Background
Infection with SARs-COV-2 results in COVID-19 disease.
Between March 2020 and August 2021, 468 COVID-19 patients confirmed by PCR or antigen test, in Yepes, Spain, received early treatment with antihistamines, adding azithromycin in selected cases. The primary endpoint is the hospitalization rate of COVID-19 patients, and the secondary endpoints are ICU admission and mortality rates. All endpoints are compared with the official Spanish rates during the time period of the study.
There were 20 hospital admissions (hospitalization rate 4,3%), 5 ICU admissions (ICU admission rate 1,1%) and 3 deaths (fatality rate of 0,6%). No patients in the study required follow up treatment, which suggest they did not develop long COVID. Results from this retrospective trail indicate that early treatment of SARS-COV-2 positive patients with antihistamines may reduce the odds of hospitalization (OR: 0.490, CI: 0.313–0.767, p-value: 0.001). Randomized controlled clinical trials are needed to further evaluate the effects of early antihistamine treatment of SARS-CoV-2 patients to prevent hospitalization, ICU admission, mortality and long-covid.
Keywords: SARS-CoV-2, Coronavirus, Azithromycin, Antihistamines
1. Introduction
In 2020, the SARS-CoV2 pandemic was declared. Since it was a new virus causing an unknown infection, doctors at different levels of care tested empiric treatments trying to reduce the severity of the infection. Despite the international efforts, early treatment options are few and with disparate success.
The National Institute of Health (NIH) recommends the use of over-the-counter antipyretics, analgesics, or antitussives for the management of symptoms for outpatients COVID-19 patients. Antihistamines fall under the umbrella of antitussives. They are a well-known class of drugs commonly used in Primary Care. Several second generation antihistamines have a good safety profile when dosed up to four times the standard dose [1]. Commonly prescribed antihistamines include cetirizine, loratadine, ebastine, dexchlorpheniramine, bilastine, levocetirizine, fexofenadine, desloratadine, mizolastine and rupatadine.
It has been hypothesized that antihistamines help damped mast cell degranulation, resulting in a reduction of the cytokine storm observed in severe COVID-19 disease [[2], [3], [4], [5]]. Two clinical studies have demonstrated utility for treating COVID-19 patients with HR1 antihistamines. Hogan et al. [6], reported a reduction in intubation and mortality for 110 hospitalized patients treated with both HR1 and HR2 receptor antagonist. Moran et al. [7] reported a reduction in hospitalization and mortality for 84 elderly out patients treated early with HR1 antagonist and azithromycin. In this study none of these high risk patients reached the endpoints of hospitalization or mortality.
The pulmonary pathological findings in COVID-19 seems to be the result from the release of multiple proinflammatory cytokines, and a key source of such cytokines and chemokines is the mast cells, which are ubiquitous in the body and especially the lungs [8,9]. It has also been described that many patients who either recovered from or had mild symptoms after covid, show diffuse, multiorgan symptoms months after the infection, including malaise, myalgias, chest tightness, brain fog and other neuropsychiatric symptoms, quite similar to those presented in mast cell activation syndrome (MCAS) [8]. It has been hypothesized that COVID-19 hyperinflammation and post-COVID-19 illness may be due to a mast cell activation syndrome [3].
Targeting cytokines during the management of COVID-19 patients could improve survival rates and reduce mortality [10]. The activation of pulmonary macrophages to viral infection induces the release of histamine. If this reaction is excessive, it will cause the release of proinflammatory cytokines, leading to the dreaded cytokine storm and ARDS aggravation. Several antihistamines have shown inhibitory properties on the production and expression of interleukins, chemokines, and other cytokines [11]. This suggests that antihistamines may help in the treatment of a COVID-19 cytokine storm through their immunomodulatory properties [6,12]. Specifically, cetirizine decreases interleukin production [13,14].
The macrolide azithromycin has previously been identified as having direct anti-inflammatory and immunomodulatory properties independent of their antibacterial properties [15]. Numerous studies describe an antiviral activity of azithromycin and other macrolides against different viruses [[16], [17], [18]]. If has been hypothesized that azithromycin affects lysosomal traffic and pH [[19], [20], [21]], interfering with ACE2 receptor and decreasing virus binding [22]. Furthermore, azithromycin has time-dependent immunomodulatory actions [15] and anti-inflammatory effects [18], reducing the production of proinflammatory cytokines IL-12 and IL-6, increasing the anti-inflammatory IL-10,[23] and provoking a global amplification of the host antiviral responses mediated by interferon [20,22]. In vitro inhibition of SARS-CoV-2 replication by azithromycin has been demonstrated [24]. Other macrolides have also shown similar properties [15,18,25,26].
