Abstract
Purpose
To determine whether reticular pseudodrusen (RPD) status and/or ARMS2/HTRA1 genotype are associated with altered geographic atrophy (GA) enlargement rate, and to analyze potential mediation of genetic effects by RPD status.
Design
Post hoc analysis of a cohort within the Age-Related Eye Disease Study 2 (AREDS2) controlled clinical trial.
Participants
771 eyes (563 participants, mean age 74.8 years) with GA.
Methods
GA area was measured by planimetry from color fundus photographs at annual visits. RPD presence was graded from fundus autofluorescence images. Mixed-model regression of square root GA area was performed according to RPD status and/or ARMS2 genotype, including mediation analysis.
Main outcome measures: change in square root GA area over time.
Results
GA enlargement was significantly faster in eyes with RPD (P<0.0001), at 0.379 (95% CI 0.329–0.430) versus 0.273 mm/year (0.256–0.289). The rate was also significantly faster in individuals carrying ARMS2 risk alleles (P<0.0001), at 0.224 (95% CI 0.198–0.250), 0.287 (0.263–0.310), and 0.307 mm/year (0.273–0.341), in those with 0–2 risk alleles, respectively. In mediation analysis, the direct effect of ARMS2 genotype on GA enlargement was 0.074 mm/year (95% CI 0.009–0.139, P=0.025), whereas the indirect effect of ARMS2 genotype via RPD status was 0.002 mm/year (95% CI −0.006–0.009, P=0.64). In eyes with incident GA, RPD presence was not associated with altered likelihood of central involvement (P=0.29) or multifocality (P=0.16) at incidence. In eyes with incident non-central GA, RPD presence was associated with faster GA progression to the central macula (P=0.009), at 157 (95% CI 126–188) versus 111 μm/year (97–125). Similar findings were observed in the AREDS, as a validation dataset.
Conclusions
GA enlargement is faster in eyes with RPD and in individuals carrying ARMS2 risk alleles. However, RPD status does not mediate the association between ARMS2 genotype and faster enlargement. RPD presence and ARMS2 genotype are relatively independent risk factors and must lead to faster enlargement by distinct mechanisms. RPD presence does not predict central involvement or multifocality at GA incidence, but is associated with faster progression towards the central macula. These findings have implications for clinical trials and clinical practice; RPD status should be considered for improved predictions of enlargement rate.
Précis
Reticular pseudodrusen presence is associated with faster geographic atrophy enlargement. The association between ARMS2/HTRA1 risk alleles and faster geographic atrophy enlargement is not mediated by reticular pseudodrusen; they are independent risk factors.
Introduction
Geographic atrophy (GA) is the defining lesion of the atrophic subtype of late age-related macular degeneration (AMD). It is estimated to affect over 5 million people worldwide and is typically bilateral and relentlessly progressive.1–3 GA enlargement rate is a primary outcome measure in many clinical trials and is recognized as a clinically important endpoint by the US Food and Drug Administration (FDA).4 Some genetic and clinical risk factors for faster or slower GA enlargement have been identified, but our understanding is imperfect and the mechanisms underlying enlargement remain poorly understood.2,3,5
Reticular pseudodrusen (RPD), also known as subretinal drusenoid deposits (SDD), are increasingly recognized as an important phenotypic feature of AMD.6–8 RPD are often poorly visible on clinical examination or color fundus photography, but can be visualized by other imaging modalities including fundus autofluorescence (FAF) and optical coherence tomography (OCT).8
In the Age-Related Eye Disease Study 2 (AREDS2), GA enlargement was significantly faster in eyes of individuals with risk alleles at ARMS2/HTRA1.2 Some previous studies have reported that GA enlargement is more rapid in eyes with RPD, though this question remains controversial3 and has not previously been analyzed in the large AREDS2 cohort. In addition, in the AREDS2, RPD presence was significantly more common in eyes of individuals with risk alleles at ARMS2/HTRA1.9 Therefore, it is possible that some or all of the faster GA enlargement observed with RPD presence is attributable to the underlying presence of ARMS2/HTRA1 risk alleles.10 Considered another way, the faster GA enlargement observed with ARMS2/HTRA1 risk alleles may in fact be mediated in part by RPD presence. Conversely, it is possible that ARMS2/HTRA1 risk alleles are causally linked to faster GA enlargement in other ways.
Understanding these relationships would be helpful for several reasons. Making accurate predictions of GA enlargement rate is critical for increased power and precision of clinical trials; in the near future, it may also be important in clinical practice.11 Addressing these questions should make it clear whether genetic testing would be required for optimal accuracy, or whether imaging features (including RPD status) should be sufficient. In addition, the biological mechanisms underlying GA enlargement are poorly understood. Insights into these questions may improve our ability to develop new therapies that target the underlying disease process, potentially personalized by disease genotype (e.g., ARMS2/HTRA1) and/or phenotype (e.g., RPD status).
Mediation analysis is ideal for examining these relationships. Mediation analysis is a statistical method that quantifies the extent to which a variable (e.g., RPD status) transmits the effect of an independent variable (e.g., ARMS2/HTRA1 genotype) to a dependent variable (e.g., GA enlargement rate).12 Hence, the total effect of an exposure on an outcome is separated into an “indirect effect” that works through a potential mediator (here, RPD status), and a “direct effect”, which is the effect of the exposure on the outcome that is not explained by the mediator under study (Figure 1).13
Figure 1.
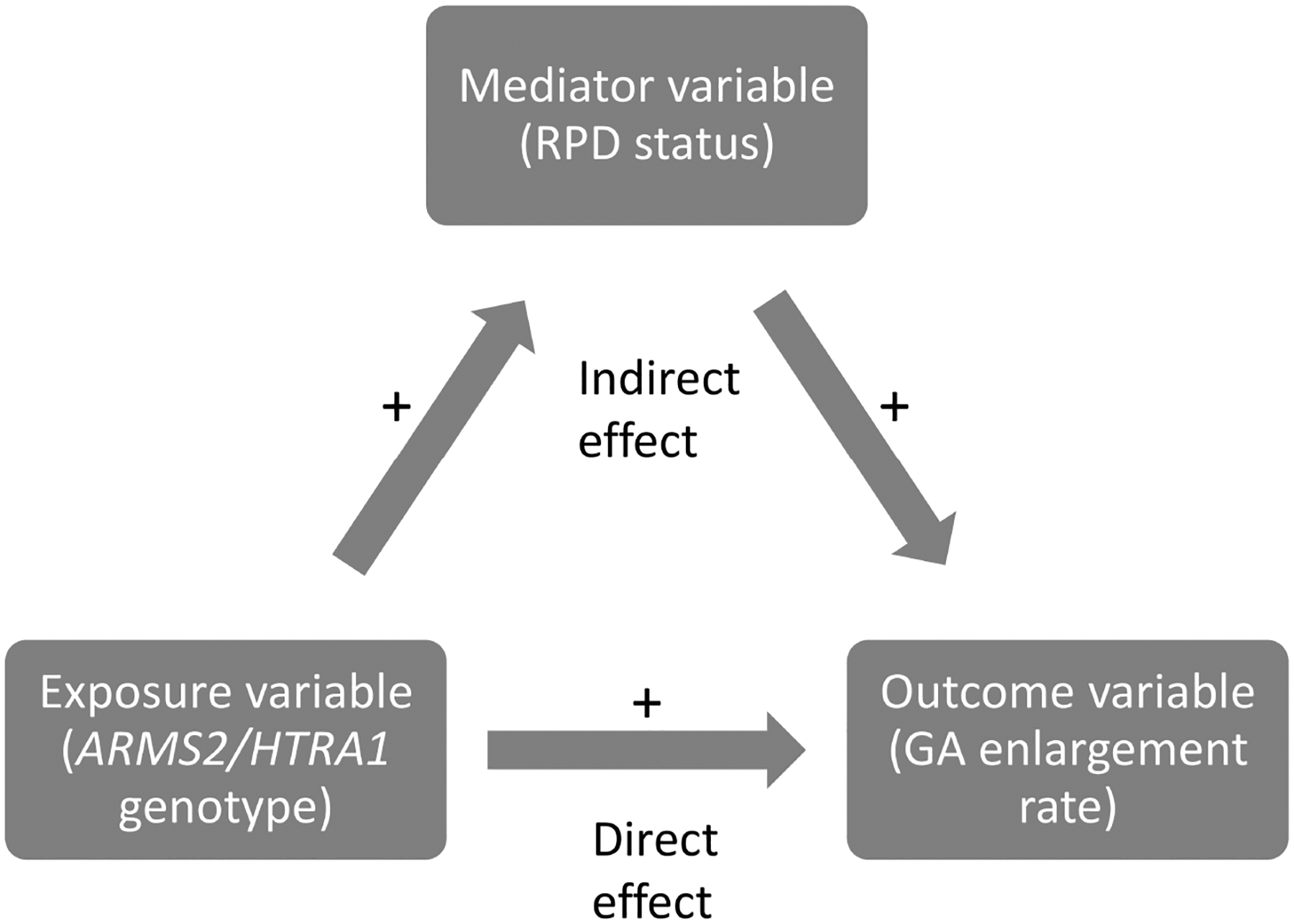
Diagram demonstrating the principles of mediation analysis.
