Abstract
The bacterial σ54 RNA polymerase holoenzyme binds to promoters as a stable closed complex that is silent for transcription unless acted upon by an enhancer-bound activator protein. Using DNA binding and transcription assays the ability of the enhancer-dependent σ54 holoenzyme to interact with promoter DNA containing various regions of heteroduplex from –12 to –1 was assessed. Different DNA regions important for stabilising σ54 holoenzyme–promoter interactions, destabilising binding, limiting template utilisation in activator-dependent transcription and for stable binding of a deregulated form of the holoenzyme lacking σ54 Region I were identified. It appears that homoduplex structures are required for early events in σ54 holoenzyme promoter binding and that disruption of a repressive fork junction structure only modestly deregulates transcription. DNA opening from –5 to –1 appears important for stable engagement of the holoenzyme following activation. The regulatory Region I of σ54 was shown to be involved in interactions with the sequences in the –5 to –1 area.
INTRODUCTION
The binding of RNA polymerases (RNAP) to promoter DNA and the subsequent DNA opening events that reveal the template strand are often subject to regulation in order to control transcription rates. The bacterial enhancer-dependent RNAP containing the σ54 subunit binds to its target promoters in a conformation that strongly restricts the amount of DNA opening occurring, producing a stable closed complex that rarely isomerises spontaneously to an open complex (1). To isomerise, the ATPase activity of specialised activator proteins targets the σ54 factor to induce conformational changes in σ54, causing it to change interactions with a DNA fork junction just downstream of the start site proximal consensus promoter element (2–4). The activator-dependent isomerisation of σ54 appears to result in some local DNA opening to about –6 that likely helps place the promoter DNA into the cleft within the RNAP core enzyme, where extra melting around the start site occurs (5). Core RNAP–promoter DNA interactions presumably stabilise the open preinitiation complex ready for initiation of transcript formation.
The use of heteroduplex DNA templates has established that activators of the σ54 holoenzyme are not simply DNA helicases and that for the σ54 holoenzyme to stably bind and utilise heteroduplex DNA for transcription the activator must hydrolyse ATP and act upon the σ54 holoenzyme to bring about a conformational change in the holoenzyme (3,4,6). The requirement for an activator protein can be bypassed in in vitro experiments where σ54 lacks its regulatory Region I to which the activator binds (7,8). In marked contrast to the wild-type σ54 holoenzyme, holoenzyme assembled with σ54 lacking Region I (ΔIσ54) can stably bind promoter DNA which is heteroduplex from –10 to –1, transcribe from it and also utilise transiently melting DNA present in supercoiled DNA templates for transcription independently of activator (6,7,9). These properties of the deregulated ΔIσ54 holoenzyme can be rationalised in terms of a loss of interactions with the repressive DNA fork junction within the closed complex and an associated increased access to single-stranded DNA-binding activities of the core RNAP enzyme (10).
Here we have examined the requirements in DNA and σ54 for regulated and deregulated interactions with promoter DNA using the wild-type σ54 holoenzyme and a deregulated form lacking Region I of σ54 (Fig. 1A). We compared the binding of the σ54 holoenzyme to homoduplex (Fig. 1B) and various heteroduplex Sinorhizobium meliloti nifH promoter probes (Table 1) in the context of wild-type σ54 and ΔIσ54. The results led to the identification of different promoter regions that contribute to the stability of initial complexes, activator-dependent binding and transcription and stable binding by the deregulated holoenzyme.
Figure 1.
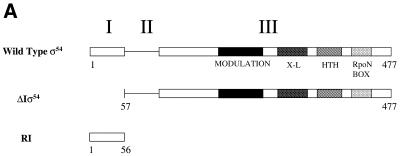
(A) Klebsiella pneumoniae σ54 and its fragments assayed. Full-length σ54 (amino acids 1–477) is divided into three regions, I–III, based on sequence alignments (24). The N-terminal Region I (amino acids 1–56) is regulatory, binds activator and is involved in polymerase isomerisation and DNA melting (2,3,5,6,8,10,25–27). Region II is acidic, variable in sequence and length and dispensable for polymerase isomerisation, but may contribute to the DNA-binding activity of the holoenzyme (9,28). The large Region III (amino acids 107–477) contains sequences important for binding core RNAP (amino acids 120–215) and DNA-binding determinants, which include a helix–turn–helix (HTH) motif (amino acids 366–386), a region that crosslinks to promoter DNA (amino acids 329–346), a conserved signature motif termed the RpoN box (amino acids 454–463) and a region (amino acids 180–306) that modulates (enhances) DNA binding (12,29–31). (B) A S.meliloti nifH promoter fragment comprising nucleotides –60 to +28 used as a basis to design heteroduplex derivatives used in this work. Consensus bases within the σ54 promoter sequence and the transcription start are indicated in bold.
Table 1. Summary of holoenzyme–DNA binding results.
