Abstract
Carotenoids have been related to a number of health benefits. Their dietary intake and circulating levels have been associated with a reduced incidence of obesity, diabetes, certain types of cancer, and even lower total mortality. Their potential interaction with the gut microbiota (GM) has been generally overlooked but may be of relevance, as carotenoids largely bypass absorption in the small intestine and are passed on to the colon, where they appear to be in part degraded into unknown metabolites. These may include apo-carotenoids that may have biological effects because of higher aqueous solubility and higher electrophilicity that could better target transcription factors, i.e., NF-κB, PPARγ, and RAR/RXRs. If absorbed in the colon, they could have both local and systemic effects. Certain microbes that may be supplemented were also reported to produce carotenoids in the colon. Although some bactericidal aspects of carotenoids have been shown in vitro, a few studies have also demonstrated a prebiotic-like effect, resulting in bacterial shifts with health-associated properties. Also, stimulation of IgA could play a role in this respect. Carotenoids may further contribute to mucosal and gut barrier health, such as stabilizing tight junctions. This review highlights potential gut-related health-beneficial effects of carotenoids and emphasizes the current research gaps regarding carotenoid—GM interactions.
Keywords: Carotenes, xanthophylls, digestion, carotenoid metabolites, gut bacteria, microbiome, mucosal layer, inflammation, oxidative stress, bactericidal effects, Bifidobacterium spp., Akkermansia spp.
Statement of significance.
This article focuses on an important gap of carotenoid research—the interactions of carotenoids with gut microbiota in the colon and potential associated health benefits. Mechanisms including altered microbiota composition, carotenoid metabolism, barrier properties, and immune-relevance are especially highlighted.
Introduction
Carotenoids are typically C40 tetraterpenoid pigments, produced by most plants, some bacteria, and fungi, but not by humans. Although there are >1100 different carotenoids, up to 50 are found in our diets and among those only a dozen appear at measurable concentrations in the human bloodstream. These pigments are among the most abundant liposoluble phytochemicals in the bloodstream (∼0.5–2 μM), despite their low daily dietary intake (ca. 5–20 mg) [1]. Some carotenoids are vitamin A precursors (i.e., provitamin A carotenoids) and following consumption they can be cleaved to produce vitamin A in the small intestine [2]. Vitamin A has well-established biological effects in eliciting cell differentiation, gap junction formation, immunity, and visual light-dark adaptation. Both provitamin A and nonprovitamin A carotenoids have also received much interest because of inverse associations between the consumption of carotenoid-rich foods and cardio-metabolic diseases [3] and total mortality [4]. These health benefits have originally been ascribed to their anti-inflammatory/antioxidant properties, as carotenoids can protect against lipid peroxidation and damage caused by reactive oxygen species (ROS) [5]. In the past decade, there has been a great interest to understand how carotenoids, or their metabolites, may interact with transcription factors, including the pro-inflammatory NF-κB and the antioxidant-related Nrf-2 [6]. They could also interact via binding to nuclear receptors, including retinoic acid receptor/retinoid X receptor (RAR/RXR) and peroxisome proliferator-activated receptors (PPARs), whose downstream effects are related to cellular differentiation and the immune response [7]. Another important aspect is the association of lutein and zeaxanthin and the reduced risk of age-related macular degeneration, the main cause of vision loss in the elderly [8].
Carotenoid absorption can be relatively low and variable, depending on the carotenoid physical-chemical structure (e.g., carotenes vs. xanthophylls), food matrix effects, co-consumption with other factors (i.e., lipids, metals, etc.), and a variety of host factors [9, 10]. Thus, the bulk of a given dose of carotenoids consumed will reach the colon. It is unclear if absorption can occur in the colon [7]. A major research gap exists also regarding carotenoid metabolism in the colon and interactions with the gut microbiota (GM), i.e., the microorganisms residing in the gut [11]. Metabolized carotenoids such as apo-carotenoids have a shorter chain length and oxygen modification, which slightly increases their solubility in an aqueous environment. In addition, their higher electrophilicity would make them suitable to interact with transcription factors [12]. Recently, dose-dependent prebiotic-like effects, including anti-inflammatory ones, of lycopene were demonstrated in a clinical trial in influencing abundances of Bifidobacterium adolescentis and Bifidobacterium longum [13]. A greater abundance of Lactobacilli spp. was also observed when lycopene was consumed. Although the authors demonstrated that lycopene supplementation improved gut metabolism, they did not provide any mechanistic basis for the prebiotic effect. Some potential interactions of carotenoids with the GM have been highlighted recently [14], emphasizing that this may constitute an under-explored pathway via which carotenoids could exert beneficial health effects. Indeed, GM composition has been associated with many chronic health complications. For instance, in some, though not all studies, obesity was associated with lower GM diversity and a lower ratio of Bacteroidetes to Firmicutes [15], with potential negative influences on resting energy expenditure [16]. GM may also trigger inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis, and certain prebiotics may ameliorate such conditions [17]. IBD is strongly related to gut barrier functionality, and carotenoids may exert positive effects here also, either via their systemic anti-inflammatory and antioxidant properties [18], via the the GM, or impacting tight junction integrity [19]. The relation of GM to many chronic diseases is not surprising, given that the GM is required for an optimal immune function expression. The immune system is hampered in germ-free animals, as shown by underdeveloped lymphatic organs and low IgA in mice [20]. Lyu et al. recently reviewed interactions between retinoic acid (the most potent form of vitamin A), astaxanthin, and the GM [21], suggesting that astaxanthin may enhance IgA production, preventing gut dysbiosis via recognizing, and coating certain bacteria and preventing their infiltration through the epithelial barrier.
Though their precise implications in disease remain often poorly understood, the crucial role of the GM in the etiology of many diseases has been emphasized, and the involvement of the gut-brain, gut-liver, and gut-heart axes have been highlighted. Carotenoids may interact with the GM in a multitude of ways (Figure 1):
-
[1)
modulate the abundance of bacteria that can either activate commensal pathways or suppress pathogenic pathways [14, 22];
-
[2)
reduce oxidative stress (OS) in the gut, which could likewise alter GM composition [23];
-
[3)
foster the production of anti-inflammatory short-chain fatty acids (SCFA) such as butyrate by altering the abundance of SCFA-producing bacteria [24], which may act on PPARγ [25], inhibiting the dysbiotic expansion of certain bacteria and being related to reduced pro-inflammatory toll-like receptor (TLR) signaling [26];
-
[4)
maintain healthy mucosa and epithelial gut barrier and tight junction integrity [27], as proposed in a recent study in the small intestine of piglets [28]; improve mucosa integrity via favorable GM composition [27];
-
[5)
influence gut metabolism and immune-related properties, such as IgA [21].
FIGURE 1.
Mechanisms by which carotenoids could interact with the gut mucosa, influencing GM and intestinal barrier properties. GPx, glutathione peroxidase; HO-1, heme-oxygenase 1; IgA, immunoglobulin A; IL-6, interleukin-6; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2–related factor 2; OPG, obligate pathogenic bacteria; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; RNS, reactive nitrogen species; SCFA, short-chain fatty acids; SOD-1, superoxide dismutase 1; TLRs, toll-like receptors; TNF-α, tumor necrosis factor alpha.
In this review, we highlight the present state of knowledge regarding carotenoid—GM interactions and emphasize possible pathways in the colon via which these pigments may contribute to health benefits, also pointing out gaps of knowledge and possible steps forward.
Brief overview of the processing of carotenoids in the stomach and small intestine
The processing and digestion of carotenoids in the upper digestive tract have been extensively reviewed elsewhere [9, [29], [30], [31]]. Herein, we briefly recapitulate the primary factors of influence on carotenoid absorption. This evidence provides sufficient background to understand why the carotenoid proportions that remain in the digestive lumen (and are available for microbial interaction in the colon) are relatively high.
Carotenoids are very hydrophobic (pKa∼10–15.6), and must be released from the food matrix and dissolved to be available for incorporation within gastric lipid droplets. The most significant factor that influences carotenoid release is the co-consumption with lipid (which provides both a medium to solubilize the carotenoid within and stimulates gastric lipase release [32, 33]). Following lipid consumption, gastric lipase cleaves free fatty acids from triglycerides, to facilitate lipid droplet emulsification [34]. Larger lipid droplets slow the rate of gastric emptying until sufficiently small droplets are achieved [35], providing additional time for both fatty acids to be cleaved from triglycerides to form smaller droplets, and for the carotenoids to be released from the matrix.
The other major factor facilitating gastric carotenoid release is the breakdown of the food matrix through preceding cooking/processing [36]. For example, Rich and colleagues [37] compared the transfer of lutein and β-carotene from raw vs. blanched and frozen spinach into lipid droplets, under gastric conditions. Both the rate of carotenoid transfer to the oil droplet, as well as the absolute concentration achieved at experiment termination at 110 min, were significantly higher (30–33x) in the blanched and frozen spinach as compared with the raw spinach. A similar trend was observed between raw and blanched carrot juice [37]. Likewise, following consumption of cooked tomato or spinach puree, a majority of the carotenoids (i.e. ∼95% of the lycopene, ∼75% of the lutein, respectively) remained embedded in the food matrix over 180 min of gastric digestion [38], with almost all freed carotenoids (5%–25%) being associated with the lipid droplet fraction. In the same study, 60% of the β-carotene in cooked carrot puree was transferred directly to gastric lipid droplets [38].
Because solubility and subsequent bioavailability are so limited, and because of implications in diseases where bioactivity would be most critical postabsorption, there is keen interest to improve carotenoid delivery. This goal can be attempted through providing supplemental doses (often administered as a super saturated, crystal-rich oil product) or through enhanced bioaccessibility. Recent strategies to improve bioaccessibility have included the addition of emulsifying agents or the formation of nanoemulsions [31, [39], [40], [41], [42], [43], [44]]. The rationale behind these approaches is essentially to deliver smaller lipid droplets or small vesicles that do not need to be micellarized or to more rapidly produce smaller lipid droplets during the digestive process [43]. Some of these approaches have been successful, whereas others have been limited, and under some circumstances may reduce carotenoid lipid droplet incorporation [40, 45]. Additionally, the complete absence of lipids or consumption of high lipid excess (i.e., >10%), can cause crystal formation and precipitation [46, 47]. In either of these instances, a high concentration remains in the gastric lumen, which may provide a source for GM interaction in the stomach.
A number of other factors further influence the proportion of carotenoids freed in the gastric compartment from foods and have more attenuated effects, as extensively reviewed previously [30]. In brief, release is enhanced in the stomach in foods with carotenoid compartmentalization within liquid-crystalline chromoplasts of the fruit or vegetable being consumed; prolonged cooking or processing times and temperatures; co-consumption with some citrus flavanones, digestible proteins, high methyl-ester pectin (relative to low methyl-ester pectin), and encapsulation. Xanthophylls are also better released relative to carotenes, all other factors being equal, because of the presence of one or more oxygen groups and thus higher polarity [29]. In contrast, additional factors that inhibit/reduce carotenoid release into the lipid droplet include compartmentalization within solid-crystalline chromoplasts of the fruit or vegetable being consumed, co-consumption with high doses of divalent metals [48], anthocyanins [49], or with naringenin [50, 51]. It is worth noting that absence of release to the lipid droplet would still leave the carotenoid present within the gastric lumen, but sequestered in crystalline form, or in a food matrix, thus limiting GM interaction.
Following gastric emptying, the digesta is met with the release of bile salts and pancreatic lipase and colipase in the duodenum. The lipase and colipase continue to facilitate fatty acid release from triglycerides and diacylglycerols, whereas the bile salts act as a natural emulsifying agent to produce smaller lipid droplets. Collectively, the free fatty acids and bile salts produce subsequently smaller lipid droplets until mixed micelles (∼4 nm in diameter) are formed [52], which also include phospholipids, monoacylglycerols, and embedded apolar compounds such as cholesterol and carotenoids.
As with the gastric compartment, lipid co-consumption is arguably the most important factor for carotenoid incorporation into micelles in the small intestine [53]. The proportion of the original carotenoid dose that ends up in the micelle is known as the “bioaccessible” fraction, which is the only proportion of the original dose thought to be available for absorption [54]. The type of lipid co-consumed strongly influences bioaccessibility, with long-chain fatty acids greatly increasing the bioaccessible (and thus bioavailable) fraction relative to medium-chain fatty acids [[55], [56], [57]]. In contrast, there is a limited influence of fatty acid saturation on bioaccessibility [55, 56]. Other factors that have a smaller but still meaningful impact on carotenoid bioaccessibility include competition with supplemental doses of carotenoids or fat-soluble vitamins E, D, and K for micellarization or uptake by the same transporters [29, 58], presence of divalent metals [48, 59, 60], and presence of certain types of proteins (i.e., β-lactoglobulin [61] and other whey proteins, caseinate, and soy proteins [62, 63]).
The concentration of carotenoids soluble in the duodenal and jejunal compartments remains fairly constant through the first 2 h of small intestinal digestion, before rising fourfold to fivefold over the next 2–3 h [64, 65]. This increase in concentration is at least partially driven by water reabsorption. Despite these increases, the bioaccessibility and subsequent bioavailability of carotenoids (i.e., removal from the intestinal lumen and ultimate release into the blood stream) on average remains low, but with high inter-individual variability, even under ideal conditions [10, 29]. Thus, a significant fraction of the original carotenoid dose remains available for interaction with the GM. The fact that so much of the carotenoids remain unabsorbed is perplexing, as fatty acids and bile salts are efficiently absorbed by transport proteins in the distal small intestine (i.e., 80%–95% fatty acids absorbed, depending on chain length and saturation, >90% of bile acids) [66, 67]. We could speculate that the reuptake of water and loss of fatty acid micelles would cause carotenoid crystallization to occur (if pure carotenoid supplement was fed), or at least partial association with digestible and indigestible fibers if the carotenoid dose was consumed as part of a fruit or vegetable preparation, and such carotenoid processing in the upper GI tract is likely to affect the GM. Considering that doses of 2–20 mg of carotenoids can be consumed from a single meal [68], significant carotenoid quantities are present in the large intestine and they may modulate the GM either directly or indirectly.
