Abstract
Age-associated cardiovascular (CV) dysfunction, namely arterial dysfunction, is a key antecedent to the development of CV disease (CVD). Arterial dysfunction with aging is characterized by impaired vascular endothelial function and stiffening of the large elastic arteries, each of which is an independent predictor of CVD. These processes are largely mediated by an excess production of reactive oxygen species (ROS) and an increase in chronic, low-grade inflammation that ultimately leads to a reduction in bioavailability of the vasodilatory molecule nitric oxide. Additionally, there are other fundamental aging mechanisms that may contribute to excessive ROS and inflammation termed the “hallmarks of aging”) these additional mechanisms of arterial dysfunction may represent therapeutic targets for improving CV health with aging. Aerobic exercise is the most well-known and effective intervention to prevent and treat the effects of aging on CV dysfunction. However, the majority of mid-life and older (ML/O) adults do not meet recommended exercise guidelines due to traditional barriers to aerobic exercise, such as reduced leisure time, motivation, or access to fitness facilities. Therefore, it is a biomedical research priority to develop and implement time- and resource-efficient alternative strategies to aerobic exercise to reduce the burden of CVD in ML/O adults. Alternative strategies that mimic or are inspired by aerobic exercise, that target pathways specific to the fundamental mechanisms of aging, represent a promising approach to accomplish this goal.
Keywords: Arterial function, Barriers to aerobic exercise, Therapeutic strategies, Older adults, Cardiovascular function
1. Introduction
The majority of the overall health care burden in the United States (US) is represented by chronic disorders, with cardiovascular disease (CVD) remaining the leading cause of morbidity and mortality in Western civilizations (Virani et al., 2020; GBD 2013 Risk Factors Collaborators, 2015). Advancing age is the strongest independent risk factor for CVD (Fig. 1) and, as such, the majority of deaths from CVD occur in mid-life and older (ML/O) adults (≥50 years of age) (Lakatta, 2003). Importantly, the projected increase in the number of ML/O adults predicts an attendant increase in CVD prevalence in the absence of effective interventions and a subsequent rise in the financial burden on our health care system (Scott et al., 2021; Heidenreich et al., 2011).
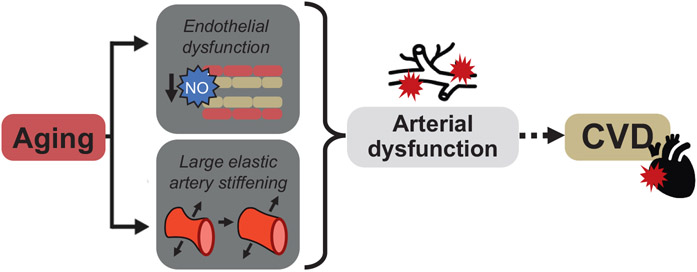
Fig. 1. Cardiovascular aging.
Aging is the primary risk factor for the development of cardiovascular diseases (CVD). This increase in risk is largely mediated by the manifestation of arterial dysfunction which is characterized by impaired endothelial function and stiffening of the large elastic arteries. NO: nitric oxide.
Arterial dysfunction is a primary antecedent and an independent predictor for the development of CVD with aging (Lakatta and Levy, 2003a; Najjar et al., 2005; Seals et al., 2014) (Fig. 1). Arterial dysfunction is also an independent predictor of many other clinical disorders common with aging including cognitive impairment and Alzheimer's Disease and related dementias (Iulita et al., 2018; Hughes et al., 2018), chronic kidney disease (Sedaghat et al., 2015), and glucose intolerance and insulin resistance (Vasan et al., 2022; Zheng et al., 2020; Tian et al., 2022; Cohen et al., n.d.).
1.1. Arterial dysfunction with aging
The age-associated increase in arterial dysfunction is, in part, characterized by the development of vascular endothelial dysfunction as indicated by a decline in endothelium-dependent dilation (EDD). The key mechanism underlying the decrease in EDD with aging is a reduction in the bioavailability of the vasodilatory and vascular-protective molecule nitric oxide (NO) (Lakatta and Levy, 2003a; Widlansky et al., 2003; Seals et al., 2011; Brandes et al., 2005) (Fig. 1). In humans, EDD is assessed in conduit (macrovascular) arteries by brachial artery flow-mediated dilation, whereas in resistance (microvascular) arteries EDD is assessed by the forearm blood flow response to brachial artery infusion of acetylcholine (ACh) (Seals et al., 2011). With aging, the EDD response to mechanical (i.e., blood flow-mediated shear stress; macrovascular) and pharmacological (i.e., ACh and insulin; microvascular) stimuli is impaired in ML/O adults compared to young adults and this age-associated difference is augmented with increasing age. These gold-standard clinical assessments of EDD in both the micro- and macro-vasculature are indicative of endothelial health and independently predict CVD risk in ML/O adults (Seals et al., 2011; Donato et al., 2015; Lind et al., 2011; Taddei et al., 2000; Yeboah et al., 2007; Yeboah et al., 2009). In rodents, corollaries to assess EDD exist, i.e., flow-mediated dilation and the change in diameter of isolated artery segments in response to chemical stimuli such as ACh or insulin (Santos-Parker et al., 2014; Wenceslau et al., 2021).
Arterial dysfunction with aging also is characterized by the development of large elastic artery stiffness (Fig. 1) which is mediated by a remodeling of the extracellular matrix (an increase in collagen deposition and elastin fragmentation and degradation) and formation of advanced glycation end-products (AGEs); the latter crosslink collagen fibers leading to a stiffer arterial wall (Lakatta and Levy, 2003a; Seals and Edward, 2014; Lakatta and Levy, 2003b; Fleenor, 2013). In humans, arterial stiffness can be assessed by measuring the time elapsed for the pressure wave produced by the contraction of the heart during systole to propagate bidirectionally to the carotid and femoral arteries through the reference standard measure of carotid-femoral pulse wave velocity (cfPWV) (Townsend et al., 2015); akin to EDD, cfPWV serves as an independent predictor of CVD risk with aging (Mitchell et al., 2010; Sutton-Tyrrell et al., 2005). Additionally, large elastic artery stiffness is also assessed by measuring arterial compliance, i.e., the ability of an artery to distend in response to a given change in intravascular pressure, in the carotid artery (opposite of arterial stiffness). In ML/O adults, cfPWV is higher, though carotid artery compliance is lower, compared to younger individuals, indicating stiffer arteries in ML/O adults. As is observed with EDD, these age-associated differences in assessments of large elastic artery stiffness are exacerbated with advancing age (Moreau et al., 2003; Tanaka et al., 2000). In rodents, arterial stiffness and carotid artery compliance are assessed by aortic PWV (Rossman et al., 2020; Gioscia-Ryan et al., 2021; Clayton et al., 2021) and changes in carotid artery dilation in response to a given increase in pressure (Fleenor et al., 2009), respectively.
The increase in large elastic artery stiffness with aging is associated with a concomitant rise in systolic blood pressure (SBP) (Fuchs and Whelton, 2020). Increased SBP is the most frequent of BP-related disorders in ML/O adults and, like the two former mechanisms of age-associated arterial dysfunction, is an independent predictor of CVD risk (Miura et al., 2001). Cross-sectional and longitudinal studies indicate that SBP increases progressively with age throughout adulthood (Franklin, 1999). In rodents, BP is measured by (1) using the noninvasive tail cuff method (Wang et al., 2017) and (2) radio telemetry (considered gold-standard in small animals, such as mice) (Wang et al., 2017). This review will focus primarily on arterial function with aging, with select discussions on BP. A more in-depth review on aging, BP, and novel interventions was previously published (Kazeminia et al., 2020).
1.2. Mechanisms underlying arterial dysfunction with aging
1.2.1. Oxidative stress and chronic low-grade inflammation
Vascular endothelial dysfunction and arterial stiffness with aging manifest in response to age-related dysfunction of molecular and cellular processes. This is primarily mediated by excess reactive oxygen species (ROS) production relative to antioxidant defenses (Fig. 2). Excess ROS reacts with NO to reduce NO bioavailability which leads to declines in EDD and increases in arterial stiffness (increased vascular smooth motor tone) (Donato et al., 2015; Seals and Edward, 2014; Donato et al., 2007; Moreau et al., 2005; Rossman et al., 2018; Eskurza et al., 2004; Wilkinson et al., 2004; Fleenor et al., 2012; Gioscia-Ryan et al., 2018). Moreover, chronic low-grade inflammation develops with aging and contributes to arterial dysfunction (Walker et al., 2014; Lesniewski et al., 2011; Jablonski et al., 2015) (Fig. 2). In turn, nuclear factor-κB (NF-κB), a pro-inflammatory transcription factor, is elevated in arteries (Donato et al., 2008) initiating a cascade of molecular events that results in adverse extracellular remodeling (an increase in collagen deposition and elastin fragmentation and degradation) (Lakatta and Levy, 2003a; Seals and Edward, 2014; Lakatta and Levy, 2003b; Fleenor, 2013) which contributes to subsequent increases in SBP with aging (Guzik and Touyz, 2017).