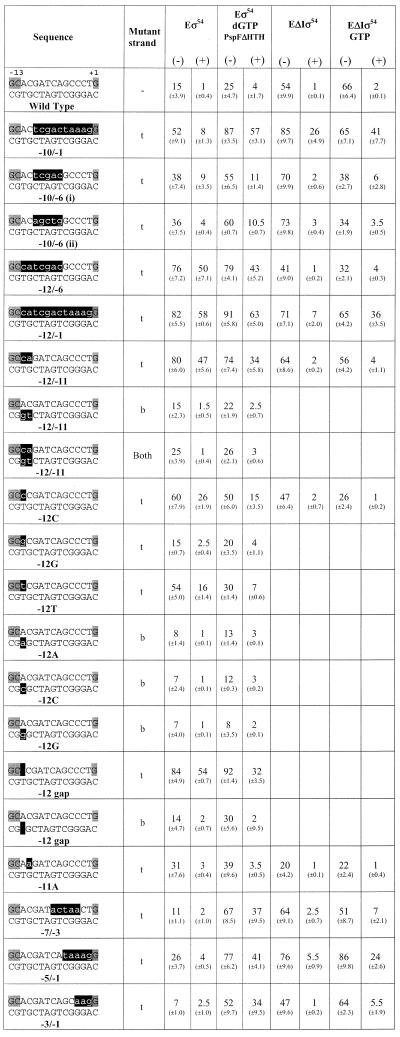
Gel mobility shift assays were conducted as described in Figures 2 and 3. The percentages of DNA bound by the wild-type σ54 holoenzyme (Eσ54) and holoenzyme containing σ54 deleted for Region I (EΔIσ54) are shown. When required, activator PspFΔHTH and hydrolysable nucleotide dGTP were included in activation assays. Data are from assays conducted in the absence (–) or presence (+) of heparin. The sequences of the S.meliloti nifH promoter probes from –14 to +1 are indicated, with the conserved GC promoter element and the transcription start highlighted in grey. The position of mismatched sequences are indicated by highlighting the mutant strand in black and positions of missing nucleotides are indicated by solid blocks in the DNA sequences. Numerical positions of mismatches are marked below the heteroduplexes. Top strand (t) and bottom strand (b) mutant sequences are also indicated. Standard deviations are shown in parentheses.
MATERIALS AND METHODS
Proteins
The Klebsiella pneumoniae σ54 protein (amino acids 1–477) and a derivative lacking Region I (amino acids 57–477, ΔIσ54) were prepared as before (11; Fig. 1A). Purified σ54 Region I (amino acids 1–56) was obtained by overproducing an N-terminal histidine-tagged fragment in Escherichia coli (12; Fig. 1A). A C-terminal deleted form of activator PspF lacking a functional DNA-binding domain, PspFΔHTH, was used in activation assays (13). Escherichia coli core RNAP was from Epicentre Technologies Corp.
DNA duplex formation
Wild-type homoduplex (Fig. 1B) or mismatched S.meliloti nifH promoter DNA heteroduplexes (Table 1) were prepared by annealing 88mer oligonucleotides (2,6). Pairs of oligonucleotides with either one strand 5′-32P-end-labeled with the unlabeled strand at a 2-fold molar excess (10 pmol in 20 µl) or for preparing unlabeled heteroduplex with both strands at equal concentration were heated at 95°C for 3 min in 10 mM Tris–HCl pH 8.0, 10 mM MgCl2 and then chilled rapidly in iced water for 5 min to allow heteroduplex formation.
Native gel mobility shift assays
Typical holoenzyme interaction assays included 100 nM core RNAP plus 200 nM σ54 or ΔIσ54 and 16 nM end-labeled duplex DNA in STA buffer (25 mM Tris–acetate pH 8.0, 8 mM Mg acetate, 10 mM KCl, 1 mM DTT, 3.5% w/v PEG 6000). When included, σ54 Region I was at 2 µM. For activation, 4 µM PspFΔHTH and 1 mM nucleotide (GTP or dGTP) were also added to DNA binding assays. Core RNAP, σ proteins and DNA were incubated at 30°C for 10 min and then, if necessary, activator and nucleotide were added for a further 10 min, followed by glycerol/bromophenol blue loading dye (final concentration 10% glycerol) and, if required, heparin (final concentration 100 µg/ml). Samples were then loaded onto 4.5% native polyacrylamide gels and run at 60 V for 80 min at room temperature in 25 mM Tris, 200 mM glycine buffer pH 8.6. Unbound DNA and protein–DNA complexes were detected by autoradiography and quantified by phosphorimager analysis. Binding assays were repeated at least twice and representative data are shown.
In vitro transcription assays
Heteroduplex promoter fragments (16 nM, unlabeled) and holoenzyme (100 nM) were incubated in a 40 µl reaction for 10 min at 30°C in STA buffer containing 40 U RNase inhibitor (RNasin; Promega) prior to addition of 1 mM GTP for [α-32P]UTP-labeled transcript assays. For activation, PspFΔHTH (4 µM) was added to allow open complexes to form. After a further 10 min, heparin (100 µg/ml) and elongation mix [ATP, CTP and UTP at 0.1 mM and 12.5 µCi [α-32P]UTP (800 Ci/mmol; Amersham)] were added to allow synthesis of transcripts, which were allowed to accumulate for 10 min before phenol extraction and precipitation. Samples were run on 15% denaturing polyacrylamide gels and transcripts were visualised using a phosphorimager.
RESULTS
Maintenance of the silent state of σ54 holoenzyme closed complexes is dependent on the integrity of promoter sequences immediately downstream of the start site proximal promoter element (3,14). In initial complexes formed between the σ54 holoenzyme and promoter DNA, the base pair downstream of the consensus GC element shows increased reactivity towards KMnO4, o-copper phenanthroline and diethylpyrocarbonate, and therefore appears to be melted out (15). Since a different set of DNA sequences proximal to the transcription start are melted in open complexes, we refer to the localised melting within closed complexes as ‘early melting’. In contrast, downstream melting of DNA during open complex formation by the σ54 holoenzyme is referred to as ‘late melting’. We used two sets of S.meliloti nifH heteroduplex promoter probes that mimic the early (mismatch sequences 1–2 bases next to conserved GC) and late (mismatch sequences between –10 and –1) melted conformations adopted by the promoter DNA to assess the binding activity and stability of the σ54 and ΔIσ54 holoenzymes (summarised in Table 1). We wished to learn how different promoter structures were used by the σ54 holoenzyme and the dependence upon the σ54 Region I sequences during transcription initiation.
