Abstract
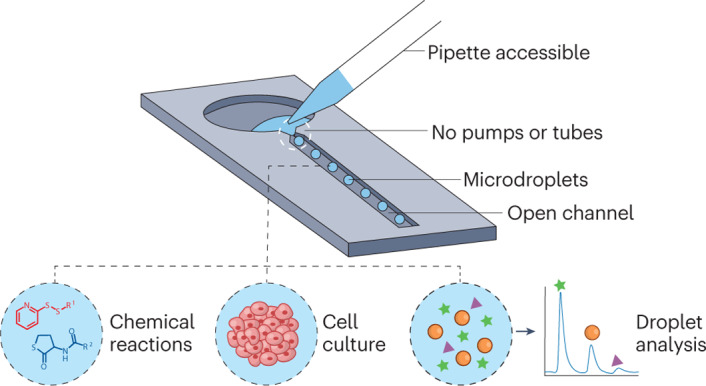
Open droplet microfluidic systems manipulate droplets on the picolitre-to-microlitre scale in an open environment. They combine the compartmentalization and control offered by traditional droplet-based microfluidics with the accessibility and ease-of-use of open microfluidics, bringing unique advantages to applications such as combinatorial reactions, droplet analysis and cell culture. Open systems provide direct access to droplets and allow on-demand droplet manipulation within the system without needing pumps or tubes, which makes the systems accessible to biologists without sophisticated setups. Furthermore, these systems can be produced with simple manufacturing and assembly steps that allow for manufacturing at scale and the translation of the method into clinical research. This Review introduces the different types of open droplet microfluidic system, presents the physical concepts leveraged by these systems and highlights key applications.
Subject terms: Microfluidics, Engineering, Lab-on-a-chip, Characterization and analytical techniques, Combinatorial libraries
Open droplet microfluidic systems have unique advantages; for example, the droplets are accessible and the systems translate well into biomedical research. This Review highlights different types of open droplet microfluidic system in chemistry and biology and discusses their physical concepts and key applications.
Introduction
Open droplet microfluidic systems use properties such as wetting, capillary force or microscale geometries (for example, channels or microstructured surfaces) to manipulate picolitre-to-microlitre-sized droplets in an open environment. In these systems at least one component of the microfluidic system (that is, the floor, ceiling or walls) is exposed to an external environment, typically through one or more air–liquid or liquid–liquid interfaces.
Research in this field is multidisciplinary; the process was started by analytical chemists and engineers as a method of manipulating small volumes of liquid in an open environment. The small volumes needed make these approaches desirable for applications that use precious samples such as rare cell types or expensive reagents. Moreover, these miniaturized technologies offer versatility, which is important for many applications, such as point-of-care and at-home sample analysis1, while also allowing researchers to study complex biological processes2,3. In the past decade, this miniaturized technology has advanced and branched out into numerous applications in chemistry and biology. The concept of open droplet microfluidics evolved from the general fields of open microfluidics and open-space microfluidics, which have been covered in previous books and reviews4–9. The terms ‘open microfluidics’ and ‘open-space microfluidics’ were originally proposed by different research groups and now these terms are often used interchangeably; both refer to systems that use channels or microstructures with at least one dimension on the micrometre (submillimetre) scale and with one side or face exposed to the outside world. Additionally, many of the concepts of open droplet microfluidics (such as the compartmentalization of experiments in droplets and the manipulation of droplets) have evolved from decades of work in closed channel droplet microfluidic systems10,11 (Fig. 1a).
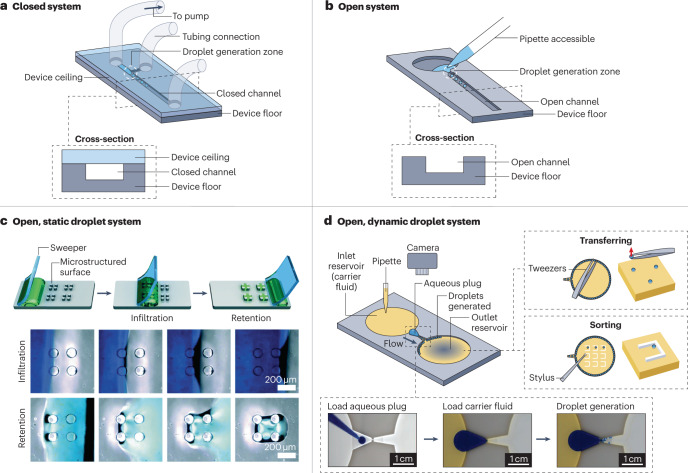
Fig. 1. Comparing closed and open channel droplet microfluidic systems.
An overview of closed and open droplet systems in which we subdivide open droplet systems into two categories: static and dynamic. a, A schematic and cross-sectional view of a closed microfluidic system in which the channel is accessible only through tubing connection ports. In this setup, droplets are generated using active pumps connected through tubing. b, A schematic and cross-sectional view of an open microfluidic system in which there is no ceiling, making the channel accessible. c, A static open droplet system that uses a sweeping method to load droplets onto the platform. Top: the stages in the sweeping process, which traps droplets within the microstructures on the platform surface. Bottom: images of the platform during the infiltration and retention stages. d, A schematic of a dynamic open droplet system that generates droplets autonomously by constricting the open channel. Bottom inset: photographs of the stages of droplet generation in the open device. Top right inset: examples of droplet manipulation using tweezers or a stylus made from polytetrafluoroethylene. Part c is reprinted with permission from ref. 18, Royal Society of Chemistry. Part d is adapted from ref. 27 under a Creative Commons licence CC BY 4.0.
Examples of open droplet microfluidic systems are freestyle fluidics12–15, droplet microarrays2,16–20, hanging drops21–23, suspended stacks3,24, acoustophoretic droplet printing25, droplets in open capillary flows26,27, exclusive liquid repellency droplets28–34, magnetic-bead-embedded droplets in an open environment35, and open digital microfluidics36,37. However, open digital microfluidics, in which a discrete droplet is moved across a surface using electrodes, will not be covered in this Review because it has been covered extensively in other review articles36–39; we note that digital microfluidics is sometimes referred to as electrowetting on dielectric (EWOD), but here we use the more general term digital microfluidics because not all digital microfluidic systems involve the EWOD mechanism.
Open systems offer many advantages over closed systems for biology and chemistry studies including accessibility, ease of fabrication and ease of use. Openness offers direct access to the droplet and enables droplet manipulations such as retrieval, sorting and extraction. Although closed droplet microfluidic systems and digital microfluidic systems have often become more complex, open droplet microfluidic systems generally take the opposite approach and simplify the mechanisms of complex tasks and functions by using passive forces such as capillarity.
This Review aims to introduce this field by broadly covering the work done and providing details of the fluidic mechanisms and biochemical and chemical applications of open droplet microfluidic systems. We first describe the different types of system (static and dynamic) and the advantages and disadvantages of open droplet microfluidic systems. We then introduce the physical principles and theory of open droplet microfluidic systems, the various system configurations and some examples of their applications. Last, we provide a perspective on potential future applications and directions for the field of open droplet microfluidics.
Open droplet microfluidic systems
In this section, we briefly explain the concept of open microfluidics (in comparison to closed microfluidics), introduce two main types of open droplet microfluidic system, and discuss the advantages and disadvantages of such systems. We note that the term ‘droplet’ is often used interchangeably with ‘plug’, as there is no well-accepted definition. In some cases, a plug is considered to be an oblong segment of liquid that fills more of a channel than a droplet, whereas droplet often refers to a volume of liquid that is approximately spherical in shape. In some cases, plugs can be used to generate droplets27.
Static and dynamic systems
Traditional microfluidics use closed channels (Fig. 1a) and often involve polydimethylsiloxane (PDMS) devices that are fabricated with soft lithography and bonded to glass, although other materials and fabrication methods, such as etching in glass, micromilling, hot embossing and injection moulding of plastics, have also been used40,41. These closed systems are usually driven by various pumps, tubing, junctions and control architecture; droplets are often generated at a high throughput (for example, thousands per second), and typically have volumes on the picolitre-to-nanolitre scale42. In the almost two decades since the emergence of closed droplet microfluidic technologies, the field has advanced from the development of proof-of-concept devices to complex studies in the chemical and biological sciences10,11. One of the most successful applications is droplet-based single-cell RNA sequencing43. More recent applications include the production of artificial cells in which droplet microfluidic technologies are used to generate and assemble unilamellar liposomes with subcellular units44. Although most research on artificial cells uses closed microfluidic systems, such research can also be combined with open platforms. For example, nanolitre aqueous droplets with lipid monolayers on their surface were generated in a closed microfluidic T-junction and then spotted onto an open substrate to form one-dimensional or two-dimensional (2D) droplet networks45. When adjacent droplets contact one another droplet interface bilayers are formed, which can simulate cellular membranes for molecule translocation analysis45.
Open microfluidic systems have at least one side or face of the device open to the air or to an immiscible liquid (Fig. 1b). In general, open droplet microfluidic systems can be categorized as static or dynamic systems. In static systems, droplets remain stationary for the majority (if not all) of the experiment, and the droplets are manipulated in the same location16–18. For example, in one such system liquid droplets generated with the sweeping method (an approach that moves a bulk liquid drop across a structured surface with an elastic sweeper) are trapped between the micropillars and are retained throughout the experiment18 (Fig. 1c). In dynamic droplet systems, droplets are moved on demand around the system for different purposes including incubation, sorting, extraction and analysis35,46. In one example, individual droplets around the perimeter of the reservoir are transported laterally to different locations within the same reservoir using a polytetrafluoroethylene (PTFE) stylus27 (Fig. 1d).
In general, static systems are simpler and more straightforward in design than dynamic systems, as the droplets are laid out in an array (sometimes called droplet microarrays16), similar to a well plate. The device surface is often patterned with hydrophilic spots where droplets are generated and located, separated by hydrophobic boundaries. Static systems are commonly used for applications such as 2D cell cultures in which adherent cells need a surface to attach to and spread out on47,48. For example, a 6 × 9 microfluidic paired-well array was used to study tumour angiogenesis47. In this example, a monolayer of endothelial cells was cocultured with 3D tumour spheroids and formed tubular structures. Besides droplet microarrays, microfluidic droplets in static open systems can also be suspended between side walls and stacked vertically3. One example is the reconfigurable stacks platform, in which each droplet in a layer forms an independent cell culture microenvironment (2D or 3D). This system was used to study the spatiotemporal dynamics of paracrine signalling by stacking the droplets to bring them into contact with one another3. Despite the simplicity of static open microfluidic systems, the throughput may be limited owing to the requirement of a relatively large device footprint to accommodate all of the droplets with sufficient spacing between droplets49, as well as a motorized stage47 or liquid handling robot50 to access droplets at different locations quickly and accurately for reagent addition and imaging.
Dynamic systems are more flexible than static systems in manipulating droplets, therefore, dynamic systems can achieve a wider range of functions. ‘Soft robots’ such as ferrofluid51 droplets actuated by programmable magnetic fields can perform multiple tasks, including the delivery of liquid cargo, manipulation of delicate objects, and navigation through narrow tubes or open channels46. Droplets can also be moved using surface modifications, as demonstrated by a bio-inspired superwettable microspine chip, which uses the principles of geometric asymmetry and surface superhydrophilicity to direct droplet self-transportation52. Regarding droplet generation, it is generally difficult for open droplet microfluidic systems to match the efficiency and high throughput of closed microfluidic systems owing to the lack of external mechanical pumps, which enable high flowrates conducive to high-throughput droplet generation (kilohertz or higher). However, a dynamic open microfluidic approach has been developed that enables similar autonomous droplet generation in open chips without the use of external forces (that is, without pumps or pipettes)27. This method (Fig. 1d) generates hundreds of microdroplets autonomously by leveraging competing hydrostatic and capillary pressure using the channel geometry27. With great functionality comes increased complexity: although dynamic open microfluidic systems are often simpler than closed microfluidic systems, they are generally more technically complicated than static systems, as they rely on external forces (such as magnetic fields35,46, styli27 and tweezers27) or pre-patterned microchannels with surface modifications52 to move the droplets individually. Moreover, if the droplets are moved across a surface, there could be residues left behind, leading to sample loss. One way to reduce this sample loss is to use the exclusive liquid repellency principle29,30,33 to optimize the contact angle between the liquid droplets and the surface, or to use a carrier fluid instead of a solid surface27,32.
