Abstract
The transcriptomic regulation induced by isotretinoin (13-cis retinoic acid) is still a matter of debate as short-term exposures of immortalized sebocytes with isotretinoin produced conflicting results. Based on translational evidence, it has been hypothesized that oral isotretinoin treatment upregulates the expression of the transcription factor p53. Twenty-five patients suffering from acne vulgaris were treated with isotretinoin (0.6 mg/kg body weight) for 6 weeks. Biopsies from back skin were taken before and after isotretinoin treatment for the determination of p53 expression by immunohistochemical staining, quantification of p53 protein concentration by enzyme-linked immunosorbent assay and TP53 gene expression by quantitative reverse transcription real time PCR. Fifteen socio-demographically cross-matched healthy volunteers served as controls. Isotretinoin treatment significantly increased the nuclear expression of p53 in sebaceous glands of treated patients compared to pre-treatment levels and p53 levels of untreated controls. Furthermore, the p53 protein and gene expression significantly increased in the skin after treatment. The magnitude of p53 expression showed an inverse correlation to acne severity score and body mass index. Under clinical conditions, isotretinoin induced the expression of p53, which controls multiple transcription factors involved in the pathogenesis of acne vulgaris including FoxO1, androgen receptor and critical genes involved in the induction of autophagy and apoptosis. Increased p53-FoxO1 signalling enhanced by systemic isotretinoin treatment explains the underlying transcriptomic changes causing sebum suppression but also the adverse effects associated with systemic isotretinoin therapy.
Keywords: Acne, Gene expression, Isotretinoin, p53, Sebaceous gland
Introduction
Acne vulgaris is a chronic inflammatory cutaneous disorder with a complex multifactorial pathogenesis depending on increased and modified sebum production, altered upper pilosebaceous duct keratinization, loss of follicular microbial diversity with aberrant biofilm-producing phylotype colonization of Cutibacterium acnes (C. acnes), and follicular as well as perifollicular inflammation [1–6].
Sebum is the secretory product of sebocytes derived from sebaceous gland holocrine secretion [6]. Exaggerated sebocyte activity stimulated by increased insulin-like growth factor 1 (IGF-1)/IGF1 receptor (IGF1R)/phosphatidylinositol-3 kinase (PI3K)/AKT [7] and androgen/androgen receptor (AR) signalling [8] enhances and modifies sebum production exhibiting higher amounts of monounsaturated pro-inflammatory fatty acids [9, 10].
On the transcriptional level, sebaceous glands of acne patients exhibit decreased nuclear expression of the transcription factors FoxO1 and FoxO3a [11–13] and increased activity of mechanistic target of rapamycin complex 1 (mTORC1) [13–15], a key regulator of sebocyte proliferation, lipogenesis, autophagy end endocrine responses in acne pathogenesis [16–18]. Of note, FoxO1 operates as a nuclear co-suppressor of AR [19]. FoxO1 and FoxO3a are extruded from the nucleus into the cytoplasm by insulin/IGF-1/AKT-mediated FoxO phosphorylation [19, 20]
Importantly, the transcription factor p53, known as the guardian of the genome [21, 22], is critically involved in the expression of FoxO1 [23], FoxO3a [24, 25], tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) [26], tumour necrosis factor receptor superfamily member 10B (TNFRSF10B; death receptor 5) [23], repression of AR [27] and IGF1R [28], and suppression of IGF-1-AKT-mTORC1 signalling [28–31], thus linking crucial transcriptional and nutrigenomic regulators involved in acne pathogenesis.
Increased mTORC1 activity promotes cell growth and anabolism [32]. Increased body mass index (BMI) has been positively associated with acne risk and severity in several studies [33–36]. Notably, p53 has been recognized as crucial player in nutrient sensing pathways and functions as a negative regulator of mTORC1 [29–31] and adipogenesis [37].
Among the various agents used for the treatment of acne vulgaris, isotretinoin (13-cis retinoic acid) is the most effective sebum-suppressive drug reducing skin surface and comedonal lipids [28, 38]. Isotretinoin is considered the first choice for the treatment of cystic acne [39, 40].
Translational evidence suggests that isotretinoin's desired anti-acne effects and its adverse effects including teratogenicity are based on isotretinoin-mediated apoptosis [41, 42]. In fact, Nelson et al. [43, 44] demonstrated in several studies that isotretinoin induces apoptosis and cell cycle arrest in human SEB-1 sebocytes [43] and increases the expression of the apoptotic protein TRAIL [44], which mediates the apoptotic effects of isotretinoin in human sebaceous glands.
Sebocytes are able to isomerize 13-cis retinoic acid to all-trans retinoic acid (ATRA) [45]. In sebocytes, isotretinoin increases the expression of cellular retinoid acid-binding protein-2 (CRABP-2) [46], which transports ARTA into the nucleus to retinoic acid receptors (RARs) regulating gene expression [47–49]. The CRABP2 gene promoter contains a TATA-box that is rapidly activated by ATRA through a retinoic acid response element (RARE) [48]. Compared to epidermis, CRABP-2 is strongly expressed in suprabasal sebocytes in isotretinoin-treated patients, promoting a preferential transport of ATRA to RARs in sebocytes [44, 50]; ATRA binding to nuclear RARs enhances the expression key transcription factors involved in apoptosis including forkhead box transcription factors FoxO1 and FoxO3a and TRAIL [51]. It has been demonstrated by Agamia et al. [53] that nuclear levels of FoxO1 and FoxO3a increased in sebaceous glands of patients with acne vulgaris after treatment with oral isotretinoin. It has been shown in epidermal keratinocytes that the expression of p53 is upregulated by ATRA exposure [54, 55]. Shi et al. [56] observed in human primary keratinocytes enhanced expression of p53, FoxO1 and p21 after isotretinoin exposure. As recently hypothesized by Melnik [57], isotretinoin-induced overexpression of p53 may also be the underlying pharmacological mode of action for sebocyte apoptosis and isotretinoin-mediated teratogenicity (neural crest cell apoptosis) [41, 42]. In fact, both isotretinoin and ATRA induce the expression of p53 and apoptosis in melanoma cells [58, 59]. Isotretinoin/ATRA-mediated upregulation of p53 in isotretinoin-treated acne patients may also be the underlying mechanism enhancing the expression of pro-apoptotic effectors including the p53-responsive genes FOXO1 [23], FOXO3A [23, 25] and TNFSF10 [26] promoting sebocyte apoptosis [26, 50, 54, 57]. Indeed, increased expression of ATRA-induced CRABP-2 and TRAIL have been demonstrated in the basal and suprabasal layers of sebaceous glands and skin during isotretinoin treatment of acne patients [44, 46, 60], where increased isotretinoin-mediated apoptosis activity has been observed [44].
The aim of this study was to assess the expression of p53 in the skin and sebaceous glands of acne patients before and during oral isotretinoin treatment to understand isotretinoin’s transcriptomic mode of action in the treatment of acne under clinical in vivo conditions.
