Precis
Significant disparities in vision health and eye care exist. To achieve health equity, we must understand the root causes and drivers of health disparities and inequities, including social determinants of health and systemic racism.
Introduction: Health Disparities in Eye and Vision Health
Health disparities exist across all fields of medicine; ophthalmology and vision health are no exception. A health disparity is a difference in health outcomes that arises from health inequities that affect medically underserved populations.1 A health inequity is the unfair distribution of health determinants, outcomes, and resources between and within different segments of a population based on social, economic, environmental, and structural factors.2 The goal of eliminating health disparities is to achieve health equity, which can be defined as a state in which every individual has a just and fair opportunity to achieve their best health. Attaining this goal requires removing social, political, and structural barriers as well as differences in health and health care–related resources, access, and use.3 The purpose of this paper is to outline the existing disparities in vision health and eye care, explore the possible reasons for these disparities, offer potential solutions, and ultimately stimulate the ophthalmology, eye care, and vision sciences community to move forward toward achieving equity in eye and vision health. Our goal is to engage our broader community in continuously narrowing health inequities to eliminate vision health disparities.
To achieve this goal of eliminating health disparities and inequities, we need to expand our traditional focus on access and use of eye care services and understand the foundational role of social determinants of health (SDOH), which are significant drivers of health disparities and inequities. The U.S. Department of Health and Human Services defines SDOH as the conditions in the environment in which people grow, live, learn, work, and age that affect health outcomes.4 These SDOH are often grouped into five domains: health care access and quality, economic stability, education access and quality, neighborhood and built environment, and social and community context.3 As such, access and use of eye care is just one of a number of key factors that drive vision health. In the larger health context, approximately 80% to 90% of a population’s health is determined by SDOH and only 10% to 20% by medical care.5 In light of these data, it is prudent for eye care providers to consider SDOH as we seek to eliminate disparities in eye care. It is also essential to understand the context in which these determinants were created, often shaped by societal factors related to socioeconomic and related factors, such as structural racism. Structural racism can be defined as differential access and distribution of opportunities, goods, and services, such as health care, by race and is increasingly recognized as a significant contributor to societal ill, including health disparities.
Part I of this Position Statement summarizes the growing body of literature examining health disparities as they relate to the field of vision health and eye care, including how these disparities manifest in the presentation of specific eye diseases. Key topics include the following:
Current state of vision health disparities and social determinants of health
Disparities in access to eye care
Health literacy and how we can improve patient education to enhance care
Effects and origins of disparities in the ophthalmology workforce
Current data sources that can be leveraged to measure progress toward the multifaceted goal of achieving equity in the field of ophthalmology
Part II provides a framework for reducing disparities in eye care, addressing issues related to access to care, patient education and health literacy, and physician workforce diversity. Finally, we discuss future areas of inquiry and how we can work together as a global community to improve eye and vision health.
PART I: WHAT WE KNOW ABOUT DISPARITIES IN VISION HEALTH
1. EPIDEMIOLOGY OF DISPARITIES BY SOCIODEMOGRAPHIC FACTORS
Visual impairment (VI) and blindness affect approximately 4.2 million persons aged 40 and older in the United States.6 Blindness in the U.S. is defined as a best-corrected visual acuity (BCVA) of 20/200 or worse or a central visual field of 20 degrees or less in the better eye. VI is typically defined as BCVA of 20/70 or worse in the better eye (although some studies use 20/40 or worse in the better eye). It has been projected that by 2050 the total number of persons affected by VI and blindness will more than double to 6.95 million.7 The prevalence of VI and many eye diseases increases with age and can vary across race and ethnicity, socioeconomic status, geographic location, and sex and gender.8 In addition, older adults with VI have been found to have greater prevalence of chronic conditions compared to those without VI.9 It is vital to understand the factors that contribute to VI and blindness to address ocular health disparities and improve health equity among all populations.
A. Race and Ethnicity
It has been well established that racial and ethnic minoritized populations are at high risk for various ocular diseases and subsequent VI and blindness.10–13 It is important to note that race is a sociopolitical construct, not a biologic determinant of disease, and is often assigned based on varying and inconsistent criteria, such as phenotype or self-report.
Nationwide estimates of incident blindness from 1968 to 1970 found rates that were up to 2.8 times higher in Black individuals than in White individuals.14 Subsequent epidemiologic studies have similarly demonstrated greater estimates of VI and blindness among Black Americans,11,15–18 as well as Hispanic Americans,10,11,17–20 Asian Americans,11,21,22 and Native Americans.18,23 Furthermore, Hispanic older adults and other racial/ethnic minorities with VI have been found to use low-vision devices at lower rates than non-Hispanic White peers.24 This disproportionate burden of VI among racial/ethnic minoritized people is of concern, given that VI has also been associated with a higher likelihood of not being employed,25 which in turn influences socioeconomic status, another important factor impacting VI and overall health states (including life expectancy).
Hispanic26–29 and Black29 adolescents have also been found to have increased estimates of VI, and they are projected to account for the highest and second-highest prevalence of VI, respectively, through 2060.28 Population rates of visual impairment are projected to continue to be higher among non-White groups.30
B. Age
Older people are disproportionately affected by VI and blindness.11,31 The number of people in the United States with VI or blindness has been steadily increasing along with our aging population, and it is estimated that this will result in a 25% increase in VI and a 21% increase in blindness by 2050.7,32 In persons aged 65 and older, the estimated prevalence of VI and blindness varies widely between studies: estimates of VI in this age group range from 2.2% to 26.6%, while estimates of blindness range from 0.6% to 16.6%.33 These variations may result from methods of estimation (e.g., patient self-reports versus examination data), survey question wording, sampling variation, or differences in data collection methodology.33
Visually impaired elderly patients face a plethora of demographic, social, and health disparities, including physical and functional disabilities, higher health care costs, poor psychological health, lower health-related quality of life, and higher medical morbidity and mortality than their non–visually impaired counterparts.9,34–37 Analysis of Medicare beneficiaries aged 65 and older demonstrates that VI is associated with hip fracture, depression, anxiety, and dementia and that these patients are less likely to have a usual source of health care.18,24,36,38 Approximately 3.6% of the U.S. elderly population live in nursing homes. Current literature shows that 63.8% to 73.0% of nursing home residents are affected by VI and blindness,37,39 and nursing home residents are three times more likely to have VI, and five times more likely to experience blindness, than individuals living in the community.40
C. Sex and Gender
According to a 2022 National Academies of Sciences, Engineering, and Medicine report, “sex is a multidimensional construct based on a cluster of anatomical and physiological traits that include external genitalia, secondary sex characteristics, gonads, chromosomes, and hormones”, while “gender is a multidimensional construct that links gender identity, which is a core element of a person’s individual identity; gender expression, which is how a person signals their gender to others through their behavior and appearance (such as hair style and clothing); and cultural expectations about social status, characteristics, and behavior that are associated with sex traits.”41 The relationship between VI and sex and gender is not as clear as the other factors considered above. Recent literature suggests that vision loss is more prevalent in females than males.7,18,31,35,38,39 No single etiology has been definitively identified for this discrepancy, but it has been proposed that the difference may be attributed, in part, to the longer life expectancy of females.7,13 In addition, known biological differences and predispositions contribute to some ocular conditions such as thyroid eye disease.13 This may help to explain why difference in vision by sex is more often reflected in the total prevalence of VI rather than incidence alone.39 Conversely, sex and gender differences in VI are not as apparent in the younger population, where the distribution by sex and gender is more similar.28 Future studies measuring differences and disparities both by sex and gender identity are necessary.
D. Underlying Factors of Social Determinants of Health
The World Health Organization defines SDOH as “the conditions in which people are born, grow, live, work and age. These circumstances are shaped by the distribution of money, power and resources at global, national and local level.”42 According to the Centers for Disease Control and Prevention (CDC), SDOH consist of the following43:
Health care access and quality (includes access to health care, health insurance, and health literacy)
Education access and quality (includes educational attainment, language and literacy, and early childhood education)
Social and community context (includes community cohesion, civic participation, workplace conditions, discrimination, and incarceration)
Economic stability (includes income, poverty, employment, food security, and housing security)
Neighborhood and built environment (includes transportation access, quality of housing, air and water quality, and crime and violence)
Furthermore, in an effort to identify the SDOH that particularly influence eye health and access to vision care, the CDC Vision Health Initiative cites factors including lower income, lower educational attainment, food insecurity, and neighborhood safety.43 In addition, the Kaiser Family Foundation identifies a specific category for food44 (which is included in the domain of economic stability by the CDC). This area warrants attention in the prevention of VI, given the importance of appropriate nutrition and micronutrients in the prevention of blinding eye diseases (e.g., vitamin A deficiency, various vitamins and zinc for AMD).
Socioeconomic status.
The association between socioeconomic status (SES) and VI has been well documented in the literature.8 In the United States, multiple studies have found associations between low income, unemployment, and less education and increased risk of VI, blindness, and sudden vision loss.10,11,45–48 Beyond that, lower income has been associated with higher rates of mortality in the United States.49 In addition to these direct socioeconomic factors, associations have been identified between lack of health insurance and low vision and lower utilization of eye and vision care.18,45,50,51 Moreover, children whose families fall below the federal poverty level are nearly twice as likely to have VI as children from families whose income was more than 200% of the poverty level.52
Higher rates of VI in the population may have further downstream socioeconomic implications. The National Health and Aging Trends Study found that near-vision loss was associated with decreased odds of having a usual source of health care, and another study found that lower family income was associated with decreased likelihood of adaptive device use by a person with VI.38,53
Geographic location.
Disparities in adult vision loss vary widely by geographic region and state.54–56 Even at and within the county level, vision loss varies significantly with geography.47 Various geographic regions in the United States have unexplained higher incidences of adult vision loss that have persisted over time.7 Americans living in urban cities have been found to have high levels of subjective VI.57 In some instances, these geographic differences are associated with poverty and income levels.47,56 Although redlining—the systematic denial of mortgages and lending bias often inflicted upon predominantly Black and poor communities—was prohibited by the Fair Housing Act of 1968, its harmful effects remain, as evidenced by the persistence of disadvantaged and often segregated communities in the United States. The impact of neighborhood-level redlining is also linked with health inequities.58,59 In one study, severe vision loss varied significantly by county and was strongly associated with area poverty levels.47 Counties with the highest levels of severe vision loss and poverty were mostly in the southern region of the United States.47 These differences also exist in childhood VI, as VI in preschool children in the U.S. varies significantly by geographic region.28
Because these several drivers of disparities in VI and blindness are inextricably connected (race and ethnicity, SES, geographic region), it may be that these geographic patterns are driven by residual or uncontrolled confounding by other related underlying SDOH and structural factors. As noted below, the relative difficulty in obtaining eye care services by ophthalmologists may also play a role.
2. EPIDEMIOLOGY OF DISPARITIES BY EYE DISEASE
Multiple studies have identified disease-specific disparities by race and ethnicity, gender, SES, geography, and other factors. Additionally, these studies have demonstrated the complexities of differentiating the natural history of disease from the disparities in diagnosis and care within these groups.
A. Cataract
Prevalence.
Early studies using data from the 1971–1972 NHANES found that the estimated prevalence of cataract was disproportionately greater in Black Americans than in White Americans.60,61 This reported disparity was later confirmed in population-based studies.62–64 Subsequent studies have also described elevated cataract prevalence in other racial/ethnic minority groups, including Hispanic65,66 and Chinese Americans.67 Other sociodemographic characteristics associated with greater cataract prevalence include female sex and gender,61,68–70 lower income,69,71,72 and lower educational attainment.60,61,72–76
Surgical treatment and outcomes.
Sociodemographic disparities for cataract surgery exist as well, and the following characteristics have been associated with lower rates of cataract surgery: Black77–86 and Hispanic79,87 race and ethnicity, lower income,77,82 rural residence,88,89 and lower educational attainment.82 Furthermore, Hispanic, Black, and Asian American patients were more likely to have complex cataract surgery than White patients.90 Worse cataract surgery outcomes have also been described for certain groups: Black patients91 and patients with intellectual disability92 are at greater risk for anterior uveitis following cataract surgery; and male,93 Black,93,94 and Native American93 patients are at greater risk for post–cataract surgery endophthalmitis. Patients who had cataract surgery through the Veterans Health Administration were found to have higher 90-day rates of secondary procedures following cataract surgery (such as vitrectomy) when compared to Medicare beneficiaries.95
B. Glaucoma
Prevalence.
Multiple reviews of age-related eye diseases have reported racial and ethnic, sex and gender, and socioeconomic disparities in glaucoma prevalence.86,96–100 Overall, there is a higher burden of glaucoma with older age and lower SES.101,102 In the United States, numerous studies have found higher prevalence of glaucoma in Black individuals compared to White individuals,103,104 although the largest demographic group with glaucoma has been predicted to shift from non-Hispanic White women to Hispanic men by 2050.105 Additionally, studies have suggested higher rates of glaucoma in Asian American patients compared to White patients.106,107
Differences in glaucoma prevalence between various groups are likely multifactorial. Racial and ethnic differences in ocular anatomy that may contribute to glaucoma risk—for example, central corneal thickness—have been identified.108–121 Although White and Black patients have similar rates of glaucoma-related blindness, Black patients undergo fewer surgeries.122 In addition to racial/ethnic disparities, ocular anatomic and hormonal differences may contribute to varying rates of glaucoma prevalence by sex and gender.123–125 Moreover, glaucoma prevalence in the Medicare population varies by region, which may suggest over- or under-diagnosis in certain areas of the U.S.126
Medication adherence and follow-up.
The reasons for poor follow-up among glaucoma patients are complex, and patient-level factors that have been identified include race and ethnicity, poor understanding of the condition, systemic comorbidities, and distance to provider.127–131 As noted earlier, structural factors and SDOH are likely play a significant role in an individual’s ability to maintain appropriate health care. Additionally, there are lower rates of ancillary glaucoma testing in Hispanic patients, in patients with Medicaid, and in certain geographic regions.132–134 Multiple sociodemographic barriers have also been associated with poor medication adherence in glaucoma patients.135–137
Surgery.
The higher rates of glaucoma surgery reported in Black patients compared to White patients may stem from underdiagnosis and later presentation in Black patients.122,138,139 Moreover, higher rates of surgical failure have been shown in Black patients than in White patients,140–142 although a review of available studies did not suggest options for primary surgical intervention for Black patients other than standard trabeculectomy.143 Recent studies have of minimally invasive glaucoma surgeries (MIGS) in non-Hispanic and Hispanic Black patients have shown good outcomes with certain MIGS procedures.144–146 Aside from race and ethnicity, potential disparities in rates of procedural glaucoma treatment and follow-up have been identified by age,147 region,148 provider type,148 and distance from provider,149 among other factors.
C. Amblyopia
Amblyopia is the leading cause of vision loss and VI among children and young adults.150,151 Early detection and treatment are essential for reducing the risk of long-term consequences and improving the overall quality of life of children.152–154 Similarly, amblyopia risk factors—strabismus and anisometropia—necessitate early diagnosis and intervention to ensure functional improvement.8,155 Population-based studies among children in the United States have found that the prevalence of amblyopia and strabismus ranges from 0.8% to 2.6% in children 30 to 71 months, and 2.1% to 3.5% in children ages 6 to 71 months, respectively.156–158 Within these findings, the prevalence of strabismus was similar in Asian American and non-Hispanic White children and was higher among older children, whereas amblyopia prevalence did not vary significantly by age.159 Black and Hispanic children have similar rates of strabismus, but significantly higher rates of amblyopia are found among Hispanic children.157 This may be related to decreased access to care.
