Abstract
Exposure to endocrine‐disrupting chemicals (EDCs) is unavoidable, which represents a public health concern given the ability of EDCs to target the ovary. However, there is a large gap in the knowledge about the impact of EDCs on ovarian function, including the process of ovulation. Defects in ovulation are the leading cause of infertility in women, and EDC exposures are contributing to the prevalence of infertility. Thus, investigating the effects of EDCs on the ovary and ovulation is an emerging area for research and is the focus of this review. The effects of EDCs on gametogenesis, uterine function, embryonic development, and other aspects of fertility are not addressed to focus on ovarian‐ and ovulation‐related fertility issues. Herein, findings from epidemiological and basic science studies are summarized for several EDCs, including phthalates, bisphenols, per‐ and poly‐fluoroalkyl substances, flame retardants, parabens, and triclosan. Epidemiological literature suggests that exposure is associated with impaired fecundity and in vitro fertilization outcomes (decreased egg yield, pregnancies, and births), while basic science literature reports altered ovarian follicle and corpora lutea numbers, altered hormone levels, and impaired ovulatory processes. Future directions include identification of the mechanisms by which EDCs disrupt ovulation leading to infertility, especially in women.
Keywords: endocrine‐disrupting chemicals, fertility, ovary, ovulation, reproduction
1. SCOPE OF REVIEW
Endocrine‐disrupting chemicals (EDCs) are exogenous chemicals that interfere with any aspect of hormone action, and exposure to EDCs can cause physiological defects and susceptibility to disease states (Gore et al., 2015; La Merrill et al., 2020). EDCs have the ability to alter the concentrations of endogenous hormones, disrupt the transport of hormones, agonize, or antagonize hormone receptors, and alter the number of hormone receptors (Gore et al., 2015; La Merrill et al., 2020). By disrupting the normal functions of the endocrine system, exposure to EDCs can potentiate risks of reproductive dysfunction (Johansson et al., 2017; Skakkebaek, 2016), metabolic and cardiovascular diseases (Heindel et al., 2017; Kahn et al., 2020; Kirkley & Sargis, 2014), cognitive defects (Braun, 2017; Ghassabian & Trasande, 2018), and certain cancers (Heindel et al., 2017; Scsukova et al., 2016).
The focus of this review is to present current literature investigating the effects of EDC exposures on ovarian‐ and ovulation‐related fertility outcomes. Among reproductive endpoints, little is known regarding the effects of EDC exposure on the ovarian control of ovulation and how these effects on the ovary can impact female fertility. As described below, the ovary is an endocrine organ, ovulation is an endocrine‐mediated process, and common mechanisms of EDC toxicities include decreased hormone synthesis and antagonism of hormone receptors. Thus, EDCs can target ovulation leading to impairments in fertility. The EDCs included in this review are phthalates, bisphenols, per‐ and poly‐fluoroalkyl substances (PFAS), flame retardants, parabens, and triclosan. These EDCs were selected due to their presence and/or persistence in the environment, ubiquitous human exposure, and links to reproductive dysfunction. The effects of EDCs on other aspects of fertility, such as gametogenesis, uterine function, placentation, fetal development, and so forth, are outside of the scope of this review but are reviewed elsewhere (Basak et al., 2020; Caserta et al., 2021; Ge et al., 2019; Gingrich et al., 2020; Gore et al., 2015; Spencer et al., 2012; Warner et al., 2021).
2. EDCs AND OVULATION‐RELATED FERTILITY ISSUES
Ovulation is the release of the egg from the ovary and is the cornerstone for female fertility. It is a hormonal process that is regulated by the hypothalamus, the anterior pituitary, and the ovary (referred to as the HPO axis). Because it is an endocrine system‐mediated process, EDCs have the potential to target the ovary and disrupt ovulation, which can cause infertility. In fact, exposure to EDCs is recognized as a contributing factor to the prevalence of infertility in women (Chiang et al., 2017; Crain et al., 2008; Foster et al., 2008; Gore et al., 2015; Rattan et al., 2017).
If women fail to ovulate, natural fertilization and subsequent pregnancy cannot occur. As such, defects in ovulation are the leading cause of infertility in women and are attributed to 30% of all cases of female infertility (National Collaborating Center & Children's, 2013). Thus, EDC‐induced impairments in the ovulatory process can lead to the negative health outcomes and economic burdens associated with infertility. Specifically, infertile women seeking to conceive experience increased stress levels, impairments in physical and mental health, and diminished social functioning, all of which result in a significant decreased quality of life (Chachamovich et al., 2010; Mousavi et al., 2013). Additionally, the diagnosis and treatment of infertility negatively impact the economy by costing society ~$5 billion annually (Macaluso et al., 2010). This burden is intensified on a personal level because infertility treatments are not usually covered by insurance, meaning couples may spend over $12,000 in out of pocket expenses per cycle of in vitro fertilization (IVF) (Katz et al., 2011). Given the negative health outcomes, the economic burdens, and the potential for EDCs to disrupt ovulation and fertility, there is a need to further understand the effects and mechanisms by which EDCs impair ovarian function and ovulation.
3. OVULATION
Egg release from the preovulatory antral follicle occurs around Day 16 of an average 29‐day menstrual cycle in women (Bull et al., 2019; Faust et al., 2019). Follicles are the functional units of the ovary that contain the oocyte (the female gamete that becomes the egg upon ovulation; [Duncan et al., 2020]) surrounded by somatic cells called granulosa cells and theca cells. Antral follicles are a mature follicle type in the ovary that also contain a fluid filled cavity called the antrum. These follicles produce abundant levels of sex steroid hormones to regulate the menstrual cycle (Duffy et al., 2019; Hannon & Flaws, 2015).
At the initiation of a new menstrual cycle, the hypothalamus and anterior pituitary secrete gonadotropin‐releasing hormone (GnRH) and follicle‐stimulating hormone (FSH), respectively. FSH acts on granulosa cells to stimulate follicle growth, development, and production of estradiol (E2; potent estrogen). Early stage antral follicles respond to FSH, and one is picked to become a dominant follicle, while the other subordinate follicles undergo apoptotic demise (referred to as atresia). The dominant, preovulatory follicle continues to produce abundant levels of E2. Now beyond a threshold level, this increase in E2 levels causes positive feedback resulting in the luteinizing hormone (LH) surge from the pituitary. The LH surge initiates the ovulatory cascade by binding to LH receptors (LHCGR) on granulosa cells. Specifically, LH action stimulates several biological processes in the follicle that are required for egg release, which are described in detail below. The LH surge also causes differentiation of an E2‐producing follicle into a predominantly progesterone (P4)‐producing corpus luteum (CL). Increased levels of P4, as well as a smaller spike of E2, from the CL are required for the initiation and maintenance of pregnancy. If pregnancy occurs, the CL remains functional and continues producing P4; however, in the absence of pregnancy, the CL undergoes regression, P4 levels decline, and the menstrual cycle starts anew (Hannon & Curry, 2018).
The periovulatory period, the time between the initiation of the LH surge and egg release, is around 36 h in women. During this period, extensive developmental changes take place in the follicle before egg release and transformation to the CL (Duffy et al., 2019). LH action through LHCGR activates numerous signaling pathways, including cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) signaling, extracellular regulated kinase 1/2 (ERK1/2) signaling, and phospholipase C/inositol triphosphate/diacylglycerol signaling (Duffy et al., 2019; Richards & Ascoli, 2018). These signaling pathways downstream of LH/LHCGR then increase the levels of genes/proteins and hormones that serve as mediators of ovulation. The most well‐documented mediators of ovulation include P4; P4 receptor; prostaglandins; epidermal growth factor (EGF)‐like peptides; EGF receptor; and numerous chemokines, cytokines, proteases, vascular growth factors, and transcription factors (Duffy et al., 2019).
LH levels dramatically decline following the apex of the surge, meaning it is the ovulatory mediators that facilitate the biological processes that are requisite for ovulation and subsequent fertility. These biological processes include: (1) leukocyte invasion for chemokine and cytokine signaling, (2) angiogenesis, (3) extracellular matrix (ECM) remodeling for follicle wall breakdown and egg release, (4) meiotic maturation of the oocyte (arrested in meiotic prophase I) to become an egg with an extruded polar body (meiotic metaphase II), (5) expansion of the cumulus oocyte complex to form a matrix for transport and fertilization, and (6) differentiation of granulosa cells to P4‐producing luteal cells (Duffy et al., 2019). Table 1 provides an overview of the most well‐established signaling pathways and ovulatory mediators (information surmised from [Duffy et al., 2019; Richards & Ascoli, 2018]). While this list is not comprehensive, blocking the synthesis and/or actions of several of these mediators can result in failure of ovulation and infertility (Duffy et al., 2019). Figure 1 summarizes antral follicle progression to the preovulatory stage, ovulation, and the follicular processes involved in egg release and CL formation.
Table 1.
Key ovulatory mediators induced by luteinizing hormone
| Mediator | Ovulatory function(s) |
|---|---|
| A disintegrin and metalloproteinase with thrombospondin motifs (ADAMs/ADAMTSs) | Follicle wall proteolysis |
| Angiopoietins (ANGTPs: ANGPT1, ANGPT2) | Angiogenesis |
| Cyclic adenosine monophosphate/protein kinase A | Signaling cascade downstream of LHCGR activation; induces ovulatory processes; and expression of ovulatory mediators |
| Epidermal growth factor (EGF)‐like factors (amphiregulin, epiregulin, betacellulin) | Cumulus oocyte complex expansion |
| Extracellular regulated kinase 1/2 (ERK1/2) | Signaling casecade downstream of LHCGR and EGF receptor activation; induces ovulatory processes; and expression of ovulatory mediators |
| Leukocyte secreted chemokines/cytokines (interleukins, CCLs, CXCLs) | Immune cell trafficking; inflammation; angiogenesis; cumulus ooctye complex expansion; follicle wall proteolysis |
| Matrix metalloproteinases (MMPs) | Follicle wall proteolysis |
| Plasminogen activator system | Follicle wall proteolysis |
| Phospholipase C/inositol triphosphate/diacylglycerol | Signaling casecade downstream of LHCGR activation; induces ovulatory processes; and expression of ovulatory mediators |
| Progesterone | Inflammation; regulator of blood flow; follicle wall proteolysis |
| Progesterone receptor | Transcriptional regulation; inflammation; follicle wall proteolysis |
| Prostaglandins (PGE2, PGF2α) | Angiogenesis; regulators of blood flow; inflammation; cumulus oocyte complex expansion; follicle wall proteolysis |
| Vascular endothelial growth factors (VEGFs: VEGFA, VEGFC, VEGFD, placental growth factor) | Angiogenesis; regulators of blood flow |
Abbreviation: LHCGR, LH receptors.
Figure 1.
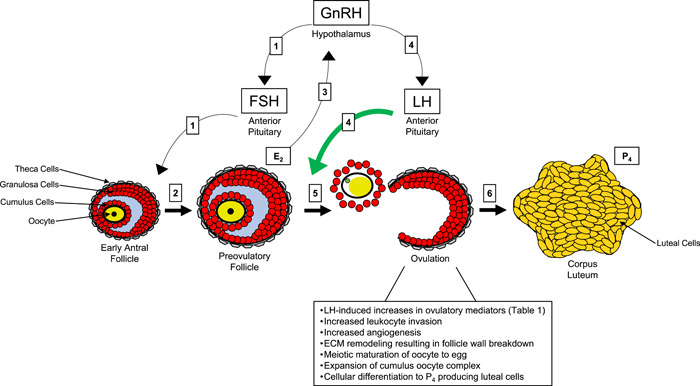
Regulation of preovulatory follicle development, ovulation, and corpus luteum (CL) formation. Boxes 1: At the initiation of the menstrual cycle, gonadotropin‐releasing hormone (GnRH) from the hypothalamus stimulates follicle‐stimulating hormone (FSH) secretion from the anterior pituitary. FSH acts on early antral follicles. The oocyte is depicted in yellow, granulosa cells in red, theca cells in gray, and follicular fluid in blue. Box 2: FSH action causes the development of a single, dominant preovulatory follicle. Box 3: The preovulatory follicle produces abundant levels of estradiol (E2), which causes positive feedback to the hypothalamus. Boxes 4: This positive feedback increases GnRH pulsatility, which initiates the luteinizing hormone (LH) surge. LH binds to LH receptors on granulosa cells of the follicle. Box 5: LH action induces the ovulatory cascade by increasing ovulatory mediators and stimulating several ovarian processes (text box below ovulation; ECM, extracellular matrix), culminating in egg release. The released egg is contained in an expanded matrix with the cumulus cells and has undergone meiotic maturation to metaphase II as depicted by germinal vesicle breakdown and the green extruded polar body. Box 6: Following release of the egg, the remnant follicular cells are now differentiated into luteal cells of the CL that predominantly produce progesterone (P4). P4 from the CL, as well as E2, are required for the initiation and maintenance of pregnancy.
Since EDCs disrupt hormonal action and ovulation is a hormonally driven process, it is imperative to understand how exposure to EDCs disrupts fertility by impairing the process of ovulation. This is supported by evidence suggesting that EDCs may contribute to the prevalence of infertility in women (Chiang et al., 2017; Crain et al., 2008; Foster et al., 2008; Gore et al., 2015; Rattan et al., 2017). Direct disruptions in the ovulatory process by EDC exposures can occur at multiple levels. EDCs can inhibit the production or decrease the levels of ovulatory mediators, such as P4, prostaglandins, and vascular endothelial growth factors. EDCs can alter the number or agonize/antagonize receptors involved in the ovulatory cascade, including P4 receptor and EGF receptor, which can impair downstream ovulatory signaling. EDCs can also interfere with the biological processes that facilitate egg release and CL formation, including cumulus oocyte complex expansion and ECM remodeling.
EDC‐induced defects in earlier stages of folliculogenesis can also indirectly affect ovulation and fertility. EDCs can deplete the primordial follicle reserve by causing atresia or premature activation, which would ultimately eliminate the number of follicles that are capable of ovulation. Further, EDCs can inhibit FSH‐dependent follicle maturation. Inhibition of preantral and early antral follicle growth and development would decrease the necessary levels of E2 and LHCGR to promote ovulation. Increased atresia at these stages would also reduce the population of follicles that could eventually ovulate. These direct and indirect effects on the ovulatory process can potentially lead to menstrual cycle irregularity with oligoovulation (infrequent ovulation) or anovulation (absence of ovulation), and ultimately subfertility or complete infertility.
4. METHODS TO INVESTIGATE THE IMPACT OF TOXICANT EXPOSURES ON OVULATION
To understand the impact of EDC exposure on ovarian function and ovulation, epidemiological and basic science approaches have been employed. Epidemiologists have used clinical data from IVF clinics, birthing registries, infertility treatment registries, and reproductive health surveys (Smarr, Sapra et al., 2017). Bench scientists have used human ovarian models and laboratory animal models to directly assess ovarian endpoints following EDC exposure in vivo and in vitro (Gore et al., 2015).
4.1. Epidemiological approaches
Studies utilizing patient populations in the IVF setting provide numerous sources of data pertaining to environmental exposures and ovarian outcomes. Women undergoing IVF are routinely subjected to blood and urine collections to assess pregnancy status. With appropriate patient consent and IRB approval, environmental health scientists can use these biological specimens to measure the levels of common EDCs, and statistical modeling can be applied to identify associations between exposure to these EDCs (or more specifically the levels of EDCs in blood/urine) and IVF outcomes (Maity et al., 2014).
IVF outcomes include clinical data captured throughout the entire course of the IVF cycle, and these outcomes can be used for analysis following patient consent and IRB approval. The initiation of an IVF cycle involves hormonal stimulation of the women to control their menstrual cycle and promote the development of several dominant antral follicles. Important measurements related to follicle health that can be collected at this timepoint include antral follicle counts via ultrasound and serum E2 levels. As the follicles reach the preovulatory stage during the IVF cycle, the patients are treated with human chorionic gonadotropin (hCG; potent and stable LH‐analogue) to initiate the ovulatory process. Just before release of the eggs from the periovulatory follicles, follicular aspiration is used to retrieve the eggs. These follicle aspirates also contain follicular fluid, which is another biological specimen by which EDCs can be measured (Y. Du et al., 2019). Important follicle and oocyte health data that can be collected here include the number of total oocytes retrieved and the number of metaphase II (meiotically matured and fertilizable) eggs retrieved. Metaphase II eggs are then fertilized in the clinic, and embryos are matured in vitro before implantation. At this stage, additional data related to oocyte competence that can be used for analysis include the number of eggs that underwent successful fertilization and the developmental quality of the embryo. Finally, implantation status (fetal production of hCG), clinical pregnancy (via ultrasound), and live births can be collected as indicators of fertility.
Additional data that can be collected from women outside of the IVF clinic include menstrual cycle length and time to pregnancy (a measure of fecundity, which is the biological ability to reproduce). Alterations in cycle length or time to pregnancy may be due to direct effects of EDCs on ovarian function or due to indirect effects on other endocrine organs. However, these defects can alter the timing of ovulation or can lead to potential ovulation and fertility issues.
4.2. Basic science approaches
Basic science approaches using in vivo and in vitro human cell/tissue and animal models allow scientists to assess the direct effects and mechanisms by which EDCs exert toxicities on ovarian function, the ovulatory process, and fertility. Following in vivo dosing of laboratory animals with EDCs, scientists can evaluate the impact of exposure on the length of the reproductive cycle, the development of antral follicles, levels of reproductive hormones, actual egg release, and transformation of the follicle to the CL. These effects are known EDC‐induced ovarian toxicities that can alter the timing of ovulation, number of ovulations in a poly‐ovulatory species, and/or hormonal‐mediated ovulatory processes, all of which can lead to potential ovulation and fertility issues (Hoyer, 2005; Mark‐Kappeler et al., 2011). It can be difficult to ascertain if the defects in fertility in these in vivo experiments are due to direct effects on the ovary or on the hypothalamic‐pituitary axis. To bypass this, scientists have injected adult animals and prepubertal animals (that do not have a mature HPO axis) with pregnant mare serum gonadotropin (PMSG; an analogue of FSH) to initiate antral follicle development and with hCG 48 h later to stimulate the ovulatory cascade, which is similar to the hormonal protocol employed in the IVF clinic (Hannon et al., 2018). This PMSG:hCG superovulation model can be used to directly assess the impact on ovulatory events in the ovary by circumventing toxicities in the hypothalamus and pituitary.
