ABSTRACT
Skin is largely composed of an epidermis that overlies a supporting dermis. Recent advancements in our understanding of how diverse groups of dermal fibroblasts regulate epidermal and hair follicle growth and differentiation have been fueled by tools capable of resolving molecular heterogeneity at a single-cell level. Fibroblast heterogeneity can be traced back to their developmental origin before their segregation into spatially distinct fibroblast subtypes. The mechanisms that drive this lineage diversification during development are being unraveled, with studies showing that both large- and small-scale positional signals play important roles during dermal development. Here, we first delineate what is known about the origins of the dermis and the central role of Wnt/β-catenin signaling in its specification across anatomical locations. We then discuss how one of the first morphologically recognizable fibroblast subtypes, the hair follicle dermal condensate lineage, emerges. Leveraging the natural variation of skin and its appendages between species and between different anatomical locations, these collective studies have identified shared and divergent factors that contribute to the extraordinary diversity of skin.
Keywords: Dermal condensate, Dermal fibroblasts, Embryo, Skin development, Skin patterning
Summary: A review of how diverse groups of dermal fibroblasts regulate epidermal and hair follicle growth and differentiation, fueled by tools capable of resolving molecular heterogeneity at the single-cell level.
INTRODUCTION
Vertebrate skin varies widely across anatomical sites in thickness, color, physical properties and patterning of appendages. The skin contains an outer epidermis largely composed of keratinocytes and an underlying dermis that houses dermal fibroblasts. The basis for the variability in skin characteristics across anatomical sites is thought to reside in the dermis (Dhouailly, 1973). Different subtypes of dermal fibroblasts produce extracellular matrix (ECM) that provides structural integrity and elasticity to skin, and promote the formation of skin appendages, including hair follicles and sweat glands. Accordingly, during wound healing, but also in chronic skin fibrosis diseases, fibroblasts are the main producers of ECM (Kokkinos et al., 2007; Sriram et al., 2015). In addition to generating skin heterogeneity by modulating ECM composition, other fibroblast populations contribute to different skin phenotypes by orchestrating the formation and maintenance of skin appendages.
Recently, the mechanisms that govern fibroblast differentiation and heterogeneity have become clearer. Across anatomical sites, dermal fibroblast progenitors are first specified from a common mesenchymal progenitor. Subsequently, dermal fibroblast progenitors further differentiate in a step-wise fashion [reviewed in detail elsewhere (Driskell and Watt, 2015; Plikus et al., 2021)] into different functional subtypes. One of the earliest morphologically distinguishable subtypes recognized is the dermal condensate (DC) population, which is crucial for hair follicle formation, and serves as a model to investigate how broad and local signals can lead to dermal lineage specification.
This Review discusses two main aspects of dermal development with emphasis on some of the key large and small-scale positional cues that regulate dermal fibroblast specification and differentiation. First, we highlight historical landmark studies that mapped the embryonic origin of dermal fibroblast precursors in chick and mouse embryos. We review how dermal fibroblasts emerge within different anatomic sites and focus on genetic studies demonstrating the role of Wnt/β-catenin signaling in dermal fibroblast specification. Second, we discuss recent genetic studies that identify dynamic biochemical and mechanical cues that regulate DC differentiation before and during hair follicle formation. Using palmoplantar and dorsal skin as examples of phenotypic variation of skin by anatomical site, we explore how the formation of DCs is modulated to obtain stark differences in skin function and appearance.
Embryonic origins and morphogenesis of dermal fibroblasts
The embryonic origin of mammalian dermal fibroblasts depends on their anatomical site (reviewed by Thulabandu et al., 2018). In the initial phase of dermal fibroblast development, progenitors emerge from different anatomical sites in the head, dorsum and ventrum, where they expand and migrate to their destination under the ectoderm to populate the skin (Fig. 1). In the head region, dermal fibroblasts of the face and head anterior to the ears are derived from cranial neural crest cells. The dermis of the posterior head region is derived from the cephalic mesoderm (Jiang et al., 2002). Dorsal and ventral dermal fibroblasts are derived from somite dermomyotomes and lateral plate mesoderm, respectively (Fig. 1A) (Atit et al., 2006; Ohtola et al., 2008). Detailed molecular understanding of how these cell populations form are still incomplete, and very little is known about the signals and cellular mechanisms that cause these large-scale, directed cellular movements. For example, Wnt11, a secreted short-range signaling molecule, is expressed in dermal fibroblast precursors in the mouse and chick dorsum and cranial sites (Goodnough et al., 2014; Houzelstein et al., 2000; Tanda et al., 1995). Wnt11 is a plausible large-scale signaling candidate because of its well-known roles in cell migration, cell shape and convergent extension movements (Garriock and Krieg, 2007; Heisenberg et al., 2000). Indeed, Wnt11 is required for oriented migration of dermal progenitors over the neural tube in chick embryos (Morosan-Puopolo et al., 2014). However, deletion of Wnt11 or Wntless, an intracellular transporter required for the secretion of all Wnt ligands in dermal precursors, showed that Wnts are dispensable for the large-scale migration of mouse cranial and dorsal dermal precursors (DiNuoscio and Atit, 2019; Goodnough et al., 2014) (Belenkaya et al., 2008). Other signals have been shown to regulate dermal fibroblast precursor proliferation, survival and differentiation from the somite and neural crest, including platelet-derived growth factor receptor α (PDGFRα) and Twist1 (Goodnough et al., 2016; Soriano, 1997; Houzelstein et al., 2000; Morosan-Puopolo et al., 2014). However, the genes and cellular mechanisms that robustly govern the large-scale migration of dermal fibroblasts precursors under the ectoderm remain to be discovered.
Fig. 1.
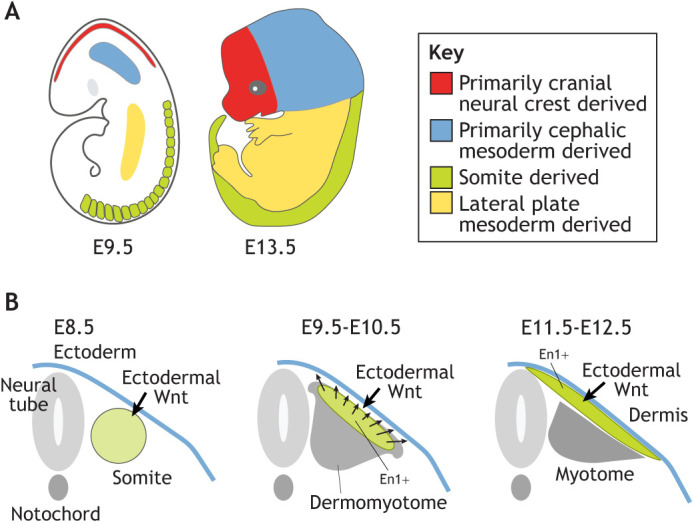
The embryonic origin of dermal fibroblasts across anatomical sites. (A) Murine dermal fibroblasts in various anatomical locations are derived from different embryonic cell types. (B) The emergence of dorsal dermal fibroblasts from the dermomyotome expressing engrailed 1 (En1) transcription factor (green) under the control of surface ectoderm Wnts. Streams of dermal fibroblasts precursors emerge from the dermomyotome and migrate under the surface ectoderm towards the neural tube and laterally in dorsum.
Dermal fibroblast specification
Lineage tracing studies of dermal fibroblasts
To understand how the dermis forms and matures, it is important to briefly review the embryology of the dermis. The dorsal trunk is one of the most extensively studied anatomical sites with regard to the emergence and specification of dermal fibroblasts from the somitic mesoderm in chick and mouse embryos (Ben-Yair and Kalcheim, 2005; Ben-Yair et al., 2003). Somites are transient structures from the mesoderm germ layer that contain the progenitors for skeletal muscle, dermis, brown fat and vertebrae (Atit et al., 2006; Ben-Yair and Kalcheim, 2005). Dermal fibroblasts emerge from the dorsal aspect of the somite: the central dermomyotome (Scaal and Christ, 2004). Lineage maps in chick embryos of somite-derived dermis show different regions of the dermomyotome giving rise to dorsal dermal fibroblasts above the neural tube and the dorsal trunk (Cheng et al., 2004; Couly and Douarin, 1988; Douarin et al., 1993; Ordahl et al., 2001). According to one study, the central dermomyotome gives rise to the dorsal dermis first and later to the dermis above the neural tube (Ben-Yair et al., 2003). Another study showed that the dorsal medial lip cells that are closer to the neural tube migrate early to form the dermis above the neural tube (Dhouailly et al., 2004; Olivera-Martinez et al., 2004a,b). Regardless of these different results from chick studies, the majority of the dorsal dermis arises from the central dermomyotome region that expresses the transcription factor engrailed 1 (En1) (Cheng et al., 2004; Olivera-Martinez et al., 2004a).
In the mouse, the central dermomyotome does not show the clear epithelial cell morphology of the chick dermomyotome. This highlights a major obstacle in mammalian dermal biology: the lack of histological and molecular markers to clearly define fibroblast subpopulations. One approach to overcome this hurdle has been to use genetic lineage tracing of En1+ cells in the central dermomyotome of mouse embryos using a tamoxifen-inducible En1Cre-estrogen receptor to genetically mark and track these cells and their progeny over time. Using this strategy, En1+ cells at embryonic day (E) 9.5 were found to contribute to dorsal dermal fibroblasts under the ectoderm and in deeper located mesenchymal cells, brown fat and trunk muscles (Atit et al., 2006). En1+ cells at E10.5 were lineage traced to the dermis above the neural tube, the superficial dermis and the deep mesenchyme in the dorsal trunk adjacent to the forelimb. Thus, En1+ cells from E9.5 and E10.5 in mouse embryos contribute to the dorsal dermis from the fetal midline to the medial one-third of the scapula (Atit et al., 2006). Consistent with this, later studies also demonstrated that dermal fibroblast subtypes arise from En1+ and PDGFRα+ precursors (Driskell et al., 2013; Jiang et al., 2018; Shook et al., 2018).