Holoenzyme binding to heteroduplex promoter probes
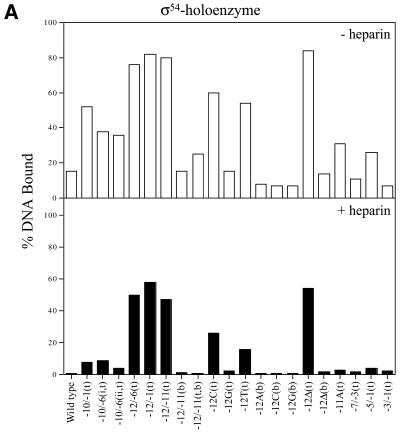
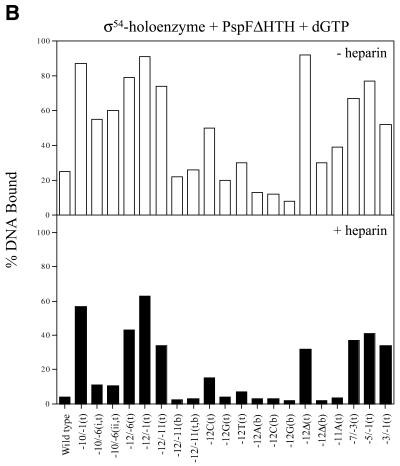
σ54 holoenzyme binding. Gel mobility shift assays with σ54 holoenzyme and a variety of early melted and late melted probes (Table 1) show a wide range of binding preferences. Heteroduplexes –10 to –1(t), –12 to –6(t), –12 to –1(t), –12 to –11(t), –12C(t) and 12T(t), with a mutant top strand (t), and a heteroduplex with the top strand –12 base missing [–12Δ(t)] gave the greatest amount of initial σ54 holoenzyme binding, with at least half of the DNA bound (Fig. 2A, open bars). The stability of the DNA-bound σ54 holoenzyme complexes was measured by means of a 5 min heparin challenge (σ54 holoenzyme complexes with homoduplex DNA are heparin sensitive). Figure 2A (black bars) shows that with heteroduplexes –12 to –6(t), –12 to –1(t), –12 to –11(t) and –12Δ(t) at least 60% of the initial σ54 holoenzyme–DNA complexes (open bars) were heparin stable. Heteroduplexes –12C(t) and –12T(t) also gave a moderate number of heparin-stable complexes formed with the σ54 holoenzyme (Fig. 2A, black bars). We carried out time course experiments to establish that the 5 min challenge represented a point in the decay curve that usefully reflected the decay and not the end-point (data not shown). Interestingly, all σ54 holoenzyme complexes formed with the early melted probes that initially gave good σ54 holoenzyme binding were markedly heparin stable independent of activation (Fig. 2A). Binding and stability appeared to be associated with the identity of the base in the upstream –12 region. When the top strand –12 position was varied against a constant wild-type bottom strand T, the preferred base for binding and stability was C compared to T or G, with G showing by far the weakest binding and stability (Fig. 2A). Removal of the top strand nucleotide at this position [–12Δ(t) probe] gave the most binding, suggesting an inhibitory role for the top strand sequence. In general, the binding patterns of σ54 holoenzyme and σ54 to probes with an unpaired nucleotide at –12 (mutant top strand) were similar, and binding was increased relative to the probes unpaired at –12 with a mutant bottom strand or homoduplex (Table 1 and data not shown; 2). Regions of heteroduplex starting from –12 and extending as far as –1 were also associated with improved σ54 and σ54 holoenzyme binding (Table 1 and data not shown; 2). An exception to this trend was binding of σ54 holoenzyme to the –12G(t) early melted probe, where binding was the same as that seen with wild-type homoduplex DNA (Table 1). Overall, the results indicate that the critical region of the heteroduplex for stable complex formation and increased σ binding is next to the consensus GC promoter region. It is striking that the two bases of the AT base pair next to the GC have opposite effects upon σ54 holoenzyme (Table 1) and σ54 binding (2).
Figure 2.
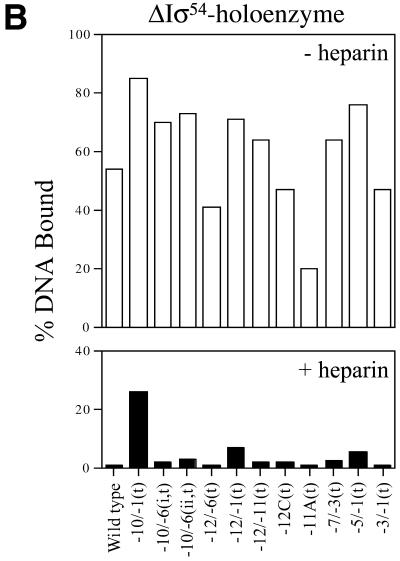
Holoenzyme binding to heteroduplex DNA. Wild-type σ54 holoenzyme (A) and ΔIσ54 holoenzyme in the absence (B) or presence (C) of the initiating nucleotide GTP, respectively, binding to the heteroduplex promoter probes described in Table 1. In a gel mobility shift assay, holoenzyme (100 nM, core RNAP:σ factor at a ratio of 1:2) was incubated with heteroduplex DNA (16 nM) for 10 min at 30°C. For (C) GTP (1 mM) was added to ΔIσ54 holoenzyme binding assays. Prior to gel loading, heparin (100 µg/ml) was added for 5 min to half of the sample. The histograms show the number of initial complexes (–heparin, open bars) and those surviving the heparin challenge (black bars). Holoenzyme binding to wild-type double-stranded promoter DNA is shown for comparison. Top strand and bottom strand mutant sequences are indicated by (t) and (b), respectively.
