Background:
Cardiac troponin (cTn) T and cTnI are considered cardiac specific and equivalent in the diagnosis of acute myocardial infarction. Previous studies suggested rare skeletal myopathies as a noncardiac source of cTnT. We aimed to confirm the reliability/cardiac specificity of cTnT in patients with various skeletal muscle disorders (SMDs).
Methods:
We prospectively enrolled patients presenting with muscular complaints (≥2 weeks) for elective evaluation in 4 hospitals in 2 countries. After a cardiac workup, patients were adjudicated into 3 predefined cardiac disease categories. Concentrations of cTnT/I and resulting cTnT/I mismatches were assessed with high-sensitivity (hs-) cTnT (hs-cTnT–Elecsys) and 3 hs-cTnI assays (hs-cTnI–Architect, hs-cTnI–Access, hs-cTnI–Vista) and compared with those of control subjects without SMD presenting with adjudicated noncardiac chest pain to the emergency department (n=3508; mean age, 55 years; 37% female). In patients with available skeletal muscle biopsies, TNNT/I1-3 mRNA differential gene expression was compared with biopsies obtained in control subjects without SMD.
Results:
Among 211 patients (mean age, 57 years; 42% female), 108 (51%) were adjudicated to having no cardiac disease, 44 (21%) to having mild disease, and 59 (28%) to having severe cardiac disease. hs-cTnT/I concentrations significantly increased from patients with no to those with mild and severe cardiac disease for all assays (all P<0.001). hs-cTnT–Elecsys concentrations were significantly higher in patients with SMD versus control subjects (median, 16 ng/L [interquartile range (IQR), 7–32.5 ng/L] versus 5 ng/L [IQR, 3–9 ng/L]; P<0.001), whereas hs-cTnI concentrations were mostly similar (hs-cTnI–Architect, 2.5 ng/L [IQR, 1.2–6.2 ng/L] versus 2.9 ng/L [IQR, 1.8–5.0 ng/L]; hs-cTnI–Access, 3.3 ng/L [IQR, 2.4–6.1 ng/L] versus 2.7 ng/L [IQR, 1.6–5.0 ng/L]; and hs-cTnI–Vista, 7.4 ng/L [IQR, 5.2–13.4 ng/L] versus 7.5 ng/L [IQR, 6–10 ng/L]). hs-cTnT–Elecsys concentrations were above the upper limit of normal in 55% of patients with SMD versus 13% of control subjects (P<0.01). mRNA analyses in skeletal muscle biopsies (n=33), mostly (n=24) from individuals with noninflammatory myopathy and myositis, showed 8-fold upregulation of TNNT2, encoding cTnT (but none for TNNI3, encoding cTnI) versus control subjects (n=16, PWald<0.001); the expression correlated with pathological disease activity (R=0.59, Pt-statistic<0.001) and circulating hs-cTnT concentrations (R=0.26, Pt-statistic=0.031).
Conclusions:
In patients with active chronic SMD, elevations in cTnT concentrations are common and not attributable to cardiac disease in the majority. This was not observed for cTnI and may be explained in part by re-expression of cTnT in skeletal muscle.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03660969.
Keywords: muscle, skeletal; myocardial infarction; myopathies, structural, congenital; troponin
Clinical Perspective.
What Is New?
High-sensitivity cardiac troponin (hs-cTn) T–Elecsys concentrations were above the upper limit of normal in >50% of patients with active chronic skeletal muscle disorder and significantly higher versus controls.
hs-cTnI concentrations measured 3 different ways were above the biological-equivalent upper limit of normal in <25% of patients and comparable to those of control subjects, thereby leading to hs-cTnT/hs-cTnI mismatches in up to 50% of patients.
hs-cTnT–Elecsys elevations in patients without cardiac disease were restricted largely to patients with active noninflammatory myopathy and myositis.
mRNA analyses in skeletal muscle biopsies showed 8-fold upregulation of TNNT2, encoding cTnT versus control subjects, and the expression correlated with circulating hs-cTnT concentrations.
What Are the Clinical Implications?
In patients presenting with suspected acute myocardial infarction, the presence of active chronic noninflammatory myopathy and myositis should be considered as a major confounder of hs-cTnT concentration but not hs-cTnI concentrations.
These patients are at risk of erroneous acute myocardial infarction diagnosis with hs-cTn when hs-cTnI is the preferred analyte.
In patients with other skeletal muscle disorder, hs-cTnT seemed to retain cardiac specificity.
Editorial, see p 1780
The troponin complex is composed of 3 isoforms (T, I and C) and is essential for contraction of striated muscle.1,2 Although the function of troponins is very similar, their amino acid sequences and configuration in cardiac and skeletal muscle differ.1,2 Cardiac troponins (cTns) are rapidly released when, for example, cardiomyocytes experience ischemic damage and have become a central component in the early diagnosis of acute myocardial infarction (AMI).3 The development and clinical implementation of high-sensitivity cTn (hs-cTn) assays have enabled precise discrimination of mild cTn elevations from normal cTn concentrations.4 In addition, hs-cTn–based rapid algorithms have substantially accelerated and facilitated the early diagnosis of AMI.5,6
Specificity issues with early iterations of the cTnT assays appeared and were believed to be attributable to a cross-reactivity between the cTnT assay and skeletal muscle epitopes.7,8 However, falsely elevated concentrations of cTnT were considered a problem solved when the latest version of the cTnT assay was used.9–15 The specificity deficit of earlier versions of the assay has been highlighted, for instance, by Ricchiuti et al,7 who found evidence of cTnT in the skeletal muscle of patients with chronic renal disease. Given the epitopes recognized by the antibodies of the commercial cTnT assay used by Ricchiuti et al in 1998 and the variable presence of these cTnT isoforms in skeletal muscle, these same authors postulated that the modified, second-generation cTnT assay would not detect these isoforms if they are released from skeletal muscle into the circulation.7
Therefore, current clinical practice guidelines consider cTnT and cTnI cardiac specific, equivalent, and interchangeable in the diagnosis of AMI.6 This concept has been challenged again by recent studies using the latest generation of the hs-cTnT assay showing evidence of re-expressed cTnT in diseased skeletal muscle of patients with neuromuscular disorders, commonly resulting in cTnT elevations but only rarely in cTnI elevations in the systemic circulation.11,12
Although previously documented, the clinical implications of these translational findings for patients presenting with skeletal muscle disorders (SMDs) and the overall population presenting with suspected AMI are incompletely understood.16 There are, for example, uncertainties related to the fact that of all studies, only 3 used the hs-cTnT assay; previous studies often had a small sample size and investigated mostly selected patients with rare neuromuscular disorders, some with cardiac involvement that possibly contributed to systemic cTnT concentrations. Furthermore, some studies had selection bias, enrolling exclusively patients with cTnT elevations instead of consecutive unselected patients, and used only 1 cTnI assay as a comparator.9,11,12,17
Therefore, we performed a prospective international multicenter study to address these uncertainties using 4 hs-cTnT/I assays in a broad population of patients presenting with muscular complaints.
Methods
The data, code, and study material that support the findings of this study are available from the corresponding author on reasonable request.
Study Design, Setting, and Patient Population
This is the primary analysis of the Heart & Muscle BASEL XII Study (Reliability of Cardiac Troponins for the Diagnosis of Myocardial Infarction in the Presence of Skeletal Muscle Disease; NCT03660969), an ongoing prospective international multicenter diagnostic study enrolling patients presenting with active chronic muscular complaints for elective ambulatory or inpatient evaluation in a neuromuscular, rheumatology, or medical service in 4 hospitals in 2 countries (Basel, Aarau, and Zürich in Switzerland and Innsbruck in Austria). Active chronic muscular complaints were defined as any symptom related to muscle disease lasting for at least 2 weeks. The study was designed to contribute to a better understanding of the reliability of cTnT and cTnI for the diagnosis of AMI in the presence of SMD. Adult patients presenting with a broad spectrum of muscular complaints, for example, muscle pain, weakness (defined as scoring ≤4 on the Medical Research Council scale for muscle strength18), atrophy, stiffness, and fasciculations, were recruited. Patients were excluded if they had acute trauma, acute medical disease such as sepsis, AMI, stroke, other acute cardiac diseases, or terminal kidney failure requiring dialysis. The study was conducted according to the principles of the Declaration of Helsinki and approved by the local ethic committees. Written informed consent was obtained from all patients. The authors designed the study, gathered and analyzed the data according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies (Table S1),19 vouched for the data and analysis, wrote the paper, and made the decision to submit the manuscript for publication. Data were entered in a dedicated RedCap database.20
Clinical Assessment
Workup for SMD was performed according to local standard operating procedures. The diagnosis of SMD was established by the treating clinicians (neurologists, rheumatologists, and internists) at the recruiting centers. All patients went through a thorough muscular and neurological diagnostic process, including laboratory testing (such as antibody screens), chest imaging, electro(neuro)myography, muscle magnetic resonance imaging, genetic testing, and muscle biopsies analyses, as clinically indicated. After workup was completed, final diagnoses were adjudicated in conjunction with a neurologist into 6 groups: noninflammatory myopathies, neuropathies, myasthenic syndromes, myositis (primary, secondary, and overlap syndromes), autoimmune diseases with muscular symptoms, and muscle symptoms of unknown origin (Table S2). Noninflammatory myopathies included myotonic dystrophy, facioscapulohumeral muscular dystrophy, limb-girdle muscular dystrophy, mitochondrial disease, and glycogen storage disease. Myositis included dermatomyositis, polymyositis, sporadic inclusion body myositis, hereditary inclusion body myositis, immune-mediated necrotizing myositis, myositis with overlap with collagenous disease, statin-induced myositis, and vasculitis.
