Abstract
Despite progress in understanding the pathological mechanisms underlying psychiatric disorders, translation from animal models into clinical use remains a significant bottleneck. Preclinical studies have implicated the orexin neuropeptide system as a potential target for psychiatric disorders through its role in regulating emotional, cognitive, and behavioral processes. Clinical studies are investigating orexin modulation in addiction and mood disorders. Here we review performance-outcome measures (POMs) arising from experimental medicine research methods which may show promise as markers of efficacy of orexin receptor modulators in humans. POMs provide objective measures of brain function, complementing patient-reported or clinician-observed symptom evaluation, and aid the translation from preclinical to clinical research. Significant challenges include the development, validation, and operationalization of these measures. We suggest that collaborative networks comprising clinical practitioners, academics, individuals working in the pharmaceutical industry, drug regulators, patients, patient advocacy groups, and other relevant stakeholders may provide infrastructure to facilitate validation of experimental medicine approaches in translational research and in the implementation of these approaches in real-world clinical practice.
Keywords: Orexin antagonist, Orexin agonist, Orexin system, Experimental medicine, Performance-outcome assessment, Cognitive task, Drug development, Psychiatry, Clinical trial
1. Introduction
There is an unmet clinical need concerning many aspects of the treatment of neuropsychiatric disorders. This is the case for disorders where current treatments already exist, like depression and anxiety disorders, and for those where pharmacological treatments are limited, such as substance use disorders (SUDs), and other disorders characterized by compulsive behavior, including eating disorders. The increasingly high prevalence and cost of these psychiatric disorders (McCrone et al., 2008; OECD, 2018; Substance Abuse and Mental Health Services Administration, 2021; The Lancet Global Health, 2020) lends urgency to the search for new treatments and raises questions about the best methodological approaches to determine their efficacy.
The orexin neuropeptide system has emerged as a potential new drug target for neuropsychiatric conditions. Although a primary function of orexin is the stabilization of wakefulness, animal studies have demonstrated that it also influences emotional, cognitive, and behavioral processes, including stress responsivity, reward processing, motivation, and feeding (Baimel et al., 2015; Boutrel et al., 2005; Harris et al., 2005; Haynes et al., 2000; Mahler et al., 2012; Tisdale et al., 2021). In humans, genetic association studies have supported links between the orexin system and specific psychiatric illnesses (Cengiz et al., 2019; Hollander et al., 2012; Nishino and Yoshida, 2003; Nishizawa et al., 2015). However, investigation of the orexin system beyond sleepwake regulation in clinical research is still at an early stage.
The orexin system’s role in diverse brain functions makes it an interesting example to illustrate how performance outcome paradigms can inform therapeutic indications for the focus of future research efforts. Performance outcomes yield measurements of physical or cognitive function, via the participant’s completion of a task according to specific instructions (Richardson et al., 2019). This can include physical tasks, such as timed walking, and/or those involving wearable technology. Another class of performance outcomes includes neuropsychological assessments that capture measurements related to cognition, perception, or decision-making, and may involve computerized testing. Traditionally these tests have been used in the laboratory and fall within experimental medicine approaches (Koychev et al., 2010). Their use in clinical research has been predicated on the notion that the resulting measures capture aspects of brain function which may otherwise be challenging for individuals to subjectively report or clinicians to observe.
This review critically evaluates experimental medicine approaches for clinical development of orexin receptor modulators as potential new treatments for some psychiatric conditions. We include a synthesis of the areas of cognition and perception in which the orexin system has been implicated, with a summary of the cognitive tasks that may be promising in evaluating the effects of orexin receptor modulation in humans. We focus on paradigms with simple technological requirements and/or limited ethical challenges for their implementation since these are likely to be applicable at multiple stages of clinical drug development. Hence, neuroimaging approaches, or those that rely on procedures such as eye tracking or administration of substances with abuse potential, are out of scope. Lastly, we summarize key challenges in the use of performance outcomes to support their use in clinical studies, drug registration, and marketing authorization.
2. Overview of the orexin system
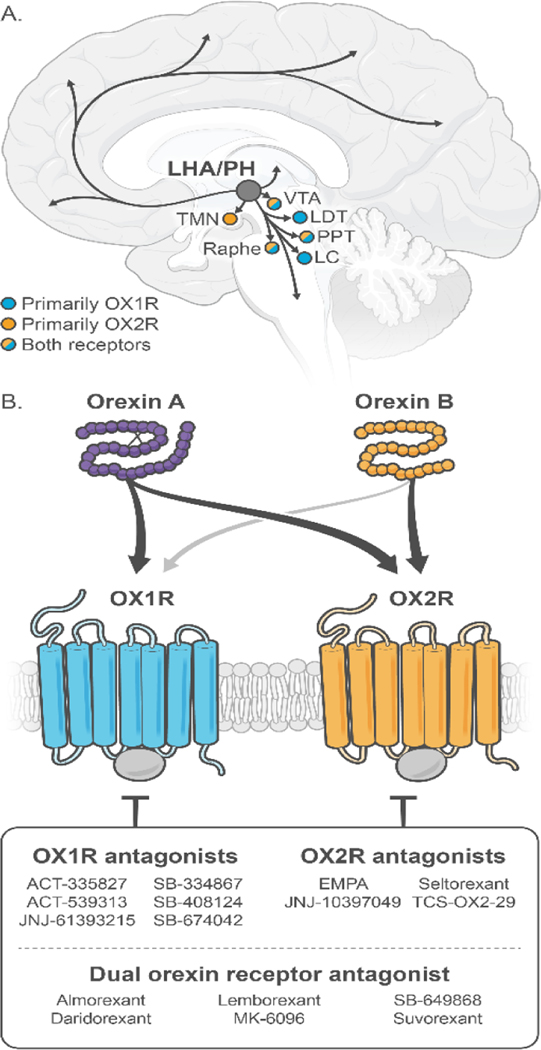
Orexins are excitatory neuropeptides produced by neurons in the perifornical area and the dorsomedial and lateral hypothalamus (LH), and their release from extensive projections of these neurons throughout the central nervous system is triggered by a variety of factors (Date et al., 1999; de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). Orexins are also known as hypocretins, which denotes their localization to cell bodies in the hypothalamus and the sequence homology with the gut peptide secretin. The two peptides termed orexin-A and orexin-B are derived by proteolytic processing from a common precursor protein called prepro-orexin, which is highly conserved across mammalian species (Soya and Sakurai, 2020). These peptides bind to and activate orexin type 1 (OX1R) and type 2 (OX2R) G-protein-coupled receptors that are expressed widely throughout the brain, consistent with a role in multiple physiological functions (Fig. 1). OX1R was shown to have a slightly greater affinity, about one order of magnitude, for orexin-A over orexin-B, whereas OX2R binds both ligands with similar affinities (de Lecea et al., 1998; Hong et al., 2021; Sakurai et al., 1998). We note, though, that these measurements were based on OXR expression and signaling in recombinant systems, which complicates a direct, quantitative comparison of ligand affinities. It might well be different in native systems (Kukkonen, 2013). OX1R and OX2R show partially overlapping expression, although some brain regions preferentially express one receptor subtype over the other. For example, OX1R appears to be selectively expressed in the locus coeruleus and cingulate cortex, while OX2R is predominantly found in the tuberomammillary nucleus, hypothalamic paraventricular nucleus, and nucleus accumbens (NAc) (Marcus et al., 2001; Matsuki and Sakurai, 2008; Mieda et al., 2011).
Figure 1.
(A) The orexin system in the human brain, with differential distribution of OX1Rs (blue) and OXR2s (orange) depicted. (B) The two orexin peptides (Orexin A and Orexin B), orexin receptors, and antagonists. OX1R, type 1 orexin receptor; OX2R, type 2 orexin receptor.
Abbreviations: LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LHA, lateral hypothalamus; OX1R, orexin type 1 receptor; OX2R, orexin type 2 receptor; PH, posterior hypothalamus; PPT, pedunculopontine nucleus; TMN, tuberomammillary nucleus; VTA, ventral tegmental area.
2.1. Multiple physiological functions
Orexin neurons stabilize wakefulness, regulate rapid eye movement sleep physiology, and are primarily active during wakefulness (Kantor et al., 2009; Lee et al., 2005; Mieda et al., 2011; Mileykovskiy et al., 2005; Sakurai, 2007). Orexin-A is released from synaptic sites during wakefulness and sleep deprivation (Blouin and Siegel, 2013; Zeitzer et al., 2003). The discovery that human narcolepsy involves selective loss of orexin neurons led to advances in understanding this condition (Mahoney et al., 2019; Nishino et al., 2000; Peyron et al., 2000; Thannickal et al., 2000), and similar findings have been observed in other mammalian species (Chemelli et al., 1999; Lin et al., 1999; Sakurai, 2013; Tisdale et al., 2021). A potential role for orexin in feeding regulation was suggested by the finding that orexin neurons are localized in the tuberal hypothalamus, a region involved in control of feeding behavior (Sakurai et al., 1998). Also, intracerebroventricular administration of orexins during the light period promotes feeding behavior in rats and mice (Haynes et al., 2002; Haynes et al., 2000; Sakurai et al., 1998; Yamanaka et al., 2000), although this could be due, at least partially, to increased wakefulness of the animals. Orexin-deficient mice, on the other hand, exhibit reduced food consumption (Hara et al., 2005; Willie et al., 2001) and studies in food-deprived rats showed that hypothalamic orexin neuronal activity was increased in response to conditioned cues, including expectation of receiving palatable chow (Choi et al., 2010; Harris et al., 2005; Petrovich et al., 2012).
Orexin neurons project from the LH to mesolimbic reward pathway areas, including the ventral tegmental area (VTA) and NAc, which are involved in behavioral responses to drugs of abuse. In line with this anatomical distribution, a role for orexins was established in regulation of motivated, emotional, reward-seeking, and addictive behaviors (Baimel et al., 2015; Boutrel et al., 2005; Harris et al., 2005; Mahler et al., 2012; Yamanaka et al., 2003). Orexin neurons are found in brain regions such as the central nucleus of the amygdala and the bed nucleus of the stria terminalis, which are associated with fear and anxiety-related behaviors. Other studies have provided evidence for a role for the orexin system in emotion, consolidation of emotional memory, anxiety, and panic-like behaviors (Chung et al., 2014; Johnson et al., 2012; Johnson et al., 2010; Li et al., 2010; Soya et al., 2013; Steiner et al., 2012). Associations between orexin neuronal activity and behavioral, neurocrine, and physiological responses to acute stress have been investigated widely and are reviewed elsewhere (Giardino and de Lecea, 2014; Sargin, 2019).
2.2. Interactions with multiple neurotransmitter systems
The role of the orexin system in sleep/wakefulness has been proposed to occur via modulation of multiple downstream arousal-promoting brain nuclei (Fig. 1). The LC receives dense extrahypothalamic projections from orexin neurons (Date et al., 1999; Hagan et al., 1999), which modulate activity of noradrenergic neurons and stimulate co-release of the excitatory neurotransmitter glutamate (Henny et al., 2010; Sears et al., 2013). In addition, orexin-A and orexin-B increase firing of serotonergic neurons in dorsal raphe nuclei and local inhibitory GABAergic input to serotonergic neurons (Brown et al., 2001; Liu et al., 2002), which, in turn, send abundant projections to pedunculopontine and laterodorsal tegmental nuclei, substantia nigra and amygdala (Sakurai, 2007). VTA dopaminergic neurons also contribute in regulating vigilance and attention (Eban-Rothschild et al., 2016; Yu et al., 2019), with modulation via arousal-related neurotransmitters such as glutamate, serotonin, and acetylcholine (Oishi and Lazarus, 2017). GABAergic neurons in the VTA become quiescent during waking, directly inhibiting LH orexin neurons (Chowdhury et al., 2019). Furthermore, activation of the orexin-basal-forebrain pathway stimulates acetylcholine release and cortical neuronal activity, contributing to attention processing with arousal (Fadel and FrederickDuus, 2008; Villano et al., 2017), and glutamatergic and GABAergic projections to LH orexin neurons further contribute to wakefulness, motivation, and other behaviors (Agostinelli et al., 2017). The importance of orexin signaling in reinforcement and reward-related processes via actions on VTA-dopamine neurons has been demonstrated in various studies, including morphine (Richardson and Aston-Jones, 2012; Taslimi et al., 2012) and cocaine (Bernstein et al., 2018; Espana et al., 2011; Espana et al., 2010) preference in self-administration animal models. Serotonergic neurons in the dorsal raphe are also active in expected/unexpected reward stimuli, including those for food, sex, and social interaction in mice (Li et al., 2016). This pathway appears to operate synergistically with the orexin-VTA pathway in modulation of reward/addiction (Li and de Lecea, 2020). Orexin neurons also project to the paraventricular thalamus which, in turn, projects to the NAc, bed nucleus of the stria terminalis, and amygdala, suggesting a role in modulation of reward/addiction processing, cognition, stress, and anxiety (Hamlin et al., 2009; Huang et al., 2006; Kirouac, 2015; Li et al., 2010). A role for orexinergic innervation in hedonic behavior is highlighted by the dense projections of orexin neurons to the ventral pallidum, a region involved in modulation of motivated behavior (Ch’ng and Lawrence, 2015; Ho and Berridge, 2013; Root et al., 2015). Other brain regions innervated by orexin neurons include: 1) insular cortex, which appears to contribute to amplification of orexin signaling in hedonic valence processing via GABAergic neurotransmission (Castro and Berridge, 2017); 2) arcuate nucleus, which mediates feeding and body-weight regulation via reciprocal orexin and neuropeptide Y connections (Fu et al., 2004; Muroya et al., 2004); and 3) local circuits within the hypothalamus containing glutamatergic and GABA-ergic neurons (Li and de Lecea, 2020).
3. Orexin receptor modulators for treatment of psychiatric disorders
More than 20 orexin receptor antagonists have been investigated in both preclinical and clinical studies for multiple indications. The dual OX1R/OX2R antagonist (DORA) almorexant was one of the first members of this new class of drugs, with sleep-promoting effects demonstrated in rats, dogs, and humans (Brisbare-Roch et al., 2007). In healthy volunteers, almorexant induced sleepiness without impairments in memory or cognitive functioning (Neylan et al., 2020). Although clinical development of almorexant was discontinued because of non-target-related safety concerns, three DORAs, suvorexant, lemborexant, and daridorexant were approved in the last decade by the US Food and Drug Administration (FDA) for the treatment of insomnia. Daridorexant is also, in 2022, the first DORA to be authorized in Europe. While the preclinical evidence has pointed to a potential role for the orexin system in feeding behavior, none of the large, longer-term clinical phase III trials involving the three FDA-approved DORAs has shown any effect on body weight (Kunz et al., 2023; Michelson et al., 2014; Yardley et al., 2021). Other DORAs such as YZJ-1139 and TS-142, as well as the selective OX2R antagonist (SO2RA) seltorexant and the selective OX1R antagonists (SO1RAs) JNJ-61393215 and ACT-539313, are in clinical phase 2 and 3 trials. As of August 2022, there are 53 completed, and 27 ongoing, clinical studies of orexin modulators registered in clinialtrials.gov. In addition to sleep disorders, potential indications such as SUDs, other addictive disorders, eating disorders, anxiety/panic disorders, and depression have received significant attention. While DORAs and SO2RAs promote sleep and should be preferentially administered at night-time, SO1RAs are largely devoid of sleep-promoting effects and can be administered during the day.
The therapeutic use of orexin receptor agonists, particularly OX2R agonists in the treatment of narcolepsy, has been supported by preclinical studies (Irukayama-Tomobe et al., 2017; Yukitake et al., 2019) and a phase 1 study of danavorexton (TAK-925) in patients with narcolepsy type 1 (NT1) showed increased wakefulness compared to placebo (Evans et al., 2019). Another OX2R agonist, TAK-994, was under investigation for excessive daytime sleepiness in NT1 and received breakthrough therapy status from the FDA in July 2021. However, the phase 2 study was stopped due to safety issues (Takeda Pharmaceutical Company Limited, 2021).
The wide-ranging therapeutic potential of orexin receptor modulators and early-stage exploration in psychiatric indications make them a strong candidate to examine experimental medicine approaches to facilitate investigation of efficacy signals.