Of the heteroduplexes tested, the early melted probes –12 to –11(b), –12A(b), –12C(b), –12G(b) and –12Δ(b), with a mutant bottom strand (b), and –12G(t) gave the lowest amounts of initial σ54 holoenzyme complexes (Fig. 2A, open bars), and these were heparin unstable (black bars). It appears that the identity of the bottom strand sequence at –12 and –11 is important for the binding of σ54 holoenzyme to promoter DNA and stability of the resulting complex. When the wild-type top strand A at position –12 was kept constant and was placed against a variable bottom strand base (A, C or G), fewer heparin-stable complexes formed and less overall binding occurred. Removal of the bottom strand nucleotide at position –12 [probe –12Δ(b)] gave the same poor binding and weak heparin stability relative to the converse arrangement of bases in the region of heteroduplex (Fig. 2A). Similarly, late melted probes –7 to –3 and –3 to –1 gave the lowest amounts of stable σ54 holoenzyme complexes, suggesting that relative to the –12 to –11 heteroduplex, these regions do not increase σ54 holoenzyme binding and stability.
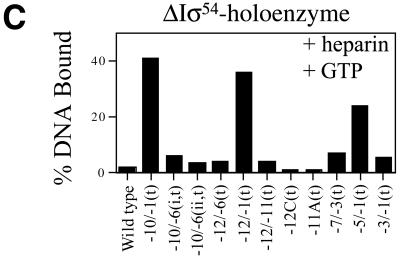
ΔIσ54 holoenzyme binding. We proposed previously that there may be regulatory cooperation between σ54 Region I and the –12 promoter region within the σ54 holoenzyme closed complex that prevents open complex formation (10,16,17). Part of the basis for this proposal was that unregulated transient melting can be caused by either removing σ54 Region I or mutations within the –12 sequences of promoter DNA (14,18). In an attempt to identify promoter regions important for the stable engagement of the σ54 holoenzyme at the promoter as occurs in an activator-independent manner, we measured the binding of ΔIσ54 holoenzyme to a range of heteroduplex probes and the stability of the resultant complexes. In the absence of the initiating nucleotide GTP, the late melted probe with heteroduplex from –10 to –1(t) gave the greatest number of initial (Fig. 2B, open bars) and heparin-stable (Fig. 2B, black bars) ΔIσ54 holoenzyme complexes. The stability of ΔIσ54 holoenzyme complexes formed on heteroduplex –10 to –1(t) was increased in the presence of GTP (compare Fig. 2B and C, black bars; see also 6), suggesting that activator-independent initiation had occurred. Binding and initiation by the ΔIσ54 holoenzyme is consistent with σ54 Region I sequences playing a role in tight binding to early melted sequences (see above) and thereby preventing activator-independent isomerisation and initiation (6,10). A similar binding pattern (+GTP) was observed with the late melted probe heteroduplex from –5 to –1(t) and the early melted probe from –12 to –1(t) (Fig. 2C). Thus, it seems that melting towards position –1 is associated with increased stability of complexes dependent upon GTP. The heteroduplex –10 to –1(t) yielded the greatest number of stable complexes independent of GTP. Strikingly, ΔIσ54 holoenzyme binding to the late melted heteroduplex –10 to –6 probes gave few heparin-stable complexes, even in the presence of GTP (compare Fig. 2B and C, black bars). This indicates that although the single-stranded DNA binding by ΔIσ54 holoenzyme associated with stable complex formation on the –10 to –1(t) probe involves interactions with sequences –10 to –6 and –5 to –1, these interactions are not simply additive (see below). Rather, the start site-proximal region of the heteroduplex makes a greater contribution.
Activator-dependent binding of holoenzymes to heteroduplex probes
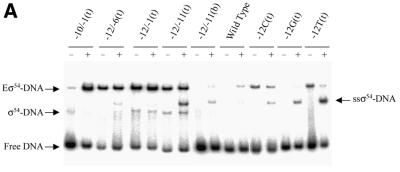
σ54 holoenzyme. To assess the ability of the σ54 holoenzyme to form activator-dependent stable complexes with heteroduplex DNA probes, binding assays were performed using the σ54-dependent activator PspFΔHTH and dGTP as the non-initiating hydrolysable nucleotide. The number of σ54 holoenzyme–heteroduplex complexes formed in the absence (–) or presence (+) of PspFΔHTH and dGTP for a sample group of probes are shown in Figure 3A and Table 1. With the early melted heteroduplexes shown, an additional activator and nucleotide-dependent supershifted complex (ssσ54–DNA) was observed, as described previously (2,5), which is a new σ54–DNA complex.
Figure 3.
Activator-dependent formation of σ54 holoenzyme–heteroduplex DNA complexes. Gel shift mobility assays were carried out as described in Figure 2 except that after σ54 holoenzyme DNA binding, activator (PspFΔHTH, 4 µM) and dGTP (1 mM) were added for a further 10 min. (A) Sample gel showing the number of heparin stable σ54 holoenzyme–heteroduplex complexes formed in the absence (–) and presence (+) of PspFΔHTH and dGTP. The sample heteroduplexes are indicated above the lanes. With certain early melted heteroduplexes an additional activator- and nucleotide-dependent supershifted complex (ssσ54–DNA) is seen that has been described previously (2,5,32). (B) Histogram showing the number of initial complexes (open bars) and the number of activator-dependent complexes surviving a 5 min heparin challenge (black bars).
