Abstract
Background:
Racial segregation is linked to poorer neighborhood quality and adverse health conditions among minorities, including worse cancer outcomes. We evaluated relationships between race, neighborhood social disadvantage, and cancer survival.
Methods:
We calculated overall and cancer-specific survival for 11,367 Non-Hispanic Black (NHB) and 29,481 Non-Hispanic White (NHW) individuals with breast, colorectal, lung, or prostate cancer using data from the Metropolitan Detroit Cancer Surveillance System. The Area Deprivation Index (ADI) was used to measure social disadvantage at the census block group level, where higher ADI is associated with poorer neighborhood factors. Associations between ADI and survival were estimated using Cox proportional hazards mixed-effects models accounting for geographic grouping and adjusting for demographic and clinical factors.
Results:
Increasing ADI quintile was associated with increased overall mortality for all four cancer sites in multivariable-adjusted models. Stratified by race, these associations remained among breast (NHW: Hazard Ratio [HR]=1.16, p<0.0001; NHB: HR=1.20, p<0.0001), colorectal (NHW: HR=1.11, p<0.0001; NHB: HR=1.09, p=0.00378), prostate (NHW: HR=1.18, p<0.0001; NHB: HR=1.18, p<0.0001), and lung cancers (NHW: HR=1.06, p<0.0001; NHB: HR=1.07, p=0.00177). Cancer-specific mortality estimates were similar to overall mortality. Adjustment for ADI substantially attenuated the effects of race on mortality for breast (overall proportion attenuated (OPA)=47%, p<0.0001; cancer-specific proportion attenuated (CSPA)=37%, p<0.0001) prostate cancer (OPA=51%, p<0.0001; CSPA=56%, p<0.0001), and colorectal cancer (OPA=69%, p=0.032; CSPA=36%, p=0.018).
Conclusions:
Area-level socioeconomic disadvantage is related to cancer mortality in a racially diverse population, impacting racial differences in cancer mortality.
Keywords: racial disparities, socioeconomic status, social disadvantage, geography
Introduction
In 2022, approximately 1.9 million people are estimated to be diagnosed with cancer and 609,360 people are estimated to die from cancer within the United States(1). Nearly half of these cancer-related deaths are due to breast, colorectal, lung, and prostate cancers. Predictors of cancer survival are complex and encompass clinical, individual, and systems-level factors, including disease stage, sex, age at diagnosis, race and ethnicity, socioeconomic status (SES), treatment, and access to quality healthcare(2). Despite major advances in treatment and improvements in overall cancer survival over the last several decades, racial disparities in cancer survival have persisted. Five-year cancer survival rates are consistently lower among Non-Hispanic Black (NHB) patients than non-Hispanic White (NHW) patients overall and in these four cancer sites specifically(3–7), even when accounting for demographic and clinical factors such as age and stage at diagnosis(8).
To identify mechanisms underlying racial disparities in cancer outcomes, it is essential to recognize that race is a social construct (9). While self-reported race in the United States is correlated with genetic ancestry(10), which itself is implicated in the etiology of various health conditions including cancer(11), our understanding of the long-lasting impact of social race-driven policies on the health of NHB cancer patients has substantially improved in recent years. For example, location-based mortgage lending bias (i.e., “redlining”), which results in residential racial segregation, is associated with increased risk for late-stage cancer diagnosis and more pronounced racial differences in cancer mortality rates(12–14). Metropolitan Detroit remains one of the most racially segregated regions in the country(15), where regions with the highest proportions of NHB residents are also the regions with the highest rates of poverty, unemployment, low education levels, and neighborhood disadvantage(16–18). Understanding the effects of neighborhood on cancer outcomes is a critical first step in the development of both community and individual interventions to increase health equity and reduce racial disparities.
Measuring social factors related to cancer survival in existing large datasets, such as population-based cancer registries, can be difficult. Historically, studies have relied on using SES to capture social and economic resources at the individual or areal level. Cancer survival rates have been shown to increase with higher individual-level SES, which is generally higher among NHW patients compared to NHB patients(19–21), resulting in some of the poorest cancer outcomes among NHB patients with low individual-level SES(22). These associations have been attributed to differences in access to high-quality healthcare resources, cost-related delays in treatment, and treatment adherence(23–25); however, individual-level measures do not capture potentially relevant contextual neighborhood factors such as the physical environment and neighborhood resources. Indeed, survival rates among cancer patients are significantly lower among those in more socially-deprived neighborhoods and among most ethnic-minority groups(26).
Racial disparities in cancer incidence and survival are a priority research area of the National Cancer Institute(27). Health equity research in recent years has brought attention to the importance of social determinants of health, which are broadly defined as the living and working conditions that affect overall health, in understanding health disparities(28). The effects of historical and contemporary structural racism disproportionately affect African Americans and have led to unequal access to work, education, housing, healthy food, and quality health care(29–31) as well as significant stressors such as low income, unemployment, lack of transportation, and single-parent homes with dependent children(32–35). Though socioeconomic status is strongly predictive of cancer outcomes(36), individual-level socioeconomic status does not capture the entirety of these disparities. Neighborhood level measures encompass unique economic, physical, and social characteristics that influence community and individual health.
The goal of this analysis was to evaluate the relationship between a robust measure of area-level social disadvantage and cancer survival among NHB and NHW cancer patients within metropolitan Detroit. To measure social deprivation, we utilized Singh’s Area Deprivation Index (ADI)(37), a validated composite tool that measures the socioeconomic deprivation of an area calculated at the census block group level using 17 indicators from the American Community Survey (ACS). These indicators encompass aspects of education, employment, housing, and income from census block groups. ADI has been associated with health outcomes and healthcare quality(38,39). Previous studies around the world have shown that increases in neighborhood deprivation are associated with increased risk for overall mortality in many major cancer sites, including lung(40–42), cervical(43,44), breast(45–47), prostate(48–50), and colorectal cancers(17,51,52). Fewer studies have observed these relationships stratified by race, and even less have looked at the role of neighborhood deprivation in the mediation of racial disparities in mortality risk. We hypothesized that higher ADI levels would be associated with higher overall and cancer-specific mortality and that ADI would partly mediate known racial disparities in cancer survival.
Materials and Methods
Identification of cancers in metropolitan Detroit
We identified all incident invasive breast, prostate, lung, and colorectal cancers diagnosed in Wayne, Oakland, and Macomb counties in Michigan between 2012–2016 of NHB or NHW race using the Metropolitan Detroit Cancer Surveillance System (MDCSS) registry database. After excluding individuals with less than one month of follow-up, our final analytic sample consisted of 40,850 individuals with invasive cancer (12,907 female breast, 6,738 colorectal, 9,862 lung, and 12,151 prostate cancers). MDCSS is a founding member of the Surveillance, Epidemiology, and End Results (SEER) Program (1) and has been continuously collecting population-based cancer data since 1973. This study was approved by the Wayne State University Institutional Review Board (IRB).
