Highlights
-
•
There is important variability in existing contouring guidelines for prostate bed radiotherapy.
-
•
We developed a contemporary ESTRO-ACROP guideline for prostate bed contouring to improve consistency in delineation.
-
•
It aimed at optimizing the therapeutic ratio by reducing the unnecessary irradiation of normal tissues.
-
•
These recommendations are generally supported by local progression patterns on MRI and PET imaging.
Keywords: Prostate cancer, Postoperative radiotherapy, Salvage radiotherapy, Adjuvant radiotherapy, Target volume delineation, Prostate cancer guidelines
Abstract
Purpose/Objective
Radiotherapy to the prostate bed is a potentially curative salvage option after radical prostatectomy. Although prostate bed contouring guidelines are available in the literature, important variabilities exist. The objective of this work is to provide a contemporary consensus guideline for prostate bed delineation for postoperative radiotherapy.
Methods
An ESTRO-ACROP contouring consensus panel consisting of 11 radiation oncologists and one radiologist, all with known subspecialty expertise in prostate cancer, was established. Participants were asked to delineate the prostate bed clinical target volumes (CTVs) in 3 separate clinically relevant scenarios: adjuvant radiation, salvage radiation with PSA progression, and salvage radiation with persistently elevated PSA. These cases focused on the presence of positive surgical margin, extracapsular extension, and seminal vesicles involvement. None of the cases had radiographic evidence of local recurrence on imaging. A single computed tomography (CT) dataset was shared via FALCON platform and contours were performed using EduCaseTM software. Contours were analyzed qualitatively using heatmaps which provided a visual assessment of controversial regions and quantitatively analyzed using Sorensen-Dice similarity coefficients. Participants also answered case-specific questionnaires addressing detailed recommendations on target delineation. Discussions via electronic mails and videoconferences for final editing and consensus were performed.
Results
The mean CTV for the adjuvant case was 76 cc (SD = 26.6), salvage radiation with PSA progression was 51.80 cc (SD = 22.7), and salvage radiation with persistently elevated PSA 57.63 cc (SD = 25.2). Compared to the median, the mean Sorensen-Dice similarity coefficient for the adjuvant case was 0.60 (SD 0.10), salvage radiation with PSA progression was 0.58 (SD = 0.12), and salvage radiation with persistently elevated PSA 0.60 (SD = 0.11). A heatmap for each clinical scenario was generated. The group agreed to proceed with a uniform recommendation for all cases, independent of the radiotherapy timing. Several controversial areas of the prostate bed CTV were identified based on both heatmaps and questionnaires. This formed the basis for discussions via videoconferences where the panel achieved consensus on the prostate bed CTV to be used as a novel guideline for postoperative prostate cancer radiotherapy.
Conclusion
Variability was observed in a group formed by experienced genitourinary radiation oncologists and a radiologist. A single contemporary ESTRO-ACROP consensus guideline was developed to address areas of dissonance and improve consistency in prostate bed delineation, independent of the indication.
There is important variability in existing contouring guidelines for postoperative prostate bed (PB) radiotherapy (RT) after radical prostatectomy. This work aimed at providing a contemporary consensus guideline for PB delineation. An ESTRO ACROP consensus panel including radiation oncologists and a radiologist, all with known subspecialty expertise in prostate cancer, delineated the PB CTV in 3 scenarios: adjuvant RT, salvage RT with PSA progression, and salvage RT with persistently elevated PSA. None of the cases had evidence of local recurrence. Contours were analysed qualitatively using heatmaps for visual assessment of controversial regions and quantitatively using Sorensen-Dice coefficient. Case-specific questionnaires were also discussed via e-mails and videoconferences for consensus. Several controversial areas of the PB CTV were identified based on both heatmaps and questionnaires. This formed the basis for discussions via videoconferences. Finally, a contemporary ESTRO-ACROP consensus guideline was developed to address areas of dissonance and improve consistency in PB delineation, independent of the indication.
Introduction
Postoperative radiotherapy to the prostate bed is a potentially curative treatment for prostate cancer patients at increased risk of local recurrence due to high-risk pathologic features (adjuvant radiotherapy) or due to biochemical, clinical, or radiological evidence of disease relapse (salvage radiotherapy) [1], [2].
Several technological advancements in radiotherapy such as image guidance and intensity modulation have allowed more precise treatments with better sparing of adjacent normal tissues. An important part of the radiotherapy planning process is the clinical definition and delineation of the target volume. In prostate cancer patients treated with prostatectomy, the accurate target volume definition can be challenging given the absence of the prostate boundaries and a variable postoperative anatomy. Despite the existence of various prostate bed contouring guidelines [3], [4], [5], [6], [7], the recommendations remain inconsistent resulting in considerable inter-observer variability in the treatment target delineation [8], [9], [10]. A contouring guideline on target volume delineation encourages a consistent application of prostate bed treatments across clinical trials, institutions, and individual clinicians. A practical guideline that is easily applicable and disseminated can improve the accuracy of radiation treatment, the reproducibility for reliable reporting and ultimately enhance oncological outcomes with reduced treatment-related toxicity.
The ESTRO-ACROP decided to develop a new consensus guideline to add to and refine the existing publications to account for more current practices, that include the use of novel imaging modalities. Therefore, this work primarily aimed at developing a contemporary consensus guideline for a standardized delineation of the prostate bed for postoperative prostate radiotherapy.
