Abstract
Molecular target inhibitors have been regularly approved by Food and Drug Administration (FDA) for tumor treatment, and most of them intervene in tumor cell proliferation and metabolism. The RAS–RAF–MEK–ERK pathway is a conserved signaling pathway that plays vital roles in cell proliferation, survival, and differentiation. The aberrant activation of the RAS–RAF–MEK–ERK signaling pathway induces tumors. About 33% of tumors harbor RAS mutations, while 8% of tumors are driven by RAF mutations. Great efforts have been dedicated to targeting the signaling pathway for cancer treatment in the past decades. In this review, we summarized the development of inhibitors targeting the RAS–RAF–MEK–ERK pathway with an emphasis on those used in clinical treatment. Moreover, we discussed the potential combinations of inhibitors that target the RAS–RAF–MEK–ERK signaling pathway and other signaling pathways. The inhibitors targeting the RAS–RAF–MEK–ERK pathway have essentially modified the therapeutic strategy against various cancers and deserve more attention in the current cancer research and treatment.
Keywords: Clinical trials, Molecular target therapy, RAS–RAF–MEK–ERK signaling pathway
Introduction
Molecular targeted therapy has been considered a valuable therapy in tumor treatment because of its small size, selective binding with a wide range of extracellular and intracellular targets, superiority over cytotoxic chemotherapy, and few side effects.1 In the last 20 years, 43 molecule target drugs have been approved by the Food and Drug Administration in the US.2 Most of these inhibitors target the kinases that regulate the cell proliferation, metabolism, and immune modulation.
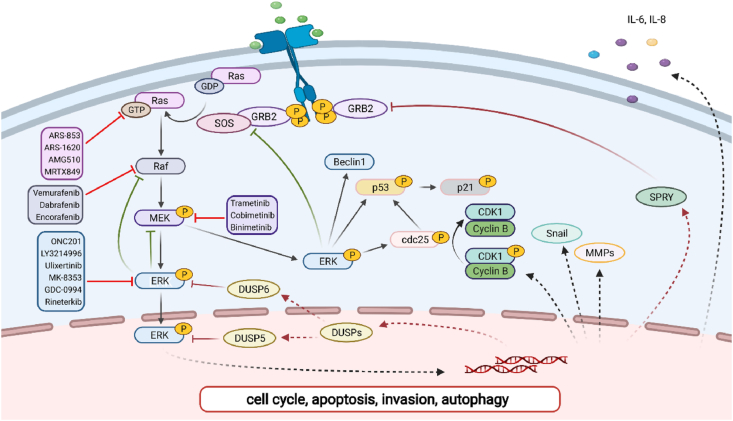
RAS–RAF–MEK–ERK is a classical pathway from the 1990s and transmits extracellular signals to specific intracellular targets (Fig. 1, created with BioRender.com).3 A vast map of substrates related to cell proliferation, differentiation, and metabolism are regulated by ERK.4, 5, 6 Statistically, 33% of RAS mutations and 8% of BRAF mutations are observed in all human cancers thus far.7 The aberrant activation of the signaling pathway contributes to tumorigenesis and tumor development.8, 9, 10 To this end, many inhibitors targeting this pathway are developed, which has essentially modified the therapeutic strategy of cancers. Potential combinations are explored in preclinical and clinical studies. In this review, we provide an overview of the current literature on the cascade activation of RAS–RAF–MEK–ERK and the development of inhibitors targeting this pathway. We hope this will throw light on the design of better therapeutic strategies.
Figure 1.
Signal transduction of RAS–RAF–MEK–ERK pathway.
Components and activation of RAS–RAF–MEK–ERK signaling pathway
Important upstream molecular marker-RAS
As an important upstream molecular marker of the RAS–RAF–MEK–ERK pathway, RAS serves as a molecular switch by binding GTP/GDP,11 and it includes four isoforms: HRAS, KRAS4A, KRAS4B, and NRAS.12 KRAS is the most frequent isoform in all human cancers.13 KRAS4A and KRAS4B are the different splicing isoforms of the same gene. When the transmembrane receptors (receptor tyrosine kinase, RTKs) are activated, the complexes of growth-factor-receptor bound protein 2 (GRB2) and son of sevenless (SOS) in the cytoplasm are recruited to the inner surface of cell membrane.14 SOS is an important guanine nucleotide exchange factor (GEF) that delivers the signal from RTK to RAS.15 Then RAS–GDP turns into RAS–GTP with the help of SOS. In this process, GRB2 acts as a bridge between RTK and SOS. After that, the intrinsic RAS-GTPase hydrolyzes RAS–GTP to inactivated RAS–GDP with the help of GTPase-activating proteins (GAPs).16
The downstream kinase of RAS–RAF
RAF, the downstream kinase of RAS, is recruited and directly phosphorylated after RAS turns on the switch. It includes three isoforms: ARAF, BRAF, and CRAF.17,18 All RAF isoforms share three conserved regions: conserved region 1 (CR1) containing RAS binding domain (RBD), conserved region 2 (CR2), which is the regulatory domain, and conserved region 3 (CR3), which is the kinase domain and contributes to the variable kinase activities (BRAF > CRAF > ARAF).19,20 Except for three catalytically competent enzymes, the RAF family also has two pseudokinases, a kinase suppressor of RAS 1/2 (KSR1/2), which generally works as a scaffold in the RAS/MAPK pathway for bridging MEK to RAF.21 RAF dimerization is widely considered as a key event in RAF activation. Active RAS induces hetero-dimerization of CRAF and BRAF, an effect that is dependent on the serine residue at position 621 of CRAF. In depth, RAS induces the CRAF–BRAF complex formation through the exposure of 14-3-3 binding sites in the COOH-terminus of CRAF.22 The subsequent research reveals that several mutants of BRAF with reduced kinase activity activate MEK by activating the CRAF.23 Wild-type B-RAF forms a complex with C-RAF in a RAS-dependent manner, while the mutants bind independently of RAS. Importantly, wild-type B-RAF can also activate C-RAF through a mechanism involving 14-3-3 mediated hetero-oligomerization and C-RAF transphosphorylation.24 Except for the kinase domain, the constitutive phosphorylation in N-terminal of BRAF also activates the CRAF while N-terminal of CRAF is dependent on MEK, suggesting a feedback mechanism and explaining a key difference between BRAF and CRAF. In addition to the hetero-dimerization of CRAF and BRAF, RAF-related pseudo-kinase KSR also heterodimerizes with RAF, thereby triggering RAF activation.25
Downstream molecular-MEK and ERK
MEK1 and MEK2, downstream kinase of RAF, are dual-specific kinase that catalyzes both tyrosine and serine/threonine residues in ERK1 and ERK2.26 The mutations of MEK that enhance MEK1 homodimerization promotes the activation of RAF kinase and catalytic activity toward the kinase ERK.27 Unlike the narrow map of substrates for MEKs, ERKs phosphorylate hundreds of substrates, including transcriptional factors and protein kinases.28 ERKs work both in the cytoplasm (BIM, MCL, RSK, and MNK) and the nucleus (CREB, FOS, MYC, JUN, MSK, ELK, and ETS).12
Feedback regulation
RAS–RAF–MEK–ERK cascade is regulated by the negative feedback, which is generally divided into short term and long term. The short-term feedback of the ERK cascade includes the direct inhibition of RAS, RAF, and MEK1 through ERK1/2.29, 30, 31, 32, 33, 34 In detail, activated ERK breaks the interaction of RAF–RAS by phosphorylating SOS, and phosphorylates the Ser/Thr sites of BRAF, thereby inhibiting the binding to the activated RAS and disrupting heterodimerization with CRAF.31 Additionally, the N-terminus of MEK is also directly phosphorylated by the activated ERK. The long-term feedback is the activation of transcriptional factors (DUSPs and SPRYs). Dual specificity protein phosphatases (DUSPs), a kind of phosphatase for ERK, are spatially located. The DUSP6 works in the cytoplasm while DUSP5 in the nucleus.35 Another one is that sprouty proteins (SPRYs) bind with Grb2 and inhibit the recruitment of the Grb2–SOS complex. In addition, SPRYs can also down-regulate the RAS–GTP by forming the juxta-cytoplasmic membrane localization of NF1.35,36
Crosstalk with PI3K pathway
The RAS–RAF–MEK–ERK pathway is also regulated by the bypath, RAS–PI3K–AKT–mTOR. Activated RAS recruits PI3K via GAB binding proteins. Subsequently, PIP3 generated through PI3K modification activates the AKT and mTOR which regulates cell growth, survival, and metabolism.37 The crosstalk between RAS/RAF/ERK and RAS/PI3K/AKT/mTOR can be summarized as cross-inhibition and pathway convergence on substrates. Cross-inhibition refers to that a member of one way negatively regulates an upstream component of another way. For example, active ERK blocks the insulin by phosphorylating GAB1 at the level of PI3K, and AKT negatively regulates ERK activation by phosphorylating RAF at Ser259.38,39 The latter crosstalk, i.e., pathway convergence including FOXO, C-MYC, BAD and GSK3, shares the same substrates activated by those signal pathways and promotes the cell survival, proliferation and metabolism.40,41
Inhibitors of RAS–RAF–MEK–ERK pathway
RAS inhibitors
The frequent mutations of RAS–RAF–MEK–ERK pathway are observed in human cancers, with 33% RAS mutations and 8% BRAF mutations, and the hyperactivity of RAS–RAF–MEK–ERK is highly related to tumor progression.7 Targeting the RAS–RAF–MEK–ERK pathway represents an attractive method for alleviating the tumor growth. At present, many inhibitors have been developed, and the discussed inhibitors are listed in the Table 1. The treatment of RAS mutant cancers is unsatisfactory because of the lack of efficient and specific targets. A targetable RAS mutation, KRASG12C, has been observed in 14% of lung cancers and 5% of colorectal cancers.95,96 Schokat group firstly reported a compound, i.e., compound 12, that could irreversibly bind to the cysteine of KRASG12C instead of glycine of KRASWT.97 Subsequently, a series of inhibitors based on the structural optimization and transformation of compound 12 are developed, such as ARS-853 (the first KRASG12C-specific compound with cellular activities, 2013) and ARS-1620 (the first KRASG12C-specific compound with in vivo activity, 2018).98,99 The KRASG12C-specific drug, AMG510 (storasib), firstly went into clinical trial in 2019 and was subsequently proved by FDA in 2021.42,43 Another KRASG12C-specific covalent inhibitor, MRTX849 (adagrasib) developed by Mirati group, also went into clinical trial in 2019.44 However, the resistance to AMG510 and MRTX849 was observed recently, resulting in a lack of efficacy, recurrence, or progression.45 At the same time, other strategies such as the destruction of RAS/effector interaction and the inhibition of RAS downstream molecules are also undergone vigorously. Therefore, we summarized the inhibitors targeting RAS downstream molecules following.
