Abstract
Objective
To estimate the effect of denosumab compared with oral bisphosphonates on reducing the risk of type 2 diabetes in adults with osteoporosis.
Design
Population based study involving emulation of a randomized target trial using electronic health records.
Setting
IQVIA Medical Research Data primary care database in the United Kingdom, 1995-2021.
Participants
Adults aged 45 years or older who used denosumab or an oral bisphosphonate for osteoporosis.
Main outcome measures
The primary outcome was incident type 2 diabetes, as defined by diagnostic codes. Cox proportional hazards models were used to estimate adjusted hazard ratios and 95% confidence intervals, comparing denosumab with oral bisphosphonates using an as treated approach.
Results
4301 new users of denosumab were matched on propensity score to 21 038 users of an oral bisphosphonate and followed for a mean of 2.2 years. The incidence rate of type 2 diabetes in denosumab users was 5.7 (95% confidence interval 4.3 to 7.3) per 1000 person years and in oral bisphosphonate users was 8.3 (7.4 to 9.2) per 1000 person years. Initiation of denosumab was associated with a reduced risk of type 2 diabetes (hazard ratio 0.68, 95% confidence interval 0.52 to 0.89). Participants with prediabetes appeared to benefit more from denosumab compared with an oral bisphosphonate (hazard ratio 0.54, 0.35 to 0.82), as did those with a body mass index ≥30 (0.65, 0.40 to 1.06).
Conclusions
In this population based study, denosumab use was associated with a lower risk of incident type 2 diabetes compared with oral bisphosphonate use in adults with osteoporosis. This study provides evidence at a population level that denosumab may have added benefits for glucose metabolism compared with oral bisphosphonates.
Introduction
Antiresorptive drugs are the most widely used treatment options for osteoporosis. Denosumab is a humanized monoclonal antibody against the receptor activator of nuclear factor κ B (RANK) ligand (RANKL) and a potent antiresorptive drug that suppresses bone resorption.1 2 Clinical guidelines have recommended denosumab for postmenopausal women, men, and people with glucocorticoid induced osteoporosis at high risk of fracture.3 4 5
Recent studies suggest an association between RANKL/RANK signaling pathway and energy metabolism. In a large population based study, higher RANKL levels were associated with a fourfold increased risk of type 2 diabetes over a five year follow-up period.6 7 Downregulation of RANKL signaling can improve glucose metabolism in both mice and humans.7 8 9 In a series of mouse models in which RANKL signaling was inhibited in the liver, hepatic insulin sensitivity and plasma glucose concentrations were improved.7 Blocking of RANKL signaling with denosumab could significantly reduce circulating dipeptidyl peptidase 4 and increase glucagon-like peptide-1 (GLP-1) levels.9 Although no randomized controlled trial has been performed in a population with diabetes, results from an observational study suggested improved glucose homeostasis in participants with type 2 diabetes or prediabetes, and a statistically significant reduction of glycated hemoglobin over 12 months among participants treated with denosumab compared with those treated with bisphosphonates or calcium plus vitamin D.9
Data on the incidence of type 2 diabetes among denosumab users is, however, scant. The largest study was a post hoc analysis performed by the Fracture REduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial.10 Although the FREEDOM trial was not adequately powered for a precise estimate, the risk of incident type 2 diabetes was lower in denosumab users compared with those receiving a placebo (hazard ratio 0.85, 95% confidence interval 0.61 to 1.17). Most clinical trials with denosumab reported no participants with diabetes (see supplemental figure 1), limiting the value of a meta-analysis. Whether denosumab reduces the risk of type 2 diabetes in the general population or in a narrower population with certain risk factors for type 2 diabetes remains unclear. In real world clinical practice, most denosumab users (about 80%) had previously used other anti-osteoporosis drugs (eg, oral bisphosphonates) before switching to denosumab. In this situation, a randomized controlled trial with a specific focus on those who switched treatment rather than those who initiated a drug is preferred because a trial that involves switching provides a pooled effect estimate of starting denosumab and stopping oral bisphosphonate on risk of type 2 diabetes. In the absence of a randomized clinical trial, this study used observational data from a real world clinical setting to estimate the effect of switching to denosumab versus continuing oral bisphosphonate on the risk of developing type 2 diabetes.11
Methods
Data source and study design
We used the IQVIA Medical Research Data (IMRD) UK primary care database as the data source. IMRD currently incorporates data from The Health Improvement Network (THIN), which is a Cegedim database. IMRD captures UK primary care records on about 18 million people from more than 800 general practitioners from 1987 to 2021. Its digitized information includes sociodemographic and anthropometric characteristics, lifestyle factors, details from visits to general practitioners (eg, disease diagnoses, drug prescriptions), diagnoses from specialist referrals and hospital admissions, and laboratory test results. A previous study showed the validity of IMRD for use in clinical and epidemiological research studies.12 13
To improve the robustness of observational analysis, we followed the target trial emulation design framework14 15 and adopted a modified new user design (or prevalent new user design used in previous literature16 17 18) to compare drug effects between denosumab and an active comparator (oral bisphosphonate) (see supplemental table 1 and supplemental figure 2). We chose oral bisphosphonate as the comparator because it has the same indication as denosumab and had been widely used before the marketing of denosumab.3 The modified new user design allowed the inclusion of almost all people who had used denosumab, including those who switched to denosumab from an oral bisphosphonate and those who initiated denosumab; these sequences of drug use represent typical clinical practice.16 17 18 19 20 This design enabled us to evaluate whether denosumab was associated with a reduced risk of type 2 diabetes in adults with osteoporosis.
Study cohort
We first selected a potentially eligible cohort, including all those who had received an antiosteoporosis drug between 1 January 1995 and 31 December 2021. The first prescription of an antiosteoporosis drug was defined as the eligible point for cohort entry. From this cohort, we chose a study cohort comprising individuals who had initiated denosumab (60 mg) or received an oral bisphosphonate (alendronate 10 mg or 70 mg, ibandronate 150 mg, risedronate 35 mg) between 1 July 2010 and 31 December 2021. We then stratified the denosumab users into two types: those who switched to denosumab (also called prevalent new users16) and those who were incident new users. Participants who switched to denosumab were those switching from an oral bisphosphonate, whereas incident new users were treatment naïve participants who initiated denosumab as their first antiosteoporosis drug. We considered the switch date or date of incident use as the index date. For every individual who switched to denosumab, we matched up to five people who continued an oral bisphosphonate and had used the oral bisphosphonate for the same duration at the time of the index date. For incident new users, we matched each denosumab user with up to five incident new users of an oral bisphosphonate in the treatment naïve populations. The modified new user design enabled us to emulate an analysis of a hypothetical trial comparing switching to denosumab or continuing an oral bisphosphonate. We excluded individuals who were younger than 45 years, had been enrolled for less than 365 days, had a diagnosis of Paget disease of bone, had a history of type 1 or type 2 diabetes, or had ever used any antidiabetes drugs before the index date.