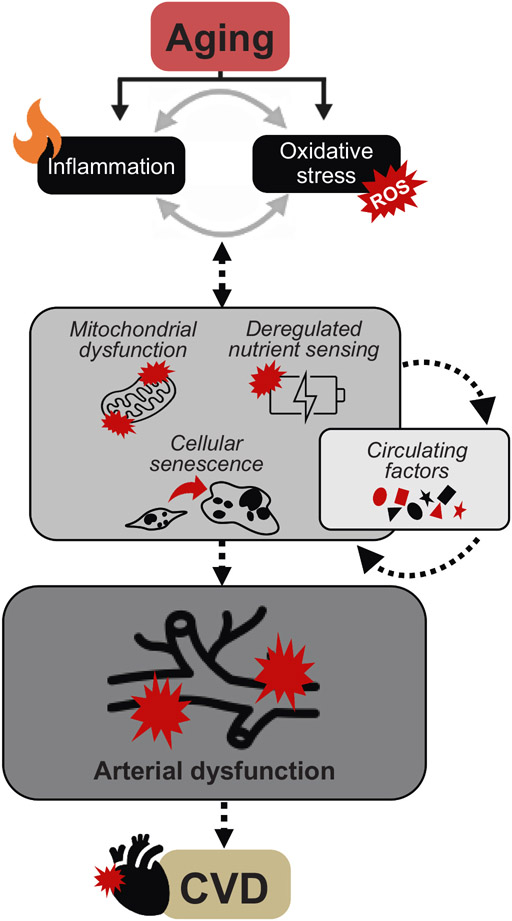
Fig. 2. Mechanisms of arterial aging.
Age-related increases in chronic, low-grade inflammation and reactive oxygen species (ROS)-mediated oxidative stress drive the hallmarks of aging including mitochondrial dysfunction, dysregulated energy sensing and cellular senescence and changes in circulating factors, another fundamental aging mechanism. Together, these upstream processes contribute to arterial dysfunction (i.e., endothelial dysfunction and large elastic artery stiffening) by reducing the bioavailability of the vasodilatory molecule nitric oxide (NO) which ultimately leads to the development of cardiovascular diseases (CVD).
Excessive ROS production and increases in chronic low-grade inflammation with aging are considered mutually reinforcing processes (Fig. 2). Excessive ROS production results in oxidative damage of endothelial cell organelle components, i.e., mitochondria, influencing an upregulation in local pro-inflammatory processes. Whereas increases in chronic low-grade inflammation promote an increase in oxidative stress in the aging vasculature leading to impaired endothelial function and increased arterial stiffness (Guzik and Touyz, 2017).
In addition to excessive ROS production and increased chronic low-grade inflammation with aging, there are other fundamental aging mechanisms some of which are termed “hallmarks of aging”, that may contribute to excess ROS and inflammation (López-Otín et al., 2013).
1.2.2. Fundamental aging mechanisms
There are a number of fundamental aging mechanisms, but herein, we will specifically discuss three “hallmarks of aging”: 1) mitochondrial dysfunction; 2) deregulated nutrient sensing; and 3) cellular senescence and the senescence associated secretory phenotype (SASP) (López-Otín et al., 2013), as well as changes in systemic circulating factors (Fig. 2).
1.2.2.1. Mitochondrial dysfunction.
Mitochondria are critical for maintaining cellular homeostasis and perform crucial signaling functions in the vasculature, many of which are mediated by the production of ROS (mtROS) at physiological levels (Jablonski et al., 2011; Pierce et al., 2011a; Dai et al., 2012). However, mitochondria become dysfunctional with aging and produce excessive levels of mtROS causing oxidative damage to mitochondrial components and declines in mitochondrial health as characterized by impaired mitochondrial fitness and quality control (James and Murphy, 2002; López-Armada et al., 2013; Seo et al., 2010). As such, mtROS is emerging as an important source of arterial oxidative stress with aging and a therapeutic target to ameliorate age-related arterial dysfunction (Fig. 2), which we have reviewed in detail previously (Rossman et al., 2020).
1.2.2.2. Deregulated nutrient sensing.
Deregulated nutrient sensing with aging (Fig. 2) is primarily characterized by changes in several key energy- and nutrient-sensing pathways, including reduced nicotinamide adenine dinucleotide (NAD+) bioavailability; reduced abundance and activation of sirtuin-1; increased abundance and activation of mammalian target of rapamycin (mTOR); and reduced activity of adenosine monophosphate-activated protein kinase (AMPK). Here, we focus on reduced NAD+ bioavailability with aging. This reduction in NAD+ bioavailability has been linked to age-related CV dysfunction (Martens et al., 2018; de Picciotto et al., 2016) and occurs by a decrease in primary NAD+ biosynthesis (Strømland et al., 2021) (NAD+ salvage pathway) and an increase in NAD+ degradation (Camacho-Pereira et al., 2016; Tarragó et al., 2018) (NAD+ consumption pathway). Therefore, reduced NAD+ bioavailability with aging appears to be a promising therapeutic target.
1.2.2.3. Cellular senescence and the SASP.
Cellular senescence is a state of essentially irreversible cell cycle arrest that, under normal physiological circumstances, is beneficial in inhibiting neoplastic growth (Demaria et al., 2017) and aids in wound healing (Demaria et al., 2014); to facilitate this process, senescent cells secrete a variety of pro-inflammatory molecules called the SASP. However, with aging there is excess accumulation of cells that enter the senescent state leading to adverse cellular and physiological function and disease (Zhang et al., 2022) (Fig. 2). In turn, these cells secrete a variety of pro-inflammatory factors, which can induce senescence in neighboring cells, further exacerbating the physiological burden of cellular senescence (Coppé et al., 2010). This process has been implicated in the age-related development of arterial dysfunction and CVD (Rossman et al., 2017; Mahoney et al., 2021; Venkatasubramanian et al., 2022). As such, targeting the increase in senescent cell burden with aging is quickly advancing as a potential interventional approach to improve age-related arterial dysfunction (Mahoney et al., 2021; Venkatasubramanian et al., 2022; Roos et al., 2016).
1.2.2.4. Circulating factors.
Changes in circulating factors with aging are likely caused by impairments in cellular and molecular processes comprising the hallmarks of aging, including those discussed above (i.e., cellular senescence and the SASP) (Fig. 2). Circulating factors are blood-borne molecules that confer functional alterations to its target cell through autocrine, paracrine, or endocrine actions. Circulating factors were initially studied in the 19th century by conducting heterochronic parabiosis experiments in mice in which the circulatory systems of two mice were adjoined to show that blood-borne factors can directly contribute to changes in physiological function (Finerty, 1952). Since then, this experimental approach has evolved to the administration of whole blood (Jeon et al., 2022), red blood cells (McCann Haworth et al., 2021), or isolation of a specific circulating factor from the plasma (Zhang et al., 2021) from one mouse to another. This design has been critical in elucidating the relation between changes in circulating factors with aging and their effects on age-associated declines in physiological function, including CV health (Kiss et al., 2020), and likely have an interactive association with the hallmarks of aging (Kiss et al., 2020) (Fig. 2). Select categories of circulating factors that have been found to change with aging and associated with elevated CVD risk include proteins (i.e., C-reactive protein; inflammatory cytokines) (Zhang et al., 2021; Lehallier et al., 2019), metabolites (i.e., trimethylamine N-oxide) (Brunt et al., 2020; Brunt et al., 2021), miRNAs (i.e., miR-151a-5p; miR-1248) (Noren Hooten et al., 2013), and lipids (i.e., oxidized low-density lipoprotein; high-density lipoprotein) (Moreau et al., 2005; Donato et al., 2008; Di Cesare et al., 2021; Eskurza et al., 2006; Jablonski et al., 2007).
Taken together, these mechanisms of age-related arterial dysfunction could be viewed as therapeutic targets for improving CV health with aging. Interventions that can target a majority, if not all these mechanisms, such as aerobic exercise, are likely to have significant biomedical impact.
