INTRODUCTION
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a debilitating urologic pain disorder that affects roughly 6% of women in the United States.[4] Care and treatment for IC/BPS represents a substantial burden on the healthcare system but these expenditures have not resulted in high patient or provider satisfaction with available treatments.[53] Difficulty treating IC/BPS stems in part from a lack of consensus amongst researchers and clinicians regarding the underlying pathophysiology of the disorder.[9] Most agree, however, that multiple overlapping mechanisms likely contribute to the disease state, and efforts are underway to improve patient phenotyping.
One such effort, driven by the NIDDK-funded Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network, has demonstrated that female IC/BPS patients with chronic overlapping pain conditions (COPCs) such as irritable bowel syndrome (IBS) and temporomandibular disorder (TMD) show heightened ex vivo cytokine release to stimulation with lipopolysaccharide (LPS), a classic agonist of one highly conserved component of the innate immune system, Toll-Like Receptor-4 (TLR4).[47; 49] In a study conducted at the University of Iowa as part of the MAPP Epidemiology Phenotyping Study (EPS),[10; 36] 66 female IC/BPS patients (40 with comorbid COPCs, 26 with IC/BPS only), a composite score of ex-vivo TLR4 stimulated cytokines (interleukins [IL] 1β and 6) from peripheral blood mononuclear cells (PBMCs) was found to be significantly elevated in those patients with comorbid COPCs. Furthermore, this composite score was associated with the spatial extent of comorbid pain measured on a comprehensive body map. In a subsample of patients who underwent experimental pain testing (n=32), greater pain sensitivity to pressure at the thumbnail was marginally associated with higher TLR4 composite scores as well.[47] Together these results were interpreted to suggest that central nervous system amplification and maintenance of pain (i.e., noicplastic pain) is associated with ex-vivo TLR4 stimulated cytokine/chemokine release in IC/BPS. This possibility is supported by a number of animal models indicating a role for TLR4 in pain augmentation in the central nervous system, and studies demonstrating that the ex-vivo peripheral and central immune responses to TLR4 stimulation are linked.[33] [15; 20]. Confirming these relationships would provide a foundation for better patient phenotyping and point to mechanistic targets for further investigation.
Following the MAPP EPS (2009–2015) a new cohort of urologic pelvic pain patients was recruited for the MAPP Research Network Symptom Phenotyping Study (SPS, 2015–2021).[8] In the MAPP SPS, a modified protocol for measuring the LPS ex-vivo cytokine/chemokine response was adopted across six recruiting sites, allowing for the opportunity to conduct a critical confirmatory analysis of the MAPP EPS findings.[8] Clinically, MAPP studies have shown that COPCs/widespread pain in IC/BPS patients is associated with greater psychosocial difficulties and worse quality of life, indicating an urgent need to establish underlying pain mechanisms in this subset of patients.[34] In the current study, we examined 135 female IC/BPS patients from the MAPP SPS to determine if ex-vivo TLR4 stimulated cytokine/chemokine release, this time measured across a larger number of cytokines/chemokines, distinguished patients with comorbid COPCs from those with IC/BPS only. We also conducted analyses to determine if the extent of widespread pain and experimental pain sensitivity were associated with this response. Our primary purpose was to determine if we could confirm the relationship between the TLR4 ex-vivo cytokine/chemokine response and characteristics of nociplastic pain.
METHODS
Sample
The MAPP Research Network SPS enrolled 620 Urologic chronic Pelvic Pain Syndrome (UCPPS) patients for longitudinal follow-up of symptoms and phenotypic characteristics (ClinicalTrials.gov Identifier: NCT02514265).[8] Due to funding limitations, only a subset of the collected biomarker samples could be analyzed. A total of 155 female IC/BPS participants were selected for biomarker analysis; a power calculation was conducted prior to selecting the number of female participants based on previous data.[47] The Cohen’s d effect size for the difference between IC/BPS only and IC/BPS + comorbid COPCs on the LPS-stimulated composite score (IL-1b+IL-6) was d= .67. Assuming two-tailed hypothesis testing, alpha=.05, and an allocation ratio of 1:2 (pelvic pain only: pelvic pain comorbid, the rough distribution in the original manuscript) 82 subjects would be required to detect the effect of interest with .80 power. We chose a larger number of samples for analysis, enough to detect an effect size of d=.50, because of the changes to the protocol (e.g., whole blood rather than PBMCs and the larger number of cytokines/chemokines tested).
The 155 individuals were selected because they provided biomarker samples at baseline, 6 months and 18 months with concurrent neuroimaging data. These longitudinal data will be analyzed as part of an ancillary R01 to the MAPP network (R01DK123164). As the primary purpose of this manuscript is to attempt a conceptual validation of MAPP EPS findings, only female patients are analyzed who also completed the full battery of COPC self-report criteria, so the final sample consisted of 135 IC/BPS participants.
Demographic information
Patient demographics were collected by self-report and Body Mass Index was calculated form height and weight.
Clinical pain, chronic overlapping pain conditions, and extent of widespread pain
Overall Pelvic Pain Severity (PPS) was calculated using a composite measure comprised of questions from the Genitourinary Pain Index (GUPI) and Interstitial Cystitis Symptom Index (ICSI) as described previously.[21]
Chronic overlapping pain conditions (COPCs) were assessed using the Complex Multi-Symptom Inventory and standardized diagnostic criteria.[59] Rather than administering all diagnostic criteria to all patients, patients first complete the screener which contains items that “trigger” full diagnostic criteria the administration of the full diagnostic criteria for COPCs that are relevant for that individual, which limits response burden by only administering relevant questionnaires. Possible diagnostic modules include chronic fatigue syndrome, irritable bowel syndrome, fibromyalgia, temporomandibular joint disorder, and migraine.[16; 19; 40; 60; 61]
Patients were asked to indicate whether they had pain or not and the severity of any pain on a 0–10 scale, using a 76-site body map adapted from the Collaborative Health Outcomes Information Registry project and further reduced to 12 non-pelvic regions for the assessment of widespread pain.[45] Sites with severity of pain rated at least 4 were counted.