Standardized cardiac assessment included a structured questionnaire for history of cardiovascular disease and cardiovascular risk factors, physical examination, 12-lead ECG, measurement of NT-proBNP (N-terminal pro-B-type natriuretic peptide) as a quantitative marker of hemodynamic cardiac stress,21 cTnT, cTnI, and cardiac imaging, including echocardiography and cardiac magnetic resonance imaging whenever indicated by clinical uncertainty about the underlying cardiac disease, including cTnT/I elevations.
Classification According to Cardiac Disease
With the use of predefined criteria, patients were centrally adjudicated into 3 categories according to the presence and extent of cardiac disease (no cardiac disease, mild cardiac disease, severe cardiac disease) by investigators blinded to cTnT/I results. According to current guidelines,6 cTnT and cTnI are quantitative markers of ongoing cardiomyocyte damage; elevations in cTnT or cTnI as a reflection of true cardiac disease may be present in a relevant proportion of patients with severe cardiac disease, are unlikely in patients with mild cardiac disease, and very unlikely in patients with no cardiac disease.
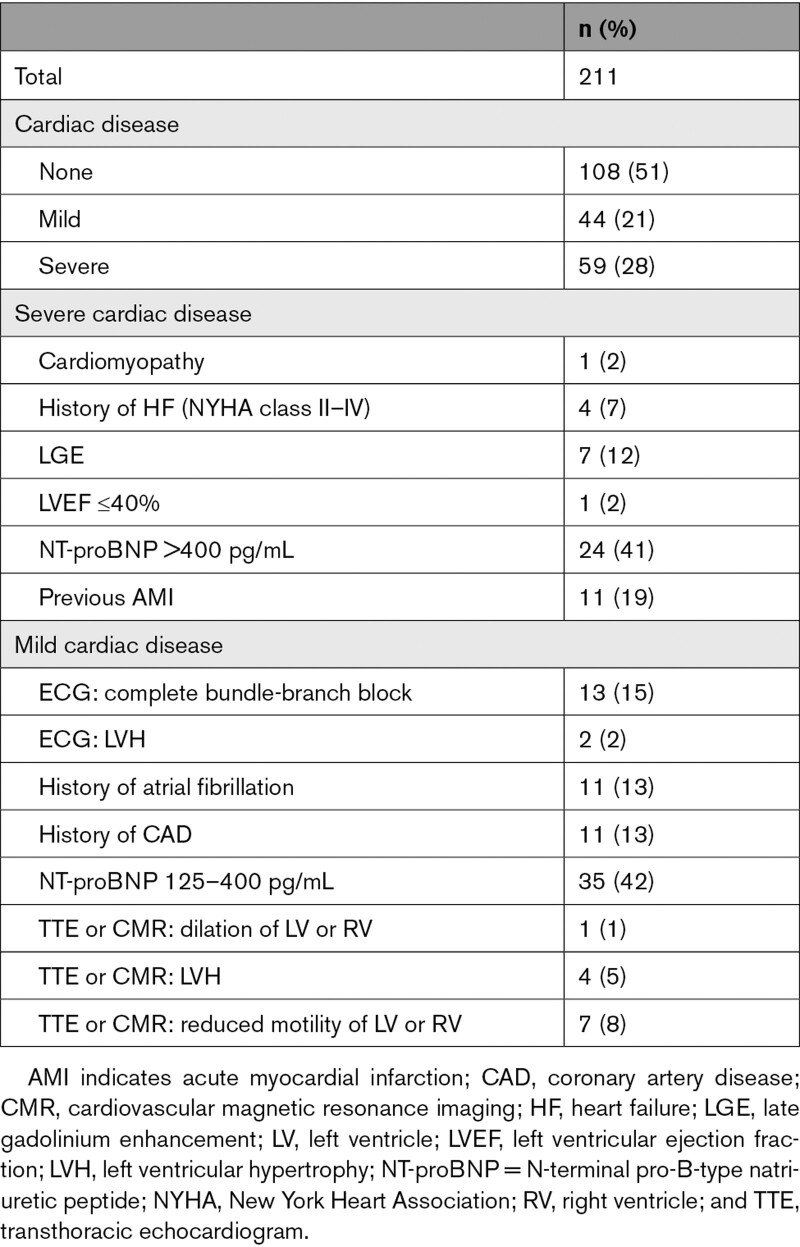
Patients with previous AMI, chronic heart failure (New York Heart Association class II or greater), severe valvular disease, left ventricular ejection fraction <40%, cardiac magnetic resonance imaging showing late gadolinium enhancement, or NT-proBNP concentrations >400 ng/L21 were classified as having severe cardiac disease. In the absence of these criteria, patients with coronary artery disease, atrial fibrillation/flutter, left or right bundle-branch block on ECG, left ventricular hypertrophy, mild to moderate valvular disease or any other structural or functional abnormality (abnormal motility, dilation) detected in echocardiography or cardiac magnetic resonance imaging, or NT-proBNP concentrations of 125 to 400 ng/L21 were classified as having mild cardiac disease. All other patients were classified as having no cardiac disease.
End Points
The primary clinical end points were systemic hs-cTnT/I concentrations, the prevalence of cTnT/I elevations in the overall cohort and in patients with no cardiac disease, and the resulting cTnT/I mismatches. Secondary end points were the patient-specific correlation of cTnT/I and creatine kinase (CK) as a quantitative marker of skeletal muscle injury. The translational end points for mRNA analyses were the gene expression in the 6 cTnT/I genes in cases versus controls and the correlation of the 3 cTnT genes with circulating hs-cTnT concentrations among cases.
Control Cohorts
hs-cTnT/I concentrations and the prevalence of hs-cTnT/I mismatches were compared with a control cohort of patients presenting to the emergency department with acute chest pain and adjudicated noncardiac cause (n=3508; mean age, 55 years; 37% women; Table S3) within an international multicenter study (APACE [Advantageous Predictors of Acute Coronary Syndromes Evaluation], ClinicalTrials.gov number NCT00470587; Methods in the Supplemental Material).
mRNA analyses were compared with a control cohort of consecutive patients free from known SMD undergoing hip replacement surgery at the University Hospital of Basel, who were asked for consent to perform an intraoperative skeletal muscle biopsy (n=16; mean age, 68 years; 44% women). No other exclusion criteria were applied. Thus, patients had cardiac and noncardiac comorbidities. Skeletal muscle tissue samples were processed by the Pathology Department of the University of Basel similarly to the skeletal muscle tissue samples obtained from patients with SMD to allow mRNA extraction and analysis.
No matching was performed between the patients with SMD and patients from the control cohorts.
Laboratory Measurements
One set of venous blood samples were drawn at enrollment with a peripheral intravenous line, and heparin plasma was then immediately processed for the measurement using the most widely applied hs-cTnT assay (Roche hs-cTnT–Elecsys) and Abbott-hs-cTnI–Architect assays or frozen at −80° C until assayed for measurement with Siemens-hs-cTnI–Dimension Vista or Beckman-hs-cTnI–Access assays. In addition, plasma CK, CK-MB isoenzyme as measured by immunoassay and plasma creatinine (Cobas automated analyzer, Roche Diagnostics), and NT-proBNP (Elecsys proBNP assay, Roche Diagnostics22) were measured. The hs-cTnT–Elecsys assay (Elecsys 2010 hs-cTnT, Roche Diagnostics) has a 99th percentile concentration (upper limit of normal [ULN]) of 14 ng/L with a coefficient of variation of 10% at 13 ng/L. Limit of blank and limit of detection have been determined to be 3 and 5 ng/L. Sex-specific ULNs were determined at 15.5 ng/L for men and 9 ng/L for women.23 The hs-cTnI–Architect assay (ARCHITECT STAT high-sensitivity troponin I, Abbott Laboratories) has a 99th percentile concentration of 26 ng/L with a coefficient of variation of <5%, a limit of blank of 1.3 ng/L, and a limit of detection of 1.9 ng/L. Sex-specific ULNs were defined at 34.2 ng/L for men and 15.6 ng/L for women.24,25
The hs-cTnI–Access assay (ACCESS hs-cTnI, Beckman Coulter) has an overall 99th percentile concentration of 18.2 ng/L with a coefficient of variation of <10%. Limit of blank and limit of detection have been determined to be 1.7 and 2.3 ng/L, respectively. Sex-specific ULNs were defined at 20.9 ng/L for men and 9.6 ng/L for women.26 The hs-cTnI–Dimension Vista has an overall 99th percentile concentration of 58.9 ng/L with a coefficient of variation of <5%, a limit of blank of 1 ng/L, and a limit of detection of 2 ng/L. Sex-specific ULNs were defined at 68 ng/L for men and 44 ng/L for women.27 The uniform and sex-specific ULNs used in the current analysis are consistent with the ULNs provided by the International Federation on Clinical Chemistry Committee on Clinical Applications of Cardiac Biomarkers.28
The different hs-cTnI assays examined use different antibody combinations and are not standardized; thus, the ratios of T/I will differ between assays.29 Therefore, we expected varying rates of hs-cTnT/I mismatches, depending on the hs-cTnI assay used.
mRNA Analysis
Muscle tissue samples were obtained during patient workup whenever clinically indicated. RNA extraction was performed by CEGAT (Tübingen, Germany) using the RNeasy Mini Kit (Qiagen, Netherlands) or RNeasy Fibrous Tissue Mini kit (Qiagen, Netherlands) after sample randomization. Libraries were prepared and RNA was sequenced as detailed in the Supplemental Material. After mapping, preprocessing, and quality control, differential gene expression (DGE) analysis was conducted on all sequenced samples using the summarized gene counts with DESeq2 version 1.28.0.30 The DGE analysis of cases was compared with that of controls. In total, 6 troponin genes were tested for DGE (TNNT2, TNNT1, TNNT3, TNNI3, TNNI1, and TNNI2). It is important to note that the entire process of RNA extraction and analysis was conducted by investigators blinded to the blood concentrations of hs-cTnT/I for all cases and controls. To correlate TNNT2 expression with disease activity, a score was derived from 18 marker variables from visual biopsy analysis by specialized pathologists at the respective centers (Methods and Figures S1 and S2 in the Supplemental Material).
