ABSTRACT
Based on a comprehensive review and critical analysis of the literature regarding the nutritional concerns of female athletes, conducted by experts in the field and selected members of the International Society of Sports Nutrition (ISSN), the following conclusions represent the official Position of the Society: 1. Female athletes have unique and unpredictable hormone profiles, which influence their physiology and nutritional needs across their lifespan. To understand how perturbations in these hormones affect the individual, we recommend that female athletes of reproductive age should track their hormonal status (natural, hormone driven) against training and recovery to determine their individual patterns and needs and peri and post-menopausal athletes should track against training and recovery metrics to determine the individuals’ unique patterns. 2. The primary nutritional consideration for all athletes, and in particular, female athletes, should be achieving adequate energy intake to meet their energy requirements and to achieve an optimal energy availability (EA); with a focus on the timing of meals in relation to exercise to improve training adaptations, performance, and athlete health. 3. Significant sex differences and sex hormone influences on carbohydrate and lipid metabolism are apparent, therefore we recommend first ensuring athletes meet their carbohydrate needs across all phases of the menstrual cycle. Secondly, tailoring carbohydrate intake to hormonal status with an emphasis on greater carbohydrate intake and availability during the active pill weeks of oral contraceptive users and during the luteal phase of the menstrual cycle where there is a greater effect of sex hormone suppression on gluconogenesis output during exercise. 4. Based upon the limited research available, we recommend that pre-menopausal, eumenorrheic, and oral contraceptives using female athletes should aim to consume a source of high-quality protein as close to beginning and/or after completion of exercise as possible to reduce exercise-induced amino acid oxidative losses and initiate muscle protein remodeling and repair at a dose of 0.32–0.38 g·kg−1. For eumenorrheic women, ingestion during the luteal phase should aim for the upper end of the range due to the catabolic actions of progesterone and greater need for amino acids. 5. Close to the beginning and/or after completion of exercise, peri- and post-menopausal athletes should aim for a bolus of high EAA-containing (~10 g) intact protein sources or supplements to overcome anabolic resistance. 6. Daily protein intake should fall within the mid- to upper ranges of current sport nutrition guidelines (1.4–2.2 g·kg−1·day−1) for women at all stages of menstrual function (pre-, peri-, post-menopausal, and contraceptive users) with protein doses evenly distributed, every 3-4 h, across the day. Eumenorrheic athletes in the luteal phase and peri/post-menopausal athletes, regardless of sport, should aim for the upper end of the range. 7. Female sex hormones affect fluid dynamics and electrolyte handling. A greater predisposition to hyponatremia occurs in times of elevated progesterone, and in menopausal women, who are slower to excrete water. Additionally, females have less absolute and relative fluid available to lose via sweating than males, making the physiological consequences of fluid loss more severe, particularly in the luteal phase. 8. Evidence for sex-specific supplementation is lacking due to the paucity of female-specific research and any differential effects in females. Caffeine, iron, and creatine have the most evidence for use in females. Both iron and creatine are highly efficacious for female athletes. Creatine supplementation of 3 to 5 g per day is recommended for the mechanistic support of creatine supplementation with regard to muscle protein kinetics, growth factors, satellite cells, myogenic transcription factors, glycogen and calcium regulation, oxidative stress, and inflammation. Post-menopausal females benefit from bone health, mental health, and skeletal muscle size and function when consuming higher doses of creatine (0.3 g·kg−1·d−1). 9. To foster and promote high-quality research investigations involving female athletes, researchers are first encouraged to stop excluding females unless the primary endpoints are directly influenced by sex-specific mechanisms. In all investigative scenarios, researchers across the globe are encouraged to inquire and report upon more detailed information surrounding the athlete’s hormonal status, including menstrual status (days since menses, length of period, duration of cycle, etc.) and/or hormonal contraceptive details and/or menopausal status.
KEYWORDS: Female athlete, Menstrual cycle, Hormonal contraceptive, Menopause, Nutrition, Exercise
1. Methods
The International Society of Sports Nutrition (ISSN) position stands are invited papers on topics the Journal of the ISSN (JISSN) Editors and Research Committee identifies as being of interest to JISSN readers. The process consists of editors and/or the ISSN Research Committee identifying a lead author or team of authors to perform a comprehensive literature review. Specifically, for this Female Athlete Position Stand, the scientific design of the studies was scrutinized for scientific validity [1, 2] as part of the inclusion criteria. After the authors develop the content of the position stand, the draft is sent to leading scholars in the field for a detailed review. Following a critical review by the scholars, the paper was revised by a team of authors, approved by the ISSN Research Committee and JISSN Editors, and published as a consensus statement and the official position of the ISSN on the topic.
2. Introduction
As even a cursory review of the history of scientific advancement would reveal, the biomedical community has long failed to give due deference to the importance of sex differences in human physiology. Males have routinely been considered the exemplar by which all measures and standards were set. The issue of male bias goes far and the literature is rife with examples of science that ignored women [3], even when women should have been a target demographic. For example, in the mid-1960’s observation that women tend to have lower rates of cardiovascular disease until their estrogen levels dropped after menopause spurred researchers to investigate whether hormone supplementation was an effective preventive treatment. The study enrolled 8,341 men and no women [4]. Similarly, the Multiple Risk Factor Intervention Trial (MRFIT) investigated dietary modifications and exercise to prevent cardiovascular disease, enrolling 13,000 male subjects and no women [5]. Given the significant sex differences from biology to behavior, excluding females means one cannot assume that any findings would apply to females. Moreover, it is possible that by doing a sex-based analysis, scientific breakthroughs could occur that could be important for all people by understanding how certain interventions vary by sex. Fortunately for all active females, the opportunities for athletic endeavors and the body of female-centric human performance research are quickly expanding. However, female-centric research studies and female subject numbers still lag significantly behind males [3,6].
While health and human performance research does target women, the focus of that research is often the influence of the menstrual cycle. One of the earliest publications regarding menstruation and physical activity was written in 1877 by Mary Putnam Jacobi, M.D., entitled “The question of rest for women during menstruation” [7]. The assumptions made from these early writings are still prevalent today that physical activity might impair reproductive function and harm the health of females. Thus, females were restricted from most organized, high intensity physical activity and sport. The study of the female menstrual cycle as it relates to sport performance began in earnest in 1953 and, as of today, ~400 studies on this topic have since been published [8]. Compared to males, the scientific design around the menstrual cycle has been deemed “complex” thus has been an obstacle to developing and funding high numbers of research studies. Yet, despite the obvious endocrinological differences between the sexes and the growing interest in menstrual cycle-specific research [9], exercise science and nutrition research continue to generalize results from male data to women [10]. Recent guidelines have been published on the scientific design and methodology relevant to the study of women’s exercise performance and nutrition [1,2,11]. Critically, these guidelines provide recommendations on the conditions under which female sex hormones need to be considered [1,8,11]. This, along with women’s increased individual interest in approaching peak mental and physical performance has resulted in a growing body of research that addresses these issues across the lifespan [12,13]. The aim of this position stand is to review this research and provide practical evidence-based female-specific recommendations for sport nutrition.
3. Overview of sex differences
The terms “sex” and “gender” have come to be used synonymously in the sport and exercise literature. However, there are two distinct concepts that should not be used interchangeably. “Sex” generally refers to the distinction between females and males based on reproductively relevant differences in chromosomes, primary and secondary sex characteristics, and endogenous hormonal profiles. “Gender” can refer to several things, including one’s self-perceived identity as a woman, man, or otherwise – a trait, which is at least partly biologically based and inherent to a person; one’s outward expression of one’s gender identity – in terms of one’s appearance and other behaviors; and the role one is expected to play in society based on one’s sex or gender identity – the specifics of which can vary across sociocultural circumstances. While discussions of gender are certainly relevant to the athletic community at large, the specific issue of nutrition among female athletes will be better informed by a restriction of the current discussion to sex and human sexual differentiation.
Sex chromosome genes and sex hormones, including estrogen, progesterone, and and androgens, contribute to the differential responses between the sexes. Following birth, significant prepubertal differences exist between the structure and function of other organ systems in boys and girls, which are eventually further emphasized by hormone activity and driven by a sex-specific reactivity to environmental stimuli, including nutrients and the diet in general. Although difficult to separate from the hormonal influences, important sex differences exist in mitochondrial function [14–16], substrate utilization, and insulin sensitivity [17–24], immune responses [25–28], muscle morphology and body composition [29–32], iron metabolism [33–40], thermoregulation [41–44], hydration [45,46]{Sims, 2008 #55, [47–53], appetite control [54–59], and energy availability and endocrine function [60–65].
3.1. Female sex hormones and differential hormone profiles
The primary female sex hormones are 17β estradiol (E2), the predominant endogenous estrogen in humans, and progesterone. Both hormones exert agonistic and antagonistic effects on metabolism and nutrient needs whereby the ratios and levels of estradiol and progesterone affect the proportions of macronutrients used as fuel, not only at rest but also during exercise. Estrogen actions in hypothalamic nuclei differentially control food intake, energy expenditure, and white adipose tissue distribution [22,66,67]. Estrogen actions in skeletal muscle, liver, adipose tissue, and immune cells are involved in insulin sensitivity as well as prevention of lipid accumulation and inflammation [20,21,67,68]. Estrogen actions in pancreatic islet β-cells also regulate insulin secretion, nutrient homeostasis, and survival [66,69,70]. Less is known of the specific mechanisms of metabolic influences of progesterone. However, progesterone has direct effects on energy expenditure through a progesterone-mediated increase in metabolic rate [71–73]; alters serum electrolyte balance through progesterone-mediated increases in aldosterone [74–77]; functions catabolically to increase amino acid oxidation and decreases muscle protein synthesis [78–81], and affects glucose metabolism through the upregulation of GLUT1 expression to increase endometrial glycolytic metabolism, attenuating skeletal and hepatic glycolytic pathways [82–85].
To better understand the potential effects of female sex hormones on metabolism and nutrient needs of female athletes, it is important to be aware of the many different hormonal environments a female athlete of reproductive age could be experiencing. For those athletes not using hormonal contraceptives (see the next section), this can range from amenorrhea (absence of menstrual cycle) to oligomenorrhea (menstrual cycle longer than 40 days, so less than 9 cycles per year) to naturally menstruating (menstrual cycle length between 21 and 40 days). And within the naturally menstruating women hormone fluctuations vary as a result of ovulatory cycles (eumenorrheic), as well as menstrual cycles with anovulation or luteal phase deficiency [2].
The menstrual cycle (MC) and its systemic effect on the body is a crucial area for research, as it has been found that women [1] frequently experience different adaptations and stress responses to their male counterparts [86,87]. For eumenorrheic women, the MC is characterized by fluctuations in several hormones, most notably the gonadal steroids, estrogen and progesterone, and is partitioned into the following phases: early follicular (EF), mid-follicular (MF), late follicular (LF), ovulation, early luteal, mid-luteal (ML), and late luteal phases (Figure 1). Throughout each phase, fluctuations in hormones trigger not only changes in the reproductive system but also in all the tissues of the body, which can have a direct effect on stress resilience, metabolism, and adaptations [1,88]. As a brief review, the length of a normal menstrual cycle is 21 to 40 days [89]. The first half of the MC is comprised of the menstrual and follicular phases during which time estrogen levels are low (early follicular/menstrual), then rise (mid follicular) and peak (late follicular) and ends with the periovulatory phase in which follicular-stimulating hormones and luteinizing hormones reach peak concentrations. After ovulation, the second half of the cycle is comprised by the early luteal (during which time estrogen level drops and then rises while progesterone rises), the mid-luteal (during which time estrogen and progesterone levels peak), and finally, the late luteal phase (during which time estrogen and progesterone levels fall). These cyclic hormone changes can affect a number of physical and psychological attributes and, ultimately, may influence sports performance, although the effects are highly individual [88,90,91].
Figure 1.
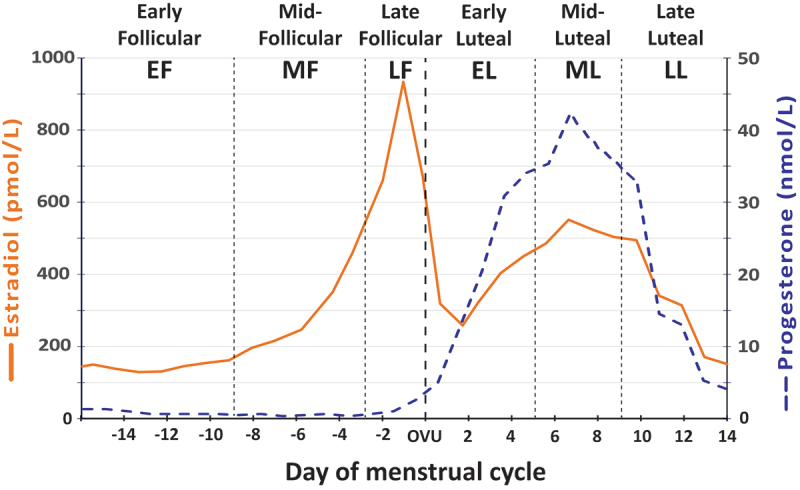
Diagram illustrating the menstrual cycle phases in a typical 28-day cycle. (from Oosthuyse et al [9]).
3.2. Hormonal contraceptives
Many female athletes decide to use hormonal contraceptives for various reasons [92]. Hormonal contraceptives (HC) contain derivatives of estrogens and progestogens, which downregulate the regular cyclic hormone activity of the hypothalamic-pituitary-gonadal axis, resulting in physiological responses that differ to those of endogenous ovarian hormones [88,93]. Ethinyl estradiol (EE) and mestranol are the two estrogens used (with ethynyl estradiol being much more frequently used), and several progestogens are currently used [94]. A full overview of the different formulations and mechanism of actions of HCs is out of scope of this position stand, thus the reader is referred to Regidor [94,95] and Benagiano [96] for in-depth detail.
Effects of synthetic/exogenous hormones on different systems of the body may require close consideration for the practitioner and athlete. For example, endogenous estrogen and progesterone have antagonistic effects on fluid balance: estrogens activate the renin–angiotensin system, stimulating the production of angiotensinogen and leading to higher levels of angiotensin, aldosterone, and sodium in plasma (sodium retention) [52], thus resulting in increased water retention. On the other hand, progesterone is a potent aldosterone antagonist [47], which stimulates the mineralocorticoid receptor preventing sodium retention. However, in OCs, the progestogens are insufficient to counteract the sodium-retaining effect of the estradiol component; consequently, the estradiol component causes fluid retention [97]. Further, Suh, Casazza et al. [98] examined the effects of oral contraceptives (OC), using a longitudinal design, on glucose flux and whole-body substrate oxidation rates during rest (90 min) and two exercise intensities [60-min leg ergometer cycling at 45% and 65% peak O2 uptake (V˙O2 peak)]. OCs significantly downregulated glucose flux during exercise, with exogenous hormones having a greater effect on exercise glucose metabolism than endogenous hormones. Thus, exogenous hormones might moderate physiological adaptations and nutritional needs in a different manner to endogenous ovarian hormones [98].
The vaginal bleeding patterns associated with continuous estrogen – progestogen (combined oral contraceptive/COC) or progestogen-only contraceptive (progestin-only pill; intrauterine device [IUD]; implants, injections) regimens are quite different from spontaneous cyclical menstrual patterns. Women using COC are subject to a different 28-day cycle wherein a 21-day intake period, characterized by constant daily doses of a synthetic estrogen (estradiol or mestranol) and one of four generations of a synthetic progesterone (termed progestin) is followed by a 7-day break inducing a withdrawal bleed (often mistaken for a real menstrual bleed) [99]. This leads to a regular pattern whereby synthetic hormone levels are high during the 21-day intake period and low during the 7-day pill break. Two major COC formulations are available: the monophasic, whereby the dosage of estrogen and progestin do not change across the active pill cycles; or multiphasic, whereby the dose of hormones varies across the active pills, particularly the progestin. The progestin provides most of the contraceptive effect and the generation, type, and dosage of the exogenous progestin determines the potency and androgenicity (e.g. Norethindrone is a first-generation drug with weak progestin changes, whereas Norgestrel and Levonorgestrel are second-generation drugs that are designed to be more potent than the 1st generation approaches and subsequently have greater androgenicity) [1,100,101]. The mechanism of action of the exogenous hormones is to cause ovarian suppression, preventing the surge of luteinizing hormone (LH) and ovulation. It is important to understand that the many different types and generations of COC formulations mean that the sparse research available in women using COC is applicable to that type of COC and not to all COC in general.
