Abstract
Memory, cognition, dementia, and neurodegeneration are complexly interlinked processes with various mechanistic pathways, leading to a range of clinical outcomes. They are strongly associated with pathological conditions like Alzheimer’s disease, Parkinson’s disease, schizophrenia, and stroke and are a growing concern for their timely diagnosis and management. Several cognition-enhancing interventions for management include non-pharmacological interventions like diet, exercise, and physical activity, while pharmacological interventions include medicinal agents, herbal agents, and nutritional supplements. This review critically analyzed and discussed the currently available agents under different drug development phases designed to target the molecular targets, including cholinergic receptor, glutamatergic system, GABAergic targets, glycine site, serotonergic targets, histamine receptors, etc. Understanding memory formation and pathways involved therein aids in opening the new gateways to treating cognitive disorders. However, clinical studies suggest that there is still a dearth of knowledge about the pathological mechanism involved in neurological conditions, making the dropouts of agents from the initial phases of the clinical trial. Hence, a better understanding of the disease biology, mode of drug action, and interlinked mechanistic pathways at a molecular level is required.
Keywords: Dementia, cognitive disorders, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, schizophrenia
1. INTRODUCTION
Memory is a cognitive process of remembering or recalling the learned or experienced scenarios [1]. Dementia and cognitive impairment cases are increasing worldwide, generating an alarming situation with an estimated to affect around 150 million humans by 2050 [2]. The reasons behind such a drastic increase may include a sedentary lifestyle, stress, and an increase in the elderly population. Cognition is the mental potential of learning processes that contribute to an individual's mental performance, including awareness, planning, perception, reasoning, and judgment. It mainly includes memory, intelligence, execution, decision making, creativity, and attention which are interconnected. Various physiological and pathological conditions, such as stress, ageing, Parkinson’s disease (PD), Alzheimer’s disease (AD), Schizophrenia, and HIV (human immunodeficiency virus), may result from cognitive impairment, developing a research thrust for understanding dementia and associated biology [3-6]. In the last few decades, the early-stage biomarkers and symptoms of dementia have become the center of research for ageing and cognitive diseases to identify reliable markers for neurodegenerative disease development [7]. Alongside, alternative treatment options in the form of cognitive enhancers are being explored. They are the drugs, nutraceuticals, and food supplements, which amplify or extend the core capacities of the brain and memory by improving the internal and external information processing systems. In the current scenario, most of the marketed cognition enhancers have a modest effect with no crisp understanding of their mode of action. In the current review, the updates regarding the pharmacological agents used for cognitive impairment, which may or may not be associated with other neurological conditions, are being focused on emphasizing the various pathways and mediators mainly involved in the memory formation process.
2. NEUROBIOLOGY AND MANAGEMENT OF DISEASE-ASSOCIATED COGNITIVE DYSFUNCTION AND DEMENTIA
Many diseases are associated with dementia and cognitive deficits. Cognitive dysfunctions occur via several pathological changes occurring in neurons due to underlying pathological conditions like Alzheimer’s disease (AD), Parkinson’s disease (PD), attention-deficit hyperactivity disorder (ADHD), and neurobiological conditions like stress, anxiety, depression, schizophrenia, etc. and atypical dementia, where pathological differentiation to a particular disease is difficult [8]. The management remains interlinked because of the coinciding clinical presentations for a major group of diseases, including several pharmacological agents and non-pharmacological approaches. Several strategies can affect cognition positively. Most interventions target disease pathology, and some affect the level of the neurotransmitter. Several pharmacological and non-pharmacological interventions like herbal medicines, pharmaceutical drugs, physical exercise, vitamins, and nutritional supplements extensively treat cognitive impairment associated with various neurological diseases. Regular exercise reduces the risk of dementia and cognitive impairment by upregulating the brain-derived neurotrophic factor (BDNF) in the hippocampus [9]. Herbal medicines, such as Ginkgo biloba, Shankhpushpi, Bacopa monniera (Bramhi), etc., have been found to enhance cognitive abilities [10]. Pharmaceutical drugs improve memory by affecting pharmacological targets like neurotransmitter levels, including monoamines, acetylcholine, gamma-aminobutyric acid (GABA), etc., or by enhancing cerebral blood flow and brain metabolism. Recent advances in understanding the pathological alterations occurring in diseased conditions and therapeutic interventions are discussed in detail.
2.1. Stroke Associated Dementia
It is estimated that approximately one-third of stroke victims will develop memory problems and serious difficulties in other aspects of performing daily activities. The memory problems scientifically called dementia can be so severe that they interfere with normal functioning; this is more common in aged or elderly patients. When dementia occurs after a stroke and no other cause can be found, it is called vascular dementia. Large strokes strategically located in certain brain areas or multiple small strokes in various brain regions can result in vascular dementia. Certain contributing factors like old age, prior cognitive complaints, a history of several strokes, or a stroke located on the left side of the brain all seem to increase the likelihood of dementia in the first year after a stroke. Vascular dementia can be caused by cerebrovascular disease or any other condition that prevents normal blood flow to the brain. Without a normal supply of blood, brain cells become deprived of the oxygen and nutrients required to function normally, and they often become so deprived that they die. The symptoms of vascular dementia and memory loss include an altered motor function like hindered gait and an inability to perform daily chores.
In contrast, cognitive alteration includes bradyphrenia and attention deficit. These symptoms may sometimes become difficult to be differentiated from those produced by AD, where age-related dementia remains a common factor [11]. Symptoms of dementia after stroke can also be masked by other more obvious stroke manifestations like paralysis, blindness, or lack of awareness. Another hurdle in identifying the symptoms of dementia after a stroke includes symptomatic similarity with depression, which is quite common after a stroke. To date, there is no specific pharmacological intervention available to reverse the memory loss that occurs after a stroke. Neurologists sometimes prescribe medications approved for AD to patients with vascular dementia, but still, we do not have studies addressing the applicability of these medications in patients with vascular dementia.
Cognitive impairments, including spatial memory and learning deficits, are common after ischemic stroke. Several pharmacological interventions have shown beneficial effects in memory impairment after cerebral ischemia. 17-beta-estradiol treatment combined with an enriched environment improved recovery of cognitive function after experimental brain ischemia, putatively through the upregulation of NGFI-A (Nerve Growth Factor Induced gene) in hippocampal subregions [12]. Minocycline treatment also had beneficial effects on memory impairment caused by cerebral focal ischemia. The improved function is associated with enhanced neurogenesis, reduced microglia activation in the dentate gyrus, and possibly an improved neural environment after chronic treatment with minocycline [13]. Treatment with D-JNKi, a peptide inhibitor of c-Jun N-terminal kinase (JNK), has also improved cognitive function in the object recognition test after transient focal cerebral ischemia in rats [14]. Coeloglossumviride (L.) Hartm. var. bracteatum (Wild.) extract (CE) treatment also attenuated learning and memory deficits, motor functional disability, and neuronal cell loss induced by global or focal cerebral ischemia. The results suggested that CE may be a potential candidate for the treatment of vascular dementia [15].
Some studies have shown that bryostatin-1, a highly potent protein kinase C (PKC) activator, interrupts pathophysiological molecular cascades and apoptosis triggered by cerebral ischemia/hypoxia, enhances neurotrophic activity, induces synaptogenesis, and preserves learning and memory capacity even four months later as well as long-term memory induced before the ischemic event [16]. Inhibiting poly (ADP-ribose) polymerase also benefits memory impairment after cerebral ischemia. Poly(ADP-ribose) polymerase-1 (PARP-1) is a coactivator of the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and is required for NF-κB mediated inflammatory responses. The brain inflammatory response induced by stroke contributes to cell death and impairs neurogenesis. Treatment with N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide (PJ34), a PARP inhibitor, improved outcomes after stroke by suppressing post-stroke inflammation [17]. A study has demonstrated that activation of the Rho/Rho-kinase pathway is related to neuronal damage and the pathogenesis of spatial learning and memory impairment in stroke. The study result further demonstrated that relative long-term hydroxyfasudil treatment improved the rats' spatial learning and memory performance [18].
2.2. Parkinson’s Disease-associated Dementia
PD is the second most common neurodegenerative disorder after AD, affecting 1% of the population by 65 years and 4–5% of the population by 85 years. PD is pathologically characterized by the degeneration of dopaminergic neurons in substantia nigra and the presence of intracytoplasmic inclusions (Lewy bodies) in the residual dopaminergic neurons. PD is predominantly a motor disorder clinically characterized by resting tremor, rigidity, hypokinesia, and postural instability. Still, dementia is the most debilitating symptom associated with disease progression and is a major concern for patients and caregivers. Dementia affects almost 31-80% of patients with PD [19, 20]. A study has reported that the cumulative incidence of dementia steadily increases with the age of PD patients and may increase to 80%-90% by the age of 90 if the patient survives. At the age of 70 years, a man with PD without dementia has a life expectancy of eight years, out of which five years would be expected to be dementia-free, and subsequent three years would be expected to be with dementia [21]. Parkinson's disease dementia (PDD) includes visual and auditory hallucinations, sensitivity to neuroleptic medications, cognitive fluctuations, and sleep disturbances. Visual hallucinations are the strongest prediction of PDD and increase the risk of dementia by 20 folds over PD without hallucination. PD's cognitive symptoms include loss of decision-making ability, inflexibility to adapting changes, disorientation in familiar surroundings, problems learning new skills, difficulty concentrating, loss of short- and long-term memory, insufficient working of sequential memory, and problems using and comprehending complex language [22, 23]. Neuropsychiatric symptoms, including depression (58%), apathy (54%), anxiety (49%), and hallucinations (44%), occur even more commonly in PDD than in PD. Sleep disorders (43%), such as rapid eye movement, sleep behavior disorder, daytime sleepiness, and sleep attacks, occur frequently. Advancing age, depression, smoking, gender, family history, and genetic alterations in α-synuclein have been reported as risk factors for PDD with clinical presentations like the onset of motor symptoms, visual hallucinations, confusion, severe hypokinesia, and speech problems [24].
Dopaminergic neurons in different brain areas play a critical role in modulating different processes related to learning and memory. Dopaminergic cells in the striatal region are involved in habitual and working memory; the dorsal and ventral hippocampus is involved in spatial memory; whereas the prefrontal cortex is involved in working memory and is found to be impaired in both extreme and deficit conditions of dopamine. Dopaminergic systems in basal ganglia [SNc (Substantia nigra pars compacta), striatum, globus pallidus, and ventral tegmental area (VTA)] are also involved in reward-seeking behavior [25, 26]. Degeneration of dopaminergic neurons in basal ganglia is mainly responsible for cognitive and visual impairment in PD. Basal ganglia act as a relay center in the brain. It receives signals from various cortex regions and relays output signals to cortex regions involved in cognitive function [27]. The visual area (area TE) in the temporal lobe receives the signal from basal ganglia and lesions in basal ganglia, thus leading to visual hallucinations. Working memory is controlled by Brodmann area 46 of the prefrontal cortex, which also receives signals from basal ganglia. A lesion in basal ganglia may lead to working memory impairment [26-28]. In addition to the dopaminergic deficit, cholinergic, noradrenergic, and serotonergic dysfunctions also occur in PDD. Dopaminergic neuronal loss in the frontal cortex leads to reduced verbal fluency, working memory, and executive function. Noradrenergic neuronal loss in locus coeruleus, cerebral cortex, and hippocampus is related to attention deficit. Serotonergic neuronal loss in cortical and subcortical areas leads to depression. Reduced levels of acetylcholine due to the destruction of the cortex causes poor performance in attention and executive function tests [29].
Though numerous medications are available to treat motor features of PD, none of them are effective in improving cognitive symptoms. These may even worsen the psychiatric symptoms of PDD. The strongest evidence for pharmacologic treatment of PDD is with cholinesterase inhibitors. The FDA has approved rivastigmine for PDD treatment. It is a centrally acting inhibitor of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). The most common adverse effects include nausea, vomiting, tremor, diarrhea, anorexia, and dizziness [30, 31]. Tacrine, donepezil, and galantamine have also been beneficial for PDD [32, 33]. However, tacrine's use is limited by poor oral bioavailability, the necessity for four-times daily dosing, and many adverse drug reactions (including nausea, diarrhea, urinary incontinence, and hepatotoxicity). The worsening of motor symptoms was observed with donepezil, and it was reported to be well tolerated with mild adverse effects like nausea and arrhythmia [34].
Anti-Parkinson agents have also shown some beneficial effects on PDD. Levodopa may benefit working memory and causes visual hallucinations, psychosis, confusion, and increased cognitive fluctuations in advanced PD. Anticho-linergic agents have been shown to induce β-amyloidosis and senile plaque formation and may even worsen the condition. Dopamine receptor agonists, pramipexole and pergolide, reduce depression but have not yet proved effective for reducing cognitive symptoms in advanced PD [35]. Surgical interventions, such as thalamotomy, thalamic stimulation (used to treat tremors), pallidotomy, and deep brain stimulation (used to treat dyskinesia), may further worsen the cognitive complications in PD. Surgery's adverse side effects include brain hemorrhage, infarction, seizures, and even death. Developments in nanotechnology (implantable carbon nanotubes and nanochips) assure greater safety and precision for delivering impulses in the substantia-nigra, reducing the side effects of surgery [36].
2.3. Brain Injury
Brain injury is considered the major cause of death and disability worldwide. Memory dysfunction is a major anomaly post-injury episode among various neurological manifes-tations. Human and animal studies suggested that critical brain regions (cortex and hippocampus) for memory function have been found disrupted in traumatic brain injury (TBI). Studies have reported that a two-fold increase may be observed in the occurrence of dementia or AD in the case of moderately severe TBI, while a four-fold rise in risk may be observed in severe TBI cases. It tends to alter the manifestations presented clinically in dementia cases and lowers the age of dementia onset. It has been found that the TBI (moderate-to-severe cases) has led to the development of proteinopathies where an excessive accumulation of amyloid protein was observed along with the tau hyperphosphorylation localized in glial cells in sulci and perivascular neurons. The brain injury may also lead to neuroinflammation due to microglia activation and disruption of the BBB. The principally injured brain regions include the orbitofrontal and anterior temporal cortex in the case of TBI.
In contrast, in other brain injuries, the frontal neocortex is involved in focal neuropathology instead of the mesolimbic or parietal region alterations observed in AD. The diffuse axonal injury leads to altered axonal transport of proteins like Aβ (Amyloid beta) and hyperphosphorylated-tau and their aggregation. The integrity of white matter is being altered, where anisotropy can be observed in the case of fibre bundles like corpus callosum and cingulum in the condition of acute brain injury. In contrast, in the case of AD and mild cognitive impairment, the whole brain white matter connectivity is disrupted, causing disturbances in the long-range tracts like uncinate fasciculus, superior longitudinal fasciculus, cingulum, inferior frontal-occipital fasciculus, and corpus callosum [37].
TBI causes a deficit in episodic memory due to physiological disruption of circuitry involving the hippocampus. Therefore, the connection between dentate gyrus, CA3, and CA1 regions can be disrupted. In the DG region, the principal cell type, i.e., granule cell, acts as a filtering media, which regulates the incoming excitability of the signals to the hippocampus. In normal conditions, the granule cells have low propensities to generate a high action potential due to strong inter-neuronal connections having GABAergic neurons. Still, in the brain injury situation, hyperexcitability is observed in the DG region, causing its hindered filtering potential; this may be due to the diminished GABAergic transmission on the granule cells [38]. Studies have also shown that the glutamatergic inputs to the somatostatin-positive interneurons increase, leading to more action potential generation [39]. The episodic memory formation and retrieval are dependent on the CA1 and CA3 areas of the hippocampus, which is also affected by brain injury. The neurons of the CA3 area are prone to death upon TBI, while the neurons in the CA1 region become hypoexcitable where the Schaeffer collaterals fibre volley amplitude is reduced with recoverable glutamatergic events from both NMDA (N-methyl-D-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors. It indicates that the glutamatergic innervation increases though the post-synaptic machinery might be disrupted [40, 41]. However, several studies reported an increase in excitability of CA1 neurons, marking a lacuna in examination studies related to experimental variability factors like time point of evaluation, injury severity, injury model, etc. The effect on working memory due to TBI can be studied by evaluating the brain's prefrontal cortex region, which is strongly connected to the hippocampus. The function of the prefrontal cortex (PFC) is affected such that an increase in the Ca+2-permeable AMPA receptors is found to be upregulated with diminished brain activity and impairment in working memory. Several potential strategies for memory dysfunction have improved post-TBI dementia, such as neural stem cell transplantation, dietary therapy with branched amino acids (glutamate precursor derivatives), and enrichment in environmental settings. These strategies have been tested in animal models; however, their clinical applicability is yet to be established [42].
2.4. Mixed Dementia
AD is the most common form of dementia in old age patients. However, it has been found that dementia in elderly patients cannot be explained by the clinical presentation of dementia found in patients with AD or even vascular dementia (VD). Such a clinical condition with overlapping symptoms and AD and VD pathologies is called mixed dementia (MD) [43]. MD has a prevalence rate of 22-24% among all the reported dementia cases [44].
The MD diagnosis has no agreement among various international referents like NINDS-AIREN (National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l’Enseigementen Neurosciences) and ADDTC (Alzheimer’s Disease Diagnostic and Treatment Centres) and also, DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) and ICD-10 (International Classification of Diseases, 10th Revision), each differing in their specific diagnostic criteria [44]. The ADDTC has used the term ‘AD with cerebrovascular disease’ instead of MD due to the second occurrence of ischemic brain disease associated with a primary systemic or brain disorder, playing a role as a causal factor for dementia. The symptoms may differ at each stage based on the severity of vascular damage and senile plaque deposition in the patient’s brain, ranging from decreased performance in cognitive activities, attention, and vasoconstriction response to spatial memory function impairment. We can also observe abnormal episodic memory and impaired thoughts, which overlap with AD clinical presentations, and impairment of executive functions and processing rate, generally observed in VD. As per ICD-10, MD diagnostic attribution depends upon the conjoining criteria for AD and VD. Even the DSM-V has mentioned MD under the umbrella ‘Major Neurocognitive Disorder with Multiple Etiologies’ term meaning that the diagnostic confirmation may depend upon the medical history, physical investigation, or laboratory evaluation stating that multiple etiological factors led to the disorder [45]. The risk factors include atherosclerosis, obesity, dyslipidemia, hypertension, diabetes, tobacco smoking, and vascular brain injury.
Dementia associated with AD and VD includes the involvement of two main theories: amyloid theory and vascular theory. Dementia, disregarding its subtype, begins to develop pathological modification several years before the first observation of clinical symptoms. The pathological changes in the vascular system begin with collagen deposition in endothelial membrane and thickening of the basal membrane of capillary beds, occurring due to a sedentary lifestyle and other conditions like hypertension, hypercholesteremia, diabetes mellitus, and cardiac disease, leading to a decreased number of terminal capillaries and blood vessels in the body and the brain as well. Several factors leading to cerebrovascular damage include macrovascular arteriosclerosis, microvascular atherosclerosis, brain infarcts, minor hemorrhage, amyloid angiopathy, and white matter lesions, all leading to the cerebrovascular disease that leads to the manifestation of cognitive decline exceeding the normal ageing, called a vascular cognitive impairment, which progresses further and impairs daily life activities. While considering the protein deposition in AD conditions, it can be majorly observed in the neocortex and hippocampus regions, including both diffuse and neuritic plaques. Also, the tau tangles are observed in a semi-quantitative amount in the hippocampus and neocortex regions [44].
The treatment includes several non-pharmacological and pharmacological interventions targeting symptomatic and preventive measures. Early-stage non-pharmacological interventions like adequate control of blood pressure, blood glucose, cholesterol, and triglyceride, along with maintaining a healthy diet and regular exercise (≥ 3 times a week for ≥ 30 minutes), would help to delay the progression of cognitive decline associated with AD and VD, aiding to maintain normalcy in daily life activities. Psychological and social assistance and support are also critical for the working group of patients [44]. However, there are no specific drugs for MD treatment, but the drugs used for AD and VD management have proven useful for MD management.
