Abstract
Aims
This study aimed to examine the clinical benefits of targeted ablation of all Premature ventricular complex (PVC) morphologies vs. predominant PVC only.
Methods and results
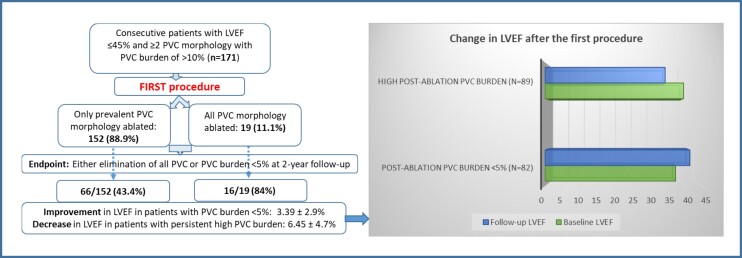
A total of 171 consecutive patients with reduced left ventricular ejection fraction (LVEF) and ≥2 PVC morphology with high burden (>10%/day) undergoing their first ablation procedure were included in the analysis. At the initial procedure, prevalent PVC alone was ablated in the majority. However, at the redo, all PVC morphologies were targeted for ablation. : At the first procedure, 152 (89%) patients received ablation of the dominant PVC only. In the remaining 19 (11%) patients, all PVC morphologies were ablated. At two years, high PVC burden was detected in 89 (52%) patients. Repeat procedure was performed in 78 of 89, where all PVC morphologies were ablated. At 5 years after the repeat procedure, 71 (91%) had PVC burden of <5% [3.8 ± 1.1% vs. 15.4 ± 4.3% in successful vs. failed subjects (P < 0.001)]. In patients with low PVC burden after the initial procedure, LVEF improved from 37.5% to 41.6% [mean difference (MD): 3.39 ± 2.9%, P < 0.001], whereas a reduction in LVEF from 39.8% to 34.5% (MD: 6.45 ± 4.7%, P < 0.001) was recorded in patients with high PVC burden. One year after the repeat procedure, LVEF improved from 36.2% to 41.7% (MD: 5.5 ± 4.3%, P < 0.001) in patients with successful ablation.
Conclusion
In this observational series, ablation of all PVC morphologies was associated with significantly lower PVC burden and improvement of LVEF at long-term follow-up, compared with ablation of the dominant morphology only.
Keywords: Premature ventricular complex (PVC), Morphology, PVC burden, LVEF, PVC ablation
Graphical Abstract
Graphical Abstract.
What’s new?
In patients presenting with Premature ventricular complexes (PVCs) with multiple morphology, ablation of all PVCs was associated with superior outcome in terms of reduction in PVC burden at long-term follow-up, compared with ablation of prevalent PVC alone.
Improvement in left ventricular ejection fraction was substantially higher in patients in whom the post-ablation PVC burden reduced to <5%.
At the repeat procedure, previously non-predominant PVCs were seen to become more frequent in the majority of patients.
Introduction
Premature ventricular complexes (PVCs) were first described by the French scientist and physiologist Étienne-Jules Marey in the late 1800s.1 With the development of Holter monitoring in 1949, further specifications, quantifications, and a better understanding of the clinical significance of this arrhythmia became possible.1 Premature ventricular complexes can result from triggered activity, automaticity, or re-entry and these can be either unifocal or originate from more than one site showing multiple morphologies.2 Although advent of procedural interventions such as catheter ablation to eliminate PVCs was late owing mostly to delay in the understanding of their clinical implications, especially in the development of cardiomyopathy, several series in recent years have reported high success rate following ablation of predominant PVCs in patients presenting with monomorphic premature complexes.1,3–7 However, the procedural success with multifocal PVCs, with most studies reporting ablation of the dominant focus only, has been seen to be inferior to those with a singular ectopic focus.4,8 Therefore, we aimed to compare the long-term improvement in the PVC burden and left ventricular ejection fraction (LVEF) following ablation of all PVC morphologies vs. the predominant morphology only, in patients presenting with pleomorphic PVCs and reduced LVEF.
Methods
In this prospective single-centre series, consecutive symptomatic patients with reduced LVEF and multiple (≥2) PVC morphology with high burden (>10%/day) undergoing their first catheter ablation were included. At the initial procedure, the decision to ablate either the predominant PVC or all PVC morphology was taken based on the frequency of non-dominant PVCs detected following ablation of the prevalent PVC. In other words, if the non-dominant PVCs were sporadic after ablation of the dominant morphology PVC, those were not targeted for ablation. During the repeat procedure, all PVC morphologies were ablated.