Alternatively, progestin and copper intrauterine devices (IUDs) do not inhibit ovulation but reduce endometrial hyperplasia, resulting in an inhospitable environment for implantation [102]. Importantly, only orally administered steroids undergo hepatic first-pass metabolism; thus, the localized progestin of the IUD exerts different metabolic effects as compared to the progestin of the COC with no changes in body composition or body mass [103]. A full review of the types and mechanisms of action for IUDs is beyond the scope of this paper and has been written elsewhere [104–106]. Although research is lacking regarding the impact of COC and IUD on metabolism and nutritional requirements, understanding the hormonal implications can help to draw inferences from research in eumenorrheic women.
3.3. Peri and post menopause
The menopausal transition, or perimenopause, is associated with profound reproductive and hormonal changes. A time-related change in the character of menstrual cycles as menopause approaches is well established, with an increasing proportion of cycles observed with prolonged follicular phases that may exhibit delayed ovulatory cycles or no ovulatory cycle altogether. Hormonal patterns during the luteal phase of the menstrual cycle also show changes with age, with greater absences of ovulatory cycles, while reductions in luteal phase progesterone become more common [107–109]. The reduction in progesterone impacts the ratio of estradiol: progesterone (E:P), invoking an imbalance, illustrated by anabolic resistance of muscle protein synthesis, insulin resistance, increased abdominal adiposity, decreased lean mass, a reduction in bone mineral density, and a decrease in energy expenditure [110,111]. The majority of body composition changes occur in the four to five years leading up to menopause (commonly defined as a period of time of 12 months of no menses), after which body composition changes are more age-related, rather than hormone-related [112]. By understanding the influence of hormone shifts on physiological parameters of female athletes during menopause transition, nutritional recommendations to improve insulin sensitivity as well as to support lean mass and bone density may positively enhance body composition, health, and performance.
4. Energy requirements: intake and availability
Energy is required by the human body for a variety of metabolic processes and physiological functions including muscular activity, heat production, nutrient and cellular transport, biological growth, and synthesis of cell components and new tissues. Thus, a primary nutritional consideration for female athletes should be achieving adequate energy availability (EA). EA is defined as the energy available for metabolic processes, after accounting for dietary energy intake and physical activity energy expenditure [61,113]. Specifically, the EA is defined as dietary energy intake (EI) minus exercise energy expenditure (EEE) corrected for fat-free mass (FFM) relative to total body mass [65,114]. It is widely accepted that sufficient energy is crucial for training consistency, particularly during intensified periods, as prolonged energy restriction can lead to impaired physiological function, maladaptation to prescribed training, and an increased risk of fatigue, illness, and injury [61,64,114–116]. In both sexes, energy homeostasis is under the control of a variety of hormones secreted from the gut, pancreas, adipose tissue, and gonads.
In this respect, the sex hormones are the primary sources of sex differences as it pertains to regulation of energy intake [117]. Estrogen, a primarily female hormone, reduces food intake and body weight and exerts an effect on meal size [118] and functions in conjunction with other circulating factors such as leptin and ghrelin to exert tonic inhibition of food intake [119]. During the late follicular phase of the menstrual cycle, estrogen is high, while progesterone concentration is low. It is during this phase that resting metabolic rate (RMR) and energy intake are lowest. Conversely, during the mid-luteal phase of the menstrual cycle, both estrogen and progesterone concentrations increase and reach a peak [58]. Accordingly, resting metabolic rates increase, while energy intakes are commonly at their highest levels [59,120]. For example, Barr et al. [121] estimated that free living energy intake increases ~ 300 kcal/day during the luteal phase of the menstrual cycle in recreationally active females.
4.1. Low energy availability
Low energy availability (LEA) is the underlying etiology of relative energy deficiency in sport (RED-S) [114] and occurs when energy intake is less than energy expenditure through exercise. As a result, the body does not have enough energy left to appropriately sustain physiological functions outside of exercise [63,64]. Research that investigates the effects of LEA typically falls into two categories: socio-psychological and physiological. Athletes are at a greater risk for LEA from sociocultural pressures, such as social media pressures, pressures from coaches/teammates/self to look a certain way, or from the prevailing belief that a lower body weight will result in greater performance outcomes [122–125]. Intentional dietary behavior modifications to achieve a certain sport esthetic may be a driver for LEA, however many athletes may unintentionally fall into LEA during phases of increased training volume or in sports with high energy expenditure [113,114,126]. Briefly, the known consequences of LEA cause a myriad of complications, which include reproductive dysfunction and impairments to skeletal, cardiovascular, gastrointestinal, endocrine, and neurological function [113,115,127,128]. Whether intentional or not, LEA may have significant detrimental effects on health and performance, both in endurance and strength-power athletes. Reviews of the current knowledge of health and performance impairments related to LEA and RED-S are discussed in detail elsewhere [114,126,129,130].
Nutrient sensing pathways are also sensitive to circulating estrogen; specifically, the regulation of hypothalamic kisspeptin neurons and the metabolic regulation of KISS1 gene expression and release. Kisspeptin is a neuropeptide involved in the regulation of reproductive function and also has a significant role in the regulation of glucose homeostasis, feeding behavior, and body composition [131]. The threshold for downregulation of kisspeptin signaling of gonadotropin-releasing hormone (GnRH) neurons has a greater sensitivity in females than in males [132], Thought to be primarily due to sex differences in the density of kisspeptinARC and kisspeptinAVPV/PeN neurons, kisspeptinAVPV/PeN neurons are also almost exclusive to the female brain [133]. Estrogen increases expression of KISS1 in kisspeptinAVPV/PeN and downregulates the expression in kisspeptinARC promoting a decrease in energy intake. Importantly, when energy deficits occur, through undernutrition or excessive energy expenditure, the KISS1 gene is downregulated resulting in subsequent repression of the GnRH neurons and downregulation of the reproductive axis [134,135].
Exercise induced energy deficits as a means to promote fat loss are more effective in men than in women [136,137]. This may be attributed in some respect to a sex disparity between the way exercise alters energy-regulating hormones and appetite. Hagobian and colleagues [57] demonstrated that in response to moderate-intensity continuous exercise with an uncompensated energy deficit, naturally menstruating women have higher concentrations of acylated ghrelin and lower concentrations of insulin, both of which stimulate energy intake. When the energy deficit was addressed, the patterns of acylated ghrelin and insulin were attenuated, but still persisted. In contrast, ghrelin concentrations did not change in men, regardless of energy status, along with a slight reduction of insulin in the energy deficit condition but not in energy balance [138]. In a follow-up study, an acute bout of moderate intensity aerobic exercise did not exert the same effects on ghrelin and insulin for women using a monophasic COC and the participants maintained an energy deficit after exercise (i.e. did not increase energy intake to match or exceed exercise expenditure) [139]. Therefore, in women, higher acylated ghrelin and lower insulin/leptin concentrations in response to physical activity may be a mechanism to oppose energy deficit, defend body fat stores, and preserve reproduction function. However, considering that the primary mechanism of action for many of COCs is to suppress ovulation via the inhibition of the hypothalamic–pituitary–adrenal (HPA) axis, hypothalamic responses to exercise and energy deficiency may be attenuated with the use of COCs [114]. Therefore, the identification of LEA in athletes using COCs can be masked, reducing practitioner and athlete ability to treat and/or prevent longer term implications of LEA.
4.2. Recommendations for prevention and treatment of LEA
Specific guidelines for an optimal EA for competitive female athletes have not been established, but conceptual models of energy availability thresholds have been proposed from well-controlled laboratory studies [63–65]. An EA of at least 45 kcal kg−1 FFM·day−1 appears to be a threshold to ensure optimal EA for physiological functions and body mass maintenance in female athletes [124]. An EA > 45 kcal kg−1 FFM·day−1 provides enough energy for weight gain and muscle hypertrophy. In clinical studies, it has been established that EA of 30 kcal kg−1 FFM·day−1 is the threshold at and below which suppressed metabolic hormones and reductions in luteinizing hormone pulsatility is observed after as little as 5 days in healthy females [64,65,129,140,141]. Planned periodization of changing EA status may be advantageous to attenuate the negative consequences of LEA and achieve optimal body composition and power-to-weight ratios for competition [142]. Continuous workload monitoring throughout the season also plays an important role in assessing stress and recovery needs, as high energy expenditure, high stress, and caloric needs often occur in the pre-season (lower fitness) and again during high travel and competition blocks [143–145]. In addition, blood biomarkers, such as cortisol, creatine kinase (CK), sex hormones, cytokines, hematological panels, and nutrient markers can be used to detect physiological responses to overall stress and identify balances between training, recovery, and performance outcomes [146–149]. Established EA thresholds may not be appropriate for reducing the risk of LEA in aging women due to the changing ratios of estrogen and progesterone in perimenopause, the subsequent absence of estrogen and progesterone in menopause and the known biological impact on resting metabolic rate and body composition [150]. As such, reducing the discrepancy between training energy expenditure and energy intake, with careful attention to nutrient timing, should be considered [151].
EA is likely to vary between training days (as a function of EEE), and it is the within-day periods of low energy intake over 24-hours that are associated with negative health indices and body composition changes despite optimal daily EA values [62,152]. Research has demonstrated that females with menstrual dysfunction exhibit greater elevations in cortisol and decreases in RMR and estradiol the longer they delay nutrition intake, post-exercise [62]. Of importance, nutrient timing has been proposed to have a significant impact on attenuating increasing health, exercise training, and recovery implications [153]. It is important to point out that quantification of EA in free-living athletes can be difficult and may have a wide margin of error. To determine the EA, the calculation requires accurately measured FFM, EI and exercise EE of the athlete, in which errors of validity and reliability will undoubtedly occur [150]. Moreover, non-purposeful physical activity expenditure needs to be included to accurately reflect perturbations in energy availability. Therefore, EA thresholds should be used to guide nutritional strategies in which the individual’s training load, nutrient timing, and manipulation of macronutrients to ensure recovery and adaptation are the forefront of the athlete’s nutritional plan.
4.3. Key recommendations
The primary consideration for female athletes, regardless of hormonal status, is to maintain an overall EA level that meets the specific goals and requirements of their individual training and performance demands.
Due to the increased energy needs in the luteal phase of the menstrual cycle, eumenorrheic athletes may consider increasing overall 24-h energy intake in this phase.
COC use can mask early signs of LEA, reducing the opportunity to identify LEA and prevent further implications.
Specific attention to nutrient timing in relation to exercise may help to reduce time spent in a catabolic state and as a result improve health, performance, and associated exercise training adaptations even in times of planned caloric deficit.
It is acknowledged that at various points in the training cycle, a caloric deficit is desired for optimizing body mass and composition, however, athletes and coaches of female athletes must understand the repercussion on the greater neuroendocrine responses to low nutrition density.
Monitoring biomarkers across training blocks and athletic seasons can provide further insight into the balance between training, recovery, and performance outcomes.
5. Sex hormone effects and substrate metabolism
Robust evidence exists that demonstrates the important roles estrogen and progesterone have in regulating substrate metabolism [20,24,66,70,80,82,84,154–159]. Estrogen, specifically 17β-estradiol, promotes lipolysis and increases fatty acid availability while decreasing the rate of gluconeogenesis with muscle and liver glycogen sparing. Although 17β-estradiol lowers mitochondrial membrane viscosity (which creates a more fluid lipid bilayer and facilitates movement and function of membrane proteins), Miotto and colleagues [14] demonstrated that female skeletal muscle has a greater abundance of the fatty acid transporter CD36, a sex difference that results in a greater membrane translocation of the CD36 protein during muscle contraction that helps to explain the heightened rates of lipid metabolism, which occur in females when compared to males during exercise [160,161]. Further, these actions are independent of hormone influences and further promote carbohydrate sparing and higher lipid oxidation during exercise [155,162,163].
Progesterone decreases the sensitivity of IGF-1 mediated uptake of glucose into skeletal muscle, but upregulates GLUT-4 and GLUT-1 translocation to enhance glycogen storage in the endometrial tissue for embryonic support [84]. Progesterone also appears to accentuate the carbohydrate-sparing actions of estrogen by decreasing hepatic glycogenolysis [157]. Progesterone also exerts influence over protein metabolism, specifically during the luteal phase, which corresponds with the previously observed increases in protein oxidation at rest and during exercise at this time in the menstrual cycle [81,164]. This increase is attributed to a greater concentration of progesterone (lowered ratio of E:P). The primary action of progesterone is to increase the net rate of protein catabolism through enhancement of plasma-free amino acid utilization by the liver, reducing plasma amino acid concentrations and increasing total urinary nitrogen excretion (without aminoaciduria) [157,165] due to increased protein biosynthesis of the endometrial lining.
Due to the aforementioned hormonal effects, research has demonstrated that sex differences and sex hormone profiles (e.g. menstrual cycle phase, hormonal contraceptive use) influence glucose kinetics and net protein balance, which may affect exercise capacity and/or performance in eumenorrheic female athletes [166,167]. For eumenorrheic women, research has demonstrated a lower rate of glucose appearance, disappearance, and total glycogen utilization in the luteal phase as compared to the follicular phase when the energy demands of exercise are high enough to exert pressure on endogenous glucose production (>50% VO2max) [168–171].
5.1. Sex mediated differences in carbohydrate metabolism
With respect to whole body carbohydrate (CHO) metabolism, eumenorrheic women have generally been observed to oxidize proportionally less CHO, more lipids, and less leucine as compared to their male counterparts both at rest and during exercise of low to moderately high intensities and durations [154,163,172–174]. The greater lipid oxidation in women during submaximal exercise appears to be from adipose tissue, whereas the main source of increased fatty acid utilization at rest is from skeletal muscle [155]. This sexual dimorphism is apparent after general adaptive changes in endurance training (decrease in muscle carbohydrate utilization and increase in lipid oxidation). In this respect, women utilize more lipid when compared to men and this is largely attributable to sex-specific hormonal influences and epigenetic adaptations to endurance exercise [86,175–177]. Moreover, in response to exercise, women maintain the same level of glycemia as men, despite having lower glucose flux [173]; indicating that women increase glucose clearance less so than men.
Exogenous ovarian hormones appear to exert greater effects on glucose flux during exercise than endogenous hormones, as decreases in rates of glucose appearance and disappearance can be observed in recently fed women using combined oral contraceptives (COC) compared to before COC use [178]. Findings from studies investigating COC use and substrate utilization will likely vary due to the use of different types of COC agents, monophasic vs. triphasic and different COC formulations as described in earlier sections of this Position Stand. Evidence shows that COCs with higher concentrations of hormones can decrease glucose tolerance, in turn augmenting insulin resistance in adult premenopausal women [179]. Additionally, increases in C-reactive protein levels, through glucose tolerance testing of Olympic female athletes on COC, have been observed, indicative of an upregulated inflammatory response [180]; with significantly higher oxidative stress in female athletes using COCs than their non-COC user counterparts irrespective of lifestyle habits [181].
Resting muscle glycogen in eumenorrheic female athletes may be reduced during the follicular phase [182,183] and carbohydrate loading (8.4–9.0 g.kg body weight−1.d−1) has been shown to increase resting muscle glycogen concentration in the mid-follicular (MF) phase of the menstrual cycle [184,185]. In contrast, CHO loading using a similar pattern and amount of carbohydrate intake during the mid-luteal (ML) phase failed to change resting muscle glycogen concentrations [182] or only documented a modest increase (13%) [186] compared to what is generally reported for male athletes (18–47%) [185,187–191] or female athletes during the follicular phase (17–31%) [184,185]. It is considered that the increased presence of progesterone in the mid-luteal phase promotes the shuttling of glucose into the liver, as well as upregulating GLUT-4 and GLUT-1 translocation to enhance glycogen storage in the endometrial tissue for embryonic support [84,157]. As a result, supercompensation of glycogen in skeletal muscle and hepatic tissue may be more difficult to attain in the mid-luteal phase without higher routine daily carbohydrate levels due to changes in insulin sensitivity and decreased sensitivity of IGF-1 mediated glucose uptake. Although the lower level of muscle glycogen storage in the mid-follicular phase of the menstrual cycle appears to be overcome by CHO loading, the greater intramuscular glycogen concentrations failed to translate into improved time trial performance [182,184].
