Abstract
UV-induced reversion of the arg4-17 ochre allele in Saccharomyces cerevisiae is largely dependent on translesion polymerase η (Rad30p), known to bypass cyclobutane-type TT dimers in an error-free fashion. arg4-17 locus reversion was predominantly due to T→C transition of T127, the 3′ T of a TT photoproduct site. This event was at least 20-fold reduced in a rad30 deletion mutant, irrespective of the status of nucleotide excision repair. These data correlate with known properties of 6–4 TT photoproducts and in vitro characteristics of polymerase η and suggest that polymerase η plays an important in vivo role in inserting G opposite the 3′ T of 6–4 TT photoproducts at this site. Alternatively, an unprecedented error-prone processing of cyclobutane-type photoproducts at this site by polymerase η must be assumed as the critical mechanism. Whereas photoreactivation results indeed hint at the latter possibility, a possible regulatory influence of reducing the overall UV damage load on the bypass probability of non-cyclobutane-type pyrimidine dimer photoproducts should not be dismissed.
INTRODUCTION
Apart from various ways of repairing DNA damage, prokaryotic and eukaryotic cells have developed mechanisms to tolerate base damage in order to restore replication competence and to increase the time window available for bona fide DNA damage repair (1). In recent years, several eukaryotic DNA polymerases have been characterized that are capable of translesion synthesis past replication-arresting DNA damage in the template strand, such as UV-induced dipyrimidine lesions (for recent reviews see 2–5). These include cyclobutane-type pyrimidine dimers (CPDs) and the less frequent pyrimidine (6–4) pyrimidone adducts (1). In yeast, polymerase ζ, a complex of proteins Rev3 and Rev7, is required for virtually all damage-induced mutagenesis and thus is considered an error-prone bypass polymerase. In vitro, polymerase ζ is indeed capable of bypassing cyclobutane-type cis-syn thymidine dimers (6,7). However, given the low bypass efficiency of polymerase ζ alone, it has been suggested that polymerase ζ is primarily required for extension of an imperfectly paired primer following the initial step of error-prone base insertion opposite a thymidine dimer, catalyzed by another as yet uncharacterized polymerase (8). In contrast, polymerase η alone can efficiently bypass a TT cyclobutane-type dimer in vitro in an error-free mode, by inserting AA as frequently as with an undamaged template (9,10). Both polymerases are evolutionarily conserved. As predicted, compromising human polymerase ζ does indeed reduce UV mutability (11). Inactivation of polymerase η is the basis for the xeroderma pigmentosum variant (XP-V) syndrome (12–14), characterized by UV sensitivity, altered UV dimer bypass, cancer susceptibility and cellular hypermutability (15–18). Yeast, human and Drosophila polymerases η have been extensively characterized in vitro and the picture of a low fidelity polymerase emerges whose active site can accommodate base pairs of imperfect Watson–Crick geometry while its base insertion preference is determined by remaining capabilities for formation of hydrogen bonds (10,19–29). Recent structure determination of yeast polymerase η has supported this assumption (30).
Given the characterized error-free bypass of UV-induced TT dimers in vitro, hypermutability of a yeast rad30 deletion mutant is predicted. However, the available in vivo studies indicate a dependency of the mutational outcome on the system used. Expected UV hypermutability has indeed been demonstrated in a forward mutation system (can1) (31) and in reversion systems (ura3-210 and ura3-364) that detect exchanges opposite TC and CC photoproduct sites (32). On the other hand, UV reversion of amber allele trp1-1 was only enhanced in a rad30 mutant if another error-free bypass pathway was inactivated at the same time (33). Even more surprising, our own data indicated that a deletion of RAD30 lowered UV-induced mutation frequencies for locus-specific reversion of the arg4-17 ochre allele (34). In this study, we present a molecular analysis of UV-induced mutations at the arg4-17 locus and correlate these findings with recent in vitro results on the bypass of photoproducts by polymerases η and ζ.
MATERIALS AND METHODS
Yeast strains
The wild-type strain used was WS3002/5 (MATa ade2-1 arg4-17 his3-Δ200 trp1-289 ura3-52 leu2-3,112). An isogenic rad30Δ::KanMX4 derivative was constructed by recombination-mediated transplacement using a RAD30 deletion fragment. The latter was isolated by PCR from an existing rad30Δ strain obtained from a yeast strain repository (Euroscarf). Replacement of RAD14 by HIS3 has been described elsewhere (35). Yeast transformation was performed by the lithium acetate method (36).
