Abstract
Background:
There is tentative evidence to support the analgesic effect of transcranial direct current stimulation (tDCS) in fibromyalgia (FM), with large variability in the effect size encountered in different clinical trials. Understanding the source of the variability and exploring how it relates to the clinical results could characterize effective neuromodulation protocols and ultimately guide care in FM pain. The primary objective of this study was to determine the effect of tDCS in FM pain as compared to sham tDCS. The secondary objective was to explore the relationship of methodology, population, and intervention factors and the analgesic effect of tDCS in FM.
Materials and Methods:
For the primary objective, a systematic review was conducted according to PRISMA guidelines. Randomized clinical trials (RCTs) investigating tDCS as an intervention for FM pain were searched at Medline, EMBASE, and the Web Of Science. Studies were excluded if they used cross-over designs, or if they did not use tDCS as an intervention for pain or did not measure clinical pain. Analysis for the main outcome was performed using a random-effects model. Risk of bias and Evidence certainty were assessed for all studies using Cochrane Risk of Bias and GRADE tools. For the secondary objective, a meta-regression was conducted to explore methodology, population, and intervention factors potentially related to the effect size.
Results:
Sixteen RCTs were included. Six studies presented a high risk of bias. Significant reduction in pain scores were found for FM (SMD=1.22; 95% CI: 0.80 to 1.65, p-value<0.001). Subgroup analysis considering tDCS as a neural target revealed no differences between common neural sites. Meta-regression revealed that the duration of the tDCS protocol in weeks was the only factor associated with the effect size (ES), where protocols that lasted four weeks or longer reported larger ES than shorter protocols.
Conclusion:
Results suggest an analgesic effect of tDCS for FM. tDCS protocols that last four weeks or more may be associated with larger effect sizes. Definite conclusions are inadequate given the large heterogeneity and limited quality of evidence of the included studies.
Keywords: Systematic review, Fibromyalgia, Transcranial Direct Current Stimulation, Meta-Analysis, Meta-Regression
INTRODUCTION
Five million people suffer from fibromyalgia (FM)1 in the United States. Annual treatment costs exceeds 29 billion dollars, with a large amount spent treating secondary symptoms2. Widespread chronic pain is the main symptom and chief complaint from FM while fatigue, impaired cognition, disrupted sleep and other somatic complaints are also common3,4. The pathophysiology of pain in FM is complex and known to be associated with central sensitization, a hyperalgesia or excessive response to painful stimulation secondary to altered nociception at the central nervous system (CNS)5. In FM and other musculoskeletal conditions, chronic pain results from an imbalance between excitatory and inhibitory pain pathways. Recent evidence suggests that pain inhibitory pathways are affected in FM. For example, patients with FM demonstrate lower conditioned pain modulation (CPM), a standardized assessment that can test pain inhibitory efficacy. Indeed, the rate of patients with FM reporting pain facilitation during CPM has been found to be significantly increased compared with that of the controls (41.7% vs 21.2%)6. Based on the current literature, there is evidence to support that for some patients with FM, the endogenous pain regulatory system is impaired7–9. There is also evidence of central nervous system changes in FM that are associated with a deficit in the inhibitory control, namely abnormal cortical excitability as expressed by decreased short intracortical inhibition and facilitation and increased resting motor threshold10 assessed by Transcranial Magnetic Stimulation. This is thought to reflect an inhibitory deficit that is associated with altered thalamic anatomy and activity11, which may result in abnormal thalamocortical circuits, as evidenced by the association between central pain and thalamic dysrhythmia12–14. Therefore, efforts are being undertaken to develop novel interventions that target these inhibitory deficits underlying pain maintenance mechanisms in patients with FM, as well as for other chronic pain conditions associated with a deficit in the regulatory pain system.
Targeted non-pharmacological therapies that modulate cortical excitability and modify activity of the CNS that modulate the descending pain inhibitory system are new research directions that could affect analgesia. Non-invasive brain stimulation (NIBS) techniques, such transcranial direct current stimulation (tDCS), are safe, cost-effective, and powerful neuromodulatory tools, which have provided pain relief in several chronic pain conditions such as FM15, knee osteoarthritis16,17, acute post-stroke pain18 and chronic pain after spinal cord injury19, among others, with absent or minimal side effects20,21. Currently, there is tentative evidence to support the analgesic effect of tDCS as compared to sham in FM, with large variability in the effect size (ES) encountered in different clinical trials22. In fact, this variability has raised questions about the validity of tDCS as an effective treatment option for pain in FM. Understanding the source of the variability and explore how it relates to the clinical results may characterize effective neuromodulation protocols and ultimately guide care in FM pain. For example, some clinical trials have compared different protocols of tDCS in an attempt to identify the protocol that produces better responses with a smaller number of sessions, consequently, minimizing the occurrence of adverse effects and reducing the total time and cost of the therapeutic intervention23. Other trials have investigated different tDCS montage, or electrode positioning, protocols in an attempt to determine the best short-term analgesic effect24. This supports that such factors may be potential sources of variability in effect. In addition to the intervention, different factors related to the study population, such as gender, and the presence of higher levels of psychosocial comorbidities including anxiety and depression, can predict outcomes following other interventions for FM pain25. Lastly, study methodology characteristics, such as inadequately powered studies, may also contribute to this observed variability in effect; and it is unknown to what degree each of these factors is contributing to it. To the best of our knowledge, no research studies, including systematic reviews, have directly explored specific factors that may be related to the analgesic effect of tDCS in FM, possibly contributing to this response variability. Identifying factors associated with tDCS clinical outcomes in FM may improve the development of current intervention protocols and help advance the design of future clinical trials for other chronic musculoskeletal pain conditions that share similar pain processing mechanisms. Although there are previous systematic reviews that have investigated the effect of tDCS on FM pain and could have explored these questions, recent clinical trials22,23,26 with conflicting results have not been included in these. In addition, recent reviews did not consider the ES calculated separately from different active tDCS intervention groups used in multi-arm intervention trials. Both of these aspects may relevantly affect a meta-analysis result.
To that end, the current study had the primary objective of determining the effect of tDCS in FM pain as compared to sham tDCS using a systematic review with meta-analysis. We hypothesize that tDCS will show a significant analgesic effect on FM pain as compared to sham tDCS. The secondary objective was to explore the relationship methodology, population, and intervention factors and the analgesic effect of tDCS in FM using meta-regression analysis. We hypothesize that factors such as the presence of higher levels of depression and anxiety, and the number of tDCS sessions will be significantly associated with the analgesic effect of tDCS in FM.
MATERIALS AND METHODS
To determine the analgesic effect of tDCS in FM, a systematic review and meta-analysis of randomized clinical trials (RCTs) was performed. To explore the relationship between the methodology, population, and intervention-related characteristics used in the clinical trials, and the analgesic effect of tDCS in FM, a meta-regression analysis was used to analyze potential sources of ES heterogeneity originating from these factors. This systematic review was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines27.
Search Strategy
Medline, EMBASE, and the Web Of Science databases were searched for potential articles. A broad search strategy was used to guarantee the detection of all potential articles (Appendix 1). Search terms included multiple variants of the terms Transcranial direct current stimulation, pain, and FM, in addition to its Medical Subject Headings (MeSH). The final search strategy was reviewed by a librarian specialized in systematic reviews and the search was performed by one author (PT), in all databases at the same day. The search inception date to all databases was February 3rd, 2022, and was performed with no language restriction. Additionally, a snowball searching method was used on any systematic reviews or randomized trials on the topic as well as verification with senior authors who were (WC, FF) experts in the field to check if any know articles were missed.
Selection criteria
Research articles investigating the effects of tDCS on FM were included if they met the following criteria: (1) utilized tDCS alone or combined with any other therapy as a treatment tool for FM pain, including exercise, cognitive-behavioral or other neurostimulation interventions (2) measured clinical pain as a primary or secondary outcome via visual analog or numeric scale or another clinical self-reported pain tool that measured pain perception; (3) were RCTs (not including cross-over designs). Journal articles were excluded if: (1) did not use sham tDCS as a comparison group; (2) did not measure clinical pain as an outcome measure; (3) animal studies; (4) oral or poster presentation abstracts.
Article Screening
The Covidence web-based systematic review software was used to organize and screen the search results. As a first level of screening, titles and abstracts were assessed to determine article eligibility. Then, full-text articles were reviewed for the inclusion and exclusion criteria. Two independent assessors (PT, KP), trained in clinical trial methodology and evidence synthesis, screened, and selected the final articles. Any non-unanimous criteria judgment among the two assessors would require a full-text review by each assessor to confirm their decision. If after full-text review, disagreement still existed, the full-text was reviewed by a senior assessor (FF) for an ultimate decision.
Data Extraction
Three authors performed the data extraction and entered it into a systematized spreadsheet that contained all the pre-determinant variables. Data on the factors related to the study methodology, population and intervention were extracted to create the variables used in the analysis (Table 1). If any study reported more than one pain outcome measure, any pain measurement used as a primary outcome of the study was prioritized and used in the meta-analysis. If pain was a secondary outcome of the study, then any continuous, ordinal pain outcome type, such as the visual analog scale or the numerical rating scale was considered before any categorical rating scale. This hierarchy was based on the common use of the visual and numerical rating scales by trialists, and to facilitate comparison among studies. If pain data was collected but not completely reported, the authors reached out to the first author of each study have the complete data necessary for the meta-analysis.
Table 1.
Considered factors related to the study methodology, population and intervention used to create the variables used in the analysis.
| Study Methodology | Population | Intervention |
|---|---|---|
| - Use of intention-to-treat analysis | - Age | - Use of tDCS with a combined intervention |
| - Size of intervention groups | - Duration of symptoms | - Type of combined intervention |
| - Dropouts in intervention groups | - Gender | - Timing of combined intervention |
| - Disease diagnostic definition criteria | - Race | - Total number of intervention sessions |
| - Blinding of study subject | - Body mass index | - Frequency of intervention sessions per week |
| - Blinding of intervention staff | - Number of subjects with another chronic illness | - Duration of tDCS intervention in weeks |
| - Baseline depression level | - Patient setting | |
| - Baseline anxiety level | - Type of tDCS montage | |
| - Baseline pain catastrophizing score | - Number of stimulation channels | |
| - Percentage of subjects using opioids | - Stimulation polarity | |
| - Percentage of subjects using gabapentinoids | - Active electrode location | |
| - Percentage of subjects using antidepressants | - tDCS current intensity parameter (mA) | |
| - Percentage of subjects using anticonvulsants | - tDCS session duration | |
| - Percentage of subjects using analgesics | - tDCS electrode surface area | |
| - Percentage of subjects using myorelaxants | ||
| - Disability score at baseline | ||
| - Sleep quality score at baseline | ||
| - Baseline pain Level (Active and Sham groups) |
As part of the data extraction, mean pain scores and respective variability estimates were collected from the included studies to be used in the meta-analysis. The web-based software WebPlotDigitizer (version 4.5) was used to extract data when articles did not include specific values in tables or in text. The primary outcome used was the mean difference in pain score between baseline and the primary time-point to which the study’s sample size was calculated for.