Table 1.
Functional difference and FDA approved treatment of discussed inhibitors.
| Inhibitors | Specific target | Mechanism | FDA approved treatment | Reference |
|---|---|---|---|---|
| ARS-853 | KRASG12C | Covalent inhibitor | _ | 42 |
| ARS-1620 | KRASG12C | Covalent inhibitor | _ | 43 |
| AMG510 | KRASG12C | Covalent inhibitor | Locally advanced or metastatic NSCLC with KRASG12C mutation | 44, 45, 46 |
| MRTX849 | KRASG12C | Covalent inhibitor | KRASG12C-mutated NSCLC | 47 |
| PLX4720 | BRAFV600E | An ATP-competitive RAF kinase inhibitor | _ | 48 |
| Vemurafenib | BRAFV600E | An ATP-competitive RAF kinase inhibitor | Metastatic melanoma with BRAFV600E mutation, Erdheim–Chester Disease with BRAFV600 mutation | 49, 50, 51 |
| Dabrafenib | BRAFV600E/K/D | An ATP-competitive RAF kinase inhibitor | Unresectable or metastatic melanoma with BRAFV600E mutation | 52, 53, 54, 55, 56, 57, 58, 59 |
| Encorafenib | BRAFV600E/K | An ATP-competitive RAF kinase inhibitor | Unresectable or metastatic melanoma with BRAFV600E/K mutation | 60 |
| Trametinib | MEK1/2 | Allosteric non-ATP competitive inhibitor | Unresectable or metastatic melanoma with BRAFV600E/K, BRAF positive anaplastic thyroid cancer, Metastatic NSCLC with BRAFV600E mutation | 61,62 |
| Binimetinib | MEK1/2 | Allosteric inhibitor | Unresectable or metastatic melanoma with BRAFV600E/K or NRAS mutation | 63,64 |
| Cobimetinib | MEK1/2 | Allosteric inhibitor | Advanced melanoma with BRAFV600E/K mutation | 65, 66, 67, 68, 69, 70, 71 |
| ONC201 | AKT/ERK | _ | Small cell lung cancer | 72, 73, 74, 75 |
| LY3214996 | ERK1/2 | Reversible ATP-competitive kinase inhibitor | _ | 76,77 |
| Ulixertinib | ERK1/2 | Reversible ATP-competitive kinase inhibitor | _ | 78, 79, 80, 81, 82 |
| MK-8353 | ERK1/2 | Reversible ATP-competitive kinase inhibitor | _ | 83, 84, 85, 86, 87 |
| GDC-0994 | ERK1/2 | Reversible ATP-competitive kinase inhibitor | _ | 88, 89, 90, 91 |
| Rineterkib | RAF/ERK1/2 | _ | _ | 92, 93, 94 |
RAF inhibitors
Compared with CRAF and ARAF, BARF has a higher mutation frequency. The mutation BRAFV600E (also named BRAFV599E, with a substitution of valine by glutamic acid at position 599) accounts for 95% of all BRAF mutations.46,47,100 This mutation shows an elevated kinase activity by stabilizing the catalytically preferred conformation. PLX4720 is the first generation BRAFV600E inhibitor that targets the active DFG motif in ATP binding pocket of BRAFV600E, which opens the door to target BRAFV600E.101 Vemurafenib (PLX4032) was subsequently derived and was approved by the United States and the European Union for the treatment of BRAFV600-mutant melanoma in 2011 and 2012, respectively.48,49,102 In clinical trials, vemurafenib suppressed the melanoma growth in a large proportion of patients, but ineffectiveness was observed after 7–8 months of treatment. Dabrafenib was another selective BRAF inhibitor after vemurafenib, and was approved for the treatment of BRAFV600-mutant melanoma in 2013.50 Compared with vemurafenib, dabrafenib not only inhibits BRAFV600E-mutant tumors, but also inhibits BRAFV600K- and BRAFV600D-mutant tumor cells, and this inhibitor particularly shows good efficacy for those tumors with BRAFV600E mutation, such as melanoma, lung cancer, biliary tract cancer and thyroid cancer.51, 52, 53, 54, 55, 56, 57 Nevertheless, the resistance to dabrafenib occurs after several months of treatment as well.58 Encorafenib is a recently approved BRAF inhibitor, and shows a longer half-time of dissociation with lower abnormal activation of the MAPK pathway.59 However, none of these inhibitors show significant benefits for KRAS-driven cancer patients, and all acquire resistance after long-term treatment.
MEK inhibitors
Although the activated mutations in MEK are relatively low in human tumors, the activity of MEK is found at high levels in more than 85% of cancers partly because of the upstream mutations of RAS or RAF. Therefore, inhibiting MEK is an attractive and novel therapeutic strategy. To this end, several combinations (MEK inhibitor and BRAF inhibitor) were approved by FDA, and those therapeutic approaches effectively halted tumor growth in preclinical models and the patients with RAS or RAF mutations.60,103 Trametinib is a non-ATP competitive inhibitor of MEK1 and MEK2 (IC50 of 0.7 nM and 0.9 nM, respectively), and was approved by the FDA in 2013 for the treatment of unresectable melanoma with BRAFV600E/K mutation.104 In 2017, the combination therapy of dabrafenib and trametinib was also approved by the FDA for the treatment of non-small cell lung cancer (NSCLC) with BRAFV600E mutation.105 Cobimetinib (GDC-0973) is an effective and highly selective allosteric MEK1/2 inhibitor.106 This drug showed a good efficacy in BRAF and KRAS mutant cell lines and in BRAFV600E/K-mutant patients.107, 108, 109, 110, 111, 112 Cobimetinib and vemurafenib were approved by the FDA and EMA (European Medical Agency) in 2015 for the treatment of BRAFV600E/K mutant and unresectable melanoma.113 Binimetinib (MEK162 or ARRY-162 or ARRY-438162) is an effective and oral MEK1/2 inhibitor (IC50 of 12 nM).114 The combined treatment of binimetinib and encorafenib was approved by the FDA in 2018 for those patients with BRAFV600E/K-mutant and unresectable melanoma. Except for tumor inhibition, the side effects of combination therapy, especially the hyperkeratosis and verrucous keratosis, is significantly lower than that of monotherapy.115 The high incidence of cutaneous side effects can be explained by the hyper-proliferation of epidermal keratinocyte, which is induced by the paradoxical activation of the MAPK pathway.61 The addition of a MEK inhibitor alleviated the side effects by inhibiting the reactivated MAPK. However, resistance also occurs in patients after a period of treatment with BRAF/MEK combination therapy.62
Mechanisms of resistance to RAF and MEK inhibitors
Drug resistances markedly block the treatment efficacy of various human cancers thus far, including the targeted therapy. The approved RAFi/MEKi described above show advantages of high selectivity, initially effective inhibition, and low side effects; however, clinical responses to those inhibitors are highly variable among patients, and resistance occurs in less than one year without other intervention treatment.65 The mechanisms of RAFi/MEKi resistance might be classified as intrinsic resistance or acquired resistance as reported.66,67 The intrinsic, i.e. innate, means that the tumors do not respond to the drug treatment; the latter, i.e., adaptive, means that the responsive tumors develop the resistance under the stress of drug treatment. However, the two forms are similar to some degree, and the main mechanisms are discussed below.
