Abstract
Lipid bilayers form the main matrix of functional cell membranes, and their dynamics underlie a host of physical and biological processes. Here we show that elastic membrane properties and collective molecular dynamics (MD) are related by the mean-square amplitudes (order parameters) and relaxation rates (correlation times) of lipid acyl chain motions. We performed all-atom MD simulations of liquid-crystalline bilayers that allow direct comparison with carbon-hydrogen (CH) bond relaxations measured with NMR spectroscopy. Previous computational and theoretical approaches have assumed isotropic relaxation, which yields inaccurate description of lipid chain dynamics and incorrect data interpretation. Instead, the new framework includes a fixed bilayer normal (director axis) and restricted anisotropic motion of the CH bonds in accord with their segmental order parameters, enabling robust validation of lipid force fields. Simulated spectral densities of thermally excited CH bond fluctuations exhibited well-defined spin-lattice (Zeeman) relaxations analogous to those in NMR measurements. Their frequency signature could be fit to a simple power-law function, indicative of nematic-like collective dynamics. Moreover, calculated relaxation rates scaled as the squared order parameters yielding an apparent modulus for bilayer bending. Our results show a strong correlation with values obtained from solid-state NMR studies of bilayers without and with cholesterol as validated by neutron spin-echo measurements of membrane elasticity. The simulations uncover a critical role of interleaflet coupling in membrane mechanics and thus provide important insights into molecular sites of emerging elastic properties within lipid bilayers.
Significance
The lipid make-up of a bilayer determines its measurable properties, but how the motions of individual molecules combine to produce these properties remains unclear. By exploiting the synergy between NMR spectroscopy and molecular dynamics (MD) simulations, we show that the lipid dynamics in a bilayer are collective yet segmental in nature and contribute directly to bilayer elasticity. Comparison between MD simulations and NMR entails an improved theoretical framework that allows the two techniques to be directly related. This study provides novel insights into the inner workings of lipid membranes while delivering a new tool for validating computational approaches against experimental data.
Introduction
Lipid membranes are ubiquitous in biology—they exhibit a large spectrum of physical properties which allows them to serve a multitude of functions (1,2). As the subjects of both experimental and theoretical investigations, they are justifiably of great contemporary interest (3,4,5,6,7). Their rich behavior is enabled by the large diversity of lipid molecules and their distinct mixing properties (8,9,10,11). The resulting structural features of the membranes such as bilayer thickness and lipid packing have been studied extensively and are known to influence membrane permeability, elasticity, and protein-membrane interactions (12,13,14,15,16,17). Lipid molecules also undergo constant thermal motions in the form of orientational fluctuations and segmental dynamics. Viewed in this context, solid-state NMR is one of the few experimental techniques that can detect and quantify these thermal motions at the level of individual lipid molecules (18,19,20,21,22,23). It is sensitive to the fluctuations of the lipid acyl chains, in particular the carbon-hydrogen (CH or deuterium) bonds. By measuring the frequency dependence of bond reorientations, i.e., their power spectrum or spectral density, analysis of the bilayer fluctuations is possible through the combination of NMR lineshape and relaxation time measurements. The NMR lineshape directly reveals the various degrees of motional averaging of the molecules and quantifies the order parameters of the carbons along the lipid chains (24,25,26). At the same time, measurements of the rate of nuclear spin relaxation of each carbon provide information on correlated CH bond fluctuations over multiple time scales (27).
Currently an extensive body of NMR data points to collective dynamics of the lipid molecules that are linked to bilayer mechanical properties (23). However, these results have been challenged by theoretical arguments stemming from the convoluted nature of the spectral density obtained from NMR relaxation measurements. One approach that has the potential to resolve this dilemma is molecular dynamics (MD) simulations. Indeed, acyl chain order parameter profiles obtained from NMR have been historically used to validate the simulation force-field parameters (7,28,29,30). These parameters govern the forces exerted on the atoms in an MD simulation that drive their dynamics, rendering comparisons between theory and experiments particularly informative. While NMR-derived order parameter profiles describe the time-averaged structural properties of the lipid molecules (31), the corresponding relaxation measurements hold the key to understanding the connection between local lipid dynamics and bilayer properties (29,32,33,34). In particular, the CH bond dynamics show both slow and fast motions, but the origins of this emerging hierarchy of motions remain hidden in the data and pose new questions. For example, are they a result of the reorientations of the individual molecules, as detailed by a so-called molecular model, or rather the concerted motions of larger lipid assemblies—in other words, collective dynamics (23,35)? And if the motions are indeed collective, do they resemble those of nematic liquid crystals where the different segments of the lipid chains reorient collectively, or smectic liquid crystals where the identity of the lipids is lost in the dynamical modes of a fluctuating two-dimensional (2D) surface? The answers to these questions will not only illuminate the relationship between lipids and bilayer dynamics but also inform the correspondence of NMR observables with various experimental techniques that measure global bilayer fluctuations, such as flicker spectroscopy (36). Experimentally validated MD simulations thus present an ideal platform for replicating NMR observables and investigating their molecular origins (29,32,37,38,39).
Here we use molecular simulations to analyze the nature of bilayer dynamics, the relationship between the magnitude of CH bond fluctuations and their relaxation rates, and the resultant elastic moduli as measured with NMR spectroscopy. To facilitate comparison with solid-state NMR data, we first develop a theoretical framework for calculating spin-lattice relaxation rates from the simulations that overcomes shortcomings of existing numerical approaches. The lipid orientational dynamics are considered for in vitro and in silico systems, together with the inherent anisotropic motion of the CH bond fluctuations. For validation, we simulated a set of experimentally well-characterized model membranes composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and cholesterol (40,41,42). Using NMR-based protocols, we calculate the spectral densities of the lipid CH bond fluctuations from the simulation trajectories and show they can be modeled by a simple power-law function. Lastly, we evaluate the simulation-derived carbon-specific order parameters and relaxation rates, which we find to follow a square-law dependence related to bilayer elasticity. Our theoretical findings validate and further clarify interpretations of NMR data while presenting a novel approach for calculating bending moduli of simulated bilayers. Importantly, the simulations identify a previously overlooked region of the bilayer where the two leaflets interdigitate that holds a key to membrane mechanics. Our methodology thus opens new avenues for the validation of lipid force fields in terms of underlying theoretical concepts of membrane elasticity for liquid-crystalline bilayers (5,38,43,44).
Materials and methods
Bilayer construction
Bilayers containing DMPC with 0, 20, 33, and 50 mol % cholesterol were constructed with the CHARMM-GUI web server (45,46,47,48,49). Each bilayer contained 100 lipids per leaflet and was hydrated with 45 water molecules per lipid with no added salt. The systems thus ranged from 46,000 to 50,000 atoms.
Simulation protocol
Each system was initially relaxed with the CHARMM-GUI 6-step equilibration protocol using NAMD version 2.12 (50) and the CHARMM36 force field for lipids (29,51). The simulation temperature was set to 317 K (44°C), the same value as used in the cognate experimental studies (34,41,52). This initial equilibration was proceeded with 1 ns of simulation following the CHARMM-GUI simulation production protocol employing a 2-fs time step, 10- to 12-Å Lennard-Jones potential with the NAMD vdwForceSwitching option enabled, and rigidbonds set to all. The particle mesh Ewald (PME) method was used for treatment of the electrostatic interactions with a grid spacing of 1 Å, and a constant temperature (317 K) and pressure (1 atm) were maintained with a Langevin thermostat and a Nose-Hoover Langevin piston, respectively. The systems were then transferred to the Summit computer cluster at Oak Ridge National Laboratory, where they were simulated for a total of 2 μs each with the OpenMM software (53). These production simulations implemented PME for electrostatic interactions and were performed at a temperature of 317 K under an NPT ensemble with semi-isotropic pressure coupling. The simulation runs utilized a 2-fs time step and Langevin dynamics with a friction parameter set to 1.0 per picosecond. Additional parameters included: EwaldErrorTolerance, 0.0005; rigidwater, True; constraints, HBonds; and ConstraintTolerance, 0.000001. The van der Waals interactions were calculated applying a cutoff distance of 12 Å and switching the potential from 10 Å. Use of the Langevin integrator for modulating the temperature in the simulations can alter the dynamics of the molecules (i.e., decrease the diffusivity) by introducing friction, which adds artificial thermal noise to the movement of the atoms, as noted in (54). The small 1.0/ps friction used here, however, is expected to have minimal effects on the fluctuations of the CH bonds and, consequently, their calculated relaxation rates.
Simulation analysis
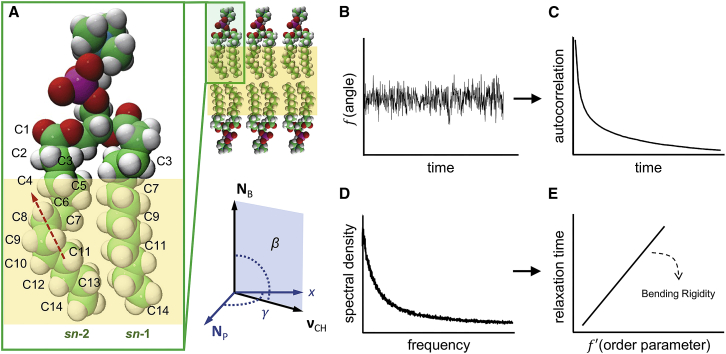
All post-processing analysis was performed with VMD (55), MATLAB, and in-house tcl and MATLAB scripts. Unless otherwise noted, calculations were done on all trajectory frames totaling more than 50,000 with an output frequency corresponding to a 40-ps time step. Each bilayer system was first centered so that in every frame the average position of the terminal methyl carbons of all lipid acyl tails (carbons C14 on the sn-1 and sn-2 chains, Fig. 1 A) was set at (x,y,z) = (0,0,0). The average area per lipid was calculated by dividing the average lateral area of the simulation box by 100, i.e., the number of lipids in one leaflet, including cholesterol. The acyl chain order parameter () at each carbon, defined as the second-rank Wigner rotation matrix element where are the Euler angles (56), was obtained with the formula
| (1) |
where is the time-dependent angle between a CH bond at this carbon and the bilayer normal (i.e., the z-dimension of the simulation box). Likewise, represents the time-dependent Euler angles, and the angular brackets denote an ensemble average over all lipids in the bilayer and all trajectory frames.
Figure 1.
Schematic representation of the simulation methodology. (A) Lipid bilayer and structure of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC). Labels indicate carbons C3, C7, C9, C11, and C14 on the sn-1 chain and all carbons on the sn-2 chain of the lipid. Carbon atoms are shown in green, hydrogen in white, oxygen in red, and phosphorus in purple. Red dashed arrow shows a representative local director vector spanning carbons C8 and C11 on the sn-2 chain. Yellow shading outlines the part of the lipid chains (in the absence of cholesterol) where the fluctuations of the CH bonds follow a square-law dependence. Shown also is an example of the angle between a CH bond vector on the lipid chain and the bilayer normal (director axis), as well as the angle describing rotation of around . (B) Fluctuations of and over time are analyzed to obtain (C) their autocorrelation function averaged across all lipids and time. Fourier transformation of the autocorrelation function gives (D) the spectral density from which (E) the NMR relaxation rate is obtained. Multiple carbon atoms on the lipid chains are used together with their respective order parameters to calculate the bilayer bending modulus. See text for details. To see this figure in color, go online.