Propensity scores
We used propensity scores to identify users of an oral bisphosphonate who were most similar to those who switched to, or initiated, denosumab.16 We considered a wide range of potential confounders. Our rationale for selecting potential confounders focused on variables associated with type 2 diabetes, which may also be associated with the drug of interest, based on current literature and expertise in the subject (see supplemental figure 3).21 Several covariates were measured at the index date: age, sex, smoking status, alcohol consumption status, body mass index (BMI), socioeconomic deprivation index (Townsend score), residence status, duration of oral bisphosphonate treatment, history of major osteoporotic fracture, comorbidities (cardiovascular disease, hypertension, hypercholesterolemia, chronic obstructive pulmonary disease, depression, prediabetes), and concomitant treatment (antihypertensive, statin, glucocorticoid, and antidepressant). We considered general health status as a potential unmeasured confounder and used common comorbidities (dementia, chronic heart failure, congestive heart disease, peripheral vascular disease, other circulation diseases, venous thromboembolism, anxiety, peptic ulcer disease, renal disease, and cancer) and related concomitant drugs (non-steroidal anti-inflammatory drug, aspirin, oral anticoagulant, and proton pump inhibitor) as proxies. We also included markers of health seeking behavior, using the number of hospital admissions and visits to doctors as proxies. For the missing values of BMI (6%), smoking status (2%), alcohol consumption status (9%), and Townsend score (13.6%), we adopted a missing indicator approach whereby missing categories were included in the primary analysis. Then we performed a sensitivity analysis with multiple imputations to examine the effect of missing information.
In a modified new user design, because denosumab users and their potential matched pairs formed clusters (see supplemental figure 4), we used conditional logistic regression to compute the propensity of switching to denosumab versus continuing an oral bisphosphonate on the basis of prespecified covariates.16 We matched individuals treated with denosumab chronologically (starting from the participant with the earliest calendar date) using a variable ratio one to many (1:5) nearest neighbor matching within a caliper to individuals treated with an oral bisphosphonate in each cluster. We set a caliper width of 0.2 standard deviations of the propensity scores on the logarithmic scale.22 Participants who were selected as the comparator group of oral bisphosphonate users were eligible for subsequent clusters. We set 10 as the maximum number of times that each participant in the oral bisphosphonate group could be used (see method section in the supplemental file for details of the matching procedure). In doing this, we created two groups of participants with the same distribution of all known baseline characteristics, such as age, sex, treatment history, and other potential confounders, emulating the randomization process of a hypothetical trial.15
Definitions of drug use
We used the British National Formulary code to define use of both denosumab and oral bisphosphonate.23 We used an as treated definition for drug use, in which participants were considered to have continually used the drug of interest if the duration of one prescription overlapped with the date of a subsequent prescription. In the case of non-overlap, we allowed for a 180 days grace period between successive prescriptions to account for adherence. Drug discontinuation was defined by a gap of more than 180 days between successive prescriptions or the initiation of another type of antiosteoporosis drug.
Main outcome measure
The primary outcome was incident type 2 diabetes, defined by diagnostic codes.24 25 The event date was defined by the date of diagnosis. In an alternative definition of incident type 2 diabetes, we defined the endpoint as any one of: a diagnostic code for type 2 diabetes; at least two prescriptions for the antidiabetes drug (two different drugs or the same drug on two different dates); or fasting blood glucose ≥7.0 mmol/L, random glucose level ≥11.1 mmol/L, glucose tolerance test result ≥11.1 mmol/L, or glycated hemoglobin A1c (HbA1c) level ≥6.5%.25 26
Follow-up
We defined the start of follow-up as the date of the first denosumab prescription for denosumab users and their matched oral bisphosphonate users. Participants were followed until the occurrence of the study outcome, discontinuation of the drug of interest, death, transfer out of primary care clinic, five years’ follow-up, or end of the study period (31 December 2021), whichever occurred first.
Statistical analysis
We used descriptive statistics to summarize the baseline characteristics of the matched study cohort. In the matched cohort, we calculated the incidence rates of type 2 diabetes, expressed as numbers of events per 1000 person years for the two groups. We used Cox proportional hazards models to estimate the hazard ratio and 95% confidence intervals of incident type 2 diabetes. The proportional hazards assumption was tested using the Kolmogorov supremum test. A robust estimator was used to estimate the variance for analyses that implemented matching with replacement.27
To test the robustness of the primary analysis, we performed extensive sensitivity analyses, including six that were prespecified and 14 that were post hoc (see method section in supplemental file). First, the primary switcher design provided a combined effect estimation of starting denosumab and stopping an oral bisphosphonate on risk of type 2 diabetes. To further test the biological impact of starting denosumab outside of stopping an oral bisphosphonate, we performed a traditional new user design only including incident new users.28 Second, to improve the comparability of participants who received denosumab or an oral bisphosphonate, we used asymmetric trimming by excluding those with a propensity score below the 2.5th and above the 97.5th centile.29 Third, we repeated the primary analysis accounting for the competing risk of death.30 Fourth, to minimize reverse causality, we introduced a six month lag period for drug use—that is, eligible participants at the start of follow-up would be considered to have not used the drug of interest until six months after the index date, and to have used the drug thereafter.31 Fifth, in the primary analysis, we included a small proportion of oral bisphosphonate users multiple times; we repeated the analysis with the algorithm of nearest neighbor matching within specified caliper widths without replacement (participants selected for the purpose of comparison were not eligible for subsequent clusters). Sixth, to reduce the unpredictable impact of covid-19, we repeated the analysis by excluding the pandemic period (from March 2020). Last, in the subpopulation of incident new users, to examine treatment effect heterogeneity between the matched population and target population, we estimated the marginal treatment effect (average treatment effect) with inverse probability treatment weighting and the conditional treatment effect (average treatment effect in those treated) with propensity score matching. To assess the impact of unmeasured confounding, we examined the potential effects of unmeasured confounding using the e-value.32
In addition, to examine the risk of type 2 diabetes between the two study groups across different patient characteristics, we performed post hoc subgroup analyses stratified by prediabetes and obesity. Prediabetes was defined by baseline impaired fasting blood glucose (5.6-6.9 mmol/L), impaired glucose tolerance (glucose tolerance test result 7.8-11.0 mmol/L), HbA1c of 5.7% to 6.4%, or a combination of these results.25 Obesity was defined as BMI ≥30.0.33 Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R-4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
No patients were involved in setting the research question, nor were they involved in the design or analysis of the study. No patients were asked to advise on the interpretation or writing up of the results. The primary obstacles to patient and public involvement were the absence of relevant training programs and the limited opportunity for face-to-face communication due to the ongoing covid-19 pandemic. However, we plan to engage the public in disseminating our research findings through various means, such as social media, newsletters, and conferences.
