Abstract
Background
It is unclear whether hepatocyte function and/or portal hypertension improves if a sustained virologic response (SVR) is achieved with direct-acting antivirals in patients with decompensated hepatitis C-related cirrhosis.
Methods
We examined the safety and efficacy of a 12-week course of sofosbuvir/velpatasvir (SOF/VEL) in 20 patients with decompensated hepatitis C-related cirrhosis. We also investigated changes in the hepatocyte receptor index (LHL15) and blood clearance index (HH15) by Tc-99 m-galactosyl human serum albumin scintigraphy, liver stiffness measurement (LSM) by transient elastography, and hepatic venous pressure gradient (HVPG) in patients who achieved an SVR at 24 weeks after treatment (SVR24).
Results
One patient discontinued treatment because of rectal variceal hemorrhage, and 19 patients completed treatment. SVR24 was achieved in 17 patients (89%). Median LHL15 increased from 0.72 pre-treatment to 0.82 after SVR24 (p = 0.012), and median HH15 decreased from 0.82 pre-treatment to 0.76 after SVR24 (p = 0.010). The percentage of patients with LSM ≥ 20 kPa was 90% before treatment and remained at 90% after SVR24. However, the percentage with severe portal hypertension (defined as HVPG ≥ 12 mmHg) decreased from 92% pre-treatment to 58% after SVR24 (p = 0.046). Patients with a decreased HVPG from pre-treatment to after SVR24 had a smaller pre-treatment spleen volume than those with an increased HVPG (median, 252 vs. 537 mL, p = 0.028).
Conclusion
Achieving SVR24 with SOF/VEL treatment in patients with decompensated hepatitis C-related cirrhosis can be expected to improve hepatocyte function and portal hypertension on short-term follow-up.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00535-023-01963-2.
Keywords: Hepatitis C, Liver cirrhosis, Portal hypertension, Sofosbuvir, Velpatasvir
Introduction
It has become clear that a high sustained virologic response (SVR) rate can be achieved even in patients with cirrhosis owing to the recent development of direct-acting antivirals (DAAs) for hepatitis C virus (HCV). In patients with compensated cirrhosis who achieve SVR, decompensation events, such as ascites, variceal exacerbation, or hepatic encephalopathy, are suppressed by improving hepatic functional reserve [1–4]. Additionally, several reports have indicated that improvement of hepatic fibrosis, portal hypertension, and prognosis can be expected in patients with compensated cirrhosis [5–8].
In 2019, sofosbuvir/velpatasvir (SOF/VEL) treatment was approved in Japan for patients with decompensated HCV-related cirrhosis. Sofosbuvir is a nonstructural protein 5B (NS5B) polymerase inhibitor, and velpatasvir is a new-generation nonstructural protein 5A (NS5A) inhibitor. Guidelines from the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and Japan Society of Hepatology (JSH) have recommended the use of fixed-dose combination therapy of these two DAAs for patients with decompensated cirrhosis [9–11]. In a phase 3 trial evaluating the efficacy of SOF/VEL treatment for HCV-infected patients with decompensated cirrhosis, the SVR rate was 92%, indicating that a high SVR rate can be expected, similar to that observed in patients with compensated cirrhosis [12]. When SVR is achieved in patients with decompensated cirrhosis, serum albumin levels increase, and an improved prognosis is expected [12, 13]. However, there is no consensus on whether hepatocyte function and/or portal hypertension improve when SVR is achieved with DAA treatment in patients with decompensated cirrhosis.
The recent Baveno VII workshop emphasized the importance of personalized care for portal hypertension, as the various disease stages are associated with differing outcomes, including the risk of death [14]. At Baveno VII, the definition and prevention of further decompensation in patients with decompensated cirrhosis were newly specified. Cirrhosis recompensation was also defined. This concept reflects at least partial regression of the structural and functional changes of cirrhosis after removing the cause of cirrhosis and is defined by the presence of three criteria: (1) removal of the primary etiology of cirrhosis, (2) resolution of ascites and encephalopathy and an absence of recurrent variceal hemorrhage, and (3) stable improvement of liver function tests. In patients with decompensated HCV-related cirrhosis, it is important to clarify the amount of recompensation achieved by eliminating HCV with DAA treatment.
In this study, we investigated changes in hepatocyte function and portal hypertension in patients with HCV-related decompensated cirrhosis before SOF/VEL treatment and after achieving SVR at 24 weeks after completing treatment (SVR24) in an attempt to clarify short-term outcomes. Our assessments included evaluation of hepatocyte receptor index (LHL15) and blood clearance index (HH15) by Tc-99 m-galactosyl human serum albumin (GSA) scintigraphy, liver stiffness measurement (LSM) by transient elastography (TE) (FibroScan®; Echosens, Paris, France), and hepatic venous pressure gradient (HVPG).
Materials and methods
Patients and study design
This is an observational study of patients with decompensated HCV-related cirrhosis who initiated SOF/VEL treatment at our institution. The patients underwent laboratory tests, computed tomography (CT), Tc-99 m-GSA scintigraphy, TE, and HVPG measurements within 3 months before SOF/VEL therapy. SOF/VEL (400/100 mg) fixed-dose combination treatment (EPUCLUSA®; Gilead Sciences, Foster City, CA, USA) was administered once daily for 12 weeks. In patients who achieved SVR24, we performed repeat laboratory tests, CT, Tc-99 m-GSA scintigraphy, TE, and HVPG measurements.
Portosystemic shunt diameter, liver volume, spleen volume, and liver-to-spleen volume ratio (L/S ratio) were measured based on pre-treatment CT data using SYNAPSE VINCENT, version 4.1, volume analyzer software (Fujifilm Medical Co., Ltd., Tokyo, Japan). HCV genotypes were identified using an HCV genotype primer kit (Institute of Immunology Co., Ltd., Tokyo, Japan), and serum HCV RNA was measured using the TaqMan HCV assay (COBAS TaqMan assay, Roche Molecular Diagnostics, Tokyo, Japan), with a lower limit of quantification of 15 IU/mL [15].
Written informed consent was obtained from all patients prior to inclusion in this study. The study was conducted in accordance with the ethical guidelines of the 1964 Declaration of Helsinki (2013 revision) and with the approval of our institution’s ethics committee (approval no. 4292).