Risk of Bias Assessment
Two independent reviewers (PM, AM) trained in clinical trials methodology used the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2)28–30 to assess and report the risk of bias of the included studies. The tool is organized into different domains of bias that are related to trial design, conduct and reporting. Bias due to deviations from the intended intervention was judged based on both the assignment and the adherence to intervention. Overall bias was categorized into low risk, some concerns and high risk. Assessors rated bias independently and crosschecked results with each other. Any conflicting results were discussed to reach an ultimate decision.
Evidence certainty
Evidence certainty and quality of the evidence were assessed by two independent assessors (PT, KP) using the ‘Grading of Recommendations Assessment, Development, and Evaluation’ (GRADE) methodology. A Summary of findings table was created.
Publication Bias
Publication bias was explored using visual analysis of the funnel plot asymmetry and tested for significance by the Egger’s test for bias and the Begg’s test for small study effect with continuity corrected p-value to confirm the presence publication bias. If bias was present, adjustment was performed using the Duval and Tweedie31 nonparametric “trim and fill” method of accounting for publication bias in meta-analysis, to re-calculate the overall ES estimate.
Statistical Analysis
Main effect
Analysis of the analgesic effect of tDCS on pain in FM was reported as a pooled effect of all selected trials. A random-effects model was used with weights based on the DerSimonian and Laird method using standardized mean differences (SMD) as it was expected that the included studies used different pain scale measurements. Results are presented using SMD with their respective 95% confidence intervals. If a clinical trial had more than one active tDCS intervention protocol being investigated, ES was calculated for all separate intervention arms and normalized by the correlation value of 0.55 to account for the assumption of independent observations32. As the articles did not provide data on group correlations for the pain outcome at baseline, the value of 0.55 was used according to previous reference33. A sensitivity analysis using correlation values of 0.25, 0.50, and 0.75 was also performed. Between-study statistical heterogeneity was examined using the I2 and Cochran’s Q test. Values of I2 greater than 50% were considered evidence for substantial heterogeneity. Lastly, descriptive statistics were used to summarize the methodological, population and intervention characteristics collected from the included studies.
Subgroup Analysis
As it was expected that studies would vary in time intervals for measuring the pain outcome score, a sensitivity analysis was planned a priori for short and moderate-term follow-up data. To that end, a short-term and moderate-term follow-up result was defined using a four-week timepoint cutoff. The 4-week cutoff was chosen based on the median of all the follow-up used in all studies. Thus, if a pain outcome score was reported less than four weeks post intervention, the ES calculated for this timepoint was used to characterize a short-term result; and scores reported after four weeks would be used as moderate term results.
Additional subgroup analysis was planned a priori considering the placement of the active electrode location for the tDCS intervention. In addition, an analysis excluding the studies which fell outside of the publication bias funnel plot was also performed, as well as an analysis considering the use of any combined intervention together with tDCS. Lastly, a subgroup analysis based on the results of risk of bias analysis comparing the classifications of “high-risk” of bias studies versus “low-risk” and “some concerns” was performed.
Meta-Regression
Data on the factors related to the study methodology, population and intervention were used to explore potential sources of ES heterogeneity and a meta-regression analysis was used for both continuous and categorical variables. To best select the variables, meta-regression models were created following a model building process intended to lower the chances of type 1 error with multiple testing. First, univariate models were used to determine the potential most significant covariates, and to eliminate non-significant variables, diminishing the number of variables for the following stages of the analysis. For that, a relaxed regression coefficient significance р-value of p<0.2 was used. The chances of type 1 error with multiple independent testing was lowered by having less variables to build the final models34. Secondly, the significant variables were included in an initial multivariate model. Then, using a backward elimination-based approach, the variable with the largest non-significant p-value was excluded from the model until a final model with only significant coefficients (p<0.05) variables was achieved.
Observations with values greater than three standard deviations above or below the mean scores were considered outliers and excluded as per the Empirical Rule34. For all meta-regression models, the assumption of linearity was assessed by visual scatterplot analysis with a superimposed regression line. Homoscedasticity was checked by comparison of scatterplots of the standardized predicted values and standardized residuals36. Normality of the residuals was checked using the Shapiro-Wilk normality test37. Durbin Watson estimate, tolerance values, Cookś distance, variance inflation factor and Standardized DFBeta values were analyzed for regression diagnostics such as multicollinearity and influential cases. Due to the exploratory nature of the meta-regression analysis, the conservative approach to correct the significance level for multiple testing was not performed. All tests were two-tailed, using a p <0.05 threshold for significance, and were performed using Stata V17 BE Statistical Software (StataCorp. 2021. College Station, TX: StataCorp LLC.)
RESULTS
The result of our search strategy revealed 418 potential articles, while one study was identified using the snowball strategy. After duplicates were removed, 314 articles were considered for the first level of screening. After all levels of screening and selection, 16 articles were selected. Figure 1 shows the CONSORT diagram for the selection of the final articles.
Figure 1.
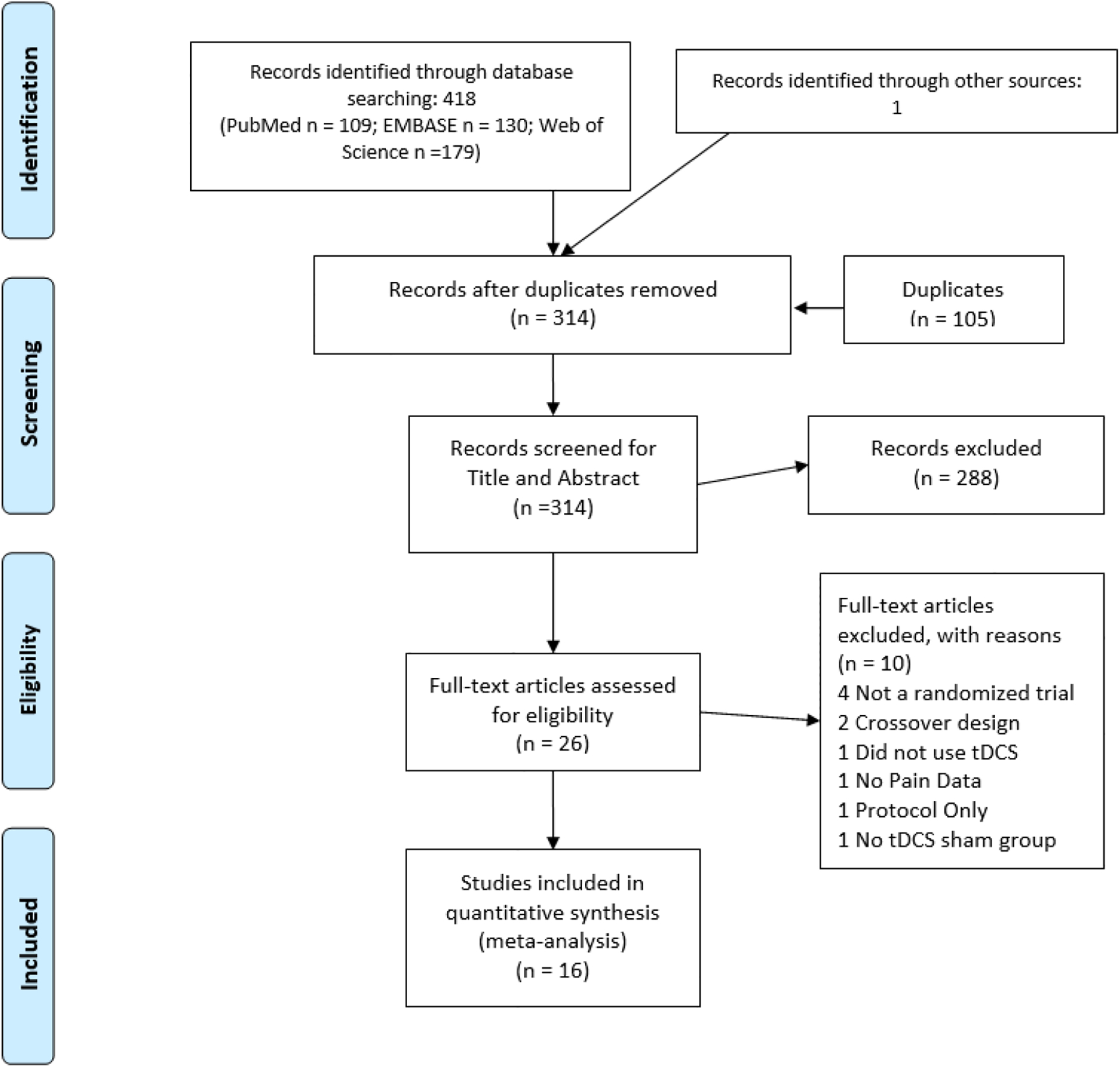
Flow diagram of study selection.
Studies were performed between 2006 and 2022 in seven different countries, 10 in Brazil23,24,26,38–44, two in Belgium45,46, and individual studies in Egypt47, Norway48, United States15, and Spain49. A total of 813 subjects were included in the analysis. Three studies45,46,48 used the pain numerical rating scale, two studies24,39 used the visual numerical scale, one study40 used the Short-Form 36 Health Questionnaire (SF36) pain subscale, while all other studies used the pain visual analog scale (10 studies). Only one multi-arm intervention study used the cathodal as the active polarity24. The two milliampere(mA) current intensity was the most used. Other current intensities included 1.5mA45,46 and 1mA43. Three studies applied tDCS in combination with other intervention39,41,44. Active electrode location varied mostly between the primary motor cortex region (M1) (11 studies)15,23,24,39,40,42–44,47–49 or the dorsolateral prefrontal cortex (DLPFC) (eight studies15,26,38,41,42,45,46,49). Some studies used both M1 and DLPFC as different active tDCS groups in the same study. The reference electrode locations varied and are listed on Table 2. Table 2 summarizes the characteristics of the selected studies used in the analysis.