Calculation of autocorrelation functions from simulation trajectories
Autocorrelation functions of the lipid CH bond fluctuations were calculated from the trajectories as follows (Fig. 1, A–C). First, the direction of each vector in the bilayer was computed as a function of time, where denotes an individual DMPC lipid, is the carbon number on the sn-1 () or sn-2 () chain, and is the hydrogen atom. These data were used to calculate three individual time series for each vector , corresponding to the second-rank Wigner rotation matrix elements with as defined in (56):
| (2) |
| (3) |
| (4) |
The other matrix elements are given by the relation where the asterisk means the complex conjugate (Eq. 4.22 of (57)). In (2), (3), (4), we have that is a function of the Euler angles describing the transformation from the principal axis system to the laboratory frame (see Fig. S1). From this point on, the subscript PL will be suppressed but implied for brevity, unless otherwise noted. Furthermore, the product of and its complex conjugate is invariant to the sign of the projection index (i.e., the result is the same regardless of whether the director vectors for the two leaflets point in the same or opposite directions), and therefore we consider only the elements with non-negative values (see Eq. 12 below and Eq. 4.31 in (57)). The angle is the angle between and the bilayer normal (where is for the lipids in the top leaflet and for the lipids in the bottom leaflet) at time , and is the time-dependent angle describing the rotation of the bond with respect to the bilayer normal (see Fig. 1 A). Thus, was calculated (in Cartesian coordinates) as the angle between the normal to the plane defined by the cross product of and , i.e., , and the -axis of the simulation box :
| (5) |
The autocorrelation function of each time series was then obtained as
| (6) |
where is the total number of frames and is the lag time (see also Eq. 13 below). Note that the second term on the right in Eq. 6, the square of the mean, vanishes for and it is thus nonzero only for the matrix element. Furthermore, for the special case of lag time , Eq. 6 becomes the variance of , given by
| (7) |
For every carbon , the autocorrelation function from Eq. 6 was averaged over its two covalently bound hydrogen atoms and all DMPC lipids in the bilayer to yield the autocorrelation function for that carbon atom:
| (8) |
Theoretical framework for calculation of NMR relaxation rates from molecular dynamics trajectories
The lipid bilayer in the MD simulations corresponds to a membrane patch whose director axis can be assumed to be the average lamellar normal, i.e., a vector parallel to the z-dimension of the simulation box. Hence, the spectral densities of the CH bond fluctuations within this director frame need to first be transformed into the so-called laboratory reference system to correspond to the experimental NMR relaxation rates, which are measured in the presence of a magnetic field (see supporting material and Fig. 4 in (58)). The director-frame spectral density functions, , are Fourier transforms of the correlation functions that characterize the internal motions within the membrane. In an NMR experiment, typically one can assume that the relaxation rates are orientationally averaged, because for the case of lipid multilamellar dispersions the molecules undergo rapid translational diffusion over all director orientations of the curved surfaces during the relaxation (≈50–100 ms and longer). Furthermore, if one assumes unrestricted isotropic motion with a single correlation time (the solution NMR limit), the relaxation rate simplifies to
| (9) |
Here is a Lorentzian reduced spectral density where is the correlation time, is the static quadrupolar coupling constant, and is the Larmor frequency of the NMR measurement (24,35) (see Eq. S21 and discussion).
However, in a simulation trajectory for a bilayer run with periodic boundary conditions and semi-isotropic pressure coupling, the lamellar normal (director) remains fixed—the assumption for unrestricted isotropic motion and, thus, Eq. 9, does not hold. Instead, to compare the simulation results to the experimental ones, the orientationally averaged but anisotropic spin-lattice relaxation rate needs to be expressed in terms of the director-frame spectral densities (see discussion and supporting material for details of the derivation):
| (10) |
where
| (11) |
and
| (12) |
The value of the numerical pre-factor in Eq. 10 is = (see supporting material), and the spectral density is defined as the two-sided Fourier transform of the correlation function . The latter decays to a zero value because the fluctuations are expressed relative to the average values of the Wigner rotation matrix elements. The director-frame correlation functions can also be written by subtracting the modulus squared of the average value to read
| (13) |
Note that for a cylindrically symmetric distribution, the last term on the right becomes where is the Kronecker delta function. For completeness, the orientationally averaged relaxation rates can also be expressed in terms of the spherical-harmonic correlation functions. The equivalent results in terms of the spherical-harmonic correlation functions and spherical-harmonic spectral densities can be found in the supporting material.
Calculation of relaxation rates from the simulation trajectories
(10), (11), (12), (13) provide the essential framework for calculating the relaxation rates from the simulation trajectories. Note that the autocorrelation function for the MD simulation trajectories in Eq. 8 is the same as the correlation function in Eq. 13. We can thus take its discrete Fourier transform to arrive at an expression for the spectral density of the bond fluctuations from the simulations:
| (14) |
In Eq. 14 with being the Larmor frequency (), is the sampling time interval, and is the time at lag .
Eq. 14 is a one-sided Fourier transform while the continuous Fourier integral in Eq. 11 goes from . We therefore need to multiply the former by a factor of two but without overcounting the element at , which thus reads
| (15) |
As mentioned above, the t = 0 element of the correlation function is the variance of the matrix element (Eq. 7). Accordingly, the second term in Eq. 15 represents a constant equal to { which is added to at every frequency. Estimation of the spectral density from the simulations thus exhibits a strong dependence on the discrete time step (sampling interval). Normally, could be as small as the time step with which atomic coordinates are written out during the simulation if every single frame is used for analysis (in our case ps). Alternatively, if every th frame is used instead, then the sampling time interval would be . Note that the simulations are run with a 2-fs time step which puts a lower bound on , but such frequent output is in general impractical for microsecond-long simulations.
Having this -dependent constant would result in an unrealistic shift of the spectral density and, consequently, the relaxation rates. Thus, to remove this apparent dependence on and recover the analytical result in Eq. 11, which is in the limit of (see discussion), we first fit to a well-defined function, and then use the fit to resample the correlation function at a much smaller . For all CH bonds, the time series of the second-rank Wigner rotation matrix elements from (2), (3), (4) follow a power law of the form: (see Figs. 2 A and S2–S4). We therefore fit for to a power-law function and use the best fit together with the variance at to resample the correlation function at a smaller interval and recover the spectral density:
| (16) |
Note that Eq. 16 is calculated at (or, equivalently, ) where is an integer and is the total sampled time of the autocorrelation function in accord with the Nyquist-Shannon sampling theorem. The choice of is constrained by the variance of which is the first and largest matrix element. Therefore, to ensure the smoothness of the reconstructed correlation function, for each carbon we set equal to the minimum value such that where . Figs. S5 and S6 show the reconstructed correlation functions and resulting carbon values (see discussion).
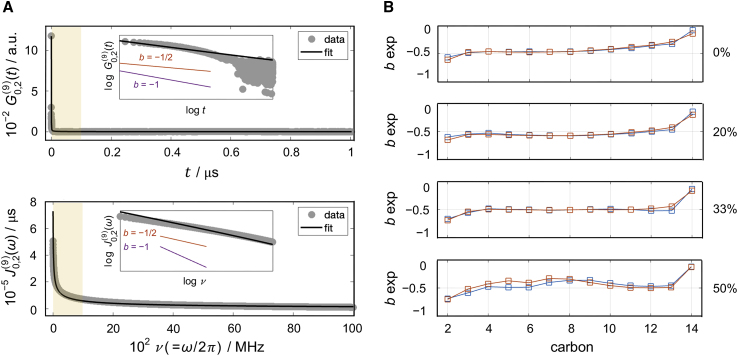
Figure 2.
Illustration of dynamics of CH bond fluctuations. (A) Example of (top) autocorrelation function and (bottom) spectral density function for carbon C9 of the sn-2 chain of DMPC. The results correspond to fluctuations of the CH bonds as described by the Wigner function. Simulation data are shown in gray and best power-law fit to the simulation results in black. Insets show expansion of the data from the highlighted region from 0 to 1000 MHz but in a log-log plot. Shown for comparison are sample functions of the form (purple) and (red). (B) Power exponents of best fits to the spectral densities for carbons C2–C14 on the sn-1 (red) and sn-2 (blue) chains of DMPC with 0, 20, 33, and 50 mol % cholesterol. The power exponents of all carbons in all simulated bilayers are close to −1/2, consistent with collective segmental dynamics. All simulations were performed at 44°C. See text for details. To see this figure in color, go online.
Accordingly, the theoretical spectral densities , and from Eq. 16 are fit to power-law functions of the form. The best fits are then used to calculate the relaxation rate of carbon following Eq. 10:
| (17) |
In the above formula , ω0 is the resonance (Larmor) frequency, and all other symbols are previously defined. The theoretical spectral densities refer to the CH fluctuations with respect to the bilayer normal (director axis) in terms of their mean-square amplitudes (variance) and reduced values. Relaxation rates are averaged over all director orientations to correspond to the actual experimental measurements. Importantly, although the relaxation rates are orientationally averaged, they are not the same as the isotropic solution NMR results found in textbooks. Use of the latter is often assumed but is inapplicable to lipid membranes, because of the underlying assumption that the fluctuations are isotropic, i.e., there is no order parameter. Rather, the CH bond fluctuations must be expressed with respect to the bilayer director and occur with restricted amplitude, as described by their orientational order parameters. Thus, the formula applicable to MD simulations and to the validation of MD force fields based on NMR relaxation is given in Eq. 17. This result is based on group theory principles (35) and is different from that obtained by application of the spherical-harmonic addition theorem (see discussion). Validation of the theoretical and experimental results is further discussed below. Note that the mean-square amplitudes are equal to the area under the spectral density in the region and they cannot be numerically determined from the integral of due to the finite sampling. Instead the mean-square amplitudes are thought of as the orientational order parameters in terms of a Clebsch-Gordon series expansion (35) and can be calculated from the raw simulation data (i.e., the time series of the Wigner rotation matrix elements from (2), (3), (4)).
Application of NMR-based approach to extract apparent bilayer bending rigidity from molecular dynamics trajectories
An apparent bending modulus can be extracted from the slope of the theoretically predicted square-law dependence between the relaxation rate and order parameter of the acyl chain carbons, as derived previously (35). Following the same NMR-based protocol, we obtain of the simulated bilayers with the formula
| (18) |
In Eq. 18 is the bilayer viscosity, is the order parameter estimated for slow motions, is an elastic constant related to and the full bilayer thickness (see below), and is a constant in units of which is obtained from the relation:
| (19) |
Here the constant is the slope of the dependence of on is the static quadrupolar coupling constant, and MHz is the deuterium Larmor frequency. The thickness which is used to obtain in Eq. 18 represents the full bilayer thickness and is calculated from the number density profile of the bilayer. Specifically, a single number density profile of all bilayer lipid atoms is calculated with the Density Profile plugin of VMD (59) for each system. The profile has two peaks corresponding to the maximum density in the hydrocarbon regions of the two leaflets and decreases to 0 at the ends of the headgroup regions where bulk water begins (see results). The thickness used in Eq. 18 is set equal to the distance between the two tails of the bilayer number density profile where the density drops below 5% of the maximum (peak) density. Table S1 lists the corresponding thicknesses for all simulated bilayers.
The full range of carbons used for the square-law fits in the simulations included C4–C13, C6–C13, C8–C13, and C10–C13 for the bilayers with 0, 20, 33, and 50 mol % cholesterol, respectively. Errors for the values obtained from the fits were calculated as the standard deviation of the slopes resulting from the best fits to the data after excluding the last 0, 1, or 2 carbon atoms with largest order parameters from each chain. Note that the terminal methyl carbon C14 was also excluded from the fits due to its different geometry.
Splay-fluctuations-based analysis of bilayer bending rigidity
The modulus can also be obtained in simulations of lipid bilayers from analysis of the fluctuations in lipid splay angles (see (60) and references therein). In brief, the procedure involves calculating the probability distribution of splay angles (i.e., the angles between the local director vectors) of neighboring pairs of lipids within a leaflet and using this distribution to obtain a potential of mean force (PMF) profile. A quadratic function is then fit to the PMF in the region of small deviations from the mean, and the coefficient of the quadratic term is the corresponding splay modulus for that lipid pair. The splay moduli of all lipid pairs are subsequently weighted and summed, revealing the leaflet value. The bilayer is then the sum of the values of the two opposed monolayers. Here, we used the OpenStructure software and algorithm presented in (61,62) to calculate with an alternative computational method from the splay moduli of DMPC, cholesterol, and DMPC-cholesterol lipid pairs in the simulation trajectories.
Analysis of local director fluctuations
Local director (LD) vectors of different lengths were defined at each carbon along the lipid acyl chains (Fig. 1 A). Each LD vector connects the carbon of interest on the sn-2 chain either to another carbon atom further up the chain on the same lipid toward the lipid headgroup with , or to the lipid phosphorus atom P (see results). The fluctuations of the orientations of an LD vector were analyzed in a manner similar to the calculation of the spectral densities described in Eqs. 8 and 15. The only differences are that all angles are defined with respect to the LD vector instead of the CH bond, the denominator in the pre-factor in Eq. 8 is instead of , and because the autocorrelation functions of the LD vector fluctuations do not follow a power law or an exponential function, no fitting and resampling of the correlation was performed. Instead, the spectral density was calculated with Eq. 15 using the sampling interval corresponding to the simulation of 40 ps.
Measurement of bending moduli with neutron spin-echo spectroscopy
To extend the results of the MD simulations and NMR analysis, complementary neutron spin-echo (NSE) measurements were performed on unilamellar vesicles extruded through a polycarbonate filter with 100-nm pore diameter (see supporting material for more details). The NSE data yielded the normalized intermediate scattering function, , for discrete q values within the accessed q range, where t is the Fourier time. For lipid membranes, the probed dynamics follow a stretched exponential function with a stretching exponent of 2/3, such that (63)
| (20) |
Fits of the intermediate scattering functions using the above equation yield the decay rate, , at individual q values. Plots of Γ(q) versus q show the typical dependence for thermally undulating elastic thin sheets predicted by Zilman and Granek (63). Using theoretical refinements by Watson and Brown (64), based on the Seifert-Langer model (65), allows calculation of a renormalized bending rigidity , where h is the height of the neutral surface from the midplane and is the monolayer area compressibility modulus. Assuming the neutral plane to be at the interface between the hydrophilic headgroups and the hydrophobic tails, these refinements result in a modified expression of the Zilman-Granek decay rates, , given by (66):
| (21) |
Here, is the thermal energy and is the solvent (i.e., D2O buffer) viscosity.