Results
Baseline characteristics
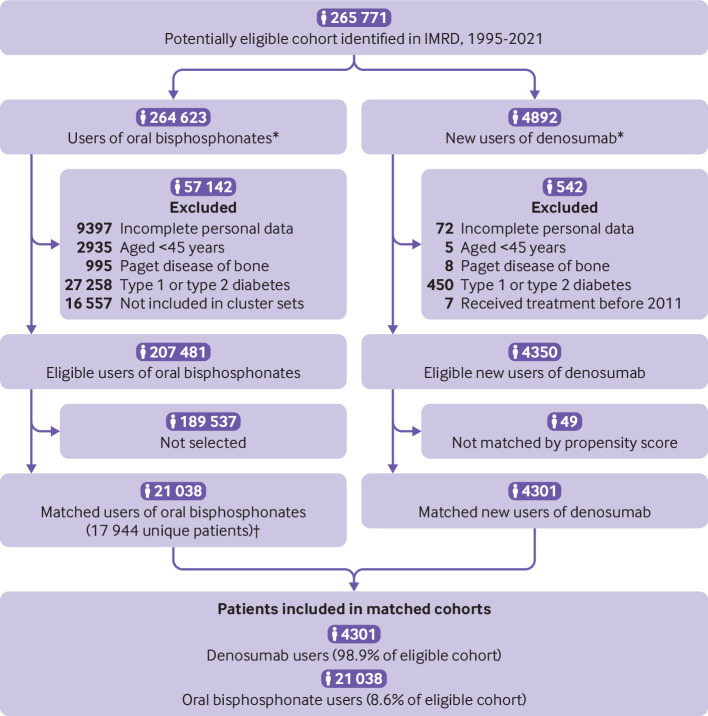
Among 4892 participants who used denosumab, 92 (1.9%) were excluded owing to incomplete personal information, age less than 45 years, or Paget disease of bone, or because they had received denosumab before 2011. A further 450 (9.2%) were excluded owing to a history of type 1 or type 2 diabetes. After exclusions, 4350 (88.9%) were eligible new users of denosumab. In the potentially eligible populations, 4350 individuals switched to, or initiated, denosumab and 207 481 initiated an oral bisphosphonate (fig 1). Those who initiated denosumab were younger than those who initiated an oral bisphosphonate (mean 69 v 72 years) and were more often women (94% v 81%). The proportion of participants with a history of major osteoporotic fracture was higher in those who initiated denosumab than in those who initiated an oral bisphosphonate (51% v 30%). Participants who initiated denosumab had a comparable prevalence for most chronic conditions but a higher comorbidity burden from peptic ulcer disease and renal disease and a higher number of hospital admissions and visits to a doctor (see supplemental table 2). Of 4350 new users of denosumab, 4301 could be matched on propensity score to 21 038 users of an oral bisphosphonate (fig 1). In the matched populations, baseline characteristics of the two groups measured at switching to, or initiating, denosumab were comparable with the standardized difference of <0.1 (table 1 and supplemental figure 5).
Fig 1.
Study flow diagram. IMRD=IQVIA Medical Research Data. *Participants could enter the study cohort a maximum of twice: first with an oral bisphosphonate and second when initiating denosumab. †Matched with replacement (also see method section in the supplemental file)
Table 1.
Baseline characteristics of matched cohorts. Values are number (percentage) unless stated otherwise
| Characteristics | Oral bisphosphonate group (n=21038) | Denosumab group (n=4301) | Standardized difference |
|---|---|---|---|
| New users: | |||
| Incident new users | 4802 (22.8) | 961 (22.3) | |
| Switched to denosumab from oral bisphosphonate | 16 236 (77.2) | 3340 (77.7) | |
| Period of cohort entry: | 0.01 | ||
| 2011-13 | 3976 (18.9) | 804 (18.7) | |
| 2014-16 | 8962 (42.6) | 1819 (42.3) | |
| 2017-19 | 6101 (29.0) | 1256 (29.2) | |
| 2020-21 | 1999 (9.5) | 422 (9.8) | |
| Mean (SD) age at cohort entry (years) | 75.7 (11.0) | 75.7 (9.9) | 0.007 |
| Women | 19 766 (94.0) | 4055 (94.3) | 0.01 |
| Residential care | 998 (4.7) | 200 (4.7) | 0.004 |
| Mean (SD) Townsend deprivation index score | 2.19 (1.47) | 2.19 (1.47) | 0.001 |
| Body mass index category: | 0.02 | ||
| Normal | 8612 (40.9) | 1766 (41.1) | |
| Obese | 2224 (10.6) | 450 (10.5) | |
| Overweight | 5466 (26.0) | 1109 (25.8) | |
| Underweight | 3470 (16.5) | 729 (16.9) | |
| Unknown | 1266 (6.0) | 247 (5.7) | |
| Smoking status: | 0.02 | ||
| Current | 1961 (9.3) | 420 (9.8) | |
| Former | 5722 (27.2) | 1169 (27.2) | |
| Never | 12 896 (61.3) | 2626 (61.1) | |
| Unknown | 459 (2.2) | 86 (2.0) | |
| Alcohol consumption status: | 0.02 | ||
| Current | 12 561 (59.7) | 2551 (59.3) | |
| Former | 778 (3.7) | 161 (3.7) | |
| Never | 5793 (27.5) | 1219 (28.3) | |
| Unknown | 1906 (9.1) | 370 (8.6) | |
| Mean (SD) duration of bisphosphonate use (years) | 5.4 (4.9) | 5.6 (5.1) | 0.04 |
| History of major osteoporotic fracture* | 10 457 (49.7) | 2169 (50.4) | 0.01 |
| Comorbidities before cohort entry: | |||
| Hypertension | 10 532 (50.1) | 2147 (49.9) | 0.003 |
| Hypercholesterolemia | 3346 (15.9) | 670 (15.6) | 0.009 |
| Chronic obstructive pulmonary disease | 4249 (20.2) | 887 (20.6) | 0.01 |
| Dementia | 1090 (5.2) | 219 (5.1) | 0.004 |
| Cerebrovascular disease | 1831 (8.7) | 369 (8.6) | 0.004 |
| Congestive heart disease | 1007 (4.8) | 217 (5.0) | 0.01 |
| Myocardial infarction | 863 (4.1) | 182 (4.2) | 0.006 |
| Chronic heart failure | 1123 (5.3) | 246 (5.7) | 0.02 |
| Peripheral vascular disease | 736 (3.5) | 152 (3.5) | 0.002 |
| Other circulation diseases | 8544 (40.6) | 1780 (41.4) | 0.02 |
| Venous thromboembolism | 1494 (7.1) | 297 (6.9) | 0.008 |
| Anxiety | 3761 (17.9) | 795 (18.5) | 0.02 |