The impact of CHO loading on the muscle glycogen content of COC users is even less clear. At this time, it is unknown what effect CHO loading may have on muscle glycogen concentration in female athletes taking COC with different concentrations of ethynyl estradiol and generations of progestins. More research is needed to address these considerations.
Achieving the high intakes of CHO (≥8 g.kg body weight−1 day−1) commonly recommended for CHO loading can be difficult for women whose habitual energy intakes are <2000 kcal.d−1 [192–194], as this dose amounts to ingesting more than 70–90% of the total energy intake as CHO for a 60-kg woman. Thus, in view of ovarian hormone effects on glucose kinetics and to ensure adequate carbohydrate availability is met, it is recommended that all women follow the recommendation to consume a pre-exercise meal or snack containing CHO three to four hours before beginning endurance exercise. This recommendation is particularly prudent during the active pill weeks of COC users and during the luteal phase of the menstrual cycle of eumenorrheic women, where there is a greater effect of sex hormone suppression of gluconeogenesis output during exercise [90,166].
5.2. Key recommendations
During all times, but especially during the follicular phase (reduced glycogen storage) and times of competitive or training scenarios where absolute glycogen availability may limit performance, eumenorrheic women should pay particular attention to consuming sufficient energy with a primary focus on consuming enough carbohydrate to support overall health as well as the duration, intensity, and environmental factors (heat, cold, altitude) of performance.
Women using COC should also ensure adequate carbohydrate availability across all phases of the active pill cycle to attenuate exogenous hormone-linked higher oxidative stress.
5.2.1. Sex mediated differences in carbohydrate metabolism - during exercise
It is well documented that carbohydrate availability during exercise is ergogenic as CHO feeding can help maintain plasma glucose and prevent hypoglycemia, spare hepatic glycogen, and delay muscle glycogen depletion [195–198]. However, in women, estrogen and muscle metabolic effects directly reduce carbohydrate utilization due to a marked hepatic glycogen sparing effect and insulin-mediated storage, thus indirectly shifting substrate utilization toward lipids across low to moderate intensity exercise. Moreover, there is a greater sensitivity in women to the lipolytic effects of catecholamines, thought to be due to an exercise-mediated upregulation of β1-(lipolysis stimulating) receptors (as compared to men) [173,199]. Recommendations for carbohydrate intake during exercise are dependent on exercise duration, absolute intensity, environmental conditions (heat, humidity, altitude), and gastrointestinal tolerance. However, these guidelines have largely been established from male data and generalized to women, with the acknowledgment that more female-centric research is needed. Based on the limited research conducted in females, several considerations exist for female athletes to improve overall carbohydrate availability for training, performance, and recovery. First, a diet high in carbohydrate (≥60% of daily energy intake) plus intake of exogenous carbohydrate during exercise has shown to increase the percentage of total energy from CHO oxidation in women, with an even greater effect than seen in men [200]. This increase was not attributable to a higher percentage of exogenous carbohydrate oxidation, but, alternatively, was attributed to a smaller decrease in endogenous CHO oxidation in eumenorrheic women during exercise [201]. Women using COC demonstrate a slightly lower reliance on exogenous CHO oxidation as compared to non-COC users [98,179,201]. Second, females exhibit slower gastric emptying and gut motility [202,203], a greater incidence of exercise-induced gastroparesis as compared to males [204]. Eumenorrheic women reportedly experience greater gastrointestinal distress across the early-follicular and late-luteal stages of the menstrual cycle as compared to other phases of the cycle [205]. Third, carbohydrate intake during exercise should be scaled according to the characteristics of the event. During sustained high-intensity sports lasting approximately one-hour, small amounts of pre-exercise carbohydrate (~30 g) [206], enhance performance. Although carbohydrate mouth rinsing has been shown to have ergogenic effects in men [207], the same does not appear to hold true for trained female athletes, in either high intensity-short duration or resistance exercises [208,209]. As observed in studies involving males, evidence in females also supports CHO ingestion rates of 30–60 g · h−1 of as an appropriate target for sports of longer duration (≥60 min) [210]. However, it has not been established if multiple transportable carbohydrate blends at high doses (up to 90 g · h−1) improve peak exogenous oxidation rates in females [201,211]. During the follicular phase in endurance-trained women, the greatest exogenous CHO oxidation and endogenous CHO sparing were observed when CHO was ingested at a rate of 60 g·h−1 with no further increases when the rate was increased to 90 g·h−1 [212]. Lastly, provision of a CHO-electrolyte beverage during endurance exercise in the heat attenuates immune disturbances compared to a placebo beverage, more so in the luteal phase of the menstrual cycle [213]. Moreover, amino acid catabolism rates appear to be reduced when CHO supplementation was provided during exercise [214].
5.3. Key recommendations
In light of the limited data available, female athletes should track their menstrual cycle/hormone status to identify any times of increased GI issues across the cycle and if carbohydrate dosing thresholds toward the upper limits of CHO ingestion (>60 g·h−1) affect GI tolerance.
A realistic starting recommendation is to ingest CHO at a rate of 30-60 g·h−1 during exercise to offset menstrual cycle effects on glucose kinetics/exercise metabolism. This will also limit potential gastrointestinal distress, immune disturbances, and protein catabolism.
The major factor limiting maximal exogenous CHO oxidation is intestinal absorption. In addition, hepatic limitations which can influence the release of CHO to systemic circulation, energy demands of exercise, gut health, and carbohydrate tolerance to intake should all be considered when developing a carbohydrate ingestion regimen.
5.3.1. Sex mediated differences in carbohydrate metabolism - post-exercise
In the acute post-exercise recovery phase, eumenorrheic women have a greater ability to maintain glycemia following prolonged exercise as compared with males irrespective of menstrual cycle phase. This is mainly due to a more immediate decrease in glucose flux at the end of exercise in women, whereas more time is required in men to re-establish basal glucose homeostasis [154]. Evidence indicates that during exercise tasks of similar relative intensity and the same duration, women display a greater capacity for lipid oxidation and regain control over glycemia and glucose flux more rapidly in recovery than do men [154,155]. Additionally, metabolic perturbations of exercise are still evident 21 hours after 60–90 min of low to moderate intensity exercise in men but are no longer apparent after 3 hours in women [155,172,215].
To the authors’ knowledge, limited investigations have examined the effect of menstrual cycle phase or hormonal contraceptives on muscle glycogen resynthesis with none in peri- and post-menopausal populations. From this limited evidence, muscle glycogen repletion is reduced in the follicular phase when compared to the luteal phase in moderately trained eumenorrheic women, suggesting an ovarian hormone influence on muscle glycogen resynthesis [216]. When compared to males, muscle glycogen resynthesis during the follicular phase of the menstrual cycle occurs at similar rates following CHO consumption of 1.2 g·kg−1 of CHO in the acute recovery period following glycogen depleting exercise [195,217]. In addition, post-exercise supplementation (1.2 g·kg−1 of CHO, 0.1 g·kg−1 of protein, and 0.02 g·kg−1 of fat), following four training sessions across a week during the follicular phase, improved time to exhaustion during endurance cycling at 75% of VO2peak. [218]. Peri- and post-menopausal athletes should adhere to the same recommendation as the effects of ovarian hormones are slight compared to sex differences in post-exercise glycemia. Moreover, due to an increase in insulin resistance associated with peri and post menopause [219]; athletes in this life phase should take advantage of the non-insulin dependent first phase of glycogen synthesis, which lasts 30–40 minutes if glycogen depletion is substantial [220]. In this respect, close consideration to nutrient timing recommendations [153] should be employed as well as other strategies to maximize glycogen recovery [221].
5.4. Key recommendations
The replenishment of endogenous glycogen stores after high volume and/or multiple sessions in a 24-hour period is of the utmost importance to optimize performance. As a result, strategies to improve carbohydrate availability can promote positive training adaptations and health.
With the limited data available, we recommend female athletes should focus on rapid consumption of at least 1.2 g·kg−1 of CHO following prolonged exercise in order to restore spent muscle glycogen.
Peri and post-menopausal women should focus on rapid consumption of CHO per above, with close consideration to nutrient timing to maximize glycogen recovery.
5.5. Sex mediated differences in protein metabolism
Skeletal muscle is viewed to be the primary reservoir for amino acids (AA), and failure to deliver adequate amino acids from the diet can challenge the body’s ability to respond to daily protein turnover, particularly muscle protein. When essential AAs are low, skeletal muscle breakdown rates are increased resulting in a release of AAs necessary to maintain proteostasis throughout the body. In contrast, when AA levels are high, muscle protein synthesis increases so that more AAs can be incorporated into muscle and other key organs and tissues. As AAs are the building blocks of proteins and play an essential role in the regulation of protein turnover, maintaining adequate protein intake is essential to ensure that a balance is maintained between the rate of muscle protein breakdown (MPB) and muscle protein synthesis (MPS) to maintain muscle mass.
To briefly review, 21 AAs are required for MPS; nine of these are essential amino acids (EAAs) as they cannot be synthesized in humans and must be supplied in the diet, while the remaining are nonessential and can be readily made by other tissues. The anabolic effects of AA ingestion on MPS are the result of the EAAs, specifically leucine, which indirectly activates the mammalian target of rapamycin (mTOR); note that although leucine triggers the essential signaling for MPS, all AAs are necessary to increase MPS in humans [222]. Feeding and exercise are potent stimuli for MPS, with the feeding-induced stimulation being transient and not solely responsible for muscle protein accretion. Exercise, primary resistance-based, enhances MPS. However, the consumption of protein post-exercise is necessary to maximize the rates of MPS and overall stimulation of skeletal muscle hypertrophy [223].
Although sex-based differences in protein metabolism are likely small compared to those observed in carbohydrate and lipid metabolism, the depth of research on female athletes is lacking. No studies are currently available that specifically address the protein requirements of female athletes across the menstrual cycle or with the use of hormonal contraceptives. In eumenorrheic women, evidence suggests protein catabolism is higher at rest and following aerobic endurance exercise in the luteal phase, when estrogen and progesterone are elevated, compared to the early follicular phase when estrogen and progesterone concentrations are low [81,164,223,224]. Moreover, it has been shown that protein oxidation during exercise appears to be greater during the mid-luteal phase [164,223] and that females require more lysine during the luteal phase than the follicular phase [81] with a lower ability to uptake and utilize amino acids for protein synthesis. Although the blood amino acid profiles of COC users are shown to differ from non-users [225], the impact of COC on protein metabolism needs at rest or in response to exercise is yet to be elucidated. The major variable in determining needs is the formulation of the contraceptive, specifically the generation of the progestin due to differences in androgenicity, and how this may influence skeletal muscle adaptations to training (e.g. first-generation progestins are derived from testosterone and have high androgenic activity, whereas third-generation progestins have been modified to have low androgenic activity). OCs also reduce endogenous levels of estrogen, progesterone, free testosterone, and insulin-growth factor-1 (IGF-1), but enhances cortisol, all of which affect the degree of anabolic responses to training [92]. For example, recent data have shown that low androgenic activity progestins impairs myofibrillar protein FSR at rest and 24 h post-endurance exercise as compared to rates observed during the follicular phase of eumenorrheic women, but they have no effect on myofibrillar protein breakdown [226]. An additional study compared the effects of two different dosages of a third-generation OC with a non-user control group on resistance training adaptations. During a 10-week supervised progressive resistance training program, a 30 μg ethynyl estradiol dose from a third-generation OC was associated with a trend toward a greater increase in muscle mass and a significantly greater increase in type I muscle fiber area compared to a 20 μg dose of ethynyl estradiol and non-users, though no changes in muscle strength related to training were found. This study highlights that the dosage provided by ethinyl estradiol may also be a key variable to consider relative to the skeletal muscle adaptations that are observed [227].
Considering that adequate dietary protein is important for supporting physiological adaptations to exercise, understanding the effects of sex differences, hormonal status, types of exercise, and training status is essential for supporting female athletes. Mercer and colleagues [228] published an in-depth review that indicates the estimated average requirement for protein intake of eumenorrheic recreationally active and/or competitive female athletes is similar for aerobic endurance exercise (1.28–1.63 g·kg−1·day−1), resistance exercise (1.49 g·kg−1·day−1) and intermittent exercise (1.41 g·kg−1·day−1), noting these requirements are within the mid-range of current sports nutrition guidelines (1.4–2.0 g·kg−1·day−1) [229,230]. Moreover, pre- and post-exercise protein intakes of 0.32–0.38 g·kg−1are recommended for beneficial adaptations in recreational and competitive female athletes. As also commonly observed and recommended in males, higher protein diets (>2.0 g·kg−1·day−1), coupled with heavy resistance training, have been shown to be important for maintaining lean mass and resting energy expenditure under periods of intentional and unintentional caloric restriction [231,232], which has been shown to be prevalent among recreationally active to elite female athletes [113,124,233–236]. If female athletes desire skeletal muscle hypertrophy, a review by Bosse and Dixon suggests that in some populations as much as a 60% increase from habitual protein intake may be needed to support this goal [237]. High protein diets (>2.2 g·kg−1·day−1) have not resulted in any adverse effects to bone mineral density or kidney function in healthy women [238,239].
As mentioned earlier, the timing of nutrient consumption around exercise directly influences performance, recovery, fat oxidation, and energy expenditure [129,153]. Female athletes often follow special diets for various reasons [113,124,125,129,233] and/or exercise in a fasted state. However, evidence indicates that for women specifically, exercising in a fasted state can blunt fat oxidation [240], whereas exercising in a fed state will result in a greater total daily energy expenditure and increased fat oxidation, potentially improving body composition [134,135]. A recent analysis suggests that consuming a bolus of protein (18 g protein, 2 g carbohydrate, 1.5 g fat) 1 hour prior to a single bout of resistance exercise (4 sets at 70–75% 1RM for nine different exercises), as opposed to consuming a bolus of carbohydrate (1 g protein, 19 g carbohydrate, 1 g fat), significantly augments energy expenditure when protein was consumed [241].
Peri-menopausal and post-menopausal athletes will need to consider daily protein intake at the mid to higher range (1.8–2.0 g·kg−1·day−1) of recommendations due to the decline of estradiol and the ensuing insulin and anabolic resistance coupled with the demands of physical activity [242–244]. Although muscle protein synthesis rates are greater in older women at rest [245], they still experience an accelerated loss of muscle mass around menopause [246]. This can be partly explained by a higher muscle protein synthesis rate in postmenopausal women, which is counteracted by an upregulation of protein breakdown [246]. Both an upregulation of stimulatory and inhibitory muscle growth regulatory genes in postmenopausal women compared with premenopausal women have also been reported [245]. An effective strategy to overcome age and hormone-mediated reductions in anabolic sensitivity in older women is the combination of resistance exercise and essential amino acid ingestion. Notably, MPS rates increased at 0–2 hours and remained elevated through 4 hours when resistance exercise was combined with ingestion of 6 to 10 grams of essential amino acids in older women [247,248].
5.5.1. Pre-sleep protein intake
Research suggests that at least 30% of the adults in the US obtain less than seven hours of sleep per night [249]. Additionally, many elite athletes fall short of their sleep requirements whereby only 3% of elite athletes reported getting enough sleep per night to feel satisfied [250]. Further, menstruating women may be at even greater risk of sleep deprivation throughout different phases of their menstrual cycle [251]. Multiple studies have reported that during the luteal phase of the menstrual cycle, an increased occurrence of sleep disturbances and decreased sleep quality result [252–254]. However, not all studies agree and some report no changes in sleep quality between the phases of the menstrual cycle [255,256]. Nevertheless, sleep deprivation after a hard bout of exercise, particularly in the evening, may lead to poor recovery, resulting in impaired muscle glycogen repletion, reduced muscle damage repair, and decreased cognitive function coupled with an increase in mental fatigue [257].
