Abstract
Poor medication adherence is a pervasive issue with considerable health and socioeconomic consequences. Although the underlying reasons are generally understood, traditional intervention strategies rooted in patient-centric education and empowerment have proved to be prohibitively complex and/or ineffective. Formulating a pharmaceutical in a drug delivery system (DDS) is a promising alternative that can directly mitigate many common impediments to adherence, including frequent dosing, adverse effects and a delayed onset of action. Existing DDSs have already positively influenced patient acceptability and improved rates of adherence across various disease and intervention types. The next generation of systems have the potential to instate an even more radical paradigm shift by, for example, permitting oral delivery of biomacromolecules, allowing for autonomous dose regulation and enabling several doses to be mimicked with a single administration. Their success, however, is contingent on their ability to address the problems that have made DDSs unsuccessful in the past.
Subject terms: Drug delivery, Pharmaceutics, Drug delivery, Outcomes research, Drug delivery
Improving medication adherence is recognized as one of the most impactful and cost-effective strategies for improving the health of the general population. Here, Baryakova and colleagues assess the potential of next-generation drug delivery systems to mitigate many common impediments to adherence and discuss the impact that drug delivery systems have had across different disease types.
Introduction
More than half of the world’s population takes at least one drug each day, and the demand for pharmaceuticals is only expected to increase as the global disease burden continues to grow1. The benefits that a drug seemingly affords in a highly controlled setting, however, will not translate to real-world use if patients do not take their medication as prescribed. Poor medication adherence is the most common reason for disparities observed between results obtained in randomized clinical trials (RCTs) and real-world outcomes2,3 and remains pervasive; estimates of non-adherence are around 50% for chronic illnesses4,5. In the United States alone, poor adherence is responsible for an estimated 125,000 deaths per year, a figure comparable with the number of deaths caused by colorectal cancer, breast cancer and prostate cancer combined6,7. Poor adherence is also estimated to cause 10% of all hospitalizations and underlie $100–300 billion of avoidable health-care costs annually owing to wasted medicine, unnecessary diagnostic procedures and excessive health-care provider utilization8–10. Rates of non-adherence are especially high among older people, who are more likely to require complicated treatment plans and suffer from cognitive and/or functional impairments (for example, dysphagia) that impede their ability to administer certain types of medication11. Owing to a globally ageing population and a worldwide shift in the general disease burden from acute to chronic conditions, the adverse effects of non-adherence are only expected to increase12.
The key reasons for poor adherence are patient forgetfulness, anxiety about treatment-associated adverse effects, low motivation due to a perceived lack of efficacy, poor health literacy and aversion to the health belief model, and stigmatization4,8. Other factors that may play a role include high prescription costs and insufficient patient–provider communication. Besides negatively impacting the health of an individual, pervasive non-adherence can have a pernicious effect on the health of a community, especially as it pertains to communicable diseases. For example, failing to complete a vaccination schedule or a course of antibiotics or antivirals as prescribed can lead to the emergence of a resistant strain of a contagious bacteria or virus. Vaccine refusal has been implicated in outbreaks of varicella, measles and pertussis, among others13.
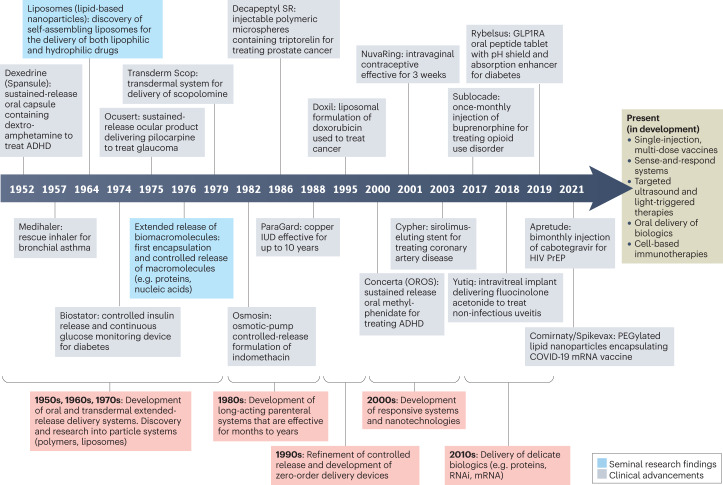
Improving adherence is recognized as one of the most impactful and cost-effective strategies for improving the health of the general population, yet it has not garnered the same attention as other approaches for improving wellness4. A mere 1% increase in drug utilization among individuals enrolled in Medicare and Medicaid in the United States is estimated to result in a $3 billion reduction in national health-care spending (0.2% of the total Medicare and Medicaid budget in 2020 (ref. 14))15,16. Traditional health-care provider-mediated strategies for improving adherence by educating and empowering patients have produced inconsistent and often underwhelming results17 (Box 1). These interventions are often too complex, requiring health-care infrastructure and/or some extent of personalization, to be cost-effective at scale. Drug delivery systems (DDSs) are promising technological alternatives that can mitigate the logistical factors negatively impacting real-world adherence. DDSs are formulations, systems or technologies used to modulate the release of a drug in the body over time and/or target the drug to a particular tissue or cell type. The first DDS, a sustained-release system delivering dextroamphetamine, was approved in 1952 (Box 2). A timeline describing the development of several key DDSs — with an emphasis on those that did (or are expected to) improve medication adherence — is provided in Fig. 1.
Fig. 1. Timeline of key delivery technologies and drug delivery systems.
This timeline illustrates examples of several key delivery technologies and drug delivery systems (DDSs) developed between the 1950s and the 2020s, many of which have improved or are expected to improve patient adherence. Several technologies currently in development that may be featured in future DDSs are also included. ADHD, attention deficit hyperactivity disorder; GLP1RA, glucagon-like peptide 1 receptor agonist; HIV, human immunodeficiency virus; IUD, intrauterine device; PrEP, pre-exposure prophylaxis.
In this Review, we first overview the fundamentals of DDSs and the mechanisms by which they can improve adherence. We then summarize the impact that DDSs have had on patient adherence across four disease and intervention types: chronic, relapsing–remitting, acute and prophylactic. Finally, we discuss the lessons that can be learned from several novel DDSs that failed to realize broad commercial success.
Box 1 Traditional interventions for improving adherence and ways to measure adherence.
Traditional strategies aimed at improving adherence include both direct and indirect methods. Examples of traditional, direct strategies for improving adherence include providing educational materials and monetary incentives, conducting motivational interviews and performing regular check-ins. Indirect strategies include strengthening patient–provider relationships and improving community-wide health literacy243. Unfortunately, there is no consensus on the efficacy of these strategies in improving medication adherence and there is evidence that their implementation can result in anywhere from a worsening to a significant improvement in rates of adherence and/or outcomes4,6,126,244–246. An analysis of data from 17 high-quality randomized clinical trials (RCTs) investigating the effect of various interventions on adherence with a low risk of bias247 revealed improvements in both medication adherence and clinical outcome in five studies, albeit only to a modest extent17. Moreover, each strategy that improved adherence consisted of a complex, multifactorial scheme, such as educational intervention from a health-care provider, rigorous counselling and/or daily treatment support.
Adherence assessments can be made using multiple methods, which can make it more difficult to compare findings between studies. In order of decreasing fidelity and cost, methods for measuring adherence include direct measures (for example, periodically measuring the concentration of a drug in a patient’s blood or urine), electronic monitoring (for example, employing a ‘smart’ container capable of sensing when medication is retrieved), secondary database measures (for example, reviewing prescription refill records), tablet counting and clinical assessment/self-reporting243,248. If using an electronic monitoring device, researchers can also gain insight into several different definitions of adherence: taking adherence (the percentage of device openings relative to the prescribed number of doses), regimen adherence (the percentage of days with the correct number of device openings per day) and timing adherence (the percentage of device openings occurring within a prescribed interval).
The two metrics most widely used to quantify adherence are the medication possession ratio and the proportion of days covered (PDC). The medication possession ratio is defined as the proportion of days’ supply obtained over a period of interest and is used approximately six times as often as the PDC10. The PDC is the proportion of days that a patient has access to their medication over a period of interest. In most instances, a patient with a medication possession ratio or PDC ≥ 0.80 is considered ‘adherent’249. Medication persistence, defined as the duration of time from treatment initiation to discontinuation, is another useful measure of medication-taking250.
It is important to note that studies often overestimate adherence owing to various confounding factors. These include ‘white coat’ adherence (patients are more adherent when under surveillance or near a clinical visit) and the ‘healthy-adherer effect’ (patients who are more adherent are also more likely to engage in health-seeking behaviour and may have better, treatment-independent outcomes). One meta-analysis considered 51 studies in which adherence to oral medications was monitored via an electronic monitoring device30. The studies that blinded patients to electronic monitoring, implemented longer follow-up periods and had a randomized trial design were associated with inferior adherence rates. As all three of these strategies are expected to increase the fidelity of the collected data, this supports the notion that adherence overestimation occurred.
Box 2 A brief history of drug delivery systems.
The earliest drug delivery systems (DDSs) were developed during the 1950s–1970s/1980s when researchers mostly sought to control the pharmacokinetics of oral and transdermal drugs. The first DDS, the Spansule sustained-release system, was approved in 1952 and consists of a gelatin capsule containing granules of the stimulant dextroamphetamine coated with a layer of natural waxes that dissolve in a composition and thickness-dependent manner251–253. Taking one Spansule capsule thus results in pharmacokinetics comparable with taking two or three immediate-release doses over the course of 12 h. Transderm Scop, the first systemic transdermal DDS, was approved in 1979 and delivers scopolamine across the skin via passive diffusion to treat nausea and vomiting. The device was recently discontinued for reasons unrelated to its efficacy or safety, but generic versions remain available. As of 2018, there were approximately 200 sustained-release oral preparations and 50 transdermal patches (both new products and generic versions) on the US market254.
Researchers developing the second generation of DDSs from the 1970s/1980s to the 2010s sought to improve and expand the use of these systems to achieve more consistent drug release rates, deliver comparatively more delicate biologics (for example, proteins, peptides and nucleic acids), utilize ‘smart’ materials capable of automatic or manual regulation and/or achieve tissue-specific targeting255. It was initially believed that large macromolecules could not withstand loading into polymeric microparticles but, in 1976, several proteins, including soybean trypsin inhibitor, lysozyme and alkaline phosphatase, were successfully encapsulated in non-inflammatory polymeric vehicles and released in a biologically active state over the course of months256. Thereafter, Lupron Depot, a formulation of poly(lactic-co-glycolic acid) microspheres encapsulating leuprolide acetate — the first long-acting injectable of its kind — was approved by the US Food and Drug Administration (FDA) in 1989 for the palliative treatment of prostate cancer. Its approved indications have since expanded to include treating endometriosis, anaemia owing to uterine fibroids and precocious puberty. Following an initial burst release, this system is capable of providing sustained release of leuprolide acetate for 1–6 months, depending on the formulation. Doxil, the first liposomal formulation of a small molecule (doxorubicin), was approved in 1995 and 13 other liposomal technologies have followed since257.
Building upon the innovations of the two prior generations, third-generation DDSs (2010s–present) are being developed to last longer and, where desirable, use less invasive technologies capable of delivering cargo with tightly controlled spatial and temporal precision. DDSs are also being employed to deliver new classes of drugs, such as nucleic acids for gene regulation and whole cells for cell-based immunotherapies22,258. Onpattro was the first RNAi therapeutic approved by the FDA in 2018; it was shown to reduce the production of the protein transthyretin in patients with hereditary transthyretin amyloidosis. rVSV-ZEBOV was the first viral vector-based vaccine approved for human use against Ebola in 2019. Most recently, Comirnaty and Spikevax were the first mRNA vaccines approved for COVID-19 vaccination in 2021.
Overview of drug delivery systems
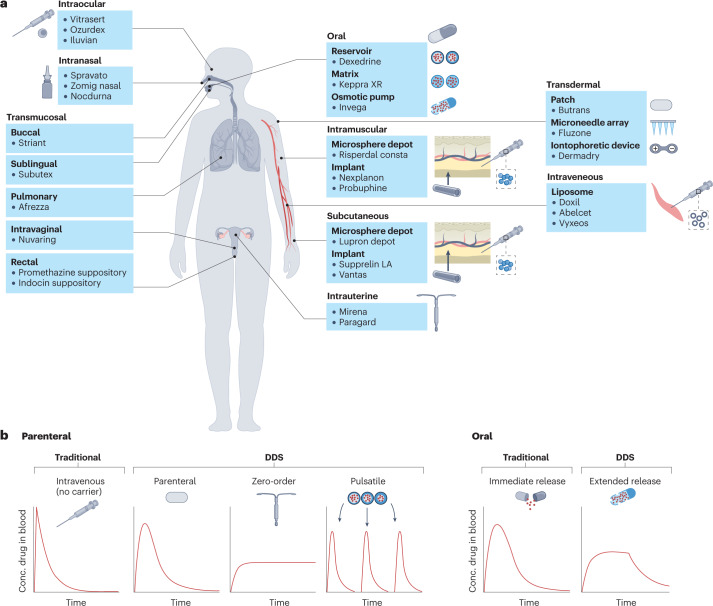
Controlled-release DDSs can be classified by numerous characteristics including their route of medication administration (for example, oral, transdermal, intravenous, intramuscular, subcutaneous, transmucosal; Fig. 2), the device type (for example, injectable microparticle depot, extended-release oral formulation, intravaginal ring) or the drug release profile afforded by the system (pulsatile, first-order, sustained, zero-order or stimuli-responsive).
Fig. 2. Drug delivery system examples and characteristics.
a, US Food and Drug Administration (FDA)-approved examples of drug delivery systems (DDSs) grouped by route of administration (oral, intramuscular, transdermal, subcutaneous, intraocular, intranasal, intrauterine and transmucosal (pulmonary, sublingual, buccal, intravaginal and rectal)). b, Pharmacokinetic profiles showing the plasma concentration of a drug following a single dose, based on the type of release. Traditional, non-DDS formulations of parenteral and oral drugs result in rapid clearance of drug from the blood, whereas some DDSs can prolong the duration over which the drug concentration remains within the therapeutic window without increasing the peak drug concentration. In the case of pulsatile release, DDSs can also allow for multiple, pre-programmed release events mimicking bolus doses of drug following a single administration.
DDSs are especially useful when the active pharmaceutical ingredient (API) has dose-limiting side effects, a narrow therapeutic window and/or a short half-life that makes maintaining the proper drug concentration difficult. Examples of DDSs that have been developed to address these issues include the liposomal formulation of the cardiotoxic chemotherapeutic doxorubicin (Doxil), a subcutaneous injectable microparticle suspension of somatotropin, a protein with a half-life of 20–30 min following intravenous injection18 (Nutropin Depot) and an extended-release oral formulation of the anticonvulsant drug phenytoin, which has a therapeutic index of only two (Phenytek capsules)19. In other instances where the payload is too fragile to survive in the body over therapeutically relevant timescales without a suitable carrier (for example, nucleic acids), a DDS such as a lipid-based nanoparticle may be required.
Chemical modifications and microenvironment modulation are two additional paradigms for improving the pharmacokinetics of an API. The former entails changing the physicochemical properties of a drug to create a new molecular entity20,21, and the latter entails changing the immediate vicinity of the drug to increase its solubility, stability and/or modulate the resulting immune response22. Although these additions may be included as part of a DDS formulation, they do not, by themselves, meet the definition of DDS used in this Review. Instead, we focus on platform technologies that can be applied to more than one API.
Limitations
Formulating APIs in DDSs is not a one-size-fits-all approach, however, and there are limitations common to certain classes of DDSs that are worth noting. For example, surgically implanting a device requires an invasive procedure and, in some instances, frequent monitoring by a health-care professional. This is also true for some state-of-the-art DDSs in preclinical development, including responsive particle systems that require external stimuli such as ultrasound or focused light to release cargo in a targeted manner, or systems that utilize instrument-mediated modes of cell transfection for gene therapy (that is, electroporation or biolistic (gene gun) delivery). Some classes of DDSs may be more likely to malfunction than their traditional alternative(s) owing to added device or usage complexity. Certain devices may also be less accessible owing to limited demand, scant coverage by insurance providers, a lack of enabling infrastructure, limited awareness among patients and providers, and/or costly premiums, especially in low and middle-income countries. These drawbacks, however, are arguably true of all nascent technologies and should diminish as further development and cost optimization enables broader adoption. Finally, some patients may express apprehension or outright refusal in favour of traditional, ‘tried and true’ methods of medication administration, depending on factors such as the severity of their disease and the device’s route of administration and usability23.
