gases [1]. COPD is a leading cause of morbidity and mortality worldwide, and is associated with a significant individual, social and economic burden and reductions in health-related quality of life (HRQoL) [2]. COPD was ranked third among the leading causes of death globally in 2019 [3] and its total direct annual costs in the European Union were estimated to be as high as €38.6 billion [4]. Alongside prominent pulmonary symptoms such as breathlessness, cough and/or sputum production, people with COPD often live with several additional comorbid symptoms and conditions [5].
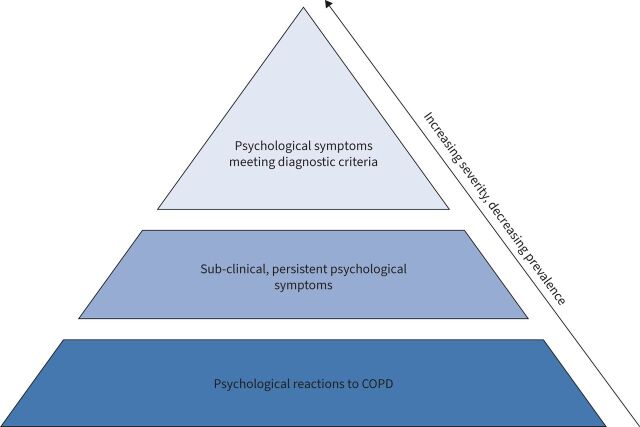
Depression and anxiety are among the most frequently observed psychological comorbidities in COPD and are associated with negative health outcomes, worse treatment adherence and greater healthcare utilisation [6, 7]. However, depression and anxiety are often overlooked in people with COPD. Only a minority receive specific treatment [8], although it is one of the most important aspects of COPD for patients [9]. Moreover, many patients do not fulfil all diagnostic criteria for a mental disorder, but still experience elevated levels of depression and anxiety symptoms (e.g. panic, worrying, lowered mood, loss of interest) as well as other emotional reactions to their illness (e.g. shame/guilt, loneliness, reduced self-esteem) [10]. In the following, “psychological distress” will be applied as a more general umbrella term for such pathological as well as nonpathological states of emotional suffering, even if they may differ in terms of phenomenology and physiological expressions (e.g. arousal levels, neuroendocrine/immunological/neural responses) [11]. Our broader view of psychological distress reflects a more inclusive model of psychological suffering in COPD (figure 1). Accordingly, it should be considered normal to experience passing periods with increased psychological distress when living with a disabling physical illness such as COPD (e.g. panic during an exacerbation; lowered mood when not being able to attend an important social event). However, if not attended to, psychological distress, including but not limited to clinical diagnoses of depression and anxiety, can become persistent and lead to considerable reductions in functioning and quality of life [6, 7]. Moreover, it has a detrimental effect on self-management.
FIGURE 1.
Psychological distress as an umbrella term for different levels of psychological suffering in COPD. This broader view of psychological distress reflects a more inclusive model of psychological suffering in COPD. It includes “normal”, passing psychological reactions to living with COPD (e.g. experiencing anxiety when there is an acute worsening of COPD; experiencing lowered mood when not being able to attend important family events; experiencing guilt when discussing smoking with the physician, etc.). Moreover, it includes more persistent, but still subclinical psychological symptoms, where anxiety, depression, self-blame, etc. do not meet the criteria for a mental disorder as well as pathological forms such as anxiety and depressive disorders. All of these levels of psychological distress can impact mental well-being, behaviour and quality of life negatively and result in nonoptimal self-management and course of COPD. The model does not attempt to make all psychological reactions pathological, but serves to make the clinician aware of the spectrum of reactions that should be assessed with the purpose of targeting management accordingly. If “normal” psychological reactions are noticed and met with appropriate support from healthcare professionals and psycho-educated family caregivers at an early stage, the spiralling effects of psychological suffering and maladaptive behaviour over time may be prevented. Thereby, psychological distress in the form of “normal” psychological reactions to COPD will be less likely to develop into anxiety and/or depressive disorders.
While there are effective pharmacological interventions available for treating depression and anxiety, trials in people with COPD have so far failed to convincingly demonstrate sustainable antidepressant and/or anxiolytic effects of selective serotonin reuptake inhibitors, benzodiazepines, tricyclic antidepressants (nortriptyline) and azapirones [12–15]. This is partly related to a lack of high-quality trials [13, 16]. Moreover, given prevalent polypharmacy and the related potential of drug interactions in people with COPD [17], plus potentially variable individual treatment preferences, nonpharmacological approaches are an important alternative or additional strategy [18].
The present narrative review aims to give a timely overview of nonpharmacological management strategies for psychological distress in COPD. Specifically, after an introduction to the prevalence and impact of psychological distress in COPD, strategies for the detection of psychological symptoms will be reviewed. Moreover, currently available treatment and supportive interventions will be presented together with available scientific evidence for their presumed benefits. Finally, current clinical challenges and future research directions will be discussed.
Prevalence and impact of psychological distress in COPD
Depression and anxiety
A large number of previous studies have focused on prevalence of depression and anxiety in people with COPD [7, 8, 19]. A meta-analysis by Yohannes et al. [20] that included 13 studies demonstrated pooled prevalence rates for symptoms of anxiety and depression in people with COPD of 36% and 40%, respectively, when assessed using questionnaire instruments. However, reported prevalence rates show substantial variation: between 8% and 80% for depression and between 2% and 96% for anxiety [21, 22]. These discrepancies partly relate to differences in the measurement instruments (e.g. self-reported questionnaires versus clinical/diagnostic interviews), populations (e.g. moderate versus severe disease severity) and/or assessment context (e.g. outpatient versus inpatient versus emergency treatment setting) [7, 23]. Moreover, the terms “psychological distress”, “anxiety” and “depression” as well as their subclinical and clinical forms are often used interchangeably, which reduces the precision of prevalence estimates. When Diagnostic and Statistical Manual of Mental Disorders (DSM) [24] or International Statistical Classification of Diseases and Related Health Problems [25] criteria-based clinical interviews are used as the gold standard of psychological diagnostics, pooled prevalence rates are typically lower [26, 27]. For example, a systematic review demonstrated prevalence rates of 26% for anxiety disorders and 19% for depression disorders across studies using clinical psychological interviews. However, these rates were still about three times higher than in non-COPD control groups [26–28].
Symptoms of depression and anxiety can have substantial negative impacts on disease management and outcomes in COPD [7, 14, 23]. Feelings of depression and anxiety in COPD have been linked to a higher symptom burden [29], higher smoking rates [30], higher exacerbation frequency [31, 32], (re)hospitalisations [33, 34] and mortality [35, 36], as well as reductions in treatment adherence [37], HRQoL [38], physical activity levels [30], functional exercise capacity [39] and less favourable outcomes of pulmonary rehabilitation (PR) [39]. Similarly, disease-specific fears (e.g. fear of breathlessness or physical activity, COPD-related anxiety) are associated with worse functional exercise capacity, HRQoL, health status, dyspnoea and physical activity as well as with reduced treatment success in people with COPD, even when controlling for levels of nonspecific psychological distress [40–45]. For example, higher disease-specific fears were associated with worse HRQoL and health status at the start, end and 6 months after an inpatient PR programme, stressing the need to target management for disease-specific fears to further improve the effects of PR [41].
Other aspects of psychological distress
While symptoms of anxiety and depression play a predominant role in the burden of psychological distress for people with COPD, other psychosocial aspects are also relevant. Due to the central role of tobacco smoking in the development of COPD, many patients who are current or former smokers feel responsible for their disease, leading to intense feelings of guilt, shame and self-blame [46, 47]. Such feelings are often mirrored in stigma and blame from others and/or society [48, 49] and can contribute to reduced treatment adherence and help-seeking, increased emotional distress, and social isolation. In turn, social isolation [50], which can also be caused by reduced functional capacity or fear-based avoidance of physical activity, can be associated with loneliness in people with COPD [51–54], which is associated with worse functional capacity, HRQoL and psychological distress [47]. However, respective prevalence estimates are often not reported, requiring further research efforts [49, 55].
COPD-related loss of functional capacity is also associated with changes in practical roles and relationships with partners, friends and/or family, which can lead to increasingly negative self-image and loss of self-esteem for many patients [56–59]. A review of patient and carer experience of breathlessness [60], one of the key symptoms of COPD, highlighted the interlinked nature of the physical, psychological, social and existential effects of breathlessness [61] on those living with it. Altogether, if the patients and/or caregivers are not supported to manage these often-interrelated psychosocial aspects of living with COPD, they can easily increase the burden of psychological distress and further decrease physical health.
Bidirectional development of COPD and psychological distress
The precise mechanisms underlying the strong associations between psychological distress and COPD are still unclear and may vary across subgroups of individuals [5, 62, 63]. These associations are likely bidirectional, with high disease burden due to COPD increasing the risk of developing high psychological distress over time, while also high psychological distress subsequently increasing the risk of developing COPD or worsening COPD symptoms by contributing to unhealthy behaviours (e.g. smoking, activity avoidance) [64–67]. A meta-analysis of respective longitudinal studies found that pre-existing COPD increased the risk of subsequently developing depression [65]. Conversely, pre-existing symptoms of depression and anxiety also increased the risk of subsequently worsening COPD outcomes, including first diagnosis and greater risk of exacerbations and mortality. According to the biopsychosocial model, depression and anxiety may affect disease severity via mechanisms associated with poor treatment adherence and self-care. In addition, several factors such as smoking behaviour [68–70], physical activity levels [71, 72], brain processes [62, 73, 74], sleeping patterns [75, 76], inflammatory processes [15, 77] and activation of cell-mediated immunity [78], fatigue [79] and genetic risk factors [80, 81], are most likely involved as contributors or moderators, requiring future systematic studies. Moreover, biological stress systems in the brain (e.g. sympathetic nervous system) are often dysregulated in individuals experiencing depression and anxiety, which might lead to increases in physical symptoms [82].
Detection and assessment of psychological symptoms in COPD
Many people with COPD experience psychological distress, though not fulfilling the diagnostic criteria for a depression and/or anxiety disorder. Routine screening and assessment of psychological distress in COPD are important to differentiate supportive psychological interventions (for nonpathological cases of psychological distress) from the specialised treatment of comorbid mental illness [83]. Such screening can be performed in the context of PR, primary care consultations, outpatient visits and/or hospital discharge.
