Abstract
The skin shows the physiological condition of the body’s organs and systems that prevent infections and physical damage. Throughout the ages, in folk medicine, phytotherapy was considered a primary form of treatment in all countries, including Kazakhstan, due to the abundance and availability of plant-based remedies. This paper discusses several medicinal plants that are traditionally used in the treatment of skin diseases in the Republic of Kazakhstan. The chemical composition of these plants was analyzed, with a particular focus on the biologically active basic compounds responsible for their therapeutic efficiency in treating skin ailments.
Keywords: medicinal plants, ethnopharmacology, skin diseases, flora of Kazakhstan, atopic dermatitis, plant drugs, anti-inflammatory activity
1. Introduction
According to the eminent scientist, philosopher, and physician Avicenna, “a doctor has three tools: the word, the plant, the knife”. The plant kingdom is recognized as the humanity’s earliest and the most ancient healing source, which people employed to treat and prevent illnesses. Tracing back through history, the most ancient documented proof of plants’ utilization in medicine dates back to a Sumerian clay slab discovered in Nagpur about 5000 years ago. This artifact included a compilation of twelve medicinal recipes that involved over 250 diverse plant species. Sumerian healers prepared powders and therapeutic infusions from plant roots and stems. Pears and figs also possessed healing properties. Additionally, they utilized dried and ground young shoots of willow and plum trees, and pine and fir trees as components in compresses and poultices. Powders from animal and mineral sources were often mixed with ones extracted from dried and crushed plants. Notably, in addition to water, wine and beer were used as solvents. Thus, at least 80 centuries ago, people employed the most simple medicinal plant-based preparations for treatment [1].
Regarding Kazakh folk medicine, it has not yet been fully researched. The traditional medicine of the Kazakh people covers not only the mere treatment of ailments but also rests on robust theoretical knowledge. Oteiboydak Tleukabyluly (1388–1478), a distinguished Kazakh healer who lived in the 15th century, wrote the ethnographic and medical book “Medical Narrative” between the years 1466 and 1473 with az-Zhanibek Khan’s order who held Oteiboydak Tleukabyluly in high esteem as a healer. Oteiboydak Tleukabyluly wrote in his book about the secrets of the healing art. This medical encyclopedia delineates the functions of various organs of the human body and provides a catalogue of the primary diseases associated with them. Furthermore, it includes a meticulous description of the methods used in traditional medicine at present, such as setting bones, listening to the pulse, and incantations. Through practical experimentation and experimentation conducted in the steppe laboratory, the healer formulated a total of 1108 different medicinal compounds, of which 858 were derived from medicinal plants, 318 were extracted from animal organs, and roughly 60 were sourced from metals. The moniker “Teacher without a teacher” was bestowed on Oteiboydak Tleukabyluly who created methods for treating 1050 different diseases [2].
At present, phytotherapy is widely practiced all over the world. According to the World Health Organization’s (WHO) global review of national policies concerning traditional, complementary, and alternative medicine, as well as the regulation of herbal medicines, there is an evident growth in the European and Asian market for herbal medicines [3].
Kazakhstan accounts for a natural flora of over 6000 plant species [4]. The exact number of medicinal plant species present in Kazakhstan remains uncertain as the list continues to expand annually. More than 150 plant species have been employed in both official and folk medicine for various ailments. This review focuses on a selection of medicinal plants growing in the territory of the Republic of Kazakhstan that have traditionally been used to alleviate skin diseases.
This study mainly discusses the plant phytochemical composition. The main components responsible for their therapeutic effects in treating dermatitis, atopic dermatitis, and eczema were analyzed.
1.1. Achillea millefolium L. Aster Family—Asteraceae
Achillea millefolium L., commonly known as common yarrow, belongs to the Asteraceae family (Asteraceae Dumort.). The plant was referred to as “venus eyelashes” during the Middle Ages due to its the feathery appearance of its leaves, while the whole plant was known as “soldier’s grass” for its use in treating wounds. There are over 100 different species of Achillea millefolium L., which are found in various regions worldwide, including North America, Europe, Asia, Australia, New Zealand, and the Middle East [5,6,7,8]. The plant is widespread in Kazakhstan and serves as a valuable source of nectar for honeybees [9].
The main components of A. millefolium are essential oils and phenolic compounds, monoterpenes, sesquiterpenes, lactones [10], amino acids, fatty acids, salicylic and succinic acids, ascorbic acid, folic acid, caffeic acid, and flavonoids [11]. The composition of the essential oil includes sesquiterpenoids: achillin, acetylbalquinolide, caryophyllene, proazulene (chamazulene); and monoterpenoids: camphor, thujol, cineole, pinene, borneol. In addition, alkaloids (the main one of which is achilein), flavonoids, including flavone glycosides apigenin and luteolin were found in the yarrow herb; also found were tannins (α-phylloquinone) and vitamins K, A, and B; amines: choline and stakhidrin; and esters (bornyl acetate, myrtenyl acetate), caryophyllene, organic acids, polyins (pontic epoxide, matrixar ester), cyclic alcohol viburnite (20%), menthol, and geraniol [12,13,14,15,16]. Yarrow also contains sterols, coumarins, the biogenic amine betaine, inulin, and other polysaccharides [17].
The bitter taste of A. millefolium can be attributed to the presence of sesquiterpene lactones in its essential oil. The quantity of essential oil produced by the plant is largely dependent on the growth stage. During the early stage of growth, the content of essential oil is 0.13%, which rises to 0.34% in the process of flowering [15,16].
In traditional medicine, yarrow has been employed to alleviate a variety of ailments including respiratory diseases (such as asthma and bronchitis), dyspepsia, skin inflammation, and headaches. The aerial part of the plant, including the leaves, stems, and inflorescences, is typically collected during the flowering phase for its usage as medicinal raw material. Yarrow is often administered as infusions, extracts, and potions to treat bleeding, flatulence, and gastrointestinal diseases [8,11,18].
A. millefolium possesses various therapeutic properties such as disinfectant, anti-inflammatory, antispasmodic, anthelmintic, antibacterial, antioxidant, and antimicrobial effects [19]. Additionally, the herb demonstrates antiulcer and anticancer activities [20], while the experimental findings suggest that yarrow may stimulate thrombocytopoiesis, leading to an increase in the number of platelets in the blood [21].
Yarrow has long been utilized in traditional medicine as an efficient remedy for various skin ailments, including acne, eczema, neurodermatitis, and urticaria. Moreover, yarrow is incorporated into medicinal preparations for vasculitis. It is administered orally to prevent the recurrence of eczema [18,22,23].
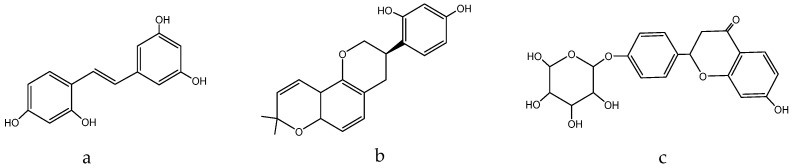
The wound-healing and anti-inflammatory effect of yarrow is due to the content of chamazulene (Figure 1) (12.34%) in it. It is known that chamazulene enhances regenerative processes, weakens allergic reactions, has a local anesthetic effect, adsorbs various poisons, softens the skin, promotes scarring, heals infected wounds, and restores damaged capillaries [24]:
Figure 1.
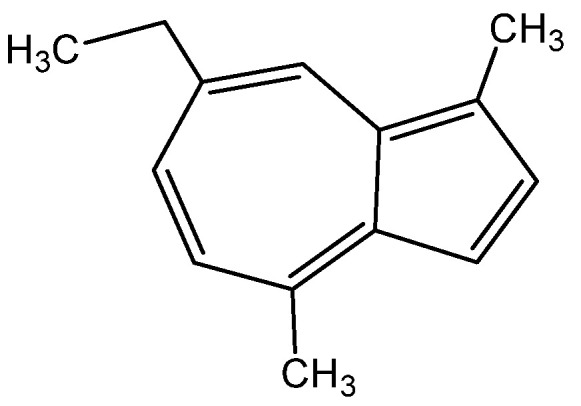
Chamazulene.
The results of the study [21] explain the mechanism of action of A. millefolium. Thus, the effect of the water–alcohol extract of Achillea millefolium (HEAML) on the proliferation and stimulation of the human skin fibroblast growth (HSF-PI-16) was studied. The extract selectively inhibited the proliferation of HSF-PI-16 cells at higher concentrations (>20.0 mg/mL) and were stimulated at lower concentrations (<20.0 mg/mL). After treating the HSF-PI-16 medium for 72 h with the extract, a significantly increased proliferation rate and stimulation in the scratch analysis was noted [25]. The activity of the plant in atopic dermatitis was also investigated. The results showed that Achillea millefolium L. significantly reduces the expression of proinflammatory cytokines in mouse macrophage cells treated with lipopolysaccharide [26].
1.2. Acorus calamus L. Aroid Family—Araceae
Acorus calamus L. (calamus) marsh is a perennial plant containing aromatic compounds and is widespread in Central Asia, India, and the Himalayas. Although its distribution has significantly diminished in Europe, it remains a common plant in the northern marshy regions with a temperate climate [27]. It is found in Asia, Europe, and North America and is known to grow in Central Kazakhstan along the banks of rivers, swamps, and lakes, sometimes forming substantial thickets.
Calamus marsh is rich in various chemical compounds, including bitter glycoside acorin, essential oil (which contains proazulene), gum, resins, ascorbic acid, tannins, starch, and mucus. The dried rhizome of Calamus marsh consists of yellow aromatic volatile oils comprising of small amounts of sesquiterpenes and their alcohols; and choline, flavone, acoradin, galangin, acolamon, and isocolamon. Furthermore, it contains cineol, limonene, terpineol, azulene, eugenol, camphene, cadinene, ethanol, galangin, magnesium, zinc, terpenes, menthol, and camphor [28].
Calamus root is considered in traditional medicine to be a therapeutic agent for a range of ailments, such as arthritis, neuralgia, diarrhea, dyspepsia, and hair loss [27,29].
The plant has been found to possess potent antioxidant, anti-inflammatory, antiulcer, antimicrobial, and wound-healing properties. It is employed in dermatology to cure pyoderma, acne vulgaris, alopecia, and eczema [30,31,32]. The advantageous effect on the skin can be attributed to the presence of β-azarone (Figure 2), a phenylpropanoid class chemical compound:
Figure 2.
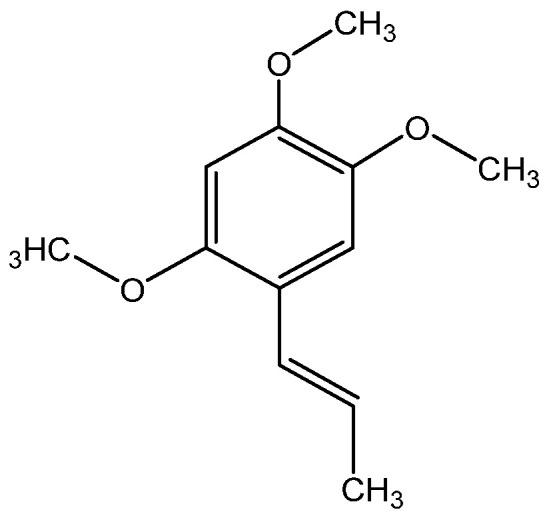
β-Azarone.
β-Azarone is known to contribute to the body’s natural defense against ultraviolet rays, but it has also been found to have carcinogenic properties and induce liver tumors. Calamus marsh, which includes varying amounts of β-azarone depending on the variety, has traditionally been used in Asian medicine for its anti-inflammatory properties, which can help alleviate skin itching, swelling, and redness. Meanwhile, European varieties of Calamus marsh are known to contain sesquiterpenoids, which possess psychoactive properties and display beneficial medicinal effects [33,34,35].
Calamus rhizomes have been found to be very useful as a topical agent in skin-related problems. The rhizomes are used in the form of powder, balms, enemas, and pills and also in ghee preparations. A tub bath in the decoction of vacha, kustha (Savccera lappa) and vidanga (Embelina ribes) is useful in curing eczema and other skin diseases [36].
1.3. Agropyron repens L. Lacquer Family—Gramineae
Agropyron repens L. is distributed widely across Europe, Asia, and Africa [37]. It can be found ubiquitously throughout Kazakhstan [38].
The chemical composition of the plant is rich in a variety of carbohydrates such as fructose, glucose, inositol, and mannitol, as well as mucous substances, pectin, triticin, thianogenic glycosides, flavonoids, saponins, essential oil, monoterpenes (such as carvacrol, carvone, transanethol, thymol, menthol), and sesquiterpenes. Moreover, the plant contains vanillin glucoside, iron, minerals, and significant quantities of silica. Among the phenolic compounds found in the plant are p-hydroxybenzoic, vanillic, and p-coumaric acids, as well as chlorogenic acid, p-hydroxycinnamic acids, and p-hydroxycinnamic acid esters. The rhizomes consist of polysaccharides, glycosides such as quercetin and luteolin, phenolic glucosides, fatty acids, and amino acids (including γ-aminobutyric acid, proline, valine, asparagine, histidine, arginine, and tryptophan) [39,40]. Furthermore, the seeds of wheatgrass contain triticin, mucus, saponins, sugar alcohols (namely, mannitol, inositol, and 2–3% of the total composition), essential oil with polyacetylenes or carvone, a small amount of vanilloside (vanillin), phenol carboxylic acids, silicic acid, and silicates [41].
Agropyron repens L. was used in folk medicine as a sedative diuretic to relieve pain and spasms in the urinary tract, and as a sedative and tonic. The traditional medicinal use of Agropyron repens L. in urolithiasis has been scientifically proved, with confirmed pharmacological effects including hypoglycemic, hypolipidemic, anti-inflammatory, and antidiabetic effects, as well as effects on motility and benefits in urinary tract infections [37,40,41,42,43].
The presence of flavonoids, alkaloids, and coumarin in the composition of this plant evidences its potential activity in the treatment of skin diseases, such as inflammatory skin diseases, atopic dermatitis, and acne [44]. Thus, in the paper [45], the effect of wheatgrass extract in a cream form on some indicators of lipid peroxidation in allergic contact dermatitis was investigated. Contact dermatitis was modeled by the double application of 0.1 mL of a 5% alcohol solution of 2.4-dinitrochlorobenzene (DNCB) on previously depilated skin areas of the lateral surface of the abdomen of experimental animals. The anti-inflammatory activity of the cream was evaluated based on the characteristics of the skin and the state of lipid peroxidation (LPO) processes, i.e., the content of oxidation products in the blood plasma—malondialdehyde (MDA), diene conjugates (DC) and the activity of the antioxidant defense enzyme catalase. According to the studies, the cream containing wheatgrass extract has anti-inflammatory activity and promotes the activation of antioxidant protection (increased catalase activity), which, in its turn, decreases the intensity of lipid peroxidation (MDA and DC levels fall). The cream accelerated recovery for 4–5 days compared to untreated rats. The anti-inflammatory effect of wheatgrass cream was comparable to that of a standard cream with glucocorticoids (Akriderm C).
1.4. Artemisia absinthium L. Aster Family—Asteraceae
Artemisia absinthium L., a plant species commonly known as wormwood, is widely distributed in Asia, the Middle East, Europe, and North Africa. It grows everywhere in Kazakhstan [46,47,48].
A. absinthium is a plant species that possesses various biologically active compounds. The grass of this plant is utilized as a source material for oil production. The oil mainly consists of thujone esters, α- and β-thujone, camphene, α-cadinene, guaiazulene, (Z)-epoxycymene, (E)-sabinyl acetate, (Z)-chrysanthenyl acetate, as well as bitter sesquiterpenoid lactones, azulene group compounds, and tannins [49]. Moreover, it contains terpenoids (such as myrcene, germacrene D, camphor, chamazulene), flavonoids (quercetin, kaempferol, apigenin, artemetin, and rutoside), phenolic acids (chlorogenic, ferulic, gallic, coffee, syringic, vanillic, and caffeoylquinic acid derivatives), and flavonoid glycosides [50]. The composition of the A. absinthium extract is dependent on the type of solvent utilized in the extraction process. The alcoholic extract, in particular, has a considerably higher concentration of flavonoids, phenols, and tannins in comparison to the aqueous and chloroform extracts [49].
For many years, A. absinthium has been used in traditional medicine to cure a wide range of ailments, particularly parasitic diseases and digestive disorders, as well as fever reduction [51]. The leaves are employed to alleviate fever, while the flowers are used to treat stomach disorders and helminthiasis. The A. absinthium tincture is highly esteemed as a tonic and digestive aid. In a published paper [52], the wormwood herb was noted for its efficiency in treating jaundice, constipation, obesity, splenomegaly, anemia, insomnia, bladder diseases, and non-healing wounds from traumas. Furthermore, the plant is utilized as a base for producing skin ointments and balms [51].
A. absinthium demonstrates various biological activities, including but not limited to antibacterial, anti-inflammatory, hepatoprotective, antidepressant, antispasmodic, and antipyretic effects [53,54]. Moreover, it exhibits antimicrobial, antiviral, antistress, hepatoprotective, antioxidant, and anticancer effects [46,55].
In the field of dermatology, the essential oil derived from A. absinthium has been shown to expedite wound healing, diminish inflammation, and exhibit antimicrobial and wound-healing properties. This effect is due to the significant content of oxygenated components in the oil, such as camphor (Figure 3a) and tirpinene-4-ol (Figure 3b), the content of which was, respectively, 47.59% and 6.36% [56]:
Figure 3.
Camphor (a) and tirpinene-4-ol (b).
Camphor induces the proliferation of primary dermal fibroblasts, maintaining or restoring collagen and elastin production in UV-exposed skin. In addition, it prevents thickening of the epidermis and subcutaneous fat layer Camphor attenuates the aging enhancement associated with β-galactosidase (SA-β-gal) activity. In addition, the oil contains chamazulene (Figure 1) (10.35%), which contributes to the manifestation of antioxidant/anti-radical activity [56,57].
1.5. Bidens tripartita L. Aster Family—Asteraceae
Bidens tripartita L. is widely distributed in the European part of the CIS, Transcaucasia, Siberia, Central Asia (excluding Turkmenistan), and the southern region of the Far East. Its range also extends to North Africa and North America [58]. In Kazakhstan, this species is ubiquitous across its regions.
B. tripartita is a plant that is rich in various biologically active compounds, including essential oil, chlorophylls, flavonoids, cinnamic acid derivatives, tannins with a high polyphenol fraction content, polysaccharides, carotenoids, ascorbic acid, coumarins, chalcones, and minerals such as Zn, Sr, Se, and Mn. Flavonoids found in the plant include luteolin, butein, sulphuretin, sulphurein, cynaroside, auron, (+)-catechin, (−)-epicatechin, rutin, myricetin, 7-hydroxyflavone, esculetin, and umbelliferone, among others [59,60,61,62,63,64,65].
In traditional medicine, the water infusion and decoction of B. tripartita have been utilized for a considerable time period in combination with baths for the treatment of scrofula, rickets, exudative diathesis, and various pustular skin diseases such as acne and boils, as well as for the management of gout, arthritis, and articular rheumatism. They are also recommended for improving appetite and digestion, and for the treatment of liver and spleen disorders, colds, bronchitis, and diabetes mellitus [66,67].
Preparations derived from B. tripartita exhibit a range of therapeutic effects, including anti-inflammatory, hemostatic, antiseptic, sedative, and wound-healing properties, lower blood pressure, and increase the amplitude of heart contractions [68,69]. The antiallergic, anti-inflammatory, diuretic, and antispasmodic effects of the alcohol extract of B. tripartita have also been proved [70,71]. The methanolic extract of B. tripartita manifests antioxidant activity against cancer cells and has the ability to inhibit key enzymes, such as α-amylase and α-glucosidase. In addition, according to evidence, the herb has antidiabetic activity, as well as antihyperglycemic and antioxidant effects [72].
The broad pharmacological effects of the plant are attributed to its abundant content of various biologically active substances. Manganese ions in the plant’s enzyme systems are believed to influence hematopoiesis, blood coagulation, endocrine gland activity, liver cell function, and blood vessel and bile duct tone, and may prevent intravascular thrombus formation and enhance antimicrobial properties of the plants [68,69]. Flavonoids in the plant are responsible for its antiallergic and diuretic effects by affecting metabolic processes. The presence of vitamin C can activate the function of the endocrine glands, improve metabolism, strengthen the immune system, and help treat viral infections. The essential oils present in the plant are effective in destroying pathogenic microflora and fungi [66,67].
The extract of B. tripartita is used in the treatment of many skin diseases: psoriasis, seborrhea, urticaria, diathesis, acne, pimples, wounds, and ulcers, as well as small cracks. The beneficial effects on the skin can be attributed to the presence of tannins. Tannins also help to get rid of increased sweating of the armpits and legs. Thus, B. tripartita is employed for making baths, lotions, and wipes to treat microbial eczema of the feet, epidermophytosis [62,66,67,73]. The mask derived from the sequence has been shown to eliminate oily sheen, tone the skin, and have a rejuvenating effect. Additionally, wiping the face with a decoction of the string has been demonstrated to reduce acne [68,69]. During diathesis, an infusion of B. tripartita (from 10–30 g of herbs) is added to the bath [74].
Khatamov et al. [75] developed and studied a new drug: a thick extract of the sum of flavonoids in the form of ointment (1, 3 and 5%) obtained from B. tripartita, which was used to cure contact allergic dermatitis experimentally caused in guinea pigs by 2,4-dinitrochlorobenzene. The study results showed that 5% ointment had the greatest therapeutic effect compared to the antihistamines Psilo-Balsam and glucocorticoid ointment Celestoderm. At the same time, the rate of reduction in the severity of skin manifestations (Ind) was the highest compared to other studied groups—37.9%.
1.6. Capsella bursa-pastoris L. Cabbage Family—Brassicaceae
Capsella bursa-pastoris L. is a wild plant with significant nutritional value that is suitable for human consumption. This plant is widely distributed across many countries, including Cyprus, Europe, Saudi Arabia, Turkey, Pakistan, India, Iraq, Iran, China, Azerbaijan, and other Asian countries [76]. It is also commonly found in various regions of Kazakhstan.
C. bursa-pastoris contains a variety of chemical components including flavonoids, polypeptides, choline, acetylcholine, histamine, tyramine, fatty acids, sterols, organic acids, amino acids, sulforaphane, vitamins [77], and various trace elements. In addition, it contains phenolic compounds, flavonoids, tannins, saponins, alkaloids, and phytosterols [76,78,79,80], as well as volatile fractions consisting mainly of terpenoids, alkane hydrocarbons (such as nonacosane), and fatty acids (including palmitic and linoleic acids) [81].
In traditional medicine, C. bursa-pastoris has been used for centuries in China and Japan as a hemostatic, diuretic, and antipyretic agent [77]. The plant has been utilized for the treatment of conditions such as edema caused by nephritis, odynuria, hemaffetia, menorrhagia, chyluria, and hypertension [82]. The entire plant is used to make tea, which has been employed as an antiscorbutic, astringent, diuretic, emmenagogue, hemostatic, hypotensive, tonic, stimulant, vasoconstrictor, and wound-healing agent. This beverage has also been considered an excellent remedy for various types of bleeding, including those originating from the stomach, lungs, uterus, and kidneys. A homeopathic remedy for nosebleeds and urolithiasis is prepared from a fresh C. bursa-pastoris plant [77].
Based on the literature, raw plant extracts and certain phytocomponents have been reported to exhibit various pharmacological effects, such as anti-inflammatory, antispasmodic, antimicrobial, hepatoprotective, cardiovascular, anticancer, sedative, and antioxidant effects [76,79,83,84,85,86]. Furthermore, these extracts have been assumed to possess infertility-reducing properties [87]. Extracts have also demonstrated inhibitory effects on acetylcholinesterase activity and significant antibacterial activity [79].
C. bursa-pastoris exhibits potent antioxidant activity attributed to its flavonoid compounds, namely quercetin, chrysoeriol, kaempferol, and isorhamnetin. In vitro studies have shown that its extracts possess antioxidant activity that prevents the development of various free radicals such as DPPH radicals, peroxyl radicals, hydroxyl radicals, and hydrogen peroxide [88]. Additionally, the plant extract has been found to have cytotoxic effects as reported by the previous studies [78]. Furthermore, a moderate hepatoprotective activity has been observed with the extract containing specific flavonoids, including 4,7-dihydroxy-5-hydroxymethyl-6,8-diprenylflavonoid, chrysoeriol-7-O-d-glucopyranoside, sinensetin, and 6,8-diprenylgalangin [89].
C. bursa-pastoris exhibits an excellent efficiency in the treatment of eczema in dermatology [90]. Moreover, preparations derived from C. bursa-pastoris have been registered and recommended by the German Institute for Pharmaceuticals and Medicines for the additional treatment of skin diseases and wounds [91]. Fumarates improve the course of psoriasis, in which both IL-12 and IL-23 promote the differentiation of pathogenic T-helpers (Th). Fumarate treatment induces IL-4-producing Th2 cells in vivo and generates type II dendritic cells (DCs) that produce IL-10 instead of IL-12 and IL-23. Type II DCs result from fumarate-induced glutathione (GSH) depletion, followed by an increase in heme oxygenase-1 (HO-1) expression and impaired STAT1 phosphorylation. The induced HO-1 breaks down, after which the N-terminal fragment of HO-1 is translocated into the nucleus and interacts with the AP-1 and NF-kB sites of the IL-23p19 promoter. This interaction prevents IL-23p19 transcription without affecting IL-12p35, whereas STAT1 inactivation prevents IL-12p35 transcription without affecting IL-23p19 [92].
1.7. Chelidonium majus L. Poppy Family—Papaveraceae
Chelidonium majus L., a plant species commonly known as greater celandine, is widely distributed across Asia, North America, and northwestern Africa [93].
The plant C. majus is known to contain a high concentration of isoquinoline alkaloids, with levels ranging from 0.27 to 2.25% in the aerial parts and 3–4% in the root. Over 70 compounds have been identified, including various alkaloids (such as chelidonin, chelerythrin, sanguinarine, berberine, protopine, allocryptopine, and koptisin), flavonoids (such as rutin, quercetin, and kaempferol), saponins, vitamins (such as vitamin A and C), mineral elements, a small amount of phytosterols (such as α-spinasterol and ergosterol), and aromatic and aliphatic acids (including chelidonic, caffeic, ferulic, polycoumaric, citric, etc.) and their derivatives. Additionally, celandine consists of polysaccharides, alcohols (1-hexocosanol, chelidoniol, and nonacosanol), choline, tyramine, histamine, and saponosides. It should be noted that a previous study provided the formulas of all organic components [94]. The content of most mineral elements in celandine ranged from 10 to 65%, where potassium (65%) and phosphorus (54%) predominated [95,96,97].
C. majus has a long history of traditional use in Europe, Asia, and Africa to treat various ailments, including those affecting the liver and bile ducts, as well as to cure skin conditions such as warts, calluses, and eczema. Additionally, the plant has been used to treat stomach ulcers, tuberculosis, skin rashes, and oral infections. In traditional Chinese medicine and homeopathy, C. majus is used to alleviate congestion, pain, swelling, and jaundice [93,98].
Celandine extracts have been found to possess a broad spectrum of pharmacological activities including anti-inflammatory, antimicrobial, anticancer, antioxidant, hepatoprotective, natriuretic, and antidiuretic effects, corroborating some of the traditional medicinal uses of C. majus. Additionally, the plant has demonstrated immunomodulatory, lipid-lowering, and radioprotective properties [94,95,98,99]. Moreover, the ethanolic extract of C. majus has been found to contain biologically active secondary metabolites with significant inhibitory effects that prevent Alzheimer’s disease [93].
The milky juice of the celandine is rich in alkaloids among which the predominant one is chelidonine (Figure 4a). Studies have demonstrated the antimicrobial, immunomodulatory, cytostatic, and cytotoxic effects of celandine alkaloids, including their anti-keratinocyte activity. Compounds such as chelidonine (Figure 4a), sanguinarine (Figure 4b), chelerythrine, coptisine, and protopin have been found to exhibit cytotoxic activity. Sanguinarine (Figure 4b) has been shown to be particularly effective at inhibiting keratinocyte growth, indicating that celandine may have potential as an additional therapy for malignant skin diseases [97].
Figure 4.
Chelidonine (a), sanguinarine (b).
1.8. Cichorium intybus L. Aster Family—Asteraceae
Cichorium intybus L., a perennial herbaceous plant belonging to the Asteraceae family, is known by various common names such as roadside grass, blue flower, roadside cornflower, bride of the sun, and sun grass. Its recognizable feature is the inflorescences-baskets that exclusively comprise reed blue flowers. However, the said baskets only open during early morning hours or in cloudy weather. The term “chicory” is derived from the Latin word, meaning “entering the fields.” Due to its therapeutic properties, this plant has earned the followings names: “king root,” “golden root,” and “cure for a hundred diseases” [100].
