Abstract
In agreement with the International Association for the Study of Pain, chronic pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. To date, there are several types of pain: nociceptive, neuropathic, and nociplastic. In the present narrative review, we evaluated the characteristics of the drugs used for each type of pain, according to guidelines, and their effects in people with comorbidity to reduce the development of severe adverse events.
Keywords: nociceptive pain, neuropathic pain, nociplastic pain, drug treatment, drug interactions, guidelines
1. Introduction
The International Association for the Study of Pain (IASP) describes chronic pain as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage [1]. Chronic pain lasting for at least 3 months may impair the social, psychological, and physical sphere of a subject, leading to serious impairment of both their autonomy and mood [2]. Social factors (e.g., job, lifestyle, and economic and religious status), self-perception, mood alterations, and physical illness are risk factors for chronic pain [3]. According to IASP recommendations, chronic pain is classified as nociceptive (involving tissue or potential tissue damage), neuropathic (involving disease or injury affecting the nervous system), and nociplastic (with no evidence of tissue or nerve damage but persistent overregulation of the nociceptive system) [3,4].
Concerning the types (mechanisms) of pain, the choices of drugs for chronic pain include non-steroidal anti-inflammatory drugs (NSAIDs) (for cyclic short-course treatment), opioids, and central nervous system (CNS)-acting drugs.
1.1. NSAIDs
In patients with chronic nociceptive pain (e.g. tendonitis, osteoarthritis, or back pain), a shorter course of NSAIDs can be considered [5,6,7]. Evaluating their pharmacodynamic differences, ibuprofen and naproxen are non-selective COX inhibitors, and celecoxib and diclofenac are COX-2 semi-selective drugs, whereas etoricoxib is a COX-2 selective inhibitor [8,9].
Acetaminophen (paracetamol), an atypical NSAID without anti-inflammatory effects, was reported to be inefficient in patients with persistent chronic low back pain [10].
1.2. Opioids
Opioids can be used in patients with nociceptive or neuropathic pain (Table 1) but not in patients with nociplastic pain [11,12] (Figure 1).
Table 1.
| Characteristic | Opioids |
|---|---|
| Weak | Codeine, tramadol, hydrocodone, and dihydrocodeine |
| Strong | Morphine, oxycodone, fentanyl, buprenorphine, hydromorphone, methadone, and tapentadol |
| Antagonist | Naloxone and naltrexone |
| Nociceptive pain | High activity: codeine, methadone, hydrocodone, hydromorphone, and morphine Low activity: tramadol, oxycodone, and fentanyl |
| Neuropathic pain | Tramadol, oxycodone, fentanyl, buprenorphine, and tapentadol |
| CYP2D6 metabolism | Codeine, tramadol, oxycodone, and hydrocodone |
| CYP3A4 metabolism | Buprenorphine, hydrocodone, methadone, oxycodone, tramadol, and fentanyl |
| Liver conjugation | Buprenorphine, codeine, hydromorphone, morphine, oxycodone, tapentadol, and tramadol |
| Mechanism of action | μ receptor full agonist (morphine, oxycodone, fentanyl, hydromorphone, hydrocodone, methadone, tapentadol, and tramadol) k receptor agonist (oxycodone) μ receptor partial agonist and k receptor antagonist (buprenorphine) SNRI activity (tapentadol and tramadol) |
| Kidney excretion | Buprenorphine (30%), codeine (90%), fentanyl (75%), hydrocodone (6.5% of the parental drug, higher quote including metabolites), hydromorphone (90%), methadone (30%), morphine (90%), oxycodone (80%), tapentadol (99%), and tramadol (90%) |
| Liver excretion | Buprenorphine (70%), codeine (10%), fentanyl (9%), hydrocodone (data not available), hydromorphone (62% of oral dose eliminated by first-pass; 1% in feces), methadone (50%), morphine (10%), oxycodone (20%), and tramadol (10%) |
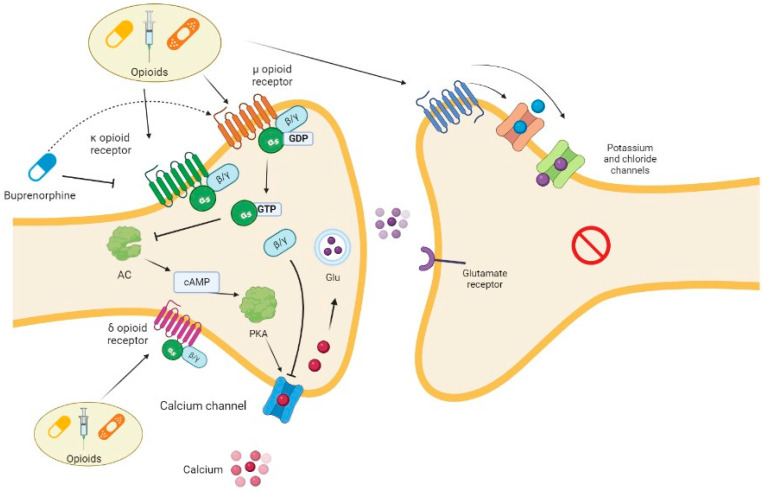
Figure 1.
Opioid mechanism of action. Opioids bind to their μ, κ, and δ receptors at presynaptic level, carrying out different actions. After the interaction with a receptor, the α subunit of protein G inhibits the pathway of AC, resulting in a reduction in calcium channel activity and then the release of glutamate. The same channel is inhibited via the βγ subunit. Buprenorphine is a particular drug since it has partial agonist activity on μ receptor and antagonist activity on κ receptors. Opioids also exert stimulating activity on calcium and chloride channels, resulting in hyperpolarization at postsynaptic level. AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; GDP, guanosine diphosphate; Glu, glutamate; GTP, Guanosine-5′-triphosphate; PKA, protein kinase A.
1.3. Central-Nervous-System-Acting Drugs
In patients with neuropathic pain, both antidepressants and antiepileptic drugs represent the first-line treatment [13].
Among tricyclic antidepressants, amitriptyline (25–125 mg/day) shows the better number needed to treat for 50% patient relief (with an NNT of 3.6), while duloxetine (30–60 mg/day) has an NNT of 6.4 [14].
Venlafaxine is not commonly used because it has low activity on the noradrenaline pathway with a dosage of lower than 150 mg per day [15]. The mechanism of action of antidepressants is summarized in Figure 2.
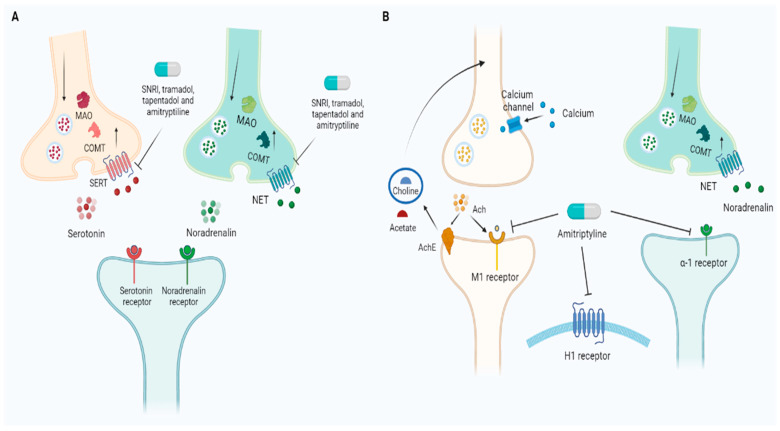
Figure 2.
Antidepressants used for pain management. (A) Amitriptyline and SNRIs inhibit SERT and NET, blocking serotonin and noradrenaline reuptake and increasing the availability of the two neurotransmitters in synaptic cleft. The same action is shared by two opioids, non-antidepressant drugs, namely, tramadol and tapentadol. (B) Nevertheless, amitriptyline is associated with several side effects according to its inhibitory action on cholinergic, adrenergic, and histaminergic pathways. Ach, acetylcholine; AchE, acetylcholinesterase; COMT, catechol-O-methyltransferase; MAO, monoamine oxidase; NET, norepinephrine transporter; SERT, serotonin transporter; SNRI, serotonin and norepinephrine reuptake inhibitors.
Gabapentin and pregabalin act on the α-2-delta subunit of voltage-gated calcium channels. The result of this action is the reduction in substance P, glutamate, and noradrenalin release [16]. Dizziness and somnolence are common adverse events that may lead to dropout. Both these drugs are excreted by the kidneys, and their dosage should be titrated carefully according to the estimated glomerular filtration rate (eGFR) [16,17].
Gabapentin has important disadvantages in comparison with pregabalin since it shows more adverse effects (leukopenia, hypertension, vasodilation, and skin reactions) and has a complex posology/titration scheme, reducing compliance [16,18,19,20].
Among antiepileptics, carbamazepine can be used for the management of chronic neuropathic pain [12], even if, usually, it is commonly prescribed for trigeminal neuralgia [21]. Carbamazepine is associated with several side effects: hematopoietic alterations, hyponatremia, neurologic effects (dizziness, somnolence, headache, and diplopia), gastrointestinal symptoms, cutaneous side effects, fatigue, and an increase in hepatic enzymes. Moreover, it is associated with teratogenic risk in pregnancy. Furthermore, it induces CYP3A4 and auto-induces its own metabolism, which is mediated by the same isoform [22,23].
Antiepileptic drugs (i.e., gabapentin and pregabalin) are indicated for neuropathic pain including postherpetic neuralgia, diabetic peripheral neuropathy, and spinal cord injury (with an NNT range of 2.9–7.7) [14]. A topical patch of 5% lidocaine (NNT = 4.4) and 9% capsaicin (NNT = 10.6) can reduce both allodynia and spontaneous pain [24,25].
For the management of nociplastic pain, commonly used drugs with neuropathic activity are, e.g., pregabalin and duloxetine [26]. The mechanisms of action of other drugs used for neuropathic pain treatment are summarized in Figure 3.
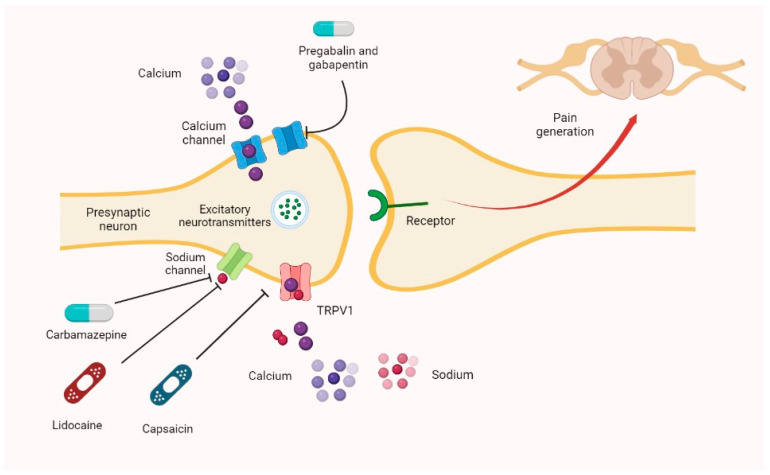
Figure 3.
Other neuropathic pain drugs’ mechanisms of action. The main principle of counteracting neuropathic pain is reducing the release of excitatory neurotransmitters in the synaptic cleft. Carbamazepine and lidocaine inhibit sodium channels, whereas capsaicin exerts its activity on TRPV1. Pregabalin and gabapentin block calcium channels in their α2δ subunit. TRPV1—transient receptor potential cation channel subfamily V member 1.
1.4. Muscle Relaxants
Muscle relaxants (e.g., cyclobenzaprine, tizanidine, thiocolchicoside, baclofen, eperisone, metaxalone, and methocarbamol) inhibit γ motoneurons and increase the activity of calcium channels and calmodulin with an increase in blood flow in the areas with muscle contraction. Moreover, each muscle relaxant has a particular mechanism of action: eperisone is a substance P antagonist; cyclobenzaprine operates via α and γ motoneurons inhibition; tizanidine is an α2 agonist; baclofen is an agonist of γ-aminobutyric acid (GABA)-B receptors; and thiocolchicoside is an agonist of GABA and glycine receptors.