Determination of Sample Size
From previous literature,11,12 we estimated the proportion of patients who would present an elevated hs-cTnT in the overall cohort to be 67% and the proportion of patients presenting an elevated hs-cTnI to be 10%. From the initial preliminary data, we conservatively predicted the proportion of patients without cardiac disease to be 25% in our cohort. We estimated that 10% of the patients would lack at least 1 of the 3 hs-cTnI measurements. For a selected power of 90% and a 2-sided type I error of 0.05, which was then adjusted for multiple testing (3 comparisons with each hs-cTnI assay), a minimal sample size of 176 patients was calculated to detect a difference in proportion of hs-cTn between the 2 assays using a McNemar test and to allow sufficient power for the population with no cardiac disease. Further details of the derivation of the sample size are given in the Supplemental Material.
Statistical Analysis
Because recent studies have reported that the approved uniform 99th percentiles of ULN are not biologically equivalent,31 biologically equivalent ULN derived from a large prospective diagnostic study with parallel measurement of the respective hs-cTnT/I assays was used for the primary analysis (Methods and Figures S3 and S4 in the Supplemental Material) for the hs-cTnI–Architect and hs-cTnI–Access assays. In brief, the biologically equivalent ULNs for hs-cTnI–Architect and hs-cTnI–Access to the ULN of 14 ng/L for hs-cTnT were derived in APACE32 and found to be 6.6 and 6.9 ng/L, respectively.31,33 For the hs-cTnI–Vista assay, the biologically equivalent ULN of 42 ng/L to the ULN of 14 ng/L for hs-cTnT was derived in a recent study of healthy individuals.27 Secondary analyses used the manufacturer-recommended and regulatory authority–approved 99th percentile ULNs described above and sex-specific cutoffs.25,26,31,34,35 To further assess the clinical implications for the early diagnosis of AMI in patients presenting with acute chest pain, the proportion of patients with hs-cTnT elevations above the rule-in cutoff of the European Society of Cardiology (ESC) 0/1h algorithm (52 ng/L) was calculated.5,6,33
Continuous variables are presented as mean±SD when normally distributed and as median with interquartile ranges (IQRs) when nonnormally distributed. Categorical variables are expressed as numbers and percentages. Independent t tests, Mann-Whitney U tests, or Kruskal-Wallis tests were applied for comparison of continuous variables, and the Fisher exact test was used for comparison of categorical variables. Comparisons of proportions were made with a 2-sample test for equality of proportions with continuity correction.
A subgroup analysis was performed to evaluate whether the prevalence of cTnT/I elevations differed among the different underlying types of muscular complaints. When hs-cTnT/I concentrations were used in statistical modeling or analyses, concentrations were transformed on the logarithmic scale to approximate normal distribution.
Correlations were assessed with the Kendall rank correlation coefficient, the coefficient of determination (R2), and P values provided by linear regressions. The Benjamini and Hochberg method was used to correct for multiple testing when appropriate.36 Statistical analyses were performed with the R statistical package (R Core Team, Vienna, Austria).
For mRNA analyses, resulting P values attained by the Wald test were also corrected for multiple testing with the Benjamini and Hochberg method. An adjusted value of P<0.05 was considered significant. A subgroup analysis on the major gene of interest, TNNT2 (cardiac type), was conducted by correlating an SMD activity score with TNNT2 gene expression while correcting for SMD pathogenesis (myopathy, myositis, or other SMD).
Results
Patient Characteristics and Assessment of Cardiac Health
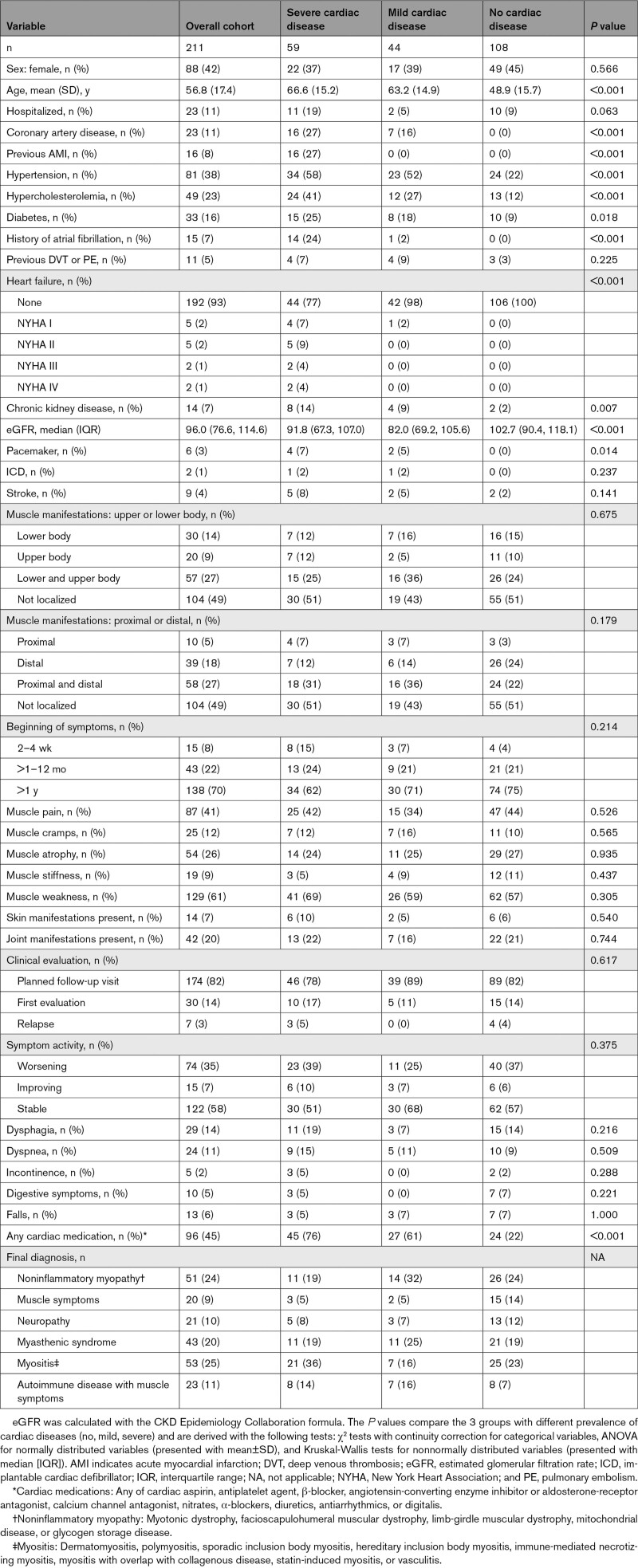
From August 2018 to October 2020, 223 patients were enrolled, and 211 patients were eligible for this analysis (Figure S5). The mean age was 57 years; 88 (42%) were women; 23 (11%) had known coronary artery disease; 16 (8%) had previous AMI; 15 (7%) had a history of atrial fibrillation; 81 (38%) had arterial hypertension; and 33 (16%) had diabetes (Table 1). Patients were recruited mainly during ambulatory evaluation of their muscle disorder (188, 89%). Most patients presented with muscle weakness (n=129, 61%) or muscle pain (n=87, 41%). Functional limitations such as dysphagia, dyspnea, incontinence, digestive symptoms, or falls were present in 2% to 14% of the patients. Echocardiography was performed in 56% of the patients; cardiac magnetic resonance imaging was performed in 22% (Table S4).
Table 1.
Baseline Characteristics
Noninflammatory myopathy, myositis, and myasthenic syndrome were the most common final diagnoses after workup (Table S2). Cardiac characterization classified 59 patients (28%) as having severe cardiac disease, 44 (21%) as having mild cardiac disease, and 108 (51%) as having no cardiac disease (Table 2).
Table 2.