C. intybus exhibits a wide geographical distribution encompassing Northern and Central Europe, Siberia, Turkey, Afghanistan, Northern and Central China, South America, South Africa, Ethiopia, Madagascar, India, Australia, and New Zealand. This herbaceous plant is capable of growing in the territories of the Commonwealth of Independent States, except the Far North region [101].
The roots of C. intybus contain 56–65% inulin (in terms of dry matter), the maximum accumulation of which is observed in autumn. Intibin glycoside gives specific bitterness to chicory roots. Proteins, sugars, pectin, and sesquiterpene lactones were also found in the roots, as well as guayanolides: cycriosides B and C, sonchuside C, tannins and resinous substances, choline, carotene, vitamins B, B2, PP and C, from mineral elements—sodium, potassium, calcium, manganese, and phosphorus, iron. Chicory roots contain taraxasterol, phenolic acids (chlorogenic, isochlorogenic, neochlorogenic, caffeic and cicoric acids) [100,102]. In the flowers of C. intybus, chicory glycoside was found, in the seeds: inulin and protocatechin aldehyde [103,104], prebiotic fructooligosaccharides, sesquiterpene lactones, caffeic acid derivatives (chicory acid, chlorogenic acid, isochlorogenic acid, dicapheoyltartaric acid), proteins, hydroxycoumarins, flavonoids, alkaloids, steroids, terpenoids, oils, volatile compounds, and vitamins [105,106]. Aliphatic compounds and their derivatives make up the main fraction; terpenoids are somewhat less common in the plant. Chicory leaves contain inulin, vitamins A, B1, B2 and C, macro- and microelements (Ca, K, Mg, Na, Fe, Cu, Mn, Zn), phenolic compounds, etc. [101].
The aerial and subterranean portions of C. intybus L. are extensively employed in traditional medicine, such as in Chinese and Mongolian practices, as an agent for modulating the immune system, promoting bile secretion, protecting the liver, and reducing blood glucose levels. The plant is documented in the Chinese Pharmacopoeia and is utilized in the formulation of homeopathic remedies in Germany. The extract of chicory herb is a constituent of the LIV-52 complex preparation from India [107].
C. intybus exhibits antiseptic and astringent properties and produces choleretic and diuretic effects. It positively influences the nervous and cardiovascular systems. Additionally, its infusion has been employed for normalizing heart rhythm. According to the literature, preparations derived from C. intybus are efficient in treating various diseases connected with the gallbladder, liver, kidneys, and urinary system. Additionally, chicory preparations have been shown to exhibit potential therapeutic benefits in managing obesity, liver diseases, atherosclerosis, hypoacid gastritis, tachycardia, arrhythmia, and nephritis. The milky juice of the plant contains bitter substances that have been found to stimulate peristalsis of the gastrointestinal tract, increase the secretion of gastric and intestinal juice, and promote regular bowel movements and appetite. According to the published literature, C. intybus has been found to possess a notable therapeutic effect in curing and preventing diabetes mellitus and in preventing it (antidiabetic effect). This effect is attributed to the presence of inulin, a natural sugar substitute that eliminates toxins and non-nutrient substances from the body. Preparations based on C. intybus exhibit diverse pharmacological activities, including anti-inflammatory, antioxidant, antiviral, choleretic, diuretic, hepatoprotective, and antibacterial effects, making them beneficial in treating colitis, gastritis, and enteritis. Decoctions of C. intybus roots have been reported to be effective in the treatment of helminthic invasion, anemia, malaria, scurvy, eczema, and tumors of the spleen [101,102,108,109,110,111]. Furthermore, some research indicates that C. intybus L. may modulate immune responses [106]. Infusions of C. intybus flowers have been found to possess antiseptic, anti-inflammatory, moisturizing, and nourishing properties, which are beneficial in treating inflammation of the skin and eyes [107].
A decoction of C. intybus L. is commonly applied externally (in the form of baths, applications, and lotions) for the treatment of various skin diseases, including but not limited to eczema, urticaria, psoriasis, seboroid dermatitis, neurodermatitis, atopic dermatitis, vitiligo, acne, and furunculosis. Additionally, the herb is known for its efficiency in the care of dry skin [101,107].
C. intybus extract has been clinically tested on volunteers as a skin UV-protecting means. The analysis results of the microrelief control area applied with sodium lauryl sulfate and causing skin damage showed there was a significant increase in roughness after 28 days of the study, while in the areas where sodium lauryl sulfate was applied on the plant extract, the roughness of the skin did not undergo any significant changes, which indicates the plant’s protective properties [112].
1.9. Equisetum arvense L. Horsetail Family—Equisetaceae
Equisetum arvense L., a herbaceous plant belonging to the Equisetaceae family, is widely distributed in North America, Europe, and Asia, including the territory of Kazakhstan [113,114].
E. arvense contains more than 210 natural compounds distributed in various organs. These compounds include alkaloids, carbohydrates, proteins and amino acids, phytosterols, saponins, sterols, ascorbic acid, silicic acid, phenolic compounds, and their glycosides, tannins, flavonoids (such as apigenin, genquanin, luteolin, kaempferol, quercetin), triterpenoids, volatile oils, and other bioactive substances [115,116].
E. arvense, a plant species from the Equisetaceae family, has been utilized in traditional medicine for its therapeutic properties. Its applications include the treatment of tuberculosis, and renal and bladder catarrh, as well as a hemostatic agent during excessive menstruation, nasal, pulmonary, and gastric bleeding, among others [117].
The water–alcohol extract of E. arvense has demonstrated various biological activities including antioxidant [118], anti-inflammatory, antibacterial, and antimicrobial effects [119]. Studies have also reported its antiproliferative activity [120], as well as antifungal, vasodilating, hepatoprotective [121], neuro- and cardioprotective, cytotoxic, and anti-cellulite properties [122,123]. Additionally, E. arvense has been traditionally used for its analgesic effects on rheumatism and frostbite, as well as its anti-inflammatory properties, which can improve blood circulation. This plant has been employed as a bath agent for skin diseases and incorporated into cosmetic products as a rejuvenating, moisturizing, anti-wrinkle, anti-acne, antiperspirant, and conditioning agent [124].
Equisetum arvense L. is recognized for its high content of silicon, a compound that is associated with promoting skin health. Silicon maintains skin firmness and elasticity, while its mild exfoliating properties allow the elimination of dead skin cells and enhancing of skin texture [125,126].
The antioxidant potential of E. arvense has been attributed to the presence of flavonoids such as quercetin (Figure 5a), kaempferol (Figure 5b), and isorhamnetin (Figure 5c) [127].
Figure 5.
Quercetin (a), kaempferol (b), and isorhamnetin (c).
Studies show that phenolic compounds in the plant reduce the formation of ROS induced by bacterial lipopolysaccharides or fungal infections due to the direct capture of free radicals or their purification through reactions with antioxidant enzymes [128]. Cosmetics containing the extract of this plant, which prevents early aging of the skin, have been actively introduced to the industry [129].
Quercetin (Figure 5a) is able to reduce inflammation, accelerate reepithelialization, and stimulate cell proliferation and the formation of granulation tissue in different experimental models of skin wounds and clinical trials. These effects are associated with their ability to decrease levels of inflammatory cytokines (IL-1β, TNF-α), cell migration (neutrophils, CD68+ macrophages), mitogen-activated protein kinases (p38p, ERK-Î2, JNK-Î2), (PEG2, leukotriene B4), inflammatory enzyme (COX-2), and transcription factor (NF-kB). In addition, quercetin promotes increased growth factors (VEGF and TGF-β1) as well as anti-inflammatory cytokines (IL-10) and antioxidant defenses (GSH, SOD, CAT). This compound also has an antifibrotic effect on second-target wounds, increasing the expression of αintergrin (a protein involved in the migration and proliferation of fibroblasts and reducing β1 integrin migration of fibroblasts and initiation of fibrosis [130].
1.10. Eryngium planum L. Seler Family—Apiaceae
The subgenera of Eryngium are predominantly distributed throughout Europe, Africa, and Asia, with certain subgenera exhibiting a widespread presence in Australia [131,132]. In Kazakhstan, Eryngium is found growing in the steppe regions of Northern Kazakhstan, as well as in the Dzungarian and Zailiyskiy Alatau mountain ranges [133].
The aerial parts of Eryngium species are characterized by the presence of saponins, flavonoids, and essential oils, while the underground parts contain triterpene saponins, monoterpene glycosides, phenolic compounds such as flavonoids and phenolic acids, coumarin derivatives, terpene aldehyde esters, essential oils, and oligosaccharides [134,135]. The isolation of eringinol from the aboveground parts of the plant was reported later [136]. Further studies on the phytochemical constituents of the plant were conducted on leaves and roots, leading to the isolation of various aglycones [136,137] and A1-barrigenol and R1-barrigenol [135].
E. planum plays a significant role in European and Asian traditional medicine for treating various inflammatory diseases. The plant’s aboveground parts are bioactive primarily due to the presence of polyphenols and saponins [136,137,138,139,140]. It has demonstrated potential for use in gastrointestinal diseases and exhibits antibacterial, analgesic, anthelmintic, anticonvulsant, and anticancer properties, thereby proving its crucial importance in ethnopharmacology [141]. The aerial part of the plant collected during flowering is used for therapeutic purposes.
According to the results obtained from HPLC-MS analysis, flavonoids, particularly rutin (Figure 6a) and isoquercetin (Figure 6b), are the major constituents of E. planum extracts [142]. Rutin is known to possess skin-toning properties and to prevent the appearance of skin diseases such as rosacea and erythema. The anti-inflammatory effects of E. planum extracts may be attributed to the synergistic activity of ursolic acid (Figure 6c) and polyphenols such as chlorogenic acid (Figure 6d) and rosmarinic acid (Figure 6e), which have previously been studied for their anti-inflammatory properties [143,144,145,146]. Notably, ursolic acid, which predominates in concentrated extracts of the plant, exhibits antioxidant, antimicrobial, anti-inflammatory, and hypoglycemic activities [147].
Figure 6.
Rutin (a), isoquercetin (b), ursolic acid (c), chlorogenic acid (d), rosmarinic acid (e).
Eryngium planum L. has potential applications in dermatology, particularly for the treatment of atrophic and purulent skin wounds when applied externally [148].
Secondary metabolites include phenolic acids, flavonoids, coumarins, and triterpenoid. Saponins isolated from E planum L. demonstrate moderate antibacterial activity and substantial antimycotic activity. It was found that phenolic compounds inhibit microbial adhesion and inactive transport protein of a cell membrane [149,150].
1.11. Glycyrrhiza glabra L. Legume Family—Fabaceae
Glycyrrhiza glabra L., commonly known as licorice, fragrant wood, or mulaiti, is a small perennial plant that grows in Eurasia, North Africa, and West Asia [151]. This plant is found ubiquitously in Kazakhstan [152,153]. The genus Glycyrrhiza is extensively distributed across the globe and has over 30 species.
The root of Glycyrrhiza glabra is a significant medicinal component due to the presence of various isolated compounds. These include triterpene saponins such as the sweet saponin glycyrrhizin, flavonoids such as liquiritin which is the primary flavonoid glycoside, rhamnoliquirilin, liquiritigenin, prenillicoflavon A, glucoliquiritin apioside, 1-methoxyphaseolin, shinpterocarpin, shinflavanone, lycopyranocoumarin, glisoflavone, lycoarylcoumarin, coumarin-GU-12, isoflavonoids, and chaconne. Among these, glycyrrhizic acid is the primary biologically active component, and it is known to be 60 times as sweet as sugar cane [151,154].
Licorice root has been employed as a therapeutic agent by both ancient and modern medical practitioners. Its oral administration has demonstrated efficiency in the treatment of various disorders including gastric, duodenal and esophageal ulcers, inflammation, laxatives, mouth ulcers, antispasmodic, antitussive, sedative, and expectorants. The herb’s constituents make it a promising candidate for curing respiratory diseases such as asthma, acute and chronic bronchitis, and chronic cough. Furthermore, it can be used in treating Addison’s disease. External application of licorice extracts has also been effective in treating inflammatory skin conditions, mouth ulcers, and maintaining oral hygiene [154,155,156].
Numerous clinical and experimental studies have shown the presence of several pharmacological properties in this substance. These properties are of great advantage, including anti-inflammatory, antiviral, antimicrobial, antioxidant, anticancer, immunomodulatory, hepatoprotective, and cardioprotective effects [154].
The ethanolic extract derived from the root of G. glabra exhibits an excellent antibacterial activity that stops the development of Propionibacterium acne and Pseudomonas aeruginosa. Due to this property G. glabra is employed in dermatology for treating skin diseases, such as dermatosis and acne [157].
Multiple studies have demonstrated the efficacy of Glycyrrhiza glabra L., which is efficient in the treatment of skin hyperpigmentation, eczema, and psoriasis, and provides skin with antioxidant properties. Due to the presence of flavonoid compounds such as oxyresveratrol (Figure 7a), glabridin (Figure 7b) and liquiritin (Figure 7c), it has a therapeutic effect [158]. The external application of skin care products containing licorice extract gives a healthy glow, as well as improves the overall quality and appearance of the skin [159]. It is achieved by the oxidative abilities of these components.
Figure 7.
Oxyresveratrol (a), glabridin (b) and liquiritin (c).
Flavonoids of Glycyrrhiza glabra: liquiritin (Figure 7c), glucoliquiritin apioside (Figure 8a), and glycyrrhizin (Figure 8b) have high skin permeability properties and are potential antioxidants. These components improve the histological properties of the dermis and epidermis and reduce the level of markers of inflammation and wrinkles [160,161]. Glycyrrhiza glabra L. also contains licochalcone A (Figure 8c), which has anti-inflammatory and antimicrobial properties and has been found to be efficient in treating acne, inflammatory skin diseases, and other skin ailments [162,163,164].
Figure 8.
Glucoliquiritin apioside (a), glycyrrhizin (b), licochalcone A (c).
1.12. Gnaphalium uliginosum L. Aster Family—Asteraceae
Gnaphalium uliginosum L. is a member of the Compositae family, a group of flowering plants, and is commonly referred to as swamp cudweed. It is widely distributed, including in Kazakhstan [165,166].
G. uliginosum is known to have a limited array of chemical constituents. It consists of approximately 125 compounds such as flavonoids, sesquiterpenes, diterpenes, triterpenes, phytosterols, anthraquinones, caffeylquinic and caffeylglucaric acids, flavonols, and carotenoids [167,168].
Marshweed, also known as G. uliginosum, has been used in traditional medicine to alleviate a variety of ailments, including gastric disorders, edema, wounds, prostatitis, lumbago, neuritis, and angina pectoris. Additionally, it has been utilized for its antihypertensive, diuretic, antipyretic, and antimalarial properties [165].
Pharmacological investigations on G. uliginosum extracts have revealed various beneficial effects, such as antioxidant [169], antibacterial, antifungal, antitussive, expectorant, antifeedant, cytotoxic, and hepatoprotective activities [170]. Additionally, this plant has anti-inflammatory, antidiabetic, and antihyperuricemic properties [165]. G. uliginosum is employed in medical practice as a hypotensive and wound-healing agent for treating hypertension, gastric ulcer, and difficult-to-heal wounds [168]. Furthermore, oil extracts derived from this plant are useful for curing laryngitis, catarrh of the upper respiratory tract, and tonsillitis [171].
In the field of dermatology, the extract derived from Gnaphalium uliginosum has been employed to treat diseases such as eczema and skin cancer [172,173].
The ointment used to treat psoriasis contains an aqueous extract of “cold pressed” Gnaphalium uliginosum L. obtained immediately after harvesting [174].
1.13. Humulus lupulus L. Hemp Family—Cannabaceae
Humulus lupulus L., commonly known as hops, is a plant species that is widely distributed in temperate regions worldwide [175,176].
H. lupulus is a plant that contains many phytochemicals, with a high concentration found in the female inflorescences from which lupulin, a yellowish-brown granular powder, is obtained. Lupulin comprises bitter resins and essential oils, imparting the characteristic aroma and flavor of hops. The primary bitter acids found in hop resin are alpha acids (humulones) and beta acids (lupulones). The essential oils contain myrcene, linalool, and geraniol, which are the most important aromatic compounds. Additionally, lupulin contains polyphenols, such as quercetin (Figure 5a), kaempferol, (Figure 5b) (see above) catechins, prenylnaringenin, hydroxycinnamic acid, and condensed tannins. Ferulic acid is the most representative compound in the phenolcarboxylic acid group. Hop seeds are rich in catechins (catechin, epicatechin), which are widely used in various industries, including pharmaceuticals, cosmetics, and nutraceuticals [175,177,178].
H. lupulus has a long history in traditional medicine, which dates back to prehistoric times. It was used to treat various ailments such as leprosy, toothache, fever, stomach issues, sleep disorders, and anxiety. Additionally, it was utilized as a bowel function enhancer and to improve the pharmaceutical properties. Due to the numerous health benefits of hop polyphenols, which include antioxidant and antimicrobial effects, they may have a therapeutic use [175,176].
Hop extract has been found to possess various pharmacological properties. For instance, it exhibits antitumor and anti-inflammatory effects, as evidenced by previous studies [179]. Moreover, the extract has been reported to possess antibacterial, anti-collagenase, and antioxidant activity [180]. Additionally, hop extract has been found to have antiallergic, antiviral, hepatoprotective, and antithrombogenic effects [181].
In dermatology, extracts of H. lupulus have been employed as an antipsoriatic medicine [179]. Furthermore, they are used in the treatment of skin inflammation diseases of adolescents, and hop cones are taken orally to cure baldness, furunculosis, lichen, and scrofula [175,180]. The plant is known to have an anti-collagenase effect on the skin; that is, it prevents the destruction of collagen fibers due to exposure to UV rays [181].
Naoto et al. tested seven natural components of hop (Humulus lupulus L.) extracts to evaluate biological activity against acne vulgaris [180]. Five strains, Propionibacterium acnes, Staphylococcus epidermidis, Staphylococcus aureus, Kocuria rhizophila, and Staphylococcus pyogenes, were selected as the main acne-causing bacteria. Hop extracts xanthohumol (Figure 9a) and the lupulones (Figure 9b) showed strong inhibitory activities against all of the strains. Although hydrogenated derivatives did not show the same level of activity, naturally occurring xanthohumol (Figure 9a), lupulones (Figure 9b), and humulones (Figure 9c) all showed moderate to strong anti-collagenase inhibitory activities:
Figure 9.
Xanthohumol (a), lupulones (b), and humulones (c).
The results of studies indicate that H. lupulus flower extract has strong antioxidant activity since it significantly reduces the production of ROS and decreases inflammation, diminishing the production of NO and the expression of COX-2 by macrophages activated by liposaccharides [182].
1.14. Juglans regia L. Walnut Family—Juglandaceae
This plant has been observed to grow in various regions across the globe, including East Asia, Europe, North Africa, and South America [183]. Its growth has also been documented in southern Kazakhstan and it is recognized as a protected species within the boundaries of the Sairam-Ugam State National Natural Park [184].
The chemical composition of walnut kernels is of significant nutritional value due to the high content of polyunsaturated fatty acids (comprising up to 75% of total content), proteins, amino acids, as well as vitamins E, C, β-carotene, and essential minerals such as potassium, calcium, magnesium, sulfur, and phosphorus [185]. Moreover, walnut is known to contain trace elements such as iron, zinc, and copper, which play a vital role in various biochemical processes within the human body [186]. The plant is also rich in fluorine salts. Walnut partition contains trace amounts of organic substances, tannins, glycosides, alkaloids, and iodine.
The chemical composition of walnut leaves is characterized by the presence of various biologically active components, including trace amounts of iodine, α- and β-hydrojuglone, polyphenols, tannins, glycosides, flavonoids, terpenoids, vitamin C, carotene, vitamin B1, essential oils, and tannins [187,188,189,190,191,192]. Among the compounds contained in walnut, polyphenolic compounds are the most important ones. They include various derivatives of chlorogenic and hydroxycinnamic acids that are the major components [193]. The study by Schwindl demonstrated that the methanolic extract derived from the leaves of Juglans regia L. includes a cumulative 40 metabolites classified under megastigmane, tetralone, phenylpropanoid, neolignan, and juglone glycosides [194].
In traditional medicine, diverse components of Juglans regia L. are utilized to cure several ailments such as diabetes, infectious diseases, and periodontal disease [195]. Furthermore, the plant is reputed to have antipyretic, analgesic, antidandruff, and burn-healing properties [196,197]. Notably, the extract of walnut shell has demonstrated notable antibacterial and antibiofilm properties, which develop resistance to coagulase-negative staphylococci [198]. Additionally, the lyophilized extract of the walnut septum has been reported to exhibit a marked antitussive, antioxidant, and anti-inflammatory effect [199].
The leaves of Juglans regia L. are traditionally used to alleviate skin inflammation and excessive sweating of the hands and feet. Moreover, they are recommended for the treatment of acne, warts, eczema, and psoriasis due to the presence of flavonoids, specifically quercetin derivatives, and tannins [200,201,202]. The high concentration of α-tocopherol in the leaves of J. regia contributes to its antioxidant effect, which promotes the repair of damaged skin and strengthens the epidermal layer [203].
1.15. Matricaria recutita L. Aster Family—Asteraceae
Matricaria chamomilla L. is a globally distributed, well known medicinal plant [204,205].
M. chamomilla contains numerous biologically active compounds, including flavonoids (such as apigenin and luteolin) and their glycosides, as well as coumarins (including gerniarin and umbelliferone) [206]. The essential oil extracted from chamomile flowers is composed of 52 different components, with the highest concentration of terpenoids, including β-farnesene, α-farnesene, α-bisabolol, chamazulene, and germacrene, as well as spiroether [204,207,208].
M. chamomilla has been widely employed in traditional medicine for treating a variety of ailments, including infections, neuropsychiatric disorders, respiratory tract, gastrointestinal, and liver diseases. Furthermore, the plant possesses sedative, antispasmodic, antiseptic, and antiemetic properties [204].
Therapeutic indications for M. chamomilla encompass a diverse array of medical conditions, including inflammatory conditions, bacterial infections, and lesions of the skin and mucous membranes such as those found in the oral cavity, gastrointestinal tract, and respiratory tract. Additionally, the plant has been employed as a remedy for spasms and ulcers of the gastrointestinal tract, insomnia, and nervous breakdown [130,209,210,211,212,213,214]. Furthermore, the plant has demonstrated pain-relieving properties [215] and wound-healing effects [216], and acted as a protective agent for the kidneys and liver [217].
M. chamomilla is regarded as a viable alternative due to a high content of bioactive secondary metabolites that can be used for the treatment of diverse skin problems, such as wounds, abscesses, and skin diseases. The plant’s therapeutic efficiency in treating skin diseases is attributed to the presence of quercetin (Figure 5a), α-bisabolol (Figure 10a), and apigenin (Figure 10b):
Figure 10.
α-Bisabolol (a), and apigenin (b).
α-Bisabolol (Figure 10a) possesses anti-inflammatory, antibacterial, and anti-irritant properties, making it suitable for use in a variety of products that protect skin from irritation caused by environmental factors. Due to its non-allergenic nature, it is widely used in hand and body lotions, aftershave creams, lipsticks, sun and after-sun care products, and baby care products [218,219]. On the other hand, apigenin (Figure 10b) has been found to alleviate the symptoms of skin inflammatory diseases by protecting skin cells from oxidative-stress-induced death. Apigenin also affects the synthesis of skin barrier factors and the influx of calcium ions. Therefore, it can potentially be used to treat skin inflammatory diseases and cancer [220].
Dos Santos et al. [130] presented a review of 20 patents using Matricaria species as an active ingredient in skin diseases. The majority of the inventions (80.00%) contained combinations of Matricaria with other plant species, including those belonging to the genus Calendula, Salviae, Eucalyptus, Urtica, and Aloe vera. On the other hand, four patents (20.00%) reported the development of bioproducts containing only species classified as chamomile, two (10.00%) with M. parthenium, and two (10.00%), M. chamomilla. Based on the information of these extracts, externally applied pharmaceutical remedies such as creams, ointments, lotions, solutions, textile dressing, and banding were developed. Capsules, granules, and alcohol dye have been developed for oral use. Regarding the skin disease treated, the selected inventions claim to treat wounds and burns, erythema and rosacea, eczema and dyshidrosis, and spots and hyperpigmentation of the skin by UV radiation. Skin peeling and damaged stratum corneum, dermatitis, hemorrhagic incision, excoriations, hand-foot syndrome, psoriasis, and acne were also mentioned.
1.16. Ononis spinosa L. Legume Family—Fabaceae
Ononis spinosa L. is widely distributed in Africa, Asia, and Europe. It is found in countries such as Algeria, Libya, Morocco, Tunisia, Afghanistan, Iran, Iraq, Palestine, Jordan, Lebanon, Syria, Turkey, Armenia, Azerbaijan, India, Denmark, Norway, Sweden, Great Britain, Austria, Belgium, Czechoslovakia, Germany, Hungary, the Netherlands, Poland, Switzerland, Estonia, Lithuania, Moldova, the European part of the Russian Federation, Albania, Bulgaria, Greece, Italy, Romania, France, Portugal, and Spain [221].
The root of O. spinosa contains a large amount of isoflavonoids, pterocarpans, and dihydroisoflavonoids, including formononetin, calicosin, pseudobaptigenin, medicarpin, maakiain, onogenin, and sativanon, with metabolites present in the form of glucosides, glucoside malonates, glucoside acetates, and free aglycones [222,223].
The roots, leaves, and flowers of O. spinosa were utilized in folk medicine for their antitussive, laxative, and diuretic properties. Infusions of the plant were employed to treat dropsy, urinary tract infections, inflammation, and rheumatism, while external applications were used to promote wound healing and alleviate skin conditions such as eczema. In Iraq, the roots were valued for their diuretic, blood purifying, laxative, and expectorant qualities [221].
Additionally, ash derived from burned samples of O. spinosa has demonstrated resistance to various Candida species [224].
Pharmacological investigations have demonstrated that O. spinosa exhibits noteworthy hepatoprotective and antitumor properties [225], and may be considered a potential therapeutic agent for treating urinary tract infections and bladder stones [222].
O. spinosa has been utilized in dermatology for its efficiency in treating skin ailments such as dermatitis (eczema) and pruritus. It also possesses wound-healing properties beneficial in the treatment of burns [226].
Ononis spinosa extract and glycerin have been clinically tested for facial laxity and wrinkles. The particular focus was made on immediate and delayed effects. Thirty-nine women used the product daily for an eight-week treatment period. Clinical assessment by experts and a new 2D imaging method (measuring the effect of an upper eyelid lift) were made at different time periods. The results showed an immediate and significant improvement in sagging and wrinkle parameters seven hours after the first application, in addition to significant long-term improvement. The lifting effect calculated from 2D images was 1.08 mm immediately after application and 1.80 mm after an eight-week treatment period. Ononis spinosa root extract inhibited hyaluronidases; Hyal-1 inhibition was a promising remedy for improving wound healing, tissue regeneration, and inducing diuresis. Two non-polar fractions of the roots of Ononis spinosa were the most active, causing inhibition of Hyal-1 by 86 ± 3% and 96 ± 13% at a concentration of 1 mg/mL, respectively. Chemical analysis revealed three main components, which were identified as onogenin, sativanon, and medikarpine. The percentages of inhibition for concentrations of 250 μM of these compounds were 25.3 ± 18, 61, 20 ± 20.6, and 22.4 ± 16, respectively. The IC50 of sativanone was determined to be 151 µM. Hot water and hydroalcoholic extracts of the Ononis spinosa root showed a moderate inhibitory effect on hyaluronidase-1 (Hyal-1) (IC50 1.36, respectively, 0.73 mg/mL), while dichloromethane extract had an inhibitory effect (Hyal-1) with IC50 190 µg/mL [221].
1.17. Onopordum acanthium L. Aster Family—Asteraceae
Onopordum acanthium L. is a widely distributed species of plants found across Africa (Algeria), Asia (Afghanistan, Iran, Iraq, Turkey, Armenia, Azerbaijan, Georgia, Russian Federation, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, China, India, Pakistan), throughout Europe, Australia, New Zealand, and North and South America (Argentina, Chile, Uruguay) [227,228].
O. acanthium is a plant species that contains various phytochemical compounds, including saponins, alkaloids, sesquiterpene lactones, flavonoids, triterpenes, sterols, nitrogen-containing compounds, phenolic acids, coumarins, inulin, soluble sugars, proteins, and oils [228]. The fatty acid composition of the plant includes palmitic, stearic, oleic, and linoleic acids [229,230]. Additionally, phenolic, triterpene, and steroid compounds were detected in the aerial parts of O. acanthium, while the roots were found to contain sesquiterpene lactones and polyacetylenes [231].
In traditional medicine, various preparations of O. acanthium, including its powder, juice, and decoction of the aerial part, were utilized as diuretics. This plant is known to stimulate the central nervous system and has demonstrated cardiotonic and hemostatic properties. Infusions of the leaves and inflorescences have also been employed to reduce swelling of various etiologies [231]. Furthermore, the extract derived from this plant has exhibited bactericidal, cardiotonic, and antitumor effects [232,233]. The extracts and isolated compounds from this plant have demonstrated a range of activities including anti-inflammatory, anti-radical, antiproliferative, and antibacterial effects [231]. Additionally, this plant can produce antioxidant and anti-inflammatory effects [234], as well as diuretic, dermatological, tonic, sedative, anticonvulsant, cardiotonic, hemostatic, and bactericidal effects, all without causing any side effects.