UV treatment
Cells were grown for 48 h to stationary phase in YPD (1% yeast extract, 2% peptone, 2% dextrose) at 30°C, washed with deionized water, briefly sonicated and plated on synthetic medium. All medium ingredients were from US Biological (Swampscott, MA) except where indicated. Solid medium contained 0.67% yeast nitrogen base without amino acids, 4% dextrose and 1.4% agar, supplemented with 740 mg/l SD-Arg mix (Bio101, Vista, CA). If required, arginine sulfate was added to a final concentration of 10 µg/ml for survival plates or 1 µg/ml for selection plates allowing a limited number of cell divisions (37). Aliquots of 8 × 106 cells were plated per mutant selection plate and appropriate dilutions of the same cell suspension were spread on plates used for determination of colony survival. Plates were irradiated with a germicidal 254 nm UV lamp and incubated for 7–10 days at 30°C to allow for red pigmentation of colonies. Only red mutant colonies were counted. For photoreactivation, plates were treated immediately after UVC treatment with 6 kJ/m2 at 360 nm (Sylvania, Blacklite Blue). Data shown represent the average of four independent experiments.
Sequence analysis
UV-induced mutant colonies were expanded in YPD and chromosomal DNA was prepared from zirconium bead lysates (38). PCR of the ARG4 region was perfomed using primers CAGAACTCTGAGATAAGACTACC (forward) and GCTTCAATACAGCAGATTTAGCG (reverse). Purified PCR products were sequenced by the Emory University Sequencing Core Facility using the listed forward primer as a sequencing primer.
RESULTS
Influence of RAD30 on UV-induced arg4-17 reversion
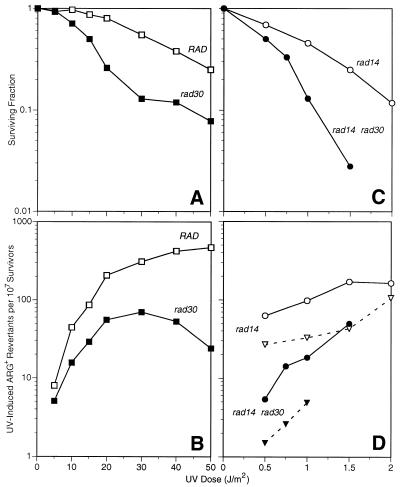
We compared UV-induced locus-specific arg4-17 reversion in a rad30 deletion mutant to an isogenic wild-type. Significantly reduced reversion frequencies were found and we confirmed our initial observations of an extended dose range (Fig. 1A and B) (34). At low doses of UV, the difference appears to be less pronounced. We observed a quantitatively similar effect of RAD30 on arg4-17 reversion in cells deleted for the nucleotide excision repair (NER) gene RAD14, involved in damage recognition (1). As compared to the rad14Δ single mutant, a rad14Δrad30Δ double mutant exhibited notably enhanced UV sensitivity of colony formation (Fig. 1C). arg4-17 reversion frequencies were determined under two conditions: on selection medium without any arginine or augmented with 1 µg/l arginine. In contrast to the wild-type, the recovery of UV-induced ARG+ revertants in NER-deficient cells is increased if selection plates are supplemented with a small amount of arginine, thus permitting a few rounds of replication on the plate (37). While confirming the published effect of enabling post-irradiation replication, a similar degree of reduced mutability in rad30Δrad14Δ versus rad14Δ was evident both on completely arginine-free and on supplemented plates (Fig. 1D).
Figure 1.
Survival (A and C) and induced arg4-17 locus-specific reversion frequencies (B and D) in UV-irradiated stationary phase cells of RAD (open squares), rad30Δ (closed squares), rad14Δ (open circles and open triangles) and rad14Δrad30Δ (closed circles and closed triangles) genotype. Mutant frequencies in NER-deficient cells (D) were measured on plates without any arginine (open and closed triangles) or with a trace amount of arginine (open and closed circles).