Cytokine/chemokines under ex-vivo stimulated and unstimulated conditions
Differences between the MAPP EPS and SPS protocols
Several important differences are noted between the MAPP EPS and MAPP SPS TLR stimulation protocols. First, in the MAPP EPS, a single site (University of Iowa) collected samples for ex vivo stimulation, while in the MAPP SPS all six recruiting sites took part. Second, in the MAPP EPS PBMCs were isolated for stimulation, while in the MAPP SPS, a commercially available whole blood ex-vivo stimulation assay was used (TruCulture, Myriad RBM) to allow all sites to collect samples in a consistent and efficient manner. In the MAPP EPS, lipopolysaccharide (LPS) concentrations of 50ng/ml were used to stimulate samples for 72 hours, while in the MAPP SPS the concentration was 100ng/ml (the standard concentration available) for an incubation period of 24 hours. For these reasons, the current study represents a conceptual validation of the MAPP EPS findings, rather than a direct replication.
SPS protocol
The TruCulture system uses vacutainers preloaded with TLR4 agonist (LPS), or control media (unstimulated condition), which are kept frozen at −20° C until being thawed for one hour at room temperature, or overnight in a standard refrigerator prior to use. Approximately 1ml of whole blood is drawn directly into each tube and then kept in a tabletop incubator at 37° C for 24 hours. After incubation, the supernatant is isolated using a valve separator included in the kit and stored in −80° C freezer for batch analysis. All samples were sent to a central biorepository at the University of Denver Anscultz Medical Campus Biorepository Core Facility under the supervision of the Director and Co-Director of the MAPP Tissue Analysis and Technology Core. 50 µL of thawed supernatant was analyzed for seven cytokine/chemokines using Luminex® Xmap technology with R&D systems high performance assays. These were monocyte chemoattractant protein-1 (MCP-1, range of assay: 3.0–1890 pg/ml), macrophage inflammatory protein 1-α (MIP-1α, 18–9840 pg/ml), IL-1β (0.34–1500 pg/ml), IL- 6 (0.95–3400, pg/ml), IL-8 (0.78–2800 pg/ml), IL-10 (0.46–2000 pg/ml), and tumor necrosis factor -α (TNF-α; 0.78–2000 pg/ml). Unstimulated samples for IL-1β, 6, 8, 10 and TNFα were diluted 2x, while the LPS condition was diluted 20x. Unstimulated samples for MCP1 and MIP-1α were diluted 4x, and the LPS condition was diluted 10x. Values below the limit of quantification (LOQ) were set to one half of the LOQ value. This family of cytokines and chemokines was selected because they represent different aspects of the inflammatory response and are all promoted by the transcriptional factor NF-ΚB, whose upregulation is a well-established consequence of TLR4 stimulation.[38]
Pressure Pain Sensitivity
As in the MAPP EPS, pressure pain sensitivity was measured using the Multimodal Automated Sensory Testing (MAST) system (Arbor Medical Innovations, Ann Arbor, MI).[24] The MAST system includes a electromechanical stimulator to deliver pressure stimuli and a touchscreen-based rating scale to capture participant responses.[22] Following a familiarization procedure to reduce testing anxiety, an ascending sequence of incremental pressure stimuli were delivered to the participants’ dominant thumbnail by a 1 cm2 rubber probe attached to the MAST stimulator. Pressure intensity started at 0.5 kgf/cm2 and increased in 0.5 kgf/cm2 steps at a ramp rate of 4.0 kgf/cm2/s. Each pressure was held constant for 5-s in duration and was separated by a 20-s inter-stimulus rest interval. Participants rated perceived pain intensity after each stimulus using a digital 0–100 NRS displayed on the touchscreen (0 = no pain; 100 = pain as bad as you could imagine). The test was completed when the participant reached his/her pain tolerance and asked that the test be stopped, the participant reported a pain intensity of ≥ 80/100, or a maximum possible pressure intensity of 10 kgf/cm2 was delivered. Data were automatically uploaded by the system to the MAPP Network Data Coordinating Center via a secure file transfer protocol for analysis. A three parameter logistic model was used to estimate the within-person inflection point on the stimulus-response curve between PPT and tolerance, referred to as Pain50 [23] To ensure standardization across sites, scripted participant instructions were used and research staff completed annual in-person training.
Statistical analyses
All analyses were performed in the R programming language, version 3.6.1.
Comparison of biomarker sample to the rest of the MAPP SPS baseline sample.
To determine if the 135 participants differed from the rest of the female IC/BPS participants in the MAPP SPS baseline sample (n=172) on variables that could plausibly influence immune parameters, we compared patient age, body mass index, depression scores, anxiety scores (Hamilton anxiety and depression scale), perceived stress (perceived stress scale), Pain50 scores, number of painful sites selected on the body map, and proportion of each sample with a COPC by t-test and Χ2 tests for continuous and categorical variables, respectively.
Comparison of IC/BPS only and IC/BPS + COPC groups
Transformation of cytokine/chemokine values for analysis
To conduct parametric analyses with appropriate covariates we proceeded to transform cytokine/chemokine values with values in the detectable range using Box-Cox transformations (all TLR4 stimulated cytokines/chemokines; unstimulated TNF-α, , IL-1β, and IL-8.)[5] The Shapiro-Wilks test statistic, where values > 0.97 indicate acceptable normality, was used to evaluate these transformations and all exceeded this threshold.
Principal components analysis
We subsequently used a principal components analysis (PCA) based on the correlation matrix between the seven transformed cytokine/chemokine values under the TLR4 stimulated condition retaining components with an eigenvalue greater than one. Factor scores for the resulting components were then extracted by the regression method. PCA has previously been used as a dimension reduction technique for inflammatory variables.[29; 31]
General linear models
The primary form of analysis was a mixed-effects linear model with the TLR4 component scores as dependent variables with patient age and body mass index included as covariates. A random intercept term was included for site of collection. The independent predictor of interest was COPC group status. We also conducted analyses with the degree of widespread pain,, and Pain50 ratings as independent predictors of the TLR4 component scores.
We recreated an inflammatory composite variable analogous to that used in the original study, which was a simple mean of the z-scores for LPS-stimulated IL-1β and IL-6 from the current study. These values were used rather than those from the MAPP EPS dataset due to the substantial differences in the protocol.
While not the focus of the current manuscript, we also repeated these analyses with the unstimulated values of TNF-α, IL-8, MCP-1, and IL-1β (transformed) as dependent variables, to determine if unstimulated values were associated with COPC status. Because a large percentage of unstimulated IL-10 (84%), IL-6 (78%), and MIP-1α (56%) values fell below the detectable limit of the assay, we did not compare these cytokines between the groups.
