Abstract
Youth-onset type 2 diabetes (T2D) is becoming increasingly prevalent, especially among Latino youth, and there is limited information on its pathophysiology and causative factors. Here, we describe findings from a longitudinal cohort study in 262 Latino children with overweight/obesity at risk of developing T2D with annual measures of oral and intravenous glucose tolerance (IVGTT), body composition, and fat distribution. Logistic binomial regression was used to identify significant predictors in those who developed T2D compared with matched control participants, and mixed-effects growth models were used to compare rates of change in metabolic versus adiposity measures between groups. Overall conversion rate to T2D at year 5 was 2% (n = 6). Rate of decline in disposition index (DI), measured with an IVGTT, over 5 years was three times higher in case patients (−341.7 units per year) compared with the extended cohort (−106.7 units per year) and 20 times higher compared with control participants (−15.2 units per year). Case patients had significantly higher annual increases in fasting glucose, hemoglobin A1c (HbA1c), waist circumference, and trunk fat, and there was an inverse correlation between rate of decline in DI and rates of increase in adiposity measures. T2D development in at-risk Latino youth is associated with a substantial and rapid decrease in DI that is directly correlated with increases in fasting glucose, HbA1c, and adiposity.
Article Highlights
Youth-onset type 2 diabetes is becoming increasingly prevalent, especially among Latino youth, and there is limited information on its pathophysiology and causative factors.
Overall conversion rate to type 2 diabetes over 5 years was 2%.
In youth who converted to type 2 diabetes, disposition index decreased rapidly by 85% compared with that in patients who did not convert during the study period.
There was an inverse correlation between rate of decline in disposition index and rates of increase in various adiposity measures.
Introduction
Youth-onset type 2 diabetes (T2D) is a growing epidemic (1). In 2021, the SEARCH for Diabetes in Youth study reported an annual percent change in prevalence of youth-onset T2D of 4.8% between 2009 and 2017 (1). T2D is disproportionately prevalent among certain racial/ethnic groups, with ∼80% of youth with T2D from ethnic minority backgrounds (31.5% Black/African American, 41.1% Latino, 6.1% American Indian, and 1.7% Asian) (1,2). Additionally, the annual percent change (from 2009 to 2017) is greatest among Asian or Pacific Islander (7.3%), Black/African American (7.1%), American Indian (4.8%), and Latino youth (3.2%) compared with non-Latino White youth (1.4%) (1). The lifetime risk of developing T2D is highest among Latino populations, with a lifetime risk of almost 50% for youth born in the year 2000 (3). Compared with adults, adolescents with T2D have greater insulin resistance and more rapid progression to β-cell destruction, contributing to early treatment failure (4–6). Despite these alarming observations and substantial health risks, there is a paucity of information on the physiological and metabolic predictors of the development of T2D in youth at highest risk (7,8).
The development of T2D arises from progression of insulin resistance and subsequent inability of β-cells to adequately compensate through an increase in insulin secretion (9–11). In youth, progression to impaired fasting glucose and conversion to T2D are marked by concurrent declines in insulin sensitivity and insulin secretion and hepatic insulin extraction (12,13). Multiple longitudinal studies have proposed that a decline in insulin sensitivity contributes to the transition from normal glucose tolerance to impaired fasting glucose and that changes in insulin secretion, with an initial increase followed by rapid decline in secretion, drive conversion to T2D (10,14,15). Additionally, greater insulin resistance has been shown to accelerate the decline in disposition index (DI) (insulin sensitivity index [SI] multiplied by the incremental area under the curve of peripheral insulin concentration [AIR]). Previous data in youth have indicated that the combination of insulin sensitivity and inability of β-cells to compensate through increasing insulin secretion predicts conversion to T2D (16–18). Interestingly, an age-related difference in the pace and progression of β-cell failure (BCF) has been observed, with a relative dominance of insulin resistance in adolescents compared with adults, requiring a high level of compensatory insulin secretion to maintain normal glucose tolerance (4,5). However, other studies have shown that increased body fat, in the absence of insulin resistance, contributes to a reduction in glucose-stimulated insulin secretion and eventual progression to T2D (9,19,20).
Given the increasing prevalence of youth-onset T2D and the aggressive pathophysiology in adolescents compared with adults, there is a great need to identify youth with increased metabolic risk early so that targeted treatment and prevention can be initiated (21). To date, most studies examining the pathophysiology of T2D in youth have been cross-sectional in nature. To study these issues in more detail, a cohort of Latino children with overweight and obesity and a positive family history of T2D was established and named SOLAR (Study of Latino Adolescents at Risk) (22,23). The overall objectives of this longitudinal study were to evaluate factors contributing to decline in DI in Latino youth during pubertal development and examine mechanisms underlying changes in DI in the progression of T2D (22,23). Previous data collected from this study group and others have suggested rapidly declining DI is the primary pathophysiological factor contributing to youth-onset conversion to T2D (24–28). However, limited longitudinal studies have examined the relationship between rate of change in DI and rates of change in adiposity measures over time in youth who convert to T2D compared with those with normoglycemia (25,28–30). Therefore, the purpose of this analysis was to examine demographic, body composition, and metabolic differences in youth who converted to T2D over the 5-year study period compared with matched control participants and an extended cohort to investigate if any of the measured outcomes were predictive of conversion. We hypothesized that youth who convert to T2D would have more severe obesity, greater visceral fat, and be more insulin resistant at baseline than nonconverters and that rates of change in DI and adiposity measures would predict conversion.