2. Aerobic exercise to promote healthy CV aging
Aerobic exercise is the most well-known and effective intervention to prevent and treat the impact of aging on CV dysfunction (Seals and Edward, 2014; Seals et al., 2018; Craighead et al., 2019) (Fig. 3). Aerobic exercise attenuates excess ROS production and chronic low-grade inflammation and favorably modifies the adverse effects induced by fundamental aging mechanisms, i.e., mitochondrial dysfunction (Durrant et al., 2009; Gioscia-Ryan et al., 2016; Lesniewski et al., 2013), deregulated nutrient sensing (de Guia et al., 2019; Ji and Yeo, 2022), cellular senescence (Rossman et al., 2017), and circulating factors (Shern-Brewer et al., 1998; Li et al., 2022) (Fig. 3). The remainder of this review will focus on the beneficial effects of aerobic exercise on CV aging because of the compelling published literature on aerobic exercise in preserving CV function and suppressing CVD risk with aging. However, we do recognize the potentially beneficial effects of resistance training on the CV aging process and direct the reader to reviews on that specific topic (Miyachi et al., 2003; Cook et al., 2006; Silva et al., 2021).
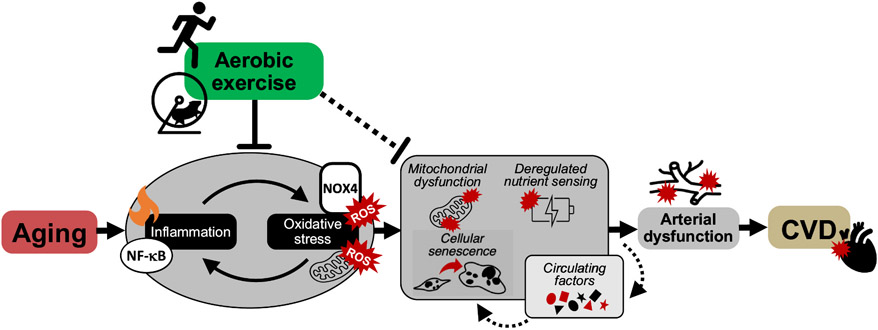
Fig. 3. Impact of aerobic exercise on arterial aging.
Aerobic exercise is the most well-known and effective intervention to prevent and treat cardiovascular diseases (CVD) with aging. Aerobic exercise suppresses excess amounts of nuclear factor-κB (NF-κB)-mediated inflammation and oxidative stress originating from mitochondrial- and NADPH oxidase (NOX4)-derived reactive oxygen species (ROS). Aerobic exercise may also favorably modulate the adverse effects induced by fundamental aging mechanisms, i.e., mitochondrial dysfunction, deregulated nutrient sensing, cellular senescence and circulating factors, to ultimately improve arterial function.
2.1. Evidence for the beneficial effects of aerobic exercise on arterial function with aging
In human cross-sectional studies, ML/O aerobic exercise-trained individuals have higher endothelial function and lower arterial stiffness versus their sedentary peers and exhibit no difference in endothelial function and arterial stiffness compared to young adults (Moreau et al., 2003; Tanaka et al., 2000; Eskurza et al., 2004; Pierce et al., 2011a; Pierce, 2018; Pierce, 2017; Laurent et al., 2011; Vlachopoulos et al., 2010; Tanaka et al., 1998). In intervention trials, aerobic exercise training (i.e., brisk walking) improves endothelial function (Pierce et al., 2011b; DeSouza et al., 2000) in ML/O men and lowers arterial stiffness (Tanaka et al., 1998; Matsubara et al., 2014; Tanahashi et al., 2014) in previously sedentary ML/O men and women.
Improvements in endothelial function with habitual aerobic exercise training in ML/O adults have been observed largely in men in our laboratory (Pierce et al., 2011a; Pierce et al., 2011b); however, there are select studies that suggest that habitual aerobic exercise is equally beneficial in improving endothelial function in otherwise healthy post-menopausal women (Lew et al., 2022; Nyberg et al., 2016; Nyberg et al., 2014). The mixed state of the literature for the efficacy of an aerobic exercise intervention to improve endothelial function in postmenopausal women suggests that a variety of factors may play a role, most notably the duration between the onset of menopause and the start of an aerobic exercise intervention (Lew et al., 2022). A recently published systematic review (Lew et al., 2022) on this topic found that, of the studies that reported time onset from menopause, an aerobic exercise intervention is effective in improving endothelial function in ML/O adult women <5 years post menopause (early) (Nyberg et al., 2016; Nyberg et al., 2014), whilst not effective >5 years post menopause (late) (Moreau et al., 2013; Gunnarsson et al., 2020; Nishiwaki et al., 2011; Hoier et al., 2021). These findings suggest that studies investigating the influence of aerobic exercise on endothelial function in postmenopausal women need to report the duration between menopause and the onset of aerobic exercise to better determine the conditions for which an aerobic exercise intervention may be effective for improving endothelial function in postmenopausal women.
Previous studies sought to determine the influence of aerobic exercise for favorably altering the fundamental mechanisms of aging to improve age-related arterial dysfunction in ML/O adults. These studies have found that ML/O aerobic exercise-trained men have higher endothelial function compared to ML/O sedentary men due to lower oxidative stress (Eskurza et al., 2004; Pierce et al., 2011a) and inflammation (Walker et al., 2014) and higher NO bioavailability (Pierce et al., 2011a); lower arterial stiffness (Jablonski et al., 2015) in ML/O aerobic exercise-trained men and women is, in part, caused by a reduction in NF-κB-mediated inflammation (Jablonski et al., 2015). Furthermore, primary arterial endothelial cells isolated from aerobic exercise-trained ML/O adults have lower abundance of several markers of cellular senescence compared to sedentary ML/O adults, which was inversely related with endothelial function (i.e., higher arterial endothelial cell senescence was related to lower endothelial function) (Rossman et al., 2017) (Fig. 3). Moreover, chronic aerobic exercise increased NAD+ bioavailability in the skeletal muscle of older adults (de Guia et al., 2019); although this study does not investigate NAD+ bioavailability in the vasculature, these findings represent an important initial study as to how aerobic exercise may modulate NAD+ bioavailability in other tissues (Fig. 3).
To gain more definitive mechanistic insight into the beneficial effects of aerobic exercise on arterial function with aging, a reverse translation approach can be employed, in which old mice are given access to a running wheel (an established preclinical model of voluntary aerobic exercise). Voluntary wheel running in old mice improves endothelial function (Fig. 3) by suppressing ROS originating from NADPH oxidase, i.e., NOX4 (Durrant et al., 2009). In one study of note, voluntary wheel running improved endothelial function in old mice by enhancing mitochondrial fitness through the suppression of excess mtROS and an increase in resistance to mitochondrial stress in arteries (Gioscia-Ryan et al., 2016). Thus, these preclinical findings elucidated sources of excessive ROS bioactivity with aging and novel mechanisms underlying the beneficial effects of habitual voluntary aerobic exercise (Fig. 3). Aerobic exercise in old mice also lowers intrinsic mechanical stiffness of the arterial wall associated with lower abundance of collagen and AGEs (Fleenor et al., 2010; Gioscia-Ryan et al., 2018).
Isolating the effect of aerobic exercise per se on the development of arterial dysfunction throughout adulthood is not practical in humans, therefore, our laboratory recently conducted a preclinical lifelong study in mice to determine whether lifelong exposure to voluntary aerobic exercise could prevent arterial dysfunction with aging (Gioscia-Ryan et al., 2021). Lifelong aerobic exercise preserved endothelial function and prevented arterial stiffness with aging even in the presence of Western diet consumption, which markedly exacerbates arterial dysfunction with aging. These favorable outcomes were mediated by preservation of NO bioavailability, prevention of excessive arterial mtROS and inflammation, and mitigating the increase in the intrinsic mechanical stiffness of the arterial wall with advancing age (Gioscia-Ryan et al., 2021) (Fig. 3).
To determine the causal role of aerobic exercise in improving age-related arterial function by targeting the hallmarks of aging (i.e., mitochondrial dysfunction, deregulated nutrient sensing, and cellular senescence), interventional and longitudinal studies are needed, which will be discussed later in this review.
Although aerobic exercise has unequivocally been shown to improve the effect of aging on arterial function by favorably modulating the fundamental mechanisms of aging, participation in aerobic exercise with aging is lacking. As such, those hurdles that hinder ML/O adult participation in aerobic exercise have been aptly coined the ‘barriers to aerobic exercise’.
3. Barriers to engaging in traditional aerobic exercise
In 2018, the Department of Health and Human Services released the Physical Activity Guideline for the US. This guideline recommends that adults should participate in at least 75 to 150 min of moderate-vigorous intensity aerobic exercise per week in addition to performing resistance training at least two times per week. ML/O adults are expected to follow the same guidelines as young adults but incorporate balance training into their exercise regimen to decrease the risk of falling. However, according to federal monitoring data, <50 % of the adult population in the US were meeting these set guidelines for physical activity (Piercy et al., 2018).