The site of origin of the PVC was assessed based on the 12-lead electrocardiogram or 12-lead Holter prior to the ablation.9,10 Premature ventricular complex was considered to be originating from the right ventricular outflow tract (RVOT) if the electrocardiogram (ECG) showed left bundle branch block (LBBB) morphology in Lead V1 with late precordial R/S transition (after Lead V3). If the site of origin was left ventricular outflow tract (LVOT), ECG presented LBBB with early precordial R/S transition (before Lead V3). Premature ventricular complexes originating from papillary muscle or fascicular system showed right bundle branch block (RBBB) morphology with R (notched) in V1 and transition to rS in V4–6.
Definitions
Premature ventricular complex with multiple morphology (Pleomorphic): Premature ventricular complexes originating from multiple sites (multifocal).
Premature ventricular complex burden: Proportion of total ventricular complexes that were PVCs/day.
Frequency: Number of PVCs in 24 h.
Predominant PVC: The most frequent of all PVC morphologies in a patient.
Reduced LVEF: ≤ 45%.
Successful ablation: Elimination of all PVCs (absence of targeted PVCs in all follow-up ECGs) or PVC burden <5% on follow-up monitoring.
Procedures were performed under conscious sedation. Heparin bolus of 10 000–12 000 units was given to maintain the activated clotting time of 300–350 s for all left-sided procedures. The right ventricle was mapped using the standard femoral approach and the left ventricle was accessed via transseptal approach or retrograde aortic approach. Intra-cardiac echocardiography was used to guide the transseptal access and monitor catheter location and 3D electroanatomic mapping was performed in all.
All patients underwent bipolar electroanatomic substrate mapping with standard scar settings of normal tissue >1.5 mV and severe scar <0.5 mV. Maps were considered complete when the entire area of interest was completely mapped and all scar borders were clearly defined.
Anatomic and activation mapping were conducted simultaneously and the earliest activation point for the specific PVC was targeted for ablation. For PVCs whose frequency prevented activation mapping, the best correlation with pace mapping was targeted for ablation. Pace mapping was performed just above the capture threshold with the coupling interval being similar to the targeted PVC. We used the automated PaSo algorithm from Biosense Webster to guide pace mapping. A median PaSo score of 95% or above was considered appropriate to deliver ablation lesions. Additionally, the paced QRS morphology and the targeted PVC morphology were visually compared for supplemental evidence.
All patients underwent radiofrequency catheter ablation with either a 3.5-mm open-irrigated ablation catheter (NaviStar ThermoCool, Biosense Webster, Diamond Bar, CA, USA) or with an open-irrigated magnetic ablation catheter (NaviStar RMT ThermoCool, Biosense Webster). The ablation parameters were the following; maximum power 45 W, 30–49 s/lesion, temperature cut-off 42°C.
Epicardial mapping either via subxiphoid access or coronary sinus cannulation was performed if needed. A focus was considered epicardial if the activation mapping was earliest or pace mapping most closely correlated with the targeted PVC morphology from the epicardium.
At the end of the ablation procedure, intravenous isoproterenol (up to 10 µg/min for 10 min) was given for induction of ectopy followed by a 20-min waiting period to ensure complete abatement of ectopic triggers. Patients were monitored overnight and were discharged the following day. During hospitalization, continuous ECG telemetry was performed to monitor the arrhythmia.
Left ventricular ejection fraction was assessed by echocardiogram at baseline and 12 months after the procedure. Premature ventricular complex burden was monitored using 7-day Holter telemetry at 3, 6, and 12 months of post-procedure and once a year for the rest of the follow-up period. If the burden was <5% at the follow-up, the procedure was considered to be effective long-term. Patient data were prospectively collected in our institutional review board approved VT registry that allows for data collection and analysis.
Statistical analysis
Clinical characteristics were summarized as frequency and percentage for categorical variables and mean ± SD for continuous variables. Student’s t-test and Fisher’s exact test were used to compare differences across groups.
Freedom from arrhythmia recurrence was the primary endpoint of the study. Assessment of PVC burden and change in LVEF at follow-up were the secondary endpoints.
Arrhythmia recurrence was compared by log-rank test. Event-free duration was defined as time from the procedure to occurrence of outcome event (arrhythmia recurrence). Patients event-free at end of follow-up were censored on date of last assessment. Premature ventricular complex burden and change in LVEF were compared between groups using two sample t-tests. All tests were two-sided, and a P-value of <0.05 was considered statistically significant.
Results
Baseline
A total of 171 consecutive patients with reduced LVEF and pleomorphic (two or more morphology) PVCs in the baseline ECG and high PVC burden (>10%), undergoing their first catheter ablation at our institution were included in this analysis. Figures 1 and 2 illustrate examples of PVCs with multiple morphology in two patients included in the current analysis.
