Abstract
To evaluate the effect of vitamin D3 supplementation on cancer mortality in the general population and on prognosis in cancer patients, a systematic review and meta-analysis of randomised, placebo-controlled trials (RCTs) and individual patient data (IPD) was conducted. Overall, 14 RCTs with a total of 104,727 participants (2015 cancer deaths) were identified and 7 RCTs, including 90 % of all study participants (n = 94,068), could be included in the IPD meta-analyses. The main meta-analysis of the 14 RCTs yielded a statistically non-significant reduction in cancer mortality by 6 % (risk ratio (RR) [95%-confidence interval (95%CI)]: 0.94 [0.86–1.02]). Subgroup analyses revealed a 12 % lower cancer mortality in the vitamin D3 group compared with the placebo group in 10 trials with a daily dosing regimen (RR [95%CI]: 0.88 [0.78–0.98]), whereas no mortality reduction was seen in 4 trials using a bolus regimen (RR [95%CI]: 1.07 [0.91–1.24]; p-value for interaction: 0.042). The IPD meta-analysis (RR [95%CI]: 0.93 [0.84; 1.02]) confirmed the finding of all trials. The IPD were used to test effect modification by age, sex, body mass index, ethnicity, baseline serum 25-hydroxyvitamin D concentration, adherence and cancer-related factors but no statistically significant findings were obtained in meta-analyses of all trials. When restricted to trials with daily dosing in a post-hoc analysis, adults aged ≥ 70 years (RR [95%CI]: 0.83 [0.77; 0.98]) and subjects with vitamin D3 therapy initiation before cancer diagnosis (RR [95%CI]: 0.87 [0.69; 0.99]) appeared to benefit most from daily vitamin D3 supplementation. Measurements of baseline 25-hydroxyvitamin D levels and inclusion of other than non-Hispanic White adults were too sparse in the trials to draw conclusions. Results for all-cause and cancer-specific survival of participants with cancer were comparable to those obtained in the general population for cancer mortality. In conclusion, vitamin D3 did not reduce cancer mortality in the main meta-analysis of all RCTs because the observed risk reduction by 6 % was not statistically significant. However, a subgroup analysis revealed that vitamin D3 administered daily, in contrast to bolus supplementation, reduced cancer mortality by 12 %.
Keywords: Vitamin D, Cancer, Mortality, Survival, Systematic review, Individual patient-data
1. Introduction
1.1. Rationale
Despite enormous efforts in prevention and therapy, cancer remains a major burden; in 2020, there were 19.3 million new cancer cases and approximately 10 million cancer deaths worldwide (International Agency for Research on Cancer, 2020). The number of new cancer diagnoses is growing due to the aging population as well as changing risk factors and is projected to reach 30.2 million new cases by 2040 (International Agency for Research on Cancer, 2022).
Vitamin D deficiency is prevalent worldwide and more common in cancer patients during cancer therapy than in the general population. The prevalence of vitamin D deficiency (defined as 25-hydroxyvitamin D (25(OH)D) levels < 30 nmol/L) in representative population samples from the United States and Europe has been reported recently as 6 % and 13 %, respectively (Cashman et al., 2016; Schleicher et al., 2016). For example, in a study with 2912 colorectal cancer patients, a much higher vitamin D deficiency prevalence of 59 % was found during or shortly after first-line treatment and, in agreement with previous observational studies, low 25(OH)D levels were strongly associated with poorer survival (Maalmi et al., 2018; Maalmi et al., 2017; Markotic et al., 2019).
From a biological perspective, it is plausible that a sufficient vitamin D status has an impact on cancer prognosis: by binding to the vitamin D receptor (VDR), the active hormone 1,25-dihydroxyvitamin D (1,25 (OH)2D) influences signaling pathways that regulate cell proliferation, differentiation, and cell survival, and thus acts as an anti-proliferative agent in many tissues and can slow the growth of malignant cells (Fleet et al., 2012). For example, animal experiments showed that 1,25 (OH)2D delays age-related changes via VDR-mediated activation of Nrf2, inhibiting oxidative stress and DNA damage, which are relevant aspects of tumorigenesis (Calabrese et al., 2010; Chen et al., 2019).
Meta-analyses of observational studies reported elevated risks of lung cancer, colorectal cancer, breast cancer, bladder carcinoma, and lymphoma in people with low serum 25(OH)D concentration (Garland and Gorham, 2017; Li et al., 2014; Zhang et al., 2015a,b). Systematic reviews further concluded that sufficient 25(OH)D levels (≥50 nmol/L) are associated with better prognosis in patients with breast and colorectal cancers, whereas there have been too few studies for other cancer sites to draw conclusions (Maalmi et al., 2018; Toriola et al., 2014; Vaughan-Shaw et al., 2017; Yao et al., 2017). Moreover, low 25(OH)D levels were substantially related to increased cancer mortality in the general population (Heath et al., 2019). Mendelian randomisation studies conducted by consortia of large cohorts from Denmark, the UK Biobank, and the CVD-EPIC study supported a causal relationship between low 25(OH)D levels and cancer mortality whereas this was not observed when also subjects with adequate 25(OH)D levels were included in the analysis, like done in an earlier Mendelian randomisation study using only the UK Biobank data (Afzal et al., 2014; Ong et al., 2018; Sofianopoulou et al., 2021).
Evidence regarding vitamin D3 and cancer mortality from randomised controlled trials (RCTs) is conflicting. Despite strong heterogeneity in study populations, intervention schemes, and other important design aspects, four out of seven previous systematic reviews and meta-analyses reported a statistically significant reduction in cancer mortality in those randomised to vitamin D3 (Bjelakovic et al., 2014; Goulão et al., 2018; Goulão et al., 2020; Guo et al., 2022; Keum et al., 2022; Keum et al., 2019; Zhang et al., 2022; Zhang et al., 2020; Zhang et al., 2019). However, none of the previous systematic reviews collected unpublished results on cancer mortality from eligible studies and individual patient data (IPD).
1.2. Objectives
We aimed to evaluate the effect of vitamin D3 supplementation on cancer mortality in the general population and on prognosis in cancer patients. Potential heterogeneity among trial results according to region, health status of the included population, vitamin D3 dose, regimen (daily or bolus), and duration of treatment was investigated. We also performed IPD subgroup analyses to shed light on potential effect modifiers, including patient characteristics (such as age, sex, body mass index (BMI), ethnicity, baseline serum 25(OH)D concentration, and adherence) and cancer-related factors.
2. Methods
2.1. Protocol and reporting checklist
This systematic review was registered in PROSPERO before data collection to preclude data-driven analyses and selective reporting (CRD42020185566). In addition, the methods, including the selection criteria, the statistical analysis, outcomes, and subgroup and sensitivity analyses, were published in advance in a study protocol (Schöttker et al., 2021). This was developed in line with the “Preferred reporting items for systematic review and meta-analysis protocols” (PRISMA-P), the Cochrane Handbook for Systematic Reviews of Interventions, and the Institute of Medicine guideline (Institute of Medicine, 2011b; Higgins et al., 2022; Moher et al., 2015; Shamseer et al., 2015). Any deviations were recorded in an amendment log, describing the exact change and rationale (supplementary table 1). Reporting is in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Individual Participant Data (PRISMA-IPD (Stewart et al., 2015); see Supplementary table 2 for completed checklist).
2.2. Search strategy
One researcher (SK) searched for eligible RCTs in MEDLINE, ISI Web of Science (WoS) and Cochrane Central Register of Controlled Trials (CENTRAL) plus appropriate systematic reviews and meta-analyses in the Cochrane Database of Systematic Reviews (CDSR) and Kleijnen Systematic Reviews (KSR) Evidence from inception to January 18, 2022. The search strings shown in Supplementary table 3 were conceived by two researchers (SK and BS) and reviewed by a specialist for systematic bibliographic searches at the Central Library of the German Cancer Research Center (Andrea Heppert). SK searched for ongoing or completed RCTs with unpublished data in the WHO’s International Clinical Trials Research Portal (ICTRP) and clinicaltrials.gov via CENTRAL. Reference lists of eligible studies were scanned to yield relevant articles via cross-referencing. No restrictions regarding time of publication, language, settings, or geographical locations were applied.
2.3. Inclusion and exclusion criteria for meta-analysis
2.3.1. Study type
Double-blind RCTs with parallel-group designs were included. Single-arm studies, observational studies (e.g., cohort and case-control studies) and other records (e.g., narrative reviews, dissertations, editorials, study protocols, clinical guidelines, commentaries, correspondences, and letters) were excluded.
2.3.2. Participants
Studies were included if they were conducted in the general population or in a population suffering from any specific chronic disease (e.g., HIV patients). Special populations such as pregnant or lactating women, infants, and COVID-19 patients were excluded. No other age restrictions were applied.
2.3.3. Interventions
Trials that used vitamin D3 and bioequivalent substances (e.g., calcitriol (i.e., 1,25(OH)2D), alfacalcidol, calcifediol (i.e., 25(OH)D) in any dose and any regimen (e.g., daily/weekly/monthly intake) for at least six months were included. Co-administration with other medications or dietary supplements (e.g., calcium or chemotherapy) was allowed if all arms received the same therapy. Studies not permitting personal/private use of vitamin D3 supplements were included as well. Trials were excluded if vitamin D3 was supplied via fortified foods, or if vitamin D2 or bioequivalent substances were used because it was already found not to affect mortality in a previous meta-analysis (Bjelakovic et al., 2014).
2.3.4. Comparators
Studies that used placebo as the comparator were included. Studies were excluded if they were designed as open-label trials, used no treatment as control or administered an active control (e.g., standard of care or lower vitamin D3 doses than the intervention dose) instead of a placebo.
2.3.5. Outcomes
Studies required at least one cancer death per arm to be eligible and were included if risk ratios for cancer mortality or cancer survival were published. Results of the intention-to-treat approach were used, including all participants randomised, when both intention-to-treat and per-protocol results were given. We prioritized unadjusted summary estimates over adjusted estimates since studies adjusted for different covariates. Published results including a subsequent follow-up were used in the meta-analyses only when results covering solely the intervention period were not available. If studies reported cancer incidence or all-cause mortality as a primary outcome or in the framework of serious adverse events, the authors were contacted to obtain unpublished data on cancer outcomes. Studies were excluded if no data on at least one of the outcomes of interest were obtainable.
2.4. Data extraction for meta-analysis
We used EndNote and Rayyan QCRI (web application) to manage citations, title/abstract screening and full-text selection (Ouzzani et al., 2016). We removed duplicates using an Excel sheet and the Bramer methods (Bramer et al., 2016). SK screened all titles and abstracts for potentially relevant RCTs and systematic reviews. SK excluded studies/reviews that did not meet the broad inclusion criteria regarding the population, intervention, comparator, and study type. In a second step, the screening for study eligibility was defined by the outcomes “cancer mortality”, “cancer survival”, “all-cause mortality” and “cancer incidence”.
To gather unpublished cancer mortality data, SK contacted authors of trials that met the inclusion criteria but reported only all-cause mortality and/or cancer incidence, had a completed, prematurely ended, unknown or ongoing status but no publication, or had unclear descriptions of the study design or intervention to determine final inclusion.
The full-text screening was conducted in duplicate by a second researcher (AZ). Furthermore, two investigators (SK and AZ) independently extracted data from included studies using standard and predefined data extraction forms. Any disagreements were resolved by consensus and third-party adjudication (BS).
2.5. Eligibility for IPD meta-analyses
If more than 20 cancer deaths were reported, studies included in the meta-analysis of all trials were additionally eligible for the IPD meta-analysis. To collect IPD, SK and BS approached the authors of eligible trials, defined conditions to use their IPD, and entered into data use agreements. To ensure the integrity of the IPD, datasets were checked for plausibility, consistency, and completeness of relevant categorical and continuous variables and compared with published results. All mortality- and survival- related outcomes were restricted to the intervention period.