Advantages and disadvantages
In general, droplet microfluidic systems (including open, closed and digital systems) are excellent for handling small volume samples. Such systems encapsulate samples in small liquid droplets that act as miniature vessels to house the samples within a discrete volume. This approach is useful for isolating the samples and is similar to depositing samples into the wells of a 96-well plate. We note that the use of high-density well plates (such as 1,536-well plates) is becoming more common for high-throughput screening and also offers advantages over small well plates (that is, plates containing 6–96 wells) such as an increased throughput and a reduction in the sample volumes required. However, open microfluidic platforms have some unique advantages over well plates; for example, they have greater flexibility in their design18,25, unique 3D geometries3,17,53, interconnected fluidic networks between compartments containing droplets3,45,53, integrated sample processing without needing a liquid-handling robot or pipettes to move the samples from well to well2,33,54, and they can be accessed through more than one air–liquid or liquid–liquid interface3.
Moreover, open droplet microfluidic systems have many advantages over conventional closed droplet microfluidic systems10,11; for example, they are convenient to access, easy to fabricate, have simple designs and facilitate optimal gas exchange between the contents of the droplets and the external environment.
First, the accessibility of open droplet systems makes it possible to directly and immediately access the contents of each droplet, unlike closed droplet microfluidic systems in which droplets can only be retrieved at a designated opening of the channel. This accessibility is immensely beneficial because researchers can access, extract, insert and analyse the samples at any time in an open system. The open droplet system also allows parallel studies to be conducted easily for experiments such as cell culture that have long incubation times, making it possible to monitor changes in multiple droplets in real time with a simple microscope setup, after which the contents of each droplet can be readily accessed if needed.
Second, there has been a great effort to simplify microfluidic devices and their operation40,55,56. Many open droplet microfluidic systems are simple in design, making them easy to fabricate and assemble. Integrating multiple open microfluidic systems to work in parallel or in series is typically less costly, less complicated and more convenient than integrating multiple closed microfluidic systems, which require additional machines external to the fluidic system. Importantly, open devices can often be fabricated in a single layer, without needing to bond multiple layers together as is required in closed systems, which enables the entire device to be produced by high-volume manufacturing methods such as injection moulding. Injection moulding allows the use of materials usually used for cell culture (such as polystyrene) and materials that are chemically resistant57.
Last, the openness of open droplet systems naturally allows gas exchange (such as the exchange of oxygen and carbon dioxide during cell culture incubation), whereas closed systems rely heavily on the gas permeability properties of the channel material(s) to allow this exchange. Moreover, the use of an open environment reduces the risk of air bubble formation and makes it easy to remove bubbles. Undesired air bubbles are a frequent and severe problem in closed microfluidic systems during long-term perfusion, because they can lead to abrupt changes in flow dynamics or to channel clogging and can cause potential cell damage and cell death53,58.
However, there are also challenges associated with open droplet microfluidic systems, such as managing evaporation, relatively low throughput and the need to carefully consider the material properties of the device (such as the wettability). Although many solutions have been proposed over years of careful development, more innovations are required to fully overcome these challenges. Another obstacle in the field is the lack of a complete theory to describe capillary-driven flows in open systems. Despite current development efforts8,59, there are still many remaining problems; for example, it is difficult to maintain capillary flow for extended periods of time (more than 1 minute).
Owing to their small volumes and the open environment, open droplet microfluidic systems can have problems with evaporation, which causes sample volumes to decrease or even dry out over time. Although this might not be an issue for devices that operate for short periods of time (that is, durations of seconds to minutes), applications such as cell culture that require incubation for days to weeks are more susceptible to evaporation. Fortunately, there are several ways to reduce evaporation, and investigations into further techniques are still under way43,60–62. Common methods include adding ‘sacrificial droplets’ (that is, excess water or buffer droplets) around the periphery of a Petri dish or container that holds the open microfluidic device, using additional secondary containment (that is, placing the primary Petri dish into a larger dish that also contains water or buffer)3,24, using under-oil systems29–31,33,47,49,50,54,63, and using automatic liquid-handling robots to increase the efficiency of reagent addition and to reduce the time that the system is exposed to air49,50,63.
Another limitation is that many open droplet systems have relatively low throughput compared with conventional closed droplet microfluidics. One reason for the lower throughput is that open droplet microfluidic systems do not typically use syringe pumps to provide the high volumetric flow rates that are usual in conventional microfluidics. Instead, open droplet microfluidic systems often use pipettes, tweezers or specialized mechanisms and device geometries to leverage gravity and capillary forces to generate and manipulate droplets. Therefore, it is important for the users to carefully consider the tradeoffs that are involved when choosing between conventional microfluidics and open droplet microfluidics. One possible workaround is to consider a hybrid system that combines both conventional microfluidics and open droplet microfluidics64.
Additionally, the material properties of the device must be carefully considered for open droplet microfluidic systems. Although material selection and properties are also important in closed droplet microfluidics, the choice of material often plays a more crucial part in the success of an open droplet system. Open microfluidics often use capillary forces, which are dependent on interfacial properties such as the contact angle and interfacial tension, both of which are affected by the device material and the carrier phase (in the case of liquid–liquid two-phase systems). Fortunately, methods such as surface functionalization, coat deposition and bonding materials of different wettability can be used to modify the interfacial properties of the device substrate to alter the wettability (that is, to alter the contact angle)29–31,33,65,66, and different carrier phases can be used to achieve the desired wettability28,30,32.
Overall, when used for the right application, open droplet microfluidic systems offer a simple design with many capabilities. Over the past decade, this technology has expanded into numerous areas of chemistry and biology including cell culture, droplet analysis, chemical synthesis and combinatorial reactions. Open droplet systems have also been interfaced with other analytical chemistry methods such as mass spectrometry20,54, electrochemical microscopy67 and DNA analysis68,69. Moreover, the open droplet microfluidics field has attracted physicists, interfacial scientists, fluid mechanicians and mass transport researchers to research its uncharacterized physical phenomena, such as wetting phenomena (for example, the dynamic contact angle and capillary filaments) in different flow regimes, the two-phase pressure drop in open microfluidic two-phase flows, capillary flow in new cross-sectional geometries, and the opportunities afforded by exclusive liquid repellency.
Theory of open droplet microfluidic systems
Here, we provide a brief overview of some of the important physical considerations in open microfluidic systems. We first introduce the basic physical concepts (such as surface tension) in microfluidic droplet systems, then discuss the flow dynamics in open microfluidic channels. Additional discussion on this topic can be found in other books and review articles5,6,8,9,70,71.
Physical concepts
In contrast to closed droplet microfluidic systems, in which droplets are usually formed and moved by forced flows, in open droplet microfluidics the applied pressure is small and surface forces at the droplet interface are the dominant forces that drive the motion of droplets. Surface forces are associated with the concept of surface tension72,73 (Box 1, Fig. 2).
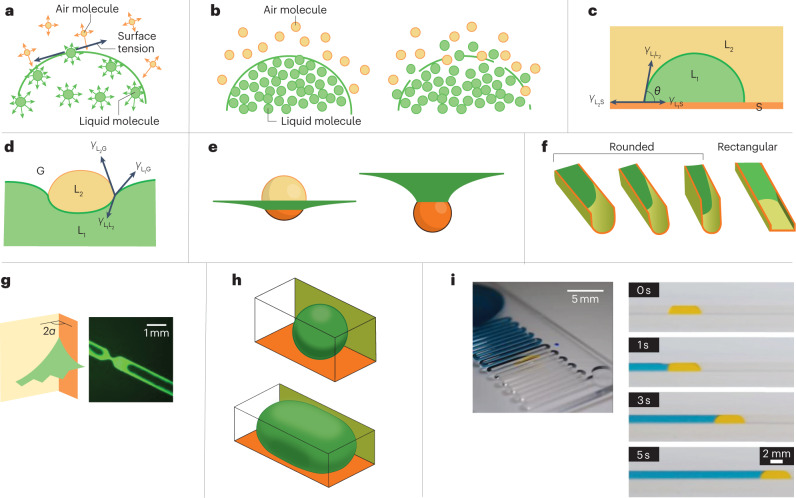
Fig. 2. Forces acting at droplet interfaces.
Key physical considerations and theory that aid the design of open droplet microfluidic systems. a, Asymmetry of molecular interactions at an air–liquid interface results in surface tension. The yellow and green arrows indicate the interactions between air and liquid molecules, respectively. b, Sketches of a sharp (left) or smeared (right) interface, corresponding to high or low surface tension, respectively. c, The Young–Dupré law for a sessile liquid droplet (L1) immersed in an immiscible liquid (L2) on a solid surface (S), where γ is the surface tension and θ is the contact angle. d, The Neumann triangle for the contact of two liquid phases (L1 and L2) and a gas phase (G). e, The dispersed phase (that is, the droplet, shown in yellow and orange) can float on (left) or be immersed in (right) a carrier fluid (green). The different locations of the droplet were calculated using the Surface Evolver software83. f, Different flow behaviours of the carrier fluid (green) in a rounded- or rectangular-bottomed open channel. Capillary filaments can form in rectangular-bottomed channels but not rounded-bottomed ones. The three rounded-bottomed channels show how the aspect (width to height) ratio of the channel affects the meniscus shape of the flow. g, A schematic (left) and photo (right) of the formation of capillary filaments by the carrier fluid in the corners of a rectangular channel, where 2α is the corner interior angle. h, Example of two-phase flow in a channel in which the dispersed liquid phase (that is, droplets and plugs) does not wet the walls75,83, produced using the Surface Evolver software84. i, Photos of a two-phase open channel flow with an aqueous plug (yellow) dispersed in a carrier fluid, 1-nonanol (blue). Liquids were dyed with food colouring for visualization. Part e is adapted with permission from ref. 114, John Wiley & Sons. Part f (left) is adapted with permission from ref. 9, IOP Publishing. Part f (right) is adapted with permission from ref. 5, Wiley-Scrivener. Part g (left) is adapted with permission from ref. 9, IOP Publishing. Part g (right) is adapted from ref. 115 under a Creative Commons licence CC BY 4.0. Part h is adapted with permission from ref. 114, John Wiley & Sons. Part i (left) is reprinted with permission from ref. 9, IOP Publishing. Part i (right) is reprinted with permission from ref. 26, American Chemical Society.
In an open droplet microfluidic system, the droplet may ‘float’ on top of or be immersed in (Fig. 2e) the carrier fluid, unlike closed channels in which the dispersed phase is always completely immersed in the carrier liquid. The relative location of the droplet mostly depends on the different surface tensions at the triple line representing the Neumann triangle (that is, the vector balance of interfacial tensions at the three-phase line)74,75 (Box 1, equation (3), Fig. 2d). For example, if the absolute values of the surface tensions (γ) between the droplet (D), carrier fluid (C) and air (A) fulfill the condition , then Neumann’s triangular equation is not fulfilled, no stable triple line can exist and the droplet sinks into the carrier fluid75.
Box 1 Surface tension forces.
In the bulk of a liquid, gas or solid, molecules have symmetrical interactions with their neighbours; however, this is not the case at interfaces (Fig. 2a). The asymmetry of the molecular interactions at the air–liquid interface induces a force called surface tension. The thickness of an interface is on the nanometre scale and depends on the surface tension (Fig. 2b). When considering microscale objects, the interface can be assumed to be infinitely thin. This assumption is useful for developing mathematical and physical concepts such as the interface curvature, the force on a curved surface and the fundamental principle of the minimization of the surface energy (theory of minimal surfaces)73. Fundamental laws such as the Laplace–Young law are deduced from these concepts73,84. The Laplace–Young law (equation (1)) states that the pressure difference, Δp, across a curved interface is equal to the product of the surface tension, γ, and the surface curvature, κ.
| 1 |
Here, R1 and R2 are the two curvature radii in two planes perpendicular to the surface. For example, in two-phase flows carrying droplets, the pressure in a spherical droplet is larger than that of the surrounding fluid and inversely proportional to the droplet radius. In the case of a sphere, R1 = R2 and the equation reduces to Δp = 2γ/R. By convention, R is positive for a convex meniscus and negative for a concave meniscus.
