Abstract
Objectives
To identify incident SARS-CoV-2 infections and inform effective mitigation strategies in university settings, we piloted an integrated symptom and exposure monitoring and testing system among a cohort of university students and employees.
Design
Prospective cohort study.
Setting
A public university in California from June to August 2020.
Participants
2180 university students and 738 university employees.
Primary outcome measures
At baseline and endline, we tested participants for active SARS-CoV-2 infection via quantitative PCR (qPCR) test and collected blood samples for antibody testing. Participants received notifications to complete additional qPCR tests throughout the study if they reported symptoms or exposures in daily surveys or were selected for surveillance testing. Viral whole genome sequencing was performed on positive qPCR samples, and phylogenetic trees were constructed with these genomes and external genomes.
Results
Over the study period, 57 students (2.6%) and 3 employees (0.4%) were diagnosed with SARS-CoV-2 infection via qPCR test. Phylogenetic analyses revealed that a super-spreader event among undergraduates in congregate housing accounted for at least 48% of cases among study participants but did not spread beyond campus. Test positivity was higher among participants who self-reported symptoms (incidence rate ratio (IRR) 12.7; 95% CI 7.4 to 21.8) or had household exposures (IRR 10.3; 95% CI 4.8 to 22.0) that triggered notifications to test. Most (91%) participants with newly identified antibodies at endline had been diagnosed with incident infection via qPCR test during the study.
Conclusions
Our findings suggest that integrated monitoring systems can successfully identify and link at-risk students to SARS-CoV-2 testing. As the study took place before the evolution of highly transmissible variants and widespread availability of vaccines and rapid antigen tests, further research is necessary to adapt and evaluate similar systems in the present context.
Keywords: COVID-19, EPIDEMIOLOGY, Public health, Infection control, Epidemiology, PUBLIC HEALTH
Strengths and limitations of this study.
The study is strengthened by rich longitudinal data including more than 117 000 daily symptom surveys, 17 000 weekly exposure surveys, 7600 quantitative PCR tests to detect active SARS-CoV-2 infection and 4900 antibody tests to detect previous infection collected from 2918 university students and employees over 3 months.
We used seroconversion data from serial antibody tests and phylogenetic analyses comparing campus viral genome sequences to a broader set of regional genomes to evaluate the extent to which the study system identified incident cases and contained an outbreak among university students.
Our identification of participants who seroconverted between baseline and endline may be incomplete due to loss to follow-up and imperfect sensitivity of SARS-CoV-2 antibody testing.
A high proportion of identified cases were traced to one outbreak, limiting the generalisability of our exploratory assessment of risk factors for incident infection.
Background
Universities have been identified as hotspots for SARS-CoV-2 transmission in the USA,1 where SARS-CoV-2 incidence is highest among young adults.2 Young adults may be less likely to adhere to social distancing guidelines and more likely to experience workplace exposure (eg, at food service or retail jobs).2 Their risk may be heightened in university settings where many live in congregate housing, interact with wide social networks, or attend large gatherings.3 Although young adults are at low risk of serious acute illness or death from COVID-19 (the disease caused by SARS-CoV-2),4 the higher likelihood of asymptomatic or mildly symptomatic infection in this age group makes young adults a key population through which SARS-CoV-2 may spread to other, more vulnerable groups.2 5 Indeed, there is evidence that transmission among university students may lead to increased COVID-19-related mortality in the surrounding counties.6–8 Although widespread vaccination has enabled campuses to return to in-person activities, the elimination of SARS-CoV-2 transmission in campus populations may be stymied by vaccine hesitancy among students and employees and breakthrough infection and subsequent transmission by vaccinated persons, particularly in the context of waning immunity and viral variants that reduce vaccine efficacy.9 10 Therefore, rapid and resource-efficient identification of incident cases in university populations is a critical first step of outbreak investigation and control, followed by isolation, case investigation and contact tracing, to minimise transmission within campus and to the broader community.
Universities have adopted a wide range of approaches for testing and outbreak mitigation.11–13 While a number of well-resourced universities have scaled up testing capacity in order to frequently test all students and employees accessing campus or living in university-affiliated housing,13 many other universities do not have well-defined testing strategies or restrict testing to those with symptoms or known exposure.12 Beyond investing in testing programmes, some universities have sought to reduce on-campus transmission by mandating the completion of self-administered symptom screening tools by students and employees. However, such tools have primarily been used to regulate daily access to campus (ie, deny entry to those who report COVID-19-like symptoms), rather than to detect emergent outbreaks among university populations. As universities resume normal operations and discontinue mitigation strategies such as masking, non-punitive, resource-efficient strategies that can both identify those who are at highest risk of infection and expediently link them to low-barrier testing services may play a key role in transitioning from a ‘one-size-fits-all’ approach of uniform testing to a sustainable monitoring paradigm.
In 2020, we piloted an integrated symptom and exposure monitoring and testing system designed to identify incident SARS-CoV-2 infections among a cohort of university students and employees.14 Here, we describe the incidence and seroprevalence of SARS-CoV-2 infection within this cohort to evaluate the extent to which incident infections were successfully detected and contained over the study period, identify sociodemographic factors associated with incident infection, and ascertain which self-reported symptoms and exposures tracked by the monitoring system were predictive of test positivity, with the ultimate objective of informing monitoring and testing strategies in university settings.
Methods
Study design and setting
The study comprised three prospective cohorts of University of California (UC), Berkeley affiliates followed from June to August 2020: students, essential workers (ie, employees working on campus in health, facilities or student services) and other employees (hereafter, ‘faculty/staff’). We report the findings according to the Strengthening the Reporting of Observational Studies in Epidemiology checklist for cohort studies.15
Throughout the study period, public health orders mandated the use of face coverings in public and upheld many restrictions set forth by earlier shelter-in-place orders, while allowing phased reopening of certain businesses and activities.16 UC Berkeley did not offer in-person classes, and on-campus work was restricted to essential workers and a small subset of faculty, staff and student researchers. Although few students were living in on-campus residence halls, many students continued to live in congregate living settings off campus, such as fraternities, sororities and cooperative housing. From June to August 2020, daily case counts in Alameda County ranged from approximately 50 to 350 (0 to 17 within the city of Berkeley).17
Participant recruitment and eligibility
The study was promoted through targeted messages from university officials to campus email listservs and social media platforms from early June to mid-July 2020. To increase reach to students expected to be at higher risk of COVID-19, we also placed flyers in congregate living settings and conducted in-person recruitment for student athletes who had resumed training on campus. Participants were eligible to enrol in the study if they were at least 18 years of age, were a current student or employee at UC Berkeley and planned to live in or near Berkeley during the summer of 2020. Specific eligibility criteria and enrolment windows varied by cohort (online supplemental table 1, online supplemental figure 1).
bmjopen-2022-063999supp001.pdf (568.1KB, pdf)
On enrolment, participants were linked to an online baseline survey that collected sociodemographic data and information about their COVID-19-related health history. Participants were then referred to a baseline testing appointment at University Health Services (UHS) which included a SARS-CoV-2 quantitative PCR (qPCR) test and blood collection for antibody testing (procedures described below). To facilitate daily temperature monitoring, study staff also provided participants with free oral thermometers on request at testing appointments. Participants who completed this appointment or a non-study qPCR test at UHS by July 20 were eligible to remain in the study. We prespecified a maximum sample size of 4000 participants across cohorts but did not reach this limit before the final day of baseline data collection.