4. Experimental medicine and neurocognitive approaches
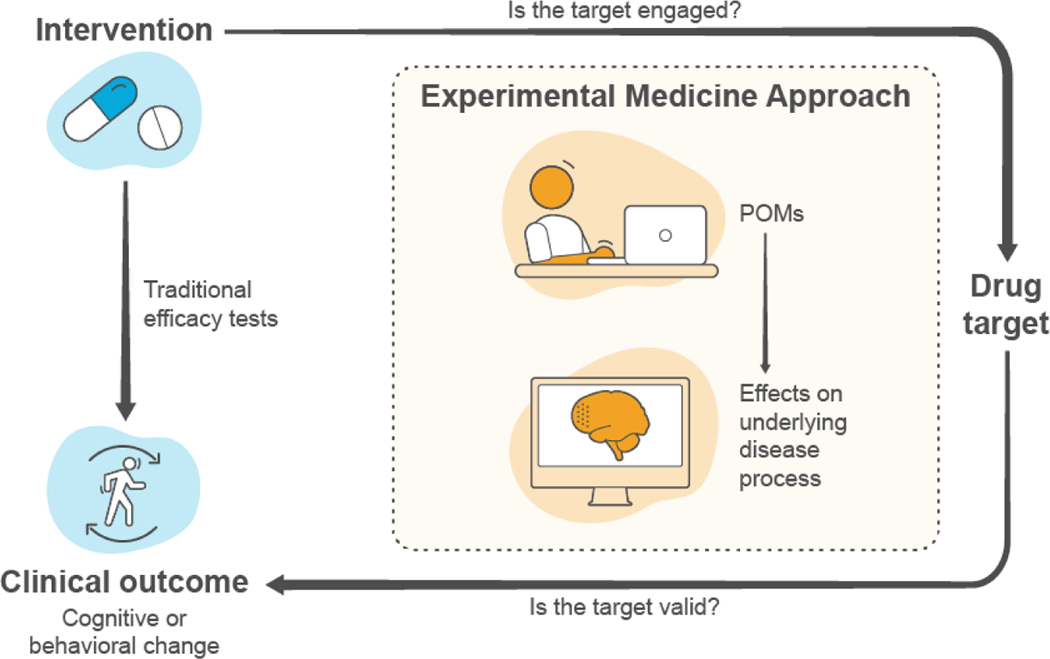
Experimental medicine (also called translational medicine) refers to studies in humans aimed at identifying disease mechanisms of action or demonstrating proof-of-concept for new discoveries or treatments (The Lancet, 2012; UK Research and Innovation, 2022). In the study of psychiatric disorders, such approaches may employ tasks that rely on specific brain circuits assumed to be modulated by compounds of interest. Thus, patients’ performance on these tasks may serve as indicators of the effects of new treatments on the underlying disease process rather than an effect on symptoms (Fig. 2) (Dawson and Goodwin, 2005). These performance-outcome measures (POMs) are often based on standardized tasks actively undertaken by a patient according to a set of instructions and may be administered by an appropriately trained individual or completed by the patient independently.
Figure 2.
Schematic of the experimental medicine approach to drug development.
POMs comprise one category of clinical outcome assessments (COAs) considered by health authorities in evaluations of clinical efficacy of new drugs. COAs include patient-, observer-, and clinician-reported outcome measures. Observer-reported measures reflect observable signs, events, or behaviors from someone other than the patient or healthcare provider, such as a parent or caregiver). Advantages of POMs are given in Box 1.
Box 1.
Advantages of POMs
| Potential advantages of POMs arising from cognitive/behavioral tasks over other outcome measures typically used in clinical trials in psychiatry include: |
| ● An objective indication of brain function and behavior |
| ● Reduced sensitivity to reporting biases and placebo effects |
| ○ Keefe et al., for example, found minimal placebo effects on performance-outcome using a cognitive test battery in a study of patients with schizophrenia (Keefe et al., 2017) |
| ● Providing a cognitive/behavioral signature similar to a molecular or imaging biomarker as an objective measure of biological state (particularly useful in the translation from preclinical to clinical research) |
| ● Mostly devoid of the expense and technical challenge of assessing neural activity directly through techniques such as functional magnetic resonance imaging (fMRI), positron emission tomography, or electroencephalography (EEG) |
Arguably the most advanced area of experimental medicine approaches showing the potential of POMs in psychiatric research has centered on emotional processing biases in depression and the effect of antidepressants in shifting these patterns. Several studies have shown that antidepressants can reduce processing of negatively-valenced stimuli and/or enhance processing of positive stimuli in healthy, depressed, and at-risk groups (Godlewska and Harmer, 2021; Harmer, 2013; Harmer et al., 2017; Warren et al., 2015). Importantly, treatment-induced changes in emotional processing may occur within days of drug administration, preceding the amelioration of depressive symptoms which usually take weeks to emerge with commonly prescribed antidepressants. Additionally, some studies have suggested that shifts in emotional processing may be predictive of the degree of symptom amelioration (Dawson et al., 2021; Shiroma et al., 2014; Tranter et al., 2009). POMs can also be used to distinguish individuals who have the pathologic behavior reflecting the disorder of interest from those who do not have it and may help identify patients who are more likely to respond to targeted therapy. Together, these findings support the potential of experimental medicine approaches to provide drug-efficacy signals which may: 1) reflect mechanism of action; 2) emerge early in the treatment course; and 3) provide a basis for defining treatment responders, informing personalizedmedicine approaches.
As an example of the above potential benefits, phase 1 studies in healthy participants assessing the pharmacodynamic effects of an investigational drug could incorporate POMs covering a range of cognitive and processing domains. Data emerging from such trials could inform decisions about appropriate indication(s), as well as enrollment criteria for later studies. In subsequent phase 2 or phase 3 studies, POMs may complement established indicators of efficacy in clarifying effects of compounds on cognition or other relevant functions. For example, clinical trials assessing SO1RA/SO2RA and DORA compounds have typically included at least one sleep assessment due to associations with the orexin system. Inclusion of POMs early in the clinical development of such compounds may identify rapid-onset effects on performance outcomes as a potential early predictor of treatment response. They may also inform whether symptom improvement is secondary to better sleep or a direct effect on the measured cognitive/perceptual domains, particularly when longitudinal collection of performance-outcome data is done. Moreover, such approaches could also identify potential markers of sustained response.
Within the last decade, transdiagnostic approaches have been put forward to enable better understanding and treatment of mental illnesses (Cuthbert, 2014; Cuthbert and Insel, 2013; Insel, 2014; Morris and Cuthbert, 2012). Experimental medicine methodologies fit well with transdiagnostic approaches because they involve indexing aspects of cognition that are relevant across the entire spectrum of human behavior, both in health and disease. Deviations from a normative behavioral pattern may be evident in multiple disorders, providing potential transdiagnostic cognitive targets. At the same time, the same aberration in cognitive or behavioral measures may also arise via different mechanisms where the condition is known to be heterogeneous, as is the case for most psychiatric disorders. One example of research in this vein has been the recent focus on anhedonia. Anhedonia is characterized by difficulties in experiencing or seeking pleasure and is a feature of multiple psychiatric disorders including depression and schizophrenia (Husain and Roiser, 2018; Šagud et al., 2019; Treadway and Zald, 2011). Anhedonia may be assessed via interviews or questionnaires, but tasks that measure willingness to exert effort to earn rewards have also been developed, providing objective performance-outcome results (Green et al., 2015). Of note, performance outcomes derived from an effort-based decision-making task were collected as secondary outcome measures in a phase 4 randomized controlled trial of the antidepressants vortioxetine and desvenlafaxine (NCT04448431; discussed in more detail below) (Clinicaltrials.gov, 2022g).
5. Experimental medicine paradigms and development of orexin receptor modulators for psychiatry
There are several questions to consider when using experimental medicine approaches with cognitive POMs in intervention studies (Box 2). Below, we address these questions to determine the most relevant and promising performance outcomes for development of orexin receptor modulators in psychiatric disorders. We focus on paradigms suitable for both small and large clinical trials, with the latter possibly requiring simpler implementation. The paradigms are organized according to the cognitive or psychological domains that they are intended to measure and highlight key preclinical work that supports the case for involvement of the orexin system.
Box 2.
Key questions when using experimental medicine approaches with cognitive POMs in intervention studies
| ● Which domains of cognition or perception are thought to be affected by the intervention, based on prior preclinical or clinical evidence? |
| ● If preclinical data exist, do the animal models have human analogues for use in clinical studies? |
| ● What is known about the target indication(s) and are the same domains of cognition known to be impaired in the patient population being studied? |
| ● Is there evidence that impairment is linked to severity of illness or outcomes in the population? |
| ● Is there evidence of test-retest reliability using the performance outcomes of interest? |
| ● Do previous results show consistency, replicability, and drug effects for shortlisted paradigms? |
| ● Are there potential implementation concerns such as technological requirements? |
| ● Are there substantial data management or analysis challenges to be overcome? |
| ● Are there potential language or cultural considerations and, if so, are there data to indicate the magnitude of these effects? |
5.1. Reward processing
Human reward processing is recognized as a multidimensional construct and likely involves distinct neural mechanisms. The National Institute of Mental Health research domain criteria differentiate between three aspects of reward processing (responsiveness, learning, and valuation), each of which is associated with multiple subdomains. For example, subdomains of reward valuation include response to delay and willingness to exert effort for rewards. Many existing human paradigms designed to measure reward processing rely on neuroimaging or EEG endpoints which can be more technically challenging, and these are discussed elsewhere (Balodis and Potenza, 2015; Meyer et al., 2021). Considering the role of the orexin system in motivated behaviors and reward-seeking, the US National Institute on Drug Abuse identified orexin receptor modulation as a high priority target for development of new medications for opioid use disorder (Rasmussen et al., 2019). In line with this, several preclinical models have been used to investigate the role of different orexin receptor antagonists in drug-seeking/drug-taking behaviors (Aston-Jones et al., 2010; Baimel et al., 2015; Plaza-Zabala et al., 2012).
5.1.1. Effort to attain rewards
Preclinical evidence supports a role for the orexin system in mediating behavior towards highly motivational reinforcers, including amount of effort expended in pursuit of rewards, such as drugs of abuse and palatable food (Baimel et al., 2015; Boutrel et al., 2013; Brodnik et al., 2015; Brown et al., 2022; Cason et al., 2010; Espana, 2012; Muschamp et al., 2014). A key paradigm which indexes effort made to attain rewards is the progressive ratio task (Roane, 2008). In human and animal versions of this task, the subject makes a sequence of effortful responses (e.g., button presses) to earn a reward. Following delivery of the reward, the number of responses required to earn subsequent rewards is increased and this continues until the participant stops responding. The schedule for the responses required to earn the reward varies but is typically increased linearly or exponentially. The key endpoint of the task is the cessation of responses.
The progressive ratio task has been used extensively for evaluating the reinforcing efficacy of drugs of abuse in preclinical studies (Stafford et al., 1998), including investigations of orexin receptor modulators on drug-seeking behavior. For example, systemic administration of the SO1RA SB-334867 compound had no effect on cocaine self-administration under fixed ratio (FR)1 or FR3 schedules of reinforcement (Smith et al., 2009). However, Hollander et al. found decreased responses for cocaine following a higher-demand FR5 schedule and by genetic deletion of OX1R (Hollander et al., 2012). Furthermore, systemic or intra-VTA injections of SB-334867 decreased the maximal effort animals expended under a progressive ratio schedule (Borgland et al., 2009; Brodnik et al., 2015).
An important challenge that comes with these reward-based paradigms is their use in large clinical trials involving administration of rewards such as substances of abuse. A recent small study of suvorexant in cocaine use disorder, employing a progressive ratio task to measure the reinforcing effects of cocaine, showed that suvorexant maintenance increased motivation to choose cocaine over money in humans (Stoops et al., 2022). These findings are incongruous with previous preclinical literature showing orexin antagonism attenuates the reinforcing and other abuse-related effects of cocaine. Rat studies have previously shown that DORAs can reduce operant responding for cocaine under a progressive ratio schedule (Gentile et al., 2018a; Prince et al., 2015). It should be noted, though, that suvorexant was given to the subjects in the human study sub-chronically, and before bedtime, while the behavioral assessments were done the next morning. Contrarily, all reported animal experiments administered the orexin antagonists acutely, directly before testing.
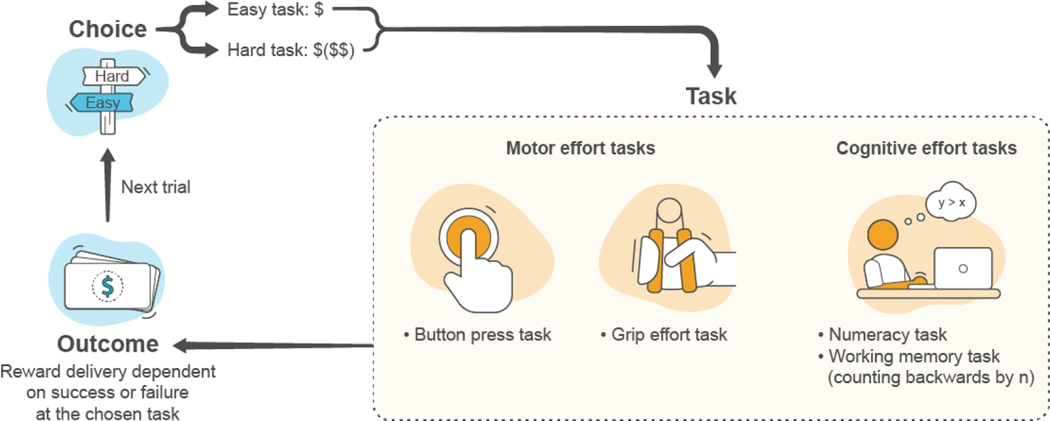
Effort-based decision-making tasks (EBDMTs) have emerged recently as another paradigm to measure willingness to work for rewards (Fig. 3) (Chong et al., 2016; Massar et al., 2018). In clinical studies employing EBDMTs, the participant chooses between difficult and easy (sub)tasks. If the participant completes their chosen task, a reward of variable value (e.g., monetary), and sometimes variable probability, is available. Analytic approaches for the tasks vary but all aim to estimate the influence of reward magnitude, reward probability, and/or expected value on choice behavior. This can be computed using a generalized estimating equation (Treadway et al., 2009) or by calculating the proportion of hard choices for each condition of interest, such as each reward level (Reddy et al., 2015).
Figure 3.
Basic trial structure of effort-based decision-making tasks. Participants choose between completing an easier task or a harder task, which may be associated with different magnitudes of reward or expected value. The participant then attempts to complete their chosen task. The kind of effort that the participant must make depends on task structure, but can e.g. be a form of motor effort or cognitive effort. Trial outcome, i.e. reward delivery, depends on whether the participant completes the task or not. The participant completes a series of trials from which indices can be extracted providing a measure of willingness to expend effort for rewards.
EBDMTs may require participants to invest motor or cognitive efforts (Culbreth et al., 2016; Reddy et al., 2018; Treadway et al., 2009). Their ecological validity is supported by data showing associations between task-performance outcome and anhedonia (Culbreth et al., 2020a; Culbreth et al., 2020b; Horan et al., 2015). The most widely used EBDMT is the effort expenditure for rewards task (EEfRT), which assesses willingness to exert motor effort (in the form of button presses) for variable monetary rewards available at different probabilities (Treadway et al., 2009). Effort-based decision-making paradigms have been used to investigate treatments for disorders characterized by motivational dysfunction, such as major depressive disorder (MDD), schizophrenia and Parkinson’s disease (Green et al., 2015; Reddy et al., 2015; Salamone et al., 2018; Salamone et al., 2016). EBDMTs have been used in other indications, including those for which orexin receptor modulators may be useful. For example, Stuppy-Sullivan and Baskin-Sommers found that individuals with severe SUD displayed decreased sensitivity to expected value information when choosing between hard and easy tasks in the EEfRT for monetary rewards. Individuals whose severity of SUD severity was related to avoiding aversive affective states were the least sensitive to expected value signals (Stuppy-Sullivan and Baskin-Sommers, 2019).
In addition to the evidence for the use of EBDMTs in SUDs, they have also been used in other indications in which orexin has been implicated. Microinjection experiments have supported a role for the orexin system in promotion of feeding, and this primarily occurs via orexin-A and OX1Rs. Recent evidence suggests that the orexin system is not indiscriminately linked to feeding but underlies seeking and consumption of palatable foods, food-seeking motivated by hunger, or seeking elicited by conditioned stimuli (Borgland, 2019; Cason and Aston-Jones, 2014; Mahler et al., 2012; Piccoli et al., 2012). Animal models have shown high levels of orexin following caloric deprivation or binge-like eating (Castro et al., 2016; Karteris et al., 2005; Olszewski et al., 2009; Thorpe et al., 2005). Moreover, orexin stimulates eating and willingness to work for palatable food (Choi et al., 2010; Valdivia et al., 2015). In an eating-disorder study, Racine et al. used a modified version of the EEfRT to examine willingness to work for food reward in individuals with binge eating disorder (BED). Participants with higher BED severity were driven more strongly by food reward, compared to those with low binge-eating levels (Racine et al., 2018).