Clinical and demographic variables
Clinical, treatment, and outcomes data were obtained from the MDCSS registry, including age at diagnosis, sex, insurance status, stage, breast cancer subtype, Gleason score (prostate cancers only), surgery type, chemotherapy, radiation, hormone therapy, vital status, cause of death, and date of last contact. For patients who had treatment data recorded in MDCSS (n=39,758, 97.3%), first course of treatment was defined as receipt of therapy for a cancer diagnosis before disease progression or recurrence and included undergoing surgery, adjuvant chemotherapy, endocrine therapy, and radiation therapy. In this analysis, we studied NHB and NHW patients as they represent the majority of the population in metropolitan Detroit and excluded other race/ethnic groups due to small sample size.
Area-level deprivation index (ADI) calculation
Address at diagnosis was geocoded using the Federal Information Processing Standard (FIPS) convention to identify census block groups for each cancer case. A total of 3,391 census block groups were identified among cases, and we calculated ADI corresponding to each block group using Michigan’s 5-year estimates (2009–2013) from the Census Bureau’s American Community Survey (ACS)(53) which were abstracted from the US census. The census-derived indicators used in the calculation of ADI include educational distribution (percentage of the population with less than 9 years and with 12 or more years of education), median family income, median home value, median gross rent, median monthly mortgage, income disparity, unemployment, percent employed person in white-collar occupation, percent families below poverty, percent population below 150% poverty threshold, single-parent household rate, homeownership rate, percent household without a telephone, percent household without a motor vehicle, percent occupied housing units without complete plumbing, and household crowding. Factor score coefficients calculated by Singh were used to weight each of the 17 census indicators and ADI for each Michigan block group was calculated(54,55). The 17 US census indicators were multiplied by the Singh’s coefficients (factor weights) and summed to obtain the base score for all block groups in Michigan. Each base score was standardized by dividing the difference between the individual block group base score (b) and Michigan block group mean (p), by Michigan block group standard deviation (Sp)
where j represents the jth block group, and k is the total number of block group in Michigan. Finally, the standardized values were adjusted to a base mean of 100 and a standard deviation of 20(55).
Statistical analyses
All data were analyzed using R statistical software (https://cran.r-project.org/). Standardized ADI scores were categorized into quintiles based on the overall population ADI distribution. Univariable associations between demographics, race, and ADI were examined using chi-squared tests and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. Cox proportional hazards regression was used to estimate associations between overall survival and cancer-specific survival. Violation of the proportional hazards assumptions were evaluated using the cox.zph function with a multiple testing p-value threshold equivalent to . For all regression models, age was included as a strata variable (breast and lung cancer models) or a covariate (prostate and colorectal cancer models) based on (1) whether they violated proportional hazards assumptions of the Cox PH models and (2) a priori evidence for being related to the outcome and to increase precision of estimates. We excluded mediators as covariates in multivariable models as identified in Figure S1 (treatment variables, insurance status, stage at diagnosis, tumor biology). Adjusted survival curves were generated by applying the “survfit” function of the R package survival to the previously fitted Cox proportional hazards models, where we specified a new data frame consisting of the median values for each of the variables in the original Cox model with indicators for ADI quintile. All statistical tests were two-sided, with a p-value of <0.05 considered to be statistically significant.
Mediation analyses
Figure S1 shows the directed acyclic graph representing our hypothesized causal relationships between race, ADI, and cancer survival as well as other key variables. We identified cancer sites for which ADI potentially attenuated the relationship between race and survival as those meeting the following criteria: (1) Race was significantly associated with survival, (2) Race and ADI were significantly associated with each other, (3) ADI was associated with survival controlling for race, and (4) the effect of race on survival while controlling for ADI decreased compared to the model without ADI. Among those cancer types for which ADI was found to attenuate the effect of race, we then implemented the “mediate” function to quantify the proportion of the total effect of race on cancer survival that was attenuated when adding ADI to the model. While treatment variables were also identified as potential mediators of the relationship between race, these variables violated the proportional hazard assumption and could not be evaluated for direct or mediation effects.
Results
A total of 40,850 invasive primary cancer cases (12,907 female breast, 6,738 colorectal, 9,862 lung, and 12,151 prostate cancers) with at least one month of follow-up were identified (Table 1). Mean age at diagnosis varied significantly by cancer type, where breast cancer cases were diagnosed younger (mean=61.4 years) and lung cancer cases were diagnosed older (mean=68.0 years) than colorectal and prostate cancer cases. No difference in sex was observed in colorectal and lung cancers, with approximately equal numbers of men and women diagnosed with each cancer type. Among colorectal and prostate cancer cases there was a higher proportion of NHB individuals (28.7% and 31.9%, respectively) than for breast and lung cancer (25.2% and 25.4%, respectively). Colorectal and lung cancer cases were much more likely to be diagnosed at distant stage (23.4% and 57.4%, respectively) than breast (6.1%) and prostate (5.8%) cancer cases. There was also significant variability in insurance status by cancer type, where prostate cancer cases were more likely to have unknown insurance status (17.8% vs. 6.0% for other sites combined) and breast cancer cases were most likely to have private insurance (46.8% vs. 30.0% for other sites combined). Breast and prostate cancer cases were more likely to live in lower ADI regions whereas colorectal and lung cancer cases were more likely to live in higher ADI regions, where higher ADI is associated with poorer neighborhood factors.
Table 1.
