Abstract
Phototherapy is an emerging approach for cancer treatment that is effective at controlling the growth of primary tumors. In the presence of light irradiation, photothermal and photodynamic agents that are delivered to tumor sites can induce local hyperthermia and the production of reactive oxygen species, respectively, that directly eradicate cancer cells. Nanoparticles, characterized by their small size and tunable physiochemical properties, have been widely utilized as carriers for phototherapeutic agents to improve their biocompatibility and tumor-targeted delivery. Nanocarriers can also be used to implement various co-delivery strategies for further enhancing phototherapeutic efficiency. More recently, there has been considerable interest in augmenting the immunological effects of nanoparticle-based phototherapies, which can yield durable and systemic antitumor responses. This review provides an overview of recent developments in using nanoparticle technology to achieve photo-immunotherapy.
Keywords: nanomedicine, phototherapy, immunotherapy, combination therapy, cancer
Graphical Abstract
The use of nanoparticle-based phototherapies has emerged as a promising strategy for cancer treatment. More recently, researchers have been interested in augmenting the immunological effects of these platforms in order to further enhance therapeutic efficacy. In this review, we discuss recent developments on the topic of nanoparticle-based photo-immunotherapy.
1. Introduction
As cancer continues to be one of the leading causes of death worldwide, significant effort has been dedicated towards developing more effective treatment modalities. Among them, phototherapies that rely on the direct application of light energy onto tumor tissues have demonstrated great promise.[1, 2] Photothermal therapy (PTT) relies on heat generation to physically ablate cancer cells, whereas photodynamic therapy (PDT) utilizes the production of reactive oxygen species (ROS) to exert cytotoxicity.[3, 4] In either case, the effects are generally mediated by a phototherapeutic agent, and an extensive body of work has been dedicated towards reducing their toxicity and improving their photoconversion efficiency.[5, 6] Along these lines, the rise of nanoparticle-based carriers has provided unique opportunities to further advance phototherapies by offering better tumor targeting, enhanced penetration, and co-delivery with other therapeutic compounds.[7] During the process of tumor destruction, phototherapies are capable of inducing immunogenic cell death (ICD), which can be characterized by the presentation or release of damage-associated molecular patterns (DAMPs) and tumor antigens.[8, 9] While this phenomenon may not be particularly potent on its own,[10, 11] there has recently been considerable interest in augmenting the immunological responses to phototherapies in order to promote systemic and durable antitumor responses.[12]
Over the past two decades, immunotherapies have proven to be viable for controlling the growth of various types of malignancies.[13, 14] Many immunotherapeutic strategies have been explored in the clinic, including vaccination, adoptive cell transfer, and checkpoint blockade.[15-17] Cancer vaccines are generally composed of select tumor antigens combined with potent immunostimulatory agents to train the immune system to better recognize and eliminate cancer cells.[18, 19] Among the cell-based therapies, the use of chimeric antigen receptor (CAR) T cells that directly recognize and kill target cells has been highly successful against some hematological malignancies.[20] Immune checkpoint blockade therapies target markers such as cytotoxic T lymphocyte-associated protein 4 (CTLA4), programmed cell death protein 1 (PD1), and programmed cell death ligand 1 (PDL1) in order to disrupt the immunosuppressive mechanisms in the tumor microenvironment, thus unleashing the intrinsic antitumor potential of the immune system.[21-23] Beyond these approaches to immunotherapy, others such as the administration of cytokines or immunological adjuvants have also demonstrated potential.
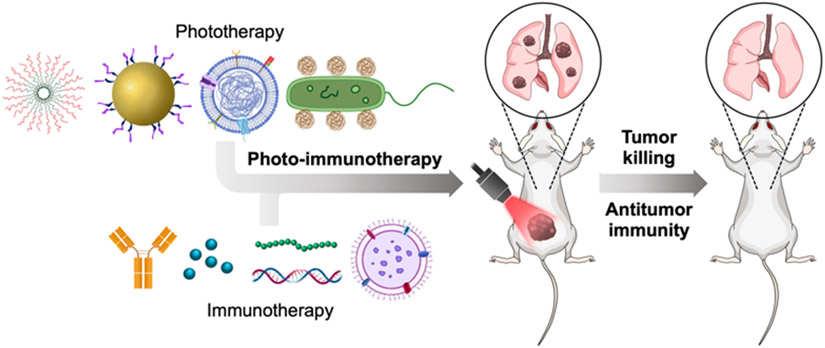
It is increasingly being understood that combinatorial approaches to tumor therapy can yield vastly superior outcomes compared with monotherapies.[24] For example, immunotherapies have demonstrated synergism with surgery, chemotherapy, and radiation therapy, among others.[25] Thus, while both phototherapies and immunotherapies have considerable utility when used in isolation, photo-immunotherapy (PIT) platforms that combine the advantages of both are becoming increasingly popular (Figure 1). In this review, we discuss the development of PTT and PDT, followed by the different approaches for augmenting their immune effects. Then, we provide a comprehensive overview of recent nanoscale phototherapeutic platforms, including those based on emerging cellular nanoparticle technologies, that have been developed to achieve improved antitumor immunity.
Figure 1.
Photo-immunotherapy for anticancer therapy. When combined, phototherapies and immunotherapies can synergize to achieve enhanced control of tumor growth, both at the primary tumor site and distant sites of metastasis.
2. Augmenting the immune response to phototherapies
2.1. Phototherapeutic modalities
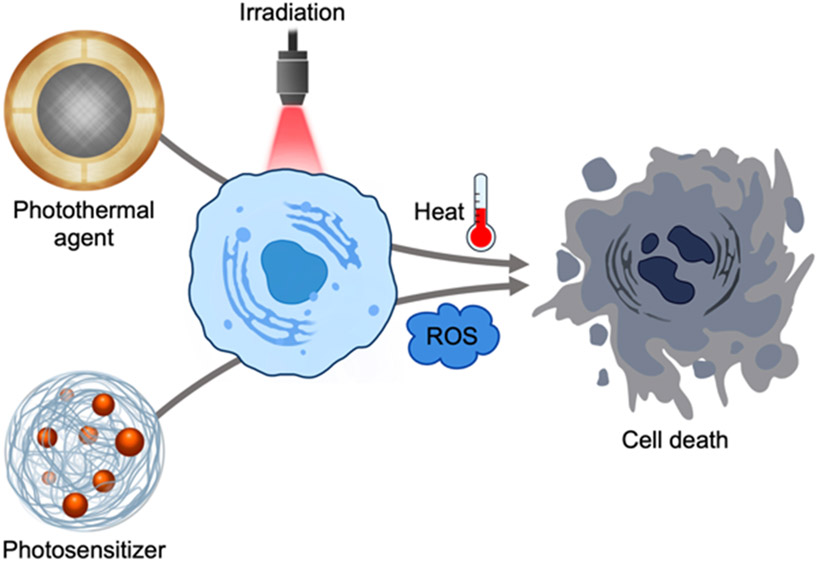
While the concepts of modern phototherapy originated in the 19th century, its roots can be traced back to ancient civilization.[26] Early examples included the consumption of Psoralea corylifolia seeds or Ammi majus extracts coupled with sunlight exposure to treat leukoderma. Modern science has identified specific agents that can produce heat for PTT or ROS for PDT under light illumination in order to cause cell death in tumor tissues (Figure 2).[27]
Figure 2.
Mechanism of action for phototherapies. Photothermal agents and photosensitizers respectively produce heat and ROS under light irradiation, which results in cell death by necrosis or apoptosis.
To achieve PTT, photothermal agents absorb the energy of photons to generate heat via localized surface plasmon resonance effects for inorganic materials or via non-radiative relaxation for organic materials.[28] The heat can then trigger cell death through either necrosis, characterized by the breakage of the plasma membrane and the presentation of DAMPs, or apoptosis, where membrane integrity is largely maintained while expressing “eat me” signals such as phosphatidylserine.[29] Overall, local hyperthermia in tumor tissues can disrupt cellular membrane, inactivate enzymes and other proteins, and affect the expression of apoptosis-related genes.[30] Compared with healthy tissues, tumor tissues are more sensitive to elevated temperatures since their heat dissipation capacity is impaired by poor capillary systems.[31] The type of laser and photothermal agent are two vital components that can be optimized to improve PTT efficacy and lower toxicity. To increase penetration depth and reduce non-desired light absorption by healthy tissues, lasers with wavelengths in biological windows (700 nm ~ 1400 nm) where normal tissues are partially transparent with low absorption and scattering have been utilized.[32] Inorganic nanoparticles such as gold nanorods, platinum nanoparticles, and carbon nanotubes have drawn attention as photothermal agents due to their high conversion efficiency and photostability.[33] However, the poor biodegradability and potential long-term toxicity of these platforms makes their clinical translation more difficult. Small molecules with photothermal conversion properties, including cyanines [e.g. indocyanine green (ICG) and IR780], porphyrins, croconaines, and diketopyrrolopyrroles, have been encapsulated into biodegradable nanoparticle formulations.[34]
PDT is regarded as a potent method for eliminating superficial tumors that relies on the production of ROS. Typically, a photosensitizer in a ground state is transformed to an excited singlet state under light illumination, after which it can then undergo intersystem crossing to a more stable excited triplet state.[35] The excited photosensitizer can then produce ROS through two different methods. In a type 1 reaction, the excited photosensitizer can directly react with substrates like cell membrane and mediate electron transfer to generate H2O2, hydroxyl radicals, and superoxide radicals.[36] In a type 2 reaction, the excited photosensitizer directly transfers energy to oxygen to produce singlet oxygen, which is highly reactive with various biological substrates.[37] These oxidative agents formed from type 1 and type 2 reactions can target different biomolecules like DNA, proteins, and lipids, mediating cellular apoptosis at lower levels and necrosis at high levels.[38] Besides directly killing tumor cells, the ROS produced by photosensitizers can also disrupt the surrounding vasculature to inhibit the transport of nutrients and oxygen.[39]
Desirable properties for PDT photosensitizers include low toxicity, optimal pharmacokinetics, good tissue penetration, high singlet oxygen yield, and tumor selectivity.[40] Earlier photosensitizers such as hematoporphyrin and its derivatives were explored for cancer therapies, although there were concerns regarding their lack of purity, poor solubility, and suboptimal absorbance spectra leading to low efficacy and unpredictable patient responses.[41-43] More recently developed photosensitizers, including chlorin e6 (Ce6), protoporphyrin IX, and phthalocyanine, are chemically pure compounds, enabling more facile quality control during large-scale production.[44] They also exhibit improved absorbance at higher wavelengths and increased singlet oxygen quantum yields. To improve their tumor accumulation and reduce their toxicity to normal cells, these photosensitizers have been conjugated with targeting moieties such as antibodies or peptides.[40] Different photosensitizer payloads have also been encapsulated into nanoparticulate carriers, which can improve their stability and solubility, avoid self-quenching effects, and increase singlet oxygen yield while also enhancing their tumor accumulation through passive and active targeting.[45]
2.2. Development of PIT
It has been reported that both PTT and PDT can elicit antitumor immunity mainly through ICD.[8, 46-49] As mentioned previously, the hyperthermia or ROS generated by these phototherapeutic modalities can result in apoptotic and necrotic cell death, during which tumor-associated antigens and DAMPs, including calreticulin (CRT), high mobility group box 1 (HMGB1), heat shock protein 70 (HSP70) and HSP90, and adenosine 5’-triphosphate (ATP), are released or presented on cell surface.[50] HMGB1 and ATP can recruit antigen-presenting cells (APCs) as “find me” signals, and these cells can be manipulated for phagocytosis by CRT as an “eat me” signal and activated by HSPs complexed with tumor-associated antigens.[51, 52] The activated APCs can then migrate to lymphoid tissues where they present tumor antigens to resident T cells that are capable of mediating tumor cell killing.[53] In addition to ICD, the disruption of the extracellular matrix and tumor vessels,[54] the release of proinflammatory cytokines such as interleukin 6 (IL6) and IL1β,[55] and the recruitment of neutrophils mediated by E-selectin and chemokines like macrophage inflammatory protein-2 have been observed to further promote antitumor immune responses during the application of phototherapies.[56]
Despite the potential for generating antitumor immunity, few clinical trials have reported potent immune responses induced by traditional phototherapies in patients.[57] Other preclinical studies have shown that many phototherapies only provide modest protection against distant tumors, tumor metastases, and tumor relapse, indicating a failed induction of systemic antitumor responses.[12] Possible reasons include the localization of immune response only to the treatment site and the presence of various immunosuppressive mechanisms, including within the tumor microenvironment.[27, 58, 59] With this in mind, many researchers are now designing phototherapeutic platforms with a specific emphasis on eliciting potent antitumor immunity. The first major strategy is to simply work on enhancing the potency of PTT or PDT, which can be done by selecting or designing more efficient phototherapeutic agents, relieving tumor hypoxia, increasing tumor accumulation, and targeting specific subcellular components such as the endoplasmic reticulum (ER) and mitochondria.[60-63] With the generation of more heat or ROS, stronger ICD effects can be elicited, resulting in elevated immune responses that are capable of providing systemic anticancer effects. The second approach involves supplementing phototherapy with an immunotherapeutic modality. Immunotherapies exploit the body’s immune system to eradicate cancer cells, and they can include cancer vaccines, checkpoint inhibitors, adoptive cell transfer, cytokines, and costimulatory receptor agonists.[64] Despite their promise, many of these approaches are not universally applicable and can demonstrate insufficient potency depending on the type of tumor being treated.[65, 66] Considering the beneficial properties of phototherapy and immunotherapy, combining both of them into a single PIT can result in considerable synergies.
3. Development of nanoparticle platforms for PIT
With their wide range of features and flexible design, nanoparticles have emerged as attractive platforms for phototherapeutic applications. In addition to being able to deliver photothermal agents or photosensitizers, many nanoparticles inherently exhibit potent photothermal or photodynamic activity.[67] With their small size, nanoparticulate platforms are able to passively accumulate at tumor sites through the enhanced permeability and retention effect.[68] Active targeting can be achieved through surface modification with various ligands, including antibodies, peptides, and aptamers, among others.[69] The use of stimuli-responsive nanomaterials can enable the controlled release of payloads within a tumor by either local or external triggers.[70] Nanocarriers are also capable of delivering compounds to alleviate the hypoxia that is characteristic of tumor microenvironments, thus further improving phototherapeutic efficacy.[71] Overall, with recent interest in the immune aspect of PTT and PDT, researchers have investigated two main methods of augmenting the immune response to nanoparticle-based phototherapies: (1) enhancement of the phototherapeutic effect, either by increasing photoconversion efficiency or improving payload delivery, and (2) direct combination with immunomodulatory agents (Table 1).
Table 1.