Patients and methods
Patients and patient samples
This study was conducted on 25 patients suffering from acne vulgaris and 15 socio-demographically cross-matched healthy volunteers who served as controls. All participants were recruited from the Dermatology Outpatient Clinic of the Alexandria Main University Hospital. Approval by ethical committee as well as written informed consent was obtained from all patients and controls. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki.
Patients presenting acne vulgaris within the age range of 17–25 years of both sexes were included. The exclusion criteria included female patients on antiandrogen therapy or with signs of hyperandrogenism (polycystic ovaries, androgenic alopecia, hirsutism), patients with a history of prior systemic retinoid intake or antibiotic therapy during the last 6 months, patients with diabetes mellitus or other endocrine diseases. Patients were not advised to change their usual dietary habits during the study. Patients were subjected to a full history, general medical examination, and dermatological examination.
Acne severity in patients was classified using a simple acne grading system [61, 62] based on the predominant lesion and the number and locations of acne lesions. It classifies acne into four grades as follows: Grade 1: comedones and occasional papules; grade 2: papules, comedones and a few pustules; grade 3: predominant pustules, nodules and abscesses; and grade 4: mainly nodules, abscesses and widespread scarring.
All patients were given oral systemic isotretinoin for treatment of acne with a dose of 0.6 mg/kg body weight/day for 6 weeks, after full routine investigations before treatment.
Skin biopsy
The procedure was explained to all patients. One 5 mm punch biopsy (for the immunohistochemical study) and two 2.5 mm punch biopsies (for ELISA and PCR) were taken from lesional skin on the back of the patient before isotretinoin treatment and another three biopsies were taken from residual non-scarred lesions on the back after 6 weeks of treatment.
The control subjects were those undergoing surgical procedure on the back recruited from the plastic surgery department. Single 5 mm punch biopsy and two 2.5 mm punch biopsies were taken from normal skin of the back.
Histopathological examination and immunohistochemical detection of p53
Skin biopsies were fixed in 10% formalin. Then tissue sections were dehydrated in a series of ascending grades of ethyl alcohol (70%, 95%, 100%). Biopsy specimens were cleared in xylene then embedded in paraffin wax, sectioned by microtome and stained with Hematoxylin and Eosin stain with addition of cover slips. Histopathological examination was performed using a light microscope; all specimens were prepared for immunohistochemical staining using mouse anti-human monoclonal p53 antibody (isotype: IgG2b). (Anti-p53 antibody) [pAb122] (ab90363) (Abcam, Cambridge, U.K.) [63].
The overall staining intensities in sebaceous gland areas of the slides stained with p53 monoclonal antibodies were scored using digital image analysis with a computer-assisted light microscope. The image of each slide was captured using a 400 × objective lens. Images were viewed and recorded using an Olympus microscope (Olympus, Centre Valley, PA, U.S.A.) equipped with a Spot digital camera (Spot Imaging Solutions, Sterling Heights, MI, U.S.A.) and MATLAB software (MathWorks, Natick, MA, U.S.A.). The mean values of each reaction were based on the mean pixel number. The integrity of the colour intensity was based on grey-level transition probabilities in digitized images from dark to light. The overall intensity of staining of slides stained with p53 monoclonal antibody was scored according to nuclear expression into 0 if staining intensity is < 10%, + 1 if staining intensity is 10% ≤ 30%, + 2 if staining intensity is 31% ≤ 50% and + 3 if staining intensity is > 50% [53].
Determination of p53 protein concentration by enzyme-linked immunosorbent assay
Skin biopsies were collected and preserved at – 80 ℃. After determination of sample weight and addition of PBS pH 7.4, samples were homogenized by hand or grinders and finally centrifuged for 3 min at a speed of 10,000 r.p.m. to remove the supernatant.
The ELISA kit (Abcam, Human p53 ELISA Kit (ab171571) was used for the determination of p53 protein. This assay is based on the principle of double-antibody sandwich technique to detect human p53 tumour protein. For further technical details, see procedure published by the manufacturer. Antibodies labelled with enzyme were added for an incubation time of 60 min at 37 ℃. After washing the plates and addition of Chromogen solution A, B, optical density (OD) values were measured for the calculation of p53 protein concentrations of the samples [64].
Determination of P53 gene expression by quantitative reverse transcription real time PCR
Total RNA was extracted from 10 mg skin tissue after lysis and homogenization, using silicate gel technique provided by the RNeasy Mini Kit (Qiagen) [65]. The concentration and purity of RNA were measured at 260, 280 and 230 nm using Nano Drop 2000c spectrophotometer (Thermo Scientific, USA). A ratio of A260/A280 = 1.8–2.1 and A260/A230 = 1.8–2.1 indicates highly pure RNA. Total RNA was reverse transcribed into cDNA using high-capacity reverse transcriptase kit (Applied Biosystems™, USA, catalog no. 4368814). To detect TP53 in tissue samples, primers had been matched to the mRNA sequences of the target genes (NCBI Blast software). GADPH was used as housekeeping gene [66].
P53
5’-AGA GTC TAT AGG CCC ACC CC-3’ (forward)
5’-GCT CGA CGC TAG GAT CTG AC-3’ (reverse)
GAPDH
5’-CAT GGG GAA GGT GAA GGT CGG A-3’ (forward)
5’-TTG GCTCCC CCC TGC AAA TGA G-3’ (reverse)
The PCR amplification was performed in a 25 µl reaction volume including SYBR green PCR Master Mix (Applied Biosystems) using ABI 7900 sequence detector (Applied Biosystems). The reaction was performed with 10 min of initial stage to activate the DNA polymerase, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Single product formation was confirmed by melting point analysis, and comparative CT method was used to calculate relative gene expression with GADPH as an endogenous control. For statistical analysis of the CT values, 2-ΔΔCT method was applied for each specific primer and real-time PCR [66].
Statistical analysis
Data were fed to the computer and analysed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp.). Shapiro–Wilk test was used to verify the normality of distribution of variables; comparisons between groups for categorical variables were assessed using χ2 test (Monte Carlo). Marginal homogeneity test was used to analyse the significance between the different stages. Mann–Whitney test was applied to compare between two groups for not normally distributed quantitative variables. Wilcoxon signed ranks test assessed for comparison between two periods for not normally distributed quantitative variables. ANOVA was used for comparing different categories. Kruskal–Wallis test was used to compare different categories for abnormally distributed quantitative variables. Pearson coefficient was used to correlate between two normally distributed quantitative variables. Significance of the obtained results was judged at the 5% level [67, 68].
Results
Patient data
This study was conducted on 40 subjects. Twenty-five patients suffering from acne vulgaris (18 males and 7 females) and 15 acne-free subjects served as controls (12 males and 3 females). The mean age of patients and controls was 20.08 ± 2.91 and 21.87 ± 3.07 years, respectively. There was a statistically significant difference between BMI in cases and controls exhibiting a mean BMI of 25.87 ± 2.47 kg/m2 in patients and 23.26 ± 3.44 kg/m2 in controls, respectively (p = 0.008*).