The embryonic origin of fibroblasts, including En1+ lineage dermal fibroblasts, have become relevant in wound healing and fibrosis in adult skin. Dermal fibroblasts from different anatomical sites vary in their scar-forming ability, as shown by genome-wide expression studies and cross-transplantation assays (Rinkevich et al., 2015). In adult dorsal skin, the En1+ fibroblasts are the source of scar formation in wound healing (Rinkevich et al., 2015). Interestingly, one group found En1− dermal fibroblasts can acquire En1 expression upon skin wounding and promote scar formation in adult mouse skin (Mascharak et al., 2021). Inducible and reversible Wnt/β-catenin signaling in En1Cre-derived dermal fibroblasts lineages causes fibrotic dermal remodeling, including ECM expansion and shrinking of dermal adipocytes in adult skin that is mediated by CD26/dipeptidyl peptidase 4 (Jussila et al., 2021). Upon withdrawal from Wnt activation, fibrotic remodeling in the dermis and dermal adipocytes was reversed in mouse skin, fully restoring skin architecture (Jussila et al., 2021). These studies demonstrate the potential presence of ‘positional memory’ that dermal fibroblasts retain in their genome (discussed below), but also the plasticity that can be induced upon a wound-healing stimulus. Understanding and exploiting these features may help translate fibroblast biology to clinical applications.
Wnt signaling in dermal fibroblast specification
In the mouse embryo, as dermal fibroblast progenitors emerge from the central dermomyotome at E10.5, they are specified to become En1+ dorsal dermal fibroblasts. Several signaling pathways are important in this early mouse and chick dermal fibroblast specification [reviewed in detail elsewhere (Olivera-Martinez et al., 2004b; Plikus et al., 2021; Thulabandu et al., 2018)]. However, the most studied signaling pathway that is vital for dermal specification and diversification to subtypes is the Wnt/β-catenin signaling pathway (Fig. 2). Studies in guard hair follicle initiation have also contributed to our understanding of dermal Wnt/β-catenin signaling (see below). Wnt signaling is activated by secreted Wnt glycoprotein ligands that bind to Frizzled and LDL-receptor related protein (LRP) 5/6 on the cell surface (Nusse and Clevers, 2017). Canonical Wnt signaling is transduced intracellularly by β-catenin translocating to the nucleus where it binds with Tcf/Lef1 transcription factors to regulate the expression of Wnt target genes in a cell type- and developmental time-dependent manner (Nusse and Clevers, 2017). Conditional mutations that lead to loss- or gain-of Wnt/β-catenin signaling in the En1+ and PDGFRα+ lineages in the somite, lateral plate mesoderm and cranial neural crest cells, have demonstrated that Wnt/β-catenin signaling is required and sufficient for dermal fibroblast specification (Atit et al., 2006; Mastrogiannaki et al., 2016; Ohtola et al., 2008; Rognoni et al., 2016; Tran et al., 2010). In the absence of Wnt/β-catenin signaling in the En1+ lineage, central dermomyotome cells express markers of the muscle lineage ectopically, indicating a cell fate change (Atit et al., 2006; Kalcheim and Ben-Yair, 2005). In the ventrum and craniofacial region, deletion of β-catenin in En1+ cells leads to ectopic expression of Sox9, a key determinant of the chondrocyte lineage (Atit et al., 2006; Ohtola et al., 2008; Tran et al., 2010). Collectively, these studies demonstrate that Wnt/β-catenin signaling is a conserved instructive signal that specifies dermal fibroblast fate at different anatomical sites and represses alternative cell fates (Atit et al., 2006; Ohtola et al., 2008; Tran et al., 2010).
Fig. 2.
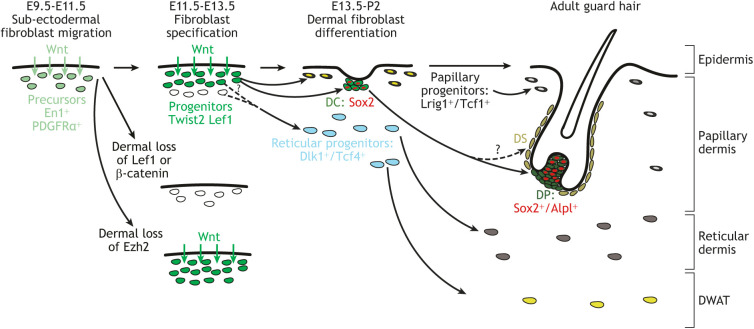
Role of Wnt signaling in mouse dermal fibroblast specification and step-wise differentiation to subtypes. Surface ectoderm Wnts are crucial for specification of dermal fibroblast precursors. In the absence of dermal β-catenin or Lef1, dermal fibroblasts progenitors are not specified. Dermal Wnt signaling is also required to generate papillary, reticular and dermal condensate (DC) lineages. Ezh2, an epigenetic regulator, is required to maintain the domain-specific Wnt signaling activation between E11.5 and E13.5. Alpl, alkaline phosphatase; DS, dermal sheath; DP, dermal papilla; DWAT, dermal white adipocytes. Hatched line with a question mark is a possible route of differentiation.
How is dermal Wnt signaling activated? Before dermal fibroblast specification, Wnt ligands are expressed in the overlying dorsal surface ectoderm and neural tube in chick and mouse embryos (Andl et al., 2002; Parr et al., 1993; Schubert et al., 2002) (Figs 1B, 2). Surgical removal of the surface ectoderm results in thinner dermis and loss of En1 expression in the chick embryo (Olivera-Martinez et al., 2004a). Additionally, deletion of Wntless in the mouse ectoderm by E9.5 leads to loss of Wnt signal transduction in the underlying mesenchyme at E11.5-12.5 and a loose dermal mesenchyme (Fig. 2) (Chen et al., 2012; Goodnough et al., 2014). Thus, ectoderm Wnt ligands expressed between E9.5 and E11 in mouse embryos are required to initiate canonical Wnt signaling in the mesenchyme to induce dermal fibroblast fate between E11.5 and E13.5 (Fig. 2).
Specification of mouse dermal fibroblasts progenitors is generally visualized by spatial expression of genetic Wnt signaling reporters and transcriptional targets of the canonical Wnt signaling pathway, such as Lef1, Axin2, Twist1 and Dermo1/Twist2 mRNA or protein between E11.5 and E13.5 (Fig. 2). But how is canonical Wnt signal transduction spatially restricted to four or five layers of dermal fibroblast progenitors between E11.5 and E13.5? The range of diffusion-mediated Wnt signaling varies widely depending on the context and there is emerging evidence that Wnt ligands can be moved from their site of synthesis via membrane extensions, exosomes and microvesicles (Parchure et al., 2018; Stewart et al., 2019; Yamashita et al., 2018). In other contexts, Wnt signaling can be regulated by epigenetic regulators, such as polycomb repressive complex 2 (PRC2) (Nehila et al., 2020). The deletion of enhancer of zeste homolog 2 (Ezh2), the key enzyme in PRC2, in dorsal dermal fibroblast precursors, leads to ectopic activation of Wnt signaling in the mesenchyme, as visualized by expansion of the Axin2-LacZ, Twist2+ and Lef1+ dermal fibroblast progenitor domain (Thulabandu et al., 2021) (Fig. 2). Thus, PRC2-Ezh2 may serve as a rheostat control of Wnt signaling and spatially restrict the domain of Wnt signaling during dermal fibroblasts progenitor fate selection (Nehila et al., 2020).
After dermal specification between E11.5 and E13.5, canonical Wnt signaling induces dermal fibroblasts to further differentiate into upper papillary and dermal condensate subtypes for the initiation of guard and secondary hair follicles after E13.5 (Atit et al., 2006). The deeper dermal fibroblast progenitors become the lower reticular progenitors and dermal white adipocytes (DWAT) (Fig. 2). One early study to examine the ontogeny of mouse dermal fibroblast subtypes assessed the expression of adult fibroblast markers in the embryonic dermis, including those of upper papillary (Tcf1 and Lrig1) and lower reticular dermal fibroblasts (Dlk1 and TCF4) (Driskell et al., 2013) (Fig. 2). Using a combination of immunohistochemistry and genetic lineage-tracing experiments, they found that the papillary and reticular dermal fibroblast subtypes could be distinguished as early as E16.5 in the mouse embryo. This landmark study, with other genetic studies, established the framework that dermal fibroblasts differentiate from a common mesenchymal cell into various subtypes before their histological distinction (Fig. 2A).