Approaches to targeting KRAS-mutant or BRAF-mutant cancers have failed, often due to the upregulation of RTK signaling. For example, an in vitro study showed that the hepatocyte growth factor (HGF) secreted by stromal cells could induce the reactivation of ERK pathways via MET receptor, therefore mediating the resistance to vemurafenib-induced RAF inhibition in BRAF melanomas.66 EGFR-mediated resistance to vemurafenib was observed in BRAF-mutant colorectal cancers and melanomas as well.68,69 In addition, the RTKs also contribute to the MEKi resistance in KRAS-mutant cancers by ERK reactivation.63,70,71,116 Therefore, blocking the signals from those activated RTKs might be a viable therapeutic approach. A recent study revealed that combining SHP099 (an inhibitor targeting SHP2 which acts between RTKs and RAS) and MEKi could re-sensitize both KRAS-mutant and wild-type KRAS tumors.64 Notably, the RAF activation is regulated by RAF dimerization upon RAS activation in wild-type BRAF cells, while the mutant RAF catalytic function is regulated by its monomer.25,117 Therefore, clinically used ATP-competitive inhibitors could cause “theoretical resistances” by stabilizing a rigid closed conformation of the kinase domain of RAF, thus promoting RAF dimerization and leading to hyperactivation of ERK pathways.118 This intrinsic resistance was observed in wild-type BRAF tumors when treated with non-saturating concentrations of ATP-competitive inhibitors.119 Besides, the alternative spliced isoform of BRAF V600E,120 and constitutive bypass signaling such as YAP1,121 amplification of cyclin D1,122 loss of PTEN,123,124 etc., were also implicated in this process.
Acquired mutations of MEK1 (Q56P, P124L and F129L)125,126 and MEK2 (MEK2Q60P)127 were reported to mediate the adaptive resistance to the combination of RAF and MET inhibitors. These acquired mutations could increase ERK phosphorylation partly via MAP3K8 activation. Acquired splicing variants of BRAFV600E (namely p61, p55, p48 and p41 based on their predicted molecular weight) exhibited enhanced dimerization that escaped the RAF inhibitor and activated the ERK signaling.128, 129, 130, 131 Acquired alterations in upstream components of the RAS–ERK pathway, such as elevated or mutant NRAS (Q61K),132, 133, 134 RTKs (such as IGF1R and PDGFRβ67,135) and its ligands, BRAF amplification,134,136,137 reduced NF1138 and even elevated other RAF proteins (such as COT that activates MEK and ERK without BRAF),139,140 and ERK-induced feedback (such as DUSPs and SPRYs) or potential MEK-induced non-ERK feedback inhibition, loss-of-function mutations in STAG2141,142 and down-regulation of BOP1 (block of proliferation 1),143 etc., are beneficial to the reactivation of the pathway, and those share similarity with the intrinsic resistance. Except for the acquired resistance of RAS–ERK pathway, acquired bypass activations should be considered as one of the vital factors of adaptive resistance to inhibitors of RAS–ERK signaling. For example, IL-6/STAT3 activation was reported to mediate the resistance to selumetinib (MEKi) in KRAS-mutant NSCLC cells.144 Mutant mediator complex subunit 12 (MED12) contributed to the resistance to MEK and BRAF inhibitors via TGF-β signaling.145 Also, the PI3K–AKT (gene mutation),146,147 WNT5A/β-catenin,148 and YAP1 signaling activations121,149 are associated with resistance and promote tumor survival.
ERK inhibitors
ERK is found in more than 85% of cancers as well, which is majorly activated by the aberrant activation of upper-stream kinase while no oncogenic ERK has been reported to our best knowledge. In contrast to the development of RAF and MEK inhibitors,62 the current progress of ERK1/2 inhibitors is relatively limited. However, several ERK inhibitors are already in the clinical trials.
ONC201 (TIC10/NSC350625) induces TRAIL-mediated apoptosis in several tumors.150 The considerable efficacy in cells and animals has led to the clinical development of ONC201. Animal studies showed no observed adverse event at 10-fold of expected therapeutic dose. After that, the first clinical trial in patients with refractory solid tumors showed that ONC201 was well tolerated, and the recommended phase II dose was 625 mg in an accelerated titration design.151 Although no objective responses by RECIST were achieved, phase I/II clinical trials demonstrating the application of ONC201 in relapsed or refractory non-Hodgkin's lymphoma, metastatic triple negative breast cancer, relapsed or refractory acute leukemia or high-risk myelodysplastic syndrome, platinum refractory or resistant ovarian cancer, recurrent glioblastoma, or metastatic colorectal cancer is in full swing. In addition, ONC201 showed a good efficacy combined with lurbinectedin (a small molecule RNA polymerase II inhibitor), and received accelerated FDA approval in 2020 for metastatic small cell lung cancer that progressed after platinum-based therapy.152 Future studies might explore the combination of ONC201 and lurbinectedin in more tumor types and the clinical translation in humans.
LY3214996 is a thieno [2,3-c] pyrrol-4-one compound, which selectively competes with the ATP of ERK, and it is synthesized by Cortez et al (United States patent US9469652. 2016.). For now, LY3214996 is applied as a single drug in healthy participant, acute myelocytic leukemia, unresectable or metastatic colorectal cancer, advanced/metastatic cancer, and pancreatic cancer (NCT04033341, NCT04081259, NCT04616183, NCT02857270, and NCT04386057). The combinations of LY3214996 with SHP2 inhibitor (RMC-4630, NCT04916236) in KRAS-mutant cancers and with CDK inhibitor (abemaciclib, NCT04534283 and NCT04391595) in recurrent glioblastoma are being conducted by several groups. Preclinical studies showed that LY3214996 exhibited the single-agent activity, and resulted in synergistic (PI3K/mTOR inhibitor) and additive (CDK4/6 inhibitor) inhibition in patient-derived xenograft models of RAS-mutant lung cancer.153 Moreover, LY3214996 enhanced the anti-tumor effects of RAF inhibitor (sorafenib) in hepatocellular carcinoma (HCC). These findings provide a theoretical basis for clinical trials of LY3214996 combined with PI3K/mTOR inhibitor, CDK4/6 inhibitor, or RAF inhibitor.154
Ulixertinib (BVD-523) is a highly selective, reversible ATP-competitive inhibitor of ERK1/2.78 Clinical trials (NCT01781429, NCT02296242, and NCT02608229) indicated that ulixertinib has an acceptable safety profile with favorable pharmacokinetics. To date, ulixertinib has been explored in patients with lymphoma or melanoma. Notably, following that pharmacologically blocking both RAS–ERK and the autophagic process might be effective for pancreatic adenocarcinoma (PDAC), the efficacy of ulixertinib with hydroxychloroquine (an autophagy inhibitor) is being explored in patients with gastrointestinal adenocarcinoma and PDAC.155,156 In addition, ulixertinib inhibits the tumor growth in NRAS- or BRAF-mutant xenograft models, and it potentiated the effects of gemcitabine, PI3K and HER inhibitors in several PDAC cell lines, and partly released ABCB1- and ABCG2-mediated chemotherapeutic drug resistance in cancer cells.78,157
MK-8353 is an oral and highly selective inhibitor of both active and inactive ERK1/2,83 and can decrease the phosphorylation of RSK (an ERK1/2 substrate) that contributes to the ERK signaling inhibition as well.84 Besides, MK-8353 is also a weak inhibitor of hERG. Preclinical studies showed the antitumor efficacy in several BRAF-mutant models. A phase I trial demonstrated antitumor activities in patients with metastatic BRAFV600-mutant melanoma.158 However, there was no efficacy in advanced solid-tumor patients with NRAS- or KRAS-mutants, including NSCLC, PDAC and colorectal cancer.158 Recently, a phase Ib trial was performed to evaluate the efficacy of MK-8353 in patients with advanced or recurrent solid tumors in combination with pembrolizumab (a humanized monoclonal anti-PD1 antibody)88 and selumetinib (a MEK1/2 inhibitor) (NCT03745989).159 Given that, further combination development of MK-8353 and selection of tumor types are currently under investigation.
GDC-0994 is an oral and highly selective inhibitor of ERK1/2 and inhibits the ERK phosphorylation, and could reduce the tumor growth in vivo and in vitro, including KRAS- and BRAF-mutant tumors.160,161 A first-in-human phase I study demonstrated that GDC-0994 inhibited the MAPK signaling ranged from 19% to 51% with an acceptable safety profile, and induced 33% of overall responses of stable disease and 4% of partial responses in patients with BRAF-mutant colorectal cancer.162 In a phase Ib study, GDC-0994 in combination with cobimetinib (a selective inhibitor of MEK1/2) was performed in patients with advanced solid tumors.72 However, the trial was restricted because of the disability to manage the overlapping adverse events and cumulative toxicity.
Rineterkib, also known as LTT-462 or ERK-IN-1, is a RAF and ERK1/2 inhibitor that has demonstrated preclinical activity in multiple MAPK activated cancer cells and xenograft models. To date, rineterkib is applied as a single drug in adult patients with advanced cancers, including ovarian neoplasms, non-small-cell lung carcinoma, melanoma, etc., in phase I dose-finding study (NCT02711345),73,74 with acceptable tolerance and limited clinical activity. Phase 1b and phase 2 clinical trials exploring the combination of LTT462 with RAF inhibitor (LXH254) in NSCLC and melanoma are ongoing (NCT02974725 and NCT04417621).75 Except of that, a phase Ib trial is exploring the efficacy of combinations of LTT462 with different RAF inhibitors in colorectal cancer (NCT04294160). A multi-arm clinical trial is exploring the efficacy of LTT462 with JAK inhibitor (ruxolitinib).