RESULTS
Demographic and clinical information is shown in Table 1. Values of stimulated and unstimulated cytokines/chemokines are shown in Table 2.
Table 1.
UCPPS symptoms and distribution of COPC types by COPC status.
| IC/BPS only(n=36) | IC/BPS+ COPC(n=99) | All(n=135) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| age | 47.21 | 16.32 | 40.97 | 14.17 | 42.63 | 14.97 |
| Body mass index | 25.64 | 5.31 | 26.96 | 5.46 | 26.61 | 5.43 |
| Age of symptom onset | 32.03 | 15.86 | 27.64 | 14.66 | 28.82 | 15.06 |
| Genitourinary pain severity | 9.26 | 5.16 | 13.48 | 6.15 | 12.35 | 6.17 |
| Urinary symptom severity | 11.68 | 3.87 | 16.45 | 5.18 | 15.18 | 5.29 |
| n | % | n | % | |||
| Chronic Overlapping Pain Conditions Fibromyalgia Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome Irritable Bowel Syndrome Temporomandibular Disorder Migraine |
0 0 0 0 0 |
0 0 0 0 0 |
10 25 57 46 53 |
10.1 25.3 57.6 46.5 53.5 |
Table 2.
Unstimulated and LPS-stimulated cytokine values by COPC status.
| IC/BPS only(n=36) | IC/BPS+ COPC(n=99) | All(n=135) | ||||
|---|---|---|---|---|---|---|
| median | 25th – 75thpercentile | median | 25th – 75thpercentile | median | 25th – 75thpercentile | |
| Unstimulated (pg/ml) | ||||||
| monocyte chemoattractant protein-1 | 118 | 78–156 | 103 | 78–148 | 109 | 78–151 |
| macrophage inflammatory protein 1-alphaa | 58 | 36–136 | 36 | 36–108 | 36 | 36–112 |
| Interleukin-1β | 1 | 1–2 | 1 | <1–3 | 1 | <1–3 |
| Interleukin-6a | 1 | 1–1 | 1 | 1–1 | 1 | 1–1 |
| Interleukin-8 | 37 | 23–68 | 37 | 37–79 | 37 | 37–77 |
| Interleukin-10a | <1 | <1−<1 | <1 | <1−<1 | <1 | <1−<1 |
| Tumor necrosis factor – α | 4 | 3–5 | 5 | 5–6 | 4 | 4–6 |
| Stimulated (pg/ml) ( | ||||||
| monocyte chemoattractant protein-1 | 1182 | 896–1658 | 1715 | 1033–2542 | 1526 | 1000–2310 |
| macrophage inflammatory protein 1-alpha | 38497 | 23818–58503 | 48184 | 37793–75389 | 45923 | 33951–72730 |
| Interleukin-1β | 5427 | 4195–9035 | 8982 | 4686–14479 | 8309 | 4316–8309 |
| Interleukin-6 | 14605 | 10209–21923 | 21218 | 15739–25878 | 20244 | 14374–25815 |
| Interleukin-8 | 11793 | 7197–15349 | 14812 | 9316–20675 | 12550 | 9004–19368 |
| Interleukin-10 | 53 | 24–83 | 68 | 41–107 | 65 | 37–98 |
| Tumor necrosis factor – α | 3807 | 2294–5648 | 4448 | 2899–5591 | 4123 | 2715–5591 |
> than 25% of values below limit of quantification
Comparison of biomarker sample to the rest of the baseline MAPP SPS cohort
There were no significant differences between the MAPP biomarker sample and the rest of the MAPP SPS baseline cohort on patient age, BMI, depression scores, anxiety scores, perceived stress scores, Pain50 scores, number of painful sites selected on the body map, and proportion of each sample with a COPC (all p < .05; data not shown).
Principal components analysis
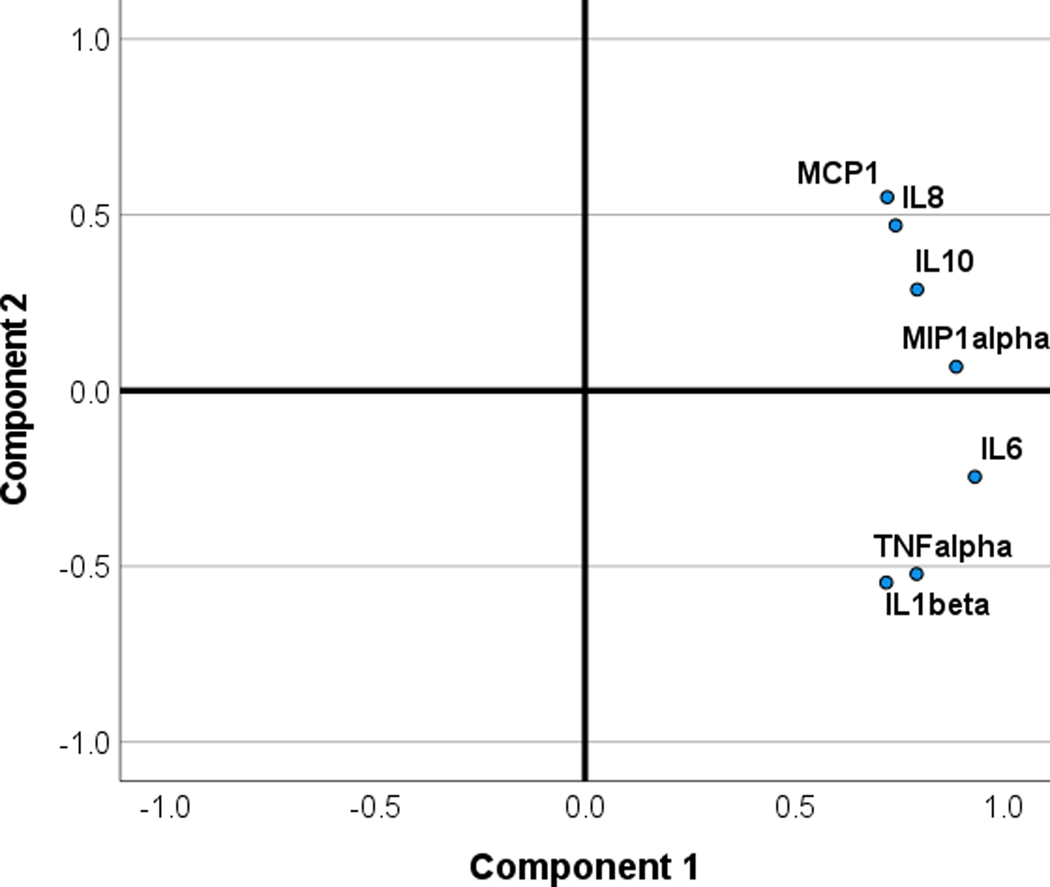
Two components were extracted, one with high positive loadings on all 7 cytokine/chemokines that explained 63.1% of the variance, and a second component with high positive loadings for MCP1, IL-8 and IL-10, a near zero loading for MIP-1α, and negative loadings for IL-6, IL-1 and TNF-α, that explained an additional 16.1% of the variance. These findings are consistent with the first component representing a global response to LPS-stimulation, and a second component more specific to anti-inflammatory activity, the regulatory function of IL-8, and chemotactic activity (MCP1). See Figure 1 for PCA loading plot.