Clinically, there was statistically improvement in acne severity after treatment among the patients (p < 0.001*). Before treatment, 12 cases (48%) were grade IV, 9 cases (36%) were grade III, and 4 cases (16%) were grade II, respectively. While after treatment, 17 patients (68%) were grade I; 8 patients (32%) were grade II. Acne severity was not correlated to the age of patients while it was significantly correlated to BMI of patients before treatment.
Laboratory findings
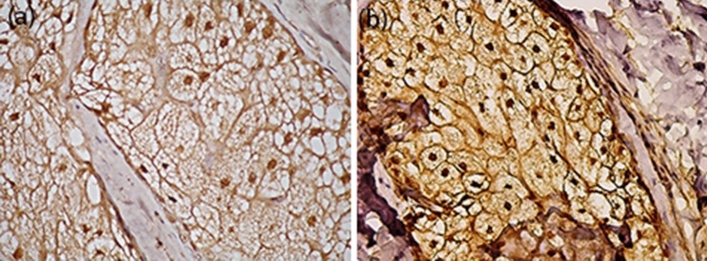
In skin biopsies taken from acne patients staining intensity of p53 increased after isotretinoin treatment compared to pre-treatment and acne-free controls. Figure 1 presents the representative immunostaining pattern with p53 antibody before and after isotretinoin treatment showing increased nuclear stain intensity in the patients’ sebaceous glands after isotretinoin treatment.
Fig. 1.
Immune staining of nuclear p53 before and after isotretinoin treatment. a p53 nuclear immune staining in sebaceous gland of acne patient before isotretinoin therapy. b Intensified nuclear p53 immune staining after 6 weeks of isotretinoin therapy
Before treatment the stain intensity of p53 was zero in 19 patients (76%), + 1 in four patients (16%) and + 2 in two patients (8%), respectively. While after treatment, the stain intensity was + 1 in five patients (20%), + 2 in eight patients (32%), and + 3 in twelve patients (48%), respectively. The difference in immunohistochemical expression of p53 before and after treatment was significant (MHp1 < 0.001*). p53 expression in the control group was significantly higher compared to the pre-treatment acne patients, (MCp2 = 0.521), while it was significantly lower when compared to the post-treatment biopsies (MCp3 < 0.001*) (Table 1).
Table 1.
Comparison between p53 expression of patients and controls
| Cases (n = 25) | Control (n = 15) | ||
|---|---|---|---|
| Before | After | ||
| P53 protein concentration by ELISA (concentration of P53/ mg protein) | |||
| Median (Min.–Max.) | 94.8 (49.9–121) | 183.3 (129.3–503) | 161 (114–202) |
| Mean ± SD | 90.78 ± 23.58 | 225.9 ± 105.3 | 160.93 ± 31.03 |
| Significance | Zp1 < 0.001*, Up2 < 0.001*, Up3 = 0.052 | ||
| P53 gene expression by RT-PCR | |||
| Median (Min.–Max.) | 0.04 (0.0–0.20) | 0.71 (0.48–0.98) | 0.27 (0.20–0.31) |
| Mean ± SD | 0.07 ± 0.06 | 0.72 ± 0.17 | 0.26 ± 0.04 |
| Significance | Zp1 < 0.001*, Up2 < 0.001*, Up3 < 0.001* | ||
| Immunohistochemical expression of P53 | |||
| Grade 0 | 19 (76.0%) | 0 (0.0%) | 10 (66.7%) |
| Grade + 1 | 4 (16.0%) | 5 (20.0%) | 2 (13.3%) |
| Grade + 2 | 2 (8.0%) | 8 (32.0%) | 3 (20.0%) |
| Grade + 3 | 0 (0.0%) | 12 (48.0%) | 0 (0.0%) |
| Significance | MHp1 < 0.001*, MCp2 = 0.521*, MCp3 < 0.001* | ||
p1: p value for comparing between before and after; p2: p value for comparing between cases (before) and control; p3: p value for comparing between cases (after) and control
SD standard deviation, U Mann–Whitney test, Z Wilcoxon signed ranks test, MH Marginal homogeneity test, MC Monte Carlo (χ2 test)
*Statistically significant at p ≤ 0.05
The mean of p53 protein concentration determined by ELISA before treatment was 90.78 ± 23.58 p53/mg protein. This increased significantly after isotretinoin treatment to 225.85 ± 105.34 p53/mg protein (Zp1 < 0.001*). The mean of p53 protein concentration in control subjects was 160.93 ± 31.03 p53/mg protein) (Up2 < 0.001*). Similarly, the mean p53 cDNA expression by PCR before treatment was 0.07 ± 0.06 that increased significantly after treatment to be 0.72 ± 0.17 (Zp1 < 0.001*). cDNA expression before treatment with isotretinoin was significantly lower than in controls (0.26 ± 0.04) (Up2 < 0.001*). After-treatment results were significantly higher than p53 baseline expression of controls. (Up3 < 0.001) (Table 1, Fig. 2).
Fig. 2.
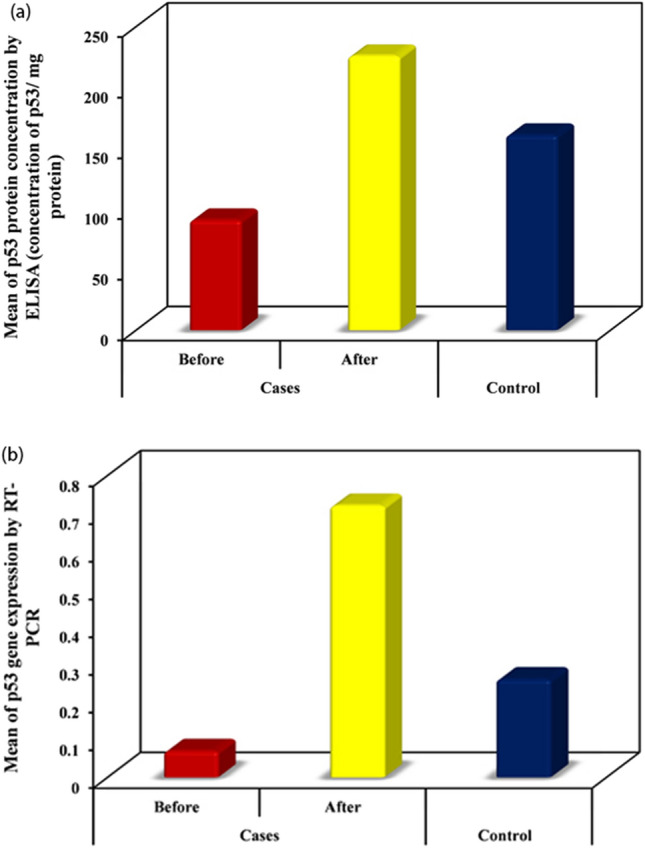
p53 protein and gene expression before and after isotretinoin therapy. a Illustrates the changes of p53 protein and b the changes of p53 gene expression of back skin biopsies of acne patients before and after 6 weeks of oral isotretinoin treatment compared to acne-free control skin biopsies
Furthermore, we could observe a negative correlation between p53 protein and gene expression with acne severity grade (Fig. 3). In addition, a negative correlation has been found between patients’ pre-treatment p53 expression and BMI (Fig. 4).