Demographic characteristics of cancer cases and the population of metropolitan Detroit
| Breast n (%) | Colorectal n (%) | Lung n (%) | Prostate n (%) | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Total | 12097 | 6738 | 9862 | 12151 | |
| Age * | |||||
| <50 | 2459 (20.3) | 847 (12.6) | 400 (4.1) | 424 (3.5) | <0.0001 |
| 50–59 | 3067 (25.4) | 1591 (23.6) | 1899 (19.3) | 2907 (23.9) | |
| 60–69 | 3226 (26.7) | 1706 (25.3) | 3158 (32.0) | 5240 (43.1) | |
| 70–79 | 2071 (17.1) | 1375 (20.4) | 2782 (28.2) | 2794 (23.0) | |
| 80+ | 1274 (10.5) | 1219 (18.1) | 1623 (16.4) | 786 (6.5) | |
| Mean (sd) | 61.4 (13.6) | 65.1 (14.4) | 68.0 (11.1) | 65.0 (9.1) | <0.0001 |
| Sex | |||||
| Male | 0 (0) | 3364 (49.9) | 4886 (49.5) | 12151 (100) | 0.64* |
| Female | 12097 (100) | 3374 (50.1) | 4976 (50.5) | 0 (0) | |
| Race | |||||
| Non-Hispanic White | 9040 (74.7) | 4803 (71.3) | 7359 (74.6) | 8279 (68.1) | <0.0001 |
| Non-Hispanic Black | 3057 (25.2) | 1935 (28.7) | 2503 (25.4) | 3872 (31.9) | |
| Stage | |||||
| Local | 7552 (63.1) | 2494 (38.5) | 1769 (18.7) | 9417 (78.8) | <0.0001 |
| Regional | 3687 (30.8) | 2473 (38.1) | 2260 (23.9) | 1843 (15.4) | |
| Distant | 725 (6.1) | 1516 (23.4) | 5418 (57.4) | 694 (5.8) | |
| Insurance | |||||
| DOD/Mil/VA | 43 (0.4) | 66 (1.0) | 160 (1.6) | 302 (2.5) | <0.0001 |
| Medicaid +/− other | 1076 (8.9) | 808 (12.0) | 1196 (12.1) | 736 (6.0) | |
| Medicare +/− other | 4373 (36.1) | 2994 (44.4) | 5179 (52.5) | 4741 (39.0) | |
| None | 132 (1.1) | 141 (2.1) | 160 (1.6) | 95 (0.8) | |
| Unknown | 806 (6.7) | 537 (8.0) | 900 (9.1) | 2161 (17.8) | |
| Private | 5667 (46.8) | 2192 (32.5) | 2266 (23.0) | 4116 (33.9) | |
| ADI | |||||
| (Low) 1 | 2749 (23.0) | 1123 (17.0) | 1254 (12.9) | 2907 (24.3) | <0.0001 |
| 2 | 2656 (22.2) | 1296 (19.6) | 1741 (18.0) | 2348 (19.7) | |
| 3 | 2385 (20.0) | 1342 (20.3) | 2069 (21.3) | 2241 (18.8) | |
| 4 | 2208 (18.5) | 1447 (21.9) | 2305 (23.8) | 2078 (17.4) | |
| (High) 5 | 1944 (16.3) | 1406 (21.2) | 2325 (24.0) | 2368 (19.8) | |
| Surgery | |||||
| No | 1007 (8.4) | 1151 (17.3) | 7482 (78.5) | 7296 (60.3) | <0.0001 |
| Yes | 11027 (91.6) | 5511 (82.7) | 2048 (21.5) | 4802 (39.7) | |
| Chemotherapy | |||||
| No | 6753 (56.4) | 3804 (57.9) | 4523 (48.0) | 11962 (99.0) | <0.0001 |
| Yes | 5214 (43.6) | 2765 (42.1) | 4892 (52.0) | 126 (1.0) | |
| Hormone therapy | |||||
| No | 4807 (39.7) | 6731 (99.9) | 9817 (99.5) | 9917 (81.6) | <0.0001 |
| Yes | 7290 (60.3) | 7 (0.1) | 45 (0.5) | 2234 (18.4) | |
| Vital Status | |||||
| Alive | 9723 (80.4) | 3675 (54.5) | 2066 (20.9) | 10143 (83.5) | <0.0001 |
| Deceased | 2374 (19.6) | 3063 (45.5) | 7796 (79.1) | 2008 (16.5) | |
| Follow-up time (median, IQR) | |||||
| Alive | 51 (30) | 49 (29) | 45 (29) | 51 (32) | <0.0001 |
| Deceased | 31 (33) | 20 (30) | 9 (19) | 33 (35) | <0.0001 |
Tested only among CRC and lung cancers
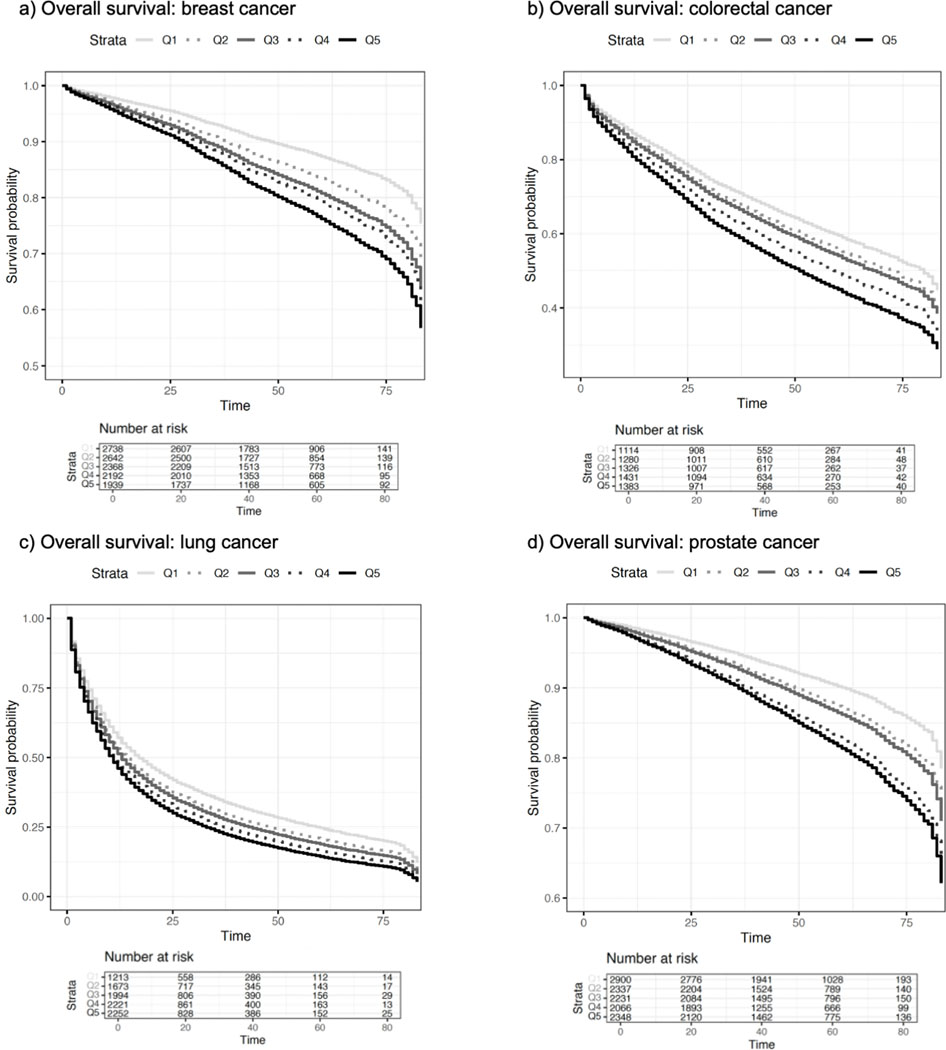
We first evaluated the relationship between ADI quintile and overall mortality (Table 2, Figure 1). Among all participants, higher ADI was associated with increased mortality for all four cancer sites, adjusting for race in the overall models, adjusting for age at diagnosis in prostate and colorectal cancer models, and including age at diagnosis as a strata variable in breast and lung cancer models. Notably, each increase in ADI quintile was associated with a 17% increase (Hazard Ratio (HR)=1.17, 95% Confidence Interval (CI) 1.13–1.21, p<0.0001) in mortality for women with breast cancer and a 16% increase (HR=1.16, 95% CI 1.12–1.21, p<0.0001) in mortality for men with prostate cancer. These associations remained when stratifying by race among breast (NHW: HR=1.16, 95% CI 1.11–1.20, p<0.0001; NHB: HR=1.20, 95% CI 1.11–1.28, p<0.0001) and prostate (NHW: HR=1.18, 95% CI 1.13–1.24, p<0.0001; NHB: HR=1.18, 95% CI 1.11–1.27, p<0.0001) cancer cases. ADI was also associated with increased overall mortality for colorectal (HR=1.11, 95% CI 1.08–1.14, p<0.0001) and lung (HR=1.07, 95% CI 1.05–1.09, p<0.0001) cancer cases. This association remained for both lung cancer cases (NHW: HR=1.06, 95% CI 1.04–1.09, p<0.0001; NHB: HR=1.07, 95% CI 1.03–1.12, p=0.00177) and colorectal cancer cases (NHW: HR=1.11, 95% CI 1.08–1.15, p<0.0001; NHB: HR=1.09, 95% CI 1.03–1.17, p=0.00378) when stratifying by race.
