Abstract
It is estimated that a majority of people who use psychostimulants, particularly methamphetamine (MA) and cocaine, experience withdrawal upon abstinence from sustained use. This review of clinical research reports the evidence regarding biomedical and behavioral treatments for psychostimulant withdrawal symptoms. It provides a framework for clinicians and scientists to increase impact on attenuating MA and cocaine withdrawal during initial and sustained abstinence. Articles reviewed included reports of controlled clinical trials (randomized or non-randomized) reporting at least one withdrawal symptom among the outcomes or specifically studying patients in withdrawal. Potential efficacy for MA withdrawal is noted for a few medications (mirtazapine, naltrexone, bupropion) and repetitive transcranial magnetic stimulation during acute (first week), early protracted (weeks 2–4) and late protracted (>4 weeks) withdrawal phases. Topiramate shows mixed evidence of efficacy for cocaine withdrawal. In general, there is inconsistent signal for biomedical and behavioral treatments on MA and cocaine withdrawal.
Keywords: withdrawal, treatment, review, methamphetamine, cocaine, amphetamine
Background
Globally, use of stimulants involves a large number of individuals, with amphetamine-type stimulants (ATS) representing the second largest class of drugs reported by individuals behind cannabis (1). Industrialized countries of Australia, United States, and Canada have shown the highest prevalence of stimulant use with significant increases in both use and mortality over the past decade (2). Globally, use of ATS is estimated at 34 million in 2020 (compared to 33 million in 2010), though market indicators from Asian countries suggest this may be higher. The highest prevalence of ATS use, especially methamphetamine (MA), is in the U.S. and Canada, followed by Australia (1, 2). From 2015 to 2019, the number of U.S. adults who reported using MA in the past year increased 43% and the number of U.S. adults with MA use disorder (MUD) increased 62% (3). The U.S. age-adjusted, MA-related overdose death rate increased 5-fold from 2012 to 2018 (4). The estimated global use of cocaine has increased from about 22 million in 2010 to over 25 million in 2020, with the highest prevalence of reported cocaine use in Oceania (Australasia, Melanesia, Micronesia, and Polynesia), U.S., and Canada. From 2010 to 2019, the prevalence of cocaine use in Australia doubled from 2.1% to 4.2% (1), with the corresponding cocaine-related mortality rate increasing from 0.10 per 100,000 to 0.23 per 100,000 (5). In U.S. adults, the prevalence of cocaine use also increased from 1.7% in to 2.1% (6), as did cocaine-related mortality from 2 per 100,000 in to 6.5 per 100,000 (7). Although reported rates of cocaine use in the U.S. are lower than in Australia, the U.S. mortality rate is greater, suggesting that cocaine use in the U.S. is more severe but less common (1) and likely to involve fentanyl use (8).
People value psychostimulants for their consistent and strong positive effects. Those effects are key reasons people who use psychostimulants initiate drug use and continue to use the drug at high doses over time. Positive physical effects of psychostimulants include increases in heart rate, blood pressure, pupil size, respiration, sensory acuity and energy and decreases of appetite, sleep and reaction time. Psychologically, psychostimulants produce increases in confidence, alertness, attention, mood, sex drive, talkativeness and energy and decreases in boredom, loneliness and timidity (9). The neurobiology supporting these positive effects include increases in dopamine availability at the synapse in areas of the brain that support functions of reward, reinforcement, memory and emotion (10). These positive effects are transient and fade with tolerance to the drugs with continued use. These strong and positive effects serve varied and different functions from sustained and high dose use of psychostimulants (additional to euphoria/getting high) in the lives of people who regularly use cocaine, crack, amphetamine and MA. These effects serve many purposes that include: (1) wakefulness at night to increase physical safety for those experiencing homelessness; (2) enhancing energy and attention to work long hours or multiple shifts; (3) enhancing sexual behaviors such as sex with drugs and supporting exchange sex; and (4) managing boredom (11–14). Soon after cessation or reduction in prolonged use of psychostimulants, many experience withdrawal symptoms that include dysphoric mood plus fatigue, vivid and unpleasant dreams, insomnia or hypersomnia, increased appetite and psychomotor retardation or agitation (15). Prevalence of MA and cocaine withdrawal is estimated to occur in most (53%-97%) people who initiate abstinence following prolonged use of psychostimulants; symptoms can last from days to months (16).
Withdrawal symptoms that emerge following reduction or discontinuation of consistent, high doses of psychostimulants can efficiently motivate individuals to use or return to use of the drugs. Representing the concept of the “dark side” of addiction, neurobiological responses to reduced or discontinued use are the foundation of the misery many experience as psychostimulant withdrawal, which is mediated by reductions of serotonin and dopamine, which corresponds with increases in corticotropic releasing factor and activity in the dynorphin-kappa receptor system (17). Symptoms are time-limited, no matter how severe. In many people’s experiences, a single use of psychostimulants instantly eliminates the negative state caused by withdrawal symptoms. The feelings of physical well-being and psychological energy that follow use defines the operant conditioning concept of negative reinforcement, i.e., the reinforcing value of the stimulant is increased in its immediate removal of the misery of withdrawal symptoms (18).
It is this negative reinforcement property that presents challenges to individuals seeking to reduce or stop psychostimulant use (19). Furthermore, decreased dopamine activity and receptor supersensitivity during psychostimulant withdrawal are biological factors that correspond with likelihood of return to use during treatment (20). This review reports on the evidence from efficacious biomedical, behavioral and psychosocial interventions for managing psychostimulant withdrawal and discusses this information relative to best practices to clinically manage these symptoms.
Dependence, withdrawal states and clinical management
The immediate withdrawal symptoms following abstinence from psychostimulants correspond with time to elimination from the body. Cocaine and crack, and their short half-lives (from 5–90 minutes), are metabolized by enzymes in the liver and blood and eliminated via the kidneys in urine, with all cocaine removed from the body within the first 24 hours (21). Metabolites of cocaine, especially benzoylecgonine, however, are longer lasting (~6 hours) with long periods to complete elimination following repeated, high-dose administrations prior to abstinence (22). Amphetamine has a long half-life (9–12 hours); MA has a short half-life (90 minutes) but metabolizes to d-amphetamine in the first pass, which has half-life of 9–12 hours (23). Significant amounts of both drugs are eliminated in urine unchanged with the remainder metabolized primarily by P450 2D6 isozyme, with drug and metabolites eliminated completely after 3–4 days.
Acute Phase.
Figure 1 depicts a timeline with three non-overlapping phases of symptoms commonly experienced following abstinence from psychostimulants. Withdrawal symptom type and severity peak after the first 2–3 days following abrupt discontinuation of stimulants; major discomfort from these symptoms usually resolves within 4–7 days. Symptoms common in this phase include: depression, anhedonia, anxiety, irritability, physical discomfort (including headaches, body aches, dental pain), fatigue, prolonged sleep, craving, and poor concentration (24, 25).
Figure 1.