Human studies support these very high concentrations, using fecal carotenoid content as a proxy for what remains in the colon. For example, following 2 weeks of daily consumption of 330 mL of tomato or carrot juice, fecal concentrations of lycopene, β-carotene, and α-carotene increased 50–60-fold in healthy men [69]. Regarding the colonic carotenoid uptake, absorption of β-carotene into human exfoliated epithelial cells of the colon was demonstrated [70], and SR-B1 (transporter responsible for uptake of some carotenoids) is also expressed in the colon [71]. Transporter expression combined with the incomplete carotenoid absorption in the small intestine suggest the colon may be a place for carotenoid uptake. Studies so far have not suggested a strong correlation between carotenoid dietary intake and colon tissue concentrations [72], though in a human trial GM composition (thought to alter absorption from the epithelium) correlated with baseline serum carotenoid concentration [73]. In mice, the reduced abundance of microbial populations in the gut led to an increase in β-carotene and vitamin A storage in the liver, though no further metabolism by GM was detected [74]. The data available are too preliminary to draw conclusions.
Apo-carotenoids during digestion
Apo-carotenoids are catabolites of carotenoids (either carotenes or xanthophylls) that contain <40 carbons. They are consumed from fresh and processed fruits and vegetables at concentrations that are 100–1000× lower than the precursor carotene concentrations in the same foods [75, 76]. These proportionally low concentrations are because apo-carotenoids (most famously strigolactones and abscisic acid but including many species that have more recently been identified) serve as potent plant hormones, whose production within the plant is regulated via carotenoid cleavage dioxygenase enzymes [77]. These enzymes produce apo-carotenoids that are ≤14 carbons in length, and serve as plant root growth regulators, aid in plant defense, provide aroma, and modulate plant stress responses [[77], [78], [79]]. This asymmetric or eccentric cleavage leaves a longer-chain (i.e., ≥26 carbon) apo-carotenoid as the corresponding “by-product.” It is these longer-chain products for which there is the most data present to-date in mammalian systems. However, both short-chain apo-carotenoids and longer-chain apo-carotenoids may have health-relevant effects in humans. For instance, abscisic acid, a C15 carbon apo-carotenoid acting as a plant hormone, has been shown to improve insulin glucose response in a murine model of diet-induced obesity [80]. Similarly, the consumption of the C20 carotenoid crocetin improved insulin resistance in a pilot study of type 2 diabetics [81]. Further potential biological effects of some of these apo-carotenoids have been reviewed by Harrison and Quadro [82].
Interest in the production of these longer-chain apo-carotenoids during digestion has arisen as the conjugated double-bond system of carotenoids is especially labile to chemical degradation, which can also produce apo-carotenoids. The gastric compartment provides a hostile environment to carotenoids, e.g., presence of oxygen, low pH, and large lipid droplets where carotenoids and unsaturated lipids co-mingle and co-oxidation can occur. Under simulated in vitro gastric conditions and in the absence of enzymes (e.g., in the presence of dioxygen, with or without iron, at the proper pH, emulsified with unsaturated lipids), some parent carotenoid loss and some apo-carotenoid production has been reported [[83], [84], [85], [86], [87], [88]]. However, both in vitro models using enzymes [84], as well as gastric and duodenal aspirates from human subjects during digestion [38, 64, 65], showed very limited (≤20%) carotenoid isomerization (the first step of carotenoid breakdown) during digestion. Additionally, no marked changes in apo-carotenoid concentrations have been observed. When lycopene or isotopically labeled β-carotene was digested alone in healthy men (n = 7), no significant change in gastric or duodenal apo-carotenoid digestate concentrations was observed, relative to the quantity of carotenoid, over 4–5 h [64, 65]. When a lycopene meal was co-digested with ferrous sulfate in healthy men (n = 7), the gastric and duodenal digestate concentrations of both lycopene and all but one species of lycopene-derived apo-carotenoids significantly decreased over 4 h, suggesting other lycopene degradation products were produced instead [65]. Notably, lycopene-derived apo-carotenoid absorption was observed in this study, as evidenced by the presence within the newly absorbed lipid-fraction within the blood and confirmed via follow-up Caco-2 experiments [65]. This evidence suggests that only a fraction of ingested lycopene-derived apo-carotenoids would remain in the GI tract for further interaction. In contrast, no isotopically labeled β-apo-carotenoids were absorbed in the previously cited work, suggesting that they would remain in the GI lumen, and could potentially elicit biological effects there [64].
Asymmetric apo-carotenoids may also be to be produced in the human body via one of the endogenously present carotenoid cleavage dioxygenases, i.e., β-carotene oxygenase 2 (BCO2). Indeed, BCO2 is expressed in a number of human tissues, including the small intestinal mucosa and the liver, although notably it is not expressed in the colon [89]. Its activity in model systems has been well characterized, with BCO2 demonstrating broad specificity but a clear preference for cleaving xanthophylls, specifically at the 9′–10′ double bond [90, 91]. BCO2 expression, which occurs in the mitochondria, increases in response to the knockout of the other main intestinal carotenoid cleavage enzyme (β-carotene oxygenase 1, BCO1) [92, 93]. Although the purpose of BCO2 expression is not fully understood, it has been postulated to serve an important role in preventing carotenoid concentrations from reaching levels which might expose the cell to increased oxidative damage [92]. Understanding the role BCO2 may play in humans is much more difficult, and very little work has been done. 13C β-carotene (which contained a baseline concentration of 13C β-apo-carotenals) was fed to healthy males, and samples of the gastric and duodenal digesta, as well as blood plasma and the newly absorbed lipid-fraction of blood were collected and isolated [64]. Notably, both 13C β-carotene and 13C β-apo-carotenals increased at largely the same rate over time in plasma (presumably as water was absorbed). Thus, there was no meaningful net change in the ratio of any of the β-apo-carotenals investigated/β-carotene, and no absorption of any 13C β-apo-carotenoids into the blood plasma except for vitamin A isoforms. This suggest that either the 13C β-apo-carotenods in the digesta were coming from the initial dose without any additional production, or it may indicate that any cleavage by BCO2 is quickly shunted toward vitamin A production, via further catabolism by BCO1.
It is also possible that apo-carotenoids could be made in other body compartments, as recently reviewed by Harrison and Kopec [94], and re-secreted into the gastrointestinal lumen with bile. Longer-chain apo-carotenoids (from dietary sources [76], or produced via BCO2 cleavage [95]) could also be cleaved further by the central, vitamin A-producing cleavage enzyme BCO1. BCO1 is expressed in some of the same tissues as BCO2, including the liver and the small intestine. BCO1 is also expressed in the stomach and the colon [89], and some evidence suggests that beyond central cleavage of β-carotene, it may also act on carotenoids such as lycopene and β-cryptoxanthin [82]. The role of intestinal BCO1 in cleavage of provitamin A, and the important effects of vitamin A on maintaining the intestinal barrier and conferring immune protection have been discussed elsewhere [91, [96], [97], [98], [99]]. The exact interplay between BCO1 and BCO2, and the relative contributions to the apo-carotenoid pool and the vitamin A pool in the human digestive lumen and within the epithelial cells themselves, remains a gap in knowledge. In addition, whether any human colonizing microbiota can synthesize apo-carotenoids remains unclear.
The biological effects of these apo-carotenoid products remain understudied; however, some apo-carotenoids have demonstrated antagonistic activity toward nuclear receptors which are stimulated by retinoic acid (the potent form of vitamin A) [7, 100]. It is also possible that apo-carotenoids produced from provitamin A carotenoids could be further cleaved or oxidized to produce vitamin A itself [101], whose functions within the GI lumen are discussed in greater detail below. Regardless, the concentrations of apo-carotenoids within the upper gastrointestinal remain relatively low in relation to the precursor carotenoids from which they are derived, and thus very low amounts would be passed on to the colon. Thus, the remainder of this review focuses on the carotenoids themselves.
Microbial carotenoid metabolism—potential breakdown and biosynthesis of carotenoids
The GM of animals and humans are complex ecosystems that support many metabolic and biological functions within their hosts. These functions range from assisting the metabolism of essential metabolites and nutrients, training the immune system to fight pathogens, and eliminating allergic stimulants, to steering the mood and behavior of its host [102]. One major symbiotic advantage of hosting microbial communities is obtaining essential amino acids and vitamins such as isoforms of all B vitamins as well as vitamin K—nutrients that the human body cannot synthesize [103]. Likewise, mammals cannot synthesize vitamin A de novo, meaning that it must be obtained either as preformed vitamin A from animal sources, or as provitamin A carotenoids from the diet and potentially from gut bacteria [21]. Although evidence of how microbes contribute to the bioavailability of carotenoids is still lacking, many lipid transporters that facilitate carotenoid uptake were found in the colon, where the highest microbial diversity resides [29]. For example, SR-B1 (scavenger receptor, class B type I) is expressed in colonic epithelial cells, suggesting that carotenoids can be taken up in the colon [7, 104]. The fraction of carotenoids remaining in the intestinal lumen could either be used by microbes to enhance lipid metabolism, to serve as antioxidants to reduce oxidative damage, or secreted in feces with the undigested fibers. Since carotenoids are important for the health of the human body, it would be desirable to understand how both the host and the GM assist each other to maximize their benefits from the ingested carotenoids. However, the mechanisms by which the carotenoids are made available for the colon host for absorption and are utilized by microbes are poorly understood. Very little on colonic metabolites has been mentioned in the literature. For E. coli, as well as lactic acid bacteria, it has been reported that the xanthophyll fucoxanthin is metabolized into fucoxanthinol, following deacetylation [105].
Engineering carotenoid biosynthesis and the applicability to the gut
In this section, we will review the current state of the art in engineering carotenoid biosynthesis and the applicability of these strategies to the gut as a novel possibility to enhance gut health and to improve carotenoid and vitamin A status. Traditionally, food has been the main source of carotenoids. However, a variety of factors, including low crop yields or lack of access to crops with high carotenoid content [106, 107], can lead to an inability to consume a sufficient amount of provitamin A carotenoid to meet vitamin A needs. Furthermore, as carotenoids find use as food colorants, cosmetics, and pharmaceutical products, new ways to synthesize, produce, and extract carotenoids have been developed [108, 109]. Recent studies have shown that carotenoid biosynthesis in microorganisms proceeds in a highly specific fashion, whereas chemical synthesis usually yields multiple geometric carotenoid isomers [110]. Using biotechnological processes, genetically engineered microorganisms (i.e., bacteria and fungi) can be easily scaled up and are not impacted by factors such as seasonal harvest fluctuations, especially in the era of climate change [109].
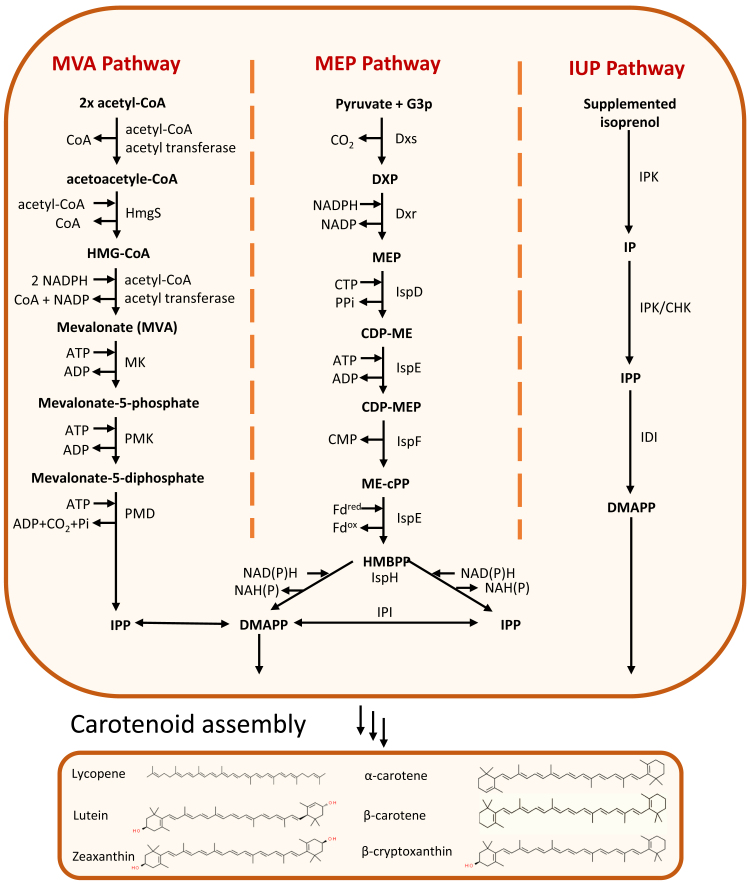
Two main pathways that are responsible for the production of carotenoids in plants and microorganisms are the mevalonate (MVA) and plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway (Figure 2) [109]. Naturally, although carotenoid-producing eukaryotes and archaea mainly follow the MVA pathway, bacteria and plant plastids mainly have the MEP pathway. To create the building blocks of any carotenoids, the MVA and MEP pathways exploit molecules that are produced by the central carbon pathway, mainly pyruvate, acetyl-CoA, and glyceraldehyde-3-phosphate (G3P). Both pathways use these substrates to create iso-pentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are then assembled into different carotenoid structures. For example, 7 IPP molecules and 1 DMAPP molecule are needed to create 1 molecule of lycopene. Overexpression of certain genes in those pathways, e.g., 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), phytoene synthase (PSY), and phytoene desaturase (CRTI) and introduction of homologs of these genes, can increase production of total carotenoids.