D. Refractive Error
Refractive error (hyperopia, myopia, astigmatism, and anisometropia) is the most common cause of correctable reduced vision in children, and the prevalence continues to increase while age of initial presentation continues to decrease.29,156,160 By 2050, it is estimated that the majority of total VI will be due to uncorrected refractive error.28 Undiagnosed and uncorrected refractive errors contribute to developmental, academic, and social challenges for children and, in some cases, permanent vision loss.151 It is estimated that 1 in 5 preschool children and 1 in 4 school-aged children in the United States have VI, but fewer than 15% of preschoolers receive an eye exam by an eye care professional, and fewer than 22% receive any type of vision screening.151 Significant variations in severity of vision loss across states and counties within those states is strongly correlated with poverty, with the highest burden of disease falling on southern states.8
The presence and type of uncorrected refractive error varies by race and ethnicity. For example, Black and Hispanic children are more likely to be myopic than White children,161 while White and Hispanic children are more likely to be hyperopic than Black children161; racial and ethnic differences exist for astigmatism as well.27,161–164 The Multi-Ethnic Pediatric Eye Disease Study found a higher prevalence of presenting refractive error–related VI in both Black children and Hispanic children than in either Asian American or non-Hispanic White children.156 Approximately 95% of first-grade students in low-income areas, 95% of whom identified as minority race or ethnicity, did not have glasses to address their decreased visual acuity, and Black and Hispanic students were less likely than non-Hispanic White students to have glasses.165 Other studies have also highlighted that the spectacle needs of Hispanic and Black children are largely unmet across all age groups, with the greatest disparity among children between the ages of 12 and 19.29,79
E. Age-Related Macular Degeneration
The prevalence of age-related macular degeneration (AMD) in the United States is predicted to double by 2050 as our aging population continues to increase. Thus, identifying modifiable risk factors is of great importance.185 Although AMD is classically associated with age over 65, female sex and gender, and White race, modifiable risk factors include smoking, waist circumference, waist-hip ratio, and cardiovascular issues such as serum cholesterol levels and hypertension.186–191 More specifically, in patients with early/intermediate disease, progression to advanced or exudative AMD is linked to long-term smoking, increased body mass index, diabetes, lower educational attainment, and use of certain anti-inflammatory medications.72,187
Black patients have a lower rate of AMD diagnosis across all Medicare ages, while Hispanic and Asian American patients under the age of 80 have similar rates of AMD as their White counterparts. After the age of 80, the incidence of AMD among the aforementioned groups decreases compared to White patients.188,192 Although several studies have detailed the higher rates of AMD in White patients, racial or ethnic minority patients with AMD often have considerably reduced visual acuity at initial presentation.193
F. Diabetic Retinopathy
As the prevalence of diabetes mellitus has increased in the United States, diabetic retinopathy (DR) has become the leading cause of legal blindness in persons aged 20 to 74.194 In addition to a diagnosis of either type 1 or type 2 diabetes, there are several other diabetes-related risk factors for DR: higher HbA1c, insulin use, duration of disease, hypertension, and elevated blood glucose.195–197 Compared to White Americans, Black and Hispanic Americans tend to have a higher and more severe disease burden but lower rates of recommended screening and eye examinations.194,195,198,199 Moreover, despite advances in therapy, Black and Hispanic patients have more severe DR at the time treatment is initiated with anti-VEGF intravitreal injections.200
One study reported a higher prevalence of DR in rural communities than in urban environments, even after adjustment for differences in access to care.201 Further investigation is warranted to determine if the increased prevalence and severity of DR in the minoritized population is confounded by structural factors and SDOH.202
G. Ocular Trauma
Epidemiology.
Ocular trauma is a leading cause of monocular blindness in the United States and is the second most-common reason for ocular-related hospitalizations.166,167 Several studies of pediatric and adult patients revealed disparities in age, sex and gender, race, ethnicity, urban or rural environment, and income or insurance status among ocular trauma cases. Approximately 35% of eye injuries in the U.S. occur in children; Black patients are at a greater risk of assault, while White patients were more likely to suffer self-inflicted or unintentional injury.168 Non-powder guns, including paintball and air guns, cause ocular injuries more frequently in older non-White children.169 Black male adolescents are disproportionately represented in sight-threatening pediatric powder firearm–associated ocular injury (FAOI).170
The prevalence of ocular injury among adults is currently estimated to be 7.5%, and most injuries occur in young males, with particularly high rates among Black and Native American patients.171–175 The incidence of open-globe injury (OGI) is highest in Black and Hispanic patients and elderly men.176 Patients on Medicare and in the lowest income quartile have the highest rates of OGI,177 and rates of hospitalization are highest for males, elderly patients, and Black patients in all eligibility groups.176 Rural patients with OGI have longer elapsed time before presentation, higher patient transfer rate, and higher rates of follow-up at another medical facility compared with patients in an urban setting.178 Several studies have demonstrated that Black patients are overrepresented among patients who experience violent, nonaccidental ocular trauma, including FAOI and assault.179,180 Studies have demonstrated that Black patients comprise up to 40–60% of patients treated for ocular trauma at hospitals throughout the US.178–180 Furthermore, Hispanic patients demonstrate a fourfold higher risk of FAOI in certain regions.181
Outcomes.
Rehabilitation after severe ocular trauma has significant impact on the ability to achieve an optimal functional outcome, which affects patients’ independence, family psychosocial stress, and ability to achieve community integration.182,183 Limited data are available on the long-term visual outcomes of ocular trauma, but it is estimated that FAOI results in permanent visual loss in 44% of cases.184 One study demonstrated that Black patients represent the majority of patients who undergo enucleation or evisceration after severe ocular trauma.179 Furthermore, the average age at eye removal is also younger among Black and Hispanic patients than among White patients.179
H. Other Ocular Conditions
Uveitis.
Sex and gender differences in the prevalence of uveitis are well established. Female patients are more likely to be diagnosed with uveitis than their male counterparts.203–206 The association between race and ethnicity and uveitis varies with the anatomic location of ocular inflammation.206 Black individuals are disproportionately affected by uveitis.203,204 Patients with younger age, lower socioeconomic status, and Medicaid insurance are more likely to be diagnosed with uveitis.135
Keratoconus.
Black and Hispanic patients have significantly higher odds of being diagnosed with keratoconus than White patients.207 Compared to people living in a rural area, those living in urban areas had higher odds of having keratoconus.207 Although there is some debate regarding the association between sex and gender and keratoconus, the Collaborative Longitudinal Evaluation of Keratoconus study found sex and gender differences in patient history, vision, and ocular symptoms in keratoconus patients.208 Finally, sex and gender, race and ethnicity, income, and education affect the treatment of patients with keratoconus.208 Male sex and gender, Black race, and lower educational attainment are associated with increased odds of receiving a penetrating keratoplasty for keratoconus.209 While scleral contact lenses have been demonstrated to be cost-effective, the price for clinical services and lens production are still high.210 Having higher net household income was associated with decreased odds of receiving a corneal transplant.209
3. DISPARITIES IN ACCESS TO VISION AND EYE CARE
Access to vision and eye care remains one of the greatest unmet health needs in the United States. Race, ethnicity, income, insurance coverage, geographic region, and educational attainment have been identified as predictors of outpatient vision care utilization. Hispanic and Black patients have fewer outpatient ophthalmologic visits than their non-Hispanic White counterparts, as do the uninsured compared to the insured, those with lower income and educational level compared to those with greater affluence and more education, and those living in the midwestern, southern, or western regions of the U.S. compared to those living in the northeastern region.211 Because comprehensive eye examinations are often not included under the umbrella of essential primary care, many Americans seek such examinations only after significant vision problems have developed.212
A. Effects of SDOH on Access to and Quality of Care
SDOH have been shown to create barriers to accessing eye and vision care and to undermine adherence to treatment.213 In any discussion of SDOH, it is important to be aware of the significant impact of structural racism, particularly in terms of neighborhood and built environments. For example, ocular hospitalizations are significantly increased in communities with worse air pollution, severe housing problems, higher rates of violent crime, increased drug poisoning deaths, and greater proportions of single parent households.214 Lower SES and poorer access to transportation, as well as crime, can impact the ability of individuals to get to and seek care, especially as fewer health care resources are available in lower SES areas.
B. Comprehensive Eye Care
Multiple barriers to comprehensive eye care services have been reported. Obstacles to care for rural and low-income populations include lack of (1) access to affordable coverage and services, (2) availability of eye care professionals, (3) knowledge about personal risks for VI/blindness, and (4) primary care physician referral to optometry or ophthalmology. There are significant missed opportunities in linking patients to eye care services; for example, 96% of respondents to a 2005 National Eye Institute survey indicated that they would be somewhat or very likely to seek an eye exam from an optometrist or ophthalmologist if recommended by their primary care physician.215
Infrastructure barriers to on-site comprehensive eye care in community health centers include inability to afford space and equipment, inadequate reimbursement from insurers, and lack of guidance for business model development.212 Barriers to obtaining eyeglasses in vulnerable patients included both internal and external factors; internal factors were related to the patient’s intrinsic motivations and experiences (past experiences, trust, misperceptions), while external factors included cost of glasses, lack of access, and lack of transportation.216 Factors that were facilitators or enablers in obtaining eye and vision care included health insurance with vision care services, diabetes education programs, personalized follow-up, screening programs targeted to high-risk groups, and mobile screening in remote areas.217 However, research has shown that access to insurance alone does not translate to increased health care utilization across different racial/ethnic groups.218 For example, one study documented that underutilization of eye care services remains an issue among low-income Black and Hispanic children even when they are enrolled in Medicaid.218 As a result, these communities are more likely to have undiagnosed and uncorrected ocular conditions.
C. Diabetes Eye Care
An analysis of 2006–2010 Behavioral Risk Factor Surveillance System data showed that among adults with diagnosed diabetes, the most commonly reported reasons for not receiving eye care in the preceding 12 months were “no need” (39.7%) and “cost or lack of insurance” (32.3%). Those who reported “no need” as a barrier were most likely to be aged 65 years or older.8,51 Unfortunately, racial and ethnic disparities in DR screening extend to younger populations as well. In a cohort study of children and adolescents with type 1 or type 2 diabetes, racial and ethnic minority youths were more likely to have DR but less likely to undergo diabetic eye examinations compared to White peers, citing issues related to transportation, lack of time, and not having been recommended to do so.219
In a systematic review of the barriers and facilitators in accessing DR screening services, patient-perceived barriers included employment, comorbidities, problems in accessing a general practitioner, and difficulty in securing appointments.217 Lack of transportation and a lack of providers in close proximity were consistently reported as barriers to diabetes care for rural residents.201 Barriers related to health care providers included long waiting time for treatment, lack of coordination between general practitioners and those screening for DR, nonadherence to practice guidelines, and lack of knowledge of DR among health professionals.217 In urban settings, greater distance from an eye care facility and poor access to public transportation were associated with lower levels of dilated eye examination adherence in patients with diabetes without a DR diagnosis.220 The authors speculated that persons with diabetes may be more responsive to transportation intervention (such as travel vouchers or arranged transportation) if coupled with proper education about diabetes.220
D. Glaucoma Care
Several studies have examined barriers to glaucoma care. In a randomized clinical trial of 906 Black patients in Philadelphia diagnosed with glaucoma, affected individuals reported forgetfulness (34.2%), lack of transportation (13.5%), and inability to miss work (7.1%) as barriers to keeping follow-up appointments.221 Participants suggested that reminder calls and assistance in transportation would help with maintenance of future appointments.221 In another study of Black patients in New Haven, factors associated with not returning for follow-up glaucoma care include no access to a car, being a current smoker, living alone, number of days between a screening and full evaluation, and younger age.222
E. Pediatric Eye Care
Barriers limiting access to eye care for children include false-negative eye-screening results at school; absence of signs, symptoms, or family history of vision problems; low SES; and health insurance status.8,86,223 Even with access to health insurance, children from less affluent households use eye care services at lower rates than those from wealthier households, and the time between visits is also greater.224 Therefore, children from less affluent communities are more likely to have underdiagnosed sight-threatening ocular diseases despite enrollment in services like Medicaid.52,225
Equal access to health insurance, which continues to improve with the expansion of Medicaid, does not result in similar rates of health care utilization across different racial and ethnic groups, leading to poorer quality of health for children from minoritized groups.218,226 Underutilization is still found in Black and Hispanic children and children from less affluent households, and studies have suggested that pediatric vision care services should be co-located with public benefit programs; other potential facilitators include increasing availability of point-of-care services, social work support, and cash incentives for follow-up care.227 Utilization of vision-related services among low-income children has also been shown to be dependent on Medicaid vision benefits for adults.228 Thus, expanding access for adults increases the opportunities for eye care providers to inform parents about the eye care needs of their children.228
4. PATIENT EDUCATION AND HEALTH LITERACY
A. Eye Health Knowledge
The peer-reviewed literature contains limited data about eye health knowledge in the United States. One study found that public awareness of glaucoma, AMD, diabetic eye disease, and low vision varied substantially by disease.229 While the majority of people who were aware of glaucoma (90%) or diabetic eye disease (51%) knew that these conditions could be treated, the majority did not know that glaucoma (92%) or DR (89%) could present with no early warning signs.229 Significant disparities in eye health literacy exist as well. Hispanic individuals were found to have the lowest eye health knowledge and least access to eye health information.229 In an online survey of 3,512 American adults conducted in 2019, only 37% knew that detectable vision loss from eye disease could in fact be asymptomatic.230
Qualitative research has also found notable gaps in patients’ understanding of eye care and risks to vision, noting that their knowledge appeared to most often stem from personal experience rather than educational materials.231 Although evidence-based eye health education programs have been developed to improve eye health literacy, it is evident that targeted campaigns and tailored educational materials are required for vulnerable groups (unemployed individuals and those with lower educational attainment), who have reduced odds of knowledge improvement through conventional programs.232
The difference in understanding may reflect differences in educational opportunity, the quality of the schooling, and the factors related to dropping out of the educational system short of graduation from high school. Lower SES populations and communities have lower levels of graduation from high school and overall educational attainment. If true reading levels are three to five grade levels less than the last year of school completed, then many individuals with potentially blinding eye diseases may have an education that enables them to read and comprehend only at an elementary school level.
B. Patient Education Preferences
Most ophthalmology patients prefer personalized education. In a survey of patients at a tertiary eye care center, patients preferred one-on-one sessions with providers as well as materials (printed and websites) recommended by their doctor. Patient age and race may affect the preferred modality for education and topic of interest.233
Effective clinician-patient communication has been proven to engage patients in their care. However, in the context of increasingly high-volume clinics, there is a tendency to resort to printed information leaflets that are not suitable for patients with VI, non-English speakers, or those with low literacy. In a systematic review assessing the use of video-based media for patient education, a majority of the studies (71%) showed a statistically significant improvement in patient comprehension after video intervention. Although more evidence is needed, the use of video-based media—and, more specifically, in the physician exam room—appears to be effective in improving patient understanding.234,235
Electronic health (e-health) systems intended for patient use (e.g., websites, apps, text messaging) are often designed without considering the needs of disadvantaged patients and their level of e-health literacy.236 Several variables, such as experience, education, numeracy, and overall health literacy, income, and rurality, are associated with e-health literacy.237 Income may present a challenge to underserved populations due to poor access to both technology and traditional health resources. Socially disadvantaged groups with less access to electronic technologies and the skills to use them are at risk of being digitally marginalized, which may reduce the effectiveness of various interventions for impacted individuals and further widen health disparities.
In addition to issues of access to technology, the content and style of e-health resources may be inappropriate for underserved groups. An assessment of online patient education materials, including those from major ophthalmologic associations, found that most are written far above the recommended reading level, and the content may be of low quality.238,239 A study of online patient information on cataracts found that commonly accessed resources are insufficient to give patients a clear and complete understanding of their condition and of the medical and surgical treatment options.240 In 2016, the American Academy of Ophthalmology performed an audit and health-literacy rewrite of its online patient education materials in response to these issues.
Mobile technology and online social networks may be underutilized as a method of providing health information to underserved minority populations; however, it is important to be aware of limitations associated with the “digital divide” in access to such technologies. A study demonstrated that among urban Black parents of children covered by public insurance, 97.0% owned a cell phone, but home internet access was more prevalent among those with higher income. Although only 17.9% of participants shared health information via texting, most expressed an interest in receiving health information or utilizing social networking to learn more about health topics.241 With regard to social networks, one study found that more than 80% of practicing medical providers agreed that social media could be an effective educational tool, but only 43% used social media for educational purposes.242
5. WORKFORCE DIVERSITY
A. Why Diversity Is Important
Workforce diversity is a critical component in providing culturally competent care to an increasingly diverse patient population, including racial/ethnic minorities, lesbian, gay, bisexual, transgender, queer (LGBTQ+) individuals, those from diverse socioeconomic backgrounds, and persons with disabilities. Thus, focusing on efforts to recruit and retain a diverse pipeline of applicants into ophthalmology is critical. Beyond increasing diversity among practicing ophthalmologists, increasing the diversity of faculty in leadership roles, journal editorial boards, and science is also necessary. Greater public visibility of individuals from groups underrepresented in medicine (URM), women, LGBTQ+, low-SES, and other minoritized persons in these roles may increase the attractiveness of the profession of ophthalmology to members of these groups.
Diversity enhances learning and communication.