Table 2.
Overall mortality associations with ADI by cancer site and race
| All participants | Non-Hispanic White | Non-Hispanic Black | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | events | Per-quintile HR (95% CI) | p-value | n | events | Per-quintile HR (95% CI) | p-value | n | events | Per-quintile HR (95% CI) | p-value | |
| Breast cancer * | 11942 | 2325 | 1.17 (1.13–1.21) | <0.0001 | 8973 | 1603 | 1.16 (1.11–1.20) | <0.0001 | 2969 | 722 | 1.20 (1.11–1.28) | <0.0001 |
| Colorectal cancer ** | 6614 | 3002 | 1.11 (1.08–1.14) | <0.0001 | 4754 | 2098 | 1.11 (1.08–1.15) | <0.0001 | 1860 | 904 | 1.09 (1.03–1.17) | 0.00378 |
| Lung cancer * | 9694 | 7663 | 1.07 (1.05–1.09) | <0.0001 | 7295 | 5734 | 1.06 (1.04–1.09) | <0.0001 | 2399 | 1929 | 1.07 (1.03–1.12) | 0.00177 |
| Prostate cancer ** | 11882 | 1893 | 1.16 (1.12–1.21) | <0.0001 | 8191 | 1174 | 1.18 (1.13–1.24) | <0.0001 | 3691 | 719 | 1.18 (1.11–1.27) | <0.0001 |
Adjusted for race (overall model only), age at diagnosis included as strata variable
Adjusted for race (overall model only), adjusted for age at diagnosis
Figure 1. Adjusted overall survival curves stratified by cancer site and ADI quintile.
Survival curves adjusted for race and age at diagnosis are shown for (a) overall survival for breast cancer, (b) overall survival for colorectal cancer, (c) overall survival for lung cancer, and (d) overall survival for prostate cancer. Individual survival curves are show in each graph for each ADI quintile (Q): solid light gray for Q1, dashed light gray for Q2, solid dark gray for Q3, dashed dark gray for Q4, and solid black for Q5. Higher ADI quintile corresponds to poorer neighborhood factors.
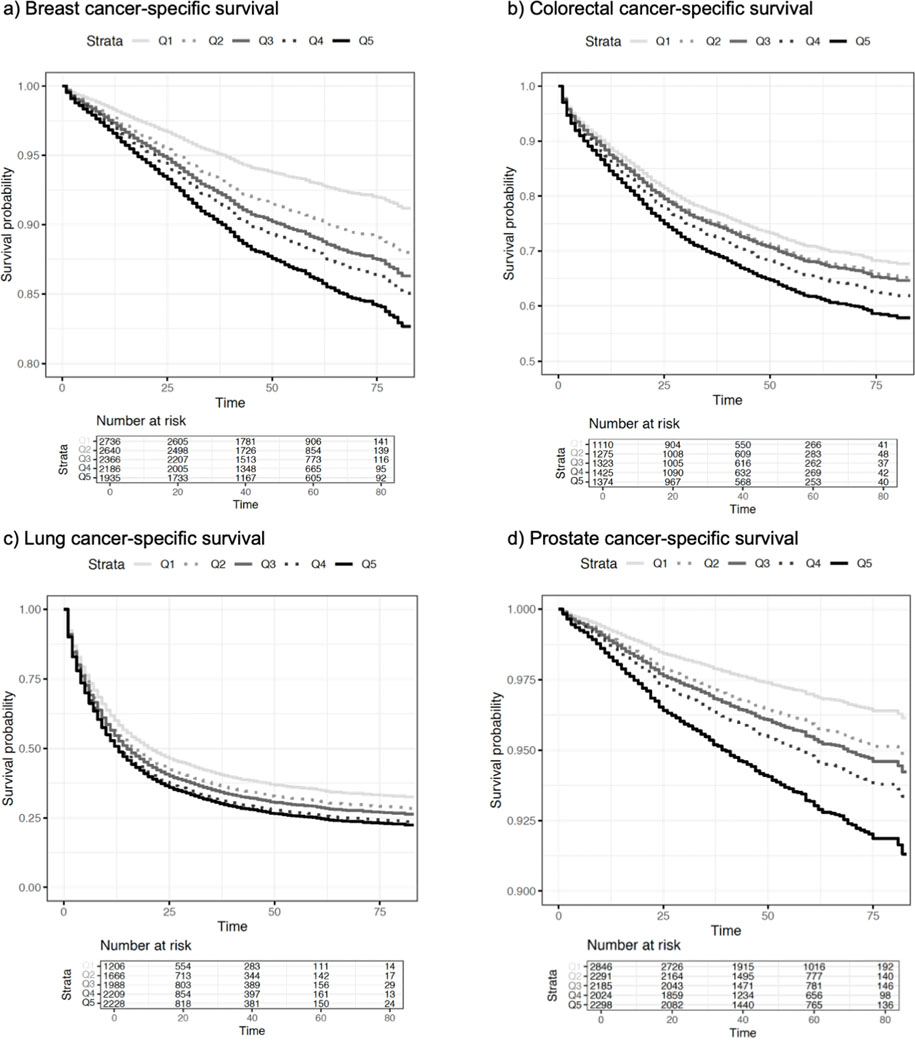
Patterns of association for ADI and cancer-specific mortality were similar to those observed in the overall mortality analyses (Table 3, Figure 2). ADI was associated with a 17% increase (HR=1.17, 95% CI 1.12–1.23, p<0.0001) in breast cancer-specific mortality, which was consistent for both NHW (HR=1.16, 95% CI 1.09–1.23, p<0.0001) and NHB women (HR=1.20, 95% CI 1.09–1.31, p=0.00013). ADI was associated with an 20% increase (HR=1.20, 95% CI 1.13–1.29, p<0.0001) in prostate cancer-specific mortality overall, where the association was stronger among NHB men (HR=1.31, 95% CI 1.15–1.49, p<0.0001) compared to NHW men (HR=1.16, 95% CI 1.07–1.27, p=0.00049). ADI associations with colorectal and lung cancer-specific mortality were also similar to overall mortality analyses, where ADI was associated with an 8% increase (95% CI 1.04–1.13, p=0.00026) in colorectal cancer-specific mortality only among NHW patients. Finally, ADI was associated with lung cancer-specific mortality overall (HR=1.06, 95% CI 1.04–1.08, p<0.0001), and the association was consistent in direction and strength among NHW (HR=1.06, 95% CI 1.04–1.08, p<0.0001) and NHB men (HR=1.07, 95% CI 1.02–1.12, p=0.00998).
Table 3.