Measurement of Tc-99 m-GSA scintigraphy
With the patients in the supine position, 185 MBq of Tc-99 m-GSA (Nihon Medi-Physics Co. Ltd., Tokyo, Japan) was injected through a cubital vein, and planar images were dynamically acquired in frames of 60 s, beginning immediately after injection and continuing for 20 min. A scintillation camera (Brightview X system; Philips Medical Systems Inc., Cleveland, OH, USA) was used to generate time-activity curves. This camera had a low-energy, high-resolution collimator and was interfaced with an extended brilliance workspace-nuclear medicine workstation. The images were constructed and analyzed using asialo analysis of the interface definition language sample application software, version 2.0.1, supplied by the Hitachi Medical Corporation (Tokyo, Japan). Time–activity curves were drawn with regions of interest set in the liver and heart on the 20-min integrated images. According to the previously reported method [16, 17], LHL15 and HH15 were determined as follows: LHL15, liver uptake of radiotracer at 15 min/(liver plus heart uptake of radiotracer at 15 min); and HH15, heart pooling of radiotracer at 15 min/heart pooling of radiotracer at 3 min.
Measurement of HVPG
A 5-French cobra-shaped Selecon MP balloon catheter (Terumo Clinical Supply Co., Ltd., Gifu, Japan) was inserted via the jugular vein or antecubital vein into the hepatic vein, and the mean free hepatic venous pressure (FHVP) was measured. The balloon was then inflated, and the average wedged hepatic venous pressure (WHVP) was determined. Both FHVP and WHVP were measured three times, and the mean values were calculated. HVPG was calculated as mean WHVP minus mean FHVP [18]. HVPG measurements were performed safely in all patients.
Statistical analysis
JMP 11.0.0 statistical software (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Continuous variables were expressed as median (interquartile range). The Mann–Whitney U test was used to compare continuous variables between 2 groups, while Fisher’s exact test or Chi-squared test was used to compare categorical variables. Correlations between continuous variables were analyzed using Spearman’s rank correlation coefficient (ρ). The Wilcoxon signed-rank test was used to compare continuous variables before and after SOF/VEL treatment, while the Bowker test was used to compare categorical variables before and after SOF/VEL treatment. p values < 0.05 were considered statistically significant.
Results
Baseline characteristics
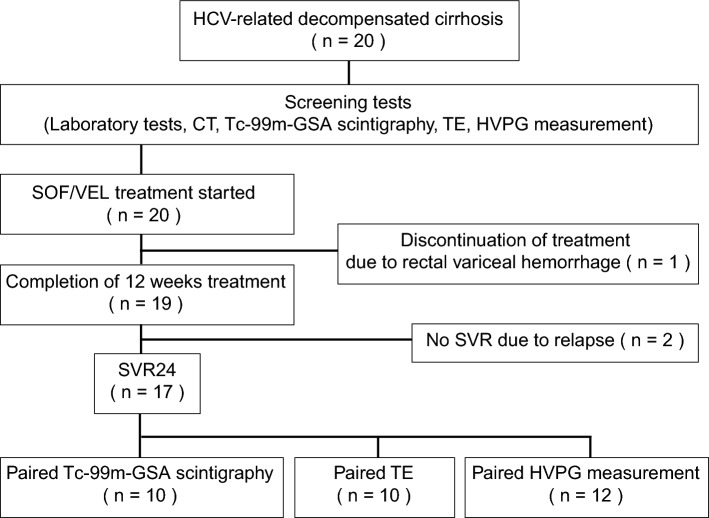
Between January 2019 and March 2021, 20 patients with decompensated HCV-related cirrhosis were enrolled and started SOF/VEL treatment. A total of 19 patients completed treatment and were observed for at least 24 weeks after the end of treatment. Because of the invasiveness of the procedures, radiation exposure, and/or retention of massive ascites, paired (before and after treatment) assessments were not performed in all patients who achieved SVR, but they were available as follows: scintigraphy, 10 patients; TE, 10 patients; and HVPG measurements, 12 patients (Fig. 1).
Fig. 1.
Study flowchart. HCV hepatitis C virus, CT computed tomography, Tc-99 m-GSA technetium-99 m-galactosyl human serum albumin, TE transient elastography, HVPG hepatic venous pressure gradient, SOF sofosbuvir, VEL velpatasvir, SVR24 sustained virologic response at 24 weeks after treatment
The clinical characteristics of all patients are summarized in Table 1. The median age was 63 years, and there were 13 males and 7 females. A total of 16 patients (80%) had esophageal varices, and 10 patients (50%) had a history of hepatocellular carcinoma (HCC). The HCV genotype was 1b in 15 patients (75%), 2a in 2 patients (10%), and 2b in 3 patients (15%). The Child–Pugh–Turcotte (CPT) category was grade B in 17 patients (85%), grade C in 3 patients (15%), and grade A in 0 patients. The median LHL15 (reflecting hepatocyte function) was 0.69, median LSM (reflecting liver stiffness) was 37 kPa, and median HVPG (reflecting portal pressure) was 17 mmHg.
Table 1.
Baseline characteristics of all patients (n = 20)
| Characteristic | Value |
|---|---|
| Sex (male/female) | 13/7 |
| Age (y) | 63 (60–72) |
| Esophageal varices (yes/no) | 16/4 |
| History of HCC (yes/no) | 10/10 |
| HCV RNA (log10 IU/mL) | 6.0 (5.7–6.2) |
| HCV genotype (1b/2a/2b) | 15/2/3 |
| CPT score | 8 (7–9) |
| CPT grade (A/B/C) | 0/17/3 |
| ALBI score | − 1.39 (− 1.11 to − 1.78) |
| ALBI grade (1/2/3) | 0/10/10 |
| LHL15 | 0.69 (0.60–0.74) |
| HH15 | 0.83 (0.79–0.85) |
| LSM (kPa) | 37 (27–45) |
| HVPG (mmHg) | 17 (13–19) |
Data are shown as numbers or median (interquartile range)
HCC hepatocellular carcinoma, HCV hepatitis C virus, CPT Child–Pugh–Turcotte, ALBI albumin–bilirubin, LHL15 hepatocyte receptor index, HH15 blood clearance index, LSM liver stiffness measurement, HVPG hepatic venous pressure gradient
Safety and efficacy of SOF/VEL treatment
During SOF/VEL treatment, the following adverse events were observed: ascites or edema in seven patients (35%), elevated serum ammonia level in three patients (15%), exacerbation or rupture of esophago-gastrointestinal varices in three patients (15%), portal thrombosis in one patient (5%), and leg cramps in one patient (5%). However, all of these events were determined to be caused by decompensated cirrhosis, not by SOF/VEL treatment. One patient discontinued treatment because of rectal variceal hemorrhage, and 19 patients completed the 12-week course of treatment. SVR24 was achieved in 17 patients (89%) (Fig. 1).