To combat these negative outcomes, data has accumulated to support the strategic manipulation of pre-sleep nutrition to meet daily caloric and protein intake needs to support the recovery demands of sport. Specifically, studies that have examined pre-sleep protein ingestion have shown efficacy in improving overnight MPS, various indices of recovery, and longitudinal improvements in strength and performance when consumed after an evening bout of exercise [258]. Recently, Apweiler et al. [259] determined that ~ 40 g pre-sleep casein protein ingestion, did not enhance functional recovery after a morning bout of exercise in healthy, active individuals. While no proper study has demonstrated that the time of day for exercise is a key influence on the potential efficacy of pre-sleep feeding strategies, it appears to be most efficacious for recovery when consumed after an evening workout when compared to a morning workout [258–260]. Recent research indicates that consuming protein before sleep may have beneficial responses in women, such as reduced muscle soreness and improvements in recovery, with no changes to lipolysis or fat oxidation [261]. Some data support an increased next-morning resting metabolic rate following pre-sleep protein ingestion; however, it is unclear if this result is positive or negative and likely depends upon the goal of the athlete [261]. Additionally, there is no difference in the morning resting metabolic rate or increases in oxygen consumption between consuming isoenergetic/isonitrogenous high-protein food (cottage cheese) versus a high-protein (casein) liquid shake [262]. It has also been shown that consuming chocolate milk before sleep increases resting and exercise carbohydrate oxidation, but this did not translate to improved next-morning running performance in female athletes [261]. However, the caloric content of the chocolate milk drink (355 ml; 180 kcal; 30 g CHO; 12 g PRO; 0 g Fat) was likely too low to have an impact on performance some eight hours post-ingestion.
Nevertheless, very little research is available that has examined the impact of pre-sleep nutrition on sleep quantity or quality, particularly amongst female athletes. Recent correlations between higher carbohydrate intake in teenage females and decreased total sleep, suggest prioritizing fats or proteins pre-sleep may be more beneficial to sleep duration [263]. Consuming foods within 4 hours of sleep, especially larger meals higher in fat, however, may also induce heart burn [264] which may be detrimental to sleep onset. Although there is the risk of disrupted sleep with pre-sleep nutrition, potential optimization of macronutrient distribution as well as size and timing of meals may decrease these negative effects while allowing for improvements in recovery and performance in women.
Pre-sleep protein ingestion has favorable outcomes in some athletes and recreationally active individuals but has yet to be elucidated in female athletes. Further, no data is available that tracks pre-sleep nutrition and internal markers of recovery such as resting heart rate or heart rate variability in athletes. These data would be intriguing due to their close association with next-day readiness to play while also offering insight for athletes to optimize pre-sleep nutrition. Moreover, these markers combined with knowledge of the menstrual cycle phases may be the next major leap forward for female athlete nutrition recommendations.
5.6. Key recommendations
Minimal research has explored the endogenous and exogenous hormonal effects on the protein needs of female athletes.
Pre-menopausal eumenorrheic and OC-using female athletes should aim to consume a source of high-quality protein at a dose of 0.32-0.38 g·kg−1 as soon after exercise as possible to replenish any exercise-induced amino acid oxidative losses and initiate muscle protein remodeling and repair.
As close to the end of exercise as possible, peri and post-menopausal athletes should aim for a bolus of high EAA (~6-10 g) containing protein food or supplement to overcome anabolic resistance.
For women at all stages of menstrual function (pre-, peri-, and post-menopausal), daily protein intake should fall within the mid-to upper ranges of current sport nutrition guidelines (1.8 – 2.2 g·kg−1·day−1). Eumenorrheic athletes in the luteal phase should consider increasing intake by ~ 12% to offset the increased protein catabolism effects of progesterone. Peri and post-menopausal women, regardless of sport, should aim for the upper end of the range. Meal dosing should be moderate in protein (∼0.3 g protein·kg−1) every 3 hours to maximize muscle protein repair and remodeling during prolonged (>24 h) recovery periods.
Pre-sleep protein ingestion has not been elucidated as specifically beneficial for female athletes in eucaloric states, however those in a low energy availability state may benefit from protein, pre-sleep, to help attenuate muscle soreness and improve recovery.
6. Special considerations
6.1. Electrolyte handling and hydration
Menstrual cycle hormones affect fluid dynamics by altering capillary permeability, vasomotor function, fluid regulatory hormone release, and plasma osmolality [265]. The elevation in plasma progesterone concentration during the luteal phase inhibits aldosterone-dependent sodium reabsorption in the kidneys due to progesterone competing with aldosterone for the mineralocorticoid receptor.
Both estrogens and progestogens can influence neural and hormonal control of thirst, fluid intake, sodium appetite, and sodium regulation. Additionally, sex differences are present in the activity and stimulus of the cell bodies of the periventricular nuclei and the supraoptic nuclei (located in the anterior hypothalamus), where arginine vasopression is synthesized [266,267]. Four studies by Stachenfeld and colleagues [48,49,53,268] and another by
Verney et al. [269] demonstrated an estrogen-associated shift to an earlier threshold in the osmotic sensitivity of thirst and release of vasopressin, indicating a smaller increase in plasma osmolality is required to trigger vasopressin release and thirst in the brain. Notably, this shift persists during OC use.
Additionally, thermoregulatory and cardiovascular capacity may be impaired at lower relative magnitudes of exercise-induced dehydration in females compared with males. Females have, on average, lower absolute total water volume as compared with males (~31 vs.~44 L) [270] even when expressed as a proportion of body mass (~49% vs. 58%) [271]. It has been suggested that the lower body water in females vs. males results in a larger proportion of a female’s total body water lost during exercise-induced dehydration. However, there appears to be equivalent losses of plasma volume between the sexes under similar exercise conditions [272,273] potentially representing sex differences in the compartmentalization of fluids. Thus, with less total body water and blood volume in conjunction with a lower proportion of total body water [274] distributed to the extracellular compartment, as compared to males, females have less absolute and relative fluid available to lose via sweating making the physiological consequences of fluid loss more severe [45].
Exercise-associated hyponatremia refers to a clinically relevant reduction in blood sodium concentrations during or up to 24 hours after physical activity. This can be a result of solute (primarily sodium) loss and/or excess fluid load [275]. Women are at greater risk for exercise-associated hyponatremia, and this risk has been primarily attributed to their lower body weight and size, excess water ingestion, and longer racing times relative to men [276]. Variations in thirst across the menstrual cycle [277] may increase the risk of hyponatremia, during prolonged races, in one phase over another if athletes are not accounting for alterations in fluid losses associated with perturbations across the menstrual cycle. While these factors may contribute to the greater incidence of hyponatremia in women, it is likely that the differential effects of female sex hormones on sodium handling also play a role.
6.2. Menopause, aging, and hydration
Independent of menopause, aging has important effects on fluid balance. Aging is associated with a higher baseline plasma osmolality, coupled with an age-related blunting of thirst sensation during exercise (and water deprivation), the usual thirst mechanism that occurs with a drop in fluid volume (dehydration) is impaired [278]. Older women are slower to excrete water (as compared to younger, premenopausal women) increasing the risk of hyponatremia [279,280]. Moreover, rehydration is a slower process with aging, primarily due to slower kidney function and hormonal responses to sodium and water flux. Estrogen-based hormone replacement therapy results in an increased basal plasma osmolality, plasma volume expansion, and an earlier osmotic threshold for arginine vasopressin release (e.g. 280 vs. 285 mOsmol/kg H2O), but a reduction in urine output, resulting in greater overall fluid retention. However, the higher overall fluid retention is not due to increased free-water retention, but rather increased sodium retention- the synthetic estrogens inducing a reduction in sodium excretion [278,281], eliciting a slight reduction in the hyponatremic risk.
7. Supplements
Dietary supplement use is the highest among females [282]. Research on collegiate female athletes suggests that more than half (65.4%) are using either traditional (single and multivitamin/mineral supplements) or non-traditional (herbals, botanicals, and other biologic and nutrient) sports supplements at least one time per month [283]. Recently, a standardized audit of the literature was conducted to determine representation for the female athlete of recognized sports supplements [284] (beta-alanine, caffeine, creatine, glycerol, nitrates/beet juice, and sodium bicarbonate) and it was determined that there is a lack of depth and elucidation of supplement use and dosages for the female athlete. Though the majority of dietary supplements have been evaluated primarily in men, the following overview introduces potential dietary supplements that may be efficacious for women based on physiological theory and sex-physiology [284].
Beta Alanine. The evidence for beta alanine (BA) determined potential erogenicity by delaying the onset of both anaerobic and aerobic fatigue allowing for longer and/or more intense training sessions, which increases the potential for greater physiological adaptations and subsequent performance [285,286]. However, lower initial muscle carnosine levels have been reported by women, suggesting women could potentially see greater benefits as compared to men. For example, Varanoske and colleagues [287] recruited 26 recreationally active participants (13 females) for 28 days of BA (6 g·d−1) supplementation. BA resulted in greater muscle carnosine concentration in females, yet no difference in fatigue attenuation was demonstrated between the females and males. Moreover, females who have greater muscle carnosine levels from higher dietary protein intake are able to delay fatigue compared to women who have lower carnosine levels [288]. Currently recommendations suggest that BA supplementation should not differ between females and males, with a total dose of 4–6 g·d−1 divided into at least 1–2 doses to increase muscle carnosine concentrations over a 4-wk timeframe [289]. For additional details, we refer interested readers to the ISSN Position Stand on Beta Alanine [290]. However, a specific dosing regimen (amount, timing, etc.) for female athletes may be required beyond what has been published in the current literature for BA and should be elucidated across pre-, peri-, and post-menopausal females to optimize supplementation effects.
Caffeine. Caffeine is one of the most widely used psychoactive compounds and reduces fatigue or improves alertness by blocking adenosine receptors in the brain. A full review of caffeine effects is beyond the scope of this position stand, thus, we refer interested readers to the ISSN Position Stand on Caffeine [291]. With regard to females, a menstrual cycle phase effect on elimination of caffeine has been demonstrated with greater accumulation and slower elimination in the luteal phase (although no phase difference in half-life) [292]. Beyond this, the evidence for caffeine erogenicity in females is mixed at best. For example, caffeine has been shown to improve strength performance in resistance-trained women [293], but erogenicity was reduced in habituated caffeine users unless ingestion of an acute dose of caffeine equivalent to the athlete’s daily caffeine ingestion is used [294,295]. Moreover, a recent systematic review [296] demonstrated that the effects of caffeine during resistance exercise may be reduced in women when compared to men ingesting the same caffeine dosage. In addition, some of the caffeine-induced stimulant effects are of smaller magnitude in women than in men [297]. It may be that the physiological responder status of caffeine has a greater implication on caffeine efficacy [298]; with a dose–response relationship relative to body weight needed to invoke effectiveness [291]. Additional research is needed to determine any sex difference or sex hormone effect on caffeine uptake, dosage, and/or efficacy.
Nitrates. Dietary nitrate is growing in popularity as a sports nutrition supplement. Inorganic nitrate is present in numerous foodstuffs and is abundant in green leafy vegetables and beetroot. Following ingestion, nitrate is converted in the body to nitrite and stored and circulated in the blood. In conditions of low oxygen availability, nitrite can be converted into nitric oxide, which is known to play a number of important roles in vascular and metabolic control [299]. Dietary nitrate supplementation has been shown to increase plasma nitrite concentration and reduce resting blood pressure [300]. Nitrate supplementation may reduce the oxygen cost of submaximal exercise and can, in male athletes, enhance exercise tolerance and performance [301]. A significant literature gap is present in nitrate/beet juice exercise research, with few studies including female participants or examining relevant outcomes exclusively in female populations. In this respect, limited evidence has demonstrated how untrained and recreationally trained females respond to nitrate supplementation. As routinely observed in males, untrained and recreationally trained females also experience a decrease in O2 costs of submaximal exercise [302,303]. Alternatively, Wickham et al. [304] investigated the effects of acute and chronic supplementation in recreationally trained COC-users and found no effect on submaximal cycling vO2 or time trial performance, despite increases in plasma [NO3–] and [NO2–] in both acute and chronic supplementation conditions. Post-menopausal women are a unique population with reduced endogenous nitric oxide bioavailability as compared to men and younger women. Recently, Proctor and colleagues investigated grip strength and force development in 13 post-menopausal women using a double-blind randomized design and found that NO3− supplementation enhanced force development and increased time to fatigue [305]. This study highlights the potential efficacy of NO3− supplementation in postmenopausal women, however future research should be conducted, in trained females controlling for hormonal status (phases of the natural and OC cycles, perimenopause, and post menopause) to determine the effects of dietary NO3– supplementation on exercise and performance metrics.
Creatine. Evidence to support the benefits of creatine supplementation for women is growing, with positive benefits related to strength, hypertrophy, performance, as well as energetic and cognitive outcomes [306]. Compared to men, females exhibit 70–80% lower endogenous intramuscular phosphocreatine stores and consume considerably lower amounts of dietary creatine [307] yet have higher reported (~10%) resting levels of intramuscular creatine concentrations [308], indicating supplementation at higher doses may be more efficacious [309]. Moreover, creatine kinase perturbations have also been shown to align with the cyclical pattern of estrogen across the menstrual cycle [310,311]. It has been suggested that supplementation in the luteal phase may be more effective for the mechanistic support of creatine supplementation with regard to muscle protein kinetics, growth factors, satellite cells, myogenic transcription factors, glycogen and calcium regulation, oxidative stress, and inflammation [312,313], but original investigations are needed to further explore this notion. Additionally, no research has investigated the relationship between hormonal contraceptive patterns and creatine kinase. Short- and long-term creatine supplementation has shown significant beneficial ergogenic outcomes in strength, hypertrophy, and exercise performance in trained and untrained female populations when compared to placebo controls [313,314]. Currently, evidence shows consistent recommended dosage amounts for males and females. In this respect, creatine supplementation typically follows a pattern of a loading dose of ~ 20 g per day for 5 days (4 × 5 g doses taken every 4 hours), followed by 3-5 g per day [306]. We refer interested readers to the ISSN Position Stand on Creatine for a full review of the mechanistic/physiological effects of creatine [315].
Iron. Iron deficiency anemia and iron deficiency are five to seven times more common in female athletes as compared to males [316]. These prevalence rates are linked to sex differences [317], poor dietary intake, exercise-induced iron losses, and menstrual cycle perturbations of iron regulation [38,318,319]. During the early follicular phase (days 0–5), lower estrogen and progesterone hormone concentrations and iron regulatory hormonal activity may facilitate increased iron absorption and recycling [38]. In the late follicular phase (days 6–14), the gradual rise in estrogen maintains low hepcidin activity, enabling iron absorption and recycling in the days following menses [320]. At ovulation, estrogen and testosterone peak, associated with increased iron uptake and erythropoiesis [321]. Following ovulation, increases in progesterone increase hepcidin expression, which limits iron utilization [322]. The increase in inflammatory markers prior to menses [1,38] can exacerbate hepcidin activity, causing further limiting of iron utilization. Previous research results demonstrate a rebound in hepcidin, serum iron, and transferrin saturation post-ovulation that stabilizes during the luteal phase [320]. Thus, iron supplementation in eumenorrheic female athletes may be more effective in the follicular vs. the luteal phase, although additional research is needed to elucidate phase-based supplementation [323]. As hormonal contraceptive use is protective against iron deficiency [324], it is not recommended for oral contraceptive users to supplement iron without medical advice. Moreover, a slower recovery of post-exercise basal hepcidin levels has been observed in post-menopausal women for up to 24 h [325], thus planning the timing of iron supplementation in postmenopausal women who exhibit iron deficiency may increase iron homeostasis in this population.
