Abstract
Introduction:
Schizophrenia and Major Depressive Disorder (MDD) are highly burdensome mental disorders, with significant cost to both individuals and society. Despite these disorders representing distinct clinical categories, they are each heterogenous in their symptom profiles, with considerable transdiagnostic features. Although movement and sleep abnormalities exist in both disorders, little is known of the precise nature of these changes longitudinally. Passively-collected longitudinal data from wearable sensors is well suited to characterize naturalistic features which may cross traditional diagnostic categories (e.g., highlighting behavioral markers not captured by self-report information).
Methods:
The present analyses utilized raw minute-level actigraphy data from three diagnostic groups: individuals with schizophrenia (N = 23), individuals with depression (N = 22), and controls (N = 32), respectively, to interrogate naturalistic behavioral differences between groups. Subjects’ week-long actigraphy data was processed without diagnostic labels via unsupervised machine learning clustering methods, in order to investigate the natural bounds of psychopathology. Further, actigraphic data was analyzed across time to determine timepoints influential in model outcomes.
Results:
We find distinct actigraphic phenotypes, which differ between diagnostic groups, suggesting that unsupervised clustering of naturalistic data aligns with existing diagnostic constructs. Further, we found statistically significant inter-group differences, with depressed persons showing the highest behavioral variability.
Limitations:
However, diagnostic group differences only consider biobehavioral trends captured by raw actigraphy information.
Conclusions:
Passively-collected movement information combined with unsupervised deep learning algorithms shows promise in identifying naturalistic phenotypes in individuals with mental health disorders, specifically in discriminating between MDD and schizophrenia.
Keywords: Unsupervised machine learning, UMAP, Depression, Schizophrenia, Actigraphy, Passive sensing
1. Introduction
MDD and schizophrenia are highly debilitating and among the most common mental health disorders worldwide, with lifetime prevalences of 16.6 % and 1 %, respectively (Kahn et al., 2015; Otte et al., 2016). Major depressive disorder (MDD) is characterized by low mood, diminished interest, impaired cognitive function, and vegetative symptoms (Otte et al., 2016). Schizophrenia, a primary psychotic disorder, is characterized by positive symptoms such as hallucinations, delusions and disorganization (Kahn et al., 2015) and negative symptoms, including low motivation, low interest, and anhedonia. Left untreated, these disorders have profoundly negative consequences for individuals, their families, and society, including increased morbidity, disability, and mortality (Walker et al., 2015). Moreover, both schizophrenia and depression are highly heterogeneous disorders that result from complex interplay between genetic and environmental risk factors (Howes and Murray, 2014; Buchanan and Carpenter, 1994; Buchsbaum and Haier, 1978; Lang et al., 2013; Carpenter and Kirkpatrick, 1988; Pine, 2019; Weissman, 1986). Therefore, it is critical to develop objective methods that are scalable and reliable to diagnose and treat MDD and schizophrenia.
Disturbances in movement have long been regarded as key diagnostic criteria in both MDD and schizophrenia. Psychomotor slowing is one of nine core symptoms of MDD, according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013); similarly, altered postural control, instability, gait, and balance defects are well established clinical characteristics of schizophrenia (Buyukdura et al., 2011; Presta et al., 2021). Research has shown that depressed and schizophrenic patients differ from normal comparison groups with regard to objectively quantified parameters of motor activity (Christina and Harold, 1997). Moreover, these psychomotor symptoms have been shown to carry unique discriminative ability compared to other symptoms. For instance, studies have found that disturbances in motor activity can uniquely distinguish specific subtypes of MDD and schizophrenia, as well as predict response to treatment (Walther et al., 2009a). Current methods of measuring motor activity in psychiatry, however, remain challenging, time-intensive, and largely limited to structured clinical observations, such as the AIMS test (Abnormal Involuntary Movement Scale), and interval self-report, such as PHQ-9 (Patient Health Questionnaire-9, Item 8) (Lane et al., 1985; Kroenke et al., 2001). Ultimately, these methods remain inadequate to capture the dynamic range of motor behavior across time, evidenced by poor validity of expert ratings (Walther et al., 2009b).
Sleep and circadian disturbances are also frequently observed in patients with MDD and schizophrenia (Wulff et al., 2010). Sleep disturbances are implicated in the neuropathologies of MDD and schizophrenia and often precede the onset of many psychiatric disorders (Wulff et al., 2010). Identification of sleep-based biomarkers may therefore prove to be useful in early detection and intervention of both psychiatric and neurodegenerative disorders (Wulff et al., 2010). Ransing et al. found that quantified parameters of sleep such as sleep efficiency were promising early prognostic biomarkers for schizophrenia and MDD (Ransing et al., 2021). Further, Afonso et al. showed that poorer sleep in schizophrenic patients also negatively impacted quality of life and subsequent treatment, such as rehabilitation strategies (Afonso et al., 2011). Despite this, little is known of the precise diagnostic or prognostic value of sleep disturbances in patients with MDD and schizophrenia. This is further complicated by medications used in depression and schizophrenia, which may impact sleep (Winokur and Kamath, 2008; Wichniak et al., 2017).
The high degree of overlap in clinical presentation and comorbidity between MDD and schizophrenia highlights the challenges in using subjective measures to diagnose and monitor psychiatric diseases. The prevalence of depression in schizophrenia is reported to be as high as 60 % (Upthegrove et al., 2016). When investigated longitudinally, up to 80 % of patients with schizophrenia have experienced at least one depressive episode; symptoms such as anhedonia, concentration difficulties, and psychomotor abnormalities are nonspecific and common in both schizophrenia and MDD. Even in the absence of comorbidity, there is significant overlap in clinical findings in patients with solely MDD and schizophrenia (e.g., low mood, diminished interest, negative symptoms). This underscores the immense clinical challenge in accurately differentiating psychiatric disorders with highly overlapping presentations, and motivates the need for objective methods that can reliably distinguish these psychiatric disorders.