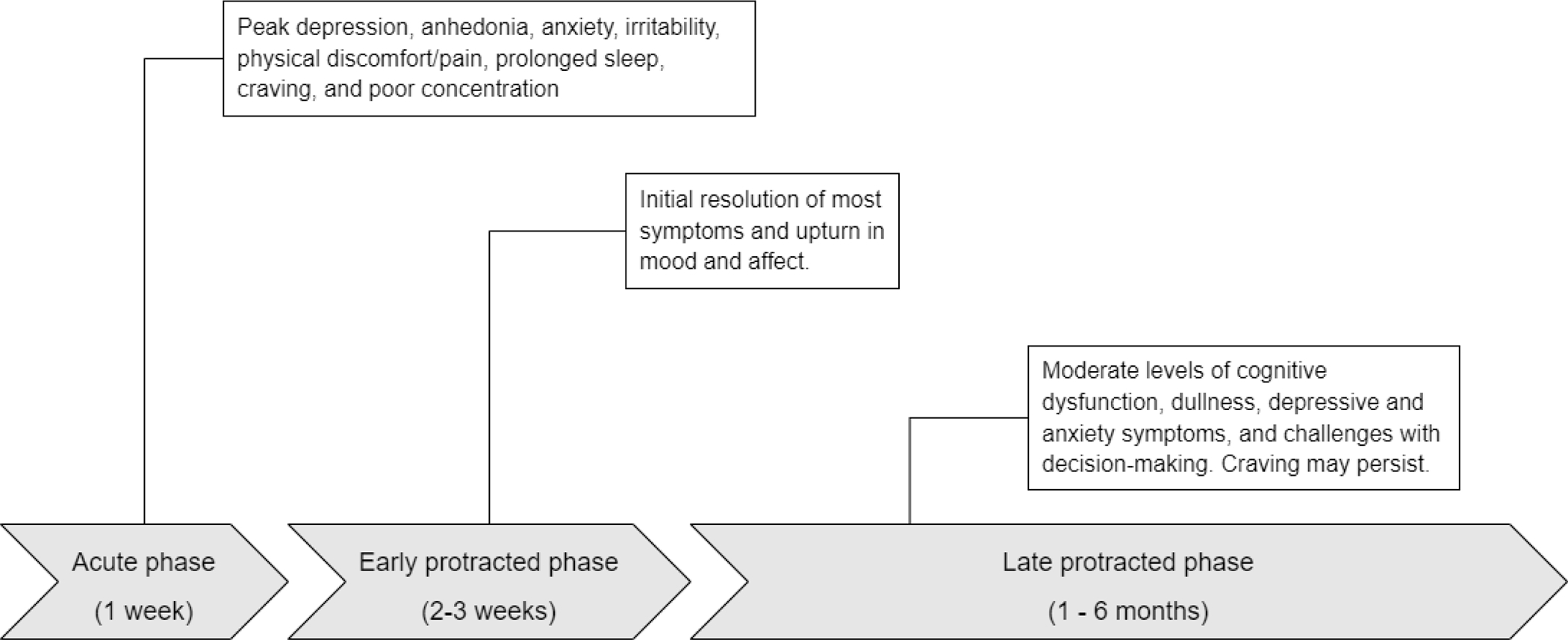
Summary of stimulant withdrawal symptoms across different phases (with timeframe following abstinence).
The difficulty for clinicians and patients in managing early withdrawal can be drawn from the neurobiology of early abstinence from stimulants. Following abstinence, the brain launches a massive inflammatory response to clear away free radicals and damaged nerve processes (particularly presynaptic axons) that result from sustained use (26). Both dopamine and serotonergic neurons are involved in this process, which occurs as kappa agonism and corticotropic releasing factor peak. As time from the last use of drug increases, withdrawal symptoms intensify. Patients feel the need to sleep, to feed and to hydrate. To sustain abstinence from stimulants, a number of executive functions are required exactly when patients feel the worst. This includes: energy and thoroughness to rid one’s environment of drug, locating and disposing of drug, drug equipment and paraphernalia, re-establishing contacts with medical, dental, work, family and social contexts. Clinicians respond and try to teach behavioral and cognitive skills to avoid lapse and or return to use while patients are experiencing withdrawal and lack of cognitive resources. Efficacious medication or interventional treatments that counter these symptoms have great value as patients have challenges in the withdrawal state in learning and implementing cognitive tasks and behavioral skills.
Early Protracted Phase.
After the first week of acute withdrawal symptoms, a protracted period emerges. Over a period of 2–3 weeks, the intensity of most withdrawal symptoms resolves and instead a period emerges where mood and affect can return to what is normal for patients. Preclinical (27) and clinical (28) evidence supports the notion of supersensitivity of dopamine receptors with abstinence from sustained exposure to stimulants, which provides a rationale why diminished dopamine tone at the receptor might be experienced more positively during the period. During weeks 1–4 after last use, people make behavioral and cognitive adjustments sufficient for many to begin to take on most activities of daily living. While craving levels peak during the first week and continue to fall with time (29, 30), brief episodes of craving can occur at any time. Some craving episodes are triggered by encountering drug cues (persons, places and things conditioned with stimulant use) (31). Cognitive-behavioral therapies for managing craving emphasize the importance of recognizing the “trigger-thought-craving-use” cycle, with lapse or return to use avoided by using techniques to interrupt the progression from trigger to thought and thought to craving. Allowing triggers and thoughts to proceed to cravings builds upon the intense subjective experience that only use will end the craving (32). A unique case of craving occurs at night for many after initiating psychostimulant abstinence in the form of “drug dreams.” In these dreams, patients see themselves getting the drug, experiencing the smells and body-feel of using stimulants, which invokes feelings of anxiety and craving upon awakening. Normalizing the nature of drug dreams in early abstinence and reminding patients to remain in bed until the craving passes can help.
Late Protracted Phase.
After the first 3–4 weeks, approximately, damaged neural systems involved in consistent, high-dose use of psychostimulants and the neurobiological processes of repair from that damage produce a new stasis. The experience of this longer, subtle phase of withdrawal is linked to mild cognitive dysfunction (33, 34), particularly in functions involving memory and executive function. Mood and affect ratings note ongoing experiences of cognitive dullness, psychostimulant craving and moderate symptoms of depression and anxiety (24, 25). Perhaps most pertinent is measured difficulties for persons with MUD in this phase who demonstrated continued deficits when making decisions about risk in the setting of recent loss in neurocognitive tasks (35).
Methods
To identify gold standard clinical practices for managing psychostimulant withdrawals, we searched PubMed, Medline, Embase, and Google Scholar from database inception to July 15, 2022, for manuscripts published in English that assessed the clinical treatment of withdrawal from MA, other ATS, and/or cocaine. We included search terms associated with psychostimulant withdrawal (“cocaine withdrawal” and “methamphetamine withdrawal”), psychostimulant treatment (“methamphetamine treatment” and “cocaine treatment”) and treatment of stimulant withdrawal (“withdrawal treatment”, “cocaine withdrawal treatment”, and “methamphetamine withdrawal treatment”). We did not include specific symptoms (e.g., anxiety, depression, craving, etc.) among our search terms, as many symptoms that occur during withdrawal are also commonplace in the cycle of active use and addiction (9), which would broaden searches beyond the scope of this review. We limited articles to reports of clinical trials (randomized or non-randomized) reporting at least one withdrawal symptom among the outcomes tested or specifically studying patients in withdrawal. Case series/studies/reports were excluded. Our search yielded 30 studies: 11 on MA withdrawal and treatment (9 biomedical, 2 behavioral) (see Table 1), 4 on ATS withdrawal and treatment (all biomedical), 12 on cocaine withdrawal and treatment (all biomedical) (see Table 2), and 2 behavioral trials with samples including both people with MUD and people with CUD. We narrated this information to improve access to clinicians interested in providing high-quality evidence-informed care.
Table 1.