2.5. Frontotemporal Dementia
The group of conditions wherein the degeneration is noted markedly in neurons of the frontal lobe, or anterior temporal lobe of the brain or both regions is termed frontotemporal dementia (FTD), leading to behavioral abnormalities and/or language impairment [42, 46]. The debilitating cognitive dysfunction is generally preceded by certain behavioral changes like decreased interpersonal conduct as irritability, impulsiveness, or lack of empathy, personal hygiene habits and language controls are affected, which then progresses to a decline in intelligence quotient (IQ), disorientation, and lately to profound dementia but the case of AD is quite different where the amnesia precedes a decline in social behavior [46]. There is no specific treatment for FTD, but symptomatic treatments are targeted. Several anti-depressants, anti-psychotic, neuropeptides, and anti-epileptic agents have been studied to manage the behavioral symptoms. Anti-depressants like paroxetine, sertraline, and citalopram trazodone have shown an improvement in repetitive behavior as well as the neuropsychiatric index (NPI). Atypical antipsychotics like quetiapine, risperidone, and aripiprazole have been shown to improve agitation behavior with no effect on NPI, whereas olanzapine has improved NPI scores. Anti-epileptic agents like valproic acid, topiramate, and carbamazepine, which have a mood-stabilizing effect, have limited evidence in clinical studies. Still, case reports prove their effectiveness for behavioral symptom management in FTD. Neuropeptide oxytocin, upon intranasal administration, showed possible trends in improvement of apathy and empathy. While discussing the management of the cognitive symptoms, AChE inhibitors are the first class of drugs due to their effective ability to treat AD-related memory impairment. Still, the use is limited as donepezil and rivastigmine have shown worsening and only a slight improvement in the NPI score, respectively. When tested for effectiveness in FTD, NMDA antagonist memantine has shown beneficial effects on daily activities and cognition. Motor symptoms management becomes important when FTD is associated with parkinsonism. The dopamine replacement therapy with levodopa/carbidopa has limited effect in this case, restricting its use only for symptomatic management [47]. Behavioral therapy is an acceptable strategy for FTD management [48].
2.6. Other Neurologically Linked Dementia/ Cognitive Dysfunction
Dementia is a complex disorder linked with several other pathological conditions like depression, schizophrenia, diabetes, etc. The characteristic pathological overlaps between the two or more conditions, leading to an insignificant diagnostic characterization of the underlying cognitive dysfunction.
Schizophrenia’s (SCZ) relationship to FTD has been studied for several decades. The phenotypic similarities in the two cases have led to being diagnosed as either Schizophrenia or FTD at the first instance but not as a co-existing disease initially. SCZ cases have altered cingulate, prefrontal, and temporal cortex regions, leading to auditory hallucinations and thought disorientation symptoms. At the same time, the FTD shows hypometabolism in frontal and temporal lobe areas, cingulate gyrus, and medial thalamus of the brain. Though Hughlings Jackson's hypothesis for the negative and positive symptoms related to SCZ has been postulated, the exact pathophysiology is still a big mystery to the researchers [49, 50]. The common pathway involved in both conditions includes the impaired GABA excitatory and inhibitory activity balance, impairing the working memory. The alteration of the dopaminergic neuron functioning in the limbic region of the brain has led the researcher to use first-generation antipsychotics to treat the cognitive dysfunction and negative symptoms in SCZ, though with a limited application due to lack of efficacy and high incidences of side effects, including dyskinesia, weight gain, extrapyramidal motor symptoms, and sedation. Second-generation antipsychotics have a higher affinity for the serotonergic (5HT2A) receptor than the dopaminergic (D2) receptor. Several other novel approaches are being studied, including NMDA-glycine site agonist, dopaminergic D1 agonist, mGluR type 2/3, or 5 and 5-HT1A receptor agonist [51, 52]. Human Immunodeficiency Virus (HIV) associated neurocognitive disorder (HAND) is a group of neurological impairments observed along with AIDS due to manifestation by HIV. HIV-associated dementia (HAD) shows manifestation in subcortical regions like deep white matter, hippocampus, basal ganglia, and hippocampus, leading to psychomotor delays, alteration in mood, anxiety levels, memory, abstraction, information processing, attention, and decisive powers [53, 54]. Based on the complexity of the viral manifestation in the brain and its effect, several targets have been focused upon for the trial of drugs and their actions. Minocycline (anti-inflammatory, tetracycline antibiotic) suppresses the JNK pathway activation, lowering the nitric oxide (NO) levels and mitogen activated protein kinase (MAPK) activation. Memantine (anti-excitotoxicity, NMDA receptor blocker) shows its effectiveness in excitotoxicity induced cognitive dysfunction. Selegiline (antioxidant and a monoamine oxidase-B inhibitor) can attenuate the oxygenated free radicals, but the effectiveness is yet to be proven clinically. The biological peptides like insulin, insulin-like growth factor - 1 (IGF-1), and fibroblast growth factor - 2 (FGF-2) are also being evaluated for their action on cognitive impairment associated with various pathological conditions, through intranasal and other routes [55, 56].
Depression is another neurological condition often linked to dementia, but the distinction is still unclear whether depression is a causative factor or a consequence of dementia [57]. The comorbidity of depression with diabetes also leads to higher cognitive deficit reports than patients with either of these conditions. Cognition decline generally starts before diabetes is diagnosed (prediabetes stage), affecting the frontal and prefrontal cortex regions and impacting working memory and executive function [58]. The pathophysiological pathway between depression, cognitive dysfunction, and type 2 diabetes is not completely understood. The involvement of microvasculature dysfunction in T2DM-linked dementia and depression is hypothesized based on available literature and studies [59].
2.7. Current Status of Drug Development for Disease-Associated Cognitive Dysfunction and Dementia
Different methods and approaches are explored to manage disease-associated cognitive dysfunction and dementia, as presented in Table 1.
Table 1.
Various interventions are used to improve disease-associated dementia or cognitive dysfunction.
| Class of Agent | Agent | Action |
|---|---|---|
| Nutrients | Acetyl-L-Carnitine Phosphatidylserine |
It provides acetyl equivalents for the production of acetylcholine [60]. Increases the production of nerve growth factor (NGF) by cultured nerve cells and helps them respond better to NGF [61]. |
| CoenzymeQ10/ Ubiquinone | In vitro research demonstrates its role in acetylcholine release and provides neuroprotection by inhibiting β amyloid and inflammation [62, 63]. | |
| α-Lipoic acid | Anti-inflammatory, antioxidant, carbonyl scavenging, and metal-chelating increase cerebral blood flow and neuroprotective properties and stimulate the ACh synthesis pathway [64]. | |
| CoenzymeQ10/ Ubiquinone | Neuroprotective action reduces brain atrophy and oxidative stress and promotes energy production [65]. | |
| Docosahexaenoic acid, ω-3 fatty acids | Increased cortico-hippocampal reduced glutathione levels and glutathione reductase activity and suppressed the increase in lipid peroxide and reactive oxygen species levels in the cerebral cortex and hippocampus of the AD model rats [66]. | |
| Vitamin B6, B12, and Folic acid | A lower level of these vitamins leads associated with cognitive decline [67]. | |
| Vitamin E | Potent reactive oxygen species scavenger affects SOD and Sir2 (silent information regulator 2), which play an important role in resistance to oxidative stress [68]. | |
| Pharmaceutical Drugs | Aducanumab | Monoclonal antibodies directed to aggregated forms of Amyloid β and reduce its buildup [69, 70]. |
| Melatonin | Improves some aspects of sleep, memory, and mood in elderly patients [71]. | |
| Nicotine | Stimulates nicotinic cholinergic receptors and has been proposed to act through modulation of signaling pathways, i.e., increased extracellular-signal-regulated kinase 1/2 (ERK1/2) and cAMP response element-binding protein (CREB) phosphorylation [72]. | |
| Caffeine | Restores brain adenosine levels, reduces the expression of both Presenilin 1 and β-secretase and reduces the Aβ production [73]. | |
| Methylphenidate, Modafinil | Affects the catecholamines, serotonin, glutamate, gamma amino-butyric acid, orexin, and histamine systems [74]. | |
| Rivastigmine | Acetylcholinesterase inhibitor and butyrylcholinesterase inhibitor [75]. | |
| Galantamine Donepezil |
Acetylcholinesterase inhibitors potentiate the α4β 2-like nAChR (nicotinic acetylcholine receptor) current at doses of 100 nM—1µM and reversibly potentiate the NMDA-induced currents mediated by c-AMP in cortical neurons [76]. Still, recent data suggested that AChEIs can have negative cognitive effects in a healthy population [77]. | |
| Ispronicline (TC-1734) |
Partial agonist of brain-selective α4β2 nicotine acetylcholine receptor [78]. | |
| Memantine, neramexane | Reversible glutamate NMDA receptor antagonist [79]. | |
| Atomoxetine | Increased acetylcholine efflux and increased cholinergic neurotransmission in cortical regions and the medial prefrontal cortex mediated through α1 and D1 receptor activation [80]. | |
| Piracetam | Enhancement of brain metabolism, modulating cholinergic and glutamatergic systems neurotransmission, improving neuroplasticity, and reducing lipofuscin (age pigment) buildup in the brain [81]. | |
| Nefiracetam | Potentiates AMPA receptor-mediated fast excitatory post-synaptic potential (fEPSP) through CaMKII (Calcium-calmodulin dependent protein kinase 2) activation and enhances NMDA receptor-dependent LTP (long-term potentiation) by enhancing post-synaptic CaMKII and protein kinase C (PKC) activities [82]. | |
| Rolipram | Phosphodiesterase type-4 inhibitor enhances LTP formation by increasing the level of c-AMP and enhancing the cAMP/PKA/CREB pathway [83]. | |
| Pharmaceutical Drugs | D-cycloserine | Facilitates declarative learning and hippocampal activity in humans; acts as a co-agonist on N-Methyl-D-Aspartate receptor [84]. |
| Antidepressant drugs | Increase synaptic plasticity in brain regions associated with mood disorders and memory, such as the hippocampus, amygdala, and prefrontal cortex [85]. | |
| Guanfacine | α-2A adrenergic agonist; increases regional cerebral blood flow in the dorsolateral prefrontal cortex of monkeys performing a spatial working memory task [86]. | |
| Tolcapone | Catechol-O-methyl transferase (COMT) inhibitor; enhances executive memory processes by increasing the amount of dopamine in the prefrontal cortex [87]. |
|
| Hydergine | Increases blood supply to the brain [88] and normalizes the brain levels of serotonin [89]. | |
| Neuropeptides | vasopressin, corticotrophin-releasing hormone (CRH), somatostatin, substance P, neuropeptide Y, and thyrotrophin-releasing hormone (TRH) show improvement in cognitive performance [90]. | |
| Pyritinol | Enhances neuronal metabolism [91]. | |
| ondansetron | Role in the enhancement of memory function in schizophrenia [92]. | |
| Nicergoline | Increases cerebral blood flow, antioxidant, and neuroprotectant properties [93]. | |
| Centrophenoxine | Increasing acetylcholinesterase activity, decreasing the deposition of the age pigment, lipofuscin; increasing the content of serotonin (5-HT) and the fluidity of brain membranes [94]. | |
| Dopaminergic agonist | Dihydrexidine shows D1/D5 receptor agonist activity, releases acetylcholine, and improves cognition in rats [95]. A-412997 shows D4 receptor agonist activity and improves cognitive performance [96]. |
|
| Glucocorticoid receptor agonist |
Dexamethasone and glucocorticoid receptor type II agonist RU 28362 show their memory-enhancing property by affecting the noradrenergic signaling and the cAMP/PKA pathway within the basolateral nucleus of the amygdala and increasing the histone protein acetylation [97]. | |
| Cytidine-5-diphosphocholine | Improves memory in cerebral stroke, AD, and other neurovascular diseases by preventing hippocampal neuronal cell death [98]. | |
| Cotinine | Reduces Aβ aggregation in the brain, prevents memory loss, and stimulates the Akt/GSK3 pathway in vivo [99]. | |
| Other Methods | Transcranial Magnetic Stimulation |
Noninvasive, nonpainful, and safe method. Improve memory by activating the hypothalamic neurons [100]. |
2.8. Drugs Acting on Cholinergic Targets
Acetylcholine has long been known for its involvement in cognitive function, particularly attention, learning, and memory. Degeneration of the cholinergic system is one of the aspects of the pathophysiology of AD. It has also been postulated to contribute to the cognitive deficits of various neuropsychiatric disorders, including schizophrenia [101]. There is ample evidence that decreased nicotinic and muscarinic acetylcholine receptors and functions in the cortex and hippocampus are associated with altered learning, memory, and cognitive functions [102]. Both ionotropic nicotinic receptors (nAChR) and metabotropic muscarinic acetylcholine receptor (mAChR) agonists have been implicated in cognitive enhancement. Besides, decreased activity of choline acetyltransferase, a biosynthetic enzyme for acetylcholine production, has been widely correlated with poor cognitive function [103].
Various drugs targeting cholinergic receptors are given in Fig. (1). Varenicline was the first nAChR agonist approved by the USFDA. It has been found to have complete agonist properties at the α7nAChR and partial agonist at α4β2 nAChR. Preclinical evidence showed that varenicline improves recognition memory in rats [104]. Activation of α7nAChR produces a procognitive and neuroprotective effect, but it has not shown significant efficacy for memory impairment associated with schizophrenia in the clinical trial phase II [105, 106]. TC-6683, AZD-1446, ABT-894/sofinicline, A-87089; ABT-089/pozanicline are α4β2 agonists which have completed phase II clinical trials for the treatment of AD, mild cognitive impairment, and attention deficit hyperactivity disorder. Dexmedetomidine is an α2-receptors agonist with sedative, anxiolytic, analgesic, and anesthetic effects. The drug currently exists in phase II clinical trial and exerts a protective effect against cognitive impairments in rats with post-traumatic stress disorder (PTSD) and in phase IV for the postoperative cognitive disorder.
Fig. (1).
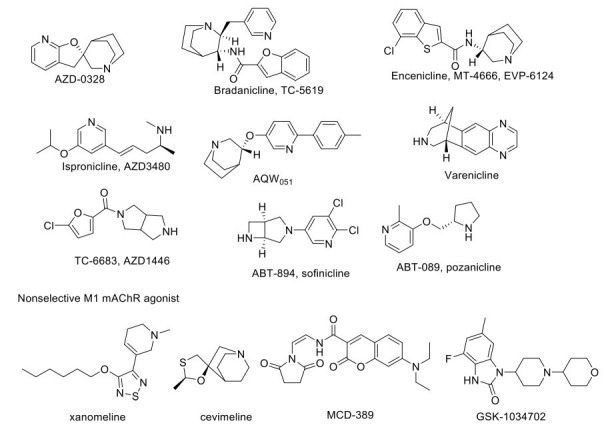
Drugs acting on cholinergic target.
M1 mAChR is the major brain postsynaptic cholinergic receptor and is localized in the cortex, hippocampus, striatum, and thalamus, regulating acetylcholine's effects. Xanomeline, a mAChR agonist (M1/M4), has been studied for clinical trial phase I and phase II for AD and schizophrenia conditions. It has shown significant improvisation of cognitive function but has several unwanted effects due to peripheral M5 antagonistic action, leading to limited patient compliance (ncats.gov.in; clinicaltrials.gov accessed on 07-May.2020). SK-1034702, an M1 receptor agonist, has completed a phase I clinical trial. Furthermore, α7 nicotinic receptor agonists play an important role in treating cognitive and perceptual disturbances in schizophrenia [107]. The investigational drug AZD-0328/ AR-R23465XX has completed phase I clinical trial in AD patients and participated in phase II trial for schizophrenia patients. However, the study has been terminated due to the inability of the drug to meet the target product profile. TC-5619/Bradanicline has completed phase II clinical trial with the result showing no benefit of the drug, whereas MT-4666/ EVP-6124/Encenicline had entered phase III clinical trial, but this study has been terminated due to gastrointestinal side effects for the treatment of AD and cognitive impairment associated with schizophrenia [108, 109]. Ispronicline (AZD3480), a selective partial agonist at the α4β2 nAChR, has completed a phase II clinical trial and shows a promising role in elderly subjects with age-associated memory impairment (AAMI). The novel α7-nACh receptor agonist, i.e., AQW051, a cognition-enhancing agent, was also found to have a beneficial effect in AD. AQW051 entered in phase II clinical trial, but later, the study was terminated. The same drug has shown positive effects in schizophrenic patients in phase II trials. Ladostigil is a reversible AChE and BuChE inhibitor with neuroprotective activity against neurodegenerative disorder. It also increases the activity of catalase and glutathione reductase while decreasing the intracellular production of ROS in a cytotoxic model of human SH-SY5Y neuroblastoma cells [110]. Ladostigil shows a potentially beneficial effect in treating AD by decreasing apoptosis via inhibition of the cleavage and prevention of caspase-3 activation. With these data, the drug made its place in phase II clinical trial and was promising in treating dementia in elderly patients. Similarly, certain other drugs like DMXAB/GTS-21 (α7nAChR agonist), ANAVEX2-73/Blarcamesine (selective sigma-1 receptor: SIGMAR1 agonist), SSR180711, and AVL-3288/CCMI (positive allosteric modulator of α7 nAChR) have shown promising activity in the animal models for cognitive diseases [111-113], and have entered into clinical trials at different phases for AD, ADHD, and schizophrenia. Other agents like selective α7 nAChR agonist A-582941 and PHA-543613 have positively affected cognitive dysfunctions related to AD [114, 115].
ABT-560 has shown promising effects in the preclinical model of cognitive deficit; anyhow, it failed to produce similar results in the clinical trial. Posiphen is currently under clinical trial phase I/II for patients with early AD (Medicines in Development for Older Americans Report 2013), which acts by blocking the translation of the SNCA gene at 5’UTR and inhibits the expression of α-synuclein protein and APP (amyloid precursor protein) gene. Finally, BMS-933043 is a novel partial agonist of α7 nACh receptor that has shown its efficacy in the cognitive impairment preclinical model and is under clinical trial phase I to treat cognitive deficits associated with schizophrenia [116].
2.9. Drugs Acting on Glutamatergic Targets
Glutamate is the major excitatory neurotransmitter in the mammalian CNS and has been recognized to play a major role in learning and memory processes. Many companies have developed agents that act on glutamate via NMDA and AMPA receptors (Fig. 2).
Fig. (2).
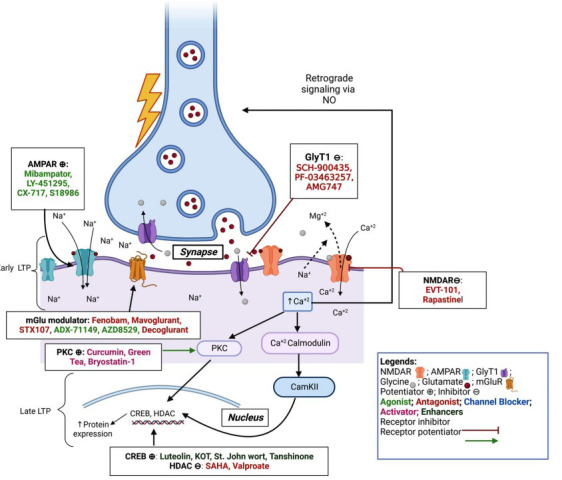
Excitotoxic pathway and drugs acting on it: NMDA, AMPA, and mGlu receptors are involved in the excitotoxicity process in cells. The early phase LTP is modulated by AMPAR (blue) action activated by Glut released from presynaptic vesicles, causing its activation and import of sodium ions, leading to depolarization. The depolarized membrane potential and Glut (red) and Gly (grey) together allow activation of NMDAR (pink) by binding to their respective binding sites on NMDAR, allowing Ca2+ ions influx. The mGluR (yellow) allows the influx of Ca2+ ions in a cell at the late phases of LTP, leading to stronger signaling in the cell. The Ca2+ ions activate Ca2+/Calmodulin protein activating protein kinase (PKC, CaKII), leading to increased mRNA and protein expression like CREB, HDAC, etc. The Ca2+ ions also activate the NOS leading to NO production and retrograde signaling and releasing Glut from presynaptic sites. The entire cycle helps in the formation of neuroprotective protein expression.