Eriodictyol (Figure 11a) and quercetin (Figure 5a) have been identified in the flowers of the plant, both of which possess potent antioxidant properties. Eriodictyol, in particular, has been found to protect skin cells from damage induced by UV radiation by inhibiting the MAPK signaling pathway, thereby exhibiting anti-aging effects [235].
Figure 11.
Eriodictyol (a) and 4β,14-dihydro-3-dehydrozaluzanin C (b).
The antitumor activity of extracts obtained from a combination of flowers and fruits, leaves, and roots of O. acanthium resistant to A431 culture (epithelial carcinoma of the skin) was examined by the authors of [236]. Aqueous, n-hexane, chloroform, and water–methanol extracts were utilized in the study. The results revealed that the chloroform extract of leaves and roots displayed the highest activity.
A number of compounds were isolated from the roots of Onopordum acanthium L., among them 4β,14-dihydro-3-dehydrozaluzanin C (Figure 11b), which showed a general antiproliferative ability comparable to that of the reference drug cisplatin in relation to epidermoid skin carcinoma.
The antiproliferative activity of compound (Figure 11b) was evaluated on epidermoid skin carcinoma cells A431 using MTT analysis [237]. The mechanism of cytotoxicity (Figure 11b) is associated with the activation of the mitochondrial pathway of cell apoptosis through the enzymes caspase-3 and caspase-9. The term “mitochondrial pathway” refers to the initiation of the apoptosis pathway in a cell as a result of a number of internal stimuli, for example, genetic damage, oxidative stress, and hypoxia. Regulation of this pathway is carried out by a group of proteins belonging to the Bcl-2 family. Bcl-2, Bcl-W, Bcl-XL, MCL-1, and Bfl-1 proteins suppress apoptosis by blocking mitochondrial release of cytochrome-C. P53-dependent pro-apoptotic proteins Bik, Bcl-Xs, Bad, Bax, Bak, Bid, Bim, and Hrk stimulate apoptosis, increasing the permeability of mitochondria and the exit from them into the cytoplasm of cytochrome-C. The ratio of pro- and anti-apoptotic proteins determines the fate of the cell. The release of cytochrome-C into the cytoplasm leads to the activation of caspase-3 through the formation of an apoptosomal complex consisting of cytochrome-c, Apaf-1 (apoptotic protease activating factor 1) and caspase-9. A number of proteins released from mitochondria into the cytoplasm can modulate apoptosis: AIF (apoptosis-inducing factor), Smac (second mitochondria-derived activator of caspase), DIABLO (direct IAP binding protein with Lowp I) and others. They bind apoptosis suppressors, proteins of the IAP family (inhibitor of apoptosis protein), which in turn are capable of inhibiting caspases-3, -7, and -9 [238,239].
O. acanthium extracts find applications in dermatology beyond skin cancer, such as in the treatment of furunculosis, purulent wounds, and lupus [240].
1.18. Orchis maculata L. Orchid Family—Orchidaceae
Spotted orchis is indigenous to countries with a cold, temperate subtropical climate, particularly in Central and Southern Europe and Asia [241]. Its distribution within Kazakhstan is primarily concentrated in the East Kazakhstan region [242].
Spotted orchis comprises a mucilaginous substance that contains polysaccharide, which decomposes to mannose, in addition to dextrin, starch, proteins, bitterness, pentoses, methylpentosans, sucrose, loroglossin glycoside, and essential oil [243,244,245,246]. Furthermore, the plant includes alkaloids, saponins, tannins, phenolic compounds (such as gallic acid, catechin, chlorogenic acid (Figure 6d), and syringic acid), terpenes, sterols, flavonoids, and anthocyanins [247,248]. O. mascula flowers’ ethanol extracts also encompass saponins, flavonoids, anthraquinone, terpenoids, tannins, cyanogenic glycosides, and cardiac glycosides [249]. These extracts exhibited a noteworthy antimicrobial effect against Salmonella paratyphi, Klebsiella oxytoca, and Staphylococcus aureus.
The spotted orchis extract has been shown to possess anti-inflammatory, antispasmodic, diuretic, enveloping, and immunomodulatory effects, as outlined in [243]. The enveloping effect can be attributed to the presence of loroglossin (Figure 12), a glycoside that protects inflamed tissues from excessive irritation [250].
Figure 12.
Loroglossin.
O. maculata contains anthocyanins and phenolic acids, which are potent antioxidants and have a nourishing impact. These compounds have the ability to inhibit collagenase, an enzyme that degrades collagen in the skin and hair. Catechin (Figure 13), for instance, influences collagen and makes it collagenase -resistant. Catechin also forms a complex with collagen, modifying its structure and making it resistant to enzyme degradation. Flavonoids, in general, contribute to scalp elasticity and nutrition, strengthen blood vessel walls, and enhance blood flow. Furthermore, polyphenols manifest antimicrobial properties, which makes them a valuable ingredient in medicines used to treat mycoses [251]:
Figure 13.
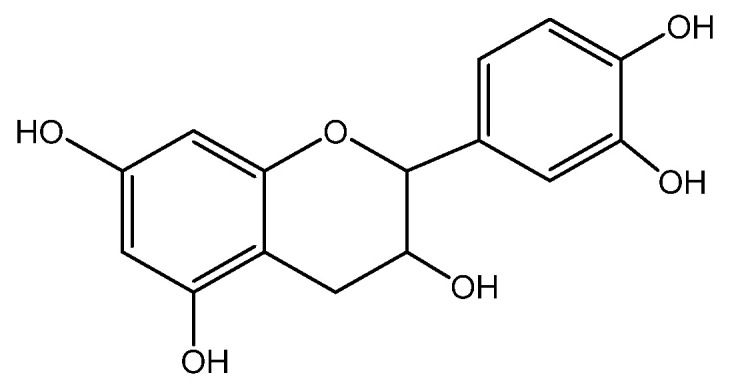
Catechin.
In dermatology, the oral use of Orchis maculata L. extract is prevalent in folk medicine for senile itching, skin tuberculosis, and other dermatoses accompanied by cachexia and chronic diseases of the respiratory and gastrointestinal tracts. The extract is also employed for the speedy healing of wounds and ulcers [244]. Additionally, cosmetic skincare products containing the extract and produced on an industrial scale are available [245].
1.19. Pastinaca sativa L. Seler Family—Apiaceae
Parsnip (Pastinaca sativa L.) is a plant species that is indigenous to Europe and Asia [103], and is also found growing in South Kazakhstan [252,253].
The root of parsnip is a rich source of numerous bioactive compounds, including coumarins, furanocoumarins, polyacetylenes, essential oils, terpenes, and flavonoids [252]. Additionally, parsnip root is rich in various minerals such as potassium, manganese, magnesium, phosphorus, zinc, and iron, as well as carotene, starch, pectin, vitamins, and sugars [254].
Parsnip has been employed in traditional medicine since antiquity. According to Avicenna’s Canon it alleviates headache, stomatitis, ophthalmitis, dermatitis, and fever [1] if it is used orally. Numerous studies have demonstrated the pharmacological effects of P. sativa on various bodily systems, including the central nervous, respiratory, gastrointestinal, hepatic, skin, cardiovascular, and genitourinary systems [252], as well as its potential in mitigating stroke, atherosclerosis, and other coronary heart diseases. Additionally, P. sativa has been shown to have positive effects on cholecystitis, constipation, anorexia, stomach pain, bladder atony, spastic enterocolitis, mild insomnia, nephritis, dysuria, renal colic, endocrine disorders such as menstrual syndrome, rheumatism, vitamin deficiency, obesity, vascular diseases, infections, loss of appetite, dysmenorrhea, fever, atherosclerosis, detoxification, anemia, and diabetes [254]. Plants containing furanocoumarins have been used to treat leprosy and vitiligo [255]. Furthermore, furanocoumarins extracted from parsnips have the ability to dilate peripheral vessels and coronary vessels of the heart, eliminate spasms of the bronchi and smooth muscles of the abdominal cavity, and have a moderate sedative effect. In addition, P. sativa exhibits antioxidant and anticytolytic activities [256].
The dried seeds of P. sativa underwent steam distillation to isolate its essential oil, which was found to contain octyl acetate (78.49%) and octyl hexanoate (6.68%) as its major constituents. Remarkably, this essential oil exhibited significant antioxidant and antimicrobial activity [257]. A large amount of vitamins A and C predominate in Pastinaca sativa. These vitamins eliminate and neutralize the free radicals responsible for body diseases (chronic diseases) and premature aging [258].
Recent studies in dermatology have shown the effects of furanocoumarins on the skin. Heraclenol (Figure 14a) and oxypeucedanine hydrate (Figure 14b) were found to have a weak stimulatory effect on melanogenesis without affecting cell proliferation. Moreover, furanocoumarins have been employed in the treatment of vitiligo and psoriasis [259]. The furanocoumarins xanthotoxin and bergapten are important components in leukoderma treatment [260].
Figure 14.
Heraclenol (a) and oxypeucedanine hydrate (b).
1.20. Plantago major L. Family—Plantaginaceae
Plantago major L. (plantain) is a well-known and widely used medicinal plant. The genus Plantago L. comprises approximately 300 diverse species that flourish in temperate areas all over the world, including 16 plant species that occur in Kazakhstan [261,262]. In arid zones, P. major is comparatively scarce and is primarily found along riverbanks and in intensely irrigated crops.
Plantain is a botanical specimen that contains diverse chemical constituents, including carbohydrates, lipids, allantoin, essential and non-essential amino acids, caffeic acid derivatives, flavonoids including baicalein, scutellarein, luteolin, baicalin, apigenin, among others; phenolcarboxylic acids and their derivatives; iridoid glycosides such as aucubin, catalpol, and aukubozid; terpenoids; and alicyclic compounds such as loliolid. Furthermore, the leaves of plantain exhibit a significant concentration of phenols and their derivatives such as ferulic acid and tyrosol, tannins, and vitamin K. The seeds of plantain contain organic acids such as succinic acid, mucus, iridoids such as aucubin, sterols such as β-sitosterol, stigmasterol, campesterol, saponins, alkaloids, tannins, flavonoids such as isoquercitrin, and fatty oil. These findings have been reported in numerous sources [263,264,265,266].
For centuries plantain had been considered to possess therapeutic properties. Various parts of the plant, including mature seeds, leaves, and juice, were used for medicinal purposes. Plantain leaves were employed in the treatment of numerous diseases, including digestive, reproductive, and circulatory ailments, as well as inflammatory skin disorders [267] and urogenital and infectious diseases [268]. Moreover, plantain was used for pain relief and to reduce fever [269].
The mucus, enzymes, and phytoncides present in psyllium provide an enveloping and mucolytic effect that restores the protective function of the ciliated epithelium in the respiratory tract, leading to increased secretion of bronchial mucus and liquefaction of sputum for easy expectoration. It is noted in [270] that plantain glycoside inhibits the cough reflex, and the hemostatic properties of plantain are due to the high content of vitamin K in it, which, along with tannins, promotes blood clotting. Psyllium is also a great antioxidant and radical scavenger with immunomodulatory effects [271].
P. major is used in various types of wound and skin diseases: deep wound, purulent wound, chronic and progressive wound, malignant wound, fire burn, erysipelas, progressive blister, pruritus, irritating urticarial, and fistula. The treatment is carried out by sprinkling the plant powder on the wound or by using a bandage covered by Plantago major together with salt or without it. It is also used for head and face skin ulcers in the same manner [272]. This plant is noted as an effective medicinal plant in the treatment of acute urticaria [273]. Ursolic acid, oleanolic acid, and α-linolenic acid are three P. major components that have shown inhibitory effects on cyclooxygenase (COX-2)-catalyzed prostaglandin production. Luteolin (one of the flavonoids) also has the ability to suppress leukocyte migration and inhibit mast cell degranulation, which all together can be considered as anti-urticaria treatment strategies [274] Polysaccharides stimulate the formation of interferon, while zinc and flavonoids aid in the normalization of phagocytosis. The combination of polysaccharides with enzymes and vitamins promotes regeneration. Plantain is also used in cosmetic dermatology to treat acne scars [275,276,277].
1.21. Ribes nigrum L. Saxifrage Family—Saxifragaceae
Ribes nigrum L. is a diminutive perennial shrub indigenous to Central Europe and North Asia that has been widely cultivated globally, including in the United States [278]. Furthermore, it is known to thrive in the territory of Kazakhstan [279].
Fresh blackcurrant fruits are known to contain a diverse range of functional and biologically active compounds, including soluble sugars, flavonoids, organic acids, vitamins, polyamino acids, macro- and microelements, and unsaturated fatty acids [280,281]. Additionally, blackcurrants are a rich source of vitamin C [282]. Anthocyanins, a group of biologically active compounds, are prominently found in blackcurrant berries, as well as in its seeds and leaves [283]. Notably, blackcurrant seed oil is a valuable source of gamma-linolenic acid (γ-C18:3), stearidonic acid (C18:4), tocochromanols (primarily γ-tocopherol and α-tocopherol), and sitosterol [280].
The fruits, leaves, and shoots of Ribes nigrum, both in fresh and dried form, have been traditionally used as a multivitamin and general tonic for hypovitaminosis and beriberi, as well as for enhancing the immune system. In folk medicine, the leaves of Ribes nigrum have been used for treating various conditions, including kidney stones, gout, cystitis, urethritis, osteochondrosis, rheumatism, muscle and joint pain, exudative diathesis, eczema, and furunculosis [284]. Additionally, Ribes nigrum is used in homeopathy [285]. In a study [286], a wide range of pharmacological actions of Ribes nigrum extract, rich in anthocyanins, were indicated. Extracts containing more than 20% anthocyanins were found to exhibit antioxidant, anti-inflammatory, and phytoestrogenic activity, anti-postprandial hyperglycemic and antidiabetic effects, and cardioprotective effects. Furthermore, the anthocyanin-rich fraction of black currant peel extract has been found to exhibit a strong cytotoxic effect on human liver cancer cells, and to have a positive effect on vision and eye health.
In the field of dermatology, blackcurrant leaves have been utilized for treating skin lesions resulting from atopic dermatitis and allergic itchy dermatoses (e.g., eczema, neurodermatitis, pruritus), while leaves and fruits have been used for curing psoriasis, scleroderma, lichen planus, vasculitis, and acne vulgaris [278,287]. Ribes nigrum may be helpful in treating various skin diseases, such as atopic dermatitis, psoriasis, and acne, owing to its higher anthocyanin content [288]. The antioxidant activity of blackcurrant, attributed to the presence of flavonoids and vitamin C, has been observed to modulate cancer and inflammation signaling pathways and absorb ultraviolet radiation [281]. Vitamin C has been shown to increase the amount of the transport protein when exposed to ultraviolet light. Furthermore, the presence of fatty acids in blackcurrant makes it therapeutically efficient for treating skin diseases [289].
The authors of [290] studied the effect of a polysaccharide (CAPS) isolated from blackcurrant (Ribes nigrum) on immunomodulation in laboratory mice. The introduction of CAPS was found out to improve the symptoms of atopic dermatitis by inhibiting the migration of mast cells into the skin of the epidermis. CAPS administration was also found to suppress immunoglobulin (IgE) overproduction and induce transcription of the IFN-γ gene in the spleen.
1.22. Rosa canina L. Rose Family—Rosaceae
Rose hips have considerable economic importance and are widespread garden plants in Europe, Asia, North America, and the Middle East. The distribution of wild roses in different regions of Kazakhstan is heterogenous. In particular, a greater range of species diversity has been observed in forest and forest-steppe zones [291]. There is a total of 21 distinct species of wild rose that grow in Kazakhstan, with five of them being present in Central Kazakhstan, including R. glabrifolia, R. laxa Retz., R. Acicularis Lindl., R. majalis Herrm. (R. cinnamomea L.), and R. pimpinellifolia L. (R. spinosissima L.) [292].
The fruits of R. canina are highly valued by the food and pharmaceutical industries due to their rich content of biologically and physiologically active compounds. These include a wide range of vitamins (C, B, P, PP, E, K), flavonoids, carotenes, carbohydrates (mono- and oligosaccharides), organic acids (tartaric, citric), polyunsaturated fatty acids, trace elements, and others [293,294]. The essential oil derived from rosehips is primarily composed of alcohols, monoterpenes, and sesquiterpenes [295]. Dog rose seeds are also a valuable source of crude oil, comprising approximately 15% of their total weight. To extract oil from the seeds, various methods are employed such as pressing, solvent extraction, ultrasonic, microwave, and sub- and supercritical fluid extraction. Rosehip oil is considered particularly valuable due to its essential fatty acid content, tocopherols, phytosterols (β-sitosterol), and phenols, which contribute to its functional properties. The primary essential fatty acids present in rosehip oil are linoleic, linolenic, and oleic acids, while the γ-tocopherol isomer of tocols is the most abundant in the oil. Among the numerous health benefits of rosehip oil, its anticancer effects are particularly noteworthy. Additionally, the therapeutic effect of rosehip oil on skin diseases makes it a preferred ingredient in cosmetics [296].
Rose hips have a well-established history in traditional medicine as a preventative and treatment remedy for colds and other infections, as well as a diuretic and therapy for various inflammatory disorders. In modern medical practice, dog rose (Rosa canina L.) is incorporated into compositions and complexes for the treatment of inflammatory diseases, including but not limited to rheumatoid arthritis, reactive arthritis, osteoarthritis, and other types of arthritis. It is also helpful in upper respiratory tract infections and psoriasis. Its other application includes the prevention of oxidative stress in the oral cavity. The unique phytochemical composition of rose hips is of huge interest because it can be considered a promising source for making functional foods, natural medicines, and cosmo-nutraceuticals. Presently, rose hips are employed as a constituent in probiotic products [295].
The rose hip extract’s antioxidant activity is predominantly attributable to its ascorbic acid and polyphenolic compounds. Moreover, the extract manifests antimutagenic and anticancer properties [294].
R. canina finds a common application in cosmetology, where it is frequently utilized in conjunction with other biologically active compounds or herbal extracts. However, there are cases when it is employed as an individual ingredient; for instance, French-patented industrial technology uses dog rose extract as an active agent for curing seborrhea together with a cosmetic skincare strategy aimed at eliminating excess sebum production and dermatological manifestations caused by it [294].
1.23. Solanum dulcamara L. Solanaceae Family—Solanaceae
Solanum dulcamara L. exhibits a wide distribution across all continents except Antarctica, with the highest concentration found in tropical and subtropical regions of Australia, Africa, and select areas of Asia, including China, India, and Japan, as well as Central and South America [297]. Notably, the plant is found ubiquitously throughout Kazakhstan.
S. dulcamara is known to contain various bioactive phytocomponents, including steroidal saponins, terpenes, flavonoids, carbohydrates (such as glucose, galactose, xylose, and rhamnose), lipids (specifically cholesterol), steroidal sapogenins (such as diosgenin, tigogenin, and yamogenin), and pigments (such as lycopene and lycoxanthin) [298]. Notably, steroid alkaloids and glycoalkaloids are the primary chemical markers for this plant genus. Additionally, S. dulcamara has been found to contain steroidal alkaloids, including solanine (Figure 15) in immature fruits, solasodine in flowers, and β-solamarin in roots [299,300].
Figure 15.
Solanine.
S. dulcamara stems have traditionally been employed in folk medicine as a narcotic agent and as a remedy for conditions such as rheumatism, migraine, and severe inflammation [301].
An ethyl acetate extract obtained from the ripe fruits of S. dulcamara demonstrates significant anti-inflammatory and antioxidant activity [302]. Moreover, S. dulcamara is reputed to possess a variety of therapeutic properties, including antimicrobial, analgesic, hepatoprotective, immunomodulatory, antitumor, and neurogenetic effects [303], as well as antioxidant [300], antihyperglycemic [304], antibacterial, and antimicrobial activity [305,306], and antirheumatic activity [307]. The aerial part of S. dulcamara is particularly rich in alkaloids, which contribute to its antibacterial activity against Streptococcus pyogenes, Staphylococcus epidermidis, and S. aureus.
S. dulcamara is a known remedy for the treatment of skin diseases and warts [308]. This plant is particularly rich in the alkaloid solanine, which is abundant in its immature fruits and has been traditionally used in Kenya to treat skin mycotic infections and other pathological diseases [309]. Saponins isolated from S. dulcamara possess remarkable antioxidant activity, as they are capable of absorbing free radicals. Due to their beneficial properties, saponins are often utilized in cosmetology, where they improve the rheological and foaming properties of body-washing products and reduce the risk of skin irritation [310]. The antioxidant properties of S. dulcamara are attributed to the presence of various phenolic compounds, flavonoids, anthocyanins, and carotenoids such as lycophyll (Figure 16), as well as hydroxy and methoxy derivatives of coumarins [311].
Figure 16.
Lycophyll.
Through non-targeted LC/MS analysis, 83 metabolites have been identified in S. dulcamara fruit extracts, including 22 polyphenolic compounds comprising of 19 phenolic acid derivatives and 3 flavonoids (namely quercetin-3-O-rutinoside and kaempferol-3-O-rutinoside), 10 amides, 16 saponins, 14 steroid alkaloids, 6 lignans, and 15 other compounds [312]. Notably, the phenolic acids in these extracts are mainly composed of chlorogenic acid (Figure 6d), caffeic acid, and p-coumaric acid.
According to the investigations of the metabolites present in S. dulcamara, unripe fruits contained a higher concentration of γ-solamarin, α-solazonin, α-solanine, abutiloside H, and solanandaine compared to ripe fruits. Moreover, methanol fruit extracts were found to exhibit significant potential in eliminating DPPH and hydroxyl radicals. Interestingly, the ability of methanol extracts to remove DPPH was found to be tissue-specific, with the outer tissue (skin) of the bittersweet fruits showing a higher antioxidant activity than the inner tissues (pulp and seeds), possibly due to the higher phenol content in the peel [312].
1.24. Sorbus aucuparia L. Rose Family—Rosaceae
Sorbus aucuparia L., a botanical species known for its nutritional and medicinal beneficial properties, is considered a valuable source of edible fruits. This plant is characterized by its ability to survive in cold and harsh environments, and is widely distributed in various regions of Northern Europe, the Caucasus, the Middle East, and East Asia [313,314,315,316].
Sorbi fructus, commonly known as Rowan fruits, serve as essential medicinal resources. The berries are harvested during their complete maturation phase, from August to September, before the advent of frost. During collection, it is advisable to exercise caution and avoid damaging the branches. The stalks of harvested raw materials are cut, and then these materials are subjected to a drying process in well-ventilated rooms or dryers, employing a temperature range of 60–80 °C [317].
This fruit, popularly referred to as a “superfruit,” contains a diverse array of phytochemicals, comprising phenolic acids: neochlorogenic and chlorogenic acids (see above), flavonoids, proanthocyanidins, iridoids, coumarins, hydrolysable tannins, carotenoids, and anthocyanins, as well as vitamins (ascorbic acid, α-tocopherol, B1, B2, P, PP, K, and folic acid) [316,318,319]. Furthermore, it is rich in various sugars, phospholipids, pectin, organic acids, bitter substances, sorbic and parasorbic acids, essential oil, and macro- and microelements. The leaves of the plant contain vitamin C and flavonoids, while rowan seeds contain fatty oil (up to 22%) and glycoside amygdalin; the bark contains tannins [320,321].
Throughout history, the fruits of S. aucuparia have been utilized in traditional medicine to alleviate ailments related to cardiovascular and digestive systems. In addition to their medicinal applications, these fruits can be eaten raw or utilized in the production of jams, syrups, and as flavoring agents in alcoholic and non-alcoholic beverages, including beer and wine [322].
The fruit extracts derived from S. aucuparia have demonstrated antioxidant [323] and antitumor activity [316]. The antioxidant activity is attributed to the presence of flavonoids, vitamins C and E [319], and anthocyanins [322,324] within their composition. Moreover, the authors of [322] reported additional pharmacological effects of the fruit extracts, including antitumor, antiproliferative, antiviral, antibacterial, antifungal, and anti-inflammatory effects.
Within the field of dermatology, Rowan berries are a valuable multivitamin raw material for the treatment of allergic diseases and other skin problems due to their wound-healing properties [325].
Sorbic and parasorbic acids, present in the fruits of mountain ash, have an antimicrobial and antifungal effect. Based on the fruits of mountain ash, an ointment is prepared that has anti-inflammatory and wound-healing properties [326].
1.25. Symphytum officinale L. Borage Family—Boraginaceae
Symphytum officinale L., commonly known as comfrey, is distributed across the humid meadow and lakeside regions of Asia, Europe, and America, as supported by reference [327]. Its occurrence has also been recorded in the northwestern and eastern regions of Kazakhstan, as documented in reference [328].
Comfrey contains various chemical compounds including phenolic compounds, flavonoids, fatty acids, polysaccharides, purine derivatives, and triterpenes [329,330,331,332].
In terms of ethnopharmacology, preparations made from the roots, leaves, or entire aerial parts of comfrey have been traditionally used since ancient times to treat various internal ailments such as respiratory, gastrointestinal, and genitourinary disorders, as well as external conditions such as bruises and tumors, through the administration of tinctures, infusions, decoctions, compresses, and ointments [333,334].
The literature indicates the potential therapeutic effects of Symphytum officinale. The plant has been reported to possess anti-inflammatory, anti-apoptotic, antitumor, neuroprotective, and antioxidant properties [335]. Furthermore, comfrey has been shown to facilitate bone regeneration [336].
Allantoin (Figure 17) and rosmarinic acid (Figure 6e), identified as active compounds in comfrey, exhibit significant skin healing properties and have been applied in the treatment of a range of skin conditions:
Figure 17.
Allantoin.
Allantoin (Figure 17) has been reported to stimulate cell proliferation and tissue repair, making it a promising therapeutic agent for wound healing. Several studies have demonstrated that allantoin (Figure 17) can accelerate the healing process of wounds, reduce inflammation, and increase skin moisture, thus exhibiting a rejuvenating effect [337,338,339]. On the other hand, rosmarinic acid (Figure 6e) (see above) possesses antimicrobial, anti-inflammatory, and antioxidant properties, which make it an effective treatment agent for various skin diseases, such as psoriasis, acne, and eczema. Studies have shown that rosmarinic acid can reduce oxidative stress and inhibit the production of inflammatory cytokines in the skin, leading to improved skin health [340].
Comfrey also contains caffeic acid (Figure 18) and chlorogenic acids (Figure 6d) (see above). In vitro analyses demonstrated that caffeic acid and chlorogenic acid accelerated the proliferative response of fibroblasts, thus enhancing wound healing:
Figure 18.
Caffeic acid.
Polyphenolic compounds present in hydroalcoholic extracts were shown to possess antioxidant and free radical scavenging properties, preventing the release of reactive species responsible for the oxidative stress and tissue damage in burns [341].
Comfrey also contains tannins and pyrrolizidine alkaloids, which contribute to its anti-inflammatory and wound-healing effects [342,343].
1.26. Tanacetum vulgare L. Aster Family—Asteraceae
Tanacetum vulgare L. is a well-known medicinal plant that is distributed across Northern Europe, North America, Russia, China, North Korea, Kazakhstan, and Japan [344,345].
T. vulgare is rich in phenolic acids, flavonoids, and their derivatives [346]. The plant contains surface flavonoids, such as the methyl esters of flavones scutellarin and 6-hydroxyluteolin, as well as vacuolar flavonoids, including apigenin and luteolin 7-glucorinides. Additionally, it contains caffeic acid, glycosides, sterols such as β-sitosterol, stigmasterol, cholesterol, and campesterol, and triterpenes such as α-amirin, β-amirin, and taraxasterol [347].
In the traditional medicine of southeastern Serbia, T. vulgare flowers are commonly used to prepare tea with various therapeutic effects such as antihelminthic, carminative, antispasmodic, abdominal organ stimulant, tonic, menstruation stimulant, antidiabetic, diuretic, and antihypertensive properties [348,349]. Apart from medicinal use, T. vulgare is also utilized in the production of balms, cosmetics, dyes, insecticides, drugs, and preservatives [350]. Furthermore, T. vulgare-based preparations have been used for the treatment of several illnesses including hysteria, migraine, neuralgia, rheumatism, renal failure, and fever [347]. The same source tells about the antibacterial, antiviral, antifungal, anti-inflammatory, and immunomodulatory activity exhibited by T. vulgare.
The bioactive components of T. vulgare, including sesquiterpene lactones, volatile oils, flavonoids, and phenolic acids, have been found to possess antioxidant, anticancer, anti-inflammatory, and antiulcer properties [349].
Taraxasterol (Figure 19a), luteolin (Figure 19b), and taraxic acid (a sesquiterpene lactone) present in T. vulgare are responsible for its anti-inflammatory and antiallergic effects, making it a potential remedy for treating skin diseases such as atopic dermatitis, eczema, and psoriasis [350,351,352].