Cancer-specific mortality associations with ADI by cancer site and race
| All participants | Non-Hispanic White | Non-Hispanic Black | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | events | Per-quintile HR (95% CI) | p-value | n | events | Per-quintile HR (95% CI) | p-value | n | events | Per-quintile HR (95% CI) | p-value | |
| Breast cancer * | 11926 | 1169 | 1.17 (1.12–1.23) | <0.0001 | 8965 | 754 | 1.16 (1.09–1.23) | <0.0001 | 2961 | 415 | 1.20 (1.09–1.31) | 0.00013 |
| Colorectal cancer ** | 6587 | 1928 | 1.08 (1.04–1.12) | <0.0001 | 4740 | 1314 | 1.08 (1.04–1.13) | 0.00026 | 1847 | 614 | 1.06 (0.99–1.14) | 0.114 |
| Lung cancer * | 9638 | 6290 | 1.06 (1.04–1.08) | <0.0001 | 7256 | 4741 | 1.06 (1.04–1.08) | <0.0001 | 2382 | 1549 | 1.07 (1.02–1.12) | 0.00998 |
| Prostate cancer ** | 11856 | 565 | 1.20 (1.13–1.29) | <0.0001 | 8176 | 344 | 1.16 (1.07–1.26) | 0.00049 | 3680 | 221 | 1.31 (1.15–1.49) | <0.0001 |
Adjusted for race (overall model only), age at diagnosis included as strata variable
Adjusted for race (overall model only), adjusted for age at diagnosis
Figure 2. Adjusted cancer-specific survival curves stratified by cancer site and ADI quintile.
Survival curves adjusted for race and age at diagnosis are shown for (a) breast cancer-specific survival, (b) colorectal cancer-specific survival, (c) lung cancer-specific survival, and (d) prostate cancer-specific survival. Individual survival curves are show in each graph for each ADI quintile (Q): solid light gray for Q1, dashed light gray for Q2, solid dark gray for Q3, dashed dark gray for Q4, and solid black for Q5. Higher ADI quintile corresponds to poorer neighborhood factors.
To understand the role of ADI in the relationship between race and cancer survival, we next evaluated whether ADI significantly attenuated the effect of race on overall and cancer-specific mortality (Table 4). NHB women with breast cancer had 66% higher mortality (HR=1.66, 95% CI 1.52–1.82, p<0.0001) compared to NHW women without adjustment for ADI. Similarly, NHB men were 69% more likely (HR=1.69, 95% CI 1.54–1.86, p<0.0001) to die from prostate cancer compared to NHW men without adjustment for ADI. NHB patients were also 28% more likely (HR=1.28, 95% CI 1.18–1.38, p<0.0001) to die from colorectal cancer and 12% more likely (HR=1.12, 95% CI 1.06–1.18 p<0.0001) to die from lung cancer comparted to NHW patients. Adjustment for ADI significantly attenuated the effects of race on overall mortality for all cancer types, except lung cancer. ADI attenuated the effect of race on overall mortality for breast cancer (proportion attenuated=47%, 95% CI 31–72%, p<0.0001), colorectal cancer (proportion attenuated=69%, 95% CI 20–445% p=0.032) and prostate cancer (proportion attenuated=51%, 95% CI 37–70%, p<0.0001).
Table 4.
Associations between race (NHB vs. NHW) and survival before and after adjustment for ADI stratified by cancer site
| Adjusted | Adjusted + ADI | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | Proportion mediated (95% CI) | p-value | |
|
| ||||||
| Overall survival | ||||||
| Breast* | 1.66 (1.52–1.82) | <0.0001 | 1.31 (1.18–1.45) | <0.0001 | 0.47 (0.31, 0.72) | <0.0001 |
| Colorectal** | 1.28 (1.18–1.38) | <0.0001 | 1.11 (1.01–1.21) | 0.025 | 0.69 (0.20, 4.45) | 0.032 |
| Lung* | 1.12 (1.06–1.18) | <0.0001 | 1.02 (0.96–1.08) | 0.51 | 0.25 (−0.04, 1.13) | 0.11 |
| Prostate** | 1.69 (1.54–1.86) | <0.0001 | 1.25 (1.12–1.40) | 0.00015 | 0.51 (0.37, 0.70) | <0.0001 |
| Cancer specific survival | ||||||
| Breast* | 1.89 (1.67–2.14) | <0.0001 | 1.50 (1.30–1.73) | <0.0001 | 0.37 (0.25, 0.53) | <0.0001 |
| Colorectal** | 1.31 (1.19–1.45) | <0.0001 | 1.19 (1.06–1.33) | 0.0029 | 0.36 (0.057, 1.16) | 0.018 |
| Lung* | 1.07 (1.01–1.14) | 0.026 | 0.98 (0.92–1.05) | 0.60 | 0.27 (−2.46, 2.84) | 0.30 |
| Prostate** | 1.75 (1.47–2.08) | <0.0001 | 1.25 (1.03–1.52) | 0.024 | 0.56 (0.34, 0.90) | <0.0001 |
Overall model adjusted for race, Age at diagnosis included as strata variable
Overall model adjusted for race, Adjusted for age at diagnosis
The effects of race on cancer-specific mortality were stronger than observed in the overall mortality analyses for all cancer types except lung cancer (Table 4). Cancer-specific mortality was 89% higher (HR=1.89, 95% CI 1.67–2.14, p<0.0001) for NHB women with breast cancer compared to NHW women. NHB men were 75% more likely (HR=1.75, 95% CI 1.47–2.08, p<0.0001) to die from prostate cancer compared to NHW men. NHB patients were 31% more likely (HR=1.31, 95% CI 1.19–1.45, p<0.0001) to die from colorectal cancer compared to NHW patients and 7% more likely (HR=1.07, CI 95% 1.01–1.14, p= 0.026) to die from lung cancer. Adjustment for ADI once again attenuated the effects of race on cancer-specific survival for breast cancer (proportion attenuated=37%, 95% CI 25% to 53%, p<0.0001), prostate cancer (proportion attenuated=56%, 95% CI 34% to 90%, p<0.0001), and colorectal cancer (proportion attenuated=36%, 95% CI 5.7–116%, p=0.018).
Discussion
The goal of this analysis was to evaluate the relationship between a neighborhood-level measure of social disadvantage and survival among NHB and NHW cancer patients within metropolitan Detroit. Overall, we found significant associations between area-level social disadvantage and both overall and cancer-specific survival across all four cancer sites. It is important to note that our observed per-quintile hazard ratios for the association between ADI and cancer mortality translate to relatively large effects when comparing individuals living in the highest ADI quintile to those in the lowest ADI quintile (ADI Q5 vs. Q1 HRs=1.26–2.07). This is particularly impactful considering that half (50.6%) of NHB residents of metropolitan Detroit reside in neighborhoods in the highest ADI quintile. In every cancer site and race stratification, all relationships regardless of significance level showed an increase in HR related to an increase in ADI quintile, as we have observed in the literature.
To the best of our knowledge, no other studies have directly estimated the effect of neighborhood deprivation in mediating the relationship between race and cancer survival. One study found that neighborhood deprivation mediates the relationship between race and stage at diagnosis for hepatocellular carcinoma(56). Another study utilizing Surveillance Epidemiology and End Results program data tested race as a mediator of the relationship of ADI on colorectal cancer, finding that race accounted for only a fifth of ADI’s indirect effect(57). Thus, our study is among the first to describe neighborhood deprivation as a substantial mediator of racial disparities in cancer survival. We saw that ADI attenuated overall mortality risk in breast, prostate, and colorectal cancers when adjusting for race, and attenuated risk of cancer-specific mortality in all three sites as well. Our mediation model of lung cancer showed an insignificant effect, which we feel may be due to the aggressiveness of the disease, lack of standardized screening, as well as differential smoking status and habits by our patients, a factor which was not captured in the model.
