Abstract
Surfactant replacement therapy is now an integral part of the care of neonates since several clinical trials of natural surfactant extracts and synthetic preparations have shown efficacy in the treatment of infants with hyaline membrane disease. In these studies, early treatment with exogenous surfactant substantially reduced mortality and the incidence of air leak, although it did not appear to reduce the incidence of other complications, in particular bronchopulmonary dysplasia. Early reports of exogenous surfactant therapy in patients with the adult respiratory distress syndrome, although promising, remain limited in number. More research is needed to improve on current modes of therapy and to investigate the possible role of surfactant in other lung diseases of both newborns and adults.
Full text
PDF
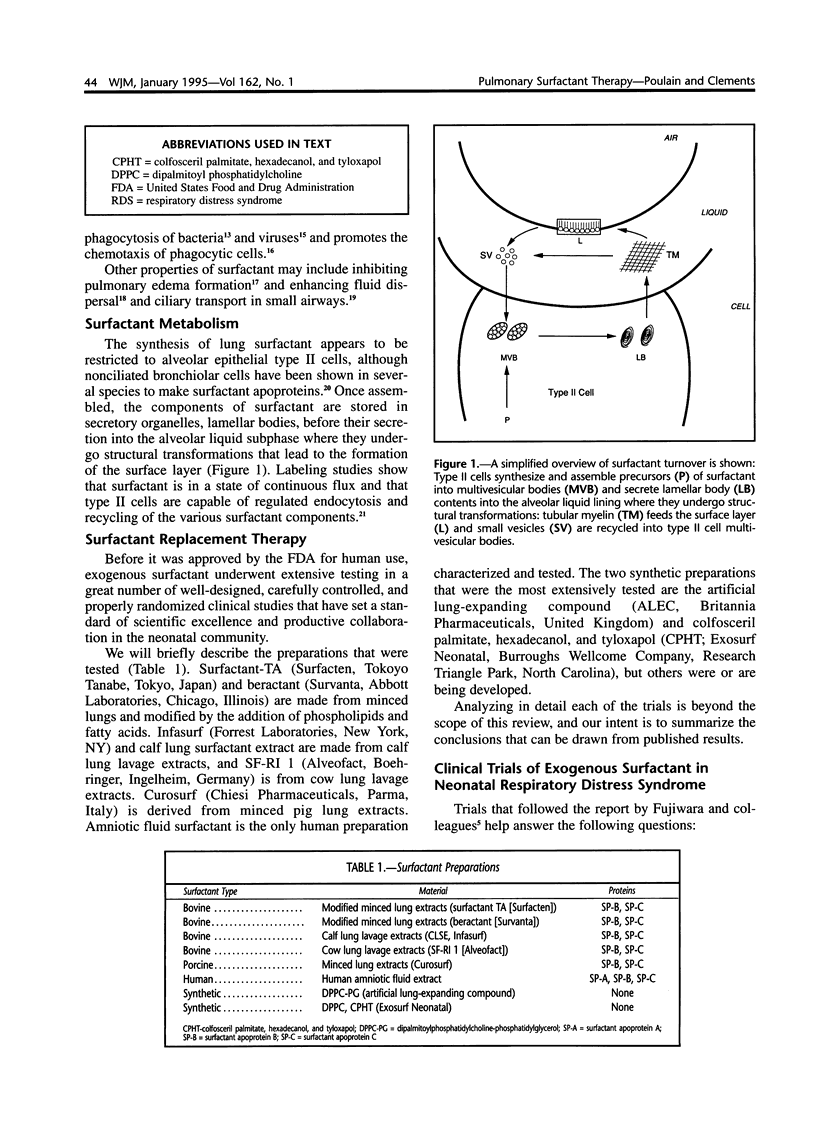
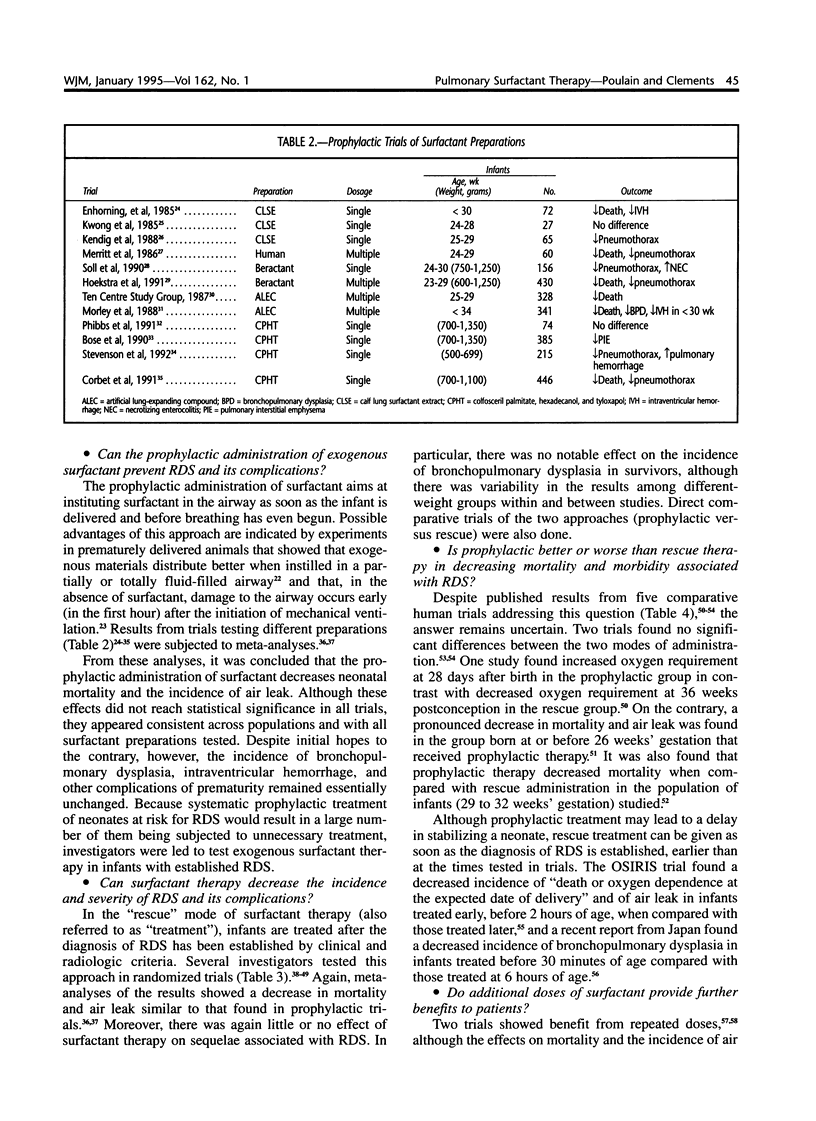
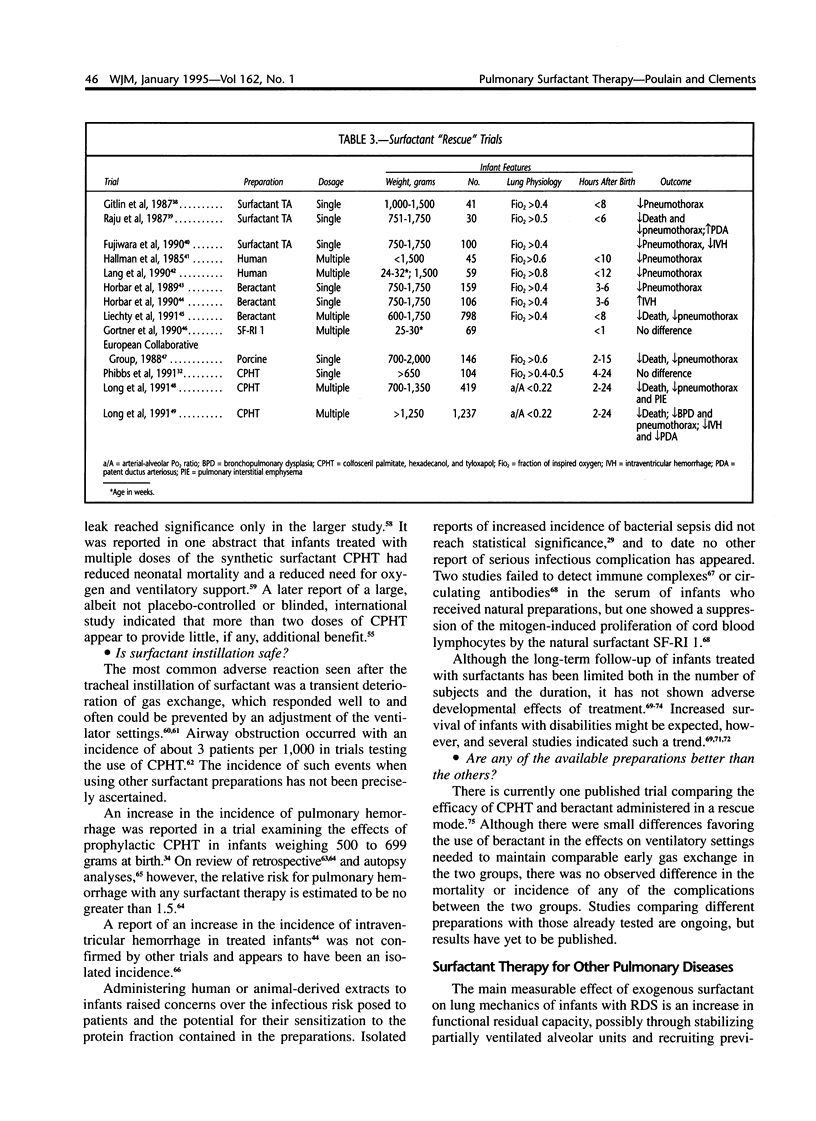
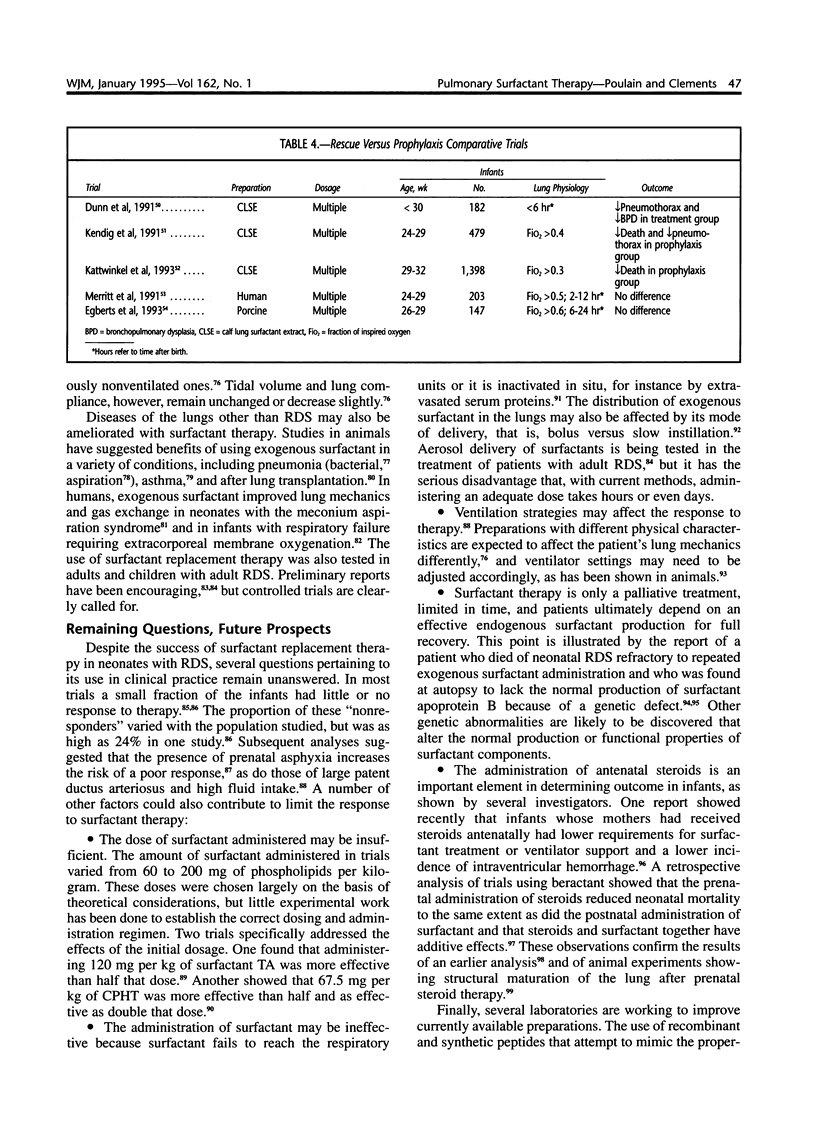



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Auten R. L., Notter R. H., Kendig J. W., Davis J. M., Shapiro D. L. Surfactant treatment of full-term newborns with respiratory failure. Pediatrics. 1991 Jan;87(1):101–107. [PubMed] [Google Scholar]
- Bartmann P., Bamberger U., Pohlandt F., Gortner L. Immunogenicity and immunomodulatory activity of bovine surfactant (SF-RI 1). Acta Paediatr. 1992 May;81(5):383–388. doi: 10.1111/j.1651-2227.1992.tb12254.x. [DOI] [PubMed] [Google Scholar]
- Berry D. D., Pramanik A. K., Philips J. B., 3rd, Buchter D. S., Kanarek K. S., Easa D., Kopelman A. E., Edwards K., Long W. Comparison of the effect of three doses of a synthetic surfactant on the alveolar-arterial oxygen gradient in infants weighing > or = 1250 grams with respiratory distress syndrome. American Exosurf Neonatal Study Group II. J Pediatr. 1994 Feb;124(2):294–301. doi: 10.1016/s0022-3476(94)70323-x. [DOI] [PubMed] [Google Scholar]
- Bose C., Corbet A., Bose G., Garcia-Prats J., Lombardy L., Wold D., Donlon D., Long W. Improved outcome at 28 days of age for very low birth weight infants treated with a single dose of a synthetic surfactant. J Pediatr. 1990 Dec;117(6):947–953. doi: 10.1016/s0022-3476(05)80143-x. [DOI] [PubMed] [Google Scholar]
- Charon A., Taeusch W., Fitzgibbon C., Smith G. B., Treves S. T., Phelps D. S. Factors associated with surfactant treatment response in infants with severe respiratory distress syndrome. Pediatrics. 1989 Mar;83(3):348–354. [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D. Pulmonary surfactant protein B (SP-B): structure-function relationships. Science. 1991 Oct 25;254(5031):566–568. doi: 10.1126/science.1948032. [DOI] [PubMed] [Google Scholar]
- Corbet A., Bucciarelli R., Goldman S., Mammel M., Wold D., Long W. Decreased mortality rate among small premature infants treated at birth with a single dose of synthetic surfactant: a multicenter controlled trial. American Exosurf Pediatric Study Group 1. J Pediatr. 1991 Feb;118(2):277–284. doi: 10.1016/s0022-3476(05)80502-5. [DOI] [PubMed] [Google Scholar]