| Depression | 3381 (16.1) | 694 (16.1) | 0.002 |
| Peptic ulcer disease | 1223 (5.8) | 268 (6.2) | 0.02 |
| Renal disease | 4494 (21.4) | 936 (21.8) | 0.01 |
| Cancer | 3388 (16.1) | 694 (16.1) | 0.001 |
| Drugs used in 2 years before cohort entry: | |||
| Non-steroidal anti-inflammatory drug | 11 460 (54.5) | 2361 (54.9) | 0.008 |
| Antihypertensive | 12159 (57.8) | 2497 (58.1) | 0.005 |
| Statin | 6958 (33.1) | 1417 (32.9) | 0.003 |
| Aspirin | 3860 (18.3) | 797 (18.5) | 0.005 |
| Oral anticoagulant | 1672 (7.9) | 333 (7.7) | 0.008 |
| Glucocorticoid | 5700 (27.1) | 1188 (27.6) | 0.01 |
| Benzodiazepine | 3416 (16.2) | 704 (16.4) | 0.004 |
| Proton pump inhibitor | 11 451 (54.4) | 2322 (54.0) | 0.009 |
| SSRI | 440 (2.1) | 100 (2.3) | 0.02 |
| Healthcare utilization in 2 years before cohort entry: | |||
| Mean (SD) No of hospital admissions | 2.1 (3.9) | 2.1 (3.5) | 0.008 |
| No of doctor visits: | 0.02 | ||
| 0-1 | 3192 (15.2) | 639 (14.9) | |
| 2-4 | 4528 (21.5) | 914 (21.3) | |
| 5-8 | 5124 (24.4) | 1042 (24.2) | |
| ≥9 | 8194 (38.9) | 1706 (39.7) | |
SSRI=selective serotonin reuptake inhibitor.
Include fractures at the hip, vertebrae, wrist, humerus, pelvis, and rib.
Primary analysis: incidence of type 2 diabetes
During five years of follow-up, the incidence of type 2 diabetes in the matched cohorts was 5.7 (95% confidence interval 4.3 to 7.3) per 1000 person years in denosumab users and 8.3 (7.4 to 9.2) per 1000 person years in oral bisphosphonates users; denosumab initiation was associated with a reduced risk of type 2 diabetes (hazard ratio 0.68, 95% confidence interval 0.52 to 0.89) (table 2 and fig 2). Using an alternative definition of diabetes by combining diagnostic codes, antidiabetes drugs, and laboratory test results, the incidence was 8.5 (95% confidence interval 6.8 to 10.4) per 1000 person years for denosumab users and 11.6 (10.6 to 12.7) per 1000 person years for oral bisphosphonate users; the rate of type 2 diabetes was reduced in denosumab users compared with oral bisphosphonate users (hazard ratio 0.73, 95% confidence interval 0.58 to 0.91) (table 2).
Table 2.
Risk of incident type 2 diabetes among participants initiating denosumab compared with propensity score matched participants using an oral bisphosphonate
| Incident type 2 diabetes by drug type | No of patients* | No of events | Person years | Incidence/1000 person years (95% CI) | Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Primary outcome† | |||||
| Oral bisphosphonate | 21 038 | 347 | 41900 | 8.3 (7.4 to 9.2) | Reference |
| Denosumab | 4301 | 60 | 10617 | 5.7 (4.3 to 7.3) | 0.68 (0.52 to 0.89) |
| Secondary outcome‡ | |||||
| Oral bisphosphonate | 21 038 | 486 | 41827 | 11.6 (10.6 to 12.7) | Reference |
| Denosumab | 4301 | 90 | 10598 | 8.5 (6.8 to 10.4) | 0.73 (0.58 to 0.91) |
CI=confidence interval.
Recipients of denosumab users were matched up to 5 oral bisphosphonate recipients with propensity scores.
Type 2 diabetes defined by diagnostic codes.
Alternative definition of type 2 diabetes using diagnostic codes, antidiabetes drug used, and laboratory test results.
Fig 2.
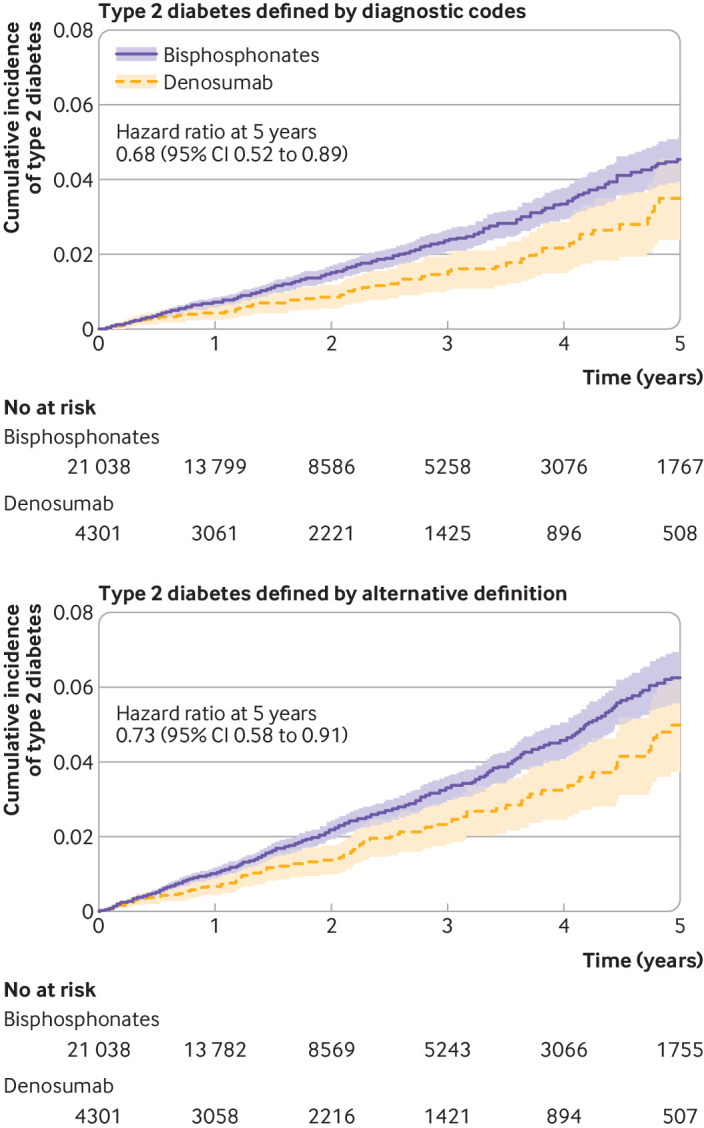
Cumulative incidence of type 2 diabetes as defined by diagnostic codes and by an alternative definition combining diagnostic codes, antidiabetes drugs, and laboratory test results among users of denosumab and matched users of bisphosphonates in IQVIA Medical Research Data. Shaded areas represent 95% confidence intervals (CIs)
To examine whether individuals at high risk of type 2 diabetes might benefit more from denosumab than from an oral bisphosphonate, we performed subgroup analyses stratified by risk factors for type 2 diabetes (table 3). The incidence of type 2 diabetes was increased in participants with prediabetes or obesity: 22.1 (95% confidence interval 19.1 to 25.4) per 1000 person years in those with prediabetes and 24.7 (20.4 to 29.7) per 1000 person years in those with obesity. In the prediabetes subgroup, denosumab was associated with a reduced risk of type 2 diabetes (hazard ratio 0.54, 95% confidence interval 0.35 to 0.82) compared with oral bisphosphonate. Results were similar in the obese subgroup (0.65, 0.40 to 1.06).
