Abstract
The Tel gene (or ETV6) is the target of the translocation (12;22)(p13;q11) in myeloid leukemia. TEL is a member of the ETS family of transcription factors and contains the pointed protein interaction (PNT) domain and an ETS DNA binding domain (DBD). By contrast to other chimeric proteins that contain TEL's PNT domain, such as TEL–platelet-derived growth factor β receptor in t(5;12)(q33;p13), MN1-TEL contains the DBD of TEL. The N-terminal MN1 moiety is rich in proline residues and contains two polyglutamine stretches, suggesting that MN1-TEL may act as a deregulated transcription factor. We now show that MN1-TEL type I, unlike TEL and MN1, transforms NIH 3T3 cells. The transforming potential depends on both N-terminal MN1 sequences and a functional TEL DBD. Furthermore, we demonstrate that MN1 has transcription activity and that MN1-TEL acts as a chimeric transcription factor on the Moloney sarcoma virus long terminal repeat and a synthetic promoter containing TEL binding sites. The transactivating capacity of MN1-TEL depended on both the DBD of TEL and sequences in MN1. MN1-TEL contributes to leukemogenesis by a mechanism distinct from that of other chimeric proteins containing TEL.
The Tel gene (or ETV6) encodes a member of the ETS family of transcription factors and is located on chromosome 12 band p13. Tel was discovered as part of a fusion gene created by a translocation (5;12) in a case of chronic myelomonocytic leukemia (20). Extensive analysis of other leukemia cases by fluorescent in situ hybridization analysis revealed that Tel is a frequent target of translocations. Over 30 different rearrangements involving Tel have been described, and over 10 of these have been cloned (45). Tel encodes two proteins, one starting at the first AUG (methionine 1) and one at the second AUG (methionine 43). Both isoforms contain an N-terminal pointed (PNT) domain involved in protein-protein interactions and a C-terminal ETS domain that binds DNA. In transient-transfection experiments, TEL appears to function as a transcriptional repressor by recruitment of histone deacetylases via the transcriptional corepressors mSin3A, SMRT, and N-CoR (8, 13, 34). In addition, the central region of TEL also contains two autonomous repression domains (34), which depend on TEL self-association.
In most cases, Tel translocations encode fusion proteins that contain the PNT domain fused to phosphotyrosine kinase (PTK) domains, such as those of platelet-derived growth factor β receptor, ABL, JAK2, NTRK3, and ARG (7, 12, 20, 21, 26, 29, 40, 41). In these fusion proteins, the PNT domain provides the oligomerization interface (24) needed for activation of the fused PTK moieties (5, 21, 47). The activated PTKs are directly responsible for the in vitro and in vivo transforming activities of these proteins (6, 21, 33, 47, 50). The PNT domain is also present in several fusions with transcription factors such as AML1, MDS1 (or EVI1), and CDX2 (9, 19, 41, 44). It is unknown at present how addition of the PNT domain alters the functions of these transcription factors or how this would confer transforming activity on the fusion proteins.
Fusions involving the ETS domain of TEL are less common, and to date only two such cases have been described: MN1-TEL and BTL-TEL (4, 10). BTL is a protein of unknown function, and it remains to be shown whether and how replacement of the TEL N-terminal sequences by those of BTL confer transforming activity on TEL. However, it is not unreasonable to speculate that the substitution would alter the transcriptional activity of TEL. We characterized the t(12;22) that occurs in acute myeloid leukemia and myelodisplastic syndrome (4) and results in expression of two different MN1-TEL fusion mRNAs. MN1-TEL type I contains almost the entire coding region of MN1 fused to TEL at a position N terminal to the PNT domain, whereas in MN1-TEL type II the fusion occurs within the PNT domain. Although the function of MN1 is unknown, the protein contains features common to many transcription factors, including its nuclear localization and its N-terminal region, which is rich in prolines and contains two polyglutamine stretches (31). The structure of MN1-TEL is reminiscent of that of EWS-FLI1, a deregulated transcription factor associated with Ewing sarcoma. Like TEL, FLI1 is a member of the ETS family of transcription factors, and the EWS-FLI1 protein retains the ETS DNA binding domain (DBD). DNA binding, in combination with EWS-provided transactivation- and transformation-specific sequences, contributes to the transforming activity of EWS-FLI in NIH 3T3 cells (1, 32, 36, 37). In this report, we provide evidence that MN1-TEL has transforming activity in NIH 3T3 fibroblasts which depends on the DNA binding activity of TEL and transactivation- and transformation-specific sequences of MN1.
MATERIALS AND METHODS
Cell lines.
The NIH 3T3 (murine fibroblast), COS-1 (simian kidney carcinoma), HeLa (human cervical carcinoma), Hep3B (human hepatocarcinoma), and 293T (human embryonic kidney) cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum.
Constructs.
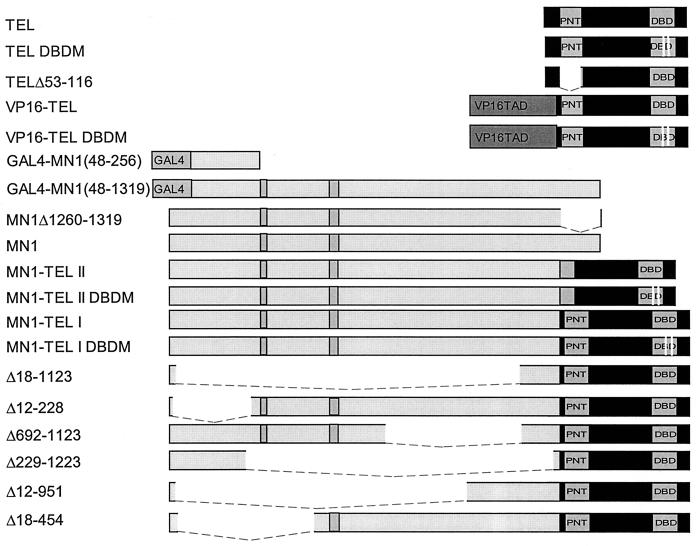
The various cDNA constructs are schematically represented below (see Fig. 2). All inserts were cloned into the cytomegalovirus (CMV) promoter containing expression vector pSCTOP (14) or into the retroviral vector pSRαMSVtkCD8. This retroviral vector allowed selection of transduced cells based on the cell surface expression of murine CD8 (23). The integrity of all mutant cDNAs was verified by sequence analysis. N-terminally tagged TEL was generated by cloning of a triple influenza hemagglutinin (HA) tag (14) into the AflIII site within the first codon of the TEL cDNA clone hpc7a (4). The deletion mutant TELΔ53-116 was generated by the in-frame deletion of the 192-bp FspI-XmnI fragment of TEL. MN1Δ1260-1319 was generated by cloning the coding region of the first exon of MN1 into the eukaryotic expression vector pCDNA3. We created a full-length MN1-TEL type I cDNA (MN1-TEL I) by a three-way ligation of the 3,736-bp SacII-NspI MN1 cDNA fragment (31), the 1,244-bp ClaI-EcoRI TEL cDNA fragment, and the NspI-ClaI MN1-TEL fusion cDNA fragment obtained from patient 2 (4). To generate MN1-TEL type II (MN1-TEL II), we ligated the same 3,736-bp SacII-NspI MN1 cDNA fragment to a 1,209-bp XmnI-EcoRI TEL cDNA fragment and to the NspI-XmnI fusion cDNA product from patient 3 (4). The various MN1-TEL type I deletion mutants were obtained by deletion of internal restriction fragments (shown in parentheses below) from the MN1 moiety. These mutants included MN1-TELΔ692-1123 (1,296-bp PmlI-SrfI), MN1-TELΔ18-1123 (3,335-bp HincII-SrfI), MN1-TELΔ12-228 (651-bp HincII), MN1-TELΔ18-454 (1,311-bp MscI), MN1-TELΔ12-951 (2,803-bp MscI-PmlI), and MN1-TELΔ229-1223 (2,985-bp HincII-Eco47III). To generate a TEL mutant that was incapable of binding DNA (TEL DBD mutant [DBDM]), we performed site-directed mutagenesis on the TEL cDNA clone hpc7a in bacteriophage M13. We substituted arginines for leucines at codons 396 and 399 by using the oligonucleotide 5′-GAGAAAATGTCCTTAGCCCTGCTCCACTACTACAA-3′ and obtained mutant phage according to the manufacturer's recommendations (Bio-Rad, Hercules, Calif.). The VP16-TEL fusion construct was created by PCR amplification of the herpes simplex virus type 1 (HSV-1) VP16 codons 413 to 489 (11) with the primers VP16COOH (5′-CCCAAGCTTGCCGCCACCATGGCCCCCCCGACCGAT-3′) and VP16BbsI (5′-CAGGCGGATCGAGTCTTCGTACTCGTCAATTCCA-3′), using pRG50 as a template. Primer VP16COOH introduces a HindIII cloning site and a Kozak consensus sequence for initiation of translation (28) and substitutes an ATG for codon 412 of VP16 to provide a translation initiation site in the VP16-TEL cDNA construct. Primer VP16BbsI contains codons 485 to 489 of VP16, followed by a BbsI restriction site. The resulting 263-bp HindIII-BbsI HSV-1 VP16 cDNA fragment was cloned into the BbsI site at codon 45 of the TEL cDNA. We created the pMSVluc reporter plasmid by cloning the 1.2-kb XhoI-HindIII fragment, containing the 5′ Moloney sarcoma virus long terminal repeat (MSV LTR) of pSRαMSVtkneo (38), into pGL2-Basic (Promega, Madison, Wis.). The 5× TEL-chloramphenicol acetyltransferase (CAT) reporter was constructed by ligating two PCR-derived fragments containing a 5× concatemerized TEL binding site and a rabbit β-globin minimal promoter containing a TATA box and a cap site. The ligated fragment was then cloned 5′ of the CAT reporter gene of pBLCAT6 (46). The GAL4 DBD MN1(48-256) and GAL4 DBD MN1(48-1319) fusion constructs were generated in pGBT9 (Clontech, Palo Alto, Calif.) and recloned into pCDNA3. The 5× GAL-luciferase construct was generated by cloning five GAL4 binding sites and the adenovirus E4 minimal promoter into pGL3-Basic (Promega).