Dermal fibroblasts are the main determinants of skin phenotype and heterogeneity
Recombination experiments in mouse and chick have demonstrated that the information to direct the epidermis towards specific phenotypes resides in the dermis (Billingham and Silvers, 1967; Hardy, 1992; Jahoda, 1992; Oliver, 1970; Reynolds et al., 1999; Sengel, 1976; Sengel and Dhouailly, 1977; Yamaguchi et al., 1999). For example, ex vivo recombination of hair follicle-forming dorsal dermis with epidermis from hairless plantar skin is sufficient to induce hair follicle growth (Dhouailly, 1973; Kollar, 1970). Heterotopic recombination experiments in duck and quail embryos further clarified that cranial neural crest cells, the progenitors to cranial dermal fibroblasts, can direct species-specific feather patterning and pigmentation (Eames and Schneider, 2005). Hox gene expression in the dermal papillae was implicated in patterning the mouse and chick skin with hair and feather, respectively (Bieberich et al., 1991; Chuong et al., 1990). Using genomic approaches to sample different anatomical sites, human dermal fibroblasts were shown to be the key cell type with consistent differences across sites (Rinn et al., 2006, 2008). Positional identities of adult human dermal fibroblasts are associated with the anatomically specific Hox gene expression initiated during embryonic development. Hox gene expression is maintained in adult skin and in vitro by epigenetic modifiers of the PRC (Chang, 2009; Chang et al., 2002; Rinn et al., 2008). Consistent with early studies in chick and mouse (Bieberich et al., 1991; Chuong et al., 1990), one group found that Hoxc cluster genes show spatial co-linearity in skin, and expression correlates with regional variation in hair growth across anatomical sites (Yu et al., 2018). They found that Hoxc genes are absent in the skin of the ear, which has resting hair follicles, whereas dorsum and tail regions with cyclically regenerating hair follicles express Hoxc4-10 and Hoxc11-13, respectively. This study also showed that single Hox genes, such as Hoxc8, can reprogram the cells of a dormant dermal papilla into a hair follicle-stimulating cells (Yu et al., 2018). Several studies support the hypothesis that a dermal ‘Hox code’ program exists and regulates site-specific epidermal differentiation (Bieberich et al., 1991; Chang et al., 2002; Chuong et al., 1990; Rinn et al., 2007, 2008; Yu et al., 2018). However, the Hox code expression is shared between similarly patterned skin at distant anatomical sites, such as the surface of the hand (palmar) versus the sole of the foot (plantar). Combined with the absence of loss-of-function data of Hox genes, there is not enough information to account for an exclusively Hox-dependent mechanism to pattern the skin. Thus, there must be other mechanisms that contribute to skin heterogeneity.
Generating skin phenotypes and heterogeneity: the role of Wnt signaling
The molecular cues that are responsible for anatomical site-specific differences of skin appear to fall on a few molecular factors in the dermis, such as Hox genes, but the downstream mediators and gene regulatory networks are only recently being elucidated. For example, Hoxc expression leads to Wnt pathway activation in the dorsal and tail skin dermal papilla (Yu et al., 2018). It has been found that expression of Wnt pathway activators, such as R-spondin2, are upregulated and the Wnt inhibitor secreted Frizzled-related protein 2 (Sfrp2) is downregulated in dorsal skin compared with the ear skin, where there are mainly resting hair follicles. How Hoxc genes control Wnt signaling and whether other downstream pathways are altered by Hoxc expression remain unknown (Millar, 2018). This is particularly relevant, because mice lacking Sfrp2 are not reported to have hair growth phenotypes (Satoh et al., 2006) and the ear skin is an environment rich in other Wnt inhibitors and bone morphogenetic proteins (BMPs) that can prevent hair growth (Wang et al., 2017).
Another class of Wnt signaling inhibitors, Dickkopff (Dkk) 1 and Dkk2, is known to contribute to skin heterogeneity (Andl et al., 2002; Zhang et al., 2008). Forced expression of Dkk1 in embryonic mouse epidermis leads to loss of hair follicle initiation, demonstrating its role in modulating Wnt signaling activity (Andl et al., 2002). Importantly, endogenous Dkk2 expression varies by anatomical region in embryonic mouse dermis. For example, Dkk2 is elevated in the dermis of plantar skin of mouse embryos, which lacks hair (Song et al., 2018). Dkk2−/− mice have sparse and small, but fully functional, ectopic hairs that are capable of cycling in the plantar skin (Song et al., 2018). Unlike mouse and human plantar skin, rabbits have hair in the plantar skin (Song et al., 2018). Consistently, Dkk2 expression is not elevated on the rabbit plantar side, suggesting evolutionary changes in cis-regulatory mechanisms. The presence of only sparse hair follicles in Dkk2−/− mice suggests that Dkk2 may cooperate with other factors that inhibit Wnt signaling to direct regional skin patterning in the dermis or epidermis. One such co-factor may be another class of Wnt inhibitor, such as sclerostin domain containing 1 (Sostdc1), which is present in the mouse embryonic plantar and dorsal skin epidermis, and expressed in Dkk2−/− mutants (Song et al., 2018).
Despite possible cooperative factors, Dkk2 expression in the dermis has a non-redundant function to prevent hair follicle initiation in the mouse embryonic plantar skin, which provides a new model with which to understand the basis for hairless versus hairy skin. Future studies using higher resolution techniques to profile hairy versus hairless mouse skin dermal fibroblasts are needed to identify and test the candidate co-factors that direct regional aspects of skin patterning.
An interesting aspect in the biology of hairless skin, such as palms, is that fibroblasts play a crucial role not only in determining whether hair follicles form or not, but also in the differentiation of the epidermis. Palmar and plantar fibroblasts can induce, in epidermal keratinocytes of hairy skin, expression of keratin 9 (KRT9), a keratin that is normally exclusively expressed in palmoplantar keratinocytes, and is an integral part of palmoplantar skin biology (Kim et al., 2016; Yamaguchi et al., 2008). Overlapping autocrine or paracrine canonical Wnt/β-catenin signaling can induce KRT9 expression in palmoplantar keratinocytes (Kim et al., 2016; Yamaguchi et al., 2008). The paradox is that, although inhibition of Wnt signaling by Dkk2 sets up the hairlessness of palmar and plantar skin, Wnt signaling is required to establish and maintain KRT9 expression (Kim et al., 2016; Rinn et al., 2008; Yamaguchi et al., 2008). This ‘paradox’ could possibly be explained by the establishment of hairlessness before expression of KRT9 in the epidermis. For an in-depth review of the timing and regulation of KRT9 expression, see Tsai et al. (2022). Evidently, the palms are informative models for studying how the formation of hair follicle DCs are suppressed.
Hair follicle formation: the dermal condensate as a model
Epithelial-mesenchymal interactions regulate the formation of skin appendages such as hair follicles, feathers, sweat glands and nails. Although the specific dermal subtypes that regulate the formation and maintenance of sweat glands and nails are less defined, the hair follicle dermal condensate (DC) is one of the most studied. The hair follicle DC is a dense cluster of quiescent fibroblasts that forms in a patterned array across the dermis in association with overlying hair follicle epithelial placodes (Fig. 3A) (Hardy, 1992; Millar, 2002; Paus et al., 1999; Saxena et al., 2019; Wessells and Roessner, 1965). Reciprocal interactions between the DC and placode result in the downward growth and differentiation of the hair follicle epithelium, whereas the DC matures into the specialized dermal papilla (DP) cell population. In adult skin, the DP engages in complex interactions with epithelial and melanocyte stem cells to regulate the cyclical regeneration of the hair follicle (Myung and Ito, 2012; Xin et al., 2016). The characteristic morphology and molecular signature of the DC in both feathers and hair follicles have made this subpopulation arguably one of the most frequently used models for studying how dermal fibroblasts differentiate to generate skin phenotypic variation by regulating skin appendage formation.
Fig. 3.
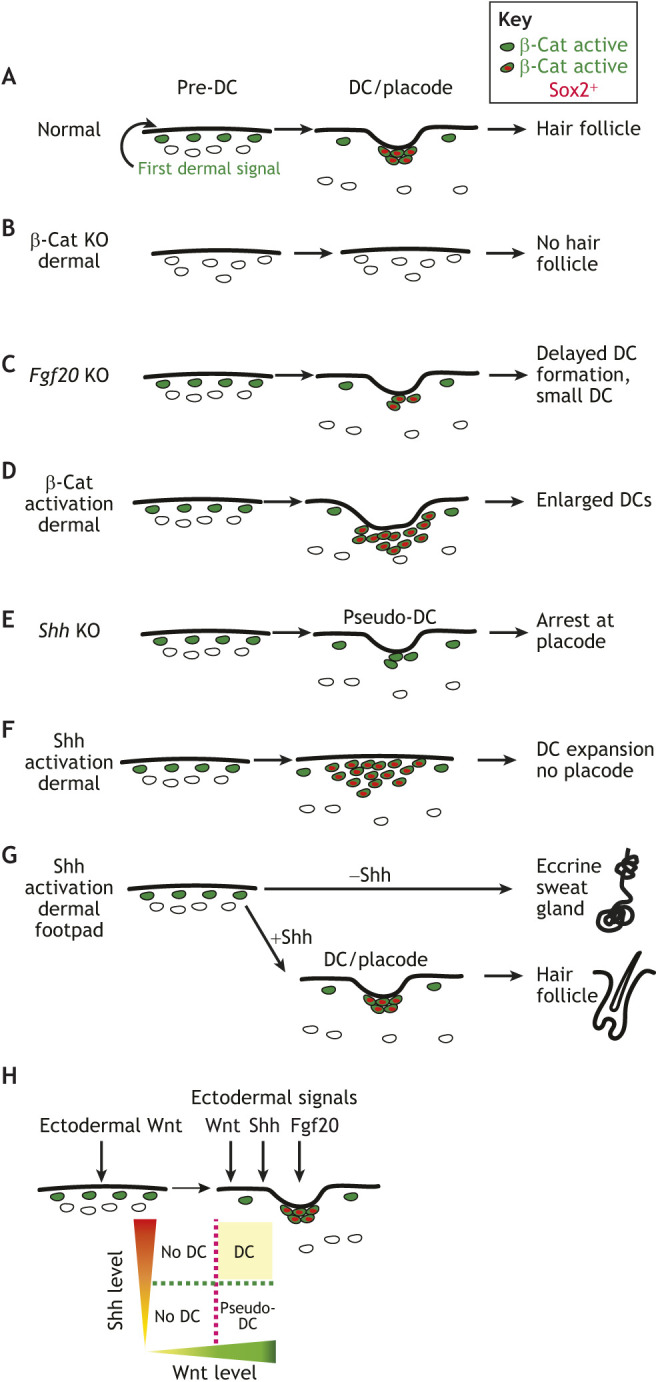
Summary of the signals involved in DC and hair follicle phenotypes. (A) In wild-type mouse skin, the elusive first dermal signal for placode initiation is dependent on canonical Wnt/β-catenin signaling. (B,D) Loss and gain of β-catenin activation in dermis corresponds to the absence and expansion of dermal condensate (DC) and placode, respectively. (C,E) Fgf20 mutants (C) have a delay in DC formation with smaller DC and hair follicle and Shh knockout mutants (E) arrest before DC formation. Fgf20 and Shh knockouts have canonical Wnt/β-catenin signaling activation in the pre-DC population. (F,G) Shh activation can expand the DC population without placodes in the dorsal skin (F) and ectopically induce hair in the foot pad (G). (H) The emerging model suggests that intersection of Wnt and Shh signaling gradients are required for DC formation.