Strategies for improving the immune response to nanoparticle-based phototherapies.
| Strategy | Examples | Ref. |
|---|---|---|
| Enhancement of phototherapeutic effect | ||
| Enhanced absorption and ROS generation efficiency | CoWO4-x nanoplatform with wide absorption spectrum. | [75] |
| p(MEO2MA-co-OEGMA)-b-pSS used to control the spacing between photosensitizers. | [60] | |
| Photosensitizer with aggregation-induced emission constructed using indole salt and pyrrolo[3,2-b]pyrrole. | [81] | |
| Relieving tumor hypoxia | Synechococcus functionalized with ICG-loaded human serum albumin nanoparticles. | [61] |
| pH-responsive nanoplatform co-loaded with Ce6 and a plasmid encoding catalase. | [101] | |
| Cu2+-doped zeolitic imidazolate framework-8 filled with oxygen and functionalized with the same MOF material loaded with Ce6 before coating with Pluronic F127. | [121] | |
| Improving tumor accumulation | Nanoparticles self-assembled from Ce6 and the amphiphilic PEG-b-poly(ε-caprolactone) block copolymer attached with ICG-conjugated poly(amidoamine) dendrimers. | [129] |
| Semiconducting polymer nanoparticles conjugated with the protease bromelain. | [131] | |
| Magnetic Fe3O4 nanoparticles coated with platelet membrane. | [147] | |
| Controlling subcellular localization | ICG-conjugated hollow gold nanospheres and hemoglobin-loaded liposomes functionalized with an ER-targeting pardaxin peptide. | [162] |
| Hafnium-iridium MOF incorporated with a zinc-phthalocyanine photosensitizer. | [166] | |
| Gold nanorods coated with a nuclear localization signal peptide attached to an amphiphilic copolymer that was then adsorbed with siRNA targeting YTHDF1. | [174] | |
| Combination of phototherapy with immune-enhancing treatments | ||
| Modulation of immune cell populations | High affinity agonistic antibody targeting the glucocorticoid-induced cancer necrosis factor receptor loaded onto polydopamine nanoparticles containing ICG and catalase. | [184] |
| CaO2 nanoparticles loaded with ultrasmall CuS-MnO2 nanoparticles and coated with hyaluronic acid. | [187] | |
| Mesoporous silica nanoparticles loaded with IR780 and metformin and sealed with CeO2 nanoparticles. | [192] | |
| Immune checkpoint blockade | Polydimethylsiloxane microneedles loaded with dextran nanoparticles co-encapsulated with anti-CTLA4 and the photosensitizer zinc phthalocyanine. | [216] |
| Au@Pt adsorbed with PDL1 antagonist peptides DPPA-1 conjugated with a tumor-homing peptide LyP-1 via an MMP2-degradable peptide linker. | [217] | |
| Semiconducting polymer nanoparticles functionalized with the small molecule IDO1 inhibitor NLG919 using a singlet oxygen-responsive linker. | [219] | |
| Enhancement of immune cell function | Nanovesicles self-assembled from CpG with black phosphorus quantum dots functionalized with PEG and hydrophobic ROS-sensitive poly(propylene sulfide). | [235] |
| Gold nanorods grafted with an imidazoquinoline-based TLR7/8 agonistic polymer. | [238] | |
| OMVs from Escherichia coli engineered with human tumor necrosis factor related apoptosis-inducing ligand and conjugated with ICG and an RGD peptide. | [246] | |
3.1. Enhancement of phototherapeutic effect
3.1.1. Enhanced absorption and ROS generation efficiency
Due to their high light conversion efficiency, various inorganic materials have been explored as novel phototherapeutic agents, including carbon dots,[72] black phosphorus nanosheets,[73] and rare earth element upconversion nanoparticles.[74] In a recent study, a CoWO4-x nanoplatform was constructed with a wide absorption spectrum, and it was capable of generating of both heat and ROS upon irradiation for dual PTT and PDT.[75] When using inorganic materials, their possible accumulation in tissues and associated toxicity risks must be considered;[76] those with good biodegradability and biocompatibility are desirable.
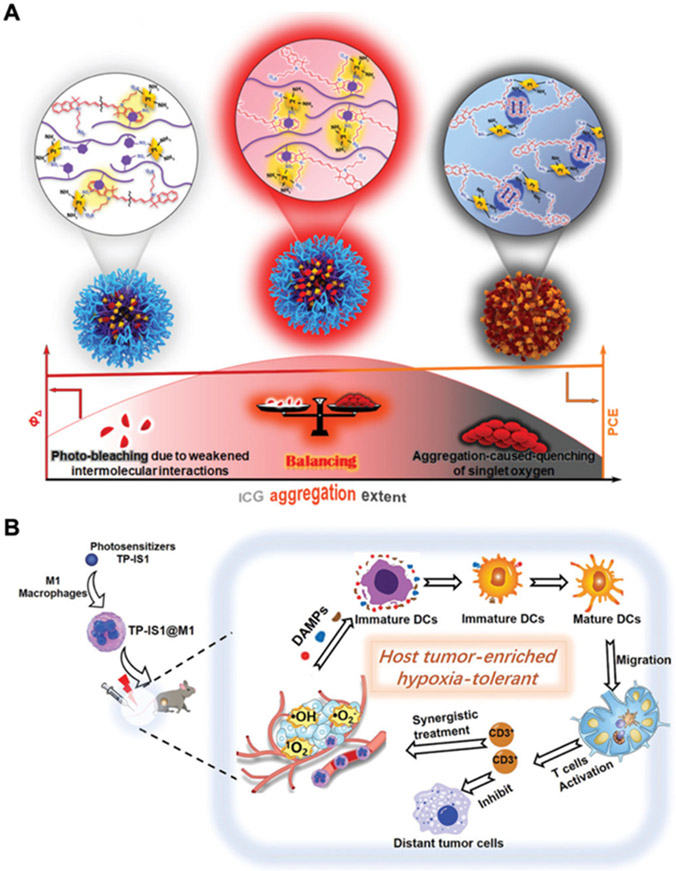
For photosensitizing agents, many candidates with potent light absorption and singlet oxygen generation efficiency have been explored.[77] Photobleaching and aggregation-caused quenching are two phenomena that often need to be overcome for phototherapy applications, and both can potentially be addressed by precisely regulating the spacing between photosensitizer molecules.[78] In one example, the polymer p(MEO2MA-co-OEGMA)-b-pSS was utilized as a spacer and used to formulate a nanocarrier to coloaded with ICG and cisplatin (Figure 3A).[60] By adjusting the ratio of the polymer to ICG, optimal aggregation of the photosensitizer could be achieved to minimize both photobleaching and quenching. The use of photosensitizers with aggregation-induced emission is another approach for overcoming quenching.[79, 80] For instance, one such agent was constructed using indole salt and pyrrolo[3,2-b]pyrrole, and it was shown to produce abundant ROS within the tumor microenvironment to enhance ICD for systemic antitumor efficacy (Figure 3B).[81]
Figure 3.
Enhancing photothermal efficiency. (A) By optimizing the spacing of a phototherapeutic agent within a nanoparticle matrix, it is possible to minimize both photobleaching and aggregation-induced quenching. Adapted with permission.[60] Copyright 2022, Wiley-VCH. (B) The hypoxia-tolerant TP-IS1 photosensitizer is loaded into M1 macrophages to enrich its tumor accumulation and elicit potent antitumor efficacy. Adapted with permission.[81] Copyright 2022, Wiley-VCH.
3.1.2. Relieving tumor hypoxia
Hypoxia in the tumor microenvironment results from an imbalance between the increased metabolic activity of cancer cells and reduced oxygen permeation due to compromised vasculature.[82] Multiple studies have identified a correlation between tumor hypoxia and features of advanced tumor progression, including angiogenesis,[83] metastasis,[84] metabolic reprogramming,[85] epigenetic reprogramming,[86] and immune suppression.[87] Tumor hypoxia has also been implicated as a cause of resistance to chemotherapy,[88] radiotherapy[89], and phototherapy, especially type 2 PDT which requires oxygen.[90] To relieve hypoxia for enhanced phototherapy, various nanoplatforms have been developed to either generate oxygen in situ or deliver exogenous oxygen.
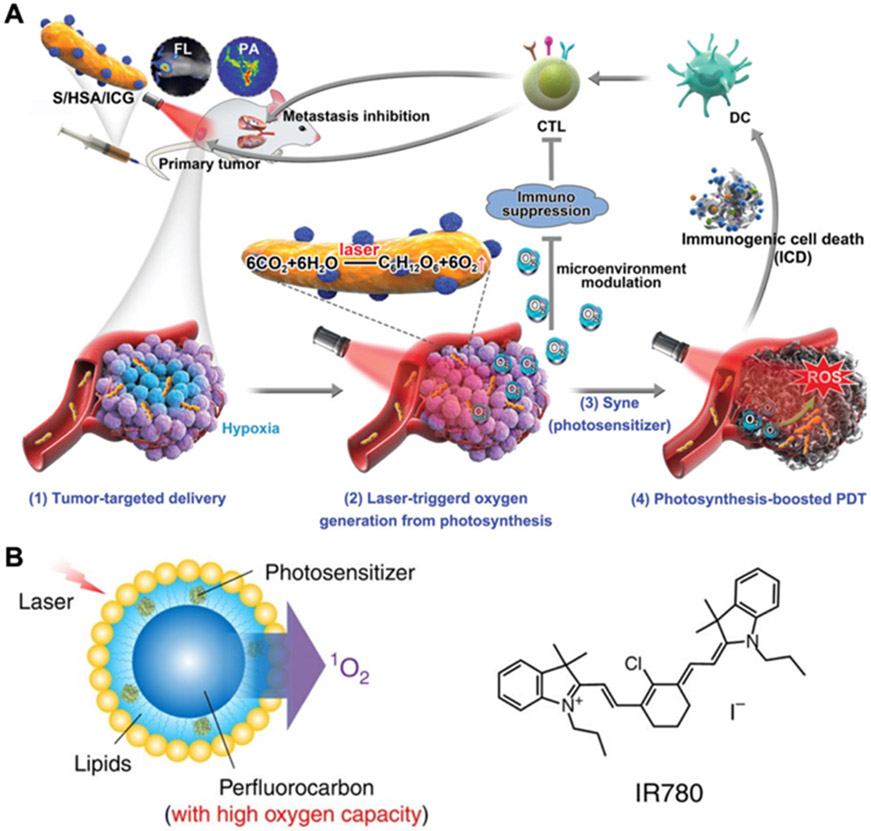
To utilize their natural oxygen production ability, living organisms such as algae[91, 92] and cyanobacteria[61, 93] have been engineered and delivered into tumors to relieve hypoxia. For instance, ICG-loaded human serum albumin nanoparticles were attached onto Synechococcus through chemical crosslinking, and the modified cyanobacteria were then injected intravenously into tumor-bearing mice (Figure 4A).[61] Compared with free nanoparticles, the final formulation showed improved tumor accumulation in both normoxic and hypoxic regions, as well as higher oxygen and singlet oxygen levels after laser irradiation. The enhanced PDT mediated by this platform significantly increased tumor CRT expression and activated dendritic cells (DCs) in the tumor microenvironment and draining lymph nodes, leading to the complete inhibition of primary 4T1 tumors and reduced metastasis. In addition to living organisms, chloroplast components have also been explored as oxygen generators. As an example, thylakoid membranes have been derived from vegetables and then coated onto various inorganic nanoparticles for phototherapeutic applications.[94, 95]
Figure 4.
Relieving tumor hypoxia. (A) An in situ photocatalyzed oxygen generation system is developed by conjugating ICG-encapsulated human serum albumin nanoparticles onto Synechococcus (S/HAS/ICG). The platform preferentially accumulates in tumors, and laser-triggered oxygen generation promotes ROS production to enhance photodynamic efficacy and antitumor immunity. Adapted with permission.[61] Copyright 2020, Wiley-VCH. (B) A perfluorocarbon nanodroplet is loaded with oxygen and the photosensitizer IR780 for oxygen self-enriching photodynamic therapy. Adapted with permission.[119] Copyright 2015, Nature Publishing Group.
Inspired by photosynthesis, novel materials have been constructed to achieve light-driven water splitting for in vivo oxygen production. For example, carbon nitride modified with carbon dots was able to produce oxygen efficiently in phosphate-buffered saline under irradiation at 630 nm.[96] After being linked with an amphipathic polymer consisting of protoporphyrin IX as a photosensitizer, the resulting nanoformulation was injected intratumorally and alleviated tumor hypoxia for enhanced antitumor efficacy. Apart from carbon nitride and its derivates, other materials including cobalt phytate,[97] graphdiyne oxide[98], and iron disulfide[99] have also been developed for in vivo water splitting.
H2O2 is often overproduced by cancer cells and can be broken down by catalase to produce oxygen, thus providing a way to mitigate tumor hypoxia.[100] Along these lines, catalase and catalase-like nanozymes have been successfully delivered into tumors. In one study, a plasmid encoding catalase was co-loaded with Ce6 into a pH-responsive nanoplatform that was able to increase ROS levels in vivo, resulting in enhanced antitumor activity.[101] To mimic catalase, various metal-based materials, including nanostructures based on palladium,[102] cobalt,[103] iridium,[104] cerium,[105] platinum,[106] gold,[107] iron[108], and manganese[109], have been reported for hypoxia alleviation. In one example, Au2Pt nanozymes possessing both catalase and peroxidase activities were synthesized and conjugated with Ce6, and the resulting platform was able to achieve both PTT and PDT when irradiated using various wavelengths of light.[110]
The direct delivery of oxygen into tumor sites is another strategy to enhance phototherapeutic efficacy, and different nanocarriers have been designed using materials capable of oxygen transportation such as hemoglobin, perfluorocarbons, and metal–organic frameworks (MOFs). As the most abundant oxygen-binding protein, hemoglobin plays a vital role in oxygen delivery among living organisms and has been used in the development of blood substitutes.[111] However, autooxidation of cell-free hemoglobin in vivo can decrease its ability to transport oxygen transportation and may cause tissue toxicity.[112] To protect it from oxidation, hemoglobin was incorporated along with the photosensitizer methylene blue into nanoparticles consisting of polydopamine, which has antioxidant properties.[113] The particles were further camouflaged with red blood cell (RBC) membrane to endow them with long circulation and low immunogenicity. After intravenous injection, the nanoparticles were able to upregulate the expression of hypoxia-inducible factor-1α within murine 4T1 tumors and inhibited their growth under irradiation.