In vitro cell and tissue culture techniques further assess the direct effects of EDC exposure on the ovary and ovulatory outcomes. Antral follicles can be developed in culture, treated with EDCs, and treated with hCG to visually quantify ovulation rates in vitro (Land et al., 2020; Y. Wang et al., 2020). Instead of utilizing the whole follicle, scientists can directly expose granulosa cells and/or cumulus oocyte complexes to EDCs. These specimens can come from follicles from adult animals or from the previously mentioned PMSG‐primed animals, where follicles are punctured to isolate granulosa cells or oocytes. Treatment with hCG and EDCs is then conducted in vitro (Hannon et al., 2018). Specific in vitro assays have also been developed to investigate the ovulatory regulation of processes such as expansion of the cumulus oocyte complex (Hsieh et al., 2007), angiogenesis (Hannon et al., 2018), leukocyte migration (Al‐Alem et al., 2015), and proteolytic activity for ECM remodeling (Curry & Smith, 2006). However, the application of EDC exposures in these models is lacking. Novel advances in bioengineering have also enabled scientists to use biomaterials, 3D printing, organ‐on‐a‐chip, and microfluidic culture platforms, all of which have abundant potential for the incorporation of toxicology studies (Xiao et al., 2017; Zubizarreta & Xiao, 2020).
Gene expression, protein localization and quantification, and enzyme activity analyses are methods that can be employed in the in vivo and in vitro models described above to elucidate the mechanisms of EDC‐induced toxicity in the ovary. Such information is required to potentially alleviate toxicities by discovering molecular targets for therapeutic interventions.
5. REVIEW CRITERIA
The following sections are grouped by EDC and contain subsections on human epidemiological data and on basic science studies. The included EDCs are considered emerging threats with evidence linking exposure to reproductive dysfunction. Studies published within the last 10 years were included, unless recent data were not available for a given EDC or a particular study published over 10 years ago specifically investigated the impact of exposure on the ovulatory process directly. In animal studies, a preference was given to studies utilizing adult mammalian/rodent models. If data were lacking, nonmammalian, in utero, neonatal, and transgenerational studies were included.
6. PHTHALATES
Phthalates are a class of several synthetic chemicals composed of alkyl diesters of phthalic acid and varying lengths of alkyl chains (Hannon & Flaws, 2015; Heudorf et al., 2007). They are used as plasticizers, solvents, and excipients in the manufacturing of common consumer goods. As plasticizers (predominantly in polyvinyl chloride), phthalates impart flexibility to food and beverage packaging, building materials, and medical supplies. Being used as solvents, phthalates are incorporated in personal care/cosmetic products. As excipients, certain phthalates are used in dietary supplements.
The widespread production and use of phthalates in a myriad of products leads to daily human exposure via oral ingestion, inhalation, and dermal absorption (Heudorf et al., 2007; Högberg et al., 2008; James‐Todd et al., 2012; Koch & Calafat, 2009). This is because phthalates are non‐covalently bound to the materials that they are manufactured into and they leach into the items humans consume and into the environment (Hannon & Flaws, 2015; Heudorf et al., 2007). As such, human exposure to phthalates is unavoidable and virtually 100% of the general population is exposed to phthalates on a daily basis (Heudorf et al., 2007; Högberg et al., 2008; James‐Todd et al., 2012; Koch & Calafat, 2009). Important for this review, women typically have an increased exposure profile to phthalates compared to men (Blount et al., 2000; Silva et al., 2004). Upon absorption, phthalates are rapidly metabolized in the gastrointestinal system. Specifically, the parent phthalate that humans are exposed to (the phthalate diester) is cleaved to its respective monoester metabolite, and it is the metabolite that is often thought of as bioactive and toxic (Koch & Calafat, 2009). Though a conclusive mechanism of endocrine disruption is not defined, phthalates have antiandrogenic, and antiestrogenic properties, have weak affinity for estrogen receptors and peroxisome proliferator‐activated receptors, and have been shown to act on the ovary (Gore et al., 2015; Hannon & Flaws, 2015; Jobling et al., 1995; Maloney & Waxman, 1999). Figure 2 depicts a brief summation of the effects of phthalates on ovarian‐ and ovulation‐related outcomes.
Figure 2.
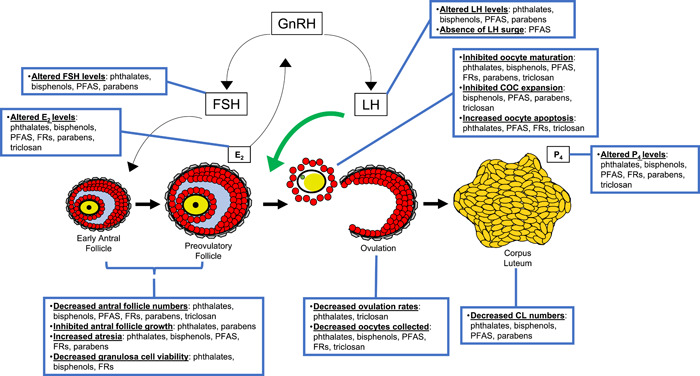
Overview of the effects of EDCs on ovarian‐ and ovulation‐related fertility outcomes. CL, corpora lutea; COC, cumulus oocyte complex; EDCs, endocrine‐disrupting chemicals; E2, estradiol; FRs, flame retardants; FSH, follicle‐stimulating hormone; GnRH, gonadotropin‐releasing hormone; LH, luteinizing hormone; PFAS, per‐ and poly‐fluoroalkyl substances; P4, progesterone.
6.1. Phthalates: epidemiological evidence
A considerable amount of epidemiological data are present in the literature linking phthalate exposure with poor IVF and fertility outcomes. In short, studies investigating IVF data have reported that increased urinary levels of phthalates were associated with lower total and mature egg yield, decreased numbers of clinical pregnancies, and decreased live births depending upon the cohort studied and metabolite measured (Begum et al., 2021; Deng et al., 2020; Hauser et al., 2016; Machtinger et al., 2018; Mínguez‐Alarcón et al., 2019). In fact, one study suggested that plasma levels of certain phthalates were increased in infertile women compared to fertile women (Pednekar et al., 2018), while another has reported that increased urinary levels of monoethyl phthalate (MEP) were associated with decreased fecundity and a longer time to pregnancy (Thomsen et al., 2017).
More specific to IVF outcomes, women with increased urinary levels of phthalate metabolites belonging to di(2‐ethylhexyl) phthalate (DEHP) had lower numbers of total and mature eggs retrieved during IVF, and decreased incidence of clinical pregnancies and live births (Hauser et al., 2016). These measured metabolites include mono(2‐ethylhexyl) phthalate (MEHP), mono(2‐ethyl‐5‐hydroxyhexyl) phthalate (MEHHP), mono(2‐ethyl‐5‐oxohexyl) phthalate (MEOHP), and mono(2‐ethyl‐5‐carboxypentyl) phthalate (MECPP). In the same study, increased urinary levels of monocarboxyisononyl phthalate, a metabolite of diisononyl phthalate (DiNP), were associated with decreased total and mature eggs retrieved (Hauser et al., 2016). In a similar study, increased urinary levels of DEHP metabolites, monobutyl phthalate (MBP), and MEP were associated with decreased numbers of total and mature eggs collected and decreased numbers of fertilized eggs, while the DEHP metabolites were additionally associated with poor quality embryos (Machtinger et al., 2018). Even a non‐phthalate replacement, di(isononyl)cyclohexane‐1,2‐dicarboxylate (DINCH), is linked to poor IVF outcomes. Increased urinary DINCH metabolites were associated with decreased levels of E2 and total number of oocytes collected (Mínguez‐Alarcón et al., 2016).
Beyond oocyte parameters, another study reported that increased urinary levels of DEHP metabolites were associated with lower probabilities of implantation, clinical pregnancy, and live birth (Mínguez‐Alarcón et al., 2019). Increased urinary levels of MBP and MEP, as well as the previously mentioned MEOHP, were inversely associated with normal fertilization odds in an additional study investigating a cohort of Chinese IVF patients (Deng et al., 2020). Following fertilization, monomethyl phthalate (MMP) and MEP levels were associated with reduced odds to gain a good quality blastocyst (Deng et al., 2020). Similarly, doublings in women's urinary MBP and monohexyl phthalate (MHxP) levels were associated with a lower likelihood of pregnancy and live birth, respectively (Begum et al., 2021). Interestingly, when follicular fluid is used as the matrix to measure phthalate levels, these associations with poor IVF outcomes are no longer present (Y. Y. Du et al., 2016). While urinary and follicular fluid levels of phthalates were correlated in these two matrices, the null associations could be due to the cohort studied and the limited number of participants (Y. Y. Du et al., 2016).
Intrafollicular dynamics, including hormone levels and follicle counts, may be a target of phthalate toxicity. Increased levels of MMP in follicular fluid were associated with decreased levels of intrafollicular E2, P4, and testosterone; increased MEP and MEHP were associated with decreased levels of P4; and, interestingly, increased levels of MBP and MEHHP were associated with increased levels of E2 (both metabolites), P4 (MEHHP), and testosterone (MEHHP) (Y. Du et al., 2019). In another study, increased urinary levels of DEHP metabolites were associated with decreases in antral follicle counts in women (Messerlian et al., 2016). Thus, the intrafollicular hormonal milieu may be altered in response to phthalate exposure either by altered steroidogenic capacity or by decreased amounts of steroidogenic follicles.
Altered menstrual cycle length has also recently been reported in the literature. Increased urinary levels of monocarboxyoctyl phthalate were associated with a shorter luteal phase; however, associations with other outcomes (follicular phase length, time to pregnancy, and early pregnancy loss) were not present (Jukic et al., 2016). Interestingly, the same study reported that DEHP metabolites were associated with reduced early pregnancy loss (Jukic et al., 2016).
6.1.1. Summary
The major findings suggest that increased exposure to phthalates is associated with reduced fecundity, poor IVF outcomes including decreased eggs collected and live births, altered sex steroid hormone levels, and disrupted menstrual cycle length. Phthalate‐induced alterations in antral follicle numbers and/or steroidogenesis can potentially disrupt the normal hormonal milieu leading to disruptions in the length of the menstrual cycle and ovulatory capacity. A strength is that several epidemiological studies using different cohorts report these findings. However, much less is known regarding the mechanism of phthalate action.
6.2. Phthalates: basic science evidence
Multiple studies in the literature have investigated the effects of phthalate exposure on general ovarian function in vivo (reviewed in Hannon & Flaws, 2015; Panagiotou et al., 2021); however, very few studies have directly assessed the effects on ovulation and fertility. In one study, adult mice orally exposed to DEHP and DiNP for 10 days had disrupted estrous cyclicity, reduced time to mating, and reduced ability to get pregnant 3‐ and 9‐months postdosing (Chiang & Flaws, 2019). This is, in part, attributed by phthalate‐induced decreases in antral follicle numbers and alterations in the levels of sex steroid hormones (Chiang et al., 2020a). When this group observed reproductive endpoints at 12‐, 15‐, and 18‐months post‐10 day dosing, the mice experienced similar disruptions in estrous cyclicity and reduced fertility (Chiang et al., 2020b). Similarly, oral exposure to dibutyl phthalate (DBP) in mice for 10 days decreased antral follicle and CL counts accompanied with disrupted estrous cyclicity and decreased serum E2 levels (Sen et al., 2015). In another study, oral DEHP exposure in mice inhibited oocyte maturation, decreased fertilization of oocytes, and increased DNA damage and apoptosis in oocytes (Lu et al., 2019). In support of these findings, some of the initial studies that investigated the effects of phthalate exposure on the ovary, albeit using non‐environmentally relevant doses, have shown that phthalate exposure inhibits ovulation and reduces fertility in rats and mice (Davis et al., 1994; Heindel et al., 1989; Sekiguchi et al., 2003).
Supporting the results mentioned in the first paragraph, several studies have reported that oral DEHP exposure in mice and rats disrupts estrous cycle length and/or the time spent in specific stages of the estrous cycle. Some studies suggest that DEHP exposure increased the amount of time spent in estrus and decreased the amount of time spent in metestrus/diestrus (Fu et al., 2021; Hannon et al., 2014, 2016), while others suggest the opposite (Hannon et al., 2016; N. Li et al., 2020), with one study reporting an overall increased length of the entire cycle (N. Li et al., 2020).
Similar to disruptions in estrous cyclicity, increased atretic demise of follicles following in vivo exposure to phthalates can also impact the ovulatory process and is shared across several studies in the literature. Using multiple strains of mice and rats, DEHP, DBP, and dimethyl phthalate (DMP) exposure induced atresia in follicles and/or granulosa cell apoptosis (Fu et al., 2021; Hannon et al., 2016; N. Li et al., 2020; Liu & Craig, 2019; Mei et al., 2019). These defects can decrease the number of follicles available to ovulate or hinder the follicles' response to the ovulatory LH stimuli by disrupting granulosa cell function.
Changes in hormone levels in response to phthalate treatment in vivo have also recently been identified in the literature. DEHP has been shown to decrease E2, P4, and testosterone levels, while also decreasing FSH and LH levels in mice and rats (Fu et al., 2021; N. Li et al., 2020). While these changes were not investigated in the context of ovulation, they imply that phthalate exposure may alter the LH surge or the ability of the follicle to respond to LH. Interestingly, exposure to DMP in mice increased E2 and LH levels but also decreased FSH levels (Mei et al., 2019). These discrepancies could be due to chemical, exposure paradigm, and/or species and strain tested. Regardless, hormonal dysregulation by phthalate exposure may contribute to potential defects in ovulation.
Studies utilizing phthalate exposures in vitro have further confirmed ovarian defects pertaining to ovulatory function. A study from the authors' laboratory used an environmentally relevant mixture of six common parent phthalates in a mouse antral follicle culture system. Findings revealed that phthalate exposure at all doses tested (1−500 μg/ml) directly decreased ovulation rates when the follicles were simultaneously treated with hCG (Land et al., 2020). By using intact follicles as a model, egg release was able to be assessed visually, and the mixture mimicked human exposure as it was derived from urinary phthalate levels in pregnant women. The authors suggested that decreased prostaglandin production, impaired P4 receptor signaling, and dysregulated ECM remodeling were the mechanisms driving the decrease in ovulation rates (Land et al., 2020). This is perhaps the first evidence suggesting that phthalate exposure directly inhibits ovulation.
Though not investigating ovulation directly, this mixture and an environmentally relevant phthalate metabolite mixture have also been used in another laboratory to assess the impacts on antral follicles. The parent and respective metabolite mixture inhibited antral follicle growth, altered sex steroid hormone levels (including decreased E2 levels), and induced oocyte fragmentation (Meling et al., 2020; C. Zhou & Flaws, 2016). The mechanisms by which the mixtures disrupted antral follicle functionality were via alterations in cell cycle regulators, apoptosis and oxidative stress response, and steroidogenic enzymes and receptors (Meling et al., 2020; C. Zhou & Flaws, 2016). Similar toxicities have been observed using the whole follicle culture system and single phthalate exposures. DEHP, MEHP, and DBP exposures decrease antral follicle growth and the production of sex steroid hormones through mechanisms involving oxidative stress, apoptosis, and inhibition of steroidogenesis and the cell cycle (Craig et al., 2013; Gupta et al., 2010; Hannon, Brannick, Wang & Flaws, 2015; Hannon, Brannick, Wang, Gupta et al., 2015; Rasmussen et al., 2017; W. Wang, Craig, Basavarajappa & Gupta et al., 2012; W. Wang, Craig, Basavarajappa & Hafner et al., 2012). These findings are all suggestive of impaired follicle development during the follicular phase, which would hinder the ability of the follicle to ovulate.
6.2.1. Summary
Studies utilizing animal models have greatly supplemented the findings presented in the epidemiology section, whereby exposure to phthalates directly targets the ovary to limit the growth, development, and steroidogenic capacity of antral follicles. A potential mechanism for these ovarian toxicities is increased oxidative stress leading to atresia. Important findings also suggest that phthalates directly decrease ovulation rates and impair meiotic maturation of the oocyte to the egg. A major strength in the basic science literature is the identification of a potential mechanism of inhibited ovulation, which includes impaired P4 receptor signaling, prostaglandin function, and remodeling of the ECM. These defects in ovarian function likely contribute to the decreases in fertility that have been reported in rodent models in vivo.
7. BISPHENOLS
Bisphenols are a group of highly produced chemicals used as plasticizers in polycarbonate plastics, the epoxy resins of aluminum cans, and in cosmetic and personal care products. The chemical structures of bisphenols contain two hydroxyphenyl functional groups linked by a methylene bridge or other functional group, depending on the specific bisphenol. Similar to phthalates, leaching of bisphenols into consumer goods leads to exposure via oral ingestion, dermal contact, and inhalation. Due to their extensive production, use, and broad applications, daily human exposure to bisphenols is ubiquitous. In fact, nearly 100% of the general population has detectible levels of several bisphenols in urinary samples, as measured in National Health and Nutrition Examination Survey (NHANES) participants (Lehmler et al., 2018).
Bisphenol A (BPA) is the most studied bisphenol. Its identification as an EDC has been thoroughly characterized given its structural similarity to E2, ability to act through E2 and other hormone/physiological receptors, and ability to cause reproductive and developmental toxicity (den Braver‐Sewradj et al., 2020; Peretz et al., 2014). Specific to this review, BPA exposure in women is associated with anovulation, lower antral follicle counts, disrupted menstrual cycle length, poor IVF outcomes (decreased total and mature eggs collected, fertilization rates, embryonic quality, and implantation rates), and infertility (Rattan et al., 2017; Ziv‐Gal & Flaws, 2016). Similar defects in ovarian and fertility outcomes are observed in animal models following exposure to BPA in vivo and in vitro (Peretz et al., 2014; Ziv‐Gal & Flaws, 2016). As such, public concern and regulatory restrictions on BPA have led to its phase out in several common consumer items. This has promoted the increased exposure to substitute BPA analogues, including bisphenol AF (BPAF), bisphenol B (BPB), bisphenol F (BPF), bisphenol M (BPM), bisphenol S (BPS), and bisphenol TMC (BPTMC) (den Braver‐Sewradj et al., 2020). However, a growing list of literature suggests that these structural analogues for BPA possess similar endocrine‐ and reproductive‐disrupting toxicities as BPA, though their effects are much less studied (den Braver‐Sewradj et al., 2020). Therefore, this review is focused on providing updates on the effects of these replacement bisphenols on ovarian and ovulatory outcomes. Figure 2 summarizes the findings from these studies.