Dermal Wnt/β-catenin signaling in hair follicle dermal condensate formation
As discussed above, early heterotopic recombination experiments using epidermis and dermis from different species showed that the dermis determines the pattern and size of epidermal skin appendages. These tissue recombination experiments have led to the working theory that a uniform ‘first dermal signal’ instructs the overlying epidermis to form patterned placodes (Fig. 3A) (Dhouailly, 1975; Jiang et al., 1999; Sengel, 1976). Placode signals then induce DC specification and DC morphogenesis. Wnt ligands and Wnt/β-catenin signaling in the epidermis are essential for hair follicle and feather placode initiation, and their inhibition results in a lack of DCs, indicating that DCs are specified by signals originating from placode cells (Chen et al., 2012; Qu et al., 2022; Sengel and Abbott, 1963; Zhang et al., 2009). Reciprocally, DCs produce several signals that regulate hair follicle formation and DC maintenance, including BMP agonists and inhibitors, and fibroblast growth factors (FGFs) (Botchkarev et al., 1999; Glover et al., 2017; Tsai et al., 2014).
The identity of a putative first dermal signal still remains poorly characterized, largely because the dermis lacks an apparent initial gene expression or morphological pattern that could explain a potential role in patterning the epidermis. Before hair follicle morphogenesis, Wnt/β-catenin signal transduction is observed across the upper dermis in response to uniformly secreted epidermal Wnt ligands (Fig. 3A). In addition to its role in dermal specification, dermal Wnt/β-catenin signaling in the upper dermis is also required for placode initiation in mice (Fig. 3B). Notably, the loss of dermal Wntless produces a mild skin phenotype with minimal effect on hair follicle formation (DiNuoscio and Atit, 2019; Fu and Hsu, 2013) making it unlikely that the first dermal signal is a Wnt ligand (Fu, 2017). Thus, it has been proposed that dermal Wnt/β-catenin signal transduction is required to transmit the first dermal signal to the epidermis (Chang et al., 2004; Chen et al., 2012; Gupta et al., 2019) (Fig. 3B,D). However, the mechanism by which dermal Wnt/β-catenin signal transduction regulates epidermal placode formation remains elusive.
The scant information regarding the early events that lead to DC genesis partly reflect the lag in genetic tools available to target dermal cells specifically. This also highlights the sparsity of landmarks within the dermis to anchor and track cell behaviors spatially and temporally before morphogenesis. Despite the availability of specific markers that characterize differentiated DC cells, the signals and cellular events that lead to DC genesis are still unknown. The expression of most DC differentiation genes is tightly coupled with DC morphogenesis and cell cycle exit, making it challenging to determine the signals and cellular events that lead to DC genesis. Recently, a placode-secreted factor, FGF20, was shown to be required for DC formation (Biggs et al., 2018; Huh et al., 2013; Mok et al., 2019). Many dermal signals, including dermal Wnt/β-catenin signal transduction, were abrogated in the absence of FGF20, and the specific progenitors that give rise to DC cells that could be interrogated were unknown. Therefore, the function of FGF20 in DC genesis remains unclear, especially as Fgf20−/− mice lack DCs in only a small subset of hair follicle types that make up the mouse hair coat (Fig. 3C).
Over the past decade, tools to genetically target dermal fibroblasts and new methods, including single-cell RNA-sequencing (scRNA-seq) and live imaging methods, have resolved cell populations and processes that were previously difficult to visualize. Together, these approaches have given new perspectives to examine DC genesis and the cell-cell interactions that couple this process to placode development. Using live imaging of embryonic skin explant cultures, some of the cell behaviors that lead to DC formation have been captured, including directed migration of committed pre-DC cells towards the DC center and nuclear shape changes, such as nuclear elongation and intercellular alignment (Biggs et al., 2018; Huh et al., 2013; Mok et al., 2019). These changes appeared to depend on placode signals and to occur after molecular differentiation and cell cycle exit, as DC markers were found to be expressed by unclustered quiescent cells shortly before the observed morphogenetic changes. Nevertheless, how a local pool of quiescent pre-DC cells is generated is unclear.
Using scRNA-seq technology to unbiasedly profile transcriptional differences between cells and to infer intermediate states that precede commitment, an inferred molecular DC differentiation trajectory was constructed (Gupta et al., 2019; Mok et al., 2019). By combining this approach with in vivo mosaic ablation of dermal β-catenin, it was shown that dermal Wnt/β-catenin signal transduction is essential for a dermal cell to acquire DC fate (Gupta et al., 2019). This result implies that dermal Wnt/β-catenin signal transduction does not solely function to communicate information to the epidermis that instructs placode initiation, but it also holds a cell-autonomous role that is essential for DC cell differentiation. Using in vivo proliferation and genetic lineage tracing assays, Wnt signaling-activated dermal cells located in a region surrounding the expanding DC were shown to divide over a short timeframe during early DC morphogenesis, giving rise to quiescent DC cells (Gupta et al., 2019; Qu et al., 2022). This finding resurrected a decades-old theory proposing that DC cells are the quiescent progeny of a highly proliferative population (Wessells and Roessner, 1965) and suggested that Wnt/β-catenin signal transduction may function in part by regulating dynamic cell cycle changes during the pre-DC-to-DC transition.
Dermal condensate genesis: the second signal
Although Wnt/β-catenin signaling by dermal progenitors is necessary for DC cell fate, Wnt signaling alone is not sufficient for DC differentiation, as expression of an activated form of β-catenin across the dermis results in larger DCs (Fig. 3D) but only where and when placodes normally form (Chen et al., 2012; Gupta et al., 2019). This finding shows that one or more placode factors must cooperate with Wnt signaling activation in dermal cells to induce DC differentiation. Combining scRNA-seq analysis with genetic mouse models, dermal sonic hedgehog (Shh) signaling has emerged as a crucial placode-dependent signal that cooperates with Wnt signaling to deterministically drive the molecular and cellular events of DC genesis (Qu et al., 2022). Specifically, Shh activation in dermal cells cooperates with dermal Wnt/β-catenin signaling to stimulate the proliferation of DC progenitors while inducing the expression of DC genes and promoting the transition to cell cycle exit (Fig. 3E). Interestingly, when high levels of Shh signaling are genetically induced across the Wnt-activated upper dermis before morphogenesis, DC differentiation genes and DC morphological changes are seen in the absence of placodes in a pattern that correlates with Wnt signaling levels (Fig. 3F). The findings from this study suggest that the spatiotemporal intersection of two gradient signals (Wnt and Shh) can determine the number of DC progenitors and their rate of transition to DC status, providing insight into mechanisms of cell fate patterning (Fig. 3H).
These studies are consistent with a similar model from studies of mouse footpads, where Shh-Wnt interactions mediate DC specification. In the mouse footpad, where sweat glands form exclusively without hair follicles, dermal Wnt/β-catenin signaling is high and the major factor that determines the formation of sweat glands versus hair follicles is the absence or presence of epidermal Shh ligand expression during a permissive phase of epithelial bud development (Lu et al., 2016) (Fig. 3G). Here, BMPs and FGFs secreted from the Wnt-activated dermis stimulate epidermal BMP and FGF signaling, which inhibit Shh expression and result in sweat gland formation. Ectopic expression of Shh during the permissive phase causes a switch from formation of sweat glands to hair follicles that are associated with DCs. Consistent with this study, it has been shown that genetic activation of Shh signaling in both the mouse plantar epidermis and dermis is sufficient to induce hair follicles in normally hairless skin. These and other studies show a crucial role for dermal Shh signaling in DC genesis. Notably, in plantar skin regions where there are no hair follicles, Dkk2 expression is high and inhibits dermal and epidermal Wnt signaling. Consequently, loss of Dkk2 leads to ectopic formation of hair follicles (Song et al., 2018) but it is still unclear whether activation of dermal or epidermal Wnt signal transduction mediates this effect. Thus, although insufficient to determine if dermal Wnt signaling provides the ‘first dermal signal’ to initiate appendage placodes, plantar skin has added considerable evidence that dermal Wnt signaling remains a candidate.
How do gradient signals converge to induce cell state transitions?