Figure 19.
Taraxasterol (a), luteolin (b).
Inulin and chlorogenic acid (Figure 6d) (see above) have demonstrated antioxidant, prebiotic, and anti-inflammatory effects, which suggest their potential use as a therapeutic approach for managing skin disorders such as acne, rosacea, and photoaging [353,354,355,356].
1.27. Taraxacum officinale Web. Family—Compositae
Taraxacum officinale Web. is a plant species commonly found in temperate climatic zones of Europe, Asia, and North America [357,358]. It can also be found in Kazakhstan, where it grows in various habitats such as wetlands, meadows, and roadsides, and occasionally in the steppes [359].
Dandelion is a plant with a rich chemical composition. Its constituents include β-carotene [360], chicory acid [361], inulin [362], sesquiterpene lactones, and triterpene compounds [363], as well as flavonoids [364] and fatty acids [365]. Dandelion also contains a variety of vitamins (A, C, D, E, and B), inositol, lecithin, and minerals, such as iron, magnesium, sodium, calcium, silicon, copper, phosphorus, zinc, and manganese [366].
According to the traditional medicine specialists’ evidence it has the tonic and diuretic properties of Taraxacum officinale, as well as its anthelmintic, anti-inflammatory, and sedative effects. Dandelion has also been shown to cure metabolic disorders and leukoformula deviations, and has been used in the treatment of hepatitis, bronchitis, pneumonia, mastitis (as a local compress), and anemia. These therapeutic effects are attributed to the various phytochemical compounds present in dandelion, including sesquiterpene lactones, triterpene compounds, flavonoids, fatty acids, and vitamins and minerals such as vitamins A, C, D, E, and B, inositol, lecithin, and minerals such as iron, magnesium, sodium, calcium, silicon, copper, phosphorus, zinc, and manganese [367,368,369,370].
Dandelion, being a versatile plant, has also found significant use in the field of dermatology owing to its potential in curing several skin diseases. Notably, Taraxacum officinale has been found to contain taraxasterol (Figure 19a), a compound that is helpful in curing melanoma [371].
Caffeic acid (Figure 18) is the predominant component of dandelion stem extract, while chlorogenic acid is predominant in dandelion leaf extract. Both extracts have the same reducing power and ability to absorb superoxide anion radical; however, the stem extract showed the strongest UVA and UVB absorption and the strongest tyrosinase inhibition. In addition, the results of molecular docking modeling have indicated that caffeic acid in the stem extract inhibits tyrosinase mainly through hydrogen bonding with its Gly165 and Pro160 residues. Thus, dandelion stem extract is a promising skin care product [372].
Additionally, the aqueous extract of dandelion has been observed to manifest high activity in inhibiting tyrosinase [373]. Dandelion extracts are commonly employed in the treatment of acne [374] and warts [375]. Furthermore, the ethyl acetate and n-butanol fractions of Taraxacum officinale Web. Have exhibited anti-inflammatory and antibacterial properties [376], the chloroform extract has been shown to possess anticancer properties [377], polyphenolic compounds in dandelion have been found to have antioxidant properties [378,379], while methanol and petroleum ether extracts have been found to have a choleretic effect [380].
1.28. Thymus serpyllum L. Lamiaceae Family—Lamiaceae
Thymus serpyllum L., commonly known as creeping thyme, Bogorodskaya grass, and thyme, is widely distributed in countries bordering with the Mediterranean, parts of Central Europe, and Asia [381]. The plant is found in the forest and forest-steppe zones of the European part of Russia, as well as in Western and Eastern Siberia, the Urals, Transbaikalia, and central regions of Kazakhstan, including the Ulytau mountains [382].
The plant is a valuable source of essential oil and pharmacologically active polyphenolic compounds, as reported in the literature [383]. Thymol is the major component of the essential oil, comprising up to 42% of the oil, alongside other constituents such as carvacrol, n-cymol, α-terpineol, and borneol. Additionally, tannins, bitterness, gum, triterpene compounds including ursolic and oleanolic acids, flavonoids, and a significant amount of mineral salts have been detected in the herb. The mature seeds of the plant have also been found to contain 33.6% fatty oil [381,384]. Thyme also exhibits a high content of flavonoid phenolic and carotenoid antioxidants, such as zeaxanthin, lutein, apigenin (Figure 10b), naringenin, and luteolin (Figure 19b) (see above), as reported previously [385].
Thymus serpyllum, a medicinal herb, is rich in oil and pharmacologically active polyphenolic compounds. It has been used in official and folk medicine to treat various ailments for many years. The herb, harvested during the flowering period, is used as a medicinal raw material after being threshed and dried in the shade or dryers at 35–40 °C. Thyme preparations have demonstrated expectorant, antimicrobial, and antifungal properties. Thyme is also used to treat a range of ailments, including sore throat, stomatitis, periodontal disease, asthma, headaches, laryngitis, and digestive system disorders [383,386]. Thyme extract has been shown to possess antitumor and antioxidant activity. Additionally, thyme is utilized as an alexiteric, emmenagogue, analgesic, and sedative, and in the form of ointments and lotions for rheumatism and skin diseases [382,387,388,389].
Due to its sedative and diuretic properties, medicines containing Thymus serpyllum can be employed for pruritic dermatoses [390]. Bulgarian herbalists think that creeping thyme can be a constituent of medicines for treating eczema, neurodermatitis, and urticaria, and can be used as an external remedy to eliminate wrinkles [391].
A study of Thymus serpyllum L. essential oil has shown that it has a strong fungistatic effect: there was almost 100% suppression of the growth of the tested fungi. Four strains of dermatomycete fungi were used in the study: Trichophyton mentagrophytes, Microsporum gypseum, Microsporum canis, and Trichophyton violaceum; two strains of mold fungi: Scopulariopsis brevicaulis, Aspergillus niger and Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH and its own isolate from dog skin (IZ 1), which causes dandruff in dogs [392].
1.29. Vaccinium myrtillus L. Cowberry Family—Ericaceae
Vaccinium myrtillus L. is a plant species that is predominantly found in forested areas in Northern Europe and North America [393], as well as in Europe, Asia, and North America [394]. Its distribution in Kazakhstan is limited to the southwestern region of Altai, situated in Eastern Kazakhstan [395].
The fruits of V. myrtillus are a rich source of bioactive compounds such as phenolic acids (chlorogenic acid being the most common), flavonoids (isoquercetin), and resveratrol in the leaf extract [393,396]. In addition, they contain polyphenols, phenolic acids, and anthocyanins [397,398]. Moreover, they are a rich source of trace elements and other phytochemicals such as organic acids, sugars, vitamins, fibers, and phenolic compounds (both anthocyanins and non-anthocyanins), glycosides (arbutin and myrtillin), peryl alcohol, resins, triterpene alcohol, pyrocatechin and pyrogallic tannins, free hydroquinone, ascorbic acid, carotene, and organic acids. They also contain retinol acetate, thiamine bromide, and pectin [399].
According to traditional medicinal practices, V. myrtillus flowers are utilized as ointments to treat a lot of skin-related diseases, including but not limited to ulcers, eczema, burns, bruises, rashes, varicose veins, and acne [393]. Moreover, this plant has demonstrated blood-glucose-lowering effects and has been shown to possess antioxidant, anti-inflammatory, and lipid-lowering properties, indicating its potential efficiency in treating chronic inflammatory diseases, including those linked to aging such as cancer and cardiovascular disease [400].
Blueberries are regarded as a valuable source of antioxidants, which explains their utilization in treating numerous ailments (e.g., inflammation, cardiovascular disease, cancer, diabetes, and aging-related diseases) linked to an increased oxidative stress [359,393,397,400].
Blueberry leaves have been found to possess hypoglycemic effects, attributed to the presence of myrtillin glycoside, which acts similarly to insulin and regulates pancreatic function. Dried blueberries are known for their astringent properties, while fresh blueberries are known to have carminative, anti-inflammatory, diuretic, hemostatic, antibiotic, and vitamin properties, and can regulate metabolism and digestive activity. In traditional medicine, blueberries have been used to treat various ailments such as bile duct and bladder stones, coughs, scurvy, and pulmonary tuberculosis. They have also been used to treat gastroenterocolitis and diarrhea, particularly in children. Due to their high content of vitamin C, blueberries have been used for scurvy treatment and external application to cure stomatitis and pharyngitis, which are accompanied by oral cavity wounds and ulcers [401,402].
The high antioxidant potential of blueberry seed oil, which contains chlorogenic acid (Figure 6d), isoquercetin (Figure 6b), and resveratrol, as well as α-linolenic, linoleic, and oleic acids, has been well identified. Furthermore, a plant extract of isoquercetin (Figure 6b) has been found to have a dose-dependent inhibitory effect on edema caused by allergic contact dermatitis [393,403].
The component composition of V. myrtillus species is represented by various groups of phenolic compounds, which are known to be effective exogenous factors of antioxidant protection [404]. Currently, the great popularity of Scots blueberries is due to the high content of anthocyanins (Figure 20) with antioxidant activity [405].
Figure 20.
Anthocyanins.
In the case of skin diseases, long-lasting wounds, and ulcers, infusions made of fruits or leaves in the form of perfumes are applied externally.
1.30. Viscum album L. Family Beltflower—Lorantliaceae
Viscum album L., commonly known as white mistletoe, is an evergreen hemiparasitic plant that grows widely in the Caucasus, Europe, and western and southern Asia [406,407].
Various chemical components have been identified in mistletoe through chemical studies, including viscotoxins (a mixture of amino acids), phenylpropanes, lignans, flavonoids, amines (viscalbin, norviscalbin, tyramine, β-phenylethylamine viscamine), α-viscol (β-amirin), β-viscol (lupeol), polysaccharides, lectins, fatty acids (oleic, linoleic, and palmitic acids), alcohols (pinit, inositol, quebrachite), resinous substances, and mineral salts [408,409,410,411]. Moreover, syringinin glycoside was detected in mistletoe bark [411]. Triterpene saponins (oleanolic and ursolic acids), vitamin C, carotene, vecerin, viscol, and choline derivatives (propionylcholine and acetylcholine) have also been found in this plant, the levels of which depend on the host tree on which the mistletoe grows, according to the authors of [412].
Viscum album L. has a rich ethnopharmacological history, with traditional uses including the treatment of various ailments such as epilepsy, anxiety, hypertension, internal bleeding, atherosclerosis, inflammation, and headaches. Additionally, it has been used as an antidote in some cultures [413,414].
Mistletoe-based preparations possess hypotensive and analgesic effects. For instance, a tincture of fresh mistletoe leaves, found in the “Akofit” preparation, is utilized to treat acute radiculitis [412]. The vasodilators “Omelen” and “Viskalen” are recommended for hypertension, while the liquid and dry extract “Reviscen” is useful for treating atherosclerosis, as it decreases blood pressure, dilates blood vessels, enhances cardiac activity, reduces nervous system excitability and intestinal atony, and acts as a hemostatic agent [415]. The active compound viscotoxin effectively cures cancer and inhibits its progression. The lectin present in mistletoe is a natural pesticide that hinders bacterial and parasitic infiltration into the body [416]. Additionally, Viscum album L. exhibits antioxidant [410], antitumor [414], antiviral, antibacterial, anti-inflammatory, antiepileptic, and immunostimulatory activity, and is also employed to treat neurological disorders [417,418,419,420]. Preparations containing white mistletoe are utilized in obstetric and gynecological practice and are prescribed for colpitis and prolonged uterine bleeding [421,422].
Studies have shown that methanol extract significantly reduces the pigmentation of primary human melanocytes. In addition, reporter promoter analysis showed that the methanol extract inhibits the transcription of microphthalmia-associated transcription factor, melanophilin, tyrosinase protein-2, and tyrosinase genes in melanoma cells [423].
The presence of viscumneoside III and viscumneoside V in Viscum album L. extract was found to significantly inhibit the expression of monocyte chemoattractant protein 1 (MCP-1). These results imply that Viscum album L. extract, as well as its active components, viscumneoside III and viscumneoside V, can regulate the production of MCP-1 and may have the ability to reduce skin toxicity induced by erlotinib by altering the activity of macrophages, without interfering with the anticancer effect of the drug [424].
Research of an alcoholic extract of mistletoe has indicated its efficiency in treating skin cancer. The extract is assumed to boost immune mechanisms, which in turn restrain the proliferation of cancerous cells. Additionally, when V. album extract was administered in combination with doxorubicin, it exhibited a synergistic effect, enhancing the antitumor impact on Ehrlich tumor cells [425]. Mistletoe has been proved as a promising treating remedy in the field of dermatology, where it has been employed to manage various cutaneous conditions such as dermatitis, age-related pigmentation, moles, acne, and papillomas, as well as psoriasis and rashes [426].
2. Discussion
In this review, we systematically summarized 30 plants of the flora of Kazakhstan, which were used traditionally for the treatment of skin diseases. As shown by analysis of the scientific literature, in Kazakhstan, these plants have been insufficiently studied to identify the pharmacological activity in the treatment of skin diseases. Earlier works [427,428] described a number of plants that were limited to the data on their use in the treatment of various types of dermatosis.
While conducting a literature search, we paid attention to the phytochemical composition of plants, especially to those secondary metabolites that condition the pharmacological effect in the treatment of skin diseases. Mechanisms of action of these biologically active compounds are described for some plants.
The Table 1 shows medicinal plants with an indication of their main biologically active substances and biological/pharmacological activity.
Table 1.
Medicinal plants used in the treatment of skin diseases.
| Family and Scientific Name | Traditional Use | Biologically Active Compounds | Biological Activity | References |
|---|---|---|---|---|
|
Аrасеае:
Acorus calamus |
pyoderma, acne vulgaris, alopecia eczema | essential oil, tannins, flavone, β-azarone, terpenes (cineol, limonene), proazulene | antioxidant, anti-inflammatory, antiulcer, antimicrobial, wound healing | [30,31,32,36] |
|
Asteraceae:
Achillea millefolium |
acne, eczema, neurodermatitis, urticarial, vasculitis |
sesquiterpenes (chamazulene) monoterpenes (camphor, thujol), flavone glycosides (apigenin, luteolin) | disinfectant, anti-inflammatory, antibacterial, antioxidant, antimicrobial, antiulcer | [18,22,23,26] |
| Artemisia absinthium | dermal fibroblasts, supporting or restoring elastin in the skin | flavonoids, phenols, and tannins | antibacterial, anti-inflammatory, antimicrobial, antiviral, antioxidant | [53,54,55,56,57] |
| Bidens tripartita L. | skin diseases such as acne and boils | essential oil, chlorophylls, flavonoids, cinnamic acid derivatives, tannins, polysaccharides, carotenoids, ascorbic acid, coumarins, chalcones, enzyme | anti-inflammatory, hemostatic, antiseptic, sedative, wound healing, antioxidant |
[72,73,74,75] |
| Cichorium intybus L. | inflammation of the skin | inulin, glycoside intibin, proteins, sugars, pectin, sesquiterpene lactones, tannins and resinous substances, choline, carotene, vitamins B, B2, PP and C, taraxasterol, phenolic acids: chlorogenic, isochlorogenic, neochlorogenic, caffeic and chicory acids | antiseptic, anti-inflammatory, moisturizing and nourishing | [101,102,108,109,110,111] |
| Gnaphalium uliginosum L. | eczema and skin cancer | flavonoids, flavonols, sesquiterpenes, diterpenes, triterpenes, phytosterols, anthraquinones, caffeylquinic and caffeylglucaric acids, and carotenoids | antioxidant, antibacterial, antifungal, | [172,173,174] |
| Matricaria recutita L. | inflammatory conditions and lesions of the skin, skin irritation | flavonoids and their glycosides, coumarins, essential oil, terpenoids | sedative, antispasmodic, antiseptic, and antiemetic properties | [22,130,220] |
| Onopordum acanthium L. | UV protection, activity against itching, wounds | saponins, alkaloids, sesquiterpene lactones, flavonoids, quercetin, triterpenoids, sterols, nitrogen-containing compounds, phenolic acids, coumarins, inulin, fatty acids, eriodictyol | antioxidant, anti-inflammatory, antibacterial | [235,236,238] |
| Tanacetum vulgare L. | skin disorders such as acne, rosacea, and photoaging | phenolic acids, flavonoids, and their derivatives, caffeic acid, glycosides, sterols, cholesterol, campesterol, triterpenes | antibacterial, antiviral, antifungal, anti-inflammatory, and immunomodulatory | [350,351,352,353,354,355,356] |
| Taraxacum officinale Web. | Acne, warts |
Taraxasterol, phenolic acids, polyphenolic compounds | Melanoma, tyrosinase inhibition, antioxidant properties | [372,373,374,375,376,377,378,379,380] |
|
Apiaceae: Eryngium planum L. |
skin wounds | flavonoids and phenolic acids, coumarin derivatives, terpene aldehyde esters, essential oils, and oligosaccharides | antioxidant, antimicrobial, anti-inflammatory | [142,144,145] |
| Pastinaca sativa L. | Stimulatory effect on melanogenesis, psoriasis, treatment of leukoderma | essential oil, heraclenol, oxypeucedanine hydrate, furanocoumarins | antioxidant | [257,258,260] |
|
Boraginaceae: Symphytum officinale L. |
therapeutic agent for wound healing, skin healing | Allantoin, rosmarinic acid, caffeic acid, chlorogenic acid | antimicrobial, anti-inflammatory, antioxidant | [335,337,340] |
|
Brassicaceae: Capsella bursa-pastoris L. |
skin diseases | flavonoids, polypeptides, choline, acetylcholine, histamine, tyramine, fatty acids, sterols, organic acids, amino acids, sulforaphane, vitamins, various trace elements | anti-inflammatory, antimicrobial, antioxidant | [88,90,91] |
|
Cannabaceae: Humulus lupulus L. |
inflammatory skin disorders in adolescents | lupulin, myrcene, linalool, kaempferol, quercetin, catechins, prenylnaringenin, geraniol, kaempferol, quercetin, catechins, prenylnaringenin | antitumor, anti-inflammatory, antiallergic, antipsoriatic, anti-collagenase | [175,180,182] |
|
Equisetaceae: Equisetum arvense L. |
skin cells and enhance skin texture | alkaloids, carbohydrates, proteins and amino acids, phytosterols, saponins, sterols, ascorbic acid, silicic acid, phenolic compounds and their glycosides, tannins, flavonoids (apigenin, genquanin, luteolin, kaempferol, quercetin), triterpenoids | antioxidant, anti-inflammatory, antibacterial, antimicrobial, antifungal | [125,126,127,128,129,130] |
|
Ericaceae: Vaccinium myrtillus L. |
skin-related ailments, eczema, burns, bruises, rashes, varicose veins, and acne | phenolic acids, flavonoids, resveratrol, polyphenols, phenolic acids, anthocyanins, organic acids, sugars, vitamins, fibers, glycosides, pyrogallic tannins, free hydroquinone, ascorbic acid, carotene, retinol acetate, thiamine bromide, pectin |
antioxidant, anti-inflammatory, lipid-lowering | [393,404,405] |
|
Fabaceae: Glycyrrhiza glabra L. |
skin diseases, skin hyperpigmentation, eczema, psoriasis, dermocosmetics |
Triterpene saponins glycyrrhizin, flavonoids, rhamnoliquirilin, liquiritigenin, lycoarylcoumarin, coumarin-GU-12, isoflavonoids, and chaconne oxyresveratrol, glabridin, liquiritin, apioside glucoliquiritin |
anti-inflammatory, antiviral, antimicrobial, antioxidant, dermatology for treating skin diseases | [154,157,160,161] |
| Ononis spinosa L. | Wound healing and eczema, dermatitis and pruritus, treatment of burns |
Isoflavonoids, pterocarpans, and dihydroisoflavonoids, comprising formononetin, calicosin, pseudobaptigenin, medicarpin, maakiain, onogenin, and sativanon, glucosides, glucoside malonates, glucoside acetates, and free aglycones | anti-inflammatory, antiviral, antimicrobial, antioxidant, anticancer, | [221,222,226] |
|
Gramineae: Agropyron repens L. |
inflammatory skin diseases, atopic dermatitis and acne | carbohydrates, pectin, triticin, thianogenic glycosides, flavonoids, saponins, essential oil, monoterpenes | skin diseases, antioxidant | [44,45] |
|
Juglandaceae: Juglans regia L. |
Skin inflammation, the treatment of acne, warts, eczema | flavonoids, quercetin, tannins, α-tocopherol | antioxidant effect | [200,201,202,203] |
|
Lamiaceae: Thymus serpyllum L. |
skin diseases | thymol, carvacrol, n-cymol, α-terpineol, borneol, ursolic, oleanolic acids, flavonoids, tannins, bitterness, gum | antioxidant, antiseptic, disinfectant | [382,390,391,392] |
|
Lorantliaceae: Viscum album L. |
skin toxicity | Lectin, viscumneoside III, viscumneoside V | antioxidant, antibacterial, antitumor | [421,423,424,426] |
|
Orchidaceae: Orchis maculata L. |
skin-preserving, | alkaloids, saponins, tannins, phenolic compounds, terpenes, sterols, flavonoids, anthocyanins | anti-inflammatory, antimicrobial, antioxidant | [244,245,251] |
|
Papaveraceae: Chelidonium majus L. |
warts, calluses, and eczema, skin rashes | Alkaloids, flavonoids, saponins, vitamins, phytosterols, aromatic and aliphatic acids, polysaccharides, alcohols, choline, tyramine, histamine, saponosides. | anti-inflammatory, antimicrobial, anticancer, antioxidant | [93,94,97] |
|
Plantaginaceae: Plantago major L. |
skin diseases, wounds, bruises, burns, and furunculosis | carbohydrates, nitrogen compounds, flavonoids, terpenoids, alicyclic compounds such as loliolid, tyrosol, tannins, vitamin K, organic acids, fatty oil. |
wound healing, antibacterial, antiviral, antioxidant | [271,274,277] |
|
Rosaceae:
Rosa sinnamotea |
Skincare–wound healing | of vitamins (C, B, P, PP, E, K), flavonoids, carotenes, carbohydrates, organic acids (tartaric, citric), polyunsaturated fatty acids, trace elements, alcohols, monoterpenes, sesquiterpenes | anti-inflammatory, antioxidant | [294,295] |
| Sorbus aucuparia L. | Skin ailments, wound-healing properties | Phenolic acids, flavonoids, proanthocyanidins, iridoids, coumarins, hydrolysable tannins, carotenoids, and anthocyanins, ascorbic acid, α-tocopherol, B1, B2, P, PP, K, and folic acid glucose, fructose, sucrose, sorbitol alcohol, phospholipids, pectin, organic acids, parasorbic acids, essential oil, macro- and microelements, glycoside amygdalin, |
anti-inflammatory, antimicrobial, antifungal effect | [320,325,326] |
|
Saxifragaceae: Ribes Nigrum L. |
Exudative diathesis, eczema, furunculosis, atopic dermatitis, allergic pruritic dermatoses: neurodermatitis, itching, psoriasis, scleroderma, lichen planus, vasculitis and acne vulgaris |
Soluble sugars, flavonoids, organic acids, vitamins, polyamino acids, macro- and microelements, and unsaturated fatty acids, gamma-linolenic acid (γ-C18:3), stearidonic acid (C18:4), tocochromanols (primarily γ-tocopherol and α-tocopherol), and sitosterol | antioxidant, antimicrobial, anti-inflammatory | [278,287,288,289,290] |
|
Solanaceae: Solanum dulcamara L. |
Treat skin mycotic infections | Solanine, saponins, phenolic compounds, flavonoids, anthocyanins, carotenoids, coumarins, phenolic acids | Antioxidant, skin diseases | [311,312] |
As we know and according to the literature data, plants Matricaria recutita, Plantago major, Chelidonium majus, Achillea millefolium, and Bidens tripartita have good potential and are used as therapeutic agents both separately and as part of phytopreparation. The high pharmacological effect is due, in most cases, to the content in plants of flavonoids (quercetin, isoquercetin, kaempferol, apigenin), phenolic acids (chlorogenic, caffeic, rosmarinic acid), tannins, and other BAS, which synergize the pharmacological action, and the pharmacological action of plant extracts is quite broad, including wound healing, antioxidant, anti-inflammatory, antimicrobial, and anticarcinogenic effects. In turn, it should be noted that there are several plant species that have been little studied for pharmacological activity in the treatment of skin diseases (Tanacetum vulgare, Gnaphalium uliginosum, etc.). These plants can be the subject of future studies, which may add to the arsenal of phytopreparations for the treatment of dermatitis, eczema, psoriasis, lichen, and other skin inflammatory processes.
3. Conclusions
In our work, we conducted a literature search, which allowed us to conclude that the medicinal plants of the flora of the Republic of Kazakhstan are rich in medicinal plants, which are widely used in medicine to create dosage forms and preparations. Most of these plants have a complex of biologically active substances that give them high biological activity. Many plants are essential for the treatment of a wide range of ailments, including skin conditions, and can be used as natural alternative medicines. In general, our results confirm the importance and value of medicinal plants of the flora of Kazakhstan for scientific and medical research.
Author Contributions
All authors conceptualized and designed this study: G.B., B.K., Y.L., I.N. and M.D.—writing—review and editing; G.B., B.K., Y.L., I.N., S.S., G.V., N.I. and M.D.—drafting and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples not available.