Results
Lipid dynamics at length scales smaller than the membrane thickness resemble nematic liquid crystals
Membrane lipids are known to diffuse rapidly within the plane of a fluid bilayer while their chains explore various rotational degrees of freedom (29,37,67,68). These thermally excited motions are governed by both bonded and nonbonded interactions and can be rather inhomogeneous along the lengths of the lipid chains due to the hydrophobic effect holding the two leaflets together (69). Nuclear spin relaxation measurements show both fast and slow components of the CH bond fluctuations at each carbon (34). Still, because the resulting signal has contributions from all lipids in the bilayer, the origins of this hierarchy of motions, and consequently the nature of the lipid dynamics, need further investigation (27,32,38,39,70). To help address this question, we sought to examine the thermal fluctuations of the CH bonds along the lipid acyl chains with all-atom MD simulations (60,67,71). As a test system we chose DMPC bilayers with increasing amounts of cholesterol that have been well characterized experimentally (34,41,52). In the past, CH bond fluctuations from simulation trajectories have been expressed in terms of spherical harmonics using spherical polar angles (72,73). However, that representation presents certain challenges (see discussion), and we sought to derive a theoretical framework centered around the so-called Wigner rotation matrix elements instead (35,74,75). The latter represent functions ((2), (3), (4)) of the Euler angles describing the CH bond orientation within the lipid membrane, where the angle is between the CH bond and the bilayer normal and the angle is for the rotation of the CH bond about the director (see Fig. 1 and Eq. 5). The autocorrelation function of the time series of each of these functions describes a different relaxation mode of the CH bond fluctuations and was found to follow a power-law dependence (see Fig. 2 A and materials and methods). Its Fourier transform yields the spectral density as a function of frequency that can be directly related to the relaxation rates measured experimentally by NMR spectroscopy (Eq. 17).
Importantly, the functional form of the spectral density contains information about the nature of the bilayer dynamics. Each spectral density function calculated from the simulations was found to follow a power-law dependence of the form (Figs. 2 A and S7–S9). This functional form has been shown to correspond to collective dynamics of the lipids, and its power-law exponent represents the dimensionality of the collective lipid interactions formulated in terms of quasi-elastic order-director fluctuations (ODF) (35). At this level, a continuum of wave-like disturbances with an exponent of −1 indicates 2D smectic-like dynamic undulations (19,75,76,77,78) while an exponent of −1/2 points to three-dimensional (3D) nematic-like fluctuations (26,35,79). Figs. 2 B and S10 show the exponent for all carbons on the sn-1 and sn-2 chains of the lipids in the simulated DMPC bilayers. As seen in the plots, the power exponents are very close to −1/2 throughout both chains in all trajectories. This result suggests that the dynamics of the lipid hydrocarbon acyl tails are collective and yet locally more segmental in nature, resembling the behavior of nematic liquid crystals at distances less than the bilayer thickness (80).
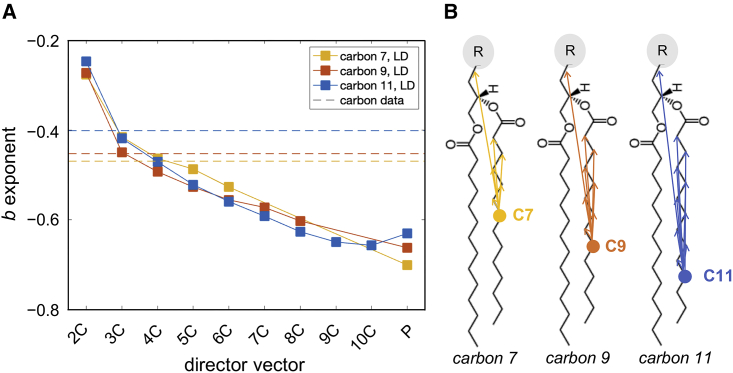
To explore this interpretation further, we compared the fluctuations of the lipid CH bonds with those of LD vectors of varying lengths (Fig. 3 A). An LD vector connects a carbon either to another carbon at increasing distance further up the chain or to the phosphate atom (red dashed arrow in Fig. 1 A and arrows in the schematic in Fig. 3 B). The spectral density of the fluctuations of each LD vector again followed a power law (Figs. S11–S13), where the fits to the power-law function yielded the corresponding b-coefficients. Fig. 3 exemplifies the relationship between these b-exponents and the lengths of the LD vectors by showing b versus length of the director vectors originating from carbons C7, C9, and C11 on the sn-2 chain of DMPC (Fig. 1 A). As seen from this figure, an increase in the length of an LD vector results in more smectic-like dynamics as the power exponent decreases, eventually approaching a value of approximately −0.7. Yet the motions of the CH bonds resemble most closely those of an LD vector of length 3–4 carbons (compare where the dashed lines cross the corresponding data points in Fig. 3 A), in agreement with a model of nematic-like dynamics of relaxation rates and order parameters, as originally proposed (35,80).
Figure 3.
Dynamics of local director (LD) vectors connecting two carbons (or a carbon and the phosphate) of a lipid. (A) Plotted are the b-exponents of the best power-law fits to the spectral densities of LD vectors originating from C7 (yellow), C9 (red), and C11 (blue) carbons on the sn-2 chain of DMPC in the cholesterol-free bilayer. The LD vectors are from two (2C) to ten (10C) carbon segments in length or extend all the way to the phosphorus atom (P). Shown for comparison are the exponents of the best power-law fits to the spectral densities of the CH bonds at C7, C9, and C11 carbons (dashed lines). (B) Schematic of all analyzed LD vectors. The CH bond fluctuations correspond most closely to LD vectors of lengths 3–4 carbons, illustrating the segmental nature of the CH bond dynamics. See text for details. To see this figure in color, go online.
Square-law dependence holds for specific carbon atoms
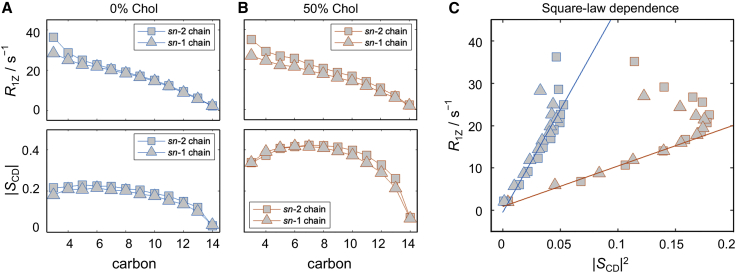
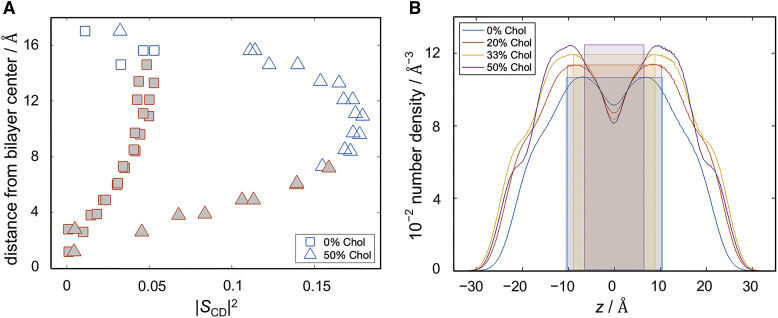
As mentioned earlier, the spectral densities of the CH bond fluctuations of a carbon atom on the lipid chains can be used to calculate the spin-lattice relaxation time of the bond fluctuations (Eq. 17 and Fig. 1). Various NMR experiments have shown that often in bilayers is dependent on the square of the order parameter, a rule thus termed the square-law (35,41,81). To understand the molecular origin of this surprising relationship—and test whether it holds in simulated bilayers—we analyzed this trend in our trajectories of DMPC bilayers with increasing amounts of cholesterol (0, 20, 33, and 50 mol %). As expected, the addition of cholesterol made the bilayer more ordered and tightly packed, decreasing the average area per lipid and increasing the bilayer thickness (Fig. S14). From this set of simulations, we calculated the spectral density profiles of the CH bonds along the two chains of DMPC in each trajectory and used them to obtain the corresponding relaxation rates at the NMR deuterium frequency of 76.8 MHz, corresponding to a magnetic field strength of 11.7 T (Eq. 17). Fig. 4 shows the relaxation rates (see materials and methods) and order parameters for all carbons in the simulated bilayers with 0 mol % and 50 mol % cholesterol, and how the two variables are functionally related to each other. From the corresponding log-log plots (Fig. S15) it becomes apparent that they exhibit a square-law relationship for a subset of the carbon atoms in the lower parts of the chains. In particular, carbons C4 through C13 in the DMPC bilayer without cholesterol and carbons within C6, C8, or C10 through C13 in the DMPC/cholesterol bilayers with 20, 33, and 50 mol % cholesterol, respectively (Figs. 4 and S15) clearly follow the square-law dependence, consistent with the experimental findings (41). The observed behavior is not chain dependent, as we see the same dependence for the sn-1 and sn-2 chains of the lipids (Fig. S15). Note that the C14 terminal methyl carbons on both chains have a different geometry and are therefore excluded from the analysis.
Figure 4.
Functional dependence of relaxation rates on CH bond order parameters in simulated bilayers. Data are shown for: (A) DMPC bilayer in the absence of cholesterol (blue) and (B) DMPC containing 50 mol % cholesterol (Chol) (red). Profiles of spin-lattice relaxation rates (R1Z, top) and order parameters (SCD, bottom) are plotted as a function of carbon position along the sn-1 and sn-2 chains of DMPC. (C) Same data but now replotted as the R1Z relaxation rate as a function of the squared SCD order parameter. Best linear fits to carbons (on both chains) C4 through C13 for 0 mol % cholesterol and C10 through C13 for 50 mol % cholesterol are shown in blue and red, respectively. Carbon atoms outside of these ranges deviate from the linear relationship. Note that the relaxation rates and order parameters exhibit a square-law dependence both with and without cholesterol. All simulations were performed at 44°C. See text for further details. To see this figure in color, go online.
Interestingly, the square-law in the simulated bilayers breaks down for carbons further up the chain (Figs. 4 and S15). These carbons are schematically illustrated in Fig. 1 A and reside at the top part of the acyl groups. To examine in more detail the differences between these carbons and the ones that follow the square-law, we calculated the average position of the carbon atoms relative to the bilayer midplane (Fig. 5 A) and compared their number density distributions (Fig. 5 B). These data show that regardless of the presence of cholesterol, the carbons that follow the square-law are consistently those in the middle of the bilayer, whose average depth within the membrane falls linearly with their squared order parameters. Stated differently, the relationship between and holds precisely for the CH bonds residing within the region of the bilayer sufficiently far from the headgroups where the two leaflets intercalate (Figs. 1 and 5). Thus, the existence of the square-law dependence uncovers the difference between interfacial (i.e., closer to the water-hydrocarbon interface) and noninterfacial (i.e., closer to the bilayer midplane) dynamics. While both are modulated by collective motions, the former are more smectic-like and the latter are more nematic-like in nature. These observations provide a possible explanation for the mechanistic origin of the square-law relationship and why it holds mainly for a subset of carbon atoms in the lipid acyl chains (see discussion).
Figure 5.
Properties of acyl carbons that follow square-law dependence of relaxation rates and order parameters in simulated bilayers. (A) Distance from the bilayer center as a function of the squared order parameter |SCD|2 for each carbon in the bilayers with 0 mol % and 50 mol % cholesterol. Filled symbols indicate the carbons used for the square-law fits. (B) Number density profiles including all atoms of the simulated bilayers with 0, 20, 33, and 50 mol % cholesterol. Color-coded highlighted areas show the regions of the carbon atoms used in the square-law fits for the corresponding bilayers. The carbons that follow the square-law dependence are within the region where lipid dynamics are influenced by interleaflet interactions. All simulations were performed at 44°C. To see this figure in color, go online.
Bilayer bending modulus emerges from the square-law dependence
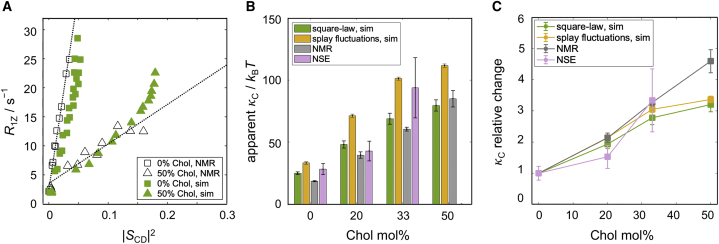
It has been previously shown that the square-law dependence, i.e., the linear relationship between and , is connected to the elastic properties of the bilayer in the liquid-crystalline state (35). In particular, the authors used the slope emerging from the fit of the data to estimate an apparent bilayer bending modulus. The calculation involved three parameters, namely the bilayer viscosity, the order parameter for slow motions, and a quadrupolar coupling constant, in addition to the bilayer thickness which is proportional to (see Eqs. 18 and 19). Accordingly, having shown that the square-law holds in our atomistic MD simulations, we followed the same protocol as in (82) to estimate of the simulated bilayers. Fig. 6 A shows the calculated values for and for DMPC with 0 mol % and 50 mol % cholesterol from the simulations (green), as well as NMR data for the identical systems together with their corresponding best linear fits (black). There is a very good agreement between theory and experiment, confirming the ability of the simulations to recapitulate the properties of the experimental model system.