Influence of photoreactivation on UV-induced arg4-17 reversion
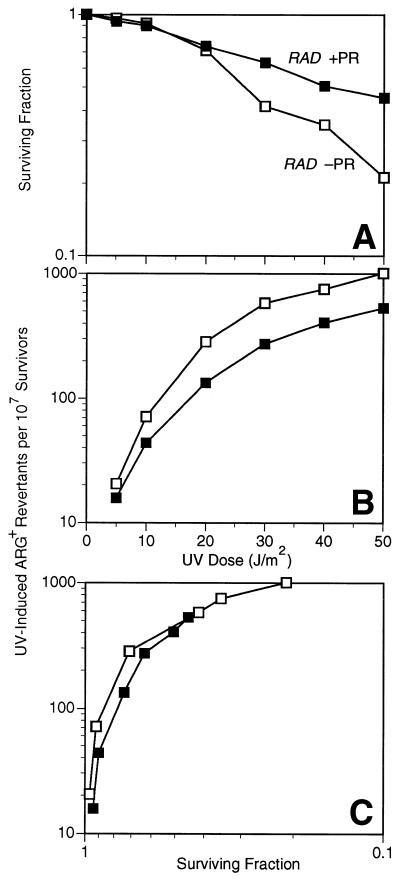
When treated with photoreactivating light after UV irradiation, survival and mutability of the arg4-17 locus were subject to substantial reactivation (Fig. 2A and B). When mutation frequencies were plotted against survival, mutation frequency values of photoreactivated and non-photoreactivated cultures seem to follow an identical curve and thus the effect of photoreactivation on arg4-17 reversion and survival appears to be quantitatively the same (Fig. 2C). No significant photoreactivation was determined in identically treated cells if the strain had been deleted for the gene PHR1 encoding yeast photolyase (not shown).
Figure 2.
Survival (A) and induced arg4-17 locus-specific reversion frequencies (B) in UV-irradiated stationary phase wild-type cells with (closed square) or without (open square) photoreactivation (PR). To compare the relative effectiveness of PR on survival versus mutagenesis, induced mutation frequencies were plotted against corresponding survival levels (C).
Sequence of UV-induced base substitutions
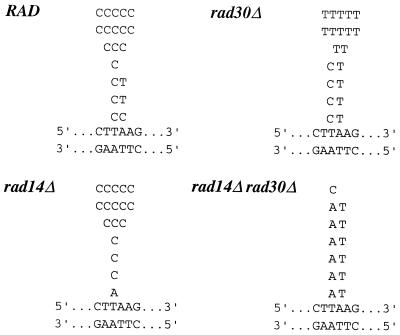
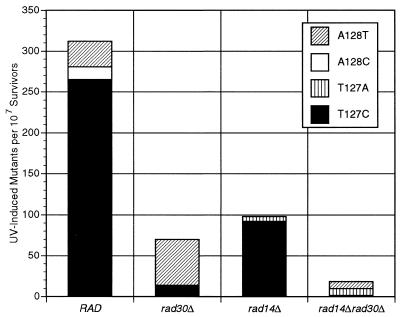
We identified the previously uncharacterized chromosomal arg4-17 ochre mutation by sequencing of a PCR product encompassing the ARG4 open reading frame. The arg4-17 mutation was mapped to codon 43 and was found to originate from an AAA→TAA exchange (lysine to ochre). In order to determine the base pair exchanges in UV-induced mutants, we sequenced this region in 20 independent mutant clones each from the wild-type and the rad30 deletion strain, induced by 30 J/m2 UV (Fig. 3). In the wild-type, by far the most frequent UV-induced base substitution was identified as a T127→C transition, the only type of event recovered at this position. Additional substitutions occurred at A128, where transversions were found. Both positions (on opposite strands) correlate with potential sites of TT photoproduct formation. The percentages of UV-induced T127 and A128 substitutions in the wild-type were 85 and 15%, respectively. In striking contrast, we found a reversed bias in the rad30 deletion mutant, with 20% T127→C transitions and 80% A128→T transversions. If one considers the different frequency levels, UV-induced base substitutions at T127 occured at an ∼20-fold lower frequency in the rad30 mutant than in the wild-type (Fig. 4). In contrast, the probability of substitutions at A128 remained essentially unchanged or may even be slightly increased.
Figure 3.
Identified base substitutions at the arg4-17 locus in UV-induced ARG+ clones isolated following treatment with 30 (RAD or rad30Δ) or 1 J/m2 (rad14 or rad14Δrad30Δ, isolated from plates with arginine supplementation). Letters above the double-stranded arg4-17 sequence reflect the types and numbers of detected substitutions. Originally, the arg4-17 mutation resulted from an AAA (Lys)→TAA (ochre) exchange, with TAA corresponding to bases 127–129 of the ARG4 open reading frame.
Figure 4.
Frequency of UV-induced substitutions at T127 and A128 in RAD and rad30Δ cells at 30 J/m2 or in rad14 and rad14Δrad30Δ cells at 1 J/m2, calculated from overall reversion frequencies (Fig. 1B), and the relative proportions of different misinsertion events (deduced from the sequenced sample shown in Fig. 3).