Fig. 3.
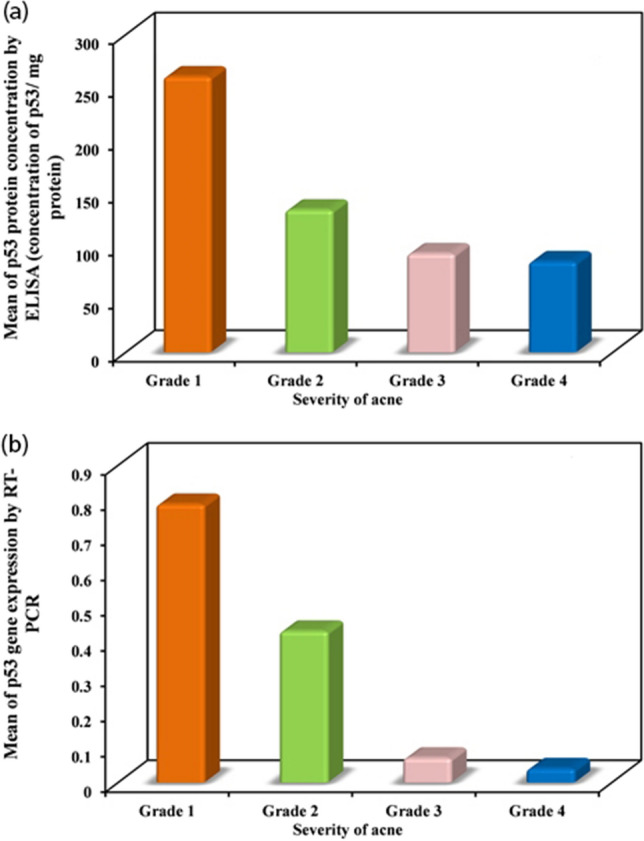
Correlation between p53 protein and gene expression with acne severity grade. a Shows a negative correlation between p53 protein expression and b an inverse relation between p53 gene expression with acne severity grade
Fig. 4.
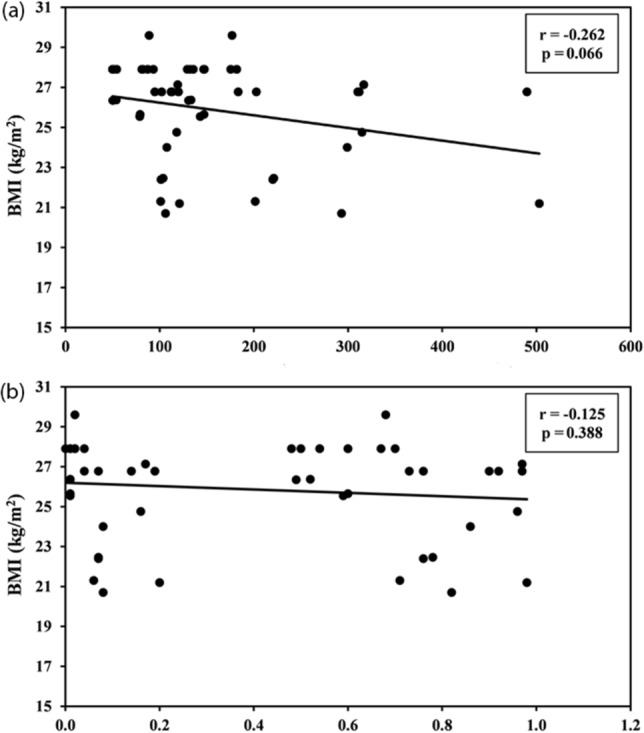
a Correlation between BMI and protein expression by ELISA in cases group (n = 50) b correlation between BMI and gene expression of p53 by RT-PCR in cases group (n = 50)
Discussion
The sebum-suppressive effect of isotretinoin has been related to sebocyte apoptosis [41–44] with isotretinoin-mediated p21-induced cell cycle arrest [43] and upregulation of pro-apoptotic transcription factors including FoxO1 [53] and FoxO3a [53] as well as the apoptosis effector TRAIL [44, 60]. The transcription factor p53, known as the guardian of the genome [69], is a key regulator of cell fate decisions including cycle control and induction of apoptosis depending on the magnitude of p53 transcription and activation. Notably, p53 promotes the expression of the cell cycle inhibitor p21 (CDKN1A) [71] and the pro-apoptotic proteins FoxO1 [23], FoxO3a [23, 25], and TRAIL [26] and inhibits anti-apoptotic pro-survival effectors such as IGF1R [28], AR [27] and survivin (BIRC5) [72], all known p53 target genes involved in acne pathogenesis. It has been demonstrated in primary human keratinocytes and melanocytes that isotretinoin and ATRA increase the expression of p53 [54–59]. According to a recent hypothesis, isotretinoin’s mode of action and its adverse effects are related to enhanced expression of p53 [57, 72]. In fact, our study provides first experimental evidence that isotretinoin significantly upregulates the expression of p53 in the skin and sebaceous glands of acne patients after 6 weeks of oral isotretinoin therapy with the commonly used daily dose of 0.6 mg/kg body weight.
Remarkably, the skin of acne patients compared to acne-free controls exhibits lower levels of p53 expression (Fig. 2), whereas after isotretinoin-treatment p53 levels significantly exceeded p53 levels in healthy skin, pointing to a strong induction of p53 by systemic and prolonged isotretinoin exposure.
It is noteworthy to mention that p53 expression is regulated by endocrine and nutrient signalling. Increased insulin and IGF-1 signalling, which both activate the kinase AKT, result in phosphorylation and activation of E3 ubiquitin ligase mouse double minute 2 (MDM2) promoting the proteasomal degradation of p53 [73, 74]. Western diet with increased insulin/IGF-1/AKT signalling [11] may thus reduce the expression of p53, the key negative regulator of mTORC1 [29–31], which exhibits increased activity in the skin and sebaceous glands of acne patients [13–17]. The fundamental ability of mTORC1 promoting cell growth and anabolism [32] may also explain the potential relation between BMI and acne risk [33–36]. In fact, p53 is not only a tumour suppressor but has been appreciated as a crucial player in nutrient sensing pathways serving as a negative regulator of mTORC1 [29–31] and adipogenesis [37].
Importantly, activated mTORC1 is a key suppressor of autophagy [75]. Remarkably, p53 not only induces apoptosis but also stimulates autophagy [76, 77]. It has recently been shown in immortalized SZ95 sebocytes that isotretinoin treatment, partly via activation of FoxO1, increased the expression of ATG5 and induced autophagy resulting in reduced sebaceous lipid accumulation [78]. Notably, p53 can activate the expression of a large set of target genes that are involved in the autophagic programme including ATG5 [79, 80]. Autophagy is required for robust p53-dependent apoptosis. Thus, autophagy and apoptosis are two closely related p53-dependent cellular responses [81, 82]. It is thus conceivable that isotretinoin induces both p53-mediated autophagy as well as p53-induced apoptosis depending on the dose and duration of isotretinoin exposure and the resulting magnitude of p53 expression.
