Abstract
Background
Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis [UC]. We evaluated the relationship between Mayo/Inflammatory Bowel Disease Questionnaire [IBDQ] scores and Work Productivity and Activity Impairment-UC [WPAI-UC] components in patients with UC.
Methods
All available pooled data from three Phase 3 tofacitinib studies [OCTAVE Induction 1 and 2 and OCTAVE Sustain] were included. Relationships were estimated using repeated measures regression models with Mayo score/subscores or IBDQ total/domain scores as a separate anchor predictor and WPAI-UC components as the outcome.
Results
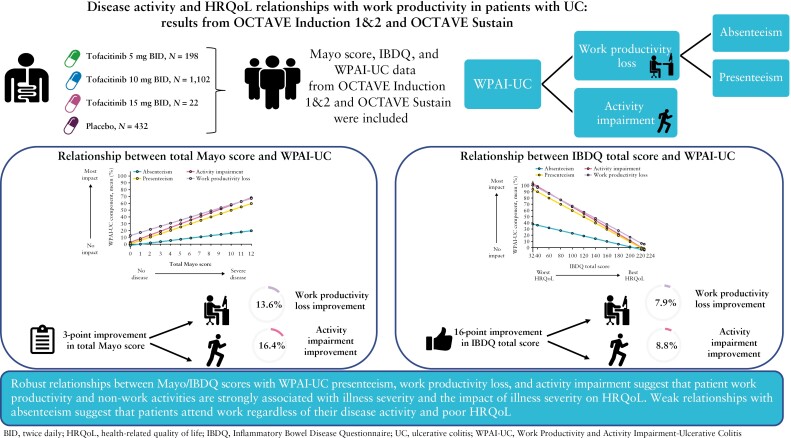
Evidence for linear relationships was confirmed between Mayo/IBDQ scores and WPAI-UC components. Robust relationships between total Mayo score/IBDQ total score and WPAI-UC presenteeism, work productivity loss, and activity impairment were observed; relationships with absenteeism were weak. Total Mayo scores of 0 and 12 corresponded, on average, to WPAI-UC component scores of < 15% and ≥ 60%, respectively, and IBDQ total scores of 224 and 32 corresponded, on average, to WPAI-UC component scores of < 6% and ≥ 90%, respectively. Presenteeism, work productivity loss, and activity impairment [all 0–100%], respectively, improved on average by 14.7, 13.6, and 16.4 percentage points for every 3-point improvement in total Mayo score, and by 8.1, 7.9, and 8.8 percentage points for every 16-point improvement in IBDQ total score.
Conclusion
Robust relationships between Mayo/IBDQ scores with WPAI-UC presenteeism, work productivity loss, and activity impairment suggest that patient productivity and non-work activities are strongly associated with disease activity and HRQoL. The weak relationships with absenteeism suggest that patients attend work regardless of their disease activity/poor HRQoL.
ClinicalTrials.gov: NCT01465763;NCT01458951;NCT01458574.
Keywords: Janus kinase inhibitors, patient-reported outcomes, quality of life, tofacitinib, ulcerative colitis, work productivity
Graphical Abstract
Graphical Abstract.
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory bowel disease [IBD] that is typified by recurrent, relapsing inflammation of the gastrointestinal tract.1 Symptoms associated with UC include urgency, increased stool frequency, diarrhoea, rectal bleeding, nausea, abdominal pain, anxiety, and fatigue.1–3 The severity of these symptoms has been associated with the impairment of health-related quality of life [HRQoL]4 and work-related outcomes such as increased rates of absenteeism, work disability, and decreased productivity.4–8 The impact of UC on work productivity and daily activities is particularly relevant because of the young age of disease onset in most cases, the severity of symptoms, and the unpredictability of disease flares.1,9–11
Clinical disease activity is usually assessed in patients with UC using a disease activity index, such as the Mayo score, and the impact of UC on their HRQoL is most commonly assessed using one of the formal instruments such as the Inflammatory Bowel Disease Questionnaire [IBDQ].12,13 However, these tools do not completely capture the impact of UC symptoms on the patient’s ability to optimally function in the work environment and in everyday life. The Work Productivity and Activity Impairment [WPAI] questionnaire is a patient-reported measure of the impact of health problems on absenteeism, presenteeism, work productivity loss [ie, overall work performance decline], and activity impairment [ie, non-work activities].14,15 The WPAI has been shown to be reliable, valid, and able to detect change and response to treatment when used across several disease areas, including UC.15
Tofacitinib is an oral small molecule Janus kinase [JAK] inhibitor for the treatment of UC. The efficacy and safety of tofacitinib for the treatment of moderately to severely active UC have been evaluated in a series of prospective clinical trials, in which all patients underwent contemporaneous assessments of clinical disease activity, HRQoL, and work productivity,16,17 generating data that enabled a detailed exploration of the relationships between these constructs. Although the WPAI specific for UC [WPAI-UC] has been evaluated in a number of clinical trials,18,19 the definitive relationships linking disease activity measures such as the Mayo score or HRQoL measures such as the IBDQ with the WPAI-UC have not been previously evaluated. Therefore, this is the first study to evaluate these relationships in patients with UC. It is of value to establish these relationships using data from a clinical trial setting in view of the limited real-world data on work impairment in patients treated with tofacitinib,20 to allow clinicians to potentially estimate a patient’s degree of work and activity impairment given their measured clinical disease activity and/or their reported HRQoL. Here, we performed a post hoc analysis to evaluate the relationships between the Mayo score [total and individual subscores] or IBDQ score [total and individual domains] and WPAI-UC components, using data from OCTAVE Induction 1 and 2 and OCTAVE Sustain.