Combination treatment of RAS–RAF–MEK–ERK pathway
Molecular target inhibitors provide a better way to customize cancer treatment for the minimum side effects on normal cells and improve efficacy. We have summarized the impact of these inhibitors alone on tumors. Next, we will describe the currently possible combination therapies including cytotoxic chemotherapy, radiotherapy, autophagy, and immunotherapy, which have been explored in preclinical experiments and clinical trials.
Combination with chemotherapy and radiotherapy
Radiotherapy (RT) is applied in approximately 50% of all cancer patients with about 40% of the curative efficacy. The primary mechanism of RT-induced anticancer toxicity is DNA double-strand breaks (DSBs), while some tumors could oppose to the efficiency through DNA damage repair. ERK and CRAF mediate RT-induced repair of DNA damage and promote tumor resistance to RT.76,77 MEK inhibitor (GSK1120212) significantly suppressed the DSB repair and sensitized the KRAS-mutant pancreatic tumor cell lines to RT. RAF inhibitors have similar effects that vemurafenib sensitized BRAFV600E thyroid cancer cells to RT by reducing the DSB repair. In contrast, PLX4720 sensitized high-grade BRAFV600E-mutant glioma cells to RT.79,80 Vemurafenib also significantly enhanced melanoma cell radio-sensitivity and synergistic tumor inhibition in melanoma xenograft models.81
Clinically, an RAF inhibitor (sorafenib) and radiotherapy were administrated to hepatocellular carcinoma patients.82 However, severe skin toxicity was observed after receiving BRAF inhibitors (vemurafenib and dabrafenib) and RT85 except for the report of Rompoti.86 And no obvious intracranial toxicity was found in combined treatment compared with that of RT alone. These phenomena might be related to the distinct metastatic locations that accepted RT. A large multi-center analysis was carried out to generate reliable data, and an acceptable toxicity was observed after radiotherapy with concomitant BRAF inhibitor therapy.87 Recently, the melanoma patients receiving interruption of vemurafenib treatment during radiation showed a prolonged survival and lower toxicity than those receiving concomitant treatment.89 The RAF/MEK inhibitors, which can penetrate the blood–brain barrier, are considered to treat melanoma brain metastasis (NCT02039947)90 combined with RT, and considerable efficacy and manageable safety were achieved. Selective BRAF and MEK inhibitors could also re-sensitize the advanced thyroid cancer cells to RT and induce a better response in patients, but the adverse events are largely remained unknown.91 These studies imply more focus on the combination in different tumors.
Chemotherapy resistance is another issue for the refractory tumors.92 The RAS–RAF–MEK–ERK pathway is widely activated in tumors, and high frequency of KRAS and BRAF mutations are observed in pancreatic cancer, melanoma and lung cancer.93,107,108 A recent study has declared the association between RAS–RAF–MEK–ERK pathway and cytotoxic chemotherapy resistance. The combination of RAF inhibitors (sorafenib) and traditional chemotherapy regimens (gemcitabine) did not show significant advantages in high-grade pancreatic cancer.93 Also, the combination of MEK inhibitor (binimetinib) and gemcitabine did not improve the disease-free survival time in cholangiocarcinoma patients. These studies suggested that combining RAS–RAF–MEK–ERK inhibitors and gemcitabine might be unable to improve the treatment outcome. However, this pathway combined with platinum-based chemotherapy has a good therapeutic effect. For example, the combination of MEK inhibitor (MEK162), bevacizumab and paclitaxel improved the treatment outcome without additional adverse events in ovarian cancer.94 Inhibition of ERK augmented the cisplatin sensitivity in squamous cell carcinoma cells and demonstrated the efficacy and acceptable tolerability in vivo.163 A phase II clinical trial evaluated the safety of a MEK inhibitor (selumetinib) combined with first-line platinum inhibitors (carboplatin and cisplatin), and suggested that selumetinib combined with platinum drugs has more potential than platinum alone in NSCLC.164
Combination with autophagy inhibitors
Recently, autophagy has been reported to act as an accomplice that contributed to the resistance to RAS–RAF–MEK–ERK pathway inhibitors especially in BRAFV600E-mutant tumors. Sorafenib, a multi-kinase inhibitor of the RAS–RAF–MEK–ERK pathway, has been applied to treat advanced HCC. But the resistance has obstructed its application, partly because of an accumulation of autophagosomes.165 Concomitant inhibition of autophagy inhibited the growth of tumors in mice, which uncovers the potential of this combination in HCC therapy. Consistent with sorafenib, resistance occurred after a period of treatment with UI-152 (an inhibitor of BRAF) in melanoma166,167; autophagy inhibition partially arrested the cell growth and promoted tumor regression after treatment with UI-152.168 The newly developed autophagy inhibitor also sensitized the BRAFV600E-mutant melanoma cells to vemurafenib.167,169 In BRAFV600E-mutant glioma cells, autophagy inhibition augmented the growth inhibition accompanied by BRAF inhibitor (UAI-201).170 Similar synergistic effects are also demonstrated in other BRAF-mutant brain tumors, thyroid cancer, pancreatic cancer and colorectal cancer.171, 172, 173, 174, 175, 176 Combining MEK inhibitors and autophagy inhibitors allowed BRAF-resistant cells to die again.177 Although the combination of inhibitors of autophagy and RAS–RAF–MEK–ERK pathway has shown good potential in cells and mouse models, the relevant clinical trials are still in an exploring phase. The above studies provide a good theoretical basis for the clinical trials that combining RAS–RAF–MEK–ERK inhibitors with autophagy inhibitors.
Combination with immune inhibitors
Immunotherapy has made remarkable progress in achieving an extension of overall survival and long-term durable remission in tumor patients, which mainly depends on memory CD8+ T cells. Recently, studies have shown that MEK inhibition could reprogram the CD8+ T cells into memory stem cells with potent antitumor ability,178 and RAF inhibition could induce PD-L1 expression of cancer cells.179, 180, 181 According to the biopsies and gene-based transcriptomic analysis, BRAF inhibitors were found to induce the exhausted CD8+ T cells in resistant melanomas.182,183 In triple-negative breast cancer cells, the MEK inhibitor significantly increased the expression of PD-L1; combining PD-L1 antibodies and MEK inhibitor enhanced the antitumor immune response in a mouse model of breast cancer and colon cancer.180,181 In pancreatic cancer, the MEK inhibitor acted on the myeloid cells of the immunosuppressive environment and enhanced the efficiency of PD-L1 antibodies.184 Genomic landscapes of gastroesophageal adenocarcinoma provided a novel insight that combined these pathway inhibitors with a specific immune checkpoint inhibitor.185 A recent study also revealed that a RAS mimetic, namely rigosertib, augmented response to checkpoint blockade by inducing the expression of CD40.186 All the evidence has driven the progress of the clinical trials that combining RAS–RAF–MEK–ERK inhibitors with immunotherapies.
Further, short-term treatment (less than 15 days) of combination of BRAF/MEK inhibitors and anti-PD-1/PD-L1 antibodies increased the immune cell infiltration and arrested the tumor growth in melanoma patients.187 However, a phase I study was terminated because of the dose-limiting hepatotoxicity in patients who received vemurafenib and ipilimumab.188 Another phase I study explored the clinical effect and safety of combination of anti-PD-L1 antibody and BRAF inhibitor (dabrafenib)/MEK inhibitor (trametinib) in BRAF-mutant and BRAF wild-type melanoma (NCT02027961).189 The clinical efficacy was considerable, and further investigations such as longer follow-up and more patients should be conducted for the safety evaluation of the concurrent treatments. Besides, a phase II study explored the efficacy of combination of anti-PD-L1 antibody (pembrolizumab) and BRAF/MEK inhibitors (dabrafenib and trametinib) in advanced melanoma patients (NCT02625337),190 and suggested a potential for optimization of the administration order in the treatment of melanoma patients.
In recent years, immune checkpoint inhibitors have uncovered a new era for lung cancer treatment,191 but the overall remission rate are still lower than 31%.192,193 Preclinical data showed that T cell activation and cytotoxic T cell-associated antigen 4 (CTLA-4) expression increased after the combination treatment of selumetinib and trametinib. In addition, pulsed MEKi therapy combined with CTLA-4 blockade prolonged the survival of tumor-bearing mice with KRAS mutation.194,195 Therefore, an initial phase Ib study was performed to explore the safety and efficacy of cobimetinib combined with atezolizumab. The median OS time for NSCLC was 13.2 months, and the ORR reached to 18%. The most common adverse events included diarrhea (67%), skin rash (48%) and fatigue (40%).196 Another ongoing phase I/II trial aims to evaluate the continuous or intermittent administration of selumetinib for immunotherapy (durvalumab/tremelimumab) in patients with NSCLC.197 Therefore, the clinical data on MEK inhibitors combined with immune checkpoint inhibitors are still not enough to determine the best remedy for NSCLC.