In clinical trials, these drugs show good clinical efficacy, although adverse drug reactions (ADRs) and drug interactions (DDIs) can limit their use [27,28,29] (Table 2), reducing adherence to the drug therapy.
Table 2.
Possible drug interactions in patients with chronic pain using analgesic or anti-inflammatory drugs.
| Drugs Used for Pain Management | Interacting Drug | Comment |
|---|---|---|
| Anticonvulsants (pregabalin and gabapentin) | CNS-depressing drugs (e.g., opioids) [19,30] and alcohol | Respiratory depression risk. If possible, avoid concomitant use, or reduce dosage. |
| Gabapentin | Antiacids containing aluminum and magnesium [19,30] | Reduction in gabapentin bioavailability. |
| Amitriptyline | CYP2D6, CYP2C19, CYP3A4, and CYP1A2 inhibitors and inducers [31] | Relevant variations in bioavailability are possible. |
| Ethanol [32] | Increase in amitriptyline concentration. | |
| QT-increasing drugs | Risk for arrhythmias [31]. | |
| Valproic acid | Increase in amitriptyline concentration. | |
| Antihypertensive drugs | Risk of further decrease in blood pressure due to α-1 receptor antagonism, but cases of hypertension have been described [31,33]. | |
| Anticholinergic drugs | Increase in the side effects related to anticholinergic actions of amitriptyline [31,33]. | |
| CNS-depressing drugs and alcohol | Increase in CNS depression [31]. | |
| L-DOPA and phenylbutazone | Reduced gastric emptying may arise: L-DOPA and phenylbutazone may be inactivated for this reason. Furthermore, L-DOPA coadministration may facilitate arrhythmias and hypotension [31]. Nevertheless, L-DOPA and amitriptyline coadministration has been associated with better molecular efficacy in Parkinson’s disease [34]. | |
| Antihistamines | Possible increase in QT interval and increased sedation [35,36,37]. | |
| Duloxetine | Other antidepressants or drugs increasing serotonin levels (e.g., tramadol and tapentadol) | Serotonin syndrome risk [38,39]. |
| CYP1A2 and CYP2D6 inhibitors or inducers | Possible variation in duloxetine levels [40]. Contraindicated if CYP1A2 inhibitors are being used in therapy [41]. | |
| CYP2D6 substrates | Increase in these drugs’ levels due to the moderate inhibitory action of duloxetine on CYP2D6 [40]. | |
| CNS-depressing drugs and alcohol | Increase in CNS depression risk [40]. | |
| Antihypertensive drugs | Increase in blood pressure due to the action on noradrenalin reuptake [42]. | |
| Anticoagulants or antiaggregant drugs | Increase in bleeding risk related to action on platelet serotonin [40,43]. | |
| Muscle relaxants | Antihypertensive drugs | Various interactions are described since baclofen, tizanidine, and cyclobenzaprine may decrease blood pressure. No alterations are described with eperisone and thiocolchicoside [44,45,46,47,48]. |
| CNS-depressing drugs and alcohol | Increased CNS depression [49]. | |
| Baclofen | Tricyclic antidepressants | Possible increase in muscular hypotonia risk [44]. |
| Carbidopa and L-DOPA | Worse control of Parkinson’s symptoms. Confusion, hallucinations, and headache [44]. | |
| Lithium | Increase in hyperkinetic symptoms [44]. | |
| Drugs decreasing renal function | Increase in baclofen levels [44]. | |
| Cyclobenzaprine | Structural analog of tricyclic antidepressants [45] | Similar pharmacodynamic actions are expected, including sedation, anticholinergic effects, and blurred vision. |
| Eperisone | Calcium antagonists [47] | Increased calcium antagonists’ effects. |
| Salicylates [47] | Reduced salicylates levels. | |
| Metaxalone | Drugs increasing serotonin levels | Possible risk of serotoninergic syndrome [50]. |
| Methocarbamol | Pyridostigmine | Decreased effect of pyridostigmine in patients with myasthenia [51]. |
| Tizanidine | CYP1A2 inhibitors and inducers [46] | Increased/decreased levels of tizanidine. Contraindicated in presence of CYP1A2 inhibitors [46]. |
| Drugs prolonging QT [46] | Risk for QT prolongations. | |
| Oral contraceptives, verapamil, and cimetidine [46,49] | Possible increase in tizanidine levels. | |
| Beta-blockers or digoxin [46] | Possible increase in hypotension and bradycardia rate. | |
| NSAIDs | CYP2C9 inhibitors/inducers [52,53] | Evaluate dosage increase/reduction. |
| Aspirin and other associated NSAIDs | Risk for reduced effect of aspirin [54]. | |
| Antihypertensive drugs due to kidney damage and inhibition of natriuretic response to diuretic response, impaired synthesis of prostaglandins, sodium and water retention, and suppression of plasma renin activity [55,56] | Increase in blood pressure levels. Minor interactions are described with calcium antagonists [53]. | |
| Anticoagulants, antiaggregant drugs, corticosteroids, SSRIs, and even nutraceuticals/supplements such as Ginkgo Biloba [57,58,59] | Increase in hemorrhagic risk. Warfarin may be released from albumin after NSAID coadministration. Moreover, concomitant CYP2C9 metabolism by the two drugs may affect their concentrations [58,59]. | |
| Lithium, methotrexate, zidovudine, and digoxin | NSAIDs may reduce kidney elimination of some substances including lithium and methotrexate [58,60]. | |
| Probenecid | Aspirin may reduce probenecid effects [58]. | |
| Nephrotoxic medications (e.g., tacrolimus, aminoglycosides, and ciclosporin) [61] | Increased nephrotoxicity. | |
| Zidovudine | Increase in NSAIDs’ plasma levels (diclofenac in particular) and toxicity in animal models [62]. In humans, naproxen modified zidovudine conversion to its glucuronidated metabolite (GZDV) with a transformation in toxic metabolites. | |
| Acetaminophen | Chloramphenicol | Possible increase in chloramphenicol half-life [63]. |
| Drugs slowing or fastening gastric emptying and cholestyramine | Possible increase or reduction in bioavailability. Cholestyramine may reduce paracetamol’s absorption [63,64]. | |
| Hepatotoxic drugs | Increased risk for transaminases increases or liver failure. Phenytoin may reduce paracetamol efficacy and increase liver failure risk [65]. | |
| Opioids | Antidiarrhoeic drugs, | Stypsis [66]. |
| CNS-depressing drugs, and alcohol | CNS depression [67,68]. | |
| CYP3A4 inhibitors/inducers | Buprenorphine, hydrocodone, methadone, oxycodone, tramadol, and fentanyl may be variously involved in these reactions with increases or reductions in drug levels [66,69,70,71,72,73]. | |
| CYP2D6 inhibitors | Codeine, tramadol, oxycodone, and hydrocodone may be involved. Codeine, a prodrug, may be counteracted in its therapeutic action [65,69,71,72,74]. | |
| Methadone | Ammonium chloride | Ammonium chloride may facilitate methadone (a weak base) elimination via its action on urine pH [66]. |
| Anticholinergic drugs | Increase in anticholinergic effects, stypsis in particular [66]. | |
| Desipramine | Increase in desipramine levels [66]. | |
| Didanosine, stavudine, and zidovudine | Methadone may reduce didanosine and stavudine bioavailability, affecting their absorptions and first pass metabolisms. Nevertheless, methadone may increase zidovudine levels, reducing glucuronidation processes and, therefore, its renal clearance [66]. | |
| Octreotide | Possible reduction in analgesic effect. | |
| P-gp inhibitors and inducers | Methadone is a P-gp substrate. Therefore, the inhibition/induction of this protein may result in variations in methadone’s serum levels [66]. | |
| Drugs prolongating QT or antiarrhythmics | Possible risk for arrhythmias [66]. | |
| Morphine | Cimetidine | Reported cases of confusion and respiratory depression [67]. |
| Diuretics | The increase in ADH may contrast the effect of diuretics [67]. | |
| Muscle relaxants/blockers and oral anticoagulants | Morphine may increase the effects of these drugs [67]. | |
| Oxycodone | Anticholinergic drugs | Increase in anticholinergic effects [73]. |
| Fentanyl, methadone, oxycodone, tapentadol, and tramadol | Drugs increasing serotonin levels | Serotonin syndrome risk. Tapentadol has minor action on serotonin reuptake; therefore, the risk of serotonin syndrome is minor, compared with tramadol [72,75]. Methadone has an elevated potential of determining this syndrome [76]. A theoretic risk is also reported for hydrocodone and buprenorphine [77,78]. |
| Tapentadol (alone) | Naproxen and probenecid | These drugs may increase tapentadol levels but without clinical significance. Due to its glucuronidation related metabolism, tapentadol shows few interactions [75,79]. |
| Tramadol (alone) | Warfarin and coumarin derivatives | Possible increase in INR [72,80]. |
| Ondansetron | Possible necessity to increase tramadol dose [72,81]. |
Even if several guidelines describe the effects of these drugs in patients with chronic pain [2,12,82,83,84], few data related to the treatment of patients with chronic pain and in polytherapy have been published. The aim of the present narrative review is to evaluate the characteristics of the drugs used for each type of chronic pain, according to the guidelines, and their effects in people with comorbidity to reduce the development of severe adverse events.
2. Materials and Methods
The PubMed, Embase, and Cochrane library databases were searched for articles published until 10 January 2023, in agreement with our recent papers [55,57,85,86,87,88]. The secondary search included articles cited in the reference lists of papers identified with the primary search. The records were first screened by title/abstract (G.M., C.V., and C.P.), and then full-text articles were retrieved for eligibility evaluation (M.E.). The remaining articles were then subject to a citation search of all reference lists (A.C., L.G., and G.D.S.). Papers were deemed eligible if they included any of the words “chronic non cancer pain”, “drug(s)”, “guidelines”, and “adverse drug reaction”. All citations were downloaded into Mendeley, and duplicates were deleted. To avoid a bias of exclusion, the full-text articles were retrieved following the first round of exclusions and were also subject to two independent eligibility reviews, this time in perfect agreement. The studies evaluated as eligible were included in the present review. We excluded manuscripts without full texts and without indications of effects on chronic pain and manuscripts not in the English language.
3. International Guidelines
The first guidelines for pain treatment are presented in the World Health Organization (WHO) guidelines that, published in 1986, do not separate between both types (acute or chronic) and the different mechanisms (nociceptive, neuropathic, or nociplastic) of pain and suggest a 3-step treatment: NSAIDs (acetaminophen and other NSAIDs; Step I), weak opioids (5 mg of codeine, tramadol, and oxycodone; Step II), or strong opioids (Step III) [89].
Other international guidelines separate the types of pain, and in the presence of chronic pain, suggest a multimodal step-by-step approach, also considering the mechanisms of the pain.
The Centers for Disease Control and Prevention (CDC) guidelines report that chronic pain should primarily be managed with non-opioid drugs [90]. When utilized for pain management, opioids should be started at the lowest effective dosage and titrated slowly [90] (Table 3).
Table 3.