Methods
An ESTRO contouring consensus panel consisting of eleven European radiation oncologists (AD, PD, VK, CC, CC, VF, PG, AGI, AZ, AB, TW) and one radiologist (VP) with known subspecialty expertise, performed a contouring exercise and delineated the required postoperative clinical target volumes (CTVs) of the prostate and seminal vesicles in the setting of three independent clinical scenarios. These cases focused on three clinical factors; presence of positive surgical margin, extracapsular extension, and seminal vesicles involvement, that are likely to impact on delineation. The consensus generating process consisted in the contouring of three CTVs by all participants. We considered that all three cases had no evidence of macroscopic local recurrence on the postoperative imaging. Recommendations for Organs at Risk (OAR) delineation have been previously published [11], therefore OAR contouring was not required. The clinical cases were as follows:
Case 1 (CTV1): 60-year-old man with a preoperative clinical T2a, Gleason 9 (5 + 4), PSA 16 ng/mL adenocarcinoma of the prostate. He underwent robotic-assisted radical prostatectomy and was found to have pathologic T3b, pN0 (12 lymph nodes removed) Gleason score 9 (5 + 4, ISUP 5) disease. Pathology revealed extracapsular extension at the right base with positive focal surgical margin at this level (R1), 3 mm extension, and an infiltration of the seminal vesicle on the right side. The patient was referred to radiation oncology 90 days after surgery with an undetectable PSA, < 0.1 ng/mL. The patient denied urinary continence but complained about erectile dysfunction.
Case 2 (CTV2): 65-year-old man with a preoperative clinical T1c, Gleason 7 (4 + 3), ISUP 3, PSA 10 adenocarcinoma of the prostate. He underwent radical retropubic prostatectomy and was found to have pathologic T2, pN0 (2 lymph nodes removed) Gleason score 7 (4 + 3), ISUP 3 disease. Pathology revealed negative surgical margins (R0). His PSA levels were undetectable (<0.1 ng/mL) after prostatectomy and remained undetectable for 2 years. After 2 years, the PSA level then rose to 0.10 ng/mL and 6 months later was 0.25 ng/mL. There was no evidence of disease on postoperative MRI and PSMA-PET scan.
Case 3 (CTV3): 70-year-old man with a preoperative clinical T3a, Gleason 8 (4 + 4), ISUP 4, PSA 20 ng/mL adenocarcinoma of the prostate. He underwent robotic-assisted radical prostatectomy and was found to have pathologic T3a, pN0 (15 lymph nodes removed), Gleason score 8 (4 + 4), ISUP 4 disease. Pathology revealed extracapsular extension at the apex with positive surgical margin at this level, 5 mm extension. PSA was detectable at 0.1 ng/mL approximately 2 months after surgery and then rose to 0.2 ng/mL 2 months later. Postoperative MRI and PSMA-PET scan did not show any evidence of disease and he was referred for assessment of salvage radiotherapy.
The computed tomography (CT) as well as postoperative magnetic resonance imaging (MRI) datasets were shared via the FALCON platform (Fellowship in Anatomic elineation and CONtouring) from ESTRO (European SocieTy for Radiotherapy and Oncology) and the software EduCaseTM from RadOnc eLearning Center, Inc. Fremont, CA, USA was used. This is a web-based contouring and analysis tool that has a graphical user interface for the management, storage, and publishing of contouring of the clinical cases. The software allows image fusion of the simulation CT scan with MRI, as well as an integrated analysis on contouring proficiency.
Multiple methods to assess the data and reach consensus were used, which included quantitative and qualitative analysis of the contours. Contour analysis was performed using the Sorensen-Dice similarity coefficient to compute the degree of association or overlay between the set of images [12]. These metrics were calculated and compared with the average contour of the group for each case.
Heat maps of all collected contours for each clinical scenario were created which provided a visual assessment of controversial regions of the prostate and seminal vesicles bed. The heatmap was created by overlaying each observer’s contours on CT scan and generating specific voxel values. A solid single colour was generated according to the number of image voxels included in the superposed contours. Based on the number of observers including those voxels in their contour set, different colours were generated and various iso-surfaces with different colours were generated. This method allows “qualitative” analysis of the geographic locations of higher and lower agreement for the identification of specific regions of controversy and subsequent discussion among the experts. All images were generated in Python version 3.7.4 (Phyton Software Foundation, USA). Two-Dimensional (2-D) images for the transversal, coronal, and sagittal view were created by overlaying the contour maps and plotting every cross-section along the X, Y, and Z axis using Matplotlib’s Pyplot module. Three-Dimensional (3-D) images were created using Plotly.graph_objects 3-D scatter function.
After all contours were submitted, 20 case-specific questions addressing detailed recommendations on target volume delineation were formulated by the leading authors (AD, PD, TW) and electronically sent to participants for discussion and consensus development. The survey also encompassed questions including but not limited to image-guidance, delineation of the bladder neck, required imaging methods, planning target volume (PTV), use of rectal spacer and endorectal balloon (Supplementary material). The twelve participants discussed discrepancies in the recommendations in multiple informal discussions by electronic mail and videoconferences. For each question, the quality of consensus in terms of percentage of agreement was measured and documented. Consensus was defined when 75% or more agreement were achieved for each recommendation as per the German S3 guidelines [13].
Results
This work was carried out by a group of eleven genitourinary radiation oncologists and one genitourinary radiologist. The radiologist’ input was not directly included in the quantitative/qualitative analysis of the contours but was included in the remaining methods for consensus.