Emulsions based on perfluorocarbons are much more effective at solubilizing certain gases compared with aqueous media, making them another potential oxygen carrier.[114] The prolonged lifetime of singlet oxygen within perfluorocarbons further underscores their utility for PDT applications.[115] To deliver perfluorocarbons, various lipid-stabilized,[116] macromolecule-stabilized,[117] and micellar[118] platforms have been explored. For example, an oxygen self-enriching approach was created by preparing IR780-loaded perfluorohexane nanoemulsions stabilized with a lipid monolayer composed of lecithin, cholesterol, and a lipid conjugated with polyethylene glycol (PEG) (Figure 4B).[119] Both in vitro and in vivo studies confirmed that the formulation could enhance the production of singlet oxygen. Compared with free IR780, improved antitumor efficacy was achieved using the nanoformulation after intratumoral or intravenous administration followed by irradiation.
MOFs, consisting of metal nodes joined with organic linkers via coordination bonds, have been widely explored in the phototherapy field since many have intrinsic photoactive properties or can be used as carriers to deliver phototherapeutic agents. By rationally selecting their surface chemistry and pore size, MOFs have been used for the selective capture and in vivo transport of oxygen.[120] In one instance, a zeolitic imidazolate framework-8 MOF doped with Cu2+ was first filled with oxygen, followed by the addition of a thin layer of the same MOF material loaded with Ce6 and then coating with Pluronic F127.[121] The nanoplatform showed high oxygen storage capacity and underwent pH-responsive release of oxygen at tumor sites to alleviate hypoxia and promote PDT. Compared with a formulation without loaded oxygen, the oxygen-loaded version exhibited significantly improved inhibition of murine 4T1 tumors under irradiation at 650 nm.
3.1.3. Improving tumor accumulation
The targeted delivery of phototherapeutic agents to tumors is desired to increase therapeutic efficacy while improving the safety of phototherapies. To achieve this, various nanoformulations have been constructed based on different strategies, including passive targeting by optimizing size and surface properties, guided targeting using various stimuli, and active targeting mediated by high-affinity ligands.[122] In terms of selecting suitable targeting ligands, there is an abundance of options, including peptides such as angiopep-2, proteins such as antibodies against human epidermal growth factor receptor 2, aptamers such as AS1411, and other molecules such as folic acid and hyaluronic acid. In one preclinical study, the PLZ4 peptide, which can bind to ανβ3 integrin, was decorated onto nanoporphyrin to enhance accumulation at the tumor site by 30 ~ 40 times.[62] This resulted in significantly improved survival in both subcutaneous and spontaneous bladder cancer models. Other examples of targeting mechanisms employed by phototherapeutic platforms include the use of albumin to promote the cellular uptake of gold nanorods,[123] IF7 peptide for annexin 1-mediated uptake,[124] arginine-glycine-aspartic acid for tumor vascular targeting via αvβ3 and αvβ5 integrins,[125] and transferrin for targeting the transferrin receptor.[126]
Even with targeting functionality, nanocarriers ideally need to penetrate deeply into tumors in order to maximize the activity of their therapeutic payloads. However, the high interstitial fluid pressure and dense extracellular matrix in the tumor microenvironment oftentimes prohibits this type of access.[127] It has been reported that positively charged nanoparticles smaller than 50 nm exhibit deeper tumor penetration, while negatively charged particles between 20 nm to 1 μm have longer blood circulation.[128] In a notable example, researchers designed a single phototherapy platform capable of taking advantage of both size-based effects by leveraging a stimuli-triggered release mechanism.[129] ICG-conjugated poly(amidoamine) dendrimers were attached to the amphiphilic PEG-b-poly(ε-caprolactone) block copolymer through an ROS-responsive thioketal linker, and the resulting construct was mixed with Ce6 and self-assembled into nanoparticles around 120 nm in size. After intravenous injection, the nanoparticles accumulated at tumor sites thanks to passive targeting, and the production of ROS under irradiation at 660 nm facilitated the release of 10-nm positively charged dendrimers capable of enhanced tumor penetration.
Modulation of the tumor microenvironment by disrupting the extracellular matrix is another potential strategy for enhancing tumor penetration. Although the hyperthermia elicited by PTT can moderately damage the extracellular matrix, additional methods have been explored to improve the efficiency of this process, including physical methods such as the use of ultrasound, enzymatic methods using hyaluronidase or other enzymes, and chemical methods like the use of cyclopamine.[130] In one instance, the protease bromelain, which can degrade collagen, was conjugated onto semiconducting polymer nanoparticles with photothermal activity (Figure 5A).[131] Under irradiation at 808 nm, enhanced tumor penetration and higher tumor temperatures were observed in mice treated with the bromelain-loaded formulation.
Figure 5.
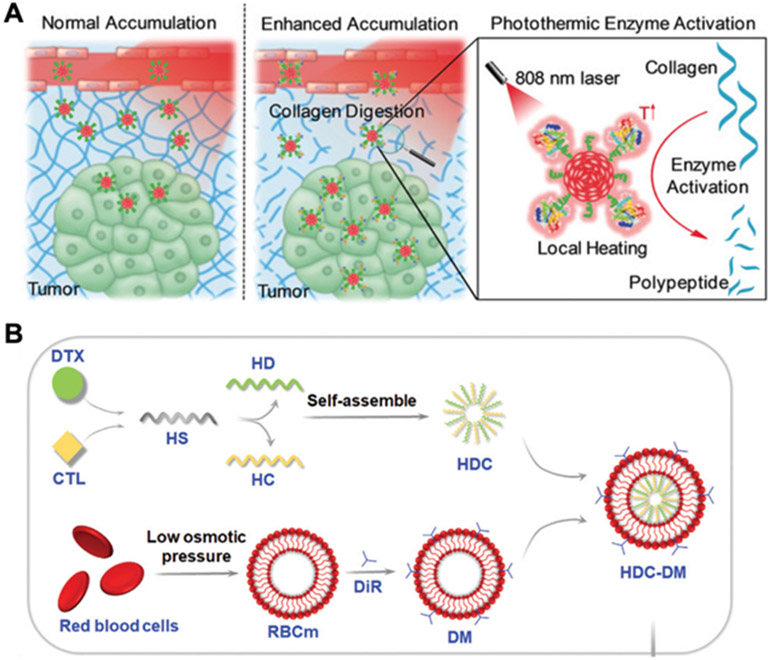
Improving tumor accumulation. (A) Semiconducting polymer nanoparticles are conjugated with the protease bromelain, which can be activated under laser irradiation to degrade collagen and enhance tumor penetration. Adapted with permission.[131] Copyright 2018, Wiley-VCH. (B) Laser-triggered drug release is realized by incorporating the lipophilic photosensitizer 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) into RBC membrane, which is coated around self-assembled nanoparticles comprised of heparan sulfate (HS) conjugated to either docetaxel (DTX, HD) or calcitriol (CTL, HC). Adapted with permission.[144] Copyright 2022, Wiley-VCH.
Cell membrane coating nanotechnology has been developed as a straightforward approach to functionalizing nanoparticles.[132-134] The resulting nanoformulations display cell-mimicking abilities that can be useful for many types of applications, including drug delivery,[135] detoxification,[136] vaccination,[133] and phototherapy.[137] To enhance nanoparticle-based phototherapy, various cell membrane coatings have been explored, such as those derived from RBCs, platelets, white blood cells, and cancer cells. RBC membrane coatings, which have been demonstrated to prolong blood circulation and improve immune evasion,[138] have been utilized to enhance tumor accumulation for both PDT and PTT nanoplatforms by passive targeting.[139] In one study, RBC membrane was used to camouflage magnetic mesoporous silica nanoparticles preloaded with the photosensitizer hypocrellin B.[140] With the help of a magnetic field, the intravenously injected formulation showed improved tumor accumulation, and significant elimination of 4T1 tumors was observed upon laser irradiation. To further improve the delivery of photosensitizers into tumors, RBC membrane can be modified with targeting ligands through lipid insertion.[141] For example, high expression of low-density lipoprotein receptor has been identified in triple-negative breast cancers, and antibodies targeting the protein have been inserted into RBC membrane using a lipid anchor with a PEG linker.[142] The engineered RBC membrane was then coated onto poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with ICG and the anti-hypoxic molecule salidroside. Compared with unmodified RBC membrane-coated nanoparticles, the modified formulation exhibited better tumor-specific delivery and tumor growth inhibition in both 4T1 and MDA-MB-231 tumor models. Despite the advantages of an RBC membrane coating, it is possible for it to hinder cellular uptake and drug release.[143] To solve this problem, RBC membrane was incorporated with a lipid photosensitizer 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide and then coated onto heparan sulfate cores conjugated with the cancer drug docetaxel and the anti-metastasis molecule calcitriol (Figure 5B).[144] After irradiation at 808 nm, the modified RBC membrane was ruptured due to the local hyperthermia induced by the photothermal agent, and the encapsulated drugs were released for better tumor penetration.
For tumor targeting, platelet membrane coatings have been utilized based on the natural interactions of their source cells with disrupted vasculature and cancer cells.[145] Similar to RBC membrane, platelet membrane also bestows nanoparticles with improved immune evasion and prolonged circulation times.[146] In one study, platelet membrane was derived and coated onto magnetic Fe3O4 nanoparticles to treat MCF-7 tumors.[147] Compared with uncoated nanoparticles, those with the membrane coating exhibited significantly prolonged blood circulation and increased accumulation at the tumors. Similarly, the homing ability of stem cells to cancers has been utilized to construct stem cell membrane-coated nanoparticles for phototherapeutic applications.[148] For example, the membrane from bone marrow-derived mesenchymal stem cells was coated onto mesoporous silica-coated upconversion nanoparticles loaded with two photosensitizers, MC540 and ZnPC.[149] The stem cell membrane coating enhanced cellular uptake by HeLa cells and facilitated tumor-specific delivery, thus leading to improved PDT efficacy. In another instance, human umbilical cord-derived mesenchymal stem cells were collected for membrane coating onto Fe3O4 nanoparticles modified with polydopamine and adsorbed with siRNA against Plk1 to elicit apoptosis.[150] Likewise, improved tumor accumulation and antitumor efficacy were observed when treating with this membrane-coated nanoformulation.
The membrane material derived from white blood cells, including macrophages, neutrophils, and T cells, have widely been utilized for improved tumor targeting due to their natural tropisms. Macrophage membrane-coated gold nanoshells showed improved binding to 4T1 cancer cells in vitro, and in vivo they demonstrated prolonged blood circulation as well as increased tumor accumulation.[151] Compared with uncoated gold nanoshells, the membrane-coated formulation showed better PTT efficacy upon irradiation at 808 nm. Owing to the affinity of neutrophils to inflammatory tissues and circulating tumor cells, neutrophil membrane-coated nanoparticles have been shown to enhance the targeting of metastatic sites.[152] Inspired by the natural infiltration of T cells into tumors, T cell membrane camouflaging has also been explored to improve tumor targeting.[153] Modified T cell membranes have been constructed to further enhance tumor accumulation. For example, a CAR T cell recognizing glypican-3 expressed in most hepatocellular carcinomas, but not in healthy cells, was generated by viral transduction, and the cell membrane was extracted for coating onto IR780-loaded mesoporous silica nanoparticles.[154] In comparison with uncoated nanoparticles, the coated formulation showed increased tumor targeting and improved PTT efficacy. In another example, primary human T cells were first metabolically engineered to express azide groups, and their membrane was coated onto ICG-loaded PLGA nanocores.[155] For in vivo application, bicyclo[6.1.0]nonyne was first injected intratumorally to label Raji tumors, and mice were then intravenously administered with the modified T cell membrane-coated nanoparticles. Due to the specific interaction between the nanoparticle’s azide groups and the bicyclo[6.1.0]nonyne on the cancer cells, strong accumulation and irradiation-induced temperature increases were achieved, resulting in complete tumor clearance.
Cancer cell membrane has been extensively used to functionalize nanoparticles for antitumor applications based on several different properties, including their homotypic binding, tumor-associated antigen presentation, and immune evasion.[132] For improved PTT, MCF-7 cancer cell membrane was first modified with PEG to shield from serum proteins and then coated onto ICG-loaded PLGA nanoparticles.[156] The resulting membrane-coated nanoformulation showed improved tumor accumulation, which helped to increase hyperthermia upon irradiation to achieve complete inhibition of tumor growth. In another example, MDA-MB-231 membrane was coated onto an ICG-loaded gold-rhodium core-shell nanostructure for enhanced PDT.[157] Similarly, the cancer membrane coating significantly improved the accumulation of ICG at the tumor site, resulting in strong antitumor efficacy. Given the fact that cancer-associated fibroblasts are the major stromal cells in the tumor environment, nanoparticles coated with their membrane have also been prepared for selective delivery to tumors by homotypic binding.[158] In one example, fibroblasts were first activated after incubation with transforming growth factor beta 1 (TGF-β1), and their membrane was then derived and cloaked onto semiconducting polymer nanoparticles with high near-infrared (NIR) absorbance.[159] Compared with uncoated and normal fibroblast membrane-coated nanoparticles, the formulation derived from activated fibroblasts exhibited significantly increased tumor accumulation and improved inhibition of 4T1 tumors.
3.1.4. Controlling subcellular localization
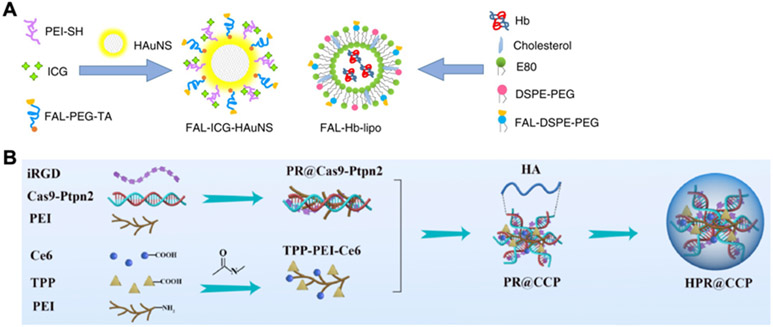
The process of ICD after phototherapy is governed by stress on the ER, which promotes the release of DAMPs such as CRT, HMGB1, and HSPs.[160] To achieve ER targeting, multiple strategies have been explored, including the use of small molecules[63, 161] and peptides.[162, 163] For example, sulfonamide derivates that can bind to the sulfonylurea receptors overexpressed on the membrane of the ER have been utilized.[63] In this case, N-tosylethylenediamine was conjugated with 4,4’,4’’,4’’’-(porphyrin-5,10,15,20-tetrayl)tetrakis(N-(2-((4-methylphenyl)sulfonamido)ethyl)benzamide to form an ER-targeting photosensitizer, which was then loaded into redox-sensitive nanoparticles. When the nanoformulation was applied against a 4T1 tumor model, the treatment successfully inhibited the growth of both primary and distant tumors, indicating the induction of systemic immune responses. In another example, an ER-targeting pardaxin peptide was linked onto ICG-conjugated hollow gold nanospheres and hemoglobin-loaded liposomes (Figure 6A).[162] Compared with those without the peptide, the modified formulations were able to significantly improve survival in both CT26 and B16 models when irradiation at 808 nm was applied.