Essentially the same effect of RAD30 on base insertion preference was found in a NER-deficient background (rad14Δ versus rad14Δrad30Δ) (Fig. 3). In rad14Δ, 16 of 17 sequenced mutations recovered at 1 J/m2 UV were T127→C transitions. In rad14Δrad30Δ, this event was found only once among 13 sequenced mutations, whereas six were A128→T transversions. Interestingly, the remaining six mutations were identified as T127→A transversions, events we have not recovered in the NER-proficient background. Again, if different frequency levels are considered, UV-induced T127→C events are specifically and drastically reduced in a rad30 mutant background (Fig. 4).
DISCUSSION
Reversion of the chromosomal ochre allele arg4-17 has been frequently used in the past for in vivo studies of damage-induced mutagenesis in the yeast Saccharomyces cerevisiae (39,40). One reason lies in the convenience of identifying tRNA suppressor mutations that constitute virtually all of the (moderate) spontaneous background and are only weakly induced by 254 nm UV treatment. These can be eliminated from analysis by use of ade2-1 as a second ochre marker in the same strain. Suppressor mutants will most likely suppress both markers and form white colonies, while locus-specific revertants develop red pigmentation due to the unsuppressed ade2-1 allele. Indeed, we never recovered red colonies that carried mutations outside the arg4-17 locus.
While we have not extensively studied the selection bias intrinsic to this mutational system, certain conclusions can be drawn from our sequence analysis. The amino acid residue position in question can apparently accommodate a variety of different amino acids and restoration of the original wild-type sequence (Lys43 = AAA) was rarely found among the sequenced mutations. We could not correlate growth characteristics on selective medium with a particular type of mutation. Importantly, an A128→G transition will result in another termination codon and will thus remain undetectable. The third base of codon 43 (A129), opposite the 5′ T of a potential TT photoproduct site, was never found to be mutated.
Inactivation of polymerase η (Rad30p) reduces UV mutability at the arg4-17 locus significantly and over a wide range of doses (Fig. 1). The apparently less pronounced difference at low UV doses may correlate with the UV inducibility of the RAD30 transcript (33,34). The mutagenic effect of RAD30 is specific for UV damage, since a similar influence was not observed for various chemical agents in the same mutational system (34; unpublished data). The influence of RAD30 is even more striking if one considers the molecular nature of the underlying base substitutions (Fig. 3). Sequence analysis indicated UV-induced misinsertion opposite the 3′ residues of two potential TT dimer sites on opposite strands. G insertion leading to a T127→C transition, by far the most frequent event in the wild-type, is reduced ∼20-fold in the rad30 deletion mutant (Fig. 4).
The notion that overall UV mutability in yeast is critically determined by CPDs is primarily supported by experiments involving photoreactivation that reduces the amount of CPDs specifically. For example, photoreactivation can dramatically lower UV-induced mutation frequencies in a forward mutation system involving multiple photodimer sites, such as the plasmid-borne SUP4o system (41). This does not preclude a locally critical importance of other photoproducts. Although by far the most frequent UV base damage, studies with defined single UV photoproducts in plasmid substrates have revealed that cis-syn cyclobutane-type TT dimers in yeast are only weakly mutagenic and are easily bypassed if compared to 6–4 dipyrimidine photoproducts (42,43). Interestingly, the event observed here, preferential G insertion opposite the 3′ T of a TT photoproduct, has been observed specifically for the bypass of 6–4 TT photoproducts in Escherichia coli (44) and in yeast (45). Recent nuclear magnetic resonance analysis has explained such insertion preference by mispair stabilization due to hydrogen bonding (46). In another yeast chromosomal forward mutation system, a substantial fraction of UV-induced substitutions were indeed T→C transitions involving the 3′ T of a TT site (47).
The observed mutational specificity and influence of bypass polymerases at the preferred arg4-17 reversion site are in excellent agreement with in vitro studies on the bypass of 6–4 TT photoproducts by purified polymerases η and ζ (19,24). Rad30p has been shown to preferentially insert G opposite the 3′ T of a 6–4 TT photoproduct and thus appears to be guided by the base pairing properties of this residue. Thus, our in vivo results appear to directly reflect these in vitro properties of purified Rad30p if 6–4 TT photoproducts at T126/T127 are assumed as the primary mutagenic lesions. G insertion opposite the 3′ T residue of the potential 6–4 TT product site is indeed largely (but not completely) dependent on RAD30, whereas other types of misinsertion that are detected at the neighboring TT site on the opposite strand are clearly not diminished.