Design considerations
Generally, there are several key design considerations that DDS development should abide by depending on the device’s intended disease target(s). Given that patients with chronic conditions are likely to use it on a frequent (often, daily) basis, reliability, affordability and ease of use generally take priority. Factors including the size of the device (if used externally), the ease of administration and the severity of rapidly onset side effects, if any, can affect its perception among patients and its clinical utility. For relapsing–remitting conditions, it may additionally be beneficial to design a device capable of accommodating medically recommended changes in treatment owing to variable disease progression. The design considerations for DDSs used to administer prophylactic medication are similar to those for DDSs intended for use in patients with chronic and relapsing–remitting conditions, given the similarity in dosing duration requirements; however, device discretion may additionally carry more weight for some prophylactic drugs, such as pre-exposure prophylaxis (PrEP) and contraceptives. Where applicable, all non-surgically implanted DDSs intended for long-term use would benefit from monitoring capabilities to track patients’ adherence and the device effectiveness. The design considerations for DDSs used to treat acute conditions include those mentioned above, but there may be additional considerations given the time-sensitive nature of the condition (as is the case with an acute infection). These may include the speed of drug delivery and the portability of the device.
Improving patient acceptability and adherence
DDSs can improve the pharmacokinetics of an API and/or enable alternative delivery routes, potentially allowing for a reduction in dosing frequency and/or abatement of adverse effects. Some DDSs can also allow for added discretion, benefitting patients who feel embarrassed by having to regularly store and take pills. These improvements, among others, can enable patients to overcome barriers to adherence such as forgetfulness, premature discontinuation and stigma-related aversion. Below, we discuss seven major ways in which DDSs can improve adherence.
Reduced dosing frequency
Patients prefer to take a drug less often and are, accordingly, more adherent when their treatment regimen aligns with their preferences24–28. There is strong evidence to support a significantly higher rate of adherence to drugs taken once daily versus those taken multiple times per day for various conditions, including bisphosphonates (BPs) for osteoporosis, angiotensin-converting enzyme inhibitors for hypertension and sulfonylureas for type 2 diabetes (T2D), among many others. On a more granular level, there is evidence to support both the notion of an inverse, monotonic relationship between dosing frequency and adherence29 as well as a subtle or insignificant difference between adherence rates for drugs taken multiple times per day30,31. In one meta-analysis, adherence rates to oral medications used to treat chronic diseases across three definitions of adherence (taking, regimen and timing; Box 1) were found to be progressively lower for regimens requiring administration of two, three and four doses per day compared with once-daily dosing regimens, with the disparity growing more pronounced as the stringency of the adherence definition increased30. In another analysis, adherence rates among patients with asymptomatic chronic diseases taking once-daily medications were significantly higher compared with rates among those taking twice-daily or thrice-daily medications26.
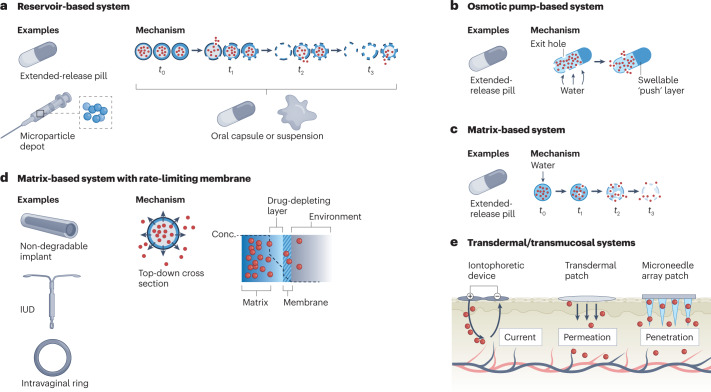
In contrast to immediate-release formulations such as capsules and intravenous injections, extended-release DDSs release a drug over a longer period of time, enabling less frequent dosing. Three common types of extended-release formulation, either for oral delivery or parenteral implantation/injection, are matrix, reservoir and osmotic-controlled systems (Fig. 3). Each system is capable of achieving delivery times of 12–24 h (if taken orally) or, potentially, years (if implanted parenterally) but is subject to trade-offs, including the rate and precision of drug release and ease of manufacturing. Some reservoir systems additionally consist of multiple types of particle, each made of biodegradable polymers of a different composition and/or thickness that allow for burst release of a drug at different times — a so-called pulsatile DDS32. These systems constitute a promising platform for addressing adherence issues with multi-dose vaccines. These are often associated with low rates of completion due, in part, to the burden of visiting a health-care provider multiple times, an adherence barrier that is heightened in low-resource settings33,34.
Fig. 3. Mechanisms of release and examples of common drug delivery systems.
a, Reservoir-based systems. These consist of a hollow, drug-filled core encapsulated by a degradable polymer. Over time, the polymeric shell degrades in a composition and thickness-dependent manner to release the drug. Using multiple types of shell allows for a fraction of the drug to release at a certain time. This technology can be used in capsules or as part of a microparticle depot suspension in oral and parenteral delivery systems, respectively. b, Osmotic pump-based systems. These systems are often used for extended-release oral delivery and consist of a water-permeable, insoluble polymer laden with drug and, often, a so-called expandable ‘push’ layer encapsulated in a hard coating. Exit holes are drilled through the coating to expose the polymer to the outside. Over time, water infiltrates the capsule and causes both layers to expand, pushing drug out through the exit holes. c, Matrix-based systems. These systems consist of drug embedded within a water-permeable, soluble monolithic matrix. Over time, water infiltrates the matrix (often, in the form of a tablet) and causes it to degrade, enabling drug release. These types of system are most often used in extended-release oral formulations. d, Matrix-based systems with a rate-limiting membrane. These consist of a drug-laden matrix core surrounded by a semi-permeable membrane that limits the rate of drug release, enabling pseudo zero-order delivery. This technology is often found in non-degradable, long-lasting implants, such as intrauterine devices (IUDs) and intravaginal rings. e, Transdermal/transmucosal systems. The three types of system shown here are iontophoretic devices, transdermal patches and microneedle array patches. Iontophoretic devices achieve delivery by creating an electric field that efficiently shuttles charged moieties across the skin barrier. Transdermal patches allow for passive delivery of molecules smaller than 500 Da. Finally, microneedle array patches penetrate the skin to deliver drug directly into the cutaneous layer.
Other extended-release DDSs include near zero-order delivery systems such as intravaginal rings, osmotic pumps, actuated pumps and implantable microchips, which are capable of providing a steady rate of drug release over a period ranging from hours to years35. Finally, nano-formulations, such as liposomes and dendrimers, can help extend the circulating time of a drug or improve its deposition characteristics, thereby increasing the therapeutic duration of the drug and reducing its required dosing frequency.
Avoidance of first-pass metabolism and accelerated onset of action
The bioavailability of oral drugs is limited by hepatic first-pass metabolism, leading to variability in the rate and extent of absorption. DDSs that avoid first-pass metabolism by using a parenteral route — such as intravenous, subcutaneous, intramuscular, transdermal, intranasal, sublingual or buccal administration — to deliver a drug directly into the bloodstream or the target site enable comparatively less material to achieve the same therapeutic effect in a well-controlled manner. They also enable a faster onset of action compared with oral delivery, which can be crucial for adherence; if patients do not immediately feel a lessening of their symptoms, they may stop taking their medication before it has a chance to exert its intended effect.
Intranasal DDSs enable a drug to access the brain via the olfactory or trigeminal nerves, bypassing hepatic first-pass metabolism, harsh gastrointestinal conditions and the blood–brain barrier to elicit the desired therapeutic effect within minutes instead of hours36,37. So-called nose-to-brain delivery systems are typically used to deliver classes of drugs such as anti-seizure medications, migraine medications, cholinesterase inhibitors and antidepressants38,39.
Rapid improvement of depressive symptoms is critical in patients with severe depression who are acutely suicidal. Accordingly, studies have shown that the short-term effects of antidepressants are predictive of long-term results40,41. Esketamine is an anaesthetic drug that is used to treat patients with treatment-resistant depression and has a quicker onset of action than traditional antidepressants. The oral bioavailability of esketamine, however, is only 8–11% and the rate of absorption appears to vary considerably between patients, possibly owing to factors such as stomach contents and gut motility42. In those taking oral esketamine, significant changes are often detected only 2–6 weeks after treatment initiation43. Intranasal esketamine (Spravato), in comparison, has a much higher bioavailability (46–54%) and is fast-acting, reaching a peak plasma concentration 20–40 min after dosing and lessening depressive symptoms as quickly as 4 h after the first dose.
Mitigation of concentration-dependent adverse effects
Experiencing adverse effects or anxiety about potential adverse effects is a major deterrent to patient adherence44–46. A high plasma concentration of certain drugs, including glucagon-like peptide 1 receptor agonists (GLP1RAs), cholinesterase inhibitors and BPs, immediately after dosing is directly correlated with the onset of gastrointestinal adverse effects such as nausea, diarrhoea and vomiting. Accordingly, these side effects are reported less frequently by patients taking long-acting formulations of these drugs than those taking short-acting formulations47–50. For patients with epilepsy, taking consecutive doses of immediate-release antiepileptic drugs (many of which have short half-lives and narrow therapeutic indices51) results in large peak-to-trough fluctuations, which may increase the risk of both seizures and concentration-dependent toxicity52. Extended-release formulations of antiepileptic drugs such as phenytoin (Dilantin) and valproate (Depakote ER), in comparison, are associated with improved tolerability, offer significant improvements in quality of life and can mitigate the effects of missed or delayed doses53.
Besides short-term adverse effects, there are other risks associated with repeated exposure to high concentrations of some drugs. These include an increased risk of developing resistance (as is the case with some antibiotics and antivirals) or developing tolerance and physical dependence (as is the case with opioids)4,54. Extended-release formulations have the potential to mitigate many of these issues by minimizing the peak-to-trough fluctuations in plasma drug concentration, enabling it to stay below toxic levels and within the therapeutic window. Because the drug metabolism rate is typically a function of concentration, these formulations have the added benefit of enabling less total drug to achieve the same therapeutic duration, potentially reducing the burden on the liver and kidneys.
Lowered barrier to continued use
Long-acting DDSs can offer a lowered barrier to continued use, benefitting patients who would otherwise prematurely stop taking a medication for the various reasons discussed below.
Patients taking fast-acting drugs may begin to feel better within a short period of time after initiating a course of medication and, considering their problem solved, fail to continue taking it as a result. This phenomenon is prevalent during the remission phase of various relapsing–remitting diseases, such as inflammatory bowel disease (IBD), relapsing–remitting multiple sclerosis and asthma. Premature discontinuation is also common in patients taking a course of antibiotics for an acute infection, which may increase the risk of developing antibiotic resistance.
Patients may also prematurely stop treatment if taking a drug with a delayed onset of action, as is common for antidepressants, antipsychotics and some immunosuppressants. In the case of depression, premature medication discontinuation contributes to its undertreatment and is a risk factor for developing a treatment-resistant form of the disease55.
Finally, patients may prematurely discontinue a drug because of its short-term side effects. Some treatment regimens for chronic diseases, such as interferon therapy in the treatment of multiple sclerosis or chronic hepatitis C, often result in flu-like symptoms that typically diminish over time56,57. The initial, acute onset of these symptoms is a deterrent to patient acceptability58, with the number and severity of symptoms inversely correlated to adherence59–61. Patients who are taking naltrexone for a substance use disorder can also initially experience unpleasant withdrawal symptoms during the first phase of treatment that can last for up to 2 weeks, depending on the substance. This often leads to premature discontinuation, a phenomenon that is common among patients receiving treatment for a substance use disorder62.
Reduced pain
Needle phobia is estimated to affect one out of every five people, and those afflicted are more likely to avoid medical treatment involving needles, including vaccination63,64. This fear is estimated to be the primary reason for non-compliance with recommended paediatric immunization schedules in 7–8% of cases65 and may account for as many as 10% and 16% of all individuals expressing COVID-19 (ref. 66) and influenza vaccine hesitancy63, respectively.
Nasal and oral vaccines are alternatives to vaccines delivered via intramuscular injection with a hypodermic needle. In a survey of parents whose children had received vaccines via both intranasal administration and intramuscular injection, a significantly larger percentage found the intranasal formulation to be more well tolerated and generally regarded it as more favourable67. However, oral vaccines are limited by challenges associated with oral delivery, such as withstanding the harsh conditions of the gastrointestinal tract and achieving sufficient absorption across the intestinal mucosal barrier despite a short residence time68. For these reasons, although they can provide mucosal immunity and are highly acceptable to patients (≥90%), oral vaccines are often unable to confer systemic immunity69.
Transdermal microneedle array patches (MAPs) are a type of DDS in preclinical development that deliver drugs to the epidermis or upper dermis, avoiding the cutaneous pain receptors found in the lower dermis and allowing for painless drug delivery. Injections with micro-sized needles are also less likely to cause serious skin irritation, redness or swelling and present a lower risk of infection than standard intramuscular injections70. Studies have shown that patient acceptability of MAPs is high, with 70–90% reporting that they would prefer to use a MAP rather than receive an intramuscular injection with a hypodermic needle71–74.
Increased cost-effectiveness
High out-of-pocket medication costs are a deterrent to patient adherence, especially in resource-limited settings75–78. DDSs have the potential to reduce the total amount of drug required to achieve a therapeutic effect by controlling the drug’s systemic concentration and rate of clearance. From a financial perspective, this benefit might be greatest for controlled-release DDSs delivering costly biologics. For example, anti-VEGF biologics are highly effective in the treatment of ocular diseases such as macular degeneration and macular oedema but are relatively expensive, and lowering the out-of-pocket costs of these treatments has been shown to significantly improve adherence79. Intravitreal bolus injections of the anti-VEGF treatments ranibizumab and aflibercept are annually estimated to use 160 and 190,000 times more drug, respectively, than that which a zero-order DDS would require80. If cost was to scale linearly with dose size, this translates to an estimated annual saving of approximately $10,000 for both treatments when formulated in a DDS, which is likely to far exceed the fabrication costs of such a device81. Moreover, controlling release over an extended duration could reduce the number of times a physician needs to administer the drug, requiring fewer doctor’s visits and reducing costs.
MAPs can also achieve dose sparing of vaccines, often requiring only 1–10% of the antigenic material that would be required in a subcutaneous or intramuscular injection due to the efficient activation of skin-resident antigen-presenting cells82,83. They may also have the potential to be stored outside the cold chain and/or self-administered, further lowering costs and improving accessibility.
Destigmatization
Stigma plays an important role in treatment acceptability and adherence for various conditions, including neuropsychiatric disorders, epilepsy, attention deficit hyperactivity disorder (ADHD) and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS)84–88.
Patients with ADHD may need to take as many as eight immediate-release methylphenidate tablets a day. This can be a source of stigma and embarrassment, especially among children who need to take one or more doses during the school day88,89. Several extended-release formulations of methylphenidate have been developed, including the osmotic controlled-release oral delivery system Concerta XL. This DDS, which consists of an immediate-release coating of methylphenidate encapsulating an extended-release osmotic pump system, produces a quick onset of action and avoids the rapid development of tolerance, extending the drug’s duration of efficacy to 10–12 h. Accordingly, results from RCTs suggest that this system leads to higher satisfaction and treatment adherence among patients over traditional immediate-release formulations90,91.
Individuals taking antiretrovirals for HIV/AIDS prophylaxis or therapy face a unique combination of stringent adherence requirements92,93 and a high rate of stigmatization, leading them to prefer comparably invasive, long-acting DDSs (for example, injectable suspensions and intravaginal rings) over short-acting oral drugs and pericoital microbicides94–96. In a survey of individuals receiving oral antiretroviral therapy, more than four fifths reported that they would definitely or probably try an injectable long-acting extended-release formulation if the dosing frequency was reduced to once monthly or better97. Accordingly, the two long-acting injectable antiretroviral therapies recently approved by the FDA have both been shown to be more effective in clinical trials over an oral PrEP alternative, ostensibly due to a demonstrable improvement in patient adherence98–100.
Trends in adherence by disease type
Adherence rates and auspicious DDS intervention strategies vary by disease type. In this section, we discuss the effects that implementation of a DDS and/or treatment modification attainable with a DDS have had on adherence in four categories of diseases and treatments: chronic, relapsing–remitting, acute and prophylactic interventions. These four categories broadly encompass most conditions that are currently treatable/addressable using DDSs and that substantially contribute to disability-adjusted life years lost worldwide. For example, cancer, diabetes, hypertensive heart disease and major depressive disorder alone (all discussed below) were collectively responsible for an estimated 15% of disability-adjusted life years lost in 2019 (refs. 101–104). In each section, we begin by discussing the impact that clinically approved DDSs have had on patients afflicted with that particular disease type and, where applicable, conclude with a brief discussion of promising DDSs in preclinical development. For further reference, a comparison of traditional versus DDS/long-acting formulations for the same API is provided in Table 1.
Table 1.