A lack of adherence to these evidence-based exercise guidelines can be attributed to various factors. According to the Center of Disease Control and Prevention there are several established barriers in meeting physical activity guidelines (CDC, 2022) (Fig. 4), including reduced leisure time, social support, motivation, and access to facilities, all of which can lead to a more sedentary lifestyle (Fig. 4). Although half of all individuals over the age of 55 are retired (Fry, n.d.) (the relative number of individuals retired only grows with advancing age [66.9 % adults 65–74; 86.7 % over the age of 75 (Fry, n.d.)]), reduced leisure time is still considered one of the most important barriers to exercise, albeit counterintuitive. Reduced leisure time is thought to be a result of ML/O adults choosing to use their extra time for other activities, such as to reconnect with family or simply not willing to participate in aerobic exercise (Ansari and Lovell, 2009; Kelly et al., 2016; Stutts, 2002; Craighead et al., 2022a). As such, it could be considered less a ‘lack of leisure time’, and more so a ‘lack of choosing’ aerobic exercise with newfound time in retirement.
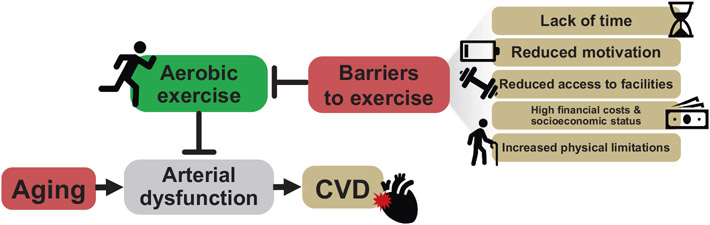
Fig. 4. Barriers to traditional aerobic exercise.
Aerobic exercise is the most well-established intervention for attenuating impairments to age-related arterial dysfunction, the primary antecedent for the development of cardiovascular diseases (CVD). Despite this, there are many barriers that limit adherence to aerobic exercise in mid-life and older adults, including lack of time, energy and motivation, access to facilities, financial costs, socioeconomic status, and physical limitations.
Another important barrier to physical activity is socioeconomic status/deprivation (Kimenai et al., 2022); socioeconomic status refers to the income, education, and occupation of a group or individual (Schultz et al., 2018), whereas socioeconomic deprivation is defined as the relative disadvantage of a group or individual in terms of access to or control of economic, material, or social opportunities (Kimenai et al., 2022). A lower socioeconomic status and/or higher socioeconomic deprivation negatively impact access to fitness facilities (Schultz et al., 2018; The BH Health Study, 2015), and leisure time (Schultz et al., 2018; The BH Health Study, 2015) and is associated with increased CVD risk (GBD 2013 Risk Factors Collaborators, 2015; Kimenai et al., 2022; Schultz et al., 2018) (Fig. 4). Furthermore, the inverse relation between lower socioeconomic status and an increase in CVD risk has grown stronger in those considered most socioeconomically vulnerable over the past 20 years (Abdalla et al., 2020). As stated previously, most ML/O adults are retired, however, a significant proportion of ML/O adults remain active in the workforce (Fry, n.d.). This could be a result of fulfillment in one's career or, more likely, the lack of financial resources to retire (Risk of Economic Hardship Among Older Adults Issue Brief, n.d.). As such, these individuals are more prone to suffer from a lower socioeconomic status and, in turn, increased CVD risk as a result of significantly heightened barriers to exercise.
Taken together, ML/O adults represent a prime candidate population for studies that investigate time and resource-efficient alternative strategies that overcome barriers to aerobic exercise. In the next part of this review, we will discuss alternative strategies to aerobic exercise for improving arterial function in ML/O adults that overcome the aforementioned barriers to exercise. Moreover, these alternative strategies need to mimic exercise and/or target specific mechanisms, such as the fundamental aging mechanisms, by which aerobic exercise elicits its beneficial effects on the vasculature to reduce risk for CVD and associated morbidities.
4. Time- and resource-efficient strategies for improving CV function
Participating in habitual aerobic exercise is the most well-known and effective intervention to prevent and treat the effects of aging on CV function (Seals and Edward, 2014; Gioscia-Ryan et al., 2021; Seals et al., 2018) (Fig. 3). This has been shown in a lifelong aerobic exercise preclinical study (Gioscia-Ryan et al., 2021) and cross-sectional (Moreau et al., 2003; Tanaka et al., 2000; Eskurza et al., 2004; Pierce et al., 2011a; Pierce, 2018; Pierce, 2017; Laurent et al., 2011; Vlachopoulos et al., 2010; Tanaka et al., 1998) and interventional (Walker et al., 2014; Pierce et al., 2011b; DeSouza et al., 2000; Matsubara et al., 2014; Tanahashi et al., 2014) clinical studies indicating that participation in habitual exercise throughout life or starting an exercise regimen late in life can reduce risk for CVD and its associated risk factors by beneficially modulating fundamental aging mechanisms (Rossman et al., 2017; Durrant et al., 2009; Gioscia-Ryan et al., 2016; Lesniewski et al., 2013; de Guia et al., 2019; Ji and Yeo, 2022; Shern-Brewer et al., 1998; Li et al., 2022) (Fig. 3).
In lieu of the benefits of exercise upon CVD risk and associated risk factors (Bennie et al., 2019), the National Institutes of Health, through the National Institute on Aging, has constructed a set of guidelines for physical activity in ML/O adults (Exercise, n.d), as stated previously. Moreover, guidelines to reduce risk of injury during exercise and to increase adherence to exercise regimens to achieve fitness-related goals are provided, in addition to guidelines for participating in exercise (Exercise, n.d.). Following these recommendations results in a 25–50 % reduction in CVD and associated risk factors compared to those individuals that did not meet any of the intended exercise prescriptions (Bennie et al., 2019). As previously discussed, ~50 % of the adult population meet the benchmark for aerobic exercise; this declines to ~33 % of adults ≥75 years of age (Bennie et al., 2019; Products - NHIS Early Release -, 2019). Adherence is further reduced by ~50 % when taking into consideration meeting both aerobic exercise and resistance training recommendations (Bennie et al., 2019).
A lack of adherence to these guidelines is likely predicated on barriers to exercise (CDC, 2022) (Fig. 4). Therefore, it is important to develop strategies that overcome these barriers to aerobic exercise to decrease the development of CVD and associated risk factors (Fig. 5).
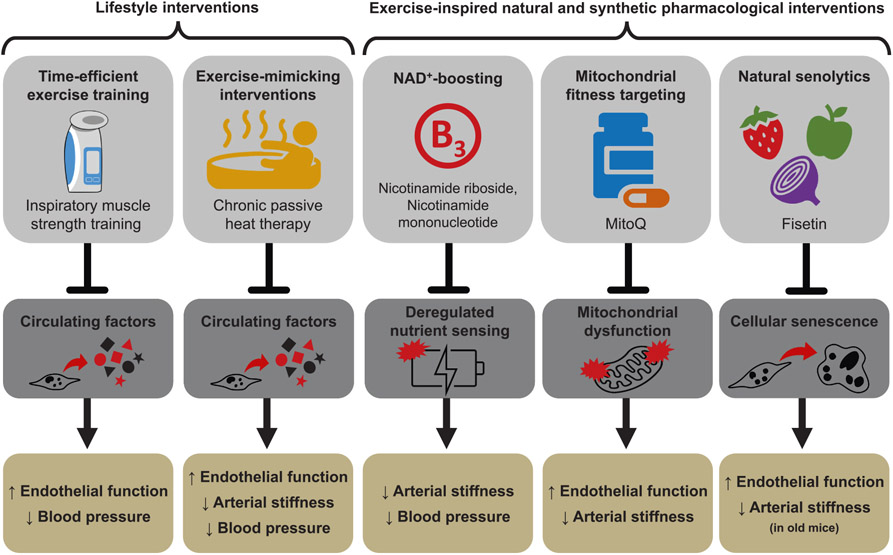
Fig. 5. Strategies, target mechanism and cardiovascular outcome.
Due to the barriers of aerobic exercise, alternative strategies such as lifestyle interventions (i.e., inspiratory muscle strength training and chronic passive heat therapy) and “exercise-inspired” pharmacological interventions (i.e., nicotinamide riboside, nicotinamide mononucleotide, mitoquinol [MitoQ] and fisetin) are being studied to promote optimal cardiovascular (CV) aging. Based on published findings, these interventions improve CV outcomes (enhanced endothelial function, reduced arterial stiffness, and/or lower blood pressure) by targeting fundamental aging mechanisms, including 1) unfavorable changes to the circulating milieu; 2) dysregulation of nutrient sensing pathways; 3) decreased mitochondrial function; and 4) increased cellular senescence burden.