Together, these findings suggest that EBDMTs may represent valuable POMs in psychiatric conditions, including those where response to reward is dampened (e.g., anhedonia) as well as conditions where incentive salience of the reward appears to be amplified (as may be the case with food in BED). Test-retest data for several EBDMTs have been reported as good to excellent in a schizophrenia study (Reddy et al., 2015). Other data support EBDMTs’ sensitivity to drug effects. For example, Wardle et al. showed that d-amphetamine administration enhanced willingness to exert effort for monetary rewards among healthy volunteers completing the EEfRT, particularly when reward probability was lower (Wardle et al., 2011). A study on acute effects of cannabis with and without cannabidiol found that the latter reduced likelihood of high-effort choices relative to placebo and increased sensitivity to expected reward value (Lawn et al., 2016). The EEfRT has been used as a secondary outcome measure in a recent phase 4 randomized controlled trial investigating the effects of vortioxetine versus desvenlafaxine in individuals with MDD and partial response to selective serotonin reuptake inhibitor monotherapy (NCT04448431) (Clinicaltrials.gov, 2022g). Results demonstrated non-inferiority of vortioxetine vs. desvenlafaxine on the primary study endpoint (Montgomery–Åsberg Depression Rating Scale [MADRS] total score) with benefits for vortioxetine in secondary endpoints including remission, daily and social functioning, and satisfaction with medication (Nordic Life Science News, 2022). Including the EEfRT in this study design may prove valuable in enabling investigators to examine drug effects on effortful choice as a complement to a clinician-rated index of anhedonia (MADRS anhedonia factor score) and potentially in relation to other collected measures of general cognition, function, and quality of life.
Implementation of EBDMTs raises several practical issues. While some versions of the task do not require specialist hardware, some do, such as devices used to measure grip force reflecting motor effort (Reddy et al., 2015), these may not be readily available at the clinical sites chosen for a study. Finally, it should be considered that most EBDMTs are >20 minutes in duration and may therefore result in participant fatigue. Further implementation issues that are relevant across reward processing tasks are highlighted in Box 3.
Box 3.
Reward processing tasks and participant reimbursement in clinical trials
| ● In some studies, participants are given actual rewards for their performance rather than merely hypothetical rewards. |
| ● Regarding ITCTs, Hamilton et al. reported that use of hypothetical rewards and real rewards results in equivalent performance outcomes (Hamilton et al., 2015c). |
| ● Such effects may vary across reward processing tasks and clinical populations. |
| ● For monetary rewards, the practicalities of reimbursement should consider standardization across different currencies and geographical locations, reflecting purchasing power parity. |
5.1.2. Delay of rewards
Delay discounting, or preference for smaller, more immediate rewards over larger, delayed rewards, represents an important aspect of how rewards are valued with respect to time and is considered a type of impulsivity (sometimes termed choice impulsivity). Higher levels of delay discounting have been linked to conditions such as SUDs, gambling disorder, BED, and obesity (Bickel et al., 2012; Carr et al., 2021; Chamorro et al., 2012; Davis et al., 2010a; Hamilton and Potenza, 2012; Kollins, 2003; Leeman and Potenza, 2012; Madden et al., 2011; Moeller et al., 2001). The findings of orexin-mediated effects on VTA dopamine neurons and orexin modulation in animal models of SUDs and BED raises the possibility that the orexin system may be involved in manifestation of impulsive behaviors. However, only one preclinical study has so far investigated orexin-receptor modulation on delay discounting, with no effect found in rats treated with suvorexant or OX1R- or OX2R-selective compounds (Gentile et al., 2018b). A small recent clinical study conducted on participants with cocaine use disorder found no effect of suvorexant maintenance in a five-trial delay-discounting task measuring discounting rates for cocaine and money (Stoops et al., 2022). Nevertheless, the relationship between orexin function and impulsivity requires further research, and use of delay-discounting and other choice-impulsivity paradigms may be important in these studies.
Delay discounting is typically assessed through behavioral inter-temporal choice tasks (ITCTs) in which the subject makes a series of choices between a smaller reward delivered quickly and a larger reward delivered after a delay. Greater degrees of discounting of larger, delayed reward reflect greater choice impulsivity. From these tasks, several endpoints can be derived, including indifference points between options, area under the curve, and percent choice of large or small rewards. Numerous ITCTs exist and include protocols which use adjusting regimes, whereby choices for smaller-sooner over larger-later rewards result in adjustment of subsequent options until an indifference point is reached (Richards et al., 1999), or where actual choice consequences are experienced (Reynolds and Schiffbauer, 2004). Some studies have implemented ITCTs with hypothetical rewards while others have delivered real rewards during or following the task. ITCTs can be employed with a variety of rewards or commodities to be discounted (e.g. money, food, or substance of abuse). Readers are referred to an in-depth review on delay discounting (Hamilton et al., 2015c).
The type of reward must be determined a priori (Box 3) as this may have impact on choice behavior and/or disease specificity. Even though most studies offer monetary rewards, particular scenarios may require other types such as food or drugs that are more specific to a disorder. Monetary rewards may have greater transdiagnostic potential though and are thus perhaps chosen for many studies.
5.2. Craving
Craving is a diagnostic criterion for SUDs (DSM-5) and also a feature of behavioral addictions, such as gambling and gaming (Yau and Potenza, 2015). While back-translation of craving from human to preclinical work is inexact, animal studies have demonstrated associations between decreased orexin activity and attenuation of drug-seeking and cue-reactivity (Bentzley and Aston-Jones, 2015; James et al., 2019; Steiner et al., 2018). This indicates that orexin modulation may reduce craving in humans and serve in treatment of SUDs and other addictions. Paradigms have been developed for performance outcomes related to craving, although the few clinical studies investigating effects of orexin receptor modulators on craving (e.g., NCT04229095, NCT03999099) have so far relied on subjective outcome measures (Clinicaltrials.gov, 2022d, e). In one small randomized controlled trial, Suchting et al. explored effects of treatment for two weeks with suvorexant versus placebo in individuals with cocaine use disorder, including effects on self-reported questionnaire scores and outcomes related to sleep, stress, and attentional bias (Suchting et al., 2020). The results suggested that suvorexant treatment reduced craving over time. This finding was supported by another randomized, double-blind, placebo-controlled clinical trial that demonstrated reduced craving on a subjective VAS in suvorexant-treated patients with opioid use disorder during buprenorphine taper (Huhn et al., 2022). In another, ongoing, small (N=20) randomized clinical trial, the effects of suvorexant versus placebo are currently being investigated in tobacco use disorder, including use of visual analogue scale measures of craving (NCT04234997) (Clinicaltrials.gov, 2022f).
5.2.1. Approach-avoidance tasks (AATs)
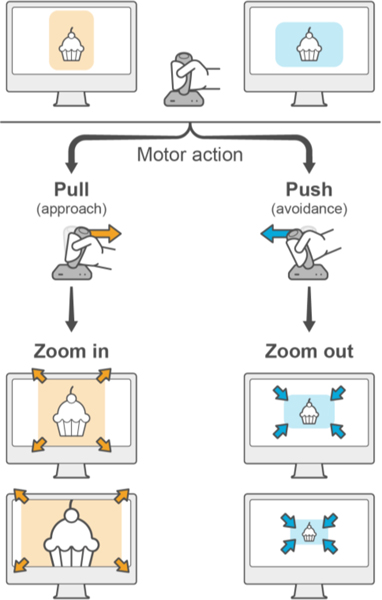
In experimental paradigms related to craving, the approach-avoidance task (AAT) measures approach versus avoidance of disease-relevant or otherwise valenced stimuli. Participants are instructed to make one type of response in reaction to images with one characteristic and to respond to images with another characteristic in a different, usually opposite, way (Fig. 4). For example, responses can be made by pulling or pushing a joystick depending on whether an image is oriented vertically or horizontally. The image size increases or decreases in response to pull or push, simulating approach or avoidance, respectively. Key POMs for a typical AAT are derivations of approach bias values, based on median reaction times. Typically, researchers derive approach bias as the difference in median reaction times between push vs. pull trials for a given stimulus category. This can be used as an outcome measure computed using only disease-relevant stimuli or contrasted between disease-relevant and non-disease-relevant stimuli (Brockmeyer et al., 2015; Sklenarik et al., 2020; Sklenarik et al., 2019; Wiers et al., 2009). In some analyses of AAT data, poorly performing participants, and/or poor-performance trials, are removed from further analysis, although working definitions of poor performance have not always been consistent across publications (Brockmeyer et al., 2015; Wiers et al., 2009). This perhaps highlights the variability that can affect some POMs and points to the need for addressing inconsistencies in analysis approaches prior to implementation in clinical trials.
Figure 4.
Basic trial structure of the Approach Avoidance Task. Participants are presented with an image which they must respond to with a motor action that simulates approach (e.g. pulling a joystick) or avoidance (e.g. pushing a joystick). The task is a measure of ‘implicit’ approach and avoidance, not explicit approach and avoidance. Participants are instructed to execute the action (“push” or “pull”) depending on an image property such as it being presented in landscape or portrait orientation, and not dependent on image content. In the example depicted, participants are instructed to pull the joystick for images in portrait orientation, and push the joystick for images in landscape orientation. Images increase in size (zoom in) or decrease in size (zoom out) in response to a “pull” or “push” action, respectively, to further simulate approach and avoidance. Approach bias values are calculated based on median reaction time differences between “push” and “pull” trials.
AATs have provided empirical evidence that systematic biases in approach and avoidance tendencies may underlie unhealthy behaviors, with greater approach biases to stimuli associated with cravings observed among individuals with addictive or compulsive disorders. For example, individuals with active tobacco use disorder have been shown to demonstrate greater approach bias for smoking cues than individuals with remitted tobacco use disorder and those who never smoked (Wiers et al., 2013), whilst participants with high levels of food craving were shown to demonstrate stronger approach tendencies towards food than those with low levels of food craving (Brockmeyer et al., 2015). Problematic pornography use, compared with non-problematic use, was associated with more than double the approach bias towards erotic stimuli among college-aged heterosexual males (Sklenarik et al., 2019), with similar findings in college-aged heterosexual females (Sklenarik et al., 2020). Supporting the ecological validity of the task are observations that approach bias correlates with self-reported craving or change in levels of craving from pre- to post-cue exposure (Brockmeyer et al., 2015; Wiers et al., 2013).
The AAT has also been used to explore approach and avoidance of social stimuli in individuals with social anxiety disorder. For example, Heuer et al. showed that highly socially anxious individuals had stronger avoidance tendencies than non-anxious individuals towards smiling and angry faces (Heuer et al., 2007).
Although joysticks can be used to measure push versus pull responses in AATs, this can introduce technical complexities as these are not typically designed for use in clinical research. Some studies have described touch-screen AATs but there are only limited data to assess the validity of this approach (Kahveci et al., 2020; Meule et al., 2020). Occasionally divergent results have been shown when different response devices are used, highlighting the need for further investigation into effects of task versions (Brockmeyer et al., 2015; Kahveci et al., 2020). Personalised versions of the task, where bespoke selections of stimuli are implemented according to individual craving profiles, may yield more sensitive and predictive measures but poses additional implementation complexity (Kahveci et al., 2020). Even without the use of bespoke stimulus sets, there can be issues around stimulus selection and validity, as described in Box 4.
Box 4.
Implementation issues for the AAT and ROC tasks
| Sourcing and selection of image stimuli |
| ● Stimulus categories like disease-relevant and neutral images should be matched on characteristics such as size, brightness, and contrast. |
| ● Cross-cultural applicability of images should be considered in international clinical trials. |
| ● Existing databases of affective images offer pictures standardized for image quality metrics and provide normative data across cultures (e.g., the Cross Cultural Food Image Database) (Toet et al., 2019). |
| ● Available information varies in different image libraries. |
| ● Some images are currently unavailable for use in commercial research. |
| Fatigue related to task duration |
| ● AATs typically take 20 minutes to complete, including practice trials (Rinck and Becker, 2007). |
| ● ROC tasks require participants to complete a cognitive reappraisal training session before the assessment task itself, which together have a duration of approximately 20–25 minutes. |
5.2.2. Regulation of craving (ROC) tasks
In contrast to AATs, regulation of craving (ROC) tasks measure cognitive downregulation and upregulation of craving. In this task, participants are presented with disease-relevant images (e.g., paraphernalia associated with substance abuse) and told to either downregulate or upregulate craving induced by the stimuli using cognitive reappraisal techniques. For example, when exposed to tobacco cues, individuals who smoke may be asked to consider how good consuming the pictured product might make them feel (upregulation) compared to the negative health impacts (downregulation). Sometimes a ‘just look’ condition is included as a no-regulation control (Sun and Kober, 2020). After each image is presented, participants are asked to rate how much they craved the item in the image on a scale from 1 (not at all) to 9 (very much). The key endpoint is the average rating of craving by regulation condition. Some researchers have also reported ‘regulatory success’, or the percent difference in averages of craving between strategy conditions and the look condition (defined as Look-Regulate / Look) (Boswell et al., 2018).
Several studies have demonstrated that ROC-related cognitive strategies impact craving in both healthy subjects and those who take substances of abuse or desire excessive food (Boswell et al., 2018; Giuliani et al., 2013; Kober et al., 2010; Strickland et al., 2016; Suzuki et al., 2020). Naqvi et al. found that individuals who drink socially compared to those with alcohol use disorder (AUD) were approximately twice as effective at reducing their alcohol craving in an ROC task. This suggested that AUD is associated with difficulties in regulating cue-induced craving (Naqvi et al., 2015). Wu et al. found that participants with internet gaming disorder had difficulty regulating gaming- and food-related craving (Wu et al., 2020a). In another study, participants with internet gaming disorder again regulated craving for gaming during ROC and emotion regulation tasks, and, under the upregulation and downregulation conditions, transcranial direct current stimulation (tDCS) of the right dorso-lateral prefrontal cortex (dlPFC) was found to increase and decrease craving, respectively (Wu et al., 2020b). A similar pattern was seen in the emotional regulation condition. Supporting the construct validity of the ROC task, Giuliani et al. found that the extent to which individuals downregulated cravings during a food version of the task was linked to other measures of food-related regulation such as the restraint subscale of the three-factor eating questionnaire (Giuliani et al., 2013). Using an ROC task that encouraged downregulation of unhealthy foods and upregulation of healthy foods, Boswell et al. found that healthy participants consumed fewer calories, supporting a role for ROC in modulating eating behaviors (Boswell et al., 2018).
5.2.3. Drug sensitivity of the AAT and ROC
Human research on sensitivity of AAT and ROC tasks to therapeutic intervention is mostly limited to studies employing behavioral rather than pharmacological interventions (Clinicaltrials.gov, 2022c; Mathew et al., 2021) or studies employing tDCS or transcranial magnetic stimulation interventions (Schluter et al., 2018; Wu et al., 2020a). Some intervention studies have used AAT or ROC tasks to drive changes in approach bias or capacity to regulate craving (Sun and Kober, 2020). For pharmacological interventions, the effects of oxytocin manipulation on AAT POMs have been investigated (Alaerts et al., 2021; Schneider et al., 2020; Yao et al., 2018) and one study is examining effects of cannabidiol versus placebo in AUD on outcomes including an alcohol AAT (NCT05387148) (Clinicaltrials.gov, 2022h). Despite a relative lack of data on drug sensitivity, AATs may be valuable in development of drugs posited to have effects on early, fast, and semi-automatic aspects of processing which bias individuals towards approach of addiction-related stimuli. In contrast, ROC tasks may be relevant to pharmacological interventions thought to enhance higher-order cognitive control (e.g., via modulation of dlPFC activity).
5.3. Anxiety and fear
5.3.1. Fear conditioning paradigms
In a typical fear conditioning task, a neutral conditioned stimulus is paired with an aversive unconditioned stimulus so that the previously neutral one becomes aversive (CS+) according to a reinforcement schedule. Generalized fear learning that occurs in response to a non-conditioned neutral stimulus (CS-) can also be measured. The reinforcement schedule of the fear conditioning phase determines whether a paired stimulus is neutral (never paired with a punisher), predictable (always or almost always paired with a punisher), or unpredictable (unreliably paired with a punisher). The fear conditioning phase is sometimes followed by an extinction phase, in which the CS+ is no longer paired with the previously paired punisher. Reinstatement protocols represent another variation involving unannounced re-exposure of original unconditioned stimulus and re-eliciting fear-related responses after successful extinction learning.