Changes in laboratory tests and endoscopy results
Serum aspartate aminotransferase (AST) (p < 0.001), alanine aminotransferase (ALT) (p < 0.001), albumin (p = 0.001), prothrombin time–international normalized ratio (p = 0.018), CPT score (p = 0.049), albumin–bilirubin (ALBI) score (p = 0.002), type 4 collagen 7S domain (p = 0.006), and mac-2 binding protein glycosylation isomer (M2BPGi) (p < 0.001) improved at SVR24, compared with pre-treatment. No patient had CPT grade A before SOF/VEL treatment, but ten patients (59%) improved to CPT grade A at SVR24 (p = 0.019) (Table 2). Of the 14 patients taking diuretics pre-treatment, 8 (57%) were able to reduce the dose of diuretics after treatment because of improved ascites or edema. In the 15 patients with esophageal varices pre-treatment, follow-up esophagogastroduodenoscopy revealed improved varices in 5 (33%), no change in varices in 5 (33%), and worsened variceal morphology in 3 (20%); 2 patients (13%) did not undergo esophagogastroduodenoscopy after viral elimination.
Table 2.
Changes in laboratory tests from before SOF/VEL treatment to after SVR24 (n = 17)
| Test | Before treatment | SVR24 | p |
|---|---|---|---|
| AST (U/L) | 47 (38–69) | 28 (25–33) | < 0.001 |
| ALT (U/L) | 34 (18–46) | 14 (11–19) | < 0.001 |
| Albumin (g/dL) | 2.8 (2.3–3.1) | 3.4 (2.7–4.0) | 0.001 |
| Total bilirubin (mg/dL) | 1.5 (1.0–2.2) | 1.3 (0.8–2.0) | 0.316 |
| PT–INR | 1.21 (1.14–1.30) | 1.15 (1.09–1.22) | 0.018 |
| Esophageal varices (yes/no/NA) | 15/2/0 | 13/1/3 | 0.392 |
| NH3 (μg/dL) | 75 (51–109) | 74 (53–89) | 0.424 |
| Ascites (no/mild/moderate) | 9/4/4 | 10/5/2 | 0.572 |
| Platelets (× 109/L) | 68 (57–108) | 79 (40–112) | 0.669 |
| CPT score | 8 (7–9) | 6 (6–9) | 0.049 |
| CPT grade (A/B/C) | 0/14/3 | 10/6/1 | 0.019 |
| ALBI score | − 1.29 (− 1.04 to − 1.82) | − 2.03 (− 1.35 to − 2.45) | 0.002 |
| ALBI grade (1/2/3) | 0/8/9 | 3/10/4 | 0.123 |
| MELD score | 10 (9–13) | 9 (8–12) | 0.211 |
| Type 4 collagen 7S (ng/mL) | 14.4 (8.8–15.7) | 9.2 (7.8–12.5) | 0.006 |
| M2BPGi (C.O.I) | 14.0 (10.2–16.2) | 7.7 (4.2–12.1) | < 0.001 |
| FIB-4 index | 7.06 (5.85–11.73) | 6.27 (4.06–10.30) | 0.057 |
Data are shown as number and median (interquartile range) and were compared using the Bowker test or Wilcoxon signed-rank test or test, respectively
SOF sofosbuvir, VEL velpatasvir, SVR sustained virologic response, AST aspartate aminotransferase, ALT alanine aminotransferase, PT prothrombin time, INR international normalized ratio, NA not assessed, NH3 ammonia, CPT Child–Pugh–Turcotte, ALBI albumin–bilirubin, MELD model for end-stage liver disease, M2BPGi mac-2 binding protein glycosylation isomer
Changes in Tc-99 m-GSA scintigraphy, LSM, and HVPG
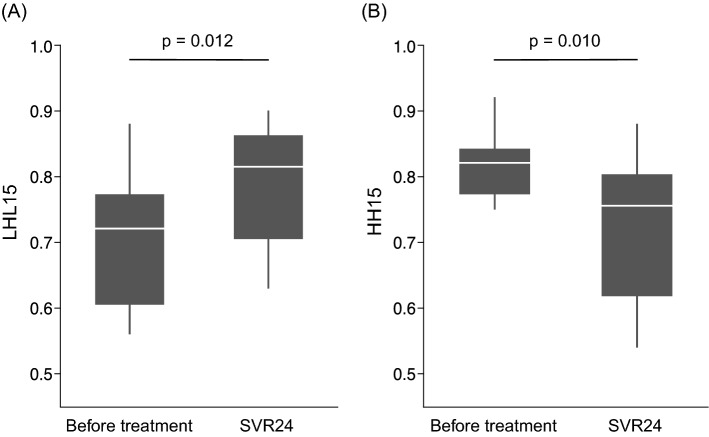
Both LHL15 and HH15 improved in nine patients (90%) and did not improve in one patient (10%) after SVR24, compared with pre-treatment. Median LHL15 increased from 0.72 pre-treatment to 0.82 after SVR24 (p = 0.012), and median HH15 decreased from 0.82 before treatment to 0.76 after SVR24 (p = 0.010) (Fig. 2). The median percentage increase in LHL15 was 14%, and the median percentage decrease in HH15 was 9%. The median percentage increase in LHL15 did not differ between patients with CPT grade B and grade C disease (p = 0.602) or between patients with and without a history of HCC (p = 0.347). Similarly, the median percentage decrease in HH15 did not differ between patients with CPT grade B and grade C disease (p = 0.794) or between patients with and without a history of HCC (p = 0.917). A representative case in which hepatocyte function improved is shown in Fig. 3, and a patient with no improvement in hepatocyte function is described in Supplementary Fig. 1.
Fig. 2.
Changes in Tc-99 m-galactosyl human serum albumin scintigraphy data before sofosbuvir/velpatasvir treatment and after sustained virologic response at 24 weeks post-treatment (SVR24). Hepatocyte receptor index (LHL15) increased from 0.72 (0.61–0.77) to 0.82 (0.71–0.86) (p = 0.012) (a), and blood clearance index (HH15) decreased from 0.82 (0.77–0.84) to 0.76 (0.62–0.80) (p = 0.010) (b), after SVR24 compared with before treatment
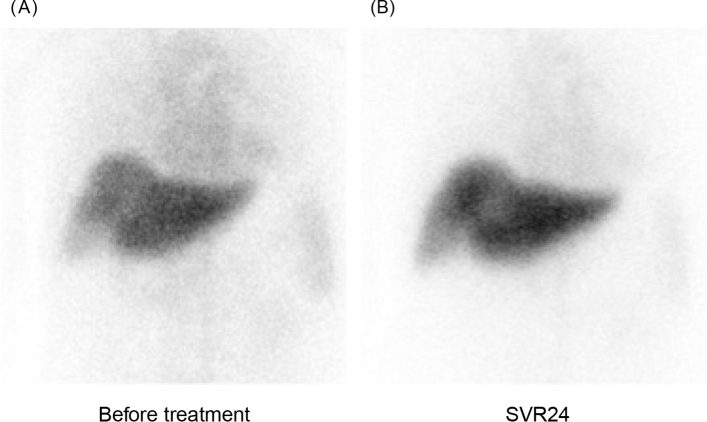
Fig. 3.