7.1. Bisphenols: epidemiological evidence
Presently, there are no epidemiological studies that have investigated the link between BPA substitutes and ovulatory defects in women. What is known is that there is a negative association between BPS exposure and semen parameters in men (Ghayda et al., 2019). The absence of studies investigating women's reproductive health is concerning and is a major weakness in the literature. This is especially true given the abundance of evidence in animal studies (discussed in detail below) that suggest that exposure to substitutes can be as, if not more, toxic for reproductive health than BPA.
7.2. Bisphenols: basic science evidence
Antral follicles and ovulation are suggested to be targets of bisphenol toxicity. Adult rats exposed to BPS, BPF, and BPB intraperitonially for 28 days had decreased antral follicle and CL counts and increased atretic follicle counts, which was attributed to oxidative stress (Ijaz et al., 2020). Decreased CL counts were also observed in rats exposed prenatally to BPS and BPF (Kaimal et al., 2021). In another prenatal exposure study, BPS decreased antral follicle counts, egg maturation rates, and fertilization rates in mice once they reached early adulthood (M. Y. Zhang, Tian et al., 2020). A study using 28‐day old mice exposed to BPS for 4 weeks reported decreased antral follicle counts, also via alterations in the oxidative stress response (Nevoral et al., 2018). When the mice were stimulated with PMSG and hCG in this study, the 10 ng/kg/day group had a decreased fertilization rate, while the 100 ng/kg/day group had an increased fertilization rate (Nevoral et al., 2018). Gestational exposure to BPS and BPE in mice resulted in alterations in estrous cyclicity, mating difficulties, and reduced pregnancy rate (Shi et al., 2019). Interestingly, decreased pregnancy rates persisted in future generations when transgenerational exposure was assessed (Shi et al., 2019). Neonatal exposure to BPS via subcutaneous injections in rats also increased atretic follicle numbers when observed after puberty and caused alterations in estrous cyclicity, increases in testosterone and E2, and decreases in LH, FSH, and P4 (Ahsan et al., 2018).
Similarly, studies utilizing human granulosa cell lines have also suggested that bisphenol exposures increase oxidative stress leading to impaired cell viability. Increased oxidative stress was observed in human KGN cells exposed to BPS, BPF, and BPAF, with BPAF exposure causing decreased cell viability (Huang et al., 2020, 2021). In another study using KGN cells, cell viability was decreased following exposure to BPAF, BPM, BPTMC, with BPM exposure also increasing oxidative stress (Rajkumar et al., 2021). BPS and BPAF exposure in another human granulosa cell line, COV434, decreased cell viability via oxidative stress, and BPS decreased the levels of VEGFA, a prominent ovulatory mediator (Bujnakova Mlynarcikova & Scsukova, 2021a).
Recent reports suggest that bisphenol exposures may directly target the ovulatory process by reducing ovulation rates, oocyte maturation, and expansion of the cumulus oocyte complex. When adult mice were dosed with BPS intraperitoneally, then were superovulated with PMSG:hCG, BPS exposure decreased the number of eggs collected leading to impaired fertility outcomes (Nourian et al., 2017). BPS exposure to porcine cumulus oocyte complexes inhibited meiotic progression to the MII stage, disrupted oocyte meiotic spindle organization and chromosome alignment, and altered hyaluronic acid levels needed for proper cumulus cell expansion (Žalmanová et al., 2017). Similarly in ovine cumulus oocyte complexes, exposure to BPS in vitro decreased the percentages of eggs that reached the MII stage during maturation (Desmarchais et al., 2020). In mouse oocytes, BPAF and BPF exposure in vitro also decreased oocyte maturation to the MII stage (BPAF) and disrupted spindle organization (both compounds) (Ding et al., 2017; L. Yang, Baumann et al., 2020). In another study, BPS exposure to bovine cumulus oocyte complexes in vitro increased oocyte spindle abnormalities and chromosome misalignment (K. A. Campen et al., 2018). Similar findings were observed in mouse oocytes that underwent in vitro maturation following gestational and lactational exposure to BPS and BPF (Nevoral et al., 2021). Although the proportion of eggs reaching the MII stage following in vitro maturation were not altered in these last two studies, these findings suggest that fertilization and/or post‐fertilization events may be impacted by BPS exposure. Though not investigating the oocyte directly, human and ovine primary theca cells exposed to BPS in vitro had enhanced gap junction intracellular communication via a mechanism involving mitogen‐activated protein kinase signaling (Gingrich et al., 2021). Because increased gap junction intracellular communication is temporospatially regulated during ovulation and theca cell communication is necessary for intrafollicular trafficking of signaling molecules that promote oocyte maturation, these findings indicate that BPS exposure may inhibit ovulation by maintaining the oocyte in meiotic arrest.
Similar to findings from BPA exposures, alterations in steroidogenesis have been reported following exposure to replacement bisphenols. In human granulosa cells obtained from women undergoing IVF, BPS exposure in vitro decreased the levels of E2 and P4 (Amar et al., 2020). Exposure to BPS, BPF, and BPB in adult rats in vivo decreased plasma levels of E2, P4, FSH, and LH while also increasing the levels of testosterone (Ijaz et al., 2020). Ovine granulosa cells exposed to BPS in vitro had decreased levels of P4 and increased levels of E2 and E2 receptors (Téteau et al., 2020). Increased levels of E2 were also seen in bovine granulosa cells exposed to BPS in vitro and in adult mice and rats exposed postnatally to BPS in vivo (Ahsan et al., 2018; K. Campen et al., 2018; Shi et al., 2017). Conversely, E2 levels and cell proliferation were decreased by BPS exposure while cell viability and metabolic activity were increased in cultured porcine granulosa cells (Berni et al., 2019). In another study utilizing cultured porcine granulosa cells, exposure to BPS, BPF, and BPAF decreased P4 levels, while BPS also decreased E2 levels, and the highest dose of BPAF decreased cell viability (Bujnakova Mlynarcikova & Scsukova, 2021b).
7.2.1. Summary
Findings from basic science studies suggest that exposure to bisphenol substitutes altered granulosa cell viability via a mechanism involving oxidative stress and altered sex steroid hormone levels. A major strength in the literature is that these findings are from studies using primary human granulosa cells and human granulosa cell lines. These alterations may contribute to the increased atresia and disrupted estrous cyclicity that is reported in the literature. Defects in ovulation include decreased CL counts, decreased oocytes collected following PMSG:hCG stimulation, impaired oocyte meiotic maturation, and inhibited cumulus oocyte complex expansion, each of which may lead to the reported decreases in fertilization rates. While mechanisms leading to ovulatory defects are largely undefined, decreases in VEGFA in the COV434 human granulosa cell line suggests that angiogenesis may be targeted by bisphenol exposure. In total, these findings highlight the need to design epidemiological studies that investigate ovarian and ovulatory outcomes in cohorts of women.
8. PFAS
PFAS are a collection of thousands of manufactured chemicals with varying lengths of carbon chains containing a perfluoroalkyl moiety (Buck et al., 2011; Calafat et al., 2019). Long chain, legacy PFAS contain eight or more carbons, have long half‐lives, and tend to persist in the environment and the human body (Buck et al., 2011; Calafat et al., 2019). Examples of long chain PFAS include perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), which are the most well‐studied PFAS in the literature. These long chain PFAS were phased out in the 2000s, leading to the increased use of short chain PFAS containing seven or less carbons and other replacement PFAS (Buck et al., 2011; Calafat et al., 2019). Examples of these include perfluorobutane sulfonate (PFBS), perfluorohexanoate (PFHxA), and GenX (ammonium salt of 2,3,3,3,‐tetrafluoro‐2‐(1,1,2,2,3,3,3‐heptafluoropropoxy)‐propanic acid) (Buck et al., 2011; Calafat et al., 2019). While these compounds are thought to decrease the environmental and human body burden of PFAS, many of these chemicals are unregulated and their effects on human health are relatively untested (Calafat et al., 2019; Pan et al., 2020; Sunderland et al., 2019).
PFAS are used as polymers and surfactants in a variety of industrial and commercial applications due to their chemical stability and resistance to degradation. Specifically, PFAS are used as coatings on textiles, non‐stick cookware, food containers, and personal care products and cosmetics (Buck et al., 2011; Calafat et al., 2019). As such, exposure to a mixture of PFAS occurs via oral ingestion and dermal contact. PFAS can also move through soils to contaminate drinking water and can bioaccumulate in food sources (Buck et al., 2011; Calafat et al., 2019). Indicative of ubiquitous exposure, serum levels of PFAS are detected in nearly all of the general population (as measured in NHANES participants) (Calafat et al., 2019). As EDCs, PFAS have been shown to disrupt ovarian function, have estrogenic properties, and have affinities for estrogen receptors and peroxisome proliferator‐activated receptors (Gore et al., 2015; Henry & Fair, 2013; Macon & Fenton, 2013). Figure 2 outlines the effects of PFAS on ovarian and ovulatory outcomes.
8.1. PFAS: epidemiological evidence
Though limited, clinical IVF and fertility data suggest an impact of PFAS exposure on reproductive outcomes. In one study, eight different PFAS chemicals were consistently measured in 90%−98% of follicular fluid samples obtained from women undergoing IVF, while in another study 15 emerging and 10 legacy PFAS were detected in 50% of follicular fluid samples (Kang et al., 2020; Y. R. Kim et al., 2020). These findings indicate that multiple PFAS can target the ovary and perhaps disrupt normal ovarian function. In another study, plasma levels of PFOA were associated with decreased numbers of total oocytes and mature eggs retrieved and decreased embryonic quality (Ma et al., 2021). Several studies have also reported an association between higher plasma levels of certain PFAS in women and longer time to pregnancy and reduced fertility/fecundity (Louis et al., 2013; Fei et al., 2009; Jørgensen et al., 2014; Vélez et al., 2015b). However, other studies have reported no associations between PFAS levels and these reproductive outcomes (Bach et al., 2015; Whitworth et al., 2016).
Limited studies have also been conducted to establish associations between PFAS and sex steroid hormone levels. In one study, there was an inverse association between serum PFOS levels and serum E2 levels in perimenopausal and menopausal women (Knox et al., 2011). In another study, serum levels of PFOS and perfluorooctane sulfonamide were inversely associated with E2 and P4 levels in saliva samples from women (Barrett et al., 2015). Interestingly, these associations were only noted in women that have not had children. This coincides with evidence suggesting that pregnancy may lower the body burden of PFAS through eliminating maternal PFAS levels via placental and breast milk transfer (Brantsæter et al., 2013; Kato et al., 2014; Sagiv et al., 2015).
There is conflicting evidence regarding the associations of PFAS levels and menstrual cycle length. Some studies report specific PFAS levels being associated with irregular length (Lum et al., 2017; Lyngsø et al., 2014; W. Zhou et al., 2017), while another study did not observe these associations (Singer et al., 2018).
8.1.1. Summary
Main findings from these epidemiological studies suggest that PFAS exposure decreased fecundity, eggs collected in IVF settings, E2 and P4 levels, and altered menstrual cycle length. Major weaknesses in the literature include an undefined mechanism for these outcomes, several conflicting findings between studies, and an overall dearth of studies investigating the impact of PFAS on ovarian‐ and ovulation‐related fertility outcomes. Studies utilizing larger cohorts that investigate associations with time to pregnancy and IVF outcomes would alleviate the present weaknesses.
8.2. PFAS: basic science evidence
Oral dosing studies in mice suggest that PFAS exposure inhibits the ovulatory process. Adult female mice exposed chronically to a low dose of PFOS (0.1 mg/kg/day) exhibited a decrease in the number of mature antral follicles and CLs in the ovary, an increase in atretic follicles in the ovary, decreases in serum E2 and P4 levels, and disruptions in reproductive cycle length (an increase time in diestrus) (Feng et al., 2015). In this study, regulation of the HPO axis appeared to be disrupted, as PFOS exposure did not induce the LH surge and decreased levels of GnRH and FSH (Feng et al., 2015). In another study, adult mice exposed to PFOS for 1 week similarly had decreased numbers of CLs, spent a prolonged time in diestrus, and failed to generate the LH surge (X. Wang et al., 2018). Similar findings have been reported in a study using oral exposure to PFOA in adult mice, where exposure diminished LH levels, decreased the number of CLs, and decreased P4 levels, leading to a prolonged time in diestrus (Y. Zhang, Cao et al., 2020). These findings suggest that ovulation is inhibited by PFOS exposure via impaired hormonal responses needed to stimulate the ovulatory LH surge.
In vitro studies have also suggested that PFAS exposure disrupts ovulatory outcomes. Perfluorononanoic acid (PFNA) exposure inhibited oocyte germinal vesicle breakdown and polar body extrusion, which are two hallmark events required for oocyte maturation to eggs, and caused early stage oocyte death via oxidative stress (Jiao et al., 2021). Another study has shown that bovine cumulus oocyte complexes exposed to PFNA during in vitro maturation had impaired expansion (Hallberg et al., 2019). Further, PFOS exposure decreased porcine oocyte maturation and viability (Domínguez et al., 2016). Outside of the oocyte compartment, exposure to PFOS or PFOA in porcine granulosa and theca cells decreased FSH and LH stimulated production of E2 and P4 (Chaparro‐Ortega et al., 2018).
8.2.1. Summary
The evidence from basic science studies suggest that altered steroidogenesis and decreased antral follicle counts potentially lead to disruptions in estrous cyclicity and increases in atresia. Specific to ovulation, PFAS exposure inhibited oocyte meiotic maturation and expansion of the cumulus oocyte complex. Further, the previously mentioned defects in ovarian function may lead to the inability to produce an LH surge resulting in decreased CL counts. However, these findings from in vivo studies do not provide evidence whether this is a direct effect on the ovary or is due to toxicities in the HPO axis. Future studies must focus on elucidating the biomolecular mechanism responsible for these ovarian and ovulatory defects as the mechanism is presently unknown.
9. FLAME RETARDANTS
Flame retardant chemicals are used abundantly in building materials, household furniture, electronics, and textiles (Darnerud et al., 2001; van der Veen & de Boer, 2012). Polybrominated diphenyl ethers (PBDEs) are considered legacy flame retardants due to their extensive use in past decades and because they have been largely phased out due to their adverse effects on human health (Darnerud et al., 2001). There are 209 different PBDE congeners, which are manufactured by bromination of diphenyl ethers containing different percentages of tetra‐, penta‐, hepta‐, octa‐, and deca‐congeners. PBDEs have since been replaced with phosphate ester flame retardants (PFRs), which were historically used as plasticizers and lubricants (van der Veen & de Boer, 2012). PFRs are esters of phosphoric acid that contain varying lengths of alkyl chains or aryl groups.
Human exposure to flame retardants is ubiquitous due to their ability to leach from products, to bioaccumulate, and to persist in the environment (Darnerud et al., 2001; van der Veen & de Boer, 2012). Daily exposure is attributed to ingestion and inhalation of dust particles, dermal absorption, and diet (Darnerud et al., 2001; van der Veen & de Boer, 2012). Though production and use of PBDEs are declining, PBDEs are still routinely measured in serum samples from participants in NHANES (Sjödin et al., 2019). As PFRs are more readily metabolized, their metabolites are consistently found in urine samples with women typically having higher levels than men (Ospina et al., 2018). Measurable levels of PBDEs are also detected in women's follicular fluid, suggesting that flame retardants can target the ovary. This is concerning because both PBDEs and PFRs have estrogenic activities, interact with pregnane X receptors, estrogen receptors, and P4 receptors, and can interfere with steroid hormone homeostasis (Bajard et al., 2021; Czerska et al., 2013; Meerts et al., 2001). Their impacts on ovarian and ovulatory endpoints are summarized in Figure 2.
9.1. PBDEs: epidemiological evidence
Associations between PBDE levels and poor IVF outcomes are conflicting in the literature. One study suggests that increased serum levels of BDE 153 and three other BDEs were associated with decreased fecundity as measured by increased time to pregnancy (Harley et al., 2010). These findings correlate with another study that showed increased follicular fluid levels of BDE 153 being associated with decreased implantation rates in an IVF setting (Johnson et al., 2012). Further, increased serum levels of PBDE congeners 17, 28, 66, and homolog triBDE were associated with increased risk of incident pregnancy loss (Choi et al., 2019). Conversely, opposite findings were observed in a later study where increased serum levels of BDE 153 and OH‐BDE metabolites were associated with an increased probability of implantation, clinical pregnancy, and live birth, suggesting a potential protective effect (Ingle et al., 2020). Interestingly, racial disparities may exist when considering the impact of PBDE exposures and female reproductive health. When stratified by race, these positive correlations remain for white women; however, increases in all PBDEs and metabolites tended to decrease all IVF outcomes in non‐white women, with a significant association between BDE 47 and decreased probability of clinical pregnancy being observed (Ingle et al., 2020). In this study, the levels of PBDEs and OH‐BDEs were higher in non‐white women when compared to white women, which is consistent with findings in the literature.
9.1.1. Summary
Epidemiological evidence suggests that exposure to PBDEs reduced fecundity. However, there is a lack of studies designed to investigate ovarian outcomes in large IVF cohorts, which is a major weakness in the literature. Further, the conflicting IVF early pregnancy findings, racial disparities, and lack of a mechanism support the need to further understand how exposure to PBDEs impacts ovarian function and ovulation in cohorts of women.
9.2. PFRs: epidemiological evidence
Even with increased exposure due to the phase out of PBDEs, there is limited information about the effects of PFRs on women's reproductive health. One study reported that increased urinary BDCIPP levels were associated with increased total oocyte yield in an IVF cohort (Carignan et al., 2017). However, increased urinary PFR metabolites in the same study were associated with decreased fertilization and implantation rates, clinical pregnancies, and live births (Carignan et al., 2017). Even when fertilization and initial pregnancy proceeded normally, increased urinary levels of DPHP and the sum of PFR metabolites were associated with increased risk of biochemical pregnancy loss (a confirmed clinical pregnancy that was never visualized on ultrasound) (Messerlian et al., 2018).