International guidelines for chronic pain. SIGN: Scottish Intercollegiate Guidelines Network; CDW: Colorado Division of Workers; AGS: American Geriatric Society; COX: cyclooxygenase; DHHS: Department of Health and Human Services; NeuPSIG: Neuropathic Pain Special Interest Group; NICE: National Institute for Health and Care Excellence; NMDA: N-methyl-D-aspartate; NSAIDs: non-steroidal anti-inflammatory drugs; OTC: over the counter; SNRI: noradrenaline–serotonin reuptake inhibitor; TCA: tricyclic antidepressant.
| STEP I | STEP II | STEP III | STEP IV | STEP V | STEP VI | |
|---|---|---|---|---|---|---|
| Nociceptive pain | ||||||
| SIGN [12] |
Paracetamol or NSAIDs | Weak opioids or topical NSAIDs | Strong opioids | |||
| CDW [49] | NSAIDs or COX-2 inhibitors | |||||
| AGS [91] | Paracetamol (up to 4 g/day) | NSAIDs | Opioids | |||
| DHHS [92] |
Ia: paracetamol Ib: ibuprofen or naproxen Ic: paracetamol plus ibuprofen or naproxen |
IIa: codeine IIb: tramadol | IIIa: low-dose morphine or buprenorphine patch (if morphine is ineffective) IIIb: high-dose morphine or 5–30 mg of oxycodone twice a day or fentanyl/buprenorphine patch if morphine is ineffective IIIc: 50 mg of tapentadol twice daily |
|||
| ESCEO [93] | Chondroitin sulfate or glucosamine sulfate | Paracetamol or topical NSAIDs | NSAIDs | Intra-articular injection of hyaluronic acid or corticosteroids | Duloxetine | Surgery |
| Neuropathic pain | ||||||
| SIGN [12] | Amitriptyline or gabapentin | Pregabalin | SNRIs | 5% lidocaine | Opioids | 9% capsaicin |
| CDW [49] | Tricyclic antidepressants | Gabapentin/pregabalin or SNRIs (duloxetine) | Other anticonvulsants | Low-dose opioids | ||
| AGS [91] | Duloxetine or pregabalin | |||||
| DHHS [92] | Amitriptyline/imipramine | Gabapentin (1st line) or pregabalin (2nd line) or 0.075% capsaicin cream | Duloxetine or lidocaine plasters (5%-700 mg/plaster) or capsaicin patch (8%-179 mg/plaster) |
|||
| Practice [94] | Duloxetine or TCA | Lidocaine or ketamine | ||||
| NICE [2]. | Antidepressants | Gabapentin or pregabalin | ||||
| NeuPSIG [14] | TCA, SNRI, or gabapentin/pregabalin | Tramadol, lidocaine, and capsaicin patches | Opioids or botulin toxin-A | |||
3.1. Neuropathic and Nociceptive Chronic Pain Treatment
The Scottish Intercollegiate Guidelines Network (SIGN) guidelines [12] suggest a seven-step treatment (Table 3) from history (Step I) to drug treatment (Step IV). To improve drug safety, the authors invite us to evaluate the mechanism of the pain (nociceptive and/or neuropathic pain) and the comorbidity. Step VII suggests an accurate follow-up for exacerbation management [12].
The American Pain Society suggests a multi-modal approach without a differentiation concerning the mechanisms of pain [95]. The authors suggest that opioids (mainly taken via the oral route and with caution in opioid-naïve patients), gabapentin and pregabalin, NSAIDs, and paracetamol are possible options [95].
The Colorado Division of Workers Compensation guidelines [49] suggest a drug reconciliation to avoid interaction or prescription errors. In patients with nociceptive pain, the authors suggest a cyclic treatment with NSAIDs for up to 7 days with non-selective NSAIDs and up to 10 days with COX-2 inhibitors. In patients with neuropathic pain, the authors suggest a four-step process (Table 3), supporting the combination of two drugs from different categories to reduce dosage and side effects (e.g., duloxetine plus pregabalin).
Concerning opioids, no evidence of the superiority of one opioid compared with other drugs has been reported. Long-acting opioids have been found to not be superior to short-acting opioids. Among these compounds, oxycodone seems to be the most abused drug.
Buprenorphine has similar efficacy in comparison with tramadol in patients with moderate–severe musculoskeletal pain and with fentanyl (regarding analgesia and sleep quality) for severe pain.
Muscle relaxants are not suggested for patients with chronic pain due to the habit-forming risk, respiratory depression, and seizure occurrence after sudden withdrawal.
Topical agents including 8% capsaicin (for postherpetic neuralgia), 5% lidocaine plasters, or 8% pump sprays (for diabetic neuropathy and post-herpetic neuralgia), and 0.1% clonidine (for diabetic peripheral neuropathy) can also be used. Among the new compounds, alpha-lipoic acid (600 mg/die for 3–5 weeks) may be used to manage neuropathic pain.
Trigger point injections (of local anesthetics with or without corticosteroids or needling alone) are a possible option for myofascial pain.
In elderly patients, the management of pain is detailed in the American Geriatric Society guidelines [91]. For chronic nociceptive pain, paracetamol (up to 4 g daily) should be the first-line medication. NSAIDs can be used in patients that have experienced failure of efficacy or the development of side effects during paracetamol treatment. Opioids can be used in patients with moderate–severe pain and functional impairment, unresponsiveness to NSAIDs, or contraindications to their use (gastritis, severe liver or renal diseases, or allergy to NSAIDs). Patients should also be assessed for the presence of drug toxicity and drug–drug interaction risks [91].
In patients with neuropathic pain or fibromyalgia, duloxetine pregabalin or gabapentin can be used even if they must be evaluated for the development of side effects; in contrast, tricyclic antidepressants should be avoided due to their high potential for side effects. Combination therapy seems to increase efficacy and reduce toxicity [91].
More recently, the Department of Health and Human Services’ best practices give further information [92], recommending non-opioid or non-pharmacologic therapeutic options in order to avoid chronic treatment with these compounds. For neuropathic pain, the first-line therapy should be chosen among anticonvulsants, SNRIs, amitriptyline, and topical analgesics (capsaicin and lidocaine). For non-neuropathic, non-cancer pain, NSAIDs and paracetamol are the first-line options. Based on patients’ responses, other medication classes include muscle relaxants [92]. Trigger point injection (dry needling injection of local anesthesia) may be useful for the management of headache-associated pain, myofascial pain, and low back pain.
3.2. Neuropathic Chronic Pain
The NeuPSIG guidelines’ last recommendations were published in 2015 [14]. A literature revision was conducted (of 229 studies), performing a meta-analysis that evaluated the number needed to treat (NNT) for 50% patient pain relief. The trial outcomes were poor or modest even for first-line drugs, which was possibly due to overestimations of the placebo effect, scarce patient profiling, and inadequate diagnostic criteria. The new recommendations are summarized in Table 3. The data for the other drugs including other antiepileptics, antidepressants, cannabinoids, tapentadol, and other topical drugs were considered inconclusive. Drugs such as levetiracetam and mexiletine are contraindicated.
The NICE guidelines provide recommendations on chronic neuropathic primary pain (including fibromyalgia). They suggest the use of antidepressants in people ≥ 18 years after the careful evaluation of risk–benefit. Pregabalin or gabapentin and local anesthetics are not suggested, except for in trials for complex regional syndrome. The contraindicated drugs are benzodiazepines, antiepileptics, corticosteroids, trigger point injections, ketamine, NSAIDs, opioids, and paracetamol [2].
The PRACTICE guidelines [94] suggest the use of anticonvulsants, SNRIs, or TCAs in patients with neuropathic pain, with low evidence for the use of SSRIs, NMDA receptor antagonists (e.g., memantine or dextromethorphan), opioids, and muscle relaxants. Topical agents such as capsaicin, lidocaine, and ketamine are also possible options for neuropathic pain. Concerning trigger point injection, it may be considered a multi-modal approach option in patients with myofascial pain (Table 3).
3.3. Nociceptive Chronic Pain
In patients with knee osteoarthrosis (nociceptive pain), the ESCEO group suggests a six-step treatment: chondroitin sulfate and glucosamine sulfate (first step) with or without topical NSAIDs or paracetamol (second step), oral NSAIDs (third step), intra-articular injection of hyaluronic acid or corticosteroids (fourth step), oral SNRI (fifth step), and, finally, surgery (final step) [93].
3.4. Nociplastic Pain
To date, there are no definitive guidelines for nociplastic pain; however, considering its pathogenetic mechanism, antidepressants and pregabalin or gabapentin can be used. NSAIDs can be indicated only in the presence of clinical evidence of inflammation, while opioids are not indicated. In fact, in these patients, it has been suggested that higher concentrations of endogenous opiates and opioid use can worsen hyperalgesia and modify sleep architecture [96,97]. For nociplastic pain (e.g., fibromyalgia, chronic back pain, and complex regional pain) a low dose of naltrexone, an opioid antagonist, by increasing the density of opioid receptors, improved the response to endogenous opiates with an improvement in clinical symptoms [98]. A similar activity could be obtained using methadone, a potent MOPR agonist and weak NMDA receptor antagonist, which seems able to reduce opioid-induced hyperalgesia [99].
4. From Guidelines to Daily Use: The Problem of Safety in Patients with Comorbidity
Since the guidelines suggest better treatments in the presence of a patient with chronic pain, it is possible that this new drug increases the risk of side effects or drug interactions [49] (Table 2).
A detailed clinical evaluation and history allow the formulation of a proper diagnosis and the description of the pain type [3]. Clinicians should be aware of contraindications for each drug and patient comorbidities since this information is essential in therapeutic decision making.
4.1. Kidney Diseases
Some drugs (e.g., gabapentin and pregabalin) need dose adjustments, whereas other molecules must be avoided, especially for long periods (NSAIDs). Amitriptyline is largely excreted by the kidneys, but its serum levels do not seem to change in chronic kidney disease (CKD), similar to carbamazepine [31,100]. Few data are available for the usage of SNRIs in CKD. Duloxetine metabolites are mainly eliminated in the urine [41,100]. A review by Davison et al. suggests that codeine, morphine, oxycodone, tramadol, and hydrocodone should be avoided in advanced CKD (Table 4). Buprenorphine, hydromorphone, fentanyl, and methadone are the suggested compounds due to their inactive metabolism and scarce kidney excretion. Moreover, none of them are significantly removed using dialysis [101].
Table 4.
Effects of analgesic drugs in patients with renal failure. eGFR: glomerular filtrate rate.
| Drug | Kidney Excretion | Effects in Patients with Renal Failure |
|---|---|---|
| Acetaminophen | 90–99% [102] | Not used with eGFR of <10 mL/min [63,100]. |
| Oxycodone | 50% | Dose adjustments [73]. Some authors consider oxycodone unsafe in patients with advanced kidney failure due to its accumulation risk, interactions, and CYP450 polymorphisms [100]. |
| Buprenorphine | 10–30 [103] | Caution with eGFR of <30 mL/min [70]. |
| Fentanyl | 10% or less of active compound and 75% of the total dose. Metabolites are excreted mainly in urine [74]. | Dose monitoring [74]. |
| Methadone | 20–50% as methadone or its metabolites [101] | Contraindicated in patients with severe kidney impairment [66]. Lower doses and longer intervals between administration in patients with kidney impairment [104]. |
| Morphine | 70–80% [105] | eGFR of 10–50 mL/min: dose reduction of 25%; eGFR of <10 mL/min: dose reduction of 50% [104]. One of the worst options in advanced kidney failure due to accumulation risk [100]. |
| Codeine | Mainly excreted in kidneys [106] | Caution is needed. Davison et al. consider codeine one of the worst options in patients with advanced kidney failure due to CYP2D6 polymorphisms and accumulation risk [100]. |
| Hydromorphone | Most of the dose; 7% unmodified drug [107] | Dose reduction [108]. |
| Hydrocodone | Eliminated with its metabolites, mainly in kidneys, percentage not available [71] | Caution/dose reduction [71,77]. Davison considers it one of the worst options in patients with advanced kidney failure, according to CYP2D6 polymorphism-related and variable responses and possible accumulation risk [100]. |
| Tapentadol | 99% [75] | Not recommended in patients with severe insufficiency [75]. |
| Tramadol | 90% [72] | Prolonged interval between doses; do not use long-release formulation [72]. Increase the interval of administration to 12 h, and limit maximum daily dose to 200 mg [109]. |
| Duloxetine | 70% [110] | eGFR of <30 mL/min: do not use [41]. |
| Amitriptyline | 95% [31] | No dose reduction [31,100,111]. |
| 5% lidocaine patch | >85% | eGFR of <30 mL/min (severe kidney impairment): caution [112]. |
| Tizanidine | 60–70% [46] | eGFR of <25 mL/min: start with 2 mg/day [46]. |
| Baclofen | 75% [44] | Start with lower dosages in all patients with mild–moderate kidney impairment, and use only if benefit outweighs the risk in those with severe kidney impairment [44]. |
| Thiocolchicoside | 20% [48] | No dose adjustments [48]. |
| Cyclobenzaprine | 80% | Low dosage [45]. |
| Eperisone | 76.6% [47] | eGFR of <25 mL/min: low dosage, max. 150 mg daily [47]. |
| Pregabalin | 99% | eGFR of 30–59 mL/min: 300 mg/daily. eGFR of 15–29 mL/min: 150 mg/daily. eGFR of <15 mL/min: 75 mg/daily [113]. |
| Gabapentin | 99% | eGFR of 30–59 mL/min: 1400 mg/daily. eGFR of 15–29 mL/min: 700 mg/daily. eGFR of <15 mL/min: 300 mg/daily [113]. |
4.2. Hepatic Failure
Paracetamol is a well-known hepatotoxic drug since all NSAIDs can induce liver injury [63,114]. Dastis et al. [114] suggested a reduction in the paracetamol dose to a maximum of 2 g/daily in patients with non-alcoholic cirrhotic liver disease, avoiding its coadministration with alcohol (due to the increased production of N-acetyl-p-benzoquinone imine (NAPQI)). However, in our opinion, this is a risk, and caution is recommended in patients with hepatic insufficiency, hepatitis, concomitant treatment altering hepatic function, a deficit in glucosium-6-phospate-dehydrogenase (GSPD), and hemolytic anemia [63]. The use of NSAIDs in cirrhosis or hepatic impairment may be very dangerous. Aspirin shows dose-dependent liver toxicity, and other drugs (nimesulide, diclofenac, and sulindac) are also associated with this effect. Nevertheless, hepatotoxicity is a class effect, which is often related to idiosyncratic mechanisms. Furthermore, NSAIDs may increase the bleeding risk and the worsening of kidney function in hepatorenal syndrome. Their interaction with diuretics used in the management of cirrhosis may further impair renal effectiveness and act as an obstacle to the management of ascites. Aspirin is contraindicated in cases of severe hepatic insufficiency. Caution and, eventually, dose adjustment are required for other NSAIDs [114,115].