The mean CTV1 (adjuvant RT) volume was 76 cc (SD = 26.6), CTV2 (salvage radiation with PSA progression) was 51.80 cc (SD = 22.7.6), and CTV3 (salvage radiation with persistently elevated PSA), 57.63 cc (SD = 25.2). Compared to the median, the mean Sorensen-Dice similarity coefficient for CTV1 was 0.60 (SD 0.10), CTV2 was 0.58 (SD = 0.12), and CTV3 0.60 (SD = 0.11). Overall, each expert contoured CTV2 and CTV3 in a similar fashion. CTV1 presented the largest volumes. This case represented the patient with pT3b disease and extra-prostatic extension (EPE) at the prostate base, and the larger volume was attributed to the clinicopathological characteristics of the case. Therefore, the group agreed to proceed with a recommendation that considers the possible clinical variations that can occur, independent of the radiotherapy timing, but with a possibility of tailoring the volume delineation according to case-specific risk factors.
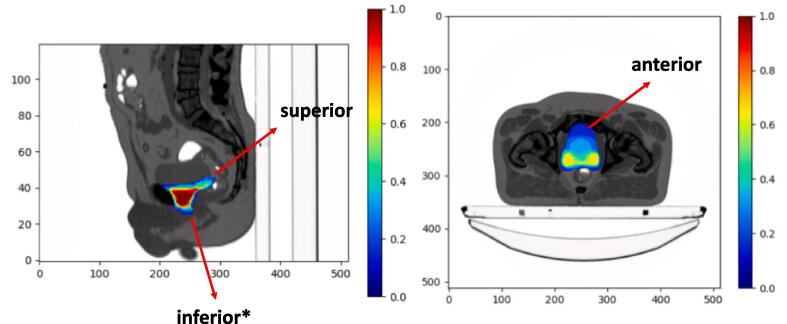
A heatmap for each clinical scenario was generated. We developed a heat map analysis of all contours to identify areas of disagreement. Supplementary Video 1 is a visual representation of consensus development with “warmer” colours, in red and orange and controversial areas in “cooler” colours, blue. Several controversial areas of the prostate bed CTV were identified based on both heatmaps and questionnaires. This formed the basis for discussions via videoconference where the panel achieved consensus on the prostate bed CTV to be used as a novel guideline for postoperative prostate cancer radiotherapy. Fig. 1 shows a heat map figure with 3 areas of greatest variability consisting of [1] the superior-most aspect at the seminal vesicles level, [2] the inferior part at the prostate apex level, and [3] anteriorly, next to the pubic symphysis.
Fig. 1.
Heatmap with areas of greatest variability in Postoperative Prostate Bed Clinical Target Delineation.
Questionnaires used in our case-specific surveys (Supplementary data) led to the recommendations that are be summarized below.
ESTRO ACROP guideline on prostate bed delineation – Summary recommendations (Table 1).
Table 1.
Summary of ESTRO-ACROP Recommendations for Postoperative Prostate Bed Clinical Target Delineation. Abbreviation: CTV = clinical target volumes.
| Border | |
|---|---|
| Inferior |
|
| Anterior |
|
| Posterior |
|
| Lateral |
|
| Superior |
|
Inferior border
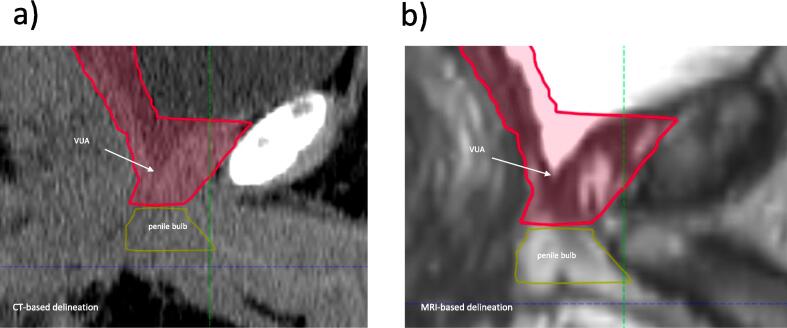
The group agreed to use the vesico-urethral anastomosis (VUA) as a landmark to delineate the inferior border of the prostate bed, and the CTV should start inferiorly 8–12 mm below the VUA. The VUA can be defined as the slice below the last slice where fluid (urine) is seen (Fig. 2a). The group does not recommend routine use of contrast for the definition of in the inferior border of the CTV. When available, a postoperative MRI can be used for the identification of the VUA (Fig. 2b). VUA positioning on MRI is reliable and has a strong correlation between readers [14]. If VUA is not identified, the group agreed that CTV should start at the slice right above the penile bulb, which is usually visible on both CT and MRI.
Fig. 2.
Inferior Border. a. CT-based delineation of the inferior border of the CTV; b. MRI-based delineation of the inferior border of the CTV. Abbreviation: CTV = clinical target volumes; CT = computed tomography; MRI = magnetic resonance imaging; VUA = vesico-urethral anastomosis.
Anterior border
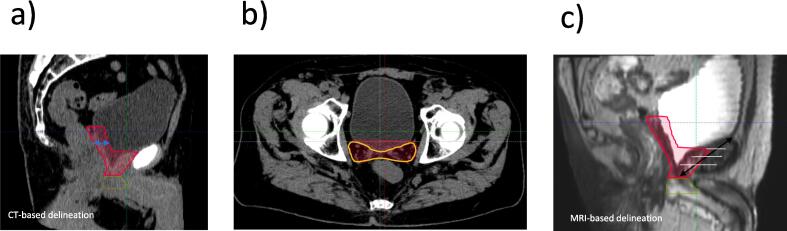
Cranially, at the level of the seminal vesicles bed, the CTV should cover 1–2 cm of the posterior bladder wall (Fig. 3a). However, the group agreed that, if daily Image-Guided Radiation Therapy (IGRT) is used and provided consistent reproducibility of the bladder, the CTV may be contoured up to the posterior margin of the bladder wall (Fig. 3b), which would avoid unnecessary irradiation of the normal bladder tissue.