Table 2.
Summary of the included RCTs that investigated the analgesic effect of tDCS on FM.
| Paper Year |
tDCS characteristics | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Active Groups (n) | Sham Group (n) | Active electrode location | Intensity (mA) | Polarity (Active) | Reference Electrode Location * | Session duration (min) | Number of sessions | Sessions / week | Duration of tDCS intervention (weeks) | Type of Combined intervention | Pain outcome used | Use of Mechanistic outcomes | Disability tool | Disability score at baseline (SD) |
|
| Bin Yoo 2018 45 | Belgium | 21 21 |
16 | OCC DLPFC + OCC |
1,5 2 |
Anode | O2 F3 |
20 | 8 | 2 | 4 | - | NRS | No | FIQ | Active OCC: 55.80 (14.45) Active OCC + DLPFC: 50.38 (14.85) Sham: 56.58 (13.86) |
| Brietzke 2019 26 | Brazil | 10 | 10 | DLPFC | 2 | Anode | F4 | 30 | 60 | 5 | 12 | - | VAS | Yes | FIQ | Active DLPFC: 71.7 (12) Sham: 63 9 (21.5) |
| Caumo 2021 38 | Brazil | 32 | 16 | DLPFC | 2 | Anode | F4 | 20 | 20 | 5 | 4 | - | VAS | Yes | FIQ | Active DLPFC: 70.2 (15.2) Shan: 65.88 (15.53) |
| Fagerlund 2014 48 | Norway | 24 | 24 | M1 | 2 | Anode | Fp2 | 20 | 5 | 5 | 1 | - | NRS | No | FIQ | Active M1: 57 (36.54) Sham: 48.41 (37.44) |
| Fregni 2006 15 | United States | 11 11 |
10 | M1 DLPFC |
2 | Anode | Fp2 | 20 | 5 | 5 | 1 | - | VAS | No | SF36-PF | Active M1: 32.1 (6.30) Active DLPFC: 30.4 (33.50) Sham: 30.3 (25.61) |
| Jales Junior 2015 43 | Brazil | 10 | 10 | M1 | 1 | Anode | Fp2 | 20 | 10 | 1 | 10 | - | VAS | Yes | SF36-PF | Active M1: 48 (16.36) Sham: 31 (23.7) |
| Khedr 2017 47 | Egypt | 18 | 18 | M1 | 2 | Anode | extra-cephalic (arm) | 20 | 10 | 5 | 2 | - | VAS | No | - | - |
| Matias 2022 44 | Brazil | 17 | 14 | M1 | 2 | Anode | Fp2 | 20 | 5 | 5 | 1 | Exercise-based | VAS | Yes | FIQ | Active M1: 67 (15.76) Sham: 73.44 (11.23) |
| Melo 2020 23 | Brazil | 13 12 |
13 | M1 | 2 | Anode | NR | 20 | 10 5 |
5 | 2 1 |
- | VAS | Yes | CIRS | - |
| Mendonça 2011 24 | Brazil | 6 6 6 6 |
6 | M1 APFC M1 APFC |
2 | Cathodal Cathodal Anode Anode |
C-T Spine Transition | 20 | 1 | 1 | 0.14 | - | VNS | Yes | - | - |
| Mendonça 2016 39 | Brazil | 15 | 15 | M1 | 2 | Anode | Fp2 | 20 | 5 | 5 | 1 | Exercise-based | VNS | Yes | SF36-PF | Active M1 + Exercise: 41 (4.8) Sham + Exercise: 45 (4.1) |
| Riberto 2011 40 | Brazil | 11 | 12 | M1 | 2 | Anode | Fp2 | 20 | 10 | 1 | 10 | - | SF36 (Pain) | No | FIQ | Active M1: 62.79 (15.03) Sham: 62.6 (18.08) |
| Samartin-Veiga 2021 49 | Spain | 34 33 33 |
30 | M1 DLPFC OIC |
2 | Anode | Fp2 Ear Lobe |
20 | 15 | 5 | 3 | - | FIQ and VAS | Yes | - | - |
| Santos 2018 41 | Brazil | 19 | 20 | DLPFC | 2 | Anode | Fp2 | 20 | 8 | 7 | 1.14 | Cognitive-behavioral based | VAS | Yes | FIQ | Active DLPFC: 63.43 (18.29) Sham: 66.16 (15.31) |
| To 2017 46 | Belgium | 15 11 |
16 | OCC DLPFC |
1.5 | Anode | O2 Fp2 |
20 | 8 | 2 | 4 | - | NRS | No | - | - |
| Valle 2009 42 | Brazil | 14 13 |
14 | M1 DLPFC |
2 | Anode | Fp2 | 20 | 10 | 5 | 2 | - | VAS | No | FIQ | Active M1: 90.95 (23.94) Active DLPFC: 92.62 (18.29) Sham: 96.28 (28.89) |
OCC: occipital region; PFC: prefrontal cortex; DLPFC: dorsolateral prefrontal cortex; M1: primary motor cortex; APFC: anterior prefrontal cortex; OIC: operculo-insular cortex; NRS: numeric rating scale; VAS: visual analog scale; VNS: vagus nerve stimulation; SF36: short form 36; FIQ: fibromyalgia impact questionnaire; CIRS: cumulative illness rating scale; ma: mili-Ampere; min: minutes; SD: standard deviation;
according to the EEG 1020 system;
NR: Not Reported
Risk of Bias Assessment
Our analysis detected varying levels of bias among the selected studies. The percentage agreement between the assessors were 95%. The percentage of studies in the classifications of high-risk, some concerns and low risk were respectively 37.5%, 37.5%, and 25%. The domains more frequently characterized as “some concerns” and “high risk” of bias were the ones evaluating the randomization (D1), deviations from intended interventions (D2), and missing outcome data (D3). Many of the studies did not properly report the methodological domains of the risk of bias tool, being characterized as “no informed”. We provide the risk of bias assessment of the included RCTs in Appendix 2.
Evidence Certainty
The GRADEpro GDT software50 was used to rate the quality of the evidence according to the GRADE criteria for the pain intensity outcome at short and moderate term follow-ups. Single randomized studies (with under 300 participants) had quality downgraded twice as it was considered inconsistent and potentially imprecise (i.e., if wide 95%CI). Presence of unclear or high risk of bias was also downgraded once according to the criteria. A “Summary of findings” table (Table 3) was created to present the result and the criteria explanation is shown in the table.
Table 3.
The GRADE “Summary of Findings” table for quality of evidence, magnitude of effect of the interventions at short- and medium to moderate-term follow-up.
| Identifying methodology, population, and intervention factors associated with the analgesic effect of tDCS in Fibromyalgia. A Systematic Review, Meta-Analysis, and Meta-Regression | ||||
| Comparison: Sham tDCS | ||||
| Outcomes | Effect size | Relative and absolute effect (average % improvement (reduction) in pain (95% CIs) in relation to post-treatment score from sham group) * * Where 95%CIs do not cross the line of no effect. | No of participants (studies) | Quality of the evidence (GRADE) |
|---|---|---|---|---|
|
Pain intensity – Short Term Effect (< 4 weeks from baseline) |
||||
| Pain intensity measured using a visual analog scale or numerical rating scale | SMD 0.81 95%CI (0.43, 1.19) |
This equates to a 27%, 95% CI (14% to 40%) reduction in pain intensity, on a 0 to 100 pain intensity scale |
770 total. 401 on active and 369 on sham. (14 Studies) |
⊕⊕◯◯ low1 |
|
Pain intensity – Moderate Term Effect (> 4 weeks from baseline) |
||||
| Pain intensity measured using a visual analog scale or numerical rating scale | SMD 1.689 95%CI (0.84, 2.54) |
This equates to a 57%, 95% CI (28% to 85%) reduction in pain intensity, on a 0 to 100 pain intensity scale |
375 total. 193 on active and 182 on sham. (7 Studies) |
⊕◯◯◯ very low2, 3, 4 |
|
GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||
Downgraded twice. Single randomized study (with under 300 participants) was considered inconsistent, imprecise (i.e., wide 95%CI) and with potential publication bias, providing low quality of evidence.
Downgraded once for inconsistency due to heterogeneity.
Downgraded once for study limitation due to high or unclear risk of bias.
Downgraded once for imprecision due to small sample size
SMD: Standardized Mean Difference; CI: confidence Interval
Main Effect Analysis
The meta-analysis for the main effect of active tDCS versus sham tDCS for pain in FM showed a positive result favoring the active tDCS intervention with a pooled ES of d=1.22 (95% CI: 0.80, 1.65, p-value<0.001, p-value < 0.001), which is considered to be large based on suggested standards51. This ES corresponds to a 41% change, which is above the threshold for the minimal clinically important difference for pain in FM (34.2%)52. Large heterogeneity was found among studies (I2=83.6%). Figure 2 shows the forest plot for the included studies.
Figure 2.