- Corbet A. Clinical trials of synthetic surfactant in the respiratory distress syndrome of premature infants. Clin Perinatol. 1993 Dec;20(4):737–760. [PubMed] [Google Scholar]
- Cotton R. B., Olsson T., Law A. B., Parker R. A., Lindstrom D. P., Silberberg A. R., Sundell H. W., Sandberg K. The physiologic effects of surfactant treatment on gas exchange in newborn premature infants with hyaline membrane disease. Pediatr Res. 1993 Oct;34(4):495–501. doi: 10.1203/00006450-199310000-00022. [DOI] [PubMed] [Google Scholar]
- Crowley P., Chalmers I., Keirse M. J. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990 Jan;97(1):11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Dunn M. S., Shennan A. T., Hoskins E. M., Lennox K., Enhorning G. Two-year follow-up of infants enrolled in a randomized trial of surfactant replacement therapy for prevention of neonatal respiratory distress syndrome. Pediatrics. 1988 Oct;82(4):543–547. [PubMed] [Google Scholar]
- Dunn M. S., Shennan A. T., Possmayer F. Single- versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks' gestation with respiratory distress syndrome. Pediatrics. 1990 Oct;86(4):564–571. [PubMed] [Google Scholar]
- Dunn M. S., Shennan A. T., Zayack D., Possmayer F. Bovine surfactant replacement therapy in neonates of less than 30 weeks' gestation: a randomized controlled trial of prophylaxis versus treatment. Pediatrics. 1991 Mar;87(3):377–386. [PubMed] [Google Scholar]
- Egberts J., de Winter J. P., Sedin G., de Kleine M. J., Broberger U., van Bel F., Curstedt T., Robertson B. Comparison of prophylaxis and rescue treatment with Curosurf in neonates less than 30 weeks' gestation: a randomized trial. Pediatrics. 1993 Dec;92(6):768–774. [PubMed] [Google Scholar]
- Enhorning G., Shennan A., Possmayer F., Dunn M., Chen C. P., Milligan J. Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics. 1985 Aug;76(2):145–153. [PubMed] [Google Scholar]
- Fuhrman B. P., Paczan P. R., DeFrancisis M. Perfluorocarbon-associated gas exchange. Crit Care Med. 1991 May;19(5):712–722. doi: 10.1097/00003246-199105000-00019. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Konishi M., Chida S., Okuyama K., Ogawa Y., Takeuchi Y., Nishida H., Kito H., Fujimura M., Nakamura H. Surfactant replacement therapy with a single postventilatory dose of a reconstituted bovine surfactant in preterm neonates with respiratory distress syndrome: final analysis of a multicenter, double-blind, randomized trial and comparison with similar trials. The Surfactant-TA Study Group. Pediatrics. 1990 Nov;86(5):753–764. [PubMed] [Google Scholar]