Research Design and Methods
Participants
The SOLAR cohort was a prospective study of Latino youth with overweight and obesity who were recruited in two waves between 2000 and 2015 (31). Participants were recruited through clinics, health fairs, newspaper announcements, and word of mouth. Inclusion criteria included 1) age between 8 and 13 years, 2) BMI ≥85th percentile based on age and sex, 3) no T1D or T2D; 4) Hispanic/Latino ethnicity (all four parents and grandparents Hispanic/Latino by self-report), 5) direct familial history of T2D; and 6) no medications known to influence insulin/glucose metabolism (e.g., antipsychotics, sedatives, hypnotics, off-label obesity medications, or insulin). Ethics approval was provided by the University of Southern California (USC) Institutional Review Board (Los Angeles, CA). Before participation, written informed assent/consent was obtained from participants and their guardians. All participants returned on an annual basis for measurement of anthropometrics and body composition, metabolic testing, physical examination, and health questionnaires. Development of T2D (n = 6) was defined, according to the American Diabetes Association, as fasting glucose ≥126 mg/dL (7.0 mmol/L) and/or 2-h glucose ≥200 mg/dL (11.1 mmol/L) and/or hemoglobin A1c (HbA1c) ≥6.5% (≥46 mmol/mol). Youth who converted to T2D with at least two annual measures were compared with control participants matched on sex, BMI, and pubertal status, with a ratio of five control participants per case patient (n = 30), who had completed at least six annual measures and then with the extended cohort of youth who had completed at least six annual measures (n = 256). Youth included in the extended cohort all had baseline insulin response measures available.
Annual Anthropometrics and Body Composition Measures
Height (by a wall-mounted stadiometer) and weight (by a balance beam medical scale) were recorded at each visit to the nearest 0.1 cm and 0.1 kg, respectively, and the average of the two measurements was used for analysis. BMI and BMI percentile for age and sex were determined based on established Centers for Disease Control and Prevention normative curves using EpiInfo 2000 (version 1.1; Centers for Disease Control and Prevention, Atlanta, GA). Waist circumference (WC) and skinfolds (axilla, chest, subscapular, superiliac, abdomen, triceps, calf, and thigh) were measured using the procedures of Lohman et al. (32). A whole-body DEXA scan was performed to determine whole-body composition using a Hologic QDR 4500 W (Bedford, MA). Intra-abdominal and subcutaneous abdominal adipose tissue were measured by MRI at the Los Angeles County/USC Imaging Science Center using a 1.5 Signa LX-Ecospeed 1.5 Tesla magnet (General Electric Company) and a single slice at the level of the umbilicus.
Annual Metabolic Testing
An oral glucose tolerance test (OGTT) was conducted using a dose of 1.75 g glucose per kilogram body weight (to a maximum of 75 g). Blood was sampled and assayed for glucose and insulin every 30 min for 2 h (0, 30, 60, 90, and 120 min relative to glucose ingestion), and indices of insulin response were measured, including area under the curve (AUC). In addition, a frequently sampled intravenous GTT (FSIVGTT) was performed within 1–2 weeks of the OGTT and after an overnight stay at the clinical research center. The FSIVGTT was conducted with a glucose dose at time zero (25% dextrose; 0.3 g/kg body weight), blood sampling at 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min, and insulin injection (0.02 units/kg body weight; Humulin R [regular insulin for human injection], Eli Lilly and Company, Indianapolis, IN) at 20 min. Values captured on the FSIVGTT for glucose (glucose oxidase method: 2700 Analyzer; YSI, Inc., Yellow Springs, OH) and insulin (ELISA; Linco, St Charles, MO) were entered into the MINMOD Millennium 2002 computer program (version 5.16) for determination of SI, AIR, and DI (SI × AIR) (33). DI was used as an index of the compensatory adaptation to insulin resistance. Blood samples during the OGTT were centrifuged, and plasma was placed on ice and analyzed within 1 h at the Los Angeles County/USC Medical Center Core Laboratory with a Dimension Clinical Chemistry system using the Hexokinase method (Dade Behring, Deerfield, IL). Blood samples from the IVGTT were centrifuged, and plasma was stored on ice before storage at 80°C. Aliquots were assayed in duplicate for glucose using the glucose oxidase method and a 2700 Analyzer (YSI, Inc.). Insulin was assayed in duplicate using an ELISA kit from Linco. Height (in meters) and weight (in kilograms) were measured at each visit and were used to calculate BMI as kilograms per meter squared.
Health Questionnaires
Participants and their guardians completed several questionnaires detailing health history, familial health history, and sociodemographic characteristics. A pediatrician performed a physical examination for determination of Tanner stage (34). Tanner staging is an objective measure of the stage of development of secondary sex characteristics during puberty. Tanner stages were classified as prepuberty (Tanner 1), early puberty (Tanner 2), puberty (Tanner 3), late puberty (Tanner 4), and postpuberty (Tanner 5). Socioeconomic status was assessed using a modified version of the Hollingshead Four-Factor Index (35).
Statistical Analysis
Participants who converted to T2D (case patients; n = 6) were compared with the extended cohort (n = 256) using ANOVA or general linear models when covariates were included. In addition, as a sensitivity analysis, case patients were compared with matched control participants (n = 30). Baseline characteristics (sex, Tanner stage, BMI, and baseline metabolic parameters) were compared between case patients and matched control participants and between case patients and the extended cohort sample using t tests. Logistic binomial regression was used to calculate propensity scores with age, sex, baseline disposition index, Tanner stage, and weight in excess of the 95th percentile (percentage BMIp95) to assess association of these factors with conversion to T2D. Case patients were matched to five (five-to-one matching) youth who did not convert to T2D during the analysis period with propensity scores similar to that of the case patient [caliper 0.007 (SD of the logit of the propensity score)]. Mixed-effects growth models were used to compare rates of change in DI in case patients versus matched control participants and the total cohort. The differences in rates of change in DI, metabolic, and anthropometric measures between case patients, control participants, and the extended cohort were examined by testing the interaction between diabetes status and time in the mixed-effects model. Analysis was performed on unadjusted data, as well as with adjustment for baseline DI. Pearson correlation coefficient was used to evaluate the association between rate of change in DI and metabolic and anthropometric measures between groups. All analyses were performed using STATA (version 17.1; STATA Corp., College Station, TX), with a two-sided type I error set at P < 0.05.