Abbreviations: RPD=reticular pseudodrusen; GA=geographic atrophy
The primary purpose of the current study was to use the AREDS2 dataset to analyze rates of GA enlargement according to (i) RPD status, (ii) ARMS2/HTRA1 genotype, and (iii) both combined, and to perform mediation analyses to assess what proportion of the faster GA enlargement associated with ARMS2/HTRA1 risk alleles may be mediated by RPD presence. In addition, controversy exists over whether, in the presence of RPD, GA might be less likely to have central involvement at onset and/or during progression.14,15 Therefore, the secondary purpose of the study was to examine both these questions in the AREDS2. Finally, the study aimed to perform similar analyses in the AREDS, as a validation dataset. By contrast, speed of GA enlargement has not been observed to vary significantly according to CFH genotype, either in focused analyses of the AREDS2 or in a genome-wide association study of a pooled dataset from four studies.2,5 Given the absence of an association between CFH genotype and GA enlargement rate, meaningful analysis of possible relationships mediated by RPD status versus other factors would not be possible.
Methods
Study procedures
The AREDS2 study design has been described previously.16 In brief, 4203 participants (aged 50 to 85 years) were recruited between 2006 and 2008 at 82 retinal specialty clinics in the United States. The inclusion criteria at enrollment were the presence of either large drusen in both eyes or late AMD in one eye and large drusen in the fellow eye. Institutional review board approval was obtained at each site and written informed consent was obtained from all participants. The research was conducted under the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. The AREDS2 participants were randomly assigned to receive the supplements that lowered risk of AMD progression in the AREDS, either alone, or with additional lutein/zeaxanthin, docosahexaenoic acid (DHA) plus eicosapentaenoic acid (EPA), or the combination. At baseline and annual visits, eye examinations were performed, and digital stereoscopic color fundus photographs were captured and graded centrally at the Wisconsin Reading Center. The randomized clinical trial lasted five years. Progression to late AMD (including GA/neovascular subtype) was defined by fundus photograph grades, together with history of intravitreal injections for neovascular AMD.16
Evaluation of geographic atrophy on color fundus photographs
The definition of GA and the methods to measure GA area and other characteristics on color fundus photographs have been described previously.2 In brief, GA was defined as a lesion equal to or larger than drusen circle I-2 (diameter 433 μm, area 0.146 mm2, i.e., 1/4 disc diameter and 1/16 disc area) at its widest diameter with at least two of the following features present: circular shape, sharp (well-demarcated) edges, and loss of the retinal pigment epithelium (RPE) (partial or complete depigmentation of the RPE, typically with exposure of underlying choroidal vessels). The configuration of GA was documented, using the definitions published by Sunness et al17, as either (1) small (single patch less than 1 disc area), (2) multifocal, (3) horseshoe or ring, (4) solid or unifocal, or (5) indeterminate. GA area (within the AREDS grid) and GA proximity to the central macula were measured.2,18 Grading for GA area measurements and other features was performed independently at the image level, i.e., the reading center graders analyzed each image independently from other images in the full time-series of images for each eye and did not have access to any accompanying clinical information.
Evaluation of reticular pseudodrusen on fundus autofluorescence images
The AREDS2 ancillary study of FAF imaging was conducted at 66 selected clinic sites, according to the availability of imaging equipment, as described previously.9 Sites were permitted to join the ancillary study at any time after FAF imaging equipment became available during the five-year study period. The FAF images were acquired from the Heidelberg Retinal Angiograph (Heidelberg Engineering, Heidelberg, Germany) and fundus cameras with autofluorescence capability by certified technicians using a standardized protocol.
The FAF images were assessed for RPD presence by graders at the Wisconsin Reading Center. The grading protocol and definitions have been described previously.9 In brief, RPD were defined as clusters of discrete round or oval lesions of hypoautofluorescence, usually similar in size, or confluent ribbon-like patterns with intervening areas of normal or increased autofluorescence; a minimum of 0.5 disc areas (approximately five lesions) was required. The reading center graders were permitted to consider the whole time series of FAF images for each eye, particularly in the case of an individual image with poor quality for assessing RPD status.9 If the first FAF image available could not be graded for RPD status, but RPD were absent in subsequent FAF images, RPD status was considered absent in the first FAF image also. If RPD status could not be graded in the first FAF image, but RPD were present or could not be graded in subsequent FAF images, no such inference was made; RPD status remained missing for the first FAF image and that time point was excluded from the analyses.
Genetic data
As part of the AREDS2, 1826 participants consented to genotype analysis. SNPs were analyzed using a custom Illumina HumanCoreExome array, as described previously.19 The SNP of interest for this study, rs10490924 at ARMS2, was that identified from previous AREDS2 analyses to be associated with increased GA enlargement rate2 and with higher likelihood of RPD presence9.
Study populations
The study population for analyses of GA enlargement according to RPD status comprised all eyes that had both (i) GA present at two or more study visits (without previous or simultaneous neovascular AMD) and (ii) RPD grading available (at least as early as the first time-point with GA present). The study population for analyses of GA enlargement according to ARMS2 genotype comprised all eyes that had both (i) GA present at two or more study visits (without previous or simultaneous neovascular AMD) and (ii) genetic data available. The study population for analyses of GA enlargement according to both RPD and ARMS2 genotype comprised the intersection of the previous two study populations (i.e., both RPD grading and genetic data available). In all cases, the study populations included eyes that had GA at baseline (i.e., prevalent GA) and those that developed GA during follow-up (i.e., incident GA).