- Fujiwara T., Maeta H., Chida S., Morita T., Watabe Y., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980 Jan 12;1(8159):55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D., Soll R. F., Parad R. B., Horbar J. D., Feldman H. A., Lucey J. F., Taeusch H. W. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987 Jan;79(1):31–37. [PubMed] [Google Scholar]
- Gortner L., Bernsau U., Hellwege H. H., Hieronimi G., Jorch G., Reiter H. L. A multicenter randomized controlled clinical trial of bovine surfactant for prevention of respiratory distress syndrome. Lung. 1990;168 (Suppl):864–869. doi: 10.1007/BF02718221. [DOI] [PubMed] [Google Scholar]
- Gunkel J. H., Banks P. L. Surfactant therapy and intracranial hemorrhage: review of the literature and results of new analyses. Pediatrics. 1993 Dec;92(6):775–786. [PubMed] [Google Scholar]
- Hallman M., Merritt T. A., Bry K., Berry C. Association between neonatal care practices and efficacy of exogenous human surfactant: results of a bicenter randomized trial. Pediatrics. 1993 Mar;91(3):552–560. [PubMed] [Google Scholar]
- Hallman M., Merritt T. A., Jarvenpaa A. L., Boynton B., Mannino F., Gluck L., Moore T., Edwards D. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr. 1985 Jun;106(6):963–969. doi: 10.1016/s0022-3476(85)80253-5. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra R. E., Jackson J. C., Myers T. F., Frantz I. D., 3rd, Stern M. E., Powers W. F., Maurer M., Raye J. R., Carrier S. T., Gunkel J. H. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics. 1991 Jul;88(1):10–18. [PubMed] [Google Scholar]
- Horbar J. D., Soll R. F., Schachinger H., Kewitz G., Versmold H. T., Lindner W., Duc G., Mieth D., Linderkamp O., Zilow E. P. A European multicenter randomized controlled trial of single dose surfactant therapy for idiopathic respiratory distress syndrome. Eur J Pediatr. 1990 Mar;149(6):416–423. doi: 10.1007/BF02009663. [DOI] [PubMed] [Google Scholar]
- Horbar J. D., Soll R. F., Sutherland J. M., Kotagal U., Philip A. G., Kessler D. L., Little G. A., Edwards W. H., Vidyasagar D., Raju T. N. A multicenter randomized, placebo-controlled trial of surfactant therapy for respiratory distress syndrome. N Engl J Med. 1989 Apr 13;320(15):959–965. doi: 10.1056/NEJM198904133201502. [DOI] [PubMed] [Google Scholar]
- Horbar J. D., Wright L. L., Soll R. F., Wright E. C., Fanaroff A. A., Korones S. B., Shankaran S., Oh W., Fletcher B. D., Bauer C. R. A multicenter randomized trial comparing two surfactants for the treatment of neonatal respiratory distress syndrome. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1993 Nov;123(5):757–766. doi: 10.1016/s0022-3476(05)80856-x. [DOI] [PubMed] [Google Scholar]
- Ikegami M., Berry D., elKady T., Pettenazzo A., Seidner S., Jobe A. Corticosteroids and surfactant change lung function and protein leaks in the lungs of ventilated premature rabbits. J Clin Invest. 1987 May;79(5):1371–1378. doi: 10.1172/JCI112964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A. H., Mitchell B. R., Gunkel J. H. Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant on preterm infants. Am J Obstet Gynecol. 1993 Feb;168(2):508–513. doi: 10.1016/0002-9378(93)90483-y. [DOI] [PubMed] [Google Scholar]