Figure 6.
Square-law dependence yields an estimate of bilayer bending rigidity. (A) Simulation (green) and NMR (black) data and best square-law fits to the experimental data for DMPC (squares) and DMPC containing 50 mol % cholesterol (triangles) in the liquid-crystalline state. Experimental data were taken from (41), and simulation data are shown for carbons C4 through C14 for DMPC and C8 through C14 for DMPC/cholesterol. (B) Comparison between the apparent bilayer bending modulus calculated from the square-law relationship (green) or splay fluctuations (yellow) in the simulations. Shown also are experimental results for obtained from the square-law relationship of NMR data (gray) and from bilayer thickness fluctuations measured with NSE (purple). All values are in units of kBT. (C) Changes in in the cholesterol-containing bilayers relative to the bilayers without cholesterol. Plotted is the ratio between the two for each of the four methods displayed in (B). Errors for the values obtained from the experimentally determined slope of the square-law relationship were calculated as the standard deviation resulting from the best fits to the data after excluding the last 0, 1, and 2 carbon atoms with largest order parameters. The corresponding errors from the square-law dependence in the simulations were calculated in a similar way (see materials and methods). All simulations were performed at 44°C. The functional dependence between relaxation rates and order parameters is directly related to the bilayer bending rigidity as validated for the full range of cholesterol concentrations. To see this figure in color, go online.
Using previously estimated values of for the bilayer viscosity, ∼0.6 for the slow order parameter, and kHz for the quadrupolar coupling constant (56), as well as the full bilayer thickness (see materials and methods and Table S1), we calculated the corresponding values as outlined in materials and methods. The resulting bending moduli are shown in Fig. 6 B and for DMPC show very good agreement with published values (Table S2). As expected, the apparent bilayer from the simulations increases with increasing cholesterol concentration, starting at 24.8 (1.1) kBT for the pure DMPC membrane and reaching 79.5 (4.5) kBT at 50 mol % cholesterol. For comparison, we used an alternative computational method to obtain from the simulation trajectories, namely by analyzing the fluctuations in lipid splay angles as described in detail in (61,62) and summarized in materials and methods. As shown in Fig. 6 B, while the absolute values are different, the two computational methods show almost identical trends with cholesterol concentration (Fig. 6 C). Plotted in the same figure are also the respective bending rigidities from NMR calculated from the slopes of the experimental data (41) with the same parameters (viscosity, slow order parameter, quadrupolar coupling constant, and thickness) used in the simulations analysis. Not surprisingly, considering the strong agreement of the raw data (Fig. 6 A), the NMR results are very close to the simulation ones (Fig. 6 B). To obtain an independent experimental validation of the bending moduli, we also used NSE spectroscopy to analyze the thickness fluctuations of extruded DMPC liposomes with 0, 20, and 33 mol % cholesterol. As shown previously and summarized in materials and methods, these thickness fluctuations are related to the bilayer bending rigidity via the Zilman-Granek theory (63). The results show a very good overall agreement with the computational methods, with a clear stiffening effect of cholesterol (Fig. 6, B and C). The relative increase in the bending constants estimated for the cholesterol-containing bilayers from the square-law relationship is slightly lower than that given by other methods. This may be due to the assumed constant value for the bilayer viscosity in Eqs. 18 and 19. However, considering the possibility that cholesterol can increase even in unsaturated membranes (83) could rectify these differences. Fig. S16 shows the bending moduli for all simulated bilayers for a range of values for calculated from published estimates for the bilayer viscosity (83,84). Knowing that the bending rigidity obtained from the slope of the square-law relationship is proportional to , the cholesterol-induced increase in bilayer viscosity would amplify the trends relative to those shown in Fig. 6 C. Consequently, the simulated relative rigidity would be closer to the experimental values, confirming the ability of the NMR-based approach to adequately report on the bilayer elasticity.
Frequency dependence of relaxation rates further informs the collective bilayer dynamics
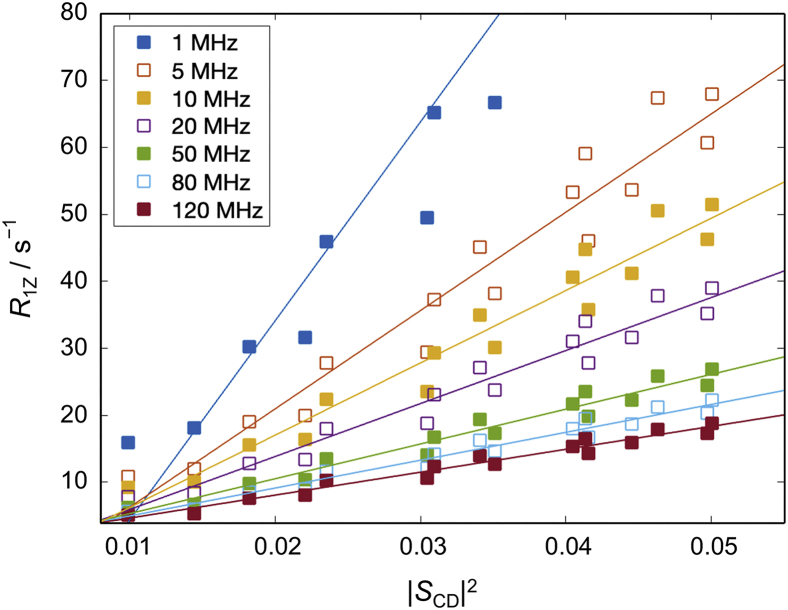
The square-law relationship between the CH bond order parameters and relaxation rates is measured at a particular Larmor frequency () (see materials and methods). To ascertain whether the trend is dependent on , we used the simulation trajectories to analyze the CH bond fluctuations for a range of Larmor frequencies. Fig. 7 shows the results calculated at different values for the DMPC bilayer in the liquid-disordered state. Regardless of the specific value of the relaxation rates and squared order parameters are linearly dependent, illustrating that the square-law relationship holds for all the accessible frequencies, as seen experimentally (85). At lower frequencies the data are more spread out, while at higher frequencies the deviation from the best fit is smaller. There is also a systematic decrease in the slope with increasing frequency both as expected theoretically and seen experimentally (85). These results demonstrate that the trend encoded by these structural and dynamic aspects of the CH bond fluctuations is not accidental but rather arises from inherent bilayer elastic properties as they emerge from the collective atomic-level interactions of the lipids.
Figure 7.
Frequency dependence of relaxation rates and order parameters for simulated bilayers. Shown are plots of the simulated 2H NMR relaxation rates of the acyl carbons of DMPC versus the squared order parameters at different Larmor frequencies (). Data are included for carbons on the sn-2 chain of DMPC in the cholesterol-free bilayer in the fluid state. Simulated results are depicted as squares, and lines indicate the corresponding best fits. Note that the slope of the square-law increases with decreasing frequency, consistent with experimental NMR data. The simulation was performed at 44°C. To see this figure in color, go online.
Discussion
It is a universally acknowledged fact that the lipid composition of a membrane affects its structure and function (9,10,83,86,87,88). However, considerably less is known about the level of cooperativity of the fluctuations of individual lipid molecules that give rise to the emerging bilayer properties. Despite the availability of an extensive amount of biophysical and NMR spectral data, interpretations have remained controversial. We were motivated by the unique atomistic level information regarding both structure and dynamics offered by the NMR approach, as well as the ongoing need to continuously validate MD force fields with experimental data (7,29). To this end, we used MD simulations to further explore the connection between lipid and bilayer dynamics, and its relation to NMR observables and bilayer elastic properties (5,38). Taking the unique features of the simulations into account, involving the fixed orientation of the bilayer normal, we derived a formulation that allows the simulation results to be directly compared with NMR measurements (34) of multilamellar vesicles (MLVs) as well as small unilamellar vesicles (SUVs) where the bilayer normal can adopt any angle with respect to the laboratory frame (magnetic field). Analysis of the simulation trajectories established the nematic-like behavior of the localized dynamics of the membrane lipids as suggested by NMR, namely their segmental 3D nature modulated by collective lipid dynamics. We saw a clear square-law relationship between the order parameters of the lipid acyl carbon atoms and their respective relaxation rates, in excellent agreement with NMR results (35,85). The atomic resolution of the simulations allowed us to further identify the region in the bilayer where the square-law dependence holds, revealing the importance of interleaflet interactions for the collective dynamics of the lipids. The apparent bilayer bending moduli emerging from the application of an NMR-based formalism to the simulation data are moreover corroborated by an alternative computational method, as well as NMR and NSE experiments (83,88). Our MD simulations thus successfully replicate NMR observables and validate their interpretation while offering new insights about their physical origins.
Theoretical framework for comparing molecular dynamics simulations with NMR measurements
There have been numerous occasions where results from MD simulations of lipid bilayers have been compared with NMR observables, such as acyl chain order parameters of the lipids or relaxation rates due to the underlying fluctuations (7,29,37,39,89). In the case of NMR spectroscopy, the elementary processes occur with correlation times near ≈ 10 ns, even though the relaxation times are in the 10–100 ms regime (weak collision limit) (34,56). Likewise, atomistic MD simulations access the same simulation timescale even though the actual time needed to perform the simulation often differs by many orders of magnitude (so-called wall clock time) (9,60,89). Thus, both NMR spectroscopy and MD simulations entail measurement times that are much longer than the actual processes of interest, in this case the lipid fluctuations, but the results describe the same underlying dynamics. The order parameters depend on a well-defined angle between the CH bond and the bilayer normal, and thus have a straightforward correspondence between the simulations and experiments. Still, the spin-lattice relaxation rate is challenging to compare between the two techniques for the following reasons: 1) there are multiple frame transformations that need to be performed to relate the orientation of a lipid CH bond to the measured relaxation rate (see Fig. 4 in (58)), and 2) NMR measurements are often performed on multilamellar lipid vesicles which have approximately spherical symmetry on average, so that the bilayer normal can adopt any angle with respect to the magnetic field axis. These aspects reside in the liquid-crystalline nature of the membrane lipids and open up the question of how to encapsulate both the lipid structure and dynamics in a fully consistent manner.
The assumption of unrestricted isotropic motion circumvents the above features, and the resulting expression for the relaxation rate simplifies to a function dependent only on a single correlation time as shown in Eq. 9 and re-derived in the supporting material. In fact, the formula in Eq. 9 (and its equivalent for the carbon-13 relaxation rates) have been the ones used in comparisons of lipid dynamics between simulations and NMR in the past (see, e.g., (32,72,73,90)). In this solution NMR approach a spherical-harmonics representation is often employed, where the CH bond fluctuations are described in terms of changes in their direction over time. The correlation function is thus written as
| (22) |
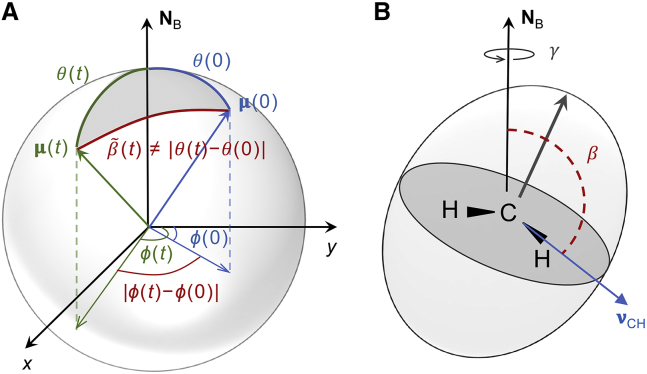
where is the second-order Legendre polynomial (analogous to in Eq. 1) and is the angle between the unit (radius) vectors of the initial and final points. The change in CH bond orientation is defined by the scalar (dot) product of the unit vector at time zero and the unit vector at time (Fig. 8 A). Fourier transformation of Eq. 22 gives , and is subsequently calculated from the solution NMR result (Eqs. 9, S22, and S23). Note that in the spherical-harmonics representation, the pre-factor in Eq. 9 may look slightly different (see supporting material and Eqs. S22 and S23). One should furthermore recall that Eq. 22 corresponds to the spherical-harmonic addition theorem, i.e., where represents one side of a spherical triangle (Fig. 8 A) (57). The other sides are the CH bond orientation (at t = 0) and the bond orientation (at time t), with | as the dihedral angle opposite to the angle. Importantly, the angle in Eq. 22 is different from the Euler angle in (2), (3), (4) because is a relative angle independent of the laboratory frame (Fig. 8 A). There is no projection axis (index) for the angular momentum, and hence Eq. 22 is applicable to solution NMR spectroscopy. For the case of solution NMR, the isotropic averaging is readily introduced leading to the well-known results in textbooks. For multi-axis or composite motions, the orientational order parameters can then be reintroduced, leading to the so-called model-free approach for proteins in solution (91) as the limit of our generalized approach published concurrently (35). This formulation has been successfully used to compare NMR relaxation results with MD simulations for proteins as well as their lipid anchors (92,93,94,95).