FIG. 2.
Schematic representation of TEL, MN1, MN1-TEL, and VP16-TEL cDNA constructs. TAD, transactivating sequences. The white lines in DBD represent mutated codons. The gray boxes in MN1 sequences represent glutamine stretches. The dashed lines represent deleted sequences.
Retroviral transduction.
Retroviruses were generated by calcium phosphate precipitation of 3 × 106 293T cells (in a 10-cm-diameter dish) with 10 μg of the appropriate pSRαMSVtkCD8-based construct and 10 μg of ecotropic replication-defective helper virus pSV-Ψ−E-MLV DNA (38). After 20 h, the precipitates were removed, and virus-containing supernatants were harvested for 42 h at 4- to 8-h intervals. The supernatants were filtered over 0.45-μm-pore-size gauze filters. We then overlaid 2 × 105 NIH 3T3 fibroblasts for 3 h (in a 10-cm-diameter dish) with 1.5 ml of high-titer supernatant that contained 8 μg of Polybrene/ml, and fresh medium was added for an additional 20 h. CD8-expressing cells were selected by fluorescence-activated cell sorting 60 h after infection.
Antibodies.
A synthetic peptide containing the 10 C-terminal amino acids of TEL was conjugated to keyhole limpet hemocyanin and injected into New Zealand White rabbits (Rockland, Gilbertsville, Pa.). Immunopurified α-TEL antibodies were obtained, using affinity purification of the polyclonal α-TEL serum over a synthetic C-terminal TEL peptide-coupled Affi-Gel 10 column (Bio-Rad). MN1-specific monoclonal antibody (MAb) 2F2 was raised against a bacterially expressed N-terminal MN1 fusion protein and will be described in detail elsewhere (A. C. Molijn and E. C. Zwarthoff, unpublished data). The α-HA1 MAb 12CA5 is directed to an influenza HA tag.
Immunofluorescence analysis.
Pools of 105 virus-infected CD8+ cells were seeded on microscope slides. After 24 h, the cells were fixed in 3% paraformaldehyde for 15 min and permeabilized with 0.2% Triton in phosphate-buffered saline (PBS) for 10 min. The fixed cells were then incubated for 2 h at room temperature with immunopurified α-TEL (1:1,250 in PBS–1% bovine serum albumin) and 2 μg of 12CA5 or α-MN1 MAb 2F2 (1:1,000 in PBS–1% bovine serum albumin). Bound antibodies were visualized with fluorescein isothiocyanate-conjugated goat anti-rabbit or Texas Red-conjugated goat anti-mouse secondary antibody. Images were obtained by confocal microscopy (Bio-Rad MRC1000 Laser Scanning confocal microscope).
Transformation assay in semisolid medium.
For each transduced construct, we plated 2 × 104 CD8-positive fibroblasts in 0.3% Noble agar in Iscove's medium supplemented with 15% fetal bovine serum in triplicate (35). Colonies were counted 21 days after the cells were plated.
Immunoprecipitation and Western blotting.
Using calcium phosphate precipitation, 2 × 105 HeLa or COS-1 cells (6-cm-diameter dish) were transfected with 10 μg of the appropriate pSCTOP-based expression vector. After 20 h, the precipitates were removed. After 36 h, the cells were metabolically labeled for 12 h with 100 μCi of [35S]methionine-[35S]cysteine in vivo labeling mix (Dupont NEN, Wilmington, Del.) or [3H]leucine (Amersham Corp., Arlington Heights, Ill.) in 1.4 ml of methionine-cysteine- or leucine-free Dulbecco's modified Eagle's medium supplemented with 8% dialyzed fetal calf serum. The labeled cells were washed twice with ice-cold PBS. Proteins from lysates were immunoprecipitated sequentially with α-HA1 and α-MN1 as described previously (15). Immunoprecipitates were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and visualized by autoradiography or were electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) for subsequent Western blot analysis with α-TEL.
Transactivation analysis.
For each expression construct, triplicate (6-cm-diameter) dishes with 1.5 × 105 NIH 3T3 cells were transfected with various amounts of the appropriate pSCTOP- or pCDNA3-based vector, 1 μg of pMSVluc, 150 ng of rat β-actin promoter-driven secreted alkaline phosphate expression construct, and 5.9 μg of pBluescript (as a carrier). The medium was changed 20 h after transfection, and luciferase assays (Promega) were performed 24 h later. Luciferase activity was measured with an Optocomp illuminometer. To control for transfection efficiency, the alkaline phosphatase activity in the medium was measured as described previously (39). Induction of transactivation equaled the corrected activity associated with insert-containing pSCTOP plasmid divided by the activity associated with empty pSCTOP. Two micrograms of the 5× TEL-CAT reporter was cotransfected with 1 μg of pSCTOP plasmid containing TEL, VP16-TEL, MN1, MN1-TEL I, or MN1-TEL I DBDM into 1.5 × 105 NIH 3T3 cells (6-cm-diameter dish). After 48 h, the cell lysates were tested for CAT activity using the Quan-T-CAT assay system (Amersham, Little Chalfont, United Kingdom), and 0.5 μg of the 5× GAL-luciferase reporter was cotransfected with 0.5 μg of pCDNA3-based GAL4-VP16TAD, GAL4-MN1(48-256), or GAL4-MN1(48-1319) into 5 × 104 Hep3B cells. After 24 h, cell lysates were tested for luciferase activity.
RESULTS
MN1-TEL I transforms NIH 3T3 fibroblasts.
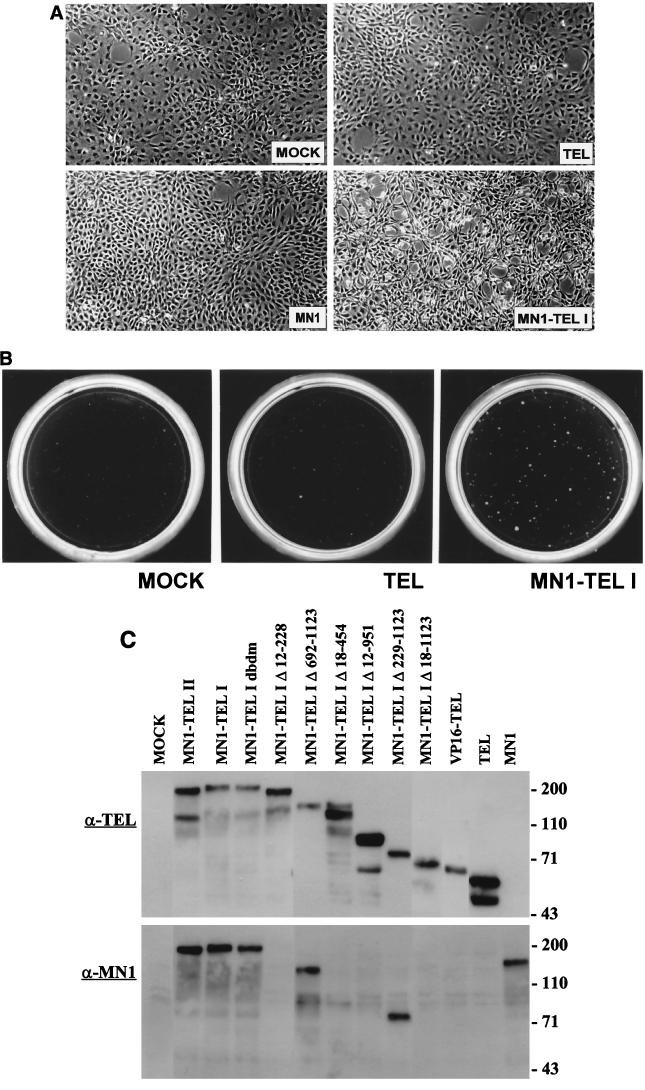
The MN1-TEL fusion protein resembles EWS-FLI1, an altered transcription factor that is associated with Ewing sarcoma and transforms NIH 3T3 cells (32, 36). We therefore used retroviral vectors to transduce MN1-TEL type I, TEL, and MN1 into an NIH 3T3 mouse fibroblast cell line that is sensitive to transformation by ETS factors and compared their transforming potentials. We used a modified retroviral transduction that allows for selection of infected NIH 3T3 cells on the basis of the cell surface expression of murine CD8 (23). Indirect immunofluorescence using TEL- and MN1-specific antibodies confirmed that more than 95% of the sorted CD8+ cells expressed the various cDNA constructs (data not shown). The morphology of the MN1-TEL I-infected cultures differed from that of the mock-, TEL-, and MN1-transduced cells (Fig. 1A). The mock-, TEL-, and MN1-infected cells grew as monolayers, whereas the cells expressing MN1-TEL I were not contact inhibited and had a more rounded-up, spiky morphology (Fig. 1A). However, TEL-infected cells grown at confluency started to reorganize into bridgelike patterns. Later, formation of cellular cords became apparent (data not shown). We recently reported the phenotypic characteristics of TEL-infected cells in detail (51).