- Jobe A. H. Pulmonary surfactant therapy. N Engl J Med. 1993 Mar 25;328(12):861–868. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M., Jacobs H., Jones S. Surfactant and pulmonary blood flow distributions following treatment of premature lambs with natural surfactant. J Clin Invest. 1984 Mar;73(3):848–856. doi: 10.1172/JCI111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari M. A., Hallman M., Eronen M., Teramo K., Virtanen M., Koivisto M., Ikonen R. S. Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomized placebo-controlled multicenter study. Pediatrics. 1994 May;93(5):730–736. [PubMed] [Google Scholar]
- Kattwinkel J., Bloom B. T., Delmore P., Davis C. L., Farrell E., Friss H., Jung A. L., King K., Mueller D. Prophylactic administration of calf lung surfactant extract is more effective than early treatment of respiratory distress syndrome in neonates of 29 through 32 weeks' gestation. Pediatrics. 1993 Jul;92(1):90–98. [PubMed] [Google Scholar]
- Kendig J. W., Notter R. H., Cox C., Aschner J. L., Benn S., Bernstein R. M., Hendricks-Munoz K., Maniscalco W. M., Metlay L. A., Phelps D. L. Surfactant replacement therapy at birth: final analysis of a clinical trial and comparisons with similar trials. Pediatrics. 1988 Nov;82(5):756–762. [PubMed] [Google Scholar]
- Kendig J. W., Notter R. H., Cox C., Reubens L. J., Davis J. M., Maniscalco W. M., Sinkin R. A., Bartoletti A., Dweck H. S., Horgan M. J. A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks' gestation. N Engl J Med. 1991 Mar 28;324(13):865–871. doi: 10.1056/NEJM199103283241301. [DOI] [PubMed] [Google Scholar]
- King R. J., Macbeth M. C. Physicochemical properties of dipalmitoyl phosphatidylcholine after interaction with an apolipoprotein of pulmonary surfactant. Biochim Biophys Acta. 1979 Oct 19;557(1):86–101. doi: 10.1016/0005-2736(79)90092-0. [DOI] [PubMed] [Google Scholar]
- King R. J. Pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):1–8. doi: 10.1152/jappl.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- Konishi M., Fujiwara T., Chida S., Maeta H., Shimada S., Kasai T., Fujii Y., Murakami Y. A prospective, randomized trial of early versus late administration of a single dose of surfactant-TA. Early Hum Dev. 1992 Jun-Jul;29(1-3):275–282. doi: 10.1016/0378-3782(92)90164-c. [DOI] [PubMed] [Google Scholar]
- Konishi M., Fujiwara T., Naito T., Takeuchi Y., Ogawa Y., Inukai K., Fujimura M., Nakamura H., Hashimoto T. Surfactant replacement therapy in neonatal respiratory distress syndrome. A multi-centre, randomized clinical trial: comparison of high- versus low-dose of surfactant TA. Eur J Pediatr. 1988 Jan;147(1):20–25. doi: 10.1007/BF00442605. [DOI] [PubMed] [Google Scholar]