The Young–Dupré law (equation (2), Fig. 2c) characterizes the contact angle, θ, between two immiscible fluids (liquid or gas phases) and a solid surface.
| 2 |
Here, L1, L2, and S refer to the two immiscible fluids and the solid surface, respectively. This equation is fundamental to a general understanding of capillary forces and it is ubiquitously used for capillary flows. In the case of open two-phase capillary flows, the Young–Dupré law is used to determine whether the discontinuous phase will wet the wall26.
Expanding on the Young–Dupré law, the Neumann triangle (equation (3), Fig. 2d) characterizes the vector balance of three interfacial tensions at the contact of three immiscible fluids74,75.
| 3 |
Here, L1, L2 and L3 refer to the three immiscible fluids, which can represent either gas or liquid phases.
Dynamics of single-phase and two-phase flows
In this section, we refer to the flows of the carrier fluid alone as single-phase flows and the flows in a droplet system, which include a dispersed phase (that is, the droplets) and carrier fluids, as two-phase flows. All droplet systems have at least two phases.
The flow of the carrier fluid is primarily determined by two laws. First, a geometrical flow condition, defined by a generalized Cassie angle (the effective contact angle for a liquid on a chemically heterogeneous surface)76 that must be smaller than π/2 (refs. 5,77). When this condition is met, the fluid will flow through the open channel. Second, the dynamics of the carrier flow is ruled by the Bosanquet equation (an application of Newton’s laws to the motion of a liquid), which states that the carrier flow inertia is the sum of the capillary force, Fcap, and the wall friction, –Fdrag (refs. 78–81):
where m and V are the mass and velocity of the fluid flowing in the channel, respectively.
At the onset of the flow, Fdrag is negligible owing to the small contact area between the liquid and the walls; this period is known as the inertial phase and has a very short duration (~0.1 s). Owing to the high inertial velocity of the fluid, the area of the wall surface that is wetted quickly increases, which causes the magnitude of Fdrag to rapidly increase; therefore, the flow enters the so-called viscous phase in which Fdrag balances Fcap. As a result, the effect of inertia decreases, and the acceleration and velocity of the flow both decrease78–82.
Depending on the wetting properties of the carrier fluid and the geometry of the channel (rounded or rectangular), carrier fluids exhibit different flow behaviours at the inner corners (or edges) of an open channel (Fig. 2f). The shape of the advancing meniscus is linked to the capillary pressure and influences the velocity of the flow. This observation is related to an important law characterizing the contact angle, θ, of the fluid in the channel and the corner half-angle (α, with the corner interior angle being 2α; Fig. 2g) known as the Concus–Finn condition83: when , the liquid phase wets the inner corners of a channel. For rectangular channels, α = π/4 and the Concus–Finn condition can be simplified to θ < π/4. Capillary filaments may form at wetted corners (Fig. 2g) and can affect the dynamics of the carrier fluid83: these filaments often precede the bulk fluid and help to move droplets in the channels. For a horizontal channel, the filament extends endlessly. When , the liquid phase does not wet the inner corners and retracts from the surface. In the case of a two-phase flow, the carrier fluid phase wets the channel walls but the dispersed liquid phase does not; as a result, the dispersed liquid detaches from the inner corners and forms droplets or plugs27,84 (Fig. 2h).
The dispersed phase (that is, the plugs or droplets) can be moved by the carrier flow in an open channel26 (Fig. 2i), allowing many plugs to be moved at the same time. Three regimes have been identified in two-phase open microfluidic flows: shift, when the plug is translated down the channel by the carrier flow; bridge, when the plug stays immobile and the carrier flow slowly flows over the bridge; and raft, when the plug moves downstream, floating on the carrier flow5,9. The flow regime present in the channel depends on the properties of the plug and the intrinsic velocity of the carrier fluid. For example, plugs that are immobilized at the bottom of the channel (bridge mode) might be removed by a high-velocity carrier flow. Moreover, because the velocity of the carrier flow in a simple uniform channel decreases as the travel distance increases, it is important that a sufficient velocity is maintained in open droplet microfluidic systems. Capillary pumping (an ensemble of microfluidic channels with tree-shaped branches) has been developed as an approach aimed at maintaining the velocity of capillary flows for as long as possible59. The capillary pump is driven by the pressure difference between the large negative pressure at the flow front and the zero pressure at the inlet reservoir and can absorb and store large volumes of liquid within the branch system.
The desired mode in an open droplet microfluidic system depends on the targeted application, for example, whether the dispersed phase must remain immobile (bridge) or be moved downstream (raft). In other words, the dispersed phase can be static and be used to transfer some chemicals to the carrier fluid or it can be moved by the carrier fluid to transport chemicals downstream.
As stated at the beginning of this section, the physics of open droplet microfluidics is a rich field, and the discussion above represents a small selection of the theory used to describe a subset of open droplet systems, which we provided to give the reader an introduction to the key concepts.
Droplet manipulation
A key component of open droplet microfluidic systems is droplet manipulation, for example, the generation, transport and merging of droplets. To demonstrate the importance of droplet manipulation, we describe a typical scenario for these systems. In this scenario, there is an open droplet microfluidic system and samples are first encapsulated in the form of a droplet. These droplets act as miniature chambers in which to conduct biological or chemical processes, allowing multiple experiments to be conducted and analysed in parallel with reduced volumes. Next, the droplets are merged with other droplets, which contain a reagent, to observe the outcome. The droplets are then transported to a storage region and undergo incubation. Next, the droplets are sorted according to their measured properties, such as fluorescence, mass or size. Finally, the droplets are split into smaller components to enable the extraction of droplet samples for chemical analysis, for example, mass spectrometry. Below, a selection of droplet manipulation methods are described to provide examples of the available options for performing each of the steps.
Droplet generation
A variety of droplet generation methods are available, providing users with flexibility in how droplets are generated to fit the needs and constraints of the desired application. Droplets are usually made by depositing the droplet using an external source such as from pipettes3,85, acoustophoretic printing25 or microfluidic channels27,64. The volume of the generated droplets ranges from 10 pl to 100 μl.
Conventional methods of generating droplets are typically fast, reliable and user-friendly. The most notable method uses a conventional closed microfluidic channel approach with a T-junction to generate droplets and then deposits them onto an open well plate64. This method generates droplets with high monodispersity (1–3%)86,87 and throughput (~1,000 droplets per second)88. Another example (Fig. 3a) uses an acoustophoretic printer to dispense droplets onto a substrate25. A similar approach uses a custom-built microfluidic printing probe, which creates liquid boundaries with hydrodynamic forces to replace the solid walls of closed microchannels89. There is also a droplet-generation method that sweeps a liquid plug over a substrate patterned with hydrophilic and hydrophobic regions, which causes droplets of volume less than 10 nl to form at the hydrophilic regions90. Compared to pipetting, these approaches substantially reduce the volume of reaction and increase throughput, giving them potential applications in cellular studies, which often require massive and complex biological experiments.
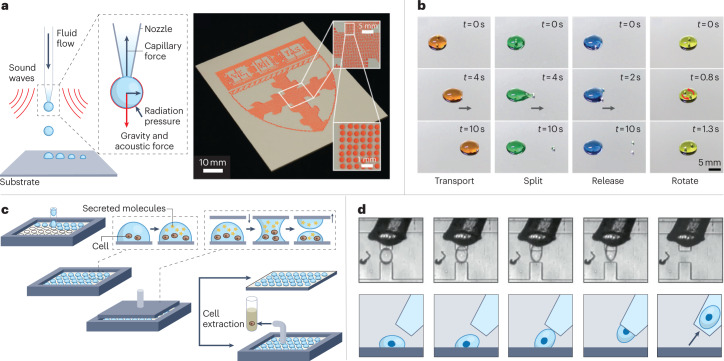
Fig. 3. Examples of droplet manipulation.
We divide droplet manipulation techniques into four categories that are typically used in droplet systems and are important for downstream applications. a, Droplet generation. Left: a schematic of acoustophoretic printing in which droplet generation is controlled by balancing the capillary force, gravity, acoustic force and radiation pressure. Right: a large-area image made from droplets produced by rasterized acoustophoretic printing. b, Droplet transportation and sorting. The use of magnetic beads to transport (orange), split (green), release (blue) and rotate (yellow) 250-μl droplets. The images show the state of the system at different times, t, into the process. The grey arrows indicate the direction that the robots are travelling in. c, Droplet splitting. The schematic for parallel plate droplet splitting. Droplets containing cells are first spotted onto a glass plate and allowed to incubate. Samples of the cell culture supernatants are extracted by applying a separate plate from above to which the droplets adhere, which is then removed to split the droplets while the cells remain in the droplets on the bottom plate owing to the effect of gravity. d, Droplet extraction. Images (top) and schematics (bottom) of the extraction of a single droplet using pipette aspiration. Part a is adapted from ref. 25, AAAS. Part b is reprinted with permission from ref. 35, AAAS. Part c is adapted with permission from ref. 20, American Chemical Society. Part d is adapted with permission from ref. 64, PNAS.
There are also newer methods of generating droplets in open systems that are fundamentally different from those described above, which do not require pre-generated droplets from a separate apparatus or pre-patterned surfaces, thus making the droplet arrangements reconfigurable. One method of generating droplets uses a stylus to split a large volume of fluid into smaller-volume droplets owing to the competing wettability of two immiscible liquids12,14,15. This method starts with a layer of fluorinated oil (FC-40) overlaid on top of a layer of aqueous solution in a Petri dish. A Teflon stylus is then inserted through the two liquid layers to bring the FC-40 into contact with the surface of the polystyrene dish, which is preferentially wetted by oil over water. The lateral movement of the stylus creates oil walls that divide the aqueous layer into small chambers in which individual reactions can occur. The oil barrier prevents contamination between samples and from the environment; the top oil layer also minimizes evaporation of the underlying aqueous phase. Furthermore, the lack of solid walls or boundaries means that the chambers can be created and reconfigured at any point, which is useful for building walls on demand to isolate living cells of interest15. Other work demonstrates a system capable of generating aqueous droplets autonomously with fluorinated oil (HFE-7500) in a carefully designed open channel geometry by using capillary and hydrostatic pressure27. The hydrostatic pressure pushes a large liquid plug into a constriction. This process causes immense fluidic pressure on the liquid plug, disrupting the plug interface, resulting in the formation of droplets (with volumes as low as 500 nl) with monodispersity of 5–7% standard deviation27. The droplets remain mobile in the carrier fluid and can be transported around or out of the system easily. These spontaneous droplet generation methods provide researchers with new ways to perform experiments without the complex external systems (such as electronics, pumps or tubing) used in conventional droplet microfluidics.
Droplet transport and sorting
Droplets are transported sequentially to multiple locations for the purpose of incubation, analysis, storage and sorting. The most straightforward method of transporting droplets is within physical channels or grooves that carry the droplets from one location to another through spontaneous capillary flow26. Other methods involve using a pipette to retrieve the droplets and dispense them to a new location64. Although these methods are relatively easy to perform and do not require special tools (only pipettes), the speed of the movement of the droplets can be hard to control and pipettes can only transport one droplet at a time.
Droplets can also be transported using magnetic forces35,46 (Fig. 3b). Some examples use magnetic beads to pull droplets35 or encapsulate samples within ferrofluid droplets46, which can be guided and pushed with magnetic forces. By using magnetic beads, droplets can not only be transported in air and on flat surfaces, but under many other conditions such as oil-in-water, gas-in-water and on inclined surfaces. Nevertheless, there are some concerns about using magnetic beads; for example, magnetic beads can be costly and could be toxic to cells, limiting the potential applications.
The use of superhydrophobic surfaces, when combined with external forces (for example, gravity91 and magnetism92,93), allows droplets to be transported efficiently owing to the limited friction. A sequence of microdroplets in individual compartments with superhydrophobic coatings can be controllably transported in the same order using gravity with a simple rocking method91. Besides hydrophobic surfaces, a slippery liquid-infused porous surface (SLIPS) has been synthesized to repel organic liquids (such as hexane and methanol); when combined with magnetic actuated organic droplets, SLIPS opens up possibilities for new applications in organic chemical reactions such as liquid–liquid extractions and polymer synthesis92.