2. Methods
2.1. Patients and study design
The full details of OCTAVE Induction 1 and 2 [NCT01465763; NCT01458951] and OCTAVE Sustain [NCT01458574] have previously been reported.16 Briefly, OCTAVE Induction 1 and 2 were two identical, randomised, placebo-controlled, 8-week, Phase 3 studies of tofacitinib for the treatment of patients with moderately to severely active UC.16 Eligible patients [≥ 18 years of age and had previously failed or were intolerant to treatment with corticosteroids, immunosuppressants, and/or tumour necrosis factor inhibitors] were assigned to receive induction therapy with tofacitinib 10 or 15 mg twice daily [BID] or placebo. Following a protocol amendment, the tofacitinib 15 mg BID dose in OCTAVE Induction 1 and 2 was discontinued. Induction responders were eligible to enter OCTAVE Sustain, a randomised, placebo-controlled, 52-week, Phase 3 maintenance study in which patients were re-randomised to receive tofacitinib 5 or 10 mg BID or placebo.
All studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided informed consent.
2.2. Endpoints
The Mayo score measures disease activity by assessing stool frequency, rectal bleeding, endoscopic disease activity, and the Physician’s Global Assessment. The total Mayo score ranges from 0 to 12 points [each subscore ranges from 0 to 3], with higher scores indicating more severe disease. An improvement of ≥ 3 points in total Mayo score was considered to indicate a clinical response to therapy. The modified Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and endoscopic subscores, with higher scores indicating more severe disease. The partial Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and Physician Global Assessment subscores, with higher scores indicating more severe disease. Mayo endoscopic disease activity was assessed prior to baseline and at Week 8 of OCTAVE Induction 1 and 2, and at Weeks 24 and 52 of OCTAVE Sustain. This allowed for the assessment of the total and modified Mayo scores. Stool frequency, rectal bleeding, and Physician Global Assessment data were collected at all study visits. This allowed for the assessment of the partial Mayo score.
Disease-specific quality of life [QoL] was measured by the IBDQ. The IBDQ is a psychometrically validated instrument for measuring the disease-specific QoL in patients with IBD. The IBDQ comprises 32 items grouped into four domains: bowel symptoms [total domain score range 10–70], systemic symptoms [total domain score range 5–35], emotional function [total domain score range 12–84], and social function [total domain score range 5–35].21 For the total score [range from 32–224] and each domain, a higher score indicates better QoL. A score of ≥ 170 corresponds to clinical remission, and an improvement of ≥ 16 points in the total IBDQ score is considered a clinically meaningful change in HRQoL.22 The IBDQ was administered at baseline, at Weeks 4 and 8 of OCTAVE Induction 1 and 2, and at Weeks 8, 16, 24, 32, 40, and 52 of OCTAVE Sustain.
Patient-perceived impact of UC on work and non-work activity was measured by the WPAI-UC, a validated instrument designed to measure the ability to work and perform regular activities.14 The WPAI-UC is a six-item questionnaire that measures impairment due to a patient’s UC in the past 7 days and generates four metrics: absenteeism [the percentage of work time missed due to the patient’s UC], presenteeism [the percentage of work time that was impaired while at work due to the patient’s UC], work productivity loss [the combination of absenteeism and presenteeism], and activity impairment [the percentage of non-work activities that were impaired due to the patient’s UC]. All WPAI-UC component scores range from 0% to 100%, with a higher percentage indicating greater impairment or less productivity/activity. The WPAI-UC was administered at baseline and Week 8 of OCTAVE Induction 1 and 2, and at Weeks 8, 16, 24, 32, 40, and 52 of OCTAVE Sustain.
2.3. Statistical analyses
Relationships between the Mayo score [total and individual subscores] or IBDQ score [total and individual domains] and WPAI-UC components were evaluated using all available data at baseline and Week 8 of OCTAVE Induction 1 and 2, and at Weeks 24 and 52 of OCTAVE Sustain. For this post hoc analysis, available data from all treatment groups [including placebo] were pooled. A repeated measures, longitudinal, regression model23,24 was used to assess the relationship between the Mayo score or IBDQ score and WPAI-UC components. In the main analysis, the Mayo score or IBDQ score was used as a continuous anchor predictor, meaning that a linear relationship was imposed between the anchor and the WPAI-UC component [as an outcome]. A sensitivity analysis was performed to evaluate the linearity assumption, using the anchor as a categorical variable. Using the anchor as a categorical variable did not impose any functional relationship between outcome and anchor.
To further evaluate, and better contrast, the relationship between individual IBDQ domains and individual WPAI-UC components using an aligned scale, the original IBDQ domain scores [calculated as a sum of items] were divided by the number of items within that domain, providing a value between 1 and 7. As an example, a maximum score of 84 from the 12 items determining emotional function, and a maximum score of 35 from the five items comprising social function, would both have a value of 7 in our analysis. Note that this mapping is a simple linear transformation of the anchor to maintain comparability between IBDQ domains and does not change the estimated outcomes for WPAI components.
3. Results
3.1. Patients
This analysis used data from 614 patients from OCTAVE Induction 1 [placebo, N = 122; tofacitinib 10 mg BID, N = 476; tofacitinib 15 mg BID, N = 16], 547 patients from OCTAVE Induction 2 [placebo, N = 112; tofacitinib 10 mg BID, N = 429; tofacitinib 15 mg BID, N = 6], and 593 patients from OCTAVE Sustain who were clinical responders in the induction studies and were re-randomised [placebo, N = 198; tofacitinib 5 mg BID, N = 198; tofacitinib 10 mg BID, N = 197] in the maintenance study. Baseline characteristics, efficacy, and safety in OCTAVE Induction 1 and 2, and OCTAVE Sustain have been previously published.16 In each analysis, the number of patients with available data varied [Supplementary Table 1].