- Kuan S. F., Rust K., Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992 Jul;90(1):97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong M. S., Egan E. A., Notter R. H., Shapiro D. L. Double-blind clinical trial of calf lung surfactant extract for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics. 1985 Oct;76(4):585–592. [PubMed] [Google Scholar]
- Lachmann B. Surfactant therapy. Resuscitation. 1989;18 (Suppl):S37–S49. doi: 10.1016/0300-9572(89)90052-x. [DOI] [PubMed] [Google Scholar]
- Lamm W. J., Albert R. K. Surfactant replacement improves lung recoil in rabbit lungs after acid aspiration. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1279–1283. doi: 10.1164/ajrccm/142.6_Pt_1.1279. [DOI] [PubMed] [Google Scholar]
- Lang M. J., Hall R. T., Reddy N. S., Kurth C. G., Merritt T. A. A controlled trial of human surfactant replacement therapy for severe respiratory distress syndrome in very low birth weight infants. J Pediatr. 1990 Feb;116(2):295–300. doi: 10.1016/s0022-3476(05)82897-5. [DOI] [PubMed] [Google Scholar]
- Liechty E. A., Donovan E., Purohit D., Gilhooly J., Feldman B., Noguchi A., Denson S. E., Sehgal S. S., Gross I., Stevens D. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics. 1991 Jul;88(1):19–28. [PubMed] [Google Scholar]
- Liu M. Y., Wang L. M., Li E., Enhorning G. Pulmonary surfactant will secure free airflow through a narrow tube. J Appl Physiol (1985) 1991 Aug;71(2):742–748. doi: 10.1152/jappl.1991.71.2.742. [DOI] [PubMed] [Google Scholar]
- Long W., Corbet A., Allen A., McMillan D., Boros S., Vaughan R., Gerdes J., Houle L., Edwards K., Schiff D. Retrospective search for bleeding diathesis among premature newborn infants with pulmonary hemorrhage after synthetic surfactant treatment. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. J Pediatr. 1992 Feb;120(2 Pt 2):S45–S48. doi: 10.1016/s0022-3476(05)81233-8. [DOI] [PubMed] [Google Scholar]
- Long W., Corbet A., Cotton R., Courtney S., McGuiness G., Walter D., Watts J., Smyth J., Bard H., Chernick V. A controlled trial of synthetic surfactant in infants weighing 1250 g or more with respiratory distress syndrome. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. N Engl J Med. 1991 Dec 12;325(24):1696–1703. doi: 10.1056/NEJM199112123252404. [DOI] [PubMed] [Google Scholar]
- Long W., Thompson T., Sundell H., Schumacher R., Volberg F., Guthrie R. Effects of two rescue doses of a synthetic surfactant on mortality rate and survival without bronchopulmonary dysplasia in 700- to 1350-gram infants with respiratory distress syndrome. The American Exosurf Neonatal Study Group I. J Pediatr. 1991 Apr;118(4 Pt 1):595–605. doi: 10.1016/s0022-3476(05)83388-8. [DOI] [PubMed] [Google Scholar]