Screening
As has been recommended for people with other chronic diseases (e.g. cystic fibrosis) [84], several different approaches to screening can be applied. Single items such as the distress thermometer [85] or the three-question anxiety and two-question depression screen from the Primary Care Evaluation of Mental Disorders (PRIME-MD) [86] can be quickly and easily administered. Nonetheless, their high sensitivity and false-positive rate can result in a higher long-term assessment burden. The Center for Epidemiological Studies Depression Scale [87] and the Brief Assessment Schedule Depression Cards [88] screening test are also often used to detect depression. Many alternative multi-item screening questionnaires have been validated and commonly applied in the COPD population, especially when time is limited, such as the Hospital Anxiety and Depression Scale (HADS) [89], the Generalized Anxiety Disorder Scale [90], the Patient Health Questionnaire [91] and the Depression, Anxiety, Stress Scale [92]. Since many symptoms of depression and anxiety overlap with somatic symptoms of COPD, such as breathlessness, loss of appetite, sleep disturbances and fatigue, screening questionnaires accounting for this overlap should be preferred, e.g. HADS [93] and the Beck Depression Inventory Fast Screen [94]. Psychological screening questionnaires specifically developed for respiratory diseases could also be applied, e.g. the Anxiety Inventory Respiratory [95] scale or the Breathlessness Beliefs Questionnaire [96]. However, considering the prevalence of multiple long-term conditions in COPD, measures that are valid and reliable across diagnoses may be particularly helpful.
Diagnostic interviewing
Potential clinical cases detected by screening should be monitored and assessed further using clinical diagnostic interviews as the gold standard. Diagnostic interviews can be performed using the Mini-International Neuropsychiatric Interview (MINI), designed for application in primary and psychiatric care, in both clinical practice and research. The MINI is organised into 19 modules exploring 17 DSM IV axis I disorders, antisocial personality disorder and suicide risk [97]. Other interviews include the Structured Clinical Interview for DSM [98] and the Anxiety Disorders Interview Schedule for DSM [99]. However, in case of limited resources and time, shorter semi-structured screening interviews such as the PRIME-MD [100] are available. The latter was developed to detect the most common DSM disorders, in both primary and tertiary care settings, and it has been extensively adopted to assess psychiatric disorders in populations with chronic illness. When the symptoms are significant, and especially in cases where depression includes self-harm, a multidisciplinary approach is essential, involving both psychopharmacology and psychotherapy, with a view to continuity of care between the hospital and the local area. If the physician is not specialised in the field of mental health and/or does not have respective diagnostic expertise, it is advisable to refer the patient to a mental health professional, who can assess which individualised path is most suitable.
To conclude from the above, the clinician is recommended to perform psychological distress screening of all patients as part of routine care (e.g. annual visits to the general practitioner or outpatient clinic, at the beginning of pulmonary rehabilitation, upon discharge from the hospital after an exacerbation). Preferably, instruments should be used that are sensitive to multiple psychological states of suffering, e.g. anxiety, depression, physical as well as social manifestations. If this initial screening reveals the need for intensified investigation, the administration of further assessment and/or diagnostic interviewing is recommended with the purpose of identifying potential cases of anxiety and/or depressive disorders.
Management of psychological distress in COPD
Over the past few decades, the management of psychological distress in COPD has improved, although there is still an urgent need for the intensified application of therapeutic approaches in clinical practice to reduce symptom progression [8, 101]. Table 1 summarises the main types of nonpharmacological management and/or supportive interventions presented in this review [102–120].
TABLE 1.
Nonpharmacological management options and their effects on symptoms of depression and anxiety in people with COPD
| Treatment options | Anxiety | Depression | |||
| Category of treatment | Specific kind of treatment | Effect | References of reviews/systematic reviews/meta-analysis | Effect | References of reviews/systematic reviews/meta-analysis |
| Psychotherapy | Cognitive behavioural therapy | + | [102] | + | [102] |
| Mindfulness-based intervention | − | [103] | − | [103] | |
| Self-management interventions | ++ | [104] | ++ | [104] | |
| Relaxation therapy | Relaxation therapy | + | [105] | + | [105] |
| Progressive muscle relaxation | + | [106] | + | [106] | |
| Mind–body exercise | Qigong | + | [107, 108] | + | [107, 108] |
| Tai chi | − | [109] | − | [109] | |
| Yoga | − | [110, 111] | − | [110, 111] | |
| Music therapy | Music therapy | + | [112] | − | [112] |
| Singing | − | [113, 114] | − | [113, 114] | |
| Integrated disease management | − | [115, 116] | − | [115, 116] | |
| Collaborative care model | − | [117] | − | [117] | |
| Pulmonary rehabilitation | ++ | [118] | +++ | [118] | |
| Telemonitoring | − | [119, 120] | − | [119, 120] | |
+++: Strong evidence for beneficial effect; ++: moderate evidence for beneficial effect; +: small/promising evidence for beneficial effect; −: no consistent evidence for beneficial effect.
Cognitive behavioural therapy (CBT)
CBT is the most studied psychological intervention in COPD [121]. Its principles were originally developed to treat depression and anxiety disorders [122, 123] and have, over the decades, been adapted and adjusted for applications in several somatic and psychiatric symptoms and settings [124, 125]. In CBT, patients learn how to cope with psychological distress by becoming aware of and targeting the closely interrelated cognitive (thinking), emotional (feeling), physical (sensing) and behavioural (doing) factors that contribute to the maintenance of psychological symptoms. For example, symptoms of anxiety are often maintained and/or increased by the patients’ catastrophic thinking and avoidance of feared situations (e.g. “I cannot handle my breathlessness, so I avoid activities that make me breathless”) [126]. Therefore, a core element in CBT for anxiety is exposure, where the patient is confronted with fear-inducing stimuli systematically and actively [127]. Likewise, symptoms of depression, such as low mood and negative automatic thinking, are often worsened by the patients’ accompanying inactivity and isolation (e.g. “I am useless, so I might as well stay here all by myself”), which is why behavioural activation is a core element in CBT for depression [128–130]. These elements are accompanied by a restructuring of dysfunctional thinking patterns by which the patient learns to modify depression and/or anxiety-induced thoughts into more realistic and less emotionally distressing thinking.
Recent meta-analyses indicate that CBT is efficacious for depression, anxiety and HRQoL in COPD, albeit yielding small effect sizes [102, 131–133] and insignificant effects when compared to other high-intensity comparators (i.e. additional activities provided on a background of usual care) [102]. Moreover, the existing randomised controlled trials are vastly heterogeneous in terms of CBT elements included treatment duration (on average weekly sessions of 60 min for at least eight meetings), intervention format (individual versus group; tele-based versus face-to-face) and healthcare professionals delivering the intervention. Additional research is therefore needed to understand optimal ways of delivering CBT to people with COPD and how to implement CBT in clinical practice.
Mindfulness/meditation and meta-cognitive therapies
Branches of CBT have also evolved to promote a more accepting mode of response to difficult thoughts and feelings, as an alternative to the restructuring elements of traditional CBT that seek to change or modify thought contents [47, 103, 134]. An example of this so-called meta-cognitive approach [135] can be seen in mindfulness-based cognitive therapy (MBCT), where CBT elements have been merged with mindfulness meditation, i.e. practising the ability to bring nonjudgemental, present-moment awareness to thoughts, feelings and bodily sensations. MBCT was originally developed for the prevention of depression relapse, but a recent trial shows MBCT to be also effective in reducing depression (but not anxiety) symptoms in COPD [136]. In a qualitative study, participants described that MBCT helped to reduce their disease-related stigma and give them a greater sense of control [137]. Other types of mindfulness/meditation-based interventions have also been tested, but a recent systematic review of five studies of mindfulness-based intervention in COPD reported nonsignificant findings for the outcomes of anxiety, stress, respiratory symptoms or other physiological outcomes [103]. These findings, however, are based on a few, relatively small studies with heterogeneous outcomes and intervention elements, and larger studies are therefore needed.
Relaxation techniques
Relaxation techniques refer to the various methods and manipulations used to reduce stress, muscle tension and anxiety in the body, increasing the patient's perception of self-control or modulating their emotions. Types of relaxation techniques include autogenic relaxation [138, 139], where both visual imagery and body awareness are used to reduce stress and improve fatigue and sleep quality, progressive muscle relaxation, involving slowly tensing and then relaxing each muscle group, reducing depression and anxiety symptoms [140], and visualisation, in which a visual journey to a peaceful, calming place or situation is taken through mental images [141]. Other relaxation techniques may include deep breathing [142], massage [143], distraction therapy [144], meditation [145], biofeedback [146], music and art therapy [112, 147], aromatherapy [148], and hydrotherapy [149]. There is some evidence for the effectiveness of relaxation techniques in COPD, although results are inconsistent due to heterogeneous methodologies, time frames, instruments and samples [150]. A meta-analysis showed slight positive effects of relaxation techniques in COPD, with small effect sizes for improving depression and anxiety, but highlighted the need for future higher-quality research, paying attention to confounders, sampling, control strategies and measurement instruments [105].
Qigong, tai chi and yoga
Recently, there has been an increase in studies evaluating the effects of ancient, Eastern, mind-body oriented movement therapies, i.e. qigong, tai chi and yoga, in COPD.
Qigong is a traditional Eastern practice involving systematic movements of the whole body in combination with specific breathing patterns that involve abdominal and pursed-lip breathing. Recent meta-analyses including mostly randomised controlled trials (RCTs) suggested that qigong in people with COPD might be effective for improving symptoms of depression and anxiety, as well as exercise capacity, breathlessness, health status, quality of life and even pulmonary function [108, 151]. However, across studies, methodological and statistical heterogeneity was high and quality was often limited. As most studies to date took place in Asian countries, cross-cultural applicability of findings is unknown.
Tai chi is a noncompetitive, self-paced system of gentle physical exercise and stretching. Tai chi has many different styles, that may focus on health maintenance or the martial arts. Recent systematic reviews and meta-analyses suggested that tai chi has some beneficial effects on functional capacity and pulmonary function [152, 153] as well as on dyspnoea, fatigue and quality of life [154]. However, potential benefits for depression and anxiety remain unclear with contrasting findings between analyses, showing no significant improvement in depressive symptoms and unstable results for anxiety symptoms [109]. This was partly related to the very low number of respective studies and high heterogeneity in methods.
Yoga aims to create a union between body, mind and spirit, as well as between the individual self and universal consciousness. Hatha yoga, which is the most widely practised and studied form of yoga in the modern world [155], stresses two main aspects, “asana” (physical postures) and “pranayama” (breathing exercises). Although systematic reviews and meta-analyses report positive effects of yoga on exercise capacity and pulmonary function in people with COPD [110, 111, 156–158], respective benefits on psychological distress and HRQoL remained unclear, and so far suggest no benefits compared to usual care for anxiety and depressive symptoms [110, 111].