FIG. 1.
Morphologic analysis and soft-agar assays of NIH 3T3 cells by retrovirus-transduced TEL, MN1, and MN1-TEL I. (A) Polyclonal populations of sorted CD8-positive NIH 3T3 cells mock infected or infected with TEL-, MN1-, or MN1-TEL I-expressing retroviruses were seeded on culture dishes. Only MN1-TEL I-infected NIH 3T3 cells were not contact inhibited and displayed an aberrant morphology. (B) MN1-TEL I-infected CD8-positive NIH 3T3 cells formed colonies in soft agar. Mock- or TEL-infected cells did not form colonies when plated in agar. The cells were seeded into 0.3% Noble agar at a density of 20,000 per plate and at a serum concentration of 15%. (C) Aliquots of cells transduced with retroviral vectors encoding all the different retroviral constructs (indicated above the lanes) used for soft-agar colony assays were lysed, and 50 μg of protein from each lysate was Western blotted and incubated with α-TEL antibody (top) or α-MN1 antibody (bottom).
To assess the transforming capacities of the various proteins, we tested the retrovirus-transduced cells for growth in soft agar. Cells transduced with MN1-TEL I, but not with TEL or MN1, formed colonies above the background level, demonstrating the transforming potential of MN1-TEL I (Fig. 1B and Table 1). Identical results were obtained in six independent experiments. Besides colonies bigger than 150 μm in diameter, as generally presented in the literature, we also show the number of smaller colonies, as it may indicate some phenotypic differences among the mutants. We also analyzed the transforming capacity of MN1-TEL II. Surprisingly, cells transduced with MN1-TEL II did not form colonies in soft agar in four independent experiments. To assure that each protein was expressed at a similar level in the transduced cells, 50 μg of protein from cellular lysates was Western blotted, and the mutant proteins were visualized with an α-TEL antibody directed against the last 10 amino acids of TEL (Fig. 1C, top) or with α-MN1 antibody (Fig. 1C, bottom). All proteins were expressed at similar levels, suggesting that the PNT domain may be important for transformation by MN1-TEL in this assay system.
TABLE 1.
Colony formation in soft agar of NIH 3T3 cells ectopically expressing MN1, TEL, and MN1-TEL
| Transduced retroviral cDNA | No. of coloniesa
|
|||||
|---|---|---|---|---|---|---|
| ≥150-μm diam
|
Total
|
|||||
| Mean | SD | n | Mean | SD | n | |
| Mock | 4 | 4 | 6 | 21 | 10 | 4 |
| TEL | 1 | 3 | 3 | 1 | 1 | 2 |
| MN1 | 0 | 0 | 2 | 5 | 2 | 2 |
| MN1-TEL I | 49 | 5 | 6 | 97 | 11 | 3 |
| MN1-TEL II | 0 | 0 | 4 | 8 | 2 | 4 |
| MN1-TEL IΔ18-1123 | 10 | 3 | 2 | 22 | 0 | 1 |
| MN1-TEL IΔ12-228 | 0 | 0 | 3 | 0 | 0 | 2 |
| MN1-TEL IΔ18-454 | 5 | 3 | 2 | 17 | 7 | 2 |
| MN1-TEL IΔ12-951 | 1 | 0 | 3 | 2 | 2 | 2 |
| MN1-TEL IΔ692-1123 | 8 | 1 | 3 | 24 | 11 | 3 |
| MN1-TEL IΔ229-1223 | 14 | 5 | 3 | 46 | 1 | 3 |
| MN1-TEL I DBDM | 8 | 4 | 5 | 45 | 14 | 3 |
| VP16-TEL | 4 | 0 | 2 | 22 | 0 | 1 |
Number of colonies formed in 0.3% Noble agar Iscove's medium (15% serum) resulting from 20,000 cells per plate. Mean and SD represent the mean number of colonies and standard deviation calculated from independent experiments (n). Each experiment was performed in triplicate.
Sequences of MN1-TEL necessary for transformation of NIH 3T3 cells.
To determine the sequences of MN1-TEL necessary for transformation of NIH 3T3 cells, we tested a series of MN1-TEL I deletion constructs (schematically presented in Fig. 2). Cells expressing mutant MN1-TEL I proteins were assayed for colony formation in semisolid medium. Deletion of most MN1 sequences (MN1-TELΔ18-1123) abolished the transforming activity of MN1-TEL I (Table 1). More subtle deletions showed that removal of amino acids 12 to 228, 18 to 454, or 12 to 951 from the N terminus of MN1-TEL I also eliminated colony formation (Table 1), although the last two mutants were cytoplasmic and therefore not expected to be informative. Removal of amino acids 229 to 1223 greatly reduced the transforming capacity of MN1-TEL I. However, a considerable increase in the number of colonies smaller than the macroscopically visible 150-μm diameter were generated (Table 1, right column), suggesting that this mutant had some, albeit impaired, growth-stimulating potential. A mutant lacking amino acids 692 to 1123 did not form colonies above the background level, indicating that amino acid sequences between 229 and 692 that contain the homopolymeric glutamine stretches negatively influenced the moderate transforming potential of MN1-TELΔ229-1223.
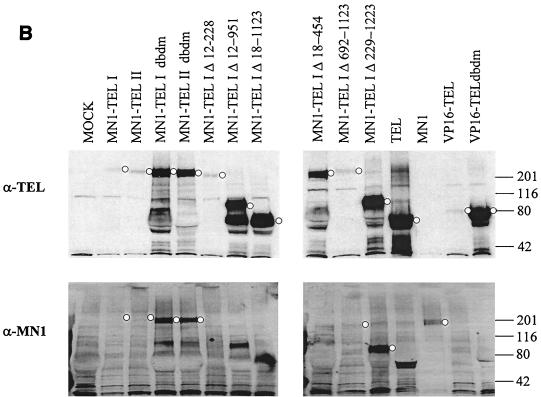
To test whether the DBD of MN1-TEL I is necessary for transformation of NIH 3T3 cells, we introduced a mutated DBD into MN1-TEL I by substitution of arginines for leucines at codons 396 and 399 of the TEL moiety. Although the subcellular expression of the mutant protein was identical to that of wild-type MN1-TEL I (see Fig. 4I and J), cells transduced with MN1-TEL I DBDM did not form colonies bigger than 150 μm in diameter (Table 1, middle columns). However, like MN1-TEL IΔ229-1223, MN1-TEL I DBDM gave rise to an increased number of smaller colonies (Table 1, right-hand columns). This result suggested that the DBD is important for MN1-TEL I-mediated transformation of NIH 3T3 cells. The mutant proteins were visualized by Western blot analysis with α-TEL antibody (Fig. 1C, top) or α-MN1 antibody (Fig. 1C, bottom). Some MN1-TEL I deletion mutants could not be visualized using the α-MN1 antibody, as they lacked its epitope (Fig. 1C, bottom).
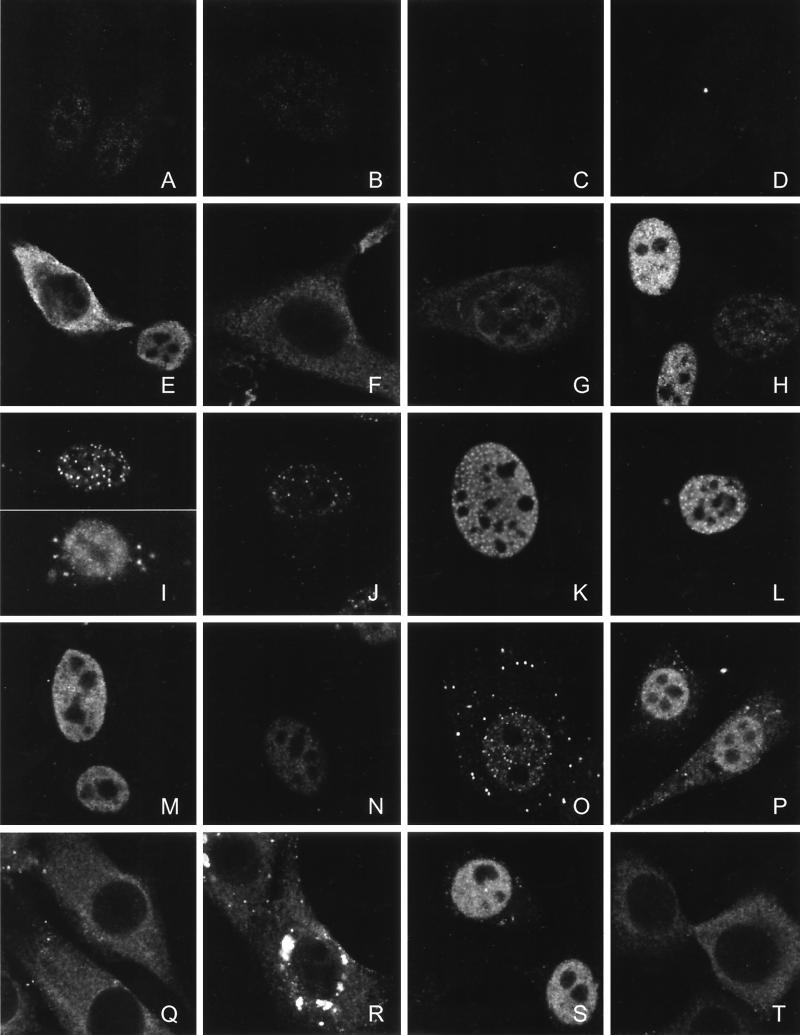
FIG. 4.