3.2. Relationships between the Mayo score and WPAI-UC
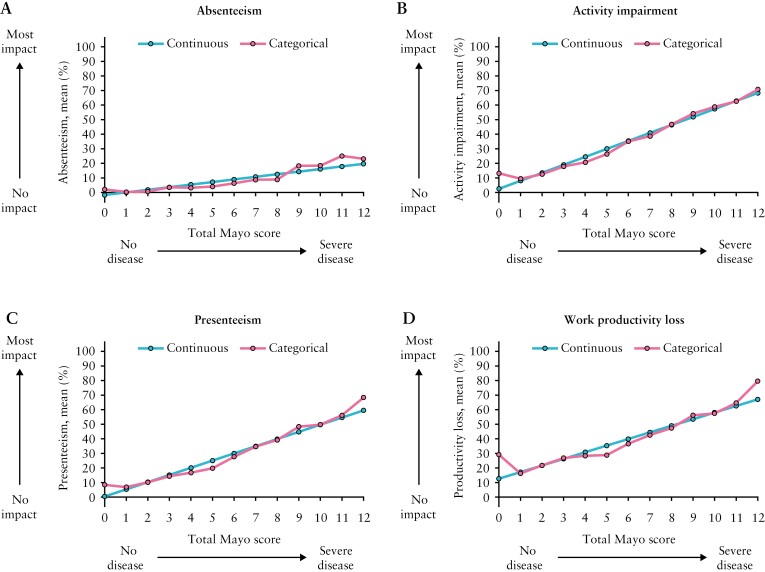
Relationships between the Mayo score and WPAI-UC components appeared similar when using the Mayo score as a continuous anchor compared with a categorical anchor, supporting the linearity assumption for the relationship between the total Mayo score and WPAI-UC components [Figure 1]. This was consistent across Mayo subscores [Supplementary Figure 1].
Figure 1.
Estimated relationship between the total Mayo score as a continuous or categorical anchor and the four WPAI-UC components; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis. Data from OCTAVE Induction 1 and 2 and OCTAVE Sustain; all treatment groups from each Phase 3 study were pooled for analysis. WPAI-UC component scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. The total Mayo score ranges from 0 to 12 points, with higher scores indicating more severe disease activity.
The estimated relationships between the total Mayo score as a continuous anchor and WPAI-UC components are shown in Figure 2A. The relationships between the total Mayo score and presenteeism, work productivity loss, and activity impairment were robust, with total Mayo scores of 0 and 12 corresponding, on average, to WPAI-UC component scores of < 15% and ≥ 60%, respectively. The relationship between total Mayo score and absenteeism was weak due to > 70% of absenteeism values represented by scores of 0.
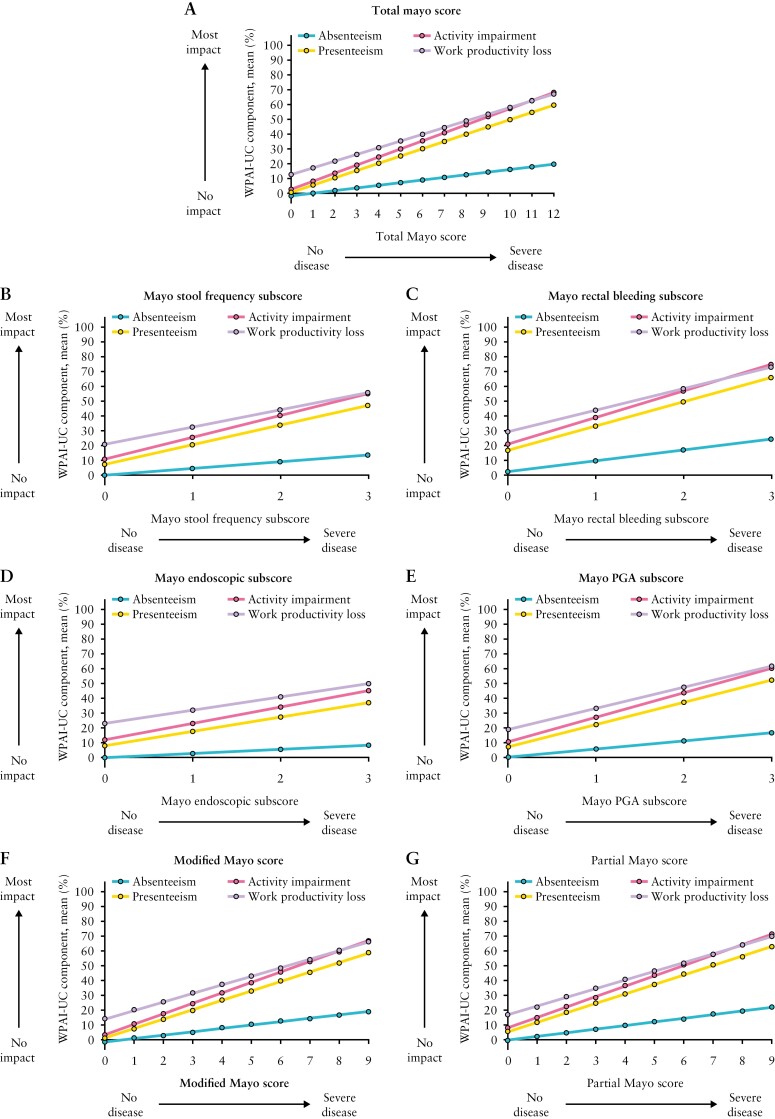
Figure 2.
Estimated relationship between total Mayo score, the four individual Mayo subscores, the modified Mayo score, and the partial Mayo score as a continuous anchor and the four WPAI-UC components; PGA, Physician Global Assessment; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis. Data from OCTAVE Induction 1 and 2 and OCTAVE Sustain; all treatment groups from each Phase 3 study were pooled for analysis. WPAI-UC component scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. The total Mayo score ranges from 0 to 12 points and each subscore ranges from 0 to 3 points; higher scores indicate more severe disease activity for both total and individual subscores. The modified Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and endoscopic subscores, with higher scores indicating more severe disease. The partial Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and PGA subscores, with higher scores indicating more severe disease.
For every 3-point improvement [meaningful change used to define clinical response] in disease activity measured by total Mayo score, presenteeism, work productivity loss, and activity impairment [95% confidence intervals (CI)] improved on average by 14.7 [13.9–15.6], 13.6 [12.5–14.7], and 16.4 [15.7–17.0] percentage points, respectively [Table 1].
Table 1.