Fig. 3.
Anterior Border. a. Cranially, CT-based delineation of the CTV covering 1–2 cm of the posterior bladder wall; b. Cranially, CT-based delineation of the CTV up to the posterior bladder wall; c. MRI-based delineation depicting the anterior border of the CTV at the posterior margin of the pubic bone up to half to two thirds of the symphysis pubis. Abbreviation: CT = computed tomography; CTV = clinical target volumes; MRI = magnetic resonance imaging.
Caudally, the anterior border should stop at the posterior margin of the pubic bone up to half to two thirds of the symphysis pubis (Fig. 3c). Of note, the antero-cranial border should not extend to the top of the symphysis pubis to avoid unnecessary irradiation of the bladder. However, if the cranial limit is located at the midpoint of the symphysis pubis on sagittal view, a proper coverage of the VUA must be ensured.
Posterior border
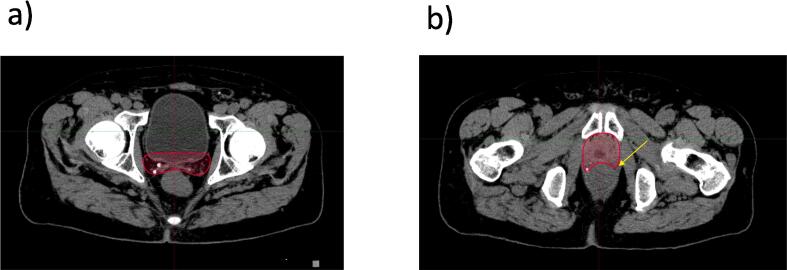
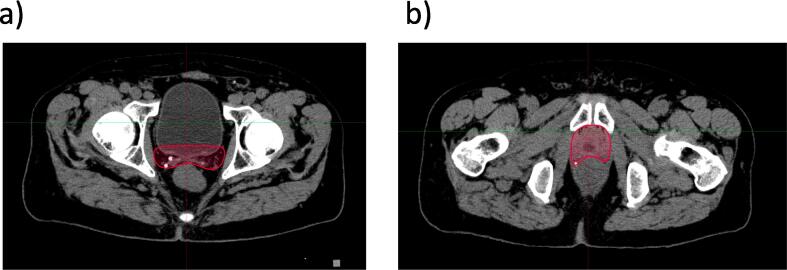
Posteriorly, the CTV should stop at the anterior rectal wall (Fig. 4a). However, at the level of the seminal vesicles bed, if the meso-rectal fascia is clearly visualized, it can be used as the posterior border of the CTV. Of note, the group agreed to include the existing surgical clips within the prostate bed CTV and the antero-lateral angles of the rectum, known to be an area at risk for recurrence (Fig. 4b).
Fig. 4.
Posterior Border. a. Cranially, CT-based delineation of the CTV up to the anterior wall of the rectum; b. Caudally, CT-based delineation of the CTV up to the anterior wall of the rectum including the existing surgical clips and the antero-lateral angles of the rectum. Arrow shows antero-lateral angles of the rectum. Abbreviation: CT = computed tomography; CTV = clinical target volumes; MRI = magnetic resonance imaging.
Lateral border
Cranially, the lateral borders of the CTV are the internal margins of the internal obturator muscles, bilaterally (Fig. 5a). The group agreed that the CTV should not be extended antero-laterally toward the region of the obturator lymph nodes to spare normal bladder irradiation. Caudally, the lateral borders are the internal margins of the internal obturator muscles or internal borders of the levator ani muscles (Fig. 5b).
Fig. 5.
Lateral Border. a. Cranially, CT-based delineation of the CTV up to the internal margins of the internal obturator muscles; b. Caudally, CT-based delineation of the CTV up to the internal margins of the internal obturator muscles. Abbreviation: CT = computed tomography; CTV = clinical target volumes.
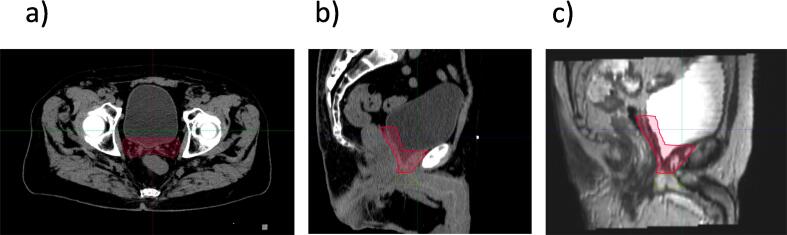
Superior border
Superiorly, the group agreed to include a “bridge” of 3–5 mm between the seminal vesicles bed or seminal vesicles remnants. In the absence of seminal vesicles invasion on pathology (pT2-pT3a), the CTV should include the region of the seminal vesicles base (lower third), i.e., up to the level of cut end of vas deferens (Fig. 6a). In the presence of seminal vesicles invasion, the CTV should ensure the inclusion of the entire seminal vesicles bed, i.e., include cranial surgical clips if present and considered relevant. When available, the use of preoperative MRI or diagnostic CT is recommended as it can help guide the delineation of the preoperative seminal vesicle bed.
Fig. 6.
Superior Border. a. Cranially, CT-based delineation of the CTV superiorly on axial view; b. CT-based delineation of the CTV on sagittal view. c. MRI-based delineation of the CTV on sagittal view. Abbreviation: CT = computed tomography; MRI = magnetic resonance image. Abbreviation: CT = computed tomography; CTV = clinical target volumes; MRI = magnetic resonance imaging.