The number of initial σ54 holoenzyme complexes (–heparin) formed with the early melted DNA probes did not increase under conditions of activation (compare Fig. 3B with 2A, open bars). Activation did not lead to increased heparin-stable complex formation on early melted probes with heteroduplex from –12 to –6(t), –12 to –1(t), –12 to –11(t), –12 to –11 (b) and –12Δ(t) (compare Fig. 3B with 2A, black bars). In contrast to early melted probes, the late melted probes with heteroduplex from –10 to –1(t), –7 to –3(t), –5 to –1(t) and –3 to –1(t) showed an increased number of initial (–heparin) and heparin–stable complexes upon activation (compare Fig. 3B with 2A). A comparison of the interactions with σ54 holoenzyme and the late melted probes with heteroduplex from –10 to –1(t), –7 to –3(t), –5 to –1(t) and –3 to –1(t) suggests that activation of the σ54 holoenzyme leads to similar levels of binding and stability (Table 1). In contrast, on the late melted probes with heteroduplex from –10 to –6, σ54 holoenzyme forms fewer activator-dependent stable complexes (Fig. 3B, black bars), suggesting that this region of heteroduplex somewhat disfavors stable complex formation. Hence, the positioning of the region of heteroduplex rather than its extent appears to play a role in the amount of binding and in how many activator-dependent complexes form. It appears that the early melted probes limit further activator-dependent stable complex formation (5). Guo et al. (3) demonstrated, using fork junction probes, that top strand sequences (in single-stranded form) from –11 to –7 were required for activator-dependent engagement of the σ54 holoenzyme with the non-template strand en route to open complex formation. In contrast, sequences from –11 to –10, when heteroduplex, do not yield extra stability under activating conditions and, in the context of a –12 to –1 region of heteroduplex, also did not yield activator-dependent increases in stable complex formation.
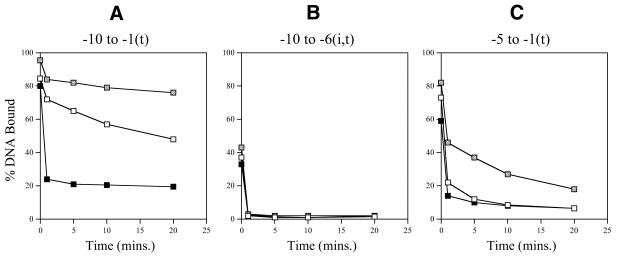
ΔIσ54 holoenzyme. We used holoenzyme lacking the regulatory σ54 Region I to elucidate the contribution of Region I to σ54 holoenzyme binding and stability on the heteroduplexes. The aim was to identify promoter regions important for the stable engagement of holoenzyme at the promoter which can occur in an activator-independent manner when σ54 Region I is removed or supplied in trans. Figure 2B shows that in the absence of the initiating nucleotide (GTP), the late melted probe with heteroduplex from –10 to –1(t) gives the greatest level of binding and heparin stability. However, late melted probes –10 to –6 (t), –7 to –3(t), –5 to –1(t) and –3 to –1(t) produced low amounts of stable complexes (Fig. 2B). The presence of the initiating nucleotide GTP results in significant levels of heparin-stable binding on the late melted heteroduplex probes –10 to –1(t), –5 to –1(t) and –12 to –1(t), indicating that activator-independent initiation had occurred (Fig. 2C). To further elucidate the contributions of σ54 Region I to σ54 holoenyzme binding to heteroduplexes –10 to –1(t), –10 to –6 (i,t) and –5 to –1(t), the stability of ΔIσ54 holoenzyme on these probes was measured in the presence of Region I peptide (residues 1–56) added in trans. As shown in Figure 4A and C, stabilisation by adding σ54 Region I in trans occurred with the late melted probes –10 to –1(t) and, to a lesser extent, –5 to –1(t) when Region I was added to the ΔIσ54 holoenzyme after DNA binding. This suggests that the single-stranded sequence from –5 to –1 interacts with σ54 Region I and that Region I increases heparin stability of the holoenzyme–DNA complex. Addition of σ54 Region I prior to ΔIσ54 holoenyzme binding to heteroduplexes (–10 to –1 and –5 to –1) resulted in a significant decrease in the heparin stability of ΔIσ54 holoenzyme–heteroduplex complexes to the level seen with the wild-type σ54 holoenzyme (Fig. 4A and C; 9). ΔIσ54 holoenzyme binding to the heteroduplex probe –10 to –6(i,t) was unchanged by the presence of σ54 Region I, suggesting that pre-opening the DNA from –10 to –6 reduces the stable engagement of both the wild-type σ54 and ΔIσ54 holoenzyme at the promoter (Fig. 4B and Table 1). In addition, the heteroduplex sequence from –10 to –6 does not appear to interact with σ54 Region I prior to binding DNA. Consistent with the requirement of σ54 Region I for stable binding to early melted DNA structures from –11 to –10, ΔIσ54 holoenzyme formed very few heparin-stable complexes with early melted probes –12 to –6(t) and –12 to –11(t) (a maximum of 4% DNA bound compared to 80% for σ54 holoenzyme), even in the presence of GTP (Table 1) (2,10).
Figure 4.
