Abstract
The main objective of this work was to explore the association of dietary phytate intake with bone mineral density (BMD) in a Mediterranean population of postmenopausal women. For this purpose, a cross-sectional analysis of 561 women aged 55–75 years with overweight/obesity and metabolic syndrome from a Mediterranean area and with data on dual-energy X-ray absorptiometry (DXA) scans in femur and lumbar spine was performed. Estimated phytate intake was calculated using a validated food frequency questionnaire. Our results indicated that phytate intake was associated with BMD [β(95%CI) per each 25 mg/100 kcal] in femoral neck [0.023(0.060–0.040) g/cm2], femoral Ward’s triangle [0.033(0.013–0.054) g/cm2], total femur [0.018(0.001–0.035) g/cm2], and all the analyzed lumbar spine sites [L1–L4: 0.033(0.007–0.059) g/cm2] after adjusting for potential confounders. The sensitivity analysis showed that phytate intake was directly associated with lumbar spine BMD in women younger than 66 years, with a body mass index higher than 32.6 kg/cm2 and without type 2 diabetes (all p-for interactions < 0.05). The overall results indicated that phytate, a substance present in food as cereals, legumes and nuts, was positively associated with BMD in Mediterranean postmenopausal women. Phytate may have a protective effect on bone resorption by adsorbing on the surfaces of HAP. Nevertheless, large, long-term, and randomized prospective clinical studies must be performed to assess the possible benefits of phytate consumption on BMD in postmenopausal women.
Keywords: phytate, bone mineral density, DXA, postmenopausal women
1. Introduction
Osteoporosis is a bone disease that develops when bone mineral density (BMD) and bone mass decrease, or when the quality and/or structure of bone changes. This can lead to a decrease in bone strength that can increase the risk of fractures, among the major public health concerns in the elderly population [1,2]. Several risk factors seem to play a role in osteoporosis development. The amount of bone mass accumulated from childhood to early adulthood is among the most important predictors of osteoporosis risk later in life [3]. Nutrition and physical activity are the most important modifiable factors with a well-known effect on bone maintenance and mass loss [4].
Postmenopausal women are at increased risk of osteoporosis because the drop of estrogen during this period leads to more bone resorption than formation, which can result in osteoporosis [5]. It is widely accepted that a balanced diet helps maintain bone health [6,7] and is important for the prevention of osteoporosis in postmenopausal women. Several studies have shown that certain nutrients, especially vitamin D and calcium, are associated with BMD [5,6,7,8]. Additional research has evaluated the relationship between bone density and other nutrients, such as essential fatty acids and vitamins [9]. A high dietary glycemic index has also been reported as a risk factor of osteoporotic fractures [10]. In addition, some studies have shown that the lower incidence of osteoporosis in some Mediterranean countries may be related to their healthy diet [11,12,13]. The Mediterranean diet is characterized by high consumption of grains, legumes, vegetables, fruits, nuts, and olive oil. The useful results of a number of its components (along with vegetables, fish, and fruits) on BMD had been formerly studied [11,12,13,14,15].
Myo-inositol hexakisphosphate calcium magnesium salt or phytin is a salt of phytic acid, an inositol ring with six phosphate groups, that acts as a reservoir for phosphorus in seeds and plant germination [16]. Phytate is a natural compound that is consumed in significant amounts (in a range of 1–2 g/day) by people on a diet rich in legumes, whole grains, and nuts. Some authors have suggested that phytate exhibits effects similar to those of bisphosphonates on bone resorption [17,18,19,20,21]. In a study with female ovariectomized rats [17], BMD values were significantly higher in both femoral bones and L4 vertebra for phytate-treated rats in comparison to rats in the non-phytate group. Phytate has been correlated with bone mass in postmenopausal subjects in a small cohort study [18,19,20]. Additionally, it has been demonstrated that phytate can act as an osteoclastogenesis inhibitor in cell and tissue studies [21]. Another study has indicated that the phytate effect on decalcification process seems to resemble that of bisphosphonates, similar to alendronate and greater than etidronate [22]. In this sense, phytate may have positive effects on bone health, with a mechanism of action on bone resorption similar to that exhibited by bisphosphonates. It could be explained, almost in part, because they have a high affinity to bind onto the calcium of hydroxyapatite crystals by chemisorption, hindering both crystallization and redissolution. Additionally, a recent in vitro study indicates that phytate can inhibit or disturb the decalcification process by adsorbing on the surfaces of hydroxyapatite (HAP) crystal and the consequent inhibition of HAP dissolution [22] and osteoclast activity [21]. Nevertheless, larger studies are needed to confirm these findings.
Our hypothesis is that an adequate daily phytate intake (0.5–1 g/day) protects against bone mass loss and an association between dietary phytate and bone parameters will be detected in our population. So, the main objective of this work is to explore the association of the dietary phytate intake with bone parameters in a Mediterranean population of women.
2. Materials and Methods
2.1. Study Design and Population
A cross-sectional analysis in a subset of women from the PREvención con DIeta MEDiterránea Plus (PREDIMED-Plus) trial was performed. The trial’s design and methods used have been described previously [23,24]. The trial design and methods are available at http://predimedplus.com (accessed on 3 April 2018). In summary, PREDIMED-Plus is a 6-year, randomized, parallel-group, multicenter, controlled study on primary prevention of cardiovascular disease that is ongoing in Spain. The trial objective is to evaluate the effect of an intensive weight-loss intervention with an energy-reduced Mediterranean diet, a behavioral support on the prevention of cardiovascular events and physical activity promotion, in comparison to usual care. The trial includes 6874 men and women, aged 55–75 with overweight/obesity (body mass index (BMI) ≥ 27 kg/m2 and <40 kg/m2) and who met at least three characteristics of the metabolic syndrome [25]. For the present study, we have included a subset of 561 women with data on bone parameters in femur and lumbar spine at baseline, coming from 4 of the 23 PREDIMED-Plus recruiting centers (7 PREDIMED-Plus centers had access to dual-energy X-ray absorptiometry scanners and 4 of them performed measurements of BMD in addition to total body composition).
2.2. Estimated Phytate Intake and Other Nutritional Variables
Estimation of phytate intake was previously described [26]. It is based on the determination of consumption of the major food sources of phytate (legumes, whole-cereals, and nuts). These were collected with a validated semi-quantitative food frequency questionnaire (FFQ) composed of 143 items [27,28], considering the serving size of each item [28], and the phytate proportion of each item based on published sources [29,30,31,32,33]. Table 1 shows estimated phytate content for standard serving sizes, based on the reported phytate content for selected items in the FFQ. FFQ data were used to determine consumption of specific food groups (vegetables and fruits (g/day) and Spanish food composition tables [34,35] were used to derive data on total energy (kcal/day) and micronutrients (calcium (mg/day), vitamin D (µg/day)) intake.
Table 1.
Estimated phytate content for standard serving sizes, based on the reported phytate content for selected items in the food frequency questionnaire (FFQ) [26,27,28,29,30,31,32,33,34,35].
| Food | Estimate mg Phytate/100 g Edible |
Serving Size (g) | Phytate per Serving (mg) |
|---|---|---|---|
| Green beans | 180 | 200 * | 360 |
| Almonds, peanuts, hazelnuts, pistachio or pine seed | 1000 | 30 | 300 |
| Walnuts | 1600 | 30 | 480 |
| Lentils | 400 | 150 * | 600 |
| Beans (pinto, kidney or lima) | 700 | 150 * | 1050 |
| Chickpeas | 400 | 150 * | 600 |
| Beans and broadbeans | 600 | 150 * | 900 |
| Whole bread | 350 | 75 | 263 |
| Whole cereals (muesli, oatmeal, all-bran) | 350 | 30 | 90 |
| Whole rice | 350 | 60 | 210 |
| Wholemeal cookies | 300 | 50 | 150 |
| Whole-wheat pasta | 300 | 60 | 180 |
* Weight after cooking.
Using average values from the International Tables of Glycemic Index [36] and glucose as the reference food, the glycemic index was estimated for each FFQ item. The glycemic index was assessed as the glycemic load of the diet divided by the grams of total carbohydrates consumed per day and expressed as percentage.