9.2.1. Summary
The findings from these limited studies suggest that PFRs may not initially impact ovarian outcomes during IVF, but PFR exposure decreased fertilization rates, pregnancy rates, and live births, even when total oocytes collected were increased. This suggests that PFRs may impact the competence of the oocyte and perhaps the early embryo. Similar to PBDEs, future studies are needed to understand the impact of PFRs on ovarian and oocyte function in women, including the need to identify mechanisms for fertilization defects and pregnancy loss.
9.3. PBDEs: basic science evidence
Ovarian and ovulatory outcomes appear to be targets for PBDE toxicities. Using PMSG primed mice, BDE 47 exposure via oral gavage decreased the number of antral follicles, the number of ovulated eggs, the number of oocytes capable of resuming meiosis to the MII stage, and increased the levels of reactive oxygen species in the oocyte leading to apoptosis (Sun et al., 2020). Oral exposure to BDE 47 in postnatal day 10 rats also decreased the number of oocytes and antral follicles via increased autophagy and apoptosis, though complete serial sectioning and follicle counting were not conducted (C. Wang et al., 2016). In contrast to the previous studies, dietary exposure to a mixture of brominated flame retardants in rats increased the number and size of antral follicles, with no impairment in fertility (Lefèvre et al., 2016). When observing the offspring exposed to this same mixture in utero and during lactation, there was an increase in the number of structurally abnormal and multi‐oocyte follicles (Allais et al., 2020).
Steroidogenesis also appears to be impacted by exposure to PBDEs. Dietary exposure to a brominated flame retardant mixture in rats decreased antioxidant enzymes and steroidogenic enzymes leading to decreased levels of 17‐hydroxypregnenolone and increased levels of testosterone (Lefèvre et al., 2016). A similar increase in androgens was seen in earlier studies using cultured porcine follicles and co‐cultures of porcine granulosa cells and theca cells with individual and mixtures of BDE 47, BDE 99, BDE 100, and BDE 209 (Gregoraszczuk et al., 2008; Karpeta & Gregoraszczuk, 2010; Karpeta et al., 2011). This same group also reported that exposure to metabolites of BDE 47 increased E2 levels by increasing aromatase activity in the cocultured porcine follicular cell model (Karpeta et al., 2013). Conversely, exposure to BDE 47 in pregnant mice reduced plasma levels of prostaglandin E2, P4, and testosterone in the dams (Y. Zhu et al., 2017). Similar decreases were observed in cultured human granulosa cells. Specifically, exposure to a follicular fluid relevant mixture of PBDEs in vitro decreased P4 and E2 levels via accelerated steroid metabolism and increased granulosa cell death via oxidative stress in a human‐derived KGN granulosa cell line (Lefevre et al., 2016).
Evidence also suggests that PBDE exposure can disrupt inflammatory control in the ovary, which is essential for proper ovulation. In KGN cells, exposure to a relevant mixture of PBDEs increased the levels of interleukin 6, which is a well‐known inflammatory ovulatory mediator involved in cumulus oocyte complex expansion and oocyte maturation (Lefevre et al., 2016). This same group identified that mural granulosa cells are more susceptible than cumulus cells to toxicities caused by PBDEs in follicular fluid from samples collected from women undergoing IVF. Specifically, levels of PBDEs in the mural cells were associated with more activated genes in pathways important for innate immunity and inflammation (Lefèvre et al., 2021). Since mural granulosa cells are responsible for producing inflammatory markers during ovulation, these findings suggest that PBDE exposure may disrupt this crucial pathway in the human ovary.
9.3.1. Summary
Contrary to epidemiology studies, there is more basic science evidence linking PBDE exposures with impaired ovarian function and ovulation. Main findings suggest that PBDEs increased granulosa cell and follicle death potentially resulting in alterations hormone levels and decreases in ovulated eggs in a PMSG primed model. Specific mechanisms related to ovulatory defects may include decreases in the levels of P4 and prostaglandin E2 and increases in interleukin 6. A major strength in the literature is that some of these mechanistic findings were from studies using human granulosa cells. While these findings suggest an imbalance in inflammation in the ovary, future studies must explore the PBDE‐induced dysregulation of these mediators, specifically in studies designed to investigate ovulation directly.
9.4. PFRs: basic science evidence
The initial studies that investigated the effects of PFRs on female reproductive health used continuous breeding protocols in mice and rats. To briefly summarize these studies conducted in the late 1980s and early 1990s, chronic exposure to high levels of tris(methylphenyl) phosphate (TMPP), tris(2‐chloroethyl)phosphate (TCEP), and butylated triphenyl phosphate (BTP) reduced the number of litters, the average litter size, and live birth rate, and some of these effects persisted into the F2 generation (Carlton et al., 1987; Chapin et al., 1988; Gulati et al., 2009; Latendresse et al., 1994).
Altered follicle counts, follicular atresia, and granulosa cell death are further evidence of PFR‐induced reproductive dysfunction. Using the human KGN granulosa cell line, exposure to multiple PFRs in vitro displayed greater cytotoxicity when compared to BDE 47, a legacy PBDE (X. Wang et al., 2022). Further, multiple PFRs in this study increased mitochondria number, decreased lysosome number, and increased the size of lipid droplets, all potentially contributing to the increase in oxidative stress (X. X. Wang et al., 2022). In in vivo studies, oral exposure to tri‐ortho‐cresyl phosphate (TOCP; an ortho‐isomer of TMPP) in mice for 3−4 weeks decreased the number of antral follicles and increased the number of abnormal/atretic follicles via increased autophagy and oxidative stress (Hu et al., 2019; J. Wang et al., 2019). The mechanism of increased autophagy leading to cell death was confirmed when granulosa cells were directly exposed to TOCP in vitro (J. Wang et al., 2019; S. Yang, Shao et al., 2020). Interestingly, studies from this same group report conflicting data regarding steroidogenesis. In the 2‐week oral exposure study, TOCP decreased serum E2 levels likely attributed to granulosa and follicle death, while the 4‐week study reported increased E2 levels and decreased P4 levels perhaps due to a compensatory shift toward E2 production (Hu et al., 2019; J. Wang et al., 2019).
9.4.1. Summary
Similar to PBDEs, main findings from studies investigating PFRs suggest that exposure caused granulosa cell and follicle death via increased oxidative stress and autophagy. These changes may lead to impaired steroidogenesis, including decreases in P4 levels. While a strength in the literature is the use of human granulosa cells to elucidate a mechanism involving oxidative strength, a major weakness is the lack of studies designed to investigate the direct effects on the ovulatory process. Antral follicle development appears to be a target of toxicity, which implies that ovulation may also be affected by PFR exposure.
10. PARABENS
Parabens are a group of alkyl esters of p‐hydroxybenzoic acid that are used as antimicrobial agents and preservatives in food products and cosmetics (Nowak et al., 2018). Because parabens are found ubiquitously in the environment, including in household dust and contaminated water sources, humans are exposed daily via ingestion, inhalation, and dermal contact (Nowak et al., 2018). Similar to phthalates and other nonpersistent EDCs, parabens are rapidly eliminated from the body; however, their constant detection frequencies in urine samples further support that human exposure is ubiquitous. In fact, measurable levels of parabens are found in almost 100% of urine samples tested depending on the specific paraben measured (Nowak et al., 2018). Adult women have also been shown to have higher levels of exposure than men (Nowak et al., 2018). Criticism of their use in everyday items is largely attributed to their estrogenic EDC‐qualities through agonism of estrogen receptors (Nowak et al., 2018). However, their effects on ovulation (briefly summarized in Figure 2) are largely unknown.
10.1. Parabens: epidemiological evidence
Human data linking paraben exposure to impaired female reproductive health are relatively lacking; however, some studies suggest defects in clinical outcomes. In one study, increased urinary levels of propylparaben (PP) produced a suggestive, but not significant, trend toward decreased antral follicle counts (Smith et al., 2013). In a different cohort, there was a significant association between increased urinary PP levels and decreased antral follicle counts, as well as decreased E2 levels (Jurewicz et al., 2020). In contrast, another study reported a positive association between urinary paraben levels and E2 levels (Pollack et al., 2018). Reflective of alterations in steroid hormone levels, increased urinary total paraben and butyl paraben (BP) levels were associated with shortened menstrual cycle length in a Japanese university cohort (Nishihama et al., 2016). When examining time to pregnancy, increased urinary levels of methyl paraben (MP) and ethyl paraben (EP) were associated with decreased fecundity (Smarr, Sundaram et al., 2017). However, these findings were not indicative of impairments in IVF outcomes, as observed in other studies. Specifically, urinary concentrations of PP, MP, and BP were not associated with changes in total and mature egg counts, embryonic quality, fertilization and implantation rates, clinical pregnancy, and live births (Mínguez‐Alarcón et al., 2019; Mínguez‐Alarcón et al., 2016).
10.1.1. Summary
Main findings suggest that paraben exposure may reduce antral follicle numbers, which potentially contributes to alterations in hormone levels, shortened menstrual cycle length, and reduced fecundity. While the reduced fecundity findings from one cohort were not correlated with poor IVF outcomes in other cohorts, the limited number of studies investigating these links is a present weakness in the literature. Future studies must utilize larger cohorts to confirm the decreased antral follicle count findings and to investigate the impact of parabens on other ovarian outcomes, including potential mechanisms of toxicity.
10.2. Parabens: basic science evidence
Similar to human studies, there is a relative lack of studies that have directly investigated the effects of paraben exposure on ovarian function and ovulation. When exposure was limited to gestation, perinatal exposure to BP in vivo reduced fertility, CL counts, and E2 and P4 levels in rats (Maske et al., 2018). Similarly, perinatal exposure to PP in mice disrupted estrous cyclicity, decreased E2 and P4 levels, and increased atretic follicles (M. Li et al., 2021). Decreased E2 levels, increased FSH and LH levels, decreased antral follicle numbers, and increased numbers of irregular follicles were also observed when young adult mice were treated with BP (Ara et al., 2021). In contrast to these findings, a multigenerational study utilizing dietary exposure to BP in rats reported no changes in fertility, fecundity, and reproductive parameters (Hubbard et al., 2020).
Studies conducted in vitro, though limited, also suggest impairments in ovarian outcomes. In a mouse antral follicle culture system, PP exposure inhibited antral follicle growth, increased the levels of E2, and increased the mRNA levels of apoptosis, cell cycle, and steroidogenic factors (Gal et al., 2019). Preantral follicles exposed to BP tended to decrease oocyte maturation, decrease E2 production, and increase steroidogenic gene expression, but these results were not statistically significant (J. H. Kim & Jee, 2020). While not affecting oocyte viability, another study suggests that MP exposure to porcine oocytes inhibited expansion of the cumulus oocyte complex and final maturation of the oocyte (Barajas‐Salinas et al., 2021). In contrast, exposure to MP and BP did not alter the levels of E2, P4, or the mRNA levels of steroidogenic factors in human cumulus cells collected from women undergoing a standardized IVF protocol (Herrera‐Cogco et al., 2020).
10.2.1. Summary
The perinatal period and young adulthood may be sensitive windows of susceptibility to paraben ovarian toxicities. Increased atresia and impaired follicle maturation likely lead to the reported decreases in the levels of E2 and P4, alterations in cyclicity, and decreases in fertility. The antral follicle also appears to be targeted by parabens, leading to decreased growth. While mechanisms for these toxicities are unknown, inhibited cumulus expansion and oocyte maturation implies that ovulatory outcomes may be disrupted by exposure to parabens. Future studies must be designed to characterize the direct effects during the ovulatory period and to elucidate the mechanisms of toxicity.
11. TRICLOSAN
5‐chloro‐2‐(2,4‐dichlorophenoxy)phenol (triclosan) is a halogenated biphenyl ether that is used as a broad spectrum antimicrobial agent. Triclosan is found in personal care products, hand sanitizer, food packaging, and medical supplies leading to daily oral and dermal exposure (Dann & Hontela, 2011; Weatherly & Gosse, 2017). Additionally, measurable levels of triclosan are detected in surface‐ and wastewater samples (Dann & Hontela, 2011; Weatherly & Gosse, 2017). While the use of triclosan was banned in certain soap products by the FDA in 2016, human exposure to triclosan is still persistent (Weatherly & Gosse, 2017). In the general population, over 74% of urine samples contain measurable levels of triclosan, with concentrations in women being higher than in men (Dann & Hontela, 2011; Weatherly & Gosse, 2017). Compared to other chemicals described herein, much less is known regarding the effects of triclosan on female reproductive health. However, the structural similarity of triclosan to known EDCs, including PDBEs and BPA, and its antiestrogenic and/or antiandrogenic effects in cell‐based assays suggest that triclosan may disrupt the endocrine and reproductive systems (Dann & Hontela, 2011; Weatherly & Gosse, 2017). Findings from studies investigating the impact of triclosan on the ovary and ovulation are outlined in Figure 2.
11.1. Triclosan: epidemiological evidence
Only a few studies have investigated associations between triclosan exposure and impaired female reproductive health. Of those, the associations are often limited to one outcome and are dependent upon the cohort studied. Increased urinary triclosan levels were associated with prolonged menstrual cycle length and decreased fecundability as measured by increased time to pregnancy and infertility risk (W. Zhu et al., 2019). When also analyzing time to pregnancy as a measure of fecundity, one study supported that increased urinary triclosan levels were associated with decreased fecundity (Vélez et al., 2015a), while another did not corroborate those findings (Smarr, Sundaram et al., 2017). Two studies have reported an association between urinary triclosan levels and decreased antral follicle counts (Jurewicz et al., 2019; Mínguez‐Alarcón et al., 2017). In support of decreased follicle counts, another study found that increased urinary triclosan levels were associated with decreased egg yield in an IVF setting, but no other fertility outcomes were impacted (Lange et al., 2015). Studies also found an association between urinary triclosan levels and decreased high quality embryo formation and implantation rates (Hua et al., 2017; Radwan et al., 2021).
11.1.1. Summary
While there are a paucity of studies investigating the impact of triclosan exposure on ovulation, the decreased antral follicle numbers may lead to menstrual cyclicity irregularities and decreased fecundity. The decreased numbers of eggs retrieved in the IVF setting suggest that triclosan may disrupt the process of follicle development, while the embryonic and early pregnancy defects suggest that the oocyte may be targeted. Future studies with larger cohorts are needed to confirm these findings, especially because these associations are often limited to one outcome and are dependent upon the cohort studied. Further, there is a lack of mechanistic evidence related to the toxicity of triclosan.
11.2. Triclosan: basic science evidence
Limited in vivo studies have been conducted to characterize triclosan‐induced defects in ovarian function and ovulation. Developmental exposure to environmentally relevant levels of triclosan decreased the amount of eggs released and fertility rates in zebrafish (Stenzel et al., 2019). In mice, a single injection of triclosan increased urinary levels of E2, which potentially can lead to excessive estrogen signaling and impaired reproductive function (Pollock et al., 2016).
Altered steroidogenesis was also observed in in vitro studies. Utilizing porcine luteal cells, triclosan exposure both increased (low doses; 1 and 10 μM) and decreased (high dose; 50 μM) P4 production (Basini et al., 2021). These findings were accompanied by inhibited cell proliferation and metabolic activity, suggestive of altered luteal functionality in response to triclosan exposure (Basini et al., 2021). In a study using the human KGN granulosa cell line, triclosan exposure altered energy metabolism by shifting pyruvate function toward steroidogenesis instead of lactate formation. This shift was suggested to be the mechanism by which triclosan increased the levels of E2 and P4 by the KGN cells (Y. Du et al., 2021). In further support of these findings, triclosan exposure in rat granulosa cells increased E2 and P4 levels via increases in steroidogenic proteins and enzymes (Chen et al., 2019).
Triclosan exposure in vitro has also been shown to directly disrupt the oocyte. During the in vitro maturation process in porcine oocytes, triclosan exposure inhibited meiotic maturation of the oocyte and expansion of the cumulus oocyte complex via a mechanism involving increased oxidative stress and apoptosis (Park et al., 2020).
11.2.1. Summary
Studies investigating the direct effects of triclosan on ovarian function and ovulation are extremely limited, which is a major weakness in the literature. Contrary to the other chemical classes, exposure may increase steroid hormone levels in preovulatory granulosa cells, including in a human granulosa cell line; although, increased steroid levels would also have negative consequences for reproductive health. Specific to the ovulatory process, egg release, expansion of the cumulus oocyte complex, final maturation of the oocyte, and CL functionality appear to be targets of triclosan exposure. These findings support the need for future studies focused directly on ovulation and identifying mechanisms of ovulatory dysfunction.
12. CONCLUSIONS AND FUTURE DIRECTIONS
A considerable amount of evidence reviewed herein suggests that exposure to EDCs can disrupt ovarian function and ovulation leading to fertility issues in women and/or female mammalian experimental models. There is compelling evidence suggesting that exposure to phthalates is associated with multiple poor IVF outcomes and inhibits ovulation in rodent models. Although IVF data are lacking with respect to exposure to BPA substitutes, multiple animal, and human cell culture studies suggest that bisphenols can cause oxidative stress leading to follicle/CL loss or cell death and can disrupt oocyte developmental competence. Considerably less is known about the effects of PFAS, PBDEs, PFRs, parabens, and triclosan, though reduced fecundity, poor IVF outcomes, altered follicle counts, and disruptions in steroidogenesis have been reported. These findings support the growing literature linking exposure to EDCs with ovulatory defects and infertility (Gore et al., 2015). However, markedly absent in these studies is the identification of potential mechanisms by which these EDCs are causing ovarian‐ and ovulation‐related fertility issues, which represents a weakness in the literature and is a necessary focus for future studies.