Comparison of traditional versus drug delivery system/long-acting formulations for the same active pharmaceutical ingredient
| DDS (API) | Type of system | Indication | Duration of effectiveness | Non-DDS alternative(s) | Mechanism(s) by which this system may improve adherence |
|---|---|---|---|---|---|
| Dexedrine Oral (dextroamphetamine), Concerta XL (methylphenidate) | Extended-release oral tablet | ADHD | 8–24 h, 1 day | Oral tablet taken 4–6 times daily (Zenzedi) or oral tablet taken 2–3 times daily (Ritalin) | Reduced dosing frequency, fewer adverse effects |
| Xeloda (capecitabine) | Extended-release oral tablet | Breast, colon and rectal cancer | 12 h | Intravenous bolus 5-fluorouracil and leucovorin administered every 3–4 weeks (Irinotecan) | Less invasive, convenience of at-home administration |
| Catapres-TTS (clonidine) | Transdermal patch | Hypertension | 1 week | Twice-daily oral tablet (Catapres Oral) | Reduced dosing frequency |
| Daytrana (methylphenidate) | Transdermal patch | ADHD | 1 day | Oral tablet taken 2–3 times daily (Ritalin) or daily (Concerta XL) | Reduced dosing frequency |
| Emsam (selegiline) | Transdermal patch | Major depressive disorder | 1 day | Oral tablets taken 2–4 times daily (for example, Marplan, Phenelzine) | Reduced dosing frequency, fewer side effects, appropriate for patients with dysphagia |
| Neupro (rotigotine) | Transdermal patch | Parkinson disease, restless legs syndrome | 1 day | Oral tablets taken 3 times daily (for example, Ropinirole, Pramipexole) | Reduced dosing frequency, fewer side effects, appropriate for patients with dysphagia |
| Exelon Patch (rivastigmine) | Transdermal patch | Alzheimer disease | 1 day | Twice-daily oral tablet (Exelon Oral) | Reduced dosing frequency, fewer adverse effects |
| Ortho Evra (ethinyl oestradiol;norelgestromin) | Transdermal patch | Contraception | 1 week | Daily oral tablet (various) | Reduced dosing frequency |
| Mirena/Kyleena/Liletta/Skyla (levonorgestrel) | IUD | Contraception | 3–7 years | Daily oral tablet (various) | Reduced dosing frequency, fewer side effects |
| Paragard (copper) | IUD | Contraception | ≤10 years | Daily oral tablet (various) | Reduced dosing frequency, fewer side effects |
| NuvaRing (etonogestrel/ethinyl oestradiol) | Intravaginal ring | Contraception | 1 month | Daily oral tablet (various) | Reduced dosing frequency |
| Norplant (levonorgestrel) | Subdermal implant | Contraception | 3 years | Daily oral tablet (various) | Reduced dosing frequency |
| Vitrasert (ganciclovir) | Intraocular, non-degradable polymeric implant | Cytomegalovirus retinitis in patients with AIDS | 5–8 months | Eye drops administered 3–5 times per day, as needed (Zirgan eye drops) | Reduced dosing frequency, reduced barrier to treatment continuation |
| Iluvien, Retisert and Yutiq (fluocinolone acetonide) | Intravitreal, non-degradable polymeric implant | Diabetic macular oedema (Iluvien) or non-infectious uveitis (Retisert and Yutiq) | 2.5 years (Retisert) up to 3 years (Iluvien and Yutiq) | Subcutaneous injection every 2 weeks for non-infectious uveitis (Humira) or intravitreal injection once every 4–8 weeks for diabetic macular oedema (Eylea, Avastin, Vabysmo, Lucentis) | Reduced dosing frequency, reduced barrier to treatment continuation, circumvention of need for monthly or bimonthly intravitreal injections at a provider’s office |
| Ozurdex (dexamethasone) | Intravitreal, extended-release degradable polymeric implant | Macular oedema | Up to 6 months | Eye drops taken 4–6 times a day, as needed (Dexamethasone Ophthalmic) | Reduced dosing frequency, reduced barrier to treatment continuation |
| Apretude (cabotegravir) | Intramuscular, extended-release injectable suspension | HIV PrEP | 2 months | Daily oral tablet (Truvada/Descovy) | Reduced dosing frequency, reduced barrier to treatment continuation, reduced perceived stigmatization |
| Cabenuva (cabotegravir; rilpivirine) | Intramuscular, extended-release injectable suspension | HIV PrEP | 1–2 months | Daily oral tablet (Truvada/Descovy) | Reduced dosing frequency, reduced barrier to treatment continuation, reduced perceived stigmatization |
| Lupron Depot (leuprolide acetate) | Subcutaneous, extended-release degradable microparticle depot | Prostate cancer, anaemia owing to uterine fibroids, endometriosis, central precocious puberty | 1–6 months, depending on formulation | Daily subcutaneous injection (Lupron injection) | Reduced dosing frequency, reduced barrier to treatment continuation, circumvention of need for daily injections |
| Vivitrol (naltrexone) | Intramuscular, extended-release degradable microparticle depot | Substance use disorder | 1 month | Daily oral tablet (Depade) | Reduced dosing frequency, reduced barrier to treatment continuation |
| Risperdal Consta (risperidone) | Intramuscular, extended-release degradable microparticle depot | Schizophrenia | 2 weeks | Daily oral tablet (Risperdal Oral) | Reduced dosing frequency, reduced barrier to treatment continuation |
| Triptodur, Fensolvi (leuprolide acetate) | Intramuscular, extended-release degradable microparticle depot | Central precocious puberty | 6 months | Daily subcutaneous injection (Lupron injection) | Reduced dosing frequency, reduced barrier to treatment continuation, circumvention of need for daily injections |
| Sustol (granisetron) | Subcutaneous, extended-release injectable degradable polymer | Prevention of nausea and vomiting associated with chemotherapy | 1 week | Twice-daily oral tablet (Zofran) | Reduced dosing frequency, alternative to oral tablets for patients with dysphagia |
| Eligard (leuprolide acetate) | Subcutaneous (in situ-forming) extended-release injectable degradable polymer | Prostate cancer | 6 months | Daily subcutaneous injection (Lupron injection) | Reduced dosing frequency, reduced barrier to treatment continuation, circumvention of need for daily injections |
| Lyxumia (lixisenatide)/Victoza (liraglutide) | Subcutaneous injection | T2D | 1 day | Twice-daily subcutaneous injection (exenatide twice daily) | Reduced dosing frequency |
| Bydureon (exenatide QW)/Eperzan and Tanzeum (albiglutide)/Ozempic, Rybelsus and Wegovy (semaglutide)/Trulicity (dulaglutide) | Subcutaneous injection | T2D | 1 week | Twice-daily subcutaneous injection (exenatide twice daily) or daily subcutaneous injection (various) | Reduced dosing frequency, fewer adverse effects owing to extended-release |
ADHD, attention deficit hyperactivity disorder; AIDS, acquired immunodeficiency syndrome; API, active pharmaceutical ingredient; DDS, drug delivery system; HIV, human immunodeficiency virus; IUD, intrauterine device; PrEP, pre-exposure prophylaxis; T2D, type 2 diabetes.
Chronic disorders
Adherence rates across chronic conditions are variable, but generally straddle 50%4. Across several conditions including hypertension, hypercholesterolaemia and heart disease, the risk of mortality in non-adherent patients is approximately twice that of adherent patients105 and rates of adverse outcomes are 1.5-fold to 5.4-fold higher106.
The potential of DDSs to improve adherence in patients with chronic conditions depends on the characteristics of the disease, the established treatment regimen and the administration parameters of the DDS. Given the low-level persistent nature of diseases such as osteoporosis, glaucoma and hypertension, long-acting injectables or implants might effectively promote adherence. In patients with a disease that can be well managed but for which no long-acting treatments currently exist, such as T2D, a DDS capable of making a seminal advancement (for example, enabling oral delivery or autonomous function) may be necessary in order to achieve widespread adoption. By contrast, for patients living with an often-debilitating disease with few treatment options, such as cancer, marginal improvements are much more meaningful and patients may be more willing to tolerate a DDS that produces adverse effects in exchange for added efficacy. For those with neuropsychiatric disorders, faster time to onset, a reduced barrier to continued use (usually in the form of an injectable or implantable DDS) and increased discretion are key characteristics.
Diabetes
Diabetes differs from many other chronic conditions in that it currently requires very frequent self-monitoring and intervention. Accordingly, poor adherence levels in patients with T2D are associated with an increased risk of hospitalization, complications, cerebrovascular disease and death107.
Several insulin analogues are commonly prescribed to patients with T2D: rapid-acting (lispro, aspart and glulisine), short-acting (regular human insulin), intermediate-acting (neutral protamine hagedorn) and long-acting (detemir and glargine). Each offers a trade-off between their onset and duration of action108. Although there is little information regarding the differences in rates of adherence between patients using different types of insulin, there is evidence that patients are more satisfied with rapid-acting analogues owing to the increased flexibility of dosing109.
Non-insulin oral and injectable antidiabetic agents, such as biguanides, sulfonylureas and GLP-1RAs, are also prescribed to some patients. There are multiple formulations of GLP-1RAs taken via subcutaneous injection twice daily, once daily or once weekly110. Persistence rates among patients with T2D taking a once-weekly GLP-1RA formulation are consistently reported to be higher than for those taking once-daily or twice-daily formulations, likely owing, in part, to a lower incidence of nausea and vomiting111–114.
Inhalable insulin was developed in the late 1990s and early 2000s as a non-invasive alternative to subcutaneous injected insulin. In clinical testing, inhalable insulin was found to have comparable efficacy and a quicker onset of action compared with subcutaneously injected insulin115. Owing to the ease of administration and convenience, patient satisfaction and acceptance was consistently reported to be higher with inhalable insulin, especially in patients whose T2D was poorly controlled by lifestyle changes and oral therapies116. However, some patients experienced side effects such as a dry cough, a mild decrease in pulmonary function and/or variable rates and extents of insulin absorption116,117. These reasons, among others, led to the eventual market failure of inhalable insulin despite its initial promise118,119.
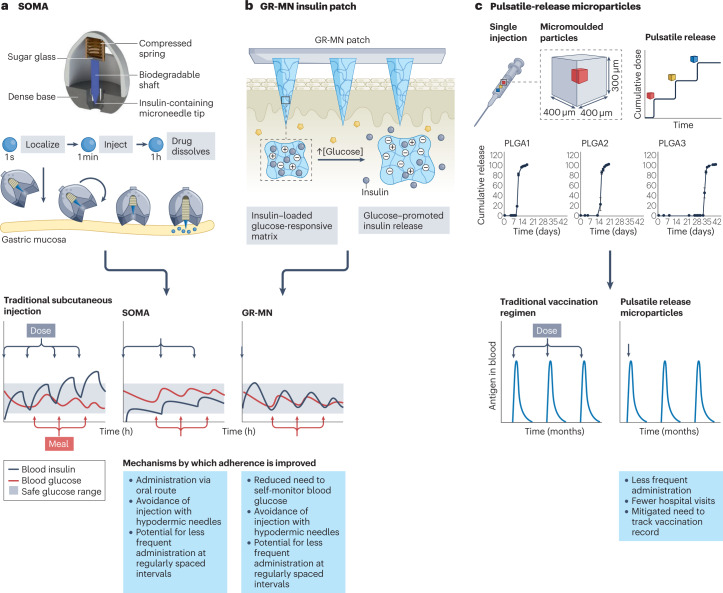
Patients with T2D are likely to understand that their condition will require near-constant surveillance and generally prefer to use less painful, short-acting insulin delivery systems for hourly maintenance of their blood glucose levels. There may be a paradigm shift in the treatment of this disease owing to two categories of novel DDSs currently in preclinical development. The first are oral delivery technologies, including an ingestible, self-righting device that can inject insulin painlessly across the gastric mucosa120, a spring-actuated microneedle injector that acts on intestinal tissue118 and a reversible intestinal permeation enhancer119. These still constitute short-acting systems that will likely require repeated daily dosing, but have the potential to change which route of administration is considered acceptable. The second are stimuli-responsive DDSs, including some MAPs121 and hydrogels122, that can autonomously sense a patient’s blood glucose and deliver the appropriate amount of insulin directly into vascularized tissue. Both types of system have the potential to help patients with T2D overcome the current challenges that impede their adherence: frequent self-monitoring and intervention, and an invasive mode of delivery due to the poor oral bioavailability and gastrointestinal permeability of antidiabetic drugs.
Cancer
Across various cancer types, patients tend to prefer oral chemotherapeutics over their parenteral counterparts (for example, in-clinic intravenous infusions and provider-administered injections) when these treatments are presented as options with equivalent efficacy123. Accordingly, home-based chemotherapies have historically resulted in increased patient satisfaction and adherence124,125. However, patients with cancer and other life-threatening illnesses are generally less willing to accept almost any reduction in efficacy or increase in toxicity, perceived or real, in exchange for an increase in convenience. Patients are sometimes also willing to tolerate more severe side effects and/or a greater number of side effects in exchange for relatively modest increases in efficacy126. In a scenario-based survey of treatment preferences among women with breast cancer, 25% of patients preferred a monthly, inpatient intramuscular injection, 63% preferred a daily tablet and 13% had no preference. However, women’s preference for injections increased from 25% to 61% and 75% when they were told to imagine a scenario where the monthly injection produced fewer hot flashes or where two monthly injections administered in the same session improved efficacy, respectively127. In another scenario-based survey of treatment preferences, approximately 70% and 74% of patients taking palliative chemotherapy were unwilling to accept the added convenience of taking a tablet at the expense of a lower response rate or shorter response duration, respectively128. One quarter and one third of patients thought that a mere 5% reduction in efficacy or one less month of response duration, respectively, was not worth the improved convenience of oral delivery. Finally, in one randomized crossover trial that employed both a scenario-based survey and a satisfaction questionnaire, 95% of patients with metastatic colorectal cancer who expressed a treatment preference initially preferred an oral therapy (capecitabine) and 5% preferred an inpatient or outpatient intravenous infusion of 5-fluorouracil/leucovorin. After receiving both types of treatment, however, the preference rate for the oral regimen dropped to only half of patients who received an outpatient intravenous infusion regimen and to two thirds of patients overall. Those who preferred the infusions generally did so because they experienced fewer side effects129.
Cancer therapies in preclinical development include particle formulations (for example, liposomes and polymeric nanoparticles) of chemotherapeutics such as paclitaxel, docetaxel and 5-fluorouracil, with or without active targeting moieties. By prolonging the elimination half-life of the drug and preferentially increasing its uptake in the desired cell population, these systems have the potential to reduce the required dosing frequency and mitigate side effects traditionally associated with non-specific chemotherapeutics and immunotherapeutics. Similarly, in situ cross-linkable hydrogels and non-degradable polymeric implants can be injected or implanted into the site of interest (when the location of the tumour burden is known) to form a slowly depleting depot that delivers drug in a localized fashion. These systems may be especially useful when the site of interest is a cavity made accessible due to a recent surgical intervention.
Osteoporosis
Bone antiresorptive agents, such as BPs and oestrogen, are first-line therapies for osteoporosis. Poor adherence, which is estimated to occur in between one third and one half of patients with osteoporosis, can significantly increase the risk of fracture and is correlated with inferior health outcomes and increased health-care costs130. Rates of treatment discontinuation are also high; up to three quarters of patients will discontinue a daily treatment regimen within the first year131. Reasons for discontinuation often include a low risk perception and/or the onset or fear of adverse effects associated with taking BPs (for example, upper gastrointestinal issues and an increased risk of oesophageal cancer)50. Upper gastrointestinal adverse effects occur in between one third and one half of patients taking oral BPs and remain the most commonly cited reason for treatment discontinuation. Reducing the dosing frequency has been shown to consistently improve adherence and persistence in patients taking BPs132–136. Two studies found that more than four out of five women with osteoporosis preferred once-weekly over once-daily BPs and believed it would help with adherence or would prefer to take it in the long term132,133. Accordingly, a higher percentage of patients were able to achieve full adherence when taking once-weekly BPs compared with once-daily BPs over a combined 8-week period133. Adherence and persistence were even higher among patients taking once-monthly BPs compared with those taking once-weekly BPs135.
DDSs in preclinical development for osteoporosis include parenteral hydroxyapatite-containing scaffolds and injectable or implantable depots137,138. These formulations may be able to address the common barriers to adherence by minimizing the need for patient intervention, abating fluctuations in dose and/or avoiding the gastrointestinal tract entirely.
Hypertension/cardiovascular disease
Poor adherence to antihypertensive therapy is associated with an increased risk of adverse outcomes, including death106,139,140. Adherence rates for common cardiovascular medicines have been reported to range from 21 to 71%141. There are several extended-release formulations of antihypertensive drugs, including nifedipine, propranolol and metoprolol/hydrochlorothiazide, which are capable of sustained drug release over 12–24 h and are taken either once or twice daily. It has consistently been shown that a reduction in the dosing frequency of chronic oral cardiovascular disease medication is associated with a significant improvement in all three types of adherence (taking, regimen and timing)28,142,143. However, because many cardiovascular medications are taken prophylactically and do not usually produce an immediately noticeable effect, many patients undervalue their importance141.