An emerging body of research has explored the use of actigraphy, a well-validated tool in sleep medicine (Smith et al., 2018), to characterize disturbances in movement and sleep patterns in both depression (Burton et al., 2013) and schizophrenia (Walther et al., 2009b). A study by Walther et al., for instance, found that objective movement parameters collected by wrist-worn device actigraphy information could reliably distinguish catatonic schizophrenia from paranoid and disorganized schizophrenia (Walther et al., 2009a). Previous work in our lab has also identified potential digital biomarkers that could predict diagnostic group status (i.e. mood disorder, control) with a high degree of accuracy (accuracy = 89 %, kappa = 0.773) (Jacobson et al., 2019). However, relevant differences in movement patterns specifically between patients with mood disorders and schizophrenia, especially over extended periods of time, are poorly understood. In addition, the extent to which naturalistic phenotypes map to DSM-defined categories has not been adequately considered. Following from this knowledge gap, our first aim (1) in this study was to determine whether unsupervised machine learning methods (using actigraphic data) would naturally differentiate MDD, schizophrenia, and non-disordered controls; in other words, would distinct actigraphic phenotypes map-on to existing diagnostic contrstucts? Our second aim if we found discriminant validity of the unsupervised approach in (1) was to characterize the distinct movement phenotypes associated with MDD, schizophrenia, and non-disordered controls. To accomplish these aims, we analyzed passively collected dense actigraphic data using an unsupervised machine learning approach. We utilized model introspection techniques to determine the respective time intervals of locomotion most important in informing our unsupervised clustering.
2. Methods
2.1. Study sample
The present work used publicly available actigraphy data collected from individuals with schizophrenia, depression, and controls (Berle et al., 2010). Participants included (N = 77) adults (including 22 with schizophrenia (Jakobsen et al., 2020), 23 with depression (Garcia-Ceja et al., 2018), and 32 healthy controls). The average age of the overall participant group was 42 years, with subgroup age averages of 46.2, 42.8, and 38.2 years for schizophrenia, depression, and control, respectively. The participants were 57 % male and 43 % female overall, with 86 %, 57 %, and 38 % men in the schizophrenia, depression, and control groups, respectively. Of those who were depressed, five participants were receiving inpatient care, while the others had outpatient care. Of those patients with schizophrenia, all were considered unable to live independently and were on antipsychotic medications, with eight participants (36 %) on clozapine to improve symptom control. The control participants comprised hospital employees (n = 23), students (n = 5), and outpatients without significant medical or psychiatric illness (n = 4) (Berle et al., 2010).
2.2. Data collection
Participants’ physical activity data was collected via an Actiwatch device, worn around the right wrist based on convenience for participants (King et al., 2005). Activity counts were recorded on minute intervals for two continuous weeks, and participants were requested to only remove the device when taking a shower (Berle et al., 2010). The original study protocol was approved by the local ethics committee (REK III, Health-West, Norway) (Berle et al., 2010).
2.3. Data preprocessing
Data handling, analysis, and visualization were completed using Python (v3.8.3) (Rossum et al., 2010). Individual participants’ raw actigraphy counts were subset to only include the first week of data collection and all participants’ were combined into a single data structure, with each row representing a given participants’ first week of raw minute-level actigraphy data (See Fig. 1A).
Fig. 1.
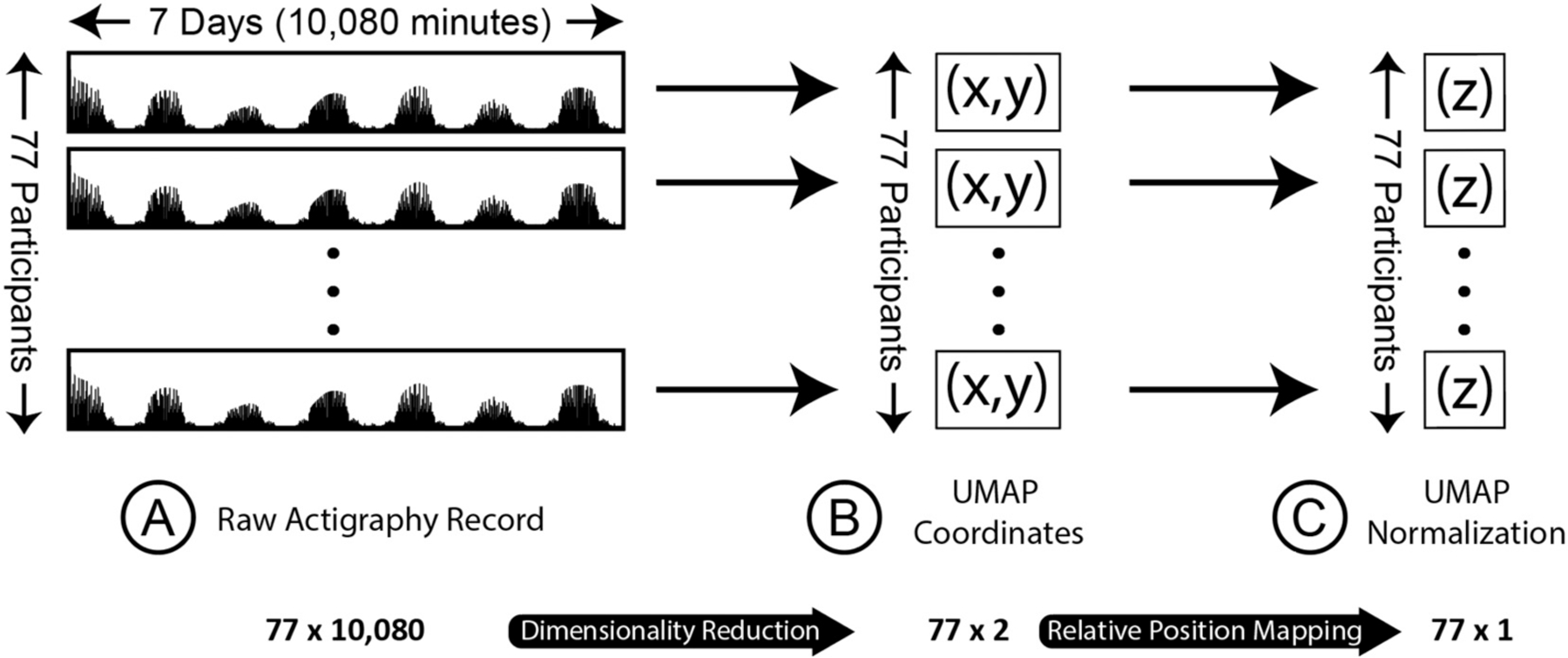
Raw actigraphy data processing and dimensionality reduction
(A) Panel A displays in schematic form raw actigraphy records for each of the 77 participants. Each participant has activity data over one week, with 10,080 min-intervals. (B) Using an unsupervised dimensionality reduction algorithm (UMAP), we reduced the 10,080 data points for each participant to two coordinates, which were plotted together on a two-dimensional cartesian coordinate plane. (C) After UMAP two-dimensional reduction, we further reduced the two-dimensional coordinates to a one-dimensional normalization score, in essence representing the participant’s 10,080 time series movement points as a single value.