Tc-99 m-galactosyl human serum albumin scintigraphic images obtained from a 65-year-old man with genotype 2b infection before sofosbuvir/velpatasvir treatment and after sustained virologic response at 24 weeks post-treatment (SVR24). Before treatment, the planar image shows weak accumulation of radiotracer in the liver and enhanced pooling in the heart; hepatocyte receptor index (LHL15) and blood clearance index (HH15) were 0.70 and 0.78, respectively (a). After SVR24, liver accumulation of radiotracer increased and cardiac pooling decreased; LHL15 and HH15 improved to 0.85 and 0.63, respectively (b). The patient’s hepatic functional reserve improved from Child–Pugh-Turcotte grade B to grade A after SVR24
LSM decreased in 6 patients (60%) and increased in 4 patients (40%) after SVR24, compared with pre-treatment. Baseline characteristics of the decreased LSM group and the increased LSM group are shown in Supplementary Table 1. Median LSM decreased from 35 kPa pre-treatment to 27 kPa after SVR24, but this decrease was not significant (p = 0.719) (Supplementary Fig. 2). The median percentage decrease in LSM was 12%. The percentage of patients with an LSM ≥ 20 kPa (defined as the cut-off value for risk of having endoscopic signs of portal hypertension according to the Baveno VI criteria [19]) was 90% before treatment and remained at 90% after SVR24 (Supplementary Fig. 3).
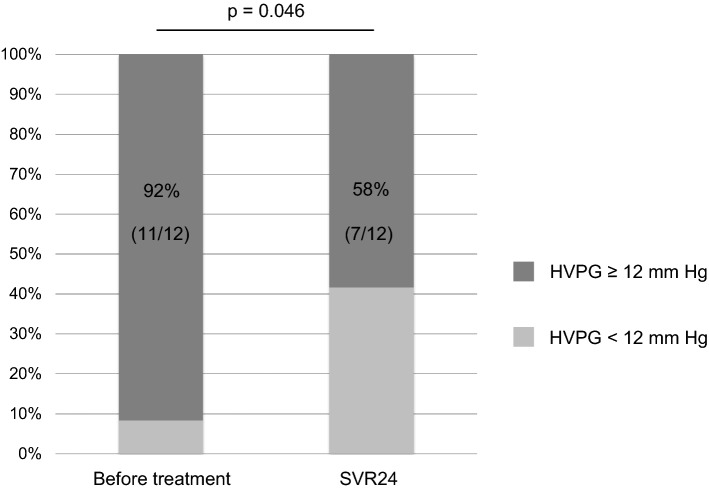
HVPG decreased in eight patients (67%) after SVR24, compared with pre-treatment. The decrease was ≥ 10% in all eight patients and ≥ 20% in five patients (42%). However, HVPG increased in four patients (33%) after SVR24, compared with pre-treatment. Median HVPG decreased from 18 mmHg pre-treatment to 15 mmHg after SVR24 (p = 0.119) (Supplementary Fig. 4), and the median percentage decrease in HVPG was 15%. The median percentage decrease in HVPG did not differ between patients with CPT grade B and grade C disease (p = 0.667) or between patients with and without a history of HCC (p = 0.223). The percentage of patients with clinically significant portal hypertension (CSPH), defined as HVPG ≥ 10 mmHg, was 92% before treatment and decreased to 75% after SVR24 (p = 0.157) (Supplementary Fig. 5). However, these results were not statistically significant. Conversely, the percentage of patients with severe portal hypertension, defined as HVPG ≥ 12 mmHg, was 92% before treatment and decreased significantly to 58% after SVR24 (p = 0.046) (Fig. 4).
Fig. 4.
Changes in percentages of patients with severe portal hypertension, defined as hepatic venous pressure gradient (HVPG) ≥ 12 mmHg, before sofosbuvir/velpatasvir treatment and after sustained virologic response at 24 weeks post-treatment (SVR24). The percentage was 92% (11/12) before treatment and significantly decreased to 58% (7/12) after SVR24 (p = 0.046)
Relationship between CT findings and changes in liver function and HVPG
We also assessed the association between pre-treatment CT findings and post-treatment changes in liver function and HVPG. The median portosystemic shunt diameter was 7 mm at baseline. There was no difference in portosystemic shunt diameter between the improved CPT score group and the unchanged or worsened CPT score group (median, 8 vs. 6 mm, p = 0.337). There was also no correlation between shunt diameter and the degree of increase in LHL15 (ρ = 0.328, p = 0.355) or decrease in HH15 (ρ = 0.065, p = 0.859). Similarly, there was no correlation between shunt diameter and pre-treatment HVPG (ρ = 0.066, p = 0.800) or the degree of decrease in HVPG (ρ = 0.155, p = 0.630).
The decreased HVPG group had a smaller spleen volume (median, 252 vs. 537 mL, p = 0.028) and larger L/S ratio (median, 4.5 vs. 1.8, p = 0.028) at baseline than the increased HVPG group. However, there were no differences in pre-treatment CPT score, ALBI score, LHL15, HH15, LSM, and HVPG between the decreased and increased HVPG groups (Table 3).
Table 3.
Baseline characteristics of the decreased HVPG group and increased HVPG group (n = 12)
| Characteristic | Decreased HVPG (n = 8) | Increased HVPG (n = 4) | p |
|---|---|---|---|
| CPT score | 8 (7–9) | 7 (7–9) | 0.413 |
| ALBI score | − 1.55 (− 1.19 to − 2.01) | − 1.25 (− 0.77 to − 1.60) | 0.235 |
| MELD score | 9 (8–12) | 11 (10–13) | 0.120 |
| Type 4 collagen 7S (ng/mL) | 11.7 (8.5–15.8) | 15.0 (14.4–16.0) | 0.235 |
| M2BPGi (C.O.I) | 13.1 (5.8–18.5) | 14.0 (9.6–17.4) | 0.734 |
| FIB-4 index | 6.60 (4.79–10.81) | 10.89 (10.07–13.55) | 0.126 |
| PSS diameter (mm) | 8 (6–9) | 7 (5–13) | 0.798 |
| Liver volume (mL) | 1044 (864–1193) | 997 (858–1247) | 0.831 |
| Spleen volume (mL) | 252 (183–343) | 537 (403–1037) | 0.028 |
| L/S ratio | 4.5 (2.7–6.0) | 1.8 (1.2–2.4) | 0.028 |
| LHL 15 | 0.72 (0.66–0.80) | 0.68 (0.60–0.77) | 0.748 |
| HH15 | 0.81 (0.73–0.85) | 0.83 (0.77–0.85) | 0.669 |
| LSM (kPa) | 30 (23–39) | 36 (33–43) | 0.364 |
| HVPG (mmHg) | 18 (14–20) | 18 (15–21) | 0.798 |
Data are shown as median (interquartile range) and were compared using the Mann–Whitney U test
HVPG hepatic venous pressure gradient, CPT Child–Pugh–Turcotte, ALBI albumin–bilirubin, MELD model for end-stage liver disease, M2BPGi mac-2 binding protein glycosylation isomer, PSS portosystemic shunt, L/S ratio liver-to-spleen volume ratio, LHL15 hepatocyte receptor index, HH15 blood clearance index, LSM liver stiffness measurement
Median spleen size changed from 365 mL before treatment to 332 mL after SVR24 (p = 0.132), and median L/S ratio changed from 2.5 at pre-treatment to 2.8 after SVR24 (p = 0.244), although these changes were not statistically significant. There was no correlation between change in spleen size and change in HVPG from before to after treatment (ρ = –0.424, p = 0.194).