However, Rad30p is incapable of extending from the inserted base, which can be accomplished by polymerase ζ (19,48). According to one study, polymerase ζ is incapable of base insertion opposite the 3′ residue (19). Another study suggested that this event is possible, but without a preference for G insertion (48). Indeed, all UV-induced arg4-17 reversion events are strictly dependent on Rev3p (40) and thus, as predicted by the in vitro study, on polymerase ζ, due to its role in extension. Interestingly, Rev1p also plays a major role in UV-induced arg4-17 reversion (40) and both Rev1p and Rev3p were indeed required for 6–4 TT photoproduct bypass if single photoproducts were assayed in duplex plasmid vectors (43).
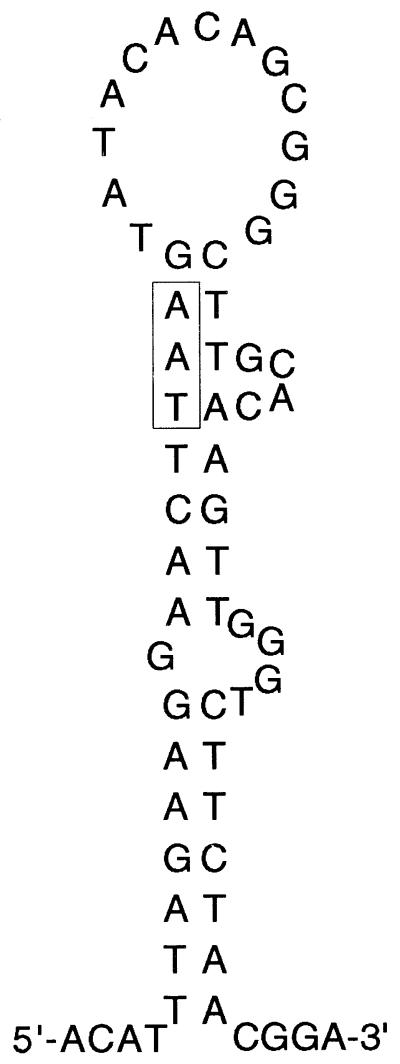
Surprisingly, the photoreactivation experiments did not confirm this interpretation. Whereas the yeast photolyase acts specifically on CPDs, UV mutability at the arg4-17 locus is subject to substantial photoreactivation in NER-proficient (Fig. 2) and NER-deficient cells (37; data not shown) and the effect is not different from the effect on overall survival. We suggest two possible explanations. (i) Whereas relevant premutagenic lesions are indeed (mostly) 6–4 TT photoproducts, the reason for reduced mutagenicity following photoreactivation treatment is a consequence of cellular regulation responding to a lowered amount of overall UV damage. For instance, photoreactivation could conceivably reduce the UV-inducible RAD30 transcript level (33,34) and thus the amount of enzyme available for mutagenic bypass. One might also want to consider that UV mutagenicity is positively influenced by checkpoint proteins (49), and their activity could be influenced by the overall damage level. (ii) CPDs constitute the relevant premutagenic lesions at T126/T127 that for some unknown reason are subject to a predominantly error-prone bypass by polymerase η. G insertion opposite the 3′ site of a cis-syn TT dimer can indeed be catalyzed in vitro (10). However, one would have to postulate that either the error-free bypass activity of polymerase η is non-functional or less active at this locus or that other normally active error-prone bypass polymerases are compromised. We noted a high potential for secondary structure within this region (Fig. 5) which may constitute an unusual environment for translesion synthesis. However, Rev3p clearly plays a major role and we also find a significant pro-mutagenic role of polymerase δ by analysis of a pol32 mutant (data not shown) (50).
Figure 5.
Hypothetical secondary structure following DNA strand separation surrounding the arg4-17 locus (indicated as boxed TAA).
The second dimer site in our system gives rise to different types of misinsertions (Fig. 2) and the fact that a deletion of rad30 leads to a slight increase in mutagenicity at this site is consistent with a role of CPDs. (However, it should be noted that G insertion opposite the 3′ T and thus the most frequent consequence of 6–4 TT photoproduct bypass is not detectable at this position, since another stop codon is created.)