Literature from a wide range of fields demonstrates that groups in professional settings benefit from greater gender and racial/ethnic diversity.243,244 For example, diverse teams produce higher-impact research publications than homogeneous teams.245–247 The American Association of Medical Colleges (AAMC) study of recent medical graduates248 found that student perceptions of learning from others who are different from themselves was positively associated with how racially and/or ethnically diverse the student body was, allowing future physicians to communicate with and treat patients from diverse backgrounds more effectively. Cultural humility training of health care professionals is associated with enhanced patient satisfaction in minority populations and better health outcomes.249,250
Diversity helps expand the knowledge base.
A recent study revealed that Black, Hispanic, and other non-White participants were underrepresented in clinical trials leading to Food and Drug Administration drug approvals compared with the expected racial/ethnic distribution based on disease burden in the United States.251 Increasing the number of underrepresented minority and women scientists would help reduce barriers to clinical trial participation in these groups.252 Though much is known about many of the factors that contribute to health disparities in the United States,253 information gaps persist because many scientific and clinical studies still do not include women and minorities in their analyses, despite National Institutes of Health requirements.251,254
Provider-patient concordance may improve outcomes.
Increasing physician diversity is an important component of reducing health care disparities, as physicians from URM backgrounds are more likely to treat underserved populations and work in underserved areas.255–257 Concordance between the physician and the patient based on racial/ethnic or gender identity has been suggested as one way to improve health outcomes for patients from minority populations. Though the data have been mixed,258 several studies have shown positive associations between physician-patient concordance and Press Ganey survey scores (a measure of patient experience)259 and with medication primary adherence260 among Black patients. In addition, a positive association between racial/ethnic concordance and the probability that a patient will seek or receive medical care was seen in Hispanic and Asian American261 patients, which may be due to lack of language or cultural barriers. Language concordance has also been shown to have positive health outcomes for Hispanic patients.262–265
A National Bureau for Economic Research study266 found that in a controlled experiment in Oakland, California, Black men would agree to more preventive services—in particular, more invasive services such as cholesterol screenings—when they were paired with a Black physician. Based on these findings, the authors calculated that it would be possible to decrease the cardiovascular mortality and life expectancy gaps between White and Black individuals by 19% and 8%, respectively. Addressing ophthalmic workforce diversity could lead to significant reductions in eye-related disparities, as underrepresented populations tend to experience visual problems such as glaucoma, diabetic retinopathy, and other visual impairments at much higher rates than White populations.267
B. Current State of Ophthalmic Workforce Diversity
Gender.
We use the term “gender” here to refer to members of the ophthalmic workforce who identify as women. Although the proportion of women in ophthalmology has risen over the last 20 years, they comprise less than 40% of entering residents in the most recent data self-reported to the American Academy of Ophthalmology; in contrast, women now constitute the majority of medical school students. Further, once women become residents, they have lower surgical volume.268,269 Women are also underrepresented in leadership positions in academic ophthalmology; department chairs (90%) and residency program directors (72%) are overwhelmingly men.270,271
Disparities are also present in practice. Women ophthalmologists are compensated significantly less than men in the first years of clinical practice, even after adjustment for the number of work, clinic, and operating days.272 Medicare data from 2012 and 2013 demonstrated that women submitted fewer charges and thus received less in collections (as low as a mean of $0.56 for women compared to $1.00 for men) from CMS.272
Fewer women are involved in industry-based research and consulting engagements, and they are paid significantly less than men for this work.273 Women are also heavily underrepresented in ophthalmic professional society boards as well as journal editorial boards.274 Disparities such as these may make it more difficult to recruit and retain women in the ophthalmic workforce.
Race and ethnicity.
Racial and ethnic disparities in ophthalmology represent an even larger gap. Underrepresented minority racial and ethnic groups include Black, Hispanic, Alaska Native, Native American, and Native Hawaiian and other Pacific Island populations. Ophthalmology faculty are less racially and ethnically diverse than graduating medical students; in particular, Hispanic, Native American, and Black faculty are underrepresented relative to each group’s proportion of the general population. Compared with 17 other clinical departments, ophthalmology has the third-lowest proportion of URM faculty (although chair positions were higher).275 In a 17-year follow-up of the National Faculty Survey of academic medicine overall, URM faculty had lower rates of peer-reviewed publications, promotion to professor, and retention.276
Sexual orientation/gender identity.
Limited information is currently available on LGBTQ+ identification among medical students, residents, and faculty physicians in general, and none within ophthalmology literature specifically. Prior research has shown that LGBTQ+ medical students are more likely to experience harassment, threats, and depression277 than non-LGBTQ+ students and are more likely to report mistreatment and burnout.278 In a survey of LGBTQ+ physicians, one-fifth of respondents reported being socially ostracized, and two-thirds reported hearing derogatory comments about LGBTQ+ individuals.279
Disability.
Disability accommodations are required by the Americans with Disabilities Act; however, policies are not always transparent in medical school education or in residency training. In addition, little attention has been focused on disability policies and accommodations for faculty or clinical practitioners.280 Although data are scarce, the prevalence of disability among medical students and professionals is not insignificant, with an estimated prevalence of 4.6%281 among medical students and 3.1% among practicing physicians.282 A higher percentage of physicians with disabilities is estimated to work in medical schools. No data are currently available on the percentage of individuals with disabilities in ophthalmology nor on educational curricula or departmental policies for their inclusion.
Socioeconomic status.
Prior research demonstrated that students from low SES backgrounds have less access to physical resources and are often mistreated by classroom teachers,283 leading them to fall behind academically. Evidence also suggests a general lack of support from teachers and other staff for low-SES students’ pursuit of STEM fields prior to medical school. Other barriers include lack of recognition of differing interests and goals for low-SES students (for example, low-SES students may be motivated by solving issues that affect their environment) and costs to improve opportunities (e.g., materials, enhanced tutoring to assist with exam taking).283 The AAMC found that applicants with a low SES had lower MCAT scores.284 Although no analysis on acceptance rates has been undertaken, a 2018 AAMC report285 demonstrated that 75% of medical school matriculants come from the top two household-income quintiles and that this distribution hasn’t changed in 30 years. Because SES information is not currently tracked beyond undergraduate medical education, little is known about SES and the ophthalmology workforce.
PART II: A FRAMEWORK FOR FUTURE APPROACHES AND RECOMMENDED STEPS TO ELIMINATE DISPARITIES IN EYE CARE
This review highlights important areas of opportunity for the American Academy of Ophthalmology, individual ophthalmologists and practices, our vision health community, and our health partners to act to reduce the impact of eye diseases and visual impairment. To this end, we have developed a framework to help our community to take action and move forward, building on the findings reported in this review and the accompanying in-depth analyses of specific areas highlighted in this report: access, workforce diversity, patient education and health literacy, and data sciences. We welcome the active engagement of our community of ophthalmologists in the United States and around the world, colleagues in optometry, other health care professionals, and our societies, organizations, and companies to further our shared goals.
The accompanying Table reflects steps that individual ophthalmologists, the American Academy of Ophthalmology, colleagues in vision care, and our health community can take to enhance ongoing efforts to redress vision health inequities to reduce health disparities. The recommendations noted below are examples of opportunities listed in the Table.
Recommendations for Reducing Disparities in Eye Health
| Ophthalmologists | AAO | Vision Community | Health Community | |
|---|---|---|---|---|
| Health Disparities | ||||
| Assess population needs | Participate in research and IRIS Registry data entry | Use IRIS Registry as part of vision health surveillance system; help define key outcomes of interest in BIPOC and U.S. populations | CDC, AAO, and partners enhance current CDC surveillance system; work to elucidate more information across all eye diseases | CDC, AAO, and partners incorporate metrics for disparities into existing national datasets; incorporate further vision health indicators into Healthy People and other programs |
| Relate visual parameters to key functions | Inquire about QOL impact of vision issues | Develop educational materials for physicians and staff | Support additional work in assessing impact on health and functioning | Include vision in chronic disease metrics; integrate vision into more population studies |
| Improve vision today by correcting refractive error | Consider venues and means for providing glasses for vulnerable populations; support and implement new ways of caring for refractive error | AAOE and AAO education and templates for enhanced refractive error correction approaches | Partner to advocate for and implement efficient and less costly means of refractive correction; work collectively with optometry | Expand Medicaid and Medicare access to include refraction and glasses; partner with NGOs to provide glasses |
| Assess impact of social determinants of health | Take enhanced social histories with patients; provide options for care; incorporate SDOH in EHR | Work to incorporate information into IRIS Registry if feasible | Support more research across NEI and other funders to intervene on SDOH | Build on relationships and knowledge in other areas; incorporate into quality metrics |
| Access to Quality Care | ||||
| Insurance | Consider expansion of accepted types of insurance including all types of Medicaid | Advocate for coverage with appropriate reimbursement | Advocate for coverage with appropriate reimbursement | Advocate for coverage with appropriate reimbursement; advocate for greater expansion of health insurance (e.g, Medicaid, state-sponsored health insurance for children, ACA) |
| Office hours | Consider expansion; can volunteer or work in FQHCs or NGOs | AAOE assistance on expanded hours / means; templates for FQHC work; DC office to work on FQHC financing and vision care | Develop partnerships with FQHCs, community organizations; expand ehealth offerings | Incorporate vision into health care sites in lower SES and communities of color |
| Travel time | Recognize the impact of distance and transportation on patients and consider these factors in scheduling; consider use of e-health when appropriate | Assist in identifying ophthalmologists with new patient openings; develop templates and action plans for e-health (telemedicine); create partnerships for care; further understand travel times in various areas | Enhance collaboration with partners for care, using variety of models; assess social determinants factors; partner with schools, teachers, and nurses for vision screening in school-based health centers | Incorporate vision services into more and different kinds of sites, e.g., through use of technology-based eye care or expanded e-health and broadband access (telemedicine); ensure equitable access to broadband |
| Return for follow-up | Recognize the importance of having patients feel welcome; become informed about ways to help patients overcome barriers | Provide IRIS Registry - based feedback to physicians; provide educational materials and templates for physicians | Collaborate on alternative means of follow-up as well as encouraging follow-up to physicians | Incorporate vision services into more and different kinds of sites |
| Adherence to therapy | Enhance adherence by using tools to help patients understand its importance to their vision health | Develop educational materials for physicians and patients; advocate for use of coaches and navigators | Collaborate on alternative means of ensuring adherence in lower SES areas and BIPOC groups | Incorporate best practices and integrate across appropriate fields of health care |
| Education | ||||
| Patient/public education | Provide in-office education, using video and other resources; use compelling, culturally appropriate stories and analogies; inform patients about reliable sources of educational materials | Maintain readability of patient education materials at 4th to 6th grade level; incorporate visual aids, picture-based instructions, and videos; assess cultural relevancy; promote research to measure health literacy, identify gaps in eye health knowledge, and evaluate interventions to improve outcomes | Provide public education that is relevant and focused on key areas; target populations at higher risk of ocular disorders and health inequities, including the elderly, people with diabetes, Black, Hispanic, and Native American people, and residents of medically underserved and rural areas | Include vision health messaging in general health |
| Ophthalmology/physician education | Pursue implicit bias and cultural humility training; develop awareness of varied SES backgrounds of patients; work with AUPO and residency programs; understand and embrace cultural shifts | Create methods for implicit bias and cultural humility training; provide information about SES backgrounds and impacts; leverage social media; provide education about language of SDOH, DEI, and disparities | Work to make implicit bias and cultural humility training a uniform standard; leverage social media; endorse evidence-based and approved consensus-based guidelines for eye exams | Educate PCPs on importance of vision screening in children/adults; promote education in ophthalmology at medical schools and in residency training programs for family medicine, internal medicine, and pediatrics |
| Workforce | ||||
| Physician | Mentor students from diverse backgrounds including URM, socioeconomically disadvantaged, LGBTQ+, and those with disabilities | Promote education in ophthalmology at medical schools; continue and enhance MOM program; collect data on workforce diversity; support URM ophthalmologists given “diversity tax” in work activities; embrace diversity opportunities for leadership | Continue and enhance MOM and Rabb-Venable programs; also reach out to lower grade levels; collaborate with AUPO to promote diversity in the applicant and faculty recruitment processes; consider additional mentorship programs including for women, LGBTQ+ students; work collectively with optometry | Work to create access to STEM in elementary school levels; partner with other organizations (e.g., ACS) |
| Staff (including technicians and assistants) | Interview and hire diverse staff; provide implicit bias training for all teammates | Develop educational materials and distribute to schools in lower SES and BIPOC areas | Develop new training approaches to increase staff from lower SES and BIPOC backgrounds | Work with larger community to enhance workforce and diversity of workforce |
| Community partners | Establish relationships with trusted local community sites/partners | Create targeted/tailored educational materials for various communities | Collaborate with other medical specialties with established community partnerships | See above; also work with DMV and other governmental agencies |
| Patient navigators/new classes | Incorporate community health workers/educators into clinical practice to serve as bridge between physician and community | Advocate for insurance reimbursement for community health worker programs | Develop new community-informed approaches to enhancing eye care access and vision health | Develop new integrated programs that communities desire |
| Data Sciences | ||||
| Surveillance system for vision and health | Participate in IRIS Registry and other studies | Enhance IRIS Registry data and continue work with CDC surveillance system (e.g., expand use of zip code and geo analyses; include variables for social deprivation index) | Help develop (with AAO) new measures and support inclusion in datasets; expand EMR use of SDOH variables, including social deprivation index | Integrate into larger surveillance system; include metrics in other systems; include focus on individuals not already in eye care system |
| Learning health system | Use IRIS Registry data and benchmarks to improve care | Fully realize potential of IRIS Registry to continuously improve care; apply AI to enhance insights | Partner with ABO, AUPO, and professional societies to facilitate integrated eye care; leverage AI and technology | Incorporate vision health and vision metrics into larger systems, with a special focus on those not already receiving regular eye care; work with ONC for health data sciences |
| Focus efforts in most needy areas | Work with local organizations to meet needs | Help develop approaches and tools to use in areas of greatest need | Use datasets to identify and target those areas with the most need; support careers of researchers in SDOH, especially those from URM backgrounds | Work to lift the health of communities using data to identify areas of greatest need; support researchers to target initiatives |
| Incorporate more social determinants | Understand and integrate impact of SDOH on patient care and outcomes | Include SDOH measures in datasets and registries | Work to identify impact of SDOH on vision health and outcomes | Integrate SDOH into larger surveillance system; include metrics such as quality of care and environmental factors; incorporate multidisciplinary approaches to examine complex etiologies of ocular trauma, such as air pollution |
Abbreviations: AAO, American Academy of Ophthalmology; AAOE, American Academy of Ophthalmic Executives; ABO, American Board of Ophthalmology; ACA, Affordable Care Act; ACS, American Chemical Society; AI, artificial intelligence; AUPO, Association of University Professors of Ophthalmology; BIPOC, Black, Indigenous, people of color; CDC, Centers for Disease Control; DEI, diversity equity, and inclusion; EHR, electronic health record; FQHC; federally qualified health center; IRIS® Registry, Intelligent Research in Sight Registry; MOM, Minority Ophthalmology Mentoring program; NEI, National Eye Institute; NGO, nongovernmental agency; ONC, Office of the National Coordinator for Health Information Technology; PCP, primary care physician; QOL, quality of life; SDOH, social determinants of health; SES, socioeconomic status; STEM, science, technology, engineering, and mathematics; URM; underrepresented in medicine.
1. IMPROVE ACCESS TO EYE CARE
A. Federally Qualified Health Centers
Federally qualified health centers (FQHCs) are uniquely poised to address the disparities in access to eye and vision care across populations. FQHCs are often the primary or only source of vision care for rural and low-income populations, but currently 70% do not have on-site eye care professionals.212 The disparities in health status that exist regionally and nationally are not found among community health center patients, even after controlling for sociodemographic factors and performing cross-sectional analysis on county-level contextual factors that influence health care utilization.286 Establishing partnerships with FQHCs to provide eye care services may help to decrease access issues in some communities and highlight opportunities for the vision health community to incorporate best practices used in FQHCs into our existing practices and care approaches.