Statistical methods: analyses of geographic atrophy enlargement by reticular pseudodrusen presence or ARMS2 genotype, considered separately
Analyses of GA enlargement were performed using methods similar to those described previously.2,20 The unit of analysis was the eye (except where stated otherwise). Mixed-model repeated-measures regression was performed with square root of GA area as the outcome measure. The square root transformation was used as this reduces the dependence of enlargement rate on baseline lesion size.2,21 The models included the variable of interest (i.e., RPD status or ARMS2 genotype), years from baseline/GA first appearance (in order to account for the repeated measures, i.e., to obtain the enlargement rate), and their interaction term. They were adjusted for age, sex, smoking status, education, and GA characteristics at baseline/first appearance (specifically central involvement, configuration, and square root of area). RPD status was considered at study baseline for eyes with prevalent GA and at first GA appearance for eyes with incident GA. To account for the correlation between both eyes of the same person and between different visits of the same eye, an unstructured and a first-order autoregressive covariance structure (UN@AR(1)), respectively, was specified.22 Regression analyses were also performed for the prevalent and the incident GA cohorts, considered separately. In sensitivity analyses, the models were also adjusted for GA involvement in the fellow eye (defined as involved if GA was present in the fellow eye at the time of baseline/first GA appearance). In other sensitivity analyses, RPD status was considered in three levels instead of two: never present, absent at baseline/first GA appearance but present later, and present at baseline/first GA appearance. Exploring whether the middle group behaved more like the first or last group (or in an intermediate way), might provide further insights into the relationship between RPD and GA enlargement. In other sensitivity analyses for the genetic analyses, the unit of analysis was the participant (as another way of avoiding potential bias from correlation between two eyes of an individual); in these cases, for participants with both eyes eligible, one eye was selected randomly.
Statistical methods: analyses of geographic atrophy enlargement by reticular pseudodrusen presence and ARMS2 genotype, considered simultaneously
Similar methods were used for analyses of GA enlargement according to both RPD status and ARMS2 genotype, considered simultaneously. In the primary analyses, the model included RPD status, ARMS2 genotype, years from baseline/GA first appearance, the interaction term between RPD status and years, and the interaction term between ARMS2 genotype and years. In secondary analyses, the model included a 4-level RPD/ARMS2 variable (specifically RPD−/GG, RPD−/GT+TT, RPD+/GG, and RPD+/GT+T), years from baseline/GA first appearance, and their interaction term. The models were adjusted for the same variables as described above.
Statistical methods: mediation analysis
As described above, mediation analysis quantifies the extent to which a variable (e.g., RPD status) transmits the effect of an independent variable (e.g., ARMS2 genotype) to a dependent variable (e.g., GA enlargement rate; Figure 1).12 It allows the decomposition of a total effect into direct and indirect effects, even in models with interactions and non-linear relationships.23 Hence, it assesses whether most of an effect is mediated through a particular mediator versus other pathways.
For the mediation analysis, the study population was the same as that in the previous analyses of GA enlargement according to both RPD and ARMS2 genotype. The outcome was defined as the GA enlargement rate, considered as a single value of the slope for each eye (i.e., the mean rate of GA enlargement, following square root transformation and adjusted for the same variables as those described above, over the full follow-up period available for that eye). The exposure was defined as ARMS2 genotype, considered in two levels, i.e., no risk alleles (GG) or 1–2 risk alleles (GT or TT). The potential mediator was defined as RPD status at baseline/first GA appearance. Using these methods, it is not possible to account for the correlation between eyes of the same person. Therefore, in sensitivity analyses, the mediation analysis was repeated with the unit of analysis as the participant; in these cases, for participants with both eyes eligible, one eye was selected randomly.
The analysis was performed according to methods described previously using the SAS macro “mediation”.23 In brief, first, the outcome variable (GA enlargement rate) was regressed on the exposure variable (ARMS2 genotype), using linear regression. Second, the potential mediator (RPD status) was regressed on the exposure variable (ARMS2 genotype), using logistic regression. The parameter estimates and covariance matrices from both models were used to calculate the mediation effects. The direct effect is defined as the relationship between the exposure (ARMS2 genotype) and the outcome (GA enlargement rate) that does not involve the mediator. The indirect effect is defined as the relationship between the exposure and the outcome that occurs through the mediator. The total effect is the sum of the direct and indirect effects.
Statistical methods: analyses of geographic atrophy progression to central involvement
The study population for analyses of GA progression to central involvement comprised all eyes that had both (i) incident non-central GA (without previous or simultaneous neovascular AMD), with at least one subsequent study visit with GA, and (ii) RPD grading available (at least as early as the first time-point with GA present). The unit of analysis was the eye. Kaplan-Meier analyses were performed for the outcome of progression to central involvement. Multivariable repeated-measures proportional hazards regression analyses were performed for the same outcome, according to RPD status (defined at the time of GA incidence). The models were adjusted for the same variables as those described above, i.e., age, sex, smoking status, education level, and GA characteristics at incidence (specifically configuration, square root of area, and proximity to central macula). Adjustment for correlation between both eyes of the same person was made by using the robust sandwich estimate for the covariance matrix in the Wald tests.24 In sensitivity analyses, RPD status was considered in three levels instead of two (as described above). In addition, in the same study population, mixed-model repeated-measures regression analyses were performed for the outcome of GA proximity to the central macula, considered during follow-up, with adjustment for the same variables as those for progression to central involvement.
Analyses of the Age-Related Eye Disease Study
Similar analyses to those described above were performed on the AREDS, as a validation dataset. FAF images and other multimodal imaging were not obtained in the AREDS, so RPD status was inferred by deep learning model-based automated grading of color fundus photographs.25,26 These methods are described in detail in the Supplementary Material.
All analyses were performed with commercially available statistical software (SAS version 9.4; SAS Institute, Cary, NC).
Results
The AREDS2 comprised 8406 eyes of 4203 participants with annual color fundus photographs over five years. The number with at least two study visits with GA (without prior or simultaneous exudative neovascular AMD) was 1222 eyes of 900 participants. Of these 1222 eyes, the number with RPD grading available (at least as early as the first time-point with GA present) and no missing covariates was 771 eyes of 563 participants. Of the 1222 eyes, the number with genetic data available was 560 eyes of 417 participants. The intersection between those two groups, i.e., with RPD grading and genetic data available, was 420 eyes of 306 participants. Their demographic, clinical, and genetic characteristics are shown in Table 1 and Supplementary Table 1.
Table 1.
Demographic, clinical, and genetic characteristics of the study populations at baseline.