FIGURE 2.
Pathways for biosynthesis of carotenoid precursors. CHK, Choline kinase; CTP, Cytidine 5'-triphosphate; DMAPP, Dimethylallyl disphosphate; Dxp, 1-Deoxy-D-xylulose 5-phosphate; Dxr, 1-Deoxy-D-xylulose 5-phosphate reductoisomerase; Dxs, 1-Deoxy-D-xylulose 5-phosphate synthase; Fd, Ferredoxin; G3P, Glyceraldehyde 3-phosphate; CMP, Cytidine monophosphate; HMBPP, E-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate; HMGR, 3-Hydroxy-3-methylglutaryl-CoA reductase; HmgS, 3-Hydroxy-3-methylglutaryl-CoA synthase; IDI, Isopentenyl diphosphate isomerase; IspD, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; IspE, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; IspH, 4-Hydroxy-3-methylbut-2-enyl diphosphate reductase; IPI, Isopentenyl diphosphate isomerase; IMO, Isomalto-oligosaccharides; IPK, Isopentenyl phosphate kinases; IPP, Isopentenyl diphosphate; IspE, CDP-methylerythritol kinase; IUP, Isopentenol utilization pathway; MK, Mevalonate kinase; MVA, Mevalonic acid; MEP, Methylerythritol 4-phosphate; PPi, Pyrophosphate; PMD, Phosphomevalonate decarboxylase; PMK, Phosphomevalonate kinase.
The use of microorganisms for producing relatively small molecules such as carotenoids is attractive because microbes can be cultured year-round and do not require large land areas or greenhouses [111]. With the availability and simplicity of genetic engineering tools to manipulate microbial genomes, as well as advances in fermentation strategies, the development of high carotenoid-producing strains is becoming increasingly possible [110]. Lycopene, β-carotene, zeaxanthin, lutein, and β-cryptoxanthin are the most relevant carotenoids related to human health and therefore most microbial engineering efforts have focused on optimizing their production (Figure 3) [112]. Some of the engineering approaches used to increase the production of these carotenoids in yeast and bacteria include: regulation of the biosynthesis pathways, enhancing enzyme expression, increasing membrane synthesis, optimization of the central metabolic pathways, rewiring central carbon metabolism [113], combinatorial assembly of different genetic parts and combinations of these methods (Table 1). Although these approaches and optimization of the MVA and MEP pathways have increased carotenoid production, the amount of IPP biosynthesis limits further optimization [109]. Therefore, a chemoenzymatic approach to increase IPP was explored [109]. Transfecting the isopentenol utilization pathway (IUP) into Escherichia coli MG1655 and Yarrowia lipolytica increased the biosynthesis of IPP by 15–100-fold and thus the production of carotenoids [114]. The IUP pathway uses the isopentenol isomer isoprenol or/and prenol, which can be supplemented in the growth media, as the main substrate for creating IPP and DMAPP molecules [114]. Compared with the MVA and MEP pathways, the IUP pathway only goes through 2 steps (MVA and MEP pathways contain 6 and 7 steps, respectively) to create one molecule of IPP, which consequently reduces byproducts and the energy expenditure required for creating carotenoid building blocks.
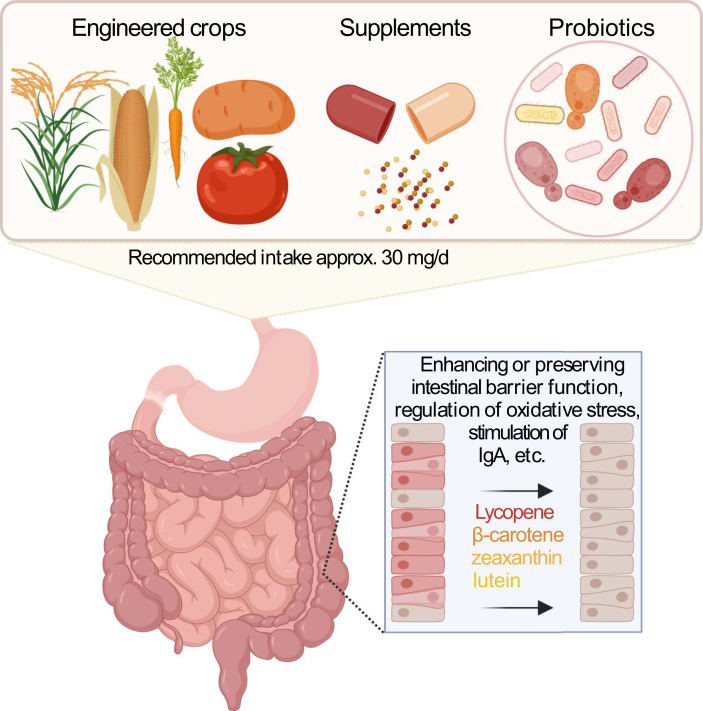
FIGURE 3.
Approaches for delivering carotenoids together with probiotics to the colon. IgA, Immunoglobulin A.
TABLE 1.
Sources of carotenoid production by microorganisms, and their delivery mechanisms. DCW = dry cell weight.
| Delivery to gut | Carotenoid | Amount | Source | Genetically modified organism | Reference |
|---|---|---|---|---|---|
| Probiotic | β-carotene | 37 μg/g | Saccharomyces boulardii | Yes | [117] |
| Probiotic | lycopene | 40.8 mg/mL | Bacillus indicus | No | [28] |
| Probiotic | lycopenoate | 23.6 mg/mL | Bacillus indicus | No | [28] |
| Probiotic | lycopene | 1.09 mg/L | Lactococcus lactis | Yes | [177] |
| Supplement | β-carotene | 7 mg/L | Corynebacterium glutamicum | No | [178] |
| Supplement | β-carotene | 6500 mg/L (90 mg/g) | Saccharomyces cerevisiae | Yes | [179] |
| Supplement | β-carotene | 4000 mg/L | Saccharomyces cerevisiae | Yes | [180] |
| Supplement | β-carotene | 2370 mg/L (73.3 mg/g) | Saccharomyces cerevisiae | Yes | [181] |
| Supplement | β-carotene | 2100 mg/L | Saccharomyces cerevisiae | Yes | [182] |
| Supplement | β-carotene | 704.1 mg/L | Blakeslea trispora | No | [183] |
| Supplement | β-carotene | 44.2 mg/g | Saccharomyces cerevisiae | Yes | [184] |
| Supplement | lycopene | 2300 mg/L | Saccharomyces cerevisiae | Yes | [185] |
| Supplement | lycopene | 500 mg/g DCW | Escherichia coli | Yes | [186] |
| Supplement | lycopene | 256 mg/L | Blakeslea trispora | No | [187] |
| Supplement | lycopene | 220 mg/L | Escherichia coli | Yes | [115] |
| Supplement | lycopene | 56 mg/g DCW | Saccharomyces cerevisiae | Yes | [188] |
| Supplement | lycopene | 10 mg/g DCW | Rhodobacter sphaeroides | Yes | [189] |
| Supplement | astaxanthin | 385.0 mg/L (7.0 mg/g) | Escherichia coli | Yes | [190] |
| Supplement | astaxanthin | 320 mg/L | Escherichia coli | Yes | [191] |
| Supplement | astaxanthin | 218 mg/L | Saccharomyces cerevisiae | Yes | [192] |
| Supplement | astaxanthin | 1.7 mg/g | Corynebacterium glutamicum | No | [193] |
| Supplement | zeaxanthin | 0.5 mg/g DCW | Xanthophyllomyces dendrorhous | Yes | [194] |
In situ production of carotenoids by probiotics in the GI tract offers a novel, potentially more sustainable approach for delivering carotenoids [[115], [116], [117]]. Probiotics are live microbes that are attractive vehicles for the engineered production of desired molecules and biologics. By delivering these molecules right where they are needed, they can ultimately reduce the cost of carotenoid production by bypassing purification and concentration steps [115, 116, 118]. Using probiotics for the production and delivery of carotenoids, such as β-carotene, could be most beneficial in developing countries where vitamin A deficiency is still prevalent, as probiotics are relatively easy to obtain [117].
Building on the progress of microbial engineering for the production of carotenoids, Durmusoglu et al. first demonstrated that the probiotic yeast Saccharomyces boulardii could produce β-carotene in the gut of gnotobiotic mice. In this study, a single dose of S. boulardii expressing heterologous β-carotene pathway was delivered to mice, and the fecal concentration of β-carotene was monitored over 2 weeks. The authors detected up to 37 μg/g of β-carotene (68 μM) in feces. Another study conducted by Stevens et al. focused on Bacillus indicus (PD01), which was isolated from the fecal matter of a healthy volunteer and found to naturally produce methyl-glycosyl-apo-8′-lycopenoate and glycosyl-apo-8′-lycopene [28]. The authors also used weaned piglets with impaired intestinal barrier function to examine the efficacy of PD01 to limit intestinal permeability or treat intestinal barrier dysfunction [119]. In a human cohort with healthy volunteers, the carotenoids produced by PD01 were observed in the plasma of the subjects, and PD01 was not found to impair intestinal barrier function, GI tract tolerance, and stool frequency or consistency [28].
Although only examined in a limited number of test subjects, these studies demonstrate that probiotics can deliver carotenoids to the GI tract and may provide a viable option to mitigate vitamin A deficiency and perhaps improve gut health. Future studies should focus on the effects of provitamin A-producing probiotics and testing carotenoid-producing probiotics in a greater diversity of subjects, and for a longer period of time.
Carotenoids and shift of gut microbiota—prebiotic-like and bactericidal effects against pathogens
About the same number of microorganisms exist in our gut as the number of human cells in our body (i.e., 1010–1012), primarily as bacteria in the colon [120, 121]. Prebiotic effects are based on preventing dysbiosis and shifting the composition of the GM toward one associated with health, by acting as a substrate for certain bacteria. In other words, prebiotics improve host health and physiology [106]. Such prebiotic effects are well recognized for several fermentable dietary fibers, such as fructooligosaccharides and galactooligosaccharides [122]. Also, some polyphenols may act similarly, since they are poorly absorbed in the small intestine and are present as glycosides that could be fermented by certain bacteria [14]. Health-beneficial bacteria include Lactobacillus and Bifidobacterium, both of which are lactic acid producers that may aid in displacing more health-detrimental bacteria, e.g., biofilm-producing Pseudomonas [123]. This displacement prevents an overgrowth of opportunistic bacterial pathogens (OBP), which is also an important function of prebiotics. Other fermentation products may confer protection, such as the short-chain fatty acid butyrate, which is associated with anti-inflammatory effects locally and systemically following mucosal uptake [124]. Some bacteria, such as Faecalibacterium prausnitzii, a dominant butyrate-producing bacteria, have been reported to be decreased under certain dysbiotic conditions, including IBD [125]. Administration of a β-carotene-rich oil (1 g. L−1 in tucuma oil rich in oleic acid) to the rumen resulted in a shift from acetate to propionate [126], which is considered even more anti-inflammatory than butyrate [127]. In addition, this β-carotene-rich oil also induced GM changes, though rumen physiology can be quite different from humans (Table 2).
TABLE 2.