Catapres-TTS is a transdermal patch containing the antihypertensive medication clonidine. In one study, 87% of surveyed patients with mild to moderate hypertension viewed the patch as a more convenient monotherapy option as compared with previously used oral alternatives. Moreover, 65% were able to achieve better treatment adherence when using the patch and physicians deemed it a satisfactory or highly satisfactory option for 80% of their patients144. For these reasons, transdermal clonidine has explicitly been recommended to patients who have difficulty adhering or are otherwise intolerant to standard oral antihypertensive medications. Several additional DDSs are in clinical trials for the treatment of atherosclerosis and other ischaemic diseases, including nanoparticle and liposomal formulations145.
Ocular disorders
Adherence among patients is negatively impacted by inaccurate beliefs about the efficacy of the medication and severity of the disease if left untreated. This is especially prevalent in patients with glaucoma as there are not usually clear markers of disease progression and the disease often causes a gradual worsening of vision that is difficult to perceive over short periods of time. Adherence rates among patients with glaucoma have been reported to be approximately 50%, and poorly adherent patients tend to exhibit higher intraocular pressure, more severe visual field loss and are more likely to go blind146.
Using topical eye drops is generally considered to be the most convenient and safe approach for treating ocular conditions, including glaucoma, and generally leads to the highest patient acceptability. However, as many as nine out of ten patients are unable to properly instil eye drops147,148, leading to an increased risk of under-medication or over-medication and, potentially, resulting in poor outcomes and unnecessarily high treatment costs. Long-acting DDSs, including clinically available injectable depots, refillable devices and in situ-forming hydrogels, are promising strategies to improve medication adherence, mainly because they minimize the need for frequent drug administration. Sustained-release ocular implants, in particular, are able to limit the adverse effects associated with both systemic exposure and the high concentrations associated with frequent intraocular injections of immediate-release drugs149. Examples of these systems include Vitrasert, a non-degradable poly(vinyl alcohol)/poly(ethylene vinyl acetate) implant that delivers ganciclovir and is used to treat viral retinitis in patients with AIDS; Iluvian, an injectable, non-degradable poly(vinyl alcohol)-based implant that delivers fluocinolone acetonide to treat diabetic macular oedema; and Ozurdex, an injectable, biodegradable poly(lactic-co-glycolic acid) implant that delivers dexamethasone for up to 6 months and is used in the management of uveitis150. Ocusert is a pilocarpine reservoir surrounded by two rate-controlling membranes that a patient inserts into their own eye socket and is capable of releasing drug at a zero-order rate for 7 days to treat glaucoma and other eye conditions. The conventional alternative, pilocarpine eye drops, needs to be administered four times per day. Given that this dosing scheme results in local concentrations that frequently fall outside the therapeutic window, patients taking these eye drops often experience more adverse effects, including blurred vision and fluctuations in intraocular pressure, than patients taking Ocusert151. This system has not achieved widespread adoption, however, partially owing to the difficulty of device insertion, resulting eye irritation and premature device ejection.
Patient acceptability of and adherence to ocular injectables and implants is generally high, provided that the system can provide sustained release over a sufficiently long duration. In a small-scale survey, patients who underwent cataract surgery received a single injection of compounded ophthalmic pharmaceuticals in one eye and followed a regimen of self-administered eye drops for up to 4 weeks in the other. There was no significant difference in outcome efficacy or self-reported pain severity between treatments, but more than 90% of patients preferred the long-lasting injection over frequent administration of eye drops152.
Several DDSs in preclinical development for ocular delivery aim to deliver biologics (for example, antibodies, neurotrophic factors and even stem cells), in the form of injections or intravitreal implants. Others include MAPs that offer the same benefits as intravitreal injections but potentially fewer risks, degradable polymeric nanoparticles that mitigate the need for a removal procedure and hydrogels and refillable port delivery systems that, following implantation, allow patients to obtain refills in a comparatively non-invasive manner at a provider’s office153.
Major psychiatric disorders
It is estimated that about half of patients with a major psychiatric disorder are non-adherent154, owing to factors including negative attitudes towards medication and/or a perceived lack of efficacy, high rates of side effects and perceived stigma. The average amount of time it takes for conventional antidepressants to take effect and achieve a full response is 14 days and 20 days, respectively41. Given the extreme risks associated with a prolonged latency period (including an increased risk of suicide), a quicker and sustained onset of action has been correlated with improved long-term outcomes155. In contrast to oral DDSs, intranasal DDSs are capable of bypassing the gastrointestinal tract and blood–brain barrier and directly reaching the brain. This type of delivery enables a rapid, localized onset of action with convenient self-administration as compared with other, fast-acting parenteral medications (for example, intravenous infusions). Intranasal esketamine (Spravato) was approved by the FDA in 2020 for the treatment of depressive symptoms in adults with treatment-resistant depression and/or major depressive disorder. In clinical trials, Spravato was shown to act quickly, lessening depressive symptoms in some patients in as quickly as 4 h and exhibiting an acceptable safety profile. In the long term, approximately half of the experimental group taking both Spravato and a standard-of-care oral antidepressant achieved clinical remission of depression after 25 days compared with one third of the placebo arm receiving only the standard-of-care oral antidepressant156,157.
Risperdal Consta is an intramuscular injection of a degradable microparticle depot containing risperidone, an antipsychotic medication used to treat schizophrenia and other mental disorders. The injection can be given every 2 weeks and is especially recommended for those who exhibit poor medication adherence to oral antipsychotics. This DDS has been shown to result in significant behavioural improvements among patients who switch158,159 and is expected to be more cost-effective than oral alternatives in the long term by preventing avoidable hospitalizations and other types of health-care spending160.
Substance use disorder
For individuals with a substance use disorder, effectively managing symptoms requires good adherence to medication over a prolonged period. Herein, DDSs have the potential to replace daily oral therapies, which are associated with notoriously poor adherence and persistence rates; for example, more than three quarters of patients discontinue oral naltrexone treatment within 6 months62. Probuphine is an FDA-approved subdermal implant capable of releasing the partial opioid agonist buprenorphine over 6 months. Given its poor oral bioavailability, no oral alternative of this drug exists, and it is often given as a sublingual tablet. The implant was developed largely to address issues with patient non-adherence and medication misuse. In a RCT setting, this system was significantly more effective than a placebo implant161 and non-inferior to daily sublingual buprenorphine, with 86% of patients receiving buprenorphine implants and 72% receiving sublingual buprenorphine maintaining opioid abstinence over a period of 6 months. An extended-release injectable suspension of naltrexone (Vivitrol) taken monthly has also been developed to address adherence issues associated with daily oral therapy. Vivitrol has been shown to result in a small but significant improvement in adherence and to be as effective as or more effective than oral naltrexone for relapse prevention in individuals with alcohol use disorder162–165. This system is also indicated for use in individuals with opioid use disorder, and a naltrexone-releasing implant (DLP-160), also capable of delivering the drug over 6 months, is in clinical development. In one clinical trial, 53% of patients receiving the naltrexone implant and an oral placebo were able to remain in treatment without relapse over 6 months, as compared with only 16% of patients receiving a placebo implant and oral naltrexone166.
Relapsing–remitting disorders
Relapsing–remitting disorders are characterized by periods of remission interspersed with occasional flare-ups. Compared with patients with chronic, symptomatic disorders, patients with relapsing–remitting disorders are more likely to exhibit disease denial and less likely to actualize the benefits of taking their medication during symptom-free periods167–170.
The rates of adherence among patients with relapsing–remitting disorders are generally poor but vary widely, with underlying reasons that tend to be complicated, multifactorial and difficult to fully address. Although the main reason for non-adherence is still forgetfulness, patients are more likely to consciously become non-adherent to their medication during symptom-free periods owing to a low perceived risk of consequences. Accordingly, patients that are well-informed, self-empowered and believe in the positive benefits of their treatment tend to exhibit higher rates of adherence170,171. Still, there are no long-acting treatment options for the diseases discussed below and developing long-acting DDSs that patients can administer less than once per week (for example, a depot injection or implant) may be valuable to some, especially in instances where the aforementioned intervention strategies are infeasible or ineffective.
Inflammatory bowel disease
The long-term risks of poor adherence in patients with IBD include an increased risk for colorectal cancer, a fivefold greater risk of recurrence and an increased risk of hospitalization and surgery172,173. Oral mesalazine is the current standard of care for the induction and maintenance of remission in patients with ulcerative colitis, the most common form of IBD174. Rates of patient adherence to ‘conventional’ regimens of oral mesalazine (taken multiple times per day) are generally poor, with adherence estimates around 40%172. The most commonly cited reason for non-adherence among patients with IBD is forgetfulness167; despite this, retrospective analyses on adherence with extended-release mesalamine taken once daily have produced mixed results. Studies have concluded that adherence with once-daily mesalamine is significantly better than175,176, slightly better than172,177 or no different to178–180 adherence with mesalamine taken multiple times per day. There is also evidence to suggest that adherence and persistence in patients with ulcerative colitis are not significantly affected by factors associated with treatment, such as drug formulations and dosing frequency, especially with longer durations of use181. There does seem to be a meaningful role for more conventional strategies, such as audio-visual reminder systems, patient education and effective patient–provider communication167,182,183. Still, multiple methods often need to be deployed concurrently with some amount of customization in order to achieve meaningful results. Because the factors underlying poor adherence to IBD treatment are complex, it may be difficult to develop a one-size-fits-all DDS or interpersonal intervention strategy for the disease.
One of the most prominent types of DDS for IBD in preclinical development are nanoparticle-based systems that target the mucus layer or intestinal epithelium. Of these, responsive systems whose degradation is triggered by an altered disease state (for example, changes to pH and microbiome composition) are also under investigation184. Other DDSs in development include probiotic bacteria ‘factories’ that produce immunomodulatory recombinant or endogenous proteins. All have the potential to enable a significant reduction in required dosing frequency when compared with the current standard of care.
Relapsing–remitting multiple sclerosis
Reported adherence rates to disease-modifying therapies (DMTs) for relapsing–remitting multiple sclerosis are variable, with estimates ranging from 36 to 87%169,185. Good medication adherence has consistently been shown to produce better outcomes, including a reduced risk of relapse, a reduced risk of hospitalization and optimized cognitive abilities57,186.
One third of patients with relapsing–remitting multiple sclerosis who report missing an injection of the DMTs interferon-β (IFNβ) or glatiramer acetate cite injection-related reasons (for example, pain at the injection site) as the cause185. In addition to obviating the need for injections, oral DMTs (mainly fingolimod, dimethyl fumarate, teriflunomide, siponimod and cladribine) are more likely to produce fewer adverse effects such as flu-like symptoms187. Despite this, there is inconsistent evidence as to whether adherence and/or persistence are higher among patients taking oral DMTs or those taking injectable DMTs. When compared with patients who self-inject DMTs, it has been reported that those taking oral fingolimod were more adherent and persistent187. Similarly, those taking oral dimethyl fumarate were reported to exhibit improved adherence that correlated with an increase in perceived effectiveness and a decrease in (predominately gastrointestinal-related) adverse effects188. By contrast, a larger retrospective study considering self-injectable DMTs and three oral DMTs (fingolimod, teriflunomide and dimethyl fumarate) reported that the route of administration did not appear to affect patient behaviour, with approximately half of patients in both groups considered adherent or persistent189. In another meta-analysis, the reported 12-month adherence rates among patients taking oral DMTs were significantly higher than among those taking injectable DMTs (53–89% versus 47–77%). However, there was no significant difference in persistence, with the mean discontinuation over 12 months being 11–33% for oral DMTs and 15–50% for injectable DMTs. Potential sources of variability in outcomes between the studies under consideration include inconsistencies in protocols, methodology and data analysis, including adherence thresholds and definitions of discontinuation190.
DDSs in development for multiple sclerosis include IFNβ nanoparticles intended for intranasal administration191. These particles were created with the intention of circumventing the blood–brain barrier and delivering IFNβ directly to the brain. By avoiding the premature degradation that IFNβ would experience upon entering systemic circulation (as is the case following intramuscular, subcutaneous or intravenous administration), lower concentrations of the drug can be used. Accordingly, this system’s comparatively non-invasive and easy delivery strategy and ability to mitigate concentration-dependent side effects are expected to improve adherence among its users.
Asthma
Owing to the episodic nature of asthma, many patients feel that it does not impact their day-to-day lives and tend to underestimate the importance of long-term treatment. Patients are also more likely to adjust dosing according to their own perceived disease burden. Rates of adherence to inhalable asthma medication across multiple classes are reported to range from 30 to 70%184,192. Predictors of adherence to long-term inhaled therapies include regular appointments with a provider and positive beliefs about the medicine and its intended effects192. A reduction in dosing frequency could also play a beneficial role193–195. DDSs for asthma currently in preclinical development include alternatives to dry powder inhalers such as liposomes, microspheres and polymeric micelles. These systems have the potential to reduce the dosing frequency by enhancing deposition efficiency and reducing the rate of clearance from the lungs196,197.
Acute illnesses
In general, there is an inverse correlation between treatment duration and adherence rate. Although patient adherence is generally higher in acute illnesses requiring short-term treatment, there remains a substantial opportunity to improve current outcomes198. For example, one third of patients are estimated to not be fully adherent when taking a short course of antibiotics and one quarter save leftover antibiotics for future use199.
Tuberculosis
In 2020, an estimated 10 million people became sick with tuberculosis191. The current standard-of-care treatment regimen for tuberculosis in adults is a 6-month course of oral antibiotics. It is estimated that 20–50% of patients do not complete their tuberculosis treatment regimen within a 2-year period200. Intervention strategies such as health education and direct supervision have generally proven ineffective at improving adherence and those that have shown some efficacy, such as monetary incentives, are feasible to implement only in an extremely limited capacity200. Simplifying and/or shortening treatment times, in comparison, is expected to significantly improve adherence rates198,201–204. Recently, a phase III RCT achieved a breakthrough in showing that an abridged 4-month antibiotic regimen was non-inferior to the standard 6-month regimen205.
Inhalable, antibiotic dry powder drug formulations are currently under investigation to improve tuberculosis treatment compared with oral, short-acting antibiotics. These systems are advantageous because they are able to achieve a high localized concentration of drug in the lungs and, potentially, reduce the risk of systemic toxicity. This may also allow a reduction in the dose amount, dosing frequency and/or treatment duration, thereby reducing the likelihood of developing multidrug-resistant tuberculosis. Liposomes are one such carrier that have been extensively investigated in the context of pulmonary delivery, including for tuberculosis. Although studies have shown that these systems — particularly those with large payload capacities206 — can provide sustained release and improve the pharmacokinetic profile of antibiotics, efforts are underway to address critical challenges, such as the co-loading of all drugs currently prescribed as part of conventional tuberculosis treatment207,208.
Prophylactics
High adherence to contraceptives and antiretroviral therapies is crucial for their efficacy given that a single instance of non-adherence could lead to unintended pregnancy or HIV transmission. Accordingly, the documented usage of long-acting contraceptives and HIV PrEP formulations provides insight into how intervention-free DDSs can maximize adherence and improve patient satisfaction and outcomes. Vaccines are a form of prophylaxis for which several long-term DDS options (for example, transdermal and microneedle patches, intranasal sprays and injectable pulsatile-release microparticles) intended to reduce administration frequency are in clinical development209,210.
In general, patients have benefited from discrete, long-acting and comparatively invasive formulations of contraceptives and HIV PrEP over shorter-acting oral alternatives. In the near future, DDSs offering additional benefits (for example, fewer side effects, dual-drug delivery or an even longer duration of action) that emerge in this space, along with the first long-acting vaccine options of their kind, are expected to further improve the health of patients and communities.
Contraceptives
Contraceptive DDSs include transdermal patches, intravaginal rings, intramuscular injectables, subdermal implants and intrauterine devices (IUDs). The latter two fall into the category of long-acting reversible contraception (LARC) as they can be removed on-demand prior to drug depletion. LARCs take the matter of adherence out of the hands of the patient almost entirely for the duration of their use (up to 3 years for an implant, 3–7 years for a hormonal IUD and 10 years for a copper IUD). Although all of the aforementioned contraceptive methods generally have a theoretical failure rate of <1% with perfect use, only IUDs attain this in practice. In comparison, the reported annual failure rates are approximately 1–2% for injectables and implants and 2–9% for non-LARC methods (tablets, patches and rings)211–213. Accordingly, it has been reported that the risk of contraceptive failure is 20-fold higher among those using tablets, patches or rings as compared with LARCs211. Continuation rates with IUDs are also generally higher; 12-month continuation rates are reported to range from 80 to 89% for IUDs, from 68 to 83% for implants and from 49 to 73% for the tablet, patch, ring and depot injection214–216. Moreover, the rates of satisfaction appear to mirror the rates of continuation and it has been reported that more than four fifths of women using a LARC were still using their chosen method after 12 months of use, in comparison with half of women using a non-LARC method214.