2.6. Statistical analyses
The computation of the summary risk ratios (RR), 95 % confidence intervals (95 % CI), the tests for heterogeneity, and publication bias were performed independently by two researchers: BS used Comprehensive Meta-Analysis 2.0 (Biostat, Englewood, NJ) and SK used the meta and metafor packages in R 4.1.3 (Balduzzi et al., 2019; Viechtbauer, 2010). The results were compared and, if there were discrepancies, the computations were checked and corrected by each analyst separately until the reasons for the inconsistencies were found and both researchers obtained the same results.
We used the DerSimonian and Laird method to fit random effects models (primary analysis) and the Mantel-Haenzel method to calculate fixed effects summary estimates (secondary analysis). Generally, results of the random effects model are shown and for the main meta-analyses, results of the fixed effects model are shown in addition (Deeks et al., 2022). Heterogeneity between studies was assessed by Cochran’s Q test, the I2 index, and tau2. Small-study effects and publication bias were evaluated via funnel plots and Egger’s test (Egger et al., 1997).
2.6.1. Meta-analyses of all trials
A meta-analysis of all trials was conducted for the outcome “cancer mortality”. To explore sources of heterogeneity, subgroup analyses regarding methodological trial differences were performed including trial duration, study population, region, dose, and treatment regimen. Pre-specified sensitivity analyses were also conducted by excluding studies with: (1) a high risk of bias; (2) not reporting intention-to-treat (ITT) results; and (3) with co-supplementation of calcium.
2.6.2. IPD meta-analyses
Unadjusted and adjusted Cox proportional hazards regression models were run with harmonized variable definitions for the obtained IPD. Analyses were not conducted in one pooled data set. Instead, a two-step approach was used for the meta-analyses, whereby the analyses were carried out on a study-specific basis, and subsequently the effect estimates were pooled using the random effects model. Five studies sent data to the German Cancer Research Center (Heidelberg, Germany) and were analysed there with the same analysis protocol independently by SK and BS using SAS 9.4 (Avenell et al., 2012; Manson et al., 2019; Scragg et al., 2018; Urashima et al., 2019; Wactawski-Wende et al., 2006). Co-authors from the FIND and D-Health studies undertook the analyses in-house using SAS code provided by BS (Neale et al., 2022; Virtanen et al., 2022).
Three main IPD meta-analyses were conducted using adjusted and unadjusted models:
Efficacy of vitamin D3 supplementation for cancer mortality reduction in the general population.
Efficacy of vitamin D3 supplementation for cancer-specific survival of cancer patients.
Efficacy of vitamin D3 supplementation for overall survival of cancer patients.
To assess cancer survival endpoints from general population cohorts, the studies were restricted to patients with a history of cancer in the five years preceding the baseline, a cancer diagnosis during the trial, or cancer death during the trial. For patients with a history of cancer in the five years preceding baseline and who died of cancer during the intervention period, the survival time was calculated from baseline to death or end of the intervention. For participants with a cancer diagnosis during the trial, the survival time was counted from the date of cancer diagnosis until death or end of the trial.
To explore sources of heterogeneity, we conducted subgroup analyses according to participant characteristics: (1) in the general population data by participant age, sex, BMI, ethnicity, baseline 25(OH)D level, cancer diagnosis before baseline, and adherence (defined as “low” if < 80 % or “high” if ≥ 80 %); and (2) in cancer patients additionally by cancer stage, cancer site, and time of cancer diagnosis.
With the exception of adherence, the factors used for the subgroup analyses were also used as covariates for the adjusted models. We further tested interactions between the treatment variable (vitamin D3 vs. placebo) and these covariates to identify potential effect modifiers. Variables with ≥ 5 % of missing data were not used in the multivariable model of the respective study but were used in subgroup analyses. No imputation of missing covariate values was done and a complete case analysis approach was applied.
2.7. Risk of bias assessment
The risk of bias assessment of included studies was conducted for the outcome “cancer mortality” by two independent reviewers (SK, AZ) using the Cochrane risk-of-bias tool for randomised trials (RoB 2) (Sterne et al., 2019). They evaluated various domains of bias including aspects of trial design, conduct, and reporting. Cases of disagreement and critical points were discussed until a consensus was reached and documented accordingly.
2.8. Strength of body of evidence
The quality of evidence for the outcomes “cancer mortality”, “overall cancer survival”, “cancer-specific survival” was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al., 2008).
3. Results
3.1. Study search and selection
The study search and selection process is summarised in Fig. 1. In our search for RCTs, we identified 3664 published articles and 899 registry records. Searches for systematic reviews and/or meta-analyses yielded 1248 potentially relevant records. After removal of duplicates and title/abstract screening, the full-text articles of 253 potentially eligible studies were identified. We identified a further 20 potentially eligible studies included in 33 previous systematic reviews. Overall, we reviewed the full-text articles of 273 studies, of which 175 studies met exclusion criteria as shown in Fig. 1 (Supplementary table 4 lists all excluded studies and reasons for exclusion).
Fig. 1.
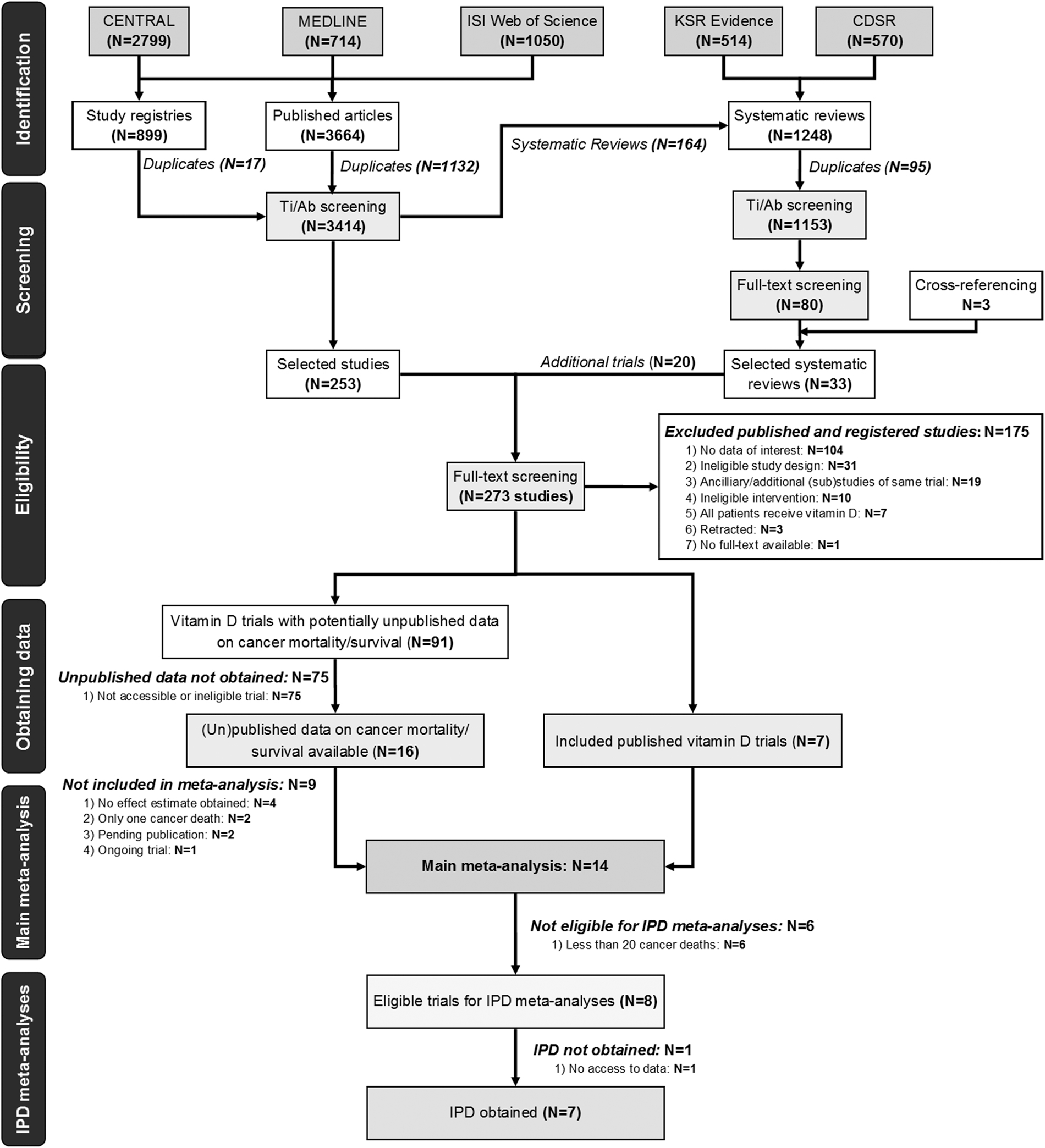
Flow diagram of study selection. Abbreviations: CENTRAL Cochrane Central Register of Controlled Trials; CDSR Cochrane Database of Systematic Reviews; IPD individual patient data; KSR Kleijnen Systematic Reviews Ltd; Ti/Ab title/abstract.
From these articles, seven trials could be included directly in the meta-analysis. We also attempted contact with authors of 91 studies with potentially unpublished data on cancer mortality/survival or to clarify uncertainties (Supplementary table 5 for authors’ (non-) responses). The authors of 16 studies responded but only seven trials met the inclusion criteria and could be included in the main meta-analysis. Based on published and acquired data, 14 RCTs were included in the main meta-analysis comparing vitamin D3 and placebo for the endpoint “cancer mortality”.
Eight trials with ≥ 20 cancer deaths were eligible for the IPD meta-analyses and seven provided data (Avenell et al., 2012; Manson et al., 2019; Neale et al., 2022; Scragg et al., 2018; Urashima et al., 2019; Virtanen et al., 2022; Wactawski-Wende et al., 2006). One trial’s data (N = 2686) have been archived and are no longer accessible (Trivedi et al., 2003). No IPD data integrity issues were identified during our analysis.
3.2. Characteristics of included studies
The complete study characteristics of the included 14 RCTs are summarized in Table 1 and Supplementary table 6. The trials comprised a total of 104,727 participants; 1928 cancer deaths occurred within the intervention period and 87 additional cancer deaths occurred up to three years after the intervention. Two studies investigated cancer survival as the primary outcome, and seven trials examined cancer mortality as a secondary outcome. Five studies were conducted in Europe, four in North America (all in the United States), two in Australia/New Zealand, two in Asia (both in Japan), and one in Africa (Tanzania). Ten trials used a daily vitamin D3 regimen ranging from 400 IU to 4000 IU daily. Four trials provided a large bolus dose of vitamin D3 intermittently (60,000 IU monthly to 100,000 IU every four months). Two trials additionally featured a high initial dose at the beginning of the intervention followed by daily dosing. The duration of vitamin D3 supplementation varied between one and seven years. Eleven studies measured the baseline 25 (OH)D in a subset or the entire population and the mean or median levels ranged from 37 to 77 nmol/L. Ten studies allowed personal vitamin D3 supplementation in the control group, ranging from 200 IU to 2000 IU daily, and one study did not provide such information.
Table 1.