Figure 1.
Principal components analysis loading plots for the cytokines/chemokines (transformed scales) under the TLR4 stimulated condition.
Comparison of IC/BPS and IC/BPS + COPC groups on TLR4 composite scores
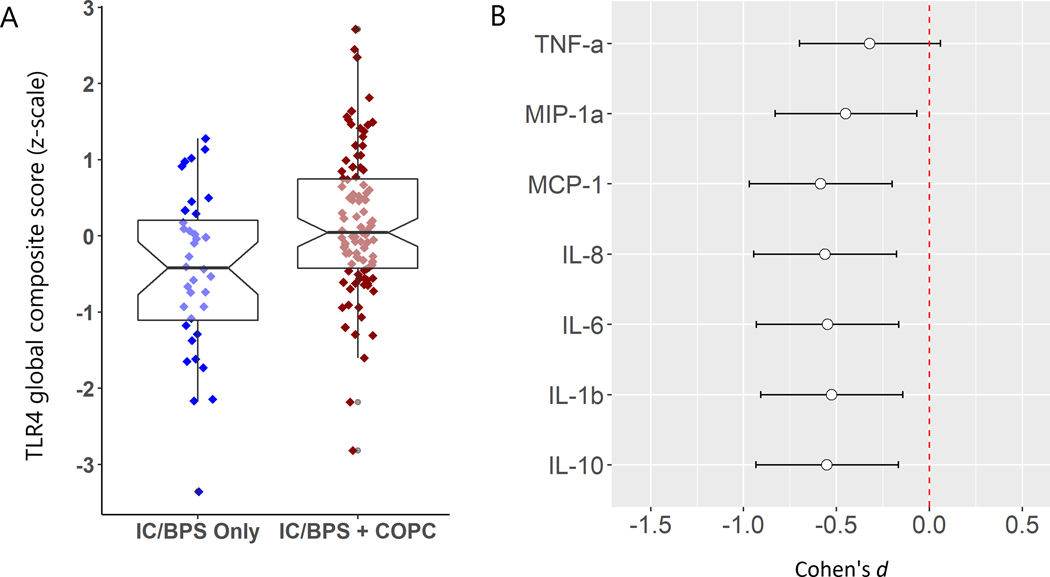
In models controlling for patient age, body mass index, and site of collection, IC/BPS + COPC patients were found to have significantly elevated TLR4 global composite scores, (p< .01), but not the TLR4 anti-inflammatory/regulatory/chemotactic composite scores (p > .05). See Fig 2a & 2b for differences in the TLR4 global composite score and Cohen’s d effect sizes for each of the seven stimulated cytokine/chemokines by COPC status. The basic difference in the TLR4 global composite score was apparent when stratified by site of collection, strengthening the generalizability of the results (Supplemental Fig 1). The z-score composite of ex-vivo TLR4 stimulated IL-6 and IL-1β was also significantly higher in the IC/BPS + COPC group (p< .05). See Table 3 for model estimates.
Figure 2.
A. ex-vivo TLR4 global composite score for female IC/BPS patients stratified by presence (n=99) or absence (n=36) of COPCs. B. Cohen’s d effect sizes for individual cytokines/chemokines on the transformed scale associated with IC/BPS only status.
Table 3.
General linear model estimates for relationship between pain variables and TLR4 stimulated composite scores.