The principles underlying modern contraceptives have remained the same for nearly 60 years as focus has largely been placed on making marginal improvements to hormonal strategies in women that are known to be effective. The future of contraceptive DDSs is towards various non-hormonal strategies in women and both hormonal and non-hormonal strategies in men. Accordingly, DDSs will likely be required to successfully deliver small molecules or delicate biologics to target locations. An example of the latter are anti-sperm antibodies capable of rapidly binding and immobilizing sperm in the female reproductive tract. These drugs must be formulated in a device, such as a vaginal film or suppository (for short-term delivery) or ring (for longer-term delivery), in order to remain stable and biologically active throughout both storage and use217. Novel DDSs such as these have the potential to improve contraceptive effectiveness, in part by mitigating or eliminating the negative side effects conventionally associated with hormonal approaches and encouraging adherence.
HIV pre-exposure prophylaxis
Stigma against HIV/AIDS is one of the most commonly reported barriers to adherence to prophylactic or therapeutic antiretroviral therapy218,219. Non-adherence is the strongest predictor of antiretroviral therapy failure and is strongly associated with an increased risk of mortality92,93. Poor adherence is also one of the primary reasons behind subpar clinical trial outcomes that have found oral PrEP to be ineffective or poorly effective. For example, in the Preexposure Prophylaxis Initiative (iPrEx) trial, daily dosing of emtricitabine and tenofovir disoproxil fumarate was initially found to reduce HIV transmission by only 44% overall220; however, when a retrospective analysis was limited to the approximately 18% of participants who were adherent (having a plasma tenofovir diphosphate concentration consistent with taking four or more doses per week221) or highly adherent (having a plasma tenofovir diphosphate concentration consistent with taking seven doses per week), the risk reduction increased to an estimated 96% and 99%, respectively222. Similar findings have been reported in other trials that initially reported an underwhelming rate of efficacy for oral PrEP drugs223.
In 2022, the FDA approved two injectables for HIV/AIDS prophylaxis and treatment: a long-acting injectable formulation of the prophylactic cabotegravir (CAB-LA) that is taken every 2 months98, and a therapeutic combination of CAB-LA and long-acting rilpivirine taken monthly99. In phase III testing of the former, patients were given either active oral Truvada and inactive CAB-LA (placebo) or inactive Truvada (placebo) and active CAB-LA. The rate of partial adherence or better among participants in the latter group was higher as determined via blood analysis. In phase III testing of CAB-LA and long-acting rilpivirine, an overwhelming majority — 91% of patients — preferred the long-acting injectable to the oral daily dosing regimen at the end of the year-long trial100. This is in alignment with results from previous, scenario-based surveys in which participants have signalled an interest in a long-acting alternative to oral PrEP and believed it would improve their adherence97,224,225.
Many DDSs currently in preclinical development for HIV are focused on increasing the duration of release for existing PrEP drugs. One class of examples are subdermal/subcutaneous implants for the extended release of drugs such as islatravir, tenofovir alafenamide, emtricitabine and cabotegravir226,227. Efforts are also underway to create novel dual-acting systems that can offer both contraception and protection against HIV228.
Multi-dose vaccines
The main barriers that parents face when vaccinating their children, aside from misconceptions about the safety of vaccines and the threat of vaccine-preventable illnesses, are forgetfulness and difficulty in tracking vaccinations229,230. Vaccines that require fewer doses and/or a shorter treatment time are associated with higher rates of completion across multiple age groups222,231,232. For example, among female patients who initiate HPV vaccination, only one third complete the vaccine regimen, and of these only two thirds receive all three doses within a valid time frame233–235. Switching to a two-dose or one-dose regimen, both of which have been shown to be effective, is expected to significantly improve adherence236–238.
Given that a majority of vaccines are administered via intramuscular injection, needle phobia is also an addressable issue that affects immunization compliance63. MAPs, a class of DDSs for single-dose or multi-dose vaccinations currently in preclinical development, can painlessly deliver a vaccine across the skin barrier into the epidermis or upper dermis using significantly less antigenic material than would be required for an intramuscular injection82,83. Tolerability studies have shown that 70–90% of participants would prefer using a MAP over receiving an intramuscular injection with a hypodermic needle71–73. There is also evidence to suggest that the added convenience of a self-administered vaccine option in the form of a MAP could improve vaccination coverage among vaccine-hesitant individuals74.
Vaccinations are different from many other types of health-care intervention in that at-home vaccination options usually do not exist and patients must visit a provider (or vice versa) in order to receive a dose. Long-acting DDSs, such as injectable pulsatile-release microparticles capable of mimicking a bolus dose multi-injection vaccine regimen, have the potential to combat under-immunization due to patient forgetfulness or negligence, or, as is often the case in the developing world, limited access to health-care providers and resources239,240.
Outlook
To have the greatest impact, DDSs must be developed in a way that aligns with the needs of patients, clinicians, pharmaceutical companies and payers. Whereas patients and clinicians typically want to achieve the best health outcomes with the fewest side effects (which should factor in the patient’s ability to adhere to the prescribed dosing regimen), pharmaceutical companies seek profit and payers aim to balance cost and patient benefits. DDSs have the potential to provide value for all stakeholders. For patients and clinicians, superior efficacy, improved convenience and reduced side effects can make DDSs valuable options. For companies, DDSs have the potential to capture an additional share of the market due to patient preference, to rescue drugs that have failed in clinical trials or to extend the effective patent lifetime for existing drugs to maintain an advantage over emerging generics. Historically, a large emphasis has been placed on developing and bringing a new drug to market whereas relatively minimal effort has been placed on developing DDSs for existing drugs with addressable flaws in their pharmacokinetic profile or route of administration. A shift in this strategy, reflecting an improved appreciation for the critical role of patient adherence, could allow insurance providers and pharmaceutical companies alike to assess the benefits of DDSs more accurately and make their development more commercially attractive (Box 3).
The nature of the existing disease treatment should be considered when creating a novel DDS. For example, patients who have taken the same medication for years may have become accustomed to the side effects and, therefore, be less receptive to a new route of administration. At present, most clinically approved DDSs are oral extended-release formulations owing to the generally high patient acceptability of this delivery route and ease of manufacturing; however, there are some limitations with this approach, such as a limited duration of action due to a short gastric residence time. Parenteral administration of injectable depots and implantable devices still constitutes a viable option for greatly reducing the required dosing frequency and improving adherence. These systems have the added benefit of being more readily compatible with biological drugs, which have gained a large share of the market over the past decade.
Novel DDSs currently in clinical or preclinical development have the potential to initiate a paradigm shift and redefine what constitutes an ‘acceptable’ treatment option in the near future. Examples of these potentially paradigm-shifting technologies include ingestible systems that make biologics orally bioavailable and/or provide sustained API release over 1 week or longer, sense-and-respond systems that autonomously regulate the concentration of a drug in circulation to minimize the need for manual intervention and pulsatile-release systems that mimic multi-dose regimens with a single injection (Fig. 4).
Fig. 4. Examples of drug delivery systems in development that could substantially improve adherence.
Medication adherence could be improved by systems that enable oral delivery of biologics, autonomously regulate the concentration of a drug in circulation or mimic a multi-dose dosing regimen with a single injection. a–c, Examples of systems in each category include the self-orienting millimetre-scale applicator (SOMA), which injects insulin across the gastric mucosa and leads to near zero-order release for hours (panel a); the glucose-responsive microneedle (GR-MN) insulin patch consisting of an array of polymeric needles that reversibly swell under hyperglycaemic conditions and collapse when blood glucose levels return to normal to modulate insulin release (panel b); and core shell poly(lactic-co-glycolic acid) (PLGA) microparticles synthesized via the stamped assembly of polymer layers microfabrication method capable of releasing active pharmaceutical ingredient cargo in discrete bursts at pre-programmed intervals (panel c). Part a adapted with permission from ref. 120, AAAS. Part b adapted from ref. 121, Springer Nature Limited. Part c is adapted from ref. 34, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Despite their advantages, DDSs still face logistical hurdles that can stymie their translation from bench to bedside, even after obtaining regulatory approval. Case studies of DDSs that have been removed from the market — inhalable insulin (Exubera and Afrezza) (Box 4), long-acting injectable human growth hormone (Nutropin Depot) (Box 5) and several iontophoretic devices (LidoSite, Ionsys and Zecuity) (Box 6) — highlight three lessons learned. First, an initial demonstration of safety is paramount to the ultimate translational success of a DDS; even if a once-flawed DDS is fixed after its initial launch or is quickly supplanted by an improved device, the perceived lack of safety among patients and clinicians can still negatively affect sales of the device and devices similar to it in the future. Second, the logistical challenges associated with large-scale manufacturing, which are typically greater for a DDS than for a stand-alone drug, should be appreciated and addressed early on to prevent potentially fatal consequences. Last, the DDS must fill an unmet or poorly met clinical need in the context of real-world use. Iontophoretic devices, for example, have thus far mainly focused on the treatment of acute symptoms such as migraine headaches, or been used to achieve dermal analgesia in a doctor’s office. In both scenarios, patient adherence is already high and viable oral and passive transdermal drug alternatives exist241,242. Moving forward, these devices may prove more commercially and clinically successful if their unique attributes are leveraged to fill a clinical gap that affects patient adherence. For example, iontophoretic devices currently in development are capable of delivering drugs into ocular tissue and directly into tumours, both of which are difficult environments to penetrate or require invasive means to do so. Thus, the types of challenge that have prevented the widespread use of once-promising DDSs are worth considering when translating new DDSs; each should be engineered to address the most pressing challenges facing the current standard-of-care treatment for the relevant disease target(s) to increase the likelihood of achieving meaningful clinical adoption.
Box 3 Creating a paradigm shift from developing new drugs to developing novel drug delivery systems.
Currently, only about 10% of new chemical entities that enter phase I clinical trials achieve US Food and Drug Administration (FDA) approval259, with the majority dropping out of the clinical pipeline owing to issues with pharmacokinetics, efficacy and safety260. An analysis of new chemical entities that failed in phase II or phase III clinical trials between 2013 and 2015 found that three quarters of these trials were discontinued, in part, owing to efficacy and safety concerns due to poor pharmacokinetics and a narrow therapeutic window, respectively261. These issues can often be addressed by formulating a drug in a controlled-release system, suggesting that a stand-alone drug that has previously failed in clinical trials could be clinically viable when combined with the proper drug delivery system (DDS).
A DDS that has the added benefit of significantly improving patient adherence can often constitute a favourable value proposition compared with the development of a new drug. For example, hypertension-related health expenditures are one of the largest costs paid out by public insurance in the United States262. Antihypertensive medications are taken by more than two thirds of US adults263 and are estimated to cost an average of $336 annually per patient264. Patients who are non-adherent to these hypertension medications are at a significantly higher risk for related morbidity and mortality265. Of the 91.7 million people taking hypertension medications in the United States263, 14.4% (13.2 million) are completely non-adherent266. In 2014, non-adherence resulted in 350,000 preventable hospitalizations at an average cost of $16,623 per patient, amounting to $5.9 billion in avoidable medical spending267. Thus, a DDS that enables these 13.2 million high-risk patients to achieve full adherence could cost up to 133% more annually ($784 total) than the standard treatment option to account for the financial benefit of avoiding unnecessary hospitalizations. This opportunity for significant cost savings is often not well conveyed to or fully appreciated by payers and providers who make these systems available to patients268. Accordingly, there is a need to instate a more holistic cost–benefit analysis when ascertaining whether the added benefits of a DDS justify the increased cost.
Box 4 Market failure of inhalable insulin.
In the years leading up to its market approval, inhalable insulin generated great interest and was widely heralded as a system with the potential to revolutionize diabetes treatment269. However, the only two inhalable insulin products to be commercialized, Exubera and Afrezza, have both experienced underwhelming market success. The Exubera device became the first US Food and Drug Administration (FDA)-approved inhalable insulin system in 2006, but was taken off the market just 1 year later270. The device was obtrusive and non-intuitive, requiring patients to convert from the often-used subcutaneous insulin ‘units’ to the non-intuitive milligrams271. Additionally, some patients who used Exubera presented with pulmonary side effects, such as a decline in lung function and/or increase in insulin antibodies, which were not as common in those taking subcutaneous insulin272. This development, in conjunction with the perceived notion that inhalable insulin slightly increased the incidence rates of lung cancer among smokers, led to the addition of a boxed warning on the prescription and increased apprehension among both patients and health-care providers270.
The only other product of this kind to emerge since the market failure of Exubera is MannKind’s Afrezza, which claimed a share of just 0.1% of the diabetes market 1 year after its launch in 2014 (refs. 270,273). Although the drug is still available and its contemporary market sales are showing an upward trend, inhalable insulin sales still account for less than 1% of total treatment visits for insulin and significantly less than 1% of the diabetes drug market274. Despite being a smaller and more intuitive device than Exubera, Afrezza still offers relatively inflexible dosing options275, leading clinicians to strongly encourage patients on Afrezza to continuously monitor their glucose levels. This requirement offsets the benefits of increased convenience and adds to the cost of disease management275,276. Additionally, Afrezza has a similar safety profile to Exubera and produces a persistent cough in more than one quarter of patients277. The fear of inhalable insulin posing a possible carcinogenic risk in general has also carried over from Exubera, despite the lack of any definitive clinical evidence271. The culmination of these factors, in addition to its high cost — which is more than twice that of injectable rapid-acting insulin278 — has led most insurance companies to list Afrezza as a non-preferred brand, imposing higher co-payments on patients and stymying interest and accessibility270.
Box 5 Market failure of long-acting injectable human growth hormone.
Recombinant human growth hormone (rhGH) is typically administered daily via subcutaneous injection in patients with growth hormone deficiency. Nutropin Depot, a twice-monthly injection of microspheres containing rhGH, was approved by the US Food and Drug Administration (FDA) in 1999 (refs. 279,280). Unfortunately, the added convenience of less frequent dosing with this drug delivery system (DDS) was partially offset by the need to deliver large volumes of the viscous formulation via multiple injections with a large-bore needle, which led to pain and left a visible bump on the (often, paediatric) patient’s skin for an extended period of time281. As is the case with most biodegradable microparticle depot formulations, Nutropin Depot also produced a large initial burst, releasing 22% of the total loaded rhGH in the first 24 h, and caused frequent injection site reactions282,283. Additionally, the bioavailability of rhGH administered via Nutropin Depot was 33–55% lower than that of rhGH administered via subcutaneous injection, requiring that 15% more rhGH was used and significantly increasing the cost of treatment284. Finally, manufacturing Nutropin Depot involved a complex and cost-intensive cryogenic freeze-drying process intended to preserve the activity of the encapsulated rhGH285. In 2004, the product comprised only $16 million (5%) of Genentech’s total growth hormone sales, but cost $4 million to manufacture and was discontinued281,286.
Box 6 Market failures of various iontophoretic devices.
Although iontophoretic devices are capable of quicker and more controlled dosing over passive transdermal formulations, many of these devices were ultimately unsuccessful owing to high costs and safety concerns287. LidoSite, an iontophoretic patch that delivers lidocaine, was discontinued in 2008 after only 2 years on the market. This device was capable of producing dermal analgesia six times faster than alternatives, but the treatment was more than ten times as expensive as the standard buffered lidocaine injection288, leading to depressed demand289,290. Similar devices, such as Lidopel and Iontocaine, were also removed from the market owing to limited demand in 2005 and 2011, respectively289,291.
Ionsys was approved for use in 2006 for the transdermal delivery of fentanyl to manage postoperative surgical pain. Clinical evaluation of the drug delivery system (DDS) found that it was effective and well tolerated292,293, yet it was removed from the market twice (temporarily in 2008 and permanently in 2017) owing to manufacturing quality concerns. The high technical complexity of the device made quality control difficult and expensive, and a corroded electronic component that could lead to the inadvertent delivery of a fatal dose of fentanyl was identified in a batch of the product shortly after its launch290. In a similar vein, reports of patients being burned and scarred by the Zecuity patch, a single-use iontophoretic device used to deliver migraine medication, led the manufacturer to temporarily suspend sales and launch an investigation in 2016, just 3 years after the initial approval of the device in 2013 (refs. 289,294). These safety concerns, in combination with the high cost of the device and marginal improvement in therapeutic efficacy over comparable low-cost alternatives, led to the permanent withdrawal of the product from the market in 2020 (refs. 294,295).