Further, several discrepancies are evident in the literature. While multiple EDCs are associated with reproductive dysfunction in epidemiological studies, other studies report null associations within the same chemical class and the outcome measured, as cited herein. Differences can be attributed to the cohort studied, the timing of enrollment of the cohort, the prospective or retrospective design of the study, or the methods of biospecimen collection. For instance, single collections of urinary samples may not accurately reflect true levels of exposure or the concentration of the EDC in the ovary (follicular fluid). Discrepancies in the animal literature are also present within these chemical classes. These differences can be due to species and strain tested, age of the animal at the start of dosing (windows of susceptibility), doses tested, and duration and route of exposure. In certain cases, inconsistent findings are evident when comparing human data with animal studies, which perhaps can be attributed to toxicokinetic (absorption, distribution, metabolism, and excretion) and overall biological differences between humans and rodents.
Human exposure to EDCs is mostly unavoidable. Even though the use of some of the chemicals included in this review have diminished or have been outright banned, humans are still being exposed daily (Young et al., 2021). Findings contained in this review support that there is an urgent need to identify how these exposures impact female reproductive health. The lack of female reproductive health studies in chemical classes such as parabens and triclosan promote avenues for further investigation. Similarly, there is a paucity of studies investigating the mechanisms by which EDCs may be disrupting the process of ovulation, especially in studies utilizing ovarian cells and tissue obtained from women. Further, IVF data and animal studies routinely focus on single chemical exposures, irrespective of human exposure to chemical mixtures.
To circumvent these limitations, several approaches can be applied. Importantly, mechanistic studies must be conducted in animal and human studies. Given that EDCs can disrupt hormone production, metabolism, and transport, future studies need to investigate how levels of P4 and prostaglandins, among additional ovulatory mediators presented in Table 1, are altered during the ovulatory period. EDCs can also alter the number of receptors and can agonize or antagonize receptors. Thus, the mechanistic focus of future investigation should be on alterations in the functions of P4 receptor, prostaglandin receptors, and receptors for other ovulatory mediators following exposure to EDCs. Elucidating the mechanisms by which EDCs impact the ovulatory process promotes the potential to circumvent EDC‐induced ovarian toxicity.
Future studies should analyze or employ exposures to mixtures of compounds within a chemical class (e.g., phthalates) as well as mixtures of different chemical classes (e.g., combined exposure to mixtures of phthalates and parabens). Dose selections should prioritize concentrations at environmentally relevant levels, and these mixtures at relevant doses can be used in human and animal cells in vitro and in animal dosing studies in vivo. In animal studies, relevant mixtures should be given via the most common route of exposure. These exposures should also be incorporated into the novel bioengineering systems that recently have been developed (biomaterials, 3D printing, organ‐on‐a‐chip, and microfluidic culture platforms) (Xiao et al., 2017; Zubizarreta & Xiao, 2020). Importantly, studies should utilize primary human tissues to increase the translational nature of this basic science, cell culture approach. This can be done by collecting primary granulosa‐lutein cells that are normally discarded from women undergoing IVF. In fact, these cultured cells respond to hCG after processing and have been shown to recapitulate ovulatory events that are observed in vivo (Hannon et al., 2018). Toxicology studies using this innovative primary human cell model are lacking. However, this approach would greatly enhance the understanding of how EDCs directly impact ovulatory events in women and is already underway in the authors' laboratory. Taken together, employing all of these concepts in future studies will enhance the translational nature and the environmental relevance of the work investigating the impact of EDCs on ovulation and fertility in women.
AUTHOR CONTRIBUTIONS
Katie L. Land: writing − original draft; methodology; writing − review & editing. Frances G. Miller: methodology; writing − review & editing. Ava C. Fugate: methodology; writing − review & editing. Patrick R. Hannon: conceptualization; funding acquisition; writing − original draft; methodology; writing − review & editing; project administration; supervision.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank all the members of Dr. Thomas Curry's, Dr. Misung Jo's, Dr. Yohan Choi's, and Dr. Patrick Hannon's laboratory for their insightful critiques of this review. This review was supported by grants R01ES033767 (P. R. H.) and R00ES028748 (P. R. H.) from the National Institute of Environmental Health Sciences.
Land, K. L. , Miller, F. G. , Fugate, A. C. , & Hannon, P. R. (2022). The effects of endocrine‐disrupting chemicals on ovarian‐ and ovulation‐related fertility outcomes. Molecular Reproduction and Development, 89, 608–631. 10.1002/mrd.23652
REFERENCES
- Ahsan, N. , Ullah, H. , Ullah, W. , & Jahan, S. (2018). Comparative effects of bisphenol S and bisphenol A on the development of female reproductive system in rats: A neonatal exposure study. Chemosphere, 197, 336–343. 10.1016/j.chemosphere.2017.12.118 [DOI] [PubMed] [Google Scholar]
- Al‐Alem, L. , Puttabyatappa, M. , Rosewell, K. , Brännström, M. , Akin, J. , Boldt, J. , Muse, K. , & Curry, T. E., Jr. (2015). Chemokine ligand 20: A signal for leukocyte recruitment during human ovulation? Endocrinology, 156(9), 3358–3369. 10.1210/en.2014-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allais, A. , Albert, O. , Lefèvre, P. L. C. , Wade, M. G. , Hales, B. F. , & Robaire, B. (2020). In utero and lactational exposure to flame retardants disrupts rat ovarian follicular development and advances puberty. Toxicological Sciences, 175(2), 197–209. 10.1093/toxsci/kfaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar, S. , Binet, A. , Téteau, O. , Desmarchais, A. , Papillier, P. , Lacroix, M. Z. , Maillard, V. , Guérif, F. , & Elis, S. (2020). Bisphenol S impaired human granulosa cell steroidogenesis in vitro. International Journal of Molecular Sciences, 21(5), 1821. 10.3390/ijms21051821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara, C. , Asmatullah, Butt, N. , Ali, S. , Batool, F. , Shakir, H. A. , & Arshad, A. (2021). Abnormal steroidogenesis, oxidative stress, and reprotoxicity following prepubertal exposure to butylparaben in mice and protective effect of Curcuma longa . Environmental Science and Pollution Research, 28(5), 6111–6121. 10.1007/s11356-020-10819-8 [DOI] [PubMed] [Google Scholar]
- Bach, C. C. , Bech, B. H. , Nohr, E. A. , Olsen, J. , Matthiesen, N. B. , Bossi, R. , Uldbjerg, N. , Bonefeld‐Jørgensen, E. C. , & Henriksen, T. B. (2015). Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environmental Research, 142, 535–541. 10.1016/j.envres.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Bajard, L. , Negi, C. K. , Mustieles, V. , Melymuk, L. , Jomini, S. , Barthelemy‐Berneron, J. , Fernandez, M. F. , & Blaha, L. (2021). Endocrine disrupting potential of replacement flame retardants—Review of current knowledge for nuclear receptors associated with reproductive outcomes. Environment International, 153, 106550. 10.1016/j.envint.2021.106550 [DOI] [PubMed] [Google Scholar]
- Barajas‐Salinas, A. , Ducolomb, Y. , Betancourt, M. , Núñez‐Macías, E. , López, A. , Barraza, J. , Quezadas‐Fuentes, J. , Bahena‐Ocampo, I. , Bonilla, E. , Retana‐Márquez, S. , Casas, E. , & Casillas, F. (2021). Effects of methylparaben on in vitro maturation of porcine oocytes. Journal of Applied Toxicology, 41(2), 330–337. 10.1002/jat.4045 [DOI] [PubMed] [Google Scholar]
- Barrett, E. S. , Chen, C. , Thurston, S. W. , Haug, L. S. , Sabaredzovic, A. , Fjeldheim, F. N. , Frydenberg, H. , Lipson, S. F. , Ellison, P. T. , & Thune, I. (2015). Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertility and Sterility, 103(5), 1261–1270. 10.1016/j.fertnstert.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak, S. , Das, M. K. , & Duttaroy, A. K. (2020). Plastics derived endocrine‐disrupting compounds and their effects on early development. Birth Defects Research, 112(17), 1308–1325. 10.1002/bdr2.1741 [DOI] [PubMed] [Google Scholar]
- Basini, G. , Bussolati, S. , Bertini, S. , Quintavalla, F. , & Grasselli, F. (2021). Evaluation of triclosan effects on cultured swine luteal cells. Animals: An Open Access Journal from MDPI, 11(3), 606. 10.3390/ani11030606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum, T. F. , Fujimoto, V. Y. , Gerona, R. , McGough, A. , Lenhart, N. , Wong, R. , Mok‐Lin, E. , Melamed, J. , Butts, C. D. , & Bloom, M. S. (2021). A pilot investigation of couple‐level phthalates exposure and in vitro fertilization (IVF) outcomes. Reproductive Toxicology, 99, 56–64. 10.1016/j.reprotox.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni, M. , Gigante, P. , Bussolati, S. , Grasselli, F. , Grolli, S. , Ramoni, R. , & Basini, G. (2019). Bisphenol S, a bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domestic Animal Endocrinology, 66, 48–56. 10.1016/j.domaniend.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Blount, B. C. , Silva, M. J. , Caudill, S. P. , Needham, L. L. , Pirkle, J. L. , Sampson, E. J. , Lucier, G. W. , Jackson, R. J. , & Brock, J. W. (2000). Levels of seven urinary phthalate metabolites in a human reference population. Environmental Health Perspectives, 108(10), 979–982. 10.1289/ehp.00108979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter, A. L. , Whitworth, K. W. , Ydersbond, T. A. , Haug, L. S. , Haugen, M. , Knutsen, H. K. , Thomsen, C. , Meltzer, H. M. , Becher, G. , Sabaredzovic, A. , Hoppin, J. A. , Eggesbø, M. , & Longnecker, M. P. (2013). Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environment International, 54, 74–84. 10.1016/j.envint.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, J. M. (2017). Early‐life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology, 13(3), 161–173. 10.1038/nrendo.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis, G. M. , Sundaram, R. , Schisterman, E. F. , Sweeney, A. M. , Lynch, C. D. , Gore‐Langton, R. E. , Maisog, J. , Kim, S. , Chen, Z. , & Barr, D. B. (2013). Persistent environmental pollutants and couple fecundity: The LIFE study. Environmental Health Perspectives, 121(2), 231–236. 10.1289/ehp.1205301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, R. C. , Franklin, J. , Berger, U. , Conder, J. M. , Cousins, I. T. , de Voogt, P. , Jensen, A. A. , Kannan, K. , Mabury, S. A. , & van Leeuwen, S. P. (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management, 7(4), 513–541. 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnakova Mlynarcikova, A. , & Scsukova, S. (2021a). Bisphenol analogs AF and S: Effects on cell status and production of angiogenesis‐related factors by COV434 human granulosa cell line. Toxicology and Applied Pharmacology, 426, 115634. 10.1016/j.taap.2021.115634 [DOI] [PubMed] [Google Scholar]
- Bujnakova Mlynarcikova, A. , & Scsukova, S. (2021b). Bisphenol analogs AF, S and F: Effects on functional characteristics of porcine granulosa cells. Reproductive Toxicology, 103, 18–27. 10.1016/j.reprotox.2021.05.004 [DOI] [PubMed] [Google Scholar]
- Bull, J. R. , Rowland, S. P. , Scherwitzl, E. B. , Scherwitzl, R. , Danielsson, K. G. , & Harper, J. (2019). Real‐world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digital Medicine, 2, 83. 10.1038/s41746-019-0152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat, A. M. , Kato, K. , Hubbard, K. , Jia, T. , Botelho, J. C. , & Wong, L. Y. (2019). Legacy and alternative per‐ and polyfluoroalkyl substances in the US general population: Paired serum‐urine data from the 2013−2014 National Health and Nutrition Examination Survey. Environment International, 131, 105048. 10.1016/j.envint.2019.105048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen, K. A. , Kucharczyk, K. M. , Bogin, B. , Ehrlich, J. M. , & Combelles, C. M. H. (2018). Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S. Human Reproduction, 33(5), 895–904. 10.1093/humrep/dey050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen, K. , Lavallee, M. , & Combelles, C. (2018). The impact of bisphenol S on bovine granulosa and theca cells. Reproduction in Domestic Animals, 53(2), 450–457. 10.1111/rda.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan, C. C. , Mínguez‐Alarcón, L. , Butt, C. M. , Williams, P. L. , Meeker, J. D. , Stapleton, H. M. , Toth, T. L. , Ford, J. B. , & Hauser, R. (2017). Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environmental Health Perspectives, 125(8), 087018. 10.1289/ehp1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, B. D. , Basaran, A. H. , Mezza, L. E. , & Smith, M. K. (1987). Examination of the reproductive effects of tricresyl phosphate administered to long‐evans rats. Toxicology, 46(3), 321–328. 10.1016/0300-483X(87)90212-5 [DOI] [PubMed] [Google Scholar]
- Caserta, D. , Costanzi, F. , De Marco, M. P. , Di Benedetto, L. , Matteucci, E. , Assorgi, C. , Pacilli, M. C. , Besharat, A. R. , Bellati, F. , & Ruscito, I. (2021). Effects of endocrine‐disrupting chemicals on endometrial receptivity and embryo implantation: A systematic review of 34 mouse model studies. International Journal of Environmental Research and Public Health, 18(13), 6840. 10.3390/ijerph18136840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachamovich, J. R. , Chachamovich, E. , Ezer, H. , Fleck, M. P. , Knauth, D. , & Passos, E. P. (2010). Investigating quality of life and health‐related quality of life in infertility: A systematic review. Journal of Psychosomatic Obstetrics & Gynecology, 31(2), 101–110. 10.3109/0167482x.2010.481337 [DOI] [PubMed] [Google Scholar]
- Chaparro‐Ortega, A. , Betancourt, M. , Rosas, P. , Vázquez‐Cuevas, F. G. , Chavira, R. , Bonilla, E. , Casas, E. , & Ducolomb, Y. (2018). Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicology in Vitro, 46, 86–93. 10.1016/j.tiv.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Chapin, R. E. , George, J. D. , & Lamb, J. C. 4th. (1988). Reproductive toxicity of tricresyl phosphate in a continuous breeding protocol in Swiss (CD‐1) mice. Fundamental and Applied Toxicology, 10(2), 344–354. 10.1016/0272-0590(88)90320-x [DOI] [PubMed] [Google Scholar]
- Chen, W. , Yang, X. , Wang, B. , Wang, L. , & Yu, X. (2019). The effects and possible mechanisms of triclosan on steroidogenesis in primary rat granulosa cells. Reproductive Toxicology, 83, 28–37. 10.1016/j.reprotox.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Chiang, C. , & Flaws, J. A. (2019). Subchronic exposure to di(2‐ethylhexyl) phthalate and diisononyl phthalate during adulthood has immediate and long‐term reproductive consequences in female mice. Toxicological Sciences, 168(2), 620–631. 10.1093/toxsci/kfz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. , Lewis, L. R. , Borkowski, G. , & Flaws, J. A. (2020a). Exposure to di(2‐ethylhexyl) phthalate and diisononyl phthalate during adulthood disrupts hormones and ovarian folliculogenesis throughout the prime reproductive life of the mouse. Toxicology and Applied Pharmacology, 393, 114952. 10.1016/j.taap.2020.114952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. , Lewis, L. R. , Borkowski, G. , & Flaws, J. A. (2020b). Late‐life consequences of short‐term exposure to di(2‐ethylhexyl) phthalate and diisononyl phthalate during adulthood in female mice. Reproductive Toxicology, 93, 28–42. 10.1016/j.reprotox.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. , Mahalingam, S. , & Flaws, J. (2017). Environmental contaminants affecting fertility and somatic health. Seminars in Reproductive Medicine, 35(3), 241–249. 10.1055/s-0037-1603569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, G. , Wang, Y. B. , Sundaram, R. , Chen, Z. , Barr, D. B. , Buck Louis, G. M. , & Smarr, M. M. (2019). Polybrominated diphenyl ethers and incident pregnancy loss: The LIFE study. Environmental Research, 168, 375–381. 10.1016/j.envres.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, Z. R. , Hannon, P. R. , Wang, W. , Ziv‐Gal, A. , & Flaws, J. A. (2013). Di‐n‐butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biology of Reproduction, 88(1), 23. 10.1095/biolreprod.112.105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain, D. A. , Janssen, S. J. , Edwards, T. M. , Heindel, J. , Ho, S. , Hunt, P. , Iguchi, T. , Juul, A. , McLachlan, J. A. , Schwartz, J. , Skakkebaek, N. , Soto, A. M. , Swan, S. , Walker, C. , Woodruff, T. K. , Woodruff, T. J. , Giudice, L. C. , & Guillette, L. J., Jr. (2008). Female reproductive disorders: The roles of endocrine‐disrupting compounds and developmental timing. Fertility and Sterility, 90(4), 911–940. 10.1016/j.fertnstert.2008.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, T., Jr. , & Smith, M. (2006). Impact of extracellular matrix remodeling on ovulation and the folliculo‐luteal transition. Seminars in Reproductive Medicine, 24(4), 228–241. 10.1055/s-2006-948552 [DOI] [PubMed] [Google Scholar]