| TLR4 global composite score | |||||
|---|---|---|---|---|---|
| estimate | S.E. | df | t value | p | |
| (Intercept) | −0.670 | 0.481 | 110.343 | −1.393 | 0.167 |
| Age (years) | 0.003 | 0.006 | 130.289 | 0.451 | 0.653 |
| BMI | 0.003 | 0.015 | 129.671 | 0.177 | 0.860 |
| COPC (yes) | 0.608 | 0.188 | 128.939 | 3.232 | 0.002 |
| (Intercept) | −0.319 | 0.479 | 97.096 | −0.667 | 0.506 |
| Age (years) | −0.001 | 0.006 | 129.277 | −0.160 | 0.873 |
| BMI | 0.005 | 0.016 | 128.691 | 0.341 | 0.734 |
| Body map sites | 0.071 | 0.032 | 125.837 | 2.258 | 0.026 |
| (Intercept) | −0.070 | 0.553 | 113.500 | −0.127 | 0.899 |
| Age (years) | −0.001 | 0.006 | 130.400 | −0.112 | 0.911 |
| BMI | 0.008 | 0.016 | 129.700 | 0.516 | 0.607 |
| Pain50 | −0.048 | 0.069 | 129.600 | −0.695 | 0.489 |
| (Intercept) | −0.574 | 0.518 | 111.100 | −1.107 | 0.270 |
| Age (years) | 0.000 | 0.006 | 130.400 | 0.054 | 0.957 |
| BMI | 0.004 | 0.016 | 129.600 | 0.268 | 0.789 |
| Pelvic Pain Severity | 0.026 | 0.016 | 127.900 | 1.613 | 0.109 |
| TLR4 anti-inflammatory, regulatory, chemotactic composite score | |||||
| (Intercept) | −1.089 | 0.494 | 114.403 | −2.205 | 0.030 |
| Age (years) | 0.006 | 0.006 | 130.623 | 1.024 | 0.308 |
| BMI | 0.025 | 0.016 | 129.622 | 1.599 | 0.112 |
| COPC (yes) | 0.263 | 0.195 | 128.789 | 1.348 | 0.180 |
| (Intercept) | 0.962 | 0.474 | 114.361 | −2.031 | 0.045 |
| Age (years) | 0.004 | 0.006 | 129.988 | 0.734 | 0.464 |
| BMI | 0.026 | 0.016 | 129.194 | 1.637 | 0.104 |
| Body map sites | 0.054 | 0.032 | 125.527 | 1.686 | 0.094 |
| (Intercept) | −1.845 | 0.520 | 124.280 | −3.549 | < 0.001 |
| Age (years) | 0.004 | 0.006 | 128.286 | 0.792 | 0.430 |
| BMI | 0.030 | 0.015 | 130.722 | 2.007 | 0.047 |
| Pain50 | 0.233 | 0.066 | 130.970 | 3.507 | < 0.001 |
| (Intercept) | −0.897 | 0.517 | 121.466 | −1.734 | 0.085 |
| Age (years) | 0.005 | 0.006 | 130.977 | 0.784 | 0.434 |
| BMI | 0.028 | 0.016 | 129.973 | 1.747 | 0.083 |
| Pelvic Pain Severity | −0.001 | 0.016 | 127.865 | −0.080 | 0.936 |
| TLR4 z-score (IL-6+IL-1β) | |||||
| (Intercept) | −0.171 | 0.491 | 93.193 | −0.349 | 0.728 |
| Age (years) | −0.001 | 0.006 | 129.598 | −0.183 | 0.855 |
| BMI | −0.008 | 0.015 | 128.996 | −0.533 | 0.595 |
| COPC (yes) | 0.491 | 0.188 | 128.121 | 2.606 | 0.010 |
Widespread pain, pressure pain sensitivity, pelvic pain severity and TLR4 composite scores
Pain50 was not associated with the TLR4 global response score (p > .05), however, higher pressure thresholds on this measure, representing less pain sensitivity, were significantly and positively associated with the TLR4 anti-inflammatory/regulatory/chemotactic score (p < .01). The number of painful regions indicated on the body map (severity ≥ 4) was positively and significantly associated with the TLR4 global composite score (p < .05), but there was no relationship with the TLR4 anti-inflammatory/regulatory/chemotactic score (p > .05). Pelvic pain severity was not associated with either composite score (p >.05).
Comparison of IC/BPS and IC/BPS + COPC groups on unstimulated cytokine/chemokine values.
There were no significant relationships between levels of unstimulated MCP1, IL-1β, TNFα, or IL-8 and COPC status (all p > .05). Model estimates are shown in Table 4.
Table 4.
General linear model estimates for COPC status and unstimulated cytokines/chemokines.
| unstimulated MCP1 | |||||
|---|---|---|---|---|---|
| (Intercept) | 2.272 | 0.069 | 124.194 | 32.945 | <0.001 |
| Age (years) | 0.002 | 0.001 | 130.995 | 2.483 | 0.014 |
| BMI | −0.001 | 0.002 | 130.323 | −0.509 | 0.611 |
| COPC (yes) | 0.017 | 0.028 | 129.987 | 0.605 | 0.546 |
| unstimulated IL-8 | |||||
| (Intercept) | 1.967 | 0.118 | 131.000 | 16.648 | <0.001 |
| Age (years) | 0.000 | 0.001 | 131.000 | 0.252 | 0.801 |
| BMI | 0.002 | 0.004 | 131.000 | 0.575 | 0.567 |
| COPC (yes) | −0.013 | 0.048 | 131.000 | −0.276 | 0.783 |
| unstimulated IL-1β | |||||
| (Intercept) | −0.402 | 0.490 | 131.000 | −0.821 | 0.413 |
| Age (years) | 0.002 | 0.006 | 131.000 | 0.308 | 0.759 |
| BMI | 0.015 | 0.016 | 131.000 | 0.930 | 0.354 |
| COPC (yes) | −0.017 | 0.198 | 131.000 | −0.084 | 0.933 |
| unstimulated TNFα | |||||
| (Intercept) | 1.093 | 0.198 | 110.610 | 5.511 | 0.000 |
| Age (years) | 0.003 | 0.002 | 130.295 | 1.099 | 0.274 |
| BMI | −0.003 | 0.006 | 129.807 | −0.406 | 0.685 |
| COPC (yes) | 0.047 | 0.077 | 129.180 | 0.611 | 0.542 |
DISCUSSION
These findings confirm a difference in immune priming between subtypes of IC/BPS patients that have localized pelvic pain versus those with comorbid pain conditions or widespread pain manifestations, as we previously demonstrated this relationship in the MAPP EPS.[47] Our findings have been expanded to include a larger number of inflammation-linked cytokines/chemokines with diverse functions, but overall the results suggest a relatively broad effect of relationship between comorbid pain and ex-vivo TLR4 immunoreactivity, as five of the seven tested cytokines/chemokines showed at least medium differences between the IC/BPS subtypes by conventional effect sizes (Cohen’s d). Furthermore, experimental pain testing suggests that there may be a mechanistic link between ex-vivo TLR4 immunoreactivity and pain sensitivity.
Ex-vivo LPS-stimulation of PBMCs, whole blood, or isolated immune cell subsets, have previously been linked to chronic and cyclic pain in a number of patient groups: chronic fatigue syndrome, mixed chronic pain samples, chronic multisite musculoskeletal pain, dysmenorrhea, and low back pain.[7; 18; 33; 52] The study in low back pain is of particular interest because some stimulated cytokines and chemokines were also higher in chronic LBP patients compared to those with acute pain.[52] While not directly analogous to the current study, this supports the use of stimulated assays for subtyping within pain cohorts. Stimulation of whole blood, as conducted in the current study, tends to show similar responses for inflammation-linked cytokines/chemokines as those obtained from stimulation of isolated cells and particularly monocytes[12; 14]. Given that our current findings are similar to those obtained in the MAPP EPS with PBMC stimulation, these results further support the idea that complex forms of chronic pain are associated with immune priming of circulating immune cells. Seen in the light of these previous studies, our results suggest that this immune priming is a common feature of what has recently been termed nociplastic pain.