Figure 8.
Schematic illustration of angles used in different approaches for analyzing CH bond fluctuations from MD simulations. (A) Spherical-harmonic representation of the orientation of the CH bond at t = and time described by unit vector and unit vector . The corresponding spherical polar coordinates are the polar angles and , respectively. The CH bond angle with respect to the bilayer normal is or . However, the angle whose fluctuations are analyzed with the use of the spherical-harmonic addition theorem (cf. Eq. 22) is different from and does not depend on the director axis. (B) Alternatively, the orientation of the CH bond is described by the Euler angles . Angles and define the orientation of with respect to the bilayer normal (director axis), and their fluctuations are analyzed in the new theoretical framework (Eq. 17). The schematic in (B) is adapted from (58). Note that use of the spherical-harmonic addition theorem is applicable to isotropic liquids with unrestricted motions, while the representation in terms of Euler angles includes the director axis (bilayer normal) and orientational order parameters of the lipids. Analysis of multiscale composite motions of liquid-crystalline membranes thus becomes possible with the latter approach. To see this figure in color, go online.
On the other hand, lipid membranes are uniaxial liquid crystals in which the fluctuations are expressed in terms of orientational order parameters with respect to the bilayer normal, as shown in Fig. 8 B (see also Fig. 1 A) (35). Hence, it is incorrect to apply the solution NMR limit to lipids in SUVs, MLVs, or planar supported bilayers. In an NMR experiment with liposomes, the bilayer normal can adopt all orientations with respect to the magnetic field and the CH bond fluctuations consequently might appear unrestricted. However, the CH bond fluctuations are not isotropic but rather anisotropic because of their inherent ordering with respect to the bilayer normal (i.e., their order parameters). That is why the analysis of solid-state NMR data assumes orientational averaging (the bilayer director can adopt all angles) but still retains information about the underlying ordering of the bonds (see supporting material). In simulations of bilayer patches, the bilayer normal is fixed but the CH bond dynamics are analogous to those in the liposomes measured experimentally with NMR spectroscopy. To connect the two approaches, we therefore derived an expression for the orientationally averaged relaxation rates, but in terms of the director frame, i.e., with respect to the local frame described by the bilayer normal (Eqs. 10 and 17). Stated differently, we take the simulation relaxation rates and then orientationally average them to compare them directly with the experimental ones. Note that Eq. 10 for the orientationally averaged relaxation rate has an equivalent formulation in terms of spherical-harmonic spectral densities (see Eq. S17). Importantly, the formulas presented here account for both the fixed bilayer normal in the simulations and the restricted anisotropic motion of the CH bond fluctuations described by segmental order parameters. They should thus be used instead of the solution NMR limit to compare simulation data with experimental NMR results.
To explicitly demonstrate the differences between the two theoretical approaches, i.e., using the spherical-harmonic addition theorem with Eq. 22 versus the Wigner rotation matrix elements and Eqs. 16 and 17, we followed (72) to calculate the 2H NMR relaxation rates corresponding to the commonly used solution NMR limit. In particular, the spectral densities were obtained from the discrete one-sided Fourier transform of the correlation function using Eq. 22, which gives
| (23) |
where is the orientational average of Eq. 22, i.e., the correlation function averaged over all bilayer orientations with respect to the laboratory frame. The relaxation rates were then calculated with Eq. 2.5 of (72) (also re-derived as Eq. S21 in supporting material):
| (24) |
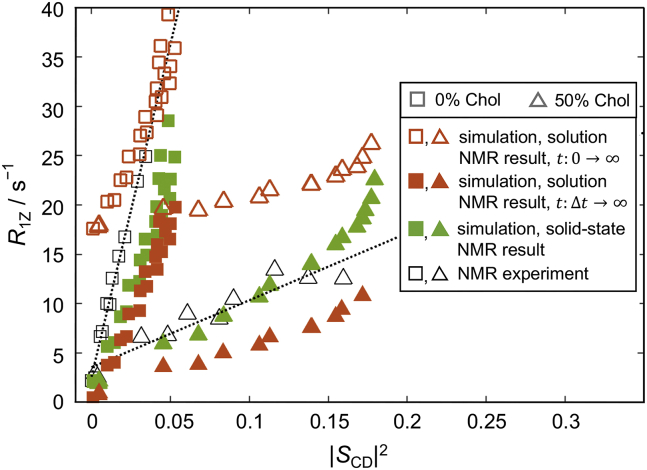
where the pre-factor in Eq. 24 is = . Accordingly, Fig. 9 shows the results from Eq. 24, noted as solution NMR, together with the results from Eq. 17, designated as solid-state NMR and the experimental data (cf. Fig. 6 A). The solution NMR relaxation rates (solid red symbols) clearly deviate from the solid-state NMR data (solid green symbols), and the deviation is larger for the more ordered bilayer with 50% cholesterol. This result illustrates the underlying assumption behind Eq. 24 that the fluctuations are isotropic, so that there is no order in the system, which is incorrect. Thus, when the order parameter is lower the two approaches are more alike, but when the order parameters are larger the differences become more pronounced.
Figure 9.
Comparison of relaxation rates from MD simulations calculated with different approaches. Shown are results from the solution NMR limit ((22), (23), (24)) (red), the new framework (Eq. 17) (green), and experiments (black). Open and solid red symbols correspond to the relaxation rates calculated from the discrete Fourier transform of the correlation function by integrating either from or from , respectively (Eq. 23). All simulations of DMPC with 0 mol % and 50 mol % cholesterol (Chol) were performed at 44°C. Quantitative agreement between the simulation and experimental relaxation rates requires consideration of both the sampling interval and whether the solution NMR or solid-state NMR limit is used. See text for details. To see this figure in color, go online.
What is more, Fig. 9 shows also how the solution NMR approach suffers from the same sampling problem due to the discrete Fourier transformation as the solid-state NMR approach (see below). At the correlation function from Eq. 22 is and when the integral in Eq. 23 goes from to , this results in a large shift in the spectral density and, correspondingly, the relaxation rates (open red symbols). If instead the element at is ignored, and the integral in Eq. 23 is evaluated from to , the relaxation rates are closer in absolute value to the experiment. Note that the shift due to the term in Eq. 23 at increases the magnitude of the relaxation rates but does not affect the slope of the square-law relationship, because for all carbons on the lipid chains. In contrast, in Eq. 15 is the mean-square amplitude or variance of the Wigner rotation matrix elements, which is different for the various carbon atoms due to their inequivalent order parameters (Fig. S14). Consequently, ignoring the element at lag time = 0 in Eq. 15 would change not only the magnitude of the values but also the slope of the square-law relationship. This aspect must be recognized in any validations of MD simulations or their force fields versus actual experimental NMR data.
The nature of lipid dynamics in biomembranes
The spectral densities of the acyl chain CH bond fluctuations hold the key to the types of motions that the lipids exhibit while interacting with other lipids in a bilayer. It was recently shown that these spectral densities (calculated using the NMR solution limit described above) can be fit to a large sum of exponential functions (68). While the respective fits are very good, likely due to the large number of fitting parameters, the results point to an extensive hierarchy of motions with unclear origins, i.e., very fast dynamics modulated by slightly slower dynamics, modulated by yet slower dynamics, and so on. Alternatively, it is shown here that the spectral densities corresponding to the NMR measurements can instead be adequately fit to a simple power-law function that has a clear physical interpretation pertaining to the nature of the underlying motions. The value of the single relevant parameter, the power exponent, indicates the degree of cooperativity of the lipid motions. For all carbons on all chains of the bilayers in the present study, we find the exponent to be around −1/2 (Figs. 2 B and S7–S9) consistent with collective segmental dynamics. This observation is further confirmed by comparison of the respective power exponents to those of LD vectors of varying lengths, whose orientations with respect to the bilayer normal fluctuate due to the thermal energy (Figs. 3 and S10). Regardless of which carbon the LD vectors originate from, their exponents decrease in an almost identical way with increasing vector length, illustrating the physical interpretation of the origins of this exponent: nematic-like motions at a value around −1/2, and smectic-like dynamics at a value approaching −1 as the limit. Our results therefore are a direct manifestation of Occam’s razor and show how the seemingly complex interplay between the lipid bond fluctuations is governed by a simple principle of segmental cooperativity.
Quantum-mechanical view of the square-law dependence
Our simulations confirm the existence of the square-law dependence in simulated bilayers, in excellent agreement with NMR results for in vitro model lipid membranes. While we can distinguish two distinct regions of the lipid chains where this relationship holds and where it breaks (Figs. 4, 5, and S15), the question remains as to the origins of this initially surprising functional correspondence between the order parameters and relaxation rates (35). Notably, the fluctuations of the CH bonds of a lipid reflect the changes in the energy of the atomic nuclei due to their magnetic or electric coupling to their surroundings. Such energy fluctuations result in transitions between different nuclear spin energy levels. The main energy levels of a nucleus in an NMR experiment are determined by the applied magnetic field (Zeeman effect), where transitions between these main energy levels (whose rate is quantified by ) are enabled by additional energy perturbations due to the interactions of the nucleus with its surroundings. Weak coupling to the surroundings results in slower and less efficient relaxation, while strong coupling gives rise to much faster relaxation rates. Thus, the rate of the relaxation depends on the strength of the energy perturbations near the resonance frequency. The transition probability between two main energy states is found to be proportional to the product of the squared matrix element of the perturbation and the density of states, according to Fermi’s golden rule. In the case of the fluctuating CH bonds, the matrix element is analogous to the mean-squared amplitude of the fluctuations, i.e., corresponding to the square of the segmental order parameter. Together with the square-law dependence, the NMR data show an additional dependence on inverse frequency in the form of a factor that multiplies or scales the squared order parameter (which is why this term appears in Eq. 19). Because the latter is analogous to the density of states in the original formulation, the square-law relationship becomes a direct manifestation of time-dependent perturbation theory and Fermi’s golden rule in spectroscopy.
Bilayer elastic properties emerging from the region of interleaflet contact
The presence of the square-law dependence shows clearly that the motional rates of lipid bond fluctuations are functionally related to their mean-squared amplitudes. Here we demonstrate that the relationship yields an estimate of the bilayer bending modulus (Fig. 6 and Table S2). The derivation relies on a single elastic constant approximation, i.e., there is no special treatment of specific deformation modes (e.g., splay, tilt, twist) as they are all approximated with a single parameter. While this formulation cannot distinguish the contribution of particular molecular motions to the membrane deformation, it is simple and general—the fact that it successfully captures both the values and trends in the bending constants makes it an advantageous descriptor of the inherent bilayer elasticity.
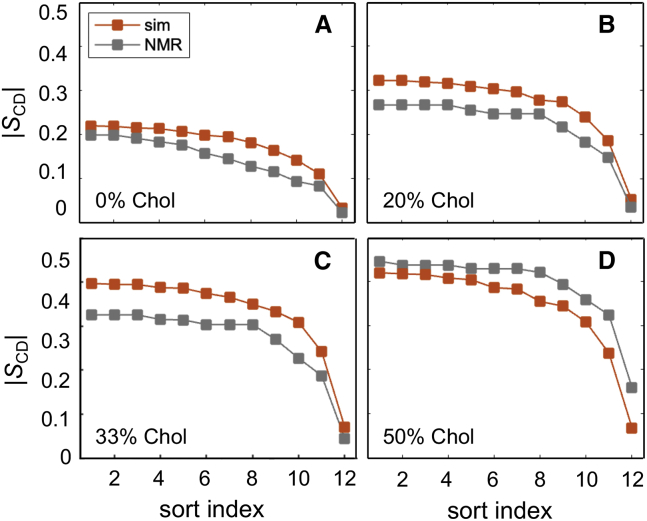
Notably, we find from our MD simulations that the collective nematic-like dynamics at the region of direct contact between the two leaflets are closely related to the bilayer bending elasticity, while outside of this region they are not (Fig. 6). This finding is unlikely to arise from simulation artifacts because: 1) available NMR data for the square-law dependence do not clearly resolve carbon atoms in the upper segments of the lipids (plateau region of order profile), and therefore it is unclear whether the bonds of these atoms follow the same dependence as in vitro systems; and 2) there is no difference in the agreement between order parameters for the upper and lower lipid chain segments obtained from NMR and the MD simulations (e.g., Fig. 10). Instead, this intriguing observation complements the relation between local thickness fluctuations in this same mid-bilayer region and the local monolayer area compressibility modulus recently reported (71). The height fluctuations of the carbon atoms inside the region of leaflet-leaflet contact were found to be decoupled from the interfacial dynamics, and the thickness fluctuations of that particular bilayer slab separately yielded the apparent area compressibility moduli of the two leaflets (71). Moreover, outside of this mid-bilayer region, extensive analysis of a large set of simulated lipid bilayers has likewise revealed a strong correlation in the height fluctuations of all carbon atoms, suggesting a direct influence of interfacial tension (occurring at the water-hydrocarbon interface) on their dynamics (Fig. 1 in (71)).