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available because they contain information that could compromise research participant privacy and consent.
Results
Participant Characteristics
A total of 262 Latino youth (mean age 11.5 ± 2 years; 41% female; 78% with a BMI >95th percentile; 68% with household education level lower than high school) were recruited between 2000 and 2015 and completed a total of 799 study visits, with a mean visit number of 3.3 (SD 1.8) for the total cohort. Most participants (73%; n = 222) completed at least five annual measurements. The overall conversion rate to T2D at year 5 was 2% (n = 6). Descriptive characteristics of the cohort at baseline were compared between case patients (n = 6), control participants (n = 30), and the extended cohort (n = 256) (Table 1). No differences in age, BMI status, household education, or Tanner stage were noted (all P > 0.05). Four boys and two girls converted to T2D. Additionally, anthropometrics, lipids, glucose homeostasis, and insulin dynamics from the baseline OGTT and FSIVGTT and body composition and body fat distribution measures were not different between groups at baseline (Tables 1 and 2).
Table 1.
Baseline characteristics for youth who converted to T2D over study period (case patients) compared with control participants and total study cohort
| Case patients (n = 6) | Control participants (n = 30) | Total cohort (n = 262) | P * | |
|---|---|---|---|---|
| Sex | ||||
| Male | 4 (67) | 16 (53) | 162 (59) | 0.7† |
| Female | 2 (33) | 14 (47) | 113 (41) | 0.3† |
| Age, years | 11.3 ± 2 | 11.2 ± 2 | 11.5 ± 2 | 0.9† |
| Weight, kg | 68.5 ± 26 | 65.8 ± 20 | 66.1 ± 20 | 0.7† |
| Height, cm | 152.5 ± 17 | 150.3 ± 13 | 151.1 ± 12 | 0.6† |
| BMI, kg/m2 | 28.3 ± 6 | 28.5 ± 5 | 28.3 ± 6 | 0.7† |
| BMI percentile | 96.9 ± 4 | 97.5 ± 3 | 96.5 ± 5 | 0.6† |
| <85th (normal) | 0 (0) | 0 (0) | 9 (3) | |
| ≥85th and ≤95th percentile (overweight) | 1 (17) | 4 (13) | 97 (35) | |
| ≥95th and 120% of 95th percentile (class I obesity) | 3 (50) | 15 (50) | 133 (48) | |
| >120% of 95th percentile (class II or III) | 2 (33) | 11 (37) | 80 (30) | |
| Tanner stage | 0.1‡ | |||
| 1 | 3 (50) | 7 (23) | 89 (32) | |
| 2 | 0 (0) | 8 (27) | 90 (33) | |
| 3 | 0 (0) | 4 (13) | 36 (13) | |
| 4 | 6 (20) | 40 (15) | ||
| 5 | 3 (50) | 5 (17) | 20 (7) | |
| Birth weight, kg | 4.0 ± 1.1 | 3.7 ± 0.8 | 3.7 ± 1.1 | 0.7† |
| Household education level | 0.2‡ | |||
| Lower than high school | 3 (50) | 17 (57) | 187 (68) | |
| Completed high school | 0 (0) | 12 (40) | 74 (27) | |
| Higher than high school | 3 (50) | 1 (3) | 14 (5) |
Data are presented as n (%) or mean ± SD. Control participants were selected with propensity score–matching sex, BMI, and pubertal status at ratio of five control participants per case patient.
Statistical comparison between group differences for case patients versus control participants.
Two-sample t test.
Fisher exact test.
Table 2.
Anthropometrics, body composition, fat distribution, and outcomes from OGTT and FSIVGTT at baseline in youth who converted to T2D over study period (case patients) compared with control participants and total study cohort
| Case patients (n = 6) | Control participants (n = 30) | Total cohort (n = 262) | P * | |
|---|---|---|---|---|
| Waist and skinfold measures | ||||
| Waist, cm | 89.5 (13.3) | 84.9 (13.1) | 89.5 (13.9) | 0.4 |
| Abdominal, mm | 31.8 (7.5) | 24.5 (10.8) | 29.0 (9.6) | 0.1 |
| Chest, mm | 13.9 (7.2) | 10.5 (6.9) | 12.6 (6.3) | 0.2 |
| Calf skinfold, mm | 18.4 (8.1) | 15.2 (8.8) | 18.0 (8.6) | 0.2 |
| Tricep skinfold, mm | 22.2 (11.6) | 15.3 (7.6) | 18.1 (8.5) | 0.1 |
| Adiposity measures via DEXA | ||||
| Total fat, kg | 26.0 (11.0) | 23.0 (8.9) | 25.3 (10.3) | 0.5 |
| Trunk fat, kg | 13.1 (5.8) | 11.0 (4.9) | 12.3 (5.4) | 0.5 |
| Total lean, kg | 39.4 (15.2) | 38.9 (12.0) | 38.0 (10.5) | 0.9 |
| Total bone, kg | 0.9 (0.1) | 0.9 (0.1) | 0.93 (0.1) | 0.9 |
| Percentage total fat | 38.8 (5.9) | 35.6 (6.8) | 38.2 (6.5) | 0.3 |
| Central adiposity via MRI | ||||
| IAAT, cm2 | 59.0 (13.0) | 40.3 (19.7) | 47.9 (21.9) | 0.1 |
| SAAT, cm2 | 371.6 (113.9) | 312.4 (145.7) | 337.0 (148.5) | 0.5 |
| IAAT to SAAT | 0.2 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.4 |
| 2-h OGTT | ||||
| Fasting glucose, mg/dL | 88.4 (4.2) | 93.5 (6.3) | 93.0 (5.9) | 0.1 |
| 2-h glucose, mg/dL | 136.2 (17.2) | 130.5 (19.4) | 123.2 (17.9) | 0.5 |
| Fasting insulin, μU/mL | 25.5 (10.2) | 20.0 (10.7) | 19.2 (11.5) | 0.2 |
| 2-h insulin, μU/mL | 184.3 (153.9) | 195.3 (150.8) | 145.6 (122.7) | 0.9 |
| Glucose AUC, mg*h/dL | 288.7 (46.4) | 281.5 (33.8) | 266.7 (32.8) | 0.7 |
| Insulin AUC, μU*h/mL | 349.1 (216.2) | 364.4 (271.1) | 307.4 (193.4) | 0.9 |
| FSIVGTT | ||||
| SI, ×10−4 min−1/(μU/mL) | 1.6 (1.0) | 1.8 (1.3) | 2.1 (1.4) | 0.4 |
| AIR, μU/mL | 1,262.0 (494.3) | 1,549.6 (1,119.9) | 1,688.6 (1,226.1) | 0.6 |
| DI, ×10−4 m−1 | 1,950.5 (1,246.9) | 1,867.9 (870.7) | 2,499.3 (1,163.2) | 0.9 |
| SG, %/min | 0.01 (0.01) | 0.02 (0.01) | 0.02 (0.02) | 0.4 |
Data are presented as mean (SD). Control participants were selected with propensity score–matching sex, BMI, and pubertal status at ratio of five control participants per case patient.
IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SG, glucose effectiveness index.
Statistical comparison between group differences for case patients versus control participants based on two-sample t test.
Insulin Response Measures
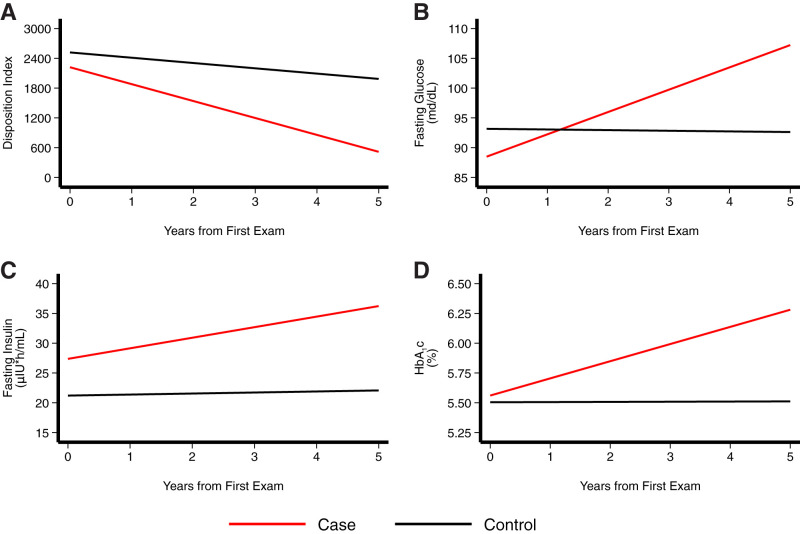
All six case patients had a >85% reduction from baseline in DI (with a 2× greater decrease in AIR), measured from FSIVGTT data, with a significantly greater increase in fasting glucose and HbA1c over time compared with the extended cohort (Table 3 and Figs. 1–3). Table 4 summarizes the mean slope for DI across the three groups, adjusted for baseline values. The mean slope for DI was −341.7 units per year in case patients compared with −15.2 units per year in control participants and −106.7 units per year in the extended cohort. The effect size for this difference is 0.76. Case patients had a 13.3% annual decline in DI compared with a 4.2% decline in matched control participants. Figure 1 summarizes the linear relationship between rate of change in DI, fasting glucose, fasting insulin, and HbA1c and time as a predictor of conversion between case patients and the extended cohort. The annual rate of increase in fasting glucose level was significantly higher in case patients (+3.8 mg/dL/year) compared with the extended cohort (−0.01 mg/dL/year; P < 0.0001) (Fig. 1). Figures 2 and 3 summarize the linear relationship between rate of change in DI and time. There was an inverse correlation between rate of change in DI and rate of change in baseline fasting glucose, insulin, 2-h insulin, glucose AUC, and insulin AUC for both case patients and the extended cohort (Supplementary Fig. 1).
Table 3.
Logistic binomial regression was used to evaluate rates of change in insulin and adiposity measures over time between case patients (n = 6) and extended cohort (n = 256)
| Outcome variable | Extended cohort | Case patients | P | ||
|---|---|---|---|---|---|
| Rate of change | SD | Rate of change | SD | ||
| Metabolic | |||||
| Fasting glucose, mg/dL | −0.01 | 0.1 | 3.8 | 0.6 | 0 |
| HbA1c, % | −0.009 | 0.005 | 0.07 | 0.03 | 0.01 |
| DI | −106.7 | 18.2 | −340.7 | 115.4 | 0.045 |
| Glucose AUC, mg × h/dL | −3.0 | 0.6 | 1.9 | 3.7 | 0.1 |
| Fasting insulin, μIU/mL | 0.3 | 0.2 | 1.8 | 1.2 | 0.2 |
| AIR, μU/mL | −58.368 | 14.4 | −161.2 | 88.4 | 0.2 |
| 2-h insulin, μIU/mL | −5.3 | 2.1 | 0.3 | 13.2 | 0.6 |
| Insulin AUC, μIU × h/mL | −7.2 | 3.1 | −0.2 | 19.1 | 0.7 |
| 2-h glucose, mg × h/dL | −1.3 | 0.4 | −0.8 | 2.3 | 0.8 |
| SG, %/min | 0 | 0 | −0.001 | 0.002 | 0.8 |
| SI, ×10−4 min−1/(μU/mL) | −0.087 | 0.02 | −0.08 | 0.1 | 0.9 |
| Body composition | |||||
| Total bone mineral density, g/cm2 | 0.05 | 0.001 | 0.04 | 0.01 | 0.01 |
| WC, cm | 1.4 | 0.1 | 2.9 | 0.6 | 0.02 |
| Total bone mineral content, g | 193.0 | 3.8 | 151.9 | 19.6 | 0.04 |
| Trunk fat, kg | 0.7 | 0.05 | 1.5 | 0.3 | 0.02 |
| Waist-to-hip ratio | −0.01 | 0.001 | −0.002 | 0.01 | 0.06 |
| Total fat mass, % | −0.8 | 0.08 | −0.02 | 0.4 | 0.07 |
| Total fat mass, kg | 1.355 | 0.1 | 2.2 | 0.4 | 0.1 |
| Total fat mass, g | 1,355.3 | 95.1 | 2,184.7 | 498.3 | 0.1 |
| SAAT, cm2 | 17.2 | 1.7 | 34.5 | 10.5 | 0.1 |
| BMI percentile | −0.5 | 0.09 | 0.05 | 0.5 | 0.2 |
| BMI, kg/m2 | 0.8 | 0.1 | 1.2 | 0.3 | 0.2 |
| Calf subcutaneous fat, g | 0.5 | 0.2 | 1.839 | 0.9 | 0.2 |
| BMI z score, SD | −0.03 | 0.005 | 0.004 | 0.03 | 0.3 |
| Excess percent of 95th percentile, % | −0.8 | 0.2 | 0.2 | 1.1 | 0.3 |
| IAAT-to-SAAT ratio | −0.01 | 0.001 | −0.02 | 0.01 | 0.6 |
| IAAT, cm2 | −1.9 | 0.3 | −1.4 | 2.2 | 0.7 |
| Abdominal subcutaneous fat, g | 3.4 | 0.2 | 3.8 | 1.1 | 0.7 |
| Chest subcutaneous fat, g | 0.9 | 0.2 | 0.8 | 0.7 | 0.8 |
Analysis was performed on unadjusted data, as well as with adjustment for age, sex, BMI, and Tanner stage. Bold font indicates significance.
IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SG, glucose effectiveness index.
Figure 1.
Annual changes in DI (A), fasting glucose (B), fasting insulin (C), and HbA1c fasting glucose (D) over 5 years in youth who converted to T2D (case patients; n = 6) compared with the extended cohort (n = 256) were examined by testing the interaction between diabetes status and time in the mixed-effects model. Analysis was performed on unadjusted data, as well as with adjustment for age, sex, BMI, and Tanner stage. In addition, adjustment for total fat, intra-abdominal adipose tissue, and other potential covariates was considered to examine whether and how much the differences in changes in insulin resistance, insulin secretion, and DI between diabetes status are mediated by changes in the covariates.
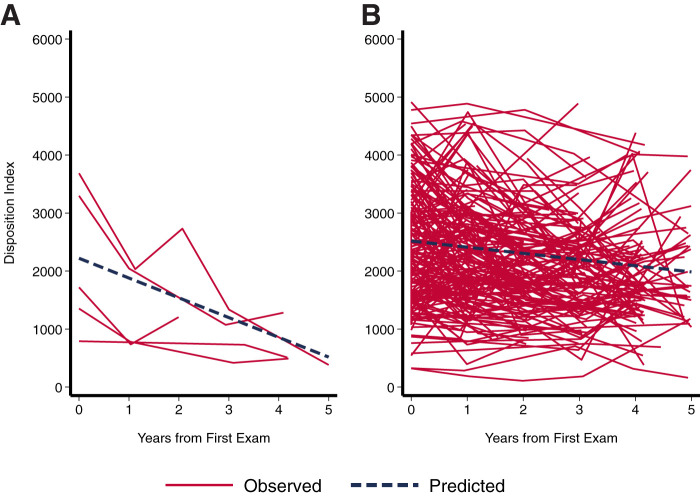
Figure 3.
Change in DI over 5 years in youth who converted to T2D (case patients; n = 6) (A) compared with the extended cohort (n = 256) (B) with mixed-effects growth models.
Table 4.
Mixed-effects growth models used to compare rate of change in DI in youth who converted to T2D over study period (case patients) compared with control participants and extended cohort
| Case patients (n = 6) | Comparison | |||||
|---|---|---|---|---|---|---|
| Control participants (n = 30) | Extended cohort (n = 256) | |||||
| Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | |
| Model 1* | −330.2 | −530.0, −130.3 | −32.2 | −123.0, 58.6 | −110.1 | −149.5, −70.7 |
| Model 2† | −341.7 | −529.3, −154.1 | −15.2 | −99.1, 68.7 | −106.7 | −142.3, −71.1 |
Total N = 262.
Unadjusted for baseline DI.
Adjusted for baseline DI.
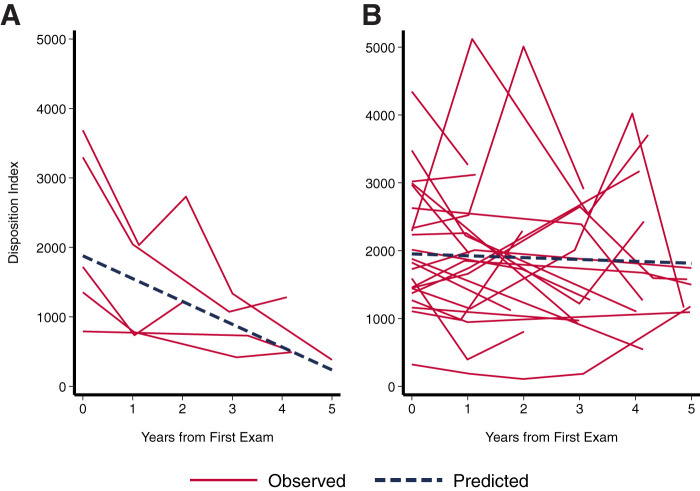
Figure 2.
Change in DI over 5 years in youth who converted to T2D (case patients; n = 6) (A) compared with control participants (n = 30) (B) with mixed-effects growth models.