Acknowledgements
K.J.M. was supported by the National Institutes of Health (NIH) (R35 GM143101). T.H.B. (Grant No. 2236422) and B.H.P. (Grant No. 1842393) were supported by the National Science Foundation (NSF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests
R.L. holds numerous patents and holds equity and consults for companies that operate in the drug delivery arena; complete details for R.L.’s competing interests can be found online (https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev). K.J.M. is an inventor on several patents and patent applications in the area of drug delivery (US20190076631A1, WO2019018301A1, US10960073B2, US 63/213,082), and was also formerly a paid consultant for Particles for Humanity, a public benefit corporation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IHME. Findings from the Global Burden of Disease Study 2017 (Institute for Health Metrics and Evaluation, 2018).
- 2.Carls GS, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469–1478. doi: 10.2337/dc16-2725. [DOI] [PubMed] [Google Scholar]
- 3.Breckenridge A, et al. Poor medication adherence in clinical trials: consequences and solutions. Nat. Rev. Drug Discov. 2017;16:149–150. doi: 10.1038/nrd.2017.1. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Adherence to Long-Term Therapies: Evidence for Action (World Health Organization, 2003).
- 5.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lars O, Terrence B. Adherence to medication. N. Engl. J. Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.CDCBreastCancer. An update on cancer deaths in the United States. Centers for Disease Control and Preventionhttps://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm (2022).
- 8.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag. Healthc. Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA. 2018;320:2461. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- 10.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8:e016982. doi: 10.1136/bmjopen-2017-016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau ETL, Steadman KJ, Cichero JAY, Nissen LM. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018;135:75–84. doi: 10.1016/j.addr.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 12.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond. Engl. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance. Pharm. Ther. 2016;41:492–506. [PMC free article] [PubMed] [Google Scholar]
- 14.CMS. NHE fact sheet. Centers for Medicare & Medicaid Serviceshttps://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet (2021).
- 15.Congressional Budget Office. Offsetting effects of prescription drug use on Medicare’s spending for medical services. Congressional Budget Officehttps://www.cbo.gov/publication/43741 (2012).
- 16.Roebuck MC, Dougherty JS, Kaestner R, Miller LM. Increased use of prescription drugs reduces medical costs in medicaid populations. Health Aff. 2015;34:1586–1593. doi: 10.1377/hlthaff.2015.0335. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwlaat R, et al. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2014;2014:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Motte S, Klinger J, Kefer G, King T, Harrison F. Pharmacokinetics of human growth hormone administered subcutaneously with two different injection systems. Arzneimittelforschung. 2001;51:613–617. doi: 10.1055/s-0031-1300089. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg RG, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin. Neuropharmacol. 2016;39:232–240. doi: 10.1097/WNF.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunaydin H, et al. Strategy for extending half-life in drug design and its significance. ACS Med. Chem. Lett. 2018;9:528–533. doi: 10.1021/acsmedchemlett.8b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AlQahtani AD, O’Connor D, Domling A, Goda SK. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed. Pharmacother. 2019;113:108750. doi: 10.1016/j.biopha.2019.108750. [DOI] [PubMed] [Google Scholar]
- 22.Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021;5:951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- 23.Wang BB, Lin MM, Nguyen T, Turalba AV. Patient attitudes toward novel glaucoma drug delivery approaches. Digit. J. Ophthalmol. 2018;24:16–23. doi: 10.5693/djo.01.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin. Ther. 2006;28:1989–1995. doi: 10.1016/j.clinthera.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38:S2–S6. doi: 10.1016/j.bone.2006.01.150. [DOI] [PubMed] [Google Scholar]
- 26.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am. J. Manag. CARE. 2009;15:12. [PubMed] [Google Scholar]
- 27.Srivastava K, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer. Adherence. 2013;7:419–434. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeda ER, et al. Impact of once- or twice-daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta-regression analysis. Int. J. Cardiol. 2016;216:104–109. doi: 10.1016/j.ijcard.2016.04.082. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg RN. Overview of patient compliance with medication dosing: a literature review. Clin. Ther. 1984;6:592–599. [PubMed] [Google Scholar]
- 30.Coleman CI, et al. Dosing frequency and medication adherence in chronic disease. J. Manag. Care Pharm. 2012;18:527–539. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001;23:1296–1310. doi: 10.1016/S0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 32.Jain D, Raturi R, Jain V, Bansal P, Singh R. Recent technologies in pulsatile drug delivery systems. Biomatter. 2011;1:57–65. doi: 10.4161/biom.1.1.17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHugh KJ, Guarecuco R, Langer R, Jaklenec A. Single-injection vaccines: progress, challenges, and opportunities. J. Control. Rel. 2015;219:596–609. doi: 10.1016/j.jconrel.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 34.McHugh KJ, et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science. 2017;357:1138–1142. doi: 10.1126/science.aaf7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laracuente M-L, Yu MH, McHugh KJ. Zero-order drug delivery: state of the art and future prospects. J. Control. Rel. 2020;327:834–856. doi: 10.1016/j.jconrel.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Veronesi MC, et al. Imaging of intranasal drug delivery to the brain. Am. J. Nucl. Med. Mol. Imaging. 2020;10:1–31. [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:103–122. doi: 10.1111/head.12505_2. [DOI] [PubMed] [Google Scholar]
- 38.Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert. Opin. Drug Deliv. 2013;10:957–972. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- 39.Vitorino C, Silva S, Bicker J, Falcão A, Fortuna A. Antidepressants and nose-to-brain delivery: drivers, restraints, opportunities and challenges. Drug Discov. Today. 2019;24:1911–1923. doi: 10.1016/j.drudis.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Szegedi A, Müller MJ, Anghelescu I, Klawe C, Benkert O. Early improvement under mirtazapine and paroxetine predicts later stable response and remission with high sensitivity in patients with major depression. J. Clin. Psychiatry. 2003;64:5288. doi: 10.4088/JCP.v64n0410. [DOI] [PubMed] [Google Scholar]
- 41.Machado-Vieira R, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010;3:19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith-Apeldoorn SY, et al. Oral esketamine for treatment-resistant depression: rationale and design of a randomized controlled trial. BMC Psychiatry. 2019;19:375. doi: 10.1186/s12888-019-2359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenblat JD, et al. Oral ketamine for depression: a systematic review. J. Clin. Psychiatry. 2019;80:13514. doi: 10.4088/JCP.18r12475. [DOI] [PubMed] [Google Scholar]
- 44.Boulet LP. Perception of the role and potential side effects of inhaled corticosteroids among asthmatic patients. Chest. 1998;113:587–592. doi: 10.1378/chest.113.3.587. [DOI] [PubMed] [Google Scholar]
- 45.Rossini M, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos. Int. 2006;17:914–921. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Gonzalez A, et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin. Rheumatol. 2008;27:883–889. doi: 10.1007/s10067-007-0816-6. [DOI] [PubMed] [Google Scholar]
- 47.Yu M, et al. Battle of GLP-1 delivery technologies. Adv. Drug Deliv. Rev. 2018;130:113–130. doi: 10.1016/j.addr.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadowsky C, Perez JAD, Bouchard RW, Goodman I, Tekin S. Switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch. CNS Neurosci. Ther. 2010;16:51–60. doi: 10.1111/j.1755-5949.2009.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: a review. Int. J. Clin. Pract. 2009;63:799–805. doi: 10.1111/j.1742-1241.2009.02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin. Proc. 2009;84:632–638. doi: 10.1016/S0025-6196(11)60752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins RJ, Garnett WR. Extended release formulations of anticonvulsant medications. CNS Drugs. 2000;14:203–212. doi: 10.2165/00023210-200014030-00003. [DOI] [Google Scholar]
- 52.Wheless JW, Phelps SJ. A clinician’s guide to oral extended-release drug delivery systems in epilepsy. J. Pediatr. Pharmacol. Ther. 2018;23:277–292. doi: 10.5863/1551-6776-23.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leppik IE, Hovinga CA. Extended-release antiepileptic drugs: a comparison of pharmacokinetic parameters relative to original immediate-release formulations. Epilepsia. 2013;54:28–35. doi: 10.1111/epi.12043. [DOI] [PubMed] [Google Scholar]
- 54.Martin C, et al. Controlled-release of opioids for improved pain management. Mater. Today. 2016;19:491–502. doi: 10.1016/j.mattod.2016.01.016. [DOI] [Google Scholar]
- 55.Stahl SM, Nierenberg AA, Gorman JM. Evidence of early onset of antidepressant effect in randomized controlled trials. J. Clin. Psychiatry. 2001;62:17–23. [PubMed] [Google Scholar]
- 56.The PRISMS (Prevention of Relapses and Disability by Interferon-β-1a Subcutaneously in Multiple Sclerosis) Study GroupThe University of British Columbia MS/MRI Analysis Group. PRISMS-4: long-term efficacy of interferon-β-1a in relapsing MS. Neurology. 2001;56:1628–1636. doi: 10.1212/WNL.56.12.1628. [DOI] [PubMed] [Google Scholar]
- 57.Kappos L, et al. Long-term subcutaneous interferon β-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67:944–953. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 58.Portaccio E, Amato MP. Improving compliance with interferon-β therapy in patients with multiple sclerosis. CNS Drugs. 2009;23:453–462. doi: 10.2165/00023210-200923060-00001. [DOI] [PubMed] [Google Scholar]
- 59.Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer. Adherence. 2020 doi: 10.2147/PPA.S8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brett Hauber A, Mohamed AF, Beam C, Medjedovic J, Mauskopf J. Patient preferences and assessment of likely adherence to hepatitis C virus treatment. J. Viral Hepat. 2011;18:619–627. doi: 10.1111/j.1365-2893.2010.01343.x. [DOI] [PubMed] [Google Scholar]
- 61.Younossi ZM, et al. Adherence to treatment of chronic hepatitis C: from interferon containing regimens to interferon and ribavirin free regimens. Medicine. 2016;95:e4151. doi: 10.1097/MD.0000000000004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J. Stud. Alcohol. Drugs. 2011;72:1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J. Adv. Nurs. 2019;75:30–42. doi: 10.1111/jan.13818. [DOI] [PubMed] [Google Scholar]
- 64.Wright S, Yelland M, Heathcote K, Ng S-K, Wright G. Fear of needles: nature and prevalence in general practice. Aust. Fam. Physician. 2009;38:172–176. [PubMed] [Google Scholar]
- 65.Taddio A, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–4812. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Freeman D, et al. Injection fears and COVID-19 vaccine hesitancy. Psychol. Med. 2021 doi: 10.1017/S0033291721002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marien A-G, et al. Parental acceptance of an intranasal vaccine: example of influenza vaccine. Arch. Pédiatrie. 2019;26:71–74. doi: 10.1016/j.arcped.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scobie HM, et al. Use of oral cholera vaccine and knowledge, attitudes, and practices regarding safe water, sanitation and hygiene in a long-standing refugee camp, Thailand, 2012–2014. PLoS Negl. Trop. Dis. 2016;10:e0005210. doi: 10.1371/journal.pntd.0005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donnelly RF, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009;26:2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coleman BL, et al. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine. 2012;30:6287–6293. doi: 10.1016/j.vaccine.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Arya J, et al. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffin P, et al. Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine. 2017;35:6676–6684. doi: 10.1016/j.vaccine.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Norman JJ, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int. J. Clin. Pract. 2011;65:314–322. doi: 10.1111/j.1742-1241.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 76.Gast A, Mathes T. Medication adherence influencing factors — an (updated) overview of systematic reviews. Syst. Rev. 2019;8:112. doi: 10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowry ADK, Shrank WH, Lee JL, Stedman M, Choudhry NK. A systematic review of adherence to cardiovascular medications in resource-limited settings. J. Gen. Intern. Med. 2011;26:1479–1491. doi: 10.1007/s11606-011-1825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care. 2004;27:384–391. doi: 10.2337/diacare.27.2.384. [DOI] [PubMed] [Google Scholar]
- 79.Jackson S, Stokes JP. Impact of out-of-pocket costs on patient initiation, adherence and persistence rates for patients treated with anti-vascular endothelial growth factor medicines. Clin. Exp. Ophthalmol. 2020;48:477–485. doi: 10.1111/ceo.13706. [DOI] [PubMed] [Google Scholar]
- 80.Rimpelä A-K, Kiiski I, Deng F, Kidron H, Urtti A. Pharmacokinetic simulations of intravitreal biologicals: aspects of drug delivery to the posterior and anterior segments. Pharmaceutics. 2019;11:9. doi: 10.3390/pharmaceutics11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiss S, et al. Real-world injection frequency and cost of ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration and diabetic macular edema. J. Manag. Care Spec. Pharm. 2020;26:253–266. doi: 10.18553/jmcp.2020.19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suh H, Shin J, Kim Y-C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccin. Res. 2014;3:42. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moon S, et al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sirey JA, et al. Stigma as a barrier to recovery: perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatr. Serv. 2001;52:1615–1620. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 85.DiIorio C, et al. The association of stigma with self-management and perceptions of health care among adults with epilepsy. Epilepsy Behav. 2003;4:259–267. doi: 10.1016/S1525-5050(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 86.Corrigan P. How stigma interferes with mental health care. Am. Psychol. 2004;59:614–625. doi: 10.1037/0003-066X.59.7.614. [DOI] [PubMed] [Google Scholar]
- 87.Fung KMT, Tsang HWH. Self-stigma of people with schizophrenia as predictor of their adherence to psychosocial treatment. Psychiatr. Rehabil. J. 2008;32:95–104. doi: 10.2975/32.2.2008.95.104. [DOI] [PubMed] [Google Scholar]
- 88.Mueller AK, Fuermaier ABM, Koerts J, Tucha L. Stigma in attention deficit hyperactivity disorder. Atten. Deficit Hyperact. Disord. 2012;4:101–114. doi: 10.1007/s12402-012-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coghill D, Seth S. Osmotic, controlled-release methylphenidate for the treatment of ADHD. Expert. Opin. Pharmacother. 2006;7:2119–2138. doi: 10.1517/14656566.7.15.2119. [DOI] [PubMed] [Google Scholar]
- 90.Steele MS, et al. A randomized, controlled, effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can. J. Clin. Pharmacol. 2006;13:e50-e62. [PubMed] [Google Scholar]
- 91.Gau SS-F, Shen H-Y, Soong W-T, Gau C-S. An open-label, randomized, active-controlled equivalent trial of osmotic release oral system methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J. Child. Adolesc. Psychopharmacol. 2006;16:441–455. doi: 10.1089/cap.2006.16.441. [DOI] [PubMed] [Google Scholar]
- 92.Gross R, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV‐1 suppression. J. Infect. Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 93.Lima VD, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J. Acquir. Immune Defic. Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Philbin MM, et al. Multisite study of women living with HIV’s perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2020;84:263–270. doi: 10.1097/QAI.0000000000002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meyers K, et al. High interest in a long-acting injectable formulation of pre-exposure prophylaxis for HIV in young men who have sex with men in NYC: a P18 cohort substudy. PLoS ONE. 2014;9:e114700. doi: 10.1371/journal.pone.0114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krovi SA, Johnson LM, Luecke E, Achilles SL, van der Straten A. Advances in long-acting injectables, implants, and vaginal rings for contraception and HIV prevention. Adv. Drug Deliv. Rev. 2021;176:113849. doi: 10.1016/j.addr.2021.113849. [DOI] [PubMed] [Google Scholar]
- 97.Williams J, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomed. 2013;8:1807–1813. doi: 10.2217/nnm.12.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landovitz RJ, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N. Engl. J. Med. 2021;385:595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swindells S, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N. Engl. J. Med. 2020;382:1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 100.Orkin C, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N. Engl. J. Med. 2020;382:1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 101.Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Global Burden of Disease 2019 Cancer Collaboration. Cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth GA, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Safiri S, et al. Prevalence, deaths and disability-adjusted-life-years (DALYs) due to type 2 diabetes and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. Front. Endocrinol. 2022;13:838027. doi: 10.3389/fendo.2022.838027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simpson SH, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gwadry-Sridhar FH, et al. A framework for planning and critiquing medication compliance and persistence research using prospective study designs. Clin. Ther. 2009;31:421–435. doi: 10.1016/j.clinthera.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Kim Y-Y, Lee J-S, Kang H-J, Park SM. Effect of medication adherence on long-term all-cause-mortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Sci. Rep. 2018;8:12190. doi: 10.1038/s41598-018-30740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int. J. Gen. Med. 2011;4:827–835. doi: 10.2147/IJGM.S26889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin. Med. Res. 2008;6:54–67. doi: 10.3121/cmr.2008.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30:202–210. doi: 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Polonsky WH, Arora R, Faurby M, Fernandes J, Liebl A. Higher rates of persistence and adherence in patients with type 2 diabetes initiating once-weekly vs daily injectable glucagon-like peptide-1 receptor agonists in US clinical practice (STAY study) Diabetes Ther. 2022;13:175–187. doi: 10.1007/s13300-021-01189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab. Syndr. Obes. Targets Ther. 2016;9:201–205. doi: 10.2147/DMSO.S99732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes. Metab. 2017;19:336–347. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 114.Patel, S. & Miles, K. What are the gastrointestinal adverse effects of the glucagon-like peptide 1 receptor agonists, and how should they be managed? Illinois ADVANCEhttps://illinoisadvance.com/what-are-gastrointestinal-adverse-effects-glucagon-peptide-1-receptor-agonists-and-how-should-they-be-managed/ (2022).