Characteristics of included studies.
| First author, year, Study ID, country | Sample size (Randomised (R), analysed (A)) | Vitamin D3 dosing regimen, galenics | Vitamin D3 daily or non-daily bolus | Intervention period [years] | No of cancer deaths in treatment period | Risk ratio for cancer mortality (95% CI) in treatment period |
|---|---|---|---|---|---|---|
|
| ||||||
| Trivedi et al. (2003) UK | R = A = 2686 | 100,000 IU Q4M (15 doses total), capsule | Bolus | 5 | 135 | 0.86 (0.61–1.20) |
| Wactawski-Wende et al. (2006), Jackson et al., (2003, 2006),Chlebowski et al. (2008),Chacko et al. (2011), WHI (NCT00000611), US | R = A = 36,282a | 400 IU/d + 1000 mg Ca/d, chewable tablet | Daily | 7 | 726 | 0.89 (0.77–1.03) |
| Avenell et al. (2012) Grant et al. (2005) RECORD (ISRCTN51647438), UK | R = A = 2675b | 800 IU/d, 1000 mg Ca/d, both/d, tablet | Daily | 2–5.2 | 88c | 0.83 (0.55–1.26)c |
| Baron et al. (2015) VitDCa Polyp Prevention Study (NCT00153816), US | R = 835d | 1000 IU/d, 1200 mg Ca/d, both/d, tablet | Daily | 3–5 | 5e | 1.44 (0.24–8.63)e |
| Martineau et al. (2015) ViDiCO (NCT00977873), UK | R = A = 240 | 120,000 IU Q2M, Vigantol oil | Bolus | 1 | 2 | 0.97 (0.06–15.29)f |
| Witte et al. (2016) VINDICATE (NCT01619891), UK | R = 223; A = 163 | 4000 IU/d, tablet | Daily | 1 | 5 g | 0.25 (0.03–2.44)g |
| Akiba et al. (2018) AMATERASU 4 (UMIN000001869), Japan | R = 155 h; A = 144 | 1200 IU/d, capsule | Daily | 1 | 2i | 1.01 (0.06–15.10)i |
| Manson et al. (2019) VITAL (NCT01169259), US | R = A = 25,871 | 2000 IU/d, n - 3 fatty acids 1 g/d, both/d, capsule | Daily | 5 | 341 | 0.83 (0.67–1.02) |
| Scragg et al. (2018) ViDA (ACTRN12611000402943) New Zealand | R = 5110; A = 5108 | Initial dose of 200,000 IU, then 100,000 IU/m, soft-gel capsule | Bolus | 3.3j | 60k | 0.99 (0.60–1.64)k |
| Urashima et al. (2019) AMATERASU 5 (UMIN000001977) Japan | R = A = 417 | 2000 IU/d, capsule | Daily | 5 | 62 | 1.09 (0.58–2.01) |
| Sudfeld et al. (2020) ToV4 (NCT01798680), Tanzania | R = A = 4000 | 50,000 IU/wk for first month of ART, then 2000 IU/d, “supplements” | Daily | 1 | 8l | 1.01 (0.25–4.02)l |
| Chatterjee et al. (2021) D2dCA (NCT01942694), US | R = A = 2385 | 4000 IU/d, soft-gel | Daily | 3 | 6 m | 0.23 (0.03–1.96)m |
| Neale et al. (2022)D-Health (ACTRN12613000743763), Australia | R = 21,315; A = 21,310 | 60,000 IU/m, gel capsule | Bolus | 5 | 452 n | 1.15 (0.96–1.39)n |
| Virtanen et al. (2022) FIND (NCT01463813), Finland | R = A = 2495 | 3200 IU/d, pills | Daily | 5 | 36 m, o | 0.90 (0.38–2.13)m, o |
| 1600 IU/d, pills | 5 | 36 m, o | 1.36 (0.63–2.97)m, o | |||
| Both intervention arms combined | 5 | 36 m, o | 1.13 (0.56–2.30)m, o | |||
Abbreviations: /d, /wk, /m per day/week/month; ART antiretroviral therapy; Ca calcium; COD cause of death; FU follow-up; m month; OS overall survival; P placebo; Q2M / Q4M every 2 / 4 months; RR relative risk; VD vitamin D3; VDS Vitamin D3 supplementation; y year
Vitamin D3 was inseparably combined with calcium.
5292 participants were randomised to vitamin D3 and calcium combined.
Derived from IPD analysis to restrict FU to intervention period. During the long-term FU, a total of 156 cancer deaths were recorded (HR (95 % CI): 0.85 (0.68–1.06)).
Regarding the two-group randomisation (2GR), women could elect to be randomly assigned to receive either calcium or calcium plus vitamin D3 (584 randomised, 540 analysed). Regarding the full factorial randomisation (FFR), all other patients were randomly assigned to receive one of the four regimens (1675 randomised, 1548 analysed). 835 refers to FFR.
Unpublished data. During the entire trial duration, 17 cancer deaths were recorded (HR (95 % CI): 0.40 (0.14–1.14)). Vitamin D3 combined with calcium yielded in 10 cancer deaths during the intervention period (HR (95% CI): 2.29 (0.59–8.86)) and a total of 30 cancer deaths during the entire trial duration (HR (95 % CI): 0.87 (0.43–1.79)).
HR extracted from “Zhangyou Guo et al. (2022) Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomised controlled trials, Critical Reviews in Food Science and Nutrition, DOI: 10.1080/10408398.2022.2056574”.
Unpublished data. 5 cancer deaths were among cause of death I, one cancer death among cause of death II. Only cause of death I was included in the analysis. Risk ratio was self-calculated based on provided data.
Eight patients from placebo arm did not receive allocated intervention.
Self-calculated based on provided clinical data.
Median
Excluded those who died of cancer diagnosed before randomisation.
Unpublished data. Note of author: Nearly all deaths were HIV related. We had eight deaths coded as attributable to cancers. However, these are based on verbal autopsy and rather incomplete medical records.
Unpublished data.
Underlying cause of death available for 889/1100. 452/889 died of cancer.
During the entire trial duration, 43 cancer deaths were recorded (HR (95% CI): 1.23 (0.59–2.56) for 1600 IU/d, 1.07 (0.50–2.28) for 3200 IU/d, 1.15 (0.60–2.21) for both dosages combined)
3.3. Main meta-analysis of all trials
3.3.1. Main pooled effect estimate
The pooled RR for vitamin D3 supplementation and cancer mortality was 0.94 (95 % CI: 0.86; 1.02, p = 0.153) in both, fixed and random-effects models (Fig. 2), with no indication of heterogeneity (Cochran’s Q = 10.96 (p = 0.614), I2 = 0 %, tau2 = 0 %). The lack of asymmetry in the funnel plot and the non-significant p-value of the Egger’s test (p = 0.600) suggested no small-study effects or publication bias (Supplementary fig. 1).
Fig. 2.
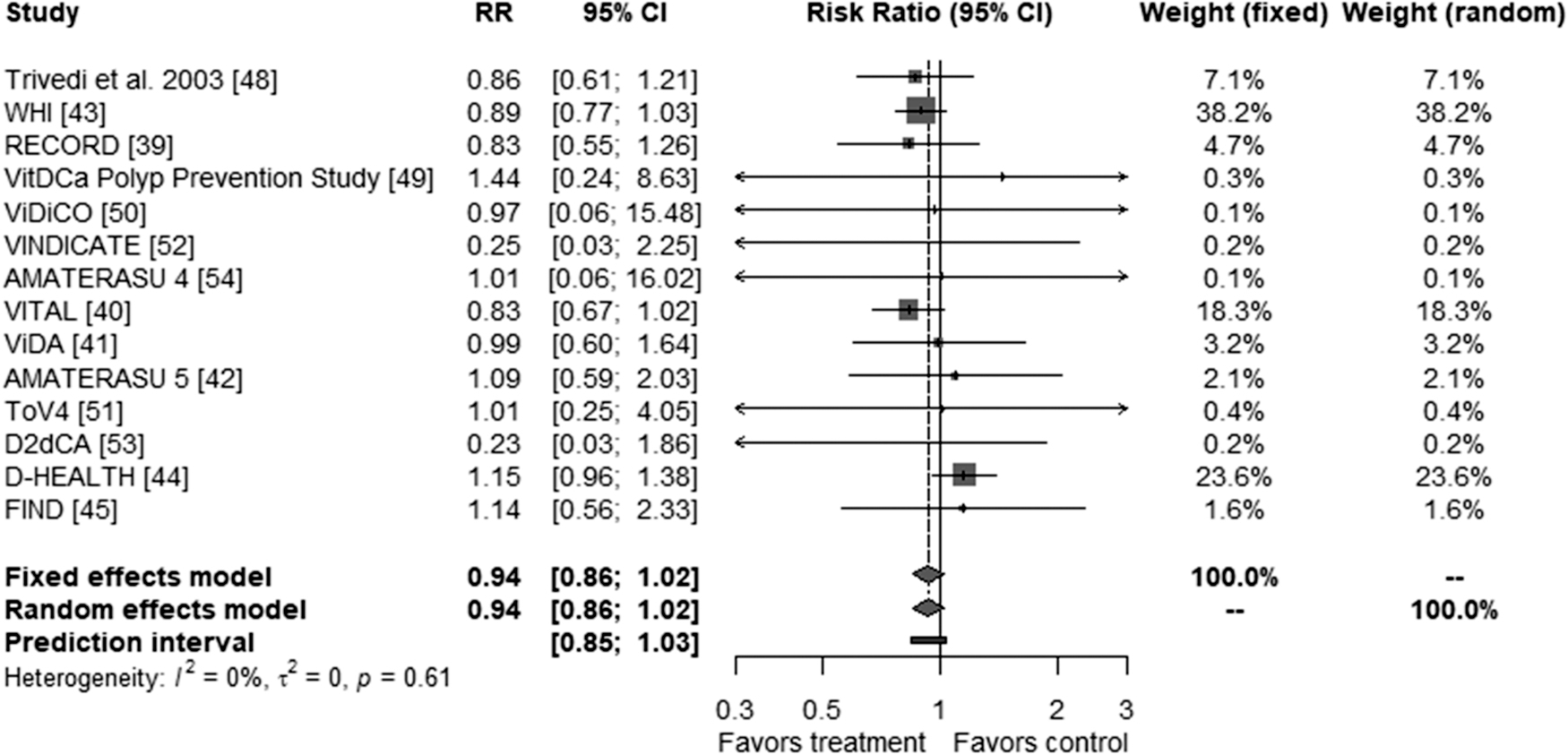
Meta-analysis of all included RCTs comparing vitamin D3 and placebo for the outcome “cancer mortality”.
3.3.2. Subgroup analyses
Fig. 3 presents the results of subgroup analyses pertinent to methodological parameters. In the ten studies using daily dosing, cancer mortality was 12% lower in the vitamin D3 group compared with the placebo group (RR [95 % CI]: 0.88 [0.78; 0.98], p = 0.019), whereas no reduction in mortality was detected in the four studies that used bolus dosing (RR [95 % CI]: 1.07 [0.91; 1.24], p = 0.411). There was a statistically significant 13 % reduction in cancer mortality among the nine RCTs conducted in the United States or Europe (RR [95% CI]: 0.87 [0.78; 0.97], p = 0.009) and no effect in studies from other regions (RR [95 % CI]: 1.12 [0.95; 1.33], p = 0.165). The tests for interaction of the treatment effect with regimen (p = 0.042) and region (p = 0.010) were statistically significant. Of note, the results of regimen and region were closely linked, since seven of the nine trials conducted in the United States or Europe used daily dosing while the two largest of the four studies from “other regions” used bolus doses. No effect modification was observed by trial duration (p = 0.584), dose (p = 0.994 for 1000–2000 IU/d; p = 0.392 for >2000 IU/d), or health status of study participants (p = 0.854).
Fig. 3.