We found that the second TLR4 component that was represented by positive relationships with anti-inflammatory cytokine IL-10, chemokine MCP1, and IL-8 (diverse functions), as well as negative relationships with classic pro-inflammatory cytokines IL-1β, IL-6, and TNFα, was associated with an ability to tolerate more pressure pain. It is worth noting that this component represented only a modest amount of the variance in the TLR4 cytokine/chemokine levels (19%). Nonetheless, the positive relationship with IL-10 and negative relationship with the pro-inflammatory group of cytokines suggest that this component may reflect a protective factor for pain sensitivity measured experimentally, though the diverse functions of IL-8 and chemotactic activity of MCP1 complicate the picture. For example, previous research has shown that increases in circulating IL-8 secondary to endotoxin (LPS) administration are associated with greater pain sensitivity.[27] Further studies are needed to parse different relationships under stimulated and unstimulated conditions with experimental pain sensitivity.
Animal models showing a state of hyperalgesia in response to LPS injections have been studied for more than 25 years.[57] It is clear from these studies that TLR4 expressed in the spinal cord plays a major role in the transition to chronic pain states: knockout of TLR4 or application of antisense oligodeoxynucleotides for TLR4 greatly attenuate behavioral assays of hyperalgesia.[51] These studies have led to great interest in therapeutics that target TLR4.[6] Given the high preponderance of chronic pain conditions in women, sex-dependent effects of TLR4 and TLR4 therapeutics have led to renewed focus on this pathway, particularly in visceral pain conditions.[15] Mechanistically, there is mounting evidence that peripheral immune cells and especially monocytes are capable of entering the CNS parenchyma and promoting long term behavioral changes.[43] Relevant to the current study, Kwok et al. demonstrated that the inflammatory response observed from spinal tissue stimulated with LPS is mirrored in PBMCs, suggesting that peripheral and central immune cells show similar immunoreactivity[33], a finding similar to that seen in our own animal models of IC/BPS symptoms.
The role of ex-vivo TLR4 immune responses in IC/BPS symptoms has been explored in recent studies using the transgenic URO-OVA mouse model of cystitis induction to induce IC/BPS symptoms. These studies observed that URO-OVA mice, a transgenic IC/BPS-like mouse model, developed pelvic/bladder pain after cystitis induction, along with altered systemic and central TLR4 activations.[11] Splenocytes from cystitis-induced URO-OVA mice produced increased proinflammatory cytokines (IL-1β, IL-6 and TNF-α) in response to LPS stimulation in vitro. Consistently, spinal tissues from cystitis-induced URO-OVA mice expressed increased mRNAs for TLR4 mediated proinflammatory cytokines (IL-6 and TNF-α). Furthermore, pain sensitization following cystitis induction was attenuated in TLR4 deficient URO-OVA mice (URO-OVATLR4−/−) despite similar levels of bladder inflammation and voiding dysfunction. Moreover, administration of TAK-242, a TLR4 antagonist[25], in cystitis-induced URO-OVA mice reduced pain sensitization, which was associated with reduction of both splenocyte production of TLR4 mediated cytokines and spinal expression of mRNAs for TLR4 mediated cytokines. This last point is critical, as it suggests that immune priming in peripheral and central compartments occur in tandem and point to the possibility that the immune priming we have demonstrated in association with nociplastic pain features in the MAPP EPS & SPS may mirror central TLR4 immunoreactivity.
These findings naturally raise the question of how immune priming occurs in IC/BPS patients. Genetic, psychosocial, and environmental factors may be responsible. IC/BPS is considerably more prevalent in first degree relatives of IC/BPS patients than in the general population, and twin studies suggest a moderate genetic contribution to IC/BPS.[54–56] Very large genetic studies would be needed to determine if polymorphisms related to innate immunity generally or TLRs specifically would be required to address this possibility, ideally with a focus on the IC/BPS + COPC subtype. Previous MAPP EPS analyses showed that IC/BPS patients with COPCs have elevated depression, anxiety, perceived stress, and are more likely to report childhood trauma, though they were not more likely to be using pain medication.[30; 48] There are well-established links between negative mood, childhood trauma, and immune responses, as well as glucocorticoid resistance.[1; 2; 41] Other studies have shown that childhood trauma is associated with increased NF-κB activity in PBMCs and LPS-stimulated PBMC cytokine/chemokine release.[17; 42] Finally, antecedent urinary tract infections have been associated with IC/BPS and likely implicate TLR4 signaling.[44; 62] These factors suggest psychosocial, environmental, and genetic vulnerabilities may interact to contribute to immune priming in IC/BPS.
Other studies have shown that basal circulating levels of inflammatory markers are associated with greater pain sensitivity,[26; 37; 46] and multiple of studies of endotoxin in human subjects show that increases in cytokines, including several measured in the current study (e.g., IL-6, TNFα, IL-8, IL-10) accompany enhanced pain sensitivity.[3; 13; 27; 58] In another recent manuscript examining plasma (unstimulated) inflammatory markers in the MAPP EPS, there were no relationships between IL-6, TNFα and widespread pain – this echoes the findings of our original MAPP EPS study showing that the TLR4 ex-vivo responses were associated with comorbid pain while plasma (unstimulated) IL-6 levels were not.[28; 47] However, exploratory analyses suggested a potential relationship between interleukin (IL)-8, Granulocyte macrophage colony-stimulating factor, and widespread pain.[28] While our findings confirm the value of TLR4 ex-vivo cytokine/chemokine responses in IC/BPS phenotypes, basal/unstimulated inflammation-linked cytokines/chemokines should continue to be explored in IC/BPS.
Clinical Relevance.
In addition to the immunological differences described here, IC/BPS patients with a high burden of COPCs or greater degrees of widespread pain show a number of pathophysiologic differences that suggest mechanistic divergence of the pain experience associated with central sensitization/nociplastic pain. IC/BPS patients with more widespread pain show distinct changes in brain structure of the supplementary motor area and functional connectivity between the primary somatosensory cortex and the salience network,[32] both experimental and self-reported sensory sensitivity,[24; 50] and are less likely to present with classic peripheral indications of IC/BPS like Hunner’s lesions.[35] Future studies attempting to delineate the mechanistic involvement of TLR4 in IC/BPS may wish to assess the impact of ex-vivo TLR4 responses on these variables. The strongest relationship between an individual cytokine under ex-vivo TLR4 stimulation and the TLR4 global composite score was IL-6; thus, LPS-stimulated IL-6 may be a useful proxy for more comprehensive assessment of cytokines.