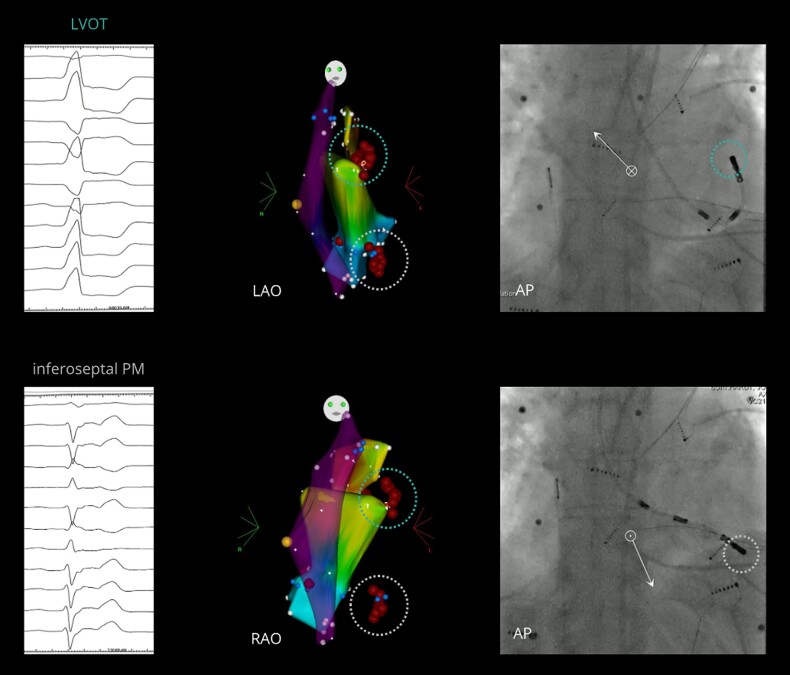
Figure 1.
Example of a patient with multiple PVCs: PVC#1 (top circle), originating from the LVOT (below the left coronary cusp, near the mitral valve annulus), and PVC#2 (bottom circle), originating from the left infero-septal PM. From left to right, ECG; Carto3 point-by-point electroanatomical maps (LAO and RAO projections) of the RVOT (partially transparent), LVOT (bottom), and aortic root/left coronary cusp (top) showing the ablation lesions at the two separate sites of successful ablation; and fluoroscopic view (AP projection) of the ablation catheter at the site of successful ablation. AP, anteroposterior; ECG, electrocardiogram; LAO, left anterior oblique; LVOT, left ventricular outflow tract; PM, papillary muscle; PVC, premature ventricular contraction; RAO, right anterior oblique; RVOT, right ventricular outflow tract.
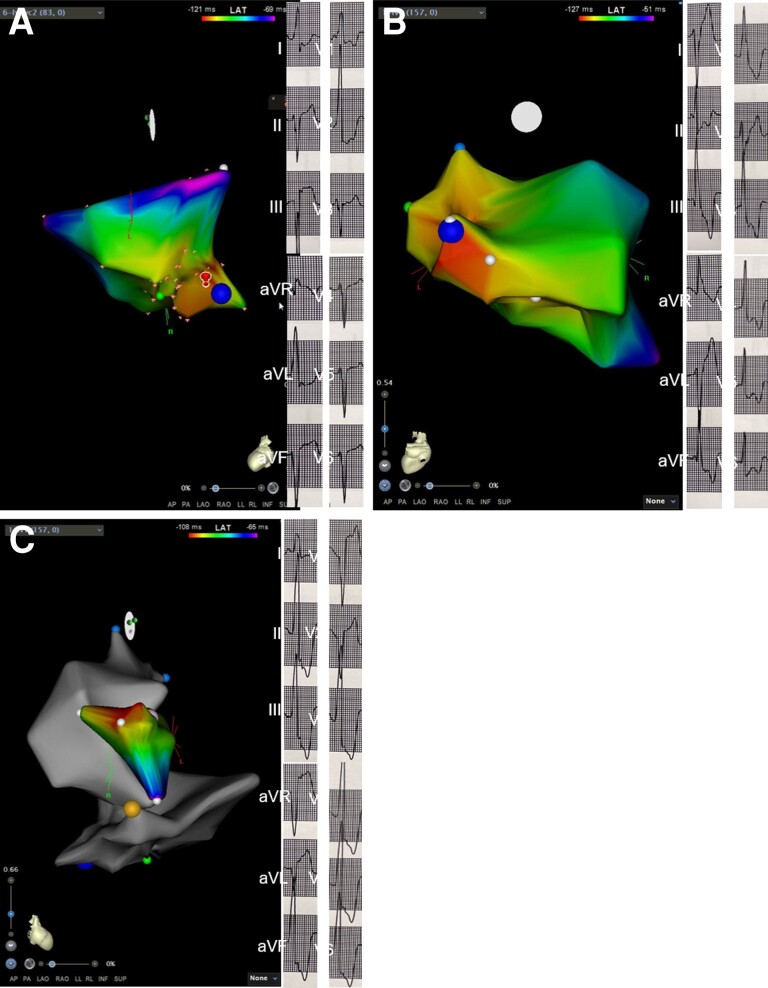
Figure 2.