Music therapy and singing
Music therapy can be passive (e.g. listening to music) or active (e.g. singing). It is an interpersonal process in which the therapist uses music to help patients improve or maintain, health, and has the potential to be both an enjoyable and low-cost intervention. Music therapy might exert beneficial effects on people with respiratory disease by improving breathing coordination and breathlessness, inducing relaxation, elevating mood and even neurotransmitter activity [147, 159, 160]. Music therapy, specifically playing wind instruments and singing, can boost lung function and promote the practice of pursed-lip breathing. In turn, breathlessness, characterised by distress and unpleasantness, may be reduced and exercise capacity improved [112]. In particular, singing can enhance mood and provide opportunities for social support and friendship [161].
A meta-analysis of randomised controlled trials that compared passive music therapy (listening to music) and mixed music therapy (combination of listening and singing) with usual care or other nonmusical types of intervention in people with COPD suggested that mixed music therapy is effective in reducing anxiety, alongside improvements in breathlessness, sleep quality and blood pressure [112]. However, although singing in people with COPD has been shown to be safe, enjoyable and lead to promising effects on physical components of quality of life and anxiety in smaller and/or qualitative studies [114, 162], a recent Cochrane review was not able to demonstrate consistent beneficial effects on depression and anxiety levels [113]. However, these results on singing and music therapy were mostly based on low-quality evidence due to the low number of studies, small sample sizes and absence of data on long-term effects, requiring future studies.
Pulmonary rehabilitation
PR is an interdisciplinary intervention based on a thorough patient assessment. Although delivery is highly varied [163], PR typically comprises exercise training, patient education and behaviour change interventions. PR aims to improve the physical and psychological condition of people with chronic respiratory disease [163] and has a substantial evidence base supporting its positive effects on quality of life, exercise capacity, symptoms (e.g. breathlessness) and hospitalisations in people with COPD [164–166]. More recently, a meta-analysis of 11 studies also demonstrated significant benefits of PR of moderate magnitude for anxiety symptoms and large magnitude for depression symptoms [118]. These benefits were not related to programme duration, age, gender or disease severity. Alongside these improvements in more general forms of depression and anxiety, recent studies further demonstrated that short, intensive 3-week inpatient PR can also improve disease-specific fears such as fear of breathlessness and fear of physical activity, with effects maintained at 6 months post-PR [40, 41].
It is currently not well understood how exactly PR impacts psychological distress, especially if no direct psychosocial support is included in some programmes. However, it is likely that most PR programmes already incorporate (hidden) aspects of CBT, e.g. goal setting, activity pacing or exposure to feared (physical) activities [102], potentially contributing to observed anxiolytic and/or antidepressant effects of PR [125, 131, 132]. In light of the current emphasis on more individualised models of patient care [167, 168], it remains to be tested which approaches and individual PR elements, in which dose, can maximise the potential benefits of PR for (which) individuals with COPD and comorbid psychological distress.
Principles of care
It can be challenging for people with COPD to actively engage in the care process, especially when treatment is characterised by multiple interventions and/or is continuous and of long duration, leading to high drop-out rates and poor adherence. This may result in worse clinical outcomes including increased exacerbation and rehospitalisation rates [169, 170]. Among the many variables that can contribute to increased or decreased levels of patient engagement are therapeutic alliance, accessibility of care and confidence that treatment will address their specific goals. For this reason, person-centred care, which focuses on patient engagement, empowerment and respect, becomes a fundamental framework for considering tools and techniques to improve engagement in treatment [171, 172]. Supported self-management in partnership with clinicians alert to the many difficulties of living with COPD, which enables patients to cope engagingly and seek help appropriately to manage their symptoms, should be encouraged [60]. Interventions for managing psychological distress described in the previous paragraphs can be integrated into the patient's care pathways in many ways, including through supported self-management and integrated models of care.
Supported self-management
Supported self-management approaches are directed towards behavioural change and comprise various components, often in heterogeneous combinations. Examples include smoking cessation, an increase in physical activity and exercise, appropriate medication use (e.g. inhaler technique), managing symptoms (e.g. breathlessness), use of energy conservation techniques (e.g. pacing), avoidance of aggravating factors (e.g. air pollution) and use of stress management techniques [173]. A recent Cochrane review demonstrated moderate evidence for beneficial effects of self-management interventions in people with COPD for reducing depression and anxiety, while also improving quality of life and respiratory-related hospital admissions [104]. Notably, another systematic review showed that self-management interventions specifically targeting mental health concerns alongside symptom management were significantly more effective in improving quality of life and other health outcomes in people with COPD than those targeting symptom management alone [174]. Similarly, evidence suggests education and action plans alone show little benefit for reducing depression or anxiety, indicating the need for more comprehensive approaches to address psychological distress [116, 175, 176].
Integrated models of care
Integrated disease management (IDM) programmes aim to combine different components of care, with healthcare providers co-operating to provide greater efficiency and quality. While a recent Cochrane review demonstrated beneficial effects across a variety of IDM programmes on quality of life, exercise capacity and number and duration of hospital admissions in people with COPD, no consistent beneficial effects were found on levels of depression and anxiety [115].
The Collaborative Care Model, instead, adopts team care, an interdisciplinary approach to deliver evidence-based diagnoses, treatment and follow-up care and is useful in supporting the management of depression in chronic conditions; although data on its efficacy, considering both depression and anxiety in general, are inconsistent. However, there are still very few studies exploring the implementation of this model. Given initial promising results, these may encourage future studies, especially involving and treating older and vulnerable patients such as those with COPD [117].
Breathlessness support services that prompt early integration of palliative care and utilise holistic (pharmacological and nonpharmacological) techniques are also a good example of integrated multidisciplinary team care. Their consideration of physical, psychological, social and spiritual needs of individual patients, their families or carers has been shown to improve distress due to breathlessness and depression symptoms [177–179]. While this evidence is predominantly in people with thoracic cancer, analysis of pooled data from three contributing trials suggests diagnosis may not be predictive of intervention response [180]. Continued calls for earlier and greater integration between respiratory and palliative care may therefore have important implications for addressing psychological distress [177, 181, 182].
Current challenges and future directions
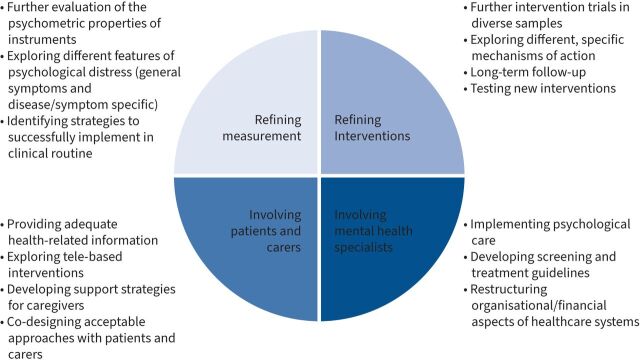
Although psychological distress is prevalent in COPD and associated with a substantial individual [38] and socio-economic burden [183, 184], it often remains undetected and untreated [185, 186]. Studies reported that in less than 44% of people with COPD, clinically relevant increased levels of depression and/or anxiety were adequately diagnosed and that only 31% of them had received any treatment for these comorbid psychological conditions [187]. These data have been subsequently confirmed [7], stressing that less than one-third of people with COPD and mental health problems receive adequate management, despite the recommendations of the Global Initiative for Chronic Obstructive Lung Disease guidelines to actively screen for and manage psychological comorbidities [188]. Hence, some important clinical and research-related challenges should be addressed in the future to optimise the management of psychological distress in COPD, which will be discussed below (figure 2).
FIGURE 2.
Future directions.
Development and implementation of valid screening instruments
As mentioned previously, prevalence estimates for psychological distress in COPD are currently compromised by confusion in terminology (i.e. “psychological distress”, “anxiety”, “depression”; “symptoms” versus “disorder”) and lack of widely accepted cut-offs for clinically significant cases. Therefore, a comprehensive evaluation of the psychometric properties of instruments measuring (specific aspects of) psychological distress in COPD is needed. Moreover, studies exploring distinctive features of psychological distress across respiratory illnesses could support more effective identification and management.
However, the fact that valid questionnaires exist does not necessarily mean that they are being used in clinical settings. A recent study explored respiratory clinicians’ perspectives of mental health in COPD [56] and identified many barriers. Firstly, despite using validated screening instruments (i.e. the HADS) it can be difficult for respiratory clinicians to differentiate mental health issues from normal psychological reactions to physical symptoms (e.g. breathlessness-related anxiety). Secondly, limited training in communication skills can hold respiratory clinicians back from initiating discussions about mental health. Thirdly, time constraints are perceived as barriers to discussing mental health, especially in primary care, and, fourthly, navigating (referral to) mental health services and fragmented care are challenging for healthcare professionals as well as patients. Nonetheless, the existing evidence also includes examples of successful implementation of psychological screening [189] and further systematic clinical implementation studies are needed in the future [190].
Refinement of interventions
While the evidence base for nonpharmacological interventions for psychological distress in COPD has grown considerably over the last decades, several issues remain. Firstly, the majority of existing RCTs yield small effect sizes, compared to treatment as usual. When comparing specific nonpharmacological interventions of comparable intensity, there tend to be no apparent differences in their effects on psychological distress. This may be explained by the common nonspecific factors of nonpharmacological interventions, such as social support in group programmes, attention from healthcare professionals and increasing physical activity by regularly attending intervention sessions [191]. Therefore, comparing the specific mechanisms of various nonpharmacological interventions, i.e. through dismantling designs [192], is needed to design more efficient intervention programmes in the future. Secondly, it is well known from studies of behavioural interventions that treatment effects tend to wane over time [132]. Many existing studies do not include follow-up measurements beyond 6 months post-treatment [132, 133, 193] and longer-term follow-ups are needed with the purpose of, firstly, identifying the most cost-efficient length and intensity of intervention programmes and, secondly, introducing booster sessions at optimal post-treatment time points. Thirdly, several nonpharmacological intervention types have not been empirically evaluated with psychological distress as the primary outcome. For example, although singing in people with COPD has been shown to be safe, enjoyable and leading to promising effects on physical components of quality of life and anxiety in smaller and/or qualitative studies [114, 162], a recent Cochrane review was not able to demonstrate consistent beneficial effects on the secondary outcomes depression and anxiety levels [113]. Moreover, low baseline levels of depression and anxiety in these samples might create floor effects, preventing further improvements in psychological distress during the intervention [162, 194], Fourthly, there is a tendency for studies of nonpharmacological interventions to cluster around culture-specific intervention types. For example, most studies of mind–body interventions, such as tai chi, qigong and yoga, have been conducted in Eastern contexts, whereas studies of CBT have predominantly been conducted in Western settings. Hence, future multi-centre studies of culturally diverse samples are needed. Finally, several psychological intervention programmes have shown promising effects on psychological distress in other somatic disease populations and may also be evaluated in people with COPD, e.g. acceptance and commitment therapy aiming to increase goal-directed and value-based behaviour [195, 196], compassion-focused therapy targeting self-criticism [197] or meta-cognitive therapy targeting rumination and worry [198], and dignity therapy targeting peoples’ sense of meaning and purpose.