Conclusions and future perspectives
Inhibitors targeting RAS–RAF–MEK–ERK exhibit good tumor-suppressive effects and biosafety. Various types of inhibitors have been approved by FDA thus far, but long-term administration leads to the emergence of drug resistance. The major mechanisms of RAFi and MEKi resistance are the re-activation of the RAS–ERK pathway and activation of the bypass signal pathway, therefore targeting the downstream molecule and bypass activation might provide an alternative strategy. At present, the exploration of inhibitors targeting ERK has been in full swing, that several inhibitors have been under evaluation in clinical trials, even so, no drugs have been approved. In addition, the combinations of ERKi with RNA polymerase II, CDK, PI3K, hydroxychloroquine, or up-stream molecules inhibitors have been explored in several clinical trials, however, severe adverse events are observed after the treatment with MEKi, which suggested that combinations with bypass signaling pathway blocking might be a better choice than the addition of the same pathway inhibitors. Except for the combinations with other targeted therapies, inhibitors of the RAS–ERK pathway alleviate resistance to traditional radiotherapy and chemotherapy and can enhance the therapeutic effects of antibodies (targeted PD-1, PD-L1, and CTLA-4). For now, the efficacy of RAFi/MEKi with traditional therapies has been well investigated and high levels of activated ERK are always observed in cisplatin and RT resistant tumor cells,198, 199, 200, 201, 202 while the combinations with ERK inhibitors are still rarely known.
Taken together, further studies should highlight the extra drug-resistance mechanisms, especially the additional activation of the bypass signaling, and focus on the therapeutic schedule for each specific tumor, including optimal combinations of inhibitors and/or clinically used drugs, suitable dosage, and even different groups of patients, therefore obtaining a considerable efficacy with minimum adverse events.
Author contributions
Yanlin Song & Zhenfei Bi: Data curation; Formal analysis; Writing – original draft. Yu Liu: Writing – review & editing. Furong Qin: Writing – review & editing. Yuquan Wei: Conceptualization; Supervision. Xiawei Wei: Conceptualization; Supervision; Validation; Writing – review editing. All authors read and approved the final manuscript.
Conflict of interests
The authors declare no competing interests.
Funding
This work is supported by the Key R&D Project of Sichuan Province, China (No. 2020YFS0553), the National Science Fund for Excellent Young Scholars, China (No. 32122052), the National Natural Science Foundation Regional Innovation and Development, China (No. U19A2003).
Acknowledgements
The authors thank the contributions of members in the laboratories, who have contributed to current and past research on molecular target therapy. Figures were produced using Biorender.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Spagnolo F., Boutros A., Tanda E., et al. The adjuvant treatment revolution for high-risk melanoma patients. Semin Cancer Biol. 2019;59:283–289. doi: 10.1016/j.semcancer.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Bedard P.L., Hyman D.M., Davids M.S., et al. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 3.Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 4.Balmanno K., Cook S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16(3):368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y., Liu W.Z., Liu T., et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 6.Asl E.R., Amini M., Najafi S., et al. Interplay between MAPK/ERK signaling pathway and microRNAs: a crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021;278:119499. doi: 10.1016/j.lfs.2021.119499. [DOI] [PubMed] [Google Scholar]
- 7.Maik-Rachline G., Hacohen-Lev-Ran A., Seger R. Nuclear ERK: mechanism of translocation, substrates, and role in cancer. Int J Mol Sci. 2019;20(5):1194. doi: 10.3390/ijms20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang Y.J., Kwon J.H., Kang S.H., et al. Increased MAPK activity and MKP-1 overexpression in human gastric adenocarcinoma. Biochem Biophys Res Commun. 1998;250(1):43–47. doi: 10.1006/bbrc.1998.9256. [DOI] [PubMed] [Google Scholar]
- 9.Fang J.Y., Richardson B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6(5):322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 10.Huynh H., Nguyen T.T., Chow K.H., et al. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. doi: 10.1186/1471-230X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F., Yang X., Geng M., et al. Targeting ERK, an Achilles' heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8(4):552–562. doi: 10.1016/j.apsb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan A.Q., Kuttikrishnan S., Siveen K.S., et al. RAS-mediated oncogenic signaling pathways in human malignancies. Semin Cancer Biol. 2019;54:1–13. doi: 10.1016/j.semcancer.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Belov A.A., Mohammadi M. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci Signal. 2012;5(249):pe49. doi: 10.1126/scisignal.2003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W.Y.C., Alvarez S., Kondo Y., et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science. 2019;363(6431):1098–1103. doi: 10.1126/science.aau5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennig A., Markwart R., Esparza-Franco M.A., et al. Ras activation revisited: role of GEF and GAP systems. Biol Chem. 2015;396(8):831–848. doi: 10.1515/hsz-2014-0257. [DOI] [PubMed] [Google Scholar]
- 17.Roskoski R., Jr. Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacol Res. 2018;135:239–258. doi: 10.1016/j.phrs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Rezaei Adariani S., Buchholzer M., Akbarzadeh M., et al. Structural snapshots of RAF kinase interactions. Biochem Soc Trans. 2018;46(6):1393–1406. doi: 10.1042/BST20170528. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J., Ng W.H., Lam P.Y.P., et al. The dimer-dependent catalytic activity of RAF family kinases is revealed through characterizing their oncogenic mutants. Oncogene. 2018;37(43):5719–5734. doi: 10.1038/s41388-018-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J., Stites E.C., Yu H., et al. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154(5):1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavoie H., Sahmi M., Maisonneuve P., et al. MEK drives BRAF activation through allosteric control of KSR proteins. Nature. 2018;554(7693):549–553. doi: 10.1038/nature25478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber C.K., Slupsky J.R., Kalmes H.A., et al. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 2001;61(9):3595–3598. [PubMed] [Google Scholar]
- 23.Wan P.T., Garnett M.J., Roe S.M., et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 24.Garnett M.J., Rana S., Paterson H., et al. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20(6):963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Rajakulendran T., Sahmi M., Lefrançois M., et al. A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461(7263):542–545. doi: 10.1038/nature08314. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Adjei A.A. The clinical development of MEK inhibitors. Nat Rev Clin Oncol. 2014;11(7):385–400. doi: 10.1038/nrclinonc.2014.83. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J., Ng W.H., Tian Z., et al. Activating mutations in MEK1 enhance homodimerization and promote tumorigenesis. Sci Signal. 2018;11(554):eaar6795. doi: 10.1126/scisignal.aar6795. [DOI] [PubMed] [Google Scholar]
- 28.Luke J.J., Ott P.A., Shapiro G.I. The biology and clinical development of MEK inhibitors for cancer. Drugs. 2014;74(18):2111–2128. doi: 10.1007/s40265-014-0315-4. [DOI] [PubMed] [Google Scholar]
- 29.Brummer T., Naegele H., Reth M., et al. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene. 2003;22(55):8823–8834. doi: 10.1038/sj.onc.1207185. [DOI] [PubMed] [Google Scholar]
- 30.Corbalan-Garcia S., Yang S.S., Degenhardt K.R., et al. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996;16(10):5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritt D.A., Monson D.M., Specht S.I., et al. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30(3):806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tassin T.C., Benavides D.R., Plattner F., et al. Regulation of ERK kinase by MEK1 kinase inhibition in the brain. J Biol Chem. 2015;290(26):16319–16329. doi: 10.1074/jbc.M115.654897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougherty M.K., Müller J., Ritt D.A., et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17(2):215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 34.Saha M., Carriere A., Cheerathodi M., et al. RSK phosphorylates SOS1 creating 14-3-3-docking sites and negatively regulating MAPK activation. Biochem J. 2012;447(1):159–166. doi: 10.1042/BJ20120938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson K.I., Brummer T., O'Brien P.M., et al. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418(3):475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 36.Ruvolo P.P. Role of protein phosphatases in the cancer microenvironment. Biochim Biophys Acta Mol Cell Res. 2019;1866(1):144–152. doi: 10.1016/j.bbamcr.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Yang J., Nie J., Ma X., et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arkun Y. Dynamic modeling and analysis of the cross-talk between insulin/AKT and MAPK/ERK signaling pathways. PLoS One. 2016;11(3):e0149684. doi: 10.1371/journal.pone.0149684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehr S., Kotzka J., Avci H., et al. Identification of major ERK-related phosphorylation sites in Gab1. Biochemistry. 2004;43(38):12133–12140. doi: 10.1021/bi049753e. [DOI] [PubMed] [Google Scholar]
- 40.Harada H., Andersen J.S., Mann M., et al. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci U S A. 2001;98(17):9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta S., Peterson T.R., Sabatini D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canon J., Rex K., Saiki A.Y., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 43.FDA approves first KRAS inhibitor: sotorasib. Cancer Discov. 2021;11(8):OF4. doi: 10.1158/2159-8290.CD-NB2021-0362. [DOI] [PubMed] [Google Scholar]