Symptom and exposure surveys
Participants received daily text messages or emails (depending on their preference specified in the baseline survey) that linked to short symptom surveys through which they reported their body temperature and any symptoms of illness. Once per week, the daily survey included a longer exposure module, which asked about recent symptoms of illness among their household member(s), potential exposure(s) to COVID-19 and activities related to potential COVID-19 risk. All surveys were administered via REDCap.18 19
Endline survey and testing
In early August, participants were sent an endline survey which collected updated information on their COVID-19 history to identify any diagnoses outside of the study. Participants in the student and essential worker cohorts were also invited to complete endline testing appointments by August 18, including a final qPCR test and blood collection.
qPCR testing
Midturbinate nasal and oral swabs were collected by UHS clinical staff and tested for SARS-CoV-2 by qPCR at the Innovative Genomics Institute (IGI).20 qPCR tests were performed at baseline for all three cohorts and at endline for the student and essential worker cohorts. Between baseline and endline testing, additional qPCR tests were performed for the following reasons:
Symptom-based or exposure-based tests triggered based on participants’ responses in daily surveys: Participants who reported COVID-19-like signs or symptoms[1] (in themselves or household member(s)) or who reported a suspected or confirmed COVID-19 case in their household were automatically notified to sign up for a qPCR test.
Random surveillance testing: A subset of participants in the student and faculty/staff cohorts who had not had a qPCR test within a week were randomly selected and emailed notifications to come in for surveillance testing in July.
Address-based surveillance testing: Participants who lived at the same address as another participant who tested positive for SARS-CoV-2 were immediately emailed surveillance testing notifications. Following an outbreak among group-housed students in early July, surveillance testing notifications were also emailed to all participants who had not been tested within the week and who reported living in fraternities, sororities or cooperative housing.
Participant-initiated testing: Participants could self-schedule study testing appointments on demand, with or without consulting a healthcare provider and regardless of exposure history.
Participants with positive qPCR test results were informed by phone by UHS clinical staff, who provided guidance on isolation and performed case investigation to identify potential contacts. Participants with negative qPCR test results were informed of their results via the UHS online patient portal.
SARS-CoV-2 sequencing and phylogenetic analyses
Viral whole genome sequencing was performed on a set of positive samples at the IGI, using previously described procedures.21 Briefly, SARS-CoV-2 RNA extracted from swabs was reverse transcribed using SuperScript IV (Invitrogen), and the viral genome was amplified from the resulting cDNA in four separate qPCR reactions using distinct primer sets tiling the SARS-CoV-2 genome. The four qPCR reactions were pooled 1:1:1:1 and diluted 1:50 in H2O. A second qPCR reaction was set up to add Nextera Unique Dual Indexing sequences to either end of the amplicons. The resulting qPCR reaction was cleaned up using 0.7x AMPureXP beads (Beckman Coulter) and quantified using a Qubit dsDNA HS Assay Kit (Thermo Fisher). The libraries were then pooled to an equimolar ratio and sequenced with a 10% PhiX spike in using a MiSeq V.3 kit at 300 bp PE reads.
Fastq sequencing files were processed through a custom pipeline using publicly available software. The reads were preprocessed by quality trimming, removing adaptors, and PhiX cleaning with BBTools,22 and then aligned to the Wuhan reference sequence (NC_045512.2) with minimap2 V.2.16-r922. ARTICv3 primers were trimmed, and the consensus sequence was built with iVar V.1.3.1, where an ‘N’ is called if the depth is less than 10 reads at any nucleotide. The genomes were then processed through the Nextstrain Auger pipeline with other genomes from GISAID to construct a maximum likelihood tree.23 24 Several phylogenies were constructed for this analysis: a tree of 7091 genomes subsampled from the worldwide genomes in GISAID at the time (approximately 200 000 genomes as of October 2020) was used to place the IGI genomes in the larger tree; a tree with all IGI genomes sequenced at the time of analysis (356 genomes); and a tree containing 500 genomes (from 1 million genomes as of April 2021) was constructed using UShER.25
Antibody testing
Up to 10 mL of blood was collected by phlebotomists via venipuncture at baseline from participants in all three cohorts and again at endline from participants in the student and essential worker cohorts. Blood was centrifuged and serum was stored at −20°C for 2–4 months before being tested at Vitalant Research Institute using the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack, which detects IgA, IgG and IgM antibodies against the SARS-CoV-2 spike protein S1 antigen and has an estimated clinical specificity of 100% and unreported sensitivity.26
Participant compensation
Participants in the student cohort received a US$50 gift card after completing baseline testing and 10 daily surveys; this incentive was conditional on daily survey completion to encourage early habit formation.27 Student participants received a second US$50 gift card at their endline testing appointment. To facilitate travel to and from UHS for testing appointments, student participants were also offered prepaid car rides via a ride-sharing app.
Participants in the essential worker cohort received a gift card worth US$1 per daily survey completed (to a maximum of US$70) after the study ended. Participants in the faculty/staff cohort were not compensated.
Statistical analyses
To identify sociodemographic factors associated with incident infection, we used Poisson regression to estimate unadjusted incidence rate ratios (IRRs) for SARS-CoV-2 infection by study cohort and within strata of sociodemographic variables self-reported in the baseline survey (eg, age, gender, housing type), setting person-months of enrolment as an offset term to account for differing lengths of follow-up.