Inclusion of and referral to mental health specialists
While several types of nonpharmacological interventions have been empirically validated, the availability of such interventions is limited in clinical practice, which is reported as a barrier to initiating discussions of mental health by respiratory clinicians [188]. An international survey among members of respiratory societies indicates that mental health specialists, e.g. psychologists and/or physicians or nurses with mental health training, are seldom represented in PR teams [199] and the provision of psychological services for people with COPD is commonly observed in primary care settings, often in the form of long waiting lists for mental health management [200].
As outlined above, there is an urgent need for psychological care in COPD and its potential value has been previously noted but rarely integrated and tested in order to optimise models of care [201, 202]. In contrast, psychological care is already embedded in cystic fibrosis (CF) care at many CF centres, in which a psychologist and/or a social worker are part of the multidisciplinary team. This model might work as well in COPD. In the CF mental health guidelines, annual mental health screening and treatment is recommended [84]. National implementation of these guidelines has been highly successful [203–205], partly due to the development of a mental health “toolbox” and additional mental health training sessions at national conferences. The International Committee on Mental Health in Cystic Fibrosis guidelines could be implemented in clinical COPD care as well if the appropriate mental health experts are integrated into the care team. Evaluating the possibilities of implementing these screening recommendations in clinical practice should be a focus of future research. Moreover, additional studies are needed to identify and address biopsychosocial factors that might influence mental health, disease severity and HRQoL in individuals with COPD.
The availability of nonpharmacological interventions to reduce psychological distress among people with COPD in the future will most likely require a substantial restructuring at the organisational level of healthcare systems, including, but not limited to 1) on-boarding of mental healthcare specialists in multidisciplinary integrated respiratory and rehabilitation teams, 2) improved referral options, and 3) increased reimbursement of management costs.
Involvement of patients and caregivers
Finally, there is a need for more careful attention to the preferences, experiences and capabilities of the individual patients and their caregivers. The often relatively low levels of health literacy in the COPD population [206] call for increased focus on dedicating time and resources to the provision of health-related information. This is especially relevant when it comes to the impact of psychological distress, as this subject can be surrounded by considerable stigma, reluctance to express it and epistemic injustice [60]. Information can be provided based on existing, evidence-based materials, e.g. the guidelines provided by Hutchinson et al. [207]. Considering challenges of experiencing “two layers of stigma” around both lung disease and mental health [188], working together with people affected by COPD to codesign acceptable therapeutic pathways and approaches will be important.
It should also be noted that social isolation and passivity are natural consequences of psychological distress in COPD – especially during epidemic periods [37, 208] – which may result in further difficulties in reaching this specific population for supportive purposes [50]. The development of tele-based treatment modalities could be a promising element in solving this problem and studies of tele-based nonpharmacological interventions do exist but are limited in sample sizes [209–211]. Nonetheless, studies directly comparing the efficiency of such interventions, compared to in-person delivery, are lacking and tele-based interventions could pose challenges for patients who 1) are less familiar with and/or have less access to technologies, 2) do not have the support of a caregiver, and 3) are beginning to experience some cognitive difficulties [129].
Additionally, there is a need to develop and evaluate screening instruments and treatment strategies for partners/caregivers of people with COPD who often suffer from psychological distress and poor quality of life as well, but are usually neglected in clinical routine [212–214].
Conclusions
Psychological distress is widespread among people with COPD and is related to worse health outcomes. It often remains underdiagnosed and untreated, intensifying the burden for patients, caregivers and family. Psychological distress encompasses many forms, including both nonpathological responses to a chronic and progressive condition, and pathological mental health conditions such as depression and anxiety, which are the most studied in COPD. Several instruments are available for detecting psychological distress in clinical practice, although implementation is currently suboptimal. Various nonpharmacological treatment options have been suggested to reduce psychological distress in COPD and, to date, PR and cognitive behavioural approaches have the strongest evidence base for improving depression and anxiety symptoms. However, optimal models of care for people with COPD experiencing high levels of distress and approaches to achieve long-term impacts across culturally diverse samples are still unknown. Strengthening the evidence for nonpharmacological interventions and routinely implementing successful approaches into clinical practice will be essential to address the unmet psychological needs of people with COPD and their families.
Points for clinical practice
Provide information (material) about psychological distress in lung diseases.
Perform routine screening for psychological distress in people with COPD.
Use validated screening questionnaires.
If significant psychological distress is detected, discuss this with the patients and their carers and perform further assessment and/or diagnostic interviewing with a mental health professional, if needed.
Consider referring people with persistent subclinical symptoms to multidisciplinary pulmonary rehabilitation or cognitive behavioral therapy.
In complex cases, consider referring to mental health care specialists for clinical diagnostic interview and/or treatment.
Footnotes
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Montes de Oca M, Laucho-Contreras ME. Smoking cessation and vaccination. Eur Respir Rev 2023; 32: 220187.
Number 2 in the Series “Nonpharmacological interventions in COPD: state of the art and future directions” Edited by Geert M. Verleden and Wim Janssens
This article has an editorial commentary: https://doi.org/10.1183/16000617.0028-2023
Conflict of interest: E. Volpato received the ERS Young Scientist Sponsorship, for her abstract entitled “Asthma expectations predict symptoms over time: a longitudinal cohort study” on the occasion of the ERS Congress 2022. She also reports personal fees from Vivisol outside the submitted work. I. Farver-Vestergaard reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Roche outside the submitted work. L.J. Brighton, J. Peters, M. Verkleij, A. Hutchinson, M. Heijmans and A. von Leupoldt have no conflict of interests.
Support statement: A. von Leupoldt is supported by research grants from the Research Foundation – Flanders (FWO), Belgium (G0C1921N; GOA3718N), an infrastructure grant from the FWO and the Research Fund KU Leuven, Belgium (AKUL/19/06; I011320N), and by the ‘‘Asthenes’’ long-term structural funding Methusalem grant (METH/15/011) by the Flemish Government, Belgium. L.J. Brighton is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King's College Hospital NHS Foundation Trust, and an NIHR research partnership (NIHR 135171). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 2.Abu Tabar N, Al Qadire M, Thultheen I, et al. . Health-related quality of life, uncertainty, and anxiety among patients with chronic obstructive pulmonary disease. F1000Res 2021; 10: 420. doi: 10.12688/f1000research.51936.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . The top 10 causes of death. 2020. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death Date last updated: 9 December 2020. Date last accessed: 26 September 2022.
- 4.Forum of International Respiratory Societies . The Global Impact of Respiratory Disease. 2nd Edn. Sheffield, European Respiratory Society, 2017. [Google Scholar]
- 5.Vanfleteren LEGW, Spruit MA, Groenen M, et al. . Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. doi: 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 6.Zareifopoulos N, Bellou A, Spiropoulou A, et al. . Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: a narrative review. COPD 2019; 16: 406–417. doi: 10.1080/15412555.2019.1679102 [DOI] [PubMed] [Google Scholar]
- 7.Maurer J, Rebbapragada V, Borson S, et al. . Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest 2008; 134: 43S–56S. doi: 10.1378/chest.08-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014; 23: 345–349. doi: 10.1183/09059180.00007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camus-García E, González-González AI, Heijmans MN, et al. . Self-management interventions for adults living with chronic obstructive pulmonary disease (COPD): the development of a core outcome set for COMPAR-EU project. PLoS One 2021; 16: e0247522. doi: 10.1371/journal.pone.0247522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerpseth H, Knutsen IR, Jensen KT, et al. . Mirror of shame: patients experiences of late-stage COPD. A qualitative study. J Clin Nurs 2021; 30: 2854–2862. doi: 10.1111/jocn.15792 [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR). 5th Edn. Washington, DC, American Psychiatric Association Publishing; 2022. [Google Scholar]
- 12.Fritzsche A, Clamor A, von Leupoldt A. Effects of medical and psychological treatment of depression in patients with COPDa review. Respir Med 2011; 105: 1422–1433. doi: 10.1016/j.rmed.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 13.Pollok J, van Agteren JE, Carson-Chahhoud KV. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018; 12: CD012346. doi: 10.1002/14651858.CD012346.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouellette DR, Lavoie K. Recognition, diagnosis, and treatment of cognitive and psychiatric disorders in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 639–650. doi: 10.2147/COPD.S123994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelgrim CE, Peterson JD, Gosker HR, et al. . Psychological co-morbidities in COPD: targeting systemic inflammation, a benefit for both? Eur J Pharmacol 2019; 842: 99–110. doi: 10.1016/j.ejphar.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 16.Usmani ZA, Carson K V, Cheng JN, et al. . Pharmacological interventions for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; 9: CD008483. doi: 10.1002/14651858.CD008483.pub2 [DOI] [PubMed] [Google Scholar]