- 115.Rave K, et al. Time–action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care. 2005;28:1077–1082. doi: 10.2337/diacare.28.5.1077. [DOI] [PubMed] [Google Scholar]
- 116.Bellary S, Barnett AH. Inhaled insulin (Exubera®): combining efficacy and convenience. Diab. Vasc. Dis. Res. 2006;3:179–185. doi: 10.3132/dvdr.2006.027. [DOI] [PubMed] [Google Scholar]
- 117.Ceglia L, Lau J, Pittas AG. Meta-analysis: efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus. Ann. Intern. Med. 2006;145:665–675. doi: 10.7326/0003-4819-145-9-200611070-00009. [DOI] [PubMed] [Google Scholar]
- 118.Abramson A, et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019;25:1512–1518. doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamson NG, et al. The strawberry-derived permeation enhancer pelargonidin enables oral protein delivery. Proc. Natl Acad. Sci. USA. 2022;119:e2207829119. doi: 10.1073/pnas.2207829119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abramson A, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611–615. doi: 10.1126/science.aau2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu J, et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020;4:499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X, et al. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017;51:294–303. doi: 10.1016/j.actbio.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 123.Eek D, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer. Adherence. 2016;10:1609–1621. doi: 10.2147/PPA.S106629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.King MT, Hall J, Caleo S, Gurney HP, Harnett PR. Home or hospital? An evaluation of the costs, preferences, and outcomes of domiciliary chemotherapy. Int. J. Health Serv. 2000;30:557–579. doi: 10.2190/CY03-EV15-K38Y-X4AA. [DOI] [PubMed] [Google Scholar]
- 125.Borras JM, et al. Compliance, satisfaction, and quality of life of patients with colorectal cancer receiving home chemotherapy or outpatient treatment: a randomised controlled trial. BMJ. 2001;322:826. doi: 10.1136/bmj.322.7290.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marshall VK. Factors associated with medication beliefs in patients with cancer: an integrative review. Oncol. Nurs. Forum. 2018;45:508–526. doi: 10.1188/18.ONF.508-526. [DOI] [PubMed] [Google Scholar]
- 127.Fallowfield L, et al. Patients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann. Oncol. 2006;17:205–210. doi: 10.1093/annonc/mdj044. [DOI] [PubMed] [Google Scholar]
- 128.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 129.Twelves C, Gollins S, Grieve R, Samuel L. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann. Oncol. 2006;17:239–245. doi: 10.1093/annonc/mdj023. [DOI] [PubMed] [Google Scholar]
- 130.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin. Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 131.Asafo-Adjei TA, Chen AJ, Najarzadeh A, Puleo DA. Advances in controlled drug delivery for treatment of osteoporosis. Curr. Osteoporos. Rep. 2016;14:226–238. doi: 10.1007/s11914-016-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simon JA, et al. Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin. Ther. 2002;24:1871–1886. doi: 10.1016/S0149-2918(02)80085-6. [DOI] [PubMed] [Google Scholar]
- 133.Kendler D, et al. Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas. 2004;48:243–251. doi: 10.1016/j.maturitas.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Ettinger MP, Gallagher R, MacCosbe PE. Medication persistence with weekly versus daily doses of orally administered bisphosphonates. Endocr. Pract. 2006;12:522–528. doi: 10.4158/EP.12.5.522. [DOI] [PubMed] [Google Scholar]
- 135.Park J-H, et al. Compliance and persistence with oral bisphosphonates for the treatment of osteoporosis in female patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2017;18:152. doi: 10.1186/s12891-017-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iglay K, et al. Systematic literature review and meta-analysis of medication adherence with once-weekly versus once-daily therapy. Clin. Ther. 2015;37:1813–1821. doi: 10.1016/j.clinthera.2015.05.505. [DOI] [PubMed] [Google Scholar]
- 137.Fisher PD, Venugopal G, Milbrandt TA, Hilt JZ, Puleo DA. Hydroxyapatite-reinforced in situ forming PLGA systems for intraosseous injection. J. Biomed. Mater. Res. A. 2015;103:2365–2373. doi: 10.1002/jbm.a.35375. [DOI] [PubMed] [Google Scholar]
- 138.Litak J, et al. Hydroxyapatite use in spine surgery — molecular and clinical aspect. Materials. 2022;15:2906. doi: 10.3390/ma15082906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shin S, et al. Effect of antihypertensive medication adherence on hospitalization for cardiovascular disease and mortality in hypertensive patients. Hypertens. Res. 2013;36:1000–1005. doi: 10.1038/hr.2013.85. [DOI] [PubMed] [Google Scholar]
- 140.Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med. Clin. North Am. 2017;101:229–245. doi: 10.1016/j.mcna.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121:1455–1458. doi: 10.1161/CIRCULATIONAHA.109.904003. [DOI] [PubMed] [Google Scholar]
- 142.Coleman CI, et al. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr. Med. Res. Opin. 2012;28:669–680. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- 143.Caldeira D, Vaz-Carneiro A, Costa J. The impact of dosing frequency on medication adherence in chronic cardiovascular disease: systematic review and meta-analysis. Rev. Port. Cardiol. Engl. Ed. 2014;33:431–437. doi: 10.1016/j.repc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 144.Hollifield J. Clinical acceptability of transdermal clonidine: a large-scale evaluation by practitioners. Am. Heart J. 1986;112:900–906. doi: 10.1016/0002-8703(86)90500-4. [DOI] [PubMed] [Google Scholar]
- 145.Kapil V, Louthan J, Hamedi N, Antoniou S, Lobo M. Transdermal clonidine in difficult-to-treat hypertension patients attending a specialist BP clinic in the United Kingdom. J. Hypertens. 2017;35:e279. doi: 10.1097/01.hjh.0000523811.90036.ac. [DOI] [Google Scholar]
- 146.Nordmann J-P, et al. Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a survey. Clin. Ophthalmol. Auckl. NZ. 2010;4:731–739. doi: 10.2147/opth.s11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gupta R, et al. Evaluating eye drop instillation technique in glaucoma patients. J. Glaucoma. 2012;21:189–192. doi: 10.1097/IJG.0b013e31820bd2e1. [DOI] [PubMed] [Google Scholar]
- 148.Choy BNK, et al. Factors associated with poor eye drop administration technique and the role of patient education among Hong Kong elderly population. J. Ophthalmol. 2019;2019:5962065. doi: 10.1155/2019/5962065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010;27:2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 150.García-Estrada P, et al. Polymeric implants for the treatment of intraocular eye diseases: trends in biodegradable and non-biodegradable materials. Pharmaceutics. 2021;13:701. doi: 10.3390/pharmaceutics13050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh RB, Ichhpujani P, Thakur S, Jindal S. Promising therapeutic drug delivery systems for glaucoma: a comprehensive review. Ther. Adv. Ophthalmol. 2020;12:2515841420905740. doi: 10.1177/2515841420905740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fisher BL, Potvin R. Transzonular vitreous injection vs a single drop compounded topical pharmaceutical regimen after cataract surgery. Clin. Ophthalmol. 2016;10:1297–1303. doi: 10.2147/OPTH.S112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kang-Mieler JJ, Rudeen KM, Liu W, Mieler WF. Advances in ocular drug delivery systems. Eye. 2020;34:1371–1379. doi: 10.1038/s41433-020-0809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Semahegn A, et al. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Syst. Rev. 2020;9:17. doi: 10.1186/s13643-020-1274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol. Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fu D-J, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I) J. Clin. Psychiatry. 2020;81:6605. doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- 157.Ionescu DF, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II) Int. J. Neuropsychopharmacol. 2021;24:22–31. doi: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Louzã MR, et al. Long-acting injectable risperidone in partially adherent and nonadherent patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2011;7:391–398. doi: 10.2147/NDT.S20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tchobaniouk LV, et al. Once-monthly subcutaneously administered risperidone in the treatment of schizophrenia: patient considerations. Patient Prefer. Adherence. 2019;13:2233–2241. doi: 10.2147/PPA.S192418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr. Dis. Treat. 2007;3:13–39. doi: 10.2147/nedt.2007.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ling W, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304:1576–1583. doi: 10.1001/jama.2010.1427. [DOI] [PubMed] [Google Scholar]
- 162.Alkermes. Opioid Dependence Pivotal Study for VIVITROL® (naltrexone for extended-release injectable suspension). Vivitrolhttps://www.vivitrolhcp.com/opioid-dependence-pivotal-study (2021).
- 163.Krupitsky EM, Blokhina EA. Long-acting depot formulations of naltrexone for heroin dependence: a review. Curr. Opin. Psychiatry. 2010;23:210–214. doi: 10.1097/YCO.0b013e3283386578. [DOI] [PubMed] [Google Scholar]
- 164.Malone M, et al. Extended-release vs. oral naltrexone for alcohol dependence treatment in primary care (XON) Contemp. Clin. Trials. 2019;81:102–109. doi: 10.1016/j.cct.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Walker JR, Korte JE, McRae-Clark AL, Hartwell KJ. Adherence across FDA-approved medications for alcohol use disorder in a veterans administration population. J. Stud. Alcohol. Drugs. 2019 doi: 10.15288/jsad.2019.80.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Krupitsky E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch. Gen. Psychiatry. 2012;69:973–981. doi: 10.1001/archgenpsychiatry.2012.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.D’incà R, et al. Risk factors for non-adherence to medication in inflammatory bowel disease patients. Aliment. Pharmacol. Ther. 2008;27:166–172. doi: 10.1111/j.1365-2036.2007.03555.x. [DOI] [PubMed] [Google Scholar]
- 168.Hawthorne AB, Rubin G, Ghosh S. Review article: medication non-adherence in ulcerative colitis—strategies to improve adherence with mesalazine and other maintenance therapies. Aliment. Pharmacol. Ther. 2008;27:1157–1166. doi: 10.1111/j.1365-2036.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 169.Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K. Facilitating medication adherence in patients with multiple sclerosis. Int. J. MS Care. 2013;15:36–45. doi: 10.7224/1537-2073.2011-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Caon C, Saunders C, Smrtka J, Baxter N, Shoemaker J. Injectable disease-modifying therapy for relapsing-remitting multiple sclerosis: a review of adherence data. J. Neurosci. Nurs. 2010;42:S5. doi: 10.1097/JNN.0b013e3181ee1240. [DOI] [PubMed] [Google Scholar]
- 171.Turner AP, Kivlahan DR, Sloan AP, Haselkorn JK. Predicting ongoing adherence to disease modifying therapies in multiple sclerosis: utility of the health beliefs model. Mult. Scler. J. 2007;13:1146–1152. doi: 10.1177/1352458507078911. [DOI] [PubMed] [Google Scholar]
- 172.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am. J. Med. 2003;114:39–43. doi: 10.1016/S0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 173.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. J. Am. Coll. Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 174.Nakase H. Optimizing the use of current treatments and emerging therapeutic approaches to achieve therapeutic success in patients with inflammatory bowel disease. Gut Liver. 2020;14:7–19. doi: 10.5009/gnl18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hawthorne BA, et al. One-year investigator-blind randomized multicenter trial comparing asacol 2.4 g once daily with 800 mg three times daily for maintenance of remission in ulcerative colitis. Inflamm. Bowel Dis. 2012;18:1885–1893. doi: 10.1002/ibd.21938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lachaine J, Yen L, Beauchemin C, Hodgkins P. Medication adherence and persistence in the treatment of Canadian ulcerative colitis patients: analyses with the RAMQ database. BMC Gastroenterol. 2013;13:23. doi: 10.1186/1471-230X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tong JL, Huang ML, Xu XT, Qiao YQ, Ran ZH. Once-daily versus multiple-daily mesalamine for patients with ulcerative colitis: a meta-analysis. J. Dig. Dis. 2012;13:200–207. doi: 10.1111/j.1751-2980.2012.00576.x. [DOI] [PubMed] [Google Scholar]
- 178.Feagan BG, MacDonald JK. Once daily oral mesalamine compared to conventional dosing for induction and maintenance of remission in ulcerative colitis: a systematic review and meta-analysis. Inflamm. Bowel Dis. 2012;18:1785–1794. doi: 10.1002/ibd.23024. [DOI] [PubMed] [Google Scholar]
- 179.Ford AC, Khan KJ, Sandborn WJ, Kane SV, Moayyedi P. Once-daily dosing vs. conventional dosing schedule of mesalamine and relapse of quiescent ulcerative colitis: systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106:2070–2077. doi: 10.1038/ajg.2011.296. [DOI] [PubMed] [Google Scholar]
- 180.Magowan SH, Kane S, Lange JL. 5-ASA prescription refill rates for ulcerative colitis are independent of formulation and dosing regimens: 1144. Am. J. Gastroenterol. 2006;101:S447. doi: 10.14309/00000434-200609001-01144. [DOI] [Google Scholar]
- 181.Kane SV, Brixner D, Rubin DT, Sewitch MJ. The challenge of compliance and persistence: focus on ulcerative colitis. J. Manag. Care Pharm. 2008;14:s2–s12. doi: 10.18553/jmcp.2008.14.S1-A.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kane SV, Accortt NA, Magowan S, Brixner D. Predictors of persistence with 5-aminosalicylic acid therapy for ulcerative colitis. Aliment. Pharmacol. Ther. 2009;29:855–862. doi: 10.1111/j.1365-2036.2009.03941.x. [DOI] [PubMed] [Google Scholar]
- 183.Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intest. Res. 2017;15:434–445. doi: 10.5217/ir.2017.15.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu AC, et al. Characteristics of new adult users of mepolizumab with asthma in the USA. BMJ Open. Respir. Res. 2021;8:e001003. doi: 10.1136/bmjresp-2021-001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Treadaway K, et al. Factors that influence adherence with disease-modifying therapy in MS. J. Neurol. 2009;256:568. doi: 10.1007/s00415-009-0096-y. [DOI] [PubMed] [Google Scholar]
- 186.Uitdehaag B, et al. Impact of exposure to interferon β-1a on outcomes in patients with relapsing-remitting multiple sclerosis: exploratory analyses from the PRISMS long-term follow-up study. Ther. Adv. Neurol. Disord. 2011;4:3–14. doi: 10.1177/1756285610391693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Agashivala N, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138. doi: 10.1186/1471-2377-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aungst A, et al. Assessing barriers to adherence with the use of dimethyl fumarate in multiple sclerosis. Clin. Drug Investig. 2020;40:73–81. doi: 10.1007/s40261-019-00866-6. [DOI] [PubMed] [Google Scholar]
- 189.Munsell M, Frean M, Menzin J, Phillips AL. An evaluation of adherence in patients with multiple sclerosis newly initiating treatment with a self-injectable or an oral disease-modifying drug. Patient Prefer. Adherence. 2016;11:55–62. doi: 10.2147/PPA.S118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mardan J, Hussain MA, Allan M, Grech LB. Objective medication adherence and persistence in people with multiple sclerosis: a systematic review, meta-analysis, and meta-regression. J. Manag. Care Spec. Pharm. 2021;27:1273–1295. doi: 10.18553/jmcp.2021.27.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.World Health Organization. Tuberculosis. WHOhttps://www.who.int/news-room/fact-sheets/detail/tuberculosis (2022).