The FFQ collected information about the food intake during the previous year. The FFQ is repeated yearly in PREDIMED-Plus participants but, given that this is a cross-sectional study using baseline data, only data of the first FFQ—administered at recruitment—is presented.
2.3. Bone Parameters’ Assessment
At baseline, bone parameters were measured by trained operators using third-generation dual-energy X-ray absorptiometry (DXA) scanners from General Electric (DXA Lunar Prodigy Primo and Lunar iDXA; GE Healthcare, Madison, WI, USA) connected with enCoreTM software. BMD was determined at the non-dominant femur (neck, Ward’s triangle, trochanter, diaphysis and total) and lumbar spine in anterior–posterior position (L1–L2, L1–L3, L1–L4, L2–L3, L2–L4, and L3–L4), and expressed in g/cm2 and in standard deviations from the young adult normal mean values (T-score), based on the Spanish reference population provided by manufacturer. In case of diaphysis, the T-score values were not available. In addition, we categorized women into two groups based on modified T-score cut-offs established by the World Health Organization (WHO) for each area [37]: low-BMD when T-score was equal or lower than −1 and normal-BMD in another case. The standard WHO categories of BMD state (normal, osteopenia, and osteoporosis) were modified due to low total number of osteoporotic cases. DXA scans, including subject positioning and daily phantom calibration, were performed following manufacturer guidelines.
2.4. Assessment of Other Variables
At baseline, trained dieticians collected information on socio-demographics, lifestyle habits and health status using general questionnaire. To categorize educational level (further education/technician, secondary education/primary education or less), and smoking behavior (never, former, current), three groups were created. Baseline prevalence of type 2 diabetes (T2D) and self-reported osteoporotic fractures was used as a dichotomous variable (yes/no). The BMI was calculated as weight (kg)/height (m) squared. Initial weight, in light clothing, and height were collected by qualified personnel using calibrated scales. The average value was utilized for analysis. Total leisure-time physical activity (MET•min/week) was determined using the Minnesota-REGICOR short physical activity questionnaire, previously validated in the Spanish population [38].
2.5. Statistical Analysis
The subset of women was separated into three groups based on their phytate tertiles: low (T1: <15 mg/100 kcal), moderate (T2: 15.0–28.4 mg/100 kcal) and high (T3: >28.4 mg/100 kcal). Unless otherwise specified, data are provided as numbers and percentages, means and standard deviations, or means and standard errors. The chi-square test was used for categorical variables, and one-way ANOVA and LSD were used as post hoc tests for quantitative data in intergroup comparisons of phytate groups.
Linear and logistic regression models were fitted to assess the associations between phytate intake with BMD expressed as absolute values (g/cm2) and T-score values (linear regression models) or low BMD status (logistic regression). For these analyses, we used tertiles of phytate intake in both linear and logistic models, considering the first tertile (low phytate intake) as the reference category. Multivariate models were adjusted for age (years), BMI (kg/m2), physical activity (MET•min/week), educational level (higher education/technician or secondary education/primary education or less), smoking status (never/former/current), T2D prevalence, self-reported osteoporotic fractures, intake of total energy (kcal/day), calcium (mg/day), vitamin D (µg/day), glycemic index, and consumption of vegetables and fruits (g/day).
Sensitivity analyses were conducted using the T-scores for lumbar spine L1–L4 measures as the outcome: these included effect modification analyses and stratification of participants by the median of age (≤66 y/>66 y), BMI (≤32.6 kg/m2/>32.6 kg/m2) and T2D prevalence (yes/no) groups. Models were adjusted by the same variables used in the main analyses. All graphs and tests yielded models that met the independence of observations, homogeneity of variance, and normality of residuals criteria.
For these analyses, we used the official PREDIMED-Plus database generated on the 22 December 2020. A two-tailed p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
3. Results
A total of 561 women were included in the present study with a mean age of 66.5 ± 4.1 years and BMI of 33.0 ± 3.6 kg/cm2. The main anthropometric, dietary and lifestyle data of participants are shown in Table 2. Women in the highest tertile of phytate intake had higher physical activity and a lower glycemic index than those in the lowest tertile. Regarding food intake, women in the top tertile consumed more vegetables, legumes and nuts and less meat, olive oil and pastries and sweets when compared to the lowest tertile.
Table 2.
Anthropometric, lifestyle and dietary characteristics of study participants according to tertiles of estimated phytate intake (mg/100 kcal·day).
| Tertile 1 | Tertile 2 | Tertile 3 | |||||
|---|---|---|---|---|---|---|---|
| <15.0 mg/100 kcal | [15.0–28.4] mg/100 kcal | >28.4 mg/100 kcal | |||||
| Variable | (n = 187) | (n = 187) | (n = 187) | p-Value | |||
| Age, years | 66.3 ± 4.1 | 67.1 ± 3.9 | 66.2 ± 4.1 | 0.062 | |||
| BMI, kg/m2 | 33.4 ± 3.5 | 32.5 ± 3.3 a | 33.1 ± 3.8 | 0.046 | |||
| Physical activity, MET•min/week | 1709 ± 1602 | 2389 ± 2153 a | 2331 ± 1738 a | <0.001 | |||
| Menopausal age, years | 48.7 ± 5.6 | 49.4 ± 5.3 | 48.6 ± 6.3 | 0.387 | |||
| Educational level, n (%) | |||||||
| Higher education | 22 | (11.8%) | 20 | (10.8%) | 28 | (15.2%) | 0.341 |
| Technician or secondary education | 43 | (23.1%) | 40 | (21.5%) | 50 | (27.2%) | |
| Primary education or less | 121 | (65.1%) | 126 | (67.7%) | 106 | (57.6%) | |
| Smoking status, n (%) | |||||||
| Never | 119 | (64.0%) | 132 | (70.6%) | 122 | (65.2%) | 0.126 |
| Former | 44 | (23.7%) | 43 | (23.0%) | 53 | (28.3%) | |
| Current | 23 | (12.4%) | 12 | (6.4%) | 12 | (6.4%) | |
| Type 2 diabetes, n (%) | 47 | (25.1%) | 46 | (24.6%) | 48 | (25.7%) | 0.972 |
| Osteporotic fractures, n (%) | 4 | (2.1%) | 5 | (2.7%) | 2 | (1.1%) | 0.523 |
| Nutrients | |||||||
| Total energy intake, kcal/day | 2376 ± 520 | 2338 ± 630 | 2250 ± 576 | 0.099 | |||
| Phytate, mg/100 kcal | 9.4 ± 3.2 | 21.1 ± 4.0 a | 40.5 ± 9.8 a,b | <0.001 | |||
| Phytate, mg/day | 225 ± 98 | 490 ± 155 a | 912 ± 331 a,b | <0.001 | |||
| Vitamin D, µg/day | 5.8 ± 3.3 | 6.3 ± 3.3 | 6.6 ± 3.5 | 0.057 | |||
| Calcium, mg/day | 1067 ± 353 | 1084 ± 377 | 1037 ± 366 | 0.454 | |||
| Phosphorous, mg/day | 1731 ± 419 | 1812 ± 479 | 1877 ± 458 a | 0.008 | |||
| Zinc, mg/day | 12.7 ± 3.1 | 13.2 ± 3.4 | 14.1 ± 3.8 a,b | 0.001 | |||
| Glycemic index | 55.5 ± 4.4 | 53.7 ± 5.0 a | 52.4 ± 6.0 a | <0.001 | |||
| Food | |||||||
| Vegetables (g/day) | 300 ± 109 | 344 ± 126 a | 354 ± 131 a | <0.001 | |||
| Fruits (g/day) | 362 ± 217 | 371 ± 238 | 353 ± 180 | 0.701 | |||
| Legumes (g/day) | 18 ± 8 | 21 ± 11 a | 21 ± 13 a | 0.007 | |||
| Cereals (g/day) | 153 ± 81 | 144 ± 78 | 152 ± 78 | 0.520 | |||
| Whole cereals (g/day) | 11 ± 22 | 43 ± 38 a | 123 ± 84 a,b | <0.001 | |||
| Dairy (g/day) | 395 ± 205 | 371 ± 216 | 363 ± 218 | 0.335 | |||
| Meat (g/day) | 159 ± 52 | 154 ± 59 | 140 ± 54 a,b | 0.004 | |||
| Olive oil (g/day) | 45 ± 15 | 42 ± 15 | 40 ± 15 a | 0.007 | |||
| Fish (g/day) | 100 ± 43 | 104 ± 44 | 104 ± 45 | 0.530 | |||
| Nuts (g/day) | 5 ± 8 | 15 ± 15 a | 25 ± 21 a,b | <0.001 | |||
| Pastries and sweets (g/day) | 34 ± 37 | 27 ± 35 a | 21 ± 25 a | 0.001 | |||
Values are expressed as the mean ± SD. The p-value was calculated with ANOVA test using LSD in the post hoc analysis. a: p < 0.05 vs. T1; b: p < 0.05 vs. T2.