| Combined cohort (prevalent and incident GA) | Combined cohort with genetic data available | Incident GA cohort | Combined cohort with genetic data available (independent of RPD data availability) | |
|---|---|---|---|---|
| Participants | 563 (771 eyes) | 306 (420 eyes) | 413 (513 eyes) | 408 (548 eyes) |
| Age (years), mean (SD) | 74.8 (7.1) | 74.1 (7.1) | 74.5 (7.1) | 73.7 (7.2) |
| Female | 326 (57.9%) | 182 (59.5%) | 240 (58.1%) | 239 (58.6%) |
| Smoking status | ||||
| Never | 233 (41.4%) | 120 (39.2%) | 175 (42.4%) | 164 (40.2%) |
| Former | 298 (52.9%) | 171 (55.9%) | 213 (51.6%) | 222 (54.4%) |
| Current | 32 (5.7%) | 15 (4.9%) | 25 (6.1%) | 22 (5.4%) |
| Education level | ||||
| High school or less | 181 (32.1%) | 105 (34.3%) | 135 (32.7%) | 140 (34.3%) |
| At least some college | 277 (49.2%) | 143 (46.7%) | 204 (49.4%) | 191 (46.8%) |
| Postgraduate | 105 (18.7%) | 58 (19.0%) | 74 (17.9%) | 77 (18.9%) |
| Follow-up (years), mean (SD)* | 3.3 (1.5) | 3.3 (1.5) | 2.5 (1.2) | 3.2 (1.5) |
| Genetics data available | 306 (54.4%) | - | 236 (57.1%) | - |
| rs10490924 ARMS2 | ||||
| Unavailable | 257 | - | 177 | - |
| GG | 105 (34.3%) | 105 (34.3%) | 79 (33.5%) | 150 (36.8%) |
| GT | 137 (44.8%) | 137 (44.8%) | 108 (45.8%) | 172 (42.2%) |
| TT | 64 (20.9%) | 64 (20.9%) | 49 (20.8%) | 86 (21.1%) |
| Eyes | 771 | 420 | 513 | 548 |
| Cohort | ||||
| Prevalent | 258 (33.5%) | 127 (30.2%) | - | 160 (29.2%) |
| Incident | 513 (66.5%) | 293 (69.8%) | - | 388 (70.8%) |
| RPD present† | 114 (14.8%) | 67 (16.0%) | 103 (20.1%) | - |
| Central/noncentral GA | ||||
| Noncentral | 515 (66.8%) | 285 (67.9%) | 346 (67.4%) | 372 (67.9%) |
| Central | 256 (33.2%) | 135 (32.1%) | 167 (32.6%) | 176 (32.1%) |
| Configuration | ||||
| Small (single patch <1DA) | 411 (53.3%) | 228 (54.3%) | 311 (60.6%) | 300 (54.7%) |
| Multifocal | 171 (22.2%) | 95 (22.6%) | 118 (23.0%) | 122 (22.3%) |
| Horseshoe or ring | 27 (3.5%) | 17 (4.0%) | 9 (1.8%) | 23 (4.2%) |
| Solid | 137 (17.8%) | 69 (16.4%) | 63 (12.3%) | 92 (16.8%) |
| Indeterminate | 25 (3.2%) | 11 (2.6%) | 12 (2.3%) | 11 (2.0%) |
| Fellow eye with GA | ||||
| Unknown | 108 | 59 | 73 | 88 |
| No | 359 (54.2%) | 200 (55.4%) | 230 (52.3%) | 258 (56.1%) |
| Yes | 304 (45.8%) | 161 (44.6%) | 210 (47.7%) | 202 (43.9%) |
| GA area (mm2), mean (SD) | 2.2 (3.1) | 2.1 (3.0) | 1.6 (2.3) | 2.0 (2.9) |
| Proximity to fovea (μm), mean (SD) | 422.3 (473.1) | 419.6 (457.4) | 431.4 (472.8) | 432.4 (480.6) |
Abbreviations: DA=disc areas; GA=geographic atrophy; RPD=reticular pseudodrusen; SD=standard deviation
follow-up from baseline (for prevalent GA) or first GA appearance (for incident GA)
defined at baseline (for prevalent GA) or at/any time prior to first GA appearance (for incident GA)
Geographic atrophy enlargement rate according to reticular pseudodrusen status
The study population for these analyses comprised 771 eyes of 563 participants (Table 1). By regression analysis, the mean change over time in square root of GA area was 0.277 mm/year (95% confidence interval 0.266–0.289). For the outcome of square root of GA area, a statistically significant interaction was observed between RPD status and years (P < 0.0001). GA enlargement was significantly faster in eyes with RPD present (Table 2). The enlargement rate was 0.269 mm/year (95% CI 0.258–0.281) in eyes with no RPD and 0.388 mm/year (95% CI 0.347–0.430) in eyes with RPD.
Table 2.
Geographic atrophy enlargement rates according to reticular pseudodrusen status.
| RPD status* | Estimate† (mm/year) |
95% CI (mm/year) |
P‡ |
|---|---|---|---|
| RPD absence (n=657 eyes) |
0.269 | 0.258–0.281 | <0.0001 |
| RPD presence (n=114 eyes) |
0.388 | 0.347–0.430 |
Abbreviations: CI=confidence interval; RPD=reticular pseudodrusen
RPD status defined at study baseline for eyes with prevalent geographic atrophy (GA) and at first GA appearance for eyes with incident GA
Mixed-model, repeated-measures regression with the square root of GA area as the dependent variable
P value for interaction between RPD status and years
Similar results were observed in analyses of the prevalent and incident GA cohorts, considered separately. In the prevalent GA cohort (n=258 eyes of 213 participants), the enlargement rates were 0.271 (95% CI 0.256–0.286) in eyes with no RPD and 0.421 mm/year (95% CI 0.347–0.495) in eyes with RPD (P < 0.0001). In the incident GA cohort (n=513 eyes of 413 participants), the equivalent rates were 0.291 (95% CI 0.269–0.312) and 0.360 mm/year (95% CI 0.310–0.410), respectively (P = 0.012).
In sensitivity analyses of the combined cohort, where the models were also adjusted for GA involvement in the fellow eye, the results were very similar. The enlargement rate was 0.258 mm/year (95% CI 0.246–0.271) in eyes with no RPD and 0.399 mm/year (95% CI 0.354–0.444) in eyes with RPD (P < 0.0001). In additional analyses of the combined cohort, RPD status was considered in three levels: never present, absent at baseline/first GA appearance but present later, and present at baseline/first GA appearance. The GA enlargement rates (in mm/year) were 0.258 (95% CI 0.245–0.272), 0.297 (95% CI 0.275–0.319), and 0.389 (95% CI 0.347–0.431), respectively (P < 0.0001), i.e., consistent with a dose-response association.
Geographic atrophy enlargement rate according to ARMS2 genotype
The study population for these analyses comprised 548 eyes of 408 participants (Table 1). Analyses similar to these have been reported previously2; however, in the previous analyses, the results were adjusted for age and sex only. For the outcome of square root of GA area, a statistically significant interaction was observed between ARMS2 genotype and years (P < 0.0001). GA enlargement was significantly faster in eyes of individuals with at least one versus no risk alleles at ARMS2 (Table 3). The enlargement rate (in mm/year) was 0.225 (0.204–0.247), 0.297 (0.276–0.318), and 0.316 (0.286–0.345) in eyes of individuals with 0 (GG), 1 (GT), and 2 (TT) risk alleles, respectively. In other analyses with the unit of analysis as the participant (n=408 eyes of 408 participants), the results were similar. The enlargement rate (in mm/year) was 0.206 (0.176–0.236), 0.286 (0.257–0.314), and 0.287 (0.246,0.329), respectively (P = 0.0002).
Table 3.
Geographic atrophy enlargement rates according to ARMS2 genotype.
| ARMS2 risk alleles | Estimate* (mm/year) |
95% CI (mm/year) |
P† |
|---|---|---|---|
| 0 (GG) (n=197 eyes) |
0.225 | 0.204–0.247 | <0.0001 |
| 1 (GT) (n=237 eyes) |
0.297 | 0.276–0.318 | |
| 2 (TT) (n=114 eyes) |
0.316 | 0.286–0.345 |
Abbreviations: CI=confidence interval
Mixed-model, repeated-measures regression with the square root of geographic atrophy area as the dependent variable
P value for interaction between ARMS2 risk alleles and years
Geographic atrophy enlargement according to reticular pseudodrusen status and ARMS2 genotype considered simultaneously
The study population for these analyses comprised 420 eyes of 306 participants (Table 1). For the outcome of square root of GA area, in a model including both interaction terms (i.e., one between RPD status and year and one between ARMS2 genotype and year), both were statistically significant (P < 0.0001, in each case). In this model, the enlargement rate remained significantly faster in eyes with RPD present (i.e., while adjusting for ARMS2 genotype): the rates were 0.273 mm/year (95% CI 0.256–0.289) in eyes with no RPD and 0.379 mm/year (95% CI 0.329–0.430) in eyes with RPD (Table 4). In the same model, the enlargement rate remained significantly faster in eyes of individuals with ARMS2 risk alleles (i.e., while adjusting for RPD status): the rates were 0.224 (95% CI 0.198–0.250), 0.287 (95% CI 0.263–0.310), and 0.307 mm/year (95% CI 0.273–0.341) in eyes of individuals with 0 (GG), 1 (GT), and 2 (TT) risk alleles, respectively (Table 4). In other analyses with the unit of analysis as the participant (n=306 eyes of 306 participants), the results were similar (Supplementary Table 2).
Table 4.