The differentiation of dermal fibroblasts during development are functionally linked to both broad positional cues (e.g. epidermal Wnt ligands) as well as local cues (e.g. placode-derived Shh ligand) and likely other factors (Chen et al., 2012; Fu and Hsu, 2013; Gupta et al., 2019). There is now more insight on how Wnt signaling might be interpreted than for Shh signaling. It is possible that different levels or duration of Wnt signaling serves to establish lineage potential or competency. Several studies have shown that expression of Wnt target genes, such as Lef1, Twist2 and Axin2, are heterogenous in the upper dermis, but it has been challenging to quantify the variation in gene expression and provide evidence that such variation is consequential (Andl et al., 2002; Chen et al., 2012; Noramly et al., 1999; Reddy et al., 2001; Zhang et al., 2009). scRNA-seq and quantitative fluorescent in situ hybridization (FISH) data from mouse embryonic skin have now established variation in expression levels of Wnt target genes in dermal fibroblasts between E13.5 and E14.5 (Gupta et al., 2019; Qu et al., 2022). How can different levels of Wnt/β-catenin signaling lead to differences in cell fate potential? Mechanistically, β-catenin has been shown to interact with epigenetic regulators, such as Prc2, to alter chromatin accessibility (Ferguson et al., 2018; Hoffmeyer et al., 2017; Jung et al., 2013), as well as directly serve as a chromatin remodeler (Iyer et al., 2018; Mosimann et al., 2009). However, this activity of β-catenin does not address how a gradient of intracellular Wnt/β-catenin signaling can result in the transcription of different target genes. Another theory to consider is the classic morphogen signal that regulates gene expression of cell fate transcription factors along a gradient. Using mouse embryos expressing an allelic series of β-catenin, it was elegantly demonstrated that known Wnt target genes are activated by different thresholds of Wnt signaling in vivo (Rudloff and Kemler, 2012). Here, Lef1 mRNA transcription requires high levels of Wnt/β-catenin signaling and Tcf4 mRNA requires lower levels in the embryo. Interestingly, Tcf4 is actually repressed by high levels of Wnt signaling. Indeed, Lef1 and Tcf4 are expressed in non-overlapping domains in the mouse dorsal and cranial dermis between E12.5 and E16.5 (Driskell et al., 2013; Goodnough et al., 2014; Thulabandu et al., 2021). Similarly, the DC differentiation process may occur along a continuum of a Wnt signaling gradient where dynamic changes in transcription factors can regulate rapid cell fate transitions (Mok et al., 2019). Given the complexity of the canonical Wnt signaling pathway, future studies are needed to determine how the duration and levels of Wnt signaling are decisively interpreted by cells in different contexts during development and regeneration.
As well as in response to Wnt signaling, changes in chromatin accessibility may also function as a mechanism for interpreting and integrating signal gradients. Significant work has shown that epidermal differentiation is regulated by epigenetic regulators and transcription factors (Adam et al., 2015, 2018; Cohen et al., 2018, 2019; Szigety et al., 2020; Zhang et al., 2009). Currently, the epigenetic regulators that affect chromatin accessibility during dermal development are poorly delineated. As single-cell multiomics and spatial transcriptomic methods expand and improve in conjunction with 3D (e.g. expansion microscopy) and 4D (e.g. optogenetic) imaging techniques, the ability to resolve specific subpopulations and the signals that give rise to diverse dermal lineages will become easier to investigate (Joshi et al., 2020; Wassie et al., 2019).
Beyond signaling molecules: mechanical force as a driver of morphogenesis
In addition to biochemical signals, the role of mechanical forces in cell fate patterning and morphogenesis is unfolding as advances in biophysical tools open the door to studying mechanobiology in skin development. A recent study provided ex vivo evidence that chick follicle patterning originates from the spontaneous self-organization of dermal fibroblasts (Palmquist et al., 2022). Using an approach similar to that described by Jiang et al. (1999), dermal fibroblasts were dissociated from E8.0 chicks once feather buds had formed. It was shown that fibroblasts could spontaneously self-organize into patterned aggregates on a collagen matrix substrate by reciprocal interactions between fibroblasts and ECM components (Jiang et al., 1999; Palmquist et al., 2022). This reciprocal interaction regulates cell contractility through calcium-dependent mechanisms, resulting in autonomous cell alignment and resolution into patterned aggregates. Presumably, subsequent signals induced by this prepattern (e.g. from the feather placode) would then stabilize these aggregates. Future work will be necessary to test these models in vivo to account for other factors, including cell proliferation, heterogenous cell types, and other ECM and biochemical components present within developing skin. Nonetheless, this study underscores the need to integrate tissue- and cell-level mechanical forces into current models of organogenesis and cell fate patterning.
The future of dermal fibroblast research
Over the past decade, significant advancements have been made to characterize fibroblast subpopulations in adult skin and how they regulate the cell behaviors and functions of epithelial, immune, vascular and neuronal cell types. This ability to dynamically regulate heterotypic cell behaviors during homeostasis and wound healing reflects the remarkable plasticity of fibroblasts to change their molecular, morphological and physical state in response to the environment. For at least some fibroblasts, the potential to adopt different cell states traces back to their embryonic origin. Hence, the mechanisms that regulate lineage diversity during development have become increasingly recognized as an unmet area of research that can guide methods to modulate fibroblasts in adult skin to promote regeneration. This is underlined by landmark work showing that embryonic programs of development can be recapitulated in adult mammalian skin after large skin wounds to reproduce many aspects of skin morphogenesis, including the formation of new hair follicles (wound-induced hair follicle neogenesis, WIHN) (Ito et al., 2007). This rare example of mammalian epimorphic regeneration has provided an invaluable model for studying mechanisms of certain aspects of skin regeneration, while also showing that adult dermal fibroblasts display remarkable plasticity to differentiate into multiple cell types, including dermal papillae, myofibroblasts and adipocytes (Lim et al., 2018; Phan et al., 2020; Plikus et al., 2017). Not surprisingly, many of the signals essential for WIHN are the same for embryonic hair follicle development, including dermal and epidermal Wnt/β-catenin and Shh signaling (Frech et al., 2022; Lim et al., 2018; Myung et al., 2012; Phan et al., 2020). The spatiotemporal dynamics of these signals and the dermal subpopulations that differentiate into neogenic DP and dermal sheath cells remain to be delineated.
Looking forward, technological advancements in imaging and single-cell multi-modality platforms hold the potential to resolve complex processes that cause divergent programs of differentiation during development. For example, 2D spatial transcriptomics is currently possible, and 3D FISH expansion microscopy continues to evolve to give a wider view of how cell transcriptional populations are organized at a global scale. Although live imaging and optogenetic tools have been useful for adult mouse and in vitro models, methods to spatiotemporally track and interrogate individual dermal cells in living embryos or skin explants are less developed. However, they hold the potential to directly trace cell states and fates over real time and space. As single-cell omics and imaging techniques continue to improve, the ability to construct a blueprint of fibroblast heterogeneity during development is on the horizon.
Additionally, emerging in vitro models of somite formation from mouse and human induced pluripotent stem cells can differentiate to express dermomyotome markers, such as doublesex, Mab3 related transcription factor 2 and paired box 3 (Brink et al., 2020; Budjan et al., 2022; Sanaki-Matsumiya et al., 2022; Tarazi et al., 2022). If these 3D models or advanced embryoids eventually proceed to the stage of dermis formation, then they will serve as powerful tools for identifying the drivers of the large scale dermal fibroblast migration and later events such as DC formation. The prospect of tractable in vitro tools to study human dermis formation is compelling and will allow the field to apply genetic and pharmacological tools to construct versatile 3D models of dermal development.
Acknowledgements
We thank Mayumi Ito, and members of the Atit and Myung labs for critical reading of the review.
Footnotes
Funding
P.M. is funded by the National Institutes of Health – National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR076420-01) and by a Doris Duke Charitable Foundation Clinical Scientist Development Award (2018102). R.A. is funded by the National Institutes of Health – National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR076938) and by the National Institutes of Health – National Institute of Dental and Craniofacial Research (R21-DE029348 and R56-DE0302026). Deposited in PMC for release after 12 months.