8. Conclusion
Although women have been underrepresented in nutrition, sport, and exercise science research, evidence continues to accumulate that highlights how sex differences and sex hormones influence the nutritional requirements to maximize health, performance, and recovery of female athletes. Due to the known wide intra-individual differences created secondary to specific hormonal profiles of female athletes, diligent tracking of menstrual cycle and hormonal contraceptive use in premenopausal women is recommended. Furthermore, attention should be paid in female athletes who may be transitioning from peri to post-menopausal status to best understand their unique patterns, which can provide insight and objective data on personalized approaches to nutritional strategies. Specific attention to overall energy status is the biggest driver in facilitating positive exercise training adaptations, promotion of optimal performance, and health outcomes. From there, key considerations should be made relative to the sex hormone's effects on metabolism. A widespread lack of studies in females assessing nutritional strategies for performance, body composition manipulation, and supplementation exists across the literature. It is our hope that this position stand will bring to light many of these key areas and motivate future scientific investigations to incorporate more females into all studies involving these outcomes.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Sims ST, Heather AK.. Myths and methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol. 2018. Oct;103(10):1309–429. [DOI] [PubMed] [Google Scholar]
- 2.Elliott-Sale KJ, Minahan CL, de Jonge X, et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021. May;51(5):843–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookshire B Science news [Internet]2016May 25.
- 4.Project CD. The coronary drug project. Initial findings leading to modifications of its research protocol. JAMA. 1970 Nov 16;214(7):1303–1313. [PubMed] [Google Scholar]
- 5.Gorder DD, Dolecek TA, Coleman GG, et al. Dietary intake in the multiple risk factor intervention trial (MRFIT): nutrient and food group changes over 6 years. J Am Diet Assoc. 1986. Jun;86(6):744–751. [PubMed] [Google Scholar]
- 6.Cowley ESO, Ross EZ, McNulty KL. “Invisible sportswomen”: the sex data gap in sport and exercise science research. Women Sport Phys Act J. 2022;29(2):146–151. [Google Scholar]
- 7.Jacobi M. The question of rest for women during menstruation. New York: GP Putnam’s sons; 1877. [Google Scholar]
- 8.Schaumberg MA, Jenkins DG, Janse de Jonge XAK, et al. Three-step method for menstrual and oral contraceptive cycle verification. J Sci Med Sport. 2017. Nov;20(11):965–969. [DOI] [PubMed] [Google Scholar]
- 9.Oosthuyse T, Strauss JA, Hackney AC. Understanding the female athlete: molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur J Appl Physiol. 2022 Nov 19. DOI: 10.1007/s00421-022-05090-3 [DOI] [PubMed] [Google Scholar]
- 10.Bruinvels G, Burden RJ, McGregor AJ, et al. Sport, exercise and the menstrual cycle: where is the research? Br J Sports Med. 2017. Mar;51(6):487–488. [DOI] [PubMed] [Google Scholar]
- 11.Janse DEJX, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exercise. 2019. Dec;51(12):2610–2617. [DOI] [PubMed] [Google Scholar]
- 12.Wohlgemuth KJ, Arieta LR, Brewer GJ, et al. Sex differences and considerations for female specific nutritional strategies: a narrative review. J Int Soc Sports Nutr. 2021 Apr 1;18(1):27. DOI: 10.1186/s12970-021-00422-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desbrow B, Burd NA, Tarnopolsky M, et al. Nutrition for special populations: young, female, and masters athletes. Int J Sport Nutr Exerc Metab. 2019 Mar 1;29(2):220–227. DOI: 10.1123/ijsnem.2018-0269 [DOI] [PubMed] [Google Scholar]
- 14.Miotto PM, McGlory C, Holloway TM, et al. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2018 Jun 1;314(6):R909–915. DOI: 10.1152/ajpregu.00025.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silaidos C, Pilatus U, Grewal R, et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol Sex Differ. 2018 Jul 25;9(1):34. DOI: 10.1186/s13293-018-0193-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr. 2015. Apr;47(1–2):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortright RN, Koves TR. Sex differences in substrate metabolism and energy homeostasis. Can J Appl Physiol. 2000. Aug;25(4):288–311. [DOI] [PubMed] [Google Scholar]
- 18.Montero D, Madsen K, Meinild-Lundby AK, et al. Sexual dimorphism of substrate utilization: differences in skeletal muscle mitochondrial volume density and function. Exp Physiol. 2018. Jun;103(6):851–859. [DOI] [PubMed] [Google Scholar]
- 19.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hevener AL, Zhou Z, Drew BG, et al. The role of skeletal muscle estrogen receptors in metabolic homeostasis and insulin sensitivity. Adv Exp Med Biol. 2017;1043:257–284. [DOI] [PubMed] [Google Scholar]
- 21.Hevener AL, Zhou Z, Moore TM, et al. The impact of ERalpha action on muscle metabolism and insulin sensitivity - Strong enough for a man, made for a woman. Mol Metab. 2018. Sep;15:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hevener AL, Ribas V, Moore TM, et al. The impact of skeletal muscle ERalpha on mitochondrial function and metabolic health. Endocrinology. 2020 Feb 1;161(2). DOI: 10.1210/endocr/bqz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009. Aug;30(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roepstorff C, Steffensen CH, Madsen M, et al. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002. Feb;282(2):E435–47. [DOI] [PubMed] [Google Scholar]
- 25.Ortona E, Pierdominici M, Rider V. Editorial: sex hormones and gender differences in immune responses. Front Immunol. 2019;10:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Nakabo S, Blanco LP, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16481–16491. DOI: 10.1073/pnas.2003603117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genolet O, Monaco AA, Dunkel I, et al. Identification of X-chromosomal genes that drive sex differences in embryonic stem cells through a hierarchical CRISPR screening approach. Genome Biol. 2021 Apr 16;22(1):110. DOI: 10.1186/s13059-021-02321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016. Oct;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 29.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010 Jan 1;40(1):41–58. [DOI] [PubMed] [Google Scholar]
- 30.Miller MS, Bedrin NG, Callahan DM, et al. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. 1985. 2013Oct 1;115(7):1004–1014. DOI: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology. 2015. Jan;30(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landen S, Hiam D, Voisin S, et al. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J Physiol. 2021 Nov 11;601:419–434. DOI: 10.1113/JP279499. [DOI] [PubMed] [Google Scholar]
- 33.Terink R, Ten Haaf D, Bongers CWG, et al. Changes in iron metabolism during prolonged repeated walking exercise in middle-aged men and women. Eur J Appl Physiol. 2018. Nov;118(11):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grubic Kezele T, Curko-Cofek B. Age-related changes and sex-related differences in brain iron metabolism. Nutrients. 2020 Aug 27;12(9). DOI: 10.3390/nu12092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badenhorst CE, Goto K, O’Brien WJ, et al. Iron status in athletic females, a shift in perspective on an old paradigm. J Sports Sci. 2021. Jul;39(14):1565–1575. [DOI] [PubMed] [Google Scholar]
- 36.Rushton DH, Dover R, Sainsbury AW, et al. Why should women have lower reference limits for haemoglobin and ferritin concentrations than men? BMJ. 2001 Jun 2;322(7298):1355–1357. DOI: 10.1136/bmj.322.7298.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Q, Jian J, Katz S, et al. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012. Jul;153(7):3170–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim M, Dawson B, Landers G, et al. Iron regulation in athletes: exploring the menstrual cycle and effects of different exercise modalities on hepcidin production. Int J Sport Nutr Exerc Metab. 2014. Apr;24(2):177–187. [DOI] [PubMed] [Google Scholar]
- 39.McKay AKA, Pyne DB, Burke LM, et al. Iron metabolism: interactions with energy and carbohydrate availability. Nutrients. 2020 Nov 30;12(12):3692. DOI: 10.3390/nu12123692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barba-Moreno L, Alfaro-Magallanes VM, de Jonge X, et al. Hepcidin and interleukin-6 responses to endurance exercise over the menstrual cycle. Eur J Sport Sci. 2020. Dec;17:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Baker FC, Siboza F, Fuller A. Temperature regulation in women: effects of the menstrual cycle. Temperature (Austin). 2020;7(3):226–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol. 2012 Dec 1;590(23):5963–5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyoho AE, Ng LJ, MacFadden L. Modeling of gender differences in thermoregulation. Mil Med. 2017. Mar;182(S1):295–303. [DOI] [PubMed] [Google Scholar]
- 44.Yanovich R, Ketko I, Charkoudian N. Sex differences in human thermoregulation: relevance for 2020 and beyond. Physiology. 2020 May 1;35(3):177–184. [DOI] [PubMed] [Google Scholar]
- 45.Wickham KA, McCarthy DG, Spriet LL, et al. Sex differences in the physiological responses to exercise-induced dehydration: consequences and mechanisms. J Appl Physiol. 1985. 2021 Aug 1;131(2):504–510. DOI: 10.1152/japplphysiol.00266.2021. [DOI] [PubMed] [Google Scholar]
- 46.Giersch GEW, Morrissey MC, Butler CR, et al. Sex difference in initial thermoregulatory response to dehydrated exercise in the heat. Physiol Rep. 2021. Jul;9(14):e14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims ST, Rehrer NJ, Bell ML, et al. Preexercise sodium loading aids fluid balance and endurance for women exercising in the heat. J Appl Physiol. 1985. 2007 Aug;103(2):534–541. DOI: 10.1152/japplphysiol.01203.2006. [DOI] [PubMed] [Google Scholar]
- 48.Stachenfeld NS, Splenser AE, Calzone WL, et al. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 1985. 2001 Oct;91(4):1893–1901. DOI: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 49.Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol. 2012 Dec 1;590(23):5949–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker LB, Munce TA, Kenney WL. Sex differences in voluntary fluid intake by older adults during exercise. Med Sci Sports Exercise. 2005. May;37(5):789–796. [DOI] [PubMed] [Google Scholar]
- 51.Eijsvogels TM, Scholten RR, van Duijnhoven NT, et al. Sex difference in fluid balance responses during prolonged exercise. Scand J Med Sci Sports. 2013. Mar;23(2):198–206. [DOI] [PubMed] [Google Scholar]
- 52.Sims ST, Rehrer NJ, Bell ML, et al. Endogenous and exogenous female sex hormones and renal electrolyte handling: effects of an acute sodium load on plasma volume at rest. J Appl Physiol. 1985. 2008 Jul;105(1):121–127. DOI: 10.1152/japplphysiol.01331.2007. [DOI] [PubMed] [Google Scholar]
- 53.Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev. 2008. Jul;36(3):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hazell TJ, Townsend LK, Hallworth JR, et al. Sex differences in the response of total PYY and GLP-1 to moderate-intensity continuous and sprint interval cycling exercise. Eur J Appl Physiol. 2017. Mar;117(3):431–440. [DOI] [PubMed] [Google Scholar]
- 55.Qian J, Morris CJ, Caputo R, et al. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23806–23812. DOI: 10.1073/pnas.1914003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 1471. 2006 Jul 29;361:1251–1263. DOI: 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagobian TA, Braun B. Physical activity and hormonal regulation of appetite: sex differences and weight control. Exerc Sport Sci Rev. 2010. Jan;38(1):25–30. [DOI] [PubMed] [Google Scholar]
- 58.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013. Dec;305(11):R1215–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas. 2012. Mar;71(3):248–256. [DOI] [PubMed] [Google Scholar]
- 60.Heikura IA, Uusitalo ALT, Stellingwerff T, et al. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018 Jul 1;28(4):403–411. DOI: 10.1123/ijsnem.2017-0313 [DOI] [PubMed] [Google Scholar]
- 61.Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports. 2015. Oct;25(5):610–622. [DOI] [PubMed] [Google Scholar]
- 62.Fahrenholtz IL, Sjodin A, Benardot D, et al. Within-day energy deficiency and reproductive function in female endurance athletes. Scand J Med Sci Sports. 2018. Mar;28(3):1139–1146. [DOI] [PubMed] [Google Scholar]
- 63.Loucks AB. Energy availability, not body fatness, regulates reproductive function in women. Exerc Sport Sci Rev. 2003. Jul;31(3):144–148. [DOI] [PubMed] [Google Scholar]
- 64.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003. Jan;88(1):297–311. [DOI] [PubMed] [Google Scholar]
- 65.Loucks AB, Verdun M. Slow restoration of LH pulsatility by refeeding in energetically disrupted women. Am J Physiol. 1998. Oct;275(4):R1218–26. [DOI] [PubMed] [Google Scholar]
- 66.Kim JH, Cho HT, Kim YJ. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014;61(11):1055–1067. [DOI] [PubMed] [Google Scholar]
- 67.Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014. Oct;35(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ropero AB, Alonso-Magdalena P, Quesada I, et al. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008. Oct;73(9–10):874–879. [DOI] [PubMed] [Google Scholar]
- 69.Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol. 2015 Dec 15;418(3):306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JQ, Brown TR, Russo J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim Biophys Acta. 2009. Jul;1793(7):1128–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Howe JCR WV, Seale JL, Seale JL. Energy expenditure by indirect calorimetry in premenopausal women: variation within one menstrual cycle. J Nutr Biochem. 1993;4(5):268–273. [Google Scholar]
- 72.Zhang S, Osumi H, Uchizawa A, et al. Changes in sleeping energy metabolism and thermoregulation during menstrual cycle. Physiol Rep. 2020. Jan;8(2):e14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benton MJ, Hutchins AM, Dawes JJ. Effect of menstrual cycle on resting metabolism: a systematic review and meta-analysis. PLoS ONE. 2020;15(7):e0236025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman AB, Zamudio S, Woodmansee W, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997. Nov;273(5):F777–82. [DOI] [PubMed] [Google Scholar]
- 75.Szmuilowicz ED, Adler GK, Williams JS, et al. Relationship between aldosterone and progesterone in the human menstrual cycle. J Clin Endocrinol Metab. 2006. Oct;91(10):3981–3987. [DOI] [PubMed] [Google Scholar]
- 76.Pechere-Bertschi A, Maillard M, Stalder H, et al. Renal segmental tubular response to salt during the normal menstrual cycle. Kidney Int. 2002. Feb;61(2):425–431. [DOI] [PubMed] [Google Scholar]
- 77.Olson BR, Forman MR, Lanza E, et al. Relation between sodium balance and menstrual cycle symptoms in normal women. Ann Intern Med. 1996 Oct 1;125(7):564–567. DOI: 10.7326/0003-4819-125-7-199610010-00005 [DOI] [PubMed] [Google Scholar]
- 78.Landau RL, Lugibihl K. The effect of progesterone on amino acid metabolism. J Clin Endocrinol Metab. 1961. Nov;21:1355–1363. [DOI] [PubMed] [Google Scholar]
- 79.Draper CF, Duisters K, Weger B, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018 Oct 1;8(1):14568. DOI: 10.1038/s41598-018-32647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faustmann G, Meinitzer A, Magnes C, et al. Progesterone-associated arginine decline at luteal phase of menstrual cycle and associations with related amino acids and nuclear factor kB activation. PLoS ONE. 2018;13(7):e0200489. DOI: 10.1371/journal.pone.0200489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kriengsinyos W, Wykes LJ, Goonewardene LA, et al. Phase of menstrual cycle affects lysine requirement in healthy women. Am J Physiol Endocrinol Metab. 2004. Sep;287(3):E489–96. [DOI] [PubMed] [Google Scholar]
- 82.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002. May;282(5):E1139–46. [DOI] [PubMed] [Google Scholar]