- Czerska, M. , Zieliński, M. , Kamińska, J. , & Ligocka, D. (2013). Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. International Journal of Occupational Medicine and Environmental Health, 26(4), 498–510. 10.2478/s13382-013-0138-7 [DOI] [PubMed] [Google Scholar]
- Dann, A. B. , & Hontela, A. (2011). Triclosan: Environmental exposure, toxicity and mechanisms of action. Journal of Applied Toxicology, 31(4), 285–311. 10.1002/jat.1660 [DOI] [PubMed] [Google Scholar]
- Darnerud, P. O. , Eriksen, G. S. , Jóhannesson, T. , Larsen, P. B. , & Viluksela, M. (2001). Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environmental Health Perspectives, 109(Suppl 1), 49–68. 10.1289/ehp.01109s149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, B. J. , Maronpot, R. R. , & Heindel, J. J. (1994). Di‐(2‐ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicology and Applied Pharmacology, 128(2), 216–223. 10.1006/taap.1994.1200 [DOI] [PubMed] [Google Scholar]
- den Braver‐Sewradj, S. P. , van Spronsen, R. , & Hessel, E. V. S. (2020). Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Critical Reviews in Toxicology, 50(2), 128–147. 10.1080/10408444.2019.1701986 [DOI] [PubMed] [Google Scholar]
- Deng, T. , Du, Y. , Wang, Y. , Teng, X. , Hua, X. , Yuan, X. , Yao, Y. , Guo, N. , & Li, Y. (2020). The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: A prospective single‐center study. Ecotoxicology and Environmental Safety, 188, 109884. 10.1016/j.ecoenv.2019.109884 [DOI] [PubMed] [Google Scholar]
- Desmarchais, A. , Téteau, O. , Papillier, P. , Jaubert, M. , Druart, X. , Binet, A. , Maillard, V. , & Elis, S. (2020). Bisphenol S impaired in vitro ovine early developmental oocyte competence. International Journal of Molecular Sciences, 21(4), 1238. 10.3390/ijms21041238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z. M. , Jiao, X. F. , Wu, D. , Zhang, J. Y. , Chen, F. , Wang, Y. S. , Huang, C. J. , Zhang, S. X. , Li, X. , & Huo, L. J. (2017). Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chemico‐Biological Interactions, 278, 222–229. 10.1016/j.cbi.2017.10.030 [DOI] [PubMed] [Google Scholar]
- Domínguez, A. , Salazar, Z. , Arenas, E. , Betancourt, M. , Ducolomb, Y. , González‐Márquez, H. , Casas, E. , Teteltitla, M. , & Bonilla, E. (2016). Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicology in Vitro, 35, 93–99. 10.1016/j.tiv.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Duffy, D. M. , Ko, C. , Jo, M. , Brannstrom, M. , & Curry, T. E. (2019). Ovulation: Parallels with inflammatory processes. Endocrine Reviews, 40(2), 369–416. 10.1210/er.2018-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, F. E. , Schindler, K. , Schultz, R. M. , Blengini, C. S. , Stein, P. , Stricker, S. A. , Wessel, G. M. , & Williams, C. J. (2020). Unscrambling the oocyte and the egg: Clarifying terminology of the female gamete in mammals. Molecular Human Reproduction, 26(11), 797–800. 10.1093/molehr/gaaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Guo, N. , Wang, Y. , Teng, X. , Hua, X. , Deng, T. , Yao, Y. , Yuan, X. , & Li, Y. (2019). Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertility and Sterility, 111(5), 953–961. 10.1016/j.fertnstert.2019.01.021 [DOI] [PubMed] [Google Scholar]
- Du, Y. , Wang, B. , Cai, Z. , Zhang, H. , Wang, B. , Liang, W. , Zhou, G. , Ouyang, F. , & Wang, W. (2021). The triclosan‐induced shift from aerobic to anaerobic metabolism link to increased steroidogenesis in human ovarian granulosa cells. Ecotoxicology and Environmental Safety, 220, 112389. 10.1016/j.ecoenv.2021.112389 [DOI] [PubMed] [Google Scholar]
- Du, Y. Y. , Fang, Y. L. , Wang, Y. X. , Zeng, Q. , Guo, N. , Zhao, H. , & Li, Y. F. (2016). Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reproductive Toxicology, 61, 142–150. 10.1016/j.reprotox.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Faust, L. , Bradley, D. , Landau, E. , Noddin, K. , Farland, L. V. , Baron, A. , & Wolfberg, A. (2019). Findings from a mobile application‐based cohort are consistent with established knowledge of the menstrual cycle, fertile window, and conception. Fertility and Sterility, 112(3), 450–457. 10.1016/j.fertnstert.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Fei, C. , McLaughlin, J. K. , Lipworth, L. , & Olsen, J. (2009). Maternal levels of perfluorinated chemicals and subfecundity. Human Reproduction, 24(5), 1200–1205. 10.1093/humrep/den490 [DOI] [PubMed] [Google Scholar]
- Feng, X. , Wang, X. , Cao, X. , Xia, Y. , Zhou, R. , & Chen, L. (2015). Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicological Sciences, 148(2), 368–379. 10.1093/toxsci/kfv197 [DOI] [PubMed] [Google Scholar]
- Foster, W. G. , Neal, M. S. , Han, M. S. , & Dominguez, M. M. (2008). Environmental contaminants and human infertility: Hypothesis or cause for concern? Journal of Toxicology and Environmental Health, Part B, 11(3−4), 162–176. 10.1080/10937400701873274 [DOI] [PubMed] [Google Scholar]
- Fu, X. , Han, H. , Li, Y. , Xu, B. , Dai, W. , Zhang, Y. , Zhou, F. , Ma, H. , & Pei, X. (2021). Di‐(2‐ethylhexyl) phthalate exposure induces female reproductive toxicity and alters the intestinal microbiota community structure and fecal metabolite profile in mice. Environmental Toxicology, 36(6), 1226–1242. 10.1002/tox.23121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal, A. , Gedye, K. , Craig, Z. R. , & Ziv‐Gal, A. (2019). Propylparaben inhibits mouse cultured antral follicle growth, alters steroidogenesis, and upregulates levels of cell‐cycle and apoptosis regulators. Reproductive Toxicology, 89, 100–106. 10.1016/j.reprotox.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Ge, W. , Li, L. , Dyce, P. W. , De Felici, M. , & Shen, W. (2019). Establishment and depletion of the ovarian reserve: Physiology and impact of environmental chemicals. Cellular and Molecular Life Sciences, 76(9), 1729–1746. 10.1007/s00018-019-03028-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian, A. , & Trasande, L. (2018). Disruption in thyroid signaling pathway: A mechanism for the effect of endocrine‐disrupting chemicals on child neurodevelopment. Frontiers in Endocrinology, 9, 204. 10.3389/fendo.2018.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayda, R. A. , Williams, P. L. , Chavarro, J. E. , Ford, J. B. , Souter, I. , Calafat, A. M. , Hauser, R. , & Mínguez‐Alarcón, L. (2019). Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environment International, 131, 105050. 10.1016/j.envint.2019.105050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich, J. , Pu, Y. , Upham, B. L. , Hulse, M. , Pearl, S. , Martin, D. , Avery, A. , & Veiga‐Lopez, A. (2021). Bisphenol S enhances gap junction intercellular communication in ovarian theca cells. Chemosphere, 263, 128304. 10.1016/j.chemosphere.2020.128304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich, J. , Ticiani, E. , & Veiga‐Lopez, A. (2020). Placenta disrupted: Endocrine disrupting chemicals and pregnancy. Trends in Endocrinology & Metabolism, 31(7), 508–524. 10.1016/j.tem.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore, A. C. , Chappell, V. A. , Fenton, S. E. , Flaws, J. A. , Nadal, A. , Prins, G. S. , Toppari, J. , & Zoeller, R. T. (2015). EDC‐2: The endocrine society's second scientific statement on endocrine‐disrupting chemicals. Endocrine Reviews, 36(6), E1–E150. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoraszczuk, E. Ł. , Rak, A. , Kawalec, K. , & Ropstad, E. (2008). Steroid secretion following exposure of ovarian follicular cells to single congeners and defined mixture of polybrominateddibenzoethers (PBDEs), p,p'‐DDT and its metabolite p,p'‐DDE. Toxicology Letters, 178(2), 103–109. 10.1016/j.toxlet.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Gulati, D. K. , Barnes, L. H. , Chapin, R. E. , & Heindel, J. (2009). Final report on the reproductive toxicity of tris(2‐chloroethyl)phosphate reproduction and fertility assessment in swiss CD‐1 mice when administered via gavage [article]. (1991) PB92‐129170 (As Cited in: Provisional Peer‐reviewed Toxicity Values for Tris(2‐chloroethyl)phosphate) (Technical Report Number DART/TER/94000396), 11–16. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85103724509%26partnerID=40%26md5=93b18e49cffdb29f6556670c925fe5f6 [Google Scholar]
- Gupta, R. K. , Singh, J. M. , Leslie, T. C. , Meachum, S. , Flaws, J. A. , & Yao, H. H. C. (2010). Di‐(2‐ethylhexyl) phthalate and mono‐(2‐ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicology and Applied Pharmacology, 242(2), 224–230. 10.1016/j.taap.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg, I. , Kjellgren, J. , Persson, S. , Örn, S. , & Sjunnesson, Y. (2019). Perfluorononanoic acid (PFNA) alters lipid accumulation in bovine blastocysts after oocyte exposure during in vitro maturation. Reproductive Toxicology, 84, 1–8. 10.1016/j.reprotox.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Hannon, P. R. , Brannick, K. E. , Wang, W. , & Flaws, J. A. (2015). Mono(2‐ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles1. Biology of Reproduction, 92(5), 120. 10.1095/biolreprod.115.129148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, P. R. , Brannick, K. E. , Wang, W. , Gupta, R. K. , & Flaws, J. A. (2015). Di(2‐ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicology and Applied Pharmacology, 284(1), 42–53. 10.1016/j.taap.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, P. R. , & Curry, T. E. (2018). Folliculogenesis. In Skinner M. K. (Ed.), Encyclopedia of reproduction (2nd ed., pp. 72–79). Academic Press. 10.1016/B978-0-12-801238-3.64628-7 [DOI] [Google Scholar]
- Hannon, P. R. , Duffy, D. M. , Rosewell, K. L. , Brännström, M. , Akin, J. W. , & Curry, T. E., Jr. (2018). Ovulatory induction of SCG2 in human, nonhuman primate, and rodent granulosa cells stimulates ovarian angiogenesis. Endocrinology, 159(6), 2447–2458. 10.1210/en.2018-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, P. R. , & Flaws, J. A. (2015). The effects of phthalates on the ovary. Frontiers in Endocrinology, 6, 8. 10.3389/fendo.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, P. R. , Niermann, S. , & Flaws, J. A. (2016). Acute exposure to di(2‐ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicological Sciences, 150(1), 97–108. 10.1093/toxsci/kfv317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, P. R. , Peretz, J. , & Flaws, J. A. (2014). Daily exposure to di(2‐ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3‐kinase signaling pathway in adult mice1. Biology of Reproduction, 90(6), 136. 10.1095/biolreprod.114.119032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, K. G. , Marks, A. R. , Chevrier, J. , Bradman, A. , Sjödin, A. , & Eskenazi, B. (2010). PBDE concentrations in women's serum and fecundability. Environmental Health Perspectives, 118(5), 699–704. 10.1289/ehp.0901450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, R. , Gaskins, A. J. , Souter, I. , Smith, K. W. , Dodge, L. E. , Ehrlich, S. , Meeker, J. D. , Calafat, A. M. , & Williams, P. L. (2016). Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the EARTH study. Environmental Health Perspectives, 124(6), 831–839. 10.1289/ehp.1509760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel, J. J. , Blumberg, B. , Cave, M. , Machtinger, R. , Mantovani, A. , Mendez, M. A. , Nadal, A. , Palanza, P. , Panzica, G. , Sargis, R. , Vandenberg, L. N. , & vom Saal, F. (2017). Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology, 68, 3–33. 10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel, J. J. , Gulati, D. K. , Mounce, R. C. , Russell, S. R. , & Lamb, J. C. 4th. (1989). Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundamental and Applied Toxicology, 12(3), 508–518. 10.1016/0272-0590(89)90024-9 [DOI] [PubMed] [Google Scholar]
- Heindel, J. J. , Skalla, L. A. , Joubert, B. R. , Dilworth, C. H. , & Gray, K. A. (2017). Review of developmental origins of health and disease publications in environmental epidemiology. Reproductive Toxicology, 68, 34–48. 10.1016/j.reprotox.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Henry, N. D. , & Fair, P. A. (2013). Comparison of in vitro cytotoxicity, estrogenicity and anti‐estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. Journal of Applied Toxicology, 33(4), 265–272. 10.1002/jat.1736 [DOI] [PubMed] [Google Scholar]
- Herrera‐Cogco, E. , López‐Bayghen, B. , Hernández‐Melchor, D. , López‐Luna, A. , Palafox‐Gómez, C. , Ramírez‐Martínez, L. , López‐Bello, E. , Albores, A. , & López‐Bayghen, E. (2020). Paraben concentrations found in human body fluids do not exert steroidogenic effects in human granulosa primary cell cultures. Toxicology Mechanisms and Methods, 30(5), 336–349. 10.1080/15376516.2020.1741052 [DOI] [PubMed] [Google Scholar]
- Heudorf, U. , Mersch‐Sundermann, V. , & Angerer, J. (2007). Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health, 210(5), 623–634. 10.1016/j.ijheh.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Högberg, J. , Hanberg, A. , Berglund, M. , Skerfving, S. , Remberger, M. , Calafat, A. M. , Filipsson, A. F. , Jansson, B. , Johansson, N. , Appelgren, M. , & Håkansson, H. (2008). Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environmental Health Perspectives, 116(3), 334–339. 10.1289/ehp.10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer, P. B. (2005). Damage to ovarian development and function. Cell and Tissue Research, 322(1), 99–106. 10.1007/s00441-005-1083-y [DOI] [PubMed] [Google Scholar]
- Hsieh, M. , Lee, D. , Panigone, S. , Horner, K. , Chen, R. , Theologis, A. , Lee, D. C. , Threadgill, D. W. , & Conti, M. (2007). Luteinizing hormone‐dependent activation of the epidermal growth factor network is essential for ovulation. Molecular and Cellular Biology, 27(5), 1914–1924. 10.1128/mcb.01919-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Liu, S. , Fu, L. , Jiang, X. , & Yang, M. (2020). Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere, 253, 126707. 10.1016/j.chemosphere.2020.126707 [DOI] [PubMed] [Google Scholar]
- Huang, M. , Li, X. , Jia, S. , Liu, S. , Fu, L. , Jiang, X. , & Yang, M. (2021). Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS‐ASK1‐JNK MAPK pathway in human granulosa cell line KGN. Environmental Pollution, 270, 116051. 10.1016/j.envpol.2020.116051 [DOI] [PubMed] [Google Scholar]
- Hua, R. , Zhou, Y. , Wu, B. , Huang, Z. , Zhu, Y. , Song, Y. , Yu, Y. , Li, H. , & Quan, S. (2017). Urinary triclosan concentrations and early outcomes of in vitro fertilization‐embryo transfer. Reproduction, 153(3), 319–325. 10.1530/rep-16-0501 [DOI] [PubMed] [Google Scholar]
- Hubbard, T. D. , Brix, A. , Blystone, C. R. , McIntyre, B. S. , Shockley, K. , Cunny, H. , Waidyanatha, S. , Turner, K. J. , McBride, S. , & Roberts, G. K. (2020). Butylparaben multigenerational reproductive assessment by continuous breeding in Hsd: Sprague Dawley SD rats following dietary exposure. Reproductive Toxicology, 96, 258–272. 10.1016/j.reprotox.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L. , Peng, T. , Huang, J. , Su, T. , Luo, R. , Zheng, Y. , Zhong, Z. , Yu, P. , Nie, K. , & Zheng, L. (2019). Tri‐ortho‐cresyl phosphate (TOCP) induced ovarian failure in mice is related to the Hippo signaling pathway disruption. Reproductive Toxicology, 83, 21–27. 10.1016/j.reprotox.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Ijaz, S. , Ullah, A. , Shaheen, G. , & Jahan, S. (2020). Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicology Mechanisms and Methods, 30(1), 60–72. 10.1080/15376516.2019.1652873 [DOI] [PubMed] [Google Scholar]
- Ingle, M. E. , Mínguez‐Alarcón, L. , Carignan, C. C. , Stapleton, H. M. , Williams, P. L. , Ford, J. B. , Moravek, M. B. , Hauser, R. , & Meeker, J. D. (2020). Exploring reproductive associations of serum polybrominated diphenyl ether and hydroxylated brominated diphenyl ether concentrations among women undergoing in vitro fertilization. Human Reproduction, 35(5), 1199–1210. 10.1093/humrep/deaa063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James‐Todd, T. , Stahlhut, R. , Meeker, J. D. , Powell, S. G. , Hauser, R. , Huang, T. , & Rich‐Edwards, J. (2012). Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001−2008. Environmental Health Perspectives, 120(9), 1307–1313. 10.1289/ehp.1104717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, X. , Liu, N. , Xu, Y. , & Qiao, H. (2021). Perfluorononanoic acid impedes mouse oocyte maturation by inducing mitochondrial dysfunction and oxidative stress. Reproductive Toxicology, 104, 58–67. 10.1016/j.reprotox.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, S. , Reynolds, T. , White, R. , Parker, M. G. , & Sumpter, J. P. (1995). A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environmental Health Perspectives, 103(6), 582–587. 10.1289/ehp.95103582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, H. K. L. , Svingen, T. , Fowler, P. A. , Vinggaard, A. M. , & Boberg, J. (2017). Environmental influences on ovarian dysgenesis—Developmental windows sensitive to chemical exposures. Nature Reviews Endocrinology, 13(7), 400–414. 10.1038/nrendo.2017.36 [DOI] [PubMed] [Google Scholar]