- 17.Hanlon P, Nicholl BI, Jani BD, et al. . Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank study. BMJ Open 2018; 8: e018404. doi: 10.1136/bmjopen-2017-018404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med 2016; 26: 16051. doi: 10.1038/npjpcrm.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schane RE, Woodruff PG, Dinno A, et al. . Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med 2008; 23: 1757–1762. doi: 10.1007/s11606-008-0749-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yohannes AM, Baldwin RC, Connolly MJ. Mood disorders in elderly patients with chronic obstructive pulmonary disease. Rev Clin Gerontol 2000; 10: 193–202. doi: 10.1017/S0959259800002100 [DOI] [Google Scholar]
- 21.Izquierdo JL, Morena D, González Y, et al. . Clinical management of COPD in a real-world setting. A big data analysis. Arch Bronconeumol 2021; 57: 94–100. doi: 10.1016/j.arbres.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Gestoso S, García-Sanz MT, Carreira JM, et al. . Impact of anxiety and depression on the prognosis of COPD exacerbations. BMC Pulm Med 2022; 22: 169. doi: 10.1186/s12890-022-01934-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yohannes AM, Willgoss TG, Baldwin RC, et al. . Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry 2010; 25: 1209–1221. doi: 10.1002/gps.2463 [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th Edn. Washington, DC, American Psychiatric Association Publishing, 2013. [Google Scholar]
- 25.World Health Organization . ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. 5th Edn. Geneva, World Health Organization, 2013. Available at: https://apps.who.int/iris/handle/10665/42980 [Google Scholar]
- 26.Baker AM, Holbrook JT, Yohannes AM, et al. . Test performance characteristics of the AIR, GAD-7, and HADS-anxiety screening questionnaires for anxiety in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2018; 15: 926–934. doi: 10.1513/AnnalsATS.201708-631OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willgoss TG, Yohannes AM. Anxiety disorders in patients with chronic obstructive pulmonary disease: a systematic review. Respir Care. 2012: 57; 550–556; doi: 10.4187/respcare.01328 [DOI] [PubMed] [Google Scholar]
- 28.Orrego C, Ballester M, Heymans M, et al. . Talking the same language on patient empowerment: development and content validation of a taxonomy of self-management interventions for chronic conditions. Health Expect 2021; 24: 1626–1638. doi: 10.1111/hex.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yohannes AM, Dryden S, Hanania NA. Validity and responsiveness of the depression anxiety stress scales-21 (DASS-21) in COPD. Chest 2019; 155: 1166–1177. doi: 10.1016/j.chest.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 30.Paine NJ, Bacon SL, Bourbeau J, et al. . Psychological distress is related to poor health behaviours in COPD and non-COPD patients: evidence from the CanCOLD study. Respir Med 2019; 146: 1–9. doi: 10.1016/j.rmed.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 31.Yohannes AM, Mülerová H, Lavoie K, et al. . The association of depressive symptoms with rates of acute exacerbations in patients with COPD: results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc 2017; 18: 955–959.e6. doi: 10.1016/j.jamda.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 32.Laurin C, Moullec G, Bacon SL, et al. . Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med 2012; 185: 918–923. doi: 10.1164/rccm.201105-0939PP [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Collet JP, Shapiro S, et al. . Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med 2008; 178: 913–920. doi: 10.1164/rccm.200804-619OC [DOI] [PubMed] [Google Scholar]
- 34.Singh G, Zhang W, Kuo YF, et al. . Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest 2016; 149: 905–915. doi: 10.1378/chest.15-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laforest L, Roche N, Devouassoux G, et al. . Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med 2016; 117: 33–39. doi: 10.1016/j.rmed.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 36.Divo M, Cote C, de Torres JP, et al. . Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 155–161. doi: 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 37.Volpato E, Toniolo S, Pagnini F, et al. . The relationship between anxiety, depression and treatment adherence in chronic obstructive pulmonary disease: a systematic review. Int J Chron Obstruct Pulmon Dis 2021; 16: 2001–2021. doi: 10.2147/COPD.S313841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blakemore A, Dickens C, Guthrie E, et al. . Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2014: 9; 501–512. doi: 10.2147/COPD.S58136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Leupoldt A, Taube K, Lehmann K, et al. . The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest 2011; 140: 730–736. doi: 10.1378/chest.10-2917 [DOI] [PubMed] [Google Scholar]
- 40.Reijnders T, Schuler M, Wittmann M, et al. . The impact of disease-specific fears on outcome measures of pulmonary rehabilitation in patients with COPD. Respir Med 2019; 146: 87–95. doi: 10.1016/j.rmed.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 41.Janssens T, Van de Moortel Z, Geidl W, et al. . Impact of disease-specific fears on pulmonary rehabilitation trajectories in patients with COPD. J Clin Med 2019; 8: 1460. doi: 10.3390/jcm8091460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keil DC, Stenzel NM, Kühl K, et al. . The impact of chronic obstructive pulmonary disease-related fears on disease-specific disability. Chron Respir Dis 2014; 11: 31–40. doi: 10.1177/1479972313516881 [DOI] [PubMed] [Google Scholar]
- 43.Fischer MJ, Scharloo M, Abbink J, et al. . Concerns about exercise are related to walk test results in pulmonary rehabilitation for patients with COPD. Int J Behav Med 2012; 19: 39–47. doi: 10.1007/s12529-010-9130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssens T, De Peuter S, Stans L, et al. . Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest 2011; 140: 618–625. doi: 10.1378/chest.10-3257 [DOI] [PubMed] [Google Scholar]
- 45.Carl J, Schultz K, Janssens T, et al. . The “can do, do do” concept in individuals with chronic obstructive pulmonary disease: an exploration of psychological mechanisms. Respir Res 2021; 22: 260. doi: 10.1186/s12931-021-01854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindqvist G, Hallberg LRM. Feelings of guilt due to self-inflicted disease. J Health Psychol 2010; 15: 456–466. doi: 10.1177/1359105309353646 [DOI] [PubMed] [Google Scholar]
- 47.Harrison SL, Robertson N, Apps L., et al. “We are not worthy” – understanding why patients decline pulmonary rehabilitation following an acute exacerbation of COPD. Disabil Rehabil 2015; 37: 750–756. doi: 10.3109/09638288.2014.939770 [DOI] [PubMed] [Google Scholar]
- 48.Plaufcan MR, Wamboldt FS, Holm KE. Behavioral and characterological self-blame in chronic obstructive pulmonary disease. J Psychosom Res 2012; 72: 78–83. doi: 10.1016/j.jpsychores.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis 2021; 16: 1647–1659. doi: 10.2147/COPD.S306874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bu F, Philip K, Fancourt D. Social isolation and loneliness as risk factors for hospital admissions for respiratory disease among older adults. Thorax 2020; 75: 597–599. doi: 10.1136/thoraxjnl-2019-214445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marty PK, Novotny P, Benzo RP. Loneliness and ED visits in chronic obstructive pulmonary disease. Mayo Clin Proc Innov Qual Outcomes 2019; 3: 350–357. doi: 10.1016/j.mayocpiqo.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reijnders T, Schuler M, Jelusic D, et al. . the impact of loneliness on outcomes of pulmonary rehabilitation in patients with COPD. COPD 2018; 15: 446–453. doi: 10.1080/15412555.2018.1471128 [DOI] [PubMed] [Google Scholar]
- 53.Scarlata S, Cardaci V, Santangelo C, et al. . Distancing measures in COVID-19 pandemic: loneliness, more than physical isolation, affects health status and psycho-cognitive wellbeing in elderly patients with chronic obstructive pulmonary disease. COPD 2021; 18: 443–448. doi: 10.1080/15412555.2021.1941834 [DOI] [PubMed] [Google Scholar]
- 54.Tumilty E, Doolan-Noble F, Latu ATF, et al. . ‘A balancing act’. Living with severe chronic obstructive pulmonary disease in Southern New Zealand: a qualitative study. J Prim Health Care 2020; 12: 166–172. doi: 10.1071/HC20007 [DOI] [PubMed] [Google Scholar]
- 55.Brighton LJ, Chilcot J, Maddocks M. Social dimensions of chronic respiratory disease: stigma, isolation, and loneliness. Curr Opin Support Palliat Care 2022; 16: 195–202. doi: 10.1097/SPC.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 56.Wang KY, Sung PY, Yang ST, et al. . Influence of family caregiver caring behavior on COPD patients’ self-care behavior in Taiwan. Respir Care 2012; 57: 263–272. doi: 10.4187/respcare.00986 [DOI] [PubMed] [Google Scholar]
- 57.Disler RT, Green A, Luckett T, et al. . Experience of advanced chronic obstructive pulmonary disease: metasynthesis of qualitative research. J Pain Symptom Manage 2014; 48: 1182–1199. doi: 10.1016/j.jpainsymman.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 58.Brighton LJ, Bristowe K, Bayly J, et al. . Experiences of pulmonary rehabilitation in people living with chronic obstructive pulmonary disease and frailty. a qualitative interview study. Ann Am Thorac Soc 2020; 17: 1213–1221. doi: 10.1513/AnnalsATS.201910-800OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Meide H, Teunissen T, Visser LH, et al. . Trapped in my lungs and fighting a losing battle. A phenomenological study of patients living with chronic obstructive and pulmonary disease. Scand J Caring Sci 2020; 34: 118–127. doi: 10.1111/scs.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchinson A, Barclay-Klingle N, Galvin K, et al. . Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J 2018; 51: 1701477. doi: 10.1183/13993003.01477-2017 [DOI] [PubMed] [Google Scholar]
- 61.Abernethy AP, Wheeler JL. Total dyspnoea. Curr Opin Support Palliat Care 2008; 2: 110–113. doi: 10.1097/SPC.0b013e328300cad0 [DOI] [PubMed] [Google Scholar]
- 62.Finnegan SL, Pattinson KTS, Sundh J, et al. . A common model for the breathlessness experience across cardiorespiratory disease. ERJ Open Res 2021; 7: 00818-2020. doi: 10.1183/23120541.00818-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Augustin IML, Franssen FME, Houben-Wilke S, et al. . Multidimensional outcome assessment of pulmonary rehabilitation in traits-based clusters of COPD patients. PLoS One 2022; 17: e0263657. doi: 10.1371/journal.pone.0263657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider C, Jick SS, Bothner U, et al. . COPD and the risk of depression. Chest 2010; 137: 341–347. doi: 10.1378/chest.09-0614 [DOI] [PubMed] [Google Scholar]
- 65.Atlantis E, Fahey P, Cochrane B, et al. . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and metaanalysis. Chest 2013; 144: 766–777. doi: 10.1378/chest.12-1911 [DOI] [PubMed] [Google Scholar]
- 66.Yohannes AM, Müllerová H, Hanania NA, et al. . Long-term course of depression trajectories in patients with COPD: a 3 year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest 2016; 149: 916–926. doi: 10.1016/j.chest.2015.10.081 [DOI] [PubMed] [Google Scholar]