- 83.Flannery CA, Choe GH, Cooke KM, et al. Insulin regulates glycogen synthesis in human endometrial glands through increased GYS2. J Clin Endocrinol Metab. 2018 Aug 1;103(8):2843–2850. DOI: 10.1210/jc.2017-01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H, Qi J, Wang Y, et al. Progesterone regulates glucose metabolism through glucose transporter 1 to promote endometrial receptivity. Front Physiol. 2020. ;11:543148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han HS, Kang G, Kim JS, et al. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016 Mar 11;48:e218. DOI: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ansdell P, Thomas K, Hicks KM, et al. Physiological sex differences affect the integrative response to exercise: acute and chronic implications. Exp Physiol. 2020. Dec;105(12):2007–2021. [DOI] [PubMed] [Google Scholar]
- 87.Oydanich M, Babici D, Zhang J, et al. Mechanisms of sex differences in exercise capacity. Am J Physiol Regul Integr Comp Physiol. 2019 Jun 1;316(6):R832–838. DOI: 10.1152/ajpregu.00394.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sims ST, Ware L, Capodilupo ER. Patterns of endogenous and exogenous ovarian hormone modulation on recovery metrics across the menstrual cycle. BMJ Open Sport Exerc Med. 2021;7(3):e001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006. May-Jun;35(3):376–384. [DOI] [PubMed] [Google Scholar]
- 90.Bruinvels G, Hackney AC, Pedlar CR. Menstrual cycle: the importance of both the phases and the transitions between phases on training and performance. Sports Med. 2022 Apr 29;52:1457–1460. DOI: 10.1007/s40279-022-01691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bruinvels G, Goldsmith E, Blagrove R, et al. Prevalence and frequency of menstrual cycle symptoms are associated with availability to train and compete: a study of 6812 exercising women recruited using the Strava exercise app. Br J Sports Med. 2021. Apr;55(8):438–443. [DOI] [PubMed] [Google Scholar]
- 92.Burrows M, Peters CE. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007;37(7):557–574. [DOI] [PubMed] [Google Scholar]
- 93.LM VL, Blanchard H, Blanchard H. Contraceptive choices and menstrual patterns in high level female athletes. Fertil Sterility. 2017;108(3):e122. [Google Scholar]
- 94.Regidor PA. Clinical relevance in present day hormonal contraception. Horm Mol Biol Clin Investig. 2018 Oct 26;37(1). DOI: 10.1515/hmbci-2018-0030. [DOI] [PubMed] [Google Scholar]
- 95.Regidor PA. The clinical relevance of progestogens in hormonal contraception: present status and future developments. Oncotarget. 2018 Oct 2;9(77):34628–34638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benagiano G, Gabelnick H, Brosens I. Long-acting hormonal contraception. Womens Health (Lond). 2015. Nov;11(6):749–757. [DOI] [PubMed] [Google Scholar]
- 97.Stachenfeld NS, Silva C, Keefe DL, et al. Effects of oral contraceptives on body fluid regulation. J Appl Physiol. 1985. 1999 Sep;87(3):1016–1025. DOI: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 98.Suh SH, Casazza GA, Horning MA, et al. Effects of oral contraceptives on glucose flux and substrate oxidation rates during rest and exercise. J Appl Physiol. 1985. 2003 Jan;94(1):285–294. DOI: 10.1152/japplphysiol.00693.2002. [DOI] [PubMed] [Google Scholar]
- 99.Hemrika DJ, Slaats EH, Kennedy JC, et al. Pulsatile luteinizing hormone patterns in long term oral contraceptive users. J Clin Endocrinol Metab. 1993. Aug;77(2):420–426. [DOI] [PubMed] [Google Scholar]
- 100.Stanczyk FZ. All progestins are not created equal. Steroids. 2003. Nov;68(10–13):879–890. [DOI] [PubMed] [Google Scholar]
- 101.Goldzieher JW, Stanczyk FZ. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008. Jul;78(1):4–9. [DOI] [PubMed] [Google Scholar]
- 102.Adeyemi-Fowode OA, Bercaw-Pratt JL. Intrauterine devices: effective contraception with noncontraceptive benefits for adolescents. J Pediatr Adolesc Gynecol. 2019. Sep;32(5S):S2–6. [DOI] [PubMed] [Google Scholar]
- 103.Lopez LM, Ramesh S, Chen M, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016. Jul;(8). DOI: 10.1002/14651858.CD008815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999. Nov;181(5 Pt 1):1263–1269. [DOI] [PubMed] [Google Scholar]
- 105.Smith-McCune K, Thomas R, Averbach S, et al. Differential effects of the hormonal and copper intrauterine device on the endometrial transcriptome. Sci Rep. 2020 Apr 23;10(1):6888. DOI: 10.1038/s41598-020-63798-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frye CA. An overview of oral contraceptives: mechanism of action and clinical use. Neurology. 2006 Mar 28;66(6 Suppl 3):S29–36. [DOI] [PubMed] [Google Scholar]
- 107.Baerwald A, Vanden Brink H, Lee C, et al. Endometrial development during the transition to menopause: preliminary associations with follicular dynamics. Climacteric. 2020. Jun;23(3):288–297. [DOI] [PubMed] [Google Scholar]
- 108.Vanden Brink H, Chizen D, Hale G, et al. Age-related changes in major ovarian follicular wave dynamics during the human menstrual cycle. Menopause. 2013. Dec;20(12):1243–1254. [DOI] [PubMed] [Google Scholar]
- 109.Baerwald A, Vanden Brink H, Hunter C, et al. Age-related changes in luteal dynamics: preliminary associations with antral follicular dynamics and hormone production during the human menstrual cycle. Menopause. 2018. Apr;25(4):399–407. [DOI] [PubMed] [Google Scholar]
- 110.Wang Q, Ferreira DLS, Nelson SM, et al. Metabolic characterization of menopause: cross-sectional and longitudinal evidence. BMC Med. 2018 Feb 6;16(1):17. DOI: 10.1186/s12916-018-1008-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med. 2010. Sep;28(5):426–434. [DOI] [PubMed] [Google Scholar]
- 112.Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019 Mar 7;4(5). DOI: 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Logue DM, Madigan SM, Melin A, et al. Low energy availability in athletes 2020: an updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients. 2020 Mar 20;12(3). DOI: 10.3390/nu12030835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mountjoy M, Sundgot-Borgen J, Burke L, et al. International olympic committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int J Sport Nutr Exerc Metab. 2018 Jul 1;28(4):316–331. DOI: 10.1123/ijsnem.2018-0136 [DOI] [PubMed] [Google Scholar]
- 115.Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004. Aug;19(8):1231–1240. [DOI] [PubMed] [Google Scholar]
- 116.Areta JL, Taylor HL, Koehler K. Low energy availability: history, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur J Appl Physiol. 2021. Jan;121(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011 Sep 26;104(4):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010 Feb 9;99(2):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mela V, Vargas A, Meza C, et al. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol. 2016. Jun;160:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bisdee JT, James WP, Shaw MA. Changes in energy expenditure during the menstrual cycle. Br J Nutr. 1989. Mar;61(2):187–199. [DOI] [PubMed] [Google Scholar]
- 121.Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995. Jan;61(1):39–43. [DOI] [PubMed] [Google Scholar]
- 122.Schofield KL, Thorpe H, Sims ST . Feminist sociology confluences with sport science: insights, contradictions, and silences in interviewing elite women athletes about low energy availability. J Sport Soc Issues. 2022;0(0):01937235211012171. [Google Scholar]
- 123.Heather AK, Thorpe H, Ogilvie M, et al. Biological and socio-cultural factors have the potential to influence the health and performance of elite female athletes: a cross sectional survey of 219 elite female athletes in aotearoa new Zealand. Front Sports Act Living. 2021. ;3:601420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slater J, Brown R, McLay-Cooke R, et al. Low energy availability in exercising women: historical perspectives and future directions. Sports Med. 2017. Feb;47(2):207–220. [DOI] [PubMed] [Google Scholar]
- 125.Wasserfurth P, Palmowski J, Hahn A, et al. Reasons for and consequences of low energy availability in female and male athletes: social environment, adaptations, and prevention. Sports Med Open. 2020 Sep 10;6(1):44. DOI: 10.1186/s40798-020-00275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stellingwerff T, Heikura IA, Meeusen R, et al. Overtraining syndrome (OTS) and relative energy deficiency in sport (RED-S): shared pathways, symptoms and complexities. Sports Med. 2021. Nov;51(11):2251–2280. [DOI] [PubMed] [Google Scholar]
- 127.Allaway HC, Southmayd EA, De Souza MJ. The physiology of functional hypothalamic amenorrhea associated with energy deficiency in exercising women and in women with anorexia nervosa. Horm Mol Biol Clin Investig. 2016. Feb;25(2):91–119. [DOI] [PubMed] [Google Scholar]
- 128.Elliott-Sale KJ, Tenforde AS, Parziale AL, et al. Endocrine effects of relative energy deficiency in sport. Int J Sport Nutr Exerc Metab. 2018 Jul 1;28(4):335–349. DOI: 10.1123/ijsnem.2018-0127 [DOI] [PubMed] [Google Scholar]
- 129.Melin AK, Heikura IA, Tenforde A, et al. Energy availability in athletics: health, performance, and physique. Int J Sport Nutr Exerc Metab. 2019 Mar 1;29(2):152–164. DOI: 10.1123/ijsnem.2018-0201 [DOI] [PubMed] [Google Scholar]
- 130.Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad–relative energy deficiency in sport (RED-S). Br J Sports Med. 2014. Apr;48(7):491–497. [DOI] [PubMed] [Google Scholar]
- 131.Hudson AD, Kauffman AS. Metabolic actions of kisspeptin signaling: effects on body weight, energy expenditure, and feeding. Pharmacol Ther. 2021. Sep;14:107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010. Jun;31(11):1984–1998. [DOI] [PubMed] [Google Scholar]
- 133.Navarro VM. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol. 2020. Aug;16(8):407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toufexis D, Rivarola MA, Lara H, et al. Stress and the reproductive axis. J Neuroendocrinol. 2014. Sep;26(9):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Castellano JM, Tena-Sempere M. Metabolic regulation of kisspeptin. Adv Exp Med Biol. 2013;784:363–383. [DOI] [PubMed] [Google Scholar]
- 136.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the midwest exercise trial. Arch Intern Med. 2003 Jun 9;163(11):1343–1350. DOI: 10.1001/archinte.163.11.1343 [DOI] [PubMed] [Google Scholar]
- 137.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57(2):36–42. [DOI] [PubMed] [Google Scholar]
- 138.Hagobian TA, Sharoff CG, Stephens BR, et al. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol. 2009. Feb;296(2):R233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hagobian TA, Yamashiro M, Hinkel-Lipsker J, et al. Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women. Appl Physiol Nutr Metab. 2013. Jan;38(1):66–72. [DOI] [PubMed] [Google Scholar]
- 140.De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998. Dec;83(12):4220–4232. [DOI] [PubMed] [Google Scholar]
- 141.Reed JL, De Souza MJ, Mallinson RJ, et al. Energy availability discriminates clinical menstrual status in exercising women. J Int Soc Sports Nutr. 2015. ;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stellingwerff T. Case study: body composition periodization in an olympic-level female middle-distance runner over a 9-year career. Int J Sport Nutr Exerc Metab. 2018 Jul 1;28(4):428–433. [DOI] [PubMed] [Google Scholar]
- 143.McFadden BA, Walker AJ, Bozzini BN, et al. Comparison of internal and external training loads in male and female collegiate soccer players during practices vs. Games. J Strength Cond Res. 2020. Apr;34(4):969–974. [DOI] [PubMed] [Google Scholar]
- 144.Bozzini BN, McFadden BA, Scruggs SK, et al. Evaluation of performance characteristics and internal and external training loads in female collegiate beach volleyball players. J Strength Cond Res. 2021 Jun 1;35(6):1559–1567. DOI: 10.1519/JSC.0000000000004051 [DOI] [PubMed] [Google Scholar]
- 145.Smith AB, Gay JL, Arent SM, et al. Examination of the prevalence of female athlete triad components among competitive cheerleaders. Int J Environ Res Public Health. 2022 Jan 26;19(3):1375. DOI: 10.3390/ijerph19031375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walker AJ, McFadden BA, Sanders DJ, et al. Biomarker rESPONSE TO A COMPETITIVE SEASON IN DIVISION I FEMALE SOCCER PLAYers. J Strength Cond Res. 2019. Oct;33(10):2622–2628. [DOI] [PubMed] [Google Scholar]
- 147.McFadden BA, Walker AJ, Arent MA, et al. Biomarkers correlate with body composition and performance changes throughout the season in women’s division I collegiate soccer players. Front Sports Act Living. 2020. ;2:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker AJ, McFadden BA, Sanders DJ, et al. Early season hormonal and biochemical changes in division i field hockey players: is fitness protective? J Strength Cond Res. 2020. Apr;34(4):975–981. [DOI] [PubMed] [Google Scholar]
- 149.Bozzini BN, McFadden BA, Elliott-Sale KJ, et al. Evaluating the effects of oral contraceptive use on biomarkers and body composition during a competitive season in collegiate female soccer players. J Appl Physiol. 1985. 2021Jun 1;130(6):1971–1982. DOI: 10.1152/japplphysiol.00818.2020. [DOI] [PubMed] [Google Scholar]
- 150.Hodson L, Harnden K, Banerjee R, et al. Lower resting and total energy expenditure in postmenopausal compared with premenopausal women matched for abdominal obesity. J Nutr Sci. 2014. ;3:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holtzman B, Ackerman KE. Recommendations and nutritional considerations for female athletes: health and performance. Sports Med. 2021. Sep;51(Suppl 1):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deutz RC, Benardot D, Martin DE, et al. Relationship between energy deficits and body composition in elite female gymnasts and runners. Med Sci Sports Exercise. 2000. Mar;32(3):659–668. [DOI] [PubMed] [Google Scholar]
- 153.Kerksick CM, Arent S, Schoenfeld BJ, et al. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2017. ;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henderson GC, Fattor JA, Horning MA, et al. Glucoregulation is more precise in women than in men during postexercise recovery. Am J Clin Nutr. 2008. Jun;87(6):1686–1694. [DOI] [PubMed] [Google Scholar]
- 155.Henderson GC, Fattor JA, Horning MA, et al. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007 Nov 1;584(Pt 3):963–981. DOI: 10.1113/jphysiol.2007.137331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roepstorff C, Thiele M, Hillig T, et al. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006 Jul 1;574(Pt 1):125–138. DOI: 10.1113/jphysiol.2006.108720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kalkhoff RK. Metabolic effects of progesterone. Am J Obstet Gynecol. 1982 Mar 15;142(6 Pt 2):735–738. [DOI] [PubMed] [Google Scholar]
- 158.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013. Jun;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vislocky LM, Gaine PC, Pikosky MA, et al. Gender impacts the post-exercise substrate and endocrine response in trained runners. J Int Soc Sports Nutr. 2008 Feb 26;5:7. DOI: 10.1186/1550-2783-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ibrahimi A, Bonen A, Blinn WD, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999 Sep 17;274(38):26761–26766. DOI: 10.1074/jbc.274.38.26761 [DOI] [PubMed] [Google Scholar]
- 161.Kiens B, Roepstorff C, Glatz JF, et al. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol. 1985. 2004 Oct;97(4):1209–1218. DOI: 10.1152/japplphysiol.01278.2003. [DOI] [PubMed] [Google Scholar]
- 162.Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014. May;44(1):S87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tarnopolsky LJ, MacDougall JD, Atkinson SA, et al. Gender differences in substrate for endurance exercise. J Appl Physiol. 1985. 1990 Jan;68(1):302–308. DOI: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 164.Lamont LS, Lemon PW, Bruot BC. Menstrual cycle and exercise effects on protein catabolism. Med Sci Sports Exercise. 1987. Apr;19(2):106–110. [PubMed] [Google Scholar]
- 165.Landau RL, Poulos JT. The metabolic influence of progestins. Adv Metab Disord. 1971;5:119–147. [DOI] [PubMed] [Google Scholar]