We also calculated IRRs comparing test positivity by recent signs/symptoms, exposures and activities reported in the daily and weekly surveys. We estimated IRRs for several temperature thresholds (ie, ≥38.0°C [100.4°F], ≥37.8°C [100.0°F], ≥37.2°C [99.0°F]) to compare to symptom-specific IRRs; however, continuous associations between temperature and positivity have been previously explored in this cohort, finding that temperature screening has low sensitivity to SARS-CoV-2 infection and, thus, limited efficacy as a primary means of detection.28 While it was not possible to isolate participants’ specific reason(s) for testing over the study period (eg, participants could receive symptom-triggered and/or exposure-triggered testing notifications over the same time window in which they completed baseline or endline testing), we linked qPCR test results to recently completed symptom and exposure surveys to identify testing appointments that took place in the days or weeks following symptom-triggered and exposure-triggered testing notifications (online supplemental figure 2). We accounted for clustered observations due to repeated tests per participant using a generalised estimating equation approach with Huber-White SE estimates and an exchangeable working correlation structure.29
Finally, to assess the extent to which the testing and monitoring system captured incident infections, we identified participants who seroconverted from having non-reactive (no antibodies detected) to reactive (antibodies detected) blood samples between baseline and endline and calculated the proportion of these participants who were also diagnosed with incident SARS-CoV-2 infection via positive qPCR test during the study period. Analyses were conducted in R V.4.2.1.30
Patient and public involvement
The study’s target population comprised university students and employees. While the study was conducted by faculty, staff and graduate students from the UC Berkeley School of Public Health, UHS and the IGI, the broader student body and university workforce were not involved in designing the study or selecting the research question, outcome measures or method of disseminating results.
Results
Participant recruitment and retention
Between 1 June 2020 and 20 July 2020, we enrolled 2180 students, 268 essential workers and 470 faculty/staff who completed at least one qPCR test or antibody test (table 1, online supplemental figure 1). The student cohort was split between undergraduate (51%) and graduate (48%) students. Nearly half (48%) of essential workers worked in health services. While 85% of essential workers were working on campus at the time of enrolment, most (78%) faculty/staff were working entirely remotely. At the time of enrolment, only 12 (0.4%) participants reported a previous COVID-19 diagnosis.
Table 1.
Baseline characteristics of participants in the Berkeley COVID-19 Safe Campus Initiative by study cohort, June–August 2020
| All | Students | Essential workers | Faculty/staff | |
| N (row %) | 2918 (100) | 2180 (74.7) | 268 (9.2) | 470 (16.1) |
| Age, mean±SD | 29.4±11.6 | 24.3±5.4 | 42.5±12.3 | 45.2±12.3 |
| Gender, n (column %) | ||||
| Man | 1177 (40.3) | 911 (41.8) | 103 (38.4) | 163 (34.7) |
| Woman | 1653 (56.6) | 1187 (54.4) | 164 (61.2) | 302 (64.3) |
| Non-binary/other | 51 (1.7) | 46 (2.1) | 1 (0.4) | 4 (0.9) |
| Race/ethnicity, n (column %)* | ||||
| American Indian/Alaska Native | 39 (1.3) | 29 (1.3) | 2 (0.7) | 8 (1.7) |
| Asian/Pacific Islander | 833 (28.5) | 703 (32.2) | 66 (24.6) | 64 (13.6) |
| Black/African American | 103 (3.5) | 83 (3.8) | 16 (6.0) | 4 (0.9) |
| Hispanic/Latine/Spanish origin | 402 (13.8) | 328 (15.0) | 39 (14.6) | 35 (7.4) |
| White | 1814 (62.2) | 1261 (57.8) | 160 (59.7) | 393 (83.6) |
| Other | 280 (9.6) | 223 (10.2) | 31 (11.6) | 26 (5.5) |
| Programme level, n (column %) | ||||
| Undergraduate | – | 1114 (51.1) | – | – |
| Graduate | – | 1039 (47.7) | – | – |
| Living at fraternity/sorority, n (column %) | – | 125 (5.7%) | – | – |
| Education, n (column %) | ||||
| High school diploma/GED | – | – | 6 (2.2) | 0 (0) |
| Some college or trade school | – | – | 59 (22.0) | 13 (2.8) |
| Bachelor’s degree | – | – | 78 (29.1) | 119 (25.3) |
| Graduate/professional degree | – | – | 121 (45.1) | 337 (71.7) |
| Department, n (column %) | ||||
| Health services | – | – | 129 (48.1) | – |
| Facilities/building services | – | – | 61 (22.8) | – |
| Student services/other | – | – | 77 (28.7) | – |
| Job title, n (column %) | ||||
| Faculty | – | – | – | 110 (23.4) |
| Staff | – | – | – | 311 (66.2) |
| Postdoctoral scholar/other | – | – | – | 49 (10.4) |
| Currently working outside the home, n (column %) | 748 (25.6) | 418 (19.2) | 228 (85.1) | 102 (21.7) |
| Pre-enrolment COVID-19 diagnosis, n (column %) | 12 (0.4) | 8 (0.4) | 1 (0.4) | 3 (0.6) |
Missing responses: n=37 gender, n=126 race/ethnicity (non-Hispanic categories), n=49 race/ethnicity (Hispanic category), n=27 programme level, n=41 fraternity/sorority, n=5 education, n=1 department, n=71 working outside home, n=43 pre-enrolment diagnosis.
*Categories not mutually exclusive.
GED, General Education Development (high school equivalency) test.
Participants provided a total of 5545 person-months of follow-up from enrolment to the end of the study (mean person-days per participant: 57, range: 32–78). Participants completed a mean of 40 daily symptom surveys and 6 weekly exposure surveys over the study period, for a total of 117 239 symptom and 17 162 exposure surveys. A subset of participants did not complete any daily symptom surveys (1.7%) or weekly exposure surveys (4.2%).
SARS-CoV-2 incidence
During the study period, participants underwent 7638 qPCR tests for active SARS-CoV-2 infection, with a mean of 2.6 tests per participant (range: 0–9). Almost all (99.9%) participants completed at least one qPCR test. Overall, 60 participants (2.1%) tested positive: 57 students, 2 essential workers and 1 faculty/staff.
Among cohorts, students were at highest risk of incident infection over the study period (IRR students vs faculty/staff: 5.8; 95% CI 1.3 to 103.0). Due to the low number of cases outside of the student cohort, we examined additional risk factors for infections among students only (table 2), finding higher rates of infection among students who were 18–19 years old (IRR vs students ≥22 years: 8.3; 95% CI 4.2 to 17.5) and undergraduates (IRR vs graduate students: 4.1; 95% CI 2.2 to 8.7). We also observed a higher incidence among white students (IRR: 3.3 vs non-white students; 95% CI 1.7 to 7.2). These associations were largely driven by an outbreak among participants living in fraternities or sororities. Nearly one-quarter of participants living in fraternities or sororities were infected with SARS-CoV-2 during the study period (IRR vs other students: 20.9; 95% CI 12.3 to 35.5) and these participants accounted for 49% of cases observed among student participants.
Table 2.