Geographic atrophy enlargement rates according to reticular pseudodrusen status and ARMS2 genotype, considered simultaneously.
| RPD status or ARMS2 risk alleles* | Estimate† (mm/year) |
95% CI (mm/year) |
P‡ |
|---|---|---|---|
| RPD absence (n=353 eyes) |
0.273 | 0.256–0.289 | <0.0001 |
| RPD presence (n=67 eyes) |
0.379 | 0.329–0.430 | |
| 0 (GG) (n=142 eyes) |
0.224 | 0.198–0.250 | <0.0001 |
| 1 (GT) (n=193 eyes) |
0.287 | 0.263–0.310 | |
| 2 (TT) (n=85 eyes) |
0.307 | 0.273–0.341 |
Abbreviations: CI=confidence interval; RPD=reticular pseudodrusen
RPD status defined at study baseline for eyes with prevalent geographic atrophy (GA) and at first GA appearance for eyes with incident GA
Mixed-model, repeated-measures regression with the square root of GA area as the dependent variable
P value for interaction between characteristic (RPD status or ARMS2 risk alleles) and years
Subsequent analyses were performed that considered eyes in several groups, based on RPD status and ARMS2 genotype simultaneously (Supplementary Table 3). For the outcome of square root of GA area, a statistically significant interaction was observed between RPD/ARMS2 group and years (P < 0.0001). The GA enlargement rate was lowest in those with RPD absence and no ARMS2 risk alleles, intermediate in those with RPD absence and ARMS2 risk alleles, and highest in those with RPD (with or without ARMS2 risk alleles), though the confidence intervals were wide in the latter group, owing to the relatively small number of eyes.
Mediation analysis
The study population for these analyses comprised the same 420 eyes of 306 participants as in the previous analyses (Table 1). The direct effect of the ARMS2 genotype (1–2 risk alleles, versus none) on the GA enlargement rate was significantly different from 0 (P = 0.025), with an estimate of 0.074 mm/year (95% CI 0.009–0.139; Table 5). The indirect effect of the ARMS2 genotype (i.e., via the potential mediator of RPD status) on the GA enlargement rate was not significantly different from 0 (P = 0.64), with an estimate of 0.002 mm/year (95% CI −0.006–0.009). Hence, the total effect of the ARMS2 genotype on the GA enlargement rate was very similar to the direct effect, at 0.076 mm/year (0.000–0.153). In other analyses with the unit of analysis as the participant (n=306 eyes of 306 participants), the results were similar (Supplementary Table 4).
Table 5.
Results of mediation analysis for the outcome of geographic atrophy enlargement rate, with ARMS2 genotype as exposure and reticular pseudodrusen status as potential mediator.
| Estimate† (mm/year) |
95% CI (mm/year) |
P | |
|---|---|---|---|
| Natural direct effect of ARMS2 genotype on enlargement rate* | 0.074 | 0.009–0.139 | 0.025 |
| Natural indirect effect of ARMS2 genotype on enlargement rate (i.e., mediated by RPD status)* | 0.002 | −0.006–0.009 | 0.64 |
| Marginal total effect of ARMS2 genotype on enlargement rate (sum of direct and indirect effects)* | 0.076 | 0.000–0.153 | 0.051 |
Abbreviations: CI=confidence interval; RPD=reticular pseudodrusen
RPD status defined at study baseline for eyes with prevalent geographic atrophy (GA) and at first GA appearance for eyes with incident GA; ARMS2 genotype considered in two levels (0 risk alleles/GG as reference and 1–2 risk alleles/GT+TT as other level)
slope of change in square root of GA area over time (mm/year), based on a single value per eye
Likelihood of geographic atrophy central involvement or multifocal configuration according to reticular pseudodrusen status
The study population for these analyses comprised 513 eyes (of 413 participants) with incident GA and known RPD status, considered at the time of GA incidence (Table 1). There was no significant difference in the likelihood of central involvement at the time of incident GA, according to RPD status (P = 0.29). The proportions of eyes with central involvement were 28.2% and 33.7%, in those with RPD present and absent, respectively. In those eyes whose GA was non-central at the time-point of GA incidence (n=346 eyes of 290 participants), there was no significant difference in the mean proximity of GA to the central macula, at that time-point (P = 0.17). The mean proximity values were 635 μm (SD 348) and 629 μm (SD 483), for eyes with RPD present and absent, respectively (i.e., just outside the central subfield, whose radius is 500 μm).
Among the same 513 eyes with incident GA and known RPD status, there was no significant difference in the proportion of eyes with multifocal GA at the time-point of GA incidence (P = 0.16), though the proportion was numerically higher in those with RPD, at 28.2% and 21.7% for those with RPD present and absent, respectively. In the subset of eyes where the GA was not multifocal at incidence (n=395 eyes of 344 participants), there was no significant difference in the rate of progression to a multifocal configuration over time, based on RPD status (P = 0.72). By proportional hazards regression, the hazard ratio associated with RPD presence was 1.10 (95% CI 0.67–1.81).
Progression from non-central geographic atrophy to central involvement according to reticular pseudodrusen status
The study population for these analyses comprised 346 eyes (of 290 participants) with incident non-central GA and known RPD status, considered at first GA appearance. Over mean follow-up time of 2.3 years (SD 1.2), the proportion of eyes that progressed to central involvement was 147 (42.5%). The Kaplan-Meier curve of progression to central involvement is shown in Supplementary Figure 1. In proportional hazards regression analyses for the outcome of progression to central involvement, eyes with RPD were not significantly more or less likely to progress to central involvement (P = 0.55). The hazard ratio associated with RPD presence was 1.14 (95% CI 0.75–1.74). However, in regression analyses for the outcome of GA proximity to the central macula, the rate of change in proximity was significantly faster in eyes with RPD (P = 0.009). The estimates for change in proximity per year were 157 μm (95% CI 126–188) and 111 μm (95% CI 97–125) in eyes with RPD present and absent, respectively, i.e., faster progression towards the central macula in eyes with RPD (Table 6).
Table 6.
Rate of change of geographic atrophy proximity to central macula per year, according to reticular pseudodrusen status, in eyes with incident non-central geographic atrophy.
| RPD status* | Estimate† (μm/year) |
95% CI (μm/year) |
P‡ |
|---|---|---|---|
| RPD absence (n=272 eyes) |
111 | 97–125 | 0.009 |
| RPD presence (n=74 eyes) |
157 | 126–188 |
Abbreviations: CI=confidence interval; RPD=reticular pseudodrusen
RPD status defined at first geographic atrophy appearance
Mixed-model, repeated-measures regression with proximity to central macula as the dependent variable
P value for interaction between RPD status and years
Analyses of the Age-Related Eye Disease Study
The results of the AREDS analyses are described in detail in the Supplementary Material.
Discussion
Main findings, interpretation, implications, and clinical importance
Our study demonstrates that GA enlargement is significantly faster in eyes with RPD. The association was not only highly statistically significant but also substantial in degree, with a difference of approximately 35% in enlargement rates. This finding was consistent in both newer (incident) and more long-standing (prevalent) GA. Interestingly, it was also evident in analyses considering RPD status in 3 levels, according to timing of presence. The dose-response association observed in those analyses suggests that the duration of time spent with RPD present alongside GA may be the most relevant factor for faster GA enlargement (rather than the ultimate RPD status of an eye or any underlying binary tendency to RPD). This finding of faster GA enlargement in eyes with RPD was replicated in the AREDS, with a similar magnitude. The replication of this result in separate analyses of an independent dataset lends support to its validity and potential generalizability. These findings are essential for planning and interpreting the results of clinical trials, for providing accurate prognostic information and making informed decisions in clinical practice (once treatments to slow GA progression are approved11), and for uncovering potential insights into biological mechanisms underlying RPD and GA progression. Specifically, incorporation of RPD status should lead to more accurate predictions of both enlargement rate and, importantly, rate of progression to central involvement.