Subcellular distribution of endogenous TEL and virus-transduced TEL, MN1, MN1-TEL, and VP16-TEL proteins. (A to C) Indirect immunofluorescence analysis of endogenous TEL using immunopurified α-TEL antibodies in NIH 3T3 cells (A) and HeLa cells (B and C) and analysis of competed α-TEL antibodies using bacterially expressed glutathione S-transferase-TEL fusion protein on HeLa cells (C). (D and H) Endogenous and exogenous expression of MN1 in NIH 3T3 cells. The distributions of virus-transduced TEL, TEL DBDM, and TELΔ53-116 (E to G), MN1-TEL I and MN1-TEL I DBDM (I and J), MN1-TEL II and MN1-TEL II DBDM (K and L), deletion mutants MN1-TEL IΔ18-1123, -Δ12-228, -Δ692-1123, -Δ229-1223, -Δ18-454, and -Δ12-951 (M to R), and VP16-TEL and VP16-TEL DBDM (S and T) in NIH 3T3 cells were analyzed using α-TEL (E to G, I, J, L, and M to T) or α-MN1(D, H, and K) antibodies. Proteins were visualized with fluorescein isothiocyanate-conjugated secondary antibody. The images were obtained by using confocal microscopy. The signals of panels A to D have been electronically amplified.
VP16-TEL does not transform NIH 3T3 fibroblasts.
To test whether MN1 confers transforming activity on TEL by addition of a strong transactivating domain, we replaced the MN1 sequences in MN1-TEL I by the acidic transactivating domain of the HSV-1 VP16 protein. The VP16-TEL fusion protein is similar in structure to VP16-FLI1, which transforms NIH 3T3 cells (32). However, as shown in Table 1, VP16-TEL failed to transform NIH 3T3 cells, despite the fact that the protein was expressed at a level similar to that of MN1-TEL I (Fig. 1C, top), indicating that addition of a strong heterologous transactivating domain alone is insufficient to confer transforming ability on TEL.
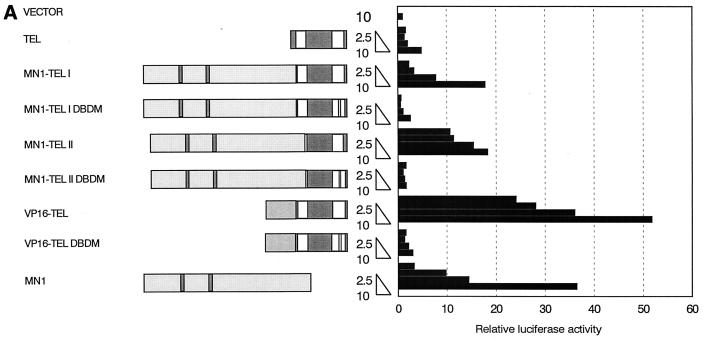
MN1-TEL transactivates the MSV LTR and a synthetic promoter containing TEL binding sites.
MN1 and TEL contribute features to MN1-TEL that are necessary for transformation of NIH 3T3 cells. We asked whether addition of MN1 (including its proline- and glutamine-rich regions) to the DBD of TEL would influence TEL's transcriptional activity. Because the MSV LTR is regulated by ETS-1 (22), we reasoned that it could also be a target of transcription regulation by TEL. This promoter linked to the luciferase gene (pMSVluc), cotransfected with increasing amounts of CMV promoter-driven TEL cDNA, resulted in a minimal (i.e., fourfold) activation of luciferase expression (Fig. 3A). However, the MN1-TEL I and II fusion proteins induced luciferase activity in a dose-dependent manner (up to 18-fold). Cotransfection of the MN1-containing construct with pMSVluc also led to dose-dependent induction of luciferase expression (Fig. 3A). These results indicated that MN1 contributed transactivating sequences to TEL and that MN1 alone can upregulate transcription. In addition, transactivation by MN1-TEL was dependent on a functional DBD of TEL, since MN1-TEL I DBDM and MN1-TEL II DBDM failed to induce expression of luciferase from the pMSVluc construct (Fig. 3A). We used the VP16-TEL expression construct as a positive control for TEL-mediated activation of pMSVluc. As shown in Fig. 3A, VP16-TEL strongly induced the expression of luciferase. Because the DNA binding mutant VP16-TEL DBDM was expressed in the cytoplasm (see Fig. 4T), we have no formal proof that transactivation induced by VP16-TEL is dependent on the DBD of TEL.
FIG. 3.
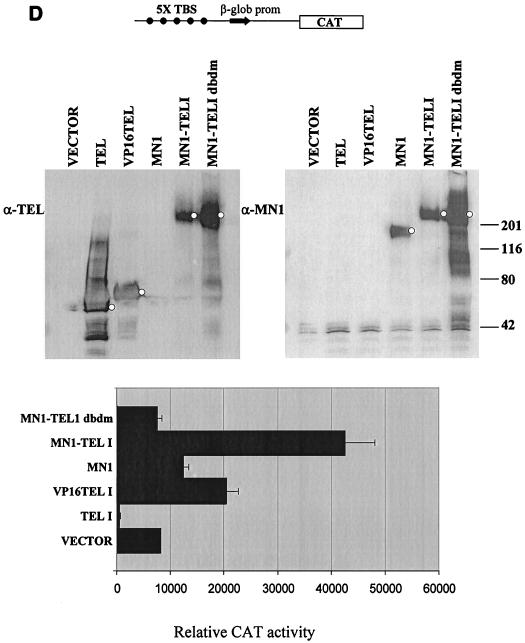
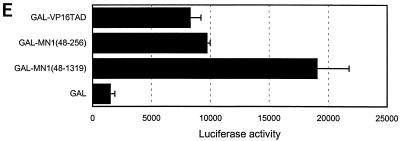
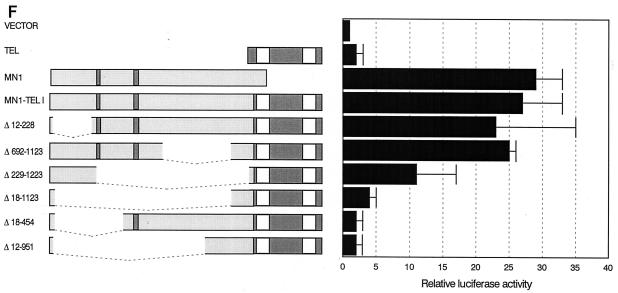
MN1 contributes transcription-activating sequences to TEL. (A) Transient-transfection experiments were performed using increasing amounts of CMV promoter-driven TEL, MN1, MN1-TEL, and VP16-TEL activator constructs (2.5 to 10 μg), as well as their respective TEL DBD mutants, with 1 μg of pMSVluc in NIH 3T3 cells. Luciferase assays were performed 24 h after removal of the calcium phosphate precipitate. The induction of luciferase (normalized to a secreted alkaline phosphatase control) is shown relative to the value of an empty vector. (B) Protein lysate (50 μg) of NIH 3T3 cells transfected in transient-transfection experiments (indicated above the lanes) was Western blotted and incubated with an α-TEL antibody (upper two blots) followed by an α-MN1 antibody (lower two blots). Bands of interest are indicated by small white circles. (C) Transient-transfection experiments using 5 μg of pCDNA3-based MN1 activator construct with 1 μg of pMSVluc in Hep3B cells. Luciferase assays were performed 40 h after removal of the calcium phosphate precipitate. The induction of luciferase is shown relative to the value of an empty vector. The mean values (+ standard deviations) of two experiments are shown. Each transfection was done in duplicate. (D) Transient-transfection experiments were performed using 1 μg of CMV promoter-driven TEL, MN1, MN1-TEL I, MN1-TEL I DBDM, and VP16-TEL activator constructs with 2 μg of 5× TEL-CAT reporter construct that contains a minimal β-globin promoter (β-glob prom) preceded by five concatemerized TEL binding sites (TBS) (CCGGAAGT) (top). In the middle is shown the relative protein expression of the different constructs used in the transient-expression assays. Each lane was loaded with 50 μg of NIH 3T3 cell lysate after transfection of each of the different effector plasmids. After Western blotting, the membrane was incubated with α-TEL antibody (left) followed by incubation with the α-MN1 antibody (right). Bands of interest are indicated by small white circles. On the bottom are shown the relative transactivations of the CAT reporter by the different constructs indicated on the left. CAT assays were performed 48 h after transfection. The values were corrected for SEAP activity derived from a cotransfected SEAP plasmid. The mean values (+ standard deviations) of four different experiments are shown. (E) Transient-transcription experiments using 0.5 μg of pCDNA3-based GAL4 DBD MN1 and VP16 fusion activator constructs with 0.5 μg of adenovirus E4 minimal-promoter-based luciferase reporter construct containing 5× GAL-responsive elements in Hep3B cells. MN1 cDNA sequences encoding amino acids 48 to 256 and 48 to 1319 or the transactivating domain of VP16 (VP16TAD) were expressed as GAL4 DBD fusion proteins. The mean values (+ standard deviations) of at least three experiments are shown. (F) Transient-transfection experiments using 3 μg of CMV promoter-driven activator constructs in NIH 3T3 cells to analyze which domains in the MN1 moiety of MN1-TEL I mediate transactivation of the MSV LTR. Normalized luciferase values relative to an empty vector are shown. The mean values (+ standard deviations) of two experiments are shown. Each transfection was performed in triplicate.