As expected for repair proteins functioning in separate competing pathways, a rad30Δrad14Δ double mutant exhibited synergistically enhanced UV sensitivity as compared to rad14Δ (Fig. 1C). Again, a lack of UV-induced T127→C transitions was observed if RAD30 was deleted in the NER-deficient strain (Fig. 3). Thus, the influence of Rad30p on mutational frequency and specificity appears to be largely independent of NER, the major error-free pathway of UV damage repair, and seems to reflect primarily the initial damage type and incidence. It is worth noting that NER is known to have a profound influence on timing of mutational fixation at the arg4-17 locus. The dependency of efficient UV-induced reversion on post-irradiation replication in NER-deficient cells has been interpreted as S phase-dependent mutational fixation, in contrast to a largely pre-S phase fixation in the wild-type, most likely during repair synthesis (37). The influence of Rad30p remains essentially the same in both situations and, in spite of different timing, the same basic molecular mechanism may be operating.
However, NER does seem to have an effect on the processes that lead to UV-induced substitution of T127, especially in the absence of polymerase η (Fig. 3). Misinsertion of T leading to a T127→A transversion is detected in NER-deficient but not NER-proficient cells. This could indicate that different polymerases are engaged in translesion synthesis that occurs in the absence of Rad30p.
In summary, the initially puzzling observation of a mutagenic activity of the ‘error-free’ bypass polymerase η in a specific reversion system could be readily explained by the known mispairing properties of 6–4 TT photoproducts and their effect on translesion synthesis in yeast. Alternatively, an unprecedented predominantly error-prone bypass of a TT CPD by polymerase η at this specific dimer site must be assumed. Whereas photoreactivation ability indeed hints at the latter possibility, we caution that an overall reduced photoproduct level may influence regulatory responses and thus indirectly reduce mutagenic bypass probabilities for non-CPD products. Further experimentation is clearly required and may even result in a general re-evaluation of photoreactivation effects in UV mutation studies in yeast.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Paul Doetsch for help with photoreactivation. This work was supported by grant no. CA87381 from the National Institutes of Health.
REFERENCES
- 1.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. American Society of Microbiology, Washington, DC.
- 2.Friedberg E.C., Feaver,W.J. and Gerlach,V.L. (2000) The many faces of DNA polymerases: strategies for mutagenesis and mutational avoidance. Proc. Natl Acad. Sci. USA, 97, 5681–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z. (2001) Translesion synthesis by the UmuC family of DNA polymerases. Mutat. Res., 486, 59–70. [DOI] [PubMed] [Google Scholar]
- 4.Woodgate R. (1999) A plethora of lesion-replicating DNA polymerases. Genes Dev., 13, 2191–2195. [DOI] [PubMed] [Google Scholar]
- 5.Johnson R.E., Washington,M.T., Prakash,S. and Prakash,L. (1999) Bridging the gap: a family of novel DNA polymerases that replicate faulty DNA. Proc. Natl Acad. Sci. USA, 96, 12224–12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson J.R., Lawrence,C.W. and Hinkle,D.C. (1996) Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science, 272, 1646–1649. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence C.W. and Hinkle,D.C. (1996) DNA polymerase ζ and the control of DNA damage induced mutagenesis in eukaryotes. Cancer Surv., 28, 21–31. [PubMed] [Google Scholar]
- 8.Johnson R.E., Washington,M.T., Haracska,L., Prakash,S. and Prakash,L. (2000) Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature, 406, 1015–1019. [DOI] [PubMed] [Google Scholar]
- 9.Johnson R.E., Prakash,S. and Prakash,L. (1999) Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science, 283, 1001–1004. [DOI] [PubMed] [Google Scholar]
- 10.Washington M.T., Johnson,R.E., Prakash,S. and Prakash,L. (2000) Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase η. Proc. Natl Acad. Sci. USA, 97, 3094–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs P.E.M., McGregor,W.G., Maher,V.M., Nisson,P. and Lawrence,C.W. (1998) A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ. Proc. Natl Acad. Sci. USA, 95, 6876–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R.E., Kondratick,C.M., Prakash,S. and Prakash,L. (1999) hRAD30 mutations in the variant form of xeroderma pigmentosum. Science, 285, 263–265. [DOI] [PubMed] [Google Scholar]
- 13.Masutani C., Araki,M., Yamada,A., Kusumoto,R., Nogimori,T., Maekawa,T., Iwai,S. and Hanaoka,F. (1999) Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass polymerase activity. EMBO J., 18, 3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masutani C., Kusumoto,R., Yamada,A., Dohmae,N., Yokoi,M., Yuasa,M., Araki,M., Iwai,S., Takio,K. and Hanaoka,F. (1999) The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature, 399, 700–704. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y.-C., Maher,V.M. and McCormick,J.J. (1991) Xeroderma pigmentosum variant cells are less likely than normal cells to incorporate dAMP opposite photoproducts during replication of UV-irradiated plasmids. Proc. Natl Acad. Sci. USA, 88, 7810–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y.-C., Maher,V.M., Mitchell,D.L. and McCormick,J. (1993) Evidence from mutation spectra that the UV hypermutability of Xeroderma pigmentosum variant cells reflects abnormal, error-prone replication on a template containing photoproducts. Mol. Cell. Biol., 13, 4276–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann A.R., Kirk-Bell,S., Arlett,C.F., Paterson,M.C., Lohman,P.H.M., de Weerd-Kastelein,E.A. and Bootsma,D. (1975) Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc. Natl Acad. Sci. USA, 72, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer K.H., Parris,C.N., Gozukara,E.M., Levy,D.D., Adelberg,S. and Seidman,M.M. (1992) Human DNA repair-deficient diseases: clinical disorders and molecular defects. In Bohr,V.A., Wassermann,K. and Kraemer,K.H. (eds), DNA Repair Mechanisms. Munksgaard, Copenhagen, Denmark, pp. 15–22.