Summary of literature on clinical management of withdrawal symptoms from methamphetamine and other amphetamine-type stimulants
| Author | Year | Journal | Design | N | Treatments | Timeframe | Withdrawal-related findings |
|---|---|---|---|---|---|---|---|
| Birath, B. et al. a | 2017 | Drug and Alcohol Dependence | Randomized placebo-controlled pilot trial | 11 | Ibudilast (0 mg, 50 mg, 100 mg each for 5 days) | 27 days hospitalized with last assessment at day 22 | Significantly reduced variability in response time to an attention task compared to placebo. |
| Black, D.S. & Amaro, H. a,† | 2019 | Behavioral Research and Therapy | Randomized-controlled trial; residential treatment setting | 200 | Mindfulness-based intervention (80 min sessions, 2 per week); psychoeducation control | 6 weeks; abstinence began upon admission to setting | Greater retention that the control group. |
| Brooks, S. et al. a | 2016 | NeuroImage: Clinical | Non-randomized controlled trial | 66 | Working memory training (30 mins per day) plus TAU; TAU | 8 weeks; no definition of start of abstinence at baseline | Increases of the bilateral basal ganglia and reduced bilateral cerebellum volume; improvements in self-reported impulsivity scores in the working memory training group, but not in the treatment-as-usual group. |
| Cruickshank, C.C. et al. a | 2008 | Drug and Alcohol Review | Randomized placebo-controlled pilot trial | 31 | Mirtazapine (30 mg nocte); placebo | Treatment during first 2 weeks of withdrawal period with follow-up at day 35 | No significant association with retention in out-patient withdrawal treatment |
| Glasner-Edwards, S. et al. a,† | 2017 | Mindfulness | Randomized-controlled trial | 63 | Mindfulness-based relapse prevention (8 weekly groups); health education (8 weekly groups) | 12-week trial; 4 weeks contingency management lead in then randomized to: 8 weeks MBRP+CM; 8 weeks HE+CM | Mindfulness-based intervention plus contingency management showed significantly greater reductions in depression and anxiety symptoms compared to Health Education for those with MDD or GAD. |
| Hester, R. et al. a | 2010 | Experimental and Clinical Psychopharmacology | Randomized placebo-controlled pilot trial | 20 | Modafinil (200 mg/d); placebo | First 7 days of withdrawal | Significantly improved verbal recall and nonsignificant—but improved—executive function and delayed memory tasks compared to placebo. |
| Heinzerling, K.G. et al. b | 2010 | Drug and Alcohol Dependence | Randomized placebo-controlled trial | 71 | Modafinil (400 mg/d); placebo | 12 weeks following randomization | No significant association with depressive symptoms, cravings, and retention |
| Jayaram-Lindström et al. b,‡ | 2008 | American Journal of Psychiatry | Randomized placebo-controlled trial | 80 | Naltrexone (50 mg/d); placebo | 12 weeks outpatient; 2 weeks abstinence prior to starting medication | Significantly greater reduction in craving |
| Kongsakin, R. et al. a,‡ | 2005 | International Clinical Psychopharmacology | Randomized placebo-controlled trial | 20 | Mirtazapine (15 mg/d titrated to 60 mg/d depending on response) | 14 days, during detoxification | Significantly greater improvement in Amphetamine Withdrawal Questionnaire scores, anxiety, and hyperarousal compared to placebo at days 3 and 14 |
| Liang, Y. et al. a | 2018 | JAMA Psychiatry (research letter) | Randomized placebo-controlled trial | 50 | Repetitive transcranial magnetic stimulation (10 Hz, left DLPFC); sham | Two sessions daily for five days with two days between sessions following initial abstinence of 1–15 days | Significantly greater reductions in withdrawal symptoms, craving, depressive symptoms, anxiety symptoms, and greater improvements in sleep quality compared to placebo. |
| McGregor, C. et al. a | 2008 | Journal of Substance Abuse Treatment | Three-group clinical trial comparing modafinil, mirtazapine, and historical treatment-as-usual | 49 | Modafinil (400 mg/d); mirtazapine (60 mg/d); periciazine (2.5–5 mg/d) | First 10 days of withdrawal | Significantly lower Amphetamine Cessation Symptom Assessment scores in modafinil and mirtazapine groups compared to periciazine group; greater hours of sleep but more sleep disturbance in the mirtazapine group compared to the modafinil group. |
| Miles, S.W. et al. b,‡ | 2013 | Addiction | Randomized-controlled trial | 79 | Extended release methylphenidate (54 mg/d); placebo | 22 weeks following randomization | No significant difference in craving compared to placebo, though retention was higher in those who received methylphenidate. |
| Rawson, R.A. et al. a | 2015 | Journal of Substance Abuse Treatment | Randomized controlled trial | 135 | Exercise (45 mins high intensity, 3 per week) plus TAU vs Health education (3 per week) plus TAU | 8 weeks, recruited upon entry in residential treatment | Significantly greater reductions in depressive and anxiety symptoms compared to health education control group. |
| Srisurapanont, M. et al. a,‡ | 1999 | Australian and New Zealand Journal of Psychiatry | Randomized-controlled trial | 44 | Amineptine (100 mg/d); placebo | 2-week inpatient trial; abstinence started < 5 days prior to randomization | Amineptine reversed decreased energy, increased appetite and craving for sleep compared to placebo |
| Su, H. et al. a | 2017 | Drug and Alcohol Dependence | Randomized placebo-controlled pilot trial | 30 | Repetitive transcranial magnetic stimulation (10 Hz, left DPLFC); sham | 3 weeks, but duration of initial abstinence not reported | Significantly greater improvements in verbal learning, memory, and social cognition compared to sham. |
| Thompson, R.G., Jr. et al. b | 2021 | Journal of Psychopharmacology | Randomized placebo-controlled pilot trial with planned crossover | 34 | d-amphetamine (60 mg/d); placebo | 4 weeks following randomization | Significant attenuation of craving compared to placebo group; heart rate elevated in placebo during week 1 |
| Trivedi, M. et al. b | 2021 | New England Journal of Medicine | Randomized placebo-controlled trial | 403 |
Bupropion (450 mg/d) with extended release naltrexone (380mg at 3 weeks); placebo | 12-week trial; assessments collected weeks 5–6 and 11–12. | Lower craving for combination treatment compared to placebo in assessment weeks |
Symptom-focused trial during the withdrawal period.
Stimulant use reduction trial reporting withdrawal symptoms.
Study participants included both people who used methamphetamine and people who used cocaine.
Study participants included those who used any amphetamine-type stimulant.
Table 2.