Moreover, it should be kept in mind that p53 is partially inactivated by simian virus 40 large T antigen in immortalized SZ95 and SEB-1 sebocytes [83, 84], which may thus not be suitable cell lines for studying p53-dependent effects of sebaceous gland regulation leading to paradoxical even acnegenic effects [85] disputed earlier [86].
There is compelling translational evidence that isotretinoin-mediated upregulation of p53 expression explains isotretinoin’s teratogenicity via p53-mediated neural crest cell apoptosis [41, 42]. Isotretinoin also induces apoptosis in primary human keratinocytes [56], melanoma cells [58, 59], rat ovarian granulosa cells [87, 88], hepatoma cells [89], associated with decreased expression of the apoptosis inhibitor survivin [89]. Increased serum levels of survivin have been reported in acne patients compared to controls [90]. Of note, survivin (BIRC5) expression is negatively regulated by p53 [91]. In accordance with these findings and our results, we conclude that isotretinoin-induced expression of p53 not only promotes sebocyte apoptosis in human sebaceous glands as the predominant sebum-suppressive effect but is also responsible for all isotretinoin’s adverse effects.
The most common mucocutaneous side effects of isotretinoin therapy, dry skin, have been related to increased expression of keratinocyte aquaporin 3 (AQP3), which damages the skin barrier and enhances transepidermal water loss causing skin dryness [92]. Notably, AQP3 is a p53 target gene [93]. Other members of the aquaporin family, AQP1 and AQP4 [94, 95] have been linked to intracranial hypertension (pseudotumor cerebri), a potential adverse effect of isotretinoin [96], and appear as well to be related to upregulated p53 [97, 98]. In addition, isotretinoin-induced hypertriglyceridemia [99] is associated with increased plasma levels of apolipoprotein B100 in very low-density lipoprotein (VLDL) and low density lipoproteins (LDL) [100]. The gene encoding apoB100 (APOB) has been identified as p53 target gene [101].
It is important to remember that retinoids induce primary and secondary transcriptional responses depending on dose and duration of retinoid exposure [51]. Sufficient nuclear transport of ATRA via CRABP2 is mandatory for ATRA-induced transcriptomic changes [51] including isotretinoin/ATRA-induced transcriptional modification resulting in sufficient sebum suppression [46]. Increased expression of CRABP2 in isotretinoin-treated sebaceous glands of patients with acne has been observed after weeks of oral isotretinoin exposure [46], whereas short-term (6 h, 24 h) isotretinoin exposure of immortalized p53-inactivated SZ95 sebocytes did neither exhibit increased CRABP2 nor upregulated p53 or FoxO1 expression [102]. This is in contrast to our in vivo findings under clinical conditions observed in patients treated with isotretinoin for 6 weeks, whose sebaceous glands are not p53-inactivated by SV40 viral transfection [53].
Notably, p53 maintains baseline expression of common tumour suppressor genes including FoxO1 [23]. Over the last 10 years, decreased FoxO1 expression has been linked to acne pathogenesis [11, 16, 53, 103], whereas isotretinoin treatment increases FoxO1 expression in sebaceous glands of acne patients [53, 104, 105]. The p53 target gene FoxO1 is a nuclear co-suppressor of multiple transcription factors critically involved in acne pathogenesis such as AR [19], SREBF1 [106], PPARA [107] and is a crucial promoter of genes involved in apoptosis. Recent evidence indicates that FoxO1 is involved in the induction of autophagy in isotretinoin-treated SZ95 sebocytes [78]. Furthermore, FoxO1 promotes the expression of GATA6, a critical transcription factor maintaining appropriate keratinocyte proliferation and differentiation of the infundibulum of the human sebaceous follicle [108], which is deficiently expressed in sebaceous follicles of acne patients linking p53-FoxO1-GATA6 deficiency to comedogenesis.
Reduced baseline expression of the tumour suppressor p53 in acne patients compared to acne-free controls may also explain the increased risk of acne patients for common p53-related malignancies such as prostate cancer [109, 110], and breast cancer [111]. Notably, there is no observed acne and very low cancer incidence in IGF-1-deficient patients with Laron syndrome [112], who exhibit higher p53-FoxO1 signalling [113]. In contrast, Western diet with high glycaemic load and milk/dairy consumption increases insulin/IGF-1 signalling promoting AKT/MDM2-mediated proteasomal degradation of p53 [50, 114, 116], whereas forced upregulation of p53-FoxO1 signalling may contribute to the tumour suppressing effect of isotretinoin in neuroblastoma [117] and retinoid chemoprevention of non-melanoma skin cancer [118].
Taken together, our study provides experimental evidence for increased nuclear expression of p53 in sebaceous glands and skin of acne patients after oral isotretinoin treatment and substantiates that enforced p53-FoxO signalling causes all desired and adverse effects of systemic isotretinoin therapy.
Author contributions
NFA, KFM, REM, EAE, RS, NE, IT and IIZ equally contributed to sample processing, immunofluorescence labelling and statistical analysis of the data. RAG performed the PCR and ELISA techniques for skin biopsies, BCM supervised the editing of the introduction and discussion section of the manuscript. All authors approved the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethics approval
Ethical committee of Alexandria University Main Hospital according to 1964 Declaration of Helsinki.
Consent to participate
Given by all study participants.