These agents increase synaptic plasticity, compensate for the loss of glutamate signaling in disease and normal ageing, and increase the production of neurotrophic factors, such as BDNF. Ampakines (e.g., LY-451395) and related agents potentiate the action of AMPA receptors to increase LTP (Fig. 3). CX516 represents a novel pharmacological intervention, showing a positive AMPA-type glutamate receptor modulatory activity, which facilitates learning and memory in animal models, and has completed the Phase II/III clinical trial showing a positive effect on the cognitive capabilities in patients with stable schizophrenia in combination with other drugs like olanzapine, clozapine or risperidone [117, 118].
Fig. (3).
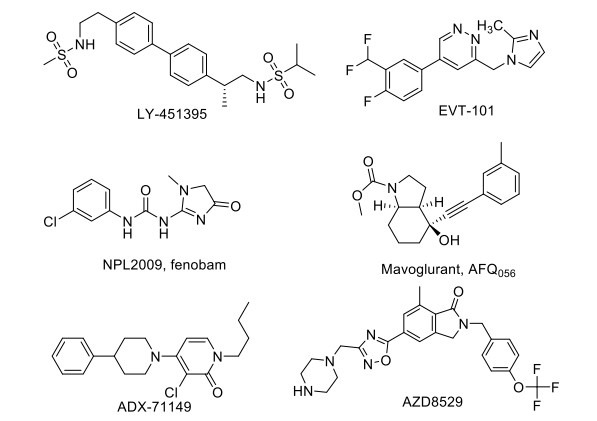
Drugs acting on Glutamatergic targets.
LY-451395 has completed a phase II clinical trial for AD, showing no significant differences between the treatment groups [119]. Other AMPA-targeted agents in different stages of the drug development process are CX-1739 and CX-717. S-18986, a novel compound that promotes long-term potentiation, facilitates post-synaptic responses by positively modulating the AMPA receptor and potentiates AMPA-induced release of noradrenaline in rat brain slices, thus slowing the progression of mild cognitive impairment [120]. It was also found to improve the oxidative stress status in the hippocampus and prelimbic cortex. Allosteric modulators of AMPA receptors can enhance the expression of BDNF mRNA in cultured primary neurons. Consequently, the long-term elevation of endogenous BDNF expression represents a potentially promising therapeutic approach for behavioral disorders with this novel drug S18986, which was under phase II clinical trial (NCT00202540), but the trial was prematurely terminated due to safety concerns regarding reporting of adverse events, including flu-like syndrome without fever, increase in a liver enzyme, and decrease in platelets (from a clinical study report) [121].
Several NMDA antagonists like memantine have been developed. EVT-101 is a potent and selective NR2B subtype-specific antagonist originating from Roche and has an improved side effect profile compared to nonselective NMDA receptor antagonists. It was in a phase II clinical trial for treatment-resistant depression, but the study was terminated due to difficulty in recruiting patients [122]. GLYX-13/ Rapastinel is a tetrapeptide (Thr-Pro-Pro-Thr) novel NMDA receptor glycine-site functional partial agonist tested in the rat. It was found to have therapeutic potential as a learning and memory enhancer due to enhancing LTP and suppressing LTD (Long term depression). Thus, extrapolated use of this agent as a nootropic and neuroprotective entity was hypothesized in-vivo. It was used for major depressive disorder and showed a positive effect in phase II clinical trial, showing amelioration of depressive symptoms. Positive allosteric modulation of mGlu5 receptors enhances synaptic plasticity and cognition [123]. NPL2009/fenobam has entered phase II of the clinical trial, showing no adverse event reported and a significant clinical effect on the Fragile X syndrome condition in its phase I result [122].
ADX-71149, a mGlu2 positive allosteric modulator (PAM), has completed a phase II clinical trial for major depressive disorder with no additional efficacy of the drug [108]. AZD8529, a novel selective PAM of mGluR2, entered in phase II clinical trial for schizophrenia and has shown no efficacy on the disease condition. Decoglurant, a negative allosteric modulator (NAM) of the mGlu2/3 receptor, has completed a phase II clinical trial with registry no. NCT01457677 and did not show any pro-cognitive or antidepressant effect on patients with major depressive disorder [124]. AFQ056, a NAM of mGlu5 receptor, has shown its efficacy in the animal model for Fragile X Syndrome but has failed to show similar action in humans in both adolescents as well as adult patients, leading to a termination of phase II clinical trial of the drug and its repurposing for cocaine use disorder. A similar agent, STX107, entered in phase II clinical trial to treat fragile X syndrome, but the study was suspended for further evaluation. Considerable pharmacological evidence suggests that activation of group II mGlu receptors can distinctly inhibit synaptic transmission and modulate presynaptic glutamate release [125, 126]. ADX-47273, a positive allosteric modulator of the mGlu5 receptor, has shown antipsychotic and improved cognitive activity in animal studies. In addition, it increased long-term potentiation and adaptive learning, which was impaired by ethanol exposure [127].
2.10. Drugs Acting on Glycine Targets
Glycine enhances excitatory neurotransmission by binding to the NMDA complex in cortical and hippocampal structures, suggesting cognition. Local glycine concentrations in the forebrain are controlled by the action of the high-affinity glycine transporter type-1 (GlyT1) (Fig. 4). Therefore, NMDA receptor function can be enhanced by increasing glycine in the synaptic space of NMDA receptors by inhibiting GlyT1. D-cycloserine, a glycine site partial agonist of NMDA receptor, has shown its efficacious action for reversing the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine)-induced memory impairment and neurodegeneration in the PD rat model [128]. Several GlyT1 inhibitors, SCH-900435, PF-03463275, JNJ17305600, AMG747, and R-231857, have been developed in different phases of the clinical trial for schizophrenia and cognitive impairment associated with schizophrenia [108]. SCH-900435/ Org 25935/ MK-8435, a glycine transporter GlyT-1 inhibitor developed by Organon International, has completed a phase II clinical trial as an adjunct therapy with the antipsychotic drug for the management of negative symptoms in schizophrenia patients but did not show any significant advantageous effect in attenuating negative symptoms or improving cognition [129]. PF-03463275 has entered a phase II clinical trial for observing the effect of GlyT1 inhibitor on patients with schizophrenia or schizoaffective disorder with a trial registry NCT01911676 [130]. AMG747 has entered the phase II clinical trial, but the study has been terminated due to toxic epidermal necrolysis observed in schizophrenia patients with negative symptoms [131].
Fig. (4).
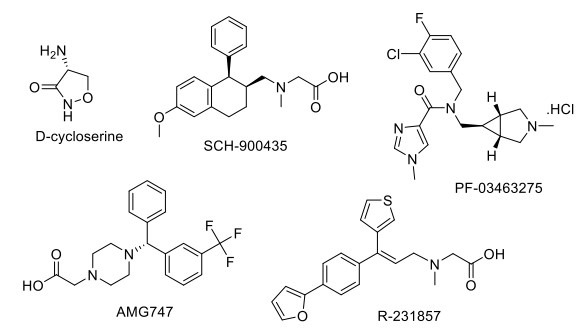
Drugs acting on Glycine target
2.11. Drugs Acting on the GABAergic System
GABA (γ-aminobutyric acid) is the principal inhibitory neurotransmitter in the mammalian CNS. It has been proposed that full-positive allosteric modulators of α2, α3, or α5 may have therapeutic potential for treating cognitive dysfunction associated with schizophrenia [123]. GABA-Aα2/3 agonist MK-O777 reverses the ketamine-induced working memory impairment, but a recent study suggested that MK-0777 has little benefit for cognitive impairment in people with schizophrenia [108]. L-830982, α2/3 agonist, is in phase II clinical trial for cognitive impairment associated with schizophrenia. The α5 subunits of the GABA receptor are localized in the hippocampus. It has been reported that an inverse agonist selective for α5 subunit-containing GABAA receptors improves memory encoding and recall but not consolidation in the Morris water maze [125]. RG1662 (α5 inverse agonist) is currently in phase I clinical trial for cognitive disorders [108]. AC-3933/radequinil, a partial inverse agonist for the benzodiazepine receptor developed by Dainippon Pharmaceutical Co. Ltd., was found to reverse the GABAergic inhibitory effect on cholinergic neurons and thus facilitate acetylcholine release in rat hippocampal slices, resulting in improved cognition. This has completed phase II clinical trial for the treatment of AD but failed to show promising efficacy. GABA-B antagonist SGS742/CGP-36742 is under a phase II clinical trial, to be used in AD, has shown promising results in a study as a therapy for cognitive impairment by reducing CREB2 (cAMP-response element binding protein 2) activity as seen in rat and improving the working memory, attention, and psychomotor speed (Fig. 5) [132].
Fig. (5).
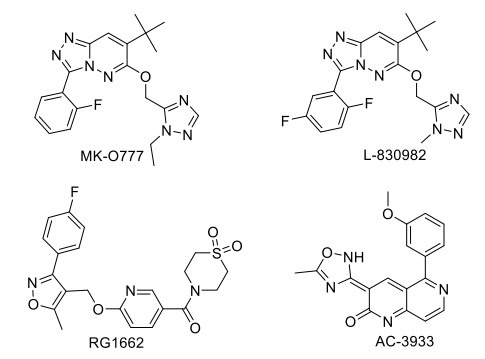
Drugs acting on GABAergic target.
2.12. Drugs Acting on PDE Targets
PDE (phosphodiesterase) inhibitors act as cognition enhancers by blocking the metabolism of cAMP and/or cGMP that enhances neuronal function in the striatum, amygdala, cortex, and hippocampus. Pfizer discovered PF-04447943 (PDE9 inhibitor) and PF-02545920 MP-10 (PDE10 inhibitor). PF-04447943 has completed a phase II clinical trial for AD, showing no improvisation of behavior and cognitive symptoms and causing gastrointestinal adverse events [133]. PF-02545920/mardepodect has completed a phase II clinical trial for schizophrenia [108]. The drug HT-0712, a PDE4 inhibitor, is currently under a phase II clinical trial and was effective in improving age-associated memory impairment and CREB-regulated genes in aged mice. Cilostazol, a potent inhibitor of PDE3, leads to phosphorylation-dependent activation of transcription factor cAMP-responsive element-binding protein (CREB), thus upregulation of Bcl-2 (B-cell lymphoma 2) and COX-2 (Cyclooxygenase-2) expressions, thereby improving cognitive impairment in post-stroke patients. The drug is under a phase IV clinical trial. It shows a positive effect on AD therapy by reducing Aβ accumulation and tau phosphorylation via a significant decrease in ApoE-mediated Aβ aggregation in a mouse model (Fig. 6) [134, 135].
Fig. (6).
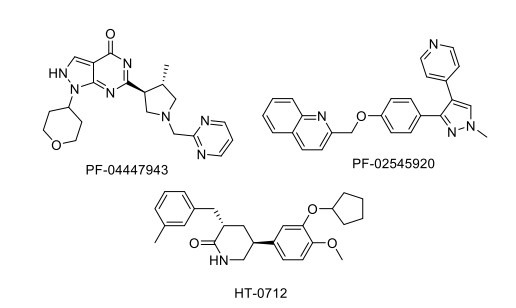
Drugs acting on PDE target.
2.13. Drugs Acting on Serotonergic Targets
Activation of the 5-HT4 (serotonin type 4) receptor leads to the secretion of a soluble non-amyloidogenic form of the amyloid precursor protein (sAPP-alpha), which has neuroprotective, neurotrophic, and cognition-enhancing effects, and it also decreases the extracellular accumulation of amyloid-beta [126]. PRX-03140, a highly selective 5-HT4 partial agonist, has been evaluated in a phase II clinical trial to treat AD at Epix Pharmaceuticals, showing a significant improvisation [136]. Dimebolin, SB-742457/ GSK-742457, SAM-531/ PF-5212365/ WAY-262531, Lu-AE-58054, and SGS-518 are developed as 5-HT6 receptor antagonists with improved affinity and better CNS penetration properties (Fig. 7). These compounds are under different clinical trial phases to treat AD and cognitive impairment associated with schizophrenia (CIAS) [108, 137]. Fluoxetine, an SSRI (Selective serotonin reuptake inhibitor), increased hippocampal population spike amplitudes and excitatory postsynaptic potential slopes in rats. Also, a few studies have been conducted where the potential for the drug to promote recovery of function is shown after DG damage [138]. Another SSRI drug, escitalopram, even after a relatively short treatment period, was effective in treating depression in the elderly and may help improve cognitive performance for social stimuli. Avagacestat (BMS-708163), a selective γ-secretase inhibitor, decreases CSF (Cerebrospinal fluid) Aβ concentrations and has completed phase II clinical trial, where it was found to be well tolerated at low doses, but statistically, insignificant improvement was observed for the treatment of patients with mild to moderate AD [139].
Fig. (7).
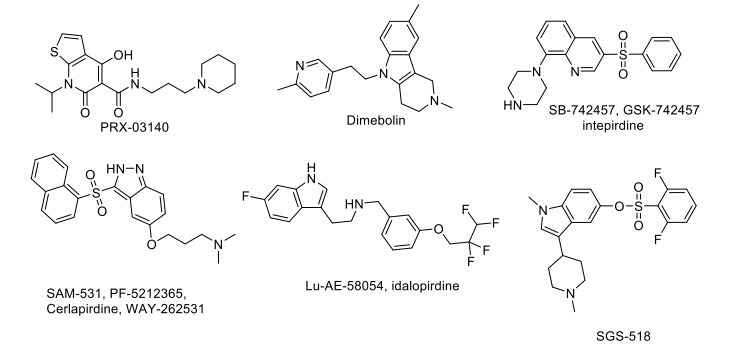
Drugs acting on Serotonergic target.
Aripiprazole is a partial agonist at D2 dopamine receptors, serotonin 5 HT1A, and 5-HT7 receptors, whereas an antagonist at serotonin 5-HT2A and 5-HT6 receptors. Administration of aripiprazole leads to an improvement in PCP (Phencyclidine or phenylcyclohexyl piperidine)-induced cognitive impairment in mice. A clinical trial of this drug reached phase IV for co-therapy with quetiapine or risperidone in schizophrenia with no significant effect on cognitive or psychiatric symptoms, but it has been well-tolerated and safe with a significant effect on prolactin levels [140]. Brexpiprazole is a novel dual serotonin-dopamine activity modulator with partial agonist activity at 5-HT1A and D2/3 receptors and possesses potent antagonist effects on 5-HT2A, α1B-, and α2C-adrenergic receptors. Brexpiprazole reverses the PCP-induced cognitive impairment in the animal model [141, 142]. Quetiapine (D2 and 5-HT2 antagonist), a new antipsychotic drug, prevents memory impairment by protecting the cultured cells against oxidative stress and decreases Aβ plaques in the brains of APP ⁄ presenilin-1 (PS-1) double-mutant mice. It also decreases the β-secretase activity and expression and the level of C99 (an APP C-terminal fragment following cleavage by β-secretase) [144]. Chronic administration of quetiapine prevents PCP-induced memory impairment. It decreases the Bcl-XL/Bax ratio in the posterior cingulate cortex, ameliorating cognitive impairment and brain apoptotic processes. The drug has reached phase IV clinical trial [143]. Lecozotan, a selective 5HT1A receptor antagonist, shows increased learning and memory in rats by stimulating the release of glutamate and acetylcholine in the hippocampus [145, 146].
2.14. Drugs Acting on Histaminergic Targets
Animal studies show that histamine has been implicated in cognitive functions. Histamine H3 receptors inhibit the release of several neurotransmitters involved in cognition. H3 receptor antagonists are currently under evaluation in a clinical trial for cognitive disorders in dementia, CIAS, narcolepsy, and excessive daytime sleepiness. H3 antagonist, thioperamide, reverses the scopolamine-induced spatial orientation, working memory, and passive avoidance impairments in animals [147]. Several H3 receptor antagonists like FUB-649, BF-2649/tiprolisant, GSK-239512, S-38093, and SAR-110894 are in phase II clinical development for the treatment of AD and CIAS (Fig. 8) [108]. H3 inverse agonist MK-0249 has completed the phase II clinical trial (NCT00420420), showing no effective improvement in cognitive deficits associated with AD [148].
Fig. (8).
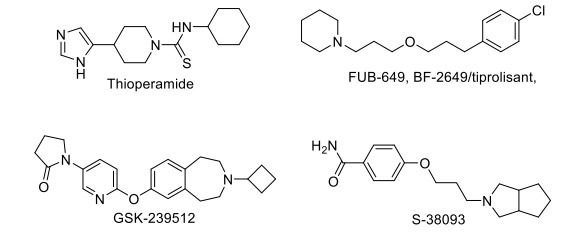
Drugs acting on Histaminergic target.
2.15. Miscellaneous Agents
APH-0703/APH-1104, a bryostatin I analogue, is a PKC modulator that activates the alpha-secretase pathway, stimulating the generation of sAPP and thus decreasing the plaque deposition and cognitive deficits in AD. This drug was approved for phase II clinical trial in 2012, but no further updates have been posted. AVN101 is a serotonin 5-HT6 receptor antagonist currently under phase II clinical trial for AD [149]. Levetiracetam is currently in phase II clinical trial (NCT01044758) as a potential drug for treating cognitive impairment in older patients, where the study proved to effectively reverse the synaptic dysfunction and deficits in learning and memory in hAPP mice. It acts by binding on SVA2, synaptic vesicle protein in the brain, and modulates neurotransmitter release [150]. Finally, an irreversible selective monoamine oxidase-B (MAO-B) inhibitor rasagiline has completed a phase II clinical trial in the treatment of dementia for elderly patients with AD, and the study showed its potential therapeutic effect on the symptomatic treatment of the cognitive disorder in patients with neurodegenerative disorders (NCT02359552) (Fig. 9) [151, 152].
Fig. (9).
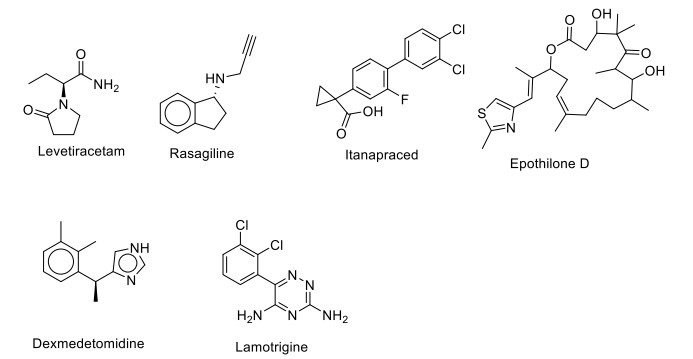
Miscellaneous agents.
In bilateral common carotid artery occlusion (BCCAO), celecoxib (NSAID: non-steroidal anti-inflammatory drug) improves behavioral alterations, glutathione defense, and attenuates acetylcholinesterase activity and TNF-α levels to be neuroprotective against ischemia-reperfusion injury-induced memory dysfunction, neuroinflammation, and oxidative damage. The drug is under phase III clinical trial to treat age-associated memory impairment (NCT00009230). Another nonsteroidal anti-inflammatory derivative, CHF5074/ itanapraced, has been reported as a γ-secretase modulator in vitro and inhibits plaque deposition, reversing memory deficit in vivo in transgenic mouse models of AD. It has completed a phase II clinical trial (NCT01303744) to treat AD in young to elderly patients. It has selectively reduced the pro-inflammatory activities of microglial cells and increased their ability to eliminate neurotoxic Aβ aggregates in the brain by phagocytosis [153-155]. Isoflavones (Novasoy, SIF) have completed a phase II clinical trial (NCT00205179) and have shown to have a good effect on the improvement of memory in older patients with memory dysfunction, mainly by showing inhibitory action on protein tyrosine kinases (EGFR/VEGFR/Her2) and modulating ERα/β, with a result of no major improvement in cognition. SIF pretreatment was found to protect the synaptic plasticity, alleviating learning and memory impairment in rats induced by Aβ1-42. These results suggested that SIF can improve long memory performance in mice. The studies showed that chronic isoflavone supplementation in males activates ERβ, thus improving cognition deficit disorder. In postmenopausal women, SIF supplementation had a positive effect on improving cognitive function and visual memory [156, 157]. The drug BMS-241027/Epothilone D/ KOS-862 has completed a phase I clinical trial (NCT01492374), and it was found that very low doses of it may be useful in the treatment of AD by increasing the stability of microtubules in older patients with taxol like mechanism (Fig. 9). The neuroprotective action of carvedilol (alpha-1 blocker) in aluminum chloride and colchicine-induced cognitive impairment and associated oxidative damage in rats has promoted its use for the treatment of AD and has completed phase IV clinical trial (NCT01354444).