- 44.Hallin J., Engstrom L.D., Hargis L., et al. The KRAS G12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10(1):54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awad M.M., Liu S., Rybkin, et al. Acquired resistance to KRAS G12C inhibition in cancer. N Engl J Med. 2021;384(25):2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer K., Giblett S., Green S., et al. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res. 2005;65(24):11493–11500. doi: 10.1158/0008-5472.CAN-05-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Nazmun N., Hassan S., et al. BRAF mutation and its inhibitors in sarcoma treatment. Cancer Med. 2020;9(14):4881–4896. doi: 10.1002/cam4.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J.T., Li L., Brafford P.A., et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23(6):820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Zhu G., Zhang Q., et al. Rational design, synthesis, and biological evaluation of Pan-Raf inhibitors to overcome resistance. Org Biomol Chem. 2017;15(16):3455–3465. doi: 10.1039/c7ob00518k. [DOI] [PubMed] [Google Scholar]
- 50.Pinchuk B., von Drathen T., Opel V., et al. Photoinduced conversion of antimelanoma agent dabrafenib to a novel fluorescent BRAF V600E inhibitor. ACS Med Chem Lett. 2016;7(10):962–966. doi: 10.1021/acsmedchemlett.6b00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulva K., Apte S., Yu A., et al. Contemporary neoadjuvant therapies for high-risk melanoma: a systematic review. Cancers. 2021;13(8):1905. doi: 10.3390/cancers13081905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Golen R.F., Dekker T.J.A. Dabrafenib plus trametinib in patients with BRAF V600E-mutated biliary tract cancer. Lancet Oncol. 2020;21(11):e515. doi: 10.1016/S1470-2045(20)30554-4. [DOI] [PubMed] [Google Scholar]
- 53.Ribeiro M.F.S.A., Knebel F.H., Bettoni F., et al. Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient. NPJ Precis Oncol. 2021;5(1):5. doi: 10.1038/s41698-021-00149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hicks H.M., McKenna L.R., Espinoza V.L., et al. Inhibition of BRAF and ERK1/2 has synergistic effects on thyroid cancer growth in vitro and in vivo. Mol Carcinog. 2021;60(3):201–212. doi: 10.1002/mc.23284. [DOI] [PubMed] [Google Scholar]
- 55.Tarhini A.A., Toor K., Chan K., et al. A matching-adjusted indirect comparison of combination nivolumab plus ipilimumab with BRAF plus MEK inhibitors for the treatment of BRAF-mutant advanced melanoma. ESMO Open. 2021;6(2):100050. doi: 10.1016/j.esmoop.2021.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Awada G., Schwarze J.K., Tijtgat J., et al. A phase 2 clinical trial of trametinib and low-dose dabrafenib in patients with advanced pretreated NRASQ61R/K/l mutant melanoma (TraMel-WT) Cancers. 2021;13(9):2010. doi: 10.3390/cancers13092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiacci E., De Carolis L., Simonetti E., et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: a pilot phase-2 clinical trial. Leukemia. 2021;35(11):3314–3318. doi: 10.1038/s41375-021-01210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proietti I., Skroza N., Michelini S., et al. BRAF inhibitors: molecular targeting and immunomodulatory actions. Cancers. 2020;12(7):1823. doi: 10.3390/cancers12071823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koelblinger P., Thuerigen O., Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol. 2018;30(2):125–133. doi: 10.1097/CCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirchberger M.C., Ugurel S., Mangana J., et al. MEK inhibition may increase survival of NRAS-mutated melanoma patients treated with checkpoint blockade: results of a retrospective multicentre analysis of 364 patients. Eur J Cancer. 2018;98:10–16. doi: 10.1016/j.ejca.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Su F., Viros A., Milagre C., et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 63.Montero-Conde C., Ruiz-Llorente S., Dominguez J.M., et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fedele C., Ran H., Diskin B., et al. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 2018;8(10):1237–1249. doi: 10.1158/2159-8290.CD-18-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan R.J., Flaherty K.T. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49(6):1297–1304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Straussman R., Morikawa T., Shee K., et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nazarian R., Shi H., Wang Q., et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prahallad A., Sun C., Huang S., et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 69.Corcoran R.B., Ebi H., Turke A.B., et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson G.R., Winter P.S., Lin K.H., et al. A landscape of therapeutic cooperativity in KRAS mutant cancers reveals principles for controlling tumor evolution. Cell Rep. 2017;20(4):999–1015. doi: 10.1016/j.celrep.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun C., Hobor S., Bertotti A., et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 2014;7(1):86–93. doi: 10.1016/j.celrep.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 72.Weekes C., Lockhart A., LoRusso P., et al. A phase Ib study to evaluate the MEK inhibitor cobimetinib in combination with the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Oncologist. 2020;25(10):833–e1438. doi: 10.1634/theoncologist.2020-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janku F., Elez E., Iyer G., et al. Phase I dose-finding study of oral ERK1/2 inhibitor LTT462 in patients (pts) with advanced solid tumors harboring MAPK pathway alterations. J Clin Oncol. 2020;38(15_suppl):3640. [Google Scholar]
- 74.Niu Y., Ji H. Current developments in extracellular-regulated protein kinase (ERK1/2) inhibitors. Drug Discov Today. 2022;27(5):1464–1473. doi: 10.1016/j.drudis.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Wolf J., Planchard D., Heist R.S., et al. 1387P Phase Ib study of LXH254+ LTT462 in patients with KRAS-or BRAF-mutant NSCLC. 2020;31:S881–S882. [Google Scholar]
- 76.Marampon F., Ciccarelli C., Zani B.M. Biological rationale for targeting MEK/ERK pathways in anti-cancer therapy and to potentiate tumour responses to radiation. Int J Mol Sci. 2019;20(10):E2530. doi: 10.3390/ijms20102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Advani S.J., Camargo M.F., Seguin L., et al. Kinase-independent role for CRAF-driving tumour radioresistance via CHK2. Nat Commun. 2015;6:8154. doi: 10.1038/ncomms9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang H., Xu M., Li L., et al. Concurrent HER or PI3K inhibition potentiates the antitumor effect of the ERK inhibitor ulixertinib in preclinical pancreatic cancer models. Mol Cancer Ther. 2018;17(10):2144–2155. doi: 10.1158/1535-7163.MCT-17-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dasgupta T., Olow A.K., Yang X., et al. Survival advantage combining a BRAF inhibitor and radiation in BRAF V600E-mutant glioma. J Neurooncol. 2016;126(3):385–393. doi: 10.1007/s11060-015-1939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robb R., Yang L., Shen C., et al. Inhibiting BRAF oncogene-mediated radioresistance effectively radiosensitizes BRAF V600E-mutant thyroid cancer cells by constraining DNA double-strand break repair. Clin Cancer Res. 2019;25(15):4749–4760. doi: 10.1158/1078-0432.CCR-18-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krayem M., Sabbah M., Najem A., et al. The benefit of reactivating p53 under MAPK inhibition on the efficacy of radiotherapy in melanoma. Cancers. 2019;11(8):1093. doi: 10.3390/cancers11081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Girard N., Mornex F. Sorafenib and radiotherapy association for hepatocellular carcinoma. Cancer Radiother. 2011;15(1):77–80. doi: 10.1016/j.canrad.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Boga S.B., Deng Y., Zhu L., et al. MK-8353: discovery of an orally bioavailable dual mechanism ERK inhibitor for oncology. ACS Med Chem Lett. 2018;9(7):761–767. doi: 10.1021/acsmedchemlett.8b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roskoski R., Jr. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol Res. 2019;142:151–168. [Google Scholar]
- 85.Satzger I., Degen A., Asper H., et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31(13):e220–e222. doi: 10.1200/JCO.2012.44.4265. [DOI] [PubMed] [Google Scholar]
- 86.Rompoti N., Schilling B., Livingstone E., et al. Combination of BRAF inhibitors and brain radiotherapy in patients with metastatic melanoma shows minimal acute toxicity. J Clin Oncol. 2013;31(30):3844–3845. doi: 10.1200/JCO.2013.50.8473. [DOI] [PubMed] [Google Scholar]
- 87.Hecht M., Zimmer L., Loquai C., et al. Radiosensitization by BRAF inhibitor therapy-mechanism and frequency of toxicity in melanoma patients. Ann Oncol. 2015;26(6):1238–1244. doi: 10.1093/annonc/mdv139. [DOI] [PubMed] [Google Scholar]
- 88.Chin H.M., Lai D.K., Falchook G.S. Extracellular signal-regulated kinase (ERK) inhibitors in oncology clinical trials. J Immunother Precis Oncol. 2019;2(1):10–16. [Google Scholar]
- 89.Hecht M., Meier F., Zimmer L., et al. Clinical outcome of concomitant vs interrupted BRAF inhibitor therapy during radiotherapy in melanoma patients. Br J Cancer. 2018;118(6):785–792. doi: 10.1038/bjc.2017.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glitza Oliva I.C., Schvartsman G., Tawbi H. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol. 2018;29(7):1509–1520. doi: 10.1093/annonc/mdy185. [DOI] [PubMed] [Google Scholar]
- 91.Jaber T., Waguespack S.G., Cabanillas M.E., et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018;103(10):3698–3705. doi: 10.1210/jc.2018-00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCubrey J.A., Steelman L.S., Kempf C.R., et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011;226(11):2762–2781. doi: 10.1002/jcp.22647. [DOI] [PubMed] [Google Scholar]
- 93.Cascinu S., Berardi R., Sobrero A., et al. Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: a GISCAD randomized phase II study. Dig Liver Dis. 2014;46(2):182–186. doi: 10.1016/j.dld.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 94.Ricci F., Guffanti F., Damia G., et al. Combination of paclitaxel, bevacizumab and MEK162 in second line treatment in platinum-relapsing patient derived ovarian cancer xenografts. Mol Cancer. 2017;16(1):97. doi: 10.1186/s12943-017-0662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan J., Dong X., Yap J., et al. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13(1):113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drosten M., Barbacid M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell. 2020;37(4):543–550. doi: 10.1016/j.ccell.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Ostrem J.M., Peters U., Sos M.L., et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patricelli M.P., Janes M.R., Li L.S., et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6(3):316–329. doi: 10.1158/2159-8290.CD-15-1105. [DOI] [PubMed] [Google Scholar]