Table 3.
Subgroup analyses stratified by risk factors for type 2 diabetes
| Subgroup analyses | No of patients | No of events | Person years | Incidence/1000 person years (95% CI) | Hazard ratio (95% CI) | P for interaction |
|---|---|---|---|---|---|---|
| Stratified by prediabetes* | 0.05 | |||||
| Prediabetes: | ||||||
| Oral bisphosphonate | 4750 | 198 | 8951 | 22.1 (19.1 to 25.4) | Reference | |
| Denosumab | 868 | 24 | 2028 | 11.8 (7.6 to 17.6) | 0.54 (0.35 to 0.82) | |
| No prediabetes: | ||||||
| Oral bisphosphonate | 16 288 | 149 | 32 949 | 4.5 (3.8 to 5.3) | Reference | |
| Denosumab | 3433 | 36 | 8589 | 4.2 (2.9 to 5.8) | 0.92 (0.65 to 1.32) | |
| Stratified by obesity† | 0.70 | |||||
| Obesity: | ||||||
| Oral bisphosphonate | 2224 | 116 | 4692 | 24.7 (20.4 to 29.7) | Reference | |
| Denosumab | 450 | 19 | 1172 | 16.2 (9.8 to 25.3) | 0.65 (0.40 to 1.06) | |
| No obesity: | ||||||
| Oral bisphosphonate | 17 548 | 218 | 34 990 | 6.2 (5.4 to 7.1) | Reference | |
| Denosumab | 3604 | 41 | 8974 | 4.6 (3.3 to 6.2) | 0.73 (0.53 to 1.01) |
CI=confidence interval.
Prediabetes defined by baseline impaired fasting blood glucose (5.6-6.9 mmol/L), or impaired glucose tolerance (7.8-11.0 mmol/L), or HbA1c of 5.7-6.4%, or a combination of these factors.
Obesity defined by body mass index ≥30.0. Patients with missing baseline body mass index were excluded from analysis.
Sensitivity analyses
We performed several sensitivity analyses (table 4). First, in the traditional new user design analysis, although the sample size was markedly reduced, participants who initiated denosumab had a reduced risk of type 2 diabetes compared with participants who initiated an oral bisphosphonate (0.35, 0.15 to 0.79) (table 4 and supplemental table 3). Second, when we used asymmetric trimming to examine the influence of participants with extreme propensity scores, the results did not change materially. No substantial changes occurred to the relative risk estimates in the other sensitivity analyses: death as a competing risk, a six month lag period for drug use, matching without replacement, multiple imputations for missing data, and excluding the covid-19 pandemic period (after March 2020). In the subpopulation of incident new users, we used inverse probability weighting to estimate the average treatment effect, and propensity score matching to estimate the average treatment effect in those treated (supplement table 4). The point estimate of the average treatment effect and average treatment effect in those treated were similar and heterogeneity was not obvious. Finally, we examined the effect of unmeasured confounding using the e-value. The e-value was 2.30 for the primary point estimate (1.50 for the confidence interval)—that is, for the observed hazard ratio of 0.68 to be explained away to the null by unmeasured confounding, these unmeasured confounders would need to be associated with both drug use and the outcome hazard ratio 2.30 each, above and beyond the measured confounders. Results from a further 14 post hoc sensitivity analyses were consistent with our primary ones, supporting the robustness of the findings (see supplemental tables 5-18).
Table 4.
Sensitivity analyses of risk of incident type 2 diabetes among particiapnts initiating denosumab compared with propensity score matched controls using an oral bisphosphonate
| No of patients | No of events | Person years | Incidence/1000 person years (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Incident new users of denosumab-oral bisphosphonate pairs | |||||
| Oral bisphosphonate | 4802 | 89 | 10 345 | 8.6 (6.9 to 10.6) | Reference |
| Denosumab | 961 | 6 | 2036 | 3.0 (1.1 to 6.4) | 0.35 (0.15 to 0.79) |
| Asymmetric trimming excluding extreme propensity scores | |||||
| Oral bisphosphonate | 20 015 | 326 | 39 961 | 8.2 (7.3 to 9.1) | Reference |
| Denosumab | 4056 | 56 | 10 049 | 5.6 (4.2 to 7.2) | 0.68 (0.52 to 0.90) |
| Death as a competing risk | |||||
| Oral bisphosphonate | 21 038 | 347 | 41 900 | 8.3 (7.4 to 9.2) | Reference |
| Denosumab | 4301 | 60 | 10617 | 5.7 (4.3 to 7.3) | 0.68 (0.52 to 0.89) |
| Six month lag period for drug use | |||||
| Oral bisphosphonate | 21 038 | 274 | 41 900 | 6.5 (5.8 to 7.4) | Reference |
| Denosumab | 4301 | 47 | 10 617 | 4.4 (3.3 to 5.9) | 0.65 (0.48 to 0.88) |
| Analysis repeated with modified matching algorithm from primary analysis* | |||||
| Oral bisphosphonate | 20 262 | 340 | 40 866 | 8.3 (7.5 to 9.3) | Reference |
| Denosumab | 4210 | 59 | 10 428 | 5.7 (4.3 to 7.3) | 0.68 (0.52 to 0.89) |
| Excluding covid-19 pandemic period† | |||||
| Oral bisphosphonate | 19 268 | 341 | 40 049 | 8.5 (7.6 to 9.5) | Reference |
| Denosumab | 3928 | 57 | 10 090 | 5.7 (4.3 to 7.3) | 0.66 (0.50 to 0.87) |
CI=confidence interval.