Dihydrexidine (DAR-0100), the first complete D1 agonist that has completed a phase II clinical trial (NCT02507206), showed improvement in cognitive activity in young and elderly individuals. TNF-alpha acts as a mediator for the disruption of synaptic memory mechanism caused by Aβ. The drug etanercept, the antagonist of TNF-alpha, which is currently under a phase II clinical trial, shows potential as an intervention for treating AD (NCT01068353). Lamotrigine (Na+ channel blocker) is used as a treatment for young to older patients suffering from memory impairment and has completed a phase IV clinical trial (NCT01142310) since a study showed that it causes the reduction of the Aβ and upregulation of BDNF and NGF in APP and PS1 mice. A novel pharmacological intervention, ST101, is under a phase II clinical trial (NCT00842673). It was found to activate T-type voltage-gated calcium channels (VGCCs), causing acetylcholine (ACh) release in the hippocampus region in mice. Currently, MEM 1003/ BAY-Z-4406, a dihydropyridine compound, has completed a phase II clinical trial for AD (NCT00257673), possibly reducing the slow after-hyperpolarization (sAHP) period and increasing cell excitability, thus improving spatial memory and attention dependent performance in aged rats. In a study, the effect of a novel L-type Ca2+ channel antagonist, MEM 1003, was evaluated on delay and trace eye blink conditioning (a simple form of associative learning) in older female New Zealand white rabbits, showing enhanced learning. In a study on rats, a bilateral injection of insulin was administered into the dorsal hippocampus, transiently enhancing the hippocampal-dependent memory. Metformin has completed a phase II clinical trial to observe the drug's effect on biomarkers of AD in older patients (NCT01965756). It is currently under phase II/III clinical trial to treat mild cognitive impairment (NCT04098666). It was found that L-methionine induces memory impairment, and metformin shows a beneficial effect in preventing this impairment by normalizing oxidative stress in the hippocampus. Metformin also prevents brain mitochondrial dysfunction, thus restoring learning behavior, which is impaired by long-term HFD consumption. The db/ db mice have increased Aβ levels, so metformin attenuated the increase of total tau, phospho-tau, and activated JNK. It also attenuated the reduction of synaptophysin in the hippocampus [158, 159]. Pioglitazone is under a clinical trial for the treatment of cognitive impairment. Various studies reported that it offered protection against scopolamine-induced memory dysfunctions by affecting the cholinergic system, possibly due to its antioxidant action and a significant neuroprotective effect, thus having therapeutic potential against AD. It also improves the morphine-induced impairment of memory acquisition through the NO pathway. In STZ rats, pioglitazone was found to have a positive effect on improving cognitive performance by lowering oxidative stress and improving cerebral glucose utilization, thereby suppressing the expression of APP, BACE1 (beta-secretase1), RAGE (Receptor for advanced glycation end-products), NF-κB-p65, and activated PPARγ (Peroxisome proliferator-activated receptor-γ) in the hippocampus and cortex. Hence, it can be exploited for dementia associated with diabetes and age-related neurodegenerative disorder. Pioglitazone treatment also reduced oxidative stress by reducing malondialdehyde (MDA) and increasing glutathione levels; these results showed its beneficial effects on cognitive impairment in MPTP-induced PD in rats [160]. Pioglitazone also reduces NMDA-mediated calcium current and transient current, thus improving cognition through the glutaminergic pathway's involvement [161, 162]. Another antidiabetic drug, rosiglitazone, counteracts the increase in IL-1β (interleukin-1β) and IFN-γ (interferon-γ) levels induced by Aβ42 oligomers in hippocampal slices on LTP, which could be the mechanisms of action of rosiglitazone on improvement in memory deficits. The chronic treatment with rosiglitazone, a high-affinity agonist at PPARγ, facilitates Aβ clearance. The study showed that an improvement in insulin resistance might justify improving cognitive performance in older individuals with mild cognitive impairment (MCI) and type 2 diabetes. The drug has completed a phase III clinical trial, and there was no statistically significant improvement in cognition or global function of patients, but the drug was found to be safe and tolerable, showing no safety concerns (NCT00348140) [163]. Exenatide (GLP-1 agonist) was found to improve the cognitive effect in PS1-KI mice by increasing the brain anaerobic glycolysis rate and improving the impaired long-term potentiation in diet-induced obesity in mice. This study can be employed on an older patient suffering from impaired cognition. It is currently in phase III clinical trial (NCT02847403) [164, 165]. The drug liraglutide is a GLP-1 agonist and is under a phase II clinical trial to be employed as a treatment for older patients who have dementia (NCT01843075) since a study has shown its effects on alleviating the hyperphosphorylation of tau and neurofilament proteins by enhancing O-glycosylation of neuronal cytoskeleton protein, improving the JNK and ERK (Extracellular-signal regulated kinases) signaling pathway, and decreasing the neural degeneration, thereby exerting the protective effects on memory impairment induced by i.c.v. injection of STZ (Streptozotocin) in mice [166-169].
EGb 761/ Tanakan® (Ginkgo biloba extract) improves cognitive abilities by increasing SOD and GSH-Px, decreasing the level of MDA, upregulating the ratio of Bcl-2/Bax, and downregulating the level of cleaved Caspase3. The antioxidant properties of EGb 761 aid in neuroprotective and neurotrophic actions on the hippocampus and explain the improvement in memory in both humans and experimental animals [170, 171]. EGb 761 inhibits Aβ oligomerization and its deposits in transgenic Caenorhabditis elegans; thus, it has a therapeutic activity for preventing and treating AD in older patients, and it is currently under phase II clinical trial (NCT03090516). However, long-term treatment with Ginkgo biloba extract in phase IV clinical trial did not reduce the risk of AD progression in patients (NCT00276510) [172]. Souvenaid, a nutritional supplement, aids in the formation of synapses, and its function is currently assessed in a phase II clinical trial. It has shown improved memory performance and brain activity in an earlier randomized clinical trial [173]. Cinacalcet is in a phase II clinical trial. Moreover, it was found to show a good effect in enhancing the memory in young individuals by allosteric activation of the calcium-sensing receptor [174].
3. NEWER TARGETS TO TREAT COGNITIVE DISORDERS AND DEMENTIA
3.1. HDAC (Histone deacetylase) Inhibitors
Epigenetic mechanisms, such as histone acetylation and DNA methylation, are important for memory processes in the adult brain. Chromatic plasticity and histone acetylation are altered in cognitive ageing, neurodegeneration, and neuropsychiatric diseases. Inhibitors of histone deacetylase (HDAC) exhibit neuroprotective potential in animal models of various brain diseases like PD, AD, stroke, schizophrenia, and Rubinstein Taybi Syndrome. HDAC inhibitors like SB (Sodium butyrate), 4-PB(4-phenylbutyrate), valproate, and SAHA are in different phases of clinical trials for the treatment of neurodegenerative diseases (Fig. 10) [175]. Valproic acid (VPA) could ameliorate spatial memory impairment and Aβ deposition via the inhibition of inflammation by reducing the increased expression in IL-1β and tumor necrosis factor-α (TNF-α) in the hippocampus and cortex of APP/ PS1 transgenic mice [176]. VPA inhibits histone deacetylase 1 (HDAC1) enzyme activation, thus attenuating the prenatal hypoxia-induced Aβ-induced memory deficits [177]. Furthermore, the significant induction of melatonin MT2 receptor expression by VPA affects the hippocampal regions involved in memory. In autism, the administration of VPA enhances the paired-pulse facilitation (PPF) and long-term potentiation (LTP), representing short- and long-term synaptic plasticity [178]. VPA was under phase IV clinical trial (NCT00375557) used in elderly patients but was withdrawn.
Fig. (10).
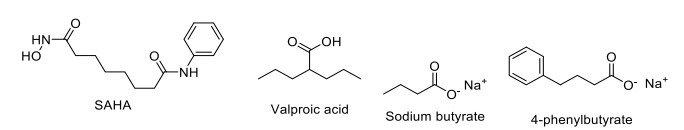
HDAC inhibitor.
3.2. cAMP-Responsive Element-Binding Protein (CREB) Enhancers
CREB/CRE mediated transcription plays a key role in nervous system development, cell survival, synaptic plasticity, and long-term memory formation [179]. Dysregulation of the CREB/CRE transcriptional cascade leads to a deleterious effect on cell survival and synaptic plasticity. Both CREB inhibition and chronic CREB activation trigger cell death via the pro-apoptotic and excitotoxic mechanisms, respectively [180, 181]. Augmentation of CREB-mediated transcription provides beneficial effects on synaptic plasticity and neuronal survival. A traditional Chinese medicinal plant, Kami-ondam-tang (KOT), significantly increased step-through latency in the passive avoidance task by increasing the expressions of phosphorylated Akt, phosphorylated CREB, and BDNF in the hippocampal CA1 and dentate gyrus [182]. Luteolin, a flavonoid, enhances long-term potentiation and memory formation by potentiating CREB signaling (Fig. 11) [183]. 73,000 compounds were screened in a quantitative high-throughput screening format by assaying CREB-mediated β-lactamase reporter gene expression, out of which 1800 compounds were found effective, enhancing the CREB-mediated gene expression phosphodiesterase-4 or protein phosphatase activity by inducing cAMP production or protein kinase A activity [184]. Tanshinone I enhances learning and memory by increasing the ERK/CREB signaling in the hippocampus [185]. N-butanolic extract of Opuntiaficus-indica var. saboten enhances long-term memory in the passive avoidance task in mice by mediating the ERK-CREB-BDNF signaling pathway and increasing immature neuronal survival [186]. In 18-month-old rats, St. John’s wort (Hypericumperforatum) reduces the aging-induced decline of spatial memory, possibly by the activation of CREB regulated genes associated with memory formation [187]. Considering evidence, CREB mediated transcription is an excellent target for the development of cognitive enhancers.
Fig. (11).
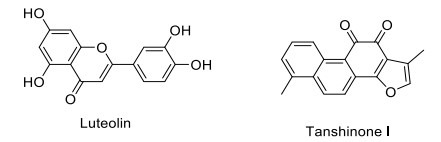
CREB enhancers.
3.3. Glycogen Synthase Kinase-3 Inhibitors
Glycogen synthase kinase-3 (GSK-3) is a multifunctional cellular serine/threonine-protein kinase that inhibits glycogen synthase and is involved in several diverse human diseases, including AD, PD, bipolar disorder (BPD), type-2 diabetes, and cancer [188-190]. GSK-3β is an important regulator of the balance between LTD and LTP, which plays an important role in learning and memory formation [191]. Lithium, a GSK-3β inhibitor, facilitates neurogenesis and reduces Aβ-induced cognitive impairment and hippocampal CA3 neuron loss [192-194]. GSK-3β-selective inhibitor SB-216763 provides only modest improvement in memory retention [194]. The phase II clinical trial of tideglusib showed the cognition-enhancing property in mild to moderate AD patients treated for 24 weeks [195]. Recently, it has been reported that curcumin improves memory and provides neuroprotection in AD by activating the Wnt/β-catenin signaling pathway by inhibiting the activity of GSK-3β (Fig. 12) [196].
Fig. (12).
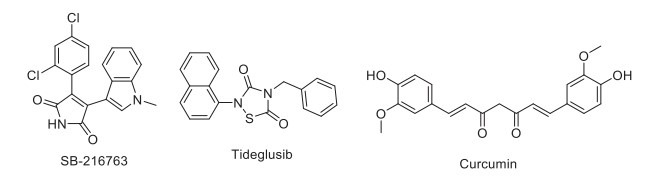
GSK3β inhibitors.
3.4. Protein Kinase C Activators
Protein kinase C (PKC) isoforms are ubiquitously expressed and activated in the central nervous system by Ca2+, phospholipids and diacylglycerol, phorbol esters, or other PKC activators (Fig. 13) [197]. PKC isoforms play an essential role in many types of learning and memory. Activation of PKC enhances synaptogenesis in the hippocampus, but overactivation of PKC leads to memory impairment [198, 199]. Intracerebroventricular (ICV) injection of PKC inhibitors causes marked memory impairment in passive avoidance and Morris water maze tasks. At the same time, curcumin-induced PKCδ degradation leads to improved spatial learning in adult and aged Wistar rats [200]. It is evident that PKCδ inhibition leads to the enhancement of polysialyltransferases (PST) activity; as a result, polysialylation of the neural cell adhesion molecule increases, which is necessary for the consolidation processes of hippocampus-based learning [201]. Activation of PKC by (–)-epigallocatechin gallate, the most active polyphenolic constituent of green tea, enhances learning and memory by facilitating presynaptic glutamate release [202]. Another natural marine product, bryostatin-1, also activates PKC, enhancing learning and memory by stimulating protein synthesis and synaptogenesis [203]. Activation of the PKC isozymes is an attractive therapeutic strategy for improving memory for future research.
Fig. (13).
Protein kinase C activators.
Exebryl-1 reduces Aβ, plaque, and tangle load in AD patients and shows marked improvement in hippocampus-dependent memory, Morris water maze learning, and probe trials [204]. Moreover, testosterone supplementation enhances cognitive function [205]. Its formulation (Androgel 1%) is in phase III clinical trial. Estradiol has also completed a phase II/III clinical trial to treat dementia in young to older patients with AD (NCT00066157). The findings showed that older men with MCI improved memory on E2 treatment. In the medial prefrontal cortex, norepinephrine, dopamine, and serotonin levels were found to decrease on chronic estrogen treatment, thus contributing to the improvement in memory. Raloxifene, a selective estrogen receptor modulator, treatment increases brain activation, thus suggesting cortical arousal where cognition domains exist.
The drug has completed a phase II clinical trial to treat AD and was found to show a positive effect on memory. Furthermore, raloxifene treatment improves cognition in young individuals with schizophrenia (NCT00368459). The cysteine protease inhibitor, E64d, improves memory impairment and reduces brain Aβ by inhibiting cathepsin B [206]. Several antibody-based products have currently reached clinical trials, such as PLY2062430 (solanezumab), AAB-001 (Bapineuzumab), GSK933776A, PF-04360365, and MABT5102A. Long-term systemic administration of antibodies against the APP beta-secretase cleavage site to transgenic mice improves cognitive functions by reducing brain inflammation and incidence of microhemorrhage [207]. Solanezumab has completed a phase III clinical trial in patients with AD. However, the drug failed to improve cognitive function significantly in mild AD [208]. Bapineuzumab underwent a phase II clinical trial showing no significant efficacy in mild to moderate AD patients [209]. An anti-Aβ antibody, GSK933776A, has also completed a phase I clinical trial in patients with AD and evidenced its target in plasma and CSF without causing brain ARIA-E/H (amyloid-related imaging abnormalities-edema or hemorrhage) [210]. PF-04360365 (Ponezumab) was found to be safe and well-tolerated but not effective in patients with mild to moderate AD in phase II clinical trial [211]. Similarly, MABT5102A (crenezumab) did not qualify the criteria to be efficacious in mild to moderate AD management in clinical trial phase II [212]. Activating the somatostatinergic nervous system in the hippocampus by FK962 ameliorates cognitive impairment in rats [213].
CONCLUSION AND FUTURE PERSPECTIVES
Cognitive impairment associated with several neurological disorders is becoming a growing concern for society as the incidences rise with time and an aging population. The molecular mechanism underlying memory formation and cognition has been extensively studied, leading to the discovery of many potential therapeutic targets to date. Still, the research continues as many stones are yet to be turned. The researchers have focused on connecting the dots between several neurological disorders and memory deficits and unveiled the neurobiological pathway of memory formation and cognition. The trends in past years have been set upon discovering pharmaceutical agents for management, and several molecules are under clinical trial for their potential use in treating neurological disorders (Table 2). The limelight has been grabbed by agents acting on the cholinergic targets, leading to the discovery of several agents like galantamine and rivastigmine donepezil, etc., widely used clinically for cognitive disorders management. Alongside, FDA approval of the newest of its class agent, aducanumab, which targets amyloid peptide aggregation, has brought hopes for more such breakthrough research and newer class agents. Researchers nowadays are also trying to target peripheral redirection and disposal of the peptide aggregated in the CNS using mAb like m266 or aggrephagy (autophagic targeting of the aggregates) or by GPCR mediated removal of aggregates. The missing bridge between efficient management of such cognitive disorder lies in the diagnosis and early-stage detection of the memory impairment due to its resemblance to general forgetfulness and amnesia, having similar clinical presentations at initial stages. When the disease leads to a progressed stage, it becomes difficult to be managed by pharmacological agents. Hence, the major focus needs to be on such early-stage biomarkers discovery. Recent studies have shown that technological advances have allowed exploring nuclear and genetic targets for developing pharmacological agents as a therapeutic alternative for disease-associated cognitive disorder and dementia management, leading to the discovery of several microRNAs and small interfering RNAs as suitable targets. As mentioned in this review, several clinical trials are going on, and efficient collaboration between the researcher, clinical researcher, and pharmaceutical industries might bring us closer to developing efficient drug targets and optimum therapy for disease-associated dementia.
Table 2.