B. Community Context and Resources
Contextual factors include demographic and social composition of communities, collective income and wealth, collective and organizational values, cultural norms, and political perspectives.286 For example, a study on the role of contextual factors showed that Black people living in counties with the highest percentages of Black individuals were more likely to obtain eye care than Black residents of counties with low percentages of Black individuals, even after controlling for individual-level effect, suggesting a synergistic effect of cultural norms.287 These findings suggest that using intensive health promotion efforts aimed at improving awareness and quality of eye care among groups at high risk for diabetes and its complications may be helpful.287 In communities with limited access to eye care, building relationships and partnering with institutions, such as community centers or faith-based organizations, that have an established, trusted community presence may decrease barriers to using eye care services.288
C. Teleophthalmology / E-Health
Expanding teleophthalmology programs in underserved areas may be another approach to increasing access to eye and vision care. In a series of focus groups and interviews with 23 type 2 diabetes patients, 50% percent of the patients reported they were willing to pay for a teleophthalmology visit, and 87% of patients were interested in a teleophthalmology visit if it was recommended by their primary care physician.289
D. Patient Education and Engagement
The importance of patient education and the role it may play in increasing utilization of existing eye and vision care cannot be overestimated, both in bringing in new patients who have not previously sought care and in enhancing follow-up care of those already in the care system. Diabetic self-management education was found to improve regular follow-up for diabetes care, including eye and vision care, in rural patients.201 A systematic review290 of the effectiveness of interventions to promote screening for DR showed that the following interventions were effective: increasing patient and provider awareness of DR, improving access to health care, implementing computer-based registration or reminder systems, fostering collaboration among local organizations that provide retinal screening, and developing a community-based health care system. A reminder was the most frequently used intervention to promote retinal screening, and it was more effective if sent to both physicians and patients.291
E. Insurance for Eye Care
Fundamentally, encouraging policymakers to expand or enhance insurance coverage of vision and eye care services can be an effective means of increasing access to care. Experts in the field have recommended expanding Medicare and Medicaid coverage to include glasses, eye health screening, and refraction in the primary care setting, with optometrists designated as primary care doctors, and increasing online access to glasses in order to reduce cost.216
State-sponsored health insurance for children has been observed to significantly increase the ability to get necessary prescriptions and eyeglasses. For example, the number of children having unmet general medical needs dropped from 20% to 2% after enrollment in North Carolina’s children’s health insurance program.292 New venues and means for providing corrective lenses to vulnerable populations are needed, as well as new ways of caring for refractive error.
2. INCREASE WORKFORCE DIVERSITY
Change requires intentional action. Education on issues such as implicit or unconscious bias, as well as cultural humility, is an important first step, not just for faculty, residents, and students, but also throughout middle and upper leadership in the field. Understanding the sex and gender, race, ethnic, and sexual orientation underrepresentation in ophthalmology and the challenges faced by these groups will help to create safe and inclusive workplace environments all ophthalmologists. More data are needed to find ways to increase the representation of individuals with disabilities in the field of ophthalmology and to enhance understanding and appropriate accommodations.
A. Diversity in Residency Programs
The diversity of students applying for ophthalmology residencies should at the outset reflect that of the pool of available medical students. To accomplish this, we should all mentor medical students from diverse backgrounds, including URM, socioeconomically disadvantaged, LGBTQ+, and those with disabilities. The AAO/AUPO Minority Ophthalmology Mentoring program and the National Medical Association’s Rabb-Venable Excellence in Ophthalmology program are examples of such initiatives. Moreover, we need to focus on defining the attributes that are most important to ophthalmic practice and research. With these attributes at the forefront, we can nurture, mentor, and select the future members of our profession by using a holistic review process, which encourages selection committees to review all of the characteristics that make an applicant unique, rather than relying on test scores or membership in honor societies, such as Alpha Omega Alpha, which in more traditional contexts have been known to maintain a status quo of structural racism.293
Individuals serving on a search or selection committee—or any ophthalmologist who is hiring other eye care providers or staff—should learn about the mechanisms and effects of implicit or unconscious bias on decision-making. This includes recognizing differences in the evaluations and letters of recommendation based on an individual’s gender, race, or ethnicity. Committee members would also benefit from learning about inequities in grading, test-taking, grants awarded, and honors received. In addition, they should become familiar with the concept of cultural humility, understanding and embracing cultural shifts. We also need to ensure that participation in hiring practices is adequately diverse.
B. Diversity in Public Representation and Honors
Diversifying the public face of ophthalmology is critical. If the invited speakers at grand rounds, state and national ophthalmology and research meetings, and corporate speakers bureaus are not diverse, it creates a barrier to attracting diverse students and trainees into our field. We can advocate for societies, leaders in academic and practice ophthalmology, and journal editorial boards to reflect at least the gender, race, and ethnicity of those who are in the field. Our society and profession must become one in which all voices are heard and valued and where all individuals feel they belong.
C. Diversity Among Staff
We must strive to increase diversity and inclusion among staff members as well. Our staff are generally the first team members our patients see; having someone who can better understand and relate to the needs of different patients can help enhance trust, create better communications, and increase the likelihood of adherence to care recommendations. Diversity among staff members brings a wider range of viewpoints and experience that help to facilitate understanding of the needs and challenges of diverse patient populations and better identify solutions for achieving optimal eye care.
3. IMPROVE EYE CARE EDUCATION FOR PATIENTS
More research is necessary on patient preferences and needs in eye care education. Exploring various methods for delivering education, such as video-based media in exam rooms, targeted and tailored educational materials, and use of mobile technology and online social networks, is necessary to increase eye care knowledge among vulnerable populations.
Among the recommendations for best practices for patient education, some are associated with enhancing knowledge and awareness among the public and others on an individual patient basis. For the public, we fully endorse the National Academy of Medicine report8 recommendation on highlighting through government agencies (as well as the American Academy of Ophthalmology and other organizations) the importance and value of vision and how best to preserve vision. This might involve making the information compelling and engaging, by using patient stories and involving family members. Use of social media channels, new technology, and other online resources can be helpful for both public implementation and individual patient education.
On the individual level, optimizing readability of our materials—ideally, with versions available at the third- to sixth-grade reading level—would be useful for our increasingly diverse population. Similarly, developing culturally as well as linguistically appropriate materials is an opportunity for the American Academy of Ophthalmology and other organizations. Personalizing and tailoring messages to specific subpopulations (intended audience) that reflect cultural as well as personal relevance is likely to be a successful approach.294
4. CREATE A CONTINUOUS IMPROVEMENT SYSTEM THROUGH DATA
Large datasets have been used to identify disparities in health care, and they can further help identify health disparities in eye care and aid in finding solutions. Below, we summarize the types of datasets in the United States, along with their strengths and weaknesses.
A. EHR-Based Datasets
The deepest phenotypic data available for ophthalmic outcomes is derived from electronic health record (EHR)-extracted datasets. In these datasets, structured data elements that are routinely entered as part of clinical care are extracted in an automated fashion for quality improvement and clinical research. For example, the American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) is a de-identified dataset collected from the majority of ophthalmic practices in the United States and includes data elements on self-reported race and ethnicity, as well as visual acuity, intraocular pressure, cup-to-disc ratio, in-office procedures, diagnostic codes, and prescription medications.
Other examples of such datasets are the Veterans Affairs (VA) National Patient Care Database, which includes EHR data from VA clinical sites, and the Sight Outcomes Research Collaborative (SOURCE) Ophthalmology Electronic Health Record Data Repository, which includes data from academic medical centers with ophthalmology departments using a common EHR system. These datasets can be harnessed to directly measure ophthalmic outcomes with respect to social determinants of health.
However, although large national EHR databases focused on ophthalmic data are powerful, they often lack the breadth of medical information needed to understand a person’s full health status. National claims datasets like the CMS Medicare Claims Datasets and commercial claims databases are able to show the totality of medical and surgical care obtained by a single individual. In addition, these databases should be updated to provide data on social determinants of health, the beneficiary’s disability status, which can be valuable and otherwise difficult to collect in routine medical datasets, and other social factors, such as social deprivation index. One particularly powerful dataset is the All-Payers Claim Databases, which not only includes commercial and Medicare claims data but also often includes elective, pediatric, and Medicaid data.
B. Other Types of Datasets
Claims- and EHR-based systems provide powerful datasets encompassing individuals who can afford medical care, but they lack information on those without medical insurance or who are not receiving medical care. Thus, disparities measured by using such datasets are biased toward the null hypothesis and may severely underestimate the true social inequities of ophthalmic care. There are a number of alternative data sources that may provide more accurate measures of disparity.
For example, the CDC, in collaboration with the National Opinion Research Center (NORC) at the University of Chicago, created the National Vision and Eye Health Surveillance System (VEHSS). This is a multisourced dataset that includes population-level screening to measure visual impairment in the United States. These datasets are extraordinarily valuable in quantifying the national trends and disparities in visual impairment accurately by including data from individuals who may not be accessing eye care services and thus would not be in EHR datasets; however, they lack more granular data on ophthalmic intervention and conditions.
Other data that are collected under a study protocol can provide valuable clues regarding those who normally do not get medical care. Studies such as the NIH All of Us and the Healthy People 2030 may also provide rich environmental, genetic, epigenetic, and medical data nationwide. These datasets also include specific survey items regarding SDOH that may not be routinely collected in the course of clinical care and therefore may not be reflected in EHR data.
C. Create a Network of Data to Address Disparities
Leveraging data is paramount to our success in addressing health disparities in ophthalmology. High-quality data allow researchers to connect and contextualize the factors that contribute to disparities and provide necessary information for achieving solutions.295 This process requires three key components: access to high-quality data, appropriate guidelines for health disparities metrics, and open data access for researchers interested in addressing health disparities.
Useful, high-quality data must include race and ethnicity as defined by participants according to the census categories, two of the indicators of SES, and appropriate ophthalmic metrics must be used to measure our progress in overcoming health disparities. If these categories are not available, the American Academy of Ophthalmology should engage with database administrators and urge them to implement best practices for improving data collection. Datasets should be inclusive, and EHRs should be expanded to incorporate SDOH variables, including social deprivation index. The Academy should strive to enhance IRIS Registry data and work closely with CDC and other surveillance systems to improve data.
The American Academy of Ophthalmology, in conjunction with the ophthalmology community, needs to define the health inequities that adversely impact disadvantaged populations along with the predictors and relevant ophthalmic data necessary to evaluate health disparities at a population level. This will define a common goal and identify specific metrics to measure progress. Ophthalmology needs to pursue open access to data, along with the associated training necessary for researchers interested in studying health disparities. There are very few health disparities researchers in ophthalmology. Black researchers are more likely to propose health disparities research348; thus, as one of the least diverse medical fields, ophthalmology is at a distinct disadvantage. The American Academy of Ophthalmology should partner with the National Eye Institute to prioritize underrepresented minority researchers and institutions with a record of health disparities research. Augmenting these researchers and institutions by providing access to data and training in advanced big data approaches, such as artificial intelligence and machine learning, will propel ophthalmology forward in addressing disparities.
5. ADDRESS GAPS IN HEALTH DISPARITIES RESEARCH DATA IN OPHTHALMOLOGY
Although this review presents substantial data on disparities in eye care, many important areas still require further exploration. First, even basic data about SDOH factors associated with eye care are lacking or minimal for at least three of the five pillars of SDOH. As such, expanded research into elements of SDOH and their relationship to disparities in eye health and care using common data definitions, metrics, and frameworks will be critical in reducing variation and, thus, disparities in care and vision health outcomes.
Analyses of SDOH will need to focus specific attention on factors associated with race and racism, on both a systemic/structural and an individual patient/provider basis, to fully comprehend and eliminate disparities in eye health and care and ultimately to eliminate inequities in vision health.
This review makes clear that data on vision health are lacking for important populations of our American family. Expanded research is needed into disparities affecting Native Americans, rural Americans, LGBTQ+, and disabled Americans, as well as those with multiple disabilities or conditions. Associated with this is a need to address how multiple factors interact to impact vision health, referred to as understanding the intersectionality of both person-centered and structural SDOH factors.
Research into developing and standardizing definitions, criteria, and patient-reported outcomes for use by our community is needed, keeping in mind the variance that exists across cultures and sociodemographic groups. The principles of community-engaged research are likely to provide useful guidance for performing our work in this area. For example, this approach may have particular importance in better understanding ocular trauma, given its intersection with many SDOH factors.
Developing a greater understanding of factors driving workforce entry and retention is necessary to advance our momentum in creating a broader and more diverse workforce for us to be successful in the future. Part of this work will be to further demonstrate how diversity can improve patient outcomes and vision health.
6. EXPAND OUR VISION AND COLLABORATIONS
A. Opportunities for Enhanced Collaboration
The proposed framework highlights the importance of working as an integrated community to address disparities in vision health and eye care. Within vision care, there are many opportunities to work together with optometry and optometrists on common issues and needs. Although an assessment of the diversity of the optometry workforce is beyond the scope of this review, we recognize that optometrists play a critically vital role in eye care delivery throughout the United States. We will continue to seek a greater understanding of the interplay between ophthalmology, optometry, and other means of care in providing access to quality eye care.
We also anticipate that new approaches to patient outreach and education (e.g., home testing for AMD or educational apps) as well as care delivery processes (e.g., e-health or home refractions) have the potential to transform care and patient engagement and knowledge. Accelerating these systems will at least partially overcome workforce supply challenges that will be exacerbated if we continue to see patients as we do today.
B. Learning From the International Community
For transformative concepts and ideas, we can collaborate closely with our international colleagues. For example, the Aravind Eye Care System and the LV Prasad Eye Institute in India are leaders in high-quality, highly efficient, sustainable eye care in less well-resourced societies. Countries like Singapore, which have strong global representation among its people, can also provide insights into care of a diverse population. Although Singapore and some other countries have developed a data-driven assessment of needs and disparities, the work we are doing in United States can also benefit international partners and countries when we share what we have learned. A dynamic collaborative approach built on data and data sciences and an appreciation for society-specific SDOH has the potential to yield synergistic results to accelerate progress in the United States.
CONCLUSION
As we continue on the journey of addressing disparities in eye care and vision health, it is imperative to know where we currently stand in order to move our field forward in an intentional and meaningful way. This paper reviews existing data on disparities in eye care, highlights opportunities to expand our understanding, and provides a framework and specific suggestions on how we can work together to achieve equity in eye care. To move toward eliminating disparities in eye care, we must improve access to eye care, increase diversity in our workforce, and enhance eye care and health care literacy in individuals and communities, all while leveraging data to improve health outcomes. We also call on our local, state, and national government officials and policymakers, as well as on community and business leaders, to address the systemic issues that drive SDOH and underlie many of the disparities in eye care and vision health. We urge all of our professional organizations to join the American Academy of Ophthalmology and our individual members to actively address disparities in eye care, thereby protecting sight and empowering lives of all of our patients.
Figure 0.
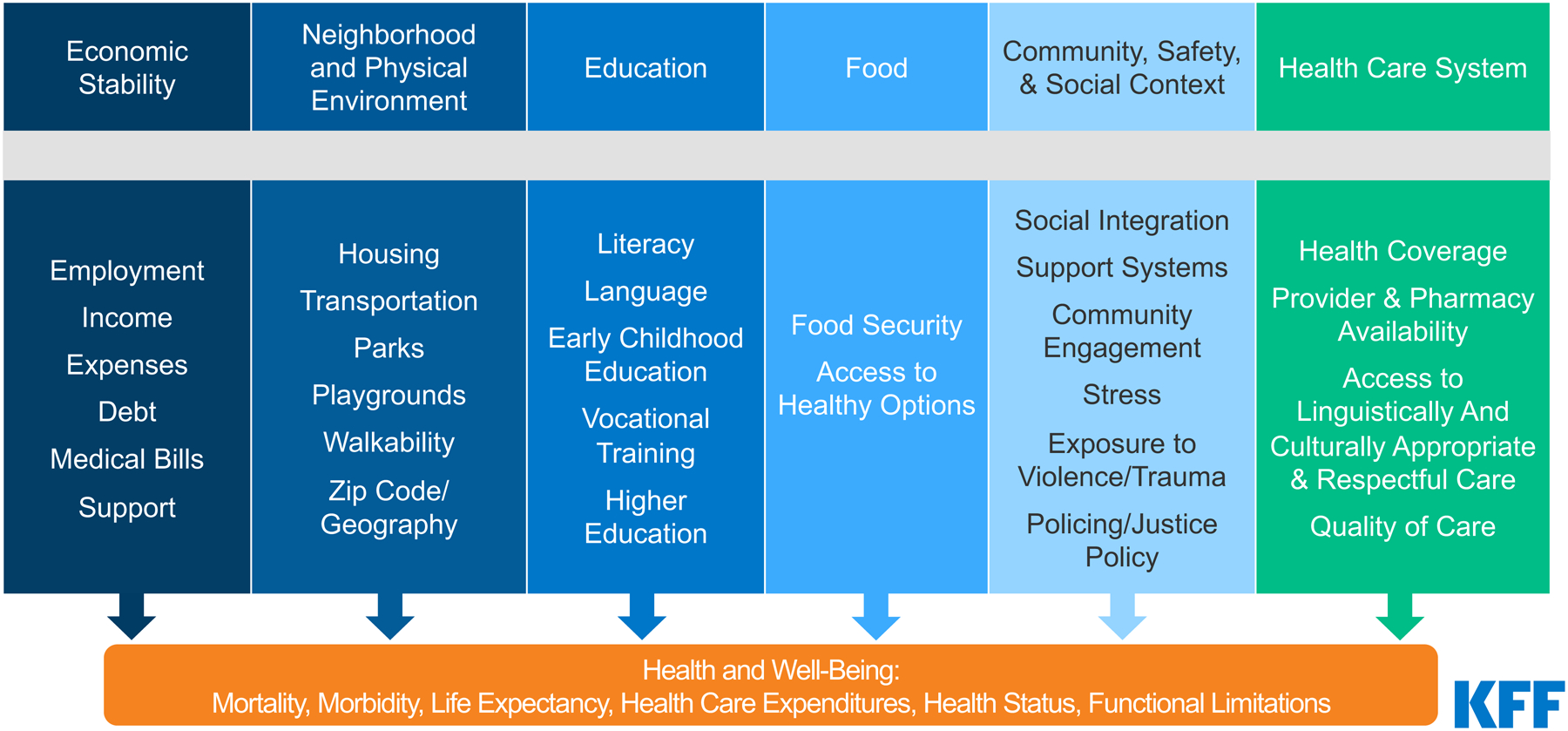
Social Determinants of Health
Example of SDOH: Air Pollution and Its Impact on Eye Disease and Eye Health.