Participants who were selected as comparators in a previous cluster were not eligible for subsequent clusters; additional inverse probability weighting analysis addressed potentially unbalanced censoring between groups (see supplemental table 16).
From March 2020.
Discussion
In this propensity score matched cohort obtained from the IMRD database in the UK, switching to, or initiating, denosumab was associated with a 32% decreased risk of type 2 diabetes compared with an oral bisphosphonate. People at high risk of type 2 diabetes (eg, those with prediabetes or obesity) who use denosumab may experience a further reduction in diabetes risk compared with those using an oral bisphosphonate.
Comparison with existing literature
Both observational studies and post hoc analysis of randomized clinical trials have examined the effect of denosumab on glycemic variables, but the results for type 2 diabetes are scant.9 10 34 35 36 37 In postmenopausal women with osteoporosis, denosumab markedly improved muscle insulin sensitivity.35 In people with type 2 diabetes or prediabetes, denosumab significantly reduced glycated hemoglobin and fasting plasma glucose levels at 12 months.9 These findings were supported by another observational study in people with type 2 diabetes, although denosumab only improved glycated hemoglobin and insulin resistance at 52 weeks and not at 26 weeks.34 Post hoc analysis of the FREEDOM trial, however, did not show improvement of glycemic variables overall but did show modestly improved fasting plasma glucose in a subgroup of women with type 2 diabetes who were not using antidiabetes drugs.8 10 None of the previous studies that have examined the effect of denosumab on type 2 diabetes had sufficient statistical power for this endpoint. In our study, we used a sophisticated study design empowered by a large electronic database and found a strong association between denosumab use and reduced risk of type 2 diabetes. This association was robust across many sensitivity analyses.
Although the current study did not examine biologic mechanisms, previous studies using genetic mouse models have shown a close relationship between RANKL inhibition and improved glucose metabolism. First, growing evidence links low grade inflammation to the development of insulin resistance and type 2 diabetes.7 35 RANKL is a potent stimulator of nuclear factor κ B, a proinflammatory master switch that modulates the level of inflammation. It has been proposed that diet induced hepatic and systemic insulin resistance may be a consequence of subacute inflammation by low level activation of nuclear factor κ B.38 Therefore, RANKL inhibition with denosumab can ameliorate subacute inflammation and improve insulin resistance.7 Second, another proposed mechanism is that RANKL inhibition could lead to the stimulation of β cell proliferation.39 Progressive β cell failure is a core pathogenic mechanism of type 2 diabetes.40 The RANKL/RANK pathway slows down β cell replication in humans. Thus, although not examined in this study, downregulation of the RANKL/RANK pathway can enhance human β cell replication, and denosumab can induce human β cell proliferation in both cell lines and genetically modified mice.39
Observational evidence suggests oral bisphosphonates may also benefit glucose metabolism.41 42 43 44 A recent meta-analysis from two post hoc analyses of randomized controlled trials and five observational studies showed that bisphosphonate use was associated with a 23% decreased risk of diabetes (relative risk 0.77, 95% confidence interval 0.65 to 0.90); although only minimal benefit (0.93, 0.74 to 1.18) in the subgroup of post hoc analyses of randomized controlled trials.45 Proposed mechanisms suggest bisphosphonates might improve glucose homeostasis by inhibiting the mevalonate pathway of endothelial cells, decreasing the number of macrophages in visceral adipose tissues, or through interaction with multiple active osteokines (eg, osteocalcin and osteopontin).42 46 47 Although more studies are needed to evaluate the exact mechanism of bisphosphonates on risk of type 2 diabetes, the use of oral bisphosphonates as compactor drug in the current study provides a more conservative estimate of the association between denosumab and risk of type 2 diabetes.
Unlike denosumab, bisphosphonates can accumulate and remain in bone for years.48 If a benefit of bisphosphonate on glucose metabolism exists, those who switched from bisphosphonates to denosumab might have persistent carry-over effects attributable to bisphosphonates. We examined the carry-over effects in a series of exploratory analyses. A subgroup of participants who switched denosumab and had previously used bisphosphonates for longer (>3 years), did not exhibit a statistically significantly larger effect than those exposed to a shorter period of previous bisphosphonates (<3 years) (see supplemental table 6). In addition, the observed effect of denosumab on risk of type 2 diabetes remained relatively stable from one year to five years (see supplemental table 18). These results do not suggest a strong carry-over effect of bisphosphonate in this study population.
Strengths and limitations of this study
The major strength of this study is that we adopted a modified new user design, which reflects real world clinical practice and could provide direct evidence for decision making. Although denosumab has been recommended as the preferred drug for postmenopausal osteoporosis, most denosumab users have a history of bisphosphonate use. In the IMRD database, 78% of denosumab users had a history of bisphosphonate use, and only 22% were treatment naïve. The classic incident new users design requires the study population to be treatment naïve, and in this study therefore only represented a small proportion of those who used denosumab and thus generalizability is limited. While many pharmacoepidemiologists strongly suggest using the new user design,28 to comprehensively evaluate the effect of denosumab on incident type 2 diabetes we used a modification of the new user design by including both treatment naïve participants and those who switched from oral bisphosphonates. The modified new user design takes advantage of treatment patterns in typical clinical practice, making it a more preferable choice than the traditional new user design.16
This study also has limitations. First, residual confounding bias (eg, family history of diabetes, causes of osteoporosis, and indication bias) remains possible in this observational study. We adopted different approaches to minimize such biases, including the use of an active comparator, prevalent new user design, propensity score matching, extensive sensitivity analyses, and quantitative bias analysis with e-values. In addition, drug use was defined by prescriptions, which might not reflect actual drug use; as a result, misclassification of drug use could bias the study findings. Such bias, if it occurred, is likely to be non-differential and would bias the observed associations toward the null. Second, we estimated the average treatment effect on treated participants, which may not coincide with the average treatment effect in the hypothetical trial. Although we did not observe obvious heterogeneity in treatment effect between the matched and overall population among incident new users (see supplemental table 4), we advise caution when extrapolating the current findings to a broader population with osteoporosis or to those who could not be matched (1.1% of the study population), which needs to be confirmed in future studies. Third, we caution against over-interpretation of the results of the subgroup analyses because they were not prespecified. Fourth, we chose continuous users of oral bisphosphonates, representing the most widely used drug pattern, as our comparator group to better inform clinical decision making. Other drug sequences (switching to intravenous bisphosphonates, romosozumab, teriparatide, selective estrogen receptor modulator) can also be used in specific clinical scenarios, and thus need to be examined in future studies. Fifth, the target trial emulation approach aims to estimate causal effects and strengthen the analysis of observational studies. However, as this study is not an experimental design, the causality of the results should be interpreted with caution. Finally, since the actual number of events in the denosumab cohort was low (table 2 and supplemental table 19) and the mean duration of follow-up was only two years, long term benefits and withdrawal effects remain to be assessed as additional real world data become available. As no denosumab intervention studies have been performed in the population with diabetes yet, this study can be viewed as hypothesis generating and an incentive for randomized controlled trials to be performed.