Opioids are also metabolized by liver cytochromes, and caution is required in clinical practice because they can induce DDIs [114], hepatic encephalopathy, and are contraindicated in patients with liver failure [73] (Table 5). Among this class, morphine (which undergoes glucuronidation and is affected later by hepatic insufficiency in comparison with the CYP450 system) and intravenous fentanyl appear to be the safest options.
Finally, muscle relaxants are variously involved in liver failure (Table 5). Metaxalone is contraindicated in patients with significantly impaired hepatic disease [49].
In this scenario, it is not futile to remember that the concomitant consumption of possible hepatotoxic drugs such as statins, antimicrobials, amiodarone, allopurinol, and contraceptives must be taken into account [88,116].
Table 5.
Effects of analgesic drugs in patients with hepatic failure.
| Drug | Biliary Excretion | Effect in Patients with Hepatic Insufficiency |
|---|---|---|
| Acetaminophen | 1–10% [102] | Contraindicated in patients with severe hepatic insufficiency. Caution is needed in the cases of mild and moderate hepatic insufficiency [63]. |
| Oxycodone | Not clearly estimated but relevant; important hepatic metabolism [73,117] | Oxycodone is contraindicated in patients with moderate–severe hepatic impairment on the label. Some authors refer to the necessity to reduce dosage and prolong intervals [73]. |
| Buprenorphine | 70% [118] | Buprenorphine should be used with caution in cases of mild–moderate hepatic impairment and is contraindicated in severe forms [70]. |
| Fentanyl | Little biliary excretion but strong hepatic metabolism [119] | Concerning fentanyl, dose reduction may be necessary [74]. |
| Hydromorphone | 1% in feces [107] | Hydromorphone is contraindicated in patients with severe hepatic impairment, whereas dose reduction is suggested in those with moderate impairment, and caution is needed in those with mild impairment. It should be avoided in patients with hepatorenal syndrome [108]. |
| Methadone | 10–45% of the metabolite [101] | Methadone is contraindicated in patients with severe hepatic impairment. Caution (lower doses and prolonged intervals between administration) is needed in those with mild–moderate illness, even if other authors describe no dose adjustments [66,104,114]. |
| Tapentadol | 1% [120] | Tapentadol is contraindicated in patients with severe hepatic impairment. No dose adjustments are required in those with mild hepatic impairment, whereas low doses and prolonged dosing intervals are recommended in patients with moderate illness [75]. |
| Tramadol | 10% [121] | Tramadol is not recommended in patients with severe hepatic impairment, and some authors suggest the prolongation of dosing intervals or dose reduction in those with mild–moderate forms [72,114]. American label suggests that the recommended dose for adult patients with severe hepatic impairment is 50 mg every 12 h [109]. |
| Hydrocodone | Low biliary excretion, data not available; relevant hepatic metabolism [71] | Dose reduction [77]. |
| Morphine and codeine | 5–10% morphine in feces [67]; similar percentages for codeine, which is then converted into morphine [106] | Morphine is contraindicated in patients with severe hepatic impairment, and dosage should be reduced by 25% in those with moderate hepatic impairment [67]. Other authors suggest dose reduction and prolongation of dose intervals for oral formulation and dose reduction only for intravenous formulation. It should be avoided in hepatorenal syndrome [114]. Codeine use is not recommended for the possible lack of analgesic effect [114]. |
| Duloxetine | 20% [110] | Duloxetine must not be used in patients with hepatopathy and alterations in hepatic function. Moreover, this drug is hepatotoxic [41,114]. |
| Amitriptyline | Small quantity [31] | Amitriptyline is contraindicated in liver diseases [31]. Other authors suggest its use with caution, even if it is worse-tolerated than nortriptyline and desipramine [114]. |
| Lidocaine 5% patch | Minor quote | Severe hepatic impairment: caution [112]. |
| Tizanidine | 20% [122] | Tizanidine is generally contraindicated in patients with relevant hepatic compromise. It should be used only if the benefit outweighs the risk [46]. |
| Baclofen | 25% [123] | Baclofen is not metabolized by liver, but it is hepatotoxic: caution is needed [44]. |
| Thiocolchicoside | 80% [48] | It may increase liver enzymes or cause hepatic damage [48]. |
| Cyclobenzaprine | Minor quote | It may increase liver enzymes or cause hepatic damage [45]. |
| Eperisone | 24.4% [47] | Eperisone is contraindicated in patients with severe hepatic failure, and caution/dose adjustment may be needed in other forms (maximum 150 mg daily dose) [47]. |
| Pregabalin | None | No dose adjustment [19,30]. |
| Gabapentin | None | No dose adjustment [19,30]. |
4.3. Hypertension
Managing patients with pain and hypertension is common. Some drugs including duloxetine (which is contraindicated in patients with uncontrolled blood pressure levels), muscle relaxants, gabapentin, NSAIDs, tramadol, and tapentadol may increase blood pressure levels [19,42,72,75,124]. Other substances such as other opioids, baclofen, cyclobenzaprine, and tizanidine may decrease blood pressure [44,46,73]. Therefore, it is important to acquire basal blood pressure levels and monitor them throughout the treatment duration.
4.4. Bone Fracture
Opioids and NSAIDs can increase the risk of bone fragility [90]. A meta-analysis by Ping et al. [125] showed an increased rate of hip fracture in opioid users. A retrospective study by George and colleagues evidenced an increased risk of non-union with COX-2 inhibitors (or other NSAIDs acting on COX-2 activity) and opioids but not with other NSAIDs [126]. The coadministration with other drugs associated with fracture risk (e.g., glucocorticoids, proton pump inhibitors, loop diuretics, nitrates, SSRI/SNRIs, or sedatives) may facilitate this eventuality [127]. Opioids may generate bone metabolism alteration through their deep effects on the endocrine system [125]. Although drugs such as proton pump inhibitors may increase fracture risk through a biochemical mechanism, opioids, other CNS-acting medications, and antihypertensive drugs (also acting on calcium excretion) determine falls associated with traumas and injuries [127].
4.5. Cardiovascular Toxicity
Heart rate and QT interval may be affected by different drugs implicated in pain control, and patients suffering from arrhythmias may suffer due to this. Amitriptyline, cyclobenzaprine, and eperisone are associated with tachycardia or palpitations [31], whereas opioids can induce both bradycardia [128] and tachycardia [72,73]. Amitriptyline and cyclobenzaprine are contraindicated in patients with cardiac insufficiency, rhythm alterations, and in the post-ischemic period [31]. Cyclobenzaprine is also contraindicated in those with hyperthyroidism, myocardial infarction, monoamine oxidase inhibitors coadministration, and arrhythmias [45].
4.6. Vertigo
Patients with pain and vertigo are not easy to treat since the use of some pain medications may increase the possibility of falls. All the CNS-acting drugs including anticonvulsants (in particular, pregabalin and gabapentin), antidepressants, opioids, and muscle relaxants share this risk, and the management of these patients may not be easy [31,41,49,90]. The resolution of the underlying vestibular pathology and the eventual choice of therapeutic options to manage vertigo must take into account possible interactions with pain medications [55]. It is interesting to observe that amitriptyline acts as an antagonist on histamine receptors, increasing somnolence [31], whereas some opioids (mainly morphine and codeine) may increase histamine release [76]. Antihistamines are part of the management of dizziness and, particularly for amitriptyline, coadministration may be a matter of interaction [55].
4.7. Respiratory Diseases
Patients with chronic obstructive pulmonary disease, asthma, and obstructive sleep apnea syndrome should avoid opioids [73,129]. The concomitant administration of CNS-depressing drugs may be dangerous in reducing the respiratory drive [130,131].
4.8. Gastrointestinal Diseases
NSAIDs are associated with gastrointestinal bleeding and ulcers. COX-2 inhibitors seem to be the safest option. However, they are associated with minor but significant gastrointestinal toxicity according to COX-2’s role in mucosal repair [132]. Amitriptyline, duloxetine, muscle relaxants, pregabalin, and gabapentin (this last drug may also produce gingivitis) are commonly associated with mild to moderate gastrointestinal symptoms including diarrhea, stypsis, dyspepsia, vomiting, and nausea [19,30,31,41,44,45,46,48]. Amitriptyline is associated with stypsis, according to its anticholinergic effect [31], and may interact with opioids. Antidepressant drugs are contraindicated in patients with pyloric stenosis and other similarly obstructive conditions [31]. Stypsis is a crucial issue in this setting since opioids are commonly associated with this side effect [133]. Using the lowest opioid dosage and managing stypsis through dietary changes, stool softeners, or, eventually, laxatives may be a proper strategy. It is important to note that tapentadol is associated with a reduced rate of constipation in comparison with other opioids. Furthermore, long-acting formulations are more probably related to this adverse event compared with short-acting ones. Opioid rotation may minimize this adverse event. The use of peripheral μ-opioid receptor antagonists (PAMORAs) such as naloxegol, naltrexone, and naldemedine is another useful option [49,134,135]. Oxycodone is a particular drug since it is a weak/moderate opioid at the dosage of 5 mg but a strong opioid at higher doses. Oxycodone was associated with a rate of constipation of 6.1% in a retrospective study by Staats and colleagues [136]. Higher dosages are generally associated with an increased probability of adverse events. Interestingly, the co-formulation of oxycodone and naloxone (a μ-opioid receptor antagonist) is a clinically relevant option to minimize gastrointestinal side effects (stypsis) and increase therapeutic adherence [137].
4.9. Sexual Dysfunctions
Pain may impair the psychosocial life of an individual, also affecting his relationships [3]. Long-term use of opioids can increase the release of prolactin (PRL) and reduce the release of GnRH (gonadotropin-releasing hormone) with the development of gynecomastia, erectile dysfunction, and reduction in sexual desire in men and amenorrhea in women. Therefore, hypogonadism and endocrine alterations are crucial issues, especially in chronic high-dose consumption [73,138]. Furthermore, amitriptyline, duloxetine, gabapentin, and pregabalin are commonly related to erectile dysfunction. These side effects have rarely been described during muscle relaxants treatment [44,45]. Other adverse events such as amenorrhea and gynecomastia are less frequent but possible with the aforementioned substances [19,30,31,41]. Patients with erectile dysfunction (e.g., with diabetic neuropathy-vasculopathy) consuming other sexuality-affecting medications (e.g., β-blockers) may require deprescription or a change in treatment. For example, nebivolol is the more indicated β-blocker in erectile dysfunction patients due to its action on nitric oxide [139]. The complaint of gynecomastia with drugs such as spironolactone, calcium antagonists, some antibiotics, efavirenz, or antipsychotics is a difficult issue [140]. Similar sexuality concerns are possible with women affected by menstrual cycle alterations [138].