Table 3 shows the BMD values (expressed in g/cm2 and T-scores) according to tertiles of phytate intake. Women in the top tertile had higher BMD values in almost all sites, both expressed as absolute values or T-scores, compared to those in the lowest tertile. Estimated phytate intake (mg/100 kcal) was also statistically higher for women with normal BMD (T-score > −1) compared to those with low BMD (T-score < −1) for Ward’s femoral triangle, lumbar spine L1–L3, L1–L4, L2–L3 and L2–L4 sites (Supplementary Figure S1).
Table 3.
Bone mineral density values (g/cm2 and T-score) according to tertiles of estimated phytate intake (mg/100 kcal·day).
| Variable | Tertile 1 <15.0 mg/100 kcal |
Tertile 2 [15.0–28.4] mg/100 kcal |
Tertile 3 >28.4 mg/100 kcal |
p-Value |
|---|---|---|---|---|
| BMD g/cm2 | ||||
| Femoral Neck, g/cm2 | 0.86 ± 0.11 | 0.86 ± 0.12 | 0.90 ± 0.12 a,b | 0.004 |
| Femoral Ward’s Triangle, g/cm2 | 0.66 ± 0.11 | 0.67 ± 0.13 | 0.71 ± 0.16 a,b | 0.000 |
| Femoral Trochanter, g/cm2 | 0.78 ± 0.10 | 0.77 ± 0.11 | 0.80 ± 0.12 b | 0.014 |
| Femoral Diaphysis, g/cm2 | 1.16 ± 0.14 | 1.16 ± 0.15 | 1.19 ± 0.16 a,b | 0.038 |
| Total Femur, g/cm2 | 0.95 ± 0.10 | 0.95 ± 0.12 | 0.98 ± 0.13 a,b | 0.010 |
| Lumbar Spine L1–L2, g/cm2 | 1.02 ± 0.15 | 1.01 ± 0.15 | 1.07 ± 0.18 a,b | 0.001 |
| Lumbar Spine L1–L3, g/cm2 | 1.06 ± 0.16 | 1.05 ± 0.15 | 1.11 ± 0.17 a,b | 0.002 |
| Lumbar Spine L1–L4, g/cm2 | 1.07 ± 0.16 | 1.08 ± 0.16 | 1.12 ± 0.17 a,b | 0.012 |
| Lumbar Spine L2–L3, g/cm2 | 1.09 ± 0.18 | 1.09 ± 0.16 | 1.13 ± 0.18 b | 0.018 |
| Lumbar Spine L2–L4, g/cm2 | 1.10 ± 0.18 | 1.11 ± 0.17 | 1.15 ± 0.18 b | 0.042 |
| Lumbar Spine L3–L4, g/cm2 | 1.12 ± 0.19 | 1.14 ± 0.18 | 1.17 ± 0.19 | 0.054 |
| BMD T–scores | ||||
| Femoral Neck | −1.00 ± 0.87 | −1.01 ± 0.99 | −0.71 ± 1.03 a,b | 0.003 |
| Femoral Ward’s Triangle | −1.82 ± 0.87 | −1.79 ± 1.03 | −1.49 ± 1.26 a,b | 0.005 |
| Femoral Trochanter | −0.09 ± 0.92 | −0.20 ± 1.00 | 0.05 ± 1.07 | 0.061 |
| Total Femur | −0.28 ± 0.84 | −0.38 ± 0.99 | −0.13 ± 1.03 a,b | 0.045 |
| Lumbar Spine L1–L2 | −1.21 ± 1.23 | −1.31 ± 1.22 | −0.77 ± 1.47 a,b | 0.001 |
| Lumbar Spine L1–L3 | −0.96 ± 1.31 | −1.01 ± 1.24 | −0.53 ± 1.45 a,b | 0.002 |
| Lumbar Spine L1–L4 | −0.91 ± 1.34 | −0.86 ± 1.33 | −0.48 ± 1.44 a,b | 0.009 |
| Lumbar Spine L2–L3 | −0.91 ± 1.49 | −0.93 ± 1.33 | −0.54 ± 1.50 ab | 0.018 |
| Lumbar Spine L2–L4 | −0.83 ± 1.49 | −0.75 ± 1.41 | −0.44 ± 1.50 a | 0.033 |
| Lumbar Spine L3–L4 | −0.65 ± 1.57 | −0.54 ± 1.50 | −0.26 ± 1.56 | 0.054 |
BMD: Bone mineral density. Values are expressed as the mean ± SD. The p-value was calculated with ANOVA using LSD in the post hoc analysis. a: p < 0.05 vs. T1; b: p < 0.05 vs. T2.
We further explored the association between phytate intake and BMD (g/cm2) values in univariate and multivariate linear regression analysis (Table 4). After adjusting for potential cofounders, phytate intake (by tertiles and per each 25 mg/100 kcal) was directly and significantly associated with BMD in femoral neck, femoral Ward’s triangle, total femur and all the analyzed lumbar spine sites but not in case of femoral trochanter (p = 0.064) and femoral diaphysis (p = 0.072).
Table 4.
Association of bone mineral density (g/cm2) with estimated phytate intake (by tertiles and per each 25 mg/100 kcal).