Association Between Rate of Change in DI and Adiposity Measures
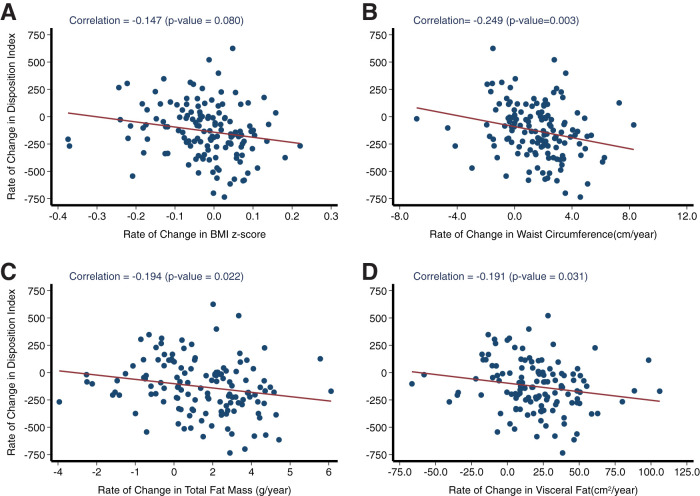
There were no significant differences in any anthropometric measures, WC, body composition data by DEXA, or abdominal fat distribution by MRI at baseline across groups (Table 2). When compared with the extended cohort, case patients had significantly greater rates of increase in trunk fat and WC over time, which were associated with a slower accrual of bone mineral content and density (Table 3). A Pearson correlation coefficient was computed to assess the linear relationship between rate of change in DI and anthropometric and body composition measures across the total cohort. There was a significant negative correlation between rate of change in DI and rates of change over time in WC (r = −0.25; P = 0.003) (Fig. 4B), total fat mass (g/year; r = −0.2; P = 0.02) (Fig. 4C), and visceral fat (cm2/year; r = −0.2; P = 0.03) (Fig. 4D). There was an inverse correlation between rate of change in DI and rates of change in BMI (as assessed by BMI z score, raw BMI, and percentage BMIp95), WC, total fat mass, subcutaneous abdominal adipose tissue, intra-abdominal adipose tissue for both case patients and the extended cohort (Table 4). Sex-specific analyses for DI and adiposity showed that rates of change in DI and adiposity measures did not differ between male and female participants.
Figure 4.
Pearson correlation coefficient was used to evaluate the association between rate of change in DI and rate of change in BMI z score (A), WC (B), total fat mass (C), and intra-abdominal adipose tissue (D) for the entire cohort (n = 262).
Discussion
The SOLAR Diabetes Project, conducted between 2000 and 2015, set out to evaluate factors contributing to the development of T2D in Latino children with overweight and obesity during pubertal growth and development. The primary aim was to examine mechanisms underlying changes in DI that might contribute to the development of T2D during this critical period of growth. In this report, we present a detailed comparison of metabolic and physiological differences in youth who converted to T2D versus control participants and the extended cohort. These results highlight four major findings: 1) a lower-than-expected conversion rate was seen, with only 2% of Latino youth with overweight and obesity and a positive family history of T2D developing T2D over 5 years; 2) a rapid decline in DI, most likely driven by a decline in AIR, was associated with conversion to T2D, suggesting limited ability for β-cell compensation; 3) youth who converted to T2D had a significantly higher annual increase in fasting glucose, HbA1c, waist circumference, and trunk fat over time than the extended cohort; and 4) there was an inverse correlation between rate of decline in DI over time and rates of increase in several adiposity measures over time. In contrast to our hypothesis, we did not find evidence that conversion to T2D was associated with baseline obesity or glycemic indices; rather, rates of increase in adiposity outcomes were negatively correlated with a rapid decline in DI and increase in fasting glucose over time.
Low Conversion Rate to T2D
Previous work from this study group reported impaired glucose tolerance in 20–30% of Latino children with overweight and obesity, as well as severe insulin resistance (14,19,23). Despite the growing prevalence of youth-onset T2D, there is a paucity of literature describing the annual rate of decline in DI in at-risk youth before conversion. Our findings show that youth who converted to T2D over the study period experienced an annual decline in DI of 13.3% compared with a decline of 4.2% in control participants. For further context, the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study reported that youth with T2D have a 20–35% annual decline in DI, whereas the SEARCH study showed rates that varied between 6 and 30% annually (36,37). Both the TODAY and SEARCH studies showed that there is great heterogeneity in the speed of DI decline; some youth have delayed progression, whereas in others progression is quite expedited (38). Conversely, adults with T2D seem to have a more consistent annual decline in DI of ∼7–8% annually (36,37). These findings have clinical implications regarding risk stratification and targeted treatment strategies for high-risk cohorts, suggesting that treatments that can slow the rate of decline in DI (e.g., target pharmacotherapies used in pediatric obesity management that increase insulin secretion and synthesis) may be beneficial in delaying or preventing the development of T2D (4,5).