The expression of the different proteins in the transient-transfection assays was also tested. The equivalent of 50 μg of protein from each of the extracts of cells transfected with 10 μg of plasmid DNA was Western blotted, and the proteins were visualized using α-TEL and α-MN1 antibodies. As shown in Fig. 3B, the levels of MN1-TEL I, MN1-TEL II, and VP16-TEL expression were similar in these experiments, but they were much lower than the level of expression of MN1-TEL DBDM I and II and MN1 (four- to fivefold), which in turn was lower than that of VP16-TEL DBDM (twofold). These differences in expression occurred despite the fact that the SEAP activities in the different samples were similar. Taking the protein expression data into account, MN1's transactivating activity is less potent than that suggested in Fig. 3A and is lower than that of MN1-TEL but is still considerable.
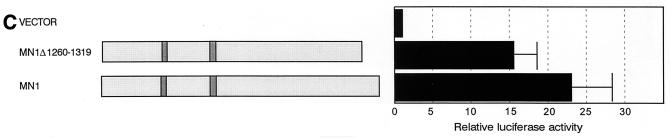
Unlike MN1 and MN1-TEL, MN1-TEL DBDM failed to induce luciferase expression (Fig. 3A). We therefore analyzed the transactivating potential of an MN1 deletion mutant, MN1Δ1260-1319, on the MSV LTR. This construct represents the MN1 sequences that are present in the MN1-TEL fusion. MN1Δ1260-1319 induced luciferase expression of the MSV LTR when transiently transfected into Hep3B cells (Fig. 3C) and NIH 3T3 cells (data not shown). These results indicated that the MN1-specific sequences contained within MN1-TEL DBDM have transactivating activity, which was inhibited by fusion to the mutated DBD of TEL.
We also tested whether MN1-TEL could transactivate a minimal promoter that was preceded by a set of five concatemerized TEL binding sites (Fig. 3D, top). The minimal promoter consisted of the TATA box and the cap site of the rabbit β-globin promoter linked to a CAT reporter gene. We determined the consensus TEL binding site, CCGGAAGT, by using selection and amplification of high-affinity binding sites from a pool of random oligonucleotides (not shown). Our consensus site was very similar to the one published recently (49). We tested the transactivating activities of MN1-TEL I, MN1-TEL I DBDM, TEL, MN1, and VP16-TEL on this promoter. The expression of the proteins during these transient-transcription experiments was analyzed (Fig. 3D, middle). MN1-TEL I was expressed at a level two- to threefold lower than those of VP16-TEL, TEL, and MN1-TEL I DBDM but at the same level as MN1. When the levels were corrected only for SEAP activity and not for protein expression levels, the promoter was substantially activated by VP16-TEL (3-fold) and MN1-TEL (5.5-fold) and less than 2-fold by MN1 (Fig. 3D, bottom). Consistent with what has been published for similar minimal promoter constructs (34), TEL inhibited the background transcription activity of this reporter and the MN1-TEL I DBDM mutant had no transactivating activity. This experiment showed that MN1-TEL transactivated this reporter via binding to the Tel consensus sites and that MN1 confers strong transcription-activating potential on TEL. MN1 alone showed a weak activating effect on basal transcription. This effect was very different from MN1's much stronger transactivation of the MSV LTR, suggesting that the latter was caused by intimate interaction of MN1 with the LTR, either by direct binding to specific sequences in the LTR or via interaction with a transcription factor that binds to the LTR.
To obtain experimental support for this concept, we fused MN1 to the GAL4 DBD and tested whether it transactivated adenovirus E4 minimal-promoter-based luciferase reporter supplemented with five GAL4-responsive elements in Hep3B cells. As shown in Fig. 3E, the GAL4 DBD fused to the transactivation domain of VP16 (GAL-VP16TAD) activated this reporter fivefold. A construct encoding the N-terminal MN1 amino acids 48 to 256 [GAL-MN1(48-256)] induced luciferase activity to a level similar to that induced by GAL-VP16TAD. A construct expressing a fusion containing all but the N-terminal 48 amino acids of MN1 [GAL-MN1(48-1319)] produced an 11-fold increase in luciferase activity, confirming that direct tethering of MN1 to a promoter potentiates its transactivating activity.
Distinct domains in MN1 mediate the transactivating capacity of MN1-TEL.
To map the sequences in MN1-TEL that transactivated the MSV LTR, we tested several MN1-TEL I deletion mutants in transient-transfection assays in NIH 3T3 cells (Fig. 3F). The relative protein expression of the mutants in these experiments is shown in Fig. 3B. MN1-TEL I, MN1-TEL II, MN1-TELΔ12-228, MN1-TELΔ692-1123, and VP16-TEL were expressed at similar low levels, whereas all other mutants were expressed at much higher levels.
Deletion of most of the MN1 moiety of MN1-TEL I (MN1–TELΔ18-1123) abolished the transactivation activity by the fusion protein (Fig. 3F). The MN1–TELΔ12-228 and MN1–TELΔ692-1123 constructs induced expression of luciferase, indicating that sequences spanning the glutamine stretches are necessary for the transactivating potential of MN1-TEL. Because MN1–TELΔ12-228 transactivated the MSV LTR but failed to transform NIH 3T3 cells, sequences within the first 228 amino acids of MN1-TEL may be essential for its transforming activity. A mutant containing this domain (MN1–TELΔ229-1223) moderately induced luciferase gene expression. Considering the fact that this mutant was expressed at a level fivefold higher than that of MN1-TEL, this indicated that these sequences possessed weak transactivation activity, which coincided with an increased number of microscopic colonies in the transformation assays induced by the molecule. Linking a domain similar to the GAL4 DBD [GAL-MN1(48-256)] and using a GAL4 reporter plasmid confirmed the presence of transactivating sequences within this N-terminal domain (Fig. 3E). Therefore, the sequences of MN1 contributing to transactivation of the MSV LTR can be divided into two subdomains, the first of which (amino acids 12 to 228) is essential for transformation while the other (amino acids 229 to 692) is required only for transactivation when analyzed in this experimental setting.
Subcellular distribution of TEL, MN1, and MN1-TEL.
To support our structure-function analyses, we determined the subcellular localization of the various TEL, MN1, and MN1-TEL proteins by indirect immunofluorescence analysis. Endogenous TEL was found predominantly in the nucleus (excluding the nucleoli) of NIH 3T3 and HeLa cells, but some protein was also detected in the cytoplasm (Fig. 4A and B). The specificity of the TEL peptide antibody was verified by competition with bacterially expressed glutathione S-transferase-TEL (Fig. 4C). By contrast, no endogenous MN1 could be detected in NIH 3T3 cells, using MN1-specific antibodies (Fig. 4D). Exogenously expressed TEL was detected in the nucleus (excluding the nucleoli) or cytoplasm or in both subcellular compartments in NIH 3T3 cells transduced with a TEL-containing retroviral vector (Fig. 4E). Interestingly, the ETS DBD mutant, TEL DBDM, was expressed exclusively in the cytoplasm (Fig. 4F). We do not know whether the arginine-to-leucine substitutions directly targeted the nuclear localization signal (NLS) of TEL or whether aberrant folding of the protein prevented nuclear transfer by masking its NLS. The deletion mutant TELΔ53-116 (which lacks most of the PNT oligomerization domain) was expressed in both the cytoplasm and the nucleus (Fig. 4G).
Exogenous MN1 was diffusely present throughout the nucleus, excluding the nucleoli (Fig. 4H), as was the deletion mutant MN1Δ1260-1319 (not shown). In contrast, α-TEL antibodies identified speckles of MN1-TEL I predominantly in the nucleus in some of the cells, while in other cells the protein was more diffusely located in the nucleus (Fig. 4I). The same pattern was found for MN1-TEL I DBDM (Fig. 4J). MN1-TEL II and MN1-TEL II DBDM were expressed in a similar pattern with α-MN1 or α-TEL antibodies, respectively (Fig. 4K and L). In double-labeling experiments, signals obtained with α-MN1 and α-TEL of MN1-TEL-transduced cells were completely overlapping (data not shown).