| Tertile 1 <15.0 mg/100 kcal |
Tertile 2 [15.0–28.4] mg/100 kcal |
Tertile 3 >28.4 mg/100 kcal |
p-Value for Trend | Phytate (per 25 mg/100 kcal) | p-Value | |
|---|---|---|---|---|---|---|
| Femoral Neck, n | 186 | 187 | 183 | 556 | ||
| Crude Model | 0 (reference) | −0.002 (−0.026–0.022) | 0.034 (0.010–0.058) | 0.005 | 0.026 (0.009–0.043) | 0.003 |
| Adjusted Model * | 0 (reference) | 0.003 (−0.021–0.026) | 0.031 (0.007–0.056) | 0.011 | 0.023 (0.006–0.040) | 0.008 |
| Femoral Ward’s Triangle, n | 186 | 187 | 183 | 556 | ||
| Crude Model | 0 (reference) | 0.005 (−0.023–0.032) | 0.050 (0.022–0.077) | <0.001 | 0.035 (0.016–0.055) | <0.001 |
| Adjusted Model * | 0 (reference) | 0.009 (−0.019–0.037) | 0.048 (0.020–0.077) | 0.001 | 0.033 (0.013–0.054) | 0.001 |
| Femoral Trochanter, n | 186 | 187 | 183 | 556 | ||
| Crude Model | 0 (reference) | −0.011 (−0.033–0.011) | 0.022 (0.000–0.044) | 0.055 | 0.018(0.002–0.034) | 0.027 |
| Adjusted Model * | 0 (reference) | −0.006 (−0.027–0.016) | 0.020 (−0.002–0.043) | 0.075 | 0.015 (−0.001–0.031) | 0.064 |
| Femoral Diaphysis, n | 178 | 184 | 180 | 542 | ||
| Crude Model | 0 (reference) | 0.000 (−0.031–0.031) | 0.035 (0.004–0.066) | 0.026 | 0.023 (0.001–0.045) | 0.041 |
| Adjusted Model * | 0 (reference) | 0.009 (−0.021–0.039) | 0.033 (0.002–0.063) | 0.035 | 0.020 (−0.002–0.041) | 0.072 |
| Total Femur, n | 178 | 184 | 180 | 542 | ||
| Crude Model | 0 (reference) | −0.004 (−0.028–0.019) | 0.030 (0.006–0.054) | 0.015 | 0.021 (0.004–0.038) | 0.015 |
| Adjusted Model * | 0 (reference) | 0.003 (−0.020–0.026) | 0.027 (0.004–0.051) | 0.023 | 0.018 (0.001–0.035) | 0.034 |
| Lumbar Spine L1–L2, n | 148 | 157 | 164 | 469 | ||
| Crude Model | 0 (reference) | −0.012 (−0.047–0.023) | 0.052 (0.017–0.088) | 0.003 | 0.043 (0.018–0.067) | 0.001 |
| Adjusted Model * | 0 (reference) | −0.004 (−0.039–0.032) | 0.043 (0.007–0.080) | 0.016 | 0.035 (0.010–0.060) | 0.006 |
| Lumbar Spine L1–L3, n | 147 | 157 | 164 | 468 | ||
| Crude Model | 0 (reference) | −0.007 (−0.043–0.029) | 0.050 (0.014–0.086) | 0.005 | 0.040 (0.015–0.065) | 0.002 |
| Adjusted Model * | 0 (reference) | 0.001 (−0.036–0.037) | 0.042 (0.005–0.079) | 0.003 | 0.033 (0.008–0.059) | 0.011 |
| Lumbar Spine L1–L4, n | 147 | 158 | 167 | 472 | ||
| Crude Model | 0 (reference) | 0.005 (−0.032–0.043) | 0.050 (0.013–0.087) | 0.007 | 0.038 (0.013–0.063) | 0,003 |
| Adjusted Model * | 0 (reference) | 0.013 (−0.024–0.050) | 0.045 (0.007–0.083) | 0.019 | 0.033 (0.007–0.059) | 0.013 |
| Lumbar Spine L2–L3, n | 167 | 171 | 176 | 514 | ||
| Crude Model | 0 (reference) | −0.003 (−0.040–0.034) | 0.044 (0.008–0.081) | 0.017 | 0.033 (0.007–0.059) | 0,012 |
| Adjusted Model * | 0 (reference) | 0.006 (−0.031–0.043) | 0.041 (0.006–0.083) | 0.032 | 0.030 (0.003–0.056) | 0.028 |
| Lumbar Spine L2–L4, n | 167 | 173 | 179 | 519 | ||
| Crude Model | 0 (reference) | 0.008 (−0.029–0.046) | 0.045 (0.007–0.082) | 0.018 | 0.033 (0.007–0.060) | 0.012 |
| Adjusted Model * | 0 (reference) | 0.017 (−0.021–0.055) | 0.044 (0.006–0.083) | 0.023 | 0.032 (0.005–0.058) | 0.019 |
| Lumbar Spine L3–L4, n | 167 | 170 | 176 | 513 | ||
| Crude Model | 0 (reference) | 0.013 (−0.026–0.053) | 0.047 (0.008–0.086) | 0.019 | 0.036 (0.008–0.063) | 0.012 |
| Adjusted Model * | 0 (reference) | 0.022 (−0.018–0.062) | 0.047 (0.006–0.087) | 0.025 | 0.034 (0.005–0.062) | 0.020 |
Linear regression models were used to evaluate the association between bone mineral density and estimated phytate intake (tertiles and per each 25 mg/100 kcal). Results are expressed as β coefficients (95% CIs). * Models adjusted for age (years), BMI (kg/m2), physical activity (MET•min/week), educational level (higher education/technician or secondary education/primary education or less), smoking status (never/former/current), type 2 diabetes prevalence, osteoporotic fractures prevalence, energy (kcal/day), calcium (mg/day), vitamin D (µg/day), glycemic index, vegetables and fruits (g/day).
Table 5 shows the beta-coefficients and 95% CI of the associations between phytate intake (by tertiles and per each 25 mg/100 kcal) and BMD T-scores values. As can be observed, after adjusting for potential confounders, phytate intake was positively and significantly associated with T-scores in femoral neck, femoral Ward’s triangle, and all the analyzed lumbar spine sites but not in case of total femur and femoral trochanter.
Table 5.
Association of bone mineral density (T-score) and estimated phytate intake (by tertiles and per each 25 mg/100 kcal).
| Tertile 1 <15.0 mg/100 kcal |
Tertile 2 [15.0–28.4] mg/100 kcal |
Tertile 3 >28.4 mg/100 kcal |
p-Value for Trend | Phytate (per 25 mg/100 kcal) | p-Value | |
|---|---|---|---|---|---|---|
| Femoral Neck, n | 185 | 187 | 182 | 554 | ||
| Crude Model | 0 (ref.) | −0.004 (−0.201–0.193) | 0.297 (0.099–0.496) | 0.004 | 0.221 (0.080–0.363) | 0.002 |
| Adjusted Model * | 0 (ref.) | 0.040 (−0.156–0.236) | 0.274 (0.072–0.475) | 0.008 | 0.200 (0.058–0.342) | 0.006 |
| Femoral Ward’s Triangle, n | 185 | 187 | 181 | 553 | ||
| Crude Model | 0 (ref.) | 0.022 (−0.194–0.238) | 0.327 (0.109–0.545) | 0.004 | 0.225 (0.070–0.380) | 0.004 |
| Adjusted Model * | 0 (ref.) | 0.053 (−0.165–0.271) | 0.319 (0.095–0.544) | 0.005 | 0.218 (0.060–0.376) | 0.007 |
| Femoral Trochanter, n | 185 | 187 | 181 | 553 | ||
| Crude Model | 0 (ref.) | −0.109 (−0.313–0.095) | 0.138 (−0.067–0.344) | 0.192 | 0.144 (−0.002–0.290) | 0.053 |
| Adjusted Model * | 0 (ref.) | −0.067 (−0.270–0.137) | 0.132 (−0.078–0.342) | 0.213 | 0.134 (−0.014–0.282) | 0.075 |
| Total Femur, n | 177 | 184 | 177 | 538 | ||
| Crude Model | 0 (ref.) | −0.094 (−0.293–0.104) | 0.156 (−0.044–0.356) | 0.128 | 0.136 (−0.006–0.278) | 0.061 |
| Adjusted Model * | 0 (ref.) | −0.034 (−0.229–0.161) | 0.152 (−0.049–0.353) | 0.133 | 0.130 (−0.011–0.271) | 0.071 |
| Lumbar Spine L1–L2, n | 148 | 157 | 164 | 469 | ||
| Crude Model | 0 (ref.) | −0.100 (−0.396–0.196) | 0.436 (0.143–0.728) | 0.003 | 0.353 (0.149–0.557) | 0.001 |
| Adjusted Model * | 0 (ref.) | −0.032 (−0.328–0.264) | 0.360 (0.059–0.661) | 0.016 | 0.291 (0.084–0.499) | 0.006 |
| Lumbar Spine L1–L3, n | 147 | 157 | 164 | 468 | ||
| Crude Model | 0 (ref.) | −0.057 (−0.359–0.245) | 0.421 (0.122–0.720) | 0.005 | 0.331 (0.123–0.539) | 0.002 |
| Adjusted Model * | 0 (ref.) | 0.009 (−0.294–0.312) | 0.355 (0.047–0.663) | 0.021 | 0.276 (0.064–0.488) | 0.011 |
| Lumbar Spine L1–L4, n | 147 | 156 | 165 | 468 | ||
| Crude Model | 0 (ref.) | 0.055 (−0.256–0.365) | 0.435 (0.129–0.742) | 0.005 | 0.333 (0.120–0.546) | 0.002 |
| Adjusted Model * | 0 (ref.) | 0.115 (−0.198–0.427) | 0.388 (0.071–0.705) | 0.015 | 0.287 (0.069–0.505) | 0.010 |
| Lumbar Spine L2–L3, n | 167 | 171 | 176 | 514 | ||
| Crude Model | 0 (ref.) | −0.020(−0.328–0.289) | 0.370 (0.064–0.676) | 0.017 | 0.276 (0.060–0.492) | 0.012 |
| Adjusted Model * | 0 (ref.) | 0.055 (−0.256–0.366) | 0.343 (0.027–0.659) | 0.032 | 0.248 (0.028–0.469) | 0.027 |
| Lumbar Spine L2–L4, n | 167 | 170 | 177 | 514 | ||
| Crude Model | 0 (ref.) | 0.0073(−0.241–0.387) | 0.389 (0.078–0.699) | 0.014 | 0.296 (0.077–0.515) | 0.008 |
| Adjusted Model * | 0 (ref.) | 0.145 (−0.171–0.462) | 0.384 (0.063–0.705) | 0.018 | 0.280 (0.056–0.504) | 0.014 |
| Lumbar Spine L3–L4, n | 167 | 170 | 176 | 513 | ||
| Crude Model | 0 (ref.) | 0.114 (−0.217–0.444) | 0.391 (0.063–0.719) | 0.019 | 0.295 (0.065–0.526) | 0.012 |
| Adjusted Model * | 0 (ref.) | 0.185 (−0.150–0.520) | 0.390 (0.049–0.073) | 0.025 | 0.281 (0.044–0.519) | 0.020 |
Linear regression models were used to evaluate the association between T-scores and estimated phytate intake (tertiles and per each 25 mg/100 kcal). Results are expressed as β coefficients (95% CIs). * Models adjusted for age (years), BMI (kg/m2), physical activity (MET•min/week), educational level (higher education/technician or secondary education/primary education or less), smoking status (never/former/current), type 2 diabetes prevalence, osteoporotic fractures prevalence, energy (kcal/day), calcium (mg/day), vitamin D (µg/day), glycemic index, vegetables and fruits (g/day).