This exploration of the effects of air pollution can serve as an example of the SDOH domain of neighborhood and built environment, as well as structural racism. Environmental exposure to outdoor and indoor air pollution is a leading global concern that can negatively impact health, cause excess mortality, and has been estimated to affect several times more individuals than previously reported.293 Racial and ethnic minoritized people in the United States are disproportionally affected by environmental inequity.294,295 For example, exposure to ambient fine particulate matter (PM2.5) is higher than average for Black, Hispanic, and Asian American individuals and lower than average for White persons based on the communities in which they live; this, in turn, reflects systematic biases in housing practices and patterns.294 Housing practices in the past have been highly influenced by racial segregation and “redlining” in the provision of mortgages by financial institutions, resulting in communities of color being concentrated in historically “less desirable” areas of housing (see “Geographic location”, for more details).
Individuals who reside in areas with higher concentrations of PM2.5 are more likely to have a glaucoma diagnosis and thinner macular ganglion cell inner plexiform layer in a dose-response fashion.296 The toxicity of PM2.5 on intraocular tissues increases oxidative stress and pyroptosis and promotes the development of ocular hypertension and glaucoma.297 Also, in older men, long-term ambient exposure to black carbon can be a risk factor for intraocular pressure (IOP)-related diseases for those susceptible to other biological stressors; IOP was greater in individuals with a high oxidative stress allelic score compared with individuals with a low score.298 In terms of indoor allergens, a study using National Health and Nutrition Examination Survey (NHANES) data found significantly higher odds of sensitization to cockroach and dog antigens among persons with glaucoma compared to those without when controlled for age, ethnicity, and steroid use.299 Contrast sensitivity impairment has been reported from environmental exposures to trichloroethylene,86 cadmium,300 and tobacco smoke.301 Understanding the impact of climate change on health, addressing the impacts of environmental inequity, and reducing pollution among all populations are essential in improving ocular health.
Financial Support:
This work was supported by National Institute for Minority Health and Health Disparities (Bethesda, MD; K23MD016430, ARE), National Institute on Aging/Michigan Center for Urban African American Aging Research (Bethesda, MD; P30AG015281, ARE), and Research to Prevent Blindness (Unrestricted Grant, ARE, VLT, ALC). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pérez-Stable EJ, Alvidrez J, Hill CV. Definitions, principles, and concepts for minority health and health disparities research. The Science of Health Disparities Research. Published online February 12, 2021:1–12. doi: 10.1002/9781119374855.ch1 [DOI] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, et al. The Root Causes of Health Inequity. National Academies Press; (US: ); 2017. [Google Scholar]
- 3.Braveman P, Arkin E, Orleans T, Proctor D, Plough A. What is health equity? And what difference does a definition make? Robert Wood Johnson Foundation. Published online 2017. [Google Scholar]
- 4.Social determinants of health. Accessed April 2, 2022. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
- 5.Hood CM, Gennuso KP, Swain GR, Catlin BB. County Health Rankings: Relationships Between Determinant Factors and Health Outcomes. Am J Prev Med. 2016;50(2):129–135. [DOI] [PubMed] [Google Scholar]
- 6.Vision impairment. Accessed April 2, 2022. http://www.visionproblemsus.org/vision-impairment.html
- 7.Varma R, Vajaranant TS, Burkemper B, et al. Visual impairment and blindness in adults in the United States. JAMA Ophthalmol. 2016;134(7):802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academies of Engineering, Sciences, and Medicine. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington, D.C., National Academies Press, 2016. [PubMed] [Google Scholar]
- 9.Crews JE, Chou CF, Sekar S, Saaddine JB. The Prevalence of Chronic Conditions and Poor Health Among People With and Without Vision Impairment, Aged ≥65 Years, 2010-2014. Am J Ophthalmol. 2017;182:18–30. [DOI] [PubMed] [Google Scholar]
- 10.Varma R, Ying-Lai M, Klein R, Azen SP. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1132–1140. [DOI] [PubMed] [Google Scholar]
- 11.Fisher DE, Shrager S, Shea SJ, et al. Visual Impairment in White, Chinese, Black, and Hispanic Participants from the Multi-Ethnic Study of Atherosclerosis Cohort. Ophthalmic Epidemiol. 2015;22(5):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Congdon N, O’Colmain B, Klaver CCW, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthal. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 13.Deng Z, Fuller-Thomson E. Temporal Trends over a Decade in Serious Vision Impairment in a Large, Nationally Representative Population-based Sample of Older Americans: Gender, Cohort and Racial/Ethnic Differences. Ophthalmic Epidemiol. Published online 2021:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Bath PE. Rationale for a program in community ophthalmology. J Natl Med Assoc. 1979;71(2):145–148. [PMC free article] [PubMed] [Google Scholar]
- 15.Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990. Feb;108(2):286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DS, West SK, Munoz B, et al. Racial variations in causes of vision loss in nursing homes: The Salisbury Eye Evaluation in Nursing Home Groups (SEEING) Study. Arch Ophthal. 2004;122(7):1019–1024. [DOI] [PubMed] [Google Scholar]
- 17.Chan T, Friedman DS, Bradley C, Massof R. Estimates of Incidence and Prevalence of Visual Impairment, Low Vision, and Blindness in the United States. JAMA Ophthalmol. 2018;136(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamedani AG, VanderBeek BL, Willis AW. Blindness and Visual Impairment in the Medicare Population: Disparities and Association with Hip Fracture and Neuropsychiatric Outcomes. Ophthalmic Epidemiol. 2019;26(4):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz B, West SK, Rodriguez J, et al. Blindness, visual impairment and the problem of uncorrected refractive error in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(3):608–614. [PubMed] [Google Scholar]
- 20.Varma R, Chung J, Foong AWP, Torres M, Choudhury F, Azen SP. Four-year incidence and progression of visual impairment in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma R, Kim JS, Burkemper BS, et al. Prevalence and Causes of Visual Impairment and Blindness in Chinese American Adults: The Chinese American Eye Study. JAMA Ophthalmol. 2016;134(7):785–793. [DOI] [PubMed] [Google Scholar]
- 22.Fuller-Thomson E, Brennenstuhl S, Hurd M. Comparison of disability rates among older adults in aggregated and separate Asian American/Pacific Islander subpopulations. Am J Public Health. 2011;101(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goins RT, Pilkerton CS. Comorbidity among older American Indians: the native elder care study. J Cross Cult Gerontol. 2010;25(4):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S, Stagg BC, Ehrlich JR. Disparities in Low-Vision Device Use Among Older US Medicare Recipients. JAMA Ophthalmol. 2018;136(12):1399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherrod CE, Vitale S, Frick KD, Ramulu PY. Association of vision loss and work status in the United States. JAMA Ophthalmol. 2014;132(10):1236–1239. [DOI] [PubMed] [Google Scholar]
- 26.Ma F, Lam BL, Lee DJ, Gómez-Marín O. Uncorrected binocular distance visual impairment in U.S. Hispanic children and adolescents. Ophthalmic Epidemiol. 2001;8(1):57–64. [DOI] [PubMed] [Google Scholar]
- 27.Margines JB, Huang C, Young A, et al. Refractive Errors and Amblyopia Among Children Screened by the UCLA Preschool Vision Program in Los Angeles County. Am J Ophthalmol. 2020;210:78–85. [DOI] [PubMed] [Google Scholar]
- 28.Varma R, Tarczy-Hornoch K, Jiang X. Visual Impairment in Preschool Children in the United States Demographic and Geographic Variations From 2015 to 2060. Published online 2016. doi: 10.1001/jamaophthalmol.2017.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Multi-Ethnic Pediatric Eye Disease Study G. Prevalence and causes of visual impairment in African-American and Hispanic preschool children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2009;116(10):1990–2000 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaxman AD, Wittenborn JS, Robalik T, et al. Prevalence of Visual Acuity Loss or Blindness in the US: A Bayesian Meta-analysis. JAMA Ophthalmol. 2021;139(7):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swenor BK, Guo X, Boland MV, Goldstein JE. Leveraging Electronic Health Records to Identify and Characterize Patients with Low Vision. Ophthalmic Epidemiol. 2019;26(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly E, Wen Q, Haddad D, O’Banion J. Effects of an Aging Population and Racial Demographics on Eye Disease Prevalence: Projections for Georgia Through 2050. Am J Ophthalmol. 2020;210:35–40. [DOI] [PubMed] [Google Scholar]
- 33.Rein DB, Lamuda PA, Wittenborn JS, et al. Vision Impairment and Blindness Prevalence in the United States: Variability of Vision Health Responses across Multiple National Surveys. Ophthalmology. 2021;128(1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews JE, Chou CF, Zhang X, Zack MM, Saaddine JB. Health-related quality of life among people aged ≥65 years with self-reported visual impairment: Findings from the 2006–2010 behavioral risk factor surveillance system. Ophthalmic Epidemiol. 2014;21(5):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaco WA, Crews JE, Nguyen ATH, Arif A. Prevalence of Vision Loss and Associations With Age-Related Eye Diseases Among Nursing Home Residents Aged ≥65 Years. J Am Med Dir Assoc. Published online 2020. doi: 10.1016/j.jamda.2020.08.03610.1016/j.jamda.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Umfress AC, Brantley MA Jr, Brantley MA Jr. Eye Care Disparities and Health-Related Consequences in Elderly Patients with Age-Related Eye Disease. Semin Ophthalmol. 2016;31(4):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35 Suppl 2:ii42–ii45. [DOI] [PubMed] [Google Scholar]
- 38.Simning A, Caprio TV, Li Y, Conwell Y. Near Vision but not Hearing Loss is Associated with Lacking a Usual Source of Health Care. J Aging Health. Published online 2021:8982643211014323–8982643211014323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai KY, Pathipati MP, Blumenkranz MS, et al. Assessment of Eye Disease and Visual Impairment in the Nursing Home Population Using Mobile Health Technology. Ophthalmic Surg Lasers Imaging Retina. 2020;51(5):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller BK, Hejkal T, Potter JF. Barriers to Vision Care for Nursing Home Residents. J Am Med Dir Assoc. 2001;2(1):15–21. [PubMed] [Google Scholar]
- 41.National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Committee on National Statistics; Committee on Measuring Sex, Gender Identity, and Sexual Orientation. Measuring Sex, Gender Identity, and Sexual Orientation. (Becker T, Chin M, Bates N, eds.). National Academies Press; (US: ); 2022. [PubMed] [Google Scholar]
- 42.Magnan S Social Determinants of Health 101 for Health Care: Five Plus Five. NAM Perspectives. 2017;7(10). doi: 10.31478/201710C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. About social determinants of health (SDOH). Published March 10, 2021. Accessed April 2, 2022 at https://www.cdc.gov/socialdeterminants/about.html
- 44.Artiga S, Hinton E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity. Published May 2018. at https://files.kff.org/attachment/issue-brief-beyond-health-care
- 45.Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999–2002 and 2005–2008. JAMA. 2012;308(22):2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tielsch JM, Sommer A, Katz J, Quigley H, Ezrine S. Socioeconomic Status and Visual Impairment Among Urban Americans. Arch Ophthal. Published online 1991. doi: 10.1001/archopht.1991.01080050051027 [DOI] [PubMed] [Google Scholar]
- 47.Kirtland KA, Saaddine JB, Geiss LS, Thompson TJ, Cotch MF, Lee PP. Geographic disparity of severe vision loss - United States, 2009–2013. MMWR Morb Mortal Wkly Rep. 2015;64(19):513–517. [PMC free article] [PubMed] [Google Scholar]
- 48.Greenberg PB, Chen AJ, Wu WC. Sudden Vision Loss and Mortality: The Jackson Heart Study. Ophthalmic Epidemiol. 2016;23(5):285–291. [DOI] [PubMed] [Google Scholar]
- 49.Chetty R, Stepner M, Abraham S, et al. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA. 2016;315(16):1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li YJ, Xirasagar S, Pumkam C, Krishnaswamy M, Bennett CL. Vision Insurance, Eye Care Visits, and Vision Impairment Among Working-Age Adults in the United States. JAMA Ophthalmol. 2013;131(4):499–506. [DOI] [PubMed] [Google Scholar]
- 51.Chou CF, Sherrod CE, Zhang X, et al. Barriers to eye care among people aged 40 years and older with diagnosed diabetes, 2006–2010. Diabetes Care. 2014;37(1):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease C, Prevention. Visual impairment and use of eye-care services and protective eyewear among children--United States, 2002. MMWR Morb Mortal Wkly Rep. 2005;54(17):425–429. [PubMed] [Google Scholar]
- 53.Gibson DM. Use of and disparities in access to adaptive devices among U.S. adults with age-related eye diseases. Prev Med Rep. 2018;12:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saydah SH, Gerzoff RB, Saaddine JB, Zhang X, Cotch MF. Eye Care Among US Adults at High Risk for Vision Loss in the United States in 2002 and 2017. JAMA Ophthalmol. 2020;138(5):479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. The State of Vision, Aging, and Public Health in America. Atlanta: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 56.Chou CF, Barker LE, Crews JE, et al. Disparities in eye care utilization among the United States adults with visual impairment: Findings from the behavioral risk factor surveillance system 2006–2009. Am J Ophthalmol. 2012;154(6 SUPPL.). doi: 10.1016/J.AJO.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 57.Goyal A, Richards C, Patel V, et al. The Vision Detroit Project: Visual Burden, Barriers, and Access to Eye Care in an Urban Setting. Ophthalmic Epidemiol. Published online 2021:1–12. [DOI] [PubMed] [Google Scholar]
- 58.Wisniewski RE. Mortgage redlining (disinvestment): the parameters of federal, state, and municipal regulation. Univ Detroit J Urban Law. 1976;54:367. [Google Scholar]