In patients with cystic fibrosis, many of whom have chronic treatment with azithromycin and other antibiotics, low morbidity and mortality related to covid disease have being registered to date, in contrast to what would be expected as a high risk pathology [27]. Thus, the Patient Registry of the European Cystic Fibrosis Society has so far collected data on 1549 cases confirmed by PCR, of which 311 were hospitalized (20,08%), 52 admitted to the ICU (3,36%) and only 23 have died (fatality rate of 1,48%) [28].
There are publications with disparate results about the effectiveness of azithromycin in COVID-19. Some of these studies included unconfirmed cases, or patients who had had symptoms for up to 14 days [[29], [30], [31]]. It is possible that these studies had poor results because patients were treated too late. The maximum effect of azithromycin would be achieved in the first days of infection [32]. In other clinical trials in which azithromycin was administered at the first symptoms, better outcomes were achieved [33,34]. Given that the immunomodulatory properties of macrolides target mainly macrophages and granulocytes, and viral drug sensitivity is maximal during early replication after internalization, macrolide therapy is possibly most effective when administered early in the course of the infection process [15]. There are more similar studies using azithromycin in COVID-19 with good results [27,35,36].
The present work is an extension of the work carried out by Moran et al. [7] This study compares the outcomes of 468 outpatient SARs-COV-2 positive subjects treated early with antihistamines in the Yepes region of Spain with Spanish national COVID-19 statistics. In selected cases azithromycin was added. Results from this study suggest that early treatment of SARs-COV-2 positive subjects with the treatment protocol outlined in Moran et al. [1] reduces the odds of being hospitalized.
2. Materials and methods
This is a retrospective observational study of 468 symptomatic patients with confirmed diagnosis of COVID-19, living in the village of Yepes, Toledo, which has a population of around 5500 inhabitants. This study was approved by the Clinical Research Ethics Committee of the IIS-FJD (Madrid). The study period runs from the beginning of March 2020 to the end of August 2021.
Comparator data was obtained from the Carlos III Health Institute [37] prepared by DATADISTA [38]. The data table ‘provinces_covid19_datos_sanidad_nueva_serie.csv’ were loaded into R version 4.0.3. Data outside of the study period of March 2020 and August 2021 were removed. The number of ‘Casos’, ‘Hospitalizados’, ‘ICI’, and ‘Fallecidos’ were summed to obtain Spanish national data for the total number of SARS-CoV-2 cases, hospital admissions, ICU admissions, and deaths. A program was written in R to compute the odds ratios of the study endpoints. A Fisher exact test was used to compute the p-value corresponding to each odds ratio.
2.1. Objective
The objective of this study is to evaluate the effectiveness of early treatment of COVID-19 in Primary Health Care using a protocol with antihistamines as the main drug. Hospitalization admission rate, ICU admission rates, and fatality rates from this case series were compared with Spanish official metrics.
2.2. Study endpoints
The primary endpoint is the hospitalization rate of COVID-19 patients, and the secondary endpoints are ICU admission and mortality rates.
2.3. Complementary diagnostic tests
Cases were confirmed by PCR, antigen test or rapid test. The rapid tests used were 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold-Based) Vazyme Biotech and BIOZEK COVID-19 IGG/IGM rapid test.
2.4. Inclusion criteria
Consecutive patients were included in this study if they had symptoms compatible with covid disease and tested positive for COVID-19 via PCR, antigen test or serology testing.
The period of the study is from March 2020 to August 2021. To avoid the potential confounding effects of vaccination on SARs-CoV-2 infection outcomes, vaccinated patients were not included in this study.
2.5. Clinical management of COVID-19 used in patients attending the Health Center of Yepes
Since the beginning of the pandemic, we have been using the same treatment regimen that we had used in the nursing homes [7], starting treatment with antihistamines every 12 h at the first symptoms (dexchlorpheniramine 2 mg, cetirizine 10 mg or loratadine 10 mg).
Early initiation of treatment is essential. In young patients, under 40 years of age, without risk factors and with few symptoms, we only prescribed antihistamines every 12 h. In patients over 40 years of age, we added azithromycin 500 mg orally for 3 days, unless symptoms persist, in which case azithromycin was prescribed for an additional 3 days.