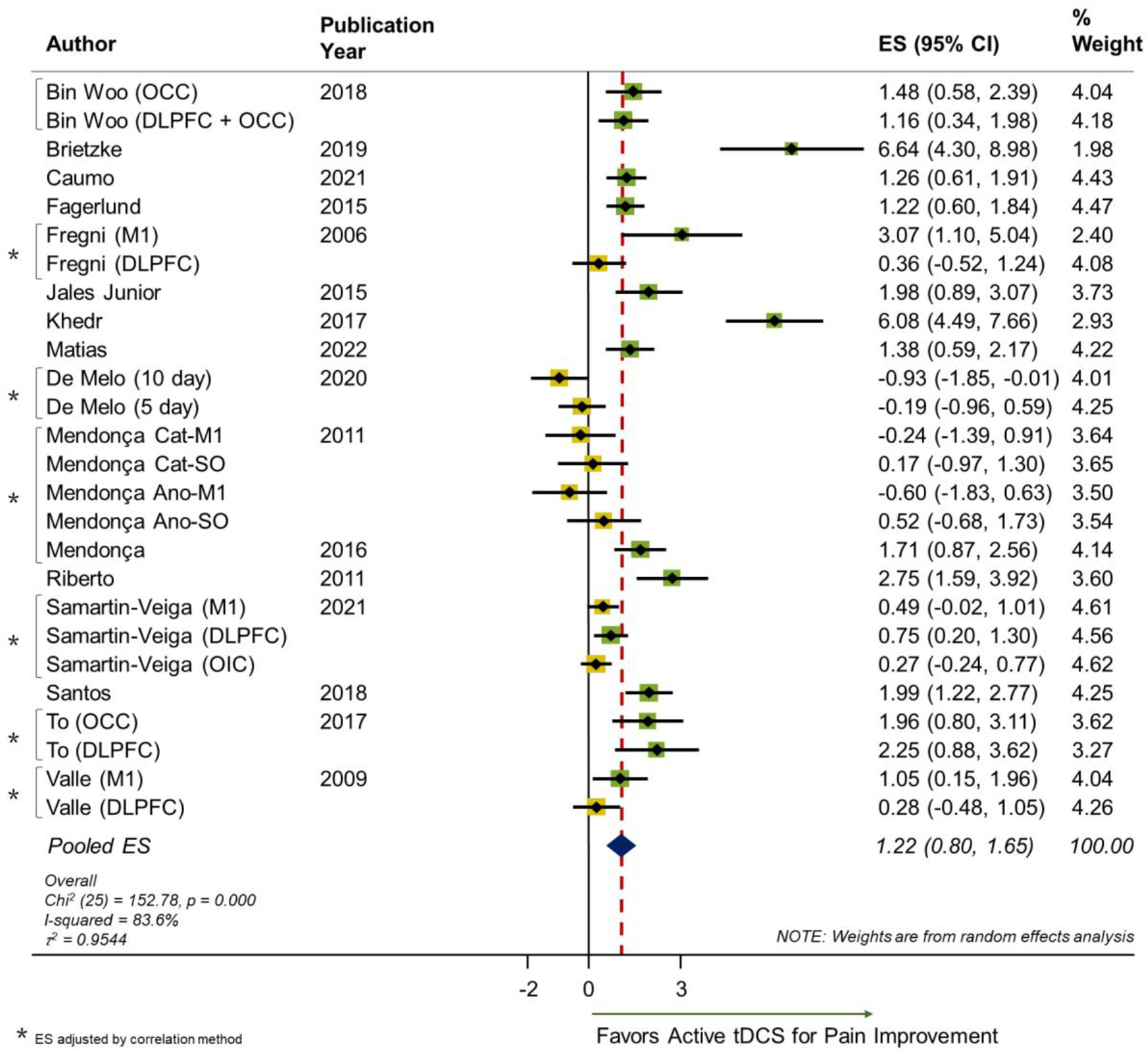
Forest plot of the main effect of tDCS on pain in FM.
OCC: occipital region; DLPFC: dorsolateral prefrontal cortex; M1: primary motor cortex; OIC: operculo-insular cortex; NRS: numeric rating scale; Cat: Cathodal; Ano: Anodal; SO: Supraorbital; ES: Effect Size; CI: Confidence Interval.
The publication bias analysis revealed an asymmetric distribution on the funnel plot suggesting a publication bias in the analysis (Appendix 3). A significant Egger’s test for bias was found t = 2.70, p-value = 0.012. Begg’s test for small study effect was statistically significant (continuity corrected p-value = 0.022) which provides evidence of publication bias. After adjustment for publication bias, the overall ES estimate was 1.161 (95% C.I. 0.51, 1.81) and still significant.
Subgroup Analysis
Short-Term versus Moderate Term Effects
As defined a priori, a sensitivity analysis according to short-term versus moderate term effects was performed. For the short-term effects of tDCS on pain, two studies40,43 did not collect pain outcomes before four weeks, and seven studies did not have moderate term data on pain15,23,24,38,41,45,46. The median timepoint for the short-term outcome results was three weeks, while for moderate term was 10 weeks. The pooled ES estimates were respectively 0.81 [95% CI: 0.43, 1.19, p-value = 0.000; I2=78.5%; Chi2 (23) = 107.21, p = 0.000; τ2 = 0.66], and 1.69 [95% CI: 0.84, 2.54, p-value = 0.000; I2=91.0%; Chi2 (9) = 100.3, p = 0.000; τ2 = 1.59]. The results suggest that moderate term effects of tDCS on pain seem to be larger than short-term effects. The forest plots are offered as a supplemental material.
Active Electrode Location
The sensitivity analysis for the main effect of tDCS versus sham tDCS for pain in FM considering the placement of the active electrode location showed no difference when M1 location was compared directly to DLPFC location. The pooled ES estimates for studies which used M1 and DLPFC were respectively 1.25 [95% CI: 0.52, 1.98, p-value = 0.000; I2=88%; Chi2 (12) = 99.71, p = 0.000; τ2 = 1.51], and 1.43 [95% CI: 0.73, 2.14, p-value = 0.000; I2=81.7%; Chi2 (7) = 38.17, p = 0.000; τ2 = 0.78] (Supplemental Material). The overlapping of the 95%CI shows that there is no advantage of using M1 or DLPFC as compared with each other.
Risk of Bias Subgroups
A total of 6 studies were classified with “high-risk” of bias (Appendix 2), while 10 studies were classified as others (“low risk” or “some concerns”). The effect sizes were respectively 1.53 [95% CI: 0.47, 2.59) and 1.07 [95% CI: 0.65, 1.49). The forest plot of this comparison analysis is provided as a supplemental material.
Presence of Combined Intervention
Three studies used a combined intervention with tDCS39,41,44 while the other 13 studies did not. The effect sizes were respectively 1.70 [95% CI: 1.24, 2.16) and 1.16 [95% CI: 0.69, 1.63). Two studies39,44 used an exercise-based type of intervention, while one study41 used a cognitive-behavioral based therapy. As for the timing of the combination, two studies41,44 combined concurrently with the tDCS stimulation, while one study39 used immediately after tDCS stimulation. The forest plot of this comparison analysis is provided as a supplemental material.
Meta-Regression
No multivariate models were significant. As FM is a disease which occurs predominantly in women gender variability not sufficient to be properly investigated as an independent variable in the meta-regression. Only one meta-regression model showed significance in univariate analysis for the variable of the duration of the tDCS intervention protocol in weeks. The direction of the regression coefficient indicated that the longer the duration of the tDCS intervention protocol in weeks, the larger the effect size (R2 = 31.3%). Figure 3 shows model information and regression plot.
Figure 3.
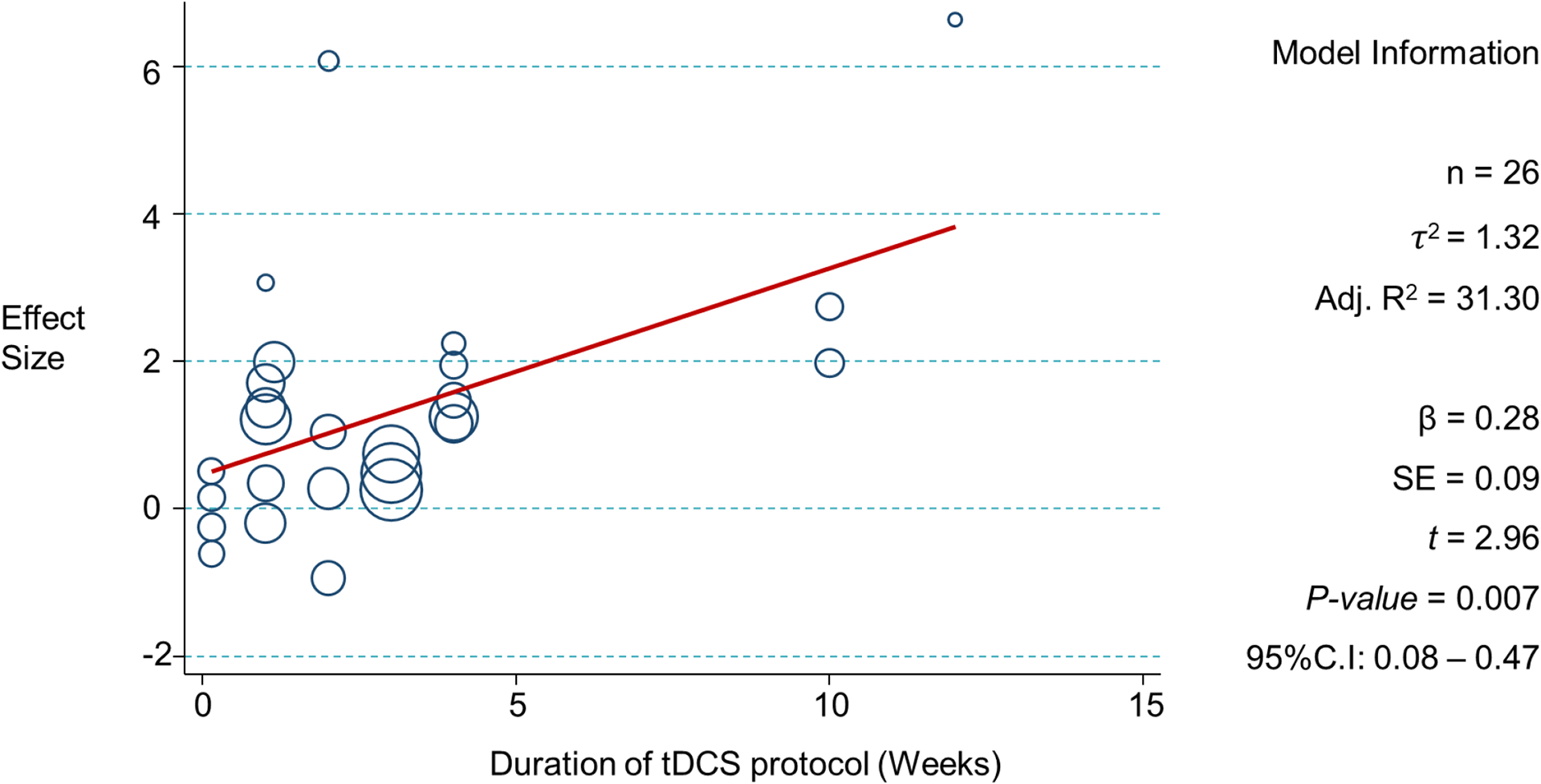
Significant univariate meta-regression model – Duration of tDCS intervention measured in weeks.
n: Number of observations; τ2 : tau-squared; Adj. R2 : adjusted r-squared; β : beta coefficient; SE : standard error; t : t statistic; 95%C.I: 95% confidence interval
After visual analysis of the regression plot, we noticed that studies with protocols lasting approximately 4–5 weeks presented higher effect sizes as compared to less than 4–5 weeks. In attempt to facilitate the clinical application of this information we have explored this data by dichotomizing the duration of the protocol variables using different cut-offs (1, 2, 3, 4 and 5 weeks) and tested for significance using meta-regression. The result was significant only for the 4-week cut-off with a regression coefficient β = 2.35 (standard error: 0.95, p=0.021, 95%C.I: 0.39, 4.30) and adjusted R2 = 22.1%. All other baseline variables were not significant, and results are shown on Appendix 4. Although the variables that represented the total number of tDCS sessions, and the tDCS session duration in minutes are shown to be significant, the p-values were above 0.05 after influential point adjustment.