Stability of ΔIσ54 holoenzyme complexes in the presence of σ54 Region I in trans. (A–C) Graphs showing the stability of ΔIσ54 holoenzyme complexes formed with late melted probes –10/–1(t), –10/–6 (i,t) and –5/–1(t), respectively, in the absence (open squares) and presence of σ54 Region I (2 µM) supplied in trans either before (black squares) and after (grey squares) heteroduplex DNA binding. Samples were taken prior to the addition of heparin (t = 0) and 1, 5, 10 and 20 min after addition of heparin (100 µg/ml) and immediately run on native gels. The percentage of heparin-resistant DNA-bound complexes remaining are plotted against time.
Transcription from heteroduplex templates
To assess whether σ54 holoenzyme–heteroduplex DNA complexes could be productive for transcription and the extent to which transcription was activator dependent, we conducted single round in vitro transcription assays. In these assays σ54 holoenzyme was initially bound to a range of heteroduplex probes either in the presence of initiating nucleotide (GTP) or in the presence of activator PspFΔHTH and initiating nucleotide. GTP also served as the hydrolysable nucleoside triphosphate used by the activator. Then a mixture of heparin and the remaining nucleotides plus [α-32P]UTP were added to allow transcripts to form. The heparin was present to prevent reinitiation and to destroy unstable complexes.
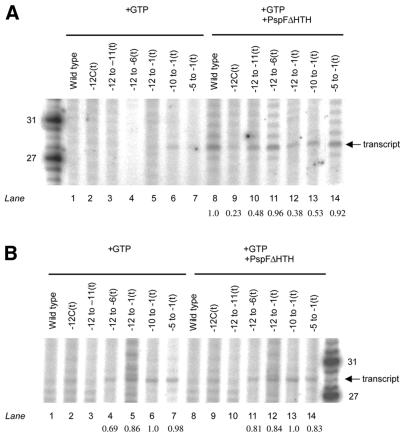
σ54 holoenzyme. Initially, our aim was to identify heteroduplex promoter regions that supported ‘regulated’, i.e. activator-dependent, transcription. First, we used heteroduplex probes –10 to –1(t) and –5 to –1(t), which yielded increases in stable σ54 holoenzyme–DNA complexes when activator and dGTP were added (compare Figs 2A and 3B). In a second set of assays we used heteroduplex probes in which no increases in stable complex formation were seen upon activation, to determine whether stable activator-independent binding led to activator-independent transcription [–12 to –6(t), –12 to –1(t), –12 to –11(t) and –12C(t)] (compare Figs 2A and 3B, black bars). Here the aim was to identify sequences which when altered by mispairing might lead to a loss of the silencing interactions associated with limiting transcription prior to activation. Figure 5A shows that no clearly distinguishable activator-independent transcription is seen from any of the heteroduplexes tested (lanes 1–7), suggesting that pre-melting the promoter DNA does not efficiently bypass the requirement for the activator by the σ54 holoenzyme for transcription initiation (4). Further, the results with heteroduplexes –12 to –6(t), –12 to –1(t), –12 to –11(t) and –12C(t) highlight that acquisition of heparin stability independent of activation and the property of transcript formation by the σ54 holoenzyme are not strongly interrelated (compare Figs 2A, black bars, and 5A, lanes 2–5). The presence of the activator PspFΔHTH resulted in an activator-dependent transcript of ∼28 bp from all the heteroduplexes tested (Fig. 5A, lanes 8–14), suggesting that the σ54 holoenzyme binds and interacts with the heteroduplex templates in a manner productive for transcription. Analysis of the gel shown in Figure 5A by densitometry suggested that the amounts of transcripts produced from the early melted heteroduplex probes –12C(t) and –12 to –11(t) (lanes 9 and 10, respectively) are 2–4-fold less than transcripts produced from the homoduplex probe, consistent with our previous observation that DNA melting within promoter complexes formed by the σ54 holoenzyme and the –12 to –11(t) early melted probe is limited upon activation (5). Control reactions with core RNAP or with [γ-32P]GTP confirmed that the transcripts are specific (data not shown).
Figure 5.
Transcription initiation from heteroduplexes by (A) the σ54 holoenzyme and (B) the ΔIσ54 holoenzyme. Activator-independent (lanes 1–7) and activator-dependent (lanes 8–14) reactions are shown. In (A) the number of transcripts formed compared to the wild-type homoduplex probe (lane 8) by the σ54 holoenzyme are shown and in (B) are compared to the late melted –10 to –1 probe by the ΔIσ54 holoenzyme (lanes 6 and 13, respectively), indicated below the lanes (values ≤0.15 are not shown).
ΔIσ54 holoenzyme. Using the ΔIσ54 holoenzyme we conducted transcription assays to determine which regions of heteroduplex best supported transcription in the absence of activator. Binding reactions included GTP for initiation, followed by the heparin nucleotide mix required for transcript elongation. Consistent with the poor stability pattern, ΔIσ54 holoenzyme did not detectably produce any transcripts from early melted heteroduplexes –12 to –11(t) and –12C(t) and the homoduplex probe (compare Figs 2C and 5B, lanes 1–3). Presence of the activator PspFΔHTH did not result in any activator-dependent transcription by the ΔIσ54 holoenzyme from these heteroduplexes (Fig. 5B, lanes 8–10). Consistent with its stability and binding patterns, the ΔIσ54 holoenzyme was active for activator-independent transcription from heteroduplexes –10 to –1(t), –12 to –1(t) and –5 to –1(t) (compare Figs 2C and 5B, lanes 5–7) and activation did not lead to any increases in transcript production (compare Fig. 5B, lanes 5–7 with 12–14). Overall, the data suggest that ΔIσ54 holoenzyme requires late melted promoter regions (between –5 and –1) to be pre-opened for transcription initiation, at least within the context of the single round transcription assays, the results of which are consistent with promoter probe binding.