- Lotze A., Knight G. R., Martin G. R., Bulas D. I., Hull W. M., O'Donnell R. M., Whitsett J. A., Short B. L. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr. 1993 Feb;122(2):261–268. doi: 10.1016/s0022-3476(06)80131-9. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Haurum J., Thiel S., Sim R. B. Interaction of C1q receptor with lung surfactant protein A. Eur J Immunol. 1992 Jun;22(6):1437–1445. doi: 10.1002/eji.1830220616. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Krstenansky J. L., Jackson R. L., Hagaman K. A., Olsen K. A., Lewis J. E. Mixtures of synthetic peptides and dipalmitoylphosphatidylcholine as lung surfactants. Am J Physiol. 1992 Mar;262(3 Pt 1):L292–L300. doi: 10.1152/ajplung.1992.262.3.L292. [DOI] [PubMed] [Google Scholar]
- Mercier C. E., Soll R. F. Clinical trials of natural surfactant extract in respiratory distress syndrome. Clin Perinatol. 1993 Dec;20(4):711–735. [PubMed] [Google Scholar]
- Merritt T. A., Hallman M., Berry C., Pohjavuori M., Edwards D. K., 3rd, Jaaskelainen J., Grafe M. R., Vaucher Y., Wozniak P., Heldt G. Randomized, placebo-controlled trial of human surfactant given at birth versus rescue administration in very low birth weight infants with lung immaturity. J Pediatr. 1991 Apr;118(4 Pt 1):581–594. doi: 10.1016/s0022-3476(05)83387-6. [DOI] [PubMed] [Google Scholar]
- Merritt T. A., Hallman M., Bloom B. T., Berry C., Benirschke K., Sahn D., Key T., Edwards D., Jarvenpaa A. L., Pohjavuori M. Prophylactic treatment of very premature infants with human surfactant. N Engl J Med. 1986 Sep 25;315(13):785–790. doi: 10.1056/NEJM198609253151301. [DOI] [PubMed] [Google Scholar]
- Morgenroth K., Bolz J. Morphological features of the interaction between mucus and surfactant on the bronchial mucosa. Respiration. 1985;47(3):225–231. doi: 10.1159/000194774. [DOI] [PubMed] [Google Scholar]
- Morley C. J., Greenough A., Miller N. G., Bangham A. D., Pool J., Wood S., South M., Davis J. A., Vyas H. Randomized trial of artificial surfactant (ALEC) given at birth to babies from 23 to 34 weeks gestation. Early Hum Dev. 1988 May;17(1):41–54. doi: 10.1016/s0378-3782(88)80056-2. [DOI] [PubMed] [Google Scholar]
- Morley C. J., Morley R. Follow up of premature babies treated with artificial surfactant (ALEC). Arch Dis Child. 1990 Jul;65(7 Spec No):667–669. doi: 10.1136/adc.65.7_spec_no.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman G. F., Bredenberg C. E. High surface tension pulmonary edema induced by detergent aerosol. J Appl Physiol (1985) 1985 Jan;58(1):129–136. doi: 10.1152/jappl.1985.58.1.129. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Grossmann G., Robertson B. Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatr Res. 1978 Apr;12(4 Pt 1):249–255. doi: 10.1203/00006450-197804000-00001. [DOI] [PubMed] [Google Scholar]
- Nogee L. M., de Mello D. E., Dehner L. P., Colten H. R. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993 Feb 11;328(6):406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function and origin of the alveolar lining layer. Nature. 1955 Jun 25;175(4469):1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- Persson A., Chang D., Rust K., Moxley M., Longmore W., Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989 Jul 25;28(15):6361–6367. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]