Transporting droplets with a PTFE stylus and tweezers using the ‘Cheerios effect’ has also been demonstrated27. The Cheerios effect is the phenomenon whereby multiple objects aggregate owing to the deformation of the interface at the surface of the liquid by the objects94. This approach is a relatively simple way to transport droplets without complex external systems; however, it might require practice for users, and the use of a PTFE stylus and tweezers may increase the risk of cross-contamination between samples. Textured surfaces on substrates can also be used to transport droplets passively by using force balances created by interfacial properties and surface geometries95. For example, droplets in a converging channel will move towards the diverging region instead of the converging region owing to the competing capillary pressures on both sides of the droplets and the droplet wettability. This approach has a low risk of contamination because it does not require the droplet to be in direct contact with a carrying object such as a stylus during transportation.
Droplet merging and splitting
Droplet merging is sometimes required to observe and analyse chemical or biological reactions. Droplets can be merged by using pipettes to fuse droplets with one another12–15,64, pricking the droplet interface with a needle27, adding magnetic beads to the droplet35, and encapsulating samples in ferrofluidic droplets46 to cause the two droplets to collide using magnetic forces. Different merging methods are needed for scientists to develop experiments for specific applications. For example, if an array of droplets needs to be combined in a high-throughput manner, it can be done by bringing two plates (each with droplets adhered at the same relative locations) face to face2,19,20. If the contents of two droplets need to be merged individually and precisely, then a magnet-assisted approach may be preferred35,46.
Droplet splitting is useful when a sample of the droplets is required for temporal analysis. Parts of the droplets can be extracted for analysis while the remaining portion of the droplets continue to undergo time evolution for later extraction and analysis. Droplets can be split by distorting the surface energy balance of the droplets using a stylus12–15, by applying pressure differences using a combination of magnetic forces and physical confinement96 or by adhering droplets onto two glass plates and then separating the plates to split the droplets20 (Fig. 3c). When the latter example was tested, samples in the separated droplets were interfaced with mass spectrometry. Furthermore, the cells in the split droplets were viable after the plate separation and could be transferred to a cultivation tube. An example of high-throughput droplet splitting was demonstrated by shearing a large droplet along the crenulation feature (that is, a series of fine notches) on the reservoir wall; the smaller daughter droplets were subsequently extracted using tweezers or a stylus27.
Droplet extraction
Droplet extraction involves extracting the whole or part of a droplet out of a system to be stored and analysed. Droplet extraction, although similar to droplet transport, is distinct because the droplets are transported out of the device. Extraction is often needed to analyse the droplet contents with external equipment. Common extraction methods involve retrieving the droplets with a pipette64 (Fig. 3d) or with a stylus or tweezers27 as described above. Furthermore, a superhydrophobic claw structure, made from a PDMS film doped with magnetic particles (carbonyl iron powder), was able to pick up droplets in a short time (response time <100 ms) without a loss of liquid97.
Applications
Continuous innovation in droplet-manipulation techniques has enabled considerable progress in applying open droplet microfluidic systems to biological and chemical research. In this section, we highlight some applications of open droplet microfluidics, including combinatorial reactions, 3D cell culture and chemical and cell analysis in droplets. We have selected work over a broad range of open droplet microfluidic systems; additional applications can be found in other reviews16,98,99. These applications demonstrate miniaturized devices that are inexpensive yet effective, and show great potential in multiple fields, such as chemistry, biochemistry, medicine, pharmaceutical science and point-of-care systems.
Combinatorial reactions and high-throughput screening
Drug development and chemical synthesis in chemistry, medicine and pharmaceutical science rely on transporting and mixing chemicals and the high-throughput screening of large compound libraries. Closed microfluidic systems have been used in the past for this application100–102; however, they require sophisticated liquid-handling techniques with complicated tubing and pumping systems, which makes it practically difficult to use such devices at a large scale. Therefore, there is a need for simple, inexpensive and efficient miniaturized methodologies for manipulating fluids for chemical synthesis and integrated biological screening. Using open droplet microfluidic systems it is possible to independently and selectively transport and manipulate droplets. Each droplet can be moved to mix or merge with other droplets in later stages of the chemical reaction, synthesis and screening27. One demonstration of precise droplet manipulation for stepwise chemical reactions uses magnetic-actuated robots35. Here, two steel beads (that is, the ‘robot’) encapsulated in liquid droplets are magnetically controlled. The adjustable structure of the robot (that is, the relative distance between the two beads) enables various droplet manipulations to be executed precisely, which is useful for controlling chemical reactions. The robot was used to split a daughter droplet (1.5 µl) from a NaOH droplet (250 µl) and transport it to an indicator (phenolphthalein) droplet (250 µl); the phenolphthalein droplet turned pink, indicating a basic environment (Fig. 4a). Subsequently, the robot entered a HCl droplet (250 µl) and brought a daughter droplet (1.5 µl) back to the indicator; the pink droplet returned to being colourless, confirming that a complete acid–base neutralization had occurred.
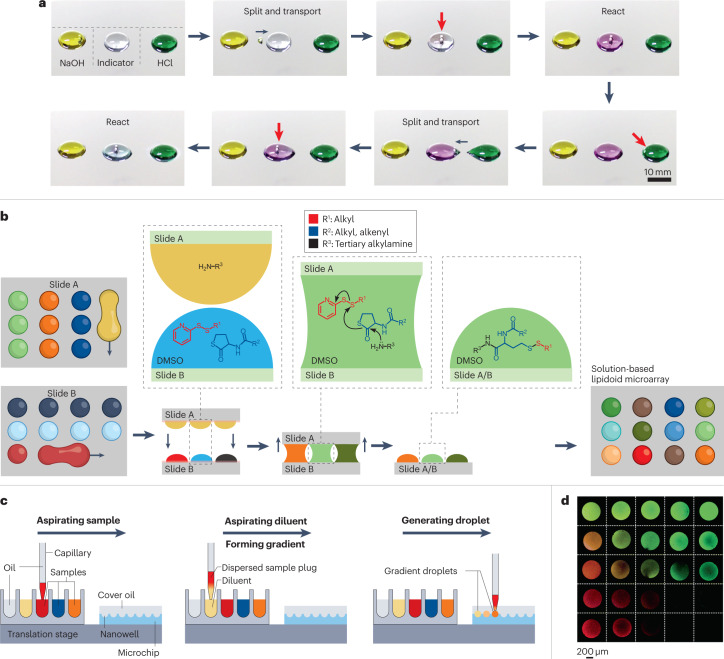
Fig. 4. Open droplet microfluidics for synthesis and combinatorial reactions.
An illustration of common workflows in open droplet microfluidic systems that minimize reagent consumption and enable parallel reactions. a, Magnetically actuated robots (that is, two steel beads), indicated by the red arrows, controlled by a magnetic control system, are programmed to manipulate droplets precisely for use in a sequential acid–base neutralization reaction. The robot is used to transport a daughter droplet from the NaOH droplet to the indicator droplet, which changes from colourless to purple, indicating that it is now alkaline. The robot then transports a daughter droplet from the HCl droplet to the indicator droplet, which returns to colourless as it is neutralized. b, An on-chip solution-based platform for compound synthesis and cellular screening (chemBIOS). A library of lipidoids is generated on-chip by sandwiching together slide A, which contains droplets of amines, and slide B, which contains mixtures of thiolactone and pyridyl disulfide derivatives dissolved in dimethyl sulfoxide (DMSO). Sandwiching the slides initiates the reaction between the reagents (shown in the expansions) to produce a microarray of different lipidoids. c, An automated microfluidic nanolitre-droplet gradient system for high-throughput screening. The three steps are aspirating the sample, aspirating the diluent to form the gradient and generating the droplet. This process leads to a sequence of droplets with a concentration gradient. d, A gradient droplet array for high-throughput screening of cellular responses to varying combinations of chemical and mechanical cues visualized with fluorescent dyes. Part a is reprinted with permission from ref. 35, AAAS. Part b is adapted from ref. 2 under a Creative Commons licence CC BY 4.0. Part c is adapted with permission from ref. 49, American Chemical Society. Part d is reprinted with permission from ref. 19, Royal Society of Chemistry.
High-throughput screening has also benefited from miniaturization using open droplet microfluidics. For example, a microassay platform (chemBIOS) compatible with solution-based organic synthesis and biological screening has been developed2. This platform comprises two patterned slides with hydrophilic spots separated by hydrophobic borders (or omniphilic spots separated by omniphobic borders), each containing an array of aqueous (or organic) droplets; these slides are then sandwiched together to initiate droplet mixing. As chemBIOS is compatible with both organic solvents and aqueous solutions, it can be used to combine the synthesis of organic compound libraries with subsequent cellular screening on the same platform, presenting a unique solution for high-throughput drug discovery2 (Fig. 4b). This work highlights the simplicity of open droplet microfluidic systems and the precise control of droplet manipulation that they offer for combinatorial reactions.
Quantitative high-throughput screening has also been demonstrated in open microfluidic systems. Using an automated microfluidic droplet handling system, picolitre-to-nanolitre-volume droplets were created on a nanowell-array chip using a unilateral dispersion-based dilution approach49 (Fig. 4c). This approach was used to load a nanowell-array chip containing 3,025 nanowells with 4-nl droplets. Compared with the traditional bilateral dispersion approach, in which an aqueous sample plug is injected into a continuous flow of diluent solution, which results in concentration gradients on both ends, the unilateral approach loads plugs of immiscible oil, sample and diluent sequentially and therefore limits the sample dispersion to only one side. This unilateral method enables a series of droplets with a concentration gradient to be deposited in a rapid and efficient manner. This technique was used in a matrix metallopeptidase 9 (MMP9) enzyme inhibitor screen comprising a library of 102 compounds and 24 concentrations and reduced the volume of reagents consumed by approximately 1,600-fold compared with a standard assay platform such as a multiwell plate.
Droplet gradients have also been created on a microgel chip19. In this method, stock droplets (containing no compounds) are first spotted onto a hydrophilic-patterned region on the microgel chip array; a reagent-containing droplet is then loaded into one stock droplet. Gradients are then rapidly formed by rolling, manually dragging an implement or moving embedded magnetic beads using a magnet19 (Fig. 4d). This technique offers an alternative approach for high-throughput screening, drug development, tissue engineering and stem cell screening and is a powerful illustration of how open droplet microfluidics can be used. This technique was used in a cell screening assay to show that the behaviour of Wnt/β-catenin signalling in RKO cells within droplets differed according to the concentration of the WNT3A protein. Moreover, activation of the signalling pathway could be detected at WNT3A concentrations of 13 ng ml–1, which was five times smaller than the detection limit based on an imaging readout19. These open droplet microfluidic systems have great potential for a low-cost, automated and high-throughput solution for chemical synthesis, drug combination screening and ultimately personalized medicine.
Three-dimensional cell culture
Cell culture models are used in many biological applications including fundamental biology studies, drug screening assays and tissue engineering. A wealth of information can be gleaned from 2D cell cultures performed on flat, cell-compatible materials including hard plastics or glass substrates, but these methods are limited in their ability to recapitulate the 3D nature of organs and biological tissue103–105. For example, liver cells lose key functional characteristics after a few days in 2D culture53. The need for cellular models that better mimic the functions and physiology of living organs and tissue has driven researchers to move from 2D to 3D cell culture platforms103,106.
Microfluidic platforms — both open and closed — are promising for use in cell culture applications because they offer precise control over biochemical gradients, biologically relevant dimensions in fabricated devices and the spatial control of cell placement107. Features such as the integration of fluid flow for perfusion can also be incorporated into microfluidic devices to enable studies that require the circulation of immune and blood cells105. Further, devices can be fabricated in optically transparent materials (such as glass, 3D-printed plastics and PDMS), making it easy to image the sample in the channel108. Droplet microfluidics is particularly advantageous for cell culture studies because cells can be isolated in separate droplets, which effectively creates individual microenvironments. Additionally, multiple conditions or treatments can be applied to the droplets to allow efficient cell-based assays or basic science studies for investigating various biological processes. Open droplet microfluidic systems offer even more flexibility than closed droplet systems because droplets are accessible at any point; for example, nutrients or treatments can be applied directly to selected droplets, which is not possible in closed droplet systems.