DISCUSSION
Transition of the closed complex to the open complex at σ54-dependent promoters is believed to involve a series of concerted changes in protein and DNA structure that are brought about by the mechano-chemical activity of the transcriptional activators that act on the σ54 holoenzyme (8). Use of a series of heteroduplex DNA templates has revealed that some regions of heteroduplex, particularly those immediately next to the conserved promoter GC element, can lead to strong stable binding of the σ54 holoenzyme to yield complexes that only transcribe rarely. This indicates that the sequences downstream of the promoter consensus GC located at –12 preferably exist as double-stranded DNA to favour the formation of open complexes. The lack of extensive reactivity to single strand reactive DNA reagents seen within activated σ54 holoenzyme–promoter complexes formed with probes heteroduplex next to the GC region supports the view that few open complexes form (5). Within closed complexes formed by the σ54 holoenzyme, local DNA opening occurs near the –12 promoter region, which if heteroduplex greatly limits transcription (5,15). This suggests that although in closed complexes there may be an initial melting near the GC promoter element, this is transient and a stable opening in this region of the promoter is disadvantageous for high rates of transcription. In stable activator-dependent open complexes that are productive for transcription, DNA opening is not evident across the promoter region immediately downstream of the GC, consistent with the view that only transient melting occurs next to the GC (15). Further, tight binding of holoenzyme to a DNA fork junction near the GC would equate with limiting transcription. The latter is consistent with the view that changing interactions between σ54 holoenzyme and promoter DNA near the GC are important in converting the silent closed complex to an open complex in which the DNA is melted out and available for transcript formation (3,5,10,16). Further, no heteroduplex DNA template we tested gave clearly distinguishable activator-independent transcription with the wild-type σ54 holoenzyme, suggesting that the repressive interactions that are thought to stabilise closed complexes were largely intact in all heteroduplexes tested (this work; 4,6) and that activator-driven changes in holoenzyme structure are important for driving open complex formation.
Binding assays with a mutant form of σ54 holoenzyme lacking the regulatory Region I of σ54 showed that this holoenzyme was able to engage with the start site sequences independently of the activator protein and associated NTP hydrolysis. Sequences heteroduplex from –10 to –1 gave the greatest levels of activator-independent stable binding and transcription, and a clear component of heteroduplex from –5 to –1 was involved, as evidenced by the binding and transcription patterns seen with such probes. The stabilising effects of σ54 Region I sequences, when added in trans to the ΔIσ54 holoenzyme after holoenzyme binding to probes heteroduplex from –10 to –1 and –5 to –1, suggest that core RNAP interactions with the –5 to –1 heteroduplex are reinforced by a binding interaction that involves σ54 Region I. Further, the stabilisation afforded by σ54 Region I suggests that Region I functions after DNA melting and interacts with promoter sequences that interact with the core RNAP enzyme in the open complex. Thus, σ54 Region I appears to function before and after DNA opening to control properties of the σ54 holoenzyme.
Region I of σ54 is proven to directly contact activators in a nucleotide-dependent reaction (8), strongly suggesting that the interactions Region I makes in closed and open complexes are subject to activator-dependent changes. In particular, changing interactions between the σ54 holoenzyme and fork junction structures appear to be early events in the pathway that leads to delivery of promoter DNA into the active site of the RNAP core enzyme (8). Later, σ54 Region I sequences appear to stabilise the melted DNA that is start site proximal. Although unrelated in primary sequence to σ54, the σ70 class of σ factors also appear to use their N-terminal Region I sequences to stabilise open complexes (19–21) and recognise a fork junction structure located at a similar position with respect to the +1 position (22,23). For both the σ54 and σ70 holoenzymes, changes in promoter interactions ∼11 bases from +1 appear important for allowing the closed to open complex transition.
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. T. Gallegos for purified σ54 Region I and M. K. Chaney for useful comments on the manuscript. This work was supported by a Wellcome trust project grant to M.B. S.R.W. was supported by a PhD studentship from the Karlsruhe LEA, Germany.