Studies with carotenoids involving effects on the GM
| Compound | Study type | Dosing and major outcomes | Main findings | Reference |
|---|---|---|---|---|
| Human studies | ||||
| β-Carotene | Observational study in subjects (n = 16) with cystic fibrosis | Associations tested between fecal microbiota and corresponding micronutrient intakes. | Intake of β-carotene (and several other antioxidants) was related to lower Bacteroides and higher Firmicutes. | [139] |
| Methyl-glycosyl-apo-8′-lycopenoate and glycosyl-apo-8′- lycopene produced by Bacillus indicus | Randomized, controlled trial in overweight/obese subjects (n =67) | Consumption of carotenoid-producing bacteria vs. placebo for 6 wk, plasma carotenoid concentrations, and colonic permeability. | 0.044 and 0.076 vs. 0 μM in controls as the sum of bacterial carotenoid compounds after 3 and 6 wk, respectively. No significant difference in gastro-duodenal or colonic permeability as determined by multi-sugar test (l-rhamnose; S/E : sucralose/erythritol). No difference in GI tolerance. | [28] |
| Lycopene | Adult subjects with obesity (n = 30), double-blinded design | 7 mg or 30 mg for 30 d as supplement, GM abundances measured. | Dose-related improvement in GM profile with enhanced fractions of, e.g., Bifidobacterium adolescentis and Bifidobacterium longum. Also related to dose-dependent variation in the blood, liver metabolism, skeletal muscle, and skin measures. | [13] |
| Animal trials | ||||
| Fucoxanthin | Male BALB/c mice (n = 40) | For 4 wk, mice were fed NCD (normal chow diet), NCD + fucoxanthin (NCDF, 125 mg/kg), HFD + fucoxanthin (HFDF, 125 mg/kg). | No difference detected between the NCD and NCDF. Firmicutes and Bacteroidetes increased in the NCDF group (26%). In the HFDF group, it increased (13%) vs. the HFD group, suggesting a positive effect of fucoxanthin. Fucoxanthin decreased abundance of Verrucomicrobia phylum. | [173] |
| β-Carotene | Piglets (n = 24) | Suckling group, weaning group, weaning + β-carotene (40 mg/kg bw.) group, and the weaning + β-carotene (80 mg/kg bw.) group, 2 wk intervention. Serum, jejunum, colon, and feces were investigated. | β-Carotene decreased phyla Bacteroidetes and the genus Prevotella, and Blautia, but increased species from the phyla Firmicutes and Parabacteroides vs. weaning animals. Spearman’s correlation analysis: revealed positive correlation between Prevotella and Blautia, and Parabacteroides and Synergistes were negatively correlated with concentrations of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α). p-75-a5 showed negative correlation with serum IL-6. | [119] |
| Lycopene | Mice (n = 10 per group) | Lycopene at 0.03% in the diet for 10 wk, together with a high fat diet. A number of behavioral tests and biomarkers were investigated. | Lycopene improved HFD-triggered memory loss. Lycopene also improved synaptic functioning. It also reduced insulin resistance, improved lipid metabolism dysfunction, and inflammatory responses in brain and liver. Finally, lycopene improved intestinal barrier integrity as shown by higher expressed claudin-1 and occludin as well as reduced circulating lipopolysaccharides. | [167] |
| Lycopene | “Cobiotic” treatment: Male Swiss albino mice, (n = 8–10 per group) | 5 and 10 mg lycopene/kg bw. and isomalto-oligosaccharides (IMO), for 12 wk. A variety of systemic markers (OS, inflammation), GM, and histopathologic examinations were carried out. | Lycopene improved SCFA concentration and ileal and colonic health. This was measured by histopathology and reduced Enterobacteriacea and increased Lactobacilli, increased total SCFA as well as improving systemic markers of OS stress and inflammation (e.g., SOD and CAT and various cytokines, respectively). Synergistic effects were observed with IMO. | [163] |
| β-Carotene | Mouse model of ulcerative colitis, number of animals nonspecified | Receiving (application mode not further specified) 5, 10, and 20 mg β-carotene/kg bw. for 28 d. | β-Carotene improved severity of UC, modulating various targets. e.g., NF-κB, cyclooxygenase- 2, interleukin 17, signal transducer and activator of transcription 3, Nrf2, matrix metalloproteinase-9, and connective tissue growth factor. Further more, β-carotene treatment maintained gut barrier integrity by increasing occludin expression. Also, β-carotene reduced plasma lipopolysaccharide levels. | [164] |
| Carotene (and proteins) | Duroc pigs (n = 32) | Pigs were fed 2 different diets for 1 mo.: a standard protein (SP) diet or SP+ carotene-enriched (CE) diet (20% of M37W-Ph3 carotenoid-enriched corn), unspecified amount carotenes. | Proteins had a stronger modifying effect than carotenes on the pig GM patterns. 160 amplicon sequences variants differed between CE and SP. | [136] |
| Methyl-glycosyl-apo-8′-lycopenoate and glycosyl-apo-8′ - lycopene produced by Bacillus indicus | Piglets (n = 16) | Either control diet or control diet with Bacillus indicus for 23 d. Barrier functionality in the gut was tested. | Supplementation with Bacillus indicus improved expression of occludin in distal small intestine and TEER in the mid colon vs. control. No differences regarding crypt depth and villus height. | [28] |
| Tomato powder (TP) rich in carotenoids | Male BCO1−/−+ BCO2−/− knockout mice (n = 18) | Mice were fed a HFD with or without dietary TP (42 g/kg diet) intervention for 24 wk. Carotenoid dose of 2.39 mg of lycopene, 0.11 mg of β-carotene/g TP | The fraction of Gram-positive bacteria was enhanced following TP; the fraction of Gram-negative bacteria was lowered accordingly. TP diminished the relative abundance of Clostridium spp. and Mucispirillum spp. | [174] |
| In vitro investigations | ||||
| Lycopene | In vitro inhibition assay: B. subtilis | Extraction from tomato paste and tests on B. subtilis, 50 μg/mL lycopene. | Inhibition against B. subtilis. | [175] |
| β-Carotene and extracts of carotenoids from red paprika, apples, oranges | In vitro inhibition assays: HSC-2, HSG, and HTLV1 | extracts of carotenoids and β-carotene from red paprika tested on infected cells (H. pylori and HIV-1 type IIIB) for 5 d. | Prevention of the development of H.pylori-associated disease, MIC50 of β-carotene and red paprika extract of > 200 μg/mL), extract from apples showed MIC50 of 36 μg/mL. | [147] |
| Tucuma oil rich in beta-carotene (1 g/L oil) | In vitro trial with rumen inoculum from donor Holstein cows | Tucuma oil at 0.5 and 1% added to diet, mostly containing oleic acid, added to rumen inocculum (n =3) during 15-d experiments. | With tucoma oil, reduced number of Fibrobacter and Rikenellacea RC9 group, and enriched Pyramidobacter, Megasphaera, Anaerovibrio, and Selenomonas. Reduced acetate and butyrate fraction of volatile fatty acids, increased fraction of propionate and valerate. | [126] |
| Extract carotenoids (from Shatian pummelo C. grandis) | In vitro inhibition assays: B. subtilis, S. aureus, E. coli, A. niger, A. flavu, P. chrysogenum, R. oryzae and S. cerevisiae | The extract was incubated for 24 h with the microorganisms tested. | Inhibition against B. subtilis, S. aureus, E. coli, A. niger, A. flavus, S. cerevisiae. | [176] |
| Annatto, carrot, corn, and tomato extracts | In vitro inhibition assays: E.coli and S. aureus | Extracts were incubated for 18–24 h with microorganisms tested. | Annatto, carrot, and tomato extracts exhibited antibacterial property for S. aureus. Annatto Extract, having the highest total carotenoid content, also exhibited the major MIC50 for S. aureus. | [149] |
GI, gastrointestinal tract; HFD, high-fat diet; MIC, Minimally inhibitory concentration; OS, Oxidative stress; TEER, transepithelial electrical resistance.
As Bacteroidetes and Firmicutes account for >90% of the phyla in the gut [128], changes in taxa that fall under these phyla are also of interest. For example, a higher ratio of Firmicutes to Bacteroidetes has been associated with obesity, and a lower one with IBD, as reviewed recently [129]. It should be noted though that until recently, bacterial populations have been primarily assessed using 16S rRNA, allowing differentiation only at the genus level. Moving forward, more insightful results may be obtained on a lower taxonomic level, adding more information about functionality but requiring more elaborated metagenomics analyses [130]. Beyond composition and abundance, a better understanding of the metabolic behavior of each bacterial species, which can radically change depending on dietary intakes [131], may better elucidate the role each microbe plays in health and disease.
There is little direct evidence that carotenoids serve as prebiotics or as direct energy sources for bacteria, and certainly other dietary factors can easily confound human studies, unless pure carotenoids are supplemented. Alcoholic or lactic acid fermentation did not result in significant losses of carotenoids [132]. In contrast, β-carotene and lycopene appeared to be produced by certain bacteria, such as Blakeslea trispora, as reviewed recently [132]. Certain bacteria and yeast were also reported to be capable of producing carotenoids, as recently summarized [133].
Nevertheless, microbial shifts were reported following intervention with carotenoids. In animal studies, capsanthin from bell pepper (>90% purity) (dose of 200 mg. kg−1 body weight) was administered to obese mice for 12 weeks [134]. Notably, the abundance of Bacteroidetes, Bifidobacterium, and Akkermansia were increased, whereas Ruminococcus and Firmicutes were reduced. In another study [135], astaxanthin from yeast (0.04% in the diet, for 8 weeks) significantly reduced OBP (i.e., Proteobacteria and Bacteroides) in β-carotene oxygenase 2 (BCO2) knockout mice, whereas strongly increasing Actinobacteria and Bifidobacterium in wild-type mice. In another animal experiment, pigs on a low-protein diet received carotenoid-fortified corn (20% of the diet, rich in zeaxanthin, ca. 10 μg. g−1 total carotenoids) or corn without carotenoids for 30 d [136]. Sequencing (16S rRNA) of feces demonstrated that, though the carotenoid-rich diet did not alter the microbiome diversity, 162 amplicon sequence variants differed in abundance in the carotenoid-treated vs. the control group, and abundance of Epulopiscium was higher in the carotenoid group. Another porcine study investigated changes related to intestinal inflammation induced by weaning: 24 piglets were distributed into a normal suckling group, and weaning piglets were randomized to receive somewhat supra-physiological carotenoid doses (either 40 mg. kg−1 body weight β-carotene or 80 mg. kg−1 body weight β-carotene) for 2 weeks [119]. Pigs receiving β-carotene had significantly decreased species from the Bacteroidetes phyla and the genus Prevotella and Blautia. The authors reasoned that reducing Prevotella especially could be of interest, because of its correlation with pro-inflammatory conditions. Furthermore, β-carotene increased abundance of the phyla Firmicutes and the genera p-75-a5 and Parabacteroides vs. the control group, believed to be because of changes in inflammatory cytokines. Parabacteroides and Synergistes correlated inversely with interleukin-1β (IL-1β), IL-6, and TNF-α concentrations, likewise p-75-a5 inversely correlated with IL-6 in serum, pointing to important activations in the immune system in young piglets. Indeed, results were related to a reduction of NF-κB in the colon, also indicative of a decreased pro-inflammatory state. GM diversity indices (i.e., Chao1 and ACE) also improved with β-carotene consumption. Summarizing these animal studies, it appears that carotenoid consumption altered the abundance of various phyla and genera, and that these changes were inversely correlated with inflammatory signaling.
In terms of human studies, consumption of a mixture of blackcurrant powder, lactoferrin, and lutein (nonspecified quantities) for 2 weeks significantly increased Bifidobacteria and Lactobacilli populations but reduced levels of β-glucuronidase (associated with colon cancer) producing Bacteroides spp. and Clostridium spp. [137] in healthy subjects. However, a similar mixture tested without lactoferrin and lutein had similar effects, suggesting that the effects may have been because of compounds in the blackcurrant extract. In a human association study, 11 taxonomic units of GM were significantly associated with serum carotenoid concentrations [73]. Specifically, serum carotenoid concentrations were negatively correlated with the abundance of Firmicutes and positively correlated with the abundance of Bacteroidetes. Similar results were found in a study with pregnant women, with plasma carotenoid concentrations positively correlating with α-diversity [138]. In cystic fibrosis patients, dietary intake of β-carotene was related to a higher Firmicutes/Bacteroides ratio [139]. Of course, these effects may be explained by carotenoids serving as a marker for fruit and vegetable, and thus fiber, intake. Most notably, in a 1-mo intervention trial, lycopene was tested in obese adults, and provided alone (7 mg or 10 mg/d) or incorporated into dark chocolate (7 mg/d), vs. respective controls (i.e., placebo, dark chocolate) [13]. Lycopene dose-dependently increased the relative abundance of Bifidobacterium adolescentis and B. longum, together with Lactobacilli. Also, dose-dependent reductions of LDL-C, LDL-peroxidase, and MDA as markers of oxidative stress (OS) were noted. Because of its low bioavailability, lycopene may reach the colon at an especially high proportion [140, 141]. The main open questions were whether lycopene directly stimulated the growth of health-beneficial bacteria or if these were indirect effects, i.e., whether the improvements in blood lipids, OS, etc., induced changes in the GM.
As carotenoids are unlikely to act as energy sources and thus as direct prebiotics for bacteria, additional modes of actions have been investigated. It is plausible that carotenoids contribute to reduced (OS) in the gut, including the colon, preventing ROS formation that may be detrimental to some bacteria [138, 142, 143]. Such effects were shown for an antioxidant peptide which decreased intracellular bacterial ROS, improving bacterial survival in the presence of antibiotics [144]. The importance of ROS for intestinal physiology, including GM and the epithelium, has been emphasized earlier. For instance, Firmicutes taxa were shown to be influenced by OS and the presence of antioxidants, with, e.g., higher numbers of Lactobacilli spp. at lower OS induced by lipoic acid [145]. Such mechanisms may be more plausible in eliciting effects on the GM, rather than through fermentation/metabolism of carotenoids, though data in this area is lacking.
Another effect by which carotenoids could alter GM composition may be because of potential bactericidal effects, though it is questionable whether sufficiently high concentrations are reached in the colon to exert such effects. Though selective suppression of pathogenic microorganisms in vivo has not been shown, carotenoids have demonstrated bactericidal properties ex vivo. For instance, an apple nonpolar (diethyl ether) extract (later diluted in DMSO) containing violaxanthin and zeaxanthin/lutein reduced H. pylori numbers at an MIC50 (50% minimum inhibitory concentration) of a carotenoid concentration of 36 μg. mL−1. Though the effects could have also been because of other apple constituents (e.g., other triterpenes), similar concentrations, though high, could be reachable in vivo after a carotenoid-rich meal or supplements, and the observed effects were not unlike the antibiotic metronidazole, also employed in this study [146]. In another study, citrus (Shatian pummelo) peel carotenoids reduced growth of especially E. coli, but also of potentially pathogenic bacteria including B. subtilis, S. aureus, Aspergillus niger, A. flavus, and Penicillium chrysogenum [147], with MIC50 of 19–140 μg. mL−1, concentrations that are achievable with carotenoid-rich meals. Lycopene in tomato oleoresin inhibited pathogenic Pseudomonas aeruginosa (MIC50: 150 μg. mL−1), whereas MIC50 for other bacteria (e.g., E. coli) were higher [148]. Carotenoid-rich extracts (annatto, carrot, tomato, ca. 0.1–1.0 mg. g−1 carotenoids) had antibacterial activity against S. aureus [149]. Fucoxanthin from algae was effective against pathogenic S. aureus at 63 μg. mL−1 concentrations. Similarly, fucoxanthinol, a metabolite of fucoxanthin in the gut, was found effective (at high concentrations of 4.25 mg. mL−1) against S. faecalis, Enterococcus sp., as well as S. aureus, and B. subtilis. This activity was similar to the positive control chloramphenicol tested at 1 mg. mL−1 [105]. Achievable carotenoid concentrations in the colon may be around 50 mg. L−1 (50 μg. mL−1), as supplements with 50 mg are commercially available and a total postprandial liquid volume of digestive fluid of ∼1L is common, not considering water uptake and concentration effects in the colon.