2.4. Uniform manifold approximation and projection (UMAP)
Uniform Manifold Approximation and Projection (UMAP) is an unsupervised, nonlinear dimensionality reduction method capable of establishing and organizing informative clusters from high-dimensional data (McInnes et al., 2018). Further, UMAP is well-suited to handle outliers and is more effective in mapping groups compared to alternative clustering techniques (Ali et al., 2019). Thus, the resulting high-dimensional minute-level actigraphy data was reduced to two dimensions via UMAP (McInnes et al., 2018), in line with recent research suggesting UMAP as an effective approach for studying comorbidities within mental health research (Sánchez-Rico and Alvarado, 2019). The reduced actigraphy data was subsequently visualized and labeled based on diagnostic group to examine higher-order relationships between individuals weekly movement patterns, and diagnostic group-level movement trends (See Fig. 1B).
2.5. UMAP euclidean distance calculation
Although the scale corresponding to the UMAP projection (X and Y coordinates) cannot be directly interpreted, quantification of the relative distance between points can still be assessed, a method previously used to quantify gene pair interactions (Dorrity et al., 2020). The euclidean distance between every point, representing an individual’s movement pattern, within a diagnostic group was calculated and averaged across the group. Average euclidean distance was compared across groups via Welch’s ANOVA (Delacre et al., 2019) followed by a Games-Howell post-hoc pairwise-comparison to directly assess diagnostic group differences (Table 1).
Table 1.
Average euclidean distance of mental health participants compared to controls.
| Group A | Group B | Group A euclidean distance (mean ± sd) | Group B euclidean distance (mean ± sd) | Games-Howell (p-value) |
|---|---|---|---|---|
| Control participants | Depressive participants | 1.41 ± 0.70 | 2.03 ± 1.02 | 0.001* |
| Control participants | Schizophrenic participants | 1.41 ± 0.70 | 1.56 ± 0.83 | 0.047* |
| Depressive participants | Schizophrenic participants | 1.56 ± 0.83 | 2.03 ± 1.02 | 0.001* |
Euclidean distance between all points (corresponding to an individual’s UMAP coordinates) within a group were calculated, and within-group average and standard deviation distance quantified. Euclidean distances across groups was compared via Welch’s ANOVA and followed by Games-Howell post-hoc pairwise-comparison to directly assess difference in average distance between the three groups.
p ≤ 0.05.
2.6. UMAP feature reduction
Coordinate pairs corresponding to the UMAP projection of individuals’ raw actigraphy data were further reduced to a single dimension (See Fig. 1C) to interrogate the relative influence of individual’s minute-level movement behavior on their overall (week-long) movement patterns using SHapley Additive exPlanation (SHAP) (Lundberg and Lee, 2017). To preserve the general relationship between diagnostic groups from the UMAP projection (e.g., individuals from the control and schizophrenia groups centralized, with individuals from the depression group on the periphery), a custom normalization equation was written to reduce the two-dimensional data accordingly (Eq. (1)).
| (1) |
In Eq. (1), individuals’ X and Y coordinates from the UMAP projection were transformed and subtracted from the total sample’s average to produce a single-dimension Z value (Fig. 3). Please see Supplementary Analysis for methods and results of implementing a machine learning approach using the X and Y coordinates from the UMAP projection and the single-dimension Z value as input features diagnostic group detection.
Fig. 3.