- 19.Johnson R.E., Haracska,L., Prakash,S. and Prakash,L. (2001) Role of DNA polymerase η in the bypass of a (6–4) TT photoproduct. Mol. Cell. Biol., 21, 3558–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haracska L., Yu,S.-L., Johnson,R.E., Prakash,L. and Prakash,S. (2000) Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nature Genet., 25, 458–461. [DOI] [PubMed] [Google Scholar]
- 21.Haracska L., Prakash,S. and Prakash,L. (2000) Replication past O6-methylguanine by yeast and human polymerase η. Mol. Cell. Biol., 20, 8001–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washington M.T., Johnson,R.E., Prakash,L. and Prakash,S. (2001) Accuracy of lesion bypass by yeast and human DNA polymerase η. Proc. Natl Acad. Sci. USA, 98, 8355–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa T., Uematsu,N., Mizukoshi,T., Iwai,S., Iwasaki,H., Masutani,C., Hanaoka,F., Ueda,R., Ohmori,H. and Todo,T. (2001) Mutagenic and nonmutagenic bypass of DNA lesions by Drosophila DNA polymerases dpolη and dpolι. J. Biol. Chem., 276, 15155–15163. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Yuan,F., Wu,X., Rechkoblit,O., Taylor,J.-S., Geacintov,N.E. and Wang,Z. (2000) Error-prone lesion bypass by human DNA polymerase η. Nucleic Acids Res., 28, 4717–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F., Zhang,Y., Rajpal,D.K., Wu,X., Guo,D., Wang,M., Taylor,J.-S. and Wang,Z. (2000) Specificity of DNA lesion bypass by the yeast DNA polymerase η. J. Biol. Chem., 275, 8233–8239. [DOI] [PubMed] [Google Scholar]
- 26.Washington M.T., Johnson,R.E., Prakash,S. and Prakash,L. (1999) Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J. Biol. Chem., 274, 36835–36838. [DOI] [PubMed] [Google Scholar]
- 27.Johnson R.E., Washington,M.T., Prakash,S. and Prakash,L. (2000) Fidelity of human polymerase η. J. Biol. Chem., 275, 7447–7450. [DOI] [PubMed] [Google Scholar]
- 28.Minko I.G., Washington,M.T., Prakash,L., Prakash,S. and Lloyd,R.S. (2001) Translesion DNA synthesis by yeast DNA polymerase η on templates containing N2-guanine adducts of 1,3-butadiene metabolites. J. Biol. Chem., 276, 2517–2522. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda T., Bebenek,K., Masutani,C., Hanaoka,F. and Kunkel,T.A. (2000) Low fidelity DNA synthesis by human DNA polymerase-η. Nature, 404, 1011–1013. [DOI] [PubMed] [Google Scholar]
- 30.Trincao J., Johnson,R.E., Escalante,C.R., Prakash,S., Prakash,L. and Aggarwal,A.K. (2001) Structure of the catalytic core of S. cerevisiae DNA polymerase η: implications for translesion synthesis. Mol. Cell, 8, 417–426. [DOI] [PubMed] [Google Scholar]
- 31.Johnson R.E., Prakash,S. and Prakash,L. (1999) Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J. Biol. Chem., 274, 15975–15977. [DOI] [PubMed] [Google Scholar]
- 32.Yu S.-L., Johnson,R.E., Prakash,S. and Prakash,L. (2001) Requirement of DNA polymerase η for error-free bypass of UV-induced CC and TT photoproducts. Mol. Cell. Biol., 21, 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald J.P., Levine,A.S. and Woodgate,R. (1997) The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics, 147, 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roush A.A., Suarez,M., Friedberg,E.C., Radman,M. and Siede,W. (1998) Deletion of the Saccharomyces cerevisiae gene RAD30 encoding an Escherichia coli DinB homolog confers UV radiation sensitivity and altered mutability. Mol. Gen. Genet., 257, 686–692. [DOI] [PubMed] [Google Scholar]