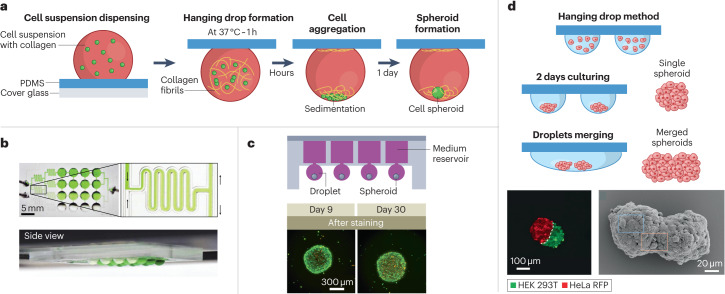
A popular open droplet microfluidic method used to create spheroids for performing 3D cell culture is the hanging drop technique103. This technique involves pipetting small volumes of a cell suspension onto a substrate such as a Petri dish, adding a cover, and then flipping the system over so that the drops are hanging or using a specialized, bottomless well to form hanging drops using liquid-handling systems21. This approach allows cells to aggregate naturally at the bottom of a spheroid under gravity. Traditionally, the hanging drop technique uses a liquid medium on a hard substrate like polystyrene103; however, the use of biocompatible materials such as PDMS has also been explored23 (Fig. 5a). The hydrophobic nature of PDMS results in the formation of droplets that are more spherical than the droplets produced on other substrates such as polystyrene. Further, the spherical shape of droplets formed on PDMS allows small volumes (1 μl) to be used while maintaining the spherical shape, which makes it possible to study rare or limited cells23. Another advance to the hanging drop technique includes work that incorporates fluidically connected open channels to create an integrated droplet network53 (Fig. 5b). The use of open channel microfluidics enables spheroids in separate droplets to be fluidically connected, creating a dynamic, integrated network for continuous cell communication, something that is not possible in traditional Petri dish set-ups.
Fig. 5. Three-dimensional cell culture and spheroid formation in open droplet systems.
Spheroids are common biomimetic culture models and open microfluidic systems enable more sophisticated fluidic manipulation of hanging drop networks. a, Liquid droplets containing cells created on a polydimethylsiloxane (PDMS) substrate. The hydrophobic nature of PDMS produces rounded droplets, and the addition of extracellular matrix components such as collagen creates an environment that is similar to those experienced by cells in the body, which encourages spheroid formation. b, A fluidically connected hanging drop network used for parallel spheroid formation. A top-down view with a close-up image of the open channels (top) and a side view (bottom). c, An integrated medium reservoir enables long-term cultures (up to 30 days) in 3D open droplet systems. Top: a cross-sectional view of the device. Bottom: fluorescence images of spheroids formed in this platform show that the cells remain viable and maintain their shape throughout a 30-day culture period; cells were stained with calcein AM and propidium iodide to indicate living (green) and dead (red) cells, respectively. d, Top: droplet merging results in multi-spheroid architectures. Bottom left: fluorescence microscopy images of spheroids containing two different cell types 24 h after merging — HEK 293T stained with a green fluorescent dye and HeLa expressing red fluorescent protein (RFP). Bottom right: scanning electron microscopy image of a multi-spheroid architecture containing HepG2 cells 24 h after merging. Part a is adapted from ref. 23 under a Creative Commons licence CC BY 4.0. Part b is reprinted from ref. 53, Springer Nature Limited. Part c is adapted with permission from ref. 22, Elsevier. Part d is adapted with permission from ref. 17, Wiley.
One persisting challenge for 3D cell culture in open droplet systems is culturing for long periods of time (>7–10 days), owing to the challenge of liquid loss by evaporation. Advances such as the addition of liquid reservoirs that continuously feed the hanging drop culture from above have extended the culture periods that can be achieved with open systems to 30 days (ref. 22) (Fig. 5c). This method allows spheroids to remain viable while maintaining their shape throughout the culture period. A different approach creates droplets in suspended wells that are open to air on two sides in a layer to form a ‘stacks’ system3 (Fig. 7c). A layer of suspended media droplets can be made to replace the old media layer to ensure that the cultures have access to fresh nutrients throughout the culture period. These innovations in open microfluidic devices open the door to longer investigations in cellular models of cancer biology, tumour progression and drug discovery.
Last, the ability to selectively merge spheroid-containing droplets is needed to create heterogenous, multi-spheroid structures for studying complex biological processes such as cancer invasion. Open microfluidic platforms are well positioned to meet this requirement as researchers can easily and selectively access droplets containing cells for manipulations. One such demonstration added a tissue sample to a droplet cell culture using forceps for dynamic co-culture experiments85. Additionally, another promising approach allows spheroids containing different cell types (such as immortalized human embryonic kidney (HEK293T) and cervical cancer (HeLa) cell lines) to form independently in separate droplets before being merged into multi-spheroid architectures, providing flexibility and control for studies in many fields including cell biology and drug discovery17 (Fig. 5d).
Droplet analysis
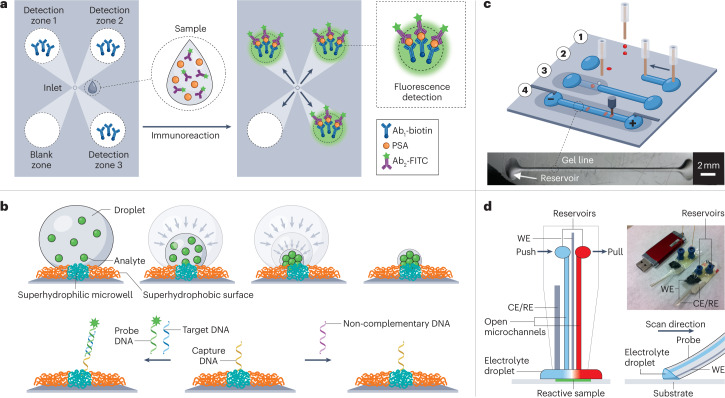
Tools or methods for performing droplet analysis based on open microfluidic technologies are becoming increasingly prevalent. The implementation of analysis can be simplified by transporting droplets using only passive forces, because no external power is needed to drive the droplet through the system4. For example, researchers have developed ways to transport droplets directionally using nanomaterials with bioinspired wettable–nonwettable (that is, superhydrophilic and superhydrophobic) micropatterned surfaces109. In one demonstration, superwettable microspine chips were used to detect prostate-specific antigen at various concentrations from serum52 (Fig. 6a). After an aqueous droplet was added to the chip inlet (a narrower and hydrophobic end of the microchannel), it was automatically transported along the open geometric-gradient microchannel to the detection zone (the wider and hydrophilic end of the channel), driven by the Laplace pressure gradient and hydrophilicity. Moreover, a sample droplet can be simultaneously analysed in the multiple detection zones of a multichannel chip, enabling rapid on-chip repeat measurements. Testing this technique with other sample types such as saliva could enable the use of the device for point-of-care applications110.
Fig. 6. Droplet analysis in open systems.
a, A multimicrochannel superwettable microspine chip for detecting prostate-specific antigen (PSA). The chip has detection zones that are pre-functionalized with biotinylated PSA antibodies (Ab1-biotin). The droplet containing target molecules and fluorescein isothiocyanate-labelled PSA antibodies (Ab2-FITC) is spontaneously transported to the detection zones to form a sandwich immunoassay for the fluorescent detection of PSA. b, The evaporation enrichment of analytes on superhydrophilic microwells with superhydrophobic substrates. Top: analytes are concentrated after liquid evaporation. Bottom: the application of the system as a biosensor for ultra-trace DNA samples. The superhydrophilic microwell is functionalized with capture DNA (middle), which only allows specific target DNA to be captured, concentrated and detected by the fluorescent probe DNA (left), but does not recognize non-complementary DNA (right). c, Illustration of a workflow for open microfluidic gel electrophoresis. The nanolitre DNA sample droplet was first spotted and dehydrated on a planar substrate (1). The droplet was then rehydrated with a liquid gel line of sieving gel matrix (2) and covered with mineral oil (3). Finally, an electric field and a detector were used to perform electrophoresis (4). Bottom: a photo of the system under oil without electrodes. d, Schematic of the push–pull probe design with two open microfluidic channels for scanning electrochemical microscopy imaging including the working electrode (WE) and the counter and reference electrodes (CE/RE). The insets show the two probes and a USB drive for size comparison (top) and a schematic of the push–pull probe for imaging (bottom). Part a is adapted with permission from ref. 52, American Chemical Society. Part b is adapted with permission from ref. 68, Wiley. Part c is adapted with permission from ref. 69, Wiley. Part d is adapted with permission from ref. 67, American Chemical Society.
There have also been advances in techniques to perform ion concentration analysis with open microfluidic devices that use only passive forces to transport the droplets. The conventional methods of analysing ions require specialized equipment (such as inductively coupled plasma mass spectrometry) and training, are expensive and can be time-consuming111. A paper-based system, which integrated a dynamic droplet system with a G-quadruplex-based luminescence switch-on assay, has been used to rapidly detect lead(II) ions in liquid solutions1. The size and portable nature of the device makes it an attractive option for point-of-care testing of drinking water or filtered river water. However, certain kinds of sample, such as seawater or sewage, must be pre-treated, as is required with most ion detection methods, which limits the potential applications of the device.
Although evaporation can be a problem in some open microfluidic systems112, it can be taken advantage of and incorporated into the design of advanced devices for some applications. A condensing-enriched strategy was applied using a superhydrophobic substrate to improve the detection sensitivity of nucleic acid, which is vital for biomedical applications68 (Fig. 6b). After the liquid droplet evaporates, a concentrated sample remains in the dried spot, thereby markedly lowering the detection limit of DNA in biological samples. The detection limit of the enriched DNA is about 10–16 M, which is four orders of magnitude lower than traditional fluorescence DNA chips. This enrichment can be used to overcome the limitations of traditional DNA microarrays for analysing ultratrace DNA samples.
One of the advantages of using droplets in open microfluidic platforms is the minimization of the footprint and amount of equipment required to perform the analysis. For example, gel electrophoresis typically requires a large number of gels and a large amount of space. These requirements have been reduced by creating an open platform that allows gel electrophoresis to be conducted using a remarkably low volume of the sample (nanolitre scale) and other materials (such as gel matrix, oil and polymer foil), without sacrificing sensitivity or resolution in the separation of a standard label-free DNA ladder69 (Fig. 6c). This multistep DNA separation and analysis technique involves spotting samples, covering and rehydrating the samples with a sieving gel matrix, and then covering the matrix with mineral oil to prevent evaporation, before applying a voltage to perform electrophoresis. This approach could also potentially be used in protein and RNA analysis.
In addition to the ability to analyse droplets within the open microfluidic platform, the miniaturized devices themselves can be used as the analytical instrument, greatly reducing the size of the equipment needed and simplifying the workflow. For example, an open microfluidic device that doubled as a USB-size probe for scanning electrochemical microscopy (SECM) has been developed67 (Fig. 6d). This configuration is more flexible than conventional SECM instruments because measurements can be carried out on dry surfaces without the need to immerse samples in an electrolyte solution and the soft material of the probe also allows measurements to be performed on tilted or even vertical substrates. The push–pull probe contains a working microelectrode, an integrated counter and reference electrode, and two open microchannels for the delivery and aspiration of the redox mediator solution at the probe tip, where a nanolitre droplet is stationed and renewed. The superhydrophilic surface can immobilize different antibodies depending on the purpose of the experiment; SECM can also be coupled with multiple analytical instruments, including mass spectrometry, high-performance liquid chromatography or capillary electrophoresis to add additional layers of information.