Classification of the Cohort in Groups of Cardiac Disease
hs-cTnT/I Concentration and hs-cTnT/I Mismatches
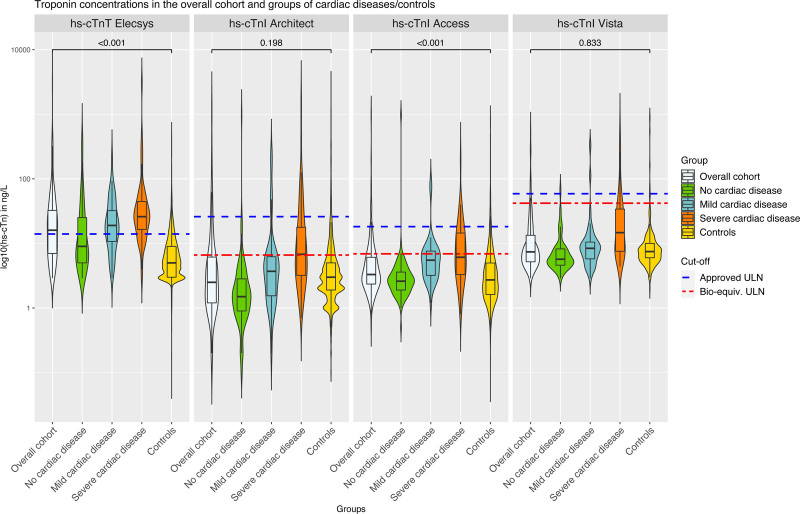
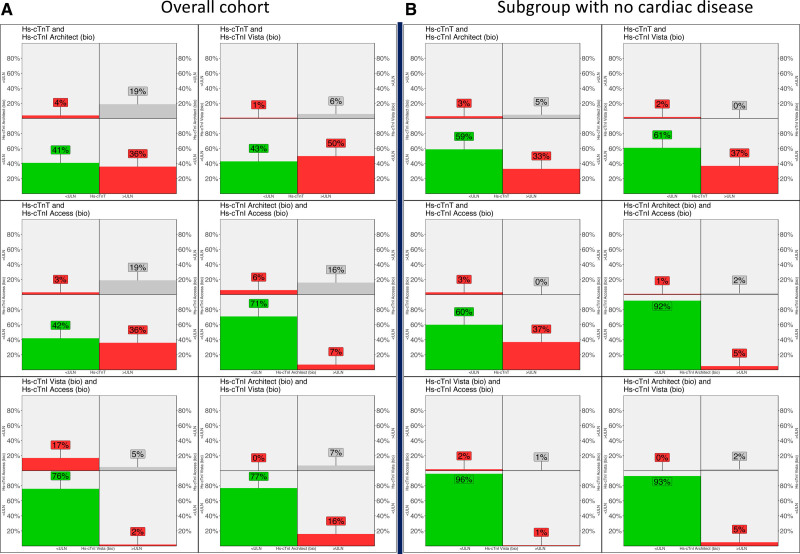
hs-cTnT/I concentrations increased significantly from patients with no cardiac disease to patients with mild cardiac disease to patients with severe cardiac disease for all assays (all PMann-Whitney<0.001). In the overall group, hs-cTnT–Elecsys concentrations were above the uniform approved ULN in 55% and significantly higher versus those of control subjects (median, 16 ng/L [IQR, 7–32.5 ng/L] versus 5 ng/L [IQR, 3–9 ng/L]; PMann-Whitney<0.001; Figure 1 and Table S5). Elevations in hs-cTnT were even above the rule-in cutoff of the ESC 0/1h algorithm and the ESC 0/2h algorithm (52 ng/L) in 34 patients (16.1%; Table S6). In contrast, hs-cTnI–Architect, hs-cTnI–Access, and hs-cTnI–Vista concentrations were above the biological-equivalent ULN in 23%, 23%, and 8% and overall comparable to those of control subjects (hs-cTnI–Architect, 2.5 ng/L [IQR, 1.2–6.2 ng/L] versus 2.9 ng/L [IQR, 1.8–5.0 ng/L]; hs-cTnI–Access, 3.3 ng/L [IQR, 2.4–6.1 ng/L] versus 2.7 ng/L [IQR, 1.6–5.0 ng/L]; and hs-cTnI–Vista, 7.4 ng/L [IQR, 5.2–13.4 ng/L] versus 7.5 ng/L [IQR, 6–10 ng/]; Table S5). This resulted in hs-cTnT/hs-cTnI-mismatches in 36% to 50% in the overall cohort and in 33% to 37% in patients without cardiac disease (Figure 2 and Table S7). These findings were confirmed when uniform approved and sex-specific ULNs were used (Figures S6–S9). In the control cohort, hs-cTnT/hs-cTnI mismatches were uncommon (4%–5% using biologically equivalent ULN; Figure S10 and Table S8).
Figure 1.
Violine plots representing the distribution of hs-cTnT/I concentrations for the 4 tested assays and across categories of cardiac disease. A single comparison using a Mann-Whitney U test was conducted between the control subjects of the APACE (Advantageous Predictors of Acute Coronary Syndromes Evaluation) cohort and the overall cohort of patients with skeletal muscle disorder. Bioequivalent and overall approved upper limits of normal (ULNs) are represented as broken lines. High-sensitivity cardiac troponin T (hs-cTnT)–Elecsys and high-sensitivity cardiac troponin I (hs-cTnI)–Architect concentrations were available in all 211 patients; hs-cTnI–Access concentrations, in 187 patients; and hs-cTnI–Vista concentrations, in 194 patients. The P values were calculating with a Wilcoxon test comparing the overall group with the control group and have been corrected for multiple testing (4 tests) with the Benjamini and Hochberg method.
Figure 2.
Interassay hs-cTnT/I mismatches using biologically equivalent ULN. For each subpanel, 2 high-sensitivity cardiac troponin T/I (hs-cTnT/I) assays are represented with their biologically equivalent assay-specific 99th percentile upper limit of normal (ULN). In each panel, the 4 quadrants represent the percentage of patients with the following constellations: green when both hs-cTnT/I assays were below the ULN, gray when both were above the ULN, and red when there was an hs-cTnT/I mismatch (with 1 of the assays above and 1 of the assays below the ULN). A, Overall cohort. B, Subgroup without cardiac disease.
Impact of Underlying Pathogenesis on CK and hs-cTnT/I Concentrations
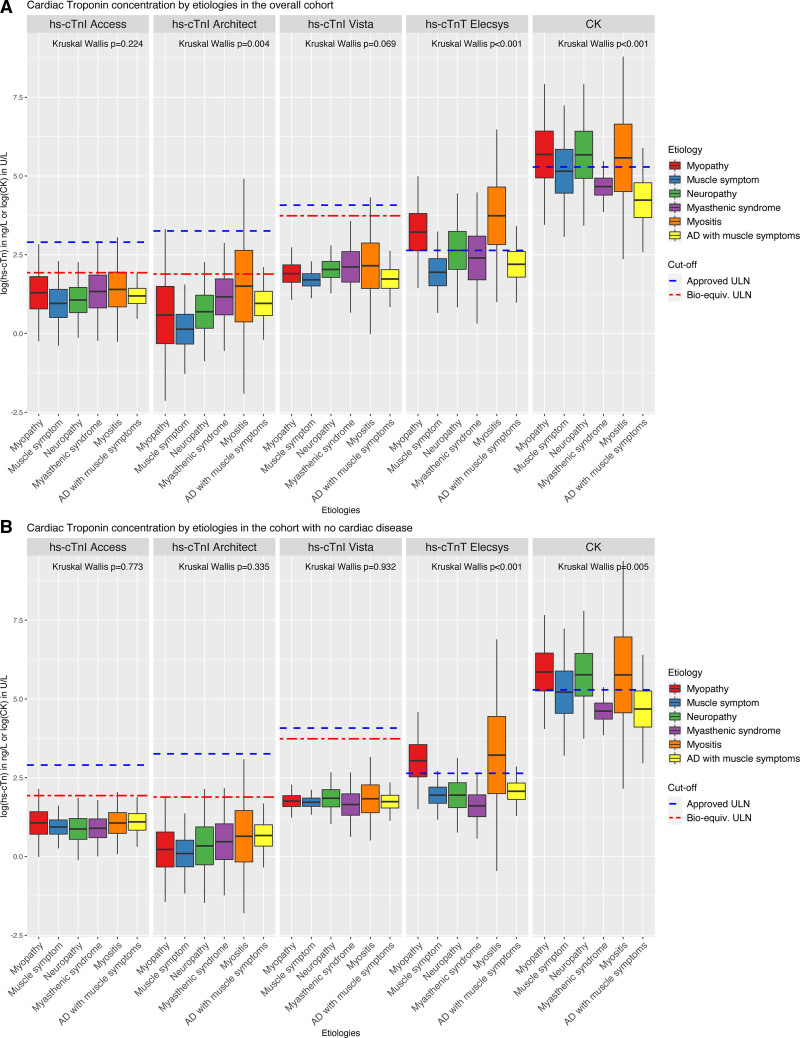
When the different types of muscle disorders were analyzed separately, relevant differences among them emerged in CK and hs-cTnT concentrations, which were not observed with the hs-cTnI Architect, hs-cTnI–Access, and hs-cTnI–Vista assays (Figure 3A). Noninflammatory myopathies and myositis had the highest CK and hs-cTnT concentrations, both in the overall cohort and in the subgroup of patients without cardiac disease (Figure 3B). hs-cTnT elevations in patients without cardiac disease were largely restricted to patients with noninflammatory myopathies and myositis. Within this subgroup of noninflammatory myopathies and myositis, 77% (in the overall cohort) and 68% (in the subgroup with no cardiac disease) presented hs-cTnT concentrations greater than uniform approved ULN, whereas only a few of these patients also showed an hs-cTnI elevation (Pz-test<0.001; Table S9, Figure 3, and Figures S11 and S12). In contrast, the vast majority of patients with neuropathies, myasthenic syndromes, and autoimmune diseases had normal hs-cTnT concentrations.
Figure 3.
Different types of skeletal muscle disorders are represented on the x axis and concentrations of the biomarkers are represented on the y axis with a logarithmic scale. Boxplots represents the interquartile range (IQR), and whiskers show the ±1.5×IQR. Bioequivalent and overall approved upper limits of normal (ULNs) are represented as broken lines. A, Overall cohort. B, Cohort without cardiac disease. The P values have been corrected for multiple testing with the Benjamini and Hochberg method. AD indicates autoimmune disease; CK, creatine kinase; and hs-cTnT/I, high-sensitivity cardiac troponin T/I.