- 67.Spathis A, Booth S, Moffat C, et al. . The breathing, thinking, functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med 2017; 27: 27. doi: 10.1038/s41533-017-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuentes-Alonso M, Lopez-Herranz M, López-de-Andrés A, et al. . Prevalence and determinants of mental health among COPD patients in a population-based sample in Spain. J Clin Med 2021; 10: 2786. doi: 10.3390/jcm10132786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathew AR, Yount SE, Kalhan R, et al. . Psychological functioning in patients with chronic obstructive pulmonary disease: a preliminary study of relations with smoking status and disease impact. Nicotine Tob Res 2019; 21: 686–690. doi: 10.1093/ntr/nty102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou P, Zhu Y, Chen P, et al. . Prevalence and correlations with depression, anxiety, and other features in outpatients with chronic obstructive pulmonary disease in China: a cross-sectional case control study. BMC Pulm Med 2012; 12: 53. doi: 10.1186/1471-2466-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanania NA, O'Donnell DE. Activity-related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. Int J Chron Obstruct Pulmon Dis 2019; 14: 1127–1138. doi: 10.2147/COPD.S188141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavoie KL, Sedeno M, Hamilton A, et al. . Behavioural interventions targeting physical activity improve psychocognitive outcomes in COPD. ERJ Open Res 2019; 5: 00013–2019. doi: 10.1183/23120541.00013-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esser RW, Stoeckel MC, Kirsten A, et al. . Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease. Front Physiol 2017; 8: 617. doi: 10.3389/fphys.2017.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esser RW, Stoeckel MC, Kirsten A, et al. . Structural brain changes in patients with COPD. Chest 2016; 149: 426–434. doi: 10.1378/chest.15-0027 [DOI] [PubMed] [Google Scholar]
- 75.Vanfleteren LE, Beghe B, Andersson A, et al. . Multimorbidity in COPD, does sleep matter? Eur J Intern Med 2020; 73: 7–15. doi: 10.1016/j.ejim.2019.12.032 [DOI] [PubMed] [Google Scholar]
- 76.Lee SH, Kim KU, Lee H, et al. . Sleep disturbance in patients with mild-moderate chronic obstructive pulmonary disease. Clin Respir J 2019; 13: 751–757. doi: 10.1111/crj.13085 [DOI] [PubMed] [Google Scholar]
- 77.Ryan R, Spathis A, Clow A, et al. . Breathlessness and inflammation: potential relationships and implications. Curr Opin Support Palliat Care 2016; 10: 242–248. doi: 10.1097/SPC.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 78.Berk M, Williams LJ, Jacka FN, et al. . So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013; 11: 200. doi: 10.1186/1741-7015-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebadi Z, Goërtz YMJ, Van Herck M, et al. . The prevalence and related factors of fatigue in patients with COPD: a systematic review. Eur Respir Rev 2021; 30: 200298. doi: 10.1183/16000617.0298-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marcolongo F, Scarlata S, Tomino C, et al. . Psycho-cognitive assessment and quality of life in older adults with chronic obstructive pulmonary disease-carrying the rs4713916 gene polymorphism (G/A) of gene FKBP5 and response to pulmonary rehabilitation. Psychiatr Genet 2022; 32: 116–124. doi: 10.1097/YPG.0000000000000308 [DOI] [PubMed] [Google Scholar]
- 81.Yohannes AM, Kohen R, Nguyen HQ, et al. . Serotonin transporter gene polymorphisms and depressive symptoms in patients with chronic obstructive pulmonary disease. Expert Rev Respir Med 2021; 15: 681–687. doi: 10.1080/17476348.2021.1865159 [DOI] [PubMed] [Google Scholar]
- 82.Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007; 29: 147–155. doi: 10.1016/j.genhosppsych.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 83.Farver-Vestergaard I, Johannesen G, ter Beek L. Occupational therapy, nutritional modulation and psychological support during pulmonary rehabilitation. In: Holland AE, Dal Corso S, Spruit MA, eds. Pulmonary Rehabilitation. Sheffield, European Respiratory Society, 2021; pp. 83–98. doi: 10.1183/2312508X.erm9321 [DOI] [Google Scholar]
- 84.Quittner AL, Abbott J, Georgiopoulos AM, et al. . International Committee On Mental Health In Cystic Fibrosis: Cystic Fibrosis Foundation And European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax. 2016; 71: 26–34. doi: 10.1136/thoraxjnl-2015-207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baken DM, Woolley C. Validation of the distress thermometer, impact thermometer and combinations of these in screening for distress. Psychooncology 2011; 20: 609–614. doi: 10.1002/pon.1934 [DOI] [PubMed] [Google Scholar]
- 86.Tamburrino MB, Lynch DJ, Nagel RW, et al. . Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry 2009; 11: 339–343. doi: 10.4088/PCC.08.m00711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eaton WW, Smith, C, Ybarra, M, et al. . Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD-R). In: Maruish ME, ed. The use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults. Mahwah, NJ, Lawrence Erlbaum Associates Publishers, 2004; pp. 363–377. [Google Scholar]
- 88.Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry 2000; 15: 1090–1096. doi: [DOI] [PubMed] [Google Scholar]
- 89.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 90.Toussaint A, Hüsing P, Gumz A, et al. . Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord 2020; 265: 395–401. doi: 10.1016/j.jad.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 91.Kroenke K, Spitzer RL, Williams JBW. The PHQ9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Osman A, Wong JL, Bagge CL, et al. . The depression anxiety stress scales-21 (DASS-21): further examination of dimensions, scale reliability, and correlates. J Clin Psychol 2012; 68: 1322–1338. doi: 10.1002/jclp.21908 [DOI] [PubMed] [Google Scholar]
- 93.Phan T, Carter O, Adams C, et al. . Discriminant validity of the Hospital Anxiety and Depression Scale, Beck Depression Inventory (II) and Beck Anxiety Inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13: 220–228. doi: 10.1177/1479972316634604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Healey AK, Kneebone II, Carroll M, et al. . A preliminary investigation of the reliability and validity of the Brief Assessment Schedule Depression Cards and the Beck Depression Inventory-Fast Screen to screen for depression in older stroke survivors. Int J Geriatr Psychiatry 2008; 23: 531–536. doi: 10.1002/gps.1933 [DOI] [PubMed] [Google Scholar]
- 95.Willgoss TG, Goldbart J, Fatoye F, et al. . The development and validation of the Anxiety Inventory for Respiratory Disease. Chest 2013; 144: 1587–1596. doi: 10.1378/chest.13-0168 [DOI] [PubMed] [Google Scholar]
- 96.De Peuter S, Janssens T, Van Diest I, et al. . Dyspnea-related anxiety: the Dutch version of the Breathlessness Beliefs Questionnaire. Chron Respir Dis 2011; 8: 11–19. doi: 10.1177/1479972310383592 [DOI] [PubMed] [Google Scholar]
- 97.Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: Suppl. 2, 22–33. [PubMed] [Google Scholar]
- 98.First MB, Spitzer RL, Gibbon M WJB. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P). New York City, Biometrics Research Department, New York State Psychiatric Institute, 1995; pp. 722. [Google Scholar]
- 99.Di Nardo PA. Reliability of DSM-III-R anxiety disorder categories. Arch Gen Psychiatry 1993; 50: 251. doi: 10.1001/archpsyc.1993.01820160009001 [DOI] [PubMed] [Google Scholar]
- 100.Spitzer RL, Williams JB, Kroenke K, et al. . Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272: 1749–1756. doi: 10.1001/jama.1994.03520220043029 [DOI] [PubMed] [Google Scholar]
- 101.Mikkelsen RL, Middelboe T, Pisinger C, et al. . Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry 2004; 58: 65–70. doi: 10.1080/08039480310000824 [DOI] [PubMed] [Google Scholar]
- 102.Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis 2020; 15: 903–919. doi: 10.2147/COPD.S178049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clari M, Conti A, Fontanella R, et al. . Mindfulness-based programs for people with chronic obstructive pulmonary disease: a mixed methods systematic review. Mindfulness 2020; 11: 1848–1867. doi: 10.1007/s12671-020-01348-z [DOI] [Google Scholar]
- 104.Schrijver J, Lenferink A, Brusse-Keizer M, et al. . Self-management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2022; 1: CD002990. doi: 10.1002/14651858.CD002990.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volpato E, Banfi P, Rogers SM, et al. . Relaxation techniques for people with chronic obstructive pulmonary disease: a systematic review and a meta-analysis. Evid Based Complement Alternat Med 2015; 2015: 628365. doi: 10.1155/2015/628365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baraniak A, Sheffield D. The efficacy of psychologically based interventions to improve anxiety, depression and quality of life in COPD: A systematic review and meta-analysis. Patient Educ Couns 2011; 83: 29–36. doi: 10.1016/j.pec.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 107.Li Z, Liu S, Wang L, et al. . Mind–body exercise for anxiety and depression in COPD patients: a systematic review and meta-analysis. Int J Environ Res Public Health 2019; 17: 22. doi: 10.3390/ijerph17010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu S, Zhang D, He Q, et al. . Efficacy of liuzijue qigong in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med 2022; 65: 102809. doi: 10.1016/j.ctim.2022.102809 [DOI] [PubMed] [Google Scholar]
- 109.Cai Q, Cai S-B, Chen J-K, et al. . Tai chi for anxiety and depression symptoms in cancer, stroke, heart failure, and chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Clin Pract 2022; 46: 101510. doi: 10.1016/j.ctcp.2021.101510 [DOI] [PubMed] [Google Scholar]
- 110.Desveaux L, Lee A, Goldstein R, et al. . Yoga in the management of chronic disease: a systematic review and meta-analysis. Med Care 2015; 53: 653–661. doi: 10.1097/MLR.0000000000000372 [DOI] [PubMed] [Google Scholar]
- 111.Reychler G, Poncin W, Montigny S, et al. . Efficacy of yoga, tai chi and qi gong on the main symptoms of chronic obstructive pulmonary disease: a systematic review. Respir Med Res 2019; 75: 13–25. doi: 10.1016/j.resmer.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 112.Huang J, Yuan X, Zhang N, et al. . Music therapy in adults with COPD. Respir Care 2021; 66: 501–509. doi: 10.4187/respcare.07489 [DOI] [PubMed] [Google Scholar]
- 113.McNamara RJ, Epsley C, Coren E, et al. . Singing for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 12; CD012296. doi: 10.1002/14651858.CD012296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lewis A, Cave P, Stern M, et al. . Singing for lung health—a systematic review of the literature and consensus statement. NPJ Prim Care Respir Med 2016; 26: 16080. doi: 10.1038/npjpcrm.2016.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poot CC, Meijer E, Kruis AL, et al. . Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021; 9: CD009437. doi: 10.1002/14651858.CD009437.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howcroft M, Walters EH, Wood-Baker R, et al. . Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: CD005074. doi: 10.1002/14651858.CD005074.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yohannes AM, Newman M, Kunik ME. Psychiatric collaborative care for patients with respiratory disease. Chest 2019; 155: 1288–1295. doi: 10.1016/j.chest.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 118.Gordon CS, Waller JW, Cook RM, et al. . Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD. Chest 2019; 156: 80–91. doi: 10.1016/j.chest.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 119.Lu J-W, Wang Y, Sun Y, et al. . Effectiveness of telemonitoring for reducing exacerbation occurrence in COPD patients with past exacerbation history: a systematic review and meta-analysis. Front Med 2021; 8: 720019. doi: 10.3389/fmed.2021.720019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song C, Liu X, Wang Y, et al. . Effects of home-based telehealth on the physical condition and psychological status of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Nurs Pract 2022; in press [ 10.1111/ijn.13062]. [DOI] [PubMed] [Google Scholar]