In this section of the review, we will discuss time- and resource-efficient therapies developed and/or advanced by our laboratory, that mimic or are inspired by traditional aerobic exercise, to foster healthy CV aging. We will also discuss how these interventions may target fundamental aging mechanisms to promote improvement in CV health with aging (Fig. 5).
4.1. High-resistance inspiratory muscle strength training
High-resistance inspiratory muscle strength training (IMST) represents a time-efficient, evidence-based alternative strategy to conventional aerobic exercise training for promoting CV health with aging (Craighead et al., 2022a; Craighead et al., 2021a) (Fig. 5). Recently, our laboratory conducted a randomized, double-blind, sham-controlled, parallel-design pilot clinical trial in ML/O adults with above-normal baseline SBP to determine whether high resistance IMST can improve CV health with aging (Craighead et al., 2021b). Briefly, we found that 30 breaths per day at 55–75 % maximal inspiratory pressure, 6 days per week, for 6 weeks (~5 min per day) increased endothelial function and decreased SBP and DBP. This protocol had outstanding adherence (95 % of subjects completed the protocol) and improved endothelial function in estrogen-deficient postmenopausal women. Moreover, the improvements in these independent predictors of CVD risk were associated with declines in oxidative stress (ROS) and chronic low-grade inflammation and changes in circulating factors that improved endothelial cell function ex vivo (Craighead et al., 2021b) (Fig. 5).
4.2. Chronic passive heat therapy
Chronic passive heat therapy is an exercise-mimicking alternative intervention to traditional aerobic exercise (Fig. 5). Passive heat therapy mimics the CV response of aerobic exercise in that a single session elicits elevated heart rate, shear rate (the rate of change of velocity at which blood flow produces force on the vessel wall), core temperature, and cardiac output in the absence of requisite coordination and weight-bearing aspects synonymous with traditional aerobic exercise. As such, chronic passive heat therapy has demonstrated systemic beneficial effects on CV health in a wide range of groups, including clinical populations at a greater risk for developing CVD (Brunt and Minson, 2021; Ely et al., 2019). Recently, our laboratory conducted a randomized, double-blind, sham-controlled, parallel-design pilot clinical trial investigating the efficacy of chronic passive heat therapy (thirty 60-minute sessions at 40.5 °C) to improve CV aging in ML/O adults (Brunt et al., 2019a). Chronic passive heat therapy was found to increase endothelial function and decrease SBP and cfPWV in ML/O adults (Brunt et al., 2019a) providing initial evidence for the efficacy of chronic passive heat therapy to improve CV function in ML/O adults. Furthermore, chronic passive heat therapy, in young adults, has shown to elicit its beneficial effects on the vasculature by favorably modulating circulating factors (Brunt et al., 2018; Brunt et al., 2019b) suggesting a similar effect to circulating factors in response to chronic passive heat therapy may exist in ML/O adults (Fig. 5).
4.3. Exercise-inspired natural and synthetic pharmacological interventions
4.3.1. Natural NAD+ boosting interventions
Deregulated nutrient sensing with aging (Fig. 2) is primarily characterized by changes in key energy- and nutrient-sensing pathways, namely reduced NAD+ bioavailability as a result of decreased NAD+ biosynthesis and increased NAD+ degradation (Strømland et al., 2021; Camacho-Pereira et al., 2016; Tarragó et al., 2018). This age-related decline in NAD+ bioavailability is ameliorated with aerobic exercise training in ML/O adults (de Guia et al., 2019) (Fig. 3). However, adherence to aerobic exercise training regimens in ML/O adults is lacking. As such, alternative strategies that increase NAD+ bioavailability and overcome the barriers to exercise represent promising therapies for treating and/or preventing arterial dysfunction with aging (Fig. 5).
Our laboratory initially investigated supplementation with a natural NAD+ boosting compound, nicotinamide mononucleotide (NMN), in drinking water to improve age-related arterial dysfunction in older mice (de Picciotto et al., 2016) (Fig. 5). It was found that old mice that received oral supplementation with 300 mg/kg/day of NMN for 8 weeks, relative to vehicle-treated animals, had higher NAD+ bioavailability in the vasculature, greater endothelial function, lower arterial ROS, reduced aortic stiffness, lower arterial collagen, and greater elastin content (de Picciotto et al., 2016). NAD+ is a potent activator of the deacetylase, SIRT-1; an increase in SIRT-1 activation and/or abundance has been implicated for improving arterial function with aging (Donato et al., 2011). As such, old mice that received oral NMN supplementation had greater SIRT-1 in the vasculature relative to vehicle-treated controls; the activation of SIRT-1 reduced the acetylated, active form of the p65 subunit of the pro-inflammatory transcription factor, NF-κB (de Picciotto et al., 2016). These findings provide compelling evidence for the relation between deregulated nutrient sensing and excess oxidative stress and inflammation in the regulation of age-related arterial dysfunction.
These findings were then translated to a randomized, double-blind, placebo-controlled crossover design pilot clinical trial in ML/O adults (Martens et al., 2018). Instead of using NMN, this pilot trial utilized another natural NAD+ boosting nutraceutical, nicotinamide riboside (NR), at 1000 mg/day for 6 weeks. Following NR supplementation, NAD+ bioavailability in peripheral blood mononuclear cells was higher and arterial stiffness (cfPWV) and SBP were lower compared to placebo supplementation. The results of this study advance the efficacy of NR supplementation as a time- and resource-efficient alternative strategy to aerobic exercise training for increasing NAD+ bioavailability to improve age-related arterial dysfunction in ML/O adults (Fig. 5).
4.3.2. Mitoquinol (MitoQ)
As previously discussed in this review, mitochondria are integral for maintaining normal cellular function in arteries (Jablonski et al., 2011; Pierce et al., 2011a; Dai et al., 2012). With aging, the mitochondria become dysfunctional and produce excessive levels of mtROS that further damage the mitochondria to result in impaired mitochondrial fitness and quality control (James and Murphy, 2002; López-Armada et al., 2013; Seo et al., 2010) (Fig. 2). We have shown that mice that undergo voluntary aerobic exercise have higher endothelial function by ameliorating excess arterial mtROS and promoting mitochondrial stress resistance in arteries (Gioscia-Ryan et al., 2016) (Fig. 3). Subsequently, time- and resource-efficient alternative strategies to aerobic exercise that show similar favorable effects on the mitochondria as aerobic exercise to improve mitochondrial function constitute an encouraging approach to treat and/or prevent age-related arterial dysfunction.
Previously, we investigated the efficacy of a synthetic pharmacological compound designed as a mitochondria-targeted antioxidant, mitoquinol (MitoQ), for improving age-related arterial function in old mice (Fig. 5). Following 4 weeks of oral supplementation with MitoQ (250 μM/day), old mice had higher endothelial function and lower arterial mtROS (Gioscia-Ryan et al., 2014). Moreover, MitoQ supplementation in old mice restored arterial stiffness back to young levels by attenuating the age-related decline in arterial elastin abundance (Gioscia-Ryan et al., 2018). These studies provided essential proof-of concept evidence for the efficacy of MitoQ to improve arterial function with aging (Fig. 5).
We then translated these findings to a randomized, double-blind, placebo-controlled crossover pilot clinical trial in which ML/O adults received 20 mg/day of MitoQ for 6 weeks (Rossman et al., 2018). Following MitoQ supplementation, ML/O adults exhibited higher endothelial function mediated, in part, by a tonic reduction in excess mtROS production and lower arterial stiffness (in those subjects exhibiting higher baseline arterial stiffness (Rossman et al., 2018)) (Fig. 5). Furthermore, 6 weeks of MitoQ supplementation in ML/O adults resulted in lower circulating oxidized low-density lipoprotein (oxLDL) (Rossman et al., 2018). As previously mentioned, circulating oxidized lipids are a candidate circulating factor that may elevate CVD risk with aging. In support of this, oxLDL increases with aging (Moreau et al., 2005; Donato et al., 2008; Jablonski et al., 2011; Eskurza et al., 2006) and is associated with endothelial dysfunction (Eskurza et al., 2006), greater mtROS (Zmijewski et al., 2005; Zheng and Lu, 2020) and higher inflammation (Valente et al., 2014). The results of this pilot clinical trial indicate that MitoQ supplementation may improve arterial dysfunction with aging by modulating several fundamental aging mechanisms and support the concept that further investigation into the efficacy of this time- and resource-efficient alternative strategy to aerobic exercise is warranted (Fig. 5).