4.10. Urinary Symptoms
Patients with benign prostatic hyperplasia (BPH) or urinary obstructive diseases may experience a worsening of their symptoms with amitriptyline, eperisone, or cyclobenzaprine [31,45,47]. Duloxetine and baclofen (also determining enuresis) are frequently associated with dysuria and pollakiuria [41,44]. Opioids have minimal anticholinergics effects, and their binding to spinal receptors may determine bladder relaxation effects and a low rate of urinary retention [141].
4.11. Other Clinical Conditions
Patients affected by glaucoma must avoid amitriptyline, whereas caution is needed with duloxetine and cyclobenzaprine [31,41,45]. Amitriptyline and baclofen are (rarely) sometimes associated with variations of glycemia [31,44].
5. Discussion
In the management of nociceptive or neuropathic pain, opioid use can induce the development of severe adverse events requiring careful monitoring [90].
Gabapentin and pregabalin, used for neuropathic and nociplastic pain, have similar indications and side effects (e.g., dizziness, diplopia, blurred vision, and psychiatric, neurological, and cutaneous adverse events). However, gabapentin is related to more side effects (infections, leukopenia, anorexia, and increased appetite) and to a more complex administration scheme [19,20]. Furthermore, these drugs seem to be ideal in poly-treated patients but have an important potential for psychiatric adverse events [142]. Pregabalin seems more effective for central neuropathic pain and fibromyalgia [143]. According to a meta-analysis by Salerno et al. [144], antidepressants seem to be more effective for relieving pain symptoms in patients with neuropathic pain with a worse functional outcome.
The concomitant administration of more than one pain drug is useful in order to reduce dosage and side effects, but clinicians must be aware of eventual interactions [12,49].
The role of transporters in DDI is complex. For example, fentanyl, methadone, and morphine are substrates of P-glycoprotein, and this may increase the risk of interactions. The presence of multiple pharmacogenomic polymorphisms may strongly influence drug response and therapeutic efficacy [145,146].
Concerning nociplastic or primary chronic pain, they are often difficult to treat. A review of non-opioid pharmacological agents for the management of various chronic pain conditions (including fibromyalgia, low back pain, and chronic headaches) reported mostly small improvements (e.g., 5–20 points on a 0–100 scale) for gabapentinoids, SNRIs, and NSAIDs for pain and function in the short term, with intermediate and long-term outcomes infrequently assessed [29]. In the SIGN guidelines, fluoxetine (and other antidepressants such as citalopram, escitalopram, and sertraline in the NICE guidelines) may be considered for chronic primary pain/fibromyalgia [12].
Opioids can be used in the management of nociceptive and neuropathic pain, but their use is related to the development of tolerance and dependence and is also contraindicated in several clinical conditions (severe respiratory insufficiency, acute alcoholism and delirium tremens, CNS drugs, children and adolescents of <16 years, breastfeeding, severe CNS compromise, paralytic ileus, acute abdominis, pulmonary heart disease, and chronic stypsis) [70]. Codeine is also contraindicated in CYP2D6 ultrarapid metabolizers [65]. Tramadol is contraindicated in epilepsy and MAOI (monoaminoxidase inhibitors) concomitant treatment [72]. Hydromorphone is also contraindicated in severe gastrointestinal stenosis, MAOI treatment, and coma [108]; methadone in cardiopathy, uncontrolled diabetes, porphyria, hypotension, hypovolemia, intracranial hypertension, or cranial traumas [66]; and morphine in intracranial hypertension, cranial traumas, uncontrolled epilepsy and convulsion, MAOI treatment, and biliary surgery [67].
Several other compounds can be used in the management of pain even if their use is not reported in guidelines.
Cannabis-based medicines hold promise but require formal studies before their widespread use in patients at high risk for adverse effects. Products are labeled on the basis of tetrahydrocannabinol and cannabidiol contents; these compounds are indicated for the management of neurodegenerative muscle diseases but not for pain even if efficacy has been suggested in patients with nociplastic and neuropathic pain [147]. A systematic review and meta-analysis by Aviram and Samuelly-Leichtag showed that cannabis may have a certain efficacy in patients with chronic pain, especially neuropathic and nociplastic pain, and that inhalation rather than oral consumption may reduce gastrointestinal adverse events [148]. Adverse effects include an increase in heart rate (and risk of myocardial infarction), dizziness, seizure, psychosis, dependency, euphoria, or other psychiatric adverse events [49].
Palmitoylethanolamide (PEA) and acetyl-L-carnitine (ALC) may be used in patients with nociplastic and neuropathic pain [149]. L-acetyl-carnitine is an endogenous substance that modulates pain via different mechanisms: stimulation of mitochondrial function and repair factors such as nerve growth factor, antioxidant activity, and activation of metabotropic glutamate receptor 2 (mGlu2). An experimental study by Cuccurazzu et al. [150] showed that ALC activates NF-kB, increasing the expression of mGlu2 and suggesting a pro-neurogenic effect. Experimental data show that ALC modulates neuropathic pain, and multiple administration is necessary to obtain analgesia [151]. Similar results were obtained by Parisi et al. [152], evidencing an excellent safety profile and good efficacy. However, Rolim and colleagues assessed the uncertainty of its efficacy in patients with diabetic neuropathy [153]. Gastrointestinal side effects are the most commonly reported side effects [154]. PEA exerts its action on the endocannabinoid system and reduces inflammation. This molecule stimulates the effects of endo- or phytocannabinoids acting on peroxisome proliferator-activated receptor α (PPAR-α), transient receptor potential vanilloid type 1 (TRPV1), and cannabinoid receptors. Moreover, it reduces the activity of inflammatory enzymes and mast cell degranulation.
Emerging treatments such as neuromodulation have theoretical potential but have little data on their effectiveness. This therapy includes deep brain and motor cortex stimulation, non-invasive treatments (transcranial magnetic stimulation, transcranial direct-current stimulation, and transcutaneous electrical nerve stimulation), and peripheral nerve stimulation. Low–moderate quality evidence is available for peripheral nerve stimulation for neuropathic pain. However, few trials have been conducted, and some of these techniques are not approved for clinical use [155].
Other procedural (exercise programs, psychological therapy, and social interventions), physical (transcutaneous electrical nerve stimulation (TENS), ultrasound, interferential therapy, manual therapy, bracing, cold, and heat), or interventional treatments (radiofrequency, acupuncture, and corticosteroid–anesthetics injection at various levels including epidural steroid injections, sacroiliac joint corticosteroid–anesthetics coadministration, botulinum injections, cryoneuroablation, thermal intradiscal procedures, peripheral nerve blocks, sympathetic nerve blocks, intrathecal medication pumps, joint injections, and vertebral augmentation) are other therapeutic opportunities depending on the clinical setting, guideline recommendations, and data from larger clinical trials [49,82,92].
Other techniques are arising. Diamagnetic therapy using pulsed magnetic fields may display an important activity on nociceptive, neuropathic, and nociplastic pain through its effect on inflammatory cytokines, neuromodulation, and neuroprotective effects. Moreover, its effect on liquids is very useful for edematous conditions [156,157,158,159]. We observed that the use of a high-intensity low-frequency magnetic field was effective in patients with ulcers and complex regional syndrome, reducing pain levels and improving their clinical status [160,161].
Oxygen–ozone therapy may be useful in the management of patients with osteoarthritis and low back pain, whereas other musculoskeletal disorders must be studied better. The mild oxidative stress induced by oxygen–ozone may activate the Nrf2 protein, through its separation from Kelch-like ECH-associated protein 1 (Keap-1). This effect leads to an increase in transcription mediated by Nrf2, which enhances genes involved in inflammation reduction and oxidative stress response. Moreover, oxygen–ozone may have an important effect on hypoxic tissues via its action on prostaglandins, nitric oxide, and adenosine, which leads to vasodilation. Lastly, the increased production of 2,3 diphosphoglycerate and lipid peroxidation may shift the hemoglobin dissociation curve to the right [162].
Finally, an important point in the management of chronic pain is represented by the biopsychosocial approach. In this model, pain is viewed as a dynamic interaction among and within the biological, psychological, and social factors unique to each person. According to this model, the treatment of the “whole” person (considering anxiety, depression, and stress) is far more important than focusing merely on a disease. This approach could be an add-on treatment to common drug use. A Cochrane review reported that cognitive behavioral therapy has small beneficial effects in reducing pain, disability, and distress, whereas the benefits from behavioral therapy were uncertain because of the poor quality of the studies included [163].
Our review has some limitations. Firstly, we did not include some non-pharmacological treatments such as acupuncture and bidimensional techniques since they were not included in the guidelines. The beginning of more solid studies showing the relevance of their biological effects is warranted. Secondly, many of the cited guidelines did not indicate the pain level/severity for which pharmacological treatment is recommended. This fact allows subjective decisions that could be a limit in clinical practice. Moreover, the synergic role of drugs and the importance of gender difference is not clarified in the clinical guidelines. In this context, the role of the volume of distribution has not been evaluated. These considerations may be key points in future recommendations to reduce drug dosage and obtain appropriate therapy. The guidelines did not establish an adequate or ideal dosage for each patient but simply describe an optimal range, including the initial dosage and maximum posology. Moreover, we did not enclose in this review chronic pain in patients with cancer or headache, and these are very important points that need a more appropriate evaluation. Finally, we did not evaluate the role of gender (e.g., economic, political, and religious status, and sex) in chronic pain as well as the role of genetic factors. To date, the guidelines do not consider this aspect; as a result, to date, it is very hard to obtain personalized therapy.
In conclusion, even if the guidelines represent an important guide for the common treatment of patients with pain, they have several limitations: (i) they do not evaluate the characteristics of each patient (age, gender, comorbidity, and polytherapy); (ii) there is no comparison with a single intervention at the time of their publications; (iii) they do not evaluate the possibility of drug interactions and genetic variability; (iv) very few guidelines evaluate nociplastic pain, and no guidelines evaluate pain combinations; and (v) no guidelines report the role of alternative treatments (other compounds or techniques). Therefore, the guidelines must be revised considering these factors and the possibility of integrating these suggestions into daily clinical activity even if there is no strong evidence for the absence of double-blind randomized clinical trials. A crucial point is the pathophysiological and diagnostic comprehension of pain and understanding the environmental, genetic, and pathological reasons that cause symptomatology. This allows physicians to make the right decisions, taking into account patients’ genders, comorbidities, and concomitant medications.
Author Contributions
Conceptualization, G.M., M.E., F.M., G.D.S., A.C. and L.G.; data curation, G.M., C.V., L.M., C.G., C.P. and L.G.; writing—original draft preparation, G.M., C.V., C.P., L.M. and L.S.; writing—review and editing, M.E., F.M., G.D.S. and L.G.; supervision, A.C. and L.G.; project administration, F.M., M.E. and L.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The Revised IASP definition of pain: Concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence (NICE) Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain. National Institute for Health and Care Excellence (NICE); London, UK: 2021. [PubMed] [Google Scholar]
- 3.Cohen S.P., Vase L., Hooten W.M. Series Chronic Pain 1 Chronic pain: An update on burden, best practices, and new advances. Lancet. 2021;397:2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 4.Fitzcharles M.-A., Cohen S.P., Clauw D.J., Littlejohn G., Usui C., Häuser W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet. 2021;397:2098–2110. doi: 10.1016/S0140-6736(21)00392-5. [DOI] [PubMed] [Google Scholar]
- 5.Gallelli L., Galasso O., Falcone D., Southworth S., Greco M., Ventura V., Romualdi P., Corigliano A., Terracciano R., Savino R., et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthr. Cartil. 2013;21:1400–1408. doi: 10.1016/j.joca.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Gallelli L., Avenoso T., Falcone D., Palleria C., Peltrone F., Esposito M., De Sarro G., Carotenuto M., Guidetti V. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313–324. doi: 10.1111/head.12162. [DOI] [PubMed] [Google Scholar]
- 7.Gallelli L., Colosimo M., Pirritano D., Ferraro M., De Fazio S., Marigliano N.M., De Sarro G. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2007;27:115–122. doi: 10.2165/00044011-200727020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cryer B., Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:413–421. doi: 10.1016/S0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 9.Walker C. Are All Oral COX-2 Selective Inhibitors the Same? A Consideration of Celecoxib, Etoricoxib, and Diclofenac. Int. J. Rheumatol. 2018;2018:1302835. doi: 10.1155/2018/1302835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado G.C., Maher C.G., Ferreira P.H., Pinheiro M.B., Lin C.W.C., Day R.O., McLachlan A.J., Ferreira M.L. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drewes A.M., Jensen R.D., Nielsen L.M., Droney J., Christrup L.L., Arendt-Nielsen L., Riley J., Dahan A. Differences between opioids: Pharmacological, experimental, clinical and economical perspectives. Br. J. Clin. Pharmacol. 2013;75:60–78. doi: 10.1111/j.1365-2125.2012.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SIGN Scottish Intercollegiate Guidelines Network. Management of Chronic Pain. [(accessed on 17 July 2022)]. Available online: http://www.sign.ac.uk/pdf/SIGN136.pdf.