DISCUSSION
Our meta-analysis included 16 RCTs with 26 different active tDCS protocol groups that were compared to sham tDCS. The field required a meta-analysis update as trials which have been published in the last five years, with varied ES, were not included in past reviews22,23,26. In addition, to the best of our knowledge, this is the first meta-analysis in the field that used the ES calculated separately for all active tDCS intervention groups used in multi-arm intervention trials. It is imperative to consider all variations of tDCS protocols as the variability in ES may be explained by differences in protocols. This updated review also included the exploration of factors related to ES variability using meta-regression analysis and revealing that the duration of the intervention protocol in weeks seemed to be a significant factor to explain the variability in the effect of tDCS on pain for FM.
Our results for the main effect of tDCS on FM pain showed a significant analgesic effect (pooled ES of d=1.22 (95% CI: 0.80, 1.65). Although this ES is larger than previously reported, our finding agree with a previous systematic review on the topic22 that reported medium to large ES (SMD= −.66), thus supporting the analgesic effect. Chaturvedi et. al. performed a systematic review also in the same topic, however no meta-analysis was made, or ES was reported. A potential reason for the ES differences of our results as compared to previous reviews may be the fact that our review included recent clinical trials with conflicting results that were not included in preceding reviews and meta-analyses. In addition, our results were calculated considering the ES calculated separately from different active tDCS intervention groups used in multi-arm intervention trials, a methodology detail that has also not been used in previous reviews. Such factors may relevantly affect a meta-analysis result. In addition, we have explored specific factors that may be related to the analgesic effect of tDCS in FM using rigorous methodology.
To our knowledge, only one previous study53 attempted to explore intervention related factors such as number of sessions, weekly frequency, and protocol duration, or different tDCS montages (M1 versus DLPFC). Hou53 and colleagues investigated the effects of different non-invasive brain stimulation treatments, including tDCS and repetitive transcranial magnetic stimulation (rTMS) as add-on treatment approaches to FM. Although the study included only five tDCS trials, the results suggested that the M1 location produced larger effect sizes as compared to DLPFC (p< 0.0001). Our subgroup analysis comparing studies with M1 and DLPFC stimulation sites did not show differences in the effect of tDCS on pain. We believe the multidimensional nature of pain in FM could be related to this finding and although the mechanisms would be different, both strategies would affect central pain processing centers. In fact, targeting M1 would lead to motor cortex excitability modulation, subsequently affecting components of sensory pain processing, ultimately facilitating the descending pain inhibitory system54. Meanwhile, targeting the DLPFC would lead to modulation of the cognitive-affective components of pain55,56 due to its connections with the limbic system structures. Upcoming mechanistic studies evaluating outcomes related to pain processing, such as quantitative sensory testing, could help clarify the effects of tDCS on different stimulation sites57. Conditioned pain modulation, for example, is believed to be a marker of the descending pain inhibitory system58 that is potentially more influenced by M1 than DLPFC stimulation59. Comparably, the effects of DLPFC stimulation on mood, fatigue, and attention, commonly impaired in patients with FM60, could also be assessed and later correlated to its effects on pain. One important consideration is that pain measurement as indexed by VAS is an unspecific measurement that is poorly correlated with mechanistic pain measurements8,61.
It is possible that the lack of differences in effect between M1 and DLPFC stimulation sites could be simply due to the lack of focality of the tDCS stimulation. Therefore, an aspect that is sometimes viewed as a disadvantage of the technique62,63 could in fact lead to positive results in this case. Targeting M1 or DLPFC would lead, therefore, to simultaneous modulating of several pain processing and affective/cognitive pathways. Here, again, future study designs comparing the two stimulation sites and their effects on different dimensions of pain would help address this potential explanation. Hou53 et. al. also explored factors related to treatment regimen such as total number of sessions, frequency, and protocol duration, however, no significant linear relationship was found. Despite the fact that our initial analysis showed that the total number of sessions and the duration of the tDCS session in minutes were significantly influencing the effect size, such variables were not relevant after controlling for influential observations.
We found that tDCS protocols that lasted four weeks or more, reported larger effect sizes than shorter protocols. These results persisted after adjusting for the number of sessions in the meta-regression model, suggesting an essential role of the session distribution across weeks more so than only the total number of sessions. Our finding is aligned with a previous mechanistic study64 that showed that the broader repetition interval of M1 tDCS (more than 24 hours) produces the strongest cortical excitability changes. Similarly, a previous tDCS trial on patients with FM reported during a longitudinal analysis of analgesic effects, that the highest clinically significant differences compared to sham tDCS, were achieved after the fourth week of stimulation (approximately after 15 sessions)65. These time-dependent phenomena are not exclusive to tDCS studies on pain populations; in fact, recent evidence-based guidelines on tDCS for neuropsychiatric conditions66 reported similar patterns for stroke recovery and depression management.
The potential underlying physiological explanation is the time-dependency of neuroplasticity. Previous studies67,68 have suggested that tDCS effects depend on the engagement of NMDA-related networks within the brain target; thus, the magnitude and duration of tDCS-induced plasticity must be related to stimulation duration and timing69. However, as it has been reported in several animal models, NMDA-related long-term potentiation and depression have a saturation effect (homeostatic plasticity) that prevents destabilization of synaptic function; this process depends on protein synthesis regulation, which requires a time window to be executed70–73. Therefore, it can be argued that some stabilization of plasticity takes place during that prolonged period between stimulation sessions, which could facilitate the cumulative gains of plasticity.
Nevertheless, we need to consider that only some studies performed protocols with a duration longer than four weeks; hence, our findings could be underpowered or driven by few influential studies and require further exploration. But it is clear that session distribution and specific timing of repetition intervals are important for optimizing the cumulative effects of tDCS protocols. As suggested by Pacheco-Barrios et al.74, there is a need for more extended protocols with an optimized architecture based on neuroplasticity physiology. For instance, including an induction phase with daily sessions (which produces an optimal target engagement64,75) for the first one or two weeks, followed by booster sessions across time76,77 to maximize cumulative plasticity and synaptic stabilization. Further studies must test methodological alternatives to increase the feasibility of these approaches, such as the inclusion of digital health methods and home-based stimulation protocols78,79.
Despite supporting evidence on the emotional and affective impact of FM and its effect on pain perception80,81, our hypothesis that the presence of higher levels of depression and anxiety, or other psychosocial related factors would be significantly associated with the analgesic effect of tDCS in FM was not confirmed as it could not be adequately tested with our data. To explore this, we extracted data on baseline levels of depression, anxiety, and pain catastrophizing. Although depression scores were reported in 14 of the 16 studies, there was small variability on levels of depression as most studies excluded subjects with severe depression. Only few studies have provided information on anxiety and pain catastrophizing, as well as other potential key factors such as the use of medications and sleep quality scores. Therefore, the available data was insufficient to construct stable meta-regression models for many of the population related variables, and model significance was likely influenced by the low number of observations. Consequently, it is not possible, or adequate, to make conclusions on the relevance of these population related factors on their influence on the analgesic effect of tDCS on pain. This finding points out the lack of consistency and structure in reporting population related factors. Future study designs are encouraged to include information and variability of such factors so its relation to tDCS clinical effect can be appropriately explored and used to diminish response variability in the field of neuromodulation.
One potentially relevant finding is related to the subgroup analysis based on the presence of a combined intervention used with tDCS. Although this comparison was not statistically significant, the three studies which used a combined intervention had a pooled ES of 1.70 [95% CI: 1.24, 2.16) and two out of the three studies used an exercise-based intervention. The studies that did not use other combined interventions had an ES of 1.16 [95% CI: 0.69, 1.63). This finding supports the use of tDCS in combination with exercises for FM pain. Research has showed that the combination of tDCS and other therapies, such as exercise, are believed to increase clinical effects on pain, as compared to tDCS alone. The evidence supports the mechanism of increasing the brain responsiveness to the corticomotor benefits of exercise or other interventions as for example increased cortical excitability, improved motor control, enhanced muscle activation82, and by adding effects on the pain system function.
Limitations
Our review and meta-regression exploration were performed using 16 RCTs with a total of 813 subjects included in the analysis. Although our analysis suggested that our review is based on low to very low evidence quality, included trials were mainly penalized for imprecision due to small sample size (under 300 participants). We agree that small and low quality RCTs may indeed present compromised results which might not represent the true effect of the intervention, or the true population characteristics. For example, small trials may have the randomization assignment compromised and suffer from potential confounding. We also acknowledge that this limitation is understandable given the challenges with recruitment and adherence in RCTs assessing NIBS in this population population83. Our results did not find any significant multivariate meta regression models. This finding should be cautiously interpreted as it is likely that potentially existing multivariate models may have been underpowered. In addition, our meta-regression analysis had an exploratory nature, and model building process was performed accordingly. Also, there is a possible influence of the risk of bias on the pooled results as the individual ES may not represent the true effect due to the study biases. Among the limitation, the comparison of different measures to assess clinical pain; the male gender not properly represented making the results generalizable only to the female population, the lack of studies using alternative cortical targets to assess the effect of tDCS, and the impossibility to assess the effect of medication should be noted and considered before any conclusion is attempted. Too, the studies selected did not included more recent approaches of transcranial electrical stimulation, such as focal-tDCS or transcranial random noise stimulation (tRNS), which perhaps may be able to exert stronger stimulation effects over the same cortical targets with reported therapeutical effects on pain84. Lastly, the sham tDCS montage could have been a limiting factor for comparison between active and sham groups for the multi-arm intervention trials. The authors caution that all inferences about any results should consider these limitations and be interpreted accordingly.