Faster GA enlargement with ARMS2 risk alleles, as observed previously in the same AREDS2 dataset2, was also highly statistically significant and substantial in degree, with a difference of approximately 35% between individuals with 0 versus 2 risk alleles. Importantly, the mediation analysis revealed that ARMS2 risk alleles were associated with faster GA enlargement in a way that was not substantially mediated by RPD presence. The “indirect effect” term was not significant and there was no signal for mediation even from the point estimate (which was 0.002 mm/year, with a relatively narrow confidence interval). Therefore, even in the case of a false negative result, any mediation by RPD status is likely to be very modest. Despite the fact that ARMS2 risk alleles are associated with RPD presence9, which is in turn associated with faster GA enlargement, this potential indirect relationship via RPD status does not appear to make any meaningful contribution. Amongst other factors, it may be relevant that the relationship between ARMS2 genotype and RPD status is not overwhelmingly strong. In previous AREDS2 analyses, many individuals with ARMS2 risk alleles never had RPD during follow-up and many individuals with RPD had no risk alleles at ARMS2.9
The results of the mediation analysis are supported by those of the other analyses that made full use of the repeated-measures data. While adjusting for RPD status, ARMS2 risk alleles are still strongly associated with faster GA enlargement (Table 4). Similarly, even after adjustment for ARMS2 genotype, RPD presence is still strongly associated with faster GA enlargement. Again, this argues against any substantial mediation by RPD status in the relationship between ARMS2 and GA enlargement rate. We note that the results of the mediation analysis and the RPD/ARMS2 combined analyses are broadly similar in the AREDS dataset, as no significant indirect effect was observed. Relatively similar estimates and P-values were obtained for the direct effect, although the P-values for AREDS and AREDS2 fell on either side of nominal significance, perhaps owing to the lower number of eyes analyzed in AREDS, the absence of configuration data in AREDS, lower accuracy of RPD grading in AREDS, or other differences between the study populations.
We conclude that RPD presence and ARMS2 risk alleles are relatively independent risk factors for GA enlargement. The enlargement rates can be considered in three main groups according to these two risk factors: slow enlargement in the RPD−/ARMS2− group, moderate enlargement in the RPD−/ARMS2+ group, and fast enlargement in the RPD+ group (Supplementary Table 3). For the purposes of clinical trials and clinical practice, it appears that imaging features are particularly important variables for accurate predictions. RPD status appears highly discriminating, though other imaging features including size, central involvement, and configuration are also associated with altered enlargement rate.2 Regarding ascertainment in clinical practice, although detailed RPD quantification typically requires expert grading on multimodal imaging, RPD presence/absence can be assessed from OCT alone, which is considered highly sensitive and specific for detection.8 This increases the accessibility of RPD detection in routine clinical practice, where multimodal imaging is not always available. Indeed, AI algorithms are becoming available to detect RPD from OCT scans alone27, as well as from FAF or color fundus photographs alone.25,26
Faster geographic atrophy enlargement and advance to central macula in eyes with reticular pseudodrusen
We did not observe that central involvement was less common at the time of GA appearance in eyes with RPD, as has been suggested anecdotally. This may be contrary to the suggestion in a previous cluster analysis, where the authors reported one GA subtype characterized by RPD presence and non-central GA (amongst other phenotypic and genetic features), though, in that study, the assessment was cross-sectional and not necessarily at the time-point of GA incidence.14,28 In the current study, the difference in risk of central involvement (as a binary variable) according to RPD status was small and non-significant. This idea was supported by similar results for proximity of GA to the central macula (as a continuous variable). Hence, even in eyes with incident non-central GA, mean GA proximity to the central macula was similar in eyes with and without RPD.
Similarly, we did not find that a multifocal GA configuration was significantly more common in eyes with RPD, as has been suggested previously in two smaller retrospective studies.29,30 At the time of GA appearance, no significant difference was present. Even in eyes without a multifocal configuration at GA incidence, there was no difference in the rate of progression to a multifocal configuration over time, according to RPD status. Overall, RPD status does not appear to be a strong predictor of central involvement or configuration at the time of GA appearance.
GA enlargement was significantly faster in eyes with RPD, as discussed above. This was true overall, in terms of GA enlargement rate considering the whole macular grid. In addition, importantly, GA progression towards the central macula was also significantly faster in eyes with RPD. GA proximity to the central macula decreased over time around 40% faster in these eyes. This finding was replicated in the AREDS, where the magnitude of the difference was even greater and statistical significance also higher (perhaps owing to increased power through longer follow-up and/or other differences in GA characteristics between the study populations). Hence, RPD presence is associated not only with faster GA enlargement overall (an FDA-approved primary outcome measure in many clinical trials4,11), but also with faster progression towards the central macula, which is typically accompanied by profoundly decreased visual acuity. This is despite the fact that RPD are much less common in the central macula.8,31 In the AREDS2, we were unable to demonstrate significantly slower progression to central involvement as a binary variable, likely owing to low power (observed as a wide confidence interval around the relevant hazard ratio) and infrequent reimaging intervals (of 12 months). However, in the AREDS, which had longer mean follow-up and lower mean baseline proximity, significantly slower progression to central involvement was observed.
Several potential reasons may help explain the association between RPD presence and faster GA enlargement. The association appears not to be explained by differences in particular anatomical GA characteristics at first appearance. For example, a higher likelihood of multifocality could have contributed to faster enlargement, through mathematical reasons alone (i.e., through the sum of multiple radii each increasing as a similar rate, as opposed to a single radius2), but this was not observed. Similarly, a higher likelihood of non-central GA could have contributed, since non-central GA has been associated with faster enlargement2, but this was not observed either. Finally, it is not explained by increased likelihood of ARMS2 risk alleles. Therefore, other factors must be responsible.
In particular, GA enlargement may occur more rapidly and preferentially into macular areas affected by RPD. This idea has some support from previous qualitative studies suggesting that macular areas with RPD may help predict future locations of GA progression.29,30 Macula areas affected by RPD are typically characterized by a thin outer retina, thin choroid, and sometimes even outer retinal atrophy (ORA)32–34, i.e., features that are usually observed in GA (though not sufficient alone for its diagnosis). Hence, the pre-existence of these features might increase the likelihood of GA, or even represent precursor lesions, since some of the anatomical criteria of GA would already be met. Specifically, GA is diagnosed on OCT by a combination of RPE loss and overlying photoreceptor degeneration (e.g., thinning of the outer nuclear layer)35, while histological analyses of retinal areas affected by RPD have suggested a loss of RPE cells (with enlarged and abnormally shaped remaining RPE cells) and outer nuclear layer thinning.8,36,37
The fact that RPD presence is associated with faster GA progression towards the central macula, despite RPD being less common in the central macula, might argue against these ideas of purely local effects. However, they could still be at least partly responsible; in this case, the final phase of enlargement (from the paracentral to the central macula) might not be faster, but progression towards the central macula could still be faster overall, considering the whole time-period of enlargement.
The alternative possibility is that GA enlargement is faster in all directions in eyes with RPD, irrespective of the precise areas affected by RPD. For example, histological analyses have shown that retinal gliosis is widespread in eyes with RPD, and not restricted to the precise areas of RPD.36 Similarly, innate immune cells such as activated microglia are believed to be involved in the pathogenesis of both RPD formation and GA progression.8,38 This potential shared pathophysiology might exhibit local or distant associations. Finally, several studies have suggested choroidal abnormalities in eyes with RPD, including a thinner choroid, lower choroidal vascularity, and/or impaired choriocapillaris flow.8,39 If choroidal vascular dysfunction plays an important role in GA progression40, then this idea might help explain faster GA enlargement in eyes with RPD. Again, this might be through choroidal abnormalities local and/or distant to the areas of RPD.