Consent for publication
Given by all patients and all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi: 10.1038/nrdp.2015.29. [DOI] [PubMed] [Google Scholar]
- 2.Clayton RW, Göbel K, Niessen CM, et al. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br J Dermatol. 2019;181:677–690. doi: 10.1111/bjd.17981. [DOI] [PubMed] [Google Scholar]
- 3.Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:1953. doi: 10.12688/f1000research.15659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337–349. doi: 10.1007/s00403-019-01908-x. [DOI] [PubMed] [Google Scholar]
- 5.Plewig G, Melnik B, Chen W. Plewig and Kligman´s Acne and Rosacea. Cham: Springer; 2019. Acne pathogenesis; pp. 45–61. [Google Scholar]
- 6.Fischer H, Fumicz J, Rossiter H, et al. Holocrine secretion of sebum is a unique DNase2-dependent mode of programmed cell death. J Invest Dermatol. 2017;137:587–594. doi: 10.1016/j.jid.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu T, Wei Z, Ju Q, et al. Sex hormones and acne: state of the art. J Dtsch Dermatol Ges. 2021;19:509–515. doi: 10.1111/ddg.14426. [DOI] [PubMed] [Google Scholar]
- 9.Zhou M, Gan Y, He C, et al. Lipidomics reveals skin surface lipid abnormity in acne in young men. Br J Dermatol. 2018;179:732–740. doi: 10.1111/bjd.16655. [DOI] [PubMed] [Google Scholar]
- 10.Okoro OE, Adenle A, Ludovici M, et al. Lipidomics of facial sebum in the comparison between acne and non-acne adolescents with dark skin. Sci Rep. 2021;11:16591. doi: 10.1038/s41598-021-96043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol. 2013;22:311–315. doi: 10.1111/exd.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirdamadi Y, Thielitz A, Wiede A, et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol Cell Endocrinol. 2015;415:32–44. doi: 10.1016/j.mce.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299–1307. doi: 10.1111/bjd.14409. [DOI] [PubMed] [Google Scholar]
- 14.Monfrecola G, Lembo S, Caiazzo G, et al. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients' skin. Exp Dermatol. 2016;25:153–155. doi: 10.1111/exd.12885. [DOI] [PubMed] [Google Scholar]
- 15.Lembo S, Di Caprio R, Balato A, et al. The increase of mTOR expression is consistent with FoxO1 decrease at gene level in acne but not in psoriasis. Arch Dermatol Res. 2020;312:77–80. doi: 10.1007/s00403-019-01959-0. [DOI] [PubMed] [Google Scholar]
- 16.Melnik BC. Acne vulgaris: an inflammasomopathy of the sebaceous follicle induced by deviated FoxO1/mTORC1 signaling. Br J Dermatol. 2016;174:1186–1188. doi: 10.1111/bjd.14564. [DOI] [PubMed] [Google Scholar]
- 17.Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29–40. doi: 10.1016/j.clindermatol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Briganti S, Flori E, Mastrofrancesco A, et al. Acne as an altered dermato-endocrine response problem. Exp Dermatol. 2020;29:833–839. doi: 10.1111/exd.14168. [DOI] [PubMed] [Google Scholar]
- 19.Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 20.Santo EE, Stroeken P, Sluis PV, et al. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013;73:2189–2198. doi: 10.1158/0008-5472.CAN-12-3767. [DOI] [PubMed] [Google Scholar]
- 21.Marcel V, Nguyen Van Long F, et al. 40 years of research put p53 in translation. Cancers (Basel) 2018;10:152. doi: 10.3390/cancers10050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laptenko O, Prives C. p53: master of life, death, and the epigenome. Genes Dev. 2017;31:955–956. doi: 10.1101/gad.302364.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappas K, Xu J, Zairis S, et al. p53 maintains baseline expression of multiple tumor suppressor genes. Mol Cancer Res. 2017;15:1051–1062. doi: 10.1158/1541-7786.MCR-17-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurinna S, Stratton SA, Tsai WW, et al. Direct activation of forkhead box O3 by tumor suppressors p53 and p73 is disrupted during liver regeneration in mice. Hepatology. 2010;52:1023–1032. doi: 10.1002/hep.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renault VM, Thekkat PU, Hoang KL, et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207–3221. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuribayashi K, Krigsfeld G, Wang W, et al. TNFSF10 (TRAIL), a p53 target gene that mediates p53-dependent cell death. Cancer Biol Ther. 2008;7:2034–2038. doi: 10.4161/cbt.7.12.7460. [DOI] [PubMed] [Google Scholar]
- 27.Alimirah F, Panchanathan R, Chen J, et al. Expression of androgen receptor is negatively regulated by p53. Neoplasia. 2007;9:1152–1159. doi: 10.1593/neo.07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci USA. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Z, Zhang H, Levine AJ, et al. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine AJ, Feng Z, Mak TW, et al. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 31.Feng Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb Perspect Biol. 2010;2:a001057. doi: 10.1101/cshperspect.a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72–82. doi: 10.1016/j.ceb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67:1129–1135. doi: 10.1016/j.jaad.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Seleit I, Bakry OA, Abdou AG, et al. Body mass index, selected dietary factors, and acne severity: are they related to in situ expression of insulin-like growth factor-1? Anal Quant Cytopathol Histpathol. 2014;36:267–278. [PubMed] [Google Scholar]
- 35.Melnik BC, John SM, Plewig G. Acne: risk indicator for increased body mass index and insulin resistance. Acta Derm Venereol. 2013;93:644–649. doi: 10.2340/00015555-1677. [DOI] [PubMed] [Google Scholar]
- 36.Sas K, Reich A. High body mass index is a risk factor for acne severity in adolescents: a preliminary report. Acta Dermatovenerol Croat. 2019;27:81–85. [PubMed] [Google Scholar]
- 37.Krstic J, Reinisch I, Schupp M, et al. p53 functions in adipose tissue metabolism and homeostasis. Int J Mol Sci. 2018;19:2622. doi: 10.3390/ijms19092622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melnik B, Kinner T, Plewig G. Influence of oral isotretinoin treatment on the composition of comedonal lipids. Implications for comedogenesis in acne vulgaris. Arch Dermatol Res. 1988;280:97–102. doi: 10.1007/BF00417712. [DOI] [PubMed] [Google Scholar]
- 39.Peck GL, Olsen TG, Yoder FW, et al. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Eng J Med. 1979;300:329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- 40.Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178:76–85. doi: 10.1111/bjd.15668. [DOI] [PubMed] [Google Scholar]
- 41.Melnik BC. Apoptosis may explain the pharmacological mode of action and adverse effects of isotretinoin, including teratogenicity. Acta Derm Venereol. 2017;97:173–181. doi: 10.2340/00015555-2535. [DOI] [PubMed] [Google Scholar]
- 42.Melnik BC. Overexpression of p53 explains isotretinoin's teratogenicity. Exp Dermatol. 2018;27:91–93. doi: 10.1111/exd.13420. [DOI] [PubMed] [Google Scholar]
- 43.Nelson AM, Gilliland KL, Cong Z, et al. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126:2178–2189. doi: 10.1038/sj.jid.5700289. [DOI] [PubMed] [Google Scholar]