An overview of the delineation prostate bed CTV slice per slice is presented in Supplementary data.
Additional consensus recommendations
Use of postoperative MRI
Although CT scan is recommended for the delineation of the prostate bed CTV, postoperative MRI with or without preoperative MRI can be helpful for guidance. The pelvic anatomy and specifically the prostate bed differs depending on the surgical technique [15]. The prostate bed is better visualized on postoperative MRI as compared to CT due to better soft tissue resolution, therefore MRI can be of value for a more accurate target definition [16], [17], [18]. If available, the co-registration of a preoperative MRI may give an insight of the extent and location of the disease which could help adjust the final prostate bed volume. The postoperative MRI acquisition protocol should include T2-weighted imaging at three orthogonal levels, a diffusion-weighted imaging with high b values and dynamic contrast enhanced imaging. Images should include the VUA, the prostatic bed, the bladder base, the levator ani, the rectum, and the residual seminal vesicles as these are common sites of relapse [19]. Of note, these prostate bed CTV recommendations can be applied to patients treated with MR-guided radiotherapy.
Expansion of the CTV at the area of positive margin
Previous guidelines [4] have recommended a supplementary 5 mm CTV expansion in the direction of microscopically involved tumor margins as reported by the pathologist (except the rectal wall). The current work did not reach consensus on the expansion of the CTV at the area of positive margin; therefore, the judicious expansion of the volume will be left to the discretion of the radiation oncologist.
PTV margins
There is no uniform recommendation for PTV margins to the prostate bed [20]. The group agreed to a minimum of 5 mm isotropic expansion of the CTV. Margins used to generate a PTV by expanding a CTV should consider the magnitude of setup errors and other uncertainties which are dependent on the institutional image guidance technique.
IGRT
The group agreed that the preferred IGRT method is cone-beam CT (CBCT), ideally daily or at a minimum of 3 times a week, with alignment to the soft tissue and/or surgical clips. Absence of image guidance or CBCT with alignment to bony anatomy are not recommended. An adequate training of radiation therapists to perform online soft tissue matching with CBCT scans is critical. The group does not recommend the use of implanted fiducial markers for postoperative radiation treatment of the prostate bed. To our knowledge, no specific guideline for postoperative IGRT is available, however the authors refer to ESTRO-ACROP guideline on IGRT for localized prostate cancer [21] for further guidance. Variations in rectum and bladder filling have greater impact on prostate bed treatment compared to primary radiotherapy of the prostate. Therefore, a protocol to consistently maintain the rectum empty and a comfortably full bladder is recommended.
Endorectal balloons
Endorectal balloons have been used to stabilize the internal anatomy of the rectum, decreasing inter- and intra-treatment target motion for prostate cancer treatment [22], [23], [24], [25]. The group does not recommend the routine use of rectal balloons in the postoperative radiation treatment of the prostate bed.
Rectal spacers
The use of a biodegradable substance in the anterior perirectal fatty space allows displacing the prostate away from the rectal wall reducing the rectal volume exposed to high level doses [26], [27]. Data is limited on the use of rectal spacers for postoperative radiation treatment of the prostate bed; therefore, the group does not recommend the use of rectal spacers in this setting.
Discussion
Postoperative radiotherapy is the only potentially curative treatment after radical prostatectomy failure or as a measure to prevent tumour recurrence. Prostate bed irradiation is associated with improved long-term oncologic outcomes compared with observation and overall has a favorable therapeutic ratio [1], [2], [28], [29]. An accurate and precise delineation of the radiotherapy target volume is of particular importance to provide reproducible and reliable reporting, maximize oncological outcomes and minimize treatment-related toxicity. Variation in contour delineation among radiation oncologists is common and can affect the resulting treatment delivery and patient outcomes [9], [30], [31], [32]. Several studies have shown that the use of guidelines and contouring atlases can improve inter-observer variability of both the target volume and OAR with additional evidence that these improvements in contour delineation can improve predicted tumour control and normal tissue complication probability [31], [32], [33], [34].
Although other consensus guidelines are available in literature, our consensus guidelines provided a few distinct recommendations as shown in Supplementary Table S1. We have attempted to refine some of the previous recommendations and optimize the therapeutic ratio by reducing the unnecessary irradiation of normal tissues. Several advancements in image guidance and radiotherapy delivery have been seen in the last decades, therefore current recommendations are intended to adapt to these improvements. One of the main innovations of this work is the recommendation of postoperative MRI for better soft tissue outlining as well as to make sure there is no residual macroscopic recurrence [18], [35]. The use of MRI possibly increases observer agreement and decreases unnecessary dose to the organs at risk in the post-operative setting [16]. Comparable to what is observed with prostate CTV delineation, there is a reduction of the overall post-operative CTV on planning MRI compared with CT. Especially the inferior border can be more clearly visualized on T2-weighted MRI sequences, leading to a shift of the CTV in caudal direction. Additionally, the anterior rectal wall is more clearly marked on MRI, resulting in minimal discrepancies between observers compared with CT-only delineation. A central review of a phase 3 trial for salvage radiotherapy using CT-based delineation showed that limited adherence to treatment protocol recommendations (e.g., overlap of the CTV with rectal wall), was associated with a higher risk of toxicity and a trend toward worse biochemical control [9]. When MRI is used to aid CT-based CTV contouring, however, it is important to ensure an appropriate image fusion which often can be challenging due to differences in bladder and rectum volumes between the imaging modalities. It is important to consider and mitigate variations in bladder and rectum filling between CT and MR imaging, and to reproduce treatment conditions. Prior to the delineation of the prostate bed both image sets should be aligned. This alignment can be initially set on bony structures, for example closure of the pubic bones, but subsequently adjusted at the level of the external urethral sphincter.