- 13.Tesfaye S., Sloan G., Petrie J., White D., Bradburn M., Julious S., Rajbhandari S., Sharma S., Rayman G., Gouni R., et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): A multicentre, double-blind, randomise. Lancet. 2022;400:680–690. doi: 10.1016/S0140-6736(22)01472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Gilron I., Haanpaa M., Hansson P., Jensen T.S., et al. Pharmacotherapy for neuropathic pain in adults: Systematic review, meta-analysis and updated NeuPSig recommendations. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade C. Augmentation of venlafaxine with bupropion: Risks associated with a triple monoamine reuptake inhibition approach to partially responsive depression. J. Clin. Psychiatry. 2013;74:119–121. doi: 10.4088/JCP.13f08348. [DOI] [PubMed] [Google Scholar]
- 16.Mathieson S., Lin C.W.C., Underwood M., Eldabe S. Pregabalin and gabapentin for pain. BMJ. 2020;369:m1315. doi: 10.1136/bmj.m1315. [DOI] [PubMed] [Google Scholar]
- 17.Roy P.J., Weltman M., Dember L.M., Liebschutz J., Jhamb M. Pain management in patients with chronic kidney disease and end-stage kidney disease. Curr. Opin. Nephrol. Hypertens. 2020;29:671–680. doi: 10.1097/MNH.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson K., Marshman L.A.G., Plummer D., Downs E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults with Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2019;76:28–34. doi: 10.1001/jamaneurol.2018.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Gabapentin. [(accessed on 24 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001771_038547_RCP.pdf&sys=m0b1l3.
- 20.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Pregabalin. [(accessed on 24 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003891_043719_RCP.pdf&sys=m0b1l3.
- 21.Di Stefano G., La Cesa S., Truini A., Cruccu G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J. Headache Pain. 2014;15:34. doi: 10.1186/1129-2377-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz B., Dimova S., Zhang Y., Chellun D., De Backer M., Gasalla T. Tolerability and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy and concomitant psychiatric conditions: Post hoc analysis of a prospective, randomized, double-blind trial. Epilepsy Res. 2020;159:106220. doi: 10.1016/j.eplepsyres.2019.106220. [DOI] [PubMed] [Google Scholar]
- 23.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Carbamazepina. [(accessed on 24 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001561_033878_RCP.pdf&sys=m0b1l3.
- 24.Derry S., Asc R., Cole P., Tan T., Ra M., Derry S., Asc R., Cole P., Tan T., Ra M. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017;1:CD007393. doi: 10.1002/14651858.CD007393.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derry S., Wiffen P.J., Moore R.A., Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014;2014:CD010958. doi: 10.1002/14651858.CD010958.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilron I., Chaparro L.E., Tu D., Holden R.R., Milev R., Towheed T., Dumerton-Shore D., Walker S. Combination of pregabalin with duloxetine for fibromyalgia: A randomized controlled trial. Pain. 2016;157:1532–1540. doi: 10.1097/j.pain.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 27.Hur G., Hwang E.K., Moon J., Ye Y., Shim J., Park H., Kang K. Oral Muscle Relaxant May Induce Immediate Allergic Reactions. Yonsei Med. J. 2012;53:863–865. doi: 10.3349/ymj.2012.53.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye A.D., Jones M.R., Viswanath O., Candido K.D., Boswell M.V., Soin A., Sanapati M., Harned M.E., Simopoulos T.T., Sudhir Diwan S.L., et al. ASIPP Guidelines for Sedation and Fasting Status of Patients Undergoing Interventional Pain Management Procedures. [(accessed on 10 December 2022)]. Available online: https://www.painphysicianjournal.com/linkout?issn=&vol=22&page=201. [PubMed]
- 29.McDonagh M.S., Selph S.S., Buckley D.I., Holmes R.S., Mauer K., Ramirez S., Hsu F.C., Dana T., Fu R., Chou R. Nonopioid Pharmacologic Treatments for Chronic Pain. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2020. [PubMed] [Google Scholar]
- 30.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Lyrica. [(accessed on 13 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003199_043740_RCP.pdf&sys=m0b1l3.
- 31.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Amitriptilina. [(accessed on 25 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_007046_019906_RCP.pdf&sys=m0b1l3.
- 32.Dorian P., Sellers E.M., Reed K.L., Warsh J.J., Hamilton C., Kaplan H.L., Fan T. Amitriptyline and ethanol: Pharmacokinetic and pharmacodynamic interaction. Eur. J. Clin. Pharmacol. 1983;25:325–331. doi: 10.1007/BF01037943. [DOI] [PubMed] [Google Scholar]
- 33.Kopera H. Anticholinergic and blood pressure effects of mianserin, amitriptyline and placebo. Br. J. Clin. Pharmacol. 1978;5:29S–34S. [PMC free article] [PubMed] [Google Scholar]
- 34.Kamińska K., Lenda T., Konieczny J., Wardas J., Lorenc-Koci E. Interactions of the tricyclic antidepressant drug amitriptyline with L-DOPA in the striatum and substantia nigra of unilaterally 6-OHDA-lesioned rats. Relevance to motor dysfunction in Parkinson’s disease. Neurochem. Int. 2018;121:125–139. doi: 10.1016/j.neuint.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Richelson E. Tricyclic antidepressants and histamine H1 receptors. Mayo Clin. Proc. 1979;54:669–674. [PubMed] [Google Scholar]
- 36.Farzam K., Tivakaran V.S. QT Prolonging Drugs. [(accessed on 14 August 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK534864/
- 37.Shah A., Yousuf T., Ziffra J., Zaidi A., Raghuvir R. Diphenhydramine and QT prolongation—A rare cardiac side effect of a drug used in common practice. J. Cardiol. Cases. 2015;12:126–129. doi: 10.1016/j.jccase.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francescangeli J., Karamchandani K., Powell M., Bonavia A. The serotonin syndrome: From molecular mechanisms to clinical practice. Int. J. Mol. Sci. 2019;20:2288. doi: 10.3390/ijms20092288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.AIFA Agenzia Italiana del Farmaco Medrol-Riassunto delle Caratteristiche del Prodotto. [(accessed on 14 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000040_014159_RCP.pdf&sys=m0b1l3.
- 40.AIFA Agenzia Italiana del Farmaco Cymbalta-Riassunto delle Caratteristiche del Prodotto. [(accessed on 14 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001230_036683_RCP.pdf&sys=m0b1l3.
- 41.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Duloxetina. [(accessed on 25 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000813_043843_RCP.pdf&sys=m0b1l3.
- 42.Park K., Kim S., Ko Y.J., Park B.J. Duloxetine and cardiovascular adverse events: A systematic review and meta-analysis. J. Psychiatr. Res. 2020;124:109–114. doi: 10.1016/j.jpsychires.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Bixby A.L., VandenBerg A., Bostwick J.R. Clinical Management of Bleeding Risk With Antidepressants. Ann. Pharmacother. 2019;53:186–194. doi: 10.1177/1060028018794005. [DOI] [PubMed] [Google Scholar]
- 44.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Baclofen. [(accessed on 14 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002322_037930_RCP.pdf&sys=m0b1l3.
- 45.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Flexiban. [(accessed on 14 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000143_025327_RCP.pdf&sys=m0b1l3.
- 46.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Navizan. [(accessed on 14 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000239_039422_RCP.pdf&retry=0&sys=m0b1l3.
- 47.AIFA Agenzia Italiana del Farmaco Expose-Riassunto delle Caratteristiche del Prodotto. [(accessed on 13 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004375_028631_RCP.pdf&sys=m0b1l3#:~:text=L’eperisonecloridratoèun,deldoloreadessaassociato.
- 48.AIFA Agenzia Italiana del Farmaco MuscoRil- Riassunto delle caratteristiche del prodotto. [(accessed on 13 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_008055_015896_RCP.pdf&sys=m0b1l3.
- 49.Colorado Division of Workers’ Compensation . Chronic Pain Disorder Medical Treatment Guideline. Colorado Division of Workers’ Compensation; Denver, CO, USA: 2017. pp. 1–178. [Google Scholar]
- 50.NIH National Institutes of Health Metaxalone-Label. [(accessed on 14 August 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b3a4f6bc-abd4-4b8e-970f-59b3aa6f17a0.
- 51.NIH National Institutes of Health Methocarbamol-Label. [(accessed on 14 August 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42c0a177-7d62-4bcf-9fce-7dd484cda4d5.
- 52.Samer C.F., Lorenzini K.I., Rollason V., Daali Y., Desmeules J.A. Applications of CYP450 testing in the clinical setting. Mol. Diagnosis Ther. 2013;17:165–184. doi: 10.1007/s40291-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., Woodruff P.G., Mauger D.T., Erzurum S.C., Johansson M.W., et al. COVID-19–related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Awa K., Satoh H., Hori S., Sawada Y. Prediction of time-dependent interaction of aspirin with ibuprofen using a pharmacokinetic/pharmacodynamic model. J. Clin. Pharm. Ther. 2012;37:469–474. doi: 10.1111/j.1365-2710.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- 55.Di Mizio G., Marcianò G., Palleria C., Muraca L., Rania V., Roberti R., Spaziano G., Piscopo A., Ciconte V., Di Nunno N., et al. Drug—Drug Interactions in Vestibular Diseases, Clinical Problems, and Medico-Legal Implications. Int. J. Environ. Res. Public Health. 2021;18:12936. doi: 10.3390/ijerph182412936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webster J. Interactions of NSAIDs with Diuretics and β-Blockers: Mechanisms and Clinical Implications. Drugs. 1985;30:32–41. doi: 10.2165/00003495-198530010-00004. [DOI] [PubMed] [Google Scholar]
- 57.Chiarella G., Marcianò G., Viola P., Palleria C., Pisani D., Rania V., Casarella A., Astorina A., Scarpa A., Esposito M., et al. Nutraceuticals for Peripheral Vestibular Pathology: Properties, Usefulness, Future Perspectives and Medico-Legal Aspects. Nutrients. 2021;13:3646. doi: 10.3390/nu13103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore N., Pollack C., Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–1075. doi: 10.2147/TCRM.S79135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalhor H.R., Taghikhani E. Probe into the Molecular Mechanism of Ibuprofen Interaction with Warfarin Bound to Human Serum Albumin in Comparison to Ascorbic and Salicylic Acids: Allosteric Inhibition of Anticoagulant Release. J. Chem. Inf. Model. 2021;61:4045–4057. doi: 10.1021/acs.jcim.1c00352. [DOI] [PubMed] [Google Scholar]
- 60.Brouwers J.R.B.J., de Smet P.A.G.M. Pharmacokinetic-Pharmacodynamic Drug Interactions with Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacokinet. 1994;27:462–485. doi: 10.2165/00003088-199427060-00005. [DOI] [PubMed] [Google Scholar]
- 61.Hersh E.V., Pinto A., Moore P.A. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin. Ther. 2007;29:2477–2497. doi: 10.1016/j.clinthera.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Radwan M.A. Zidovudine, Diclofenac and Ketoprofen Pharmacokinetic Interactions in Rats. J. Pharm. Pharmacol. 2010;52:665–669. doi: 10.1211/0022357001774507. [DOI] [PubMed] [Google Scholar]
- 63.AIFA . Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Tachipirina. AIFA; Rome, Italy: 2022. [Google Scholar]
- 64.Dordoni B., Willson R.A., Thompson R.P.H., Williams R. Reduction of Absorption of Paracetamol by Activated Charcoal and Cholestyramine: A Possible Therapeutic Measure. Br. Med. J. 1973;3:86–87. doi: 10.1136/bmj.3.5871.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.AIFA Agenzia Italiana del Farmaco Paracetamolo e Codeina-Riassunto delle Caratteristiche del Prodotto. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002753_037351_RCP.pdf&sys=m0b1l3.