References
- Adam, R. C., Yang, H., Rockowitz, S., Larsen, S. B., Nikolova, M., Oristian, D. S., Polak, L., Kadaja, M., Asare, A., Zheng, D.et al. (2015). Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature 521, 366-370. 10.1038/nature14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam, R. C., Yang, H., Ge, Y., Lien, W.-H., Wang, P., Zhao, Y., Polak, L., Levorse, J., Baksh, S. C., Zheng, D.et al. (2018). Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell 22, 398-413.e7. 10.1016/j.stem.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl, T., Reddy, S. T., Gaddapara, T. and Millar, S. E. (2002). WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643-653. 10.1016/S1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Atit, R., Sgaier, S. K., Mohamed, O. A., Taketo, M. M., Dufort, D., Joyner, A. L., Niswander, L. and Conlon, R. A. (2006). Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164-176. 10.1016/j.ydbio.2006.04.449 [DOI] [PubMed] [Google Scholar]
- Belenkaya, T. Y., Wu, Y., Tang, X., Zhou, B., Cheng, L., Sharma, Y. V., Yan, D., Selva, E. M. and Lin, X. (2008). The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell 14, 120-131. 10.1016/j.devcel.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Ben-Yair, R. and Kalcheim, C. (2005). Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 132, 689-701. 10.1242/dev.01617 [DOI] [PubMed] [Google Scholar]
- Ben-Yair, R., Kahane, N. and Kalcheim, C. (2003). Coherent development of dermomyotome and dermis from the entire mediolateral extent of the dorsal somite. Development 130, 4325-4336. 10.1242/dev.00667 [DOI] [PubMed] [Google Scholar]
- Bieberich, C. J., Ruddle, F. H. and Stenn, K. S. (1991). Differential expression of the Hox 3.1 gene in adult mouse skin. Ann. N. Y. Acad. Sci. 642, 346-353; discussion 353-4. 10.1111/j.1749-6632.1991.tb24400.x [DOI] [PubMed] [Google Scholar]
- Biggs, L. C., Mäkelä, O. J., Myllymäki, S.-M., Roy, R. D., Närhi, K., Pispa, J., Mustonen, T. and Mikkola, M. L. (2018). Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. Elife 7, e36468. 10.7554/eLife.36468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham, R. E. and Silvers, W. K. (1967). Studies on the conservation of epidermal specificies of skin and certain mucosas in adult mammals. J. Exp. Med. 125, 429-446. 10.1084/jem.125.3.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev, V. A., Botchkareva, N. V., Roth, W., Nakamura, M., Chen, L. H., Herzog, W., Lindner, G., Mcmahon, J. A., Peters, C., Lauster, R.et al. (1999). Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1, 158-164. 10.1038/11078 [DOI] [PubMed] [Google Scholar]
- Brink, S. C., Van Den, Alemany, A., Batenburg, V., Van Moris, N., Blotenburg, M., Vivié, J., Baillie-Johnson, P., Nichols, J.et al. (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405-409. 10.1038/s41586-020-2024-3 [DOI] [PubMed] [Google Scholar]
- Budjan, C., Liu, S., Ranga, A., Gayen, S., Pourquié, O. and Hormoz, S. (2022). Paraxial mesoderm organoids model development of human somites. Elife 11, e68925. 10.7554/eLife.68925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. Y. (2009). Anatomic demarcation of cells: genes to patterns. Science 326, 1206-1207. 10.1126/science.1175686 [DOI] [PubMed] [Google Scholar]
- Chang, H. Y., Chi, J.-T., Dudoit, S., Bondre, C., Van De Rijn, M. , Botstein, D. and Brown, P. O. (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 99, 12877-12882. 10.1073/pnas.162488599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.-H., Jiang, T.-X., Lin, C.-M., Burrus, L. W., Chuong, C.-M. and Widelitz, R. (2004). Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech. Dev. 121, 157-171. 10.1016/j.mod.2003.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Jarrell, A., Guo, C., Lang, R. and Atit, R. (2012). Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 139, 1522-1533. 10.1242/dev.076463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L., Alvares, L. E., Ahmed, M. U., El-Hanfy, A. S. and Dietrich, S. (2004). The epaxial–hypaxial subdivision of the avian somite. Dev. Biol. 274, 348-369. 10.1016/j.ydbio.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Chuong, C. M., Oliver, G., Ting, S. A., Jegalian, B. G., Chen, H. M. and Robertis, E. M. D. (1990). Gradients of homeoproteins in developing feather buds. Development 110, 1021-1030. 10.1242/dev.110.4.1021 [DOI] [PubMed] [Google Scholar]
- Cohen, I., Zhao, D., Bar, C., Valdes, V. J., Dauber-Decker, K. L., Nguyen, M. B., Nakayama, M., Rendl, M., Bickmore, W. A., Koseki, H.et al. (2018). PRC1 fine-tunes gene repression and activation to safeguard skin development and stem cell specification. Cell Stem Cell 22, 726-739.e7. 10.1016/j.stem.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, I., Zhao, D., Menon, G., Nakayama, M., Koseki, H., Zheng, D. and Ezhkova, E. (2019). PRC1 preserves epidermal tissue integrity independently of PRC2. Gene Dev. 33, 55-60. 10.1101/gad.319939.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly, G. and Douarin, N. M. L. (1988). The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development 103, 101-113. 10.1242/dev.103.Supplement.101 [DOI] [PubMed] [Google Scholar]
- Dhouailly, D. (1973). Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J. Embryol. Exp. Morphol. 30, 587-603. [PubMed] [Google Scholar]
- Dhouailly, D. (1975). Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux's Arch. Dev. Biol. 177, 323-340. 10.1007/BF00848183 [DOI] [PubMed] [Google Scholar]
- Dhouailly, D., Olivera-Martinez, I., Fliniaux, I., Missier, S., Viallet, J. P. and Thelu, J. (2004). Skin field formation: morphogenetic events. Int. J. Dev. Biol. 48, 85-91. 10.1387/ijdb.15272373 [DOI] [PubMed] [Google Scholar]
- Dinuoscio, G. and Atit, R. P. (2019). Wnt/β-catenin signaling in the mouse embryonic cranial mesenchyme is required to sustain the emerging differentiated meningeal layers. Genesis 57, e23279-e23212. 10.1002/dvg.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douarin, N. M. L., Ziller, C. and Couly, G. F. (1993). Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev. Biol. 159, 24-49. 10.1006/dbio.1993.1219 [DOI] [PubMed] [Google Scholar]
- Driskell, R. R. and Watt, F. M. (2015). Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 25, 92-99. 10.1016/j.tcb.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Driskell, R. R., Lichtenberger, B. M., Hoste, E., Kretzschmar, K., Simons, B. D., Charalambous, M., Ferron, S. R., Herault, Y., Pavlovic, G., Ferguson-Smith, A. C.et al. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277-281. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames, B. F. and Schneider, R. A. (2005). Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development 132, 1499-1509. 10.1242/dev.01719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, J., Devarajan, M., Dinuoscio, G., Saiakhova, A., Liu, C.-F., Lefebvre, V., Scacheri, P. C. and Atit, R. P. (2018). PRC2 is dispensable in vivo for β-catenin-mediated repression of chondrogenesis in the mouse embryonic cranial mesenchyme. G3 8, 491-503. 10.1534/g3.117.300311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech, S., Forsthuber, A., Korosec, A., Lipp, K., Kozumov, V. and Lichtenberger, B. M. (2022). Hedgehog signaling in papillary fibroblasts is essential for hair follicle regeneration during wound healing. J. Invest. Dermatol. 142, 1737-1748.e5. 10.1016/j.jid.2021.11.026 [DOI] [PubMed] [Google Scholar]
- Fu, J. (2017). Wnt and the first dermal signal initiating embryonic hair development: a mini-review. Reprod. Dev. Med. 1, 120-122. 10.4103/2096-2924.216857 [DOI] [Google Scholar]
- Fu, J. and Hsu, W. (2013). Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J. Invest. Dermatol. 133, 890-898. 10.1038/jid.2012.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock, R. J. and Krieg, P. A. (2007). Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Dev. Biol. 304, 127-140. 10.1016/j.ydbio.2006.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, J. D., Wells, K. L., Matthäus, F., Painter, K. J., Ho, W., Riddell, J., Johansson, J. A., Ford, M. J., Jahoda, C. A. B., Klika, V.et al. (2017). Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS Biol. 15, e2002117. 10.1371/journal.pbio.2002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough, L. H., Dinuoscio, G. J., Ferguson, J. W., Williams, T., Lang, R. A. and Atit, R. P. (2014). Distinct requirements for cranial ectoderm and mesenchyme-derived wnts in specification and differentiation of osteoblast and dermal progenitors. PLoS Genet. 10, e1004152. 10.1371/journal.pgen.1004152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough, L. H., Dinuoscio, G. J. and Atit, R. P. (2016). Twist1 contributes to cranial bone initiation and dermal condensation by maintaining Wnt signaling responsiveness. Dev. Dyn. 245, 144-156. 10.1002/dvdy.24367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, K., Levinsohn, J., Linderman, G., Chen, D., Sun, T. Y., Dong, D., Taketo, M. M., Bosenberg, M., Kluger, Y., Choate, K.et al. (2019). Single-cell analysis reveals a hair follicle dermal niche molecular differentiation trajectory that begins prior to morphogenesis. Dev. Cell 48, 17-31.e6. 10.1016/j.devcel.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, M. H. (1992). The secret life of the hair follicle. Trends Genet. 8, 55-61. 10.1016/0168-9525(92)90350-D [DOI] [PubMed] [Google Scholar]
- Heisenberg, C.-P., Tada, M., Rauch, G.-J., Saúde, L., Concha, M. L., Geisler, R., Stemple, D. L., Smith, J. C. and Wilson, S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76-81. 10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- Hoffmeyer, K., Junghans, D., Kanzler, B. and Kemler, R. (2017). Trimethylation and acetylation of β-catenin at lysine 49 represent key elements in ESC pluripotency. Cell Rep. 18, 2815-2824. 10.1016/j.celrep.2017.02.076 [DOI] [PubMed] [Google Scholar]