- 121.Farver-Vestergaard I, Danielsen JTT, Løkke A, et al. . Psychosocial intervention in chronic obstructive pulmonary disease: meta-analysis of randomized controlled trials. Psychosom Med 2022; 84: 347–358. doi: 10.1097/PSY.0000000000001043 [DOI] [PubMed] [Google Scholar]
- 122.Hynninen MJ, Bjerke N, Pallesen S, et al. . A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med 2010; 104: 986–994. doi: 10.1016/j.rmed.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 123.Coventry PA, Gellatly JL. Improving outcomes for COPD patients with mild-to-moderate anxiety and depression: A systematic review of cognitive behavioural therapy. Br J Health Psychol 2008; 13: 381–400. doi: 10.1348/135910707X203723 [DOI] [PubMed] [Google Scholar]
- 124.Kapella MC, Herdegen JJ, Perlis ML, et al. . Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis 2011; 6: 625–635. doi: 10.2147/COPD.S24858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yohannes AM, Junkes-Cunha M, Smith J, et al. . Management of dyspnea and anxiety in chronic obstructive pulmonary disease: a critical review. J Am Med Dir Assoc 2017; 18: 1096.e1–1096.e17. doi: 10.1016/j.jamda.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 126.Bove DG, Overgaard D, Lomborg K, et al. . Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open 2015; 5: e008031. doi: 10.1136/bmjopen-2015-008031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barrera TL, Grubbs KM, Kunik ME, et al. . A review of cognitive behavioral therapy for panic disorder in patients with chronic obstructive pulmonary disease: the rationale for interoceptive exposure. J Clin Psychol Med Settings 2014; 21: 144–154. doi: 10.1007/s10880-014-9393-4 [DOI] [PubMed] [Google Scholar]
- 128.Doyle C, Bhar S, Fearn M, et al. . The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol 2017; 22: 542–556. doi: 10.1111/bjhp.12245 [DOI] [PubMed] [Google Scholar]
- 129.You E, Doyle C, Dunt D, et al. . Study protocol for a randomized controlled trial of telephone-delivered cognitive behavior therapy compared with befriending for treating depression and anxiety in older adults with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 327–334. doi: 10.2147/COPD.S100859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heslop-Marshall K, Baker C, Carrick-Sen D, et al. . Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res 2018; 4: 00094-2018. doi: 10.1183/23120541.00094-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pollok J, van Agteren JE, Esterman AJ, et al. . Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2019; 3: CD012347. doi: 10.1002/14651858.CD012347.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Usmani ZA, Carson KV, Heslop K, et al. . Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 3: CD010673. doi: 10.1002/14651858.CD010673.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma RC, Yin YY, Wang YQ, et al. . Effectiveness of cognitive behavioural therapy for chronic obstructive pulmonary disease patients: a systematic review and metaanalysis. Complement Ther Clin Pract 2020; 38: 101071. doi: 10.1016/j.ctcp.2019.101071 [DOI] [PubMed] [Google Scholar]
- 134.López-Lois B, González-Barcala FJ, Facal D. Application of mindfulness techniques in patients with asthma or COPD. J Asthma 2021; 58: 1237–1246. doi: 10.1080/02770903.2020.1776729 [DOI] [PubMed] [Google Scholar]
- 135.Norman E, Pfuhl G, Sæle RG, et al. . Metacognition in psychology. Rev Gen Psychol 2019; 23: 403–424. doi: 10.1177/1089268019883821 [DOI] [Google Scholar]
- 136.Farver-Vestergaard I, O'Toole MS, O'Connor M, et al. . Mindfulness-based cognitive therapy in COPD: a cluster randomised controlled trial. Eur Respir J 2018; 51: 1702082. doi: 10.1183/13993003.02082-2017 [DOI] [PubMed] [Google Scholar]
- 137.Malpass A, Kessler D, Sharp D, et al. . MBCT for patients with respiratory conditions who experience anxiety and depression: a qualitative study. Mindfulness 2015; 6: 1181–1191. doi: 10.1007/s12671-014-0370-7 [DOI] [Google Scholar]
- 138.Akgün Şahin Z, Dayapoğlu N. Effect of progressive relaxation exercises on fatigue and sleep quality in patients with chronic obstructive lung disease (COPD). Complement Ther Clin Pract 2015; 21: 277–281. doi: 10.1016/j.ctcp.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 139.Seyedi Chegeni P, Gholami M, Azargoon A, et al. . The effect of progressive muscle relaxation on the management of fatigue and quality of sleep in patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial. Complement Ther Clin Pract 2018; 31: 64–70. doi: 10.1016/j.ctcp.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 140.Lolak S, Connors GL, Sheridan MJ, et al. . Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother Psychosom 2008; 77: 119–125. doi: 10.1159/000112889 [DOI] [PubMed] [Google Scholar]
- 141.Blake RL, Vandiver TA, Braun S, et al. . A randomized controlled evaluation of a psychosocial intervention in adults with chronic lung disease. Fam Med 1990, 22: 365–370. [PubMed] [Google Scholar]
- 142.Leelarungrayub J, Puntumetakul R, Sriboonreung T, et al. . Preliminary study: comparative effects of lung volume therapy between slow and fast deep-breathing techniques on pulmonary function, respiratory muscle strength, oxidative stress, cytokines, 6minute walking distance, and quality of life in persons with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 3909–3921. doi: 10.2147/COPD.S181428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kütmeç Yilmaz C, Duru Aşiret G, Çetinkaya F. The effect of back massage on physiological parameters, dyspnoea, and anxiety in patients with chronic obstructive pulmonary disease in the intensive care unit: a randomised clinical trial. Intensive Crit Care Nurs 2021; 63: 102962. doi: 10.1016/j.iccn.2020.102962 [DOI] [PubMed] [Google Scholar]
- 144.Russo P, Prinzi G, Ksialiou A, et al. . Action plans and coping strategies in elderly COPD patients influence the result of pulmonary rehabilitation: an observational study. Eur J Phys Rehabil Med 2017; in press [ 10.23736/S1973-9087.17.04501-4]. [DOI] [PubMed] [Google Scholar]
- 145.Chan RR, Lehto RH. The experience of learning meditation and mind/body practices in the COPD population. Explore 2016; 12: 171–179. doi: 10.1016/j.explore.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 146.Kaja R, Vaiyapuri A, Sirajudeen MS, et al. . Biofeedback flutter device for managing the symptoms of patients with COPD. Technol Health Care 2020; 28: 477–485. doi: 10.3233/THC-202222 [DOI] [PubMed] [Google Scholar]
- 147.Horuz D, Kurcer MA, Erdoğan Z. The effect of music therapy on anxiety and various physical findings in patients with COPD in a pulmonology service. Holist Nurs Pract 2017; 31: 378–383. doi: 10.1097/HNP.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 148.Withy K, Glickman-Simon R. Tai chi, qigong and COPD, flaxseeds and post-menopausal symptoms, aromatherapy and post-operative nausea, acupuncture and ankle sprains, zinc, and the common cold. Explore 2014; 10: 132–135. doi: 10.1016/j.explore.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 149.Felcar JM, Probst VS, de Carvalho DR, et al. . Effects of exercise training in water and on land in patients with COPD: a randomised clinical trial. Physiotherapy 2018; 104: 408–416. doi: 10.1016/j.physio.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 150.Halpin D, Hyland M, Blake S, et al. . Preference for different relaxation techniques by COPD patients: comparison between six techniques. Int J Chron Obstruct Pulmon Dis 2016; 11: 2315–2319. doi: 10.2147/COPD.S113108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang T, Zhou R, Wang T, et al. . Effects of traditional mind–body movement therapy on chronic cardiopulmonary dyspnoea: a systematic review and meta-analysis. Thorax 2022; 78: 69–75. doi: 10.1136/thoraxjnl-2021-218030 [DOI] [PubMed] [Google Scholar]
- 152.Ngai SP, Jones AY, Tam WWS. Tai chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2016; 2016; CD009953. doi: 10.1002/14651858.CD009953.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo C, Xiang G, Xie L, et al. . Effects of tai chi training on the physical and mental health status in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Thorac Dis 2020; 12: 504–521. doi: 10.21037/jtd.2020.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo JB, Chen BL, Lu YM, et al. . Tai chi for improving cardiopulmonary function and quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil 2016; 30: 750–764. doi: 10.1177/0269215515604903 [DOI] [PubMed] [Google Scholar]
- 155.Muktibodhananda S. Hatha Yoga Pradipika. Bihar, Bihar School of Yoga, 2012, p. 642. [Google Scholar]
- 156.Jayawardena R, Ranasinghe P, Ranawaka H, et al. . Exploring the therapeutic benefits of pranayama (yogic breathing): a systematic review. Int J Yoga 2020; 13: 99–110. doi: 10.4103/ijoy.IJOY_37_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cramer H, Haller H, Klose P, et al. . The risks and benefits of yoga for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil 2019; 33: 1847–1862. doi: 10.1177/0269215519860551 [DOI] [PubMed] [Google Scholar]
- 158.Li C, Liu Y, Ji Y, et al. . Efficacy of yoga training in chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Complement Ther Clin Pract 2018; 30: 33–37. doi: 10.1016/j.ctcp.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 159.von Leupoldt A, Taube K, Schubert-Heukeshoven S, et al. . Distractive auditory stimuli reduce the unpleasantness of dyspnea during exercise in patients with COPD. Chest 2007; 132: 1506–1512. doi: 10.1378/chest.07-1245 [DOI] [PubMed] [Google Scholar]
- 160.Keeler JR, Roth EA, Neuser BL, et al. . The neurochemistry and social flow of singing: bonding and oxytocin. Front Hum Neurosci 2015; 9: 518. doi: 10.3389/fnhum.2015.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu H, Song M, Zhai ZH, et al. . Group singing improves depression and life quality in patients with stable COPD: a randomized community-based trial in China. Qual Life Res 2019; 28: 725–735. doi: 10.1007/s11136-018-2063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaasgaard M, Rasmussen DB, Andreasson KH, et al. . Use of singing for lung health as an alternative training modality within pulmonary rehabilitation for COPD: a randomised controlled trial. Eur Respir J 2022; 59: 2101142. doi: 10.1183/13993003.01142-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spruit MA, Singh SJ, Garvey C, et al. . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 164.Puhan MA, Gimeno-Santos E, Cates CJ, et al. . Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: CD005305. doi: 10.1002/14651858.CD005305.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McCarthy B, Casey D, Devane D, et al. . Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 23: CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Osadnik CR, Singh S. Pulmonary rehabilitation for obstructive lung disease. Respirology 2019; 24: 871–878. doi: 10.1111/resp.13569 [DOI] [PubMed] [Google Scholar]