Correlation Between hs-cTnT/I and CK as a Quantitative Indicator of Muscle Damage
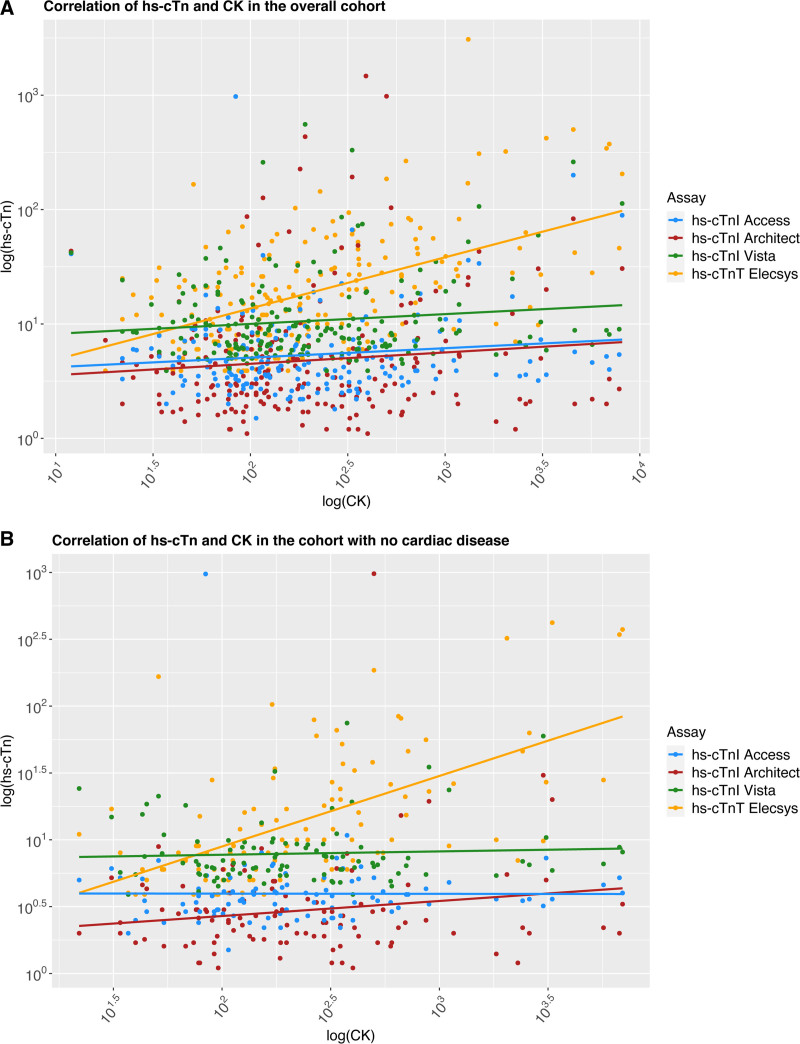
In the overall cohort, hs-cTnI concentrations did not correlate with CK or CK-MB concentrations, whereas hs-cTnT concentrations showed a positive significant correlation with CK and CK-MB concentrations (eg, with CK, R=0.33 and R=0.43 in subgroup of patients without cardiac disease; both Ptau-statistic<0.001; Figure 4A and 4B, Figure S13, and Tables S10 and S11).
Figure 4.
Correlation between creatine kinase and hs-cTn. Correlation between CK and hs-cTn in (A) the overall cohort and (B) patients with no cardiac disease. Biomarkers have been log-transformed to approximate normal distribution. CK indicates creatine kinase; and hs-cTnT/I, high-sensitivity cardiac troponin T/I.
Muscle Tissue mRNA Analysis
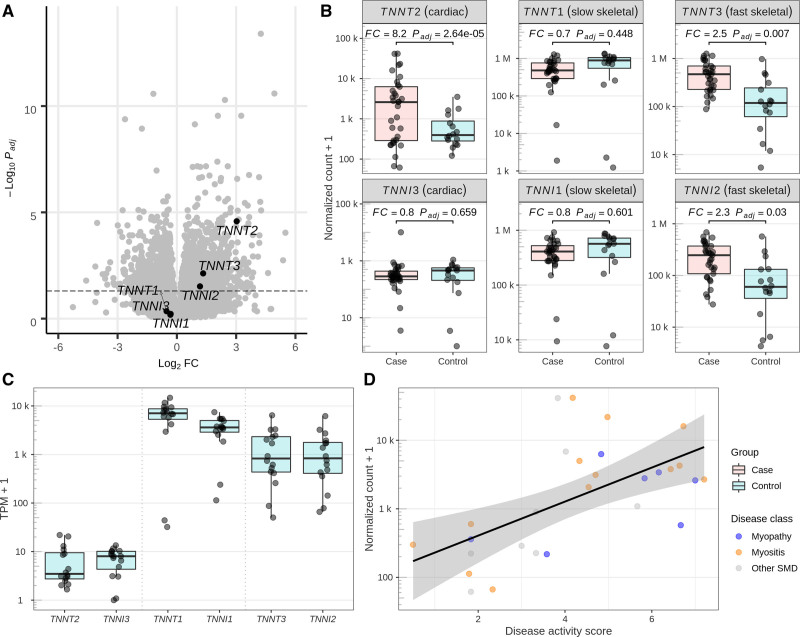
Muscle biopsies from diseased skeletal muscle were available for 33 patients (mean age, 59 years; 39% women; Table S12). DGE analysis showed significant upregulation of the gene TNNT2 coding for cTnT (top 96 differentially expressed gene; 8-fold change compared with controls; Pt-statistic<0.001) and fast skeletal muscle TNNT3 and TNNI2 genes (Figure 5A and 5B). Both cardiac TNNT2 and TNNI3 genes were expressed at low levels in control muscle biopsies, but the level of expression stayed largely below the expression of skeletal muscle troponin genes (Figure 5C). There was a significant positive correlation between the TNNT2 gene and an SMD activity score based on pathological features of the diseased skeletal muscle biopsies independently of disease origin, with higher disease activity score showing higher TNNT2 upregulation (R=0.59, Pt-statistic<0.001; Figure 5D).
Figure 5.
mRNA analyses of muscle biospies. A, Differential gene expression (DGE) results from a case/control study comparing skeletal muscle biopsies from patients with (33 cases) and without (16 controls) skeletal muscle disorder (SMD). After correction for multiple testing with the Benjamini and Hochberg procedure, 847 of 17 124 protein-coding genes were upregulated and 966 were downregulated at a significance level of 𝛼 = 0.05. Three of 6 genes of the troponin gene family show a significant upregulation (TNNT2, top 96 differentially expressed gene [DEG]; TNNT3, top 881 DEG; TNNI2, top 1821 DEG). B, Detailed DGE results for 6 genes of the Troponin gene family. Fold changes (FCs) and significance levels are concordant among the slow (TNNT3 and TNNI2) and fast (TNNT1, TNNI1) skeletal muscle gene pairs but not for the cardiac gene pair (TNNT2 and TNNI3). C, Base-level expression of the troponin gene family in skeletal muscle. Cardiac genes TNNT2 and TNNI3 exhibit an expression of 6 and 6.3 transcripts per million (TPM), ranking among the top 39% and 38% expressed protein-coding genes in the DGE analysis. Fast and slow skeletal muscle genes TNNT1, TNNI1, TNNT3, and TNNI2 exhibit a mean expression of 6848, 3614, 1590, and 1418 TPM (all top 0.1%). D, Variation of TNNT2 expression in the case samples (n=28 after filtering for missingness in marker variables for disease activity) can be explained by biopsy-specific disease activity. Linear regression shows a significant positive correlation (R=0.59, P<0.001) between a disease activity score derived from 14 disease activity markers and normalized counts. The score remains significant (P=0.001) after adjustment for disease class (myopathy [n=7], myositis [n=13], other SMD [n=8]). Conversely, the case subset showed borderline significant differences between disease classes after adjustment for disease activity score in a likelihood ratio test (P=0.069). A detailed description of the score calculation is available in the Supplement Material.
Correlation Between Normalized Count of cTnT Gene Expression and Circulating hs-cTnT
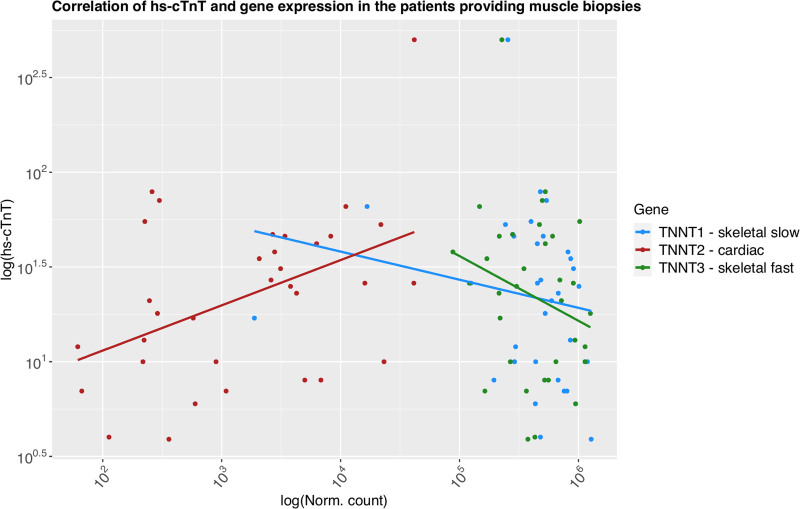
Among the 33 patients providing muscle tissue, circulating hs-cTnT concentrations significantly positively correlated with normalized TNNT2 (R=0.26, Pt-statistic=0.032; Figure 6 and Table S15) expression but not with TNNT1 or TNNT3.
Figure 6.
Correlation between normalized gene expression of the 3 cTnT genes and circulating hs-cTnT concentrations. High-sensitivity cardiac troponin T (hs-cTnT) concentrations and normalized gene expression have been log-transformed to approximate normal distribution.