In very high-risk patients (such as immunocompromised, morbidly obese, chronic respiratory or cancer patients), instead of azithromycin we added levofloxacin every 12 h for a week. The rest of the recommendations are the same that we used in nursing homes. In all cases, patients are re-evaluated every 24 or 48 h until recovery.
Close contacts are treated prophylactically with antihistamines at the same dose as symptomatic patients.
2.6. Study population
The study population includes people who had not received a SARs-CoV-2 vaccine living in the community, in the village of Yepes, Toledo, who tested positive for SARS-CoV-2 between March 2020 and August 2021.
All patients with symptoms were prescribed treatment with antihistamines, adding azithromycin in 289 patients (62%). The most commonly prescribed antihistamine was cetirizine (46.6%), due its safety profile. Cetirizine can be used in all age groups, and has a low percentage of adverse effects and drug interactions. The second and third antihistamines most commonly prescribed were dexchlorpheniramine (35.7%) and ebastine (1.7%).
3. Results
From March 2020 to August 2021, 468 covid patients who lived in the community were attended. All of them were confirmed as positive by antigen test or PCR.
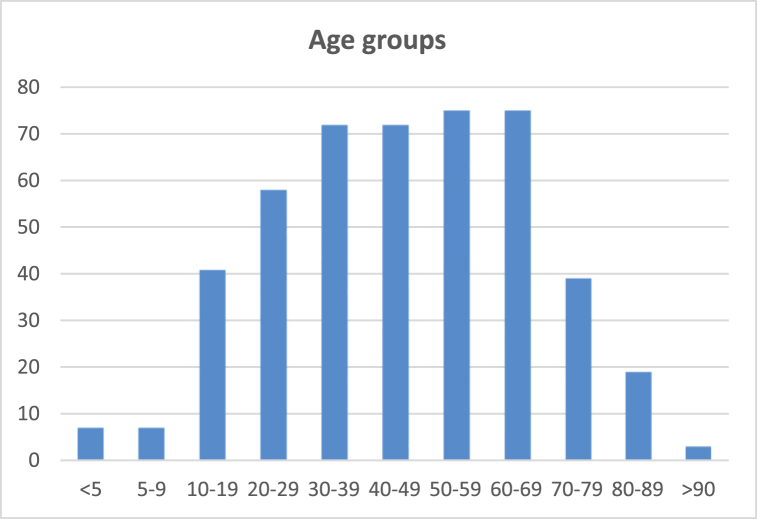
Fig. 1 shows a histogram of the ages of all study subjects. The study included subject aged zero to ninety-six. The mean age was 46 years and median age was 47. The study population included 136 patient who were 60 or older (29%).
Fig. 1.
Histogram showing the patient population age distribution. One hundred and thirty six patients were 60 or older.
Table 1 shows the prevalence of risk factors in the patient population. The most frequent risk factor was hypertension, suffered by 102 patients (22%), followed by obesity (68 patients, 15%). 28 patients had diabetes mellitus (6%), 17 had COPD/asthma (4%) and 13 had cancer (3%).
Table 1.
Risk factors associated with hospital admission, intensive care unit (ICU) admission and in-hospital death among patients hospitalized with COVID-19.
| Risk Factor | All Subjects | Hospital |
ICU |
Death |
|||
|---|---|---|---|---|---|---|---|
| Nº | % | Nº | % | Nº | % | ||
| Cancer | 13 | 1 | 7,7 | 0 | 0,0 | 1 | 7,7 |
| Hypertension | 102 | 12 | 11,8 | 2 | 2,0 | 0 | 0,0 |
| DM | 28 | 4 | 14,3 | 1 | 3,6 | 0 | 0,0 |
| Obesity | 68 | 7 | 10,3 | 3 | 4,4 | 0 | 0,0 |
| EPOC/ASTHMA | 17 | 3 | 16,7 | 1 | 5,6 | 1 | 5,6 |
This study included 13 patients whom were previously diagnosed with cancer. Table 3 shows the sex, age, antihistamine prescribed, and cancer type for this subset of patients. Marked in grey is the only patient with cancer who died.
Table 3.