Neuropsychological models posit that fear-related disorders are associated with enhanced fear learning, overgeneralization of fear learning to a CS-, and/or impaired extinction. Fear is linked with highly probable, clearly defined, imminent or certain punishments, whereas anxiety is more closely related to responses of low probability, distal or otherwise uncertain punishments (Bradford et al., 2014). Concerning this difference, specific experimental protocols have been developed to investigate processes relevant to fear versus anxiety (Davis et al., 2010b), social anxiety (Lissek et al., 2008), and intrusions (Wegerer et al., 2013). Orexin modulators have been posited as potential treatments for anxiety and fear-related disorders, and therefore fear-conditioning paradigms may prove to be valuable in testing for potential anxiolytic efficacy and cognitive mechanisms of action.
Based on current preclinical evidence, the fear conditioning phenomena and performance outcomes most reliably associated with orexin modulation are: 1) expression of fear, 2) extinction, and 3) reinstatement. Orexin signaling contributes to consolidation and extinction of aversive memories, with excitatory effector neurons in the amygdala involved in regulation of emotional behavior and fear memory (Sargin, 2019). This was demonstrated by pre-treatment of rats with the SO1RA SB-334867 before conditioning, which impaired fear memory when assessed 24 hours afterwards (Sears et al., 2013). Furthermore, SB-334867 administration immediately after conditioning reduced fear memory, but not when administered 4 hours after conditioning (Flores et al., 2014). This supports the case that OX1R is involved during the acquisition/early-consolidation phase of aversive memories. Also, SB-334867 enhanced fear extinction, and administration of orexin-A impaired this process (Flores et al., 2014). The latter study also showed that rats with poor extinction of cue-induced freezing had a higher percentage of activated orexin neurons in the hypothalamus, suggesting that increased orexin neuronal activation may be associated with impaired ability to overcome traumatic experiences (Sargin, 2019; Sharko et al., 2017).
In another study, Chen et al. showed that systemic administration of the DORA TCS-1102 attenuated acute foot-shock-stress-induced fear responses assessed by freezing and induced anxiolytic effects in a subgroup of rats exhibiting high levels of immobility to a novel non-conditioned context the day after the foot-shock episode. In addition, increases in prepro-orexin mRNA levels were correlated with the amount of time rats spent freezing (Chen et al., 2014). Rats pre-treated with SB-334867 were also shown to display less avoidance from cat odor compared to vehicle-treated rats, but no difference in behavior was observed when another type of stressor based on environmental novelty (elevated-plus maze test) was used (Staples and Cornish, 2014). Finally, there is still limited preclinical evidence that the orexin system may play a role in over-generalization of fear. Viviani et al. found that 3-week treatment with the DORA almorexant attenuated over-generalized contextual fear in rats at 5- and 17-weeks washout, compared with the positive anxiolytic control of sertraline (Viviani et al., 2015).
The consensus of studies has led to the idea that the orexin system is involved in specific forms of stress. For example, Furlong et al. showed that wakefulness, exploration, and conditioned fear elicited c-fos expression in orexin neurons, while restraint stress did not (Furlong et al., 2009). Also, rodent studies have shown that only behavioral or physiological responses to specific acute stressors appear to be mediated by orexin, including foot shock, shock-associated or novel context, short-term forced swimming, food restriction, panic-like states, and social stress (Carrive, 2013; Johnson et al., 2012; Yeoh and Wilkinson, 2014). All of these acute stressors evoke adaptive coping responses, such as escape attempts, non-specific aggression-submission, novelty exploration, or freezing in a shock-associated context (Mahler et al., 2014).
Thus far, no clinical studies have tested the effects of orexin modulators on fear conditioning. Unconditioned stimuli in human fear-conditioning protocols are commonly electrical shocks or other painful or aversive stimuli such as human screams, and POMs are often physiological responses including fear-potentiated startle, skin conductance, heart rate, pupil dilation, or neurological imaging measures. Technical requirements may make these setups unfeasible for some clinical trials, and delivery of pain stimuli may raise ethical issues, especially in vulnerable groups. Subjective response to the CS+ and CS- can be collected as POMs, posing lesser implementation issues, although the relative sensitivity of subjective versus physiological responses is unclear. Fear conditioning tasks can also be long in duration to generate reliable learning or extinction for all stimuli. In addition, some analysis procedures incorporate confirmation that individuals have learned the stimulus associations. This may be important in intervention studies, given that pharmacological enhancement of extinction has been observed only in participants who demonstrated low levels of fear compared to those with high fear at the end of the exposure (Smits et al., 2014; Smits et al., 2013a; Smits et al., 2013b; Telch et al., 2014). However, there are no standardized criteria for this performance-outcome-based exclusion and enforcing such criteria can potentially exclude some endpoints or participants, thereby jeopardizing the generalizability of findings.
Although test-retest coefficients for fear-conditioning paradigms are generally high (Torrents-Rodas et al., 2014), reliability should be ascertained for specific endpoints, time intervals and fear-conditioning-paradigm specifications for which prior data are not always available. Lonsdorf et al. provided detailed information about methodological issues for implementing fear-conditioning paradigms in humans, including task design and analysis considerations (Lonsdorf et al., 2017). Also see Haaker et al. for a more specific focus on reinstatement studies in humans (Haaker et al., 2014).
5.3.2. Other paradigms related to fear and anxiety
Two other paradigms are thought to provide objective measures related to anxiety and fear. These are the cold pressor test and CO2 challenge. These have already been used in clinical investigations of orexin receptor modulators (cold pressor test: NCT02785406, NCT04234997; CO2 challenge: NCT02593682, Kaufmann et al., NCT02812251, NCT03007693) (Clinicaltrials.gov, 2022a, b, f; Kaufmann et al., 2021; Salvadore et al., 2020). However, as these tests depend primarily on responses to physiological challenges rather than responses based on cognitive processing, they are not considered further in this review.
5.3.3. Appetitive and drug cue extinction
Human paradigms that measure appetitive conditioning, for instance with substances of abuse, are of potential relevance for evaluating the efficacy of orexin receptor antagonists given evidence linking orexin modulation with cue-based learning (Cole et al., 2020; Cole et al., 2015; Keefer et al., 2016; Pantazis et al., 2021; Sharko et al., 2017) and the potential of orexin receptor modulators to treat conditions such as SUDs and eating disorders (Han et al., 2020; Steffen et al., 2006; Zarrabian et al., 2020). Appetitive and drug cue associations have been under-researched (Konova and Goldstein, 2019) and nobody has yet evaluated the effects of orexin modulation on appetitive, non-fear domains of extinction in humans. Nevertheless, preclinical work has suggested some similarities between fear and non-fear extinction (Millan et al., 2011; Peters et al., 2009). Additional grounding studies are warranted exploring non-fear extinction in SUDs, eating disorders, gambling disorder, and other conditions characterized by compulsive choice.
5.4. Emotional processing – negative and positive emotional biases
Several paradigms have been developed to measure processing of emotional information. For behavioral measures, processing of emotional stimuli can take different forms, including accuracy in classifying stimuli as positive or negative, speed of categorization, memory for stimuli, or spatial-attentional biases towards emotionally-valenced stimuli. These tasks have been shown to provide measures of emotional processing biases present in mood disorders like depression. Furthermore, many antidepressant compounds reduce processing of negative stimuli and/or enhance processing of positive stimuli. An example of the former would be decreased recall of negative self-referential words, and that of the latter is increased accuracy in identifying happy facial expressions.
Reduced orexin system function has been identified in association with depressive-like behavior in rats with hormonal and behavioral features similar to those observed in patients with depression (Sargin, 2019), and in socially defeated rats that display increased immobility in the forced swim test (Lutter et al., 2008). In adult male diurnal Nile grass rats, LH orexin immunoreactivity was attenuated in a model of seasonal affective disorder using dim light conditions (Deats et al., 2014). Chronically restrained mice that show multiple measures of depressive-like behavior had increased orexin mRNA levels in the amygdala and knocking down orexin expression reversed these behaviors (Kim et al., 2015). OX1R knockout mice showed decreased immobility time in a forced swim test study, an indication of reduced “depressive-like” behavior (Abbas et al., 2015). Also, pharmacological blockade with the DORA almorexant during unpredictable chronic mild stress decreased subsequent immobility in the tail-suspension test as an indication of antidepressant-like effects similar to the serotonin specific reuptake inhibitor fluoxetine, and restoration of the associated stress-induced HPA axis impairment (Nollet et al., 2012).
As orexin receptor modulators are posited to have a role in ameliorating symptoms of depression and anxiety, we focus here on the emotion-processing POMs which have been used the most in experimental medicine studies of antidepressant and anxiolytic compounds. These are tasks of facial expression recognition, emotional categorization, recall and recognition, and dot-probe tasks (Table 1). Several studies have suggested that emotion-processing patterns deviate significantly in patients with psychiatric disorders compared to healthy controls. MDD is the most intensively studied and has consistently been associated with increased processing of negative versus positive emotional information (Panchal et al., 2019; Warren et al., 2015). In anxiety studies, the findings have not been consistent. For example, some studies using recognition of facial expressions have shown that highly anxious individuals are more sensitive than healthy individuals to fearful or threatening faces, and more likely to interpret neutral or ambiguous faces as threatening (Bell et al., 2011; Doty et al., 2013; Gutiérrez-García and Calvo, 2017; Heuer et al., 2010; Richards et al., 2002; Surcinelli et al., 2006). Others reported either no case-control differences (Cooper et al., 2008; Jusyte and Schonenberg, 2014; Philippot and Douilliez, 2005) or reduced sensitivity to negative facial expression in anxiety (Jarros et al., 2012; Montagne et al., 2006). Some of the heterogeneity may be driven by variability in the study populations and paradigms used, and/or inconsistencies in measuring anxiety state versus anxiety trait.
Table 1.
Experimental medicine tasks that provide measures of emotional processing in humans
| Task and aspect of emotional processing measured | Task description | Key performance outcome(s) | Potential implementation and analytics issues |
|---|---|---|---|
|
Facial Expression Recognition Tasks
Measuring perception / identification of affective information (facial emotional expression) |
On each trial participants are presented with an image of a face showing a facial expression and must indicate the expression shown. | Accuracy, reaction time, misclassifications, target sensitivity, and response bias, by category of facial expression. | Many versions of the task exist, and it is unclear to what extent previous findings generalize across task version. Cognitive demand of task is modest, although some longer task designs can potentially lead to fatigue. Cross-cultural validity and standardization is a consideration for facial expression image libraries, given observations that accuracy for emotion recognition is higher for faces from member of the same cultural group (Elfenbein and Ambady, 2003) in a way that may interact with effects of diagnosis (Hunter et al., 2009; Kang et al., 2019). Task can yield a relatively high number of POMs, leading to potential multiple comparison issues at statistical analysis. There is little consistency in the literature regarding the precise endpoints that show case-control or drug effects; this may depend on population and drug mechanism of action. |
|
Emotional Categorization Tasks
Measuring speed of processing of affective information |
On each trial participants are presented with a stimulus (e.g. a personality characteristic word such as “hostile” or “honest”) and asked to categorize it as positive or negative. | Reaction time to correctly classifying positive vs. negative stimuli. Less commonly, accuracy for positive / negative stimuli, and frequency of classifying stimuli as positive / negative. | Stimuli need to be standardized across cultures / languages. Note, emotional categorization tasks can be viewed as a necessary precursor to tasks of emotional memory and emotional recall (see following two rows). Duration of these individual is typically relatively short (in the range of 5–10 minutes); however, the combined duration of the series of tasks may result in fatigue in some study designs and populations. |
|
Emotional Recall Tasks
Measuring free-recall memory for affective information |
Participants are asked to freely recall (e.g. writing or typing) as many positive and negative stimuli as possible, previously presented in e.g. an emotional categorization task. | Number of stimuli (e.g. words) correctly recalled, for positive and negative stimuli. | Some versions of the task implement a “surprise” free recall procedure, and for repeated-measures study designs, the surprise element may be necessarily lost. See also implementation issues associated with Emotional Categorization Tasks (above). |
|
Emotional Recognition Memory Tasks
Measuring recognition memory for affective information |
On each trial participants are presented with either a stimulus that was previously presented to them (for example in an emotional categorization task), or a previously unseen (novel) stimuli. For each stimulus, participants are required to indicate whether it was previously presented. | Number of stimuli correctly identified as previously presented; errors of omission; false alarms (errors of commission); target sensitivity; and response bias; for positive and negative stimuli. | See implementation issues associated with Emotional Categorization Tasks (above). |
|
Dot Probe Task
Attention to, or spatial orientation to, affective information |
On each trial participants are presented with two stimuli on the computer screen, which are replaced by a target pair of dots in the spatial location of one of the initially presented stimuli. The participant responds by indicating whether the dots are vertically or horizontally aligned. Typically there is a manipulation between the two stimuli that were initially presented. For example, one may be disease-relevant (e.g., a fearful face) and the other may be a control stimulus (e.g., a happy or neutral face). The reaction time to respond to the dots can be used as a measure of attention to the disease-relevant vs. control stimuli. Experimental stimuli can be masked or unmasked (allowing the assessment of very fast, automatic orienting/attention to masked emotional stimuli). | Difference in reaction time to target, when target is in the same spatial location as the disease-relevant stimuli vs. control stimuli. | May require specialized hardware, because of high temporal precision required for delivering stimuli (including, in some task designs, masking stimuli) and recording responses. Task duration typically 5–10 minutes. |
There have been no meta-analyses of emotion-processing patterns in SUDs, eating disorders, or other conditions of compulsive or addictive behaviors. However, difficulties recognizing disgust and tendencies to interpret non-angry faces as angry ones in bulimia nervosa have been observed (Dapelo et al., 2017), suggesting nuanced aberrancies in processing negative emotional information. In another study, people with binge drinking showed impairments in recognizing all expressions compared to healthy individuals, and extent of impairment was related to alcohol consumption (Lannoy et al., 2018). These findings suggest that general cognitive or emotional difficulties are associated with binge drinking, rather than specific difficulties in processing negative versus positive information.
The dot-probe task has conceptual roots in neuropsychological models of anxiety, measuring attention and orientation to negative or threatening information. While socially anxious individuals preferentially allocate attention towards threatening faces, a meta-analysis of dot-probe studies in anxiety found heterogenous results, suggesting that measuring this bias may depend on type and duration of reference stimulus, and clinical level of social anxiety (Bantin et al., 2016).
Dot-probe results in studies of eating disorders have also been equivocal. A version of this task which employed disease-relevant food stimuli did not differentiate between healthy individuals and those with either bulimia nervosa or BED (Leslie et al., 2019). Furthermore, Svaldi et al. reported that adults with obesity with or without BED showed similar levels of food-stimuli cueing in a task similar to the dot-probe (Svaldi et al., 2015). However, using a spatial cueing paradigm similar to the dot-probe, Schmitz et al. found that patients with BED showed more priming to food compared to weight-matched healthy individuals, which correlated with reported severity of binge-eating symptoms (Schmitz et al., 2014). Thus, the validity of the dot-probe task to measure clinically relevant aspects of cognitive dysfunction in eating disorders is unclear.
Evidence in the field of SUDs and other compulsive behaviors may be more informative. Lubman et al. used a pictorial dot-probe task to investigate attentional biases to stimuli associated with drug use in opioid use disorder. Participants with this disorder had faster reaction times to probes that replaced drug pictures rather than neutral pictures, consistent with an attentional bias to drug-related stimuli (Lubman et al., 2000). Along the same lines, Townshend et al. found that individuals with heavy but non-dependent social drinking showed attentional biases towards alcohol-related stimuli compared with those with occasional social drinking in a dot-probe task that employed alcohol-related pictures and words as primes (Townshend and Duka, 2001). Attentional biases to sexual cues during performance of a dot-probe task have also been reported in men with compulsive sexual behaviors (Mechelmans et al., 2014).
Tasks of emotional processing are sensitive to antidepressant compounds, in healthy volunteers and participants with depression (Warren et al., 2015). This is based on data collected in tasks of facial expression recognition, emotional categorization, and emotional recall. However, few studies have investigated aspects of task validity, such as test-retest reliability. Adams et al. found no effect of repeat testing in healthy controls on emotional word categorization, recall or recognition, with limited effects of repeated testing on facial expression recognition one week after baseline. Although performance outcomes in a facial expression recognition test (FERT) was better after one week compared to baseline, there was no effect of repeat testing on relative accuracy for specific emotions (Adams et al., 2016). Furthermore, Thomas et al. reported no effect of session number on performance outcomes in healthy individuals in an emotional categorization task and found that practice effects had stabilized after two sessions for facial expression recognition, emotional word recall, and emotional word recognition tasks (Thomas et al., 2016).
See Table 1 for details of implementation issues and considerations for each of the emotional processing tasks considered above.