Cell analysis
Cell analysis is an important aspect of cell culture, enabling researchers to measure cell health, function and death as experimental outcomes. Despite concerns about evaporation and detection limits owing to the small volumes, a wide range of cell analysis techniques can be used with open droplet microfluidic systems. Researchers have found ways to control evaporation in long-term cultures and to detect molecules using sensitive analysis methods or by pooling multiple microcultures. As the sensitivity of instruments and specificity of reagents continue to increase, more of the analytical methods used in macroscale cell culture (for example, in a Petri dish or well plate) can be readily applied — with little or no modification — to microscale experiments. Advances in dynamic open systems could also make it possible to apply droplet microfluidic technologies developed for closed systems (such as flow cytometry and single-cell RNA sequencing) to open systems. Currently, frequently used cell analysis methods in open droplet microfluidic systems include microscopy2,19,24,29,31,47,53, enzyme assays29,49,53, gene expression analysis3,33,50,63 and protein expression analysis3,24,30,47,54.
Many cellular techniques rely on fluorescence microscopy for detection. Because most open droplet microfluidic systems are simple and stand-alone, they can be placed inside standard labware (such as a Petri dish) for imaging without needing specialized holders. For example, fluorescence images of a solution-based on-chip microarray platform were used for reverse cell transfection screening of a library of lipoplexes containing plasmid DNA. These images were taken by placing the cell-seeded slide in a Petri dish, where the lid was prepared with phosphate buffered saline (PBS)-soaked sterile tissue to prevent evaporation2. The efficiency of cell transfection was evaluated using the ratio of the number of green (transfected with green fluorescent protein) cells to the total number of cells. Using this approach, the authors identified the lipidoid that was the most efficient for cell transfection out of the synthesized library of 25 lipoplexes, with a transfection efficiency of 52 ± 4%.
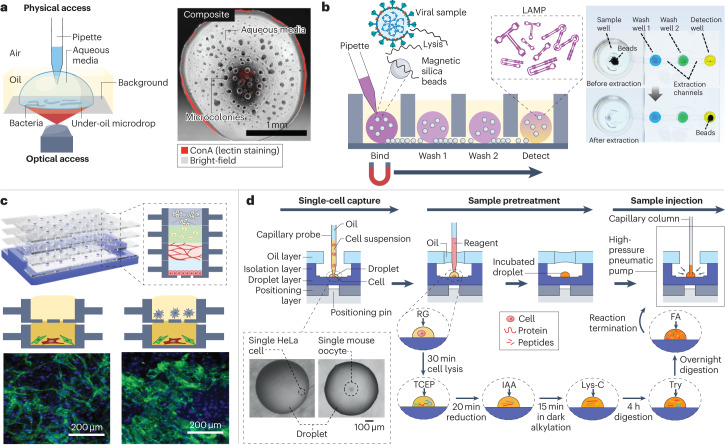
To monitor the behaviour of live cells over time, time-lapsed images (brightfield, phase-contrast or fluorescence) can be recorded throughout an experiment31,47,53. In one study, an under-oil open microfluidic system was used to study the motility of microcolonies self-assembled from the gliding bacteria Flavobacterium johnsoniae31 (Fig. 7a). Wild-type or mutated strains of bacterial cells were encapsulated in under-oil sessile microdrops on a glass substrate. The open system enabled physical access from above with a pipette and optical access from below with a microscope. The pipette accessibility made it easy to add reagents (such as fluorescent lectin dyes for staining the extracellular polysaccharide matrix in the F. johnsoniae microcolonies). The microscope accessibility made it possible to monitor the structure and growth dynamics of the microcolonies using a multi-photon and bright-field microscope, respectively31.
Fig. 7. Cell analysis in open droplet systems.
Open droplet microfluidic platforms enable more flexibility and control in cell culture experiments, and many of the platforms are compatible with conventional in vitro experimental readouts. a, Left: a schematic of an under-oil open microfluidic system used to study the growth dynamics and microcolony motility of wild-type Flavobacterium johnsoniae with physical and optical access. Right: a composite bright-field and fluorescent Concanavalin A (ConA) lectin-stained image of a microdrop obtained with this system 12 h after the culture growth was initiated. b, Left: a schematic of the oil-immersed lossless total analysis system (OIL-TAS) used to perform integrated RNA extraction and detection of SARS-CoV-2. The detection step uses loop-mediated isothermal amplification (LAMP). Right: images of a unit of the OIL-TAS device before and after extraction in which the wells were coloured for visualization. c, Top: a reconfigurable multilayer suspended cell-culture system (‘stacks’) for studying spatiotemporal paracrine signalling across different culture microenvironments. Bottom: immunocytochemistry images show the difference in morphology of the endothelial structures (green) in the endothelial–fibroblast mixture layer when macrophages are absent (left) and present (right) in the top layer. d, Single-cell proteomic analysis using a nanolitre-scale oil–air–droplet chip with a self-aligning monolithic device. The pretreatment of the sample involves the addition of RapiGest (RG), tris(2-carboxyethyl)-phosphine (TCEP), iodoacetamide (IAA), endoproteinase Lys-C, trypsin (Try) and formic acid (FA) to extract proteins from a single cell and digest them into peptides. The sample is then injected into a capillary liquid chromatography column for subsequent liquid chromatography tandem mass spectrometry analysis. The inset shows microscopy images of droplets containing a single cell. Part a is adapted from ref. 31 under a Creative Commons licence CC BY 4.0. Part b is adapted from ref. 33 under a Creative Commons licence CC BY 4.0. Part c is reprinted from ref. 3, Springer Nature Limited. Part d is adapted from ref. 54, American Chemical Society.
Another type of readout that can be achieved in open droplet cell culture systems is enzyme assays. Cellular adenosine triphosphate (ATP) content is a hallmark for cell viability and is usually measured using a luminescence-based enzyme assay29,53. Enzyme kinetics can also be studied in open droplet systems using a readout of fluorescence intensities49. For example, live cell imaging and an ATP assay were employed to quantify the effect of pro-drug (cyclophosphamide) bioactivation on the inhibition of tumour spheroid growth in a hanging drop network53. To measure the ATP content, spheroids were harvested individually from the bottom of the drop using a pipette or simultaneously brought into contact with the wells of a 384-well plate. The open system’s pipette accessibility provides the flexibility to retrieve any droplet of interest at any time; its compatibility with standard well plates allows the system to be readily used in existing downstream instrumental analysis at high throughput53.
Gene expression measurement enables the detection of early changes in cellular functions. Polymerase chain reaction (PCR) is a molecular biology technique that is widely used to amplify nucleic acids for DNA and mRNA measurements; both quantitative PCR3,33,50 and digital PCR63 have been used, directly or with modifications, in microscale open droplet systems. However, the stringent temperature requirements and sample processing steps for PCR assays make PCR difficult to use in point-of-care applications. Alternatively, loop-mediated isothermal amplification (LAMP)-based assays often have more lenient hardware and sample preparation requirements, and are therefore simpler and more cost-effective than PCR assays for use in diagnostic open microfluidic devices113. Recently, a LAMP assay was integrated with an oil-immersed lossless total analysis system for the detection of SARS-CoV-2 (ref. 33) (Fig. 7b). The system combined the advantages of exclusive liquid repellency, exclusion-based sample preparation and LAMP with a colorimetric readout. After pipetting aqueous droplets containing the sample, lysis buffer and magnetic beads into oil-filled wells, RNA extraction and detection can be performed on the same device using a magnetic extractor33.
Proteins are the ultimate product of gene expression and play a vital part in all aspects of biology. Although secretory protein levels can be measured by immunoassays, such as enzyme-linked immunosorbent assays or Luminex multiplex immunoassays24, detecting protein expression levels inside cells (such as the subcellular localization) often requires immunocytochemistry staining3,24,30,47. Cell–cell signalling has been investigated in a reconfigurable stacks system using proof-of-concept angiogenesis models with immunocytochemistry readouts3 (Fig. 7c). Each layer was first prepared individually and then vertically assembled into stacks sequentially using a holder. The stacked microenvironments were fluidically connected by open and suspended droplets and channels; three types of open microfluidic channel were integrated into each layer to enable horizontal (within a layer) and vertical (across layers) flow, allowing different interactions with the droplet in the culture well. A cover glass was fixed to the bottom of the suspended device before imaging under a standard inverted fluorescence microscope. The study revealed that a mixed layer of endothelial cells and fibroblasts formed a substantially different network of vessel-like structures (stained with an endothelial marker, CD31) when a layer of macrophages was added to or removed from the stacked multi-culture system3.
Proteomics is the quantitative study of all proteins, often based on liquid chromatography tandem mass spectrometry. A nanolitre-scale oil–air–droplet system was developed for single cell proteomic analysis54 (Fig. 7d), in which a layer of oil was pinned above the aqueous droplet with some air in between. The semi-open design minimizes evaporation and allows access into the droplet for reagent addition and sample recovery. With the help of a self-aligning monolithic device, the multistep sample pretreatment was completed within a single cell droplet, which was then injected into a capillary liquid chromatography column for liquid chromatography tandem mass spectrometry proteomic analysis. The performance of the oil–air–droplet chip-based system was tested using low-input samples (such as 100 HeLa cells or a single mouse oocyte), and the system demonstrated key advantages over traditional tube-based systems including minimal sample loss, high sequence coverage, enriched hydrophobic proteins and efficient enzymatic digestion54.
Outlook
Overall, open droplet microfluidic systems with their open accessibility, minimal setup and simple design offer tremendous potential when used for appropriate applications. The systems can use many droplet-manipulation techniques and have shown promising results in applications ranging from chemical reactions to cell culture and analysis.
Looking ahead, we envision future open droplet microfluidic systems that can conduct complicated tasks while maintaining a simple design and small footprint. Solving the challenges associated with these systems, such as under-characterized wetting phenomena, the risk of evaporation and low throughput, will encourage further developments in the field of open droplet microfluidics and reveal new applications. Testing new material and liquid combinations and studying the underlying physical principles will enable the flow rate and throughput of these systems to be increased. Several approaches have been used to mitigate evaporation, but best practice remains to be characterized and standardized. The integration of parallelization and automation, which should increase the throughput of open microfluidic systems and reduce the risk of evaporation by minimizing the exposure time, has yet to be fully explored. With advances in the sensitivity and specificity of instruments and assays available, more of the analysis techniques used in macroscale systems could be applied directly to microfluidic droplets. In the next decade, we expect to see the field of open droplet microfluidics not only expand in breadth — with novel system design and development — but also mature in depth — to improve and implement existing tools to study complex chemical and biological problems.
Acknowledgements
The authors thank the following funding sources: the National Institutes of Health (R35GM128648 to A.B.T.), Howard Hughes Medical Institute Gilliam Fellowship (to T.L.v.N.), and the American Society of Nephrology KidneyCure Predoctoral Fellowship (to Y.Z.).
Glossary
- Acoustophoretic droplet printing
A droplet generation and patterning method that uses a well-controlled, highly localized acoustic pressure to eject monodisperse droplets of materials in air with a wide range of compositions and properties.
- Capillary flows
The spontaneous movements of liquids in narrow spaces based on capillary action.
- Droplet microarrays
A microfluidic platform based on chemically patterned liquid-repellent boundaries (for example, hydrophilic–superhydrophobic patterning) to generate microlitre-to-nanolitre-sized droplets in a 2D array.
- Exclusive liquid repellency
A regime of liquid repellency in multi-liquid-phase systems in which the dispersed liquid phase is completely repelled by the solid surface.
- Ferrofluid
A colloidal suspension of ferromagnetic nanoparticles that is often controlled by electromagnetic actuations. It can consist of maghemite (Fe2O3, γ-Fe2O3), magnetite (FeO·Fe2O3), or ferrite accompanied by metals such as Ni, Co, Mg and Zn.
- Freestyle fluidics
A microfluidic approach in which liquids are drawn on flat unpatterned substrates and confined by immiscible fluid walls and ceilings.
- Hanging drops
A spheroid formation method in which a drop of liquid containing cells or microorganisms is suspended from an inverted surface.
- Open microfluidics
Also termed open-space microfluidics, these are microfluidic systems that use channels or microstructures with at least one dimension on the submillimetre scale and with one side or face exposed to the outside world.
- Spheroid
A spherical cell aggregate that is self-assembled during three-dimensional cell culture.
- Traditional microfluidics
Microfluidic systems that use closed channels with a designated inlet and outlet, usually driven by various pumps, tubing, junctions and control architecture.