Regarding the anatomical landmarks, previous guidelines [3], [5] recommend that the anterior border of the CTV should extend superiorly to the top of symphysis pubis, consequently covering an important portion of the normal bladder. In the absence of radiologic evidence of macroscopic disease, the normal bladder at the level of the top of symphysis pubis is unlikely to harbor microscopic disease and therefore should not be considered part of the prostate bed CTV. This work, therefore, recommends that the prostate bed CTV should extend up to half or two-thirds of the symphysis pubis on sagittal view (Fig. 3c). Anatomical patterns of progression in the prostatic fossa on MRI and PET imaging have shown that recurrences at the cranial aspect of the posterior edge of the pubic bone are rare [36], [37]. Two recent 68 Ga-PSMA datasets [38], [39] lead to similar insights for developing new contouring guidelines. Together, more than 250 patients with local recurrences on the 68 Ga-PSMA PET/CTs after prostatectomy showed the importance of including the antero-lateral angles of the rectum, including more than 5 mm below the VUA; and the possibility of excluding the CTV at the cranial aspect of the posterior edge of the pubic bone. However, it is important to note that some recurrences at the anterior portion of the CTV may not be well visualized in the 68 Ga-PSMA PET/CT due to the urinary activity in the bladder.
Yet, postoperative variations of the supero-inferior positioning of the VUA exist, and radiation oncologists must use their best judgment for the adequate coverage of the VUA, which is a common site of prostate bed recurrences [40], [41]. In addition, patterns of recurrence on PET scan have shown that the anterolateral angles of the rectum is a common site of disease, therefore we concur with the Groupe Francophone de Radiothérapie Urologique (GFRU) [42] recommendation on extending the posterior border of the contour to include this area.
Considering the anterior margin of the CTV cranially, we recommend that the CTV should be contoured up to the outer margin of the bladder wall as, by definition, intravesical tissue is not at risk for recurrence due to micro-metastatic disease. However, it is important to note that the postero-superior part of the CTV has greater motion, and a larger antero-posterior PTV expansion of the upper prostate bed has been previously suggested [43].Therefore, daily IGRT and consistent reproducibility of bladder and rectum volumes should be ensured to safely reduce the anterior coverage of the CTV more cranially.
This is the first guideline for postoperative RT to use a qualitative analysis through heatmaps which provided a visual assessment of controversial regions of contouring. It is important to mention that these guidelines were developed in the setting of adjuvant or early salvage radiotherapy without the evidence of local disease on mpMRI, PET-CT or PET-MRI. The delineation of target volumes in the setting of visible disease is the subject of a separate work by the same ESTRO-ACROP team. These guidelines were developed in the context of conventionally fractionated treatment, and recommendations on alternate fractionation schedules (e.g., moderate, or extreme hypofractionation) are beyond the scope of this work. We believe our recommendations would still be applicable irrespective of the fractionation regime.
Future research should focus on the validation of the reproducibility of these recommendations and on its potential impact for treatment planning and clinical outcomes. Moreover, these recommendations could be an opportunity for the development and deployment of knowledge-based planning and artificial intelligence contouring solutions.
Conclusion
This work showed variability in prostate bed delineation in a group formed by radiation oncologists and a radiologist, all with expertise in prostate cancer. A single contemporary ESTRO-ACROP consensus guideline was developed to address areas of dissonance, promote standardization, improve previous recommendations, and increase consistency in prostate bed delineation, independent of the indication.
Disclaimer
ESTRO cannot endorse all statements or opinions made on the guidelines. Regardless of the vast professional knowledge and scientific expertise in the field of radiation oncology that ESTRO possesses, the Society cannot inspect all information to determine the truthfulness, accuracy, reliability, completeness or relevancy thereof. Under no circumstances will ESTRO be held liable for any decision taken or acted upon as a result of reliance on the content of the guidelines.
The component information of the guidelines is not intended or implied to be a substitute for professional medical advice or medical care. The advice of a medical professional should always be sought prior to commencing any form of medical treatment. To this end, all component information contained within the guidelines is done so for solely educational and scientific purposes. ESTRO and all of its staff, agents and members disclaim any and all warranties and representations with regards to the information contained on the guidelines. This includes any implied warranties and conditions that may be derived from the aforementioned guidelines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the support of Dr. Radka Stoyanova, Ph.D., and her team for creating the heatmaps for the qualitative analysis of the contours. We thank Eralda Azizaj from ESTRO for the administrative support. We also thank Miika Palmu for providing organizational and technical support for using the FALCON platform and EduCaseTM software. Finally, we acknowledge the comprehensive review of these recommendations by Prof. Peter Hoskin, Prof. Piet Ost and Prof. Thomas Zilli. We sincerely appreciate their valuable comments and suggestions, which helped us in improving the quality of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100638.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tilki D., Chen M.-H., Wu J., Huland H., Graefen M., Wiegel T., et al. Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol. 2021;39(20):2284–2293. doi: 10.1200/JCO.20.03714. [DOI] [PubMed] [Google Scholar]