Drugs under clinical trial.
| Drug Name | Clinical Trial ID | Target | Indication | Trial Stage | Outcome/ Termination Reason | Refs. |
|---|---|---|---|---|---|---|
| Cholinergic Receptor | ||||||
| TC-6683/ AZD-1446 | NCT01012375 | α4β2 agonist nAChR | ADHD | Phase IIb | No significant effect | [214] |
| ABT-894 | NCT00429091 | ADHD | Phase II | Positive result | [215] | |
| A-87089/ ABT-089 Pozanicline |
NCT00555204 | AD | Phase II | Futility criterion-related termination | [216] | |
| Ispronicline/ AZD3480/ TC-1734-112 |
NCT00109564 | α4β2nAChR partial agonist | Age associated memory impairment | Phase IIb | Positive effect | [217] |
| Xanomeline | NCT03697252 | M1/M4mAChR agonist | Schizophrenia | Phase II | Positive results | [218] |
| GSK-1034702 | NCT01371799 | M1agonsit | Cognitive dysfunction | Phase I | Completed | [219] |
| Varenicline | NCT00802919 | α7nAChR agonist | Schizophrenia | Phase II | No significant effect | [220] |
| DMXBA/ GTS-21 | NCT00100165/ NCT00419445 | Schizophrenia/ ADHD | Phase II | No effect on cognition significantly but the negative symptoms were ameliorated | [221] | |
| MT-4666/ Encenicline | NCT02327182 | AD | Phase III Terminated |
GI side effects | [222] | |
| AZD-0328 | NCT00669903 | Schizophrenia | Phase II Terminated |
Inability to meet the target product profile | [223] | |
| AQW051 | NCT00825539 | Schizophrenia | Phase II | Positive effect | [224] | |
| TC-5619 | NCT01488929 | Schizophrenia | Phase II | No benefit of the drug | [225] | |
| SSR180711 | NCT00602680 | α7nAChR Partial agonist | Mild AD | Phase II Terminated |
Insufficient expected benefit-risk | Clinicaltrials.gov |
| AVL-3288/ CCMI | NCT02978599 | PAM of α7nAChR | Schizophrenia | Phase I | No efficacy of the compound | [226] |
| Ladostigil | NCT01429623 | Reversible AchE and BuChE inhibitor | MCI | Phase II | No effect on cognition but reduced brain and hippocampal atrophy | {Schneider, 2019 #14} [227] |
| Posiphen | NCT02925650 | APP 5’UTR region inhibitor | Early AD | Phase I/II Recruiting |
NA | Clinicaltrials.gov.in |
| ANAVEX2-73, blarcamesine | NCT02756858 | SIGMAR1 agonist | Mild to moderate AD | Phase II/ III | NA | [228] |
| Glutamatergic System | ||||||
| CX516/ Mibampator | NCT00235352 | AMPAR potentiator | Stable Schizophrenia | Phase II/III | Positive Effects on cognition | [229] |
| LY-451395 | NCT00843518 | AD | Phase II | No significant improvement | [119] | |
| S 18986 | NCT00202540 | Behavioral disorder | Phase II Terminated |
ADR reported | [230] | |
| GLYX-13/ Rapastinel | NCT01234558 | NMDA antagonist | Major depressive disorder | Phase II | Amelioration of depressive symptoms | [231] |
| NPL2009/fenobam | NCT00637221 | NAM of mGlu5 | fragile X syndrome | Phase I/ II | No ADR and clinical efficacy proved | [232] |
| AFQ056/ Mavoglurant | NCT01433354 | Phase II Terminated |
Negative outcomes | [233] | ||
| STX107 | NCT01325740 | Phase II Suspended |
NA | Clinicaltrials.gov.in | ||
| ADX-71149/ JNJ-40411813 | NCT01582815 | PAM of mGlu2 | Major Depressive Disorder | Phase II | No significant effect | [234] |
| AZD8529 | NCT00921804 | Schizophrenia | Phase II | No efficacy | [235] | |
| Decoglurant | NCT01457677 | NAM of mGlu2/3 | Major Depressive Disorder | Phase II | No significant pro-cognitive effect for test compound | [124] |
| Glycine | ||||||
| SCH-900435/ Org 25935/ MK-8435 | NCT00725075 | GlyT1 inhibitors | Negative symptoms of Schizophrenia | Phase II Completed |
No significant improvement | [236] |
| PF-03463275 | NCT01911676 | Phase II Recruiting |
NA | Clinicaltrials.gov.in | ||
| AMG747 | NCT01568229 | Phase II Terminated |
Toxic Epidermal Necrolysis ADR reported for test compound | [131] | ||
| GABAnergic System | ||||||
| MK-O777/ L-830982/ TPA023 | NCT00129441 | GABA-Aα2/3 agonist | Schizophrenia | Phase II | Little benefit for cognitive impairment | [237-239] |
| RG1662/ Basmisanil/ RO5186582 | NCT02024789 | GABA-Aα5 inverse agonist/ NAM | Cognitive impairment associated with Down’s Syndrome | Phase II | No efficacy | [240] |
| AC-3933/ Radequinil | NCT00359944 | partial inverse agonist for the BDZ receptor | AD | Phase II | Lack of efficacy | [241] |
| SGS742/ CGP-36742 | NCT00093951 | GABA-B antagonists | AD | Phase II | Improved working memory and psychomotor speed with test compound | Clinicaltrials.gov.in |
| Tramiprosate/ ALZ-801 |
NCT04770220 | GABA mimetic action/ Aβ42 stabilization | AD | Phase II | Safe and well-tolerated; Reduction in CSF Aβ42 levels | [70, 242] |
| PDE Inhibitors | ||||||
| PF-04447943 | NCT00930059 | PDE9 inhibitor | AD | Phase II | No clinical improvisation | [133] |
| PF-02545920/ Mardepodect | NCT01175135 | PDE 10A inhibitor | Schizophrenia | Phase II | No effect | [243, 244] |
| HT-0712 | NCT02013310 | PDE4 inhibitor | AAMI | Phase II | Improved memory and cognition | [245] |
| Cilostazol | NCT01409564 | PDE3 inhibitor | AD | Phase IV | Delays the regional cerebral metabolism | [246] |
| Serotonergic System | ||||||
| PRX-03140 | NCT00384423 | 5-HT4 partial agonist | AD | Phase II | Clinically efficacious | Clinicaltrials.gov.in |
| SB-742457/ Intepirdine/ RVT-101/ GSK-742457 | NCT02585934 | - | Mild to moderate AD | Phase III Completed |
Efficacious | [247] |
| SAM-531/ Cerlapirdine/ PF-5212365/ WAY-262531 | NCT00895895 | Phase II Terminated |
NA | Clinicaltrials.gov.in | ||
| Lu-AE-58054/ Idalopirdine | NCT02006654 | Phase III Completed |
No cognitive improvement | [248] | ||
| Avagacestat BMS-708163 | NCT00810147 | selective γ-secretase inhibitor | Mild to moderate AD | Phase II Completed |
Well tolerated but test drug not significantly efficacious | [139] |
| Aripiprazole/ Abilify/ OPC 14597 | NCT00325689 | partial agonist at D2 receptors | Schizophrenia | Phase IV Completed |
No psychiatric advantage | [249] |
| Others | ||||||
| Levetiracetam | NCT01044758 | SVA2 binding modulates NT release | MCI | Phase II Completed |
Reverses synaptic dysfunction | [250] |
| Rasagiline | NCT02359552 | MAO-B inhibitor | Dementia with AD | Phase II | Potential effect | [251] |
| Celecoxib | NCT00009230 | NSAID | AAMI | Phase III | - | [252] |
| CHF5074/ Itanapraced | NCT01303744 | AD | Phase II | - | [153] | |
| BMS-241027/ Epothilone D | NCT01492374 | Microtubule stabilization | AD | Phase I | Effective | [253] |
| Dihydrexidine/ DAR-0100 | NCT02507206 | Full D1 agonist | Cognitive deficit | Phase II | Improved cognition | [254] |
| Etanercept | NCT01068353 | TNF-alpha antagonist | AD | Phase II | Potential intervention | [255] |
| Lamotrigine | NCT01142310 | Na+ channel blocker | Memory Impairment in AD | Phase IV Completed |
Improved cognitive function | [256] |
| ST101 | NCT00842673 | T-type voltage gated Ca+2 channel blocker | Memory deficits | Phase II | - | [257] |
| Metformin |
NCT01965756 NCT04098666 |
AMPK | AD and MCI |
Phase II Phase II/III |
- | [258] |
| Exenatide | NCT02847403 | GLP-1 agonist | Cognitive impairment | Phase III | - | [259] |
| Liraglutide | NCT01843075 | GLP-1 agonist | Dementia | Phase II | - | [260] |
| EGb 761 | NCT03090516 | Antioxidant | AD | Phase II/III Recruiting |
NA | [261] |
| Raloxifene | NCT00368459 | SERM | AD | Phase II | Improved cognition | [262] |
| PQ912 (Varoglutamstat) | NCT03919162 | Glutaminyl cyclase inhibitor | Early AD | Phase II | Safe and well-tolerated, efficacy result not published | [263] |
Fig. (14).
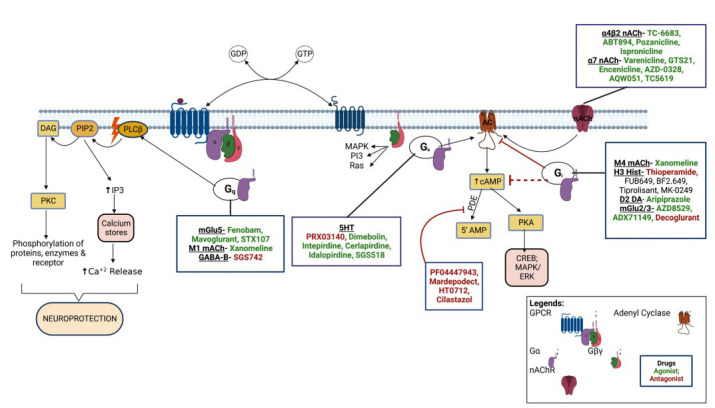
Drugs acting on ionic and metabotropic targets. The metabotropic targets include GPCRs (Blue) with Gs, Gi, and Gq subtypes. When stimulated by ligand, the receptor activates the G-protein, dissociating the Gα (Purple) subunit and activating the secondary messenger. The Gq protein activates PLC in the membrane, which causes the conversion of phospho-inositol biphosphate (PIP2) to diacylglycerol (DAG). The PIP2 is converted to Inositol triphosphate (IP3), which stimulates the release of Ca+2 from the calcium stores like the endoplasmic reticulum. At the same time, the DAG activates PKC, leading to phosphorylation of proteins helping in neuroprotection. The G-βγ (Green, Pink) can also activate pathways like MAPK, PI3, Ras, etc. The Gq protein includes receptors like mGlu5, M1mACh, and GABA-B. The Gs type of GPCR activates the adenyl cyclase (AC, Brown) in the membrane, increasing the cAMP levels, activating protein kinase (PKA), and increasing protein expression by activating CREB MAPK, ERK, etc. This includes receptors like 5HT4 and 5HT6 involved in cognitive processes in CNS. The cAMP is converted to 5’ AMP by phosphodiesterase enzyme, leading to its inactivation. The Gi subtype of GPCR tends to block the AC receptor inhibiting the cAMP cycle. It includes receptors like M4mACh, Histamine H3, Dopamine D2, and mGlu2/3 involved in the memory and cognition processes of the brain. The ionic nACh receptor acts by the movement of sodium and potassium ions through the channels, leading to modulation of cellular processes by acting upon AC. The nACh receptor includes α7 and α4β2 receptors.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- PD
Parkinson’s Disease
- AD
Alzheimer’s Disease
- HIV
Human Immunodeficiency Virus
- ADHD
Attention-Deficit Hyperactivity Disorder
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Arlt S. Non-Alzheimer’s disease-related memory impairment and dementia. Dialogues Clin. Neurosci. 2013;15(4):465–473. doi: 10.31887/DCNS.2013.15.4/sarlt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C., Duering M., Hachinski V., Joutel A., Pendlebury S.T., Schneider J.A., Dichgans M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 4.Landrø N.I. Memory function in schizophrenia. Acta Psychiatr. Scand. Suppl. 1994;384:87–94. doi: 10.1111/j.1600-0447.1994.tb05896.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaul M., Garden G.A., Lipton S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 6.Ringman J.M., Cummings J.L. Current and emerging pharmacological treatment options for dementia. Behav. Neurol. 2006;17(1):5–16. doi: 10.1155/2006/315386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen R.C., Caracciolo B., Brayne C., Gauthier S., Jelic V., Fratiglioni L. Mild cognitive impairment: a concept in evolution. J. Intern. Med. 2014;275(3):214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljubenkov P.A., Geschwind M.D. Dementia. Semin. Neurol. 2016;36(4):397–404. doi: 10.1055/s-0036-1585096. [DOI] [PubMed] [Google Scholar]
- 9.Griesbach G.S., Hovda D.A., Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A., Shanker G., Nath C., Pal R., Singh S., Singh H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002;73(4):893–900. doi: 10.1016/S0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 11.Romay M.C., Toro C., Iruela-Arispe M.L. Emerging molecular mechanisms of vascular dementia. Curr. Opin. Hematol. 2019;26(3):199–206. doi: 10.1097/MOH.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Söderström I., Strand M., Ingridsson A.C., Nasic S., Olsson T. 17beta-estradiol and enriched environment accelerate cognitive recovery after focal brain ischemia. Eur. J. Neurosci. 2009;29(6):1215–1224. doi: 10.1111/j.1460-9568.2009.06662.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Fan Y., Won S.J., Neumann M., Hu D., Zhou L., Weinstein P.R., Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 14.Esneault E., Castagne V., Moser P., Bonny C., Bernaudin M. D-JNKi, a peptide inhibitor of c-Jun N-terminal kinase, promotes functional recovery after transient focal cerebral ischemia in rats. Neuroscience. 2008;152(2):308–320. doi: 10.1016/j.neuroscience.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Ma B., Li M., Nong H., Shi J., Liu G., Zhang J. Protective effects of extract of Coeloglossum viride var. bracteatum on ischemia-induced neuronal death and cognitive impairment in rats. Behav. Pharmacol. 2008;19(4):325–333. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PubMed] [Google Scholar]
- 16.Sun M.K., Alkon D.L. Synergistic effects of chronic bryostatin-1 and alpha-tocopherol on spatial learning and memory in rats. Eur. J. Pharmacol. 2008;584(2-3):328–337. doi: 10.1016/j.ejphar.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Kauppinen T.M., Suh S.W., Berman A.E., Hamby A.M., Swanson R.A. Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J. Cereb. Blood Flow Metab. 2009;29(4):820–829. doi: 10.1038/jcbfm.2009.9. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., He Z., Guo L., Wang H. Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of rho-kinase. Cell. Mol. Neurobiol. 2008;28(5):757–768. doi: 10.1007/s10571-007-9157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams-Gray C.H., Foltynie T., Brayne C.E., Robbins T.W., Barker R.A. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 20.Bronnick K., Emre M., Lane R., Tekin S., Aarsland D. Profile of cognitive impairment in dementia associated with Parkinson’s disease compared with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2007;78(10):1064–1068. doi: 10.1136/jnnp.2006.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buter T.C., van den Hout A., Matthews F.E., Larsen J.P., Brayne C., Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 22.Williams-Gray C.H., Foltynie T., Lewis S.J., Barker R.A. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20(6):477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Galvin J.E., Pollack J., Morris J.C. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67(9):1605–1611. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 24.Rongve A., Aarsland D. Management of Parkinson’s disease dementia: practical considerations. Drugs Aging. 2006;23(10):807–822. doi: 10.2165/00002512-200623100-00004. [DOI] [PubMed] [Google Scholar]
- 25.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67(1):53–83. doi: 10.1016/S0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 26.Brown L.L., Schneider J.S., Lidsky T.I. Sensory and cognitive functions of the basal ganglia. Curr. Opin. Neurobiol. 1997;7(2):157–163. doi: 10.1016/S0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 27.Israel Z., Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci. Biobehav. Rev. 2008;32(3):367–377. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Leyden J., Kleinig T. The role of the basal ganglia in data processing. Med. Hypotheses. 2008;71(1):61–64. doi: 10.1016/j.mehy.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2(4):229–237. doi: 10.1016/S1474-4422(03)00351-X. [DOI] [PubMed] [Google Scholar]
- 30.Emre M., Aarsland D., Albanese A., Byrne E.J., Deuschl G., De Deyn P.P., Durif F., Kulisevsky J., van Laar T., Lees A., Poewe W., Robillard A., Rosa M.M., Wolters E., Quarg P., Tekin S., Lane R. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 31.Moretti R., Torre P., Vilotti C., Antonello R.M., Pizzolato G. Rivastigmine and Parkinson dementia complex. Expert Opin. Pharmacother. 2007;8(6):817–829. doi: 10.1517/14656566.8.6.817. [DOI] [PubMed] [Google Scholar]
- 32.Werber E.A., Rabey J.M. The beneficial effect of cholinesterase inhibitors on patients suffering from Parkinson’s disease and dementia. J. Neural Transm. (Vienna) 2001;108(11):1319–1325. doi: 10.1007/s007020100008. [DOI] [PubMed] [Google Scholar]
- 33.Aarsland D., Hutchinson M., Larsen J.P. Cognitive, psychiatric and motor response to galantamine in Parkinson’s disease with dementia. Int. J. Geriatr. Psychiatry. 2003;18(10):937–941. doi: 10.1002/gps.949. [DOI] [PubMed] [Google Scholar]
- 34.Cummings J.L. Cholinesterase inhibitors for treatment of dementia associated with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2005;76(7):903–904. doi: 10.1136/jnnp.2004.061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shohamy D., Myers C.E., Geghman K.D., Sage J., Gluck M.A. L-dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia. 2006;44(5):774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N., Pillay V., Choonara Y.E. Advances in the treatment of Parkinson’s disease. Prog. Neurobiol. 2007;81(1):29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Mendez M.F. What is the Relationship of Traumatic Brain Injury to Dementia? J. Alzheimers Dis. 2017;57(3):667–681. doi: 10.3233/JAD-161002. [DOI] [PubMed] [Google Scholar]
- 38.Parga B.A., Logsdon A.F., Banks W.A., Ransom C.B. Traumatic Brain Injury Broadly Affects GABAergic Signaling in Dentate Gyrus Granule Cells. eNeuro. 2021;8(3):ENEURO.0055-20.2021.. doi: 10.1523/ENEURO.0055-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carron S.F., Yan E.B., Alwis D.S., Rajan R. Differential susceptibility of cortical and subcortical inhibitory neurons and astrocytes in the long term following diffuse traumatic brain injury. J. Comp. Neurol. 2016;524(17):3530–3560. doi: 10.1002/cne.24014. [DOI] [PubMed] [Google Scholar]
- 40.Yan H., Feng Y., Wang Q. Altered Effective Connectivity of Hippocampus-Dependent Episodic Memory Network in mTBI Survivors. Neural Plast. 2016;2016:6353845. doi: 10.1155/2016/6353845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas C. Marković D.; Giza, C.C. Alterations in mesoscopic oscillations affecting episodic memory following developmental traumatic brain injury. Exp. Neurol. 2018;300:259–273. doi: 10.1016/j.expneurol.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Paterno R., Folweiler K.A., Cohen A.S. Pathophysiology and treatment of memory dysfunction after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2017;17(7):52. doi: 10.1007/s11910-017-0762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langa K.M., Foster N.L., Larson E.B. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 44.Custodio N., Montesinos R., Lira D., Herrera-Pérez E., Bardales Y., Valeriano-Lorenzo L. Mixed dementia: A review of the evidence. Dement. Neuropsychol. 2017;11(4):364–370. doi: 10.1590/1980-57642016dn11-040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fierini F. Mixed dementia: Neglected clinical entity or nosographic artifice? J. Neurol. Sci. 2020;410:116662. doi: 10.1016/j.jns.2019.116662. [DOI] [PubMed] [Google Scholar]
- 46.Bhidayasiri R. Atypical dementia: when it is not Alzheimer’s disease. J. Med. Assoc. Thai. 2007;90(10):2222–2232. [PubMed] [Google Scholar]
- 47.Tsai R.M., Boxer A.L. Therapy and clinical trials in frontotemporal dementia: past, present, and future. J. Neurochem. 2016;138(Suppl. 1):211–221. doi: 10.1111/jnc.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulkey M. Understanding Frontotemporal Disease Progression and Management Strategies. Nurs. Clin. North Am. 2019;54(3):437–448. doi: 10.1016/j.cnur.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Cipriani G., Danti S., Nuti A., Di Fiorino M., Cammisuli D.M. Is that schizophrenia or frontotemporal dementia? Supporting clinicians in making the right diagnosis. Acta Neurol. Belg. 2020;120(4):799–804. doi: 10.1007/s13760-020-01352-z. [DOI] [PubMed] [Google Scholar]
- 50.Harciarek M., Malaspina D., Sun T., Goldberg E. Schizophrenia and frontotemporal dementia: shared causation? Int. Rev. Psychiatry. 2013;25(2):168–177. doi: 10.3109/09540261.2013.765389. [DOI] [PubMed] [Google Scholar]
- 51.Uehara T., Sumiyoshi T., Kurachi M. New pharmacotherapy targeting cognitive dysfunction of schizophrenia via modulation of GABA neuronal function. Curr. Neuropharmacol. 2015;13(6):793–801. doi: 10.2174/1570159X13666151009120153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper J.J., Ovsiew F. The relationship between schizophrenia and frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 2013;26(3):131–137. doi: 10.1177/0891988713490992. [DOI] [PubMed] [Google Scholar]