- Houzelstein, D., Chéraud, Y., Auda-Boucher, G., Fontaine-Pérus, J. and Robert, B. (2000). The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development 127, 2155-2164. 10.1242/dev.127.10.2155 [DOI] [PubMed] [Google Scholar]
- Huh, S.-H., Närhi, K., Lindfors, P. H., Häärä, O., Yang, L., Ornitz, D. M. and Mikkola, M. L. (2013). Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Gene Dev. 27, 450-458. 10.1101/gad.198945.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, M., Yang, Z., Andl, T., Cui, C., Kim, N., Millar, S. E. and Cotsarelis, G. (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316-320. 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- Iyer, L. M., Nagarajan, S., Woelfer, M., Schoger, E., Khadjeh, S., Zafiriou, M. P., Kari, V., Herting, J., Pang, S. T., Weber, T.et al. (2018). A context-specific cardiac β-catenin and GATA4 interaction influences TCF7L2 occupancy and remodels chromatin driving disease progression in the adult heart. Nucleic Acids Res. 46, 2850-2867. 10.1093/nar/gky049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda, C. A. (1992). Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development 115, 1103-1109. 10.1242/dev.115.4.1103 [DOI] [PubMed] [Google Scholar]
- Jiang, T. X., Jung, H. S., Widelitz, R. B. and Chuong, C. M. (1999). Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development 126, 4997-5009. 10.1242/dev.126.22.4997 [DOI] [PubMed] [Google Scholar]
- Jiang, X., Iseki, S., Maxson, R. E., Sucov, H. M. and Morriss-Kay, G. M. (2002). Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 241, 106-116. 10.1006/dbio.2001.0487 [DOI] [PubMed] [Google Scholar]
- Jiang, D., Correa-Gallegos, D., Christ, S., Stefanska, A., Liu, J., Ramesh, P., Rajendran, V., Santis, M. M. D., Wagner, D. E. and Rinkevich, Y. (2018). Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat. Cell Biol. 20, 422-431. 10.1038/s41556-018-0073-8 [DOI] [PubMed] [Google Scholar]
- Joshi, J., Rubart, M. and Zhu, W. (2020). Optogenetics: background, methodological advances and potential applications for cardiovascular research and medicine. Front. Bioeng. Biotechnol. 7, 466. 10.3389/fbioe.2019.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H.-Y., Jun, S., Lee, M., Kim, H.-C., Wang, X., Ji, H., Mccrea, P. D. and Park, J.-I. (2013). PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation. Mol. Cell 52, 193-205. 10.1016/j.molcel.2013.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila, A. R., Zhang, B., Caves, E., Kirti, S., Steele, M., Hamburg-Shields, E., Lydon, J., Ying, Y., Lafyatis, R., Rajagopalan, S.et al. (2021). Skin fibrosis and recovery is dependent on Wnt activation via DPP4. J. Invest. Dermatol. 142, 1597-1606.e9. 10.1016/j.jid.2021.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim, C. and Ben-Yair, R. (2005). Cell rearrangements during development of the somite and its derivatives. Curr. Opin. Genet. Dev. 15, 371-380. 10.1016/j.gde.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Kim, D., Hossain, M. Z., Nieves, A., Gu, L., Ratliff, T. S., Oh, S. M., Park, A., Han, S., Yang, N. B., Qi, J.et al. (2016). To control site-specific skin gene expression, autocrine mimics paracrine canonical Wnt signaling and is activated ectopically in skin disease. Am. J. Pathol. 186, 1140-1150. 10.1016/j.ajpath.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos, M. I., Wafai, R., Wong, M. K., Newgreen, D. F., Thompson, E. W. and Waltham, M. (2007). Vimentin and epithelial-mesenchymal transition in human breast cancer – observations in vitro and in vivo. Cells Tissues Organs 185, 191-203. 10.1159/000101320 [DOI] [PubMed] [Google Scholar]
- Kollar, E. J. (1970). The induction of hair follicles by embryonic dermal papillae. J. Invest. Dermatol. 55, 374-378. 10.1111/1523-1747.ep12260492 [DOI] [PubMed] [Google Scholar]
- Lim, C. H., Sun, Q., Ratti, K., Lee, S.-H., Zheng, Y., Takeo, M., Lee, W., Rabbani, P., Plikus, M. V., Cain, J. E.et al. (2018). Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 9, 4903. 10.1038/s41467-018-07142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C. P., Polak, L., Keyes, B. E. and Fuchs, E. (2016). Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 354, aah6102. 10.1126/science.aah6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak, S., Desjardins-Park, H. E., Davitt, M. F., Griffin, M., Borrelli, M. R., Moore, A. L., Chen, K., Duoto, B., Chinta, M., Foster, D. S.et al. (2021). Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki, M., Lichtenberger, B. M., Reimer, A., Collins, C. A., Driskell, R. R. and Watt, F. M. (2016). β-catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of theReticular dermis. J. Invest. Dermatol. 136, 1130-1142. 10.1016/j.jid.2016.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, S. E. (2002). Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, 216-225. 10.1046/j.0022-202x.2001.01670.x [DOI] [PubMed] [Google Scholar]
- Millar, S. E. (2018). Hox in the niche controls hairy-geneity. Cell Stem Cell 23, 457-458. 10.1016/j.stem.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Mok, K.-W., Saxena, N., Heitman, N., Grisanti, L., Srivastava, D., Muraro, M. J., Jacob, T., Sennett, R., Wang, Z., Su, Y.et al. (2019). Dermal condensate niche fate specification occurs prior to formation and is placode progenitor dependent. Dev. Cell 48, 32-48.e5. 10.1016/j.devcel.2018.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosan-Puopolo, G., Balakrishnan-Renuka, A., Yusuf, F., Chen, J., Dai, F., Zoidl, G., Lüdtke, T. H.-W., Kispert, A., Theiss, C., Abdelsabour-Khalaf, M.et al. (2014). Wnt11 is required for oriented migration of dermogenic progenitor cells from the dorsomedial lip of the avian dermomyotome. PLoS One 9, e92679. 10.1371/journal.pone.0092679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann, C., Hausmann, G. and Basler, K. (2009). β-Catenin hits chromatin: regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 10, 276-286. 10.1038/nrm2654 [DOI] [PubMed] [Google Scholar]
- Myung, P. and Ito, M. (2012). Dissecting the bulge in hair regeneration. J. Clin. Invest. 122, 448-454. 10.1172/JCI57414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung, P. S., Takeo, M., Ito, M. and Atit, R. P. (2012). Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 133, 31-41. 10.1038/jid.2012.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehila, T., Ferguson, J. W. and Atit, R. P. (2020). Polycomb repressive complex 2: a dimmer switch of gene regulation in calvarial bone development. Curr. Osteoporos Rep. 18, 378-387. 10.1007/s11914-020-00603-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noramly, S., Freeman, A. and Morgan, B. A. (1999). beta-catenin signaling can initiate feather bud development. Development 126, 3509-3521. 10.1242/dev.126.16.3509 [DOI] [PubMed] [Google Scholar]
- Nusse, R. and Clevers, H. (2017). Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985-999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Ohtola, J., Myers, J., Akhtar-Zaidi, B., Zuzindlak, D., Sandesara, P., Yeh, K., Mackem, S. and Atit, R. (2008). β-Catenin has sequential roles in the survival and specification of ventral dermis. Development 135, 2321-2329. 10.1242/dev.021170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, R. F. (1970). The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J. Embryol. Exp. Morphol. 23, 219-236. [PubMed] [Google Scholar]
- Olivera-Martinez, I., Thelu, J. and Dhouailly, D. (2004a). Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int. J. Dev. Biol. 48, 93-101. 10.1387/ijdb.15272374 [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez, I., Viallet, J. P., Michon, F., Pearton, D. J. and Dhouailly, D. (2004b). The different steps of skin formation in vertebrates. Int. J. Dev. Biol. 48, 107-115. 10.1387/ijdb.15272376 [DOI] [PubMed] [Google Scholar]
- Ordahl, C. P., Berdougo, E., Venters, S. J. and Denetclaw, W. F. (2001). The dermomyotome dorsomedial lip drives growth and morphogenesis of both the primary myotome and dermomyotome epithelium. Development 128, 1731-1744. 10.1242/dev.128.10.1731 [DOI] [PubMed] [Google Scholar]
- Palmquist, K. H., Tiemann, S. F., Ezzeddine, F. L., Yang, S., Pfeifer, C. R., Erzberger, A., Rodrigues, A. R. and Shyer, A. E. (2022). Reciprocal cell-ECM dynamics generate supracellular fluidity underlying spontaneous follicle patterning. Cell 185, 1960-1973.e11. 10.1016/j.cell.2022.04.023 [DOI] [PubMed] [Google Scholar]
- Parchure, A., Vyas, N. and Mayor, S. (2018). Wnt and hedgehog: secretion of lipid-modified morphogens. Trends Cell Biol. 28, 157-170. 10.1016/j.tcb.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr, B. A., Shea, M. J., Vassileva, G. and Mcmahon, A. P. (1993). Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119, 247-261. 10.1242/dev.119.1.247 [DOI] [PubMed] [Google Scholar]
- Paus, R., Müller-Röver, S., Veen, C. V. D., Maurer, M., Eichmüller, S., Ling, G., Hofmann, U., Foitzik, K., Mecklenburg, L. and Handjiski, B. (1999). A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol. 113, 523-532. 10.1046/j.1523-1747.1999.00740.x [DOI] [PubMed] [Google Scholar]
- Phan, Q. M., Fine, G. M., Salz, L., Herrera, G. G., Wildman, B., Driskell, I. M. and Driskell, R. R. (2020). Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife 9, e60066. 10.7554/eLife.60066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus, M. V., Guerrero-Juarez, C. F., Ito, M., Li, Y. R., Dedhia, P. H., Zheng, Y., Shao, M., Gay, D. L., Ramos, R., Hsi, T.-C.et al. (2017). Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748-752. 10.1126/science.aai8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus, M. V., Wang, X., Sinha, S., Forte, E., Thompson, S. M., Herzog, E. L., Driskell, R. R., Rosenthal, N., Biernaskie, J. and Horsley, V. (2021). Fibroblasts: origins, definitions, and functions in health and disease. Cell 184, 3852-3872. 10.1016/j.cell.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, R., Gupta, K., Dong, D., Jiang, Y., Landa, B., Saez, C., Strickland, G., Levinsohn, J., Weng, P., Taketo, M. M.et al. (2022). Decomposing a deterministic path to mesenchymal niche formation by two intersecting morphogen gradients. Dev. Cell 57, 1053-1067.e5. 10.1016/j.devcel.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, S., Andl, T., Bagasra, A., Lu, M. M., Epstein, D. J., Morrisey, E. E. and Millar, S. E. (2001). Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107, 69-82. 10.1016/S0925-4773(01)00452-X [DOI] [PubMed] [Google Scholar]