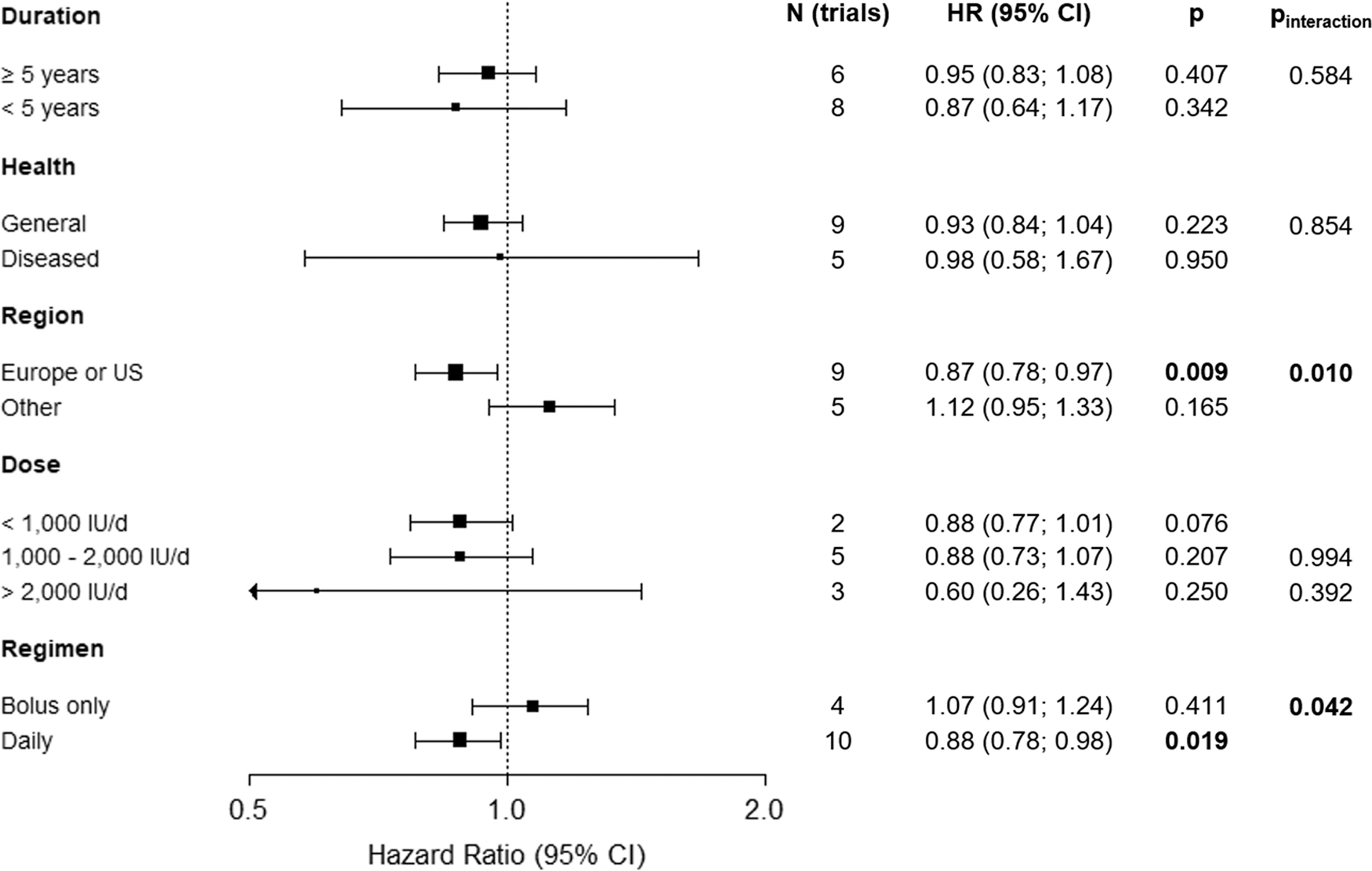
Subgroup analyses of vitamin D3 supplementation and cancer mortality by duration of intervention, health status, region, dose and regimen in all trials. Note: The FIND study appears twice in the subgroup analysis towards dose. The treatment arm with 1600 IU counted towards moderate dose and the one with 3200 IU counted towards high dose.
3.3.3. Risk of bias assessment
Of the 14 RCTs, eight studies had a low risk of bias and one study a high risk of bias (due to the ascertainment of cancer data, see footnote “k” in Table 1). Five studies were rated as having “some concerns” exclusively in the “Selection of the Reported Results” category, which was due to the outcome data used for the meta-analysis being obtained from the authors and not reported in the publication (Supplementary fig. 2).
3.3.4. Sensitivity analyses
The sensitivity analyses are summarized in Supplementary fig. 3. When only trials with a low risk of bias (n = 8) were considered, the effect estimate remained similar (RR [95% CI]: 0.94 [0.85; 1.03], p = 0.183). This was also the case when only trials reporting the intention-to-treat results were pooled (RR [95% CI]: 0.94 [0.86; 1.03], p = 0.161). When the large Women’s Health Initiative (WHI) trial (Wactawski-Wende et al., 2006), the only study that used vitamin D3 along with calcium, was removed, the summary RR increased from 0.94 to 0.97 (95% CI: 0.86; 1.08; p = 0.559).
3.4. Main IPD meta-analyses
3.4.1. Cancer mortality in the general population
Six of the seven studies included in the IPD meta-analyses were performed in the general population and could be included in the analysis on cancer mortality (Ntotal= 93,651, including 1683 cancer deaths during the intervention period (Avenell et al., 2012; Manson et al., 2019; Neale et al., 2022; Scragg et al., 2018; Virtanen et al., 2022; Wactawski-Wende et al., 2006)) The study participants’ characteristics are shown in Supplementary table 7. These six trials contributed 89.6 % to the weight of the meta-analysis of all 14 trials on the association of vitamin D3 supplementation with cancer mortality, and thus it was not surprising that the hazard ratio (HR) point estimate in the IPD meta-analysis (HR [95%CI]: 0.93 [0.84; 1.02], p = 0.125) was almost identical to that for all trials (RR [95%CI]: 0.94 [0.86; 1.02], p = 0.153). Fig. 4 panel A shows the forest plot of this IPD meta-analysis with unadjusted effect estimates. Details about the individual study results and the meta-analysis with the multivariable model, which yielded almost the same pooled effect estimate, can be found in Supplementary table 8.
Fig. 4.
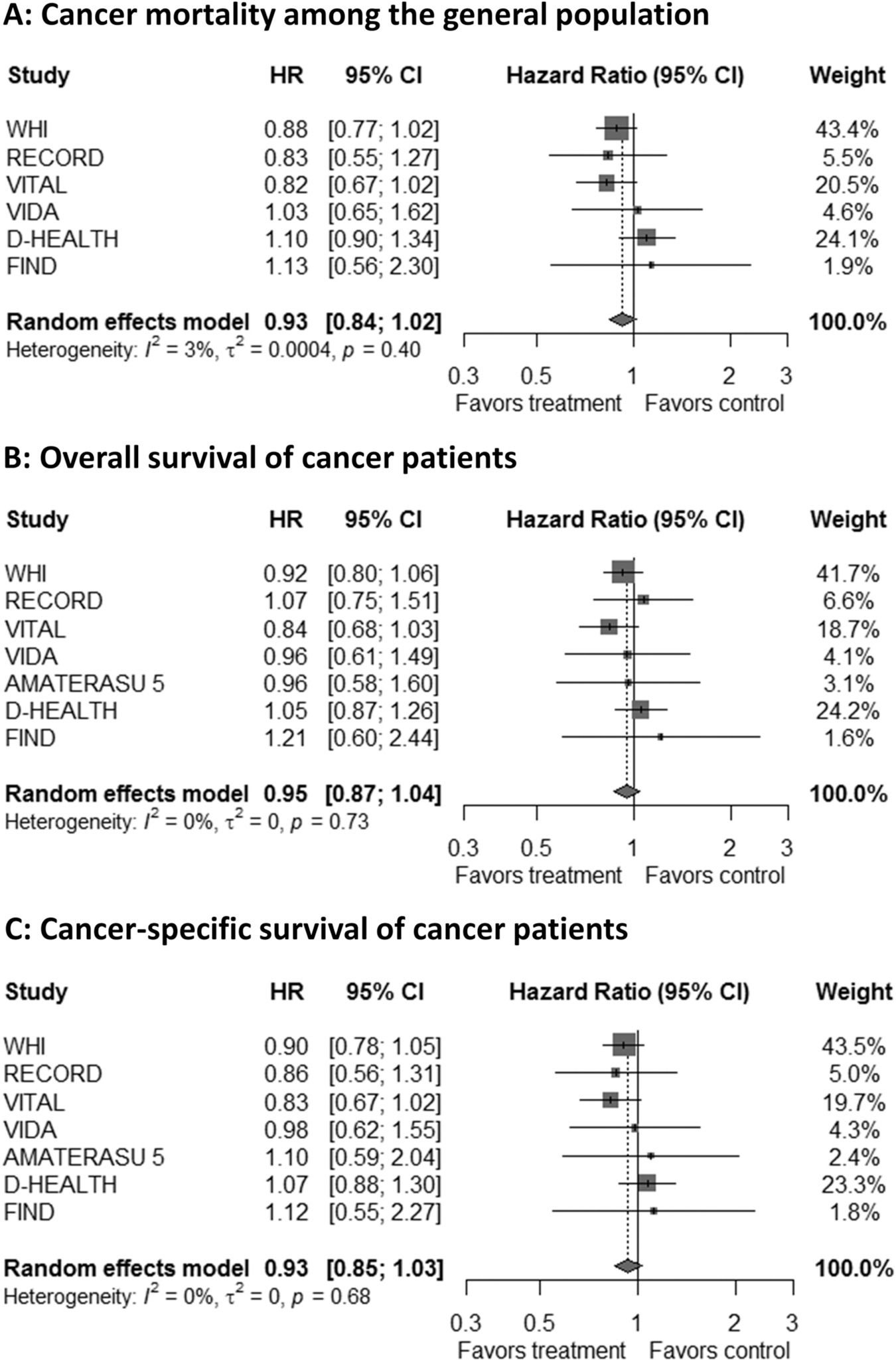
IPD meta-analyses of RCTs comparing vitamin D3 and placebo for the outcome “cancer mortality” in the general population (panel A) and for the outcomes “overall and cancer-specific survival” in cancer patients (panel B and C). Note: Unadjusted results are shown. The adjusted results are almost identical (Supplementary tables 8, 10 and 11).
3.4.2. Cancer survival
All seven studies included in the IPD analyses contributed to the meta-analysis of overall survival (Ntotal=7528, including 1932 cancer deaths during the intervention period) and cancer-specific survival (Ntotal=7513, including 1726 cancer deaths during the intervention period) among patients with cancer (of which most were diagnosed after randomisation and only a few up to 5 years prior to study enrolment). The patient characteristics of the study populations are provided in Supplementary table 9. In unadjusted models, vitamin D3 supplementation was associated with a statistically non-significant 5% improved overall survival (HR [95 % CI]: 0.95 [0.87; 1.04], p = 0.270) and 7 % improved cancer-specific survival (HR [95% CI]: 0.93 [0.85; 1.03], p = 0.151). Fig. 4 panels B and C show the corresponding forest plots; additional details, including adjusted effect estimates, are presented in Supplementary tables 10 and 11.
3.5. IPD subgroup analyses
3.5.1. Cancer mortality in the general population
Fig. 5 illustrates the main results of the IPD subgroup analyses; details of the individual trial results and interaction tests are shown in Supplementary tables 12A and B and 13 A and B, respectively. None of the subgroup analyses showed a statistically significant effect of vitamin D3 supplementation on cancer mortality (Fig. 5, panel A). However, statistically significant findings were observed in a post-hoc analysis when trials were restricted to those with a daily vitamin D3 dosing regimen (Fig. 5, panel B). Statistically significant cancer mortality reductions by vitamin D3 supplementation were observed among adults aged ≥ 70 years (HR [95 % CI]: 0.83 [0.69; 0.99], p = 0.043), men (HR [95 % CI]: 0.73 (0.56; 0.96), p = 0.024), non-Hispanic Whites (HR [95 % CI]: 0.84 [0.74; 0.95], p = 0.007), and individuals with no history of cancer prior to the trial (HR [95 % CI]: 0.87 [0.77; 0.98], p = 0.022). However, the interaction terms of these factors with the treatment group were not statistically significant. BMI, baseline 25(OH)D level, and adherence had no impact on the results. It should be mentioned that the number of 25(OH)D measurements at baseline was small and could only be used for our analysis from two trials. Moreover, only n = 3535 (17 %) of the participants with 25(OH)D measurements had vitamin D insufficiency (25(OH)D < 50 nmol/L).
Fig. 5.