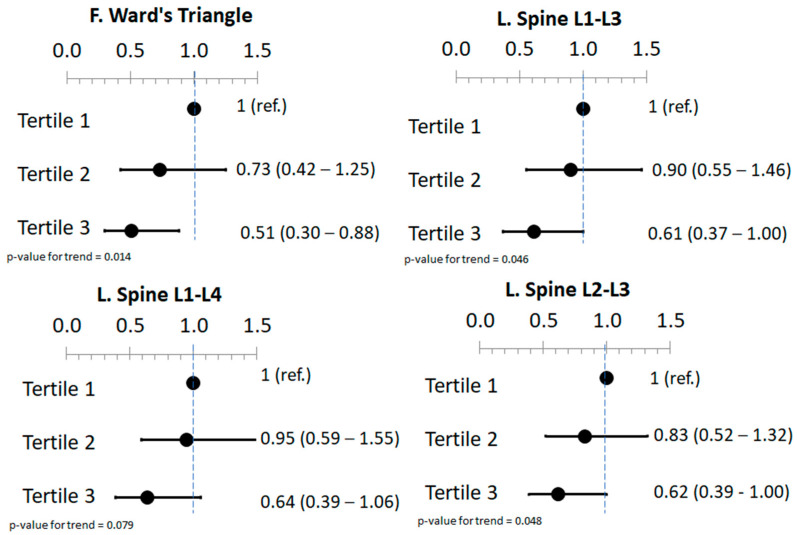
Binary logistic regression was also performed to study the association between phytate intake and low BMD defined as a T-score ≤ 1 (Supplementary Table S1). After adjusting for potential cofounders, phytate intake was inversely associated with low BMD status in femoral Ward’s triangle, lumbar spine L1–L3 and lumbar spine L2–L3 sites but not in the rest of the analyzed sites (Figure 1).
Figure 1.
Forest plot of low bone mineral density (T-score ≤ −1) and tertiles of estimated phytate intake. Values are expressed as the adjusted odds ratio (95% CI). Models were adjusted for age (years), BMI (kg/m2), physical activity (MET•min/week), educational level (higher education/technician or secondary education/primary education or less), smoking status (never/former/current), type 2 diabetes prevalence, osteoporotic fractures prevalence, energy (kcal/day), calcium (mg/day), vitamin D (µg/day), glycemic index, vegetables and fruits (g/day). This figure is a summary of the Supplementary Table S1.
Finally, we carried out stratified analyses by age, BMI and T2D for the association between phytate intake and the T-score in lumbar spine L1–L4, which is the most common measure used in the diagnosis of osteopenia or osteoporosis in lumbar spine (Table 6). Phytate intake was associated with T-score values in women younger than 66 years, with a BMI higher than 32.6 kg/cm2 and with no T2D (p for all interactions < 0.05).
Table 6.
Association of bone mineral density (T-score) in lumbar spine L1–L4 and estimated phytate intake (by tertiles and per each 25 mg/100 kcal) by subgroups.
| Tertile 1 <15.0 mg/100 kcal |
Tertile 2 [15.0–28.4] mg/100 kcal |
Tertile 3 >28.4 mg/100 kcal |
p-Value for Trend | Phytate (per 25 mg/100 kcal) | p-Value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤66 years | 72 | 69 | 100 | 241 | ||
| 0 (ref.) | 0.149 (−0.316–0.615) | 0.860 (0.424–1.296) | <0.001 | 0.580 (0.260–0.901) | <0.001 | |
| >66 years | 75 | 87 | 65 | 227 | ||
| 0 (ref.) | −0.015 (−0.452–0.421) | −0.274 (−0.757–0.210) | 0.279 | −0.021 (−0.443–0.400) | 0.920 | |
| p for interaction | 0.021 | 0.008 | ||||
| BMI | ||||||
| ≤32.6 kg/cm2 | 66 | 82 | 78 | 226 | ||
| 0 (ref.) | 0.081 (−0.370–0.533) | 0.185 (−0.283–0.654) | 0.432 | 0.221 (−0.151–0.593) | 0.243 | |
| >32.6 kg/cm2 | 84 | 74 | 87 | 242 | ||
| 0 (ref.) | 0.181 (−0.262–0.624) | 0.607 (0.168–1.046) | 0.007 | 0.425 (0.080–0.770) | 0.016 | |
| p for interaction | <0.001 | <0.001 | ||||
| Type 2 Diabetes | ||||||
| No | 112 | 120 | 121 | 353 | ||
| 0 (ref.) | 0.332 (−0.017–0.681) | 0.528 (0.170–0.887) | 0.004 | 0.332 (0.083–0.581) | 0.009 | |
| Yes | 35 | 36 | 44 | 115 | ||
| 0 (ref.) | −0.511 (−1.209–0.188) | 0.078 (−0.583–0.739) | 0.697 | 0.321 (−0.135–0.776) | 0.165 | |
| p for interaction | 0.023 | 0.010 |
Linear regression models were used to evaluate the association between T-score L1–L4 and estimated phytate intake (tertiles and per each 25 mg/100 kcal). Results are expressed as β coefficients (95% CIs). Models were adjusted for age (years), BMI (kg/m2), physical activity (MET•min/week), educational level (higher education/technician or secondary education/primary education or less), smoking status (never/former/current), type 2 diabetes prevalence, osteoporotic fractures prevalence, energy (kcal/day), calcium (mg/day), vitamin D (µg/day), glycemic index, vegetables and fruits (g/day). p-value of interaction with estimated phytate intake was indicated in italics.
4. Discussion
The present study reported a positive association between phytate intake and BMD in a sample of postmenopausal women with overweight/obesity and metabolic syndrome from Spain.
Our results are in accordance with previous observational studies that indicate that phytate intake has a positive association with bone health. Body weight and low phytate intake were risk factors for BMD of the lumbar vertebrae and neck of the femur in a cross-sectional investigation of postmenopausal women [20]. In another study in postmenopausal women, the 10-year fracture probability in the low-phytate group was significantly higher than in the high-phytate group, both in major osteoporotic and hip fracture [18]. Phytate, in an in vitro study, inhibited hydroxyapatite (HAP) dissolution in a concentration-dependent manner by adsorbing phytate in HAP sur-faces, and this effect was comparable to alendronate but greater than that of etidronate [22]. Moreover, it has been shown that phytate inhibits osteoclastogenesis in human primary osteoclast cell line and in RAW 264.7 monocyte/macrophage mouse cell line [21]. Last but not least, it has been noted that phytate consumption produces phytate hydrolysates (InsP5, InsP4, InsP3, InsP2) by intestinal phosphatase activity [30] that may also have a significant impact on bone resorption by adsorbing in crystal surfaces of HAP [39]. Altogether, phytate may therefore offer, by adhering to HAP surfaces and reducing osteoclast activity, a unique type of nutraceutical or therapeutic agent of the decalcification process.