We then analyzed the localization of the MN1-TEL I deletion constructs. The MN1-TELΔ18-1123 (Fig. 4M), MN1-TELΔ12-228 (Fig. 4N), MN1-TELΔ692-1123 (Fig. 4O), and MN1-TELΔ229-1223 (Fig. 4P) constructs were all solely or predominantly expressed in the nucleus. In contrast, MN1-TELΔ18-454 and MN1-TELΔ12-951 were expressed in the cytoplasm. MN1-TELΔ12-951 displayed diffuse cytoplasmic staining (Fig. 4Q), whereas MN1-TELΔ18-454 was also expressed in large perinuclear plaques (Fig. 4R). VP16-TEL was present in the nucleus (Fig. 4S), but VP16-TEL DBDM (like TEL DBDM) localized to the cytoplasm (Fig. 4T). These results demonstrate that MN1-TEL is expressed diffusely in the nucleus and in distinct speckles, supporting the possibility that MN1-TEL may act as an aberrant transcription regulator. Furthermore, analysis of the subcellular localization of the deletion mutants was a crucial control for the correct interpretation of our functional assays. Despite the presence of an alleged NLS in the DBD, as this region is highly conserved between ETS factors and is essential for the nuclear localization of ETS-1 (3, 52), some mutants did not localize to the nucleus and therefore were not expected to be active in transcription assays.
The PNT domain in MN1-TEL does not mediate homotypic interactions.
The PNT domain defines a specific protein interaction interface that mediates oligomerization of TEL (24). It also mediates oligomerization of TEL-ABL and TEL-platelet-derived growth factor β receptor fusion proteins, which is essential for the activation of their intrinsic tyrosine kinase activity (5, 21, 47). Although the junction in MN1-TEL I occurs 5′ of the PNT domain, the fusion in MN1-TEL II occurs within the PNT domain. This suggests that homotypic interaction of the PNT domain could be functionally impaired in MN1-TEL I.
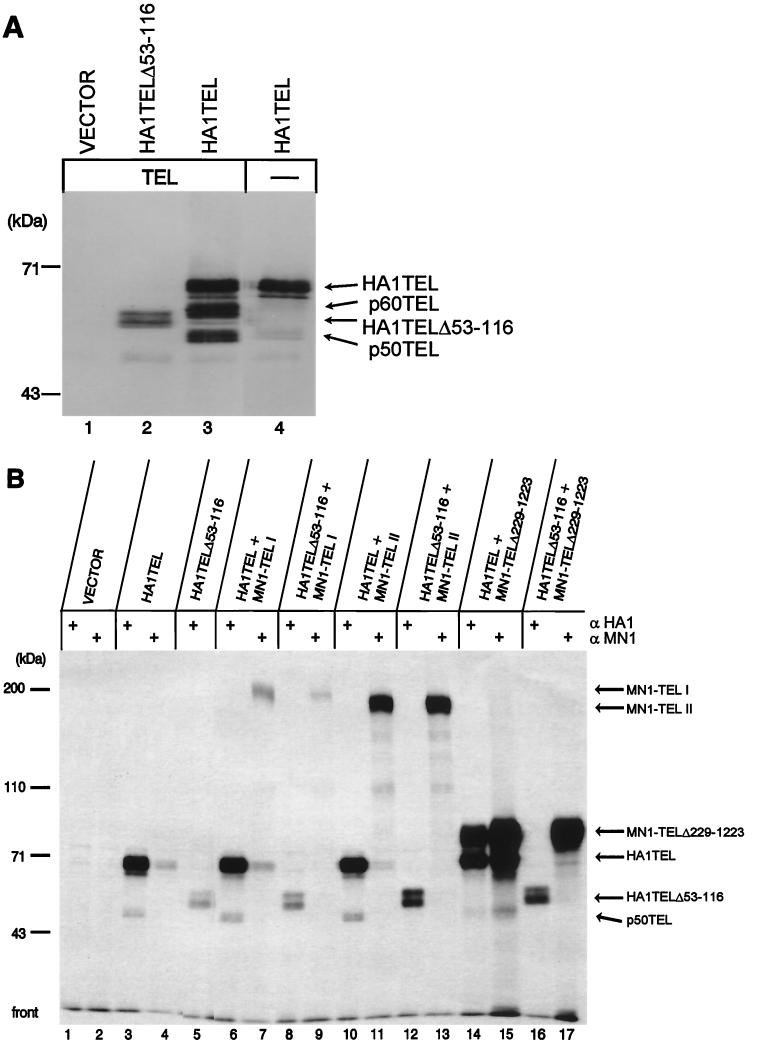
To visualize TEL's homotypic interaction, we used the HA1-specific MAb to immunoprecipitate complexes from HeLa cells cotransfected with expression plasmids encoding TEL and HA1-tagged TEL or HA1-tagged TELΔ53-116. The subcellular localizations of the proteins were identical in transiently transfected HeLa cells and in virus-transduced NIH 3T3 cells (data not shown). Immunoprecipitated complexes using α-HA1 were separated on an SDS–10% polyacrylamide gel and electroblotted. By using Western blot analysis with α-TEL serum, a doublet of 67 kDa (p67HA1TEL [Fig. 5A, lane 3]) and 58 kDa (p58HA1TELΔ53-116 [Fig. 5A, lane 2]) was recognized. The identities of p67HA1TEL and p58HA1TELΔ53-116 were confirmed by subsequent Western blot analysis with α-HA1 (data not shown). Furthermore, only p67HA1TEL coprecipitated TEL (a doublet of 60 kDa and a doublet of 50 kDa [Fig. 5A, lane 3]), detected with α-TEL antiserum. To determine whether HA1TEL coimmunoprecipitated endogenous TEL from HeLa cells, a similar experiment was performed in which only HA1TEL was transfected. Proteins of 60 and 50 kDa were detected by α-TEL serum (Fig. 5A, lane 4). Our results suggest that there may be multiple modified forms of two distinct TEL proteins, which is in accordance with observations reported by Poirel, Bernard, and coworkers (43).
FIG. 5.
TEL's PNT oligomerization domain is nonfunctional in MN1-TEL. (A) HeLa cells were transiently transfected with expression plasmids encoding HA1TELΔ53-116, HA1TEL, and TEL as indicated above the lanes. Proteins were immunoprecipitated with α-HA1. Complexes were separated on an SDS–10% polyacrylamide gel and electroblotted. The proteins were visualized by Western blot analysis with α-TEL antiserum. HA1TELΔ53-116, HA1TEL, and coprecipitating proteins are indicated by arrows on the right. A molecular mass standard is on the left. (B) COS-1 cells were (co)transfected with expression plasmids encoding HA1TEL or HA1TELΔ53-116 and MN1-TEL I, MN1-TEL II, and MN1-TEL IΔ229-1223 as indicated above the lanes. Following metabolic labeling with [3H]leucine, the proteins were immunoprecipitated with α-HA1 followed by immunoprecipitation with α-MN1 and analyzed on an SDS–polyacrylamide gel. The immunoprecipitates were visualized by autoradiography. HA1TEL, HA1TELΔ53-116, and coimmunoprecipitating proteins are indicated by arrows on the right. A molecular mass standard is on the left. +, present; −, absent.
To study whether the PNT domain in MN1-TEL I is functional, we performed a similar cotransfection experiment. COS-1 cells were (co)transfected with either HA1TEL or HA1TELΔ53-116 in the presence of MN1-TEL I, MN1-TEL II, or MN1-TEL IΔ229-1223. The cells were labeled with [3H]leucine, and HA1-tagged proteins were immunoprecipitated with α-HA1 (Fig. 5B). Interestingly, in COS-1 cells only endogenous p50TEL was coprecipitated with transfected human HA1-tagged TEL (Fig. 5B, lanes 3, 6, 10, 14, and 15). In cells cotransfected with HA1TEL and MN1-TEL I, no 200-kDa MN1-TEL I was immunoprecipitated with α-HA1 (Fig. 5B, lane 6). This was not caused by a lack of MN1-TEL I expression, because in a sequential immunoprecipitation of the lysate with α-MN1, MN1-TEL I protein was precipitated (Fig. 5B, lane 7). Similarly, HA1TEL did not coimmunoprecipitate MN1-TEL II (Fig. 5B, lane 10). These results indicate that although the PNT domain is present in MN1-TEL I, it did not physically interact with HA1TEL or with endogenous simian TEL, possibly due to steric hindrance by the bulky MN1 moiety of the fusion protein. To test this possibility, we analyzed whether a substantial deletion of MN1 sequences would allow interaction of MN1-TEL I with HA1TEL and cotransfected HA1TEL and MN1-TEL IΔ229-1223. Endogenous simian p50TEL and a protein of the expected size of MN1-TELIΔ229-1223 (approximately 75 kDa) were coprecipitated with HA1TEL (Fig. 5B, lane 14). Similarly, α-MN1 precipitated MN1-TEL I Δ229-1223, p50TEL, and HA1TEL (Fig. 5B, lane 15). As expected, transfected HA1TELΔ53-116 did not coimmunoprecipitate MN1-TEL IΔ229-1223 with α-HA1 (Fig. 5B, lane 16). In addition, p50TEL did not coprecipitate with MN1-TEL IΔ229-1229 with α-MN1 (Fig. 5B, lane 17), suggesting that the affinity of this deletion mutant for endogenous simian TEL is low and that the PNT domain within HA1TEL mediates the formation of a trimeric complex with p50TEL and MN1-TEL IΔ229-1223. Overall, these results indicate that homotypic interaction via the PNT domain has been significantly reduced or eliminated in the MN1-TEL I fusion protein.