Strengths and Limitations.
The current study benefits from several strengths, including the collection of data at multiple sites in geographically diverse regions of the country, deep symptom-based phenotyping of the patient population, and an a priori framework for pursuing the differences of interest. There are limitations as well. Only five COPCs were assessed in the current study, while up to ten are recognized by the NIH pain consortium,[39] and the study considered only female patients. Future explorations of the relationship between inflammatory markers and comorbid pain in IC/BPS will benefit from more granular explorations of inflammatory pathways using proteomic and/or transcriptomic approaches to identify cellular sources, intracellular signaling pathways, and characterization of Toll-like receptor type and density on cell surfaces. These analyses will help to delineate potential peripheral-CNS crosstalk that promotes sensitization beyond the relatively non-specific relationships encountered here.
Conclusions.
Heightened ex-vivo TLR4 immune responses are a feature of IC/BPS + COPC female patients. These responses may be mechanistically linked to nociplastic pain in IC/BPS and should be investigated further for potential clinical applications.
Supplementary Material
Acknowledgements:
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) [DK082315 (Andriole, G; Lai, H), DK082316 (Landis, J), DK082325 (Buchwald, D), DK082333 (Lucia, M), DK082342 (Klumpp, D; Schaeffer A), DK082344 (Kreder, K), DK082345 (Clauw, D; Clemens, JQ), DK082370 (Mayer, E; Rodriguez L), DK103227 (Moses, M), DK103260 (Anger, J; Freeman, M), DK103271 (Nickel, J), and DK123164 (Harte, SE)].
Footnotes
The authors have no conflicts of interest to disclose.
*See MAPP masthead
REFERENCES
- [1].Alexander N, Kirschbaum C, Wankerl M, Stauch BJ, Stalder T, Steudte-Schmiedgen S, Muehlhan M, Miller R. Glucocorticoid receptor gene methylation moderates the association of childhood trauma and cortisol stress reactivity. Psychoneuroendocrinology 2018;90:68–75. [DOI] [PubMed] [Google Scholar]
- [2].Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry 2016;21:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit JS, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain 2012;153:794–799. [DOI] [PubMed] [Google Scholar]
- [4].Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Box GEP, Cox DR. An Analysis of Transformations. Journal of the Royal Statistical Society Series B (Methodological) 1964;26:211–252. [Google Scholar]
- [6].Bruno K, Woller SA, Miller YI, Yaksh TL, Wallace M, Beaton G, Chakravarthy K. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain 2018;159:1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chao CC, Janoff EN, Hu SX, Thomas K, Gallagher M, Tsang M, Peterson PK. Altered cytokine release in peripheral blood mononuclear cell cultures from patients with the chronic fatigue syndrome. Cytokine 1991;3:292–298. [DOI] [PubMed] [Google Scholar]
- [8].Clemens JQ, Kutch JJ, Mayer EA, Naliboff BD, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Clauw DJ, Harte SE, Schrepf AD, Williams DA, Andriole GL, Lai HH, Buchwald D, Lucia MS, van Bokhoven A, Mackey S, Moldwin RM, Pontari MA, Stephens-Shields AJ, Mullins C, Landis JR. The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn 2020;39:1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, Harte SE, Kutch JJ, Lai HH, Martucci KT, Moldwin R, Naliboff BD, Pontari MA, Sutcliffe S, Landis JR, Group MRNS. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol 2019;16:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ, Group MRNS. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cui X, Jing X, Lutgendorf SK, Bradley CS, Schrepf A, Erickson BA, Magnotta VA, Ness TJ, Kreder KJ, O’Donnell MA. Cystitis-induced bladder pain is Toll-like receptor 4 dependent in a transgenic autoimmune cystitis murine model: a MAPP Research Network animal study. American Journal of Physiology-Renal Physiology 2019;317:F90–F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Damsgaard CT, Lauritzen L, Calder PC, Kjær TM, Frøkiær H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines—a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. Journal of immunological methods 2009;340:95–101. [DOI] [PubMed] [Google Scholar]
- [13].de Goeij M, van Eijk LT, Vanelderen P, Wilder-Smith OH, Vissers KC, van der Hoeven JG, Kox M, Scheffer GJ, Pickkers P. Systemic inflammation decreases pain threshold in humans in vivo. PloS one 2013;8:e84159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Groote D, Zangerlé P-F, Gevaert Y, Fassotte M-F, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992;4:239–248. [DOI] [PubMed] [Google Scholar]
- [15].Dodds KN, Beckett EA, Evans SF, Grace PM, Watkins LR, Hutchinson MR. Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl Psychiatry 2016;6:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121:953–959. [DOI] [PubMed] [Google Scholar]
- [17].Geiger ML, Boeck C, Koenig AM, Schury K, Waller C, Kolassa S, Karabatsiakis A, Kolassa I-T. Investigating the effects of childhood maltreatment on pro-inflammatory signaling: The influence of cortisol and DHEA on cytokine secretion ex vivo. Mental Health & Prevention 2019;13:176–186. [Google Scholar]
- [18].Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Dekker J, Penninx B. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain 2014;155:1605–1612. [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez YM, Schiffman E, Gordon SM, Seago B, Truelove EL, Slade G, Ohrbach R. Development of a brief and effective temporomandibular disorder pain screening questionnaire: reliability and validity. J Am Dent Assoc 2011;142:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, Williams DA, Clemens JQ, Afari N, Tu F, Lloyd RB, Patrick DL, Mullins C, Kusek JW, Sutcliffe S, Hong BA, Lai HH, Krieger JN, Bradley CS, Kim J, Landis JR. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol 2016;195:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Harte SE, Mitra M, Ichesco EA, Halvorson ME, Clauw DJ, Shih AJ, Kruger GH. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Med Biol Eng Comput 2013;51:633–644. [DOI] [PubMed] [Google Scholar]
- [23].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai H, Sutcliffe S, Hartounian S, Halvorson M, Harris RE, Ichesco E, Naliboff B, Farrar J, Tu F, Landis JR, Clauw DJ, Network ftMR. Quantitative assessment of non-pelvic pain sensitivity in urological chronic pelvic pain syndrome: a MAPP Research Network study. Pain In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N. Quantitative assessment of non-pelvic pressure pain sensitivity in urological chronic pelvic pain syndrome: a MAPP research network study. Pain 2019;160:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl) sulfamoyl] cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Molecular pharmacology 2006;69:1288–1295. [DOI] [PubMed] [Google Scholar]