- 35.Siede W., Friedberg,A.S., Dianova,I. and Friedberg,E.C. (1994) Characterization of G1 checkpoint control in the yeast Saccharomyces cerevisiae following exposure to DNA-damaging agents. Genetics, 138, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gietz R.D. and Schiestl,R.H. (1995) Transforming yeast with DNA. Methods Mol. Cell. Biol., 5, 255–269. [Google Scholar]
- 37.Kilbey B.J., Brychcy,T. and Nasim,A. (1978) Initiation of UV mutagenesis in Saccharomyces cerevisiae. Nature, 274, 889–891. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser C., Michaelis,S. and Mitchell,A. (1994) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Resnick M.A. (1969) Induction of mutations in Saccharomyces cerevisiae by ultraviolet light. Mutat. Res., 7, 315–332. [DOI] [PubMed] [Google Scholar]
- 40.Lemontt J.F. (1971) Mutants of yeast defective in mutation induction by ultraviolet light. Genetics, 68, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong J.D. and Kunz,B.A. (1992) Photoreactivation implicates cyclobutane dimers as the major UVB lesions in yeast. Mutat. Res., 268, 83–94. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence C.W., Gibbs,P.E.M., Borden,A., Horsfall,M.J. and Kilbey,B.J. (1993) Mutagenesis induced by single UV photoproducts in E. coli and yeast. Mutat. Res., 299, 157–164. [DOI] [PubMed] [Google Scholar]
- 43.Nelson J.R., Gibbs,P.E.M., Nowicka,A.M., Hinkle,D.C. and Lawrence,C.W. (2000) Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol., 37, 549–554. [DOI] [PubMed] [Google Scholar]
- 44.LeClerc J.E., Borden,A. and Lawrence,C.W. (1991) The thymine-thymine pyrimidine-pyrimidone(6–4) photoproduct is highly mutagenic and specifically introduces 3′ thymine-to-cytosine transitions in Escherichia coli. Proc. Natl Acad. Sci. USA, 88, 9685–9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibbs P.E.M., Borden,A. and Lawrence,C.W. (1995) The T–T pyrimidine (6–4) pyrimidone UV photoproduct is much less mutagenic in yeast than in Escherichia coli. Nucleic Acids Res., 23, 1919–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.-H., Hwang,G.-S. and Choi,B.-S. (1999) Solution structure of a DNA decamer duplex containing the stable 3′ TG base pair of the pyrimidine(6–4)pyrimidone photoproduct [(6–4) adducts]: implications for the highly specific 3′T→C transition of the (6–4) adducts. Proc. Natl Acad. Sci. USA, 96, 6632–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee G.S.-F., Savage,E.A., Ritzel,R.G. and von Borstel,R.C. (1988) The base-alteration spectrum of spontaneous and ultraviolet radiation-induced forward mutations in the URA3 locus of Saccharomyces cerevisiae. Mol. Gen. Genet., 214, 396–404. [DOI] [PubMed] [Google Scholar]
- 48.Guo D., Wu,X., Rajpal,D.K., Taylor,J.-S. and Wang,Z. (2001) Translesion synthesis by yeast DNA polymerase ζ from templates containing lesions of ultraviolet radiation and acetylaminofluorene. Nucleic Acids Res., 29, 2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulovich A.G., Armour,C.D. and Hartwell,L.H. (1998) The Saccharomyces cerevisiae RAD9, RAD17, RAD24 and MEC3 genes are required for tolerating irreparable, ultraviolet-induced DNA damage. Genetics, 150, 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang M.-E., de Calignon,A., Nicolas,A. and Galibert,F. (2000) POL32, a subunit of the Saccharomyces cerevisiae DNA polymerase δ, defines a link between DNA replication and the mutagenic repair bypass pathway. Curr. Genet., 38, 178–187. [DOI] [PubMed] [Google Scholar]