Discussion
Management of decompensated cirrhosis focuses on preventing cirrhosis progression. The most important strategy involves suppression of the etiologic factors that have caused liver inflammation and subsequent fibrosis [20]. In patients with HCV-related decompensated cirrhosis, SVR is required to reduce liver inflammation and improve liver function. Several studies have been previously published regarding the efficacy and outcomes of SOF/VEL treatment in patients with decompensated cirrhosis [21–23]. While high SVR rates have been reported with DAA treatment for decompensated cirrhosis, the effects of SVR on hepatocyte function and portal hypertension have not been heretofore studied in detail [12, 13]. This is the first study reporting hepatocyte function before and after DAA treatment using Tc-99 m-GSA scintigraphy in patients with HCV-related cirrhosis. Furthermore, since we targeted only patients with decompensated cirrhosis (CPT grades B or C), this study evaluated changes in LSM and HVPG before and after treatment in patients with more advanced liver disease than those included in previous studies [24–28].
Regarding the efficacy of SOF/VEL treatment for patients with decompensated HCV-related cirrhosis, SVR12 rates were 83% to 92% in a phase 3 study [12, 29] and 90% to 93% in real-world data [13, 30]. In our study, the SVR24 rate was 89%, which was comparable to these previous results. Prior studies reported that adverse events associated with SOF/VEL treatment were nonspecific, such as fatigue, headache, nausea, and appetite loss [29, 30]. In this study, ascites, edema, elevated serum ammonia level, and exacerbation of esophago-gastrointestinal varices were observed during SOF/VEL treatment, but these adverse events are often experienced during cirrhosis decompensation itself. They can be managed by implementing usual cirrhosis treatment and do not require SOF/VEL-specific treatment. Our results, therefore, suggest that it is safe to administer SOF/VEL, even in patients with decompensated cirrhosis.
It is well known that antiviral treatment improves liver function indices, such as serum albumin, CPT score, and ALBI score, but it is necessary to verify whether this is due to improvement of hepatocyte function or the effects of other factors, such as improved nutrition, biliary stenosis, or cholestasis. Tc-99 m-GSA scintigraphy results directly reflect hepatocyte function and are less likely to be affected by extrahepatic factors since GSA specifically binds to asialoglycoprotein receptors (ASGPRs) on the surface of each hepatocyte [16, 17]. Kira et al. found that patients with chronic hepatitis C treated with interferon-α exhibited improved Tc-99 m-GSA scintigraphy data after SVR [31]. Similarly, Ishii et al. described a patient with chronic hepatitis C whose Tc-99 m-GSA scintigraphy results improved with low-dose long-term interferon-α treatment [32]. No previously published study used Tc-99 m-GSA scintigraphy to evaluate hepatocyte function in patients with hepatitis C treated with DAAs. We found that SOF/VEL improved both LHL15 and HH15, indicating that this combination treatment improves hepatocyte function in the short term, even in patients with decompensated cirrhosis. ASGPRs may play a role in this improved hepatocyte function. ASGPRs are expressed in a polar manner on the sinusoidal and basolateral surfaces of the hepatocyte plasma membrane [33, 34]. Expression of ASGPRs is altered by various cytokines, and ASGPR expression on the sinusoidal surface is decreased in cirrhosis [35, 36]. Suppression of hepatitis by DAA treatment may reduce inflammatory cytokines and preserve ASGPR function, thereby restoring the differentiation and proliferation potential of residual hepatocytes and improving the ability to synthesize albumin, prothrombin, and other substances. Decompensated cirrhosis often develops in patients with esophagogastric varices and/or HCC, and effective treatment for these patients can be indicated if hepatic function is preserved. Although longer observation periods are required to assess the impact of improved hepatocyte function on longer-term outcomes of patients with HCV-related decompensated cirrhosis, SOF/VEL treatment should be considered if esophagogastric varices and/or HCC are controlled.
We found that AST, ALT, albumin, CPT score, and ALBI score improved after SVR24, as in previous reports [12, 13, 30], and that liver fibrosis markers, such as type 4 collagen 7S and M2BPGi, also improved. Serum markers for liver fibrosis have been previously reported to improve with achieving SVR in chronic hepatitis and compensated cirrhosis [37–39]. However, fibrosis markers elevate because of not only liver fibrosis but also liver inflammation [39–41]. Poordad et al. reported that 71% of patients with cirrhosis had a histologically proven downgraded METAVIR score at 52 weeks after DAA treatment [42]. In contrast, we previously reported that decreased serum type 4 collagen 7S after SVR was related to an improved liver inflammatory response, while no significant regression of histologic fibrosis stage was noted at 41 ± 20 weeks after DAA treatment in patients with chronic hepatitis C [43]. Our current results also showed that LSM did not improve at SVR24. Taken together, these results suggest that regression of liver fibrosis is limited or absent in decompensated cirrhosis in the short term, although we did not examine liver histology to confirm this.
Previous studies have shown that HVPG decreases when SVR is achieved in patients with HCV-related cirrhosis [24–28]. Mandorfer et al. analyzed 60 patients with cirrhosis treated with DAAs and found that HVPG decreased significantly from 13.1 mmHg pre-treatment to 10.4 mmHg after SVR, but the amount of HVPG decrease was small in patients with CPT stage B disease [26]. In their study of 226 patients with CSPH, Lens et al. found that the median HVPG decreased from 15 mmHg at baseline to 13 mmHg at SVR24, but CSPH persisted in the majority of patients at this time [27], as well as in a follow-up study conducted 96 weeks after treatment [28]. In those studies, the proportion of patients with CPT grade A cirrhosis was high. In contrast, all patients in our study had CPT grade B or C decompensated cirrhosis, so baseline HVPG was high (17 mmHg). CSPH represents the risk of clinical decompensation [44], and HVPG ≥ 12 mmHg is the cut-off value associated with a high risk of variceal bleeding and other complications of portal hypertension [45, 46]. In addition, a reduction in HVPG to ≤ 12 mmHg or by ≥ 20% from baseline was previously shown to be protective against the development of variceal bleeding and associated with a decreased incidence of decompensation [47, 48]. In our study, 42% of patients exhibited a decrease in HVPG by ≥ 20%, and the percentage of patients with severe portal hypertension (HVPG ≥ 12 mmHg) decreased significantly from 92% before treatment to 58% after SVR. However, 75% of our study cohort of patients with CPT grade B or C cirrhosis continued to have CSPH after SVR24, which was similar to the results reported by Lens et al. whose study also included CPT grade A patients [27]. Thus, our results indicate that careful follow-up is required to monitor for the development of liver-related complications, regardless of whether SVR is achieved.