Electroanatomic map showing three PVC morphologies (A: postero-medial papillary muscle, B: antero-lateral papillary muscle, and C: left coronary cusp) in Participant II.
Conventional mapping was performed manually in 119 (69.6%) patients and with the robotic system (Stereotaxis, St. Louis, MO, USA) in the remaining participants. Baseline and procedural characteristics of the study population are presented in Table 1. Majority (83.7%) of the patients had two PVC morphology detected. Premature ventricular complex burden at baseline was 23.5 ± 8% (median 24.3, IQR 15.6–28.6). Average burden of predominant PVC was 21.6 ± 10.0% (median 20.6, IQR 15.2–27.3) and non-predominant PVC was 7.5 ± 9.6% (median 7.8, IQR 4.5–10.1). The distribution of the site of origin of PVCs was as following: RVOT (n = 110, 64.3%) and papillary muscle (n = 51, 29.8%). The remaining were from the left ventricle: coronary cusp (38/45, 84.4%), left ventricular (LV) summit (41/45, 91.1%), subaortic valvular region (37/45, 82.2%), and coronary venous system (33/45, 73.3%). The mean pre-QRS activation time was −23 ± 6 ms. Left ventricular ejection fraction was <40% in 48 (28%) patients.
Table 1.
Baseline characteristics of the study population
| Demographics and clinical characteristics | N = 171 |
|---|---|
| Age | 66 ± 12 |
| Male | 135 (79%) |
| LVEF | 38.6 ± 6.11 |
| LVEF (<40%) | 86 (50.3%) |
| Hypertension | 112 (65.5%) |
| Type II diabetes | 36 (21%) |
| Coronary artery disease | 82 (48%) |
| Obstructive sleep apnoea | 37 (21.6%) |
| Number of PVC morphology | 3.1 ± 1.2 |
| 2 morphologies | 143 (83.7%) |
| 3 morphologies | 24 (14%) |
| ≥4 morphologies | 4 (2.3%) |
| Sites of origin | |
| Right ventricle | 110 (64.3%) |
| Left ventricle | 45 (26.3%) |
| Papillary muscle | 51 (29.8%) |
| PVC burden | 23.5 ± 8% |
| Mean duration of PVC before ablation (years) | 2.9 ± 1.4 |
| Procedure time (min) | 97.6 ± 23.4 |
LVEF, left ventricular ejection fraction; PVC, premature ventricular complex.
Premature ventricular complexes originated from within the scar in 9/54 (16.7%), adjacent to the scar in 13/54 (24.1%), and away from the scar in 49 (90.7%) patients.
Baseline medications included beta-blockers (97, 56.7%), flecainide (27, 15.8%), dofetilide (14, 8.2%), dilitiazem or verapamil (28, 16.3%), sotalol (11, 6.4%), and amiodarone (9, 5.2%).
First procedure
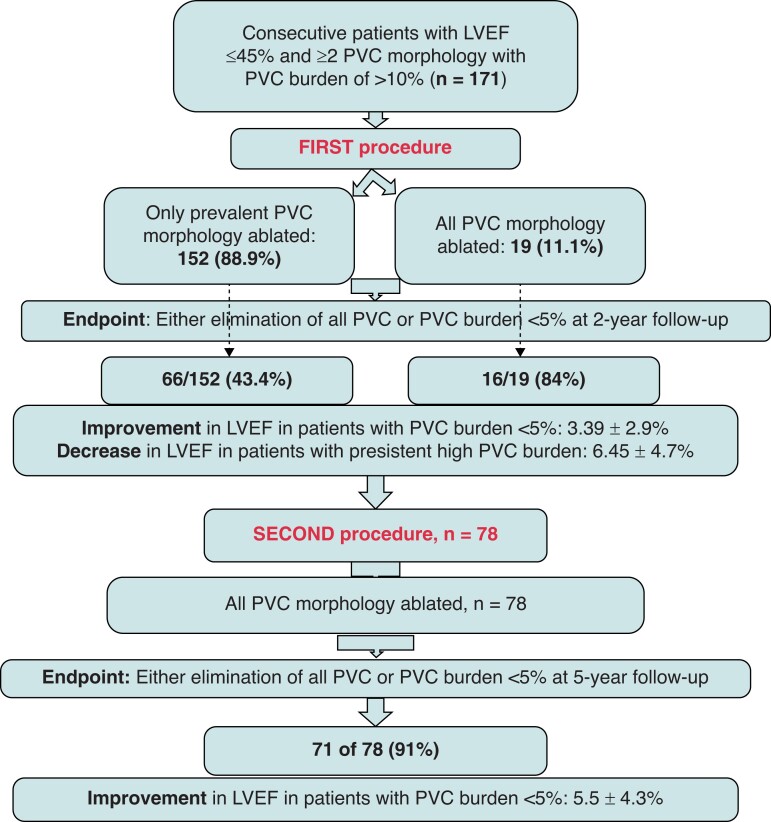
In 152 (88.9%) patients, only the prevalent PVC morphology was ablated. In the remaining 19 subjects, all PVC morphologies were targeted for ablation. Site of origin of the predominant PVC was epicardial in 11 (6.4%) patients. Total mean fluoro time was 17 ± 3.8 min and radiofrequency time was 7 ± 3 min. Myocardial scar was detected in 54 (31.6%) patients of which 33 (61%) had LVEF <40%.