- 59.Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-Level Redlining and Lending Bias Are Associated with Breast Cancer Mortality in a Large and Diverse Metropolitan Area. Cancer Epidemiol Biomarkers Prev. 2021;30(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiller R, Sperduto RD, Ederer F. Epidemiologic associations with cataract in the 1971–1972 National Health and Nutrition Examination Survey. Am J Epidemiol. 1983;118(2):239–249. [DOI] [PubMed] [Google Scholar]
- 61.Hiller R, Sperduto RD, Ederer F. Epidemiologic associations with nuclear, cortical, and posterior subcapsular cataracts. Am J Epidemiol. 1986;124(6):916–925. [DOI] [PubMed] [Google Scholar]
- 62.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996;103(11):1721–1726. [DOI] [PubMed] [Google Scholar]
- 63.West SK, Munoz B, Schein OD, Duncan DD, Rubin GS. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project. Am J Epidemiol. 1998;148(11):1033–1039. [DOI] [PubMed] [Google Scholar]
- 64.Friedman DS, Munoz B, Roche KB, et al. Poor uptake of cataract surgery in nursing home residents: the Salisbury Eye Evaluation in Nursing Home Groups study. Arch Ophthal. 2005;123(11):1581–1587. [DOI] [PubMed] [Google Scholar]
- 65.Broman AT, Hafiz G, Munoz B, et al. Cataract and barriers to cataract surgery in a US Hispanic population: Proyecto VER. Arch Ophthalmol. 2005;123(9):1231–1236. [DOI] [PubMed] [Google Scholar]
- 66.Richter GM, Chung J, Azen SP, Varma R, Los Angeles Latino Eye Study G. Prevalence of visually significant cataract and factors associated with unmet need for cataract surgery: Los Angeles Latino Eye Study. Ophthalmology. 2009;116(12):2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varma R, Sun J, Torres M, et al. Prevalence of Lens Opacities in Adult Chinese Americans: The Chinese American Eye Study (CHES). Invest Ophthalmol Vis Sci. 2016;57(15):6692–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology. 1992;99(4):546–552. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez J, Sanchez R, Munoz B, et al. Causes of blindness and visual impairment in a population-based sample of U.S. Hispanics. Ophthalmology. 2002;109(4):737–743. [DOI] [PubMed] [Google Scholar]
- 70.Ryskulova A, Turczyn K, Makuc DM, Cotch MF, Klein RJ, Janiszewski R. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 national health interview survey. Am J Public Health. 2008;98(3):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein BE, Klein R, Lee KE, Meuer SM. Socioeconomic and lifestyle factors and the 10-year incidence of age-related cataracts. Am J Ophthalmol. 2003;136(3):506–512. [DOI] [PubMed] [Google Scholar]
- 72.Klein R, Klein BE, Jensen SC, Moss SE. The relation of socioeconomic factors to the incidence of early age-related maculopathy: the Beaver Dam eye study. Am J Ophthalmol. 2001;132(1):128–131. [DOI] [PubMed] [Google Scholar]
- 73.Klein R, Klein BE, Jensen SC, Moss SE, Cruickshanks KJ. The relation of socioeconomic factors to age-related cataract, maculopathy, and impaired vision. The Beaver Dam Eye Study. Ophthalmology. 1994;101(12):1969–1979. [DOI] [PubMed] [Google Scholar]
- 74.Bochow TW, West SK, Azar A, Munoz B, Sommer A, Taylor HR. Ultraviolet light exposure and risk of posterior subcapsular cataracts. Arch Ophthalmol. 1989;107(3):369–372. [DOI] [PubMed] [Google Scholar]
- 75.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. 1977;106(1):33–41. [DOI] [PubMed] [Google Scholar]
- 76.Leske MC, Chylack LT Jr, Wu SY. The Lens Opacities Case-Control Study. Risk factors for cataract. Arch Ophthalmol. 1991;109(2):244–251. [DOI] [PubMed] [Google Scholar]
- 77.Javitt JC, Kendix M, Tielsch JM, et al. Geographic variation in utilization of cataract surgery. Med Care. 1995;33(1):90–105. [DOI] [PubMed] [Google Scholar]
- 78.Kauh CY, Blachley TS, Lichter PR, et al. Geographic Variation in the Rate and Timing of Cataract Surgery Among US Communities. JAMA Ophthalmol. 2016;134(3):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu M, Wang SY, Singh K, Lin SC. Racial disparities in uncorrected and undercorrected refractive error in the United States. Invest Ophthalmol Vis Sci. 2014;55(10):6996–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schein OD, Cassard SD, Tielsch JM, et al. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shahbazi S, Studnicki J, Warner-Hillard CW. A Cross-Sectional Retrospective Analysis of the Racial and Geographic Variations in Cataract Surgery. PLoS One. 2015;10(11):e0142459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharkness CM, Hamburger S, Kaczmarek RG, et al. Racial differences in the prevalence of intraocular lens implants in the United States. Am J Ophthalmol. 1992;114(6):667–674. [DOI] [PubMed] [Google Scholar]
- 83.Wang F, Javitt JC, Tielsch JM. Racial variations in treatment for glaucoma and cataract among Medicare recipients. Ophthalmic Epidemiol. 1997;4(2):89–100. [DOI] [PubMed] [Google Scholar]
- 84.Williams A, Sloan FA, Lee PP. Longitudinal rates of cataract surgery. Arch Ophthalmol. 2006;124(9):1308–1314. [DOI] [PubMed] [Google Scholar]
- 85.Wu AM, Wu CM, Tseng VL, et al. Characteristics Associated With Receiving Cataract Surgery in the US Medicare and Veterans Health Administration Populations. JAMA Ophthalmol. 2018;136(7):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Cotch MF, Ryskulova A, et al. Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. Am J Ophthalmol. 2012;154(6 Suppl):S53–S62 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone JS, Fukuoka H, Weinreb RN, Afshari NA. Relationship Between Race, Insurance Coverage, and Visual Acuity at the Time of Cataract Surgery. Eye Contact Lens. 2018;44(6):393–398. [DOI] [PubMed] [Google Scholar]
- 88.Lee CS, Su GL, Baughman DM, et al. Disparities in delivery of ophthalmic care; An exploration of public Medicare data. PLoS One. 2017;12(8):e0182598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McElroy JA, Klein BE, Lee KE, et al. Place-based exposure and cataract risk in the Beaver Dam cohort. J Environ Health. 2014;76(6):34–40. [PMC free article] [PubMed] [Google Scholar]
- 90.Mahr MA, Hodge DO, Erie JC. Racial/ethnic differences in rates of complex cataract surgery among United States Medicare beneficiaries. J Cataract Refract Surg. 2018;44(2):140–143. [DOI] [PubMed] [Google Scholar]
- 91.Reddy AK, Patnaik JL, Miller DC, et al. Risk Factors Associated with Persistent Anterior Uveitis after Cataract Surgery. Am J Ophthalmol. 2019;206:82–86. [DOI] [PubMed] [Google Scholar]
- 92.Wu TT, Amini L, Leffler CT, et al. Cataracts and cataract surgery in mentally retarded adults. Eye Contact Lens. 2005;31(2):50–53. [DOI] [PubMed] [Google Scholar]
- 93.Keay L, Gower EW, Cassard SD, et al. Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology. 2012;119(5):914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.West ES, Behrens A, McDonnell PJ, et al. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112(8):1388–1394. [DOI] [PubMed] [Google Scholar]
- 95.French DD, Margo CE, Campbell RR. Comparison of complication rates in veterans receiving cataract surgery through the Veterans Health Administration and Medicare. Med Care. 2012;50(7):620–626. [DOI] [PubMed] [Google Scholar]
- 96.Zambelli-Weiner A, Crews JE, Friedman DS. Disparities in adult vision health in the United States. Am J Ophthalmol. 2012;154(6 Suppl):S23–S30.e1. [DOI] [PubMed] [Google Scholar]
- 97.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5–ORSF13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang X, Beckles GL, Chou CF, et al. Socioeconomic disparity in use of eye care services among US adults with age-related eye diseases: National Health Interview Survey, 2002 and 2008. JAMA Ophthalmol. 2013;131(9):1198–1206. [DOI] [PubMed] [Google Scholar]
- 99.Norris KL, Beckles GL, Chou CF, et al. Association of Socioeconomic Status with Eye Health Among Women With and Without Diabetes. J Womens Health. 2016;25(3):321–326. [DOI] [PubMed] [Google Scholar]
- 100.Varma R, Mohanty SA, Deneen J, et al. Burden and predictors of undetected eye disease in Mexican-Americans: the Los Angeles Latino Eye Study. Med Care. 2008;46(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu J, Yu X, Ping X, et al. Socioeconomic disparities in the global burden of glaucoma: an analysis of trends from 1990 to 2016. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):587–594. [DOI] [PubMed] [Google Scholar]
- 102.Wang W, He M, Li Z, et al. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019;97(3):e349–e355. [DOI] [PubMed] [Google Scholar]
- 103.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 104.Friedman DS, Jampel HD, Muñoz B, et al. The Prevalence of Open-angle Glaucoma Among Blacks and Whites 73 Years and Older: The Salisbury Eye Evaluation Glaucoma Study. Arch Ophthal. 2006;124(11):1625–1630. [DOI] [PubMed] [Google Scholar]
- 105.Vajaranant TS, Wu S, Torres M, et al. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol. 2012;154(2):303–314 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sales CS, Lee RY, Agadzi AK, Hee MR, Singh K, Lin SC. Open-angle glaucoma in Filipino and white Americans: a comparative study. J Glaucoma. 2014;23(4):246–253. [DOI] [PubMed] [Google Scholar]
- 107.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118(6):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gracitelli CPB, Zangwill LM, Diniz-Filho A, et al. Detection of Glaucoma Progression in Individuals of African Descent Compared With Those of European Descent. JAMA Ophthalmol. 2018;136(4):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bowd C, Zangwill LM, Weinreb RN, et al. Racial Differences in Rate of Change of Spectral-Domain Optical Coherence Tomography-Measured Minimum Rim Width and Retinal Nerve Fiber Layer Thickness. Am J Ophthalmol. 2018;196:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Girkin CA, Fazio MA, Bowd C, et al. Racial Differences in the Association of Anterior Lamina Cribrosa Surface Depth and Glaucoma Severity in the African Descent and Glaucoma Evaluation Study (ADAGES). Invest Ophthalmol Vis Sci. 2019;60(13):4496–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rhodes LA, Huisingh CE, Quinn AE, et al. Comparison of Bruch’s Membrane Opening Minimum Rim Width Among Those With Normal Ocular Health by Race. Am J Ophthalmol. 2017;174:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skaat A, De Moraes CG, Bowd C, et al. African Descent and Glaucoma Evaluation Study (ADAGES): Racial Differences in Optic Disc Hemorrhage and Beta-Zone Parapapillary Atrophy. Ophthalmology. 2016;123(7):1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tamimi EA, Pyne JD, Muli DK, et al. Racioethnic Differences in Human Posterior Scleral and Optic Nerve Stump Deformation. Invest Ophthalmol Vis Sci. 2017;58(10):4235–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen RI, Barbosa DT, Hsu CH, Porco TC, Lin SC. Ethnic differences in trabecular meshwork height by optical coherence tomography. JAMA Ophthalmol. 2015;133(4):437–441. [DOI] [PubMed] [Google Scholar]
- 115.Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma. 2014;23(9):606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aghaian E, Choe JE, Lin S, et al. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004;111(12):2211–2219. [DOI] [PubMed] [Google Scholar]
- 117.Lee RY, Huang G, Cui QN, et al. Association of lens vault with narrow angles among different ethnic groups. Curr Eye Res. 2012;37(6):486–491. [DOI] [PubMed] [Google Scholar]
- 118.Lee RY, Kao AA, Kasuga T, et al. Ethnic variation in optic disc size by fundus photography. Curr Eye Res. 2013;38(11):1142–1147. [DOI] [PubMed] [Google Scholar]
- 119.Lee RY, Huang G, Porco TC, et al. Differences in iris thickness among African Americans, Caucasian Americans, Hispanic Americans, Chinese Americans, and Filipino-Americans. J Glaucoma. 2013;22(9):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seider MI, Lee RY, Wang D, et al. Optic disk size variability between African, Asian, white, Hispanic, and Filipino Americans using Heidelberg retinal tomography. J Glaucoma. 2009;18(8):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haseltine SJ, Pae J, Ehrlich JR, et al. Variation in corneal hysteresis and central corneal thickness among black, hispanic and white subjects. Acta Ophthalmol. 2012;90(8):e626–e631. [DOI] [PubMed] [Google Scholar]
- 122.Devgan U, Yu F, Kim E, et al. Surgical undertreatment of glaucoma in black beneficiaries of medicare. Arch Ophthalmol. 2000;118(2):253–256. [DOI] [PubMed] [Google Scholar]
- 123.Khachatryan N, Pistilli M, Maguire MG, et al. Primary Open-Angle African American Glaucoma Genetics (POAAGG) Study: gender and risk of POAG in African Americans. PLoS One. 2019;14(8):e0218804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tehrani S Gender difference in the pathophysiology and treatment of glaucoma. Curr Eye Res. 2015;40(2):191–200. [DOI] [PubMed] [Google Scholar]
- 125.Vajaranant TS, Nayak S, Wilensky JT, et al. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010;21(2):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cassard SD, Quigley HA, Gower EW, et al. Regional variations and trends in the prevalence of diagnosed glaucoma in the Medicare population. Ophthalmology. 2012;119(7):1342–1351. [DOI] [PubMed] [Google Scholar]
- 127.Murakami Y, Lee BW, Duncan M, et al. Racial and ethnic disparities in adherence to glaucoma follow-up visits in a county hospital population. Arch Ophthalmol. 2011;129(7):872–878. [DOI] [PubMed] [Google Scholar]
- 128.Rothman AL, Stoler JB, Vu DM, et al. A Geodemographic Service Coverage Analysis of Travel Time to Glaucoma Specialists in Florida. J Glaucoma. 2020;29(12):1147–1151. [DOI] [PubMed] [Google Scholar]
- 129.Zhao D, Guallar E, Bowie JV, et al. Improving Follow-up and Reducing Barriers for Eye Screenings in Communities: The SToP Glaucoma Study. Am J Ophthalmol. Published online 2018. doi: 10.1016/j.ajo.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 130.Zheng CX, Hu WD, Tran J, et al. Barriers to Receiving Follow-Up Eye Care and Detection of Non-Glaucomatous Ocular Pathology in the Philadelphia Glaucoma Detection and Treatment Project. J Community Health. 2016;41(2):359–367. [DOI] [PubMed] [Google Scholar]
- 131.Lee BW, Murakami Y, Duncan MT, et al. Patient-related and system-related barriers to glaucoma follow-up in a county hospital population. Invest Ophthalmol Vis Sci. 2013;54(10):6542–6548. [DOI] [PubMed] [Google Scholar]
- 132.Elam AR, Andrews C, Musch DC, et al. Large Disparities in Receipt of Glaucoma Care between Enrollees in Medicaid and Those with Commercial Health Insurance. Ophthalmology. 2017;124(10):1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stein JD, Talwar N, Laverne AM, et al. Racial disparities in the use of ancillary testing to evaluate individuals with open-angle glaucoma. Arch Ophthal. 2012;130(12):1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elam AR, Blachley TS, Stein JD. Geographic Variation in the Use of Diagnostic Testing of Patients with Newly Diagnosed Open-Angle Glaucoma. Ophthalmology. 2016;123(3):522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Juzych MS, Randhawa S, Shukairy A, et al. Functional health literacy in patients with glaucoma in urban settings. Arch Ophthalmol. 2008;126(5):718–724. [DOI] [PubMed] [Google Scholar]