Mean percentage point [95% CI] change in WPAI-UC components associated with 1-point or 3-point changes in the total Mayo score, Mayo subscores, modified Mayo score, and partial Mayo score [main model with Mayo score as a continuous anchor].
| WPAI-UC component [percentage points] | ||||
|---|---|---|---|---|
| Absenteeism | Presenteeism | Work productivity loss | Activity impairment | |
| Total Mayo score | ||||
| 3 points | 5.3 [4.6–6.1] |
14.7 [13.9–15.6] |
13.6 [12.5–14.7] |
16.4 [15.7–17.0] |
| Mayo stool frequency subscore | ||||
| 1 point | 4.5 [3.8–5.2] |
13.3 [12.4–14.1] |
11.7 [10.5–12.8] |
14.8 [14.1–15.5] |
| Mayo rectal bleeding subscore | ||||
| 1 point | 7.3 [6.3–8.4] |
16.4 [15.2–17.5] |
14.5 [13.2–15.9] |
17.9 [17.1–18.8] |
| Mayo endoscopic subscore | ||||
| 1 point | 2.8 [2.1–3.5] |
9.7 [8.7–10.7] |
9.0 [7.5–10.5] |
11.1 [10.2–12.0] |
| Mayo PGA subscore | ||||
| 1 point | 5.5 [4.7–6.3] |
15.0 [14.1–16.0] |
14.3 [13.0–15.6] |
16.5 [15.8–17.3] |
| Modified Mayo score | ||||
| 3 points | 6.9 [5.9–7.9] |
19.2 [18.0–20.3] |
17.5 [15.9–19.0] |
21.3 [20.4–22.2] |
| Partial Mayo score | ||||
| 3 points | 7.4 [6.4–8.3] |
19.2 [18.2–20.2] |
17.6 [16.2–19.0] |
21.1 [20.3–21.9] |
Data from OCTAVE Induction 1 and 2 and OCTAVE Sustain; all treatment groups from each Phase 3 study were pooled for analysis. WPAI-UC component scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. The total Mayo score ranges from 0 to 12 points and each subscore ranges from 0 to 3 points; higher scores indicate more severe disease activity for both total and individual subscores. The modified Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and endoscopic subscores, with higher scores indicating more severe disease. The partial Mayo score ranges from 0 to 9 points and is the sum of stool frequency, rectal bleeding, and PGA subscores, with higher scores indicating more severe disease.
CI, confidence interval; PGA, Physician Global Assessment; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis.
Similarly, the relationship between individual Mayo subscores and presenteeism, work productivity loss, and activity impairment were robust and the relationship with absenteeism was weak [Figure 2B–E]. The relationship between the modified Mayo and partial Mayo scores and presenteeism, work productivity loss, and activity impairment were also robust [Figure 2F–G].
The Mayo rectal bleeding subscore had the strongest association with WPAI-UC components, and the Mayo endoscopic subscore had the weakest association. For example, for every 1-point improvement in Mayo rectal bleeding subscore, presenteeism, work productivity loss, and activity impairment [95% CI] improved on average by 16.4 [15.2–17.5], 14.5 [13.2–15.9], and 17.9 [17.1–18.8] percentage points, respectively; corresponding values for a 1-point improvement in Mayo endoscopic subscore were 9.7 [8.7–10.7], 9.0 [7.5–10.5], and 11.1 [10.2–12.0] percentage points, respectively. For every 3-point improvement in modified Mayo score, presenteeism, work productivity loss, and activity impairment improved on average by 19.2 [18.0–20.3], 17.5 [15.9–19.0], and 21.3 [20.4–22.2] percentage points, respectively. For every 3-point improvement in partial Mayo score, presenteeism, work productivity loss, and activity impairment improved on average by 19.2 [18.2–20.2], 17.6 [16.2–19.0], and 21.1 [20.3–21.9] percentage points, respectively [Table 1].
3.3. Relationships between the IBDQ score and WPAI-UC
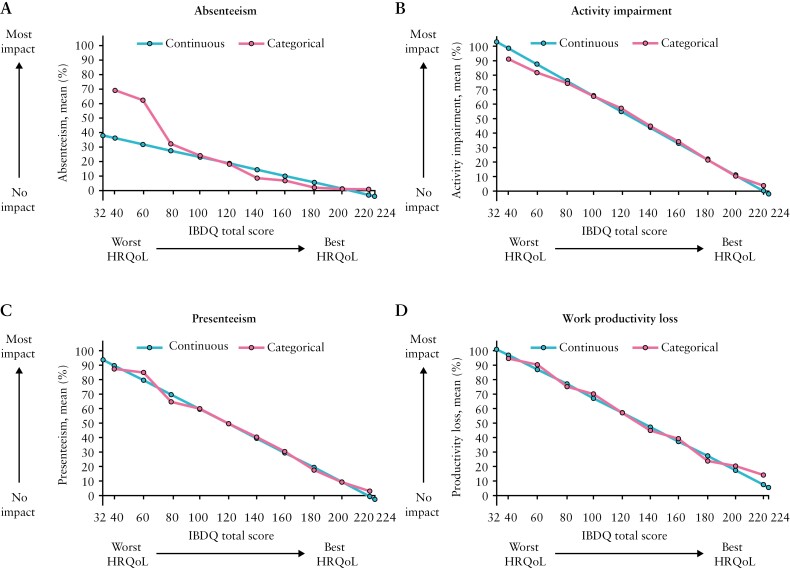
Relationships between the IBDQ total score and WPAI-UC were similar when using the IBDQ total score as a continuous anchor compared with a categorical anchor [Figure 3], supporting the linearity assumption, with the exception of absenteeism. This was generally consistent across IBDQ domains [Supplementary Figure 2].
Figure 3.