Summary of literature on clinical management of cocaine withdrawal symptoms
| Authors | Year | Journal | Design | N | Treatment | Timeframe | Withdrawal-related findings |
|---|---|---|---|---|---|---|---|
| Arndt, I.O. et al. | 1992 | Archives of General Psychiatry | Randomized placebo-controlled trial | 59 | Desipramine hydrochloride (50 mg/d, increasing 50mg every 2–4 days to 250–300mg/d); matching placebo | 12-week medication phase, followed by 1, 3, and 6 post-treatment recontact | Significantly greater improvements in psychiatric status compared to placebo. |
| Campbell, J. et al. | 2003 | American Journal on Addictions | Randomized placebo-controlled trial | 57 | Desipramine (50 mg/d increasing to 200 mg/d); carbamazepine (initiated at 200 mg/d increasing to 800 mg/d); matching placebo | 8 weeks outpatient following randomization | Desipramine showed significantly greater reductions in depressive symptoms and irritability compared other groups. |
| Dackis et al. | 2005 | Neuropsychopharmacology | Randomized placebo-controlled trial | 62 | Modafinil (400 mg/d); matching placebo; all with CBT | 8 weeks following randomization | Significantly greater abstinence compared to placebo, but no significant differences in craving or withdrawal. |
| Dackis et al. | 2012 | Journal of Substance Abuse Treatment | Randomized placebo-controlled trial | 210 | Modafinil (0 mg/day, 200 mg/day, or 400 mg/day) for 8 weeks; all with CBT | 8 weeks following randomization; 3 months post-treatment follow-up | No significant differences in cocaine use, craving, withdrawal, retention, or abstinence |
| Gawin, F.H., et al. | 1989 | Archives of General Psychiatry | Randomized placebo-controlled trial | 72 | Desipramine hydrochloride (2.5 mg/kg); active placebo (atropine 0.1 mg) | 6 weeks outpatient following randomization | Significantly greater reductions in craving compared to lithium and placebo. |
| Johnson, B.A. et al. | 2013 | JAMA Psychiatry | Randomized placebo-controlled trial | 142 | Topiramate (50 mg/d increasing to 300 mg/d in weeks 6–12); matching placebo; all with cognitive behavioral therapy (CBT); | 12 weeks following randomization | Significantly greater reductions in craving, symptoms of cocaine dependence, and cocaine use (confirmed by urine tests) compared to placebo. |
| Kampman , K.M. et al. | 2006 | Drug and Alcohol Dependence | Randomized placebo-controlled trial | 199 | Amantadine (300 mg/day); propranolol (100 mg/day); amantadine (300 mg/d) + propranolol (100 mg/d); matching placebo | 10 weeks with 2-week baseline phase and 8-week treatment phase | No significant difference in retention or abstinence compared to placebo among those entering the study with severe cocaine withdrawal; changes in withdrawal symptoms not among the reported outcomes. |
| Kampman, K.M. et al. | 2013 | Drug and Alcohol Dependence | Randomized placebo-controlled trial | 170 | Topiramate (300 mg/d); placebo; all with psychotherapy | 13 weeks; 3-day abstinence prior to treatment | No significant differences in reducing craving or cocaine use. |
| McDowell, D. et al. | 2005 | Drug and Alcohol Dependence | Randomized placebo-controlled trial | 111 | Desipramine (50 mg/d increased by 50 mg every 4 days up to 300 mg/d); matching placebo; all with psychosocial treatment | 12 weeks after 1-week single blind placebo phase and randomization | Significantly greater improvements in depressive symptoms compared to placebo. |
| Nuijten, M. et al. | 2014 | Drug and Alcohol Dependence | Randomized controlled trial | 74 | Topiramate (200 mg/d) + CBT; CBT only | 12 weeks after randomization | No significant differences in improving craving, physical health, or mental health. |
| Umbricht et al. | 2014 | Drug and Alcohol Dependence | Randomized placebo-controlled trial | 171 | 2x2 factorial design of topiramate (ascending by 25 mg to 300 mg/d by week 13, tapered weeks 21–23) vs. placebo, plus contingency management vs. non-contingent rewards; all participants in methadone maintenance | 31 weeks total, with treatment from weeks 5–23 following methadone induction and placebo lead-in | No significant differences in reduced craving, depression, or anxiety. |
| Terraneo et al. | 2016 | European Neuropsychopharmacology | Randomized controlled trial | 32 | Repetitive transcranial magnetic stimulation (15 Hz, left DLPFC) | 29 days following randomization, 63-day follow-up post-treatment | Significantly greater reductions in craving and higher number of negative urine tests compared to control. |
Note: Articles listed are stimulant use reduction trials reporting withdrawal symptoms.
Gold-standard current practice
There are no pharmacological or other biomedical treatments approved by the U.S. Food and Drug Administration that attenuate withdrawal symptoms in people who are recently abstinent from ongoing use of ATS or cocaine. Psychosocial/behavioral interventions—such as contingency management, community reinforcement, and cognitive behavioral therapy—remain the standard and most reliably available practices for treatment of MUD (36) and CUD (37). However, these approaches largely target abstinence without addressing withdrawal management. The advantages of outpatient versus inpatient treatment for psychostimulant disorders may vary by patient. In general, acute withdrawal symptoms from MA and cocaine are safe to manage at home (38), and those who have limited transportation options, hold a daytime job, attend school, or have privacy concerns may find outpatient treatment more appealing and accessible (39). In instances where patients have severe polysubstance use, severe psychiatric symptoms, or difficult life circumstances like homelessness or domestic violence, inpatient treatment may offer a safer setting for withdrawal management (38, 40).
For this review, differences in trial design prevent using meta analyses techniques to compare outcomes across studies; as well, studies in pharmacotherapies are generally underpowered and yield inconsistent results. Clinical trials for psychostimulant withdrawal provide assessments of treatments instituted in the first few weeks of treatment or abstinence, while abstinence-focused treatment studies often, but not always, measure withdrawal symptoms over periods of 8–16 weeks.
Of the 9 biomedical trials for MA identified by this review, 6 focused on symptoms during a period of MA withdrawal, while the other 3 trials primarily tested for efficacy for MA reduction with one or more withdrawal symptoms as secondary outcomes. For ATS, there were 2 biomedical withdrawal trials and 2 treatment trials for ATS reduction. All 13 of the biomedical trials for CUD identified for this review were efficacy trials for reducing cocaine use with one or more withdrawal symptoms as secondary outcomes. A listing of the studies in this review are provided in Tables 1 and 2 for ATS/MA and cocaine withdrawal treatments, respectively. In lieu of combining findings across studies, details about the treatment, the dose, the length of the trial, and the withdrawal symptoms that showed significant findings compared to other randomized conditions are provided.
Withdrawal Symptoms.
From the review, three reports of four MA treatments report significant reductions of standard measures of withdrawal symptoms over placebo or sham conditions: Two early pilots of mirtazapine (15–60mg/d for 14 days; (41) and 60mg/d for 10 days (38)), one of modafinil 400mg for 10 days (38) and one trial of repetitive transcranial magnetic stimulation (rTMS) (10HZ at left DPLFC, two 5-day sessions over 12 days (42)). A third trial of mirtazapine (30mg/nocte for 14 days (43) showed no differences in withdrawal symptoms, though in this study all medications were self-administered at night and medication adherence problems may have interfered. Another early trial of amineptine, a tricyclic antidepressant (100mg/d for 14 days (44)) showed significant improvements in withdrawal symptoms of decreased energy, decreased appetite and cravings for sleep. Amineptine was removed from the market in 2004 for misuse liability and safety concerns and remains a Schedule I drug in the U.S. and Schedule II drug in many countries (45), all of which stopped interest in continued evaluation of this medication. This remains one of the few evaluations of a psychostimulant to treat psychostimulant withdrawal.
Craving.
Craving is not only a withdrawal symptom for psychostimulants, but is now a diagnostic criteria for addiction disorders in DSM-5 nosology (15) and therefore also a part of the pattern of use (46, 47). Three medication studies showed significant reductions in craving ratings for MA: d-amphetamine 60mg/d for four weeks (48), combination of extended release naltrexone (380mg every 3 weeks) and bupropion (450mg q d; (49)) and rTMS (10HZ at left DPLFC, two 5-day sessions over 12 days (42)). Of interest, significant increase in heart rate for placebo over 60mg d-amphetamine were reported in week 1 of the 4-week trial of d-amphetamine (48), though otherwise the medication was tolerated well. A randomized controlled trial of naltrexone (50mg/d) for amphetamine use disorder showed significantly greater reductions in craving ratings compared to placebo (50). Of interest, participants randomized to this trial had to achieve two weeks of amphetamine abstinence at randomization. For cocaine withdrawal, two treatment trials reported significant craving reductions over placebo: a trial of desipramine (2.5 mg/kg/d for 6 weeks (51) and a 12-week trial of topiramate (300mg/d at steady state (52). For topiramate, a fructopyranose derivative that increases GABA(A) concentrations and antagonizes glutamate, findings from this trial were not replicated in other trials (53–55). A randomized placebo-controlled trial of modafinil (400mg/d) for CUD indicated significantly lower use compared to placebo, but no significant differences in craving or withdrawal (56), while a larger subsequent trial showed no differences in cocaine use, craving, withdrawal, retention, or abstinence even when accounting for dosage (57).
Depression and Anxiety Symptoms.