- 166.Willett HN, Koltun KJ, Hackney AC. Influence of menstrual cycle estradiol-β-17 fluctuations on energy substrate utilization-oxidation during aerobic, endurance exercise. Int J Environ Res Public Health. 2021 Jul 5;18(13):7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010 Mar 1;40(3):207–227. [DOI] [PubMed] [Google Scholar]
- 168.Chappell S, Hackney AC. Associations between menstrual cycle phase, physical activity level and dietary macronutrient intake. Biol Sport. 1997;14(4):251–258. [PMC free article] [PubMed] [Google Scholar]
- 169.Hackney AC, McCracken-Compton MA, Ainsworth B. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int J Sport Nutr Exerc Metab. 1994;4(3):299–308. [DOI] [PubMed] [Google Scholar]
- 170.Devries MC, Hamadeh MJ, Phillips SM, et al. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1120–1128. DOI: 10.1152/ajpregu.00700.2005 [DOI] [PubMed] [Google Scholar]
- 171.Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol. 2001;90(2):447–453. [DOI] [PubMed] [Google Scholar]
- 172.Horton TJ, Pagliassotti MJ, Hobbs K, et al. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1985. 1998Nov;85(5):1823–1832. DOI: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 173.Horton TJ, Grunwald GK, Lavely J, et al. Glucose kinetics differ between women and men, during and after exercise. J Appl Physiol. 1985. 2006 Jun;100(6):1883–1894. DOI: 10.1152/japplphysiol.01431.2005. [DOI] [PubMed] [Google Scholar]
- 174.Hedrington MS, Davis SN. Sexual dimorphism in glucose and lipid metabolism during fasting, hypoglycemia, and exercise. Front Endocrinol. 2015;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab. 2001. Jun;280(6):E898–907. [DOI] [PubMed] [Google Scholar]
- 176.Bagley L, Slevin M, Bradburn S, et al. Sex differences in the effects of 12 weeks sprint interval training on body fat mass and the rates of fatty acid oxidation and VO2max during exercise. BMJ Open Sport Exerc Med. 2016;2(1):e000056. DOI: 10.1136/bmjsem-2015-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Landen S, Voisin S, Craig JM, et al. Genetic and epigenetic sex-specific adaptations to endurance exercise. Epigenetics. 2019. Jun;14(6):523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suh S-H, Casazza G, Horning M, et al. Effects of oral contraceptives on glucose flux and substrate oxidation rates during rest and exercise. J Appl Physiol. 2003. ;94:285–294. [DOI] [PubMed] [Google Scholar]
- 179.Silva-Bermudez LS, Toloza FJK, Perez-Matos MC, et al. Effects of oral contraceptives on metabolic parameters in adult premenopausal women: a meta-analysis. Endocr Connect. 2020. Oct;9(10):978–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Larsen B, Cox A, Colbey C, et al. Inflammation and oral contraceptive use in female athletes before the rio olympic games. Front Physiol. 2020. ;11:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cauci S, Buligan C, Marangone M, et al. Oxidative stress in female athletes using combined oral contraceptives. Sports Med Open. 2016. Dec;2(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McLay RT, Thomson CD, Williams SM, et al. Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab. 2007. Apr;17(2):189–205. [DOI] [PubMed] [Google Scholar]
- 183.Hackney AC. Effects of the menstrual cycle on resting muscle glycogen content. Horm Metab Res. 1990. Dec;22(12):647. [DOI] [PubMed] [Google Scholar]
- 184.Paul D, Mulroy S, Horner J, et al. Carbohydrate-loading during the follicular phase of the menstrual cycle: effects on muscle glycogen and exercise performance. Int J Sport Nutr Exerc Metab. 2001. ;11:430–441. [DOI] [PubMed] [Google Scholar]
- 185.Tarnopolsky M, Zawada C, Richmond L, et al. Gender differences in carbohydrate loading are related to energy intake. J Appl Physiol. 2001. ;91:225–230. [DOI] [PubMed] [Google Scholar]
- 186.Walker J, Heigenhauser G, Hultman E, et al. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol. 2000. ;88:2151–2158. [DOI] [PubMed] [Google Scholar]
- 187.Sherman W, Costill D, Fink W, et al. The effect of exercise-diet manipulation on muscle glycogen and its subsequent utilisation during performance. Int J Sports Med. 1981. ;2:114–118. [DOI] [PubMed] [Google Scholar]
- 188.Rauch L, Rodger I, Wilson G, et al. The effects of carbohydrate loading on muscle glycogen content and cycling performance. Int J Sport Nutr. 1995. ;5:25–36. [DOI] [PubMed] [Google Scholar]
- 189.Hawley J, Palmer G, Noakes T. Effects of 3 days of carbohydrate supplementation on muscle glycogen content and utilisation during a 1-h cycling performance. Eur J Appl Physiol. 1997;75:407–412. [DOI] [PubMed] [Google Scholar]
- 190.Burke L, Hawley J, Schabort E, et al. Carbohydrate loading failed to improve 100-km cycling performance in a placebo-controlled trial. J Appl Physiol. 2000. ;88:1284–1290. [DOI] [PubMed] [Google Scholar]
- 191.Rauch H, St Clair Gibson A, Lambert E, et al. A signaling role for muscle glycogen in the regulation of pace during prolonged exercise. Br J Sports Med. 2005. ;39:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tarnopolsky M, Atkinson S, Phillips S, et al. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol. 1995;75:2134–2141. [DOI] [PubMed] [Google Scholar]
- 193.Wismann J, Willoughby D. Gender differences in carbohydrate metabolism and carbohydrate loading. J Int Soc Sports Nutr. 2006;31(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sedlock D. The latest on carbohydrate loading: a practical approach. Curr Sports Med Rep. 2008;7(4):209–213. [DOI] [PubMed] [Google Scholar]
- 195.Burke LM, Loucks AB, Broad N. Energy and carbohydrate for training and recovery. J Sports Sci. 2006. Jul;24(7):675–685. [DOI] [PubMed] [Google Scholar]
- 196.Burke LM, Hawley JA, Wong SH, et al. Carbohydrates for training and competition. J Sports Sci. 2011;29(1):S17–27. DOI: 10.1080/02640414.2011.585473 [DOI] [PubMed] [Google Scholar]
- 197.Burke LM, Hawley JA, Jeukendrup A, et al. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab. 2018 Sep 1;28(5):451–463. DOI: 10.1123/ijsnem.2018-0289 [DOI] [PubMed] [Google Scholar]
- 198.Moore DR, Sygo J, Morton JP. Fuelling the female athlete: carbohydrate and protein recommendations. Eur J Sport Sci. 2022. May;22(5):684–696. [DOI] [PubMed] [Google Scholar]
- 199.Boschmann M, Rosenbaum M, Leibel RL, et al. Metabolic and hemodynamic responses to exercise in subcutaneous adipose tissue and skeletal muscle. Int J Sports Med. 2002. Nov;23(8):537–543. [DOI] [PubMed] [Google Scholar]
- 200.Riddell MC, Partington SL, Stupka N, et al. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance trained athletes. Int J Sport Nutr Exerc Metab. 2003. Dec;13(4):407–421. [DOI] [PubMed] [Google Scholar]
- 201.Tremblay J, Peronnet F, Massicotte D, et al. Carbohydrate supplementation and sex differences in fuel selection during exercise. Med Sci Sports Exercise. 2010. Jul;42(7):1314–1323. [DOI] [PubMed] [Google Scholar]
- 202.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003. Jan;38(1):36–42. [DOI] [PubMed] [Google Scholar]
- 203.Mori H, Suzuki H, Matsuzaki J, et al. Gender difference of gastric emptying in healthy volunteers and patients with functional dyspepsia. Digestion. 2017;95(1):72–78. DOI: 10.1159/000452359 [DOI] [PubMed] [Google Scholar]
- 204.Gonzalez Z, Loganathan P, Sarosiek I, et al. Gender-related differences in gastroparesis. Am J Med Sci. 2020. Nov;360(5):474–483. [DOI] [PubMed] [Google Scholar]
- 205.Bernstein MT, Graff LA, Avery L, et al. Gastrointestinal symptoms before and during menses in healthy women. BMC Womens Health. 2014 Jan 22;14:14. DOI: 10.1186/1472-6874-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee CL, Cheng CF, Astorino TA, et al. Effects of carbohydrate combined with caffeine on repeated sprint cycling and agility performance in female athletes. J Int Soc Sports Nutr. 2014. ;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beaven CM, Maulder P, Pooley A, et al. Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Appl Physiol Nutr Metab. 2013. Jun;38(6):633–637. [DOI] [PubMed] [Google Scholar]
- 208.Chryssanthopoulos C, Ziaras C, Oosthuyse T, et al. Carbohydrate mouth rinse does not affect performance during a 60-min running race in women. J Sports Sci. 2018. Apr;36(7):824–833. [DOI] [PubMed] [Google Scholar]
- 209.Karayigit R, Forbes SC, Naderi A, et al. Different doses of carbohydrate mouth rinse have no effect on exercise performance in resistance trained women. Int J Environ Res Public Health. 2021 Mar 26;18(7):3463. DOI: 10.3390/ijerph18073463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baur DA, Saunders MJ. Carbohydrate supplementation: a critical review of recent innovations. Eur J Appl Physiol. 2021. Jan;121(1):23–66. [DOI] [PubMed] [Google Scholar]
- 211.Rowlands DS, Swift M, Ros M, et al. Composite versus single transportable carbohydrate solution enhances race and laboratory cycling performance. Appl Physiol Nutr Metab. 2012. Jun;37(3):425–436. [DOI] [PubMed] [Google Scholar]
- 212.Wallis G, Yeo S, Blannin A, et al. Dose-response effects of ingested carbohydrate on exercise metabolism in women. Med Sci Sports Exercise. 2007;39(1):131–138. DOI: 10.1249/01.mss.0000241645.28467.d3 [DOI] [PubMed] [Google Scholar]
- 213.Hashimoto H, Ishijima T, Hayashida H, et al. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J Int Soc Sports Nutr. 2014. ;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bailey S, Zacher C, Mittleman K. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J Appl Physiol. 2000;88:690–697. [DOI] [PubMed] [Google Scholar]
- 215.Phelain JF, Reinke E, Harris MA, et al. Postexercise energy expenditure and substrate oxidation in young women resulting from exercise bouts of different intensity. J Am Coll Nutr. 1997. Apr;16(2):140–146. [DOI] [PubMed] [Google Scholar]
- 216.Nicklas B, Hackney A, Sharp R. The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med. 1989;10:264–269. [DOI] [PubMed] [Google Scholar]
- 217.Tarnopolsky MA, Bosman M, Macdonald JR, et al. Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. J Appl Physiol. 1985. 1997 Dec;83(6):1877–1883. DOI: 10.1152/jappl.1997.83.6.1877. [DOI] [PubMed] [Google Scholar]
- 218.Roy B, Luttmer K, Bosman M, et al. The influence of post-exercise macronutrient intake on energy balance and protein metabolism in active females participating in endurance training. Int J Sport Nutr Exerc Metab. 2002. ;12:172–188. [DOI] [PubMed] [Google Scholar]
- 219.Yu W, Zhou G, Fan B, et al. Temporal sequence of blood lipids and insulin resistance in perimenopausal women: the study of women’s health across the nation. BMJ Open Diabetes Res Care. 2022. Mar;10(2):e002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ivy JL, Katz AL, Cutler CL, et al. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol. 1985. 1988 Apr;64(4):1480–1485. DOI: 10.1152/jappl.1988.64.4.1480. [DOI] [PubMed] [Google Scholar]
- 221.Burke LM, van Loon LJC, Hawley JA, et al. Postexercise muscle glycogen resynthesis in humans. J Appl Physiol. 1985. 2017 May 1;122(5):1055–1067. 10.1152/japplphysiol.00860.2016 [DOI] [PubMed] [Google Scholar]
- 222.Zaromskyte G, Prokopidis K, Ioannidis T, et al. Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: a systematic review. Front Nutr. 2021. ;8:685165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Burd NA, Tang JE, Moore DR, et al. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol. 1985. 2009 May;106(5):1692–1701. DOI: 10.1152/japplphysiol.91351.2008. [DOI] [PubMed] [Google Scholar]
- 224.Phillips SM, Atkinson SA, Tarnopolsky MA, et al. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol. 1985. 1993 Nov;75(5):2134–2141. DOI: 10.1152/jappl.1993.75.5.2134. [DOI] [PubMed] [Google Scholar]
- 225.Eisenhofer G, Peitzsch M, Kaden D, et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017. Jul;470:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hansen M, Langberg H, Holm L, et al. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand J Med Sci Sports. 2011. Feb;21(1):62–72. [DOI] [PubMed] [Google Scholar]
- 227.Dalgaard LB, Dalgas U, Andersen JL, et al. Influence of oral contraceptive use on adaptations to resistance training. Front Physiol. 2019. ;10:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mercer D, Convit L, Condo D, et al. Protein requirements of pre-menopausal female athletes: systematic literature review. Nutrients. 2020 Nov 16;12(11). DOI: 10.3390/nu12113527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement nutrition and athletic performance. Med Sci Sports Exerc. 2016. Mar;48(3):543–568. [DOI] [PubMed] [Google Scholar]
- 230.Jager R, Kerksick CM, Campbell BI, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 2017. ;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Houltham SD, Rowlands DS. A snapshot of nitrogen balance in endurance-trained women. Appl Physiol Nutr Metab. 2014. Feb;39(2):219–225. [DOI] [PubMed] [Google Scholar]
- 232.Carbone JW, McClung JP, Pasiakos SM. Recent advances in the characterization of skeletal muscle and whole-body protein responses to dietary protein and exercise during negative energy balance. Adv Nutr. 2019 Jan 1;10(1):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kuikman MA, Mountjoy M, Burr JF. Examining the relationship between exercise dependence, disordered eating, and low energy availability. Nutrients. 2021 Jul 28;13(8). DOI: 10.3390/nu13082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Slater J, McLay-Cooke R, Brown R, et al. Female recreational exercisers at risk for low energy availability. Int J Sport Nutr Exerc Metab. 2016. Oct;26(5):421–427. [DOI] [PubMed] [Google Scholar]
- 235.Black K, Slater J, Brown RC, et al. Low energy availability, plasma lipids, and hormonal profiles of recreational athletes. J Strength Cond Res. 2018. Oct;32(10):2816–2824. [DOI] [PubMed] [Google Scholar]
- 236.Sharps FRJ, Wilson LJ, Graham CA, et al. Prevalence of disordered eating, eating disorders and risk of low energy availability in professional, competitive and recreational female athletes based in the United Kingdom. Eur J Sport Sci. 2021. Jul;16:1–7. [DOI] [PubMed] [Google Scholar]
- 237.Bosse JD, Dixon BM. Dietary protein to maximize resistance training: a review and examination of protein spread and change theories. J Int Soc Sports Nutr. 2012 Sep 8;9(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Antonio J, Ellerbroek A, Evans C, et al. High protein consumption in trained women: bad to the bone? J Int Soc Sports Nutr. 2018. ;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Antonio J, Ellerbroek A, Silver T, et al. A high protein diet (3.4 g/kg/d) combined with a heavy resistance training program improves body composition in healthy trained men and women–a follow-up investigation. J Int Soc Sports Nutr. 2015. ;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Stannard SR, Buckley AJ, Edge JA, et al. Adaptations to skeletal muscle with endurance exercise training in the acutely fed versus overnight-fasted state. J Sci Med Sport. 2010. Jul;13(4):465–469. [DOI] [PubMed] [Google Scholar]
- 241.Hackney KJ, Bruenger AJ, Lemmer JT. Timing protein intake increases energy expenditure 24 h after resistance training. Med Sci Sports Exercise. 2010. May;42(5):998–1003. [DOI] [PubMed] [Google Scholar]
- 242.Gould LM, Gordon AN, Cabre HE, et al. Metabolic effects of menopause: a cross-sectional characterization of body composition and exercise metabolism. Menopause. 2022 Feb 28;29(4):377–389. DOI: 10.1097/GME.0000000000001932 [DOI] [PubMed] [Google Scholar]
- 243.Agostini D, Zeppa Donati S, Lucertini F, et al. Muscle and bone health in postmenopausal women: role of protein and vitamin d supplementation combined with exercise training. Nutrients. 2018 Aug 16;10(8). DOI: 10.3390/nu10081103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Phillips SM, Chevalier S, Leidy HJ. Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab. 2016. May;41(5):565–572. [DOI] [PubMed] [Google Scholar]
- 245.Smith GI, Reeds DN, Hall AM, et al. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ. 2012 May 23;3(1):11. DOI: 10.1186/2042-6410-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ. 2019 Aug 28;10(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bukhari SS, Phillips BE, Wilkinson DJ, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab. 2015 Jun 15;308(12):E1056–65. DOI: 10.1152/ajpendo.00481.2014 [DOI] [PubMed] [Google Scholar]