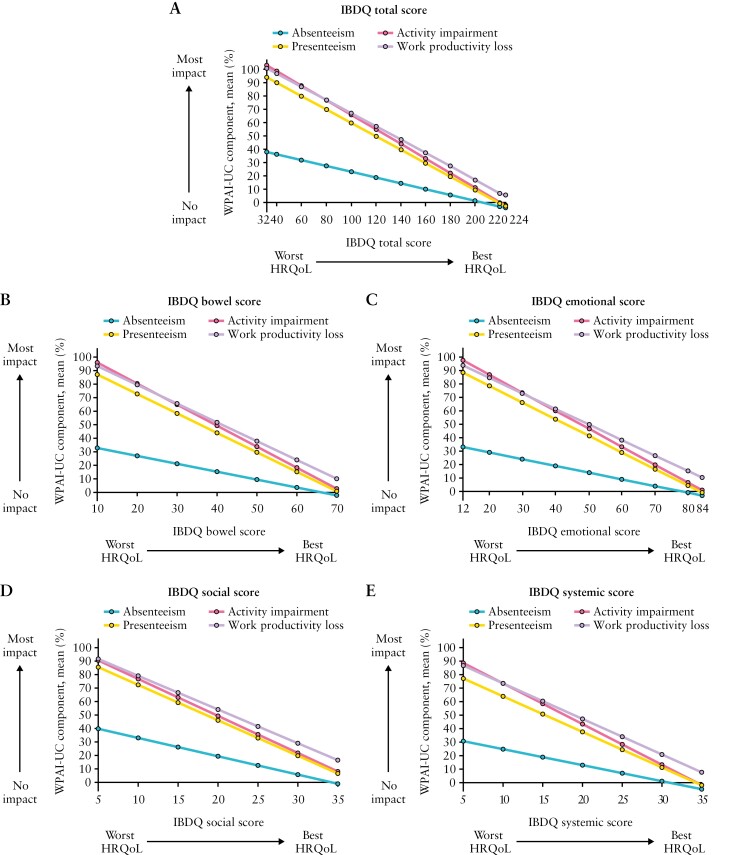
Estimated relationship between the IBDQ total score as a continuous or categorical anchor and the four WPAI-UC components; HRQoL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis. Data from OCTAVE Induction 1 and 2 and OCTAVE Sustain; all treatment groups from each Phase 3 study were pooled for analysis. WPAI-UC component scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. The IBDQ total score ranges from 32 to 224 and includes four domains: bowel [total domain score range 10–70], emotional [total domain score range 12–84], social [total domain score range 5–35], and systemic [total domain score range 5–35]. For the total score [range 32–224] and each domain, a higher score indicates a better HRQoL.
Figure 4 shows the relationship between the IBDQ total score and WPAI-UC components. The relationships between the IBDQ total score and presenteeism, work productivity loss, and activity impairment were robust, with IBDQ total scores of 224 and 32 corresponding on average to WPAI-UC component scores of < 6% and ≥ 90%, respectively. The relationship between IBDQ total score and absenteeism was weak due to > 70% of observations reporting no days of work lost. For every 16-point improvement in IBDQ total score [clinically meaningful change used to define response], presenteeism, work productivity loss, and activity impairment [95% CI] improved on average by 8.1 [7.8–8.4], 7.9 [7.5–8.4], and 8.8 [8.5–9.0] percentage points, respectively. An IBDQ total score of ≥ 170 [corresponds to clinical remission] was associated with an average work productivity loss no greater than 35%. Similar trends were observed between individual IBDQ domains and WPAI-UC components [Figure 4]. Poor HRQoL as indicated by the lowest IBDQ score in each domain was associated with an average work productivity loss of > 85%.
Figure 4.
Estimated relationship between the IBDQ total score or the four individual IBDQ domain scores as a continuous anchor and the four WPAI-UC components; HRQoL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis. Data from OCTAVE Induction 1 and 2 and OCTAVE Sustain; all treatment groups from each Phase 3 study were pooled for analysis. WPAI-UC scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. The IBDQ total score ranges from 32 to 224 and includes four domains: bowel [total domain score range 10–70], emotional [total domain score range 12–84], social [total domain score range 5–35], and systemic [total domain score range 5–35]. For the total score [range 32–224] and each domain, a higher score indicates a better HRQoL.
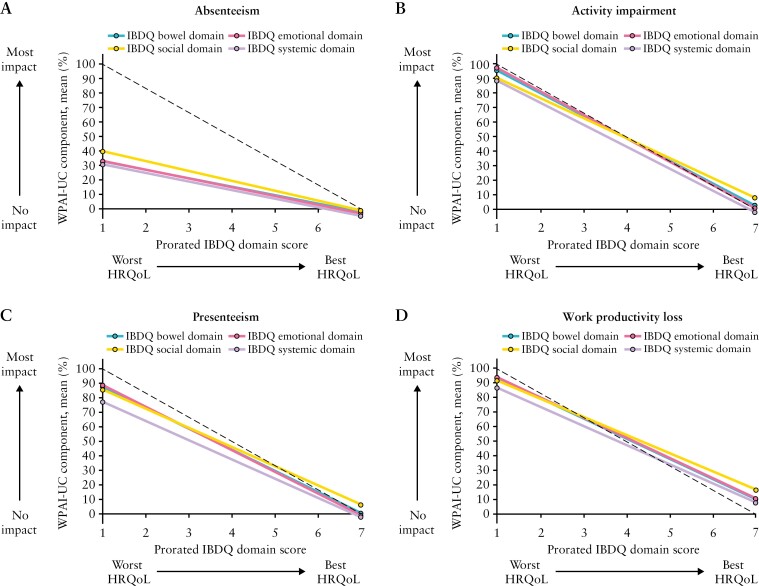
The relationships between all four IBDQ domains and any one of the WPAI-UC components were evaluated by prorating the IBDQ domain scores on a scale of 1 to 7. All four IBDQ domains had a similar relationship with any one of the WPAI-UC components [Figure 5]. The IBDQ bowel, emotional, and social domains had the strongest associations with work productivity loss, and the systemic domain had the weakest association with work productivity loss.
Figure 5.
Relationship between the four IBDQ domains and each individual WPAI-UC component [main model with IBDQ domain score as a continuous anchor]; HRQoL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; WPAI-UC, Work Productivity and Activity Impairment-Ulcerative Colitis. WPAI-UC scores are expressed as percentages, with a higher percentage indicating greater impairment and less productivity. IBDQ domain scores were prorated and converted to represent the mean of the items within a range of 1 to 7, and a higher score indicates a better HRQoL. The dotted line represents a perfect relationship [maximum y corresponds to minimum x, and minimum y to maximum x].