Bivariate associations between sociodemographic characteristics and SARS-CoV-2 incidence among student participants in the Safe Campus Initiative, June–August 2020
| Cases, N (row %) | Non-cases, N (row %) | IRR (95% CI) | |
| Overall* | 57 (2.6) | 2120 (97.4) | – |
| Age | |||
| 18–19 years | 21 (8.0) | 243 (92.0) | 8.3 (4.2 to 17.5) |
| 20–21 years | 24 (3.8) | 607 (96.2) | 4.1 (2.1 to 8.6) |
| ≥22 years | 12 (0.9) | 1270 (99.1) | Reference |
| Gender | |||
| Woman | 37 (3.1) | 1147 (96.9) | 1.5 (0.8 to 2.6) |
| Man | 19 (2.1) | 892 (97.9) | Reference |
| Non-binary/other | 0 (0) | 46 (100) | – |
| Race/ethnicity† | |||
| American Indian/Alaska Native | 0 (0) | 29 (100) | – |
| Asian/Pacific Islander | 11 (1.6) | 691 (98.4) | 0.5 (0.2 to 0.9) |
| Black/African American | 1 (1.2) | 82 (98.8) | 0.4 (0.0 to 2.0) |
| Hispanic/Latine/Spanish origin | 7 (2.1) | 320 (97.9) | 0.8 (0.3 to 1.7) |
| White | 45 (3.6) | 1216 (96.4) | 3.3 (1.7 to 7.2) |
| Other | 4 (1.8) | 217 (98.2) | 0.6 (0.2 to 1.6) |
| Programme level | |||
| Undergraduate | 46 (4.1) | 1067 (95.9) | 4.1 (2.2 to 8.7) |
| Graduate | 10 (1.0) | 1027 (99.0) | Reference |
| Living at fraternity/sorority | 28 (22.4) | 97 (77.6) | 20.9 (12.3 to 35.5) |
| Currently working outside the home | 6 (1.4) | 410 (98.6) | 0.5 (0.2 to 1.1) |
*N=2177 students with at least one qPCR test for SARS-CoV-2 during the study period.
†Not mutually exclusive; all participants not included in specified racial/ethnic category served as reference for each comparison.
IRR, incidence rate ratio; qPCR, quantitative PCR.
Phylogenetic analysis
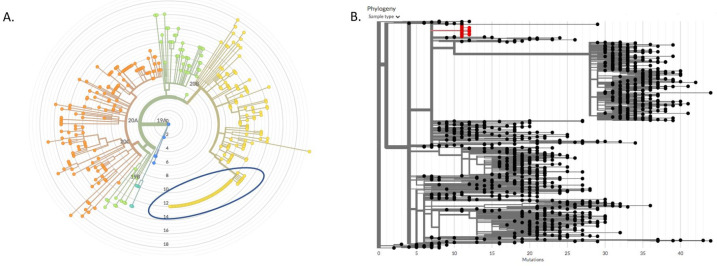
We retrieved whole viral genome sequences for 35 of the 60 positive samples from this study, 29 (83% of cases with sequenced samples, 48% of all cases among study participants) of which were found to be part of a campus super-spreader event involving a total of 57 campus-affiliated individuals with samples sequenced by IGI (figure 1A). Most (69%) study participants within this cluster lived at one of two residences, with likely a single participant originating the super-spreader event. The cluster of genomes was defined by three mutations (A6360G, C24502A and G110083T), two of which were extremely rare at the time of the outbreak. The combination of the three variants was only found in four genomes outside of this cluster (two in the UK and two in Florida) by October 2020, making it a strong phylogenetic signature.
Figure 1.
Phylogeny of outbreak-associated strain of SARS-CoV-2 among participants in the Safe Campus Initiative. A. A maximum likelihood phylogeny constructed from 357 genomes sequenced by the Innovative Genomics Institute between May and July 2020 constructed using Nextstrain. Branch lengths represent divergence from Wuhan reference genome at center. Blue circle marks cluster of identical genomes from a campus super-spreader event. B. A 1,057 node subtree of a neighbor-joining tree constructed with all SARS-CoV-2 sequences to date (constructed using UShER with over 1 million genomes in April 2021), showing the most similar genomes to the super-spreader event cluster (in red). There are no descendant branches from the cluster, demonstrating that the outbreak was contained and the lineage died out.
Phylogenetic analysis demonstrated that the cluster remained confined to campus, as this signature was not observed in any genomes from samples in the surrounding communities or California state in the months following the super-spreader event. When the trio of mutations was searched in a phylogeny constructed from over 1.2 million genomes worldwide using UShER in April 2021,25 no descendent leaves were found in the tree under the cluster (figure 1B), indicating that the lineage died out after the super-spreader event.
Factors associated with test positivity
At least one symptom survey was completed in the 7 days before sample collection for 88% of tests (n=6668), including 72% of tests (n=5465) that had symptom data from the day of sample collection. Of the 52 cases who completed at least one survey during the week before their positive sample was collected (mean: 4 surveys), 23 cases (44%) had reported at least 1 of the 10 COVID-19 signs/symptoms that triggered a notification for them to test. Test positivity was 12.7 times higher among participants who had a recent symptom-triggered notification (95% CI 7.4 to 21.8) (table 3). Notification-triggering symptoms most strongly associated with test positivity included loss of sense of taste or smell and feeling feverish. Weakness, sweats or chills, and swollen glands were the non-triggering symptoms most strongly associated with test positivity.
Table 3.