Racial and financial inequalities in access to high-quality health care are likely to play a role in our observed associations between ADI, race, and cancer mortality, and indeed, much of the existing literature on neighborhood features in cancer outcomes has highlighted the importance of access to high-quality health care(28). NHB patients are more likely to live in high-poverty areas than NHW patients(58), where those living in high-poverty areas are more likely to be diagnosed with advanced-stage disease(59). Stage at diagnosis is strongly linked to cancer treatment efficacy, access to care, and availability of treatments in the patient’s area due to transportation issues(60,61). A recent spatial analysis of colorectal cancer mortality in Virginia found that in addition to socioeconomic status and rurality, the density of primary care physicians (PCPs) was inversely associated with colorectal cancer mortality(62). Further, the strength of association between PCP density and mortality varied geographically, highlighting the importance of considering contextual factors such as rurality when evaluating care access.
Our findings are consistent with the existing literature demonstrating that factors such as area-level poverty, neighborhood disadvantage, residential segregation, rural/urban status, racial/ethnic composition of residents, commuting and traffic patterns, and residential mobility are associated with both stage at diagnosis and survival(16–18,45,63). We also observed substantial variability in the strength of effect of ADI and mortality by race and cause of death. This may be reflecting the fact that quantitative measures of neighborhood deprivation may capture distinct neighborhood features across racially segregated areas, and previous research has shown that the impact of neighborhood and specific neighborhood characteristics such as transportation access, poverty levels, prevalence of homeownership, and unemployment rates on cancer mortality often vary by race and/or ethnicity(21,64–66).
Our results are also consistent with existing studies comparing cancer mortality risk to area-level socioeconomic status by race. A study on breast cancer and socioeconomic status found that African American women with breast cancer were more likely to live in areas of lower neighborhood socioeconomic status (nSES) compared to non-Hispanic white women and both had increased risk of all-cause mortality as their nSES decreased(67). However, the cancer-specific mortality risk for decreased nSES was not as pronounced of a relationship for the non-Hispanic white women compared to the African American women, which is a trend we noticed in our study. Another study focused on prostate cancer found that decreased neighborhood quality increased the prostate cancer mortality risk for non-Hispanic white men, but the association was not as clear for African American men, our study also showed a weaker relationship for prostate cancer-specific mortality, but in both races studied(68). Our data on lung cancer showed similar trends but lower statistical significance than the literature, whereas other studies found significance in lung cancer mortality at all increases in socioeconomic deciles, where black participants saw a higher increase in risk from the least deprived to the second least deprived decile than their white counterparts(69). Lastly, similar results of colorectal cancer mortality associations were observed in the literature for white patients, but other studies have found a stronger relationship between socioeconomic deprivation and colorectal cancer mortality for African American patients than we observed, again likely partly due to a different breakdown in deprivation groups(69).
Finally, while neighborhood disadvantage may impact cancer survival through its influence on the health behaviors and health conditions of residents(70), there is consistent evidence not only that neighborhood effects are independent of individual-level characteristics but that the effects of neighborhood on cancer survival may be stronger than for many individual behaviors(50,71–73). A multilevel analysis of the California Collaborative Prostate Cancer Study using data from 1,800 prostate cancer cases found a joint effect between lower neighborhood SES and lower individual-level education on mortality, and further adjustment for behavioral, hospital, and food environment characteristics did not substantially dampen the effect of neighborhood SES(50). Similarly, a study of more than 6,000 breast cancer cases across 900 census block groups found that neighborhood disadvantage was significantly associated with both obesity and late-stage diagnosis, but that obesity did not have significant mediating or indirect effects on mortality itself(73). These findings suggest that investment in disadvantaged neighborhoods, beyond a focus on individual-level interventions alone, has the potential to substantially improve cancer outcomes and reduce health disparities.
There are several strengths to our study. Beginning with sample size, we were able to complete a large analysis including over 40,000 participants from the population-based Metropolitan Detroit Cancer Surveillance System (MDCSS), which increases the generalizability and validity of our results. Additionally, southeast Michigan is a racially diverse area which makes it an excellent population to study African American cancer survivorship compared to Non-Hispanic Whites with ample power. The results of this study can likely be generalized to other highly segregated metropolitan areas of the United States, such as New Orleans, Baton Rouge, Pine Bluff, Chicago, and Memphis, which also have large black populations with high poverty rates. Poverty rate is an important variable in the calculation of ADI, and we found that the Non-Hispanic Black patients skewed towards higher scores of deprivation in all cancer sites, compared to Non-Hispanic White patients who skewed towards scores of lower deprivation. We also utilized ADI, which is a validated measure used to quantify neighborhood deprivation and SES adversity that addresses the impact of social determinants such as material deprivation, healthcare access, and utility(74,75). It uses block group-level census data to provide a robust picture of small area-level variation in SES, which accurately describes the most accessible neighborhood resources to an individual. Additionally, ADI is a standardized measure used to capture social disadvantage. Thus, studies that also utilize ADI, especially in these areas of high segregation, would be directly comparable.
However, some limitations come with the use of small-area level measures like the ADI. Since ADI focuses on the current living situation, it does not consider the deprivation that occurs throughout the person’s lifetime. ADI does not measure other key factors that are important social determinants of health such as food insecurity, insurance coverage, access to transportation, distance to travel, language preference, health literacy and numeracy, cultural practices, and immigration status. However, despite the limitations, ADI is crucial to improving survival rates as it factors in social support, exposure to violence and fear, access to quality nutrition and transportation, environmental toxicant, and pollutant exposure to help identify individuals who are at high risk. Lastly, we did not study the effects of various health behaviors which could impact cancer outcomes, including comorbidities, dietary habits, stress, or other lifestyle factors. Though these are important factors to consider, likely, many of these factors are well captured in the ADI measure due to patient access to health-promoting resources. We will explore these factors related to ADI in later work to better understand the behavioral factors related to neighborhood quality.
In summary, ADI is related to increased risk for overall and cancer-specific mortality at four common cancer sites in a racially diverse population. In some cancer sites, ADI mediates the relationship between race and mortality risk, which can differ by race. These results highlight the importance of neighborhood impact on cancer outcomes. Understanding the relationship between race, ADI, and cancer survivorship can be used to improve patient outcomes. By understanding the needs of cancer patients, we can help create community-based outreach programs to assist in improving their survival.
Supplementary Material
Impact:
Understanding the role of neighborhood quality in cancer survivorship could improve community-based intervention practices.
Acknowledgements
K.S. Purrington, E.S. Stoffel, L.S. Rozek, E.S. Peters, and T.A. Hastert received funding from the National Cancer Institute (R01CA259420). A.G. Schwartz and J.J. Ruterbusch received funding from the National Institutes of Health Center Grant (P30CA022453) and a National Cancer Institute Health and Human Services contract (HHSN261201300011I).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest with this work.