4. Discussion
This post hoc analysis evaluated the relationships between the Mayo score, the IBDQ score, and work impairment and reduction of daily non-work activities as analysed by the WPAI-UC. In this study, which used data from patients who participated in the tofacitinib UC clinical trials, OCTAVE Induction 1 and 2, and OCTAVE Sustain, we showed robust relationships between both the Mayo score [total and individual subscores] and the IBDQ score [total and individual domains] with the WPAI-UC components of presenteeism, work productivity loss, and activity impairment. The robust relationships were also observed when using the modified Mayo and partial Mayo scores. This analysis is distinctive in that it examines the joint relationship of WPAI-UC with the Mayo score, and separately with the IBDQ, which is pooled across treatment groups.
The results presented here demonstrated markedly close relationships between the Mayo score or the IBDQ score and the WPAI-UC presenteeism, work productivity loss, and activity impairment, irrespective of whether the Mayo score or IBDQ score was used as a categorical or continuous anchor. For both the Mayo score and the IBDQ score, approximately linear relationships with WPAI-UC components were observed. These robust relationships suggest that patient productivity and non-work activities are strongly associated with illness severity and impact of illness severity on HRQoL. The robust relationship between the modified Mayo score and partial Mayo score with the WPAI-UC is important for clinicians, as these scores are often used in clinical practice instead of the total Mayo score, to avoid the requirement for both an endoscopy and Physician Global Assessment.25 It has been suggested that the Physician Global Assessment subscore can be a limitation of the total Mayo score due to its subjective nature. As such, the US Food and Drug Administration guidance now recommends the use of the modified Mayo score to assess disease activity in certain circumstances, including primary endpoints in clinical trials in patients with UC. In exploratory analyses such as those presented here, the Physician Global Assessment subscore can be considered.26
The relationships between the Mayo score or IBDQ score and absenteeism were weak. This is likely related to the skewed distribution observed for absenteeism, with the majority [≥ 70%] of patient responses indicating that they had missed zero days of work in the past 7 days due to their UC. One possible explanation for this finding is that the majority of patients with UC continue to work at all levels of disease activity and are reluctant to miss work, regardless of symptom severity or a poor HRQoL, although absenteeism appears to be more common in patients with higher Mayo scores and/or low self-reported HRQoL. However, these data have suggested that the individual absenteeism WPAI-UC component alone is a less informative outcome than the WPAI-UC components taken together, and therefore all components should be evaluated as a whole to understand the full implications for patients with UC. A systematic literature review of the WPAI in patients with UC noted that measurement properties were weaker for absenteeism relative to the other three components, and this was attributed to the highly skewed distribution of absenteeism scores.15 For example, in one study of patients with mildly to moderately active UC, 73% of patients had responses of zero for absenteeism,19 which is consistent with what was observed in our study [72–73%].
The WPAI outcome of work productivity loss encapsulates absenteeism and presenteeism, and therefore can be viewed as the most all-encompassing WPAI outcome. In this study, a robust relationship between the IBDQ total score and work productivity loss was observed and could be construed as a near-perfect relationship between anchor and outcome. In contrast, a study in Austria of patients with IBD reported only a moderate correlation between HRQoL as assessed by the short IBDQ and work productivity loss.27 However, it should be noted that the patient population in the Austrian study included patients with UC and Crohn’s disease, and the IBDQ used in the study was composed of only 10 questions, rather than the full, 32-item questionnaire. Interpretation of the relationships between the total Mayo score or IBDQ total score and work productivity loss shows that patients’ work lives are affected by disease activity and HRQoL, but patients in clinical remission [defined in the OCTAVE programme as a total Mayo score of ≤ 2, with no individual subscore > 1 and a rectal bleeding subscore of 0] or those with an IBDQ total score of ≥ 170 still report more than 20% work productivity loss. Similar findings have previously been reported from a global UC Narrative survey, completed by physicians and patients, which examined numerous aspects of UC such as diagnosis, treatment, and impact on patient quality of life. In this survey, 81% of patients who viewed themselves as being in remission [defined as UC being controlled with few to no symptoms] agreed that UC affected their work and, of those who were employed, a mean of 7.3 working days were missed in the past 12 months.9 It should be considered that the strong relationship between the WPAI and IBDQ is likely because patients are known to respond to questions around workplace productivity in a similar way to how they respond to questions about overall well-being.28
Previously, a significant, but not strong, correlation between IBDQ total scores and clinical efficacy in both OCTAVE Induction 1 and 2 and in OCTAVE Sustain has been reported.29 In our analysis, out of the Mayo subscores, rectal bleeding had the closest relationship with WPAI-UC components; and in general, all four of the IBDQ domains had a close relationship with WPAI-UC components [except absenteeism]. The data reported here suggest that Mayo subscores such as rectal bleeding or stool frequency only partially explain work productivity loss, whereas the IBDQ bowel domain generally captures a more comprehensive clinical picture by collecting all bowel symptoms beyond rectal bleeding and stool frequency [ie, loose stools, abdominal bloating and pain, excessive flatulence, urge to defaecate, and nausea] related to work productivity loss. From a clinical perspective, bowel symptoms such as urgency and abdominal pain may be expected to affect work productivity loss and activity impairment more than mental health or general health perception. While our data showing the relationship between IBDQ and WPAI-UC suggest that there are only minor differences between the bowel and emotional domains, the results from the global UC Narrative survey demonstrated that of the patients satisfied with their UC medication, the top three reasons for satisfaction were less frequent flares, less abdominal pain, and less urgency to go to the bathroom.9 In contrast, a study in patients with irritable bowel syndrome [IBS] showed that there was a stronger association between IBS symptoms [abdominal pain, frequency of pain, severity of pain, severity of distention, bowel habit dissatisfaction, and daily life interference] and the presenteeism and activity impairment WPAI-UC components, than the association between gastrointestinal anxiety and the presenteeism and activity impairment WPAI-UC components.30
The findings of this study are particularly important to employers. If patients are attending work despite their poor disease activity and HRQoL, as the weak relationship with absenteeism would suggest, this serves as a reminder to employers that accommodations most likely may need to be made for their employees with UC. A recent study reporting on workplace challenges for patients with IBD suggested required accommodations included remote working opportunities and sufficient paid sick leave, as well as flexible hours.31 Our findings are also relevant to clinicians, as they provide a basis on which to estimate a patient’s degree of work and activity impairment based on their measured clinical disease activity [and their reported HRQoL, although clinicians may not routinely measure IBDQ in clinical practice]. If clinicians can recommend flexible or remote working to their patients’ employers, this may result in improved work productivity in some patients, which will be of relevance to employers. The benefits of remote working for people with chronic conditions have been demonstrated in a recent study in a cohort of working adults with musculoskeletal conditions, which showed that improvements in health and well-being were achieved through working at home.32 It is of note that patient advocacy groups are likely to play an important role in facilitating the change to working practices for patients with UC in individual countries. These groups have the ability to exert their advocacy work on research, government, and employment organisations.33 The impact of such patient groups has been demonstrated by the rewrite of the UK IBD standards, which was directed by a multidisciplinary working group of IBD stakeholders which included patients.34
One strength of our study is that the data included those collected at baseline and during OCTAVE Induction 1 and 2 and OCTAVE Sustain, and those from patients treated with tofacitinib or placebo; this meant that Mayo, IBDQ, and WPAI-UC scores spanned the respective ranges and increased the validity of the analyses and the credibility of the results. However, it should be noted that there were relatively few observations at the extreme ends of all ranges. Overall, the real value of this work is being able to quantify the expected impact of a clinical response on workplace productivity to clinicians and employers, as well as insurers and other health care payers.