In patients with compensated cirrhosis, Tsuji et al. found that larger-diameter portosystemic shunts were associated with the development of portal hypertension-related events and impaired improvement of the liver function after DAA treatment [49]. Similarly, in 72 patients with decompensated cirrhosis treated with SOF/VEL, Takaoka et al. reported that a large portosystemic shunt (diameter ≥ 6 mm) was associated with less improvement in CPT scores [50]. In the current study, we identified no association between portosystemic shunt diameter and improvement in CPT scores, Tc-99 m-GSA scintigraphy results, or HVPG. Nevertheless, we did find that patients with a decreased HVPG after SVR24 had a smaller pre-treatment spleen volume. When the spleen is large, splenic arterial blood flow increases, resulting in increased splenic venous flow and worsened portal hypertension [51]. However, we found no association between a decreased HVPG and the pre-treatment HVPG value. One explanation may be that splenic venous flow secondary to splenomegaly does not decrease at only 24 weeks after completing treatment, so a reduction in HVPG would not be observed. Another possible explanation is that, like vascular endothelial cells, the spleen produces endothelin-1, which activates hepatic stellate cells and generates constriction of hepatic sinusoids, resulting in increased intrahepatic vascular resistance [52]. Recovery of hepatocyte function with SOF/VEL treatment may improve the hepatic microcirculatory environment, but HVPG may not decrease in patients with splenomegaly possibly because of high amounts of endothelin-1 produced by the enlarged spleen.
This study has several limitations. First, it was conducted with an observational design at a single center, so the data may be heterogeneous or insufficient. Second, the statistical power of this study may be low, and selection bias cannot be excluded because the number of patients enrolled was small, and not all patients underwent paired (pre- and post-treatment) tests. Furthermore, multivariate analyses could not be performed because of the small number of patients; therefore, it is difficult to assess the role of potential confounding factors. Third, the observation period of this study was short because data were assessed at SVR24, so the effects of SOF/VEL treatment on longer-term prognosis with regard to liver-related complications and survival are unclear. Further validation studies with a larger cohort are required to resolve these concerns in the future.
In conclusion, a 12-week course of SOF/VEL treatment for decompensated cirrhosis can eliminate HCV at a high SVR, although patients must be closely monitored for the development of complications of cirrhosis. Our results also suggest that SOF/VEL treatment in patients with decompensated HCV-related cirrhosis may restore hepatocyte function and reduce the proportion of patients with severe portal hypertension. However, the effects of SOF/VEL treatment on prognosis in terms of liver-related complications and survival requires further studies with longer-term follow-up.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 A 72-year-old man with hepatitis C genotype 1b infection whose hepatocyte function did not improve. His pre-treatment data were as follows: Child-Pugh-Turcotte (CPT) score, 7; hepatocyte receptor index (LHL15), 0.78; blood clearance index (HH15), 0.75; liver stiffness measurement (LSM), 43 kPa; and hepatic venous pressure gradient (HVPG), 17 mmHg. Before treatment, the planar image of Tc-99m-galactosyl human serum albumin (GSA) scintigraphy showed weak accumulation of radiotracer in the liver and enhanced pooling in the heart (a). After sustained virologic response at 24 weeks post-treatment (SVR24), all of the abovementioned values were worse: CPT score, 9; LHL15, 0.73; HH15, 0.79; LSM, 75 kPa; and HVPG, 22 mmHg. Accumulation of radiotracer in the liver remained weak, and pooling of tracer in the heart did not decrease (b). CT performed after SVR24 showed recurrent hepatocellular carcinoma lesions in the right lobe, which were enhanced in the arterial phase and washed out in the late phase (arrow) (c) (PPTX 425 KB)
Supplementary file2 Liver stiffness measurement (LSM) was 35 (27–39) kPa before sofosbuvir/velpatasvir treatment and decreased to 27 (21–44) kPa after sustained virologic response at 24 weeks post-treatment (SVR24). This decrease was not statistically significant (p=0.719) (PPTX 73 KB)
Supplementary file3 Percentage of patients with a liver stiffness measurement (LSM) ≥ 20 kPa was 90% (9/10) before sofosbuvir/velpatasvir treatment and remained at 90% (9/10) after sustained virologic response at 24 weeks post-treatment (SVR24) (p=1.000) (PPTX 60 KB)
Supplementary file4 Hepatic venous pressure gradient (HVPG) was 18 (15–20) mmHg before sofosbuvir/velpatasvir treatment and decreased to 15 (9–22) mmHg after sustained virologic response at 24 weeks post-treatment (SVR24). However, this decrease was not statistically significant (p=0.119) (PPTX 76 KB)
Supplementary file5 Percentage of patients with clinically significant portal hypertension (CSPH), defined as a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, was 92% (11/12) before sofosbuvir/velpatasvir treatment and decreased to 75% (9/12) after sustained virologic response at 24 weeks post-treatment (SVR24). However, the decrease was not statistically significant (p=0.157) (PPTX 60 KB)
Acknowledgements
The authors sincerely thank Ms. Sanae Deguchi, Ayano Fujikawa, and Rie Yasuda for their assistance with data collection, and the staff in the department of Interventional Radiology and Nuclear Medicine in our institution for their cooperation. The authors also thank BioScienceWriters (https://www.bioscience writers.com/) for English language editing.
Author contributions
KK designed the study, collected and analyzed data, and wrote the manuscript. ME, SUK, and AT were involved in the clinical data analysis and data interpretation. YYM, NO, HM, RK, EK, AH, and HF were involved in patient enrollment. KK and AY performed hepatic venous pressure gradient measurements. AY, SH, and JK performed Tc-99 m-galactosyl human serum albumin scintigraphy and data processing. NK supervised the study and edited the manuscript.
Funding
This study was partially supported by Japan Society for the Promotion of Science KAKENHI Grant Number JP20K08338 and a Research Grant from the Japan Agency for Medical Research and Development (no. JP22fk0210113).