Three trials of desipramine report significant improvement along depressive symptoms over placebo for cocaine use disorder. This includes two trials in primary cocaine use disorder (desipramine 200mg/d for 8 weeks (58) and up to 300mg/d for 12 weeks (59)) and a single report in methadone maintained participants with cocaine use disorder for desipramine (250–300mg/d for 12 weeks (60)) in improving psychiatric status. As well, desipramine at 200mg for 8 weeks (58) reduced symptoms of irritability, which is a component of depressive disorder. These studies point to desipramine efficacy as an antidepressant, however, there are no findings to point to desipramine in producing cocaine abstinence.
Behavioral and neuromodulation interventions show some consistency in reducing these symptoms with initiation of stimulant abstinence. Depressive symptoms are reduced significantly with two 5-session courses of rTMS following MA abstinence (42). Using behavioral techniques, depressive and anxiety symptoms in abstinence from MA use are reduced with three times per week of 45 minutes of intensive exercise in a residential treatment setting (61). For people living with major depressive and general anxiety disorders, eight 60-minute weekly mindfulness relapse prevention group sessions reduced ratings of depression and anxiety symptoms over a health education group in the setting of a 12-week contingency management program (62). Related to this, 30 minutes per day in working memory training plus treatment as usual compared to treatment as usual alone for MUD yielded differential changes in fMRI measures of volume in bilateral basal ganglia and cerebellum and improvements in self-reported ratings of impulsivity (63).
Cognition.
Findings are not strong regarding cognitive improvements in the trials reviewed. Still there are indications that point to some interventions that can improve aspects of cognitive functioning, all in evaluations of MA treatments. Five sessions of rTMS (10HZ at DLPFC (64)) increased measures of verbal learning, memory and social cognition compared to sham procedures. Seven days of modafinil (200 mg/d) increased measures of verbal recall (65). As well, five days of 100mg the phosphodiesterase inhibitor, ibudilast, produced significant improvement in attention tasks over placebo (66). Of these, only rTMS is being investigated for its efficacy to reduce withdrawal symptoms. The National Institute on Drug Abuse is currently supporting a Clinical Trials Network study on rTMS for MUD and CUD (Protocol ID: CTN-0108) (67).
Sleep.
This review identified two findings, both in studies of MA treatments, that affected measures of sleep. First, rTMS delivered in two sets of five sessions produced reports of higher sleep quality compared to sham (42). In the second, 10 days of 60mg mirtazapine/d produced more sleep hours, but more sleep disturbance compared to 10 days of 200mg modafinil/d or to placebo (38).
Retention.
Though retention is not part of addressing withdrawal symptoms per se, it remains that one cannot engage a patient who is not in the room. In a trial of extended-release methylphenidate (54mg/d) for ATS (including MA) no significant difference was observed in reducing craving ratings compared to placebo, but the medication did produce significantly better retention (68). In a parallel group randomized controlled trial of women in residential treatment (of whom 76% used MA and 13% used cocaine), those assigned to the MBI showed significantly greater retention than the control group and a significant dose response on improved distress tolerance and positive affect (69).
Weighing risks versus benefits.
Although the efficacy of any particular treatment for managing psychostimulant withdrawals appears limited, the benefits of introducing treatment whether biomedical or behavioral poses low risk relative to the potential benefits of quitting or reducing use. As stated previously, the withdrawal syndrome for MA and cocaine are largely non-life threatening, with the acute symptoms resolving in 4–7 days. Support for antidepressant medications is based on 8–12 week trials; it is not known whether short-term use of antidepressant during acute withdrawal would show significant benefits. Furthermore, commonplace withdrawal symptoms like depression and anxiety are often already part of the typical highs and lows experienced with regular MA (70) or cocaine use (71). Even if treatment does not result in complete, sustained abstinence, reducing use to a lower frequency or dose may reduce the psychosocial problems, the risk of developing chronic health conditions, and the risk of acquiring infectious diseases associated with the drug (14). The alternative to treatment could mean continued use at high, frequent levels, which could lead to HIV infection, HIV disease progression in people with HIV, development of chronic diseases like heart disease (72) and pulmonary hypertension (73), overdose (3), and major psychiatric illness such as psychosis (74, 75).
Complex cases
Psychostimulant use disorders can be complicated by co-use of other substances, especially opioids such as fentanyl (76) and heroin (76–78), as psychostimulant-opioid co-use has increased in various nations over the past 6 years. If a patient uses both psychostimulants and opioids, individualized assessment will be needed to determine severity and in turn, which is the highest priority to treat first. Treatment with buprenorphine or methadone should be maintained at levels that minimize opioid withdrawal (79).
As previously noted, patients with severe psychiatric conditions may benefit from inpatient treatment to ensure safety and access to mental health care (38, 40). Depressive symptoms often co-occur during MUD and CUD, so treatments with efficacy for reducing depressive symptoms in MUD and CUD patients could be considered, such as desipramine (58–60), rTMS (42), or behavioral approaches such as exercise or mindfulness-based relapse prevention (61, 62). If a patient presents with psychotic symptoms, clinically distinguishing between primary psychosis and psychostimulant-induced psychosis is needed to inform treatment plans (80). For those with a primary psychosis diagnosis, treatment plans will include long-term neuroleptic medication, psychotherapy, family interventions, housing services, employment counseling, and possibly benzodiazepines to manage severe psychotic symptoms and agitation during acute withdrawal (80). Attaining abstinence or reducing psychostimulant use is recommended as the first line of treatment of both primary psychosis or psychostimulant-induced psychosis (80), as psychostimulant misuse can cause psychotic symptoms (81, 82). For psychostimulant-induced psychosis, psychotic symptoms resolve with abstinence by about 1 week, whereas primary psychosis will require continued care with antipsychotic medication (80).
In pregnant persons, psychostimulant use can lead to gestational hypertension, preeclampsia, and psychiatric issues, as well as pre-term birth, reduced birth weight, head circumference, and birth length, trouble feeding, insomnia, and muscle tone abnormalities in infants (83, 84). To improve maternal and infant health outcomes, non-pharmacological substance use treatment is typically recommended to address withdrawal and to promote abstinence; however, abrupt abstinence is not recommended (85). Psychiatric medication may be considered for pregnant persons with severe psychiatric symptoms, especially during the withdrawal period (85).
Chronic MA and cocaine use are risk factors for HIV infection (86) and barriers to adequate treatment in people with HIV (87–89). Those with HIV should receive viral load and CD4 quantification to evaluate for viral suppression and immune functioning. Providers should ensure that the patient has a current prescription and access to antiretroviral therapy, as a well as provide HIV medication adherence counseling since adherence can be a challenge for those using psychostimulants (90).
Post-detoxification prognosis
Severity of withdrawal symptoms declines significantly after the acute phase and in the following 2–3 weeks during the early protracted phased. The late protracted phase can extend from 1 to 6 months after initial abstinence. Risk of returning to use within 1 year after treatment discharge is high, with only 23–39% sustained abstinence in those treated for MUD (91, 92) and about 33% sustained abstinence in those treated for CUD (93). Factors associated with returning to use include injecting drugs (91), having a parent with alcohol or substance use disorder (92), low striatal dopamine (DA D2) receptor availability (20), and experience of stress (93).
Relapse prevention
Longer duration of initial treatment is recommended, as it corresponds to lower chance to returning to use (92). Additionally, research suggests that participation in psychosocial care including Twelve-Step programs (e.g., Alcoholics Anonymous, Narcotics Anonymous) or behavioral treatment (e.g., individual or group therapy) also significantly lowers risk of returning to use (91, 92). Social support quality and social network size are also protective against using in the year after treatment, so it is important to help patients establish a supportive network in their recovery following treatment completion (93).