This study has some limitations. These analyses were post hoc and were based on data from OCTAVE Induction 1 and 2 and OCTAVE Sustain clinical trials, which may not be fully generalisable to patients with UC in clinical practice. Moreover, these data were obtained from multiple countries globally where working conditions may vary due to regional differences in work regulations and customs. For example, it has been found that IBD-related absenteeism was 24.6% in the UK35 but only 8.1% in Japan,10 despite similar rates of presenteeism [34.1% and 28.1%, respectively].10,35 Furthermore, the WPAI-UC questionnaire responses are a patient’s reflection of how they feel their UC has impacted them, and there are limitations on what can be interpreted from a single question response. In addition, data were not well distributed for some variables. In some analyses, using the anchor as a continuous variable resulted in predicted [estimated] values below 0% and above 100% for WPAI-UC components at the extreme values of the anchor. This was due to the small number of available observations at the extremes of the range and to imposing a linear relationship. When the anchor was used as a categorical variable, as expected, there were no such occurrences.
In conclusion, to our knowledge, this is the first study to directly measure and quantify relationships between Mayo score, IBDQ score, and WPAI-UC components. These findings provide insight into the relationships of a frequently used disease activity measure [the Mayo score] and an HRQoL instrument [IBDQ] with work productivity and activity impairment. Characterising and quantifying this relationship will help inform health care providers on the impact of UC on a patient’s work and non-work activities.
Supplementary Material
Acknowledgements
The authors would like to thank the patients, investigators, and study teams involved in the OCTAVE studies. Medical writing support, under the direction of the authors, was provided by Helen Findlow, PhD, and Caitlin Duncan, PhD, CMC Connect, IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice [GPP 2022] guidelines [Ann Intern Med 2022;175:1298–304].
Contributor Information
Laura Targownik, Division of Gastroenterology and Hepatology, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada.
Marla C Dubinsky, Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Flavio Steinwurz, Unit of Inflammatory Bowel Disease, Hospital Israelita Albert Einstein, São Paulo, Brazil.
Andrew G Bushmakin, Pfizer Inc, Groton, CT, USA.
Joseph C Cappelleri, Pfizer Inc, Groton, CT, USA.
Elaine Tai, Pfizer Canada Inc, Kirkland, QC, Canada.
Sean Gardiner, Pfizer Inc, New York, NY, USA.
Peter Hur, Pfizer Inc, New York, NY, USA.
Julian Panés, Formerly: Department of Gastroenterology, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Funding
These studies were sponsored by Pfizer.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data; see [https://www.pfizer.com/science/clinical-trials/trial-data-and-results] for more information.
Conflict of Interest
LT has received research funding from AbbVie Canada, Amgen Canada, Gilead Canada, Pfizer Canada, Roche Canada, Sandoz Canada, and Takeda Canada; and has served as an advisory board member for AbbVie Canada, Amgen Canada, Celltrion Canada, JAMP Canada, Janssen Canada, Merck Canada, Pfizer Canada, Roche Canada, Sandoz Canada, and Takeda Canada. MCD reports consulting fees from AbbVie, Arena, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Pfizer Inc, Prometheus Labs, Takeda, and UCB; and is a shareholder of Trellus Health. FS has served as an advisory board member for Pfizer Inc; and has received consulting and speaker fees from AbbVie, Amgen, Celltrion, Eurofarma, Ferring Pharmaceuticals, Janssen, Sandoz, Takeda, and UCB. ET, AGB, JCC, SG, and PH are employees and shareholders of Pfizer Inc. JP reports personal fees from AbbVie, Arena, Athos, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Galapagos, Genentech/Roche, GSK, Immunic, Janssen, Mirum, Morphic, Nestlé, Origo, Pandion, Pfizer Inc, Progenity, Revolo, Takeda, Theravance Biopharma, and Wassermann; and has received grant support from AbbVie and Pfizer Inc.