Discussion
This prospective multicenter study evaluated cTnT and cTnI concentrations using 4 widely applied hs-cTnT/I assays in a broad population of patients presenting with skeletal muscle symptoms. Those were compared with a large control cohort of patients adjudicated to have noncardiac causes of acute chest pain to assess possible implications for the diagnosis of AMI or other cardiac diseases and cardiovascular risk stratification. We report 8 major findings.
First, ≈50% of patients with SMD had mild or severe cardiac disease. Most of the cardiac abnormalities were related to common cardiac disorders such as coronary artery disease, which are associated with an increased risk of future AMI, further documenting the clinical relevance of reliable AMI diagnosis in this population. Second, hs-cTnT/I serum concentrations significantly increased from patients with no cardiac disease to those with mild cardiac disease to those with severe cardiac disease for all assays. Accordingly, cardiomyocyte injury resulting from cardiac disease was a major contributor to hs-cTnI and hs-cTnT concentrations also in patients with SMD. Third, hs-cTnT–Elecsys concentrations were above the uniform approved ULN in 55% and significantly higher compared with control subjects (median, 16 ng/L versus 5 ng/L; P<0.001). In contrast, hs-cTnI–Architect, hs-cTnI–Access, and hs-cTnI–Vista concentrations were above the biological-equivalent ULN in 23%, 23%, and 8% and overall comparable to those of control subjects. These findings were confirmed with uniform approved and sex-specific ULNs. Fourth, elevations in hs-cTnT/I concentrations were most often mild. However, 16.1% of patients in the overall cohort and 12.9% in the subgroup without cardiac disease had hs-cTnT concentrations above the rule-in cutoff of the ESC 0/1h algorithm and ESC 0/2h algorithm (52 ng/L).5,6,33 Therefore, the proportion of patients with SMD who possibly are misclassified by the rule-in cutoff of the ESC 0/1h algorithm or ESC 0/2h algorithm seemed even higher compared with more common populations with increased baseline hs-cTnT/I concentrations such as patients with renal dysfunction and the elderly.37,38 Fifth, hs-cTnT elevations in patients without cardiac disease were restricted largely to patients with noninflammatory myopathy and myositis, whereas the vast majority of patients with neuropathies, myasthenic syndromes, autoimmune diseases, or other causes of skeletal muscle symptoms had hs-cTnT concentrations within the normal range. Sixth, hs-cTnT, but not hs-cTnI, showed a significant positive correlation with CK, a biomarker of skeletal muscle damage, providing further support for the concept that damaged skeletal muscle is the origin of some of the systemic hs-cTnT concentration.9,11,12 Seventh, in the subgroup of patients with skeletal muscle biopsies available, mRNA analyses in diseased skeletal muscle showed 8-fold upregulation of TNNT2, encoding cTnT, compared with control subjects without SMD undergoing hip replacement. The expression strongly correlated with pathological disease activity, thereby suggesting active chronic SMD as a significant contributor to the systemic hs-cTnT concentration. This assumption was further strengthened by a positive and significant correlation between TNNT2 gene expression and circulating hs-cTnT concentrations. Eighth, in contrast, no evidence of upregulation/re-expression in diseased skeletal muscle was found for cTnI.
These findings extend and corroborate results from previous studies, including 3 studies using the hs-cTnT assay.9,12,17 Among 27 ambulatory patients with skeletal myopathies and muscle dystrophies, hs-cTnT concentrations were elevated in 18 patients (67%), with a median of 21 ng/L (IQR, 11–38 ng/L), whereas cTnI was elevated in only 1 patient (4%).11 Among 74 patients with hereditary and acquired skeletal myopathies, hs-cTnT concentrations were elevated in 69%, with a median of 24 ng/L (IQR, 11–54 ng/L), whereas hs-cTnI was elevated in 4% of patients.12 In 122 patients with Pompe disease, hs-cTnT concentrations were elevated in 82% of patients (median, 27 ng/L), whereas hs-cTnI concentrations were normal in all patients. All 3 studies found elevated systemic concentration of hs-cTnT, but not hs-cTnI, and, in part, evidence for some RNA or protein expression on skeletal muscular tissue level.
From these consistent findings, the following insights emerge. First, in the presence of active chronic SMD from 2 categories, noninflammatory myopathy and myositis, including statin-induced myopathy, hs-cTnT loses cardiac specificity as diseased skeletal muscle contributes to the systemic hs-cTnT concentration. Second, according to mRNA analysis, re-expression of cTnT during chronic repair mechanisms in the diseased skeletal muscle appears to be the underlying pathophysiology.7,9–13,39,40 Third, re-expression of cTnT during skeletal muscle repair mechanisms seems to be time dependent. It was present in this study of patients with active chronic SMD with ongoing skeletal muscle damage and repair lasting for weeks to months. In contrast, no evidence was found in patients with acute rhabdomyolysis, an in vivo model of acute skeletal muscle damage of several days’ duration, because no correlation was observed between hs-cTnT and CK concentrations. Accordingly, hs-cTnT/I mismatches were uncommon in acute rhabdomyolysis.41,42 Fourth, other SMD categories, including neuropathies, myasthenic syndromes, autoimmune diseases, or other causes of skeletal muscle symptoms, do not seem to be relevant sources of systemic hs-cTnT concentration. Fifth, although a small number of patients with SMD also showed elevations in hs-cTnI, the absence of an increase in cTnI mRNA at the tissue level and the absence of correlation with CK highlighted in both this and previous studies clearly argue against a skeletal muscle origin.7,17 Alternative explanations need to be considered in these patients such as analytic interference attributable to heterophilic antibodies, autoantibodies, or the formation of macrotroponin complexes, which seem to affect hs-cTnI more commonly than hs-cTnT.43–47 The interpretation of CK-MB is difficult because it has cardiac and skeletal muscle sources and is re-expressed in diseased skeletal muscle.3
Contrary to what was expected, the prevalence of hs-cTnT/I mismatch was higher in the overall group, with about half having documented cardiac disease compared with the subgroup without cardiac disease (36%–50% versus 33%–37%). This indicates that to some degree preferential release of cTnT versus cTnI from cardiomyocytes attributable to chronic cardiac disease also may have contributed to hs-cTnT/I mismatch. The exact pathophysiology underlying this differential release is largely unknown.48 An alternative explanation is that differences in renal function between the overall group and patients with no cardiac disease could have led to differences in clearance between the hs-cTnT and hs-cTnI circulating concentrations.
These findings have clinical implications. In patients presenting with suspected AMI and without ST-segment elevation, the presence of active chronic noninflammatory myopathy or myositis as a possible important confounder of hs-cTnT concentrations must be actively assessed in institutions using hs-cTnT as their standard of care in the emergency department because the risk of erroneous AMI diagnosis is increased in these patients. If no SMD or SMD other than these 2 categories is present, no change in their standard of care seems necessary. If patient history reveals active chronic noninflammatory myopathy or myositis, hs-cTnI rather than hs-cTnT should be measured as an alternative, if available. If hs-cTnI is not available, resampling at 1 or 2 hours would be mandatory to differentiate AMI with its rise within 1 or 2 hours versus noncardiac causes of chest pain with usually stable hs-cTnT concentrations.5,6,33 A similar change in management should be considered in other acute disorders in which an elevated hs-cTnT concentration is associated with a change in management such as rhythm monitoring or escalation of therapy as in pericarditis/myocarditis and in patients with acute pulmonary embolism. Although the prevalence of patients with active chronic noninflammatory myopathy or myositis in previous diagnostic studies deriving rapid hs-cTnI–based triage algorithms likely was very small, our findings provide further support for the more sophisticated methodology of using 2 adjudicated final diagnoses: 1 using serial measurements of hs-cTnT and 1 of hs-cTnI.49,50 Last, our results highlight the need for future hs-cTnT assays to ensure that their antibodies do not cross-react with the troponin T form found in diseased skeletal muscle.
Several limitations of the present study merit consideration. First, although being the largest study performed to date, the sample size of some disease types was only modest. Second, this study included 3 widely applied hs-cTnI assays. Although the findings were quite consistent among the different hs-cTnI assays, studies including other clinically used hs-cTnI assays seem warranted to explore their reliability in patients with SMD. Given the relevant differences in the antibody combination used in these different immunoassays,27,35 different findings may emerge. Third, we may have misclassified a small number of patients as having no cardiac disease because symptoms, signs, ECG, and NT-proBNP concentrations were available in all of these patients but cardiac imaging was available in only a subset. Fourth, over the span of their lifetime, 10% to 20% of patients with noninflammatory myopathies and myositis seem to develop clinically apparent cardiac involvement.51–53 Therefore, in a small proportion of patients with these underlying causes, despite normal findings in cardiac imaging, subtle microscopic cardiomyocyte injury may already have been present and contributed to the high prevalence of hs-cTnT elevation. Future studies including long-term follow-up are necessary to provide an additional domain assessing the biological significance of elevated hs-cTnT concentrations in these patients. Fifth, it is impossible to precisely quantify the proportion of the systemic hs-cTnT concentration that was contributed by the diseased skeletal muscle versus cardiomyocyte injury in the 2 affected SMD categories. The modest correlation between TNNT2 gene expression and circulating hs-cTnT concentrations and the persistent association between the extent of cardiac disease and hs-cTnT concentration suggest that cardiomyocyte injury remained the dominant source. Sixth, because of the absence of serial assessments, we cannot comment on the exact time point at which cTnT start to be re-expressed in noninflammatory myopathies and myositis. Longitudinal studies are required to quantify the time to re-expression.