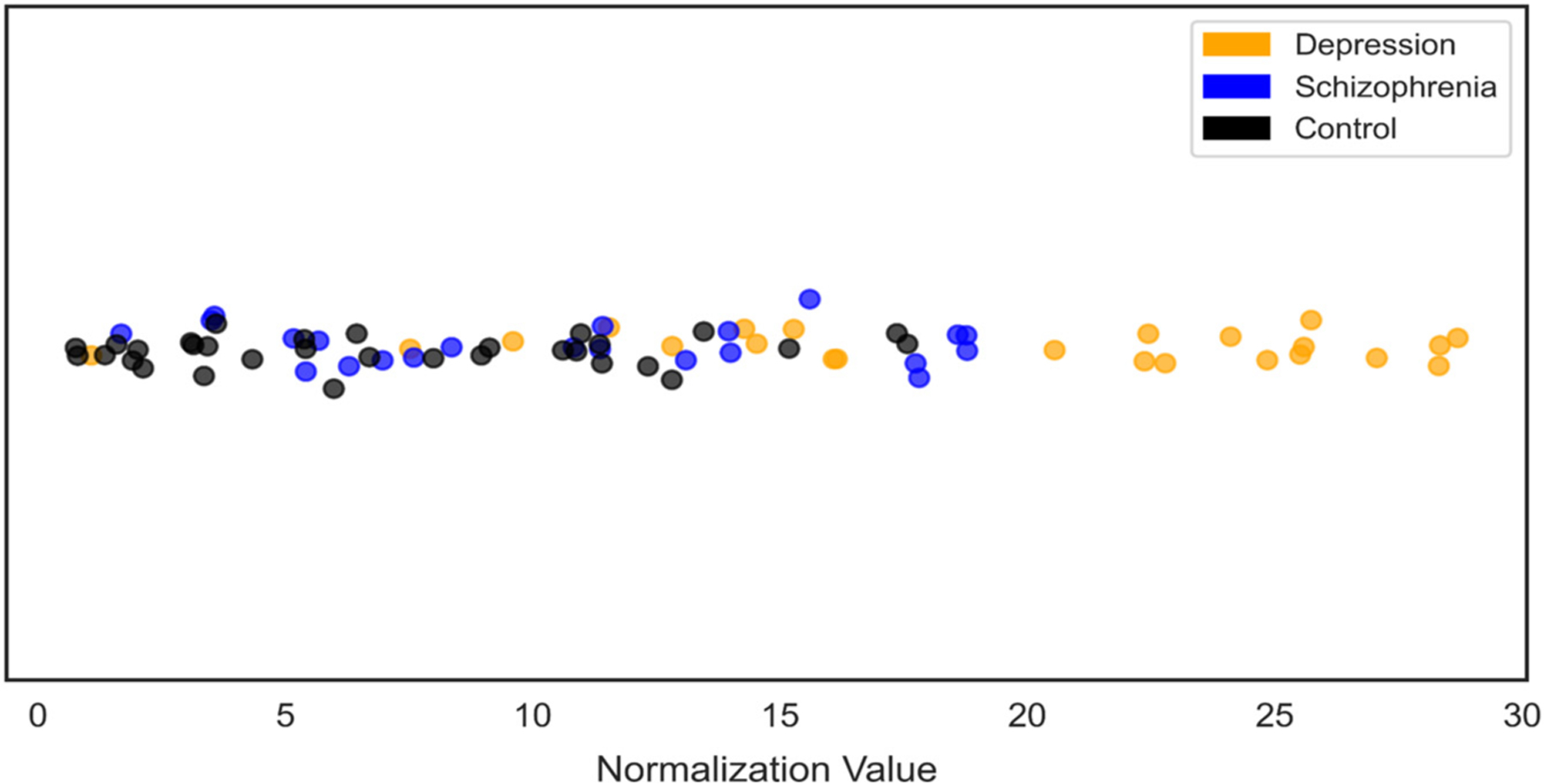
One-dimensional representation of UMAP coordinates
UMAP coordinates reduced to a single dimension for all participants raw actigraphy data, color-coded by group.
2.7. Data explainability
SHAP was implemented to aid in behavioral pattern interpretability by evaluating the most influential times of day in a participants weekly movement pattern. Intuitively, SHAP allows for model introspection by iteratively perturbing the input data and assessing how this affects the output (Lundberg and Lee, 2017). In the present analyses, SHAP values represent how the model establishes the latent features that define cluster membership. By expressing the variation of the two-dimensional space in one-dimension, we were able to construct single shap values per person per feature (minute-level actigraphy data) to represent how the model informed the latent space. These values were visualized in averaged two-hour intervals against the individual’s raw actigraphy data to map the influential features to the corresponding movement patterns.
3. Results
We present descriptive findings from our observations of the following: (1) the UMAP projection plot, which displays dimensionally reduced raw actigraphy data, and corresponding euclidean distance metrics (2) the non-reduced raw actigraphy plots. Additionally, the performance of a supervised machine learning approach using the movement-derived clustering coordinates (X, Y, and Z values) to detect an individual’s diagnostic group is provided in Supplementary Analysis: Modeling Results.
3.1. General UMAP trends
UMAP displays each subject in a dimensionally reduced feature space, with each subject displayed as a single point in two-dimensional space. Thus, each subject’s position (Xi, Yi) in the two-dimensional space is representative of that subject’s raw movement over one week. It follows that subjects whose points are closer together in the two-dimensional space have movement that is more similar. With such a reduction, we make possible detection of relative similarities between subjects, with unbiased clustering of movement-similar participants, regardless of their respective diagnoses. Notably, we find that subjects with a common diagnosis do, in fact, tend to cluster. Specifically, participants with schizophrenia and controls tend to cluster together, centrally in the two-dimensional space (Fig. 2). Depressed participants (both inpatient and outpatient) tend to occupy the periphery, encircling the schizophrenic patients and controls. Controls and schizophrenic patients are quite difficult to disentangle, visually, though can be distinguished positionally from depressed patients. To quantify the coordinate dispersion between diagnostic groups, euclidean distance between points within a diagnostic group was assessed (Table 1). Confirming qualitative assessment of the UMAP projections, the average distance between any two individuals within the same diagnostic group was greatest in the depression group. Notably, pairwise comparisons found a statistically significant difference between average distance in every group (Table 1).
Fig. 2.