- 136.Blumberg DM, Prager AJ, Liebmann JM. Variation in Prescription Drug Coverage Enrollment Among Vulnerable Beneficiaries With Glaucoma Before and After the Implementation of Medicare Part D. JAMA Ophthalmol. 2016;134(2):212–220. [DOI] [PubMed] [Google Scholar]
- 137.Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122(7):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ostermann J, Sloan FA, Herndon L, et al. Racial differences in glaucoma care: the longitudinal pattern of care. Arch Ophthalmol. 2005;123(12):1693–1698. [DOI] [PubMed] [Google Scholar]
- 139.Javitt JC, McBean AM, Nicholson GA, et al. Undertreatment of glaucoma among black Americans. N Engl J Med. 1991;325(20):1418–1422. [DOI] [PubMed] [Google Scholar]
- 140.Ederer F, Gaasterland DA, Dally LG, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004;111(4):651–664. [DOI] [PubMed] [Google Scholar]
- 141.Ishida K, Netland PA. Ahmed Glaucoma Valve implantation in African American and white patients. Arch Ophthalmol. 2006;124(6):800–806. [DOI] [PubMed] [Google Scholar]
- 142.Edmiston AM, SooHoo JR, Seibold LK, et al. Postoperative Inflammation After Endoscopic Cyclophotocoagulation: Racial Distribution and Effect on Outcomes. J Glaucoma. 2018;27(3):266–268. [DOI] [PubMed] [Google Scholar]
- 143.Taubenslag KJ, Kammer JA. Outcomes Disparities between Black and White Populations in the Surgical Management of Glaucoma. Semin Ophthalmol. 2016;31(4):385–393. [DOI] [PubMed] [Google Scholar]
- 144.Laroche D, Nkrumah G, Ng C. Real-World Retrospective Consecutive Study of Ab Interno XEN 45 Gel Stent Implant with Mitomycin C in Black and Afro-Latino Patients with Glaucoma: 40% Required Secondary Glaucoma Surgery at 1 Year. Middle East Afr J Ophthalmol. 2019;26(4):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laroche D, Nkrumah G, Ng C. Real-world efficacy of the Hydrus microstent in Black and Afro-Latinx patients with glaucoma: a retrospective study. Ther Adv Ophthalmol. 2020;12:2515841420964311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Laroche D, Nkrumah G, Ugoh P, et al. Real World Outcomes of Kahook Dual Blade Goniotomy in Black and Afro-Latinx Adult Patients with Glaucoma: A 6-Month Retrospective Study. J Natl Med Assoc. 2021;113(2):230–236. [DOI] [PubMed] [Google Scholar]
- 147.Schultz NM, Wong WB, Coleman AL, et al. Predictors, Resource Utilization, and Short-term Costs of Laser Trabeculoplasty Versus Medication Management in Open-Angle Glaucoma. Am J Ophthalmol. 2016;168:78–85. [DOI] [PubMed] [Google Scholar]
- 148.Lee AY, Lee CS, Pieters M, et al. Differences in Tertiary Glaucoma Care in the Veterans Affairs Health Care System. JAMA Ophthalmol. 2018;136(11):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Funk IT, Strelow BA, Klifto MR, et al. The Relationship of Travel Distance to Postoperative Follow-up Care on Glaucoma Surgery Outcomes. J Glaucoma. 2020;29(11):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fu Z, Hong H, Su Z, et al. Global prevalence of amblyopia and disease burden projections through 2040: a systematic review and meta-analysis. Br J Ophthalmol. 2020;104(8):1164–1170. [DOI] [PubMed] [Google Scholar]
- 151.American Optometric Association. Evidence-Based Clinical Practice Guideline: Comprehensive Pediatric Eye and Vision Examination. doi: 10.37685/uiwlibraries.2575-7717.2.2.1007 [DOI] [Google Scholar]
- 152.Holmes JM, Lazar EL, Melia BM, et al. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129(11):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holmes JM, Levi DM. Treatment of amblyopia as a function of age. Vis Neurosci. 2018;35:E015. [DOI] [PubMed] [Google Scholar]
- 154.Wen G, McKean-Cowdin R, Varma R, et al. General health-related quality of life in preschool children with strabismus or amblyopia. Ophthalmology. 2011;118(3):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grossman DC, Curry SJ, Owens DK, et al. Vision Screening in Children Aged 6 Months to 5 Years: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318(9):836–844. [DOI] [PubMed] [Google Scholar]
- 156.Tarczy-Hornoch K, Cotter SA, Borchert M, et al. Prevalence and causes of visual impairment in Asian and non-Hispanic white preschool children: Multi-ethnic Pediatric Eye Disease Study. Ophthalmology. 2013;120(6):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months the multi-ethnic pediatric eye disease study. Ophthalmology. 2008;115(7):1229–1236 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128–2134 e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, et al. Prevalence of amblyopia or strabismus in asian and non-Hispanic white preschool children: multi-ethnic pediatric eye disease study. Ophthalmology. 2013;120(10):2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kleinstein RN, Sinnott LT, Jones-Jordan LA, et al. New cases of myopia in children. Arch Ophthalmol. 2012;130(10):1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Borchert MS, Varma R, Cotter SA, et al. Risk factors for hyperopia and myopia in preschool children the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118(10):1966–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ying GS, Maguire MG, Cyert LA, et al. Prevalence of vision disorders by racial and ethnic group among children participating in head start. Ophthalmology. 2014;121(3):630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of myopia and hyperopia in 6- to 72-month-old african american and Hispanic children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2010;117(1):140–147 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fozailoff A, Tarczy-Hornoch K, Cotter S, et al. Prevalence of astigmatism in 6- to 72-month-old African American and Hispanic children: the Multi-ethnic Pediatric Eye Disease Study. Ophthalmology. 2011;118(2):284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kodjebacheva G, Brown ER, Estrada L, Yu F, Coleman AL. Uncorrected refractive error among first-grade students of different racial/ethnic groups in southern California: results a year after school-mandated vision screening. J Public Health Manag Pract. 2011;17(6):499–505. [DOI] [PubMed] [Google Scholar]
- 166.USEIR. US Eye Injury Registry. Accessed April 4, 2022 at https://useir.org
- 167.Iftikhar M, Junaid N, Lemus M, et al. Epidemiology of Primary Ophthalmic Inpatient Admissions in the United States. Am J Ophthalmol. 2018;185:101–109. [DOI] [PubMed] [Google Scholar]
- 168.Gise R, Truong T, Parsikia A, et al. Visual Pathway Injuries in Pediatric Ocular Trauma-A Survey of the National Trauma Data Bank From 2008 to 2014. Pediatr Neurol. 2018;85:43–50. [DOI] [PubMed] [Google Scholar]
- 169.Lee R, Fredrick D. Pediatric eye injuries due to nonpowder guns in the United States, 2002–2012. J AAPOS. 2015;19(2):163–168 e1. [DOI] [PubMed] [Google Scholar]
- 170.Weiss R, He C, Gise R, et al. Patterns of Pediatric Firearm-Related Ocular Trauma in the United States. JAMA Ophthalmol. Published online 2019. doi: 10.1001/jamaophthalmol.2019.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McGwin G Jr, Xie A, Owsley C. Rate of eye injury in the United States. Arch Ophthalmol. 2005;123(7):970–976. [DOI] [PubMed] [Google Scholar]
- 172.Kuhn F, Maisiak R, Mann L, et al. The Ocular Trauma Score (OTS). Ophthalmol Clin North Am. 2002;15(2):163–165, vi. [DOI] [PubMed] [Google Scholar]
- 173.Kuhn F, Morris R, Witherspoon CD, et al. Epidemiology of blinding trauma in the United States Eye Injury Registry. Ophthalmic Epidemiol. 2006;13(3):209–216. [DOI] [PubMed] [Google Scholar]
- 174.Katz J, Tielsch JM. Lifetime prevalence of ocular injuries from the Baltimore Eye Survey. Arch Ophthalmol. 1993;111(11):1564–1568. [DOI] [PubMed] [Google Scholar]
- 175.Savar A, Andreoli MT, Kloek CE, et al. Enucleation for open globe injury. Am J Ophthalmol. 2009;147(4):595–600 e1. [DOI] [PubMed] [Google Scholar]
- 176.Chen G, Sinclair SA, Smith GA, et al. Hospitalized ocular injuries among persons with low socioeconomic status: a medicaid enrollees-based study. Ophthalmic Epidemiol. 2006;13(3):199–207. [DOI] [PubMed] [Google Scholar]
- 177.Siddiqui N, Chen EM, Parikh R, et al. Epidemiology of United States Inpatient Open Globe Injuries from 2009–2015. Ophthalmic Epidemiol. Published online 2021:1–10. [DOI] [PubMed] [Google Scholar]
- 178.Fu R, Kancherla S, Eller AW, et al. Characteristics and Outcomes of Open Globe Trauma in the Urban versus Rural Population: A Single Center Retrospective Review. Semin Ophthalmol. 2018;33(4):566–570. [DOI] [PubMed] [Google Scholar]
- 179.Gauthier AC, Oduyale OK, Fliotsos MJ, et al. Clinical Characteristics and Outcomes in Patients Undergoing Primary or Secondary Enucleation or Evisceration After Ocular Trauma. Clin Ophthalmol. 2020;14:3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rk H Ocular injuries from violence treated at an inner-city hospital. J Trauma. 1994;37(1):5–8. [DOI] [PubMed] [Google Scholar]
- 181.Truong T, Poulsen DM, Parsikia A, et al. Firearm-associated ocular injuries: analysis of national trauma data. Arquivos Brasileiros de Oftlmologia. Published online 2020. doi: 10.5935/0004-2749.20210006 [DOI] [PubMed] [Google Scholar]
- 182.Colantonio A, Ratcliff G, Chase S, et al. Long-term outcomes after moderate to severe traumatic brain injury. Disabil Rehabil. 2004;26(5):253–261. [DOI] [PubMed] [Google Scholar]
- 183.Vangel SJ Jr, Rapport LJ, Hanks RA. Effects of family and caregiver psychosocial functioning on outcomes in persons with traumatic brain injury. J Head Trauma Rehabil. 2011;26(1):20–29. [DOI] [PubMed] [Google Scholar]
- 184.Chopra N, Gervasio KA, Kalosza B, et al. Gun trauma and ophthalmic outcomes. Eye. 2018;32(4):687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–540. [DOI] [PubMed] [Google Scholar]
- 186.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. [DOI] [PubMed] [Google Scholar]
- 187.Clemons TE, Milton RC, Klein R, et al. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–380. [DOI] [PubMed] [Google Scholar]
- 189.Frank RN, Puklin JE, Stock C, et al. Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109–115; discussion 115–117. [PMC free article] [PubMed] [Google Scholar]
- 190.Seddon JM, Cote J, Davis N, et al. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121(6):785–792. [DOI] [PubMed] [Google Scholar]
- 191.Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vanderbeek BL, Zacks DN, Talwar N, et al. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011;152(2):273–282 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Patel SN, Wu C, Obeid A, et al. Sociodemographic Factors in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127(2):280–282. [DOI] [PubMed] [Google Scholar]
- 194.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Elam AR, Lee PP. High-Risk Populations for Vision Loss and Eye Care Underutilization: A Review of the Literature and Ideas on Moving Forward. Surv Ophthalmol. 2013;58(4). doi: 10.1016/j.survophthal.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 196.Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563. [DOI] [PubMed] [Google Scholar]
- 197.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105(6):998–1003. [DOI] [PubMed] [Google Scholar]
- 198.Nsiah-Kumi P, Ortmeier SR, Brown AE. Disparities in diabetic retinopathy screening and disease for racial and ethnic minority populations--a literature review. J Natl Med Assoc. 2009;101(5):430–437. [DOI] [PubMed] [Google Scholar]
- 199.Harris MI, Klein R, Cowie CC, et al. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care. 1998;21(8):1230–1235. [DOI] [PubMed] [Google Scholar]
- 200.Malhotra NA, Greenlee TE, Iyer AI, et al. Racial, Ethnic, and Insurance-Based Disparities Upon Initiation of Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema in the US. Ophthalmology. Published online 2021. doi: 10.1016/j.ophtha.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 201.Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes: disparities across rural America. J Community Health. 2010;35(4):365–374. [DOI] [PubMed] [Google Scholar]
- 202.Signorello LB, Schlundt DG, Cohen SS, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Merrill PT, Kim J, Cox TA, et al. Uveitis in the southeastern United States. Curr Eye Res. 1997;16(9):865–874. [DOI] [PubMed] [Google Scholar]
- 204.Chauhan K, Scaife S, Rosenbaum JT. Uveitis and health disparities: results from the National Inpatient Sample. Br J Ophthalmol. 2019;103(9):1301–1305. [DOI] [PubMed] [Google Scholar]
- 205.Sen HN, Davis J, Ucar D, et al. Gender disparities in ocular inflammatory disorders. Curr Eye Res. 2015;40(2):146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Y, Amin S, Lung KI, et al. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: A claims-based analysis. PLoS One. 2020;15(8):e0237995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Woodward MA, Blachley TS, Stein JD. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus: An Analysis of a Nationwide Heath Care Claims Database. Ophthalmology. 2016;123(3):457–465 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fink BA, Wagner H, Steger-May K, et al. Differences in keratoconus as a function of gender. Am J Ophthalmol. 2005;140(3):459–468. [DOI] [PubMed] [Google Scholar]
- 209.Sarezky D, Orlin SE, Pan W, et al. Trends in Corneal Transplantation in Keratoconus. Cornea. 2017;36(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shepard DS, Razavi M, Stason WB, et al. Economic appraisal of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2009;148(6):860–868.e2. [DOI] [PubMed] [Google Scholar]
- 211.Rasendran C, Tye G, Knusel K, et al. Demographic and Socioeconomic Differences in Outpatient Ophthalmology Utilization in the United States. Am J Ophthalmol. 2020;218:156–163. [DOI] [PubMed] [Google Scholar]
- 212.Shin P, Finnegan B. Assessing the need for on-site eye care professionals in community health centers. Policy Brief George Wash Univ Cent Health Serv Res Policy. Published online 2009:1–23. [PubMed] [Google Scholar]
- 213.Centers for Disease Control and Prevention. Social determinants of health, health equity, and vision loss. Published June 15, 2021. Accessed April 2, 2022. https://www.cdc.gov/visionhealth/determinants/index.html
- 214.French DD, Wang A, Prager AJ, et al. Association of the Robert Wood Johnson Foundations’ Social Determinants of Health and Medicare Ocular Hospitalizations: A Cross Sectional Data Analysis. Ophthalmology and therapy. 2019;8(4):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.National Eye Health Education Program, National Eye Institute, Lions Club International Foundation. 2005 Survey of Public Knowledge, Attitudes, and Practices Related to Eye Health and Disease: Executive Summary. March 2008.