Taken together, carotenoids and carotenoid-rich extracts have been associated with a GM microbial profile that is more closely correlated with positive health attributes; however, what remains to be elucidated are 1) the underlying mechanism(s) and 2) whether changes in GM were a cause of, and not the consequence of, other favorable physiological changes observed.
Carotenoids and IgA
An important factor for gut health is also the host’s immune system, and provitamin A carotenoid metabolites can interact via RAR/RXR nuclear receptors that elicit immune system responses [150]. IgA is the most abundant antibody isotype [151], expressed mainly in the mucosa and the gut. SlgA is the secreted form of IgA, which is formed by the concerted activity of plasma cells and endothelial cells. This results in secreted IgA and a free secretory component (SC) that are released into the colon by transcytosis. For further information, the reader is referred to a more detailed review [151]. SlgA has been reported to protect against enteric pathogens and toxins [152], whereas IgA can bind to specific microbiota, contributing to the gut homeostasis [153]. SC protects immunoglobulins from their proteolytic degradation by proteases. SC also contributes to activating recognition functions on SlgA and soluble IgM (SIgM), resulting in the binding and removal of bacteria [154]. IgA dysfunction has been reported to disturb GM homeostasis, reducing the abundance of Bifidobacterium and Lactobacilli and increasing Gammaproteobacteria [21]. De-regulated gut IgA expression has also been related to many allergies [155].
It is known that retinoids can have cell proliferative effects, and β-carotene (50 mg/kg feed) was shown in weanling mice to increase IgA antibody-secreting cells (ASC), an effect mainly attributed to all-trans retinoic acid [156]. Certainly, all-trans retinoic acid, the most potent form of vitamin A, can bind to retinoic acid receptor β (RARβ) to produce IgA in the mucosa, which may confer some protection, among other possible mechanisms [157]. Similar results were reported earlier by Nishiyama et al. [158]. In this study, pregnant mice received 50 mg. kg−1 β-carotene in the diet 6.5 d postcoitus (dpc) to 14 d postpartum (dpp). In the offspring, IgA concentrations were significantly higher in the stomach, serum, and feces of mice receiving β-carotene vs. a control group. Of note, mice have a much higher cleavage efficiency to convert β-carotene to vitamin A than humans [159]. This further supports vitamin A as the main agonist behind IgA production in this model. Similarly, it is known that mice deficient in vitamin A have compromised IgA secretion of the colonic mucosa [160].
The interaction between carotenoids, GM, and IgA has been reviewed by Lyu et al. [21]. Both astaxanthin and retinoic acid were shown to enhance IgA production, preventing gut dysbiosis presumably via recognition and coating of pathogenic bacteria, preventing their infiltration through the epithelial barrier. However, the mechanism(s) remain unclear. For example, in weanling mice, astaxanthin-enriched yeast (120 mg/(kg. d) feed) increased IgA ASC after 7 d and enhanced small intestinal IgA mRNA expression after 14 d (colonic effects were not studied) [135]. The authors reasoned that astaxanthin could be conferring protection via ROS scavenging properties. However, it was also noted that the yeast alone could have had some IgA stimulatory effects, and this effect was not controlled for. Another study feeding mice with astaxanthin (at 200 mg/(kg. d) body weight for 10 d) or no carotenoids reported reduced bacterial loads of H. pylori in the stomach, reduced mucosal inflammation, and changes in T-lymphocyte response in the astaxanthin animals as compared with controls. This supports a role for astaxanthin in perhaps modulating IgA, though IgA was not measured in this study [161]. In a recent human study with young soccer players [162], astaxanthin supplementation (4 mg/d for 90 d) reduced CRP, and neutrophil levels in the blood compared with a placebo-receiving group. Salivary SlgA concentrations and secretion rate were also significantly increased by ∼20%. In summary, both retinoic acid and astaxanthin consumption favorably appear to regulate IgA and SlgA expression, effects that have been associated with a more favorable GM composition in animal studies.
Carotenoids and gut barrier
Another remarkable property of carotenoids is their influence on gut barrier function, especially gut mucosa integrity and maintenance of tight gap junction functionality. Stevens et al. [28] fed the carotenoid-producing strain Bacillus indicus to piglets, for 23 d. Following necropsy, enhanced expression of tight junction proteins (Tjp1 and occludin) in the mid and distal small intestine, and improved colonic transepithelial electrical resistance were reported, whereas crypt depth and villus height were not influenced. The same bacterial strain fed to obese-overweight humans for 6 weeks was not shown to impact excretion of certain sugars (an objective measure of intestinal barrier function) [28], but other measures of intestinal permeability (e.g., those assessed in the piglet study) were not investigated.
Earlier studies on mice with β-carotene and lycopene likewise showed positive effects on gut barrier functionality. In one of the studies [163], Swiss albino mice received a high-fat diet with or without lycopene (5 or 10 mg/(kg. d) for 12 weeks), provided in combination with or without a prebiotic (isomalto-oligosaccharides, IMO). Lycopene alone improved ileal and colonic health. This was measured by histopathology and reduced Enterobacteriaceae abundance, increased Lactobacilli abundance, increased total SCFA concentrations, and improved systemic markers of OS and inflammation (i.e., increased concentrations of enzymes such as SOD and reduced cytokines, respectively). Additive effects were observed when lycopene + IMO was fed. In another study by Trivedi and Jena [164], β-carotene was given at 3 different doses (0, 5, 10, 20 mg/(kg. d) body weight for 21 d) to mice with induced ulcerative colitis (UC). Compared with controls, β-carotene reduced systemic markers of OS and inflammation, and increased occludin expression in the colon. This was accompanied by reduced plasma lipopolysaccharide concentrations, which also suggested that barrier integrity improved. Colon length and histology scores based on optical appearances were also improved dose-dependently with increasing β-carotene intakes. The authors hypothesized that the increase in Nrf2 expression related to OS prevention contributed to the mucosal protection. Vitamin A and β-carotene also contributed to gut-tight junction functionality in infants of HIV-positive mothers [165]. In this study, mothers received 1.5 mg retinyl palmitate and 30 mg β-carotene daily, in addition to 60 mg retinyl palmitate at delivery. Improved gut barrier function was demonstrated via mannitol and lactulose appearance in urine after an oral challenge. Because these women were at increased risk of vitamin A depletion and deficiency, the important role of vitamin A in mucus production from goblet cells was likely at least partially responsible for the effects observed [166]. In a study by Wang et al. [167], mice received 0.03% lycopene in a high-fat diet for 10 weeks. Lycopene was shown to improve intestinal barrier properties as shown by occludin and claudin-1 in the jejunum (colon was not measured), but also decreased plasma lipopolysaccharide levels, relative to a control group.
An earlier study has highlighted that gut bacteria may reduce the mucosal lining if dietary fiber is low [27], as these glycoproteins could serve as an alternative energy source. Likewise, bacterial shifts may also lead to changes in species important for the maintenance of the mucus layer, i.e., depending on the abundance and activity of mucin degraders. A reduced thickness of the mucus layer has been associated with an increased risk of allergen uptake, as well as the development of colitis and infections, as reviewed elsewhere [168]. A damaged mucus layer in the GI tract impacts microbial colonization and stability by changing the adherence and the organization of the GM and therefore changing the composition of communities [169]. Therefore, changes such as reductions in Akkermansia muciniphila could be important for optimal mucosa integrity and gut health [170]. Utilization of certain carotenoids has been shown to reduce the abundance of Akkermansia, which impacts the integrity of the gut mucosa, as reviewed previously [14]. Lactobacillus spp., Lactobacillus reuteri, and Bifidobacterium longum have also been reported to contribute to increased mucus layer thickness and growth [171]. Djuric et al. [73] found in a human study that carotenoids in plasma correlated with 11 operational taxonomic units, including a positive association with Bacteroidetes and a negative association with Firmicutes. It is also important to consider that carotenoids may merely serve as an indicator of a healthy diet. For example, the Mediterranean diet, which is carotenoid-rich, was also generally found to be associated with lower presence of Firmicutes and Lachnospiraceae [172]. Thus, though a direct relationship between carotenoid consumption and gut mucosa thickness is still missing, these associations merit further study to establish if such a causal relationship exists. In summary, several carotenoids may improve gut barrier functionality via increasing expression of occludin and claudin-1, increased mucus production, altered GM, and potentially antioxidant and anti-inflammatory effects.
In conclusion, there is mounting evidence that carotenoids can contribute to colonic health. Due to limited absorption in the small intestine, the majority of carotenoids are passed on to the colon, where they may act via different mechanisms. Though carotenoids are unlikely to present any significant source of energy for bacteria, potential mechanisms include alterations of the GM composition, likely via direct bactericidal effects, effects on IgA, or altering local OS levels. Additional effects such as improving tight junction integrity are also likely. Gaps in our knowledge include elucidating causal relationships between carotenoids and the mechanisms discussed herein. They also include questions on the effects of apo-carotenoids on GM, the structure and potential function of bacterially-derived metabolites, and also to what extent carotenoids or apo-carotenoid metabolites could be absorbed from the colon. A rather novel approach is the colonization of the GM with carotenoid-producing strains, which could be a promising alternative to consuming carotenoid-rich food items for carotenoid delivery. More studies on the relationship of carotenoids to the GM and colon-related health are needed.
Author disclosures
The authors report no conflict of interest.
Acknowledgments and Author Contributions
All authors were involved in the conception and the writing of the manuscript. The authors’ responsibilities were as follows: AE and TB designed the outline of the manuscript and wrote large parts of it; REK wrote the chapter on carotenoid processing during digestion and on apo-carotenoids and further proofread the manuscript; ISA and NC contributed in writing several sub-chapters regarding carotenoid microbiota metabolism and synthesis; all authors validated the final content, have read and approved the final version.
Contributor Information
Abdulkerim Eroglu, Email: aeroglu@ncsu.edu.
Torsten Bohn, Email: Torsten.Bohn@lih.lu.
Funding
I.S.A. was supported by NCSU CBE startup funds and the Ministry of Higher Education - Oman. R.E.K. received salary support from USDA National Institute of Food and Agriculture, Hatch project W5122. A.E. received funds from the USDA NIFA AFRI Grant Award, Number: 2022-67018-37188.