5.5. Response inhibition
Response inhibition refers to withholding of actions that are inappropriate in certain contexts and deleterious to goal-directed behavior. Tasks of response inhibition involve control of attention, behavior, thoughts, and/or emotions to override predispositions or contextual lures (Diamond, 2013). Decreased response inhibition is associated with impulsivity which, in turn, is associated with clinical indications linked to the orexin system, including SUDs and eating disorders, characterized by problematic behavioral patterns (Hamilton et al., 2015b; Moeller et al., 2001). In line with this, recent preclinical studies have found that increased activation of medial hypothalamic orexin neurons was correlated with higher accuracy in a palatable-food go/no-go (GNG) task (Freeman and Aston-Jones, 2020), while suvorexant reduced cocaine-induced premature responding in the 5-choice serial reaction time task that assesses response impulsivity (Gentile et al., 2018b).
Several experimental medicine tasks provide measures of response inhibition in humans. Here we focus on the GNG, stop signal (SS), and Stroop tasks, as these are technically less challenging to implement and can easily incorporate affective or disease-relevant stimuli (Table 2). The basic framework of the GNG and SS tasks can be employed in both preclinical and clinical research. The GNG task has shown cross-species consistency in studies on neurobiological underpinnings of impulsivity and effects of drug manipulation (Eagle et al., 2008; Hamilton et al., 2015a). For example, rat studies have implicated serotonin as a modulator of impulsivity (Harrison et al., 1999), whilst in human research fMRI effects during no-go (response inhibition) trials are differentially affected by administration of citalopram vs. tryptophan depletion (interventions posited to increase and decrease serotonin activity, respectively) (Macoveanu et al., 2013). The SS task has also shown some cross-species consistency in neural circuitry. For example, noradrenaline reuptake inhibitors improve SS task performance in both humans and animals (Chamberlain et al., 2006; Robinson et al., 2008). Similar brain areas have been implicated across species, with rat-lesion models and fMRI studies suggesting prefrontal cortical involvement in SS task performance (Aron et al., 2007; Bari et al., 2011).
Table 2.
Experimental medicine tasks that provide measures of response inhibition in humans
| Task | Task description | Key performance outcomes | Potential implementation and analytics issues |
|---|---|---|---|
|
|
|
|
|
| Go/No-Go task / Affective Go/No-Go task * | In the classic Go/No-Go task the participant must respond rapidly to a cue (e.g., X) but withhold a response when a no-go stimuli is shown (e.g., O). Typically target stimuli are presented more frequently than non-target stimuli (e.g., at a ratio of 3:1) in order to elicit prepotent responses to the target stimuli. Go/No-Go tasks that include affective or disease-relevant stimuli have been developed (Hege et al., 2015; Lyu et al., 2017; Mobbs et al., 2011). False alarms are the key performance outcome associated with response inhibition. Other measures include errors of omission, hits, successfully inhibited responses, and reaction times for hits. Some researchers calculate target sensitivity and/or response bias, although these appear to be less favored in the literature (Meule, 2017), possibly because interpretation is more unclear. Where different conditions have been employed, endpoints are explored by task condition, e.g. false alarms to affective stimuli vs. false alarms to neutral stimuli. Task is often quite long (approximately 15 minutes) to allow for a sufficient number of false alarms (Hege et al., 2015; Lyu et al., 2017). If adopting an affective version of the Go/No-Go, affective stimuli can be paired with the Go or the No-Go response, or both, and the task can take a block design or a mixed trials design. Block designs can allow investigation of the participants' ability to shift between rules (Meule, 2017). However, this can extend task duration and increase participant burden. Stop Signal Reaction Time task / Affective Stop Signal Reaction Time Task* Participants | False alarms are the key performance outcome associated with response inhibition. Other measures include errors of omission, hits, successfully inhibited responses, and reaction times for hits. Some researchers calculate target sensitivity and/or response bias, although these appear to be less favored in the literature (Meule, 2017), possibly because interpretation is more unclear. Where different conditions have been employed, endpoints are explored by task condition, e.g. false alarms to affective stimuli vs. false alarms to neutral stimuli. | Task is often quite long (approximately 15 minutes) to allow for a sufficient number of false alarms (Hege et al., 2015; Lyu et al., 2017). If adopting an affective version of the Go/No-Go, affective stimuli can be paired with the Go or the No-Go response, or both, and the task can take a block design or a mixed trials design. Block designs can allow investigation of the participants’ ability to shift between rules (Meule, 2017). However, this can extend task duration and increase participant burden. |
| Stop Signal Reaction Time task / Affective Stop Signal Reaction Time Task * | Participants perform a continuous or repetitive motor response, for example repeatedly pressing a button or touching a screen, in response to a ‘go’ cue. On some trials the ‘go’ cue is proceeded by a ‘stop’ cue indicating that the participant should withhold the response. The task can be modified to include affective stimuli, for example by presenting affective images prior to the go cue (Kalanthroff et al., 2013) or using affective stimuli as the go or stop cues (Svaldi et al., 2014). | Stop signal reaction time (SSRT), which is a measure of the speed of inhibiting the response after the stop cue has been given. Since correct inhibitions result in a lack of response the SSRT cannot be directly measured, and therefore mathematical models such as the independent race model (Logan and Cowan, 1984) are used to estimate the SSRT based on other endpoints such as the RT on unsuccessful stop trials, RTs on go trials, and the probability of making a false response. | There can be challenges when estimating the SSRT. Researchers must ensure that task design is appropriate for the given population, generating sufficient number of both successful and unsuccessful stop trials. Analysts must ensure that the underlying assumptions of the mathematical model are met before estimating the SSRT, otherwise estimates and therefore conclusions may be unreliable. See Verbruggen et al. for a comprehensive review of challenges in the design and analysis of the Stop Signal Task (Verbruggen et al., 2019). Task duration is approximately 10 minutes. |
| Stroop / Emotional Stroop * | Participants must respond to one feature of presented word stimuli, which is incongruent, on some trials, with the semantic properties of the word presented. For example the word ‘blue’ is presented in red color font, and the participant must name the color and inhibit the prepotent response to read the word. In the affective version of the Stroop, and those based on substances of abuse, disease-relevant word stimuli may produce attentional biases towards or away from the stimuli, compared to neutral word stimuli. | Accuracy and reaction time (for correct responses) for each trial type (e.g., incongruent vs congruent stimuli, or diseaserelevant vs. neutral). | As with the Affective Go/No-Go task, the Emotional Stroop can be implemented in either a block design or a mixed design. Stroop tasks are usually implemented with either button-press responses or vocal responses. However, we are not aware of comparative research which compares and contrasts these response types. Finally, there is a choice of whether or not to use masked stimuli. Duration of task incorporating several stimulus categories is roughly 5–10 minutes. |
If using image stimuli, there are considerations to be made about sourcing and selecting images (see main text).
Response inhibition tasks were designed to gauge rapid response impulsivity as specific measures of response inhibition subtypes (Fineberg et al., 2014; Hamilton et al., 2015b). Schachar et al. suggested that the GNG task provides a measure of action restraint (preventing action before initiation) while the SS task assesses action cancellation (inhibiting response during execution) (Schachar et al., 2007). This distinction is supported by a meta-analysis of fMRI results revealing distinctions in the neural networks recruited by GNG and SS tasks (Swick et al., 2011). This has led to recommendations that researchers consider using both tasks for assessments of response inhibition (Hamilton et al., 2015a). For many clinical trials, this may be limited by participant burden. It may also be important to determine if specific aspects of response impulsivity are aberrant in the population under study and/or have an established association with clinically relevant measures before initiating clinical testing.
The basic Stroop and emotional Stroop tests assess response inhibition and attentional biases, such as orienting towards and/or avoidance of stimuli, as well as cognitive conflict and interference impulsivity. Therefore, Stroop tasks may be considered not to be specific measures of response inhibition, but rather of assessing cognitive control (Bustamante et al., 2021; Starzomska, 2017).
The classical GNG, SS, and Stroop tasks have been adapted to include affective or disease-relevant stimuli to increase ecological validity and sensitivity in specific clinical populations. Some researchers have speculated that disease- or symptom-specific stimuli may be important to isolate distinct differences that contribute to the emergence and maintenance of psychiatric problems. One example of this is the use of food-related stimuli in eating-disorder populations (Berner et al., 2017). Limited systematic research comparing disease-relevant and neutral stimuli in response-inhibition tasks has suggested greater impairments in inhibitory control when disease-relevant stimuli are used in some groups (Wu et al., 2013). On the other hand, a study by Lyu et al. found no difference in false alarm rate (response inhibition) when comparing women who binge eat and weight-matched individuals without binge eating, when performing a GNG task incorporating food (disease-relevant) stimuli and household object (neutral) stimuli (Lyu et al., 2017). We suggest that, where possible, both disease-relevant and neutral stimuli should be used to determine specific versus generalized effects of orexin receptor modulation.
A meta-analysis of GNG- and SS-related POMs in patients with heavy substance use or addiction-like behaviors found significant case-control differences for heavy use/addiction to cocaine, tobacco, and alcohol and gambling (Smith et al., 2014). A meta-analysis of the emotional Stroop task found that individuals with post-traumatic stress disorder (PTSD), MDD and anxiety disorders showed greater interference by disease-related stimuli, compared with healthy individuals (Joyal et al., 2019). Furthermore, people with PTSD and affective disorders showed more interference from positive and negative stimuli, respectively. Wu et al. conducted a meta-analysis of neuropsychological studies on inhibitory control in bulimic-type eating disorders, considering both general and disease-specific stimuli. This included studies using the GNG, SS, and Stroop tasks, which identified significant deficits in response inhibition across bulimic-type eating-disorder groups for general stimuli and greater impairments in inhibitory control in bulimia nervosa for disease-relevant stimuli (Wu et al., 2013). However, a recent meta-analysis by Cury et al. found impaired working memory in individuals with obesity and BED in comparison to those with obesity without BED (Cury et al., 2020).
Some studies have reported drug sensitivities of response inhibition tasks. In a randomized controlled trial of treatment-resistant depression, in which patients received 0.5 mg/kg ketamine, 0.2 mg/kg ketamine or saline infusion, Chen et al. found an increase in GNG correct responses and a decrease in omission rate in the high-dose ketamine group at day 14 post-infusion. Also, the improvement in sustained attention and response control was negatively associated with depressive symptoms in the high-dose ketamine group, suggesting improved cognitive function (Chen et al., 2018). Nathan et al. examined effects of the GSK598809 dopamine D3 receptor antagonist versus placebo on attentional bias to rewarding food cues in individuals with overweight and obesity and binge and emotional eating. In a Stroop task with food stimuli, the GSK598809 treatment had no effects on attentional bias, although bias scores were inversely correlated with a measure of eating restraint and allowed identification of low- and high-restraint subpopulations (Nathan et al., 2012). Pringle et al. assessed the effects of acute low-dose acute tryptophan depletion (ATD) in females who were dieting. While ATD had no significant effect on mood or hunger, it led to increased interference during the processing of negative stimuli in a masked version of the emotional Stroop test, including stimuli related to eating, weight, and shape (Pringle et al., 2012). As another example of drug sensitivity, Franken et al. found that after a single oral dose of haloperidol versus placebo, detoxified heroin-dependent patients’ performance outcome was better in an emotional Stroop task, which included heroin-related or neutral words (Franken et al., 2004).
Other studies have reported the ecological validity of response inhibition tasks, modified to involve presentation of disease-specific stimuli. For example, individuals with obesity and BED made more errors in a food- and body-specific version of the GNG, compared to individuals with obesity alone (Mobbs et al., 2011). Decreased response inhibition in a food GNG correlated with trait-like attentional impulsiveness in BED (Hege et al., 2015), helping validate the GNG as a measure of impulsivity in this population. Another study found alcohol-specific impairments in response inhibition in individuals with binge drinking (Czapla et al., 2015). The same study showed that false alarms to alcohol stimuli were a significant predictor of binge drinking, and this had greater predictive power than general impulsivity scales. These findings support the sensitivity of disease-specific GNG tasks in identifying disease-relevant impulsivity differences.
The response inhibition tasks described above are often associated with few implementation complexities, although sourcing and selection of image stimuli may be subject to the same issues as described for the AAT and ROC (see previous section). Table 2 also provides more detail about implementation issues for each of the response inhibition tasks considered here.
6. Limitations and challenges
This review supports the proposition that experimental medicine approaches can be valuable in at least two aspects of drug development: 1) translation of preclinical findings to relevance in humans, and 2) characterization of the effects of treatments in clinical trials. However, there are still limitations and challenges for each of these applications.
6.1. Translating research from animals to humans for drug development: POMs considerations
The ‘fail-fast’ approach is predicated on the goal of eliminating early candidate drugs that are unlikely to be successful. We posit that experimental medicine approaches could bridge the translational gap of limited predictive utility of animal models in psychiatry, given the subjective nature and heterogeneity of many psychiatric disorders. The methods used to assess target engagement and treatment effects should be founded on sound understanding of the fundamental biology and pathology of the disorder in question (Gould and Manji, 2004). Unfortunately, knowledge of underlying molecular factors contributing to disease and mechanisms remains inadequate and has not materialized into new and efficacious treatments for psychiatric disorders, compared to other disease areas. While some of the POMs described above could be linked to dysregulation of various neurotransmitter systems, use of these measures can lead to wrong conclusions about drug effects if knowledge is not available regarding how and when a specific behavioral change impacts the disease. There is also the requirement of knowing how these POMs signal dysregulation of the orexin system. In addition, disease heterogeneity may impact the interpretation of these outcomes, as may also be the case with clinician- and patient-reported outcomes. Evaluation within clearly defined patient and control groups may constitute a critical first step to establishing assay sensitivity, independent of intervention effects. Thus, variables such as age, sex/gender, disease presentation, medical history, cultural diversity, and genetic background should be considered when designing these studies. Other important challenges include demonstrating reproducibility of findings and taking into account standardized implementation of the POMs and appropriate statistical analyses. The potential advantage of trans-diagnostic relevance of some of these POMs may need to be balanced against the targeting of more pronounced manifestations of an outcome of interest, when researching a specific condition.
6.2. Characterization of treatment effects and clinical benefit
A key challenge to wider use and acceptance of POMs to support drug development and eventual approval is the lack of formal scientific guidance. Despite the current lack of specific guidance on evidentiary requirements for POMs in drug development and registration, many of the principles described in the FDA Patient-Reported Outcome guidance are applicable, including endpoint modeling, choice of instrument, content validity, test-retest reliability, ability to detect change, ceiling/floor effects, other psychometric properties, clinical trial design, and data analysis (U. S. Food & Drug Administration, 2009). Since COAs are intended to measure treatment effects on how a patient feels or functions, it should be clear what aspect of the condition the POM represents. Hence, the concept of interest and context of use in which the measure is applied are critical to the choice of paradigm and corresponding assessment. A consistent understanding of assessment instructions by trial subjects and investigators and unambiguous interpretation of scores are further elements of content validity that should be demonstrable in association with the behavioral outcome. In addition, it should be determined if the results obtained with POMs stand alone or if additional data from other COAs are needed to interpret the findings.
Establishment of clinically meaningful within-patient change is another important aspect of COA development and validation and may challenge POMs. For example, depressed patients often do not respond to the first antidepressant prescribed, resulting in multiple attempts to find a treatment at a suitable dose that reduces symptoms. As a result, for some patients it can take months before symptoms are reduced. A personalized medicine approach offers a means of reducing this delay by utilizing early biomarkers of treatment response. Browning et al. assessed the clinical effectiveness of such an approach using a predictive algorithm based on the FERT combined with measures of subjective symptoms to guide antidepressant treatment (Browning et al., 2019). They conducted a multi-center, open-label, randomized controlled trial with 913 medication-free depressed patients assigned to have their antidepressant treatment guided by the predictive algorithm (PReDicT arm) or treatment as usual. There was a significantly greater reduction in anxiety symptoms at week 8 and a greater improvement in functional outcome at week 24 in the PReDicT arm, showing that POMs can also be deployed to improve real-world outcomes.
Normative data for disorders with many phenotypes may not exist or otherwise be difficult to generate. Relevance to patient day-to-day activities is often used to gauge clinical meaningfulness, and the behavioral concepts assessed by POMs may not be linked directly to these or may be associated with other activities that are difficult to disentangle. There are also operational challenges that come with implementation of POMs across different age groups and in global clinical trials with culturally diverse populations. Cognitive behavior is influenced by environmental and socio-economic factors, and ensuring consistency in implementation and interpretation of POMs needs to go beyond simple language barriers to cross-cultural adaptation. Finally, feasibility of implementation also depends on complexity, duration and frequency of the task/assessment, and may involve complex considerations for some psychiatric conditions.