Funding Statement
Grant financing of scientific and (or) scientific and technical projects of the Republic of Kazakhstan for 2021–2023 with a period of implementation of 36 months on the topic: “Phytochemical composition and development of drugs for the treatment of skin diseases”, IRN AP09057982.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kelly K. History of Medicine. Early Civilizations Prehistoric Times to 500 C.E. Infobase Publishing; New York, NY, USA: 2009. [(accessed on 14 May 2023)]. pp. 29–50. (Facts on File) Available online: https://kupdf.net/download/the-history-of-medicine-2009_5af98606e2b6f51b389016b7_pdf. [Google Scholar]
- 2.Jumagaliyeva K.V., Sarmurzina N., Kairgalieva G.K. History of traditional medicine of the Kazakh people. J. Samara Sci. Cent. RAS Hist. Sci. 2020;1:117–126. doi: 10.37313/2658-4816-2020-2-1-117-126. (In Russian) [DOI] [Google Scholar]
- 3.World Health Organization . WHO Monographs on Medicinal Plants Widespread in CGM (NHH) World Health Organization; Geneva, Switzerland: 2010. p. 455. (In Russian) [Google Scholar]
- 4.Grudzinskaya L.M., Gemejiyeva N.G., Karzhaubekova Z.Z. The Kazakhstan medicinal flora survey in a leading families volume. Bull. Karaganda Univ. Ser. Biol. Med. Geogr. 2020;4:39–51. doi: 10.31489/2020BMG4/39-51. [DOI] [Google Scholar]
- 5.Gubanov I.A., Kiseleva K.V., Novikov V.S., Tikhomirov V.N. Illustrated determinant of plants of Central Russia. Mosc. Assoc. Sci. Publ. CMC Inst. Technol. Res. 2004;3:11. (In Russian) [Google Scholar]
- 6.Dekker J. The Evolutionary Ecology of Weeds and Invasive Plants. [(accessed on 14 May 2023)];Evolut. Ecol. 2010 197 Available online: https://e.eruditor.one/file/2696081/ [Google Scholar]
- 7.Kurbanov S.A. Agriculture: A Textbook for Universities. Yurayt Publishing House; Moscow, Russia: 2023. p. 252. [Google Scholar]
- 8.Ivanović M., Grujić D., Cerar J., Islamčević Razboršek M., Topalić-Trivunović L., Savić A., Kolar M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants. 2022;11:724. doi: 10.3390/antiox11040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nesterova S.G., Ogar N.P., Inelova Z.A., Karamanidi E.E. The family spectrum of the flora of the Toraigyr mountains. Bull. Treas. Biol. Ser. 2012;54:7–10. (In Russian) [Google Scholar]
- 10.Karami P., Zandi M., Ganjloo A. Evaluation of physicochemical, mechanical, and antimicrobial properties of gelatin-sodium alginate-yarrow (Achillea millefolium L.) essential oil film. J. Food Process. Preserv. 2022;46:16632. doi: 10.1111/jfpp.16632. [DOI] [Google Scholar]
- 11.Ayoobi F., Shamsizadeh A., Fatemi I., Vakilian A., Allahtavakoli M., Hassanshahi G., Moghadam-Ahmadi A. Bio-effectiveness of the main flavonoids of Achillea millefolium in the pathophysiology of neurodegenerative disorders—A review. Iran. J. Basic Med. Sci. 2017;20:604. doi: 10.22038/IJBMS.2017.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiseleva T.L. Kinetic synergism in phytotherapy: Traditional drugs from the point of view of modern scientific concepts. Tradit. Med. 2011;2:50–57. (In Russian) [Google Scholar]
- 13.Musaev F.A., Zakharova O.A., Musaeva R.F. Medicinal Plants (Textbook) [(accessed on 14 May 2023)];Int. J. Exp. Educ. 2014 :77–78. Available online: https://expeducation.ru/ru/article/view?id=6220. (In Russian) [Google Scholar]
- 14.Anishchenko L.V. Encyclopedia of Medicinal Plants. AST; Moscow, Russia: 2017. p. 208. (In Russian) [Google Scholar]
- 15.Aslanova D., Karomatov I.D. Yarrow is common in folk and scientific herbal medicine. Biol. Integr. Med. 2018;1:167–186. (In Russian) [Google Scholar]
- 16.Vazirinejad R., Ayoobi F., Arababadi M.K., Eftekharian M.M., Darekordi A., Goudarzvand M., Shamsizadeh A. Effect of aqueous extract of Achillea millefolium on the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Indian J. Pharmacol. 2014;46:303. doi: 10.4103/0253-7613.132168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale S., Colanero S., Placidi M., Di Emidio G., Tatone C., Amicarelli F., D’Alessandro A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules. 2022;27:3566. doi: 10.3390/molecules27113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadhosseini M., Sarker S.D., Akbarzadeh A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017;199:257–315. doi: 10.1016/j.jep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Masłowski M., Aleksieiev A., Miedzianowska J., Strzelec K. Potential application of peppermint (Mentha piperita L.), german chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.) as active fillers in natural rubber biocomposites. Int. J. Mol. Sci. 2021;22:7530. doi: 10.3390/ijms22147530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali S.I., Gopalakrishnan B., Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017;31:1140–1161. doi: 10.1002/ptr.5840. [DOI] [PubMed] [Google Scholar]
- 21.Applequist W.L., Moerman D.E. Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ. Bot. 2011;65:209–225. doi: 10.1007/s12231-011-9154-3. [DOI] [Google Scholar]
- 22.Maver T., Maver U., Stana Kleinschek K., Smrke D.M., Kreft S. A Review of Herbal Medicines in Wound Healing. Int. J. Dermatol. 2015;54:740–751. doi: 10.1111/ijd.12766. [DOI] [PubMed] [Google Scholar]
- 23.Shi C., Wang C., Liu H., Li Q., Li R., Zhang Y., Liu Y., Shao Y., Wang J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020;8:182. doi: 10.3389/fbioe.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakhomova E.E., Pakhomova A.E., Pakhomova Y.V., Karabintseva N.O., Ovsyanko E.V. Evaluation of wound healing, antimicrobial, anti-inflammatory effects of essential oils. J. Sib. Med. Sci. 2015;6:70. [Google Scholar]
- 25.Ghobadian Z., Ahmadi M.R.H., Rezazadeh L., Hosseini E., Kokhazadeh T., Ghavam S. In vitro evaluation of Achillea millefolium on the production and stimulation of human skin fibroblast cells (HFS-PI-16) Med. Arch. 2015;69:212. doi: 10.5455/medarh.2015.69.212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngo H.T., Hwang E., Kang H., Park B., Seo S.A., Yi T.H. Anti-inflammatory effects of Achillea millefolium on atopic dermatitis-like skin lesions in NC/Nga mice. Am. J. Chin. Med. 2020;48:1121–1140. doi: 10.1142/S0192415X2050055X. [DOI] [PubMed] [Google Scholar]
- 27.Abdul-hafiz I.Y., Egorov M.A., Suchenko L.T. Antibacterial activity of essential oil and alcohol extracts of air marsh (Acorus calamus) and camel thorn (Alhagi pseudalhagi), collected in the Astrakhan region. Vestn. Altai State Agrar. Univ. 2011;3:50–53. (In Russian) [Google Scholar]
- 28.Guryev A.M., Pozhan I.S. Research of the Chemical Composition of Rhizomes Acorus calamus L. Sciences about Man; Tomsk, Russia: 2003. p. 197. Collection of Articles on the Materials of the Fourth Congress of Young Scientists and Specialists. [Google Scholar]
- 29.Kim H., Han T.H., Lee S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009;122:149–156. doi: 10.1016/j.jep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Khwairakpam A.D., Damayenti Y.D., Deka A., Monisha J., Roy N.K., Padmavathi G., Kunnumakkara A.B. Acorus calamus: A bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018;29:107–122. doi: 10.1515/jbcpp-2016-0132. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A. Medicinal properties of Acorus calamus. J. Drug Deliv. Therapeutics. 2013;3:143–144. doi: 10.22270/jddt.v3i3.528. [DOI] [Google Scholar]
- 32.Yende S., Harle U., Rajgure D., Tuse T., Vyawahare N. Pharmacological profile of Acorus calamus: An overview. Pharmacogn. Rev. 2008;2:23. [Google Scholar]
- 33.Singh R., Sharma P.K., Malviya R. Pharmacological Properties and Ayurvedic Value of Indian Buch Plant (Acorus calamus): A Short Review. [(accessed on 14 May 2023)];Adv. Biol. Res. 2011 5:145–154. Available online: http://www.idosi.org/abr/5/3.pdf. [Google Scholar]
- 34.Marongiu B., Piras A., Porcedda S., Scorciapino A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005;53:7939–7943. doi: 10.1021/jf051100x. [DOI] [PubMed] [Google Scholar]
- 35.Balakumbahan R., Rajamani K., Kumanan K. Acorus calamus: An overview. J. Med. Plants Res. 2010;4:2740–2745. [Google Scholar]
- 36.Rajput S.B., Tonge M.B., Karuppayil S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine. 2014;21:268–276. doi: 10.1016/j.phymed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Chamorro M.M.A., Collado S.A.V., Márquez D. Effectiveness of Using Renalof in the Elimination of Kidney Stones under 10 mm Located in the Renal-Ureteral Tract. Open J. Nephrol. 2021;11:78. doi: 10.4236/ojneph.2021.111007. [DOI] [Google Scholar]
- 38.Atabayeva S., Sarsenbayev B., Prasad M.N.V., da Silva J.A.T., Kenzhebayeva S., Usenbekov B., Kotuhov Y. Accumulation of Trace Metals in Grasses of Kazakhstan: Relevance to Phytostabilization of Mine Waste and Metal-Smelting Areas. AAJPSB Spec. Issue Kazakhstan Plant Sci. Biotechnol. 2010;1:91–97. [Google Scholar]
- 39.Neagu E., Păun G., Moroeanu V., Ungureanu O., Radu G.L. Antioxidant and Antidiabetic Properties of Polyphenolic-Rich Extracts of Apium graveolens and Agropyrum repens. Rev. Roum. Chim. 2019;64:909–913. doi: 10.33224/rrch/2019.64.10.10. [DOI] [Google Scholar]
- 40.Bortolami M., Di Matteo P., Rocco D., Feroci M., Petrucci R. Metabolic Profile of Agropyron repens (L.) P. Beauv. Rhizome Herbal Tea by HPLC-PDA-ESI-MS/MS Analysis. Molecules. 2022;27:4962. doi: 10.3390/molecules27154962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsubanova N.A., Barska A.V., Cherniavski E.S. Clinical efficiency of preparations based on medical plant raw materials in the treatment of urolithiasis. Fam. Med. 2019;81:80–87. doi: 10.30841/2307-5112.1.2019.172217. [DOI] [Google Scholar]
- 42.Al-Snafi A.E. Chemical constituents and pharmacological importance of Agropyron repens—A review. Res. J. Pharmacol. Toxicol. 2015;1:37–41. [Google Scholar]
- 43.Beydokthi S.S., Sendker J., Brandt S., Hensel A. Traditionally used medicinal plants against uncomplicated urinary tract infections: Hexadecyl coumaric acid ester from the rhizomes of Agropyron repens (L.) P. Beauv. with Antiadhesive Activity against Uropathogenic E. coli. Fitoterapia. 2017;117:22–27. doi: 10.1016/j.fitote.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Anghel N., Melinte V. Polysaccharide-Based Matrix Doped with Plant Extract for Medical and Cosmetic Applications. Cellul. Chem. Technol. 2022;56:283–291. doi: 10.35812/CelluloseChemTechnol.2022.56.25. [DOI] [Google Scholar]
- 45.Petrova A.P., Krasnov E.A., Saprykina E.V., Subbotina Y.A., Ermilova E.V. The Chemical Composition of Wheat Grass and the Study of Its Antioxidant Activity in Allergic Contact Dermatitis. Chem. Pharm. J. 2009;43:30–32. doi: 10.1007/s11094-009-0231-1. (In Russian) [DOI] [Google Scholar]
- 46.Yousefi M., Zahedi S., Reverter M., Adineh H., Hoseini S.M., Van Doan H., Hoseinifar S.H. Enhanced growth performance, oxidative capacity and immune responses of common carp, cyprinus carpio fed with Artemisia absinthium extract-supplemented Diet. Aquaculture. 2021;545:737167. doi: 10.1016/j.aquaculture.2021.737167. [DOI] [Google Scholar]
- 47.Kabdulkarimova K.K., Dinzhumanova R., Olzhayeva R., Karimova A.A., Uzbekova S.I., Orazalina A., Lauenova S.A. Determination of the chemical composition and antioxidant activity of Artemisia vulgaris and Artemisia absinthium growing in the conditions of the Semey Region. Open Access Maced. J. Med. Sci. 2022;10:1512–1519. doi: 10.3889/oamjms.2022.10503. [DOI] [Google Scholar]
- 48.Dyusebaeva M.A., Kurmanbaeva A.K., Nurlybekova A.K., Aisa H.A., Jenis J. Amino-acid and fatty-acid compositions of two Artemisia species. Chem. Nat. Compd. 2018;54:1208–1210. doi: 10.1007/s10600-018-2599-1. [DOI] [Google Scholar]
- 49.Szopa A., Pajor J., Klin P., Rzepiela A., Elansary H.O., Al-Mana F.A., Ekiert H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants. 2020;9:1063. doi: 10.3390/plants9091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batiha G.E.S., Olatunde A., El-Mleeh A., Hetta H.F., Al-Rejaie S., Alghamdi S., Rivero-Perez N. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium) Antibiotics. 2020;9:353. doi: 10.3390/antibiotics9060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amidon C., Barnett R., Cathers J., Chambers B., Hamilton L., Kellett A., Kennel E., Montowski J., Thomas M.A., Watson B. In: Artemisia—An Essential Guide from the Herb Society of America. Caroline A., Thomas M., Kennel E., editors. The Herb Society of America; Kirtland, OH, USA: 2014. [Google Scholar]
- 52.Ahamad J. A Pharmacognostic Review on Artemisia absinthium. Int. Res. J. Pharm. 2019;10:25–31. doi: 10.7897/2230-8407.10015. [DOI] [Google Scholar]
- 53.Bordean M.E., Muste S., Marțiș G.S., Mureșan V., Buican B.C. Health effects of wormwood (Artemisia absinthium L.): From Antioxidant to Nutraceutical. J. Agroalim. Proc. Technol. 2021;27:211–218. [Google Scholar]
- 54.Hbika A., Daoudi N.E., Bouyanzer A., Bouhrim M., Mohti H., Loukili E.H., Kouda A., Tahiri M., Zaid A. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics. 2022;14:481. doi: 10.3390/pharmaceutics14030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hbika A., Bouyanzer A., Saadi M., El Ammari L., Benali M., Majidi L., Zarrouk A. Structural Study and Thermal Stability of Artemetin Extracted from Artemisia absinthium L. Chem. Data Collect. 2022;40:100880. doi: 10.1016/j.cdc.2022.100880. [DOI] [Google Scholar]
- 56.Benkhaled A., Boudjelal A., Napoli E., Baali F., Ruberto G. Phytochemical Profile, Antioxidant Activity and Wound Healing Properties of Artemisia absinthium Essential Oil. Asian Pac. J. Trop. Biomed. 2020;10:496. doi: 10.4103/2221-1691.294089. [DOI] [Google Scholar]
- 57.Tran T.A., Ho M.T., Song Y.W., Cho M., Cho S.K. Camphor Induces Proliferative and Anti-senescence Activities in Human Primary Dermal Fibroblasts and Inhibits UV-Induced Wrinkle Formation in Mouse Skin. Phytother. Res. 2015;12:1917–1925. doi: 10.1002/ptr.5484. [DOI] [PubMed] [Google Scholar]
- 58.Karolina Ś., Elżbieta S., Jan O., Joanna K.A. Micelle mediated extraction as a new method of obtaining the infusion of Bidens tripartite. Acta Biochim. Pol. 2016;63:543–548. doi: 10.18388/abp.2015_1223. [DOI] [PubMed] [Google Scholar]
- 59.Uysal S., Ugurlu A., Zengin G., Baloglu M.C., Altunoglu Y.C., Mollica A., Mahomoodally M.F. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem. Toxicol. 2018;111:525–536. doi: 10.1016/j.fct.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 60.Calitz C., Plessis L., Gouws C., Steyn D., Steenekamp J., Muller C., Hamman S. Herbal hepatotoxicity: Current status, examples, and challenges. Expert Opin Drug Metab. Toxicol. 2015;11:1551–1565. doi: 10.1517/17425255.2015.1064110. [DOI] [PubMed] [Google Scholar]
- 61.Boyko N.N., Bondarev A.V., Zhilyakova E.T., Pisarev D.I., Novikov O.O. Phytodrugs, analysis of Russian Federation pharmaceutical market. Research Result. Med. Pharm. 2017;3:30–38. (In Russian) [Google Scholar]
- 62.Oproshanskaya T.V. Fatty acids from Bidens tripartita HERB. Chem. Nat. Comp. 2015;51:944–945. doi: 10.1007/s10600-015-1455-9. [DOI] [Google Scholar]
- 63.Rodin M.N., Bokov D.O., Kovaleva T.Y., Bobkova N.V., Sergunova E.V., Strelyaeva A.V., Bobkova N.V., Sergunova E.V., Strelyaeva A.V., Khasanova S.R. Composition of biologically active compounds, biological and pharmacological activity of the three-part beggarticks (Bidens tripartita L.) Nveo—Nat. Volatiles Essent. Oils J. 2021;8:11039–11053. [Google Scholar]
- 64.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharm. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomczykowa M., Wróblewska M., Winnicka K., Wieczorek P., Majewski P., Celi’nska-Janowicz K., Sawczuk R., Miltyk W., Tryniszewska E., Tomczyk M. Novel gel formulations as topical carriers for the essential oil of Bidens tripartita for the treatment of candidiasis. Molecules. 2018;23:2517. doi: 10.3390/molecules23102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karazhan N.V., Buzuk G.N. Comparative study of morphological and anatomical-diagnostic signs of species of Bur-marigold herb. Pharm. Bull. 2013;1:12–19. [Google Scholar]
- 67.Tomczykowa M., Leszczyńska K., Tomczyk M., Tryniszewska E., Kalemba D. Composition of the Essential Oil of Bidens tripartita L. Roots and Its Antibacterial and Antifungal Activities. J. Med. Food. 2011;4:428–433. doi: 10.1089/jmf.2010.0066. [DOI] [PubMed] [Google Scholar]
- 68.Andrew C. Encyclopedia of Herbal Medicine. Dorling Kindersley; London, UK: 2016. p. 336. [Google Scholar]
- 69.Arkhipov O.A., Zhuravleva V.V., Alexandrova M.V., Alexandrov T.V. Safety of Herbal Medicines: Clinical and Pharmacological Aspects Demidova. Sci. Cent. Expert Eval. Med. Prod. 2020;8:165–177. doi: 10.30895/2312-7821-2020-8-4-165-177. [DOI] [Google Scholar]
- 70.Mironov A.N., Sakaeva I.V., Sakanyan E.I., Korsun L.V., Mochikina O.A. Current approaches to standartization of herbal substasnce. Vedomosti Nauchnogo tsentra ekspertizy sredstv meditsinskogo primeneniya. Bull. Sci. Cent. Expert Eval. Med. Prod. 2013;2:52–56. (In Russian) [Google Scholar]
- 71.Sambukova T.V., Ovchinnikov B.V., Ganapolski V.P., Yatmanov A.N., Shabanov P.D. Prospects for phytopreparations use in modem pharmacology. Obzory po klinicheskoy farmakologii i lekarstvennoy terapii. Rev. Clin. Pharmacol. Drug Ther. 2017;15:56–63. doi: 10.17816/RCF15256-63. (In Russian) [DOI] [Google Scholar]
- 72.Orhan N., İçöz Ü.G., Altun L., Aslan M. Anti-hyperglycaemic and antioxidant effects of Bidens tripartita and quantitative analysis on its active principles. Iran. J. Basic Med. Sci. 2016;19:1114–1124. doi: 10.22038/ijbms.2016.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olisova O.Y., Snarskaya E.S., Gladko V.V., Burova E.P. Russian traditional medicine in dermatology. Clin Dermatol. 2018;36:325–337. doi: 10.1016/j.clindermatol.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Kaskoniene V., Kaškonas P., Maruška A., Ragažinskienė O. Essential oils of Bidens tripartita L. collected during period of 3 years composition variation analysis. Acta Physiol. Plant. 2012;7:1056–1064. [Google Scholar]
- 75.Khatamov H.M., Suyarov A.A., Kireev V.V., Ziyadullaev S.H., Muhtorov S.M., Alimzhanova L.I. Efficiency of a dense extract of the sum of fl avonoids in the form of ointment at treatment contact allergic dermatitis in experiment. Immunology. 2020;41:269–273. doi: 10.33029/0206-4952-2020-41-3-269-273. (In Russian) [DOI] [Google Scholar]
- 76.Dar M.A., Ahad P., Masoodi M.H., Mir S.R., Akbar S. Edible Plants Health Diseases: Volume II: Phytochemical and Pharmacological Properties. Springer; Berlin/Heidelberg, Germany: 2022. Lady’s Purse (Capsella bursa-pastoris L.): Current Perspective on Its Ethnopharmacological, Therapeutic Potential, and Phytochemistry; pp. 425–455. [Google Scholar]
- 77.Al-Snafi A.E. The chemical constituents and pharmacological effects of Capsella bursa-pastoris—A review. Int. J. Pharmacol. Toxicol. 2015;5:76–81. [Google Scholar]
- 78.Riaz I., Bibi Y., Ahmed N. Evaluation of nutritional, phytochemical, antioxidant and cytotoxic potential of Capsella bursa-pastoris, a wild vegetable from potohar region of Pakistan. Kuwait J. Sci. 2021;48:1–11. doi: 10.48129/kjs.v48i3.9562. [DOI] [Google Scholar]
- 79.Grosso C., Vinholes J., Silva L.R., Pinho P.G.d., Gonçalves R.F., Valentão P., Jäger A.K., Andrade P.B. Chemical composition and biological screening of Capsella bursa-pastoris. Rev. Bras. Farmacogn. 2011;21:635–643. doi: 10.1590/S0102-695X2011005000107. [DOI] [Google Scholar]
- 80.Cha J.M., Kim D.H., Lee T.H., Subedi L., Kim S.Y., Lee K.R. Phytochemical Constituents of Capsella bursa-pastoris and Their Anti-inflammatory Activity. Nat. Prod. Sci. 2018;24:132–138. doi: 10.20307/nps.2018.24.2.132. [DOI] [Google Scholar]
- 81.Sushchuk N.A., Kolesnik Y.S., Kislichenko V.S., Kuznecova V.Y. Investigation of the component composition of volatile fractions of shepherd’s purse grass and black currant buds. Bull. Tajik Natl. Univ. Nat. Sci. Ser. 2013;1/3:84–88. (In Russian) [Google Scholar]
- 82.Song N., Xu W., Guan H., Liu X., Wang Y., Nie X. Several flavonoids from Capsella bursa-pastoris (L.) Medic. Asian J. Tradit. Med. 2007;2:218–222. [Google Scholar]
- 83.Xie L.K., Xu X.J., Wu X., Wang M.-J., Gao C.-F., Wang D.-M., Ren S.-M., Pan Y.-N., Liu X.-Q. Capsella bursa-pastoris (L.) Medic. extract alleviate cataract development by regulating the mitochondrial apoptotic pathway of the lens epithelial cells. J. Ethnopharmacol. 2022;284:114783. doi: 10.1016/j.jep.2021.114783. [DOI] [PubMed] [Google Scholar]
- 84.Hasan R.N., Ali M.R., Shakier S.M., Khudhair A.M., Hussin M.S., Kadum Y.A., Mohammed A.I., Abbas A.A. Antibacterial activity of aqueous and alcoholic extracts of Capsella Bursa against selected pathogenic bacteria. Am. J. BioScience. 2013;1:6–10. doi: 10.11648/j.ajbio.20130101.12. [DOI] [Google Scholar]
- 85.Cha J.M., Suh W.S., Lee T.H., Subedi L., Kim S.Y., Lee K.R. Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-inflammatory Activity. Molecules. 2017;22:1023. doi: 10.3390/molecules22061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wani M.A., Jan N., Qazi H.A., Andrabi K.I., John R. Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol. Plant. 2018;40:1–14. doi: 10.1007/s11738-018-2747-z. [DOI] [Google Scholar]
- 87.Lee K.E., Shin J., Hong I.S., Cho N.P., Cho S.D. Effect of methanol extracts of Cnidium officinale Makino and Capsella bursa-pastoris on the apoptosis of HSC-2 human oral cancer cells. Exp. Ther. Med. 2013;5:789–792. doi: 10.3892/etm.2012.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kubínová R., Spačková V., Svajdlenka E., Lučivjanská K. Antioxidant activity of extracts and HPLC analysis of flavonoids from Capsella bursa-pastoris (L.) Medik. Ceska A Slov. Farm. Cas. Ceske Farm. Spol. A Slov. Farm. Spol. 2013;62:174–176. [PubMed] [Google Scholar]
- 89.Ma Q., Guo Y., Wei R., Sang Z., Liu W., Gao L., Liu T. Flavonoids from Capsella bursa-pastoris and their hepatoprotective activities in vitro. Rev. Bras. Farmacogn. 2016;26:710–713. doi: 10.1016/j.bjp.2016.06.006. [DOI] [Google Scholar]
- 90.Rahman I.U., Afzal A., Iqbal Z., Ijaz F., Ali N., Bussmann R.W. Traditional and ethnomedicinal dermatology practices in Pakistan. Clin. Dermatol. 2018;36:310–319. doi: 10.1016/j.clindermatol.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 91.Schulz H. Utilisation of plant genetic resources for valuable raw materials in foods, cosmetics, and pharmaceutical products. Schr. Zu Genet. Ressour. 2003:182–191. [Google Scholar]
- 92.Ghoreschi K., Brück J., Kellerer C., Deng C., Deng C., Peng H., Rothfuss O., Hussain R.Z., Gocke A.R., Respa A., et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011;24:2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shivraj H.N., Hui W., Arti N., Xianmin L., Huilin D., Baskar V., Elwira S., Gansukh E., Guoyin K. Comparative analysis of metabolic variations, antioxidant potential and cytotoxic effects in different parts of Chelidonium majus L. Food Chem. Toxicol. 2021;156:112483. doi: 10.1016/j.fct.2021.112483. [DOI] [PubMed] [Google Scholar]
- 94.Maji A.K., Banerji P. Chelidonium majus L. (Greater Celandine)—A Review on Its Phytochemical and Therapeutic Perspectives. [(accessed on 14 May 2023)];Int. J. Herb. Med. 2015 3:10–27. doi: 10.22271/flora.2015.v3.i1.03. Available online: https://www.florajournal.com/vol3issue1/may2015/2-6-10.1.pdf. [DOI] [Google Scholar]
- 95.Heba F., Gomaa N.N., Fadl W.M.A., Elmashad D.M.A., Fathia A.M., Khaled G.A. Protective efficiency of Chelidonium majus extract against hepatoimmune and DNA changes induced by aflatoxin B1. J. Appl. Pharm. Sci. 2022;12:140–149. doi: 10.7324/JAPS.2022.120315. [DOI] [Google Scholar]
- 96.Maciej S., Sławomir D., Beata P., Kamil S., Ireneusz S., Daniel Z., Rob V., Sylwia Z., Paweł K., Magdalena W. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules. 2022;27:2815. doi: 10.3390/molecules27092815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nawrot J., Wilk J.M., Nawrot S., Nawrot K., Wilk B., Dawid P.R., Urbanska M., Micek I., Nowak G., Gornowicz P.J. Milky sap of greater celandine (Chelidonium majus L.) and anti-viral properties. Int. Ional J. Environ. Res. Public Health. 2020;17:1540. doi: 10.3390/ijerph17051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jyoti B.S. Chelidonium majus L.—A review on pharmacological activities and clinical effects. Glob. J. Res. Med. Plants Indig. Med. 2013;2:238. [Google Scholar]
- 99.Madjeed H.K., Dawood S.H., Hameed N.M., Mahdi R.A., Alkhafaje W.K., Mahdi R.A., Alkhafaje W.K., Salaam A.E., Hussein H.A., Hmod F.K., et al. Investigation of in vitro Cytotoxicity of Chelidonium majus against Leishmania major. Arch. Razi Inst. 2022;77:1211–1214. doi: 10.22092/ARI.2022.358758.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Janda K., Gutowska I., Geszke-Moritz M., Jakubczyk K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules. 2021;26:1814. doi: 10.3390/molecules26061814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Katiyar P., Kumar A., Mishra A.K., Dixit R.K., Kumar A., Kumar R., Gupta A.K. Kasni (Cichorium intybus L.) A propitious traditional medicinal herb. Int. J. Pharmacogn. 2015;8:368–380. [Google Scholar]
- 102.Laurenov G.V., Lavrenov V.K. Encyclopedia of Medicinal Plants. Volume 2. Publishing House “Donechchina”; Donetsk, Ukraine: 2016. p. 1440. [Google Scholar]
- 103.Khaled N.R., Monica B. Antimicrobial and antioxidant effects of Cichorium intybus aerial parts and Chemical profile. Egyp. J Chem. Artic. 2021;64:4689–4696. doi: 10.21608/ejchem.2021.83913.4112. [DOI] [Google Scholar]
- 104.Cicillin A. Medicinal Plants in and around the Country. Litres; Moscow, Russia: 2014. [(accessed on 14 May 2023)]. p. 4966. Complete Encyclopedia. Available online: https://www.tursar.ru/page-joy.php?j=1650. [Google Scholar]
- 105.Harsahay M., Basant B., Swati A., Madhu B. Evaluation of phytochemicals, antioxidant property and effects of Cichorium intybus cultivated at foothill area of Uttarakhand on hyperglycemic rats. IP Int. J. Comp. Adv. Pharm. 2022;7:54–64. [Google Scholar]