DISCUSSION
We studied the transforming and transactivating potentials of MN1-TEL, encoded by t(12;22)(p13;q11), which is associated with human myeloid leukemia. We used a modified retroviral transduction system to analyze MN1-TEL's transforming potential in NIH 3T3 cells. This method allows for selection of infected NIH 3T3 cells on the basis of cell surface expression of murine CD8 (23). To show the transforming activity of MN1-TEL, we used an NIH 3T3 subline that is sensitive to transformation by ETS factors (36). This line is likely to contain an activating mutation in a pathway downstream of RAS (L. van Rompaey and G. Grosveld, unpublished results) that cooperates with MN1-TEL in transformation. Standard NIH 3T3 cells cannot be transformed by MN1-TEL, as they cannot be transformed by EWS-FLI1 (32, 36, 37). We only consider colonies of ≥150-μm diameter truly transformed, because such colonies are obtained when the cells are transduced with a retroviral vector encoding oncogenic RAS. In addition, smaller colonies (<150-μm diameter) appear, and their numbers increase as the number of true transformants increases. Although they do reflect an increased growth capacity of a certain mutant, we do not consider these colonies transformed. We are confident that the NIH 3T3 subline reflects the transforming activity of MN1-TEL because bone marrow cells transduced with MN1-TEL retroviral vectors show a dramatically increased self-renewal capacity (van Rompaey and Grosveld, unpublished), confirming that MN1-TEL also has growth-promoting potential in another assay system. Surprisingly MN1-TEL type II did not induce colony formation of NIH 3T3 cells in soft-agar assays. MN1-TEL type II may fail to induce transformation as a result of changes in the secondary or tertiary structure of the protein. This may cause MN1-TEL I and II to differ in their abilities to interact with factors essential for colony formation of the NIH 3T3 cells in soft-agar assays. These factors cannot include TEL, because MN1-TEL was inhibited in its PNT-mediated homotypic interaction with TEL, which eliminated the possibility that TEL positively contributes to the transforming activity of MN1-TEL. Differences in potencies of colony formation in soft-agar assays are not unique to MN1-TEL isoforms because similar differences have been reported for the two alternative E2A-PBX1 chimeric proteins, associated with t(1;19) in pre-B ALL. The two forms, p85 and p77, display different levels of tumorigenicity in different assays. NIH 3T3 cells expressing either isoform were equally potent in tumor formation in nude mice, but only the p77 variant efficiently produced colonies in soft-agar assays (25). As mentioned above, we are assessing the transforming potential of MN1-TEL I and II in other assay systems, such as transgenic mice and retroviral-vector-transduced bone marrow. These approaches may reveal whether the two molecules indeed display a difference in transforming activity or whether the results of our soft agar assays reflect the limits of this test system.
Recently, it was determined that MN1 functions as a strong transcriptional coactivator (E. Zwarthoff, unpublished results), explaining its strong upregulation of the MSV LTR. Thus, fusion of this molecule to TEL changes it from a repressor (on the 5× TEL-CAT reporter) or a weak activator (on the pMSV-Luc reporter) into a transcription factor with strong transactivating activity, which is in accordance with the results presented here. This change is similar to that caused by the fusion of EWS to FLI1 (37) or of FKHR to PAX3 (2). Due to these similarities, it is important to determine whether it is just the addition of strong transactivating sequences that renders these fusion proteins oncogenic or whether the addition of sequences with other activities is also important. Analysis of EWS-FLI1 and PAX3-FKHR suggests that both transcriptional and additional activities are important (30, 32). Deletion of the N-terminal MN1 sequences (amino acids 12 to 228) from MN1-TEL abolished its transforming activity. On the other hand, fusion of these sequences alone to TEL (MN1-TEL IΔ229-1223) was not sufficient to render the fusion protein fully transforming and resulted in a threefold reduction in the number of colonies in soft-agar assays. When correlating the transforming activity of MN1-TEL mutants with their transactivating activity, the two activities seem to overlap only partially. In contrast with its effect on transformation, deletion of amino acids 12 to 228 had a minor impact on transient transactivation of the MSV LTR. Fusion of these sequences alone to TEL, or of a comparable fragment to the GAL4 DBD, confirmed their moderate transactivation activities. Because the fragment did display transactivating activity, we cannot exclude the possibility that this activity contributes to transformation. The sequences with the strongest transactivating activities in the context of the MSV LTR were comprised within amino acids 228 to 692. These sequences contain two glutamine stretches and proline-rich sequences that might function as transactivators (31). Glutamine stretches can form β-sheets that mediate protein-protein interaction by functioning as polar zippers (42, 48), and such sequences in SP1 have been demonstrated to interact with the basal transcription machinery (18). Interestingly, the presence of this strong activation domain in MN1-TEL is not sufficient for transformation because the transforming activity of MN1-TEL IΔ12-228 or -Δ692-1123 was abolished or considerably diminished, respectively. The notion that addition of a strong transactivating sequence alone to TEL is insufficient to render it transforming is further supported by our VP16-TEL mutant, which strongly transactivated the MSV LTR but failed to transform NIH 3T3 cells. We conclude that MN1 does not contribute to transformation solely by the addition of its transactivating sequences to TEL but also by the addition of sequences that are not involved in transcription control. This conclusion is further supported by the fact that sequences contained within amino acids 692 to 1123 contributed to MN1-TEL's transforming activity while their deletion had little if any negative effect on the transactivation activity of MN1-TEL. We hypothesize that these sequences could mediate interaction with other proteins that directly or indirectly affect normal cell cycle arrest via cell-cell contact inhibition. Further studies are required to verify this hypothesis. We infer that similar interactions might also be responsible for the fact that MN1-TEL I DBDM maintained partial transforming activity despite the fact that the molecule would be unable to bind to TEL DNA binding sites. We think that this explanation is more likely than the possibility that the fusion protein would still be capable of coactivating transcription factors to which MN1 is normally recruited, because MN1-TEL1 DBDM failed to transactivate the MSV LTR. It is likely that the fusion of TEL to MN1 has a major effect on the folding of the MN1 moiety and hence prevents its recruitment by transcription factors with which MN1 normally interacts.
We confirmed that TEL interacts with itself via the PNT domain (24). In contrast, no heterodimerization between MN1-TEL and TEL was observed, suggesting that the PNT homotypic oligomerization domain in MN1-TEL is nonfunctional. This may be of functional significance, because the PNT-mediated homotypic interaction is necessary for the recruitment of the transcriptional corepressors mSin3A, SMRT, and N-CoR (8, 13, 34). Therefore, TEL will not be able to attenuate the transcriptional activity of MN1-TEL via the recruitment of corepressors and histone deacetylases and may represent an additional level at which the fusion protein evades normal regulation.
In conclusion, our data demonstrate that TEL obtains transforming activity by fusion to MN1, and the functions that contribute to this activity are a gain in transactivating activity, binding to DNA, and other as-yet-undefined functions of MN1.
ACKNOWLEDGMENTS
We thank J. Boer and M. Fornerod for many helpful discussions of the work, R. Ashmun for fluorescence-activated cell sorter analysis, L. Shapiro for the pGL2 luciferase vectors, D. Bar-Sagi for RASV12, P. O'Hare for pRG50, A. G. Jochemsen for adenovirus E4 minimal promoter, D. Baltimore for the 293T cells, C. Denny for NIH 3T3 cells, D. Afar and O. Witte for the retroviral production protocols, A. Frazier for editing the manuscript, and C. Hill for secretarial assistance.
These studies were supported by the NIH Cancer Center CORE Grant CA-21765 (G.C.G.) and by the Associated Lebanese Syrian American Charities (ALSAC) of St. Jude Children's Research Hospital. G.C.G. and M.F.R. are supported by NCI grants CA72996-03 and CA56819-08, respectively.