Rapid Declines in DI and Glycemic Status in Those Developing T2D
Both adult and pediatric data have demonstrated that change in DI over time is a key pathophysiological component of conversion to T2D (10,27,39). In alignment with this concept, the current analysis showed that youth who developed T2D had a three times greater rate of decrease in DI compared with control participants. Of note, although not statically significant, youth who developed T2D had a less healthy metabolic profile at baseline than the other groups, as indicated by a higher fasting insulin level with associated higher 2-h glucose level and lower SI, AIR, and DI at baseline. Furthermore, consistent with previous literature, in this cohort, there was an inverse correlation between rate of decline in DI over time and fasting glucose, insulin, 2-h glucose and insulin, and glucose and insulin AUC concentrations (26,28,30). Youth who developed T2D had greater increases in fasting glucose and HbA1c over time compared with the extended cohort. The current findings further reinforce previous cross-sectional data from this cohort, which show that both impaired fasting glucose and impaired glucose tolerance were associated with declining DI (13,14). Consistent with the present data, past analyses from this cohort and others have shown that youth with obesity and normal glucose tolerance who have a glucose level at the upper limit of normal, both fasting and stimulated (fasting glucose between 90 and 100 mg/dL, 1-h glucose >155 mg/dL, and 2-h glucose between 120 and 140 mg/dL), have a greater likelihood of developing impaired fasting glucose and T2D over time compared with youth with obesity with fasting glucose between 70 and 80 mg/dL and 2-h glucose between 100 and 119 mg/dL (15–17,40–42). A recent pediatric study investigating the association between clamp DI and glucose AUC showed that there was a significant inverse relationship between clamp DI and glucose AUC and that a 30–75% improvement in DI was associated with restoration of glycemia in youth with obesity (15,43). These findings are consistent with the theory that rate of change in DI seems to be the differentiating factor between normoglycemia and T2D and that baseline glycemic status is an early predictor of future rapid decline and thus development of T2D. It is important to highlight the clinical indications of these findings for youth at risk of T2D. The DI measured in this study was obtained from an IVGTT, which is impractical to collect in the clinical setting, and we failed to see any relationships with OGTT measures. However, we were limited to the use of a traditional OGTT sampling protocol, whereas there is growing evidence to suggest that frequently sampled OGTT protocols that include early measures of the insulin and C-peptide response may allow for more accurate modeling of glucose-stimulated insulin secretion (44,45) and be more sensitive than oral disposition index in detecting dysglycemia. We therefore recommend that future studies include OGTTs with rapid initial sampling, because this may be a more cost-effective and practical option to identify a screening tool that is accurate and able to be disseminated in clinical practice.
Correlation Between Rapid Decline in DI and Increased Adiposity Over Time
Obesity is associated with increased insulin concentrations during both fasting and stimulated states. Previous studies have proposed that increased insulin secretion contributes to insulin resistance and ultimately conversion to T2D in individuals with obesity (46,47). However, this hypothesis has been challenged by recent work that has shown that increased insulin secretion in individuals with obesity is associated with increased adiposity and is not only a compensatory response to insulin resistance but also an independent risk factor for conversion to T2D (45). There is evidence in adult cohorts that the association between excess adiposity and insulin resistance results from an inability of peripheral adipose tissue to store excess energy intake, which leads to ectopic fat deposition that alters metabolic pathways and thus disturbs and ultimately destroys β-cells (48,49). Previous work from this study group has shown that total fat mass was independently and positively related to fasting insulin and negatively related to insulin sensitivity but was not related to AIR in this at-risk youth cohort (19,23,50). Although not statistically significant, given the small conversion rate in this study, youth who converted to T2D tended to have higher adiposity across all metrics at baseline compared with control participants, and there was an inverse linear relationship between rate of change in DI and adiposity measures across the total cohort. Given the close correlation between general adiposity and ectopic fat stores, it can be postulated that increasing overall adiposity is a surrogate for increased ectopic fat deposition, supporting the hypothesis that accumulation of ectopic fat could be an important factor contributing to BCF; however, those metrics were not measured in the current study. When comparing the inverse correlation between rate of change in DI and adiposity measures between case patients and control participants, those youth who converted to T2D had much stronger correlations across all adiposity measures over time. Case patients had significantly greater rates of increase in trunk fat and WC over time, consistent with previous literature on youth in obesity; they were also found to have slower accrual of bone mineral content and density over the study period. This strong correlation matches what the RISE (Restoring Insulin Secretion) study previously demonstrated: that youth with T2D have more severe obesity than older adults with T2D (37,51). Our findings further reinforce the relationship between rates of increase in adiposity measures and decline in DI over time in high-risk cohorts.
Limitations
To our knowledge, this is the first prospective longitudinal study in Latino youth at risk of T2D to examine change in insulin sensitivity indexes and adiposity over time in relation to development of T2D. We focused on a high-risk cohort, assessed diabetes risk using robust measures of insulin sensitivity and secretion from the FSIVGTT to estimate DI, controlled for the potential confounding effects of maturation and body composition, and used powerful statistical modeling techniques to account for the variance of change across time. These findings are essential to inform future trials investigating the pathophysiology of youth-onset T2D and design interventions that aim to restore BCF in the hope of preventing or delaying conversion in this high-risk cohort. Despite these strengths, we acknowledge potential limitations that should be considered. First, given the longitudinal nature of the study, not all participants were available for every year of testing, so controlling for missing data by linear mixed modeling was necessary. In addition, because only annual measures were obtained, we could not determine exactly when pubertal progression was occurring or how that might relate to change in metabolic risk. This is an important component of the multifactorial pathophysiology that heralds youth-onset conversion to T2D and requires further investigation. However, considering the relative lack of longitudinal data in this high-risk cohort and the increased efforts in developing targeted interventions to preserve BCF, we believe our data still provide much-needed information and potential guidance concerning power calculations in designing such trials. Another limitation of the study is the small sample size of those converting to T2D. Because of the low conversion rate, we opted to evaluate baseline differences and rates of change in insulin indexes and adiposity. Future studies will need to recruit much larger cohorts observed over longer periods to definitively determine how to predict the development of overt diabetes in youth. Lastly, a major limitation of our current study is that the cohort was limited to Latino youth with obesity and a positive family history of T2D, making it difficult to generalize our findings to other segments of the population at risk of developing pediatric T2D. Additional longitudinal reference data in other cohorts would be useful for comparative purposes. The present information might prove beneficial in designing large-scale interventional trials in youth with diverse ethnic backgrounds.
Conclusions
These findings suggest that development of T2D in Latino youth with obesity is preceded by a substantial and rapid decrease in DI, increases in fasting glucose and HbA1c, and increased adiposity measures. These results suggest that it is the rapid decline in DI and the rate of adiposity increase that seem to be the differentiating factors in the development of T2D in this age group.
Article Information
Acknowledgments. The authors acknowledge all the families who participated in this study, as well as Quintilla Avilla, Christina Ayala, Rosa Rangel, and numerous project coordinators and clinical research staff, without whom this study would not have been possible.