4.3.3. Senolytics
Under normal physiological conditions, the senescent cell program and the associated SASP execute beneficial physiological processes to facilitate health, however, with aging there is an excess accumulation of senescent cells and secretion of SASP factors leading to the development of arterial dysfunction (Rossman et al., 2017; Mahoney et al., 2021; Venkatasubramanian et al., 2022) (Fig. 2). In turn, habitual aerobic exercise can reduce the burden of excess cellular senescence and preserve endothelial function in older-trained ML/O adults (Rossman et al., 2017) (Fig. 3). However, given the low adherence rates emphasized above, developing and implementing alternative strategies to aerobic exercise to reduce the senescent cell burden with aging and to improve arterial function is required (Fig. 5).
The traditional approach to ameliorate the adverse physiological effects of excess senescent cells utilizes a synthetic pharmacological/chemotherapeutic senolytic (a molecule that can clear senescent cells), dasatanib, in combination with a flavonoid antioxidant, quercetin (D + Q). Old mice treated with D + Q demonstrated higher endothelial function and associated lower senescent cell burden in a variety of tissues (Roos et al., 2016). This treatment has since been translated to human clinical trials to treat other clinical populations (none associated with CVD) (Justice et al., 2019; Hickson et al., 2019), however, the broad translatability of D + Q to ML/O adults free of overt CVD for improving arterial function is limited by the adverse safety profile of dasatanib.
Our laboratory is advancing natural, safer, and more generalizable senolytics to improve arterial function in ML/O adults free of clinical disease, with one such senolytic being fisetin. Fisetin is a flavonoid found in a variety of fruits, vegetables and nuts that extends lifespan and reduces the senescent cell burden in mice (not quantified in arteries) (Yousefzadeh et al., 2018). Initial data from our laboratory suggest that intermittent oral supplementation with fisetin (100 mg/kg/day; 1 week on-2 weeks off–1 week on) results in higher endothelial function as a result of direct suppression of cellular senescence in arteries (Mahoney et al., 2022) (Fig. 5). As a next step, a pilot clinical trial in ML/O adults investigating fisetin as a natural and safe alternative treatment paradigm to improve arterial aging by reducing cellular senescence is required.
5. Potential strategies, research gaps, and future directions
5.1. Advancing efficacy of alternative strategies to aerobic exercise
Aerobic exercise remains the most effective intervention to improve arterial function with aging (Seals and Edward, 2014; Seals et al., 2018) (Fig. 3). However, more than half of the ML/O adults in the US do not meet the recommended guidelines for aerobic exercise (Bennie et al., 2019; Products - NHIS Early Release -, 2019), which is likely a result of barriers to aerobic exercise and other related limitations (CDC, 2022; Kimenai et al., 2022) (Fig. 4). In this review, we provide evidence-based alternative strategies that mimic exercise or target fundamental aging mechanisms known to be influenced by aerobic exercise (Fig. 5), however, the efficacy of these alternative strategies is not yet established and requires further translation prior to their administration as viable therapies to reduce arterial dysfunction with aging in ML/O adults (Table 1).
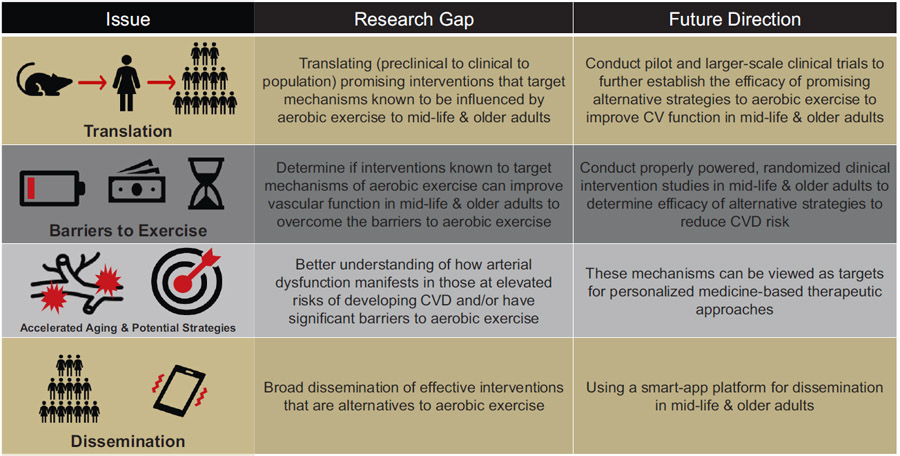
Table 1.
Potential strategies, research gaps, and future directions to overcome traditional barriers to exercise to reduce cardiovascular disease risk.
|
Although there has been extensive research on the effects of aerobic exercise on healthy cardiovascular (CV) aging, several important gaps remain to explore alternative strategies to overcome traditional barriers to aerobic exercise in groups that are vulnerable to CV diseases (CVD). These include, but are not limited to: 1) translating new and effective “exercise-inspired” interventions; 2) conducting intervention studies to establish the efficacy for alternative strategies to exercise to improve arterial function and reduce CVD risk in mid-life & older adults; 3) identifying key mechanisms of CV aging in populations that exhibit increased risk for CVD; and 4) disseminating effective implementation strategies for CV-targeted interventions to improve public health outcomes.
As such, our laboratory is currently conducting large-scale clinical trials (Table 1) to further establish the efficacy of several of these alternative strategies to improve CV function in ML/O adults and include: (1) high-resistance inspiratory muscle strength training (NCT04848675) (Craighead et al., 2022b), (2) chronic passive heat therapy (NCT05300971), (3) nicotinamide riboside (NCT03821623) (Freeberg et al., 2022) and (4) MitoQ (NCT04851288) (Murray et al., 2022).
5.2. Translation to groups most affected by barriers to exercise
Earlier in this review, we established that aerobic exercise interventions are effective in improving arterial function to reduce CVD risk in ML/O adults, however traditional barriers to exercise (CDC, 2022), as well as other less recognized barriers to exercise, such as socioeconomic status/deprivation (Kimenai et al., 2022), reduce adherence to and participation in aerobic exercise regimens in ML/O adults. In turn, lower aerobic exercise results in an increased risk for the development of CVD and associated risk factors (Fig. 4). In addition to ML/O adults, there are racial, ethnic, and other marginalized groups (i.e., American Indians and Alaskan Natives, African Americans, Hispanics, South Asians, LGBTQ+ (Carnethon et al., 2017; Rodriguez et al., 2014; Volgman et al., 2018; Caceres et al., 2020; Breathett et al., 2020)) that have shown improvements in CV outcomes in response to aerobic exercise interventions (Conn et al., 2012). However, these groups exhibit earlier onset of or elevated risk for CVD and associated risk factors compared to age-matched Non-Hispanic Whites. This phenomenon may be a result of increased vulnerability to barriers to aerobic exercise, most notably socioeconomic status/deprivation (Kimenai et al., 2022) (Table 1). As such, it is an important future research direction and biomedical research priority to determine whether time- and resource-efficient interventions known to target specific mechanisms of aerobic exercise that have shown efficacy in ML/O adults (Fig. 5) can favorably influence arterial function in the groups most vulnerable to present barriers (Table 1). Per NIH guidelines, subject participation in large clinical trials should reflect the general racial/ethnic and sex/gender demographics of the US (Guidelines for the Review of Inclusion, 2019); in addition to meeting this important requirement, properly powered studies to investigate these strategies designed specifically to target improving CV function in these groups, respectively, are needed (Table 1).
In doing so, the scientific community will obtain a better understanding of how arterial dysfunction manifests in those at elevated risk of developing CVD and/or have significant barriers to aerobic exercise. Results from ongoing investigations in ML/O adults and future investigations in vulnerable populations will establish alternative strategies for obtaining aerobic exercise-related benefits to improve overall CV function and health, as well as provide mechanisms that can be viewed as targets for personalized medicine-based therapeutic approaches (Table 1). Moreover, the development of broad dissemination tactics, i.e., a smart-phone app-based approach, to extend the access to and understanding of effective interventions that are alternatives to aerobic exercise could be highly effective (Table 1).
Acknowledgements
The authors thank the various past and present members of the Integrative Physiology of Aging Laboratory at the University of Colorado Boulder who have contributed to and/or are currently contributing to the body of work presented in this review. At the same time, authors thank https://www.flaticon.com/, which was the source of some of the images presented in the figures.