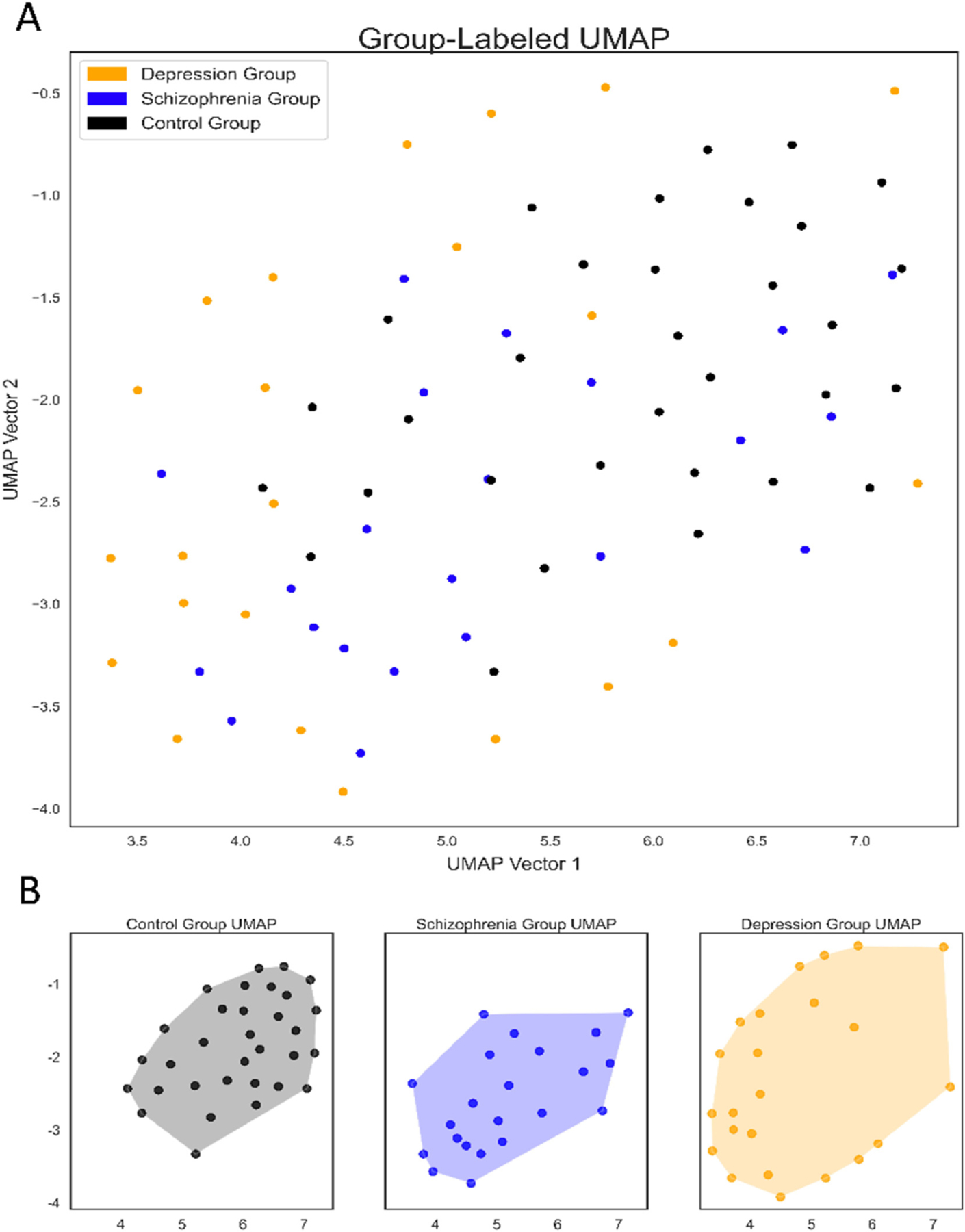
UMAP representation of raw actigraphy data by group
(A) UMAP representation of all participants raw actigraphy data, color-coded by group (B) UMAP representation of raw actigraphy data separated and color-coded by group.
3.2. General actigraphy plot trends
To visualize daily behavioral patterns influential in characterizing overall movement, three representative individuals from each diagnostic group were chosen to allow for qualitative interpretation of the general daily movement patterns and influential time periods identified via SHAP (Fig. 4).
Fig. 4.
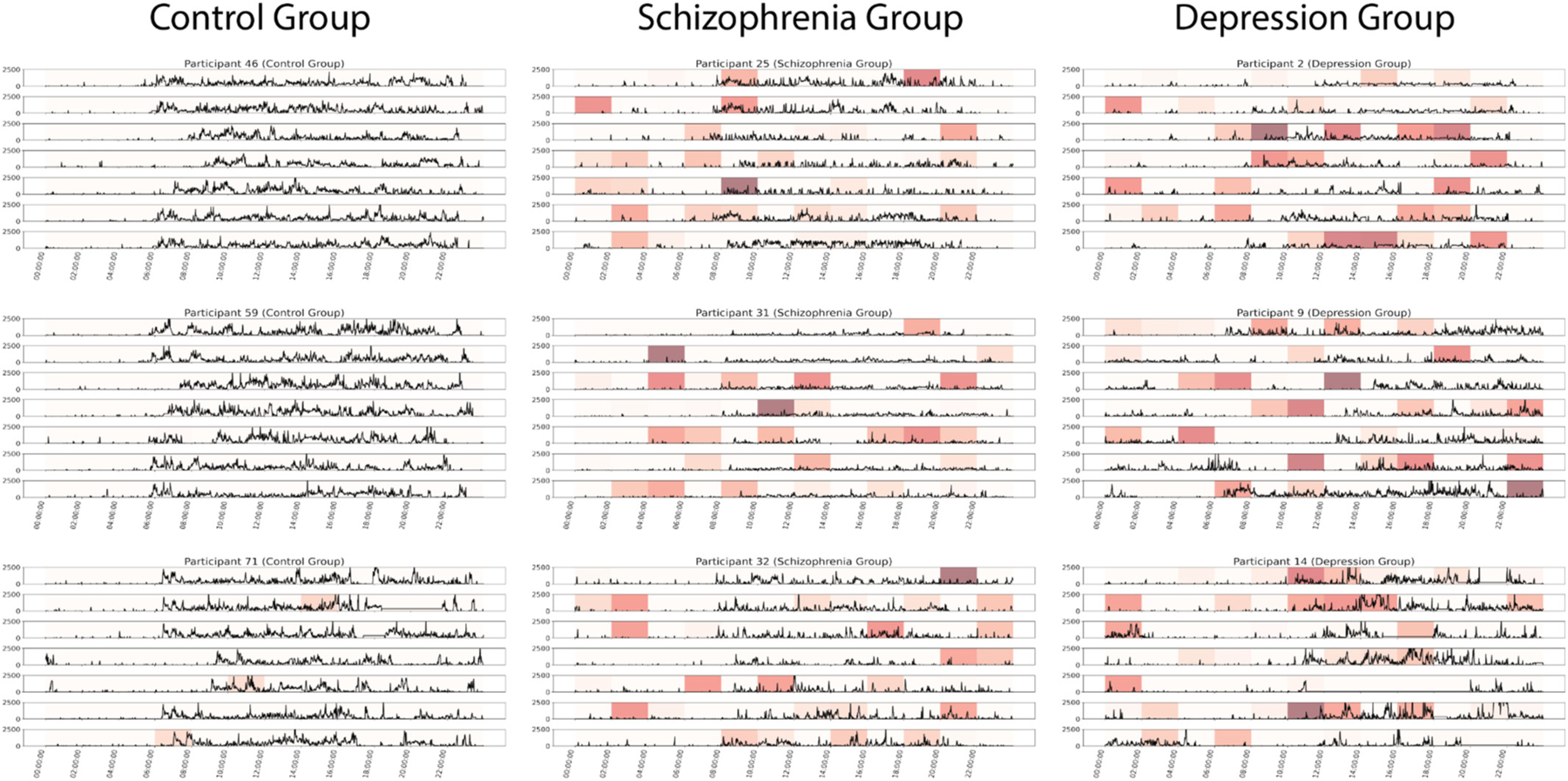
Representative group activity patterns with corresponding SHAP values
Representative participants picked from the control, schizophrenia, and depression group(s). Participants were selected from the central regions of their cluster on the UMAP to avoid outliers.
3.2.1. Control group
Unique to the control group, a consistent diurnal pattern was observed, with daily movement between 8:00 AM to 10:00 PM, and routine sleep. Interestingly, due to the structured movement patterns of individuals in the control group across the week, SHAP values were generally low, suggesting that no time windows were of notable influence in characterizing the daily movement patterns (Fig. 4).
3.2.2. Schizophrenia group
Compared to the control group, SHAP suggested more influential behavioral trends evidenced of decreased diurnal regularity, most notably short instances of activity observed in early morning hours (12:00 AM - 6:00 AM). Further, the overall amplitude of the representative actigraphy output is notably lower compared to the representative control group (Fig. 4).
3.2.3. Depression group
As evidenced by the representative individual’s actigraphy patterns, participants diagnosed with major depressive disorder display highly erratic daily movement, many of which are determined by SHAP as influential to the individual’s overall activity pattern. Relative to the control and schizophrenia groups, participants with depression exhibited much less regularity and routine in their activity and displayed very little interdaily or intradaily stability. Further, sleep patterns appeared volatile, with inconsistent ‘wake up’ times, as determined by notable increase in actigraphy amplitude. Overall, representative participants from the depression group exhibit low levels of routineness and intensity in their movement patterns, and are most differentiated from the control group.
4. Discussion
4.1. Overview