Figure 6.
Controlling subcellular localization. (A) ER-targeted delivery of phototherapy agents or oxygen-delivering hemoglobin is achieved using the pardaxin peptide (FAL). Adapted with permission.[162] Copyright 2019, Nature Publishing Group. (B) Mitochondria-targeted delivery of the photosensitizer Ce6 and a CRISPR-Cas9 plasmid against Ptpn2 is achieved using the targeting ligand triphenylphosphonium (TPP). Adapted with permission.[168] Copyright 2020, Elsevier Ltd.
As the energy factory of the cell and an important regulator of redox and calcium signaling, the mitochondria have been widely explored as a target for cancer therapy.[164] Due to their high sensitivity to hyperthermia and ROS, mitochondria-targeting has the potential to increase the efficacy of phototherapies.[165, 166] When compared with targeting to other intracellular organelles such as the ER and lysosomes, the mitochondria-specific localization of a photosensitizer was shown to elicit the highest degree of apoptosis and ROS levels in tumor cells.[167] To target mitochondria, cationic and lipophilic constructs, including delocalized lipophilic cations, peptides, and liposomes, have been utilized.[164] For example, triphenylphosphonium and the photosensitizer Ce6 were conjugated onto polyethylenimine for mitochondria-targeted delivery (Figure 6B).[168] Compared with free Ce6, the targeted formulation enhanced the generation of singlet oxygen and induced apoptosis of B16F10 cells. Positively charged and lipophilic nanoscale MOFs can sometimes have an intrinsic affinity to mitochondria. In one study, a hafnium-iridium MOF was constructed as a mitochondria-targeting carrier and incorporated with a zinc-phthalocyanine photosensitizer.[166] In comparison to free photosensitizer, the nanoformulation induced higher levels of singlet oxygen, leading to higher expression of CRT and better inhibition of MC38 and CT26 tumor growth.
The nucleus has long been regarded as an important target for anticancer drugs that regulate proliferation, metabolism, and the cell cycle.[169] The ROS and heat generated by phototherapeutic modalities can efficiently damage nuclear components, providing a promising means of achieving anticancer efficacy.[170] However, multiple barriers hinder localization to the nucleus, including endolysosomal degradation and selective entry through nuclear pore complexes.[171, 172] To enhance nuclear accumulation, nanoformulations can be passively targeted by enhancing their hydrophilicity and reducing their size, or they can be actively delivered with the help of certain targeting ligands such as TAT peptides and the AS1411 aptamer.[173] In addition to the direct destruction of nuclear components, nucleus-targeting nanocarriers can also be used to modulate gene function to augment phototherapies. In one study, gold nanorods with NIR-II photothermal activity were coated with a nuclear localization signal peptide attached to an amphiphilic copolymer that was then adsorbed with siRNA targeting YTHDF1.[174] Delivery of the siRNA significantly reduced HSP70 expression, which increased the sensitivity of the tumor cells to hyperthermia.
3.2. Combination of phototherapy with immune-enhancing treatments
3.2.1. Modulation of immune cell populations
The infiltration of immunosuppressive cells, including Tregs, M2-polarized tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), can contribute to the immunosuppressive environment of tumors and decrease the effectiveness of cancer treatments.[175] Tregs inhibit antitumor immune responses by secreting inhibitory cytokines such as IL10 and TGF-β to impede the functions of DCs and CD8+ T cells, and they can also directly regulate effector T cells and natural killer (NK) cells using granzymes and perforin.[176] As one of the most abundant immune cells in tumors, TAMs are derived from recruited peripheral monocytes and tissue-resident macrophages.[177] They are divided into two groups: M1 type TAMs can mediate the elimination of cancer cells and M2 type TAMs can aid in tumor immunosuppression. By secreting anti-inflammatory cytokines like IL10, expressing co-inhibitory markers like PDL1, and releasing proteins such as matrix metalloproteinases (MMPs) and vascular endothelial growth factor, M2 TAMs have been reported to play important roles in tumor progression and metastasis.[178] MDSCs are generated from myeloid progenitor cells in the bone marrow that subsequently accumulate in tumors.[179] They can potently mediate immune suppression by interacting with other immunosuppressive cells and inhibiting effector T cells through ROS, nitric oxide, and adenosine production.[180]
Although phototherapy can eliminate local tumors and elicit ICD, its ability to promote strong antitumor immunity can be restricted by the presence of immunosuppressive cells.[181] As a result, synergistic therapies combining phototherapy and the depletion of immunosuppressive cells have been explored. Since Tregs are essential in maintaining immune tolerance to self-antigens, their depletion should only occur within tumors to avoid systemic toxicity and autoimmunity.[182] Along these lines, CD25 is a potential target for the selective depletion of effector Tregs. Glucocorticoid-induced cancer necrosis factor receptor is a co-stimulatory marker expressed on the surface of Tregs that can be used to block their immunosuppressive functions.[183] Based on this, a high affinity agonistic antibody was loaded onto photothermally active polydopamine nanoparticles containing ICG as a photosensitizer and catalase to alleviate hypoxia (Figure 7A).[184] After treatment using the nanoformulation, the tumor Treg population was significantly decreased and more infiltration of cytotoxic T cells was observed, which resulted in potent antitumor efficacy in a 4T1 bilateral model. In another study, an antibody against the same marker was covalently conjugated onto PLGA nanoparticles loaded with IR780 and imatinib, which can help to downregulate the Treg phenotype.[185] After intravenous administration, the nanoformulation exhibited prolonged circulation and enhanced accumulation at the tumor site. Upon laser irradiation, signs of antitumor immunity were observed, with increases in mature DC and cytotoxic T cell infiltration, along with a reduction in Tregs.
Figure 7.
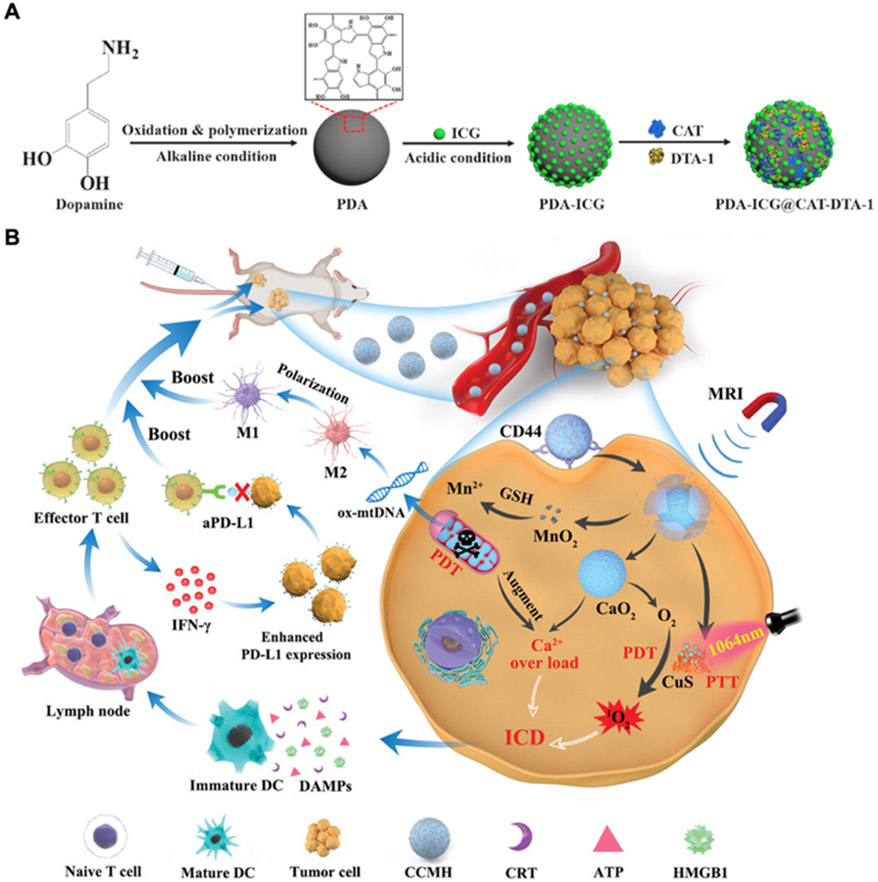
Modulation of immune cell populations for PIT. (A) Polydopamine (PDA) nanoparticles functionalized with ICG are further modified with catalase (CAT) to relieve tumor hypoxia and the agonistic antibody DTA-1 against glucocorticoid-induced tumor necrosis factor receptor family–related protein to target Tregs. Adapted with permission.[184] Copyright 2021, Elsevier Ltd. (B) Hyaluronic acid-camouflaged CaO2 nanoparticles functionalized with ultrasmall CuS-MnO2 nanoparticles can facilitate the repolarization of M2 macrophages to M1 macrophages by promoting the release of oxidatively damaged mitochondrial DNA (ox-mtDNA). Adapted with permission.[187] Copyright 2022, Wiley-VCH.
The repolarization of TAMs from an M2 to M1 phenotype is another viable strategy for promoting antitumor immunity. It has been reported that damage of mitochondrial DNA caused by ROS during PDT can mediate this repolarization process.[186] Inspired by this finding, a nanoplatform for enhanced PDT was constructed by loading ultrasmall CuS-MnO2 nanoparticles onto CaO2 nanoparticles, followed by coating with hyaluronic acid for tumor cell targeting (Figure 7B).[187] After cellular uptake, the CuS component mediated NIR-triggered PTT and PDT, which was aided by the increased oxygen generated from CaO2 and reduced glutathione facilitated by MnO2. In vitro results with 4T1 cells confirmed mitochondrial injury and increased expression of CRT, HMGB1, ATP, and HSP70, which promoted the M2 to M1 transformation of macrophages. After intravenous administration, the nanoplatform elicited DC maturation, cytotoxic T cell and NK cell infiltration, and Treg downregulation, enabling it to inhibit the growth of both primary and distant 4T1 tumors. The delivery of specific agents capable of macrophage repolarization has also been tested in combination with phototherapy. BLZ-945, which can block the pathway between macrophage-colony stimulating factor and its receptor, can downregulate M2 markers on TAMs and increase the infiltration of CD8+ T cells.[188] In one study, BLZ-945 and ICG were loaded into ruthenium nanoparticles modified with MMP-responsive triglyceride monostearates, and the resulting formulation was able to mediate M2 to M1 repolarization while showing potent antitumor efficacy in a CT26 mouse model.[189] Inspired by reports that NK cells can promote the M1 polarization of TAMs, NK cell membrane was isolated and coated onto a polymeric core loaded with a photosensitizer.[190] Compared with uncoated nanoparticles, those with the NK membrane coating exhibited increased tumor accumulation and enhanced M1 polarization capabilities. With irradiation under a 660 nm laser, the formulation was able to eliminate primary tumors and had potent effects against distant tumors in a bilateral 4T1 model.
Multiple strategies targeting MDSCs have been developed to alleviate tumor immunosuppression, including specific depletion, inhibition of immunosuppressive functions, blockade of migration and recruitment into tumors, promotion of differentiation, and hindering metabolism.[175] Hypoxia has been reported as an important factor to prevent the differentiation of MDSCs and maintain their accumulation within tumor sites.[191] Along these lines, an oxygen-producing nanoformulation was constructed by loading IR780 and metformin into mesoporous silica nanoparticles and sealing the pores with CeO2 nanoparticles.[192] After uptake into cancer cells, the CeO2 nanoparticles were able to react with endogenous H2O2 to produce oxygen, and the released metformin inhibited mitochondrial respiration for reduced oxygen consumption. This helped to decrease the MDSC population after treatment, contributing to the infiltration of CD8+ T cells and the inhibition of tumor growth in both subcutaneous and metastasis models of B16F10 melanoma. Inhibition of phosphodiesterase 5 can lead to the reduced recruitment of MDSCs and TAMs in tumors through downregulation of nitric oxide synthase 2 and arginase 1.[193] In one study, the inhibitor tadalafil and the photosensitizer ICG were mixed to prepare nanoparticles based on coordination bonds with ferric ions.[194] Ultimately, the nanoformulation inhibited the infiltration of MDSCs and M2 TAMs while increasing mature DC and cytotoxic T cell populations.
3.2.2. Immune checkpoint blockade
As a vital part of immune homeostasis, immune checkpoints can help to moderate T cell activation and prevent the occurrence of autoimmunity.[195] However, cancer cells are able to take advantage of these mechanisms to acquire resistance against immune detection and elimination.[196] To address this challenge, various antibody and small molecule therapeutics capable of blocking immune checkpoints have been developed.[197] An antibody targeting CTLA4 was the first immune checkpoint blockade therapy approved by the United States Food and Drug Administration (FDA) against advanced melanoma. CTLA4 is a transmembrane glycoprotein expressed on T cells that competes with the co-stimulatory receptor CD28 to recognize the ligands CD80 and CD86.[198] Blockade of CTLA4 enhances immune responses broadly through the improvement of helper T cell activity and the inhibition of Tregs.[199] PD1 is expressed on T cells, as well as activated B cells and NK cells,[200] while its ligand PDL1 can be highly expressed on tumor cells for immune evasion.[201] Interaction between the two markers inhibits T cell activation and can promote the apoptosis of infiltrated T cells.[202] To block this immunosuppressive pathway, various antibodies targeting PD1 and PDL1 have been developed as treatments against different types of tumors. Overexpressed in tumor cells and DCs, indoleamine 2,3-dioxygenase 1 (IDO1) is a cytosolic enzyme that helps to construct an immunosuppressive tumor microenvironment by increasing the kynurenine to tryptophan ratio, which suppresses the antitumor functions of NK cells and promotes the infiltration of Tregs.[203, 204] As high IDO1 activity is correlated with poor prognoses in various types of cancers,[205, 206] various drugs are being explored to target this pathway.[207] Another mechanism that is actively being investigated involves CD47, which is expressed by cancer cells to inhibit the functions of myeloid cells through binding to signal-regulatory protein α.[208] The blockade of CD47 can lead to increased elimination of tumor cells,[209] and various therapies targeting this pathway are being tested in clinical trials.[210]
As the ICD induced by phototherapy often fails to elicit potent systemic antitumor immunity, the combination of phototherapy with immune checkpoint blockades has been explored as a solution. It should be noted that the increased expression of checkpoint markers such as IDO1 and PDL1 during phototherapy can further attenuate antitumor efficacy.[211] A simple method to achieve this type of PIT is to administer immune checkpoint treatments as a supplement to phototherapies. For example, a plasma membrane-targeting peptide was conjugated with the photosensitizer protoporphyrin IX via a hydrophilic PEG chain and a hydrophobic alkyl chain, and the resulting construct was then self-assembled into nanoparticles.[212] After cellular uptake, the nanoparticles migrated to the plasma membrane with the help of farnesyltransferase and promoted enhanced release of intracellular DAMPs such as ATP and HMGB1 upon irradiation at 660 nm. In vivo, treatment with the nanoparticles in combination with anti-PD1 increased the infiltration of effector T cells and lead to the eradication of primary tumors as well as the inhibition of distant tumors. To enhance the antitumor activity of anti-PDL1, a nanoscale MOF was constructed by combining Fe3O clusters and 5,10,15,20-tetra(p-benzoato)porphyrin as a photosensitizer for oxygen-replenishing PDT.[213] The platform enabled the degradation of endogenous H2O2 to produce oxygen through an Fe3O-mediated Fenton reaction, which led to increased singlet oxygen production under light irradiation. When treating with a combination of the MOFs administered intratumorally and intraperitoneal anti-PDL1, potent antitumor responses against both primary and distant tumors were observed in a CT26 model.