Comparison with literature
We are not aware of any previous studies analyzing GA enlargement rate according to both RPD status and genotype, considered simultaneously, either by mediation analysis or by stratified analyses. A small number of smaller studies have examined GA enlargement rate according to RPD status, using several different methodologies, with mixed results.3 In one early study of 16 eyes over follow-up of 18 months, the GA enlargement rate did not differ significantly between according to RPD status, though the analyses did not include square root transformation or adjustment for multiple variables associated with altered enlargement rate.41 In an early retrospective study of 50 eyes over mean follow-up of 27 months, with a very high prevalence of RPD, subsequent GA enlargement (considered qualitatively as present or absent) was significantly more common in macular grid zones (range 0–5) with RPD presence at baseline; the proportion of zones affected by RPD had borderline nominal significance for an association with faster GA enlargement (without square root transformation), in a model that also included baseline GA area.29 Similarly, in another early retrospective study of 126 eyes over mean follow-up of 20 months, with RPD prevalence of 94% at baseline, GA enlargement (considered qualitatively) was significantly more common in macular grid zones (range 0–5) with RPD presence.30
In a more recent study of 181 eyes over median follow-up of 30 months, RPD presence was associated with faster GA enlargement (following square root transformation) of around 10%, in a final model that also included FAF pattern, hyperreflective foci concentration, fellow eye GA status, and interaction terms between hyperreflective foci and FAF pattern and between age and square root of GA area.42 Similarly, in another study of 83 eyes over median follow-up of 12 months, where 71% of eyes were graded as having RPD, RPD presence was associated with faster GA enlargement (without the square root transformation), though only by approximately 5%.43 In a recent retrospective study of 39 eyes over median follow-up of 3 years with GA, RPD presence was considered as one of five lesion types preceding GA development locally; the RPD precursor type was associated with the fastest subsequent enlargement rate (following square root transformation), though the models were not adjusted for other important variables such as baseline area, focality, and central involvement.44
Overall, of the small number of previous studies in this area, the majority have suggested that RPD presence may be associated with faster GA enlargement, consistent with the results of the current study. However, most of these previous studies have been either small in number, relatively short in follow-up, considered GA enlargement qualitatively by region, contained few eyes without RPD for comparison, have not used the square root transformation or other means to decrease dependence on baseline lesion size, and/or been limited by adjustment for very few other variables known to be associated with altered enlarged rate. The study that had fewest of these limitations42 had results most similar to those of the current study.
Previous studies have examined genetic risk factors for altered GA enlargement rate (i.e., irrespective of RPD status). In analyses of the same AREDS2 dataset, GA enlargement was significantly faster in individuals with ARMS2 risk alleles, C3 non-risk alleles, and APOE non-risk alleles.2 In a genome-wide association study that combined several datasets, GA enlargement was significantly faster in individuals with variants at PRMT6 and LSS.5 In both sets of analyses, no significant association was observed between CFH genotype and GA enlargement rate. For this reason, mediation analysis to explore relationships via RPD status versus other potential mediators would not be meaningful.
Strengths and limitations
The strengths of this study include the characteristics of the AREDS2 dataset in containing a large number of eyes with the combination of data on GA enlargement rate, RPD status, and genetic data. This permitted detailed analyses to understand the complex relationship between ARMS2 genotype, RPD status, and GA enlargement; in particular, mediation analysis was ideally suited to examining these associations. Other strengths include prospective and standardized collection of imaging and data at regular time-points, reading center measurements of GA area (together with important characteristics such as configuration and proximity), reading center grading for RPD status, long follow-up time, and adjustment for multiple factors known to be associated with altered enlargement rate. Finally, the use of the AREDS as a second and independent dataset to validate the findings was an additional strength.
The study limitations include post hoc hypothesis generation, the lack of genetic data in some participants, and the possibility of residual or unmeasured confounding. The GA area and proximity data were from planimetry of color fundus photographs, but GA is thought to be detected earlier, and perhaps with less variability of area measurements, on FAF.45 However, previous studies have demonstrated high correlations between color fundus photography and FAF in the measurement of both GA area and enlargement rate.45–47 The AREDS analyses were limited by the absence of reading center grading of RPD presence; RPD status was therefore inferred by deep learning-based grading of the fundus photographs. However, the ground truth of the algorithm’s training was from reading center grading of FAF images (leading to high specificity of grading from fundus photographs alone, in a previous study25). The current study considered RPD status in a binary way (i.e., present/absent), since image grading was performed in that way. Hence, we were unable to analyze potential associations according to RPD area or number of lesions. While this approach may be sufficient to capture differences in enlargement rate for many purposes, we aim to perform future studies that incorporate quantitative metrics of RPD status. The dataset included a wide spectrum of GA area sizes, but contained more eyes with relatively smaller baseline GA area, compared to those often enrolled in clinical trials.11 Although the findings were consistent in two datasets and should be widely generalizable, they may be most representative of eyes with relatively smaller GA lesions.
Mediation analysis may carry its own potential limitations. No software or widely accepted method exists for mediation analysis of longitudinal data (i.e., with repeated measures for each subject). Hence, in this study, the repeated-measures data on GA area were converted into an individual slope of GA enlargement over time for each eye, and the slopes used as the outcome variable in mediation analysis. This might decrease the precision of the results, though sensitivity analyses showed that both mediation analysis and repeated-measures regression generated very similar estimates for the degree of faster GA enlargement by ARMS2 genotype. Mediation analysis usually specifies that the potential mediator should be relatively rare (e.g., <10% frequency); in this study, the frequency of RPD presence was 16%. The proportion of the mediated effect (i.e., natural indirect effect: marginal total effect) is considered unstable with a sample size less than 500; the number here was 420 eyes. However, these two considerations appear more relevant for avoiding the possibility of false positive findings; it seems unlikely that they would substantially alter the main negative finding, of no or minimal mediation through RPD status.
Conclusions
GA enlargement is significantly faster in eyes with RPD presence, with a substantial difference in enlargement rates. Therefore, in clinical practice, noting RPD status in eyes with GA should improve the accuracy of prognostic information provided to patients. In eyes with RPD, the GA is likely to enlarge more rapidly; in the case of non-central GA, the atrophy is likely to progress faster towards the central macula. GA enlargement is also significantly faster in individuals with ARMS2 risk alleles, but not in a way that is mediated by RPD presence. Therefore, RPD presence and ARMS2 risk alleles appear to be relatively independent risk factors for faster GA enlargement; ARMS2 risk alleles must lead to faster GA enlargement in other ways. Since there is no significant mediation or interaction between ARMS2 genotype and RPD status for GA enlargement rate, assessing RPD status is helpful by itself for prognostic information; in many cases, genetic testing may not be required to identify eyes at high risk of fast enlargement. Hence, imaging features appear particularly important in predicting GA enlargement rate, and RPD are observed with high sensitivity on standard-of-care modalities (i.e., OCT). RPD status is not a strong predictor of central involvement or GA configuration at the time of GA appearance. However, the faster GA enlargement observed in eyes with RPD is accompanied by faster progression over time towards the central macula. Overall, these findings have important implications for both clinical trials and clinical practice, where RPD status should be considered for more accurate prognostic information on enlargement rate. They also provide insights into the biology underlying the complex relationship between GA, genotype, and RPD.
Supplementary Material
Financial support
This research was supported by the Intramural Research Program of the National Eye Institute (including award ZIAEY000546) and the National Library of Medicine, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland. It was also supported by contracts from the National Eye Institute (contract NOI-EY-0-2127 for AREDS and contracts HHS-N-260-2005-00007-C and ADB contract N01-EY-5-0007 for AREDS2). Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke. The sponsor and funding organization participated in the design and conduct of the study, data collection, management, analysis, and interpretation, and preparation, review and approval of the manuscript. AD was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., to the University of Wisconsin Madison Department of Ophthalmology and Visual Sciences.