Conclusions
hs-cTnT elevations are common in patients with active chronic noninflammatory myopathy and myositis, but not with other SMDs, and in part are attributable to upregulation and thus re-expression of TNNT2 in diseased skeletal muscle. In contrast, no evidence of upregulation/re-expression in diseased skeletal muscle was found for cTnI. Therefore, in patients with active chronic noninflammatory myopathy and myositis, cTnI is the preferred analyte for assessing cardiac health in general and the presence of AMI.
Article Information
Sources of Funding
The study was supported by research grants from the Swiss Heart Foundation, University of Basel, University Hospital of Basel, Abbott, Roche, and Siemens.
Disclosures
Dr Jeanne du Fay de Lavallaz has received research support from the Swiss Heart Foundation. Dr Nestelberger has received research support from the Swiss National Science Foundation (P400PM_191037/1), the Prof Dr Max Cloëtta Foundation, the Margarete und Walter Lichtenstein-Stiftung (3MS1038), and the University Hospital Basel, as well as speaker honoraria/consulting honoraria from Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics, and Orion Pharma, outside the submitted work. Dr Boeddinghaus has received research grants from the University of Basel and the Division of Internal Medicine, the Swiss Academy of Medical Sciences, and the Gottfried and Julia Bangerter-Rhyner-Foundation, and speaker honoraria from Siemens, Roche, Ortho Clinical Diagnostics, and Quidel Corp, outside the submitted work. Dr Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, the University of Basel, Abbott, Beckman Coulter, Idorsia, Novartis, Ortho Clinical Diagnostics, Quidel, Roche, and Siemens, as well as speaker honoraria/consulting honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Idorsia, Novartis, Osler, Roche, and Sanofi, outside the submitted work. Dr Maurer has grant/research support from the Prof Max Cloetta Foundation, AbbVie, Protagen, and Novartis Biomedical Research and received speaker fees from Boehringer-Ingelheim, as well as congress support from Pfizer, Roche, Actelion, Mepha, and MSD. In addition, Dr. Maurer has a patent mir-29 for the treatment of systemic sclerosis issued (US8247389, EP2331143), all outside the submitted work. Dr Gualandro received research grants from Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, Brasil and consulting honoraria from Roche, outside the submitted work. Dr Lopez-Ayala has received research support from the Swiss Heart Foundation (FF20079). Dr Puelacher reports research funding from Roche Diagnostics, the University of Basel, and the University Hospital Basel, outside of the submitted work. Dr Sinnreich has received financial support from Roche from 2015 to 2019 for a research collaboration unrelated to the current work. The other authors report no conflicts. The hs-cTn assays investigated were donated by the manufacturers, who had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Supplemental Material
Expanded Methods
Supplemental data on the APACE control cohort
Cohort details
Derivation of bioequivalent cut-offs in the control cohort
Sample size calculation
RNA-seq experiment
Sample randomization
RNA extraction
Library preparation and RNA sequencing
RNA-seq computational analysis
Mapping and preprocessing
Quality control
Differential gene expression
TNNT2 vs disease activity correlation analysis
Figures S1–S13
Tables S1–S15
Appendix and contributing authors
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AMI
- acute myocardial infarction
- APACE
- Advantageous Predictors of Acute Coronary Syndromes Evaluation
- BASEL XII
- Reliability of Cardiac Troponins for the Diagnosis of Myocardial Infarction in the Presence of Skeletal Muscle Disease
- CK
- creatine kinase
- cTnT
- cardiac troponin T
- cTnI
- cardiac troponin I
- DGE
- differential gene expression
- ESC
- European Society of Cardiology
- hs-cTnT
- high-sensitivity cardiac troponin T
- hs-cTnI
- high-sensitivity cardiac troponin I
- IQR
- interquartile range
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- SMD
- skeletal muscle disorder
- ULN
- upper limit of normal
J. du Fay de Lavallaz and A. Prepoudis contributed equally.
A complete list of the BASEL XII Investigators is given in the Supplemental Material.
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.058489.
For Sources of Funding and Disclosures, see page 1777.
Contributor Information
Jeanne du Fay de Lavallaz, Email: Jeanne.duFaydelavallaz@usb.ch.
Alexandra Prepoudis, Email: alexandra.prepoudis@usb.ch.
Maria Janina Wendebourg, Email: Janina.wendebourg@usb.ch.
Eva Kesenheimer, Email: Eva.kesenheimer@usb.ch.
Diego Kyburz, Email: diego.kyburz@usb.ch.
Thomas Daikeler, Email: thomas.nestelberger@usb.ch.
Philip Haaf, Email: philip.haaf@usb.ch.
Julia Wanschitz, Email: Julia.Wanschitz@i-med.ac.at.
Wolfgang N. Löscher, Email: wolfgang.loescher@i-med.ac.at.
Bettina Schreiner, Email: Bettina.Schreiner@usz.ch.
Mira Katan, Email: mira.katan@usz.ch.
Hans H. Jung, Email: hans.jung@usz.ch.
Britta Maurer, Email: britta.maurer@insel.ch.
Angelika Hammerer-Lercher, Email: Angelika.Hammerer@ksa.ch.
Agnes Mayr, Email: a.mayr@i-med.ac.at.
Danielle M. Gualandro, Email: Danielle.Gualandro@usb.ch.
Annemarie Acket, Email: annemarie.acket@gmail.com.
Christian Puelacher, Email: christian.mueller@usb.ch.
Jasper Boeddinghaus, Email: jasper.boeddinghaus@usb.ch.
Thomas Nestelberger, Email: thomas.nestelberger@usb.ch.
Pedro Lopez-Ayala, Email: p.lopezayala@gmail.com.
Noemi Glarner, Email: noemi.glarner@usb.ch.
Samyut Shrestha, Email: samyut.shrestha@usb.ch.
Robert Manka, Email: robert.manka@usz.ch.
Joanna Gawinecka, Email: joanna.gawinecka@usz.ch.
Salvatore Piscuoglio, Email: Salvatore.Piscuoglio@usb.ch.
John Gallon, Email: john.gallon@unibas.ch.
Sophia Wiedemann, Email: sophia.j.wiedemann@gmail.com.
Michael Sinnreich, Email: Michael.sinnreich@usb.ch.
References
- 1.Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323–333. doi: 10.1080/15216540216037 [DOI] [PubMed] [Google Scholar]
- 2.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853 [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 4.Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, Plein S, Mueller C, Haaf P. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017;12:147–155. doi: 10.1007/s11739-017-1612-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann JT, Twerenbold R, Ojeda F, Sörensen NA, Chapman AR, Shah ASV, Anand A, Boeddinghaus J, Nestelberger T, Badertscher P, et al. Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med. 2019;380:2529–2540. doi: 10.1056/NEJMoa1803377 [DOI] [PubMed] [Google Scholar]
- 6.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 7.Ricchiuti V, Voss EM, Ney A, Odland M, Anderson PA, Apple FS. Cardiac troponin T isoforms expressed in renal diseased skeletal muscle will not cause false-positive results by the second generation cardiac troponin T assay by Boehringer Mannheim. Clin Chem. 1998;44:1919–1924. [PubMed] [Google Scholar]
- 8.Apple FS, Ricchiuti V, Voss EM, Anderson PA, Ney A, Odland M. Expression of cardiac troponin T isoforms in skeletal muscle of renal disease patients will not cause false-positive serum results by the second generation cardiac troponin T assay. Eur Heart J. 1998;19(suppl N):N30–N33. [PubMed] [Google Scholar]
- 9.Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. 2011;58:1819–1824. doi: 10.1016/j.jacc.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 10.Lindberg C, Klintberg L, Oldfors A. Raised troponin T in inclusion body myositis is common and serum levels are persistent over time. Neuromuscul Disord. 2006;16:495–497. doi: 10.1016/j.nmd.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Rittoo D, Jones A, Lecky B, Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. J Am Coll Cardiol. 2014;63:2411–2420. doi: 10.1016/j.jacc.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 12.Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, Radl R, Beer M, Polacin M, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018;71:1540–1549. doi: 10.1016/j.jacc.2018.01.070 [DOI] [PubMed] [Google Scholar]
- 13.Erlacher P, Lercher A, Falkensammer J, Nassonov EL, Samsonov MI, Shtutman VZ, Puschendorf B, Mair J. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin Chim Acta. 2001;306:27–33. doi: 10.1016/s0009-8981(01)00392-8 [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal R, Lebiedz-Odrobina D, Sinha A, Manadan A, Case JP. Serum cardiac troponin T, but not troponin I, is elevated in idiopathic inflammatory myopathies. J Rheumatol. 2009;36:2711–2714. doi: 10.3899/jrheum.090562 [DOI] [PubMed] [Google Scholar]
- 15.McLaurin MD, Apple FS, Voss EM, Herzog CA, Sharkey SW. Cardiac troponin I, cardiac troponin T, and creatine kinase MB in dialysis patients without ischemic heart disease: evidence of cardiac troponin T expression in skeletal muscle. Clin Chem. 1997;43(pt 1):976–982. [PubMed] [Google Scholar]