Author contributions
Y.Z., J.W.K., T.L.v.N., W-c.T., J.B. and S.T. researched data for the article. All authors contributed substantially to the discussion of content. Y.Z., J.W.K., T.L.v.N., W-c.T., J.B., S.T., E.B. and A.B.T. wrote sections of the manuscript. E.B. and A.B.T. edited the manuscript. Y.Z., T.L.v.N., W-c.T., J.B. and A.B.T. revised the manuscript during the review process. Y.Z. and J.W.K. contributed equally to this article.
Peer review
Peer review information
Nature Reviews Chemistry thanks Elisabeth Verpoorte, Darius Rackus and the other, anonymous, reviewer for their contribution to the peer review of this work.
Competing interests
A.B.T. has ownership in Stacks to the Future. S.T. and E.B. have ownership in Stacks to the Future, Tasso and Salus Discovery. Y.Z., J.W.K., T.L.v.N., W-c.T. and J.B. have no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuting Zeng, Jian Wei Khor.
Contributor Information
Erwin Berthier, Email: erwin.berthier@gmail.com.
Ashleigh B. Theberge, Email: abt1@uw.edu
References
- 1.Sun H, et al. A suspending-droplet mode paper-based microfluidic platform for low-cost, rapid, and convenient detection of lead(II) ions in liquid solution. Biosens. Bioelectron. 2018;99:361–367. doi: 10.1016/j.bios.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 2.Benz M, Molla MR, Böser A, Rosenfeld A, Levkin PA. Marrying chemistry with biology by combining on-chip solution-based combinatorial synthesis and cellular screening. Nat. Commun. 2019;10:2879. doi: 10.1038/s41467-019-10685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, et al. Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling. Nat. Biomed. Eng. 2019;3:830–841. doi: 10.1038/s41551-019-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaigala GV, Lovchik RD, Delamarche E. Microfluidics in the ‘open space’ for performing localized chemistry on biological interfaces. Angew. Chem. Int. Edn. 2012;51:11224–11240. doi: 10.1002/anie.201201798. [DOI] [PubMed] [Google Scholar]
- 5.Berthier, J., Brakke, K. A. & Berthier, E. Open Microfluidics (Wiley-Scrivener, 2016).
- 6.Delamarche, E. & Kaigala, G. V. Open-Space Microfluidics: Concepts, Implementations, Applications (Wiley-VCH, 2018).
- 7.Oliveira NM, Vilabril S, Oliveira MB, Reis RL, Mano JF. Recent advances on open fluidic systems for biomedical applications: a review. Mater. Sci. Eng. C. 2019;97:851–863. doi: 10.1016/j.msec.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Berthier E, Dostie AM, Lee UN, Berthier J, Theberge AB. Open microfluidic capillary systems. Anal. Chem. 2019;91:8739–8750. doi: 10.1021/acs.analchem.9b01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthier, J., Theberge, A. B. & Berthier, E. Open-Channel Microfluidics: Fundamentals And Applications (IOP Publishing, 2019).
- 10.Ding Y, Howes PD, deMello AJ. Recent advances in droplet microfluidics. Anal. Chem. 2020;92:132–149. doi: 10.1021/acs.analchem.9b05047. [DOI] [PubMed] [Google Scholar]
- 11.Shang L, Cheng Y, Zhao Y. Emerging droplet microfluidics. Chem. Rev. 2017;117:7964–8040. doi: 10.1021/acs.chemrev.6b00848. [DOI] [PubMed] [Google Scholar]
- 12.Soitu C, et al. Jet-printing microfluidic devices on demand. Adv. Sci. 2020;7:2001854. doi: 10.1002/advs.202001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh EJ, et al. Microfluidics with fluid walls. Nat. Commun. 2017;8:816. doi: 10.1038/s41467-017-00846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soitu C, et al. Microfluidic chambers using fluid walls for cell biology. Proc. Natl Acad. Sci. USA. 2018;115:E5926–E5933. doi: 10.1073/pnas.1805449115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soitu C, et al. Raising fluid walls around living cells. Sci. Adv. 2019;5:eaav8002. doi: 10.1126/sciadv.aav8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W, Ueda E, Levkin PA. Droplet microarrays: from surface patterning to high-throughput applications. Adv. Mater. 2018;30:e1706111. doi: 10.1002/adma.201706111. [DOI] [PubMed] [Google Scholar]
- 17.Cui H, et al. Assembly of multi‐spheroid cellular architectures by programmable droplet merging. Adv. Mater. 2021;33:2006434. doi: 10.1002/adma.202006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park D, et al. Microstructure guided multi-scale liquid patterning on an open surface. Lab Chip. 2018;18:2013–2022. doi: 10.1039/C7LC01288H. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, et al. Rapid generation of hybrid biochemical/mechanical cues in heterogeneous droplets for high-throughput screening of cellular responses. Lab Chip. 2021;21:2691–2701. doi: 10.1039/D1LC00209K. [DOI] [PubMed] [Google Scholar]
- 20.Haidas D, Napiorkowska M, Schmitt S, Dittrich PS. Parallel sampling of nanoliter droplet arrays for noninvasive protein analysis in discrete yeast cultivations by MALDI-MS. Anal. Chem. 2020;92:3810–3818. doi: 10.1021/acs.analchem.9b05235. [DOI] [PubMed] [Google Scholar]
- 21.Tung Y-C, et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–478. doi: 10.1039/C0AN00609B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun B, Zhao Y, Wu W, Zhao Q, Li G. A superhydrophobic chip integrated with an array of medium reservoirs for long-term hanging drop spheroid culture. Acta Biomater. 2021;135:234–242. doi: 10.1016/j.actbio.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Kuo CT, et al. Three-dimensional spheroid culture targeting versatile tissue bioassays using a PDMS-based hanging drop array. Sci. Rep. 2017;7:4363. doi: 10.1038/s41598-017-04718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry SB, Gower MS, Su X, Seshadri C, Theberge AB. A modular microscale granuloma model for immune-microenvironment signaling studies in vitro. Front. Bioeng. Biotechnol. 2020;8:931. doi: 10.3389/fbioe.2020.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foresti D, et al. Acoustophoretic printing. Sci. Adv. 2018;4:eaat1659. doi: 10.1126/sciadv.aat1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JJ, et al. Droplet behavior in open biphasic microfluidics. Langmuir. 2018;34:5358–5366. doi: 10.1021/acs.langmuir.8b00380. [DOI] [PubMed] [Google Scholar]
- 27.Khor JW, Lee UN, Berthier J, Berthier E, Theberge AB. Interfacial tension driven open droplet microfluidics. Adv. Mater. Interf. 2023 doi: 10.1002/admi.202202234. [DOI] [Google Scholar]
- 28.Li C, et al. Double-exclusive liquid repellency (double-ELR): an enabling technology for rare phenotype analysis. Lab Chip. 2018;18:2710–2719. doi: 10.1039/C8LC00584B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juang DS, Lang JM, Beebe D. Volumeless reagent delivery: a liquid handling method for adding reagents to microscale droplets without increasing volume. Lab Chip. 2022 doi: 10.1039/d1lc00906k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, et al. Exclusive liquid repellency: an open multi-liquid-phase technology for rare cell culture and single-cell processing. ACS Appl. Mater. Interf. 2018;10:17065–17070. doi: 10.1021/acsami.8b03627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, et al. Social motility of biofilm-like microcolonies in a gliding bacterium. Nat. Commun. 2021;12:5700. doi: 10.1038/s41467-021-25408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, et al. Under oil open-channel microfluidics empowered by exclusive liquid repellency. Sci. Adv. 2020;6:eaay9919. doi: 10.1126/sciadv.aay9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juang DS, et al. Oil immersed lossless total analysis system for integrated RNA extraction and detection of SARS-CoV-2. Nat. Commun. 2021;12:4317. doi: 10.1038/s41467-021-24463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, et al. Under-oil autonomously regulated oxygen microenvironments: a Goldilocks Principle-based approach for microscale cell culture. Adv. Sci. 2022;9:e2104510. doi: 10.1002/advs.202104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li A, et al. Programmable droplet manipulation by a magnetic-actuated robot. Sci. Adv. 2020;6:eaay5808. doi: 10.1126/sciadv.aay5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jebrail MJ, Bartsch MS, Patel KD. Digital microfluidics: a versatile tool for applications in chemistry, biology and medicine. Lab Chip. 2012;12:2452–2463. doi: 10.1039/c2lc40318h. [DOI] [PubMed] [Google Scholar]
- 37.Choi K, Ng AHC, Fobel R, Wheeler AR. Digital microfluidics. Annu. Rev. Anal. Chem. 2012;5:413–440. doi: 10.1146/annurev-anchem-062011-143028. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Kim CJ. Current commercialization status of electrowetting-on-dielectric (EWOD) digital microfluidics. Lab Chip. 2020;20:1705–1712. doi: 10.1039/D0LC00144A. [DOI] [PubMed] [Google Scholar]
- 39.Pang L, Ding J, Liu XX, Fan SK. Digital microfluidics for cell manipulation. TrAC Trends Anal. Chem. 2019;117:291–299. doi: 10.1016/j.trac.2019.06.008. [DOI] [Google Scholar]
- 40.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 41.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 42.Teh S-Y, Lin R, Hung L-H, Lee AP. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- 43.Streets AM, et al. Microfluidic single-cell whole-transcriptome sequencing. Proc. Natl Acad. Sci. USA. 2014;111:7048–7053. doi: 10.1073/pnas.1402030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss M, et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 2018;17:89–96. doi: 10.1038/nmat5005. [DOI] [PubMed] [Google Scholar]
- 45.Bachler S, Haidas D, Ort M, Duncombe TA, Dittrich PS. Microfluidic platform enables tailored translocation and reaction cascades in nanoliter droplet networks. Commun. Biol. 2020;3:76–9. doi: 10.1038/s42003-020-01489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan X, Dong X, Karacakol AC, Xie H, Sitti M. Reconfigurable multifunctional ferrofluid droplet robots. Proc. Natl Acad. Sci. USA. 2020;117:27916–27926. doi: 10.1073/pnas.2016388117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du X, et al. Droplet array-based 3D coculture system for high-throughput tumor angiogenesis assay. Anal. Chem. 2018;90:3253–3261. doi: 10.1021/acs.analchem.7b04772. [DOI] [PubMed] [Google Scholar]
- 48.Efremov AN, Stanganello E, Welle A, Scholpp S, Levkin PA. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell-cell communication. Biomaterials. 2013;34:1757–1763. doi: 10.1016/j.biomaterials.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 49.Wei Y, Zhu Y, Fang Q. Nanoliter quantitative high-throughput screening with large-scale tunable gradients based on a microfluidic droplet robot under unilateral dispersion mode. Anal. Chem. 2019;91:4995–5003. doi: 10.1021/acs.analchem.8b04564. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, et al. Printing 2-dimentional droplet array for single-cell reverse transcription quantitative PCR assay with a microfluidic robot. Sci. Rep. 2015;5:9551. doi: 10.1038/srep09551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Sun L, Yu Y, Zhao Y. Flexible ferrofluids: design and applications. Adv. Mater. 2019;31:1–35. doi: 10.1002/adma.201903497. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, et al. Bioinspired superwettable microspine chips with directional droplet transportation for biosensing. ACS Nano. 2020;14:4654–4661. doi: 10.1021/acsnano.0c00324. [DOI] [PubMed] [Google Scholar]
- 53.Frey O, Misun PM, Fluri DA, Hengstler JG, Hierlemann A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014;5:4250. doi: 10.1038/ncomms5250. [DOI] [PubMed] [Google Scholar]
- 54.Li ZY, et al. Nanoliter-scale oil-air-droplet chip-based single cell proteomic analysis. Anal. Chem. 2018;90:5430–5438. doi: 10.1021/acs.analchem.8b00661. [DOI] [PubMed] [Google Scholar]
- 55.Walsh DI, Kong DS, Murthy SK, Carr PA. Enabling microfluidics: from clean rooms to makerspaces. Trends Biotechnol. 2017;35:383–392. doi: 10.1016/j.tibtech.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsao C-W. Polymer microfluidics: simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines. 2016;7:225. doi: 10.3390/mi7120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee UN, et al. Fundamentals of rapid injection molding for microfluidic cell-based assays. Lab Chip. 2018;18:496–504. doi: 10.1039/C7LC01052D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, et al. Systematic prevention of bubble formation and accumulation for long-term culture of pancreatic islet cells in microfluidic device. Biomed. Microdevices. 2012;14:419–426. doi: 10.1007/s10544-011-9618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JJ, Berthier J, Kearney KE, Berthier E, Theberge AB. Open-channel capillary trees and capillary pumping. Langmuir. 2020;36:12795–12803. doi: 10.1021/acs.langmuir.0c01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berthier E, Warrick J, Yu H, Beebe DJ. Managing evaporation for more robust microscale assays: Part 1. Volume loss in high throughput assays. Lab Chip. 2008;8:852–859. doi: 10.1039/b717422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berthier E, Warrick J, Yu H, Beebe DJ. Managing evaporation for more robust microscale assays: Part 2. Characterization of convection and diffusion for cell biology. Lab Chip. 2008;8:860–864. doi: 10.1039/b717423c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun SH, et al. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal. Chem. 2007;79:1126–1134. doi: 10.1021/ac061990v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu WW, Zhu Y, Feng YM, Fang J, Fang Q. Droplet-based multivolume digital polymerase chain reaction by a surface-assisted multifactor fluid segmentation approach. Anal. Chem. 2017;89:822–829. doi: 10.1021/acs.analchem.6b03687. [DOI] [PubMed] [Google Scholar]
- 64.Cole RH, et al. Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells. Proc. Natl Acad. Sci. USA. 2017;114:8728–8733. doi: 10.1073/pnas.1704020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Liang Y, Zhou F, Liu W. Extreme wettability and tunable adhesion: biomimicking beyond nature? Soft Matter. 2012;8:2070–2086. doi: 10.1039/C1SM07003G. [DOI] [Google Scholar]
- 66.Jothi Prakash CG, Prasanth R. Approaches to design a surface with tunable wettability: a review on surface properties. J. Mater. Sci. 2021;56:108–135. doi: 10.1007/s10853-020-05116-1. [DOI] [Google Scholar]
- 67.Momotenko D, Cortes-Salazar F, Lesch A, Wittstock G, Girault HH. Microfluidic push–pull probe for scanning electrochemical microscopy. Anal. Chem. 2011;83:5275–5282. doi: 10.1021/ac2006729. [DOI] [PubMed] [Google Scholar]
- 68.Xu LP, et al. Ultratrace DNA detection based on the condensing-enrichment effect of superwettable microchips. Adv. Mater. 2015;27:6878–6884. doi: 10.1002/adma.201502982. [DOI] [PubMed] [Google Scholar]
- 69.Gutzweiler L, et al. Open microfluidic gel electrophoresis: rapid and low cost separation and analysis of DNA at the nanoliter scale. Electrophoresis. 2017;38:1764–1770. doi: 10.1002/elps.201700001. [DOI] [PubMed] [Google Scholar]
- 70.Berthier, J. & Silberzan, P. Microfluidics for Biotechnology 2nd edn, 483 (Artech House, 2009).