Funding
Work from the authors' research was supported by U.S. National Institutes of Health Awards T32 DK007135 (KOM), F31 HL165885 (SAM), R01 AG061514 (DRS), R01 AG071506 (DRS), R21 AG061677 (DRS), R01 AG066730 (DRS), R01 AG055822 (DRS), K99 HL159241 (ZSC).
References
- Abdalla SM, Yu S, Galea S, 2020. Trends in cardiovascular disease prevalence by income level in the United States. JAMA Netw. Open 3, e2018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari WE, Lovell G, 2009. Barriers to exercise in younger and older non-exercising adult women: a cross sectional study in London, United Kingdom. Int. J. Environ. Res. Public Health 6, 1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennie JA, De Cocker K, Teychenne MJ, Brown WJ, Biddle SJH, 2019. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. Adults. Int. J. Behav. Nutr. Phys. Act 16, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R, 2005. Endothelial aging. Cardiovasc. Res 66, 286–294. [DOI] [PubMed] [Google Scholar]
- Breathett K, et al. , 2020. Cardiovascular health in american indians and Alaska natives: a scientific statement from the American Heart Association. Circulation 141, e948–e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, Minson CT, 2021. Heat therapy: mechanistic underpinnings and applications to cardiovascular health. J. Appl. Physiol 1985 (130), 1684–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, Wiedenfeld-Needham K, Comrada LN, Minson CT, 2018. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J. Physiol 596, 4831–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, et al. , 2019. Passive heat therapy lowers systolic blood pressure and improves vascular endothelial function in healthy older adults. FASEB J. 33, 829.2–829.2. [Google Scholar]
- Brunt VE, et al. , 2019. Serum from young, sedentary adults who underwent passive heat therapy improves endothelial cell angiogenesis via improved nitric oxide bioavailability. Temperature (Austin) 6, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, et al. , 2020. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, et al. , 2021. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience 43, 377–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres BA, et al. , 2020. Assessing and addressing cardiovascular health in LGBTQ adults: a scientific statement from the American Heart Association. Circulation 142, e321–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, et al. , 2016. CD38 dictates age-related NAD decline and mitochondrial dysfunction through a SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon MR, et al. , 2017. Cardiovascular health in african americans: a scientific statement from the American Heart Association. Circulation 136, e393–e423. [DOI] [PubMed] [Google Scholar]
- CDC, 2022. Overcoming Barriers to Physical Activity. Centers for Disease Control and Prevention. https://www.cdc.gov/physicalactivity/basics/adding-pa/barriers.html. [Google Scholar]
- Clayton ZS, et al. , 2021. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am. J. Physiol. Heart Circ. Physiol 321, H185–H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JB et al. n.d.Arterial stiffness and diabetes risk in Framingham Heart Study and UK Biobank. Circ. Res 0, 10.1161/CIRCRESAHA.122.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Phillips LJ, Ruppar TM, Chase J-AD, 2012. Physical activity interventions with healthy minority adults: meta-analysis of behavior and health outcomes. J. Health Care Poor Underserved 23, 59–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JN, et al. , 2006. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am. J. Physiol. Heart Circ. Physiol 290, H1596–H1600. [DOI] [PubMed] [Google Scholar]
- Coppé J-P, Desprez P-Y, Krtolica A, Campisi J, 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead DH, et al. , 2019. Time-efficient physical training for enhancing cardiovascular function in midlife and older adults: promise and current research gaps. J. Appl. Physiol 1985 (127), 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead DH, Freeberg KA, McCarty NP, Seals DR, 2021. Time-efficient, high-resistance inspiratory muscle strength training for cardiovascular aging. Exp. Gerontol 154, 111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead DH, et al. , 2021. Time-efficient inspiratory muscle strength training lowers blood pressure and improves endothelial function, NO bioavailability, and oxidative stress in Midlife/Older adults with above-Normal blood pressure. J. Am. Heart Assoc 10, e020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead DH, Freeberg KA, Maurer GS, Myers VH, Seals DR, 2022. Translational potential of high-resistance inspiratory muscle strength training. Exerc. Sport Sci. Rev 50, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead D, et al. , 2022. Inspiratory muscle strength training for lowering blood pressure and improving endothelial function in postmenopausal women: comparison with “standard of care” aerobic exercise. Front. Physiol 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D-F, Rabinovitch PS, Ungvari Z, 2012. Mitochondria and cardiovascular aging. Circ. Res 110, 1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, et al. , 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, et al. , 2017. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, et al. , 2000. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Di Cesare F, Luchinat C, Tenori L, Saccenti E, 2021. Age- and sex-dependent changes of free circulating blood metabolite and lipid abundances, correlations, and ratios. J. Gerontol. A Biol. Sci. Med. Sci 77, 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, et al. , 2007. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR, 2008. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, et al. , 2011. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol 589, 4545–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Morgan RG, Walker AE, Lesniewski LA, 2015. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol 89, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, et al. , 2009. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J. Physiol 587, 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely BR, et al. , 2019. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol 317, R630–R640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR, 2004. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol 556, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Kahn ZD, Seals DR, 2006. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J. Physiol 571, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How Older Adults Can Get Started With Exercise. n.d. National Institute on Aging; https://www.nia.nih.gov/health/how-older-adults-can-get-started-exercise. [Google Scholar]
- Finerty JC, 1952. Parabiosis in physiological studies. Physiol. Rev 32, 277–302. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, 2013. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 4, 76–83. [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR, 2010. Arterial stiffening with ageing is associated with transforming growth factor-(β1-related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol 588, 3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML, Sindler AL, 2012. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, 1999. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J. Hypertens. Suppl 17, S29–S36. [PubMed] [Google Scholar]
- Freeberg KA, et al. , 2022. Nicotinamide riboside supplementation for treating elevated systolic blood pressure and arterial stiffness in midlife and older adults. Front. Cardiovasc. Med 9, 881703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs FD, Whelton PK, 2020. High blood pressure and cardiovascular disease. Hypertension 75, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Risk Factors Collaborators, 2015. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, et al. , 2014. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol 592, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, et al. , 2016. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8, 2897–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, et al. , 2018. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J. Appl. Physiol 1985 (124), 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, et al. , 2021. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J. Physiol 599, 911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guia RM, et al. , 2019. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol. Rep 7, e14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson TP, et al. , 2020. Hypertension is associated with blunted NO-mediated leg vasodilator responsiveness that is reversed by high-intensity training in postmenopausal women. Am. J. Phys. Regul. Integr. Comp. Phys 319, R712–R723. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Touyz RM, 2017. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70, 660–667. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, et al. , 2011. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944. [DOI] [PubMed] [Google Scholar]
- Hickson LJ, et al. , 2019. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoier B, et al. , 2021. Aerobic high-intensity exercise training improves cardiovascular health in older post menopausal women. Front. Aging 2, 667519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, et al. , 2018. Arterial stiffness and dementia pathology. Neurology 90, e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iulita MF, Noriega de la Colina A, Girouard H, 2018. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J. Neurochem 144, 527–548. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ, 2007. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J. Appl. Physiol 1985 (103), 1715–1721. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR, 2011. 25-hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, et al. , 2015. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. J. Hypertens 33, 2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Murphy MP, 2002. How mitochondrial damage affects cell function. J. Biomed. Sci 9, 475–487. [DOI] [PubMed] [Google Scholar]
- Jeon OH, et al. , 2022. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat. Metab 10.1038/s42255-022-00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Yeo D, 2022. Maintenance of NAD+ homeostasis in skeletal muscle during aging and exercise. Cells 11, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, et al. , 2019. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeminia M, et al. , 2020. The effect of exercise on the older Adult’s blood pressure suffering hypertension: systematic review and meta-analysis on clinical trial studies. Int. J. Hypertens 2020, 2786120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, et al. , 2016. Barriers and facilitators to the uptake and maintenance of healthy behaviours by people at mid-life: a rapid systematic review. PLoS One 11, e0145074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimenai DM, et al. , 2022. Socioeconomic deprivation: an important, largely unrecognized risk factor in primary prevention of cardiovascular disease. Circulation 146, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, et al. , 2020. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience 42, 727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D, 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a ‘set up’ for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D, 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107, 346–354. [DOI] [PubMed] [Google Scholar]
- Laurent P, et al. , 2011. Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J. Am. Soc. Hypertens 5, 85–93. [DOI] [PubMed] [Google Scholar]