Bivariate associations between prospectively monitored symptoms and exposures and SARS-CoV-2 qPCR test positivity among participants in the Safe Campus Initiative, June–August 2020
| Test positivity, % (+ tests/all tests) | IRR (95% CI) | |
| Overall* | 0.8 (60/7615) | – |
| Signs/symptoms within 7 days of test | ||
| No | 0.3 (18/5489) | Reference† |
| Yes (any) | 2.9 (34/1179) | 8.8 (5.0, 15.5) |
| Temperature ≥38.0°C‡ | 0.0 (0/10) | 0.0 (0.0, 0.0) |
| Temperature ≥37.8°C | 10.5 (2/19) | 13.2 (3.4, 50.9) |
| Temperature ≥37.2°C | 2.9 (12/417) | 4.3 (2.2, 8.2) |
| Feeling feverish‡ | 15.3 (11/72) | 24.6 (13.2, 45.8) |
| Dry cough‡ | 5.6 (7/126) | 8.1 (3.7, 17.7) |
| Coughing up mucus‡ | 5.5 (5/91) | 7.7 (3.1, 19.0) |
| Unusual chest pain or pressure‡ | 9.7 (6/62) | 13.9 (6.1, 31.6) |
| Difficulty breathing‡ | 5.6 (1/18) | 7.2 (1.0, 49.8) |
| Shortness of breath‡ | 8.9 (4/45) | 12.3 (4.6, 32.9) |
| Unexplained trouble thinking/concentrating‡ | 7.6 (5/66) | 10.7 (4.4, 26.1) |
| Loss of sense of taste‡ | 42.9 (3/7) | 58.3 (23.7, 143.4) |
| Loss of sense of smell‡ | 33.3 (4/12) | 46.2 (19.2, 111.4) |
| Any notification-triggering symptom‡ | 5.9 (23/393) | 12.7 (7.4, 21.8) |
| Loss of appetite | 10.3 (6/58) | 14.8 (6.5, 33.9) |
| Fatigue | 3.5 (13/371) | 5.7 (3.0, 10.6) |
| Trouble sleeping | 5.1 (7/136) | 7.5 (3.4, 16.4) |
| Headache | 4.7 (14/300) | 7.8 (4.3, 14.3) |
| Runny, blocked or painful sinuses | 5.2 (14/267) | 8.8 (4.8, 16.2) |
| Sneezing | 1.9 (2/106) | 2.5 (0.6, 10.1) |
| Swollen, red or painful eyes | 8.6 (5/58) | 12.1 (4.9, 30.1) |
| Sore throat | 3.1 (8/258) | 4.5 (2.1, 9.5) |
| Stomach pain | 5.8 (5/86) | 8.1 (3.3, 20.1) |
| Diarrhoea | 4.9 (4/82) | 6.7 (2.5, 18.2) |
| Nausea or vomiting | 3.3 (3/90) | 4.5 (1.4, 14.2) |
| Body aches or muscle pain | 8.2 (12/146) | 13.4 (7.2, 25.2) |
| Sweats or chills | 11.5 (10/87) | 18.0 (9.2, 35.2) |
| Swollen glands | 12.2 (5/41) | 17.3 (7.2, 41.2) |
| Weakness | 13.5 (10/74) | 21.2 (11.0, 40.9) |
| Exposures within 14 days before test | ||
| No | 0.3 (15/4319) | Reference† |
| Yes (any) | 3.4 (17/506) | 9.6 (4.8, 19.2) |
| Suspected or confirmed COVID-19 case in household‡ | 7.4 (7/95) | 13.9 (6.1, 31.8) |
| Close contact with suspected or confirmed case outside household | 3.5 (5/144) | 6.0 (2.3, 15.4) |
| Household member with new COVID-19-like symptoms‡ | 4.4 (5/114) | 7.6 (3.0, 19.6) |
| Household member with any new symptoms of illness | 2.6 (9/347) | 5.0 (2.3, 10.8) |
| Any notification-triggering exposure‡ | 5.1 (9/177) | 10.3 (4.8, 22.0) |
| Activities within 14 days before test | ||
| No | 0.4 (3/678) | Reference† |
| Yes (any) | 0.7 (29/4145) | 1.6 (0.5, 5.1) |
| Spent time at another residence | 1.1 (26/2327) | 4.6 (1.9, 11.3) |
| Had visitors at own residence | 1.0 (22/2205) | 2.6 (1.2, 5.5) |
| Attended gathering of >10 people | 2.8 (19/672) | 9.0 (4.5, 18.1) |
| Worked outside the home | 0.5 (10/2152) | 0.6 (0.3, 1.2) |
| Used public restroom | 0.7 (12/1833) | 1.0 (0.5, 2.0) |
| Used public transportation | 0.6 (4/703) | 0.8 (0.3, 2.4) |
| Participated in group sports | 1.6 (4/257) | 2.5 (0.9, 7.2) |
*Excluding resamples, same-day retests and repeated positives; includes N=2914 participants with at least one qPCR test for SARS-CoV-2 during the study period.
†Reference group for ‘yes (any)’ comparisons; reference groups for specific symptoms/exposures/activities were those who did not report that symptom/exposure/activity.
‡Reporting triggered notification to test.
IRR, incidence rate ratio; qPCR, quantitative PCR.
Participants completed at least one weekly exposure survey in the 14 days before sample collection for 63% of tests (n=4825). Of the 32 cases who had recently completed an exposure survey at the time of sample collection, 9 (28%) reported a potential household exposure that triggered a notification for them to test (table 3). Test positivity was 10.3 times higher among participants who had a recent exposure-triggered notification (95% CI 4.8 to 22.0). Test positivity was also significantly higher among participants who reported recent engagement in ‘higher risk’ social activities, most notably attending a gathering of more than 10 people (IRR 9.0; 95% CI 4.5 to 18.1).
SARS-CoV-2 seroprevalence
Only 18 (0.6%) of 2877 participants who provided blood samples at baseline had SARS-CoV-2 antibodies (table 4), all but one of them students. Most participants with antibodies at baseline either suspected past infection (22%), had been previously diagnosed (22%) or had a positive qPCR test the day blood was drawn (11%). Most (85%) participants in the student and essential worker cohorts provided blood samples at both baseline and endline (mean interval between samples: 48 days). Among 2076 participants with baseline and endline blood samples, 33 (1.6%) seroconverted from non-reactive at baseline to reactive at endline, 30 of whom (91%) were also diagnosed via qPCR test during the study. Of the three participants who seroconverted without a positive qPCR test, two self-reported suspected past infection (one before baseline, one during the study period), while the third did not suspect past infection and had four negative qPCR tests over 40 days of study participation.
Table 4.
Seroprevalence of SARS-CoV-2 antibodies among participants in the Safe Campus Initiative, June–August 2020
| Baseline, N (%) | Endline, N (%) | Both, N (%) | |
| Serostatus—cross-sectional* | |||
| Reactive | 18 (0.6) | 48 (2.3) | – |
| Non-reactive | 2859 (99.4) | 2039 (97.7) | – |
| Serostatus—longitudinal† | |||
| Non-reactive → non-reactive | – | – | 2029 (97.7) |
| Non-reactive → reactive | – | – | 33 (1.6) |
| Reactive → non-reactive | – | – | 0 (0) |
| Reactive → reactive | – | – | 14 (0.7) |
| Serostatus—previous qPCR positive‡ | |||
| Reactive | – | 34 (82.9) | – |
| Non-reactive | – | 7 (17.1) | – |
*N=2888 participants who provided at least one blood sample.
†N=2076 participants who provided blood samples at baseline and endline.
‡N=41 participants who provided an endline blood sample ≥7 days after SARS-CoV-2 infection identified via positive qPCR test.
qPCR, quantitative PCR.
Of the 60 participants with incident SARS-CoV-2 infection during the study period, 41 (68%) provided an endline blood sample at least 1 week after the date of their first positive qPCR test (mean time between positive qPCR test and blood sample: 36 days; range 13–52 days). Of these, 34 (83%) were reactive (table 4).
Discussion
This study provides a model of a voluntary, incentivised system to identify and link at-risk students to SARS-CoV-2 testing. While the incidence and seroprevalence of SARS-CoV-2 were generally low in this cohort of university students and employees in the summer of 2020, we observed the highest incidence among undergraduate students living in congregate settings, with nearly half of cases found to be associated with a super-spreader event.