- 44.Nelson AM, Cong Z, Gilliland KL, et al. TRAIL contributes to the apoptotic effect of 13-cis retinoic acid in human sebaceous gland cells. Br J Dermatol. 2011;165:526–533. doi: 10.1111/j.1365-2133.2011.10392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukada M, Schröder M, Roos TC, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115:321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 46.Sitzmann JH, Bauer FW, Cunliffe WJ, et al. In situ hybridization analysis of CRABP II expression in sebaceous follicles from 13-cis retinoic acid-treated acne patients. Br J Dermatol. 1995;133:241–248. doi: 10.1111/j.1365-2133.1995.tb02622.x. [DOI] [PubMed] [Google Scholar]
- 47.Napoli JL. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther. 2017;173:19–33. doi: 10.1016/j.pharmthera.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei LN. Cellular retinoic acid binding proteins: Genomic and non-genomic functions and their regulation. Subcell Biochem. 2016;81:163–178. doi: 10.1007/978-94-024-0945-1_6. [DOI] [PubMed] [Google Scholar]
- 49.Lixa C, Clarkson MW, Iqbal A, et al. Retinoic acid binding leads to CRABP2 rigidification and dimerization. Biochemistry. 2019;58:4183–4194. doi: 10.1021/acs.biochem.9b00672. [DOI] [PubMed] [Google Scholar]
- 50.Melnik BC. Mechanism of action of isotretinoin, chapt. 4. In: Karadag AS, Aksoy B, Parish LC, editors. Retinoids in dermatology. Boca Raton: CRC Press; 2019. pp. 1–13. [Google Scholar]
- 51.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakoe Y, Sakoe K, Kirito K, et al. FOXO3A as a key molecule for all-trans retinoic acid-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia. Blood. 2010;115:3787–3795. doi: 10.1182/blood-2009-05-222976. [DOI] [PubMed] [Google Scholar]
- 53.Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleo-cytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344–1351. doi: 10.1111/exd.13787. [DOI] [PubMed] [Google Scholar]
- 54.Mrass P, Rendl M, Mildner M, et al. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: a possible explanation for tumor preventive action of retinoids. Cancer Res. 2004;64:6542–6548. doi: 10.1158/0008-5472.CAN-04-1129. [DOI] [PubMed] [Google Scholar]
- 55.Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427–439. doi: 10.1002/jcp.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi G, Liao PY, Cai XL, et al. FoxO1 enhances differentiation and apoptosis in human primary keratinocytes. Exp Dermatol. 2018;27:1254–1260. doi: 10.1111/exd.13775. [DOI] [PubMed] [Google Scholar]
- 57.Melnik BC. p53: key conductor of all anti-acne therapies. J Transl Med. 2017;15:195. doi: 10.1186/s12967-017-1297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Rosdahl I. Expression profiles of p53, p21, bax and bcl-2 proteins in all-trans-retinoic acid treated primary and metastatic melanoma cells. Int J Oncol. 2004;25:303–308. [PubMed] [Google Scholar]
- 59.Guruvayoorappan C, Pradeep CR, Kuttan G. 13-cis-retinoic acid induces apoptosis by modulating caspase-3, bcl-2, and p53 gene expression and regulates the activation of transcription factors in B16F–10 melanoma cells. J Environ Pathol Toxicol Oncol. 2008;27:197–207. doi: 10.1615/JEnvironPatholToxicolOncol.v27.i3.40. [DOI] [PubMed] [Google Scholar]
- 60.Kelhälä HL, Fyhrquist N, Palatsi R, et al. Isotretinoin treatment reduces acne lesions but not directly lesional acne inflammation. Exp Dermatol. 2016;25:477–478. doi: 10.1111/exd.12971. [DOI] [PubMed] [Google Scholar]
- 61.Tutakne MA, Chari KVR. Acne, rosacea and perioral dermatitis. In: Valia RG, Valia AR, editors. IADVL Textbook and atlas of dermatology. 2. Mumbai: Bhalani Publishing House; 2003. pp. 689–710. [Google Scholar]
- 62.Ruixing Yu, Fei W, Ning X, Cui Y. Inter-rater variability and consistency within four acne grading systems recommended in China, USA, and Europe. J Cosmet Dermatol. 2022;00:1–7. doi: 10.1111/jocd.15178. [DOI] [PubMed] [Google Scholar]
- 63.Shi SR, Guo J, Cote RJ, et al. Sensitivity and detection efficiency of a novel two-step detection system (PowerVision) for immunohistochemistry. Appl Immunohistochem. 1999;7:201. doi: 10.1097/00022744-199909000-00005. [DOI] [Google Scholar]
- 64.Liu Y, Yan J, Wang F. Effects of TACE combined with precise RT on p53 gene expression and prognosis of HCC patients. Oncol Lett. 2018;16:5733–5738. doi: 10.3892/ol.2018.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohtani S, Kagawa S, Tango Y, et al. Quantitative analysis of p53-targeted gene expression and visualization of p53 transcriptional activity following intratumoral administration of adenoviral p53 in vivo. Mol Cancer Ther. 2004;3:93–100. doi: 10.1158/1535-7163.93.3.1. [DOI] [PubMed] [Google Scholar]
- 66.Arya M, Shergill IS, Williamson M, et al. Basic principles of real-time quantitative PCR. Expert Rev Mol Diagn. 2005;5:209–219. doi: 10.1586/14737159.5.2.209. [DOI] [PubMed] [Google Scholar]
- 67.Kotz S, Balakrishnan N, Read CB, et al. Encyclopedia of statistical sciences. 2. Hoboken: Wiley Interscience; 2006. [Google Scholar]
- 68.Kirkpatrick LA, Feeney BC. A Simple guide to IBM SPSS statistics: for version 20.0. Student. Belmont: Wadsworth, Cengage Learning; 2013. [Google Scholar]
- 69.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 70.Hafner A, Bulyk ML, Jambhekar A, et al. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20:199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- 71.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 72.Plewig G, Melnik B, Chen W. Plewig and Kligman´s Acne and Rosacea. Cham: Springer; 2019. Acne Therapy; pp. 223–292. [Google Scholar]
- 73.Haupt Y, Maya R, Kazaz A, et al. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 74.Ogawara Y, Kishishita S, Obata T, et al. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 75.Rabanal-Ruiz Y, Otten EG, Korolchuk VI. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017;61:565–584. doi: 10.1042/EBC20170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6:a026120. doi: 10.1101/cshperspect.a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kenzelmann Broz D, Spano Mello S, Bieging KT, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27:1016–1031. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo SH, Jung JY, Park K, et al. Autophagy regulates lipid production and contributes to the sebosuppressive effect of retinoic acid in human SZ95sebocytes. J Dermatol Sci. 2020;98:128–136. doi: 10.1016/j.jdermsci.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 80.Lima S, Takabe K, Newton J, et al. TP53 is required for BECN1- and ATG5-dependent cell death induced by sphingosine kinase 1 inhibition. Autophagy. 2018;14:942–957. doi: 10.1080/15548627.2018.1429875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan YJ, Zong WX. The cellular decision between apoptosis and autophagy. Chin J Cancer. 2013;32:121–129. doi: 10.5732/cjc.012.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 83.McCormick F, Clark R, Harlow E, et al. SV40 T antigen binds specifically to a cellular 53 K protein in vitro. Nature. 1981;292:63–65. doi: 10.1038/292063a0. [DOI] [PubMed] [Google Scholar]
- 84.Dobbelstein M, Roth J. The large T antigen of simian virus 40 binds and inactivates p53 but not p73. J Gen Virol. 1998;79(Pt 12):3079–3083. doi: 10.1099/0022-1317-79-12-3079. [DOI] [PubMed] [Google Scholar]