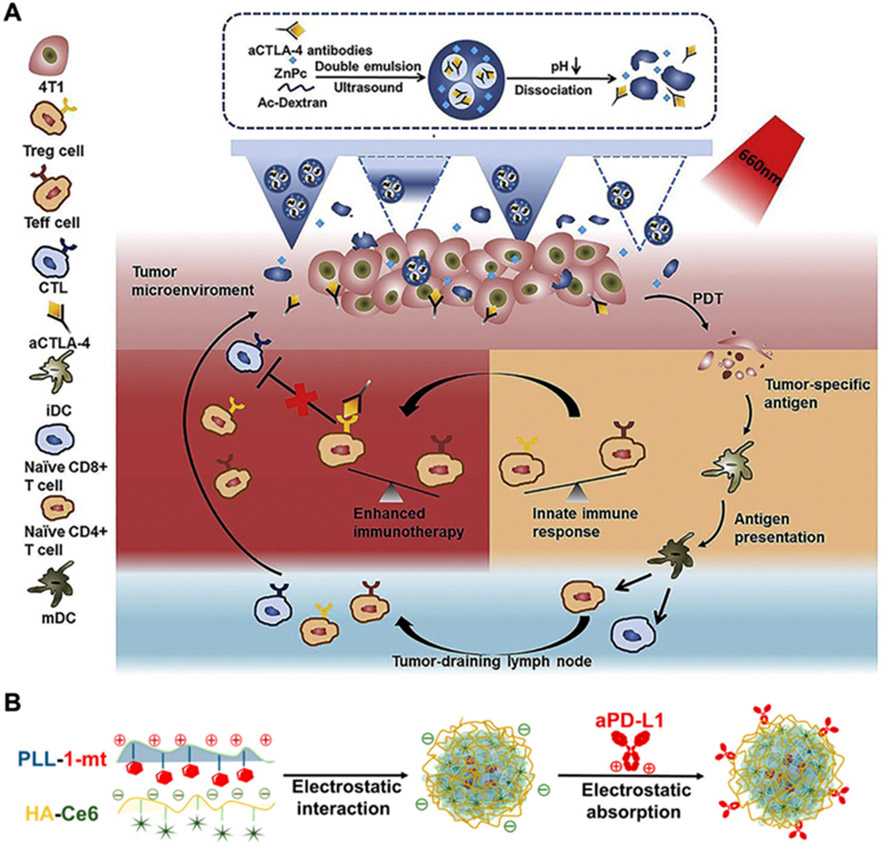
Compared with the systemic administration of immune checkpoint blockades, local delivery via intratumoral administration or intradermal injection proximal to the tumor site have been reported to elicit stronger antitumor immunity.[214] Along these lines, PEG-modified black phosphorus nanoparticles have been injected intratumorally along with anti-CD47 to elicit synergistic antitumor effects.[215] In comparison with either nanoparticle-mediated PTT or anti-CD47 alone, the combination therapy promoted the repolarization of TAMs to an M1 phenotype, increased the infiltration of CD4+ and CD8+ T cells, and elevated the serum level of proinflammatory cytokines, thus leading to potent efficacy in a bilateral A20 murine tumor model. The co-delivery of immune checkpoint blockades and phototherapeutic agents is another potential strategy to improve the accumulation of both payloads at tumor sites for PIT. In one study, anti-CTLA4 and the photosensitizer zinc phthalocyanine were co-encapsulated into dextran nanoparticles using a double emulsion method, and the resulting formulation was further loaded into polydimethylsiloxane microneedles (Figure 8A).[216] After application onto tumors, the combination therapy significantly increased the infiltration of cytotoxic T cells and helper T cells, along with promoting the release of the proinflammatory tumor necrosis factor α (TNFα).
Figure 8.
Immune checkpoint blockade for PIT. (A) Microneedle delivery of photosensitizers and anti-CTLA4 loaded within double emulsion nanoparticles enables synergistic antitumor therapy. Adapted with permission.[216] Copyright 2020, Elsevier Ltd. (B) A 3-in-1 platform for delivering the photosensitizer Ce6 and two immune checkpoint inhibitors [dextro-1-methyl tryptophan (1-mt) and anti-PDL1] is constructed via electrostatic interactions. Adapted with permission.[221] Copyright 2019, American Chemical Society.
In addition to antibodies, checkpoint blockade can also be achieved using peptides, siRNA, and small molecules, all of which can be co-delivered with phototherapeutic agents for PIT. For example, the PDL1 antagonist peptide DPPA-1 was conjugated with a tumor-homing peptide LyP-1 via an MMP2-degradable peptide linker.[217] After adsorption onto Au@Pt nanoparticles with photothermal properties, the resulting nanoformulation was administered intravenously and accumulated at the tumor site where the elevated levels of MMP2 degraded the linker. The released DPPA-1 blocked PDL1 on the tumor cell surface while the exposed LyP-1 peptide then targeted the nanoparticles for cellular uptake for enhanced PTT. Compared with a formulation without DPPA-1, the final nanoformulation elicited more potent infiltration of cytotoxic T cells in both primary and distant tumors, better downregulation of Tregs, and increased levels of proinflammatory cytokines in the serum. In another study, MnO2 nanoparticles loaded with ICG and coated with CaCO3 were constructed, followed by the adsorption of anti-PDL1 siRNA through electrostatic interactions.[218] The nanoplatform had a size of around 125 nm with a zeta potential about −10 mV, and it was able to silence the expression of PDL1 in Lewis lung carcinoma cells in a dose-dependent manner. In vivo, the nanoparticles enhanced mature DC and T cell infiltration, leading to potent antitumor efficacy in a subcutaneous tumor model of Lewis lung carcinoma.
Multiple small molecules have been developed as IDO1 inhibitors, which can be loaded into nanoparticulate delivery systems for combination treatment with phototherapy. For instance, the inhibitor NLG919 was conjugated onto semiconducting polymer nanoparticles through a singlet oxygen-responsive linker.[219] Cellular experiments with 4T1 cells confirmed the production of singlet oxygen and inhibition of IDO1-mediated kynurenine metabolism. After intravenous injection, the nanoparticles accumulated at the tumor site, enabling local hyperthermia and ROS generation after NIR irradiation. In comparison to control nanoparticles without the inhibitor, the NLG919-conjugated nanoparticles decreased the kynurenine to tryptophan ratio in primary tumors and increased the infiltration of cytotoxic T cells into distant tumors, leading to overall control of tumor growth and a reduction in lung metastasis. Besides chemical conjugation, the encapsulation of IDO1 inhibitors into nanoparticles has also been explored. In one study, a porous nanoscale MOF composed of a chlorin derivative was constructed, and an analogue of epacadostat was loaded into the MOF as an IDO1 inhibitor.[220] Synergistic antitumor efficacy was achieved after nanoparticle administration followed by laser irradiation, with potent abscopal effects against distant tumors observed in both CT26 and MC38 models.
The rational combination of different immune checkpoint blockades has the potential to greatly improve antitumor immunity without increasing toxicity. For example, a three-in-one platform was constructed by the assembly of the IDO1 inhibitor dextro-1-methyl tryptophan conjugated to polylysine, Ce6-loaded hyaluronic acid, and anti-PDL1 through electrostatic interactions (Figure 8B).[221] Upon in vivo application, the hyaluronic acid was degraded by hyaluronidase expressed in the tumor microenvironment to release anti-PDL1 and enhance tumor elimination by effector cytotoxic T cells. After cellular uptake, the IDO1 inhibitor was released to further alleviate tumor immunosuppression, and the photosensitizer Ce6 enabled PDT that induced ICD. By promoting multiple phases of the antitumor immune response, including antigen presentation and lymphocyte activation, the platform was able to eradicate both primary and distant tumors as well as inhibit pulmonary metastasis.
3.2.3. Enhancement of immune cell function
Immunological adjuvants are bioactive molecules capable of eliciting potent innate and adaptive immune responses.[222] When combined with tumor antigens as a vaccine, adjuvants can help to induce potent tumor-specific immunity.[223] Adjuvants come in a multitude of forms and work by a variety of different mechanisms.[224] As a type of pattern recognition receptors, Toll-like receptors (TLRs) are expressed abundantly by some immune cells to recognize pathogen-associated molecular patterns (PAMPs), which subsequently leads to the induction of proinflammatory responses.[225] There are nine TLRs that have been extensively studied, with some located on the plasma membrane and others expressed in the endosomal compartment.[226] Examples of common TLR agonists include polyinosinic-polycytidylic acid for TLR3, monophosphoryl lipid A derived from lipopolysaccharide for TLR4, flagellin for TLR5, imidazoquinolines for TLR7 and/or TLR8, and CpG oligonucleotides for TLR9.[224] In contrast to TLR agonists, alum is a classical inorganic adjuvant first reported in 1926 and has different forms, including aluminum phosphate and aluminum hydroxide.[133] Alum activates caspase-1 and induces IL1β release through the inflammasome pathway for T helper 2-biased immune responses.[227] Chitosan is a biopolymer derived from chitin of insects and crustaceans, and it has been utilized as both a carrier and adjuvant to elicit potent humoral and cellular immune responses,[228] although its mechanism of action is still not entirely clear.[229] Cell-derived components, including intracellular proteins such as HSPs, various cell-derived exosomes, and bacterial cell membranes have also been explored as adjuvants to elicit antitumor immunity.[230-232]
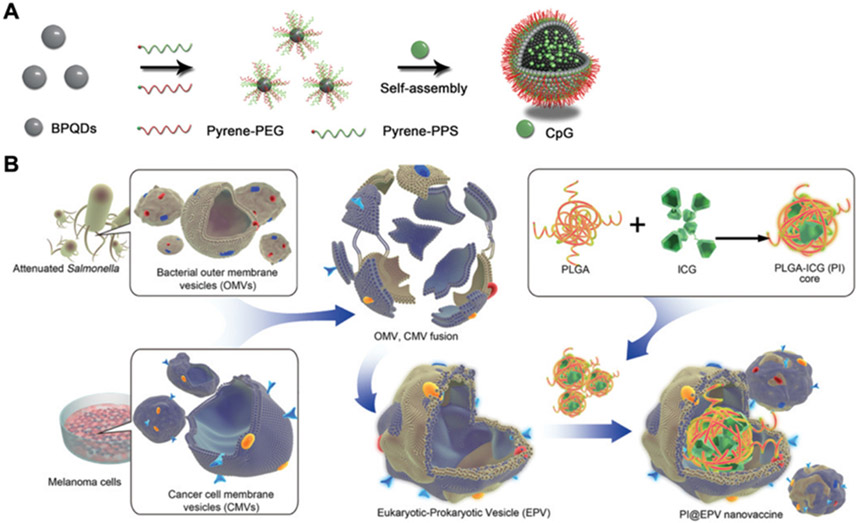
CpG oligonucleotides can be divided into four types based on their motifs and structures,[233] and they have been widely explored for augmenting immune responses to cancer.[234] In one study, black phosphorus quantum dots were functionalized with PEG and hydrophobic ROS-sensitive poly(propylene sulfide) for self-assembly with CpG into a larger nanovesicle structure (Figure 9A).[235] Under NIR irradiation, the hydrophobic polymer was degraded, leading to the disassembly of the nanostructure into its constituent black phosphorus quantum dot building blocks for CpG release. Compared with single treatments using either CpG only or black phosphorus nanovesicles alone, the combination therapy elicited the highest level of proinflammatory cytokines, including IL6, IL12 and TNFα, in the serum, along with the strongest efficacy against primary tumors, distant tumors, and metastases in a 4T1 mouse model. In addition to loading CpG into nanoparticles, DNA hydrogels consisting of CpG have been proposed as another platform for facilitating nanoparticle-based PIT. For example, a hexapod-like DNA structure containing CpG sequences was mixed with oligodeoxynucleotide-modified gold nanorods and self-assembled into a nanocomposite hydrogel.[236] The resulting hydrogel formulation demonstrated both photothermal properties and immunostimulatory activity. After intratumoral injection, antigen-specific antibodies and T cell responses were generated to inhibit tumor growth in a murine model.
Figure 9.
Enhancement of immune cell functions for PIT. (A) An NIR/ROS-responsive platform is constructed by functionalizing black phosphorus quantum dots (BPQDs) with PEG and poly(propylene sulfide) (PPS), and CpG is encapsulated inside to promote immune simulation. Adapted with permission.[235] Copyright 2019, Wiley-VCH. (B) A tumor vaccine is developed using an OMV and cancer cell hybrid membrane coated around ICG-loaded PLGA nanoparticles. The OMV membrane serves as the immune adjuvant, while the cancer cell membrane provides the tumor antigen material. Adapted with permission.[250] Copyright 2020, Wiley-VCH.
Small molecule adjuvants like imidazoquinolines can be either directly loaded into nanoformulations or conjugated onto polymers for incorporation by self-assembly. For example, magnetic Fe3O4 nanoparticles were first loaded with ICG through electrostatic interactions and then coated with PEG conjugated with polyphenols that were preloaded with R837.[237] With the direction of a magnetic field, the dual-payload nanoparticles accumulated more at tumor sites and were degraded to release the adjuvant upon laser irradiation. As a result, the combination therapy significantly enhanced the maturation of DCs and the proliferation of T cells in lymph nodes, inhibiting the growth of 4T1 tumors in both subcutaneous and metastasis models. In another study, an imidazoquinoline was conjugated onto a polymeric backbone to create a new TLR7/8 agonistic polymer, which was then grafted onto gold nanorods.[238] An R9 peptide protected by PEG via an MMP2-sensitive peptide was also conjugated onto the nanoparticles. In the tumor microenvironment, the R9 peptide was exposed, enabling it to nonspecifically capture tumor antigens. Using this formulation for tumor treatment, increased apoptosis and enhanced infiltration of cytotoxic T cells and NK T cells were observed.