CONCLUSION
Our study provides an updated systematic review and meta-analysis with meta-regression methodology on the clinical analgesic effect of tDCS in FM. The main effect of active tDCS versus sham tDCS for clinical pain in FM was confirmed to have a positive result favoring the active tDCS, with a large ES. Our findings also suggest that tDCS protocols that last four weeks or more may be associated with larger effect sizes than shorter protocols, and time-dependency neuroplasticity is discussed as a potential underlying physiological cause. The offered conclusions should be interpreted with caution due to the risk of bias and low levels of certainty found in our analysis and considering the lack of robust studies. Lastly, future trials must be thorough to methodological details such as randomization process, deviations from intended interventions, missing data, and reporting results to minimize bias.
Supplementary Material
Source of financial support:
Research reported in this publication was supported by the National Institutes of Health under Award Number R01 AT009491-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix 1.
Search strategy.
| Database | Search Terms | Results |
|---|---|---|
|
MEDLINE PubMed |
TDCS “noninvasive brain stimulation”[Title/Abstract] OR “non-invasive brain stimulation”[Title/Abstract] OR “neuromodulation”[Title/Abstract] OR “NIBS”[Title/Abstract] OR “motor cortex stimulation”[Title/Abstract] OR “Transcranial Electric Stimulation”[Title/Abstract] OR “transcranial direct current stimulation”[MeSH Terms] OR “transcranial direct current stimulation”[Title/Abstract] OR “tdcs”[All Fields] OR “transcranial direct current stimulation”[All Fields] |
18,246 |
|
Fibromyalgia: “Fibromyalgia”[MeSH Terms] OR “Fibromyalgia”[All Fields] OR “fatigue syndrome, chronic”[MeSH Terms] OR “chronic fatigue syndrome”[All Fields] OR “Myofascial Pain Syndromes”[MeSH Terms] OR “Myofascial Pain Syndrome”[All Fields] OR “Trigger Points”[MeSH Terms] OR “Trigger Points”[All Fields] OR “Trigger Point Pain”[All Fields] OR “Fibromialgia”[All Fields] OR “Widespread chronic pain”[All Fields] OR “widespread pain”[All Fields] |
27,761 | |
| TDCS and FIBROMYALGIA | 109 | |
| EMBASE |
TDCS ‘transcranial direct current stimulation’/exp OR ‘noninvasive brain stimulation’:ab,ti OR ‘noninvasive brain stimulation’/exp OR ‘non-invasive brain stimulation’:ab,ti OR ‘transcranial direct current stimulation’:ab,ti OR ‘neuromodulation’:ab,ti OR ‘neuromodulation’/exp OR ‘nibs’:ab,ti OR ‘tdcs’:ab,ti OR ‘transcranial direct current stimulator’ |
59,921 |
|
FIBROMYALGIA ‘fibromyalgia’/exp OR ‘fibromyalgia’:ab,ti OR ‘myofascial pain’/exp OR ‘myofascial pain syndrome’/exp OR ‘myofascial pain’:ab,ti OR ‘trigger point pain’ OR ‘trigger point’:ab,ti OR ‘chronic fatigue syndrome’/exp OR ‘chronic fatigue syndrome’:ab,ti OR ‘chronic fatigue’:ab,ti OR ‘widespread chronic pain’ OR ‘widespread chronic pain’:ab,ti |
45,271 | |
| TDCS and FIBROMYALGIA | 490 | |
|
EMBASE FILTER by Study Type: clinical trial + randomized controlled trial + randomized controlled trial topic |
130 | |
| WEB OF SCIENCE |
TDCS transcranial direct current stimulation OR noninvasive brain stimulation OR noninvasive brain stimulation OR non-invasive brain stimulation OR transcranial direct current stimulation OR neuromodulation OR neuromodulation OR nibs OR tdcs |
32,311 |
|
FIBROMYALGIA Fibromyalgia OR chronic fatigue syndrome OR Myofascial Pain Syndrome OR Myofascial Pain OR Trigger Points OR trigger point pain OR Fibromialgia OR chronic fatigue syndrome OR Widespread chronic pain OR Widespread pain |
65,205 | |
| TDCS and FIBROMYALGIA | 267 | |
|
WeB of Science Filter - Document Type
Articles |
179 |
Appendix 2.
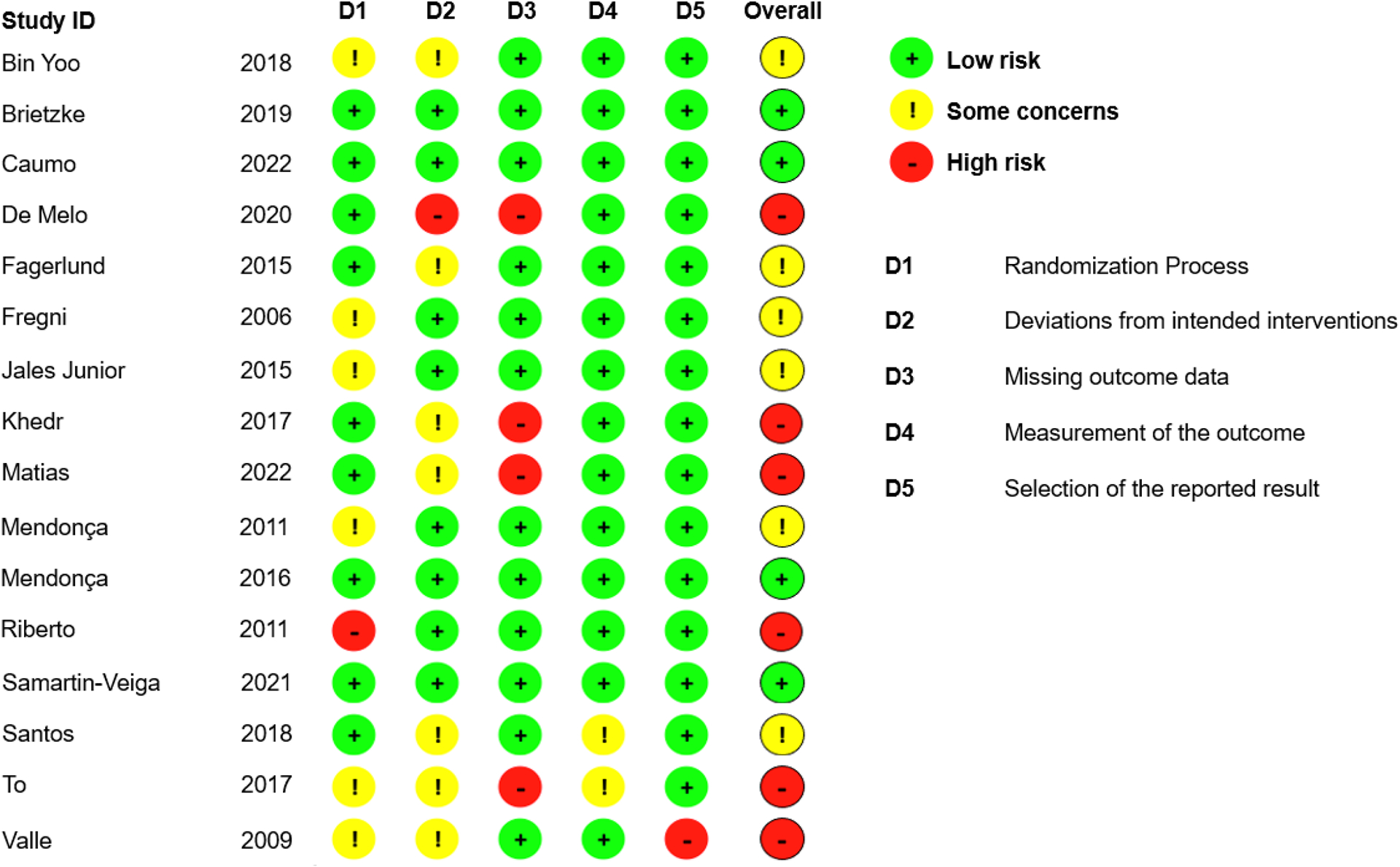
Risk of bias assessment of the included studies.
Appendix 3.
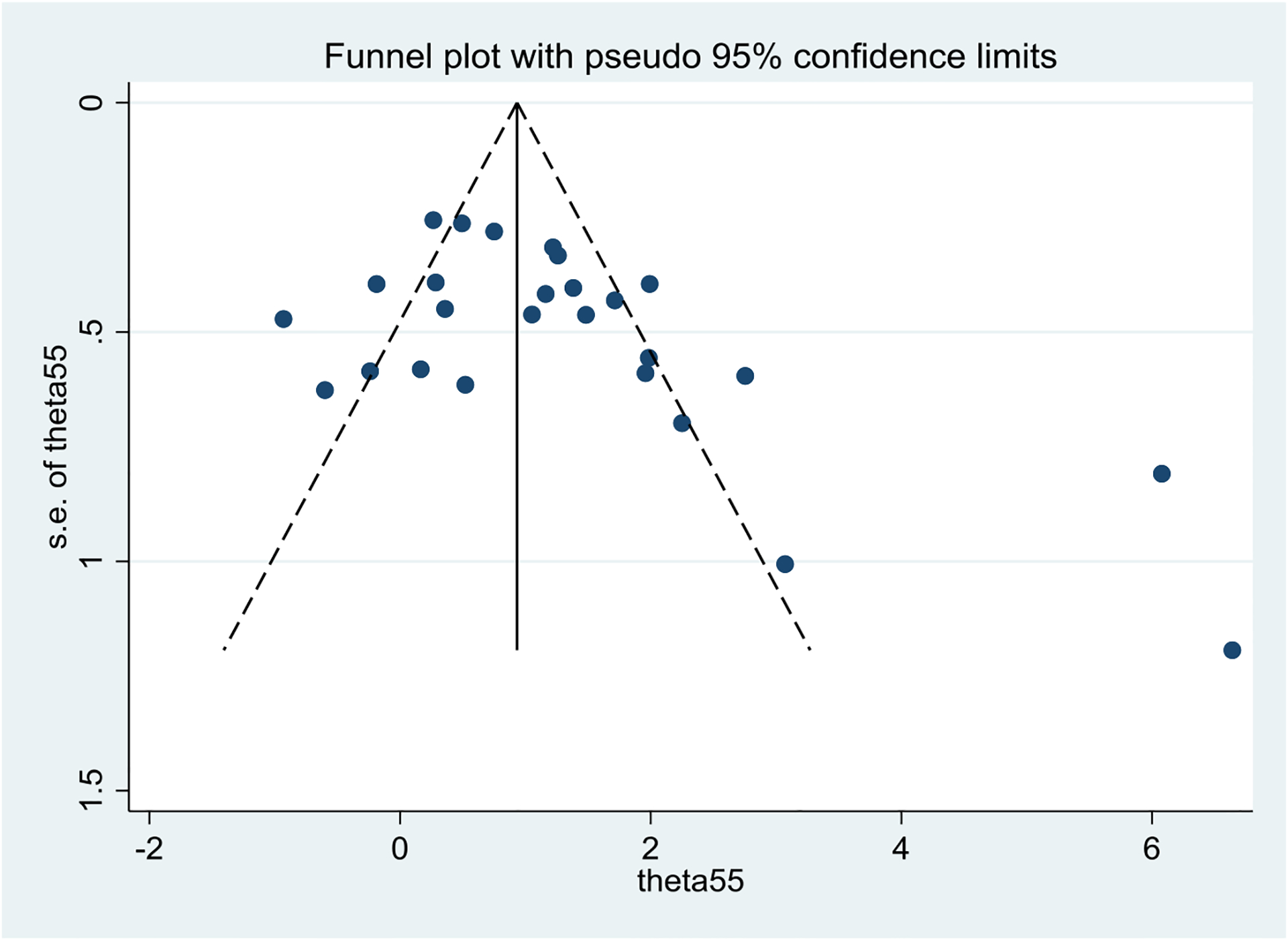
Funnel plot for publication bias analysis.