New Developments
Two early pilots of mirtazapine for MA withdrawal showed some signal, though at doses that are twice that found efficacious to reduce MA use in treatment trials (30mg/d; (94, 95)). Intriguing evidence supports brief courses (5 sessions or 10 sessions over 12 days) of rTMS for reducing MA withdrawal symptoms; longer treatment with rTMS showed signal for improving related symptoms including craving, depression and anxiety symptoms and sleep disturbance. The past signal for amineptine, a tricyclic antidepressant with strong psychostimulant properties, to reduce withdrawal symptoms corresponds in some ways with recent evaluation of d-amphetamine to ameliorate discomfort of initial amphetamine abstinence. Additional work with amineptine is unlikely given the warnings about the drug’s safety and misuse liability profile. A key trial evaluating the value of using a psychostimulant in initial abstinence to treat acute phase withdrawal symptoms is in the field in Australia at this time (96).
Findings also underscore the strong correlation between measurement of withdrawal from psychostimulants and that of craving for psychostimulants upon initiation of abstinence in treatment studies. As noted, craving is not only a symptom, but a diagnostic criterion for addiction in DSM-5TR and a non-essential, but key feature to diagnose substance dependence using ICD-11 (97). Clinical trials of treatments to reduce MA and cocaine use have included craving measures, and the best evidence of potential medications that might reduce craving as part of withdrawal symptoms upon abstinence include naltrexone (oral or extended release), bupropion for MA and topiramate for cocaine. Desipramine shows some signal for reducing craving for cocaine during treatment, but there is consistent literature documenting a very weak (if any) signal for desipramine to reduce cocaine use.
Other symptoms that correspond with psychostimulant withdrawal, especially affective symptoms of depression and anxiety show response to approved medications and behavioral therapies used to treat these disorders. Desipramine reduces depression in those with chronic MA and cocaine use. Behavioral therapies including mindfulness (particularly in women and in those with existing affective disorders) and rTMS reduce self-reports of depression and anxiety symptoms during withdrawal. These treatments may have important links to decisions to remain in treatment to stop (or reduce) psychostimulant use, especially as acute and early protracted withdrawal periods progress.
Small pilot studies of rTMS, ibudilast and modafinil report signal for these treatments to improve select areas of cognitive function in the acute abstinence phase for MA use. For both ibudilast and modafinil, these early signals on improving cognitive function, however, have no signal for reducing MA use in larger randomized controlled trials (98, 99). The use of rTMS, however, does have reliable signal over the short term in reducing key symptoms of MA withdrawal; an ongoing trial will report on its efficacy as a treatment to reduce MA use. Acute withdrawal from psychostimulants affects sleep architecture and rTMS shows some signal to enhance sleep quality; high dose mirtazapine increases sleep hours, but also sleep disruptions.
Conclusion
Findings from this review present limited evidence supporting a use of small set of pharmacological, neurobiological and behavioral treatments to use in clinical settings for patients experiencing symptoms of psychostimulant withdrawal during acute and early protracted abstinence. The evidence supporting these treatments is mixed, with many studies being underpowered, and for treatments showing initial signals, findings often were not replicated in subsequent trials.
Drawing upon the evidence is limited by the varieties in trial design length, in underlying neurobiology for ATS and cocaine use disorders, and in measures used to define withdrawal. Yet the signals across studies indicate there may be one or a few medications or neurobiological treatments, particularly for acute and early protracted phases of MA withdrawal, that may be useful for reducing withdrawal symptoms. Use of one or more comprehensive, validated measure of psychostimulant withdrawal symptoms in clinical trials would increase precision and replicability in detecting a signal that of a treatment that could be used in clinics. As improvements in precision for measuring outcomes (and in monitoring medication adherence) continue in psychostimulant treatment trials, capturing withdrawal symptoms during corresponding phases—1) the acute experience when the body eliminates the drug and metabolites; 2) the early protracted period during neurobiological shifts toward new stasis; 3) and the late protracted period during longer-term brain and behavioral adjustments to long-term abstinence—will reduce error and increase potential for identifying consistent signal. After 30 years, efforts to use science to identify treatments with signal have yielded a few interventions that should be evaluated as agents to reduce psychostimulant withdrawal symptoms, as well as some behavioral and neuromodulation interventions that provide some functional benefits through withdrawal beyond comfort care.
Acknowledgements:
This work was supported by the National Institute on Drug Abuse grant (K01DA051329, PI: Li); Center for HIV Identification, Prevention and Treatment Services, National Institute of Mental Health grant (P30MH58107; PI: Shoptaw); NIDA Clinical Trials Network (UG1DA020024, PI: Trivedi).
Footnotes
Conflict of Interest:
We declare no conflicts of interests.
References
- 1.United Nations Office on Drugs and Crime. Drug Market Trends: Cocaine, Amphetamine-type Stimulants, New Psychoactive Substances. World Drug Report; [Internet]. 2022. Available from: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2022.html. [Google Scholar]
- 2.Farrell M, Martin NK, Stockings E, Bórquez A, Cepeda JA, Degenhardt L, et al. Responding to global stimulant use: challenges and opportunities. The Lancet. 2019;394(10209):1652–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Jones CM, Einstein EB, Volkow ND. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry. 2021;78(12):1329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B, Cotto J, Etz K, Einstein EB, Compton WM, Volkow ND. Methamphetamine Overdose Deaths in the US by Sex and Race and Ethnicity. JAMA Psychiatry. 2021;78(5):564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man N, Chrzanowska A, Price O, Bruno R, Dietze PM, Sisson SA, et al. Trends in cocaine use, markets and harms in Australia, 2003–2019. Drug and Alcohol Review. 2021;40(6):946–56. [DOI] [PubMed] [Google Scholar]
- 6.Mustaquim D, Jones CM, Compton WM. Trends and correlates of cocaine use among adults in the United States, 2006–2019. Addict Behav. 2021;120:106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano M, Oh S, Salas-Wright CP, Vaughn MG. Cocaine use and overdose mortality in the United States: Evidence from two national data sources, 2002–2018. Drug Alcohol Depend. 2020;214:108148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan ML, Shamasunder S, Colon-Berezin C, Kunins HV, Paone D. Increased Presence of Fentanyl in Cocaine-Involved Fatal Overdoses: Implications for Prevention. Journal of urban health : bulletin of the New York Academy of Medicine. 2019;96(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarone D, Shoptaw S. Understanding Stimulant Use and Use Disorders in a New Era. Med Clin North Am. 2022;106(1):81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díaz RM, Heckert AL, Sánchez J. Reasons for stimulant use among lation gay men in San Francisco: A comparison between methamphetamine and cocaine users. J Urban Health. 2005;82(1):i71–i8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halkitis PN, Fischgrund BN, Parsons JT. Explanations for methamphetamine use among gay and bisexual men in New York City. Subst Use Misuse. 2005;40(9–10):1331–45. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict Behav. 1998;23(2):201–7. [DOI] [PubMed] [Google Scholar]
- 14.Shoptaw S, Li MJ, Javanbakht M, Ragsdale A, Goodman-Meza D, Gorbach PM. Frequency of reported methamphetamine use linked to prevalence of clinical conditions, sexual risk behaviors, and social adversity in diverse men who have sex with men in Los Angeles. Drug Alcohol Depend. 2022;232:109320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision,,. 5th ed. Washington, DC: American Psychiatric Publishing, Inc.; 2022. [Google Scholar]
- 16.Zhao J, Kral AH, Simpson KA, Ceasar RC, Wenger LD, Kirkpatrick M, et al. Factors associated with methamphetamine withdrawal symptoms among people who inject drugs. Drug Alcohol Depend. 2021;223:108702. [DOI] [PubMed] [Google Scholar]
- 17.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark MR, Treisman GJ. Chronic pain and addiction: Karger Medical and Scientific Publishers; 2011. [Google Scholar]