Cell membrane coating technology has been explored as an emerging strategy to elicit potent immune responses through the presentation of tumor antigens, activation of APCs with PAMPs, and direct activation of T cells with co-stimulatory factors.[239-242] Taking advantage of the ability of DCs to present antigens and prime T cells, the membrane from mature bone marrow DCs was coated onto nanoparticles loaded with the photothermal agent IR-797.[243] The nanoplatform retained the expression of major histocompatibility complex I and II, as well as the co-stimulatory factors CD80 and CD86, enabling it to specifically activate T cells ex vivo. Additionally, treatment with the nanoparticles decreased the expression of HSPs in 4T1 cells, making them more sensitive to mild hyperthermia. Upon irradiation at 808 nm, potent antitumor immunity and significant inhibition of both primary and secondary tumors were achieved in a 4T1 model. Besides using DC membrane, it is possible to construct artificial APCs directly from cancer cells by engineering them to express co-stimulatory markers.[242] Along these lines, a formulation for PIT was developed by expressing the co-stimulatory marker CD86 and the immune checkpoint blockade anti-lymphocyte activating 3 on 4T1 membrane, which was then used to camouflage a photosensitizer-containing nanoparticle.[244] Compared with uncoated nanoparticles, the final nanoformulation elicited a potent abscopal effect and promoted long-term immune memory when irradiated at 660 nm.
Bacterial membranes, particularly outer membrane vesicles (OMVs) derived from Gram-negative bacteria, can contain multiple PAMPs and thus have been explored as natural adjuvants.[245] In one study, OMVs from Escherichia coli engineered with human tumor necrosis factor related apoptosis-inducing ligand were collected and conjugated with ICG and an RGD peptide targeting αvβ3 integrin.[246] After topical administration, the OMVs could penetrate through the stratum corneum thanks to their nanoscale size and lipid composition, thus accumulating at the tumor site. After irradiation at 808 nm, the hyperthermia induced by the formulation synergized with the apoptosis elicited by the engineered ligand for enhanced antitumor efficacy. Another study reported the utilization of OMV-coated nanoparticles capable of hitchhiking on neutrophils due to their PAMP recognition ability.[247] Upon adoptive transfer, the loaded neutrophils were able to navigate to tumors that had been subjected to PTT, subsequently releasing the ingested nanoparticles for synergistic antitumor therapy.
Hybrid membranes have been constructed by fusing two or more membranes from different cell types together.[248, 249] In an example for PIT, OMVs derived from attenuated Salmonella were selected for their adjuvant properties and fused with B16F10 membrane as a tumor antigen source (Figure 9B).[250] The hybrid membrane was coated onto ICG-loaded PLGA nanoparticles, and the maintenance of protein markers from both source membranes was confirmed. In vitro, the hybrid membrane-coated nanoparticles were able to enhance DC maturation and increase T cell proliferation. As a nanovaccine, the nanoparticles offered significant protection specifically against B16F10 tumor growth. When applied therapeutically against B16F10 tumors, the hybrid membrane-coated nanoparticles showed the best efficacy in combination with laser irradiation.
4. Conclusions
Phototherapy has emerged as a promising approach for cancer treatment that relies on the local generation of heat or ROS to induce tumor cell death. To enhance the antitumor effects of phototherapies, researchers have recently begun to focus on generating stronger systemic immune responses. By optimizing the potency of phototherapeutic platforms, it is possible to enhance immune-related effects such as ICD, and this can be further combined with specific immunotherapeutic modalities to create novel PIT approaches. With the rise of nanotechnology, various nanoparticle-based platforms have been designed to boost photothermal and photodynamic efficiency through approaches such as payload co-delivery, tumor or organelle targeting, and oxygen supplementation. While phototherapy and immunotherapy can be applied separately in order to achieve synergistic antitumor effects, all-in-one PIT platforms are becomingly increasingly popular, particularly as our ability to fabricate multifunctional nanoparticle platforms improves. To enhance the broad applicability of PIT, an emphasis should be placed on improving penetration depth, which would provide access to non-superficial tumors. Additionally, combination with other therapeutic modalities such as radiotherapy or chemotherapy may further boost the therapeutic effects, and the use of more biodegradable and biocompatible components will help to mitigate any safety issues. Overall, continued research on novel strategies for PIT will yield platforms with higher efficacy and better safety that may eventually help to improve the outlook of cancer patients in the clinic.
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-21-1-0010 and the National Institutes of Health under Award Number R21AI159492.
Biographies

Zhongyuan Guo is a graduate researcher in the Department of NanoEngineering at the University of California San Diego. He received his B.S. in Pharmaceutical Science at Fudan University in 2019. His research involves utilizing biomimetic nanoparticles against infectious diseases, cancers, and neurotoxins.

Audrey T. Zhu is an undergraduate researcher in the Department of NanoEngineering at the University of California San Diego. She will receive her B.S. in Biology at University of California San Diego in 2025. Her research is focused on creating biomimetic nanoparticles as vaccines against cancer and infectious diseases.

Ronnie H. Fang is an Associate Project Scientist in the Department of NanoEngineering at the University of California San Diego. He received his Ph.D. in NanoEngineering at the University of California San Diego. His research is focused on leveraging biomimetic nanoparticles for drug delivery and immunoengineering applications.

Liangfang Zhang is a Professor in the Department of NanoEngineering, Chemical Engineering Program and Moores Cancer Center at the University of California San Diego. He received his Ph.D. in Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign. His research aims to create cutting-edge biomimetic nanotechnologies and exploit them for various biomedical applications with a particular focus on biomimetic nanodelivery and biological neutralization.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Shi H, Sadler PJ, Br. J. Cancer 2020, 123, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pivetta TP, Botteon CEA, Ribeiro PA, Marcato PD, Raposo M, Nanomaterials 2021, 11, 3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doughty ACV, Hoover AR, Layton E, Murray CK, Howard EW, Chen WR, Materials 2019, 12, 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham TC, Nguyen VN, Choi Y, Lee S, Yoon J, Chem. Rev 2021, 121, 13454. [DOI] [PubMed] [Google Scholar]
- 5.Bilici K, Cetin S, Aydindogan E, Yagci Acar H, Kolemen S, Front. Chem 2021, 9, 707876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao D, Guo X, Zhang X, Chen S, Wang Y, Chen T, Huang G, Gao Y, Tian Z, Yang Z, Mater. Today Bio 2020, 5, 100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Shi J, Nie W, Wang S, Liu G, Cai K, Adv. Healthc. Mater 2021, 10, 2001207. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues MC, Morais JAV, Ganassin R, Oliveira GRT, Costa FC, Morais AAC, Silveira AP, Silva VCM, Longo JPF, Muehlmann LA, Pharmaceutics 2022, 14, 1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan X, Chan C, Lin W, Angew. Chem. Int. Ed. Engl 2019, 58, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lou J, Aragaki M, Bernards N, Chee T, Gregor A, Hiraishi Y, Ishiwata T, Leung C, Ding L, Kitazawa S, Koga T, Sata Y, Ogawa H, Chen J, Kato T, Yasufuku K, Zheng G, Biomaterials 2023, 292, 121918. [DOI] [PubMed] [Google Scholar]
- 11.Huang PY, Zhu YY, Zhong H, Chen PL, Shi QY, Chen JY, Lai JM, Tu YF, Liu SW, Liu LH, Biomater. Sci 2022, 10, 1267. [DOI] [PubMed] [Google Scholar]
- 12.Zou J, Li L, Yang Z, Chen X, Nanophotonics 2021, 10, 3229. [Google Scholar]
- 13.Mellman I, Coukos G, Dranoff G, Nature 2011, 480, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farkona S, Diamandis EP, Blasutig IM, BMC Med. 2016, 14, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonaguro L, Tagliamonte M, Vaccines 2020, 8, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Donnell JS, Teng MWL, Smyth MJ, Nat. Rev. Clin. Oncol 2019, 16, 151. [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Taube JM, Pardoll DM, Science 2020, 367, 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanath DI, Liu HC, Huston DP, Chua CYX, Grattoni A, Biomaterials 2022, 280, 121297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang J, Holay M, Park JH, Fang RH, Zhang J, Zhang L, Theranostics 2019, 9, 7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterner RC, Sterner RM, Blood Cancer J 2021, 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patsoukis N, Wang Q, Strauss L, Boussiotis VA, Sci. Adv 2020, 6, eabd2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, Hirsch FR, Nat. Rev. Clin. Oncol 2021, 18, 345. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuiki N, Schwab C, Grimbacher B, Immunol. Rev 2019, 287, 33. [DOI] [PubMed] [Google Scholar]
- 24.Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H, Oncotarget 2017, 8, 38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, Li Z, Pan CX, J. Hematol. Oncol 2021, 14, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniell MD, Hill JS, Aust. N. Z. J. Surg 1991, 61, 340. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Lovell JF, Yoon J, Chen X, Nat. Rev. Clin. Oncol 2020, 17, 657. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zhang W, Ji W, Wang J, Wang N, Wu W, Wu Q, Hou X, Hu W, Li L, J. Mater. Chem. B 2021, 9, 7909. [DOI] [PubMed] [Google Scholar]
- 29.Melamed JR, Edelstein RS, Day ES, ACS Nano 2015, 9, 6. [DOI] [PubMed] [Google Scholar]
- 30.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H, Crit. Rev. Oncol. Hematol 2002, 43, 33. [DOI] [PubMed] [Google Scholar]
- 31.Bian W, Wang Y, Pan Z, Chen N, Li X, Wong W-L, Liu X, He Y, Zhang K, Lu Y-J, ACS Appl. Nano Mater 2021, 4, 11353. [Google Scholar]
- 32.Jaque D, Martinez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodriguez E, Garcia Sole J, Nanoscale 2014, 6, 9494. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Ning C, Zhou Z, Yu P, Zhu Y, Tan G, Mao C, Prog. Mater. Sci 2019, 99, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS, Chem. Soc. Rev 2018, 47, 2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolmans DE, Fukumura D, Jain RK, Nat. Rev. Cancer 2003, 3, 380. [DOI] [PubMed] [Google Scholar]
- 36.Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z, Pharmaceutics 2021, 13, 1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CN, Hsu R, Chen H, Wong TW, Molecules 2020, 25, 5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison RR, Moghissi K, Clin. Endosc 2013, 46, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabrowski JM, Arnaut LG, Photochem. Photobiol. Sci 2015, 14, 1765. [DOI] [PubMed] [Google Scholar]
- 40.Mfouo-Tynga IS, Dias LD, Inada NM, Kurachi C, Photodiagnosis Photodyn. Ther 2021, 34, 102091. [DOI] [PubMed] [Google Scholar]
- 41.Pushpan SK, Venkatraman S, Anand VG, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar TK, Curr. Med. Chem. Anticancer Agents 2002, 2, 187. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor AE, Gallagher WM, Byrne AT, Photochem. Photobiol 2009, 85, 1053. [DOI] [PubMed] [Google Scholar]
- 43.Kou J, Dou D, Yang L, Oncotarget 2017, 8, 81591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chilakamarthi U, Giribabu L, Chem. Rec 2017, 17, 775. [DOI] [PubMed] [Google Scholar]
- 45.Yang H, Liu R, Xu Y, Qian L, Dai Z, Nano-Micro Lett. 2021, 13, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Liu X, Liu X, Yu J, Bai X, Wu X, Guo X, Liu Z, Liu X, Front. Immunol 2022, 13, 955920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L, Cell Death Dis. 2020, 11, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, Zhang Y, Li X, Zhao Y, Li M, Jiang W, Tang X, Dou J, Lu L, Wang F, Wang Y, ACS Nano 2019, 13, 11967. [DOI] [PubMed] [Google Scholar]
- 49.Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV, J. Immunother. Cancer 2021, 9, e001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P, Nat. Rev. Cancer 2012, 12, 860. [DOI] [PubMed] [Google Scholar]