Appendix 4.
Univariate models for all explored variables.
| Study Methodology Related Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95%CI | |||||||||
| Variables | n | τ2 | Adj. R2 | β | SE | t | p | LB | UP |
| Use of intention-to-treat analysis | 22 | 2.53 | 8.14 | 0.30 | 0.91 | 0.33 | 0.75 | −1.60 | 2.19 |
| Size of active intervention group | 26 | 2.05 | 6.87 | 0.00 | 0.04 | −0.09 | 0.93 | −0.08 | 0.07 |
| Size of sham intervention group | 26 | 2.05 | 6.72 | 0.00 | 0.05 | 0.09 | 0.93 | −0.09 | 0.10 |
| # of Dropouts in active intervention group | 22 | 2.40 | 1.88 | 0.19 | 0.22 | −0.86 | 0.40 | −0.64 | 0.27 |
| # of Dropouts in sham intervention group | 22 | 2.41 | 2.48 | −0.18 | 0.21 | −0.82 | 0.42 | −0.62 | 0.27 |
| Dropout % in active intervention group | 24 | 2.04 | 1.79 | −0.05 | 0.05 | −1.02 | 0.32 | −0.15 | 0.05 |
| Dropout % in sham intervention group | 24 | 2.11 | 1.54 | −0.04 | 0.05 | −0.85 | 0.41 | −0.14 | 0.06 |
| Blinding of participants | 26 | 2.05 | 6.89 | 0.13 | 1.64 | 0.08 | 0.94 | −3.24 | 3.52 |
| Blinding of intervention staff | 26 | 1.93 | 0.47 | 1.58 | 1.66 | 0.95 | 0.35 | −1.85 | 5.02 |
| Population Related Factors | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Group | Sham Group | |||||||||||||||||
| 95%CI | 95%CI | |||||||||||||||||
| Variables | n | τ2 | Adj. R2 | β | SE | t | p | LB | UP | n | τ2 | Adj. R2 | β | SE | t | p | LB | UP |
| Average age | 23 | 1.83 | 1.15 | −0.06 | 0.06 | −1.04 | 0.31 | −0.19 | 0.06 | 23 | 1.76 | 4.52 | −0.09 | 0.08 | −1.20 | 0.24 | −0.26 | 0.07 |
| Duration of symptoms (months) | 8 | 2.99 | 0.64 | −0.01 | 0.01 | −1.06 | 0.33 | −0.05 | 0.02 | 8 | 2.95 | 0.73 | −0.01 | 0.01 | −1.10 | 0.31 | −0.04 | 0.02 |
| Percentage of women | 26 | 1.91 | 0.36 | −0.02 | 0.02 | −0.85 | 0.40 | −0.05 | 0.02 | 26 | 1.80 | 6.38 | −0.02 | 0.02 | −1.25 | 0.23 | −0.06 | 0.02 |
| Body mass index | 7 | 2.64 | 17.21 | −0.73 | 0.51 | −1.43 | 0.21 | −2.04 | 0.58 | 7 | 3.91 | 22.42 | 0.37 | 0.60 | 0.61 | 0.57 | −1.18 | 1.91 |
| Number of subjects with another chronic illness | 6 | 6.42 | 21.10 | 0.10 | 0.19 | 0.51 | 0.63 | −0.43 | 0.63 | 6 | 6.70 | 26.46 | 0.07 | 0.19 | 0.35 | 0.74 | −0.46 | 0.60 |
| Baseline depression level | 14 | 2.48 | 2.18 | −0.81 | 0.75 | −1.09 | 0.30 | −2.44 | 0.81 | 14 | 2.48 | 2.18 | −0.08 | 0.75 | −1.09 | 0.30 | −2.44 | 0.81 |
| Baseline anxiety level | 7 | 3.33 | 6.21 | −0.76 | 0.84 | −0.90 | 0.41 | −2.93 | 1.41 | 7 | 3.31 | 5.36 | −0.73 | 0.80 | −0.92 | 0.40 | −2.79 | 1.33 |
| Baseline pain catastrophizing score | 5 | 3.70 | 33.52 | 0.17 | 0.23 | 0.75 | 0.51 | −0.55 | 0.89 | 5 | 3.86 | 39.35 | −0.19 | 0.33 | −0.58 | 0.61 | −1.23 | 0.85 |
| Percentage of subjects using opioids† | ||||||||||||||||||
| Disability score at baseline | 14 | 0.62 | 7.92 | −0.02 | 0.25 | −0.78 | 0.45 | −0.07 | 0.04 | 14 | 0.65 | 4.07 | −0.02 | 0.23 | −0.97 | 0.35 | −0.07 | 0.03 |
| Sleep quality score at baseline | 7 | 2.90 | 8.46 | 0.01 | 0.08 | 1.14 | 0.31 | −0.12 | 0.31 | 7 | 3.10 | 15.95 | 0.09 | 0.09 | 0.99 | 0.37 | −0.15 | 3.91 |
Calculation not possible due to low number of observations
| Intervention Related Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95%CI | |||||||||
| Variables | n | τ2 | Adj. R2 | β | SE | t | p | LB | UP |
| Presence of tDCS with a combined intervention ‡ | |||||||||
| Total number of intervention sessions | 26 | 1.45 | 24.40 | 0.09 | 0.03 | 2.97 | 0.007† | 0.03 | 0.15 |
| # of Intervention sessions above/below 8 (median) | 26 | 2.01 | 4.60 | 0.56 | 0.64 | 0.87 | 0.39 | −0.77 | 1.89 |
| Frequency of intervention sessions per week | 26 | 2.04 | 6.30 | 0.09 | 0.17 | 0.52 | 0.61 | −0.27 | 0.45 |
| Duration of tDCS intervention (in weeks) | 26 | 1.32 | 31.30 | 0.28 | 0.09 | 2.96 | 0.007* | 0.08 | 0.47 |
| Patient setting (inpatient/ outpatient/ home) | 26 | 1.69 | 12.09 | −1.99 | 0.96 | −2.06 | 0.05 | −3.98 | 0.00 |
| Active electrode location | 26 | 2.03 | 5.91 | 0.08 | 0.34 | 0.23 | 0.82 | −0.63 | 0.79 |
| Use of a combined intervention with tDCS | 26 | 2.00 | 4.19 | 0.46 | 0.98 | 0.47 | 0.64 | −1.57 | 2.49 |
| tDCS duration of session (in minutes) | 26 | 1.34 | 30.11 | 0.55 | 0.18 | 3.08 | 0.005† | 0.18 | 0.92 |
Not significant after influential point adjustment;
Statistically significant at 0.01 level;
Calculation not possible due to low number of observations
This study was carried out at the Neuromodulation Center and Center for Clinical Research Learning, Spaulding Rehabilitation Hospital and Massachusetts General Hospital, Charlestown, MA, USA.