- 53.Eggers C., Arendt G., Hahn K., Husstedt I.W., Maschke M., Neuen-Jacob E., Obermann M., Rosenkranz T., Schielke E., Straube E. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017;264(8):1715–1727. doi: 10.1007/s00415-017-8503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Liu M., Lu Q., Farrell M., Lappin J.M., Shi J., Lu L., Bao Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology. 2020;95(19):e2610–e2621. doi: 10.1212/WNL.0000000000010752. [DOI] [PubMed] [Google Scholar]
- 55.Lindl K.A., Marks D.R., Kolson D.L., Jordan-Sciutto K.L. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J. Neuroimmune Pharmacol. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambrosius B., Gold R., Chan A., Faissner S. Antineuroinflammatory drugs in HIV-associated neurocognitive disorders as potential therapy. Neurol. Neuroimmunol. Neuroinflamm. 2019;6(3):e551. doi: 10.1212/NXI.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennett S., Thomas A.J. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Black S., Kraemer K., Shah A., Simpson G., Scogin F., Smith A. Diabetes, depression, and cognition: A recursive cycle of cognitive dysfunction and glycemic dysregulation. Curr. Diab. Rep. 2018;18(11):118. doi: 10.1007/s11892-018-1079-0. [DOI] [PubMed] [Google Scholar]
- 59.van Sloten T.T., Sedaghat S., Carnethon M.R., Launer L.J., Stehouwer C.D.A. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livingston G.A., Sax K.B., McClenahan Z., Blumenthal E., Foley K., Willison J., Mann A.H., James I.M., Acetyl L. Carnitine in dementia. Int. J. Geriatr. Psychiatry. 1991;6(12):853–860. doi: 10.1002/gps.930061205. [DOI] [Google Scholar]
- 61.Rampello L., Giammona G., Aleppo G., Favit A., Fiore L. Trophic action of acetyl-L-carnitine in neuronal cultures. Acta Neurol. (Napoli) 1992;14(1):15–21. [PubMed] [Google Scholar]
- 62.Pedata F., Giovannelli L., Spignoli G., Giovannini M.G., Pepeu G. Phosphatidylserine increases acetylcholine release from cortical slices in aged rats. Neurobiol. Aging. 1985;6(4):337–339. doi: 10.1016/0197-4580(85)90013-2. [DOI] [PubMed] [Google Scholar]
- 63.Hashioka S., Han Y.H., Fujii S., Kato T., Monji A., Utsumi H., Sawada M., Nakanishi H., Kanba S. Phosphatidylserine and phosphatidylcholine-containing liposomes inhibit amyloid beta and interferon-gamma-induced microglial activation. Free Radic. Biol. Med. 2007;42(7):945–954. doi: 10.1016/j.freeradbiomed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Holmquist L., Stuchbury G., Berbaum K., Muscat S., Young S., Hager K., Engel J., Münch G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol. Ther. 2007;113(1):154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Beal M.F. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004;36(4):381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 66.Hashimoto M., Hossain S., Shimada T., Sugioka K., Yamasaki H., Fujii Y., Ishibashi Y., Oka J., Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002;81(5):1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 67.Tucker K.L., Qiao N., Scott T., Rosenberg I., Spiro A. III High homocysteine and low B vitamins predict cognitive decline in aging men: the veterans affairs normative aging study. Am. J. Clin. Nutr. 2005;82(3):627–635. doi: 10.1093/ajcn/82.3.627. [DOI] [PubMed] [Google Scholar]
- 68.Aiguo Wu. Zhe Ying; Gomez-Pinilla, F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil. Neural Repair. 2010;24(3):290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cummings J., Aisen P., Lemere C., Atri A., Sabbagh M., Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res. Ther. 2021;13(1):98. doi: 10.1186/s13195-021-00838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tolar M., Abushakra S., Hey J.A., Porsteinsson A., Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 2020;12(1):95. doi: 10.1186/s13195-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jean-Louis G., von Gizycki H., Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J. Pineal Res. 1998;25(3):177–183. doi: 10.1111/j.1600-079X.1998.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 72.Bitner R.S., Bunnelle W.H., Anderson D.J., Briggs C.A., Buccafusco J., Curzon P., Decker M.W., Frost J.M., Gronlien J.H., Gubbins E., Li J., Malysz J., Markosyan S., Marsh K., Meyer M.D., Nikkel A.L., Radek R.J., Robb H.M., Timmermann D., Sullivan J.P., Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J. Neurosci. 2007;27(39):10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arendash G.W., Schleif W., Rezai-Zadeh K., Jackson E.K., Zacharia L.C., Cracchiolo J.R., Shippy D., Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142(4):941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 74.Minzenberg M.J., Carter C.S. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 75.Giacobini E., Spiegel R., Enz A., Veroff A.E., Cutler N.R. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J. Neural Transm. (Vienna) 2002;109(7-8):1053–1065. doi: 10.1007/s007020200089. [DOI] [PubMed] [Google Scholar]
- 76.Narahashi T., Moriguchi S., Zhao X., Marszalec W., Yeh J.Z. Mechanisms of action of cognitive enhancers on neuroreceptors. Biol. Pharm. Bull. 2004;27(11):1701–1706. doi: 10.1248/bpb.27.1701. [DOI] [PubMed] [Google Scholar]
- 77.Balsters J.H., O’Connell R.G., Martin M.P., Galli A., Cassidy S.M., Kilcullen S.M., Delmonte S., Brennan S., Meaney J.F., Fagan A.J., Bokde A.L., Upton N., Lai R., Laruelle M., Lawlor B., Robertson I.H. Donepezil impairs memory in healthy older subjects: behavioural, EEG and simultaneous EEG/fMRI biomarkers. PLoS One. 2011;6(9):e24126. doi: 10.1371/journal.pone.0024126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunbar G., Boeijinga P.H., Demazières A., Cisterni C., Kuchibhatla R., Wesnes K., Luthringer R. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology (Berl.) 2007;191(4):919–929. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- 79.Zoladz P.R., Campbell A.M., Park C.R., Schaefer D., Danysz W., Diamond D.M. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol. Biochem. Behav. 2006;85(2):298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Tzavara E.T., Bymaster F.P., Overshiner C.D., Davis R.J., Perry K.W., Wolff M., McKinzie D.L., Witkin J.M., Nomikos G.G. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol. Psychiatry. 2006;11(2):187–195. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- 81.Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005;11(2):169–182. doi: 10.1111/j.1527-3458.2005.tb00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moriguchi S., Shioda N., Han F., Narahashi T., Fukunaga K. CaM kinase II and protein kinase C activations mediate enhancement of long-term potentiation by nefiracetam in the rat hippocampal CA1 region. J. Neurochem. 2008;106(3):1092–1103. doi: 10.1111/j.1471-4159.2008.05440.x. [DOI] [PubMed] [Google Scholar]
- 83.Gong B., Vitolo O.V., Trinchese F., Liu S., Shelanski M., Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 2004;114(11):1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Onur O.A., Schlaepfer T.E., Kukolja J., Bauer A., Jeung H., Patin A., Otte D.M., Shah N.J., Maier W., Kendrick K.M., Fink G.R., Hurlemann R. The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol. Psychiatry. 2010;67(12):1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 85.Monleón S., Vinader-Caerols C., Arenas M.C., Parra A. Antidepressant drugs and memory: insights from animal studies. Eur. Neuropsychopharmacol. 2008;18(4):235–248. doi: 10.1016/j.euroneuro.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Avery R.A., Franowicz J.S., Studholme C., van Dyck C.H., Arnsten A.F. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23(3):240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 87.Liljequist R., Haapalinna A., Ahlander M., Li Y.H., Männistö P.T. Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav. Brain Res. 1997;82(2):195–202. doi: 10.1016/S0166-4328(97)80989-8. [DOI] [PubMed] [Google Scholar]
- 88.Emmenegger H., Meier-Ruge W. The actions of Hydergine on the brain. A histochemical, circulatory and neurophysiological study. Pharmacology. 1968;1(1):65–78. doi: 10.1159/000135946. [DOI] [PubMed] [Google Scholar]
- 89.Markstein R. Hydergine: interaction with the neurotransmitter systems in the central nervous system. J. Pharmacol. 1985;16(Suppl. 3):1–17. doi: 10.1007/978-1-4612-5058-6_30. [DOI] [PubMed] [Google Scholar]
- 90.Bennett G.W., Ballard T.M., Watson C.D., Fone K.C. Effect of neuropeptides on cognitive function. Exp. Gerontol. 1997;32(4-5):451–469. doi: 10.1016/S0531-5565(96)00159-3. [DOI] [PubMed] [Google Scholar]
- 91.Hindmarch I., Coleston D.M., Kerr J.S. Psychopharmacological effects of pyritinol in normal volunteers. Neuropsychobiology. 1991;24(3):159–164. doi: 10.1159/000119478. [DOI] [PubMed] [Google Scholar]
- 92.Levkovitz Y., Arnest G., Mendlovic S., Treves I., Fennig S. The effect of Ondansetron on memory in schizophrenic patients. Brain Res. Bull. 2005;65(4):291–295. doi: 10.1016/j.brainresbull.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 93.Fioravanti M., Flicker L. Efficacy of nicergoline in dementia and other age associated forms of cognitive impairment. Cochrane Database Syst. Rev. 2001;(4):CD003159. doi: 10.1002/14651858.CD003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stancheva S.L., Alova L.G. Effect of centrophenoxine, piracetam and aniracetam on the monoamine oxidase activity in different brain structures of rats. Farmakol. Toksikol. 1988;51(3):16–18. [PubMed] [Google Scholar]
- 95.Steele T.D., Hodges D.B., Jr, Levesque T.R., Locke K.W., Sandage B.W., Jr The D1 agonist dihydrexidine releases acetylcholine and improves cognition in rats. Ann. N. Y. Acad. Sci. 1996;777(1):427–430. doi: 10.1111/j.1749-6632.1996.tb34457.x. [DOI] [PubMed] [Google Scholar]
- 96.Woolley M.L., Waters K.A., Reavill C., Bull S., Lacroix L.P., Martyn A.J., Hutcheson D.M., Valerio E., Bate S., Jones D.N.C., Dawson L.A. Selective dopamine D4 receptor agonist (A-412997) improves cognitive performance and stimulates motor activity without influencing reward-related behaviour in rat. Behav. Pharmacol. 2008;19(8):765–776. doi: 10.1097/FBP.0b013e32831c3b06. [DOI] [PubMed] [Google Scholar]
- 97.Roozendaal B., Quirarte G.L., McGaugh J.L. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15(3):553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 98.Takasaki K., Uchida K., Fujikawa R., Nogami A., Nakamura K., Kawasaki C., Yamaguchi K., Morita M., Morishita K., Kubota K., Katsurabayashi S., Mishima K., Fujiwara M., Iwasaki K. Neuroprotective effects of citidine-5-diphosphocholine on impaired spatial memory in a rat model of cerebrovascular dementia. J. Pharmacol. Sci. 2011;116(2):232–237. doi: 10.1254/jphs.11013FP. [DOI] [PubMed] [Google Scholar]
- 99.Echeverria V., Zeitlin R., Burgess S., Patel S., Barman A., Thakur G., Mamcarz M., Wang L., Sattelle D.B., Kirschner D.A., Mori T., Leblanc R.M., Prabhakar R., Arendash G.W. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J. Alzheimers Dis. 2011;24(4):817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 100.Hamani C., McAndrews M.P., Cohn M., Oh M., Zumsteg D., Shapiro C.M., Wennberg R.A., Lozano A.M. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann. Neurol. 2008;63(1):119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 101.Hampel H., Toschi N., Babiloni C., Baldacci F., Black K.L., Bokde A.L.W., Bun R.S., Cacciola F., Cavedo E., Chiesa P.A., Colliot O., Coman C.M., Dubois B., Duggento A., Durrleman S., Ferretti M.T., George N., Genthon R., Habert M.O., Herholz K., Koronyo Y., Koronyo-Hamaoui M., Lamari F., Langevin T., Lehéricy S., Lorenceau J., Neri C., Nisticò R., Nyasse-Messene F., Ritchie C., Rossi S., Santarnecchi E., Sporns O., Verdooner S.R., Vergallo A., Villain N., Younesi E., Garaci F., Lista S. Revolution of Alzheimer precision neurology. passageway of systems biology and neurophysiology. J. Alzheimers Dis. 2018;64(s1):S47–S105. doi: 10.3233/JAD-179932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stip E., Chouinard S., Boulay L.J. On the trail of a cognitive enhancer for the treatment of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):219–232. doi: 10.1016/j.pnpbp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Rollema H., Hajós M., Seymour P.A., Kozak R., Majchrzak M.J., Guanowsky V., Horner W.E., Chapin D.S., Hoffmann W.E., Johnson D.E., McLean S., Freeman J., Williams K.E. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem. Pharmacol. 2009;78(7):813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 105.Thomsen M.S., Hansen H.H., Timmerman D.B., Mikkelsen J.D. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr. Pharm. Des. 2010;16(3):323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 106.Roncarati R., Scali C., Comery T.A., Grauer S.M., Aschmi S., Bothmann H., Jow B., Kowal D., Gianfriddo M., Kelley C., Zanelli U., Ghiron C., Haydar S., Dunlop J., Terstappen G.C. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J. Pharmacol. Exp. Ther. 2009;329(2):459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- 107.Martin L.F., Kem W.R., Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl.) 2004;174(1):54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 108.Wallace T.L., Ballard T.M., Pouzet B., Riedel W.J., Wettstein J.G. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol. Biochem. Behav. 2011;99(2):130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 109.Schuster R.M., Pachas G.N., Stoeckel L., Cather C., Nadal M., Mischoulon D., Schoenfeld D.A., Zhang H., Ulysse C., Dodds E.B., Sobolewski S., Hudziak V., Hanly A., Fava M., Evins A.E. Phase IIb trial of an α7 nicotinic receptor partial agonist with and without nicotine patch for withdrawal-associated cognitive deficits and tobacco abstinence. J. Clin. Psychopharmacol. 2018;38(4):307–316. doi: 10.1097/JCP.0000000000000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bar-Am O., Weinreb O., Amit T., Youdim M.B. The novel cholinesterase-monoamine oxidase inhibitor and antioxidant, ladostigil, confers neuroprotection in neuroblastoma cells and aged rats. J. Mol. Neurosci. 2009;37(2):135–145. doi: 10.1007/s12031-008-9139-6. [DOI] [PubMed] [Google Scholar]
- 111.Maurice T. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behav. Brain Res. 2016;296:270–278. doi: 10.1016/j.bbr.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 112.Reggiani A.M., Simoni E., Caporaso R., Meunier J., Keller E., Maurice T., Minarini A., Rosini M., Cavalli A. In vivo characterization of ARN14140, a memantine/galantamine-based multi-target compound for Alzheimer’s disease. Sci. Rep. 2016;6(1):33172. doi: 10.1038/srep33172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takata K., Amamiya T., Mizoguchi H., Kawanishi S., Kuroda E., Kitamura R., Ito A., Saito Y., Tawa M., Nagasawa T., Okamoto H., Sugino Y., Takegami S., Kitade T., Toda Y., Kem W.R., Kitamura Y., Shimohama S., Ashihara E. Alpha7 nicotinic acetylcholine receptor-specific agonist DMXBA (GTS-21) attenuates Aβ accumulation through suppression of neuronal γ-secretase activity and promotion of microglial amyloid-β phagocytosis and ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2018;62:197–209. doi: 10.1016/j.neurobiolaging.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 114.Medeiros R. Castello, N.A.; Cheng, D.; Kitazawa, M.; Baglietto-Vargas, D.; Green, K.N.; Esbenshade, T.A.; Bitner, R.S.; Decker, M.W.; LaFerla, F.M. α7 Nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am. J. Pathol. 2014;184(2):520–529. doi: 10.1016/j.ajpath.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 115.Sadigh-Eteghad S., Talebi M., Mahmoudi J., Babri S., Shanehbandi D. Selective activation of α7 nicotinic acetylcholine receptor by PHA-543613 improves Aβ25-35-mediated cognitive deficits in mice. Neuroscience. 2015;298:81–93. doi: 10.1016/j.neuroscience.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 116.King D., Iwuagwu C., Cook J., McDonald I.M., Mate R., Zusi F.C., Hill M.D., Fang H., Zhao R., Wang B., Easton A.E., Miller R., Post-Munson D., Knox R.J., Gallagher L., Westphal R., Molski T., Fan J., Clarke W., Benitex Y., Lentz K.A., Denton R., Morgan D., Zaczek R., Lodge N.J., Bristow L.J., Macor J.E., Olson R.E. BMS-933043, a Selective α7 nAChR partial agonist for the treatment of cognitive deficits associated with schizophrenia. ACS Med. Chem. Lett. 2017;8(3):366–371. doi: 10.1021/acsmedchemlett.7b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krintel C., Harpsøe K., Zachariassen L.G., Peters D., Frydenvang K., Pickering D.S., Gajhede M., Kastrup J.S. Structural analysis of the positive AMPA receptor modulators CX516 and Me-CX516 in complex with the GluA2 ligand-binding domain. Acta Crystallogr. D Biol. Crystallogr. 2013;69(Pt 9):1645–1652. doi: 10.1107/S0907444913011839. [DOI] [PubMed] [Google Scholar]
- 118.Tatsukawa T., Raveau M., Ogiwara I., Hattori S., Miyamoto H., Mazaki E., Itohara S., Miyakawa T., Montal M., Yamakawa K. Scn2a haploinsufficient mice display a spectrum of phenotypes affecting anxiety, sociability, memory flexibility and ampakine CX516 rescues their hyperactivity. Mol. Autism. 2019;10:15. doi: 10.1186/s13229-019-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trzepacz P.T., Cummings J., Konechnik T., Forrester T.D., Chang C., Dennehy E.B., Willis B.A., Shuler C., Tabas L.B., Lyketsos C. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer’s disease. Int. Psychogeriatr. 2013;25(5):707–719. doi: 10.1017/S1041610212002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao D., Xie F., Xu X., Zhou X. The impact and mechanism of ampakine CX1739 on protection against respiratory depression in rats. Future Med. Chem. 2020;12(23):2093–2104. doi: 10.4155/fmc-2020-0256. [DOI] [PubMed] [Google Scholar]
- 121.Yefimenko N., Portero-Tresserra M., Martí-Nicolovius M., Guillazo-Blanch G., Vale-Martínez A. The AMPA receptor modulator S18986 in the prelimbic cortex enhances acquisition and retention of an odor-reward association. Neurosci. Lett. 2013;548:105–109. doi: 10.1016/j.neulet.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 122.Liu W., Jiang X., Zu Y., Yang Y., Liu Y., Sun X., Xu Z., Ding H., Zhao Q. A comprehensive description of GluN2B-selective N-methyl-D-aspartate (NMDA) receptor antagonists. Eur. J. Med. Chem. 2020;200:112447. doi: 10.1016/j.ejmech.2020.112447. [DOI] [PubMed] [Google Scholar]
- 123.Ayala J.E., Chen Y., Banko J.L., Sheffler D.J., Williams R., Telk A.N., Watson N.L., Xiang Z., Zhang Y., Jones P.J., Lindsley C.W., Olive M.F., Conn P.J. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34(9):2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Umbricht D., Niggli M., Sanwald-Ducray P., Deptula D., Moore R., Grünbauer W., Boak L., Fontoura P. Randomized, double-blind, placebo-controlled trial of the mGlu2/3 negative allosteric modulator decoglurant in partially refractory major depressive disorder. J. Clin. Psychiatry. 2020;81(4):18m12470. doi: 10.4088/JCP.18m12470. [DOI] [PubMed] [Google Scholar]