- 51.Chang M, Hou Z, Wang M, Li C, Lin J, Adv. Mater 2021, 33, 2004788. [DOI] [PubMed] [Google Scholar]
- 52.Castano AP, Mroz P, Hamblin MR, Nat. Rev. Cancer 2006, 6, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asadzadeh Z, Safarzadeh E, Safaei S, Baradaran A, Mohammadi A, Hajiasgharzadeh K, Derakhshani A, Argentiero A, Silvestris N, Baradaran B, Cancers 2020, 12, 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Q, Huang Z, Duan Y, Fu Z, Bin L, Bioconjug. Chem 2020, 31, 1268. [DOI] [PubMed] [Google Scholar]
- 55.Yom SS, Busch TM, Friedberg JS, Wileyto EP, Smith D, Glatstein E, Hahn SM, Photochem. Photobiol 2003, 78, 75. [DOI] [PubMed] [Google Scholar]
- 56.Nowis D, Stoklosa T, Legat M, Issat T, Jakobisiak M, Golab J, Photodiagnosis Photodyn. Ther 2005, 2, 283. [DOI] [PubMed] [Google Scholar]
- 57.Hameed S, Mo S, Mustafa G, Bajwa SZ, Khan WS, Dai Z, Adv. Ther 2019, 3, 1900101. [Google Scholar]
- 58.Hunt DW, Levy JG, Expert Opin. Investig. Drugs 1998, 7, 57. [DOI] [PubMed] [Google Scholar]
- 59.Zhang P, Xiao Y, Sun X, Lin X, Koo S, Yaremenko AV, Qin D, Kong N, Farokhzad OC, Tao W, Med 2022, DOI: 10.1016/j.medj.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H, Xu J, Feng C, Ren J, Bao L, Zhao Y, Tao W, Zhao Y, Yang X, Adv. Mater 2022, 34, 2106390. [DOI] [PubMed] [Google Scholar]
- 61.Liu L, He H, Luo Z, Zhou H, Liang R, Pan H, Ma Y, Cai L, Adv. Funct. Mater 2020, 30, 1910176. [Google Scholar]
- 62.Zhu Z, Ma AH, Zhang H, Lin TY, Xue X, Farrukh H, Zhu S, Shi W, Yuan R, Cao Z, Chittepu V, Prabhala R, Li Y, Lam KS, Pan CX, Clin. Cancer Res 2022, 28, 4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng H, Zhou Z, Yang W, Lin LS, Wang S, Niu G, Song J, Chen X, Nano Lett. 2020, 20, 1928. [DOI] [PubMed] [Google Scholar]
- 64.Riley RS, June CH, Langer R, Mitchell MJ, Nat. Rev. Drug Discov 2019, 18, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A, Cell 2017, 168, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I, Clin. Cancer Res 2016, 22, 1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang L, Zhang A, Zhang Z, Zhao Q, Li J, Mei Y, Yin Y, Wang W, Cancer Commun. 2022, 42, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK, Mater. Sci. Eng. C Mater. Biol. Appl 2019, 98, 1252. [DOI] [PubMed] [Google Scholar]
- 69.Pearce AK, O'Reilly RK, Bioconjug. Chem 2019, 30, 2300. [DOI] [PubMed] [Google Scholar]
- 70.Pham SH, Choi Y, Choi J, Pharmaceutics 2020, 12, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Li J, Wang Y, Gong X, Xu X, Wang J, Li Y, Sha X, Zhang Z, Control J. Release 2020, 319, 25. [DOI] [PubMed] [Google Scholar]
- 72.Xu N, Du J, Yao Q, Ge H, Li H, Xu F, Gao F, Xian L, Fan J, Peng X, Carbon 2020, 159, 74. [Google Scholar]
- 73.Wang J, Zhang H, Xiao X, Liang D, Liang X, Mi L, Wang J, Liu J, Acta Biomater. 2020, 107, 260. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Xie Y, Wang M, Li C, Front. Chem 2020, 8, 596658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H, Yang Q, Guo W, Lin H, Zhang F, Zhao J, Ma T, Zhao L, Xu N, Wang R, Chem. Eng. J 2020, 385, 123979. [Google Scholar]
- 76.De Matteis V, Toxics 2017, 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie Z, Fan T, An J, Choi W, Duo Y, Ge Y, Zhang B, Nie G, Xie N, Zheng T, Chen Y, Zhang H, Kim JS, Chem. Soc. Rev 2020, 49, 8065. [DOI] [PubMed] [Google Scholar]
- 78.Luby BM, Walsh CD, Zheng G, Angew. Chem. Int. Ed. Engl 2019, 58, 2558. [DOI] [PubMed] [Google Scholar]
- 79.Liu L, Wang X, Wang LJ, Guo L, Li Y, Bai B, Fu F, Lu H, Zhao X, ACS Appl. Mater. Interfaces 2021, 13, 19668. [DOI] [PubMed] [Google Scholar]
- 80.Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D, Tang BZ, Chem. Commun 2001, 2001, 1740. [DOI] [PubMed] [Google Scholar]
- 81.Cui S, Dai S, Lin N, Wu X, Shi J, Tong B, Liu P, Cai Z, Dong Y, Small 2022, 18, 2203825. [DOI] [PubMed] [Google Scholar]
- 82.Fukumura D, Jain RK, Microvasc. Res 2007, 74, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lv X, Li J, Zhang C, Hu T, Li S, He S, Yan H, Tan Y, Lei M, Wen M, Zuo J, Genes Dis. 2017, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rankin EB, Giaccia AJ, Science 2016, 352, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schito L, Rey S, Trends Cell Biol. 2018, 28, 128. [DOI] [PubMed] [Google Scholar]
- 86.Thienpont B, Steinbacher J, Zhao H, D'Anna F, Kuchnio A, Ploumakis A, Ghesquiere B, Van Dyck L, Boeckx B, Schoonjans L, Hermans E, Amant F, Kristensen VN, Peng Koh K, Mazzone M, Coleman M, Carell T, Carmeliet P, Lambrechts D, Nature 2016, 537, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar V, Gabrilovich DI, Immunology 2014, 143, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin X, Gong L, Peng Y, Li L, Liu G, Aging 2021, 13, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL, Cancer Res. 2001, 61, 2911. [PubMed] [Google Scholar]
- 90.Hong L, Li J, Luo Y, Guo T, Zhang C, Ou S, Long Y, Hu Z, Biomolecules 2022, 12, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou TJ, Xing L, Fan YT, Cui PF, Jiang HL, Control J. Release 2019, 307, 44. [DOI] [PubMed] [Google Scholar]
- 92.Qiao Y, Yang F, Xie T, Du Z, Zhong D, Qi Y, Li Y, Li W, Lu Z, Rao J, Sun Y, Zhou M, Sci. Adv 2020, 6, eaba5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong D, Li W, Qi Y, He J, Zhou M, Adv. Funct. Mater 2020, 30, 1910395. [Google Scholar]
- 94.Zheng D, Li B, Xu L, Zhang QL, Fan JX, Li CX, Zhang XZ, ACS Nano 2018, 12, 6218. [DOI] [PubMed] [Google Scholar]
- 95.Cheng Y, Zheng R, Wu X, Xu K, Song P, Wang Y, Yan J, Chen R, Li X, Zhang H, Adv. Healthc. Mater 2021, 10, 2001666. [DOI] [PubMed] [Google Scholar]
- 96.Zheng DW, Li B, Li CX, Fan JX, Lei Q, Li C, Xu Z, Zhang XZ, ACS Nano 2016, 10, 8715. [DOI] [PubMed] [Google Scholar]
- 97.Yang Z, Liu X, Wang X, Wang P, Ruan S, Xie A, Shen Y, Zhu M, Chem. Eng. J 2020, 387, 124113. [Google Scholar]
- 98.Jiang W, Zhang Z, Wang Q, Dou J, Zhao Y, Ma Y, Liu H, Xu H, Wang Y, Nano Lett. 2019, 19, 4060. [DOI] [PubMed] [Google Scholar]
- 99.Li M, Lin H, Qu F, Chem. Eng. J 2020, 384, 123374. [Google Scholar]
- 100.Yang N, Xiao W, Song X, Wang W, Dong X, Nano-Micro Lett. 2020, 12, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J, Xiao Z, Chen G, Li T, Peng Y, Shuai X, Nano Today 2022, 43, 101390. [Google Scholar]
- 102.Liu J, Wang L, Shen X, Gao X, Chen Y, Liu H, Liu Y, Yin D, Liu Y, Xu W, Cai R, You M, Guo M, Wang Y, Li J, Li Y, Chen C, Nano Today 2020, 34, 100907. [Google Scholar]
- 103.Mu J, Zhang L, Zhao M, Wang Y, J. Mol. Catal. A Chem 2013, 378, 30. [Google Scholar]
- 104.Zhen W, Liu Y, Lin L, Bai J, Jia X, Tian H, Jiang X, Angew. Chem. Int. Ed. Engl 2018, 57, 10309. [DOI] [PubMed] [Google Scholar]
- 105.Yao C, Wang W, Wang P, Zhao M, Li X, Zhang F, Adv. Mater 2018, 30, 1704833. [DOI] [PubMed] [Google Scholar]
- 106.Dong P, Wang W, Pan M, Yu W, Liu Y, Shi T, Hu J, Zhou Y, Yu S, Wang F, Liu X, ACS Appl. Mater. Interfaces 2021, 13, 16075. [DOI] [PubMed] [Google Scholar]
- 107.Liu CP, Wu TH, Liu CY, Chen KC, Chen YX, Chen GS, Lin SY, Small 2017, 13, 1700278. [DOI] [PubMed] [Google Scholar]
- 108.Hou H, Huang X, Wei G, Xu F, Wang Y, Zhou S, ACS Appl. Mater. Interfaces 2019, 11, 29579. [DOI] [PubMed] [Google Scholar]
- 109.Chen M, Song J, Zhu J, Hong G, An J, Feng E, Peng X, Song F, Adv. Healthc. Mater 2021, 10, 2101049. [DOI] [PubMed] [Google Scholar]
- 110.Wang M, Chang M, Chen Q, Wang D, Li C, Hou Z, Lin J, Jin D, Xing B, Biomaterials 2020, 252, 120093. [DOI] [PubMed] [Google Scholar]
- 111.Mairbaurl H, Weber RE, Compr. Physiol 2012, 2, 1463. [DOI] [PubMed] [Google Scholar]
- 112.Alayash AI, Nat. Rev. Drug Discov 2004, 3, 152. [DOI] [PubMed] [Google Scholar]
- 113.Liu WL, Liu T, Zou MZ, Yu WY, Li CX, He ZY, Zhang MK, Liu MD, Li ZH, Feng J, Zhang XZ, Adv. Mater 2018, 30, 1802006. [DOI] [PubMed] [Google Scholar]
- 114.Krafft MP, Curr. Opin. Pharmacol 2020, 53, 117. [DOI] [PubMed] [Google Scholar]
- 115.Day RA, Sletten EM, Curr. Opin. Colloid Interface Sci 2021, 54, 101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu M, Xu X, Cai Y, Zou L, Shuai X, Biomaterials 2018, 175, 61. [DOI] [PubMed] [Google Scholar]
- 117.Hu D, Zhong L, Wang M, Li H, Qu Y, Liu Q, Han R, Yuan L, Shi K, Peng J, Qian Z, Adv. Funct. Mater 2019, 29, 1806199. [Google Scholar]
- 118.Liu Z, Xue Y, Wu M, Yang G, Lan M, Zhang W, Biomacromolecules 2019, 20, 4563. [DOI] [PubMed] [Google Scholar]
- 119.Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y, Nat. Commun 2015, 6, 8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sutton AL, Melag L, Sadiq MM, Hill MR, Angew. Chem. Int. Ed. Engl 2022, 61, e202208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie Z, Liang S, Cai X, Ding B, Huang S, Hou Z, Ma P, Cheng Z, Lin J, ACS Appl. Mater. Interfaces 2019, 11, 31671. [DOI] [PubMed] [Google Scholar]
- 122.Chitgupi U, Qin Y, Lovell JF, Nanotheranostics 2017, 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li D, Zhang M, Xu F, Chen Y, Chen B, Chang Y, Zhong H, Jin H, Huang Y, Acta Pharm. Sin. B 2018, 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo Z, Li M, Zhou M, Li H, Chen Y, Ren X, Dai Y, Nanomedicine 2019, 19, 49. [DOI] [PubMed] [Google Scholar]
- 125.Song C, Zhang Y, Li C, Chen G, Kang X, Wang Q, Adv. Funct. Mater 2016, 26, 4192. [Google Scholar]
- 126.Zhu M, Sheng Z, Jia Y, Hu D, Liu X, Xia X, Liu C, Wang P, Wang X, Zheng H, ACS Appl. Mater. Interfaces 2017, 9, 39249. [DOI] [PubMed] [Google Scholar]
- 127.Su YL, Hu SH, Pharmaceutics 2018, 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duan X, Li Y, Small 2013, 9, 1521. [DOI] [PubMed] [Google Scholar]
- 129.Wang K, Tu Y, Yao W, Zong Q, Xiao X, Yang RM, Jiang XQ, Yuan Y, ACS Appl. Mater. Interfaces 2020, 12, 6933. [DOI] [PubMed] [Google Scholar]
- 130.Chen Q, Liu G, Liu S, Su H, Wang Y, Li J, Luo C, Trends Pharmacol. Sci 2018, 39, 59. [DOI] [PubMed] [Google Scholar]
- 131.Li J, Xie C, Huang J, Jiang Y, Miao Q, Pu K, Angew. Chem. Int. Ed. Engl 2018, 57, 3995. [DOI] [PubMed] [Google Scholar]
- 132.Fang RH, Kroll AV, Gao W, Zhang L, Adv. Mater 2018, 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo Z, Kubiatowicz LJ, Fang RH, Zhang L, Adv. Ther 2021, 4, 2100072. [Google Scholar]
- 134.Fang RH, Gao W, Zhang L, Nat. Rev. Clin. Oncol 2023, 20, 33. [DOI] [PubMed] [Google Scholar]
- 135.Park JH, Jiang Y, Zhou J, Gong H, Mohapatra A, Heo J, Gao W, Fang RH, Zhang L, Sci. Adv 2021, 7, eabf7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang D, Ai X, Duan Y, Xian N, Fang RH, Gao W, Zhang L, ACS Nano 2022, 16, 19145. [DOI] [PubMed] [Google Scholar]
- 137.Zhen X, Cheng P, Pu K, Small 2019, 15, 1804105. [DOI] [PubMed] [Google Scholar]
- 138.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L, Proc. Natl. Acad. Sci. U. S. A 2011, 108, 10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng BD, Xiao MT, Colloids Surf. B Biointerfaces 2022, 220, 112895. [DOI] [PubMed] [Google Scholar]
- 140.Xuan M, Shao J, Zhao J, Li Q, Dai L, Li J, Angew. Chem. Int. Ed. Engl 2018, 57, 6049. [DOI] [PubMed] [Google Scholar]
- 141.Fang RH, Hu CM, Chen KN, Luk BT, Carpenter CW, Gao W, Li S, Zhang DE, Lu W, Zhang L, Nanoscale 2013, 5, 8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan Y, He Y, Zhao X, Pan Y, Meng X, Lv Z, Hu Z, Mou X, Cai Y, Adv. Healthc. Mater 2022, 11, 2200962. [DOI] [PubMed] [Google Scholar]
- 143.Liu W, Ruan M, Wang Y, Song R, Ji X, Xu J, Dai J, Xue W, Small 2018, 14, 1801754. [Google Scholar]
- 144.Wu T, Lang T, Zheng C, Yan W, Li Y, Zhu R, Huang X, Xu H, Li Y, Yin Q, Adv. Funct. Mater 2023, 33, 2212109. [Google Scholar]
- 145.Li B, Chu T, Wei J, Zhang Y, Qi F, Lu Z, Gao C, Zhang T, Jiang E, Xu J, Xu J, Li S, Nie G, Nano Lett. 2021, 21, 2588. [DOI] [PubMed] [Google Scholar]
- 146.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L, Nature 2015, 526, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rao L, Bu L-L, Meng Q-F, Cai B, Deng W-W, Li A, Li K, Guo S-S, Zhang W-F, Liu W, Sun Z-J, Zhao X-Z, Adv. Funct. Mater 2017, 27, 1604774. [Google Scholar]
- 148.Zhang W, Huang X, Mater. Today Bio 2022, 16, 100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao C, Lin Z, Wu Z, Lin X, He Q, ACS Appl. Mater. Interfaces 2016, 8, 34252. [DOI] [PubMed] [Google Scholar]
- 150.Mu X, Li J, Yan S, Zhang H, Zhang W, Zhang F, Jiang J, ACS Biomater. Sci. Eng 2018, 4, 3895. [DOI] [PubMed] [Google Scholar]
- 151.Xuan M, Shao J, Dai L, Li J, He Q, ACS Appl. Mater. Interfaces 2016, 8, 9610. [DOI] [PubMed] [Google Scholar]
- 152.Kang T, Zhu Q, Wei D, Feng J, Yao J, Jiang T, Song Q, Wei X, Chen H, Gao X, Chen J, ACS Nano 2017, 11, 1397. [DOI] [PubMed] [Google Scholar]