Meaning of the study
Osteoporosis and diabetes mellitus are major global health problems with a high prevalence. Of the 7808 postmenopausal women with osteoporosis in the FREEDOM trial, 24.7% had diabetes or prediabetes,8 whereas in the population older than 60 years with prediabetes in another study, nearly 60% of women and 40% of men had osteoporosis or osteopenia.49 Drawing on previous experimental and preclinical research, this study provides population level evidence that denosumab use for osteoporosis in adults may simultaneously reduce the risk of type 2 diabetes. These findings have important implications for tailoring individualized drug management of osteoporosis.
Conclusions
In adults with osteoporosis, denosumab was associated with a reduced risk of type 2 diabetes compared with an oral bisphosphonate. As a considerable proportion of people with osteoporosis are at high risk of type 2 diabetes (eg, those with prediabetes or obesity), the risk of type 2 diabetes might be reduced in these individuals when using denosumab compared with oral bisphosphonates.
What is already known on this topic
Downregulation of the receptor activator of nuclear factor κ B ligand (RANKL) signaling can improve glucose metabolism
Both observational studies and post hoc analyses of randomized clinical trials have shown the benefit of denosumab on glycemic variables
Whether denosumab, a humanized monoclonal antibody against RANKL, reduces the risk of type 2 diabetes, however, remains unclear
What this study adds
Switching to, or initiating, denosumab was associated with a 32% decreased risk of type 2 diabetes compared with using an oral bisphosphonate
Individuals at high risk of type 2 diabetes (eg, those with prediabetes or obesity) who use denosumab may experience a further reduction in diabetes risk compared with those who use bisphosphonates
Acknowledgments
We thank Yuqing Zhang (epidemiologist, Massachusetts General Hospital, Harvard Medical School) for his methodological and statistical support of this work. IMRD is a registered trademark of Cegedim in the United Kingdom and other countries. Reference made to the IMRD database is intended to be descriptive of the data asset licensed by IQVIA.
Web extra.
Extra material supplied by authors
Supplementary information: Additional figures 1-5, methods, tables 1-19, and references
Contributors: GL (lei_guanghua@csu.edu.cn), PT (pftang301@126.com), and DHS (dsolomon@bwh.harvard.edu) are joint corresponding authors and contributed equally to this work. HL, GL, PT, and DHS conceived and designed the study. HL, XL, and JW acquired and analyzed the data under the supervision of GL. HL, SSZ, and DHS prepared the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript. GL had full access to the data and takes responsibility for the integrity of the study. The corresponding authors (GL, PT, and DHS) attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. GL, PT, and DHS are the guarantors of this manuscript.
Funding: DHS is supported by the National Institutes of Health (NIH, P30 AR072577). SKT is supported by NIH (K23 AR075070 and L30 AR070514). SSZ is supported by a National Institute for Health and Care Research clinical lectureship. GL is supported by the National Natural Science Foundation of China (NSFC, 81930071 and U21A20352) and the National Key Research and Development Program (2022YFC3601900). PT is supported by the National NSFC (81972102). CZ is supported by the National NSFC (82072502). HL is supported by The Excellent Young Scholar Training Program from The Chinese PLA General Hospital (YQPY2020001), Beijing Association for Science and Technology (BYESS2022021), and China Post-doctoral Science Foundation (2020M682593). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; DHS receives salary support from unrelated research contracts to his institution from Abbvie, Amgen, CorEvitas, Janssen, and ModernaTx; SSZ receives lecture honorariums and participates on the medical advisory board from Union Chimique Belge; KY receives consulting fees from OM1; and no other relationships or activities that could appear to have influenced the submitted work.
DHS, PT, and GL (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. For inquiries about methodology details, please contact HL (houchenlyu@gmail.com).
Dissemination to participants and related patient and public communities: Findings from this study will be disseminated through the websites of the authors’ institutes alongside the publication of this manuscript. Social media will be used to draw attention to the work and stimulate debate about its findings.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the scientific review committee of IQVIA and the institutional review board at Xiangya Hospital (21SRC059, 2018091077) with waiver of informed consent. This work uses deidentified data provided by patients as a part of their routine primary care.
Data availability statement
No additional data available. Applications to access data can be made via https://www.the-health-improvement-network.com .
References
- 1. Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756-65. 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 2. Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 2012;11:401-19. 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab 2019;104:1595-622. 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 4. Gregson CL, Armstrong DJ, Bowden J, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2022;17:58. 10.1007/s11657-022-01061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol 2017;69:1521-37. 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 6. Bonora E, Kiechl S, Willeit J, et al. Bruneck study . Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes 2004;53:1782-9. 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 7. Kiechl S, Wittmann J, Giaccari A, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med 2013;19:358-63. 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 8. Napoli N, Pannacciulli N, Vittinghoff E, et al. Effect of denosumab on fasting glucose in women with diabetes or prediabetes from the FREEDOM trial. Diabetes Metab Res Rev 2018;34:e2991. 10.1002/dmrr.2991. [DOI] [PubMed] [Google Scholar]
- 9. Weivoda MM, Chew CK, Monroe DG, et al. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat Commun 2020;11:87. 10.1038/s41467-019-14003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz AV, Schafer AL, Grey A, et al. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J Bone Miner Res 2013;28:1348-54. 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- 11. Hernán MA. Methods of Public Health Research - Strengthening Causal Inference from Observational Data. N Engl J Med 2021;385:1345-8. 10.1056/NEJMp2113319. [DOI] [PubMed] [Google Scholar]
- 12. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393-401. 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 13. Myland M, O’Leary C, Bafadhal B, et al. IQVIA Medical Research Data (IMRD). In: Sturkenboom M, Schink T, eds. Databases for Pharmacoepidemiological Research. Springer International Publishing, 2021: 67-76, 10.1007/978-3-030-51455-6_4. [DOI] [Google Scholar]