- Johnson, P. I. , Altshul, L. , Cramer, D. W. , Missmer, S. A. , Hauser, R. , & Meeker, J. D. (2012). Serum and follicular fluid concentrations of polybrominated diphenyl ethers and in‐vitro fertilization outcome. Environment International, 45, 9–14. 10.1016/j.envint.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, K. T. , Specht, I. O. , Lenters, V. , Bach, C. C. , Rylander, L. , Jönsson, B. A. , Lindh, C. H. , Giwercman, A. , Heederik, D. , Toft, G. , & Bonde, J. P. (2014). Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environmental Health, 13, 116. 10.1186/1476-069x-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic, A. M. , Calafat, A. M. , McConnaughey, D. R. , Longnecker, M. P. , Hoppin, J. A. , Weinberg, C. R. , Wilcox, A. J. , Baird, D. D. , Calafat, A. M. , McConnaughey, D. R. , Longnecker, M. P. , Hoppin, J. A. , Weinberg, C. R. , Wilcox, A. J. , & Baird, D. D. (2016). Urinary concentrations of phthalate metabolites and bisphenol A and associations with follicular‐phase length, luteal‐phase length, fecundability, and early pregnancy loss. Environmental Health Perspectives, 124(3), 321–328. 10.1289/ehp.1408164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz, J. , Radwan, M. , Wielgomas, B. , Karwacka, A. , Klimowska, A. , Kałużny, P. , Radwan, P. , & Hanke, W. (2020). Parameters of ovarian reserve in relation to urinary concentrations of parabens. Environmental Health, 19(1), 26. 10.1186/s12940-020-00580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz, J. , Wielgomas, B. , Radwan, M. , Karwacka, A. , Klimowska, A. , Dziewirska, E. , Korczak, K. , Zajdel, R. , Radwan, P. , & Hanke, W. (2019). Triclosan exposure and ovarian reserve. Reproductive Toxicology, 89, 168–172. 10.1016/j.reprotox.2019.07.086 [DOI] [PubMed] [Google Scholar]
- Kahn, L. G. , Philippat, C. , Nakayama, S. F. , Slama, R. , & Trasande, L. (2020). Endocrine‐disrupting chemicals: Implications for human health. The Lancet Diabetes & Endocrinology, 8(8), 703–718. 10.1016/s2213-8587(20)30129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimal, A. , Al Mansi, M. H. , Dagher, J. B. , Pope, C. , Varghese, M. G. , Rudi, T. B. , Almond, A. E. , Cagle, L. A. , Beyene, H. K. , Bradford, W. T. , Whisnant, B. B. , Bougouma, B. D. K. , Rifai, K. J. , Chuang, Y. J. , Campbell, E. J. , Mandal, A. , MohanKumar, P. S. , & MohanKumar, S. M. J. (2021). Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere, 276, 130118. 10.1016/j.chemosphere.2021.130118 [DOI] [PubMed] [Google Scholar]
- Kang, Q. , Gao, F. , Zhang, X. , Wang, L. , Liu, J. , Fu, M. , Zhang, S. , Wan, Y. , Shen, H. , & Hu, J. (2020). Nontargeted identification of per‐ and polyfluoroalkyl substances in human follicular fluid and their blood‐follicle transfer. Environment International, 139, 105686. 10.1016/j.envint.2020.105686 [DOI] [PubMed] [Google Scholar]
- Karpeta, A. , Barc, J. , Ptak, A. , & Gregoraszczuk, E. L. (2013). The 2,2',4,4'‐tetrabromodiphenyl ether hydroxylated metabolites 5‐OH‐BDE‐47 and 6‐OH‐BDE‐47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology, 305, 65–70. 10.1016/j.tox.2012.10.021 [DOI] [PubMed] [Google Scholar]
- Karpeta, A. , & Gregoraszczuk, E. (2010). Mixture of dominant PBDE congeners (BDE‐47, ‐99, ‐100 and ‐209) at levels noted in human blood dramatically enhances progesterone secretion by ovarian follicles. Endocrine Regulations, 44(2), 49–55. 10.4149/endo_2010_02_49 [DOI] [PubMed] [Google Scholar]
- Karpeta, A. , Rak‐Mardyła, A. , Jerzak, J. , & Gregoraszczuk, E. L. (2011). Congener‐specific action of PBDEs on steroid secretion, CYP17, 17β‐HSD and CYP19 activity and protein expression in porcine ovarian follicles. Toxicology Letters, 206(3), 258–263. 10.1016/j.toxlet.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Kato, K. , Wong, L. Y. , Chen, A. , Dunbar, C. , Webster, G. M. , Lanphear, B. P. , & Calafat, A. M. (2014). Changes in serum concentrations of maternal poly‐ and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003−2006. Environmental Science & Technology, 48(16), 9600–9608. 10.1021/es501811k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, P. , Showstack, J. , Smith, J. F. , Nachtigall, R. D. , Millstein, S. G. , Wing, H. , Eisenberg, M. L. , Pasch, L. A. , Croughan, M. S. , & Adler, N. (2011). Costs of infertility treatment: Results from an 18‐month prospective cohort study. Fertility and Sterility, 95(3), 915–921. 10.1016/j.fertnstert.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H. , & Jee, B. C. (2020). Effects of butylparaben supplementation on in vitro development of mouse preantral follicle. Reproductive Sciences, 27(6), 1365–1371. 10.1007/s43032-020-00159-w [DOI] [PubMed] [Google Scholar]
- Kim, Y. R. , White, N. , Bräunig, J. , Vijayasarathy, S. , Mueller, J. F. , Knox, C. L. , Harden, F. A. , Pacella, R. , & Toms, L. M. L. (2020). Per‐ and poly‐fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environmental Research, 190, 109963. 10.1016/j.envres.2020.109963 [DOI] [PubMed] [Google Scholar]
- Kirkley, A. G. , & Sargis, R. M. (2014). Environmental endocrine disruption of energy metabolism and cardiovascular risk. Current Diabetes Reports, 14(6), 494. 10.1007/s11892-014-0494-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, S. S. , Jackson, T. , Javins, B. , Frisbee, S. J. , Shankar, A. , & Ducatman, A. M. (2011). Implications of early menopause in women exposed to perfluorocarbons. The Journal of Clinical Endocrinology & Metabolism, 96(6), 1747–1753. 10.1210/jc.2010-2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, H. M. , & Calafat, A. M. (2009). Human body burdens of chemicals used in plastic manufacture. Philosophical Transactions of the Royal Society, B: Biological Sciences, 364(1526), 2063–2078. 10.1098/rstb.2008.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill, M. A. , Vandenberg, L. N. , Smith, M. T. , Goodson, W. , Browne, P. , Patisaul, H. B. , Guyton, K. Z. , Kortenkamp, A. , Cogliano, V. J. , Woodruff, T. J. , Rieswijk, L. , Sone, H. , Korach, K. S. , Gore, A. C. , Zeise, L. , & Zoeller, R. T. (2020). Consensus on the key characteristics of endocrine‐disrupting chemicals as a basis for hazard identification. Nature Reviews Endocrinology, 16(1), 45–57. 10.1038/s41574-019-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land, K. L. , Lane, M. E. , Fugate, A. C. , & Hannon, P. R. (2020). Ovulation is inhibited by an environmentally relevant phthalate mixture in mouse antral follicles in vitro. Toxicological Sciences, 179, 195–205. 10.1093/toxsci/kfaa170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, A. , Carignan, C. C. , Minguez‐Alarcon, L. , Williams, P. , Calafat, A. M. , Toth, T. L. , & Hauser, R. (2015). Triclosan exposure and treatment outcomes in women undergoing in vitro fertilization. Fertility and Sterility, 104(3) (Suppl), e86. 10.1016/j.fertnstert.2015.07.264 [DOI] [Google Scholar]
- Latendresse, J. R. , Brooks, C. L. , Flemming, C. D. , & Capen, C. C. (1994). Reproductive toxicity of butylated triphenyl phosphate and tricresyl phosphate fluids in F344 rats. Fundamental and Applied Toxicology, 22(3), 392–399. 10.1006/faat.1994.1044 [DOI] [PubMed] [Google Scholar]
- Lefèvre, P. L. C. , Berger, R. G. , Ernest, S. R. , Gaertner, D. W. , Rawn, D. F. K. , Wade, M. G. , Robaire, B. , & Hales, B. F. (2016). Exposure of female rats to an environmentally relevant mixture of brominated flame retardants targets the ovary, affecting folliculogenesis and steroidogenesis. Biology of Reproduction, 94(1), 9. 10.1095/biolreprod.115.134452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre, P. L. C. , Nardelli, T. C. , Son, W. Y. , Sadler, A. R. , Rawn, D. F. K. , Goodyer, C. , Robaire, B. , & Hales, B. F. (2021). Polybrominated diphenyl ethers in human follicular fluid dysregulate mural and cumulus granulosa cell gene expression. Endocrinology, 162(3). 10.1210/endocr/bqab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre, P. L. C. , Wade, M. , Goodyer, C. , Hales, B. F. , & Robaire, B. (2016). A mixture reflecting polybrominated diphenyl ether (PBDE) profiles detected in human follicular fluid significantly affects steroidogenesis and induces oxidative stress in a female human granulosa cell line. Endocrinology, 157(7), 2698–2711. 10.1210/en.2016-1106 [DOI] [PubMed] [Google Scholar]
- Lehmler, H. J. , Liu, B. , Gadogbe, M. , & Bao, W. (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in US adults and children: The National Health and Nutrition Examination Survey 2013−2014. ACS Omega, 3(6), 6523–6532. 10.1021/acsomega.8b00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Zhou, S. , Wu, Y. , Li, Y. , Yan, W. , Guo, Q. , Xi, Y. , Chen, Y. , Li, Y. , Wu, M. , Zhang, J. , Wei, J. , & Wang, S. (2021). Prenatal exposure to propylparaben at human‐relevant doses accelerates ovarian aging in adult mice. Environmental Pollution, 285, 117254. 10.1016/j.envpol.2021.117254 [DOI] [PubMed] [Google Scholar]
- Li, N. , Zhou, L. , Zhu, J. , Liu, T. , & Ye, L. (2020). Role of the 17β‐hydroxysteroid dehydrogenase signalling pathway in di‐(2‐ethylhexyl) phthalate‐induced ovarian dysfunction: An in vivo study. Science of the Total Environment, 712, 134406. 10.1016/j.scitotenv.2019.134406 [DOI] [PubMed] [Google Scholar]
- Liu, X. , & Craig, Z. R. (2019). Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovary. Biology of Reproduction, 101(4), 854–867. 10.1093/biolre/ioz122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum, K. J. , Sundaram, R. , Barr, D. B. , Louis, T. A. , & Buck Louis, G. M. (2017). Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: Findings from a prospective pregnancy study. Epidemiology, 28(1), 90–98. 10.1097/ede.0000000000000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z. , Zhang, C. , Han, C. , An, Q. , Cheng, Y. , Chen, Y. , Meng, R. , Zhang, Y. , & Su, J. (2019). Plasticizer bis(2‐ethylhexyl) phthalate causes meiosis defects and decreases fertilization ability of mouse oocytes in vivo. Journal of Agricultural and Food Chemistry, 67(12), 3459–3468. 10.1021/acs.jafc.9b00121 [DOI] [PubMed] [Google Scholar]
- Lyngsø, J. , Ramlau‐Hansen, C. H. , Høyer, B. B. , Støvring, H. , Bonde, J. P. , Jönsson, B. A. G. , Lindh, C. H. , Pedersen, H. S. , Ludwicki, J. K. , Zviezdai, V. , & Toft, G. (2014). Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: A cross‐sectional study. Human Reproduction, 29(2), 359–367. 10.1093/humrep/det390 [DOI] [PubMed] [Google Scholar]
- Macaluso, M. , Wright‐Schnapp, T. J. , Chandra, A. , Johnson, R. , Satterwhite, C. L. , Pulver, A. , Berman, S. M. , Wang, R. Y. , Farr, S. L. , & Pollack, L. A. (2010). A public health focus on infertility prevention, detection, and management. Fertility and Sterility, 93, 16.e1–10. 10.1016/j.fertnstert.2008.09.046 [DOI] [PubMed] [Google Scholar]
- Machtinger, R. , Gaskins, A. J. , Racowsky, C. , Mansur, A. , Adir, M. , Baccarelli, A. A. , Calafat, A. M. , & Hauser, R. (2018). Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environment International, 111, 23–31. 10.1016/j.envint.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon, M. B. , & Fenton, S. E. (2013). Endocrine disruptors and the breast: Early life effects and later life disease. Journal of Mammary Gland Biology and Neoplasia, 18(1), 43–61. 10.1007/s10911-013-9275-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity, A. , Williams, P. L. , Ryan, L. , Missmer, S. A. , Coull, B. A. , & Hauser, R. (2014). Analysis of in vitro fertilization data with multiple outcomes using discrete time‐to‐event analysis. Statistics in Medicine, 33(10), 1738–1749. 10.1002/sim.6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney, E. K. , & Waxman, D. J. (1999). Trans‐activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicology and Applied Pharmacology, 161(2), 209–218. 10.1006/taap.1999.8809 [DOI] [PubMed] [Google Scholar]
- Mark‐Kappeler, C. J. , Hoyer, P. B. , & Devine, P. J. (2011). Xenobiotic effects on ovarian preantral follicles. Biology of Reproduction, 85(5), 871–883. 10.1095/biolreprod.111.091173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maske, P. , Dighe, V. , & Vanage, G. (2018). n‐butylparaben exposure during perinatal period impairs fertility of the F1 generation female rats. Chemosphere, 213, 114–123. 10.1016/j.chemosphere.2018.08.130 [DOI] [PubMed] [Google Scholar]
- Ma, X. , Cui, L. , Chen, L. , Zhang, J. , Zhang, X. , Kang, Q. , Jin, F. , & Ye, Y. (2021). Parental plasma concentrations of perfluoroalkyl substances and in vitro fertilization outcomes. Environmental Pollution, 269, 116159. 10.1016/j.envpol.2020.116159 [DOI] [PubMed] [Google Scholar]
- Meerts, I. A. , Letcher, R. J. , Hoving, S. , Marsh, G. , Bergman, A. , Lemmen, J. G. , van der Burg, B. , & Brouwer, A. (2001). In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environmental Health Perspectives, 109(4), 399–407. 10.1289/ehp.01109399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, Y. , Rongshuang, M. , Ruizhi, Z. , Hongyuan, H. , Qiyue, T. , & Shuhua, Z. (2019). Effects of dimethyl phthalate (DMP) on serum sex hormone levels and apoptosis in C57 female mice. International Journal of Endocrinology and Metabolism, 17(2), e82882. 10.5812/ijem.82882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling, D. D. , Warner, G. R. , Szumski, J. R. , Gao, L. , Gonsioroski, A. V. , Rattan, S. , & Flaws, J. A. (2020). The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicology and Applied Pharmacology, 388, 114875. 10.1016/j.taap.2019.114875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian, C. , Souter, I. , Gaskins, A. J. , Williams, P. L. , Ford, J. B. , Chiu, Y. H. , Calafat, A. M. , & Hauser, R. (2016). Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Human Reproduction, 31(1), 75–83. 10.1093/humrep/dev292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian, C. , Williams, P. L. , Mínguez‐Alarcón, L. , Carignan, C. C. , Ford, J. B. , Butt, C. M. , Meeker, J. D. , Stapleton, H. M. , Souter, I. , & Hauser, R. (2018). Organophosphate flame‐retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertility and Sterility, 110(6), 1137–1144. 10.1016/j.fertnstert.2018.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez‐Alarcón, L. , Chiu, Y. H. , Messerlian, C. , Williams, P. L. , Sabatini, M. E. , Toth, T. L. , Ford, J. B. , Calafat, A. M. , & Hauser, R. (2016). Urinary paraben concentrations and in vitro fertilization outcomes among women from a fertility clinic. Fertility and Sterility, 105(3), 714–721. 10.1016/j.fertnstert.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez‐Alarcón, L. , Christou, G. , Messerlian, C. , Williams, P. L. , Carignan, C. C. , Souter, I. , Ford, J. B. , Calafat, A. M. , Hauser, R. , Keller, M. G. , Ye, X. , Zhou, X. , & Jia, T. (2017). Urinary triclosan concentrations and diminished ovarian reserve among women undergoing treatment in a fertility clinic. Fertility and Sterility, 108(2), 312–319. 10.1016/j.fertnstert.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez‐Alarcón, L. , Messerlian, C. , Bellavia, A. , Gaskins, A. J. , Chiu, Y. H. , Ford, J. B. , Azevedo, A. R. , Petrozza, J. C. , Calafat, A. M. , Hauser, R. , & Williams, P. L. (2019). Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environment International, 126, 355–362. 10.1016/j.envint.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez‐Alarcón, L. , Souter, I. , Chiu, Y. H. , Williams, P. L. , Ford, J. B. , Ye, X. , Calafat, A. M. , Hauser, R. , & Earth Study, T. (2016). Urinary concentrations of cyclohexane‐1,2‐dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non‐phthalate plasticizer di(isononyl)cyclohexane‐1,2‐dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center. Environmental Research, 151, 595–600. 10.1016/j.envres.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi, S. A. , Masoumi, S. Z. , Keramat, A. , Pooralajal, J. , & Shobeiri, F. (2013). Assessment of questionnaires measuring quality of life in infertile couples: A systematic review. Journal of Reproduction & Infertility, 14(3), 110–119. [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre, W. , & Children's, H. (2013). National Institute for Health and Clinical Excellence: Guidance. In Fertility: Assessment and treatment for people with fertility problems. Royal College of Obstetricians & Gynaecologists (UK) National Collaborating Centre for Women's and Children's Health.