Electroanatomic map in Figure 2 demonstrates ablation points in the coronary cusp and papillary muscle area in one of the study participants.
At 2 years after the first procedure, 82 (48%) patients had either elimination of all PVCs or the PVC burden was reduced to <5%. This included 16 of the 19 (84%) subjects that received ablation of all PVC morphologies and 66 of the 152 (43.4%) patients in whom only predominant PVC was targeted for ablation (P = 0.0008). High PVC burden was persistent in the remaining 89/171 (52%) patients (3/19 (15.7%) receiving ablation of all PVC morphology vs. 86/152 (56.5%) that had only the prevalent PVC ablated, P < 0.001).
The success rate was similar between patients with vs. without myocardial scar [25/54 (46.3%) vs. 57/117 (48.7%), P = 0.76].
Repeat procedure
Repeat procedure was performed in 78 of 89 (87.6%) patients. Return of the predominant PVC that was ablated in the 1st procedure was detected in 6 (7.7%), previously non-predominant PVCs were seen to become more frequent in 73 (93.6%), and new PVCs were observed in 5 (6.4%) patients. All received ablation of both dominant and non-dominant PVC morphology at the repeat procedure.
At 5 years of follow-up after the repeat procedure, 71 of 78 (91%) had either all PVC eliminated or the burden was <5%. Premature ventricular complex burden was 3.8 ± 1.1% (median 3.7, IQR 2.6–4.8) vs. 15.4 ± 4.3% (median 14.8, IQR 12.61–18.38) in successful vs. failed subjects (P < 0.001).
Effect of ablation on left ventricular ejection fraction
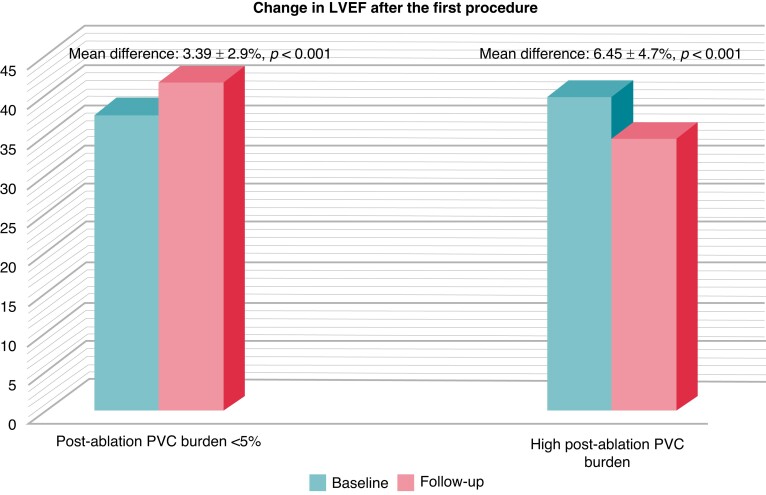
In patients with successful ablation after the initial procedure, LVEF improved from 37.5% to 41.6% [mean difference (MD): 3.39 ± 2.9%, P < 0.001], whereas a reduction in LVEF from 39.8% to 34.5% (MD: 6.45 ± 4.7%, P < 0.001) was recorded in patients with high PVC burden (Figure 3).
Figure 3.
Bar diagram shows change in LVEF after the first procedure. In patients with successful ablation after the initial procedure, LVEF improved from 37.5% to 41.6% [mean difference (MD): 3.39 ± 2.9%, P < 0.001], whereas a reduction in LVEF from 39.8% to 34.5% (MD: 6.45 ± 4.7%, P < 0.001) was recorded in patients with high PVC burden. LVEF, left ventricular ejection fraction; PVC, premature ventricular complex.
Of the 82 patients that were PVC-free at follow-up after the initial procedure, LVEF was normalized to >50% in 9 (10.9%), and improved ≥10% in 55 (67%) patients.
After the repeat ablation, LVEF improved from 36.2% to 41.7% (MD: 5.5 ± 4.3%, P < 0.001) in the 71 patients that either remained PVC free or had the PVC burden <5% [LVEF normalized in 8 (11.2%) and an improvement of ≥10% was documented in 54 (76%)]. Of the 25 patients with scar that had successful ablation of PVC, LVEF was normalized in none (0%), improved >10% in 22 (88%), and <10% in 3 (12%). Of the 57 patients with successful ablation and no scar, LVEF improved in all (100%); normalized in 9 (15.8%), improved >10% in 33 (57.9%), and improved <10% in the remaining 15 patients.