Conflict of Interest Statement: All authors declare that they have no conflict of interest.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken). 2010;62:460–464. 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 3.Duruturk N, Tuzun EH, Culhaoglu B. Is balance exercise training as effective as aerobic exercise training in fibromyalgia syndrome? Rheumatol Int. 2015;35:845–854. 10.1007/s00296-014-3159-z. [DOI] [PubMed] [Google Scholar]
- 4.de la Coba P, Montoro CI, Reyes Del Paso GA, Galvez-Sánchez CM. Algometry for the assessment of central sensitisation to pain in fibromyalgia patients: a systematic review. Ann Med. 2022;54:1403–1422. 10.1080/07853890.2022.2075560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijs J, Van Houdenhove B, Oostendorp RAB. Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man Ther. 2010;15:135–141. 10.1016/j.math.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain. 2016;157:1704–1710. 10.1097/j.pain.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 7.Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, Stroman PW. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp. 2016;37:1349–1360. 10.1002/hbm.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uygur-Kucukseymen E, Castelo-Branco L, Pacheco-Barrios K, et al. Decreased neural inhibitory state in fibromyalgia pain: a cross-sectional study. Neurophysiol Clin. 2020;50:279–288. 10.1016/j.neucli.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco-Barrios K, Lima D, Pimenta D, et al. Motor cortex inhibition as a fibromyalgia biomarker: a meta-analysis of transcranial magnetic stimulation studies. Brain Netw Modul. 2022;1:88–101. 10.4103/2773-2398.348254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010;149:495–500. 10.1016/j.pain.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Henderson LA, Peck CC, Petersen ET, et al. Chronic pain: lost inhibition? J Neurosci. 2013;33:7574–7582. 10.1523/jneurosci.0174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walton KD, Llinás RR. Central pain as a thalamocortical dysrhythmia: a thalamic efference disconnection? In: Kruger L, Light AR, eds. Translational Pain Research: From Mouse to Man. CRC Press/Taylor & Francis; 2010. [PubMed] [Google Scholar]
- 13.Alshelh Z, Di Pietro F, Youssef AM, et al. Chronic neuropathic pain: it’s about the rhythm. J Neurosci. 2016;36:1008–1018. 10.1523/jneurosci.2768-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 15.Fregni F, Gimenes R, Valle AC, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 16.da Graca-Tarragó M, Lech M, Angoleri LDM, et al. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res. 2019;12:209–221. 10.2147/JPR.S181019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang WJ, Bennell KL, Hodges PW, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: a pilot randomised controlled trial. PLoS One. 2017;12:e0180328. 10.1371/journal.pone.0180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 2017;20:215–222. 10.1111/ner.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract. 2016;2:19–25. 10.1016/j.cnp.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd DM, Wittkopf PG, Arendsen LJ, Jones AKP. Is transcranial direct current stimulation (tDCS) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. J Pain. 2020;21:1085–1100. 10.1016/j.jpain.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 23.de Melo GA, de Oliveira EA, Dos Santos Andrade SMM, Fernández-Calvo B, Torro N. Comparison of two tDCS protocols on pain and EEG alpha-2 oscillations in women with fibromyalgia. Sci Rep. 2020;10:18955. 10.1038/s41598-020-75861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendonca ME, Santana MB, Baptista AF, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain. 2011;12:610–617. 10.1016/j.jpain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 25.de Rooij A, van der Leeden M, Roorda LD, Steultjens MP, Dekker J. Predictors of outcome of multidisciplinary treatment in chronic widespread pain: an observational study. BMC Musculoskelet Disord. 2013;14:133. 10.1186/1471-2474-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brietzke AP, Zortea M, Carvalho F, et al. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain. 2020;21:212–224. 10.1016/j.jpain.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Accessed November 14, 2022. http://www.handbook.cochrane.org. [Google Scholar]
- 29.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane; 2022. Accessed August 12, 2022. https://training.cochrane.org/handbook/current/chapter-08 [Google Scholar]
- 30.Risk of Bias 2 (RoB 2) tool. Cochrane Methods Accessed August 12, 2022. https://methods.cochrane.org/risk-bias-2 [Google Scholar]
- 31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheung MWL. A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol Rev. 2019;29:387–396. 10.1007/s11065-019-09415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Fan S, Xu Y, Cui L. Non-invasive brain stimulation for fatigue in multiple sclerosis patients: a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;36, 101375. 10.1016/j.msard.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Altman N, Krzywinski M. P values and the search for significance. Nat Methods. 2017;14:3–4. 10.1038/nmeth.4120. [DOI] [Google Scholar]
- 35.Galvan MC, Pyrczak F. Writing Empirical Research Reports. A Basic Guide for Students of the Social and Behavioral Sciences. Routledge; 2014. Accessed August 12, 2022. https://www.taylorfrancis.com/books/mono/10.4324/9781315265926/writing-empiricalresearch-reports-fred-pyrczak [Google Scholar]
- 36.Osborne JW, Waters E. Four assumptions of multiple regression that researchers should always test 2002;8:6. 10.7275/r222-hv23. [DOI] [Google Scholar]
- 37.Yap BW, Sim CH. Comparisons of various types of normality tests. J Stat Comput Simul. 2011;81:2141–2155. 10.1080/00949655.2010.520163. [DOI] [Google Scholar]
- 38.Caumo W, Alves RL, Vicuña P, et al. Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: a randomized, double-blind sham-controlled study. J Pain. 2022;23:641–656. 10.1016/j.jpain.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. 2016;10:68. 10.3389/fnhum.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. 10.2174/1874312901105010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos VSDSD, Zortea M, Alves RL, et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: a randomized clinical trial. Sci Rep. 2018;8:12477. 10.1038/s41598-018-30127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valle A, Roizenblatt S, Botte S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2:353–361. [PMC free article] [PubMed] [Google Scholar]
- 43.Jales LH Jr, Costa MdDL, Jales Neto LH, Ribeiro JPM, Freitas WJSdN, Teixeira MJ. Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy. Rev Dor. 2015;16:37–42. 10.5935/1806-0013.20150008. [DOI] [Google Scholar]
- 44.Matias MGL, Germano Maciel D, França IM, et al. Transcranial direct current stimulation associated with functional exercise program for treating fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. 2022;103:245–254. 10.1016/j.apmr.2021.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Yoo HB, Ost J, Joos W, Van Havenbergh T, De Ridder D, Vanneste S. Adding prefrontal transcranial direct current stimulation before occipital nerve stimulation in fibromyalgia. Clin J Pain. 2018;34:421–427. 10.1097/AJP.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 46.To WT, James E, Ost J, Hart J Jr, De Ridder D, Vanneste S. Differential effects of bifrontal and occipital nerve stimulation on pain and fatigue using transcranial direct current stimulation in fibromyalgia patients. J Neural Transm (Vienna). 2017;124:799–808. 10.1007/s00702-017-1714-y. [DOI] [PubMed] [Google Scholar]
- 47.Khedr EM, Omran EAH, Ismail NM, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 2017;10:893–901. 10.1016/j.brs.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156:62–71. 10.1016/j.pain.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 49.Samartin-Veiga N, Pidal-Miranda M, González-Villar AJ, et al. Transcranial direct current stimulation of 3 cortical targets is no more effective than placebo as treatment for fibromyalgia: a double-blind sham-controlled clinical trial. Pain. 2022;163:e850–e861. 10.1097/j.pain.0000000000002493. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Routledge; 1988. 10.4324/9780203771587. [DOI] [Google Scholar]
- 51.Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res (Hoboken). 2011;63:821–826. 10.1002/acr.20449. [DOI] [PubMed] [Google Scholar]
- 52.Chaturvedi R, Kulandaivelan S, Malik M, Joshi S. Effect of transcranial direct current stimulation (TDCS) on pain in fibromyalgia-systematic review based on Prisma guidelines. Int J Physiol Nutr Phys Educ. 2018;3:858–862. [Google Scholar]
- 53.Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford). 2016;55:1507–1517. 10.1093/rheumatology/kew205. [DOI] [PubMed] [Google Scholar]
- 54.Brighina F, Curatolo M, Cosentino G, et al. Brain modulation by electric currents in fibromyalgia: a structured review on non-invasive approach with transcranial electrical stimulation. Front Hum Neurosci. 2019;13:40. 10.3389/fnhum.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva AF, Zortea M, Carvalho S, et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7:135. 10.1038/s41598-017-00185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Lopez A, Vanderhasselt MA, Allaert J, Baeken C, De Raedt R. Neurocognitive mechanisms behind emotional attention: inverse effects of anodal tDCS over the left and right DLPFC on gaze disengagement from emotional faces. Cogn Affect Behav Neurosci. 2018;18:485–494. 10.3758/s13415-018-0582-8. [DOI] [PubMed] [Google Scholar]
- 57.Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. 2019;160:1920–1932. 10.1097/j.pain.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14:339. 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Castelo-Branco L, Uygur Kucukseymen E, Duarte D, et al. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia-targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ Open. 2019;9:e032710. 10.1136/bmjopen-2019-032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Häuser W, Ablin J, Fitzcharles MA, et al. Fibromyalgia. Nat Rev Dis Primers. 2015;1, 15022. 10.1038/nrdp.2015.22. [DOI] [PubMed] [Google Scholar]
- 61.Pacheco-Barrios K, Pinto CB, Saleh Velez FG, et al. Structural and functional motor cortex asymmetry in unilateral lower limb amputation with phantom limb pain. Clin Neurophysiol. 2020;131:2375–2382. 10.1016/j.clinph.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bikson M, Datta A. Guidelines for precise and accurate computational models of tDCS. Brain Stimul. 2012;5:430–431. 10.1016/j.brs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Alam M, Truong DQ, Khadka N, Bikson M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys Med Biol. 2016;61:4506–4521. 10.1088/0031-9155/61/12/4506. [DOI] [PubMed] [Google Scholar]
- 64.Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol. 2010;103:1735–1740. 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- 65.Castillo-Saavedra L, Gebodh N, Bikson M, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17:14–26. 10.1016/j.jpain.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24:256–313. 10.1093/ijnp/pyaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 68.Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gartside IB. Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature. 1968;220:382–383. 10.1038/220382a0. [DOI] [PubMed] [Google Scholar]
- 72.Kamikubo Y, Egashira Y, Tanaka T, Shinoda Y, Tominaga-Yoshino K, Ogura A. Longlasting synaptic loss after repeated induction of LTD: independence to the means of LTD induction. Eur J Neurosci. 2006;24:1606–1616. 10.1111/j.1460-9568.2006.05032.x. [DOI] [PubMed] [Google Scholar]
- 73.Shinoda Y, Kamikubo Y, Egashira Y, Tominaga-Yoshino K, Ogura A. Repetition of mGluR-dependent LTD causes slowly developing persistent reduction in synaptic strength accompanied by synapse elimination. Brain Res. 2005;1042:99–107. 10.1016/j.brainres.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 74.Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Rev Med Devices. 2020;17:879–898. 10.1080/17434440.2020.1816168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012;5:208–213. 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, et al. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: a randomized factorial trial. Neurorehabil Neural Repair. 2021;35:704–716. 10.1177/15459683211017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castelo-Branco L, Fregni F. Home-based transcranial direct current stimulation (tDCS) to prevent and treat symptoms related to stress: a potential tool to remediate the behavioral consequences of the COVID-19 isolation measures? Front Integr Neurosci. 2020;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pacheco-Barrios K, Cardenas-Rojas A, de Melo PS, et al. Home-based transcranial direct current stimulation (tDCS) and motor imagery for phantom limb pain using statistical learning to predict treatment response: an open-label study protocol. Princ Pract Clin Res. 2021;7:8–22. 10.21801/ppcrj.2021.74.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toussaint LL, Vincent A, McAllister SJ, Oh TH, Hassett AL. A comparison of fibromyalgia symptoms in patients with healthy versus depressive, low and reactive affect balance styles. Scand J Pain. 2014;5:161–166. 10.1016/j.sjpain.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galvez-Sánchez CM, Duschek S, Reyes Del Paso GA. Psychological impact of fibromyalgia: current perspectives. Psychol Res Behav Manag. 2019;12:117–127. 10.2147/PRBM.S178240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br J Sports Med. 1998;32:20–24. 10.1136/bjsm.32.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardenas-Rojas A, Castelo-Branco L, Pacheco-Barrios K, et al. Recruitment characteristics and non-adherence associated factors of fibromyalgia patients in a randomized clinical trial: a retrospective survival analysis. Contemp Clin Trials Commun. 2021;24:100860. 10.1016/j.conctc.2021.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curatolo M, La Bianca G, Cosentino G, et al. Motor cortex tRNS improves pain, affective and cognitive impairment in patients with fibromyalgia: preliminary results of a randomised sham-controlled trial. Clin Exp Rheumatol. 2017;35(suppl 105):100–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