- Nevoral, J. , Havránková, J. , Kolinko, Y. , Prokešová, Š. , Fenclová, T. , Monsef, L. , Žalmanová, T. , Petr, J. , & Králíčková, M. (2021). Exposure to alternative bisphenols BPS and BPF through breast milk: Noxious heritage effect during nursing associated with idiopathic infertility. Toxicology and Applied Pharmacology, 413, 115409. 10.1016/j.taap.2021.115409 [DOI] [PubMed] [Google Scholar]
- Nevoral, J. , Kolinko, Y. , Moravec, J. , Žalmanová, T. , Hošková, K. , Prokešová, Š. , Klein, P. , Ghaibour, K. , Hošek, P. , Štiavnická, M. , Řimnáčová, H. , Tonar, Z. , Petr, J. , & Králíčková, M. (2018). Long‐term exposure to very low doses of bisphenol S affects female reproduction. Reproduction, 156(1), 47–57. 10.1530/rep-18-0092 [DOI] [PubMed] [Google Scholar]
- Nishihama, Y. , Yoshinaga, J. , Iida, A. , Konishi, S. , Imai, H. , Yoneyama, M. , Nakajima, D. , & Shiraishi, H. (2016). Association between paraben exposure and menstrual cycle in female university students in Japan. Reproductive Toxicology, 63, 107–113. 10.1016/j.reprotox.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Nourian, A. , Soleimanzadeh, A. , Shalizar Jalali, A. , & Najafi, G. (2017). Effects of bisphenol‐S low concentrations on oxidative stress status and in vitro fertilization potential in mature female mice. Veterinary Research Forum: An International Quarterly Journal, 8(4), 341–345. [PMC free article] [PubMed] [Google Scholar]
- Nowak, K. , Ratajczak–Wrona, W. , Górska, M. , & Jabłońska, E. (2018). Parabens and their effects on the endocrine system. Molecular and Cellular Endocrinology, 474, 238–251. 10.1016/j.mce.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Ospina, M. , Jayatilaka, N. K. , Wong, L.‐Y. , Restrepo, P. , & Calafat, A. M. (2018). Exposure to organophosphate flame retardant chemicals in the US general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environment International, 110, 32–41. 10.1016/j.envint.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotou, E. M. , Ojasalo, V. , & Damdimopoulou, P. (2021). Phthalates, ovarian function and fertility in adulthood. Best Practice & Research Clinical Endocrinology & Metabolism, 35(5), 101552. 10.1016/j.beem.2021.101552 [DOI] [PubMed] [Google Scholar]
- Pan, Y. , Wang, J. , Yeung, L. W. Y. , Wei, S. , & Dai, J. (2020). Analysis of emerging per‐ and polyfluoroalkyl substances: Progress and current issues. TrAC, Trends in Analytical Chemistry, 124, 115481. 10.1016/j.trac.2019.04.013 [DOI] [Google Scholar]
- Park, H. J. , Song, B. S. , Kim, J. W. , Yang, S. G. , Kim, S. U. , & Koo, D. B. (2020). Exposure of triclosan in porcine oocyte leads to superoxide production and mitochondrial‐mediated apoptosis during in vitro maturation. International Journal of Molecular Sciences, 21(9), 3050. 10.3390/ijms21093050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pednekar, P. P. , Gajbhiye, R. K. , Patil, A. D. , Surve, S. V. , Datar, A. G. , Balsarkar, G. D. , Chuahan, A. R. , & Vanage, G. R. (2018). Estimation of plasma levels of bisphenol‐A & phthalates in fertile & infertile women by gas chromatography‐mass spectrometry. The Indian Journal of Medical Research, 148(6), 734–742. 10.4103/ijmr.IJMR_2077_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz, J. , Vrooman, L. , Ricke, W. A. , Hunt, P. A. , Ehrlich, S. , Hauser, R. , Padmanabhan, V. , Taylor, H. S. , Swan, S. H. , VandeVoort, C. A. , & Flaws, J. A. (2014). Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environmental Health Perspectives, 122(8), 775–786. 10.1289/ehp.1307728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack, A. Z. , Mumford, S. L. , Krall, J. R. , Carmichael, A. E. , Sjaarda, L. A. , Perkins, N. J. , Kannan, K. , & Schisterman, E. F. (2018). Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach. Environment International, 120, 137–144. 10.1016/j.envint.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, T. , Greville, L. J. , Tang, B. , & deCatanzaro, D. (2016). Triclosan elevates estradiol levels in serum and tissues of cycling and peri‐implantation female mice. Reproductive Toxicology, 65, 394–401. 10.1016/j.reprotox.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Radwan, P. , Wielgomas, B. , Radwan, M. , Krasiński, R. , Klimowska, A. , Zajdel, R. , Kaleta, D. , & Jurewicz, J. (2021). Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environmental Science and Pollution Research, 28(10), 12993–12999. 10.1007/s11356-020-11287-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar, A. , Luu, T. , Beal, M. A. , Barton‐Maclaren, T. S. , Robaire, B. , & Hales, B. F. (2021). Elucidation of the effects of bisphenol A and structural analogs on germ and steroidogenic cells using single cell high‐content imaging. Toxicological Sciences, 180(2), 224–238. 10.1093/toxsci/kfab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, L. M. , Sen, N. , Vera, J. C. , Liu, X. , & Craig, Z. R. (2017). Effects of in vitro exposure to dibutyl phthalate, mono‐butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biology of Reproduction, 96(5), 1105–1117. 10.1095/biolreprod.116.144691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan, S. , Zhou, C. , Chiang, C. , Mahalingam, S. , Brehm, E. , & Flaws, J. A. (2017). Exposure to endocrine disruptors during adulthood: Consequences for female fertility. Journal of Endocrinology, 233(3), R109–R129. 10.1530/joe-17-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, J. S. , & Ascoli, M. (2018). Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends in Endocrinology & Metabolism, 29(5), 313–325. 10.1016/j.tem.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Sagiv, S. K. , Rifas‐Shiman, S. L. , Webster, T. F. , Mora, A. M. , Harris, M. H. , Calafat, A. M. , Ye, X. , Gillman, M. W. , & Oken, E. (2015). Sociodemographic and perinatal predictors of early pregnancy per‐ and polyfluoroalkyl substance (PFAS) concentrations. Environmental Science & Technology, 49(19), 11849–11858. 10.1021/acs.est.5b02489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scsukova, S. , Rollerova, E. , & Bujnakova Mlynarcikova, A. (2016). Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone‐related cancer. Reproductive Biology, 16(4), 243–254. 10.1016/j.repbio.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Sekiguchi, S. , Ito, S. , & Honma, T. (2003). Experimental model to study reproductive toxicity of chemicals using induced ovulation in immature F344 rats. Industrial Health, 41(3), 287–290. [DOI] [PubMed] [Google Scholar]
- Sen, N. , Liu, X. , & Craig, Z. R. (2015). Short term exposure to di‐n‐butyl phthalate (DBP) disrupts ovarian function in young CD‐1 mice. Reproductive Toxicology, 53, 15–22. 10.1016/j.reprotox.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, M. , Sekulovski, N. , MacLean, J. A., 2nd , & Hayashi, K. (2017). Effects of bisphenol A analogues on reproductive functions in mice. Reproductive Toxicology, 73, 280–291. 10.1016/j.reprotox.2017.06.134 [DOI] [PubMed] [Google Scholar]
- Shi, M. , Sekulovski, N. , MacLean, J. A. , Whorton, A. , & Hayashi, K. (2019). Prenatal exposure to bisphenol A analogues on female reproductive functions in mice. Toxicological Sciences, 168(2), 561–571. 10.1093/toxsci/kfz014 [DOI] [PubMed] [Google Scholar]
- Shi, M. , Whorton, A. E. , Sekulovski, N. , MacLean, J. A. , & Hayashi, K. (2019). Prenatal exposure to bisphenol A, E, and S induces transgenerational effects on female reproductive functions in mice. Toxicological Sciences, 170(2), 320–329. 10.1093/toxsci/kfz124 [DOI] [PubMed] [Google Scholar]
- Silva, M. J. , Barr, D. B. , Reidy, J. A. , Malek, N. A. , Hodge, C. C. , Caudill, S. P. , Brock, J. W. , Needham, L. L. , & Calafat, A. M. (2004). Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999−2000. Environmental Health Perspectives, 112(3), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, A. B. , Whitworth, K. W. , Haug, L. S. , Sabaredzovic, A. , Impinen, A. , Papadopoulou, E. , & Longnecker, M. P. (2018). Menstrual cycle characteristics as determinants of plasma concentrations of perfluoroalkyl substances (PFASs) in the Norwegian mother and child cohort (MoBa study). Environmental Research, 166, 78–85. 10.1016/j.envres.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin, A. , Jones, R. S. , Wong, L. Y. , Caudill, S. P. , & Calafat, A. M. (2019). Polybrominated diphenyl ethers and biphenyl in serum: Time trend study from the National Health and Nutrition Examination Survey for years 2005/06 through 2013/14. Environmental Science & Technology, 53(10), 6018–6024. 10.1021/acs.est.9b00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek, N. E. (2016). A brief review of the link between environment and male reproductive health: Lessons from studies of testicular germ cell cancer. Hormone Research in Paediatrics, 86(4), 240–246. 10.1159/000443400 [DOI] [PubMed] [Google Scholar]
- Smarr, M. M. , Sapra, K. J. , Gemmill, A. , Kahn, L. G. , Wise, L. A. , Lynch, C. D. , Factor‐Litvak, P. , Mumford, S. L. , Skakkebaek, N. E. , Slama, R. , Lobdell, D. T. , Stanford, J. B. , Jensen, T. K. , Boyle, E. H. , Eisenberg, M. L. , Turek, P. J. , Sundaram, R. , Thoma, M. E. , & Buck Louis, G. M. (2017). Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Human Reproduction, 32(3), 499–504. 10.1093/humrep/dew361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr, M. M. , Sundaram, R. , Honda, M. , Kannan, K. , & Louis, G. M. B. (2017). Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples' fecundity. Environmental Health Perspectives, 125(4), 730–736. 10.1289/ehp189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. W. , Souter, I. , Dimitriadis, I. , Ehrlich, S. , Williams, P. L. , Calafat, A. M. , & Hauser, R. (2013). Urinary paraben concentrations and ovarian aging among women from a fertility center. Environmental Health Perspectives, 121(11–12), 1299–1305. 10.1289/ehp.1205350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, T. E. , Dunlap, K. A. , & Filant, J. (2012). Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Molecular and Cellular Endocrinology, 354(1−2), 34–53. 10.1016/j.mce.2011.09.035 [DOI] [PubMed] [Google Scholar]
- Stenzel, A. , Wirt, H. , Patten, A. , Theodore, B. , & King‐Heiden, T. (2019). Larval exposure to environmentally relevant concentrations of triclosan impairs metamorphosis and reproductive fitness in zebrafish. Reproductive Toxicology, 87, 79–86. 10.1016/j.reprotox.2019.05.055 [DOI] [PubMed] [Google Scholar]
- Sunderland, E. M. , Hu, X. C. , Dassuncao, C. , Tokranov, A. K. , Wagner, C. C. , & Allen, J. G. (2019). A review of the pathways of human exposure to poly‐ and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology, 29(2), 131–147. 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M. H. , Li, X. H. , Xu, Y. , Xu, Y. , & Sun, S. C. (2020). Exposure to PBDE47 affects mouse oocyte quality via mitochondria dysfunction‐induced oxidative stress and apoptosis. Ecotoxicology and Environmental Safety, 198, 110662. 10.1016/j.ecoenv.2020.110662 [DOI] [PubMed] [Google Scholar]
- Téteau, O. , Jaubert, M. , Desmarchais, A. , Papillier, P. , Binet, A. , Maillard, V. , & Elis, S. (2020). Bisphenol A and S impaired ovine granulosa cell steroidogenesis. Reproduction, 159(5), 571–583. 10.1530/rep-19-0575 [DOI] [PubMed] [Google Scholar]
- Thomsen, A. M. L. , Riis, A. H. , Olsen, J. , Jönsson, B. A. G. , Lindh, C. H. , Hjollund, N. H. , Jensen, T. K. , Bonde, J. P. , & Toft, G. (2016). Female exposure to phthalates and time to pregnancy: A first pregnancy planner study. Human Reproduction, 32(1), 232–238. 10.1093/humrep/dew291 [DOI] [PubMed] [Google Scholar]
- van der Veen, I. , & de Boer, J. (2012). Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 88(10), 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Vélez, M. P. , Arbuckle, T. E. , & Fraser, W. D. (2015a). Female exposure to phenols and phthalates and time to pregnancy: The maternal‐infant research on environmental chemicals (MIREC) study. Fertility and Sterility, 103(4), 1011–1020. 10.1016/j.fertnstert.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Vélez, M. P. , Arbuckle, T. E. , & Fraser, W. D. (2015b). Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Human Reproduction, 30(3), 701–709. 10.1093/humrep/deu350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Zhang, S. , Ma, R. , Zhang, X. , Zhang, C. , Li, B. , Niu, Q. , Chen, J. , Xia, T. , Li, P. , Zhao, Q. , Dong, L. , Xu, C. , & Wang, A. (2016). Roles of endoplasmic reticulum stress, apoptosis and autophagy in 2,2',4,4'‐tetrabromodiphenyl ether‐induced rat ovarian injury. Reproductive Toxicology, 65, 187–193. 10.1016/j.reprotox.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Ruan, W. , Huang, B. , Shao, S. , Yang, D. , Liu, M. , Zeng, L. , Wei, J. , & Chen, J. (2019). Tri‐ortho‐cresyl phosphate induces autophagy of mouse ovarian granulosa cells. Reproduction, 158(1), 61–69. 10.1530/rep-18-0456 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Craig, Z. R. , Basavarajappa, M. S. , Gupta, R. K. , & Flaws, J. A. (2012). Di (2‐ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicology and Applied Pharmacology, 258(2), 288–295. 10.1016/j.taap.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Craig, Z. R. , Basavarajappa, M. S. , Hafner, K. S. , & Flaws, J. A. (2012). Mono‐(2‐ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles1. Biology of Reproduction, 87(6), 152. 10.1095/biolreprod.112.102467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Bai, Y. , Tang, C. , Cao, X. , Chang, F. , & Chen, L. (2018). Impact of perfluorooctane sulfonate on reproductive ability of female mice through suppression of estrogen receptor α‐activated kisspeptin neurons. Toxicological Sciences, 165(2), 475–486. 10.1093/toxsci/kfy167 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Luu, T. , Beal, M. A. , Barton‐Maclaren, T. S. , Robaire, B. , & Hales, B. F. (2022). The effects of organophosphate esters used as flame retardants and plasticizers on granulosa, leydig, and spermatogonial cells analyzed using high‐content imaging. Toxicological Sciences, 186, 269–287. 10.1093/toxsci/kfac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Xu, J. , Stanley, J. E. , Xu, M. , Brooks, B. W. , Scott, G. I. , Chatterjee, S. , Zhang, Q. , Zelinski, M. B. , & Xiao, S. (2020). A closed vitrification system enables a murine ovarian follicle bank for high‐throughput ovotoxicity screening, which identifies endocrine disrupting activity of microcystins. Reproductive Toxicology, 93, 118–130. 10.1016/j.reprotox.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, G. R. , Dettogni, R. S. , Bagchi, I. C. , Flaws, J. A. , & Graceli, J. B. (2021). Placental outcomes of phthalate exposure. Reproductive Toxicology, 103, 1–17. 10.1016/j.reprotox.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly, L. M. , & Gosse, J. A. (2017). Triclosan exposure, transformation, and human health effects. Journal of Toxicology and Environmental Health, Part B, 20(8), 447–469. 10.1080/10937404.2017.1399306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth, K. W. , Haug, L. S. , Sabaredzovic, A. , Eggesbo, M. , & Longnecker, M. P. (2016). Brief report: Plasma concentrations of perfluorooctane sulfonamide and time‐to‐pregnancy among primiparous women. Epidemiology, 27(5), 712–715. 10.1097/ede.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S. , Coppeta, J. R. , Rogers, H. B. , Isenberg, B. C. , Zhu, J. , Olalekan, S. A. , McKinnon, K. E. , Dokic, D. , Rashedi, A. S. , Haisenleder, D. J. , Malpani, S. S. , Arnold‐Murray, C. A. , Chen, K. , Jiang, M. , Bai, L. , Nguyen, C. T. , Zhang, J. , Laronda, M. M. , Hope, T. J. , … Woodruff, T. K. (2017). A microfluidic culture model of the human reproductive tract and 28‐day menstrual cycle. Nature Communications, 8, 14584. 10.1038/ncomms14584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Baumann, C. , De La Fuente, R. , & Viveiros, M. M. (2020). Mechanisms underlying disruption of oocyte spindle stability by bisphenol compounds. Reproduction, 159(4), 383–396. 10.1530/rep-19-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Shao, S. , Huang, B. , Yang, D. , Zeng, L. , Gan, Y. , Long, D. , Chen, J. , & Wang, J. (2020). Tea polyphenols alleviate tri‐ortho‐cresyl phosphate‐induced autophagy of mouse ovarian granulosa cells. Environmental Toxicology, 35(4), 478–486. 10.1002/tox.22883 [DOI] [PubMed] [Google Scholar]
- Young, A. S. , Herkert, N. , Stapleton, H. M. , Cedeño Laurent, J. G. , Jones, E. R. , MacNaughton, P. , Coull, B. A. , James‐Todd, T. , Hauser, R. , Luna, M. L. , Chung, Y. S. , & Allen, J. G. (2021). Chemical contaminant exposures assessed using silicone wristbands among occupants in office buildings in the USA, UK, China, and India. Environment International, 156, 106727. 10.1016/j.envint.2021.106727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žalmanová, T. , Hošková, K. , Nevoral, J. , Adámková, K. , Kott, T. , Šulc, M. , Kotíková, Z. , Prokešová, Š. , Jílek, F. , Králíčková, M. , & Petr, J. (2017). Bisphenol S negatively affects the meotic maturation of pig oocytes. Scientific Reports, 7(1), 485. 10.1038/s41598-017-00570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. Y. , Tian, Y. , Yan, Z. H. , Li, W. D. , Zang, C. J. , Li, L. , Sun, X. F. , Shen, W. , & Cheng, S. F. (2020). Maternal bisphenol S exposure affects the reproductive capacity of F1 and F2 offspring in mice. Environmental Pollution, 267, 115382. 10.1016/j.envpol.2020.115382 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Cao, X. , Chen, L. , Qin, Y. , Xu, Y. , Tian, Y. , & Chen, L. (2020). Exposure of female mice to perfluorooctanoic acid suppresses hypothalamic kisspeptin‐reproductive endocrine system through enhanced hepatic fibroblast growth factor 21 synthesis, leading to ovulation failure and prolonged dioestrus. Journal of Neuroendocrinology, 32(5), e12848. 10.1111/jne.12848 [DOI] [PubMed] [Google Scholar]
- Zhou, C. , & Flaws, J. A. (2016). Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicological Sciences, 156, kfw245. 10.1093/toxsci/kfw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Zhang, L. , Tong, C. , Fang, F. , Zhao, S. , Tian, Y. , Tao, Y. , & Zhang, J. (2017). Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environmental Health Perspectives, 125(6), 067012. 10.1289/ehp1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Zhou, W. , Huo, X. , Zhao, S. , Gan, Y. , Wang, B. , Cheng, W. , Ouyang, F. , Wang, W. , Tian, Y. , & Zhang, J. (2019). Triclosan and female reproductive health: A preconceptional cohort study. Epidemiology, 30(Suppl 1), S24–S31. 10.1097/ede.0000000000001011 [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Tan, Y. Q. , & Leung, L. K. (2017). Exposure to 2,2',4,4'‐tetrabromodiphenyl ether at late gestation modulates placental signaling molecules in the mouse model. Chemosphere, 181, 289–295. 10.1016/j.chemosphere.2017.04.089 [DOI] [PubMed] [Google Scholar]
- Ziv‐Gal, A. , & Flaws, J. A. (2016). Evidence for bisphenol A‐induced female infertility: A review (2007−2016). Fertility and Sterility, 106(4), 827–856. 10.1016/j.fertnstert.2016.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubizarreta, M. E. , & Xiao, S. (2020). Bioengineering models of female reproduction. Bio‐Design and Manufacturing, 3(3), 237–251. 10.1007/s42242-020-00082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