- 19.Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Kosten T. Cocaine Withdrawal Symptoms Predict Medication Response in Cocaine Users. The American Journal of Drug and Alcohol Abuse. 2006;32(4):617–27. [DOI] [PubMed] [Google Scholar]
- 20.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17(9):918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone EJ, Tsadik A, Oyler J, Darwin WD. Cocaine Metabolism and Urinary Excretion After Different Routes of Administration. Ther Drug Monit. 1998;20(5). [DOI] [PubMed] [Google Scholar]
- 22.Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24(7):467–77. [DOI] [PubMed] [Google Scholar]
- 23.Musshoff F Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine. Drug metabolism reviews. 2000;32(1):15–44. [DOI] [PubMed] [Google Scholar]
- 24.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320–9. [DOI] [PubMed] [Google Scholar]
- 25.Mancino MJ, Gentry BW, Feldman Z, Mendelson J, Oliveto A. Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: a pilot field study. The American journal of drug and alcohol abuse. 2011;37(2):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B, Yun J, Park B. Methamphetamine-Induced Neuronal Damage: Neurotoxicity and Neuroinflammation. Biomolecules & therapeutics. 2020;28(5):381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ujike H, Okumura K, Zushi Y, Akiyama K, Otsuki S. Persistent supersensitivity of sigma receptors develops during repeated methamphetamine treatment. Eur J Pharmacol. 1992;211(3):323–8. [DOI] [PubMed] [Google Scholar]
- 28.Strickland JC, Gipson CD, Dunn KE. Dopamine Supersensitivity: A Novel Hypothesis of Opioid-Induced Neurobiological Mechanisms Underlying Opioid-Stimulant Co-use and Opioid Relapse. Frontiers in psychiatry. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: A prospective study of mood, sleep and withdrawal symptoms. Drug Alcohol Depend. 2000;59(3):277–86. [DOI] [PubMed] [Google Scholar]
- 31.Bruehl AM, Lende DH, Schwartz M, Sterk CE, Elifson K. Craving and Control: Methamphetamine Users’ Narratives. J Psychoactive Drugs. 2006;38(sup3):385–92. [DOI] [PubMed] [Google Scholar]
- 32.Merikle EP. The Subjective Experience of Craving: An Exploratory Analysis. Subst Use Misuse. 1999;34(8):1101–15. [DOI] [PubMed] [Google Scholar]
- 33.Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. Journal of studies on alcohol and drugs. 2010;71(3):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat. 2004;27(1):59–66. [DOI] [PubMed] [Google Scholar]
- 35.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and Functional Neuroimaging Evidence for Prefrontal Dysfunction in Methamphetamine-Dependent Subjects. Neuropsychopharmacology. 2002;26(1):53–63. [DOI] [PubMed] [Google Scholar]
- 36.Paulus MP, Stewart JL. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry. 2020;77(9):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kampman KM. The treatment of cocaine use disorder. Sci Adv. 2019;5(10):eaax1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008;35(3):334–42. [DOI] [PubMed] [Google Scholar]
- 39.Pennay AE, Lee NK. Barriers to methamphetamine withdrawal treatment in Australia: findings from a survey of AOD service providers. Drug Alcohol Rev. 2009;28(6):636–40. [DOI] [PubMed] [Google Scholar]
- 40.Lisa P, Felicia K, Laura H, Daniela K, Marlies R, Stefanie N, et al. Associations between methamphetamine use, psychiatric comorbidities and treatment outcome in two inpatient rehabilitation centers. Psychiatry Res. 2019;280:112505. [DOI] [PubMed] [Google Scholar]
- 41.Kongsakon R, Papadopoulos KI, Saguansiritham R. Mirtazapine in amphetamine detoxification: a placebo-controlled pilot study. Int Clin Psychopharmacol. 2005;20(5). [DOI] [PubMed] [Google Scholar]
- 42.Liang Y, Wang L, Yuan TF. Targeting Withdrawal Symptoms in Men Addicted to Methamphetamine With Transcranial Magnetic Stimulation: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(11):1199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, et al. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug and Alcohol Review. 2008;27(3):326–33. [DOI] [PubMed] [Google Scholar]
- 44.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine Withdrawal: II. A Placebo-Controlled, Randomised, Double-Blind Study of Amineptine Treatment. Aust N Z J Psychiatry. 1999;33(1):94–8. [DOI] [PubMed] [Google Scholar]
- 45.Drug Enforcement Administration U.S. Department of Justice. 21 CFR Part 1308 - Schedules of Controlled Substances: Placement of Amineptine in Schedule I. Federal Register Online via the Government Publishing Office. 2021;86(138):38619–24. [Google Scholar]
- 46.Preston KL, Vahabzadeh M, Schmittner J, Lin J-L, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berl). 2009;207(2):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galloway GP, Singleton EG. How Long Does Craving Predict Use of Methamphetamine? Assessment of Use One to Seven Weeks after the Assessment of Craving. Substance Abuse: Research and Treatment. 2008;1:SART.S775. [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson RG Jr, Oliveto A, Thostenson JD, Wilson MP, McGaugh J, Mancino MJ. Utility of a controlled amphetamine withdrawal paradigm among adults who use methamphetamine: A pilot clinical trial. Journal of Psychopharmacology. 2021;35(11):1420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi MH, Walker R, Ling W, Dela Cruz A, Sharma G, Carmody T, et al. Bupropion and Naltrexone in Methamphetamine Use Disorder. N Engl J Med. 2021;384(2):140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the Treatment of Amphetamine Dependence: A Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2008;165(11):1442–8. [DOI] [PubMed] [Google Scholar]
- 51.Gawin FH, Kleber HD, Byck R, Rounsaville BJ, Kosten TR, Jatlow PI, et al. Desipramine Facilitation of Initial Cocaine Abstinence. Arch Gen Psychiatry. 1989;46(2):117–21. [DOI] [PubMed] [Google Scholar]
- 52.Johnson BA, Ait-Daoud N, Wang XQ, Penberthy JK, Javors MA, Seneviratne C, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338–46. [DOI] [PubMed] [Google Scholar]
- 53.Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nuijten M, Blanken P, van den Brink W, Hendriks V. Treatment of crack-cocaine dependence with topiramate: a randomized controlled feasibility trial in The Netherlands. Drug Alcohol Depend. 2014;138:177–84. [DOI] [PubMed] [Google Scholar]
- 55.Umbricht A, DeFulio A, Winstanley EL, Tompkins DA, Peirce J, Mintzer MZ, et al. Topiramate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug Alcohol Depend. 2014;140:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A Double-Blind, Placebo-Controlled Trial of Modafinil for Cocaine Dependence. Neuropsychopharmacology. 2005;30(1):205–11. [DOI] [PubMed] [Google Scholar]
- 57.Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell J, Nickel EJ, Penick EC, Wallace D, Gabrielli WF, Rowe C, et al. Comparison of Desipramine or Carbamazepine to Placebo for Crack Cocaine-Dependent Patients. Am J Addict. 2003;12(2):122–36. [PubMed] [Google Scholar]
- 59.McDowell D, Nunes EV, Seracini AM, Rothenberg J, Vosburg SK, Ma GJ, et al. Desipramine treatment of cocaine-dependent patients with depression: A placebo-controlled trial. Drug Alcohol Depend. 2005;80(2):209–21. [DOI] [PubMed] [Google Scholar]
- 60.Arndt IO, Dorozynsky L, Woody GE, McLellan AT, O’Brien CP. Desipramine Treatment of Cocaine Dependence in Methadone-Maintained Patients. Arch Gen Psychiatry. 1992;49(11):888–93. [DOI] [PubMed] [Google Scholar]
- 61.Rawson RA, Chudzynski J, Gonzales R, Mooney L, Dickerson D, Ang A, et al. The Impact of Exercise On Depression and Anxiety Symptoms Among Abstinent Methamphetamine-Dependent Individuals in A Residential Treatment Setting. J Subst Abuse Treat. 2015;57:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glasner-Edwards S, Mooney LJ, Ang A, Garneau HC, Hartwell E, Brecht M-L, et al. Mindfulness-Based Relapse Prevention for Stimulant Dependent Adults: A Pilot Randomized Clinical Trial. Mindfulness. 2017;8(1):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brooks SJ, Burch KH, Maiorana SA, Cocolas E, Schioth HB, Nilsson EK, et al. Psychological intervention with working memory training increases basal ganglia volume: A VBM study of inpatient treatment for methamphetamine use. NeuroImage: Clinical. 2016;12:478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su H, Zhong N, Gan H, Wang J, Han H, Chen T, et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend. 2017;175:84–91. [DOI] [PubMed] [Google Scholar]
- 65.Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489–97. [DOI] [PubMed] [Google Scholar]
- 66.Birath JB, Briones M, Amaya S, Shoptaw S, Swanson A-N, Tsuang J, et al. Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend. 2017;178:386–90. [DOI] [PubMed] [Google Scholar]
- 67.National Institute on Drug Abuse. Transcranial Magnetic Stimulation for the Treatment of Methamphetamine/Cocaine Use Disorder (CTN-108). 2021. Available from: https://nida.nih.gov/about-nida/organization/cctn/ctn/research-studies/transcranial-magnetic-stimulation-treatment-methamphetaminecocaine-use-disorder.