- 216.Killeen OJ, Cho J, Newman-Casey PA, Kana L, Woodward MA. Barriers and Facilitators to Obtaining Eyeglasses for Vulnerable Patients in a Michigan Free Clinic. Optom Vis Sci. 2021;98(3):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Piyasena MMPN, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One. 2019;14(4):e0198979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hall AG. Medicaid’s impact on access to and utilization of health care services among racial and ethnic minority children. J Urban Health. 1998;75(4):677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thomas CG, Channa R, Prichett L, et al. Racial/Ethnic Disparities and Barriers to Diabetic Retinopathy Screening in Youths. JAMA Ophthalmol. 2021;139(7):791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee DJ, Kumar N, Feuer WJ, et al. Dilated eye examination screening guideline compliance among patients with diabetes without a diabetic retinopathy diagnosis: the role of geographic access. BMJ Open Diabetes Research & Care. 2014;2(1):e000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hark LA, Radakrishnan A, Madhava M, et al. Awareness of ocular diagnosis, transportation means, and barriers to ophthalmology follow-up in the Philadelphia Telemedicine Glaucoma Detection and Follow-up Study. Soc Work Health Care. 2019;58(7):651–664. [DOI] [PubMed] [Google Scholar]
- 222.Gwira JA, Vistamehr S, Shelsta H, et al. Factors Associated with Failure to Follow Up after Glaucoma Screening: A Study in an African American Population. Ophthalmology. 2006;113(8):1315–1319.e1. [DOI] [PubMed] [Google Scholar]
- 223.Frazier M, Garces I, Scarinci I, Marsh-Tootle W. Seeking eye care for children: perceptions among Hispanic immigrant parents. J Immigr Minor Health. 2009;11(3):215–221. [DOI] [PubMed] [Google Scholar]
- 224.Stein JD, Andrews C, Musch DC, et al. Sight-Threatening Ocular Diseases Remain Underdiagnosed Among Children Of Less Affluent Families. Health Aff. 2016;35(8):1359–1366. [DOI] [PubMed] [Google Scholar]
- 225.Ehrlich JR, Anthopolos R, Tootoo J, et al. Assessing Geographic Variation in Strabismus Diagnosis among Children Enrolled in Medicaid. Ophthalmology. 2016;123(9):2013–2022. [DOI] [PubMed] [Google Scholar]
- 226.Lee H, Porell FW. The Effect of the Affordable Care Act Medicaid Expansion on Disparities in Access to Care and Health Status. Med Care Res Rev. 2020;77(5):461–473. [DOI] [PubMed] [Google Scholar]
- 227.Lee B Reducing Disparities in Pediatric Vision Care Utilization by Strategically Addressing Access. JAMA Ophthalmol. 2019;137(2):127–128. [DOI] [PubMed] [Google Scholar]
- 228.Lipton BJ. Association Between Changes in Parental Medicaid Vision Coverage and Child Use of Vision Care. Acad Pediatr. 2021;21(1):101–108. [DOI] [PubMed] [Google Scholar]
- 229.National Eye I. Survey Shows Americans Lack Critical Facts about Maintaining Eye Health: Disparities are Greatest among Hispanics. Published online 2008. https://www.nih.gov/news-events/news-releases/survey-shows-americans-lack-critical-facts-about-maintaining-eye-health
- 230.Survey Reveals Most Americans Know a Lot Less About Eye Health Than They Think They Do: Here’s Why That’s a Problem - American Academy of Ophthalmology. https://www.aao.org/newsroom/news-releases/detail/survey-reveals-most-americans-know-less-eye-health
- 231.Irving EL, Sivak AM, Spafford MM. “I can see fine”: patient knowledge of eye care. Ophthalmic Physiol Opt. 2018;38(4):422–431. [DOI] [PubMed] [Google Scholar]
- 232.Rhodes LA, Huisingh CE, McGwin G Jr, et al. Eye Care Quality and Accessibility Improvement in the Community (EQUALITY): impact of an eye health education program on patient knowledge about glaucoma and attitudes about eye care. Patient Relat Outcome Meas. 2016;7:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rosdahl JA, Swamy L, Stinnett S, et al. Patient education preferences in ophthalmic care. Patient Prefer Adherence. 2014;8:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Farwana R, Sheriff A, Manzar H, et al. Watch this space: a systematic review of the use of video-based media as a patient education tool in ophthalmology. Eye. 2020;34(9):1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Davis SA, Carpenter DM, Blalock SJ, et al. Glaucoma Patient Preferences for Video Education on Eye Drop Technique. Optom Vis Sci. 2019;96(5):325–330. [DOI] [PubMed] [Google Scholar]
- 236.Christina, Beauchamp A, Elsworth GR, et al. Applying the Electronic Health Literacy Lens: Systematic Review of Electronic Health Interventions Targeted at Socially Disadvantaged Groups. J Med Internet Res. 2020;22(8):e18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chesser A, Burke A, Reyes J, et al. Navigating the digital divide: A systematic review of eHealth literacy in underserved populations in the United States. https://doi.org/103109/175381572014948171. 2015;41(1):1–19. [DOI] [PubMed] [Google Scholar]
- 238.Edmunds MR, Barry RJ, Denniston AK. Readability Assessment of Online Ophthalmic Patient Information. JAMA Ophthalmol. 2013;131(12):1610–1616. [DOI] [PubMed] [Google Scholar]
- 239.Huang G, Fang CH, Agarwal N, et al. Assessment of Online Patient Education Materials From Major Ophthalmologic Associations. JAMA Ophthalmol. 2015;133(4):449–454. [DOI] [PubMed] [Google Scholar]
- 240.Patel AJ, Kloosterboer A, Yannuzzi NA, et al. Evaluation of the Content, Quality, and Readability of Patient Accessible Online Resources Regarding Cataracts. https://doi.org/101080/0882053820211893758. 2021;36(5–6):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mitchell SJ, Godoy L, Shabazz K, et al. Internet and Mobile Technology Use Among Urban African American Parents: Survey Study of a Clinical Population. J Med Internet Res. 2014;16(1). doi: 10.2196/JMIR.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pizzuti AG, Patel KH, McCreary EK, et al. Healthcare practitioners’ views of social media as an educational resource. PLoS One. 2020;15(2). doi: 10.1371/JOURNAL.PONE.0228372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hong L, Page SE. Groups of diverse problem solvers can outperform groups of high-ability problem solvers. Proceedings of the National Academy of Sciences. 2004;101(46):16385–16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sommers SR. INTERPERSONAL RELATIONS AND GROUP PROCESSES On Racial Diversity and Group Decision Making: Identifying Multiple Effects of Racial Composition on Jury Deliberations. Published online 2006. doi: 10.1037/0022-3514.90.4.597 [DOI] [PubMed] [Google Scholar]
- 245.Phillips KW, Northcraft GB, Neale MA. Surface-Level Diversity and Decision-Making in Groups: When Does Deep-Level Similarity Help?: http://dx.doi.org/101177/1368430206067557. 2006;9(4):467–482. [Google Scholar]
- 246.Campbell LG, Mehtani S, Dozier ME, et al. Gender-Heterogeneous Working Groups Produce Higher Quality Science. PLoS One. 2013;8(10):e79147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Freeman RB, Huang W. Collaborating with People Like Me: Ethnic Coauthorship within the United States. https://doi.org/101086/678973. 2015;33(S1):S289–S318. [Google Scholar]
- 248.Morrison E, Grbic D. Dimensions of Diversity and Perception of Having Learned from Individuals from Different Backgrounds: The Particular Importance of Racial Diversity. Acad Med. 2015;90(7):937–945. [DOI] [PubMed] [Google Scholar]
- 249.Horvat L, Horey D, Romios P, et al. Cultural competence education for health professionals. Cochrane Database Syst Rev. 2014;2014(5). doi: 10.1002/14651858.CD009405.PUB2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nielsen MW, Alegria S, Börjeson L, et al. Opinion: Gender diversity leads to better science. Proceedings of the National Academy of Sciences. 2017;114(8):1740–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Geller SE, Koch A, Pellettieri B,et al. Inclusion, Analysis, and Reporting of Sex and Race/Ethnicity in Clinical Trials: Have We Made Progress? J Womens Health. 2011;20(3):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity. Communities in Action: Pathways to Health Equity. Published online January 11, 2017:1–558. [PubMed] [Google Scholar]
- 253.Govere L, Govere EM. How Effective is Cultural Competence Training of Healthcare Providers on Improving Patient Satisfaction of Minority Groups? A Systematic Review of Literature. Worldviews Evid Based Nurs. 2016;13(6):402–410. [DOI] [PubMed] [Google Scholar]
- 254.Chen MS Jr., Lara PN, Dang JHT, et al. Twenty Years Post-NIH Revitalization Act: Renewing the Case for Enhancing Minority Participation in Cancer Clinical Trials. Cancer. 2014;120(0 7):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Walker KO, Moreno G, Grumbach K. The Association Among Specialty, Race, Ethnicity, and Practice Location Among California Physicians in Diverse Specialties. J Natl Med Assoc. 2012;104(0):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Marrast LM, Zallman L, Woolhandler S, et al. Minority Physicians’ Role in the Care of Underserved Patients: Diversifying the Physician Workforce May Be Key in Addressing Health Disparities. JAMA Intern Med. 2014;174(2):289–291. [DOI] [PubMed] [Google Scholar]
- 257.Xierali IM, Nivet MA, Wilson MR. Current and Future Status of Diversity in Ophthalmologist Workforce. JAMA Ophthalmol. 2016;134(9):1016–1023. [DOI] [PubMed] [Google Scholar]
- 258.Meghani SH, Brooks JM, Gipson-Jones T, et al. Patient–provider race-concordance: does it matter in improving minority patients’ health outcomes? Ethn Health. 2009;14(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Takeshita J, Wang S, Loren AW, et al. Association of Racial/Ethnic and Gender Concordance Between Patients and Physicians With Patient Experience Ratings. JAMA Network Open. 2020;3(11):e2024583–e2024583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Adamson AS, Glass DA, Suarez EA. Patient-provider race and sex concordance and the risk for medication primary nonadherence. J Am Acad Dermatol. 2017;76(6):1193–1195. [DOI] [PubMed] [Google Scholar]
- 261.Ma A, Sanchez A, Ma M. The Impact of Patient-Provider Race/Ethnicity Concordance on Provider Visits: Updated Evidence from the Medical Expenditure Panel Survey. Journal of racial and ethnic health disparities. 2019;6(5):1011–1020. [DOI] [PubMed] [Google Scholar]
- 262.Dunlap JL, Jaramillo JD, Koppolu R, et al. The effects of language concordant care on patient satisfaction and clinical understanding for Hispanic pediatric surgery patients. J Pediatr Surg. 2015;50(9):1586–1589. [DOI] [PubMed] [Google Scholar]
- 263.Fernandez A, Schillinger D, Warton EM, et al. Language barriers, physician-patient language concordance, and glycemic control among insured Latinos with diabetes: the Diabetes Study of Northern California (DISTANCE). J Gen Intern Med. 2011;26(2):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to Cardiovascular Disease Medications: Does Patient-Provider Race/Ethnicity and Language Concordance Matter? J Gen Intern Med. 2010;25(11):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yusuf R, Chen E, Nwanyanwu K, et al. Neighborhood Deprivation and Adherence to Initial Diabetic Retinopathy Screening. Ophthalmology Retina. 2020;4(5):550–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Alsan M, Garrick O, Graziani G. Does Diversity Matter for Health? Experimental Evidence from Oakland. Am Econ Rev. 2019;109(12):4071–4111. [Google Scholar]
- 267.Aguwa UT, Srikumaran D, Brown N, et al. Improving Racial Diversity in the Ophthalmology Workforce: A Call to Action for Leaders in Ophthalmology. Am J Ophthalmol. 2021;223:306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gong D, Winn BJ, Beal CJ, et al. Gender Differences in Case Volume Among Ophthalmology Residents. JAMA Ophthalmol. 2019;137(9):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lin F, Oh SK, Gordon LK, et al. Gender-based differences in letters of recommendation written for ophthalmology residency applicants. BMC Medical Education 2019 19:1. 2019;19(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kloosterboer A, Yannuzzi NA, Gedde SJ, et al. Residency Program Directors of United States Ophthalmology Programs: A Descriptive Analysis. Am J Ophthalmol. 2020;209:71–76. [DOI] [PubMed] [Google Scholar]
- 271.Dotan G, Qureshi HM, Gaton DD. Chairs of United States Academic Ophthalmology Departments: A Descriptive Analysis and Trends. Am J Ophthalmol. 2018;196:26–33. [DOI] [PubMed] [Google Scholar]
- 272.Reddy AK, Bounds GW, Bakri SJ, et al. Differences in Clinical Activity and Medicare Payments for Female vs Male Ophthalmologists. JAMA Ophthalmol. 2017;135(3):205–213. [DOI] [PubMed] [Google Scholar]
- 273.Reddy AK, Bounds GW, Bakri SJ, et al. Representation of Women With Industry Ties in Ophthalmology. JAMA Ophthalmol. 2016;134(6):636–643. [DOI] [PubMed] [Google Scholar]
- 274.Camacci ML, Lu A, Lehman EB, et al. Association between Sex Composition and Publication Productivity of Journal Editorial and Professional Society Board Members in Ophthalmology. JAMA Ophthalmol. 2020;138(5):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fairless EA, Nwanyanwu KH, Forster SH, et al. Ophthalmology Departments Remain Among the Least Diverse Clinical Departments at United States Medical Schools. Ophthalmology. 2021;128(8):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kaplan SE, Raj A, Carr PL, et al. Race/ethnicity and success in academic medicine: Findings from a longitudinal multi-institutional study. Ophthal Plast Reconstr Surg. 2017;33(5):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Przedworski JM, Dovidio JF, Hardeman RR, et al. A comparison of the mental health and well-being of sexual minority and heterosexual first-year medical students: A report from the medical student CHANGE study. Acad Med. 2015;90(5):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Samuels EA, Boatright DA, Wong AH, et al. Association Between Sexual Orientation, Mistreatment, and Burnout Among US Medical Students. JAMA network open. 2021;4(2):e2036136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Eliason MJ, Dibble SL, Robertson PA. Lesbian, gay, bisexual, and transgender (LGBT) physicians’ experiences in the workplace. J Homosex. 2011;58(10):1355–1371. [DOI] [PubMed] [Google Scholar]
- 280.Meeks LM, Case B, Plegue M, et al. National Prevalence of Disability and Clinical Accommodations in Medical Education. Journal of medical education and curricular development. 2020;7:238212052096524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Meeks LM, Case B, Herzer K, et al. Change in Prevalence of Disabilities and Accommodation Practices Among US Medical Schools, 2016 vs 2019. JAMA. 2019;322(20):2022–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nouri Z, Dill MJ, Conrad SS, et al. Estimated Prevalence of US Physicians With Disabilities. JAMA Network Open. 2021;4(3):e211254–e211254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Major J, Godwin A. Towards Making the Invisible Engineer Visible: A Review of Low-Socioeconomic Students’ Barriers Experiencing College STEM Education. Proceedings - Frontiers in Education Conference, FIE. 2019;2018-October. doi: 10.1109/FIE.2018.8659241 [DOI] [Google Scholar]
- 284.Grbic D, Jones DJ, Case ST. The Role of Socioeconomic Status in Medical School Admissions: Validation of a Socioeconomic Indicator for Use in Medical School Admissions. Acad Med. 2015;90(7):953–960. [DOI] [PubMed] [Google Scholar]
- 285.An Updated Look at the Economic Diversity of U.S. Medical Students. AAMC. Accessed April 4, 2022. https://www.aamc.org/data-reports/analysis-brief/report/updated-look-economic-diversity-us-medical-students [Google Scholar]
- 286.Proser M, Shin P. The role of community health centers in responding to disparities in visual health. Optometry. 2008;79(10):564–575. [DOI] [PubMed] [Google Scholar]
- 287.Varma R, Jiang X. The Role of Contextual Factors in Racial/Ethnic Disparities in Eye Care Use. JAMA Ophthalmol. 2016;134(10):1167–1168. [DOI] [PubMed] [Google Scholar]
- 288.Elam AR, Lee PP. Barriers to and Suggestions on Improving Utilization of Eye Care in High-Risk Individuals: Focus Group Results. International Scholarly Research Notices. Published online 2014. doi: 10.1155/2014/527831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ramchandran RS, Yilmaz S, Greaux E, et al. Patient perceived value of teleophthalmology in an urban, low income US population with diabetes. PLoS One. 2020;15(1). doi: 10.1371/JOURNAL.PONE.0225300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang J, Liu Z, Hu S, et al. Meta-Analysis of the Pharmacogenetics of ARMS2 A69S Polymorphism and the Response to Advanced Age-Related Macular Degeneration. Ophthalmic Res. 2021;64(2):192–204. [DOI] [PubMed] [Google Scholar]
- 291.Zhang X, Norris SL, Saadine J, et al. Effectiveness of interventions to promote screening for diabetic retinopathy. Am J Prev Med. 2007;33(4):318–335. [DOI] [PubMed] [Google Scholar]
- 292.Slifkin RT, Freeman VA, Silberman P. Effect of the North Carolina State Children’s Health Insurance Program on Beneficiary Access to Care. Arch Pediatr Adolesc Med. 2002;156(12):1223–1229. [DOI] [PubMed] [Google Scholar]
- 293.Boatright D, Ross D, O’Connor P, et al. Racial Disparities in Medical Student Membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177(5):659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Newman-Casey PA, Niziol LM, Lee PP, et al. The Impact of the Support, Educate, Empower Personalized Glaucoma Coaching Pilot Study on Glaucoma Medication Adherence. Ophthalmol Glaucoma. 2020;3(4):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Dankwa‐ Mullan I, Zhang X, Le P, et al. Applications of Big Data Science and Analytic Techniques for Health Disparities Research. The Science of Health Disparities Research. Published online February 12, 2021:221–242. [Google Scholar]
- 296.Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. 2018;115(38): 9592e9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tessum CW, Paolella DA, Chambliss SE, Apte JS, Hill JD, Marshall JD. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Science Advances. 2021;7(18):4491e4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tessum CW, Apte JS, Goodkind AL, et al. Inequity in consumption of goods and services adds to racialeethnic disparities in air pollution exposure. Proceedings of the National Academy of Sciences. 2019;116(13):6001e6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chua SYL, Khawaja AP, Morgan J, et al. The Relationship Between Ambient Atmospheric Fine Particulate Matter (PM2.5) and Glaucoma in a Large Community Cohort. Invest Ophthalmol Vis Sci. 2019;60(14):4915e4923. [DOI] [PubMed] [Google Scholar]
- 300.Li L, Xing C, Zhou J, et al. Airborne particulate matter (PM2.5) triggers ocular hypertension and glaucoma through pyroptosis. Particle and Fibre Toxicology 2021 18:1. 2021;18(1):1e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nwanaji-Enwerem JC, Wang W, Nwanaji-Enwerem O, et al. Association of Long-term Ambient Black Carbon Exposure and Oxidative Stress Allelic Variants with Intraocular Pressure in Older Men. JAMA Ophthalmol. 2019;137(2):129e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tseng VL, Lee GY, Shaikh Y, et al. The Association Between Glaucoma and Immunoglobulin E Antibody Response to Indoor Allergens. Am J Ophthalmol. 2015;159(5):986e993.e1. [DOI] [PubMed] [Google Scholar]
- 303.Reif JS, Burch JB, Nuckols JR, et al. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environ Res. 2003;93(3):248e258. [DOI] [PubMed] [Google Scholar]
- 304.Paulsen AJ, Schubert CR, Johnson LJ, et al. Association of Cadmium and Lead Exposure With the Incidence of Contrast Sensitivity Impairment Among Middle-aged Adults. JAMA Ophthalmol. 2018;136(12):1342e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fernandes TP, Silverstein SM, Almeida NL, Santos NA. Visual impairments in tobacco use disorder. Psychiatry Res. 2019;271:60e67. [DOI] [PubMed] [Google Scholar]