- 248.Ispoglou T, Deighton K, King RF, et al. Novel essential amino acid supplements enriched with L-leucine facilitate increased protein and energy intakes in older women: a randomised controlled trial. Nutr J. 2017 Nov 28;16(1):75. DOI: 10.1186/s12937-017-0298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Du C, Tucker RM, Yang CL. How are you sleeping? Why nutrition professionals should ask their patients about sleep habits. J Am Nutr Assoc. 2022. Feb;23:1–11. [DOI] [PubMed] [Google Scholar]
- 250.Sargent C, Lastella M, Halson SL, et al. How much sleep does an elite athlete need? Int J Sports Physiol Perform. 2021 Dec 1;16(12):1746–1757. DOI: 10.1123/ijspp.2020-0896 [DOI] [PubMed] [Google Scholar]
- 251.Romans SE, Kreindler D, Einstein G, et al. Sleep quality and the menstrual cycle. Sleep Med. 2015. Apr;16(4):489–495. [DOI] [PubMed] [Google Scholar]
- 252.Brown SG, Morrison LA, Calibuso MJ, et al. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48(4):429–444. DOI: 10.1080/03630240802575179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004. Feb;56(2):239–243. [DOI] [PubMed] [Google Scholar]
- 254.Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997. May;16(3):209–214. [DOI] [PubMed] [Google Scholar]
- 255.Hachul H, Andersen ML, Bittencourt L, et al. A population-based survey on the influence of the menstrual cycle and the use of hormonal contraceptives on sleep patterns in Sao Paulo, Brazil. Int J Gynaecol Obstet. 2013. Feb;120(2):137–140. [DOI] [PubMed] [Google Scholar]
- 256.Stanicic A, Jokic-Begic N. Psychophysical characteristics of the premenstrual period. Coll Antropol. 2010. Dec;34(4):1421–1425. [PubMed] [Google Scholar]
- 257.Nedelec M, Halson S, Abaidia AE, et al. Stress, sleep and recovery in elite soccer: a critical review of the literature. Sports Med. 2015. Oct;45(10):1387–1400. [DOI] [PubMed] [Google Scholar]
- 258.Abbott W, Brett A, Cockburn E, et al. Presleep casein protein ingestion: acceleration of functional recovery in professional soccer players. Int J Sports Physiol Perform. 2019 Mar 1;14(3):385–391. DOI: 10.1123/ijspp.2018-0385 [DOI] [PubMed] [Google Scholar]
- 259.Apweiler E, Wallace D, Stansfield S, et al. Pre-bed casein protein supplementation does not enhance acute functional recovery in physically active males and females when exercise is performed in the morning. Sports (Basel). 2018 Dec 28;7(1). DOI: 10.3390/sports7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.West DWD, Abou Sawan S, Mazzulla M, et al. Whey protein supplementation enhances whole body protein metabolism and performance recovery after resistance exercise: a double-blind crossover study. Nutrients. 2017 Jul 11;9(7). DOI: 10.3390/nu9070735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ormsbee MJ, Gorman KA, Miller EA, et al. Nighttime feeding likely alters morning metabolism but not exercise performance in female athletes. Appl Physiol Nutr Metab. 2016. Jul;41(7):719–727. [DOI] [PubMed] [Google Scholar]
- 262.Leyh SM, Willingham BD, Baur DA, et al. Pre-sleep protein in casein supplement or whole-food form has no impact on resting energy expenditure or hunger in women. Br J Nutr. 2018. Nov;120(9):988–994. [DOI] [PubMed] [Google Scholar]
- 263.Al-Disi D, Al-Daghri N, Khanam L, et al. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J. 2010;57(10):915–923. DOI: 10.1507/endocrj.K10E-145 [DOI] [PubMed] [Google Scholar]
- 264.Shibli F, Skeans J, Yamasaki T, et al. Nocturnal gastroesophageal reflux disease (GERD) and sleep: an important relationship that is commonly overlooked. J Clin Gastroenterol. 2020. Sep;54(8):663–674. [DOI] [PubMed] [Google Scholar]
- 265.Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women. Comprehensive Physiology: John Wiley & Sons, Inc.; 2011. [DOI] [PubMed] [Google Scholar]
- 266.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84(12):4637–4644. [DOI] [PubMed] [Google Scholar]
- 267.Sar M, Stumpf W. Simultaneous localization of [3 H] estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci Lett. 1980;17(1):179–184. [DOI] [PubMed] [Google Scholar]
- 268.Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. 2002. Oct;283(4):E711–21. [DOI] [PubMed] [Google Scholar]
- 269.Verney EB. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci. 1947;135(878):25–106. [PubMed] [Google Scholar]
- 270.Ritz P, Vol S, Berrut G, et al. Influence of gender and body composition on hydration and body water spaces. Clin Nutr. 2008. Oct;27(5):740–746. [DOI] [PubMed] [Google Scholar]
- 271.Driscoll RL, McCarthy DG, Palmer MS, et al. Mild dehydration impaired intermittent sprint performance and thermoregulation in females. Appl Physiol Nutr Metab. 2020. Sep;45(9):1045–1048. [DOI] [PubMed] [Google Scholar]
- 272.Logan-Sprenger HM, Heigenhauser GJ, Killian KJ, et al. Effects of dehydration during cycling on skeletal muscle metabolism in females. Med Sci Sports Exercise. 2012. Oct;44(10):1949–1957. [DOI] [PubMed] [Google Scholar]
- 273.Logan-Sprenger HM, Heigenhauser GJ, Jones GL, et al. Increase in skeletal-muscle glycogenolysis and perceived exertion with progressive dehydration during cycling in hydrated men. Int J Sport Nutr Exerc Metab. 2013. Jun;23(3):220–229. [DOI] [PubMed] [Google Scholar]
- 274.Bhave G, Neilson EG. Body fluid dynamics: back to the future. J Am Soc Nephrol. 2011. Dec;22(12):2166–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25(4):303–320. 10.1097/JSM.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 276.Almond CS, Shin AY, Fortescue EB, et al. Hyponatremia among runners in the boston marathon. N Engl J Med. 2005;352(15):1550–1556. DOI: 10.1056/NEJMoa043901 [DOI] [PubMed] [Google Scholar]
- 277.Vokes TJ, Weiss NM, Schreiber J, et al. Osmoregulation of thirst and vasopressin during normal menstrual cycle. Am J Physiol. 1988. Apr;254(4 Pt 2):R641–7. [DOI] [PubMed] [Google Scholar]
- 278.Stachenfeld NS, Dipietro L, Palter SF, et al. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol Regul Integr Comp Physiol. 1998;274(1):R187–195. DOI: 10.1152/ajpregu.1998.274.1.R187 [DOI] [PubMed] [Google Scholar]
- 279.Rosner MH, Bennett B, Hew-Butler T, et al. Exercise-associated hyponatremia. In: Simon Eeditor. Hyponatremia: evaluation and Treatment. New York, NY: Springer New York; 2013. pp. 175–192. [Google Scholar]
- 280.Stachenfeld NS. Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci. 2014. May;21(5):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Stachenfeld NS, Splenser AE, Calzone WL, et al. Selected contribution: sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001;91(4):1893–1901. DOI: 10.1152/jappl.2001.91.4.1893 [DOI] [PubMed] [Google Scholar]
- 282.Garthe I, Maughan RJ. Athletes and supplements: prevalence and perspectives. Int J Sport Nutr Exerc Metab. 2018 Mar 1;28(2):126–138. [DOI] [PubMed] [Google Scholar]
- 283.Herbold NH, Visconti BK, Frates S, et al. Traditional and nontraditional supplement use by collegiate female varsity athletes. Int J Sport Nutr Exerc Metab. 2004. Oct;14(5):586–593. [DOI] [PubMed] [Google Scholar]
- 284.Smith ES, McKay AKA, Kuikman M, et al. Auditing the representation of female versus male athletes in sports science and sports medicine research: evidence-based performance supplements. Nutrients. 2022 Feb 23;14(5):953. DOI: 10.3390/nu14050953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Glenn JM, Smith K, Moyen NE, et al. Effects of acute beta-alanine supplementation on anaerobic performance in trained female cyclists. J Nutr Sci Vitaminol (Tokyo). 2015;61(2):161–166. DOI: 10.3177/jnsv.61.161 [DOI] [PubMed] [Google Scholar]
- 286.Smith AE, Stout JR, Kendall KL, et al. Exercise-induced oxidative stress: the effects of beta-alanine supplementation in women. Amino Acids. 2012. Jul;43(1):77–90. [DOI] [PubMed] [Google Scholar]
- 287.Varanoske AN, Hoffman JR, Church DD, et al. Beta-Alanine supplementation elevates intramuscular carnosine content and attenuates fatigue in men and women similarly but does not change muscle l-histidine content. Nutr Res. 2017. Dec;48:16–25. [DOI] [PubMed] [Google Scholar]
- 288.Varanoske AN, Hoffman JR, Church DD, et al. Influence of skeletal muscle carnosine content on fatigue during repeated resistance exercise in recreationally active women. Nutrients. 2017 Sep 7;9(9). DOI: 10.3390/nu9090988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Harris RC, Tallon MJ, Dunnett M, et al. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006. May;30(3):279–289. [DOI] [PubMed] [Google Scholar]
- 290.Trexler ET, Smith-Ryan AE, Stout JR, et al. International society of sports nutrition position stand: beta-alanine. J Int Soc Sports Nutr. 2015. ;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Guest NS, VanDusseldorp TA, Nelson MT, et al. International society of sports nutrition position stand: caffeine and exercise performance. J Int Soc Sports Nutr. 2021 Jan 2;18(1):1. DOI: 10.1186/s12970-020-00383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lane JD, Steege JF, Rupp SL, et al. Menstrual cycle effects on caffeine elimination in the human female. Eur J Clin Pharmacol. 1992;43(5):543–546. DOI: 10.1007/BF02285099 [DOI] [PubMed] [Google Scholar]
- 293.Goldstein E, Jacobs PL, Whitehurst M, et al. Caffeine enhances upper body strength in resistance-trained women. J Int Soc Sports Nutr. 2010 May 14;7:18. DOI: 10.1186/1550-2783-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Filip-Stachnik A, Wilk M, Krzysztofik M, et al. The effects of different doses of caffeine on maximal strength and strength-endurance in women habituated to caffeine. J Int Soc Sports Nutr. 2021 Mar 30;18(1):25. DOI: 10.1186/s12970-021-00421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Filip-Stachnik A, Krzysztofik M, Del Coso J, et al. Acute effects of two caffeine doses on bar velocity during the bench press exercise among women habituated to caffeine: a randomized, crossover, double-blind study involving control and placebo conditions. Eur J Nutr. 2022. Mar;61(2):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mielgo-Ayuso J, Marques-Jimenez D, Refoyo I, et al. Effect of caffeine supplementation on sports performance based on differences between sexes: a systematic review. Nutrients. 2019 Sep 30;11(10). DOI: 10.3390/nu11102313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Adan A, Prat G, Fabbri M, et al. Early effects of caffeinated and decaffeinated coffee on subjective state and gender differences. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Oct 1;32(7):1698–1703. DOI: 10.1016/j.pnpbp.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 298.Guest N, Corey P, Vescovi J, et al. Caffeine, CYP1A2 genotype, and endurance performance in athletes. Med Sci Sports Exercise. 2018. Aug;50(8):1570–1578. [DOI] [PubMed] [Google Scholar]
- 299.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001. Jan;81(1):209–237. [DOI] [PubMed] [Google Scholar]
- 300.Gao C, Gupta S, Adli T, et al. The effects of dietary nitrate supplementation on endurance exercise performance and cardiorespiratory measures in healthy adults: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2021 Jul 9;18(1):55. DOI: 10.1186/s12970-021-00450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jones AM. Influence of dietary nitrate on the physiological determinants of exercise performance: a critical review. Appl Physiol Nutr Metab. 2014. Sep;39(9):1019–1028. [DOI] [PubMed] [Google Scholar]
- 302.Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010. Oct;299(4):R1121–31. [DOI] [PubMed] [Google Scholar]
- 303.Wylie LJ, Ortiz de Zevallos J, Isidore T, et al. Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: acute vs. chronic supplementation. Nitric Oxide. 2016 Jul 1;57:30–39. DOI: 10.1016/j.niox.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 304.Wickham KA, McCarthy DG, Pereira JM, et al. No effect of beetroot juice supplementation on exercise economy and performance in recreationally active females despite increased torque production. Physiol Rep. 2019. Jan;7(2):e13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Proctor DN, Neely KA, Mookerjee S, et al. Inorganic nitrate supplementation and blood flow restricted exercise tolerance in post-menopausal women. Nitric Oxide. 2022 May 1;122-123:26–34. DOI: 10.1016/j.niox.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Smith-Ryan AE, Cabre HE, Eckerson JM, et al. Creatine supplementation in women’s health: a lifespan perspective. Nutrients. 2021 Mar 8;13(3). DOI: 10.3390/nu13030877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. [DOI] [PubMed] [Google Scholar]
- 308.Forsberg AM, Nilsson E, Werneman J, et al. Muscle composition in relation to age and sex. Clin Sci (Lond). 1991. Aug;81(2):249–256. [DOI] [PubMed] [Google Scholar]
- 309.Mihic S, MacDonald JR, McKenzie S, et al. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med Sci Sports Exercise. 2000. Feb;32(2):291–296. [DOI] [PubMed] [Google Scholar]
- 310.Ellery SJ, Walker DW, Dickinson H. Creatine for women: a review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapy. Amino Acids. 2016. Aug;48(8):1807–1817. [DOI] [PubMed] [Google Scholar]
- 311.Bundey S, Crawley JM, Edwards JH, et al. Serum creatine kinase levels in pubertal, mature, pregnant, and postmenopausal women. J Med Genet. 1979. Apr;16(2):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chilibeck PD, Kaviani M, Candow DG, et al. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. 2017. ;8:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Volek JS, Rawson ES. Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition. 2004. Jul-Aug;20(7–8):609–614. [DOI] [PubMed] [Google Scholar]
- 314.Williams T, Walz E, Lane AR, et al. The effect of estrogen on muscle damage biomarkers following prolonged aerobic exercise in eumenorrheic women. Biol Sport. 2015. Sep;32(3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Buford TW, Kreider RB, Stout JR, et al. International society of sports nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr. 2007 Aug 30;4:6. DOI: 10.1186/1550-2783-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.DellaValle DM, Haas JD. Impact of iron depletion without anemia on performance in trained endurance athletes at the beginning of a training season: a study of female collegiate rowers. Int J Sport Nutr Exerc Metab. 2011. Dec;21(6):501–506. [DOI] [PubMed] [Google Scholar]
- 317.Murphy WG, Tong E, Murphy C. Why do women have similar erythropoietin levels to men but lower hemoglobin levels? Blood. 2010 Oct 14;116(15):2861–2862. [DOI] [PubMed] [Google Scholar]
- 318.Peeling P, Dawson B, Goodman C, et al. Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exercise. 2009. May;41(5):1138–1145. [DOI] [PubMed] [Google Scholar]
- 319.Peeling P, Dawson B, Goodman C, et al. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol. 2009. May;106(1):51–59. [DOI] [PubMed] [Google Scholar]
- 320.Laine F, Angeli A, Ropert M, et al. Variations of hepcidin and iron-status parameters during the menstrual cycle in healthy women. Br J Haematol. 2016. Dec;175(5):980–982. [DOI] [PubMed] [Google Scholar]
- 321.Angeli A, Laine F, Lavenu A, et al. Joint model of iron and hepcidin during the menstrual cycle in healthy women. Aaps J. 2016. Mar;18(2):490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Li X, Rhee DK, Malhotra R, et al. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J Clin Invest. 2016. Jan;126(1):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pedlar CR, Brugnara C, Bruinvels G, et al. Iron balance and iron supplementation for the female athlete: a practical approach. Eur J Sport Sci. 2018. Mar;18(2):295–305. [DOI] [PubMed] [Google Scholar]
- 324.Sim M, Dawson B, Landers G, et al. Interleukin-6 and hepcidin levels during hormone-deplete and hormone-replete phases of an oral contraceptive cycle: a pilot study. Ann Nutr Metab. 2017;70(2):100–105. DOI: 10.1159/000465530 [DOI] [PubMed] [Google Scholar]
- 325.Alfaro-Magallanes VM, Benito PJ, Rael B, et al. Menopause delays the typical recovery of pre-exercise hepcidin levels after high-intensity interval running exercise in endurance-trained women. Nutrients. 2020 Dec 17;12(12). DOI: 10.3390/nu12123866 [DOI] [PMC free article] [PubMed] [Google Scholar]