- Phibbs R. H., Ballard R. A., Clements J. A., Heilbron D. C., Phibbs C. S., Schlueter M. A., Sniderman S. H., Tooley W. H., Wakeley A. Initial clinical trial of EXOSURF, a protein-free synthetic surfactant, for the prophylaxis and early treatment of hyaline membrane disease. Pediatrics. 1991 Jul;88(1):1–9. [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Raju T. N., Langenberg P. Pulmonary hemorrhage and exogenous surfactant therapy: a metaanalysis. J Pediatr. 1993 Oct;123(4):603–610. doi: 10.1016/s0022-3476(05)80963-1. [DOI] [PubMed] [Google Scholar]
- Raju T. N., Vidyasagar D., Bhat R., Sobel D., McCulloch K. M., Anderson M., Maeta H., Levy P. S., Furner S. Double-blind controlled trial of single-dose treatment with bovine surfactant in severe hyaline membrane disease. Lancet. 1987 Mar 21;1(8534):651–656. doi: 10.1016/s0140-6736(87)90414-4. [DOI] [PubMed] [Google Scholar]
- Richman P. S., Batcher S., Catanzaro A. Pulmonary surfactant suppresses the immune lung injury response to inhaled antigen in guinea pigs. J Lab Clin Med. 1990 Jul;116(1):18–26. [PubMed] [Google Scholar]
- Rider E. D., Jobe A. H., Ikegami M., Sun B. Different ventilation strategies alter surfactant responses in preterm rabbits. J Appl Physiol (1985) 1992 Nov;73(5):2089–2096. doi: 10.1152/jappl.1992.73.5.2089. [DOI] [PubMed] [Google Scholar]
- Robertson B., Curstedt T., Tubman R., Strayer D., Berggren P., Kok J., Koppe J., van Sonderen L., Halliday H., McClure G. A 2-year follow up of babies enrolled in a European multicentre trial of porcine surfactant replacement for severe neonatal respiratory distress syndrome. Collaborative European Multicentre Study Group. Eur J Pediatr. 1992 May;151(5):372–376. doi: 10.1007/BF02113261. [DOI] [PubMed] [Google Scholar]
- Shaffer T. H., Wolfson M. R., Clark L. C., Jr Liquid ventilation. Pediatr Pulmonol. 1992 Oct;14(2):102–109. doi: 10.1002/ppul.1950140208. [DOI] [PubMed] [Google Scholar]
- Soll R. F., Hoekstra R. E., Fangman J. J., Corbet A. J., Adams J. M., James L. S., Schulze K., Oh W., Roberts J. D., Jr, Dorst J. P. Multicenter trial of single-dose modified bovine surfactant extract (Survanta) for prevention of respiratory distress syndrome. Ross Collaborative Surfactant Prevention Study Group. Pediatrics. 1990 Jun;85(6):1092–1102. [PubMed] [Google Scholar]
- Speer C. P., Robertson B., Curstedt T., Halliday H. L., Compagnone D., Gefeller O., Harms K., Herting E., McClure G., Reid M. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics. 1992 Jan;89(1):13–20. [PubMed] [Google Scholar]
- Ueda T., Ikegami M., Rider E. D., Jobe A. H. Distribution of surfactant and ventilation in surfactant-treated preterm lambs. J Appl Physiol (1985) 1994 Jan;76(1):45–55. doi: 10.1152/jappl.1994.76.1.45. [DOI] [PubMed] [Google Scholar]