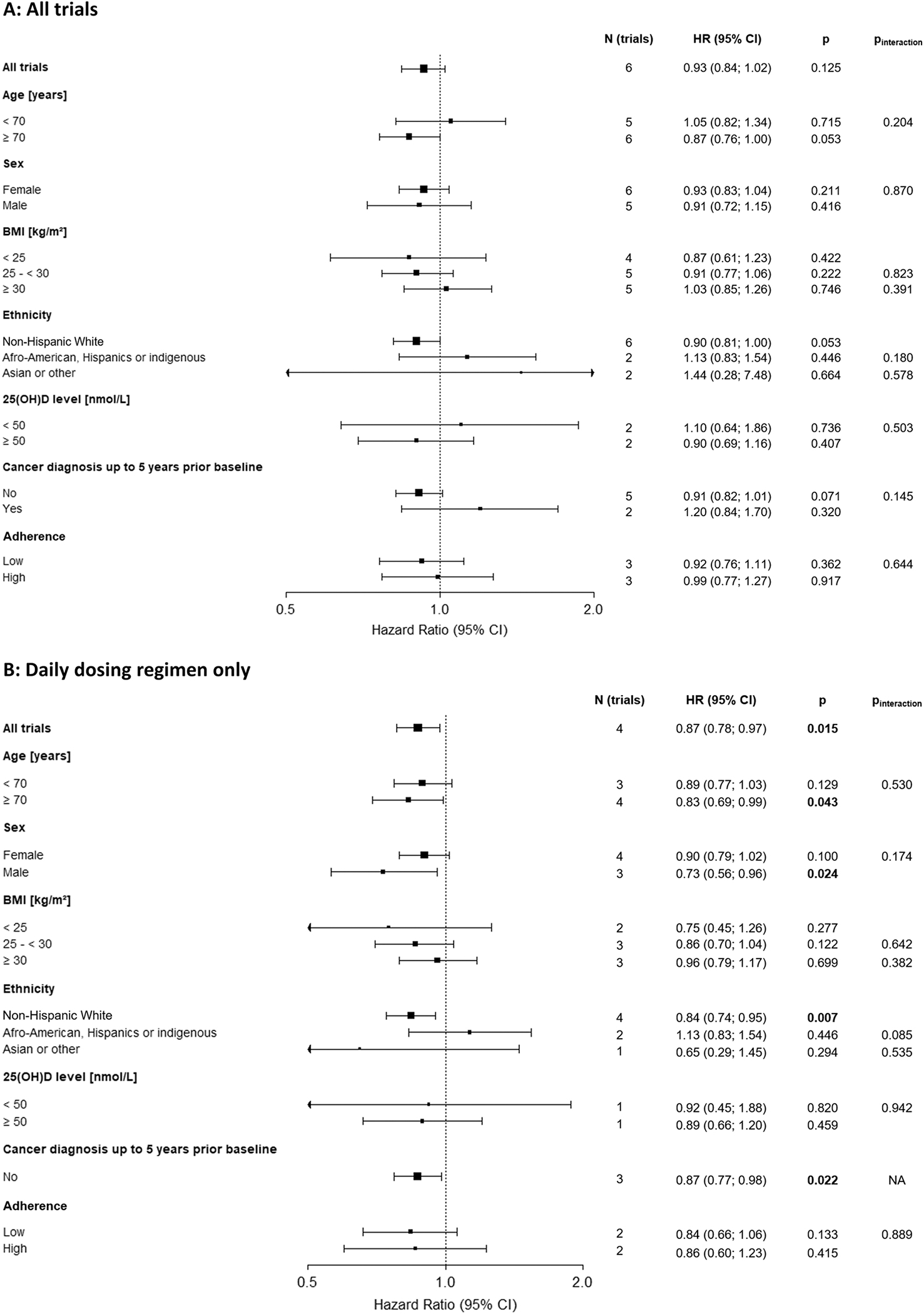
IPD subgroup analyses of vitamin D3 supplementation and cancer mortality in the general population by age, sex, BMI, ethnicity, vitamin D baseline level, cancer diagnosis in five years prior baseline, and adherence in all trials (panel A, main analysis) and restricted to trials with a daily dosing regimen (panel B, post-hoc analysis). Note: No studies available for “Cancer diagnosis up to 5 years prior baseline” = yes.
3.5.2. Cancer survival
Fig. 6 shows the pooled effect estimates of the IPD subgroup analyses for cancer-specific survival of cancer patients. Supplementary table 14A and B shows the individual study results and Supplementary table 15A and B presents the tests for interaction with vitamin D3. Results were similar to those observed for cancer mortality in the general population. In the main analysis, none of the meta-analyses of all trials showed statistically significant vitamin D3 effects on cancer survival except the subgroup conducted with patients free of cancer at baseline: HR [95 % CI]: 0.88 [0.79; 0.99], p = 0.030 (Fig. 6, panel A). Yet, when the trials were restricted to those with a daily dosing regimen in a post-hoc analysis, the effect estimates among all trials (HR [95 % CI]: 0.89 [0.80; 0.99], p = 0.040) and among non-Hispanic Whites were statistically significant while the results for patients free of cancer at baseline remained the same (HR [95 % CI]: 0.88 [0.79; 0.99], p = 0.032, Fig. 6, panel B). In contrast to the results for cancer mortality in the general population, there was some evidence of effect for cancer survival among adults aged ≥ 70 years (HR [95 % CI]: 0.85 [0.71; 1.01], p = 0.065) and men (HR [95 % CI]: 0.79 [0.61; 1.02], p = 0.069). Similarly, there was a suggestion of effect among prostate (HR [95 % CI]: 0.30 [0.08; 1.07], p = 0.064) and colorectal cancer patients (HR [95% CI]: 0.72 [0.51; 1.02], 0.061), whereas no vitamin D3 effects were observed for cancer survival among breast and lung cancer patients. Only two trials had data on cancer stage, which provided too few patients to draw conclusions from this subgroup analysis. All interaction terms of population characteristics with the treatment group were not statistically significant (but were also underpowered).
Fig. 6.
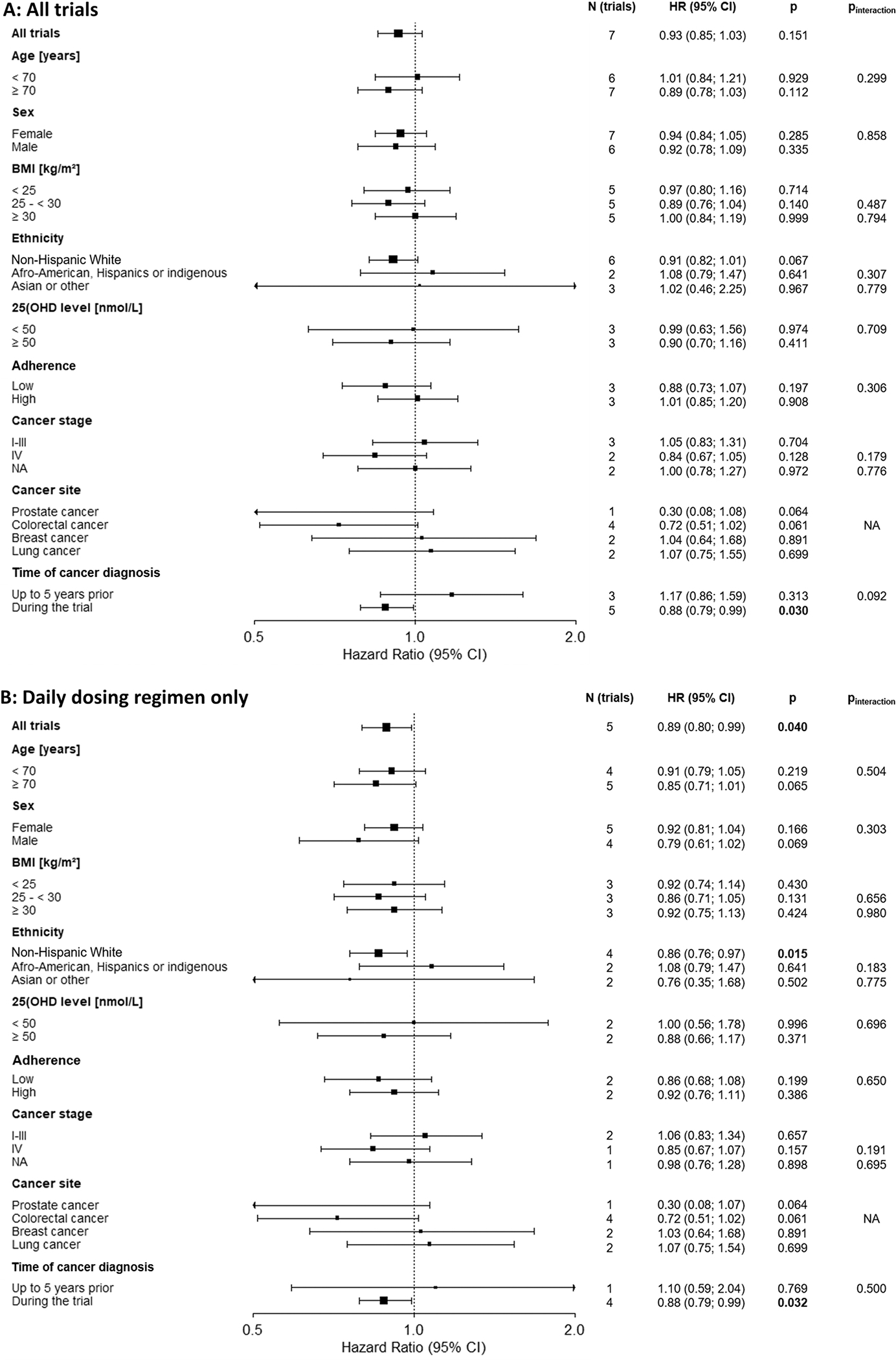
IPD subgroup analyses of vitamin D3 supplementation and cancer-specific survival in the cancer population by age, sex, BMI, ethnicity, vitamin D baseline level, adherence, cancer stage, cancer site, time of cancer diagnosis in all trials (panel A, main analysis) and restricted to trials with a daily dosing regimen (panel B, post-hoc analysis).
3.6. Strength of evidence (GRADE)
Based on the GRADE approach, the quality of evidence was assessed as high for all outcomes (supplementary table 16). The “inconsistency” domain was not downgraded, although recent trials published since 2018 have suggested a trend toward lack of efficacy of vitamin D3 supplementation on cancer mortality compared to older studies for the following reasons: (I) the studies using bolus vitamin D3 treatment are among the new studies; (II) some new studies allowed personal use of vitamin D3 up to 2000 IU/d (Neale et al., 2022; Virtanen et al., 2022) and, even if prohibited, the increased awareness of health effects by vitamin D3 in the last decade might have led to increased self-medication with vitamin D3 over time, which could align effects in the placebo group with those in the intervention group. The domain “imprecision” was not downgraded because wide confidence intervals were found primarily in studies with unpublished data and small case numbers.
4. Discussion
4.1. Summary of main findings
This systematic review and IPD meta-analysis observed that, overall, vitamin D3 supplementation resulted in a statistically non-significant 6% reduction of cancer mortality in the general population, 5% improved overall survival of cancer patients and 7% improved cancer-specific survival of cancer patients. The relationship with cancer mortality was stronger and statistically significant when the analysis was restricted to trials with a daily vitamin D3 dosing regimen (reduction by 12%). Subgroup analysis with IPD of trials with daily vitamin D3 treatment revealed statistically significant efficacy for cancer mortality among adults aged ≥ 70 years, males, non-Hispanic Whites, and participants free of cancer at initiation of treatment. However, tests for interaction by these factors were not significant and these results must be interpreted with caution because they were post-hoc analyses and confidence intervals overlapped (see below).
4.2. Comparison with other systematic reviews
Previous meta-analyses reporting statistically significant effects of vitamin D3 supplementation on cancer mortality did not include the recently published D-Health trial (Neale et al., 2022), which applied a bolus dosing regime, had a negative finding and contributed 23.6% of the weight to our meta-analysis of all trials (Bjelakovic et al., 2014; Guo et al., 2022; Keum et al., 2019; Zhang et al., 2019; Zhang et al., 2020). Our non-significant pooled effect estimate of all trials (RR [95% CI]: 0.94 [0.86; 1.02]) is comparable to the most recent systematic review by Zhang et al. (RR [95% CI]: 0.96 [0.80–1.16]), which also included the D-Health trial but not the WHI trial because of co-administration of calcium (Zhang et al., 2022). Thus, their result is similar to our sensitivity analysis excluding trials with co-administration of calcium (HR [95% CI]: 0.97 [0.86; 1.08]). However, it is debatable whether it is necessary to exclude the WHI trial because it is unclear whether calcium supplementation has an impact on cancer mortality. A meta-analysis of RCTs found no effect of calcium on cancer mortality at trial-level (HR [95%CI]: 0.96 [0.74; 1.24]) or patient-level (HR [95%CI]: 0.98 [0.74; 1.29]) and, moreover, no biologically plausible explanation is currently available for an effect of calcium supplementation on cancer mortality (Bristow et al., 2013; Yang et al., 2016).