Patient population details for study participants with cancer. The row highlighted grey corresponds to the one subject with cancer who reached the endpoint of death.
| Sex | Age | Antihistamine | Cancer |
|---|---|---|---|
| M | 88 | Ebastine | Prostate |
| M | 86 | Dexchlorpheniramine | Prostate |
| F | 83 | Dexchlorpheniramine | Breast |
| M | 80 | Dexchlorpheniramine | Colon |
| M | 71 | Cetirizine | Prostate |
| M | 70 | Dexchlorpheniramine | Colon |
| F | 65 | Dexchlorpheniramine | Breast |
| F | 64 | Ebastine | Breast |
| M | 59 | Cetirizine | Prostate |
| M | 58 | Cetirizine | Gastric |
| M | 56 | Cetirizine | Prostate |
| F | 46 | Levocetirizine | Breast |
| F | 23 | Cetirizine | Breast |
A breakdown of the number of patients who reached or did not reach the study endpoints for both the study and comparator groups are shown in Table 4.
Table 4.
The number and percentages of patients in the study and Spanish national statistics whom reached the study endpoints.
| Event | Nº |
% |
Nº |
% |
||
|---|---|---|---|---|---|---|
| Study Event | Study No Event | Yepes | Control Event | Control No Event | Spain | |
| Hospitalization | 20 | 448 | 4,27 | 395.070 | 4.338.540 | 8,35 |
| ICU | 5 | 463 | 1,07 | 37.372 | 4.696.238 | 0,79 |
| Death | 3 | 465 | 0,64 | 82.738 | 4.650.872 | 1,75 |
The odds ratio of reaching the study endpoints are shown in Table 5. There is a statistically significant decrease in the odds of hospitalization for the study group compared with national statistics. The odds ratio for ICU admission and death is not significant.
Table 5.
Odds ratios, 95% confidence intervals and fisher exact test p-values for each study endpoint.
| Event | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Hospitalization | 0.49 | 0.31–0.77 | 0.00078 |
| ICU | 1.36 | 0.56–3.28 | 0.42725 |
| Death | 0.36 | 0.12–1.12 | 0.07476 |
There were 20 hospital admissions, with a mean age of 64 and an average hospital stay of 12.7 days. Most of these patients had as risk factors hypertension (12 patients) or obesity (7 patients). Four patients did not have any risk factor in their medical registries. Five patients were admitted to the ICU due to bilateral pneumonia caused by SARS-CoV-2 infection. Hypertension and obesity were the most frequent risk factors. Detailed data are shown in Table 2.
Table 2.
Clinical features of patients who required hospital and ICU admission.
| Age | Sex | Treatment |
Risk factors |
Hospital stay |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antihistamine | Azithromycin | Cancer | Hypertension | DM | Obesity | EPOC/ASTHMA | Total admission time (days) | ICU stay (days) | Exitus | |||
| 96 | M | Dexchlorpheniramine | 1 | 1 | 8 | |||||||
| 85 | M | Hydroxyzine | 1 | 1 | 17 | |||||||
| 80 | M | Dexchlorpheniramine | 1 | 1 | 1 | 2 | 1 | |||||
| 78 | M | Dexchlorpheniramine | 1 | 1 | 1 | 5 | ||||||
| 76 | F | Loratadine | 1 | 1 | 1 | 31 | 24 | |||||
| 75 | M | Cetirizine | 1 | 1 | 1 | 1 | 6 | |||||
| 72 | M | Ebastine | 1 | 1 | 1 | 5 | ||||||
| 69 | F | Cetirizine | 1 | 11 | ||||||||
| 67 | M | Cetirizine | 1 | 1 | 1 | 1 | 48 | 29 | ||||
| 67 | F | Cetirizine | 1 | 1 | 8 | |||||||
| 65 | M | Dexchlorpheniramine | 1 | 4 | ||||||||
| 61 | M | Cetirizine | 1 | 1 | 35 | 34 | 1 | |||||
| 56 | M | Cetirizine | 1 | 1 | 8 | |||||||
| 53 | F | Cetirizine | 1 | 1 | 11 | |||||||
| 51 | F | Cetirizine | 1 | 4 | ||||||||
| 49 | M | Cetirizine | 1 | 1 | 6 | |||||||
| 47 | F | Ebastine | 1 | 8 | ||||||||
| 47 | F | Cetirizine | 1 | 1 | 1 | 7 | ||||||
| 39 | F | Dexchlorpheniramine | 1 | 1 | 16 | 10 | ||||||
| 38 | F | Ebastine | 1 | 14 | 5 | |||||||
| Total | 17 | 1 | 12 | 4 | 7 | 3 | 2 | |||||
| Mean | 64 | 12,7 | 20,4 | |||||||||
Three deaths, occurred between March and May 2021: an 87-year-old woman in a previous terminal condition who died at home after a rapid evolution of the disease, an 80-year-old man with colon cancer and hypertension (died in hospital, no admission at ICU) and a 61-year-old man with asthma (died in ICU).