Our aim in the present analysis was to better understand distinct movement patterns in patients with depression and schizophrenia. To do this, we used passively collected movement data with an unsupervised machine learning algorithm to detect differences in movement between controls, patients with depression, and patients with schizophrenia. We began with descriptive pattern analysis of all participants’ raw activity data over one week’s duration, looking specifically for differences between depression, schizophrenia, and control groups. Though we observed differences, this was a difficult task due to the size of the feature space (recall a total of 10,080 values for each participant’s 7-day activity record), the number of participants, and the variability between participants, even in the same group. To address this challenge and to further explicate similarities and differences in the activity data across groups, we used an unsupervised machine learning algorithm to reduce the feature space from 10,080 values, to two values, which we plotted on a two-dimensional cartesian coordinate system. In order to better understand the relative feature importances of the raw actigraphy data, we leveraged SHAP (detailed in methods) to determine the effect of micro-perturbations of a single activity point on a final normalized Z-score.
4.2. Raw actigraphy
On qualitative analysis of raw activity records, control participants were more likely to have regular sleep and wake times, consistent daily activity patterns, and stable activity amplitudes throughout the day. Depressed patients, in contrast, had more irregular daily schedules, more erratic sleep, often later bedtimes and later wake times in the morning. Perhaps counterintuitively, schizophrenic patients had more regular sleep and wake times, more similar to control participants than depressed patients; however, these findings align with the original work which identified a more structural behavioral pattern in schizophrenia than in depression (Berle et al., 2010). Furthermore, these findings are consistent with sleep disturbance, a core diagnostic feature of MDD (American Psychiatric Association, 2013), which may include insomnia, hypersomnia, or some combination. Similarly, lower amplitude activity observed in schizophrenic patients is consistent with known movement abnormalities (e.g., catatonic behavior, psychomotor slowing) associated with schizophrenia (American Psychiatric Association, 2013; Morrens et al., 2007) and its treatment (Miller et al., 2008). More regular nighttime sleep and lower activity amplitude in the schizophrenic group (compared to the depression group) may be tied to antipsychotics (recall 36 % on clozapine and all on some antipsychotic), many of which, including clozapine, have sedating effects (Miller, 2004).