REFERENCES
- 1.Bailly R, Bosselut R, Zucman J, Cormier F, Delattre O, Roussel M, Thomas G, Ghysdael J. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol Cell Biol. 1994;14:3230–3241. doi: 10.1128/mcb.14.5.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennicelli J L, Edwards R H, Barr F G. Mechanism for transcriptional gain of function resulting from chromosomal translocation in alveolar rhabdomyosarcoma. Proc Natl Acad Sci USA. 1996;93:5455–5459. doi: 10.1073/pnas.93.11.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boskulos K E, Pognonec P, Rabault B, Begue A, Ghysdael J. Definition of an Ets1 protein domain required for nuclear localization in cells and DNA-binding activity in vitro. Mol Cell Biol. 1989;9:5718–5721. doi: 10.1128/mcb.9.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, Geurts van Kessel A, Riegman P, Lekanne Deprez R, Zwarthoff E, Hagemeijer A, Grosveld G. Translocation (12;22)(p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11. Oncogene. 1995;10:1511–1519. [PubMed] [Google Scholar]
- 5.Carroll M, Tomasson M H, Barker G F, Golub T R, Gilliland D G. The TEL/platelet-derived growth factor β receptor (PDGFβR) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGFβR kinase-dependent signaling pathways. Proc Natl Acad Sci USA. 1996;93:14845–14850. doi: 10.1073/pnas.93.25.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carron C, Cormier F, Janin A, Lacronique V, Giovannini M, Daniel M T, Bernard O, Ghysdael J. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95:3891–3899. [PubMed] [Google Scholar]
- 7.Cazzaniga G, Tosi S, Aloisi A, Giudici G, Daniotti M, Pioltelli P, Kearney L, Biondi A. The tyrosine kinase ab1-related gene ARG is fused to ETV6 in an AML-M4Eo patient with a t(1;12)(q25;p13): molecular cloning of both reciprocal transcripts. Blood. 1999;94:4370–4373. [PubMed] [Google Scholar]
- 8.Chakrabarti S R, Nucifora G. The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSIN3A. Biochem Biophys Res Commun. 1999;264:871–877. doi: 10.1006/bbrc.1999.1605. [DOI] [PubMed] [Google Scholar]
- 9.Chase A, Reiter A, Burci L, Cazanigga G, Biondi A, Pickard J, Roberts I, Goldman J, Cross N. Fusion of ETV6 to the caudal-related homeobox gene CDX2 in acute myeloid leukemia with the t(12;13)(p13;q12) Blood. 1999;93:1025–1031. [PubMed] [Google Scholar]
- 10.Cools J, Bilhou-Nadera C, Wlodarska I, Cabrol C, Talmant P, Bernard P, Hagemeijer A, Marynen P. Fusion of a novel gene, BTL, to ETV6 in acute myeloid leukemias with t(4;12)(q11–q12;p13) Blood. 1999;94:1820–1824. [PubMed] [Google Scholar]
- 11.Dalrymple M, McGeoch D, Davison A, Preston C. DNA sequence of the herpes simplex virus type 1 gene whose product is responsible for transcriptional activation of immediate early promoters. Nucleic Acids Res. 1985;13:7865–7879. doi: 10.1093/nar/13.21.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, Kudoh S, Tanaka K, Setoyama M, Nagamura F, Asano S, Kamada N. Fusion of ETV6 to neurotrophin-5 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93:1355–1363. [PubMed] [Google Scholar]
- 13.Fenrick R, Amann J M, Lutterbach B, Wang L, Westendorf J J, Downing J R, Hiebert S W. Both TEL and AML-1 contribute repression domains to the t(12;21) fusion protein. Mol Cell Biol. 1999;19:6566–6574. doi: 10.1128/mcb.19.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornerod M, Boer J, van Baal S, Jaeglé M, Von Lindern M, Murti K G, Davis D, Bonten J, Buijs A, Grosveld G. Relocation of the carboxyterminal part of CAN from the nuclear envelope to the nucleus as a result of leukemia-specific chromosome rearrangements. Oncogene. 1995;10:1739–1748. [PubMed] [Google Scholar]
- 15.Fornerod M, Boer J, van Baal S, Morreau H, Grosveld G. Interaction of cellular proteins with the leukemia specific fusion proteins DEK-CAN and SET-CAN and their normal counterpart, the nucleoporin CAN. Oncogene. 1996;13:1801–1808. [PubMed] [Google Scholar]
- 16.Gerber H, Seipel K, Georgiev O, Hofferer M, Hug M, Rusconi S, Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994;263:808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- 17.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 18.Gill G, Pascal E, Tseng Z H, Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci USA. 1994;91:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golub T R, Barker G F, Bohlander S K, Hiebert S W, Ward D C, Bray-Ward P, Morgan E, Raimondi S C, Rowley J D, Gilliland D G. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golub T R, Barker G F, Lovett M, Gilliland D G. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 21.Golub T R, Goga A, Barker G F, Afar D E, McLaughlin J, Bohlander S K, Rowley J D, Witte O N, Gilliland D G. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol. 1996;16:4107–4116. doi: 10.1128/mcb.16.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunther C, Nye J, Bryner R, Graves B. Sequence-specific DNA binding of the proto-oncoprotein ETS-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990;4:667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Roussel M F, Kato J, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2671–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jousset C, Carron C, Boureux A, TranQuang C, Oury C, Dusanter-Fourt I, Charon M, Levin J, Bernard O, Ghysdael J. A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL-PDGFRβ oncoprotein. EMBO J. 1997;16:69–82. doi: 10.1093/emboj/16.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamps M P, Look A T, Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991;5:358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- 26.Knezevich S, McFadden D, Tao W, Lim J, Sorensen P. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 27.Kodandapani R, Pio F, Ni C-Z, Piccialli G, Clemsz M, McKercher S, Maki R A, Ely K R. A new pattern for helix-turn-helix recognition revealed by the PU.1 ETS-domain-DNA complex. Nature. 1996;380:456–460. doi: 10.1038/380456a0. [DOI] [PubMed] [Google Scholar]
- 28.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–231. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacronique V, Boureux A, Valle V, Poirel H, Quang C, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard O. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 30.Lam P Y, Sublett J E, Hollenbach A D, Roussel M. The oncogenic potential of the Pax3-FKHR fusion protein requires the Pax3 homeodomain recognition helix but not the Pax3 paired-box DNA binding domain. Mol Cell Biol. 1999;19:594–601. doi: 10.1128/mcb.19.1.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lekanne Deprez R H, Riegman P H, Groen N A, Warringa U L, van Biezen N A, Molijn A C, Bootsma D, de Jong P J, Menon A G, Kley N A, Seizinger B R, Zwarthoff E C. Cloning and characterization of MN1, a gene from chromosome 22q11, which is disrupted by a balanced translocation in a meningioma. Oncogene. 1995;10:1521–1528. [PubMed] [Google Scholar]
- 32.Lessnick S L, Braun B S, Denny C T, May W A. Multiple domains mediate transformation by the Ewing's sarcoma EWS-FLI-1 fusion gene. Oncogene. 1995;10:423–431. [PubMed] [Google Scholar]
- 33.Liu Q, Schwaller J, Kutok J, Cain D, Aster J C, Williams I R, Gilliland D G. Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15)(p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 2000;19:1827–1838. doi: 10.1093/emboj/19.8.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez R G, Carron C, Oury C, Gardellin P, Bernard O, Ghysdael J. TEL is a sequence-specific transcriptional repressor. J Biol Chem. 1999;274:30132–30138. doi: 10.1074/jbc.274.42.30132. [DOI] [PubMed] [Google Scholar]
- 35.Lugo T, Witts O. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989;9:1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May W A, Gishizky M I, Lessnick S L, Lunsford L B, Lewis B C, Delattre O, Zucman J, Thomas G, Denny C T. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA. 1993;90:5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May W A, Lessnick S L, Braun B S, Klemsz M, Lewis B C, Lunsford L B, Hromas R, Denny C T. The Ewing's sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. 1993;13:7393–7398. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller A J, Young J C, Pendergast A, Pondel M, Landau N R, Littman D R, Witte O N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991;11:1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen R D, Bortner D M, Ostrowski M C. RAS oncogene activation of a VL30 transcriptional element is linked to transformation. Mol Cell Biol. 1990;10:1–9. doi: 10.1128/mcb.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulos P, Ridge S A, Boucher C A, Stocking C, Wiedemann L M. The novel activation of ABL by fusion to an ets-related gene. Cancer Res. 1995;55:34–38. [PubMed] [Google Scholar]
- 41.Peeters P, Raynaud S, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, van Rompaey L, Baens M, van der Berghe H, Marynen P. Fusion of TEL, the ETS variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;12;15) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 42.Perutz M F, Johnson T, Suzuki M, Finch J T. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci USA. 1994;91:5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poirel H, Oury C, Caron C, Duprez E, Laabi Y, Romana S, Tsapis A, Machauffe M, Le Coniat M, Berger R, Ghysdael J, Bernard O. The TEL gene products: nuclear phosphoproteins with DNA binding properties. Oncogene. 1997;14:349–357. doi: 10.1038/sj.onc.1200829. [DOI] [PubMed] [Google Scholar]
- 44.Romana S P, Machauffe M, Le Coniat M, Chumakov I, Le Paslier D, Berger R, Bernard O A. The t(12;21) of acute lymphoblastic leukemia results in a TEL-AML1 gene fusion. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- 45.Rowley J. The role of chromosome translocations in leukemogenesis. Semin Hematol. 1999;36:59–72. [PubMed] [Google Scholar]
- 46.Scholer H R, Hatzopoulos A K, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjoblom T, Boureux A, Ronnstrand L, Heldin C H, Ghysdael J, Ostman A. Characterization of the chronic myelomonocytic leukemia associated TEL-PDGFβR fusion protein. Oncogene. 1999;18:7055–7062. doi: 10.1038/sj.onc.1203190. [DOI] [PubMed] [Google Scholar]
- 48.Stott K, Blackburn J M, Butler P J, Perutz M. Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc Natl Acad Sci USA. 1995;92:6509–6513. doi: 10.1073/pnas.92.14.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szymczyna B R, Arrowsmith C H. DNA binding specificity studies of four ETS proteins support an indirect read-out mechanism of protein-DNA recognition. J Biol Chem. 2000;275:28363–28370. doi: 10.1074/jbc.M004294200. [DOI] [PubMed] [Google Scholar]
- 50.Tomasson M H, Williams I R, Hasserjian R, Udomsakdi C, McGrath S M, Schwaller J, Druker B, Gilliland D G. TEL/PDGFβR induces hematologic malignancies in mice that respond to a specific tyrosine kinase inhibitor. Blood. 1999;93:1707–1714. [PubMed] [Google Scholar]
- 51.Van Rompaey L, Dou W, Buijs A, Grosveld G. Tel, a frequent target of leukemic translocations, induces cellular aggregation and influences expression of extracellular matrix components. Neoplasia. 1999;1:526–536. doi: 10.1038/sj.neo.7900064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wasylyk B, Hahn S L, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]