At the time of the study, many infection control strategies centred on symptomatic testing, reducing the likelihood of identifying asymptomatic, mildly symptomatic and presymptomatic infections. Our approach sought to integrate symptom-based monitoring with exposure monitoring, random surveillance testing and targeted surveillance testing in the context of an outbreak. Within this cohort, we previously demonstrated the acceptability of our low-barrier SARS-CoV-2 mitigation approach and the limitations of temperature monitoring as a tool for case identification.14 28 The present analysis builds on these contributions by triangulating prospective qPCR testing data with phylogenetic analyses of positive samples and serial antibody testing to evaluate whether case identification and containment were achieved. In doing so, we found evidence that the system successfully identified a high proportion of incident SARS-CoV-2 cases among participants and may have mitigated community transmission after an outbreak. Specifically, 91% of participants with newly-identified antibodies for SARS-CoV-2 at the end of the study had also been diagnosed with incident infection via qPCR test during the study period. While a sizeable cluster of cases among participants was traced to a single super-spreader event, the associated cluster lineage was successfully contained without spreading beyond campus. As the outbreak unfolded, the system also allowed for rapid real-time response (ie, surveillance testing notifications to students living in congregate housing) and offered a readily accessible, incentivised entry point for testing for students concerned about potential exposure.
Although some universities have adopted punitive measures intended to prevent transmission by controlling student behaviour (eg, suspending students for hosting gatherings),31–33 this approach has been criticised for its potential to reduce students’ trust and cooperation.34–36 Instead of punishing or shaming students who fail to adhere to public health guidance, some epidemiologists have called for a harm-reduction approach that supports and engages students as part of the solution.34–36 This study reinforces the potential to integrate voluntary testing and risk monitoring systems to support targeted case identification, as evidenced by the significantly higher positivity rates found among participants whose self-reported symptoms and exposures triggered notifications to test. Our findings also support increased outreach to groups of students at highest risk, particularly younger students in congregate housing.
This study is strengthened by rich longitudinal data, including symptom and exposure tracking, qPCR testing, and seroprevalence data from more than 2000 participants. The study population comprised a broad sample of university affiliates, both students and employees, with strong representation of university subpopulations perceived to be at higher risk of infection (eg, undergraduates, essential healthcare workers). As on-campus activities were severely restricted throughout the study period (all classes were held online, and few students were living in residence halls), this study cannot provide insight into SARS-CoV-2 transmission risks related to on-campus student activities. Nevertheless, as 73% of UC Berkeley undergraduate students lived off campus before the pandemic,37 systems to detect off-campus (ie, community and household) transmission remain important for SARS-CoV-2 monitoring efforts among students. Additionally, all participants in the essential workers cohort and a subset of participants in the faculty/cohort were working on campus during the study period, further motivating efforts to monitor incidence in this population.
There remain several limitations. We observed relatively few SARS-CoV-2 cases during the study period. Accordingly, although many associations are statistically significant, our estimates are imprecise (ie, have wide CIs) and must be interpreted with caution. This study took place before the development of highly transmissible variants, such as Delta and Omicron, and before vaccine roll-out. Observed associations between symptoms and positivity may also differ among those who have been infected by more recent variants and/or vaccinated. Further research is necessary to adapt and evaluate similar systems in the context of both heightened transmissibility and more prevalent natural and vaccine-induced immunity. Additionally, a high proportion of identified cases were traced to one outbreak, limiting the generalisability of our exploratory assessment of risk factors for incident infection. There was also anecdotal evidence that the outbreak prompted exposed students to enrol as study participants.14 While this self-referral into the study is likely to increase selection bias, it also illustrates the utility of implementing non-stigmatising, incentivised testing approaches to increase testing uptake among at-risk students. Finally, our identification of participants who seroconverted between baseline and endline may be incomplete due to loss to follow-up and imperfect sensitivity of SARS-CoV-2 antibody testing.
By integrating symptom and exposure monitoring systems with low-barrier testing, we identified incident SARS-CoV-2 infections to reduce transmission within a university setting. Our study contributes to a growing body of literature on novel, integrated SARS-CoV-2 surveillance strategies in university settings.38–44 While there have been seismic shifts in the SARS-CoV-2 pandemic since 2020, universities continue to grapple with how best to mitigate on-campus spread in the face of emerging variants, incomplete vaccination coverage, breakthrough infections and decreased reliance on other mitigation strategies (eg, masking, remote learning).45 46 In light of universities’ resource constraints and persistently high case counts, incentivised approaches may not be feasible or sustainable in many settings. Thus, further research is needed to identify and test non-monetary incentives and other behavioural nudge strategies that encourage students and other campus community members to actively participate in public health efforts to combat the pandemic. The lessons learnt through this study may inform the design of future adaptive strategies, ideally building beyond symptom/exposure monitoring and qPCR testing to integrate complementary interventions such as rapid antigen self-testing and vaccination promotion.
Supplementary Material
Acknowledgments
We are grateful for the contributions of an exceptional team of graduate student researchers (Mariah De Zuzuarregui, Darren Frank, Sarah Gomez-Aladino, Ariel Muñoz, Ruben Prado, Lawrence Tello, Emily Wang, and Sabrina Williamson) and our collaborators at UC Berkeley’s University Health Services (including but not limited to: Judith Sansone, Melody Heller, Holly Stern, Tyler Crooks, Desi Gallardo, Jeff Kreutzen, Rebecca Stephenson, Lisa Polley, and Melissa Hennings), the Innovative Genomics Institute (including but not limited to: Fyodor Urnov, Shana McDevitt, Ariana Hirsch, Alexander Ehrenberg, Jennifer A Doudna, and the other members of the IGI SARS-CoV-2 testing consortium: M Amen, Kerrie W Barry, John M Boyle, Cara E Brook, Seunga Choo, L T Cornmesser, David J Dilworth, Indro Fedrigo, Skyler E Friedline, Thomas G W Graham, Ralph Green, Jennifer R Hamilton, Megan L Hochstrasser, Dirk Hockemeyer, Netravathi Krishnappa, Azra Lari, Hanqin Li, Enrique Lin-Shiao, Tianlin Lu, Elijah F Lyons, Kevin G Mark, Lisa Argento Martell, A Raquel O Martins, Patrick S Mitchell, Erica A Moehle, Christine Naca, Divya Nandakumar, Elizabeth O’Brien, Derek J Pappas, Kathleen Pestal, Diana L Quach, Benjamin E Rubin, Rohan Sachdeva, Elizabeth C Stahl, Abdullah Muhammad Syed, I-Li Tan, Amy L Tollner, Connor A Tsuchida, C Kimberly Tsui, Timothy K Turkalo, M Bryan Warf, Oscar N Whitney, and Lea B Witkowsky), and Vitalant Research Institute (including but not limited to: Mars Stone, Chloe Thorbrogger, Alice Lee, and Heather Tanner). The author would also like to thank Sandra McCoy, Stefano Bertozzi, and Lauren Ralph for their feedback on this manuscript.