4.3. Discussion of UMAP coordinate reduction
Upon UMAP reduction of the participant’s raw actigraphy data, we found that participants with like pathologies clustered together (Fig. 2). Schizophrenic participants and control participants clustered near each other, centrally, on the cartesian coordinate system. Depressed participants, in contrast, tended to cluster more peripherally, positionally distinct from both the schizophrenic and control groups. The significance of this separation should not be underestimated, in particular because it occurred by an unsupervised method, agnostic to disorder labels. In other words, the separation we observe reflects naturalistic patterns in the raw movement data, which happen to align with DSM-based pathology labels. We hypothesize that the pattern of clustering (e.g., controls and individuals with schizophrenia appearing more similar to each other and distinct from depression group) is due to the greater regularity of sleep-wake cycles in controls and schizophrenic patients, compared to depressed patients.
4.4. Dimension reduction explainability and introspection
In order to better understand the most influential time points in UMAP feature reduction, we utilized SHAP. The results of the SHAP analysis for all participants are included as a supplement to the present work (Supplemental Files 1–4). Across all groups, overnight and morning events seemed to have high importance for the unsupervised algorithm. Among controls, we noticed a characteristic short double-peak activity burst within the first hour of waking (e.g., prominent in Fig. 4), delineating sleep from wakefulness. This was not generally present in depressed or schizophrenic participants, who showed more irregular or attenuated sleep wake boundaries, heavily detected by SHAP. This may be reflective of more irregular sleep, slowed wake times, and reduced morning activity.
4.5. Implications and importance
This study is the first of its kind to utilize naturalistic, passively collected movement data with an unsupervised machine learning approach to better understand multi-disorder motor differences. Our choice of an unsupervised algorithm in this study was driven by our exploratory aim, that is, to understand disorder movement patterns and whether these movement patterns “map on” to existing disorder classifications. An exploratory, unsupervised approach such as this allows for naturalistic clustering, agnostic to disorder labels (i.e., schizophrenia, depression, control). In doing so, we can discover naturalistic patterns in the data, unbiased by existing disorder labels. Our results indicate that naturalistic patterns do, in fact, partially map on to disorder labels. Perhaps counterintuitively, we observe severely ill schizophrenic patients bare greater activity-based likeness to healthy controls, than to depressed patients. Collectively, our approach aims to explore the movement-related dimension of psychopathology, specifically, the degree to which movement patterns may detect or distinguish categorical psychiatric disorders. This, in effect, serves to empirically validate traditional mental health nosology against naturalistic and highly dimensional passively-collected movement information. We see practical importance in this validation for two reasons. First, such work is a necessary step toward a dimensional understanding of psychopathology, which accounts for the complexity and heterogeneity across and within diagnostic categories. Second, such research forms the empirical basis for automated and scalable technologies, with the potential to provide greater public access to improved mental health assessment. Though additional research is needed, our results are promising and do suggest the exploratory utility of passive time series with ML methods to (1) further characterize behavioral patterns with high temporal resolution and (2) to understand the degree to which naturalistic behaviors map to existing disease classifications.
4.6. Limitations
We present several important limitations to our work, which include (1) a small sample, drawn from a single institution, which limits generalizability of our results; (2) within group and between-group differences may be confounded by demographic characteristics such as age, medication, disorder type, and gender; (3) as noted by the authors of the original paper, the depression and schizophrenia sample severity used in the study likely deviate from that of their representative diagnostic groups (i.e., depressed patients in the study likely have lower than average disorder severity, while schizophrenic patients likely have higher than average disorder severity) (Berle et al., 2010). Comparison of the present approach in an independent participant population, or a larger study using more heterogenous and representative subgroups capable of being matched by disorder-related characteristics such as disorder severity, duration of illness, and medication use are needed to produce more generalizable results. A final limitation inherent in the exploratory unsupervised machine learning approach is the difficulty in identifying those features most predictive of a particular category. While we may make inferences based on the unsupervised clustering, we cannot determine with certainty which features are most predictive of a particular disorder.
4.7. Conclusion
The present work demonstrates the potential for unsupervised deep learning methods combined with passively collected data to identify naturalistic behavioral phenotypes of mental health pathology. The work is exploratory in that it investigates the likeness of these empirically derived behavioral phenotypes to traditional diagnostic constructs (e.g., MDD and schizophrenia). Our findings suggest that diagnostic constructs do have distinguishable naturalistic phenotypes, and our unsupervised methods allow the data to “speak for themselves,” unbiased by these construct labels. Our findings show promise for future research aimed at more fully understanding behavioral dimensions of psychiatric pathology through dense longitudinal data.
Supplementary Material
Role of the funding source
This work was supported by the National Institute of Mental Health (NIMH) and the National Institute of General Medical Sciences (NIGMS) (grant number 1 R01 MH123482–01) and the National Institute on Drug Abuse (grant number 5 P30 DA029926).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2022.08.013.
CRediT authorship contribution statement
GP, MH, and NJ contributed to conceptualization and design of the study. GP and MN implemented the deep learning modeling and analyses. All authors wrote sections of the manuscript and contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
None.