- 2.Cornford P., van den Bergh R.C.N., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol. 2021;79(2):263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Michalski J.M., Lawton C., El Naqa I., Ritter M., O'Meara E., Seider M.J., et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–368. doi: 10.1016/j.ijrobp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poortmans P., Bossi A., Vandeputte K., Bosset M., Miralbell R., Maingon P., et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84(2):121–127. doi: 10.1016/j.radonc.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Wiltshire K.L., Brock K.K., Haider M.A., Zwahlen D., Kong V., Chan E., et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69(4):1090–1099. doi: 10.1016/j.ijrobp.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 6.Robin S., Jolicoeur M., Palumbo S., Zilli T., Crehange G., De Hertogh O., et al. Prostate Bed Delineation Guidelines for Postoperative Radiation Therapy: On Behalf Of The Francophone Group of Urological Radiation Therapy. Int J Radiat Oncol Biol Phys. 2021;109(5):1243–1253. doi: 10.1016/j.ijrobp.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Sidhom M.A., Kneebone A.B., Lehman M., Wiltshire K.L., Millar J.L., Mukherjee R.K., et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2008;88(1):10–19. doi: 10.1016/j.radonc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Malone S., Croke J., Roustan-Delatour N., Belanger E., Avruch L., Malone C., et al. Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. Int J Radiat Oncol Biol Phys. 2012;84(3):725–732. doi: 10.1016/j.ijrobp.2011.12.081. [DOI] [PubMed] [Google Scholar]
- 9.Beck M., Sassowsky M., Schär S., Mathier E., Halter M., Zwahlen D.R., et al. Adherence to Contouring and Treatment Planning Requirements Within a Multicentric Trial: Results of the Quality Assurance of the SAKK 09/10 trial. Int J Radiat Oncol Biol Phys. 2022;113(1):80–91. doi: 10.1016/j.ijrobp.2021.12.174. [DOI] [PubMed] [Google Scholar]
- 10.Ost P., De Meerleer G., Vercauteren T., De Gersem W., Veldeman L., Vandecasteele K., et al. Delineation of the postprostatectomy prostate bed using computed tomography: interobserver variability following the EORTC delineation guidelines. Int J Radiat Oncol Biol Phys. 2011;81(3):e143–e149. doi: 10.1016/j.ijrobp.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Salembier C., Villeirs G., De Bari B., Hoskin P., Pieters B.R., Van Vulpen M., et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127(1):49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Taha A.A., Hanbury A. An efficient algorithm for calculating the exact Hausdorff distance. IEEE Trans Pattern Anal Mach Intell. 2015;37(11):2153–2163. doi: 10.1109/TPAMI.2015.2408351. [DOI] [PubMed] [Google Scholar]
- 13.[Available from: https://www.krebsgesellschaft.de/gcs/german-cancer-society/guidelines.html.
- 14.Lim Joon D., Lim A., Schneider M., Hiew C.-Y., Lawrentschuk N., Sengupta S., et al. Prostate cancer post-prostatectomy radiotherapy: CT vs MRI for vesico-urethral anastomosis target delineation. Radiother Oncol. 2017;125(1):113–117. doi: 10.1016/j.radonc.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Lopes Dias J., Lucas R., Magalhães Pina J., João R., Costa N.V., Leal C., et al. Post-treated prostate cancer: normal findings and signs of local relapse on multiparametric magnetic resonance imaging. Abdom Imaging. 2015;40(7):2814–2838. doi: 10.1007/s00261-015-0473-1. [DOI] [PubMed] [Google Scholar]
- 16.Sefrova J., Odrazka K., Paluska P., Belobradek Z., Brodak M., Dolezel M., et al. Magnetic resonance imaging in postprostatectomy radiotherapy planning. Int J Radiat Oncol Biol Phys. 2012;82(2):911–918. doi: 10.1016/j.ijrobp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Croke J., Malone S., Roustan Delatour N., Belanger E., Avruch L., Morash C., et al. Postoperative radiotherapy in prostate cancer: the case of the missing target. Int J Radiat Oncol Biol Phys. 2012;83(4):1160–1168. doi: 10.1016/j.ijrobp.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Panebianco V., Villeirs G., Weinreb J.C., Turkbey B.I., Margolis D.J., Richenberg J., et al. Prostate Magnetic Resonance Imaging for Local Recurrence Reporting (PI-RR): International Consensus -based Guidelines on Multiparametric Magnetic Resonance Imaging for Prostate Cancer Recurrence after Radiation Therapy and Radical Prostatectomy. Eur Urol Oncol. 2021;4(6):868–876. doi: 10.1016/j.euo.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Mertan F.V., Greer M.D., Borofsky S., Kabakus I.M., Merino M.J., Wood B.J., et al. Multiparametric Magnetic Resonance Imaging of Recurrent Prostate Cancer. Top Magn Reson Imaging. 2016;25(3):139–147. doi: 10.1097/RMR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilotte F., Antoine M., Bobin M., Latorzeff I., Supiot S., Richaud P., et al. Post-Prostatectomy Image-Guided Radiotherapy: The Invisible Target Concept. Front Oncol. 2017;7 doi: 10.3389/fonc.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghadjar P., Fiorino C., Munck Af Rosenschold P., Pinkawa M., Zilli T., van der Heide U.A. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother Oncol. 2019;141:5–13. doi: 10.1016/j.radonc.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Jameson M.G., De Leon J., Windsor A.A., Cloak K., Keats S., Dowling J.A., et al. Endorectal balloons in the post prostatectomy setting: do gains in stability lead to more predictable dosimetry? Radiother Oncol. 2013;109(3):493–497. doi: 10.1016/j.radonc.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Smeenk R.J., van Lin E.N., van Kollenburg P., McColl G.M., Kunze-Busch M., Kaanders J.H. Endorectal balloon reduces anorectal doses in post-prostatectomy intensity-modulated radiotherapy. Radiother Oncol. 2011;101(3):465–470. doi: 10.1016/j.radonc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Leon J.F., Jameson M.G., Windsor A., Cloak K., Keats S., Vial P., et al. Superior target volume and organ stability with the use of endorectal balloons in post-prostatectomy radiotherapy. J Med Imaging Radiat Oncol. 2015;59(4):507–513. doi: 10.1111/1754-9485.12300. [DOI] [PubMed] [Google Scholar]