Twitter: @ShelleyFacente
Collaborators: The IGI SARS-CoV-2 testing consortium (Fyodor Urnov, Shana McDevitt, Ariana Hirsch, Alexander Ehrenberg, Jennifer A Doudna; M Amen, Kerrie W Barry, John M Boyle, Cara E Brook, Seunga Choo, L T Cornmesser, David J Dilworth, Indro Fedrigo, Skyler E Friedline, Thomas G W Graham, Ralph Green, Jennifer R Hamilton, Megan L Hochstrasser, Dirk Hockemeyer, Netravathi Krishnappa, Azra Lari, Hanqin Li, Enrique Lin-Shiao, Tianlin Lu, Elijah F Lyons, Kevin G Mark, Lisa Argento Martell, A Raquel O Martins, Patrick S Mitchell, Erica A Moehle, Christine Naca, Divya Nandakumar, Elizabeth O’Brien, Derek J Pappas, Kathleen Pestal, Diana L Quach, Benjamin E Rubin, Rohan Sachdeva, Elizabeth C Stahl, Abdullah Muhammad Syed, I-Li Tan, Amy L Tollner, Connor A Tsuchida, C Kimberly Tsui, Timothy K Turkalo, M Bryan Warf, Oscar N Whitney, and Lea B Witkowsky)
Contributors: LAH performed statistical analyses and wrote the first draft of the manuscript. SW performed phylogenomic analyses and prepared associated figures and paragraphs. ALR and MLP designed the study and provided input on the manuscript. LJP, SNF, AH, GN, the IGI SARS-CoV-2 Testing Consortium, CDG and MPB provided feedback on the study design and manuscript. YL assisted with data analyses. LAH is responsible for the overall content as the guarantor.
Funding: The study was funded by private donors who had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Competing interests: Vitalant Research Institute, of which Dr. Michael Busch is Director, receives research funding and free assay kits from Ortho Clinical Diagnostics. Dr. Busch does not receive salary support or personal compensation from Ortho Clinical Diagnostics. The remaining authors declare no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Signs or symptoms that triggered a testing notification when reported were: temperature of ≥38.0°C (100.4°F), dry cough (without mucus), coughing up mucus, feeling feverish, unusual pain or pressure in the chest, difficulty breathing, shortness of breath, unexplained trouble thinking or concentrating, loss of sense of taste or loss of sense of smell.
Contributor Information
Collaborators: the IGI SARS-CoV-2 Testing Consortium, Fyodor Urnov, Shana McDevitt, Ariana Hirsch, Alexander Ehrenberg, Jennifer A Doudna, M Amen, Kerrie W Barry, John M Boyle, Cara E Brook, Seunga Choo, LT Cornmesser, David J Dilworth, Indro Fedrigo, Skyler E Friedline, Thomas G W Graham, Ralph Green, Jennifer R Hamilton, Megan L Hochstrasser, Dirk Hockemeyer, Netravathi Krishnappa, Azra Lari, Hanqin Li, Enrique Lin-Shiao, Tianlin Lu, Elijah F Lyons, Kevin G Mark, Lisa Argento Martell, A Raquel O Martins, Patrick S Mitchell, Erica A Moehle, Christine Naca, Divya Nandakumar, Elizabeth O’Brien, Derek J Pappas, Kathleen Pestal, Diana L Quach, Benjamin E Rubin, Rohan Sachdeva, Elizabeth C Stahl, Abdullah Muhammad Syed, I-Li Tan, Amy L Tollner, Connor A Tsuchida, C Kimberly Tsui, Timothy K Turkalo, M Bryan Warf, Oscar N Whitney, and Lea B Witkowsky
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All study activities were approved by the University of California, Berkeley Committee for the Protection of Human Subjects (#2020-06-13349, #2020-05-13261, #2020-04-13238). Participants gave informed consent to participate in the study before taking part.
References
- 1. Tracking the coronavirus at U.S. colleges and universities. The New York Times 2020. Available: https://www.nytimes.com/interactive/2020/us/covid-college-cases-tracker.html [Google Scholar]
- 2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1404–9. 10.15585/mmwr.mm6939e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvatore PP, Sula E, Coyle JP, et al. Recent increase in COVID-19 cases reported among adults aged 18-22 years - United States, May 31-September 5, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1419–24. 10.15585/mmwr.mm6939e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossen LM, Branum AM, Ahmad FB, et al. Excess deaths associated with COVID-19, by age and race and ethnicity - United States, January 26-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1522–7. 10.15585/mmwr.mm6942e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A 2020;117:17513–5. 10.1073/pnas.2008373117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ivory D, Gebeloff R, Mervosh S. Young people have less Covid-19 risk, but in college towns, deaths rose fast. The New York Times 2020. Available: https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html [Google Scholar]
- 7. Andersen MS, Bento AI, Basu A, et al. College openings, mobility, and the incidence of COVID-19 cases. MedRxiv 2020:2020.09.22.20196048. 10.1101/2020.09.22.20196048 [DOI] [Google Scholar]
- 8. Leidner AJ, Barry V, Bowen VB, et al. Opening of large institutions of higher education and county-level COVID-19 incidence - United States, July 6-September 17, 2020. MMWR Morb Mortal Wkly Rep 2021;70:14–9. 10.15585/mmwr.mm7001a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255–63. 10.15585/mmwr.mm7107e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasmin F, Najeeb H, Moeed A, et al. COVID-19 vaccine hesitancy in the United States: a systematic review. Front Public Health 2021;9:770985.:770985. 10.3389/fpubh.2021.770985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booeshaghi AS, Tan F, Renton B, et al. Markedly heterogeneous COVID-19 testing plans among US colleges and universities. medRxiv [Preprint] 2020. 10.1101/2020.08.09.20171223 [DOI]
- 12. Nadworny E, McMinn S. Even in COVID-19 hot spots, many colleges aren’t aggressively testing students. NPR 2020. Available: https://www.npr.org/2020/10/06/919159473/even-in-covid-hot-spots-many-colleges-arent-aggressively-testing-students [Google Scholar]
- 13. Schultes O, Clarke V, Paltiel AD, et al. COVID-19 testing and case rates and social contact among residential college students in Connecticut during the 2020-2021 academic year. JAMA Netw Open 2021;4:e2140602. 10.1001/jamanetworkopen.2021.40602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Packel L, Reingold A, Hunter L, et al. Piloting an integrated SARS-CoV-2 testing and data system for outbreak containment among college students: a prospective cohort study. PLOS ONE 2021;16:e0245765. 10.1371/journal.pone.0245765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007;18:800–4. 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 16. Health Officer Order Documents . City of Berkeley. 2020. Available: https://berkeleyca.gov/safety-health/covid-19/covid-19-restrictions/health-officer-order-documents
- 17. COVID 19 daily cases and deaths. Alco Data Portal 2023. Available: https://data.acgov.org/datasets/f6cabaab66ea43a585bfd83e0a3bcc63_0/ [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) -- a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208.:S1532-0464(19)30126-1. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. IGI Testing Consortium . Blueprint for a pop-up SARS-CoV-2 testing lab. Nat Biotechnol 2020;38:791–7. 10.1038/s41587-020-0583-3 [DOI] [PubMed] [Google Scholar]
- 21. Gohl DM, Garbe J, Grady P, et al. A rapid, cost-effective tailed amplicon method for sequencing SARS-CoV-2. BMC Genomics 2020;21:863. 10.1186/s12864-020-07283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bushnell B. BBMap. 2021. Available: https://sourceforge.net/projects/bbmap
- 23. Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018;34:4121–3. 10.1093/bioinformatics/bty407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill 2017;22:30494. 10.2807/1560-7917.ES.2017.22.13.30494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turakhia Y, Thornlow B, Hinrichs AS, et al. Ultrafast sample placement on existing trees (UShER) empowers real-time phylogenetics for the SARS-CoV-2 pandemic. BioRxiv 2020:2020.09.26.314971. 10.1101/2020.09.26.314971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortho Clinical Diagnostics . Instructions for use: VITROS immunodiagnostic products anti-SARS-CoV-2 total reagant pack, VITROS immunodiagnostic products anti-SARS-CoV-2 total calibrator. 2020. Available: https://www.fda.gov/media/136967/download [Accessed 7 Jan 2021].