- 66.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Metadone. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000549_029610_RCP.pdf&sys=m0b1l3.
- 67.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Morfina Cloridrato. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000141_030677_RCP.pdf&sys=m0b1l3.
- 68.Inturrisi C.E., Jamison R.N. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 69.Overholser B.R., Foster D.R. Opioid pharmacokinetic drug-drug interactions. Am. J. Manag. Care. 2011;17:276–287. [PubMed] [Google Scholar]
- 70.AIFA Agenzia Italiana del Farmaco Buprenorfina-Riassunto delle Caratteristiche del Prodotto. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002322_039747_RCP.pdf&retry=0&sys=m0b1l3.
- 71.Cardia L., Calapai G., Quattrone D., Mondello C., Arcoraci V., Calapai F., Mannucci C., Mondello E. Preclinical and clinical pharmacology of hydrocodone for chronic pain: A mini review. Front. Pharmacol. 2018;9:1122. doi: 10.3389/fphar.2018.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Contramal. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000045_028853_RCP.pdf&sys=m0b1l3.
- 73.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Ossicodone. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000549_043927_RCP.pdf&sys=m0b1l3.
- 74.AIFA Agenzia Italiana del Farmaco Fentanyl-Riassunto delle Caratteristiche del Prodotto. [(accessed on 14 July 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002838_035693_RCP.pdf&sys=m0b1l3.
- 75.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Palexia. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003822_037148_RCP.pdf&sys=m0b1l3.
- 76.Baldo B.A. Toxicities of opioid analgesics: Respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch. Toxicol. 2021;95:2627–2642. doi: 10.1007/s00204-021-03068-2. [DOI] [PubMed] [Google Scholar]
- 77.NIH National Institutes of Health Hydrocodone and Acetaminophen-Label. [(accessed on 16 August 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f505b2a-45a2-4d34-96f6-dedb574cb508.
- 78.NIH National Institutes of Health Buprenorphine-Label. [(accessed on 16 August 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=17ad1a5b-e89f-d5a3-d15b-2eb48bcded7d#Section_7.
- 79.Dickenson A.H., Kress H.G. Tapentadol: A new option for the treatment of cancer and noncancer pains. J. Pain Res. 2019;12:1509–1511. doi: 10.2147/JPR.S190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabbe J.R., Sims P.J., Sims M.H. Tramadol-warfarin interaction. Pharmacotherapy. 1998;18:871–873. [PubMed] [Google Scholar]
- 81.Stevens A.J., Woodman R.J., Owen H. The effect of ondansetron on the efficacy of postoperative tramadol: A systematic review and meta-Analysis of a drug interaction. Anaesthesia. 2015;70:209–218. doi: 10.1111/anae.12948. [DOI] [PubMed] [Google Scholar]
- 82.American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine- Practice guidelines for chronic pain management. Anesthesiology. 2010;112:810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 83.National Institute for Health and Care Excellence . Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings. National Institute for Health and Care Excellence (NICE); London, UK: 2013. pp. 1–36. [Google Scholar]
- 84.HCANJ Pain Management Guideline. [(accessed on 17 July 2022)]. Available online: https://www.hcanj.org/files/2013/09/Pain-Management-Guidelines-_HCANJ-May-12-final.pdf.
- 85.Pullano S.A., Marcianò G., Bianco M.G., Oliva G., Rania V., Vocca C., Cione E., De Sarro G., Gallelli L., Romeo P., et al. FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering. 2022;9:503. doi: 10.3390/bioengineering9100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muraca L., Scuteri A., Burdino E., Marcianò G., Rania V., Catarisano L., Casarella A., Cione E., Palleria C., Colosimo M., et al. Effectiveness and Safety of a New Nutrient Fixed Combination Containing Pollen Extract plus Teupolioside, in the Management of LUTS in Patients with Benign Prostatic Hypertrophy: A Pilot Study. Life. 2022;12:965. doi: 10.3390/life12070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marcianò G., Roberti R., Palleria C., Mirra D., Rania V., Casarella A., De Sarro G., Gallelli L. SARS-CoV-2 Treatment: Current Therapeutic Options and the Pursuit of Tailored Therapy. Appl. Sci. 2021;11:7457. doi: 10.3390/app11167457. [DOI] [Google Scholar]
- 88.Marcianò G., Palleria C., Casarella A., Rania V., Basile E., Catarisano L., Vocca C., Bianco L., Pelaia C., Cione E., et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals. 2022;15:589. doi: 10.3390/ph15050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J., Bauer B.A., Wahner-Roedler D.L., Chon T.Y., Xiao L. The modified WHO analgesic ladder: Is it appropriate for chronic non-cancer pain? J. Pain Res. 2020;13:411–417. doi: 10.2147/JPR.S244173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dowell D., Haegerich T.M., Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 91.American Geriatric Society Pharmacological Management of Persistent Pain in Older Persons. J. Am. Geriatr. Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 92.U.S. Department of Health and Human Services . Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. U.S. Department of Health & Human Services; Washington, DC, USA: 2019. p. 116. [Google Scholar]
- 93.Kucharz E.J., Szántó S., Ivanova Goycheva M., Petronijević M., Šimnovec K., Domżalski M., Gallelli L., Kamenov Z., Konstantynowicz J., Radunović G., et al. Endorsement by Central European experts of the revised ESCEO algorithm for the management of knee osteoarthritis. Rheumatol. Int. 2019;39:1117–1123. doi: 10.1007/s00296-019-04332-6. [DOI] [PubMed] [Google Scholar]
- 94.Rosenquist R.W., Benzon H.T., Connis R.T., De Leon-Casasola O.A., Glass D.D., Korevaar W.C., Mekhail N.A., Merrill D.G., Nickinovich D.G., Rathmell J.P., et al. American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine Practice guidelines for chronic pain management. Anesthesiology. 2010;112:995–1004. doi: 10.1097/00000542-199704000-00032. [DOI] [PubMed] [Google Scholar]
- 95.Chou R., Gordon D.B., De Leon-Casasola O.A., Rosenberg J.M., Bickler S., Brennan T., Carter T., Cassidy C.L., Chittenden E.H., Degenhardt E., et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive commi. J. Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Xia W.S., Peng Y.N., Tang L.H., Jiang L.S., Yu L.N., Zhou X.L., Zhang F.J., Yan M. Spinal ephrinB/EphB signalling contributed to remifentanil-induced hyperalgesia via NMDA receptor. Eur. J. Pain. 2014;18:1231–1239. doi: 10.1002/j.1532-2149.2014.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosen I.M., Aurora R.N., Kirsch D.B., Carden K.A., Malhotra R.K., Ramar K., Abbasi-Feinberg F., Kristo D.A., Martin J.L., Olson E.J., et al. Chronic opioid therapy and sleep: An American academy of sleep medicine position statement. J. Clin. Sleep Med. 2019;15:1671–1673. doi: 10.5664/jcsm.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palareti G., Legnani C., Cosmi B., Antonucci E., Erba N., Poli D., Testa S., Tosetto A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016;38:42–49. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- 99.Axelrod D.J., Reville B. Using methadone to treat opioid-induced hyperalgesia and refractory pain. J. Opioid Manag. 2007;3:113–114. doi: 10.5055/jom.2007.0048. [DOI] [PubMed] [Google Scholar]
- 100.Davison S.N. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin. J. Am. Soc. Nephrol. 2019;14:917–931. doi: 10.2215/CJN.05180418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dean M. Opioids in renal failure and dialysis patients. J. Pain Symptom Manage. 2004;28:497–504. doi: 10.1016/j.jpainsymman.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 102.Siegers C.P., Loeser W., Gieselmann J., Oltmanns D. Biliary and renal excretion of paracetamol in man. Pharmacology. 1984;29:301–303. doi: 10.1159/000138026. [DOI] [PubMed] [Google Scholar]
- 103.Kacinko S.L., Jones H.E., Johnson R.E., Choo R.E., Concheiro-Guisan M., Huestis M.A. Urinary excretion of buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide in pregnant women receiving buprenorphine maintenance treatment. Clin. Chem. 2009;55:1177–1187. doi: 10.1373/clinchem.2008.113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.NIH National Institutes of Health Methadone-Label. [(accessed on 17 August 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=802ab399-479b-4271-a2a7-07aadde91cff.
- 105.Hoskin P.J., Hanks G.W. Morphine: Pharmacokinetics and clinical practice. Br. J. Cancer. 1990;62:705–707. doi: 10.1038/bjc.1990.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NIH National Institutes of Health Acetaminophen and Codeine. [(accessed on 17 September 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=97b0ee29-d08e-4d9b-82ed-3ce287dbec28.
- 107.Abi-aad K.R., Derian A. Hydromorphone. [(accessed on 17 September 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470393/
- 108.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Jurnista. [(accessed on 16 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001445_037396_RCP.pdf&sys=m0b1l3.
- 109.Dhesi M., Maldonado K.A., Maani C.V. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2023. Tramadol. [PubMed] [Google Scholar]
- 110.NIH National Institutes of Health Duloxetine. [(accessed on 17 September 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dde979d-b6f8-41d1-96fb-325c75ea3a74#section-12.3.
- 111.Nagler E.V., Webster A.C., Vanholder R., Zoccali C. Antidepressants for depression in stage 3–5 chronic kidney disease: A systematic review of pharmacokinetics, ef fi cacy and safety with recommendations by European Renal Best Practice (ERBP)*. Nephrol. Dial. Transpl. 2012;27:3736–3745. doi: 10.1093/ndt/gfs295. [DOI] [PubMed] [Google Scholar]
- 112.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Lidocaina. [(accessed on 17 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000045_040335_RCP.pdf&sys=m0b1l3#:~:text=Lidocainacontenutaneicerottidi,quindilariduzionedeldolore.
- 113.Raouf M., Atkinson T.J., Crumb M.W., Fudin J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J. Pain Res. 2017;10:275–278. doi: 10.2147/JPR.S130942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dastis S.N., Rahier J., Lerut J., Geubel A.P. Liver transplantation for nonsteroidal anti-inflammatory drug-induced liver failure: Nimesulide as the first implicated compound. Eur. J. Gastroenterol. Hepatol. 2007;19:919–922. doi: 10.1097/MEG.0b013e3282eeb4cc. [DOI] [PubMed] [Google Scholar]
- 115.AIFA Agenzia Italiana del Farmaco Aspirina . Riassunto delle Caratteristiche del Prodotto. AIFA; Rome, Italy: 2021. [Google Scholar]
- 116.Björnsson E.S. Hepatotoxicity by drugs: The most common implicated agents. Int. J. Mol. Sci. 2016;17:224. doi: 10.3390/ijms17020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huddart R., Clarke M., Altman R.B., Klein T.E. PharmGKB summary: Oxycodone pathway, pharmacokinetics. Pharm. Genom. 2018;28:230–237. doi: 10.1097/FPC.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ling W. Buprenorphine for opioid dependence. Expert Rev. Neurother. 2009;9:609–616. doi: 10.1586/ern.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.EMA European Medicines Agenecy Effentora-Summary of Product Characteristics. [(accessed on 16 September 2022)]. Available online: https://www.ema.europa.eu/en/documents/product-information/effentora-epar-product-information_en.pdf.
- 120.Fidman B., Nogid A. Role of tapentadol immediate release (Nucynta) in the management of moderate-to-severe pain. Pharm. Ther. 2010;35:330–334. [Google Scholar]
- 121.Faria J., Barbosa J., Moreira R., Queirós O., Carvalho F., Dinis-Oliveira R.J. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur. J. Pain. 2018;22:827–844. doi: 10.1002/ejp.1196. [DOI] [PubMed] [Google Scholar]
- 122.NIH National Institutes of Health Tizanidine. [(accessed on 17 September 2022)]; Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7f39499a-98cf-4d13-9b07-ccb5c22e43c8.