References
- 1.Cancer Facts & Figures 2022. | American Cancer Society [Internet]. [cited 2022 Sep 27]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html
- 2.Moke DJ, Tsai K, Hamilton AS, Hwang A, Liu L, Freyer DR, et al. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019;3:pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5014–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards TB, Henley SJ, Puckett MC, Weir HK, Huang B, Tucker TC, et al. Lung Cancer Survival in the United States by Race and Stage (2001–2009): Findings From the CONCORD-2 Study. Cancer. 2017;123:5079–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele CB, Li J, Huang B, Weir HK. Prostate Cancer Survival in the United States by Race and Stage (2001–2009): Findings From the CONCORD-2 Study. Cancer. 2017;123:5160–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J-X, Gong Y, Ling H, Hu X, Shao Z-M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat. 2019;173:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Facts & Figures for African American/Black People [Internet]. [cited 2022 Sep 27]. Available from: https://www.cancer.org/research/cancer-facts-statistics/cancer-facts-figures-for-african-americans.html
- 8.Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, et al. Charting the Future of Cancer Health Disparities Research: A Position Statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. American Association for Cancer Research; 2017;77:4548–55. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham BA. Race: A Starting Place. AMA J Ethics. American Medical Association; 2014;16:472–8. [DOI] [PubMed] [Google Scholar]
- 10.Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, et al. Race and Genetic Ancestry in Medicine — A Time for Reckoning with Racism. N Engl J Med. Massachusetts Medical Society; 2021;384:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KK, Rishishwar L, Ban D, Nagar SD, Mariño-Ramírez L, McDonald JF, et al. Association of Genetic Ancestry and Molecular Signatures with Cancer Survival Disparities: A Pan-Cancer Analysis. Cancer Res. 2022;82:1222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Bemanian A, Beyer KMM. Housing Discrimination, Residential Racial Segregation, and Colorectal Cancer Survival in Southeastern Wisconsin. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2017;26:561–8. [DOI] [PubMed] [Google Scholar]
- 13.Beyer KMM, Zhou Y, Laud PW, McGinley EL, Yen TWF, Jankowski C, et al. Mortgage Lending Bias and Breast Cancer Survival Among Older Women in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39:2749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyer KMM, Laud PW, Zhou Y, Nattinger AB. Housing discrimination and racial cancer disparities among the 100 largest US metropolitan areas. Cancer. 2019;125:3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Most to Least Segregated Metro Regions | Othering & Belonging Institute [Internet]. [cited 2022 Apr 15]. Available from: https://belonging.berkeley.edu/most-least-segregated-metro-regions
- 16.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177:537–48. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin SS. Social determinants of colorectal cancer risk, stage, and survival: a systematic review. Int J Colorectal Dis. 2020;35:985–95. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020;8:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcelo A, Duffett-Leger L, Pastor-Valero M, Pereira J, Colugnati FAB, Trapido E. The role of education on Cancer amenable mortality among non-Hispanic blacks & non-Hispanic whites in the United States (1989–2018). BMC Cancer. 2021;21:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor JM, Sedghi T, Dhodapkar M, Kane MJ, Gross CP. Factors Associated With Cancer Disparities Among Low-, Medium-, and High-Income US Counties. JAMA Netw Open. 2018;1:e183146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol. Wolters Kluwer; 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr. 2014;2014:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29:1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabroff KR, Reeder-Hayes K, Zhao J, Halpern MT, Lopez AM, Bernal-Mizrachi L, et al. Health Insurance Coverage Disruptions and Cancer Care and Outcomes: Systematic Review of Published Research. JNCI J Natl Cancer Inst. 2020;112:671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M. Radiation Therapy Noncompliance and Clinical Outcomes in an Urban Academic Cancer Center. Int J Radiat Oncol Biol Phys. Elsevier; 2016;95:563–70. [DOI] [PubMed] [Google Scholar]
- 26.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. Hindawi; 2017;2017:e2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annual Report to the Nation 2021. [Internet]. SEER. [cited 2022 Sep 27]. Available from: https://seer.cancer.gov/report_to_nation/ [Google Scholar]
- 28.Obeng-Gyasi S, Obeng-Gyasi B, Tarver W. Breast Cancer Disparities and the Impact of Geography. Surg Oncol Clin N Am. 2022;31:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odoms-Young AM. Examining the Impact of Structural Racism on Food Insecurity: Implications for Addressing Racial/Ethnic Disparities. Fam Community Health. 2018;41:S3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Implicit Bias and Racial Disparities in Health Care [Internet]. [cited 2022 Apr 15]. Available from: https://www.americanbar.org/groups/crsj/publications/human_rights_magazine_home/the-state-of-healthcare-in-the-united-states/racial-disparities-in-health-care/
- 31.Race in Education [Internet]. Cent. Educ. Train. Employ. [cited 2022 Apr 15]. Available from: https://cete.osu.edu/initiatives/racial-equity-diversity-and-inclusion-redi-initiative/race-in-education/
- 32.Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL. Using Index of Concentration at the Extremes as Indicators of Structural Racism to Evaluate the Association with Preterm Birth and Infant Mortality—California, 2011–2012. J Urban Health. 2019;96:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace M, Crear-Perry J, Richardson L, Tarver M, Theall K. Separate and unequal: Structural racism and infant mortality in the US. Health Place. 2017;45:140–4. [DOI] [PubMed] [Google Scholar]
- 34.Ross CL, Leigh NG. Planning, Urban Revitalization, and the Inner City: An Exploration of Structural Racism. J Plan Lit. SAGE Publications Inc; 2000;14:367–80. [Google Scholar]
- 35.Baker RS, O’Connell HA. Structural racism, family structure, and Black–White inequality: The differential impact of the legacy of slavery on poverty among single mother and married parent households. J Marriage Fam [Internet]. [cited 2022 May 24];n/a. Available from: 10.1111/jomf.12837 [DOI] [Google Scholar]
- 36.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. [DOI] [PubMed] [Google Scholar]
- 37.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg KA, Dalton JE, Gunzler DD, Coulton CJ, Freedman DA, Krieger NI, et al. The ADI-3: a revised neighborhood risk index of the social determinants of health over time and place. Health Serv Outcomes Res Methodol. 2021;21:486–509. [Google Scholar]
- 39.Maroko AR. Integrating Social Determinants of Health With Treatment and Prevention: A New Tool to Assess Local Area Deprivation. Prev Chronic Dis [Internet]. 2016. [cited 2022 Jan 28];13. Available from: https://www.cdc.gov/pcd/issues/2016/16_0221.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairfield KM, Black AW, Ziller EC, Murray K, Lucas FL, Waterston LB, et al. Area Deprivation Index and Rurality in Relation to Lung Cancer Prevalence and Mortality in a Rural State. JNCI Cancer Spectr. 2020;4:pkaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Sundquist J, Zöller B, Sundquist K. Neighborhood Deprivation and Lung Cancer Incidence and Mortality: A Multilevel Analysis from Sweden. J Thorac Oncol. Elsevier; 2015;10:256–63. [DOI] [PubMed] [Google Scholar]