Declarations
Conflict of interest
NK received lecture fees and research funding from Gilead Sciences, AbbVie, and MSD, and a scholarship donation from AbbVie. AT received research funding from Gilead Sciences. HF received research funding from MSD. SUK received research funding from Bristol-Myers and MSD, and scholarship donation from AbbVie. All other authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ogawa E, Kawano A, Ooho A, et al. Long-term hepatic function of patients with compensated cirrhosis following successful direct-acting antiviral treatment for hepatitis C virus infection. J Gastroenterol Hepatol. 2022;37:371–377. doi: 10.1111/jgh.15703. [DOI] [PubMed] [Google Scholar]
- 2.Nahon P, Bourcier V, Layese R, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152:142–56.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SA, Pollock KG, Barclay ST, et al. Real-world impact following initiation of interferon-free hepatitis C regimens on liver-related outcomes and all-cause mortality among patients with compensated cirrhosis. J Viral Hepat. 2020;27:270–280. doi: 10.1111/jvh.13232. [DOI] [PubMed] [Google Scholar]
- 4.Krassenburg LAP, Maan R, Ramji A, et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053–1063. doi: 10.1016/j.jhep.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Everson GT, Balart L, Lee SS, et al. Histological benefits of virological response to peginterferon alfa-2a monotherapy in patients with hepatitis C and advanced fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2008;27:542–551. doi: 10.1111/j.1365-2036.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwabl P, Mandorfer M, Steiner S, et al. Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment Pharmacol Ther. 2017;45:139–149. doi: 10.1111/apt.13844. [DOI] [PubMed] [Google Scholar]
- 7.Rincon D, Ripoll C, Lo lacono O, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–2274. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagaoki Y, Imamura M, Teraoka Y, et al. Impact of viral eradication by direct-acting antivirals on the risk of hepatocellular carcinoma development, prognosis, and portal hypertension in hepatitis C virus-related compensated cirrhosis patients. Hepatol Res. 2020;50:1222–1233. doi: 10.1111/hepr.13554. [DOI] [PubMed] [Google Scholar]
- 9.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org. Accessed 29 Jul 2022
- 10.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology Japan society of hepatology guidelines for the management of hepatitis C virus infection: 2019 update. Hepatol Res. 2020;50:791–816. doi: 10.1111/hepr.13503. [DOI] [PubMed] [Google Scholar]
- 12.Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54:87–95. doi: 10.1007/s00535-018-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahata Y, Hikita H, Mochida S, et al. Sofosbuvir plus velpatasvir treatment for hepatitis C virus in patients with decompensated cirrhosis: a Japanese real-world multicenter study. J Gastroenterol. 2021;56:67–77. doi: 10.1007/s00535-020-01733-4. [DOI] [PubMed] [Google Scholar]
- 14.de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII–renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozuka R, Hai H, Motoyama H, et al. The presence of multiple NS5A RASs is associated with the outcome of sofosbuvir and ledipasvir therapy in NS5A inhibitor-naïve patients with chronic HCV genotype 1b infection in a real-world cohort. J Viral Hepat. 2018;25:535–542. doi: 10.1111/jvh.12850. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki N, Shiomi S, Iwata Y, et al. Clinical usefulness of scintigraphy with 99mTc-galactosyl-human serum albumin for prognosis of cirrhosis of the liver. J Nucl Med. 1999;40:1652–1656. [PubMed] [Google Scholar]
- 17.Kotani K, Kawabe J, Higashiyama S, et al. Heterogeneous liver uptake of Tc-99m-GSA as quantified through SPECT/CT helps to evaluate the degree of liver fibrosis: a retrospective observational study. Medicine (Baltimore) 2018;97:e11765. doi: 10.1097/MD.0000000000011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto A, Kawada N, Jogo A, et al. Utility of minimally invasive measurement of hepatic venous pressure gradient via the peripheral antecubital vein. Gut. 2021;70:1199–1201. doi: 10.1136/gutjnl-2020-322367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Franchis R, Faculty BVI. Expanding consensus in portal hypertension: report of the baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the liver EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Atsukawa M, Tsubota A, Kondo C, et al. Time-course changes in liver functional reserve after successful sofosbuvir/velpatasvir treatment in patients with decompensated cirrhosis. Hepatol Res. 2022;52:235–246. doi: 10.1111/hepr.13739. [DOI] [PubMed] [Google Scholar]
- 22.Tahata Y, Hikita H, Mochida S, et al. Liver-related events after direct-acting antiviral therapy in patients with hepatitis C virus-associated cirrhosis. J Gastroenterol. 2022;57:120–132. doi: 10.1007/s00535-021-01845-5. [DOI] [PubMed] [Google Scholar]
- 23.Sano T, Amano K, Ide T, et al. A combination of hepatic encephalopathy and body mass index was associated with the point of no return for improving liver functional reserve after sofosbuvir/velpatasvir treatment in patients with hepatitis C virus-related decompensated cirrhosis. Hepatol Res. 2022 doi: 10.1111/hepr.13837.(inpress). [DOI] [PubMed] [Google Scholar]
- 24.Diez C, Berenguer J, Ibanez-Samaniego L, et al. Persistence of clinically significant portal hypertension after eradication of hepatitis C virus in patients with advanced cirrhosis. Clin Infect Dis. 2020;71:2726–2729. doi: 10.1093/cid/ciaa502. [DOI] [PubMed] [Google Scholar]
- 25.Afdhal N, Everson GT, Calleja JL, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24:823–831. doi: 10.1111/jvh.12706. [DOI] [PubMed] [Google Scholar]
- 26.Mandorfer M, Kozbial K, Schwabl P, et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Lens S, Alvarado-Tapias E, Mariño Z, et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153:1273–83.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Lens S, Baiges A, Alvarado-Tapias E, et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol. 2020;73:1415–1424. doi: 10.1016/j.jhep.2020.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Curry MP, O'Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 30.Tada T, Kurosaki M, Nakamura S, et al. Real-world clinical outcomes of sofosbuvir and velpatasvir treatment in HCV genotype 1- and 2-infected patients with decompensated cirrhosis: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Med Virol. 2021;93:6247–6256. doi: 10.1002/jmv.27157. [DOI] [PubMed] [Google Scholar]