- Reynolds, A. J., Lawrence, C., Cserhalmi-Friedman, P. B., Christiano, A. M. and Jahoda, C. A. B. (1999). Trans-gender induction of hair follicles. Nature 402, 33-34. 10.1038/46938 [DOI] [PubMed] [Google Scholar]
- Rinkevich, Y., Walmsley, G. G., Hu, M. S., Maan, Z. N., Newman, A. M., Drukker, M., Januszyk, M., Krampitz, G. W., Gurtner, G. C., Lorenz, H. P.et al. (2015). Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Bondre, C., Gladstone, H. B., Brown, P. O. and Chang, H. Y. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, e119. 10.1371/journal.pgen.0020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Kertesz, M., Wang, J. K., Squazzo, S. L., Xu, X., Brugmann, S. A., Goodnough, L. H., Helms, J. A., Farnham, P. J., Segal, E.et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311-1323. 10.1016/j.cell.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Wang, J. K., Allen, N., Brugmann, S. A., Mikels, A. J., Liu, H., Ridky, T. W., Stadler, H. S., Nusse, R., Helms, J. A.et al. (2008). A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 22, 303-307. 10.1101/gad.1610508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni, E., Gomez, C., Pisco, A. O., Rawlins, E. L., Simons, B. D., Watt, F. M. and Driskell, R. R. (2016). Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143, 2522-2535. 10.1242/dev.131797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudloff, S. and Kemler, R. (2012). Differential requirements for beta-catenin during mouse development. Development 139, 3711-3721. 10.1242/dev.085597 [DOI] [PubMed] [Google Scholar]
- Sanaki-Matsumiya, M., Matsuda, M., Gritti, N., Nakaki, F., Sharpe, J., Trivedi, V. and Ebisuya, M. (2022). Periodic formation of epithelial somites from human pluripotent stem cells. Nat. Commun. 13, 2325. 10.1038/s41467-022-29967-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, W., Gotoh, T., Tsunematsu, Y., Aizawa, S. and Shimono, A. (2006). Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development 133, 989-999. 10.1242/dev.02274 [DOI] [PubMed] [Google Scholar]
- Saxena, N., Mok, K. and Rendl, M. (2019). An updated classification of hair follicle morphogenesis. Exp. Dermatol. 28, 332-344. 10.1111/exd.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaal, M. and Christ, B. (2004). Formation and differentiation of the avian dermomyotome. Anat. Embryol. 208, 411-424. 10.1007/s00429-004-0417-y [DOI] [PubMed] [Google Scholar]
- Schubert, F. R., Mootoosamy, R. C., Walters, E. H., Graham, A., Tumiotto, L., Munsterberg, A. E., Lumsden, A. and Dietrich, S. (2002). Wnt6 marks sites of epithelial transformations in the chick embryo. Mech. Dev. 114, 143-148. 10.1016/S0925-4773(02)00039-4 [DOI] [PubMed] [Google Scholar]
- Sengel, P. (1976). Morphogenesis of Skin. Cambridge: Cambridge University Press. [Google Scholar]
- Sengel, P. and Abbot, U. K. (1963). In vitro studies with scaleless mutant: interactions during feather and scale differentiation. J. Hered. 54, 255-262. 10.1093/oxfordjournals.jhered.a107261 [DOI] [PubMed] [Google Scholar]
- Sengel, P. and Dhouailly, D. (1977). Tissue Interactions in Amniote skin Development in Cell Interactions in Differentiation (ed. Karkinen-Jaaskelainen M. and Saxin L.). Academic Press. [Google Scholar]
- Shook, B. A., Wasko, R. R., Rivera-Gonzalez, G. C., Salazar-Gatzimas, E., López-Giráldez, F., Dash, B. C., Muñoz-Rojas, A. R., Aultman, K. D., Zwick, R. K., Lei, V.et al. (2018). Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362, eaar2971. 10.1126/science.aar2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y., Boncompagni, A. C., Kim, S.-S., Gochnauer, H. R., Zhang, Y., Loots, G. G., Wu, D., Li, Y., Xu, M. and Millar, S. E. (2018). Regional control of hairless versus hair-bearing skin by Dkk2. Cell Rep. 25, 2981-2991.e3. 10.1016/j.celrep.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, P. (1997). The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124, 2691-2700. 10.1242/dev.124.14.2691 [DOI] [PubMed] [Google Scholar]
- Sriram, G., Bigliardi, P. L. and Bigliardi-Qi, M. (2015). Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 94, 483-512. 10.1016/j.ejcb.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Stewart, R. A., Ramakrishnan, A.-B. and Cadigan, K. M. (2019). Diffusion and function of Wnt ligands. PLoS Genet. 15, e1008154. 10.1371/journal.pgen.1008154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigety, K. M., Liu, F., Yuan, C. Y., Moran, D. J., Horrell, J., Gochnauer, H. R., Cohen, R. N., Katz, J. P., Kaestner, K. H., Seykora, J. T.et al. (2020). HDAC3 ensures stepwise epidermal stratification via NCoR/SMRT-reliant mechanisms independent of its histone deacetylase activity. Gene Dev. 34, 973-988. 10.1101/gad.333674.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda, N., Ohuchi, H., Yoshioka, H., Noji, S. and Nohno, T. (1995). A chicken WNT gene, WNT-11, is involved in dermal development. Biochem. Biophys. Res. Commun. 211, 123-129. 10.1006/bbrc.1995.1786 [DOI] [PubMed] [Google Scholar]
- Tarazi, S., Aguilera-Castrejon, A., Joubran, C., Ghanem, N., Ashouokhi, S., Roncato, F., Wildschutz, E., Haddad, M., Oldak, B., Gomez-Cesar, E.et al. (2022). Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 185, 3290-3306.e25. 10.1016/j.cell.2022.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulabandu, V., Chen, D. and Atit, R. P. (2018). Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev. Dev. Biol. 7, e307. 10.1002/wdev.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulabandu, V., Nehila, T., Ferguson, J. W. and Atit, R. P. (2021). Dermal EZH2 orchestrates dermal differentiation and epidermal proliferation during murine skin development. Dev. Biol. 478, 25-40. 10.1016/j.ydbio.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T. H., Jarrell, A., Zentner, G. E., Welsh, A., Brownell, I., Scacheri, P. C. and Atit, R. (2010). Role of canonical Wnt signaling/beta-catenin via Dermo1 in cranial dermal cell development. Development 137, 3973-3984. 10.1242/dev.056473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, S.-Y., Sennett, R., Rezza, A., Clavel, C., Grisanti, L., Zemla, R., Najam, S. and Rendl, M. (2014). Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 385, 179-188. 10.1016/j.ydbio.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, J., Rostom, M. and Garza, L. A. (2022). Understanding and Harnessing Epithelial–Mesenchymal Interactions in the Development of Palmoplantar Identity. J. Invest. Dermatol. 142, 282-284. 10.1016/j.jid.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Oh, J. W., Lee, H.-L., Dhar, A., Peng, T., Ramos, R., Guerrero-Juarez, C. F., Wang, X., Zhao, R., Cao, X.et al. (2017). A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. Elife 6, e22772. 10.7554/eLife.22772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie, A. T., Zhao, Y. and Boyden, E. S. (2019). Expansion microscopy: principles and uses in biological research. Nat. Methods 16, 33-41. 10.1038/s41592-018-0219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells, N. K. and Roessner, K. D. (1965). Nonproliferation in dermal condensations of mouse vibrissae and pelage hairs. Dev. Biol. 12, 419-433. 10.1016/0012-1606(65)90007-2 [DOI] [PubMed] [Google Scholar]
- Xin, T., Greco, V. and Myung, P. (2016). Hardwiring stem cell communication through tissue structure. Cell 164, 1212-1225. 10.1016/j.cell.2016.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, Y., Itami, S., Tarutani, M., Hosokawa, K., Miura, H. and Yoshikawa, K. (1999). Regulation of keratin 9 in nonpalmoplantar keratinocytes by palmoplantar fibroblasts through epithelial–mesenchymal interactions. J. Invest. Dermatol. 112, 483-488. 10.1046/j.1523-1747.1999.00544.x [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., Passeron, T., Hoashi, T., Watabe, H., Rouzaud, F., Yasumoto, K., Hara, T., Tohyama, C., Katayama, I., Miki, T.et al. (2008). Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/ β–catenin signaling in keratinocytes. FASEB J. 22, 1009-1020. 10.1096/fj.07-9475com [DOI] [PubMed] [Google Scholar]
- Yamashita, Y. M., Inaba, M. and Buszczak, M. (2018). Specialized intercellular communications via cytonemes and nanotubes. Annu. Rev. Cell Dev. Biol. 34, 59-84. 10.1146/annurev-cellbio-100617-062932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z., Jiang, K., Xu, Z., Huang, H., Qian, N., Lu, Z., Chen, D., Di, R., Yuan, T., Du, Z.et al. (2018). Hoxc-dependent mesenchymal niche heterogeneity drives regional hair follicle regeneration. Cell Stem Cell 23, 487-500.e6. 10.1016/j.stem.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Andl, T., Yang, S. H., Teta, M., Liu, F., Seykora, J. T., Tobias, J. W., Piccolo, S., Schmidt-Ullrich, R., Nagy, A.et al. (2008). Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135, 2161-2172. 10.1242/dev.017459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Tomann, P., Andl, T., Gallant, N. M., Huelsken, J., Jerchow, B., Birchmeier, W., Paus, R., Piccolo, S., Mikkola, M. L.et al. (2009). Reciprocal requirements for EDA/EDAR/NF-κB and Wnt/β-catenin signaling pathways in hair follicle induction. Dev. Cell 17, 49-61. 10.1016/j.devcel.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]