- 106.Perović J., Šaponjac V.T., Kojić J., Krulj J., Moreno D.A., Garcia-Viguera C., Bodroza-Solarov M., Ilic N. (Cichorium intybus L.) as a food ingredient-Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021;336:127676. doi: 10.1016/j.foodchem.2020.127676. [DOI] [PubMed] [Google Scholar]
- 107.Khayrullina Z.A., Canarian A.V. Phytochemical composition of chicory products (Cichoriumintybus L.) J. Bull. Int. Cold Acad. 2016:21–25. [Google Scholar]
- 108.Süntar I., Akkol E.K., Keles H., Yesilada E., Sarker S.D., Baykal T. Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: Stepwise isolation of an active component by in vivo bioassay and its mode of activity. J. Ethnopharmacol. 2012;143:299–309. doi: 10.1016/j.jep.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 109.Popova E.A., Shatalova T.A., Michnik L.A., Michnik O.V., Hayrapetova A.Y. Study of sales of medicinal plants by retail pharmacies and level of their consumption in sanatoriums on kmv. Mod. Prob. Sci. Edu. 2015;3:263. [Google Scholar]
- 110.Lebeda A.F., Giurenko N.I., Isaikina A.P., Sobko V.G. Med. Plants. ACT-Press; Moscow, Russia: 2010. p. 494. The Most Complete Encyclopedia. [Google Scholar]
- 111.Migliorini A.A., Piroski C.S., Daniel T.G., Cruz T.M., Escher G.B., Carmo M.A.V., Azevedo L., Marques M.B., Granato D. Neiva Red Chicory (Cichorium Intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019;84:990–1001. doi: 10.1111/1750-3841.14506. [DOI] [PubMed] [Google Scholar]
- 112.Maia Campos P.M.B.G., Mercurio D.G., Melo M.O., Closs-Gonthier B. Cichorium intybus root extract: A “vitamin D-like” active ingredient to improve skin barrier function. J. Dermatol. Treat. 2017;28:78–81. doi: 10.1080/09546634.2016.1178695. [DOI] [PubMed] [Google Scholar]
- 113.Makia R., Al Halbosiy M.M., Al Mashhadani M.H. Pharmacology of the species Equisetum (Equisetum arvense) GSC Biol. Pharm. Sci. 2022;18:290–294. doi: 10.30574/gscbps.2022.18.2.0060. [DOI] [Google Scholar]
- 114.Galina S. Wild medical plants in the phytocenoses of the Northern Kazakhstan. Med. Health Sci. J. 2012;13:128. [Google Scholar]
- 115.Amber N.P., Iris L., Dunja Š., Bernd M.L. Differential Accumulation of Metabolites and Transcripts Related to Flavonoid, Styrylpyrone, and Galactolipid Biosynthesis in Equisetum Species and Tissue Types. Metabolites. 2022;12:403. doi: 10.3390/metabo12050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Botirov E.H., Bonacheva V.M., Kolomiets N.E. Chemical Composition and Biological Activity of Metabolites of Plants of the Genus Equisetum L. Chemistry of Plant Raw Materials. Food and Agriculture Organization of the United Nations; Rome, Italy: 2021. pp. 5–26. [DOI] [Google Scholar]
- 117.Raghda M., Khulood W.A., Mohammad M.F., Mohammed H.A. Phytochemistry of the Genus Equisetum (Equisetum arvense) GSC Biol. Pharm. Sci. 2022;18:283–289. doi: 10.30574/gscbps.2022.18.2.0059. [DOI] [Google Scholar]
- 118.Nagai T., Myoda T., Nagashima T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005;91:389–394. doi: 10.1016/j.foodchem.2004.04.016. [DOI] [Google Scholar]
- 119.Niko R., Gordana S., Radosav P. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 2006;20:85–88. doi: 10.1002/ptr.1815. [DOI] [PubMed] [Google Scholar]
- 120.Dragana D.Č., Jasna M.Č., Gordana M.B., Sonja M.D., Gordana S.Ć., Vesna T.T., Bratislav T.S. Antioxidative and Antiproliferative Activities of Different Horsetail (Equisetum arvense L.) Extracts. J. Med. Food. 2010;13:452–459. doi: 10.1089/jmf.2008.0159. [DOI] [PubMed] [Google Scholar]
- 121.Bhragual D.D., Kumar N., Garg V.K., Sharma P.K. Review on plants havin g hepatoprotective activity. J. Pharm. Res. 2010;3:2077–2082. [Google Scholar]
- 122.Aldaas S. Ph.D. Thesis. King Abdullah University of Science and Technology (KAUST); Thuwal, Saudi Arabia: 2011. [(accessed on 14 May 2023)]. Cytotoxic and Antibacterial Activity of an Extract from a Saudi Traditional Medicinal Plant Equisetum arvense. Available online: https://core.ac.uk/download/pdf/132719332.pdf. [Google Scholar]
- 123.Zia-Ur-Rehman, Gurgul A., Youn I., Maldonado A., Wahid F., Che C.T., Khan T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. and evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022;29:103271. doi: 10.1016/j.sjbs.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Navdeep S.S., Sarabjit K., Divneet C. Equietum arvense: Pharmacology and phytochemistry—A review. Asian J. Pharm. Clin. Res. 2010;3:146–150. [Google Scholar]
- 125.Hayat A., Temamogullari F., Yilmaz R., Karabulut O. Effect of Equisetun arvense on wound contraction of Full-Thicnes Skin Wounds in Rabbits. J. Anim. Vet. Adv. 2011;10:81–83. [Google Scholar]
- 126.Wang L., Zhang L., Zheng G., Luo H., El-Kott A.F., El-Kenawy A.E. Equisetum arvense L. aqueous extract: A novel chemotherapeutic supplement for treatment of human colon carcinoma. Arch. Med. Sci. 2021:1–7. doi: 10.5114/aoms/99839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carneiro D.M., Jardim T.V., Araújo Y.C.L., Arantes A.C., de Sousa A.C., Barroso W.K.S., Sousa A.L.L., da Cunha L.C., Cirilo H.N.C., Bara M.T.F., et al. Equisetum arvense: New evidences supports medical use in daily clinic. Pharmacogn. Rev. 2019;13:50–58. doi: 10.5530/phrev.2019.2.4. [DOI] [Google Scholar]
- 128.Oh H., Kim D.H., Cho J.H., Kim Y.C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J. Ethnopharmacol. 2004;95:421–424. doi: 10.1016/j.jep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 129.Kim H.S., Kim Y.J., Lee S.I., Chang I.S., Kang H.H., Lee O.S. 2005 Cosmetic composition containing as available ingredient the extracts of Equisetum arvense L. WO2007004771A1. KR Patent. 2005 June 30;
- 130.Dos Santos D.S., Barreto R.D.S.S., Serafini M.R., Gouveia D.N., Marques R.S., de Carvalho Nascimento L., de Carvalho Nascimento J., Guimaraes A.G. Phytomedicines Containing Matricaria Species for the Treatment of Skin Diseases: A Biotechnological Approach. Fitoterapia. 2019;138:104267. doi: 10.1016/j.fitote.2019.104267. [DOI] [PubMed] [Google Scholar]
- 131.Wörz A., Diekmann H. Classification and evolution of the genus Eryngium, L. (Apiaceae-Saniculoideae): Results of fruit anatomical and petal morphological studies. Plant Divers. Evol. 2010;128:387–408. doi: 10.1127/1869-6155/2010/0128-0018. [DOI] [Google Scholar]
- 132.Wörz A. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae) Bot. Jahrbücher Syst. Pflanzengesch. Pflanzengeogr. 2005:253–259. doi: 10.1127/0006-8152/2005/0126-0253. [DOI] [Google Scholar]
- 133.Amantayeva M.E., Kozhanova K.K. The study of plants of the genus Eryngium as promising sources for obtaining phytosubstances. Bull. KazNMU. 2019;1:449–451. (In Russian) [Google Scholar]
- 134.Kartal M., Mitaine-Offer A.C., Abu-Asaker M., Miyamoto T., Calis I., Wagner H., Lacaille-Dubois M.A. Two new triterpene saponins from Eryngium campestre. Chem. Pharm. Bull. 2005;53:1318–1320. doi: 10.1248/cpb.53.1318. [DOI] [PubMed] [Google Scholar]
- 135.Dalar A., Türker M., Zabaras D., Konczak I. Phenolic composition, antioxidant and enzyme inhibitory activities of Eryngium bornmuelleri leaf. Plant Foods Hum. Nutr. 2014;69:30–36. doi: 10.1007/s11130-013-0393-6. [DOI] [PubMed] [Google Scholar]
- 136.Erdem S.A., Nabavi S.F., Orhan I.E., Daglia M., Izadi M., Nabavi S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015;23:1–22. doi: 10.1186/s40199-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colloca C.B., Espinar L.A., Sosa V.E. Triterpenoid saponins from Eryngium agavifolium. NPAIJ. 2014;10:61–68. [Google Scholar]
- 138.Conea S., Vlase L., Chirila I. Comparative study on the polyphenols and pectin of three Eryngium species and their antimicrobial activity. Cellul. Chem. Technol. 2016;27:363. doi: 10.3390/molecules27020363. [DOI] [Google Scholar]
- 139.Kikowska M., Budzianowski J., Krawczyk A., Thiem B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012;34:2425–2433. doi: 10.1007/s11738-012-1011-1. [DOI] [Google Scholar]
- 140.Kowalczyk M., Masullo M., Thiem B., Piacente S., Stochmal A., Oleszek W. Three new triterpene saponins from roots of Eryngium planum. Nat. Prod. Res. 2014;28:653–660. doi: 10.1080/14786419.2014.895722. [DOI] [PubMed] [Google Scholar]
- 141.Rodrigues T.L., Silva M.E., Gurgel E.S., Oliveira M.S., Lucas F.C. Eryngium foetidum L. (Apiaceae): A literature review of traditional uses, chemical composition, and pharmacological activities. Evid.-Based Complement. Altern. Med. 2022;2022:15. doi: 10.1155/2022/2896895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paun G., Neagu E., Moroeanu V., Albu C., Savin S., Lucian Radu G. Chemical and bioactivity evaluation of Eryngium planum and Cnicus benedictus polyphenolic-rich extracts. BioMed Res. Int. 2019;2019:10. doi: 10.1155/2019/3692605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chockalingam N., Muruhan S. Anti-inflammatory properties of rosmarinic acid—A review. Int. J. Res. Pharm. Sci. 2017;8:656–662. [Google Scholar]
- 144.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., Xia F.F., Modarresi-Ghazani F., et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 145.Pan M.H., Lai C.S., Ho C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- 146.Danciu C., Avram S., Pavel I.Z., Ghiulai R., Dehelean C.A., Ersilia A., Minda D., Petrescu C., Moaca E., Soica C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets. 2018;19:841–853. doi: 10.2174/1389450118666171109150731. [DOI] [PubMed] [Google Scholar]
- 147.Kashyap D., Tuli H.S., Sharma A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 148.Kuatbay E., Ustenova G., Arykbaeva A. Prospects of the flat-leaved bluebird (Eryngium planum L.) In the prevention and treatment of dermatological diseases. Bull. Bashkir State Med. Univ. 2019;4:173–178. (In Russian) [Google Scholar]
- 149.Kikowska M., Dlugaszewska J., Kubicka M.M., Kedziora I., Budzianowski J., Thiem B. In vitro antimicrobial activity of extracts and their fractions from three Eryngium L. species. Herba Pol. 2016;62:67–77. doi: 10.1515/hepo-2016-0012. [DOI] [Google Scholar]
- 150.Wang P., Su Z., Yuan W., Deng G., Li S. Phytochemical constituents and pharmacological activities of Eryngium L. (Apiaceae) Pharm. Crops. 2012;3:99–120. doi: 10.2174/2210290601203010099. [DOI] [Google Scholar]
- 151.El-Saber Batiha G., Magdy Beshbishy A., El-Mleeh A., Abdel-Daim M.M., Prasad Devkota H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae) Biomolecules. 2020;10:352. doi: 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ishmuratova M.Y., Imanbayeva A.A., Tuyakova A.T., Kopbaeva G.B. Study of common licorice (Glycyrrhiza glabra) reserves in Atyrau and Western-Kazakhstan regions. Biosci. Biotechnol. Res. Asia. 2016;13:1429. doi: 10.13005/bbra/2286. [DOI] [Google Scholar]
- 153.Alexyuk P.G., Bogoyavlenskiy A.P., Alexyuk M.S., Turmagambetova A.S., Zaitseva I.A., Omirtaeva E.S., Berezin V. Adjuvant activity of multimolecular complexes based on Glycyrrhiza glabra saponins, lipids, and influenza virus glycoproteins. Arch. Virol. 2019;164:1793–1803. doi: 10.1007/s00705-019-04273-2. [DOI] [PubMed] [Google Scholar]
- 154.Khan Ahmadi M.M., Naghdi Badi H., Akhondzadeh S., Khalighi-Sigaroodi F., Mehrafarin A., Shahriari S., Hajiaghaee R. A Review on Medicinal Plant of Glycyrrhiza glabra L. J. Med. Plants. 2013;12:1–12. [Google Scholar]
- 155.Wang K.L., Yu Y.C., Chen H.Y., Chiang Y.F., Ali M., Shieh T.M., Hsia S.M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy-and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites. 2022;12:535. doi: 10.3390/metabo12060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parvaiz M., Hussain K., Khalid S., Hussnain N., Iram N., Hussain Z., Ali M.A. A review: Medicinal importance of Glycyrrhiza glabra L. (Fabaceae family) Global J. Pharmacol. 2014;8:8–13. doi: 10.5829/idosi.gjp.2014.8.1.81179. [DOI] [Google Scholar]
- 157.Anagha K., Manasi D., Priya L., Meera M. Antimicrobial activity of yashtimadhu (Glycyrrhiza glabra L.)—A review. Int. J. Curr. Microbiol. App. Sci. 2014;3:329–336. [Google Scholar]
- 158.Sukirti U., Ashoke G., Singh V. Research Article Hair Growth Promotant Activity of Petroleum Ether Root Extract of Glycyrrhiza glabra L. (Fabaceae) in Female Rats Tropical. J. Pharm. Res. 2012;11:753–758. doi: 10.4314/tjpr.v11i5.8. [DOI] [Google Scholar]
- 159.Panichakul T., Rodboon T., Suwannalert P., Tripetch C., Rungruang R., Boohuad N., Youdee P. Additive effect of a combination of Artocarpus lakoocha and Glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals. 2020;13:310. doi: 10.3390/ph13100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nukebay A.K. Application in medicine of extracts isolated from liqorice root Glycyrrhiza glabra L.; Proceedings of the Conference Pharmaceutical Science and Practice: Problems, Achievements, Development Prospects; Kharkov, Ukraine. 15–16 April 2021; Kharkov, Ukraine: National University of Pharmacy, Kharkov; 2021. pp. 108–109. (In Russian) [Google Scholar]
- 161.Fatoki T.H., Ajiboye B.O., Aremu A.O. In Silico Evaluation of the Antioxidant, Anti-Inflammatory, and Dermatocosmetic Activities of Phytoconstituents in Licorice (Glycyrrhiza glabra L.) Cosmetics. 2023;10:69. doi: 10.3390/cosmetics10030069. [DOI] [Google Scholar]
- 162.Cerulli A., Masullo M., Montoro P., Piacente S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and their constituents as active cosmeceutical ingredients. Cosmetics. 2022;9:7. doi: 10.3390/cosmetics9010007. [DOI] [Google Scholar]
- 163.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baumann L.S. Less-known botanical cosmeceuticals. Dermatol. Ther. 2007;20:330–342. doi: 10.1111/j.1529-8019.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 165.Zheng X., Wang W., Piao H., Xu W., Shi H., Zhao C. The genus Gnaphalium L. (Compositae): Phytochemical and pharmacological characteristics. Molecules. 2013;18:8298–8318. doi: 10.3390/molecules18078298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pozdnyakova Y., Omarova G., Murzatayeva A. Wild Plants of Central Kazakhstan with Antibiotic Properties and Effect. Int. J. Agric. Biol. 2022;27:259–269. doi: 10.17957/IJAB/15.1924. [DOI] [Google Scholar]
- 167.Wang L.J., Su S., Wu J., Du H., Li S.S., Huo J.W., Wang L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chem. 2014;160:357–364. doi: 10.1016/j.foodchem.2014.03.081. [DOI] [PubMed] [Google Scholar]
- 168.Olennikov D.N., Chirikova N.K., Kashchenko N.I. Spinacetin, a new caffeoylglycoside, and other phenolic compounds from Gnaphalium uliginosum. Chem. Nat. Compd. 2015;51:1085–1090. doi: 10.1007/s10600-015-1498-y. [DOI] [Google Scholar]
- 169.Sharonova N.L., Terenzhev D.A., Bushmeleva K.N., Gumerova S.K., Lyubina A.P., Fitsev I.M., Belov T.G. Phytochemical Contents, Antimicrobial and Antioxidant Properties of Gnaphalium uliginosum L. Ethanolic Extract and Essential Oil for Agricultural Uses. Asian J. Chem. 2019;11:2672–2678. [Google Scholar]
- 170.Lubsandorzhieva P.B., Rendyuk T.D., Dargaeva T.D., Ferubko E.V. Pharmacognostic Study of Collection and Study of its Hepatoprotective Activity. Pharmacogn. J. 2021;13:713–721. doi: 10.5530/pj.2021.13.91. [DOI] [Google Scholar]
- 171.Shikov A.N., Kundracikova M., Palama T.L., Pozharitskaya O.N., Kosman V.M., Makarov V.G., Verpoorte R. Phenolic constituents of Gnaphalium uliginosum L. Phytochem. Lett. 2010;3:45–47. doi: 10.1016/j.phytol.2009.11.002. [DOI] [Google Scholar]
- 172.Goun E.A., Petrichenko V.M., Solodnikov S.U., Suhinina T.V., Kline M.A., Cunningham G., Miles H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002;81:337–342. doi: 10.1016/S0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 173.Sõukand R., Kalle R., Pieroni A. Homogenisation of biocultural diversity: Plant ethnomedicine and its diachronic change in Setomaa and Võromaa, Estonia, in the last century. Biology. 2022;11:192. doi: 10.3390/biology11020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Deev M.V., Schmidt S.V. 2004 “Antipsoriaz” Cream. 2 246 935 C1. RU Patent. 2004 April; (In Russian)
- 175.Korpelainen H., Pietiläinen M. Hop (Humulus lupulus L.): Traditional and present use, and future potential. Econ. Bot. 2021;75:302–322. doi: 10.1007/s12231-021-09528-1. [DOI] [Google Scholar]
- 176.Bizaj K., Škerget M., Košir I.J., Knez Ž. (Humulus lupulus L.) Essential Oils and Xanthohumol Derived from Extraction Process Using Solvents of Different Polarity. Horticulturae. 2022;8:368. doi: 10.3390/horticulturae8050368. [DOI] [Google Scholar]
- 177.Balciunaitiene A., Viskelis P., Viskelis J., Streimikyte P., Liaudanskas M., Bartkiene E., Streimikyte P., Liaudanskas M., Bartkiene E., Lele V. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes. 2021;9:1304. doi: 10.3390/pr9081304. [DOI] [Google Scholar]
- 178.Astray G., Gullón P., Gullón B., Munekata P.E.S., Lorenzo J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020;10:5074. doi: 10.3390/app10155074. [DOI] [Google Scholar]
- 179.Zita H., Marie-Luise F., Fabian G., Martin H., Birgit H., Anja C., Kay S., Christoph M.S., Ute W. The Anti-Inflammatory Effect of Humulus lupulus Extract in vivo Depends on the Galenic System of the Topical Formulation. Pharmaceuticals. 2022;15:350. doi: 10.3390/ph15030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Natarajan P., Katta S., Andrei I., Ambati V.B.R., Leonida M., Haas G.J. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine. 2008;15:194–201. doi: 10.1016/j.phymed.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 181.Yamaguchi N., Satoh-Yamaguchi K., Ono M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine. 2009;16:369–376. doi: 10.1016/j.phymed.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 182.Guiomar L.S.L. Ph.D. Thesis. Universidade Beira Interior; Covilhã, Portugal: 2020. Evaluation of Humulus lupulus L. Therapeutic Properties for the Treatment of Skin Diseases. [Google Scholar]
- 183.Taha N.A., Al-Wadaan M.A. Significance and use of walnut, Juglans regia Linn: A review. Adv. J. Microbiol. Res. 2021;15:1–10. [Google Scholar]
- 184.Dzhangaliev A.D., Salova T.N., Turekhanova R.M. Wild Fruit Plants of Kazakhstan. KazgosINTI; Almaty, Kazakhstan: 2001. p. 135. [Google Scholar]
- 185.Abdallah I.B., Tlili N., Martinez-Force E., Rubio A.G., PerezCamino M.C., Albouchi A., Boukhchina S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food. Chem. 2015;173:972–978. doi: 10.1016/j.foodchem.2014.10.095. [DOI] [PubMed] [Google Scholar]
- 186.Makarenkova O.G., Shevyakova L.V., Bessonov V.V. Natural trace elements of nuts are an integral part of a healthy diet. Nutr. Issues. 2016;85:202. (In Russian) [Google Scholar]
- 187.Bennacer A., Sahir-Halouane F., Aitslimane-Aitkaki S., Oukali Z., Oliveira I.V., Rahmouni N., Aissaoui M. Structural characterization of phytochemical content, antibacterial, and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal. Agric. Biotechnol. 2022;40:102304. doi: 10.1016/j.bcab.2022.102304. [DOI] [Google Scholar]
- 188.Vasipov V.V., Vytovtov A.A. Walnut (Juglans regia L.)—A promising source of biologically active substances. Food Ecol. Qual. 2016;1:223–228. (In Russian) [Google Scholar]
- 189.Ivanova R.A., Elisovetskaya D.S. Medicinal Plants: Biodiversity, Technology, Application. GSAU; Grodno, Russia: 2014. [(accessed on 14 May 2023)]. Antioxidant Activity of Extracts from Various Types of Unripe Nuts Juglans Spp. pp. 129–131. Available online: https://www.ggau.by/downloads/prints/lekarstwennyje_trawy.pdf#page=130. (In Russian) [Google Scholar]
- 190.Gupta A., Behl T., Panichayupakaranan P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019;16:100142. doi: 10.1016/j.obmed.2019.100142. [DOI] [Google Scholar]
- 191.Paudel P., Satyal P., Dosoky N.S., Maharjan S., Setzer W.N. Juglans regia and J. nigra, two trees important in traditional medicine: A comparison of leaf essential oil compositions and biological activities. Nat. Prod. Commun. 2013;8:1481–1486. doi: 10.1177/1934578X1300801038. [DOI] [PubMed] [Google Scholar]
- 192.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 193.Bittner Fialová S., Rendeková K., Mučaji P., Nagy M., Slobodníková L. Antibacterial activity of medicinal plants and their constituents in the context of skin and wound infections, considering European legislation and folk medicine—A review. Int.J. Mol. Sci. 2021;22:10746. doi: 10.3390/ijms221910746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schwindl S., Kraus B., Heilmann J. Phytochemical study of Juglans regia L. leaves. Phytochemistry. 2017;144:58–70. doi: 10.1016/j.phytochem.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 195.Boulfia M., Lamchouri F., Toufik H. Mineral analysis, in vitro evaluation of alpha-amylase, alpha-glucosidase, and beta-galactosidase inhibition, and antibacterial activities of Juglans regia L. bark extracts. BioMed Res. Int. 2021:14. doi: 10.1155/2021/1585692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Khattak P., Khalil T.F., Bibi S., Jabeen H., Muhammad N., Khan M.A., Liaqat S. Juglans regia (Walnut Tree) Bark in Dentistry: Walnut Tree Bark in Dentistry. Pak. BioMed. J. 2022;5:152–156. doi: 10.54393/pbmj.v5i2.201. [DOI] [Google Scholar]
- 197.Al-Snafi A.E. Chemical Constituents, Nutritional, Pharmacological and Therapeutic Importance of Juglans regia—A Review. [(accessed on 14 May 2023)];IOSR J. Pharm. 2018 8:1–21. Available online: http://med.utq.edu.iq/wp-content/uploads/sites/7/2021/07/chemical-constituents-nutritional-pharmcological-and-therapeutic-importance-of-juglans-regia-a-review.pdf. [Google Scholar]
- 198.Acquaviva R., D’Angeli F., Malfa G.A., Ronsisvalle S., Garozzo A., Stivala A., Ragusa S., Nicolosi D., Salmeri M., Genovese C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021;35:2076–2081. doi: 10.1080/14786419.2019.1650352. [DOI] [PubMed] [Google Scholar]
- 199.Fizeșan I., Rusu M.E., Georgiu C., Pop A., Ștefan M.G., Muntean D.M., Popa D.S. Antitussive, antioxidant, and anti-inflammatory effects of a walnut (Juglans regia L.) septum extract rich in bioactive compounds. Antioxidants. 2021;10:119. doi: 10.3390/antiox10010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Amel B., Saida C.H. Contribution to the Ethnobotanical, Phytochemical, Antimicrobial and Antioxidant Study of the Leaves Aqueous Extract of the Common Walnut” Juglans regia L. Int. J. Pharmacol. Phytochem. Ethnomedicine. 2016;7:41–52. [Google Scholar]
- 201.Hussain S.Z., Naseer B., Qadri T., Fatima T., Bhat T.A. Fruits Grown in Highland Regions of the Himalayas: Nutritional & Health Benefits. Springer; Berlin/Heidelberg, Germany: 2021. Walnut (Juglans regia)-Morphology, Taxonomy, Composition and Health Benefits; pp. 269–281. [DOI] [Google Scholar]
- 202.Santos A., Barros L., Calhelha R.C., Dueñas M., Carvalho A.M., Santos-Buelga C., Ferreira I. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crops Prod. 2013;51:430–436. doi: 10.1016/j.indcrop.2013.10.003. [DOI] [Google Scholar]
- 203.Pereira J.A., Oliveira I., Sousa A., Ferreira I.C., Bento A., Estevinho L. Bioactive properties and chemical bookcomposition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 204.Al-Snafi A., Esteves da Silva J.C., Charfi S., Candela Castillo M.E., Lamarti A., Arnao M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life. 2022;12:479. doi: 10.3390/life12040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Makubaeva A.I., Adekenova A.S., Rakhataeva A., Mamyrkhan H. Therapeutic and Cosmetic Agents Based on Biologically Active Substances of Matricaria chamomilla L. and Hypericum perforatum L. Chem. J. Kazakhstan. 2020;4:105–112. [Google Scholar]
- 206.Höferl M., Wanner J., Tabanca N., Ali A., Gochev V., Schmidt E., Kaul V.K., Singh V., Jirovetz L. Biological activity of Matricaria chamomilla essential oils of various chemotypes. Planta Med. Int. Open. 2020;7:114–121. doi: 10.1055/a-1186-2400. [DOI] [Google Scholar]
- 207.Obead A.R. Novelty effect of extract of alcohol for Matricaria chamomilla on bacterial growth. Plant Arch. 2019;19:1850–1852. [Google Scholar]
- 208.Almosawi M.B.H. A study of chemical composition and effective materials in chamomile flowers (Matricaria chamomilla) Plant Arch. 2020;20:311–312. [Google Scholar]
- 209.Asgharzade S., Rabiei Z., Rafieian-Kopaei M. Effects of Matricaria chamomilla Extract on Motor Coordination Impairment Induced by Scopolamine in Rats. Asian Pac. J. Trop. Biomed. 2015;5:829–833. doi: 10.1016/j.apjtb.2015.06.006. [DOI] [Google Scholar]
- 210.Golkhani S., Vahdati A., Modaresi M., Edalatmanesh M.A. The Effects of Matricaria chamomilla Extract during Neonatal Period of Rats on Pituitary-Gonadal Hormone Axis and Changes in Testicular Tissue of Male Progenies. Middle East J. Fam. Med. 2017;15:126–132. doi: 10.5742/MEWFM.2017.92991. [DOI] [Google Scholar]
- 211.Rafraf M., Zemestani M., Asghari-Jafarabadi M. Effectiveness of Chamomile Tea on Glycemic Control and Serum Lipid Profile in Patients with Type 2 Diabetes. J. Endocrinol. Investig. 2015;38:163–170. doi: 10.1007/s40618-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 212.Bayliak M.M., Dmytriv T.R., Melnychuk A.V., Strilets N.V., Storey K.B., Lushchak V.I. Chamomile as a Potential Remedy for Obesity and Metabolic Syndrome. EXCLI J. 2021;20:1261. doi: 10.17179/excli2021-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Awaad A.A., El-Meligy R.M., Zain G.M., Safhi A.A., Al Qurain N.A., Almoqren S.S., Zain Y.M., Sesh Adri V.D., Al-Saikhan F.I. Experimental and Clinical Antihypertensive Activity of Matricaria chamomilla Extracts and Their Angiotensin-Converting Enzyme Inhibitory Activity. Phytother. Res. 2018;32:1564–1573. doi: 10.1002/ptr.6086. [DOI] [PubMed] [Google Scholar]
- 214.Silveira E.S., Bezerra S.B., Ávila K.S., Rocha T.M., Pinheiro R.G., de Queiroz M.G.R., Leal L.K.A. Gastrointestinal effects of standardized brazilian phytomedicine (arthur de carvalho drops®) containing Matricaria recutita, Gentiana lutea and Foeniculum vulgare. Pathophysiology. 2019;26:349–359. doi: 10.1016/j.pathophys.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 215.Saidi R., Heidari H., Sedehi M., Safdarian B. Evaluating the Effect of Matricaria chamomilla and Melissa officinalis on Pain Intensity and Satisfaction with Pain Management in Patients after Orthopedic Surgery. J. Herbmed Pharmacol. 2020;9:339–345. doi: 10.34172/jhp.2020.43. [DOI] [Google Scholar]