- 31.Kira T, Tomiguchi S, Takahashi M, et al. Correlation of 99mTc-GSA hepatic scintigraphy with liver biopsies in patients with chronic active hepatitis type C. Radiat Med. 1999;17:125–130. [PubMed] [Google Scholar]
- 32.Ishii R, Togashi H, Iwaba A, et al. (99m)Tc-GSA SPECT analysis was clinically useful to evaluate the effect of interferon in a patient with interferon non-responsive chronic hepatitis C. Ann Nucl Med. 2011;25:520–523. doi: 10.1007/s12149-011-0484-0. [DOI] [PubMed] [Google Scholar]
- 33.Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 34.Rigopoulou EI, Roggenbuck D, Smyk DS, et al. Asialoglycoprotein receptor (ASGPR) as target autoantigen in liver autoimmunity: lost and found. Autoimmun Rev. 2012;12:260–269. doi: 10.1016/j.autrev.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Treichel U, Paietta E, Poralla T, et al. Effects of cytokines on synthesis and function of the hepatic asialoglycoprotein receptor. J Cell Physiol. 1994;158:527–534. doi: 10.1002/jcp.1041580319. [DOI] [PubMed] [Google Scholar]
- 36.Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702–706. doi: 10.1002/hep.1840150425. [DOI] [PubMed] [Google Scholar]
- 37.Miyaki E, Imamura M, Hiraga N, et al. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol Res. 2016;46:758–764. doi: 10.1111/hepr.12621. [DOI] [PubMed] [Google Scholar]
- 38.Kikukawa K, Uchida-Kobayashi S, Tamori A, et al. Serum Mac-2-binding protein glycosylation isomer predicts esophagogastric varices in cirrhotic patients with chronic hepatitis C virus infection treated with IFN-free direct-acting antiviral agent: M2BPGi levels predict varices in SVR patients. Ann Hepatol. 2020;19:367–372. doi: 10.1016/j.aohep.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067. doi: 10.1016/j.jhepr.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa WF, Andrade VG, Braz AMM, et al. Cirrhosis regression after SVR with indirect methods of fibrosis analysis: how far is it real? Clin Exp Med. 2022;22:269–275. doi: 10.1007/s10238-021-00749-1. [DOI] [PubMed] [Google Scholar]
- 41.Morio K, Imamura M, Daijo K, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol. 2017;52:1252–1257. doi: 10.1007/s00535-017-1345-y. [DOI] [PubMed] [Google Scholar]
- 42.Poordad F, Castro RE, Asatryan A, et al. Long-term safety and efficacy results in hepatitis C virus genotype 1-infected patients receiving ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in the TOPAZ-I and TOPAZ-II trials. J Viral Hepat. 2020;27:497–504. doi: 10.1111/jvh.13261. [DOI] [PubMed] [Google Scholar]
- 43.Enomoto M, Ikura Y, Tamori A, et al. Short-term histological evaluations after achieving a sustained virologic response to direct-acting antiviral treatment for chronic hepatitis C. United Eur Gastroenterol J. 2018;6:1391–1400. doi: 10.1177/2050640618791053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 45.Abraldes JG, Tarantino I, Turnes J, et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 46.La Mura V, Garcia-Guix M, Berzigotti A, et al. A prognostic strategy based on stage of cirrhosis and HVPG to improve risk stratification after variceal bleeding. Hepatology. 2020;72:1353–1365. doi: 10.1002/hep.31125. [DOI] [PubMed] [Google Scholar]
- 47.D’Amico G, Garcia-Pagan JC, Luca A, et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611–1624. doi: 10.1053/j.gastro.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634–1641. doi: 10.1136/gutjnl-2012-304038. [DOI] [PubMed] [Google Scholar]
- 49.Tsuji S, Uchida Y, Uemura H, et al. Involvement of portosystemic shunts in impaired improvement of liver function after direct-acting antiviral therapies in cirrhotic patients with hepatitis C virus. Hepatol Res. 2020;50:512–523. doi: 10.1111/hepr.13471. [DOI] [PubMed] [Google Scholar]
- 50.Takaoka Y, Miura K, Morimoto N, et al. Real-world efficacy and safety of 12-week sofosbuvir/velpatasvir treatment for patients with decompensated liver cirrhosis caused by hepatitis C virus infection. Hepatol Res. 2021;51:51–61. doi: 10.1111/hepr.13576. [DOI] [PubMed] [Google Scholar]
- 51.Bolognesi M, Merkel C, Sacerdoti D, et al. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002;34:144–150. doi: 10.1016/S1590-8658(02)80246-8. [DOI] [PubMed] [Google Scholar]
- 52.Nagasue N, Dhar DK, Yamanoi A, et al. Production and release of endothelin-1 from the gut and spleen in portal hypertension due to cirrhosis. Hepatology. 2000;31:1107–1114. doi: 10.1053/he.2000.6596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 A 72-year-old man with hepatitis C genotype 1b infection whose hepatocyte function did not improve. His pre-treatment data were as follows: Child-Pugh-Turcotte (CPT) score, 7; hepatocyte receptor index (LHL15), 0.78; blood clearance index (HH15), 0.75; liver stiffness measurement (LSM), 43 kPa; and hepatic venous pressure gradient (HVPG), 17 mmHg. Before treatment, the planar image of Tc-99m-galactosyl human serum albumin (GSA) scintigraphy showed weak accumulation of radiotracer in the liver and enhanced pooling in the heart (a). After sustained virologic response at 24 weeks post-treatment (SVR24), all of the abovementioned values were worse: CPT score, 9; LHL15, 0.73; HH15, 0.79; LSM, 75 kPa; and HVPG, 22 mmHg. Accumulation of radiotracer in the liver remained weak, and pooling of tracer in the heart did not decrease (b). CT performed after SVR24 showed recurrent hepatocellular carcinoma lesions in the right lobe, which were enhanced in the arterial phase and washed out in the late phase (arrow) (c) (PPTX 425 KB)
Supplementary file2 Liver stiffness measurement (LSM) was 35 (27–39) kPa before sofosbuvir/velpatasvir treatment and decreased to 27 (21–44) kPa after sustained virologic response at 24 weeks post-treatment (SVR24). This decrease was not statistically significant (p=0.719) (PPTX 73 KB)
Supplementary file3 Percentage of patients with a liver stiffness measurement (LSM) ≥ 20 kPa was 90% (9/10) before sofosbuvir/velpatasvir treatment and remained at 90% (9/10) after sustained virologic response at 24 weeks post-treatment (SVR24) (p=1.000) (PPTX 60 KB)
Supplementary file4 Hepatic venous pressure gradient (HVPG) was 18 (15–20) mmHg before sofosbuvir/velpatasvir treatment and decreased to 15 (9–22) mmHg after sustained virologic response at 24 weeks post-treatment (SVR24). However, this decrease was not statistically significant (p=0.119) (PPTX 76 KB)
Supplementary file5 Percentage of patients with clinically significant portal hypertension (CSPH), defined as a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, was 92% (11/12) before sofosbuvir/velpatasvir treatment and decreased to 75% (9/12) after sustained virologic response at 24 weeks post-treatment (SVR24). However, the decrease was not statistically significant (p=0.157) (PPTX 60 KB)