- 192.Lindsay JT, Heaney LG. Nonadherence in difficult asthma — facts, myths, and a time to act. Patient Prefer. Adherence. 2013;7:329–336. doi: 10.2147/PPA.S38208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guest JF, Davie AM, Ruiz FJ, Greener MJ. Switching asthma patients to a once-daily inhaled steroid improves compliance and reduces healthcare costs. Prim. Care Respir. J. 2005;14:88–98. doi: 10.1016/j.pcrj.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Navaratnam P, Friedman HS, Urdaneta E. Treatment with inhaled mometasone furoate reduces short-acting β2 agonist claims and increases adherence compared to fluticasone propionate in asthma patients. Value Health. 2011;14:339–346. doi: 10.1016/j.jval.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 195.Price D, et al. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm. Med. 2010;10:1. doi: 10.1186/1471-2466-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mehta P. Dry powder inhalers: a focus on advancements in novel drug delivery systems. J. Drug Deliv. 2016;2016:e8290963. doi: 10.1155/2016/8290963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mishra B, Singh J. Novel drug delivery systems and significance in respiratory diseases. Target. Chronic Inflamm. Lung Dis. Using Adv. Drug Deliv. Syst. 2020 doi: 10.1016/B978-0-12-820658-4.00004-2. [DOI] [Google Scholar]
- 198.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther. Clin. Risk Manag. 2008;4:269–286. doi: 10.2147/TCRM.S1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kardas P, Devine S, Golembesky A, Roberts C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int. J. Antimicrob. Agents. 2005;26:106–113. doi: 10.1016/j.ijantimicag.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 200.Volmink J, Garner P. Interventions for promoting adherence to tuberculosis management. Cochrane Database Syst. Rev. 2000;2000:CD000010. doi: 10.1002/14651858.CD000010. [DOI] [PubMed] [Google Scholar]
- 201.Verma M, Furin J, Langer R, Traverso G. Making the case: developing innovative adherence solutions for the treatment of tuberculosis. BMJ Glob. Health. 2019;4:e001323. doi: 10.1136/bmjgh-2018-001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat. Med. 2007;13:290–294. doi: 10.1038/nm0307-290. [DOI] [PubMed] [Google Scholar]
- 203.Zumla AI, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect. Dis. 2014;14:327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 204.Abu-Raddad LJ, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl Acad. Sci. USA. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dorman SE, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N. Engl. J. Med. 2021;384:1705–1718. doi: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Muttil P, et al. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. 2007;32:140–150. doi: 10.1016/j.ejps.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 207.Pham D-D, Fattal E, Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015;478:517–529. doi: 10.1016/j.ijpharm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 208.Buya AB, et al. Application of lipid-based nanocarriers for antitubercular drug delivery: a review. Pharmaceutics. 2021;13:2041. doi: 10.3390/pharmaceutics13122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim, Y. C., Jarrahian, C., Zehrung, D., Mitragotri, S. & Prausnitz, M. R. in Intradermal Immunization (ed. Teunissen, M. B. M.) 77–112 (Springer, 2012). [DOI] [PMC free article] [PubMed]
- 210.Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur. J. Pharm. Biopharm. 2014;88:8–27. doi: 10.1016/j.ejpb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 211.Winner B, et al. Effectiveness of long-acting reversible contraception. N. Engl. J. Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 212.Polis, C. B. et al. Contraceptive Failure Rates in the Developing World: An Analysis of Demographic and Health Survey Data in 43 Countries. Technical Report (Guttmacher Institute, 2016).
- 213.Kost K, Singh S, Vaughan B, Trussell J, Bankole A. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21. doi: 10.1016/j.contraception.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Peipert JF, et al. Continuation and satisfaction of reversible contraception. Obstet. Gynecol. 2011;117:1105–1113. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wong RC, Bell RJ, Thunuguntla K, McNamee K, Vollenhoven B. Implanon users are less likely to be satisfied with their contraception after 6 months than IUD users. Contraception. 2009;80:452–456. doi: 10.1016/j.contraception.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 216.Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69:407–412. doi: 10.1016/j.contraception.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 217.Johnston DS, Goldberg E. Preclinical contraceptive development for men and women. Biol. Reprod. 2020;103:147–156. doi: 10.1093/biolre/ioaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Katz IT, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J. Int. AIDS Soc. 2013;16:18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kalichman SC, Katner H, Banas E, Hill M, Kalichman MO. HIV-related stigma and non-adherence to antiretroviral medications among people living with HIV in a rural setting. Soc. Sci. Med. 2020;258:113092. doi: 10.1016/j.socscimed.2020.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Grant RM, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Anderson PL, et al. Emtricitabine–tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci. Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nelson JC, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a Vaccine Safety Datalink Study. Am. J. Public Health. 2009;99:S389–S397. doi: 10.2105/AJPH.2008.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nelson AG, et al. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. J. Control. Rel. 2015;219:669–680. doi: 10.1016/j.jconrel.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Meyers K, et al. Interest in long-acting injectable PrEP in a cohort of men who have sex with men in China. AIDS Behav. 2018;22:1217–1227. doi: 10.1007/s10461-017-1845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Philbin MM, et al. Interest in long-acting injectable pre-exposure prophylaxis (LAI PrEP) among women in the Women’s Interagency HIV Study (WIHS): a qualitative study across six cities in the United States. AIDS Behav. 2021;25:667–678. doi: 10.1007/s10461-020-03023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Johnson LM, et al. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV pre-exposure prophylaxis (PrEP) Pharmaceutics. 2019;11:315. doi: 10.3390/pharmaceutics11070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matthews RP, et al. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial. Nat. Med. 2021;27:1712–1717. doi: 10.1038/s41591-021-01479-3. [DOI] [PubMed] [Google Scholar]
- 228.Sitruk-Ware R, Nath A, Mishell DR. Contraception technology: past, present and future. Contraception. 2013;87:319–330. doi: 10.1016/j.contraception.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Abahussin AA, Albarrak AI. Vaccination adherence: review and proposed model. J. Infect. Public Health. 2016;9:781–789. doi: 10.1016/j.jiph.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 230.Hargreaves AL, et al. Adherence to timely vaccinations in the United States. Pediatrics. 2020;145:e20190783. doi: 10.1542/peds.2019-0783. [DOI] [PubMed] [Google Scholar]
- 231.Matthews I, Dawson H. The impact of dosing schedules on the success of vaccination programmes in elderly populations: a summary of current evidence. Hum. Vaccines Immunother. 2018;14:1957–1962. doi: 10.1080/21645515.2018.1467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Packnett E, et al. Meningococcal-group B (MenB) vaccine series completion and adherence to dosing schedule in the United States: a retrospective analysis by vaccine and payer type. Vaccine. 2019;37:5899–5908. doi: 10.1016/j.vaccine.2019.06.065. [DOI] [PubMed] [Google Scholar]
- 233.Widdice LE, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127:77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liu G, Kong L, Du P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res. 2016;2:1–8. doi: 10.1016/j.pvr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hudson SM, Rondinelli J, Glenn BA, Preciado M, Chao C. Human papillomavirus vaccine series completion: qualitative information from providers within an integrated healthcare organization. Vaccine. 2016;34:3515–3521. doi: 10.1016/j.vaccine.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 236.Iversen O-E, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]
- 237.Kreimer AR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16:775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Barnabas RV, et al. Efficacy of single-dose human papillomavirus vaccination among young African women. NEJM Evid. 2022;1:EVIDoa2100056. doi: 10.1056/EVIDoa2100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tzeng SY, et al. Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release. J. Control. Rel. 2016;233:101–113. doi: 10.1016/j.jconrel.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guarecuco R, et al. Immunogenicity of pulsatile-release PLGA microspheres for single-injection vaccination. Vaccine. 2018;36:3161–3168. doi: 10.1016/j.vaccine.2017.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rapoport A, Winner P. Nasal delivery of antimigraine drugs: clinical rationale and evidence base. Headache. 2006;46:S192–S201. doi: 10.1111/j.1526-4610.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 242.Achar S, Kundu S. Principles of office anesthesia: part I. Infiltrative anesthesia. Am. Fam. Physician. 2002;66:91–95. [PubMed] [Google Scholar]
- 243.Lam WY, Fresco P. Medication adherence measures: an overview. BioMed. Res. Int. 2015;2015:1–12. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Marengo MF, Suarez-Almazor ME. Improving treatment adherence in patients with rheumatoid arthritis: what are the options? Int. J. Clin. Rheumatol. 2015;10:345–356. doi: 10.2217/ijr.15.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert. Rev. Clin. Immunol. 2012;8:337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 246.Jaleel A, Saag KG, Danila MI. Improving drug adherence in osteoporosis: an update on more recent studies. Ther. Adv. Musculoskelet. Dis. 2018;10:141–149. doi: 10.1177/1759720X18785539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Whalley Buono E, et al. Coming full circle in the measurement of medication adherence: opportunities and implications for health care. Patient Prefer. Adherence. 2017;11:1009–1017. doi: 10.2147/PPA.S127131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Haynes RB, et al. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2:757–764. doi: 10.1161/01.HYP.2.6.757. [DOI] [PubMed] [Google Scholar]
- 250.Cramer JA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 251.Park H, Otte A, Park K. Evolution of drug delivery systems: from 1950 to 2020 and beyond. J. Control. Rel. 2022;342:53–65. doi: 10.1016/j.jconrel.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hillery, A. M. & Park, K. Drug Delivery: Fundamentals and Applications 2nd edn (CRC, 2016).
- 253.Tulloch SJ, Zhang Y, McLean A, Wolf KN, Mays DA. SLI381 (Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparison of fasted, fed, and sprinkled administration. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002;22:1405–1415. doi: 10.1592/phco.22.16.1405.33687. [DOI] [PubMed] [Google Scholar]
- 254.Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10:263. doi: 10.3390/pharmaceutics10040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yun YH, Lee BK, Park K. Controlled drug delivery: historical perspective for the next generation. J. Control. Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 257.Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27:1372. doi: 10.3390/molecules27041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26:5905. doi: 10.3390/molecules26195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mullard A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016;15:447–447. doi: 10.1038/nrd.2016.136. [DOI] [PubMed] [Google Scholar]
- 260.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 261.Harrison RK. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016;15:817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 262.Dieleman JL, et al. US Health care spending by payer and health condition, 1996–2016. JAMA. 2020;323:863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.CDC. Estimated Hypertension Prevalence, Treatment, and Control Among U.S. Adults. Million Heartshttps://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html (2021).
- 264.Park C, et al. The uses and expenses of antihypertensive medications among hypertensive adults. Res. Soc. Adm. Pharm. 2020;16:183–189. doi: 10.1016/j.sapharm.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 265.Gupta P, et al. Non-adherence to heart failure medications predicts clinical outcomes: assessment in a single spot urine sample by liquid chromatography–tandem mass spectrometry (results of a prospective multicentre study) Eur. J. Heart Fail. 2021;23:1182–1190. doi: 10.1002/ejhf.2160. [DOI] [PubMed] [Google Scholar]
- 266.Kulkarni S, Rao R, Goodman JDH, Connolly K, O’Shaughnessy KM. Nonadherence to antihypertensive medications amongst patients with uncontrolled hypertension: a retrospective study. Medicine. 2021;100:e24654. doi: 10.1097/MD.0000000000024654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kumar N, et al. Thirty-day readmissions after hospitalization for hypertensive emergency. Hypertension. 2019;73:60–67. doi: 10.1161/HYPERTENSIONAHA.118.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Miller BS, Velazquez E, Yuen KCJ. Long-acting growth hormone preparations — current status and future considerations. J. Clin. Endocrinol. Metab. 2020;105:e2121–e2133. doi: 10.1210/clinem/dgz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Harsch IA, Hahn EG, Ficker JH. Inhalable insulin: the breakthrough in insulin therapy? Ann. Saudi Med. 2001;21:45–48. doi: 10.5144/0256-4947.2001.45. [DOI] [PubMed] [Google Scholar]
- 270.Oleck J, Kassam S, Goldman JD. Commentary: why was inhaled insulin a failure in the market? Diabetes Spectr. Publ. Am. Diabetes Assoc. 2016;29:180–184. doi: 10.2337/diaspect.29.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Greene SF, Nikula KJ, Poulin D, McInally K, Reynolds JA. Long-term nonclinical pulmonary safety assessment of Afrezza, a novel insulin inhalation powder. Toxicol. Pathol. 2021;49:334–348. doi: 10.1177/0192623320960420. [DOI] [PubMed] [Google Scholar]
- 272.Fineberg SE, Kawabata T, Finco-Kent D, Liu C, Krasner A. Antibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trial. J. Clin. Endocrinol. Metab. 2005;90:3287–3294. doi: 10.1210/jc.2004-2229. [DOI] [PubMed] [Google Scholar]
- 273.Feuerstein, A. MannKind’s Afrezza lags behind biggest failure in inhaled insulin. TheStreethttps://www.thestreet.com/investing/stocks/mannkinds-afrezza-lags-behind-biggest-failure-in-inhaled-insulin-13203821 (2015).
- 274.Sarkar S, Heyward J, Alexander GC, Kalyani RR. Trends in insulin types and devices used by adults with type 2 diabetes in the United States, 2016 to 2020. JAMA Netw. Open. 2021;4:e2128782. doi: 10.1001/jamanetworkopen.2021.28782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pettus J, Santos Cavaiola T, Edelman SV. Recommendations for initiating use of Afrezza inhaled insulin in individuals with type 1 diabetes. Diabetes Technol. Ther. 2018;20:448–451. doi: 10.1089/dia.2017.0463. [DOI] [PubMed] [Google Scholar]
- 276.Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2021;38:e14528. doi: 10.1111/dme.14528. [DOI] [PubMed] [Google Scholar]
- 277.Bode BW, et al. Inhaled technosphere insulin compared with injected prandial insulin in type 1 diabetes: a randomized 24-week trial. Diabetes Care. 2015;38:2266–2273. doi: 10.2337/dc15-0075. [DOI] [PubMed] [Google Scholar]
- 278.Al-Tabakha MM. Future prospect of insulin inhalation for diabetic patients: the case of Afrezza versus Exubera. J. Control. Rel. 2015;215:25–38. doi: 10.1016/j.jconrel.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 279.FDA. Drug Approval Package: Nutropin Depot (Somatropin [rDNA Origin] Injection) NDA# 21-075. US Food and Drug Administrationhttps://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21075_Nutropin.cfm (1999).
- 280.Reiter EO, et al. A multicenter study of the efficacy and safety of sustained release GH in the treatment of naive pediatric patients with GH deficiency. J. Clin. Endocrinol. Metab. 2001;86:4700–4706. doi: 10.1210/jcem.86.10.7932. [DOI] [PubMed] [Google Scholar]
- 281.Lal RA, Hoffman AR. Long-acting growth hormone preparations in the treatment of children. Pediatr. Endocrinol. Rev. 2018;16:162–167. doi: 10.17458/per.vol16.2018.lh.longactingghpreparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cawley P, Wilkinson I, Ross RJ. Developing long-acting growth hormone formulations. Clin. Endocrinol. 2013;79:305–309. doi: 10.1111/cen.12240. [DOI] [PubMed] [Google Scholar]
- 283.Johnson OL, et al. The stabilization and encapsulation of human growth hormone into biodegradable microspheres. Pharm. Res. 1997;14:730–735. doi: 10.1023/A:1012142204132. [DOI] [PubMed] [Google Scholar]
- 284.Cleland JL, Daugherty A, Mrsny R. Emerging protein delivery methods. Curr. Opin. Biotechnol. 2001;12:212–219. doi: 10.1016/S0958-1669(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 285.Desai K-GH, Schwendeman SP. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Rel. 2013;165:62–74. doi: 10.1016/j.jconrel.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.L.A. Times Archives. Genentech to Stop Selling Type of Growth Hormone. Los Angeles Timeshttps://www.latimes.com/archives/la-xpm-2004-jun-02-fi-genen2-story.html (2 June 2004).
- 287.Wang Y, Zeng L, Song W, Liu J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: an overview of recent progress. Drug Deliv. Transl. Res. 2022;12:15–26. doi: 10.1007/s13346-021-00898-6. [DOI] [PubMed] [Google Scholar]
- 288.Pershad J, Steinberg SC, Waters TM. Cost-effectiveness analysis of anesthetic agents during peripheral intravenous cannulation in the pediatric emergency department. Arch. Pediatr. Adolesc. Med. 2008;162:952–961. doi: 10.1001/archpedi.162.10.952. [DOI] [PubMed] [Google Scholar]
- 289.Bakshi P, Vora D, Hemmady K, Banga AK. Iontophoretic skin delivery systems: success and failures. Int. J. Pharm. 2020;586:119584. doi: 10.1016/j.ijpharm.2020.119584. [DOI] [PubMed] [Google Scholar]
- 290.Yeoh T. Current landscape and trends in transdermal drug delivery systems. Ther. Deliv. 2012;3:295–297. doi: 10.4155/tde.12.11. [DOI] [PubMed] [Google Scholar]
- 291.Miller K. Transdermal patches: past, present and future. Ther. Deliv. 2015;6:639–641. doi: 10.4155/tde.15.16. [DOI] [PubMed] [Google Scholar]
- 292.Viscusi ER, Ding L, Itri LM. The efficacy and safety of the fentanyl iontophoretic transdermal system (IONSYS®) in the geriatric population: results of a meta-analysis of phase III and IIIb trials. Drugs Aging. 2016;33:901–912. doi: 10.1007/s40266-016-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Poplawski S, et al. Use of fentanyl iontophoretic transdermal system (ITS) (IONSYS®) in the management of patients with acute postoperative pain: a case series. Pain. Ther. 2016;5:237–248. doi: 10.1007/s40122-016-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Center for Drug Evaluation and Research. FDA evaluating the risk of burns and scars with Zecuity (sumatriptan) migraine patch. FDAhttps://www.fda.gov/drugs/drug-safety-and-availability/fda-evaluating-risk-burns-and-scars-zecuity-sumatriptan-migraine-patch (2020).
- 295.Loder EW, Rayhill M, Burch RC. Safety problems with a transdermal patch for migraine: lessons from the development, approval, and marketing process. Headache. 2018;58:1639–1657. doi: 10.1111/head.13424. [DOI] [PubMed] [Google Scholar]