- 216.Niknam S., Tofighi Z., Faramarzi M.A., Abdollahifar M.A., Sajadi E., Dinarvand R., Toliyat T. Polyherbal Combination for Wound Healing: Matricaria Chamomilla L. and Punica Granatum L. DARU J. Pharm. Sci. 2021;29:133–145. doi: 10.1007/s40199-021-00392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hassan D. Amerolative Influence of Chamomile (Matricaria recutita L.) on Synthetic Food Additive Induced Probable Toxicity in Male Albino Rats. J. Food Dairy Sci. 2021;12:161–170. doi: 10.21608/JFDS.2021.80037.1022. [DOI] [Google Scholar]
- 218.Gomes-Carneiro M.R., Dias D.M., De-Oliveira A.C.A.X., Paumgartten F.J. Evaluation of Mutagenic and Antimutagenic Activities of α-Bisabolol in the Salmonella/Microsome Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005;585:105–112. doi: 10.1016/j.mrgentox.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 219.Tai Y., Wang H., Yao P., Sun J., Guo C., Jin Y., Yang L., Chen Y., Shi F., Yu L., et al. Biosynthesis of α-Bisabolol by Farnesyl Diphosphate Synthase and α-Bisabolol Synthase and Their Related Transcription Factors in Matricaria recutita L. Int. J. Mol. Sci. 2023;24:1730. doi: 10.3390/ijms24021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yoon J.H., Kim M.Y., Cho J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023;24:1498. doi: 10.3390/ijms24021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Al-Snafi A.E. The traditional uses, constituents and pharmacological effects of Ononis spinosa. IOSR J. Pharm. 2020;10:53–59. [Google Scholar]
- 222.Gampe N., Darcsi A., Kursinszki L., Béni S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. J. Chromatogr. B. 2018;1091:21–28. doi: 10.1016/j.jchromb.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 223.Gampe N., Darcsi A., Lohner S., Béni S., Kursinszki L. Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC–MS/MS and NMR. J. Pharm. Biomed. Anal. 2016;123:74–81. doi: 10.1016/j.jpba.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 224.Altuner E.M., Çeter T., Lşlek C. Investigation of antifungal activity of Ononis spinosa L. ASH used for the therapy of skin infections as folk remedies. Mikrobiyoloji Bul. 2010;44:633–639. [PubMed] [Google Scholar]
- 225.Thuwaini M.M. Natural sources as promising future anticancer therapies—A review. GSC Biol. Pharm. Sci. 2022;19:84–113. doi: 10.30574/gscbps.2022.19.2.0178. [DOI] [Google Scholar]
- 226.Stojković D., Dias M.I., Drakulić D., Barros L., Stevanović M., CFR Ferreira I., Soković M.D. Methanolic extract of the herb Ononis spinosa L. is an antifungal agent with no cytotoxicity to primary human cells. Pharmaceuticals. 2020;13:78. doi: 10.3390/ph13040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dimitrova-DyulgerovA I., Stoyanova A. Lipid Composition of Carduus Thoermeri Weinm. Onopordum acanthium L. and Silybum marianum L., Growing in Bulgaria. [(accessed on 14 May 2023)];Bulg. J. Agricult. Sci. 2014 20:622–627. Available online: https://www.agrojournal.org/20/03-18.pdf. [Google Scholar]
- 228.Al-Snafi A.E. Constituents and pharmacology of Onopordum acanthium. IOSR J. Pharm. 2020;10:7–14. [Google Scholar]
- 229.Bruno M., Maggio A., Rosselli S., Safder M., Bancheva S. The metabolites of the genus Onopordum (Asteraceae): Chemistry and biological properties. Curr. Org. Chem. 2011;15:888–927. doi: 10.2174/138527211794518880. [DOI] [Google Scholar]
- 230.Tonguc M.U.H.A.M.M.E.T., ERBAŞ S. Evaluation of fatty acid compositions and some seed characters of common wild plant species of Turkey. Turk. J. Agric. For. 2012;36:673–679. doi: 10.3906/tar-1201-22. [DOI] [Google Scholar]
- 231.Garsiya E.R., Konovalov D.A., Shamilov A.A., Glushko M.P., Orynbasarova K.K. Traditional medicine plant, Onopordum acanthium L. (Asteraceae): Chemical composition and pharmacological research. Plants. 2019;8:40. doi: 10.3390/plants8020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mobli M., Qaraaty M., Amin G., Haririan I., Hajimahmoodi M., Rahimi R. Scientific evaluation of medicinal plants used for the treatment of abnormal uterine bleeding by Avicenna. Arch. Gynecol. Obstet. 2015;292:21–35. doi: 10.1007/s00404-015-3629-x. [DOI] [PubMed] [Google Scholar]
- 233.Mamedov N., Mehdiyeva N.P., Craker L.E. Medicinal plants used in traditional medicine of the Caucasus and North America. J. Med. Act. Plants. 2015;4:42–66. doi: 10.7275/R51834DS. [DOI] [Google Scholar]
- 234.Ryzhov V.M., Belchenko A.S. Issues of Diagnostics of Prickly Tartar Fruit (Onopordum acanthium L.) as a Promising Medicinal Plant Raw Material. [(accessed on 3 April 2023)];Proc. Samara Sci. Cent. Russ. Acad. Sci. 2014 16:1025–1029. Available online: https://cyberleninka.ru/article/n/issledovanie-perspektivy-kompleksnoy-pererabotki-nadzemnoy-chasti-tatarnika-kolyuchego-onopordum-acanthium-l. (In Russian) [Google Scholar]
- 235.Sharifi N., Souri E., Ziai S.A., Amin G., Amini M., Amanlou M. Isolation, identification and molecular docking studies of a new isolated compound, from Onopordon acanthium: A novel angiotensin converting enzyme (ACE) inhibitor. J. Ethnopharmacol. 2013;148:934–939. doi: 10.1016/j.jep.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 236.Csupor-Löffler B., Hajdú Z., Réthy B., Zupkó I., Máthé I., Rédei T., Hohmann J. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part II. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009;23:1109–1115. doi: 10.1002/ptr.2755. [DOI] [PubMed] [Google Scholar]
- 237.Csupor-Löffler B., Zupkó I., Molnár J., Forgo P., Hohmann J. Bioactivity-guided isolation of antiproliferative compounds from the roots of Onopordum acanthium. Nat. Prod. Commun. 2014;9:337–340. doi: 10.1177/1934578X1400900313. [DOI] [PubMed] [Google Scholar]
- 238.Polyakova V.S., Nikolaeva T.V., Setko N.P., Voronina L.G. The Role of Apoptosis Induced by Heavy Metals in the Development of Autoimmune Diseases. [(accessed on 3 April 2023)];Mod. Probl. Sci. Educ. 2016 6 Available online: https://science-education.ru/ru/article/view?id=26018. (In Russian) [Google Scholar]
- 239.Bowen A.R., Hanks A.N., Murphy K.J., Florell S.R., Grossman D. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasia. Am. J. Dermatopathol. 2004;26:177–181. doi: 10.1097/00000372-200406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Naumov S.Y., Vatanskaya I.Y. Medicinal Plants in the Flora of the Volga-Akhtuba Floodplain. [(accessed on 3 April 2023)];Sci. Notes Cape Martian Nat. Reserve. 2017 8:112–117. Available online: https://cyberleninka.ru/article/n/lekarstvennye-rasteniya-vo-flore-volgo-ahtubinskoy-poymy. (In Russian) [Google Scholar]
- 241.Vakhrameeva M.G., Denisova L.V., Nikitina S.V., Samsonov S.K. Orchidei of Our Country. Science; Moscow, Russia: 1991. p. 224. [Google Scholar]
- 242.Teoh E.S. Medicinal Orchids of Asia. Springer; Berlin/Heidelberg, Germany: 2016. Sources of medicinal orchids and conservation; pp. 691–727. [DOI] [Google Scholar]
- 243.Khadartsev A.A., Sukhiy G.T., Volochaeva M.V., Platonov V.V., Dunaeva I.V. Chromato-mass spectrometry of ethanol extract of spotted (orcmaculate, orcmacular family) Her. New Med. Technol. 2019;4:1–20. doi: 10.24411/2075-4094-2019-16354. [DOI] [Google Scholar]
- 244.Arora M., Mahajan A., Sembi J.K. A Review on phytochemical and pharmacological potential of family Orchidaceae. Int. J. Pharm. Pharm. Res. 2017;8:9–24. doi: 10.7897/2230-8407.0810176. [DOI] [Google Scholar]
- 245.Brinkmann J.A. Quick Scan of Orchidaceae Species in European Commerce as Components of Cosmetic. Food Med. Prod. 2014;1:22. [Google Scholar]
- 246.Yapo B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011;86:373–385. doi: 10.1016/j.carbpol.2011.05.065. [DOI] [Google Scholar]
- 247.Loseva A.I., Pozdnyakova A.V., Prosekov A.Y., Ostapova E.V., Al’tshuler O.G. Callus Orchis maculata L. as a source of bioactive substances: Biotechnology of cultivation. Bull. SUSU. Ser. Food Biotechnol. 2021;9:13–22. [Google Scholar]
- 248.Al-Snafi A.E. Pharmacological potential of Orchis mascula—A review. IOSR J. Pharm. 2020;10:1–6. [Google Scholar]
- 249.Rajamalar P., Kavisri M., Elangovan M., Vairamani S., Shanmugam A., Elumalai P., Seedevi P. Chemical characterization of Orchis mascula and its antibacterial efficiency against clinical isolated human pathogenic bacteria. Biomass convers. Biorefinery. 2022;1:9. doi: 10.1007/s13399-022-03022-x. [DOI] [Google Scholar]
- 250.Filippava S.N., Ditchenko T.I., Lohvina H.O., Yurin V.M. Development of an effective method for deposition of callus cultures of valuable medicinal plants. Proc. BSU. 2015;10:205–220. (In Russian) [Google Scholar]
- 251.Gantait S., Das A., Mitra M., Chen J.T. Secondary metabolites in orchids: Biosynthesis, medicinal uses, and biotechnology. S. Afr. J. Bot. 2021;139:338–351. doi: 10.1016/j.sajb.2021.03.015. [DOI] [Google Scholar]
- 252.Kenari H.M., Kordafshari G., Moghimi M., Eghbalian F., TaherKhani D. Review of pharmacological properties and chemical constituents of Pastinaca sativa. J. Pharmacopunct. 2021;24:14. doi: 10.3831/KPI.2021.24.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kupriyanov A.N., Klyuykov E.V., Ukrainskaya U.A., Kupriyanov O.A. Review of umbrella species (Apiaceae Lindl.) of the Kazakh upland. Bot. Stud. Sib. Kazakhstan. 2017;23:3–29. [Google Scholar]
- 254.Augustin I.F., Butnariu M. A review about Pastinaca sativa L. ssp. sylvestris [Mill.] secondary metabolite diversity and inducibility. J. Appl. Biotechnol. Bioeng. 2022;9:5–6. doi: 10.15406/jabb.2022.09.00277. [DOI] [Google Scholar]
- 255.Averill K.M., DiTommaso A. Wild parsnip (Pastinaca sativa): A troublesome species of increasing concern. Weed Technol. 2007;21:279–287. doi: 10.1614/WT-05-186.1. [DOI] [Google Scholar]
- 256.Winter J.C., Thieme K., Eule J.C., Saliu E.M., Kershaw O., Gehlen H. Photodermatitis and Ocular Changes in Nine Horses after Ingestion of Wild Parsnip (Pastinaca sativa) [(accessed on 14 May 2023)];BMC Vet. Res. 2022 18:80. doi: 10.1186/s12917-022-03162-2. Available online: https://link.springer.com/article/10.1186/s12917-022-03162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Symonenko N., Shpychak O., Mishchenko O., Kyslychenko V., Shpychak T., Grashchenkova S. Antioxidant and anti-cytolytic activity of parsnip (Pastinaca sativa L.) herb thick extract in conditions of catecholamine myocardiodystrophy in rats. Sci. Rise Pharm. Sci. 2022;1:70–76. doi: 10.15587/2519-4852.2022.253546. [DOI] [Google Scholar]
- 258.Matejić J.S., Džamić A.M., Mihajilov-Krstev T., Ranđelović V.N., Krivošej Z.Đ., Marin P.D. Antimicrobial potential of essential oil from Pastinaca sativa L. Biol. Nyssana. 2014;5:31–35. [Google Scholar]
- 259.Jianu C., Goleț I., Stoin D., Cocan I., Lukinich-Gruia A.T. Antioxidant activity of Pastinaca sativa L. ssp. sylvestris [Mill.] Rouy and Camus essential oil. Molecules. 2020;25:869. doi: 10.3390/molecules25040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Waksmundzka-Hajnos M., Petruczynik A., Dragan A., Wianowska D., Dawidowicz A.L., Sowa I. Influence of the extraction mode on the yield of some furanocoumarins from Pastinaca sativa fruits. J. Chromatogr. B. 2004;800:181–187. doi: 10.1016/j.jchromb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 261.Ji X., Hou C., Guo X. Physicochemical Properties, Structures, Bioactivities and Future Prospective for Polysaccharides from Plantago L. (Plantaginaceae): A Review. Int. J. Biol. Macromol. 2019;135:637–646. doi: 10.1016/j.ijbiomac.2019.05.211. [DOI] [PubMed] [Google Scholar]
- 262.Baitenov M.S. Flora of Kazakhstan. Volume 2. Gylym; Almaty, Kazakhstan: 2001. pp. 190–191. (In Russian) [Google Scholar]
- 263.Haddadian K.K., Haddadian K.K., Zahmatkash M. A review of Plantago plant. Indian J. Tradit Know. 2014;13:5. [Google Scholar]
- 264.Kassaw E., Yohannes T., Bizualem E. In vitro antibacterial activity of Plantago lanceolata against some selected standard pathogenic bacterial. Int. J. Biotechnol. 2018;7:44–50. doi: 10.18488/journal.57.2018.71.44.50. [DOI] [Google Scholar]
- 265.Nazarizadeh A., Mikaili P., Moloudizargari M., Aghajanshakeri S., Javaherypour S. Therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. J. Basic Appl. Sci. Res. 2013;3:212–221. [Google Scholar]
- 266.Abate L., Bachheti R.K., Tadesse M.G., Bachheti A. Ethnobotanical Uses, Chemical Constituents, and Application of Plantago lanceolata L. J. Chem. 2022;2022:1532031. doi: 10.1155/2022/1532031. [DOI] [Google Scholar]
- 267.Arslan E., Aygan A., Kocabaş Y.Z. Antimicrobial Activity of Plantago major Grown in Kahramanmaraş Against Bacteria Causing Hospital Infections. Ecology. 2018 (In Turkish) [Google Scholar]
- 268.Kartini K., Wati N., Gustav R., Wahyuni R., Anggada Y.F., Hidayani R., Raharjo A., Islamie R., Putra S.E.D. Wound Healing Effects of Plantago major Extract and Its Chemical Compounds in Hyperglycemic Rats. Food Biosci. 2021;41:100937. doi: 10.1016/j.fbio.2021.100937. [DOI] [Google Scholar]
- 269.Iskandarova S.F., Murotov S.B. Determination of biologically active substances of a dry extract obtained on the basis of plantain leaves. Sci. Time. 2018;2:48–51. (In Russian) [Google Scholar]
- 270.Núñez Guillén M.E., da Silva Emim J.A., Souccar C., Lapa A.J. Analgesic and Anti-Inflammatory Activities of the Aqueous Extract of Plantago major L. Int. J. Pharmacogn. 1997;35:99–104. doi: 10.1076/phbi.35.2.99.13288. [DOI] [Google Scholar]
- 271.Najafian Y., Hamedi S.S., Farshchi M.K., Feyzabadi Z. Plantago major in Traditional Persian Medicine and Modern Phytotherapy: A Narrative Review. Electron. Physician. 2018;10:6390. doi: 10.19082/6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Samuelsen A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A Review. J. Ethnopharmacol. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Adom M.B., Taher M., Mutalabisin M.F., Amri M.S., Kudos M.B.A., Sulaiman M.W.A.W., Sengupta P., Susanti D. Chemical Constituents and Medical Benefits of Plantago major. Biomed. Pharmacother. 2017;96:348–360. doi: 10.1016/j.biopha.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 274.Nemereshina O.N., Gusev N.F., Malkova T.L. Biologically active substances of the large plantain (Plantago major L.) of the steppe zone. News Orenbg. State Agrar. Univ. 2018;3:113–117. (In Russian) [Google Scholar]
- 275.Yazdian M.A., Gheisari M., Khodadoost M., Barikbin B., Yazdian M., Askarfarashah M., Kamalinejad M. Evaluation of Plantago major aqueous extract in treatment of acute urticarial. Int. J. Biosci. 2014;5:182–188. [Google Scholar]
- 276.Yazdian M.A., Khodadoost M., Gheisari M., Kamalinejad M., Ehsani A.H., Barikbin B. A Hypothesis on the Possible Potential of Plantago major in the Treatment of Urticaria. Hong Kong Med. J. 2014;3:123–126. doi: 10.31661/gmj.v3i2.244. [DOI] [Google Scholar]
- 277.Pasalar M., Tabatabaei F., Bradley R., Tajadini H., Kamali M., Hasheminasab F.S., Parvizi M.M. Mechanistic support of traditional Persian medicine for the treatment of acne vulgaris: A scoping review. J. Cosmet. Dermatol. 2022;6:2338–2348. doi: 10.1111/jocd.14464. [DOI] [PubMed] [Google Scholar]
- 278.Gopalan A., Reuben S.C., Ahmed S., Darvesh A.S., Hohmann J., Bishayee A. The health benefits of blackcurrants. Food Funct. 2012;3:795–809. doi: 10.1039/c2fo30058c. [DOI] [PubMed] [Google Scholar]
- 279.Magazhanov Z.M., Bektursunova M.Z. Research on biologically active substances of some fruit crops growing in the southeast of Kazakhstan. Food Process. Tech. Technol. 2016;43:30–35. [Google Scholar]
- 280.Pieszka M., Migdał W., Gąsior R., Rudzińska M., Bederska-Łojewska D., Pieszka M., Szczurek P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015:2015. doi: 10.1155/2015/659541. [DOI] [Google Scholar]
- 281.Ma E.Z., Khachemoune A. Flavonoids and their therapeutic applications in skin diseases. Arch. Dermatol. Res. 2023;315:321–331. doi: 10.1007/s00403-022-02395-3. [DOI] [PubMed] [Google Scholar]
- 282.Rani L., Sharma N., Singh S., Grewal A.S. Therapeutic potential of vitamin c: An overview of various biological activities. Int. J. Pharm. Qual. Assur. 2019;10:605–612. doi: 10.25258/ijpqa.10.4.8. [DOI] [Google Scholar]
- 283.Teleszko M., Wojdylo A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods. 2015;14:736–746. doi: 10.1016/j.jff.2015.02.041. [DOI] [Google Scholar]
- 284.Popova T.S., Popov D.M., Tereshina N.S. The Study of Flavonoids of Buds and Leaves of Black Currant by HPLC. [(accessed on 14 May 2023)];Pharmaciya. 2015 1:13–15. Available online: https://pharmaciyajournal.ru/sites/default/files/fulltext-pdf/25419218-2015-01-04.pdf. (In Russian) [Google Scholar]
- 285.Mikhailova I.V., Filippova Y.V., Kuzmicheva N.A., Vinokurova N.V., Ivanova E.V., Voronkova I.P. Black currant as a promising source of polyphenolic antioxidants. Int. Res. J. 2021;109:28–32. (In Russian) [Google Scholar]
- 286.Cao L., Park Y., Lee S., Kim D.O. Extraction, identification, and health benefits of anthocyanins in blackcurrants (Ribes nigrum L.) Appl. Sci. 2021;11:1863. doi: 10.3390/app11041863. [DOI] [Google Scholar]
- 287.Staszowska-Karkut M., Materska M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa) Nutrients. 2020;12:463. doi: 10.3390/nu12020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nanashima N., Horie K., Maeda H., Tomisawa T., Kitajima M., Nakamura T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients. 2018;10:495. doi: 10.3390/nu10040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Balić A., Vlašić D., Žužul K., Marinović B., Bukvić Mokos Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020;21:741. doi: 10.3390/ijms21030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ashigai H., Komano Y., Wang G., Kawachi Y., Sunaga K., Yamamoto R., Takata R., Miyake M., Yanai T. Effect of administrating polysaccharide from black currant (Ribes nigrum L.) on atopic dermatitis in NC/Nga mice. Biosci. Microbiota Food. Health. 2018;37:19–24. doi: 10.12938/bmfh.17-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Aidarkhanova G.S. Biodiversity and ecological safety of rose hips (Rosa L.) in East Kazakhstan; Proceedings of the International Scientific Conference “Perspectives of Medicinal Plant Science”; Mosocw, Russian. 1–2 November 2018; Moscow, Russia: VILAR; 2018. pp. 101–105. [Google Scholar]
- 292.Ikhsanov Y.S., Tasmagambetova G.E., Litvinenko Y.A., Burasheva G.S., Seitimova G.A. Phytochemical composition of lipophilic fraction of plants of the plant Rosa canina L. genus Rosa. Proc. Natl. Acad. Sci. Repub. Kazakhstan Chem. Technol. Ser. 2020;2:69–74. doi: 10.32014/2020.2518-1491.25. [DOI] [Google Scholar]
- 293.Kizatova M., Serik B. Chemical composition and application of dog rose hips in various industries. Med. Pharm. 2023;140:533–536. [Google Scholar]
- 294.Roman I., Stănilă A., Stănilă S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013;7:73. doi: 10.1186/1752-153X-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ahmad N., Anwar F. Essential oils in Food Preservation, Flavor and Safety. Academic Press; Cambridge, MA, USA: 2016. Rose hip (Rosa canina L.) oils; pp. 667–675. [DOI] [Google Scholar]
- 296.Kiralan M., Yildirim G. Fruit Oils: Chemistry & Functionality. Springer Nature; Basel, Switzerland: 2019. Rosehip (Rosa canina L.) Oil; pp. 803–814. [DOI] [Google Scholar]
- 297.Patel P., Prasad A., Srivastava K., Singh S.S., Chakrabarty D., Misra P. Updates on steroidal alkaloids and glycoalkaloids in Solanum spp.: Biosynthesis, in vitro production and pharmacological values. Stud. Nat. Prod. Chem. 2021;69:99–127. doi: 10.1016/B978-0-12-819487-4.00012-4. [DOI] [Google Scholar]
- 298.Isabelle P., Monica B. Highlighting the compounds with pharmacological activity from some medicinal plants from the area of Romania. Med. Aromat. Plants. 2021;10:370. [Google Scholar]
- 299.Marzouk A.M., Deans S.G., Nash R.J., Gray A.I. Genetic Transformation. INTECH Open Access Publisher; Rijeka, Croatia: 2011. Transformed root cultures of Solanum dulcamara L.: A model for studying production of secondary metabolites; pp. 271–290. [Google Scholar]
- 300.Zha X., Sun H., Hao J., Zhang Y. Efficient Synthesis of Solasodine, O-Acetylsolasodine, and Soladulcidine as Anticancer Steroidal Alkaloids. Chem. Biodivers. 2007;4:25–31. doi: 10.1002/cbdv.200790003. [DOI] [PubMed] [Google Scholar]
- 301.Popova V.T., Stoyanova M.A., Ivanova T.A., Stoyanova A.S., Dimitrova-Dyulgerova I.Z. Phytochemical composition of leaves and stems of Solanum nigrum L. and Solanum dulcamara L. (Solanaceae) from Bulgaria. IOP Conf. Ser. Mater. Sci. Eng. 2021;1031:1. doi: 10.1088/1757-899X/1031/1/012091. [DOI] [Google Scholar]
- 302.Morais M.G., Saldanha A.A., Azevedo L.S., Mendes I.C., Rodrigues J.P.C., Amado P.A., dos Santos Lima L.A.R. Antioxidant and anti-inflammatory effects of fractions from ripe fruits of Solanum lycocarpum St. Hil. (Solanaceae) and putative identification of bioactive compounds by GC–MS and LC-DAD-MS. Food Res. Int. 2022;156:111145. doi: 10.1016/j.foodres.2022.111145. [DOI] [PubMed] [Google Scholar]
- 303.Kowalczyk T., Merecz-Sadowska A., Rijo P., Mori M., Hatziantoniou S., Górski K., Sitarek P. Hidden in plants—A review of the anticancer potential of the Solanaceae family in in vitro and in vivo studies. Cancers. 2022;14:1455. doi: 10.3390/cancers14061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sabudak T., Kaya O., Cukurova E. A new biflavonoid from Solanum dulcamara L. and investigation of anti-hyperglycaemic activity of its fruit extract. Nat. Prod. Res. 2015;29:308–314. doi: 10.1080/14786419.2014.928878. [DOI] [PubMed] [Google Scholar]
- 305.Kumar P., Sharma B., Bakshi N. Biological activity of alkaloids from Solanum dulcamara L. Nat. Prod. Res. 2009;23:719–723. doi: 10.1080/14786410802267692. [DOI] [PubMed] [Google Scholar]
- 306.Fallahzadeh A.R., Mohammadi S. Assessment of the antinociceptive, anti-inflammatory, and acute toxicity effects of Solanum dulcamara essential oil in male mice. J. Babol Univ. Med. Sci. 2020;22:162–168. doi: 10.22088/jbums.22.1.162. [DOI] [Google Scholar]
- 307.Gutiérrez-Grijalva E.P., López-Martínez L.X., Contreras-Angulo L.A., Elizalde-Romero C.A., Heredia J.B. Plant alkaloids: Structures and bioactive properties. Plant-Deriv. Bioact. Chem. Mode Action. 2020:85–117. doi: 10.1007/978-981-15-2361-8_5. [DOI] [Google Scholar]
- 308.Mutlu E.C., Turker A.U. Efficient plant regeneration of bittersweet [Solanum dulcamara L.], a medicinal plant. [(accessed on 14 May 2023)];Acta Soc. Bot. Pol. 2008 77:275–280. Available online: file:///C:/Users/user/Downloads/Efficient_plant_regeneration_of_bit%20.pdf. [Google Scholar]
- 309.Neha T., Verma S.K. Aspects of Phenolic Compounds in Pharmacological Activities of Solanum Family. Mol. Biol. 2020;9:1–5. doi: 10.37421/mbl.2020.09.235. [DOI] [Google Scholar]
- 310.Nizioł-Łukaszewska Z., Bujak T. Saponins as natural raw materials for increasing the safety of bodywash cosmetic use. J. Surfactants Deterg. 2018;21:767–776. doi: 10.1002/jsde.12168. [DOI] [Google Scholar]
- 311.Khalighi S.F., Ahvazi M., Yazdani D., Kashefi M. Cytotoxicity and antioxidant activity of five plant species of Solanaceae family from Iran. J. Med. Plants. 2012;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 312.Milutinović M., Nakarada Đ., Božunović J., Todorović M., Gašić U., Živković S., Mišić D. Solanum dulcamara L. Berries: A Convenient Model System to Study Redox Processes in Relation to Fruit Ripening. Antioxidants. 2023;12:346. doi: 10.3390/antiox12020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Minkhaidarov V.Y. Medicinal & Food Plants of the Far East. PGSHA; Ussuriysk, Russia: 2015. 329p. (In Russian) [Google Scholar]
- 314.Shchulipenko I.M., Shchulipenko L.I. Green pharmacy of nature: Past and present. Phytotherapy. 2010;4:5–9. (In Ukrainian) [Google Scholar]
- 315.McAllister H. The Genus Sorbus: Mountain Ash and Other Rowans. Royal Botanic Gardens, Kiew; Richmond, VA, USA: Surrey, UK: 2005. [Google Scholar]
- 316.Lykholat Y.L., Didur O.O., Khromykh N.O., Davydov V.R., Borodai Y.S., Kravchuk K.V., Lykholat T.Y. Comparative analysis of the antioxidant capacity and secondary metabolites accumulation in the fruits of rowan (Sorbus aucuparia L.) and some closely related species. Ecol. Noospherology. 2021;32:3–8. doi: 10.15421/032101. [DOI] [Google Scholar]
- 317.Chikov P.S. Medicinal Plants. Medicine; Moscow, Russia: 2002. 496p. (In Russian) [Google Scholar]
- 318.Isaikina N.V., Kalinkina G.I., Razina T.G., Zueva E.P., Rybalkina O.I., Ulrich A.V., Fedorova E.P., Shilova A.V. Sorbus aucuparia L. fruit is a source of the drug for increasing the efficiency of tumor chemotherapy. Rus. J. Bioorg. Chem. 2018;44:899–905. doi: 10.1134/S1068162018070038. [DOI] [Google Scholar]
- 319.Šavikin K.P., Zdunić G.M., Krstić-Milošević D.B., Šircelj H.J., Stešević D.D., Pljevljakušić D.S. Sorbus aucuparia and Sorbus aria as a source of antioxidant phenolics, tocopherols, and pigments. Chem. Biodivers. 2017;14:1700329. doi: 10.1002/cbdv.201700329. [DOI] [PubMed] [Google Scholar]
- 320.Yakovlev G.P., Pancake K.F. Medicinal Plant Material, Pharmacognosy. SpecLit.; St. Petersburg, Russia: 2004. 765p. (In Russian) [Google Scholar]
- 321.Bussmann R.W., Paniagua Z., Narel Y., Sikharulidze S., Kikvidze Z., Kikodze D., Tchelidze D., Batsatsashvili K., Robbie E. Plants in the spa–the medicinal plant market of Borjomi, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017;16:25–34. [Google Scholar]
- 322.Popoviciu D.R., Negreanu-Pîrjol T. Carotenoid, Flavonoid and Total Phenolic Content of Sorbus torminalis Fruits. Rom. Arab. Int. J. Geobiodivers. 2019;3:20–25. [Google Scholar]
- 323.Sirotina K., Kazimova K., Shcherbakova Y., Akhmadullina F., Nikitin E. Study of the antioxidant activity of rowan extracts (Sorbus aucuparia) by biotesting method. IOP Conf. Ser. Earth Environ. Sci. 2022;949:012032. doi: 10.1088/1755-1315/949/1/012032. [DOI] [Google Scholar]
- 324.Razina T.G., Zueva E.P., Ulrich A.V., Rybalkina O.I., Tchaikovsky A.V., Isaikina N.V., Kalinkina G.I., Zhdanov V.V., Zyuz’Kov G.N. Antitumor effects of Sorbus aucuparia L. extract highly saturated with anthocyans and their mechanisms. Bull. Exp. Biol. Med. 2016;162:93–97. doi: 10.1007/s10517-016-3554-4. [DOI] [PubMed] [Google Scholar]