- 27. Mantzari E, Vogt F, Shemilt I, et al. Personal financial incentives for changing habitual health-related behaviors: a systematic review and meta-analysis. Prev Med 2015;75:75–85.:S0091-7435(15)00072-9. 10.1016/j.ypmed.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Facente SN, Hunter LA, Packel LJ, et al. Feasibility and effectiveness of daily temperature screening to detect COVID-19 in a prospective cohort at a large public university. BMC Public Health 2021;21:1693. 10.1186/s12889-021-11697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 30. R Core Team . R: A language and environment for statistical computing. 2021. Available: https://www.R-project.org/
- 31. Gross EL. Ohio State University suspends 228 students for violating Covid-19 guidelines. Forbes 2020. Available: https://www.forbes.com/sites/elanagross/2020/08/24/ohio-state-university-suspends-228-students-for-violating-covid-19-guidelines/ [Google Scholar]
- 32. Giuliani-Hoffman F. Tulane students warned of suspension or expulsion for partying in groups larger than 15. CNN 2020. Available: https://www.cnn.com/2020/07/09/us/tulane-students-group-gathering-coronavirus-trnd/index.html [Google Scholar]
- 33. Treisman R. More than 200 Ohio State University students suspended for violating pandemic rules. NPR 2020. Available: https://www.npr.org/sections/coronavirus-live-updates/2020/08/25/906039378/more-than-200-ohio-state-university-students-suspended-for-violating-pandemic-ru [Google Scholar]
- 34. Marcus J, Gold J. Colleges are getting ready to blame their students. The Atlantic 2020. Available: https://www.theatlantic.com/ideas/archive/2020/07/colleges-are-getting-ready-blame-their-students/614410/ [Google Scholar]
- 35. Marcus J. Quarantine fatigue is real. The Atantic 2020. Available: https://www.theatlantic.com/ideas/archive/2020/05/quarantine-fatigue-real-and-shaming-people-wont-help/611482/ [Google Scholar]
- 36. Marcus J, Baral S, et al . University leaders should take a more humane approach to students during COVID-19 (opinion). Inside Higher Ed 2020. Available: https://www.insidehighered.com/views/2020/10/15/university-leaders-should-take-more-humane-approach-students-during-covid-19 [Google Scholar]
- 37. University of California, Berkeley . UC Berkeley Office of Undergraduate Admissions. Housing | UC Berkeley. 2021. Available: https://admissions.berkeley.edu/housing
- 38. Petros BA, Paull JS, Tomkins-Tinch CH, et al. Multimodal surveillance of SARS-CoV-2 at a university enables development of a robust outbreak response framework. Med (N Y) 2022;3:883–900.S2666-6340(22)00404-4. 10.1016/j.medj.2022.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karthikeyan S, Nguyen A, McDonald D, et al. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. MSystems 2021;6:e00793-21. 10.1128/mSystems.00793-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Currie DW, Moreno GK, Delahoy MJ, et al. Interventions to disrupt coronavirus disease transmission at a university, Wisconsin, USA, August-October 2020. Emerg Infect Dis 2021;27:2776–85. 10.3201/eid2711.211306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pollock BH, Kilpatrick AM, Eisenman DP, et al. Safe reopening of college campuses during COVID-19: the University of California experience in fall 2020. PLOS ONE 2021;16:e0258738. 10.1371/journal.pone.0258738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Towers SA, Gemechu NB, Nagaraj NC, et al. SARS-CoV-2 surveillance and outbreak response on an urban American college campus. J Am Coll Health 2022:1–9. 10.1080/07448481.2022.2034834 [DOI] [PubMed] [Google Scholar]
- 43. McDonnell KA, Vyas A, Castel A, et al. Keeping it close: the role of a campus COVID support team (CCST) in sustaining a safe and healthy university campus during COVID-19. Public and Global Health [Preprint] 2022. 10.1101/2022.05.01.22274540 [DOI]
- 44. Meredith GR, Diel DG, Frazier PI, et al. Routine surveillance and vaccination on a university campus during the spread of the SARS-CoV-2 Omicron variant. JAMA Netw Open 2022;5:e2212906. 10.1001/jamanetworkopen.2022.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorin J, Gardner A. Colleges let students go unmasked even with Covid bearing down. Bloomberg 2021. Available: https://www.bloomberg.com/news/articles/2021-11-23/colleges-drop-indoor-mask-rules-despite-holiday-covid-threat [Google Scholar]
- 46. Saul S, Hartocollis A. Some colleges loosen rules for a virus that won’t go away. The New York Times 2022. Available: https://www.nytimes.com/2022/01/16/us/politics/colleges-covid-coronavirus.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063999supp001.pdf (568.1KB, pdf)
Data Availability Statement
Data are available on reasonable request.