- 125.Collinson N., Atack J.R., Laughton P., Dawson G.R., Stephens D.N. An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology (Berl.) 2006;188(4):619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- 126.King M.V., Marsden C.A., Fone K.C.F. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol. Sci. 2008;29(9):482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Marszalek-Grabska M., Gibula-Bruzda E., Bodzon-Kulakowska A., Suder P., Gawel K., Talarek S., Listos J., Kedzierska E., Danysz W., Kotlinska J.H. ADX-47273, a mGlu5 receptor positive allosteric modulator, attenuates deficits in cognitive flexibility induced by withdrawal from ‘binge-like’ ethanol exposure in rats. Behav. Brain Res. 2018;338:9–16. doi: 10.1016/j.bbr.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Ho Y.J., Ho S.C., Pawlak C.R., Yeh K.Y. Effects of D-cycloserine on MPTP-induced behavioral and neurological changes: potential for treatment of Parkinson’s disease dementia. Behav. Brain Res. 2011;219(2):280–290. doi: 10.1016/j.bbr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 129.Lidö H.H., Jonsson S., Hyytiä P., Ericson M., Söderpalm B. Further characterization of the GlyT-1 inhibitor Org25935: anti-alcohol, neurobehavioral, and gene expression effects. J. Neural Transm. (Vienna) 2017;124(5):607–619. doi: 10.1007/s00702-017-1685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.D’Souza D.C., Carson R.E., Driesen N., Johannesen J., Ranganathan M., Krystal J.H., Yale Gly T.S.G. Dose-related target occupancy and effects on circuitry, behavior, and neuroplasticity of the glycine transporter-1 inhibitor PF-03463275 in healthy and schizophrenia subjects. Biol. Psychiatry. 2018;84(6):413–421. doi: 10.1016/j.biopsych.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dunayevich E., Buchanan R.W., Chen C.Y., Yang J., Nilsen J., Dietrich J.M., Sun H., Marder S. Efficacy and safety of the glycine transporter type-1 inhibitor AMG 747 for the treatment of negative symptoms associated with schizophrenia. Schizophr. Res. 2017;182:90–97. doi: 10.1016/j.schres.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 132.Vogel K.R., Pearl P.L., Theodore W.H., McCarter R.C., Jakobs C., Gibson K.M. Thirty years beyond discovery--clinical trials in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. J. Inherit. Metab. Dis. 2013;36(3):401–410. doi: 10.1007/s10545-012-9499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schwam E.M., Nicholas T., Chew R., Billing C.B., Davidson W., Ambrose D., Altstiel L.D. A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr. Alzheimer Res. 2014;11(5):413–421. doi: 10.2174/1567205011666140505100858. [DOI] [PubMed] [Google Scholar]
- 134.McHutchison C., Blair G.W., Appleton J.P., Chappell F.M., Doubal F., Bath P.M., Wardlaw J.M. Cilostazol for Secondary Prevention of Stroke and Cognitive Decline: Systematic Review and Meta-Analysis. Stroke. 2020;51(8):2374–2385. doi: 10.1161/STROKEAHA.120.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Motta N.A.V., Autran L.J., Brazão S.C., Lopes R.O., Scaramello C.B.V., Lima G.F., Brito F.C.F. Could cilostazol be beneficial in COVID-19 treatment? Thinking about phosphodiesterase-3 as a therapeutic target. Int. Immunopharmacol. 2021;92:107336. doi: 10.1016/j.intimp.2020.107336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen F., Smith J.A., Chang R., Bourdet D.L., Tsuruda P.R., Obedencio G.P., Beattie D.T. 5-HT(4) receptor agonist mediated enhancement of cognitive function in vivo and amyloid precursor protein processing in vitro: A pharmacodynamic and pharmacokinetic assessment. Neuropharmacology. 2011;61(1-2):69–79. doi: 10.1016/j.neuropharm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 137.Millan M.J., Dekeyne A., Gobert A., Brocco M., Mannoury la Cour C., Ortuno J.C., Watson D., Fone K.C.F. Dual-acting agents for improving cognition and real-world function in Alzheimer’s disease: Focus on 5-HT6 and D3 receptors as hubs. Neuropharmacology. 2020;177:108099. doi: 10.1016/j.neuropharm.2020.108099. [DOI] [PubMed] [Google Scholar]
- 138.Xu F., Zhang G., Yin J., Zhang Q., Ge M.Y., Peng L., Wang S., Li Y. Fluoxetine mitigating late-stage cognition and neurobehavior impairment induced by cerebral ischemia reperfusion injury through inhibiting ERS-mediated neurons apoptosis in the hippocampus. Behav. Brain Res. 2019;370:111952. doi: 10.1016/j.bbr.2019.111952. [DOI] [PubMed] [Google Scholar]
- 139.Coric V., Salloway S., van Dyck C.H., Dubois B., Andreasen N., Brody M., Curtis C., Soininen H., Thein S., Shiovitz T., Pilcher G., Ferris S., Colby S., Kerselaers W., Dockens R., Soares H., Kaplita S., Luo F., Pachai C., Bracoud L., Mintun M., Grill J.D., Marek K., Seibyl J., Cedarbaum J.M., Albright C., Feldman H.H., Berman R.M. Targeting prodromal alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72(11):1324–1333. doi: 10.1001/jamaneurol.2015.0607. [DOI] [PubMed] [Google Scholar]
- 140.Gómez-Revuelta M., Pelayo-Terán J.M., Juncal-Ruiz M., Ortiz-García de la Foz V., Vázquez-Bourgon J., González-Pinto A., Crespo-Facorro B. Long-Term Antipsychotic Effectiveness in First Episode of Psychosis: A 3-Year Follow-Up Randomized Clinical Trial Comparing Aripiprazole, Quetiapine, and Ziprasidone. Int. J. Neuropsychopharmacol. 2018;21(12):1090–1101. doi: 10.1093/ijnp/pyy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frampton J.E. Brexpiprazole: A Review in Schizophrenia. Drugs. 2019;79(2):189–200. doi: 10.1007/s40265-019-1052-5. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe Y., Yamada S., Otsubo T., Kikuchi T. Brexpiprazole for the Treatment of Schizophrenia in Adults: An Overview of Its Clinical Efficacy and Safety and a Psychiatrist’s Perspective. Drug Des. Devel. Ther. 2020;14:5559–5574. doi: 10.2147/DDDT.S240859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han D., Shi S., Luo H. The therapeutic effect of quetiapine on cognitive impairment associated with 5-HT1A presynaptic receptor involved schizophrenia. J. Integr. Neurosci. 2019;18(3):245–251. doi: 10.31083/j.jin.2019.03.186. [DOI] [PubMed] [Google Scholar]
- 144.He J., Luo H., Yan B., Yu Y., Wang H., Wei Z., Zhang Y., Xu H., Tempier A., Li X., Li X.M. Beneficial effects of quetiapine in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging. 2009;30(8):1205–1216. doi: 10.1016/j.neurobiolaging.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Schechter L.E., Smith D.L., Rosenzweig-Lipson S., Sukoff S.J., Dawson L.A., Marquis K., Jones D., Piesla M., Andree T., Nawoschik S., Harder J.A., Womack M.D., Buccafusco J., Terry A.V., Hoebel B., Rada P., Kelly M., Abou-Gharbia M., Barrett J.E., Childers W. Lecozotan (SRA-333): a selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. J. Pharmacol. Exp. Ther. 2005;314(3):1274–1289. doi: 10.1124/jpet.105.086363. [DOI] [PubMed] [Google Scholar]
- 146.Labie C., Lafon C., Marmouget C., Saubusse P., Fournier J., Keane P.E., Le Fur G., Soubrié P. Effect of the neuroprotective compound SR57746A on nerve growth factor synthesis in cultured astrocytes from neonatal rat cortex. Br. J. Pharmacol. 1999;127(1):139–144. doi: 10.1038/sj.bjp.0702545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Komater V.A., Buckley M.J., Browman K.E., Pan J.B., Hancock A.A., Decker M.W., Fox G.B. Effects of histamine H3 receptor antagonists in two models of spatial learning. Behav. Brain Res. 2005;159(2):295–300. doi: 10.1016/j.bbr.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 148.Egan M., Yaari R., Liu L., Ryan M., Peng Y., Lines C., Michelson D. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr. Alzheimer Res. 2012;9(4):481–490. doi: 10.2174/156720512800492530. [DOI] [PubMed] [Google Scholar]
- 149.Ivachtchenko A.V., Lavrovsky Y., Okun I. AVN-101: A multi-target drug candidate for the treatment of CNS disorders. J. Alzheimers Dis. 2016;53(2):583–620. doi: 10.3233/JAD-151146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alrabiah H. Levetiracetam. Profiles Drug Subst. Excip. Relat. Methodol. 2019;44:167–204. doi: 10.1016/bs.podrm.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 151.Hattori N., Takeda A., Takeda S., Nishimura A., Kitagawa T., Mochizuki H., Nagai M., Takahashi R. Rasagiline monotherapy in early Parkinson’s disease: A phase 3, randomized study in Japan. Parkinsonism Relat. Disord. 2019;60:146–152. doi: 10.1016/j.parkreldis.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 152.Chang Y., Wang L.B., Li D., Lei K., Liu S.Y. Efficacy of rasagiline for the treatment of Parkinson’s disease: an updated meta-analysis. Ann. Med. 2017;49(5):421–434. doi: 10.1080/07853890.2017.1293285. [DOI] [PubMed] [Google Scholar]
- 153.Ross J., Sharma S., Winston J., Nunez M., Bottini G., Franceschi M., Scarpini E., Frigerio E., Fiorentini F., Fernandez M., Sivilia S., Giardino L., Calza L., Norris D., Cicirello H., Casula D., Imbimbo B.P. CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebo-controlled study. Curr. Alzheimer Res. 2013;10(7):742–753. doi: 10.2174/13892037113149990144. [DOI] [PubMed] [Google Scholar]
- 154.Ronsisvalle N., Di Benedetto G., Parenti C., Amoroso S., Bernardini R., Cantarella G. CHF5074 protects SH-SY5Y human neuronal-like cells from amyloidbeta 25-35 and tumor necrosis factor related apoptosis inducing ligand toxicity in vitro. Curr. Alzheimer Res. 2014;11(7):714–724. doi: 10.2174/1567205011666140618104430. [DOI] [PubMed] [Google Scholar]
- 155.Porrini V., Lanzillotta A., Branca C., Benarese M., Parrella E., Lorenzini L., Calzà L., Flaibani R., Spano P.F., Imbimbo B.P., Pizzi M. CHF5074 (CSP-1103) induces microglia alternative activation in plaque-free Tg2576 mice and primary glial cultures exposed to beta-amyloid. Neuroscience. 2015;302:112–120. doi: 10.1016/j.neuroscience.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 156.Gleason C.E., Fischer B.L., Dowling N.M., Setchell K.D., Atwood C.S., Carlsson C.M., Asthana S. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J. Alzheimers Dis. 2015;47(4):1009–1019. doi: 10.3233/JAD-142958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roozbeh N., Kashef R., Ghazanfarpour M., Kargarfard L., Darvish L., Khadivzadeh T., Dizavandi F.R., Afiat M. Overview of the Effect of Herbal Medicines and Isoflavones on the Treatment of Cognitive Function. J. Menopausal Med. 2018;24(2):113–118. doi: 10.6118/jmm.2018.24.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Z., Zhang B., Wang X., Zhang X., Yang Q.X., Qing Z., Zhang W., Zhu D., Bi Y. Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: Insights from clinical and functional neuroimaging studies. Diabetes Care. 2019;42(7):1274–1283. doi: 10.2337/dc18-2584. [DOI] [PubMed] [Google Scholar]
- 159.Li J., Deng J., Sheng W., Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol. Biochem. Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chou P.S., Ho B.L., Yang Y.H. Effects of pioglitazone on the incidence of dementia in patients with diabetes. J. Diabetes Complications. 2017;31(6):1053–1057. doi: 10.1016/j.jdiacomp.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 161.Tseng C.H. Pioglitazone reduces dementia risk in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Clin. Med. 2018;7(10):310. doi: 10.3390/jcm7100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tseng C.H. Dementia risk in type 2 diabetes patients: Acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 2020;11(3):658–667. doi: 10.14336/AD.2019.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harrington C., Sawchak S., Chiang C., Davies J., Donovan C., Saunders A.M., Irizarry M., Jeter B., Zvartau-Hind M., van Dyck C.H., Gold M. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr. Alzheimer Res. 2011;8(5):592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- 164.Mullins R.J., Mustapic M., Chia C.W., Carlson O., Gulyani S., Tran J., Li Y., Mattson M.P., Resnick S., Egan J.M., Greig N.H., Kapogiannis D. A pilot study of exenatide actions in Alzheimer’s disease. Curr. Alzheimer Res. 2019;16(8):741–752. doi: 10.2174/1567205016666190913155950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.An J., Zhou Y., Zhang M., Xie Y., Ke S., Liu L., Pan X., Chen Z. Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2019;370:111932. doi: 10.1016/j.bbr.2019.111932. [DOI] [PubMed] [Google Scholar]
- 166.Femminella G.D., Frangou E., Love S.B., Busza G., Holmes C., Ritchie C., Lawrence R., McFarlane B., Tadros G., Ridha B.H., Bannister C., Walker Z., Archer H., Coulthard E., Underwood B.R., Prasanna A., Koranteng P., Karim S., Junaid K., McGuinness B., Nilforooshan R., Macharouthu A., Donaldson A., Thacker S., Russell G., Malik N., Mate V., Knight L., Kshemendran S., Harrison J., Hölscher C., Brooks D.J., Passmore A.P., Ballard C., Edison P. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials. 2019;20(1):191. doi: 10.1186/s13063-019-3259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duarte A.I., Candeias E., Alves I.N., Mena D., Silva D.F., Machado N.J., Campos E.J., Santos M.S., Oliveira C.R., Moreira P.I. Liraglutide protects against brain amyloid-β1-42 accumulation in female mice with early Alzheimer’s disease-like pathology by partially rescuing oxidative/nitrosative stress and inflammation. Int. J. Mol. Sci. 2020;21(5):E1746. doi: 10.3390/ijms21051746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sharma D., Verma S., Vaidya S., Kalia K., Tiwari V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018;108:952–962. doi: 10.1016/j.biopha.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 169.Yaribeygi H., Rashidy-Pour A., Atkin S.L., Jamialahmadi T., Sahebkar A. GLP-1 mimetics and cognition. Life Sci. 2021;264:118645. doi: 10.1016/j.lfs.2020.118645. [DOI] [PubMed] [Google Scholar]
- 170.Lopez O.L., Chang Y., Ives D.G., Snitz B.E., Fitzpatrick A.L., Carlson M.C., Rapp S.R., Williamson J.D., Tracy R.P., DeKosky S.T., Kuller L.H. Blood amyloid levels and risk of dementia in the Ginkgo Evaluation of Memory Study (GEMS): A longitudinal analysis. Alzheimers Dement. 2019;15(8):1029–1038. doi: 10.1016/j.jalz.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wan W., Zhang C., Danielsen M., Li Q., Chen W., Chan Y., Li Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016;81:92–100. doi: 10.1016/j.exger.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 172.Vellas B., Coley N., Ousset P-J., Berrut G., Dartigues J-F., Dubois B., Grandjean H., Pasquier F., Piette F., Robert P., Touchon J., Garnier P., Mathiex-Fortunet H., Andrieu S. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851–859. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 173.Scheltens P., Twisk J.W., Blesa R., Scarpini E., von Arnim C.A., Bongers A., Harrison J., Swinkels S.H., Stam C.J., de Waal H., Wurtman R.J., Wieggers R.L., Vellas B., Kamphuis P.J. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J. Alzheimers Dis. 2012;31(1):225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 174.Wallace H.J., Wallace I.R., McCaffrey P. Cognitive decline reversed by cinacalcet. QJM. 2015;108(1):59–61. doi: 10.1093/qjmed/hcs081. [DOI] [PubMed] [Google Scholar]
- 175.Fischer A., Sananbenesi F., Mungenast A., Tsai L.H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol. Sci. 2010;31(12):605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Xuan A.G., Pan X.B., Wei P., Ji W.D., Zhang W.J., Liu J.H., Hong L.P., Chen W.L., Long D.H. Valproic acid alleviates memory deficits and attenuates amyloid-β deposition in transgenic mouse model of Alzheimer’s disease. Mol. Neurobiol. 2015;51(1):300–312. doi: 10.1007/s12035-014-8751-4. [DOI] [PubMed] [Google Scholar]
- 177.Wang Z., Zhang X.J., Li T., Li J., Tang Y., Le W. Valproic acid reduces neuritic plaque formation and improves learning deficits in APP(Swe)/PS1(A246E) transgenic mice via preventing the prenatal hypoxia-induced down-regulation of neprilysin. CNS Neurosci. Ther. 2014;20(3):209–217. doi: 10.1111/cns.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin H.C., Gean P.W., Wang C.C., Chan Y.H., Chen P.S. The amygdala excitatory/inhibitory balance in a valproate-induced rat autism model. PLoS One. 2013;8(1):e55248. doi: 10.1371/journal.pone.0055248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21(1):127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 180.Ao H., Ko S.W., Zhuo M. CREB activity maintains the survival of cingulate cortical pyramidal neurons in the adult mouse brain. Mol. Pain. 2006;2:15. doi: 10.1186/1744-8069-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Valor L.M., Jancic D., Lujan R., Barco A. Ultrastructural and transcriptional profiling of neuropathological misregulation of CREB function. Cell Death Differ. 2010;17(10):1636–1644. doi: 10.1038/cdd.2010.40. [DOI] [PubMed] [Google Scholar]
- 182.Hong J.G., Kim D.H., Park S.J., Kim J.M., Cai M., Liu X., Lee C.H., Ryu J.H. The memory-enhancing effects of Kami-ondam-tang in mice. J. Ethnopharmacol. 2011;137(1):251–256. doi: 10.1016/j.jep.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 183.Xu B., Li X.X., He G.R., Hu J.J., Mu X., Tian S., Du G.H. Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2010;627(1-3):99–105. doi: 10.1016/j.ejphar.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 184.Xia M., Huang R., Guo V., Southall N., Cho M.H., Inglese J., Austin C.P., Nirenberg M. Identification of compounds that potentiate CREB signaling as possible enhancers of long-term memory. Proc. Natl. Acad. Sci. USA. 2009;106(7):2412–2417. doi: 10.1073/pnas.0813020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim D.H., Kim S., Jeon S.J., Son K.H., Lee S., Yoon B.H., Cheong J.H., Ko K.H., Ryu J.H. Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathway. Br. J. Pharmacol. 2009;158(4):1131–1142. doi: 10.1111/j.1476-5381.2009.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim J.M., Kim D.H., Park S.J., Park D.H., Jung S.Y., Kim H.J., Lee Y.S., Jin C., Ryu J.H. The n-butanolic extract of Opuntia ficus-indica var. saboten enhances long-term memory in the passive avoidance task in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(6):1011–1017. doi: 10.1016/j.pnpbp.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 187.Trofimiuk E., Holownia A., Braszko J.J. Activation of CREB by St. John’s wort may diminish deletorious effects of aging on spatial memory. Arch. Pharm. Res. 2010;33(3):469–477. doi: 10.1007/s12272-010-0318-y. [DOI] [PubMed] [Google Scholar]
- 188.Rylatt D.B., Aitken A., Bilham T., Condon G.D., Embi N., Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur. J. Biochem. 1980;107(2):529–537. doi: 10.1111/j.1432-1033.1980.tb06060.x. [DOI] [PubMed] [Google Scholar]
- 189.Kockeritz L., Doble B., Patel S., Woodgett J.R. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr. Drug Targets. 2006;7(11):1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 190.Frame S., Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359(Pt 1):1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peineau S., Bradley C., Taghibiglou C., Doherty A., Bortolotto Z.A., Wang Y.T., Collingridge G.L. The role of GSK-3 in synaptic plasticity. Br. J. Pharmacol. 2008;153(S1) Suppl. 1:S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rockenstein E., Torrance M., Adame A., Mante M., Bar-on P., Rose J.B., Crews L., Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007;27(8):1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toledo E.M., Inestrosa N.C. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry. 2010;15(3):272–285, 228. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- 194.Dash P.K., Johnson D., Clark J., Orsi S.A., Zhang M., Zhao J., Grill R.J., Moore A.N., Pati S. Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One. 2011;6(9):e24648. doi: 10.1371/journal.pone.0024648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lovestone S., Boada M., Dubois B., Hüll M., Rinne J.O., Huppertz H-J., Calero M., Andrés M.V., Gómez-Carrillo B., León T., del Ser T. A phase II trial of tideglusib in Alzheimer’s disease. J. Alzheimers Dis. 2015;45(1):75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 196.Zhang X., Yin W.K., Shi X.D., Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur. J. Pharm. Sci. 2011;42(5):540–546. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 197.Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 1977;252(21):7603–7609. doi: 10.1016/S0021-9258(17)41009-X. [DOI] [PubMed] [Google Scholar]