The bisphosphonate is currently among the most popular therapies for osteoporosis to lower the risk of fracture and reduce bone resorption. These are effective drugs for bone disorders characterized by increased bone resorption, such as Paget’s disease, osteoporosis, hypercalcemia of cancer, multiple myeloma, and bony metastases [40,41]. The bisphosphonates are adsorbed very effectively to HAP, the crystalline form of calcium and phosphate in bone. Bisphosphonates are analogues of pyrophosphate which have potent inhibitory effects on bone resorption. Pyrophosphate is an important endogenous body’s regulator of calcification. Within humans, pyrophosphate is released as a product of many synthetic reactions, and it has been detected in many tissues, including blood and urine [42,43]. However, it was demonstrated that pyrophosphate could inhibit pathological calcification only when it was injected rather than ingested. For this reason, the use of pyrophosphate as a drug was constrained by its pharmacokinetics because it was hydrolyzed and inactivated when was administered orally [42,43]. Consequently, bisphosphonates were found to be chemically stable analogues of pyrophosphate that could inhibit pathological calcification and bone resorption when they were administered orally [44,45]. Similar to their natural analogue pyrophosphate, bisphosphonates have a very high affinity for bone mineral because they bind to hydroxyapatite crystals. Therefore, the retention of bisphosphonates on bone surface depends on the availability of hydroxyapatite binding sites. To date, many studies using in vitro systems, animal models, and clinical trials have shown that a variety of bisphosphonates can inhibit bone resorption [44,45].
Similar to bisphosphonates and pyrophosphate, phytate can act as inhibitor of both calcification and decalcification processes. In this sense, phytate has also demonstrated to inhibit the formation of pathological calcifications (such as renal calculi [39,46,47], dental calculi [48], and cardiovascular calcification [49,50,51]). Moreover, some studies in animals and cells indicate that phytate may also provide protection against cancer [52], Parkinson’s disease [53], cognitive degeneration [54] and diabetes-related diseases [55,56]. Phytate is natural polyphosphate readily accessible to people consuming a balanced diet (0.5–1 g/day) through nuts, legumes and cereals. Interestingly, the Mediterranean diet results in consumption of approximately 1 g of phytate/day [26]. All mammalian fluids, tissues, and organs contain phytate [57,58], and its contents rely on the exogenous supply either orally [57,58] or topically [59,60]. In this way, after 22 days without phytate in the diet, the urine content of phytate is undetectable [57,58]. It is understood that phytate’s capacity to form complexes with iron, zinc, copper, magnesium, and calcium may reduce these minerals’ bioavailability from food [57]. Foods of plant origin have a high phytate content expressed as magnesium, potassium and calcium salts. Together, all these salts are called phytates, but they have a different solubility and ability to complex other divalent cations [30,61]. In this sense, the different phytate salts can modify the bioavailability of the different ions such as calcium, iron and zinc to a different extent [30,62,63]. For this reason, phytate has been labeled as anti-nutrients that chelate metal ions and thus reduce their bioavailability. It is important to remark that this antinutritional effect only occur in unbalanced diets when the mineral intake is very low and dietary phytate is very high [30,64]. In this sense, some authors have indicated that this negative effect on mineral bioavailability may appear in growing children [65], adolescents and pregnant mothers who consume diets rich in phytates (cereal-based foods) and low in minerals [32,66]. Almost all of these cases are reported in low-income countries [67] as a consequence of the adherence to diets that do not agree with the mineral recommended intakes. So, under non-varied and non-balanced dietary conditions, phytate may affect the bioavailability of iron, zinc and calcium [30,32,62,63,64,65,66,67]. Nevertheless, no negative effects on mineral status have been observed in mineral balanced diets (as Mediterranean diet) with adequate amounts of phytate (0.5–1 g/day) [26,57,58,59,60]. Furthermore, a recent paper has indicated that a phytate consumption higher than 307 mg/day was associated with a normal lumbar BMD (t-score > −1) in postmenopausal women [22]. Consequently, a healthy and balanced diet with the right amounts of these trace minerals may prevent the loss of bone mass and prevent trace metals deficiencies [57,58,59,60].
Our study has some limitations, the first is the cross-sectional nature, which precludes conclusions regarding the temporal nature of our findings and no causality can be established. Even though we found that phytate intake is associated with BMD, we cannot confirm which one is the cause and which is the effect; and we cannot confirm that a higher phytate consumption is associated with a lower risk of fracture or osteoporosis. For these reasons, prospective studies are needed to establish the time sequence in the relationship between them and clinically relevant findings. The second limitation is that our participants are Mediterranean postmenopausal women with over-weight/obesity and metabolic syndrome, and our results cannot be generalized to other populations. Another limitation is that phytate intake is an “estimated measure”, although it has been calculated as previously described [26] based on the FFQ consumption data. On the other hand, the major strengths are the use of DXA scan for BMD determination, the control for many potential cofounders and the inclusion of sensitivity analyses.
5. Conclusions
In conclusion, our results show that phytate intake, a natural product present in legumes, nuts and whole cereals, is positively associated with BMD in Mediterranean postmenopausal women.
We hypothesize that eating a diet rich in phytate may prevent or alleviate disorders that result in bone mass loss, such as osteoporosis. The mechanism of action of phytate on bone resorption could be explained by its capacity to adsorb on the surfaces of hydroxyapatite (HAP) crystal and the consequent inhibition of HAP dissolution [22] and osteoclast activity [21]. However, to evaluate the impact of phytate consumption on BMD, large, lengthy, and randomized prospective clinical trials must be carried out.
Acknowledgments
We are grateful to the PREDIMED-Plus volunteers for the participation and personnel, investigators, and primary care centers for their contribution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15071791/s1, Table S1: Odds ratio and 95% CIs between low bone mineral density (T-score ≤ −1) and estimated phytate intake (by tertiles and per each 25 mg/100 kcal). Figure S1: Estimated phytate intake (mg/100 kcal) for normal (T-score > −1) and low (T-score < −1). Bone Mineral density of (A) femoral neck, Ward’s triangle, trochanter and total femur; (B) lumbar spine L1, L2, L3 and L4; and (C) lumbar spine L1–L2, L1–L3, L1–L4, L2–L3, L2–L4 and L3–L4. Values are expressed as the mean ± SE. * p-value < 0.05 vs. corresponding group of normal BMD (t-Student for independent samples).