No adverse effects were recorded. None of our surviving patients have required medical attention for sequelae or symptoms of long COVID.
Close contacts were also treated with antihistamines, and none of them subsequently consulted for symptoms compatible with covid.
4. Discussion
This study design was motivated by promising results using antihistamines and azithromycin to successfully treat 84 high risk elderly patients [7]. When this treatment protocol was expanded to treat COVID-19 patients in the general population, clinical improvements were typically observed 24–48 h after treatment regardless of the disease severity. A minority of patients required complementary treatment due to more serious or persistent respiratory symptoms: other antibiotics (levofloxacin or moxifloxacin), corticosteroids, home oxygen therapy, bronchodilators, etc.
When a patient was diagnosed, we began simultaneously to treat prophylactically close contacts with an antihistamine (one dose every 12 h). After 1 month none of them had consulted for symptoms of covid disease, what made us think that antihistamines could have avoided contagion and the spread of the infection to other members of the families.
After all this time using our protocol, a statistically significant reduction in the risk of hospital admission among patients treated with antihistamines, regardless of age and comorbidities was observed.
For those patients who required hospitalization, once admitted their medication was withdrawn and hospital protocols were applied. Several of the admitted patients had various comorbidities. The treatment prior to hospitalization may have influenced a better subsequent evolution.
In order to avoid severe disease, it is important to develop a proper strategy to treat patients early. It is hypothesized that this treatment protocol is effective at treating COVID-19 due to the antiviral and the immunomodulatory properties of antihistamines and azythromycin, and these drugs act synergistically in the early stages of the disease, so it is essential to start the treatment as soon as possible. The effectiveness of this combined treatment is probably more limited in advanced stages.
The reduction in hospitalization using the protocol previously described here and in Morán et al. [7], suggest that future evaluation of this treatment protocol in randomized controlled clinical trials are merited.
The positive results in this population have contributed to replacing the initial fear of the virus with a respect similar to that of any other pathogen, so that these patients can lead a normal life in the presence of SARS-CoV-2.
4.1. Limitations
This study has the limitations of all observational studies. These types of studies are currently used on a large scale as a basis for pharmacoepidemiological research in Primary Care [39]. In addition, using national Spanish statistics as a proxy for a control group is a limitation. Data in the control group such as patient comorbidities, vaccination status, and treatment protocol does not exist. Therefore, it is unknown how well of a control this group truly is. Randomized, placebo-controlled trials are recommended as a next step to evaluate the efficacy of this treatment protocol for the early management of SARS-CoV-2 infection.
5. Conclusions
COVID-19 disease continues to challenge our lives. Despite the high vaccination rates in Spain, many patients with a complete vaccination schedule develop the disease and die. [40] There is, therefore, an unmet medical need, effective drugs that can be used early in Primary Care and prevent progression to serious disease. As we have seen, recent publications compile successful experiences in treating covid patients with antihistamines and other medications used in allergies.
As we previously reported similarly in the Yepes nursing homes, early treatment of covid patients with antihistamines for 30 days at the Primary Health Care level, associating azithromycin for 3–6 days in selected cases, had excellent outcomes in patients living in the community, regardless of patient's age and risk factors. As none of the close contacts who took antihistamines developed the disease, we believe that antihistamines may have played a role as a preventive drug for COVID-19 which should be studied.
After one year and a half using this safe and inexpensive treatment protocol in our Health Center, with a very low mortality rate compared to the official figures, we strongly recommend trying this treatment with COVID-19 patients in Primary Care. Treatment should be started when the first symptoms appear. Antihistamines and azithromycin are drugs with a good safety profile and an extensive experience of use, good tolerance, low cost and wide availability, so this treatment regimen may respond to the global therapeutic needs for COVID-19 for all age groups. Clinical trials are necessary to determine its effectiveness.
Author contribution statement
JIMB: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
JAB: Performed the experiments; Contributed reagents, materials, analysis tools or data.