- 198.Hama H., Hara C., Yamaguchi K., Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41(3):405–415. doi: 10.1016/S0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- 199.Brennan A.R., Yuan P., Dickstein D.L., Rocher A.B., Hof P.R., Manji H., Arnsten A.F.T. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol. Aging. 2009;30(5):782–792. doi: 10.1016/j.neurobiolaging.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takashima A., Yokota T., Maeda Y., Itoh S. Pretreatment with caerulein protects against memory impairment induced by protein kinase C inhibitors in the rat. Peptides. 1991;12(4):699–703. doi: 10.1016/0196-9781(91)90122-6. [DOI] [PubMed] [Google Scholar]
- 201.Conboy L., Foley A.G., O’Boyle N.M., Lawlor M., Gallagher H.C., Murphy K.J., Regan C.M. Curcumin-induced degradation of PKC delta is associated with enhanced dentate NCAM PSA expression and spatial learning in adult and aged Wistar rats. Biochem. Pharmacol. 2009;77(7):1254–1265. doi: 10.1016/j.bcp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 202.Chou C.W., Huang W.J., Tien L.T., Wang S.J. (-)-Epigallocatechin gallate, the most active polyphenolic catechin in green tea, presynaptically facilitates Ca2+-dependent glutamate release via activation of protein kinase C in rat cerebral cortex. Synapse. 2007;61(11):889–902. doi: 10.1002/syn.20444. [DOI] [PubMed] [Google Scholar]
- 203.Alkon D.L., Sun M.K., Nelson T.J. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol. Sci. 2007;28(2):51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 204.Snow A.D., Cummings J., Lake T., Hu Q., Esposito L., Cam J., Hudson M., Smith E., Runnels S. Exebryl-1: a novel small molecule currently in human clinical trials as a disease-modifying drug for the treatment of Alzheimer’s disease. Alzheimers Dement. 2009;5(4) Suppl. 1:418. doi: 10.1016/j.jalz.2009.04.925. [DOI] [Google Scholar]
- 205.Cherrier M.M., Matsumoto A.M., Amory J.K., Asthana S., Bremner W., Peskind E.R., Raskind M.A., Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64(12):2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 206.Hook G., Hook V., Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-β and improves memory deficits in Alzheimer’s disease animal models by inhibiting cathepsin B, but not BACE1, β-secretase activity. J. Alzheimers Dis. 2011;26(2):387–408. doi: 10.3233/JAD-2011-110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rakover I., Arbel M., Solomon B. Immunotherapy against APP beta-secretase cleavage site improves cognitive function and reduces neuroinflammation in Tg2576 mice without a significant effect on brain abeta levels. Neurodegener. Dis. 2007;4(5):392–402. doi: 10.1159/000103250. [DOI] [PubMed] [Google Scholar]
- 208.Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., Case M., Dean R.A., Hake A., Sundell K., Poole Hoffmann V., Carlson C., Khanna R., Mintun M., DeMattos R., Selzler K.J., Siemers E. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med. 2018;378(4):321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 209.Blennow K., Zetterberg H., Rinne J.O., Salloway S., Wei J., Black R., Grundman M., Liu E., Investigators A. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch. Neurol. 2012;69(8):1002–1010. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 210.Andreasen N., Simeoni M., Ostlund H., Lisjo P.I., Fladby T., Loercher A.E., Byrne G.J., Murray F., Scott-Stevens P.T., Wallin A., Zhang Y.Y., Bronge L.H., Zetterberg H., Nordberg A.K., Yeo A.J., Khan S.A., Hilpert J., Mistry P.C. First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer’s disease: a randomized, placebo-controlled study. PLoS One. 2015;10(3):e0098153. doi: 10.1371/journal.pone.0098153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Landen J.W., Andreasen N., Cronenberger C.L., Schwartz P.F., Börjesson-Hanson A., Östlund H., Sattler C.A., Binneman B., Bednar M.M. Ponezumab in mild-to-moderate Alzheimer’s disease: Randomized phase II PET-PIB study. Alzheimers Dement. (N. Y.) 2017;3(3):393–401. doi: 10.1016/j.trci.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu Z., Zhang A., Sun H., Han Y., Kong L., Wang X. Two decades of new drug discovery and development for Alzheimer’s disease. RSC Advances. 2017;7(10):6046–6058. doi: 10.1039/C6RA26737H. [DOI] [Google Scholar]
- 213.Tokita K., Inoue T., Yamazaki S., Wang F., Yamaji T., Matsuoka N., Mutoh S. FK962, a novel enhancer of somatostatin release, exerts cognitive-enhancing actions in rats. Eur. J. Pharmacol. 2005;527(1-3):111–120. doi: 10.1016/j.ejphar.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 214.Jucaite A., Öhd J., Potter A.S., Jaeger J., Karlsson P., Hannesdottir K., Boström E., Newhouse P.A., Paulsson B. A randomized, double-blind, placebo-controlled crossover study of α4β 2* nicotinic acetylcholine receptor agonist AZD1446 (TC-6683) in adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl.) 2014;231(6):1251–1265. doi: 10.1007/s00213-013-3116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bain E.E., Robieson W., Pritchett Y., Garimella T., Abi-Saab W., Apostol G., McGough J.J., Saltarelli M.D. A randomized, double-blind, placebo-controlled phase 2 study of α4β2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38(3):405–413. doi: 10.1038/npp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lenz R.A., Pritchett Y.L., Berry S.M., Llano D.A., Han S., Berry D.A., Sadowsky C.H., Abi-Saab W.M., Saltarelli M.D. Adaptive, dose-finding phase 2 trial evaluating the safety and efficacy of ABT-089 in mild to moderate Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2015;29(3):192–199. doi: 10.1097/WAD.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 217.Dunbar G.C., Inglis F., Kuchibhatla R., Sharma T., Tomlinson M., Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI). J. Psychopharmacol. 2007;21(2):171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- 218.Shekhar A., Potter W.Z., Lightfoot J., Lienemann J., Dubé S., Mallinckrodt C., Bymaster F.P., McKinzie D.L., Felder C.C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165(8):1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 219.Nathan P.J., Watson J., Lund J., Davies C.H., Peters G., Dodds C.M., Swirski B., Lawrence P., Bentley G.D., O’Neill B.V., Robertson J., Watson S., Jones G.A., Maruff P., Croft R.J., Laruelle M., Bullmore E.T. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int. J. Neuropsychopharmacol. 2013;16(4):721–731. doi: 10.1017/S1461145712000752. [DOI] [PubMed] [Google Scholar]
- 220.Smith R.C., Amiaz R., Si T.M., Maayan L., Jin H., Boules S., Sershen H., Li C., Ren J., Liu Y., Youseff M., Lajtha A., Guidotti A., Weiser M., Davis J.M. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia: A double-blind randomized trial. PLoS One. 2016;11(1):e0143490. doi: 10.1371/journal.pone.0143490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Freedman R., Olincy A., Buchanan R.W., Harris J.G., Gold J.M., Johnson L., Allensworth D., Guzman-Bonilla A., Clement B., Ball M.P., Kutnick J., Pender V., Martin L.F., Stevens K.E., Wagner B.D., Zerbe G.O., Soti F., Kem W.R. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Keefe R.S., Meltzer H.A., Dgetluck N., Gawryl M., Koenig G., Moebius H.J., Lombardo I., Hilt D.C. Randomized, double-blind, placebo-controlled study of encenicline, an α7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40(13):3053–3060. doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arneric S.P., Sher E. Nicotinic Receptors. Springer; 2014. Current and future trends in drug discovery and development related to nicotinic receptors. pp. 435–461. [DOI] [Google Scholar]
- 224.Feuerbach D., Pezous N., Weiss M., Shakeri-Nejad K., Lingenhoehl K., Hoyer D., Hurth K., Bilbe G., Pryce C.R., McAllister K., Chaperon F., Kucher K., Johns D., Blaettler T., Lopez Lopez C. AQW051, a novel, potent and selective α7 nicotinic ACh receptor partial agonist: pharmacological characterization and phase I evaluation. Br. J. Pharmacol. 2015;172(5):1292–1304. doi: 10.1111/bph.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walling D., Marder S.R., Kane J., Fleischhacker W.W., Keefe R.S., Hosford D.A., Dvergsten C., Segreti A.C., Beaver J.S., Toler S.M., Jett J.E., Dunbar G.C. Phase 2 Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) in Negative and Cognitive Symptoms of Schizophrenia. Schizophr. Bull. 2016;42(2):335–343. doi: 10.1093/schbul/sbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kantrowitz J.T., Javitt D.C., Freedman R., Sehatpour P., Kegeles L.S., Carlson M., Sobeih T., Wall M.M., Choo T.H., Vail B., Grinband J., Lieberman J.A. Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology. 2020;45(8):1339–1345. doi: 10.1038/s41386-020-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schneider L.S., Geffen Y., Rabinowitz J., Thomas R.G., Schmidt R., Ropele S., Weinstock M. Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial. Neurology. 2019;93(15):e1474–e1484. doi: 10.1212/WNL.0000000000008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hampel H., Williams C., Etcheto A., Goodsaid F., Parmentier F., Sallantin J., Kaufmann W.E., Missling C.U., Afshar M. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: Analysis of the blarcamesine (ANAVEX2-73) Phase 2a clinical study. Alzheimers Dement. (N. Y.) 2020;6(1):e12013. doi: 10.1002/trc2.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Goff D.C., Lamberti J.S., Leon A.C., Green M.F., Miller A.L., Patel J., Manschreck T., Freudenreich O., Johnson S.A. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2008;33(3):465–472. doi: 10.1038/sj.npp.1301444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bernard K., Danober L., Thomas J.Y., Lebrun C., Muñoz C., Cordi A., Desos P., Lestage P., Morain P. DRUG FOCUS: S 18986: A positive allosteric modulator of AMPA-type glutamate receptors pharmacological profile of a novel cognitive enhancer. CNS Neurosci. Ther. 2010;16(5):e193–e212. doi: 10.1111/j.1755-5949.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Preskorn S., Macaluso M., Mehra D.O., Zammit G., Moskal J.R., Burch R.M. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015;21(2):140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 232.Berry-Kravis E., Hessl D., Coffey S., Hervey C., Schneider A., Yuhas J., Hutchison J., Snape M., Tranfaglia M., Nguyen D.V., Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Berry-Kravis E., Des Portes V., Hagerman R., Jacquemont S., Charles P., Visootsak J., Brinkman M., Rerat K., Koumaras B., Zhu L., Barth G.M., Jaecklin T., Apostol G., von Raison F. Mavoglurant in fragile X syndrome: Results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 2016;8(321):321ra5. doi: 10.1126/scitranslmed.aab4109. [DOI] [PubMed] [Google Scholar]
- 234.Kent J.M., Daly E., Kezic I., Lane R., Lim P., De Smedt H., De Boer P., Van Nueten L., Drevets W.C., Ceusters M. Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;67:66–73. doi: 10.1016/j.pnpbp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 235.Litman R.E., Smith M.A., Doherty J.J., Cross A., Raines S., Gertsik L., Zukin S.R. AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: A proof of principle study. Schizophr. Res. 2016;172(1-3):152–157. doi: 10.1016/j.schres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 236.Schoemaker J.H., Jansen W.T., Schipper J., Szegedi A. The selective glycine uptake inhibitor org 25935 as an adjunctive treatment to atypical antipsychotics in predominant persistent negative symptoms of schizophrenia: results from the GIANT trial. J. Clin. Psychopharmacol. 2014;34(2):190–198. doi: 10.1097/JCP.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 237.Kraus M.S., Gold J.M., Barch D.M., Walker T.M., Chun C.A., Buchanan R.W., Csernansky J.G., Goff D.C., Green M.F., Jarskog L.F., Javitt D.C., Kimhy D., Lieberman J.A., McEvoy J.P., Mesholam-Gately R.I., Seidman L.J., Ball M.P., Kern R.S., McMahon R.P., Robinson J., Marder S.R., Keefe R.S.E. The characteristics of cognitive neuroscience tests in a schizophrenia cognition clinical trial: Psychometric properties and correlations with standard measures. Schizophr. Res. Cogn. 2019;19:100161. doi: 10.1016/j.scog.2019.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Buchanan R.W., Keefe R.S., Lieberman J.A., Barch D.M., Csernansky J.G., Goff D.C., Gold J.M., Green M.F., Jarskog L.F., Javitt D.C., Kimhy D., Kraus M.S., McEvoy J.P., Mesholam-Gately R.I., Seidman L.J., Ball M.P., McMahon R.P., Kern R.S., Robinson J., Marder S.R. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol. Psychiatry. 2011;69(5):442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Atack J.R. Subtype-selective GABA(A) receptor modulation yields a novel pharmacological profile: the design and development of TPA023. Adv. Pharmacol. 2009;57:137–185. doi: 10.1016/S1054-3589(08)57004-9. [DOI] [PubMed] [Google Scholar]
- 240.Potier M-C., Reeves R.H. Intellectual disabilities in Down syndrome from birth and throughout life: Assessment and treatment. Front. Behav. Neurosci. 2016;10:120. doi: 10.3389/fnbeh.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hatayama Y., Hashimoto T., Kohayakawa H., Kiyoshi T., Nakamichi K., Kinoshita T., Yoshida N. In vivo pharmacological characterization of AC-3933, a benzodiazepine receptor partial inverse agonist for the treatment of Alzheimer’s disease. Neuroscience. 2014;265:217–225. doi: 10.1016/j.neuroscience.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 242.Manzano S., Agüera L., Aguilar M., Olazarán J. A review on tramiprosate (homotaurine) in Alzheimer’s disease and other neurocognitive disorders. Front. Neurol. 2020;11:614. doi: 10.3389/fneur.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.DeMartinis N., III, Lopez R.N., Pickering E.H., Schmidt C.J., Gertsik L., Walling D.P., Ogden A. A proof-of-concept study evaluating the phosphodiesterase 10A inhibitor PF-02545920 in the adjunctive treatment of suboptimally controlled symptoms of schizophrenia. J. Clin. Psychopharmacol. 2019;39(4):318–328. doi: 10.1097/JCP.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 244.Walling D.P., Banerjee A., Dawra V., Boyer S., Schmidt C.J., DeMartinis N. Phosphodiesterase 10A inhibitor monotherapy is not an effective treatment of acute schizophrenia. J. Clin. Psychopharmacol. 2019;39(6):575–582. doi: 10.1097/JCP.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 245.Prickaerts J., Heckman P.R.A., Blokland A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2017;26(9):1033–1048. doi: 10.1080/13543784.2017.1364360. [DOI] [PubMed] [Google Scholar]
- 246.Lee J.Y., Lee H., Yoo H.B., Choi J.S., Jung H.Y., Yoon E.J., Kim H., Jung Y.H., Lee H.Y., Kim Y.K. Efficacy of cilostazol administration in Alzheimer’s disease patients with white matter lesions: a positron-emission tomography study. Neurotherapeutics. 2019;16(2):394–403. doi: 10.1007/s13311-018-00708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Maher-Edwards G., Zvartau-Hind M., Hunter A.J., Gold M., Hopton G., Jacobs G., Davy M., Williams P. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr. Alzheimer Res. 2010;7(5):374–385. doi: 10.2174/156720510791383831. [DOI] [PubMed] [Google Scholar]
- 248.Atri A., Frölich L., Ballard C., Tariot P.N., Molinuevo J.L., Boneva N., Windfeld K., Raket L.L., Cummings J.L. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients With Alzheimer disease: Three randomized clinical trials. JAMA. 2018;319(2):130–142. doi: 10.1001/jama.2017.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Li H., Luo J., Wang C., Xie S., Xu X., Wang X., Yu W., Gu N., Kane J.M. Efficacy and safety of aripiprazole in Chinese Han schizophrenia subjects: a randomized, double-blind, active parallel-controlled, multicenter clinical trial. Schizophr. Res. 2014;157(1-3):112–119. doi: 10.1016/j.schres.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 250.Schoenberg M.R., Rum R.S., Osborn K.E., Werz M.A. A randomized, double-blind, placebo-controlled crossover study of the effects of levetiracetam on cognition, mood, and balance in healthy older adults. Epilepsia. 2017;58(9):1566–1574. doi: 10.1111/epi.13849. [DOI] [PubMed] [Google Scholar]
- 251.Weintraub D., Hauser R.A., Elm J.J., Pagan F., Davis M.D., Choudhry A., Investigators M. Rasagiline for mild cognitive impairment in Parkinson’s disease: A placebo-controlled trial. Mov. Disord. 2016;31(5):709–714. doi: 10.1002/mds.26617. [DOI] [PubMed] [Google Scholar]
- 252.Follow-up evaluation of cognitive function in the randomized Alzheimer’s disease anti-inflammatory prevention trial and its follow-up study. Alzheimers Dement. 2015;11(2):216–25.e1. doi: 10.1016/j.jalz.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bakota L., Brandt R. Tau biology and tau-directed therapies for Alzheimer’s disease. Drugs. 2016;76(3):301–313. doi: 10.1007/s40265-015-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rosell D.R., Zaluda L.C., McClure M.M., Perez-Rodriguez M.M., Strike K.S., Barch D.M., Harvey P.D., Girgis R.R., Hazlett E.A., Mailman R.B., Abi-Dargham A., Lieberman J.A., Siever L.J. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology. 2015;40(2):446–453. doi: 10.1038/npp.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Butchart J., Brook L., Hopkins V., Teeling J., Püntener U., Culliford D., Sharples R., Sharif S., McFarlane B., Raybould R., Thomas R., Passmore P., Perry V.H., Holmes C. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology. 2015;84(21):2161–2168. doi: 10.1212/WNL.0000000000001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Suzuki H., Gen K. Clinical efficacy of lamotrigine and changes in the dosages of concomitantly used psychotropic drugs in Alzheimer’s disease with behavioural and psychological symptoms of dementia: a preliminary open-label trial. Psychogeriatrics. 2015;15(1):32–37. doi: 10.1111/psyg.12085. [DOI] [PubMed] [Google Scholar]
- 257.Gauthier S., Rountree S., Finn B., LaPlante B., Weber E., Oltersdorf T. Effects of the acetylcholine release agent ST101 with donepezil in Alzheimer’s disease: A randomized phase 2 study. J. Alzheimers Dis. 2015;48(2):473–481. doi: 10.3233/JAD-150414. [DOI] [PubMed] [Google Scholar]
- 258.Lin Y., Wang K., Ma C., Wang X., Gong Z., Zhang R., Zang D., Cheng Y. Evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front. Aging Neurosci. 2018;10:227. doi: 10.3389/fnagi.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Perna S., Mainardi M., Astrone P., Gozzer C., Biava A., Bacchio R., Spadaccini D., Solerte S.B., Rondanelli M. 12-month effects of incretins versus SGLT2-Inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with Type-2 diabetes mellitus. Clin. Pharmacol. 2018;10:141–151. doi: 10.2147/CPAA.S164785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mansur R.B., Ahmed J., Cha D.S., Woldeyohannes H.O., Subramaniapillai M., Lovshin J., Lee J.G., Lee J-H., Brietzke E., Reininghaus E.Z., Sim K., Vinberg M., Rasgon N., Hajek T., McIntyre R.S. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study. J. Affect. Disord. 2017;207:114–120. doi: 10.1016/j.jad.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 261.Demarin V. Bašić Kes, V.; Trkanjec, Z.; Budišić M.; Bošnjak Pašić M.; Črnac, P.; Budinčević H. Efficacy and safety of Ginkgo biloba standardized extract in the treatment of vascular cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial. Neuropsychiatr. Dis. Treat. 2017;13:483–490. doi: 10.2147/NDT.S120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Henderson V.W., Ala T., Sainani K.L., Bernstein A.L., Stephenson B.S., Rosen A.C., Farlow M.R. Raloxifene for women with Alzheimer disease: A randomized controlled pilot trial. Neurol. 2015;85(22):1937–1944. doi: 10.1212/WNL.0000000000002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scheltens P., Hallikainen M., Grimmer T., Duning T., Gouw A.A., Teunissen C.E., Wink A.M., Maruff P., Harrison J., van Baal C.M., Bruins S., Lues I., Prins N.D. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimers Res. Ther. 2018;10(1):107. doi: 10.1186/s13195-018-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