Funding. This work was supported in part by grants from the National Center for Advancing Translational Sciences (UL1TR001855), the National Institute for Diabetes Digestive and Kidney Diseases (R01 DK059211 and K23DK134801), and the National Institute on Minority Health and Health Disparities of the U.S. National Institutes of Health (P50 MD017344).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors conceptualized and designed the study, drafted the initial manuscript, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. M.I.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22325254.
References
- 1. Lawrence JM, Divers J, Isom S, et al.; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021;326:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler AM. Social determinants of health and racial/ethnic disparities in type 2 diabetes in youth. Curr Diab Rep 2017;17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arslanian SA, El Ghormli L, Kim JY, et al.; RISE Consortium . OGTT glucose response curves, insulin sensitivity, and β-cell function in RISE: comparison between youth and adults at randomization and in response to interventions to preserve β-cell function. Diabetes Care 2021;44:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RISE Consortium; RISE Consortium Investigators . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults: The RISE consortium. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clin Proc 2014;89:806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeitler P. Progress in understanding youth-onset type 2 diabetes in the United States: recent lessons from clinical trials. World J Pediatr 2019;15:315–321 [DOI] [PubMed] [Google Scholar]
- 8. Yang W, Wang C. Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:2014–2015 [DOI] [PubMed] [Google Scholar]
- 9. Chen ME, Chandramouli AG, Considine RV, Hannon TS, Mather KJ. Comparison of β-cell function between overweight/obese adults and adolescents across the spectrum of glycemia. Diabetes Care 2018;41:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010;33:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 12. Ball GDC, Weigensberg MJ, Cruz ML, Shaibi GQ, Kobaissi HA, Goran MI. Insulin sensitivity, insulin secretion and β-cell function during puberty in overweight Hispanic children with a family history of type 2 diabetes. Int J Obes 2005;29:1471–1477 [DOI] [PubMed] [Google Scholar]
- 13. Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab 2004;89:207–212 [DOI] [PubMed] [Google Scholar]
- 14. Weigensberg MJ, Ball GDC, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care 2005;28:2519–2524 [DOI] [PubMed] [Google Scholar]
- 15. Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016;39:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JY, Goran MI, Toledo-Corral CM, Weigensberg MJ, Choi M, Shaibi GQ. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care 2013;36:1681–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peña A, Kim JY, Reyes JA, et al. Changes in OGTT-derived biomarkers in response to lifestyle intervention among Latino adolescents with obesity. Pediatr Obes 2022;17:e12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Utzschneider KM, Younes N, Rasouli N, et al. Shape of the OGTT glucose response curve: relationship with β-cell function and differences by sex, race, and BMI in adults with early type 2 diabetes treated with metformin. BMJ Open Diabetes Res Care 2021;9:e002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alderete TL, Toledo-Corral CM, Goran MI. Metabolic basis of ethnic differences in diabetes risk in overweight and obese youth. Curr Diab Rep 2014;14:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ. Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol 2015;52:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah AS, Nadeau KJ. The changing face of paediatric diabetes. Diabetologia 2020;63:683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 23. Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah AS, Nadeau KJ, Dabelea D, Redondo MJ. Spectrum of phenotypes and causes of type 2 diabetes in children. Annu Rev Med 2022;73:501–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 26. Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tfayli H, Lee S, Arslanian S. Declining β-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 2010;33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JY, Tfayli H, Bacha F, Lee S, Gebara N, Arslanian S. β-cell impairment and clinically meaningful alterations in glycemia in obese youth across the glucose tolerance spectrum. Metabolism 2020;112:154346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alderete TL, Habre R, Toledo-Corral CM, et al. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes 2017;66:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obes Res 2001;9:432–442 [DOI] [PubMed] [Google Scholar]
- 33. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pols H. August Hollingshead and Frederick Redlich: poverty, socioeconomic status, and mental illness. Am J Public Health 2007;97:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dabelea D, Mayer-Davis EJ, Andrews JS, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 2012;55:3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis 2014;5:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arslanian S, El Ghormli L, Bacha F, et al.; TODAY Study Group . Adiponectin, insulin sensitivity, β-cell function, and racial/ethnic disparity in treatment failure rates in TODAY. Diabetes Care 2017;40:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tfayli H, Lee SJ, Bacha F, Arslanian S. One-hour plasma glucose concentration during the OGTT: what does it tell about β-cell function relative to insulin sensitivity in overweight/obese children? Pediatr Diabetes 2011;12:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soltero EG, Ayers SL, Avalos MA, et al. Theoretical mediators of diabetes risk and quality of life following a diabetes prevention program for Latino youth with obesity. Am J Health Promot 2021;35:939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arslanian S, El Ghormli L, Kim JY, et al. The shape of the glucose response curve during an oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 2019;42:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012;35:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittendorfer B, Patterson BW, Smith GI, Yoshino M, Klein S. β cell function and plasma insulin clearance in people with obesity and different glycemic status. J Clin Invest 2022;132:e154068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Vliet S, Koh HE, Patterson BW, et al. Obesity is associated with increased basal and postprandial β-cell insulin secretion even in the absence of insulin resistance. Diabetes 2020;69:2112–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sbraccia P, D’Adamo M, Guglielmi V. Is type 2 diabetes an adiposity-based metabolic disease? From the origin of insulin resistance to the concept of dysfunctional adipose tissue. Eat Weight Disord 2021;26:2429–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vajravelu ME, Kindler JM, Zemel BS, et al. Visceral adiposity is related to insulin sensitivity and inflammation in adolescents with obesity and mild sleep disordered breathing. J Pediatr Endocrinol Metab 2022;35:1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining β-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lotta LA, Gulati P, Day FR, et al.; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium . Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Toledo-Corral CM, Vargas LG, Goran MI, Weigensberg MJ. Hemoglobin A1c above threshold level is associated with decreased β-cell function in overweight Latino youth. J Pediatr 2012;160:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arslanian SA, Kahn SE, Buchanan TA, et al.; RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]