- 25.Smeenk R.J., Teh B.S., Butler E.B., van Lin E.N., Kaanders J.H. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. Radiother Oncol. 2010;95(3):277–282. doi: 10.1016/j.radonc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Mariados N., Sylvester J., Shah D., Karsh L., Hudes R., Beyer D., et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Karsh L.I., Gross E.T., Pieczonka C.M., Aliotta P.J., Skomra C.J., Ponsky L.E., et al. Absorbable Hydrogel Spacer Use in Prostate Radiotherapy: A Comprehensive Review of Phase 3 Clinical Trial Published Data. Urology. 2018;115:39–44. doi: 10.1016/j.urology.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Zaorsky N.G., Calais J., Fanti S., Tilki D., Dorff T., Spratt D.E., et al. Salvage therapy for prostate cancer after radical prostatectomy. Nat Rev Urol. 2021;18(11):643–668. doi: 10.1038/s41585-021-00497-7. [DOI] [PubMed] [Google Scholar]
- 29.Tendulkar R.D., Agrawal S., Gao T., Efstathiou J.A., Pisansky T.M., Michalski J.M., et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol. 2016;34(30):3648–3654. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
- 30.Berry S.L., Boczkowski A., Ma R., Mechalakos J., Hunt M. Interobserver variability in radiation therapy plan output: Results of a single-institution study. Pract Radiat Oncol. 2016;6(6):442–449. doi: 10.1016/j.prro.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segedin B., Petric P. Uncertainties in target volume delineation in radiotherapy - are they relevant and what can we do about them? Radiol Oncol. 2016;50(3):254–262. doi: 10.1515/raon-2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hague C., Beasley W., Dixon L., Gaito S., Garcez K., Green A., et al. Use of a novel atlas for muscles of mastication to reduce inter observer variability in head and neck radiotherapy contouring. Radiother Oncol. 2019;130:56–61. doi: 10.1016/j.radonc.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Vinod S.K., Min M., Jameson M.G., Holloway L.C. A review of interventions to reduce inter-observer variability in volume delineation in radiation oncology. J Med Imaging Radiat Oncol. 2016;60(3):393–406. doi: 10.1111/1754-9485.12462. [DOI] [PubMed] [Google Scholar]
- 34.Lin D., Lapen K., Sherer M.V., Kantor J., Zhang Z., Boyce L.M., et al. A Systematic Review of Contouring Guidelines in Radiation Oncology: Analysis of Frequency, Methodology, and Delivery of Consensus Recommendations. Int J Radiat Oncol Biol Phys. 2020;107(4):827–835. doi: 10.1016/j.ijrobp.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson N.L., Sala E., Benz M., Landa J., Scardino P., Scher H.I., et al. Combined Whole Body and Multiparametric Prostate Magnetic Resonance Imaging as a 1-Step Approach to the Simultaneous Assessment of Local Recurrence and Metastatic Disease after Radical Prostatectomy. J Urol. 2017;198(1):65–70. doi: 10.1016/j.juro.2017.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miralbell R., Vees H., Lozano J., Khan H., Mollà M., Hidalgo A., et al. Endorectal MRI assessment of local relapse after surgery for prostate cancer: A model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys. 2007;67(2):356–361. doi: 10.1016/j.ijrobp.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Kudchadker R., Choi S., Pettaway C.A., Choi H., Hobbs B.D., et al. Local recurrence map to guide target volume delineation after radical prostatectomy. Pract Radiat Oncol. 2014;4(6):e239–e246. doi: 10.1016/j.prro.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Sonni I., Dal Pra A., O'Connell D.P., Ells Z., Benz M., Nguyen K., et al. PSMA PET/CT-based Atlas for Prostatic Bed Recurrence after Radical Prostatectomy: Clinical Implications for Salvage Radiation Therapy Contouring Guidelines. J Nucl Med. 2023 doi: 10.2967/jnumed.122.265025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horsley P.J., Koo C.M., Eade T., Hsiao E., Emmett L., Brown C., et al. Mapping of Local Recurrences After Radical Prostatectomy Using 68-Gallium-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography: Implications for Postprostatectomy Radiation Therapy Clinical Target Volumes. Int J Radiat Oncol Biol Phys. 2023;115(1):106–117. doi: 10.1016/j.ijrobp.2022.05.044. [DOI] [PubMed] [Google Scholar]
- 40.Silverman J.M., Krebs T.L. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR Am J Roentgenol. 1997;168(2):379–385. doi: 10.2214/ajr.168.2.9016212. [DOI] [PubMed] [Google Scholar]
- 41.Connolly J.A., Shinohara K., Presti J.C., Jr., Carroll P.R. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 1996;47(2):225–231. doi: 10.1016/S0090-4295(99)80421-X. [DOI] [PubMed] [Google Scholar]
- 42.Robin S., Jolicoeur M., Palumbo S., Zilli T., Crehange G., Hertogh O., et al. Prostate bed delineation guidelines for postoperative radiation therapy: On behalf of the francophone group of urological radiation therapy. Int J Radiat Oncol Biol Phys. 2021;109(5):1243–1253. doi: 10.1016/j.ijrobp.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Bell L.J., Cox J., Eade T., Rinks M., Herschtal A., Kneebone A. Determining optimal planning target volume and image guidance policy for post-prostatectomy intensity modulated radiotherapy. Radiat Oncol. 2015;10:151. doi: 10.1186/s13014-015-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.