PFS and KVGH: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
PFS was supported under Air Force Contract No. FA8702-15-D-0001. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the U.S. Air Force.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgements
We thank Ma del Mar Cruz Acquaroni (MD) for her support and suggestions on improving this manuscript.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COPD
Chronic obstructive pulmonary disease
- DM
Diabetes mellitus
- HR1
Histamine receptor 1
- HR2
Histamine receptor 2
- HT
Hypertension
- ICU
Intensive Care Unit
- IL
Interleukin
- MCAS
Mast cell activation syndrome
- NIH
National Institute of Health
- PCR
Polymerase chain reaction
- SpO2
Oxygen saturation (SpO2) levels
References
- 1.Cataldi M., Maurer M., Taglialatela M., Church M.K. Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin. Exp. Allergy. 2019;49(12):1615–1623. doi: 10.1111/cea.13500. [DOI] [PubMed] [Google Scholar]
- 2.Malone R.W., Tisdall P., Fremont-Smith P., et al. COVID-19: famotidine, histamine, mast cells, and mechanisms. Front. Pharmacol. 2021;12(March:1–21. doi: 10.3389/fphar.2021.633680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afrin L.B., Weinstock L.B., Molderings G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020;100(January):327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti P., Caraffa A., Tetè G., et al. Mast cells activated by sars-cov-2 release histamine which increases il-1 levels causing cytokine storm and inflammatory reaction in covid-19. J. Biol. Regul. Homeost. Agents. 2020;34(5):1629–1632. doi: 10.23812/20-2EDIT. [DOI] [PubMed] [Google Scholar]
- 5.Ricke D.O. Hyperactivated mast cells pathogenesis hypothesis for COVID-19 cutaneous manifestations. JID Innov. 2021;1(4) doi: 10.1016/j.xjidi.2021.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan R.B., Hogan R.B., Cannon T., et al. Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Puml. Pharm. Ther, 2020;63 doi: 10.1016/j.pupt.2020.101942. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco Ji Morán, Alvarenga Bonilla J.A., Homma S., Suzuki K., Fremont-Smith P., Gómez de las Heras K.V. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care – a retrospective observational study in elderly patients. Pulm. Pharmacol. Ther. 2021 doi: 10.1016/j.pupt.2021.101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theoharides T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2021;126(January):217–218. doi: 10.1016/j.anai.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang H.W., Kanegasaki S., Jin F., et al. A common signaling pathway leading to degranulation in mast cells and its regulation by CCR1-ligand. Allergy Eur. J. Allergy Clin. Immu. 2020;75(6):1371–1381. doi: 10.1111/all.14186. [DOI] [PubMed] [Google Scholar]
- 10.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11(June:1–4. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aydin S., Aydin S. 2020. Could Antihistamines Help in the Treatment and Spread of COVID-19 Via Re-Modulating Cytokines and by Reducing Sneezing ? pp. 172–173. 4(4) [Google Scholar]
- 12.Eldanasory O.A., Eljaaly K., Memish Z.A., Al-Tawfiq J.A. Travel Med Infect Dis; 2020;37(August. Histamine Release Theory and Roles of Antihistamine in the Treatment of Cytokines Storm of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosch A., Borgmann S., Rihoux J.-P., König W. Effect of the H1 receptor antagonist cetirizine on the stimulated expression of adhesion molecules and the activation of NFκB in human endothelial cells. Int. Arch. Allergy Immunol. 2001;124(1–3):362–364. doi: 10.1159/000053758. [DOI] [Google Scholar]
- 14.Arnold R., Rihoux J.P., König W. Cetirizine counter-regulates interleukin-8 release from human epithelial cells (A549) Clin. Exp. Allergy. 1999;29(12):1681–1691. doi: 10.1046/j.1365-2222.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 15.Kricker J.A., Page C.P., Gardarsson F.R., Baldursson O., Gudjonsson T., Parnham M.J. Nonantimicrobial actions of macrolides: overview and perspectives for future development. Pharmacol. Rev. 2021;73(4):233–262. doi: 10.1124/pharmrev.121.000300. [DOI] [PubMed] [Google Scholar]
- 16.Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6(June:1–11. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Xia S., Zou P., Lu L. Erythromycin estolate inhibits zika virus infection by blocking viral entry as a viral inactivator. Viruses. 2019;11(11) doi: 10.3390/v11111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohe M., Shida H., Jodo S., et al. Macrolide treatment for COVID-19: will this be the way forward? Biosci. Trends. 2020:4–5. doi: 10.5582/bst.2020.03058. [DOI] [PubMed] [Google Scholar]