- 99.Janes M.R., Zhang J., Li L.S., et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172(3):578–589. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Davies H., Bignell G.R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 101.Tsai J., Lee J.T., Wang W., et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ascierto P.A., Kirkwood J.M., Grob J.J., et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dummer R., Schadendorf D., Ascierto P.A., et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(4):435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- 104.Kakadia S., Yarlagadda N., Awad R., et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095–7107. doi: 10.2147/OTT.S182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gilmartin A.G., Bleam M.R., Groy A., et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17(5):989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 106.Sarkisian S., Davar D. MEK inhibitors for the treatment of NRAS mutant melanoma. Drug Des Devel Ther. 2018;12:2553–2565. doi: 10.2147/DDDT.S131721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amaral T., Nouri N., Garbe C. The safety and efficacy of cobimetinib for the treatment of BRAF V600E or V600K melanoma. Expert Rev Anticancer Ther. 2016;16(7):705–715. doi: 10.1080/14737140.2016.1192469. [DOI] [PubMed] [Google Scholar]
- 108.Eagles J.R., Jimeno A. Cobimetinib: inhibiting MEK1/2 in BRAF V600-mutant melanoma. Drugs Today. 2016;52(11):593–605. doi: 10.1358/dot.2016.52.11.2542234. [DOI] [PubMed] [Google Scholar]
- 109.Hatzivassiliou G., Haling J.R., Chen H., et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501(7466):232–236. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 110.Larkin J., Ascierto P.A., Dréno B., et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 111.Ribas A., Gonzalez R., Pavlick A., et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–965. doi: 10.1016/S1470-2045(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 112.Grimaldi A.M., Simeone E., Ascierto P.A. Vemurafenib plus cobimetinib in the treatment of mutated metastatic melanoma: the CoBRIM trial. Melanoma Manag. 2015;2(3):209–215. doi: 10.2217/mmt.15.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andrlová H., Zeiser R., Meiss F. Cobimetinib (GDC-0973, XL518) Recent Results Cancer Res. 2018:177–186. doi: 10.1007/978-3-319-91442-8_12. Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- 114.Shirley M. Encorafenib and binimetinib: first global approvals. Drugs. 2018;78(12):1277–1284. doi: 10.1007/s40265-018-0963-x. [DOI] [PubMed] [Google Scholar]
- 115.Russo I., Zorzetto L., Frigo A.C., et al. A comparative study of the cutaneous side effects between BRAF monotherapy and BRAF/MEK inhibitor combination therapy in patients with advanced melanoma: a single-centre experience. Eur J Dermatol. 2017;27(5):482–486. doi: 10.1684/ejd.2017.3069. [DOI] [PubMed] [Google Scholar]
- 116.Manchado E., Weissmueller S., Morris J.P., 4th, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534(7609):647–651. doi: 10.1038/nature18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freeman A.K., Ritt D.A., Morrison D.K. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol Cell. 2013;49(4):751–758. doi: 10.1016/j.molcel.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lavoie H., Thevakumaran N., Gavory G., et al. Inhibitors that stabilize a closed RAF kinase domain conformation induce dimerization. Nat Chem Biol. 2013;9(7):428–436. doi: 10.1038/nchembio.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poulikakos P.I., Zhang C., Bollag G., et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin L., Asthana S., Chan E., et al. Mapping the molecular determinants of BRAF oncogene dependence in human lung cancer. Proc Natl Acad Sci U S A. 2014;111(7):E748–E757. doi: 10.1073/pnas.1320956111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kitajima S., Asahina H., Chen T., et al. Overcoming resistance to dual innate immune and MEK inhibition downstream of KRAS. Cancer Cell. 2018;34(3):439–452. doi: 10.1016/j.ccell.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smalley K.S., Lioni M., Dalla Palma M., et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7(9):2876–2883. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paraiso K.H., Xiang Y., Rebecca V.W., et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Catalanotti F., Cheng D.T., Shoushtari A.N., et al. PTEN loss-of-function alterations are associated with intrinsic resistance to BRAF inhibitors in metastatic melanoma. JCO Precis Oncol. 2017;1 doi: 10.1200/PO.16.00054. PO.16.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Emery C.M., Vijayendran K.G., Zipser M.C., et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang H., Daouti S., Li W.H., et al. Identification of the MEK1(F129L) activating mutation as a potential mechanism of acquired resistance to MEK inhibition in human cancers carrying the B-RafV600E mutation. Cancer Res. 2011;71(16):5535–5545. doi: 10.1158/0008-5472.CAN-10-4351. [DOI] [PubMed] [Google Scholar]
- 127.Wagle N., Van Allen E.M., Treacy D.J., et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4(1):61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poulikakos P.I., Rosen N. Mutant BRAF melanomas: dependence and resistance. Cancer Cell. 2011;19(1):11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 129.Poulikakos P.I., Persaud Y., Janakiraman M., et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480(7377):387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vido M.J., Le K., Hartsough E.J., et al. BRAF splice variant resistance to RAF inhibitor requires enhanced MEK association. Cell Rep. 2018;25(6):1501–1510. doi: 10.1016/j.celrep.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moriceau G., Hugo W., Hong A., et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer Cell. 2015;27(2):240–256. doi: 10.1016/j.ccell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaplan F.M., Kugel C.H., 3rd, Dadpey N., et al. SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J Biol Chem. 2012;287(50):41797–41807. doi: 10.1074/jbc.M112.390906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi H., Hugo W., Kong X., et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson D.B., Menzies A.M., Zimmer L., et al. Acquired BRAF inhibitor resistance: a multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer. 2015;51(18):2792–2799. doi: 10.1016/j.ejca.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villanueva J., Vultur A., Lee J.T., et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corcoran R.B., Dias-Santagata D., Bergethon K., et al. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3(149):ra84. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Long G.V., Fung C., Menzies A.M., et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun. 2014;5:5694. doi: 10.1038/ncomms6694. [DOI] [PubMed] [Google Scholar]
- 138.Van Allen E.M., Wagle N., Sucker A., et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doudican N.A., Orlow S.J. Inhibition of the CRAF/prohibitin interaction reverses CRAF-dependent resistance to vemurafenib. Oncogene. 2017;36(3):423–428. doi: 10.1038/onc.2016.214. [DOI] [PubMed] [Google Scholar]
- 140.Johannessen C.M., Boehm J.S., Kim S.Y., et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li C., Scott D.A., Hatch E., et al. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development. 2007;134(1):167–176. doi: 10.1242/dev.02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen C.H., Kim S.H., Trousil S., et al. Loss of cohesin complex components STAG2 or STAG3 confers resistance to BRAF inhibition in melanoma. Nat Med. 2016;22(9):1056–1061. doi: 10.1038/nm.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gupta R., Bugide S., Wang B., et al. Loss of BOP1 confers resistance to BRAF kinase inhibitors in melanoma by activating MAP kinase pathway. Proc Natl Acad Sci U S A. 2019;116(10):4583–4591. doi: 10.1073/pnas.1821889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee H.J., Zhuang G., Cao Y., et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26(2):207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 145.Huang S., Hölzel M., Knijnenburg T., et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151(5):937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Irvine M., Stewart A., Pedersen B., et al. Oncogenic PI3K/AKT promotes the step-wise evolution of combination BRAF/MEK inhibitor resistance in melanoma. Oncogenesis. 2018;7(9):72. doi: 10.1038/s41389-018-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Catalanotti F., Solit D.B., Pulitzer M.P., et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013;19(8):2257–2264. doi: 10.1158/1078-0432.CCR-12-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anastas J.N., Kulikauskas R.M., Tamir T., et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Investig. 2014;124(7):2877–2890. doi: 10.1172/JCI70156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin L., Sabnis A.J., Chan E., et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47(3):250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Greer Y.E., Lipkowitz S. TIC10/ONC201: a bend in the road to clinical development. Oncoscience. 2015;2(2):75–76. doi: 10.18632/oncoscience.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stein M.N., Bertino J.R., Kaufman H.L., et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23(15):4163–4169. doi: 10.1158/1078-0432.CCR-16-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liguori N.R., Sanchez Sevilla, Uruchurtu A., et al. Preclinical studies with ONC201/TIC10 and lurbinectedin as a novel combination therapy in small cell lung cancer (SCLC) Am J Cancer Res. 2022;12(2):729–743. [PMC free article] [PubMed] [Google Scholar]