Figure 10.
Comparison of DMPC order profiles obtained from simulations and NMR spectroscopy. Acyl chain order parameters are shown for DMPC bilayers containing (A) 0 mol %, (B) 20 mol %, (C) 33 mol %, and (D) 50 mol % cholesterol. The results are plotted as a function of sort index (i.e., in descending order) either calculated from simulations (red) or reported from NMR (gray). Simulation data are average from the two chains. Experimental data were taken from (34) with the 0 mol % cholesterol values interpolated from the data at 30°C, 50°C, and 60°C. All simulations were performed at 44°C. Validation of lipid force fields with NMR data requires consideration of both order parameters and relaxation rates. See text for details. To see this figure in color, go online.
The existence of two functionally decoupled bilayer regions can then be explained by variations in the conformational dynamics of the lipid chains along the bilayer normal. In particular, since the chains are effectively tethered to the aqueous interface, their extent (travel) along the bilayer normal (director) depends on the numbers of gauche versus trans isomers. The more gauche isomers, the shorter the chain extension along the director and vice versa. However, the 3D volume must still be occupied, and hence the shorter chain extensions must be compensated by greater disorder of the surrounding chains (96) to fill the volume at liquid hydrocarbon density (80). Accordingly, we propose that the bilayer can be subdivided into two regions (Fig. 1 A). The outermost bilayer region corresponds to the so-called plateau of the NMR order parameter profiles (56), coming from the top part of the chains where backfolding and interleaflet penetration are minimal. In contrast, the innermost or mid-bilayer region is where the chain terminations become appreciable and the segmental disorder reaches a maximum (71,96). To a first approximation, we can then consider the outer and mid-bilayer regions separately for the analysis of bilayer mechanics.
Emerging within the mid-bilayer region, the thickness and lipid chain CH bond fluctuations have a hitherto unrecognized connection to the elastic moduli of the membrane. An intricate relationship exists for both bending and compressibility between the noninterfacial segmental dynamics modulated by interleaflet coupling and the energy to locally bend or stretch the bilayer. These mechanical constants have been traditionally obtained from global bilayer properties (e.g., thermal vesicle shape fluctuations or response to induced out-of-equilibrium deformations, flicker spectroscopy) in terms of underlying theoretical concepts of membrane elasticity (5,38,43,44,97). This analysis treats the membrane as a 2D flexible surface (a thin sheet) that exhibits smectic-like dynamics at longer length scales. In addition, our results point to an alternative contribution from the more localized nematic-like lipid dynamics at the bilayer midplane, which appear to have a major role in the observed elastic behavior at shorter length scales. Both approaches at the global and more local scales have been shown to yield similar results for some bilayers (e.g., saturated lipids with and without cholesterol) but contradictory results for other types of bilayers (e.g., di-unsaturated lipids with cholesterol (83)). The sources of these discrepancies may lie in the timescales and length scales of the underlying dynamics (83,88) and are the subject of ongoing debate.
Validation of molecular dynamics simulations with experimental NMR data
As discussed above, the proper comparison between relaxation rates obtained from NMR and calculated from MD simulations requires special considerations which are included in the theoretical framework presented here. The new formulation allows us to comment on a few key points pertaining to theoretical MD simulations and experimental NMR relaxation studies. Here we recall that the simulation data are always discrete in nature; that is, the atomic coordinates are collected at some fixed time sampling interval (). As a result, calculation of the spectral densities is inevitably done via a discrete (one-sided) Fourier transform of the correlation function (Eq. 15). This discrete transformation as illustrated in Eq. 15 produces a constant equal to the product of the autocorrelation at zero lag time (which is the variance of the data, from Eq. 7) and the sampling interval. This constant is added to the spectral density at every frequency, and consequently it shifts the calculated relaxation rates in a -dependent manner as an inevitable result of the discrete Fourier transform. Theoretically, the spectral density goes to infinite frequency, and its integral is equal to as can also be obtained from the second- and fourth-order Legendre polynomials (⟨ and ) via a Clebsch-Gordan series expansion (see Table V in (75)). Analytically, in the Fourier time domain, the correlation function goes to zero and equals the initial value. However, that limit is impossible to reach either in simulations or experiments—therefore, we can only access a small window or bandwidth of the spectral density in the Fourier space.
Even then, we managed to alleviate the dependence on the discrete sampling interval by taking advantage of the fact that the correlation functions of the CH bond fluctuations, expressed in terms of the second-rank Wigner rotation matrix elements, follow a power-law relation. As explained in materials and methods, this approach allowed us to fit the correlation functions and use the best fits to resample them at smaller time intervals. Correspondingly, we found the smallest that preserved the smoothness of the correlation function for each carbon, as shown in Fig. S6. These values correspond to the fastest rotations of the CH bonds that contribute to the spectral density and are in the range of 0.5–30 ps. The results are in excellent agreement with the 5- to 20-ps correlation times predicted to arise from the bilayer microviscosity and account for the apparent enhancement in relaxation of lipid bilayers versus hydrocarbon fluids as measured with NMR spectroscopy (23,80). If a larger sampling time step were used (such as 40 ps due to the output frequency in our simulations), it would effectively mask these rapid bond rotations, while an output frequency corresponding to the femtosecond regime would be impractical for long MD simulations. Mathematical tricks for resampling of the correlation functions are thus necessary to help bridge the gap between the discrete and continuum representations, although an exact correspondence can never be achieved.
Another important observation from our analysis is that the square-law dependence can be used in a model-free way, as illustrated by Fig. 6 A, to validate lipid force fields against NMR relaxation data. While the order parameters contain information about the average structure of the lipid chains—and therefore provide a static picture of their conformations—the spin-lattice relaxation rates describe the CH bond dynamics that produce these conformations, thus complementing the structural data. To substantiate this point, Fig. 10, A–D shows a comparison between the theoretically simulated and experimental order parameter profiles for DMPC with various mole fractions of cholesterol. Although the DMPC lipids in the presence of 0, 20, and 33 mol % cholesterol in the simulations are more ordered than in the experiment, both theory and experiment are in remarkable agreement in that we see smaller slopes of their square-law dependences corresponding to higher effective bending rigidities of the simulated bilayers (Fig. 6 B). This feature is expected from the known dependence of on lipid packing (and, consequently, order): the more disordered the lipids the lower the value (60,83). Interestingly, the simulated bilayer with 50 mol % cholesterol has slightly lower order parameters than the NMR measurements, and its square-law slope matches closely the experimental one for carbons C10 through C14 (cf. Fig. 6 A). Therefore, while the overall trends in the deviations from experimental data are similar across the order parameters and relaxation rates, there may be differences from the ability of the force fields to capture the elasticity of the bilayer or its average structure, thus highlighting a path forward toward their improvements. For this to occur, the validation of lipid dynamics in the form of CH bond relaxation rates, as measured with NMR, becomes critical.
Lastly, it is worth noting that simulations are often validated against experimental data where differences in the types of samples and analysis for the various techniques may produce seemingly contradictory structural and/or dynamical features. One example is the recently reported problem in the lack of correspondence between the order parameters of sphingomyelin bilayers obtained from NMR and the bilayer structural properties (area per lipid and thickness) obtained from small-angle scattering techniques (6). Because the analysis of any experimental data adds another set of variables to its interpretation, a model-free comparison of the raw data is always optimal for a bias-free comparison. However, whenever there is a mismatch in the structure resolved by two approaches, the simulated system will inevitably fail to reproduce certain experimental observables.
Conclusions
Experimentally observed relationships between the relaxation rates of CH bond fluctuations and their order parameters or the frequency at which they are measured have raised questions about the cooperativity of lipid motions in a bilayer and their link to membrane elasticity. We have shown here that the local movements of the lipid acyl chains in the region of interleaflet contact are captured by MD simulations that are indicative of collective segmental motions analogous to nematic liquid crystals. For this, we developed a new theoretical framework that allows direct comparison of the relaxation rates calculated from the simulations with those measured with NMR experiments by accounting for the inherent differences between the two approaches. Following an NMR-based formalism for liquid-crystalline bilayers, we found that the square-law dependence between the rate and amplitude of the lipid CH bond fluctuations yields a frequency-independent estimate of an apparent bilayer bending modulus, which for DMPC bilayers follows the expected trends with addition of cholesterol. Our results are fully in line with NMR observables and their interpretation and offer an alternative protocol for extracting mechanical properties of the simulated membranes. In the future, it will be interesting to explore whether our analysis is applicable to bilayers with mono- and polyunsaturated lipids, as well as more complex lipid mixtures in terms of establishing the universality of bilayer functional properties.
Author contributions
M.D. and M.F.B. developed the concept and designed the research. G.K. produced the simulation trajectories and M.D. performed all computational analysis. R.A. performed the NSE experiments, including data collection and analysis. M.D., G.K., R.A., and M.F.B. wrote and edited the manuscript.
Acknowledgments
Klaus Gawrisch is acknowledged with gratitude for sharing his eternal insight and friendly collegiality over the years. We thank Steven Abel, Frederick Heberle, Kayla Sapp, and Alexander Sodt for helpful advice and insightful discussions on the Fourier analysis of time autocorrelation data from the simulations. We also gratefully acknowledge Andrew Erly and Trivikram Molugu for valuable discussions on the interpretation of simulation results. M.D. was supported by NIH postdoctoral fellowship F32GM134704. R.A. acknowledges NSF support through grant MCB 2137154. G.K. is supported by the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute of Computational Biomedicine at Weill Cornell Medical College through the 1923 Fund. M.F.B. acknowledges NIH support through grant R01EY026041 and NSF through grants MCB 1817862 and CHE 1904125. Access to the NGA-NSE beamline was provided by the Center for High Resolution Neutron Scattering, a partnership between the National Institute of Standards and Technology and the National Science Foundation under agreement no. DMR-1508249. MD simulations were performed using the Oak Ridge Leadership Computing Facility (Summit allocation BIP109) at the Oak Ridge National Laboratory (supported by the Office of Science of the US Department of Energy under contract no. DE-AC05-00OR22725).
Declaration of interests
The authors declare no competing interests.
Editor: Richard Pastor.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.12.007.
Contributor Information
Milka Doktorova, Email: md4xm@virginia.edu.
Michael F. Brown, Email: mfbrown@u.arizona.edu.