- 167.Houben-Wilke S, Augustin IM, Vercoulen JH, et al. . COPD stands for complex obstructive pulmonary disease. Eur Respir Rev 2018; 27: 180027. doi: 10.1183/16000617.0027-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Agusti A, Bel E, Thomas M, et al. . Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 169.Usmani OS, Lavorini F, Marshall J, et al. . Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res 2018; 19: 10. doi: 10.1186/s12931-017-0710-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jansen EM, van de Hei SJ, Dierick BJH, et al. . Global burden of medication non-adherence in chronic obstructive pulmonary disease (COPD) and asthma: a narrative review of the clinical and economic case for smart inhalers. J Thorac Dis 2021; 13: 3846–3864. doi: 10.21037/jtd-20-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lopez-Campos JL, Ruiz-Duque B, Carrasco-Hernandez L, et al. . Integrating comorbidities and phenotype-based medicine in patient-centered medicine in COPD. J Clin Med 2020; 9: 2745. doi: 10.3390/jcm9092745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weldam SWM, Schuurmans MJ, Zanen P, et al. . The effectiveness of a nurse-led illness perception intervention in COPD patients: a cluster randomised trial in primary care. ERJ Open Res 2017; 3: 00115–2016. doi: 10.1183/23120541.00115-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bourbeau J, Lavoie K, Sedeno M. Comprehensive self-management strategies. Semin Respir Crit Care Med 2015; 36: 630–638. doi: 10.1055/s-0035-1556059 [DOI] [PubMed] [Google Scholar]
- 174.Newham J, Presseau J, Heslop-Marshall K, et al. . Features of self-management interventions for people with COPD associated with improved health-related quality of life and reduced emergency department visits: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2017; 12: 1705–1720. doi: 10.2147/COPD.S133317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Walters JA, Turnock AC, Walters EH, et al. . Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010; 12: CD005074. doi: 10.1002/14651858.CD005074.pub3 [DOI] [PubMed] [Google Scholar]
- 176.Blackstock F, Webster K. Disease-specific health education for COPD: a systematic review of changes in health outcomes. Health Educ Res 2006; 22: 703–717. doi: 10.1093/her/cyl150 [DOI] [PubMed] [Google Scholar]
- 177.Brighton LJ, Tunnard I, Farquhar M, et al. . Recommendations for services for people living with chronic breathlessness in advanced disease: Results of a transparent expert consultation. Chron Respir Dis 2019; 16: 147997311881644. doi: 10.1177/1479973118816448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brighton LJ, Miller S, Farquhar M, et al. . Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019; 74: 270–281. doi: 10.1136/thoraxjnl-2018-211589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Higginson IJ, Bausewein C, Reilly CC, et al. . An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2: 979–987. doi: 10.1016/S2213-2600(14)70226-7 [DOI] [PubMed] [Google Scholar]
- 180.Brighton LJ, Gao W, Farquhar M, et al. . Predicting outcomes following holistic breathlessness services: a pooled analysis of individual patient data. Palliat Med 2019; 33: 462–466. doi: 10.1177/0269216319830299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maddocks M, Lovell N, Booth S, et al. . Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017; 390: 988–1002. doi: 10.1016/S0140-6736(17)32127-X [DOI] [PubMed] [Google Scholar]
- 182.Corner J, Plant H, A'Hern R, et al. . Non-pharmacological intervention for breathlessness in lung cancer Palliat Med 1996; 10: 299–305. doi: 10.1177/026921639601000405 [DOI] [PubMed] [Google Scholar]
- 183.Schwab P, Dhamane A, Hopson S, et al. . Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2017; 12: 735–744. doi: 10.2147/COPD.S112256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wacker M, Kitzing K, Jörres R, et al. . The contribution of symptoms and comorbidities to the economic impact of COPD: an analysis of the German COSYCONET cohort. Int J Chron Obstruct Pulmon Dis 2017; 12: 3437–3448. doi: 10.2147/COPD.S141852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim HFS, Kunik ME, Molinari VA, et al. . Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics 2000; 41: 465–471. doi: 10.1176/appi.psy.41.6.465 [DOI] [PubMed] [Google Scholar]
- 186.Hanania NA, Müllerova H, Locantore NW, et al. . Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med 2011; 183: 604–611. doi: 10.1164/rccm.201003-0472OC [DOI] [PubMed] [Google Scholar]
- 187.Kunik ME. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005; 127: 1205–1211. doi: 10.1378/chest.127.4.1205 [DOI] [PubMed] [Google Scholar]
- 188.Wang J, Willis K, Barson E, et al. . The complexity of mental health care for people with COPD: a qualitative study of clinicians’ perspectives. NPJ Prim Care Respir Med 2021; 31: 40. doi: 10.1038/s41533-021-00252-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hardy S, Smart D, Scanlan M, et al. . Integrating psychological screening into reviews of patients with COPD. Br J Nurs 2014; 23: 832–836. doi: 10.12968/bjon.2014.23.15.832 [DOI] [PubMed] [Google Scholar]
- 190.Damschroder LJ, Aron DC, Keith RE, et al. . Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4: 50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mulhall P, Criner G. Non-pharmacological treatments for COPD. Respirology 2016; 21: 791–809. doi: 10.1111/resp.12782 [DOI] [PubMed] [Google Scholar]
- 192.Guidi J, Brakemeier EL, Bockting CLH, et al. . Methodological recommendations for trials of psychological interventions. Psychother Psychosom 2018; 87: 276–284. doi: 10.1159/000490574 [DOI] [PubMed] [Google Scholar]
- 193.Connolly MJ, Yohannes AM. The impact of depression in older patients with chronic obstructive pulmonary disease and asthma. Maturitas 2016; 92: 9–14. doi: 10.1016/j.maturitas.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 194.Harrison SL, Greening NJ, Williams JEA, et al. . Have we underestimated the efficacy of pulmonary rehabilitation in improving mood? Respir Med 2012; 106: 838–844. doi: 10.1016/j.rmed.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 195.Graham CD, Gouick J, Krahé C, et al. . A systematic review of the use of acceptance and commitment therapy (act) in chronic disease and long-term conditions. Clin Psychol Rev 2016; 46: 46–58. doi: 10.1016/j.cpr.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 196.Brożek B, Fopka-Kowalczyk M, Łabuś-Centek M, et al. . Dignity therapy as an aid to coping for COPD patients at their end-of-life stage. Adv Respir Med 2019; 87: 135–145. doi: 10.5603/ARM.a2019.0021 [DOI] [PubMed] [Google Scholar]
- 197.Gilbert P. Compassion Focused Therapy: Distinctive Features. 1st Edn. New York, Taylor & Francis; 2010. doi: 10.4324/9780203851197 [DOI] [Google Scholar]
- 198.Fisher P, Wells A. Metacognitive Therapy. The CBT Distinctive Features Series. New York, Taylor & Francis; 2009. [Google Scholar]
- 199.Spruit MA, Pitta F, Garvey C, et al. . Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014; 43: 1326–1337. doi: 10.1183/09031936.00145613 [DOI] [PubMed] [Google Scholar]
- 200.Yohannes AM. General practitioners views and experiences in managing depression in patients with chronic obstructive pulmonary disease. Expert Rev Respir Med 2012; 6: 589–595. doi: 10.1586/ers.12.64 [DOI] [PubMed] [Google Scholar]
- 201.Roberts NJ, Ward M, Patel I, et al. . Reflections on integrated care from those working in and leading integrated respiratory teams. London J Prim Care 2018; 10: 24–30. doi: 10.1080/17571472.2017.1421020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lunn S, Restrick L, Stern M. Managing respiratory disease. Chron Respir Dis 2017; 14: 45–53. doi: 10.1177/1479972316688914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Verkleij M, de Winter D, Hurley MA, et al. . Implementing the International Committee on Mental Health in Cystic Fibrosis (ICMH) guidelines: screening accuracy and referral-treatment pathways. J Cyst Fibros 2018; 17: 821–827. doi: 10.1016/j.jcf.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 204.Abbott J, Havermans T, Jarvholm S, et al. . Mental health screening in cystic fibrosis centres across Europe. J Cyst Fibros 2019; 18: 299–303. doi: 10.1016/j.jcf.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 205.Quittner AL, Abbott J, Hussain S, et al. . Integration of mental health screening and treatment into cystic fibrosis clinics: evaluation of initial implementation in 84 programs across the United States. Pediatr Pulmonol 2020; 55: 2995–3004. doi: 10.1002/ppul.24949 [DOI] [PubMed] [Google Scholar]
- 206.Jeganathan C, Hosseinzadeh H. The role of health literacy on the self-management of chronic obstructive pulmonary disease: a systematic review. COPD 2020; 17: 318–325. doi: 10.1080/15412555.2020.1772739 [DOI] [PubMed] [Google Scholar]
- 207.Hutchinson A, Barclay N, Galvin K, et al. . Living well with breathlessness: how clinicians can help. Br J Gen Pract 2019; 69: 26–27. 10.3399/bjgp19X700505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tsutsui M, Gerayeli F, Sin DD. Pulmonary rehabilitation in a post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis 2021; 16: 379–391. doi: 10.2147/COPD.S263031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 5: CD01142. doi: 10.1002/14651858.CD011425.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moayeri F, Dunt D, Hsueh YS, et al. . Cost-utility analysis of telephone-based cognitive behavior therapy in chronic obstructive pulmonary disease (COPD) patients with anxiety and depression comorbidities: an application for willingness to accept concept. Expert Rev Pharmacoecon Outcomes Res 2019; 19: 331–340. doi: 10.1080/14737167.2019.1536550 [DOI] [PubMed] [Google Scholar]
- 211.Farver-Vestergaard I, O'Connor M, Smith NC, et al. . Tele-delivered mindfulness-based cognitive therapy in chronic obstructive pulmonary disease: a mixed-methods feasibility study. J Telemed Telecare 2019; 25: 468–475. doi: 10.1177/1357633X18780563 [DOI] [PubMed] [Google Scholar]
- 212.Micklewright K, Farquhar M. Does the carer support needs assessment tool cover the established support needs of carers of patients with chronic obstructive pulmonary disease? A systematic literature search and narrative review. Palliat Med 2020; 34: 1305–1315. doi: 10.1177/0269216320939243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nakken N, Janssen DJA, van den Bogaart EHA, et al. . Informal caregivers of patients with COPD: home sweet home? Eur Respir Rev 2015; 24: 498–504. doi: 10.1183/16000617.00010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fernández-García S, Represas-Represas C, Ruano-Raviña A, et al. . Sociodemographic and clinical variables related to the overburden of the informal caregivers of patients hospitalized for chronic obstructive pulmonary disease exacerbations. Int J Chron Obstruct Pulmon Dis 2021; 16: 1119–1126. doi: 10.2147/COPD.S301637 [DOI] [PMC free article] [PubMed] [Google Scholar]