Author Contributions
Conceptualization, P.S., R.M.P., J.K. and D.R.; methodology, P.S., R.M.P., J.K. and D.R.; software, P.S., R.M.P., J.K. and D.R.; validation, P.S, R.M.P., J.K. and DR; formal analysis, P.S., J.K. and D.R.; investigation, P.S., R.M.P., J.K., D.R., F.G., A.C-B., I.A., J.S.-S., V.M., M.R.-C., N.B., J.F.G.-G., A.G. and J.A.M.; resources, J.K., D.R., I.A., J.S.-S., V.M., M.R.-C., N.B., J.F.G.-G., A.G. and J.A.M.; data curation, J.K., D.R., I.A., J.S.-S., V.M., M.R.-C., N.B., J.F.G.-G., A.G. and J.A.M.; writing—original draft preparation, P.S., R.M.P., J.K., D.R., F.G. and A.C-B.; writing—review and editing, P.S., J.K., D.R., I.A., J.S.-S., V.M., M.R.-C., N.B., J.F.G.-G., A.G. and J.A.M.; visualization, P.S., R.M.P., J.K., D.R., F.G. and A.C.-B.; supervision, D.R. and F.G.; project administration, P.S., R.M.P., J.K. and D.R.; funding acquisition, F.G. and D.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. The PREDIMED-Plus study protocol was approved by the Research Ethic Committees from all the participating centers and the trial was registered at the International Standard Randomized Controlled Trial (ISRCTN) with the code 89898870. All participants provided written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings of this study are available from PREDIMED-Plus trial Steering Committee. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of PREDIMED-Plus trial Steering Committee.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the ‘National Institutes of Health’ [NIH 1R01DK127601] granted to MR-C and JS-S; ‘European Research Council’ [Advanced Research Grant 2014–2019; agreement #340918] granted to MÁM-G; and the ‘Spanish National Health Institute of Health Carlos III (ISCIII)’, through ‘CIBEROBN’ and ‘Fondo de Investigación para la Salud’ (FIS), which is co-funded by the ‘European Regional Development Fund’ [PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462,PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722,PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919,PI14/00853,PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662,PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366,PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508,PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032,PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158, PI20/0471]; the ‘Especial Action Project’ entitled: Implementación y evaluación de una intervención intensive sobre la actividad física Cohorte PREDIMEDPlus grant to JS-S; the ‘Recercaixa’ [number 2013ACUP00194] grant to JS-S; grants from the ‘Consejería de Salud de la Junta de Andalucía’ (PI0458/2013, PS0358/2016,PI0137/2018); the [PROMETEO/2017/017] grant from the ‘Generalitat Valenciana’; the SEMERGEN grant. Juan de la Cierva-Incorporación research grant [IJC2019-042420-I] of the ‘Spanish Ministry of Economy, Industry and Competitiveness’ and ‘European Social Funds’ to JK. This work was also partially supported by ‘ICREA’ under the ICREA Academia programme. None of the funding sources took part in the design, collection, analysis, interpretation of the data, or writing the report, or in the decision to submit the manuscript for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis J.A., Cooper C., Rizzoli R., Reginster J.Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019;30:3. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen L.R., Hou P.H., Chen K.H. Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients. 2019;11:2848. doi: 10.3390/nu11122848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji M.X., Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015;1:9–13. doi: 10.1016/j.cdtm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpouzos A., Diamantis E., Farmaki P., Savvanis S., Troupis T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017;2017:4218472. doi: 10.1155/2017/4218472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashman K.D. Diet, Nutrition, and Bone Health. J. Nutr. 2007;137:2507S–2512S. doi: 10.1093/jn/137.11.2507S. [DOI] [PubMed] [Google Scholar]
- 8.Sunyecz J.A. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 2008;4:827–836. doi: 10.2147/TCRM.S3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orchard T.S., Pan X., Cheek F., Ing S.W., Jackson R.D. A systematic review of omega-3 fatty acids and osteoporosis. Br. J. Nutr. 2012;107:S253–S260. doi: 10.1017/S0007114512001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García Gavilán J.F., Bulló M., Camacho-Barcia L., Rosique-Esteban N., Hernández-Alonso P., Basora J., Martínez-González M.A., Estruch R., Fitó M., Salas-Salvadó J. Higher dietary glycemic index and glycemic load values increase the risk of osteoporotic fracture in the PREvención con DIeta MEDiterránea (PREDIMED)-Reus trial. Am. J. Clin. Nutr. 2018;107:1035–1042. doi: 10.1093/ajcn/nqy043. [DOI] [PubMed] [Google Scholar]
- 11.Kontogianni M.D., Melistas L., Yannakoulia M., Malagaris I., Panagiotakos D.B., Yiannakouris N. Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition. 2009;25:165–171. doi: 10.1016/j.nut.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Rivas A., Romero A., Mariscal-Arcas M., Monteagudo C., Feriche B., Lorenzo M.L., Olea F. Mediterranean diet and bone mineral density in two age groups of women. Int. J. Food Sci. Nutr. 2013;64:155–161. doi: 10.3109/09637486.2012.718743. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Rey J., Roncero-Martín R., Rico-Martín S., Rey-Sánchez P., Pedrera-Zamorano J.D., Pedrera-Canal M., López-Espuela F., Lavado García J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients. 2019;11:555. doi: 10.3390/nu11030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderon-Garcia J.F., Moran J.M., Roncero-Martin R., Rey-Sanchez P., Rodriguez-Velasco F.J., Pedrera-Zamorano J.D. Dietary habits, nutrients and bone mass in Spanish premenopausal women: The contribution of fish to better bone health. Nutrients. 2012;5:10–22. doi: 10.3390/nu5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Gavilán J.F., Bulló M., Canudas S., Martínez-González M.A., Estruch R., Giardina S., Fitó M., Corella D., Ros R.E., Salas-Salvadó J. Extra virgin olive oil consumption reduces the risk of osteoporotic fractures in the PREDIMED trial. Clin. Nutr. 2018;37:329–335. doi: 10.1016/j.clnu.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Raboy V. Myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry. 2003;64:1033–1043. doi: 10.1016/S0031-9422(03)00446-1. [DOI] [PubMed] [Google Scholar]
- 17.Grases F., Sanchis P., Prieto R.M., Perelló J., López-González Á.A. Effect of tetracalcium dimagnesium phytate on bone characteristics in ovariectomized rats. J. Med. Food. 2010;13:1301–1306. doi: 10.1089/jmf.2009.0152. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez A.A.L., Grases F., Mari B., Tomas-Salva M., Rodriguez A. Urinary phytate concentration and risk of fracture determined by the FRAX index in a group of postmenopausal women. Turk. J. Med. Sci. 2019;49:458–463. doi: 10.3906/sag-1806-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-González A.A., Grases F., Roca P., Mari B., Vicente-Herrero M.T., Costa-Bauzá A. Phytate (myo-inositol hexaphosphate) and risk factors for osteoporosis. J. Med. Food. 2008;11:747–752. doi: 10.1089/jmf.2008.0087. [DOI] [PubMed] [Google Scholar]
- 20.López-González A.A., Grases F., Monroy N., Marí B., Vicente-Herrero M.T., Tur F., Perelló J. Protective effect of myo-inositol hexaphosphate (phytate) on bone mass loss in postmenopausal women. Eur. J. Nutr. 2013;52:717–726. doi: 10.1007/s00394-012-0377-6. [DOI] [PubMed] [Google Scholar]
- 21.Arriero M.M., Ramis J.M., Perelló J., Monjo M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE. 2012;7:e43187. doi: 10.1371/journal.pone.0043187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchis P., López-González Á.A., Costa-Bauzá A., Busquets-Cortés C., Riutord P., Calvo P., Grases F. Understanding the Protective Effect of Phytate in Bone Decalcification Related-Diseases. Nutrients. 2021;13:2859. doi: 10.3390/nu13082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-González M.A., Buil-Cosiales P., Corella D., Bulló M., Fitó M., Vioque J., Romaguera D., Martínez J.A., Wärnberg J., López-Miranda J., et al. Cohort profle: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019;48:387–388. doi: 10.1093/ije/dyy225. [DOI] [PubMed] [Google Scholar]
- 24.Sayón-Orea C., Razquin C., Bulló M., Corella D., Fitó M., Romaguera D., Vioque J., Alonso-Gómez A.M., Wärnberg J., Martínez J.A., et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients With Metabolic Syndrome Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA. 2019;322:1486–1499. doi: 10.1001/jama.2019.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 26.Prieto R.M., Fiol M., Perello J., Estruch R., Ros E., Sanchis P., Grases F. Effects of Mediterranean diets with low and high proportions of phytate-rich foods on the urinary phytate excretion. Eur. J. Nutr. 2010;49:321–326. doi: 10.1007/s00394-009-0087-x. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Ballart J.D., Piñol J.L., Zazpe I., Corella D., Carrasco P., Toledo E., Perez-Bauer M., Martínez-González M.A., Salas-Salvadó J., Martín-Moreno J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010;103:1808–1816. doi: 10.1017/S0007114509993837. [DOI] [PubMed] [Google Scholar]