- 153.Köhler J., Zhao Y., Li J., et al. ERK inhibitor LY3214996-based treatment strategies for RAS-driven lung cancer. Mol Cancer Ther. 2021;20(4):641–654. doi: 10.1158/1535-7163.MCT-20-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ma Y., Xu R., Liu X., et al. LY3214996 relieves acquired resistance to sorafenib in hepatocellular carcinoma cells. Int J Med Sci. 2021;18(6):1456–1464. doi: 10.7150/ijms.51256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bryant K.L., Stalnecker C.A., Zeitouni D., et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25(4):628–640. doi: 10.1038/s41591-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang D., Kroemer G., Kang R. Oncogenic KRAS blockade therapy: renewed enthusiasm and persistent challenges. Mol Cancer. 2021;20(1):128. doi: 10.1186/s12943-021-01422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ji N., Yang Y., Lei Z.N., et al. Ulixertinib (BVD-523) antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Biochem Pharmacol. 2018;158:274–285. doi: 10.1016/j.bcp.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 158.Moschos S.J., Sullivan R.J., Hwu W.J., et al. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018;3(4):92352. doi: 10.1172/jci.insight.92352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Markham A., Keam S.J. Selumetinib: first approval. Drugs. 2020;80(9):931–937. doi: 10.1007/s40265-020-01331-x. [DOI] [PubMed] [Google Scholar]
- 160.Blake J.F., Burkard M., Chan J., et al. Discovery of (S)-1-(1-(4-chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem. 2016;59(12):5650–5660. doi: 10.1021/acs.jmedchem.6b00389. [DOI] [PubMed] [Google Scholar]
- 161.Kirouac D.C., Schaefer G., Chan J., et al. Clinical responses to ERK inhibition in BRAF(V600E)-mutant colorectal cancer predicted using a computational model. NPJ Syst Biol Appl. 2017;3:14. doi: 10.1038/s41540-017-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Varga A., Soria J.C., Hollebecque A., et al. A first-in-human phase I study to evaluate the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Clin Cancer Res. 2020;26(6):1229–1236. doi: 10.1158/1078-0432.CCR-19-2574. [DOI] [PubMed] [Google Scholar]
- 163.Kong L.R., Chua K.N., Sim W.J., et al. MEK inhibition overcomes cisplatin resistance conferred by SOS/MAPK pathway activation in squamous cell carcinoma. Mol Cancer Ther. 2015;14(7):1750–1760. doi: 10.1158/1535-7163.MCT-15-0062. [DOI] [PubMed] [Google Scholar]
- 164.Goffin J.R., Nicholas G., Mates M., et al. Canadian Cancer Trials Group (CCTG) IND215: a phase Ib study of Selumetinib in patients with untreated advanced or metastatic NSCLC who are receiving standard chemotherapy regimens. Investig New Drugs. 2019;37(3):498–506. doi: 10.1007/s10637-018-0680-z. [DOI] [PubMed] [Google Scholar]
- 165.Shimizu S., Takehara T., Hikita H., et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer. 2012;131(3):548–557. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 166.Ahn J.H., Lee M. Autophagy-dependent survival of mutant B-Raf melanoma cells selected for resistance to apoptosis induced by inhibitors against oncogenic B-Raf. Biomol Ther. 2013;21(2):114–120. doi: 10.4062/biomolther.2013.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.BRAF inhibitor resistance can be overcome by blocking autophagy. Cancer Discov. 2014;4(5):OF10. doi: 10.1158/2159-8290.CD-RW2014-059. [DOI] [PubMed] [Google Scholar]
- 168.Ma X.H., Piao S.F., Dey S., et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Investig. 2014;124(3):1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao Y., Wang W., Min I., et al. BRAF V600E-dependent role of autophagy in uveal melanoma. J Cancer Res Clin Oncol. 2017;143(3):447–455. doi: 10.1007/s00432-016-2317-y. [DOI] [PubMed] [Google Scholar]
- 170.Ahn J.H., Lee Y.W., Ahn S.K., et al. Oncogenic BRAF inhibitor UAI-201 induces cell cycle arrest and autophagy in BRAF mutant glioma cells. Life Sci. 2014;104(1–2):38–46. doi: 10.1016/j.lfs.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 171.Mulcahy Levy J.M., Zahedi S., Griesinger A.M., et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife. 2017;6:e19671. doi: 10.7554/eLife.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sueda T., Sakai D., Kawamoto K., et al. BRAF V600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci Rep. 2016;6:18949. doi: 10.1038/srep18949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qian X., Tan C., Yang B., et al. Astaxanthin increases radiosensitivity in esophageal squamous cell carcinoma through inducing apoptosis and G2/M arrest. Dis Esophagus. 2017;30(6):1–7. doi: 10.1093/dote/dox027. [DOI] [PubMed] [Google Scholar]
- 174.Kinsey C.G., Camolotto S.A., Boespflug A.M., et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25(4):620–627. doi: 10.1038/s41591-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W., Kang H., Zhao Y., et al. Targeting autophagy sensitizes BRAF-mutant thyroid cancer to vemurafenib. J Clin Endocrinol Metab. 2017;102(2):634–643. doi: 10.1210/jc.2016-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Strohecker A.M., White E. Targeting mitochondrial metabolism by inhibiting autophagy in BRAF-driven cancers. Cancer Discov. 2014;4(7):766–772. doi: 10.1158/2159-8290.CD-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martin S., Dudek-Perić A.M., Maes H., et al. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemurafenib-resistant melanoma cells. Biochem Pharmacol. 2015;93(3):290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 178.Verma V., Jafarzadeh N., Boi S., et al. MEK inhibition reprograms CD8(+) T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol. 2021;22(1):53–66. doi: 10.1038/s41590-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Atefi M., Avramis E., Lassen A., et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20(13):3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Loi S., Dushyanthen S., Beavis P.A., et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22(6):1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ebert P.J.R., Cheung J., Yang Y., et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44(3):609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 182.Hugo W., Shi H., Sun L., et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell. 2015;162(6):1271–1285. doi: 10.1016/j.cell.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kakavand H., Rawson R.V., Pupo G.M., et al. PD-L1 expression and immune escape in melanoma resistance to MAPK inhibitors. Clin Cancer Res. 2017;23(20):6054–6061. doi: 10.1158/1078-0432.CCR-16-1688. [DOI] [PubMed] [Google Scholar]
- 184.Zhang Y., Velez-Delgado A., Mathew E., et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66(1):124–136. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang J.Y., Xiu J., Baca Y., et al. Distinct genomic landscapes of gastroesophageal adenocarcinoma depending on PD-L1 expression identify mutations in RAS-MAPK pathway and TP53 as potential predictors of immunotherapy efficacy. Ann Oncol. 2021;32(7):906–916. doi: 10.1016/j.annonc.2021.03.203. [DOI] [PubMed] [Google Scholar]
- 186.Yan C., Saleh N., Yang J., et al. Novel induction of CD40 expression by tumor cells with RAS/RAF/PI3K pathway inhibition augments response to checkpoint blockade. Mol Cancer. 2021;20(1):85. doi: 10.1186/s12943-021-01366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Deken M.A., Gadiot J., Jordanova E.S., et al. Targeting the MAPK and PI3K pathways in combination with PD1 blockade in melanoma. OncoImmunology. 2016;5(12):e1238557. doi: 10.1080/2162402X.2016.1238557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Atkins M.B., Larkin J. Immunotherapy combined or sequenced with targeted therapy in the treatment of solid tumors: current perspectives. J Natl Cancer Inst. 2016;108(6):djv414. doi: 10.1093/jnci/djv414. [DOI] [PubMed] [Google Scholar]
- 189.Ribas A., Algazi A., Ascierto P.A., et al. PD-L1 blockade in combination with inhibition of MAPK oncogenic signaling in patients with advanced melanoma. Nat Commun. 2020;11(1):6262. doi: 10.1038/s41467-020-19810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang M., Liu Y., Cheng Y., et al. Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment. Biochim Biophys Acta Rev Cancer. 2019;1871(2):199–224. doi: 10.1016/j.bbcan.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 191.Han J., Liu Y., Yang S., et al. MEK inhibitors for the treatment of non-small cell lung cancer. J Hematol Oncol. 2021;14(1):1. doi: 10.1186/s13045-020-01025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 193.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Choi H., Deng J., Li S., et al. Pulsatile MEK inhibition improves anti-tumor immunity and T cell function in murine Kras mutant lung cancer. Cell Rep. 2019;27(3):806–819. doi: 10.1016/j.celrep.2019.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Poon E., Mullins S., Watkins A., et al. The MEK inhibitor selumetinib complements CTLA-4 blockade by reprogramming the tumor immune microenvironment. J Immunother Cancer. 2017;5(1):63. doi: 10.1186/s40425-017-0268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hellmann M.D., Kim T.W., Lee C.B., et al. Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol. 2019;30(7):1134–1142. doi: 10.1093/annonc/mdz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gaudreau P.O., Lee J.J., Heymach J.V., et al. Phase I/II trial of immunotherapy with durvalumab and tremelimumab with continuous or intermittent MEK inhibitor selumetinib in NSCLC: early trial report. Clin Lung Cancer. 2020;21(4):384–388. doi: 10.1016/j.cllc.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang J., Zhou J.Y., Wu G.S. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67(24):11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 199.Li J., Ye T., Liu Y., et al. Transcriptional activation of Gstp1 by MEK/ERK signaling confers chemo-resistance to cisplatin in lung cancer stem cells. Front Oncol. 2019;9:476. doi: 10.3389/fonc.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Z., Zhou W., Zhang Y., et al. ERK regulates HIF1α-mediated platinum resistance by directly targeting PHD2 in ovarian cancer. Clin Cancer Res. 2019;25(19):5947–5960. doi: 10.1158/1078-0432.CCR-18-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hu T., Zhou R., Zhao Y., et al. Integrin α6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci Rep. 2016;6:33376. doi: 10.1038/srep33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Paramanantham A., Jung E.J., Go S.I., et al. Activated ERK signaling is one of the major hub signals related to the acquisition of radiotherapy-resistant MDA-MB-231 breast cancer cells. Int J Mol Sci. 2021;22(9):4940. doi: 10.3390/ijms22094940. [DOI] [PMC free article] [PubMed] [Google Scholar]