References
- Afonso P, Brissos S, Figueira ML, Paiva T, 2011. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep–wake cycles measured by actigraphy. Psychiatry Res 189, 62–66. [DOI] [PubMed] [Google Scholar]
- Ali M, Jones MW, Xie X, Williams M, 2019. TimeCluster: dimension reduction applied to temporal data for visual analytics. Vis. Comput 35, 1013–1026. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders American Psychiatric Association. 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Berle JO, Hauge ER, Oedegaard KJ, Holsten F, Fasmer OB, 2010. Actigraphic registration of motor activity reveals a more structured behavioural pattern in schizophrenia than in major depression. BMC Res. Notes 3, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Carpenter WT, 1994. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J. Nerv. Ment. Dis 182, 193–204. [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, 1978. Biological homogeneity, symptom heterogeneity, and the diagnosis of Schizophrenia*. Schizophr. Bull 4, 473–475. [DOI] [PubMed] [Google Scholar]
- Burton C, et al. , 2013. Activity monitoring in patients with depression: a systematic review. J. Affect. Disord 145, 21–28. [DOI] [PubMed] [Google Scholar]
- Buyukdura JS, McClintock SM, Croarkin PE, 2011. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT, Kirkpatrick B, 1988. The heterogeneity of the long-term course of schizophrenia. Schizophr. Bull 14, 645–652. [DOI] [PubMed] [Google Scholar]
- Christina S, Harold S, 1997. Psychomotor symptoms of depression. Am. J. Psychiatry 154, 4–17. [DOI] [PubMed] [Google Scholar]
- Delacre M, Leys C, Mora YL, Lakens D, 2019. Taking parametric assumptions seriously: arguments for the use of Welch’s F-test instead of the classical F-test in one-way ANOVA. Int. Rev. Soc. Psychol 32, 13. [Google Scholar]
- Dorrity MW, Saunders LM, Queitsch C, Fields S, Trapnell C, 2020. Dimensionality reduction by UMAP to visualize physical and genetic interactions. Nat. Commun 11, 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ceja E, et al. , 2018. Mental health monitoring with multimodal sensing and machine learning: a survey. Pervasive Mob. Comput 51, 1–26. [Google Scholar]
- Howes OD, Murray RM, 2014. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet 383, 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NC, Weingarden H, Wilhelm S, 2019. Digital biomarkers of mood disorders and symptom change. Npj Digit. Med 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen P, et al. , 2020. PSYKOSE: a motor activity database of patients with schizophrenia In: 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS). IEEE, pp. 303–308. 10.1109/CBMS49503.2020.00064. [DOI] [Google Scholar]
- Kahn RS, et al. , 2015. Schizophrenia. Nat. Rev. Dis. Primer 1, 15067. [DOI] [PubMed] [Google Scholar]
- King M, Jaffre M, Morrish E, Shneerson J, Smith I, 2005. The validation of a new actigraphy system for the measurement of periodic leg movements in sleep. Sleep Med 6, 507–513. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Glazer WM, Hansen TE, Berman WH, Kramer SI, 1985. Assessment of tardive dyskinesia using the abnormal involuntary movement scale. J. Nerv. Ment. Dis 173, 353–357. [DOI] [PubMed] [Google Scholar]
- Lang FU, Kösters M, Lang S, Becker T, Jäger M, 2013. Psychopathological long-term outcome of schizophrenia – a review. Acta Psychiatr. Scand 127, 173–182. [DOI] [PubMed] [Google Scholar]
- Lundberg SM, Lee S-I, 2017. A unified approach to interpreting model predictions. In: Advances in Neural Information Processing Systems, pp. 4765–4774. [Google Scholar]
- McInnes L, Healy J, Saul N, Großberger L, 2018. UMAP: uniform manifold approximation and projection. J. Open Source Softw 3, 861. [Google Scholar]
- Miller DD, 2004. Atypical antipsychotics: sleep, sedation, and efficacy. Prim. Care Companion J. Clin. Psychiatry 6, 3–7. [PMC free article] [PubMed] [Google Scholar]
- Miller DD, et al. , 2008. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br. J. Psychiatry 193, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Sabbe B, 2007. Psychomotor slowing in schizophrenia. Schizophr. Bull 33, 1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, et al. , 2016. Major depressive disorder. Nat. Rev. Dis. Primer 2, 16065. [DOI] [PubMed] [Google Scholar]
- Pine DS, 2019. Heterogeneity in major depressive disorder: lessons from developmental research on irritability. Am. J. Psychiatry 176, 331–332. [DOI] [PubMed] [Google Scholar]
- Presta V, et al. , 2021. Posture and gait in the early course of schizophrenia. PLOS ONE 16, e0245661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransing R, Patil P, Patil S, Agrawal S, 2021. Comparison of actigraphy indices among patients with depression and schizophrenia: a preliminary study. J. Fam. Med. Prim. Care 10, 3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossum G.van, Drake FL, Van Rossum G, 2010. The Python Language Reference Python Software Foundation. [Google Scholar]
- Sánchez-Rico M, Alvarado JM, 2019. A machine learning approach for studying the comorbidities of complex diagnoses. Behav. Sci 9, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, et al. , 2018. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med 14, 1209–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Marwaha S, Birchwood M, 2016. Depression and schizophrenia: cause, consequence or trans-diagnostic issue? Schizophr. Bull sbw097 10.1093/schbul/sbw097. [DOI] [PMC free article] [PubMed]
- Walker ER, McGee RE, Druss BG, 2015. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, et al. , 2009. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology 60, 80–86. [DOI] [PubMed] [Google Scholar]
- Walther S, Koschorke P, Horn H, Strik W, 2009. Objectively measured motor activity in schizophrenia challenges the validity of expert ratings. Psychiatry Res 169, 187–190. [DOI] [PubMed] [Google Scholar]
- Weissman MM, 1986. Understanding the clinical heterogeneity of major depression using family data. Arch. Gen. Psychiatry 43, 430. [DOI] [PubMed] [Google Scholar]
- Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W, 2017. Effects of antidepressants on sleep. Curr. Psychiatry Rep 19, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur A, Kamath J, 2008. The effect of typical and atypical antipsychotic drugs on sleep of schizophrenic patients. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ (Eds.), Serotonin and Sleep: Molecular, Functional and Clinical Aspects Birkhäuser, pp. 587–610. 10.1007/978-3-7643-8561-3_24. [DOI] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG, 2010. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci 11, 589–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