- 42.Riaz SP, Horton M, Kang J, Mak V, Lüchtenborg M, Møller H. Lung Cancer Incidence and Survival in England: An Analysis by Socioeconomic Deprivation and Urbanization. J Thorac Oncol. 2011;6:2005–10. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Sundquist J, Calling S, Zöller B, Sundquist K. Neighborhood deprivation and risk of cervical cancer morbidity and mortality: A multilevel analysis from Sweden. Gynecol Oncol. Elsevier; 2012;127:283–9. [DOI] [PubMed] [Google Scholar]
- 44.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–7. [DOI] [PubMed] [Google Scholar]
- 45.Shariff-Marco S, DeRouen MC, Yang J, Jain J, Nelson DO, Weden MM, et al. Neighborhood archetypes and breast cancer survival in California. Ann Epidemiol. 2021;57:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharyya O, Li Y, Fisher JL, Tsung A, Eskander MF, Hamad A, et al. Low neighborhood socioeconomic status is associated with higher mortality and increased surgery utilization among metastatic breast cancer patients. The Breast. 2021;59:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrijvers CTM, Mackenbach JP, Lutz J-M, Quinn MJ, Coleman MP. Deprivation and survival from breast cancer. Br J Cancer. Nature Publishing Group; 1995;72:738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.K CM, Oral E, Rung AL, Trapido EJ, Rozek LS, Fontham ETH, et al. Neighborhood deprivation and risk of mortality among men with prostate cancer: Findings from a long-term follow-up study. The Prostate. 2022;82:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Sundquist K, Sundquist J. Neighborhood deprivation and prostate cancer mortality: a multilevel analysis from Sweden. Prostate Cancer Prostatic Dis. Nature Publishing Group; 2012;15:128–34. [DOI] [PubMed] [Google Scholar]
- 50.DeRouen MC, Schupp CW, Koo J, Yang J, Hertz A, Shariff-Marco S, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol. 2018;53:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen L, Behrens G, Finke I, Maier W, Gerken M, Pritzkuleit R, et al. Area-Based Socioeconomic Inequalities in Colorectal Cancer Survival in Germany: Investigation Based on Population-Based Clinical Cancer Registration. Front Oncol [Internet]. 2020. [cited 2022 Sep 21];10. Available from: 10.3389/fonc.2020.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lian M, Schootman M, Doubeni CA, Park Y, Major JM, Torres Stone RA, et al. Geographic Variation in Colorectal Cancer Survival and the Role of Small-Area Socioeconomic Deprivation: A Multilevel Survival Analysis of the NIH-AARP Diet and Health Study Cohort. Am J Epidemiol. 2011;174:828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bureau UC. American Community Survey (ACS) [Internet]. Census.gov. [cited 2022 Jun 15]. Available from: https://www.census.gov/programs-surveys/acs [Google Scholar]
- 54.Kind AJH, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oluyomi AO, Mohammadi KA, El-Serag HB, Thrift AP. Mediating Effects of Neighborhood-Level Socioeconomic Deprivation on the Association Between Race/Ethnicity and Advanced Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2022;31:1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu K-X, Yuan W-J, Huang C-H, Xiao L, Xiao R-S, Zeng P-W, et al. Socioeconomic deprivation and survival outcomes in patients with colorectal cancer. Am J Cancer Res. 2022;12:829–38. [PMC free article] [PubMed] [Google Scholar]
- 58.factsheet-erm.pdf [Internet]. [cited 2022 Mar 27]. Available from: https://www.apa.org/pi/ses/resources/publications/factsheet-erm.pdf
- 59.Association of Census-Tract Poverty Level and Early Stage Cancer Diagnosis in Wisconsin, 2012–2016 - Data Bulletin from the Wisconsin Cancer Reporting System (WCRS). :4. [Google Scholar]
- 60.Jiang C, Deng L, Wang Q, Perimbeti S, Han X. Transportation barriers to health care and mortality risk among the U.S. adults with history of cancer. J Clin Oncol. Wolters Kluwer; 2021;39:121–121. [Google Scholar]
- 61.Jiang C, Yabroff KR, Deng L, Wang Q, Perimbeti S, Shapiro CL, et al. Self-reported Transportation Barriers to Health Care Among US Cancer Survivors. JAMA Oncol [Internet]. 2022. [cited 2022 Apr 14]; Available from: 10.1001/jamaoncol.2022.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thatcher EJ, Camacho F, Anderson RT, Li L, Cohn WF, DeGuzman PB, et al. Spatial analysis of colorectal cancer outcomes and socioeconomic factors in Virginia. BMC Public Health. 2021;21:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeRouen MC, Yang J, Jain J, Weden MM, Gomez SL, Shariff-Marco S. Disparities in Prostate Cancer Survival According to Neighborhood Archetypes, A Population-Based Study. Urology. 2021;S0090–4295(21)00674–9. [DOI] [PubMed] [Google Scholar]
- 64.Lynch SM, Handorf E, Sorice KA, Blackman E, Bealin L, Giri VN, et al. The effect of neighborhood social environment on prostate cancer development in black and white men at high risk for prostate cancer. PloS One. 2020;15:e0237332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao L, Ladabaum U, Gomez SL, Cheng I. Colorectal cancer mortality among Hispanics in California: differences by neighborhood socioeconomic status and nativity. Cancer. 2014;120:3510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shariff-Marco S, Yang J, John EM, Kurian AW, Cheng I, Leung R, et al. Intersection of Race/Ethnicity and Socioeconomic Status in Mortality After Breast Cancer. J Community Health. 2015;40:1287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, et al. Impact of Neighborhood and Individual Socioeconomic Status on Survival after Breast Cancer Varies by Race/Ethnicity: The Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2014;23:793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. 2009;20:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I—All Cancers and Lung Cancer and Part II—Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardman R, Begg S, Spelten E. What impact do chronic disease self-management support interventions have on health inequity gaps related to socioeconomic status: a systematic review. BMC Health Serv Res. 2020;20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng E, Soulos PR, Irwin ML, Cespedes Feliciano EM, Presley CJ, Fuchs CS, et al. Neighborhood and Individual Socioeconomic Disadvantage and Survival Among Patients With Nonmetastatic Common Cancers. JAMA Netw Open. 2021;4:e2139593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiese D, Stroup AM, Crosbie A, Lynch SM, Henry KA. The Impact of Neighborhood Economic and Racial Inequalities on the Spatial Variation of Breast Cancer Survival in New Jersey. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2019;28:1958–67. [DOI] [PubMed] [Google Scholar]
- 73.DeGuzman PB, Cohn WF, Camacho F, Edwards BL, Sturz VN, Schroen AT. Impact of Urban Neighborhood Disadvantage on Late Stage Breast Cancer Diagnosis in Virginia. J Urban Health Bull N Y Acad Med. 2017;94:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mora J, Krepline AN, Aldakkak M, Christians KK, George B, Hall WA, et al. Adjuvant therapy rates and overall survival in patients with localized pancreatic cancer from high Area Deprivation Index neighborhoods. Am J Surg. Elsevier; 2021;222:10–7. [DOI] [PubMed] [Google Scholar]
- 75.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an Area Deprivation Index Measuring Patient Socio-economic Status in an Integrated Health System: Implications for Population Health. EGEMs Gener Evid Methods Improve Patient Outcomes. Ubiquity Press; 2016;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.