The main findings are summarized in a flow chart in Figure 4.
Figure 4.
Flowchart shows the main results summarized.
Complications
No major complications such as phrenic nerve palsy, coronary vessel damage, pericardial effusion, or stroke/transient ischaemic attacks were reported peri-procedurally following the first and the repeat procedure. Access-site haematoma were reported in 4 (2.3%) cases after the first procedure that were conservatively managed. After the repeat procedure, one case of access-site haematoma was reported in Groups 1 and 2 minor vascular complications in Group 2 patients.
Discussion
To the best of our knowledge, this is the first study that directly compared the benefits of targeting all PVC morphologies vs. prevalent morphology only, in patients presenting with multifocal PVCs. Our main findings were the following: (i) significantly lower PVC burden was observed at long-term follow-up when all PVC morphologies were ablated vs. only the prevalent one, (ii) safety of the procedure was demonstrated by very low complication rate and no major complications, and (iii) improvement in LVEF was substantially higher in patients after complete elimination of all PVC morphologies or reduction in the burden to <5%.
In order to understand the clinical significance of the above findings, the first and foremost question that is needed to be addressed is why it is important to eliminate all PVC morphologies (originating from more than one site) and not just the prevalent form. Based on available evidences, there are multiple reasons that justify ablation of all PVC morphologies.
Multifocal premature ventricular complexes are known to be associated with poor cardiovascular outcome
Premature ventricular complexes with multiple morphology (multiform) can have a single focus of origin (unifocal) and yet produce distinctly different morphologies owing to variable exit pathways and pattern of propagation or these can have multiple origin sites (multifocal PVC). In our series, all patients had PVCs originating from multiple locations. The presence of multiform PVCs and the number of PVC morphologies were reported to be directly correlated with left ventricular dysfunction, cardiovascular (CV) adverse events, all-cause mortality, new-onset atrial fibrillation and heart failure, hospitalization, and cerebral thrombo-embolic events.8,11,12 Pleomorphic PVCs might be associated with underlying subclinical heart disease that led to subsequent CV complications in these patients.8
Ablation success is evidently affected by the presence of premature ventricular complexes with multiple morphology
Catheter ablation is a preferred therapeutic option in patients presenting with high burden of PVCs and signs of cardiomyopathy.13 In a survey conducted by the European Heart Rhythm Association on the management of idiopathic monomorphic PVC, 99% of responders considered catheter ablation as an important option with some opting for it as the first-line treatment.13 Moreover, the majority of the participants was in accordance with therapeutic intervention for PVC burden exceeding 10% in a 24-h Holter ECG recording.13 However, PVCs often arise from multiple locations and the presence of pleomorphic PVCs is known to affect ablation outcomes.4 In a series of patients with 30% presenting with pleomorphic PVCs, Sheldon et al.4 observed the presence of PVCs with multiple morphology as an independent predictor of unsuccessful ablation, although most of the recurrences were detected to be due to re-emergence of the originally targeted predominant PVC morphology. One plausible explanation for this observation is that the pleomorphic PVCs described in this study were most likely PVCs with single origin but multiple exit sites and/or were originating from locations that were challenging to ablate (papillary muscle, LV summit).9 Recurrence after successful ablation is possible due to remodelling of the arrhythmogenic substrate or reconnection to exit sites.9 In addition, PVCs originating from papillary muscle are known to be associated with higher failure rate although these can be successfully eliminated in the hands of skilled operators resulting in freedom from recurrent ventricular arrhythmia at long-term follow-up.14 Sheldon et al.4 described the other reason of high failure rate to be the ‘frequency cut-off’ for non-prevalent PVCs of ≥ 156 beats differentiating between ineffective and effective ablation procedure. Moreover, they observed the transformation of previously detected non-predominant PVCs to become the dominant PVC at recurrence, when those were not targeted during the prior procedure.4 In agreement with their results, we observed non-frequent PVCs at the baseline procedure to become more frequent and contributing to the maintenance of a high burden, if only prevalent PVC was ablated at the initial procedure. Of note, our results demonstrated significantly higher success rate in the group that had all PVC morphologies targeted for ablation compared with those where only predominant PVC was ablated and non-predominant PVCs were not addressed. Thus, whenever warranted, the operators should consider ablating all PVC morphologies instead of targeting the dominant only.