- 123.Ghanavatian S., Derian A. Baclofen. [(accessed on 17 September 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK526037/
- 124.Szeto C.C., Sugano K., Wang J.G., Fujimoto K., Whittle S., Modi G.K., Chen C.H., Park J.B., Tam L.S., Vareesangthip K., et al. Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: Joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut. 2020;69:617–629. doi: 10.1136/gutjnl-2019-319300. [DOI] [PubMed] [Google Scholar]
- 125.Ping F., Wang Y., Wang J., Chen J., Zhang W., Zhi H., Liu Y. Opioids increase hip fracture risk: A meta-analysis. J. Bone Miner. Metab. 2017;35:289–297. doi: 10.1007/s00774-016-0755-x. [DOI] [PubMed] [Google Scholar]
- 126.George M.D., Baker J.F., Leonard C.E., Mehta S., Miano T.A., Hennessy S. Risk of Nonunion with Nonselective NSAIDs, COX-2 Inhibitors, and Opioids. J. Bone Jt. Surg.—Am. Vol. 2020;102:1230–1238. doi: 10.2106/JBJS.19.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Emeny R.T., Chang C.H., Skinner J., O’Malley A.J., Smith J., Chakraborti G., Rosen C.J., Morden N.E. Association of Receiving Multiple, Concurrent Fracture-Associated Drugs with Hip Fracture Risk. JAMA Netw. Open. 2019;2:e1915348. doi: 10.1001/jamanetworkopen.2019.15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L., Setoguchi S., Cabral H., Jick S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J. Intern. Med. 2013;273:511–526. doi: 10.1111/joim.12035. [DOI] [PubMed] [Google Scholar]
- 129.Dam V.C.J., Schrier V.D.R., Velzen V.M., Lemmen V.M., Simons P., Kuijpers K.W.K., Jansen S., Kowal M.A., Olofsen E., Kramers C., et al. Inhaled Δ9-tetrahydrocannabinol does not enhance oxycodone- induced respiratory depression: Randomised controlled trial in healthy volunteers. Br. J. Anaesth. 2023;130:485–493. doi: 10.1016/j.bja.2022.12.018. [DOI] [PubMed] [Google Scholar]
- 130.Lakkad M., Martin B., Li C., Harrington S., Dayer L., Painter J.T. The use of gabapentinoids and opioids and risk of developing opioid-induced respiratory depression among older breast cancer survivors with neuropathic pain. J. Cancer Surviv. 2023 doi: 10.1007/s11764-023-01338-9. [DOI] [PubMed] [Google Scholar]
- 131.Jungquist C.R., Flannery M., Perlis M.L., Grace J.T. Original Article Relationship of Chronic Pain and Opioid Use with Respiratory Disturbance during Sleep. Pain Manag. Nurs. 2012;13:70–79. doi: 10.1016/j.pmn.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 132.Wallace J.L. COX-2: A pivotal enzyme in mucosal protection and resolution of inflammation. Sci. World J. 2006;6:577–588. doi: 10.1100/tsw.2006.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farmer A.D., Holt C.B., Downes T.J., Ruggeri E., Del Vecchio S., De Giorgio R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 2018;3:203–212. doi: 10.1016/S2468-1253(18)30008-6. [DOI] [PubMed] [Google Scholar]
- 134.Nero R., Allen B., Hailu K., Noor R., Theiss K. Impact of oral naloxegol vs subcutaneous methylnaltrexone in treatment of opioid-induced constipation in the hospital setting. Am. J. Health Syst. Pharm. 2022 doi: 10.1093/ajhp/zxac356. [DOI] [PubMed] [Google Scholar]
- 135.Takemura M., Niki K., Miyaguchi S., Ueda M. Naldemedine-laxative combination: Retrospective inpatient study. BMJ Support. Palliat. Care. 2022 doi: 10.1136/spcare-2022-003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Staats P.S., Markowitz J., Schein J. Incidence of Constipation Associated with Long-acting Opioid Therapy: A Comparative Study. South. Med. J. 2004;97:129–134. doi: 10.1097/01.SMJ.0000109215.54052.D8. [DOI] [PubMed] [Google Scholar]
- 137.Leppert W., Zajaczkowska R., Wordliczek J. The role of oxycodone/naloxone in the management of patients with pain and opioid-induced constipation. Expert Opin. Pharmacother. 2019;20:511–522. doi: 10.1080/14656566.2018.1561863. [DOI] [PubMed] [Google Scholar]
- 138.Mccabe M.P., Sharlip I.D., Atalla E., Balon R., Fisher A.D., Laumann E., Lee S.W., Lewis R., Segraves R.T. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016;13:135–143. doi: 10.1016/j.jsxm.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 139.Gungor G., Perk H., Soyupek S., Baykal B., Demir M., Sezer M.T. Nebivolol protects erectile functions compared to Metoprolol in hypertensive men with atherogenic, venogenic, psychogenic erectile dysfunction. Eur. J. Intern. Med. 2022;103:69–75. doi: 10.1016/j.ejim.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 140.Trinchieri A., Perletti G., Magri V., Stamatiou K., Trinchieri M., Montanari E. Drug-induced gynecomastia: A systematic review and meta-analysis of randomized clinical trials. Arch. Ital. Urol. Androl. 2021;93:489–496. doi: 10.4081/aiua.2021.4.489. [DOI] [PubMed] [Google Scholar]
- 141.Doherty R.J., Wahood W., Yolcu Y.U., Zreik J., Goyal A., Gazelka H.M., Habermann E.B., Sebastian A., Bydon M. Chronic opioid use is associated with increased postoperative urinary retention, length of stay and non-routine discharge following lumbar fusion surgery. Clin. Neurol. Neurosurg. 2020;197:106161. doi: 10.1016/j.clineuro.2020.106161. [DOI] [PubMed] [Google Scholar]
- 142.Kela I., Kakarala C.L., Hassan M., Belavadi R., Gudigopuram S.V.R., Raguthu C.C., Gajjela H., Sange I. Chronic Pain: A Complex Condition With a Multi-Tangential Approach. Cureus. 2021;13:e19850. doi: 10.7759/cureus.19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wiffen P.J., Derry S., Moore R.A., Aldington D., Cole P., Rice A.S.C., Lunn M.P.T., Hamunen K., Haanpaa M., Kalso E.A. Antiepileptic drugs for neuropathic pain and fibromyalgia—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2013;2013:CD010567. doi: 10.1002/14651858.CD010567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salerno S.M., Browning R., Jackson J.L. The effect of antidepressant treatment on chronic back pain: A meta-analysis. Arch. Intern. Med. 2002;162:19–24. doi: 10.1001/archinte.162.1.19. [DOI] [PubMed] [Google Scholar]
- 145.Slepukhina M.A., Ivashchenko D.V., Sheina M.A., Muradian A.A., Blagovestnov D.A., Sychev D.A. Pain pharmacogenetics. Drug Metab. Pers. Ther. 2020;35:20202939. doi: 10.1515/dmpt-2020-2939. [DOI] [PubMed] [Google Scholar]
- 146.Hooten W.M., Hu D., Cunningham J.M. Effects of the ABCB1 c.3435C>T (rs1045642) Polymorphism on Heat Pain Perception in Opioid-Free Adults With Chronic Pain. Anesth. Analg. 2021;133:1028–1035. doi: 10.1213/ANE.0000000000005629. [DOI] [PubMed] [Google Scholar]
- 147.Bhaskar A., Bell A., Boivin M., Briques W., Brown M., Clarke H., Cyr C., Eisenberg E., Ferreira R., Silva D.O., et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021;3:22. doi: 10.1186/s42238-021-00073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aviram J., Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20:E755–E796. doi: 10.36076/ppj.20.5.E755. [DOI] [PubMed] [Google Scholar]
- 149.Freo U., Brugnatelli V., Turco F., Zanette G. Analgesic and Antidepressant Effects of the Clinical Glutamate Modulators Acetyl- L -Carnitine and Ketamine. Front. Neurosci. 2021;15:584649. doi: 10.3389/fnins.2021.584649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cuccurazzu B., Bortolotto V., Valente M.M., Ubezio F., Koverech A., Canonico P.L., Grilli M. Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology. 2013;38:2220–2230. doi: 10.1038/npp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiechio S., Copani A., Iv R.W.G., Nicoletti F. Acetyl-L-carnitine in neuropathic pain: Experimental data. CNS Drugs. 2007;21:31–38. doi: 10.2165/00023210-200721001-00005. [DOI] [PubMed] [Google Scholar]
- 152.Parisi S., Ditto M.C., Borrelli R., Fusaro E. Efficacy of a fixed combination of palmitoylethanolamide and acetyl-l-carnitine (PEA+ALC FC) in the treatment of neuropathies secondary to rheumatic diseases. Minerva Med. 2021;112:492–499. doi: 10.23736/S0026-4806.21.07486-3. [DOI] [PubMed] [Google Scholar]
- 153.Rolim L.C.S.P., da Silva E.M.K., Flumignan R.L.G., Abreu M.M., Dib S.A. Acetyl-l-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2019;2019:CD011265. doi: 10.1002/14651858.CD011265.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.AIFA Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Nicetile. [(accessed on 19 August 2022)]; Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004375_025369_RCP.pdf&sys=m0b1l3.
- 155.Knotkova H., Hamani C., Sivanesan E., Le Beuffe M.F.E., Moon J.Y., Cohen S.P., Huntoon M.A. Neuromodulation for chronic pain. Lancet. 2021;397:2111–2124. doi: 10.1016/S0140-6736(21)00794-7. [DOI] [PubMed] [Google Scholar]
- 156.Premi E., Benussi A., La Gatta A., Visconti S., Costa A., Gilberti N., Cantoni V., Padovani A., Borroni B., Magoni M. Modulation of long-term potentiation-like cortical plasticity in the healthy brain with low frequency-pulsed electromagnetic fields. BMC Neurosci. 2018;19:34. doi: 10.1186/s12868-018-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wuschech H., von Hehn U., Mikus E., Funk R.H. Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics. 2015;36:576–585. doi: 10.1002/bem.21942. [DOI] [PubMed] [Google Scholar]
- 158.Maestú C., Blanco M., Nevado A., Romero J., Rodríguez-Rubio P., Galindo J., Lorite J.B., De Las Morenas F., Fernández-Argüelles P. Reduction of pain thresholds in fibromyalgia after very low-intensity magnetic stimulation: A double-blinded, randomized placebo-controlled clinical trial. Pain Res. Manag. 2013;18:101–107. doi: 10.1155/2013/270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weintraub M.I., Cole S.P. Pulsed Magnetic Field Therapy in Refractory Neuropathic Pain Secondary to Peripheral Neuropathy: Electrodiagnostic Parameters—Pilot Study. Neurorehabil. Neural Repair. 2004;18:42–46. doi: 10.1177/0888439003261024. [DOI] [PubMed] [Google Scholar]
- 160.Roberti R., Marcianò G., Casarella A., Rania V., Palleria C., Muraca L., Citraro R., De Sarro G., Serra R., Romeo P., et al. High-Intensity, Low-Frequency Pulsed Electromagnetic Field as an Odd Treatment in a Patient with Mixed Foot Ulcer: A Case Report. Reports. 2022;5:3. doi: 10.3390/reports5010003. [DOI] [Google Scholar]
- 161.Roberti R., Marcianò G., Casarella A., Rania V., Palleria C., Vocca C., Catarisano L., Muraca L., Citraro R., Romeo P., et al. Diamagnetic Therapy in a Patient with Complex Regional Pain Syndrome Type I and Multiple Drug Intolerance: A Case Report. Reports. 2022;5:18. doi: 10.3390/reports5020018. [DOI] [Google Scholar]
- 162.De Sire A., Agostini F., Lippi L., Mangone M., Marchese S., Cisari C., Bernetti A., Invernizzi M. Oxygen—Ozone Therapy in the Rehabilitation Field: State of the Art on Mechanisms of Action, Safety andEffectiveness in Patients with Musculoskeletal Disorders. Biomolecules. 2021;11:356. doi: 10.3390/biom11030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de C Williams A.C., Fisher E., Hearn L., Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2020;8:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.