References
- 1.Böhm V., Lietz G., Olmedilla-Alonso B., Phelan D., Reboul E., Bánati D., et al. From carotenoid intake to carotenoid blood and tissue concentrations – implications for dietary intake recommendations. Nutr Rev. 2021;79(5):544–573. doi: 10.1093/nutrit/nuaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross A.C., Harrison E.H. In: Handbook of Vitamins. JWS Janos Zempleni, JF Gregory III, PJ Stover. Harrison AC Ross, Earl H., editors. CRC Press; 2013. Vitamin A: nutritional aspects of retinoids and carotenoids. [Google Scholar]
- 3.Sugiura M., Nakamura M., Ogawa K., Ikoma Y., Yano M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res Care. 2015;3(1) doi: 10.1136/bmjdrc-2015-000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buijsse B., Feskens E.J., Schlettwein-Gsell D., Ferry M., Kok F.J., Kromhout D., et al. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (Seneca) Am J Clin Nutr. 2005;82(4):879–886. doi: 10.1093/ajcn/82.4.879. [DOI] [PubMed] [Google Scholar]
- 5.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26(6):459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34(11):907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Bohn T., Desmarchelier C., El S.N., Keijer J., van Schothorst E., Rühl R., et al. Beta-carotene in the human body: metabolic bioactivation pathways - from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78(1):68–87. doi: 10.1017/S0029665118002641. [DOI] [PubMed] [Google Scholar]
- 8.Lem D.W., Davey P.G., Gierhart D.L., Rosen R.B. A systematic review of carotenoids in the management of age-related macular degeneration. Antioxidants (Basel, Switzerland) 2021;10(8) doi: 10.3390/antiox10081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmarchelier C., Borel P. Overview of carotenoid bioavailability determinants: from dietary factors to host genetic variations. Trends Food Sci Technol. 2017;69(Part B):270–280. [Google Scholar]
- 10.Haskell M.J. The challenge to reach nutritional adequacy for vitamin A: beta-carotene bioavailability and conversion--evidence in humans. Am J Clin Nutr. 2012;96(5):1193S. doi: 10.3945/ajcn.112.034850. 203S. [DOI] [PubMed] [Google Scholar]
- 11.Bohn T. Bioactivity of carotenoids – chasms of knowledge. Int J Vitam Nutr Res. 2017;87(1–2):5–9. doi: 10.1024/0300-9831/a000400. [DOI] [PubMed] [Google Scholar]
- 12.Linnewiel-Hermoni K., Motro Y., Miller Y., Levy J., Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic Biol Med. 2014;75:105–120. doi: 10.1016/j.freeradbiomed.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Wiese M., Bashmakov Y., Chalyk N., Nielsen D.S., Krych Ł., Kot W., et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. BioMed Res Int. 2019 doi: 10.1155/2019/4625279. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingeo G., Brito A., Samouda H., Iddir M., La Frano M.R., Bohn T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020;11(10):8444–8471. doi: 10.1039/d0fo01483d. [DOI] [PubMed] [Google Scholar]
- 15.Crovesy L., Masterson D., Rosado E.L. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251–1262. doi: 10.1038/s41430-020-0607-6. [DOI] [PubMed] [Google Scholar]
- 16.John G.K., Mullin G.E. The gut microbiome and obesity. Curr Oncol Rep. 2016;18(7):45. doi: 10.1007/s11912-016-0528-7. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L., Wen X.L. Gut microbiota and inflammatory bowel disease: the current status and perspectives. World J Clin Cases. 2021;9(2):321–333. doi: 10.12998/wjcc.v9.i2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohn T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants (Basel) 2019;8(6):E179. doi: 10.3390/antiox8060179. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J., Balbuena E., Miller B., Eroglu A. The role of β-carotene in colonic inflammation and intestinal barrier integrity. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.723480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyu Y., Wu L., Wang F., Shen X., Lin D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp Biol Med (Maywood). 2018;243(7):613–620. doi: 10.1177/1535370218763760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciano F., Vajro P. In: Gastrointestinal tissue. Gracia-Sancho J., Salvadó J., editors. Academic Press; 2017. Chapter 8 - Oxidative stress and gut microbiota; pp. 113–123. [Google Scholar]
- 24.McLoughlin R.F., Berthon B.S., Jensen M.E., Baines K.J., Wood L.G. Short-chain fatty acids, prebiotics, Synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(3):930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- 25.Byndloss M.X., Olsan E.E., Rivera-Chávez F., Tiffany C.R., Cevallos S.A., Lokken K.L., et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dana N., Vaseghi G., Haghjooy Javanmard S. Crosstalk between peroxisome proliferator-activated receptors and toll-like receptors: a systematic review. Adv Pharm Bull. 2019;9(1):12–21. doi: 10.15171/apb.2019.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353. doi: 10.1016/j.cell.2016.10.043. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens Y., Pinheiro I., Salden B., Duysburgh C., Bolca S., Degroote J., et al. Effect of a carotenoid-producing Bacillus strain on intestinal barrier integrity and systemic delivery of carotenoids: a randomised trial in animals and humans. J Funct Foods. 2021;80 [Google Scholar]
- 29.Reboul E. Mechanisms of carotenoid intestinal absorption: where do we stand? Nutrients. 2019;11(4) doi: 10.3390/nu11040838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopec R.E., Failla M.L. Recent advances in the bioaccessibility and bioavailability of carotenoid and effects of other dietary lipophiles. J Food Compost Anal. 2018;68:16–30. [Google Scholar]
- 31.Soukoulis C., Bohn T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit Rev Food Sci Nutr. 2018;58(1):1–36. doi: 10.1080/10408398.2014.971353. [DOI] [PubMed] [Google Scholar]
- 32.Iddir M., Porras Yaruro J.F., Larondelle Y., Bohn T. Gastric lipase can significantly increase lipolysis and carotenoid bioaccessibility from plant food matrices in the harmonized INFOGEST static in vitro digestion model. Food Funct. 2021;12(19):9043–9053. doi: 10.1039/d1fo00786f. [DOI] [PubMed] [Google Scholar]
- 33.Saqui-Salces M., Dowdle W.E., Reiter J.F., Merchant J.L. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 2012;26(8):3127–3139. doi: 10.1096/fj.11-197426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gargouri Y., Pieroni G., Riviere C., Sauniere J.F., Lowe P.A., Sarda L., et al. Kinetic assay of human gastric lipase on short- and long-chain triacylglycerol emulsions. Gastroenterology. 1986;91(4):919–925. doi: 10.1016/0016-5085(86)90695-5. [DOI] [PubMed] [Google Scholar]
- 35.Hussein M.O., Hoad C.L., Wright J., Singh G., Stephenson M.C., Cox E.F., et al. Fat emulsion intragastric stability and droplet size modulate gastrointestinal responses and subsequent food intake in young adults. J Nutr. 2015;145(6):1170–1177. doi: 10.3945/jn.114.204339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verkempinck S., Pallares Pallares A., Hendrickx M., Grauwet T. Processing as a tool to manage digestive barriers in plant-based foods: recent advances. Curr Opin Food Sci. 2020;35:1–9. [Google Scholar]
- 37.Rich G.T., Bailey A.L., Faulks R.M., Parker M.L., Wickham M.S., Fillery-Travis A. Solubilization of carotenoids from carrot juice and spinach in lipid phases: I. Modeling the gastric lumen. Lipids. 2003;38(9):933–945. doi: 10.1007/s11745-003-1147-0. [DOI] [PubMed] [Google Scholar]
- 38.Tyssandier V., Reboul E., Dumas J.F., Bouteloup-Demange C., Armand M., Marcand J., et al. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G913–G923. doi: 10.1152/ajpgi.00410.2002. [DOI] [PubMed] [Google Scholar]
- 39.Salvia-Trujillo L., McClements D.J. Improvement of beta-carotene bioaccessibility from dietary supplements using excipient nanoemulsions. J Agric Food Chem. 2016;64(22):4639–4647. doi: 10.1021/acs.jafc.6b00804. [DOI] [PubMed] [Google Scholar]
- 40.Goupy P., Genot C., Hammaz F., Halimi C., Caris-Veyrat C., Borel P. Mechanisms governing the transfer of pure and plant matrix carotenoids toward emulsified triglycerides. Mol Nutr Food Res. 2020;64(7) doi: 10.1002/mnfr.201900911. [DOI] [PubMed] [Google Scholar]
- 41.Dai L., Zhou L., Zhou H., Zheng B., Ji N., Xu X., et al. Comparison of lutein bioaccessibility from dietary supplement-excipient nanoemulsions and nanoemulsion-based delivery systems. J Agric Food Chem. 2021;69(46):13925–13932. doi: 10.1021/acs.jafc.1c05261. [DOI] [PubMed] [Google Scholar]
- 42.Yi J., Fan Y., Zhang Y., Zhao L. Characterization of catechin-α-lactalbumin conjugates and the improvement in β-carotene retention in an oil-in-water nanoemulsion. Food Chem. 2016;205:73–80. doi: 10.1016/j.foodchem.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Salvia-Trujillo L., Verkempinck S.H., Sun L., Van Loey A.M., Grauwet T., Hendrickx M.E. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: influence of emulsion droplet size. Food Chem. 2017;229:653–662. doi: 10.1016/j.foodchem.2017.02.146. [DOI] [PubMed] [Google Scholar]
- 44.Li Z., Zhong S., Meshram N., Kopec R. The influence of increasing lecithin concentrations on carotenoid bioaccessibility and cell uptake. Curr Dev Nutr. 2022;6(Supplement_1):61. [Google Scholar]
- 45.Tan Y., Zhang Z., Liu J., Xiao H., McClements D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): oil. Food Funct. 2020;11(11):9936–9946. doi: 10.1039/d0fo01505a. [DOI] [PubMed] [Google Scholar]
- 46.Tan Y., Zhang Z., Zhou H., Xiao H., McClements D.J. Factors impacting lipid digestion and β-carotene bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): oil droplet concentration. Food Funct. 2020;11(8):7126–7137. doi: 10.1039/d0fo01506g. [DOI] [PubMed] [Google Scholar]
- 47.Böhm V. Intestinal absorption of lycopene from different types of oleoresin capsules. J Food Sci. 2002;67(5):1910–1913. [Google Scholar]
- 48.Biehler E., Hoffmann L., Krause E., Bohn T. Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J Nutr. 2011;141(10):1769–1776. doi: 10.3945/jn.111.143388. [DOI] [PubMed] [Google Scholar]
- 49.Phan M.A.T., Bucknall M.P., Arcot J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem. 2019;276:402–409. doi: 10.1016/j.foodchem.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Nie M., Zhang Z., Liu C., Li D., Huang W., Liu C., et al. Hesperetin and hesperidin improved β-carotene incorporation efficiency, intestinal cell uptake, and retinoid concentrations in tissues. J Agric Food Chem. 2019;67(12):3363–3371. doi: 10.1021/acs.jafc.9b00551. [DOI] [PubMed] [Google Scholar]
- 51.Reboul E., Thap S., Tourniaire F., André M., Juhel C., Morange S., et al. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamins C and E) on lutein absorption. Br J Nutr. 2007;97(3):440–446. doi: 10.1017/S0007114507352604. [DOI] [PubMed] [Google Scholar]
- 52.Hernell O., Staggers J.E., Carey M.C. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29(8):2041–2056. doi: 10.1021/bi00460a012. [DOI] [PubMed] [Google Scholar]
- 53.Yao Y., Tan P., Kim J.E. Effects of dietary fats on the bioaccessibility and bioavailability of carotenoids: a systematic review and meta-analysis of in vitro studies and randomized controlled trials. Nutr Rev. 2022;80(4):741–761. doi: 10.1093/nutrit/nuab098. [DOI] [PubMed] [Google Scholar]
- 54.Reboul E., Richelle M., Perrot E., Desmoulins-Malezet C., Pirisi V., Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem. 2006;54(23):8749–8755. doi: 10.1021/jf061818s. [DOI] [PubMed] [Google Scholar]
- 55.Nagao A., Kotake-Nara E., Hase M. Effects of fats and oils on the bioaccessibility of carotenoids and vitamin E in vegetables. Biosci Biotechnol Biochem. 2013;77(5):1055–1060. doi: 10.1271/bbb.130025. [DOI] [PubMed] [Google Scholar]
- 56.Huo T., Ferruzzi M.G., Schwartz S.J., Failla M.L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55(22):8950–8957. doi: 10.1021/jf071687a. [DOI] [PubMed] [Google Scholar]
- 57.Borel P., Tyssandier V., Mekki N., Grolier P., Rochette Y., Alexandre-Gouabau M.C., et al. Chylomicron beta-carotene and retinyl palmitate responses are dramatically diminished when men ingest beta-carotene with medium-chain rather than long-chain triglycerides. J Nutr. 1998;128(8):1361–1367. doi: 10.1093/jn/128.8.1361. [DOI] [PubMed] [Google Scholar]
- 58.Goncalves A., Roi S., Nowicki M., Dhaussy A., Huertas A., Amiot M.J., et al. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155–160. doi: 10.1016/j.foodchem.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Corte-Real J., Iddir M., Soukoulis C., Richling E., Hoffmann L., Bohn T. Effect of divalent minerals on the bioaccessibility of pure carotenoids and on physical properties of gastro-intestinal fluids. Food Chem. 2016;197(A):546–553. doi: 10.1016/j.foodchem.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 60.Borel P., Desmarchelier C., Dumont U., Halimi C., Lairon D., Page D., et al. Dietary calcium impairs tomato lycopene bioavailability in healthy humans. Br J Nutr. 2016;116(12):2091–2096. doi: 10.1017/S0007114516004335. [DOI] [PubMed] [Google Scholar]
- 61.Mensi A., Borel P., Goncalves A., Nowicki M., Gleize B., Roi S., et al. β-lactoglobulin as a vector for β-carotene food fortification. J Agric Food Chem. 2014;62(25):5916–5924. doi: 10.1021/jf501683s. [DOI] [PubMed] [Google Scholar]
- 62.Iddir M., Dingeo G., Porras Yaruro J.F., Hammaz F., Borel P., Schleeh T., et al. Influence of soy and whey protein, gelatin and sodium caseinate on carotenoid bioaccessibility. Food Funct. 2020;11(6):5446–5459. doi: 10.1039/d0fo00888e. [DOI] [PubMed] [Google Scholar]
- 63.Iddir M., Pittois D., Guignard C., Weber B., Gantenbein M., Larondelle Y., et al. Whey- and soy protein isolates added to a carrot-tomato juice alter carotenoid bioavailability in healthy adults. Antioxidants (Basel). 2021;10(11) doi: 10.3390/antiox10111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopec R.E., Caris-Veyrat C., Nowicki M., Gleize B., Carail M., Borel P. Production of asymmetric oxidative metabolites of [13C]-β-carotene during digestion in the gastrointestinal lumen of healthy men. Am J Clin Nutr. 2018;108(4):803–813. doi: 10.1093/ajcn/nqy183. [DOI] [PubMed] [Google Scholar]