7. Conclusions and future perspectives
There remains an unmet medical need in the treatment of neuropsychiatric disorders. Despite progress in understanding the biological underpinnings of these disorders, translation of basic neuroscience findings to development of novel and improved therapies has been slow to materialize. In this review, we have described how experimental medicine approaches can identify POMs to provide an indication of efficacy throughout the drug development process, using modulators of the orexin system as an example. Preclinical studies have implicated the orexin system in emotional, cognitive, and behavioral processes, including stress responses, reward processing, motivations, feeding, and sleep. The first clinical investigations of orexin receptor antagonists in psychiatric disorders, besides insomnia, have started, and some initial promising results in the fields of MDD and SUDs have emerged. The investigation of the effects of orexin receptor agonists in the clinic is still in its infancy and may hold great promise for the future.
Use of POMs may facilitate research on the orexin system and other targets in the field of psychiatry by providing more objective measures of brain function, complementing the standard patient-reported or clinician-observed changes in symptoms. While there are still challenges surrounding the development, validation, and operationalization of these POMs arising from experimental medicine approaches, we encourage the inclusion of these measures in studies investigating the effects of novel drugs for neuropsychiatric disorders. Since these assessments are intended to measure treatment effects at the symptom level, the concept of interest and context of use are important in the choice of paradigms and corresponding POMs. This requires understanding of assessment instructions by study participants and investigators, and unambiguous interpretation of assessment scores, depending on the types of behavioral outcomes being assessed.
Wider use and acceptability of POMs generated by experimental-medicine approaches will benefit from collaborative partnership models. Public-private partnerships (PPPs) such as NIMH-MATRICS and the EU’s Innovative Medicines Initiative (https://www.imi.europa.eu/) can offer an arena for cooperation between diverse stakeholders including not only pharmaceutical companies and Universities, but also small- and medium-sized enterprises (SMEs), regulatory bodies, health-technology-assessment organizations and patient groups (de Vrueh et al., 2019).
PPPs are seen as well-suited to pre-competitive research initiatives (de Vrueh and Crommelin, 2017) such as those that working towards the validation of POMs. PPPs in the area of CNS research have in some cases demonstrated impact in, for example, the form of tools, standards, platforms, and cohort datasets (de Vrueh and Crommelin, 2017; Innovative medicines initiative, 2016; O’Rourke et al., 2022). However, translation of research into tangible benefits that meet industry partner needs, sustainability, administrative burden, and management of non-performing partners can be issues of concern (O’Rourke et al., 2022). Furthermore, the timelines associated with PPPs – often playing out over the course of 5 or more years, not including application timelines – can be at odds with the more rapid pace of change in the objectives of industry partners. The IMI-funded PRISM project initially included seven pharmaceutical partners (https://prism-project.eu/en/prism-study/), whilst the follow-on PRISM2 project has only one (https://prism-project.eu/en/prism-study/), possibly reflecting change in business objectives of these companies over the projects’ lifetime. An alternative model for collaborative partnership which can offer more agility is the SME-led private precompetitive consortia, such the Reward Task Optimization consortium (https://www.p1vital.com/research/rtoc/). Although associated with different strengths and weaknesses, these different frameworks represent important opportunities for technical validation of experimental-medicine approaches for research or drug-registration purposes, and also adoption of such approaches in real-world clinical practice.
Highlights.
Translation from animal models to clinical use in psychiatry remains challenging
Experimental medicine approaches may support investigation of efficacy in humans
Preclinical studies suggest potential of orexin modulation in psychiatric disorders
Experimental medicine approaches in studies of orexin modulation are reviewed
Validation and operationalization are key challenges to more widespread adoption
Acknowledgements
The authors thank Anne Sayers (MA(Cantab)) of Idorsia Pharmaceuticals Ltd. for providing editorial support, which was funded by Idorsia Pharmaceuticals Ltd. in accordance with Good Publications Practice (GPP 2022) guidelines.
Funding
Idorsia Pharmaceuticals Ltd. provided funding to support the preparation of this article. Dr. Potenza’s involvement was supported by the NIH grant R01 DK121551.
Footnotes
Declaration of interest
Amy C. Beckenstrom: Part-time employee of P1vital Ltd.
Preciosa M. Coloma: Full-time employee of, and owns shares at, Idorsia Pharmaceuticals Ltd.
Gerard R. Dawson: Full-time employee of P1vital Ltd.
Ailidh K. Finlayson: Part-time employee of P1vital Ltd.
Asad Malik: Full-time employee of P1vital Ltd.
Anke Post: None
Michel Alexander Steiner: Full-time employee of, and owns shares at, Idorsia Pharmaceuticals Ltd.
Marc N. Potenza: Dr. Potenza has consulted for Opiant Therapeutics, Game Day Data, Baria-Tek, the Addiction Policy Forum, AXA and Idorsia Pharmaceuticals; has been involved in a patent application with Yale University and Novartis; has received research support from Mohegan Sun Casino and the Connecticut Council on Problem Gambling; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for and/or advised gambling and legal entities on issues related to impulsive, compulsive and addictive disorders; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts
Conflict of interest statement
Dr. Potenza discloses the following. Dr. Potenza has consulted for Opiant Therapeutics, Game Day Data, Baria-Tek, the Addiction Policy Forum, AXA and Idorsia Pharmaceuticals; has been involved in a patent application with Yale University and Novartis; has received research support from Mohegan Sun Casino and the Connecticut Council on Problem Gambling; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for and/or advised gambling and legal entities on issues related to impulsive, compulsive and addictive disorders; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr Coloma and Dr Steiner are full-time employees of, and own shares at, Idorsia Pharmaceuticals Ltd.
Dr Malik and Dr Dawson are full-time employees of P1vital Ltd.
Dr Beckenstrom and Dr Finlayson are part-time employees of P1vital Ltd.
The other authors do not report disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T, 2015. Comprehensive Behavioral Analysis of Male Ox1r (−/−) Mice Showed Implication of Orexin Receptor-1 in Mood, Anxiety, and Social Behavior. Front Behav Neurosci 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T, Pounder Z, Preston S, Hanson A, Gallagher P, Harmer CJ, McAllister-Williams RH, 2016. Test-retest reliability and task order effects of emotional cognitive tests in healthy subjects. Cogn Emot 30, 1247–1259. [DOI] [PubMed] [Google Scholar]
- Agostinelli LJ, Ferrari LL, Mahoney CE, Mochizuki T, Lowell BB, Arrigoni E, Scammell TE, 2017. Descending projections from the basal forebrain to the orexin neurons in mice. J Comp Neurol 525, 1668–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K, Taillieu A, Daniels N, Soriano JR, Prinsen J, 2021. Oxytocin enhances neural approach towards social and non-social stimuli of high personal relevance. Sci Rep 11, 23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA, 2007. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27, 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA, 2010. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res 1314, 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL, 2015. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172, 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN, 2015. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry 77, 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantin T, Stevens S, Gerlach AL, Hermann C, 2016. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. J Behav Ther Exp Psychiatry 50, 40–51. [DOI] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW, 2011. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci 31, 9254–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Bourke C, Colhoun H, Carter F, Frampton C, Porter R, 2011. The misclassification of facial expressions in generalised social phobia. J Anxiety Disord 25, 278–283. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Crosby RD, Cao L, Engel SG, Lavender JM, Mitchell JE, Wonderlich SA, 2017. Temporal associations between affective instability and dysregulated eating behavior in bulimia nervosa. J Psychiatr Res 92, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DL, Badve PS, Barson JR, Bass CE, Espana RA, 2018. Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine. Addict Biol 23, 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM, 2012. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther 134, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Siegel JM, 2013. Relation of melanin concentrating hormone levels to sleep, emotion and hypocretin levels. Sleep 36, 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, 2019. Releasing the brake on eating: Obesity alters neuronal gene expression and activity that may influence overeating. Science 364, 1233–1234. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell RG, Sun W, Suzuki S, Kober H, 2018. Training in cognitive strategies reduces eating and improves food choice. Proc Natl Acad Sci U S A 115, E11238-E11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L, 2005. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, Halfon O, 2013. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Magruder KP, Korhumel RA, Curtin JJ, 2014. Using the threat probability task to assess anxiety and fear during uncertain and certain threat. J Vis Exp, 51905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F, 2007. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13, 150–155. [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Hahn C, Reetz C, Schmidt U, Friederich HC, 2015. Approach bias and cue reactivity towards food in people with high versus low levels of food craving. Appetite 95, 197–202. [DOI] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, Espana RA, 2015. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behav Brain Res 291, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL, 2001. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40. [DOI] [PubMed] [Google Scholar]
- Brown RM, Dayas CV, James MH, Smith RJ, 2022. New directions in modelling dysregulated reward seeking for food and drugs. Neurosci Biobehav Rev 132, 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Kingslake J, Dourish CT, Goodwin GM, Harmer CJ, Dawson GR, 2019. Predicting treatment response to antidepressant medication using early changes in emotional processing. Eur Neuropsychopharmacol 29, 66–75. [DOI] [PubMed] [Google Scholar]
- Bustamante L, Lieder F, Musslick S, Shenhav A, Cohen J, 2021. Learning to Overexert Cognitive Control in a Stroop Task. Cogn Affect Behav Neurosci 21, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MM, Wiedemann AA, Macdonald-Gagnon G, Potenza MN, 2021. Impulsivity and compulsivity in binge eating disorder: A systematic review of behavioral studies. Prog Neuropsychopharmacol Biol Psychiatry 110, 110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, 2013. Orexin, orexin receptor antagonists and central cardiovascular control. Front Neurosci 7, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G, 2014. Role of orexin/hypocretin in conditioned sucroseseeking in female rats. Neuropharmacology 86, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, AstonJones G, 2010. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav 100, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC, 2017. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci U S A 114, E9125–E9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Terry RA, Berridge KC, 2016. Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’. Neuropsychopharmacology 41, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz M, Karaj V, Kocabasoğlu N, Gozubatik-Celik G, Dirican A, Bayoglu B, 2019. Orexin/hypocretin receptor, Orx(1), gene variants are associated with major depressive disorder. Int J Psychiatry Clin Pract 23, 114–121. [DOI] [PubMed] [Google Scholar]
- Ch’ng SS, Lawrence AJ, 2015. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: A characterisation of OX1R-eGFP mice. J Chem Neuroanat 66-67, 1–9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ, 2006. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311, 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro J, Bernardi S, Potenza MN, Grant JE, Marsh R, Wang S, Blanco C, 2012. Impulsivity in the general population: a national study. J Psychiatr Res 46, 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell TE, Lee CE, Richardson JA, Williams SC, Xiong Y, Kisanuki YY, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M, 1999. Narcolepsy in orexin Knockout Mice: Molecular Genetics of Sleep Regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, Cheng CM, Su TP, 2018. Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord 241, 1–7. [DOI] [PubMed] [Google Scholar]
- Chen X, Liao Z, Wong YT, Guo Y, He J, 2014. Time course of the dependence of associative memory retrieval on the entorhinal cortex. Neurobiol Learn Mem 116, 155–161. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC, 2010. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167, 11–20. [DOI] [PubMed] [Google Scholar]
- Chong TTJ, Bonnelle V, Husain M, 2016. Chapter 4 - Quantifying motivation with effort-based decision-making paradigms in health and disease, in: Studer B, Knecht S. (Eds.), Progress in Brain Research. Elsevier, pp. 71–100. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Matsubara T, Miyazaki T, Ono D, Fukatsu N, Abe M, Sakimura K, Sudo Y, Yamanaka A, 2019. GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Kim JG, Kim JW, Kim HW, Yoon BJ, 2014. Orexin administration to mice that underwent chronic stress produces bimodal effects on emotion-related behaviors. Regul Pept 194–195, 16–22. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov, 2022a. NCT02593682. The Role of Orexin in Human Panic Disorder. URL: https://clinicaltrials.gov/ct2/show/NCT02593682. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022b. NCT02785406. Role of the Orexin Receptor System in Stress, Sleep and Cocaine Use. URL: https://clinicaltrials.gov/ct2/show/NCT02785406. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022c. NCT03557710. Devaluing Foods to Change Eating Behavior. URL: https://clinicaltrials.gov/ct2/show/NCT03557710. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022d. NCT03999099. Targeting Orexin to Treat Nicotine Dependence. URL: https://clinicaltrials.gov/ct2/show/NCT03999099. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022e. NCT04229095. Medication Development in Alcoholism: Suvorexant Versus Placebo. URL: https://clinicaltrials.gov/ct2/show/NCT04229095. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022f. NCT04234997. Suvorexant to Reduce Symptoms of Nicotine Use. URL: https://clinicaltrials.gov/ct2/show/NCT04234997. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022g. NCT04448431. Comparison of Vortioxetine and Desvenlafaxine in Adult Patients Suffering From Depression (VIVRE). URL: https://clinicaltrials.gov/ct2/show/NCT04448431. Accessed September 15, 2022.