- 153.Kang M, Hong J, Jung M, Kwon SP, Song SY, Kim HY, Lee JR, Kang S, Han J, Koo JH, Ryu JH, Lim S, Sohn HS, Choi JM, Doh J, Kim BS, Adv. Mater 2020, 32, 2003368. [DOI] [PubMed] [Google Scholar]
- 154.Ma W, Zhu D, Li J, Chen X, Xie W, Jiang X, Wu L, Wang G, Xiao Y, Liu Z, Wang F, Li A, Shao D, Dong W, Liu W, Yuan Y, Theranostics 2020, 10, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han Y, Pan H, Li W, Chen Z, Ma A, Yin T, Liang R, Chen F, Ma Y, Jin Y, Zheng M, Li B, Cai L, Adv. Sci 2019, 6, 1900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P, Gao G, Pan H, Liu L, Ma A, Cui H, Ma Y, Cai L, ACS Nano 2016, 10, 10049. [DOI] [PubMed] [Google Scholar]
- 157.Wang J, Sun J, Hu W, Wang Y, Chou T, Zhang B, Zhang Q, Ren L, Wang H, Adv. Mater 2020, 32, 2001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Biffi G, Tuveson DA, Physiol. Rev 2021, 101, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J, Zhen X, Lyu Y, Jiang Y, Huang J, Pu K, ACS Nano 2018, 12, 8520. [DOI] [PubMed] [Google Scholar]
- 160.Liu Y, Jia H-R, Han X, Wu F-G, Smart Mater. Med 2021, 2, 334. [Google Scholar]
- 161.Li Y, Zhang X, Wan X, Liu X, Pan W, Li N, Tang B, Adv. Funct. Mater 2020, 30, 2000532. [Google Scholar]
- 162.Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, Zhu C, Yuan X, Zhang J, Luo Z, Du Y, Li Q, Lou Y, Qiu Y, You J, Nat. Commun 2019, 10, 3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wan J, Sun L, Wu P, Wang F, Guo J, Cheng J, Wang C, Polym. Chem 2018, 9, 1206. [Google Scholar]
- 164.Gao Y, Tong H, Li J, Li J, Huang D, Shi J, Xia B, Front. Bioeng. Biotechnol 2021, 9, 720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tong H, Gao Y, Li J, Li J, Huang D, Shi J, Santos HA, Xia B, Part. Part. Syst. Charact 2021, 38, 2100013. [Google Scholar]
- 166.Nash GT, Luo T, Lan G, Ni K, Kaufmann M, Lin W, J. Am. Chem. Soc 2021, 143, 2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Y-H, Jia H-R, Wang H-Y, Hua X-W, Bao Y-W, Wu F-G, J. Control. Release 2022, 351, 692. [DOI] [PubMed] [Google Scholar]
- 168.Yang C, Fu Y, Huang C, Hu D, Zhou K, Hao Y, Chu B, Yang Y, Qian Z, Biomaterials 2020, 255, 120194. [DOI] [PubMed] [Google Scholar]
- 169.Pan L, Liu J, Shi J, Chem. Soc. Rev 2018, 47, 6930. [DOI] [PubMed] [Google Scholar]
- 170.Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD, Redox Biol. 2019, 25, 101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu J, Li M, Luo Z, Dai L, Guo X, Cai K, Nano Today 2017, 15, 56. [Google Scholar]
- 172.Zimmerli CE, Allegretti M, Rantos V, Goetz SK, Obarska-Kosinska A, Zagoriy I, Halavatyi A, Hummer G, Mahamid J, Kosinski J, Beck M, Science 2021, 374, eabd9776. [DOI] [PubMed] [Google Scholar]
- 173.Long X, Zhang X, Chen Q, Liu M, Xiang Y, Yang Y, Xiao Z, Huang J, Wang X, Liu C, Nan Y, Huang Q, Front. Pharmacol 2022, 13, 905375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Du Y, Dai X, Han M, Wang Z, Wang Y, Shi Z, Yan F, Feng S, Chem. Eng. J 2023, 453, 139635. [Google Scholar]
- 175.Tie Y, Tang F, Wei YQ, Wei XW, J. Hematol. Oncol 2022, 15, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li C, Jiang P, Wei S, Xu X, Wang J, Mol. Cancer 2020, 19, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang L, Zhang Y, J. Hematol. Oncol 2017, 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ngambenjawong C, Gustafson HH, Pun SH, Adv. Drug Deliv. Rev 2017, 114, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kumar V, Patel S, Tcyganov E, Gabrilovich DI, Trends Immunol. 2016, 37, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vetsika EK, Koukos A, Kotsakis A, Cells 2019, 8, 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gao J, Wang WQ, Pei Q, Lord MS, Yu HJ, Acta Pharmacol. Sin 2020, 41, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tanaka A, Sakaguchi S, Cell Res. 2017, 27, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Riccardi C, Ronchetti S, Nocentini G, Expert Opin. Ther. Targets 2018, 22, 783. [DOI] [PubMed] [Google Scholar]
- 184.Sun Q, Yang Z, Lin M, Peng Y, Wang R, Du Y, Zhou Y, Li J, Qi X, Biomaterials 2021, 269, 120648. [DOI] [PubMed] [Google Scholar]
- 185.Ou W, Jiang L, Thapa RK, Soe ZC, Poudel K, Chang JH, Ku SK, Choi HG, Yong CS, Kim JO, Theranostics 2018, 8, 4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang H, Guo Y, Wei C, Hu P, Shi J, Adv. Mater 2021, 33, 2008065. [DOI] [PubMed] [Google Scholar]
- 187.Huang C, Lin B, Chen C, Wang H, Lin X, Liu J, Ren Q, Tao J, Zhao P, Xu Y, Adv. Mater 2022, 34, 2207593. [DOI] [PubMed] [Google Scholar]
- 188.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG, Cancer Res. 2014, 74, 5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Y, Wen Y, Chen X, Zhu X, Yu Q, Gong Y, Yuan G, Liu J, Qin X, J. Mater. Chem. B 2019, 7, 6210. [DOI] [PubMed] [Google Scholar]
- 190.Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P, Cai L, ACS Nano 2018, 12, 12096. [DOI] [PubMed] [Google Scholar]
- 191.Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, Ng IO, Wong CC, Nat. Commun 2017, 8, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zuo H, Hou Y, Yu Y, Li Z, Liu H, Liu C, He J, Miao L, ACS Appl. Mater. Interfaces 2020, 12, 55723. [DOI] [PubMed] [Google Scholar]
- 193.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I, J. Exp. Med 2006, 203, 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang T, Xiong H, Ma X, Gao Y, Xue P, Kang Y, Sun ZJ, Xu Z, Small Methods 2021, 5, 2100115. [DOI] [PubMed] [Google Scholar]
- 195.Kalbasi A, Ribas A, Nat. Rev. Immunol 2020, 20, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Toor SM, Sasidharan Nair V, Decock J, Elkord E, Semin. Cancer Biol 2020, 65, 1. [DOI] [PubMed] [Google Scholar]
- 197.Zappasodi R, Merghoub T, Wolchok JD, Cancer Cell 2018, 33, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM, Science 2011, 332, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP, J. Exp. Med 2009, 206, 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pardoll DM, Nat. Rev. Cancer 2012, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T, J. Exp. Med 2000, 192, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blank C, Mackensen A, Cancer Immunol. Immunother 2007, 56, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, Wainwright DA, Clin. Cancer Res 2015, 21, 5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P, Cell Death Differ. 2002, 9, 1069. [DOI] [PubMed] [Google Scholar]
- 205.Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G, Gyorffy B, Brastianos PK, Binder DC, Sosman JA, Giles FJ, James CD, Horbinski C, Stupp R, Wainwright DA, Clin. Cancer Res 2017, 23, 6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hascitha J, Priya R, Jayavelu S, Dhandapani H, Selvaluxmy G, Sunder Singh S, Rajkumar T, Clin. Biochem 2016, 49, 919. [DOI] [PubMed] [Google Scholar]
- 207.Tang K, Wu YH, Song Y, Yu B, J. Hematol. Oncol 2021, 14, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Logtenberg MEW, Scheeren FA, Schumacher TN, Immunity 2020, 52, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Matlung HL, Szilagyi K, Barclay NA, van den Berg TK, Immunol. Rev 2017, 276, 145. [DOI] [PubMed] [Google Scholar]
- 210.Maute R, Xu J, Weissman IL, Immunooncol. Technol 2022, 13, 100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.He M, Yang T, Wang Y, Wang M, Chen X, Ding D, Zheng Y, Chen H, Adv. Healthc. Mater 2021, 10, 2002104. [DOI] [PubMed] [Google Scholar]
- 212.Zhang C, Gao F, Wu W, Qiu WX, Zhang L, Li R, Zhuang ZN, Yu W, Cheng H, Zhang XZ, ACS Nano 2019, 13, 11249. [DOI] [PubMed] [Google Scholar]
- 213.Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W, J. Am. Chem. Soc 2018, 140, 5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Francis DM, Manspeaker MP, Schudel A, Sestito LF, O'Melia MJ, Kissick HT, Pollack BP, Waller EK, Thomas SN, Sci. Transl. Med 2020, 12, eaay3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie Z, Peng M, Lu R, Meng X, Liang W, Li Z, Qiu M, Zhang B, Nie G, Xie N, Zhang H, Prasad PN, Light Sci. Appl 2020, 9, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen SX, Ma M, Xue F, Shen S, Chen Q, Kuang Y, Liang K, Wang X, Chen H, J. Control. Release 2020, 324, 218. [DOI] [PubMed] [Google Scholar]
- 217.Yang Q, Peng J, Shi K, Xiao Y, Liu Q, Han R, Wei X, Qian Z, J. Control. Release 2019, 308, 29. [DOI] [PubMed] [Google Scholar]
- 218.Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J, Alfranca G, Sun X, Ma L, de la Fuente JM, Song J, Ni J, Cui D, Theranostics 2019, 9, 6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li J, Cui D, Huang J, He S, Yang Z, Zhang Y, Luo Y, Pu K, Angew. Chem. Int. Ed. Engl 2019, 58, 12680. [DOI] [PubMed] [Google Scholar]
- 220.Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, Lin W, J. Am. Chem. Soc 2016, 138, 12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Q, Zhang D, Zhang J, Jiang Y, Song A, Li Z, Luan Y, Nano Lett. 2019, 19, 6647. [DOI] [PubMed] [Google Scholar]
- 222.Vermaelen K, Front. Immunol 2019, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hammerich L, Binder A, Brody JD, Mol. Oncol 2015, 9, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Banstola A, Jeong JH, Yook S, Acta Biomater. 2020, 114, 16. [DOI] [PubMed] [Google Scholar]
- 225.Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Oncoimmunology 2012, 1, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pulendran B, P SA, O'Hagan DT, Nat. Rev. Drug Discov 2021, 20, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Franchi L, Nunez G, Eur. J. Immunol 2008, 38, 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dmour I, Islam N, Int. J. Biol. Macromol 2022, 200, 498. [DOI] [PubMed] [Google Scholar]
- 229.Li X, Xing R, Xu C, Liu S, Qin Y, Li K, Yu H, Li P, Carbohydr. Polym 2021, 264, 118050. [DOI] [PubMed] [Google Scholar]
- 230.Tsan MF, Gao B, Am. J. Physiol. Cell Physiol 2004, 286, C739. [DOI] [PubMed] [Google Scholar]
- 231.Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L, J. Immunol 2014, 193, 1006. [DOI] [PubMed] [Google Scholar]
- 232.Zhou J, Karshalev E, Mundaca-Uribe R, Esteban-Fernandez de Avila B, Krishnan N, Xiao C, Ventura CJ, Gong H, Zhang Q, Gao W, Fang RH, Wang J, Zhang L, Adv. Mater 2021, 33, 2103505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kayraklioglu N, Horuluoglu B, Klinman DM, Methods Mol. Biol 2021, 2197, 51. [DOI] [PubMed] [Google Scholar]
- 234.Chen W, Jiang M, Yu W, Xu Z, Liu X, Jia Q, Guan X, Zhang W, Int. J. Nanomedicine 2021, 16, 5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li Z, Hu Y, Fu Q, Liu Y, Wang J, Song J, Yang H, Adv. Funct. Mater 2019, 30, 1905758. [Google Scholar]
- 236.Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, Imahori H, Umeki Y, Shiomi T, Takakura Y, Nishikawa M, Biomaterials 2017, 146, 136. [DOI] [PubMed] [Google Scholar]
- 237.Zhang F, Lu G, Wen X, Li F, Ji X, Li Q, Wu M, Cheng Q, Yu Y, Tang J, Mei L, J. Control. Release 2020, 326, 131. [DOI] [PubMed] [Google Scholar]
- 238.Liu X, Su Q, Song H, Shi X, Zhang Y, Zhang C, Huang P, Dong A, Kong D, Wang W, Biomaterials 2021, 275, 120921. [DOI] [PubMed] [Google Scholar]
- 239.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O'Connor DE, Zhang L, Nano Lett. 2014, 14, 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L, Nano Lett. 2015, 15, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Zhang L, Adv. Mater 2017, 29, 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, Yu CL, Duan Y, Gao W, Fang RH, Zhang L, Adv. Mater 2020, 32, 2001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sun Z, Deng G, Peng X, Xu X, Liu L, Peng J, Ma Y, Zhang P, Wen A, Wang Y, Yang Z, Gong P, Jiang W, Cai L, Biomaterials 2021, 279, 121228. [DOI] [PubMed] [Google Scholar]
- 244.Sun Z, Liu J, Li Y, Lin X, Chu Y, Wang W, Huang S, Li W, Peng J, Liu C, Cai L, Deng W, Sun C, Deng G, Adv. Mater 2023, 35, 2208555. [DOI] [PubMed] [Google Scholar]
- 245.Holay M, Guo Z, Pihl J, Heo J, Park JH, Fang RH, Zhang L, ACS Appl. Bio Mater 2021, 4, 3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Peng LH, Wang MZ, Chu Y, Zhang L, Niu J, Shao HT, Yuan TJ, Jiang ZH, Gao JQ, Ning XH, Sci. Adv 2020, 6, eaba2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li M, Li S, Zhou H, Tang X, Wu Y, Jiang W, Tian Z, Zhou X, Yang X, Wang Y, Nat. Commun 2020, 11, 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhao Y, Li A, Jiang L, Gu Y, Liu J, Biomacromolecules 2021, 22, 3149. [DOI] [PubMed] [Google Scholar]
- 249.Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, Zhang L, Adv. Mater 2017, 29, 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J, Chu PK, Bai H, Tang G, Adv. Mater 2020, 32, 1908185. [DOI] [PubMed] [Google Scholar]