- 19.Homolak J., Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents. 2020;(xxxx) doi: 10.1016/j.ijantimicag.2020.106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin. Pharmacol. Ther. 2020;0(0):1–11. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherrmann J. Intracellular ABCB1 as a possible mechanism to explain the synergistic effect of hydroxychloroquine-azithromycin combination in COVID-19 therapy. AAPS J. 2020;22(4):1–6. doi: 10.1208/s12248-020-00465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mégarbane B., Scherrmann J.M. Hydroxychloroquine and azithromycin to treat patients with COVID-19: both friends and foes? J. Clin. Pharmacol. 2020;60(7):808–814. doi: 10.1002/jcph.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy B.S., Sundareshan V., Cory T.J., Hayes D., Anstead M.I., Feola D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61(3):554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 24.Touret F., Gilles M., Barral K., et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaya M., Shinya K., Hatachi Y., et al. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Therapeut. 2010;333(1):81–90. doi: 10.1124/jpet.109.162149. [DOI] [PubMed] [Google Scholar]
- 26.Min J.Y., Jang Y.J. Macrolide therapy in respiratory viral infections. Mediat. Inflamm. 2012;2012 doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondejar-Lopez P., Quintana-Gallego E., Giron-Moreno R.M., et al. Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: incidence and results of the national CF-COVID19-Spain survey. Respir. Med. 2020;170(May) doi: 10.1016/j.rmed.2020.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Cystic Fibrosis Society . 2021. COVID-19 in People with CF in Europe. Data up to 15 Nov 2021.https://www.ecfs.eu/covid-cf-project-europe Published. [Google Scholar]
- 29.Butler C.C., Dorward J., Yu L.M., et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinks T.S.C., Cureton L., Knight R., et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir. Med. 2021;9(10):1130–1140. doi: 10.1016/S2213-2600(21)00263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furtado R.H.M., Berwanger O., Fonseca H.A., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Million M., Lagier J.C., Tissot-Dupont H., et al. Early combination therapy with hydroxychloroquine and azithromycin reduces mortality in 10,429 COVID-19 outpatients. Rev. Cardiovasc. Med. 2021;22(3):1063–1072. doi: 10.31083/J.RCM2203116. [DOI] [PubMed] [Google Scholar]
- 33.Rashad A., Nafady A., Hassan M.H., et al. Therapeutic efficacy of macrolides in management of patients with mild COVID-19. Sci. Rep. 2021;11(1):1–7. doi: 10.1038/s41598-021-95900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekhavati E., Jafari F., Seyedalinaghi S., et al. Safety and effectiveness of azithromycin in patients with COVID-19: an open-label randomised trial. Int. J. Antimicrob. Agents. 2020;56(January doi: 10.1016/j.ijantimicag.2020.106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;(xxxx) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz R.A., Suskind R.M. Azithromycin and COVID-19: prompt early use at first signs of this infection in adults and children, an approach worthy of consideration. Dermatol. Ther. 2020;33(4):2–4. doi: 10.1111/dth.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centro Nacional de Epidemiología - instituto de Salud Carlos III - ministerio de Sanidad. COVID-19 en España. 2022. https://cnecovid.isciii.es/covid19/#documentación-y-datos Published.
- 38.Datadista. No title. https://github-com.translate.goog/datadista/datasets?_x_tr_sl=es&_x_tr_tl=en&_x_tr_hl=en&_x_tr_pto=op (wapp)
- 39.Maciá-Martínez M.-A., Gil M., Huerta C., et al. Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP): a data resource for pharmacoepidemiology in Spain. Pharmacoepidemiol. Drug Saf. 2020;29(10):1236–1245. doi: 10.1002/pds.5006. [DOI] [PubMed] [Google Scholar]
- 40.General de Sanidad Secretaria. Actualización No524. Enfermedad Por El Coronavirus (COVID-19). 16.12.2021 - Centro de Coordinación de Alertas y Emergencias Sanitarias. 2021. http://www.Sanidad.Gob.Es/Profesionales/SaludPublica/Ccayes/AlertasActual/NCov/Documentos/Actualizacion_524_COVID-19https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_44_COVID_1200.pdf