- Clinicaltrials.gov, 2022h. NCT05387148. The Efficacy and Neurobehavioural Mechanism of Cannabidiol (CBD) for Alcohol Dependence. URL: https://clinicaltrials.gov/ct2/show/NCT05387148. Accessed September 15, 2022. [Google Scholar]
- Cole S, Keefer SE, Anderson LC, Petrovich GD, 2020. Medial Prefrontal Cortex Neural Plasticity, Orexin Receptor 1 Signaling, and Connectivity with the Lateral Hypothalamus Are Necessary in Cue-Potentiated Feeding. J Neurosci 40, 1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD, 2015. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Sci Rep 5, 16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RM, Rowe AC, Penton-Voak IS, 2008. The role of trait anxiety in the recognition of emotional facial expressions. J Anxiety Disord 22, 1120–1127. [DOI] [PubMed] [Google Scholar]
- Culbreth A, Westbrook A, Barch D, 2016. Negative symptoms are associated with an increased subjective cost of cognitive effort. J Abnorm Psychol 125, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Moran EK, Kandala S, Westbrook A, Barch DM, 2020a. Effort, avolition and motivational experience in schizophrenia: Analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin Psychol Sci 8, 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Westbrook A, Braver TS, Barch DM, 2020b. Effort in daily life: relationships between experimental tasks and daily experience. Motiv Sci 6, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury MEG, Berberian A, Scarpato BS, Kerr-Gaffney J, Santos FH, Claudino AM, 2020. Scrutinizing Domains of Executive Function in Binge Eating Disorder: A Systematic Review and Meta-Analysis. Front Psychiatry 11, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, 2014. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology 51, 1205–1206. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla M, Simon JJ, Friederich HC, Herpertz SC, Zimmermann P, Loeber S, 2015. Is binge drinking in young adults associated with an alcohol-specific impairment of response inhibition? Eur Addict Res 21, 105–113. [DOI] [PubMed] [Google Scholar]
- Dapelo MM, Surguladze S, Morris R, Tchanturia K, 2017. Emotion Recognition in Face and Body Motion in Bulimia Nervosa. Eur Eat Disord Rev 25, 595–600. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M, 1999. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A 96, 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C, 2010a. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite 54, 208–213. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010b. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Goodwin GM, 2005. Experimental medicine in psychiatry. J Psychopharmacol 19, 565–566. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Post A, Smart TS, Browning M, Harmer CJ, 2021. Accuracy in recognising happy facial expressions is associated with antidepressant response to a NOP receptor antagonist but not placebo treatment. J Psychopharmacol 35, 1473–1478. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg EKF, Gautvik VT, Bartlett FSI, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrueh RLA, Crommelin DJA, 2017. Reflections on the Future of Pharmaceutical Public-Private Partnerships: From Input to Impact. Pharm Res 34, 1985–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrueh RLA, de Vlieger JSB, Crommelin DJA, 2019. Editorial: Public-Private Partnerships as Drivers of Innovation in Healthcare. Front Med 6, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Lonstein JS, Yan L, 2014. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 272, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Annu Rev Psychol 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty TJ, Japee S, Ingvar M, Ungerleider LG, 2013. Fearful face detection sensitivity in healthy adults correlates with anxiety-related traits. Emotion 13, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW, 2008. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 199, 439–456. [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L, 2016. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19, 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N, 2003. Universals and Cultural Differences in Recognizing Emotions. Current Directions in Psychological Science 12, 159–164. [Google Scholar]
- Espana RA, 2012. Hypocretin/orexin involvement in reward and reinforcement. Vitam Horm 89, 185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR, 2011. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 214, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR, 2010. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Hazel J, Faessel H, Wu J, Hang Y, Alexander R, Rosen L, Hartman D, 2019. Results of a phase 1, 4-period crossover, placebo-controlled, randomized, single dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-925, a novel orexin 2 receptor agonist, in sleep-deprived healthy adults, utilizing modafinil as an active comparator. Sleep Medicine 64. [Google Scholar]
- Fadel J, Frederick-Duus D, 2008. Orexin/hypocretin modulation of the basal forebrain cholinergic system: insights from in vivo microdialysis studies. Pharmacol Biochem Behav 90, 156–162. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Shekar S, Gorwood PA, Voon V, Morein-Zamir S, Denys D, Sahakian BJ, Moeller FG, Robbins TW, Potenza MN, 2014. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 19, 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F, 2014. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology 39, 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM, Stam CJ, Van den Brink W, 2004. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol 14, 503–508. [DOI] [PubMed] [Google Scholar]
- Freeman LR, Aston-Jones G, 2020. Activation of Medial Hypothalamic Orexin Neurons during a Go/No-Go Task. Brain Res 1731, 145928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN, 2004. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci 24, 8741–8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P, 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30, 1603–1614. [DOI] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Barker DJ, Shaw JK, España RA, Muschamp JW, 2018a. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol 23, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, Muschamp JW, 2018b. Effects of Suvorexant, a Dual Orexin/Hypocretin Receptor Antagonist, on Impulsive Behavior Associated with Cocaine. Neuropsychopharmacology 43, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L, 2014. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol 29, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Calcott RD, Berkman ET, 2013. Piece of cake. Cognitive reappraisal of food craving. Appetite 64, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Harmer CJ, 2021. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology (Berl) 238, 1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Manji HK, 2004. The molecular medicine revolution and psychiatry: bridging the gap between basic neuroscience research and clinical psychiatry. J Clin Psychiatry 65, 598–604. [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, Gold JM, 2015. Effort-Based Decision Making: A Novel Approach for Assessing Motivation in Schizophrenia. Schizophr Bull 41, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-García A, Calvo MG, 2017. Social anxiety and threat-related interpretation of dynamic facial expressions: Sensitivity and response bias. Personality and Individual Differences 107, 10–16. [Google Scholar]
- Haaker J, Golkar A, Hermans D, Lonsdorf TB, 2014. A review on human reinstatement studies: an overview and methodological challenges. Learn Mem 21, 424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DNC, Smith MI, Piper DC, Hunter AJ, Porter R, Upton N, 1999. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A 96, 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Littlefield AK, Anastasio NC, Cunningham KA, Fink LHL, Wing VC, Mathias CW, Lane SD, Schütz CG, Swann AC, Lejuez CW, Clark L, Moeller FG, Potenza MN, 2015a. Rapid-response impulsivity: definitions, measurement issues, and clinical implications. Personal Disord 6, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schutz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG, 2015b. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal Disord 6, 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schütz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG, 2015c. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal Disord 6, 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Potenza MN, 2012. Relations among delay discounting, addictions, and money mismanagement: implications and future directions. Am J Drug Alcohol Abuse 38, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP, 2009. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci 29, 802–812. [DOI] [PubMed] [Google Scholar]
- Han Y, Yuan K, Zheng Y, Lu L, 2020. Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders. Neurosci Bull 36, 432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Yanagisawa M, Sakurai T, 2005. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett 380, 239–242. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, 2013. Emotional processing and antidepressant action. Curr Top Behav Neurosci 14, 209–222. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ, 2017. How do antidepressants work? New perspectives for refining future treatment approaches. The Lancet Psychiatry 4, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G, 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW, 1999. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go:no-go conditional visual discrimination. Behav Brain Res 100, 99–112. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Chapman H, Taylor C, Moore GBT, Cawthorne MA, Tadayyon M, Clapham JC, Arch JRS, 2002. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept 104, 153–159. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter R, Arch JRS, 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96, 45–51. [DOI] [PubMed] [Google Scholar]
- Hege MA, Stingl KT, Kullmann S, Schag K, Giel KE, Zipfel S, Preissl H, 2015. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int J Obes (Lond) 39, 353–360. [DOI] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE, 2010. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169, 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer K, Lange WG, Isaac L, Rinck M, Becker ES, 2010. Morphed emotional faces: emotion detection and misinterpretation in social anxiety. J Behav Ther Exp Psychiatry 41, 418–425. [DOI] [PubMed] [Google Scholar]
- Heuer K, Rinck M, Becker ES, 2007. Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behav Res Ther 45, 2990–3001. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC, 2013. An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ, 2012. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Byrne NJ, Zamlynny B, Tummala S, Xiao L, Shipman JM, Partridge AT, Minnick C, Breslin MJ, Rudd MT, Stachel SJ, Rada VL, Kern JC, Armacost KA, Hollingsworth SA, O’Brien JA, Hall DL, McDonald TP, Strickland C, Brooun A, Soisson SM, Hollenstein K, 2021. Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation. Nat Commun 12, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM, Marder SR, Wynn JK, Young JW, Green MF, 2015. Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 2—External Validity and Correlates. Schizophr Bull 41, 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN, 2006. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 95, 1656–1668. [DOI] [PubMed] [Google Scholar]
- Huhn AS, Finan PH, Gamaldo CE, Hammond AS, Umbricht A, Bergeria CL, Strain EC, Dunn KE, 2022. Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Sci Transl Med 14, eabn8238. [DOI] [PubMed] [Google Scholar]
- Hunter LR, Buckner JD, Schmidt NB, 2009. Interpreting facial expressions: the influence of social anxiety, emotional valence, and race. J Anxiety Disord 23, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Roiser JP, 2018. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci 19, 470–484. [DOI] [PubMed] [Google Scholar]
- Innovative medicines initiative, 2016. IMI Socio-economic Impact Assessment Expert Group - Final Report. URL: https://www.imi.europa.eu/sites/default/files/uploads/documents/reference-documents/SocioeconomicImpactAssessment_FINALMay2016.pdf. Accessed February 2, 2023
- Insel TR, 2014. The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. Am J Psychiatry 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, Kawabe Y, Uchida S, Nakajima R, Saitoh T, Kanda T, Vogt K, Sakurai T, Nagase H, Yanagisawa M, 2017. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A 114, 5731–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G, 2019. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry 85, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarros RB, Salum GA, Belem da Silva CT, Toazza R, de Abreu Costa M, Fumagalli de Salles J, Manfro GG, 2012. Anxiety disorders in adolescence are associated with impaired facial expression recognition to negative valence. J Psychiatr Res 46, 147–151. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A, 2012. Orexin, stress, and anxiety/panic states. Prog Brain Res 198, 133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, TraskmanBendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal M, Wensing T, Levasseur-Moreau J, Leblond J, A TS, Fecteau S, 2019. Characterizing emotional Stroop interference in posttraumatic stress disorder, major depression and anxiety disorders: A systematic review and meta-analysis. PLoS One 14, e0214998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusyte A, Schonenberg M, 2014. Subliminal cues bias perception of facial affect in patients with social phobia: evidence for enhanced unconscious threat processing. Front Hum Neurosci 8, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahveci S, Meule A, Lender A, Blechert J, 2020. Food approach bias is moderated by the desire to eat specific foods. Appetite 154, 104758. [DOI] [PubMed] [Google Scholar]
- Kalanthroff E, Cohen N, Henik A, 2013. Stop feeling: inhibition of emotional interference following stop-signal trials. Front Hum Neurosci 7, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Kim G, Kim H, Lee SH, 2019. The Influence of Anxiety on the Recognition of Facial Emotion Depends on the Emotion Category and Race of the Target Faces. Exp Neurobiol 28, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE, 2009. Orexin Neurons Are Necessary for the Circadian Control of REM Sleep. Sleep 32, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS, 2005. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab 288, E1089–1100. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Ort M, Golor G, Kornberger R, Dingemanse J, 2021. Multiple-dose clinical pharmacology of the selective orexin-1 receptor antagonist ACT-539313. Prog Neuropsychopharmacol Biol Psychiatry 108, 110166. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, Marder S, Hilt DC, Umbricht D, 2017. Placebo Response and Practice Effects in Schizophrenia Cognition Trials. JAMA Psychiatry 74, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer SE, Cole S, Petrovich GD, 2016. Orexin/hypocretin receptor 1 signaling mediates Pavlovian cue-food conditioning and extinction. Physiol Behav 162, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, Lee EH, Kim SW, Lee JK, Kang HS, Han PL, 2015. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis 79, 59–69. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, 2015. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev 56, 315–329. [DOI] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN, 2010. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend 106, 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, 2003. Delay discounting is associated with substance use in college students. Addictive Behaviors 28, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Konova AB, Goldstein RZ, 2019. The emerging neuroscience of appetitive and drug cue extinction in humans. Psychopharmacology (Berl) 236, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koychev I, El-Deredy W, Haenschel C, Deakin JF, 2010. Visual information processing deficits as biomarkers of vulnerability to schizophrenia: an event-related potential study in schizotypy. Neuropsychologia 48, 2205–2214. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, 2013. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol 304, C2–32. [DOI] [PubMed] [Google Scholar]
- Kunz D, Dauvilliers Y, Benes H, Garcia-Borreguero D, Plazzi G, Seboek Kinter D, Coloma P, Rausch M, Sassi-Sayadi M, Thein S, 2023. Long-Term Safety and Tolerability of Daridorexant in Patients with Insomnia Disorder. CNS Drugs 37, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S, Maurage P, D’Hondt F, Billieux J, Dormal V, 2018. Executive Impairments in Binge Drinking: Evidence for a Specific Performance-Monitoring Difficulty during Alcohol-Related Processing. Eur Addict Res 24, 118–127. [DOI] [PubMed] [Google Scholar]
- Lawn W, Freeman TP, Pope RA, Joye A, Harvey L, Hindocha C, Mokrysz C, Moss A, Wall MB, Bloomfield MA, Das RK, Morgan CJ, Nutt DJ, Curran HV, 2016. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology (Berl) 233, 3537–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE, 2005. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 25, 6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN, 2012. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl) 219, 469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M, Leppanen J, Paloyelis Y, Treasure J, 2019. The influence of oxytocin on eating behaviours and stress in women with bulimia nervosa and binge eating disorder. Mol Cell Endocrinol 497, 110354. [DOI] [PubMed] [Google Scholar]
- Li SB, de Lecea L, 2020. The hypocretin (orexin) system: from a neural circuitry perspective. Neuropharmacology 167, 107993. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ, 2010. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 212, 251–265. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M, 2016. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7, 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E, 1999. The Sleep Disorder Canine Narcolepsy Is Caused by a Mutation in the Hypocretin (Orexin) Receptor 2 Gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C, 2008. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry 165, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK, 2002. Hypocretins (Orexins) Regulate Serotonin Neurons in the Dorsal Raphe Nucleus by Excitatory Direct and Inhibitory Indirect Actions. J Neurosci 22, 9453–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, 1984. On the Ability to Inhibit Thought and Action: A Theory of an Act of Control. Psychol Rev 91, 295–327. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O, Meir Drexler S, Meulders A, Nees F, Pittig A, Richter J, Romer S, Shiban Y, Schmitz A, Straube B, Vervliet B, Wendt J, Baas JMP, Merz CJ, 2017. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev 77, 247–285. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF, 2000. Attentional bias for drug cues in opiate dependence. Psychol Med 30, 169–175. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM, 2008. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11, 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Z, Zheng P, Chen H, Jackson T, 2017. Approach and inhibition responses to external food cues among average-weight women who binge eat and weight-matched controls. Appetite 108, 367–374. [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Hornboll B, Elliott R, Erritzoe D, Paulson OB, Siebner H, Knudsen GM, Rowe JB, 2013. Serotonin 2A receptors, citalopram and tryptophandepletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology 38, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Francisco MT, Brewer AT, Stein JS, 2011. Delay discounting and gambling. Behav Processes 87, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17, 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G, 2012. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res 198, 79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE, 2019. The neurobiological basis of narcolepsy. Nat Rev Neurosci 20, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Massar SAA, Csatho A, Van der Linden D, 2018. Quantifying the Motivational Effects of Cognitive Fatigue Through Effort-Based Decision Making. Front Psychol 9, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AS, Rech MA, Lee HJ, 2021. Evaluating the role of Approach-Avoidance Training on action-tendencies in individuals with skin-picking disorder: A preliminary randomized experiment. J Behav Addict 10, 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Sakurai T, 2008. Orexins and Orexin Receptors: From Molecules to Integrative Physiology. Results Probl Cell Differ 46, 27–55. [DOI] [PubMed] [Google Scholar]
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S, 2008. Paying the price: The cost of metnal health care in England to 2026. King’s Fund, London. [Google Scholar]
- Mechelmans DJ, Irvine M, Banca P, Porter L, Mitchell S, Mole TB, Lapa TR, Harrison NA, Potenza MN, Voon V, 2014. Enhanced attentional bias towards sexually explicit cues in individuals with and without compulsive sexual behaviours. PLoS One 9, e105476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, 2017. Reporting and Interpreting Task Performance in Go/No-Go Affective Shifting Tasks. Front Psychol 8, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Richard A, Lender A, Dinic R, Brockmeyer T, Rinck M, Blechert J, 2020. Measuring approach-avoidance tendencies towards food with touchscreen-based arm movements. Psychol Res 84, 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GM, Marco-Pallares J, Boulinguez P, Sescousse G, 2021. Electrophysiological underpinnings of reward processing: Are we exploiting the full potential of EEG? Neuroimage 242, 118478. [DOI] [PubMed] [Google Scholar]
- Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, Walsh JK, Krystal AD, Benca RM, Cohn M, Lines C, Roth T, Herring WJ, 2014. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol 13, 461–471. [DOI] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T, 2011. Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci 31, 6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM, 2005. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP, 2011. Extinction of drug seeking. Behav Brain Res 217, 454–462. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M, 2011. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite 57, 263–271. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC, 2001. Psychiatric Aspects of Impulsivity. Am J Psychiatry 158, 1783–1793. [DOI] [PubMed] [Google Scholar]
- Montagne B, Schutters S, Westenberg HG, van Honk J, Kessels RP, de Haan EH, 2006. Reduced sensitivity in the recognition of anger and disgust in social anxiety disorder. Cogn Neuropsychiatry 11, 389–401. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN, 2012. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 14, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T, 2004. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 19, 1524–1534. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA Jr., 2014. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A 111, E1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Ochsner KN, Kober H, Kuerbis A, Feng T, Wall M, Morgenstern J, 2015. Cognitive regulation of craving in alcohol-dependent and social drinkers. Alcohol Clin Exp Res 39, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PJ, O’Neill BV, Mogg K, Bradley BP, Beaver J, Bani M, Merlo-Pich E, Fletcher PC, Swirski B, Koch A, Dodds CM, Bullmore ET, 2012. The effects of the dopamine D(3) receptor antagonist GSK598809 on attentional bias to palatable food cues in overweight and obese subjects. Int J Neuropsychopharmacol 15, 149–161. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Richards A, Metzler TJ, Ruoff LM, Varbel J, O’Donovan A, Sivasubramanian M, Motraghi T, Hlavin J, Batki SL, Inslicht SS, Samuelson K, Morairty SR, Kilduff TS, 2020. Acute cognitive effects of the hypocretin receptor antagonist almorexant relative to zolpidem and placebo: a randomized clinical trial. Sleep 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E, 2000. Hypocretin (orexin) deficiency in human narcolepsy. The Lancet 355, 39–40. [DOI] [PubMed] [Google Scholar]
- Nishino S, Yoshida Y, 2003. History and perspectives of hypocretin/orexin research in sleep medicine. Sleep and Biological Rhythms 1, 43–54. [Google Scholar]
- Nishizawa D, Kasai S, Hasegawa J, Sato N, Yamada H, Tanioka F, Nagashima M, Katoh R, Satoh Y, Tagami M, Ujike H, Ozaki N, Inada T, Iwata N, Sora I, Iyo M, Yamada M, Kondo N, Won MJ, Naruse N, Uehara-Aoyama K, Itokawa M, Ohi K, Hashimoto R, Tanisawa K, Arai T, Mori S, Sawabe M, Naka-Mieno M, Yamada Y, Yamada M, Sato N, Muramatsu M, Tanaka M, IrukayamaTomobe Y, Saito YC, Sakurai T, Hayashida M, Sugimura H, Ikeda K, 2015. Associations between the orexin (hypocretin) receptor 2 gene polymorphism Val308Ile and nicotine dependence in genome-wide and subsequent association studies. Mol Brain 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S, 2012. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37, 2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Life Science News, 2022. Malin Otmani. New results from Lundbeck’s vortioxetine trial. URL: https://nordiclifescience.org/new-results-from-lundbecks-vortioxetine-trial/. Accessed January 31, 2023.