- 16.Rubini Gimenez M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Schaefer M, Zellweger C, Moehring B, Stallone F, Sou SM, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014;35:2303–2311. doi: 10.1093/eurheartj/ehu188 [DOI] [PubMed] [Google Scholar]
- 17.Wens SC, Schaaf GJ, Michels M, Kruijshaar ME, van Gestel TJ, In ‘t Groen S, Pijnenburg J, Dekkers DH, Demmers JA, Verdijk LB, et al. Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ Cardiovasc Genet. 2016;9:6–13. doi: 10.1161/CIRCGENETICS.115.001322 [DOI] [PubMed] [Google Scholar]
- 18.Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka-Moser V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40:665–671. doi: 10.2340/16501977-0235 [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. ; REDCap Consortium. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, et al. ; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 22.Sokoll LJ, Baum H, Collinson PO, Gurr E, Haass M, Luthe H, Morton JJ, Nowatzke W, Zingler C. Multicenter analytical performance evaluation of the Elecsys proBNP assay. Clin Chem Lab Med. 2004;42:965–972. doi: 10.1515/CCLM.2004.157 [DOI] [PubMed] [Google Scholar]
- 23.Rubini Giménez M, Twerenbold R, Boeddinghaus J, Nestelberger T, Puelacher C, Hillinger P, Wildi K, Jaeger C, Grimm K, Heitzelmann KF, et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin T in suspected myocardial infarction. JAMA Cardiol. 2016;1:912–920. doi: 10.1001/jamacardio.2016.2882 [DOI] [PubMed] [Google Scholar]
- 24.Abbott Laboratories. ARCHITECT High Sensitive Troponin I: Troubleshooting Guide. 2015. [Google Scholar]
- 25.Krintus M, Kozinski M, Boudry P, Capell NE, Köller U, Lackner K, Lefèvre G, Lennartz L, Lotz J, Herranz AM, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med. 2014;52:1657–1665. doi: 10.1515/cclm-2014-0107 [DOI] [PubMed] [Google Scholar]
- 26.Pretorius CJ, Tate JR, Wilgen U, Cullen L, Ungerer JPJ. A critical evaluation of the Beckman Coulter Access hsTnI: analytical performance, reference interval and concordance. Clin Biochem. 2018;55:49–55. doi: 10.1016/j.clinbiochem.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 27.Apple FS, Wu AHB, Sandoval Y, Sexter A, Love SA, Myers G, Schulz K, Duh SH, Christenson RH. Sex-specific 99th percentile upper reference limits for high sensitivity cardiac troponin assays derived using a universal sample bank. Clin Chem. 2020;66:434–444. doi: 10.1093/clinchem/hvz029 [DOI] [PubMed] [Google Scholar]
- 28.IFCC Committee on Clinical Applications of Cardiac Bio-markers. High-sensitivity cardiac troponin I and T assay analytical characteristics designated by manufacturer IFCC Committee on Clinical Applications of Cardiac Bio-Markers (C-CB) V082318. 2019. Accessed December 10, 2021. http://www.ifcc.org/media/477441/high-sensitivity-cardiac-troponin-i-and-t-assay-analytical-characteristics-designated-by-manufacturer-v08232018.pdf
- 29.Ungerer JP, Marquart L, O’Rourke PK, Wilgen U, Pretorius CJ. Concordance, variance, and outliers in 4 contemporary cardiac troponin assays: implications for harmonization. Clin Chem. 2012;58:274–283. doi: 10.1373/clinchem.2011.175059 [DOI] [PubMed] [Google Scholar]
- 30.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildi K, Gimenez MR, Twerenbold R, Reichlin T, Jaeger C, Heinzelmann A, Arnold C, Nelles B, Druey S, Haaf P, et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation. 2015;131:2032–2040. doi: 10.1161/CIRCULATIONAHA.114.014129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steyerberg EW, Harrell FE, Jr. Regression modeling strategies: with applications, to linear models, logistic and ordinal regression, and survival analysis, 2nd ed. Heidelberg: Springer. Biometrics. 2016;72(3):1006–1007. doi: 10.1111/biom.12569 [Google Scholar]
- 33.Twerenbold R, Costabel JP, Nestelberger T, Campos R, Wussler D, Arbucci R, Cortes M, Boeddinghaus J, Baumgartner B, Nickel CH, et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol. 2019;74:483–494. doi: 10.1016/j.jacc.2019.05.046 [DOI] [PubMed] [Google Scholar]
- 34.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654 [DOI] [PubMed] [Google Scholar]
- 35.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73–81. doi: 10.1373/clinchem.2016.255109 [DOI] [PubMed] [Google Scholar]
- 36.Noble WS. How does multiple testing correction work? Nat Biotechnol. 2009;27:1135–1137. doi: 10.1038/nbt1209-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boeddinghaus J, Nestelberger T, Twerenbold R, Neumann JT, Lindahl B, Giannitsis E, Sörensen NA, Badertscher P, Jann JE, Wussler D, et al. ; APACE, BACC, and TRAPID-AMI Investigators. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur Heart J. 2018;39:3780–3794. doi: 10.1093/eurheartj/ehy514 [DOI] [PubMed] [Google Scholar]
- 38.Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Puelacher C, Sabti Z, Rubini Gimenez M, Tschirky S, du Fay de Lavallaz J, et al. 0/1-Hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation. 2018;137:436–451. doi: 10.1161/CIRCULATIONAHA.117.028901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messner B, Baum H, Fischer P, Quasthoff S, Neumeier D. Expression of messenger RNA of the cardiac isoforms of troponin T and I in myopathic skeletal muscle. Am J Clin Pathol. 2000;114:544–549. [PubMed] [Google Scholar]
- 40.Fisher C, Agrawal S, Wong WM, Fahie-Wilson M, Dasgupta B. Clinical observations on the significance of raised cardiac troponin-T in patients with myositis of varying etiologies seen in rheumatology practice. Clin Rheumatol. 2010;29:1107–1111. doi: 10.1007/s10067-010-1511-6 [DOI] [PubMed] [Google Scholar]
- 41.du Fay de Lavallaz J, Zehntner T, Puelacher C, Walter J, Strebel I, Rentsch K, Boeddinghaus J, Nestelberger T, Twerenbold R, Mueller C. Rhabdomyolysis: a noncardiac source of increased circulating concentrations of cardiac troponin T? J Am Coll Cardiol. 2018;72:2936–2937. doi:10.1016/j.jacc.2018.09.050 [DOI] [PubMed] [Google Scholar]
- 42.Giger RD, du Fay de Lavallaz J, Prepoudis A, Stoll T, Lopez-Ayala P, Glarner N, Boeddinghaus J, Puelacher C, Nestelberger T, Mueller C. Rhabdomyolysis: a noncardiac source of increased circulating concentrations of cardiac troponin T? J Am Coll Cardiol. 2020;76:2685–2687. doi: 10.1016/j.jacc.2020.08.088 [DOI] [PubMed] [Google Scholar]
- 43.Lewis JG, Connolly AJL, Ploeg H, Phillips IJ, King RI, Elder PA, Florkowski CM. Grossly elevated false-positive high-sensitivity troponin I due to heterophilic antimouse IgG1 antibodies. J Appl Lab Med. 2020;5:815–817. doi: 10.1093/jalm/jfaa024 [DOI] [PubMed] [Google Scholar]
- 44.Strasser B, Tomasits J, Fellner A, Lambert T. Troponin interference with special regard to macrocomplex formation. Clin Chem Lab Med. 2022;60:162–168. doi:10.1515/cclm-2021-0841 [DOI] [PubMed] [Google Scholar]
- 45.Herman DS, Kavsak PA, Greene DN. Variability and error in cardiac troponin testing: an ACLPS critical review. Am J Clin Pathol. 2017;148:281–295. doi: 10.1093/ajcp/aqx066 [DOI] [PubMed] [Google Scholar]
- 46.Lam L, Aspin L, Heron RC, Ha L, Kyle C. Discrepancy between cardiac troponin assays due to endogenous antibodies. Clin Chem. 2020;66:445–454. doi: 10.1093/clinchem/hvz032 [DOI] [PubMed] [Google Scholar]
- 47.Leuschner F, Li J, Göser S, Reinhardt L, Ottl R, Bride P, Zehelein J, Pfitzer G, Remppis A, Giannitsis E, et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29:1949–1955. doi: 10.1093/eurheartj/ehn268 [DOI] [PubMed] [Google Scholar]
- 48.Mair J, Lindahl B, Hammarsten O, Müller C, Giannitsis E, Huber K, Möckel M, Plebani M, Thygesen K, Jaffe AS. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care. 2018;7:553–560. doi: 10.1177/2048872617748553 [DOI] [PubMed] [Google Scholar]
- 49.Boeddinghaus J, Nestelberger T, Koechlin L, Wussler D, Lopez-Ayala P, Walter JE, Troester V, Ratmann PD, Seidel F, Zimmermann T, et al. ; APACE Investigators. Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol. 2020;75:1111–1124. doi: 10.1016/j.jacc.2019.12.065 [DOI] [PubMed] [Google Scholar]
- 50.Koechlin L, Boeddinghaus J, Nestelberger T, Lopez-Ayala P, Wussler D, Shrestha S, Resa T, Wildi K, Bakula A, Frey S, et al. ; APACE Investigators. Performance of the ESC 0/2h-algorithm using high-sensitivity cardiac troponin I in the early diagnosis of myocardial infarction. Am Heart J. 2021;242:132–137. doi: 10.1016/j.ahj.2021.08.008 [DOI] [PubMed] [Google Scholar]
- 51.Gupta R, Wayangankar SA, Targoff IN, Hennebry TA. Clinical cardiac involvement in idiopathic inflammatory myopathies: a systematic review. Int J Cardiol. 2011;148:261–270. doi: 10.1016/j.ijcard.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 52.Lilleker JB, Vencovsky J, Wang G, Wedderburn LR, Diederichsen LP, Schmidt J, Oakley P, Benveniste O, Danieli MG, Danko K, et al. ; all EuroMyositis contributors. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis. 2018;77:30–39. doi: 10.1136/annrheumdis-2017-211868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology. 2006;45(suppl 4):18–21. doi: 10.1093/rheumatology/kel311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.