4.3. Effect modification by vitamin D3 dosing regimen
Our results showing efficacy of daily, but not bolus, vitamin D3 supplementation in reducing cancer mortality are consistent with previous meta-analyses on cancer mortality or all-cause mortality (Guo et al., 2022; Keum et al., 2022; Keum et al., 2019; Zhang et al., 2022; Zhang et al., 2019). However, by including more trials than these previous meta-analyses, we were able to detect statistically significant effect modification by treatment regimen for the first time with statistical significance (pinteraction=0.042). The pattern of intake could be important for a favourable steady state of the bioavailability of the active 1,25 (OH)2D hormone. Daily administration counteracts the fast excretion of vitamin D from the circulation (Hollis and Wagner, 2013; Keum et al., 2022). Moreover, the enzymes CYP27B1 (converts 25(OH)D to 1,25 (OH)2D) and CYP24A1 (inactivates 25(OH)D and 1,25(OH)2D) follow first-order reaction kinetics (Vieth, 2009). This means that doubling the concentration of the precursor doubles the yield of the product, unlike other steroid hormones (e.g., cortisol, oestrogen, testosterone) that follow zero-order kinetics (Vieth, 2020). Intermittent, non-physiologically large vitamin D3 bolus doses may lead to unstable cycling of 25(OH)D and 1,25(OH)2D levels in blood because the system needs time to adapt to the large doses (Hollis and Wagner, 2013; Keum et al., 2019; Vieth, 2020). In the long run, intermittent bolus regimens at weekly or larger intervals can lead to an up-regulation of countervailing factors (e.g., 24-hydroxylase (CYP24A1), 24,25(OH)2D and fibroblast growth factor 23), all of which ultimately leads to lower synthesis or higher degradation of 1,25(OH)2D levels (Mazess et al., 2021). Bolus doses, unlike daily doses, failed to reduce C-reactive protein response and actually elevated anti-inflammatory cytokines and doubled the risk of hypercalcemia in previous studies (Krishnan et al., 2012; Martineau et al., 2017; Mazess et al., 2021).
4.4. Effect modification by study region
The absence of an effect of vitamin D3 supplementation on cancer mortality in the meta-analysis of trials not conducted in the United States or Europe was mainly driven by the D-Health and ViDA studies, which were conducted in Australia and New Zealand, respectively. According to nationally representative surveys with standardised 25(OH)D assays, the prevalence of vitamin D deficiency (defined as 25(OH)D < 30 nmol/L) is lower in Australia (4.7%) and New Zealand (4.9%) than in Europe (e.g., 15.0% in Germany) but not much lower than in the United States (5.0%) (Cashman, 2022). The latter can be explained by higher food fortification with vitamin D in the United States outweighing the lower UV-B radiation compared to Oceania (Cashman, 2021). Thus, the high UV-B radiation and low prevalence of vitamin D deficiency in Oceania, could explain a lower efficacy of vitamin D3 supplementation in Oceania compared to Europe but not to the US. However, as the efficacy of vitamin D3 supplementation on cancer mortality was the same in European (RR [95%CI]: 0.87 [0.68–1.10]) and US studies (RR [95% CI]: 0.87 [0.77–0.98]), it is more likely that it was not the study region that led to the null findings in the two studies from Oceania but rather the fact that both used a bolus vitamin D3 regimen.
4.5. Discussion of IPD subgroup analyses
4.5.1. Ethnicity
The subgroup analyses for ethnicity need to be interpreted with caution due to small sample sizes for non-White ethnicities. Overall, 1437 cancer deaths were included in the subgroup analysis for non-Hispanic Whites, 161 for African Americans, Hispanics, or indigenous people, and 42 for Asians and other ethnicities (supplementary table 12B). As skin pigmentation has an influence on vitamin D synthesis and genetic variations with relevance for the biosynthesis of the vitamin D binding protein have been observed, which could have an influence on the 25(OH)D bioavailability (Jarrett and Scragg, 2020), results from non-Hispanic Whites should not be generalized to other ethnicities. Instead, further trials should be conducted with study participants from other ethnic backgrounds.
4.5.2. Age
Our IPD subgroup analysis restricted to studies applying a daily regimen in a post-hoc analysis is the first to show a statistically significant vitamin D3 effect distinctly for those aged 70 years or older for cancer mortality (HR [95% CI]: 0.83 [0.69; 0.99], p = 0.043). However, the vitamin D3 effect in people aged younger than 70 years was not much different from the one in the older age group and the confidence intervals widely overlapped (HR [95% CI]: 0.89 [0.77; 1.03], p = 0.129). Nevertheless, a somewhat higher vitamin D3 efficacy in the older age group is plausible because the efficiency to synthesize vitamin D in the skin declines with ageing (Chalcraft et al., 2020). Furthermore, the older population is often found to be more homebound due to lower mobility and/or disabilities, further limiting sun exposure (Institute of Medicine, 2011a, 2011b). In addition, statins belong to typically prescribed co-medications due to cardiovascular co-morbidities and may reduce vitamin D synthesis (Robien et al., 2013).
4.5.3. Sex
Among males, we observed a statistically significant efficacy of vitamin D supplementation on cancer mortality in the post-hoc IPD meta-analysis of trials with daily vitamin D dosing regimen (HR [95% CI]: 0.73 [0.56; 0.96], p = 0.024). However, the effect in women was not suggestive, with clear overlap of the confidence intervals (HR [95% CI]: 0.90 [0.79; 1.02], p = 0.100). Thus, we believe there is insufficient evidence of sex differences in the results.
4.5.4. BMI
Body weight could have a role in the efficacy of vitamin D3 supplementation because vitamin D metabolites are stored in adipose tissue. As a consequence, obese individuals usually have lower serum 25(OH)D levels than non-obese people and require higher vitamin D3 doses to achieve adequate 25(OH)D levels (Jansen and Svendsen, 2014). Interestingly, the recent meta-analysis of Keum et al. observed a significant reduction of cancer incidence and cancer mortality by daily vitamin D supplementation in participants with BMI < 25 kg/m2 but not in those with higher BMI (Keum et al., 2022). Furthermore, a secondary analysis of the VITAL trial observed a strong and statistically significant effect of vitamin D3 in the reduction of the incidence of advanced (metastatic or fatal) cancer (HR [95%CI]: 0.62 [0.45–0.86]) in individuals with BMI < 25 kg/m2, whereas no effects were observed among those with BMI 25 - < 30 (HR [95%CI]: 0.89 [0.68–1.17]) or BMI ≥ 30 (HR [95%CI]: 1.05 [0.74–1.49]) (Chandler et al., 2020). We observed the same trend among trials with daily dosing regimen in a post-hoc analysis: point estimates were also lower for the BMI < 25 kg/m2 group (HR: 0.75) than in the groups with a BMI between 25 and 30 kg/m2 (HR: 0.86) and a BMI ≥ 30 kg/m2 (HR: 0.96). However, our results were not statistically significant although we included more trials than Keum et al. Future studies with more statistical power would be needed to elucidate whether daily vitamin D supplementation is more effective for cancer mortality in non-obese individuals.
4.5.5. Timing of cancer diagnosis and initiation of vitamin D3 supplementation
For cancer survival, a statistically significant effect was observed if the cancer was diagnosed during the trial (HR [95% CI]: 0.88 [0.79; 0.99], p = 0.030), but not if it was diagnosed up to five years prior to the trial (HR [95% CI]: 1.17 [0.86; 1.59], p = 0.313). Thus, it could be important that vitamin D3 treatment is initiated early, ideally before cancer diagnosis. The most relevant times for cancer survival are before diagnosis (because this is relevant for the stage at cancer detection) and during cancer therapy (since this time decides on the efficacy and tolerance of the cancer therapy). It is plausible that taking vitamin D3 in these times is most relevant because vitamin D3 has been ascribed anti-proliferative and anti-inflammatory effects in cancer patients (Krishnan et al., 2012). The former mechanism could reduce tumor size before diagnosis and the latter improve cancer treatment tolerance.
4.5.6. Cancer stage
The overall association between vitamin D3 supplementation and cancer stage is biologically plausible as the vitamin D receptor is also present in malignant cells, enabling vitamin D3 to slow tumor progression by promoting cell differentiation and inhibiting metastasis (Kim and Giovannucci, 2020). We observed a HR < 1.0 for stage IV cancer based on two studies but the results were not statistically significant (HR [95% CI]: 0.84 [0.67; 1.05], p = 0.13). There is epidemiological evidence that late stages of colorectal cancer are associated with vitamin D deficiency, which is consistent with the previously reported finding and again encourages vitamin D3 supplementation (Negri et al., 2020). In contrast, the stage-specific data are conflicting for breast and prostate cancer (Negri et al., 2020).
4.5.7. Cancer site
None of the meta-analyses for overall survival of prostate, colorectal, breast, and lung cancer patients were statistically significant in the IPD analysis. However, it should be noted that overall prostate cancer and colorectal cancer survival narrowly missed statistical significance, whereas overall breast and lung cancer survival were unrelated to vitamin D supplementation. Future studies restricted to specific cancer sites are clearly needed and they might find differences in vitamin D3 efficacy for cancer survival according to cancer sites (Sluyter et al., 2021). While the IPD meta-analysis on prostate cancer survival is based on only one study, the data availability is currently best for colorectal cancer with data from four RCTs. Taken together with evidence from observational studies, which have shown a statistically significant association of higher circulating 25(OH)D concentration as well as sun exposure with lower colorectal cancer risk (Grant, 2014; Grant and Garland, 2006; McCullough et al., 2019), a beneficial role of vitamin D3 supplementation for colorectal cancer patients seems likely.
4.5.8. Baseline 25(OH)D level
Our IPD analyses did not show stronger effects in participants with vitamin D insufficiency (25(OH)D < 50 nmol/L) at baseline although this would be expected given the L-shaped association of 25(OH)D levels with cancer mortality reported from cohort studies (Brenner et al., 2017; Heath et al., 2019). The very low number of people with 25(OH)D levels < 50 nmol/L that could be used for the meta-analysis on cancer mortality may best explain this finding (ntotal = 3535, ncases =55).
None of the trials included in this systematic review restricted recruitment to people with vitamin D insufficiency. In the three studies, in which 25(OH)D levels were measured in subgroups, most participants had adequate 25(OH)D levels at baseline > 50 nmol/L (Manson et al., 2019; Scragg et al., 2018; Urashima et al., 2019). It is highly likely that more than half of the study population included in this systematic review had no chance to benefit from a vitamin D3 intervention because they already had a sufficient vitamin D status at baseline. This is the major limitation of the current evidence base, as treatment of people without low vitamin D status may have led to a substantial underestimation of the potential efficacy of vitamin D3 supplementation (Brenner et al., 2017). A much higher vitamin D3 efficacy could be expected from trials with initial restriction to people with vitamin D insufficiency (Pilz et al., 2022; Rejnmark et al., 2017; Sluyter et al., 2021; Wyse et al., 2021; Zgaga et al., 2022).
4.6. Strength of the vitamin D3 dose
For daily dosing regimens and cancer mortality, we observed no efficacy differences between low doses of < 1000 IU/d and average doses of 1000 – 2000 IU/d. The point estimate of the HR was lower at high doses > 2000 IU/d but the confidence interval was wide and we cannot conclude that a higher dose has greater efficacy. It would be of interest to see future studies using a dose of 2000 IU/d or higher targeted to participants with initial vitamin D deficiency (see protocol of the VICTORIA trial for example (Schöttker et al., 2020 )). The lack of an observation of a dose-response relationship in the currently available trials is in agreement with former systematic reviews and meta-analyses (Guo et al., 2022; Keum et al., 2019).
As an efficacy of low-dose vitamin D supplements for cancer mortality cannot be excluded, self-medication with vitamin D in the placebo group should be excluded as much as tolerated by study participants and ethically feasible in all future trials. However, this is challenging or even impossible for trials, which run for several years. In 10 of the 14 trials in the main meta-analysis, self-medication was allowed, which may have reduced the relative risk estimate between the vitamin D3 and placebo group.