- O’Rourke D, Coll-Padros N, Bradshaw A, Killin L, Pradier L, Georges J, Dawoud DM, Steukers L, Diaz C, 2022. The Innovative Medicines Initiative neurodegeneration portfolio: From individual projects to collaborative networks. Front Neurol 13, 994301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2018. Promoting mental health in Europe: Why and how? URL: https://www.oecd-ilibrary.org/content/component/health_glance_eur-2018-4-en. Accessed January 31, 2023. [Google Scholar]
- Oishi Y, Lazarus M, 2017. The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci Res 118, 66–73. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, Levine AS, 2009. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides 30, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal P, Kaltenboeck A, Harmer CJ, 2019. Cognitive emotional processing across mood disorders. CNS Spectr 24, 54–63. [DOI] [PubMed] [Google Scholar]
- Pantazis CB, Gonzalez LA, Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF, 2021. Cues conditioned to withdrawal and negative reinforcement: Neglected but key motivational elements driving opioid addiction. Sci Adv 7, eabf0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ, 2009. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ, 2012. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience 224, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E, 2000. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6, 991–997. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot P, Douilliez C, 2005. Social phobics do not misinterpret facial expression of emotion. Behav Res Ther 43, 639–652. [DOI] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merlo-Pich E, Di Fabio R, Corsi M, 2012. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology 37, 1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Maldonado R, Berrendero F, 2012. The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol 45, 424–439. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA, 2015. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci 6, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle A, Cooper MJ, Browning M, Harmer CJ, 2012. Effects of low dose tryptophan depletion on emotional processing in dieters. Eat Behav 13, 154–157. [DOI] [PubMed] [Google Scholar]
- Racine SE, Horvath SA, Brassard SL, Benning SD, 2018. Effort expenditure for rewards task modified for food: A novel behavioral measure of willingness to work for food. Int J Eat Disord. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, White DA, Acri JB, 2019. NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44, 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, Lyons N, Marder SR, Treadway MT, Wynn JK, Young JW, Green MF, 2015. Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 1-Psychometric Characteristics of 5 Paradigms. Schizophr Bull 41, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Reavis EA, Wynn JK, Green MF, 2018. Pupillary responses to a cognitive effort task in schizophrenia. Schizophr Res 199, 53–57. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R, 2004. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes 67, 343–356. [DOI] [PubMed] [Google Scholar]
- Richards A, French CC, Calder AJ, Webb B, Fox R, Young AW, 2002. Anxiety-related bias in the classification of emotionally ambiguous facial expressions. Emotion 2, 273–287. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H, 1999. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. J Exp Anal Behav 71, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E, Burnell J, Adams HR, Bohannon RW, Bush EN, Campbell M, Chen WH, Coons SJ, Papadopoulos E, Reeve BR, Rooks D, Daniel G, 2019. Developing and Implementing Performance Outcome Assessments: Evidentiary, Methodologic, and Operational Considerations. Ther Innov Regul Sci 53, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Aston-Jones G, 2012. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci 32, 3809–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, Becker ES, 2007. Approach and avoidance in fear of spiders. J Behav Ther Exp Psychiatry 38, 105–120. [DOI] [PubMed] [Google Scholar]
- Roane HS, 2008. On the applied use of progressive-ratio schedules of reinforcement. J Appl Behav Anal 41, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW, 2008. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33, 1028–1037. [DOI] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC, 2015. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šagud M, Šimunović Filipčić I, Jakšić N, Šimunić L, Jezernik D, Tudor L, Madžarac Z, Stefanović I, Kosanović Rajačić B, Mihaljević-Peleš A, Vuksan-Ćusa B, Kudlek Mikulić S, Pivac N, 2019. Anhedonia in Schizophrenia: Mini-Review. Psychiatr Danub 31, 143–147. [PubMed] [Google Scholar]
- Sakurai T, 2007. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8, 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2013. Orexin deficiency and narcolepsy. Curr Opin Neurobiol 23, 760–766. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Ferrigno S, Yang JH, Rotolo RA, Presby RE, 2018. The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation. Pharmacol Rev 70, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn S, Lopez Cruz L, San Miguel N, Alatorre L, 2016. The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behav Processes 127, 3–17. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Bonaventure P, Shekhar A, Johnson PL, Lord B, Shireman BT, Lebold TP, Nepomuceno D, Dugovic C, Brooks S, Zuiker R, Bleys C, Tatikola K, Remmerie B, Jacobs GE, Schruers K, Moyer J, Nash A, Van Nueten LGM, Drevets WC, 2020. Translational evaluation of novel selective orexin-1 receptor antagonist JNJ-61393215 in an experimental model for panic in rodents and humans. Transl Psychiatry 10, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargin D, 2019. The role of the orexin system in stress response. Neuropharmacology 154, 68–78. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C, 2007. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol 35, 229–238. [DOI] [PubMed] [Google Scholar]
- Schluter RS, van Holst RJ, Goudriaan AE, 2018. Repetitive transcranial magnetic stimulation (rTMS) in alcohol dependence: study protocol of a randomized controlled clinical trial of efficacy and working mechanisms. BMC Psychiatry 18, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Naumann E, Trentowska M, Svaldi J, 2014. Attentional bias for food cues in binge eating disorder. Appetite 80, 70–80. [DOI] [PubMed] [Google Scholar]
- Schneider I, Boll S, Volman I, Roelofs K, Spohn A, Herpertz SC, Bertsch K, 2020. Oxytocin Normalizes Approach-Avoidance Behavior in Women With Borderline Personality Disorder. Front Psychiatry 11, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE, 2013. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci U S A 110, 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Fadel JR, Kaigler KF, Wilson MA, 2017. Activation of orexin/hypocretin neurons is associated with individual differences in cued fear extinction. Physiol Behav 178, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma PR, Thuras P, Johns B, Lim KO, 2014. Emotion recognition processing as early predictor of response to 8-week citalopram treatment in late-life depression. Int J Geriatr Psychiatry 29, 1132–1139. [DOI] [PubMed] [Google Scholar]
- Sklenarik S, Potenza MN, Gola M, Astur RS, 2020. Approach bias for erotic stimuli among heterosexual female college students who use pornography. Addict Behav 108, 106438. [DOI] [PubMed] [Google Scholar]
- Sklenarik S, Potenza MN, Gola M, Kor A, Kraus SW, Astur RS, 2019. Approach bias for erotic stimuli in heterosexual male college students who use pornography. J Behav Addict 8, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM, 2014. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 145, 1–33. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, Tuerk P, Shiekh M, Rosenfield B, Hofmann SG, Powers MB, 2014. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry 75, 840–846. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Simon NM, Pollack MH, Hofmann SG, 2013a. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res 47, 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Pollack MH, Tart CD, 2013b. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry 73, 1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Sakurai T, 2020. Evolution of Orexin Neuropeptide System: Structure and Function. Front Neurosci 14, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T, 2013. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J Neurosci 33, 14549–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR, 1998. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 139, 169–184. [DOI] [PubMed] [Google Scholar]
- Staples LG, Cornish JL, 2014. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of Trial 1 and Trial 2 effects. Horm Behav 65, 294–300. [DOI] [PubMed] [Google Scholar]
- Starzomska M, 2017. Applications of the dot probe task in attentional bias research in eating disorders: A review. Psicológica 38, 283–346. [Google Scholar]
- Steffen KJ, Roerig JL, Mitchell JE, Uppala S, 2006. Emerging drugs for eating disorder treatment. Expert Opin Emerg Drugs 11, 315–336. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, Jenck F, 2012. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology (Berl) 223, 465–475. [DOI] [PubMed] [Google Scholar]
- Steiner N, Rossetti C, Sakurai T, Yanagisawa M, de Lecea L, Magistretti PJ, Halfon O, Boutrel B, 2018. Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology 133, 395–403. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Strickland JC, Hatton KW, Hays LR, Rayapati AO, Lile JA, Rush CR, 2022. Suvorexant maintenance enhances the reinforcing but not subjective and physiological effects of intravenous cocaine in humans. Pharmacol Biochem Behav 220, 173466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Reynolds AR, Stoops WW, 2016. Regulation of cocaine craving by cognitive strategies in an online sample of cocaine users. Psychol Addict Behav 30, 607–612. [DOI] [PubMed] [Google Scholar]
- Stuppy-Sullivan A, Baskin-Sommers A, 2019. Evaluating dysfunction in cognition and reward among offenders with antisocial personality disorder. Personal Disord 10, 416426. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2021. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21–07-01–003, NSDUH Series H-56), Rockville, MD. [Google Scholar]
- Suchting R, Yoon JH, Miguel GGS, Green CE, Weaver MF, Vincent JN, Fries GR, Schmitz JM, Lane SD, 2020. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Res 1731, 146359. [DOI] [PubMed] [Google Scholar]
- Sun W, Kober H, 2020. Regulating food craving: From mechanisms to interventions. Physiol Behav 222, 112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surcinelli P, Codispoti M, Montebarocci O, Rossi N, Baldaro B, 2006. Facial emotion recognition in trait anxiety. J Anxiety Disord 20, 110–117. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Mell MM, O’Malley SS, Krystal JH, Anticevic A, Kober H, 2020. Regulation of Craving and Negative Emotion in Alcohol Use Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 5, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Naumann E, Biehl S, Schmitz F, 2015. Impaired Early-Response Inhibition in Overweight Females with and without Binge Eating Disorder. PLoS One 10, e0133534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Naumann E, Trentowska M, Schmitz F, 2014. General and food-specific inhibitory deficits in binge eating disorder. Int J Eat Disord 47, 534–542. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U, 2011. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56, 1655–1665. [DOI] [PubMed] [Google Scholar]
- Takeda Pharmaceutical Company Limited, 2021. Takeda Provides Update on TAK-994 Clinical Program. URL: https://www.takeda.com/newsroom/newsreleases/2021/taked-aprovides-update-on-tak-994-clinical-program/. Accessed September 15, 2022.
- Taslimi Z, Arezoomandan R, Omranifard A, Ghalandari-Shamami M, Riahi E, Vafaei AA, Rashidy-Pour A, Haghparast A, 2012. Orexin A in the ventral tegmental area induces conditioned place preference in a dose-dependent manner: involvement of D1/D2 receptors in the nucleus accumbens. Peptides 37, 225–232. [DOI] [PubMed] [Google Scholar]
- Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, Gonzalez-Lima F, 2014. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. Am J Psychiatry 171, 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM, 2000. Reduced Number of Hypocretin Neurons in Human Narcolepsy. Neuron 27, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet, 2012. Translational research and experimental medicine in 2012. The Lancet 379. [DOI] [PubMed] [Google Scholar]
- The Lancet Global Health, 2020. Mental health matters. Lancet Glob Health 8, e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Higgs S, Dourish CT, 2016. Test-retest reliability and effects of repeated testing and satiety on performance of an Emotional Test Battery. J Clin Exp Neuropsychol 38, 416–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Teske JA, Kotz CM, 2005. Orexin A-induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol 289, R367–R372. [DOI] [PubMed] [Google Scholar]
- Tisdale RK, Yamanaka A, Kilduff TS, 2021. Animal models of narcolepsy and the hypocretin/orexin system: Past, present, and future. Sleep 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toet A, Kaneko D, de Kruijf I, Ushiama S, van Schaik MG, Brouwer AM, Kallen V, van Erp JBF, 2019. CROCUFID: A Cross-Cultural Food Image Database for Research on Food Elicited Affective Responses. Front Psychol 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrents-Rodas D, Fullana MA, Bonillo A, Andión O, Molinuevo B, Caseras X, Torrubia R, 2014. Testing the temporal stability of individual differences in the acquisition and generalization of fear. Psychophysiology 51, 697–705. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T, 2001. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 157, 67–74. [DOI] [PubMed] [Google Scholar]
- Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM, 2009. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord 118, 87–93. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH, 2009. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 4, e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2011. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35, 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Food & Drug Administration, 2009. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. URL: https://www.fda.gov/media/77832/download. Accessed September 15, 2022.
- UK Research and Innovation, 2022. Experimental medicine. URL: https://www.ukri.org/what-we-offer/browse-our-areas-of-investment-and-support/experimental-medicine/. Accessed September 15, 2022.
- Valdivia S, Cornejo MP, Reynaldo M, De Francesco PN, Perello M, 2015. Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology 60, 206–216. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Band GP, Beste C, Bissett PG, Brockett AT, Brown JW, Chamberlain SR, Chambers CD, Colonius H, Colzato LS, Corneil BD, Coxon JP, Dupuis A, Eagle DM, Garavan H, Greenhouse I, Heathcote A, Huster RJ, Jahfari S, Kenemans JL, Leunissen I, Li CR, Logan GD, Matzke D, Morein-Zamir S, Murthy A, Pare M, Poldrack RA, Ridderinkhof KR, Robbins TW, Roesch M, Rubia K, Schachar RJ, Schall JD, Stock AK, Swann NC, Thakkar KN, van der Molen MW, Vermeylen L, Vink M, Wessel JR, Whelan R, Zandbelt BB, Boehler CN, 2019. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villano I, Messina A, Valenzano A, Moscatelli F, Esposito T, Monda V, Esposito M, Precenzano F, Carotenuto M, Viggiano A, Chieffi S, Cibelli G, Monda M, Messina G, 2017. Basal Forebrain Cholinergic System and Orexin Neurons: Effects on Attention. Front Behav Neurosci 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Haegler P, Jenck F, Steiner MA, 2015. Orexin neuropeptides contribute to the development and persistence of generalized avoidance behavior in the rat. Psychopharmacology (Berl) 232, 1383–1393. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H, 2011. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci 31, 16597–16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MB, Pringle A, Harmer CJ, 2015. A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc Lond B Biol Sci 370, 20140213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegerer M, Blechert J, Kerschbaum H, Wilhelm FH, 2013. Relationship between fear conditionability and aversive memories: evidence from a novel conditioned-intrusion paradigm. PLoS One 8, e79025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Kuhn S, Javadi AH, Korucuoglu O, Wiers RW, Walter H, Gallinat J, Bermpohl F, 2013. Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology (Berl) 229, 187–197. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E, 2009. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav 8, 101–106. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M, 2001. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24, 429–458. [DOI] [PubMed] [Google Scholar]
- Wu LL, Potenza MN, Zhou N, Kober H, Shi XH, Yip SW, Xu JH, Zhu L, Wang R, Liu GQ, Zhang JT, 2020a. A role for the right dorsolateral prefrontal cortex in enhancing regulation of both craving and negative emotions in internet gaming disorder: A randomized trial. Eur Neuropsychopharmacol 36, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Zhu L, Shi XH, Zhou N, Wang R, Liu GQ, Song KR, Xu LX, Potenza MN, Zhang JT, 2020b. Impaired regulation of both addiction-related and primary rewards in individuals with internet gaming disorder. Psychiatry Res 286, 112892. [DOI] [PubMed] [Google Scholar]
- Wu M, Hartmann M, Skunde M, Herzog W, Friederich HC, 2013. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One 8, e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai A, Matsuzaki I, Miwa Y, Goto K, Sakurai T, 2000. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res 859, 404–409. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T, 2003. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochemical and Biophysical Research Communications 303, 120–129. [DOI] [PubMed] [Google Scholar]
- Yao S, Zhao W, Geng Y, Chen Y, Zhao Z, Ma X, Xu L, Becker B, Kendrick KM, 2018. Oxytocin Facilitates Approach Behavior to Positive Social Stimuli via Decreasing Anterior Insula Activity. Int J Neuropsychopharmacol 21, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J, Kärppä M, Inoue Y, Pinner K, Perdomo C, Ishikawa K, Filippov G, Kubota N, Moline M, 2021. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med 80, 333–342. [DOI] [PubMed] [Google Scholar]
- Yau YH, Potenza MN, 2015. Gambling disorder and other behavioral addictions: recognition and treatment. Harv Rev Psychiatry 23, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh SY, Wilkinson P, 2014. Acute psychosocial stress does not increase dysfunctional attitudes. Psychiatr Danub 26 Suppl 1, 240–245. [PubMed] [Google Scholar]
- Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, Ba W, Miracca G, Wang D, Li L, Guo J, Chen M, Li Y, Yustos R, Vyssotski AL, Burdakov D, Yang Q, Dong H, Franks NP, Wisden W, 2019. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukitake H, Fujimoto T, Ishikawa T, Suzuki A, Shimizu Y, Rikimaru K, Ito M, Suzuki M, Kimura H, 2019. TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol Biochem Behav 187, 172794. [DOI] [PubMed] [Google Scholar]
- Zarrabian S, Riahi E, Karimi S, Razavi Y, Haghparast A, 2020. The potential role of the orexin reward system in future treatments for opioid drug abuse. Brain Res 1731, 146028. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E, 2003. Circadian and Homeostatic Regulation of Hypocretin in a Primate Model: Implications for the Consolidation of Wakefulness. J Neurosci 23, 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]