- 325.KC B., Gyawali S., Luintel S., Sharma H.P., Kunwar R.M., Bussmann R.W., Paniagua-Zambrana N.Y. Ethnobotany of the Himalayas. Springer; Cham, Switzerland: 2021. Sorbus cuspidata (Spach) Hedl Rosaceae; pp. 1917–1926. [DOI] [Google Scholar]
- 326.Koromatov I.D., Rasulova H.N. Healing properties of mountain ash. Biol. Integr. Med. 2017;7:133–139. (In Russian) [Google Scholar]
- 327.Salehi B., Sharopov F., Boyunegmez T.T., Ozleyen A., Rodríguez-Pérez C., Ezzat S.M., Martins N. Symphytum species: A comprehensive review on chemical composition, food applications and phytopharmacology. Molecules. 2019;24:2272. doi: 10.3390/molecules24122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Prozorova T.A., Chernykh I.B. Forage Plants of Kazakhstan. Pavlodar; Almaty, Kazakhstan: 2004. p. 170. (In Russian) [Google Scholar]
- 329.Mahmoudzadeh E., Nazemiyeh H., Valizadeh H., Khaleseh F., Mohammadi S., Hamedeyazdan S. Nanoencapsulation of n-butanol extract of Symphytum kurdicum and Symphytum asperrimum: Focus on phytochemical analysis, anti-oxidant and antibacterial activity. Iran. J. Basic Med. Sci. 2022;25:364. doi: 10.22038/IJBMS.2022.62032.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nastić N., Borrás-Linares I., Lozano-Sánchez J., Švarc-Gajić J., Segura-Carretero A. Comparative assessment of phytochemical profiles of comfrey (Symphytum officinale L.) root extracts obtained by different extraction techniques. Molecules. 2020;25:837. doi: 10.3390/molecules25040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Cao Z., Guo Y., Liu Z., Zhang H., Zhou H., Shang H. Ultrasonic enzyme-assisted extraction of comfrey (Symphytum officinale L.) polysaccharides and their digestion and fermentation behaviors in vitro. Process Biochem. 2022;112:98–111. doi: 10.1016/j.procbio.2021.11.008. [DOI] [Google Scholar]
- 332.Vanitha A., Kavinprashantha R., Mugendhira-na S., Shashikanth J. Conservation Of Symphytum officinale L. At Cmprh Garden, Emerald. J. Univ. Shanghai Sci. Technol. 2022;24:261–272. doi: 10.51201/JUSST/22/0133. [DOI] [Google Scholar]
- 333.Sowa I., Paduch R., Strzemski M., Zielińska S., Rydzik-Strzemska E., Sawicki J., Wójciak-Kosior M. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018;32:605–609. doi: 10.1080/14786419.2017.1326492. [DOI] [PubMed] [Google Scholar]
- 334.Trifan A., Zengin G., Sinan K.I., Skalicka-Woźniak K., Minceva M., Luca S.V. Symphytum ibericum Steven: LC–HRMS/MS-based phytochemical profile, in vitro antioxidant and enzyme inhibitory potential. Chem. Biol. Technol. Agric. 2022;9:1–12. doi: 10.1186/s40538-022-00308-0. [DOI] [Google Scholar]
- 335.Le V., Dolganyuk V., Sukhikh A., Babich O., Ivanova S., Prosekov A., Dyshlyuk L. Phytochemical analysis of Symphytum officinale root culture extract. Appl. Sci. 2021;11:4478. doi: 10.3390/app11104478. [DOI] [Google Scholar]
- 336.Vaezi S., Haghighi H.M., Farzad S.A., Arabzadeh S., Kalalinia F. Bone Regeneration by Homeopathic Symphytum officinale. Regen. Eng. Transl. Med. 2020;7:548–555. doi: 10.1007/s40883-020-00181-z. [DOI] [Google Scholar]
- 337.Seigner J., Junker-Samek M., Plaza A., D ‘Urso G., Masullo M., Piacente S., de Martin R. A Symphytum officinale root extract exerts anti-inflammatory properties by affecting two distinct steps of NF-κB signaling. Front. Pharmacol. 2019;10:289. doi: 10.3389/fphar.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Colobatiu L., Gavan A., Potarniche A.V., Rus V., Diaconeasa Z., Mocan A., Mihaiu M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019;145:104369. doi: 10.1016/j.reactfunctpolym.2019.104369. [DOI] [Google Scholar]
- 339.Zanfirescu A., Marineci C.D., Păun G., Ungureanu O., Neagu E., Chiriță C., Negreș S. Chitosan supports containing Impatiens noli-tangere and Symphytum officinale hydroalcoholic extracts in burns treatment: Antimicrobial and healing effects. Farmacia. 2021;69:948–953. doi: 10.31925/farmacia.2021.5.18. [DOI] [Google Scholar]
- 340.Grollier J.-F., Allec J., Fourcadier C., Rosenbaum G., Darmenton P. Cosmetic Compositions for the Treatment of the Hair and Skin Contain in the form of a Powder Particles Resulting from the Pulverization of at Least One Plant Substance and a Cohesion Agent. 4,569,839. U.S. Patent. 1986 February 11;
- 341.Habtemariam S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2016 doi: 10.1155/2016/2680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Middleton E., Jr., Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 343.Mahmoudzadeh E., Nazemiyeh H., Hamedeyazdan S. Anti-inflammatory Properties of the Genus Symphytum L.: A Review. Iran. J. Pharm. Res. 2022;21:e123949. doi: 10.5812/ijpr.123949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Uehara A., Akiyama S., Iwashina T. Foliar flavonoids from Tanacetum vulgare var. boreale and their geographical variation. Nat. Prod. Commun. 2015;10:403–405. doi: 10.1177/1934578X1501000307. [DOI] [PubMed] [Google Scholar]
- 345.Aidarbayeva D.K., Sholpankulova G., Jarylkapova S., Shokanova A. Natural resources of some medicinal plants of Kazakhstan. Int. Multidiscip. Sci. GeoConference SGEM. 2018;18:385–391. doi: 10.5593/sgem2018/6.2/S25.051. [DOI] [Google Scholar]
- 346.Räisänen R., Primetta A., Nikunen S., Honkalampi U., Nygren H., Pihlava J.M., von Wright A. Examining safety of biocolourants from fungal and plant sources-examples from Cortinarius and Tapinella, Salix and Tanacetum spp. and Dyed Woollen Fabrics. Antibiotics. 2020;9:266. doi: 10.3390/antibiotics9050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Vilhelmova N., Simeonova L., Nikolova N., Pavlova E., Gospodinova Z., Antov G., Nikolova I. Antiviral, cytotoxic and antioxidant effects of Tanacetum vulgare L. Crude Extract In Vitro. Folia Med. 2020;62:172–179. doi: 10.3897/folmed.62.e49370. [DOI] [PubMed] [Google Scholar]
- 348.Aćimović M., Puvača N. Tanacetum vulgare L.—A Systematic Review. J. Agron. Technol. Eng. Manag. 2020;3:416–422. [Google Scholar]
- 349.Ivănescu B., Tuchiluș C., Corciovă A., Apetrei C., Mihai C.T., Gheldiu A.-M., Vlase L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia. 2018;66:282–288. [Google Scholar]
- 350.Devrnja N., Anđelković B., Aranđelović S., Radulovic S., Sokovic M., Krstić-Milošević D., Ristić M., Galic D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017;111:212–221. doi: 10.1016/j.sajb.2017.03.028. [DOI] [Google Scholar]
- 351.Zengin G., Cvetanović A., Gašić U., Stupar A., Bulut G., Senkardes I., Dogan A., Sinan K.I., Uysal S., Aumeeruddy-Elalfi Z., et al. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020;146:112202. doi: 10.1016/j.indcrop.2020.112202. [DOI] [Google Scholar]
- 352.Boisnic S., Branchet M.C., Soto P. Inulin and dermatology. J. Cosmet. Dermatol. 2018;17:968–971. doi: 10.1111/jocd.12528. [DOI] [PubMed] [Google Scholar]
- 353.Choi J.H., Shin K.M., Kim N.Y., Hong J.P., Lee Y.S., Kim H.J., Park H.J., Lee K.T. Taraxinic acid, a hydrolysate of sesquiterpene lactone glycoside from the Taraxacum coreanum NAKAI, induces the differentiation of human acute promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2002;25:1446–1450. doi: 10.1248/bpb.25.1446. [DOI] [PubMed] [Google Scholar]
- 354.Nguyen D.T.C., Nguyen T.T., Le H.T.N., Nguyen T.T.T., Bach L.G., Nguyen T.D., Vo D.V.N., Tra T.V. The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture: A review, Environ. Chem. Lett. 2021;19:3701–3726. doi: 10.1007/s10311-021-01266-z. [DOI] [Google Scholar]
- 355.Babich O., Larina V., Krol O., Ulrikh E., Sukhikh S., Gureev M.A., Prosekov A., Ivanova S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics. 2023;15:616. doi: 10.3390/pharmaceutics15020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chowdhury A.R., Sharma S., Mandal S., Goswami A., Mukhopadhyay S., Majumder H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002;366:653–661. doi: 10.1042/bj20020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Biel W., Jaroszewska A., Łysoń E., Telesiński A. The chemical composition and antioxidant properties of common dandelion leaves compared to Sea buckthorn. Can. J. Plant Sci. 2017;97:1165–1174. doi: 10.1139/CJPS-2016-0409. [DOI] [Google Scholar]
- 358.Modaresi M., Resalatpour N. The effect of Taraxacum officinale hydroalcoholic extract on blood cells in mice. Adv. Hematol. 2012;2012:653412. doi: 10.1155/2012/653412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gemejiyeva N.G., Grudzinskaya L.M. Vegetation of Central Asia and Environs. Springer; Berlin/Heidelberg, Germany: 2018. [(accessed on 14 May 2023)]. Current State and Prospects for Studies on the Diversity of Medicinal Flora in Kazakhstan; pp. 239–262. Available online: https://link.springer.com/chapter/10.1007/978-3-319-99728-5_9. [Google Scholar]
- 360.Khoo H.-E., Prasad K.N., Kong K.-W., Jiang Y., Ismail A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules. 2011;16:1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Kenny O., Smyth T.J., Hewage C.M., Brunton N.P. Quantitative UPLC-MS/MS analysis of chlorogenic acid derivatives in antioxidant fractionates from dandelion (Taraxacum officinale) root. Int. J. Food Sci. Technol. 2015;50:766–773. doi: 10.1111/ijfs.12668. [DOI] [Google Scholar]
- 362.Roberfroid M.B. Concepts in functional foods: The case of inulin and oligofructose. J. Nutr. 1999;129:1398S–1401S. doi: 10.1093/jn/129.7.1398S. [DOI] [PubMed] [Google Scholar]
- 363.Kisiel W., Barszcz B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia. 2000;71:269–273. doi: 10.1016/S0367-326X(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 364.Jedrejek D., Lis B., Rolnik A., Stochmal A., Olas B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019;126:233–247. doi: 10.1016/j.fct.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 365.Zhang Y., Hu Y.F., Li W., Xu G.Y., Wang K.R., Li L., Wu J.S. Updates and advances on pharmacological properties of Taraxacum mongolicum Hand.-Mazz and its potential applications. Food Chem. 2022;373:131380. doi: 10.1016/j.foodchem.2021.131380. [DOI] [PubMed] [Google Scholar]
- 366.Ata S., Farooq F., Javed S. Elemental profile of 24 common medicinal plants of Pakistan and its direct link with traditional uses. J. Med. Plants Res. 2011;5:6164–6168. doi: 10.5897/JMPR11.866. [DOI] [Google Scholar]
- 367.Sweeney B., Vora M., Ulbricht C., Basch E. Evidence-based systematic review of dandelion (Taraxacum officinale) by natural standard research collaboration. J. Herb. Pharmacother. 2005;5:79–93. doi: 10.1080/J157v05n01_09. [DOI] [PubMed] [Google Scholar]
- 368.Modaresi M. A comparative analysis of the effects of garlic, elderberry and black seed extract on the immune system in mice. J. Anim. Vet. Adv. 2012;11:458–461. doi: 10.3923/javaa.2012.458.461. [DOI] [Google Scholar]
- 369.Blumental M., Cladbery A., Brinkman J. Herbal Medicine: Expanded Commission E Monographs. Integrative Medicine Communications; Newton, MA, USA: 2000. [Google Scholar]
- 370.Mahesh A., Jeyachandran R., Cindrella L., Thangadurai D., Veerapur V., Muralidhara Rao D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. Acta Biol. Hung. 2010;61:175–190. doi: 10.1556/ABiol.61.2010.2.6. [DOI] [PubMed] [Google Scholar]
- 371.Liu W., Yu Q., Wang F., Li Y., Zhang G., Tao S. Taraxasterol attenuates melanoma progression via inactivation of reactive oxygen species-mediated PI3K/Akt signaling pathway. Hum. Exp. Toxicol. 2022;41:1–8. doi: 10.1177/09603271211069034. [DOI] [PubMed] [Google Scholar]
- 372.Jovanović M., Poljački M., Mimica-Dukić N., Boža P., Vujanović L.J., Ðuran V., Stojanović S. Sesquiterpene lactone mix patch testing supplemented with dandelion extract in patients with allergic contact dermatitis, atopic dermatitis and non-allergic chronic inflammatory skin diseases. Contact Dermat. 2004;51:101–110. doi: 10.1111/j.0105-1873.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 373.Im D.Y., Lee K.I. Nitric oxide production inhibitory and scavenging activity and tyrosinase inhibitory activity of extracts from Taraxacum officinale and Taraxacum coreanum. Korean J. Med. Crop. Sci. 2011;19:362–367. doi: 10.7783/KJMCS.2011.19.5.362. [DOI] [Google Scholar]
- 374.Kadeeja Sinoobiya T.T., Shijikumar P.S., Sirajudheen M.K., Baboo R. A Review on Pharmacological Activity of Dandelion Plant. [(accessed on 14 May 2023)];Int. J. Pharm. Pharm. Res. 2020 18:18–30. Available online: https://ijppr.humanjournals.com/wp-content/uploads/2020/07/2.Kadeeja-Sinoobiya.T.T-Shijikumar-P-S-Sirajudheen-M-K-RV-Celestin-Baboo.pdf. [Google Scholar]
- 375.Singh A., Malhotra S., Subban R. Dandelion (Taraxacum officinale)-Hepatoprotective Herb with Therapeutic Potential. [(accessed on 14 May 2023)];Pharmacogn. Rev. 2008 2:163. Available online: https://www.phcogrev.com/sites/default/files/PhcogRev-2-3-163.pdf. [Google Scholar]
- 376.Jeon H.J., Kang H.J., Jung H.J., Kang Y.S., Lim C.J., Kim Y.M., Park E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008;115:82–88. doi: 10.1016/j.jep.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 377.Al-Eisawi Z., Abderrahman S.M., Al-Khalaf I.F., Al-Abbassi R., Bustanji Y.K. Taraxacum officinale Extracts Exhibit Safe and Selective Anticancer Activity. Nat. Prod. J. 2022;12:69–77. doi: 10.2174/2210315510999201109202255. [DOI] [Google Scholar]
- 378.Epure A., Parvu A., Vlase L., Benedec D., Hanganu D., Vlase A., Oniga I. Polyphenolic compounds, antioxidant activity and nephroprotective properties of Romanian Taraxacum officinale. Farmacia. 2022;70:47–53. doi: 10.31925/farmacia.2022.1.7. [DOI] [Google Scholar]
- 379.Pfingstgraf I.O., Taulescu M., Pop R.M., Orăsan R., Vlase L., Uifalean A., Pârvu A.E. Protective effects of Taraxacum officinale L. (Dandelion) root extract in experimental acute on chronic liver failure. Antioxidants. 2021;10:504. doi: 10.3390/antiox10040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Schütz K., Carle R., Schieber A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006;107:313–323. doi: 10.1016/j.jep.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 381.Jovanović A.A., Ðordević V.B., Zdunić G.M., Pljevljakušić D.S., Šavikin K.P., Godevac D.M., Bugarski B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017;179:369–380. doi: 10.1016/j.seppur.2017.01.055. [DOI] [Google Scholar]
- 382.Orazbayeva P.Z., Akhmetalimova A.M., Ivasenko S.A., Loseva I.V., Ishmuratova M.Y. Distribution of some plants of the Thyme genus on the territory of Central Kazakhstan. Mod. Asp. Use Plant Raw Mater. Raw Mater. Nat. Orig. Med. 2017;2017:170–172. (In Russian) [Google Scholar]
- 383.Jovanović A.A., Balanč B., Petrović P., Pravilović R., Djordjević V. Pharmacological potential of Thymus serpyllum L. (wild thyme) extracts and essential oil: A review. J. Eng. Process. Manag. 2021;13:32–41. doi: 10.7251/JEPM2102032J. [DOI] [Google Scholar]
- 384.Khudonogova E.G., Kiseleva T.V. The content of essential oils in the aboveground part of creeping thyme. Sib. Bull. Agric. Sci. 2010;7:110–113. (In Russian) [Google Scholar]
- 385.Konovalov D.A., Orobinskaya V.N., Pisarenko O.N. Antioxidants of fruits and vegetables. Mod. Sci. Innov. 2013;4:76–83. (In Russian) [Google Scholar]
- 386.Goncharova T.A. Encyclopedia of Medicinal Plants. Volume 1. M.: Publishing house of MSP; Moscow, Russia: 2001. pp. 528–560. (In Russian) [Google Scholar]
- 387.Chaadaeva H.H., Boitsova O.A. Anatomical features of the structure of Thymus serpyllum L., growing on the territory of the Orel region. Sci. Notes Orel State Univ. Ser. Nat. Tech. Med. Sci. 2010;2:134–141. (In Russian) [Google Scholar]
- 388.Bazuk A.G., Yurchenko R.A., Vinarsky V.A., Buzuk G.N. Comparative pharmacognostic analysis of Thyme herb. Bull. Pharm. 2011;3:19–24. (In Russian) [Google Scholar]
- 389.Jarić S., Mitrović M., Pavlović P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015;2015:101978. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Tadele A., Urga K., Gemeda N., Lemma H., Melaku D., Mudie K. Antimicrobial activity of topical formulations containing Thymus vulgaris essential oil on major pathogens causing skin diseases. Ethiop. Pharm. J. 2009;26:103–110. doi: 10.4314/epj.v26i2.43041. [DOI] [Google Scholar]
- 391.Udintsev S.N., Zhilyakova T.P., Melnikov D.P. Vegetable feed additives prospects for the use of Grass and Thyme meal. Pig Breed. 2010;5:18–21. (In Russian) [Google Scholar]
- 392.Michalczyk A., Ostrowska P. Essential oils and their components in combating fungal pathogens of animal and human skin. J. Med. Mycol. 2021;31:101118. doi: 10.1016/j.mycmed.2021.101118. [DOI] [PubMed] [Google Scholar]
- 393.Tadić V.M., Nešić I., Martinović M., Rój E., Brašanac V.S., Maksimović S., Žugić A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants. 2021;10:465. doi: 10.3390/antiox10030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Podwyszynska M., Mynett K., Markiewicz M., Pluta S., Marasek C.A. Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.) Agronomy. 2021;11:2584. doi: 10.3390/agronomy11122584. [DOI] [Google Scholar]
- 395.Kubentayev S.A., Suleimenov A.N., Kotukhov J.A., Danilova A.N., Sumbembayev A.A. Phytocenotic characteristics and stocks of the main medicinal plants of the South-Western Altai (East Kazakhstan) Eur. J. BioSci. 2018;12:355–368. [Google Scholar]
- 396.Tung Y.T., Wu M.F., Lee M.C., Wu J.H., Huang C.C., Huang W.C. Antifatigue Activity and Exercise Performance of Phenolic-Rich Extracts from Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus. Nutrients. 2019;11:1715. doi: 10.3390/nu11081715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Musilova J., Frankova H., Lidikova J., Vollmannová A., Bojňanská T., Jurítková J. The content of bioactive substances and their antioxidant effects in the European blueberry (Vaccinium myrtillus L.) influenced by different ways of their processing. J. Food Process. Preserv. 2022;46:16549. doi: 10.1111/jfpp.16549. [DOI] [Google Scholar]
- 398.Chehri A., Yarani R., Yousefi Z., Shakouri S.K., Ostadrahimi A., Mobasseri M., Araj-Khodaei M. Phytochemical and pharmacological anti-diabetic properties of bilberries (Vaccinium myrtillus), recommendations for future studies. Prim. Care Diabetes. 2022;16:27–33. doi: 10.1016/j.pcd.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 399.Pires T.C., Caleja C., Santos-Buelga C., Barros L., Ferreira I.C. Vaccinium myrtillus L. fruits as a novel source of phenolic compounds with health benefits and industrial applications—A review. Curr. Pharm. Des. 2020;26:1917–1928. doi: 10.2174/1381612826666200317132507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Govindaraghavan S. Pharmacopeial HPLC identification methods are not sufficient to detect adulterations in commercial bilberry (Vaccinium myrtillus) extracts. Anthocyanin profile provides additional clues. Fitoterapia. 2014;99:124–138. doi: 10.1016/j.fitote.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 401.Güder A., Gür M., Engin M.S. Antidiabetic and antioxidant properties of bilberry (Vaccinium myrtillus Linn.) fruit and their chemical composition. J. Agric. Sci. Technol. 2015;17:387–400. [Google Scholar]
- 402.Drozd J., Anuszewska E. Bilberry plant—Prospects of new applications in prevention and supportive treatment of civilisation diseases. Prz. Med. Uniw. Rzesz. Inst. Leków. 2013;2:226–235. [Google Scholar]
- 403.Kitagawa S., Yoshii K., Morita S.Y., Teraoka R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011;59:793–796. doi: 10.1248/cpb.59.793. [DOI] [PubMed] [Google Scholar]
- 404.Menshikova E.B., Lankan V.Z., Kandalinzeva N.V. Phenolic Antioxidants in Biology and Medicine: Structure, Properties, Mechanisms of Action. Lap Lambert Academic Publishing; Saarbrücken, Germany: 2012. p. 492. [Google Scholar]
- 405.Kurkin V.A., Ryazanova T.K. New Approaches in the Field of Standardization of Raw Materials and Preparations of Chernika Common. Volume 1. Izvestia of the Samara Science Center of the Russian Academy of Sciences; Samara, Russia: 2011. p. 13. [Google Scholar]
- 406.Yakimenko O.V., Grigorievskaya A.Y., Ternovets M.A. Mistletoe Viscum album L. (Loranthaceae) and “Witch’s Broom” (Proliferation). Series: Geography. Geoecology. Bull. VSU. 2019;2:82–85. (In Russian) [Google Scholar]
- 407.Kleszken E., Timar A.V., Memete A.R., Miere F., Vicas S.I. On overview of bioactive compounds, biological and pharmacological effects of mistletoe (Viscum album L.) Pharmacophore. 2022;13:10–26. doi: 10.51847/Tmo2sXGQRs. [DOI] [Google Scholar]
- 408.Peñaloza E., Holandino C., Scherr C., Araujo P.I.P.d., Borges R.M., Urech K., Baumgartner S., Garrett R. Comprehensive Metabolome Analysis of Fermented Aqueous Extracts of Viscum album L. by Liquid Chromatography–High Resolution Tandem Mass Spectrometry. Molecules. 2020;25:4006. doi: 10.3390/molecules25174006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Vergara-Barberán M., Lerma-García M.J., Nicoletti M., Simó-Alfonso E.F., Herrero-Martínez J.M., Fasoli E., Righetti P.G. Proteomic fingerprinting of mistletoe (Viscum album L.) via combinatorial peptide ligand libraries and mass spectrometry analysis. J. Proteom. 2017;164:52–58. doi: 10.1016/j.jprot.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 410.Orhan D.D., Küpeli E., Yesilada E., Ergun F. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z. Für Nat. C. 2006;61:26–30. doi: 10.1515/znc-2006-1-205. [DOI] [PubMed] [Google Scholar]
- 411.Blinova K.F. Botanical-Pharmacognostic Dictionary: A Reference Guide. Higher School; Moscow, Russia: 2013. p. 117. (In Russian) [Google Scholar]
- 412.Kyosev P. Medicinal Plants: The Most Complete Reference Book. Eksmo; Moscow, Russia: 2011. p. 888. (In Russian) [Google Scholar]
- 413.Majeed M., Rehman R.U. Phytochemistry, Pharmacology, and Toxicity of an Epiphytic Medicinal Shrub Viscum album L. (White Berry Mistletoe) Med. Aromat. Plants Healthc. Ind. Appl. 2021:287–301. doi: 10.1007/978-3-030-58975-2_12. [DOI] [Google Scholar]
- 414.Jäger T., Holandino C., Melo M.N.D.O., Peñaloza E.M.C., Oliveira A.P., Garrett R., Baumgartner S. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants. 2021;10:1726. doi: 10.3390/plants10081726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Golovkin B.N., Rudenskaya R.N., Trofimova I.A., Schroeter A.I., Semikhov V.F. Biologically Active Substances of Plant Origin. Science; Moscow, Russia: 2002. p. 202. (In Russian) [Google Scholar]
- 416.Kwon Y.S., Chun S.Y., Kim M.K., Nan H.Y., Lee C., Kim S. Mistletoe extract targets the STAT3-FOXM1 pathway to induce apoptosis and inhibits metastasis in breast cancer cells. Am. J. Chin. Med. 2021;49:487–504. doi: 10.1142/S0192415X21500221. [DOI] [PubMed] [Google Scholar]
- 417.Pietrzak W., Nowak R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules. 2021;26:3741. doi: 10.3390/molecules26123741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Menke K., Schwermer M., Eisenbraun J., Schramm A., Zuzak T.J. Anticancer effects of Viscum album Fraxini extract on medulloblastoma cells in vitro. Complement. Med. Res. 2021;28:15–22. doi: 10.1159/000507318. [DOI] [PubMed] [Google Scholar]
- 419.Szurpnicka A., Zjawiony J.K., Szterk A. Therapeutic potential of mistletoe in CNS-related neurological disorders and the chemical composition of Viscum species. J. Ethnopharmacol. 2019;231:241–252. doi: 10.1016/j.jep.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 420.Turova A.D. Medicinal Plants and Their Use. Medicine; Moscow, Russia: 2013. p. 203. (In Rissian) [Google Scholar]
- 421.Sayakova G.M., Khamitova A.E., Olataeva Z.N. Creation of New Dosage Forms from Domestic Plant Materials of Thick-Fruited Sophora (Sophora pachycarpa) and White Mistletoe (Viscum album) as Promising Sources of Biologically Active Substances. [(accessed on 14 May 2023)];Bull. KazNMU. 2018 4:217–220. Available online: https://cyberleninka.ru/article/n/sozdanie-novyh-lekarstvennyh-form-iz-otechestvennogo-rastitelnogo-syrya-sofory-tolstoplodnoy-soph-ra-pachyc-rpa-i-omely-beloy-viscum-album/viewer. (In Russian) [Google Scholar]
- 422.Köse B., Erentürk S. Drying characteristics of mistletoe (Viscum album L.) in convective and UV combined convective type dryers. Ind. Crops Prod. 2010;32:394–399. doi: 10.1016/j.indcrop.2010.06.008. [DOI] [Google Scholar]
- 423.Hah Y.S., Kim E.J., Goo Y.M., Kil Y.S., Sin S.M., Kim S.G., Yoon T.J. Depigmenting Effects of Mistletoe (Viscum album var. coloratum) Extracts. J. Life Sci. 2022;32:355–361. doi: 10.5352/JLS.2022.32.5.355. [DOI] [Google Scholar]
- 424.Harati K., Behr B., Daigeler A., Hirsch T., Jacobsen F., Renner M., Becerikli M. Curcumin and Viscum album extract decrease proliferation and cell viability of soft-tissue sarcoma cells: An in vitro analysis of eight cell lines using real-time monitoring and colorimetric assays. Nutr. Cancer. 2017;69:340–351. doi: 10.1080/01635581.2017.1263349. [DOI] [PubMed] [Google Scholar]
- 425.Choi H.J., Park S.J., Choi Y.N., Kim S.D., Kwag E.B., Song S.Y., Park J.H., Kim J.K., Seo C., Choi J.J., et al. Selective Immune Modulating Activities of Viscum album and Its Components; A Possibility of Therapeutics on Skin Rash Induced by EGFR Inhibitors. Integr. Cancer Ther. 2022;21:1–11. doi: 10.1177/15347354221118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Palfi M.C., Racea R.C., Drăghici G., Seclaman E.P., Munteanu M., Mușat O., Ungureanu E., Milcu A., Boruga M., Rusu L., et al. Polyphenols Content and In Vitro Antitumor Activity of Hydroalcoholic Extract of Viscum album in Two Pigmented and Unpigmented Skin Cancer Cell Lines. Pharmaceuticals. 2022;70:5. doi: 10.31925/farmacia.2022.5.5. [DOI] [Google Scholar]
- 427.Mamedov N., Gardner Z., Craker L. Medicinal Plants Used in Russia and Central Asia for the Treatment of Selected Skin Conditions. J. Herbs Spices Med. Plants. 2005;11:191–222. doi: 10.1300/J044v11n01_07. [DOI] [Google Scholar]
- 428.Ustenova G.O., Beisembayeva U.T., Tuleeva A.M. The use of medicinal plants of the flora of Kazakhstan in the treatment of dermatoses. Bull. KazNMU. 2014:207–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.