- 85.Burney W, Bosanac SS, Nguyen C, et al. Short-term exposure of human sebocytes to 13-cis-retinoic acid induces acnegenic changes. Br J Dermatol. 2018;179:1201–1202. doi: 10.1111/bjd.16837. [DOI] [PubMed] [Google Scholar]
- 86.Melnik BC, John SM, Agamia NF, et al. Isotretinoin's paradoxical effects in immortalized sebocytes. Br J Dermatol. 2019;180:957–958. doi: 10.1111/bjd.17579. [DOI] [PubMed] [Google Scholar]
- 87.Abali R, Yuksel MA, Aktas C, et al. Decreased ovarian reserve in female Sprague-Dawley rats induced by isotretinoin (retinoic acid) exposure. Reprod Biomed Online. 2013;27:184–191. doi: 10.1016/j.rbmo.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Abdelhamed A, Ezz El-Dawla R, Karadag AS, et al. The impact of isotretinoin on the pituitary-ovarian axis: an interpretative review of the literature. Reprod Toxicol. 2021;104:85–95. doi: 10.1016/j.reprotox.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 89.Arce F, Gätjens-Boniche O, Vargas E, et al. Apoptotic events induced by naturally occurring retinoids ATRA and 13-cis retinoic acid on human hepatoma cell lines Hep3B and HepG2. Cancer Lett. 2005;229:271–281. doi: 10.1016/j.canlet.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 90.Assaf HA, Abdel-Maged WM, Elsadek BE, et al. Survivin as a novel biomarker in the pathogenesis of acne vulgaris and its correlation to insulin-like growth factor-I. Dis Markers. 2016;2016:7040312. doi: 10.1155/2016/7040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mirza A, McGuirk M, Hockenberry TN, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- 92.Xing F, Liao W, Jiang P, et al. Effect of retinoic acid on aquaporin 3 expression in keratinocytes. Genet Mol Res. 2016;15:15016951. doi: 10.4238/gmr.15016951. [DOI] [PubMed] [Google Scholar]
- 93.Choudhary V, Olala LO, Kagha K, et al. Regulation of the glycerol transporter, aquaporin-3, by histone deacetylase-3 and p53 in keratinocytes. J Invest Dermatol. 2017;137:1935–1944. doi: 10.1016/j.jid.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stiebel-Kalish H, Eyal S, Steiner I. The role of aquaporin-1 in idiopathic and drug-induced intracranial hypertension. Med Hypotheses. 2013;81:1059–1062. doi: 10.1016/j.mehy.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Kerty E, Heuser K, Indahl UG, et al. Is the brain water channel aquaporin-4 a pathogenetic factor in idiopathic intracranial hypertension? Results from a combined clinical and genetic study in a Norwegian cohort. Acta Ophthalmol. 2013;91:88–91. doi: 10.1111/j.1755-3768.2011.02231.x. [DOI] [PubMed] [Google Scholar]
- 96.Roytman M, Frumkin A, Bohn TG. Pseudotumor cerebri caused by isotretinoin. Cutis. 1988;42:399–400. [PubMed] [Google Scholar]
- 97.Yan JH, Khatibi NH, Han HB, et al. p53-induced uncoupling expression of aquaporin-4 and inwardly rectifying K+ 4.1 channels in cytotoxic edema after subarachnoid hemorrhage. CNS Neurosci Ther. 2012;18:334–342. doi: 10.1111/j.1755-5949.2012.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koral L, Ovali MA, Tufekcioglu NK, et al. The role of AQP3 and AQP4 channels in cisplatin-induced cardiovascular edema and the protective effect of melatonin. Mol Biol Rep. 2021;48:7457–7465. doi: 10.1007/s11033-021-06763-6. [DOI] [PubMed] [Google Scholar]
- 99.Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981–985. doi: 10.1056/NEJM198510173131604. [DOI] [PubMed] [Google Scholar]
- 100.Melnik B, Bros U, Plewig G. Characterization of apoprotein metabolism and atherogenic lipoproteins during oral isotretinoin treatment. Dermatologica. 1987;175(Suppl 1):158–168. doi: 10.1159/000248880. [DOI] [PubMed] [Google Scholar]
- 101.Ashur-Fabian O, Har-Zahav A, Shaish A, et al. apoB and apobec1, two genes key to lipid metabolism, are transcriptionally regulated by p53. Cell Cycle. 2010;9:3761–3770. doi: 10.4161/cc.9.18.12993. [DOI] [PubMed] [Google Scholar]
- 102.Kovács D, Hegyi K, Szegedi A, et al. Isotretinoin is indirectly effective in sebocytes. Br J Dermatol. 2020;182:1052–1054. doi: 10.1111/bjd.18562. [DOI] [PubMed] [Google Scholar]
- 103.Melnik BC. FoxO1—the key for the pathogenesis and therapy of acne? J Dtsch Dermatol Ges. 2010;8:105–114. doi: 10.1111/j.1610-0387.2010.07344.x. [DOI] [PubMed] [Google Scholar]
- 104.Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141–165. doi: 10.4161/derm.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Y, Zhang J, Guo H, et al. Transcriptome comparison of isotretinoin-effective and isotretinoin-ineffective severe acne vulgaris patients. J Cosmet Dermatol. 2021;20:261926. doi: 10.1111/jocd.13898. [DOI] [PubMed] [Google Scholar]
- 106.Deng X, Zhang W, O-Sullivan I, et al. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J Biol Chem. 2012;287:20132–20143. doi: 10.1074/jbc.M112.347211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fan W, Imamura T, Sonoda N, et al. FOXO1 trans represses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem. 2009;284:12188–12197. doi: 10.1074/jbc.M808915200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oulès B, Philippeos C, Segal J, et al. Contribution of GATA6 to homeostasis of the human upper pilosebaceous unit and acne pathogenesis. Nat Commun. 2020;11:5067. doi: 10.1038/s41467-020-18784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sutcliffe S, Giovannucci E, Isaacs WB, et al. Acne and risk of prostate cancer. Int J Cancer. 2007;121:2688–2692. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ugge H, Udumyan R, Carlsson J, et al. Acne in late adolescence and risk of prostate cancer. Int J Cancer. 2018;142:1580–1585. doi: 10.1002/ijc.31192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murphy JD, Sandler D, White AJ, et al. Severe acne and risk of breast cancer. Breast Cancer Res Treat. 2019;177:487–495. doi: 10.1007/s10549-019-05302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950–954. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 113.Werner H, Laron Z. Role of the GH-IGF1 system in progression of cancer. Mol Cell Endocrinol. 2020;518:111003. doi: 10.1016/j.mce.2020.111003. [DOI] [PubMed] [Google Scholar]
- 114.Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from Laron syndrome. Nutr Metab (Lond) 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371–388. doi: 10.2147/CCID.S69135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melnik BC. Milk disrupts p53 and DNMT1, the guardians of the genome: implications for acne vulgaris and prostate cancer. Nutr Metab (Lond) 2017;14:55. doi: 10.1186/s12986-017-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hadjidaniel MD, Reynolds CP. Antagonism of cytotoxic chemotherapy in neuroblastoma cell lines by 13-cis-retinoic acid is mediated by the antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2010;9:3164–3174. doi: 10.1158/1535-7163.MCT-10-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bettoli V, Zauli S, Virgili A. Retinoids in the chemoprevention of non-melanoma skin cancers: why, when and how. J Dermatolog Treat. 2013;24:235–237. doi: 10.3109/09546634.2012.746634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.