4.7. Strengths and limitations
This is the first systematic review and meta-analysis on the efficacy of vitamin D3 supplementation for cancer mortality and survival using IPD. All major trials contributed IPD except a single older one (Trivedi et al., 2003), making the IPD analyses representative for the overall available evidence in this field. Furthermore, the acquisition of previously unpublished data is a strength of this systematic review, as it reduced selective reporting biases that were listed as limitations in previous systematic reviews. The final number of 14 RCTs included in meta-analyses for the endpoint “cancer mortality” involved 104,727 randomised participants including 1928 cancer deaths, which led to a high statistical power and precision of the pooled effect estimates, and allowed the conduction of subgroup analyses, which were nonetheless underpowered. No signs of heterogeneity or publication bias were detected.
A further strength of our systematic review is that we included exclusively double-blind and placebo-controlled randomised trials. We meticulously followed guidelines such as the PRISMA-IPD statement (supplementary table 2), registered the systematic review before any data collection occurred, published a protocol (Schöttker et al., 2021), recorded all deviations to ensure transparency (supplementary table 1), and evaluated the strength of evidence according to the GRADE approach. Moreover, data extraction, risk assessment and all statistical analyses were performed by two independent researchers.
However, our systematic review and IPD meta-analysis also has limitations. As anticipated in the protocol, the sample size was limited for certain subgroup analyses, such as non-White ethnicities, baseline 25 (OH)D levels, cancer stage and cancer sites, and sometimes the studies contributed not to all subgroup meta-analyses for the same factor (e.g. if only women were included in the trial, the study could not contribute to the subgroup analysis on males), making it challenging to draw firm conclusions. The IPD subgroup analyses have the further limitation that the additional meta-analyses restricted to trials with daily vitamin D3 dosing regimen were not specified in the review protocol and have to be reported as post-hoc analyses, which have been influenced by the knowledge of the review authors that statistically significant findings could only be observed in this subgroup of trials.
Despite the high response rate and excellent collaboration with authors from around the globe, we lacked replies from 30 studies and did not find an appropriate contact for ten studies. In most cases, these were studies that dated back more than 15 years and whose authors had moved on or retired, or whose data were stored in inaccessible archives. Thus, selective reporting bias cannot be completely excluded, but it is likely negligible because the results of the additional trial data obtained were equally distributed and did not all point into either a favourable or unfavourable direction for vitamin D.
5. Conclusions
The conclusion of the main meta-analysis of all RCTs is that vitamin D3 supplementation did not reduce cancer mortality because the 6% reduction of cancer mortality was not statistically significant: HR 0.94 (95% CI: 0.86; 1.02). However, we believe that the arguments for an efficacy of daily (as compared to bolus) vitamin D treatment regimens are convincing. Indeed, restricting the IPD meta-analysis to trials with daily dosing regimen yielded a statistically significant 13% cancer mortality reduction and 11% increased cancer-specific survival. As these effect estimates are based on untargeted vitamin D3 supplementation of individuals with and without vitamin D insufficiency, the potential in a situation where only patients with low vitamin D status are treated is likely to be substantially underestimated. Furthermore, our findings suggest that, for the health outcome “cancer survival”, starting vitamin D3 treatment before or at least shortly after a cancer diagnosis could be beneficial, which was not done for all cancer patients in the large trials, which recruited from the general population. Considering the very likely underestimation of vitamin D3 effects in the currently available trials by not focussing on subjects with low 25(OH)D levels and allowing vitamin D self-medication to the control group, the almost negligible risk of adverse events from vitamin D3 supplementation at reasonable doses and the very low treatment costs, we believe that vitamin D is an underutilised medication for cancer patients and should be considered for use in addition to the primary cancer therapy when low serum 25 (OH)D levels justify its use.
Supplementary Material
Acknowledgments
Sabine Kuznia (SK) and Ben Schöttker (BS) thank all collaborators who provided IPD and all authors who shared unpublished data and/or additional information (e.g., Prof. Alison Avenell (School of Medicine, Medical Sciences and Nutrition, University of Aberdeen) and Dr. David Cooper (HSRU, University of Aberdeen) for providing IPD from the RECORD trial). SK thanks Andrea Heppert (Central Library of the German Cancer Research Center) for the detailed review of the search strategy as well as Carissa Reid and Vivienn Weru (both from the Department of Biostatistics at the German Cancer Research Center) for their support in R.
Funding
This project was supported by a grant from the non-profit organization “Deutsche Krebshilfe” (grant no. 70114605).
AMATERASU was supported by the Ministry of Education, Culture, Sports, Science, and Technology in the Japan-Supported Program for the Strategic Research Foundation at Private Universities, funding from the International University of Health and Welfare Hospital, and Jikei University School of Medicine.
The D-Health Trial is funded by National Health and Medical Research Council.
FIND was supported by funding from the Academy of Finland (#137826), University of Eastern Finland, Juho Vainio Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and Finnish Cultural Foundation.
The United Kingdom Medical Research Council funded the central organization of the RECORD Trial (Grant G9706483), and Shire Pharmaceuticals Group plc funded the drugs, which were co-funded and manufactured by Nycomed AS.
ViDA was supported by grant 10/400 from the Health Research Council of New Zealand (Drs Scragg, Khaw, Toop, Lawes, and Camargo) and by the Accident Compensation Corporation of New Zealand.
VITAL was supported by grants (U01 CA138962 and R01 CA138962) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple institutes, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Mental Health, and others.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A short list of WHI investigators can be found in supplementary table 17 and the full list of all the investigators who have contributed to WHI science can be accessed at: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Long-List.pdf.
Neither the project funder nor the study sponsors had a role in the study design in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We hereby confirm the independence of researchers from funders and that all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Declaration of Competing Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years except for Julie E. Buring who declares an association to Pharmavite. All authors further declare no other relationships or activities that could appear to have influenced the submitted work.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.arr.2023.101923.
Data Availability
The authors do not have permission to share data.
References
- Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG, 2014. Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts. BMJ 349, g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba T, Morikawa T, Odaka M, Nakada T, Kamiya N, Yamashita M, Yabe M, Inagaki T, Asano H, Mori S, Tsukamoto Y, Urashima M, 2018. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 24, 4089–4097. [DOI] [PubMed] [Google Scholar]
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA, 2012. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD Trial). J. Clin. Endocrinol. Metab. 97, 614–622. [DOI] [PubMed] [Google Scholar]
- Balduzzi S, Rucker G, Schwarzer G, 2019. How to perform a meta-analysis with R: a practical tutorial. Evid. -Based Ment. Health 22, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, Beck GJ, Bresalier RS, Burke CA, Church TR, Cruz-Correa M, Figueiredo JC, Goodman M, Kim AS, Robertson DJ, Rothstein R, Shaukat A, Seabrook ME, Summers RW, 2015. A trial of calcium and vitamin d for the prevention of colorectal adenomas. N. Engl. J. Med. 373, 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C, 2014. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T, 2016. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 104, 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Jansen L, Saum KU, Holleczek B, Schottker B, 2017. Vitamin D supplementation trials aimed at reducing mortality have much higher power when focusing on people with low serum 25-hydroxyvitamin D concentrations. J. Nutr. 147, 1325–1333. [DOI] [PubMed] [Google Scholar]
- Bristow SM, Bolland MJ, MacLennan GS, Avenell A, Grey A, Gamble GD, Reid IR, 2013. Calcium supplements and cancer risk: a meta-analysis of randomised controlled trials. Br. J. Nutr. 110, 1384–1393. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP, 2010. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal 13, 1763–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman KD, 2021. Global view of per capita daily vitamin D supply estimates as proxy measures for vitamin D intake data. JBMR 5, e10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman KD, 2022. 100 years of vitamin D: global differences in vitamin D status and dietary intake: a review of the data. Endocr. Connect. 11, e210282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GBM, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M, 2016. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko SA, Song YQ, Manson JE, Van Horn L, Eaton C, Martin LW, McTiernan A, Curb JD, Wylie-Rosett J, Phillips LS, Plodkowski RA, Liu SM, 2011. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr. 94, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcraft JR, Cardinal LM, Wechsler PJ, Hollis BW, Gerow KG, Alexander BM, Keith JF, Larson-Meyer DE, 2020. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients 12, 2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PD, Chen WY, Ajala ON, Hazra A, Cook N, Bubes V, Lee IM, Giovannucci EL, Willett W, Buring JE, Manson JE, 2020. Effect of vitamin D3 supplements on development of advanced cancer: a secondary analysis of the VITAL Randomized clinical trial. JAMA Netw. Open 3, e2025850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R, Fuss P, Vickery EM, LeBlanc ES, Sheehan PR, Lewis MR, Dolor RJ, Johnson KC, Kashyap SR, Nelson J, Pittas AG, Grp, Dd.R., 2021. Vitamin D supplementation for prevention of cancer: the D2d cancer outcomes (D2dCA) ancillary study. J. Clin. Endocrinol. Metab. 106, 2767–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L, Goltzman D, Miao D, 2019. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 18, e12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA, Investig, Ws.H.I., 2008. Calcium plus vitamin D supplementation and the risk of breast cancer. J. Natl. Cancer Inst. 100, 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J, Higgins J, Altman D, (editors), 2022. Available from www.training.cochrane.org/handbook. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet JC, DeSmet M, Johnson R, Li Y, 2012. Vitamin D and cancer: a review of molecular mechanisms. Biochem. J. 441, 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CF, Gorham ED, 2017. Dose-response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: a meta-analysis. J. Steroid Biochem. Mol. Biol. 168, 1–8. [DOI] [PubMed] [Google Scholar]
- Goulão B, Stewart F, Ford JA, MacLennan G, Avenell A, 2018. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am. J. Clin. Nutr. 107, 652–663. [DOI] [PubMed] [Google Scholar]
- Goulão B, Stewart F, Ford JA, MacLennan G, Avenell A, 2020. Corrigendum to: cancer and vitamin D supplementation: systematic review and meta-analysis. Am J Clin Nutr 2018;107:652–63. Am. J. Clin. Nutr. 111, 729–730. [DOI] [PubMed] [Google Scholar]
- Grant AM, Anderson FH, Avenell A, Campbell MK, Cooper C, Donaldson C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA, McPherson GC, MacLennan GS, McDonald AM, Grant M, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA, Grp RT, 2005. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365, 1621–1628. [DOI] [PubMed] [Google Scholar]
- Grant WB, 2014. Solar ultraviolet irradiance and cancer incidence and mortality. In: Reichrath J (Ed.), Sunlight, Vitamin D and Skin Cancer. Springer, New York, pp. 52–62. [Google Scholar]
- Grant WB, Garland CF, 2006. The association of solar ultraviolet B (UVB) with reducing risk of cancer: Multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res 26, 2687–2699. [PubMed] [Google Scholar]
- Guo ZY, Huang M, Fan DD, Hong Y, Zhao M, Ding R, Cheng Y, Duan SG, 2022. Association between vitamin D supplementation and cancer incidence and mortality: a trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr 1–15. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Grp GW, 2008. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AK, Kim IY, Hodge AM, English DR, Muller DC, 2019. Vitamin D status and mortality: a systematic review of observational studies. Int. J. Environ. Res. Public Health 16, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, 2022. Available from www.training.cochrane.org/handbook. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. [Google Scholar]
- Hollis BW, Wagner CL, 2013. The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 98, 4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, 2011b. Finding What Works in Health Care: Standards for Systematic Reviews. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209518/. Accessed 23 August 2022. The National Academies Press, Washington (DC). [PubMed] [Google Scholar]
- Institute of Medicine. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al. , 2011a. Dietary Reference Intakes for Calcium and Vitamin D. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56050/. Accessed 4 July 2022., Washington (DC). [Google Scholar]
- International Agency for Research on Cancer. Estimated number of new cases from 2020 to 2040, Both sexes, age [0–85+]. Available from: https://gco.iarc.fr/tomorrow/en/dataviz/isotype. Accessed 2 June 2022.