Supporting material
References
- 1.Zimmerberg J., Gawrisch K. The physical chemistry of biological membranes. Nat. Chem. Biol. 2006;2:564–567. doi: 10.1038/nchembio1106-564. https://www.ncbi.nlm.nih.gov/pubmed/17051226 [DOI] [PubMed] [Google Scholar]
- 2.Harayama T., Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018;19:281–296. doi: 10.1038/nrm.2017.138. https://www.ncbi.nlm.nih.gov/pubmed/29410529 [DOI] [PubMed] [Google Scholar]
- 3.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18216768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heberle F.A., Marquardt D., et al. Pabst G. Subnanometer structure of an asymmetric model membrane: interleaflet coupling influences domain properties. Langmuir. 2016;32:5195–5200. doi: 10.1021/acs.langmuir.5b04562. http://www.ncbi.nlm.nih.gov/pubmed/27128636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allolio C., Haluts A., Harries D. A local instantaneous surface method for extracting membrane elastic moduli from simulation: comparison with other strategies. Chem. Phys. 2018;514:31–43. doi: 10.1016/j.chemphys.2018.03.004. [DOI] [Google Scholar]
- 6.Doktorova M., Kučerka N., et al. Heberle F.A. Molecular structure of sphingomyelin in fluid phase bilayers determined by the joint analysis of small-angle neutron and X-ray scattering data. J. Phys. Chem. B. 2020;124:5186–5200. doi: 10.1021/acs.jpcb.0c03389. https://www.ncbi.nlm.nih.gov/pubmed/32468822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y., Krämer A., et al. Pastor R.W. CHARMM36 lipid force field with explicit treatment of long-range dispersion: parametrization and validation for phosphatidylethanolamine, phosphatidylglycerol, and ether lipids. J. Chem. Theor. Comput. 2021;17:1581–1595. doi: 10.1021/acs.jctc.0c01327. https://www.ncbi.nlm.nih.gov/pubmed/33620194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honerkamp-Smith A.R., Veatch S.L., Keller S.L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta. 2009;1788:53–63. doi: 10.1016/j.bbamem.2008.09.010. https://www.ncbi.nlm.nih.gov/pubmed/18930706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sodt A.J., Sandar M.L., et al. Lyman E. The molecular structure of the liquid-ordered phase of lipid bilayers. J. Am. Chem. Soc. 2014;136:725–732. doi: 10.1021/ja4105667. https://www.ncbi.nlm.nih.gov/pubmed/24345334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorent J.H., Levental K.R., et al. Levental I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020;16:644–652. doi: 10.1038/s41589-020-0529-6. https://www.ncbi.nlm.nih.gov/pubmed/32367017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levental I., Levental K.R., Heberle F.A. Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol. 2020;30:341–353. doi: 10.1016/j.tcb.2020.01.009. https://www.ncbi.nlm.nih.gov/pubmed/32302547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorent J.H., Diaz-Rohrer B., et al. Levental I. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 2017;8:1219. doi: 10.1038/s41467-017-01328-3. https://www.ncbi.nlm.nih.gov/pubmed/29089556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doktorova M., Heberle F.A., et al. Dick R.A. Cholesterol promotes protein binding by affecting membrane electrostatics and solvation properties. Biophys. J. 2017;113:2004–2015. doi: 10.1016/j.bpj.2017.08.055. https://www.ncbi.nlm.nih.gov/pubmed/29117524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doktorova M., Heberle F.A., et al. Andersen O.S. Gramicidin increases lipid flip-flop in symmetric and asymmetric lipid vesicles. Biophys. J. 2019;116:860–873. doi: 10.1016/j.bpj.2019.01.016. https://www.ncbi.nlm.nih.gov/pubmed/30755300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghysels A., Krämer A., et al. Pastor R.W. Permeability of membranes in the liquid ordered and liquid disordered phases. Nat. Commun. 2019;10:5616. doi: 10.1038/s41467-019-13432-7. https://www.ncbi.nlm.nih.gov/pubmed/31819053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley E.G., Butler P.D., et al. Nagao M. Scaling relationships for the elastic moduli and viscosity of mixed lipid membranes. Proc. Natl. Acad. Sci. USA. 2020;117:23365–23373. doi: 10.1073/pnas.2008789117. https://www.ncbi.nlm.nih.gov/pubmed/32883879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballweg S., Sezgin E., et al. Ernst R. Regulation of lipid saturation without sensing membrane fluidity. Nat. Commun. 2020;11:756. doi: 10.1038/s41467-020-14528-1. https://www.ncbi.nlm.nih.gov/pubmed/32029718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufourc E.J., Mayer C., et al. Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys. J. 1992;61:42–57. doi: 10.1016/S0006-3495(92)81814-3. https://www.ncbi.nlm.nih.gov/pubmed/1540698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisz K., Gröbner G., et al. Kothe G. Deuteron nuclear magnetic resonance study of the dynamic organization of phospholipid/cholesterol bilayer membranes: molecular properties and viscoelastic behavior. Biochemistry. 1992;31:1100–1112. doi: 10.1021/bi00119a019. [DOI] [PubMed] [Google Scholar]
- 20.Nowacka A., Mohr P.C., et al. Topgaard D. Polarization transfer solid-state NMR for studying surfactant phase behavior. Langmuir. 2010;26:16848–16856. doi: 10.1021/la102935t. https://www.ncbi.nlm.nih.gov/pubmed/20925371 [DOI] [PubMed] [Google Scholar]
- 21.Leftin A., Job C., et al. Brown M.F. Solid-state 13C NMR reveals annealing of raft-like membranes containing cholesterol by the intrinsically disordered protein α-synuclein. J. Mol. Biol. 2013;425:2973–2987. doi: 10.1016/j.jmb.2013.04.002. https://pubmed.ncbi.nlm.nih.gov/23583776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leftin A., Molugu T.R., et al. Brown M.F. Area per lipid and cholesterol interactions in membranes from separated local-field 13C NMR spectroscopy. Biophys. J. 2014;107:2274–2286. doi: 10.1016/j.bpj.2014.07.044. https://pubmed.ncbi.nlm.nih.gov/25418296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown M.F. In: Characterization of Biological Membranes: Structure and Dynamics. Nieh M., Heberle F., Katsaras J., editors. De Gruyter, Berlin; 2019. 7. Collective dynamics in lipid membranes; pp. 231–268. [DOI] [Google Scholar]
- 24.Douliez J.-P., Léonard A., Dufourc E.J. Restatement of order parameters in biomembranes: calculation of CC bond order parameters from CD quadrupolar splittings. Biophys. J. 1995;68:1727–1739. doi: 10.1016/S0006-3495(95)80350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douliez J.-P., Ferrarini A., Dufourc E.-J. On the relationship between CC and CD order parameters and its use for studying the conformation of lipid acyl chains in biomembranes. J. Chem. Phys. 1998;109:2513–2518. doi: 10.1063/1.476823. [DOI] [Google Scholar]
- 26.Brown M.F., Chan S.I. Bilayer membranes: deuterium carbon-13 NMR. eMagRes. 2007 doi: 10.1002/9780470034590.emrstm0023. [DOI] [Google Scholar]
- 27.Brown M.F., Thurmond R.L., et al. Beyer K. Elastic deformation of membrane bilayers probed by deuterium NMR relaxation. J. Am. Chem. Soc. 2002;124:8471–8484. doi: 10.1021/ja012660p. https://www.ncbi.nlm.nih.gov/pubmed/12105929 [DOI] [PubMed] [Google Scholar]
- 28.Vermeer L.S., de Groot B.L., et al. Czaplicki J. Acyl chain order parameter profiles in phospholipid bilayers: computation from molecular dynamics simulations and comparison with 2H NMR experiments. Eur. Biophys. J. 2007;36:919–931. doi: 10.1007/s00249-007-0192-9. https://www.ncbi.nlm.nih.gov/pubmed/17598103 [DOI] [PubMed] [Google Scholar]
- 29.Klauda J.B., Venable R.M., et al. Pastor R.W. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. https://www.ncbi.nlm.nih.gov/pubmed/20496934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira T.M., Coreta-Gomes F., et al. Topgaard D. Cholesterol and POPC segmental order parameters in lipid membranes: solid state 1H–13C NMR and MD simulation studies. Phys. Chem. Chem. Phys. 2013;15:1976–1989. doi: 10.1039/c2cp42738a. https://pubmed.ncbi.nlm.nih.gov/23258433 [DOI] [PubMed] [Google Scholar]
- 31.Mallikarjunaiah K.J., Kinnun J.J., et al. Brown M.F. Flexible lipid nanomaterials studied by NMR spectroscopy. Phys. Chem. Chem. Phys. 2019;21:18422–18457. doi: 10.1039/c8cp06179c. https://www.ncbi.nlm.nih.gov/pubmed/31410425 [DOI] [PubMed] [Google Scholar]
- 32.Pastor R.W., Venable R.M., Feller S.E. Lipid bilayers, NMR relaxation, and computer simulations. Acc. Chem. Res. 2002;35:438–446. doi: 10.1021/ar0100529. https://www.ncbi.nlm.nih.gov/pubmed/12069629 [DOI] [PubMed] [Google Scholar]
- 33.Soubias O., Gawrisch K. Docosahexaenoyl chains isomerize on the sub-nanosecond time scale. J. Am. Chem. Soc. 2007;129:6678–6679. doi: 10.1021/ja068856c. https://www.ncbi.nlm.nih.gov/pubmed/17477528 [DOI] [PubMed] [Google Scholar]
- 34.Leftin A., Brown M.F. An NMR database for simulations of membrane dynamics. Biochim. Biophys. Acta. 2011;1808:818–839. doi: 10.1016/j.bbamem.2010.11.027. https://www.ncbi.nlm.nih.gov/pubmed/21134351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown M.F. Theory of spin-lattice relaxation in lipid bilayers and biological membranes. 2H and 14N quadrupolar relaxation. J. Chem. Phys. 1982;77:1576–1599. doi: 10.1063/1.443940. [DOI] [Google Scholar]
- 36.Usery R.D., Enoki T.A., et al. Feigenson G.W. Membrane bending moduli of coexisting liquid phases containing transmembrane peptide. Biophys. J. 2018;114:2152–2164. doi: 10.1016/j.bpj.2018.03.026. https://www.ncbi.nlm.nih.gov/pubmed/29742408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber T., Rajamoorthi K., et al. Brown M.F. Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by 2H NMR and molecular dynamics simulations. J. Am. Chem. Soc. 2002;124:298–309. doi: 10.1021/ja011383j. https://pubmed.ncbi.nlm.nih.gov/11782182 [DOI] [PubMed] [Google Scholar]
- 38.Venable R.M., Brown F.L.H., Pastor R.W. Mechanical properties of lipid bilayers from molecular dynamics simulation. Chem. Phys. Lipids. 2015;192:60–74. doi: 10.1016/j.chemphyslip.2015.07.014. https://www.ncbi.nlm.nih.gov/pubmed/26238099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith A.A., Vogel A., et al. Huster D. A method to construct the dynamic landscape of a bio-membrane with experiment and simulation. Nat. Commun. 2022;13:108. doi: 10.1038/s41467-021-27417-y. https://www.ncbi.nlm.nih.gov/pubmed/35013165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trouard T.P., Nevzorov A.A., et al. Brown M.F. Influence of cholesterol on dynamics of dimyristoylphosphatidylcholine bilayers as studied by deuterium NMR relaxation. J. Chem. Phys. 1999;110:8802–8818. doi: 10.1063/1.478787. [DOI] [Google Scholar]
- 41.Martinez G.V., Dykstra E.M., et al. Brown M.F. NMR elastometry of fluid membranes in the mesoscopic regime. Phys. Rev. E. 2002;66:050902. doi: 10.1103/PhysRevE.66.050902. https://www.ncbi.nlm.nih.gov/pubmed/12513460 [DOI] [PubMed] [Google Scholar]
- 42.Martinez G.V., Dykstra E.M., et al. Brown M.F. Lanosterol and cholesterol-induced variations in bilayer elasticity probed by 2H NMR relaxation. Langmuir. 2004;20:1043–1046. doi: 10.1021/la036063n. https://www.ncbi.nlm.nih.gov/pubmed/15803674 [DOI] [PubMed] [Google Scholar]
- 43.Watson M.C., Brandt E.G., et al. Brown F.L.H. Determining biomembrane bending rigidities from simulations of modest size. Phys. Rev. Lett. 2012;109:028102. doi: 10.1103/PhysRevLett.109.028102. https://www.ncbi.nlm.nih.gov/pubmed/23030207 [DOI] [PubMed] [Google Scholar]
- 44.Johner N., Harries D., Khelashvili G. Curvature and lipid packing modulate the elastic properties of lipid assemblies: comparing HII and lamellar phases. J. Phys. Chem. Lett. 2014;5:4201–4206. doi: 10.1021/jz5022284. https://www.ncbi.nlm.nih.gov/pubmed/26278954 [DOI] [PubMed] [Google Scholar]
- 45.Jo S., Kim T., et al. Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. https://www.ncbi.nlm.nih.gov/pubmed/18351591 [DOI] [PubMed] [Google Scholar]
- 46.Brooks B.R., Brooks C.L., et al. Karplus M. CHARMM: the biomolecular simulation program. J. Comput. Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. https://pubmed.ncbi.nlm.nih.gov/19444816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu E.L., Cheng X., et al. Im W. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 2014;35:1997–2004. doi: 10.1002/jcc.23702. https://www.ncbi.nlm.nih.gov/pubmed/25130509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J., Cheng X., et al. Im W. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theor. Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. https://www.ncbi.nlm.nih.gov/pubmed/26631602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J., Patel D.S., et al. Im W. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theor. Comput. 2019;15:775–786. doi: 10.1021/acs.jctc.8b01066. https://www.ncbi.nlm.nih.gov/pubmed/30525595 [DOI] [PubMed] [Google Scholar]