- Lehallier B, et al. , 2019. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med 25, 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, et al. , 2011. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead box O phosphorylation. J. Gerontol. A Biol. Sci. Med. Sci 66, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, et al. , 2013. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp. Gerontol 48, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew LA, Ethier TS, Pyke KE, 2022. The impact of exercise training on endothelial function in postmenopausal women: a systematic review. Exp. Physiol 107, 1388–1421. [DOI] [PubMed] [Google Scholar]
- Li VL, et al. , 2022. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Berglund L, Larsson A, Sundström J, 2011. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123, 1545–1551. [DOI] [PubMed] [Google Scholar]
- López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN, 2013. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13, 106–118. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney S, et al. , 2021. Late-life treatment with the senolytic ABT-263 reverses aortic stiffening and improves endothelial function with aging. FASEB J. 35. [Google Scholar]
- Mahoney SA, et al. , 2022. Fisetin supplementation improves age-related vascular endothelial function by suppressing cellular senescence and mitochondrial oxidative stress. FASEB J. 36. [Google Scholar]
- Martens CR, et al. , 2018. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun 9, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, et al. , 2014. Aerobic exercise training increases plasma klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol 306, H348–H355. [DOI] [PubMed] [Google Scholar]
- McCann Haworth SM, et al. , 2021. Red blood cells from patients with pre-eclampsia induce endothelial dysfunction. J. Hypertens 39, 1628–1641. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, et al. , 2010. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation 121, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, et al. , 2001. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago heart association detection project in industry. Arch. Intern. Med 161, 1501–1508. [DOI] [PubMed] [Google Scholar]
- Miyachi M, et al. , 2003. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41, 130–135. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H, 2003. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc. Res 57, 861–868. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR, 2005. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Stauffer BL, Kohrt WM, Seals DR, 2013. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J. Clin. Endocrinol. Metab 98, 4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, et al. , 2022. Mitochondrial-targeted antioxidant supplementation for improving age-related vascular dysfunction in humans: a study protocol. Front. Physiol 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG, 2005. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46, 454–462. [DOI] [PubMed] [Google Scholar]
- Nishiwaki M, et al. , 2011. Vascular adaptations to hypobaric hypoxic training in postmenopausal women. J. Physiol. Sci 61, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, et al. , 2013. Age-related changes in microRNA levels in serum. Aging (Albany NY) 5, 725–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, et al. , 2014. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am. J. Phys. Regul. Integr. Comp. Phys 306, R510–R517. [DOI] [PubMed] [Google Scholar]
- Nyberg M, et al. , 2016. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training. Hypertension 68, 1011–1020. [DOI] [PubMed] [Google Scholar]
- de Picciotto NE, et al. , 2016. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, 2017. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr. Hypertens. Rep 19, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, 2018. Initiating life-long aerobic exercise 4–5 days per week before or near age 50 years: is this the ‘holy-grail’ of preventing age-related central artery stiffness? J. Physiol 596, 2635–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, et al. , 2011. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR, 2011. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin. Sci. (Lond.) 120, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy KL, et al. , 2018. The physical activity guidelines for Americans. JAMA 320, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Products - NHIS Early Release - 2018, 2019. https://www.cdc.gov/nchs/nhis/releases/released201905.htm.
- Fry R Amid the Pandemic, a Rising Share of Older U.S. Adults are Now Retired. n.d. Pew Research Center; https://www.pewresearch.org/fact-tank/2021/11/04/amid-the-pandemic-a-rising-share-of-older-u-s-adults-are-now-retired/. [Google Scholar]
- Risk of Economic Hardship Among Older Adults Issue Brief. n.d. ASPE; https://aspe.hhs.gov/reports/risk-economic-hardship-among-older-adults-issue-brief-0. [Google Scholar]
- Rodriguez CJ, et al. , 2014. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States. Circulation 130, 593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, et al. , 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, et al. , 2017. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Phys. Heart Circ. Phys 313, H890–H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, et al. , 2018. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71, 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Gioscia-Ryan RA, Clayton ZS, Murphy MP, Seals DR, 2020. Targeting mitochondrial fitness as a strategy for healthy vascular aging. Clin. Sci 134, 1491–1519. [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, LaRocca TJ, Seals DR, 2014. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv. Physiol. Educ 38, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz WM, et al. , 2018. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 137, 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Ellison M, Sinclair DA, 2021. The economic value of targeting aging. Nat. Aging 1, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Edward F, 2014. Adolph distinguished lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J. Appl. Physiol 117, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ, 2011. Aging and vascular endothelial function in humans. Clin. Sci. (Lond.) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ, 2014. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29, 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Brunt VE, Rossman MJ, 2018. Keynote lecture: strategies for optimal cardiovascular aging. Am. J. Physiol. Heart Circ. Physiol 315, H183–H188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat S, et al. , 2015. Arterial stiffness and decline in kidney function. Clin. J. Am. Soc. Nephrol 10, 2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, et al. , 2010. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell Sci 123, 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shern-Brewer R, Santanam N, Wetzstein C, White-Welkley J, Parthasarathy S, 1998. Exercise and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol 18, 1181–1187. [DOI] [PubMed] [Google Scholar]
- Silva JKTNF, Menêses AL, Parmenter BJ, Ritti-Dias RM, Farah BQ, 2021. Effects of resistance training on endothelial function: a systematic review and meta-analysis. Atherosclerosis 333, 91–99. [DOI] [PubMed] [Google Scholar]
- Strømland Ø, Diab J, Ferrario E, Sverkeli LJ, Ziegler M, 2021. The balance between NAD+ biosynthesis and consumption in ageing. Mech. Ageing Dev 199, 111569. [DOI] [PubMed] [Google Scholar]
- Stutts WC, 2002. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 50, 499–507. [PubMed] [Google Scholar]
- Sutton-Tyrrell K, et al. , 2005. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111, 3384–3390. [DOI] [PubMed] [Google Scholar]
- Taddei S, et al. , 2000. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101, 2896–2901. [DOI] [PubMed] [Google Scholar]
- Tanahashi K, et al. , 2014. Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am. J. Hypertens 27, 415–421. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR, 1998. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler. Thromb. Vasc. Biol 18, 127–132. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. , 2000. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102, 1270–1275. [DOI] [PubMed] [Google Scholar]
- Tarragó MG, et al. , 2018. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 27, 1081–1095.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The BH Health Study, 2015. de S. Andrade AC et al. Social context of neighborhood and socioeconomic status on leisure-time physical activity in a brazilian urban center. Cad. Saúde Pública 31, 136–147. [DOI] [PubMed] [Google Scholar]
- Tian X, et al. , 2022. Hypertension, arterial stiffness, and diabetes: a prospective cohort study. Hypertension 79, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend RR, et al. , 2015. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension 66, 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AJ, Irimpen AM, Siebenlist U, Chandrasekar B, 2014. OxLDL induces endothelial dysfunction and death via TRAF3IP2. Inhibition by HDL3 and AMPK activators. Free Radic. Biol. Med 70, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, et al. , 2022. Arterial stiffness and long-term risk of health outcomes: the Framingham heart study. Hypertension 79, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian R, et al. , 2022. Cellular senescence and the associated secretome contribute to age-related vascular dysfunction. FASEB J. 36. [Google Scholar]
- Virani SS, et al. , 2020. Heart disease and stroke Statistics-2020 update: a report from the American Heart Association. Circulation 141, e139–e596. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C, 2010. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol 55, 1318–1327. [DOI] [PubMed] [Google Scholar]
- Volgman AS, et al. , 2018. Atherosclerotic cardiovascular disease in south asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation 138, e1–e34. [DOI] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR, 2014. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor-κB. Clin. Sci. (Lond.) 127, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Thatcher SE, Cassis LA, 2017. Measuring blood pressure using a noninvasive tail cuff method in mice. Methods Mol. Biol 1614, 69–73. [DOI] [PubMed] [Google Scholar]
- Wenceslau CF, et al. , 2021. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol 321, H77–H111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Vita JA, 2003. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol 42, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Franklin SS, Cockcroft JR, 2004. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 44, 112–116. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM, 2007. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the cardiovascular health study. Circulation 115, 2390–2397. [DOI] [PubMed] [Google Scholar]
- Yeboah J, et al. , 2009. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, et al. , 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2021. Circulating pro-inflammatory exosomes worsen stroke outcomes in aging. Circ. Res 129, e121–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD, 2022. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 10.1111/febs.16350. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lu C, 2020. Oxidized LDL causes endothelial apoptosis by inhibiting mitochondrial fusion and mitochondria autophagy. Front. Cell Dev. Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng M, et al. , 2020. Arterial stiffness preceding diabetes: a longitudinal study. Circ. Res 127, 1491–1498. [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, et al. , 2005. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol 289, H852–861. [DOI] [PubMed] [Google Scholar]
- Guidelines for the Review of Inclusion, 8, 2019. [Google Scholar]