REFERENCES
- 1.Buck M., Gallegos,M.-T., Studholme,D.J., Guo,Y. and Gralla,J.D. (2000) The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol., 15, 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon W.V., Gallegos,M.-T. and Buck,M. (2000) Isomerization of a binary sigma-promoter DNA complex by transcription activators. Nature Struct. Biol., 7, 594–601. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y., Lew,C.M. and Gralla,J.D. (2000) Promoter opening by σ54 and σ70 RNA polymerases: σ factor-directed alterations in the mechanism and tightness of control. Genes Dev., 14, 2242–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedel A. and Kustu,S. (1995) The bacterial enhancer-binding protein NTRC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev., 9, 2042–2052. [DOI] [PubMed] [Google Scholar]
- 5.Cannon W., Gallegos,M.-T. and Buck,M. (2001) DNA melting within a binary σ54-promoter DNA complex. J. Biol. Chem., 276, 386–394. [DOI] [PubMed] [Google Scholar]
- 6.Cannon W., Gallegos,M.-T., Casaz,P. and Buck,M. (1999) Amino-terminal sequences of σN (σ54) inhibit RNA polymerase isomerization. Genes Dev., 13, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J.T., Syed,A. and Gralla,J.D. (1997) Multiple pathways to bypass the enhancer requirement of sigma 54 RNA polymerase: roles for DNA and protein determinants. Proc. Natl Acad. Sci. USA, 94, 9538–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaney M., Grande,R., Wigneshweraraj,S.R., Cannon,W., Casaz,P., Gallegos,M.-T., Schumacher,J., Jones,S., Elderkin,S., Dago,A.E. et al. (2001) Binding of transcriptional activators to sigma 54 in the presence of the transition state analog ADP-aluminium fluoride: insights into activator mechanochemical action. Genes Dev., 15, 2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon W., Chaney,M. and Buck,M. (1999) Characterisation of holoenzyme lacking σN Regions I and II. Nucleic Acids Res., 27, 2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y., Wang,L. and Gralla,J.D. (1999) A fork junction DNA–protein switch that controls promoter melting by the bacterial enhancer-dependent sigma factor. EMBO J., 18, 3736–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon W., Missailidis,S., Smith,C., Cottier,A., Austin,S., Moore,M. and Buck,M. (1995) Core RNA polymerase and promoter DNA interactions of purified domains of σN: bipartite functions. J. Mol. Biol., 248, 781–803. [DOI] [PubMed] [Google Scholar]
- 12.Gallegos M.-T. and Buck,M. (1999) Sequences in σ54 determining holoenzyme formation and properties. J. Mol. Biol., 288, 539–553. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic G., Weiner,L. and Model,P. (1996) Identification, nucleotide sequence and characterization of PspF, the transcriptional activator of the Escherichia coli stress-induced psp operon. J. Bacteriol., 178, 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L. and Gralla,J.D. (1998) Multiple in vivo roles for the –12-region elements of sigma 54 promoters. J. Bacteriol., 180, 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris L., Cannon,W., Claverie-Martin,F., Austin,S. and Buck,M. (1994) DNA distortion and nucleation of local DNA unwinding within σ54 (σN) holoenzyme closed promoter complexes. J. Biol. Chem., 269, 11563–11571. [PubMed] [Google Scholar]
- 16.Wigneshweraraj S.R., Chaney,M.K., Ishihama,A. and Buck,M. (2001) Regulatory sequences in sigma 54 localise near the start of DNA melting. J. Mol. Biol., 306, 681–701. [DOI] [PubMed] [Google Scholar]
- 17.Chaney M. and Buck,M. (1999) The σ54 DNA-binding domain includes a determinant of enhancer responsiveness. Mol. Microbiol., 33, 1200–1209. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.T., Syed,A. and Gralla,J.D. (1995) Converting Escherichia coli RNA polymerase into an enhancer-responsive enzyme: role of an NH2-terminal leucine patch in σ54. Science, 270, 992–994. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin N.E. and Dombroski,A.J. (2001) Isolation and characterization of mutations in region 1.2 of Escherichia coliσ70. Mol. Microbiol., 42, 427–437. [DOI] [PubMed] [Google Scholar]
- 20.Vuthoori S., Bowers,C.W., McCracken,A., Dombroski,A.J. and Hinton,D.M. (2001) Domain 1.1 of the sigma(70) subunit of Escherichia coli RNA polymerase modulates the formation of stable polymerase/promoter complexes. J. Mol. Biol., 309, 561–572. [DOI] [PubMed] [Google Scholar]
- 21.Wilson C. and Dombroski,A.J. (1997) Region 1 of σ70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J. Mol. Biol., 267, 60–74. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y. and Gralla,J.D. (1998) Promoter opening via a DNA fork junction binding activity. Proc. Natl Acad. Sci. USA, 95, 11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.J. and Gralla,J.D. (2001) Sigma38 (rpoS) RNA polymerase promoter engagement via –10 region nucleotides. J. Biol. Chem., 276, 30064–30071. [DOI] [PubMed] [Google Scholar]
- 24.Merrick M.J. (1993) In a class of its own—the RNA polymerase sigma factor sigma 54 (sigma N). Mol. Microbiol., 10, 903–909. [DOI] [PubMed] [Google Scholar]
- 25.Gallegos M.T., Cannon,W. and Buck,M. (1999) Functions of the σ54 Region I in trans and implications for transcription activation. J. Biol. Chem., 274, 25285–25290. [DOI] [PubMed] [Google Scholar]
- 26.Syed A. and Gralla,J.D. (1998) Identification of an N-terminal region of sigma 54 required for enhancer responsiveness. J. Bacteriol., 180, 5619–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasse-Dwight S. and Gralla,J.D. (1990) Role of eukaryotic-type functional domains found in the prokaryotic enhancer receptor factor σ54. Cell, 62, 945–954. [DOI] [PubMed] [Google Scholar]
- 28.Southern E. and Merrick,M. (2000) The role of Region II in the RNA polymerase σ factor σN (σ54). Nucleic Acids Res., 28, 2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon W., Claverie-Martin,F., Austin,S. and Buck,M. (1994) Identification of a DNA-contacting surface in the transcription factor sigma-54. Mol. Microbiol., 11, 227–236. [DOI] [PubMed] [Google Scholar]
- 30.Taylor M., Butler,R., Chambers,S., Casimiro,M., Badii,F. and Merrick,M.J. (1996) The RpoN-box motif of the RNA polymerase sigma factor σN plays a role in promoter recognition. Mol. Microbiol., 22, 1045–1054. [DOI] [PubMed] [Google Scholar]
- 31.Cannon W.V., Chaney,M.K., Wang,X. and Buck,M. (1997) Two domains within sigmaN (sigma54) cooperate for DNA binding. Proc. Natl Acad. Sci. USA, 94, 5006–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallegos M.-T. and Buck,M. (2000) Sequences in σ54 Region I required for binding to early melted DNA and their involvement in sigma-DNA isomerisation. J. Mol. Biol., 297, 849–859. [DOI] [PubMed] [Google Scholar]