- International Agency for Research on Cancer . Globocan 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed: 2 June 2022.
- Jackson RD, LaCroix AZ, Cauley JA, McGowan J, 2003. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann. Epidemiol. 13, S98–S106. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SAA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D, 2006. Calcium plus Vitamin D Supplementation and the Risk of Fractures. N. Engl. J. Med 354, 669–683. [DOI] [PubMed] [Google Scholar]
- Jansen RB, Svendsen OL, 2014. The effect of oral loading doses of cholecalciferol on the serum concentration of 25-OH-Vitamin-D. Int. J. Vitam. Nutr. Res. 84, 45–54. [DOI] [PubMed] [Google Scholar]
- Jarrett P, Scragg R, 2020. Evolution, prehistory and vitamin D. Int. J. Environ. Res. Public Health 17, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E, 2019. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann. Oncol. 30, 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum N, Chen QY, Lee DH, Manson JE, Giovannucci E, 2022. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: a meta-analysis of randomised controlled trials. Br. J. Cancer 127, 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Giovannucci E, 2020. Vitamin D status and cancer incidence, survival, and mortality. In: Reichrath J (Ed.), Sunlight, Vitamin D and Skin Cancer. Springer International Publishing, Cham, pp. 39–52. [Google Scholar]
- Krishnan AV, Trump DL, Johnson CS, Feldman D, 2012. The role of vitamin D in cancer prevention and treatment. Rheum. Dis. Clin. N. Am. 38, 161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MA, Chen PZ, Li JQ, Chu RA, Xie D, Wang H, 2014. Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 99, 2327–2336. [DOI] [PubMed] [Google Scholar]
- Maalmi H, Walter V, Jansen L, Boakye D, Schöttker B, Hoffmeister M,Brenner H, 2018. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients 10, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalmi H, Walter V, Jansen L, Chang-Claude J, Owen RW, Ulrich A, Schöttker B, Hoffmeister M, Brenner H, 2017. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 32, 961–971. [DOI] [PubMed] [Google Scholar]
- Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, Grp VR, 2019. Vitamin D supplements and prevention of cancer and cardiovascular disease. New Engl. J. Med 380, 33–44. [DOI] [PubMed] [Google Scholar]
- Markotic A, Langer S, Kelava T, Vucic K, Turcic P, Tokic T, Stefancic L, Radetic E, Farrington S, Timofeeva M, Rudan I, Campbell H, Dunlop M, Kirac I, Zgaga L, 2019. Higher post-operative serum vitamin D level is associated with better survival outcome in colorectal cancer patients. Nutr. Cancer 71, 1078–1085. [DOI] [PubMed] [Google Scholar]
- Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, Islam K, McLaughlin D, Bhowmik A, Timms PM, Rajakulasingam RK, Rowe M, Venton TR, Choudhury AB, Simcock DE, Wilks M, Degun A, Sadique Z, Monteiro WR, Corrigan CJ, Hawrylowicz CM, Griffiths CJ, 2015. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet. Resp. Med. 3, 120–130. [DOI] [PubMed] [Google Scholar]
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Kumar GT, Urashima M, Camargo CA, 2017. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356 i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B, 2021. Vitamin D: bolus is bogus—a narrative review. JBMR 5, e10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, Eliassen AH, Zeleniuch-Jacquotte A, Agnoli C, Albanes D, Barnett MJ, Buring JE, Campbell PT, Clendenen TV, Freedman ND, Gapstur SM, Giovannucci EL, Goodman GG, Haiman CA, Ho GYF, Horst RL, Hou T, Huang WY, Jenab M, Jones ME, Joshu CE, Krogh V, Lee IM, Lee JE, Mannisto S, Le Marchand L, Mondul AM, Neuhouser ML, Platz EA, Purdue MP, Riboli E, Robsahm TE, Rohan TE, Sasazuki S, Schoemaker MJ, Sieri S, Stampfer MJ, Swerdlow AJ, Thomson CA, Tretli S, Tsugane S, Ursin G, Visvanathan K, White KK, Wu K, Yaun SS, Zhang XH, Willett WC, Gail MH, Ziegler RG, Smith-Warner SA, 2019. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J. Natl. Cancer Inst. 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P, 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale RE, Baxter C, Romero BD, McLeod DSA, English DR, Armstrong BK, Ebeling PR, Hartel G, Kimlin MG, O’Connell R, van der Pols JC, Venn AJ, Webb PM, Whiteman DC, Waterhouse M, 2022. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 10, 120–128. [DOI] [PubMed] [Google Scholar]
- Negri M, Gentile A, de Angelis C, Monto T, Patalano R, Colao A, Pivonello R, Pivonello C, 2020. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients 12, 1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JS, Gharahkhani P, An JY, Law MH, Whiteman DC, Neale RE, MacGregor S, 2018. Vitamin D and overall cancer risk and cancer mortality: a Mendelian randomization study. Hum. Mol. Genet 27, 4315–4322. [DOI] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A, 2016. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Trummer C, Theiler-Schwetz V, Grubler MR, Verheyen ND, Odler B, Karras SN, Zittermann A, Marz W, 2022. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 14, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejnmark L, Bislev LS, Cashman KD, Eirisksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips P, Pilz S, van Schoor NM, Kiely M, Jorde R, 2017. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One 12, e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robien K, Oppeneer SJ, Kelly JA, Hamilton-Reeves JM, 2013. Drug-vitamin D interactions: a systematic review of the literature. Nutr. Clin. Pract. 28, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, Johnson CL, Pfeiffer CM, 2016. National estimates of serum total 25-Hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. J. Nutr. 146, 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttker B, Kuznia S, Brenner H, 2021. Efficacy of vitamin D3 supplementation on cancer mortality in the general population and the prognosis of patients with cancer: protocol of a systematic review and individual patient data meta-analysis of randomised controlled trials. BMJ Open 11, e041607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttker B, Kuznia S, Laetsch DC, Czock D, Kopp-Schneider A, Caspari R, Brenner H, 2020. Protocol of the VICTORIA study: personalized vitamin D supplementation for reducing or preventing fatigue and enhancing quality of life of patients with colorectal tumor - randomized intervention trial. BMC Cancer 20, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg R, Khaw KT, Toop L, Sluyter J, Lawes CMM, Waayer D, Giovannucci E, Camargo CA, 2018. Monthly High-Dose Vitamin D Supplementation and Cancer Risk A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol 4 e182178–e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349, g7647. [DOI] [PubMed] [Google Scholar]
- Sluyter JD, Manson JE, Scragg R, 2021. Vitamin D and clinical cancer outcomes: a review of meta-analyses. JBMR 5, e10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofianopoulou E, Kaptoge SK, Afzal S, Jiang T, Gill D, Gundersen TE, Bolton TR, Allara E, Arnold MG, Mason AM, Chung R, Pennelis LAM, Shi FC, Sun LL, Willeit P, Forouhi NG, Langenberg C, Sharp SJ, Panico S, Engstrom G, Melander O, Tong TYN, Perez-Comago A, Norberg M, Johansson I, Katzke V, Srour B, Sanchez MJ, Redondo-Sanchez D, Olsen A, Dahm CC, Overvad K, Brustad M, Skeie G, Moreno-Iribas C, Onland-Moret NC, van der Schouw YT, Tsilidis KK, Heath AK, Agnoli C, Krogh V, de Boer IH, Kobylecki CJ, Colak Y, Zittermann A, Sundstrom J, Welsh P, Weiderpass E, Aglago EK, Ferrari P, Clarke R, Boutron MC, Severi G, MacDonald C, Providencia R, Masala G, Zamora-Ros R, Boer J, Verschuren WMM, Cawthon P, Schierbeck LL, Cooper C, Schulze MB, Bergmann MM, Hannemann A, Kiechl S, Brenner H, van Schoor NM, Albertorio JR, Sacerdote C, Linneberg A, Karhus LL, Huerta JM, Joergensen LMC, Ben-Shlomo Y, Lundqvist A, Gallacher J, Sattar N, Wood AM, Wareham NJ, Nordestgaard BG, Di Angelantonio E, Danesh J, Butterworth AS, Burgess S, Collaboration ERF, 2021. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 9, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernan MA, Hopewell S, Hrobjartsson A, Junqueira DR, Juni P, Kirkham JJ, Lasserson T, Li TJ, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT, 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, 14898. [DOI] [PubMed] [Google Scholar]
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF, Grp P-ID, 2015. Preferred reporting items for a systematic review and meta-analysis of individual participant data The PRISMA-IPD statement. JAMA 313, 1657–1665. [DOI] [PubMed] [Google Scholar]
- Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga N, Hong B, Wang M, Fawzi WW, 2020. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV 7, e463–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriola AT, Nguyen N, Scheitler-Ring K, Colditz GA, 2014. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol. Biomark. Prev. 23, 917–933. [DOI] [PubMed] [Google Scholar]
- Trivedi DP, Doll R, Khaw KT, 2003. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima M, Ohdaira H, Akutsu T, Okada S, Yoshida M, Kitajima M, Suzuki Y, 2019. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers the AMATERASU randomized clinical trial. JAMA 321, 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Shaw PG, O’Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG, Zgaga L, 2017. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br. J. Cancer 116, 1092–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W, 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. [Google Scholar]
- Vieth R, 2009. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res 29, 3675–3684. [PubMed] [Google Scholar]
- Vieth R, 2020. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 74, 1493–1497. [DOI] [PubMed] [Google Scholar]
- Virtanen JK, Nurmi T, Aro A, Bertone-Johnson ER, Hypponen E, Kroger H, Lamberg-Allardt C, Manson JE, Mursu J, Mantyselka P, Suominen S, Uusitupa M, Voutilainen A, Tuomainen TP, Hantunen S, 2022. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial. Am. J. Clin. Nutr. 115, 1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE, 2006. Calcium plus vitamin D supplementation and the risk of colorectal cancer. New Engl. J. Med. 684–696. [DOI] [PubMed] [Google Scholar]
- Witte KK, Byrom R, Gierula J, Paton MF, Jamil HA, Lowry JE, Gillott RG, Barnes SA, Chumun H, Kearney LC, Greenwood JP, Plein S, Law GR, Pavitt S, Barth JH, Cubbon RM, Kearney MT, 2016. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF The VINDICATE Study. J. Am. Coll. Cardiol. 67, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse J, Mangan R, Zgaga L, 2021. Power determination in vitamin D randomised control trials and characterising factors affecting it through a novel simulation-based tool. Sci. Rep. 11, 10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BY, Campbell PT, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, Flanders WD, McCullough ML, 2016. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am. J. Clin. Nutr, 103, 886–894. [DOI] [PubMed] [Google Scholar]
- Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TYD, Hong CC, McCann SE, Tang L, Davis W, Liu S, Quesenberry CP, Lee MM, Ambrosone CB, Kushi LH, 2017. Association of serum level of vitamin d at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol. 3, 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaga L, Shraim R, Bolger E, Wyse J, 2022. Statistical power in vitamin D randomized control trials investigating biomarkers as continuous outcomes. J. Steroid Biochem. Mol. Biol. 222, 106148. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wen XH, Zhang YG, Wei XL, Liu TY, 2015a. Vitamin D deficiency and increased risk of bladder carcinoma: a meta-analysis. Cell. Physiol. Biochem. 37, 1686–1692. [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Wang SH, Che XY, Li XH, 2015b. Vitamin D and lung cancer risk: a comprehensive review and meta-analysis. Cell. Physiol. Biochem. 36, 299–305. [DOI] [PubMed] [Google Scholar]
- Zhang RJ, Zhang Y, Liu ZR, Pei YY, Xu P, Chong W, Hai Y, He L, He Y, Yu JY, Wang JJ, Fang F, Peng XC, 2022. Association between Vitamin D supplementation and cancer mortality: a systematic review and meta-analysis. Cancers (Basel) 14, 3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fang F, Tang JJ, Jia L, Feng YN, Xu P, 2020. Corrigendum to: association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 2019 (366), 14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fang F, Tang JJ, Jia L, Feng YN, Xu P, Faramand A, 2019. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 366, 14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.