- 65.Kopec R.E., Caris-Veyrat C., Nowicki M., Bernard J.P., Morange S., Chitchumroonchokchai C., et al. The effect of an iron supplement on lycopene metabolism and absorption during digestion in healthy humans. Mol Nutr Food Res. 2019;63(22) doi: 10.1002/mnfr.201900644. [DOI] [PubMed] [Google Scholar]
- 66.McKimmie R.L., Easter L., Weinberg R.B. Acyl chain length, saturation, and hydrophobicity modulate the efficiency of dietary fatty acid absorption in adult humans. Am J Physiol Gastrointest Liver Physiol. 2013;305(9):G620–G627. doi: 10.1152/ajpgi.00258.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dawson P.A., Lan T., Rao A. Bile acid transporters. J Lipid Res. 2009;50(12):2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.USDA . USDA; 2015. National Nutrient Database for Standard Reference Release 28.http://ndb.nal.usda.gov/ndb/foods/show [Internet] Available from: [Google Scholar]
- 69.Briviba K., Schnäbele K., Rechkemmer G., Bub A. Supplementation of a diet low in carotenoids with tomato or carrot juice does not affect lipid peroxidation in plasma and feces of healthy men. J Nutr. 2004;134(5):1081–1083. doi: 10.1093/jn/134.5.1081. [DOI] [PubMed] [Google Scholar]
- 70.Gireesh T., Sudhakaran P.R. In vitro uptake of beta-carotene by human exfoliated colonic epithelial cells. Int J Food Sci Nutr. 2009;60(2):109–118. doi: 10.1080/09637480701610525. [DOI] [PubMed] [Google Scholar]
- 71.Levy E., Ménard D., Suc I., Delvin E., Marcil V., Brissette L., et al. Ontogeny, immunolocalisation, distribution and function of SR-BI in the human intestine. J Cell Sci. 2004;117(2):327–337. doi: 10.1242/jcs.00856. [DOI] [PubMed] [Google Scholar]
- 72.Sen A., Ren J., Ruffin M.T., Turgeon D.K., Brenner D.E., Sidahmed E., et al. Relationships between serum and colon concentrations of carotenoids and fatty acids in randomized dietary intervention trial. Cancer Prev Res (Phila). 2013;6(6):558–565. doi: 10.1158/1940-6207.CAPR-13-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Djuric Z., Bassis C.M., Plegue M.A., Ren J., Chan R., Sidahmed E., et al. Colonic mucosal bacteria are associated with inter-individual variability in serum carotenoid concentrations. J Acad Nutr Diet. 2018;118(4):606–616. doi: 10.1016/j.jand.2017.09.013. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grolier P., Borel P., Duszka C., Lory S., Alexandre-Gouabau M.C., Azais-Braesco V., et al. The bioavailability of alpha- and beta-carotene is affected by gut microflora in the rat. Br J Nutr. 1998;80(2):199–204. [PubMed] [Google Scholar]
- 75.Kopec R.E., Riedl K.M., Harrison E.H., Curley R.W., Jr., Hruszkewycz D.P., Clinton S.K., et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58(6):3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleshman M.K., Lester G.E., Riedl K.M., Kopec R.E., Narayanasamy S., Curley R.W., Jr., et al. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of beta-carotene bioaccessibility and bioavailability. J Agric Food Chem. 2011;59(9):4448–4454. doi: 10.1021/jf200416a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreno J.C., Mi J., Alagoz Y., Al-Babili S. Plant apocarotenoids: from retrograde signaling to interspecific communication. Plant J. 2021;105(2):351–375. doi: 10.1111/tpj.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J.Y., Lin P.Y., Al-Babili S. On the biosynthesis and evolution of apocarotenoid plant growth regulators. Semin Cell Dev Biol. 2021;109:3–11. doi: 10.1016/j.semcdb.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Koschmieder J., Wüst F., Schaub P., Álvarez D., Trautmann D., Krischke M., et al. Plant apocarotenoid metabolism utilizes defense mechanisms against reactive carbonyl species and xenobiotics. Plant Physiol. 2021;185(2):331–351. doi: 10.1093/plphys/kiaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leber A., Hontecillas R., Tubau-Juni N., Zoccoli-Rodriguez V., Goodpaster B., Bassaganya-Riera J. Abscisic acid enriched fig extract promotes insulin sensitivity by decreasing systemic inflammation and activating LANCL2 in skeletal muscle. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-67300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behrouz V., Dastkhosh A., Hedayati M., Sedaghat M., Sharafkhah M., Sohrab G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: a pilot study. Diabetol Metab Syndr. 2020;12:59. doi: 10.1186/s13098-020-00568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrison E.H., Quadro L. Apocarotenoids: emerging roles in mammals. Annu Rev Nutr. 2018;38:153–172. doi: 10.1146/annurev-nutr-082117-051841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goupy P., Reynaud E., Dangles O., Caris-Veyrat C. Antioxidant activity of (all-E)-lycopene and synthetic apo-lycopenoids in a chemical model of oxidative stress in the gastro-intestinal tract. New J Chem. 2012;36(3):575–587. [Google Scholar]
- 84.Kopec R.E., Gleize B., Borel P., Desmarchelier C., Caris-Veyrat C. Are lutein, lycopene, and beta-carotene lost through the digestive process? Food Funct. 2017;8(4):1494–1503. doi: 10.1039/c7fo00021a. [DOI] [PubMed] [Google Scholar]
- 85.Kanner J., Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31(11):1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 86.Sy C., Caris-Veyrat C., Dufour C., Boutaleb M., Borel P., Dangles O. Inhibition of iron-induced lipid peroxidation by newly identified bacterial carotenoids in model gastric conditions: comparison with common carotenoids. Food Funct. 2013;4(5):698–712. doi: 10.1039/c3fo30334a. [DOI] [PubMed] [Google Scholar]
- 87.Vulcain E., Goupy P., Caris-Veyrat C., Dangles O. Inhibition of the metmyoglobin-induced peroxidation of linoleic acid by dietary antioxidants: action in the aqueous vs. lipid phase. Free Radic Res. 2005;39(5):547–563. doi: 10.1080/10715760500073865. [DOI] [PubMed] [Google Scholar]
- 88.Sy C., Dangles O., Borel P., Caris-Veyrat C. Iron-induced oxidation of (all-E)-beta-carotene under model gastric conditions: kinetics, products, and mechanism. Free Radic Biol Med. 2013;63:195–206. doi: 10.1016/j.freeradbiomed.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 89.Lindqvist A., He Y.G., Andersson S. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem. 2005;53(11):1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 90.Kiefer C., Hessel S., Lampert J.M., Vogt K., Lederer M.O., Breithaupt D.E., et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276(17):14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 91.von Lintig J., Moon J., Lee J., Ramkumar S. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(11) doi: 10.1016/j.bbalip.2019.158580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amengual J., Lobo G.P., Golczak M., Li H.N., Klimova T., Hoppel C.L., et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25(3):948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maeda T., Perusek L., Amengual J., Babino D., Palczewski K., von Lintig J. Dietary 9-cis-β,β-carotene fails to rescue vision in mouse models of Leber congenital amaurosis. Mol Pharmacol. 2011;80(5):943–952. doi: 10.1124/mol.111.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrison E.H., Kopec R.E. Enzymology of vertebrate carotenoid oxygenases. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(11) doi: 10.1016/j.bbalip.2020.158653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly M.E., Ramkumar S., Sun W., Colon Ortiz C., Kiser P.D., Golczak M., et al. The biochemical basis of vitamin A production from the asymmetric carotenoid β-cryptoxanthin. ACS Chem Biol. 2018;13(8):2121–2129. doi: 10.1021/acschembio.8b00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ross A.C., Restori K.H. In: Diet, immunity and inflammation. PC Calder. Yaqoob P., editor. Woodhead Publishing; 2013. Chapter 8 - Vitamin A and the immune system; pp. 221–243. [Google Scholar]
- 97.Harrison E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta. 2012;1821(1):70–77. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Menning A., Loddenkemper C., Westendorf A.M., Szilagyi B., Buer J., Siewert C., et al. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol. 2010;40(9):2539–2548. doi: 10.1002/eji.200939938. [DOI] [PubMed] [Google Scholar]
- 99.Widjaja-Adhi M.A.K., Palczewski G., Dale K., Knauss E.A., Kelly M.E., Golczak M., et al. Transcription factor ISX mediates the cross talk between diet and immunity. Proc Natl Acad Sci U S A. 2017;114(43):11530–11535. doi: 10.1073/pnas.1714963114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eroglu A., Hruszkewycz D.P., dela Sena C., Narayanasamy S., Riedl K.M., Kopec R.E., et al. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287(19):15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bandara S., Thomas L.D., Ramkumar S., Khadka N., Kiser P.D., Golczak M., et al. The structural and biochemical basis of apocarotenoid processing by β-carotene Oxygenase-2. ACS Chem Biol. 2021;16(3):480–490. doi: 10.1021/acschembio.0c00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferreiro A., Crook N., Gasparrini A.J., Dantas G. Multiscale evolutionary dynamics of host-associated microbiomes. Cell. 2018;172(6):1216–1227. doi: 10.1016/j.cell.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hill M.J. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6(Suppl 1) doi: 10.1097/00008469-199703001-00009. S43–5. [DOI] [PubMed] [Google Scholar]
- 104.Nagao A. Absorption and function of dietary carotenoids. Forum Nutr. 2009;61:55–63. doi: 10.1159/000212738. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z., Sun X., Sun X., Wang S., Xu Y. Fucoxanthin Isolated from Undaria pinnatifida Can InterAct with Escherichia coli and lactobacilli in the Intestine and Inhibit the Growth of Pathogenic Bacteria. J Ocean Univ China. 2019;18(4):926–932. [Google Scholar]
- 106.Hutkins R.W., Krumbeck J.A., Bindels L.B., Cani P.D., Fahey G., Jr., Goh Y.J., et al. Prebiotics: why definitions matter. Curr Opin Biotechnol. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li M.Y., Xu Z.S., Tian C., Huang Y., Wang F., Xiong A.S. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci Rep. 2016;6 doi: 10.1038/srep23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dias M.G., Olmedilla-Alonso B., Hornero-Méndez D., Mercadante A.Z., Osorio C., Vargas-Murga L., et al. Comprehensive database of carotenoid contents in Ibero-American foods. A valuable tool in the context of functional foods and the establishment of recommended intakes of bioactives. J Agric Food Chem. 2018;66(20):5055–5107. doi: 10.1021/acs.jafc.7b06148. [DOI] [PubMed] [Google Scholar]
- 109.Li C., Swofford C.A., Sinskey A.J. Modular engineering for microbial production of carotenoids. Metab Eng Commun. 2020;10 doi: 10.1016/j.mec.2019.e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saini R.K., Keum Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: an updated review of critical issues. J Ind Microbiol Biotechnol. 2019;46(5):657–674. doi: 10.1007/s10295-018-2104-7. [DOI] [PubMed] [Google Scholar]
- 111.Choi K.R., Yu H.E., Lee S.Y. Microbial food: microorganisms repurposed for our food. Microb Biotechnol. 2022;15(1):18–25. doi: 10.1111/1751-7915.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C., Zhao S., Shao X., Park J.B., Jeong S.H., Park H.J., et al. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb Cell Fact. 2019;18(1):55. doi: 10.1186/s12934-019-1105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meadows A.L., Hawkins K.M., Tsegaye Y., Antipov E., Kim Y., Raetz L., et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 2016;537(7622):694–697. doi: 10.1038/nature19769. [DOI] [PubMed] [Google Scholar]
- 114.Chatzivasileiou A.O., Ward V., Edgar S.M., Stephanopoulos G. Two-step pathway for isoprenoid synthesis. Proc Natl Acad Sci U S A. 2019;116(2):506–511. doi: 10.1073/pnas.1812935116. 201812935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heavey M.K., Durmusoglu D., Crook N., Anselmo A.C. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol. 2022;40(3):354–369. doi: 10.1016/j.tibtech.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sola-Oladokun B., Culligan E.P., Sleator R.D. Engineered probiotics: applications and biological containment. Annu Rev Food Sci Technol. 2017;8:353–370. doi: 10.1146/annurev-food-030216-030256. [DOI] [PubMed] [Google Scholar]
- 117.Durmusoglu D., Al’Abri I.S., Collins S.P., Cheng J., Eroglu A., Beisel C.L., et al. In situ biomanufacturing of small molecules in the mammalian gut by probiotic Saccharomyces boulardii. ACS Synth Biol. 2021;10(5):1039–1052. doi: 10.1021/acssynbio.0c00562. [DOI] [PubMed] [Google Scholar]
- 118.Durmusoglu D., Catella C.M., Purnell E.F., Menegatti S., Crook N.C. Design and in situ biosynthesis of precision therapies against gastrointestinal pathogens. Curr Opin Physiol. 2021;23 [Google Scholar]
- 119.Li R., Li L., Hong P., Lang W., Hui J., Yang Y., et al. β-carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Anim Biosci. 2021;34(7):1221–1234. doi: 10.5713/ajas.19.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 2016;14(8) doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collins S., Reid G. Distant site effects of ingested prebiotics. Nutrients. 2016;8(9) doi: 10.3390/nu8090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hughes R.L., Alvarado D.A., Swanson K.S., Holscher H.D. The prebiotic potential of inulin-type fructans: a systematic review. Adv Nutr. 2021;13(2):492–529. doi: 10.1093/advances/nmab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shokri D., Khorasgani M.R., Mohkam M., Fatemi S.M., Ghasemi Y., Taheri-Kafrani A. The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob Proteins. 2018;10(1):34–42. doi: 10.1007/s12602-017-9267-9. [DOI] [PubMed] [Google Scholar]
- 124.Liu H., Wang J., He T., Becker S., Zhang G., Li D., et al. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9(1):21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Eeckhaut V., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 126.Ramos A.F.O., Terry S.A., Holman D.B., Breves G., Pereira L.G.R., Silva A.G.M., et al. Tucumã oil shifted ruminal fermentation, reducing methane production and altering the microbiome but decreased substrate digestibility within a RUSITEC fed a mixed hay - concentrate diet. Front Microbiol. 2018;9:1647. doi: 10.3389/fmicb.2018.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 129.Stojanov S., Berlec A., Štrukelj B. The Influence of probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and inflammatory bowel disease. Microorganisms. 2020;8(11) doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heintz-Buschart A., Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018;26(7):563–574. doi: 10.1016/j.tim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 131.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mapelli-Brahm P., Barba F.J., Remize F., Garcia C., Fessard A., Mousavi Khaneghah A., et al. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: an overview. Trends Food Sci Technol. 2020;99:389–401. [Google Scholar]