- 50.Phillips J.C., Hardy D.J., et al. Tajkhorshid E. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020;153:044130. doi: 10.1063/5.0014475. https://www.ncbi.nlm.nih.gov/pubmed/32752662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J.B., Rogaski B., Klauda J.B. Update of the cholesterol force field parameters in CHARMM. J. Phys. Chem. B. 2012;116:203–210. doi: 10.1021/jp207925m. https://www.ncbi.nlm.nih.gov/pubmed/22136112 [DOI] [PubMed] [Google Scholar]
- 52.Nevzorov A.A., Trouard T.P., Brown M.F. Lipid bilayer dynamics from simultaneous analysis of orientation and frequency dependence of deuterium spin-lattice and quadrupolar order relaxation. Phys. Rev. E. 1998;58:2259–2281. doi: 10.1103/PhysRevE.58.2259. [DOI] [Google Scholar]
- 53.Eastman P., Swails J., et al. Pande V.S. OpenMM 7: rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 2017;13:e1005659. doi: 10.1371/journal.pcbi.1005659. https://www.ncbi.nlm.nih.gov/pubmed/28746339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krämer A., Ghysels A., et al. Pastor R.W. Membrane permeability of small molecules from unbiased molecular dynamics simulations. J. Chem. Phys. 2020;153:124107. doi: 10.1063/5.0013429. https://www.ncbi.nlm.nih.gov/pubmed/33003739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:27–38. doi: 10.1016/0263-7855(96)00018-5. http://www.ncbi.nlm.nih.gov/pubmed/8744570 [DOI] [PubMed] [Google Scholar]
- 56.Molugu T.R., Lee S., Brown M.F. Concepts and methods of solid-state NMR spectroscopy applied to biomembranes. Chem. Rev. 2017;117:12087–12132. doi: 10.1021/acs.chemrev.6b00619. https://www.ncbi.nlm.nih.gov/pubmed/28906107 [DOI] [PubMed] [Google Scholar]
- 57.Rose M.E. John Wiley & Sons, New York; 1957. Elementary Theory of Angular Momentum. [Google Scholar]
- 58.Kinnun J.J., Mallikarjunaiah K.J., et al. Brown M.F. Elastic deformation and area per lipid of membranes: atomistic view from solid-state deuterium NMR spectroscopy. Biochim. Biophys. Acta. 2015;1848:246–259. doi: 10.1016/j.bbamem.2014.06.004. https://pubmed.ncbi.nlm.nih.gov/24946141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giorgino T. Computing 1-D atomic densities in macromolecular simulations: the density profile tool for VMD. Comput. Phys. Commun. 2014;185:317–322. doi: 10.1016/j.cpc.2013.08.022. [DOI] [Google Scholar]
- 60.Doktorova M., Harries D., Khelashvili G. Determination of bending rigidity and tilt modulus of lipid membranes from real-space fluctuation analysis of molecular dynamics simulations. Phys. Chem. Chem. Phys. 2017;19:16806–16818. doi: 10.1039/c7cp01921a. https://www.ncbi.nlm.nih.gov/pubmed/28627570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johner N., Harries D., Khelashvili G. Implementation of a methodology for determining elastic properties of lipid assemblies from molecular dynamics simulations. BMC Bioinf. 2016;17:161. doi: 10.1186/s12859-016-1003-z. https://www.ncbi.nlm.nih.gov/pubmed/27071656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johner N., Harries D., Khelashvili G. Erratum to: implementation of a methodology for determining elastic properties of lipid assemblies from molecular dynamics simulations. BMC Bioinf. 2016;17:236. doi: 10.1186/s12859-016-1091-9. https://www.ncbi.nlm.nih.gov/pubmed/27301431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zilman A.G., Granek R. Undulations and dynamic structure factor of membranes. Phys. Rev. Lett. 1996;77:4788–4791. doi: 10.1103/PhysRevLett.77.4788. https://www.ncbi.nlm.nih.gov/pubmed/10062631 [DOI] [PubMed] [Google Scholar]
- 64.Watson M.C., Brown F.L.H. Interpreting membrane scattering experiments at the mesoscale: the contribution of dissipation within the bilayer. Biophys. J. 2010;98:L9–L11. doi: 10.1016/j.bpj.2009.11.026. https://www.ncbi.nlm.nih.gov/pubmed/20303849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seifert U., Langer S.A. Viscous modes of fluid bilayer membranes. Europhys. Lett. 1993;23:71–76. doi: 10.1209/0295-5075/23/1/012. [DOI] [Google Scholar]
- 66.Nagao M., Kelley E.G., et al. Butler P.D. Probing elastic and viscous properties of phospholipid bilayers using neutron spin echo spectroscopy. J. Phys. Chem. Lett. 2017;8:4679–4684. doi: 10.1021/acs.jpclett.7b01830. https://www.ncbi.nlm.nih.gov/pubmed/28892394 [DOI] [PubMed] [Google Scholar]
- 67.Högberg C.J., Lyubartsev A.P. A molecular dynamics investigation of the influence of hydration and temperature on structural and dynamical properties of a dimyristoylphosphatidylcholine bilayer. J. Phys. Chem. B. 2006;110:14326–14336. doi: 10.1021/jp0614796. https://www.ncbi.nlm.nih.gov/pubmed/16854139 [DOI] [PubMed] [Google Scholar]
- 68.Antila H.S., M Ferreira T., et al. Miettinen M.S. Using open data to rapidly benchmark biomolecular simulations: phospholipid conformational dynamics. J. Chem. Inf. Model. 2021;61:938–949. doi: 10.1021/acs.jcim.0c01299. https://www.ncbi.nlm.nih.gov/pubmed/33496579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978;200:1012–1018. doi: 10.1126/science.653353. https://www.ncbi.nlm.nih.gov/pubmed/653353 [DOI] [PubMed] [Google Scholar]
- 70.Klauda J.B., Eldho N.V., et al. Pastor R.W. Collective and noncollective models of NMR relaxation in lipid vesicles and multilayers. J. Phys. Chem. B. 2008;112:5924–5929. doi: 10.1021/jp075641w. https://www.ncbi.nlm.nih.gov/pubmed/18179193 [DOI] [PubMed] [Google Scholar]
- 71.Doktorova M., LeVine M.V., et al. Weinstein H. A new computational method for membrane compressibility: bilayer mechanical thickness revisited. Biophys. J. 2019;116:487–502. doi: 10.1016/j.bpj.2018.12.016. https://pubmed.ncbi.nlm.nih.gov/30665693/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindahl E., Edholm O. Mesoscopic undulations and thickness fluctuations in lipid bilayers from molecular dynamics simulations. Biophys. J. 2000;79:426–433. doi: 10.1016/S0006-3495(00)76304-1. https://pubmed.ncbi.nlm.nih.gov/10866968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindahl E., Edholm O. Molecular dynamics simulation of NMR relaxation rates and slow dynamics in lipid bilayers. J. Chem. Phys. 2001;115:4938–4950. doi: 10.1063/1.1389469. [DOI] [Google Scholar]
- 74.Trouard T.P., Alam T.M., Brown M.F. Angular dependence of deuterium spin-lattice relaxation rates of macroscopically oriented dilauroylphosphatidylcholine in the liquid-crystalline state. J. Chem. Phys. 1994;101:5229–5261. doi: 10.1063/1.467378. [DOI] [Google Scholar]
- 75.Nevzorov A.A., Brown M.F. Dynamics of lipid bilayers from comparative analysis of 2H and 13C nuclear magnetic resonance relaxation data as a function of frequency and temperature. J. Chem. Phys. 1997;107:10288–10310. doi: 10.1063/1.474169. [DOI] [Google Scholar]
- 76.Marqusee J.A. Dynamics of late stage phase separations in two dimensions. J. Chem. Phys. 1984;81:976–981. doi: 10.1063/1.447698. [DOI] [Google Scholar]
- 77.Rommel E., Noack F., et al. Kothe G. Proton spin relaxation dispersion studies of phospholipid membranes. J. Phys. Chem. 1988;92:2981–2987. doi: 10.1021/j100321a053. [DOI] [Google Scholar]
- 78.Althoff G., Stauch O., et al. Kothe G. Transverse nuclear spin relaxation studies of viscoelastic properties of membrane vesicles. II. Experimental results. J. Phys. Chem. B. 2002;106:5517–5526. doi: 10.1021/jp012829l. [DOI] [Google Scholar]
- 79.Nevzorov A.A., Trouard T.P., Brown M.F. Correlation functions for lipid membrane dynamics obtained from NMR spectroscopy. Phys. Rev. E. 1997;55:3276–3282. doi: 10.1103/PhysRevE.55.3276. [DOI] [Google Scholar]
- 80.Brown M.F., Ribeiro A.A., Williams G.D. New view of lipid bilayer dynamics from 2H and 13C NMR relaxation time measurements. Proc. Natl. Acad. Sci. USA. 1983;80:4325–4329. doi: 10.1073/pnas.80.14.4325. https://www.ncbi.nlm.nih.gov/pubmed/6576340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams G.D., Beach J.M., et al. Brown M.F. Dependence of deuterium spin-lattice relaxation rates of multilamellar phospholipid dispersions on orientational order. J. Am. Chem. Soc. 1985;107:6868–6873. doi: 10.1021/ja00310a021. [DOI] [Google Scholar]
- 82.Brown M.F., Thurmond R.L., et al. Beyer K. Composite membrane deformation on the mesoscopic length scale. Phys. Rev. E. 2001;64:010901. doi: 10.1103/PhysRevE.64.010901. [DOI] [PubMed] [Google Scholar]
- 83.Chakraborty S., Doktorova M., et al. Ashkar R. How cholesterol stiffens unsaturated lipid membranes. Proc. Natl. Acad. Sci. USA. 2020;117:21896–21905. doi: 10.1073/pnas.2004807117. https://www.ncbi.nlm.nih.gov/pubmed/32843347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zgorski A., Pastor R.W., Lyman E. Surface shear viscosity and interleaflet friction from nonequilibrium simulations of lipid bilayers. J. Chem. Theor. Comput. 2019;15:6471–6481. doi: 10.1021/acs.jctc.9b00683. https://www.ncbi.nlm.nih.gov/pubmed/31476126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown M.F. Theory of spin-lattice relaxation in lipid bilayers and biological membranes. Dipolar relaxation. J. Chem. Phys. 1984;80:2808–2831. doi: 10.1063/1.447030. [DOI] [Google Scholar]
- 86.Teague W.E., Jr., Soubias O., et al. Gawrisch K. Elastic properties of polyunsaturated phosphatidylethanolamines influence rhodopsin function. Faraday Discuss. 2013;161:383–395. doi: 10.1039/c2fd20095c. https://www.ncbi.nlm.nih.gov/pubmed/23805751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown M.F. Soft matter in lipid-protein interactions. Annu. Rev. Biophys. 2017;46:379–410. doi: 10.1146/annurev-biophys-070816-033843. https://www.ncbi.nlm.nih.gov/pubmed/28532212 [DOI] [PubMed] [Google Scholar]
- 88.Ashkar R., Doktorova M., et al. Brown M.F. Reply to Nagle et al.: the universal stiffening effects of cholesterol on lipid membranes. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2102845118. https://www.ncbi.nlm.nih.gov/pubmed/33952694 e2102845118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eldho N.V., Feller S.E., et al. Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid–differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 2003;125:6409–6421. doi: 10.1021/ja029029o. http://www.ncbi.nlm.nih.gov/pubmed/12785780 [DOI] [PubMed] [Google Scholar]
- 90.Pastor R.W., Venable R.M., et al. Szabo A. A simulation based model of NMR T1 relaxation in lipid bilayer vesicles. J. Chem. Phys. 1988;89:1128–1140. doi: 10.1063/1.455219. [DOI] [Google Scholar]
- 91.Lipari G., Szabo A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J. Am. Chem. Soc. 1982;104:4546–4559. doi: 10.1021/ja00381a009. [DOI] [Google Scholar]
- 92.Prompers J.J., Brüschweiler R. Reorientational eigenmode dynamics: a combined MD/NMR relaxation analysis method for flexible parts in globular proteins. J. Am. Chem. Soc. 2001;123:7305–7313. doi: 10.1021/ja0107226. https://www.ncbi.nlm.nih.gov/pubmed/11472158 [DOI] [PubMed] [Google Scholar]
- 93.Vogel A., Tan K.T., et al. Huster D. Flexibility of ras lipid modifications studied by 2H solid-state NMR and molecular dynamics simulations. Biophys. J. 2007;93:2697–2712. doi: 10.1529/biophysj.107.104562. https://www.ncbi.nlm.nih.gov/pubmed/17557790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vogel A., Reuther G., et al. Huster D. Backbone conformational flexibility of the lipid modified membrane anchor of the human N-Ras protein investigated by solid-state NMR and molecular dynamics simulation. Biochim. Biophys. Acta. 2010;1798:275–285. doi: 10.1016/j.bbamem.2009.09.023. https://www.ncbi.nlm.nih.gov/pubmed/19819220 [DOI] [PubMed] [Google Scholar]
- 95.Gu Y., Li D.W., Brüschweiler R. NMR order parameter determination from long molecular dynamics trajectories for objective comparison with experiment. J. Chem. Theor. Comput. 2014;10:2599–2607. doi: 10.1021/ct500181v. https://www.ncbi.nlm.nih.gov/pubmed/26580780 [DOI] [PubMed] [Google Scholar]
- 96.Petrache H.I., Dodd S.W., Brown M.F. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by 2H NMR spectroscopy. Biophys. J. 2000;79:3172–3192. doi: 10.1016/S0006-3495(00)76551-9. https://www.ncbi.nlm.nih.gov/pubmed/11106622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 1973;28c:693–703. doi: 10.1515/znc-1973-11-1209. https://pubmed.ncbi.nlm.nih.gov/4273690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.