High premature ventricular complex burden and higher prevalence of multifocal premature ventricular complexes increase the risk of cardiomyopathy
The concept of PVC-induced cardiomyopathy was first proposed by Duffee and co-workers, who observed a small group of patients with cardiomyopathy recovering normal LV function after pharmacological suppression of frequent PVCs.15 Premature ventricular complex ablation currently holds a Class 1 indication for treating high-burden symptomatic drug-refractory PVCs, as high PVC burden is known to increase the risk of cardiomyopathy in addition to affecting the quality of life and overall well-being because of the symptoms.15 Although PVC burden of >24% was identified by one study as a cut-off for risk of cardiomyopathy, findings from other studies showed the burden as low as 5–6% to be associated with increased risk of the same.16,17 Higher prevalence of multiform PVCs was also reported to be associated with reduced LVEF in a retrospective series.18 Regardless of whether the pleomorphic PVCs were the cause or the manifestation of a pre-existing cardiomyopathy, several trials have demonstrated significant reduction in PVC burden and improvement in LVEF following catheter ablation, in agreement with our current findings.3,5 It is noteworthy to mention here that in patients with no structural heart disease, survival analysis did not show any difference in mortality between the strata when patients were classified according to PVC burden.19
Of note, studies have reported improvement in NYHA class from 3.0 to 2.0, enhancement in the efficacy of cardiac resynchronization therapy in non-responders and improvement in survival in patients with PVC cardiomyopathy following substantial betterment in LVEF after successful PVC ablation.20,21
Premature ventricular complexes with multiple morphologies have been shown to be associated with underlying arrhythmogenic substrate
In a prospective series, Oebel et al.22 showed the presence of RBBB pattern and multiple PVC morphology to be independent predictors of underlying ventricular fibrotic substrate identified by cardiac magnetic resonance imaging. In agreement with our findings, PVCs were reported to be terminated equally successfully in patients with structural substrate and those without.22,23 Baser et al.24 observed patients with multiple PVCs to experience recurrence of arrhythmia and reappearance of cardiomyopathy significantly more often than those without, as non-dominant PVCs were not targeted for ablation in the earlier procedure.
Lastly, we encountered very few procedural complications as well as no major adverse events at long-term follow-up after the PVC ablation targeting all morphologies. Our findings were in agreement with earlier reports.3
Limitations
We acknowledge few limitations of the current study. (i) It was a single-centre non-randomized analysis. However, consecutive patients were included and the procedural and follow-up data were collected prospectively. In addition, our institution is a high-volume centre with a wide diversity of patient population receiving interventions for their arrhythmic disorders. (ii) Cardiac magnetic resonance imaging was not performed routinely that could have provided more information regarding the underlying fibrotic substrate. However, we did not observe any correlation between presence of scar and ablation success. (iii) The sample size was relatively small. A larger population comparing the benefits of ablation of all sites vs. just the prevalent location in patients presenting with PVCs originating from multiple sites will be more elucidative. (iv) Spontaneous resolution of PVCs has been reported to happen over time. However, in our patients with long history of drug-refractory symptomatic PVCs, the possibility of spontaneous resolution was very unlikely.
Conclusion
In this prospective series of patients with multiple PVC morphologies, ablation of the prevalent PVCs alone was associated with effective reduction of PVC burden to <5% in only 43% of patients, whereas with ablation of all PVC morphologies, the same outcome was achieved in 84% of cases. Furthermore, persistence of high PVC burden was associated with significant worsening of the LVEF. In subjects with reduction in PVC burden to <5%, we observed a substantial improvement in the left ventricular ejection fraction at 1 year of follow-up.
Contributor Information
Sanghamitra Mohanty, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
John D Burkhardt, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Luigi Di Biase, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA; Department of Electrophysiology, Albert Einstein College of Medicine, 111 East 210th street, Bronx, NY 10467, USA.
Prasant Mohanty, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Sai Shishir Shetty, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Carola Gianni, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Domenico G Della Rocca, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Karim K Baho, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Trevor Morris, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Angel Mayedo, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Bryan MacDonald, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Amin Al-Ahmad, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Mohamed Bassiouny, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Gerald Joseph Gallinghouse, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Rodney Horton, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA.
Andrea Natale, Department of Electrophysiology, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, TCAI: 1015 east 32nd street, suite 408, Austin, TX-78705, USA; Interventional Electrophysiology, Scripps Clinic, 3811 Valley Centre Dr., SD, CA 92130, USA; Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109, USA.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
De-identified and analysed data will be available for research purpose only upon reasonable request to the corresponding author.