- 133.Ram S., Mitra M., Shah F., Tirkey S.R., Mishra S. Bacteria as an alternate biofactory for carotenoid production: a review of its applications, opportunities and challenges. J Funct Foods. 2020;67 [Google Scholar]
- 134.Wu T., Gao Y., Hao J., Geng J., Zhang J., Yin J., et al. Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice. Food Res Int. 2020;128 doi: 10.1016/j.foodres.2019.108774. [DOI] [PubMed] [Google Scholar]
- 135.Nagayama T., Sugimoto M., Ikeda S., Kume S. Effects of astaxanthin-enriched yeast on mucosal IgA induction in the jejunum and ileum of weanling mice. Anim Sci J. 2014;85(4):449–453. doi: 10.1111/asj.12154. [DOI] [PubMed] [Google Scholar]
- 136.González-Prendes R., Pena R.N., Solé E., Seradj A.R., Estany J., Ramayo-Caldas Y. Modulatory Effect of Protein and carotene Dietary Levels on Pig gut microbiota. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-51136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Molan A.L., Liu Z., Plimmer G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother Res. 2014;28(3):416–422. doi: 10.1002/ptr.5009. [DOI] [PubMed] [Google Scholar]
- 138.Schmidt K.M., Haddad E.N., Sugino K.Y., Vevang K.R., Peterson L.A., Koratkar R., et al. Dietary and plasma carotenoids are positively associated with alpha diversity in the fecal microbiota of pregnant women. J Food Sci. 2021;86(2):602–613. doi: 10.1111/1750-3841.15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li L., Krause L., Somerset S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin Nutr. 2017;36(4):1097–1104. doi: 10.1016/j.clnu.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 140.Böhm V., Bitsch R. Intestinal absorption of lycopene from different matrices and interactions to other carotenoids, the lipid status, and the antioxidant capacity of human plasma. Eur J Nutr. 1999;38(3):118–125. doi: 10.1007/s003940050052. [DOI] [PubMed] [Google Scholar]
- 141.Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. 2008;4(4):240–258. [Google Scholar]
- 142.Klimenko N.S., Tyakht A.V., Popenko A.S., Vasiliev A.S., Altukhov I.A., Ischenko D.S., et al. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients. 2018;10(5):576. doi: 10.3390/nu10050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lv Z., Wang Y., Yang T., Zhan X., Li Z., Hu H., et al. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 2016;59(2):113–121. doi: 10.3164/jcbn.15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sannasimuthu A., Sharma D., Paray B.A., Al-Sadoon M.K., Arockiaraj J. Intracellular oxidative damage due to antibiotics on gut bacteria reduced by glutathione oxidoreductase-derived antioxidant molecule GM15. Arch Microbiol. 2020;202(5):1127–1133. doi: 10.1007/s00203-020-01825-y. [DOI] [PubMed] [Google Scholar]
- 145.Qiao Y., Sun J., Ding Y., Le G., Shi Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl Microbiol Biotechnol. 2013;97(4):1689–1697. doi: 10.1007/s00253-012-4323-6. [DOI] [PubMed] [Google Scholar]
- 146.Molnár P., Kawase M., Satoh K., Sohara Y., Tanaka T., Tani S., et al. Biological activity of carotenoids in red paprika, Valencia orange and Golden delicious apple. Phytother Res. 2005;19(8):700–707. doi: 10.1002/ptr.1735. [DOI] [PubMed] [Google Scholar]
- 147.Tao N., Gao Y., Liu Y., Ge F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am Eur J Agric Environ Sci. 2010;7:110–115. [Google Scholar]
- 148.Ranjbar A., Ranjbar E. Antimicrobial property of lycopene oleoresin on some food pathogens running head: lycopene oleoresin antibacterial potent (Brief report) Food Sci Technol Res;J. 2016;(12):382–387. [Google Scholar]
- 149.Natividad L.R., Rafael R.R. Carotenoid analyses and antibacterial assay of annato (Bixa orellana L.), carrot (Daucus carota L.), corn (Zea mays L.) and tomato (Solanum Lycopersicum L.) extracts. Res J rec sci. 2014;3:40–45. [Google Scholar]
- 150.Bohn T., Bonet M.L., Borel P., Keijer J., Landrier J.F., Milisav I., et al. Mechanistic aspects of carotenoid health benefits - where are we now? Nutr Res Rev. 2021;34(2):276–302. doi: 10.1017/S0954422421000147. [DOI] [PubMed] [Google Scholar]
- 151.Pabst O., Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13(1):12–21. doi: 10.1038/s41385-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mantis N.J., Forbes S.J. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol Invest. 2010;39(4–5):383–406. doi: 10.3109/08820131003622635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huus K.E., Bauer K.C., Brown E.M., Bozorgmehr T., Woodward S.E., Serapio-Palacios A., et al. Commensal bacteria modulate immunoglobulin A binding in response to host nutrition. Cell Host Microbe. 2020;27(6):909–921. doi: 10.1016/j.chom.2020.03.012. e5. [DOI] [PubMed] [Google Scholar]
- 154.Stadtmueller B.M., Huey-Tubman K.E., López C.J., Yang Z., Hubbell W.L., Bjorkman P.J. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. eLife. 2016;5 doi: 10.7554/eLife.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo J., Ren C., Han X., Huang W., You Y., Zhan J. Role of IgA in the early-life establishment of the gut microbiota and immunity: implications for constructing a healthy start. Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nishida K., Sugimoto M., Ikeda S., Kume S. Effects of supplemental β-carotene on mucosal IgA induction in the jejunum and ileum of mice after weaning. Br J Nutr. 2014;111(2):247–253. doi: 10.1017/S0007114513002195. [DOI] [PubMed] [Google Scholar]
- 157.Seo G.Y., Jang Y.S., Kim J., Choe J., Han H.J., Lee J.M., et al. Retinoic acid acts as a selective human IgA switch factor. Hum Immunol. 2014;75(8):923–929. doi: 10.1016/j.humimm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 158.Nishiyama Y., Sugimoto M., Ikeda S., Kume S. Supplemental β-carotene increases IgA-secreting cells in mammary gland and IgA transfer from milk to neonatal mice. Br J Nutr. 2011;105(1):24–30. doi: 10.1017/S0007114510003089. [DOI] [PubMed] [Google Scholar]
- 159.Lee C.M., Boileau A.C., Boileau T.W., Williams A.W., Swanson K.S., Heintz K.A., et al. Review of animal models in carotenoid research. J Nutr. 1999;129(12):2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 160.Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 161.Bennedsen M., Wang X., Willén R., Wadström T., Andersen L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol Lett. 1999;70(3):185–189. doi: 10.1016/s0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 162.Baralic I., Andjelkovic M., Djordjevic B., Dikic N., Radivojevic N., Suzin-Zivkovic V., et al. Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/783761. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh D.P., Khare P., Zhu J., Kondepudi K.K., Singh J., Baboota R.K., et al. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int J Obes (Lond). 2016;40(3):487–496. doi: 10.1038/ijo.2015.197. [DOI] [PubMed] [Google Scholar]
- 164.Trivedi P.P., Jena G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur J Nutr. 2015;54(4):639–652. doi: 10.1007/s00394-014-0745-5. [DOI] [PubMed] [Google Scholar]
- 165.Filteau S.M., Rollins N.C., Coutsoudis A., Sullivan K.R., Willumsen J.F., Tomkins A.M. The effect of antenatal vitamin A and beta-carotene supplementation on gut integrity of infants of HIV-infected South African women. J Pediatr Gastroenterol Nutr. 2001;32(4):464–470. doi: 10.1097/00005176-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 166.Fan X., Liu S., Liu G., Zhao J., Jiao H., Wang X., et al. Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLOS ONE. 2015;10(9) doi: 10.1371/journal.pone.0139131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang J., Wang Z., Li B., Qiang Y., Yuan T., Tan X., et al. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut-liver-brain axis. Int J Obes (Lond). 2019;43(9):1735–1746. doi: 10.1038/s41366-018-0277-9. [DOI] [PubMed] [Google Scholar]
- 168.Parrish A., Boudaud M., Kuehn A., Ollert M., Desai M.S. Intestinal mucus barrier: a missing piece of the puzzle in food allergy. Trends Mol Med. 2022;28(1):36–50. doi: 10.1016/j.molmed.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Hansson G.C. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15(1):57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van der Lugt B., van Beek A.A., Aalvink S., Meijer B., Sovran B., Vermeij W.P., et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1-/Δ7 mice. Immun Ageing. 2019;16:6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paone P., Cani P.D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gutiérrez-Díaz I., Fernández-Navarro T., Sánchez B., Margolles A., González S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 2016;7(5):2347–2356. doi: 10.1039/c6fo00105j. [DOI] [PubMed] [Google Scholar]
- 173.Guo B., Yang B., Pang X., Chen T., Chen F., Cheng K.W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019;10(9):5644–5655. doi: 10.1039/c9fo01018a. [DOI] [PubMed] [Google Scholar]
- 174.Xia H., Liu C., Li C.C., Fu M., Takahashi S., Hu K.Q., et al. Dietary tomato powder inhibits high-fat diet-promoted hepatocellular carcinoma with alteration of gut microbiota in mice lacking carotenoid cleavage enzymes. Cancer Prev Res (Phila). 2018;11(12):797–810. doi: 10.1158/1940-6207.CAPR-18-0188. [DOI] [PubMed] [Google Scholar]
- 175.Dhanawade S.S., Sakhare A.V. Isolation of lycopene from tomato and study of its antimicrobial activity. Int J Sci Res. 2014;3:671. 3. [Google Scholar]
- 176.NengGuo T., YuMei G., YueJin L., Fei G. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am Eurasian J Agric Environ Sci. 2010;7:110–115. [Google Scholar]
- 177.Wu J., Tian X., Xu X., Gu X., Kong J., Guo T. Engineered probiotic Lactococcus lactis for lycopene production against ROS Stress in intestinal epithelial cells. ACS Synth Biol. 2022;11(4):1568–1576. doi: 10.1021/acssynbio.1c00639. [DOI] [PubMed] [Google Scholar]
- 178.Henke N.A., Heider S.A.E., Hannibal S., Wendisch V.F., Peters-Wendisch P. Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front Microbiol. 2017;8:633. doi: 10.3389/fmicb.2017.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Larroude M., Celinska E., Back A., Thomas S., Nicaud J.M., Ledesma-Amaro R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol Bioeng. 2018;115(2):464–472. doi: 10.1002/bit.26473. [DOI] [PubMed] [Google Scholar]
- 180.Gao S., Tong Y., Zhu L., Ge M., Zhang Y., Chen D., et al. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab Eng. 2017;41:192–201. doi: 10.1016/j.ymben.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 181.Ma T., Shi B., Ye Z., Li X., Liu M., Chen Y., et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng. 2019;52:134–142. doi: 10.1016/j.ymben.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 182.Zhou C., Li Z., Wiberley-Bradford A.E., Weise S.E., Sharkey T.D. Isopentenyl diphosphate and dimethylallyl diphosphate/isopentenyl diphosphate ratio measured with recombinant isopentenyl diphosphate isomerase and isoprene synthase. Anal Biochem. 2013;440(2):130–136. doi: 10.1016/j.ab.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 183.Mantzouridou F., Roukas T., Achatz B. Effect of oxygen transfer rate on β-carotene production from synthetic medium by Blakeslea trispora in shake flask culture. Enzyme Microb Technol. 2005;37(7):687–694. [Google Scholar]
- 184.Wu T., Ye L., Zhao D., Li S., Li Q., Zhang B., et al. Membrane engineering - A novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab Eng. 2017;43(A):85–91. doi: 10.1016/j.ymben.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 185.Kang W., Ma T., Liu M., Qu J., Liu Z., Zhang H., et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nat Commun. 2019;10(1):4248. doi: 10.1038/s41467-019-12247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Coussement P., Bauwens D., Maertens J., De Mey M. Direct combinatorial pathway optimization. ACS Synth Biol. 2017;6(2):224–232. doi: 10.1021/acssynbio.6b00122. [DOI] [PubMed] [Google Scholar]
- 187.Mantzouridou F.T., Naziri E. Scale translation from shaken to diffused bubble aerated systems for lycopene production by Blakeslea trispora under stimulated conditions. Appl Microbiol Biotechnol. 2017;101(5):1845. doi: 10.1007/s00253-016-7943-4. 56. [DOI] [PubMed] [Google Scholar]
- 188.Chen Y., Xiao W., Wang Y., Liu H., Li X., Yuan Y. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact. 2016;15(1):113. doi: 10.1186/s12934-016-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Su A., Chi S., Li Y., Tan S., Qiang S., Chen Z., et al. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J Agric Food Chem. 2018;66(23):5879–5885. doi: 10.1021/acs.jafc.8b00855. [DOI] [PubMed] [Google Scholar]
- 190.Park S.Y., Binkley R.M., Kim W.J., Lee M.H., Lee S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab Eng. 2018;49:105–115. doi: 10.1016/j.ymben.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 191.Zhang C., Seow V.Y., Chen X., Too H.P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat Commun. 2018;9(1):1858. doi: 10.1038/s41467-018-04211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin J., Wang Y., Yao M., Gu X., Li B., Liu H., et al. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol Biofuels. 2018;11:230. doi: 10.1186/s13068-018-1227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Henke N.A., Wiebe D., Pérez-García F., Peters-Wendisch P., Wendisch V.F. Coproduction of cell-bound and secreted value-added compounds: simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour Technol. 2018;247:744–752. doi: 10.1016/j.biortech.2017.09.167. [DOI] [PubMed] [Google Scholar]
- 194.Pollmann H., Breitenbach J., Sandmann G. Engineering of the carotenoid pathway in Xanthophyllomyces dendrorhous leading to the synthesis of zeaxanthin. Appl Microbiol Biotechnol. 2017;101(1):103–111. doi: 10.1007/s00253-016-7769-0. [DOI] [PubMed] [Google Scholar]