- Vaucher Y. E., Harker L., Merritt T. A., Hallman M., Gist K., Bejar R., Heldt G. P., Edwards D., Pohjavuori M. Outcome at twelve months of adjusted age in very low birth weight infants with lung immaturity: a randomized, placebo-controlled trial of human surfactant. J Pediatr. 1993 Jan;122(1):126–132. doi: 10.1016/s0022-3476(05)83505-x. [DOI] [PubMed] [Google Scholar]
- Vilallonga F. Surface of L-alpha-diphalmitoyl lecithin at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):290–300. doi: 10.1016/0005-2736(68)90114-4. [DOI] [PubMed] [Google Scholar]
- Ware J., Taeusch H. W., Soll R. F., McCormick M. C. Health and developmental outcomes of a surfactant controlled trial: follow-up at 2 years. Pediatrics. 1990 Jun;85(6):1103–1107. [PubMed] [Google Scholar]
- Waring A., Taeusch W., Bruni R., Amirkhanian J., Fan B., Stevens R., Young J. Synthetic amphipathic sequences of surfactant protein-B mimic several physicochemical and in vivo properties of native pulmonary surfactant proteins. Pept Res. 1989 Sep-Oct;2(5):308–313. [PubMed] [Google Scholar]
- Weaver T. E., Whitsett J. A. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J. 1991 Jan 15;273(Pt 2):249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Hull W. M., Luse S. Failure to detect surfactant protein-specific antibodies in sera of premature infants treated with survanta, a modified bovine surfactant. Pediatrics. 1991 Apr;87(4):505–510. [PubMed] [Google Scholar]
- Wright J. R., Dobbs L. G. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Youmans D. C. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am J Physiol. 1993 Apr;264(4 Pt 1):L338–L344. doi: 10.1152/ajplung.1993.264.4.L338. [DOI] [PubMed] [Google Scholar]
- Zola E. M., Overbach A. M., Gunkel J. H., Mitchell B. R., Nagle B. T., DeMarco N. G., Henwood G. A., Gold A. J. Treatment Investigational New Drug experience with Survanta (beractant). Pediatrics. 1993 Mar;91(3):546–551. [PubMed] [Google Scholar]
- van Houten J., Long W., Mullett M., Finer N., Derleth D., McMurray B., Peliowski A., Walker D., Wold D., Sankaran K. Pulmonary hemorrhage in premature infants after treatment with synthetic surfactant: an autopsy evaluation. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. J Pediatr. 1992 Feb;120(2 Pt 2):S40–S44. doi: 10.1016/s0022-3476(05)81232-6. [DOI] [PubMed] [Google Scholar]
- van Iwaarden F., Welmers B., Verhoef J., Haagsman H. P., van Golde L. M. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990 Jan;2(1):91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- van Iwaarden J. F., van Strijp J. A., Ebskamp M. J., Welmers A. C., Verhoef J., van Golde L. M. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am J Physiol. 1991 Aug;261(2 Pt 1):L204–L209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]