- 71.Folch, A. Introduction to BioMEMS (CRC Press, 2012).
- 72.van der Waals JD. The thermodynamic theory of capillarity under the hypothesis of a continuous variation of density. J. Stat. Phys. 1979;20:197–200. doi: 10.1007/BF01011513. [DOI] [Google Scholar]
- 73.Widom B. Capillarity and wetting phenomena: drops, bubbles, pearls, waves. Phys. Today. 2004;57:66–67. doi: 10.1063/1.1878340. [DOI] [Google Scholar]
- 74.David R, Dobson SM, Tavassoli Z, Cabezas MG, Neumann AW. Investigation of the Neumann triangle for dodecane liquid lenses on water. Colloids Surf. A. 2009;333:12–18. doi: 10.1016/j.colsurfa.2008.09.018. [DOI] [Google Scholar]
- 75.Neumann, F. Vorlesungen über die theorie der Capillarität (B.G. Teubner, 1894).
- 76.Henderson JR. Statistical mechanics of Cassie’s law. Mol. Phys. 2000;98:677–681. doi: 10.1080/00268970009483335. [DOI] [Google Scholar]
- 77.Gibbs, J. W. Scientific Papers (Dover Publications, 1961).
- 78.Rideal EK. On the flow of liquids under capillary pressure. Phil. Mag. Ser. 1922;6:1152–1159. doi: 10.1080/14786441008634082. [DOI] [Google Scholar]
- 79.Washburn EW. The dynamics of capillary flow. Phys. Rev. 1921;17:273–283. doi: 10.1103/PhysRev.17.273. [DOI] [Google Scholar]
- 80.Lucas R. Ueber das Zeitgesetz des kapillaren Aufstiegs von Flüssigkeiten. Kolloid-Z. 1918;23:15–22. doi: 10.1007/BF01461107. [DOI] [Google Scholar]
- 81.Bosanquet CH. On the flow of liquids into capillary tubes. Lond. Edinb. Dubl. Phil. Mag. J. Sci. 1923;45:525–531. doi: 10.1080/14786442308634144. [DOI] [Google Scholar]
- 82.Fries N, Dreyer M. The transition from inertial to viscous flow in capillary rise. J. Colloid Interf. Sci. 2008;327:125–128. doi: 10.1016/j.jcis.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Concus P, Finn R. On the behavior of a capillary surface in a wedge. Proc. Natl Acad. Sci. USA. 1969;63:292–299. doi: 10.1073/pnas.63.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young T. An essay on the cohesion of fluids. Phil. Trans. R. Soc. Lond. 1805;95:65–87. [Google Scholar]
- 85.De Groot TE, Veserat KS, Berthier E, Beebe DJ, Theberge AB. Surface-tension driven open microfluidic platform for hanging droplet culture. Lab Chip. 2016;16:334–344. doi: 10.1039/C5LC01353D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baroud CN, Gallaire F, Dangla R. Dynamics of microfluidic droplets. Lab Chip. 2010;10:2032–2045. doi: 10.1039/c001191f. [DOI] [PubMed] [Google Scholar]
- 87.Gai Y, Khor JW, Tang SKY. Confinement and viscosity ratio effect on droplet break-up in a concentrated emulsion flowing through a narrow constriction. Lab Chip. 2016;16:3058–3064. doi: 10.1039/C6LC00478D. [DOI] [PubMed] [Google Scholar]
- 88.Anna SL, Bontoux N, Stone HA. Formation of dispersions using ‘flow focusing’ in microchannels. Appl. Phys. Lett. 2003;82:364–366. doi: 10.1063/1.1537519. [DOI] [Google Scholar]
- 89.Juncker D, Schmid H, Delamarche E. Multipurpose microfluidic probe. Nat. Mater. 2005;4:622–627. doi: 10.1038/nmat1435. [DOI] [PubMed] [Google Scholar]
- 90.Ueda E, Geyer FL, Nedashkivska V, Levkin PA. DropletMicroarray: facile formation of arrays of microdroplets and hydrogel micropads for cell screening applications. Lab Chip. 2012;12:5218–5224. doi: 10.1039/c2lc40921f. [DOI] [PubMed] [Google Scholar]
- 91.Oomen PE, Mulder JPSH, Verpoorte E, Oleschuk RD. Controlled, synchronized actuation of microdroplets by gravity in a superhydrophobic, 3D-printed device. Anal. Chim. Acta. 2017;988:50–57. doi: 10.1016/j.aca.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Agrawal P, Salomons TT, Chiriac DS, Ross AC, Oleschuk RD. Facile actuation of organic and aqueous droplets on slippery liquid-infused porous surfaces for the application of on-chip polymer synthesis and liquid-liquid extraction. ACS Appl. Mater. Interf. 2019;11:28327–28335. doi: 10.1021/acsami.9b08849. [DOI] [PubMed] [Google Scholar]
- 93.Agrawal P, Bachus KJ, Carriere G, Grouse P, Oleschuk RD. An investigation into the kinematics of magnetically driven droplets on various (super)hydrophobic surfaces and their application to an automated multi-droplet platform. Anal. Bioanal. Chem. 2019;411:5393–5403. doi: 10.1007/s00216-018-1378-y. [DOI] [PubMed] [Google Scholar]
- 94.Vella D, Mahadevan L. The “Cheerios effect”. Am. J. Phys. 2005;73:817–825. doi: 10.1119/1.1898523. [DOI] [Google Scholar]
- 95.Li J, et al. Oil droplet self-transportation on oleophobic surfaces. Sci. Adv. 2016;2:e1600148. doi: 10.1126/sciadv.1600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hutama TJ, Oleschuk RD. Magnetically manipulated droplet splitting on a 3D-printed device to carry out a complexometric assay. Lab Chip. 2017;17:2640–2649. doi: 10.1039/C7LC00629B. [DOI] [PubMed] [Google Scholar]
- 97.Su Y, et al. Magnetism-actuated superhydrophobic flexible microclaw: from spatial microdroplet maneuvering to cross-species control. ACS Appl. Mater. Interf. 2021;13:35165–35172. doi: 10.1021/acsami.1c09142. [DOI] [PubMed] [Google Scholar]
- 98.Sun L, et al. Tailoring materials with specific wettability in biomedical engineering. Adv. Sci. 2021;8:2100126. doi: 10.1002/advs.202100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong Z, Fang Q. Automated, flexible and versatile manipulation of nanoliter-to-picoliter droplets based on sequential operation droplet array technique. TrAC Trends Anal. Chem. 2020;124:115812. doi: 10.1016/j.trac.2020.115812. [DOI] [Google Scholar]
- 100.Marchand G, et al. Organic synthesis in soft wall-free microreactors: real-time monitoring of fluorogenic reactions. Anal. Chem. 2008;80:6051–6055. doi: 10.1021/ac800855u. [DOI] [PubMed] [Google Scholar]
- 101.Keng PY, et al. Micro-chemical synthesis of molecular probes on an electronic microfluidic device. Proc. Natl Acad. Sci. USA. 2012;109:690–695. doi: 10.1073/pnas.1117566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Y, et al. Facile high throughput wet-chemical synthesis approach using a microfluidic-based composition and temperature controlling platform. Front. Chem. 2020;8:579828. doi: 10.3389/fchem.2020.579828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 104.Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 106.Laschke MW, Menger MD. Life is 3D: boosting spheroid function for tissue engineering. Trends Biotechnol. 2017;35:133–144. doi: 10.1016/j.tibtech.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 107.van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr. Opin. Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 108.Castiaux AD, Spence DM, Martin RS. Review of 3D cell culture with analysis in microfluidic systems. Anal. Methods. 2019;11:4220–4232. doi: 10.1039/C9AY01328H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, Xu LP, Zhang X, Wang S. Bioinspired wettable-nonwettable micropatterns for emerging applications. J. Mater. Chem. B. 2020;8:8101–8115. doi: 10.1039/D0TB01382J. [DOI] [PubMed] [Google Scholar]
- 110.Shiiki N, et al. Association between saliva PSA and serum PSA in conditions with prostate adenocarcinoma. Biomarkers. 2011;16:498–503. doi: 10.3109/1354750X.2011.598566. [DOI] [PubMed] [Google Scholar]
- 111.Wilschefski SC, Baxter MR. Inductively coupled plasma mass spectrometry: introduction to analytical aspects. Clin. Biochem. Rev. 2019;40:115–133. doi: 10.33176/AACB-19-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kachel S, et al. Evaporation from open microchannel grooves. Lab Chip. 2014;14:771–778. doi: 10.1039/C3LC50892G. [DOI] [PubMed] [Google Scholar]
- 113.Zhang H, et al. LAMP-on-a-chip: revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019;113:44–53. doi: 10.1016/j.trac.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berthier, J. & Brakke, K. A. The Physics Of Microdroplets (John Wiley & Sons, 2012).
- 115.Berthier J, Brakke KA, Gosselin D, Huet M, Berthier E. Metastable capillary filaments in rectangular cross-section open microchannels. AIMS Biophys. 2014;1:31–48. doi: 10.3934/biophy.2014.1.31. [DOI] [Google Scholar]