- 28.De La Fuente-Arrillaga C., Vazquez Ruiz Z., Bes-Rastrollo M., Sampson L., Martinez-González M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010;13:1364–1372. doi: 10.1017/S1368980009993065. [DOI] [PubMed] [Google Scholar]
- 29.Harland B.F., Oberleas D. Phytate in foods. World Rev. Nutr. Diet. 1987;52:235–259. doi: 10.1159/000415199. [DOI] [PubMed] [Google Scholar]
- 30.Schlemmer U., Frølich W., Prieto R.M., Grases F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Rev. 2009;53:S330–S375. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- 31.Harland B.F., Smikle-Williams S., Oberleas D. High performance liquid chromatography analysis of phytate (IP6) in selected foods. J. Food Comp. Anal. 2004;17:227–233. doi: 10.1016/j.jfca.2003.08.005. [DOI] [Google Scholar]
- 32.Chan S.S.L., Ferguson E.L., Bailey K., Fahmidab U., Harpera T.B., Gibsona R.S. The concentrations of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indonesia. J. Food Comp. Anal. 2007;20:609–617. doi: 10.1016/j.jfca.2007.03.003. [DOI] [Google Scholar]
- 33.Plaami S. Myo-inositol phosphates: Analysis, content in foods and effects in nutrition. LWT Food Sci. Technol. 1997;30:633–647. doi: 10.1006/fstl.1997.0246. [DOI] [Google Scholar]
- 34.Mataix J. Tablas de Composición de Alimentos. 4th ed. Universidad de Granada; Granada, Spain: 2003. [Google Scholar]
- 35.Moreiras O., Carvajal A., Cabrera L. Tablas de Composición de Alimentos Food Composition Tables. 9th ed. Ediciones Pirámide; Madrid, Spain: 2005. [Google Scholar]
- 36.Atkinson F.S., Foster-Powell K., Brand-Miller J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Prevention and management of osteoporosis. World Health Organ. Tech. Rep. Ser. 2003;921:161–164. [PubMed] [Google Scholar]
- 38.Molina L., Sarmiento M., Peñafiel J., Donaire D., Garcia-Aymerich J., Gomez M., Ble M., Ruiz S., Frances A., Schröder H., et al. Validation of the regicor short physical activity questionnaire for the adult population. PLoS ONE. 2017;12:168148. doi: 10.1371/journal.pone.0168148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grases F., Costa-Bauza A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules. 2019;24:4434. doi: 10.3390/molecules24244434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleisch H.A. Bisphosphonates: Preclinical aspects and use in osteoporosis. Ann. Med. 1997;29:55–62. doi: 10.3109/07853899708998743. [DOI] [PubMed] [Google Scholar]
- 41.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleisch H., Bisaz S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am. J. Physiol. 1962;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- 43.Fleisch H., Neuman W.F. Mechanism of calcification: Role of collagen, polyphosphates, and phosphatases. Am. J. Phisiol. 1961;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 44.Fleisch H., Russell R.G., Francis M.D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969;165:1262–1264. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- 45.Russell R.G., Muhlbauer R.C., Bisaz S., Williams D.A., Fleisch H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif. Tissue Res. 1970;6:183–196. doi: 10.1007/BF02196199. [DOI] [PubMed] [Google Scholar]
- 46.Pujol A., Sanchis P., Grases F., Masmiquel L. Phytate Intake, Health and Disease: Let Thy Food Be Thy Medicine and Medicine Be Thy Food. Antioxidants. 2023;12:146. doi: 10.3390/antiox12010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grases F., Isern B., Sanchis P., Perello J., Torres J.J., Costa-Bauza A. Phytate acts as an inhibitor in formation of renal calculi. Front. Biosci. 2007;12:2580–2587. doi: 10.2741/2256. [DOI] [PubMed] [Google Scholar]
- 48.Grases F., Perello J., Sanchis P., Isern B., Prieto R.M., Costa-Bauzá A., Santiago C., Ferragut M.L., Frontera G. Anticalculus effect of a triclosan mouthwash containing phytate: A doubleblind, randomized, three-period crossover trial. J. Periodontal. Res. 2009;44:616–621. doi: 10.1111/j.1600-0765.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 49.Grases F., Sanchis P., Perello J., Isern B., Prieto R.M., Fernandez-Palomeque C., Saus C. Phytate reduces age-related cardiovascular calcification. Front. Biosci. 2008;13:7115–7122. doi: 10.2741/3214. [DOI] [PubMed] [Google Scholar]
- 50.Sanchis P., Buades J.M., Berga F., Gelabert M.M., Molina M., Íñigo M.V., García S., Gonzalez J., Bernabeu M.R., Costa-Bauzá A., et al. Protective Effect of Myo-Inositol Hexaphosphate (Phytate) on Abdominal Aortic Calcification in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2016;26:226–236. doi: 10.1053/j.jrn.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Palomeque C., Grau A., Perelló J., Sanchis P., Isern B., Prieto R.M., Costa-Bauzá A., Caldés O.J., Bonnin O., Garcia-Raja A., et al. Relationship between Urinary Level of Phytate and Valvular Calcification in an Elderly Population: A Cross-Sectional Study. PLoS ONE. 2015;10:0136560. doi: 10.1371/journal.pone.0136560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vucenik I., Shamsuddin A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: From laboratory to clinic. J. Nutr. 2003;133:3778–3784. doi: 10.1093/jn/133.11.3778S. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q., Kanthasamya A.G., Reddy M.B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245:101–108. doi: 10.1016/j.tox.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Larvie D.Y., Armah S.M. Estimated Phytate Intake Is Associated with Improved Cognitive Function in the Elderly, NHANES 2013–2014. Antioxidants. 2021;10:1104. doi: 10.3390/antiox10071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchis P., Rivera R., Berga F., Fortuny R., Adrover M., Costa-Bauza A., Grases F., Masmiquel L. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci. Rep. 2018;8:9619. doi: 10.1038/s41598-018-27853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omoruyi F.O., Stennett D., Foster S., Dilworth L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules. 2020;25:1720. doi: 10.3390/molecules25071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grases F., Simonet B.M., Prieto R.M., March J.G. Variation of InsP4, InsP5, InsP6 levels in tissues and biological fluids depending on dietary phytate. J. Nutr. Biochem. 2001;12:595–601. doi: 10.1016/S0955-2863(01)00178-4. [DOI] [PubMed] [Google Scholar]
- 58.Grases F., Simonet B.M., Vucenik I., Prieto R.M., Costa-Bauzá A., March J.G., Shamsuddin A.M. Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. BioFactors. 2001;15:53–61. doi: 10.1002/biof.5520150105. [DOI] [PubMed] [Google Scholar]
- 59.Grases F., Isern B., Perelló J., Sanchis P., Prieto R.M. Absorption of myo-inositol hexakisphosphate (InsP6) through the skin: Study of the matrix effects. Mechanism of phytate topical absorption. Front. Biosci. 2005;10:799–802. doi: 10.2741/1573. [DOI] [PubMed] [Google Scholar]
- 60.Grases F., Isern B., Perelló J., Sanchis P., Prieto R.M., Costa-Bauzá A. Absorption of myo-inositol hexakisphosphate (InsP6) through the skin in humans. Pharmazie. 2006;61:652. [PubMed] [Google Scholar]
- 61.Weaver C.M., Kannan S. In: Phytate and mineral bioavailability. Food Phytates. 1st ed. Reddy N.R., Sathe S.K., editors. CRC Press; Boca Raton, FL, USA: 2001. pp. 227–240. [Google Scholar]
- 62.Zhang Y.Y., Stockmann R., Ng K., Ajlouni S. Crit Revisiting phytate-element interactions: Implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Rev. Food Sci. Nutr. 2022;62:1696–1712. doi: 10.1080/10408398.2020.1846014. [DOI] [PubMed] [Google Scholar]
- 63.Castro-Alba V., Lazarte C.E., Bergenståhl B., Granfeldt Y. Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci. Nutr. 2019;7:2854–2865. doi: 10.1002/fsn3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim O.H., Booth C.J., Choi H.S., Lee J., Kang J., Hur J., Jung W.J., Jung Y.S., Choi H.J., Kim H., et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. eLife. 2020;9:e52709. doi: 10.7554/eLife.52709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aggarwal V., Seth A., Aneja S., Sharma B., Sonkar P., Singh S., Marwaha R.K. Role of calcium deficiency in development of nutritional rickets in indian children: A case control study. J. Clin. Endocrinol. Metab. 2012;97:3461–3466. doi: 10.1210/jc.2011-3120. [DOI] [PubMed] [Google Scholar]
- 66.Al Hasan S.M., Hassan M., Saha S., Islam M., Billah M., Islam S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr. 2016;2:24. doi: 10.1186/s40795-016-0064-8. [DOI] [Google Scholar]
- 67.Gibson R.S., Bailey K.B., Gibbs M., Ferguson E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010;31:S134–S146. doi: 10.1177/15648265100312S206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from PREDIMED-Plus trial Steering Committee. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of PREDIMED-Plus trial Steering Committee.