Author Contributions
ET, AB, and JCC designed the post hoc analysis. AB and JCC performed the statistical analysis. All authors contributed to the interpretation of the data and the development of the manuscript, and critically reviewed/revised the manuscript for important intellectual content. All authors approved the final version of the manuscript before submission. Some of the data from this manuscript have been presented at the United European Gastroenterology Week [UEGW] congress, Vienna, Austria, October 8–11, 2022, and the American Congress of Gastroenterology [ACG] Annual Meeting, Charlotte, USA, October 21–26, 2022. An infographic plain language summary of this paper is available at: 10.25454/pfizer.figshare.21299151
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F.. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nag A, Romero B.. Development and content validation of patient-reported outcomes tools for ulcerative colitis and Crohn’s disease in adults with moderate-to-severe disease. Health Qual Life Outcomes 2022;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonefjall B, Simren M, Lasson A, Ohman L, Strid H.. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United Eur Gastroenterol J 2018;6:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gibson PR, Vaizey C, Black CM, et al. Relationship between disease severity and quality of life and assessment of health care utilisation and cost for ulcerative colitis in Australia: A cross-sectional, observational study. J Crohns Colitis 2014;8:598–606. [DOI] [PubMed] [Google Scholar]
- 5. Boonen A, Dagnelie PC, Feleus A, et al. The impact of inflammatory bowel disease on labor force participation: Results of a population sampled case-control study. Inflamm Bowel Dis 2002;8:382–9. [DOI] [PubMed] [Google Scholar]
- 6. Neovius M, Arkema EV, Blomqvist P, Ekbom A, Smedby KE.. Patients with ulcerative colitis miss more days of work than the general population, even following colectomy. Gastroenterology 2013;144:536–43. [DOI] [PubMed] [Google Scholar]
- 7. Siebert U, Wurm J, Gothe RM, et al. ; Swiss IBD Cohort Study Group. Predictors of temporary and permanent work disability in patients with inflammatory bowel disease: Results of the Swiss Inflammatory Bowel Disease Cohort Study. Inflamm Bowel Dis 2013;19:847–55. [DOI] [PubMed] [Google Scholar]
- 8. van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group. Risk factors of work disability in patients with inflammatory bowel disease–a Dutch nationwide web-based survey: Work disability in inflammatory bowel disease. J Crohns Colitis 2014;8:590–7. [DOI] [PubMed] [Google Scholar]
- 9. Dubinsky MC, Watanabe K, Molander P, et al. Ulcerative Colitis Narrative global survey findings: The impact of living with ulcerative colitis – a patients’ and physicians’ view. Inflamm Bowel Dis 2021;27:1747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamabe K, Liebert R, Flores N, Pashos CL.. Health-related quality of life outcomes and economic burden of inflammatory bowel disease in Japan. ClinicoEconomics Outcomes Res 2019;11:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh S, Mitchell R.. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations [EFCCA] patient survey. J Crohns Colitis 2007;1:10–20. [DOI] [PubMed] [Google Scholar]
- 12. Alrubaiy L, Rikaby I, Dodds P, Hutchings HA, Williams JG.. Systematic review of health-related quality of life measures for inflammatory bowel disease. J Crohns Colitis 2015;9:284–92. [DOI] [PubMed] [Google Scholar]
- 13. Pabla BS, Schwartz DA.. Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin North Am 2020;49:671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reilly MC, Zbrozek AS, Dukes EM.. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEcon 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- 15. Yarlas A, Maher SM, Bayliss MS, Lovley A, Cappelleri JC, DiBonaventura MD.. Psychometric validation of the work productivity and activity impairment questionnaire in ulcerative colitis: Results from a systematic literature review. J Patient Rep Outcomes 2018;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: Final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022;55:464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of adalimumab on clinical outcomes and health-related quality of life among patients with ulcerative colitis in a clinical practice setting: Results from InspirADA. J Crohns Colitis 2017;11:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yarlas A, Yen L, Hodgkins P.. The relationship among multiple patient-reported outcomes measures for patients with ulcerative colitis receiving treatment with MMX® formulated delayed-release mesalamine. Qual Life Res 2015;24:671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas PWA, den Broeder N, Derikx M, et al.. Impact of biological therapies and tofacitinib on real-world work impairment in inflammatory bowel disease patients: a prospective study. Inflamm Bowel Dis, Feb 24; doi: 10.1093/ibd/izac002. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804–10. [PubMed] [Google Scholar]
- 22. Irvine EJ. Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 1999;28:S23–7. [DOI] [PubMed] [Google Scholar]
- 23. Cappelleri JC, Zou KH, Bushmakin AG, et al.. Patient-reported Outcomes: Measurement, Implementation and Interpretation. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 24. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 25. Naegeli AN, Hunter T, Hoskin B, et al. Full, partial, and modified permutations of the Mayo score: characterizing clinical and patient-reported outcomes in ulcerative colitis patients. Crohns Colitis 360 2021;3:otab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research [CDER]. Ulcerative Colitis: Clinical Trial Endpoints . Guidance fIndustry. 2016. http://www.fda.gov/downloads/Drugs/Guidances/UCM515143.pdf Accessed July 8, 2021. [Google Scholar]
- 27. Walter E, Hausberger SC, Groß E, Siebert U.. Health-related quality of life, work productivity and costs related to patients with inflammatory bowel disease in Austria. J Med Econ 2020;23:1061–71. [DOI] [PubMed] [Google Scholar]
- 28. Calvet X, Argüelles-Arias F, López-Sanromán A, et al. Patients’ perceptions of the impact of ulcerative colitis on social and professional life: Results from the UC-LIFE survey of outpatient clinics in Spain. Patient Prefer Adherence 2018;12:1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panés J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: Health-related quality of life in phase 3, randomised, controlled induction and maintenance studies. J Crohns Colitis 2018;12:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asa F, Hans T, Sofie J, Magnus S.. Work productivity and activity impairment in irritable bowel syndrome [IBS]: a multifaceted problem. Am J Gastroenterol 2018;113:1540–9. [DOI] [PubMed] [Google Scholar]
- 31. Cheng L, Jetha A, Cordeaux E, Lee K, Gignas MAM.. Workplace challenges, supports, and accommodations for people with inflammatory bowel disease: a scoping review. Disabil Rehabil 2021,. Sep 24; doi: 10.1080/09638288.2021.1979662. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. Morton LK, Stelfox K, Beasley M, et al. Enabling work participation for people with musculoskeletal conditions: lessons from work changes imposed by COVID-19: A mixed-method study. BMJ Open 2022;12:e057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nijsten T, Bergstresser PR.. Patient advocacy groups: let’s stick together. J Invest Dermatol 2010;130:1757–9. [DOI] [PubMed] [Google Scholar]
- 34. Kapasi R, Glatter J, Lamb CA, et al. Consensus standards of healthcare for adults and children with inflammatory bowel disease in the UK. Frontline Gastroenterol 2020;11:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaizey CJ, Gibson PR, Black CM, et al. Disease status, patient quality of life and healthcare resource use for ulcerative colitis in the UK: an observational study. Frontline Gastroenterol 2014;5:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data; see [https://www.pfizer.com/science/clinical-trials/trial-data-and-results] for more information.