References
- 1. Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular Complex induced cardiomyopathy. Curr Probl Cardiol 2015;40:379–422. [DOI] [PubMed] [Google Scholar]
- 2. Bagliani G, Della Rocca DG, De Ponti R, Capucci A, Padeletti M, Natale A. Ectopic beats: insights from timing and morphology. Card Electrophysiol Clin 2018;10:257–75. [DOI] [PubMed] [Google Scholar]
- 3. Latchamsetty R, Yokokawa M, Morady F, Kim HM, Mathew S, Tilz Ret al. . Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 2015;1:116–23. [DOI] [PubMed] [Google Scholar]
- 4. Sheldon SH, Latchamsetty R, Morady F, Bogun F. Catheter ablation in patients with pleomorphic, idiopathic, premature ventricular complexes. Heart Rhythm 2017;14:1623–8. [DOI] [PubMed] [Google Scholar]
- 5. Zhong L, Lee YH, Huang XM, Asirvatham SJ, Shen WK, Friedman PAet al. . Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187–93. [DOI] [PubMed] [Google Scholar]
- 6. Lamba J, Redfearn DP, Michael KA, Simpson CS, Abdollah H, Baranchuk A. Radiofrequency catheter ablation for the treatment of idiopathic premature ventricular contractions originating from the right ventricular outflow tract: a systematic review and meta-analysis. Pacing Clin Electrophysiol 2014;37:73–8. [DOI] [PubMed] [Google Scholar]
- 7. Kany S, Alken FA, Schleberger R, Baran J, Luik A, Haas Aet al. . Bipolar ablation of therapy-refractory ventricular arrhythmias: application of a dedicated approach. Europace 2022;24:959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin CY, Chang SL, Lin YJ, Lo LW, Chung FP, Chen YYet al. . Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol 2015;180:80–5. [DOI] [PubMed] [Google Scholar]
- 9. Noheria A, Deshmukh A, Asirvatham SJ. Ablating premature ventricular complexes: justification, techniques, and outcomes. Methodist Debakey Cardiovasc J 2015;11:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson RD, Kumar S, Parameswaran R, Wong G, Voskoboinik A, Sugumar Het al. . Differentiating right- and left-sided outflow tract ventricular arrhythmias: classical ECG signatures and prediction algorithms. Circ Arrhythm Electrophysiol 2019;12:e007392. [DOI] [PubMed] [Google Scholar]
- 11. Gallagher MM, Padula M, Sgueglia M, Santini L, Voci P, Mahon NGet al. . Electrocardiographic markers of structural heart disease and predictors of death in 2332 unselected patients undergoing outpatient Holter recording. Europace 2007;9:1203–8. [DOI] [PubMed] [Google Scholar]
- 12. Ephrem G, Levine M, Friedmann P, Schweitzer P. The prognostic significance of frequency and morphology of premature ventricular complexes during ambulatory Holter monitoring. Ann Noninvasive Electrocardiol 2013;18:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorgente A, Farkowski MM, Iliodromitis K, Guerra JM, Jubele K, Chun JKRet al. . Contemporary clinical management of monomorphic idiopathic premature ventricular contractions: results of the European Heart Rhythm Association survey. Europace 2022;24:1006–14. [DOI] [PubMed] [Google Scholar]
- 14. Santoro F, Di Biase L, Hranitzky P, Sanchez JE, Santangeli P, Perini APet al. . Ventricular fibrillation triggered by PVCs from papillary muscles: clinical features and ablation. J Cardiovasc Electrophysiol 2014;25:1158–64. [DOI] [PubMed] [Google Scholar]
- 15. Callans DJ. Premature ventricular contraction-induced cardiomyopathy. Arrhythm Electrophysiol Rev 2017;6:153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han FT. Empiric ablation of asymptomatic PVCs when there is greater than 20% burden but normal left ventricular function—an argument in support of catheter ablation. Heart Rhythm O2 2021;2:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire Cet al. . Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865–9. [DOI] [PubMed] [Google Scholar]
- 18. Deyell MW, Park KM, Han Y, Frankel DS, Dixit S, Cooper JMet al. . Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm 2012;9:1465–72. [DOI] [PubMed] [Google Scholar]
- 19. Scorza R, Jonsson M, Friberg L, Rosenqvist M, Frykman V. Prognostic implication of premature ventricular contractions in patients without structural heart disease. Europace 2022;euac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huizar JF, Fisher SG, Ramsey FV, Kaszala K, Tan AY, Moore Het al. . Outcomes of premature ventricular contraction–cardiomyopathy in the veteran population: a secondary analysis of the CHF-STAT study. JACC Clin Electrophysiol 2021;7:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakkireddy D, Di Biase L, Ryschon K, Biria M, Swarup V, Reddy YMet al. . Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol 2012;60:1531–9. [DOI] [PubMed] [Google Scholar]
- 22. Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann Aet al. . ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol 2017;28:1316–23. [DOI] [PubMed] [Google Scholar]
- 23. Penela D, Martínez M, Fernández-Armenta J, Aguinaga L, Tercedor L, Ordóñez Aet al. . Influence of myocardial scar on the response to frequent premature ventricular complex ablation. Heart 2019;105:378–83. [DOI] [PubMed] [Google Scholar]
- 24. Baser K, Bas HD, LaBounty T, Yokokawa M, Good E, Latchamsetty Ret al. . Recurrence of PVCs in patients with PVC-induced cardiomyopathy. Heart Rhythm 2015;12:1519–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified and analysed data will be available for research purpose only upon reasonable request to the corresponding author.