- [26].Iordanova Schistad E, Kong XY, Furberg AS, Backryd E, Grimnes G, Emaus N, Rosseland LA, Gordh T, Stubhaug A, Engdahl B, Halvorsen B, Nielsen CS. A population-based study of inflammatory mechanisms and pain sensitivity. Pain 2020;161:338–350. [DOI] [PubMed] [Google Scholar]
- [27].Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, Olgart Hoglund C, Axelsson J. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav Immun 2015;46:35–43. [DOI] [PubMed] [Google Scholar]
- [28].Karshikoff B, Martucci KT, Mackey S. Relationship Between Blood Cytokine Levels, Psychological Comorbidity, and Widespreadness of Pain in Chronic Pelvic Pain. Front Psychiatry 2021;12:651083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Keenan-Devlin LS, Caplan M, Freedman A, Kuchta K, Grobman W, Buss C, Adam EK, Entringer S, Miller GE, Borders AEB. Using principal component analysis to examine associations of early pregnancy inflammatory biomarker profiles and adverse birth outcomes. Am J Reprod Immunol 2021;86:e13497. [DOI] [PubMed] [Google Scholar]
- [30].Krieger JN, Stephens AJ, Landis JR, Clemens JQ, Kreder K, Lai HH, Afari N, Rodriguez L, Schaeffer A, Mackey S, Andriole GL, Williams DA, Network MR. Relationship between chronic nonurological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. J Urol 2015;193:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kumar RG, Rubin JE, Berger RP, Kochanek PM, Wagner AK. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain Behav Immun 2016;53:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, Network MR. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158:1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kwok YH, Tuke J, Nicotra LL, Grace PM, Rolan PE, Hutchinson MR. TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers. PloS one 2013;8:e77799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lai HH, Jemielita T, Sutcliffe S, Bradley CS, Naliboff B, Williams DA, Gereau RW, Kreder K, Clemens JQ, Rodriguez LV. Characterization of whole body pain in urological chronic pelvic pain syndrome at baseline: a MAPP research network study. The Journal of urology 2017;198:622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lai HH, Newcomb C, Harte S, Appleby D, Ackerman AL, Anger JT, Nickel JC, Gupta P, Rodriguez LV, Landis JR, Clemens JQ, Network MR. Comparison of deep phenotyping features of UCPPS with and without Hunner lesion: A MAPP-II Research Network Study. Neurourol Urodyn 2021;40:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ, Group MRNS. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008;42:145–151. [DOI] [PubMed] [Google Scholar]
- [39].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain 2016;17:T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olesen J, Steiner TJ. The International classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry 2004;75:808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 2020;87:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 2012;26:13–17. [DOI] [PubMed] [Google Scholar]
- [43].Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015;289:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rudick CN, Billips BK, Pavlov VI, Yaggie RE, Schaeffer AJ, Klumpp DJ. Host-pathogen interactions mediating pain of urinary tract infection. J Infect Dis 2010;201:1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Scherrer KH, Ziadni MS, Kong JT, Sturgeon JA, Salmasi V, Hong J, Cramer E, Chen AL, Pacht T, Olson G, Darnall BD, Kao MC, Mackey S. Development and validation of the Collaborative Health Outcomes Information Registry body map. Pain Rep 2021;6:e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schistad EI, Stubhaug A, Furberg AS, Engdahl BL, Nielsen CS. C-reactive protein and cold-pressor tolerance in the general population: the Tromso Study. Pain 2017;158:1280–1288. [DOI] [PubMed] [Google Scholar]
- [47].Schrepf A, Bradley CS, O’Donnell M, Luo Y, Harte SE, Kreder K, Lutgendorf S, Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research N. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun 2015;49:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schrepf A, Naliboff B, Williams DA, Stephens-Shields AJ, Landis JR, Gupta A, Mayer E, Rodriguez LV, Lai H, Luo Y, Bradley C, Kreder K, Lutgendorf SK, Network MR . Adverse Childhood Experiences and Symptoms of Urologic Chronic Pelvic Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Study. Ann Behav Med 2018;52:865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schrepf A, O’Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S, Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research N. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain 2014;155:1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE, Network MR. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. Pain 2018;159:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 2005;102:5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain. Clin J Pain 2019;35:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tung A, Hepp Z, Bansal A, Devine EB. Characterizing Health Care Utilization, Direct Costs, and Comorbidities Associated with Interstitial Cystitis: A Retrospective Claims Analysis. J Manag Care Spec Pharm 2017;23:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tunitsky E, Barber MD, Jeppson PC, Nutter B, Jelovsek JE, Ridgeway B. Bladder pain syndrome/interstitial cystitis in twin sisters. J Urol 2012;187:148–152. [DOI] [PubMed] [Google Scholar]
- [55].Warren JW, Jackson TL, Langenberg P, Meyers DJ, Xu J. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology 2004;63:17–21. [DOI] [PubMed] [Google Scholar]
- [56].Warren JW, Keay SK, Meyers D, Xu J. Concordance of interstitial cystitis in monozygotic and dizygotic twin pairs. Urology 2001;57:22–25. [DOI] [PubMed] [Google Scholar]
- [57].Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res 1994;654:15–26. [DOI] [PubMed] [Google Scholar]
- [58].Wegner A, Elsenbruch S, Maluck J, Grigoleit JS, Engler H, Jager M, Spreitzer I, Schedlowski M, Benson S. Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun 2014;41:46–54. [DOI] [PubMed] [Google Scholar]
- [59].Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am 2009;35:339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Seminars in arthritis and rheumatism 2016;46:319–329. [DOI] [PubMed] [Google Scholar]
- [61].Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia--I: examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 2011;152:291–299. [DOI] [PubMed] [Google Scholar]
- [62].Yang MH, Huang JY, Chen SL, Wei JC. Association of Interstitial Cystitis/Bladder Pain Syndrome with Stress-Related Diseases: A Nationwide Population-Based Study. J Clin Med 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.