- 68.Miles SW, Sheridan J, Russell B, Kydd R, Wheeler A, Walters C, et al. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7):1279–86. [DOI] [PubMed] [Google Scholar]
- 69.Black DS, Amaro H. Moment-by-Moment in Women’s Recovery (MMWR): Mindfulness-based intervention effects on residential substance use disorder treatment retention in a randomized controlled trial. Behav Res Ther. 2019;120:103437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug and alcohol review. 2008;27(3):253–62. [DOI] [PubMed] [Google Scholar]
- 71.Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48(1):43–51. [DOI] [PubMed] [Google Scholar]
- 72.Kaye S, McKetin R, Duflou J, Darke S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction. 2007;102(8):1204–11. [DOI] [PubMed] [Google Scholar]
- 73.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130(6):1657–63. [DOI] [PubMed] [Google Scholar]
- 74.Brady KT, Lydiard RB, Malcolm R, Ballenger JC. Cocaine-induced psychosis. The Journal of clinical psychiatry. 1991. [PubMed] [Google Scholar]
- 75.Grant KM, LeVan TD, Wells SM, Li M, Stoltenberg SF, Gendelman HE, et al. Methamphetamine-associated psychosis. J Neuroimmune Pharmacol. 2012;7(1):113–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Twillman RK, Dawson E, LaRue L, Guevara MG, Whitley P, Huskey A. Evaluation of Trends of Near-Real-Time Urine Drug Test Results for Methamphetamine, Cocaine, Heroin, and Fentanyl. JAMA Network Open. 2020;3(1):e1918514–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giang LM, Li MJ, Okafor CN, Diep NB, Shoptaw SJ. Correlates of methamphetamine use severity among patients receiving methadone maintenance treatment for opioid use disorder in Vietnam. J Subst Abuse Treat. 2022;132:108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones CM, Underwood N, Compton WM. Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction. 2020;115(2):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs Methadone Maintenance Treatment for Concurrent Opioid Dependence and Cocaine Abuse. Arch Gen Psychiatry. 1997;54(8):713–20. [DOI] [PubMed] [Google Scholar]
- 80.Glasner-Edwards S, Mooney LJ. Methamphetamine Psychosis: Epidemiology and Management. CNS Drugs. 2014;28(12):1115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roncero C, Daigre C, Gonzalvo B, Valero S, Castells X, Grau-López L, et al. Risk factors for cocaine-induced psychosis in cocaine-dependent patients. Eur Psychiatry. 2013;28(3):141–6. [DOI] [PubMed] [Google Scholar]
- 82.McKetin R, Lubman DI, Baker AL, Dawe S, Ali RL. Dose-Related Psychotic Symptoms in Chronic Methamphetamine Users: Evidence From a Prospective Longitudinal Study. JAMA Psychiatry. 2013;70(3):319–24. [DOI] [PubMed] [Google Scholar]
- 83.Gorman MC, Orme KS, Nguyen NT, Kent EJ, Caughey AB. Outcomes in pregnancies complicated by methamphetamine use. Am J Obstet Gynecol. 2014;211(4):429.e1–e7. [DOI] [PubMed] [Google Scholar]
- 84.Perez FA, Blythe S, Wouldes T, McNamara K, Black KI, Oei JL. Prenatal methamphetamine—impact on the mother and child—a review. Addiction. 2022;117(1):250–60. [DOI] [PubMed] [Google Scholar]
- 85.World Health Organization. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. 2014. [PubMed] [Google Scholar]
- 86.Grov C, Westmoreland D, Morrison C, Carrico AW, Nash D. The crisis we are not talking about: one-in-three annual HIV seroconversions among sexual and gender minorities were persistent methamphetamine users. Journal of acquired immune deficiency syndromes (1999). 2020;85(3):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dash S, Balasubramaniam M, Villalta F, Dash C, Pandhare J. Impact of cocaine abuse on HIV pathogenesis. Front Microbiol. 2015;6:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Passaro RC, Pandhare J, Qian H-Z, Dash C. The complex interaction between methamphetamine abuse and HIV-1 pathogenesis. J Neuroimmune Pharmacol. 2015;10(3):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li MJ, Su E, Garland WH, Oksuzyan S, Lee S-J, Kao UH, et al. Trajectories of Viral Suppression in People Living With HIV Receiving Coordinated Care: Differences by Comorbidities. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2020;84(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKetin R, Kothe A, Baker AL, Lee NK, Ross J, Lubman DI. Predicting abstinence from methamphetamine use after residential rehabilitation: Findings from the Methamphetamine Treatment Evaluation Study. Drug and Alcohol Review. 2018;37(1):70–8. [DOI] [PubMed] [Google Scholar]
- 92.Brecht M-L, Herbeck D Time to relapse following treatment for methamphetamine use: A long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McMahon RC. Personality, stress, and social support in cocaine relapse prediction. J Subst Abuse Treat. 2001;21(2):77–87. [DOI] [PubMed] [Google Scholar]
- 94.Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, et al. Mirtazapine to Reduce Methamphetamine Use: A Randomized Controlled Trial. Arch Gen Psychiatry. 2011;68(11):1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coffin PO, Santos GM, Hern J, Vittinghoff E, Walker JE, Matheson T, et al. Effects of Mirtazapine for Methamphetamine Use Disorder Among Cisgender Men and Transgender Women Who Have Sex With Men: A Placebo-Controlled Randomized Clinical Trial. JAMA Psychiatry. 2020;77(3):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ezard N, Clifford B, Dunlop A, Bruno R, Carr A, Liu Z, et al. Safety and tolerability of oral lisdexamfetamine in adults with methamphetamine dependence: a phase-2 dose-escalation study. BMJ Open. 2021;11(5):e044696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.World Health Organization. International Classification of Diseases, Eleventh Revision (ICD-11). Geneva: World Health Organization; 2022. Available from: https://icd.who.int [Google Scholar]
- 98.Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109(1–3):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heinzerling KG, Briones M, Thames AD, Hinkin CH, Zhu T, Wu YN, et al. Randomized, Placebo-Controlled Trial of Targeting Neuroinflammation with Ibudilast to Treat Methamphetamine Use Disorder. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2020;15(2):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