- 14. Zhao SS, Lyu H, Solomon DH, Yoshida K. Improving rheumatoid arthritis comparative effectiveness research through causal inference principles: systematic review using a target trial emulation framework. Ann Rheum Dis 2020;79:883-90. 10.1136/annrheumdis-2020-217200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016;183:758-64. 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suissa S, Moodie EEM, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 2017;26:459-68. 10.1002/pds.4107. [DOI] [PubMed] [Google Scholar]
- 17. Douros A, Dell’Aniello S, Yu OHY, Filion KB, Azoulay L, Suissa S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ 2018;362:k2693. 10.1136/bmj.k2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douros A, Lix LM, Fralick M, et al. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis : A Multicenter Cohort Study. Ann Intern Med 2020;173:417-25. 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 19. Yu OHY, Dell’Aniello S, Shah BR, et al. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Sodium-Glucose Cotransporter 2 Inhibitors and the Risk of Below-Knee Amputation: A Multicenter Observational Study. Diabetes Care 2020;43:2444-52. 10.2337/dc20-0267. [DOI] [PubMed] [Google Scholar]
- 20. Fisher A, Fralick M, Filion KB, et al. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Sodium-glucose co-transporter-2 inhibitors and the risk of urosepsis: A multi-site, prevalent new-user cohort study. Diabetes Obes Metab 2020;22:1648-58. 10.1111/dom.14082. [DOI] [PubMed] [Google Scholar]
- 21. Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ 2017;359:j5019. 10.1136/bmj.j5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyu H, Yoshida K, Zhao SS, et al. Delayed Denosumab Injections and Fracture Risk Among Patients With Osteoporosis : A Population-Based Cohort Study. Ann Intern Med 2020;173:516-26. 10.7326/M20-0882. [DOI] [PubMed] [Google Scholar]
- 24. Wright AK, Kontopantelis E, Emsley R, et al. Life Expectancy and Cause-Specific Mortality in Type 2 Diabetes: A Population-Based Cohort Study Quantifying Relationships in Ethnic Subgroups. Diabetes Care 2017;40:338-45. 10.2337/dc16-1616. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes Association Professional Practice Committee . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl 1):S17-38. 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 26. Movahedi M, Beauchamp ME, Abrahamowicz M, et al. Risk of Incident Diabetes Mellitus Associated With the Dosage and Duration of Oral Glucocorticoid Therapy in Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1089-98. 10.1002/art.39537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC, Cafri G. Variance estimation when using propensity-score matching with replacement with survival or time-to-event outcomes. Stat Med 2020;39:1623-40. 10.1002/sim.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 2015;11:437-41. 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crump RK, Hotz VJ, Imbens GW, et al. Dealing with limited overlap in estimation of average treatment effects. Biometrika 2009;96:187-99. 10.1093/biomet/asn055. [DOI] [Google Scholar]
- 30. Schuster NA, Hoogendijk EO, Kok AAL, Twisk JWR, Heymans MW. Ignoring competing events in the analysis of survival data may lead to biased results: a nonmathematical illustration of competing risk analysis. J Clin Epidemiol 2020;122:42-8. 10.1016/j.jclinepi.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 31. Lee DH, Rezende LFM, Ferrari G, et al. Physical activity and all-cause and cause-specific mortality: assessing the impact of reverse causation and measurement error in two large prospective cohorts. Eur J Epidemiol 2021;36:275-85. 10.1007/s10654-020-00707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017;167:268-74. 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 33. ElSayed NA, Aleppo G, Aroda VR, et al. on behalf of the American Diabetes Association . 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023;46(Suppl 1):S128-39. 10.2337/dc23-S008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abe I, Ochi K, Takashi Y, et al. Effect of denosumab, a human monoclonal antibody of receptor activator of nuclear factor kappa-B ligand (RANKL), upon glycemic and metabolic parameters: Effect of denosumab on glycemic parameters. Medicine (Baltimore) 2019;98:e18067. 10.1097/MD.0000000000018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest 2019;129:3214-23. 10.1172/JCI125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lasco A, Morabito N, Basile G, et al. Denosumab Inhibition of RANKL and Insulin Resistance in Postmenopausal Women with Osteoporosis. Calcif Tissue Int 2016;98:123-8. 10.1007/s00223-015-0075-5. [DOI] [PubMed] [Google Scholar]
- 37. Passeri E, Benedini S, Costa E, Corbetta S. A Single 60 mg Dose of Denosumab Might Improve Hepatic Insulin Sensitivity in Postmenopausal Nondiabetic Severe Osteoporotic Women. Int J Endocrinol 2015;2015:352858. 10.1155/2015/352858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-kappaB. Nat Med 2005;11:183-90. 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kondegowda NG, Fenutria R, Pollack IR, et al. Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-κB Ligand Pathway. Cell Metab 2015;22:77-85. 10.1016/j.cmet.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 2020;16:349-62. 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 41. Chan D-C, Yang R-S, Ho C-H, Tsai YS, Wang JJ, Tsai KT. The use of alendronate is associated with a decreased incidence of type 2 diabetes mellitus--a population-based cohort study in Taiwan. PLoS One 2015;10:e0123279. 10.1371/journal.pone.0123279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toulis KA, Nirantharakumar K, Ryan R, Marshall T, Hemming K. Bisphosphonates and glucose homeostasis: a population-based, retrospective cohort study. J Clin Endocrinol Metab 2015;100:1933-40. 10.1210/jc.2014-3481. [DOI] [PubMed] [Google Scholar]
- 43. Vestergaard P. Risk of newly diagnosed type 2 diabetes is reduced in users of alendronate. Calcif Tissue Int 2011;89:265-70. 10.1007/s00223-011-9515-z. [DOI] [PubMed] [Google Scholar]
- 44. Viggers R, Al-Mashhadi Z, Starup-Linde J, Vestergaard P. The Efficacy of Alendronate Versus Denosumab on Major Osteoporotic Fracture Risk in Elderly Patients With Diabetes Mellitus: A Danish Retrospective Cohort Study. Front Endocrinol (Lausanne) 2022;12:826997. 10.3389/fendo.2021.826997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen P-W, Su H-Y, Tu Y-K, et al. Association of bisphosphonates with diabetes risk and glycemic control: a meta-analysis. Osteoporos Int 2023;34:387-97. 10.1007/s00198-022-06616-3. [DOI] [PubMed] [Google Scholar]
- 46. Zhou R, Guo Q, Xiao Y, et al. Endocrine role of bone in the regulation of energy metabolism. Bone Res 2021;9:25. 10.1038/s41413-021-00142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panagiotakou A, Yavropoulou M, Nasiri-Ansari N, et al. Extra-skeletal effects of bisphosphonates. Metabolism 2020;110:154264. 10.1016/j.metabol.2020.154264. [DOI] [PubMed] [Google Scholar]
- 48. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83:1032-45. 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen C, Chen Q, Nie B, et al. Trends in Bone Mineral Density, Osteoporosis, and Osteopenia Among U.S. Adults With Prediabetes, 2005-2014. Diabetes Care 2020;43:1008-15. 10.2337/dc19-1807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional figures 1-5, methods, tables 1-19, and references
Data Availability Statement
No additional data available. Applications to access data can be made via https://www.the-health-improvement-network.com .