Abbreviations
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- CI
confidence interval
- DA
disc area
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAF
fundus autofluorescence
- FDA
Food and Drug Administration
- GA
geographic atrophy
- OCT
optical coherence tomography
- RPD
reticular pseudodrusen
- RPE
retinal pigment epithelium
- SD
standard deviation
- SDD
subretinal drusenoid deposit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
No conflicting relationship exists for any author.
References
- 1.Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119(3):571–580. [DOI] [PubMed] [Google Scholar]
- 2.Keenan TD, Agron E, Domalpally A, et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125(12):1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleckenstein M, Mitchell P, Freund KB, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(3):369–390. [DOI] [PubMed] [Google Scholar]
- 4.Csaky K, Ferris F 3rd, Chew EY, Nair P, Cheetham JK, Duncan JL. Report From the NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases. Invest Ophthalmol Vis Sci. 2017;58(9):3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grassmann F, Harsch S, Brandl C, et al. Assessment of Novel Genome-Wide Significant Gene Loci and Lesion Growth in Geographic Atrophy Secondary to Age-Related Macular Degeneration. JAMA Ophthalmol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018;63(6):782–815. [DOI] [PubMed] [Google Scholar]
- 7.Agron E, Domalpally A, Cukras CA, et al. Reticular Pseudodrusen: the Third Macular Risk Feature for Progression to Late Age-related Macular Degeneration. Ophthalmology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Fletcher EL, Kumar H, Greferath U, Guymer RH. Reticular pseudodrusen: A critical phenotype in age-related macular degeneration. Prog Retin Eye Res. 2021:101017. [DOI] [PubMed] [Google Scholar]
- 9.Domalpally A, Agron E, Pak JW, et al. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology. 2019;126(12):1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan TD, Agron E, Chew EY, Group AR. Reply. Ophthalmology. 2019;126(5):e40–e41. [DOI] [PubMed] [Google Scholar]
- 11.Keenan TDL. Local Complement Inhibition for Geographic Atrophy in Age-Related Macular Degeneration: Prospects, Challenges, and Unanswered Questions. Ophthalmology Science. 2021;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKinnon DP. Introduction to statistical mediation analysis. Routledge; 2012. [Google Scholar]
- 13.MacKinnon DP, Pirlott AG. Statistical approaches for enhancing causal interpretation of the M to Y relation in mediation analysis. Pers Soc Psychol Rev. 2015;19(1):30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biarnes M, Colijn JM, Sousa J, et al. Genotype- and Phenotype-Based Subgroups in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmol Retina. 2020;4(12):1129–1137. [DOI] [PubMed] [Google Scholar]
- 15.Keenan TDL, Oden NL, Agron E, et al. Cluster Analysis and Genotype-Phenotype Assessment of Geographic Atrophy in Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report 25. Ophthalmol Retina. 2021;5(11):1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AREDS2 Research Group, Chew EY, Clemons T, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness JS, Bressler NM, Tian Y, Alexander J, Applegate CA. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40(8):1761–1769. [PubMed] [Google Scholar]
- 18.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci. 2013;54(7):4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agron E, Mares J, Chew EY, Keenan TDL, Group AR. Adherence to a Mediterranean Diet and Geographic Atrophy Enlargement Rate: Age-Related Eye Disease Study 2 Report 29. Ophthalmol Retina. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmol. 2013;131(1):110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galecki AT. General class of covariance structures for two or more repeated factors in longitudinal data analysis. Communications in Statistics - Theory and Methods. 1994;23(11):3105–3119. [Google Scholar]
- 23.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei LJ, Lin DY, Weissfeld L Regression-analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 25.Chen Q, Keenan TDL, Allot A, et al. Multimodal, multitask, multiattention (M3) deep learning detection of reticular pseudodrusen: Toward automated and accessible classification of age-related macular degeneration. J Am Med Inform Assoc. 2021;28(6):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan TDL, Chen Q, Peng Y, et al. Deep Learning Automated Detection of Reticular Pseudodrusen from Fundus Autofluorescence Images or Color Fundus Photographs in AREDS2. Ophthalmology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz R, Khalid H, Liakopoulos S, et al. A deep learning framework for the detection and quantification of drusen and reticular pseudodrusen on optical coherence tomography. 2022; https://arxiv.org/abs/2204.02406. Accessed 05/11/2022. [DOI] [PMC free article] [PubMed]
- 28.Keenan TDL. The Hitchhiker’s Guide to Cluster Analysis: Multi Pertransibunt et Augebitur Scientia. Ophthalmol Retina. 2020;4(12):1125–1128. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Blonska AM, Pumariega NM, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flamendorf J, Agron E, Wong WT, et al. Impairments in Dark Adaptation Are Associated with Age-Related Macular Degeneration Severity and Reticular Pseudodrusen. Ophthalmology. 2015;122(10):2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang TT, Keenan TD, Agron E, et al. Macular Thickness in Intermediate Age-Related Macular Degeneration Is Influenced by Disease Severity and Subretinal Drusenoid Deposit Presence. Invest Ophthalmol Vis Sci. 2020;61(6):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33(9):1800–1808. [DOI] [PubMed] [Google Scholar]
- 35.Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018;125(4):537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greferath U, Guymer RH, Vessey KA, Brassington K, Fletcher EL. Correlation of Histologic Features with In Vivo Imaging of Reticular Pseudodrusen. Ophthalmology. 2016;123(6):1320–1331. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Messinger JD, Kar D, Duncan JL, Curcio CA. Biometrics, Impact, and Significance of Basal Linear Deposit and Subretinal Drusenoid Deposit in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2021;62(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Wong WT. Innate Immunity in Age-Related Macular Degeneration. Adv Exp Med Biol. 2021;1256:121–141. [DOI] [PubMed] [Google Scholar]
- 39.Keenan TD, Klein B, Agron E, Chew EY, Cukras CA, Wong WT. Choroidal Thickness and Vascularity Vary with Disease Severity and Subretinal Drusenoid Deposit Presence in Nonadvanced Age-Related Macular Degeneration. Retina. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelfand BD, Ambati J. A Revised Hemodynamic Theory of Age-Related Macular Degeneration. Trends Mol Med. 2016;22(8):656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg JS, Auge J, Jaffe GJ, et al. Longitudinal analysis of reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(6):4054–4060. [DOI] [PubMed] [Google Scholar]
- 42.Bui PTA, Reiter GS, Fabianska M, et al. Fundus autofluorescence and optical coherence tomography biomarkers associated with the progression of geographic atrophy secondary to age-related macular degeneration. Eye (Lond). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiter GS, Told R, Schranz M, et al. Subretinal Drusenoid Deposits and Photoreceptor Loss Detecting Global and Local Progression of Geographic Atrophy by SD-OCT Imaging. Invest Ophthalmol Vis Sci. 2020;61(6):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiele S, Nadal J, Pfau M, et al. Prognostic value of intermediate age-related macular degeneration phenotypes for geographic atrophy progression. Br J Ophthalmol. 2021;105(2):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domalpally A, Danis R, Agron E, et al. Evaluation of Geographic Atrophy from Color Photographs and Fundus Autofluorescence Images: Age-Related Eye Disease Study 2 Report Number 11. Ophthalmology. 2016;123(11):2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaspan BL, Williams DF, Holz FG, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9(395). [DOI] [PubMed] [Google Scholar]
- 47.Khanifar AA, Lederer DE, Ghodasra JH, et al. Comparison of color fundus photographs and fundus autofluorescence images in measuring geographic atrophy area. Retina. 2012;32(9):1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


